Classification of products under the Food and Drugs Act (F&DA)
Classification is the first step in any regulatory process at Health Canada. The Food and Drugs Act (F&DA) and its regulations serve as a basis for the classification of drugs, devices, food, and cosmetics. The definitions of "food," "drug," "cosmetic" and "device" in section 2 of the F&DA, and the definition of "natural health product" (NHP) in section 1 of the Natural Health Products Regulations, are fundamental to the classification of these products.
The four main sets of regulations that may apply to a product under the F&DA are:
- the Cosmetic Regulations;
- the Food and Drug Regulations;
- the Medical Devices Regulations; and
- the Natural Health Products Regulations
The following diagram provides a visual overview of the regulations that apply to each product line:
Figure 1
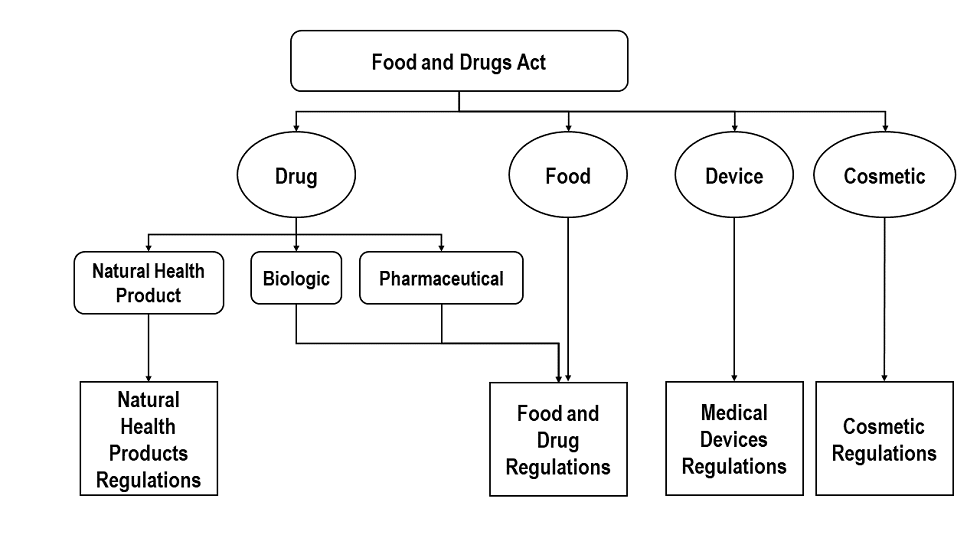
Text description: Figure 1
Regulations that apply to natural health products, biologic drugs, pharmaceutical drugs, food, medical devices and cosmetics products under the Food and Drugs Act.
As illustrated, the main subsets of drug products include NHPs, biologics, and pharmaceuticals. These subsets are important to distinguish, as NHPs fall under the Natural Health Products Regulations, and biologics, pharmaceuticals and hard surface disinfectants fall under the Food and Drug Regulations.
For certain novel drugs and devices for which the current regulations cannot appropriately accommodate, Health Canada's new framework for Advanced Therapeutic Products (ATPs) provides a pathway that is separate from the existing drug and device regulations. This pathway allows for more flexibility in the risk-based market authorization of these products.
How are products under the F&DA classified?
Most classification decisions are made by the appropriate program area upon submission of required information, or application for market authorization. However, there are instances where the classification of a product is not immediately apparent, or where a product meets multiple definitions. These products are known as 'interface products'. Examples of product interfaces include: the drug-medical device interface, cosmetic-drug interface, and the food-natural health product (NHP) interface.
Requests for the classification of products at the drug-medical device interface can be sent to the Office of Science (OoS) in the Bureau of Policy, Science and International Programs (BPSIP) within the Therapeutic Products Directorate of the Health Products and Food Branch (HPFB). The OoS primarily provides recommendations on the classification of products as drugs (i.e., pharmaceutical, biologic, or natural health products), medical devices, or drug-medical device combinations. When necessary, the OoS also provides advice on the classification of other products that are regulated by Health Canada. The in-house classification process involves rigorous research, analysis, and internal consultation with different areas within the Branch and Department. When needed, the OoS will engage the Therapeutic Products Classification Committee (TPCC) for guidance and/or concurrence on the classification recommendation. For questions on the classification of other products/interfaces, please see the contact information provided below.
Classification criteria
Relevant Acts and regulations are the central frameworks for product classification at Health Canada. For example, the definitions outlined in section 2 of the F&DA are the foundation of classification for drugs, devices, food, and cosmetics. In order to be classified as one of the four product types outlined in the F&DA, a product must meet one of these definitions. Classification is done on a case-by-case basis in accordance with a list of set criteria. The criteria used for classification vary depending on the interface or product type. Generally, four important criteria for classification include:
- Representation (e.g., any written claims, pictures, symbols, indications, conditions of use, etc.)
- Product purpose and intended use (i.e., the role of the product in fulfilling its claimed effect)
- Composition (i.e., the ingredients, components, structure of the product, and their contribution to the claimed effect)
- Format (e.g., products in conventional food formats such as prepackaged, ready-to-consume beverage products, bars, cereals, etc. versus dosage formats such as tablets or capsules)
More detailed information on the three interfaces and the criteria used for classification can be found in the following guidance documents:
- Classification of Products at the Drug-Medical Device Interface
- Classification of Products at the Cosmetic-Drug Interface
- Classification of Products at the Food-Natural Health Product Interface: Products in Food Formats
The new ministerial schedule
In June of 2019, the Budget Implementation Act (BIA) received royal assent. Consequently, sub-section 2.4 and a new Schedule A of the F&DA were put in place to create new Ministerial authorities. These new authorities allow the Minister to determine a single set of regulations that would apply to a product that simultaneously meets more than one of the definitions outlined in the F&DA (i.e., drug, food, device, or cosmetic). Schedule A is intended to improve consistency, predictability, and transparency of classification decisions for industry stakeholders. Any change to Schedule A would be published in the Canada Gazette.
Contact information
For classification requests on:
- Health products at the drug-medical device interface or a drug-medical device combination product:
- Please contact the Office of Science within the Therapeutic Products Directorate
- Cosmetics or drugs
- For cosmetic-related questions, please contact the Cosmetics Program
- For drug-related questions, please contact the Office of Science
- Natural Health Products (NHPs) or food
- For NHP-related questions, please contact the Natural and Non-Prescription Health Products Directorate (NNHPD)
- For food-related questions, please contact the Submission Management and Information Unit (SMIU)
- Medical Devices (i.e., class of device):
For device-related questions, please contact the Medical Devices Directorate
For information on the regulation of HPFB's product lines:
- Prescription drugs
- Please contact the Therapeutic Products Directorate
- Non-prescription drugs and natural health products
- Please contact the Natural and Non-Prescription Health Products Directorate
- Biologics, radiopharmaceuticals and genetic therapies
- Please contact the Biologic and Radiopharmaceutical Drugs Directorate
- Food products
- Please contact the Food Directorate
- Medical devices
- Please contact the Medical Devices Directorate
- Veterinary drugs
- Please contact the Veterinary Drugs Directorate
For information on the regulation of other product lines:
- Cosmetics
- Please contact the Cosmetics Program
- Tobacco products
- Please contact the Tobacco Control Directorate
- Pest Control Products
- Please contact the Screening and Regulatory Guidance (Pest Management Regulatory Agency)
- Consumer Products
- Please contact the Consumer Product Safety Program
Tools & resources:
- Drug and Health Product Portal
- Funding & fees
- Device advice: e-learning tool
- Special Access Programme (drugs)
- Licensed natural health products database
- Natural health products ingredients database (NHPID)
- Guidelines for the nonprescription and cosmetic industry regarding non-therapeutic advertising and labelling claims
- Drug product database
- For inquiries, please contact the Therapeutics Products Directorate (TPD)
- The prescription drug list (PDL)
- For inquiries, please contact the Prescription Drug Status Committee
- The Drugs and health products contact directory
Page details
- Date modified: