Data on cannabis adverse reactions: 2020 annual report
Download in PDF format
(706.5 KB, 27 pages)
Organization: Health Canada
Date published: December 2022
Cat.: H131-1E-PDF
ISBN: 2817-0555
Pub.: 220663
Adverse reactions associated with cannabis reported to Health Canada between January 1, 2020 - December 31, 2020
Contents
- Key highlights
- 1.0 Introduction
- 2.0 Adverse reactions with cannabis
- 2.1 Adverse reaction cases associated with cannabis as a substance
- 2.2 Adverse reaction cases associated with legal cannabis products for medical or non-medical purposes
- 2.3 Demographics
- 2.4 Suspected cannabis products
- Sex and Gender Based Analysis Plus (SGBA plus) of 2020 Adverse Reaction Data
- 3.0 Clinical evaluation of serious and medically important cases
- 3.1 Summary of serious and medically important adverse reactions
- 3.2 Important identified risks during the reporting period
- 3.3 Important potential risks during the reporting period
- 3.4 Missing information during the reporting period
- 4.0 Note to readers
- 5.0 Reporting an adverse reaction involving a cannabis product
- 6.0 Contact us
Key highlights
- This is the second annual report pertaining to adverse reaction reports associated with cannabis that are submitted to the Canada Vigilance Database and monitored and analysed by the Controlled Substances and Cannabis Branch within Health Canada.
- The purpose of this report is to provide an overview of the adverse reaction reports collected by Health Canada during the second year since the coming into force of the Cannabis Act and its Regulations, covering the reporting period of January 1, 2020 to December 31, 2020, with comparisons to the first year of legalization (October 17, 2018 to December 31, 2019).
- Between January 1, 2020 to December 31, 2020, 287 adverse reaction reports were reported to Health Canada's Canada Vigilance Database associated with cannabis as a substance (including cases of polysubstance use).
- Of the 159 adverse reaction cases associated with legal cannabis products in 2020, 121 were reported to Health Canada as serious with 'other' (medically important condition) as the most frequently reported reason for seriousness.
- The majority of 2020 cases involving legal cannabis products originated from consumers (88%) and were submitted to Health Canada by cannabis licence holders (86%); 11% were reported to Health Canada by health care practitioners.
- In 2020, cases involving legal cannabis products often involved adults aged 65-74 years (22%) and females (57%).
- In 2020, most cases involved legal cannabis products for medical purposes (77%) and frequently involved ingestible liquid extracts (75%) (for example, ingestible oils and softgels).
- In 2020, the most frequently reported medical events across all cases involving legal cannabis products were hallucination (n=52); dizziness (n=13); nausea (n=12); euphoric mood (n=10); feeling abnormal (n=10); and insomnia (n=10).
- Certain medical events were more frequently reported with THC-dominant or THC-leaning legal cannabis products, whereas others were more frequently reported with CBD-dominant or CBD-leaning legal cannabis products.
- Some findings have changed while others have remained consistent between the first and second years following the coming into force of the Cannabis Act. However, conclusions are only preliminary at this time and further years of data are required to conduct proper trend analysis and to assess any long-term changes in findings.
- In 2020, there was one suspected case of vaping-associated lung illness (VALI) that was reported as involving legal cannabis products and two suspected cases that were reported as involving undefined cannabis. However, no cases were established to be a confirmed or probable case according to the case definition established by the Public Health Agency of Canada.
- Health Canada will continue to monitor, assess and report on adverse reactions associated with cannabis, and findings will be used to inform evidence-based information on health and safety risks with cannabis, including risk communications and educational resources.
1.0 Introduction
This report describes the findings of the domestic case reports of adverse reactions associated with cannabis submitted to Health Canada's Canada Vigilance Database and analyzed by the Office of Cannabis Science and Surveillance of the Controlled Substances and Cannabis Branch (CSCB). This work forms part of the Vigilance Framework for Cannabis that has been in place since the coming into force of the Cannabis Act and Cannabis Regulations (October 17, 2018).
This second annual report presents a summary of all domestic case reports of adverse reactions reported to Health Canada between January 1, 2020 and December 31, 2020, suspected of being associated with a cannabis product as defined under the Cannabis Act or its Regulations, intended for human consumption:
A cannabis product means cannabis of one of the classes set out in Schedule 4 to the Act — or a cannabis accessory that contains such cannabis — after it has been packaged and labelled for sale to a consumer at the retail level. It does not include:
- cannabis that is intended for an animal
- a cannabis accessory that contains cannabis that is intended for an animal
- health products containing cannabis or for use with cannabis
As outlined in Section 248 of the Cannabis Regulations, licence holders (LHs) who sell or distribute a cannabis product must within 15 days after becoming aware of a serious adverse reaction to the cannabis product, provide Health Canada with a detailed report containing all information in their possession that is associated with the use of the cannabis product by the individual who experienced the reaction:
- An adverse reaction is defined as "a noxious and unintended response to a cannabis product"
- A serious adverse reaction is defined as "a noxious and unintended response to a cannabis product that requires inpatient hospitalization or a prolongation of existing hospitalization, causes congenital malformation, results in persistent or significant disability or incapacity, is life-threatening or results in death"
Consumers, patients, health care practitioners (HCPs), medical cannabis clinics and other reporters such as provincial and territorial authorized retailers submit adverse reaction reports with cannabis products on a voluntary basis to Health Canada. Reports may also be received from market authorization holders of licenced health products that submit adverse reaction reports for other suspect health products (such as prescription or non-prescription drugs, or natural health products) in which cannabis is also identified as co-suspect.
Adverse reactions with cannabis may involve cannabis that is: (i) a cannabis product produced by a federal LH; (ii) cultivated in the home Footnote 1; (iii) undefined (cannabis as a substance not otherwise specified); or (iv) from illegal sources.
For the purposes of this report, case reports involving a legal cannabis product (that is, identifiable by product brand name and/or LH) are classified according to the intended use of the cannabis product(s) as described in the case report:
- Cannabis use for medical purposes includes case reports described as having a medical authorization document; or, a reported medical or therapeutic purpose, without mention of a medical authorization document. This definition aligns with the definition of medical use within the Canadian Cannabis Survey. This is broader in scope than the regulatory definition of cannabis for medical purposes under the Cannabis Regulations; that is, a medical document from a HCP (physician, nurse practitioner) authorizing the use of cannabis for medical purposes.
- Cannabis use for non-medical purposes: If there is no reported medical or therapeutic indication or reason for use provided in the case report, minimal details or the intended use is for non-medical purposes, the case report is classified as 'non-medical use of cannabis'.
Adverse reaction case reports are collected and housed in the Canada Vigilance Database. The majority of adverse reaction reports submitted to Health Canada are reported spontaneously (report type=spontaneous) from consumers, patients, HCPs or from LHs (referred to as market authorization holder in the Canada Vigilance Database). However, reports may also originate from studies (report type= study), including real-world observational studies, human studies involving cannabis that fall outside of the definition of a clinical trial, or other organized data collection systems (for example, surveys of patients or HCPs).
Health Canada conducts near-time monitoring, detection, assessment and associated activities for cases of adverse reactions involving cannabis products as part of the Vigilance Framework for Cannabis. Health Canada also monitors cases involving cannabis as a substance for broader issues of public health importance such as vaping-associated lung illness (VALI), cases involving pediatric populations, and other potential emerging safety issues.
The purpose of this report is to provide a brief summary of adverse reaction reports involving cannabis, as well as a descriptive analysis of domestic adverse reaction data associated with cannabis products regulated under the Cannabis Act and the Cannabis Regulations, submitted to Health Canada between January 1, 2020 and December 31, 2020. This reporting period is reflective of the second full year of legalization and regulation of cannabis for non-medical purposes for adults in Canada, and the first year after the implementation of amendments to the Cannabis Regulations that authorized the legal sale of new classes of cannabis products (namely cannabis extracts [other than ingestible cannabis oils], edible cannabis and topicals).
In addition to the descriptive analysis of cases from the 2020 reporting period, inter-report comparisons to the previous reporting period (October 17, 2018 to December 31, 2019) have been made, where appropriate. These comparisons are largely descriptive and differences have not been tested for significance in most cases due to the limited nature of the data. Therefore, caution should be taken with the interpretation of these changes in results over the two reporting periods.
This report does not cover adverse reaction data associated with health products, including drugs containing cannabis, which are regulated under the Food and Drugs Act and its Regulations. A summary of adverse reactions associated with other health products received by Canada Vigilance in 2020 are described in Health Canada's InfoWatch Newsletter: Adverse reactions to health products - annual report 2020.
1.1 Considerations
Certain caveats should be considered when interpreting the adverse reaction data in this report:
- Adverse reactions are generally spontaneously reported to Health Canada and cannot be used to determine the incidence or prevalence of adverse reactions to cannabis in the general population.
- Serious adverse reactions have a greater representation in this dataset as LHs have a regulatory obligation to report these to Health Canada under the Cannabis Regulations. The submission of non-serious adverse reactions by LHs to Health Canada as individual case reports is voluntary Footnote 2; therefore, these cases are likely underreported and underrepresented in this dataset.
- Reporting of adverse reactions by consumers, HCPs, hospitals, medical cannabis clinics and retailers is voluntary for cannabis products. Therefore, both serious and non-serious cases from these sources are likely underreported.
- Individuals experiencing serious outcomes or using cannabis products for medical purposes may be more motivated to report or seek out medical attention.
- Several factors may influence the number of cases reported to Health Canada such as: consumer/patient medical history; reason for cannabis use; length of time a product is on the market; media coverage; awareness, motivation and ability to report; and nature of reports (for example, spontaneous reports versus studies or other organized data-collection systems).
- This report includes information on cannabis for medical and non-medical purposes; however, the number of cases elsewhere (for example, online databases, other reports) may not directly align with what is presented in this current annual summary report due to different dates of extraction from the Canada Vigilance Database.
2.0 Adverse reactions with cannabis
2.1 Adverse reaction cases associated with cannabis as a substance
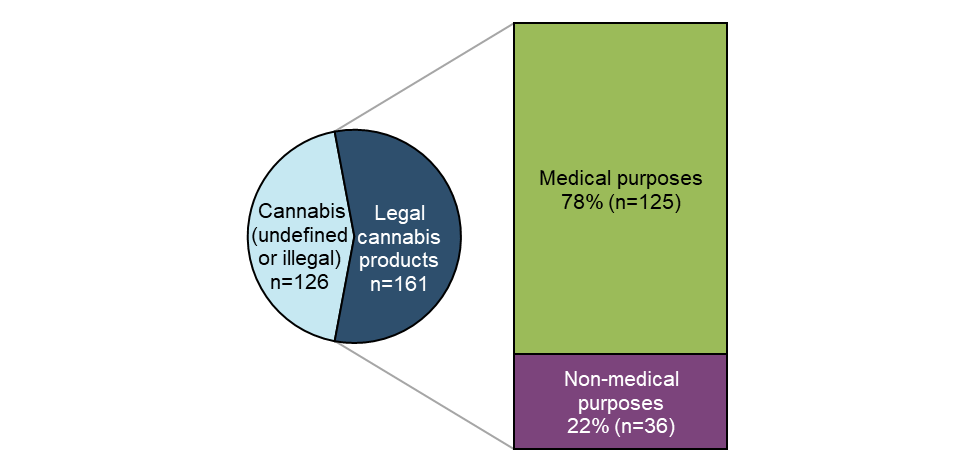
Figure 1 - Text description
| Total number of reports | Proportion (%) of all reports | |
|---|---|---|
| Undefined or illegal cannabis | 126 | 44 |
| Legal cannabis product - Medical purposes | 125 | 44 |
| Legal cannabis product - Non-medical purposes | 36 | 13 |
| Grand total | 287 | 100 |
Caveat(s):
- Reports are classified for internal reporting purposes within Canada Vigilance depending on whether they report using legal cannabis products used for either for medical or non-medical purposes (one classification per case); cases that do not involve cannabis products from legal sources are not assigned a classification and remain classified as cannabis as a substance only (undefined or illegal cannabis).
- This figure represents all reports submitted to Canada Vigilance Database, including duplicates where the case details are the same, but reporters differ (n=17).
A total of 287 reports associated with cannabis as a suspect substance were reported to Health Canada between January 1, 2020 and December 31, 2020. More than half of these reports involved legal cannabis products (56%, n=161), of which most were reported as being used for medical purposes (78%, n=125). Of the cases involving illegal or undefined cannabis (n=126) (for example, reports involving 'cannabis' or 'marijuana' without specific details), 27% (n=34) involved additional co-suspect substances (that is, polysubstance use) or health products.
There were three suspected cases of VALI involving cannabis that were reported to the Canada Vigilance Database in 2020. One of these cases was suspected of involving multiple legal cannabis products while the other two cases were suspected of involving undefined or illegal cannabis. However, none of these reports met the case definition of a confirmed or probable case of VALI as defined by the Public Health Agency of Canada (PHAC). A full epidemiological analysis of all confirmed and probable cases of VALI reported to PHAC between September 2019 and December 2020 is now available. More information on VALI in Canada can be found on PHAC's VALI webpage.
In addition, Health Canada is aware of and has been monitoring reports of accidental ingestion of cannabis occurring in the pediatric population (under 18 years of age), which included undefined and illegal cannabis. These case reports have originated from multiple Canadian surveillance systems including the Canadian Surveillance System for Poison Information and the Canadian Paediatric Surveillance Program's study on non-medical cannabis use in children and youth. In August 2020, Health Canada issued a public advisory on the issue of accidental ingestion of edible cannabis by children. An updated public advisory was issued on April 8, 2022.
At the time of data extraction, 44 cases of adverse reactions to cannabis involving the pediatric population had been reported to the Canada Vigilance Database for 2020. None of the pediatric cases of accidental ingestion in this dataset were confirmed to have involved legal cannabis products.
Case reports involving cannabis products for self-reported medical and non-medical purposes from the legal marketplace form the basis for the remaining portions of this Annual Report.
2.2 Adverse reaction cases associated with legal cannabis products for medical or non-medical purposes
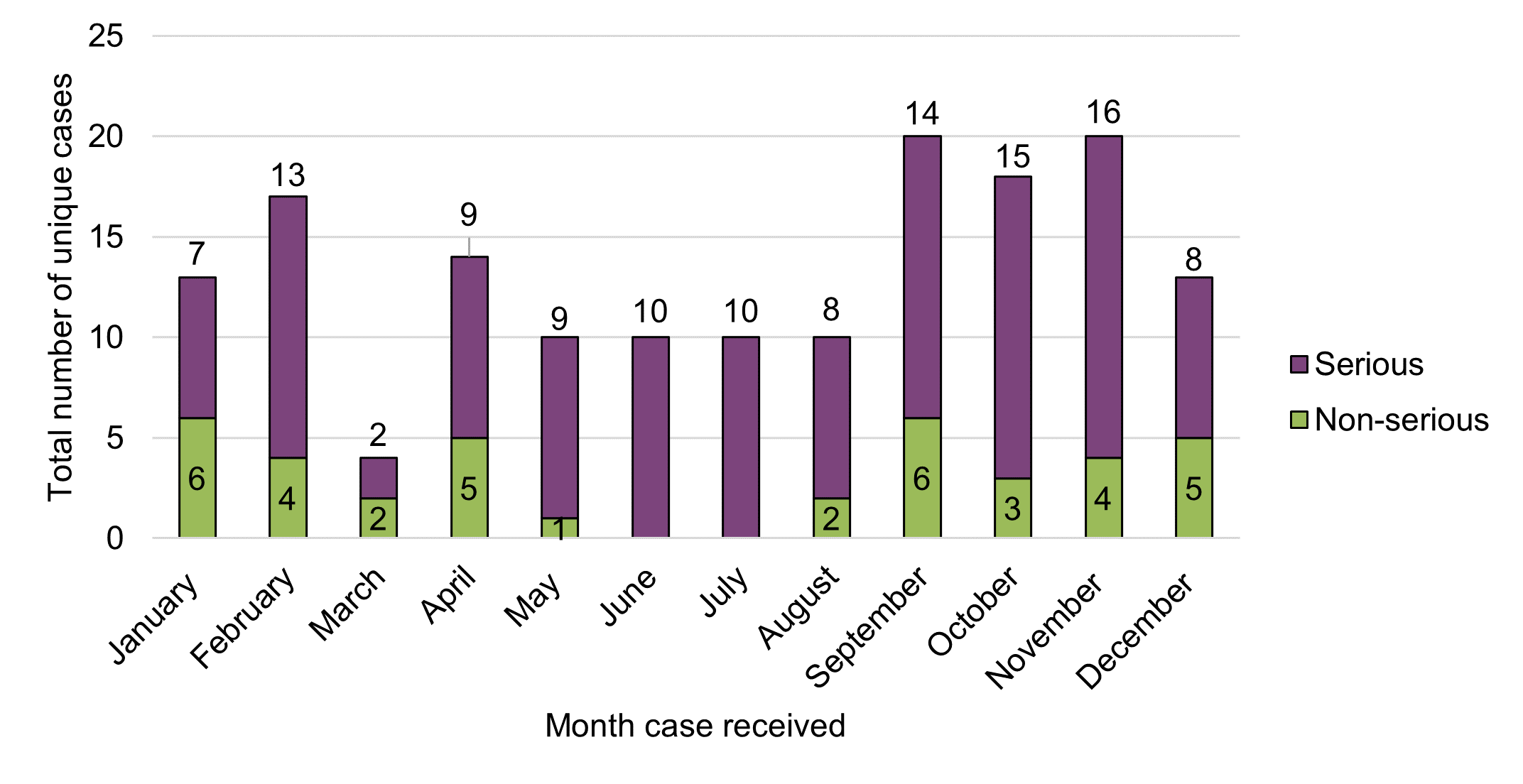
Figure 2 - Text description
| Month case received | Total number of unique cases by seriousness | ||
|---|---|---|---|
| Non-serious | Serious | Grand total | |
| January | 6 | 7 | 13 |
| February | 4 | 13 | 17 |
| March | 2 | 2 | 4 |
| April | 5 | 9 | 14 |
| May | 1 | 9 | 10 |
| June | 0 | 10 | 10 |
| July | 0 | 10 | 10 |
| August | 2 | 8 | 10 |
| September | 6 | 14 | 20 |
| October | 3 | 15 | 18 |
| November | 4 | 16 | 20 |
| December | 5 | 8 | 13 |
| Grand total | 38 | 121 | 159 |
Caveat(s):
- Cases are presented according to the initial date of receipt by the Canada Vigilance Database. The actual date of the adverse reaction may not align with the month that the case was received (lag time between event and reporting).
- Seriousness is based on the initial report and may be subject to change if additional information is submitted to Health Canada.
There were no apparent temporal trends observed in the total number of adverse reaction cases involving legal cannabis products reported to Health Canada in 2020 in relation to the number of serious cases and non-serious cases on a cumulative basis, or month-over-month. The average number of cases per month overall during the reporting period was approximately 13, ranging from four to 20 cases per month.
There were some descriptive trends observed across reporting periods. Compared to the previous reporting period (October 2018 – December 2019), the total number of cases overall and the average number of cases per month, increased in 2020 (total cases: from 151 to 159; average cases per month: from 10 to 13) despite the previous reporting period having covered a longer reporting period (14.5 months vs. 12 months). Similarly, the total number of serious cases reported to Health Canada increased by 57% between the reporting periods, from 77 cases in 2018-2019 to 121 cases in 2020. In contrast, the total number of non-serious adverse reaction reports decreased by 49%, from 74 in 2018-2019 to 38 in 2020.
As noted below, the increase in the number of serious cases may be explained by an increase in the number of medically important cases. Licence holders may submit medically important cases as serious reports ("other medically important condition") as encouraged in Health Canada's Cannabis adverse reaction reporting guide for licence holders and consistent with the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) guidelines for expedited reporting (E2A), of which Health Canada is a member.
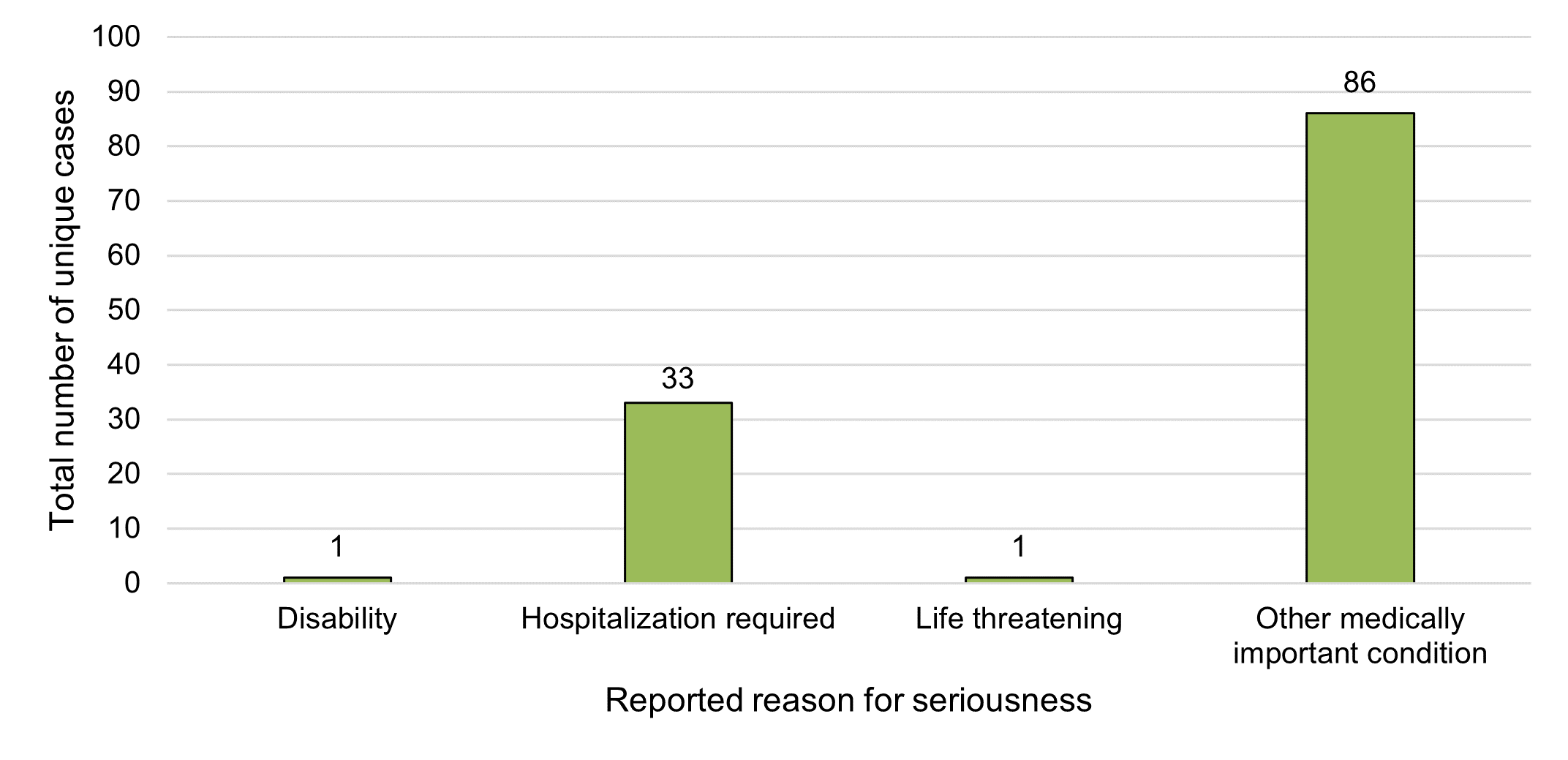
Figure 3 - Text description
| Reported reason for seriousness | Total number of unique cases |
|---|---|
| Disability | 1 |
| Hospitalisation required | 33 |
| Life threatening | 1 |
| Other medically important condition | 86 |
| Grand total | 121 |
Caveat(s):
- Each serious case may have more than one reason for seriousness as the reporter can select multiple reasons.
In 2020, each serious case reported to Health Canada involved just one response for seriousness, for a total of 121 responses for seriousness, spanning four categories, selected by reporters (Figure 3). The most frequently selected category of reason for seriousness among 2020 cases was "other medically important condition" (71%, n=86). "Other medically important condition" includes events that are not immediately life threatening or result in death or hospitalization but may jeopardize the patient or may require intervention (for example, ambulatory services, emergency department visits, outpatient visits with a HCP or at-home medical interventions) to prevent a serious outcome. In the previous reporting period, 35% (n=27) of cases were reported as "other medically important condition". The most common reported reason for seriousness in the previous reporting period was "hospitalization required" (55%, n=43), which represented 27% (n=33) of serious cases in 2020.
Similar to the previous reporting period, there were no fatalities reported among cases involving legal cannabis products used for reported medical or non-medical purposes in 2020. One case was reported as 'life threatening' (a decrease from seven cases during the previous reporting period) and one case reported 'disability' (the same as the previous year).
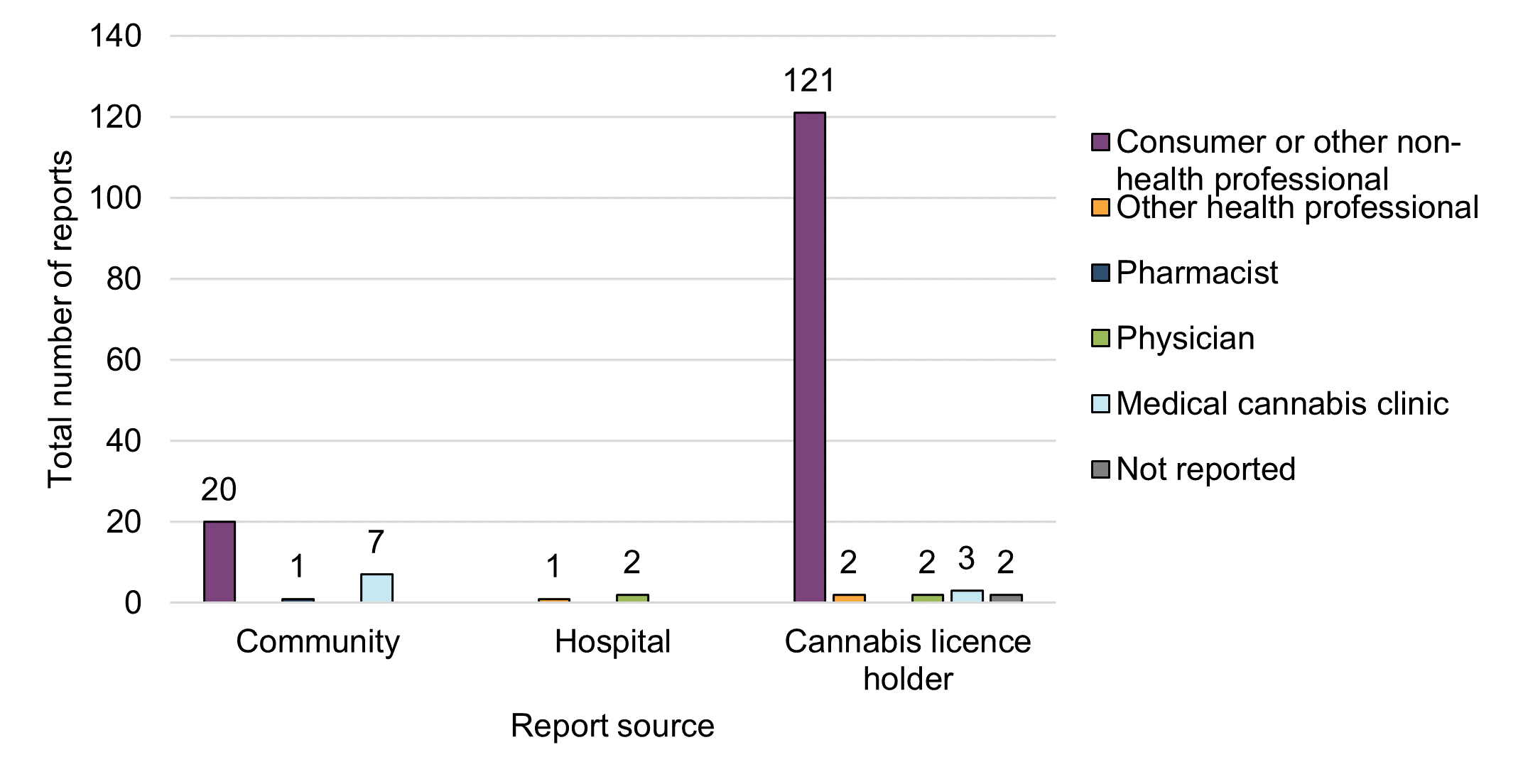
Figure 4 - Text description
| Report source | Initial reporter type | ||||||
|---|---|---|---|---|---|---|---|
| Consumer or other non-health professional | Other health professional | Pharmacist | Physician | Medical cannabis clinic | Not reported | Grand total | |
| Community | 20 | 0 | 1 | 0 | 7 | 0 | 28 |
| Hospital | 0 | 1 | 0 | 2 | 0 | 0 | 3 |
| Cannabis licence holder | 121 | 2 | 0 | 2 | 3 | 2 | 130 |
| Grand total | 141 | 3 | 1 | 4 | 10 | 2 | 161 |
Caveat(s):
- This figure includes duplicates where the case details are the same, but reporters differ (n=2).
- Report source is reflective of where the report was submitted from to Health Canada (community, hospital, or cannabis licence holder [referred to as "market authorization holder" in the Canada Vigilance Database]).
- For purposes of this figure, each report source is further subdivided according to initial reporter type (consumer, HCPs [physician, pharmacist, other], medical cannabis clinic).
- Reports from HCPs are considered 'medically confirmed' as per international guidelines Footnote 3; therefore, are distinct from consumer reports.
- Medical cannabis clinic reports are considered 'medically confirmed' as they fall under the scope of a medical practice and medical oversight by clinics who have patients using cannabis for medical purposes.
The majority of the adverse reaction reports involving legal cannabis products used for reported medical or non-medical purposes were submitted to Health Canada by cannabis LHs (81%, n=130), followed by voluntary community reporters (17%, n=28).
Most adverse reaction reports that were submitted to Health Canada by LHs and others from the community arose from two main initial reporter types: consumers (88%, n=141) and HCPs (11%, n=18). The majority of the HCP reports came from medical cannabis clinics (n=10). These descriptive reporting trends are consistent with observations from the previous reporting period in that consumers appear to be more inclined to report adverse reactions directly to the LH who then submit to Health Canada as per their reporting obligations. Health care practitioners tend to report on behalf of their patients directly to Health Canada. The number of reports of adverse reactions that identify legal cannabis products from hospitals remained limited in 2020 (2%, n=3).
Under the Protecting Canadians from Unsafe Drugs Act, as of December 16, 2019, hospitals are required to submit all serious adverse reactions to Health Canada involving suspect drugs (including drugs containing cannabis) with or without other suspect products. Reports involving a co-suspect drug and co-suspect cannabis are required to be submitted to Health Canada. However, under the Cannabis Act and its Regulations, hospitals are voluntary reporters and submit adverse reactions to Health Canada involving only cannabis as a sole suspect product on a voluntary basis.
2.3 Demographics
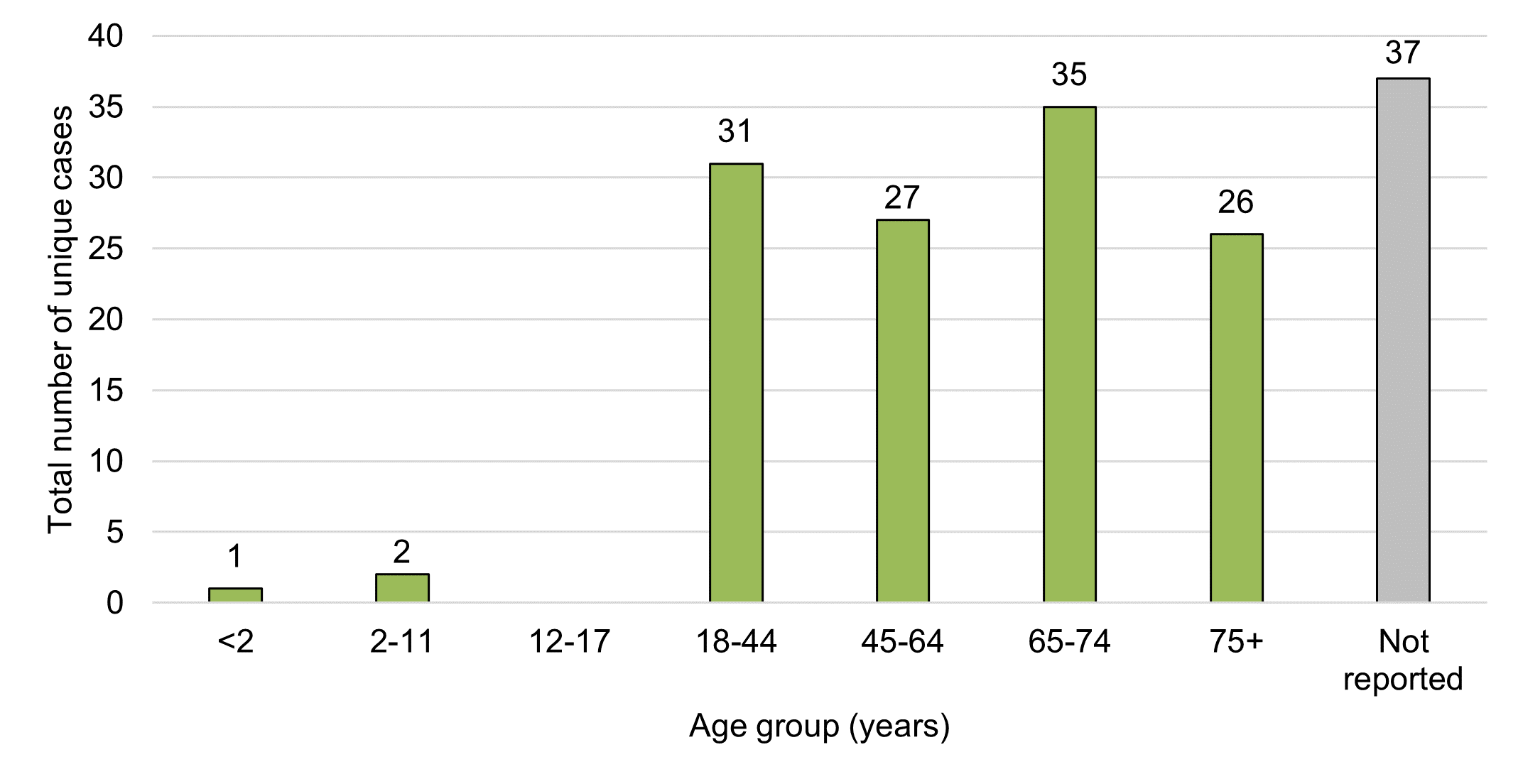
Figure 5 - Text description
| Age group (years) | Total number of unique cases |
|---|---|
| <2 | 1 |
| 02-11 | 2 |
| 12-17 | 0 |
| 18-44 | 31 |
| 45-64 | 27 |
| 65-74 | 35 |
| 75+ | 26 |
| Not reported | 37 |
| Grand total | 159 |
Caveat(s):
- In cases where the year of birth and the date of reaction are listed in the report, then the age is calculated.
- In cases where the year of birth is listed without a date of reaction, the date the report was submitted is used to calculate the age.
The average age in cases involving adverse reactions to legal cannabis products and reported to Health Canada in 2020 was approximately 59 years (95% CI: 56-63) with a range of five months to 91 years. This did not differ significantly from the previous reporting period where the average age was 56 years (95% CI: 52-59) with a range of four years to 93 years.
When classified using the World Health Organization's Vigilyze database (Vigibase) age groupings, most cases involved persons aged 65-74 years (22%, n=35). This differs from the previous reporting period where 18-44 years was the most common age group (25%, n=37). Adverse reaction cases in 2020 equally involved adults aged <65 years and those aged ≥65 years (38% or n=61 each). Almost a quarter of 2020 cases lacked age-related information (23%, n=37), similar to the previous reporting period (22%, n=33).
These data reflect a shift from the previous reporting period where persons aged <65 years represented 50% (n=75) of all 2018-2019 cases as compared to those aged ≥65 years, which represented 28% (n=43) of adverse reaction cases in 2018-2019. In general, there was a more even distribution of cases across these age groupings in 2020 compared to the previous reporting period. In both reporting periods only a small number of pediatric cases (that is, individuals <18 years of age) were reported to Health Canada involving legal cannabis products for medical purposes (n=2 in 2018-2019; n=3 in 2020).
Despite an even split of total adverse reaction cases across younger and older age groups in 2020, serious cases were slightly more represented in older adults (≥65 years). Of the serious cases (n=121) reported to Health Canada in 2020, 44% (n=53) occurred in older adults, and 40% (n=48) occurred in adults <65 years. The age group '≥65 years' represents aging adults in which polypharmacy and/or pre-existing health conditions or comorbidities are more common, which may also increase the risk of adverse reactions.
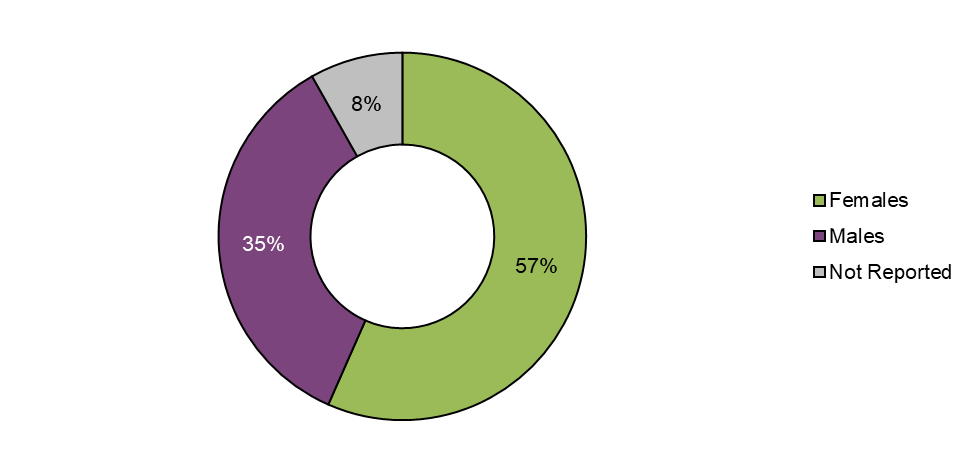
Figure 6 - Text description
| Sex | Total number of unique cases | Proportion (%) of total |
|---|---|---|
| Females | 90 | 57 |
| Males | 56 | 35 |
| Not reported | 13 | 8 |
| Grand total | 159 | 100 |
More than half of the adverse reaction cases reported to Health Canada in 2020 involved females (57%, n=90), while about a third involved males (35%, n=56) and 8% (n=13) were missing sex. The distribution of cases by sex was similar to the previous reporting period (females: 55%; males: 39%). The number of cases missing a sex classification (that is, not reported) increased from 6% in 2018-2019 to 8% in 2020.
Sex (and age) may be missing due to several reasons, including consumers not wanting to disclose this information to LHs or Health Canada and/or cases originating from other reporting forms such as the Cannabis Reporting Form, which do not capture this type of demographic information.
2.4 Suspected cannabis products
The majority of adverse reaction cases reported to Health Canada in 2020 associated with legal cannabis products identified cannabis as a sole suspect product (94%, n=150), meaning that no other products were reported as co-suspects. Products may include prescription or non-prescription drugs, natural health products or other types of health products regulated under the Food and Drugs Act. However, the majority of cases (96%, n=153) included at least one concomitant product (that is, a product that is used at the time of the adverse reaction but not considered suspect). Concomitant products may be prescription or non-prescription drugs, vaccines, biologics, medical devices, natural health products, other regulated substances (for example, alcohol, tobacco, other vaping products (nicotine or flavours)) and / or illegal substances.
A small proportion of cases 17%, (n= 27) reported to Health Canada in 2020 had two or more suspect cannabis products reported, with a range of two to four cannabis products. Comparatively, the previous reporting period had 25 case reports with two or more suspect cannabis products, ranging from two to seven cannabis products. Of note, all suspect products are based on the suspicion of the reporter and the involvement of other products, substances or factors cannot always be ruled out. These considerations form part of the clinical evaluation in the clinical summary portion of this report.
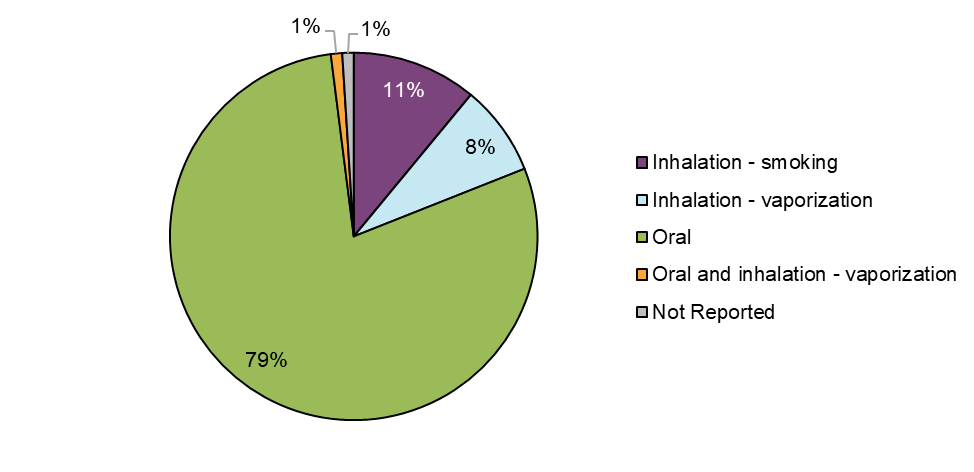
Figure 7 - Text description
| Route of administration | Total number of unique cases | Proportion (%) of total |
|---|---|---|
| Inhalation - smoking | 18 | 11 |
| Inhalation - vaporization | 13 | 8 |
| Oral | 125 | 79 |
| Oral and inhalation - vaporization | 1 | 1 |
| Not Reported | 2 | 1 |
| Grand total | 159 | 100 |
Caveat(s):
- This figure describes route of administration of suspect cannabis products in cases, which is coded separately from dosage form (that is, a cannabis oil product may be ingested, applied topically, etc.).
- Oral administration refers to consuming cannabis by mouth and may involve ingestion, buccal administration, sublingual administration, etc. meaning that absorption may occur at the level of the gastrointestinal tract as well directly into the bloodstream through oral mucosal tissue.
- Inhalation refers to consuming cannabis through the respiratory tract and may involve smoking or vaporization, among others.
- Cases may involve more than one suspect cannabis product; therefore, multiple routes of administration may appear for a single case.
Suspect cannabis products associated with adverse reaction cases were most frequently consumed via the oral route as cannabis extracts (79%, n=125) followed by inhalation (19%, n=31), of which 11% involved smoking dried cannabis (n=18) and 8% (n=13) involved vaporization. Most of these included inhalation of vaporized cannabis extracts (85%, n=11), followed by inhalation of vaporized dried cannabis (15%, n=2). One case involved both oral and inhalation routes of administration. Comparisons between the two reporting periods are limited as cannabis extracts (excluding cannabis oils for ingestion) were not legally available in the marketplace in the previous reporting period.
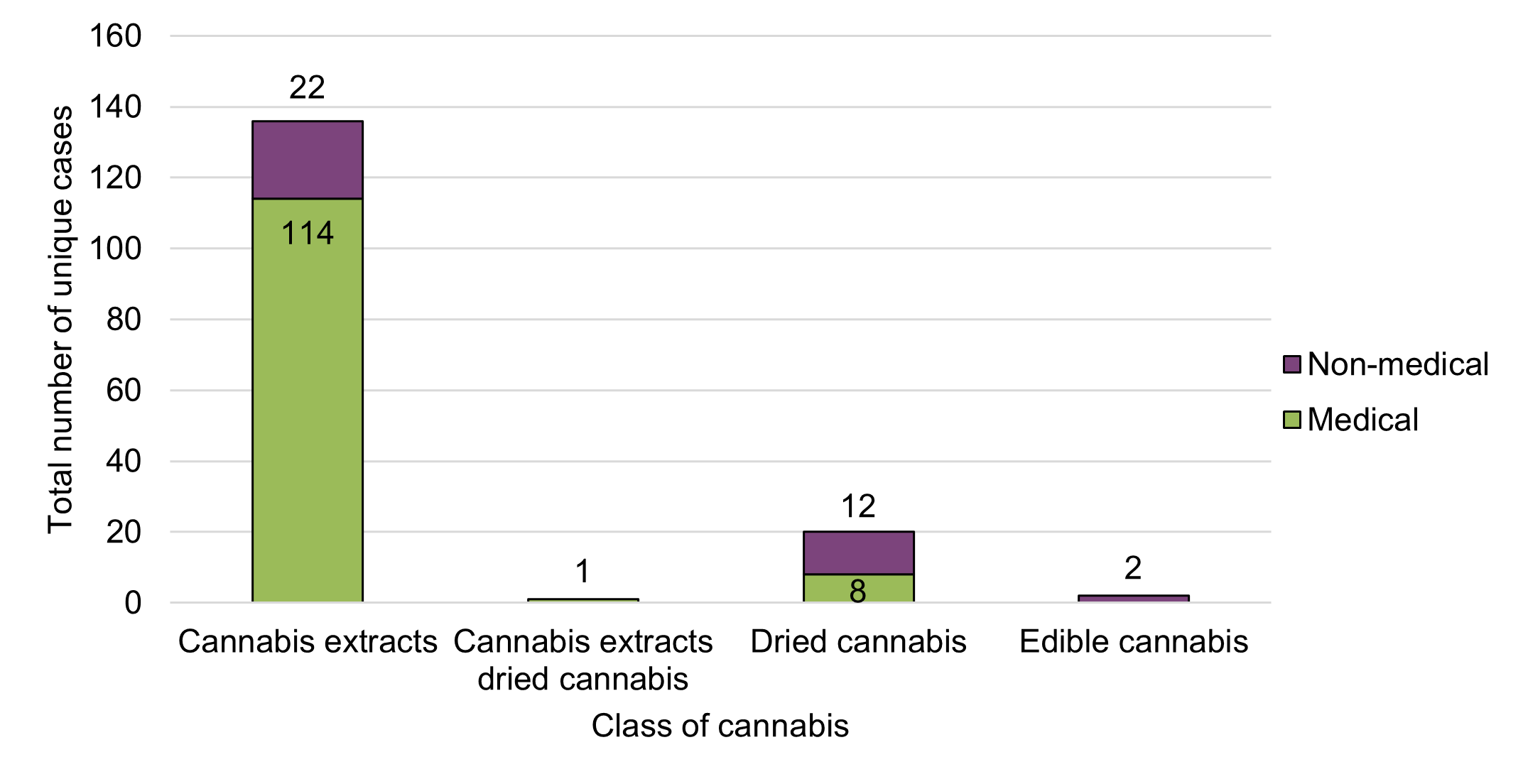
Figure 8 - Text description
| Cannabis product class | Reason for use | ||
|---|---|---|---|
| Medical | Non-medical | Grand total | |
| Cannabis extracts | 114 | 22 | 136 |
| Cannabis extracts and dried cannabis | 1 | 0 | 1 |
| Dried cannabis | 8 | 12 | 20 |
| Edible cannabis | 0 | 2 | 2 |
| Grand total | 123 | 36 | 159 |
The majority of adverse reaction cases reported to Health Canada in 2020 were associated with cannabis products used for medical purposes (77%, n=123), relative to non-medical purposes (23%, n=36). The majority of the cases involving cannabis products for medical purposes were serious (86%, n=106). In contrast, the majority of cases involving cannabis products for non-medical purposes were non-serious (58%, n=21).
Cases were associated with three classes of legal cannabis products: cannabis extracts (n=136), dried cannabis (n=20) and edible cannabis (n=2) (Figure 8). No cases in 2020 were reported as being associated with cannabis topicals or fresh cannabis. The majority of cases involving cannabis extract products were reported as being used for medical purposes (84%, n=114). Conversely, 60% (n=12) of cases involving dried cannabis were reported as being used for non-medical purposes. Cannabis extracts were associated with 91% (n=117) of serious and 68% (n=25) of non-serious cases in 2020. These descriptive observations are consistent with observations from the previous reporting period despite the expansion and availability of other legal cannabis products as of late 2019 (edible cannabis products, cannabis extracts [other than oils] and cannabis topicals).
Of note, some consumers or patients may preferentially select ingestible cannabis oil products for one or more reasons such as: to avoid inhaling cannabis; for the ability to draw or titrate a specific measured dose; for duration of effect; for cannabinoid concentration or other reason(s). However, cannabis products consumed via the oral route (ingested) are subject to first-pass metabolism in the liver and have a longer time to onset and duration of effect, which may increase the risk of possible interactions between cannabis and health products or other substances if used concomitantly, thereby contributing to an increased risk of experiencing adverse reactions.
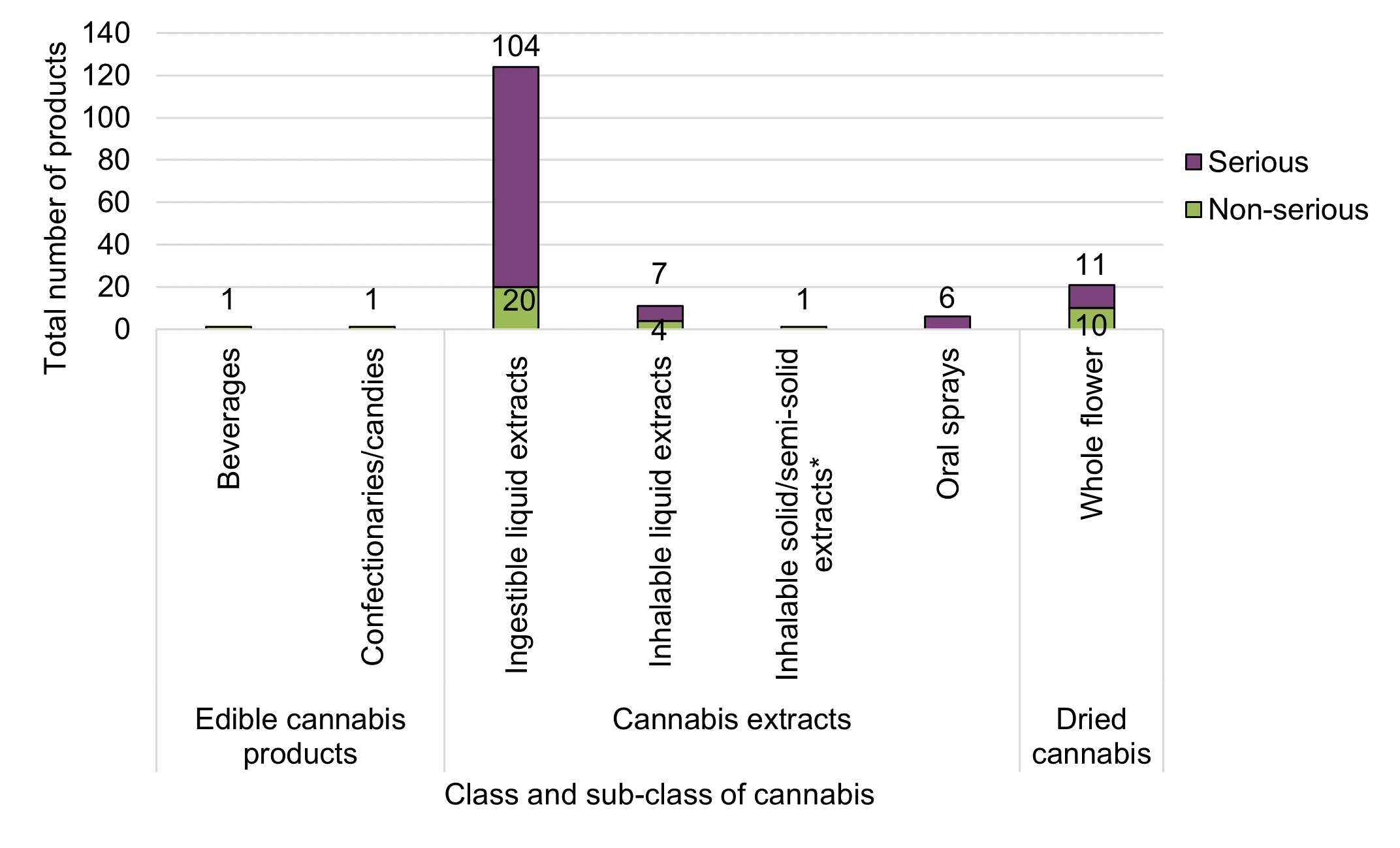
* Includes chemical extracts created through the use of solvents and physical extracts created through the use of pressure or cold/ice.
Figure 9 - Text description
| Cannabis product class | Cannabis product subclass | Total number of products by seriousness | ||
|---|---|---|---|---|
| Non-serious | Serious | Grand total | ||
| Cannabis edibles | Beverages | 1 | 0 | 1 |
| Confectionaries/candies (e.g., mints, chocolates) | 1 | 0 | 1 | |
| Cannabis extracts | Ingestible liquid extracts (e.g., bottled oils, capsules, tinctures) | 20 | 104 | 124 |
| Inhalable liquid extracts (e.g., vaping liquids) | 4 | 7 | 11 | |
| Inhalable solid/semi-solid extracts (e.g., rosin, honey, budder, shatter, hash)Footnote * | 1 | 0 | 1 | |
| Oral sprays | 0 | 6 | 6 | |
| Dried cannabis | Whole flower | 10 | 11 | 21 |
| Grand total | 37 | 128 | 165 | |
|
||||
Caveat(s):
- This figure was developed by Health Canada through manual categorization of cases according to their sub-class of suspect cannabis product(s).
- Cases may have multiple suspected cannabis products reported across different sub-classes of cannabis. For this reason, the total number reflected in the table according to count of suspect products by sub-classes and seriousness may exceed the total number of unique cases.
- Cannabis extracts as a class involves a diverse group of product forms including oral liquids/drops, softgels, capsules/tablets, sublingual sprays, dissolvable strips or highly concentrated extracts like shatter, wax, rosin, resin or vaping liquids. Dried cannabis includes whole dried flower, milled flower, and pre-rolls. Edibles includes food-like formats (chocolate, confectionary, mints) and beverages.
- Inhalable solid or semi-solid extracts can include extracts produced via chemical or physical methods.
- Oral sprays (ingestible) are noted separately from ingestible liquid extracts based to their intended sublingual/oro-mucosal use (that is inside the cheek) that may absorb directly into the bloodstream.
As noted above, cannabis extracts were the most frequently reported class of cannabis products among adverse reaction cases. Within the category of cannabis extracts, ingestible oils in liquid form (bottled oils with dropper) and in softgel capsules were most frequently involved, representing 65% (n=88) and 26% (n=36) of adverse reaction cases involving cannabis extracts. This compares to inhalable liquid extracts (vaping liquids [8%, n=11] or inhalable solid/semi-solid extracts (rosin [<1%, n=1]). Generally, cases involving liquid cannabis extracts consumed orally were 'CBD-dominant' or 'CBD-leaning' and cases involving dried cannabis products were typically 'THC-dominant'Footnote 4.
Consistent with the previous reporting period, cases involving older adults (≥65 years) exclusively reported use of cannabis extracts, whereas cases involving younger adults reported use of both dried cannabis and cannabis extracts. When broken down by sub-product type, only young to middle aged adult groups (18-64 years) were involved in adverse reaction cases with vaping liquids, while older adults (≥65 years) were more frequently involved in adverse reaction cases with ingestible oils in liquid form and softgel capsules.
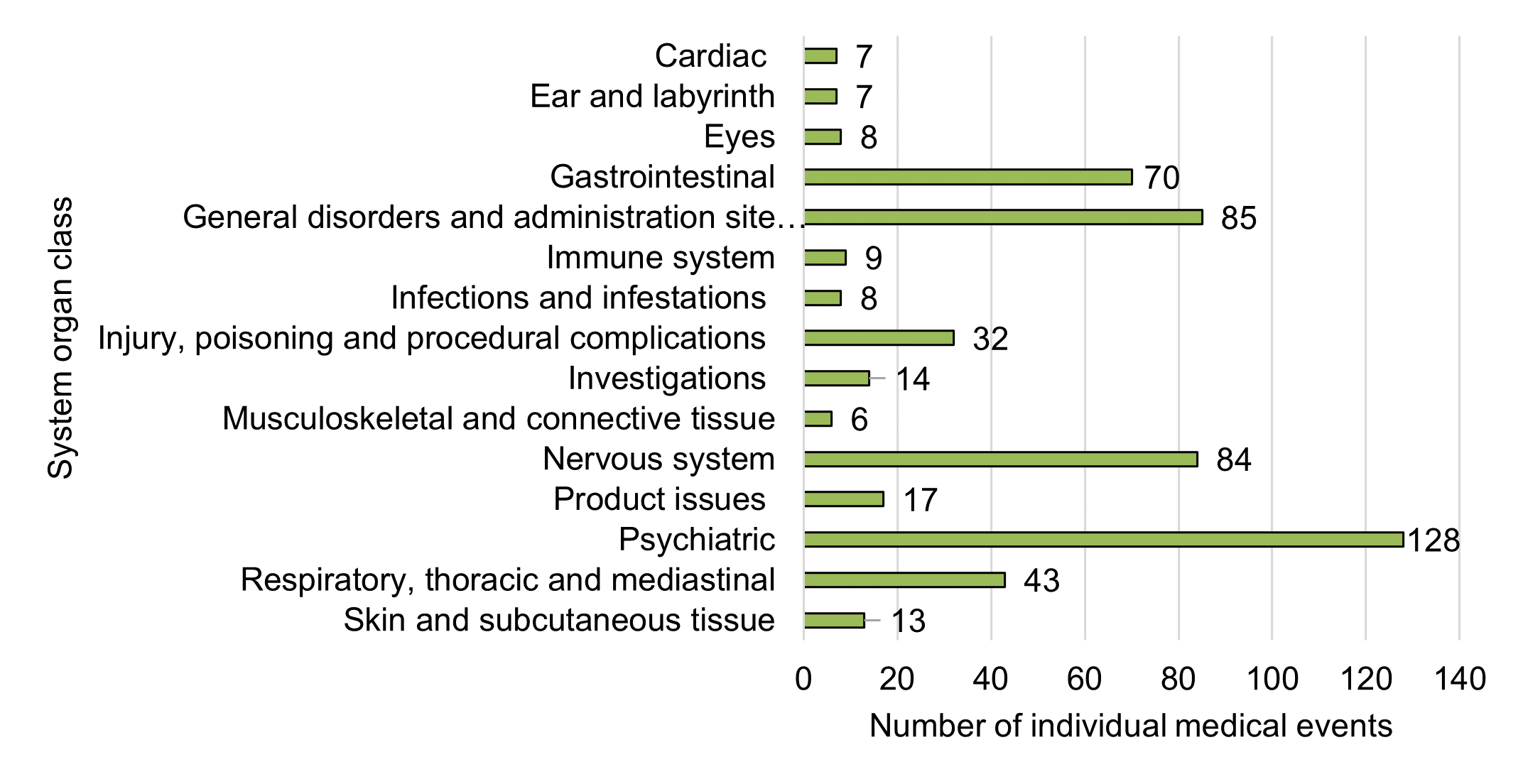
Figure 10 - Text description
| System organ class | Number of individual medical events |
|---|---|
| Cardiac disorders | 7 |
| Ear and labyrinth disorders | 7 |
| Eye disorders | 8 |
| Gastrointestinal disorders | 70 |
| General disorders and administration site conditions | 85 |
| Immune system disorders | 9 |
| Infections and infestations | 8 |
| Injury, poisoning and procedural complications | 32 |
| Investigations | 14 |
| Musculoskeletal and connective tissue disorders | 6 |
| Nervous system disorders | 84 |
| Product issues | 17 |
| Psychiatric disorders | 128 |
| Respiratory, thoracic and mediastinal disorders | 43 |
| Skin and subcutaneous tissue disorders | 13 |
Caveat(s):
- This figure focuses on the top 15 System Organ Classes (SOCs) reported across all adverse reaction cases.
- Each case may describe one or more individual medical event reflective of signs, symptoms, diseases, diagnoses, investigations, and procedures.
- Events are coded according to MedDRA, which provides standardized medical terminology in hierarchical groupings. The highest level grouping is the SOC.
- One adverse reaction case may be represented across multiple SOCs, and is influenced by how individual medical events (signs, symptoms, observations or diagnostics) are reported.
Overall, 557 individual medical events (representing 248 unique types of individual medical event categories) were reported across 159 cases in 2020. When grouped at the broadest grouping level (that is, SOC) the five most frequent categories were:
- Psychiatric disorders (23%, n=128)
- General disorders and administration site conditions (15%, n=85)
- Nervous system disorders (15%, n=84)
- Gastrointestinal disorders (13%, n=70)
- Respiratory, thoracic and mediastinal disorders (8%, n=43)
These top five SOCs for 2020 are consistent with the top five SOCs reported in the previous reporting period; however, nervous system disorders were previously the most frequently reported SOC, followed by psychiatric disorders.
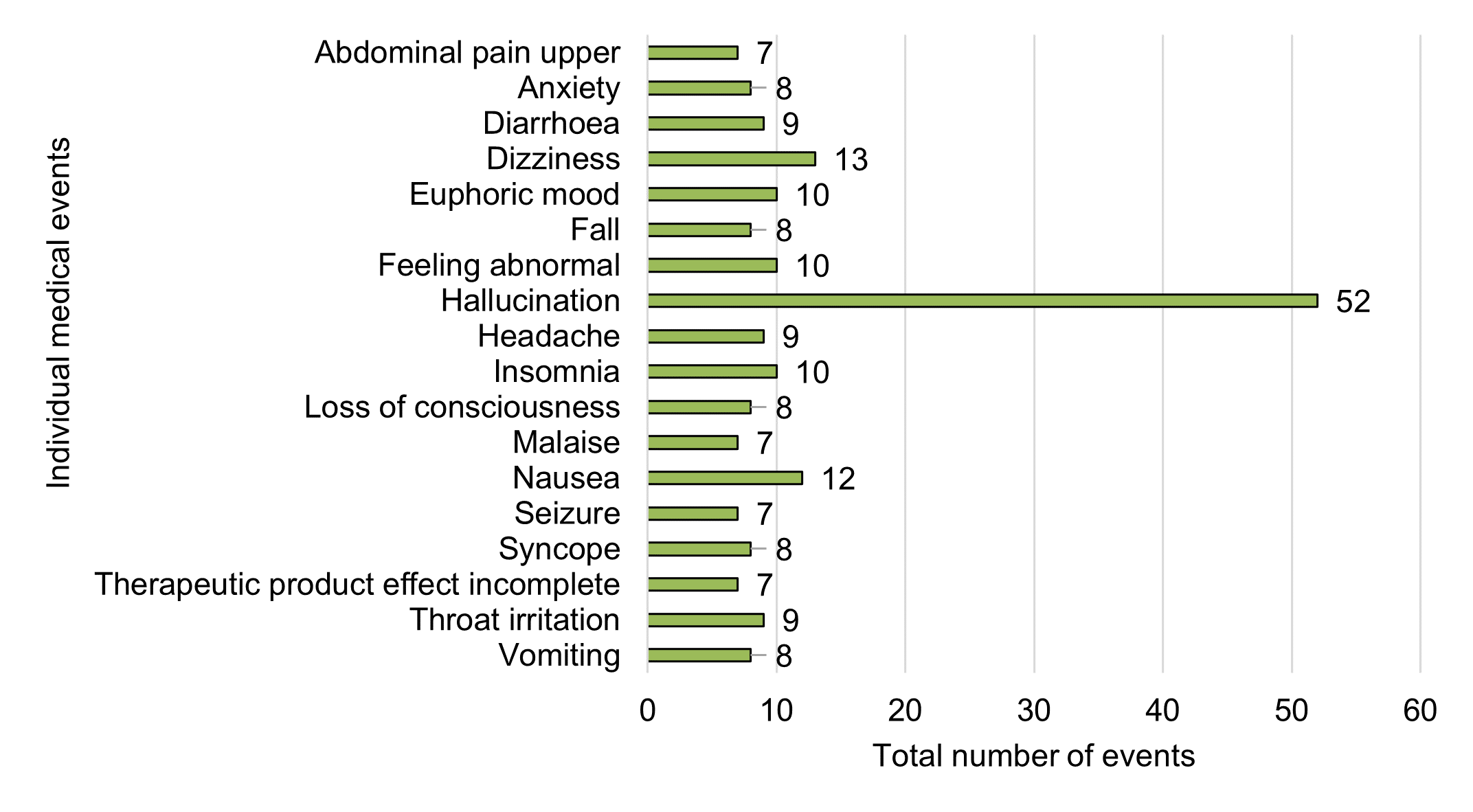
Figure 11 - Text description
| Individual medical event | Total number of events |
|---|---|
| Abdominal pain upper | 7 |
| Anxiety | 8 |
| Diarrhoea | 9 |
| Dizziness | 13 |
| Euphoric mood | 10 |
| Fall | 8 |
| Feeling abnormal | 10 |
| Hallucination | 52 |
| Headache | 9 |
| Insomnia | 10 |
| Loss of consciousness | 8 |
| Malaise | 7 |
| Nausea | 12 |
| Seizure | 7 |
| Syncope | 8 |
| Therapeutic product effect incomplete | 7 |
| Throat irritation | 9 |
| Vomiting | 8 |
Caveat(s):
- This figure focuses on the top 15 individual medical events most frequently reported across all adverse reaction cases. More than 15 events are reflected in this figure due to several events being observed an equal number of times. Other individual medical events reported less frequently do not appear in this figure.
- Individual medical events are coded using MedDRA terminology based on the verbatim described in the case report.
- Each case can have multiple individual medical events reported therefore the number of individual medical events exceeds the total number of unique cases.
- Several types of hallucination were combined to create an all-inclusive hallucination category. These included auditory hallucination, visual hallucination, mixed hallucination, hypnagogic hallucination and pseudohallucination.
As noted in Figure 11, the most frequently reported individual medical events in 2020 were:
- Hallucination (n=52)
- Dizziness (n=13)
- Nausea (n=12)
- Euphoric mood (n=10)
- Feeling abnormal (n=10)
- Insomnia (n=10)
In the previous reporting period, headache was the most frequently reported individual medical event, followed by nausea and hallucination.
Other reported events of interest included drug interactions (n=5) and suspected product issues (n=4). These two types of events have decreased since the previous reporting period (from n=8 cases each). It should be noted that it is often difficult to validate drug interaction as a medical event due to limitations in the quantity and quality of information provided. Some cases may be suggestive of drug interaction (through signs and symptoms) but may not specifically report the term, whereas others may report it but do not provide sufficient information to discern if an interaction actually occurred. Of the five cases of potential drug interaction identified in 2020, only one case was medically confirmed (reported by a HCP). Suspected product issues also often lack in detail making it difficult to confirm that a product quality issue was involved in the adverse reaction. Any suspected quality complaints are referred to CSCB's Compliance Directorate for further review.
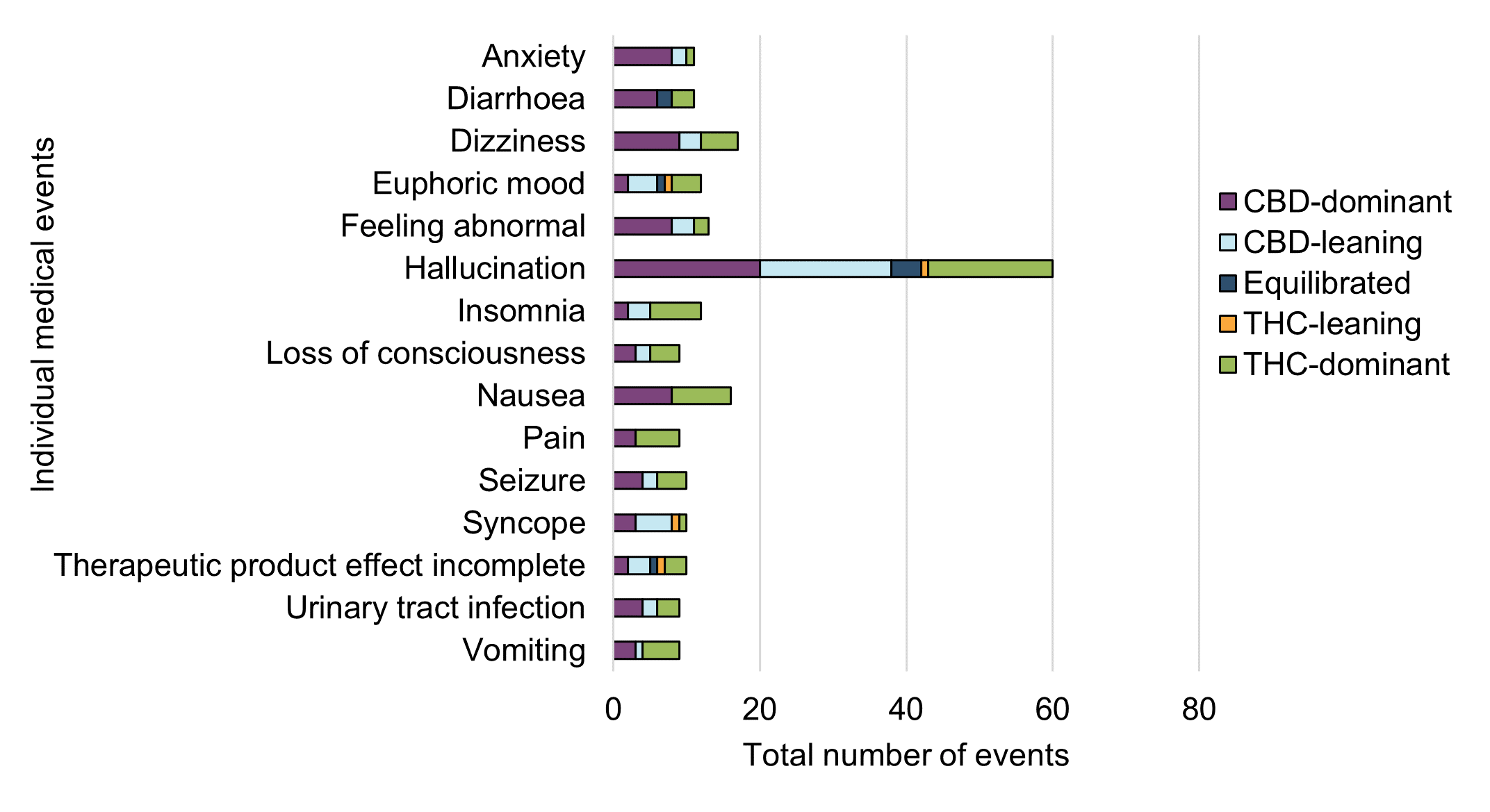
Figure 12 - Text description
| Individual medical event | Cannabinoid dominance | Grand Total (excluding unclassified cases) | ||||
|---|---|---|---|---|---|---|
| CBD-dominant | CBD-leaning | Equilibrated | THC-leaning | THC-dominant | ||
| Anxiety | 8 | 2 | 0 | 0 | 1 | 11 |
| Diarrhoea | 6 | 2 | 0 | 3 | 11 | |
| Dizziness | 9 | 3 | 0 | 0 | 5 | 17 |
| Euphoric mood | 2 | 4 | 1 | 1 | 4 | 12 |
| Feeling abnormal | 8 | 3 | 0 | 0 | 2 | 13 |
| Hallucination | 20 | 18 | 4 | 1 | 17 | 60 |
| Insomnia | 2 | 3 | 0 | 0 | 7 | 12 |
| Loss of consciousness | 3 | 2 | 0 | 0 | 4 | 9 |
| Nausea | 8 | 0 | 0 | 0 | 8 | 16 |
| Pain | 3 | 0 | 0 | 0 | 6 | 9 |
| Seizure | 4 | 2 | 0 | 0 | 4 | 10 |
| Syncope | 3 | 5 | 0 | 1 | 1 | 10 |
| Therapeutic product effect incomplete | 2 | 3 | 1 | 1 | 3 | 10 |
| Urinary tract infection | 4 | 2 | 0 | 0 | 3 | 9 |
| Vomiting | 3 | 1 | 0 | 0 | 5 | 9 |
Caveat(s):
- This figure focuses on the top 15 medical events after stratification by cannabinoid dominance. This figure excludes cases without sufficient information for assignment of cannabinoid dominance (that is, unclassified), therefore, the top 15 events in this figure may differ from those observed in Figure 11. Other individual medical events were reported during the reporting period, but do not appear in this figure.
- This figure was manually created by Health Canada by classifying each suspect cannabis product to a cannabinoid dominance based on available product details and assigning all individual medical events within a case to all reported suspect cannabis products and their cannabinoid dominance (weighted equally for all events). Therefore, this may over-estimate the correlation between cannabinoid dominance and individual medical events and is not reflective of causality.
Overall, CBD-dominant or leaning products were more frequently reported than THC-dominant or leaning products across all adverse reaction case reports in 2020. This is consistent with observations from the previous reporting period where adverse reaction data were mostly associated with cannabis oil products that were CBD-dominant or leaning.
As highlighted in Figure 12, certain individual medical events were more frequently reported with THC-dominant or leaning products, whereas others were more frequently reported with CBD-dominant or leaning products. For example, insomnia and pain were more frequently reported with THC-dominant or leaning products, whereas anxiety and diarrhoea were more frequently reported with CBD-dominant or leaning products. Dizziness, loss of consciousness, syncope and hallucination were also more frequently reported with CBD-dominant or leaning products.
It is important to note that these are reported events only and other factors may be contributing to these events including: the age and health status of patients (including pre-existing health conditions and use of concomitant medications); prior exposure to cannabis (for example, cannabis naïve consumers); dosage; route of administration; and knowledge or awareness of effects of cannabis and cannabinoids. Further years of data are required to draw firmer conclusions.
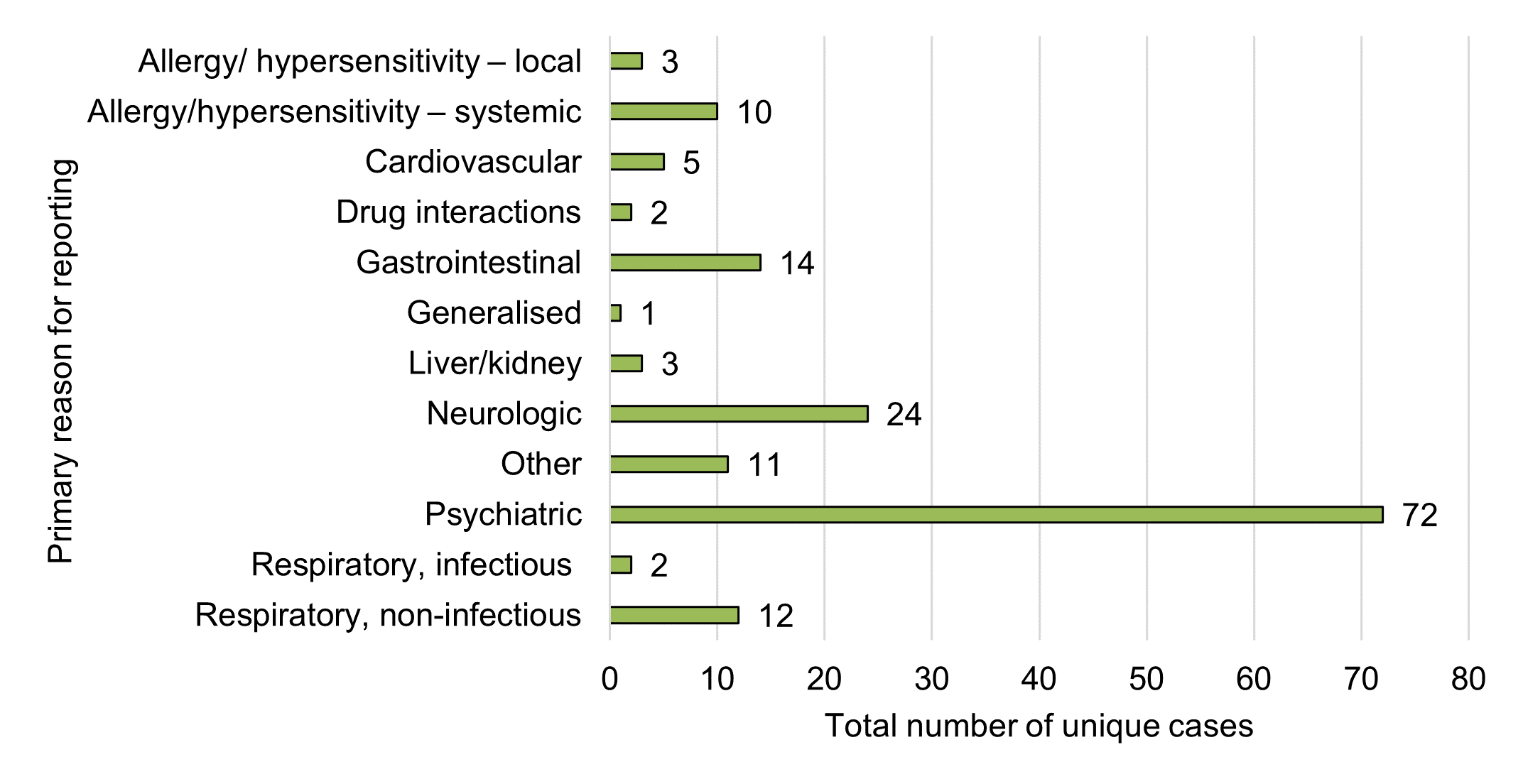
Figure 13 - Text description
| Primary reason for reporting | Total number of unique cases |
|---|---|
| Allergy/ hypersensitivity – local | 3 |
| Allergy/hypersensitivity – systemic | 10 |
| Cardiovascular condition(s) | 5 |
| Drug interaction(s) | 2 |
| Gastrointestinal condition(s) | 14 |
| Generalised condition(s) | 1 |
| Liver/kidney condition(s) | 3 |
| Neurologic condition(s) | 24 |
| Other | 11 |
| Psychiatric condition(s) | 72 |
| Respiratory, infectious condition(s) | 2 |
| Respiratory, non-infectious condition(s) | 12 |
| Grand total | 159 |
Caveat(s):
- This data was manually created by Health Canada by assigning one primary medical reason at the case level based on the overall details (including verbatim narrative), rather than by individual medical event level (frequency).
- If more than one reason existed, the event with greater severity was selected.
As described above, breakdowns of individual medical events by SOC is useful for overall data presentation; however, it is not always sufficient to represent clinical conditions or events that may have multi-system involvement (that is, involve multiple SOCs), which is important for ongoing monitoring and detection of new safety signals. As such, Health Canada also conducts a manual review of case-level details in order to assign a primary reason for reporting to each case. This allows Health Canada to identify and highlight cases of clinical interest that may span across multiple SOCsFootnote 5, or that may fall under generalized SOC categories that lack specificityFootnote 6. This aids in signal monitoring and case identification for further assessment.
Using this methodology, psychiatric, neurologic (nervous system), gastrointestinal and allergy / hypersensitivity conditions (systemic and localized) were the most common primary reasons for reporting at the case level, which tends to be generally consistent with the SOC groupings (according to frequency of individual medical events in Figure 11).
Respiratory (non-infectious) conditions were among the top five primary reasons for reporting in 2020. However, this category was not among the top five reasons for the previous reporting period, and was not readily observable in the individual medical events by SOC (frequency). More than half of these respiratory (non-infectious) cases (58%, n=7) involved dried cannabis (whole flower). Another four cases involved inhaled cannabis extracts (vaping liquids, n=3; rosin, n=1), which became legally available during this reporting period. One additional case involved an ingestible cannabis extract (oil). All cases were considered non-serious.
While drug interaction was reported as an individual medical event in five cases (does not appear among the top 15 events in Figure 11), it was only designated as a primary reason for reporting in just two cases in Figure 13 due to a lack of detailed information in the three remaining cases. Similarly, product quality was not identified as a primary reason for reporting in any of the cases but was listed as an individual medical event in one case, due to the limited amount of detail and data available.
Sex and Gender Based Analysis Plus (SGBA plus) of 2020 Adverse Reaction Data
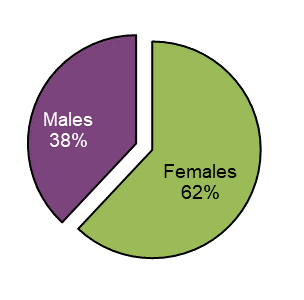
Figure 14 - Text description
| Sex | Total number of unique cases | Proportion (%) of total |
|---|---|---|
| Females | 90 | 62 |
| Males | 56 | 38 |
| Grand total | 146 | 100 |
A total of 146 adverse reaction cases included a designation for sex. Of these cases 62% (n=90) involved females. Females were involved in 66% of all serious cases with a reported sex while 89% of females and 68% of males used cannabis for reported medical purposes.
Females involved in adverse reaction cases associated with cannabis products were older than males. The average age of females was 64.2 years (95% CI: 60.6-67.9) and 51.2 years for males (95% CI: 44.4-58.0).
<65 years:
Females, n=31; males, n=28
≥65 years:
Females, n=46; males, n=15
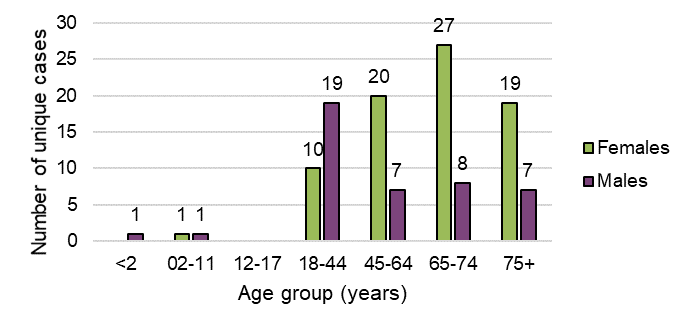
Figure 15 - Text description
| Age group (years) | Total number of unique cases | |
|---|---|---|
| Females | Males | |
| <2 | 0 | 1 |
| 02-11 | 1 | 1 |
| 12-17 | 0 | 0 |
| 18-44 | 10 | 19 |
| 45-64 | 20 | 7 |
| 65-74 | 27 | 8 |
| 75+ | 19 | 7 |
| Grand total | 77 | 43 |
Adverse reaction cases involving inhaled dried cannabis (whole flower) and vaping liquids more frequently involved males than females. Conversely, ingestible cannabis extracts in liquid format (bottled oils), softgel capsules and oral sprays more frequently involved females than males.
Females were more frequently involved in adverse reaction cases where multiple suspect cannabis products were reported than males (16 vs. 9 cases with multiple products, respectively).
Top three individual medical events reported, by sex:
| Females | Males |
|---|---|
| HallucinationFootnote * (n=37) | HallucinationFootnote * (n=14) |
| Dizziness (n=12) | Fall (n=5) |
| Nausea (n=9) | Psychotic disorder (n=5) |
|
|
3.0 Clinical evaluation of serious and medically important cases
3.1 Summary of serious and medically important adverse reactions
- All adverse reactions cases that were reported as serious to Health Canada were assessed for causality (n=121). An additional four cases that were reported as non-serious but deemed medically important by CSCB were also assessed for causality, for an overall total of 125 cases in scope for causality assessment during the reporting period.
| Causality Assigned | Number of cases |
|---|---|
| Certain | 0 |
| Probable | 11 |
| Possible | 95 |
| Unlikely | 5 |
| Unassessable | 14 |
| Grand total | 125 |
- The causality of adverse reactions is based on the information reported in the cases and through follow-up where possible. Cases may lack sufficient information to assess the causal association between event(s) and exposure to the cannabis products. Causality assessment is a clinical investigation of cases and is a routine practice in pharmacovigilance to determine the likelihood of association between the product(s) and the adverse reaction(s). This practice supports the identification of potential safety concerns that may require further investigation or actions by the regulatory authority and/or LH.
- Overall, the majority of the cases were assigned a causality of 'Possible' (76%, n=95), meaning there was a reasonable possibility that the cannabis product may have contributed to the adverse reaction but the contribution of other factors could not be ruled out (for example, concomitant medications, co-morbidities, etc.).
- There were 11 cases assigned a causality of 'Probable' (9%), meaning there was sufficient information to judge that the cannabis product probably contributed to the adverse reaction and the contribution of other factors was considered to be unlikely.
- There were 14 cases determined to be 'Unassessable' (11%), meaning there was insufficient information to establish a causal association between the cannabis product and the reported adverse reaction.
- There were five cases assigned a causality of 'Unlikely' (4%), meaning that the cannabis product was judged to not play a causative role in the reported adverse reaction (other probable factors identified).
- No cases were assigned a causality of 'Certain', for which a stringent level of evidence is required, including both laboratory and medical confirmation. As well, the case must lack any other alternative explanations.
- Among the 11 cases assigned a causality of 'Probable', all involved legal cannabis extract products for ingestion (softgel capsules, n=5; oils, n=6). The suspect cannabis products had varying concentrations of cannabinoids (5 CBD-dominant, 2 CBD-leaning, 2 THC-dominant, 1 THC-leaning and 1 equilibrated product) and other ingredients (for example, carriers). Among these cases, 10 out of 11 reported cannabis as a sole suspect product, meaning that no other health products were reported as co-suspects.
- Among the 'Probable' cases, most of the patients had pre-existing medical conditions and reported using cannabis products for medical purposes (for example, pain related to cancer, arthritis, and other chronic disorders) (82%, n=9). However, details of the cases supported that there was a probable association to the use of the cannabis products (for example, acceptable time to onset between exposure and the events stopping when the product is discontinued [that is, positive de-challenge]).
3.2 Important identified risks during the reporting period
- During this reporting period, none of the serious or medically important cases identified were classified as new important risks by Health Canada.
- Most serious or medically important cases with 'Probable' causality were considered to involve known identified risks (that is, characterised in Health Canada's Information for Health Care Professionals document; last updated: Spring 2018). These included:
- Hypersensitivity /allergic reactions (for example, localised or systemic hypersensitivity reactions such as pruritus, urticaria, swelling/oedema, anaphylaxis)
- Psychiatric reactions (for example, panic, anxiety, hallucinations)
- Neurologic reactions (for example, headache, light-headedness)
- Gastrointestinal reactions (for example, nausea, vomiting, stomach pain)
3.3 Important potential risks during the reporting period
'Important potential risks'Footnote 7 observed in adverse reaction data with cannabis products during this reporting period included:
- Risk of high intraocular pressure/aggravation of glaucoma associated with the use of CBD-dominant cannabis products: This signal was considered to be new/unexpected (that is, not previously well characterised). Therefore, an in-depth assessment (case series) was conducted, which included a review of both domestic and foreign case reports of adverse reactions as well as data from the published literature (case reports and scientific literature). Health Canada's review of the available information found that there is currently limited case-level evidence of aggravated glaucoma or increased ocular pressure (n=3 domestic cases) with oral consumption of CBD-dominant cannabis products. No dose-response can be determined from the domestic cases, which lacked in important details (that is, concomitant medications and medical history). However, limited published evidence from small clinical studies suggests that oral administration of THC may cause a transient reduction in intra-ocular pressure (IOP) while oral administration of CBD, in contrast, may cause a transient increase in IOP, as described in the Information for Health Care Professionals document. At this time, only a potential risk can be established between the use of these cannabis products and the risk of increased intraocular pressure/aggravation of glaucoma. Although evidence is limited, it is considered an important health effect and further evidence may strengthen this safety signal.
- Risk of cannabis – drug interaction: During this reporting period, there were five cases involving a suspected cannabis-drug interaction; however, three cases did not have sufficient information to discern if an interaction was involved. In the two remaining cases, only one was medically confirmed which included a suspected interaction between an equilibrated cannabis oil product and metronidazole. This interaction was assessed to be 'Possible' by both the reporting physician and Health Canada. The second case was not medically confirmed, but involved a suspected interaction between a CBD-leaning cannabis softgel product and lorazepam. This interaction was assessed to be 'Possible' due to an acceptable temporal relationship and pharmacological plausibility. This report was missing important details to assign a stronger causal association and fully elucidate whether or not a drug interaction actually occurred. However, data from the scientific literature indicate that cannabinoids can modulate drug-metabolizing enzymes (for example, THC as a CYP1A2 inducer and CBD as a potent inhibitor of CYP3A4 and CYP2D6). Therefore, there is a possible risk of interaction between cannabinoids and certain medications that may vary in clinical significance depending on product (for example, concentration of THC and CBD), dose, and route of administration and patient risk factors. As outlined in Health Canada's Information for Health Care Professionals document, impairment from THC can be exacerbated with co-consumption of other central nervous system depressants. As such, the signal of cannabis-drug interaction remains an important potential risk that Health Canada continues to monitor in the future.
- Risk of hallucination involving CBD products: While hallucination is known to be associated with THC due to its psychotropic properties as described in the Information for Health Care Professionals document, cases of hallucinations following the use of CBD-dominant or CBD-leaning products are considered to be new/unexpected since CBD is not known to be psychotropic. During this reporting period, there were 34 suspected cases of hallucination (auditory hallucination, visual hallucination, mixed hallucination, hypnagogic hallucination or pseudohallucination) involving CBD-dominant or CBD-leaning products. It is important to note that although these products are CBD-dominant or CBD-leaning, they may still contain small amounts of THC and dose/frequency of use may increase exposure to THC in sufficient quantities to trigger a psychotropic effect such as hallucination. The majority of these cases occurred in adults aged 65 years and older with reported pre-existing conditions, and other risk factors may be involved in these cases. At the time of this report, CSCB was reviewing this signal.
3.4 Missing information during the reporting period
This section highlights potential risks identified on a preliminary basis with limited information available, and continue to be monitored.
- Aggravation of autoimmune disorder: There was one reported case involving aggravation of autoimmune disorder; however, limited information was available for further assessment.
4.0 Note to readers
Adverse reaction reports with cannabis submitted to Health Canada are received and entered into the Canada Vigilance Database. The Marketed Health Products Directorate (MHPD) of the Health Products and Food Branch (HPFB) collects, monitors and analyses adverse reactions submitted to the Canada Vigilance Database, amongst other activities, and codes and houses adverse reaction reports for cannabis. The Controlled Substances and Cannabis Branch (CSCB) is responsible for the monitoring, detection, prioritization, evaluation and aggregate reporting of adverse reactions associated with cannabis (pharmacovigilance).
Voluntary reports from the public may be received via the online reporting form, through the toll-free number or through the printable form via electronic fax or mailing to Health Canada. Mandatory reports are submitted by LHs in order to meet their regulatory reporting obligations for serious adverse reactions under the Cannabis Regulations and are submitted through fax or mail, unless the company is registered to submit electronic reports directly to the Canada Vigilance Database (specific format must be met). Cannabis complaints and/or product quality issues may also be referred via the Cannabis Reporting Form from Health Canada. Incidents involving cannabis accessories (for example, mechanical, physical or electrical issue or failure of a cannabis accessory and associated injuries) can be reported via the Consumer Product Incident Reporting Form from the Healthy Environments and Consumer Safety Branch.
All cannabis adverse reaction cases are coded in the following manner:
- Case reports are translated into electronic data into the Canada Vigilance Database. All individual medical events are coded using the Medical Dictionary for Regulatory Activities (MedDRA), which is developed, maintained and updated by the ICH as an international set of standardized medical terms for symptoms, signs, diseases, syndromes and diagnoses.
- Case reports involving cannabis as a substance in a suspected role are coded as 'cannabis sativa' at the active ingredient level, irrespective of the identity of the cannabis product (legal, illegal, unspecified, undetermined).
- Case reports involving a legal cannabis product in a suspected role (identified either by product name or LH) are classified according to the intended use: cannabis product used for medical purposes ('medical cannabis') or used for non-medical purposes ('non-medical cannabis'), based on the information in the report. Cannabis use for medical purposes includes reports described having a medical authorization document; or, a reported medical or therapeutic purpose or indication, without mention of a medical authorization document. If there is no reported reason for use provided in the report, minimal details or intended use for non-medical purposes, the report is classified as 'non-medical cannabis'.
- Case reports are coded as serious as reported if at least one criterion for seriousness is selected: death, life-threatening, admitted to the hospital, lengthened hospital stay, disability, or birth defect. Serious - other medically important condition may also be selected by the reporter.
- According to international pharmacovigilance guidelines (ICH guidelinesFootnote 8), medically important conditions may also be considered serious under certain circumstances and therefore are an option to select when reporting an adverse reaction to Health Canada, and any adverse reaction case identified as such are further reviewed. However, these cases fall outside of the regulatory definition of a serious adverse reaction under the Cannabis Regulations.
- Cannabinoid dominance is a value assigned by Health Canada to each individual suspect cannabis product across all cases, based on available information. In the event that the concentrations are missing, using the product name the cannabinoid concentrations are verified from online information using the LH's website, provincial store websites, or other available resources. In the event that a product is not able to be identified (for example, Unknown Cannabis Oil by LH) and the cannabinoid concentrations are not reported, then the cannabinoid dominance is assigned as "unassessable".
The criteria used for assigning cannabinoid dominance is as follows:
- "THC-dominant": THC:CBD ratio greater than 1.5:1
- "THC-leaning": THC:CBD ratio in between 1.5:1 and 1.2:1
- "Equilibrated": THC:CBD ratio in between 1.2:1 and 1:1.2
- "CBD-leaning": THC:CBD ratio in between 1:1.2 and 1:1.5
- "CBD-dominant": THC:CBD ratio greater than 1:1.5
Health Canada conducts routine monitoring, detection, assessment and associated activities for all cannabis adverse reaction reports, which involves:
- Screening of all new cannabis case reports to ensure they are coded appropriately according to MedDRA; classified as either cannabis for medical or non-medical purposes (legal classes); and, product names are accurate.
- Case reports involving a suspected non-compliance (that is visual mould present, metallic taste, unusual odour, etc.) are referred to the Compliance Directorate for verification.
- Non-serious reports are screened by Health Canada and those deemed to be medically important events are included for further assessment (causality assessment).
- All serious and medically important case reports undergo further investigation and assessment:
- follow-up is conducted for additional information on product details or clinical details of cases to aid in assessment
- cursory causality assessment is conducted on all routine serious and medically significant cases
- all fatal and life-threatening cases are considered priority reports and undergo a comprehensive individual causality assessment
- causality assessment is based primarily on the World Health Organization (WHO-UMC) causality system
- Any cases involving new or unexpected adverse reactions of interest undergo preliminary assessment to determine if they should be further evaluated (signal prioritization).
- A case series evaluation (signal assessment) is conducted in the event of a cluster or related cases involving new adverse reactions of interest. These comprehensive assessments involve the determination of biological plausibility based on published literature, domestic as well as international adverse reaction data (WHO Vigibase).
5.0 Reporting an adverse reaction involving a cannabis product
Licence holders must submit serious adverse reaction reports, as defined by the Cannabis Regulations, involving a cannabis product and are encouraged to voluntarily submit non-serious adverse reaction reports involving a cannabis product. More information can be found in the Cannabis adverse reaction reporting guide for licence holders.
Consumers and HCPs are encouraged to report any adverse reaction to a cannabis product directly to CSCB. Consumers and health care practitioners may also send a report to the LH of the cannabis product.
6.0 Contact us
Any questions or comments on this report, including any requests for the data used to support this report, should be directed to cannabis_oss-cannabis_bss@hc-sc.gc.ca.
Footnotes
- Footnote 1
-
Under the Cannabis Act and its Regulations adults are allowed to legally grow up to a maximum of four cannabis plants. This is in addition to any plants that may be authorized for personal and designated production for medical purposes. However, rules surrounding home growing for non-medical purposes may vary based on the rules and regulations of individual provinces or territories.
- Footnote 2
-
As per Section 248 of the Cannabis Regulations, all adverse reactions, including non-serious adverse reactions, must be maintained in an annual summary report by the LH, which can be requested by Health Canada.
- Footnote 3
-
World Health Organization (2012). Safety monitoring of medicinal products. Reporting system for the general public. Available from: https://www.who.int/publications/i/item/9789241503198.
- Footnote 4
-
CBD-dominant, CBD-leaning, THC-dominant, THC-leaning, and equilibrated categories were manually assigned by Health Canada. Please refer to Section 4: Note to Readers for further details.
- Footnote 5
-
For example, allergy or hypersensitivity reactions may span multiple SOCs, including SOC skin and subcutaneous disorders; SOC immune system disorders; and may involve others such as SOC cardiac disorders, SOC investigations, and SOC gastrointestinal disorders in instances of anaphylaxis.
- Footnote 6
-
For example, drug interaction is classified under the SOC 'general disorders and administration site conditions'.
- Footnote 7
-
Risk for which there is suspicion of an association but no confirmation based on current information, requiring ongoing monitoring.
- Footnote 8
-
https://www.ema.europa.eu/en/documents/scientific-guideline/international-conference-harmonisation-technical-requirements-registration-pharmaceuticals-human-use_en-12.pdf
Page details
- Date modified:
