Updated draft assessment - NMP and NEP
Official title: Updated draft assessment - NMP and NEP
Chemical Abstracts Service Registry Numbers
(872-50-4 and 2687-91-4)
Environment and Climate Change Canada
Health Canada
January 2024
Synopsis
Pursuant to section 68 of the Canadian Environmental Protection Act, 1999 (CEPA), the Minister of the Environment and the Minister of Health have conducted an assessment of two substances, N-methyl-2-pyrrolidone (NMP) and N-ethyl-2-pyrrolidone (NEP). The Chemical Abstracts Service Registry Numbers (CAS RNFootnote 1 ), their Domestic Substances List (DSL) names, their common names, and abbreviations are listed in the table below. A draft screening assessment for NMP and NEP was published in February 2017. New information subsequently became available regarding exposure to products containing NMP and NEP available to consumers, which had the potential to alter assessment conclusions. As a result, the draft assessment was updated.
| CAS RN | DSL name | Common name (abbreviation) |
|---|---|---|
| 872-50-4 | 2-Pyrrolidinone, 1-methyl- | N-Methylpyrrolidone (NMP) |
| 2687-91-4 | 2-Pyrrolidinone, 1-ethyl- | N-Ethylpyrrolidone (NEP) |
NMP and NEP are anthropogenic substances that do not occur naturally in the environment. According to information submitted in response to a CEPA section 71 survey, NMP is used in chemical manufacturing and automotive, aircraft, and transportation sectors in Canada, including the manufacture of agricultural products, electrical and electronic products, metal and mining products, paper products, mixtures or manufactured items, and plastic and rubber materials. Products in Canada that contain NMP, and that may be available to the general population, include adhesives and sealants, auto interior cleaners, cleaning and degreasing products, paints and coatings, and paint removers. NMP may be present in certain personal care products, including as a non-medicinal ingredient in pharmaceuticals and in a limited number of nail care, synthetic nail or eyelash adhesives and adhesive removers, and hair products. NMP is also present as a formulant in certain registered pest control products. According to information submitted in response to a CEPA section 71 survey, NEP is used in chemical manufacturing as well as in manufacturing adhesives and sealants, paints and coatings, and plastic and rubber materials in Canada. NEP was not identified in any products available to Canadian consumers.
According to information submitted in response to a CEPA section 71 survey, NMP was imported into Canada in a quantity range of 100 000 kg to 1 000 000 kg, and NEP was imported in a quantity range of 1 000 kg to 10 000 kg during the 2011 calendar year. Neither substance was reported to be manufactured in Canada in quantities above the survey reporting threshold of 100 kg. Given the similar physical-chemical properties of these substances, they may be used interchangeably.
The ecological risks of NMP and NEP were characterized using the ecological risk classification of organic substances (ERC), which is a risk-based approach that employs multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. Hazard profiles are based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Metrics considered in the exposure profiles include potential emission rate, overall persistence, and long-range transport potential. A risk matrix is used to assign a low, moderate, or high level of potential concern for substances on the basis of their hazard and exposure profiles. Based on the outcome of the ERC analysis, NMP and NEP are considered unlikely to be causing ecological harm.
Considering all available lines of evidence presented in this updated draft assessment, there is low risk of harm to the environment from NMP and NEP. It is proposed to conclude that NMP and NEP do not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
For the general population of Canada, the predominant source of exposure to NMP is from the use of products available to consumers that contain this substance. Dermal and inhalation routes of exposure may both contribute to potential NMP exposure. Estimates of potential exposure to NMP were derived for environmental media (air), use of adhesives, driveway sealer, and personal care products.
Estimates of exposure were not derived for NEP as no consumer uses or releases of NEP to the environment were identified. As exposure to NEP for the general population is not expected, the potential risk to human health is considered to be low.
NMP and NEP have been reviewed internationally by the US Environmental Protection Agency (US EPA) and the European Chemicals Agency (ECHA), respectively. Both NMP and NEP have been classified by ECHA as suspected reproductive toxicants (category 1B). A reproductive 1B classification also includes adverse affects to a conceptus, before or after birth.
In laboratory studies, NMP was identified as a reproductive and developmental toxicant. Margins between levels of exposure of NMP to the general population and the critical effect level were considered inadequate to address uncertainties in the health effects and exposure data from the intermittent use of deck construction adhesives. Margins for all other uses were considered adequate.
The human health assessment took into consideration those groups of individuals within the Canadian population who, due to greater susceptibility or greater exposure, may be more vulnerable to experiencing adverse health effects. Consideration of laboratory studies on rats suggests that pregnant people and the pre-natal life stage were found to be susceptible to developmental effects of NMP during acute exposures to NMP. People of reproductive age were found to be susceptible to reproductive effects (that is, reduced fertility or fecundity) during chronic exposure to NMP. Compared with adolescents and adults, one-year-old infants were found to have higher exposures to NMP from estimated NMP levels in air. In the assessment of exposure from products, exposure estimates were highest for teenage females. All of these populations were taken into consideration when addressing the potential harm to human health.
Considering all the information presented in this updated draft assessment, it is proposed to conclude that NMP meets the criteria under paragraph 64(c) of CEPA as it is entering or may enter the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health. Considering all the information presented in this updated draft assessment, it is proposed to conclude that NEP does not meet the criteria under paragraph 64(c) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore proposed to conclude that NMP meets one or more of the criteria set out in section 64 of CEPA. It is also proposed to conclude that NEP does not meet any of the criteria set out in section 64 of CEPA.
It is also proposed that NMP does not meet the persistence and bioaccumulation criteria as set out in the Persistence and Bioaccumulation Regulations of CEPA.
1. Introduction
Pursuant to section 68 of the Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999), the Minister of the Environment and the Minister of Health conducted an assessment of two substances, N-methyl-2-pyrrolidone (NMP) and N-ethyl-2-pyrrolidone (NEP) to determine whether these substances present or may present a risk to the environment or to human health. NMP was identified as a priority for assessment as it met categorization criteria as described in ECCC, HC (modified 2017). NEP did not meet categorization criteria; however, it was determined to be a priority as a result of the approach described for Identification of Risk Assessment Priorities (Environment Canada, Health Canada 2014; ECCC, HC 2015). Given the similarity in their structures, NEP could be used as a substitute for NMP in commercial products.
A draft screening assessment for NMP and NEP was published in February 2017 (ECCC, HC 2017). Significant new information subsequently became available regarding exposure to products available to consumers containing NMP and NEP. As a result, this draft screening was updated and is presented here. This updated draft assessment includes consideration of information on chemical properties, environmental fate, hazards, uses, and exposures, including additional information submitted by stakeholders. Relevant data were identified up to August 2021. Empirical data from key studies as well as results from models were used to reach proposed conclusions. When available and relevant, information presented in assessments from other jurisdictions was considered.
NMP and NEP have also been reviewed internationally. A risk assessment of NEP was conducted by the European Chemicals Agency (ECHA). In addition, NMP was identified in the Regulatory Cooperation Council’s Assessment Collaboration Framework - Canada-United States Rolling Workplan as a candidate for risk assessment work sharing (ECCC, HC [modified 2018]). NMP was also assessed by the US Environmental Protection Agency (US EPA) as part of the work plan of chemicals for further assessment under the Toxic Substances Control Act. These assessments undergo rigorous review and approval processes. Assessment activities conducted by ECHA and the US EPA have therefore been used to inform the health effects characterization in this assessment.
This updated draft assessment was prepared by staff in the CEPA Risk Assessment Program at Health Canada and Environment and Climate Change Canada and incorporates input from other programs within these departments. The ecological portion of this assessment is based on the ERC document (published July 30, 2016), which was subject to an external review as well as a 60-day public comment period. Additionally, the first draft of this screening assessment (published February 2017) was subject to a 60-day public comment period. While external comments were taken into consideration, the final content and outcome of this updated draft assessment remain the responsibility of Health Canada and Environment and Climate Change Canada.
Assessments focus on information critical to determining whether substances meet the criteria as set out in section 64 of CEPA by considering scientific information, including information, if available, on subpopulations who may have greater susceptibility or greater exposure, vulnerable environments and cumulative effectsFootnote 2 , and by incorporating a weight of evidence approach and precautionFootnote 3 . This updated draft assessment presents the critical information and considerations on which the proposed conclusions are based.
2. Identity of substances
The Chemical Abstracts Service Registry Numbers (CAS RN), Domestic Substances List (DSL) names, common names, and abbreviations for NMP and NEP are presented in Table 2‑1.
| CAS RN | Abbreviation | DSL name (common name) |
Chemical structure and molecular formula |
|---|---|---|---|
| 872-50-4 | NMP | 2-Pyrrolidinone, 1-methyl- (N-methylpyrrolidone) |
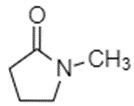 C5H9NO |
| 2687-91-4 | NEP | 2-Pyrrolidinone, 1-ethyl- (N-ethylpyrrolidone) |
 C6H11NO |
3. Physical and chemical properties
Summaries of physical and chemical property data for NMP and NEP are presented in Table 3‑1 and Table 3‑2. Additional physical and chemical properties are presented in ECCC (2016b).
| Property | Value | Type of data | Key reference(s) |
|---|---|---|---|
| Physical state | Liquid | Observed at 25°C | SDS 2021 |
| Melting point (°C) | -24 | Measured | ChemIDplus 1993 SDS 2021 |
| Vapour pressure (Pa) | 46 | Measured at 25°C | ChemIDplus 1993 |
| Henry’s law constant (Pa·m3/mol) | 3.2 × 10-4 | Measured at 25°C | ChemIDplus 1993 |
| Water solubility (mg/L) | 1 × 106 | Measured at 25°C | ChemIDplus 1993 |
| Log Kow (dimensionless) | -0.380 | Measured at 32°C | Sasaki et al. 1988 |
Abbreviation: KOW, octanol-water partition coefficient
| Property | Value | Type of data | Key reference |
|---|---|---|---|
| Physical state | Liquid | Observed at 20°C | ECHA 2021a |
| Melting point (°C) | -100 to -120 | Measured | ECHA 2021a |
| Vapour pressure (Pa) | 18 | Measured at 20°C | ECHA 2021a |
| Water solubility (mg/L) | 1 × 106 | Measured at 23°C | ECHA 2021a |
4. Sources and uses
NMP and NEP are anthropogenic substances that do not occur naturally in the environment.
NMP is miscible with water and most common organic solvents. The substance is primarily used as a solvent or formulating agent in agricultural chemicals, electronic cleaning, engineering plastics, industrial/domestic cleaning, paints and coatings, paint removers, and the petroleum industry, in both industrial and consumer products (IPCS 2001; US EPA 2020; ECHA 2021a). NMP is also used as an intermediate in the pharmaceutical industry (IPCS 2001). In cosmetics, NMP can function as a solvent, surfactant (SCCS 2011), or vehicle to enhance the dermal absorption of other cosmetic ingredients (IPCS 2001; SCCS 2011).
No consumer uses or releases of NEP to the environment in Canada were identified. NEP has previously been marketed as a substitute for NMP (ECHA 2011a; Silberzahn 2013; FMI 2015; BASF 2021). According to FMI (2015), while both substances may be used interchangeably due to their similar physical-chemical properties, scrutiny of NMP by regulatory bodies is expected to result in a reduced future demand for NEP from commercial manufacturers, distributers, and retailers (particularly for NMP uses being regulated or restricted).
According to Environment and Climate Change Canada’s National Pollutant Release Inventory (NPRI), 23 500 kg of NMP were reported to be released on-site to air and 300 kg released to “all media” from various Canadian companies during the 2019 reporting year (NPRI 2020). No occurrence data or records of NMP releases to water were identified. Release information for NEP was unavailable as NPRI reporting is not required for this substance.
According to information submitted in response to a CEPA section 71 survey, between 100 000 kg and 1 000 000 kg of NMP, and between 1 000 kg and 10 000 kg of NEP were reported to be imported into Canada in 2011 (Canada 2012). Neither NMP nor NEP were reported to be manufactured in Canada above the reporting threshold (Environment Canada 2013).Footnote 4
Uses identified for NMP and NEP in Canada for 2011 are based on consumer and commercial codes submitted in response to a CEPA section 71 survey (Canada 2012) and are summarized in Table 4‑1
| Use | NMP | NEP |
|---|---|---|
| Adhesives and sealants | Ya | Yb |
| Agricultural products | Yb | N |
| Automotive, aircraft, and transportation | Yb | N |
| Chemical manufacturing | Yb | Yb |
| Electrical and electronic products | Yb | N |
| Metal and mining products | Yb | N |
| Paints and coatings | Ya | Yb |
| Paper products, mixtures, or manufactured items | Yb | N |
| Plastic and rubber materials | Yb | Yb |
Source: Environment Canada (2013)
Abbreviations: Y = use was reported for this substance; N = use was not reported for this substance.
a Reported to have consumer uses
b Reported to have commercial uses only
NMP was also identified in other products intended for consumers, which are summarized in Table 4‑2. No information was received regarding additional uses in Canada for NEP
| Use | Details |
|---|---|
| Medicinal or non-medicinal ingredients in disinfectant, human, or veterinary drug productsa | Non-medicinal ingredient in antipsychotic, antineoplastic, or antibacterial agents |
| Notified to be present in cosmetics under the Cosmetic Regulationsb | Notified as present in certain nail care, cosmetic adhesives and adhesive removers, and hair products |
| Formulant in registered pest control productsc | Approved formulant in certain registered products |
a Personal communication, email from the Therapeutic Products Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated June 2021; unreferenced.
b Personal communication, email from the Consumer and Hazardous Products Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated September 2021; unreferenced.
c Personal communication, email from the Health Canada Pest Management Regulatory Agency, to the Existing Substances Risk Assessment Bureau, Health Canada, dated June 2021; unreferenced.
In Canada, while NMP has not been identified as having direct uses in the manufacture of food packaging applications, it may be present as a by-product of other components that are used in the manufacture of some food packaging materials (personal communication, email from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated June 2021; unreferenced).
The US EPA (2020) final report on NMP identified widespread uses of products available to consumers that have also been confirmed in Canada on the basis of publicly available safety data sheets (SDSs). These uses include adhesives and sealants (SDS 2015c, 2015d, 2019a, 2020b), automotive interior cleaning products (SDS 2015b), cleaning and degreasing products (SDS 2018a, 2020a), paints and coatings (SDS 2015a), and paint and coating removers (SDS 2018b, 2019b, 2019c, 2023).
Outside Canada, NEP has reported uses in adhesives and sealants, air care products (for example, air fresheners), anti-freeze products, coating products, inks and toners, leather treatment products, lubricants and greases, non-metal-surface treatment products, polishes and waxes, and washing and cleaning products available to consumers (ECHA 2021a). However, the availability of these products in Canada could not be confirmed.
5. Environmental fate and behaviour
5.1 Environmental persistence
According to models used in ERC (ECCC 2016b), NMP and NEP are not expected to persist in air, water, sediment, or soil.
5.2 Potential for bioaccumulation
Given their low log Kow and low bioconcentration factors (ECCC 2016b), NMP and NEP are not expected to significantly bioaccumulate in organisms.
6. Potential to cause ecological harm
6.1 Characterization of ecological risk
The ecological risks of NMP and NEP were characterized using the ecological risk classification of organic substances (ERC) approach (ECCC 2016a). The ERC is a risk-based approach that considers multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. The various lines of evidence are combined to discriminate between substances of lower or higher potency and lower or higher potential for exposure in various media. This approach reduces the overall uncertainty with risk characterization compared to an approach that relies on a single metric in a single medium (for example, median lethal concentration) for characterization. The following summarizes the approach, which is described in detail in ECCC (2016a).
Data on physical-chemical properties, fate (chemical half-lives in various media and biota, partition coefficients, and fish bioconcentration), acute fish ecotoxicity, and chemical import or manufacture volume in Canada were collected from the scientific literature, from available empirical databases (for example, OECD QSAR Toolbox 2014), from responses to surveys issued pursuant to section 71 of CEPA, or they were generated using selected (quantitative) structure-activity relationship ([Q]SAR) or mass-balance fate and bioaccumulation models. These data were used as inputs to other mass-balance models or to complete the substance hazard and exposure profiles.
Hazard profiles were based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Exposure profiles were also based on multiple metrics, including potential emission rate, overall persistence, and long-range transport potential. Hazard and exposure profiles were compared to decision criteria in order to classify the hazard and exposure potentials for each organic substance as low, moderate, or high. Additional rules were applied (for example, classification consistency, margin of exposure) to refine the preliminary classifications of hazard or exposure.
A risk matrix was used to assign a low, moderate, or high classification of potential risk for each substance on the basis of its hazard and exposure classifications. ERC classifications of potential risk were verified using a two-step approach. The first step adjusted the risk classification outcomes from moderate or high to low for substances that had a low estimated rate of emission to water after wastewater treatment, representing a low potential for exposure. The second step reviewed low risk potential classification outcomes using relatively conservative, local-scale (that is, in the area immediately surrounding a point source of discharge) risk scenarios, designed to be protective of the environment, to determine whether the classification of potential risk should be increased.
ERC uses a weighted approach to minimize the potential for both over- and under- classification of hazard and exposure, and of subsequent risk. The balanced approaches for dealing with uncertainties are described in greater detail in ECCC (2016a). The following describes two of the more substantial areas of uncertainty. Error with empirical or modelled acute toxicity values could result in changes in classification of hazard, particularly metrics relying on tissue residue values (that is, mode of toxic action), many of which are predicted values from (Q)SAR models (OECD QSAR Toolbox 2014). However, the impact of this error is mitigated by the fact that overestimation of median lethality will result in a conservative (protective) tissue residue value used for critical body residue analysis. Error with underestimation of acute toxicity will be mitigated through the use of other hazard metrics such as structural profiling of mode of action, reactivity, and/or estrogen binding affinity. Changes or errors in chemical quantity could result in differences in classification of exposure as the exposure and risk classifications are highly sensitive to emission rate and use quantity. The ERC classifications thus reflect exposure and risk in Canada on the basis of what is estimated to be the current use quantity and may not reflect future trends.
Critical data and considerations used to develop the substance-specific profiles for NMP and NEP as well as the hazard, exposure, and risk classification results are presented in ECCC (2016b).
On the basis of low hazard and low exposure classifications, according to information considered under ERC, NMP and NEP were classified as having a low potential for ecological risk. It is unlikely that these substances are resulting in concerns for the environment in Canada.
7. Potential to cause harm to human health
7.1 Exposure assessment
Potential exposures to NMP are based on measured and modelled concentrations of NMP in environmental media (air) and products available to consumers.
No reports of NEP concentrations measured in environmental media in Canada, or elsewhere, were identified. However, potential exposure to NEP is expected to be lower than exposure estimates for NMP as NEP import quantities into Canada are 10 to 1000 times lower in comparison to NMP import quantities (Environment Canada 2013). No information regarding NEP levels in products available to Canadian consumers was identified. NEP-containing cosmetics, including those identified in ECCC, HC (2017), are no longer available in Canada.
NMP and NEP were detected in a first trimester maternal urine study in Canada. Sampling occurred from 2008 to 2011. Detection rates are shown in Table 7‑1 (personal communication, email from the Environmental and Radiation Health Sciences Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated March 2022; unreferenced). Results of the Canadian biomonitoring study are preliminary; only the metabolite detection frequencies are currently available.
| Substance | Metabolite | Detection rate |
|---|---|---|
| NMP | 2-Hydroxy-N-methylsuccinimide | 92% |
| NMP | 5-Hydroxy-N-methyl-2-pyrrolidone | 81% |
| NEP | 2-Hydroxy-N-ethylsuccinimide | 46% |
| NEP | 5-hydroxy-N-ethyl-2-pyrrolidone | 42% |
7.1.1 Environmental media and food
Limited environmental monitoring data relevant to current exposures in Canada have been identified for NMP. NMP has been monitored in indoor air samples collected as part of the Canadian Health Measures Survey (CHMS) Cycle 2 (2009–2011), where concentrations ranged from less than 0.15 µg/m3 (detection limit in field blanks) to 1.18 μg/m3, with a detection frequency of 37% (personal communication, Zhu 2012 raw data; unreferenced; Patry-Parisien et al. 2013).
NMP was also included in a study by the National Research Council (NRC) of Canada on chemicals released to indoor air of residences from building materials and furnishings (Won and Lusztyk 2011). NMP releases were detected from only 2 of the 66 materials studied in chamber air at concentrations ranging from 0.84 µg/m3 to 1.54 µg/m3. The materials associated with NMP releases were carpet and rubber-based flooring. In the same report, concentrations of NMP in air and dust collected from homes in a Quebec City field study were also reported. Levels of NMP in air ranged from 0.39 µg/m3 to 6.26 μg/m3 (detection frequency of 9%), and NMP levels in dust ranged from 0.21 µg/g to 47.4 µg/g (detection frequency of 15%). Based on these levels, exposure to NMP from dust is expected to be negligible.
While no outdoor air monitoring has been identified in the literature, NMP has been reported to be released to air from point sources (NPRI 2020). Maximum NMP levels in ambient air near point source releases are estimated to be 1.07 µg/m3 over a one-year period using SCREEN3 (2013) dispersion exposure modelling (see Appendix A for input parameters). Maximum acute NMP levels in ambient air near point source releases using SCREEN3 modelling are estimated to be 5.33 µg/m3.
A measured indoor air level of 6.26 µg/m3 (Won and Lusztyk 2011) was chosen to estimate upper-bounding daily intake from air. One-year old infants had the greatest estimated exposure to NMP via inhalation (4.55 µg/kg bw/day). Exposure estimates for all age groups are presented in Appendix B.
No data are available on levels of NMP, if any, in food. In Canada, NMP has been identified as a possible by-product during the manufacture of certain food packaging materials, some of which may have direct contact with food. However, dietary exposure, if any, from the presence of NMP as a by-product in food packaging applications is expected to be very low (personal communication, email from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated June 2021; unreferenced).
7.1.2 Products available to consumers
NMP is used in a variety of products available to consumers that may result in exposure. A number of these products identified in Canada were also assessed in a report by the US EPA (2020), which was used to inform potential exposure to NMP since the use scenarios developed for American users were considered to be relevant and protective of Canadian users. NMP concentrations used to model exposure in US EPA (2020) were similar to, or exceeded, maximum NMP concentrations identified in Canadian products. The maximum NMP concentrations in products available to consumers and the estimated exposure to NMP from their use are shown in Table 7‑2.
| Product category | Maximum NMP concentration reported in Canada | Maximum NMP concentration used to model exposure in US EPA (2020) | Total exposure, Cmax (mg/L)a |
|---|---|---|---|
| Adhesives | >85% (SDS 2015d) | 85% | 18.63 |
| Automotive interior cleaners | <1% (SDS 2015d) | 5% | 0.26 |
| Engine cleaners and degreasers | 40% (SDS 2018a) | 40% | 1.68 |
| Paints and coatingsb | 10% (SDS 2015a) | 8.25% | 0.37 |
| Paint and coating removers | 55% (SDS 2018b, 2019b, 2019c, 2023) | 60% | 2.01 |
| Personal care products | 10%c | Not evaluated | Not evaluated |
| Sealants for use on eavestroughs and automotive seams | 1% (SDS 2019a, 2020b) |
0.8% | 0.07 |
| Sealants for use on residential driveways | 1% (SDS 2015c) | Not evaluated | Not evaluated |
a Systemic exposure for US consumers that considers the sum of potential exposure from dermal and inhalation routes of exposure. Exposure estimates are shown for the age group with the highest exposure (females aged 14 to 18 years old) (US EPA 2020). Cmax is the peak, or maximum, blood concentration of NMP.
b US EPA (2020) identified NMP in consumer paints and coatings at concentrations as great as 10%, but evaluated exposure using “an average high-end [NMP concentration] of 8.25%.”
c Canadian concentrations reported in a personal communication, email from the Consumer and Hazardous Products Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated September 2021; unreferenced.
The greatest potential exposure evaluated in US EPA (2020) that was relevant to Canadian consumers was from an adhesive product used in outdoor deck construction. The product (SDS 2015d) has an NMP concentration greater than 85% and is available to the general public in Canada and the US. Potential exposure was estimated using the Consumer Exposure Model (CEM 2019), and the results were subsequently integrated into a physiologically-based pharmacokinetic (PBPK) model to derive peak, or maximum, blood concentrations of NMP (denoted as Cmax). The PBPK model was based on a human model published by Poet et al. (2010 as cited in US EPA 2015) that was further assessed and validated by the US EPA. To note, the PBPK model was calibrated and validated based on several human toxicokinetic studies (Poet et al. 2010 as cited in US EPA 2015). The greatest estimated exposure to NMP from this use was for a female teenager. Estimated exposure was reported as a Cmax in the range of 4.08 mg/L to 18.63 mg/L for moderate and high intensity uses, respectively. High and medium intensity use can be described as using upper-bounding and central-tendency parameters, respectively. For the adhesive scenario, this includes adjustments to factors such as duration of use and mass of product used per event; both estimates used the maximum NMP concentration reported. For details on specific use scenario parameters, refer to US EPA (2020).
Products identified in Canada for sealing eavestroughs, automotive seams, and asphalt driveways (SDS 2015c, 2019a, 2020b) had NMP concentrations of 1% or less. The deck construction adhesive scenario is considered sufficient to address risk associated with uses involving eavestrough and automobile sealants as the product amounts used in these projects are similar, and the concentration of NMP is much lower compared to the adhesive product.
Use of driveway sealing products involves significantly larger amounts of product compared to sealants for eavestroughs or automotive repair. This use was not identified in the US EPA assessment. A use scenario for driveway sealing products was developed using the CEM on the basis of a modified use scenario for varnishes and floor finishes products. NMP exposure was estimated for a female teenager applying sealant to a 7.6 m x 12.2 m (25 foot x 40 foot) driveway with a water-based driveway sealant containing NMP at a concentration of 1% (SDS 2015c). Product amount required was 23.45 L per application (TDS 2021). Each application was simulated to last 2 hours. The first application was modelled using the CEM, and then the exposure estimate was scaled (doubled) outside of the CEM to simulate a 2-coat use scenario. To simulate exposure outdoors, the working environment was modelled to have a volume of 93 m3 (that is, a box with a base equal to the driveway surface area and 2 m in height) with an air exchange rate of 402 exchanges per hour (CEM default). This air exchange rate is equivalent to a 3 km per hour breeze across a driveway 7.5 m in width. The maximum NMP air concentration (calculated at 30-second intervals) was estimated to be 1.7 mg/m3. Total exposure to NMP (via dermal and inhalation routes) from use of this product is estimated to be 0.13 mg/kg bw/event for 16- to 20-year-old females. The input parameters used to estimate NMP exposure using the CEM can be found in Appendix C.
Based on notifications submitted to Health Canada under the Cosmetic Regulations, NMP may be present in adhesives for synthetic eyelashes and nails at concentrations of up to 10% and 3%, respectively, cosmetic adhesive removers (up to 1%), nail polish (up to 0.3%), and hair conditioner (up to 1%) (personal communication, email from the Consumer and Hazardous Products Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated September 2021; unreferenced). Nail polish and adhesives and adhesive removers intended for synthetic nails are products considered to result in intermittent or acute exposure to NMP.
Estimates of potential exposure for cosmetics were modelled using ConsExpo Web (2020) unless otherwise noted and are shown in Table 7‑3. Only estimated exposures for 14- to 18-year-old females are presented as this was the population with the highest estimated exposure. Input parameters for ConsExpo modelling are listed in Appendix C. Potential exposure to NMP from use of nail polishes was not estimated as NMP concentrations in nail adhesive products were an order of magnitude greater. Given the similarity in use patterns for the two products, exposure scenarios for nail adhesive products are considered to be representative and protective of potential exposure from use of nail polishes.
| Product scenarioa | Product concentrationb | Dermal exposure | Inhalation exposure | Total exposurec |
|---|---|---|---|---|
| Hair conditioner |
1% | 0.02 | 0.01 | 0.03 |
| Eyelash adhesived | 10% | 0.02 | Not determined | 0.02 |
| Nail adhesive |
3% | 0.15 | <0.001 | 0.15e |
| Nail adhesive remover | 1% | 1.0 | <0.001 | 1.0e |
a Exposure simulated using ConsExpo Web (2020) unless otherwise noted. Input parameters are listed in Appendix C.
b Personal communication, email from the Consumer and Hazardous Products Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated September 2021; unreferenced.
c Systemic exposure that considers the sum of potential exposure from dermal and inhalation routes of exposure. Exposure estimates are shown for the age group with the highest exposure (females aged 14 to 18 years old).
d Exposure estimated using product amount assumed to be 0.012 g (for both eyes), and frequency of use once per day (Lim et al. 2014 as cited in ECCC, HC 2019). Dermal absorption is assumed to be 100%. Therefore, inhalation exposure was not determined in order to avoid double counting total estimated exposure.
e Exposure estimate was reported as mg/kg bw/event as exposure to synthetic nail adhesive and remover products is considered to be intermittent.
7.1.3 Consideration of subpopulations who may have greater exposure
There are groups of individuals within the Canadian population who, due to greater exposure, may be more vulnerable to experiencing adverse health effects. Exposure estimates are routinely assessed by age to take into consideration physical and behavioural differences during different stages of life. In the assessment of exposure from environmental media (air), young children had higher exposure to NMP than adults. In the assessment of exposure from products, exposure estimates were highest for teenage females.
7.2 Health effects assessment
The US EPA (2020, 2022) and ECHA (2011a, 2021a) summarized the health effects information and characterized hazards related to NMP and NEP, respectively; therefore, these sources inform the health effects assessment for the respective substances, including the selection of critical effects and points of departure. A literature search was conducted for the period from November 2010 to June 2021 for NEP, and from March 2014 to June 2021 for NMP. No studies that could result in a different health effects characterization from those of the US EPA (2020) and ECHA (2021a) were identified.
Both NMP and NEP have been classified by ECHA as a suspected reproductive toxicant (category 1B) (ECHA 2021a, 2021b). Additionally, NMP is on the ECHA candidate list of substances of very high concern for authorization; the reason for its inclusion is toxic for reproduction (ECHA 2011b).
NMP
This section summarizes only the critical effects and corresponding points of departure identified by the US EPA (2020). The US EPA assessment summarizes other endpoints that were considered, where studies were available.
The US EPA (2020) selected reproductive and developmental toxicity (that is, reduced fertility and post-implantation loss) as the most appropriate critical effects for human hazard assessment and risk characterization. Reduced male fertility was identified as the most sensitive endpoint for chronic exposures; this was protective of reduced female fecundity as well. Evidence suggests that developmental effects, such as fetal resorptions and mortality, can occur after single exposures at a developmentally critical period (US EPA 1991; Van Raaij et al. 2003; Davis et al. 2009). Therefore, the US EPA (2020) specifically selected increased post-implantation loss (this includes fetal resorptions and fetal mortality) to assess risks from acute exposures and reduced male fertility to evaluate risks from chronic exposures.
Exxon (1991 as cited in US EPA 2020) was selected as the most representative study for chronic toxicity as it is a high quality, two-generation reproductive toxicity study. Male and female Sprague-Dawley rats were exposed to doses of 50, 160, or 500 mg/kg bw/day before mating, throughout gestation and lactation in the first generation, and during development and mating in the second generation. For developmental toxicity, the NOAEL of 160 mg/kg bw/day was based on significant decreases in offspring survival and growth rates, as well as an increase in stillborn pups at 500 mg/kg bw/day in both generations. For reproductive toxicity, the LOAEL of 50 mg/kg bw/day (lowest dose) was based on significantly decreased male fertility and female fecundity in the second generation, which were 18% to 28% and 18% to 20% relative to the controls, respectively. Reduced male fertility was measured as the most sensitive hazard endpoint and is protective of the other endpoints considered, such as reduced female fecundity, reduced fetal body weight, and stillbirths. Reproductive toxicity is further supported by effects seen in the second generation at 500 mg/kg bw/day. Specifically, a greater incidence of smaller testes and effects on the ovaries and uterus, including decreased numbers of corpora lutea and decreased implantation sites, were reported by Exxon (1991 as cited in US EPA 2020).
Of the high quality studies assessed by the US EPA (evaluation methods for data quality are determined according to US EPA [2018]) for acute toxicity scenarios, increased post-implantation loss was demonstrated by an oral developmental study (Saillenfait et al. 2002). In the oral study, rats (Sprague-Dawley) were administered NMP via gavage at 0, 125, 250, 500, or 750 mg/kg bw/day from gestational day (GD) 6 to 20 (Saillenfait et al. 2002). A developmental toxicity NOAEL was identified at 125 mg/kg bw/day based on statistically significant reduced fetal body weight at 250 mg/kg bw/day and higher in a dose-related manner (reduction of 10%, 30%, and 47% at 250, 500, and 750 mg/kg, respectively). In an earlier US EPA risk evaluation for NMP (US EPA 2015), the reduction of fetal body weight was previously considered to be a hazard endpoint for chronic toxicity as reduced fetal body weight is unlikely to occur following a single, acute exposure.
Fetal resorption and fetal malformations were observed in the same study at 500 mg/kg bw/day and 750 mg/kg bw/day in a dose-dependent manner. Although fetal resorptions and fetal malformations were considered statistically significant at 500 mg/kg bw/day and 750 mg/kg bw/day, increased mean percentage of dead fetuses per litter (fetal mortality), considered alone, was not statistically significant at any dose. However, when considering post-implantation loss, which is measured as the occurrence of increased fetal resorptions paired with increased fetal mortality in the original study, there is a statistically significant increase at 500 mg/kg bw/day and 750 mg/kg bw/day. Therefore, a NOAEL for post-implantation loss was identified at 250 mg/kg bw/day. A maternal toxicity NOAEL was also identified at 250 mg/kg bw/day based on significantly reduced food consumption and significantly reduced absolute body weight gain (subtracting gravid uterine weight) compared to controls at 500 mg/kg bw/day and 750 mg/kg bw/day (Saillenfait et al. 2002). It is plausible that fetal toxicity can occur without attribution to a secondary effect of maternal toxicity since there is toxicokinetic evidence for NMP to cross a placenta via oral or inhalation routes of exposure in pregnant rats (ECHA 2013; US EPA 2020).
Additionally, doses from an inhalation study similar in study design were used to increase the strength of dose-response modelling to derive a point of departure for acute exposure based on post-implantation loss observed in the oral Saillenfait et al. study in 2002 (Saillenfait et al. 2003). In the inhalation study, Sprague-Dawley rats were exposed (whole body) to NMP vapors at 0, 30, 60 or 120 ppm (approximately equivalent to 0, 122, 243 or 487 mg/m3), 6 hours per day, from GD 6 to 20 (Saillenfait et al. 2003). A developmental toxicity NOAEC was identified at 243 mg/m3 based on a statistically significant reduction of 5% to 6% fetal body weight compared to controls at 487 mg/m3. No significant decrease in overall weight gain during pregnancy was reported after the deduction of gravid uterine weight, although transient reduction in weight gain was reported to be significant at doses of 243 mg/m3 and 487 mg/m3 during the first half of exposure. Fetal resorption was not observed in this inhalation study. However, increased fetal resorption was observed in an earlier NMP inhalation study (exposure of 6 hours daily during pre-mating, mating, and gestation days 1 to 20) at the highest dose of 472 mg/m3; this finding was reported as biologically significant but not statistically significant (Dupont 1990 as cited in US EPA 2015). Doses from the oral and inhalation developmental toxicity studies can be combined based on PBPK-derived internal dose metrics to expand the range of doses in the dose-response model. This is possible due to a similar study design, exposure duration, and rat strain in the above oral and inhalation studies (Saillenfait et al. 2002, 2003; US EPA 2020).
NMP was identified as the proximate toxicant rather than its metabolites. The US EPA (2020) used a modified version of a validated PBPK model (Poet et al. 2010 as cited in US EPA 2015) to convert exposure concentrations of NMP identified in the above oral and inhalation animal studies to internal doses, and then applied benchmark dose (BMD) modelling to the internal doses to generate the appropriate point of departure (POD) for acute and chronic exposure scenarios. The POD internal dose is the benchmark dose lower confidence limit (BMDL), using a benchmark response (BMR) of 1% relative deviation for increased post-implantation losses for the acute exposure model and a BMR of 10% extra risk for the chronic exposure model (US EPA 2020).
The US EPA (2020) used PBPK modelling to convert the internal dose to an external rat oral equivalent dose. From this value, allometric scaling was used to convert this rat external dose to an external human equivalent dose (HED), which is applicable for all routes of exposure (with a conservative assumption of 100% absorption).
Table 7‑4 provides an overview of points of departure and human equivalent doses identified by the US EPA (2020, 2022) for both acute and chronic exposures.
| Scenario | Endpoint | Internal dose PODa (rat oral equivalent dose) | Human equivalent doseb | Reference for modelled data |
|---|---|---|---|---|
| Acute | Developmental: post-implantation loss (resorptions and fetal mortality) |
437 mg/L blood Cmax (418 mg/kg bw/day) |
105 mg/kg bw/dayc | Saillenfait et al. 2002, 2003 |
| Chronic | Reproductive: decreased male fertility |
183 hr-mg/L blood AUC (28 mg/kg bw/day) |
6.5 mg/kg bw/dayd | Exxon 1991 as cited in US EPA 2020 |
a Derived using the PBPK model. This single POD is designed to evaluate combined exposures via inhalation, dermal contact, and vapor through skin.
b Oral HED derived from rat oral equivalent dose using the rat PBPK model for a drinking water scenario (US EPA 2022).
c Allometric scaling done with the assumption that a female teen body weight is 65.9 kg to reflect a pregnancy scenario.
d Allometric scaling done with the assumption that an adult male body weight is 80 kg to reflect an adult exposure scenario.
NEP
ECHA (2011a) identified developmental toxicity as the critical effect for the risk characterization of NEP. NMP and NEP are structurally similar, with one methyl group difference on the side chain (that is, NMP has a methyl group, while NEP has an ethyl group attached to the heterocyclic nitrogen). The similarity of these two substances is also reflected in their activity in different toxicological studies. For example, both NMP and NEP produced negative results in several genotoxicity tests (for example, Ames test, in vitro mammalian gene mutation), while showing similar developmental effects (that is, increased fetal resorptions and reduced body weight) in rats via the oral route (ECHA 2011a; US EPA 2020). Based on these similarities, ECHA (2011a) considered health effects information for NMP to be applicable to the evaluation of NEP health effects for reproductive toxicant classification.
No consumer exposures were found for NEP; therefore, a hazard assessment is not warranted.
7.2.1 Consideration of subpopulations who may have greater susceptibility
There are groups of individuals within the Canadian population who, due to greater susceptibility, may be more vulnerable to experiencing adverse health effects. The potential for susceptibility during different life stages or by sex are considered on the basis of the available studies. In this assessment, developmental and reproductive studies were evaluated to assess the potential for susceptibility during these critical life stages (in utero and postnatal) and by sex. Laboratory studies in animals have found both males and females to be susceptible to chronic adverse health effects of NMP. A reproductive effect for males based on decreased fertility was used as the critical health effect to characterize risk to the general population from chronic exposure to NMP. This is protective of adverse effects seen in the same study, where female rats displayed reduced fecundity at a similar dose. A developmental effect based on post-implantation loss was used as the critical health effect to characterize risk from acute exposure to NMP. Overall, people of reproductive age and the pre-natal life stage were identified as the most susceptible populations for adverse health effects based on reproductive (reduced fertility) and developmental (post-implantation loss) endpoints used as the critical effects to characterize risk from exposure to NMP.
7.3 Characterization of risk to human health
Both NMP and NEP have been classified by ECHA as a suspected reproductive toxicant (category 1B) (ECHA 2021a, 2021b).
The critical effect of acute exposures to NMP is related to post-implantation loss in rats, which was considered significant with a NOAEL of 250 mg/kg bw/day. After using PBPK modelling to convert this to an internal dose and BMD modelling to select the BMR of 1%, a Cmax of 437 mg/L was identified as the acute POD. The corresponding external human equivalent dose was identified to be 105 mg/kg bw.
The critical effect of chronic exposures to NMP is related to reduced male fertility in a multi-generational rat study, which was identified at the lowest dose tested (LOAEL of 50 mg/kg bw/day). Following the application of the PBPK model and BMD modelling to identify a BMR of 10% extra risk, an internal dose area under the curve (AUC) of 183 hr-mg/L blood was selected as the chronic POD. This was protective of the internal dose AUC of 202 hr-mg/L blood associated with reduced female fecundity within the same study. The corresponding external human equivalent dose for the internal dose AUC of 183 hr-mg/L blood was identified to be 6.5 mg/kg bw/day.
A target margin of exposure (MOE) for NMP of 30 (3x interspecies extrapolation and 10x intraspecies variation) was identified by the US EPA (US EPA 2020) and is used in this assessment. The 3x uncertainty factor accounts for toxicodynamic differences between species, and the interspecies toxicokinetic differences were already accounted for in the PBPK modelling.
Potential exposure to NMP from environmental media was expected to occur via inhalation. On the basis of maximal measured NMP levels in air (6.26 µg/m3), the highest estimated intake via inhalation was 4.55 µg/kg bw/day for 1-year-old infants. In comparison to a chronic human equivalent dose of 6.5 mg/kg bw/day, the MOE is greater than 1400, and is considered adequate to address uncertainties in the health effects and exposure datasets used to characterize risk. Potential dietary exposure to NMP, if any, from food packaging applications is not expected to be significant.
NMP is a component found in a variety of products available to consumers in Canada, including automotive interior cleaners, engine cleaners and degreasers, paints and coatings, and paint and coating removers. The US EPA (2020) derived potential exposure scenarios to NMP from use of these products based on combined inhalation, dermal, and vapor-through-skin exposures of consumers. Exposure values (that is, applied doses) were converted to estimates of internal dose using PBPK modelling, and then an MOE was derived based on the ratio of the internal acute POD of 437 mg/L (blood) to the estimated peak exposure (Cmax, mg/L) for the sensitive life stage. Estimated MOEs for these uses as reported in US EPA (2020) are considered applicable to Canadian consumers and are considered adequate to address uncertainties in the health effects and exposure datasets used to characterize risk (summarized in Table 7‑5).
Certain consumer adhesive products used in deck construction with NMP concentrations greater than 85% were identified in US EPA (2020) and are also available in Canada (SDS 2015d). MOEs were derived by the US EPA for teenage females (the population with the highest estimated exposure) by comparing exposure to an internal acute POD of 437 mg/L. The resulting MOEs ranged from 23 (high-intensity use) to 107 (medium-intensity use). The MOE for the high-intensity use of this deck construction adhesive is considered inadequate to address uncertainties in the health effects and exposure datasets used to characterize risk for females of childbearing age.
Canadian consumers may be exposed to NMP when using certain driveway sealers containing this substance at a concentration of up to 1% (SDS 2015c). The resulting MOEs for females 16 years old, or older, are summarized in Table 7‑5 and are considered adequate to address uncertainties in the health effects and exposure data sets used to characterize risk for both age groups, with females aged 16 to 20 years old having the greatest estimated exposure.
Consumer exposure to NMP through the use of certain nail and hair care products, and cosmetic adhesives and adhesive removers was identified. MOEs for the age group with the highest potential exposure from use of these products (females aged 14 to 18 years) are shown in Table 7‑5, and all of them are considered adequate to address uncertainties in the health effects and exposure data used to characterize risk as these values will be protective of other populations.
| Exposure scenario | Total exposure estimatea |
Critical effect levelb |
MOEc |
|---|---|---|---|
| Adhesives for use in deck construction | 18.63 mg/L | 437 mg/L | 23 |
| Automotive interior cleaners | 0.26 mg/L | 437 mg/L | 1680 |
| Engine cleaners and degreasers | 1.68 mg/L | 437 mg/L | 260 |
| Paints and coatings | 0.37 mg/L | 437 mg/L | 1180 |
| Paint and coating removers | 2.01 mg/L | 437 mg/L | 217 |
| Sealants for use on eavestroughs and automotive seams | 0.07 mg/L | 437 mg/L | 6240 |
| Sealants for use on residential driveways | 0.13 mg/kg bw/eventd | 105 mg/kg bw/daye | 807 |
| Hair conditioner | 0.03 mg/kg bw/day | 6.5 mg/kg bw/dayf | 217 |
| Eyelash adhesive | 0.02 mg/kg bw/day | 6.5 mg/kg bw/dayf | 325 |
| Nail adhesive | 0.15 mg/kg bw/event | 105 mg/kg bw/daye | 700 |
| Nail adhesive remover | 1.0 mg/kg bw/event | 105 mg/kg bw/daye | 105 |
a Exposure estimates adopted from US EPA (2020) and reported as maximum NMP concentration estimated in blood (Cmax), except for driveway sealant (CEM 2019), hair conditioner, eyelash adhesive, and nail care products (ConsExpo Web 2020), which were derived by staff within the CMP. All exposure estimates shown are for the age group with the highest potential for exposure (females 14 to 18 years old) and include exposure from both inhalation and dermal routes where relevant.
b Critical effect level for increased post-implantation loss, including fetal resorptions and mortality observed in short-term (exposure during gestational days 6 to 20), oral and inhalation developmental toxicity studies (Saillenfait et al. 2002, 2003; US EPA 2022), unless otherwise noted.
c Target MOE of 30 was identified by the US EPA (3x interspecies extrapolation and 10x intraspecies variation) (US EPA 2020) and is used in this assessment. The 3x uncertainty factor accounts for toxicodynamic differences between species, and the interspecies toxicokinetic differences were already accounted for in the PBPK modelling.
d Exposure estimated using the CEM (2019). Input parameters available in Appendix C.
e Human equivalent dose derived from a rat PBPK model (Cmax = 437 mg/L). See US EPA (2022) for further discussion.
f Critical effect level for decreased male fertility observed in a two-generation, oral reproductive toxicity study (Exxon 1991 as cited in US EPA 2020) and reported as a human equivalent dose derived from a rat PBPK model (183 hr‑mg/L area under the curve) (US EPA 2022).
No NEP-containing products available to consumers in Canada were identified, and there is insufficient information (that is, no environmental release data, or Canadian environmental monitoring studies) to estimate exposure levels or determine associated MOEs to persons in Canada.
While exposure to the general population to NEP is not a concern at current levels, this substance is considered to have a health effect of concern based on reproductive toxicity. Therefore, there may be a concern if exposures were to increase.
7.4 Uncertainties in evaluation of risk to human health
Single exposures are being compared to effects seen in rats exposed from gestational days 6 through 20. There is uncertainty associated with this as it is not fully understood how the timing of the acute exposures can contribute to the critical effect of post-implantation loss. This uncertainty could lead to an overestimation of risk.
8. Conclusion
Considering all available lines of evidence presented in this updated draft assessment, there is low risk of harm to the environment from NMP and NEP. It is proposed to conclude that NMP and NEP do not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
Considering all the information presented in this updated draft assessment, it is proposed to conclude that NMP meets the criteria under paragraph 64(c) of CEPA as it is entering or may enter the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health. Considering all the information presented in this updated draft assessment, it is proposed to conclude that NEP does not meet the criteria under paragraph 64(c) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore proposed to conclude that NMP meets one or more of the criteria set out in section 64 of CEPA. It is also proposed to conclude that NEP does not meet any of the criteria set out in section 64 of CEPA.
It is also proposed that NMP does not meet the persistence and bioaccumulation criteria as set out in the Persistence and Bioaccumulation Regulations of CEPA.
References
[BASF] Badische Anilin und SodaFabrik. 2021. Badische Anilin und SodaFabrik product finder database; search results for CAS RN 2687-94-4. Ludwigshafen (DE): BASF (DE). [updated date unknown; accessed 2021 Aug 17].
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C. 1999, c.33.
Canada [Dept. of the Environment]. 2012. Canadian Environmental Protection Act, 1999: Notice with respect to certain substances on the Domestic Substances List [PDF]. Canada Gazette, Part I, vol. 146, no. 48, Supplement.
[CEM] Consumer Exposure Model [human exposure estimation model]. 2019. Ver. 2.1. Washington (DC): US EPA Office of Pollution Prevention and Toxics.
ChemIDplus [database]. 1993. Bethesda (MD): US National Library of Medicine. [accessed 2021 Aug 12].
ConsExpo Web [consumer exposure web model]. 2020. Ver. 1.0.7. Bilthoven (NL): Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment].
Curry P, Kramer G, Newhook R, Somers D, Tracy B, Oostdam J. 1993. Reference values for Canadian populations. Prepared by the Environmental Health Directorate Working Group on reference values. Health Canada. 1988 [updated in 1993].
Davis A, Gift JS, Woodall GM, Narotsky MG, Foureman GL. 2009. The role of developmental toxicity studies in acute exposure assessments: analysis of single-day vs. multiple-day exposure regimens. Regul Toxicol Pharmacol. 54(2):134-142.
[ECCC] Environment and Climate Change Canada. 2016a. Science approach document: ecological risk classification of organic substances. Ottawa (ON): Government of Canada.
[ECCC] Environment and Climate Change Canada. 2016b. Supporting documentation: data used to create substance-specific hazard and exposure profiles and assign risk classifications. Gatineau (QC): ECCC. Information in support of the science approach document: ecological risk classification of organic substances. Available from: substances@ec.gc.ca.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2015. Identification of risk assessment priorities: results of the 2015 review. Ottawa (ON): Government of Canada. [accessed 2021 Aug 11].
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2017. Draft screening assessment: 2-pyrrolidinone, 1-methyl- (NMP) and 2-pyrrolidinone, 1-ethyl- (NEP). Ottawa (ON): Government of Canada [accessed 2021 Aug 12].
[ECCC, HC] Environment and Climate Change Canada, Health Canada. [modified 2017 Mar 12]. Categorization of chemical substances. Ottawa (ON): Government of Canada. [accessed 2022 Mar 31].
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2017a. [modified 2018 Sep 7]. Appendix 1: Assessment Collaboration Framework - Canada-United States Rolling Workplan. Ottawa (ON): Government of Canada. [accessed 2022 Mar 31].
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2019. Screening assessment of the carboxylic acid anhydrides group. Ottawa (ON): Government of Canada [accessed 2021 Aug 25].
[ECHA] European Chemicals Agency. 2011a. Committee for Risk Assessment RAC: opinion proposing harmonised classification and labelling at community level of n-ethyl-2-pyrrolidone (NEP). Helsinki (FI): ECHA Committee for Risk Assessment. [adopted 2011 Nov 29; accessed 2021 Aug 17]. Report No. ECHA/RAC/ CLH-O-0000002192-83-01/F.
[ECHA] European Chemicals Agency. 2011b. Inclusion of substances of very high concern in the candidate list (Decision of the European Chemicals Agency). Helsinki (FI): Director of Regulatory Affairs. [validity 2011 Jun 20; accessed 2022 May 25]. Document No. ED/31/2011.
[ECHA] European Chemicals Agency. 2013. Annex XV restriction report. Proposal for a restriction: N‑methylpyrrolidone (NMP) [PDF]. [accessed 2022 Mar 28].
[ECHA] European Chemicals Agency [database]. 2021a. Brief profile: 1-methyl-2-pyrrolidone; CAS RN 872-50-4. Helsinki (FI): ECHA. [updated 2021 Dec 23; accessed 2021 Dec 29].
[ECHA] European Chemicals Agency [database]. 2021b. Brief profile: 1-ethylpyrrolidin-2-one; CAS RN 2687-91-4. Helsinki (FI): ECHA. [updated 2021 Dec 23; accessed 2021 Dec 29].
Environment Canada. 2013. DSL Inventory Update data collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain substances on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
Environment Canada, Health Canada. 2014. Approach for identification of chemicals and polymers as risk assessment priorities under Part 5 of the Canadian Environmental Protection Act, 1999 (CEPA 1999). Ottawa (ON): Government of Canada. [accessed 2021 Dec 29].
Ficheux AS, Chevillotte G, Wesolek N, Morriset T, Dornic N, Bernard A, Bertho A, Romanet A, Leroy L, Mercat AC, et al. 2016. Consumption of cosmetic products by the French population. Second part: amount data. Food Chem Toxicol. 90:130-141.
Ficheux AS, Morriset T, Chevillotte G, Postic C, Roudot AC. 2014. Probabilistic assessment of exposure to nail cosmetics in French consumers. Food Chem Toxicol. 66:36-43.
[FMI] Future Market Insights. 2015. N-ethyl-2-pyrrolidone market: global industry analysis and opportunity assessment 2015-2025. Dubai (AE): FMI [accessed 2021 Aug 17].
Health Canada. 1998. Exposure factors for assessing total daily intake of priority substances by the general population of Canada. Unpublished report. Ottawa (ON): Government of Canada.
Health Canada. 2015. Food Consumption Table derived from Statistics Canada’s 2004 Canadian Community Health Survey, Nutrition, Share file. Ottawa (ON): Government of Canada. [accessed 2021 Aug 25].
Health Canada. 2021. [modified Jun 25]. Canadian exposure factors used in human health risk assessments. Ottawa (ON): Government of Canada. [accessed 2021 Aug 25].
[IPCS] International Programme on Chemical Safety. 2001. Concise international chemical assessment document 35: N-Methyl-2-pyrrolidone. Geneva (CH): United Nations Environment Programme; International Labour Organization; World Health Organization. [accessed 2021 Aug 12]. On the cover: First draft prepared by Dr Bengt Åkesson, Department of Occupational & Environmental Health, University Hospital, Lund, Sweden.
Loretz LJ, Api AM, Babcock L, Barraj LM, Burdick J, Cater KC, Jarrett G, Mann S, Pan YHL, Re TA, et al. 2008. Exposure data for cosmetic products: facial cleanser, hair conditioner, and eye shadow. Food Chem Toxicol. 46(5):1516-1524.
[NPRI] National Pollutant Release Inventory. 2020. NPRI Data Search: NPRI datasets: NPRI-INRP_releasesrejets_1993-present [dataset; CSV]. Ottawa (ON): Government of Canada. Search results for CAS RN 872-50-4. [modified 2020 Oct 6; accessed 2021 Jul 6].
OECD QSAR Toolbox [read-across tool]. 2014. Version 3.3. Paris (FR): Organisation for Economic Co-operation and Development, Laboratory of Mathematical Chemistry.
Patry-Parisien J, Zhu J, Wong SL. 2013. Implementation of the indoor air component of cycle 2 of the Canadian Health Measures Survey (CHMS). Components of Statistics Canada Catalogue no. 82-003-X Health Reports.
Saillenfait AM, Gallissot F, Langonné I, Sabaté JP. 2002. Developmental toxicity of n-methyl-2-pyrrolidone administered orally to rats. Food Chem Toxicol. 40(11):1705-1712.
Saillenfait AM, Gallissot F, Morel G. 2003. Developmental toxicity of n-methyl-2-pyrrolidone in rats following inhalation exposure. Food Chem Toxicol. 41(4):583-588.
Sasaki H, Kojima M, Mori Y, Nakamura J, Shibasaki J. 1988. Enhancing effect of pyrrolidone derivatives on transdermal drug delivery. I. Int J Pharm. 44(1-3):15-24.
[SCCS] Scientific Committee on Consumer Safety. 2011. Opinion on n-methyl-2-pyrrolidone (NMP) [PDF]. Brussels (BE): SCCS, Public Health and Risk Assessment Directorate. 10th plenary meeting, 2011 Mar 22. [updated date unknown; accessed 2021 Aug 11].
SCREEN3 [computer model]. 2013. Ver. 3.5.0. Research Triangle Park (NC): US EPA Office of Air Quality Planning and Standards.
[SDS] Safety Data Sheet. 2015a. Nano Defence Premium Clear Floor Finish [PDF]. Concord (ON): Rust-Oleum Consumer Brands Canada. [updated 2015 Jul 8; accessed 2015 Jul 23].
[SDS] Safety Data Sheet. 2015b. Mothers Leather Cleaner [PDF]. Huntington Beach (CA): Mothers Polishes Waxes Cleaners. [updated 2015 May 24; accessed 2021 Jun 21].
[SDS] Safety Data Sheet. 2015c. Epoxy Driveway Filler Sealer [PDF]. Concord (ON): Rust-Oleum Consumer Brands Canada. [updated 2015 Jun 8; accessed 2015 Jun 23].
[SDS] Safety Data Sheet. 2015d. Azek Adhesive [PDF]. Scranton (PA): Azek Building Products. [updated 2015 Nov 20; accessed 2021 Jun 23].
[SDS] Safety Data Sheet. 2018a. Slide Resin Remover Aerosol [PDF]. Wheeling (IL): Slide Products Inc. [updated 2018 Aug 13; accessed 2021 Jun 11].
[SDS] Safety Data Sheet. 2018b. Blue Bear Brush and Spray Cleaner - Kleen Again [PDF]. Bloomington (IL): Franmar Chemical Inc. [updated 2018 Aug 2; accessed 2021 Jun 23].
[SDS] Safety Data Sheet. 2019a. Leak Seal Clear [PDF]. Vernon Hills (IL): Rust-Oleum Corporation. [updated 2019 Jul 2; accessed 2021 Jun 23].
[SDS] Safety Data Sheet. 2019b. CRC Graffiti Remover [PDF]. Toronto (ON): CRC Canada Co. [updated 2019 Aug 29; accessed 2021 Jun 11].
[SDS] Safety Data Sheet. 2019c. 690PB Lead Paint Remover [PDF]. Bloomington (IL): Franmar Chemical Inc. [updated 2019 May 21; accessed 2021 Jun 11].
[SDS] Safety Data Sheet. 2020a. Gunk Hydroseal II Heavy Duty Parts Cleaner [PDF]. Indian Trail (NC): Blumenthal Brands Integrated, LLC. [updated 2020 Mar 25; accessed 2021 Jun 11].
[SDS] Safety Data Sheet. 2020b. 3M MSP Seam Sealer Gray. London (ON): 3M Canada Company. [updated 2020 Oct 16; accessed 2021 Jun 15].
[SDS] Safety Data Sheet. 2021. 1-Methyl-2-pyrrolidinone [PDF]. Oakville (ON): MilliporeSigma Canada Ltd. [accessed 12 Aug 21].
[SDS] Safety Data Sheet. 2023. Graffiti Remover 20-47 [PDF]. Windsor (ON): Seymour of Sycamore. [updated 2023 Jan 9; accessed 2023 Apr 6].
Silberzahn J. 2013. Is NMP still needed in coating removers? JOT Int Surf Technol. 6(2):24-25.
[TDS] Technical Data Sheet. 2021. Epoxy Driveway Resurfacer and Sealer [PDF]. Concord (ON): Rust-Oleum Consumer Brands Canada. [updated date unknown, revision No. 032513; accessed 2021 Jun 23].
[US EPA] US Environmental Protection Agency. 1991. Guidelines for developmental toxicity risk assessment. Washington (DC): US EPA Office of Research and Development, Risk Assessment Forum. [updated 1991 Dec 5; accessed 2021 Aug 11]. Report No. EPA/600/FR-91/001.
[US EPA] US Environmental Protection Agency. 1992. Screening procedures for estimating the air quality impact of stationary sources. Research Triangle Park (NC): US EPA, Office of Air and Radiation, Office of Air Quality Planning and Standards. EPA-454/R-92-019.
[US EPA] US Environmental Protection Agency. 1999. Estimates of stack heights and exit gas velocities for TRI facilities in OPPT’s Risk-Screening Environmental Indicators Model. Washington (DC): US EPA, Office of Pollution Prevention and Toxics.
[US EPA] US Environmental Protection Agency. 2011. Chapter 6: inhalation rates. Exposure factors handbook 2011 edition (final). Washington (DC): US EPA, Office of Research and Development. [accessed 2021 Dec 30]. Report No. EPA/600/R-09/052F.
[US EPA] US Environmental Protection Agency. 2015. TSCA work plan chemical risk assessment n-methylpyrrolidone: paint stripper use CASRN: 872-50-4 [PDF]. Washington (DC): US EPA, Office of Chemical Safety and Pollution Prevention. [updated 2015 Mar 23; accessed 2021 Aug 17].
[US EPA] US Environmental Protection Agency. 2018. Application of systematic review in TSCA risk evaluations [PDF]. Washington (DC): US EPA, Office of Chemical Safety and Pollution Prevention. [updated 2018 May 31; accessed 2022 May 25].
[US EPA] US Environmental Protection Agency. 2020. Risk evaluation for n-methylpyrrolidone (2-pyrrolidinone, 1-methyl-) (NMP) [PDF]. Washington (DC): US EPA, Office of Chemical Safety and Pollution Prevention. [updated 2020 Dec; accessed 2021 Jun 1]. Report No. EPA-740-R1-8009.
[US EPA] US Environmental Protection Agency. 2022. Draft TSCA screening level approach for assessing ambient air and water exposures to fenceline communities version 1.0 [PDF]. [updated 2022 Jan; accessed 2022 Feb 3]
Van Raaij MTM, Janssen PAH, Piersma AH. 2003. The relevance of developmental toxicity endpoints for acute limit setting. Bilthoven (NL): Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment]. [updated 2003 Jun 5; accessed 2021 Aug 11]. RIVM Report 601900004.
Won D, Lusztyk E. 2011. Chemicals Management Plan Health Canada moderate priorities: data gathering on chemicals released to indoor air of residences from building materials and furnishings. Ottawa (ON): Health Canada. Report No. B3332.2 [issued 2011 Mar].
Wu XM, Bennett DH, Ritz B, Cassady DL, Lee K, Hertz-Picciotto I. 2010. Usage pattern of personal care products in California households. Food Chem Toxicol. 48(11):3109-3119.
Appendix A. Input parameters to SCREEN3 for dispersion modelling and estimated NMP concentrations in the vicinity of industrial point source releases
| Input parameter | Parameter value |
|---|---|
| Source type | Point |
| Emission ratea (g/s) | 0.603 |
| Source release heightb (m) | 15 |
| Stack inside diameterb (m) | 3 |
| Stack gas exit velocityc (m/s) | 4 |
| Emission temperatured (K) | 1273 |
| Ambient air temperatured (K) | 293 |
| Receptor heighte (m) | 2 |
| Urban-rural optionb | Urban |
| Consider building downwash | No |
| Consider terrain above stack height | No |
| Consider terrain above stack base | No |
| Meteorologyd | Full |
| Minimum and maximum distance to useb (m) | 500 (minimum), 1800 (maximum) |
| Adjustment factor for annual exposuref | 0.2 |
a Based on on-site releases to air for 2019 (NPRI 2020) and assumed to operate 16.5 h/day, 6 days/week, 50 weeks/year.
b Estimated from Google maps.
c Professional judgement; using the median exit gas velocity reported in US EPA (1999) resulting in the greatest release estimate.
d Default value in SCREEN3.
e Curry et al (1993). Representative of the breathing zone of an adult.
f An adjustment factor of 0.2 is used to estimate maximum NMP concentration over a one-year period, based on the resultant SCREEN3 output (which is an estimate for a 1-hour period). This factor takes into account temporal variations in wind and meteorological conditions (US EPA 1992 [modified]).
Appendix B. Estimates of daily intake of NMP via inhalation by various age groups
| - | 6 to 11 monthsa | 1 yearb | 2 to 3 yearsc | 4 to 8 yearsd | 9 to 13 yearse | 14 to 18 yearsf | 19+ yearsg |
|---|---|---|---|---|---|---|---|
| Total intakeh | 3.71 | 4.55 | 3.84 | 3.02 | 2.07 | 1.60 | 1.28 |
a Assumed to weigh 9.1 kg (Health Canada 2015), and to breathe 5.4 m3 of air per day (US EPA 2011 [modified]).
b Assumed to weigh 11 kg (Health Canada 2015), and to breathe 8.0 m3 of air per day (US EPA 2011 [modified]).
c Assumed to weigh 15 kg (Health Canada 2015), and to breathe 9.2 m3 of air per day (US EPA 2011 [modified]).
d Assumed to weigh 23 kg (Health Canada 2015), and to breathe 11.1 m3 of air per day (US EPA 2011 [modified]).
e Assumed to weigh 42 kg (Health Canada 2015), and to breathe 13.9 m3 of air per day (US EPA 2011 [modified]).
f Assumed to weigh 62 kg (Health Canada 2015), and to breathe 15.9 m3 of air per day (US EPA 2011 [modified]).
g Assumed to weigh 74 kg (Health Canada 2015), and to breathe 15.1 m3 of air per day (US EPA 2011 [modified]).
h Won and Lusztyk (2011) measured NMP in indoor air samples in Canada. An NMP concentration of 6.26 µg/m3 was used to estimate potential NMP exposure from ambient and indoor air. Canadians are expected to spend 3 hours outdoors and 21 hours indoors per day (Health Canada 1998).
Appendix C. Parameters to estimate human exposure to products available to consumers in Canada
NMP exposure to humans from the use of driveway sealing products was estimated using CEM (2019). Parameters used to estimate dermal and inhalation exposure are listed in Table C-1. Consumer exposure to NMP from the use of certain personal care products was estimated using ConsExpo Web (2020). Parameters used to estimate dermal and inhalation exposure are listed in Table C-2.
| Location required input value | Parameter | Approach description |
|---|---|---|
| Chemical of interest | N-methyl-2-pyrrolidone | Not applicable |
| CAS RN | 872-50-4 | Not applicable |
| CEM (2019) model scenario | Varnishes and floor finishes | Chosen because the driveway sealer is applied similarly to a floor finish; manufacturer recommends product be applied directly from pail with a roller |
| CEM model scenario - dermal | Permeability | Not applicable |
| Product user(s) | Females of reproductive age: 16 to 20 years old and 21+ years olda | Not applicable |
| Activity pattern | Not applicable | User stays at home the entire day |
| Product/article environment of use | Simulate an outside scenario | Not applicable |
| Scenario | Weight fraction of chemical (-) | Single: 0.01 (SDS 2015c) |
| Scenario | Background air concentration (mg/m3) | 0 |
| Select pathways | Inhalation and dermal | Not applicable |
| Modelling options (user-defined emission rates, near-field zone, dermal absorption, or permeability) | E2, Let CEM estimate emission rate, P_INH2, P-DER2b | Used near-field scenario model user exposure from product use |
| Chemical properties | Vapour pressure (torr) | 0.345 |
| Chemical properties | Molecular weight (g/mol) | 99.1 |
| Chemical properties | Saturation concentration in air (mg/m3) | 1.84E+03 |
| Log octanol-water partition coefficient | Not applicable | -0.38 |
| Water solubility | mg/mL | 1.0E+03 |
| Henry’s Law coefficient | atm/M | 3.2E-09 |
| Gas phase mass transfer coefficient | m/hr | 3.04, set to same value as outdoor adhesive scenario in US EPA (2020) |
| Emission factor | µg/m2/hr | 1 600 000, CEM default |
| Product/article properties | Density of product/article (g/cm3) | 1.29 (SDS 2015c) |
| Product/article properties | Product dilution fraction (-) | Fixed at 1 (that is, no dilution) |
| Product/article properties | Frequency of use - acute (events/day) | Parameter fixed at 1 (and scaled outside CEM to simulate 2 coats applied on same day) |
| Product/article properties | Duration of use - acute (min) | 120 |
| Product/article properties | Mass of product used - acute (g/use) | 23 450, reported coverage is 450–650 sq ft per 10 L. Assumed practical coverage is 550 sq ft. Sources: coverage (TDS 2021); density (SDS 2015c) |
| Aerosol fraction | Not applicable | Not applicable |
| Fraction product ingested | Not applicable | Not applicable |
| Product/article properties | Skin permeability coefficient (cm/hr) | 0.000239, CEM estimate |
| Environment inputs | Building volume (m3) | Not applicable |
| Environment inputs | Use environment volume (m3) | 186, equal to a box representing 2 m in height above a 93 m2 driveway |
| Environment inputs | Air exchange rate, zone 1 (per hour) | 100, to simulate an outdoor use scenario |
| Environment inputs | Air exchange rate, zone 2 (per hour) | Not applicable |
| Environment inputs | Interzone ventilation rate (m3/hr) | 0, to simulate an outdoor use scenario |
| Air exchange rate, near-field boundary | hr-1 | 100, to simulate an outdoor use scenario |
| Near field-Far field volume | m3 | 1 |
| Body weight | 74 kg (females 21+ years old) and 65.9 kg (females 16 to 20 years old) |
US statistics were modelled to allow a comparison with their exposure modelling of adhesives used outside. |
| Inhalation rate during use | 0.67 m3/hr (both age groups) | Not applicable |
| Inhalation rate after use | 0.635 m3/hr (21+ years old) and 0.57 m3/hr (16 to 20 years old) | Not applicable |
| SA-BW Ratio | 6.01 cm2/kg (21+ years old) and 6.30 cm2/kg (16 to 20 years old) |
Not applicable |
| Use Start Time | 9:00 AM | Not applicable |
a Age groups were selected to be consistent with those used by the US EPA.
| Parameter | Value |
|---|---|
| Substance name | N-methyl-2-pyrrolidone |
| CAS RN | 872-50-4 |
| Molecular weight (g/mol) | 99.1 |
| Log Kow | -0.38 |
| Populationa | Varies |
| Body weighta (kg) | Varies |
| Frequencyb (per day) | Varies |
| Product amount (g) | Variesc |
| Weight fraction substance | 0.01 (hair conditioner) 0.03 (nail adhesive) 0.01 (nail adhesive remover) |
| Inhalation exposure model | Exposure to vapour - evaporation |
| Exposure duration (hour) | Variesd |
| Product in pure form | No |
| The product is used in dilution | No |
| Room volume (m³) | 10 (hair conditioner) 1 (nail care products) |
| Ventilation rate (per hour) | 2 |
| Inhalation rate a (m³/day) | Variesa |
| Limit concentration to saturated air concentration | No |
| Inhalation absorption model | Yes |
| Absorption fraction | 1 |
| Dermal exposure model | Direct contact - instant application |
| Exposed area (cm²) | Variesa, e |
| Dermal absorption model | Yes |
| Absorption fraction | 1 |
Exposure modelling software: ConsExpo Web (2020)
Abbreviation: KOW, octanol-water partition coefficient
a Females 16 to 20 years old assumed to weigh 65.9 kg and have an inhalation rate of 16.08 m3/day; females 21 years and older assumed to weigh 74 kg and have an inhalation rate of 16.08 m3/day to allow for comparison between CEPA-derived and US EPA-derived risk estimates (US EPA 2020).
b Mean or median frequency for hair conditioner, 14 to 18 years: 0.7 times per day (Wu et al. 2010); 19 years old and older: 1.1 times per day (Loretz et al. 2008). Cosmetic adhesive and adhesive remover scenarios were evaluated per event.
c Hair conditioner product amount is 10 g for 14 to 18 years old (Ficheux et al. 2016), 13.1 g for 19 years old and older (Loretz et al. 2008). Nail adhesive product amount is 0.33 g/event; nail adhesive remover is 6.59 g/event (Ficheux et al. 2014).
d Hair conditioner is a “wash-off” product. Exposure is assumed to be 24 hours per day with a retention factor of 0.01 applied outside of ConsExpo Web modelling to account for exposure occurring after washing the product off. Duration is 24 hours for users 14 to 18 years old; 21.81 hours for users 19 years old and older. Nail adhesive duration is 18 minutes (application time until adhesive has cured); nail adhesive remover duration is 30 minutes.
e Hair conditioner modelled using a surface area equal to the combined surface areas of half the surface area of the head and half the surface area of two hands (Health Canada 2021). Surface area for nail care products was chosen from Ficheux et al. (2014).
Page details
- Date modified:
