CATMAT Statement: Japanese encephalitis


Download this article as a PDF (285 KB - 13 pages)
Published by: The Public Health Agency of Canada
Issue: Volume 37 ACS-1
Date published: April 2011
ISSN: 1481-8531
Submit a manuscript
About CCDR
Browse
Volume 37 ACS-1, April 2011
An Advisory Committee Statement (ACS)
Committee to Advise on Tropical Medicine and Travel (CATMAT)*†
Statement on Protection Against Japanese Encephalitis
Contributors
*Members: Dr. P.J. Plourde (Chair); Dr. C. Beallor; Dr. A. Boggild; Dr. J. Brophy; Dr. M. Crockett; Dr. W. Ghesquiere; Ms. A. Henteleff; Dr. A. McCarthy; Dr. K. L. McClean
Ex-Officio Representatives: Dr. G. Brunette, Dr. J. Creaghan, Dr. P. Charlebois; Dr. M. Tepper; Dr. P. McDonald; Dr. J. Given
Liaison Representatives: Dr. C. Greenaway; Dr. A. Pozgay; Dr. C. Hui; Dr. P. Teitelbaum
Member Emeritus: Dr. C. William L. Jeanes
Consultant: Dr. S. Schofield
† This Statement was prepared by Dr. M. Tepper and Dr. S. Schofield and approved by CATMAT.
DOI
https://doi.org/10.14745/ccdr.v37i00a01
Table of Contents
- Preamble
- Introduction
- Clinical Picture
- Epidemiology
- Personal Protective Measures
- Japanese Encephalitis Vaccine
- CATMAT Recommendations
- Appendix 1: Risk for Japanese Encephalitis by Country
- Appendix 2: Geographic Distribution of Japanese Encephalitis
- Appendix 3: Factors to Consider when Evaluating a Traveller's Risk for Japanese Encephalitis Virus
- Appendix 4: Strength and Quality of Evidence
- References
Preamble
The Committee to Advise on Tropical Medicine and Travel (CATMAT) provides the Public Health Agency of Canada (PHAC) with ongoing and timely medical, scientific, and public health advice relating to tropical infectious disease and health risks associated with international travel. PHAC acknowledges that the advice and recommendations set out in this statement are based upon the best current available scientific knowledge and medical practices, and is disseminating this document for information purposes to both travellers and the medical community caring for travellers.
Persons administering or using drugs, vaccines, or other products should also be aware of the contents of the product monograph(s) or other similarly approved standards or instructions for use. Recommendations for use and other information set out herein may differ from that set out in the product monograph(s) or other similarly approved standards or instructions for use by the licensed manufacturer(s). Manufacturers have sought approval and provided evidence as to the safety and efficacy of their products only when used in accordance with the product monographs or other similarly approved standards or instructions for use.
Introduction
Japanese encephalitis (JE) is caused by a flavivirus transmitted by Culex mosquitoes. It is one of the most important causes of viral encephalitis worldwide, with an estimated minimum of 50,000 cases, 10,000 deaths and 15,000 cases of long-term neuro-psychiatric sequelae annually(1). JE occurs in many areas of Asia, especially in the south-east, and in parts of the western Pacific (see Appendixes 1 and 2)(1). The risk to the susceptible resident population in rural areas with active JE transmission may be 0.1-2 cases per 100,000 per week(2) . There is no specific treatment for JE(3) , although an efficacious vaccine(4,5) and use of personal protective measures (PPMs)(6) will provide substantial protection against infection and disease.
Clinical Picture
In only a small proportion of humans infected with JE virus does symptomatic disease develop (reported range: 1/25 to 1/1,000)(7) after an incubation period of 5-15 days. The case-fatality ratio among symptomatic infections with JE is about 20-30% and, among survivors, 30-50% have longterm neurological or psychological sequelae(3). In endemic areas, disease occurs primarily in children(7) ; by adulthood, most persons have serologic evidence of previous JE infection.
Epidemiology
JE is principally a threat in rural agricultural areas. The virus is maintained in an enzootic cycle that prototypically involves Culex mosquitoes and wild birds (e.g. Ardeids such as egrets and herons)(4) . Secondary epizootic cycles may lead to infection of incidental hosts, such as humans and horses, and often involve pigs as amplifying hosts.
The main vector of JE is Culex mosquitoes, of which one of the most important is Culex tritaeniorhynchus, a species that tends to bite primarily at dusk and dawn, outdoors more than indoors and non-human mammals preferentially. Larvae of this species and many other Culex develop in standing water habitats, of which rice fields can be an important site because they may support very high larval populations(4).
Two epidemiologic patterns of JE occur. In temperate areas, JE is transmitted sporadically with periodic seasonal (summer and fall) epidemics, whereas in subtropical/tropical areas transmission can occur through much of the year(1,4). See Appendix 1 for a country-by-country description of JE risk and Appendix 2 for a map of JE risk areas.
Large-scale JE vaccine programs (targeted at children), changes in animal husbandry practices and increased urbanization have led to a substantial reduction in human cases of JE in some countries(1,4), e.g. Japan and Korea. However, JE may still present a risk to non-immune persons (e.g. travellers) in these areas because transmission may persist in a zoonotic cycle.
There have been few cases of JE reported among Western travellers. In the literature, only 55 cases of travel-associated JE among persons from nonendemic countries were reported in the 35 year period from 1973 to 2008; cases were identified as 33 tourists, nine expatriates, six soldiers and seven with unknown travel status(8). In the published data from the GeoSentinel Surveillance Network of more than 40 specialized travel and tropical medicine clinics in six continents and involving almost 50,000 patients, no case of JE has been reported(9,10) ; however, only a proportion of travellers in the Network visited JE risk areas. A possible case of JE was reported in a Canadian returning from Manchuria (northeastern China) in 1982(11) ; this may be the only Canadian case reported. Some travel-associated cases have involved minimal rural exposure, e.g. one night in a rural area(12). An overall estimate of the risk of clinical JE among short-term travellers to Asia is less than 1 per million(3), but in highly endemic areas during the transmission season risk may reach similar levels to the susceptible resident population (0.1-2 per 100,000 per week)(2).
Developing a precise risk estimate for an unprotected traveller for JE exposure/acquisition is very difficult and entails issues regarding the destination, the duration of travel, the season of travel and specific activities; see Appendix 3 for guidance regarding evaluation of a traveller's risk for JE.
Personal Protective Measures
Insect repellents that contain DEET (N,N-diethyl-m-toluamide)(13,14), permethrin-treated bed nets(6,15) and permethrin-treated clothing(16) have been shown to be efficacious in preventing the bites of Culex mosquitoes. Indeed, use of treated bed nets alone has been shown to achieve a substantial reduction in JE disease(6). Overall, well-used PPMs are expected to substantially reduce the risk of exposure to JE(17) (and other arthropod-borne diseases, e.g. dengue); such reduction might be of the order of 90%.
Japanese Encephalitis Vaccine
Until recently, the only JE vaccine available in Canada was the inactivated mouse brain-derived vaccine JE-VAX®, which was produced by Biken (the Research Foundation for Microbial Diseases of Osaka University) and distributed through Sanofi Pasteur Limited. However, JE-VAX® is no longer produced or marketed and, hence, is no longer available in Canada.
An inactivated Vero cell culture-derived vaccine, IXIARO®, manufactured by Intercell AG and marketed in Canada by Novartis Pharmaceuticals Canada Inc, was approved for sale in Canada in December 2009(18) ; this is the only JE vaccine currently available in Canada. It is important that the product monograph for IXIARO®(19) be read by those who prescribe and/or inoculate this vaccine. Some important features of IXIARO® include:
- Administration: IXIARO® is given intramuscularly with a primary series being two doses, of 6 ug each, at time 0 and 28 days. There is no "rapid/accelerated" schedule available.
- Age approved for: IXIARO®is only approved for use in persons 18 years or older(18,19). This age-specific approval and the unavailability of JE-VAX®* creates a substantial problem in providing vaccine-induced protection for those under 18 years of age. There is currently no satisfactory solution for protection of persons under 18 years of age against JE. Data from a very small number of children (40-50) vaccinated with 2 doses of IXIARO® indicated protective antibody response similar to adults and without unexpected adverse events(3,19,20) . Half dosing (3 ug vs 6 ug) has also been considered in younger children(20). The US Food and Drug Administration (FDA) approved IXIARO for use in those 17 years of age and older(21) while the European Medicines Agency (EMA)(22), as with Health Canada(18), has approved use in those 18 years of age and older. Presuming travel cannot be avoided or deferred, travellers under 18 years old should be advised to assiduously use PPMs. Regarding vaccination, it is acknowledged that several approaches might be considered, e.g., use of IXIARO® "off label" or vaccination with products available in the risk area; however, such approaches need to balance critically the risks and benefits.
- Efficacy/effectiveness: IXIARO® is approved for use based on non-inferiority of applicable serologic response compared to JE-VAX®(19,23) and to the World Health Organization threshold of neutralizing antibiodies of ≥ 1:10(1) and not on demonstrated disease prevention in humans. However, licensing bodies in Canada (Health Canada(18)) and elsewhere (e.g. United States/FDA(21), European Union/EMA(22)) are satisfied that the data are sufficiently robust to associate the demonstrated serologic response with disease prevention.
- Adverse events: To date, based on the relatively small number of vaccinees in clinical trials and early use, IXIARO® is not associated with a worrisome adverse event profile compared to either JE-VAX® or placebo(3,24) . However, only with post-marketing surveillance will it become possible to evaluate the full adverse event profile, in particular for rarer adverse events.
- Pregnancy and breastfeeding: There are no data related to safety (or efficacy) of IXIARO® in pregnant women or nursing mothers(3,19) and, hence, the recommendation is to use this vaccine in such groups only if the risk of disease outweighs the (unknown) risk to the woman and/or her fetus/nursing infant(3,19). Preclinical studies of IXIARO® in pregnant rats did not show evidence of harm to the mother or fetus ascribed to the vaccine(19).
- Antibody response: A single dose of IXIARO® induces sufficient protective antibodies in 30% of vaccinees at 10 days after the vaccination and in 40% of vaccinees at 28 days post-vaccination(3,19). A second dose of vaccine given at 28 days after the first dose induces antibodies in about 95% of vaccinees at 28 days after the second dose(3,19,23). Antibodies usually do not appear until about 7 days after the vaccine dose(19); hence, vaccination in sufficient time in advance of entering a JE risk area is preferred. If there is insufficient time to provide the two dose vaccination series, a single dose might be considered since a substantial minority of vaccinees may develop protective antibodies and/or the single dose might "prime" the vaccinee's immune system to respond to natural exposure to JE in a timely fashion. Vaccination with two doses of vaccine at the same time might increase the seroconversion rate to 60% at 10 days post-vaccination(25); however, such dosing would be "off label".
- Booster dosing: In clinical trials to date, the IXIARO®-induced protective antibody level declines over time with 80-95% of fully immunized vaccinees maintaining an adequate level at 6 months after the first dose and 60-80% maintaining adequate antibodies at 12 months after the first dose(3,19,26,27) . A "booster dose" of vaccine induces an adequate protective antibody level in those who have lost protective antibodies at 12 months after their first dose(3,19,26). A precise recommended booster interval for IXIARO® cannot be derived at this time. For information, the EMA recommends a booster dose between 12-24 months after the primary series prior to re-exposure to JE or at 12 months if at continuing risk (e.g. residing in endemic areas)(22); these intervals appear reasonable.
- Interchangability: There is no data related to the interchangability of IXIARO® with JE-VAX®, either in primary series or in booster dosing(3). In the absence of data, if a patient has received JE-VAX® more than 3 years ago and still needs (or needs again) JE protection, then the use of two doses of IXIARO® (i.e. a primary series) is recommended. IXIARO® and JE-VAX® target different JE strains, i.e. SA14-14-2 (IXIARO®) and Nakayama-NIH (JE-VAX®). This difference in target strains may account for some of the differences in the comparison of antibody levels between the two vaccines; the clinical significance of these differences is not known.
- Concomitant administration: Only one other vaccine (HAVRIX®) has been studied in concomitant administration with IXIARO® resulting in the expected antibody responses for each vaccine(3,19,28). There was an increase in reported pain, redness and swelling in the concomitant group as opposed to the single vaccine groups. It is a general approach in immunization that there is no contraindication to the use of multiple vaccines at the same session provided the vaccines are given "separately", e.g. separate syringe, separate site/limb(29).
- Further studies: Further clinical trials should clarify many of the issues noted in the preceding subparagraphs, e.g. use in children, rapid scheduling, booster dosing.
- Decision to recommend: The decision to recommend JE vaccine should take into account highly variable transmission intensities, alternative preventive tactics (such as PPMs), individual risk-tolerances, and cost-effectiveness considerations. Given the significant cost of the vaccine, generally IXIARO® might not be considered particularly cost-effective where transmission intensity is low to very low and/or where other preventive tactics including PPMs are fastidiously used(3).
*Discussion with sanofi pasteur in Canada and in the United States (US) indicates that importing JE-VAX® from the US-held supply is not an option (the US-held supply is specifically for persons under 17 years of age and expires in the spring of 2011).
CATMAT Recommendations
| CATMAT Recommendations | EBM rating(30) |
|---|---|
1. Properly used PPMs(6,13,14,15,16,17) reduce the risk of JE and are recommended. When the risk of JE infection is low, the use of PPMs is expected to reduce the already small risk of JE to a level at which IXIARO® provides little additional benefit, e.g. at a risk of 1/10,000 of JE without any protection, 90% effective PPMs reduce the residual risk that can be addressed by IXIARO® to 1/100,000. |
BI |
2. IXIARO® is expected to be efficacious in preventing JE. |
AI |
3. Generally, IXIARO® is recommended for travel to JE endemic/epidemic areas during the risk season: |
|
i. For all travellers who spend more than a cumulative total of 30 days in rural areas (or in urban areas known to be endemic or epidemic for JE); this might include longer term travellers or expatriates who, while based in urban areas, might be expected to make intermittent short trips to rural areas of risk; |
CIII |
ii. For travellers who will spend less than a cumulative total of 30 days in rural areas (or in urban areas known to be endemic or epidemic for JE) if they expect to have substantial cumulative activity outdoors (or indoors if the indoor area does not exclude mosquitoes), especially during the evening/night. |
CIII |
4. Generally, IXIARO® use is not recommended for JE endemic/epidemic areas during the risk season: |
|
i. For travellers whose entire itinerary will be in urban areas (unless the urban areas are known to be endemic or epidemic for JE); |
CIII |
ii. For travellers whose visits to rural areas (or urban areas known to be endemic or epidemic for JE) will be during the daytime only. |
CIII |
5. If there is insufficient time before entering a JE risk situation to allow for the usual dosing schedule (0, 28 days), a single dose of IXIARO® can be considered provided the patient understands that protection against JE cannot be reliably expected. Alternatively, "off label" use of two doses of IXIARO at the same time might be considered; however, this approach needs to balance critically the risks and benefits. |
CIII |
6. The pediatric traveller, especially the longer term traveller, to areas endemic for JE may well be at risk for JE infection and to have serious complications with such infection. Although there is no licensed pediatric JE vaccine in Canada, potential options include the "off label" use of IXIARO® or the use of licensed JE vaccines at the risk destination; however, these approaches need to balance critically the risks and benefits. |
CIII |
Appendix 1
Risk for Japanese Encephalitis, by Country†(2)
NOTE: Since the situation regarding JE risk may change over time, it is recommended that travel health practitioners access up-to-date risk information, e.g. at the US CDC website2.
| Country | Affected Areas | Transmission Season | Comments |
|---|---|---|---|
| Australia | Outer islands of Torres Strait |
December to May; all human cases reported from February to April |
One human case reported from north Queensland mainland. |
| Bangladesh | Little data; probably widespread |
Unknown; most human cases reported from May to October |
One outbreak of human disease reported from Tangail District in 1977. Sentinel surveillance has identified human cases in Chittagong, Khulna, and Rajshahi divisions, and Mymensingh district. |
| Bhutan | No data |
No data |
|
| Brunei | No data; presumed to be endemic countrywide |
Unknown; presumed year-round transmission |
|
| Burma (Myanmar) |
Limited data; presumed to be endemic countrywide |
Unknown; most human cases reported from May to October |
Outbreaks of human disease documented in Shan State. JEV antibodies documented in animals and humans in other areas. |
| Cambodia | Presumed to be endemic countrywide |
Probably year round with peaks reported from May to October |
Sentinel surveillance has identified human cases in at least 14 provinces including Phnom Penh, Takeo, Kampong, Cham, Battambang, Svay Rieng, and Siem Reap. |
| China | Human cases reported from all provinces except Xizang (Tibet), Xinjiang, and Qinghai. |
Most human cases reported from April to October |
Highest rates reported from the southwest and south central provinces. |
| India | Human cases reported from all states except Dadra, Daman, Diu, Gujarat, Himachal, Jammu, Kashmir, Lakshadweep, Meghalaya, Nagar Haveli, Punjab, Rajasthan, and Sikkim |
Most human cases reported from May to October especially in northern India. The season may be extended or year round in some areas especially in southern India. |
Highest rates of human disease reported from the states of Andhra Pradesh, Assam, Bihar, Goa, Haryana, Karnataka, Kerala, Tamil Nadu, Uttar Pradesh, and West Bengal. |
| Indonesia | Presumed to be endemic countrywide |
Human cases reported year round; peak season varies by island |
Sentinel surveillance has identified human cases in Bali, Kalimantan, Java, Nusa Tenggara, Papua, and Sumatra. |
| Japan ‡ | Rare-sporadic cases on all islands except Hokkaido. Enzootic activity ongoing |
Most human cases reported from May to October |
Large number of human cases reported until routine JE vaccination introduced in 1968. Most recent small outbreak reported from Chugoku district in 2002. Sporadic cases reported among U.S. military personnel on Okinawa. Enzootic transmission without human cases observed on Hokkaido |
| Korea, North | No data |
No data |
|
| Korea, South ‡ | Rare sporadic cases countrywide. Enzootic activity ongoing |
Most human cases reported from May to October |
Large number of human cases reported until routine JE vaccination introduced in 1968. Highest rates of disease were reported from the southern provinces. Last major outbreak reported in 1982. Vaccine not routinely recommended for travel limited to Seoul or other major cities. |
| Laos | No data; presumed to be endemic countrywide |
Presumed to be May to October |
|
| Malaysia | Endemic in Sarawak; sporadic cases or outbreaks reported from all states of Peninsula, and probably Sabah |
Year-round transmission |
Most human cases from reported from Penang and Sarawak. Vaccine not routinely recommended for travel limited to Kuala Lumpur or other major cities. |
| Mongolia | Not considered endemic |
|
|
| Nepal | Endemic in southern lowlands (Terai). Sporadic cases or outbreaks reported from the Kathmandu valley |
Most human cases reported from May to November |
Highest rates of human disease reported from western Terai districts, including Bankey, Bardia, Dang, and Kailali. Vaccine not routinely recommended for travel limited to high-altitude areas. |
| Pakistan | Limited data; human cases reported from around Karachi |
Most human cases reported from May to October |
|
| Papua New Guinea | Limited data; sporadic human cases reported from Western, Gulf, and South Highland Provinces |
Unknown |
A case of JE was reported from near Port Moresby in 2004. Human cases documented in Papua Indonesia. |
| Philippines | Limited data; presumed to be endemic on all islands |
Unknown; probably year-round |
Outbreaks reported in Nueva Ecija, Luzon, and Manila. |
| Russia | Rare human cases reported from the Far Eastern maritime areas south of Khabarousk |
Most human cases reported from July to September |
|
| Singapore | Rare sporadic human cases reported |
Year-round transmission |
Vaccine not routinely recommended. |
| Sri Lanka | Endemic countrywide except in mountainous areas |
Year-round with variable peaks based on monsoon rains |
Highest rates of human disease reported from Anuradhapura, Gampaha, Kurunegala, Polonnaruwa, and Puttalam districts. |
| Taiwan ‡ | Rare sporadic human cases island-wide |
Most human cases reported from May to October |
Large number of human cases reported until routine JE vaccination introduced in 1968. Vaccine not routinely recommended for travel limited to Taipei or other major cities. |
| Thailand | Endemic countrywide; seasonal epidemics in the northern provinces |
Year-round with seasonal peaks from May to October, especially in the north |
Highest rates of human disease reported from the Chiang Mai Valley. Sporadic human cases reported from Bangkok suburbs. |
| Timor-Leste | Limited data; anecdotal reports of sporadic human cases |
No data |
|
| Vietnam | Endemic countrywide; seasonal epidemics in the northern provinces |
Year-round with seasonal peaks from May to October, especially in the north |
Highest rates of disease in the northern provinces around Hanoi and northwestern provinces bordering China. |
| Western Pacific Islands | Outbreaks of human disease reported in Guam in 1947–1948 and Saipan in 1990 |
Unknown; most human cases reported from October to March |
Enzootic cycle might not be sustainable; outbreaks may follow introductions of JE virus. |
†Data are based on published reports and personal correspondence. Risk assessments should be performed cautiously because risk can vary within areas and from year to year, and surveillance data regarding human cases and JE virus transmission are incomplete.
‡In some endemic areas, human cases among residents are limited because of vaccination or natural immunity. However, because JE virus is maintained in an enzootic cycle between animals and mosquitoes, susceptible visitors to these areas still may be at risk for infection.
Appendix 2
Geographic distribution of Japanese Encephalitis(2)
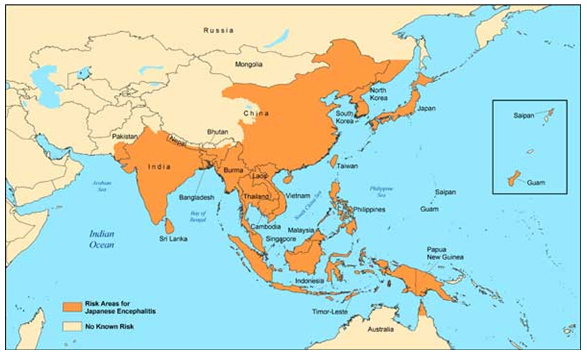
Geographic map of South East Asia indicating risk areas for Japanese Encephalitis including part of Australia, Bangladesh, Brunei, Cambodia, part of China, part of India, Indonesia, Japan, North Korea, South Korea, Laos, Malaysia, Myanmar, part of Nepal, part of Pakistan, Papua New Guinea, Philippines, part of Russia, Singapore, Sri Lanka, Taiwan, Thailand, Timor-Leste, Vietnam, and Western Pacific Islands of Guam and Saipan.
Appendix 3
Factors to Consider when Evaluating a Traveller's Risk for Japanese Encephalitis Virus Exposure(3)
Destination
JE occurs in areas throughout most of Asia and parts of the western Pacific.
The highest risk of JEV exposure occurs in rural agricultural areas, often associated with rice production and flooding irrigation.
JE can occur in large, focal outbreaks indicating extensive active JEV transmission in that area.
Duration of travel
Most reported travel-associated JE cases have occurred among expatriates or long-term travellers (i.e., ≥1 month).
Although no specific duration of travel puts a traveller at risk for JE, a longer itinerary increases the likelihood that a traveller might be exposed to a JEV-infected mosquito.
Season
In most temperate areas of Asia, JEV transmission is seasonal, and human disease usually peaks in summer and fall.
In the subtropics and tropics, JEV transmission patterns vary, and human disease can be sporadic or occur year-round.
Activities
The mosquitoes that transmit JEV feed on humans most often in the outdoors, with peak feeding times after sunset and again after midnight.
Extensive outdoor activities (e.g., camping, hiking, trekking, biking, fishing, hunting, or farming), especially during the evening or night, increase the risk of being exposed to a JEV-infected mosquito.
Accommodations with no air conditioning, screens, or bed nets increase the risk of exposure to mosquitoes that transmit JEV and other vector-borne diseases (e.g., dengue and malaria).
Use of Personal Protective Measures
Use of PPMs, e.g. bed net, repellent, clothing treatment, are expected to provide substantial protection against the bites of the mosquito that transmits JE. The level of personal compliance with PPMs can significantly alter the risk of exposure to JE.
Appendix 4
Strength and Quality of Evidence(30)
| Categories for the Strength of Each Recommendation | |
|---|---|
| CATEGORY | DEFINITION |
| A | Good evidence to support a recommendation for use. |
| B | Moderate evidence to support a recommendation for use. |
| C | Poor evidence to support a recommendation for or against use. |
| D | Moderate evidence to support a recommendation against use. |
| E | Good evidence to support a recommendation against use. |
| Categories for the Quality of Evidence on Which Recommendations are Made | |
| GRADE | DEFINITION |
| I | Evidence from at least one properly randomized, controlled trial. |
| II | Evidence from at least one well-designed clinical trial without randomization, from cohort or case-controlled analytic studies, preferably from more than one centre, from multiple time series, or from dramatic results in uncontrolled experiments. |
| III | Evidence from opinions or respected authorities on the basis of clinical experience, descriptive studies, or reports of expert committees. |
References
1 World Health Organization. WHO Position Paper: Japanese encephalitis vaccines. Wkly Epidemiol Record 2006;81:331-40..
2 Centers for Disease Control and Prevention. CDC health information for international travel 2010. Atlanta: U.S. Department of Health and Human Services, Public Health Service; 2009.
3 Advisory Committee on Immunization Practices (ACIP). Japanese encephalitis vaccines. MMWR 2010;59(RR-1).
4 Vaughn DW, Hoke CH. The epidemiology of Japanese encephalitis: Prospects for prevention. Epidemiol Rev 1992;14:197-221.
5 Hoke CH, Nisalak A, Sangawhipa N et al. Protection against Japanese encephalitis by inactivated vaccines. N Engl J Med 1988;319:608-14.
6 Luo D, Zhang K, Song J, Yao R et al. The protective effect of bed nets impregnated with pyrethroid insecticides and vaccination against Japanese encephalitis. Trans R Soc Trop Med Hyg 1994;88:632-34.
7 Solomon T, Nguyen MD, Kneen R, Gainsboroug M et al. Neurological aspects of tropical disease: Japanese encephalitis. J Neurol Neurosurg Psychiatry 2000;68:405-15.
8 Hills SL, Griggs AC and Fischer M. Japanese encephalitis in travelers from non-endemic countries, 1973-2008. Am J Trop Med Hyg 2010;82(5):930-36.
9 Freedman DO, Weld LH, Kozarsky PE, Fisk T et al. Spectrum of disease and relation to place of exposure among ill returned travelers. N Engl J Med 2006;354:119-30.
10 Boggild AK, Castelli F, Gautret P, Torresi J et al. Vaccine preventable diseases in returned travelers: results from the GeoSentinel Surveillance Network. Vaccine 2010;28(46):7389-95.
11 Artsob H, Spence L. Imported arbovirus infections in Canada, 1974-89. Can J Infect Dis 1991;2:95-100.
12 Centers for Disease Control and Prevention. Japanese encephalitis in a US traveler returning from Thailand, 2004. MMWR 2005;54(5):123-25.
13 Frances SP, Eamsila C, Pilakasiri C et al. Effectiveness of repellent formulations containing DEET against mosquitoes in northeastern Thailand. J Am Mosq Control Assoc 1996;12:331-33.
14 Thavara U, Tawatsin A, Chompoosri J et al. Laboratory and field evaluations of the insect repellent 3535 (ethyl butylacetylaminopriopionate) and DEET against mosquito vectors in Thailand. J Am Mosq Control Assoc 2001;17:190-95.
15 Jinjiang X, Meiluan Z, Xinfu L et al. Evaluation of permethrin-impregnated mosquito-nets against mosquitoes in China. Med Vet Entomol 1988;2:247-51.
16 Harbach RE, Tang DB, Wirtz RA et al. Relative repellency of two formulations of N,N-diethyl-3-methylbenzamide (DEET) and permethrin-treated clothing against Culex sitiens and Aedes vigilax in Thailand. J Am Mosq Control Assoc 1990;6:641-44.
17 Committee to Advise on Tropical Medicine and Travel (CATMAT). Statement on personal protective measures to prevent arthropod bites. CCDR 2005;31(ACS-4).
18 Health Canada. Notice of Decision for IXIARO® [Internet]. 2009 Dec 2 [cited 2010 Jul 12]. Available from: http://www.hc-sc.gc.ca/dhp-mps/prodpharma/sbd-smd/phase1-decision/drug-med/nd_ad_2009_ixiaro_120330-eng.php
19 Intercell AG. Product monograph: IXIARO® : Japanese encephalitis vaccine (inactivated, adsorbed; suspension for injection [Internet]. 2009 Sep 15 [cited 2010 Jul 13]. Available from: http://www.novartis.ca/downloads/en/products/ixiaro_monograph_en.pdf
20 Kaltenbock A, Dubischar-Kastner K, Schuller E, Datla M et al. Immunogenicity and safety of IXIARO (IC51) in a Phase II study in healthy Indian children between 1 and 3 years of age. Vaccine 2010;28(3):834-39.
21 US Food and Drug Administration. March 30, 2009 Approval letter [for IXIARO®] [Internet]. 2009 Mar 30 [updated 2009 Apr 30; cited 2010 Jul 13]. Available from: http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm142577.htm
22 European Medicines Agency. European public assessment report: IXIARO [Internet]. 2010 [updated 2010 Apr 20; cited 2010 Jul 30]. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000963/
human_med_000862.jsp&murl=menus/medicines/medicines.jsp&mid=WC0b01ac058001d125
23 Tauber E, Kollaritsch H, Korinek M, Rendi-Wagner P et al. Safety and immunogenicity of a Vero-cell-derived inactivated Japanese encephalitis vaccine: a non-inferiority phase III, randomised controlled trial. Lancet 2007;370:1847-53.
24 Tauber E, Kollaritsch H, von Sonnenburg F, Lademann M et al. Randomized, double-blind, placebo-controlled Phase 3 trial of the safety and tolerability of IC51, and inactivated Japanese encephalitis vaccine. JID 2008;198:493-99.
25 Schuller E, Klade CS, Wolfl G, Kaltenbock A et al. Comparison of a single, high-dose vaccination regimen to the standard regimen for the investigational Japanese encephalitis vaccine, IC51: a randomized, observer-blind, controlled Phase 3 study. Vaccine 2009;27(15):2188-93.
26 Dubischar-Kaster K, Eder S, Buerger V, Gartner-Woelfl G et al. Long-term immunity and immune response to a booster dose following vaccination with the inactivated Japanese encephalitis vaccine IXIARO, IC51. Vaccine 2010;28(32):5197-202.
27 Schuller E, Jilma B, Voicu V, Golor G et al. Long-term immunogenicity of the new Vero cell-derived inactivated Japanese encephalitis virus vaccine IC51: six and 12 month results of a multicenter follow-up phase 3 study. Vaccine 2008;26(34):4382-86.
28 Kaltenbock A, Dubischar-Kastner K, Eder G, Jilg W et al. Safety and immunogenicity of concomitant vaccination with the cell-culture based Japanese encephalitis vaccine IC51 and the hepatitis A vaccine HAVRIX 1440 in healthy subjects: a single-blind, randomized, controlled phase 3 study. Vaccine 2009;27(33):4483-89.
29 Public Health Agency of Canada. Canadian immunization guide, 7th ed., 2006.
30 Committee to Advise on Tropical Medicine and Travel. Evidence-based medicine. CCDR 1994;20:145-47.
Search CCDR
Page details
- Date modified: