How Health Canada inspects medical device establishments: About inspections
On this page
- How inspections work
- Health Canada’s authority to inspect
- Where and when inspections take place
- Inspection process
- How Health Canada enforces compliance
How inspections work
Health Canada may inspect anyone who has a medical device establishment licence (MDEL) to ensure they comply with the Act and the Regulations. Those inspections support our national compliance and enforcement program.
During the inspection, inspectors assess whether your establishment (company) complies with the parts of the Act and the Regulations that apply to you. Companies are manufacturers, importers, distributors, persons, partnerships and associations.
Following the inspection, inspectors rate companies as compliant or non-compliant with the Act and Regulations. The inspector determines the rating based on the risk associated with the inspection’s findings.
- Compliant: At the time of the inspection, the establishment has demonstrated that the activities it conducts are in compliance with the Act and the Regulations. A compliant rating does not mean that there are no observations or that corrective actions are not required.
- Non-compliant: At the time of the inspection, the establishment has not demonstrated that the activities it conducts are in compliance with the Act and the Regulations.
For information about the risk ratings, consult:
Focus of inspections
The activities you carry out under your MDEL are the focus of the inspection.
Health Canada inspectors will look at your procedures and processes to detect and respond to risks to safety and effectiveness. They will also look at the label and licence requirements that govern your actions to import, advertise and sell medical devices.
Separate inspections, called audits, assess your compliance with the quality management system standard (ISO 13485) needed to get a medical device licence. These audits are carried out by auditing organizations of the Medical Device Single Audit Program (MDSAP). This type of inspection applies to you if you:
- design and/or manufacture Class II, III or IV devices
- have a valid ISO 13485 quality management system certificate issued by an MDSAP auditing organization
In spite of the audit of your quality management system, Health Canada inspectors may look to see that any Class II, III or IV device you import or sell:
- has a medical device licence
- is labelled properly
Health Canada’s authority to inspect
Under section 22(1) of the Act, Health Canada appoints inspectors to enforce the Act and ensure compliance. Section 23 of the Act also outlines the powers given to inspectors so they can perform their role.
While inspectors focus on MDEL activities, section 23 gives them the power to examine and take action against anything that is not compliant with the Act and Regulations. Inspectors decide on the course of action to take based on the risk the deviation, deficiency or failure poses to health and safety.
Section 23(3) of the Act allows an inspector to enter any place as described in section 23(1) by accessing it remotely by means of telecommunication. The same principles apply to inspections conducted remotely as to in-person inspections.
When entry to the place is in-person, you may or may not be given notice before the inspection. However, when entering remotely by telecommunication, you, the owner or person in charge, will be made aware of the entry. The Policy on accessing the premises of a regulated party remotely to verify compliance (POL-0138) provides additional information about conducting inspections by means of telecommunications.
Where and when inspections take place
Inspections are conducted on-site or remotely by means of telecommunication. On-site inspections are carried out in-person at your establishment or third-party location. Remote inspections are conducted using a means of telecommunication such as videoconferences. An inspection may include both a remote entry and an on-site visit.
Health Canada inspects licensed companies located inside Canada (referred to as “domestic inspections”) and outside of Canada (“foreign inspections”).
We schedule inspections of companies in Canada using a risk-based approach. It includes factors such as compliance history and medical device classification.
Types of inspections
There are 5 types of inspections:
New inspection:
- first inspection of a newly licensed company to verify if its activities comply with the Act and Regulations
Regular:
- most common type of inspection
- routinely scheduled after a company has completed the first inspection
- inspector assesses all the requirements of the Act and Regulations
- refer to Checklist of the provisions of the Regulations that apply to you, for more information
Reassessment:
- inspection of a company that received a compliant rating with observations
- inspector decides that it needs to be inspected sooner than regularly scheduled
- usually takes place within 12 months of the previous inspection
Re-inspection:
- inspection of a company that received a non-compliant rating during a previous inspection
- inspector focuses on the observations identified during the previous inspection when a non-compliant rating occurred
- usually takes place within 12 months of the previous inspection
Targeted inspection:
- (unplanned) inspection conducted following the receipt of information or intelligence from other sources (for example, regulatory partners, consumers, internal branch partners)
- after a preliminary evaluation, the information may seem to indicate that an unacceptable risk to the health and safety of Canadians related to MDEL activities may be present
Amount of time inspections take
Inspections of small companies are generally completed in 1 day. Inspections of larger companies may take from 2 to 5 days.
Note: Inspections will take a different form if you run a “virtual” or online company and do not rely on physical buildings to store your medical devices.
If you operate at more than 1 location, the inspector may also inspect the other sites listed on your MDEL application. This will add more time to your inspection since inspections are typically carried out by a single inspector.
If needed, the lead inspector may ask other inspectors to join the inspection. Also, subsection 23(7) of the Act allows inspectors to be accompanied by any other individual that the inspector believes is necessary to help them exercise their powers or perform their duties.
Inspection process
Inspectors carry out inspections using the following process.
Medical Device Inspection Process
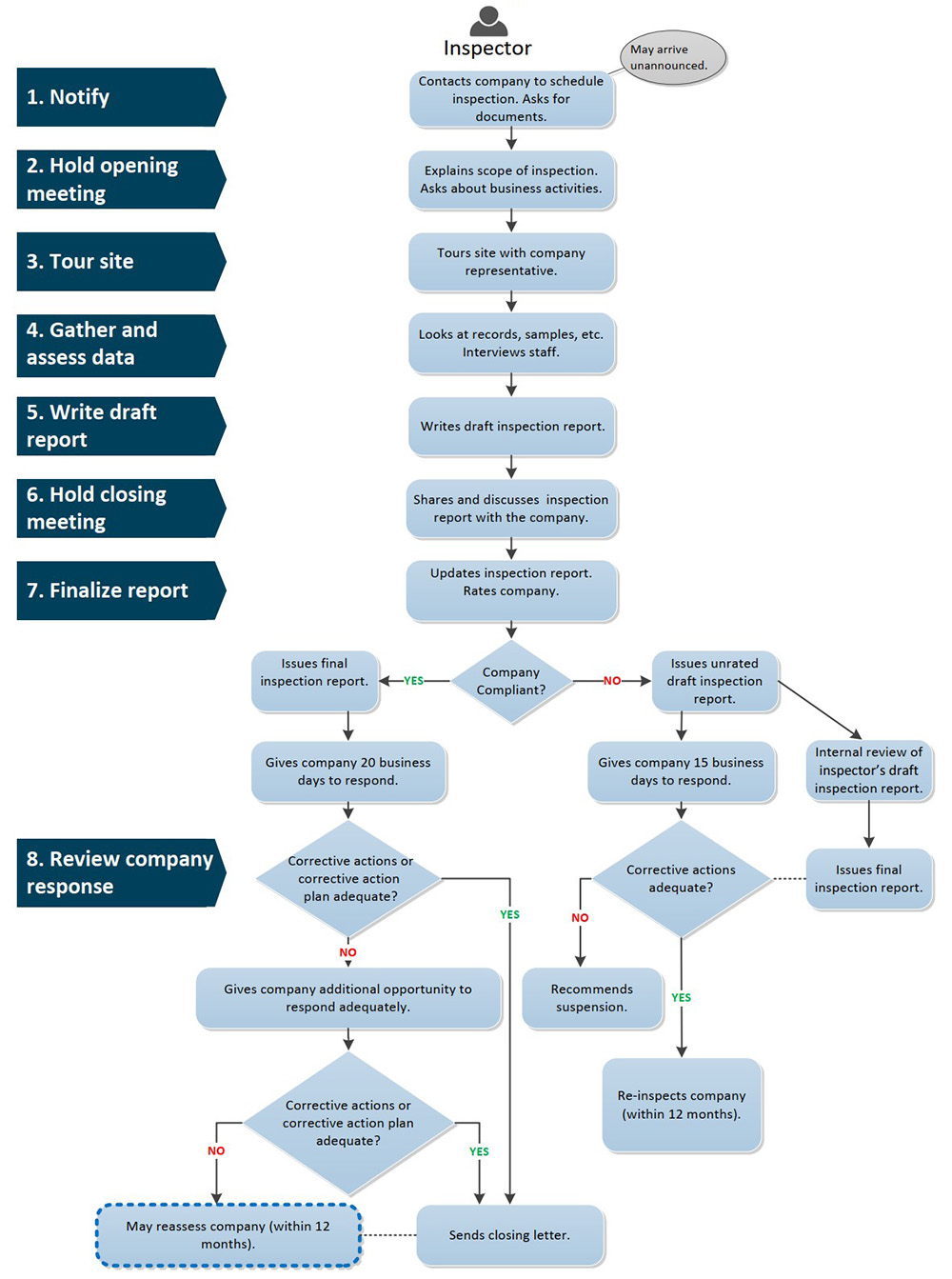
Text description
This flowchart is divided into two sections. The left section indicates the steps required in the inspection process and the right section describes the tasks taken by an inspector.
- Step 1 is entitled “Notify”. In this step, the Inspector contacts the company to schedule an inspection and also asks for documents. The inspector may also arrive unannounced at the company.
- Step 2 is entitled “Hold opening meeting”. The inspector explains to the company the scope of the inspection. The inspector also asks about business activities. This step may be performed in person or remotely.
- Step 3 is entitled “Tour site”. The inspector tours the site with a company representative. The tour may also be conducted using videoconference for a remote inspection.
- Step 4 is entitled “Gather and assess data”. The inspector looks at records, samples, etc. and interviews staff.
- Step 5 is entitled “Write draft report”. The inspector writes a draft inspection report.
- Step 6 is entitled “Hold closing meeting”. The inspector shares and discusses the inspection report with the company.
- Step 7 is entitled “Finalize report”. The inspector updates the inspection report and determines the inspection rating. This part is split into two streams, one where the company is compliant, and the other for a non-compliant company. If the rating is compliant, a final inspection report is issued to the company and they are given 20 business days to respond to the inspection report. If the inspector proposes a non-compliant inspection rating, a draft unrated inspection report is issued to the company and the company is given 15 business days to respond. During this 15-day period, an internal review of the inspector’s draft unrated inspection report is conducted by Health Canada. The final inspection report, with a confirmed inspection rating, is issued to the company following this review.
- Step 8 is entitled “Review company response”. The inspector reviews the company’s response to the inspection report. For a company given a compliant rating, if their corrective actions or corrective action plan is adequate, the inspector sends a closing letter to conclude the inspection. If the corrective actions or the corrective action plan is not adequate, the inspector gives the company additional opportunity to respond in a satisfactory manner. If the corrective actions or the corrective action plan is then adequate, a closing letter is sent to the company. If the corrective actions or the corrective action plan is then inadequate, the company may be reassessed within 12 months.
For a company given a non-compliant rating, if the company’s corrective actions for deficiencies which contributed to a non-compliant rating are considered adequate, the company will be scheduled for a re-inspection within 12 months. If the company’s corrective actions for deficiencies which contributed to a non-compliant rating are inadequate, the inspector recommends the suspension of the company’s establishment licence.
This concludes the flow chart.
Step 1: Notify company and schedule inspection
The inspector will contact you by telephone or email before the inspection to:
- describe the purpose of the inspection, confirm the type of inspection and specify whether your inspection will be conducted on-site or remotely by videoconferencing
- verify information about your MDEL such as your activities, types and classes of devices you offer for sale, type of customers you sell devices to and any other sites involved with the activities of your MDEL
- determine whether medical devices will be available on-site and, if not, ask for samples to help verify label and device licence
- confirm if your company is located at a private dwelling
- ask for details on the accessibility of your records (electronic or paper) and about your company’s technology capabilities for remote access
- confirm the date(s) and location(s) for the on-site inspection or designated dates and timeframes for virtual meetings if your inspection will be conducted remotely
Note: The inspector may choose to arrive:
- without notice if it will result in a more accurate picture of your compliance
- unannounced if unable to reach you by phone or email
The inspector may also ask you to provide:
- a list of all medical devices you are currently selling
- the names of manufacturers for the devices you are selling
- the names of your suppliers for the devices you are selling
- copies of the procedures you have attested to having put in place in your MDEL application or most recent annual licence renewal (refer to checklist for inspections for the procedures you will be expected to make available)
Once the inspection date has been determined, the inspector will confirm the details in writing.
Note: When reviewing your procedures, the inspector will look to see that they:
- define how to complete a task or tasks
- explain how to do the work, who should do it and under what circumstances
- identify who has been assigned any authority or responsibility and define what that authority or responsibility is
- identify the supplies, materials, documents and records needed to carry out the work
Step 2: Hold opening inspection meeting
The inspector will cover the following points during the opening meeting:
- describe the objectives of the inspection program
- outline the scope of the inspection
- confirm company information such as contact person and contact information
- get an understanding of the nature of your company’s activities
- outline the steps of the inspection process
- explain the risk rating system for observations and the overall inspection rating
- inform you about the posting of findings on the medical device inspections webpage
Step 3: Tour the site
The inspector will ask for a tour (in-person or virtual) of your site to get an overview of the activities and devices available for sale. During the tour, the inspector may want to look at the following areas:
- shipping and receiving
- warehousing or storage
- manufacturing
- testing or quality control laboratory
- returns or servicing
- quarantine
- sales and marketing departments
The inspector may also interview staff in company areas.
Step 4: Gather and assess data
The inspector will assess your compliance based on the interviews, tour and review of company procedures. The inspector will also collect and review samples of your company’s records and medical devices. The review will take into account your company’s product lines and the classification of your devices (Class I, II, III or IV).
The number of samples looked at will depend on several factors:
- the controls you have in place and their capability
- the degree of compliance you have shown in previous inspections
- the number of devices offered for sale
Health Canada considers the data looked at (for example, interviews, tour, samples) as “objective evidence” of your company’s compliance at a specific moment in time. This is because the evidence is based on facts the inspector:
- gets through observation, measurement, testing or other means
- can prove true
When looking at this evidence, the inspector will note a deviation, deficiency or failure to comply with the Act and/or Regulations as an observation. Inspectors rate observations as:
- Risk 1: critical (high risk)
- Risk 2: major (medium risk)
- Risk 3: minor (low risk)
Note: For more information about the risk ratings, consult the following document:
Throughout the inspection, the inspector will keep you informed of deviations, deficiencies or failures. If a deviation, deficiency or failure poses a critical or major risk and may contribute to a non-compliant rated inspection, the inspector will bring it to your attention at once. The inspector will ask you to act immediately to address those observations.
The inspector may also take compliance and enforcement actions. The inspector will make sure that any such actions comply with the following documents:
- Compliance and enforcement policy for health products (POL-0001)
- Guidance on medical device compliance and enforcement (GUI-0073)
Note: You are encouraged to take action to correct any deviations, deficiencies or failures the inspector points out to you. If corrected before the closing meeting, the inspector will indicate this in the inspection report.
Step 5: Write draft inspection report
The inspector will draft an inspection report based on the evidence of your company’s compliance. The inspection report will include the risk ratings of any deviations, deficiencies or failures noted during the inspection. It will also include your company’s rating if the inspector found your activities compliant with the Act and Regulations. A non-compliant rating will not appear on the draft inspection report.
Note: Health Canada will make sure that anything seen as non-compliant is well defined, clear and supported by the Act and Regulations. For what you must comply with based on your activities (manufacturing, importing or distributing), refer to the checklist of the provisions of the Regulations that apply to you.
Step 6: Hold closing meeting
The inspector will hold a closing meeting with you. At the closing meeting, the inspector will:
- provide a copy of the draft inspection report based on the assessment of your activities
- verify that you understand the observations and risk ratings
- discuss with you the records and documents that may be needed to meet the requirements of the Act and/or Regulations
- discuss the steps and actions needed to correct any deviations, deficiencies or failures noted during the inspection and prevent them from occurring again
Note: As part of our commitment to openness and transparency, Health Canada will post the initial findings on the medical device inspections webpage.
If the inspector recommends a non-compliant rating, the inspector will issue a letter with the draft inspection report and ask that you respond within 15 business days. The letter will:
- ask you to take immediate corrective and preventive actions for critical and major observations that have contributed to the proposed non-compliant rating
- notify you that Health Canada will conduct a review of the inspector’s draft inspection report to confirm or change the non-compliant rating in the final inspection report
You will be expected to explain the actions you have taken to address observations rated as critical (Risk 1) and major (Risk 2) that have contributed to the proposed non-compliant rating. We encourage you to ask questions so that you fully understand the inspector’s findings.
Step 7: Finalize inspection report
The inspector will update the report to note any deviations, deficiencies or failures you have corrected. The inspector will then send you the final inspection report confirming the rating of the inspection with a cover letter. The cover letter will explain any next steps you are expected to take and provide deadlines. It will also tell you how to let Health Canada know if you disagree with anything in the inspection report.
Note: As part of our commitment to openness and transparency, Health Canada will post an inspection report card on the medical device inspections web page. The report card summarizes the findings of the inspection.
You will be expected to respond within the following timeframe:
- Compliant rating: respond within 20 business days from when the final inspection report was issued
- Non-compliant rating: respond within 15 business days from when the draft inspection report was issued
Note: A condition of getting an MDEL is having written procedures in place for various activities. For the licence requirements, including the mandatory procedures, refer to sections 44 to 51 on establishment licences.
When you completed the application for the licence, you were asked to confirm that you had the required written procedures in place.
During the inspection, a written procedure is considered missing if:
- you made a false attestation by ticking the box on your MDEL application or annual licence renewal that says you have a specific procedure in place but it was not available at the time of the inspection or
- a procedure that you are required to have based on your activities was not available at the time of the inspection
- for example, if you are conducting installations of medical devices of Class II, III or IV, you must have a written procedure for installing medical devices as per paragraph 45(i) of the Regulations (even if you have not ticked the box on the application or annual licence renewal)
You must produce a missing procedure within the timeline provided in the inspection report.
The response you send to Health Canada will vary depending on whether you receive a compliant or non-compliant rating.
If your company receives a compliant rating, you are expected to respond with a corrective action plan that:
- lists the steps and actions you have taken to correct each observation and prevent them from occurring again
- provides a timeline for completing the corrective and preventive actions that cannot be taken immediately
- includes documents that support corrective and preventive actions you have taken
Actions are considered adequate if they are correcting the deficiencies and preventing them from occurring again.
Note: For help with preparing your corrective action plan, consult Corrective action plan template. If you were asked to include documents that support your corrective action plan, the inspector will also review and assess those documents. If inadequate, you will be expected to resubmit new documents. The inspector may reassess your company if the documents resubmitted are still inadequate. The reassessment is normally conducted within 12 months.
If your company receives a non-compliant rating, you are expected to respond by:
- listing the steps and actions you have taken to address all observations rated as critical (Risk 1) and major (Risk 2) that contributed to the non-compliant rating
- including documents that support corrective and preventive actions you have taken
- corrective actions are considered adequate if they are correcting the deficiency and preventing them from occurring again
- providing a corrective action plan with timelines for completing any actions to address remaining observations
Step 8: Review company response and action taken
The inspector will review your corrective actions and/or written corrective action plan to the inspection report to ensure they are adequate.
Company with compliant rating:
If your corrective actions and/or corrective action plan are:
- Adequate: The inspector will send you a letter formally closing the inspection.
- Inadequate: You will be given additional opportunity to respond. If your corrective actions and/or corrective action plan remain inadequate, the inspector may reassess your company. The reassessment is normally conducted within 12 months.
If you do not provide corrective actions or a corrective action plan (you don’t respond), additional compliance and enforcement actions may be taken. This is in accordance with the following documents:
- Compliance and enforcement policy for health products (POL-0001)
- Guidance on medical device compliance and enforcement (GUI-0073)
Company with non-compliant rating:
If you took corrective actions to address the non-compliant rating, and the corrective actions are:
- adequate: The inspector will schedule a re-inspection within 12 months to evaluate the actions you have taken.
- inadequate: The inspector will recommend a suspension of your licence to the Medical Devices Establishment Licence Unit.
The inspector will notify the Medical Devices Establishment Licence Unit whether your response is adequate or inadequate.
Note: Health Canada will send you a “proposal to suspend” letter if:
- your company receives a non-compliant rating and
- corrective and preventive actions taken are not addressing the observations
You will be given a chance to respond to the proposal to suspend your licence.
How Health Canada enforces compliance
In keeping with the Compliance and enforcement policy for health products (POL-0001), Health Canada prefers that you agree to comply voluntarily by taking corrective actions. But if you are unwilling or unable to do so, we will enforce compliance.
In deciding on what action to enforce, we will consider the following factors:
- risk to health
- your cooperation during and after the inspection, as well as your written response to the inspection
- your compliance history
If you are a company in Canada, we can:
- request a stop sale of your product
- request a recall of your product
- order a recall of your product
- order you to provide information or documents
- detain your product imports
- take control of your non-compliant items (administrative seizure)
- suspend your licence
- recommend prosecution to the Department of Justice
If you are a company outside of Canada, we can:
- request a stop sale of your product
- request a recall of your product
- suspend your licence
- recommend a refusal of entry of your product into Canada
Any compliance and enforcement actions taken by inspectors will be consistent with the following documents:
- Compliance and enforcement policy for health products (POL-0001)
- Guidance on medical device compliance and enforcement (GUI-0073)
Inspectors can take compliance and enforcement actions at any time.
Page details
- Date modified: