Guidelines: Mumps in Canada


Download this article as a PDF (651 KB - 49 pages)
Published by: The Public Health Agency of Canada
Issue: Volume 36 Supplement 1
Date published: January 2010
ISSN: 1481-8531
Submit a manuscript
About CCDR
Browse
Volume 36 Supplement 1, January 2010
Supplement
Guidelines for the Prevention and Control of Mumps Outbreaks in Canada
DOI
https://doi.org/10.14745/ccdr.v36i00as1
Table of Contents
- Introduction
- Objectives
- Epidemiology of Mumps in Canada
- Definitions
- Laboratory Guidelines for the Diagnosis of Mumps
- Management
- Immunization
- Strategic Risk Communications
- References
- Appendix 1: Persons and Organizations involved in Developing and Reviewing the Guidelines
- Appendix 2: Key Recommendations for the Prevention and Control of Mumps Outbreaks
- Appendix 3: Sample Mumps Outbreak Case Report and Follow-up Form
- Appendix 4: Laboratory Guidelines for the Diagnosis of Mumps
- Appendix 5: Algorithms for the Health-Care Setting
- Appendix 6: Case Study – Communications in Nova Scotia during the 2007 Mumps Outbreak
1.0 Introduction
1.1 Background
Mumps is an acute viral disease characterized by fever, swelling, and tenderness of one or more of the salivary glands. Symptoms include fever, headache, muscle ache, and swelling and tenderness of the salivary glands at the angle of the jaw (parotid glands). Rarely, mumps infection can lead to meningitis, inflammation of the testicles or ovaries, inflammation of the pancreas, and transient or permanent hearing loss. The severity of illness in recent outbreaks has been low, as there have been few hospitalizations and no deaths reported.
Since the approval of the vaccine against mumps in 1969, the number of reported mumps cases in Canada has decreased by more than 99% (from an average of 34,000 cases reported per year in the early 1950s to fewer than 400 cases per year in the early 1990s). A further reduction in incidence was observed following the introduction of the routine second dose of the measles, mumps, and rubella (MMR) vaccine in most provinces and territories for measles control.
As a result of the prolonged outbreak of mumps in the Maritime provinces in 2007 and the increased risk among susceptible populations (among whom mumps cases are expected), these guidelines have been compiled to assist public health officials and clinicians in the public health management of mumps cases and their contacts during outbreaks.
1.2 Guidelines Development and Approval Process
A task group of federal, provincial, and territorial (FPT) partners was assembled by the Centre for Immunization and Respiratory Infectious Diseases (CIRID) of the Public Health Agency of Canada (PHAC). The task group brought together public health expertise from across the country, including representation from jurisdictions that had direct experience with or were directly affected by the outbreaks. National teleconferences were held to address outbreak issues and the development of this document. Comments on the various chapters of the guidelines were collected and integrated by CIRID staff on an ongoing basis. The process involved the establishment of links with the Canadian Immunization Committee (CIC) Mumps Immunization Program Option process.
The complete set of guidelines was approved by all members of the FPT task group and reviewed by the CIC, which reports to the Communicable Disease Control Expert Group. The Council of Chief Medical Officers of Health (CCMOH) and Council of the Public Health Network have endorsed this document. The participants involved in the national consensus process are listed in Appendix 1.
2.0 Objectives
These guidelines are based on national and international expertise, outbreak experiences, and best practice. At the request of CIC and the CCMOH, they were prepared primarily to assist Canadian public health authorities in their investigation and management of mumps outbreaks. They are intended to provide consistent case and contact definitions and, consequently, to improve reporting and surveillance information that will guide future outbreak management.
Depending on the epidemiology of an outbreak (e.g., age groups and settings affected), public health authorities may need to adapt the guidelines and key recommendations to accommodate their local public health protocol and response.
These outbreak guidelines address the following:
- case and contact definitions;
- reporting and surveillance;
- laboratory diagnosis of mumps;
- public health response to cases and contacts;
- outbreak control in community and health care settings;
- immunization; and
- communication strategies.
All key recommendations are highlighted in boxes throughout the document.
Appendix 2 provides the full set of recommendations, for easy reference.
3.0 Epidemiology of Mumps in Canada
3.1 Prior to 2007
The number of reported mumps cases has decreased from an average of 34,000 cases reported per year in the early 1950s to fewer than 400 cases per year in the early 1990s. During the period 2000–2006, an average of 79 cases were reported annually, ranging from 28 in 2003 to 202 in 2002(1). From 1996 to 2006, Canada had five outbreaks, with the number of cases ranging from 13 to 193 (Table 1). These outbreaks primarily involved pre-school or school-aged children, adolescents, and young adults(2-5).
| Province | Year(s) | Number of cases | Affected age group(s) |
|---|---|---|---|
| British Columbia(2) | 1996 | 83 | 15–24 |
| Québec(3) | 1998–1999 | 37 | 0.9–42 (average 10) |
| Alberta(4) | 2001–2002 | 193 | School-age / Pre-school |
| Nova Scotia(5) | 2005 | 13 | 13–19 |
| Nova Scotia(5) | 2005 | 19 | 20–27 |
Over time, the age distribution of mumps cases in Canada has changed. The proportion of reported cases aged 20 years and older increased from 14% in 1988–1990 to 64% in 2003–2005(6). Conversely, the proportion of cases aged 1–9 decreased from 49% to 17% during the same period(6).
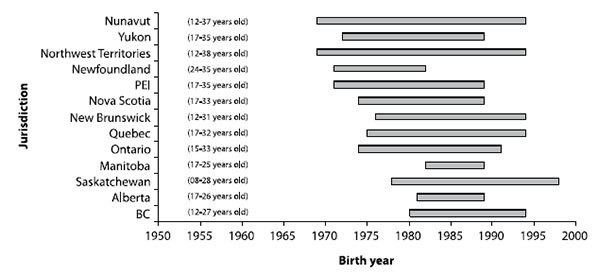
On the basis of the community epidemiology of mumps, most people born in Canada before 1970 are immune to mumps, as they were likely exposed to the wild mumps virus that was circulating during their childhood. In the majority of jurisdictions, most people born between 1990 and 1994 (depending on the province/territory of residence) have been offered two doses of mumpscontaining vaccine following the introduction of a second dose of MMR vaccine for measles control in 1996 and1997, either during a mass campaign or as part of the routine schedule. This left a possibly susceptible cohort of people born between 1970 through 1990 (to a lesser extent through 1994) who were offered only one dose of mumps-containing vaccine and who are not assumed to have natural immunity (Figure 1). It is important to note that the age at which natural immunity tomumps can be assumed to have been acquired is not known with certainty and that some individuals born before 1970 may still be susceptible to mumps. See section 7.1 for more information on mumps vaccine and immunization.
Figure 1. Canadian cohorts offered one dose of mumps-containing vaccine by jurisdiction and birth year (age in 2007)
3.2 2007 Outbreak
As of March 5, 2008, 1,284 confirmed cases of mumps had been reported in Canada with symptom onset in 2007. The vast majority of cases (1,159; 90%) were from Nova Scotia, New Brunswick, and Alberta (Figure 2 and Table 2). The majority (58%) of cases occurred in persons aged 20–29 (Figure 3), many of whom were college or university students (50% when age information was known). Both sexes were equally affected.
The particular susceptibility among those who are college/university-aged is multifactorial. They are too young for natural immunity and too old for inclusion in routine two-dose MMR immunization programs. Mumps has a fairly long infectious period (up to 16 days) and a long incubation period (14 to 25 days), and 20% to 30% of infectious cases show no signs or symptoms.
In addition, the very social and mobile lifestyles of this age group appear to be facilitating disease transmission and interfering with control measures. Young people in this age group tend not to adhere to isolation requests, and they generally do not participate when immunization is offered. Furthermore, post-secondary students often share living/ sleeping arrangements, many are involved in competitive sports, and many frequent bars/pubs/ nightclubs, as well as travel during school holidays and breaks. Additional cases in this demographic group, and possibly other jurisdictions, would not be unexpected.
Immunization history was known for less than half of the mumps cases (586; 46%) reported in 2007. Of these, 45 (8%) had received two or more doses, 430 (73%) had received one dose, and 111 (19%) had received no doses of mumpscontaining vaccine.
Figure 2. Confirmed* mumps cases in Canada epi-year 2007 (onset December 31, 2006, to December 29, 2007) (n = 1,219**)
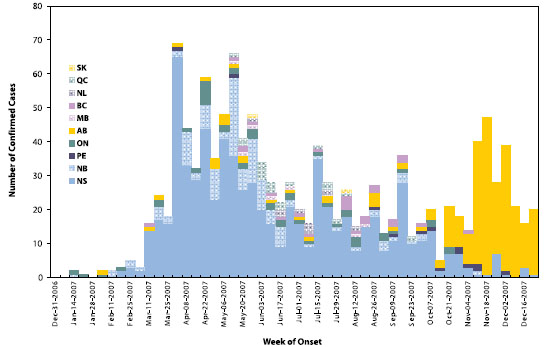
* A confirmed case is either a laboratory-confirmed case OR clinically compatible and linked to a laboratory-confirmed case as of 5 March 2008
** Remainder of the 1,284 confirmed cases that were reported are missing onset dates.
| Province/territory | Case count | % Male |
|---|---|---|
| Nova Scotia | 777 | 49 |
| Alberta | 258 | 58 |
| New Brunswick | 124 | 57 |
| Ontario | 48 | 33 |
| British Columbia | 25 | 44 |
| Québec | 20 | 55 |
| Prince Edward Island | 13 | 73 |
| Newfoundland and Labrador | 10 | 40 |
| Manitoba | 7 | 57 |
| Saskatchewan | 2 | 50 |
| Nunavut | 0 | 0 |
| Northwest Territories | 0 | 0 |
| Yukon Territory | 0 | 0 |
| National total | 1,284 | 51 |
| * Confirmed cases are either laboratory-confirmed OR clinically compatible and linked to a laboratory-confirmed case as of March 5, 2008. | ||
Figure 3. Proportion of reported mumps cases by age, Canada; onset December 31, 2006, to December 29, 2007 (n = 1,284)
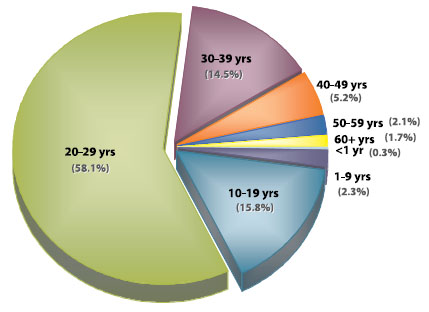
Source: Centre for Immunization and Respiratory Infectious Diseases, Public Health Agency of Canada.
The data on mumps hospitalizations and complications are incomplete. Complications were noted in approximately 8% of reported cases in 2007. There were reports of orchitis (76), oophoritis (9), hearing loss (8), mastitis (3), meningitis (1), encephalitis (1), pancreatitis (1), and nephritis (1). Less than 2% of cases resulted in visits to hospital emergency departments, overnight observation, or hospital admission.
Identifying the virus strain is useful for differentiating vaccine and wild-type strains, linking cases, linking outbreaks, tracking importations, and documenting the elimination of a particular strain from a geographic area. The viral strain in the two 2007 Canadian outbreaks (Maritimes and Alberta) is identical to the strain (genotype G) detected in the 2005–2006 Nova Scotia outbreaks, the U.S. multi-state outbreak in 2006, and the U.K. epidemic in 2004–2006. In the U.S. outbreak, there were over 6,500 cases reported in 45 states.
In the U.K., the epidemic peaked in 2005 with more than 50,000 mumps notifications, the majority being 15–24 years old.
4.0 Definitions
4.1 National Case Definition
Mumps is a reportable disease in all provinces and territories and notifiable at the national level. A revision of the national case definition for mumps is to be published in Spring 2009. The current national notifiable disease case definitions can be found at http://www.phac-aspc.gc.ca.
4.2 Outbreak Definition
An outbreak can be defined in many ways. When an increased number of cases are reported for a particular disease, it is important to determine that it is a true outbreak by considering factors such as historical disease activity, seasonal events, and changes in surveillance, reporting, and/or diagnostic procedures. Furthermore, what is considered an outbreak may vary across jurisdictions. Table 3 provides a working definition of mumps outbreaks.
| Outbreak | Confirmed cases in excess of what is expected in the jurisdiction over a given period of time. |
|---|
Household links would generally not be considered to be an outbreak, but that determination is up to the discretion of the jurisdiction.
4.3 Case and Contact Definitions
During increased mumps activity or outbreaks, the definitions in Table 4 of a confirmed case, clinical/probable case, and contact should be used. Section 5.0 and Appendix 4 have details on preferred clinical specimens and interpretation of laboratory results. Final case classification should be based on all available information.
| Confirmed case | Any one of the following in the absence of recent immunization (i.e., in the previous 28 days):
|
|---|---|
| Clinical/probable case | Acute onset of unilateral or bilateral parotitis lasting longer than 2 days without other apparent cause |
| Contact | Any of the following during the infectious period (i.e., approximately 7 days before to 5 days after symptom onset):
Refer to Section 6.3.3 if the contact is a health care worker. |
4.4 Surveillance and Reporting
In Canada, the reporting of notifiable diseases is mandated by provincial/territorial legislation and regulation. The list of reportable diseases varies slightly by province and territory. Reporting by the provinces and territories to the federal level is voluntary. The Notifiable Diseases Reporting System (NDRS) is the national, passive surveillance system used to monitor more than 40 nationally notifiable infectious diseases. Since data submission to the NDRS is voluntary, there may be inconsistencies in disease case counts, variability in the frequency of data submission, and incomplete coverage (i.e. number of provinces/territories submitting). Probable cases of mumps are not nationally notifiable. Each province or territory has procedures in place for the rapid notification of cases to medical officers of health and timely reporting to the appropriate provincial or territorial public health authorities.
Most jurisdictions rely on passive surveillance for the identification of cases. When an increase in the incidence of mumps is suspected in a particular area, enhanced surveillance of cases by collection of more detailed epidemiologic, clinical, and laboratory information is encouraged; active surveillance and rapid entry into an electronic reporting system might also be considered. Detailed epidemiologic information helps identify susceptible groups and determine associations that will permit targeted interventions. The types of information to collect include demographic and clinical information (including hospitalization and complications), immunization history, laboratory results, exposure (e.g., household, school/educational institution, occupational, mass gatherings) and recent travel. Other information relevant to the outbreak may be social or cultural settings. A sample case report and follow-up form (based on those used in previous outbreaks) are included in Appendix 3.
During outbreaks, clinical specimens can be forwarded by the provincial laboratory to the National Microbiology Laboratory (NML) at PHAC for molecular characterization and strain identification, which can distinguish between vaccine and wild types of the mumps virus. Strain identification can also be used to link cases or outbreaks and to track importations.
When multiple jurisdictions are involved, it is not always clear who should report a case. Case reporting is important for describing and monitoring the epidemiology, impact, and spread of an outbreak. Therefore, during outbreaks, the jurisdiction that initially identifies and primarily handles the case is asked to report it. This decision rule is flexible and should be evaluated for each case, considering place of residence, travel itinerary, and the public health response or intervention that was implemented.
Mumps cases that are confirmed as per the outbreak case definition (Table 4) should remain part of routine provincial/territorial and national reporting of mumps. However, it is important to note that, while outbreak definitions may differ from routine surveillance definitions, they will have increased sensitivity because of increased disease activity.
5.0 Laboratory Guidelines for the Diagnosis of Mumps
The clinical and laboratory diagnosis of mumps can be difficult. Proper specimen collection and transportation, along with appropriate laboratory testing and cautious interpretation of results, are important in determining a mumps diagnosis. This section is based on recent experiences in mumps diagnostics in both Canada and the United States. A comprehensive description of mumps diagnostics can be found in the Manual of Clinical Microbiology(7).
Of the currently available tests, reverse transcriptase polymerase chain reaction (RT-PCR) is preferred for mumps virus detection. A summary of the laboratory diagnostics for mumps is shown in Table 5. The full version of the laboratory guidelines for the diagnosis of mumps (revised in 2007) can be found in Appendix 4.
| Specimen collection | Buccal swab or collection of saliva from the buccal cavity for reverse transcriptase polymerase chain reaction (RT-PCR) assay collected within the first 3–5 days of symptom onset is the preferred specimen. Buccal specimens should be collected using a swab approved for virus isolation and placed in virus transport media. Swabs may be dacron, nylon, and rayon tipped, and either flocked or non-flocked. Calcium alginate swabs are not acceptable, as they inhibit PCR reactions. Charcoal swabs or swabs in Ames media used for swabbing for bacterial pathogens (such as group A Streptococcus) are not acceptable. Swabs with wooden or aluminum shafts are also not acceptable. Mumps virus has been detected in the urine by culture up to 14 days after the onset of symptoms. However, experiences in the Nova Scotia and U.S. outbreaks suggest that mumps virus cannot be detected in the urine with the same sensitivity as in oral specimens. The first (acute) serum specimen should be collected as soon as possible upon presentation with mumps symptoms. A second (convalescent) serum specimen should be collected at least 10 days (ideally) and up to 3 weeks after the first sample. |
|---|---|
| Serology | Testing for mumps-specific IgM-class antibody has suboptimal sensitivity for the diagnosis of acute mumps in a partially immunized population (may be detectable in only 30% of acute cases). In addition, without an established epidemiologic link to a confirmed case or without travel history to an area with known/likely mumps activity, one should be cautious of false-positive IgM results. Seroconversion (i.e., negative to positive result) or a fourfold or greater rise in titre between the acute and convalescent sera is indicative of an acute mumps infection. The presence of mumps-specific IgG, as determined using an enzyme immunoassay (EIA), does not necessarily predict the presence of neutralizing antibodies and, thus, immunity. Conversely, the absence of detectable mumps IgG using EIA may reflect the lower sensitivity of the EIA in comparison to a more sensitive assay, such as a neutralization assay, in which IgG may be detectable. |
| Contact | The RT-PCR assay is reliable for the definitive diagnosis of mumps infection, but its sensitivity can be influenced by the following:
Only molecular methods (i.e., genotyping) can be used to distinguish between vaccine and wild types of the virus. Virus genotyping is useful for differentiating vaccine and wild-type strains, linking cases, linking outbreaks, tracking importations, and documenting the elimination of a particular strain from a geographic area. |
| Interpretation of laboratory results | Testing by RT-PCR and IgM-class antibody detection is not sufficiently sensitive to rule out mumps infection, particularly if the specimen was collected 4–5 days after symptom onset. In order to properly interpret laboratory results and to assess the performance of mumps diagnostic assays, both clinical and epidemiologic information need to be considered along with the laboratory information (e.g., prior vaccination history, travel history, timing of sample collection relative to onset of symptoms). Therefore, communication and information sharing between public health and the laboratory are essential. |
| NML services | Mumps virus detection, isolation, and genotyping are available at the National Microbiology Laboratory. The online guide to services is available at http://www.nml-lnm.gc.ca/english/guide/default.asp. |
6.0 Management
6.1 Spread and Control
Mumps is generally spread by close face-to-face contact. Infection occurs through direct contact with saliva or respiratory droplets from the nose or throat, spread through coughing, sneezing, sharing drinks, or kissing, or from contact with any surface that has been contaminated with the mumps virus(8-11).
The incubation period for mumps ranges from 14 to 25 days(8-11). Once an individual is infected, mumps can be communicable from 7 days before to 5 days after the onset of parotitis (swelling of the parotid gland)(12). A recent review of the scientific evidence suggests that, while the mumps virus can be isolated from saliva or respiratory secretions ≥5 days after parotitis onset, the risk of transmission after 5 days is considered low, due to decreasing viral load(12). Approximately 20% to 30% of mumps infections can be asymptomatic, and these cases can also be infectious(8,11). High childhood immunization rates in Canada have resulted in a dramatic reduction in rates of mumps infection. Under-immunized and unimmunized children and young adults remain the groups at highest risk of infection. Immunity is generally lifelong and develops after either inapparent (asymptomatic) or clinical infections. Mumps immunization is further discussed in Section 7.
The public health response to increased mumps activity includes managing cases, contact identification and management; identifying social networks when individual follow-up is not feasible; and maintaining/enhancing surveillance for further cases and disease outcomes (e.g., hospitalizations, complications). Generally, a mumps outbreak is controlled by the following methods(13-15):
- defining the at-risk populations and transmission settings;
- preventing further transmission through isolation of cases and contact education/ awareness;
- protecting susceptible populations with immunization (where no contraindication to MMR vaccine exists); and
- good risk communication.
6.2 Case Management
There is no specific or prophylactic treatment for mumps; all confirmed and clinical cases of mumps should be offered supportive care. Cases should be encouraged to practise good hand hygiene, avoid sharing drinking glasses or utensils, and cover coughs and sneezes with a tissue or forearm.
Clinical cases should be advised to stay home from school or post-secondary educational institutions, child care facilities, workplaces, and other group settings for 5 days from symptom onset. Self-isolation will prevent exposure of susceptible individuals to the virus. CDC has revised their recommendation for self-isolation from 9 days to 5 days, citing new information about the period of communicability of mumps(12). Although the mumps virus has been isolated from respiratory secretions >5 days after parotitis onset, the risk of transmission 5 days after parotitis onset is low(12). During recent mumps outbreaks in Nova Scotia (2007), Iowa (2006), and the United Kingdom (2006), local public health authorities found that there were compliance issues with the 9-day self-isolation requests.
Cases in health care facilities should be managed with droplet precautions (in addition to routine practice) until 5 days after symptom onset.
| Case management (clinical cases should be managed as confirmed cases until laboratory evidence suggests otherwise) |
|
|---|
6.3 Contact Management (Refer to Section 6.4.3 for management of contacts who are health care workers)
Contacts of mumps cases (as defined in Section 4.3) who are considered susceptible to mumps infection include the following:
- those born in Canada in 1970 or later who did not receive two doses of mumpscontaining vaccine (at least 4 weeks apart) after their first birthday;
- those who have not had laboratoryconfirmed mumps; and
- those who do not have documented immunity to mumps(16).
Immunization of mumps-susceptible contacts with MMR vaccine should be considered. However, immunization after exposure may not prevent infection. Passive immunization with immunoglobulin is not effective in preventing mumps. In addition, isolation of mumps-susceptible contacts is not required. On the basis of the epidemiology of the outbreak, susceptible groups should be targeted for immunization, especially those at greatest risk of exposure. Mumps immunization is further discussed in Section 7.0.
Public health capacity during the 2007 outbreak in the Maritimes was quickly overwhelmed by the resources required for individual contact tracing and management. At the start of an outbreak, individual contacts can be managed either directly by public health authorities or indirectly by asking cases to disseminate information to their contacts. Depending on the age groups and settings involved in the outbreak, alternative follow-up mechanisms may be considered to effectively reach large numbers of contacts and other at-risk groups. Examples of alternatives that were used are letters or cards to copy and distribute, the Internet or established e-mail distribution lists, public service announcements, press releases, and a toll-free telephone number.
The logistics of providing immunization to susceptible contacts and at-risk populations should be carefully considered. Some of the issues encountered in managing previous out-breaks included vaccine supply and acquisition costs, low uptake by the college/university-aged cohort, accurate determination of susceptible groups complicated by poor or non-existent immunization records, and vaccine administration and related costs.
To minimize the spread of the virus and the impact on vulnerable groups, contacts with serious mumps-like symptoms should be advised to call ahead before visiting their health care provider. In the event of a large community outbreak where Public Health has set up triage centres, it is appropriate for potential cases to be redirected to one of these centres. For an individual who has developed mild mumps-like symptoms not requiring medical attention, a call to Public Health would ensure that they are included in case counts for the outbreak.
Contacts that are in a health care facility should be managed using droplet precautions for the duration of their period of communicability.
| Contact management | Regardless of the mechanism, the dissemination of information to contacts should include
Offer immunization to susceptible groups as defined by the epidemiology of the outbreak; recognize that immunization may not prevent disease if the individual is already infected. Previous outbreaks have indicated that immunization uptake is low. |
|---|
6.4 Exposure Settings
The management, prevention, and control of mumps may be specific to the exposure settings affected. Management of mumps cases and contacts in three of the commonly experienced high-risk exposure settings are described below.
6.4.1 Gatherings
Gatherings apply to events of all sizes, in both private and public forums. Gatherings include social or religious functions, sports activities, organized shopping excursions, concerts, conferences, and meetings, as well as public transit. During an outbreak, events need not be cancelled, although jurisdictions may consider postponing gatherings that may pose a risk for transmission or involve vulnerable populations (e.g., well-baby clinics).
It is prudent for organizers to use these opportunities to inform participants about the potential for disease transmission and methods to minimize the spread of the disease, including immunization, practising good hand hygiene, avoiding sharing drinking glasses or utensils, covering coughs and sneezes with a tissue or forearm, and staying home when ill(17). Because of the slight but real risk of infection, exposure settings should be widely communicated to the public. Further details on risk communications are found in Section 8.0.
| Gatherings | During an outbreak, events need not be cancelled. Public exposure settings should be communicated to the public, and event organizers should advise participants of the following:
|
|---|
6.4.2 Schools/Educational Institutions
Mumps cases should be excluded from school, day care, child care, or the workplace until 5 days after the onset of symptoms. Caregivers are to be advised to keep the child away from other susceptible children and adults for the period of exclusion. Schools/educational institutions may already have exclusion policies in place; this varies depending on the affected jurisdictions. The risk of exposure should also be communicated to all staff, students, and families.
| Schools/educational institutions | Encourage schools/educational institutions to practise good general hygiene to prevent disease spread (e.g., practise good hand hygiene, avoid sharing food/drink/utensils, cover coughs and sneezes with a tissue or forearm, and stay home when ill). If a case is identified, notify staff, students, and families. Refer to Section 4.3 for the definitions of contacts of cases. |
|---|
6.4.3 Health Care Settings
Health care settings include acute care and long- term care facilities, as well as home care. In these settings, a health care worker has the potential to acquire or transmit an infectious agent during the course of his or her work. Examples include nurses, physicians, support staff, home-care workers, emergency responders, students, and volunteers.
There is a small body of literature describing the impact of mumps—both isolated cases and outbreaks—in the health care setting. According to experience with mumps in hospitals during a Tennessee outbreak in 1986–1987, the introduction of mumps by either employees or patients is likely during an epidemic(18).
During the recent Nova Scotia outbreak and as of December 2007, mumps was diagnosed in 37 health care workers (personal communication: S. Clay, Nova Scotia Department of Health Promotion and Protection, Halifax, 2007) It was difficult to distinguish community versus occupational exposure, but, in the region with the majority of cases, most of the health care worker cases were related to community exposures with no clearly documented cross-transmissions to other health care workers or patients (personal communication: L. Johnston, Nova Scotia's Capital District Health Authority). During the Iowa outbreak, there were no cases of mumps among exposed, non-immune health care workers (unpublished data: D. Diekema, 17th Annual Scientific Meeting of the Society for Healthcare Epidemiology of America, Baltimore, 2007). Evidence that mumps can have an impact in the workplace is presented in a report of a Chicago outbreak that documented 119 cases of mumps among employees and their household contacts in three Chicago workplaces(19).
The clinical diagnosis of mumps can be difficult, even in the outbreak setting (see section 5.0), as up to 30% of mumps infections are sub-clinical(20), and a number of other infectious agents can cause mumps-like illness(21). Additionally, many Canadian physicians in practice today will never have seen a case of mumps. Physicians who are familiar with mumps are a definite asset to an occupational health program. A mumps diagnosis may not be easy to make or to exclude. This difficulty becomes an important one when managing potential mumps cases and exposures in the health care setting.
While mumps is largely a self-limited illness, a small number of affected individuals will experience complications or chronic consequences of acute mumps. There are limited data on whether hospitalized or immunocompromised patients experience increased or more severe complications from mumps.
The available evidence indicates that there is a population of health care workers that is susceptible to mumps. Serologic testing during the 2007 Nova Scotia outbreak found that 83.4% of those born before 1970 and 67.7 % of those born after had laboratory evidence of immunity (unpublished data: S. Clay, 34th Annual Conference of the Association for Professionals in Infection Control and Epidemiology, San Jose, 2007). A community mumps outbreak can have considerable impact on health care settings and health care capacity. Factors contributing to the potential for mumps transmission in health care settings are as follows: the long infectious and incubation periods; a high proportion of sub-clinical and misdiagnosed cases; and a sizable population of susceptible health care workers. During a community outbreak, health care workers may be exposed in workplace settings in addition to their community exposures. In one hospital during the Iowa outbreak, there were 31 exposure events involving more than 600 health care workers (unpublished data: D. Diekema, 17th Annual Scientific Meeting of the Society for Healthcare Epidemiology of America, Baltimore, 2007). In Nova Scotia, one region evaluated 2,400 health care workers for reported contacts and furloughed 261 (personal communication: B. Walker, Nova Scotia's Capital District Health Authority). A number of the exposures involved co-workers at meetings.
To minimize disruption in the health care setting, both the U.S. Centers for Disease Control and Prevention (CDC) and PHAC have guidelines for mumps management(22,23). In addition, the National Advisory Committee on Immunization (NACI) addresses the immunization of health care workers in some of its statements(1). All authorities emphasize the importance of assessing immunity and providing two-dose MMR vaccine to health care workers where indicated before an outbreak occurs. This strategy will result in minimal disruption to health care facilities during a community outbreak. Exclusion of health care workers who are contacts from work should be balanced with the availability of human resources and should consider the outbreak epidemiology.
Assessing the evidence of immunity can be challenging. NACI suggests cautious application of the natural immunity assumption to high-risk adults like health care workers and military personnel(16). This is further supported by Nova Scotia's serologic results, which suggest that approximately 15% of those born before 1970 may not be immune to mumps. It is therefore recommended that birth in Canada before 1970 not be taken as evidence of immunity for health care workers and that even birth before 1957 offers only presumptive evidence of immunity(22). Furthermore, a self-reported history of mumps is not acceptable as proof of immunity. A positive IgG result may not necessarily indicate immunity, although a negative result may indicate that antibody levels are simply too low to be detected by commercially available assays. See Section 5.0 and Appendix 4 for further details on IgG tests and interpretation of test results.
In addition to the recommendations for health care settings (Table 10), algorithms to assist with the management of health care workers who are close contacts of a case of mumps and the assessment of health care workers for susceptibility to mumps are outlined in Appendix 5 (figures A and B). Management strategies in health care settings should take into account the epidemiology of the outbreak and the composition of the patient population.
| Health care settings | Health care settings include those related to acute care, long-term care, and home care. Some health care settings may not have occupational health and infection prevention and control departments. When these are mentioned, they refer to the individual(s) responsible for occupational health and infection prevention and control for that health care setting. A health care worker (HCW) is an individual who may have the potential to acquire or transmit an infectious agent during the course of his or her work in the health care setting (e.g., nurses, physicians, students, volunteers, home-care workers, emergency responders, and support staff ). Pre-placement of HCWs
Existing HCWs
HCWs who are cases
HCWs who are contacts For contact in the community (see Section 4.3) and contact in the health care setting (if unprotected face-to-face interaction within 1 metre of an infectious mumps case)
|
|---|
6.5 Travellers
When a case of mumps is being investigated, the travel history as a potential risk factor should be considered both within and outside of Canada. The provincial/territorial health authority that identifies an infectious traveller should advise the provincial/territorial health authority of the area of residence of the case and of any known contacts, so that the authorities may follow up accordingly. The identifying health authority should also report the information to PHAC.
When cases or contacts are from a different country, the identifying provincial/territorial health authority should notify PHAC, which will contact the appropriate authority of the affected country. When international travellers associated with mumps cases or contacts are identified by the Quarantine Service or Duty Officers at an international port of entry, PHAC will notify the appropriate provincial/territorial or international public health authority.
When multiple jurisdictions are involved, it is not always clear who should report a case. Case reporting is important for describing and monitoring the epidemiology, impact, and spread of an outbreak. Therefore, during outbreaks, the jurisdiction that initially identifies and primarily handles the case is asked to report it. This decision rule is flexible and should be evaluated for each case, considering place of residence, travel itinerary, and the public health response or intervention that was implemented.
6.5.1 Airplanes
PHAC, the CDC, the World Health Organization (WHO), and the International Air Transport Association have guidelines on when and how to notify passengers and flight crew after they have been exposed to certain infectious diseases aboard international commercial aircraft(24-27). These guidelines apply mainly to highly communicable or virulent diseases such as tuberculosis, measles, or meningococcal disease, as well as other conditions listed in the Quarantine Act(28).
The appropriate public health response to exposure and transmission of mumps during commercial air travel varies. During the 2006 mumps outbreak in Iowa, the CDC initiated contact tracing of passengers sitting near an infectious passenger for flights of 5 hours or longer. The U.K. and Canada, on the other hand, generally do not follow up on the reported exposure of mumps on aircraft. In Canada, if a traveller infected with mumps has travelled by air during the infectious period (7 days before onset of symptoms to 5 days after the onset of symptoms), the local public health authorities and PHAC should be consulted. However, contact tracing through a passenger manifest is not necessary since the guidelines for tuberculosis, measles, and meningococcal disease may not apply to less infectious and self-limited diseases like mumps. In addition, follow-up is not performed, as there is no treatment or prophylactic intervention for mumps, and the passenger manifests are difficult to obtain and/or are often incomplete.
Communication of the traveller's itinerary should be considered by the overseeing public health authority so that other jurisdictions are aware of the potential exposure, as they may have different protocols or be assessing changes in their own mumps activity. Non-nominal travel details can be shared with public health professionals across the country through the Canadian Integrated Outbreak Surveillance Centre, a secure, Webbased, alerting application.
In Canada, airlines can refuse permission to board to individuals who appear to have an infectious disease.
6.5.2 Cruise Ships
Respiratory tract infections are frequent in cruise ship settings. In the event of an identified mumps outbreak, the cruise ship's health services would have responsibility for the traveller's health during the cruise and would follow up with contacts according to the conveyance operator's policy.
Ninety-six hours before port arrival, ships are to report to Canadian port authorities as to the presence and status of anyone aboard with certain communicable diseases. If a condition of quarantine or public health concern is suspected, the port authority will notify National Quarantine Services (PHAC) to meet the ship upon arrival; PHAC will then alert the provinces and territories if an outbreak is confirmed. In Canada, cruise lines can refuse permission to board to individuals who appear to have an infectious disease.
In Canada, cruise lines can refuse permission to board to individuals who appear to have an infectious disease.
| Travellers | Travellers should ensure that their routine immunizations are up to date. As mumps is transmitted through infected oral/nasal secretions, travellers should protect themselves and others by practising good hand hygiene, coughing or sneezing into a tissue or forearm, and avoiding sharing food, drinks, or utensils. In Canada, individuals can be refused permission to board an aircraft or cruise ship if they appear to have an infectious disease. Travellers with symptoms of mumps, including fever, should postpone travel until they are better. When provincial/territorial borders are crossed, the province or territory where the case was diagnosed should alert other provinces/territories and the Public Health Agency of Canada (Centre for Immunization and Respiratory Infectious Diseases). When international borders are crossed, the province or territory where the case was diagnosed should alert the Public Health Agency of Canada (Centre for Immunization and Respiratory Infectious Diseases) which will, in turn, notify the appropriate international authorities. |
|---|
7.0 Immunization
7.1 Mumps-Containing Vaccine and Immunization Programs in Canada
The mumps vaccine is a live, attenuated virus vaccine and is available in the combined form with measles and rubella vaccine. The Merck MMR vaccine, using the Jeryl-Lynn mumps virus strain, has been used in Canada since its approval in the 1970s. There are two different mumps-containing vaccines currently available in Canada: M-M-R® II, manufactured by Merck Frosst Canada Ltd., and Priorix®, manufactured by GlaxoSmithKline Inc. Both products contain the Jeryl-Lynn mumps virus strain. There is currently no single-component mumps-containing vaccine available in Canada(1,16).
Trivirix®, manufactured by Institut Armand Frappier/Smith Kline, which uses the Urabe Am9 mumps strain, was licensed in the mid-1980s, but it was withdrawn in the late 1980s because of an association between the Urabe Am9 strain and aseptic meningitis.
By 1983, all provinces and territories were routinely immunizing infants with MMR vaccine. To eliminate measles, a two-dose MMR schedule was implemented in 1996 to decrease the proportion of children susceptible as a result of primary vaccine failure. Most provinces and territories conducted measles catch-up campaigns in 1996– 1997. Some jurisdictions used a measles-only vaccine, whereas others used a measles-rubella vaccine for catch-up. Two provinces (Nova Scotia and New Brunswick) did not conduct a measles catch-up campaign. All provinces/territories now use an MMR vaccine in their routine two-dose programs. The immunization schedule in all provinces and territories offers a first dose of MMR vaccine at 12 months of age. Ten provinces/ territories offer the second dose at 18 months of age, and the other three (Nova Scotia, Manitoba, and Alberta) offer it at 4–6 years of age.
While there are several genotypes (strains) of the mumps virus, it is traditionally accepted that mumps viruses belong to a single serogroup. There is evidence that the immunity induced by one mumps virus strain protects against infection by other strains(7). Rubin and colleagues(29) showed that sera from 74 different people who tested positive for mumps antibodies by a commercial enzyme immunoassay were able to neutralize mumps viruses belonging to two different genotypes. Only 10% of sera could neutralize only one virus and not the other. These results suggest that although the differences in neutralization titres provide evidence for some antigenic variation, the fact that 90% of the sera could neutralize both viruses supports the historical view that all mumps viruses belong to a single serotype.
Conversely, there are some data to suggest that the immune response directed against one genotype of mumps may not provide absolute protection against infection with mumps viruses of other genotypes(30). These results have shown that neutralization antisera generated by the vaccine containing the Jeryl-Lynn vaccine strain (genotype A) may not protect against infections with mumps virus from the C and D genotype lineage(31).
As described in Section 3.0, the viral strain in the two 2007 Canadian outbreaks (Maritimes and Alberta), the 2005–2006 Nova Scotia outbreaks, the U.S. multi-state outbreak in 2006, and the 2004–2006 U.K. epidemic was identical. This G genotype is not unusual or rare and, like the rest of known genotypes of mumps, it has been circulating globally for decades or longer. Genotypes currently identified include A to L(32).
The MMR vaccines are safe, immunogenic, and effective and are recommended by NACI and PHAC for primary immunization against measles, mumps, and rubella. The combined MMR vaccine should be used even in individuals who may have prior immunity to components of the vaccine, and it can be used to immunize susceptible adults against mumps.
Mumps immunization after exposure to mumps virus may not prevent the disease. Should the exposure not result in an infection, the vaccine should confer protection against future exposures. If indicated, a second dose of MMR vaccine can be given 1 month or more after the first dose(1).
It is unknown whether primary vaccine failure or waning immunity has been the major risk factor for mumps in vaccinated individuals and mumps outbreaks in the recent past(16). In controlled clinical trials, one dose of mumps vaccine was 95% efficacious in preventing mumps disease(33). However, observational studies conducted during mumps outbreaks have demonstrated lower estimates of vaccine effectiveness, usually around 70% to 80% with single-dose regimens(34-39).
Mumps outbreaks have been reported in school populations in the United States with very high (greater than 95%) coverage with single-dose mumps-containing vaccine, suggesting that one dose of mumps-containing vaccine is not sufficient to prevent mumps outbreaks in the school setting(16,39,40). A two-dose measles, mumps, and rubella immunization schedule used in Finland resulted in higher mumps-specific antibody levels, a higher seropositivity rate, and slower decline of antibody levels(41).
The duration of vaccine-induced immunity is unknown. There are many studies demonstrating a drop in antibody levels over time (i.e., waning immunity)(16,38-40,42-44). The length of antibody persistence is unknown in settings with high vaccine coverage but low or no circulating wild virus, and no data are currently available correlating specific antibody titres with susceptibility to mumps.
Immunization history was known for less than half of the mumps cases reported in Canada in 2007 (n = 586). Of those, 45 (8%) had received two or more doses, 430 (73%) had received one dose, and 111 (19%) had received no doses of mumps-containing vaccine.
7.2 National Advisory Committee on Immunization: Statement on Mumps Vaccine
NACI publishes detailed recommendations pertaining to the use of vaccines in Canada(1). These recommendations are contained in the Canadian Immunization Guide and are updated as new information becomes available. Updated and recent immunization statements are available at http:// www.phac-aspc.gc.ca/naci-ccni/index-eng.php.
NACI issued a revised statement for mumps- containing vaccine in August 2007 as a result of recent mumps outbreaks in Canada and internationally, and after reviewing data on vaccine effectiveness and waning immunity. Its previous recommendation for routine, one-dose mumps immunization was changed to two doses for infants and children. In addition, two-dose mumps immunization is now recommended for certain adult high-risk groups, including secondary and post-secondary students, military personnel, and health care workers(16). NACI suggests consideration of a single dose of MMR vaccine for high-risk adults like health care workers and military personnel born before 1970.
It is expected that large outbreaks of mumps will occur less frequently as the NACI two-dose recommendations are implemented. Cases that do occur may result in transmission of mumps, most likely among children and young adults who have not received two doses of mumps-containing vaccine or who have not had natural mumps disease. A dose of mumps-containing vaccine is therefore recommended for susceptible (i.e., those born in or after 1970 who received only one dose of a mumps-containing vaccine), at-risk populations during outbreaks. At-risk populations will need to be defined by the age groups and settings involved in the outbreak. No more than two doses of MMR vaccine given after the first birthday are currently recommended(16).
7.3 Outbreaks
7.3.1 Immunization of Susceptible Populations
In response to recent mumps outbreaks in Canada, several jurisdictions have undertaken immunization campaigns targeting populations that may be susceptible to mumps. Susceptible populations include those born in Canada in 1970 or later who did not receive two doses of mumps-containing vaccine (at least 4 weeks apart) after their first birthday and who have not had laboratory- confirmed mumps or who do not have documented immunity to mumps. The exact age of the cohorts varies by jurisdiction, depending on when one- and two-dose mumps containing immunization programs were introduced (Figure 1). For those individuals not born in Canada, susceptibility may be determined on the basis of immunization documentation. Those who do not have documented receipt of two doses of mumps-containing vaccine should be considered susceptible.
Jurisdictions contemplating an immunization campaign for susceptible groups as part of their outbreak control strategy should consider using the epidemiology of the outbreak to define their target susceptible group. In previous outbreaks, uptake has been found to be low, particularly among post-secondary student and young adult populations. If the outbreak strategy includes offering immunization to this group, specific approaches to increase coverage should be considered. These might include partnering with student health centres, offering incentives, and providing vaccine in settings where students congregate, such as residences, student centres, bars, and clubs(10).
As with any new immunization campaign, supply of vaccine should be coordinated in consultation with FPT counterparts (see Section 7.4 on Vaccine Supply).
7.3.2 Community Contacts of Cases
Immunization of susceptible contacts of cases may not prevent disease if an individual is already infected(10). It may be considered if repeated exposure to mumps is expected.
However, experience during recent outbreaks has been that public health capacity was quickly overwhelmed by the resources required for individual contact tracing and management. The logistics of providing immunization to susceptible contacts and population groups should be carefully considered. Some of the issues encountered in managing previous outbreaks included vaccine supply and acquisition costs, low uptake by the university-aged cohort, accurate determination of susceptible groups complicated by poor or non-existent immunization records, vaccine administration, and associated costs.
7.3.3 Health Care Workers Who Are Contacts of Cases
Health care workers who are the contacts of a confirmed case should have their immune status reviewed. If they have two documented doses of mumps-containing vaccine or documentation of antibody to mumps, then they can be considered immune and can return to work immediately. If they have one documented dose of mumps- containing vaccine, a dose of MMR vaccine should be provided and they can return to work immediately. If they have an undocumented immune history, it is recommended that mumps IgG be tested and one dose of MMR vaccine be provided. While waiting for the serology results, the health care worker should be excluded from work for the period of communicability, which starts on day 10 after the first exposure. If IgG serology is positive, then the health care worker can be considered immune and return to work. A second dose of MMR vaccine should be administered 28 days after the first for adequate measles protection. If IgG serology is negative, then the health care worker should be considered susceptible. A second dose of MMR vaccine should be provided 28 days after the first was given, and exclusion from work should continue from day 10 after the first exposure until day 26 after the last exposure.
7.4 Vaccine Supply
The status of MMR vaccine supply should be considered before undertaking immunization initiatives as part of the outbreak response. As with any new immunization initiative, vaccine supply should be coordinated in consultation with FPT counterparts through the Vaccine Supply Working Group and the CIC. While MMR vaccine supply has been stable in recent years, factors such as introduction of mumps-containing immunization catch-up programs in some jurisdictions, measles immunization programs, and possible introduction of MMR-V (measles, mumps, rubella, and varicella) vaccine could all affect the availability of MMR vaccine.
In the event of an actual or projected shortage of MMR vaccine during an outbreak, the identification of priority groups may be necessary. In the United Kingdom, the following priority order was considered: routine immunization for infants and children, immunization of rubella-susceptible women of child-bearing age, and immunization of susceptible (measles, mumps or rubella) health care workers, followed by immunization of other susceptible individuals as defined by the epidemiology of the outbreak(45). Prioritization should take place in consultation with the Vaccine Supply Working Group.
8.0. Strategic Risk Communications
8.1 Background
Strategic risk communications are collaborative, two-way processes between stakeholders and decision-makers that build trust and a shared understanding of the risk. This results in risk mitigation strategies that are grounded in the social and cultural realities of the situation.
In outbreaks, strategic risk communication plays a key role in encouraging behavioural changes within the community that can contribute to limiting the spread of infectious diseases (e.g., social distancing, immunization, hygiene practices). There will be a high demand for information from the media, the public, those in the health care sector in particular, those who are infected, and those at high risk of infection. It is important to balance the needs of each of these groups. The health care sector and at-risk populations must be communications priorities, but the media can also help to disseminate public health messages to secondary audiences.
A risk communications strategy allows public health authorities and other organizations to set communications objectives, identify stakeholders, and develop plans, activities, and messages appropriate for each stakeholder group. An understanding of stakeholder attitudes, perceptions, and behaviours is needed for communications to be effective. This understanding can be gained through research, but if time does not permit it may be more informally assessed through available knowledge and informal consultation.
Communications lessons learned during the Nova Scotia outbreak in 2007 are included in Appendix 6.
8.2 Best Practices in Outbreak Communications
Strategic risk communications are a critical component of integrated risk management during an infectious disease outbreak. The goal is to help decision-makers and stakeholders make well-informed decisions leading to responsible and ethical risk management. To facilitate the implementation of strategic risk communications, the Communications Directorates at PHAC and Health Canada have developed a Strategic Risk Communications Framework and Handbook(46).
The handbook outlines five guiding principles for strategic risk communications, which align with checkpoints that the WHO developed for best practices in infectious disease outbreaks. These WHO checkpoints focus on building trust and are good to keep in mind when managing communications during an outbreak(47):
Strategic risk communications are essential for integrated risk management
Involve communications managers early and ensure that they remain part of the team throughout the process. Collaborate on developing an opportunity statement that defines the scope of the risk as it pertains to key stakeholders, and identify the opportunity to mitigate that risk. Establish the desired behavioural outcomes, which should be measurable and form the basis of all communications objectives.
Stakeholders are a focal point
Stakeholders are an invaluable source of information, knowledge, expertise, and insight. Decisions must consider stakeholders' perception of risks and benefits. Involve stakeholders (e.g., university students, administration, health care professionals) as soon as possible to better focus the risk management and communications approach. For example, involve stakeholders to help assess the barriers that different groups might have to following public health advice, like getting immunized or staying home when sick.
Decisions are evidence-based
Decisions should draw equally on scientific evidence and social science research concerning the attitudes and beliefs of the key stakeholders. For example, if the scientific advice is that university students are at high risk of mumps infection, social scientific evidence is needed to inform the development of effective means to reach that stakeholder group and reduce the risk. Social science research includes public opinion and focus group research, as well as information gleaned from any other available knowledge (literature review, behaviour trend analysis, etc).
Transparency
Communicate openly to stakeholders about risks and benefits. Make the methods and plans used in risk management understandable and accessible. Be clear about what gaps in knowledge remain and what is being done to address them. Announce outbreaks early to control rumours and establish leadership. Even with incomplete information, a government presence early on helps to build public trust. Leave room for the unexpected, and never make promises (e.g. "We've already seen the worst of it"). Outbreaks are unpredictable, so spokespersons should not be overconfident or mislead the public.
Continuous Evaluation
Set clear, measurable objectives from the outset. Measure the outcomes against the objectives on an ongoing basis to monitor continuous improvement. Adjust the strategy when necessary to meet goals as well as time and cost efficiencies.
8.3 Networking and Collaboration
The communications responsibilities during an outbreak are primarily managed at the local and provincial levels. It is the responsibility of each province and territory to communicate about the situation within its jurisdiction. Mumps is a nationally notifiable disease, so PHAC can provide information from a national perspective. PHAC can also facilitate the sharing of key messages, communications materials, and best practices through established groups like the CIC and the Pan-Canadian Public Health Network.
At the provincial/territorial level, government communicators can maximize their effectiveness by working with communicators from professional associations and health care facilities. Working groups can be established to share messages and communications products. Professional associations can help get messages on the outbreak and on diagnostic testing out to health care providers and health care facilities. They can work with government communicators to promote immunization clinics. Across the country, provincial/ territorial governments can work with each other to establish best practices as well as share messages and communications products where appropriate.
| Communication strategies | In outbreaks, strategic communications play a key role in successfully managing the risk. It is important that risk managers and communicators collaborate to identify the desired behavioural changes that will reduce the risk among stakeholders and to identify the barriers that may discourage change, in order to develop efficient strategies for risk mitigation. The goal of strategic risk communications is to establish trust with the affected stakeholders in order to encourage them to make behavioural changes that will reduce their risk. The best way to do this is to involve stakeholders early on and be transparent with all information. Communications activities include identifying spokespersons to speak to the media about the issue and developing media lines, backgrounders, and question and answer content. Sharing key messages, communication materials, and best practices across all jurisdictions involved is essential for managing an outbreak. |
|---|
References
- National Advisory Committee on Immunization. Canadian immunization guide. 7th ed. Ottawa: Public Health Agency of Canada, 2006.
- Bell A, Fyfe M, Bigham M et al. Outbreak of mumps among young adults – Vancouver, British Columbia. CCDR 1997;23(22):169-72.
- Bruneau A, Duchesne C. Outbreak of mumps, Montreal, October 1998 to March 1999 – with a particular focus on a school. CCDR 2000;26(8):69-71.
- Alberta Health and Wellness. Public health notifiable disease management guidelines (June 2005). http://www.health.gov.ab.ca/ professionals/NotifiableDiseases.html. Date of access: November 23, 2007.
- Watson-Creed G, Saunders A, Scott J et al. Two successive outbreaks of mumps in Nova Scotia among vaccinated adolescents and young adults. CMAJ 2006;175(5):483-88.
- Centre for Immunization and Respiratory Infectious Diseases, Public Health Agency of Canada. Analysis of data from the National Notifiable Diseases Reporting System. Ottawa: Public Health Agency of Canada, 2007.
- Leland DS. Parainfluenza and mumps viruses. In: Murray PR, Baron EJ, Jorgensen JH et al., eds. Manual of clinical microbiology. 9th ed. Washington DC: ASM Press, 2007.
- Robertson S. Mumps. In: Heymann DL, ed. Control of communicable diseases manual. 18th ed. Washington DC: American Public Health Association, 2004;376-79.
- American Academy of Pediatrics. Mumps. In: Pickering LK, ed. Red Book: 2003 Report of the Committee on Infectious Diseases. 26th ed. Elk Grove Village: American Academy of Pediatrics, 2003:439-43.
- Zimmerman L, Reef S, Wharton M. Mumps. In: Wharton M, Hughes H, Reilly M, eds. Manual for the surveillance of vaccine-preventable diseases. 3rd ed. Atlanta: Centers for Disease Control and Prevention, 2002:7-1–7-12.
- Centers for Disease Control and Prevention. Mumps. In: Atkinson W, Hamborsky J, McIntyre L et al., eds. Epidemiology and prevention of vaccine-preventable diseases. 10th ed. Washington DC: Public Health Foundation, 2007;149-58.
- Seward et al. Updated Recommendations for the Duration of Isolation Precautions for Persons with Mumps. MMWR 2008; 57:1103-5.
- Nova Scotia Health Promotion and Protection. Mumps outbreak case management and surveillance guidelines, May 2007. Version: May 14, 2007. Nova Scotia Health Promotion and Protection, 2007.
- Iowa Department of Public Health. Mumps. Revision 5/5/2006. Iowa Department of Public Health, 2006.
- Centers for Disease Control and Prevention. CDC health update – corrected: multi-state mumps outbreak. Health Alert Network, April 26, 2006.
- National Advisory Committee on Immunization. Statement on mumps vaccine. CCDR 2007;33(ACS-8):1-10.
- Iowa Department of Public Health. Public health bulletin: Mass gathering policy – mumps. Iowa Department of Public Health, 18 April, 2006.
- Wharton M, Cochi SI, Hutcheson RH et al. Mumps transmission in hospitals. Arch Intern Med 1990;150:47-9.
- Kaplan KM, Marder DC, Cochi SL et al. Mumps in the workplace: further evidence of the changing epidemiology of a childhood vaccinepreventable disease. JAMA 1988;260:1434-38.
- Gupta RK, Best J, MacMahon E. Mumps and the UK epidemic 2005. BMJ 2005;330:1132-35.
- Davidkin I, Jokinen S, Paananen A et al. Etiology of mumps-like illnesses in children and adolescents vaccinated for measles, mumps, and rubella. J Infect Dis 2005;191:719-23.
- Centers for Disease Control and Prevention. Prevention and control of mumps in healthcare settings. http://www.cdc.gov/mumps/prev-control-settings/index.html. Date of access: October 31, 2007.
- Public Health Agency of Canada. Prevention and control of occupational infections in health care. CCDR 2002;28(S1):1-276.
- Public Health Agency of Canada. Guidelines for reporting a case of infectious tuberculosis on an aircraft (draft). Ottawa: Public Health Agency of Canada, 2007.
- Centers for Disease Control and Prevention. Exposure to mumps during air travel – United States, April 2006. MMWR 2006;55(14):401-02.
- World Health Organization. Tuberculosis and air travel: guidelines for prevention and control. 2nd ed. World Health Organization, 2006.
- International Air Transport Association. Emergency response plan: a template for air carriers – public health emergency. International Air Transport Association, 2005.
- Quarantine Act, RSC 2005, c20, s82. Repeals RSC 1985, cQ-I.
- Rubin S, Mauldin J, Chumakov K et al. Serological and phylogenetic evidence of monotypic immune responses to different mumps virus strains. Vaccine 2006;24:2662-68.
- Nöjd J, Tecle T, Samuelsson A et al. Mumps virus neutralizing antibodies do not protect against reinfection with a heterologous mumps virus genotype. Vaccine 2001;19:1727-31.
- Örvell C, Alsheikhly AR, Kalantari M et al. Characterization of genotype-specific epitopes of the HN protein of mumps virus. J Gen Virol 1997;78:3187-93.
- Jin L, Rima B, Brown D et al. Proposal for genetic characterisation of wild-type mumps strains: preliminary standardisation of the nomenclature. Arch Virol 2005;150:1903–09.
- Plotkin S. Mumps vaccine. In: Plotkin S, Orenstein W & Offit P, eds. Vaccines. 4th ed. WB Saunders Company, 2003;441-60.
- Cohen C, White JM, Savage EJ et al. Vaccine effectiveness estimated, 2004-2005 mumps outbreak, England. Emerg Infect Dis 2007;13(1):12-17.
- Gay N, Miller, Hesketh L et al. Mumps surveillance in England and Wales supports introduction of two-dose vaccination schedule. Commun Dis Rep Rev 1997;7:R21-6.
- Harling R, White J, Ramsay M et al. The effectiveness of the mumps component of the MMR vaccine: a case-control study. Vaccine 2005;23:4070-74.
- Wharton M, Cochi S, Hutcheson R et al. A large outbreak of mumps in the postvaccine era. J Infect Dis 1988;158(6):1253-60.
- Vandermeulen C, Roelants M, Vermoere M et al. Outbreak of mumps in a vaccinated child population: a question of vaccine failure? Vaccine 2004;22:2713-16.
- Hersh B, Fine P, Kent W et al. Mumps outbreak in a highly vaccinated population. J Pediatr 1991;119:187-93.
- Briss P, Fehrs L, Parker R et al. Sustained transmission of mumps in a highly vaccinated population: assessment of primary vaccine failure and waning vaccine-induced immunity. J Infect Dis 1994;169:77-82.
- Peltola H, Heinonen O, Valle M et al. The elimination of indigenous measles, mumps and rubella from Finland by a 12 year, two-dose vaccination program. N Engl J Med 1994;331:1397-402.
- Pebody R, Gay N, Hesketh L et al. Immunogenicity of second dose measles-mumps-rubella (MMR) vaccine and implications for serosurveillance. Vaccine 2002;20:1134–40.
- Davidkin I, Malle M, Julkunen I. Persistence of anti-mumps virus antibodies after a two-dose MMR vaccination: a nine-year follow-up. Vaccine 1995;13(16):1617-22.
- Park DW, Nam MH, Kim JY et al. Mumps outbreak in a highly vaccinated school population: assessment of secondary vaccine failure using IgG avidity measurements. Vaccine 2007;25(24):4665-70.
- Health Protection Agency. MMR vaccination: Priorities for use in mumps outbreak. <www.hpa.org.uk/infections/topics_az/mumps/images/MMRpriorities.pdf>. Date of access: September 18, 2007.
- Health Canada and the Public Health Agency of Canada. Strategic risk communications framework and handbook. Thorne Butte: Decision Partners Inc. 2006.
- World Health Organization. Outbreak communication: best practices for communicating with the public during an outbreak. World Health Organization, 2005.
Appendix 1: Persons and Organizations Involved in Developing and Reviewing the Guidelines
| Newfoundland Labrador Department of Health and Community Services | |
| Butler, Gillian | Disease Control Nurse Specialist |
| O'Keefe, Cathy | Director, Disease Control |
| Yetman, Marion | Infection Control Nurse Specialist |
| Prince Edward Island Department of Health | |
| Neatby, Anne | Coordinator, Communicable Disease and Immunization Programs |
| Sweet, Lamont | Chief Health Officer |
| Nova Scotia Department of Health Promotion and Protection | |
| Clay, Susan | Field Epidemiologist, Population Health Assessment and Surveillance |
| Coombs, Ann | Field Surveillance Officer |
| Sarwal, Shelly | Medical Officer of Health |
| New Brunswick Department of Health | |
| Akwar, Holy T. | Director of Communicable Disease Epidemiology |
| Cochrane, Lynn | Senior Program Advisor, Office of the Chief Medical Officer of Health |
| Dhaliwal, Jastej | Communicable Disease Epidemiologist |
| Giffin, Scott | Medical Officer of Health |
| Schellenberg, Gwyn | Coordinator and Public Health Nurse |
| Ministère de la Santé et des Services sociaux du Québec | |
| Landry, Monique | Médecin conseil, Direction de la protection de la santé publique |
| Ontario Ministry of Health and Long-Term Care | |
| Dolman, Sharon | Nurse Epidemiologist |
| Manitoba Health | |
| Dyck, Myrna | Epidemiologist |
| Hilderman, Tim | Medical Officer of Health, and CDC Branch Acting Medical Director |
| Long, Michelle | Vaccine Preventable Diseases Program Specialist |
| Richards, Lisa | Community Health Sciences Resident |
| Whitlock, Mandy | Project Epidemiologist |
| Saskatchewan Ministry of Health | |
| Bangura, Helen | Communicable Disease Epidemiologist |
| Findlater, Ross | Chief Medical Health Officer |
| Levett, Paul | Assistant Clinical Director, Saskatchewan Disease Control Laboratory |
| Sly, Lisa | Communicable Disease Consultant |
| Tuchscherer, Rosalie | Public Health Nursing Consultant |
| Alberta Health and Wellness | |
| Lachance, Lisa | Communicable Disease Nurse Consultant |
| Smith, Susan E. | Communicable Disease Nurse Consultant |
| St.Jean, Theresa | Manager, Communicable Disease Control |
| British Columbia Centre for Disease Control | |
| Anderson, Maureen | Surveillance Epidemiologist |
| Harry, Regina | Communications |
| Naus, Monika | Director, Immunization Programs & Associate Director, Epidemiology Services |
| Nunavut Department of Health and Social Services | |
| Palacios, Carolina | Communicable Disease Consultant |
| Northwest Territories Department of Health and Social Services | |
| Case, Cheryl | Communicable Disease Specialist, Population Health |
| White, Wanda | Communicable Disease Specialist, Population Health |
| Yukon Health and Social Services | |
| Larke, Bryce | Medical Health Officer |
| Infection Control | |
| Johnston, Lynn | Hospital Epidemiologist, QE II Health Science Centre, Nova Scotia |
| Chair, Public Health Agency of Canada Infection Control Guidelines Steering Committee | |
| O'Neil, Laurie | Nurse Consultant, Nosocomial and Occupational Infections Section, Public Health Agency of Canada |
| Laboratory Guidelines | |
| Tipples, Graham | Director, Surveillance and Reference Services, National Microbiology Laboratory, Public Health Agency of Canada |
| Tsang, Raymond | Chief of Laboratory for Pathogenic Neisseria and Vaccine Preventable Bacterial Diseases, National Microbiology Laboratory, Public Health Agency of Canada |
| Fonseca, Kevin | Clinical Virologist and Program Leader Virology, Alberta Provincial Laboratory for Public Health |
| Hatchette, Todd | Director of Virology and Immunology, QE II Health Science Centre, Nova Scotia |
| Public Health Agency of Canada | |
| Hickey, Raymonde | Senior Travel Health Nurse, Centre for Emergency Preparedness and Response |
| Welsh, Frank | Director, Office of Emergency Preparedness, Centre for Emergency Preparedness and Response |
| Badger, Gillian | Communications Advisor, Communications Directorate |
| McGihon, Julie | Senior Communications Advisor, Communications Directorate |
| Dalloo, Adrian | Field Epidemiologist, Centre for Immunization and Respiratory Infectious Diseases |
| Desai, Shalini | Medical Expert, Centre for Immunization and Respiratory Infectious Diseases |
| Harris, Tara | Program Evaluation Officer, Centre for Immunization and Respiratory Infectious Diseases |
| Law, Barbara | Interim Director, Vaccine Preventable Diseases and Vaccine Safety, Centre for Immunization and Respiratory Infectious Diseases |
| Lipskie, Tammy | Epidemiologist, Centre for Immunization and Respiratory Infectious Diseases |
| Macey, Jeannette | Acting Head, Vaccine Preventable Disease Surveillance, Centre for Immunization and Respiratory Infectious Diseases |
| Moffatt, Carolyn | Project Officer, Centre for Immunization and Respiratory Infectious Diseases |
| Tam, Theresa | Director, (former) Immunization and Respiratory Infections Division |
| Thom, Alan | Vaccine Supply Officer, Centre for Immunization and Respiratory Infectious Diseases |
| Varughese, Paul | Senior Science Advisor, Centre for Immunization and Respiratory Infectious Diseases |
| Writers/Editors: | Tammy Lipskie, Michael Garner, Adrian Dalloo, Shalini Desai |
|---|---|
| Acknowledgements: | The following individuals are recognized for developing various sections of the guidelines: Graham Tipples (Section 5.0, Appendix 4); Laurie O'Neil & Lynn Johnston (Section 6.3.3, Appendix 5); Raymonde Hickey (Section 6.4); Tara Harris (Section 7.0); Julie McGihon & Gillian Badger (Section 8.0, Appendix 6) |
| Approved by: | Arlene King, Director General, Centre for Immunization and Respiratory Infectious Diseases, Public Health Agency of Canada. Canadian Immunization Committee. Communicable Disease Control Expert Group, Council of the Public Health Network. Council of Chief Medical Officers of Health. |
Appendix 2: Key Recommendations for the Prevention and Control of Mumps Outbreaks
| Section(s) | Key Recommendations |
|---|---|
| 4.2 | Outbreak Definition Confirmed cases in excess of what is expected in the jurisdiction over a given period of time. |
4.3 |
Case Definitions In the absence of recent immunization (i.e. in the previous 28 days): Confirmed Case (any one of the following):
Clinical Case / Probable Case
Refer to Section 5.0 and Appendix 4 for details on preferred clinical specimens and interpretation of laboratory results. |
4.3 |
Contact Definition Any of the following during the infectious period (i.e. approximately 7 days before to 5 days after symptom onset):
Refer to Section 6.3.3 if the contact is a health care worker. |
6.1 |
Case Management Clinical cases should be managed as confirmed cases until laboratory evidence suggests otherwise.
|
6.2 |
Contact Management (community contacts; health care workers who are contacts are addressed separately in section 6.4.3) At the start of the outbreak, individual contacts can be managed either directly/individually or indirectly using the case to disseminate information to their contacts. Depending on the epidemiology of the outbreak, alternative follow-up mechanisms (e.g. letter, Internet, public service announcement, press release, toll-free telephone number) should be considered to reach contacts and other at-risk groups. Regardless of the mechanism, the dissemination of information to contacts should include
Offer immunization to susceptible groups as defined by the epidemiology of the outbreak; recognize that immunization may not prevent disease if the individual is already infected, and previous outbreak experiences have found uptake to be low |
6.3.1 |
Gatherings During an outbreak, events need not be cancelled. However, because of the slight but real risk of exposure, public exposure settings should be communicated to the public and gathering organizers should advise participants of the following:
|
6.3.2 |
Schools/Educational Institutions Encourage schools/educational institutions to practise general good hygiene to prevent disease spread (e.g. use good hand hygiene, avoid sharing food/drink/utensils, cover coughs and sneezes with a tissue or forearm, and stay home when ill). If a case is identified, notify staff, students and families. Refer to Section 4.3 for defining contacts of cases. |
6.3.3 |
Health Care Settings (include acute care, long-term care and home care) |
7.3.3 |
Some health care settings may not have occupational health and infection prevention and control departments. When occupational health and infection prevention and control are referred to, they mean the individual(s) responsible for occupational health and infection prevention and control for that health care setting. Definitions: A health care worker (HCW) is an individual who may have the potential to acquire or transmit an infectious agent during the course of his or her work in the health care workplace (e.g. nurses, physicians, students, volunteers, home care workers, emergency responders and support staff). Pre-placement of HCWs
Existing HCWs Occupational Health should provide MMR to all HCWs unless the individual has
HCWs who are cases
HCWs who are contacts
|
| 6.4 | Travellers Travellers should ensure that their routine immunizations are up to date. As mumps is transmitted through infected oral/nasal secretions, travellers should protect themselves and others by practicing good hand hygiene and coughing or sneezing into a tissue or forearm. They should avoid sharing food, drinks or utensils. In Canada, individuals can be refused permission to board an aircraft or cruise ship if they appear to have an infectious disease. Travellers with symptoms of mumps, including fever, should postpone travelling until they are better. When provincial/territorial borders are crossed, the province or territory where the case was diagnosed should alert other provinces/territories and the Public Health Agency of Canada (Centre for Immunization and Respiratory Infectious Diseases). When cases or contacts are from a different country, the identifying provincial/territorial health authority should notify PHAC, which will contact the appropriate authority of the affected country. When mumps cases or contacts are identified in international travellers by the Quarantine Service or Duty Officers at an international port of entry, PHAC will notify the appropriate provincial/territorial or international public health authority. Airplanes: Individual follow-up is not recommended, although notification of implicated public health authorities is suggested as other jurisdictions may have different protocols. Cruise Ships: The cruise ship's health services would have the responsibility for the traveller's health during the cruise and would follow up with contacts according to the conveyance operator's policy. |
7.0 |
Immunization (summarized from previous sections) |
7.3.2 |
Community contacts of cases |
6.2 |
|
7.3.3 |
HCWs who are contacts of cases |
6.3.3 |
|
| 8.0 | Strategic Risk Communications In outbreaks, strategic communications play a key role in successfully managing the risk. It is important that risk managers and communicators collaborate to identify the desired behavioural changes that will reduce the risk among stakeholders and to identify the barriers that may discourage change, in order to develop efficient strategies for risk mitigation. |
8.1 |
The goal of strategic risk communications is to establish trust with the affected stakeholders in order to encourage them to make behavioural changes to reduce their risk. The best way to do this is to involve stakeholders early on and to be transparent with all information. |
8.2 |
Communications activities include identifying spokespersons to speak to the media about the issue and developing media lines, backgrounders, and question and answer content. Sharing key messages, communication materials and best practices across all jurisdictions involved is essential for managing an outbreak. |
Appendix 3: Sample Mumps Outbreak Case Report and Follow-up Form
The case report form can be downloaded and filled out electronically.
Appendix 3: Sample Mumps Outbreak Case Report and Follow-up Form ( PDF Version 3 Pages - 54 KB)
Appendix 4: Laboratory Guidelines for the Diagnosis of Mumps
1.0 Introduction
The purpose of these laboratory guidelines is to provide information on the collection, transportation, laboratory testing, and interpretation of laboratory test results of specimens for suspected mumps cases. A comprehensive description of mumps diagnostics can be found in the Manual of Clinical Microbiology(1). The information presented here is based on recent experiences in mumps diagnostics both in Canada and in the United States.
2.0 Summary
- The reverse transcriptase polymerase chain reaction (RT-PCR) assay is reliable for the definitive diagnosis of an acute mumps infection, but its sensitivity can be influenced by the following:
- timing of the specimen collection in relation to onset of illness;
- specimen integrity (rapid specimen processing).
- Buccal swab or saliva from the buccal cavity collected within the first 3 to 5 days of parotitis or symptom onset is the preferred specimen.
- Testing for mumps-specific IgM class antibody has been shown to be poorly predictive for the diagnosis of acute mumps in a partially immunized population (may be detectable in only 30% of acute cases).
- Collection of an acute serum specimen and a convalescent serum specimen 10 to 14 days later may show a seroconversion for IgM and/or IgG antibody in those cases in which the mumps RT-PCR assay and IgM antibody were negative or indeterminate at onset of illness, thus identifying additional cases.
- Testing by RT-PCR and IgM class antibody detection is not sufficiently reliable to rule out mumps infection
- In the absence of another diagnosis for parotitis, persons with symptoms clinically compatible with mumps (clinical illness) AND an established epidemiologic link to a laboratory-confirmed case should be reported as confirmed cases (epidemiologically confirmed case). As well, those with clinical illness but no established epidemiologic links should be managed (for public health purposes) as a probable mumps case, particularly during periods of known outbreak activity.
3.0 National Surveillance Case Definition for Mumps2
- Confirmed Case
Laboratory confirmation of infection* in the absence of recent history of administration of mumps vaccine by- isolation of mumps virus from an appropriate clinical specimen
OR - detection of mumps virus RNA†
OR - significant rise or seroconversion in mumps IgG antibody titre by a standard serologic assay
OR - detection of mumps IgM class antibody in a person with compatible illness
OR - clinical illness‡ in a person who can be epidemiologically linked to a laboratory-confirmed case
- isolation of mumps virus from an appropriate clinical specimen
- Probable Case
Clinical illness‡ in the absence of appropriate laboratory tests and no epidemiological link to a laboratory-confirmed case
4.0 Specimen Collection
4.1 For virus detection (RT-PCR) or isolation (culture)
Buccal swabs or saliva swabs particularly in the area around Stensen's duct adjacent to the swollen salivary glands are optimal (see <http://www.cdc.gov/nip/diseases/mumps/detection_IL.pdf>).
Buccal specimens should be collected using a swab approved for virus isolation and placed in virus transport media. Swabs may be dacron, nylon, or rayon tipped, and either flocked or non-flocked. Calcium alginate swabs are not acceptable as they inhibit PCR reactions. Charcoal swabs or swabs in Ames media used for swabbing for bacterial pathogens (such as group A Streptococcus) are not acceptable. Swabs with wooden or aluminum shafts are also not acceptable. Oral samples should be collected within 5 days of onset of symptoms. Swabs should be placed in standard viral transport media (VTM). Swabs must be left in VTM for a minimum of 1 hour to allow the virus to elute.
Typically, mumps has been found in the urine by culture up to 14 days after the onset of symptoms. However, experiences in the Nova Scotia and U.S. outbreaks suggest that mumps cannot be detected in the urine with the same sensitivity as in oral specimens. Limited data suggest that virus can be detected in the urine slightly later (> 4 days post-onset) than in oral specimens.
Procedure:
- A minimum volume of 50 mL urine should be collected in a sterile container.
- Centrifuge urine at 2500 x g for 15 minutes at 4°C.
- Resuspend the sediment in 2 mL of residual urine before processing.
4.2 Serology
The first (acute) serum specimen should be collected as soon as possible upon presentation of mumps. A second (convalescent) serum specimen should be collected ideally at least 10 days after the first sample.
4.3 Specimen Storage and Transport
Unprocessed samples can be shipped at 4ºC within 48 hours of collection. If subsequent testing is delayed, processed samples can be frozen at -70°C and shipped on dry ice. Transport urine, buccal or saliva samples, and sera as diagnostic specimens, category B.
5.0 Serology
5.1 IgM
Although the presence of mumps-specific IgM antibodies is typically indicative of a primary acute mumps infection, recent data show that in a partially vaccinated population (recipients have received only one dose of mumps-containing vaccine) the IgM antibody response is delayed or absent. In addition, when the prevalence of mumps is low, the positive predictive value of the IgM test is correspondingly lower, and the potential for generating false-positive results is considerably greater. In sporadic suspected mumps cases without an established epidemiologic link to a confirmed case or without travel history to an area with known/likely mumps activity, one should be suspicious of positive IgM results, which are potentially false positives. In such sporadic suspected mumps cases, additional laboratory tests, such as paired acute/convalescent IgG serology or detection of mumps virus by RT-PCR, should be highly encouraged to confirm the mumps infection and subsequent genotyping of the strain if positive by RT-PCR.
5.2 IgG
The presence of mumps-specific IgG is indicative of a recent or prior exposure to mumps virus. Seroconversion (i.e., negative to positive result) or a fourfold or greater rise in titre between the acute and convalescent sera is indicative of an acute mumps infection. However, this does require a delay in the collection of the second (convalescent) serum sample of 10 days or more after the collection of the first (acute) serum sample. When using enzyme immunoassays (EIA) for IgG titre determination, it is important to do end-point titrations as opposed to single dilution runs of a sample in order to conclusively determine the fourfold or greater difference in titres between acute and convalescent sera.
Limitations to IgG serology:
- Serology cannot differentiate between vaccine and wild-type mumps strain exposure.
- The presence of mumps-specific IgG, as determined using an EIA, does not necessarily predict the presence of neutralizing antibodies and thus "immunity." Conversely, the absence of detectable mumps IgG using EIA may reflect the lower sensitivity of the EIA in comparison to a more sensitive assay, such as a neutralization assay, in which IgG may be detectable.
- A single serum sample tested for mumps-specific IgG has no value in diagnosing an acute mumps infection.
- Experience from the recent outbreaks in the United States suggests that when investigating suspected mumps in previously vaccinated individuals, in particular those who have received two doses of mumps-containing vaccine, the absorbance or quotient EIA values of IgG class antibody to mumps in the first (acute) serum may be quite high, and thus documentation of a fourfold or greater rise in titre/absorbance quotient may not be possible. Moreover, detection of appreciable levels of IgG class antibody in the acute serum does not rule out mumps infection.
The National Microbiology Laboratory can assist with the determination of mumps IgG titres if requested.
6.0 Mumps Virus Detection
6.1 Reverse transcriptase polymerase chain reaction (RT-PCR)
Because of the limited sensitivity of IgM testing discussed above, RT-PCR has been employed as the primary diagnostic approach for the laboratory confirmation of mumps cases.
Mumps virus is an RNA virus, and thus RT-PCR is the common approach for mumps virus detection. Commercial mumps RT-PCR assays are not available, and so conventional hemi-nested3 and real-time in-house assays have been developed and validated. The National Microbiology Laboratory uses the LightCycler real-time PCR platform for both the CDC Taqman probe-based method for detection of the SH gene, as well as the real-time method developed by Uchida et al.4 based on detection of the F gene. Appropriate positive and negative controls are essential for inclusion in RT-PCR runs to control for inhibition, extraction and contamination issues. Protocols and controls for mumps RT-PCR are available from the National Microbiology Laboratory on request.
Although the analytic sensitivity of RT-PCR is between 10 and 100 genome copies, the overall clinical sensitivity is affected by pre-analytical factors (see Section 3.0 above), namely the following:
- specimen type and quality;
- timing of specimen collection, in relation to onset of illness;
- rapid transportation of the specimen to the laboratory;
- rapid processing of specimens:
- It is recommended that specimens for virus detection be processed within 48 hours after sample collection. Further delays result in significant reduction in sensitivity.
- appropriate storage of specimens; and
- avoid freeze thawing unprocessed specimens.
Mumps cases with a history of immunization may also present challenges for RT-PCR. Such cases may have a shorter period of viral shedding and shed potentially lower amounts of virus, resulting in a higher incidence of false-negative results by the RT-PCR assay.
6.2 Mumps virus isolation
Mumps virus can be isolated in several cell lines, including primary monkey kidney, human neonatal kidney, HeLa, and Vero cells. Cytopathogenic effect is usually detected after 6 to 8 days post-inoculation. Accordingly, the RT-PCR assay is a more rapid approach for diagnostic purposes. Refer to the Manual of Clinical Microbiology1 for further details on mumps virus isolation protocols.
6.3 Mumps virus genotyping
Mumps genotyping has been standardized3 and is available at the National Microbiology Laboratory. Mumps virus genotyping is useful for molecular epidemiologic purposes such as differentiating vaccine and wild-type strains, especially if the individual developed a mumps-compatible presentation within 28 days of receiving the vaccine. In addition, genotyping can be helpful in linking cases, linking outbreaks, tracking importations, and documenting the elimination of a particular strain from a geographic area.
7.0 Interpretation of Laboratory Results
In an outbreak, a negative test, either RT-PCR or IgM antibody, cannot be interpreted to rule out mumps infection. In the absence of another diagnosis to rule out mumps infection, persons with clinically compatible mumps (clinical illness) AND an established epidemiologic link to a laboratory-confirmed case should be reported as confirmed cases (clinical confirmed case). As well, those with clinical illness but no established epidemiologic links should be managed (for public health purposes) as a probable mumps case, particularly during periods of known outbreak activity.
In order to properly interpret laboratory results and to assess the performance of mumps diagnostic assays, both clinical and epidemiologic information needs to be considered along with the laboratory information. As outlined above, prior vaccination history, travel history, and timing of sample collection relative to disease onset are all factors that must be considered in the interpretation of laboratory results for the purpose of confirming mumps cases. As such, communication and information sharing between public health and the laboratory are essential.
8.0 Mumps Testing at the National Microbiology Laboratory
Information on mumps testing available at the National Microbiology Laboratory can be found through the online guide to services at http://www.nml-lnm.gc.ca/english/guide/default.asp.
9.0 References
- Leland DS. 2007. Parainfluenza and mumps viruses. In: PR Murray, EJ Baron, JH Jorgensen et al, eds., ML Landry and MA Pfaller (ed.), Manual of clinical microbiology, 9th ed, Washington, D.C.: ASM Press, 2007.
- Laboratory Centre for Disease Control. Case definitions for diseases under national surveillance. CCDR 2000;26(S3). <http://www.phac-aspc.gc.ca/publicat/ccdr-rmtc/00vol26/26s3/index.html>.
- Jin L, B Rima, D Brown et al. Proposal for genetic characterisation of wild-type mumps strains: preliminary standardisation of the nomenclature. Arch Virol 2005;150:1903-9.4.
- Uchida K, M Shinohara, S Shimada et al. Rapid and sensitive detection of mumps virus RNA directly from clinical samples by real-time PCR. J Med Virology 2005;75:470–4.
10.0 Contributors/Acknowledgements
Graham Tipples, Jennifer Beirnes, and Joanne Hiebert (Viral Exanthemata Section, National Microbiology Laboratory, Public Health Agency of Canada)
Todd Hatchette and Janice Pettipas (QEII Health Sciences Centre, Halifax, Nova Scotia)
Kevin Fonseca (ProvLab Alberta, Calgary, Alberta)
Martin Petric (BC-CDC, Vancouver, British Columbia)
Jeannette Macey, Shelley Deeks, and Tammy Lipskie (Centre for Immunization and Respiratory Infectious Diseases, Public Health Agency of Canada)
William Bellini, Paul Rota, and Jennifer Rota (Measles, Mumps, Rubella and Herpes Branch, Centers for Disease Control and Prevention, Atlanta, Georgia, USA)
11.0 Contact Information, Public Health Agency of Canada
11.1 National Microbiology Laboratory
Graham Tipples, PhD, FCCM
Director, Surveillance and Reference Services
Chief, Viral Exanthemata Section
1015 Arlington Street
Winnipeg, MB, Canada R3E 3R2
Phone: (204) 789-6080
Fax: (204) 789-7039
Pager: (204) 935-4712
Email: Graham.Tipples@phac-aspc.gc.ca
Joanne Hiebert or Jennifer Beirnes
Technicians
Viral Exanthemata Section
Phone: (204) 789-6082 or (204) 789-7055
Fax: (204) 789-5009
Email: Joanne.Hiebert@phac-aspc.gc.ca or Jennifer.Beirnes@phac-aspc.gc.ca
11.2 Centre for Immunization and Respiratory Infectious Diseases
The Public Health Agency of Canada continues to post general information about mumps and a national summary of the outbreak(s) on its Web site: www.phac-aspc.gc.ca/mumps-oreillons/index.html.
Until further notice, please notify the Centre for Immunization and Respiratory Infectious Diseases (which compiles the national summary) of any confirmed mumps cases in your jurisdiction that are linked to the outbreaks.
*A laboratory-confirmed case does not have to meet the clinical illness description.
† The Canadian Public Health Laboratory Network has endorsed the addition of mumps RT-PCR testing as a standard approach for mumps virus RNA detection.
‡ Clinical illness is characterized by acute onset of unilateral or bilateral tender, self-limited swelling of the parotid or other salivary gland, lasting > 2 days, and without other apparent cause.
Appendix 5: Algorithms for the Health Care Setting
Figure A: Management of health-care workers who are close contacts of a case of mumps
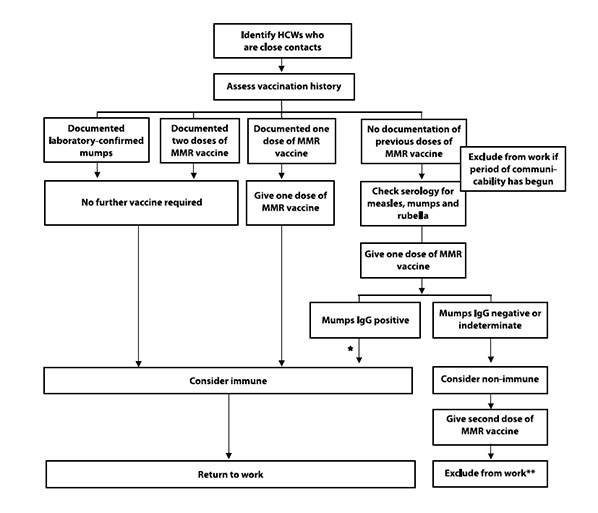
* May need a second dose of MMR vaccine for measles protection.
** Contacts should be excluded from day 10 after the first contact of a case to day 26 after the last contact with a case (where day of exposure is day 1). The HCW may have returned to work prior to receiving the second dose of MMR.
Figure B: Assessing health care workers for susceptibility to mumps
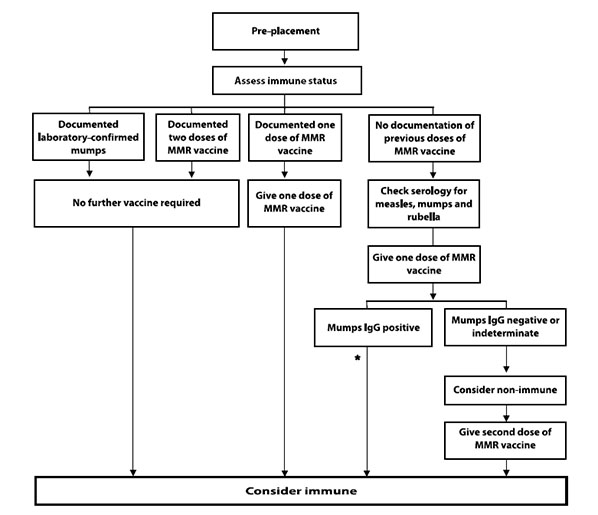
* May need a second dose of MMR vaccine for measles protection.
Appendix 6: Case Study – Communications in Nova Scotia during the 2007 Mumps Outbreak
More than half (59%) of the people affected in the Nova Scotia mumps outbreak of 2007 were between the ages of 20 and 29. Most were post-secondary students and, because of their lifestyle, the disease spread primarily in social situations like bars and shared housing. Because this population was the most at risk to acquire and spread the infection, post-secondary students were a clear target audience for public health messages.
In order to reduce the rate of transmission and limit the number of cases, it was important that this group receive a second dose of MMR vaccine, practice social distancing and hygiene measures if they were already infected, and stop risky behaviour, like sharing drinks.
However, communicators at Nova Scotia's department of Health Promotion and Protection found that traditional means of communication (news releases, fact sheets, etc.) were ineffective in reaching this audience. During the mumps outbreaks in Nova Scotia, communicators used alternative means to reach this audience:
- LCD/TV displays run by Volt Media on university campuses.
- Messages on university Web sites and through local students' unions.
- Very edgy posters for use on campus and in bus shelters near campus bus stops (<http://www.gov.ns.ca/hpp/mumps/students-parents.html>). The advertising encouraged immunization and explained some of the risks of mumps.
- It was also suggested that this kind of messaging be used in university student hot-spots like bars, restaurants, and coffee shops.
- Development of groups raising awareness of the outbreak on social networking Web sites such as Facebook.
Messages to encourage immunization of post-secondary students were also aimed at their parents through the following:
- Advertising in daily and weekly newspapers urging parents to get their children immunized if they were post-secondary students.
- Letters sent home from post-secondary registrars urging students to be immunized before coming to campus and supplying information on access to on-campus immunization clinics.
Just as there was a cohort of young adults who had not received two doses of MMR vaccine, there was also a cohort of health care workers who had never diagnosed or seen a case of mumps. Communicators at Nova Scotia's department of Health Promotion and Protection worked with communicators at Doctors Nova Scotia (a professional association) to distribute to their members fact sheets and videos on how to diagnose mumps.
A third target audience was the media, and they had a high demand for information. The resulting media coverage allowed public health messages to reach secondary audiences like the parents of young adults at risk of mumps infection, health care workers, and the public. The media were very receptive to interviews with the Chief Medical Officer of Health, and there were frequent media briefings to provide updates on the number of cases, age groups, and geographic areas affected by the outbreak.
During the peak of the outbreak, media briefings were restricted to once a week as the statistics were changing so rapidly that reporting more frequently would have been ineffective. After the peak period, statistics were still made available each Friday. However, numbers were not proactively distributed to the media. Instead, a note to editors was issued inviting them to contact the department of Health Promotion and Protection if they were interested in the latest statistics.
Overall, it was found to be important to segment the audiences and deliver messages in a suitable way for each. Some audiences to consider in a mumps outbreak are the at-risk population, parents/legal guardians, school administrators, health care workers, and the media.
Search CCDR
Page details
- Date modified: