Switching a medicinal ingredient from prescription to non-prescription status draft guidance document: Applying for a switch
On this page
- 6. Classification of a product resulting from a successful switch
- 7. Understanding the overall switch processes
- 8. Requesting pre-submission meeting
- 9. Assembling NDS or SNDS (all switches)
- 10. Formatting and filing an NDS or SNDS (all switches)
- 11. Paying fees (all switches)
- 12. Health Canada assesses the NDS or SNDS
- 13. Health Canada consults the public
- 14. Health Canada announces its intent to amend the PDL
- 15. Health Canada issues a DIN (Rx to NPD switches only)
- 16. Filing a Product Licence Application (Rx to NHP switches only)
- 17. Health Canada verifies the PLA
- 18. Health Canada amends the PDL and issues the product authorization
6. Classification of a product resulting from a successful switch
The applicant needs to determine whether their proposed product would be classified as an NHP or NPD, if the switch were successful. This determination will assist the applicant in identifying which process, the Rx to NHP switch process or the Rx to NPD switch process, applies to their situation.
The applicant should verify whether, following a successful switch, the proposed product would meet the definition of an NHP as set out in the NHPR. If so, a successful switch results in the product being classified as an NHP. Otherwise, it is classified as an NPD under the FDR.
When considering whether the proposed product’s ingredients are acceptable in an NHP, the applicant should consult the definition of “natural health product” in the NHPR, Schedule 1 and 2 of the NHPR as well as the Natural Health Products Ingredients Database (NHPID).
7. Understanding the overall switch processes
This section provides an overview of the switch processes for Rx to NPD and Rx to NHP switches and is followed by sections 8 to 19 that provide additional guidance on the steps of the process.
7.1 Process 1: A successful Rx to NPD switch
The following is the main process for an Rx to NPD switch that leads to issuance or update of a product authorization:
- The applicant assembles the pre-submission meeting data package for Health Canada and requests a pre-submission meeting. (See section 8 for further details.)
- The applicant meets with Health Canada for a pre-submission meeting to present and discuss the data package for the proposed switch. This meeting may lead the applicant to conduct further studies.
- The applicant assembles the final version of the NDS or SNDS including the necessary data on safety, efficacy and quality; product labelling; and the “PDL Principles and Factors Assessment”. (Section 9)
- The applicant files the NDS or SNDS with Health Canada in the appropriate format and pays the applicable fees. (Sections 10 and 11)
- Health Canada screens the submission for completeness. If there are no deficiencies, the submission proceeds into review.
- Health Canada assesses the submission including the information submitted in the PDL Principles and Factors Assessment. If Health Canada’s assessment is positive, the process continues. (Section 12)
- Health Canada posts a public “Notice of Consultation” on the canada.ca Website outlining its proposal to remove the medicinal ingredient, or remove the medicinal ingredient for certain conditions of use, from the PDL and puts the NDS or SNDS on switch hold. (Section 13)
- Health Canada reviews the comments provided by the public and other stakeholders during the PDL consultation. (Section 13)
- After analysis of the comments, if Health Canada decides to proceed, Health Canada posts a “Notice of Intent to Amend” which outlines when the amendment to the PDL will occur. (Section 14)
- Health Canada issues the Drug Identification Number (DIN) for the proposed product if required. (Section 15)
- Health Canada amends the PDL and posts a “Notice of Amendment” to that effect. (Section 18)
- Health Canada issues a Notice of Compliance (NOC) for the NPD. (Section 18.1)
- If in addition to the NOC, the appropriate DEL(s) have been issued to those conducting activities related to the product (e.g. fabricate, import), the product can be sold in Canada in accordance with the FDR. (Section 19.4.1)
Flowchart 1: A successful Rx to NPD switch

Text description
The steps for the process just described (section 7.1) are illustrated in a flowchart. Additionally, the image shows that all steps of this process fall under the FDR and steps 7, 8, 9 and 11 are part of the PDL process.
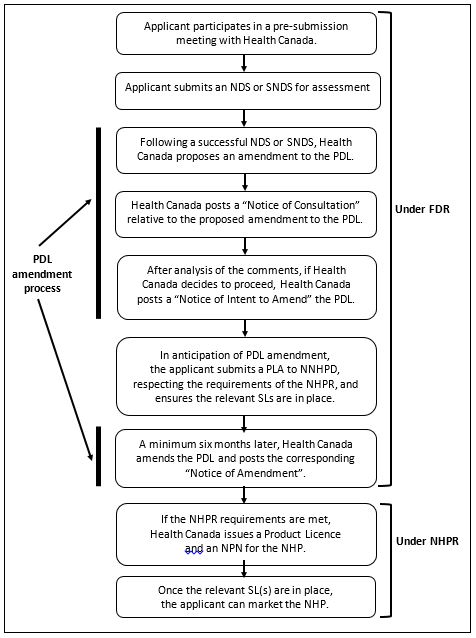
7.2 Process 2: A successful Rx to NHP switch
The following is the main process for an Rx to NHP switch that leads to the issuance of a product authorization. Note that the first nine steps of this process are the same as those for an Rx to NPD switch.
- The applicant assembles the pre-submission meeting data package for Health Canada and requests a pre-submission meeting. (See section 8 further details.)
- The applicant meets with Health Canada for a pre-submission meeting to present and discuss the data package for the proposed switch. This meeting may lead the applicant to conduct further studies.
- The applicant assembles the final version of the NDS or SNDS including the necessary data on safety, efficacy and quality; product labelling; and the “PDL Principles and Factors Assessment”. (Section 9)
- The applicant files the NDS or SNDS with Health Canada in the appropriate format and pays the applicable fees. (Section 10 and 11)
- Health Canada screens the submission for completeness. If there are no deficiencies, the submission proceeds into review.
- Health Canada assesses the submission including the information submitted in the PDL Principles and Factors Assessment. If Health Canada’s assessment is positive, the process continues. (Section 12)
- Health Canada posts a public “Notice of Consultation” on the Canada.ca Website outlining its proposal to remove the medicinal ingredient, or remove the medicinal ingredient for certain conditions of use, from the PDL and puts the NDS or SNDS on switch hold. (Section 13)
- Health Canada reviews the comments provided by the public and other stakeholders during the consultation.(Section 13)
- After analysis of the comments, if Health Canada decides to proceed, Health Canada posts a “Notice of Intent to Amend” which outlines when the amendment to the PDL will occur. (Section 14)
- The applicant then files a PLA in accordance with the NHPR reflecting the NDS and SNDS information in anticipation of the PDL amendment. (Section 16)
- Health Canada verifies the PLA. (Section 17)
- Health Canada amends the PDL and posts a “Notice of Amendment”. (Section 18)
- Health Canada issues a Notice of Non-Compliance (NON) for the NDS or SNDS and, if applicable, cancels the DIN(s) because the product is no longer a drug under the FDR. (Section 18.2)
- If the applicant has satisfied the requirements of the NHPR, Health Canada issues the Product Licence and the Natural Product Number (NPN) for the product. (Section 18.2)
- If in addition to the Product Licence and NPN, the appropriate SL has been issued to those conducting activities related to the product (i.e. manufacture, import, package and/or label), the product can be sold in Canada in accordance with the NHPR. (Section 19.4.2)
Flowchart 2: Successful Rx to NHP switch
Text description
The steps for the process just described (section 7.2) are illustrated in a flowchart. Additionally, the image shows that steps 1- 9, 12 and 13 of this process fall under the FDR; steps 14 and 15 are under the NHPR; and steps 7, 8, 9, and 12 are part of the PDL process.
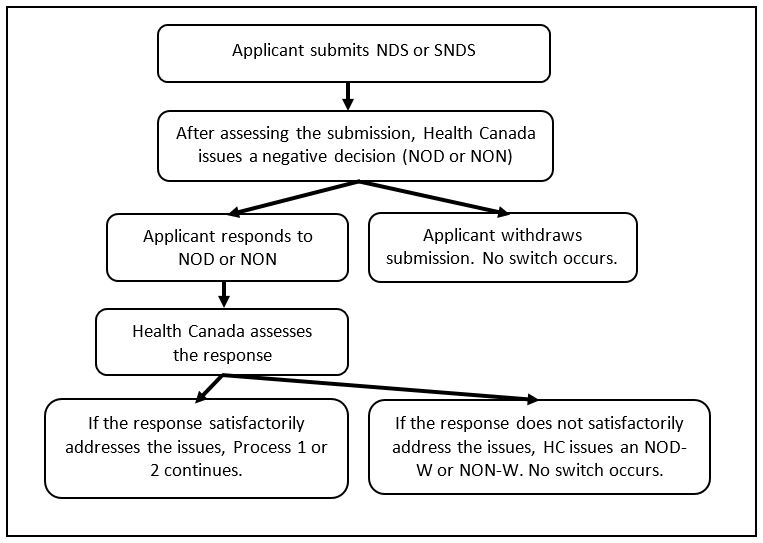
7.3 Process 3: The assessment of submission leads to a negative decision
Not all switch submissions will be successful. Process 3 outlines the steps that would occur if the applicant has not successfully demonstrated to Health Canada in the NDS or SNDS that
- the product has met the safety, efficacy, and quality requirements; and/or,
- the PDL principles and factors do not apply to the product.
Process 3:
- If the applicant does not successfully demonstrate the above, Health Canada issues a Notice of Deficiency (NOD) or a NON.
- The applicant responds to the NOD/NON or withdraws the submission.
- If the applicant responds, Health Canada assesses the response.
- If the response does not satisfactorily address the issues, Health Canada issues a NOD-Withdrawal (NOD-W) or NON-Withdrawal (NON-W). There is no change to the PDL.
- If the response satisfactorily addresses the issues, the switch process (as described in Process 1 for successful Rx to NPD switches or Process 2 for successful Rx to NHP switches) would continue.
For more information on NODs, NONs, NOD-Ws and NON-Ws, please consult the guidance document “Management of Drug Submissions and Applications”.
Some other examples of where a switch may fail include the following:
- an incomplete NDS or SNDS package
- significant stakeholder objections being raised during the PDL consultation which cannot be appropriately addressed otherwise (e.g. additional data demonstrating new safety concerns / need for practitioner oversight)
- for Rx to NHP switches, an incomplete PLA and/or a failure to meet the requirements of the NHPR in the second part of the switch process
Flowchart 3: Assessment of submission leading to a negative decision
Text description
The steps described at the beginning of section 7.3 are illustrated in a flowchart.
8. Requesting a pre-submission meeting
Prior to filing an NDS or an SNDS, the applicant is strongly encouraged to request a pre-submission meeting with Health Canada to discuss questions the applicant has related to the adequacy of their evidence in support of the proposed switch. For example, before undertaking clinical trials or consumer use studies, the applicant should meet with Health Canada. Note that it is possible for a company to have more than one pre-submission meeting.
For information on pre-submission meetings for an NDS or SNDS, the applicant should consult section 7 of the guidance document “Management of Drug Submissions and Applications”.
| Type of Switch: | In the pre-submission meeting, the applicant will meet with: |
|---|---|
Rx to NHP switches |
|
Rx to NPD switches |
|
9. Assembling NDS or SNDS (all switches)
Submission Type
The applicant assembles an NDS or an SNDS requesting the switch. The type of submission required depends on the specific situation.
Situations requiring an NDS
- If the proposed switch relates to a currently authorized “Division 1” prescription drug, the applicant files an NDS relative to the proposed non-prescription status product, as this switch represents a change in the conditions of use (namely, the sale in a non-prescription setting without practitioner oversight) as per C.08.002 of the FDR.
- If there is no existing currently authorized prescription drug, the applicant files an NDS as per C.08.002 of FDR.
- If the proposed switch would result in:
- the applicant’s currently-authorized “Division 8” prescription drug becoming an NHP or NPD with changes to the conditions of use relative to those authorized for prescription drug; and
- the prescription drug remaining on the market for some of its other conditions of use;
then the applicant files an NDS, as per C.08.002 of FDR, relative to the proposed non-prescription status product as it will be an additional product introduced to the market. In this way, future changes to the non-prescription status product can be tracked against the new authorization separate from the prescription drug authorization. (Note that if the switch is successful, the applicant will also file an SNDS relative to their currently authorized prescription drug to reflect the removal of some of its conditions of use.)
Situations requiring an SNDS
- If the proposed switch would result in:
- the applicant’s currently-authorized “Division 8” prescription drug becoming an NHP or NPD, with or without changes to the conditions of use, and
- the prescription drug no longer existing on the market,
then the applicant files an SNDS, as per C.08.003 of the FDR.
Submission content
In the NDS or SNDS, the applicant includes the following content:
- the necessary information on safety, efficacy and quality of the proposed product
- the applicant’s PDL Principles and Factors Assessment
- the proposed labelling
9.1 Evidence of safety, efficacy and quality
For both Rx to NHP and Rx to NPD switches, the applicant files an NDS or SNDS in which the applicant provides evidence to demonstrate the safety, efficacy and quality of the proposed product. The amount of evidence will depend on the type of switch the applicant is proposing, as outlined below.
9.1.1 The applicant is proposing a switch of an authorized prescription drug without changes
Generally, in this type of switch, the safety, efficacy and quality of the product have already been demonstrated in the submission(s) for the authorized prescription drug. Therefore, the applicant generally submits less evidence than for other types of switches. (The only condition of use that is changing is the context in which the product is sold.)
At a minimum, the applicant provides the following:
- the most recent Health Canada authorized Product Monograph or Prescribing Information for the prescription drug along with an annotated version of the proposed changes
- any available post-market information
- any more recent clinical trial data, if available, investigating the safety of the drug under similar conditions of use along with the appropriate clinical and non-clinical overviews/summaries
- consumer use studies
9.1.2 The applicant is proposing the switch of an authorized prescription drug that includes changes to its conditions of use
The conditions of use of an authorized prescription drug are specified in the Product Monograph or Prescribing Information. If the applicant is proposing changes to the conditions of use as part of the switch then additional evidence will be required. Examples of how the proposed NHP or NPD could differ from the authorized prescription drug include changes to the indication, maximum single and/or daily dose, strength of the dosage unit, route of administration, dosage form, manufacturing, formulation and target population.
The nature of the changes to the product and the conditions of use will determine what evidence is required. If applicable, the applicant can build on the data previously submitted to Health Canada for the authorized prescription drug.
The applicant is encouraged to seek guidance from the NNHPD for Rx to NPD switches and from the relevant TPD review bureau for Rx to NHP switches regarding the need for, and scope of, the data that would be required.
9.1.3 The applicant is proposing a switch and does not own a related authorized prescription drug
In these instances, the applicant provides a full data package to demonstrate the safety, efficacy and quality of the proposed product.
9.1.4 Outdated data
Applicants should be aware that if they are relying in their submission on safety, efficacy or quality studies that were generated by investigations that do not meet present day standards for safety, efficacy or quality assessments, then additional data may be required. Applicants are encouraged to discuss this type of issue with Health Canada in a pre-submission meeting.
9.1.5 Further information
For more information on the evidence of safety, efficacy and quality that is required, the applicant should consult the following:
- the applicable guidance documents that are available on the Website “Guidance Documents – Applications and submissions – Drug products”
- Health Canada (e.g., in a pre-submission meeting)
- the sections 9.1.1 to 9.1.4 of this document
9.2 PDL principles and factors assessment
The applicant follows the guidance provided in Appendix B, C and D when completing their PDL Principles and Factors Assessment. This is one of the key elements for a successful switch. The template for the assessment document is found in Appendix E. The applicant includes the completed assessment in the NDS or SNDS.
9.3 Labelling for inclusion in the NDS or SNDS
For both Rx to NHP switches and Rx to NPD switches, the applicant follows all the requirements regarding labelling of NPDs when preparing the labels for inclusion in the NDS or SNDS.
The applicant can consult the Government of Canada Website for relevant guidance documents on labelling.
As discussed in Appendix C, the applicant conducts their consumer use studies using a label that closely reflects the final label that consumers will see on the market. This will help achieve the objective of having the data from the consumer use studies accurately reflect how well consumers will be able to understand and apply the ‘final’ labelling information.
For Rx to NPD switches, the labelling must include a Canadian Drug Facts Table (CDFT). For information on CDFT formats and flexibilities, the applicant can consult the guidance document “Labelling Requirements for Non-prescription Drugs”.
For Rx to NHP switches, the applicant has two options:
- The applicant can use labelling with a product facts table or drug facts table in their consumer use studies and for inclusion in the NDS or SNDS, if this Facts Table will be included on the final NHP label.
- The applicant can use labelling without a product facts table or drug facts table in their consumer use studies and for the NDS or SNDS, if the applicant is not intending on having a Facts Table on the final NHP label.
10. Formatting and filing an NDS or SNDS (all switches)
In terms of the format of the submission, the applicant follows the instructions in section 8 of “Management of Drug Submissions and Applications” as well as the guidance documents referenced therein. Furthermore, the applicant includes the PDL Principles and Factors Assessment in Module 1.0.7, the consumer use studies in Module 5 and the summary of the consumer use studies in Module 2.
If there is an authorized prescription drug that is being switched and it was authorized as the result of a paper-based submission, Health Canada encourages the applicant to re-submit the evidence pertaining to authorization of the prescription drug in electronic format to expedite the review.
Information on submission filing procedures are outlined in the guidance document “Management of Drug Submissions and Applications”.
11. Paying fees (all switches)
All applicants pay the cost recovery fees for the assessment of the information submitted in support of their NDS or SNDS. Note that it is the content of the NDS and SNDS that determines the size of the fee and associated performance standard, not whether it is an NDS or SNDS. For example, in 2021, the fee was $224, 242 for a switch that required clinical or non-clinical data as well as chemistry and manufacturing data and did not include a new active substance. The relevant fees for product assessment are found in Schedule 1 of the “Fees in Respect of Drugs and Medical Devices Order”, SOR/2019-124.
For more information on fees, refer to the guidance document “Fees for the Review of Human Drugs and Disinfectant Submissions and Applications”. Note that at the top of this Website, the applicant will find links (the boxes) to other sections of the document which provide information on the fee categories and fee mitigation measures.
12. Health Canada assesses the NDS or SNDS
Health Canada assesses the NDS or SNDS, including the applicant’s PDL Principles and Factors Assessment, to determine if the applicant has successfully demonstrated that:
- the product meets the safety, efficacy and quality requirements of the FDR for product authorization; and
- the PDL principles and factors do not apply to the product.
Specifically, the assessment officers for NPDs in NNHPD assesses the NDSs or SNDSs for Rx to NPD switches and the assessment officers in relevant bureaux in TPD assesses the NDS or SNDS for Rx to NHP switches.
The performance standards for the assessment of the NDS or SNDS under the FDR are outlined in Appendix 3 of the guidance document “Management of Drug Submissions and Applications”.
13. Health Canada consults the public
If the assessment described in section 12 comes to a positive conclusion, Health Canada starts the PDL amendment process.
More information on the PDL and the PDL amendment process can be found in the guidance document entitled “Questions and Answers - Prescription Drug List”.
For switches, Health Canada consults the public and other stakeholders on amendments to the PDL by posting a “Notice of Consultation” to the canada.ca Website. In the Notice of Consultation, Health Canada outlines the proposed amendment to remove the medicinal ingredient or remove the ingredient for certain conditions of use from the PDL. In the second scenario, for example, a medicinal ingredient can be removed from the PDL for only some indications or at lower doses.
At the same time, Health Canada places the NDS or SNDS on switch hold (that is, a temporary pause on the progress of the submission) pending the outcome of the consultation and PDL amendment process.
After the public consultation, Health Canada analyzes the comments received. Depending on the nature of the comments and the issues raised, the analysis could result in Health Canada deciding to:
- proceed,
- modify, or
- no longer pursue the proposed amendment.
If the results of the analysis supports proceeding, the next step will be to publish a Notice of Intent to Amend.
If the proposal needs modification, Health Canada continues the PDL amendment process with a modified version of the proposed amendment or conducts a new consultation depending on the nature of the modification. In the past, modifications have ranged from minor changes in the wording of the qualifier to significant re-working of the proposal. Health Canada communicates these plans to the applicant prior to publishing the Notice of Intent to Amend or the new Notice of Consultation.
If the analysis results in Health Canada deciding not to pursue the amendment, Health Canada communicates with the applicant and issues a notice to the public indicating that Health Canada will not amend the PDL.
14. Health Canada announces its intent to amend the PDL
When the analysis of the consultation comments results in Health Canada moving to the next stage of the PDL process, Health Canada posts a “Notice of Intent to Amend”. This Notice specifies the date when the amendment of the PDL will occur, typically after a minimum six-month transition period. The transition period is in accordance with the international Technical Barriers to Trade (TBT) Agreement and allows market authorization holders of other affected products time to comply with the upcoming new regulatory requirements (e.g., revise labelling).
15. Health Canada issues a DIN (Rx to NPD switches only)
After Health Canada posts the Notice of Intent to Amend the PDL, Health Canada issues the applicant a Drug Notification Form (DNF) with the assigned DIN, if applicable. A new DIN is required along with the NOC, if any of the following apply:
- A DIN has not been previously assigned to the product.
- The applicant is requesting that the switch occur for certain conditions of use of the prescription drug, such that after the switch there will be both the prescription drug and the NPD on the market. The new DIN would be relative to the new NPD.
- A DIN was previously assigned, but the switch changes one or more of the drug characteristics listed in paragraphs C.01.014.1(2)(a) to (f) of the FDR and after the switch there will only be the NPD on the market.
Relative to the last situation, note that the applicant submits a “Notification of discontinuation of sale” to Health Canada for the previously assigned DIN. The notification must be sent within 30 days of the cessation of sale. Health Canada then cancels the previously assigned DIN.
16. Filing a Product Licence Application (Rx to NHP switches only)
After Health Canada posts the Notice of Intent to Amend, the applicant files a second time. This time the applicant submits a web-based Natural Health Product Licence Application (web PLA) to obtain a Product Licence and the NPN. This filing can be either during or after the transition period as Health Canada is willing to contemplate the PLA prior to the PDL amendment, however decisions will only occur under NHPR after the PDL is amended.
The applicant files a web PLA as outlined in the “Natural Health Products Management of Applications Policy”. There are no fees for the assessment of the PLA.
The applicant completes the web PLA Form by accurately reflecting the information in the labelling that was finalized during the NDS or SNDS assessment. Note that the Product Monograph constitutes part of the labelling. Additionally, the applicant indicates in the cover letter of the web PLA Form that “Evidence in support of the NHP Product Licence is contained in the switch submission control # [insert control number assigned to the NDS or SNDS].” The applicant does not need to resubmit evidence that was submitted as part of the NDS or SNDS, nor the PDL Principles and Factors Assessment.
17. Health Canada verifies the PLA
NNHPD in Health Canada verifies the web PLA. If the applicant does all of the following:
- applies within 60 days of the publication of the Notice of Intent to Amend;
- appropriately reflects the final labelling from the NDS or SNDS in the web PLA form; and
- complies with all the requirements of the NHPR,
NNHPD can then issue the Product Licence and the NPN when the PDL is amended. An applicant who submits the web PLA any later than this may not receive their NPN until some time after the PDL amendment occurs.
18. Health Canada amends the PDL and issues the product authorization
Once the transition period is over, Health Canada amends the PDL and posts the “Notice of Amendment” on the canada.ca Website to announce that the amendment to the PDL has occurred.
18.1 Rx to NPD switches
At this time, Health Canada issues the applicant an NOC for the NPD.
18.2 Rx to NHP switches
At this time, Health Canada issues the applicant a NON with respect to the NDS or SNDS on switch hold and, if applicable, cancels the DIN(s) because the product is no longer a drug regulated under the FDR (refer to paragraph C.01.014.6 (1) (c) of the FDR). It is now a product subject to the NHPR.
The applicant then has the option to withdraw the submission or respond to the NON acknowledging the product is no longer a drug under the FDR. In the latter case, upon receiving a response to the NON, Health Canada issues the NON-W re-iterating the product is no longer a drug under the FDR.
Additionally, if all the conditions set out in section 17 of this guidance document are met, Health Canada issues a Product Licence and NPN for the proposed NHP.
Page details
- Date modified: