Infants and children hospitalized with respiratory syncytial virus

 Download this article as a PDF
Download this article as a PDFPublished by: The Public Health Agency of Canada
Issue: Volume 47-9: FluWatchers: A Crowdsourcing Approach
Date published: September 2021
ISSN: 1481-8531
Submit a manuscript
About CCDR
Browse
Volume 47-9: FluWatchers: A Crowdsourcing Approach
Rapid Communication
Burden of illness in infants and young children hospitalized for respiratory syncytial virus: A rapid review
Aireen Wingert1, Jennifer Pillay1, Dorothy L Moore2, Samantha Guitard1, Ben Vandermeer1, Michele P Dyson1, Angela Sinilaité2, Matthew Tunis2, Lisa Hartling1
Affiliations
1 Alberta Research Centre for Health Evidence, Department of Pediatrics, Faculty of Medicine and Dentistry, University of Alberta, Edmonton, AB
2 Public Health Agency of Canada, Ottawa, ON
Correspondence
Suggested citation
Wingert A, Pillay J, Moore DL, Guitard S, Vandermeer B, Dyson MP, Sinilaité A, Tunis M, Hartling L. Burden of illness in infants and young children hospitalized for respiratory syncytial virus: A rapid review. Can Commun Dis Rep 2021;47(9):381–96. https://doi.org/10.14745/ccdr.v47i09a05
Keywords: respiratory syncytial virus, disease burden, hospitalization, systematic review
Abstract
Respiratory syncytial virus (RSV) infections are common among young children and represent a significant burden to patients, their families and the Canadian health system. Here we conduct a rapid review of the burden of RSV illness in children 24 months of age or younger. Four databases (Medline, Embase, Cochrane Database of Clinical Trials, ClinicalTrials.gov from 2014 to 2018), grey literature and reference lists were reviewed for studies on the following: children with or without a risk factor, without prophylaxis and with lab-confirmed RSV infection. Of 29 studies identified, 10 provided within-study comparisons and few examined clinical conditions besides prematurity. For infants of 33–36 weeks gestation (wGA) versus term infants, there was low-to-moderate certainty evidence for an increase in RSV-hospitalizations (n=599,535 infants; RR 2.05 [95% CI 1.89–2.22]; 1.3 more per 100 [1.1–1.5 more]) and hospital length of stay (n=7,597 infants; mean difference 1.00 day [95% CI 0.88–1.12]). There was low-to-moderate certainty evidence of little-to-no difference for infants born at 29–32 versus 33–36 wGA for hospitalization (n=12,812 infants; RR 1.20 [95% CI 0.92–1.56]). There was low certainty evidence of increased mechanical ventilation for hospitalized infants born at 29–32 versus 33–35 wGA (n=212 infants; RR 1.58, 95% CI 0.94–2.65). Among infants born at 32–35 wGA, hospitalization for RSV in infancy may be associated with increased wheeze and asthma-medication use across six-year follow-up (RR range 1.3–1.7). Children with versus without Down syndrome may have increased hospital length of stay (n=7,206 children; mean difference 3.00 days, 95% CI 1.95–4.05; low certainty). Evidence for other within-study comparisons was of very low certainty. In summary, prematurity is associated with greater risk for RSV-hospitalization and longer hospital length of stay, and Down syndrome may be associated with longer hospital stay for RSV. Respiratory syncytial virus-hospitalization in infancy may be associated with greater wheeze and asthma-medication use in early childhood. Lack of a comparison group was a major limitation for many studies.
Introduction
Respiratory syncytial virus (RSV) infections are common among young childrenFootnote 1Footnote 2, presenting as bronchiolitis, pneumonia, or other respiratory morbidityFootnote 3. Hospitalization due to RSV is a significant burden for patients, families and the Canadian health systemFootnote 4.
Increased risk for RSV-hospitalization has been associated with age younger than one year. Footnote 3Footnote 5, prematurityFootnote 6, chronic lung diseaseFootnote 7, congenital heart diseaseFootnote 8, other chronic conditions including cystic fibrosis, immunodeficiencyFootnote 9Footnote 10Footnote 11Footnote 12 and residence in Indigenous, northern or remote communitiesFootnote 13. These populations may also have higher rates of admission to intensive care units (ICU), requirements for respiratory support, and higher mortality attributable to RSV Footnote 9. RSV-hospitalization in the first two years of life has also been associated with wheezing in childhoodFootnote 14Footnote 15Footnote 16.
While no active vaccines exist for RSV prophylaxis, the monoclonal antibody palivizumab (Synagis®, AstraZeneca) has demonstrated effectiveness in preventing RSV-hospitalization among some high risk populationsFootnote 17Footnote 18. However, while efficacy of palivizumab (PVZ) in clinical trials appears to be high for children with some underlying clinical conditions, real-world evidence from observational studies is less certainFootnote 2, with wide variations in effectiveness. Due to the high numbers needed to treat in order to prevent hospitalization and the relatively high cost of PVZ, most jurisdictions use the intervention sparingly for select groups at highest risk of severe disease. Additionally, RSV vaccine development has been well under way, with some vaccine candidates undergoing phase 3 clinical trialsFootnote 19. There is currently no global consensus on RSV risk groups and variable policies exist even within Canada.
The objective of this rapid review is to address the following question: What is the burden of RSV illness including long-term sequelae among children 24 months of age and younger without prophylaxis, and with or without risk factors for severe RSV disease, and for immunocompromised children younger than 18 years of age?
Findings from the review will help inform updated recommendations of Canada's National Advisory Committee on Immunization on the use of PVZ prophylaxis to prevent severe consequences of RSV infection. This evidence base will also be relevant for future deliberations on program design for anticipated RSV vaccines and newer monoclonal antibodiesFootnote 19.
Methods
This review was guided by methods for reviews of interventionsFootnote 20, overall prognosisFootnote 21, and risk of future event (prognosis)Footnote 22; a protocol was developed a priori (Supplement 1), and reviewed and approved by the National Advisory Committee on Immunization RSV Working Group. In light of the restricted literature search timeframe of interest to the review commissioners, we refer to the undertaken work as a rapid review.
Literature search
Searches were conducted on September 6, 2018, in Medline, Embase, Cochrane Database of Clinical Trials, ClinicalTrials.gov, and websites of international public health authorities (Supplement 2). Limits were applied for date of publication (January 1, 2014 to September 6, 2018) and language (English or French). The date limit was aimed at capturing outcomes just before and after significant changes in clinical practice stemming from the revised recommendations for PVZ prophylaxis by the American Academy of PediatricsFootnote 23 as well as the Canadian Paediatric SocietyFootnote 2.
Study selection and eligibility criteria
Two reviewers independently screened titles and abstracts followed by full texts. Discrepancies were resolved by discussions.
Studies conducted in Organisation for Economic Co-operation and Development (OECD) countries, including observational studies and placebo groups of controlled trials were eligible for inclusion. Studies reporting on children 24 months of age and younger, with or without a risk factor of interest, or immunocompromised children 18 years of age and younger without PVZ prophylaxis and with lab-confirmed RSV infection were eligible. Children without RSV infection were eligible as a comparator group for long-term outcomes. Short-term outcomes included RSV-hospitalization, hospital length of stay, ICU admission and length of stay, oxygen support and duration, mechanical ventilation and duration, extracorporeal membrane oxygenation and duration, case fatality (death due to RSV), and complications from RSV infection (e.g. secondary infection). Long-term outcomes (minimum one-year follow-up) included self-reported, parent-reported or physician-diagnosed recurrent wheeze, atopic asthma, deterioration of pulmonary or cardiac function, and impaired growth or development. Detailed inclusion and exclusion criteria are in Supplement 3.
Data extraction, synthesis/analysis and risk of bias assessment
One reviewer extracted data with second-reviewer verification.
For dichotomous outcomes, we extracted the number of events and the number analysed in each eligible group, or relative measures (e.g. odds ratio) if crude events were not reported. For continuous outcomes, mean values for each time-point, and change scores, including standard deviations or measures of variability were extracted. Risk ratio (RR) with 95% confidence interval (CI) and mean difference (MD) were used for comparisons between groups.
Our primary interest was using data from studies that reported on two or more groups, either having different risk factors, or a risk group versus healthy term infants (within-study/direct comparisons). For similar comparisons reported by more than one study, data were pooled using the DerSimonian Laird random effects model inverse variance method with Mantel-Haenszel weighting. Risk differences were used when rare or zero events appeared in at least one study group. We also made comparisons between short-term outcomes in risk groups and healthy term infants reported by different studies (between-study/indirect comparisons). We used the double-arc sine transformation to pool single-group proportions across multiple studies. When no comparison was made, we report event proportions for the single group in these studies.
For outcomes where estimates were statistically significant, we calculated the absolute risk differenceFootnote 24.
Analyses were performed using Excel, Review Manager (version 5.3) and STATA (version 14.2).
Two reviewers independently assessed the risk of bias for each study, using a modified tool based on the Quality Assessment Tool for Observational Cohort and Cross-sectional Studies and the Quality In Prognosis Studies (QUIPS) tool (Supplement 4). Disagreements were resolved via consensus or third-reviewer consultation.
Certainty of evidence
Two reviewers independently assessed the certainty of evidence for each outcome (as high, moderate, low, or very low) from within-study comparisons (direct evidence), with disagreements resolved through consensus. The approach followed principles of the Grading of Recommendations Assessment, Development and Evaluation working group and considerations for a body of evidence that examines risk of future events (prognosis) (Supplement 5)Footnote 21.
Results
Study selection and characteristics
Twenty-nine cohort studies were included (Figure 1, Table 1, and Supplements 6 and 7)Footnote 13Footnote 25Footnote 26Footnote 27Footnote 28Footnote 29Footnote 30Footnote 31Footnote 32Footnote 33Footnote 34Footnote 35Footnote 36Footnote 37Footnote 38Footnote 39Footnote 40Footnote 41Footnote 42Footnote 43Footnote 44Footnote 45Footnote 46Footnote 47Footnote 48Footnote 49Footnote 50Footnote 51Footnote 52; of these, 10 reported at least one within-study comparison Footnote 26Footnote 27Footnote 28Footnote 31Footnote 32Footnote 36Footnote 37Footnote 42Footnote 50Footnote 52. Twelve studies were conducted in the United StatesFootnote 25Footnote 26Footnote 33Footnote 34Footnote 36Footnote 38Footnote 42Footnote 45Footnote 46Footnote 47Footnote 49Footnote 50, three each, in CanadaFootnote 13Footnote 29Footnote 48 and the NetherlandsFootnote 30Footnote 43Footnote 52, two each, in FinlandFootnote 27Footnote 28, FranceFootnote 35Footnote 37 and JapanFootnote 40Footnote 41 and one each in ChileFootnote 44, DenmarkFootnote 31, IrelandFootnote 39, SpainFootnote 32 and multiple countriesFootnote 51. ThirteenFootnote 13Footnote 25Footnote 26Footnote 29Footnote 30Footnote 31Footnote 32Footnote 37Footnote 43Footnote 45Footnote 46Footnote 51Footnote 52 studies had some form of industry funding. Three papers reported on the same study: primary publication by Ambrose et al.Footnote 25, with associated publications by Franklin et al.Footnote 53 and Simões et al.Footnote 54.
Figure 1: Flow diagram of study selection
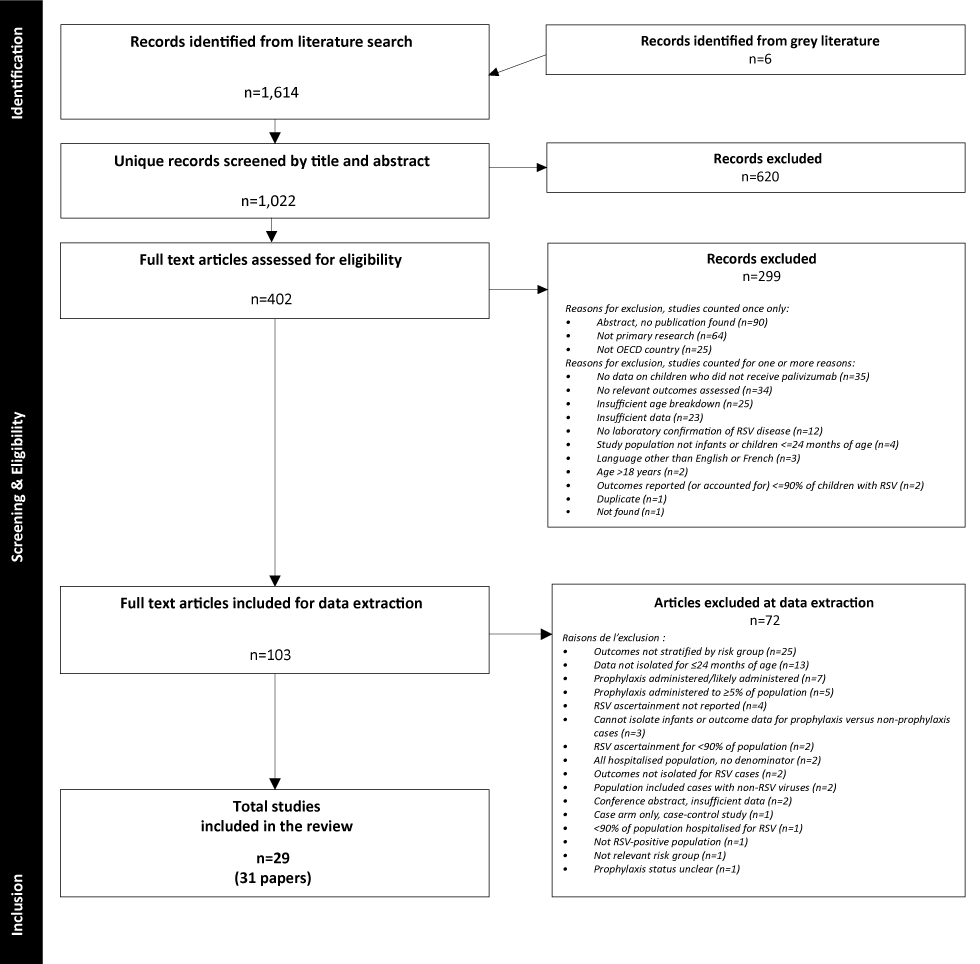
Text description: Figure 1
The diagram shows the flow of study selection, beginning with 1,620 records identified from the databases and grey literature. After duplicates were removed, 1,022 unique records were screened at title and abstract, resulting in 402 articles after excluding records (n=620) that did not meet eligibility criteria. Full-text screening resulted in 103 eligible articles (299 records were excluded), and 72 were further excluded at data extraction. A total of 31 articles reporting on 29 studies were included in the review. Reasons for exclusion at full text and at data extraction are listed in their respective boxes.
Study design & setting (no. of studies) |
Risk groups (no. of studiesFootnote a) |
RSV infection (no. of studies) |
Short-term outcomes (no. of studiesFootnote a) |
Long-term outcomes and follow-up (no. of studiesFootnote a) |
Risk of bias by outcome (no. of studiesFootnote a) |
|---|---|---|---|---|---|
Study design:
|
At-risk:
|
Age at RSV:
|
Incidence of RSV-hospitalization (n=23) Hospital LOS (n=16) ICU admission (n=13) ICU LOS (n=5) Oxygen therapy (n=6) Oxygen therapy duration (n=5) MV (n=15) MV duration (n=4) Case fatality (n=7) |
Wheeze:
|
Incidence of RSV-hospitalization:
|
Eighteen studiesFootnote 25Footnote 26Footnote 29Footnote 30Footnote 33Footnote 34Footnote 35Footnote 36Footnote 37Footnote 39Footnote 40Footnote 41Footnote 42Footnote 43Footnote 45Footnote 48Footnote 50Footnote 51 included children either not given or not considered for PVZ prophylaxis prior to RSV-hospitalization; four studiesFootnote 13Footnote 32Footnote 46Footnote 47 reported prophylaxis among less than 5% of the applicable population with RSV, and seven studiesFootnote 27Footnote 28Footnote 31Footnote 38Footnote 44Footnote 49Footnote 52 were considered by clinical judgement to not have included children who received prophylaxis. One study included some children who may have received prophylaxisFootnote 34.
We included three studies of children with RSV older than 24 months of age to capture evidence for immunocompromised populations: children three years old or younger with Down syndrome with or without known risk factors for RSVFootnote 50, and younger than 18 years old with liver transplantationFootnote 38 and sickle cell diseaseFootnote 49.
Studies of at-risk populations reporting short-term outcomes included the following: infants with premature birth (eleven studies)Footnote 25Footnote 26Footnote 30Footnote 32Footnote 36Footnote 37Footnote 42Footnote 43Footnote 47Footnote 48Footnote 51; cystic fibrosis (two studies)Footnote 29Footnote 39; one study each for congenital cystic lung diseaseFootnote 40, childhood interstitial lung diseaseFootnote 35, Down syndromeFootnote 50, sickle cell diseaseFootnote 49, acute leukemiaFootnote 41 and prior liver transplantFootnote 38; and children residing in remote geographic locations (two studies)Footnote 13Footnote 46.
Seven studies reporting short-term outcomes included data on healthy term infants hospitalized for RSVFootnote 33Footnote 37Footnote 42Footnote 44Footnote 45Footnote 50Footnote 52.
Six studies reported on long-term outcomes: healthy term infants with versus without RSV in infancyFootnote 27Footnote 28Footnote 31Footnote 52, premature infants with versus without RSV-hospitalization in infancyFootnote 32, and premature versus term infants hospitalized for RSV in their first RSV seasonFootnote 37.
Risk of bias
Risk of bias ratings are in Table 1 and Supplements 4 and 6. Studies that reported incidence of RSV-hospitalization were at moderate-to-high risk of bias, mainly due to lack of blinding to childrens’ risk status by healthcare providers that may have influenced admission to hospital. For other short-term outcomes, studies were mostly at moderate risk of biasFootnote 25Footnote 26Footnote 29Footnote 33Footnote 35Footnote 39Footnote 45Footnote 46Footnote 47. Two studies were at high risk of bias due to concerns in more than one domainFootnote 41Footnote 44. Nearly all reported long-term outcomesFootnote 27Footnote 28Footnote 32Footnote 37Footnote 52 were at moderate risk of bias, arising from lack of blinding for patient or parent-reported outcomes and/or potential selection biases.
Short-term outcomes from within-study comparisons
Table 2 summarizes evidence for short-term outcomes from within-study comparisons. Here we do not report further on findings having very low certainty of evidence.
Outcome |
Comparator 1 | Comparator 2 | Study design (no. of studies); Sample size |
Absolute difference (95% CI) | Relative risk (95% CI) |
Certainty of evidence | Conclusion | |
|---|---|---|---|---|---|---|---|---|
| Comparator 2 risk | Absolute risk differenceFootnote a | |||||||
| RSV-hospitalization | ||||||||
| At-risk population | Prematurity: 29–32 wGA | Prematurity: 33–36 wGA | RC36 (n=1); 12,812 |
4.2 per 100 | NS | RR 1.20 (0.92, 1.56) |
Moderate to lowFootnote bFootnote cFootnote d | Little to no difference For RSV-hospitalization in their first RSV season among infants born premature at 29–32 wGA vs. 33–36 wGA |
| At-risk vs. not-at-risk population | Prematurity: 33–36 wGA | Term: ≥37 wGA | RC42 (n=1); 599,535 |
1.2 per 100 | 1.3 more per 100 (1.1 to 1.5 more) |
RR 2.05 (1.89, 2.22) |
Moderate to lowFootnote bFootnote cFootnote d | Increase RSV-hospitalization by age <24 months among infants born premature (33–36 wGA) vs. at term Among this group, infants born at 33–34 wGA had highest incidence density for RSV hospitalization at 6–12 months of age (adjusted hazard ratio [aHR] 1.74 [1.17–2.58], p<0.05) and 12–24 months of age (aHR 1.96 [1.26–3.05], p<0.05) compared to term infants |
| At-risk vs. not-at-risk population | Prematurity: <33 wGA | Term: 39–41 wGA | RFUPC37 (n=1); 443 |
1.5 per 100 | 4.3 more per 100 (0.2 to 18 more) |
RR 3.88 (1.13, 13.30) |
Very lowFootnote bFootnote cFootnote e | Very uncertain For RSV-hospitalization in their first RSV season among infants born at <33 wGA vs. at term |
| Hospital length of stay, mean days | ||||||||
| At-risk population | Prematurity: 29–32 wGA | Prematurity: 33–35 wGA | PC26 (n=1); 212 |
MD 4.00 (1.54, 6.46) |
N/A | Very lowFootnote bFootnote cFootnote e | Very uncertain For hospital length of stay among infants born premature at 29–32 wGA vs. 33–35 wGA and hospitalized for RSV at <12 months |
|
| At-risk vs. not-at-risk population | Prematurity: 33–36 wGA | Term: ≥37 wGA | RC42 (n=1); 7,597 |
MD 1.00 (0.88, 1.12) |
N/A | Moderate to lowFootnote bFootnote cFootnote d | Small increase For hospital length of stay among infants born premature at 33–36 wGA vs. at term and hospitalized for RSV at <24 months |
|
| At-risk vs. not-at-risk population | Down syndrome | No Down syndrome | RC50 (n=1); 7,206 |
MD 3.00 (1.95, 4.05) |
N/A | LowFootnote bFootnote c | Small increase For hospital length of stay for RSV among infants with vs. without Down syndrome and hospitalized for RSV at <3 years |
|
| Hospital length of stay, <1 day vs. ≥1 day | ||||||||
| At-risk population | Prematurity: 29–32 wGA | Prematurity: 33–36 wGA | RC36 (n=1); 542 |
13.9 per 100 | NS | <1 day: RR 0.86 (0.41, 1.78) |
LowFootnote cFootnote e | Little to no difference For hospital length of stay <1 day among infants born premature at 29–32 wGA vs. 33–35 wGA and hospitalized in their first RSV season |
| At-risk population | Prematurity: 29–32 wGA | Prematurity: 33–36 wGA | RC36 (n=1); 542 |
86.1 per 100 | NS | ≥1 day: RR 1.02 (0.93, 1.13) |
LowFootnote cFootnote e | Little to no difference For hospital length of stay ≥1 day among infants born premature at 29–32 wGA vs. 33–36 wGA and hospitalized in their first RSV season |
| ICU admission, among RSV-hospitalized population | ||||||||
| At-risk population | Prematurity: 29–32 wGA | Prematurity: 33–35 wGA | PC26 (n=1); 212 |
50.4 per 100 | NS | RR 1.03 (0.79, 1.34) |
Low to very lowFootnote bFootnote cFootnote dFootnote e | Little to no difference/ very uncertain For ICU admission among infants born premature at 29–32 wGA vs. 33–35 wGA and hospitalized for RSV at <12 months |
| ICU length of stay, mean days | ||||||||
| At-risk population | Prematurity: 29–32 wGA | Prematurity: 33–35 wGA | PC26 (n=1); 169 |
MD 2.00 (-0.28, 4.28) |
N/A | Low to very lowFootnote bFootnote cFootnote dFootnote e | Small increase/very uncertain For ICU length of stay among infants born premature at 29–32 wGA or at 33–35 wGA and hospitalized for RSV at <12 months |
|
| Mechanical ventilation, among RSV-hospitalized population | ||||||||
| At-risk population | Prematurity: 29–32 wGA | Prematurity: 33–35 wGA | PC26 (n=1); 212 |
17.1 per 100 | NS | RR 1.58 (0.94, 2.65) |
LowFootnote cFootnote e | Small increase For mechanical ventilation among infants born premature at 29–32 wGA vs. 33–35 wGA and hospitalized for RSV at <12 months |
| Mechanical ventilation, among ICU population | ||||||||
| At-risk population | Prematurity: 29–32 wGA | Prematurity: 33–35 wGA | PC26 (n=1); 108 |
33.9 per 100 | NS | RR 1.54 (0.99, 2.40) |
Very lowFootnote cFootnote eFootnote f | Very uncertain For mechanical ventilation therapy among infants born premature at 29–32 wGA vs. 33–35 wGA and admitted to ICU for RSV at <12 months |
| Mechanical ventilation therapy duration, mean days | ||||||||
| At-risk population | Prematurity: 29–32 wGA | Prematurity: 33–35 wGA | PC26 (n=1); 45 |
MD 2.00 (-1.21, 5.21) | N/A | Very lowFootnote cFootnote eFootnote f | Very uncertain For duration of mechanical ventilation therapy among infants born premature at 29–32 wGA vs. 33–35 wGA and hospitalized for RSV at <12 months |
|
| Case fatality, among RSV-hospitalized population | ||||||||
| At-risk population | Prematurity: 29–32 wGA | Prematurity: 33–35 wGA | PC26 (n=1); 212 |
0 per 100 | NS | RR 4.13 (0.17, 100.30) |
Very lowFootnote cFootnote eFootnote f | Very uncertain For death due to RSV among infants born premature at 29–32 wGA vs. 33–35 wGA and hospitalized for RSV at <12 months |
| Case fatality, among ICU population | ||||||||
| At-risk population | Prematurity: 29–32 wGA | Prematurity: 33–35 wGA | PC26 (n=1); 108 |
0 per 100 | NS | RR 4.02 (0.17, 96.53) |
Very lowFootnote cFootnote eFootnote f | Very uncertain For death due to RSV among infants born premature at 29–32 wGA vs. 33–35 wGA and admitted to ICU for RSV at <12 months |
Different degrees of prematurity: One study found little-to-no difference in RSV-hospitalization during their first RSV season for infants born at 29–32 compared with 33–36 weeks’ gestation (wGA)Footnote 36. This study found little-to-no difference for hospital stay of less than one day versus one day or more between infants born at 29–32 or 33–36 wGAFootnote 36.
Another study of infants born at 29–32 versus 33–35 wGA who were hospitalized for RSV in the first year of life found little-to-no difference for ICU admission, but longer (although not statistically significant) ICU length of stay among the infants born at 29–32 wGAFootnote 26. There was a greater need for mechanical ventilation for hospitalized infants born at 29–32 wGA versus 33–35 wGAFootnote 26.
There were no studies of premature infants born before 29 wGA.
Premature versus term infants: One study found that being born late-premature (33–36 wGA) versus at term was associated with increased RSV-hospitalization in the first two years of lifeFootnote 42. The same study found slightly longer hospitalization for the preterm groupFootnote 42.
Down syndrome: One study comparing children with Down syndrome without additional risk factors for RSV versus healthy children, all followed to three years of age, reported a higher hospitalization rate in those with Down syndromeFootnote 50. There was a discrepancy between the text and tables for RSV-hospitalization rates in these groups that could not be resolved due to unsuccessful attempts to contact the study authorsFootnote 50. This study also found that RSV was associated with longer hospital length of stay among children with Down syndrome without other risk factors versus children without Down syndromeFootnote 50. For all cases of Down syndrome, including those with known risk factors for RSV, the authors conclude that Down syndrome is independently associated, after adjusting for known risk factors for RSV disease, with an increased risk for RSV-hospitalizationFootnote 50. Of note, data on children younger than 24 months of age without risk factors were not isolated from those with additional risk factors, and therefore, were not used in our analysis.
Select short-term outcome comparisons: Other data
Supplement 8 contains single risk group data and pooled analyses (when appropriate). Data for between-study comparisons are in Supplement 9. Findings for select outcomes are reported below; data on other short-term outcomes are in Supplement 8.
Single group proportions for RSV-hospitalizations were 5.1% in the first six months of life and 3.3% in the first two years of life for infants 29 to younger than 33 wGAFootnote 36Footnote 37 and 32/33 to 35 wGAFootnote 25Footnote 30Footnote 32Footnote 36Footnote 42Footnote 43Footnote 48Footnote 51, respectively; 5.3% two years post-transplantation, 8.3% in the first two years of life, 12.3% in the first two years of life and 30.0% in the first or second RSV season for liver transplantFootnote 38, congenital cystic lung diseaseFootnote 40, cystic fibrosisFootnote 29Footnote 39 and childhood interstitial lung diseaseFootnote 35, respectively. The 95% confidence intervals for the latter three conditions were very wide. The pooled proportion for healthy term infants was 1.2% in the first two years of lifeFootnote 37Footnote 42Footnote 45Footnote 52.
Case fatality rate attributable to RSV for those hospitalized were 1.1 % (n=89), 2.5% (n=80), 4.4% (n=135) and 40.0% (n=10) for infants of 29–32 wGAFootnote 26, children residing in remote geographic areaFootnote 46, children with liver transplantFootnote 38 and with leukemiaFootnote 41, respectively. Most studies reported no attributable deaths. Many studies of clinical conditions contained very small sample sizes.
Complications
One study reported on complications associated with RSV-hospitalization (Supplement 10).
Long-term outcome comparisons from within-study comparisons
Table 3, Table 4 and Table 5 summarize evidence for long-term outcomes from within-study comparisons. No study reported on growth or impaired development. Here we do not report on findings having very low certainty evidence.
| Outcome | Comparator 1 | Comparator 2 | FU | Study design (no. of studies); Sample size |
Absolute difference (95% CI) | Relative risk (95% CI) |
Certainty of evidence | Conclusion | |
|---|---|---|---|---|---|---|---|---|---|
| Comparator 2 risk | Absolute risk differenceNote de bas de page a | ||||||||
| Simple wheeze; parent and/or physician-reported | |||||||||
| At-risk with RSV-H vs. at-risk without RSV-H | Prematurity: 32–35 wGA, RSV-H <12 months of age |
Prematurity: 32–35 wGA, No RSV-H <12 months of age |
During 6th y | PC32 (n=1); 434 |
14 per 100 | NS | RR 1.16 (0.70, 1.93) |
LowFootnote bFootnote c | Little to no difference For parent/physician-reported simple wheeze (episodes <3 within 12 months) during the 6th year among infants born premature (32–35 wGA) with vs. without hospitalization for RSV at <12 months |
| At-risk with RSV-H vs. at-risk without RSV-H | Prematurity: 32–35 wGA, RSV-H <12 months of age |
Prematurity: 32–35 wGA, No RSV-H <12 months of age |
Across 2–6 y | PC32 (n=1); 474 |
49 per 100 | 18 more per 100 (7–30 more) |
RR 1.36 (1.15, 1.60) |
LowFootnote bFootnote c | Small increase For parent/physician-reported simple wheeze (episodes <3 within 12 months) from 2–6 years among infants born premature (32–35 wGA) with vs. without hospitalization for RSV at <12 months |
| At-risk with RSV-H vs. not-at-risk with RSV-H | Prematurity: <33 wGA & RSV-H | Term: 39–41 wGA & RSV-H | 1 y | RFUPC37 (n=1); 17 |
67 per 100 | NS | RR 0.54 (0.18, 1.55) |
Very lowFootnote bFootnote cFootnote d | Very uncertain For parent and physician-reported simple wheeze (episodes <3 in 12 months) within one year among premature (<33 wGA) vs. term infants with hospitalization in their first RSV season |
| Recurrent wheeze; parent and/or physician-reported | |||||||||
| At-risk with RSV-H vs. at-risk without RSV-H | Prematurity: 32–35 wGA, RSV-H <12 months of age |
Prematurity: 32–35 wGA, No RSV-H <12 months of age |
During 6th y | PC32 (n=1); 434 |
10 per 100 | NS | RR 1.28 (0.71, 2.32) |
LowFootnote bFootnote c | Little to no difference For parent/physician-reported recurrent wheeze (≥3 episodes within 12 months) during the 6th year among infants born premature (32-35 wGA) with vs. without hospitalization for RSV at <12 months |
| At-risk with RSV-H vs. at-risk without RSV-H | Prematurity: 32–35 wGA, RSV-H <12 months of age |
Prematurity: 32–35 wGA, No RSV-H <12 months of age |
Across 2–6 y | PC32 (n=1); 422 |
27 per 100 | 19 more per 100 (7-35 more) |
RR 1.70 (1.27, 2.29) |
LowFootnote bFootnote c | Small increase For parent/physician-reported recurrent wheeze (≥3 episodes within 12 months) from 2-6 years among infants born premature (32-35 wGA) with vs. without hospitalization for RSV at <12 months |
| At-risk with RSV-H vs. not-at-risk with RSV-H | Prematurity: <33 wGA & RSV-H | Term: 39–41 wGA & RSV-H | 1 y | RFUPC37 (n=1); 17 |
0 per 100 | NS | RR 0.80 (0.04, 16.14) |
Very lowFootnote bFootnote cFootnote d | Very uncertain For parent and physician-reported recurrent wheeze ≥3 episodes in 12 months) within one year among premature (<33 wGA) vs. term infants with hospitalization in their first RSV season |
| Any/all wheeze; parent and/or physician-reported | |||||||||
| At-risk with RSV-H vs. at-risk without RSV-H | Prematurity: 32–35 wGA, RSV-H <12 months of age |
Prematurity: 32–35 wGA, No RSV-H <12 months of age |
During 6th y | PC32 (n=1); 434 |
21 per 100 | NS | RR 1.12 (0.75, 1.67) |
LowFootnote bFootnote c | Little to no difference For parent/physician-reported any/all wheeze (any within 12 months) during the 6th year among infants born premature (32–35 wGA) with vs. without hospitalization for RSV at <12 months |
| At-risk with RSV-H vs. at-risk without RSV-H | Prematurity: 32–35 wGA, RSV-H <12 months of age |
Prematurity: 32–35 wGA, No RSV-H <12 months of age |
Across 2–6 y | PC32 (n=1); 412 |
54 per 100 | 17 more per 100 (5–31 more) |
RR 1.31 (1.10, 1.57) |
LowFootnote bFootnote c | Small increase For parent/physician-reported any/all wheeze (any within 12 months) from 2–6 years among infants born premature (32–35 wGA) with vs. without hospitalization for RSV at <12 months |
| At-risk with RSV-H vs. not-at-risk with RSV-H | Prematurity: <33 wGA & RSV-H | Term: 39–41 wGA & RSV-H | 1 y | RFUPC37 (n=1); 17 |
67 per 100 | NS | RR 0.64 (0.24, 1.75) |
Very lowFootnote bFootnote cFootnote d | Very uncertain For parent and physician-reported any/all wheeze within one year among premature (<33 wGA) vs. term infants with hospitalization in their first RSV season |
| Severe wheeze; parent or physician-reported | |||||||||
| At-risk with RSV-H vs. at-risk without RSV-H | Prematurity: 32–35 wGA, RSV-H <12 months of age |
Prematurity: 32–35 wGA, No RSV-H <12 months of age |
During 6th y | PC34 (n=1); 434 |
9 per 100 | NS | RR 0.91 (0.44, 1.88) |
LowFootnote bFootnote c | Little to no difference For parent/physician-reported severe wheeze (≥1 hospitalization or ≥3 medical attendances or medication for three consecutive months or five cumulative months) during the 6th year among infants born premature (32–35 wGA) with vs. without hospitalization for RSV at <12 months |
| At-risk with RSV-H vs. at-risk without RSV-H | Prematurity: 32–35 wGA, RSV-H <12 months of age |
Prematurity: 32–35 wGA, No RSV-H <12 months of age |
Across 2–6 y | PC34 (n=1); 427 |
24 per 100 | 14 more per 100 (3–29 more) |
RR 1.59 (1.13, 2.24) |
LowFootnote bFootnote c | Small increase For parent/physician-reported severe wheeze (≥1 hospitalization or ≥3 medical attendances or medication for three consecutive months or five cumulative months) from 2–6 years among infants born premature (32–35 wGA) with vs. without hospitalization for RSV at <12 months |
| At-risk with RSV-H vs. not-at-risk with RSV-H | Prematurity: <33 wGA & RSV-H | Term: 39–41 wGA & RSV-H | 1 y | RFUPC37 (n=1); 17 |
0 per 100 | NS | RD 0.00 (-0.34, 0.34) |
Very lowFootnote bFootnote cFootnote d | Very uncertain For physician-reported severe wheeze (hospitalization for wheeze in 12 months) within one year among premature (<33 wGA) vs. term infants with hospitalization in their first RSV season |
| Wheeze duration (days per month post-RSV); parent-reported | |||||||||
| Not-at-risk population | RSV-positive, hospitalized | RSV-positive, non-hospitalized | 1 y | PC52 (n=1); 90 |
MD 0.70 (-0.94, 2.34) |
N/A | Very lowFootnote bFootnote cFootnote d | Very uncertain For parent-reported days with wheeze at one year among hospitalized vs. non-hospitalized healthy term infants positive for RSV at <12 months |
|
Outcome |
Comparator 1 | Comparator 2 | FU | Study design (no. of studies); Sample size |
Absolute difference (95% CI) | Relative risk (95% CI) |
Certainty of evidence | Conclusion | |
|---|---|---|---|---|---|---|---|---|---|
| Comparator 2 risk | Absolute risk differenceFootnote a | ||||||||
| Asthma; physician-diagnosed | |||||||||
| Not-at-risk population | RSV infection in first year of life | Infection with a respiratory pathogen other than RSV in first year of life | 7 y | PC31 (n=1); 329 |
12 per 100 | 15 more per 100 (4–35 more) |
RR 2.33 (1.35, 4.05) Adjusted for total number of respiratory episodes: OR 1.26 (0.54, 2.91), p=0.59 |
Very lowFootnote bFootnote cFootnote dFootnote e | Very uncertain For physician-diagnosed asthma at seven years of age among healthy infants with RSV vs. a different respiratory pathogen in the first year of life |
| Not-at-risk population | RSV-H | No RSV-H | 28–31 y | PC28 (n=1); 129 |
13 per 100 | NS | RR 1.82 (0.84, 3.94) |
Very lowFootnote bFootnote cFootnote e | Very uncertain For physician-diagnosed asthma at 28–31 years of age among term infants with vs. without hospitalization for RSV at age <24 months |
| Asthma; self-reported | |||||||||
| Not-at-risk population | RSV-H | No RSV-H | 17–20 y; 28–31 y |
PC27,28 (n=2); 203 |
15 per 100 | 19 more per 100 (0.1-60 more) |
RR 2.28 (1.01, 5.12) |
LowFootnote bFootnote e | Small increase For self-reported asthma in adulthood (17–31 years of age) among infants with vs. without hospitalization for RSV at age <24 months |
| Asthma medication (bronchodilator) | |||||||||
| At-risk with RSV-H vs. at-risk without RSV-H | Prematurity: 32-35 wGA, RSV-H |
Prematurity: 32–35 wGA, No RSV-H |
Across 2–6 y | PC32 (n=1); 487 |
17 per 100 | 8 more per 100 (4–13 more) |
RR 1.48 (1.23, 1.77) |
LowFootnote cFootnote e | Small increase Parent-reported bronchodilator use from 2–6 years of age among infants born premature (32–35 wGA) with vs. without hospitalization for RSV at <12 months |
| Not-at-risk population | RSV-H | No RSV-H | 28–31 y | PC28 (n=1); 129 |
14 per 100 | 16 more per 100 (1-47 more) |
RR 2.17 (1.08, 4.34) |
Very lowFootnote bFootnote cFootnote e | Very uncertain For self-reported bronchodilator use in adulthood (28–31 years of age) among term infants with vs. without hospitalization for RSV at age <24 months |
| Asthma medication (inhaled CS) | |||||||||
| At-risk with RSV-H vs. at-risk without RSV-H | Prematurity: 32–35 wGA, RSV-H |
Prematurity: 32–35 wGA, No RSV-H |
Across 2–6 y | PC32 (n=1); 487 |
16 per 100 | 10 more per 100 (2-22 more) |
RR 1.65 (1.13, 2.40) |
LowFootnote cFootnote e | Small increase Parent-reported ICS use from 2–6 years of age among infants born premature (32–35 wGA) with hospitalization for RSV at <12 months |
| Not-at-risk population | RSV-H | No RSV-H | 28–31 y | PC28 (n=1); 129 |
11 per 100 | NS | RR 1.56 (0.62, 3.89) |
Very lowFootnote bFootnote cFootnote e | Very uncertain For self-reported ICS use in adulthood (28–31 years of age) among term infants with vs. without hospitalization for RSV at age <24 months |
| Asthma medication (oral CS) | |||||||||
| At-risk with RSV-H vs. at-risk without RSV-H | Prematurity: 32–35 wGA, RSV-H |
Prematurity: 32–35 wGA, No RSV-H |
Across 2–6 y | PC32 (n=1); 487 |
11 per 100 | 8 more per 100 (0.6–19 more) |
RR 1.71 (1.06, 2.74) |
LowFootnote cFootnote e | Small increase Parent-reported oral CS use from 2–6 years of age among infants born premature (32–35 wGA) with vs. without hospitalization for RSV at <12 months |
| Asthma medication (leukotriene antagonist) | |||||||||
| At-risk with RSV-H vs. at-risk without RSV-H | Prematurity: 32–35 wGA, RSV-H |
Prematurity: 32–35 wGA, No RSV-H |
Across 2–6 y | PC32 (n=1); 487 |
6 per 100 | 10 more per 100 (3–22 more) |
RR 2.52 (1.43, 4.42) |
LowFootnote cFootnote e | Increased Parent-reported leukotriene antagonist use from 2–6 years of age among infants born premature (32–35 wGA) with vs. without hospitalization for RSV at <12 months |
Outcome |
Comparator 1 | Comparator 2 | FU | Study design (no. of studies); Sample size |
Absolute difference (95% CI) | Relative risk (95% CI) |
Certainty of evidence | Conclusion | |
|---|---|---|---|---|---|---|---|---|---|
| Comparator 2 risk | Absolute risk differenceFootnote a | ||||||||
| Lung function: FEV1 Z-score ranking [-2,-1] | |||||||||
| At-risk with RSV-H vs. at-risk without RSV-H | Prematurity: 32–35 wGA, RSV-H |
Prematurity: 32–35 wGA, No RSV-H |
During 6th y | PC32 (n=1); 243 |
21 per 100 | NS | RR 0.83 (0.45, 1.53) |
LowFootnote bFootnote c | Little to no difference For forced expiratory volume in one second (Z-score rank of [-2, -1], considered extreme range) during the 6th year of age among children hospitalized with RSV at <12 months |
| Lung function (FEV1 pre-BD, mean % of predicted) | |||||||||
| Not-at-risk population | RSV-H | No RSV-H | 17–20 y; 28–31 y |
PC27,28 (n=2); 202 |
MD -7.63 (-11.35, -3.91) |
N/A | LowFootnote cFootnote d | Small decrease For forced expiratory volume in one second (mean % of predicted, pre-bronchodilation test) in adulthood (17–31 years of age) among infants with vs. without hospitalization for RSV at age <24 months |
|
| Lung function (FEV1, change in mean % predicted) | |||||||||
| Not-at-risk population | RSV-H | No RSV-H | 17–20 y; 28–31 y |
PC27,28 (n=2); 202 |
MD 0.81 (-0.67, 2.30) |
N/A | LowFootnote cFootnote d | Little to no difference For forced expiratory volume in one second (change in mean % predicted, pre vs. post-bronchodilation test) in adulthood (17–31 years of age) among infants with vs. without hospitalization for RSV at age <24 months |
|
| Lung function (FVC pre-BD, mean % of predicted) | |||||||||
| Not-at-risk population | RSV-H | No RSV-H | 17–20 y; 28–31 y |
PC27,28 (n=2); 202 |
MD -4.74 (-7.80, -1.67) |
N/A | LowFootnote cFootnote d | Small decrease For forced vital capacity (mean % of predicted, pre-bronchodilation test) in adulthood (17–31 years of age) among infants with vs. without hospitalization for RSV at age <24 months |
|
| Lung function (FVC, change in mean % predicted) | |||||||||
| Not-at-risk population | RSV-H | No RSV-H | 17–20 y | PC27 (n=1); 74 |
MD 0.60 (-0.67, 1.87) |
N/A | Very lowFootnote cFootnote dFootnote e | Very uncertain For forced vital capacity (change in mean % predicted, pre vs. post-bronchodilation test) in adulthood (17–20 years of age) among infants with vs. without hospitalization for RSV at age <24 months |
|
| Lung function (FEV1/FVC pre-BD, % of predicted) | |||||||||
| Not-at-risk population | RSV-H | No RSV-H | 17–20 y; 28–31 y |
PC27,28 (n=2); 202 |
MD -3.20 (-9.07, 2.67) |
N/A | Very lowFootnote bFootnote cFootnote d | Very uncertain For FEV1/FVC (mean % of predicted, pre-bronchodilation test) in adulthood (17–31 years of age) among infants with vs. without hospitalization for RSV at age <24 months |
|
| Lung function (FEV1/FVC, change in mean % predicted) | |||||||||
| Not-at-risk population | RSV-H | No RSV-H | 17–20 y | PC27 (n=1); 74 |
MD -0.20 (-2.71, 2.31) |
N/A | Very lowFootnote bFootnote cFootnote e | Very uncertain For FEV1/FVC (change in mean % predicted, pre vs. post-bronchodilation test) in adulthood (17–20 years of age) among infants with vs. without hospitalization for RSV at age <24 months |
|
| Lung function (FENO, mean ppb) | |||||||||
| Not-at-risk population | RSV-H | No RSV-H | 17–20 y; 28–31 y |
PC27,28 (n=2); 202 |
MD -1.00 (-14.49, 12.49) |
N/A | LowFootnote bFootnote c | Little to no difference For fractional exhaled nitric oxide (mean ppb) in adulthood (17–31 years of age) among infants with vs. without hospitalization for RSV at age <24 months |
|
| Lung function (MEF50 pre-BD, mean % of predicted) | |||||||||
| Not-at-risk population | RSV-H | No RSV-H | 17–20 y | PC27 (n=1); 74 |
MD -4.00 (-14.95, 6.95) |
N/A | Very lowFootnote bFootnote cFootnote dFootnote e | Very uncertain For maximum expiratory flow after 50% of expired FVC (change in mean % predicted, pre-bronchodilation test) in adulthood (17–20 years of age) among infants with vs. without hospitalization for RSV at age <24 months |
|
| Lung function (MEF50, change in mean % predicted) | |||||||||
| Not-at-risk population | RSV-H | No RSV-H | 17–20 y | PC27 (n=1); 74 |
MD 3.70 (-5.42, 12.82) |
N/A | Very lowFootnote bFootnote cFootnote dFootnote e | Very uncertain For maximum expiratory flow after 50% of expired FVC (change in mean % predicted, pre vs. post-bronchodilation test) in adulthood (17–20 years of age) among infants with vs. without hospitalization for RSV at age <24 months |
|
Prematurity: One study enrolled premature (32–35 wGA) infants with or without hospitalization for RSV infection before 12 months of age to examine several long-term outcomesFootnote 32. Data were collected through telephone calls every four months and annual visits until the 6th year of life. The authors analysed data both across the five years and only within the 6th year. All findings offered low certainty evidence.
Across years two through six, associations were found between RSV-hospitalization and increased risk for parent or physician-reported simple wheeze, recurrent wheeze, severe wheeze and any/all wheezeFootnote 32. When examining the 6th year only, there was little-to-no difference in parent or physician-reported simple wheeze, recurrent wheeze, severe wheeze and any/all wheezeFootnote 32. This study also compared groups for parent-reported asthma-associated medication use across years two through six. There were associations with increased risk from RSV-hospitalization for use of bronchodilators, inhaled corticosteroids, oral corticosteroids and leukotriene antagonistsFootnote 32. Through lung function testing using spirometry, there was little-to-no difference in severe respiratory morbidity at six years of age between groupsFootnote 32.
RSV without risk factors: Pooled data from two studies of children with versus without RSV-hospitalization at younger than 24 months of age found low certainty of an increase in self-reported asthma in adulthoodFootnote 27Footnote 28. Of note, there was no difference in physician-diagnosed asthma (considered more reliable than patient-reported asthma)Footnote 28, but certainty of evidence was very low for this outcome. An association was also found between RSV and lower pre-bronchodilation mean percent of predicted forced expiratory volume in one second (FEV1), but there was little-to-no difference in change in FEV1 from pre to post-bronchodilationFootnote 27Footnote 28. The RSV was associated with lower predicted forced vital capacity, but not fractional exhaled nitric oxideFootnote 27Footnote 28.
Long-term outcome comparisons: Other data
Supplement 11 contains single group data and pooled analyses (when appropriate). We did not conduct analyses for between-study comparator groups, since single studies that contributed to each long-term outcome were represented among within-study comparisons.
Discussion
Summary of findings and limitations
Few studies contributed data for within-study comparisons of outcomes of interest. There was moderate-to-low certainty that RSV is associated with a small increase in hospitalization and length of stay among moderate-to-late preterm (33–36 wGA) compared with term infants. There was moderate-to-low certainty evidence for no significant differences in hospitalizations between infants born at 29–32 wGA versus 33–36 wGA. Low certainty of evidence was found for a slight increase in mechanical ventilation among those born at 29–32 wGA versus 33–35 wGA and hospitalized for RSV prior to 12 months of age. Low certainty evidence was found for increased hospital length of stay among children younger than three years of age with versus without Down syndrome. There was low certainty evidence for increased wheezing and asthma medication use from two to six years of age among RSV-hospitalized versus non-hospitalized premature (32–35 wGA) infants, although there was little-to-no difference for these outcomes in the 6th year of follow-up. Low certainty evidence was found for decreased lung function measurements before bronchodilation but changes in measurements after bronchodilation did not differ between groups. Very low certainty evidence was found for other long-term outcomes comparing different risk groups.
Single studies contributed data for most outcomes, where populations with rare conditions (e.g. cystic fibrosis) often represent small/under-powered sample sizes, precluding investigation of heterogeneity among studies for important population and RSV characteristics, or consistency in findings. The paucity of studies on clinical conditions other than prematurity is a limitation of the evidence base. We also did not find studies of premature infants born before 29–30 weeks gestation, or of children with chronic lung disease of prematurity or congenital heart disease, groups for whom prophylaxis is now recommended in the United States and in CanadaFootnote 2Footnote 23.
Retrospective study designs utilizing older data (i.e. pre-2014) were included, and may reflect different practices (e.g. prophylaxis, RSV-testing, standard of care) over time and across countries and settings. Detection of RSV infection may be impacted by variation in testing methods, including types of tests and indications for testing, and seasonal and geographic variability. Among tested individuals, the proportion of patients with viral or bacterial co-infections may be an important confounder in etiology of outcome severity. Lack of blinding of healthcare providers to risk status may influence rates of hospitalization and possibly other care parameters, particularly among children with known RSV risk factors.
Comparison with other reviews
A series of systematic reviews of publications from 1995 to 2015 found that RSV-hospitalization is associated with significant morbidity among children younger than 18 years old in Western countries (Canada, United States, Europe), particularly for young children with prematurity, chronic lung disease of prematurity and congenital heart diseaseFootnote 6Footnote 7Footnote 8Footnote 9. Whereas the current work focused on children younger than two years of age with a single risk factor, these reviews also included studies of children up to 18 years of age. Our review scope searched comparatively more recent publications (2014–2018) and covered a broader geographic area by including high-income (OECD) countries.
Future research
Based on current evidence, there is a need for studies to focus on the burden of RSV disease among children with underlying chronic conditions, for some of which data on risk are contradictory or non-existing. Assessments of current RSV surveillance activities in Canada have identified data gaps for particular populations, including children with underlying medical conditions and those living in Indigenous, northern or remote communitiesFootnote 19. Gaps will need to be filled in preparation for monitoring of RSV vaccine effectiveness in the future.
Conclusion
Prematurity is associated with increased risk for RSV-hospitalization in infancy and increased hospital length of stay, and may be associated with increased wheeze and asthma-medication use at up to six years of age. Down syndrome may be associated with longer hospital length of stay. We are very uncertain about evidence from other within-study comparisons. Very few studies included a comparison group.
Authors’ statement
- AW — Conceptualization, methodology, analysis, writing–original draft, review and editing
- JP — Conceptualization, methodology, analysis, writing–review and editing
- DLM — Conceptualization, methodology, analysis, writing–review and editing
- SG — Analysis, writing–review and editing
- BV — Analysis, writing–review and editing
- MPD — Conceptualization, methodology, analysis
- AS — Conceptualization, methodology, analysis, writing–review and editing
- MT — Conceptualization, methodology, writing–review and editing
- LH — Conceptualization, methodology, writing–review and editing
Competing interests
AW, JP, SG, BV, MPD and LH report grants from the Public Health Agency of Canada during the conduct of the study.
Acknowledgments
The authors would like to thank Canada’s National Advisory Committee on Immunization RSV Working Group (Drs. D Moore, J Papenburg, J Robinson, M Salvadori, W Vaudry, R Stirling, A Sinilaité, and R Pless) for their advice during initial study conception on strategic framing of the risk groups and for reviewing the search parameters. The authors thank Ms. L Gamble, librarian (Health Library of Health Canada and Public Health Agency of Canada), for conducting the literature search; Mr. S Duchesne-Belanger for assistance with title/abstract screening; and Dr. A Gates and Ms. S Rahman for assistance with data verification. The authors gratefully acknowledge Drs. K Backman and K Bønnelykke for providing study data.
Funding
This work was conducted for the National Advisory Committee for Immunization, under contract to the Public Health Agency of Canada (contract #4600001536). The views of the funding body have not influenced the content of the review. The views expressed are those of the authors and do not necessarily represent those of the Public Health Agency of Canada. LH is supported by a Tier 1 Canada Research Chair in Knowledge Synthesis and Translation.
Supplemental material
These documents can be accessed on the Supplemental material file.
Supplement 1: Methods
Supplement 2: Search strategy
Supplement 3: Inclusion/exclusion criteria
Supplement 4: Methodological quality assessments
Supplement 5: Certainty of evidence assessments
Supplement 6: Characteristics of included studies
Supplement 7: Outcomes of included studies
Supplement 8: Single group proportions for short-term outcomes
Supplement 9: Summary of evidence for short-term outcomes—between-study
Supplement 10: Complications
Supplement 11: Single group proportions for long-term outcomes
References
- Footnote 1
-
Buchan SA, Chung H, Karnauchow T, McNally JD, Campitelli MA, Gubbay JB, Katz K, McGeer AJ, Richardson DC, Richardson SE, Simor A, Smieja M, Zahariadis G, Tran D, Crowcroft NS, Rosella LC, Kwong JC. Characteristics and outcomes of young children hospitalized with laboratory-confirmed influenza or respiratory syncytial virus in Ontario, Canada, 2009-2014. Pediatr Infect Dis J 2019;38(4):362-9. https://doi.org/10.1097/INF.0000000000002164
- Footnote 2
-
Robinson JL, Le Saux N; Canadian Paediatric Society, Infectious Diseases and Immunization Committee. Preventing hospitalizations for respiratory syncytial virus infection. Paediatr Child Health 2015;20(6):321-33. https://doi.org/10.1093/pch/20.6.321
- Footnote 3
-
Barr R, Green CA, Sande CJ, Drysdale SB. Respiratory syncytial virus: diagnosis, prevention and management. Ther Adv Infect Dis 2019;6:2049936119865798. https://doi.org/10.1177/2049936119865798
- Footnote 4
-
Mitchell I, Defoy I, Grubb E. Burden of respiratory syncytial virus hospitalizations in Canada. Can Respir J 2017;2017:4521302. https://doi.org/10.1155/2017/4521302
- Footnote 5
-
Pisesky A, Benchimol EI, Wong CA, Hui C, Crowe M, Belair MA, Pojsupap S, Karnauchow T, O'Hearn K, Yasseen AS 3rd, McNally JD. Incidence of hospitalization for respiratory syncytial virus infection amongst children in Ontario, Canada: A Population-Based Study Using Validated Health Administrative Data. PLoS One 2016;11(3):e0150416. https://doi.org/10.1371/journal.pone.0150416
- Footnote 6
-
Figueras-Aloy J, Manzoni P, Paes B, Simões EA, Bont L, Checchia PA, Fauroux B, Carbonell-Estrany X. Defining the Risk and Associated Morbidity and Mortality of Severe Respiratory Syncytial Virus Infection Among Preterm Infants Without Chronic Lung Disease or Congenital Heart Disease. Infect Dis Ther 2016;5(4):417-52. https://doi.org/10.1007/s40121-016-0130-1
- Footnote 7
-
Paes B, Fauroux B, Figueras-Aloy J, Bont L, Checchia PA, Simões EA, Manzoni P, Carbonell-Estrany X. Defining the risk and associated morbidity and mortality of severe respiratory syncytial virus infection among infants with chronic lung disease. Infect Dis Ther 2016;5(4):453-71. https://doi.org/10.1007/s40121-016-0137-7
- Footnote 8
-
Checchia PA, Paes B, Bont L, Manzoni P, Simões EA, Fauroux B, Figueras-Aloy J, Carbonell-Estrany X. Defining the risk and associated morbidity and mortality of severe respiratory syncytial virus infection among infants with congenital heart disease. Infect Dis Ther 2017;6(1):37-56. https://doi.org/10.1007/s40121-016-0142-x
- Footnote 9
-
Bont L, Checchia PA, Fauroux B, Figueras-Aloy J, Manzoni P, Paes B, Simões EA, Carbonell-Estrany X. Defining the epidemiology and burden of severe respiratory syncytial virus infection among infants and children in western countries. Infect Dis Ther 2016;5(3):271-98. https://doi.org/10.1007/s40121-016-0123-0
- Footnote 10
-
Kristensen K, Hjuler T, Ravn H, Simões EA, Stensballe LG. Chronic diseases, chromosomal abnormalities, and congenital malformations as risk factors for respiratory syncytial virus hospitalization: a population-based cohort study. Clin Infect Dis 2012;54(6):810-7. https://doi.org/10.1093/cid/cir928
- Footnote 11
-
Kua KP, Lee SW. Systematic review of the safety and efficacy of palivizumab among infants and young children with cystic fibrosis. Pharmacotherapy 2017;37(6):755-69. https://doi.org/10.1002/phar.1936
- Footnote 12
-
Manzoni P, Figueras-Aloy J, Simões EA, Checchia PA, Fauroux B, Bont L, Paes B, Carbonell-Estrany X. Defining the incidence and associated morbidity and mortality of severe respiratory syncytial virus infection among children with chronic diseases. Infect Dis Ther 2017;6(3):383-411. https://doi.org/10.1007/s40121-017-0160-3
- Footnote 13
-
Banerji A, Panzov V, Young M, Robinson J, Lee B, Moraes T, Mamdani M, Giles BL, Jiang D, Bisson D, Dennis M, Morel J, Hall J, Hui C, Paes B, Mahony JB. Hospital admissions for lower respiratory tract infections among infants in the Canadian Arctic: a cohort study. CMAJ Open 2016;4(4):E615-22. https://doi.org/10.9778/cmajo.20150051
- Footnote 14
-
Driscoll AJ, Arshad SH, Bont L, Brunwasser SM, Cherian T, Englund JA, Fell DB, Hammitt LL, Hartert TV, Innis BL, Karron RA, Langley GE, Mulholland EK, Munywoki PK, Nair H, Ortiz JR, Savitz DA, Scheltema NM, Simões EA, Smith PG, Were F, Zar HJ, Feikin DR. Does respiratory syncytial virus lower respiratory illness in early life cause recurrent wheeze of early childhood and asthma? Critical review of the evidence and guidance for future studies from a World Health Organization-sponsored meeting. Vaccine. 2020:2435-48. https://doi.org/10.1016/j.vaccine.2020.01.020
- Footnote 15
-
Jalink MB, Langley JM, Dodds L, Andreou P. Severe respiratory syncytial virus infection in preterm infants and later onset of asthma. Pediatr Infect Dis J 2019;38(11):1121-5. https://doi.org/10.1097/INF.0000000000002432
- Footnote 16
-
Shi T, Ooi Y, Zaw EM, Utjesanovic N, Campbell H, Cunningham S, Bont L, Nair H; RESCEU Investigators. Association between respiratory syncytial virus-associated acute lower respiratory infection in early life and recurrent wheeze and asthma in later childhood. J Infect Dis 2020;222 Suppl 7:S628-33. https://doi.org/10.1093/infdis/jiz311
- Footnote 17
-
Andabaka T, Nickerson JW, Rojas-Reyes MX, Rueda JD, Bacic Vrca V, Barsic B. Monoclonal antibody for reducing the risk of respiratory syncytial virus infection in children. Cochrane Database Syst Rev 2013;4(4):CD006602. https://doi.org/10.1002/14651858.CD006602.pub4
- Footnote 18
-
Robinson KA, Odelola OA, Saldanha IJ. Palivizumab for prophylaxis against respiratory syncytial virus infection in children with cystic fibrosis. Cochrane Database Syst Rev 2016;7(7):CD007743. https://doi.org/10.1002/14651858.CD007743.pub6
- Footnote 19
-
Killikelly A, Shane A, Yeung MW, Tunis M, Bancej C, House A, Vaudry W, Moore D, Quach C. Gap analyses to assess Canadian readiness for respiratory syncytial virus vaccines: report from an expert retreat. Can Commun Dis Rep 2020;46(4):62-8. https://doi.org/10.14745/ccdr.v46i04a02
- Footnote 20
-
Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions: Cochrane, version 6.2 (updated 2021-02; accessed 2021-02-24). www.training.cochrane.org/handbook
- Footnote 21
-
Iorio A, Spencer FA, Falavigna M, Alba C, Lang E, Burnand B, McGinn T, Hayden J, Williams K, Shea B, Wolff R, Kujpers T, Perel P, Vandvik PO, Glasziou P, Schunemann H, Guyatt G. Use of GRADE for assessment of evidence about prognosis: rating confidence in estimates of event rates in broad categories of patients. BMJ 2015;350:h870. https://doi.org/10.1136/bmj.h870
- Footnote 22
-
Foroutan F, Guyatt G, Zuk V, Vandvik PO, Alba AC, Mustafa R, Vernooij R, Arevalo-Rodriguez I, Munn Z, Roshanov P, Riley R, Schandelmaier S, Kuijpers T, Siemieniuk R, Canelo-Aybar C, Schunemann H, Iorio A. GRADE Guidelines 28: Use of GRADE for the assessment of evidence about prognostic factors: rating certainty in identification of groups of patients with different absolute risks. J Clin Epidemiol 2020;121:62-70. https://doi.org/10.1016/j.jclinepi.2019.12.023
- Footnote 23
-
American Academy of Pediatrics Committee on Infectious Diseases; American Academy of Pediatrics Bronchiolitis Guidelines Committee. Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics 2014;134(2):415-20. https://doi.org/10.1542/peds.2014-1665
- Footnote 24
-
Murad MH, Montori VM, Walter SD, Guyatt GH. Estimating risk difference from relative association measures in meta-analysis can infrequently pose interpretational challenges. J Clin Epidemiol 2009;62(8):865-7. https://doi.org/10.1016/j.jclinepi.2008.11.005
- Footnote 25
-
Ambrose CS, Anderson EJ, Simões EA, Wu X, Elhefni H, Park CL, Sifakis F, Groothuis JR. Respiratory syncytial virus disease in preterm infants in the U.S. born at 32-35 weeks gestation not receiving immunoprophylaxis. Pediatr Infect Dis J 2014;33(6):576-82. https://doi.org/10.1097/INF.0000000000000219
- Footnote 26
-
Anderson EJ, Krilov LR, DeVincenzo JP, Checchia PA, Halasa N, Simões EA, Domachowske JB, Forbes ML, Pannaraj PS, McBride SJ, McLaurin KK, Kumar VR, Ambrose CS. SENTINEL1: an observational study of respiratory syncytial virus hospitalizations among U.S. infants born at 29 to 35 weeks' gestational age not receiving immunoprophylaxis. Am J Perinatol 2017;34(1):51-61. https://doi.org/10.1055/s-0036-1584147
- Footnote 27
-
Backman K, Ollikainen H, Piippo-Savolainen E, Nuolivirta K, Korppi M. Asthma and lung function in adulthood after a viral wheezing episode in early childhood. Clin Exp Allergy 2018;48(2):138-46. https://doi.org/10.1111/cea.13062
- Footnote 28
-
Backman K, Piippo-Savolainen E, Ollikainen H, Koskela H, Korppi M. Adults face increased asthma risk after infant RSV bronchiolitis and reduced respiratory health-related quality of life after RSV pneumonia. Acta Paediatr 2014;103(8):850-5. https://doi.org/10.1111/apa.12662
- Footnote 29
-
Bjornson C, Chan P, Li A, Paes B, Lanctôt KL, Mitchell I. Palivizumab prophylaxis for respiratory syncytial virus in infants with cystic fibrosis: is there a need? Eur J Clin Microbiol Infect Dis 2018;37(6):1113-8. https://doi.org/10.1007/s10096-018-3225-7
- Footnote 30
-
Blanken MO, Korsten K, Achten NB, Tamminga S, Nibbelke EE, Sanders EA, Smit HA, Groenwold RH, Bont L; Dutch RSV Neonatal Network. Population-attributable risk of risk factors for recurrent wheezing in moderate preterm infants during the first year of life. Paediatr Perinat Epidemiol 2016;30(4):376-85. https://doi.org/10.1111/ppe.12295
- Footnote 31
-
Bønnelykke K, Vissing NH, Sevelsted A, Johnston SL, Bisgaard H. Association between respiratory infections in early life and later asthma is independent of virus type. J Allergy Clin Immunol 2015;136(1):81-86.e4. https://doi.org/10.1016/j.jaci.2015.02.024
- Footnote 32
-
Carbonell-Estrany X, Pérez-Yarza EG, García LS, Guzmán Cabañas JM, Bòria EV, Atienza BB; IRIS (Infección Respiratoria Infantil por Virus Respiratorio Sincitial) Study Group. Long-term burden and respiratory effects of respiratory syncytial virus hospitalization in preterm infants-the SPRING study. PLoS One 2015;10(5):e0125422. https://doi.org/10.1371/journal.pone.0125422
- Footnote 33
-
Caserta MT, Qiu X, Tesini B, Wang L, Murphy A, Corbett A, Topham DJ, Falsey AR, Holden-Wiltse J, Walsh EE. Development of a Global Respiratory Severity Score for Respiratory Syncytial Virus Infection in Infants. J Infect Dis 2017;215(5):750-6. https://doi.org/10.1093/infdis/jiw624
- Footnote 34
-
Chu PY, Hornik CP, Li JS, Campbell MJ, Hill KD. Respiratory syncytial virus hospitalisation trends in children with haemodynamically significant heart disease, 1997-2012. Cardiol Young 2017;27(1):16-25. https://doi.org/10.1017/S1047951116000470
- Footnote 35
-
Drummond D, Thumerelle C, Reix P, Fayon M, Epaud R, Clement A, Mahloul M, Habouria D, Delacourt C, Hadchouel A. Effectiveness of palivizumab in children with childhood interstitial lung disease: the French experience. Pediatr Pulmonol 2016;51(7):688-95. https://doi.org/10.1002/ppul.23354
- Footnote 36
-
Farber HJ, Buckwold FJ, Lachman B, Simpson JS, Buck E, Arun M, Valadez AM, Ruiz T, Alonzo J, Henry A, Cos-Okpalla N, Nguyen K, Brendel W, Small J, Glomb WB. Observed effectiveness of palivizumab for 29-36-week gestation infants. Pediatrics 2016;138(2):e20160627. https://doi.org/10.1542/peds.2016-0627
- Footnote 37
-
Fauroux B, Gouyon JB, Roze JC, Guillermet-Fromentin C, Glorieux I, Adamon L, Di Maio M, Anghelescu D, Miloradovich T, Escande B, Elleau C, Pinquier D. Respiratory morbidity of preterm infants of less than 33 weeks gestation without bronchopulmonary dysplasia: a 12-month follow-up of the CASTOR study cohort. Epidemiol Infect 2014;142(7):1362-74. https://doi.org/10.1017/S0950268813001738
- Footnote 38
-
Feldman AG, Sundaram SS, Beaty BL, Kempe A. Hospitalizations for Respiratory Syncytial Virus and Vaccine-Preventable Infections in the First 2 Years After Pediatric Liver Transplant. J Pediatr 2017;182:232-238.e1. https://doi.org/10.1016/j.jpeds.2016.12.021
- Footnote 39
-
Groves HE, Jenkins L, Macfarlane M, Reid A, Lynn F, Shields MD. Efficacy and long-term outcomes of palivizumab prophylaxis to prevent respiratory syncytial virus infection in infants with cystic fibrosis in Northern Ireland. Pediatr Pulmonol 2016;51(4):379-85. https://doi.org/10.1002/ppul.23376
- Footnote 40
-
Hama I, Takahashi S, Nakamura T, Ito Y, Kawasaki K, Sago H. Risk of respiratory syncytial virus infection in infants with congenital cystic lung disease. Pediatr Int 2015;57(2):253-7. https://doi.org/10.1111/ped.12544
- Footnote 41
-
Hatanaka M, Miyamura T, Koh K, Taga T, Tawa A, Hasegawa D, Kajihara R, Adachi S, Ishii E, Tomizawa D. Respiratory syncytial virus infection in infants with acute leukemia: a retrospective survey of the Japanese Pediatric Leukemia/Lymphoma Study Group. Int J Hematol 2015;102(6):697-701. https://doi.org/10.1007/s12185-015-1890-1
- Footnote 42
-
Helfrich AM, Nylund CM, Eberly MD, Eide MB, Stagliano DR. Healthy Late-preterm infants born 33-36+6 weeks gestational age have higher risk for respiratory syncytial virus hospitalization. Early Hum Dev 2015;91(9):541-6. https://doi.org/10.1016/j.earlhumdev.2015.06.009
- Footnote 43
-
Korsten K, Blanken MO, Nibbelke EE, Moons KG, Bont L; Dutch RSV Neonatal Network. Prediction model of RSV-hospitalization in late preterm infants: an update and validation study. Early Hum Dev 2016;95:35-40. https://doi.org/10.1016/j.earlhumdev.2016.01.020
- Footnote 44
-
Luchsinger V, Ampuero S, Palomino MA, Chnaiderman J, Levican J, Gaggero A, Larrañaga CE. Comparison of virological profiles of respiratory syncytial virus and rhinovirus in acute lower tract respiratory infections in very young Chilean infants, according to their clinical outcome. J Clin Virol 2014;61(1):138-44. https://doi.org/10.1016/j.jcv.2014.06.004
- Footnote 45
-
McLaurin KK, Farr AM, Wade SW, Diakun DR, Stewart DL. Respiratory syncytial virus hospitalization outcomes and costs of full-term and preterm infants. J Perinatol 2016;36(11):990-6. https://doi.org/10.1038/jp.2016.113
- Footnote 46
-
O'Brien KL, Chandran A, Weatherholtz R, Jafri HS, Griffin MP, Bellamy T, Millar EV, Jensen KM, Harris BS, Reid R, Moulton LH, Losonsky GA, Karron RA, Santosham M; Respiratory Syncytial Virus (RSV) Prevention study group. Efficacy of motavizumab for the prevention of respiratory syncytial virus disease in healthy Native American infants: a phase 3 randomised double-blind placebo-controlled trial. Lancet Infect Dis 2015;15(12):1398-408. https://doi.org/10.1016/S1473-3099(15)00247-9
- Footnote 47
-
Rajah B, Sánchez PJ, Garcia-Maurino C, Leber A, Ramilo O, Mejias A. Impact of the Updated Guidance for Palivizumab Prophylaxis against Respiratory Syncytial Virus Infection: A Single Center Experience. J Pediatr 2017;181:183-188.e1. https://doi.org/10.1016/j.jpeds.2016.10.074
- Footnote 48
-
Ryan VM, Langley JM, Dodds L, Andreou P. Estimating respiratory syncytial virus-associated hospitalization in the first year of life among infants born at 32-35 weeks of gestation. Pediatr Infect Dis J 2016;35(8):851-5. https://doi.org/10.1097/INF.0000000000001186
- Footnote 49
-
Sadreameli SC, Reller ME, Bundy DG, Casella JF, Strouse JJ. Respiratory syncytial virus and seasonal influenza cause similar illnesses in children with sickle cell disease. Pediatr Blood Cancer 2014;61(5):875-8. https://doi.org/10.1002/pbc.24887
- Footnote 50
-
Stagliano DR, Nylund CM, Eide MB, Eberly MD. Children with Down syndrome are high-risk for severe respiratory syncytial virus disease. J Pediatr 2015;166(3):703-9.e2. https://doi.org/10.1016/j.jpeds.2014.11.058
- Footnote 51
-
Straňák Z, Saliba E, Kosma P, Posfay-Barbe K, Yunis K, Farstad T, Unnebrink K, van Wyk J, Wegzyn C, Notario G, Kalus S, Campbell FJ. Predictors of RSV LRTI hospitalization in infants born at 33 to 35 weeks gestational age: a large multinational study (PONI). PLoS One 2016;11(6):e0157446. https://doi.org/10.1371/journal.pone.0157446
- Footnote 52
-
Zomer-Kooijker K, Uiterwaal CS, van der Gugten AC, Wilbrink B, Bont LJ, van der Ent CK. Decreased lung function precedes severe respiratory syncytial virus infection and post-respiratory syncytial virus wheeze in term infants. Eur Respir J 2014;44(3):666-74. https://doi.org/10.1183/09031936.00009314
- Footnote 53
-
Franklin JA, Anderson EJ, Wu X, Ambrose CS, Simões EA. Insurance status and the risk of severe respiratory syncytial virus disease in United States preterm infants born at 32-35 weeks gestational age. Open Forum Infect Dis 2016;3(3):ofw163. https://doi.org/10.1093/ofid/ofw163
- Footnote 54
-
Simões EA, Anderson EJ, Wu X, Ambrose CS. Effects of chronologic age and young child exposure on respiratory syncytial virus disease among US preterm infants born at 32 to 35 weeks gestation. PLoS One 2016;11(11):e0166226. https://doi.org/10.1371/journal.pone.0166226
Page details
- Date modified:
