ARCHIVED - Statement on conjugate meningococcal vaccine for serogroups A, C, Y and W135
Canada Communicable Disease Report
Volume 33 • ACS-3
1 May 2007
An Advisory Committee Statement (ACS)
National Advisory Committee on Immunization (NACI)*†
PDF Version
24 Pages -576 KB
Preamble
The National Advisory Committee on Immunization (NACI) provides the Public Health Agency of Canada with ongoing and timely medical, scientific and public health advice relating to immunization. The Public Health Agency of Canada acknowledges that the advice and recommendations set out in this statement are based upon the best current available scientific knowledge and is disseminating this document for information purposes. People administering the vaccine should also be aware of the contents of the relevant product monograph(s). Recommendations for use and other information set out herein may differ from that set out in the product monograph(s) of the Canadian manufacturer(s) of the vaccine(s). Manufacturer(s) have sought approval of the vaccine(s) and provided evidence as to its safety and efficacy only when it is used in accordance with the product monographs. NACI members and liaison members conduct themselves within the context of the Public Health Agency of Canada's Policy on Conflict of Interest, including yearly declaration of potential conflict of interest.
Introduction
Menactra™ is a quadrivalent protein-polysaccharide conjugate vaccine produced by sanofi pasteur limited that provides protection against meningococcal serogroups A, C, Y and W135. It was approved for use in Canada for persons 2 to 55 years of age in May 2006. The polysaccharide of each meningococcal serogroup in Menactra™ is individually conjugated to a diphtheria toxoid protein carrier. One dose of 0.5 mL contains 4 μg of each of the polysaccharides for serogroups A, C, Y and W135 along with a total of 48 μg of diphtheria toxoid protein carrier.
The serogroup coverage provided by Menactra™ is the same as that of Menomune®, the currently available quadrivalent polysaccharide meningococcal product, which is also produced by sanofi pasteur. In addition, there are three monovalent meningococcal C conjugate products currently available in Canada: Menjugate® (Novartis Vaccines), NeisVac-C™ (GlaxoSmithkline), and Meningitec™ (Wyeth Canada).
As additional information on Menactra™ becomes available and the epidemiology of invasive meningococcal disease (IMD) changes, the recommendations in this statement will be reviewed.
Epidemiology of meningococcal disease in Canada
IMD is endemic in Canada. It has periods of increased activity occurring roughly every 10 to 15 years with no consistent pattern. IMD incidence varies considerably, different serogroups affecting different age groups and geographic locations at various times. The last major epidemic of meningococcal disease occurred in 1940-1943 (serogroup A), when the peak incidence was close to 13 per 100,000 population per year. Since then, the overall incidence of disease has remained at ≤ 2.1 per 100,000 per year (range 0.5 to 2.1 per 100,000 per year).
Between 1995 and 2004, the overall Canadian incidence of IMD remained at or below 1.1 per 100,000 per year (range 0.6 to 1.1 per 100,000 per year). Overall, the average annual incidence rate is highest among children < 1 year of age (9.2 per 100,000) and then declines as age increases, except for a smaller peak in the 15 to 19 year age group (2.0 per 100,000). An average of 244 cases of meningococcal disease have been reported annually. Disease occurs year round, but there is seasonal variation with the majority
of cases occurring in the winter months. Of the 2,437 cases reported between 1995 and 2004, infants < 1 year of age, who make up approximately 1% of the Canadian population, accounted for 13% of cases (mean 32 cases per year). Children and adolescents aged 1 to 4 years, 5 to 9 years, 10 to 14 years and 15 to 19 years accounted for 15% (mean 37 cases per year), 5% (mean 13 cases per year), 6% (mean 13 cases per year) and 17% (mean 42 cases per year) respectively. Adults ≥ 20 years of age accounted for 43% of cases. The overall case fatality rate (CFR) for this period was 9%.
Of the small numbers of Neisseria meningitidis isolates characterized from 1971 to 1974, serogroups A and C were the most frequently identified. From 1975 to 1989 serogroup B predominated1. In 1986 a new clone of serogroup C, serotype 2a characterized by multi-locus enzyme electrophoresis as electrophoretic type 15 (ET-15), was identified in Canada for the first time2. Since then, serogroups B and C have been responsible for most of the cases of endemic IMD in Canada. However, serogroup C isolates have almost exclusively been responsible for clusters or outbreaks in schools and communities. Sporadic cases of serogroup C are caused by a wide variety of strains, whereas clusters or outbreaks are generally caused by a single circulating strain. Figure 1 shows the annual incidence rate by serogroup for 1995-2004. The proportion of reported cases for which serogroup data are available has risen over time, from 60% in 1985 to 88% in 1992 and 94% in 2004. Table 1 summarizes the epidemiology of serogroups A, B, C, Y and W135 in Canada.
Figure 1. Incidence rates of invasive meningococcal disease by serogroup and year, 1995-2004
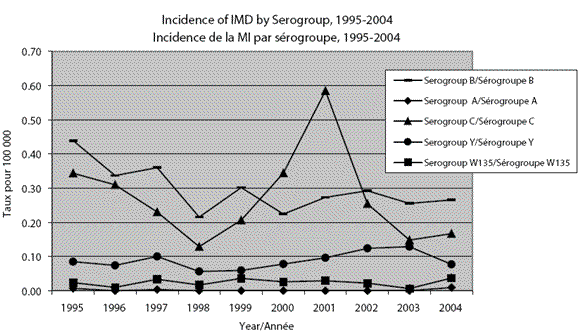
Table 1. Summary of the epidemiology of meningococcal disease in Canada between 1995 and 2004 by serogroup
| Serogroup |
Average annual number of cases (range) |
Average annual rate |
Median age |
Case fatality rate |
Comments |
| C |
84 cases |
0.27/100,000 |
19 years |
13% |
Rates declining likely as a result of serogroup C conjugate vaccinationprograms |
| B |
93 cases |
0.30/100,000 |
11 years |
6% |
Rates have been relatively stable over time |
| Y |
28 cases |
0.09/100,000 |
45 years |
7% |
Rates have been relatively stable over time |
| W135 |
9 cases |
0.03/100,000 |
19 years |
8% |
|
| A |
0.5 cases |
0.002/100,000 |
41 years |
No deaths reported |
Mainly a risk for travellers to meningococcal-endemic areas |
*Data related to serogroup unknown or not typeable not shown
Serogroup C epidemiology
From 1995 to 2004, there were an average of 84 serogroup C cases reported per year in Canada, with a peak incidence in 2001 of 0.58 per 100,000 population. The highest average annual incidence rates for serogroup C are seen among infants < 1 year of age (0.90 per 100,000) and adolescents 15 to 19 years of age (0.90 per 100,000). An annual average of 3.1 (range 1 to 8 cases) and 18.7 (range 6 to 40 cases) cases were reported for these age groups respectively.
The emergence of the serogroup C ET-15 clone was associated with localized outbreaks and periods of elevated activity during 1989-1993 and 2000-20012,3. Recent data suggest that incidence rates of serogroup C are decreasing. This is expected as immunization programs are implemented. The average incidence rates for serogroup C disease during 1995-2001 and 2002-2004 were 0.31 per 100,000 and 0.19 per 100,000 respectively. However, as the epidemiology of IMD is unpredictable, more years of data are needed to determine whether this reduction will be sustained.
Serogroup B epidemiology
There has been less fluctuation in the incidence of serogroup B disease over time in Canada than serogroup C. From 1995 to 2004, an average of 93 serogroup B cases were reported per year, resulting in an average annual incidence of 0.30 per 100,000 population (range 0.21 to 0.44). Although children < 5 years of age constitute 5.9% of the total population, this age group accounts for the greatest proportion (43.5%) of serogroup B cases. Young children have the highest average annual incidence (per 100,000 population), with rates of 6.40 among infants < 1 year of age and 1.21 among children 1 to 4 years of age.
Serogroup Y epidemiology
The incidence of serogroup Y remained relatively stable in Canada between 1995 and 2004, at an average of 0.09 cases per 100,000 population. The average number of cases per year was 28. Serogroup Y disease has tended to affect older individuals (median 45 years of age).
Serogroup A and serogroup W135 epidemiology
Neither serogroup A nor serogroup W135 meningococcal disease is commonly reported in Canada. Between 1995 and 2004, there were a total of five cases of invasive serogroup A disease reported (range 0 to 2 cases per year) with an average incidence of 0.002 per 100,000 population. There were no deaths attributed to serogroup A disease. During the same period, a total of 90 cases of invasive serogroup W135 disease were reported with an average incidence of 0.03 per 100,000 population.
Serogroup A and W135 remain risks for travellers to meningococcal- endemic areas and to Mecca, Saudi Arabia, during the Hajj. The traditional endemic or hyperendemic areas of the world (the “meningitis belt”) include the savannah areas of sub-Saharan Africa extending from Gambia and Senegal in the west to Ethiopia and Western Eritrea in the east.
The annual average risk of IMD was estimated in a study by De Wals at al4. Age- and serogroup-specific annual attack rates were derived from Public Health Agency of Canada surveillance data for the 1995-2001 period and adjusted for under-diagnosis, under-reporting and unknown serogroups. The estimated annual average risk vaccine preventable IMD risk in the period before the implementation of immunization programs against serogroup C was 5.7 per million, with serogroup-specific risks as follows: 3.9 per million for serogroup C, 1.4 per million for serogroup Y, 0.4 per million for serogroup W135 and close to zero for serogroup A.
Comparison of meningococcal vaccines currently available in Canada
Two categories of meningococcal vaccine are currently available in Canada - polysaccharide vaccines and protein-polysaccharide conjugate vaccines (hereafter referred to as conjugate vaccines). Polysaccharide vaccines are not recognized by T cell receptors. The T cell independent response produced by polysaccharide vaccines makes these vaccines poorly immunogenic in children < 2 years of age(5), which results in poor effectiveness6. Polysaccharide vaccines also do not induce immunologic memory, and they have limited duration of protection7,8. Reimmunization is recommended at 6 months to ≤ 5 years depending on the age of initial vaccination if the risk is ongoing9. Polysaccharide vaccines have a limited effect on asymptomatic carriage, and the effect on herd immunity, if present, does not appear to be long lasting10,11. Polysaccharide vaccines can induce hyporesponsiveness such that repeated doses of polysaccharide vaccines do not achieve geometric mean titres (GMTs) as high as those achieved after the initial dose8,12,13. This raises concerns that vaccinated individuals will also have a suboptimal immune response when exposed to N. meningitidis, resulting in increased susceptibility to disease. A possible increase in susceptibility was noted in children ≤ 5 years of age at 3 to 5 years after vaccination with polysaccharide vaccine in a mass immunization campaign in Quebec. For children 2 to 5 years of age at vaccination, the vaccine effectiveness 3 to 5 years later was - 73.8% (95% confidence interval [CI], - 1956.2% to 85.3%), and for children < 2 years of age at vaccination the effectiveness was - 390.5% (95% CI, - 4599.2% to 48.8%) 3 to 5 years later14. However, the clinical significance of immunologic hyporesponsiveness is not known.
In contrast, conjugate vaccines do induce a T cell antibody response and therefore generate antibodies with better functional activity15 , which are immunogenic in young children, induce immunologic memory8,16,17 and decrease N. meningitidis carriage18. In the United Kingdom (UK) this reduction in carriage has been shown to induce herd immunity: a 67% reduction (95% CI, 52% to 77%) in meningococcal C disease was noted in unvaccinated children when comparing the time frame before and after implementation of the mass campaign, in which more than 80% of the target population was vaccinated19. As well, conjugate vaccines do not induce hyporesponsiveness and can overcome the hyporesponsiveness induced by polysaccharide vaccines. However, the GMTs tend to be somewhat lower following conjugate vaccination in those who previously received a polysaccharide vaccine as compared with those vaccinated only with a conjugate vaccine13,20,21. Younger children previously vaccinated with polysaccharide vaccines who did not achieve a satisfactory response after one dose of conjugate vaccine did mount a satisfactory response after a second dose of conjugate vaccine20.
Three different carrier proteins have been used to manufacture conjugate vaccines: CRM197 (Meningitec™ and Menjugate®), tetanus toxoid (NeisVac-C™) and diphtheria toxoid (Menactra™). CRM197 is a non-toxic mutant of diphtheria toxin isolated from culture of Corynebacterium diphtheriae22. The diphtheria toxoid carrier used in Menactra™ is a toxin produced by C. diphtheriae that has been detoxified with formaldehyde. The same carrier protein is used in diphtheria-toxoid-containing vaccines such as DTaP-Polio, Td and dTap, but the diphtheria toxoid in Menactra™ is not an immunizing agent.
With the approval of Menactra™, six different meningococcal products are approved for use in Canada. Table 2 provides a comparison of the available products.
Table 2. Comparison of the meningococcal vaccine products approved for use in Canada
| Name |
Type of vaccine |
Manufacturer |
Concentration of polysaccharide(s) |
Protein carrier |
Administration |
Schedule |
| Menactra™ |
Conjugate A, C, Y, W135 |
Sanofi Pasteur |
4 μg of each serogroup |
48 μg of diphtheria toxoid |
0.5 mL IM |
2-55 years of age: 1 dose |
| Meningitec™ |
Conjugate C |
Wyeth Canada |
10 μg |
15 μg CRM197 |
0.5 mL IM |
Infants: 3 doses starting no earlier than 2 months and separated by at least 1 month, with 1 dose after 5 months of age; Infants 4 to 11 months not previously vaccinated: 2 doses at least 1 month apart; Children ≥ 1 year of age, adolescents and adults: 1 dose |
| Menjugate® |
Conjugate C |
Novartis Vaccines |
10 μg |
12.5-25 μg CRM197 |
0.5 mL IM |
Infants: 3 doses starting no earlier than 2 months and separated by at least 1 month, with 1 dose after 5 months of age; Infants 4 to 11 months not previously vaccinated: 2 doses at least 1 month apart; Children ≥ 1 year of age, adolescents and adults: 1 dose |
| Neis Vac-C™ |
Conjugate C |
GlaxoSmithKline |
10 μg |
10-20 μg tetanus toxoid |
0.5 mL IM |
Infants: 2 doses starting no earlier than 2 months of age and separated by at least 2 months, with 1 dose after 5 months of age; Children ≥ 1 year of age, adolescents and adults: 1 dose |
| Menomune® |
Polysaccharide A, C |
Sanofi Pasteur |
50 μg of each serogroup |
Not applicable |
0.5 mL SQ |
Children ≥ 2 years of age, adolescents and adults: 1 dose;repeat at interval based on age if at ongoing risk; can be used for children ≥ 3 months of age for serogroup A protection |
| Menomune® |
Polysaccharide A, C, Y, W135 |
Sanofi Pasteur |
50 μg of each serogroup |
Not applicable |
0.5 mL SQ |
Children ≥ 2 years of age, adolescents and adults: 1 dose;repeat at interval based on age if at ongoing risk; can be used for children ≥ 3 months of age for serogroup A protection |
| IM - intramuscularly |
||||||
Before 2001, polysaccharide vaccines (Menomune® A/C/Y/W135 and Menomune® A/C) were used to control outbreaks of serogroup C disease. Menomune® A/C/Y/W135 was used for travellers, those with high-risk medical conditions, military personnel and certain laboratory workers. Monovalent meningococcal C conjugate vaccines were first approved for use in Canada in 2001. Routine meningococcal C conjugate programs have been implemented in all jurisdictions in Canada beginning in 2002.Programs vary by jurisdiction; further information can be found at http://www.phac-aspc.gc.ca/im/ptimprog-progimpt/index-eng.php.
As seen in Table 2, Menactra™ contains a lower concentration of polysaccharide for serogroup C than the three monovalent conjugate C products (4 μg compared with 10 μg) and a substantially lower concentration than Menomune® (50 μg per dose). Three published studies assessed the optimal dose of polysaccharide in a conjugate quadrivalent meningococcal vaccine comparing 1 μg, 4 μg and 10 μg for each serogroup. A study in infants found no statistical difference in antibody concentrations between the three doses and no statistically significant immunologic advantage of increasing the dose above 4 μg. In these infants, higher rates of local reactions were observed with the 10 μg dose23. The 4 μg dose was found to be immunologically optimal in a study in toddlers24. Among adults, the results showed a dose-related effect for serogroups A, C and W135, the 10 μg dose providing the best immunologic response for these serogroups25.
Immunogenicity, efficacy and effectiveness of meningococcal vaccines
Regulatory approval of conjugated vaccines for meningococcal disease has been based primarily on short-term immunogenicity studies26. Data from efficacy and effectiveness studies have been difficult to obtain because of the relative rarity of meningococcal disease. Serum bactericidal antibody (SBA) levels are the standard measure used to determine susceptibility and immunity to IMD. Traditionally, human serum has provided the complement source for this assay. Using human serum complement, a titre of ≥ 1:4 has been found to correlate with protection against serogroup C meningococcal disease27. Baby rabbit serum (rSBA), which is more readily available, has replaced human complement in more recent studies. A titre of ≥ 1:8 has been proposed as a correlate of short-term immunity using baby rabbit complement28. There are no immunologic correlates of protection for the other three vaccine
preventable serogroups. GMTs are also used as a measure of immune response to meningococcal vaccine. In 1976, the WHO Expert Committee on Biological Standardisation recommended that for a meningococcal polysaccharide vaccine to be acceptable, ≥ 90% of the immunized subjects must have at least a 4-fold rise (increase of ≥ 2 dilutions) in SBA titre when tested against target strains by the specified SBA assay29. Although immunogenicity studies likely predict short-term effectiveness, their ability to determine long-term effectiveness is uncertain. Additionally, immunogenicity studies do not predict the impact of vaccination on carriage and herd immunity.
Recently, several published studies have assessed the effectiveness of meningococcal C conjugate vaccines for outbreak management followed by introduction into the routine schedule in Quebec30, the UK31 and Spain32. These studies have all shown excellent effectiveness in the first year after the mass immunization program. Two of them also provide data on longer-term follow-up31,32. These studies raise concerns about the long-term effectiveness of the vaccine among children who received the meningococcal C conjugate vaccine as young infants without a booster dose.
Previously in the UK, infants received the meningococcal C conjugate vaccine at 2, 3 and 4 months of age. Trotter et al. found that at more than 1 year after scheduled vaccination, effectiveness in the children vaccinated as infants with this schedule had fallen to - 81% (95% CI, - 7430% to 71%). Those vaccinated at 5 to 11 months of age and 1 to 2 years of age had better long-term protection, showing vaccine effectiveness at more than 1 year of 82% (95% CI, - 8% to 97%) and 61% (95% CI, - 327% to 94%) respectively. Even in these older age groups, the confidence intervals
were very wide and included zero, suggesting that long-term efficacy may be low31. In Spain, infants were vaccinated with meningococcal C conjugate vaccine at 2, 4 and 6 months of age. At more than 1 year after vaccination, the effectiveness in those given this schedule was 78% (95% CI, 3.1% to 95.0%). Children vaccinated at 7 months to 5 years of age had greater protection, with a vaccine effectiveness at > 1 year after vaccination of 94.3% (95% CI, 71.2% to 98.8%)32.
Immune memory has been demonstrated for meningococcal C conjugate vaccines as indicated by increases in titres upon challenge with polysaccharide or meningococcal C conjugate vaccines21,33. However, it is not known whether immune memory is sufficient to protect against IMD, which has a short incubation period (range 2 to 10 days, commonly 3 to 4 days34). It may be that, because of the short incubation period, circulating antibodies are required for protection. It is possible that decreases in meningococcal disease as a result of vaccination may lead to decreased natural boosting opportunities, decreased circulating antibodies and increased susceptibility with time. Consequently, booster doses of meningococcal C conjugate vaccine may be needed in the future26. A modeling study by De Wals et al. reported that with a schedule of one dose of meningococcal C conjugate vaccine at 12 months of age, a booster dose would be indicated if meningococcal C protection waned at 3% or more per year. The authors suggest that the optimal age for this booster dose would be around 12 years of age, given the current epidemiology of serogroup C meningococcal disease in Canada35.
Immunogenicity and duration of protection of Menactra™
Menactra™ was approved for use mainly on the basis of demonstration of immunologic non-inferiority to Menomune® for all four serogroups. Studies have been conducted in three age groups: children 2 to 10 years of age36,37, adolescents 11 to 18 years of age36,38,39 and adults 18 to 55 years of age36,38. Studies are also being conducted in infants23, although the vaccine is not currently approved for use in this age group. No studies have been conducted that directly compare Menactra™ with the currently approved meningococcal C conjugate vaccines. As well, in none of the above-mentioned studies was a distinction made between the immune response in naïve individuals and in those who were already primed following asymptomatic nasopharyngeal carriage of N. meningitidis or infection with another organism inducing cross reaction.
Several measures of immunity have been used in Menactra™ studies. The main outcome measure has been the percentage of participants who achieved a ≥ 4-fold rise in rSBA titres from before immunization to 28 days after immunization as compared with Menomune®. Additional outcome measures have included comparison of Menactra™ and Menomune® in terms of the GMTs for each serogroup; seroconversion, defined as the percentage of individuals whose titre changed from < 1:8 before vaccination to ≥ 1:32 at 28 days after vaccination; reverse cumulative distribution curves comparing the percentage of people who achieved a range of titres 28 days after receiving Menactra™ or Menomune®; and serogroup-specific IgG and IgM antibodies as measured by ELISA36,38.
Adults 18 to 55 years of age: Menactra™ was found to be immunologically non-inferior to Menomune® in a randomized control trial that enrolled 2,554 adults 18 to 55 years of age. However, in this trial the percentage of individuals who achieved a ≥ 4-fold rise in rSBA at 28 days after vaccination was lower for all four serogroups in the Menactra™ group than in the Menomune ® group: serogroup C, 88.5% versus 89.7%; serogroup A, 80.5% versus 84.6%; serogroup Y, 73.5% versus 79.4%; and serogroup W135, 89.4% versus 94.4%. GMTs were also somewhat lower for Menactra™ than Menomune® for all four serogroups36,38. A lot-consistency study in this age group found that all three studied lots were immunogenic, but the rSBA responses to serogroups C and Y showed some variability38.
Adolescents 11 to 18 years of age: Menactra™ was found to be immunologically non-inferior to Menomune® in a randomized control trial that enrolled 881 adolescents 11 to 18 years of age. The percentage of individuals achieving a ≥ 4-fold rise in rSBA titres at 28 days after vaccination was similar between the Menactra™ and Menomune® groups for all serogroups: serogroup C, 91.7% versus 88.7%; serogroup A, 92.7% versus 92.4%; serogroup Y, 81.8% versus 80.1%; and serogroup W135, 96.7% versus 95.3% respectively. GMTs were somewhat higher for Menactra™ than Menomune® for serogroups A, C and Y and slightly lower for serogroup W13536,38,39.
Three years after vaccinating their original series, Keyserling et al. revaccinated a subset of the original subjects with Menactra™ (76 and 77 adolescents who had originally received Menactra™ and Menomune® respectively). Before revaccination, the group that originally received Menactra™ had higher GMTs for all serogroups than the group that received Menomune®. On days 8 and 28 after revaccination, the GMTs were substantially higher in the Menactra™ group than the Menomune® group39.
Children 2 to 10 years of age: Menactra™ was found to be immunologically non-inferior to Menomune® in a randomized controlled trial by Pichichero et al., which enrolled 1,398 children 2 to 10 years of age. The percentage of individuals achieving a ≥ 4-fold rise in rSBA titres at 28 days after vaccination was higher for the Menactra™ group than the Menomune® group for all serogroups: serogroup C, 73.4% versus 68.9%; serogroup A, 87.7% versus 83.8%; serogroup Y, 56.6% versus 45.6%; and serogroup W135, 91.0% versus 85.4%. GMTs were higher for Menactra™ than Menomune® for all four serogroups at both 28 days and 6 months after vaccination36,37.
Although this study suggests good immunogenicity with Menactra™ as compared with Menomune® in children 2 to 10 years of age, it should be noted that the effectiveness of Menomune® in this age group is lower than at older ages. De Wals et al. assessed the impact of a mass immunization campaign using Menomune® in response to an outbreak of serogroup C meningococcal disease in Quebec in the winter of 1992-1993. Age-adjusted vaccine effectiveness in the 5 years after vaccination was 41% (95% CI, - 106% to 79%) for children 2 to 9 years of age. By comparison, adolescents 10 to 14 years of age showed vaccine effectiveness of 75% (95% CI, - 17% to 93%), and persons 15 to 20 years of age demonstrated vaccine effectiveness of 83% (95% CI, 39% to 96%)6. In addition, Tables 3 and 4 demonstrate that children 2 to 10 years of age vaccinated with Menactra™ have lower GMTs and a lower percentage demonstrating a ≥ 4-fold rise in antibody response compared with older age groups. These data suggest that effectiveness in children 2 to 10 years of age may be lower and/or of shorter duration than at older age groups.
A series of studies assessing the immunity and duration of protection of Menactra™ in children 2 to 3 years of age were conducted on a subset of children from the Pichichero et al. study. The number of children enrolled in these studies who received Menactra™ ranged from 30 to 90, and some of the studies included the same subjects in their analyses40-42.
In one study, Menactra™ was found to induce bactericidal antibody titres against serogroup C (defined as a ≥ 1:4 titre using a human complement source) in 50% of 30 children 1 month after Menactra™. After 6 months, 40% of the 30 children had a bactericidal antibody titre of ≥ 1:440. In another publication, antibody concentrations in 48 children were measured 2 to 3 years after Menactra™ when the children were between 4 and 5 years of age. This study found that only 14.6% of vaccinated children had bactericidal antibody titres against serogroup C 2 to 3 years after vaccination with Menactra™41. Similarly, 40 children who were 2 years of age when they received Menactra™ were followed up for a mean interval of 2.4 years to assess persistence of immunity to serogroups A, Y and W135. Less than half had persistent bactericidal antibodies at a titre of ≥ 1:4. Specifically, 15% had a titre of ≥ 1:4 for serogroup A, 32.5% for serogroup Y and 45% for serogroup W13542.
Another follow-up study was conducted on 92 children from the Pichichero et al. study. This study examined immune memory in children 2 to 3 years after they had been vaccinated with Menactra™ at 2 to 3 years of age. Very high GMTs for all serogroups were found 8 and 28 days after re-vaccination with a challenge of one-tenth of a dose of Menomune®, suggesting induction of immune memory in young children. Although GMTs were much higher in the group that had previously received Menactra™, the previously unvaccinated control group also had high GMTs at eight and 28 days after the challenge Menomune® dose36.
A randomized controlled study that enrolled 103 children 2 to 4 years of age assessed the immunogenicity of Menactra™ compared with a control vaccine (against Haemophilus influenzae type b) in those children who had received meningococcal C conjugate vaccine at least 1 year earlier. A high percentage of participants who had received Menactra™ achieved a ≥ 4-fold response for all serogroups at 28 days after vaccination: serogroup C, 93.2%; serogroup A, 97.7%; serogroup Y, 79.6%; and serogroup W135, 97.7%. The GMTs were high for all serogroups and were very high for serogroups C and A. This study suggests that Menactra™ can be used to boost the immune response in children who have previously received meningococcal C conjugate vaccine36,43.
In Canada, three monovalent meningococcal C conjugate products are approved for use in the 2 to 10-year age group. Some evidence of the effectiveness of these vaccines in this age group is now available. Trotter et al. demonstrated an effectiveness of 93% (95% CI, 78% to 98%) more than 1 year after vaccination in children vaccinated at 3 to 4 years of age(31). Similarly, Larrauri et al. showed that the meningococcal C conjugate vaccine was 94.3% effective (95% CI, 71.2% to 98.8%) in children vaccinated at 7 months to 5 years of age more than 1 year after vaccination32. Given the uncertainties regarding the immunogenicity of Menactra™ among children 2 to 10 years of age and the evidence of effectiveness of meningococcal C conjugate vaccines, additional studies will be required to ensure that Menactra™ provides comparable protection to that of the meningococcal C conjugate vaccines in this age group.
Table 3. Geometric mean titres by age at 28 days after vaccination with one dose of Menactra™
| Age group |
2 to 10-year-olds 36,37 |
11 to 18-year-olds 36,38,39 |
18 to 55-year-olds 36,38 |
| Serogroup C |
354 |
1,924 |
3,231 |
| Serogroup A |
1,700 |
5,483 |
3,897 |
| Serogroup Y |
637 |
1,322 |
1,750 |
| Serogroup W 135 |
750 |
1,407 |
1,271 |
Table 4. Percentage of participants who achieved a ≥ 4-fold rise in antibody titres from before vaccination to 28 days after vaccination with one dose of Menactra™
| Age group |
2 to 10-year-olds 36,37 |
11 to 18-year-olds 36,38,39 |
18 to 55-year-olds 36,38 |
| Serogroup C |
73.4% |
91.7% |
88.5% |
| Serogroup A |
87.7% |
92.7% |
80.5% |
| Serogroup Y |
56.6% |
81.8% |
73.5% |
| Serogroup W 135 |
91.0% |
96.7% |
89.4% |
Summary
Menactra™ has been shown to be non-inferior to Menomune® in all age groups, although the immune response to Menactra™ is lower in the group 2 to 10 years old than at older ages. The immunogenicity of Menactra™ has not been compared with meningococcal C conjugate products, so non-inferiority to these products cannot be determined. Immunogenicity data alone are insufficient to predict vaccine effectiveness and herd immunity. Additional studies are needed to evaluate vaccine effectiveness, vaccine impact on nasopharyngeal carriage, indirect effects of vaccine on disease rates among unvaccinated populations and non-inferiority to meningococcal C conjugate vaccines. It is expected that MenactraTM will provide longer protection than polysaccharide vaccine, but as is the case with meningococcal C conjugate vaccines studies are needed to determine the duration of protection with Menactra™.
Description of Menactra™
One 0.5 mL dose of Menactra™ contains 4 μg each of serogroups A, C, Y and W135 capsular polysaccharide antigens, individually conjugated to diphtheria toxoid protein. Each dose contains a total of 48 μg of diphtheria toxoid protein carrier. Menactra™ is a sterile, clear to slightly turbid liquid. It should be checked for discoloration and particulate matter before use. There are no preservatives or adjuvants in Menactra™. It is supplied in both prefilled syringes and single-dose vials. The prefilled syringes contain no latex, but there is latex in the stopper of the vials.
Dosage and route of administration
Menactra™ is administered as a single intramuscular (IM) injection of 0.5 mL. The deltoid region is the preferred location for administration.
A study was conducted of 100 people who inadvertently received Menactra™ subcutaneously. The rates of adverse events were similar in the group that had received the vaccine subcutaneously to those in retrospective controls who had received the vaccine through the IM route. The percentage of subjects with rSBA titres ≥ 1:8 was similar in the two groups, although the GMTs were lower for serogroups A, C and Y when the vaccine was administered subcutaneously. It is therefore not felt to be necessary to repeat the dose of Menactra™ if it is inadvertently administered subcutaneously44.
Vaccination with Menactra™ does not change the need for routine diphtheria immunizations.
Storage requirements
Menactra™ should be maintained between +2o C and +8o C during transportation and storage, and should not be frozen. It should be protected from light.
Simultaneous administration with other vaccines
A study that enrolled 945 adults aged 18 to 55 years assessed immunologic response to the concomitant administration of typhoid vaccine and Menactra™ as compared with the administration of typhoid vaccine followed after 1 month by Menactra™. No difference was noted in the immunologic response to either vaccine in the two groups36,38. See the Adverse reactions section for information on the safety of the coadministration of typhoid vaccine and Menactra™.
The concomitant administration of Td and Menactra™ was compared with the administration of Td followed after 1 month by Menactra™ in a study involving 1,010 adolescents 11 to 17 years old36,38. This study found that the GMTs for diphtheria were much higher in the group that received both vaccines at the same visit. The concomitant group also had much higher GMTs for all four meningococcal serogroups. As well, the proportion achieving a ≥ 4-fold rise in titres was higher in the concomitant group for serogroups C, Y and W135 but not for serogroup A. The GMTs in the group that received Td followed by Menactra™ were similar for serogroups C, Y and W135 and higher for serogroup A compared with the titres obtained in another study in this age group of Menactra™ alone36,38,39. However, the Td followed by Menactra™ group had lower percentages of individuals achieving a ≥ 4-fold rise in titres at day 28 after vaccination for serogroups C, Y and W135 (82.4%, 65.1% and 87,7% respectively) than the Menactra™ alone group. This would suggest that it may be preferable to administer Td with Menactra™ if both products are needed. The tetanus GMTs were similar between the two groups. See the Adverse reactions section for information on the safety of the coadministration of Td and Menactra™.
No studies have assessed the safety, effectiveness or immunogenicity of concomitant administration of Menactra™ and other vaccines, such as dTap, hepatitis B, hepatitis A or human papillomavirus. In general, more than one vaccine can be administered at the same visit using separate needles, syringes and injection sites45.
Revaccination
The need for reimmunization with additional doses of MenactraTM is currently unknown, as there are insufficient data to predict persistence of immunity and long-term effectiveness. Ongoing monitoring will be needed to determine whether revaccination or booster doses are required.
Individuals who previously received polysaccharide meningococcal vaccine should receive Menactra™ if the risk of meningococcal disease is ongoing. Revaccination with Menactra™ after polysaccharide vaccine in those with ongoing risk should be considered 1 to 2 years later if the polysaccharide vaccine was received at 13 to 23 months of age; 2 to 3 years later if the polysaccharide vaccine was received at 2 to 5 years of age; and 5 years later if the polysaccharide vaccine was received at ≥ 6 years of age. Since an adequate response to conjugate C vaccine has been observed with a delay of 6 months after immunization with purified polysaccharide vaccine in adults13, this remains the recommended minimum interval to receive Menactra™ after having received a polysaccharide vaccine, until further data are available.
Individuals who have received meningococcal C conjugate vaccine can receive Menactra™, and similarly those who have received Menactra™ can receive meningococcal C conjugate vaccine. Although no data exist, an interval of 1 month between products is recommended according to expert opinion.
Adverse reactions
The safety of Menactra™ has been assessed in the following three age groups: 2 to 10 years36,37, 11 to 18 years36,38,39 and 18 to 55 years36,38. In persons 11 to 55 years of age, six clinical trials assessed safety in a total of 7,640 participants who had received Menactra™36. Three clinical trials assessed safety in over 2,400 children 2 to 10 years of age who received Menactra™36. Severe local and systemic reactions were uncommon.
Local reactions: In general, local reactions tend to occur more often following Menactra™ than Menomune®. This may be due to the diphtheria toxoid carrier protein in Menactra™ or to its IM administration rather than the subcutaneous administration of Menomune®. In a summary of clinical trials, injection site pain within 7 days following Menactra™ was reported in 56.3% of persons 1 to 55 years of age38. In children 2 to 10 years of age, pain was reported in 48.1% of Menactra™ recipients in one study37 and 39.7% in another36.
In adults, pain occurred more commonly at the typhoid vaccine injection site (75.2%) than the Menactra™ injection site (46.5%) when these two vaccines were administered at the same visit36,38. Similarly when Menactra™ and Td were administered at the same visit to children aged 11 to 17, more local reactions were reported from the Td injection site than the Menactra™ site (70.9% reported pain from the Td site versus 52.9% from the Menactra™ site)36,38. Reactions were no more common at the Menactra™ injection site when the Td and Menactra™ were administered at the same visit than when Menactra™ was administered 1 month after Td. No studies have assessed the impact of administering Menactra™ followed by Td or dTap. These combinations have the potential to result in increased local reactions because of the concentration of diphtheria toxoid in Menactra™.
Systemic reactions: The frequency of systemic reactions was similar in both the Menactra™ and Menomune® groups36-39. In a summary of studies, headache and fatigue were the most commonly reported adverse reactions within 7 days of vaccination. Headache was reported in 40.3% of those aged 15 to 2538, 37.4% of those aged 26 to 55(38) and 44.8% of those aged 11 to 18(39) who had received Menactra™. In these same age groups fatigue was reported in 34.0%38, 29.1%38 and 28.2%39 respectively. The most frequently reported adverse effects within 7 days of vaccination in those aged 2 to 19 years were fussiness (35.2%) and drowsiness (26.0%)37. No serious systemic reactions attributable to the vaccine were reported in these trials.
A study that concomitantly administered typhoid vaccine and Menactra™ to adults compared the rates of adverse events with those of a group that received typhoid vaccine followed after 1 month by Menactra™36,38. Systemic reactions such as headache and fatigue occurred more often in the group that received concomitant typhoid vaccine and Menactra™, but the rates of these adverse events were similar to those reported after the typhoid vaccine was given alone36. In a study in 11 to 17-year-olds, adverse systemic reactions were reported more frequently after Menactra™ and Td had been administered concomitantly than when Menactra™ had been administered 1 month after Td. However, in general the rates of adverse events in the Menactra™ and Td group were similar to those reported in other studies after Menactra™ alone38. As well, headache and fatigue, the most common reactions, occurred at a rate in the concomitant group similar to that reported after the Td vaccine alone36.
Guillain-Barré Syndrome (GBS): Post-marketing surveillance using the Vaccine Adverse Event Reporting System (VAERS) in the United States has identified 17 cases of GBS occurring within 6 weeks of receipt of Menactra™. The cases of GBS occurred between 2 and 33 days after vaccination with a mean of 15.7 days and a median of 14 days. The cases were reported to be significantly clustered around days 9 to 15, and this clustering was more than would be expected by chance (p = 0.012). No cases reported symptoms compatible with Campylobacter jejuni, a leading cause of GBS. One stool culture and one serologic test for C. jejuni were negative. Fifteen of the 17 cases occurred in adolescents between the ages of 11 and 19 years, which is the age group most commonly being vaccinated with Menactra™ in the United States. The other two cases were 30 and 43 years of age.
An analysis of the risk of GBS in the 11 to 19 age group was conducted. Background rates of GBS in this age group were determined from two sources, the Healthcare Cost and Utilization Project (HCUP) and the Vaccine Safety Datalink (VSD). The rate of GBS from VAERS was calculated per person-month based on the number of doses of vaccine distributed to that age group. Using background GBS rates from HCUP (0.11 per 100,000 person-months in 11 to 19-year-olds), the VAERS reported rate of GBS was 1.78 times higher (95% CI, 1.02 to 2.85). Using background rates from VSD (0.11 per 100,000 person-months) and adjusting for seasonal variation, the VAERS reported rate of GBS was 1.77 times higher (95% CI, 0.96 to 3.07).
Using the relative risk of GBS after vaccination of 1.78 based on the HCUP data, the Centers for Disease Control and Prevention (CDC) in the United States estimated that 1.25 (95% CI, 0.058 to 5.99) excess cases of GBS can be anticipated for every 1 million doses of vaccine distributed to persons 11 to 19 years of age. The CDC indicated that substantial uncertainty exists regarding this risk estimate. Because of the limitation of the data indicating a small risk of GBS and the ongoing risk of meningococcal disease, the CDC did not make any changes to their recommendation to use Menactra™ to vaccinate adolescents, college freshmen living in dormitories and others at high risk of meningococcal disease. The CDC recommended that Menactra™ not be used in those with a past history of GBS, although people at especially high risk of meningococcal disease, such as some microbiologists, might consider immunization46. It should be noted that monovalent meningococcal C conjugate vaccines are not licensed in the United States, and therefore Menactra™ is the only conjugate meningococcal vaccine available there.
Health care providers should report cases of GBS temporally associated with Menactra™ to their local public health authority.
Contraindications and precautions
Menactra™ should not be used in anyone who has experienced an anaphylactic reaction to a previous meningococcal vaccine or any of its components. Individuals with a prior history of anaphylaxis to other meningococcal or diphtheria-containing vaccines should be evaluated in an attempt to determine the component responsible for the allergic reaction. Individuals with a known anaphylactic allergy to latex should not receive Menactra ™ that has come from the vial, since the vial stopper contains latex. The prefilled syringe preparation does not contain latex.
Although the risk of recurrent GBS is unknown, caution should be used in administering Menactra™ to an individual with a previous episode of GBS especially when alternate products are available. For individuals aged ≥ 2 years with a history of GBS, a polysaccharide vaccine is preferred for short-term protection as no association with GBS has been described for any meningococcal polysaccharide vaccine, despite extensive use for more than 30 years. For extended protection against serogroup C IMD, a monovalent conjugate C product is preferred. For extended protection against serogroups A, Y and W135, the risk of recurrence of GBS should be balanced against the risk of IMD and the patient appropriately informed.
Menactra™ has not been studied in immunocompromised individuals. As with other inactivated vaccines, its effectiveness may be decreased in these individuals.
Menactra™ has not been studied in pregnant or breastfeeding women so should only be used if the benefits outweigh the risks.
Recommended usage
Preamble: IMD is relatively uncommon in Canada with an annual rate since 1995 of ≤ 1.1 per 100,000 per year. The burden of meningococcal disease is mainly related to serogroups C and B. No vaccine is currently available in Canada to protect against serogroup B disease. The burden of disease from serogroups Y, W135 and A is very small and has remained relatively stable in Canada over the years. It should be noted, however, that the epidemiology of meningococcal serogroups changes with time and within specific geographic locations, and these serogroups could become more common in the future. As well, the median age of people affected by serogroups Y and A is older (44 and 45 years respectively), which means that routine immunization programs aimed at children and adolescents may not have a significant impact on the burden of disease caused by these serogroups. Three very effective monovalent meningococcal C conjugate vaccines are available in Canada, although the duration of protection of these vaccines remains uncertain. Protection for those vaccinated in early infancy appears to be low 1 or more years after vaccination. However, good effectiveness of > 1 year's duration has been demonstrated in those vaccinated at older ages31,32. The effectiveness of Menactra™ has not been assessed and its immunogenicity has not been compared with meningococcal C conjugate vaccines.
Vaccination against serogroups A, Y and W135 will result in only a limited decrease in the burden of IMD given that these serogroups are relatively uncommon. Furthermore, concerns regarding GBS possibly associated with Menactra™, which is estimated to result in 1.25 excess cases of GBS per 1,000,000 doses distributed, warrants a cautious approach to the use of this vaccine in Canada, as alternatives, such as meningococcal C conjugate vaccines, are available. Careful attention should be paid to post-marketing surveillance in Canada and the United States to better understand the possible relation between Menactra™ and GBS.
Because of the relative rarity of serogroups A, Y and W135 in the population and the possible risk of GBS related to Menactra™, the use of this vaccine should be considered only in individuals or circumstances when serogroups A, Y or W135 occur with increased frequency. In these groups, Menomune® could also be considered, although Menomune® produces less than optimal response in children < 10 years of age and induces immunologic hyporesponsiveness; the clinical significance of this is not known. Therefore, Menactra™ is recommended in high-risk groups when long-term protection is needed and when repeated vaccination with Menomune® would have been required if this product had been used.
High-risk use: Menactra™ is recommended for persons 2 to 55 years of age in the following high-risk groups:
- persons with anatomic or functional asplenia (see below for information regarding splenectomy);
- persons who have complement, properdin or factor D deficiencies;
- travellers for whom meningococcal vaccine is indicated or required, including pilgrims to the Hajj in Mecca. Vaccine should be provided at least 2 weeks before departure if possible (see below for further advice to travelers);
- research, industrial and clinical laboratory personnel who are routinely exposed to N. meningitidis;
- military recruits.
NACI has assessed that there is fair rationale to recommend Menactra™ as outlined above - strength of recommendation B (Table 5). The available evidence comes from immunogenicity studies in healthy people, and so it has been assessed as a level of evidence of II-2 for healthy people and level of evidence of III for immunocompromised people. One published study supports the possible association of GBS and Menactra™ in adolescents 11 to 19 years of age. The risk in the above groups has not been studied so the level of evidence to support a possible association in these groups is assessed as III.
Table 5. Quality and strength of evidence
| Level of evidence47,48
Strength of recommendations
|
The following describes the preferred products for contact follow-up and outbreak management:
- Close contacts of persons with meningococcal disease caused by sero- groups A, C, Y or W135 (see below for the definition of close contacts)
- For susceptible contacts of persons with serogroup C meningococcal disease, meningococcal C conjugate vaccine is preferred.
- For susceptible contacts of serogroup A, Y and W135, Menactra™ is preferred if the recipient is between 2 and 10 years of age. For older age groups, either Menactra™ or Menomune® can be used, unless the individual is likely to require long-term protection or meningococcal vaccine again in the future, in which case Menactra™ is preferred.
NACI has assessed that there is fair rationale to recommend the use of Menactra™ as outlined above - strength of recommendation B (Table 5). Menactra™ has not been specifically studied in close contacts so the level of evidence is assessed as III.
- To control outbreaks caused by serogroups A, C, Y or W135. Consultation with public health officials and/or experts in communicable disease is important in the assessment and control of meningococcal disease outbreaks in various settings, and reference to published guidelines should be made 49.
- For control of outbreaks caused by serogroup C menin- gococcal disease, meningococcal C conjugate vaccine is preferred unless there are indications for broader serogroup protection.
- For control of outbreaks caused by serogroups A, Y and W135, Menactra™ is preferred.
NACI has assessed that there is fair rationale to recommend the use of Menactra™ as outlined above - strength of recommendation B (Table 5). Menactra™ has not been specifically studied for outbreak control so the level of evidence is assessed as III.
People with human immunodeficiency virus (HIV) are likely at increased risk of meningococcal disease50. Although the effectiveness of Menactra™ in individuals with HIV is not known, the vaccine can be considered for this group.
NACI has assessed that there is insufficient evidence to make a recommendation for Menactra™ for people with HIV - strength of recommendation I (Table 5). Menactra™ has not been specifically studied in HIV-positive individuals so the level of evidence is assessed as III.
Because of the proven effectiveness of meningococcal C conjugate vaccines in children aged 2 to 10 years and the possibility of lower effectiveness with Menactra™, a dose of meningococcal C conjugate vaccine is also appropriate for high-risk individuals, as outlined above, who are 2 to 10 years of age and receive Menactra™. Meningococcal C conjugate vaccines and Menactra™ should be given with at least a 1 month interval between each product. Menactra™, which provides a broader spectrum of serogroup protection, should be administered first.
NACI has assessed that there is fair rationale for the above recommendation - strength of recommendation B (Table 5). There have been no specific studies of the above strategy, so the level of evidence is assessed as III.
Menactra™ is not currently approved for use in children < 2 years of age. Further studies on immunogenicity and safety are required in this age group. Menactra™ should be given to high-risk children as outlined above once they turn 2 years of age.
NACI has assessed that there is fair rationale to recommend against the use of Menactra™ in children < 2 years of age - strength of recommendation D (Table 5). Menactra™ has not been specifically studied in this age group, so the level of evidence is assessed as III.
For adults ≥ 56 years of age, Menactra™ can be considered if indicated.
NACI has assessed that there is insufficient evidence to make a recommendation regarding the use of Menactra™ in adults ≥ 56 years of age - strength of recommendation I (Table 5). Menactra™ has not been specifically studied in this age group, so the level of evidence is assessed as III.
Splenectomy: When elective surgical splenectomy is being planned, Menactra™ and all other recommended vaccines should be administered at least 2 weeks before removal of the spleen, if possible. If this is not possible, as in an emergency splenectomy, Menactra™ and other vaccines should be given 2 weeks after splenectomy. If the patient is discharged earlier and there is a concern that he/she might not return, vaccination should be carried out before discharge51.
Advice to travellers: Health care providers advising Canadian travellers should remain current with global meningococcal activity. The Committee to Advise on Tropical Medicine and Travel (CATMAT) provides guidelines for health care providers counselling Canadian international travellers on meningococcal vaccination. In deciding on the need for immunization, there should be particular consideration of the destination to be visited, the nature and duration of exposure, and the age and health of the traveller. Current meningococcal outbreak information can be obtained through the Public Health Agency of Canada, Travel Medicine Program (http://www.travelhealth.gc.ca)
and the World Health Organization (WHO) (http://www.who.int/csr/disease/meningococcal/en/).
Close contacts: Close contacts of individuals with meningococcal infections are at increased risk of IMD; this risk is greatest for household contacts. The vaccination status of close contacts, including the type of meningococcal vaccine, the number of doses and age at vaccine administration should be determined. Vaccination of susceptible close contacts, in addition to chemoprophylaxis, should be considered when the serogroup is vaccine preventable, as it may further reduce the risk of subsequent meningococcal disease; vaccination should be carried out as soon as possible. The increased risk of disease for household contacts persists for up to 1 year after disease in the index case and beyond any protection from antibiotic chemoprophylaxis. In general, this prolonged risk is not seen among other contacts who do not have ongoing exposure.
The following individuals are considered close contacts for whom immunoprophylaxis and chemoprophylaxis should be given:
- household contacts of a case;
- persons who share sleeping arrangements with the case;
- persons who have direct contaminations of their nose or mouth with the oral/nasal secretions of a case (e.g. kissing on the mouth, shared cigarettes, shared drinking bottles);
- children and staff in child care and nursery school facilities.
The following individuals are close contacts who should receive only chemoprophylaxis (not immunoprophylaxis):
- health care workers who have had intensive unprotected contact (without wearing a mask) with infected patients (i.e. intubating, resuscitating or closely examining the oropharynx);
- airline passengers sitting immediately on either side of the case (but not across the aisle) when the total time spent aboard the aircraft was at least 8 hours.
Routine use: For most jurisdictions, the current epidemiology of serogroups A, Y and W135 does not support the routine use of Menactra™. Furthermore, the possible association between GBS and Menactra™ warrants further monitoring, both because of the potential seriousness of GBS and because adverse events associated with a particular vaccine potentially jeopardize the public's confidence in all vaccines.
NACI has assessed that there is insufficient evidence to make a recommendation on the routine use of Menactra™ – strength of recommendation I (Table 5). As further information becomes available, this recommendation may need to be revisited. The available evidence comes from immunogenicity studies in healthy people, so it has been assessed as a level of evidence II-2. One published study supports the possible association of GBS and Menactra™ in adolescents 11 to 19 years of age, so the level of evidence for the association in this age group is assessed as II-3.
Recommendations for the use of Menactra™ may change in the future on the basis of the following considerations:
- the results of ongoing monitoring regarding the association of Menactra™ and GBS;
- increases in the incidence of serogroups A, Y and/or W135 IMD;
- the need for adolescent booster doses of meningococcal vaccine in those vaccinated in childhood with meningococcal C conjugate vaccine.
Careful monitoring of the above parameters is required to determine the appropriate future use of Menactra™.
A summary of the recommended meningococcal vaccine by age group and risk category is provided in Table 6.
Table 6. Summary of meningococcal vaccine recommendations by age group and risk category
| Risk category |
0 to < 2 years |
2 to 10 years |
11 to 24 years |
25 to 55 years |
≥ 56 years |
| High risk |
Conjugate C followed by Menactra™ after |
Menactra™ followed by conjugate C in |
Menactra™ |
Menactra™ |
Menactra™ or MenomuneMD |
| Routine use |
Conjugate C |
Conjugate C |
Conjugate C unless local epidemiology warrants use of Menactra™ |
Not recommended |
Not recommended |
Simultaneous administration with other vaccines
If both Td and Menactra™ are needed, it may be preferable to administer both products at the same visit, because increased immunogenicity has been found when compared with Td followed by Menactra™. It is uncertain whether increased immunogenicity will also occur when Menactra™ and other Td-containing products, such as dTap, are administered at the same visit.
NACI has assessed that there is insufficient evidence to make a recommendation for the use of Menactra™ as outlined above – strength of Recommendation I (Table 5). Menactra™ has not been specifically studied with dTap, so the level of evidence is assessed as III.
Recommended research priorities
Research to address the following outstanding questions will assist with further recommendations regarding the use of Menactra™:
- the relation between Menactra™ and GBS;
- the effectiveness of Menactra™ and the duration of protection;
- comparison of the immunogenicity of Menactra™ and meningococcal C conjugate vaccines;
- the impact of Menactra™ on meningococcal carriage and herd immunity;
- the safety, immunogenicity and effectiveness of Menactra™ in children < 2 years of age and adults ≥ 56 years;
- the safety, immunogenicity and effectiveness of Menactra™ in certain high-risk groups, such as the immunocompromised;
- the safety and immunogenicity of concomitant adminis- tration with other vaccines, such as dTap, human papillomavirus, hepatitis A and hepatitis B;
- the safety and immunogenicity of administering Menactra™ followed by dTap or Td.
References
Varughese PV, Carter AO. Meningococcal disease in Canada. Surveillance summary to 1987. CDWR 1989;15:89-96.
Whalen CM, Hockin JC, Ryan A et al. The changing epidemiology of invasive meningococcal disease in Canada, 1985 through 1992: Emergence of a virulent clone of Neisseria meningitidis. JAMA 1995;273:390-94.
Squires SG, Deeks SL, Tsang RSW. Enhanced surveillance of invasive meningococcal disease in Canada: 1 January, 1999, through 31 December, 2001. CCDR 2004;30:17-28.
De Wals P, Coudeville L, Trottier P et al. Vaccinating adolescents against meningococcal disease in Canada: A cost-effectiveness analysis. (Submitted for publication) 2007.
Lebel MH, Tapeiro BF, Saintonge F. Immunogenicity of bivalent AC polysaccharide meningococcal vaccine in children aged 6 through 24 months. JAMA 2001;285:1578-79.
De Wals P, De Serres G, Niyonsenga T. Effectiveness of a mass immunization campaign against serogroup C meningococcal disease in Quebec. JAMA 2001;285:177-81.
Lepow ML, Goldschneider I, Gold R et al. Persistence of antibody following immunization of children with group A and C meningococcal polysaccharide vaccines. Pediatrics 1977;60:673-80.
MacDonald NE, Halperin SA, Law BJ et al. Induction of immunologic memory by conjugated vs plain meningococcal C polysaccharide vaccine in toddlers: A randomized controlled trial. JAMA 1998;280:1685-89.
National Advisory Committee on Immunization (NACI). Meningococcal vaccine. In: Canadian immunization guide, 7th ed. Ottawa: Public Health Agency of Canada, 2007:237-50.
Hassan-King MKA, Wall RA, Greenwood BM. Meningococcal carriage, meningococcal disease and vaccination. J Infect 1988;16:55-9.
De Wals P, Erickson L. Economic analysis of the 1992-1993 mass immunization campaign against serogroup C meningococcal disease in Quebec. Vaccine 2002;20:2840-44.
Granoff DM, Gupta RK, Belshe RB et al. Induction of immunologic refractoriness in adults by meningococcal C polysaccharide vaccination. J Infect Dis 1998;178:870-4.
Richmond P, Kaczmarski E, Burrow R et al. Meningococcal C polysaccharide vaccine induces immunologic hyporesponsiveness in adults that is overcome by meningococcal C conjugate vaccine. J Infect Dis 2000;181:761-4.
De Wals P, Deceuninck G, De Serres G et al. Effectiveness of serogroup C meningococcal polysaccharide vaccine: Results from a case-control study in Quebec. Clin Infect Dis 2005;40:1116-22.
Harris S, Finn A, Granoff DM. Disparity in functional activity between serum anticapsular antibodies induced in adults by immunization with an investigational group A and C Neisseria meningitidis-diphtheria toxoid conjugate vaccine and by a polysaccharide vaccine. Infect Immun 2003;71:3402-8.
MacLennan JM, Shackley F, Heath PT et al. Safety, immunogenicity, and induction of immunologic memory by a serogroup C meningococ- cal conjugate vaccine in infants: A randomized controlled. JAMA 2000;283:2795-801.
Kelly DF, Snape MD, Cutterbuck EA et al. CRM197-conjugated serogroup C meningococcal capsular polysaccharide, but not the native polysaccharide, induces persistent antigen-specific memory B cells. Blood 2006;108:2642-7.
Maiden MCJ, Stuart JM. Carriage of serogroup C meningococci 1 year after meningococcal C conjugate polysaccharide vaccination. Lancet 2002;359:1829-30.
Ramsay ME, Andrew NJ, Trotter CL et al. Herd immunity from meningococcal serogroup C conjugate vaccination in England: Database analysis. BMJ 2003;326:365-6.
Borrow R, Goldblatt D, Andrews N et al. Influence of prior meningococcal C polysaccharide vaccination on the response and generation of memory after meningococcal C conjugate vaccination in young children. J Infect Dis 2001;184:377-80.
McVernon J, MacLennan J, Buttery J et al. Safety and immuno- genicity of meningococcus serogroup C conjugate vaccine administered as a primary or booster vaccination to healthy four-year-old children. Pediatr Infect Dis J 2002;21:747-53.
Jódar L, Griffiths E, Feavers I. Scientific challenges for the quality control and production of group C meningococcal conjugate vaccines. Vaccine 2004;22:1047-53.
Rennels M, King J, Ryall R et al. Dosage escalation, safety and immunogenicity study of four dosages of a tetravalent meningococcal polysaccharide diphtheria toxoid conjugate vaccine in infants. Ped Infect Dis J 2004;23:429-35.
Rennels M, King J, Ryall R et al. Dose escalation, safety and immunogenicity study of a tetravalent meningococcal polysaccharide diphtheria conjugate vaccine in toddlers. Ped Infect Dis J 2002;21:978-9.
Campbell JD, Edelman R, King JC et al. Safety, reactogenicity and immunogenicity of a tetravalent meningococcal polysaccharide-diphtheria toxoid conjugate vaccine given to healthy adults. J Infect Dis 2002;186:1848-51.
Snape MD, Pollard AJ. Meningococcal polysaccharide-protein conjugate vaccines. Lancet Infect Dis 2005;5:21-30.
Goldschneider I, Gotschlich EC, Artenstein MS. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med 1969;129:1307-26.
Borrow R, Balmer P, Miller E. Meningococcal surrogates of protection–serum bactericidal antibody activity. Vaccine 2005;23:2222-7.
World Health Organization. Requirements for meningococcal polysaccharide vaccine. Geneva: World Health Organization; 1976. Report No. 594.
De Wals P, Deceuninck G, Boulianne N et al. Effectiveness of a mass immunization using serogroup C meningococcal conjugate vaccine. JAMA 2004;292:2491-4.
Trotter CL, Andrews NJ, Kaczmarski EB et al. Effectiveness of meningococcal serogroup C conjugate vaccine 4 years after introduction. Lancet 2004;364:365-7.
Larrauri A, Cano R, Garcia M et al. Impact and effectiveness of meningococcal C conjugate vaccine following its introduction in Spain. Vaccine 2005;23:4097-100.
Borrow R, Goldblatt D, Andrews N et al. Antibody persistence and immunological memory at age 4 years after meningococcal group C conjugate vaccination in children in the United Kingdom. J Infect Dis 2002;186:1353-7.
Meningococcal infection – meningococcal meningitis. In:Heymann DL, eds. Control of communicable disease manual, 18th ed. Washington: American Public Health Association, 2004:359-66.
De Wals P, Trottier P, Pepin J. Relative efficacy of different immunization schedules for the prevention of serogroup C meningococcal disease: A model- based evaluation. Vaccine 2006;24:3500-4.
Sanofi pasteur Limited. Menactra™ product monograph. Toronto, Ont.: sanofi pasteur Limited; 2006.
Pichichero M, Casey J, Blatter M et al. Comparative trial of the safety and immunogenicity of quadrivalent (A, C, Y, W-135) meningococcal polysaccharide-diphtheria conjugate vaccine versus quadrivalent polysaccharide vaccine in two- to ten-year-old children. Ped Infect Dis J 2005;24:57-62.
US Food and Drug Administration. Product approval information ,– licensing action. URL: < http://www.fda. gov/cber/products/mpdtave011405.htm >. Rockville, MD: US Food and Drug Administration, Center for Biologics Evaluation and Research, 2005.
Keyserling H, Papa T, Koranyi K et al. Safety, immunogenicity,and immune memory of a novel meningococcal (groups A, C, Y, and W-135) polysaccharide diphtheria toxoid conjugate vaccine (MCV-4) in healthy adolescents. Arch Pediatr Adolesc Med 2005;159:907-13.
Granoff DM, Harris SL. Protective activity of group C anticapsular antibodies elicited in two-year-olds by an investigational quadrivalent Neisseria meningitidis-diphtheria toxoid conjugate vaccine. Pediatr Infect Dis J 2004;23:490-97.
Granoff DM, Morgan A, Welsch J. Persistence of group C anticapsular antibodies two to three years after immunization with an investigational quadrivalent Neisseria meningitidis-diphtheria toxoid conjugate vaccine. Pediatr Infect Dis J 2005;24:132-36.
Granoff DM, Morgan A, Welsch J. Immunogenicity of an investigational quadrivalent Neisseria meningitidis-diph-theria toxoid conjugate vaccine in 2-year old children. Vaccine 2005;23:4307-14.
El Bashir H, Heath PT, Papa T et al. Antibody responses to meningococ- cal (groups A, C, Y and W135) polysaccharide diphtheria toxoid conjugate vaccine in children who previously received meningococcal C conjugate vaccine. Vaccine 2006;24:2544-9.
Centers for Disease Prevention and Control. Inadvertent misadministration of meningococcal conjugate vaccine – United States, June-August 2005. MMWR 2006;55:1016-7.
National Advisory Committee on Immunization (NACI). Vaccine administration practices. In: Canadian immunization guide, 7th ed. Ottawa: Public Health Agency of Canada, 2007:38-44.
Centers for Disease Prevention and Control. Update: Guillain-Barre syndrome among recipients of Menactra meningococcal conjugate vaccine – United States, June 2005-September 2006. MMWR 2006;55:1120-4.
Canadian Task Force on the Periodic Health Examination. New grades for recommendations from the Canadian Task Force on Preventive Health Care. Can Med Assoc J 2003;169:207-8.
Canadian Task Force on the Periodic Health Examination. The Canadian guide to clinical preventive health care. Ottawa: Minister of Supply and Services Canada, 1994. Cat. no. H21-117/1994E.
Public Health Agency of Canada. Guidelines for the prevention and control of meningococcal disease. CCDR 2005;31(S1):1-20.
Stephens DS, Hajjeh RA, Baughman WS et al. Sporadic meningococcal disease in adults: Results of a 5-year population-based study. Ann Intern Med 1995;123:937-40.
National Advisory Committee on Immunization (NACI). Immunization of immunocompromised persons. In: Canadian immunization guide, 7th ed. Ottawa: Public Health Agency of Canada, 2007:117-30.
* Members:Dr. M. Naus (Chairperson), Dr. S. Deeks (Executive Secretary), Dr. K. Laupland, Dr. S. Dobson, Dr. B. Duval, Dr. J. Embree, Ms. A. Hanrahan, Dr. J. Langley, Dr. A. McGeer, Dr. S. McNeil, Dr. M.-N. Primeau, Dr. B. Tan, Dr. B. Warshawsky.
Liaison Representatives: Ms. S. Callery (CHICA), Dr. J. Carsley (CPHA), Dr. J. Smith (CDC), Dr. D. Money (SOGC), Ms. E. Holmes (CNCI), Dr. B. Larke (CCMOH), Dr. B. Law (ACCA), Dr. M. Salvadori (CPS), Dr. S. Rechner (CFPC), Dr. J. Salzman (CATMAT), Dr. D. Scheifele (CAIRE), Dr. P. Orr (AMMI Canada).
Ex-Officio Representatives: Dr. H. Rode (BGTD), Dr. M. Lem (FNIHB), Dr. J. Anderson (DND).
†This statement was prepared by Bryna Warshawsky, Philippe De Wals, Shelley Deeks and Christine Navarro; it was approved by NACI and the Public Health Agency of Canada.
Page details
- Date modified:
