Page 11: Guidelines for Canadian Drinking Water Quality: Guideline Technical Document – Toluene, Ethylbenzene and the Xylenes
10.0 Classification and assessment
The assessments of toluene, ethylbenzene and xylenes are combined into one supporting document, as co-exposure to these compounds (along with benzene) is likely during a contamination event in drinking water. Although all three compounds show some overlap in health effects (i.e., neurological impairment), they were not assessed as a mixture, given that the modes of action involved in the most sensitive health effects are different for each compound. It is unclear whether current PBPK models are adequate for characterizing interactions from oral exposure to mixtures of toluene, ethylbenzene and xylenes (as well as benzene) (ATSDR, 2004).
The results of PBPK model simulations and experimental exposures to mixtures of toluene, ethylbenzene and xylenes (with benzene) in rats and humans indicate that inhalation exposure to mixtures containing each component at a concentration of approximately 20 ppm is unlikely to result in biologically significant increased blood levels of these chemicals compared with exposure to each component individually (Tardif et al., 1997; Haddad et al., 1999, 2000, 2001). For these reasons, toluene, ethylbenzene and xylenes were modelled separately, with no metabolic interactions considered, and three separate maximum acceptable concentrations (MACs) are established.
10.1 Toluene
Toluene is an extensively used solvent found in paints, paint thinners, lacquers and adhesives. It is also found in gasoline and used as an intermediate in chemical synthesis. The health effects of toluene have been studied in humans in several occupational environments in which toluene use is predominant, including printing, painting, the rubber industry and shoe manufacturing. These studies have revealed an array of neurological effects, including loss of colour vision and disturbances in memory, concentration and cognitive function in general, upon long-term inhalation of toluene. Studies of oral exposure in animals support adverse neurological effects as a critical endpoint of toluene toxicity, as shown by altered behaviour, changes in neurotransmitter levels and brain necrosis. The International Agency for Research on Cancer (IARC, 1999) has determined that toluene is not classifiable as to its carcinogenicity to humans (Group 3). Consequently, Health Canada has focused on neurological endpoints in determining the risk associated with toluene exposure via drinking water.
Due to the number of epidemiological studies available and to the availability of human PBPK models to estimate oral doses, the risk assessment of toluene was based on human data. No chronic oral studies in animals pertaining to neurological effects were identified. Neurological endpoints were determined to be the most significant adverse effect, as they were consistently observed in occupationally exposed humans as well as in experimental animals exposed via oral and inhalation administration. Several occupational studies were identified that had adequate exposure information and examined chronic neurological effects. These included studies of auditory and visual adverse effects (Nakatsuka et al., 1992; Abbate et al., 1993; Vrca et al., 1995; Zavalic et al., 1998; Cavalleri et al., 2000) as well as other neurobehavioural and neurophysiological alterations (Foo et al., 1990; Murata et al., 1993; Boey et al., 1997; Eller et al., 1999; Neubert et al., 2001; Seeber et al., 2004, 2005).
Two studies that examined the same population of exposed individuals within 14 rotary printing plants stood out in particular (Seeber et al., 2004, 2005). These studies covered all of the aforementioned neurological endpoints, including vibration thresholds, colour discrimination, auditory thresholds, attention (symbol–digit substitution, switching attention and simple reaction), memory (digit span forward and backward, immediate and delayed reproduction of pictures) and psychomotor functions (steadiness, line tracing, aiming, tapping, pegboard). Moreover, the neurological effects were investigated in terms of length of exposure, with an average of 21 years as a lifetime-weighted average and an average of 6 years as a current exposure level. The shorter-term data were more relevant in the selection of a point of departure, as toluene levels were measured four times over the period of 5 years directly in the breathing environment of workers over full days, whereas long-term data were estimated using a job exposure matrix. In addition to adequate exposure monitoring, the Seeber et al. (2004, 2005) studies had a large sample size, a reference group from the same population as the exposed group, and appropriate controls for age, education and alcohol intake. None of the endpoints investigated within these studies was indicative of an adverse effect following exposure to toluene. As such, a NOAEL of 26 ppm or 98 mg/m3 (as an average of highly exposed individuals) was retained as the point of departure. It should be noted that all effects investigated in other epidemiological studies were observed at concentrations that exceeded 26 ppm. Although the true NOAEL for neurological endpoints may be higher than 26 ppm, we considered 26 ppm as the most appropriate point of departure based on available studies.
In order to determine an oral dose from an inhalation study, PBPK modelling was employed to estimate an internal toluene blood concentration of 0.0075 mg/L following inhalation exposure. This internal dose was then inputted into the human PBPK model in order to determine the external oral dose required to result in a similar blood concentration, assuming consumption of 1.5 L of drinking water per day. The resulting human external dose from drinking water was determined to be 0.097 mg/kg bw per day. Since 21 years of exposure to a lifetime-weighted average concentration of 45 ppm or 170 mg/m3 did not reveal any effects on any of the neurological endpoints assessed by Seeber et al. (2004, 2005), the addition of an uncertainty factor for the use of a short-term study was deemed unnecessary. Moreover, studies of toluene included chronic studies in two species, developmental toxicity studies in two species as well as a two-generation reproductive toxicity study. As such, the database of information was determined to be adequate, and thus no additional uncertainty factor was added with regards to database adequacy. However, an uncertainty factor was added to account for intraspecies variability. Taking this into consideration, the tolerable daily intake (TDI) was calculated as follows:
Equation 1
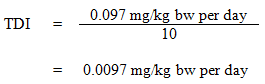
where:
- 0.097 mg/kg bw per day is the external oral dose required to give a blood concentration that is equivalent to the NOAEL of 26 ppm (Seeber et al., 2004, 2005); and
- 10 is the uncertainty factor to account for intraspecies variability.
Equation 1 - Text Description
The TDI was employed to calculate the MAC, as follows:
Equation 2
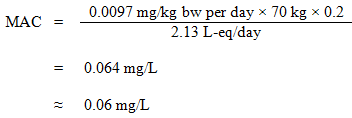
where:
- 0.0097 mg/kg bw per day is the TDI derived above;
- 70 kg is the average body weight of an adult;
- 0.2 is the default allocation factor for drinking water, used as a "floor value", since drinking water is not a major source of exposure, and there is evidence of widespread presence in at least one of the other media (air, food, soil, or consumer products) (Krishnan and Carrier, 2013); and
- 2.13 L-eq is the daily volume of water consumed by an adult, accounting for multiple routes of exposure (see Section 5.6).
Equation 2 - Text Description
10.2 Ethylbenzene
Ethylbenzene is primarily used as an intermediate in the production of styrene, but also occurs in gasoline and as a component of mixed xylenes. The health effects of ethylbenzene have been studied in experimental animals, with similar outcomes occurring in all species. However, effects in humans are relatively unknown, due to the lack of occupational settings in which exposure to ethylbenzene is predominant. Animal data have identified liver and kidney as the primary targets of ethylbenzene. Inhalation and ingestion of ethylbenzene in rats and mice lead to enlarged liver and kidney and increased severity of nephropathy. Chronic studies of exposure to ethylbenzene by inhalation and ingestion suggest that exposure may lead to tumour formation at various sites. Mechanistically, it is suggested that ethylbenzene-induced carcinogenesis may result from excessive tissue damage and proliferation in the absence of genotoxicity. Due to sufficient evidence of carcinogenicity in experimental animals but inadequate data in humans, ethylbenzene was classified as possibly carcinogenic to humans (Group 2b) by the International Agency for Research on Cancer (IARC, 2000). Health Canada has considered both cancer and non-cancer endpoints in deriving a health-based value (HBV) for ethylbenzene.
10.2.1 Cancer risk assessment
There is significant evidence of ethylbenzene-induced carcinogenesis in experimental animals. Inhalation of ethylbenzene is carcinogenic in animals, producing tumours in the kidney (male and female rats), lung (male mice), liver (female mice) and Leydig cells (male rats). In rats, renal cancers through exacerbation of chronic progressive nephropathy, Leydig cell tumours through the perturbation of serum testosterone levels and liver tumours through phenobarbital-like induction are all considered to be not relevant to humans (see Section 9.3). The potential role for oxidative metabolites in the mode of action of ethylbenzene-induced lung tumours in mice, however, suggests that these tumours may be relevant to humans. The mode of action for lung tumours suggests that there is a threshold below which tumours are not expected to be observed. As a result, Health Canada deemed it appropriate to determine an HBV for lung tumours using a tolerable daily intake approach.
The NTP (1999) study was determined to be the most appropriate animal study for the derivation of an HBV for lung tumour development from ethylbenzene exposure. A mouse PBPK model was employed in order to adjust for an oral dose that is relevant to humans. The cumulative lifetime internal blood concentration of ethylbenzene (in milligrams of parent compound per litre of blood) generated by the mouse PBPK model was determined to be the most appropriate dose metric to represent the concentration of ethylbenzene in the lung. This dose metric is supported by the proposed mode of action for lung tumours, which suggests that ethylbenzene metabolism occurs in the lung, generating toxic metabolites leading to potential oxidative stress and the possible promotion of lung tumours (see Section 9.3). As ethylbenzene generates several oxidative stress–inducing metabolites, the parent compound level in blood was selected in order to establish the most conservative estimate of oxidative stress in lung. PBPK modelling allows for the use of an inhalation study instead of an ingestion study and accounts for metabolic differences between animals and humans as well as metabolic differences between high and low exposure levels. Although it is likely that the additional lung tumours in this experimental model are due to the inhalation route of exposure, ethylbenzene is also expected to exert its toxicity systemically. The use of blood concentrations provides a more conservative estimate of cancer risk than the internal dose in the lung compartment and is more appropriate for the derivation of a drinking water guideline. Consequently, blood concentrations have been used as the dose metric in the cancer risk assessment. The external doses associated with lung tumours in mice from the NTP (1999) study were inputted into the mouse PBPK model in order to estimate the cumulative lifetime internal blood concentrations of ethylbenzene (in mg/L blood). Using the log logistic model (as the best fit model) from the U.S. EPA's BMD software (U.S. EPA, 2010), these internal doses were then analysed to determine the most appropriate point of departure for inputting into the human model; the animal point of departure was determined to be the lower 95% confidence limit of the BMD corresponding to a 10% increase in lung tumours (BMDL10) of 1.43 mg of ethylbenzene per litre of blood. This internal dose was then inputted into the human PBPK model in order to determine the human external dose required to give a blood concentration similar to that in the mouse, assuming 1.5 L consumption of drinking water per day and 70 years of exposure. The resulting external human oral dose was determined to be 10.17 mg/kg bw per day. Using this human external dose, the TDI for ethylbenzene can be determined as follows:
Equation 3
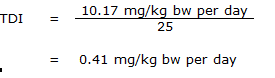
where:
- 10.17 mg/kg bw per day is the BMDL10 for lung tumours in mice observed in the NTP (1999) study; and
- 25 is the uncertainty factor accounting for intraspecies variability (10) and interspecies variability (2.5) (see below).
Equation 3 - Text Description
The interspecies uncertainty factor can be divided in two components: a toxicokinetic (delivered dose) component (x4) and a toxicodynamic (differential tissue sensitivity) component (x2.5) (IPCS, 2005). PBPK modelling accounts for differences in the toxicokinetics between animals and humans; as a result, the toxicokinetic component of the interspecies uncertainty factor (x4) can be removed from the TDI calculation. As the toxicodynamic variation (relating to tissue sensitivity) between animals and humans for ethylbenzene is not well known, the toxicodynamic portion of the interspecies uncertainty factor was retained for determining the TDI.
The TDI was employed to calculate an HBV, as follows:
Equation 4

where:
- 0.41 mg/kg bw per day is the TDI as derived above;
- 70 kg is the average body weight of an adult;
- 0.2 is the default allocation factor for drinking water, used as a "floor value", since drinking water is not a major source of exposure, and there is evidence of widespread presence in at least one of the other media (air, food, soil, or consumer products) (Krishnan and Carrier, 2013); and
- 2.15 L-eq/day is the daily volume of water consumed by an adult, accounting for multiple routes of exposure (see Section 5.6).
Equation 4 - Text Description
10.2.2 Non-cancer risk assessment
Due to the abundance of animal studies and the lack of epidemiological studies, animal data were considered in the non-cancer risk assessment of ethylbenzene. Oral and inhalation studies, including one study spanning two years of exposure by inhalation in mice, were shown to target the liver and kidneys of exposed rodents, as demonstrated by increased weights of these organs. Chronic exposures to ethylbenzene produced lesions in mouse liver, lung, thyroid and pituitary gland and in rat kidney, prostate gland, bone marrow and liver. Ethylbenzene is not considered to be teratogenic, is not a reproductive toxicant, is not selectively toxic to the developing nervous system and is not harmful to the immune system. Based on an evaluation of all the non-cancer data in mice and rats, a NOAEL of 75 ppm or 326 mg/m3 was identified from the NTP (1999) study for hyperplasia of the pituitary gland and liver cellular alterations in mice. To adjust for an oral dose that is relevant to humans, PBPK modelling was employed to estimate internal blood and liver concentrations in the mouse of 0.324 and 0.08 mg/L, respectively. The liver level is chosen to calculate the HBV, since it is lower than the blood concentration associated with the NOAEL. This internal dose was then inputted into the human PBPK model in order to determine the human external dose required to give a blood concentration similar to that in the rat, assuming 1.5 L consumption of drinking water per day. The resulting human external dose corresponding to a liver concentration of 0.08 mg/L is 0.54 mg/kg bw per day. This value was used to derive the TDI:
Equation 5

where:
- 0.54 mg/kg bw per day is the human external oral dose required to give a blood concentration equivalent to the NOAEL of 75 ppm based on hyperplasia of the pituitary gland and liver cellular alterations in mice (NTP 1999); and
- 25 is the uncertainty factor to account for intraspecies variability (x10) and interspecies variability (x2.5) (see Section 10.2.1).
Equation 5 - Text Description
The TDI was employed to calculate an HBV, as follows:
Equation 6
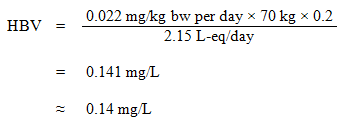
where:
- 0.022 mg/kg bw per day is the TDI derived above;
- 70 kg is the average body weight of an adult;
- 0.2 is the default allocation factor for drinking water, used as a "floor value", since drinking water is not a major source of exposure, and there is evidence of widespread presence in at least one of the other media (air, food, soil, or consumer products) (Krishnan and Carrier, 2013); and
- 2.15 L-eq/day is the daily volume of water consumed by an adult, accounting for multiple routes of exposure (see Section 5.6).
Equation 6 - Text Description
10.2.3 Comparison of cancer and non-cancer risk assessments
In Section 10.2.1, using a TDI (threshold) approach, an HBV for ethylbenzene that is protective of lung tumours was determined to be 3 mg/L. In Section 10.2.2, also using a TDI approach, an HBV of 0.14 mg/L was determined to be protective of hyperplasia of the pituitary gland and liver cellular alterations. As the non-cancer risk assessment resulted in a more conservative HBV for ethylbenzene, the MAC of 0.14 mg/L is deemed to be protective of both cancer and non-cancer health effects.
10.3 Xylenes
Xylenes (o-, m- and p-isomers) are used as industrial solvents, synthetic intermediates and solvents in paints, coatings, adhesive removers and paint thinners; they are also a naturally occurring component of petroleum. There is insufficient information from both animal and epidemiological studies to determine whether xylenes are carcinogenic in humans; both IARC and the U.S. EPA consider xylenes as not classifiable with respect to human carcinogenicity. The primary health effects following exposure to xylenes are effects on the central nervous system by all routes of exposure, effects on the respiratory tract following inhalation exposure and hepatic, renal and body weight effects following higher oral exposures. The scientific literature indicates that the isomers of xylene have displayed similar toxicokinetic properties and toxicological effects, with no single isomer consistently exhibiting any greater potency for a given health endpoint. Thus, studies of both mixtures and individual isomers were considered for risk assessment. Due to the lack of evidence of tumour formation and the non-genotoxic mode of action of xylenes, the MAC for xylenes is derived using non-cancer endpoints.
Few studies were available for a risk characterization of xylenes, and most of these lacked adequate dose–response characterization or demonstrated no adverse effects upon exposure. No suitable oral exposure studies were identified in animals or humans. Among the endpoints investigated, neurobehavioural alterations were the most consistently observed. One study in particular examined the performance of male rats on the rotarod test, as a measure of motor coordination disturbances (indicative of adverse neuromuscular effects), after a 3-month inhalation exposure to 50 and 100 ppm (or 217 and 434 mg/m3) m-xylene (Korsak et al., 1994). The tests were performed 24 hours after the last exposure, thus allowing complete excretion of m-xylene from the animals. This study was selected as the most appropriate, given that it is a well-controlled animal study that employed multiple doses with no co-exposure to other chemicals. Furthermore, the established lowest-observed-adverse-effect concentration (LOAEL) of 100 ppm was further supported by a 6-month study in rats demonstrating decreased performance on the rotarod test at the same concentration (Korsak et al., 1992). The Korsak et al. (1992) study was not considered in the derivation of the point of departure, since only one dose was studied.
Since the central nervous system is a major target of xylenes in various exposure scenarios, the inhalation route of exposure was considered to be appropriate for deriving a MAC. The increase in failures on the rotarod test showed a clear dose–response relationship in the studied animals. Information considered necessary for proper BMD analysis was not available. However, clearly significant effects were observed at the 100 ppm concentration. As such, the 50 ppm dose was retained as the point of departure.
In order to adjust for an oral dose that is relevant to humans, PBPK modelling was employed to estimate internal blood concentrations of 0.1380 mg/L for the 50 ppm concentration in the rat exposed for 3 months. This internal dose was then inputted into the human PBPK model in order to determine the human external doses required to give blood concentrations similar to those in the rat, assuming 1.5 L consumption of drinking water. The resulting human external doses corresponding to blood m-xylene concentrations of 0.14 and 0.40 mg/L were determined to be 1.00 and 2.91 mg/kg bw per day, respectively.
Uncertainty factors considered in deriving the TDI include interspecies and intraspecies variability as well as the use of a subchronic study instead of a chronic study. Because the effects on the rotarod test observed in this study were consistent 6 months after exposure, the uncertainty factor for use of a subchronic study was reduced. The TDI was calculated as follows:
Equation 7

where:
- 1.00 mg/kg bw per day is the human external oral dose required to give a blood concentration equivalent to a NOAEL of 50 ppm in the rat; and
- 75 is the uncertainty factor to account for intraspecies variability (10), interspecies variability (2.5) (see Section 10.2.1) and the use of a subchronic study instead of a chronic study (3).
Equation 7 - Text Description
The TDI was employed to calculate the MAC, as follows:
Equation 8
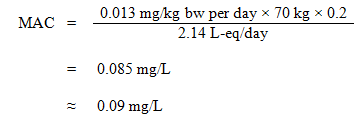
where:
- 0.013 mg/kg bw per day is the TDI derived above;
- 70 kg is the average body weight of an adult;
- 0.2 is the default allocation factor for drinking water, used as a "floor value", since drinking water is not a major source of exposure, and there is evidence of widespread presence in at least one of the other media (air, food, soil, or consumer products) (Krishnan and Carrier, 2013); and
- 2.14 L-eq/day is the daily volume of water consumed by an adult, accounting for multiple routes of exposure (see Section 5.6).
Equation 8 - Text Description
10.4 International considerations
This section presents the various drinking water guidelines and standards from other international organizations. Variation in these limits can be attributed simply to the year of assessment, or to differing policies and approaches including the choice of key study, as well as the use of different consumption rates, body weights, and allocation factors.
10.4.1 Toluene
The U.S. EPA has established a maximum contaminant level (MCL) for toluene in drinking water of 1.0 mg/L (1000 µg/L) based on a NOAEL of 312 mg/kg bw/day (adjusted to 223 mg/kg bw/day to adjust from five to seven exposure days per week) for increased kidney and liver weight observed in a 13-week oral gavage study in rats (NTP, 1989). An uncertainty of 1000 is applied (100 for interspecies and intraspecies variation and 10 for database insufficiencies and contradictions in the immunotoxicity data). An allocation factor for drinking water of 20% was employed in deriving the final guideline value.
The WHO (2004) established a drinking water guideline for toluene of 0.7 mg/L (700 µg/L). This guideline is based on a LOAEL of 312 mg/kg bw per day for marginal hepatotoxic effects observed in a 13-week gavage study in mice (NTP, 1990), correcting for 5 days/week dosing and using an uncertainty factor of 1000 (100 for interspecies and intraspecies variation and 10 for the use of a short-term study and the use of a LOAEL instead of a NOAEL). An allocation factor for drinking water of 10% was employed in deriving the final guideline value.
The California EPA (OEHHA, 1999) developed a non-mandatory public health goal (PHG) of 0.15 mg/L (150 µg/L) for toluene in drinking water based on a subchronic study (Hsieh et al., 1989) in which toluene was administered to mice via drinking water. Significantly increased liver weights (hepatomegaly) and decreased thymus weights were observed at a treatment level of 105 mg/kg bw per day, but not at 22 mg/kg bw per day. From this study, a NOAEL of 22 mg/kg bw per day was identified. Due to the volatility of toluene, a relative source contribution of 40% and an adult drinking water consumption rate of 4 L/day were assumed. A factor of 1000 (10-fold for interspecies variation, 10-fold for human variability and 10-fold to account for the use of a subchronic study for determining a lifetime value) was used to account for uncertainty in the PHG calculation.
10.4.2 Ethylbenzene
The U.S. EPA has established an MCL for ethylbenzene in drinking water of 0.7 mg/L (700 µg/L), based on a NOAEL of 136 mg/kg bw per day for histopathological changes in liver and kidney observed in a limited 6-month study in rats (Wolf et al., 1956), correcting for 5 days/week dosing and using an uncertainty factor of 1000 (100 for interspecies and intraspecies variation and 10 for the use of a short-term study). An allocation factor for drinking water of 20% was employed in deriving the final guideline value.
The WHO (2003a) drinking water guideline for ethylbenzene of 0.3 mg/L (300 µg/L) is based on a NOAEL of 136 mg/kg bw/day for hepatotoxicity and nephrotoxicity observed in a limited 6-month study in rats (Wolf et al., 1956), correcting for 5 days/week dosing and using an uncertainty factor of 1000 (100 for interspecies and intraspecies variation and 10 for database deficiencies and the use of a short-term study). An allocation factor for drinking water of 10% was employed in deriving the final guideline value.
The California EPA (OEHHA, 1997a) established a non-mandatory PHG of 0.3 mg/L (300 µg/L) for ethylbenzene in drinking water based on non-carcinogenic effects observed in experimental animals. The NTP (1996) study, a preliminary draft of NTP (1999) study available at the time of assessment, provided evidence of hepatotoxicity in mice exposed to 250 ppm ethylbenzene in air for 2 years. A NOAEL for hepatotoxicity was determined to be 75 ppm from the NTP (1999) study, corresponding to a daily dose of 49 mg/kg bw. For the calculation of the PHG, factors accounting for uncertainty in interspecies extrapolation, potentially sensitive human subpopulations and the potential for a severe effect (cancer) were incorporated, for a cumulative uncertainty factor of 1000.
10.4.3 Xylenes
The U.S. EPA (1987) established a maximum contaminant level (MCL) for total xylenes in drinking water of 10.0 mg/L based on a NOAEL of 250 mg/kg-day for reduced body weight and decreased survival in male rats from a 2-year gavage study (NTP, 1986). The NOAEL was adjusted for dosing 5 days/week to 179 mg/kg bw/day and an uncertainty factor of 100 (for intra- and inter-species variation) was applied to arrive at an oral RfD of 2 mg/kg bw/day. A relative source contribution (RSC) of 20% was applied in deriving the final MCL.
The WHO (2003b) drinking water guideline for xylenes of 0.5 mg/L (500 µg/L) is based on a NOAEL of 250 mg/kg bw per day for decreased body weight in a 103-week gavage study in rats (NTP, 1986), correcting for 5 days/week dosing and using an uncertainty factor of 1000 (100 for interspecies and intraspecies variation and 10 for the limited toxicological endpoints). An allocation factor for drinking water of 10% was employed in deriving the final guideline value.
The California EPA (OEHHA, 1997b) developed a non-mandatory PHG of 1.8 mg/L for individual xylene isomers or the sum of xylene isomers in drinking water based on neurotoxic effects in chronic exposures to xylene in humans as reported in Uchida et al. (1993). The LOAEL from this study of 7.5 mg/kg bw per day was divided by a factor of 30 (3 for extrapolation from a LOAEL to a NOAEL and 10 for potential variations in sensitivity among humans), then further divided by 2 to account for extra exposure by the inhalation route from the household water supply and corrected for an assumed relative source contribution from drinking water of 40%.
Page details
- Date modified: