Growth and tolerance clinical trial protocol – healthy term newborn infants
A guidance document for infant formula manufacturers
2021
Table of contents
- 1.0 Introduction
- 2.0 Definitions
- 3.0 Protocol for growth and tolerance clinical trials to support term infant formula premarket submissions
- 3.1 Registration
- 3.2 Study title
- 3.3 Abstract
- 3.4 Introduction and background
- 3.5 Study design
- 3.5.1 Description of study design
- 3.5.2 Study objectives
- 3.5.3 Choice of non-inferiority /superiority margin
- 3.5.4 Subject eligibility
- 3.5.5 Sample size
- 3.5.6 Intervention
- 3.5.7 Study duration
- 3.5.8 Assignment
- 3.5.9 Blinding
- 3.5.10 Data collection and outcomes
- 3.5.11 Adverse events
- 3.5.12 Stopping rules, discontinuation, withdrawals and dropouts
- 3.5.13 Quality control procedures
- 3.5.14 Independent Data Monitoring Committee (IDMC)
- 3.5.15 Protocol violation
- 3.5.16 Protocol amendment
- 3.5.17 Pre-planned statistical analyses
- 3.6 Study results
- 3.7 Discussion and conclusions
- 4.0 Ethical standard for investigators
- 5.0 Glossary and references
- Appendix 1. CONSORT 2010 checklist of information to include when reporting a randomised trial
1.0 Introduction
All infant formulas must be safe and fit for human consumption as per Section 4.1 of the Food and Drugs Act. These foods, intended for the infant population, are regulated as per the Food and Drug Regulations (FDR) Part B Division 25, and require a premarket assessment by Health Canada.
Petitioners must submit a premarket notification for a new infant formula or one that has undergone a major change in composition, manufacturing, or packaging. Notifications must include the evidence relied upon to establish that the infant formula is safe, nutritionally adequate and will promote acceptable growth and development in infants when consumed in accordance with the directions for use.
The objective of this document is to provide guidance to manufacturers on the appropriate design of a clinical study of growth and tolerance. Manufacturers are encouraged to consult with Health Canada on the clinical protocol for any planned clinical studies, and to schedule a pre-submission meeting to review the evidence required for premarket assessment, since requirements may vary.
2.0 Definitions
- Healthy growth
Encompasses all aspects of physical growth and normal development, including maturation of organ systems and achievement of normal functional development of motor, neurocognitive, and immune system (1).
- Healthy term infant
Means infant delivered between gestational age of 37 to 42 weeks with birth weight of ≥ 2.5 kilograms with no disease (1).
- Human milk substitute (FDR B.25.001)
Means any food that is represented (a) for use as a partial or total replacement for human milk and intended for consumption by infants, or (b) for use as an ingredient in a food referred to in paragraph (a).
- Infant formula (FDR B.25.001)
Is the common name of a human milk substitute.
- Major Change for a human milk substitute (FDR B.25.001)
Means, in respect of a human milk fortifier or a human milk substitute, any change of an ingredient, the amount of an ingredient or the processing or packaging of the human milk fortifier or human milk substitute where the manufacturer's experience or generally accepted theory would predict an adverse effect on the levels or availability of nutrients in, or the microbiological or chemical safety of, the human milk fortifier or human milk substitute.
Examples of major changes for a human milk substitute:
- substantial change in processing or packaging of the human milk substitute change of or a new manufacturing facility
- addition of a new macronutrient source (protein, fat, or carbohydrate)
- substantial change in the amount of protein, fat, or carbohydrate
- addition of a new ingredient or novel food
- change in the amount or the source of vitamins or minerals, which, in the manufacturer's experience or generally accepted theory, would predict an adverse effect on:
- the levels or availability of the nutrients, or
- the microbiological or chemical safety
For more information about the scientific evidence needed to establish the nutritional adequacy of a new human milk substitute or one that has undergone a major change, please refer to the guide, Scientific Evidence Requirements for Nutritional Adequacy- Healthy term newborn infants.
- New human milk substitute (Infant Formula) (FDR B.25.001)
Means a human milk substitute that is:
- manufactured for the first time, or
- sold in Canada for the first time, or
- manufactured by a person who manufactures it for the first time.
- Normal physical growth
The World Health Organization's Child Growth Standards (2006) are the standards used for physical growth assessment.
3.0 Protocol for growth and tolerance clinical trials to support term infant formula premarket submissions
The following sections discuss all the general requirements for a clinical trial protocol for a growth and tolerance study in healthy term newborn infants. The study report should follow the format specified in Appendix 1 (CONSORT 2010 checklist).
3.1 Registration
Registration of clinical trials should meet the following requirements:
- The protocol should be registered on a clinical trial registry. Examples of acceptable trial primary registries are available on the WHO website (2);
- The registry should include all information and criteria specified in the World Health Organization Trial Registration Data Set (3);
- Registration number and name of trial registry should be reported;
- Registration should indicate where the full trial protocol can be accessed (4);
- Although changes in protocol after commencement are greatly discouraged, the clinical trial's registration must be updated when amendments or protocol violations are made after the trial commencement and details should be clearly described in the trial report (5).
3.2 Study title
The study title should specify that the study is a randomized, non-inferiority trial in healthy term newborn infants (5,6).
3.3 Abstract
The protocol and study report should include an abstract that describes the trial objectives, design and methodology used in data collection and analysis, and study findings/conclusions (7).
3.4 Introduction and background
The introduction should include the following information:
- name and description of the investigational product(s), including the physical form (e.g. powder, concentrated liquid, etc.) and confirmation that the concurrent control is appropriate;
- rationale for the clinical trial and a clearly stated study question;
- description of the population sample to be studied and its similarity with the target population
- reference to literature and data that are relevant to the trial, including background for the trial
- a summary of findings from relevant clinical and non-clinical studies;
- a summary of known and potential risk and benefits, if any, to human subjects, especially infants
3.5 Study design
3.5.1 Description of study design
The study should be designed based on the gold standard for clinical trials: a prospective, randomized, well-controlled, double-blind, parallel and single/multi-center study (8). The inclusion of a schematic diagram of the trial design, procedures, and stages is required.
3.5.2 Study objectives
The objectives of the study should be to assess the effects of the experimental or test formula on measures of growth and tolerance. The control used for comparison is a marketed infant formula in Canada, that has been clinically tested and proven safe and nutritionally adequate, and to an acceptable international growth standard (8,9).
3.5.2.1 Primary objective
The primary objective of the trial should be to assess the nutritional adequacyFootnote 1 as measured by weight gain rate over a 16 weeks interval starting during the first 14 days of life, expressed as grams per day in healthy term newborn infants fed the experimental formula as compared to infants fed a concurrent control of a marketed infant formula in Canada (9).
- Footnote 1
-
For more information on the evidence required by Health Canada to support the nutritional adequacy of term infant formula, please refer to Scientific evidence requirements for nutritional adequacy of a term infant formula – A guidance document for infant formula manufacturers.
3.5.2.2 Secondary objectives
The secondary objectives should be to assess and compare the growth and safety measures between the test, control and breast-fed groups. The breast-fed group should be considered as a reference group. As the growth of breast-fed infants is the gold standard for growth, comparing the growth of infants in the test group to those in the breast-fed group would provide additional information to evaluate the physiologic significance of an effect of a new formula substance on growth (10).
The following growth and safety parameters should be the secondary objectives:
- comparison of the plotted group means attained weight, length and head circumference at pre-specified intervals with the growth percentiles of the WHO Child Growth Standards, 2006 (11);
- rates of gain in length and in head circumference, expressed as cm/week;
- comparison of standardized, plotted group average z-scores (and their 95% confidence intervals) for weight-, length-, and head circumference-for-age, and weight-for length based on the WHO Child Growth Standards (2006) by sex;
- tolerance (including but not limited to colic, fussiness, cramps, sleeping patterns, vomiting, regurgitation, constipation, diarrhea and stool characteristics);
- average daily volume of formula intake together with information on the methods used to ascertain formula intake;
- types and incidence of adverse events and serious adverse events;
- any planned biochemical tests (the need for such tests depends on the nature of the formulation and would be determined on a case-by-case basis).
3.5.3 Choice of non-inferiority /superiority margin
At least one adequately powered clinical study in the target population should be conducted to assess the safety and nutritional adequacy of the test formula. The design of the clinical trial depends on many factors and the study purposes.
A non-inferiority trial, with a predefined margin of non-inferiority (MNI), is highly recommended when the objective of the study is to assess safety and nutritional adequacy in infants fed the test formula as compared to those fed the commercial control formula (6,8). If any additional testing of the efficacy of the test formula is planned (e.g., to support a health claim), a superiority analysis is also required (8,12).
The test hypothesis concerning non-inferiority of growth rates in infants fed the test formula compared to those fed the control formula should be clearly stated, specifying the MNI, which should be clinically justified (6).
It is necessary to define the non-inferiority MNI in advance. The determination of the MNI is based on a combination of both statistical reasoning and clinical judgment. Include a rationale and/or scientific literature to justify the choice of margin in the protocol. The margin chosen for a non-inferiority trial must not be greater than the smallest effect size of the control product. Magnitude and variability of the effect size of the control should be derived from historical trials and should be taken into consideration (13). Furthermore, the selected MNI should reflect uncertainties in the evidence on which the choice is based, be suitably conservative and account for variability as well (6,13). Rationale and/or scientific literature to justify the choice of such margin should be included in the protocol.
In 1988, the American Academy of Pediatrics (AAP) Task Force, based on the 1987 Neilson's data, recommended that a feeding-related difference in weight gain of more than 3 g/day over a 3.5 month period would be considered nutritionally and clinically significant, and the standard deviation of gain in weight on a sex-specific and formula-specific basis for this 3.5-month interval was about 4.5 g/day. In the past decades, several international guidelines and clinical trials have chosen the difference of 3 g/day in weight gain as a clinically acceptable MNI (e.g., -3 g/day) (1,8,14,15,16,17). Health Canada's internal systematic review and meta-analysis using pooled data from 20 recent clinical studies of growth in infants (18), as well as the Guo, et.al., 1991 study (19) show that the MNI of -3 g/day is reasonable and acceptable (refer to Figure 1). The standard deviation of weight gain in infants over the 3.5-month period is, however, about 6.0 g/day, which is higher than the (1988) AAP's value of 4.5 g/day.
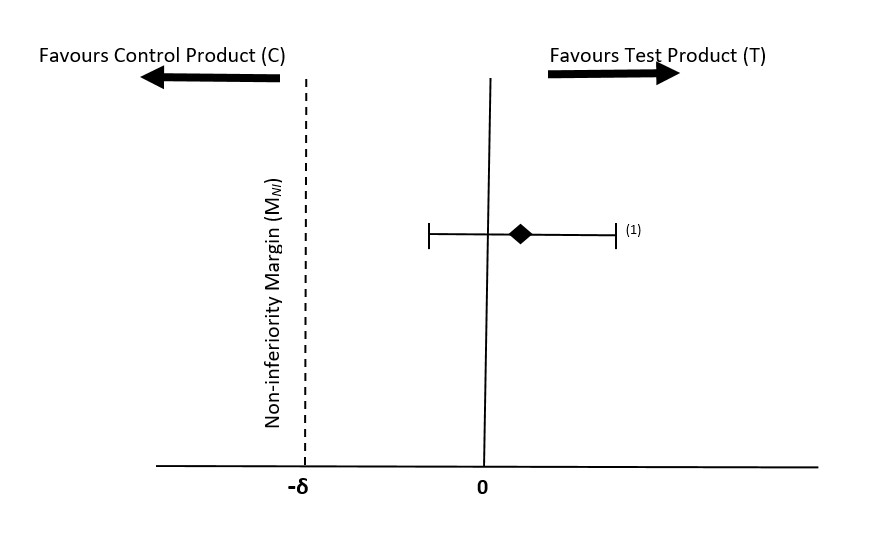
Figure 1 - Text description
Comparison of clinically acceptable MNI of –δ and estimated mean difference in the rate of weight gain (T-C) and its 95% confidence interval. Test product (T) is non-inferior to the control product (C), but not superior(1) (20).
3.5.4 Subject eligibility
At study entry, subjects should be healthy, full-term newborn infants, 0-14 days of age, and the mother must have already decided not to breastfeed the infants in the formula-fed groups. Both male and female infants should be included in the study in numbers that conform to the sample size calculation. Methods of recruitment, clinical settings and geographical locations where the eligibility criteria are to be collected should be reported (21). Dates defining the periods of recruitment and follow-up, and information on how these subjects are intended to be followed-up should be provided.
The following inclusion and exclusion criteria should be applied:
Inclusion criteria
- healthy newborn;
- singleton birth, term infant (37-42 weeks gestation);
- birth weight of 2490-4200 grams;
- 0-14 days of age at enrolment;
- signed consent form obtained from parent/legal guardian;
- exclusive formula feeding before initiation of study and mothers agree to completely refrain from smoking during entire study period;
- exclusive breast-feeding from birth for the breast-fed group and mothers agree to breast-feed during the entire study period and completely refrain from smoking during the entire study period.
Exclusion criteria
- history of underlying disease or condition or congenital malformation that, in the opinion of the investigator, is likely to interfere with the normal growth and development or the evaluation of the participant;
- participation in another clinical study that has not been approved by the sponsor;
- have a family history of cow's milk protein intolerance/allergy;
- have a maternal history with known adverse effects on the fetus and/or the newborn infant, such as diabetes, active tuberculosis, perinatal infection, or substance abuse.
3.5.5 Sample size
The sample size calculation should be based on the primary outcome, with methodologies/criteria specially designed for a non-inferiority trial. Determination of the appropriate sample size depends on various factors including the choice of MNI and statistical power.
As discussed in Section 3.5.3, the MNI of -3 g/day in infant weight gain rate over a 3.5-month interval beginning during the first 2 weeks of life is considered a clinically acceptable margin. The standard deviation of weight gain rate on a sex-specific and formula-specific basis for a 3.5- month period is about 6.0 g/day (19, 22). Recent meta-analyses also show that the difference in weight gain between test formula-fed and concurrent control formula-fed infants over the first 3.5 months of life is not statistically significant (10).
Therefore, it is recommended that sample size should be calculated to test if the experimental formula is inferior to the control formula. With the MNI of -3 g/day, and assuming the expected mean difference in weight gain over 3.5 months between the test and the control groups is zero and the common standard deviation of 6.0 g/ day, the number of subjects of a specified sex needed in each study arm to detect that the test formula is non-inferior to the control formula in weight gain rate with a power of 90% and alpha of 0.025 is 42 subjects for each sex (n = 84 per group). With an anticipated dropout rate of 25%, a minimum required sample size would be 112 subjects per group. Calculation of the statistical power at the end of the study is also required. Since it is well established that the growth of male and female infants are different, subjects should be stratified by sex and growth must be assessed separately (23).
3.5.6 Intervention
Precise details of the intervention intended for each group should be reported. It is recommended that subjects be assigned in a balanced ratio to the following groups:
- Test product
The test product should be the one proposed for the Canadian market. It should, comply with the requirements under Section B.25.046 of the FDR.
- Control product
A control should be included in the study. It is recommended that a Canadian commercial infant formula be used as a concurrent control.
Note: In the case of an infant formula with an added new ingredient, the test and control products should be identical except for the new ingredient.
- Comparison
The protocol should identify and describe any differences in composition and nutritional profiles, including the energy density of the test and control formulas.
- Reference group
A concurrent reference group of breast-fed infants should be included.
3.5.7 Study duration
Subjects should exclusively receive the assigned formula (approximately 80-90% of daily intake) during the peak growth rate period (from ≤14 days of age for at least 16 weeks, 112 days). Long-term follow-up is strongly encouraged in certain cases to confirm the long-term safety and nutritional effects of formulas with new/novel added ingredients (such as probiotics). Health Canada may also request longer-term post marketing surveillance where appropriate.
3.5.8 Assignment
Participant allocation to the treatment or control groups should be randomized using concealed allocation and following the CONSORT guidelines (6,7). Randomization reduces selection bias by controlling for known and unknown confounding factors in the study population. The randomization process includes the following aspects (7, 21):
- Sequence generation
Describe the method used to generate the random allocation sequence. Adequate methods include the use of a random-number table or a computerized random number generator (6). Subjects' randomization should be stratified based on birth weight category, center (in multicenter trial) and sex since boys have a different growth rate than girls.
- Allocation concealment
The sequence of random allocation should be concealed until recruitment is complete, irrevocable, and interventions have been assigned. For example, treatment allocation could be concealed by using a computerized randomization tool or enclosing assignments in sequentially numbered, opaque, sealed containers or sealed and stapled opaque envelopes (21).
Good allocation concealment mechanisms, which incorporate external involvement are recommended (e.g., the use of a pharmacy or central telephone randomization system are two common techniques) (21).
- Randomization implementation
The protocol should include detailed information on all personnel who are responsible for generating the random sequence, enrolling participants and allocating participants to each study arm (21). Any deviation from random allocation should be described in the study report.
3.5.9 Blinding
Study participants (parents/caregivers), investigators and all other study personnel should be blinded to treatment assignment. The test and control products should be identical in appearance (e.g. packaging and texture), taste and smell (21). Detailed information should be given on how products are labelled (e.g. by individual subject codes), who has access to the product codes, whether there are any predefined circumstances in which the blinding could be broken and who from the team of investigators would be un-blinded in case of such a need. In addition, blinding should be maintained until the study is completed. Outcomes assessors should be blinded at all times.
3.5.10 Data collection and outcomes
3.5.10.1 Collection schedule
Describe the data collection schedule. Subjects in the test and control groups should be enrolled and followed in parallel. Medical history prior to the first study visit should be gathered from medical records. Data on concomitant medications should be collected and compared between groups. An appropriate schedule for data collection is presented in Table 1, below.
Type of Data |
Enrollment visit1 |
Study day visit2 |
||||
|---|---|---|---|---|---|---|
Day 14 |
Day 28 |
Day 56 |
Day 84 |
Day 112 |
||
Enrollment/ randomization |
Collect basic data set (above) x |
|||||
Demographic data |
x |
|||||
Weight, length, and head circumference |
x |
x |
x |
x |
x |
x |
Interval history3 |
x |
x |
x |
x |
x |
|
Adverse events, verified by a medical professional |
x |
x |
x |
x |
x |
|
1 Day of birth is day zero (0) of life (enrollment 0-14 days of age); enrollment may be up to day 14 of age.
2 Visit window ± 3 days.
3 Collection of the parents' diaries documenting intakes and tolerance data, and any adverse event or reaction, collected over at least the last 3 days before each visit.
Standardized procedures and calibrated instruments should be used in collecting measurement data. Study personnel training and quality assurance procedures should be in place to ensure measurements are as precise and accurate as possible across participants, study sites, and time of data recording. In cases where incomplete data are collected, the extent and impact of the missing data should be described in the study report and the approach chosen to handle the missing data should be reported.
3.5.10.2 Collection of basic demographic dataset
In order to allow a comprehensive scientific assessment of the study, the following basic information should be recorded for each participant:
- infant sex;
- method of delivery (vaginal or C-section);
- single birth, twins or triplet;
- birth weight in grams;
- gestation in completed weeks;
- birth weight per gestation (e.g., small for gestational age, adequate for gestational age);
- anthropometry at baseline including body weight, length and head circumference in absolute values and z-scores, together with a documentation of the growth standard used to calculate the z-scores. Please refer to the following links to guide on measuring weight, length, and head circumference of infants:
- age at recruitment, randomization, baseline (that is, at the start of the intervention) and at each assessment point;
- race and/or ethnicity;
- maternal and paternal age, education and body mass index (BMI);
- history of smoking, alcohol intake and medications use during pregnancy;
- socioeconomic status (when possible).
3.5.10.3 Collection of intake data
Parents should record formula consumption over at least the last 3 days before each study visit and the average intake should be calculated by the study coordinator. Proper systems should be in place to assess compliance with the intake of the assigned formula during the study. This data is important for the interpretation of trial results.
3.5.10.4 Outcome measurements
The following outcome parameters must be measured over the duration of at least 112 days. At minimum, measurements are required to be taken at the following key time points: at enrollment, study day 0, 14, 28, 56, 84 and 112 (9,23). The study visits should be conducted as close as possible to those planned in the protocol with a visit window of ±3 days at most. Any methods used to enhance the quality of measurements (such as, multiple observations, training of assessors) should be reported.
- Primary outcome
Rate of weight gain over the course of the study expressed as grams per day between study day 0 and the end of 16 weeks (i.e., study completion) (23).
- Secondary outcomes
The following growth and safety outcomes should be compared between the test and control groups:
- achieved body weight, length, and head circumference of each participant at each study visit;
- rate of gain in length (millimeters per day) and in head circumference (centimeters per week) between study day 0 and the end of the 16 weeks (study completion);
- standardized plotted z-scores for weight-for-age, length-for-age, head circumference-for-age, and weight-for-length, together with methodologies used to calculate the z-scores based on the WHO Child Growth Standards (2006), of each participant and group average (and their 95% confidence intervals) at baseline and at each study visit;
- interval weight gain (grams per day) at pre-specified intervals;
- plotted individual raw and group means growth data against the growth percentiles of the WHO Child Growth Standards (2006);
- average daily formula intake volume in milliliters;
- nutrient biochemical indices/biomarker measures, when appropriate/case-by-case (e.g., serum ferritin level when a new source or level of iron is used in the formula, and blood urea nitrogen when a new source or level of protein is used in the formula);
- acceptance/tolerance: parents diaries and physicians' appraisal of tolerance (fussiness, colic, sleeping patterns, cramps, regurgitation, diarrhea, constipation and stool characteristics), respiratory and dermatologic manifestations;
- number of participants with food allergy and/or gastrointestinal symptoms (such as hives, swelling of the body or face, vomiting);
- records of all adverse events and serious adverse events recorded in daily diaries by the parents/caregivers and verified by a medical professional;
- frequencies and severity of adverse events and serious adverse events, as well as the total number of participants with any adverse event.
3.5.11 Adverse events
Accurate and clear information on adverse events and side effects occurring during the study or during a pre-specified follow-up period must be carefully described and reported whether or not it is immediately associated with the study treatment (24).
The protocol to ascertain possible adverse events and side effects should be defined a priori and reported. This should include the duration and frequency of monitoring, a case definition for common or anticipated adverse events with standardized criteria, checklists or validated scales/scores for diagnosis, where appropriate. The person(s) who detected or confirmed the adverse event must also be documented (e.g., parents, investigator, clinical personnel, sponsor) as well as how the information was collected (e.g., questionnaires, diaries, passive or active surveillance). When pertinent, the method for dealing with and counting recurrent events should also be documented. Any trial stoppage guidelines related to adverse events should be defined and described.
In case of adverse events, the following information should be documented in the medical records and confirmed by medical professionals for each case, as appropriate:
- subject number, date and duration of event;
- description of the event;
- severity (mild, moderate, severe);
- seriousness and frequency;
- potential attribution to the study feeding;
- action taken and details of any medications used;
- participant withdrawal following adverse event.
3.5.12 Stopping rules, discontinuation, withdrawals and dropouts
The protocol should provide details of the procedure to monitor serious adverse events and should indicate the stopping rules, discontinuation criteria or withdrawal criteria for individual subjects, parts of trial and entire trial, and the plan to follow-up withdrawals and dropout (24).
Mechanisms should be in place to reduce withdrawals from the study as much as possible for reasons other than anticipated or unexpected adverse events. For all participants who withdraw from the study prior to the final study visit, the principal investigator should make reasonable attempts to follow up to determine the reasons for dropping-out. The following details of subjects who fail to comply, or who withdraw or dropout should be carefully captured and reported:
- detailed description of participants who have failed to comply, and those who withdrew or dropped-out;
- description of reason(s) for withdrawal or dropping-out from study;
- date and age when stopped participating as per protocol;
- details of formula intakes before dropping out or withdrawal;
- length of time subject was in the study before dropping out;
- reason(s) for non-compliance;
- age at withdrawal or dropout from the study.
3.5.13 Quality control procedures
The protocol should provide a description of the quality control procedures with written standard operating procedures (SOP) to ensure that the trial is conducted and data is generated, documented (recorded), and reported in compliance with the protocol, good clinical practice (GCP), and the applicable regulatory requirement(s) (25). The SOP also ensures that the recruitment into the trial is conducted properly to minimize potential sources of selection bias and all outcome measurements collected during the study are accurate, reproducible, valid and reliable.
3.5.14 Independent Data Monitoring Committee (IDMC)
The sponsor should establish an IDMC to periodically assess the progress of the clinical trial, safety data and efficacy parameters, and to recommend to the sponsor whether to continue the trial, modify or terminate the trial. The IDMC should have written operating procedures and maintain records of all its meetings, including interim results. The Committee should include a biostatistician with knowledge of clinical trial design and analysis; clinical trial scientists knowledgeable in the field; and a physician (5, 25).
3.5.15 Protocol violation
Protocol violations should be reported in the study report and the registry should be updated. The study report should document the assessment of the magnitude of these violations and how much influence they might have on the interpretation of the results (5).
3.5.16 Protocol amendment
Protocol deviations or any significant amendment(s) required during the study should be reported in the study report and study protocol, and the registry should be updated, specifically noting that a change was made. The rationale for any significant amendment and its influence on the interpretation of the results should be assessed, reported in the study report and protocol, and the study registration updated (5).
3.5.17 Pre-planned statistical analyses
Overall, the statistical analysis and methods used should be in line with generally accepted scientific principles (12).
The protocol must clearly state the test hypotheses and/or the treatment effects which are to be estimated in order to satisfy the primary and secondary objectives of the trial. The statistical methods to be used to accomplish these tasks must be clearly described for the primary as well as the secondary analyses and the underlying statistical model must be made clear a priori. A description must be given of any intentions to use baseline data to improve precision or to adjust estimates for potential baseline differences, for example by means of analysis of covariance.
The study power and the statistical significance level must be reported with the expected attrition rate, which should be accounted for during sample size calculation. Calculation of the statistical power at the end of the study is also required (9). Timing of any planned interim analyses and the criteria for the termination of the trial must be indicated.
The criteria for inclusion/exclusion of subjects in the per-protocol (PP) analysis must be planned a priori in the protocol. Detailed description of reasons, method used and rationale for any potential data exclusion and/or adjustment (e.g. deleting a partial data, replacing missing values, adjusting invalid values) from the analysis must be clearly discussed and described in the study report.
Results must be provided for comparisons between the test and control groups for all outcome variables assessed. Growth patterns of the study groups must also be compared with acceptable growth standards [26]. In particular, the following information should be provided (8):
- descriptive and inferential statistics (such as, means, standard deviations, 95% confidence intervals and measures of statistical significance) for each measured outcome for each group at the beginning of the study and at every visit throughout the study should be reported and analysed; both PP and intention-to-treat (ITT) analyses should be performed (8). Non-inferiority and the robustness of the results are clearly established when both PP and ITT analyses demonstrate lack of statistically significant difference between test and control formula-fed groups. Discrepancies in the results of the 2 analyses indicate the possibility of exclusion bias, suggesting that the reasons that participants are not included in the PP analysis is somehow related to the treatment;
- estimates of effect size (e.g., difference in weight gain between the test and control groups) must be accompanied with its inferences (precision);
- the number of infants analysed at each time point for each analysis;
- statistical models and covariates used in the analysis, with appropriate justification for their use
- the presentation and interpretation of the results using confidence interval (CI) in relation to the MNI;
- the results of both the adjusted and unadjusted analysis;
- for each sex, plotted mean achieved body weight, length, and head circumference and their 95% confidence intervals against age by study group;
- for each sex, plotted mean weight-for-age, length-for-age, head circumference-for-age and weight-for-length z-scores, based on the WHO Child Growth Standards (2006), for each study group over the study period;
- total number and reasons for dropouts or withdrawals of infants from the study by investigators for each study group, together with an assessment/discussion of the impact of dropouts/withdrawals on the study results;
- if there is a plan to conduct a subgroup analysis or to adjust for any confounders at the analysis stage, details should be specified a priori in the protocol.
- Handling of missing data
If imputation (replacing the missing values with estimates) of missing data is foreseen, information on the robustness of the assumptions made should be reported. Detailed explanations should be provided in the study report as to how such estimations or derivations were done; number and reasons for missing values in each group; what underlying assumptions were made, and the impacts of imputations on the study conclusions.
- Multiple comparisons
For studies for which an adjustment for multiple comparisons is needed, the pre-planned approach towards adjusting for multiplicity should be specified.
3.6 Study results
The study results must be reported in a transparent and reproducible manner in line with the CONSORT Statement (7).
3.6.1 Participants flow chart and analysis of withdrawals and dropouts
A flow chart is strongly recommended, as it is useful in illustrating participants flow throughout the study, from recruitment to study completion. Any deviation from planned protocol should be described together with reasons. For each study arm, the following detailed information should be indicated in the flow chart:
- total number of subjects randomised, receiving intended intervention, completing the study protocol, and being analysed for the primary and secondary outcomes;
- total number of subjects withdrawing or dropping-out from the study and being excluded from the primary and secondary analyses of outcomes.
A table showing baseline demographic and clinical characteristics for each group should be included.
3.6.2 Baseline analyses
Include results of analyses comparing the socio-demographic characteristics and anthropometric measurements at birth and baseline between the test and control groups in the report (refer to Basic dataset in Section 3.5.10.2).
3.6.3 Analyses of primary and secondary outcomes
Include results derived from the PP and ITT analyses of the primary and secondary outcome parameters (listed under "Outcome measurements" Section 3.5.10.4) between the following study arms in the final report:
- test formula group versus control group;
- test formula group versus breast-fed reference group, and control group versus breast-fed reference group.
In particular, clearly report the following findings:
- number of participants analysed at each predefined time point in each of the PP and ITT analyses
- descriptive statistics including mean, median, and its inferences for each outcome variable by sex and study arm;
- mean z-scores for weight, length, head circumference-for- age, weight-for-length and its inferences by sex and study group;
- achieved weight, length and head circumference at each study visit and over the study duration by group;
- estimates of significant mean differences, their inferences and p-values in each assessed outcome across the above groups, as well as detailed discussions regarding those significant differences.
The WHO Child Growth Standards (2006) should be used to compare study group's mean attained weight, length, and head circumference at each study visit and over the duration of the study (from ≤14 of age to the study completion).
- Individual growth charts
The individual anthropometric data should be plotted on the WHO Child Growth Standards (2006) in order to trace the growth performance of each individual. Individual growth charts must be provided in the infant formula premarket notification.
- Z-scores
The individual anthropometric measurements can be transformed to z-scores based on the standard growth charts. These z-scores indicate the distance and direction of an observation away from the population mean (standard deviations above and below the mean).
Group z-scores for weight, length, head circumference-for-age and weight-for-length for each sex should be plotted on the WHO Child Growth Standards.
3.6.4 Analyses of adverse events
Detailed information, listed under adverse events Section 3.5.11, for subjects with incidents of adverse events should be interpreted by comparing the occurrence of adverse events in each study arm, withdrawals resulting from these events, and also by assessing the severity against the number of events. It is strongly recommended that the clinical assessment of adverse events and its attribution to the assigned treatment be conducted in a blinded manner by an independent medical professional. These data should inform a balanced assessment of harms and benefits.
If further examination is required to assess the relationship between an occurrence of a serious adverse event and the intervention, all pertinent examinations or laboratory findings must be noted with their results in the case report form or attached to a follow-up file.
3.7 Discussion and conclusions
Discussion and interpretation of the study results should clearly distinguish between clinical and statistical significance. The authors' conclusions should be in line with the reported study results taking the non-inferiority hypotheses into account. It should be indicated whether the conclusion relating to non-inferiority is based on PP or ITT analysis or both, and whether the conclusions are consistent with both analyses. They should include a balanced assessment of the benefits and harms with an emphasis on study limitations, and should consider any other relevant evidence including consistency or inconsistency with other trials, sources of potential bias or imprecision, lack of or partial blinding, dangers associated with multiplicity of analyses and outcomes and generalizability (external validity, applicability of the study findings).
4.0 Ethical standard for investigators
4.1 Informed consent (International Conference on Harmonisation (ICH) E 6 (R1))
Each potential subject (in this case parents or caregivers) must be adequately informed of the aims, methods, sources of funding, any possible conflicts of interest, institutional affiliations of the researcher, the anticipated benefits and potential risks of the study and the discomfort it may entail, post-study provisions and any other relevant aspects of the study. Parents or caregivers must be informed of the right to refuse to participate in the study or to withdraw consent to participate at any time without reprisal (25).
The study is to be conducted in accordance with the ethical principles and rules that have their origin in the declaration of Helsinki and its subsequent amendments and should be consistent with good clinical practice (GCP) and by any applicable regulatory requirements.
The protocol, any amendments and the informed consent process should receive Institutional Review Board (IRB) approval/favourable opinion prior to initiation. The institution granting the approval should be reported. The name of any contract research organisation that has been tasked to carry out the work should be provided.
Investigational products should be manufactured, handled and stored in accordance with applicable good manufacturing practices (GMPs). They should be used in accordance with the approved protocol.
4.2 Funding, sponsorship and conflict of interest
The protocol should include information regarding funding, sponsors, institutional affiliations, potential conflicts of interest, incentives for subjects and information regarding provisions for treating and/or compensating subjects who are harmed as a consequence of participation in the research study. The exact role and contribution of the funder and sponsor to the study should be disclosed (e.g. in the design, conduct, analysis and/or reporting) (5).
An investigator who is independent of the breastmilk substitute industry should take overall responsibility for the conduct of the trial, planning and conduct of statistical analyses, decision to publish, reporting and interpretation of the trial findings (5).
4.3 Investigators credentials
The protocol should describe the necessary qualifications and experience of the investigators.
5.0 Glossary and references
5.1 Glossary
Adjusted analysis
Usually refers to attempts to control for baseline imbalances between groups in important patient characteristic. Sometimes used to refer to adjustment of p-value to take account of multiple testing (27).
Adverse effect
Is an adverse event for which the causal relation between the intervention and the event is at least a reasonable possibility by it (24).
Adverse event
An adverse outcome that occurs during or after the use of a drug or other intervention but is not necessarily caused by it (27).
Adverse event grading of mild, moderate and severe
This is commonly used in non-oncology studies. The definition of the mild, moderate, and severe may be different from one study protocol to another. The severity (intensity) of each adverse event including serious adverse events are assigned to one of the following categories:
- mild: an event that is easily tolerated by the subject, causing minimal discomfort and not interfering with everyday activities
- moderate: an event that is sufficiently discomforting to interfere with normal everyday activities
- severe: an event that prevents normal everyday activities
OR
- mild: awareness of sign or symptom, but easily tolerated
- moderate: discomfort sufficient to cause interference with normal activities
- severe: incapacitating, with inability to perform normal activities (28)
Adverse reaction
Events for which a causality link to the intervention is well established and strong enough (sensitive and specific) to warrant attribution of the event to the intervention (24).
Allocation concealment
Is a technique used to ensure an unpredictable sequence of assignments. It is a critical process that prevents foreknowledge of treatment assignment and thus shields those who enroll participants from being influenced by this knowledge. The decision to accept or reject a participant should be made, and informed consent should be obtained from the participant, in ignorance of the next assignment in the sequence (27).
Allocation sequence
A list of interventions, randomly ordered, used to assign sequentially enrolled participants to intervention groups (27).
Attrition
The loss of participants during the course of a study (also called loss to follow up). Participants that are lost during the study are often call dropouts (29).
Bias
Systematic distortion of the estimated intervention effect away from the "truth" caused by inadequacies in the design, conduct, or analysis of a trial (27).
Blinding
The process of preventing those involved in a trial from knowing to which comparison group a particular participant belongs. It is the practice of keeping the trial participants, care providers, data collectors, and sometimes those analyzing data unaware of which intervention is being administered to which participant (27).
Clinical trial
An experiment to compare the effects of two or more healthcare interventions. Clinical trial is an umbrella term for a variety of designs of healthcare trials, including uncontrolled trials, controlled trials, and randomized controlled trials (29).
Clinically significant
A result (e.g., a treatment effect) that is large enough to be of practical importance to patients and healthcare providers. This is not the same thing as statistically significant. Assessing clinical significance takes into account factors such as the size of a treatment effect, the severity of the condition being treated, the side effects of the treatment, and the cost (29).
Confidence interval
A measure of the precision of an estimated value. The interval represents the range of values, consistent with the data that is believed to encompass the "true" value with high probability (usually 95%). The confidence interval is expressed in the same units as the estimate. Wider intervals indicate lower precision; narrow intervals indicate greater precision (27).
Confounded comparison
A comparison between two treatment groups that will give a biased estimate of the effect of treatment due to the study design. For a comparison to be not confounded, the two treatment groups must be treated identically apart from the randomized treatment (29).
Confounder
A factor that is associated with both an intervention (or exposure) and the outcome of interest (29).
Control
In a controlled trial: A participant in the arm that acts as a comparator for one or more experimental interventions. Controls may receive placebo, no treatment, standard treatment, or an active intervention, such as a standard drug;
In statistics: To adjust for, or take into account, extraneous influences or observations (29).
Effect size
A generic term for the estimate of effect of treatment for a study;
A dimensionless measure of effect that is typically used for continuous data when different scales (e.g., for measuring pain) are used to measure an outcome and is usually defined as the difference in means between the intervention and control groups divided by the standard deviation of the control or both groups (29).
Efficacy
The extent to which an intervention produces a beneficial result under ideal conditions. Clinical trials that assess efficacy are sometimes called explanatory trials and are restricted to participants who fully co-operate (29).
Exclusive breastfeeding (EBF)
Breastfeeding with no supplemental liquid or solid foods other than medications or vitamins (30).
External validity
The extent to which the results of a trial provide a correct basis for generalization to other circumstances (27).
Generalisability, generalisation
The extent to which the findings of a clinical trial can be reliably extrapolated from the subjects who participated in the trial to a broader patient population and a broader range of clinical settings (12).
Hypothesis test
A statistical procedure to determine whether to reject a null hypothesis on the basis of the observed data (29).
Imputation
A procedure for entering a value for a specific data item where the response is missing or unusable. This is done by changing some of the responses or assigning values when they are missing on the record being edited to ensure that estimates are of high quality and that a plausible, internally consistent record is created (31).
Intention-to-treat analysis
A strategy for analysing data from a randomised controlled trial. All participants are included in the arm to which they were allocated, whether or not they received (or completed) the intervention given to that arm. Intention-to-treat analysis prevents bias caused by the loss of participants, which may disrupt the baseline equivalence established by randomisation and which may reflect non-adherence to the protocol. The term is often misused in trial publications when some participants were excluded (29).
Interim analysis
Any analysis intended to compare treatment arms with respect to efficacy or safety at any time prior to the formal completion of a trial (12).
Internal validity
The extent to which the design and conduct of a study are likely to have prevented bias (29).
Mean
An average value, calculated by adding all the observations and dividing by the number of observations (29).
Multicenter trial
A clinical trial conducted according to a single protocol but at more than one site, and therefore, carried out by more than one investigator (12).
Multiplicity
The proliferation of possible comparisons in a trial. Common sources of multiplicity are multiple outcomes, outcomes assessed at several time points after the intervention, subgroup analyses, or multiple intervention groups (27).
Non-inferiority margin (MNI)
The margin is the largest difference that can be judged as being clinically acceptable and should be smaller than differences observed in superiority trials of the active comparator (12).
Non-inferiority trial
A trial with the primary objective of showing that the response to the investigational product is not clinically inferior to a comparative agent (active or a placebo control) (12), a one-sided version of an equivalence trial (29).
Null hypothesis
In simplest terms, the null hypothesis states that the factor of interest (e.g., treatment) has no impact on outcome (e.g., risk of death) (29).
One-tailed test
A hypothesis test in which the values for which we can reject the null hypothesis are located entirely in one tail of the probability distribution. Testing whether one treatment is better than another (rather than testing whether one treatment is either better or worse than another) would be a one-tailed test (29).
P-value
The probability (ranging from almost zero to one) that the results observed in a study (or results more extreme) could have occurred by chance if in reality the null hypothesis was true.
Percentiles
Are the most commonly used clinical indicator to assess the size and growth patterns of an individual infant. Percentiles rank the position of an individual by indicating what percent of the reference population the individual would equal or exceed. Percentiles are less useful at the extremes (>99 and < 1) compared to z-scores (32).
Per-protocol
An analysis of the subset of participants from a randomized controlled trial who complied with the protocol sufficiently to ensure that their data would be likely to exhibit the effect of treatment. This subset may be defined after considering exposure to treatment, availability of measurements and absence of major protocol violations. The per protocol analysis strategy may be subject to bias as the reasons for non-compliance may be related to treatment (29).
Power
The probability that a trial will detect as statistically significant an intervention effect of a specified size. The pretrial size is often chosen to give the trial the desired power (12).
Precision [In statistics]
A measure of the likelihood of random errors in the results of a study, meta-analysis or measurement. The greater the precision, the less random error. Confidence intervals around the estimate of effect from each study are one way of expressing precision, with a narrower confidence interval meaning more precision (29)..
Primary outcome
The outcome deemed of greatest importance (29).
Prospective study
In evaluations of the effects of healthcare interventions, a study in which people are identified according to current risk status or exposure, and followed forwards through time to observe outcome (29).
Protocol deviation
A protocol deviation occurs when, without significant consequences, the activities on a study diverge from the Institutional Review Board-approved protocol, for example, missing a visit window because the subject is traveling. Not as serious as a protocol violation (33).
Protocol violation
A divergence from the protocol that materially: reduces the quality or completeness of the data,
makes the Informed Consent Form inaccurate, or impacts a subject's safety, rights, or welfare (33).
Safety
Substantive evidence of an absence of harm. The term is often misused when there is simply absence of evidence of harm (24).
Selection bias
Systematic error in creating intervention groups, causing them to differ with respect to prognosis. That is, the groups differ in measured or unmeasured baseline characteristics because of the way in which participants were selected for the study or assigned to their study groups. The term is also used to mean that the participants are not representative of the population of all possible participants (12).
Serious adverse event (in the context of a clinical trial)
When in the view of either the investigator or sponsor it results in any of the following outcomes: Death, a life threatening adverse event, inpatient hospitalization or prolongation of existing hospitalization, a persistence or significant incapacity or substantial disruption of the ability to conduct normal life functions (25).
Side effect
Is an adverse event for which the causal relation between the intervention and the event is at least a reasonable possibility by it (24).
Standard deviation
A measure of the spread or dispersion of a set of observations, calculated as the average difference from the mean value in the sample (29).
Stratified randomization
Random assignment within groups defined by participant characteristics, such as age or disease severity, intended to ensure good balance of these factors across intervention groups (27).
Superiority trial
A trial with the primary objective of showing that the response to the investigational product is superior to a comparative agent (active or placebo control) (12).
Type I error
A conclusion that a treatment works, when it actually does not work. The risk of a Type I error is often called alpha. In a statistical test, it describes the chance of rejecting the null hypothesis when it is in fact true (also called false positive) (29).
Type II error
A conclusion that there is no evidence that a treatment works, when it actually does work. The risk of a Type II error is often called beta. In a statistical test, it describes the chance of not rejecting the null hypothesis when it is in fact false. The risk of a Type II error decreases as the number of participants in a study increases (also called false negative) (29).
Z-score
Is the deviation of the value for an individual from the mean value of the reference population divided by the standard deviation for the reference population (32).
5.2 References
- Institute of Medicine (US) Committee on the Evaluation of the Addition of Ingredients New to Infant Formula. (2004). Infant formula: evaluating the safety of new ingredients. Washington (DC). National Academies Press.
- World Health Organization (WHO). (2021). WHO primary registries. [Online]. Available: https://www.who.int/clinical-trials-registry-platform/network/primary-registries.
- World Health Organization (WHO). (2009). International clinical trials registry platform (ICTRP). [Online]. Available: https://www.who.int/clinical-trials-registry-platform/network/registry-criteria.
- Boutron I, Altman DG, Moher D, Schulz KF, Ravaud P; CONSORT NPT Group. (2017). CONSORT Statement for Randomized Trials of Nonpharmacologic Treatments: A 2017 Update and a CONSORT Extension for Nonpharmacologic Trial Abstracts. Ann Intern Med. 67(1):40-47..
- Jarrold, K., Helfer, B., Eskander, M., Crawley, H., Trabulsi, J., Caulfield, L. E., Duffy, G., Garcia-Larsen, V., Hayward, D., Hyde, M., Jeffries, S., Knip, M., Leonardi-Bee, J., Loder, E., Lodge, C. J., Lowe, A. J., McGuire, W., Osborn, D., Przyrembel, H., Renfrew, M. J., … Boyle, R. J. (2020). Guidance for the Conduct and Reporting of Clinical Trials of Breast Milk Substitutes. JAMA pediatrics, 174(9), 874–881.
- Piaggio G, Elbourne DR, Pocock SJ, Evans SJ, Altman DG; CONSORT Group. (2012). Reporting of noninferiority and equivalence randomized trials: extension of the CONSORT 2010 statement. JAMA. 308(24), 2594-604.
- Schulz KF, Altman DG, Moher D; CONSORT Group. (2010). CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. J Clin Epidemiol. 63(8), 834-40.
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies). (2017). Scientific and technical guidance for the preperation and presentation of an application for authorisation of an infant and/or follow-on formula manufacturer from protein hydrolysates. EFSA Journal, 15(5), 4779.
- Food and Drug Administration. (2014). Current good manufacturing practices, quality control procedures, quality factors, notification requirements, and records and reports, for infant formula. [Online]. Available: https://www.federalregister.gov/documents/2014/06/10/2014-13384/currentgood-manufacturing-practices-quality-control-procedures-quality-factors-notification.
- Wallingford J and Barber C. (2019). A review of studies on the growth of infants fed infant formula. Current Developments in Nutrition, 3(9), nzz095.
- Center for Diseases Control and Prevention. (2010). WHO Growth Standards are recommended for use in the U.S. for infants and children 0 to 2 years of age. [Online]. Available: https://www.cdc.gov/growthcharts/who_charts.htm.
- International Conference on Harmonisation (ICH) of Technical Requirements for Registration of Pharmaceuticals for Human Use. (1998). ICH Harmonised Tripartite Guideline, Statistical Principles For Clincal Trials E9. [Online]. Available: https://database.ich.org/sites/default/files/E9_Guideline.pdf.
- U.S. Food and Drug Administration. (2016). Non-inferiority clinical trials to establish effectiveness. [Online]. Available: https://www.fda.gov/media/78504/download.
- European Commission. (2003). Report of the Scientific Committee on Food on the revision of essential requirements of infant formulae and follow-on formulae. [Online]. Available: Report of the Scientific Committee on Food on the Revision of Essential Requirements of Infant Formulae and Follow-on Formulae (europa.eu).
- Puccio G, Alliet P, Cajozzo C, Janssens E, Corsello G, Sprenger N, Wernimont S, Egli D, Gosoniu L Steenhout P. (2017). Effects of infant formula with human milk oligosaccharides on growth and morbidity: A randomized multicenter trial. Journal of pediatric gastroenterology and nutrition, 64(4), 624-631.
- Béghin L, Marchandise X, Lien E, Bricout M, Bernet J, Lienhardt J, Jeannerot F, Menet V, Requillart J, Marx J, De Groot N, Jaeger J, Steenhout P, Turck D. (2019). Growth, stool consistency and bone mineral content in healthy term infants fed sn-2-palmitate-enriched starter infant formula: A randomized, double-blind, multicentre clinical trial. Clinical Nutrition, 38(3),1023-1030.
- Kouwenhoven S, Antl N, Finken M, Twisk J, van der Beek EM, Abrahamse-Berkeveld M, van de Heijning B, Schierbeek H, Holdt LM, van Goudoever JB, Koletzko BV. (2020). A modified low-protein infant formula supports adequate growth in healthy, term infants: a randomized, double-blind, equivalence trial. The American Journal of Clinical Nutrition, 111(5),962-974.
- Chen L and Nguyen L. Meta-Analysis for non-inferiority margins of weigh gains between formula-fed and breast-fed infant. Manuscript in preparation.
- Guo S, Roche A, Fomon S, Nelson S, Chumlea W, Rogers R, Baumgartner R, Ziegler E, Siervogel R. (1991). Reference data on gains in weight and length during the first two years of life. The Journal of Pediatrics, 119(3), 355-362.
- Schumi J and Wittes J. (2011). Through the looking glass: understanding non-inferiority. Trials, 12(106), 1-12.
- Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, Elbourne D, Egger M, Altman DG. (2010). CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. Journal of clinical epidemiology, 63(8),e1-e37.
- Nelson S, Rogers R, Ziegler E, Fomon S. (1989). Gain in weight and length during early infancy. Early Human Development, 19(4). 223-239.
- American Academy of Pediatrics Committee on Nutrition. (1988). Clinical testing of infant formulas with respect to nutritional suitability for term infants, report to the FDA.
- Ioannidis JPA, Evans SJW, Gøtzsche PC, O'Neill RT, Altman DG, Schulz K, Moher D for the CONSORT group. (2004). Better reporting of harms in randomized trials: an extension of the CONSORT statement. Ann Intern Med, 141(10), 781-8.
- International Conference on Harmonisation (ICH) of Technical Requirements for Registration of Pharmaceuticals for Human Use, "ICH harmonised guideline for good clinical practice E 6 (R2)," 2016. [Online]. Available: https://database.ich.org/sites/default/files/E6_R2_Addendum.pdf.
- World Health Organization (WHO). (2006). Multicentre growth reference study group, WHO child growth standard based on length/heigh, weight and age. Acta Paediatrica, 76-85.
- Altman DG, Schulz KF, Moher D, Egger M, Davidoff F, Elbourne PC, GØtzsche PC, Lang T for the CONSORT Group. (2001). The revised CONSORT statement for reporting randomized trials: explanation and elaboration. Annals of internal medicine, 134(8), 663-694.
- Deng C. (2018). Grading the severity of AEs and its impact on AE reporting [Online]. Available: http://onbiostatistics.blogspot.com/2018/05/grading-severity-of-aes-and-its-impact.html.
- Cochrane Collaboration. (2019). Glossary of Cochrane Terms [Online]. Available: https://epoc.cochrane.org/sites/epoc.cochrane.org/files/public/uploads/SURE-Guides-v2.1/Collectedfiles/source/glossary.html
- World Health Organization (WHO), "Optimal feeding of low-birth-weight infants," 2006 [Online]. Available: https://apps.who.int/iris/bitstream/handle/10665/43602/9789241595094_eng.pdf;jsessionid=80736F92F581729831081B1CA71D704B?sequence=1
- Organisation for Economic Co-operation and Development, "Economic Commission of Europe of the United Nations (UNECE) Glossary of terms on statistical data editing, conference of European statisticians, methodological material.," 2000 [Online]. Available: https://stats.oecd.org/glossary/detail.asp?ID=3462.
- National Center for Health Statistics [Online]. Available: https://www.cdc.gov/nchs/index.htm
- Bhatt A. (2012). Protocol deviation and violation. Perspectives in Clinical Research, 3(3): 117
Section/Topic |
Item No |
Checklist item |
|---|---|---|
Title |
1a |
Identification as a randomized trial in the title |
Abstract |
1b |
Structured summary of trial design, methods, results, and conclusions (for specific guidance see CONSORT for abstracts (21,31)) |
Introduction |
||
Background and objectives |
2a |
Scientific background and explanation of rationale |
2b |
Specific objectives or hypotheses |
|
Methods |
||
Trial design |
3a |
Description of trial design (such as parallel, factorial) including allocation ratio |
3b |
Important changes to methods after trial commencement (such as eligibility criteria), with reasons |
|
Participants |
4a |
Eligibility criteria for participants |
4b |
Settings and locations where the data were collected |
|
Interventions |
5 |
The interventions for each group with sufficient details to allow replication, including how and when they were actually administered |
Outcomes |
6a |
Completely defined pre-specified primary and secondary outcome measures, including how and when they were assessed |
6b |
Any changes to trial outcomes after the trial commenced, with reasons |
|
Sample size |
7a |
How sample size was determined |
7b |
When applicable, explanation of any interim analyses and stopping guidelines |
|
Randomization |
||
Sequence generation |
8a |
Method used to generate the random allocation sequence |
8b |
Type of randomization; details of any restriction (such as blocking and block size) |
|
Allocation concealment mechanism |
9 |
Mechanism used to implement the random allocation sequence (such as sequentially numbered containers), describing any steps taken to conceal the sequence until interventions were assigned |
Implementation |
10 |
Who generated the random allocation sequence, who enrolled participants, and who assigned participants to interventions |
Blinding |
11a |
If done, who was blinded after assignment to interventions (e.g., participants, care providers, those assessing outcomes) and how |
11b |
If relevant, description of the similarity of interventions |
|
Statistical methods |
12a |
Statistical methods used to compare groups for primary and secondary outcomes |
12b |
Methods for additional analyses, such as subgroup analyses and adjusted analyses |
|
Results |
||
Participant flow (a diagram is strongly recommended) |
13a |
For each group, the numbers of participants who were randomly assigned, received intended treatment, and were analyzed for the primary outcome |
13b |
For each group, losses and exclusions after randomization, together with reasons |
|
Recruitment |
14a |
Dates defining the periods of recruitment and follow-up |
14b |
Why the trial ended or was stopped |
|
Baseline data |
15 |
A table showing baseline demographic and clinical characteristics for each group |
Numbers analysed |
16 |
For each group, number of participants (denominator) included in each analysis and whether the analysis was by original assigned groups |
Outcomes and estimation |
17a |
For each primary and secondary outcome, results for each group, and the estimated effect size and its precision (such as 95% confidence interval) |
17b |
For binary outcomes, presentation of both absolute and relative effect sizes is recommended |
|
Ancillary analyses |
18 |
Results of any other analyses performed, including subgroup analyses and adjusted analyses, distinguishing pre-specified from exploratory |
Harms |
19 |
All important harms or unintended effects in each group (for specific guidance see CONSORT for harms (28)) |
Discussion |
||
Limitations |
20 |
Trial limitations, addressing sources of potential bias, imprecision, and, if relevant, multiplicity of analyses |
Generalisability |
21 |
Generalisability (external validity, applicability) of the trial findings |
Interpretation |
22 |
Interpretation consistent with results, balancing benefits and harms, and considering other relevant evidence |
Other information |
||
Registration |
23 |
Registration number and name of trial registry |
Protocol |
24 |
Where the full trial protocol can be accessed, if available |
Funding |
25 |
Sources of funding and other support (such as supply of drugs), role of funders |
1CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials (7) – Recommend reading in conjunction with the CONSORT 2010 Explanation and Elaboration or important clarifications on all the items
Page details
- Date modified: