Draft Health Canada IMDRF table of contents for medical device applications guidance
(PDF version, 650 KB, 15 pages)
On this page
- 1. Introduction and background
- 2. Guidance for implementation
- 3. Filing process
- 4. Resources, tools and classification matrices
- 5. Access to information
- 6. Contact information
1. Introduction and background
1.1 Purpose
The International Medical Device Regulators Forum (IMDRF) is a voluntary group of regulators committed to the acceleration of medical device regulatory harmonization and convergence. It was founded in 2011 and is the successor to the Global Harmonization Task Force. The Table of Contents (ToC) format was developed by the IMFDR to provide a globally harmonized structure and has been adopted by Health Canada for medical device regulatory activities. Health Canada is adopting the IMDRF ToC formats to encourage and support the global convergence of format for medical device applications. It is expected that use of the ToC will reduce time and costs for both industry and the regulator, and will ultimately result in timely access to medical devices for Canadians.
This guidance incorporates content from the IMDRF ToC Documents (In Vitro Diagnostic Medical Device Market Authorization Table of Contents (IVD MA ToC) and Non-In Vitro Diagnostic Device Market Authorization Table of Contents (nIVD MA ToC)) published by IMDRF and regional guidance specific to the Canadian context.
The guidance is intended to support manufacturers and regulatory correspondents in the creation of an information package for submission to Health Canada in support of medical device regulatory activities.
1.2 Scope and application
This guidance is intended to aid manufacturers and regulatory correspondents in understanding the structure, content, and assembly and system requirements for various Health Canada medical device regulatory activities.
1.2.1 Structure guidance
The ToC structure requirements apply to information packages created in support of the following:
- Pre-Market:
- New and Amendment Class 2, Class 3 and Class 4 medical device licence applications for In-Vitro Diagnostic Devices (IVD) and Non-In Vitro Diagnostic Device (nIVD)
- All medical device Private Label licence applications
- All Fax-back (Minor Change) applications
- All Screening Deficiency Responses, Clarification Responses, and Additional Information Responses associated with those activities listed above
- Post-Market:
- Responses to Class 2, Class 3 and Class 4 post-market requests issued under the Medical Devices Regulations (e.g. Responses to Section 39 or Section 36 of the Medical Devices Regulations)
- Responses to all Classes (1 to 4) for IVD and nIVD Post-Market Request from the Marketed Health Products Directorate
- All Clarification and Additional Information Responses associated with those activities listed above
1.2.2 Content guidance
This guidance defines the content requirements applicable to applications for medical device licences and the other regulatory transactions and activities described in Section 1.2.1 Structure Guidance above. For additional information on the legal requirements to support medical device licence application, please refer to the Medical Devices Regulations.
1.2.3 Assembly and system requirements guidance
This guidance is intended to define the system requirements for all ToC submissions, focusing primarily on file format, file name, path length, and submission media filesystem concerns. This guidance also provides instructions for assembling a ToC-compliant information package.
1.3 Policy objectives
This guidance document is intended to facilitate the creation and submission to Health Canada of ToC-based medical device regulatory information.
1.4 Policy statements
This guidance is to be used in the preparation of medical device related regulatory transactions.
1.5 Abbreviations and acronyms
- HPFB
- Health Products & Food Branch
- IMDRF
- International Medical Device Regulators Forum
- IVD
- In vitro diagnostic
- MHPD
- Marketed Heath Products Directorate
- nIVD
- non-in vitro diagnostic
- PIS
- Proprietary Information Submission
- RPS
- Regulated Product Submission
- ToC
- Table of Contents
- TPD
- Therapeutic Products Directorate
1.6 Definitions
Summary
A summary should include a brief synopsis of the (1) purpose, (2) methods, (3) acceptance criteria, (4) results and (5) discussion and conclusions. Outliers and deviations should be reported with the results. Results should be stated quantitatively with appropriate statistical context where applicable (e.g. value ± SD, confidence intervals, etc.).
The summary should specifically address:
- Why the characteristic being evaluated is of interest;
- Why the particular methods are being used to evaluate the characteristic, if applicable including why a regional or harmonized/recognized standard/guidance has or has not been complied with;
- How the stated acceptance and sample size are scientifically supported;
- What device was tested and how it relates to the devices that will be marketed;
- Why the tested components are representative of the range of devices that will be marketed;
- Whether the summary has been previously submitted and reviewed by the regulator, including identification of the device and the reference number for the submission; and
- Whether the testing has been conducted in-house or by a 3rd party.
Full report
Typically includes a complete, detailed description of the objective of the assessment, the methods and procedures, study endpoint(s), pre-defined pass/fail criteria, deviations, results, discussion and conclusions, and may include data. Complete, detailed support of method selection, worst case justification, study endpoint selection, and pass/fail criteria should be included.
In vitro diagnostic device (IVDD)
An in vitro Diagnostic Device is a medical device that is intended to be used in vitro for the examination of specimens taken from the body.
Manufacturer
A Manufacturer is a person who sells a medical device under their own name, or under a trade-mark, design, trade name or other name or mark owned or controlled by the person, and who is responsible for designing, manufacturing, assembling, processing, labelling, packaging, refurbishing or modifying the device, or for assigning to it a purpose, whether those tasks are performed by that person or on their behalf.
Proprietary information submissions
Proprietary Information Submissions are used when firms that manufacture or process the device under contract to the manufacturer elect to submit all or a portion of the manufacturing information applicable to their facility directly to the Medical Device Bureau (MDB) in the form of a Proprietary Information Submission. The manufacturer or device sponsor should inform these firms of the need to provide detailed information on the device. Manufacturers referencing information held in a Proprietary Information Submission submitted by another company must obtain permission from the owner of the file each time the file is accessed. The letter of permission should indicate the extent of information to be considered for each application.
Recall
A recall in respect of a medical device that has been sold, means any action taken by the manufacturer, importer or distributor of the device to recall or correct the device, or to notify its owners and users of its defectiveness or potential defectiveness, after becoming aware that the device:
- may be hazardous to health;
- may fail to conform to any claim made by the manufacturer or importer relating to its effectiveness, benefits, performance characteristics or safety; or,
- may not meet the requirements of the Food and Drugs Act or the Medical Devices Regulations.
2. Guidance for implementation
2.1 IMDRF ToC folder structure
The IMDRF ToC structure is defined by IMDRF. The headings are to be structured in a folder structure mimicking the hierarchy described. In order to reduce the file path lengths, names have been shortened in some cases. Further discussion and details regarding the folder structure is included in the Health Canada Adapted Assembly and Technical Guide. Folder structure templates are available and must be followed; see Section 4 - Resources, Tools, and Classification Matrices below for further details.
2.2 Heading classifications and content guidance
As the IMDRF ToC is comprehensive in nature, not all headings are required for Health Canada. The ToC documents are therefore intended to work together with a separate document created for each participating jurisdiction – a Classification Matrix. Headings that are required are defined in the Health Canada classification matrices and depend on the submission type.
Heading classifications are used to define content requirements for various different application types in the documentation that follows. Complete definitions and discussion of heading classifications is provided in the Health Canada Adapted Assembly and Technical Guide and familiarization is strongly recommended. For convenience, brief definitions are provided below:
- R - Required. Any folder that is established as Required must not be deleted. Content must be submitted in this folder.
- NR - Not Required. Any folder that is established as Not Required must be deleted.
- CR - Conditionally Required. Any folder that is established as Conditionally Required needs a determination against the conditions by the applicant. Specific conditions are defined by Health Canada for each CR heading and can be found in the detailed content guidance.
- O - Optional. Any folder that is established as Optional requires a decision by the applicant and then must be deleted if not populated.
- OR - Optional but Recommended. Any folder that is established as Optional but Recommended requires a decision by the applicant and then must be deleted if not populated.
2.2.1 Class 3 and 4
Guidance regarding the content of an application is presented based on the content requirement defined by IMDRF as well as any additional regional guidance. This guidance has been arranged by type of device application as follows:
- Class 3 nIVD Applications Content and Classification Guidance
- Class 4 nIVD Applications Content and Classification Guidance
- Class 3 IVD Applications Content and Classification Guidance
- Class 4 IVD Applications Content and Classification Guidance
Note: Heading numbers that are not required by Health Canada are excluded from the content guidance and templates (e.g. 1.01 - Cover Letter is followed by 1.03 - List of Terms/Acronyms as 1.02 - Submission Table of Contents is not required by Health Canada).
Chapter 3 (Non-clinical evidence) incorporates Overview folders and Custom headings. Overview folders have been created in the folder template where the IMDRF ToC guidance indicates a requirement for content at a parent folder. This folder structure was created to ensure the sequence of information presented is maintained in a Windows environment. For example, in the nIVD structure, Section 3.05.06 Biocompatibility & Toxicology Evaluation, there is a sub-folder named “3.05.06.00-Overview” in the template. The content prescribed by the IMDRF guidance for Section 3.05.06-Biocompatibility & Toxicology Evaluation should be placed in this folder. Each specific study/piece of evidence should have its own Custom heading. Within each custom heading are a summary and, when required, full report files. Guidance for each of these folders/headings should be strictly followed. Further details are provided in the Health Canada Adapted Assembly and Technical Guide.
When presenting a summary for sections in Chapter 3, you should follow the detailed definitions of a summary as described in Section 1.6-Definitions above.
Do not repeat identical content in multiple sections of your submission.
The IMDRF ToC structure and content guidance were developed primarily for Class 3 and Class 4 medical device licence applications. Other transaction types (i.e. Class II, Post-Market, Fax-back (Minor Change), Private Labels) typically require a very limited subset of IMDRF ToC headings and the requirements for these submissions types are discussed within this document in Sections 2.2.2 and 2.2.4 below.
2.2.2 Class 2, private label, fax-backs (minor change)
Class 2, Private label, and Fax-backs (minor change) have significantly different evidentiary requirements from Class 3 and 4 applications. While the ToC is used for these, only select folders are required and content guidance is specific to this context.
Note: No distinction is made between nIVD and IVD for applications of these types.
A sample folder structure for Class 2/Private Label/Fax-backs (Minor Change) applications is shown below:
Figure 1 - Screenshot depicting the folder structure for Class 2/Private Label/Fax-backs (Minor Change) applications

Text Description
The root folder is “Licence Name” and sub-folders includes 1-REG ADMIN, 2-CONTEXT, 5-LABELLING sub folders that are applicable as defined in the table below
The classifications for the headings within Class 2/Private Label/Fax-back (Minor Change) applications are shown in the table below.
Note: Classifications are defined in Section 2.0.
| Folder | Licence | Private Label | Fax-Back (Minor Change) | ||||
|---|---|---|---|---|---|---|---|
| Class 2 | Class 2, 3, 4 | Class 3/4 | All Classes (except addition to Class 3/4) | All Classes | |||
| New | Amend | New & Amend | Add New Device Identifier | Add, Delete or Change Device Identifier | Change to Licence/ Device Name | Change to Manufacturer’s Name/ Address | |
| 1.01 | CR | CR | CR | CR | CR | CR | CR |
| 1.04 | R | R | R | R | R | R | R |
| 1.06 | R | NR | NR | NR | NR | NR | R |
| 1.09 | CR | CR | CR | CR | CR | CR | CR |
| 1.14 | NR | NR | R | R | R | R | R |
| 2.04.04 | NR | O | NR | R | NR | NR | NR |
| 5.02 | CR | CR | CR | O | O | O | O |
| 5.03 | CR | CR | CR | O | O | O | O |
| 5.04 | CR | CR | CR | O | O | O | O |
| 5.05 | CR | CR | CR | O | O | O | O |
| 5.06 | CR | CR | CR | O | O | O | O |
| 5.07 | CR | CR | CR | O | O | O | O |
| 5.08 | CR | CR | CR | O | O | O | O |
| 5.09 | CR | CR | CR | O | O | O | O |
| 5.10 | CR | CR | CR | O | O | O | O |
For detailed content guidance and conditions refer to the following:
- Class 2 Licence Application Content and Classifications Guidance
- Private Label Application Content and Classification Guidance
- Fax-back (Minor Change) Application Content and Classification Guidance
2.2.3 Responses to additional information or screening deficiency letters
Responses to requests from Health Canada must clearly identify the Application Number of the associated application. Responses to Screening Deficiency Letters, Clarification Requests, and Additional Information Letters must be provided in a question and answer format and be accompanied by a copy of the original Health Canada letter. This information is to be filed within Section “1.01-Cover Letter” and the substantial supporting information must be structured using the same format as the initial application.
Note: Your Additional Information response should not contain information that was previously submitted within the same application. Only submit any documents that have been modified and place them in the appropriate folders with a clear reference to the changes outlined in the cover letter.
2.2.4 Combination products
A combination product is a therapeutic product that combines a drug component and a device component (which by themselves would be classified as a drug or a device), such that the distinctive nature of the drug component and device component is integrated in a singular product.
For further information on combination products and how they are classified please refer to the policy on Drug/Medical Device Combination Products.
Combination products classified as devices are regulated under the Medical Devices Regulations and applications can be created following the IMDRF ToC format.
For further guidance please contact the Medical Devices Bureau at devicelicensing-homologationinstruments@hc-sc.gc.ca.
2.2.5 Market health products directorate (MHPD) post-market responses
Post-market interactions refer to communication with the Marketed Health Product Directorate (MHPD) where evidentiary requirements differ significantly from licence applications. While the ToC is used for these interactions, only select folders are required and content guidance is specific to this context.
A sample folder structure for MHPD transactions is shown below:
Figure 2 - Screenshot depicting the folder structure for MHPD transactions
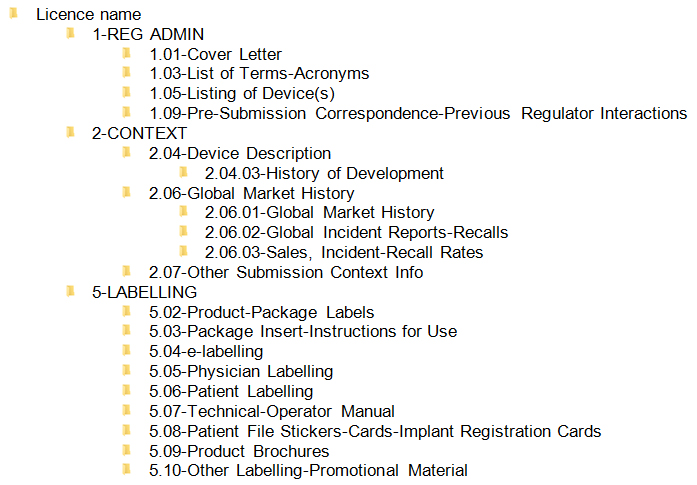
Text Description
Folders include 1-REG ADMIN, 2-CONTEXT, 5-LABELLING sub folders that are defined in the Marketed Health Products Directorate Post-Market Submission Guidance.
Note: Responses to the Medical Devices Bureau Section 36 Letter and Section 39 Letter should follow the format of an AI/deficiency response as discussed in Section 2.2.3 Responses to Additional Information or Screening Deficiency Letters.
For more detailed guidance for post-market ToC submissions, refer to the Marketed Health Products Directorate Post-Market Submission Guidance.
2.3 How-to and system requirements
Specific guidelines for building an application in the IMDRF ToC format, including system requirements are detailed in the Health Canada Adapted Assembly and Technical Guide for IMDRF Table of Contents Submissions. It is essential that readers are familiar with this guidance before building an application in this format.
3. Filing process
3.1 Transmission Options
3.1.1 Physical Media
Submissions should be provided on media, unless specified otherwise. Media should be sent to the appropriate address as indicated the Contact Information Section below.
The media formats acceptable when providing electronic ToC based submissions are:
- Compact Disc-Recordable (CD-R) conforming to the Joliet specification
- Digital Versatile Disc-Random Access Memory (DVD-RAM) Universal Disc Format (UDF) standard
- Single and dual layer Recordable Digital Versatile Discs
- Single and dual layer Blu-ray discs
- Universal Serial Bus (USB) 2.0 or 3.0 drive
- Portable External Hard Drive with USB 2.0 or 3.0 interfaces
The media are to be labelled with the following information:
- Manufacturer’s name;
- Device name;
- “Protected B”[1];
- Virus free certification, the software used for the virus check and the date of the virus definition file(s);
- Date of application; and
- An identifying number for each media and total number of media provided (e.g. Disc 1 of 2).
Subsequent to burning the CD/DVD or transferring data to a drive, stakeholders should ensure that all files can be opened, no files are corrupt, and that “Thumb.db” files are removed.
Important notes:
- Media should be scanned using current virus-scanning software and should be certified virus-free.
- Manufacturers should place all documents in as few CDs or DVDs as possible.
- Duplicate copies of the physical media are not required.
- Media will not be returned.
3.1.2 Email
Any ToC-based submission may be submitted to Health Canada via email provided:
- The manufacturer accepts the risk of transmitting their business information through email.
- The submission does not exceed the 20 megabytes.
- The manufacturer has packaged the submission as a zipped file that is not password protected.
Important notes:
- The submission should still follow all other guidance regarding assembly of ToC-based submission and structure of information.
- A duplicate copy should not be provided by mail.
- The body of the email should only contain the zipped submission; no other documents or related information should be included.
3.2 Where to Submit
3.2.1 Licence applications and Section 36 and 39 responses
All Medical Device Licensing related interactions including responses to Letters issued under Section 36 or 39 of the Medical Device Regulations should be directed to:
Device Licensing Services Division
Medical Devices Bureau
11 Holland Avenue
Tower A, Second Floor,
Postal Locator: 3002A
Ottawa, ON, Canada
K1A 0K9
Telephone: 613-957-7285
Email: devicelicensing-homologationinstruments@hc-sc.gc.ca
3.2.2 Responses to requests from the MHPD
All requests received from the Marketed Health Products Directorate should be directed to:
Marketed Pharmaceuticals and Medical Devices Bureau
Marketed Health Products Directorate
Health Canada
Postal Locator 1912A
200 Eglantine Driveway
Ottawa, ON Canada
K1A 0K9
Telephone: 613-948-8523
Fax: 613-952-6011
Email: hc.mpmdb.rpm-bppmmc.gpr.sc@canada.ca
4. Resources, tools and classification matrices
4.1 Resources
Further detailed guidance documents for medical devices are available and should be consulted when composing medical device regulatory submission. For a complete listing, please refer to Guidance Documents – Medical devices.
4.2 Tools
The following additional tools are available to aid in creating applications:
- Folder templates – these are empty folder structures that use the defined abbreviated folder names
- Folder based samples – these samples are the folder structure templates above, with files added within the structure. The files include content guidance and classifications. These are for use by users who want to view the content guidance in an alternative format and can be used similar to the folder templates above, to build submissions. Important: Do not include any of the sample files in your submission.
- Controlled vocabulary – this listing is for users who might want a precise listing of abbreviated folder names. These are intended for users who will not be using the folder templates above, but have their own submission building software to configure with accurate folder names.
4.3 Classification matrices
Classification matrices are detailed tabular listings of heading classification created for various submission types. These are intended to provide users with a bird's eye view of submission requirements based on the submission type and are also intended for users who have their own submission building software to configure.
- C3/CIV nIVD Classification Matrix [Excel]
- C3/CIV IVD Classification Matrix [Excel]
- C2/Fax-back (Minor Change) Classification Matrix [Excel]
5. Access to information
Information provided to Health Canada by manufacturers is subject to the provisions of the Access to Information Act. Trade secrets or confidential scientific, technical, commercial, or financial information is protected from disclosure by this Act. According to TPD policy, information regarding medical device regulatory activities that have been received or are being processed is also considered confidential. Once a licence has been issued, basic information about a device, such as that listed in section 32(1) of the Regulations, is considered public information.
6. Contact information
Any questions or concerns related to this guidance document or its use should be directed to:
Device Licensing Services Division
Medical Devices Bureau
11 Holland Avenue
Tower A, Second Floor,
Postal Locator: 3002A
Ottawa, ON, Canada
K1A 0K9
Telephone: 613-957-7285
Email: meddevices-instrumentsmed@hc-sc.gc.ca
The email subject line should be: “IMDRF ToC Question(s)”
Page details
- Date modified: