Pan-Canadian Action Plan on Antimicrobial Resistance
Download in PDF format
(3 MB, 42 pages)
Organization: Public Health Agency of Canada
Date published: 2023-06-22
Cat.: HP40-337/2024E-PDF
ISBN: 978-0-660-69438-2
Pub.: 230659
Related links
Table of contents
- Ministers' message
- Land acknowledgement
- Acknowledgements
- Executive summary
- Section 1: The global and domestic AMR landscape
- Section 2: Action plan development and engagement process
- Section 3: A 5-year pan-Canadian commitment to address AMR
- Section 4: Implementing the action plan
- Annexes
Ministers' message
As the federal, provincialFootnote 1 and territorial Ministers of Health and Agriculture, we are pleased to release the Pan-Canadian Action Plan on Antimicrobial Resistance (the "action plan"). The action plan is a five-year (2023 to 2027) blueprint to coordinate an accelerated pan-Canadian response to address antimicrobial resistance (AMR), one of the major health threats of our time.
Antimicrobials help form the foundation of modern healthcare. Without effective antimicrobials, common infections could become life-threatening and treatments such as surgical procedures, joint replacements, and chemotherapy may no longer be possible. Effective antimicrobials are also critical for animal health. In addition to pet owners, farmers often use antimicrobials to care for animals or support the health of plants and crops. As a result, action to reduce AMR and preserve antimicrobial effectiveness helps to minimize an economic threat to the agriculture industry and maintain food security.
Over the course of this action plan, federal, provincial and territorial governments will work together and with Indigenous Peoples and partners across multiple sectors to implement ten shared priority actions. These actions span the areas of research and innovation, surveillance, stewardship, infection prevention and control and leadership.
The action plan builds on previous experience. For years, health professionals, the agriculture industry, researchers and many other groups and sectors in Canada have made progress to detect, understand and act against AMR.
However, more must be done. Increased commitment and action are required to address the growing threat AMR poses to health, agriculture and economies in Canada and around the world. Our objective as leaders of health and agriculture in Canada is to build on our collective past experiences to implement concrete activities that support the priority actions in this action plan and accelerate our response on AMR.
While governments have a responsibility to address AMR, contributions from many partners across several sectors are required to achieve our goal. We call on all relevant One Health partners to join us and work together to improve the appropriate use of antimicrobials and reduce the spread of AMR.
The action plan represents a significant milestone in our collective responses to AMR in Canada. We look forward to continuing to work together to strengthen AMR preparedness and response for years to come.
Land acknowledgement
We respectfully recognize and acknowledge that the lands on which we developed this action plan are the homelands of First Nations, Inuit, and Métis Peoples. We acknowledge our privilege to live and work on these lands and continue in striving to foster respectful partnerships with Indigenous Peoples and working collaboratively to advance reconciliation in Canada.
As this is a Pan-Canadian Action Plan, we invite you to take some time to reflect on the Indigenous lands which you call home and on the important historical impacts of colonialism that continue to generate inequities between Indigenous and non-Indigenous Peoples.
Acknowledgements
Federal, provincial and territorial Ministers of Health and Agriculture would like to thank all of those who participated in the development of this action plan. Your contributions have together helped provide Canada with a blueprint for collaboratively addressing the serious challenges presented by AMR.
The action plan would not have been possible without the dedication of the FPT Steering Committee, which consisted of government representatives from the public health, human and animal health, agriculture, and agri-food sectors.
Prior to the COVID-19 pandemic, the FPT Steering Committee led over 2 years of engagement and dialogue between Canada's leading experts in the human and animal health, agriculture and agri-food sectors. The FPT Steering Committee was supported by 4 expert task groups on surveillance, infection prevention and control (IPC), antimicrobial stewardship, and research and innovation. Co-chairs provided leadership to each of the task groups, which were made up of members of the medical and veterinary communities, industry, and academia.
In 2022, the Public Health Agency of Canada (PHAC) AMR Task Force resumed efforts to update and finalize the action plan under the guidance of the FPT Steering Committee. The final action plan reflects to the extent possible the collective contributions, dedication, and expertise of the FPT Steering Committee, task groups, organizations and individuals across Canada.
Executive summary
Antimicrobial resistance (AMR) is a growing threat to human and animal health. Nearly 15 people in Canada per day were estimated to have lost their lives to antimicrobial-resistant infections in 2018.Endnote 1 The costs to the healthcare system and Canada's GDP are already significant – an estimated $1.4 billion and $2.0 billion, respectively, in 2018.Endnote 1 Essential medical interventions such as organ transplantations, joint replacements and chemotherapy are becoming riskier as the antimicrobials used to prevent and treat infectious complications from these interventions are losing their effectiveness.
Antimicrobials also help protect animal health and welfare, and play a critical role in Canada's agriculture and food production systems. Increasing AMR can impact the health of food-animals and lead to reduced productivity and food safety concerns. An effective response to AMR requires joint One Health action across sectors and jurisdictions, both in Canada and globally.
The Pan-Canadian Action Plan on Antimicrobial Resistance (the "action plan") has been developed in collaboration with federal, provincial and territorial (FPT) partners, and responds to calls to action from industry, academia and other partners across One Health sectors. Considerations for mitigating the impacts of AMR on Indigenous populations were also discussed during engagement with Indigenous partners, including National Indigenous Organizations and other Indigenous health organizations and associations. Continued engagement with Indigenous Peoples, including through a distinctions-based approach, will help inform AMR actions that recognize and respond to the unique cultures, contexts, needs and priorities of First Nations, Inuit and Métis Peoples.
The action plan establishes FPT commitments on AMR over the next 5 years (2023 to 2027). Ten priority actions will guide Canada's multi-sectoral and multi-jurisdictional efforts across 5 pillars: research and innovation; surveillance; stewardship; infection prevention and control (IPC); and leadership (Figure 1).
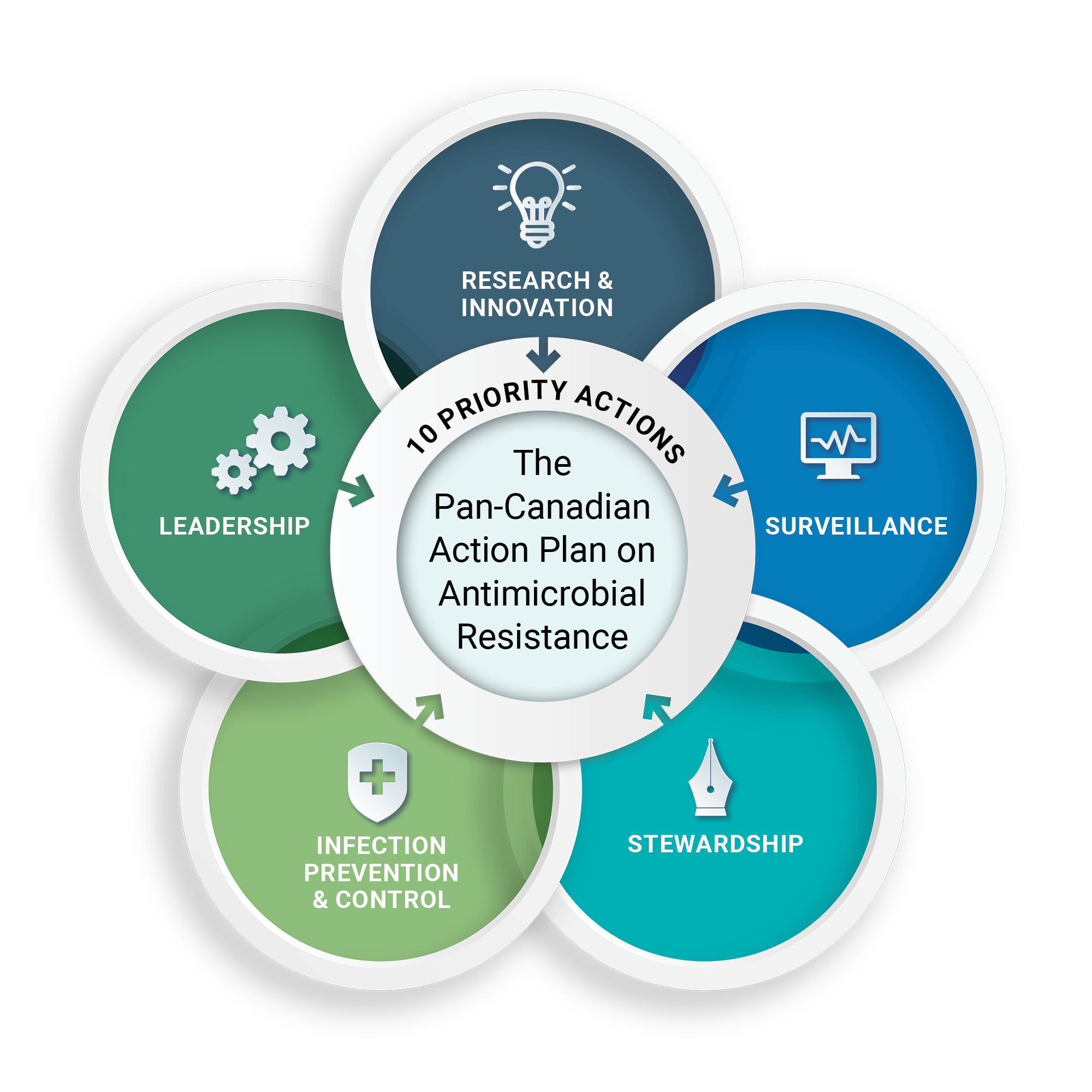
Figure 1: Text description
Figure 1 shows the action plan as composed of 5 interlocking pillars (Research and Innovation, Surveillance, Stewardship, Infection Prevention and Control, and Leadership) which outline 10 priority actions. The figure demonstrates that actions across pillars are mutually reinforcing and designed as a suite of actions that will together have the greatest impact in the Canadian context.
Partners involved in the development of the action plan recognize that sustained long-term commitment is required beyond the 5-year period of the action plan. Moving forward, progress made against the 10 priority actions will be monitored and reported publicly. Monitoring progress will promote continuous improvement, help partners and stakeholders adjust activities over time and identify new priorities over the course of the 5-year timeframe of this action plan and beyond.
A One Health response to AMR
Drug-resistant microorganisms are found in humans, animals, crops, and food. They are present in plants, soil, water, and other elements of the environment and may be transferred between and among different species, affecting the health of humans, companion animals, food-producing animals, and wildlife. Similarly, antimicrobials administered to humans or animals can make their way into the environment, further accelerating the development of AMR in water and soil.
There is global consensus that AMR must be addressed by adopting a One Health approach (Figure 2). This approach means that actions to limit the emergence and spread of AMR must consider each sector and the close interplay between humans, animals, crops, and their shared environment.
Action related to AMR and the environment is an increasing area of priority. Efforts within the human and animal health spheres have often taken precedence. Going forward, it will be important to work more closely with the environment sector to identify and mitigate AMR reservoirs and pathways for the transmission of medically-significant drug-resistant organisms through soil, water, flora, and fauna.
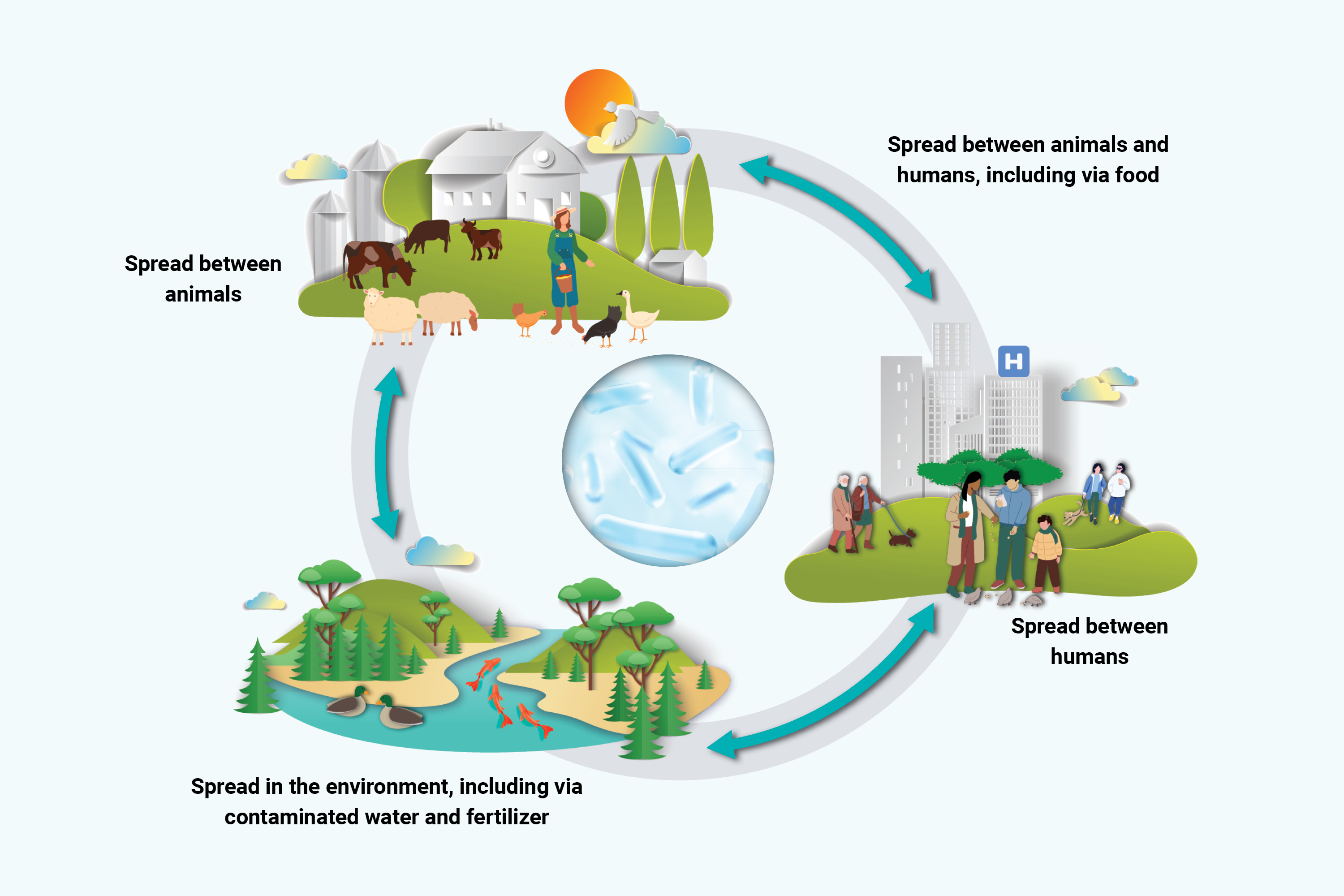
Figure 2: Text description
Figure 2 represents how human health, animal health, and the environment are linked to the issue of AMR. In a continuous circle, you can see how AMR is spread between:
- humans
- animals and humans including via food
- animals
- the environment, including via contaminated water and fertilizers
Section 1 of the action plan provides an overview of AMR and its impacts in Canada and globally as of late-2022. This section outlines the rationale for a collaborative One Health approach across jurisdictions and sectors to address AMR.
Section 2 describes how the action plan was developed and what it sets out to achieve.
Section 3 introduces 10 priority actions across 5 pillars: research and innovation, surveillance, stewardship, IPC and leadership.
Section 4 includes an overview of partners and stakeholders involved in Canada's AMR response. It describes how implementation of the action plan will be monitored.
Section 1: The global and domestic AMR landscape
Antimicrobial resistance (AMR) is a serious threat to human and animal health. Antimicrobial drugs, including antibiotics, antivirals, antifungals and antiparasitics, are a cornerstone of modern medicine. They can prevent and treat common and life-threatening infections as well as those that can complicate routine medical interventions. Antimicrobials also have an important role in protecting the health and welfare of food-producing and companion animals, and are used to support plant and crop health.
Bacteria, viruses and other microorganisms that can cause infections are evolving in ways that make the antimicrobials designed to treat them less effective.Endnote 2 This is a natural process that can be accelerated by the overuse and misuse of antimicrobials. Infections that were once treatable are becoming more difficult or impossible to resolve without the development of new treatment options and strategies to mitigate increasing AMR. AMR can affect anyone, anywhere, yet certain regions of the world and some population groups shoulder a greater burden.
The global AMR context
AMR is on the rise globally. The overuse and misuse of antimicrobials in human health, animal health and agriculture; the global movement of people, animals and goods; and socio-economic and health system disparities both in Canada and globally, have created the conditions for AMR to accelerate and spread. At the same time, the pace of development of new antimicrobials and antimicrobial alternatives are not keeping up with the rise of AMR, giving health providers fewer effective options to treat infections in humans and animals.
AMR is a leading cause of death worldwide. An estimated 4.95 million deaths were associated with bacterial AMR in 2019, including 1.27 million directly attributable deaths.Endnote 3 That is more than the number of deaths caused by HIV/AIDS or malaria in 2019.Endnote 3 The World Health Organization declared AMR a top 10 public health threat facing humanity.Endnote 4
Antimicrobial-resistant infections also impact human health in some countries and regions more than others. In 2019, mortality rates from antimicrobial-resistant infections were highest in low- and middle-income countries (LMICs).Endnote 3 The countries with the highest burden of AMR had more than 4 times the number of AMR-attributable deaths compared to countries with the lowest burden.Endnote 3 AMR also disproportionately impacts certain population groups, such as communities with low access to primary healthcare and inadequate sanitation infrastructure and housing.
The global burden of disease in animals due to AMR is not well-understood. The World Organisation for Animal Health (WOAH) is supporting efforts to improve our understanding in this spaceEndnote 5, and supports global One Health AMR action through the implementation of their strategy on AMR.Endnote 6 Similar to the inequitable global burdens of AMR on human health, the impact of AMR on animal health may pose a particular threat to the livelihoods of livestock farmers in low-income countries.Endnote 7 The World Bank estimated that in a high-impact AMR scenario global livestock production could decrease by up to 7.5% by 2050 due to impacts on animal productivity and exports, including an up to 11% decline in low-income countries.Endnote 8
The growing threat of AMR has been met by several international calls to action and commitments. Both G7 and G20 fora have called upon member countries to commit to increased and coordinated action on AMR, including in areas such as research and development, stewardship and surveillance (for example, see 2022 G7 Leaders' Communiqué).Endnote 9 The growing urgency for action on AMR is also recognized as 1 of the 6 priorities in the One Health Joint Plan of ActionEndnote 10 launched in October 2022 by the Quadripartite – the Food and Agriculture Organization of the United Nations (FAO), the United Nations Environment Programme (UNEP), the World Health Organization (WHO), and the WOAH.
The global urgency for action on AMR is expected to only increase. Canada must be prepared to bolster its AMR prevention, preparedness and response efforts, both globally and at-home.
The domestic AMR context
AMR poses growing challenges to the health and wellbeing of Canadians. The Council of Canadian Academies' (CCA) 2019 report, When Antibiotics Fail: The Expert Panel on the Socio-Economic Impacts of Antimicrobial Resistance in Canada estimated that 26% of bacterial infections in humans were resistant to first-line antimicrobials in 2018.Footnote 2 The CCA further estimated that drug-resistant bacterial infections were associated with the deaths of over 14,000 people in Canada in 2018. Of these deaths, 5,400 could be considered directly attributable to AMR itself, and preventable if first-line antimicrobials had worked.
The CCA's Expert Panel considers it a likely scenario that the proportion of infections resistant to first-line antimicrobials in Canada will grow from 26% in 2018 to 40% by the year 2050. In this scenario the number of deaths in Canada attributable to AMR would increase to 13,700 per year. As AMR increases over time, more infections will no longer be treatable by first-line antimicrobials. This means that, in future years, there will be increasingly fewer treatment choices for common infections and essential medical interventions such as surgeries, cancer treatments and organ transplantation will become riskier to perform. The increased burden of AMR can be expected to create significant pressures on both community-based and hospital healthcare delivery.Endnote 1
AMR also has significant economic impacts. In 2018, AMR was estimated to have reduced GDP by $2.0 billion due to impacts on labour productivity. If resistance to first-line antimicrobials grows to 40%, AMR could reduce GDP by $21 billion per year due to impacts on labour productivity, with a cumulative GDP decline of $388 billion by 2050.Endnote 1
The development and spread of drug-resistant infections in animal populations can also harm animal health and welfare, impacting both farming productivity and food security. Canada is a major producer of food-animals which must be kept healthy to maintain a supply of safe animal-origin products for domestic and international markets. Importers, or other purchasers of these commodities, must be confident that food products are safe for consumers. The combined impact of 40% resistance to first-line antimicrobials on human labour productivity and on animal productivity could cost the animal farming industry $225 billion between 2020 and 2050.Endnote 1
The threats AMR pose to health and economy are significant but can be mitigated through concerted action. Canada is committed to coordinated multi-sectoral action using a One Health approach to mitigate the continued spread of AMR, preserve the effectiveness of antimicrobials, and protect the health of people, animals, and their shared environment.
AMR and antimicrobial use trends in Canada
Human health
Canada's surveillance systems provide a growing understanding of AMR and antimicrobial use (AMU) trends in human and animal health sectors. In human health, AMU is trending downward overall with a more than 25% decrease in both community and healthcare sectors from 2017 to 2021.Endnote 11 The most pronounced decrease in community AMU coincided with the start of the broader COVID-19 pandemic response (March to April 2020), and as of April 2022 has remained below pre-pandemic levels.Endnote 11 Some international reports have noted an increase in inappropriate prescribing in the hospital setting during the COVID-19 pandemic.Endnote 12 In Canada, the overall effect of pandemic-related factors on the appropriateness of hospital-based AMU has yet to be determined but continues to be an important area of research.
Despite the overall downward trend in AMU in the human health sector, recent data note several areas of concern.Endnote 11 From 2018 to 2021, nearly a quarter of antibiotic prescriptions in hospitals were considered inappropriate. The community-based use of carbapenems, which are a group of antibiotics often considered last-resort treatments, increased by 21.4% from 2017 to 2021, suggesting either inappropriate use of these precious medicines or a decreased effectiveness of first line antimicrobials. Compared to 30 European countries Canada was the 10th lowest consumer of antimicrobials in 2020, but still consumed 50% more antimicrobials than the country with the lowest consumption (Netherlands).Footnote 3 These data and others demonstrate the critical need for strengthened stewardship practices in human health.
AMR rates continued to increase for most priority pathogens from 2016 to 2020Endnote 11, with some changes in trends following the start of the COVID-19 pandemic, which were likely driven by decreased admissions and heightened IPC practices. Following a decrease in many key antimicrobial-resistant organisms' infection rates at the onset of the pandemic, preliminary surveillance findings from 2021 suggest that some rates have returned to pre-pandemic levels (2019). AMR surveillance data also highlight missed opportunities for prevention. For example, the rate of invasive pneumococcal disease increased from 2016 to 2020, including the rate of infections caused by a form of the bacteria that is preventable with a vaccine available in Canada.Endnote 11
Animal health, agriculture and agri-food
Antimicrobials are essential to keep animals healthy and to help maintain a safe and secure food supply. Some antimicrobials used in veterinary medicine are considered important in human medicine. These are known as medically important antimicrobials (MIAs) and include Categories I, II, and III under Health Canada's categorization systemEndnote 13 and first-in-class antimicrobials not yet categorized.Endnote 14
In 2020, 82% of all MIAs sold by volume in Canada were intended for use in food producing animals and horses.Endnote 11 Less than 1% of antimicrobials were sold for use in plants/crops and companion animals (that is, cats and dogs), with the remaining 17% sold or purchased for human use.Endnote 11 It is important to note that there are substantially more animals than people in Canada. Taking populations and estimated weights into account, approximately 1.8 times more MIAs were intended for use in production animals (food animals plus horses) than in people.Footnote 4 The types and dosing of MIAs used in most production animals are often different from those used in humans, making it challenging to understand the comparison of sales data based solely on volume. Overall sales of MIAs across production animals in Canada decreased by 7% (by kg) from 2018 to 2021Endnote 15, with variations in sales trends by animal species (for example, farmed livestock, aquaculture and horses). These AMU trends coincided with the implementation of veterinary drug regulatory changes and policy interventions in 2017 to 2018, which included making MIAs available by prescription only and removing growth promotion claims from all MIAs.
Sentinel farm surveillance of AMU on broiler chicken, grower-finisher pig and turkey farms also indicated a decrease in AMU from 2016 to 2020.Endnote 11 More antimicrobials were reported to be used for disease prevention purposes (primarily for prevention of enteric diseases) than for disease treatment (respiratory, enteric, septicemia or lameness) in 2020, which is similar to previous years.Endnote 11 The decreasing trend in AMU was accompanied by a decrease in AMR in bacteria from samples from the same sentinel farms, using resistance to 3 or more classes of antimicrobials for Escherichia coli as the AMR indicator.Endnote 11
Advancements have been made to keep animals healthy and reduce the use of MIAs through, for example, federal initiatives to improve access to antimicrobial alternatives. There have also been sector-specific initiatives to eliminate the preventive use of third generation cephalosporins (and other antimicrobial classes in some cases). Decisions related to the access and use of antimicrobials in the animal sector are complex, species-specific, and influenced by numerous factors including animal husbandry practices, animal disease conditions and market supply and demand.
AMR poses a challenge to sustaining the important domestic and global contributions of the agriculture industry. From 2012 to 2050, gross global agricultural output is estimated to grow between 40% and 54% to meet food demand, depending on the scenario as analyzed by the FAO.Endnote 16 The need for safe and effective antimicrobials in agriculture is clear, and in Canada the quantity of MIAs sold for use in production animals (in mg/population correction unit (PCU)Footnote 5) is higher than the median reported by other countries using similar metrics, though there are important differences across antimicrobial classes sold and production practices.Endnote 11 The agriculture sector will be a critical partner in helping balance the need to preserve the effectiveness of antimicrobials for human and animal health with the need for safe and affordable food products.
AMR and Indigenous Peoples in Canada
A key theme heard during engagement with Indigenous partners, including National Indigenous Organizations and other Indigenous organizations and associations, is that the impact of AMR should not be viewed as a standalone health issue. Efforts to mitigate the impacts of AMR require an understanding of broader social, health and economic factors that can impact diverse Indigenous communities and the capacity to address AMR in an environment of competing health and social priorities. For example, inequitable access to housing, healthcare and social services can contribute to broader disparities in infectious disease prevalence and exposure risks to antimicrobial-resistant organisms. Recent investments made to address broader issues impacting some Indigenous Peoples – such as in housing and clean drinking water – in addition to more community-based health programming and access to Traditional Healers can further support AMR mitigation efforts.
Available disease-specific data suggest some Indigenous communities have a disproportionate burden of community-acquired resistant infections. Elevated rates of community-acquired methicillin resistant Staphylococcus aureus (CA-MRSA) have been identified in remote Indigenous communities, with causes potentially related to structural factors such as crowded living conditions.Endnote 17Endnote 18 While CA-MRSA does not always lead to illness, if spread to others and left untreated, CA-MRSA can cause serious health complications such as bloodstream infections.Endnote 19
There are some gaps in knowledge with respect to the drivers and impacts of AMR across Indigenous populations. Strengthened research led by or in partnership with Indigenous communities would improve understandings of the burden of AMR and prevalence of inappropriate AMU, and subsequently enable implementation of community-led interventions.
The action plan will help guide collaboration with Indigenous partners to co-develop AMR actions that recognize the unique cultures, contexts, needs and priorities of First Nations, Inuit and Métis Peoples. Strengthened collaboration with FPT, First Nations, Inuit and Métis partners, including through a distinctions-based approach, to bridge knowledge gaps and guide action plan implementation is a first step in this process.
Incorporating lessons learned from the COVID-19 pandemic
The responses to the COVID-19 pandemic across Canada reinforce the need for collaboration to accelerate progress on AMR. The AMR response, like the COVID-19 pandemic, requires robust multi-sectoral and intergovernmental coordination that recognizes the interconnectedness of our health, economic and social systems. The collaborative approach taken by FPT governments helped inform public communications, bolster surveillance and data systems, and ensure the timely procurement and equitable rollout of vaccines and other therapeutic treatments.
Jurisdictions are already applying the infrastructure built through the COVID-19 response to AMR. For example, FPT and municipal governments, along with academia, worked together to establish pan-Canadian wastewater surveillance for the SARS-CoV-2 virus that causes COVID-19.Endnote 20 Wastewater data helped inform public health action across the country and has been made publicly available through online dashboards, such as the COVID-19 wastewater surveillance dashboard.Endnote 21 The infrastructure and experience developed through COVID-19 wastewater surveillance is now being applied to help early detection of other public health threats, including AMR.
The COVID-19 pandemic also created an unprecedented urgent need for access to health products, such as COVID-19 vaccines. FPT and municipal governments and other partners responded with unprecedented regulatory and logistical agility to support and promote the safe and timely access to critical COVID-19 medical countermeasures. It is important to draw on these lessons learned and apply them within the AMR context given the need to secure access to new antimicrobials and other innovative health products to prevent, detect and treat antimicrobial-resistant infections.
Learning from the COVID-19 pandemic response, Canada's AMR interventions should adopt an equity lens and include measures for sectors and population groups that face the greatest risks to antimicrobial-resistant infections and antimicrobial misuse. For example, antimicrobial-resistant infections and inappropriate prescribingEndnote 22Endnote 23 may pose a particular threat to residents of long-term care facilities, where resistant organisms imported from acute-care hospitals can spread between residents who are at higher risk of severe outcomes from these infections.Endnote 24 An equity lens will guide the implementation of activities across the action plan to help address inequities in how AMR impacts some populations.
Section 2: Action plan development and engagement process
Overview of action plan development and engagement
The action plan provides a 5-year (2023 to 2027) blueprint for strengthening Canada's collective AMR preparedness and response across the One Health spectrum. Prior to the COVID-19 pandemic, extensive engagement informed the development of a draft action plan, including with AMR researchers, experts, FPT governments, other public and private sector partners and stakeholders. This work was led by an FPT AMR Steering Committee and supported by 4 task groups covering the areas of surveillance, stewardship, IPC, and research and innovation. While the COVID-19 pandemic response delayed completion and release of the action plan, progress on several AMR actions continued across Canada during this time.
In 2021, the PHAC established a dedicated Task Force on AMR. One of the Task Force's priorities was resuming the work to update and finalize the action plan with FPT partners, including re-establishing the FPT AMR Steering Committee to guide the completion of the action plan. The final action plan is informed by the lessons learned from the COVID-19 response, along with best practices from Canadian and international efforts to address AMR.
The action plan reflects the commitment of health and agriculture leaders in governments across Canada and responds to calls to action from non-governmental organizations, the healthcare and veterinary sectors, the scientific and research community, and industry to work together to combat the growing threat of AMR. The action plan will also help drive forward strengthened collaboration with the environmental sector in pursuit of a comprehensive One Health approach to AMR.
Annex 1 provides further details on the governance structures that supported action plan development.
Principles informing the action plan
The action plan builds on a solid AMR policy foundation in Canada. In 2014, the Government of Canada released a Federal Framework for Action on Antimicrobial Resistance and Use, followed by the Federal Action Plan on Antimicrobial Resistance and Use in Canada in 2015. In 2017, the Government of Canada collaborated with provinces, territories, and other partners and stakeholders to release Tackling Antimicrobial Resistance and Antimicrobial Use: A Pan-Canadian Framework for Action ("the framework"). The framework introduced 4 core components of an AMR response in Canada (surveillance, IPC, stewardship, and research and innovation) and called for the development of a subsequent action plan. The Pan-Canadian Action Plan on AMR builds on the 4 components of the framework and introduces a new leadership pillar in recognition of the need for strong domestic and global leadership on AMR.
The action plan and its implementation will be guided by the following principles:
- One Health – actions recognize and consider each sector and the interplay between human health, animal health and their shared environment
- Equity – AMR disproportionately impacts certain populations and at-risk demographics. Tailored approaches are necessary to ensure programs and initiatives are equitable and mitigate disproportionate impacts of AMU and AMR
- Collaboration (domestic & international)– no one entity or order of government can effectively address AMR alone. Coordination across jurisdictions, sectors, partners and the public is essential to effectively address AMR
- Momentum – implementation should build on existing successes to achieve continuous improvement toward stated outcomes
Section 3: A 5-year pan-Canadian commitment to address AMR
Pillars of action
The action plan outlines 10 priority actions that will guide FPT governments' AMR efforts across 5 pillars: research and innovation, surveillance, stewardship, IPC, and leadership. Actions were prioritized by FPT governments based on relevance to current health contexts, feasibility of implementation and for ensuring greatest impact on AMR across Canada within the 5-year timeline of the action plan. Jurisdictions across Canada will implement actions within their areas of responsibility and based on respective needs and capacity.
While the 10 priority actions have been grouped into 5 distinct pillars for the purposes of this action plan, they are mutually reinforcing and designed as a suite of actions that will together have the greatest impact in the Canadian context. The implementation of any one priority action is expected to have horizontal benefits for others. For example, progress in implementing the surveillance pillar will be integral for informing parallel action in stewardship and IPC. Efforts to enhance governance and coordination on AMR will be essential for ensuring progress made in any one sector or jurisdiction is harnessed within the larger pan-Canadian AMR response.
Pillar 1: Research and innovation
Research and innovation are critical for improving our understanding of AMR in different settings (including healthcare systems, agriculture and agri-food systems and the environment), establishing effective population-level interventions (such as legislation, policies, guidelines and educational approaches to stewardship and IPC), and stimulating the discovery of new antimicrobials, diagnostics, and alternatives to antimicrobials (for example, vaccines, adjuvants, phage therapy, and probiotics) in human and animal health. Numerous research disciplines, including social sciences, medical and life sciences, among others, support AMR mitigation efforts and health product discovery.
There are several unique challenges in the antimicrobial development pipeline that limit innovation and access to novel antimicrobials for Canadians. The high cost of drug discovery and development, low valuation and the closely stewarded use of new antimicrobials ultimately result in low return on investment for industry. This low return on investment has disincentivized investment in antimicrobial R&D and contributes to the limited development of new antimicrobials. Large pharmaceutical companies have widely vacated the antimicrobial development field. The rate of the development of resistance to antibiotics has also continued to accelerateEndnote 25, placing additional pressures on the small and medium sized companies that are driving the innovation pipeline.Endnote 26
Canada is already falling behind international counterparts in bringing new antimicrobials to the domestic market. From 2010 to 2019, the Government of Canada secured access to only 2 out of the 18 new antibiotics launched worldwideEndnote 27 (a third antibiotic was launched in the Canadian market in 2021). This means physicians and healthcare professionals in Canada do not have ready access to the full complement of globally-available antimicrobials, limiting treatment options for Canadians. Domestic pressures contributing to Canada's limited access to new antimicrobials include our small market share, federated healthcare system, and a procurement and reimbursement model for which responsibility is distributed across FPT jurisdictions, including hospitals and pharmacies.
Canada, in close collaboration with international partners, must continue to strengthen its multi-disciplinary research and innovation on AMR to support mitigation efforts and help secure access to antimicrobials and other health-related products. This includes improving access to veterinary biologics, such as vaccines, for use in animals. Improved access to effective veterinary biologics and other non-antimicrobial options are important additional tools for producers to keep animals healthy, reducing the need to use antimicrobials.
Desired outcome 1: Improved, sustainable access to antimicrobials, diagnostics, and alternatives to antimicrobials to better mitigate AMR
Action: Develop and implement economic and/or regulatory incentives to support innovation and facilitate sustainable access to new and existing antimicrobials, diagnostics, and alternatives to antimicrobials.
New investments, market incentive models (for example, new approaches to valuation/reimbursement), and collaborative approaches to research and innovation will help ensure sustainable development and access to new and existing antimicrobials, diagnostics and alternatives to antimicrobials in both human and veterinary medicine. "Pull" incentives are one method for incentivizing antimicrobial development and sustaining commercialization and market access. In contrast, "push" incentives are upstream measures that attract and support R&D efforts. Both push and pull incentives are necessary to sustain antimicrobial innovationEndnote 28 (Figure 3). Canada's approach to supporting innovation and access will be informed by external expert advice and engagement with countries and international organizations who have implemented similar approaches.
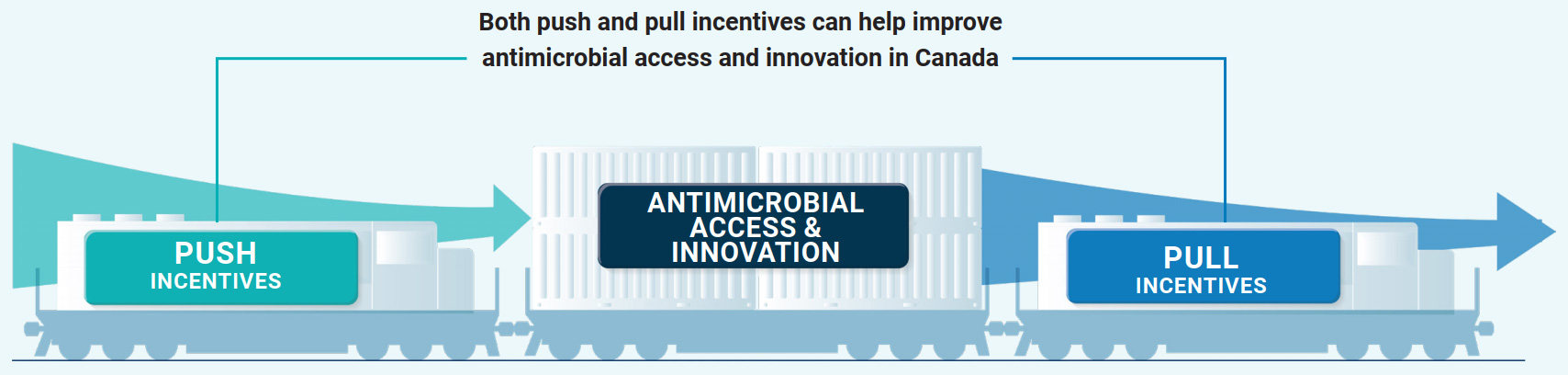
Figure 3: Text description
Figure 3 shows 3 separate railcars on a train track. The middle railcar is labelled "antimicrobial access and innovation". It is being pushed by a railcar labelled "push incentives", and pulled by a railcar labelled "pull incentives". Push incentives are upstream measures that attract and support research and development. Research grants are one example of push incentives. Pull incentives can help create a sustainable commercial market by rewarding successful antimicrobial innovation with market incentives. This figure demonstrates how both push and pull incentives can help improve antimicrobial access and innovation in Canada.
Our efforts are building on a solid foundation. Health Canada has taken steps to support access to new antimicrobial drugs through its regulatory levers, including collaboration with other international regulators and the use of the Pathogens of Interest list as a selection criterion for priority regulatory review.Endnote 29 Agriculture and Agri-Food Canada (AAFC) is supporting research to accelerate innovation in the agriculture and agri-food sector through the Agri-Science program.Endnote 30 Canada also has a strong and collaborative research and innovation culture with expertise in drug discovery, microbiology, alternatives to antimicrobials, diagnostics, and vaccine research. Academic institutions, governments, non-governmental organizations and industry researchers are making important contributions to protect the health of humans and animals against AMR.
Desired outcome 2: Expand scientific knowledge base and tools to inform effective AMR/AMU interventions
Action: Develop a One Health, national research strategy for combatting AMR across all action plan pillars.
Research that translates directly into action is key for supporting implementation of all areas of the action plan. A national research strategy will set the foundation for improved coordination of research efforts and will help identify priority research areas across disciplines. It will also help build a program of work that responds to specific stakeholder needs across action plan pillars, including stewardship, IPC and surveillance.
The research strategy is one next step in strengthening research on AMR in Canada. Since 2000, AMR has been a key research priority for the Canadian Institutes of Health Research (CIHR). In 2016 CIHR established the AMR Research Initiative, which provides $1.8 million in annual grant funds.Endnote 31 This funding is in addition to investigator-initiated research projects funded through open competitions, along with funds to support international partnerships and research on AMR.
There are many potential areas for further research to fill gaps in knowledge. In human healthcare and animal production systems, research can support the development and uptake of diagnostics and other technologies that support antimicrobial stewardship, including point-of-care tools. There are also opportunities to improve and adapt the tools we already have. For example, research into new methodologies and strategies can support the earlier detection and containment of antimicrobial-resistant organisms across One Health sectors. Improved evidence on the dynamics of community and environmental transmission can support responses that are evidence-based, effective and equitable. Additional research will also help identify innovative approaches to improve IPC, biosecurity and livestock management practices in agricultural settings, and IPC practices in healthcare and community settings.
Societal, organizational and behavioural forces can contribute to inappropriate AMU.Endnote 32 Investments are needed in social, behavioural and implementation science, including structural and population-level interventions, to better understand and influence the behaviours of prescribers, producers, patients and the public. Improved knowledge mobilization across research disciplines can further extend the reach and impact of AMR research in Canada.
The development of a national research strategy is a key step in mobilizing Canada's research community in support of the action plan's shared priorities. Such a strategy will need to ensure that the full research cycle is optimized: from evidence synthesis of existing data, development of new knowledge and technologies, and mobilization of knowledge to practice. Meaningful engagement with diverse impacted populations, including Indigenous partners and people with lived/living experience will inform development, implementation and evaluation of the strategy.
Pillar 2: Surveillance
Surveillance underpins Canada's ability to detect, understand and take necessary action to respond to emerging public health threats. Surveillance is closely linked to other pillars such as research and innovation, stewardship, and IPC, as it supports and informs actions, reveals trends and gaps, and helps measure the effect of interventions. All partners in the AMR response rely on data from existing surveillance systems to inform and support evidence-based decision making.
Desired outcome 1: Canada has robust, integrated One Health AMR/AMU surveillance infrastructure with data that are accessible, reliable, timely, nationally representative and capable of detecting emerging threats
Action: Expand sources, coverage and integration of AMR and AMU surveillance data, including the use of modern laboratory technologies and standardized reporting, to help monitor AMR/AMU across One Health sectors, with specific focus on improving data from the environment; transmission pathways between sectors; and population groups disproportionately impacted by AMR and inappropriate AMU.
Canada has a strong AMR/AMU surveillance foundation. Many surveillance collaborations already exist between partners across FPT jurisdictions and industry sectors. The implementation of evidence-based interventions and policies that address AMR and inappropriate AMU across One Health sectors requires timely, comprehensive and representative data that are integrated at the national level and accessible to partners, while respecting jurisdictional roles and data-sharing agreements.
Investments committed through the Government of Canada's Budget 2021 have helped to address gaps in the human health, animal health and agri-food sectors, however there continues to be areas for improvement. Current or planned efforts to expand One Health AMR surveillance data include bolstering representation of hospital data in rural and remote regions, testing wastewater for antimicrobials, initiating AMR surveillance in long-term care homes, and expanding surveillance across the food chain, among others.
The modernization of surveillance infrastructure is also integral to a coordinated response to AMR. For example, genomic sequencing played an important role in informing COVID-19 response efforts. Canada's expertise in genomics can be used to help determine the origin and spread of AMR threats across Canada. Collaboration across jurisdictions and sectors to standardize data/isolate collection, laboratory methods, analysis and reporting methods would further improve the quality and interoperability of AMR data and complement investments in modern laboratory technologies.
Case study: AMRNet
The Antimicrobial Resistance Network (AMRNet) is a data-driven One Health approach to AMR surveillance. The laboratory-based system captures information on antimicrobial susceptibility testing from clinical and veterinary laboratories. AMRNet will allow Canada to better detect the emergence and spread of AMR between sectors, to integrate AMR data across humans and animals, and to provide rich data to the WHO's Global Antimicrobial Resistance and Use Surveillance System. AMRNet is poised to build antimicrobial stewardship capacity at local levels by providing standardized regional antibiograms and trend analyses to local teams. In 2021, AMRNet covered approximately 40% of the Canadian population and is working with provincial and territorial partners to expand across the country.
Desired outcome 2: Canada has a comprehensive understanding of AMR and AMU trends at national, regional and local levels to support evidence-based decision-making and to monitor the impacts of interventions
Action: Work with partners to:
- establish baselines and targets for national, provincial and territorial levels of AMR and appropriate AMU in human health
- establish baselines, goals and measures of progress for increasing appropriate AMU and reducing AMR in the agriculture and agri-food sectors
An effective pan-Canadian response to AMR rests on an understanding of current AMR and AMU trends throughout Canada. Baselines, targets and other measurements of progress can help identify patterns, gaps and successes which in turn foster a culture of continuous improvement. Baselines can be used to help jurisdictions and sectors identify which interventions are having the greatest impact on reducing AMR and improving the appropriate use of antimicrobials. Targets, goals and other measures of progress are key for mobilizing joint action and for understanding progress throughout Canada over time compared to baselines.
Efforts to expand surveillance data across One Health sectors will help bolster the evidence base for developing baselines, targets and/or other measures of progress. Collaboration with partners is required to understand what data gaps need to be addressed to ensure baselines and other metrics can be adapted to different sectors and be used to inform actions. The process to develop baselines and other metrics will similarly require a collaborative approach with partners. Lessons learned in this process can inform how data gaps in other sectors, such as the environment, can be addressed in the future.
Global attention is turning to similar monitoring approaches. Many countries currently have established, or are planning to establish baselines, performance indicators and targets for infection incidence rates and AMU in humans and animals. In 2022, G7 countries agreed to establish national measurable targets on AMR, as well as AMU in human health, by the end of 2023.Endnote 33 The approach to goals and targets in Canada will be informed by both the domestic context and by the international experiences of others.
Pillar 3: Stewardship
AMR emerges naturally over time even when antimicrobials are used appropriately. When antimicrobials are used inappropriately in humans and animals, however, the emergence and spread of AMR can be accelerated. This means that, in some cases, antimicrobials lose their effectiveness faster. Antimicrobial stewardship (AMS) is a system-wide approach that recognizes the role of patients, prescribers, producers and the public in promoting appropriate AMU. It includes coordinated interventions designed to promote, improve, monitor, and evaluate appropriate AMU to preserve antimicrobial effectiveness while promoting and protecting human and animal health.
In human health, there are differences in antimicrobial prescribing across some populations. From 2016 to 2022, females received nearly 40% more community antimicrobial prescriptions than males.Endnote 11 In 2022, people aged 80 and older in Canada received more than 4 times the number of prescriptions in the community compared to people 18 and youngerEndnote 11, with inappropriate use in long-term care facilities of particular concern. In hospital settings, nearly a quarter of antibiotic prescriptions were considered inappropriate from 2018 to 2021.Endnote 11
In companion and food-producing animals, it is important to promote appropriate AMU to preserve the effectiveness of existing antimicrobials against potential diseases and disease outbreaks. For food-producing animals specifically, preserving antimicrobial effectiveness and mitigating AMR is important to help maintain food safety and security. The potential for AMU on plants/crops to contribute to AMR is a growing area of concern and requires greater research and surveillance to effectively inform stewardship priorities.
AMS programs in human health, animal health and agriculture should reflect, where possible, known disparities in prescribing patterns, inappropriate, misuse or overuse, as well as local-level AMR trends. This will require close collaboration with surveillance and research sectors as well as coordination with other partners to address gaps in knowledge, promote the development and implementation of targeted stewardship initiatives, scale up best practices and raise awareness.
Desired outcome 1: Prescribers and other professionals in Canada have the resources, training, and tools to facilitate appropriate AMU in humans and animals
Action: Develop, implement and promote guidelines/standards for appropriate AMU in humans and animals through policy and regulatory initiatives, monitoring and educational interventions/accreditation requirements for health professionals and prescribers.
Stewardship programs are critical for facilitating appropriate AMU. There are many successful local AMS initiatives in jurisdictions across Canada, however many of these have not yet reached the scale required to optimize AMU across entire systems or regions. Evidence-based guidance on antimicrobial treatment therapy and the appropriate use of diagnostics should be tailored to local AMR risks and burdens where possible. Information about inappropriate AMU need to be readily available, updated and accessible at point-of-care to prescribers and stewardship programs to support effective AMS practices. AMS programs can be further supported by policy and regulatory initiatives, such as through updates to antimicrobial labels that help foster appropriate use.
Enhancing antimicrobial stewardship by strengthening the Veterinary Drug Regulatory Framework
In 2017 to 2018, Health Canada introduced multiple regulatory and policy changes to strengthen the oversight and responsible use of MIAs in animals. These included improved oversight on importation, requiring mandatory sales reporting, making these antimicrobials available by prescription only, phasing out their use for growth promotion purposes and adding prudent use statements on their labels.
The development and dissemination of guidelines and other appropriate stewardship tools and standards will allow stakeholders to integrate stewardship programs into their daily operations. Even within a given sector, understanding of what constitutes 'appropriate use' varies, contributing to a lack of clarity for prescribers and to challenges in monitoring prescribing patterns. Improved sources, access and integration of AMR and AMU surveillance data, clinical research focused on defining appropriate use, and greater collaboration between partners is required to achieve consensus on this important issue.
Success box: Firstline app
Vancouver-based start-up Firstline is working with the WHO to connect frontline health providers with clinical decision-making and prescribing support at point of care. Firstline has also partnered with the Canadian Veterinary Medical Association to make prescribing guidelines available to veterinarians through the Firstline platform. Canada has invested $2.2 million in the tool to continue its digital innovation and reach more providers and patients.
To increase the understanding and application of stewardship principles among prescribers and other health professionals, AMR and AMU should consistently be made a core component of professional education, training and ongoing development in the human health and veterinary sectors and in agriculture and agri-food practices. Professional associations and licensing bodies can help establish professional standards and certifications, as well as equip their members with the training, information and tools they require to fulfill their stewardship role. For example, monitoring tools can provide feedback mechanisms to prescribers on antimicrobial prescribing appropriateness. Educational programs that have been deployed in the human health sector can be adapted for the veterinary medicine sector for companion and food-producing animals, and vice-versa, thereby leveraging existing programs for greater impact.
Desired outcome 2: Canadians understand the importance of the appropriate use of antimicrobials
Action: Foster understanding of the risks of AMR and the importance of appropriate use of antimicrobials in humans and animals amongst the public, patients and producers through awareness/education campaigns, feedback mechanisms and policy and regulatory initiatives.
According to public opinion research conducted between 2019 and 2022Endnote 34, only 25% of Canadians are familiar with the term "antimicrobial resistance", and 33% incorrectly believe that antibiotics kill viruses. These findings highlight the need for targeted initiatives to increase public awareness and understanding of AMR. Clear messaging can raise awareness about how to manage common infections, when to access medical and veterinary care, and how to use antimicrobials appropriately. Governments in Canada and other partners can build on communications approaches from the COVID-19 pandemic to emphasize preventative public health measures, such as hand washing, food safety tips to reduce foodborne illnesses, and the importance of being immunized against vaccine-preventable diseases.
Going forward, awareness and educational materials should be tailored to the contexts, expectations and needs of the target audience. This requires co-developing material and messaging with FPT health professionals, Indigenous partners, childhood educators, elder care providers, social and behavioural science experts, and animal owners or producers. To ensure initiatives reach intended audiences and result in behaviour change, it is also critical to measure and adjust interventions appropriately.
In addition to awareness and education campaigns, feedback mechanisms aligned with AMU surveillance systems could empower producers with improved appropriate use information and context.
Pillar 4: Infection prevention and control
Antimicrobials are essential tools for modern-day medicine and animal care. They will continue to be used in human and animal health and AMR will occur naturally over time. As such, strategies to prevent the transmission of antimicrobial-resistant pathogens to vulnerable populations are critical to mitigate the impact of AMR. In healthcare settings, IPC measures can prevent and/or mitigate the risk of healthcare-associated infections (HAI) caused by antimicrobial-resistant pathogens. Resistant infections in the acute and long-term care health settings are associated with increased mortality and increased cost to the health care system. In Canada, the average cost of treating antimicrobial-resistant infections in hospitals was estimated to be $18,000 per patient in 2018.Endnote 1
In the community, basic measures such as hand washing, avoiding congregate settings when ill, and vaccination are important and cost-effective measures for preventing infections. Improving adult vaccine uptake in the community can help reduce the burden of vaccine-preventable diseases, including for populations at higher risk of severe complications.
Approaches to promote animal health and protection against animal disease combine routine IPC measures, vaccination, veterinary oversight and biosecurity measures. Biosecurity plays an important role in agri-food systems, and when combined with good manufacturing practices and food safety measures, can help reduce the spread of pathogenic microorganisms to other animals, workers and consumers.
Desired outcome 1: Canada has infection prevention programs in place across community and institutional health sectors, including for populations disproportionately impacted by AMR
Action: Increase effective implementation of infection prevention measures, particularly for populations disproportionately impacted by AMR such as remote, northern and isolated communities, First Nations, Inuit and Métis populations, long-term care residents, and hospitalized patients by developing, updating and promoting uptake of guidelines/best practices for human health.
Building on progress made during the COVID-19 pandemic, there is an opportunity to further strengthen infection prevention measures in acute, continuing-care and long-term care health settings, community care settings, and/or for populations disproportionately impacted by AMR. Strategies that can help prevent the transmission of antimicrobial-resistant pathogens include addressing information gaps, updating and promoting uptake of infection prevention guidelines, sharing knowledge and evidence, and scaling up best practices.
IPC guidelines should be updated and implemented with the latest evidence on antimicrobial-resistant pathogens. This will require close collaboration with health system partners and with surveillance system programs such as the Canadian Nosocomial Infection Surveillance Program. The Standards Council of Canada, Canadian Standards Association and Health Standards Organization have developed national long-term care standards, which are one step to help protect seniors living in long-term care from the threat of infections, including AMR infections.
The development of community-based infection prevention guidelines should consider the larger social, health and economic conditions that put some populations at greater risk for disease transmission, such as crowded living conditions and access to healthcare, which contribute to the larger challenge of health inequity. The spread of some antimicrobial-resistant pathogens is a particular issue in congregate living settings, such as shelters for people experiencing homelessness, and in crowded living situations that exist for some Indigenous populations. Community-based infection prevention guidelines should be developed and implemented in collaboration with these populations, adapted to their unique needs and environments, and informed by local-level AMR research and surveillance.
Improved adult vaccine uptake based on immunization guidelines and coverage goals can further bolster community-based infection prevention measures. Canada falls far short of national vaccination coverage goals for the pneumococcal vaccineEndnote 35 and influenza vaccine.Endnote 36 Improving uptake of these vaccines and others will reduce the overall burden of these diseases in adults, including reducing hospitalizations and associated AMU.
Desired outcome 2: Improved animal health and food safety along the farm-to-fork continuum to prevent and limit the spread of infection and foodborne pathogens
Action: Support the increased implementation of enhanced IPC, biosecurity, and food safety protocols across the agriculture and agri-food sectors, prioritizing sound animal husbandry, access to veterinary care, and access to additional health and nutritional aids to promote animal health.
The spread of antimicrobial-resistant pathogens can affect the health and welfare of animals. Along the farm-to-fork continuum, resistant organisms or genes encoding for AMR can be passed to other animals or humans through direct contact with another person or animal, consumption and handling of contaminated food or water and direct contact with contaminated soil. Efforts to address AMR and inappropriate AMU in agriculture and agri-food, such as through enhanced housing, management and improved biosecurity and IPC systems, can therefore support the health of both animals and humans by addressing the conditions that lead to disease spread.
Enhancing access to veterinary care and on-farm food safety programs, developing, implementing, and promoting the uptake of standards, and improving vaccination strategies can all play a significant role in improving IPC and biosecurity while helping secure food production. The Hazard Analysis and Critical Control Point (HACCP) system, for example, provides guidance and monitoring for food safety and manufacturing throughout the food production process. Adherence to HACCP is required for all federal and many provincial food processors. Additional tools that could support improved animal health and food safety include public education campaigns, highlighting the potential risk of foodborne pathogens, and sharing research information or best practices on reducing antimicrobial-resistant organisms during food processing.
Pillar 5: Leadership
There is strong commitment among FPT governments to mobilize efforts on AMR across One Health sectors. COVID-19 demonstrated the importance of pan-Canadian collaboration, coordination and collective action to address public health threats. The federal government has clear international leadership, convenor, health promotion and protection roles. Provinces and territories similarly have clear areas of responsibility across pillars, such as the delivery of healthcare services, health promotion and protection. Indigenous partners lead the development and implementation of AMR activities that respond to the needs and cultures of First Nations, Inuit and Métis Peoples.
Many jurisdictions and sectors across Canada have been making strides on AMR for years and have established comprehensive networks focused on their area of responsibility. Despite the progress made, there are missed opportunities for collaboration, coordination and leveraging of resources and best practices. Strengthened domestic governance is essential to provide the structure and mechanisms that enable diverse partners to deliver on the action plan's common agenda.
The complex challenge of AMR demands action at both domestic and international levels. AMR organisms do not respect national borders. Limited access to appropriate antimicrobials, vaccines and other medical countermeasures in one region can accelerate the global spread of disease and AMR, and cause illnesses and deaths that could otherwise have been prevented. Similarly, the overuse or inappropriate use of antimicrobials in one area can lead to their reduced effectiveness worldwide. Joint action by all countries is required to slow the spread of AMR and preserve the effectiveness of our life-saving antimicrobials. Canada is well-positioned to leverage areas of strength to shape and drive progress on global health priorities.
Desired outcome 1: The Pan-Canadian Action Plan is implemented through coordinated, multi-sectoral domestic action
Action: Build on existing One Health AMR governance structures to create a "network of networks" with inclusive representation to support action plan implementation and share progress and lessons learned within and across the 5 pillars of action, prioritizing strengthened FPT, First Nations, Inuit and Métis collaboration to co-develop AMR actions.
The foundation for implementing the action plan already exists in Canada. Within and across the action plan's pillars of action, there are many well-established networks advancing respective priorities on AMR. Improved AMR governance to implement the action plan will build on existing effective networks and promote a shift from siloed activities to collective impact.
To strengthen a pan-Canadian One Health approach on AMR, a new "network of networks" governance approach will enable partners committed to the shared agenda in the action plan to contribute to concrete activities in support of implementation. Mutually reinforcing activities across actions will be prioritized to promote broader system change. Shared measurements and enhanced information sharing across actions will also support continuous improvement.
FPT governments will collaborate on the design and implementation of the "network of networks" model. This includes identifying the coordination and enabling activities required to support partners in action implementation. FPT governments will take steps, in both health and agriculture sectors, to ensure appropriate coordination mechanisms are in place within jurisdictions to drive action across sectors and pillars of action.
An equity lens will inform how the governance is structured and the overall implementation approach. One step in this process is to ensure the governance structure is inclusive to populations disproportionately impacted by AMR. The governance approach will prioritize strengthened collaboration with FPT, First Nations, Inuit and Métis partners to co-develop AMR actions, including through a distinctions-based approach.
Desired outcome 2: Canada's contributions and partnerships strengthen joint global action to mitigate the threat of AMR
Action: Increase Canada's contributions to global efforts to advance key bilateral and multilateral commitments by prioritizing:
- generating improved data/evidence on AMR/AMU and strengthening surveillance systems and data standards
- expanding efforts to support low- and middle-income countries by advancing equitable access, stewardship and IPC initiatives
The action plan has described the interconnected global threat AMR poses to health and economies across Canada. Mitigating this risk requires coordinated global action to detect, prevent and respond to emerging AMR threats across One Health sectors. Recent global commitments and calls to action on AMR include strengthening surveillance and monitoring, creating favourable economic conditions to address antimicrobial market failures, developing AMR and AMU targets, spurring research and innovation, supporting equitable and affordable access to antimicrobials, and developing and implementing national action plans on AMR, among others. Canada will continue to play a leadership role in key international fora such as the Global AMR Research and Development Hub, and work with international partners to advance domestic priorities and global commitments on AMR.
There are specific opportunities where Canada can leverage existing strengths to advance global AMR objectives. Canada has strong technical knowledge and experience with integrated AMR/AMU surveillance systems and research. Domestically, this knowledge and experience is critical for growing the evidence base on AMR. Globally, Canada contributes technical advice and leadership to the development of international surveillance standards and guidelines (for example, the Codex Alimentarius Guidelines on integrated monitoring and surveillance of foodborne antimicrobial resistanceEndnote 37). Canada is also a longstanding contributor to the Joint-Programming Initiative on AMR, an international One Health research platform that has been at the forefront of the creation of a global virtual network of researchers, facilities and infrastructure of cross-cutting AMR priorities.
Equity is a key principle that informed the development of this action plan. Championing equity-based global AMR action includes prioritizing improved antimicrobial access, stewardship and IPC initiatives for LMICs. This approach aligns with lessons learned from the global COVID-19 response. Canada will continue to promote a focus on equity in key fora, such as the G20, and work with international partners to support LMIC AMR response capacity through initiatives such as SECURE, jointly led by the Global Antibiotic Research & Development Partnership and the WHO.
Success box: Financial contribution to SECURE
SECURE is a new global initiative to expand access to essential, life-saving antibiotics for countries and populations in need and to ensure their appropriate use. Canada has joined international partners with a $300,000 contribution to help address the burden of infectious diseases and AMR in LMICs and increase global AMR preparedness.
Section 4: Implementing the action plan
Key partner roles and responsibilities
The action plan is designed to encourage jurisdictions, sectors, and disciplines across Canada to easily identify how and where they can best contribute to collective efforts to address AMR and inappropriate AMU within their unique contexts and for their specific stakeholders.
In Canada, responsibility for human and animal health and welfare, food safety, and the environment is shared between different orders of government. Although FPT governments, as well as Indigenous partners, have distinct roles and responsibilities, these roles complement one another and are often implemented in collaboration. Each order of government has a specific role to play to ensure that antimicrobials are used appropriately and to mitigate the spread of AMR. Effective implementation of the action plan relies on jurisdictions and partners working together within their respective areas of responsibility to advance the shared FPT priority actions.
- The federal role in AMR includes promoting human and animal health; national surveillance of AMR and AMU; liaising with international organizations; delivering healthcare services to some populations (for example, on-reserve First Nations communities); supporting AMR awareness and education, including federally-employed healthcare providers and their patients; facilitating research and innovation to understand AMR and find new treatment options and tools; ensuring the safety, efficacy and quality of products to treat, prevent and detect infection through the regulatory review and approval of antimicrobial drugs, vaccines, diagnostic tests and alternatives to antimicrobials for human and animal use; establishing policies, standards and guidance on human and animal health; ensuring the safety of the Canadian food supply through policy development, surveillance, inspection, and import control; and by setting biosecurity standards.
- Provinces and territories are responsible for healthcare settings and the delivery of healthcare services; surveillance of AMR and AMU; establishing policies and standards for healthcare settings; promoting human and animal health; education and raising awareness regarding appropriate AMU; immunization programs; supporting research and academic initiatives; managing surveillance systems for monitoring the prevention and control of diseases; approving antimicrobials for medical formularies (that is, funded pharmaceuticals); establishing and implementing relevant provincial/territorial regulations for the distribution, dispensing and use of antimicrobials in veterinary medicine; setting IPC measures; and undertaking surveillance in healthcare settings.
- Indigenous partners identify the priorities of First Nations, Inuit, and Métis Peoples with respect to AMR and develop and implement policies, strategies, programs, and services to address these priorities in ways that respond to the needs and cultures of Indigenous Peoples, including recognizing the value of Indigenous healing practices and existing community expertise in research, surveillance, and AMR prevention, treatment, and care.
Stakeholders have a range of roles and responsibilities related to AMR. The expertise of these groups has been instrumental in developing the action plan. Their expertise, energy, investment, and vision will be critical in sharing data and building strong and reliable tools, systems, and processes to address AMR and AMU in each sector:
- Professional associations and licensing bodies (for example, medical and allied professionals, veterinary medical associations and regulatory colleges) establish standards and certification for their professions and prescribing guidelines in addition to playing roles in raising awareness, training, etc. Professional associations and licensing bodies have unique insights into the implications of AMR and AMU within their particular contexts or jurisdictions
- Human and animal health professionals diagnose infections, and prescribe and dispense medications; contribute to surveillance; educate patients and owners of animals; and establish IPC programs, including biosecurity
- Individual healthcare settings (for example, hospitals, community practices, long-term care facilities, and veterinary clinics and hospitals) implement IPC measures, promote appropriate AMU, and perform surveillance
- Life sciences firms and the pharmaceutical industry undertake research and development in antimicrobials, diagnostics and alternatives to antimicrobials, including vaccines, or partner with academia to facilitate the commercialization of research innovations
- The agriculture and agri-food industries advance appropriate AMU, promote IPC and good animal management practices, and establish some control programs in farmed animal production to protect the health of animals and to preserve the quality of the food supply
- Non-governmental organizations take action in the human and animal health, agriculture and agri-food sectors to advance AMR issues (for example, collect information on AMR and AMU, deliver education and awareness programs, address sector specific issues)
- The environment sector (for example, wastewater treatment facilities, water use management professionals) conduct water quality monitoring, water use management, and wastewater treatment, and support monitoring and surveillance activities to identify AMR and AMU trends in marine sediments and the environment. Canada's One Health approach to AMR will be improved via a greater understanding of the roles of partners working in the environment sector
- The academic sector undertakes research to better understand AMR and to discover solutions to mitigate resistance. Educational institutions develop and/or administer curricula regarding AMR and AMU for health professionals and other allied professionals involved in the care of humans and animals; and for elementary, secondary and post-secondary students. Research and innovation provide evidence to help drive the work of governments and industry, while education and training make knowledge accessible
- Members of the public protect themselves and others from infections and have a responsibility to use antimicrobials as recommended. Individual members of society – both in their professional capacities as doctors, nurses, dentists, pharmacists, health support workers, daycare workers, veterinarians, farmers, and countless other professions, and as members of the public – have tremendous potential to halt the spread of AMR by remaining informed, practising good infection prevention measures, and committing to appropriate AMU in their immediate spheres of influence
- Key populations and most-affected communities: Some populations face disproportionate risks and burdens of AMR pathogens. Best practices in infectious disease prevention, treatment, and care include working with the communities and community leadership disproportionately affected by AMR
Governance
One of the action plan's 10 priority actions is to build on existing One Health AMR governance in support of action plan implementation. The PHAC AMR Task Force will work closely with the FPT AMR Steering Committee, First Nations, Inuit and Métis partners, and other partners across sectors to develop an effective network of networks approach for supporting the successful implementation of the action plan, building on best practices and evidence.Endnote 38 This will include putting in place the mechanisms and supports that enable partners to deliver on each of the 10 distinct actions while facilitating knowledge-sharing across sectors and jurisdictions.
A compendium document will also be published that outlines roles and responsibilities and initial activities underway or planned by FPT governments to implement the action plan. Strengthened governance will support expanded engagement with jurisdictional and sectoral partners to support comprehensive multi-sectoral implementation and monitoring over the action plan's 5-year timeframe.
Monitoring progress
As part of action plan implementation, PHAC will work with jurisdictional partners and other stakeholders, as appropriate, to develop and implement an approach for monitoring and reporting on progress. Regular public reporting on progress will help partners adjust interventions over time and ensure best practices are shared with domestic and international stakeholders. Capturing progress achieved in each of the 10 priority actions will also help Canada establish long-term priorities on AMR beyond the 5 years of this action plan. Several sources of data can support monitoring efforts. Program and policy implementation milestones, AMR and AMU surveillance metrics, and/or stakeholder feedback, among others, can together provide a window into the implementation progress of each of the 10 priority actions over the 5 years of this action plan.
The action plan will be considered a living document over its 5-year implementation and remain flexible to reflect new evidence, challenges and/or resources.
Conclusion
AMR is an increasing public health threat that can affect anyone, anywhere and at any time. The impacts of AMR can be mitigated, but the solutions require decisive, concerted and sustained action. The success of the action plan relies on collaborative action to tackle AMR between FPT governments, Indigenous partners and other sectoral stakeholders.
With widespread recognition of the threat posed by AMR and increasing momentum and calls to actions both domestically and globally, this One Health action plan provides a 5-year roadmap for increased, meaningful and impactful action to address AMR.
Annexes
Annex 1: Development of the action plan
The development of the action plan started prior to the COVID-19 pandemic and was supported by a dedicated FPT AMR governance structure. An FPT AMR Steering Committee led the development of the action plan and guided the work of 4 task groups covering the areas of surveillance, stewardship, IPC, and research and innovation. The task groups were made up of members of the medical and veterinary communities, industry, and academia. Extensive engagement and expertise went into the action plan in this pre-COVID-19 period, however capacity constraints caused by the COVID-19 pandemic delayed its completion.
In 2022, the PHAC AMR Task Force resumed efforts to complete the action plan, building on prior efforts and updating to reflect the current context and pandemic lessons learned. The PHAC AMR Task Force was guided by a renewed FPT AMR Steering Committee, along with a One Health federal interdepartmental AMR governance structure. To the extent possible, the completed action plan builds on the efforts, engagement and expertise contributed prior to the COVID-19 pandemic. Additional engagement, including with an Advisory Group on Antimicrobial Resistance, industry representatives and Indigenous partners and health organizations, further informed action plan development.
Continued engagement with Indigenous Peoples and other partners across jurisdictions and sectors is essential to enable action plan implementation.
Governance structures supporting action plan development
A multi-faceted governance structure supported development of the action plan. The main components include:
FPT AMR Steering Committee: Comprised of senior level FPT policy representatives from the public health, healthcare, animal health, agriculture and agri-food sectors, the steering committee led the development of the action plan and guided the work of the task groups prior to the COVID-19 pandemic. This committee was renewed in 2022 to resume efforts to complete the action plan.
Task groups: Prior to the COVID-19 pandemic, 4 expert task groups were responsible for providing advice and recommendations in the areas of surveillance, stewardship, IPC, and research and innovation to the FPT AMR Steering Committee on the development of both the Pan-Canadian Framework and the action plan. They were comprised of government representatives and external subject matter experts from industry, academia and medical and veterinary communities.
Other governance structures: Several established structures supported the work of the FPT AMR Steering Committee and contributed to the development of the action plan. These structures helped ensure the action plan was developed in collaboration with the public health, healthcare, animal health, agriculture and agri-food sectors to enable collaborative and cohesive action on AMR.
Health
- FPT Ministers of Health
- Council of Deputy Ministers of Health
- Public Health Network Council
- Council of Chief Medical Officers of Health
Agriculture/Agri-food
- FPT Ministers of Agriculture
- Deputy Ministers of Agriculture
- FPT Regulatory Assistant Deputy Ministers Committee
- FPT Policy Assistant Deputy Ministers Committee
- Council of Chief Veterinary Officers and Veterinary Registrars
- Food Safety Committee
- Animal Health Canada
Annex 2: List of acronyms
- AAFC
- Agriculture and Agri-Food Canada
- AMR
- Antimicrobial resistance
- AMRNet
- Antimicrobial Resistance Network
- AMS
- Antimicrobial stewardship
- AMU
- Antimicrobial use
- CA-MRSA
- Community-acquired methicillin resistant Staphylococcus aureus
- CCA
- Council of Canadian Academies
- CIHR
- Canadian Institutes of Health Research
- FAO
- Food and Agriculture Organization of the United Nations
- FPT
- Federal/Provincial/Territorial
- HACCP
- Hazard Analysis and Critical Control Points
- HAI
- Healthcare associated infection
- IPC
- Infection prevention and control
- LMICs
- Low- and middle-income countries
- MIA
- Medically important antimicrobial
- PCU
- Population correction unit
- PHAC
- Public Health Agency of Canada
- R&D
- Research and development
- UNEP
- United Nations Environment Programme
- WHO
- World Health Organization
- WOAH
- World Organisation for Animal Health
Page details
- Date modified:
