Labelling requirements for non-prescription drugs guidance document
Foreword
Guidance documents are meant to provide assistance to industry and health care professionals on how to comply with governing statutes and regulations. They also provide assistance to staff on how Health Canada’s mandates and objectives should be implemented in a manner that is fair, consistent and effective.
Guidance documents are administrative instruments not having force of law and, as such, allow for flexibility in approach. These alternate approaches to the principles and practices described in this document may be acceptable provided they are supported by adequate justification. In order to support the uniformity of labelling information, sponsors are strongly encouraged to implement the content and format described in this guidance document. Alternate approaches should be discussed in advance with the relevant program area to avoid the possible finding that applicable statutory or regulatory requirements have not been met.
As a corollary to the above, it is equally important to note that Health Canada reserves the right to request information or material, or define conditions not specifically described in this document, in order to allow the Department to adequately assess the safety, efficacy or quality of a therapeutic product. Health Canada is committed to ensuring that such requests are justifiable and that decisions are clearly documented.
This document should be read in conjunction with the accompanying notice and the relevant sections of other applicable guidance documents.
Please note, Health Canada has taken a first step to simplify market access for non-prescription drugs. For more information visit, Non-prescription Drug Action Plan.
Health Canada acknowledges the Institute for Safe Medication Practice for the important contributions made with respect to the development of several key guidance documents related to the safe and effective use of self-care products, i.e., the Good Label and Packages Practice for Non-prescription Drugs and Natural Health Products.
Table of contents
- Labelling requirements for non-prescription drugs
- Canadian Drug Facts Table for non-prescription drugs
- 2.1 General principles
- 2.2 Content
- 2.3 Design and format
- 2.4 Application of graduated flexibilities
- 2.5 Tailored flexibilities for Category IV products, mouthwashes and toothpastes
- 2.6 Labelling for low risk non-prescription drugs (i.e., Self-Care Framework Category 1 products)
- 2.7 Innovative labels
- Canadian Drug Facts Tables for active ingredients
Labelling requirements for non-prescription drugs
1.1 Background
The safe use of non-prescription drugs depends on consumers being able to identify the desired product and also being able to understand and act upon the information presented. The label and package are the first points of interaction between a health product and a consumer or healthcare professional. Prior to the implementation of the Plain Language Labelling (PLL) initiative, the general practice in Canada was to present key information within blocks of text on the product label. This sometimes made it difficult for the consumer to easily identify information necessary for appropriate selection and proper use of the product. In some cases, the information appeared in small type, with poor contrast between the label text and the background.
All of these factors can prevent the consumer from finding the information needed to make informed decisions in a timely manner, particularly at the time of selecting the product. Important product information should be placed in a consistent location on the label and be easy to read and understand. The aging of the population and the significant increase in the number of non-prescription drugs on the market add to the importance of addressing these issues.
As part of Health Canada's PLL initiative, the outer label of non-prescription drugs is required by regulations to display a table containing specific information, per section C.01.004.02 (1). The purpose of the Canadian Drug Facts Table (CDFT) is to display the information required by the regulations in a standardized, easy-to-read format in order to enhance the safe and effective use of non-prescription drugs. The concept is similar to that of the Nutrition Facts Table for foods in Canada and the Drug Facts box required by the Food and Drug Administration for over-the-counter (OTC) drugs in the United States.
The CDFT will enable consumers and healthcare professionals to:
- compare different products, specifically where there may be similarities in the name, packaging, or ingredients, to help in selection of the product most suitable for their needs or symptoms
- identify the same medicinal ingredient in multiple products, to avoid the potential for unintentional overdose
- quickly locate the directions for safe use and associated warnings
- quickly locate the list of product ingredients, to avoid the potential for allergic reactions
This guidance document provides prescriptive information on the design specifications, required sections of the CDFT, and labelling content. Sponsors seeking approval for a non-prescription drug product must adhere to the principles, format specifications, and content indicated in this guidance document. While consideration may be given to statements to the effect of those listed in this guidance document, labelling statements must be presented using the plain language principles outlined in the Good Label and Package Practices Guide for Non-prescription Drugs and Natural Health Products and this guidance document.
1.2 Scope
The requirement for a CDFT applies to all non-prescription drugs available for self-selection. The following four product categories are excluded from this requirement:
- drugs on the federal Prescription Drug List;
- biologics and radiopharmaceuticals;
- drugs that are permitted to be sold without a prescription but that are administered only under the direction of a healthcare professional (e.g., nitroglycerin, insulin, injectable epinephrine for anti-allergic purposes); and
- drugs that are represented as being solely for use as disinfectants on hard non-porous surfaces.
1.3 Required labelling information and associated regulations
The following provides a summary of labelling requirements for non-prescription drugs; additional information can be found in the Guidance Document: Labelling of Pharmaceutical Drugs for Human Use
- 1.3.1 The principal display panel (that is (i.e.), main panel) is the main product display surface visible to the user under normal or customary conditions of display or use. Pursuant to the Regulations, the principal display panel of an inner and outer label must normally show the following information:
- 1.3.1.1 The brand name of the drug product or if no brand name exists the proper or common name of the drug product, per C.01.004 (1)(a)(i);
- 1.3.1.2 The proper or common name of the drug product, in a font size not less than half the size used for the brand name, to a minimum size of 6 point (per Health Canada interpretation of paragraphs A.01.017 (a) and (b)) immediately before or after the brand name, per C.01.004 (1)(a)(i) and C.01.004 (1)(a)(ii);
- 1.3.1.3 The standard for the drug product, if any, per C.01.004 (1)(a)(iii-iv);
- 1.3.1.4 The notation "sterile (stérile)," if required by the Regulations, per C.01.004 (1)(a)(v);
- 1.3.1.5 The symbol corresponding to the appropriate schedule or to a drug containing an ingredient listed in the Prescription Drug List (if applicable), per C.01.004 (1)(b); and
- 1.3.1.6 The Drug Identification Number (DIN), per C.01.005 (1).
- 1.3.2 Pursuant to the Regulations, the following information must be displayed on any panel of the inner and outer labels:
- 1.3.2.1 The name and address of the manufacturer/sponsor, per C.01.004 (1)(c)(i) and of the distributor if the manufacturer/sponsor is not Canadian, per C.01.004.01 (2);
- 1.3.2.2 The lot number, per C.01.004 (1)(c)(ii);
- 1.3.2.3 The expiration date, per C.01.004 (1)(c)(v);
- 1.3.2.4 Adequate directions for use of the drug product, per C.01.004 (1)(c)(iii); and
- 1.3.2.5 A quantitative list of the medicinal ingredients of the drug product, per C.01.004 (1)(c)(iv).
- 1.3.3 The following additional information is to be displayed on any panel of the outer label:
- 1.3.3.1 The net amount of the drug product in the container in terms of weight, measure or number (for example (e.g., number of tablets), per C.01.004 (2)(a);
- 1.3.3.2 A quantitative list of all preservatives in parenteral preparations and of all mercurial preservatives in any drug product containing mercury or a salt or derivative thereof as a preservative, per C.01.004 (2)(b) and C.01.004 (2)(c); and
- 1.3.3.3 A bilingual table, or one table in English and another in French, per C.01.004.02 (1), that contains only the following information:
- 1.3.3.3.1 Adequate directions for use of the drug, per C.01.004.02 (1)(a);
- 1.3.3.3.2 A quantitative list of the drug's medicinal ingredients, by their proper names, or if they have no proper names, by their common names, per C.01.004.02 (1)(b);
- 1.3.3.3.3 A qualitative list of the drug's non-medicinal ingredients, in alphabetical order or in descending order of quantity, preceded by the heading that distinguishes them from medicinal ingredients, per C.01.004.02 (1)(c); and
- 1.3.3.3.4 Contact information, in the form of a telephone number, email address, website, postal address or the like that enables communication with a contact person in Canada with a statement to the effect that adverse events associated with the use of the product may be reported to the contact person, per C.01.004.02 (1)(d).
- 1.3.4 The following additional information is to be displayed on any panel of the inner label:
- 1.3.4.1 Adequate directions for use of the drug, per C.01.004.03 (a);
- 1.3.4.2 A quantitative list of the drug's medicinal ingredients, by their proper names, or if they have no proper names, by their common names, per C.01.004.03 (b); and
- 1.3.4.3 Contact information, in the form of a telephone number, email address, website, postal address or the like that enables communication with a contact person in Canada with a statement to the effect that adverse events associated with the use of the product may be reported to the contact person, per C.01.004.03 (c).
- 1.3.5 In some cases, a non-prescription drug may be available for sale with only one label (i.e., when there is no outer carton included). In these cases, the label must contain all of the information required by the Regulations for both the inner and outer labels, per section C.01.006.
1.4 Labelling considerations and interpretations of plain language labelling regulations
The 2014 Regulations Amending the Food and Drug Regulations (commonly known as the PLL Regulations) impose certain requirements with respect to the format and display of the prescribed information. This section of the Guidance describes Health Canada's interpretation of compliance with the PLL Regulations.
- 1.4.1 Any information that is required by the Regulations, and the adequate directions for use, must be equally displayed on labelling in both English and French, per A.01.015 (1) and A.01.015 (2).
Per paragraph A.01.017 (a) and A.01.017 (b) of the Regulations, labelling components must be represented in the following manner:
- 1.4.2 All information required by the Regulations on both inner and outer labels must employ:
- 1.4.2.1 A non-decorative, sans-serif font (Brand name, Expiration date, and Lot number may employ a non-decorative serif font that does not impair legibility of the information)
- 1.4.2.2 Minimum font size of 6 points
- 1.4.2.3 Adequate contrast to prominently display required information
- 1.4.2.4 Plain language
Refer to the specific formatting instructions for the CDFT (Section 2), Expiration dates (Section 1.4.3) and Lot numbers (Section 1.4.4).
- 1.4.3 Expiration date:
The expiration date must be included on both inner and outer labels, using one of the acceptable formats listed below:
EXP 2020-JA-11
EXP 11-JA-2020
EXP 2020-JA
EXP JA-2020
EXP 2020-01
EXP 01-2020
EXP 01-31-2020 (when only the last day of the corresponding month is used)
EXP 31-01-2020 (when only the last day of the corresponding month is used)
EXP 2020-01-31 (when only the last day of the corresponding month is used)Note: Dashes (-), slashes (/), or spaces are acceptable to separate information within the expiration date.
Expiration date formats that are not acceptable include:
EXP JA-20
EXP 20-JA
EXP 2020-01-21 (when the last day of the corresponding month is not used)
EXP 11-01-2020 (when the last day of the corresponding month is not used)Expiration dates must be presented using non-decorative font in minimum size of 6 points (1/12 of an inch).
- 1.4.4 Lot numbers:
Lot numbers must be expressed on both inner and outer labels, preceded by the designation "Lot number", "Lot No.", "Lot", "LOT", or "(L)", per A.01.014
- 1.4.5 Other labelling considerations
- 1.4.5.1 Contrast must be maximized for all information; reflective substrates are not permitted in locations where information required by the regulations is displayed.
- 1.4.5.2 Foil may be used for select packaging applications (e.g., blister packaging and pouches), however sponsors must ensure that the legibility of the prescribed information is maximized (i.e., dark print ink must be used, foils must be legible and complete after blisters have been pierced, etc.). Sponsors should consider backing the foil blisters with more legible substrates.
- 1.4.5.3 Products packaged in a pressurized container must include the required labelling per sections A.01.061, A.01.062, and A.01.063 of the Regulations.
- 1.4.5.4 Product packaging and labelling must comply with section A.01.065 of the Regulations regarding security packaging and the labelling of security features.
- 1.4.5.5 Product formulation, packaging and labelling must comply with sections C.01.021 to C.01.027 of the Regulations regarding limits of drug dosage
- 1.4.5.6 Product packaging and labelling must comply with sections C.01.028 to C.01.035 of the Regulations regarding cautionary statement and child resistant packages
Canadian Drug Facts Table for non-prescription drugs
This section of the guidance document outlines the design and format for the standard CDFT and available flexibilities to reduce the need for package size increases or innovative labels while respecting the PLL Regulations. Formatting specifications are presented for the following:
- Standard CDFT format
- CDFT with graduated flexibilities
- CDFT with tailored flexibilities for Category IV products, mouthwashes and toothpastes
- Labelling for Low Risk Non-prescription Drugs (i.e., Self-Care Framework Category 1 Products), to be available as of December 2018 (refer to Section 2.6 of this document)
- CDFT for products using innovative labels
In conjunction with this guidance document, sponsors should consult the Good Label and Package Practices Guide for Non-prescription Drugs and Natural Health Products and the Guidance Document: Electronic Canadian Drug Facts Table (eCDFT) Technical Standards.
2.1 General principles
A consistent order and format must be used for the CDFT. The information should be written for a grade 6 to grade 8 reading level, avoiding technical language and using short sentences or bullet form wherever possible.
At the time of selection and purchase, the CDFT must be visible on the outer label of the retail package or, if there is no outer label, on the immediate container label. If the CDFT is displayed on an innovative label, the label must be accessible to the consumer prior to purchase.
2.2 Content
Table 1 describes the content to be included under each heading and subheading within the CDFT. The title, headings and subheadings must appear as listed in Table 1. The order of the content must be respected as outlined below. However, if a heading or subheading is not applicable, it should be omitted.
- 2.2.1 Promotional wording must be avoided to ensure adequate space for the required information (e.g., 'fast and effective relief of pain' can be rephrased as 'pain relief'.)
- 2.2.2 Pictograms or graphic images (including the UPC code) must not appear within the CDFT, other than symbols required by the Regulations (e.g., red octagon preceding specific warnings) or for a telephone or telephone receiver before the 'Questions?' heading. Note: it is acceptable to modify the shape of the CDFT to accommodate other labelling elements outside of the CDFT (e.g. a UPC), conditional that this does not reduce legibility.
The content of the CDFT must be populated per the labelling requirements identified in Section 3 of this guidance document, which aligns existing safety information with plain language content principles. A statement to the effect of the content included in Section 3 may be acceptable.
The use of graduated flexibilities does not change the order of the information presented in the CDFT, however this will mean that some products will have a complete CDFT on the outer label or package whereas others may have some information on a package insert and/or a URL. All situations require that the information important to appropriate selection of the product is available on the outer label or package.
Title/heading/subheading |
Description and comments regarding content |
|
|---|---|---|
1.a |
Drug Facts |
The titles to be used are 'Drug Facts' and 'Info-médicament'. If the table is split, each subsequent component of the table must include the title 'Drug Facts (continued)' or 'Info-médicament (suite)'. |
1.b |
Prompts to an eCDFT must appear underneath the title; the statement "For full table, visit / Pour le tableau complet, visitez www.websitename.com" must be used (see Figure 4). If the complete product information is included on the outer label, the statement “For electronic version, visit / Pour la version électronique, visitez: www.websitename.com” may be used. |
|
1.c |
Active ingredient(s) |
Include in this section a quantitative list of the product's active ingredients by their proper names or, if they have no proper names, by their common names. The heading must also include the dosage unit (e.g., "in each tablet", "w/w", etc.) |
1.d |
Purpose(s) |
Indicate the general pharmacological category or principal intended action of the product or, where the product contains more than one active ingredient, the general pharmacological categories or the principal intended actions of each active ingredient. |
1.e |
Use(s) |
List in this section the indication(s) for use of the product. |
1.f |
Warnings |
Warning statements are to be included in this section in the specific order (sequence) as listed below. Only the subheadings that apply to the product should be used. Warning statements must comply with pertinent regulations (see Section 1) and other Health Canada labelling guidance, which may require the use of octagonal symbols. |
1.f.1 |
For external use only |
Include warning statements regarding route of administration when applicable. No text is required other than the warning statement. |
1.f.2 |
Reye's syndrome |
Include in this section the statement regarding Reye's syndrome when applicable. |
1.f.3 |
Allergy alert |
List in this section any specific allergic reaction warnings (e.g., eggs, milk, mustard, peanuts, seafood [fish, crustaceans and shellfish], sesame, soy, sulphites, tree nuts, wheat), known cross-allergenicity with other drugs (e.g., ASA with other salicylates) or asthma warnings. |
1.f.4 |
Flammability warning |
Include in this section flammability warnings with appropriate signal words when applicable. |
1.f.5 |
Choking |
Include in this section choking warnings (e.g., water-soluble gum warning) when applicable. |
1.f.6 |
Alcohol warning |
Include in this section these types of warnings when applicable. |
1.f.7 |
Sore throat warning |
Include in this section this type of warning when applicable. |
1.f.8 |
Dosage warning |
Include in this section dosage warning, such as "taking more than the recommended dose in 24 hours can be harmful", when applicable. |
1.f.9 |
Sexually transmitted diseases (STDs) alert |
Include in this section warnings such as "this product does not protect against HIV/AIDS or other STDs" when applicable. |
1.f.10 |
Do not use |
In this section, list all contraindications for use with the product. This is reserved for situations in which consumers should not use the product unless a prior diagnosis has been made by a doctor, or for situations in which certain consumers should not use the product under any circumstances, regardless of whether a doctor or healthcare professional is consulted. |
1.f.11 |
Ask a doctor or pharmacist before use if you |
In this section, list all warnings for persons with pre-existing conditions or when experiencing particular symptoms. List all drug-drug, drug-NHP and drug-food interaction warnings. Include a statement here regarding use in pregnancy and breastfeeding. For oral health products, the subheading may be "Ask a dentist or pharmacist before use if you" and "Consultez un dentiste ou un pharmacien avant l'utilisation si vous" |
1.f.12 |
When using this product |
In this section, list the side effects that the consumer may experience and identify the substances (e.g., alcohol, sedatives) or activities (e.g., operating machinery, driving a car) that should be avoided while using the product. |
1.f.13 |
Stop use and ask a doctor if |
In this section, list any signs of toxicity or other adverse reactions that would necessitate immediately discontinuing use of the product. For oral health products, the subheading may be "Stop use and ask a dentist if" and "Cessez d'utiliser et consultez un dentiste si" |
1.f.14 |
Other warnings |
Include here any other required warnings that do not fit under the other warnings subheadings. Include a statement to indicate that additional information is available on other parts of the label and package insert, if applicable. |
1.f.15 |
Keep out of reach of children. |
Include in this section a general warning and a warning about accidental overdose or ingestion. For example, "In case of overdose, call a poison control centre or get medical help right away." |
1.g |
Directions |
Provide in this section the directions for use, which could include dose instructions, duration, route of administration, maximum daily dose. |
1.h |
Other information |
This should be limited to storage instructions, any special instructions (e.g., for disposal), and a list of certain ingredients (e.g., calcium, magnesium, potassium and sodium content). |
1.i |
Inactive ingredients |
Provide in this section a qualitative list of inactive ingredients listed either in alphabetical order or in descending order of predominance by their proportion in the product. Any information required by regulation related to preservatives (e.g., mercurial) should appear here. This section could also be used to indicate whether the product is gluten-free, lactose-free, etc., when appropriate. For Category IV products, mouthwashes, and toothpastes: inactive ingredients may be represented on labels using the International Nomenclature of Cosmetic Ingredients (INCI), as long as the ingredient is listed in the Natural Health Products Ingredient Database (NHPID). |
1.j |
Questions? |
Provide in this section the sponsor's contact information for consumers to obtain answers to questions about the product or to report concerns or adverse events (adverse reactions or medication incidents) associated with the product. Contact information may be the full numeric representation of a telephone number, an email address, website address, postal address or any other information that enables communication with a contact person in Canada. This section may also include a symbol of a telephone receiver ( or ), however the symbol cannot replace the heading “Questions?”. |
2.3 Design and format
Table 2 presents the format for the standard CDFT. Certain modifications to the standard format are permitted, as described in Section 2.4: Application of Graduated Flexibilities.
Refer to Figure 1 for an example of the standard CDFT, labelled with the design specifications.
| Label/ design element | Standard Canadian Drug Facts Table format | |
|---|---|---|
| 2.a | Principal display panel ("branding panel") | If space permits, two branding panels can be used (i.e., one in English and one in French). |
| 2.b | Font style |
|
| 2.c | Font weight | Boldface font is required for the table title 'Drug Facts', the headings and the subheadings. No other bolding is permitted unless required by the Regulations. Refer to the permitted fonts above for details of acceptable weights for regular and bolded type. |
| 2.d | Font size |
*If Helvetica Neue is not available, an acceptable font as per 'Font Style' above must be substituted. |
| 2.e | Print colour |
|
| 2.f | Title: Drug Facts |
|
| 2.g | Title: Drug Facts (continued) |
|
| 2.h | Headings (e.g., uses, warnings, directions) |
|
| 2.i | Subheadings (e.g., do not use, alcohol warning) |
|
| 2.j | Bullets | Solid square or solid round bullets are acceptable. They must be set to a minimum width of 3 points or be of a size consistent with the CDFT text. |
| 2.k | Bulleted text |
|
| 2.l | Box frame, rules and hairlines |
|
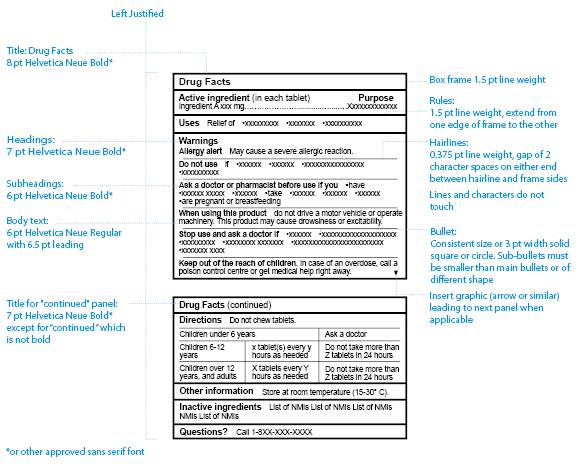
Figure 1: Format specifications of a standard CDFT - text equivalent
Figure 1, Format Specifications of a Standard CDFT, is a unilingual sample of a Canadian drug facts table that shows formatting specifications.
There are 2 panels, one with the title Drug Facts and one with the title Drug Facts (continued).
The headings in the table are, in order: Active ingredient (in each tablet), Purpose, Uses, Warnings, Directions, Other information, Inactive ingredients, and Questions with a question mark. All headings are bolded and start with a capital letter.
Under the heading Warnings, there are subheadings: Allergy alert, Do not use, Ask a doctor or pharmacist before use if you, When using this product, Stop use and ask a doctor if, and Keep out of the reach of children. All headings are bolded and start with a capital letter.
Headings are separated from each other by a horizontal heavy line, and subheadings in the warnings section are separated by horizontal hairlines. Each heading or subheading starts on a new line.
There is mock text after each heading and subheading, in short sentences or bullet points, except in the directions section which is presented in the form of an embedded table.
Specifications for font type and size, line weights and text alignment are shown, with a dotted line pointing out each design element in the table.
2.3.1 CDFT formatting requirements
2.3.1.1 General requirements
2.3.1.1.1 Information about the 'Active ingredients' and 'Purpose' should appear on the same horizontal line (with the exception of flexibilities for Category IV products, mouthwash and toothpaste, in which 'Purpose' can be removed). When not feasible, these headings must be presented in blocks of text to clearly organize the information, for example:
Recommended:

Text equivalent
This image shows the placement of bilingual headings on the same line. The text shows, from left to right, the heading Active ingredient (in each tablet), slash, Ingrédient actif (dans chaque comprimé), followed by the heading Purpose, slash, Utilité, on the right side of the same line. On the following line, the ingredient (Dimenhydrinate XX mg) appears with the purpose (Anti-nauseant/ antinauséeux) on the right side of the same line.
Accepted:

Text equivalent
This image shows the placement of bilingual headings on separate lines. The text shows, from left to right, the heading Active ingredient (in each tablet), slash, then Purpose on the right side. On the next line, the heading Ingrédient actif (dans chaque comprimé) appears, followed by Utilité on the right side. On the following line, the ingredient (Dimenhydrinate XX mg) appears with the purpose (Anti-nauseant/ antinauséeux) on the right side of the same line.
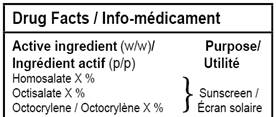
2.3.1.1.2 When more than one active ingredient has the same purpose, the information should be presented in a manner that readily associates each active ingredient with its purpose (by using brackets, dot leaders, or other graphical features). For example:

Text equivalent
This image shows an excerpt from a sample bilingual drug facts table, showing multiple active ingredients which have the same purpose. The multiple ingredients on the left are linked to the single purpose on the right, by a large bracket.
2.3.1.1.3 Similarly, when space does not permit the purpose or name of an active ingredient to be displayed on a single line, it may spill into a second line if it remains legible, for example:
Text equivalent
This image shows an excerpt from a sample bilingual drug facts table, showing multiple active ingredients which have the same purpose. The ingredients on the left are linked to the single purpose on right by a large bracket. The purpose is displayed on two lines.

Text equivalent
This image shows an excerpt from a sample bilingual drug facts table, showing multiple active ingredients which have the same purpose. The ingredients on the left are linked to the single purpose on right by a large bracket. The purpose is displayed on two lines, as is the English and French name of an ingredient.
2.3.1.1.4 To arrange additional text related to a single bulleted statement, use sub-bulleted statements. Sub-bullets should be clearly different (i.e., a different shape or smaller size than the main bullets, and must not be hollow). For example:

Text equivalent
This image gives an example of how to treat a complex bulleted list.
The first bulleted item in the list of uses is set off with a filled-in round bullet. It says “temporarily relieves pain and itching due to.” The 2 sub-bullets under this first bulleted item are indented further and use a filled-in square bullet. They read “insect bites” and “minor skin irritation,” and complete the thought of the first bulleted item.
The second bulleted item in the list of uses is also set off with a filled-in round bullet. It says “dries the oozing and weeping of.” The 3 sub-bullets under this second bulleted item are also indented further and use a filled-in square bullet. They read “poison ivy,” “poison oak” and “poison sumac.” They complete the thought of the second bullet.
2.3.1.1.5 Directions can be presented in a table format, as bulleted statements, or as a combination of a table and bulleted statements. For products with directions for use in more than two age groups or populations, these instructions should appear in an embedded table format, to make it easier for the consumer to read (refer to Figure 1: Format Specifications of a Standard CDFT).
2.3.1.1.6 Fractions may be expressed in numeric notation or as text (e.g., "½" or "one-half"). The text for fractions should be in the same type style and type size used for the other text in the CDFT.
2.3.1.2 Bilingual information
2.3.1.2.1 Type specifications (e.g., sizes, styles) must be consistent across both languages.
2.3.1.2.2 When possible, the information should be organized by language for better readability (refer to Figure 2: Canadian Drug Facts Tables (Unilingual Layout: English and French).

Figure 2: Canadian Drug Facts Tables (unilingual layout: English and French) - text equivalent
Figure 2, Canadian Drug Facts Tables (Unilingual Layout, English and French), shows two side-by-side samples of the same drug facts table. On the left is a unilingual English table with the title Drug Facts. On the right is a unilingual French table with the title Info-médicament.
The headings in the table are, in order: Active ingredient (in each xx mL), Purpose, Uses, Warnings, Directions, Other information, Inactive ingredients, and Questions with a question mark.
Under the heading Warnings, there are subheadings: Ask a doctor or pharmacist before use if you, When using this product, and Keep out of the reach of children. All headings are bolded and start with a capital letter.
The headings in the French table are Ingrédients actifs (dans chaque xx mL), Utilité, Usages, Mises en garde, Mode d'emploi, Autres renseignements, Ingrédients inactifs, and Questions with a question mark.
Under the heading Mises en garde, there are subheadings : Consultez un médecin ou un pharmacien avant l'utilisation si vous, Lorsque vous utilisez ce produit, and Garder hors de la portée des enfants. All subheadings are bolded and start with a capital letter.
Each heading or subheading starts on a new line. Headings are separated from each other by a horizontal heavy line, and each subheading in the warnings section is separated from the next by a horizontal hairline.
There is mock text after each heading and subheading, in short sentences or bullet points.
Note: Figure 2 is not drawn to scale.
2.3.1.2.3 A bilingual CDFT may be the preferred format when space on the label is limited. Separate blocks of English and French text may alternate (as in Figure 3: Canadian Drug Facts Table (Bilingual Layout: Alternating English and French) or they can be combined (as in Figure 4: Canadian Drug Facts Table (Bilingual Layout: Combined English and French) within the same table.

Figure 3: Canadian Drug Facts Table (bilingual layout: alternating English and French) - Text equivalent
Figure 3, Canadian Drug Facts Table (Bilingual Layout: Alternating English and French), is a sample drug facts table that shows information in a bilingual layout with alternating blocks of English and French text.
At the top of the drug facts table is the title Drug Facts / Info-médicament.
The headings are, in order from top to bottom of the table, Active ingredient (in each xx mL), Ingrédient actif (dans chaque xx mL), Purpose, Utilité, Uses, Usages, Warnings, Mises en garde, Directions, Mode d'emploi, Other information, Autres renseignements, Inactive ingredients, Ingrédients inactifs, and Questions with a question mark. All headings are bolded and start with a capital letter.
Each English heading is followed by mock text. On a new line immediately underneath is the corresponding French heading and mock text. Each bilingual pair of headings is separated from the next pair of headings by a horizontal heavy line.
Under the Warnings heading, there are subheadings and mock text. The subheadings are: Ask a doctor or pharmacist before use if you, When using this product, and Keep out of the reach of children. All subheadings are bolded and start with a capital letter. Each subheading is separated from the next by a horizontal hairline.
Immediately following the last English subheading in the warnings section is the French heading Mises en garde, the French subheadings and mock text. Each subheading is separated from the next by a horizontal hairline. The french subheadings are Consultez un médecin ou un pharmacien avant l'utilisation si vous, Lorsque vous utilisez ce produit, and Garder hors de la portée des enfants.
All mock text is in short sentences or bullet form. All headings and subheading start on a new line.
Note: Figure 3 is not drawn to scale.
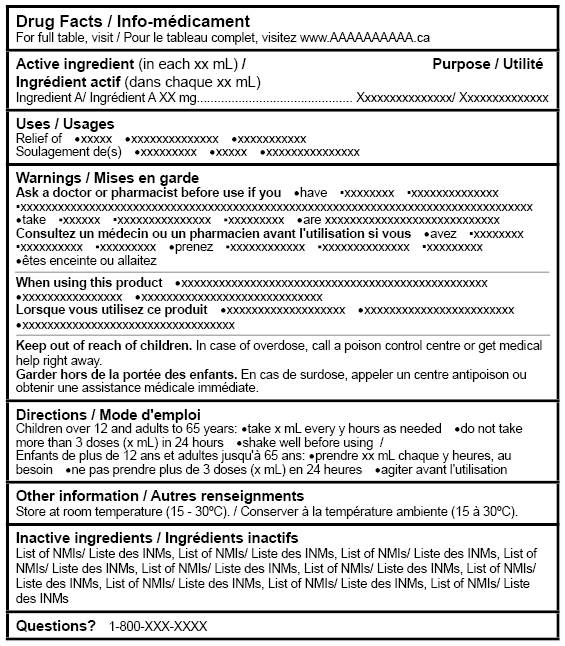
Figure 4: Canadian Drug Facts Table (bilingual layout: combined English and French) - Text equivalent
Figure 4, Canadian drug facts table (bilingual layout: combined English and French), is a sample drug facts table showing information in a bilingual layout that combines English and French text.
At the top of the drug facts table is the title Drug Facts / Info-médicament.
The headings are presented together as English heading, slash, French heading. Each heading has mock text in English and then in French, separated by a forward slash.
The headings are, in order from top to bottom of the table, Active ingredient(in each xx mL) / Ingrédient actif (dans chaque xx mL), Purpose / Utilité, Uses / Usages, Warnings / Mises en garde, Directions / Mode d'emploi, Other information / Autres renseignements, Inactive ingredients / Ingrédients inactifs, and Questions with a question mark. All headings are bolded and start with a capital letter.
Each heading starts with a capital letter. Each bilingual pair of headings is bolded and starts on a new line. Each bilingual pair of headings is separated from the next pair of headings by a horizontal heavy line.
Under the Warnings / Mises en garde heading, there are subheadings and mock text.
First, the English subheading with its corresponding mock text appears, followed immediately by the French subheading with its corresponding mock text. The subheadings are, in order from top to bottom of the table, Ask a doctor or pharmacist before use if you, Consultez un médecin ou un pharmacien avant l'utilisation si vous, When using this product, Lorsque vous utilisez ce produit, Keep out of the reach of children, and Garder hors de la portée des enfants.
Each subheading is bolded, starts with a capital letter and starts on a new line. Each pair of subheadings is separated from the next by a horizontal hairline.
All mock text is in short sentences or bullet form.
Note: Figure 4 is not drawn to scale.
2.3.1.2.4 English and French text should be organized so that the information can be easily read in the language of choice. Warning statements with many bullets are more readable if grouped together in the same language, rather than alternating English and French statements for each bullet. For example:
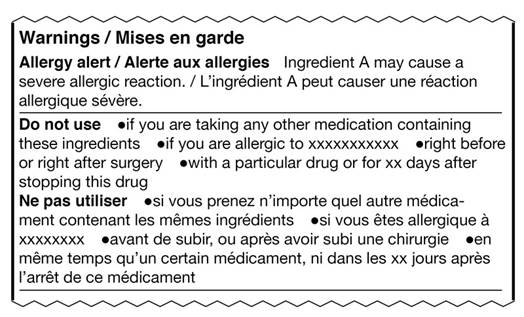
Text equivalent
This image shows an excerpt from a sample drug facts table. It shows two ways of laying out information in a bilingual format. The heading is, Warnings / Mises en garde. The subheading Allergy alert / Alerte aux allergies is followed by short sample text in English, then French. The English text is separated from the French text by a forward slash. The next subheading, Do not use, is followed by longer mock text in English. The French subheading Ne pas utiliser is on a new line followed by its mock text. Each subheading and its bilingual content is separated from the next subheading by a horizontal hairline. Each heading and subheading is bolded and starts with a capital letter. Each heading and subheading starts on a new line.
2.3.1.3 Display considerations
2.3.1.3.1 Orientation:
In general, a vertical presentation of the table is recommended, to enhance readability and facilitate product comparison. However, an alternative orientation (refer to Figure 6: Canadian Drug Facts Table (Bilingual Layout with Alternative Orientation) may help to maximize available label space and, in some instances, may improve readability (e.g., in the case of elongated packages, such as packages for topical ointments).
2.3.1.3.2 Splitting the table:
Where there is insufficient room on a single face of a package, the CDFT may be split over more than one face. However, the overall format and sequence of the information should remain the same. Depending on the layout, sections may continue on subsequent panels. Consider whether starting the CDFT on a side panel may make the table easier to follow (refer to Figure 5: Canadian Drug Facts Table Bilingual layout: Multiple Panels and Figure 6: Canadian Drug Facts Table (Bilingual Layout with Alternative Orientation). The sequence of CDFT panels cannot be interrupted by branding/other panels, except when separate unilingual CDFTs are applied on opposing panels (i.e. an English CDFT and a French CDFT can stand alone with a branding/ other panel between them).
If the table is split, a mechanism should be provided to allow the consumer to clearly follow the contents of the CDFT from the beginning to the end (i.e., it should be clear where the table starts and ends). A graphic symbol, such as an arrow, should be used to signal continuation of the CDFT to the next panel. The title 'Drug Facts (continued)' must appear at the top of the next panel, although with the application of graduated flexibilities this may be omitted.
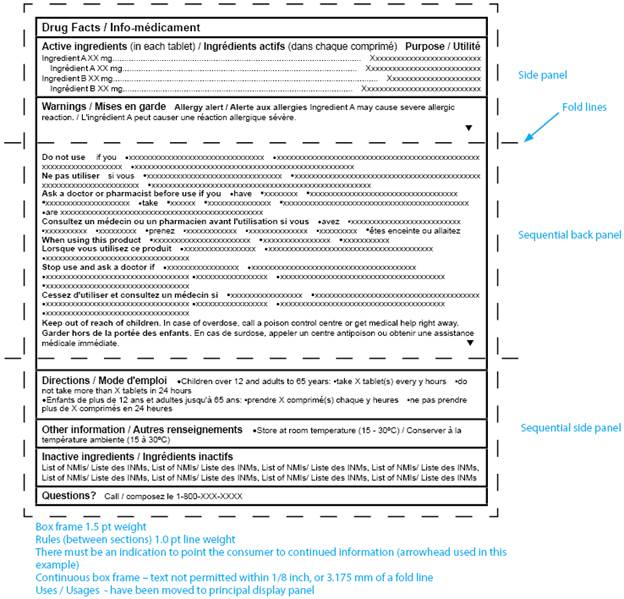
Figure 5: Canadian Drug Facts Table (bilingual layout: multiple panels) - Text equivalent
Figure 5, Canadian Drug Facts Table (Bilingual layout: Multiple Panels), is a sample drug facts table that spans across 3 adjacent panels. The text is laid out in "landscape" orientation. There is one rectangular panel. It is surrounded by a second, slightly larger rectangle where the sides have dotted lines. There are 2 horizontal dotted lines that run through both rectangles, located about 1.5 inches from the top and 1.5 inches from the bottom. The dotted lines are meant to simulate the fold lines of a 3-dimensional box.
The title of the table is Drug Facts, slash, Info-médicament.
The information is presented in a combined bilingual format.
The headings are, in order from top to bottom of the table, Active ingredients (in each tablet) / Ingrédients actifs (dans chaque comprimé), Purpose / Utilité, Uses / Usages, Warnings/ Mises en garde, Directions/ Mode d'emploi, Other information / Autres renseignements, Inactive ingredients/ Ingrédients inactifs, and Questions with a question mark. Each pair of headings is bolded and starts on a new line. Each heading starts with a capital letter.
Under the Warnings / Mises en garde heading, the English and French subheadings appear in alternating format. The subheadings are, in order from top to bottom of the table, Allergy alert / Alerte aux allergies, Do not use, Ne pas utiliser, Ask a doctor or pharmacist before use if you, Consultez un médecin ou un pharmacien avant l'utilisation si vous, When using this product, Lorsque vous utilisez ce produit, Stop use and ask a doctor if, Cessez d'utiliser et consultez un médecin si, Keep out of reach of children, and Garder hors de la portée des enfants.
Immediately following each subheading is the corresponding mock text. Each subheading is bolded, starts with a capital letter and starts on a new line. A Level 1 flexibility is used which removes horizontal hairlines.
All mock text is in short sentences or bullet form.
Note: Figure 5 is not drawn to scale.
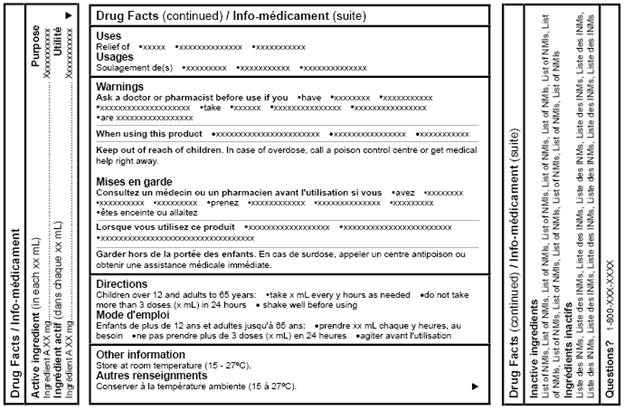
Figure 6: Canadian Drug Facts Table (bilingual layout with alternative orientation) - Text equivalent
Figure 6, Canadian Drug Facts Table (Bilingual Layout with Alternative Orientation), is a sample drug facts table showing information in a bilingual layout that alternates English and French text. There are 3 panels, one large panel and 2 smaller, narrow ones. The panels simulate the sides of a rectangular package. Both of the smaller panels are oriented at a 90 degree angle to the large panel.
On the left, the drug facts table begins with the title Drug Facts / Info-médicament. The middle and right-side panels are titled Drug Facts (continued) / Info-médicament (suite).
The headings are presented in alternating format, in order from top to bottom of the table, Active ingredient (in each xx mL), Ingrédient actif (dans chaque xx mL), Purpose, Utilité, Uses, Usages, Warnings, Mises en garde, Directions, Mode d'emploi, Other information, Autres renseignements, Inactive ingredients, Ingrédients inactifs, and Questions with a question mark. Each heading is bolded, starts with a capital letter and starts on a new line.
Under the Warnings / Mises en garde heading, the English and French subheadings appear in alternating format. The subheadings are, in order from top to bottom of the table, Ask a doctor or pharmacist before use if you, When using this product, and Keep out of reach of children, followed by the French subheadings Consultez un médecin ou un pharmacien avant l'utilisation si vous, Lorsque vous utilisez ce produit, and Garder hors de la portée des enfants.
Immediately following each subheading is the corresponding mock text.
All mock text is in short sentences or bullet form.
Note: Figure 6 is not drawn to scale.
2.3.1.3.3 Using columns:
When two or more columns appear on the same side of a package, the first column should be positioned to the left of a second column and so on. Columns should be approximately the same size. If two or more columns are placed on the same side of a package, the right side of the first column and the left side of the second column may share a common vertical box frame extending from the top to the bottom of the CDFT (see example below). This also applies to the right side of the second column and the left side of the third column, if a third column is used.

Text equivalent
This image shows an excerpt from a sample drug facts table showing information laid out in a 2-column format. The columns are beside each other and separated by a heavy vertical line. The left-hand column contains the English title, Drug Facts; the headings Active ingredients, Purpose, Uses and Warnings; and under Warnings, the subheadings Ask a doctor or pharmacist before use if you, When using this products, and Keep out of reach of children. Each heading or subheading has associated mock text.
The right-hand column contains the French title (Info-médicament); the headings Ingrédients actifs, Utilité, Usages and Mises en garde; and under Mises en garde, the subheadings Consultez un médecin ou un pharmacien avant l’utilisation si vous, Lorsque vous utilisez ce produit, and Gardez hors de la portée des enfants. Each heading or subheading has associated mock text.
Each heading and subheading is bolded and starts with a capital letter. Each heading and subheading starts on a new line.
A graphic symbol (e.g., an arrow) should be used at the bottom of the first column (and at the bottom of subsequent columns, where needed) to signal continuation of the CDFT to the next column. The title 'Drug Facts (continued)' must appear at the top of each subsequent column, although with the application of graduated flexibilities this may be omitted.
2.4 Application of graduated flexibilities
All products under the scope of the regulations must apply the CDFT format. However, some products and package sizes may not present sufficient space to accommodate the standard format of the CDFT on the label.
In some cases, an innovative label may be the most effective way to accommodate the space requirements of the CDFT. This determination should be made early in the label and package development process. In other cases, a modified format with graduated flexibilities may be considered according to the criteria outlined in Table 3: Canadian Drug Facts Table format with graduated flexibilities and Table 4: Tailored flexibilities for Category IV products, mouthwashes and toothpastes. The purpose of the available flexibilities is to gain space on the label and in some cases, may prevent the need for innovative labels or increased package sizes.
The inclusion of a URL directing consumers to an eCDFT is optional for all products.
2.4.1 Branding
Sponsors using two branding panels may apply any one or more of the Level 1 flexibilities indicated in Table 3. Sponsors are not permitted to apply flexibilities beyond those in Level 1 if they choose to retain two branding panels in the design of their packaging, i.e., use of a condensed font is not permitted.
When a single bilingual branding panel is used, sponsors may apply additional flexibilities (Levels 2 to 4). Sponsors are not required to apply all Level 1 flexibilities prior to moving to Level 2 (font modifications).
2.4.2 Moving information from the CDFT
Except for Category IV products, and uniquely for Self-Care Framework Category 1 products as of December 1st 2018, any information removed from the CDFT (after application of graduated flexibilities) must appear on a package insert, following the same specifications as the standard CDFT. This does not apply to ‘Uses’, ‘Inactive ingredients’ or product storage information captured in ‘Other information’ since these elements are required by the Regulations to appear on the outer label.
Category IV products as well as mouthwashes and toothpastes that limit information in the CDFT on the physical label (as per the Minimum Information Requirements, see Table 4) are required to include either a package insert (following the same specifications as the standard CDFT), or a URL linking consumers to a complete CDFT (as outlined in Guidance Document: Electronic Canadian Drug Facts Table (eCDFT) Technical Standards).
For Division 8 drugs, the CDFT is required to be located on the outer label. However, if the packaging cannot fit the standard CDFT, available flexibilities should be applied or an innovative label can be used. A package insert (Part III of the Product Monograph) is required to be included in the packaging for all Division 8 drugs. If the Part III of the Product Monograph includes the information removed from the CDFT, a complete CDFT is not required to be added to the insert.
Please refer to Section 2.6 of this document for details on the new flexibility to be made available for products falling in Category 1 as defined in the context of the Self-Care Framework.
Table 3: Canadian Drug Facts Table format with graduated flexibilities
Level 1 flexibilities available for products with two unilingual branding panels
- Move content from 'Uses' heading to the principal display panel (with conditions, see below)
- Remove hairlines from 'Warnings' section
- Remove 'Drug Facts (continued)' title (with conditions, see below)
- Begin text immediately following 'Warnings' section heading
- Reduce weight of rules to 1.0 point
- Continuous box frame (with conditions, see below)
Sponsors are not required to apply all flexibilities in conjunction.
For products with two unilingual branding panels, sponsors are only permitted to apply the flexibilities described above.
Level 1 |
Sponsors are not required to apply all Level 1 flexibilities in conjunction, or to apply all of Level 1 prior to applying Level 2. |
|---|---|
Level 2 - Use of a condensed font |
|
Level 3 - Move storage information and inactive ingredients |
|
Level 4 - Move 'point of use' warning information |
|
Conditions
- Content from the 'Uses' heading can be moved from the CDFT to the principal display panel. However, uses cannot be combined with non-approved statements that are promotional in nature.
- Removal of the 'Drug Facts (continued)' heading must be accompanied by a strategy to point the consumer to continued information (e.g., arrowhead).
- Where multiple panels are sequential, sponsors are permitted to use a single, continuous box frame rather than a frame around each individual panel. Text is not permitted within 3.175 mm (1/8 inch) of a fold line. Note that when applying a continuous box frame, all panels of the CDFT must maintain a consistent orientation.
- Moving point-of-use warning information from the outer label to a package insert is permitted only after applying the flexibilities included in Levels 1 to 3. Sponsors may use Level 4 flexibilities while keeping the list of inactive ingredients in the CDFT if moving the inactive ingredients provides no additional space on the label, subject to review.
2.5 Tailored flexibilities for Category IV products, mouthwashes and toothpastes
Category IV products as well as mouthwashes and toothpastes that limit information in the CDFT on the physical label (as per the Minimum Information Requirements below) are required to include either a package insert (following the same specifications as the standard CDFT), or a URL linking consumers to a complete CDFT. The inclusion of a URL is optional for products that include a complete CDFT on the physical label or within an insert. Information on the standards to be used in applying a URL in order to enable consumers to access a complete electronic version of the CDFT can be found in the Guidance Document: Electronic Canadian Drug Facts Table (eCDFT) Technical Standards.
Sponsors may apply any of the flexibilities described in Table 4 to the CDFT on the outer label. Note that conditions for the use of a condensed font remain the same as previously indicated for the CDFT format with graduated flexibilities, meaning that sponsors must merge two unilingual branding panels to a single bilingual branding panel prior to adopting a condensed font. Sponsors may also use the flexibilities described in Table 4 when using an innovative label, however sponsors may not remove point-of-use warning information.
The flexibilities listed under Table 4: Tailored Flexibilities for Category IV Products, Mouthwashes and Toothpastes, will be superseded by those listed under Table 5: Labelling for Low-Risk Non-prescription Drugs (i.e., Self-Care Framework Category 1 Products), as of December 1, 2018.
Table 4: Tailored flexibilities for Category IV products, mouthwashes and toothpastes
Available formatting flexibilities (url/ package insert optional)
- Remove hairlines from 'Warnings' section
- Remove Drug Facts (continued) title (with conditions, see below)
- Begin text immediately following 'Warnings' section heading
- Reduce weight of rules to 1.0 point
- Continuous box frame (with conditions, see below)
- Use of a condensed font is permitted (see Level 2 flexibilities in Table 3)
- The character width may be reduced by no more than 10% horizontally (i.e., 90% horizontal scale).
- Move the list of 'Inactive ingredients' to elsewhere on the outer label or on a tag attached to the package. The 'Inactive ingredients' heading must remain in the CDFT with a note directing the consumer to the location of this information
Minimum information requirements (URL/ package insert required)
The CDFT on the outer label of Category IV products, mouthwashes and toothpastes must include the following minimum information:
- 'Active ingredients' ('Purpose' is not required)
- 'Uses' (if not present on the principal display panel)
- Point-of-selection warnings, i.e., information that is needed by the consumer when selecting a product for purchase, such as:
- 'Warning' statements regarding route of administration
- 'Do not use' subsection
- 'Ask a doctor or pharmacist before use if you' subsection
- Warnings regarding drowsiness or excitability
- 'Directions'
- 'Inactive ingredients'
- 'Questions?' and contact information
- A statement directing consumers to a URL or package insert where they can view a complete CDFT.
Sponsors may choose to include the 'Keep out of reach of children' warning statement in the CDFT or may place it elsewhere on the outer label. Should a sponsor wish to include any other 'point-of-use' information on the outer label (e.g., 'When using this product'), the information must be presented in the CDFT format.
Conditions
- Removal of the 'Drug Facts (continued)' heading must be accompanied by a strategy to point the consumer to continued information (e.g., arrowhead).
- Where multiple panels are sequential, flexibilities allow for the use of a single, continuous box frame rather than a frame around each individual panel. Text is not permitted within 3.175 mm (1/8 inch) of a fold line. Note that when applying a continuous box frame, all panels of the CDFT must maintain a consistent orientation..
- Content from the 'Uses' heading can be moved from the CDFT to the principal display panel. However, uses cannot be combined with non-approved statements that are promotional in nature.
- Excluding the ‘Purpose’ and/or moving the ‘Uses’ to the principal display panel does not necessitate a URL/package insert.
2.6 Labelling for low risk non-prescription drugs (i.e., Self-Care Framework Category 1 products)
Updates to the Regulations, including changes to labelling requirements, will be made in the context of the Self-Care Framework implementation plan. One such change will be the voluntary tabular format of the CDFT for those products that are low-risk and have cosmetic-like claims.
Effective December 2018, select Category IV products, mouthwashes, and toothpastes that are considered to be low risk and that align with the applicable monograph, will be considered as Category 1 products under the Self Care Framework. These products will not be required to present the labelling information in tabular format and will have access to the flexibilities listed in Table 5.
Only those products that fully align with the following monographs or combinations thereof (to be updated by December 2018) will be subject to the flexibilities described in Table 5:
- Acne therapy
- Anti-dandruff products
- Antiseptic skin cleansers (domestic/personal care use)
- Athlete's foot treatments
- Diaper rash products
- Oral health
- Medicated skin care products
- Primary sunscreen
- Secondary sunscreen
Any other Category IV products that do not fully align with the above listed monographs or combinations thereof will not have access to the Category 1 flexibilities presented in Table 5, nor the Category IV products, mouthwashes, and toothpastes flexibilities presented in Table 4, as of December 1st, 2018.
Table 5: Labelling for low risk non-prescription drugs (i.e., Self-Care Framework Category 1 products)
Available formatting flexibilities
- Remove Drug Facts title and Drug Facts (continued) title
- Remove rules, hairlines, and/ or box frame
- Remove 'Purpose' of active ingredients
- Move 'Uses' to principal display panel
- Begin text immediately following section headings and subheadings
- Use of a condensed font is permitted (see Level 2 flexibilities in Table 3)
- The character width may be reduced by no more than 10% horizontally (i.e., 90% horizontal scale).
- Sequence of the information can be altered
- Sections may continue on subsequent panels, however the 'Warnings' section must be continual (i.e., all warnings must be kept together).
- Move the list of 'Inactive ingredients' to a tag attached to the package or a URL with optional package insert. The 'Inactive ingredients' heading must remain on the outer label with a note immediately following the heading directing consumers where they can view a complete CDFT.
- Move point-of-use warnings to a URL with optional package insert, with a statement to the effect of "For complete warnings, visit …" directing consumers to where they can view a complete CDFT (this statement must immediately precede any remaining warning information, and must be kept together with the 'Warnings' section). Warning statements regarding route of administration, drowsiness or excitability, and those following the 'Do not use' and 'Ask a doctor or pharmacist before use if you' subheading must always appear on the outer label.
Minimum formatting requirements
- Text: 6 point sans serif font, 6.5 point leading minimum
- Headings and subheadings left justified and in bold font style
- 'Warnings' must be kept together
- For optimal contrast, all text must be printed with a 100% line black on white. Where a line black is not available, the colour must be displayed in the strongest contrasting colour being used (100% screen black, dark blue, dark brown, dark green and dark purple are acceptable).
The inclusion of a URL directing consumers to an eCDFT is optional for products presenting complete product information. If space is limited, sponsors may move select point-of-use warnings and/or the list of inactive ingredients to an eCDFT, conditional that statements are included to direct consumers to the complete product information (in the form of an eCDFT) on a URL and optional package insert. These statements must be kept together with their respective headings and associated text. When both the inactive ingredients and warnings appear on the same panel of the label, sponsors may combine these statements while complying with the individual requirements (see example below).

Text Equivalent
This image shows an excerpt from a sample drug facts table for a Self-Care Framework Category 1 product. The image shows sample statements for directing consumers to additional information. The bilingual heading Inactive ingredients / Ingrédients inactifs appears, followed by For a list of inactive ingredients and complete warnings, visit / Pour la liste des ingredients inactifs et toutes les mises en garde, visiter: www. URL.com .
On the following line, the heading Warnings / Mises en garde appears, followed by the warning statement For external use only/ Pour usage externe seulement.
2.7 Innovative labels
The choice to use an innovative label may be made early in the package and label development process, or when graduated flexibilities have been applied and space is still lacking. Examples of innovative labels include peel-back, fold-out, etc. Novel label formats must comply with applicable regulations and guidance documents. URLs or QR codes are not considered to be innovative labels.
2.7.1 General principles
2.7.1.1 An innovative label must be accessible to the consumer at the time of product selection, before purchase. The CDFT must be accessible without destroying or compromising the integrity of the label or the package.
2.7.1.2 The 'Drug Facts' title, and the 'Active ingredients' and 'Purposes' sections of the CDFT must be visible at the time of product selection prior to manipulation of the innovative label.
2.7.1.3 For Category IV products, mouthwashes, and toothpastes, the tailored flexibilities may be combined with innovative labels; in these cases, the 'Drug Facts' title and the 'Active ingredients' sections of the CDFT must be visible at the time of product selection prior to manipulation of the innovative label.
2.7.1.4 A statement directing the consumer to the continuation of the table on the innovative label must also be visible.
2.7.2 Permitted flexibilities
Sponsors are permitted to apply the flexibilities listed in Table 3, 4, and 5 to their innovative labels, however flexibilities that remove the list of inactive ingredients or point-of-use warning information from the CDFT are not permitted.
Table 6: Flexibilities available for innovative labels
Sponsors are not required to move to a single branding panel before applying these flexibilities.
- Move content from the 'Uses' heading to the principal display panel (with conditions, see below)
- Remove hairlines from 'Warnings' section
- Remove 'Drug Facts (continued)' title (with conditions, see below)
- Begin text immediately following 'Warnings' section heading
- Reduce weight of rules to 1.0 point
- Continuous box frame (with conditions, see below)
- Use of a condensed font is permitted (see level 2 flexibilities in Table 3)
- The character width may be reduced by no more than 10% horizontally (i.e., 90% horizontal scale).
Conditions
- Content from the 'Uses' heading can be moved from the CDFT to the principal display panel. However, uses cannot be combined with non-approved statements that are promotional in nature.
- Removal of the 'Drug Facts (continued)' heading must be accompanied by a strategy to point the consumer to continued information (e.g., arrowhead).
- Where multiple panels are sequential, flexibilities allow for the use of a single, continuous box frame rather than a frame around each individual panel. Text is not permitted within 3.175 mm (1/8 inch) of a fold line. All panels of the CDFT must maintain a consistent orientation.
3. Canadian Drug Facts Tables for active ingredients
Guidance document: Drug Facts Table for non-prescription drugs
Page details
- Date modified: