Archived Consultation Summary: Notice of Intent – Potential Measures to Reduce the Impact of Vaping Products Advertising on Youth and Non-users of Tobacco Products
Table of contents
- Executive summary
- Introduction
- Who responded?
- What we heard
Executive summary
On February 5, 2019, Health Canada released a consultation paper entitled "Notice of Intent - Potential Measures to Reduce the Impact of Vaping Products Advertising on Youth and Non-users of Tobacco Products" (NOI) to seek feedback on selected regulatory measures under consideration. The NOI included measures to restrict the placement and the content of advertisements and other forms of promotions, such as prohibiting the display of vaping products at retail where youth have access.
A total of 321 submissions were received from a wide variety of respondents. Respondents were categorized under the following groups: general public; academia; associations of health professionals; consumer associations; local and regional health authorities; non-governmental organizations (NGOs); municipalities; provincial and territorial governments; manufacturers; retailer associations; vaping industry associations and vape shop owners.
The majority of the respondents supported restrictions on the promotion of vaping products. NGOs, associations of health professionals, local and regional health authorities, municipalities and the general public called for stricter regulations similar to those for tobacco products.
Comments from persons who formerly smoked described how vaping products helped them quit smoking and improved their health. They commented that Health Canada's proposed approach reinforces the misconception that vaping products are as harmful as tobacco products.
In a joint submission, all thirteen provincial and territorial governments strongly recommended immediate regulatory measures to reduce youth uptake of vaping products. They suggested that all forms of vaping advertising and promotion restrictions should align with those in place for tobacco products.
Responses from the vaping industry were divergent. Vape shop owners and vaping industry associations supported the proposed restrictions on advertising and on the display of vaping products at points of sale where youth have access. Retailer associations and larger manufacturers (multinational manufacturers that manufacture both tobacco and vaping products), were against these proposals. In general, the vape shop owners, vaping industry associations and vaping manufacturers were supportive of the proposed restrictions on the placement of advertisements in public places, broadcast media and in publications.
With regard to the regulatory measures under consideration concerning the content of advertising, most respondents were supportive of measures that would require advertisements to carry a health warning about nicotine addiction. However, industry respondents did not support the other proposed health warning about the chemicals released by vaping products.
Academia commented that the measures under consideration should aim to minimize the potential harms to youth while ensuring that these products remain attractive to persons who smoke.
Comments received through this consultation will be carefully considered as Health Canada continues to work on new regulations to restrict the promotion of vaping products.
Introduction
The consultation paper entitled "Notice of Intent - Potential Measures to Reduce the Impact of Vaping Products Advertising on Youth and Non-users of Tobacco Products" was published on the Government of Canada's website on February 5, 2019, providing a 45-day-comment period that closed on March 22, 2019.
The Notice of Intent (NOI) described regulatory measures under consideration to help reduce the impact of vaping product advertising on youth and non-users of tobacco products. Such measures, if adopted, would be made as regulations under the authority of the Tobacco and Vaping Products Act (TVPA). The NOI sought comments from interested parties and the general public in three main areas, namely:
- Restrictions on the placement of advertisements in various locations, namely
- at point of sale where youth have access
- in public places
- in broadcast media
- in publications
- Restrictions on the content of advertisements by
- requiring the display of a health warning
- restricting its visual content
C) Restrictions on other forms of retail promotions, such as the display of vaping products.
Who responded?
A total of 321 submissions were received. Respondents were categorized under the following groups: general public; academia; associations of health professionals; consumer associations; local and regional health authorities; non-governmental organizations (NGOs); manufacturers; municipalities; provincial and territorial governments; manufacturers; retailer associations; vaping industry associations and vape shop owners. Retailer associations represent convenience stores owners. The majority of the submissions, 187 comments, were from the general public. The bar chart below shows the breakdown of the number of submissions by group:
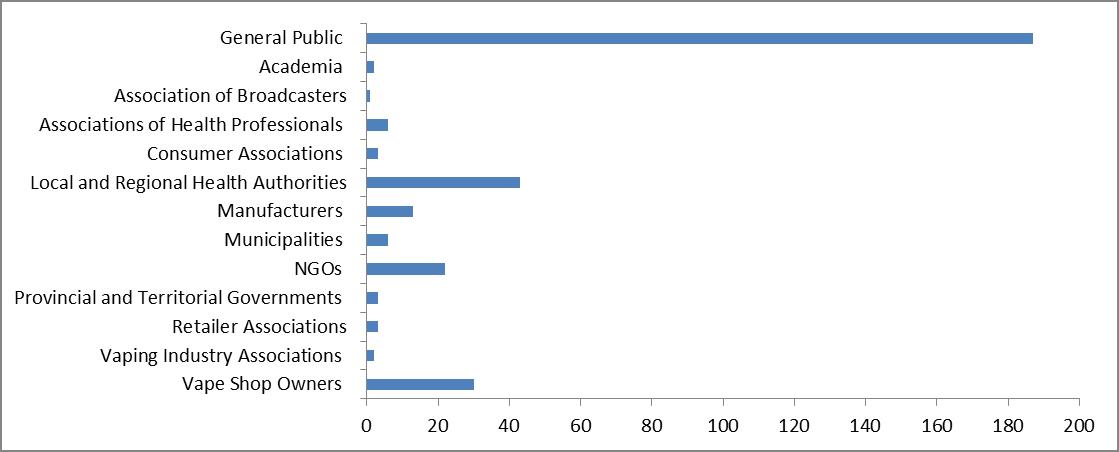
The breakdown of the number of submissions by group - Text equivalent
| Respondent group | Number of submissions |
|---|---|
| Vape Shop Owners | 30 |
| Vaping Industry Associations | 2 |
| Retailer Associations | 3 |
| Provincial and Territorial Governments | 3 |
| NGOs | 22 |
| Municipalities | 6 |
| Manufacturers | 13 |
| Local and Regional Health Authorities | 43 |
| Consumer Associations | 3 |
| Associations of Health Professionals | 6 |
| Association of Broadcasters | 1 |
| Academia | 2 |
| General Public | 187 |
| Total | 321 |
What we heard
This document first highlights the general comments on the proposal to restrict vaping product promotions. It then describes the specific comments on each proposed regulatory measure set out in the NOI.
General comments on the proposed regulatory measures
The majority of the respondents supported further restrictions on the promotion of vaping products. NGOs, associations of health professionals, local and regional health authorities, provincial and territorial governments, municipalities and, the general public, called for stricter regulations similar to those for tobacco products. Most NGOs called for a comprehensive prohibition of advertising in public places, in broadcast media, in publications including on the internet and at point of sale where youth have access. They suggested that there should be a general prohibition on advertising in the legislation with exceptions for brand preference advertising and brand information advertising in publications that are addressed and sent to an adult who is identified by name or in signs in a place where young persons are not permitted by law. They also commented that the regulatory process is too lengthy and therefore inappropriate to deal with the urgent issue of youth uptake of vaping products and suggested that legislative amendment of the TVPA would be a faster route. NGOs referred to the challenges encountered in the past with regards to partial bans on tobacco products advertising and suggested that a comprehensive ban on advertising is the most appropriate way to protect youth from the inducements to use vaping products.
Among the general public, persons who formerly smoked described how vaping products helped them quit smoking and improved their health. They commented that Health Canada's proposed approach reinforces the misconception that vaping products are as harmful as tobacco products if Health Canada pursues regulatory actions that align with those for tobacco products. Others mentioned that the current regulatory regime for vaping product promotions is fine and requires no change. One respondent suggested that the Government of Canada is not considering the role of promotion being an integral part of a harm reduction strategy in allowing vital information on vaping products to be communicated to persons who smoke and to prevent youth from smoking. One submission pointed out that the overwhelming cause of nicotine or tobacco-related harm is from smoking and that policy makers should consider the interactions between smoking and vaping in both adults and adolescents. The respondent concluded that excessive risk-averse policies towards vaping may trigger unintended consequences that could harm both adults and adolescents by obstructing the migration of those who currently smoke to vaping.
In a joint submission, all thirteen provincial and territorial governments strongly supported immediate regulatory measures to reduce youth uptake of vaping products. They suggested that all forms of advertising and promotion restrictions should align with those in place for tobacco products. One province specifically suggested that Health Canada continue to collaborate with provincial governments on the warnings regarding the health hazards of using vaping products so that the messaging balances the use of vaping products as a cessation tool for tobacco users with prevention and protection measures for youth and non-users of tobacco products.
The respondents from academia suggested that the regulations on the advertising of vaping products should aim to minimize the potential harms to youth, while ensuring that these products remain attractive to persons who smoke. One academic proposed that Health Canada implement and strongly enforce a comprehensive ban on the advertising of vaping products in media and locations where youth and young people have access and are likely to be exposed to the marketing of these products. The academic also called for dedicated funding for research and monitoring of peer-reviewed literature to better understand the impact of advertising of vaping products for promoting the potential cessation and harm reduction benefits of these products to persons who smoke.
Consumer associations voiced their concerns that the proposals would curtail the rights of consumers, especially to those who smoke. They argued that vaping products, which are a less harmful product than cigarettes, are relatively new and therefore persons who smoke should have access to information on them. They were skeptical that advertising and display of vaping products were the cause of youth uptake of these products.
The association of broadcasters suggested that Health Canada issue guidelines that would provide industry with information on how to assess advertising with respect to the prohibitions in the TVPA. These guidelines would be based on criteria that evaluated whether advertisements are appealing to youth, contain lifestyle elements as per the definition in the TVPA, use testimonials or endorsements or include descriptions of flavours that would be considered appealing to young people. The guidelines could include supplementary criteria consistent with and adapted from other legislation that restricts advertising to children such as those required by the Alcohol Code or in the Quebec children's advertising framework. The guidelines should clarify that while specific legislative provisions must be considered individually, supplementary criteria are to be considered collectively and in context. A pre-clearance system administered by an advertising self-regulatory body was proposed to ensure compliance with the rules, and was suggested that it would be a more effective approach than enacting regulations.
Other suggestions
Numerous respondents provided suggestions and comments on other possible measures to reduce youth uptake of vaping products (setting a limit on nicotine concentration, restrictions on flavours, vaping product design, further restrictions on access to vaping products, etc.). Those comments will be included in the summary of a separate consultation, launched by Health Canada on April 11, 2019 with the release of a document entitled Reducing Youth Access and Appeal of Vaping Products: Potential Regulatory Measures.
Specific comments on the proposed regulatory measures
A: Placement of advertisements
A1: Point of sale
Proposal: Vaping product advertisements would not be permitted at any point of sale where youth is allowed access, including online. However, signs that indicate the availability and price of vaping products could be displayed under certain conditions. As well, catalogues or pamphlets that provide information on the brands of vaping products available would be allowed at any point of sale, provided that they are not publicly displayed and are only made available to an adult customer upon request. These restrictions would not apply at points of sale where youth do not have access (e.g. a vape shop that does not allow youth on its premises or on its website), as long as the advertising material cannot be seen from the outside of these places
With respect to advertising at points of sale where youth have access, vape shop owners and vaping industry associations were supportive of the proposed restrictions. They mentioned that several provinces have already implemented restrictions on advertising at retail where youth have access. However, one vaping industry association commented that a complete prohibition on advertising in public places would have a negative impact on persons who smoke.
Most of the larger manufacturers (multinational manufacturers that manufacture both tobacco and vaping products) and all of the retailer associations were against the proposed advertising restrictions at point of sale. They stated that the proposed restrictions would limit considerably the communication of the availability of vaping products as a less harmful alternative to persons who smoke. One retailer association suggested that if advertisements were allowed in the area where tobacco products are sold at points of sale where youth have access, they would be supportive of such regulations. They felt strongly that the same restrictions on the placement of advertisements should equally apply to vape shops where youth do not have access since not doing so puts other retailers at a competitive disadvantage.
With respect to websites where vaping products are sold, manufacturers suggested that the proposed regulations should clearly indicate the criteria for determining whether youth do not have reasonable access that would allow them to place advertisements. One manufacturer asked whether an age-gate verification, as currently used on websites for online retailers, would be sufficient or an age-gate in combination with third party age verification would be required in the proposed regulations. However, the manufacturer mentioned that third party age-verification to access a website may have the unintended consequence of preventing customers who smoke from switching to vaping products.
A2: Public places
Proposal: Vaping product advertisements (e.g. signs) would not be permitted in certain public places where youth have access such as shopping malls; recreation, arts and cultural facilities; parks; in public transit vehicles and stations; billboards and other outdoor physical supports for commercial advertising.
In general, most respondents were supportive of the proposal to restrict advertisements in public places or did not express any opposition to the proposal. Several vape shop owners proposed that broad, national brand-specific advertising campaigns in public venues should be prohibited except in age-restricted locations. One manufacturer suggested that all outdoor advertising be prohibited within 500 feet of any schools, youth-oriented facilities and childcare facilities. However, one manufacturer commented that if advertising in public places would be severely restricted that they should be allowed to communicate at retail with persons who smoke to let them know that vaping products are a less harmful alternative to smoking.
A3: Broadcast media
Proposal: Vaping product advertisements would not be permitted in broadcast media during or adjacent to (within 30 minutes before or after) all children's and youth-oriented programming at all times of day and night and on all channels.
Some vape shop owners mentioned that advertisements of vaping products in broadcast media should be restricted to adult programming. Another suggested that advertisements should only be allowed after prime-time and that this needs to be specified in regulations. Some vape shop owners felt that the proposed criteria to prohibit promotion during "youth-oriented" programming could be problematic as it would only capture children's programs. Instead, they suggested that advertisements only be permitted after prime time (to be specified) or be limited to adult viewing time.
One vaping industry association suggested that broad, national brand-specific advertising campaigns in all media or in public places should be prohibited except in age-restricted locations.
Most manufacturers were supportive of the proposal, with the following comments:
- Advertising must not be directed at young persons through the selection of media or the context in which they appear. No media should display vaping products advertisements if more than 25% of the audience is below 25 years old.
- Smaller manufacturers suggested that the content of advertising should be limited to name of the store and the products they sell. No brand specific advertisements in broadcast media should be allowed.
A4: Publications
Proposal: Advertisements of vaping products would not be permitted in children's and youth-oriented publications. This would include electronic publications such as websites and social media platforms.
Most manufacturers supported the proposal to restrict the placement of advertisements in youth-oriented publications with the following comments:
- "Youth-oriented" should be clearly defined in the regulations, especially with regard to online publications such as websites and social media platforms.
- The majority of youth obtain information on vaping products online or on platforms such as Instagram or YouTube, which are challenging to regulate.
- One large manufacturer commented that the proposal to restrict advertising in publications online did not go far enough and that the enforcement of the proposed measure would be difficult.
- Another large manufacturer suggested that advertising on social media should be completely banned.
- One large manufacturer mentioned that they place advertisements in printed media only if they are assured of at least 75% adult readership.
Certain vape shop owners mentioned that businesses should be allowed to use signs/billboards to advertise the company name, location, website, phone number and hours of operation. They also indicated that such signs should be allowed to display authorized comparative health effect statements. One vaping industry association mentioned that Facebook and other social media platforms are critically important to ensure that adults who smoke /vapers have access to information and support. Several vape shop owners mentioned that communication through social media is important for their business to reach out to adults who smoke. They do so by implementing measures that restrict the social media platform to communicate with adults only.
B: Content of advertisements
B1: Content of advertisements- Health warnings
Proposal: In order to enhance public awareness of the health hazards of using vaping products, Health Canada is considering requiring that advertisements include a health warning. The content, format, size and the manner of display of the health warning would be prescribed by regulations. Where the advertisement only has an audio content, the applicable health warning would have to be read.
The proposed health warning on nicotine addictiveness received widespread support. With regards to the proposed text of the proposed health warning, several NGOs warned that the proposed wording "should not be used by youth" was counterproductive and should not be used as the wording could induce adolescents to try vaping products. There were also suggestions that alternate warnings be considered that warn about harms of nicotine exposure, such as the risk of damage to the developing adolescent brains and the risk of vaping leading to tobacco use.
Numerous vape shop owners and most manufacturers expressed reservations on the statement "Vaping products also release chemicals that can harm your health." They mentioned that it is factually ambiguous and could misinform Canadians or discourage persons who smoke from switching to vaping products. Some suggested that "can" be replaced by "may".
Several manufacturers and vape shop owners stated the proposed warnings should be attributed to Health Canada, a respected source of information.
Manufacturers, vape shop owners and vaping industry associations also suggested that proposed comparative health effect statements could be authorized by Health Canada to provide balanced messaging for people who smoke.
The association of broadcasters suggested that the health warnings be no longer than 25 words and readable in 5 seconds or less, to permit reasonable use on audio media such as the radio. Furthermore, the association commented that Health Canada should consider the advertising of non-substance specific vaping devices through an approved generic warning, such as "Vaping devices may release chemicals that can harm your health. Youth should not vape. Health Canada."
B2: Content of advertisements- Restrict the visual content
Proposal: Another measure under consideration is to restrict the visual content of advertisements to only text and illustrations or images of the vaping product or its package.
NGOs supported this proposal.
The association of broadcasters pointed out that the proposed restrictions on visual content of advertisements would make it very difficult to create TV advertising.
One manufacturer supported the proposed restrictions on the content of advertising to limit youth uptake, however did not support a prohibition of advertising at point of sale. Another manufacturer stated that that the existing prohibitions in the TVPA appropriately restrict the visual content of advertisements to the extent necessary to achieve the TVPA's objectives. Restricting the visual content of vaping product advertisements would limit the ability to communicate to Canadians that vaping products are a less harmful alternative to combustible tobacco products.
Some vape shop owners mentioned that similar restrictions proposed for vaping products do not apply to other products that are detrimental to youth such as alcohol.
C: Other forms of retail promotion
Proposal: Health Canada is considering measures to restrict the display of vaping products at points of sale. Such restrictions would not apply at points of sale where youth do not have access (e.g. a vape shop that does not allow youth on its premises or that blocks access to its website to youth), as long as the products cannot be seen from the outside of these places.
Most of the larger manufacturers and the retailer associations were strongly against these proposals, stating that such restrictions are not reflective of a balanced approach given the harm reduction potential of vaping products compared to tobacco products. Furthermore, they stated that the proposed restrictions would considerably limit communication about the availability of vaping products as a less harmful alternative to persons who smoke, especially when they are about to purchase tobacco products. While one manufacturer supported the proposal to prohibit the display of vaping products at retail locations where youth have access, the manufacturer requested that Health Canada consider allowing a manufacturer, wholesaler or distributor to display vaping products.
Retailer associations felt strongly that the same rules that apply to vape shops that do not allow youth access, should apply to them. Having different rules for retailers puts them at a competitive disadvantage.
Smaller manufacturers, vape shop owners and the vaping industry associations were in favour of a display ban at point of sale where youth have access. They mentioned that certain provinces have already implemented such measures.
Conclusion
Health Canada would like to thank everyone who submitted their feedback to this consultation. Comments received as part of this consultation will be taken into careful consideration in the development of regulations on vaping product promotions. The public and interested parties will have an opportunity to provide comments at the time of the pre-publication of the proposed regulations, and their accompanying Regulatory Impact Analysis Statement, in the Canada Gazette, Part I.