ARCHIVED - Formative Evaluation of the Canadian HIV Vaccine Initiative
Context and Overview of CHVI
In 2007, the CHVI was established as Canada’s contribution to the Global HIV Vaccine Enterprise. It is a collaborative effort between the GoC and the Bill & Melinda Gates Foundation and part of Canada’s global commitment to accelerate the development of safe, effective, affordable and globally accessible HIV vaccines.
2.1 CHVI Context
The CHVI was created to build on Canada’s strengths to support the Global HIV Vaccine Enterprise (Global Enterprise).1 The Global Enterprise, founded in 2004, is an alliance of independent organizations, governments, and stakeholders dedicated to the development of preventive HIV vaccines. It developed and implemented a shared Scientific Strategic Plan (SSP), which focused on expanding research, increasing global capacity for manufacturing high quality clinical trial lots for HIV vaccines, building capacity to conduct large-scale clinical trials, reinforcing developing country expertise for reviewing and approving clinical trials and assessing results and establishing strategies to manage intellectual property issues.
Canadian HIV researchers, with representatives from the private and public sectors, met with Bill & Melinda Gates Foundation officials in October 2005 to explore how Canada could contribute to the SSP to further progress towards the development and delivery of HIV vaccines. This meeting and subsequent interdepartmental discussions culminated in the signing of a Memorandum of Understanding (MOU) between the Minister of Health, the Minister of International Cooperation, the Ministry of Industry and the Bill & Melinda Gates Foundation in August 2006. This MOU focused on delivering results in areas that harmonize Canada’s areas of HIV vaccine expertise with the gaps identified in the SSP.
The CHVI is the vehicle to implement this MOU. It represents a coordinated domestic and international contribution to global HIV vaccine efforts and is linked to international bodies such as the International AIDS Vaccines Initiative, the World Health Organization and the Joint United Nations Program on AIDS. Policy authority was granted in January 2007 and a joint announcement on the initiative was made on 20 February 2007, by the Bill & Melinda Gates Foundation and the Prime Minister.
2.2 CHVI Objectives and Structure
2.2.1 CHVI Objectives
The CHVI is a six-year (2007/08 to 2012/13) $139M initiative. Its objectives and program activities reflect four guiding principles: (1) strategic coordination and integration, (2) multi-sectoral collaboration and engagement, (3) promotion of human rights and global access, and (4) accountability and transparency.2 The overall purpose of the CHVI is to implement the MOU between the GoC and the Bill & Melinda Gates Foundation by mobilizing domestic and international resources to contribute to the global efforts to accelerate the development of effective and globally accessible HIV vaccines.3 More specifically, the CHVI objectives are to:
- Strengthen HIV vaccine discovery and social research capacity;
- Strengthen clinical trial capacity and networks, particularly in low and middle-income countries (LMICs);
- Increase global pilot scale manufacturing capacity for HIV vaccine clinical trial lots;
- Strengthen policy and regulatory approaches for HIV vaccines, particularly in LMICs;
- Promote the community and social aspects of HIV vaccine research and delivery; and
- Ensure horizontal collaboration within the CHVI and with domestic and international collaborators.4
The CHVI was expected to strengthen both domestic and international capacity and expertise and achieve the following results:
- Increased HIV research and capacity in Canada and in LMICs;
- Strengthened clinical trial capacity in LMICs;
- Canadian-based pilot scale manufacturing facility to produce clinical trial lots for clinical trials to be performed mostly in LMICs where the incidence and prevalence of HIV/AIDS are the highest;
- Enhanced policy and regulatory expertise in Canada and particularly in LMICs where clinical trials will be conducted and vaccines will ultimately be made available; and
- Expanded capacity to integrate community and social dimensions into HIV vaccines development and delivery efforts.5
The CHVI logic model is provided in Appendix A. The outcomes reflected in the logic model are similar but not identical to these results statements.
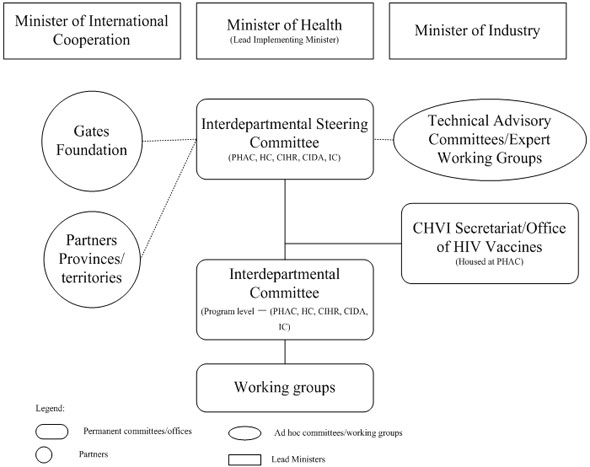
2.2.2 CHVI Governance Structure
Five federal departments and agencies are implementing the CHVI in collaboration with the Bill & Melinda Gates Foundation. These partners planned to collaborate with each other to deliver on the objectives of the CHVI, in line with their own mandates. Exhibit 2.1 outlines the roles of each department and agency.
The Minister of Health (as the Minister for PHAC), in consultation with the Ministers of Industry and International Cooperation, is the lead Minister for the CHVI. The governance bodies included (see also Exhibit 2.2):6
- Interdepartmental Steering Committee composed of representatives from IC, CIDA, HC, CIHR and PHAC. The Committee is co-chaired by PHAC and CIDA. The Steering Committee is responsible for providing strategic directions and priorities, reviewing progress, and establishing and maintaining linkages with identified collaborators (such as the Bill & Melinda Gates Foundation);
- Interdepartmental Committee (program level) reports to the Interdepartmental Steering Committee. It is co-chaired by PHAC and CIDA and includes representatives from all participating departments/agencies. Its role is to ensure effective coordination of the CHVI at the working level, through sharing information, coordinating program planning and policy development activities, and monitoring and reporting on CHVI; and
- CHVI Secretariat, housed at PHAC, works in close collaboration with participating departments/agencies to ensure a coordinated cohesive approach. The Secretariat provides operational support, coordination and planning of activities for the interdepartmental committees and contributes to partnership and stakeholder engagement. It is also responsible for monitoring, reporting and evaluation.
Exhibit 2.1: Roles of Partner Departments and Agencies in CHVI7
Expert working groups, identified by the Steering Committee, were formed on specific issues, as required, to engage both domestic and international key stakeholders. One such expert working group was set up for the review of the applications for the Pilot Scale Manufactory Capacity for Clinical Trial Lots component. In addition, the program-level Interdepartmental Committee has established working groups for specific tasks (e.g. communications) and ad hoc working groups have been established for the design of most grant or contribution programs.
The Results-based Management Accountability Framework/Results-Based Audit Framework (RMAF/RBAF)8 foresaw also the establishment of an Advisory Committee to ensure active involvement by governments, the private sector, international stakeholders, people living with HIV/AIDS, researchers and NGOs and relevant stakeholders. Although a Chair for the committee was announced, at the time of this evaluation, no further action had been taken on this Committee.
In addition, the development of a formal federal/provincial/territorial (F/P/T) engagement strategy was planned, in order to build on existing F/P/T networks for informing provincial and territorial collaborators about the CHVI. This was expected also to serve as a forum to explore opportunities for collaboration.
Text Equivalent
ARCHIVED - Formative Evaluation of the Canadian HIV Vaccine Initiative
Lead Minister
The Minister of Health, in consultation with the Minister of Industry and Minister of International Cooperation, will be the lead implementing Minister for the CHVI for the purposes of overall coordination while the Ministers will each be accountable to Parliament for their funding contribution to the CHVI.
Departmental/Agency Roles
Departments and agencies will collaborate to deliver on the goals of the CHVI in line with their respective mandates. Industry Canada will assist with industry-related issues such as the appropriate engagement of potential private sector collaborators. Health Canada will apply its wide range of expertise, including vaccine-related policy, regulations and protocols; facilitating collaborative networks of specialists; and enhancing international collaborations. PHAC will contribute its public health scientific, policy and program expertise, building on past HIV/AIDS and vaccines initiatives and serve as the lead agency for the CHVI. CIHR will provide linkages to the Canadian research community, as well as bring critical expertise in peer review mechanisms and related professional support services to identify and fund eligible HIV vaccines projects. CIDA will provide the effective linkages to international development efforts and ensure consistency with Canada’s international commitments. All departments/agencies will ensure the CHVI’s efforts are coordinated within the global HIV/AIDS research community. Moreover, CIDA will provide strategic guidance to ensure that the goals of the CHVI promote the development and delivery of HIV vaccines that benefit the needs of the highly endemic HIV/AIDS countries in the developing world
CHVI Steering Committee
In support of the Ministers, coordination for the Government of Canada will be provided by an interdepartmental steering committee consisting of Industry Canada, the Canadian International Development Agency, Health Canada, the Canadian Institutes of Health Research, and the Public Health Agency of Canada. The steering committee will be co-chaired by CIDA and PHAC.
Following approval of the MC, the steering committee will be established and terms of reference will be developed to reflect its key responsibilities including:
- reviewing submissions to Cabinet committees;
- developing the Terms of Reference for the steering committee;
- reviewing implementation, governance and delivery structures;
- providing ongoing strategic direction and priority-setting;
- ensuring a coordinated approach to stewardship of resources and due diligence;
- monitoring and reporting on performance and finances;
- reviewing all reports, to minister and for parliamentary reporting purposes as required;
- reviewing evaluation frameworks;
- ensuring alignment of program efforts with broader domestic and international agendas;
- developing an engagement strategy for new collaborators;
- selecting lead departments/agencies for project/programs;
- approving terms of reference for the CHVI Advisory Committee;
- reviewing the development of Minister-Minister MOUs related to funding transfers; and
- providing input on CHVI communications strategies and plans.
A key responsibility of the Steering Committee will be to maintain active communications and linkages with identified collaborators such as the Gates Foundation and other potential new collaborators. While these collaborators will not be formally represented on the Steering Committee, which is comprised solely of participating departments and agencies, the Steering Committee will maintain links to the collaborators. In addition, these collaborators may also play a role in the CHVI Advisory Committee and expert working groups as appropriate.
CHVI Secretariat
A secretariat will be established at the Public Health Agency of Canada, which will work in close collaboration with other involved departments/agencies to provide operational support, including monitoring and evaluation. The secretariat will assist the Steering Committee in achieving its mandate.
Collaborators
As outlined in the MOU, the Gates Foundation is contributing funding towards the construction of a pilot scale clinical trial lot manufacturing facility. The Gates Foundation will share decision-making responsibilities with the Government of Canada (through the Steering Committee) on all aspects of the facility (i.e. development of request for proposals, establishment of review criteria, review and selection process).
The CHVI will also enable wider participation and support from other potential collaborators (e.g. governments, non-governmental and private sector organizations) on HIV vaccine- related initiatives in which they share common goals with the Government of Canada and the Gates Foundation. A formal federal/provincial/territorial engagement strategy will be undertaken to seek provincial and territorial collaborators. Following the announcement of the CHVI, the Minister of Health will write to his provincial and territorial counterparts to explore their interest in participating in the CHVI. Existing F/P/T networks will also be utilized to inform provincial and territorial officials about the CHVI which may also serve as a forum to explore opportunities for collaboration (e.g. discovery research networks, Public Health Network). Specific agreements outlining areas of collaboration and respective roles and responsibilities with provinces and territories will be put into place on a case-by-case basis.
Specific mechanisms will be established to deliver the obligations outlined in specific collaboration agreements. Financial management will be in accordance with existing federal policies and procedures.
CHVI Advisory Committee
Active involvement by governments, the private sector, international stakeholders, people living with HIV/AIDS, researchers and NGOs and relevant stakeholders will be critical to the success of the CHVI and will be realized through the creation of an advisory committee. Linkages to the Global Enterprise Secretariat will also be established for the advisory committee to ensure ongoing alignment with global efforts. Expert working groups will be formed on specific issues, as required, which may include participation by advisory committee members and/or other key stakeholders, both domestic and international.
The steering committee will identify key issues for input from the advisory committee, including:
- advising the steering committee in CHVI program priorities and implementation;
- linking with national and international efforts;
- forming expert working groups, as instructed by the steering committee; and
- communicating and broad information sharing.
2.3 CHVI Components
2.3.1 Description of CHVI Components
In order to fulfill its objectives, the CHVI has four program components:
- Discovery and social research;
- Clinical trial capacity building and networks;
- Pilot scale manufacturing capacity for clinical trial lots; and
- Policy and regulatory issues, community and social dimensions.
The planning, coordination and evaluation activities of the Program were considered into a fifth component for the purposes of the allocation of CHVI resources; however, it is not considered a programming component. The purpose of, and activities carried out in, each component are described in greater detail in the following sections.
Discovery and Social Research
The objective of the Discovery and Social Research component is to promote greater collaboration between researchers in Canada and LMICs who are working in HIV vaccine discovery and social research. To maximize the potential for important scientific discoveries, a multi-pronged approach is being used to support creativity through a series of research grants available to both individual investigators and collaborative teams. These include:
- Catalyst Grants provide short-term seed money to support innovative HIV vaccine-related research activities that are expected to contribute to the development of new proposals, tools, techniques, inventions or methodologies.
- Operating Grants provide funding for Canadian researchers with an interest in basic and social research related to HIV vaccines, to enhance research in HIV prevention and build future Canadian research capacity in the field.
- Emerging Team Grants support the work of Canadian research teams and their efforts to strengthen capacity, develop expertise and strategies for knowledge translation and exchange, provide a superior training and mentoring environment, and create mechanisms for individual investigators and teams funded under the initiative to network and share information with one another.
- Large Team Grants support the work of teams of Canadian and LMIC researchers in their efforts to contribute important knowledge to the global search for HIV vaccines, build capacity (human and infrastructure) for HIV vaccines discovery and related social research in Canada and in LMICs, provide opportunities for new and young investigators and create mechanisms for teams funded under the Initiative to network and share information with one another.
CIHR has the lead on the Discovery and Social Research component. Funding for the Large Team Grants comes jointly from CIHR and CIDA.
Clinical Trial Capacity Building and Networks
The purpose of this component is to strengthen the capacity of researchers and research institutions to conduct high-quality clinical trials and build site capacity to undertake clinical trials of HIV vaccines and other preventive technologies in LMICs. Funds were to be channelled through the Global Health Research Initiative (GHRI) for "Capacity Building" and "Synergy and Networking" grants to researchers and research institutions, particularly in LMICs.
CIDA has the lead on this component and is providing the $16M for the component. The funds are to be transferred to IDRC, which will administer the grants through its regular GHRI.
Pilot Scale Manufacturing Capacity for Clinical Trial Lots
The purpose of this component was to increase the global capacity to produce HIV vaccines for use in clinical trials to be conducted mostly in, and for the benefit of, LMICs.9 This was expected to be implemented through the establishment of a dedicated pilot scale manufacturing facility in Canada to produce candidates for clinical trials. A not-for-profit corporation (with private sector and other partners) would build and operate the facility. The identification of the team to create this facility was ongoing during the data collection for this evaluation. PHAC had the lead on this component; however funding was also to come from CIDA, IC and the Bill & Melinda Gates Foundation. The total funding was $89M.
Policy and Regulatory Issues, Community and Social Dimensions
The purpose of this component is to address policy, regulatory, community and social dimensions related to the development of a safe, effective, affordable and globally accessible HIV vaccine. It includes a number of related activities:
- Strengthening of vaccine policy approaches that promote global access to HIV vaccines;
- Enhancement of the regulatory pathway and processes for HIV vaccines in LMICs;
- Collaboration with partners in Canada and in LMICs in advancing legal, ethical and human rights dimensions of HIV vaccines; and
- Strengthening existing mechanisms to support community involvement in vaccine research and development, clinical trials and activities related to public awareness and education.
PHAC, CIDA and HC are engaged in this component.
Planning, Coordination and Evaluation
The last component – although not strictly a program component – covers the CHVI planning, coordination and evaluation. Its purpose is to ensure effective strategic planning, scientific oversight, coordination and evaluation to meet CHVI objectives. Tasks include:
- Establishing and maintaining the CHVI governance structure;
- Monitoring key trends in HIV vaccine research and development;
- Enhancing Canada’s contribution to the global efforts by mobilizing expertise, partnerships, resources and liaising with domestic and international stakeholders;
- Establishing new partnerships and promote stakeholder engagement in the CHVI;
- Raising the profile of the CHVI among stakeholders in Canada and internationally and to communicate progress;
- Providing secretariat support to the Initiative, including its Interdepartmental Steering Committee and multi-stakeholder advisory committee; and
- Reporting on CHVI progress.
PHAC has the lead on this component and is responsible for funding the planning, coordination and evaluation activities. However, all partner departments/agencies contribute to the component, primarily through their participation in the governance structures.
2.4 CHVI Financial Profile
Exhibits 2.3 and 2.4 reflect the CHVI financial profile. Exhibit 2.3 reflects the initial total allocation for the Initiative – $85M in new funding, $26M in funding that was reprofiled from PHAC’s Federal Initiative for HIV/AIDS and $28M from the Bill & Melinda Gates Foundation, for a total of $139M.
CIDA is the largest contributor to the Initiative, with contributions to four components, accounting for 43% of the total investment (including the Bill & Melinda Gates Foundation) and 53% of the GoC investment.
The actual expenditures, compared to the amounts allocated in the approved program authority document, are shown in Exhibit 2.4. This exhibit also reflects the CHVI Secretariat’s revised plan for the Initiative, if it is extended to 2015/16. No authorities are yet in place for the continuance of the Initiative beyond the five-year timeframe. (Note that Exhibit 2.4 does not include the expected $28 M from the Bill & Melinda Gates Foundation.)
Analysis of the differences between the approved commitments, on a year by year basis, and actual expenditures, reflects the significant delays in the implementation of the CHVI:
- The first year of the Initiative (2007/08) was planned to be a development year and, as a result, expenditures were expected to be lower than in the following years. Yet, the actual expenditures were considerably lower than the modest amount planned – 63% of the $1.5 million planned for the year;
- Expenditures for the first two years of full programming (2008/09 ad 2009/10) reflect significant under-spending, compared to approved commitments. In 2008/09, actual expenditures represented only 11% of planned expenditures and in 2009/10, the revised planned expenditures will be only 15% of the approved commitments for that year; and
- All program components (excluding Planning, Coordination and Evaluation) saw major lapses in spending compared to approved commitments for these two years.
These financial figures reflect the delays in implementation of the Initiative.
| Program Area | 2007-08 | 2008-09 | 2009-10 | 2010-11 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Planned | Actual | Planned | Actual | Planned | Revised Plan | Planned | Revised Plan | ||
Source: CHVI Secretariat, December 2009 * Does not include an additional $495,850 provided by PHAC to support the Global HIV Vaccine Enterprise Note: All figures for future spending of GoC departments were based on projections made for the original 5-year duration of the initiative. |
|||||||||
| Discovery and Social Research | CIHR/ IRSC |
500,000 | 110,000 | 2,000,000 | 682,624 | 2,000,000 | 1,416,942 | 2,000,000 | 1,254,019 |
| CIDA | 0 | 0 | 2,400,000 | 0 | 2,400,000 | 0 | 2,400,000 | 1,200,000 | |
| Clinical Trial Capacity Building and Networks | CIDA | 0 | 0 | 3,200,000 | 388,163 | 3,200,000 | 1,000,000 | 3,200,000 | 3,500,000 |
| Policy and Regulatory Issues, Community and Social Dimensions | Health Canada | 200,000 | 180,000 | 200,000 | 0 | 200,000 | 0 | 200,000 | 260,000 |
| PHAC | 223,600 | 46,600 | 1,185,000 | 341,627 | 1,185,000 | 1,455,373* | 1,435,000 | 1,185,000 | |
| CIDA | 0 | 0 | 500,000 | 0 | 500,000 | 400,000 | 500,000 | 400,000 | |
| Planning, Coordination and Evaluation | PHAC | 426,000 | 426,000 | 738,000 | 738,000 | 738,000 | 738,000 | 738,000 | 738,000 |
| Non- facility sub-total |
1,349,600 | 762,600 | 10,223,000 | 2,150,414 | 10,223,000 | 5,010,315 | 10,473,000 | 8,537,019 | |
| Pilot Scale Manufacturing Capacity for Clinical Trial Lots | PHAC | 206,400 | 206,400 | 238,000 | 238,000 | 8,738,000 | 238,000 | 8,738,000 | 8,738,000 |
| Industry Canada | 0 | 0 | 3,250,000 | 0 | 3,250,000 | 3,250,000 | 3,250,000 | ||
| CIDA | 0 | 0 | 2,750,000 | 0 | 12,250,000 | 12,250,000 | 12,250,000 | ||
| Total | 1,556,000 | 969,000 | 16,461,000 | 2,388,414 | 34,461,000 | 5,248,315 | 34,711,000 | 32,775,019 | |
| Program Area | 2007-08 | 2008-09 | 2009-10 | 2010-11 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Planned | Actual | Planned | Actual | Planned | Revised Plan | Planned | Revised Plan | ||
Source: CHVI Secretariat, December 2009 * Does not include an additional $495,850 provided by PHAC to support the Global HIV Vaccine Enterprise Note: All figures for future spending of GoC departments were based on projections made for the original 5-year duration of the initiative. This table is being included for the purposes of the evaluation and will not be used to inform future spending by departments under the renewed CHVI. |
|||||||||
| Discovery and Social Research | CIHR/ IRSC |
2,000,000 | 2,243,353 | 1,500,000 | 1,753,743 | 1,550,000 | 1,550,000 | 560,681 | 10,000,000 |
| CIDA | 2,400,000 | 2,400,000 | 2,400,000 | 2,400,000 | 2,400,000 | 3,600,000 | 0 | 12,000,000 | |
| Clinical Trial Capacity Building and Networks | CIDA | 3,200,.000 | 3,500,000 | 3,200,000 | 3,500,000 | 4,111,837 | 0 | 0 | 16,000,000 |
| Policy and Regulatory Issues, Community and Social Dimensions | Health Canada | 200,000 | 280,000 | 0 | 280,000 | 0 | 0 | 0 | 1,000,000 |
| PHAC | 1,435,000 | 1,435,000 | 0 | 1,000,000 | 0 | 0 | 0 | 5,463,600 | |
| CIDA | 500,000 | 400,000 | 0 | 400,000 | 400,000 | 0 | 0 | 2,000,000 | |
| Planning, Coordination and Evaluation | PHAC | 738,000 | 738,000 | 0 | 0 | 0 | 0 | 0 | 3,378,000 |
| Non- facility sub-total |
10,473,000 | 10,996,353 | 7,100,000 | 9,333,743 | 8,461,837 | 5,150,000 | 560,681 | 49,841,600 | |
| Pilot Scale Manufacturing Capacity for Clinical Trial Lots | PHAC | 238,000 | 8,738,000 | 0 | 0 | 0 | 0 | 0 | 18,158,400 |
| Industry Canada | 3,250,000 | 3,250,000 | 0 | 3,250,000 | 3,250,0000 | 0 | 0 | 13,000,000 | |
| CIDA | 2,750,000 | 12,250,000 | 0 | 5,500,000 | 0 | 0 | 30,000,000 | ||
| Total | 16,711,000 | 35,234,353 | 7,100,000 | 18,083,743 | 11,711,837 | 5,150,000 | 560,681 | 111,000,000 | |
- This description of the context draws extensively from the “Integrated Result-Based Management and Accountability Framework and Risk-based Audit Framework”, undated, p. 6. This framework accompanied a document that was approved in June 2007.
- CHVI Website (http://www.chvi-icvv.gc.ca/gp-ld-eng.html)
- “Integrated Result-Based Management and Accountability Framework and Risk-based Audit Framework” (RMAF/RBAF), undated, p. 7
- CHVI Website (http://www.chvi-icvv.gc.ca/gp-ld-eng.html)
- RMAF/RBAF, p. 6 – 7
- RMAF/RBAF, p. 9 – 10.
- Canadian HIV Vaccine Initiative Annual Report, 2008-2009, March 30th, 2009 draft
- An RMAF is “A document which outlines the rationale, theory, resources and governance and accountability structures of a program policy or initiative and sets out a plan to measure, monitor and report on results throughout the lifecycle of the policy, program or initiative.”, “Results-Based Management Lexicon”, Treasury Board Secretariat, http://www.tbs-sct.gc.ca/cee/pubs/lex-eng.asp, Accessed March 2010. An RBAF “provides for identification of level‑of‑program monitoring and of sources of risk; assessment of the likelihood and impact of those risks, including the underlying assumptions made; and a discussion of risk mitigation actions (including management controls) taken and planned.”, “A Guide to Preparing Treasury Board Submissions, Appendix D: More Information on the "Remarks" Section”, http://www.tbs-sct.gc.ca/pubs_pol/opepubs/TBM_162/gptbs-gppct09-eng.asp. Accessed March 2010
- On 19 February 2010, the decision not to proceed with the facility was announced jointly by the GoC and the Gates Foundation. At the time of finalizing this report, the evaluation team was aware that a recommendation to this effect had been made. However, this occurred after the completion of the data collection for this evaluation. See http://www.chvi-icvv.gc.ca/index-eng.html Accessed April 2010
- Not including the $28M contribution from the Gates Foundation
Page details
- Date modified: