Screening assessment — sodium cyclamate and cyclohexylamine
Official title: Screening assessment — Sulfamic acid, cyclohexyl-, monosodium salt (sodium cyclamate) and cyclohexanamine (cyclohexylamine)
Chemical Abstracts Service Registry Numbers:
139-05-9
108-91-8
Environment and Climate Change Canada
Health Canada
April 2022
Cat. No.: En84-289/2022E-PDF
ISBN 978-0-660-42342-5
Synopsis
Pursuant to section 74 of the Canadian Environmental Protection Act, 1999 (CEPA), the Minister of the Environment and the Minister of Health have conducted a screening assessment of two substances: sulfamic acid, cyclohexyl-, monosodium salt (sodium cyclamate) and cyclohexanamine (cyclohexylamine). These substances were identified as priorities for assessment as they met categorization criteria under subsection 73(1) of CEPA. The Chemical Abstracts Service Registry Numbers (CAS RNFootnote 1 ), their Domestic Substances List (DSL) names, and their common names and abbreviations are listed in the table below.
| CAS RN | DSL name | Common name (abbreviation) |
|---|---|---|
| 139-05-9 | Sulfamic acid, cyclohexyl-, monosodium salt | Sodium cyclamate |
| 108-91-8 | Cyclohexanamine | Cyclohexylamine (CHA) |
Sodium cyclamate and cyclohexylamine do not naturally occur in the environment. According to information submitted in response to a CEPA section 71 survey, no manufacturing quantity was reported for sodium cyclamate or for cyclohexylamine above the reporting threshold of 100 kg in Canada in 2011. The import quantities were reported in a range of 100 000 kg to 1 000 000 kg for sodium cyclamate and a total of 871 518 kg for cyclohexylamine.
In Canada, sodium cyclamate is primarily used as a table-top sweetener, and as a non-medicinal ingredient for the purpose of sweetening in drugs, including natural health products. It is not a permitted food additive in Canada, nor has it been identified as being used as a component in the manufacture of food packaging materials. Cyclohexylamine is primarily used as a corrosion inhibitor in water treatment, but it is also a boiler-cleaning agent, and may be used in cosmetics, as a formulant in pesticides, as a component in the manufacture of food packaging materials, incidental additives used in food processing establishments, and in other products available to consumers.
The ecological risks of sodium cyclamate and cyclohexylamine were characterized using the ecological risk classification of organic substances (ERC), which is a risk-based approach that employs multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. Hazard profiles are based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Metrics considered in the exposure profiles include potential emission rate, overall persistence, and long-range transport potential. A risk matrix is used to assign a low, moderate or high level of potential concern for substances on the basis of their hazard and exposure profiles. Based on the outcome of the ERC analysis, sodium cyclamate and cyclohexylamine are considered unlikely to be causing ecological harm.
Considering all available lines of evidence presented in this screening assessment, there is low risk of harm to the environment from sodium cyclamate and cyclohexylamine. It is concluded that sodium cyclamate and cyclohexylamine do not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
Exposure of the general population of Canada to sodium cyclamate can result from its use as a table-top sweetener and from drinking water. Exposure can also result from the use as a non-medicinal ingredient for the purpose of sweetening in drugs, including natural health products.
Exposure of the general population to cyclohexylamine can result from drinking water and food. While there is no potential for direct food contact associated with its uses as a component in the manufacture of food packaging materials, there is potential for dietary exposure from the use of the substance as a boiler water additive in food processing establishments. The general population may also be exposed to cyclohexylamine from use of cosmetics such as aerosol hairsprays and from the use of firespace gel fuel canisters.
Laboratory studies with sodium cyclamate were limited in quality, but indicated potential effects on the testes after a lifetime of high daily oral doses. Given the limited quality of the studies, data from sodium cyclamate’s metabolite, cyclohexylamine, or its analogue, cyclohexylamine hydrochloride, were used to inform selected critical health effects of sodium cyclamate.
For sodium cyclamate and cyclohexylamine, comparisons of levels of oral, dermal and inhalation exposures to the general population and levels at which critical health effects were observed, results in margins of exposure considered adequate to address uncertainties in the health effects and exposure databases.
Considering all the information presented in this screening assessment, it is concluded that sodium cyclamate and cyclohexylamine do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
Therefore, it is concluded that sodium cyclamate and cyclohexylamine do not meet any of the criteria set out in section 64 of CEPA.
1. Introduction
Pursuant to section 74 of the Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999), the Minister of the Environment and the Minister of Health have conducted a screening assessment of sulfamic acid, cyclohexyl-, monosodium salt (sodium cyclamate) and cyclohexanamine (cyclohexylamine or CHA) to determine whether these substances present or may present a risk to the environment or to human health. Cyclohexanamine was moved from the Aliphatic Amines Group to the assessment of sulfamic acid, cyclohexyl-, monosodium salt, since CHA is a metabolite of sodium cyclamate in mammals and CHA data were used to assess the risk to human health of both substances. These substances were identified as priorities for assessment as they met categorization criteria under subsection 73(1) of CEPA (ECCC, HC [modified 2017]).
The ecological risks of sodium cyclamate and CHA were characterized using the ecological risk classification of organic substances (ERC) approach (ECCC 2016a). The ERC describes the hazard of a substance using key metrics including mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity and considers the possible exposure of organisms in the aquatic and terrestrial environments on the basis of such factors as potential emission rates, overall persistence and long-range transport potential in air. The various lines of evidence are combined to identify substances as warranting further evaluation of their potential to cause harm to the environment or as having a low likelihood of causing harm to the environment.
Sodium cyclamate in association with cyclamate and its salts have been reviewed by, the Joint (Food and Agriculture Organization/World Health Organization [FAO/WHO]) Expert Committee on Food Additives (JECFA). Cyclamate and its salts were also assessed by the International Agency for Research on Cancer (IARC). The documents from both of these organizations also reviewed CHA as it is a significant metabolite of cyclamates and its salts. These assessments undergo rigorous review. Health Canada and Environment and Climate Change Canada consider these assessments to be reliable. Sodium cyclamate was also assessed by the European Scientific Committee on Food (SCF).
This screening assessment includes consideration of information on chemical properties, environmental fate, hazards, uses and exposures, including additional information submitted by stakeholders. Relevant data for sodium cyclamate and CHA were identified up to October 2018. Empirical data from key studies as well as results from models were used to reach conclusions. When available and relevant, information presented in assessments from other jurisdictions was considered.
This screening assessment was prepared by staff in the CEPA Risk Assessment Program at Health Canada and Environment and Climate Change Canada and incorporates input from other programs within these departments. The ecological and human health portions of this assessment have undergone external review and/or consultation. Comments on the technical portions relevant to human health were received from Dr. Lynne Haber, Dr. Jennifer Seed, and Dr. Pamela Williams through the University of Cincinnati Risk Science Center. The ecological portion of this assessment is based on the ERC document (published July 30, 2016), which was subject to an external review as well as a 60-day public comment period. dditionally, the draft of this screening assessment (published December 14, 2019) was subject to a 60-day public comment period. While external comments were taken into consideration, the final content and outcome of the screening assessment remain the responsibility of Health Canada and Environment and Climate Change Canada.
This screening assessment focuses on information critical to determining whether substances meet the criteria as set out in section 64 of CEPA by examining scientific information and incorporating a weight of evidence approach and precautionFootnote 2 . This screening assessment presents the critical information and considerations on which the conclusions are based.
2. Identity of substances
The CAS RNFootnote 3 , Domestic Substance List (DSL) names, common names and abbreviations for sodium cyclamate and CHA are presented in Table 2‑1.
| CAS RN | DSL name (common name; abbreviation) |
Chemical structure and molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
| 139-05-9 | Sulfamic acid, cyclohexyl-, monosodium salt (sodium cyclamate) | 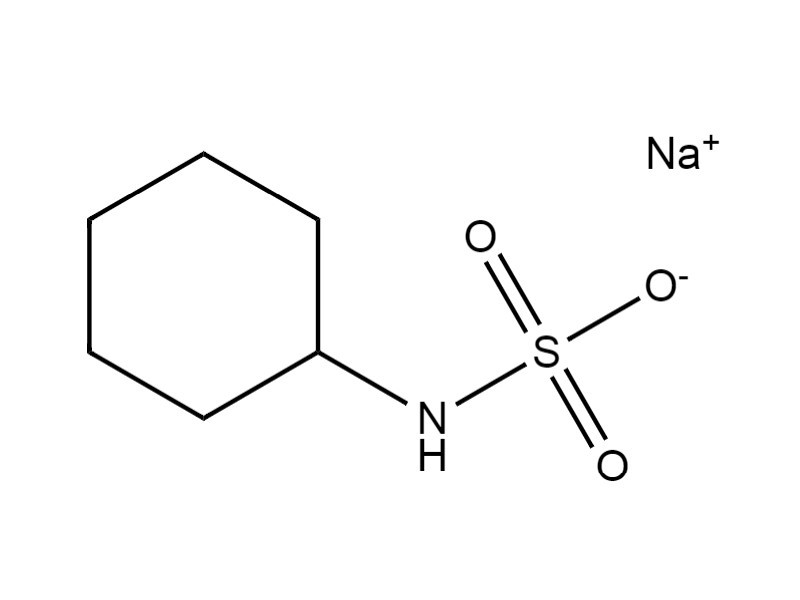 C6H12NNaO3S C6H12NNaO3S |
201.2 |
| 108-91-8 | Cyclohexanamine (cyclohexylamine; CHA) | 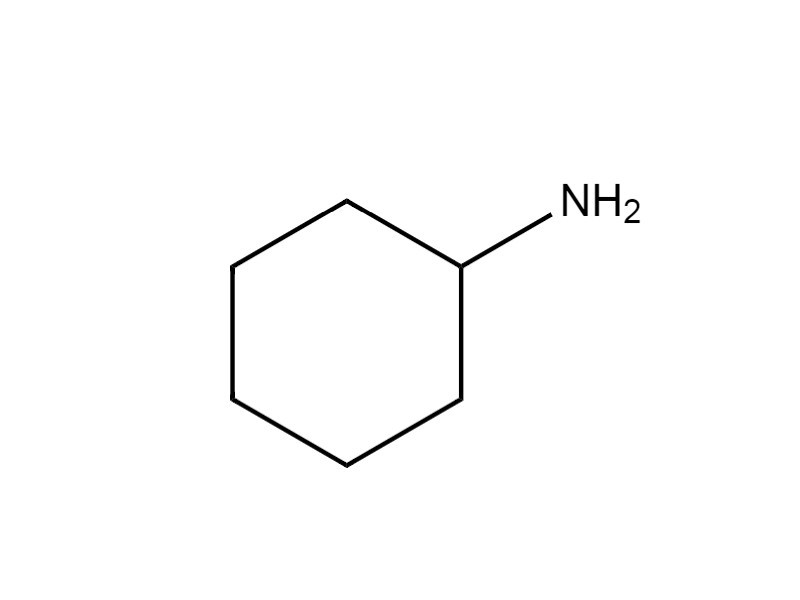 C6H13N C6H13N |
99.2 |
Cyclamate can refer to cyclamic acid (CAS RN 100-88-9), sodium cyclamate (CAS RN 139-05-9), or calcium cyclamate (CAS RN 139-06-0) (Lawrence 2003). In this assessment, cyclamate refers to the cyclamate moiety of sodium cyclamate or the cyclamate anion that forms when sodium cyclamate dissociates (the dissociation will mainly result in the cyclamate anion and the sodium cation).
2.1 Selection of analogues
A read-across approach using data from analogues was used to inform the human health assessment.
Analogues selected were structurally and/or functionally similar to substances in this assessment (similar physical-chemical properties, toxicokinetics), and were associated with relevant empirical data that could be used to read-across to substances in this assessment.
Specifically, cyclohexylamine hydrochloride (CHA HCl; CAS RN 4998-76-9) was used to inform the assessment of health effects of CHA (details are provided in Appendix A). Health effects information on CHA and CHA HCl was also used to inform the characterization of critical health effects of sodium cyclamate.
3. Physical and chemical properties
A summary of physical and chemical property data for sodium cyclamate and CHA are presented in Table 3‑1. Additional physical and chemical properties are reported in ECCC (2016b).
| Property | Sodium cyclamate | CHA |
|---|---|---|
| Physical state | White crystalsa | Liquide |
| Melting point (°C) | 300b | -17.7f |
| Vapour pressure (Pa) | 7.08 × 10−5 [modelled]c |
1430g |
| Henry’s law constant (atm·m3/mol) | 1.70 × 10−8 [modelled]c |
4.16 x 10-6 g |
| Water solubility (mg/L) | 1.0 × 106 [modelled]c |
1.0 × 106 g (miscible) |
| Log Kow (dimensionless) | -1.61 [modelled]c |
3.7e,h |
| pKa (dimensionless) | 1.71 [modelled]d |
10.68e |
| Log Koc (dimensionless) | 1.079 [modelled]d |
1.606 [modelled]i |
Abbreviations: Kow, octanol–water partition coefficient; pKa, acid dissociation constant; Koc, organic carbon–water partition coefficient.
a PubChem (2004- )
b US EPA [updated 2018]
c EPI Suite (c2000-2012)
d HSDB (2012)
e ECHA (c2007-2017)
f Carswell and Morrill (1937)
g HSDB (2005)
h At temperature of 25°C and pH of 6.8
i ChemSpider (2015): Log Koc is predicted by EPISuite
4. Sources and uses
Sodium cyclamate and CHA do not occur naturally in the environment.
Sodium cyclamate and CHA have been included in a survey issued pursuant to section 71 of CEPA (Canada 2012). Table 4‑1 presents a summary of information reported on the total manufacture and total import quantities for sodium cyclamate and CHA.
| Substance | Total manufacturea | Total importsa (kg) | Reporting year |
|---|---|---|---|
| Sodium cyclamate | NR | 100 000 to 1 000 000 | 2011 |
| CHA | NR | 871 518 | 2011 |
Abbreviations: NR, Not Reported above the reporting threshold of 100 kg.
a Values reflect quantities reported in response to a CEPA section 71 survey (Canada 2012). See survey for specific inclusions and exclusions (schedules 2 and 3).
Table 4-2 presents a summary of the major uses of sodium cyclamate and CHA according to information submitted in response to a CEPA section 71 survey (Environment Canada 2013).
| Major usesa | Sodium cyclamate | CHA |
|---|---|---|
| Food and beverages | Y | N |
| Water treatment | N | Y |
| Personal care products | N | Y |
Abbreviations: Y, use was reported for this substance; N, use was not reported for this substance.
a Non-confidential uses reported in response to a CEPA section 71 survey (Canada 2012). See survey for specific inclusions and exclusions (schedules 2 and 3).
| Use | Sodium cyclamate | CHA |
|---|---|---|
| Food-related uses other than food additivea | Y | N |
| Incidental additivea, b | N | Y |
| Food packaging materialsa | N | Y |
| Medicinal or non-medicinal ingredients in disinfectant, human or veterinary drug productsc | Y | N |
| Medicinal or non-medicinal ingredients in licensed natural health productsd | Y | N |
| Notified to be present in cosmetics under the Cosmetic Regulationse | N | Y |
| Active ingredient or formulant in registered pest control productsf | N | Y |
Abbreviations: Y, use was reported for this substance; N, use was not reported for this substance.
a Sodium cyclamate, personal communication, e-mails from Food Directorate (FD), Health Canada (HC), to Existing Substances Risk Assessment Bureau (ESRAB), Health Canada (HC), dated Sept. 11 and Oct. 23, 2018; unreferenced; Health Canada established an acceptable daily intake (ADI) of 11 mg/kg bw/day, expressed as cyclamic acid (personal communication, e-mails from FD, HC to ESRAB, HC, dated Oct. 24, 2018; unreferenced). CHA, personal communication, e-mail from FD, HC to ESRAB, HC dated January 10, 2017; unreferenced.
b While not defined under the Food and Drugs Act (FDA), incidental additives may be regarded, for administrative purposes, as those substances which are used in food processing plants and which may potentially become adventitious residues in foods (e.g. cleaners, sanitizers).
c Sodium cyclamate is used as a non-medicinal ingredient for the purpose of sweetening in drugs, personal communication, e-mails from Therapeutic Products Directorate (TPD), HC to ESRAB, HC, dates ranging from May 3 to Sept. 17, 2018; unreferenced.
d Sodium cyclamate is a non-medicinal ingredient in natural health products, personal communication, e-mails from Natural and Non-Prescription Health Products Directorate (NNHPD), HC to ESRAB, HC, dates ranging from May 3 to Sept. 18, 2018; unreferenced.
e CHA, personal communication, e-mails from Consumer and Hazardous Products Safety Directorate (CHPSD), HC, to ESRAB, HC, dated Dec. 14, 2016 and Dec. 14, 2017; unreferenced
f Sodium cyclamate, personal communication, e-mail from Pest Management Regulatory Agency (PMRA), HC to ESRAB, HC, dated April 5, 2018; unreferenced. CHA is a formulant, personal communication, e-mail from PMRA, HC to ESRAB, HC, dated Dec. 21, 2016; unreferenced.
In Canada, sodium cyclamate is primarily used as a table-top sweetener (Canada 1978b, Canada 2012). Under Part E of the Food and Drug Regulations, cyclohexyl sulfamic acid (cyclamic acid) or any of its salts (e.g., sodium cyclamate) may be sold as a sweetener for personal use provided it is labelled to state that it should be used only on the advice of a physician and provided its energy value is labelled (personal communication, e-mail from FD, HC, to ESRAB, HC, dated Nov. 9, 2018; unreferenced). Sodium cyclamate is used as a non-medicinal ingredient for the purpose of sweetening in drugs, including natural health products (personal communication, e-mails from NNHPD and TPD, HC to ESRAB, HC, dates ranging from May 3 to Sept. 18, 2018; adi).
In Canada, CHA is used as a corrosion inhibitor in water treatment, a boiler-cleaning agent, a processing aid, and in cosmetics (Environment Canada 2013). More specifically, CHA is in aerosol hairsprays (personal communication, e-mails from CPSD, HC, to ESRAB, HC, dated Dec 14, 2016 and Dec 14, 2017; unreferenced) and firespace gel fuel canisters (e.g., for a fireplace, fire bowl, or lantern) (MSDS 2018). CHA may be used in food packaging materials as a component in the external layer of pipes intended for the transfer of beverages, and in the non-food contact layer of polyethylene-coated paper/paperboard used to package beverages in Canada. CHA is an incidental additive which may be used in food processing establishments where it may be used as a boiler water additive, and can be present in the resulting steam that may be in contact with food (personal communication, e-mail from FD, HC to ESRAB, HC, dated Jan 10, 2017; unreferenced). CHA is a pesticide formulant (personal communication, e-mail from PMRA, HC to ESRAB, HC, dated Dec 21, 2016; unreferenced).
5. Potential to cause ecological harm
5.1 Characterization of ecological risk
The ecological risks of sodium cyclamate and CHA were characterized using the ecological risk classification of organic substances (ERC) approach (ECCC 2016a). The ERC is a risk-based approach that considers multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. The various lines of evidence are combined to discriminate between substances of lower or higher potency and lower or higher potential for exposure in various media. This approach reduces the overall uncertainty with risk characterization compared to an approach that relies on a single metric in a single medium (e.g., median lethal concentrations) for characterization. The following summarizes the approach, which is described in detail in ECCC (2016a).
Data on physical-chemical properties, fate (chemical half-lives in various media and biota, partition coefficients, and fish bioconcentration), acute fish ecotoxicity, and chemical import or manufacture volume in Canada were collected from the scientific literature, available empirical databases (e.g., OECD QSAR Toolbox 2014), and from responses to surveys conducted under section 71 of CEPA, or they were generated using selected (quantitative) structure-activity relationship ([Q]SAR) or mass-balance fate and bioaccumulation models. These data were used as inputs to other mass-balance models or to complete the substance hazard and exposure profiles.
Hazard profiles were based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Exposure profiles were also based on multiple metrics, including potential emission rate, overall persistence, and long-range transport potential. Hazard and exposure profiles were compared to decision criteria in order to classify the hazard and exposure potentials for each organic substance as low, moderate, or high. Additional rules were applied (e.g., classification consistency, margin of exposure) to refine the preliminary classifications of hazard or exposure.
A risk matrix was used to assign a low, moderate or high classification of potential risk for each substance on the basis of its hazard and exposure classifications. ERC classifications of potential risk were verified using a two-step approach. The first step adjusted the risk classification outcomes from moderate or high to low for substances that had a low estimated rate of emission to water after wastewater treatment, representing a low potential for exposure. The second step reviewed low risk potential classification outcomes using relatively conservative, local-scale (i.e., in the area immediately surrounding a point source of discharge) risk scenarios, designed to be protective of the environment, to determine whether the classification of potential risk should be increased.
ERC uses a weighted approach to minimize the potential for both over- and under- classification of hazard and exposure, and of subsequent risk. The balanced approaches for dealing with uncertainties are described in greater detail in ECCC (2016a). The following describes two of the more substantial areas of uncertainty. Error with empirical or modelled acute toxicity values could result in changes in classification of hazard, particularly metrics relying on tissue residue values (i.e., mode of toxic action), many of which are predicted values from (Q)SAR models (OECD QSAR Toolbox 2014). However, the impact of this error is mitigated by the fact that overestimation of median lethality will result in a conservative (protective) tissue residue value used for critical body residue analysis. Error with underestimation of acute toxicity will be mitigated through the use of other hazard metrics such as structural profiling of mode of action, reactivity and/or estrogen binding affinity. Changes or errors in chemical quantity could result in differences in classification of exposure as the exposure and risk classifications are highly sensitive to emission rate and use quantity. The ERC classifications thus reflect exposure and risk in Canada based on what is estimated to be the current use quantity, and may not reflect future trends.
Critical data and considerations used to develop the substance-specific profiles for sodium cyclamate and CHA, and the hazard, exposure and risk classification results are presented in ECCC (2016b).
On the basis of low hazard and low exposure classifications according to information considered under ERC, sodium cyclamate and CHA were classified as having a low potential for ecological risk. It is unlikely that these substances are resulting in concerns for the environment in Canada.
6. Potential to cause harm to human health
6.1 Exposure assessment
6.1.1 Environmental media and food
Environmental media, sodium cyclamate
Sodium cyclamate is expected to dissociate and be present as the cyclamate anion and sodium cation in the environment, as indicated by its pKa value. With its low vapour pressure, volatilization of the cyclamate anion from water and soil surfaces is not expected. Also, cyclamate anion is not expected to adsorb more strongly to soils than its neutral counterparts, and it is not expected to adsorb to suspended solids and sediment in water based on the estimated Koc (HSDB 2012).
No measured concentrations of sodium cyclamate in air, soil, or dust were identified in Canada. Concentrations were modelled in environmental media using ChemCAN (2003) based on commercial quantities of sodium cyclamate submitted in Canada in response to a CEPA section 71 survey (Environment Canada 2013). Total daily intakes of sodium cyclamate via ambient air, indoor air, soil and dust were estimated to result in negligible exposure.
Sodium cyclamate has been detected in the form of cyclamate anion in drinking water and surface water in Canada. The concentrations of cyclamate anion in water are estimates of sodium cyclamate levels that have dissociated in water, but these concentrations may also include contributions of cyclamate anions from other cyclamate salts, such as calcium cyclamate.
In 2011, cyclamate was measured at concentrations ranging from 0.09 to 4.1 µg/L with a median value of 0.17 µg/L in groundwater samples (8% detection frequency) collected from 59 domestic wells located in southern portion of the Nottawasaga River watershed in Ontario (Spoelstra et al. 2017). Spoelstra et al. (2013) also measured cyclamate at a mean concentration of 0.2 µg/L and a maximum concentration of 0.88 µg/L in 57 surface water samples from the Grand River watershed in Ontario during the 2007 to 2009 period. The maximum concentration of cyclamate reported in Canada from Spoelstra et al. (2017) (4.1 µg/L) was selected for characterizing exposure to sodium cyclamate via drinking water. The highest daily intake of sodium cyclamate from drinking water is estimated to be 5.4 x 10-4 mg/kg bw/day in formula-fed infants aged 0 to 5 months (Appendix B).
Sodium cyclamate (based on cyclamate anion levels) has been measured in ambient air, soil, dust and surface water in other countries (Gan et al. 2014; Berset et al. 2012; Scheurer et al. 2009; Arbeláez et al. 2015; Lange et al. 2012; Sang et al. 2014; Tran et al. 2014; Watanabe et al. 2016; Gan et al. 2013; Edwards et al. 2017; Yang et al. 2018). These measurements are not expected to be reflective of Canadian environmental concentrations due to differences in manufacturing activities and environmental conditions.
Environmental media, CHA
CHA is expected to be present in protonated form as a cation in the environment, as indicated by its pKa value. As such, adsorption to soils and suspended soils in water is likely (PhysProp c2013). CHA is associated with a high vapour pressure and will exist as a vapour in the atmosphere if released to air (PhysProp c2013). CHA is expected to exist in the cationic form in surface waters, and volatilization from water surfaces is not expected as the cationic form of CHA is not expected to be volatile. However, CHA may volatilize from dry soil surfaces based upon its vapour pressure (HSDB 2005).
No measured concentrations of CHA in air, soil, or dust were identified in Canada. Concentrations were modelled in these environmental matrices using ChemCAN (2003) and based on commercial quantities of CHA reported in Canada in response to a CEPA section 71 survey (Environment Canada 2013). Estimated total daily intakes of CHA via ambient air, indoor air, soil and dust, resulted in negligible exposure.
No Canadian data on concentrations of CHA in drinking water or surface water were identified. Estimated concentrations in surface water were derived with the New Substances Assessment and Control Bureau (NSACB) Environmental Assessment Unit (EAU) Drinking Water Workbook using the industrial release scenario (Health Canada, in-house model unpublished; see Table B-1, Appendix B for details). The resulting 50th percentile predicted environmental concentration was 13 µg/L, which resulted in a conservative daily intake of 1.7 x 10-3 mg/kg bw/day for formula-fed infants aged 0 to 5 months.
Food, sodium cyclamate
Food consumption data from the Canadian Community Health Survey (CCHS) (Statistics Canada 2015), a national 24-hour dietary recall survey, were used to assess exposure of the general population to sodium cyclamate. Over 20 000 respondents were surveyed on the first 24-hour recall, and approximately one third of respondents were surveyed on a second, non-consecutive day in order to provide a means to measure day-to-day variability in consumption, which is required to calculate usual intakes.
Respondents included children, people with and without diabetes, as well as pregnant and breastfeeding women. Approximately 2% of the surveyed adults reported consumption of table-top sweeteners containing sodium cyclamate, with the median age of 62 years. No one below age 19, or who was pregnant (n=114) reported consumption of sodium cyclamate. Only 1 of the 187 breastfeeding women surveyed reported consuming cyclamate-containing table-top sweeteners. Therefore, ‘eaters only’ daily dietary exposures were estimated for adults age 19 and over, who reported consuming sodium cyclamate-based table-top sweeteners on at least one day of the CCHS survey.
The assessment assumed a sodium cyclamate content of 32% in table-top sweetener, based on the most commonly used brand of cyclamate sweetener, out of three brands containing from 30% to 34% sodium cyclamate (personal communication, e-mail from FD HC to ESRAB HC, dated November 9, 2018). As Health Canada’s acceptable daily intake (ADI) associated with this compound is defined on a cyclamic acid basis, the quantity of sodium cyclamate was in turn converted to a quantity of cyclamic acid using the relative molecular weights of sodium cyclamate and cyclamic acid.
Dietary exposure to cyclamic acid was estimated for 'eaters only', by multiplying the sodium cyclamate concentration (32%) by the quantity of those sweeteners consumed by individual survey respondents (personal communication, e-mail from FD, HC, to ESRAB, HC, dated November 2, 2018; unreferenced) and then applying the molecular weight ratio to convert this to cyclamic acid. This yielded a full distribution of cyclamic acid exposure estimates for each of the included subpopulations. An adjustment to yield 'usual intakes' was then applied in order to generate exposure estimates that are more reflective of the typical, long-term dietary exposure to cyclamic acid. Details on the parameters used and adjustment approach are found in Appendix C.
Mean and 90th percentile estimates of eaters-only, usual dietary exposure to sodium cyclamate (as cyclamic acid) are presented in Table 6‑1. The usual exposure was estimated both for the general population (including those with diabetes), and for individuals with diabetes as a subpopulation hypothesized to consume higher quantities of table-top sweeteners. In general, women consumed approximately 19% to 22% more cyclamate than men on a body weight basis.
| Population | Male mean dietary exposure (mg/kg bw/day) | Female mean dietary exposure (mg/kg bw/day) | Male 90th percentile dietary exposure (mg/kg bw/day) | Female 90th percentile dietary exposure (mg/kg bw/day) |
|---|---|---|---|---|
| General Population (adults, 19+ years) | 6.53 | 8.00 | 12.63 | 14.98 |
| Population with Diabetes (adults, 19+ years) | 6.28 | 7.67 | 12.15 | 14.57 |
For the general population 19 years of age and above, 2.14% of respondents reported consumption of cyclamate on either day of recall; among the subpopulation of respondents with diabetes, 9.68% reported consumption of cyclamate. However, usual exposure estimates for individuals with diabetes were quantitatively similar to or slightly lower than those of the general population, suggesting that while more people with diabetes may consume cyclamate, the amount they consume per day is not any higher.
Food, CHA
In Canada, CHA may be present in certain food packaging materials as a result of its use as a component in external layer of pipes (at a maximum level of 25 ppm) intended for the transfer of beverages and in the non-food contact layer of paper/paperboard intended for use in contact with beverages. There is no potential for direct food contact associated with these uses since CHA may be present in the external layer of pipes, and in the paper/paperboard, a polyethylene coating acts as a barrier from direct food contact (Personal communication, e-mail from FD, HC, to ESRAB, HC, dated January 10, 2017; unreferenced).
CHA may also be present as an incidental additive used in food processing establishments in Canada where it is used as a boiler water additive (BWA). The maximum concentration of CHA allowed in boiler water systems is 10 ppm in the steam. Steam treated with CHA is not considered to be acceptable for use in the processing of milk and milk products. Health Canada had no objection to the use of blends of restricted chemicals including CHA as a BWA, provided that the total amine concentration of steam does not exceed 25 ppm (Health Canada 2010). This use results in direct food contact with a conservative estimate of a probable daily intake of 5.7 µg/kg bw/day for adults (Personal communication, e-mail from FD, HC to ESRAB, HC, dated Jan 10, 2017; unreferenced).
6.1.2 Products available to consumers
Sodium cyclamate
Potential exposure to sodium cyclamate from its use as non-medicinal ingredient for the purpose of sweetening in drugs, including natural health products was estimated based on conservative assumptions. Details are presented in Appendix D. Estimates for scenarios that result in the highest level of potential oral, dermal, or inhalation exposure (referred to as sentinel scenarios) for relevant age groups are presented in Table 6‑2.
| Duration and route of exposure | Product scenario | Age group | Maximum quantity per dosage unit or concentration | Exposure estimate (mg/kg bw/day) |
|---|---|---|---|---|
| Daily oral | Non-medicinal ingredient or the purpose of sweeteninga | Adults (19+ years) | 70 mg/dose (1.4%) | 2.84 |
| Daily oral | Non-medicinal ingredient or the purpose of sweeteninga | Toddlers (1 year) | 1.5% | 1.36 |
| Daily buccal | Mouthwashb | Teens (14-18 years) | 0.5% | 0.69 |
| Daily inhalation | Respirator solution to treat bronchospasmb, c, d | Children (5-8 years) | 3 mg/dose | 0.034 |
| Intermittent oral | Chest congestion relief syrupb | Children (12-13 years) | 1.5% | 14.3 |
| Intermittent buccal | Buccal anesthetic solutionb | Toddlers (2-3 years) | 2% | 7.2 |
| Intermittent dermal | Topical anesthetic solution for skin pain reliefb | Children (3-8 years) | 2% | 0.38 |
a Personal communication, e-mails from NNHPD, HC to ESRAB, HC, dates ranging from May 3 to Sept. 18, 2018; unreferenced.
b Personal communication, e-mails from TPD, HC to ESRAB, HC, dates ranging from May 3 to Sept. 17, 2018; unreferenced.
c Inhalation exposure estimates shown here are the internal dose on day of exposure (mg/kg bw/day) estimated using ConsExpo Web (2018) and parameters outlined in Appendix D. Internal dose was selected to compare with oral hazard endpoints.
d A respirator solution for the treatment of severe bronchospasm associated with exacerbations of chronic bronchitis and bronchial asthma. A prepared solution is administered using a respirator or nebulizer.
CHA
CHA is found in aerosol hairspray products in Canada up to concentrations of 0.3% (personal communication, e-mails from CPSD, HC, to ESRAB, HC, dated December 8 and 14, 2016; unreferenced). It is also found in firespace gel fuel canisters at a maximum concentration of 0.3% (MSDS 2018). Inhalation and dermal exposure estimates for aerosol hairspray products and inhalation exposure estimates for firespace gel fuel canisters were derived using ConsExpo Web (2018). Estimates for scenarios that result in the highest level of potential exposure (referred to as sentinel scenarios) for relevant age groups are presented in Table 6‑3. Details are presented in Appendix D.
| Product scenario (age group) | Maximum concentration | Inhalation exposureb | Dermal exposurec | Exposure estimated |
|---|---|---|---|---|
| Daily use of aerosol hairspray (adults, 19+ years)a | 0.3% | 0.00075 mg/kg bw/day | 0.013 mg/kg bw/day | 0.014 mg/kg bw/day |
| Per event use of aerosol hairspray (children, 4-8 years)a | 0.3% | 0.0012 mg/kg bw | 0.026 mg/kg bw | 0.027 mg/kg bw |
| Per event use of firespace gel fuel canisters (toddlers, 1 year)e,f | 0.3% | 3 mg/kg bw | N/A | N/A |
Abbreviation: N/A, Not Applicable
a Personal communication, e-mail from CPSD, HC, to ESRAB, HC, dated December 14, 2016; unreferenced.
b Inhalation exposure estimates shown here are the internal dose on day of exposure (mg/kg bw/day) estimated using ConsExpo Web (2018) and parameters outlined in Appendix D. Internal dose was selected to compare with oral hazard endpoints.
c Dermal exposure was calculated as a dose (mg/kg bw/day) using parameters outlined in Appendix D.
d Combined dose (mg/kg bw/day) from dermal and inhalation exposure.
e MSDS (2018).
f When this product is used indoors (e.g., by an adult), toddlers can be exposed to CHA released in the indoor air.
Although dermal and inhalation exposure are both expected from use of hair spray, the high vapour pressure of CHA may result in a short retention time on the skin, therefore consideration of both inhalation and dermal routes of exposure is considered to be a conservative approach.
6.2 Health effects assessment
Sodium cyclamate in association with cyclamate and its salts was reviewed by the European Scientific Committee on Food (SCF 2000), the JECFA (1970, 1982), and the US FDA (1980). Cyclamates and its salts, including sodium cyclamate, are not classifiable as to their carcinogenicity in humans (Group 3; inadequate evidence in humans and inadequate evidence in animals) (IARC 1999). Information from these reviews and additional literature were taken into consideration in this assessment.
As a metabolite of cyclamate and its salts, CHA was also reviewed in these documents. CHA was also evaluated by the European Union (ECHA 2017) and the US EPA (1988). The European Commission classifies CHA as a reproductive toxicant (Repr 2) (EU 2008).
Studies with sodium cyclamate were limited. Data from CHA, the primary metabolite in humans who can convert sodium cyclamate to CHA (Bopp et al. 1986), and cyclohexylamine hydrochloride (CHA HCl) were used to inform the assessment of health effects of sodium cyclamate and CHA.
Toxicokinetics of sodium cyclamate
The toxicokinetics of sodium cyclamate was reviewed by Bopp et al. (1986). When sodium cyclamate was orally administrated to humans, peak plasma levels occurred between 6 and 8 hours. The half-life was determined to be 8 hours; this correlated with the half-lives of 6.6 and 8.8 hours determined in rats and dogs, respectively. In humans 37% of the quantity of cyclamates ingested is absorbed from the gastrointestinal tract, leaving 63% unabsorbed, based on unpublished information submitted to HC (personal communication, e-mail from FD, HC to ESRAB, HC, dated Nov. 20, 2018; unreferenced). Absorption of cyclamate from the gastrointestinal tract of rats and dogs is similar (33 to 40%), and higher in monkeys (62 to 66%) (Parekh et al. 1970; Bopp et al. 1986).
Rats and dogs orally dosed with sodium cyclamate showed that cyclamate distributes to most tissues in the body with highest levels in the kidneys and lowest in the brain. Cyclamate may also cross the placenta and enter the fetuses of rats, monkeys and humans. It has also been found in the milk of rats, dogs and pigs (Bopp et al. 1986).
Oral or subcutaneous dosing of sodium cyclamate results in cyclamate in the gastrointestinal environment of pigs (Collings 1989), suggesting that sodium cyclamate dissociates into cyclamate in the gastrointestinal tract of mammals. Cyclamate in the gastrointestinal tract can then be microbially biotransformed in mammals by a bacterial enzyme, sulfamatase, through hydrolysis of cyclamate into CHA (Bopp et al. 1986). In several published studies, most humans were unable to convert cyclamate to CHA, with approximately 10% to 30% of over 1000 human subjects able to generally convert 0.1 to 8% cyclamate to CHA, with a minority converting up to 60% of ingested cyclamate (IARC 1980). Similarly, in the few monkeys tested for CHA levels in a limited dietary monkey study, most monkeys did not convert cyclamate to CHA after 12 years of repeated dietary dosing, with a minority converting up to 1% sodium cyclamate to CHA (Thorgeirsson et al. 1994). In contrast, most rats (up to 92%) in multiple oral (dietary, gavage, drinking water) studies, converted up to 35% cyclamate to CHA, similar to 30% in humans (Wallace et al. 1970; Renwick and Williams 1972a; Bickel et al. 1974; Bopp et al. 1986). CHA is the major metabolite in humans who can convert cyclamate to CHA (Bopp et al. 1986).
Individually, human non-converters generally remained non-converters (Renwick et al. 2004), but conversion rates could increase with repeated cyclamate intake or decrease with cessation of intake (Buss et al. 1992; Renwick et al. 2004). Overall, JECFA (1982) estimated 30% CHA can be formed by microbial biotransformation of cyclamate in the gastrointestinal tract of all mammalian species studied including humans (IARC 1999; SCF 1985 cited in SCF 2000). Although there is variability in conversion rates between and within converters of cyclamate to CHA, estimating complete conversion of all ingested cyclamate to CHA is a conservative assumption. If it is assumed that in humans 63% of sodium cyclamate is not absorbed in the gastrointestinal system, then 63% dissociated cyclamate is available for 30% conversion to CHA (SCF 1985 cited in SCF 2000; Baines and DiNovi 2010), then up to 19% of the ingested dose of sodium cyclamate may convert to CHA.
In experimental animals orally dosed with sodium cyclamate, cyclamate absorbed from the gastrointestinal tract is primarily excreted in the urine, and less than 1% of the dose is secreted in the bile of rats and dogs (Bopp et al. 1986). Twenty-four men ingesting 70.5 to 226 mg/kg bw/day of sodium cyclamate (capsules administered with meals 3 times/day) for 30.4 weeks had a wide interindividual variation of CHA urinary excretion, with a mean of 17.2% (0.25% to 75.4%); when one subject receiving 141 mg/kg bw/day was analyzed over the course of the study, there was also a variation of 0.21 to 19% urinary excretion of CHA with no consistent relation between excretion of CHA and cyclamate (Wills et al. 1981). This may indicate that conversion of sodium cyclamate to CHA varies amongst and within humans, and supports the estimate of 19% as the portion of the ingested dose of sodium cyclamate that may be converted to CHA in humans.
Toxicokinetics of CHA
When CHA is orally administrated to humans, rats and dogs, peak blood or plasma levels occurred between 1 and 2 hours and the half-life ranged from 3 to 5 hours (ECHA 2017). After a single oral radiolabelled dose of CHA, most of the parental compound was excreted unchanged in the urine of animals (90% or more) and humans (86% to 95%) (Bopp et al. 1986, Renwick and Williams 1972b; Eichelbaum et al. 1974; ECHA 2017).
Orally administered CHA in the rat showed the highest concentrations of CHA in the lungs, spleen, liver, adrenal glands, heart, gastrointestinal tract and kidneys (unpublished report cited in both Bopp et al. 1986 and ECHA 2017).
In the rat kidney, CHA is metabolized mainly by hydroxylation of the cyclohexane ring, and in the human kidney, it is metabolized by deamination. In both cases, CHA forms minor metabolites including cyclohexanone, cyclohexanol, trans-cyclohexane-1,2-diol and N-hydrocyclohexylamine (Gaunt et al. 1974; Golberg et al. 1969; IARC 1999). CHA was demonstrated to diffuse across the placental membrane in pregnant monkeys and human women given an intravenous dose of CHA (ECHA 2017; Pitkin et al. 1969; Pitkin et al. 1970). Consequently, the developing fetus may be exposed to maternal CHA in pregnant women who can convert cyclamate to CHA.
CHA is excreted mostly unchanged in the urine (IARC 1999). CHA metabolites are also excreted in the urine (Golberg et al. 1969; IARC 1999; Renwick and Williams 1972b; Gaunt et al. 1974).
Health effect studies using sodium cyclamate
In a two-year chronic toxicity/carcinogenicity study, although there were increased papillary carcinomas in the bladder in rats fed 2500 mg/kg bw/day 10:1 sodium cyclamate and sodium saccharin mixture (Oser et al. 1975), the role of sodium cyclamate was confounded by the mixture and this tumour type was not considered toxicologically relevant to humans (US NTP 2016).
In a lifetime study, monkeys (cynomolgus, rhesus and African green) were fed sodium cyclamate in a vitamin mixture applied in sandwiches at 0, 100 or 500 mg/kg bw/day (11/5, 5/5, 8/3 males/females, respectively) five times per week from a few days after birth and continuing for up to 24 years. There were no treatment-related effects observed in females at any dose. Most treated monkeys died during the last 10 years of the study due to circumstances not related to treatment. After 23 years (two years after the average lifespan of 21.3 years in control monkeys), testicular effects were observed in 2/5 males at 500 mg/kg bw/day (focal germ cell aplasia or Sertoli-cell only tubules in both monkeys mixed with areas showing normal spermatogenesis, and chronic inflammation of the testis in one of the two). At 12 years of age, when monkeys are considered to be of reproductive age, measures of testicular size and morphology, semen analysis and serum testosterone and gonadotropin levels showed no differences between treated and control monkeys (as reported in Thorgeirsson et al. 1994). Further, testicular biopsies from treated monkeys showed no histological differences from control monkeys at 12 years of age (Takayama et al. 2000). Unfortunately, a full detailed analysis of testicular effects was only conducted at the end of life. The study authors did not consider the testicular effects to be due to cyclamate per se, but did not exclude a role for CHA based on the correlation of higher CHA levels in the plasma, testes and urine of a monkey with testicular effects (CHA levels were not measured in the other monkey with the same effect). Complete necropsies and histopathological analysis of major organs and tissues were conducted and there was no evidence of carcinogenicity in either sex. (Takayama et al. 2000). While this study highlighted testicular effects to be of interest for sodium cyclamate, it was not selected as a critical study due to confounding factors including a limited number of animals and limited analysis of testicular effects at 12 years, and a varicella infection during 1 year of the study (as per Thorgeirsson et al. 1994), which resulted in 2 mortalities.
Results from in vitro genotoxicity studies conducted with sodium cyclamate were mixed. There was negative mutagenicity in a host-mediated assay using Salmonella (S.) typhimurium (mice injected intraperitoneally with S. typhimurium were exposed to sodium cyclamate by subcutaneous injection), positive clastogenicity in Chinese hamster lung cell and human skin fibroblast chromosome aberration studies and equivocal clastogenicity in human lymphocytes, positive DNA damage/repair in a cell transformation assay using rat bladder cells (IARC 1999), but negative DNA damage/repair in rat hepatocytes (Jeffrey and William 2000 cited in HSDB 2012). A Comet assay conducted on human colon cancer cell lines and human embryonic kidney cells showed minimal, if any, DNA damage (van Eyk 2015).
However, all in vivo genotoxicity studies using sodium cyclamate showed negative results. In vivo mutation studies were negative in Chinese hamsters and mice (IARC 1999). HSDB (2012) also reported negative chromosome aberration in an in vivo study using Chinese hamsters. In humans dosed orally with 70 mg/kg bw/day of sodium cyclamate for 4 days, the peripheral blood lymphocytes were negative for chromosomal aberration (IARC 1999).
In a limited multi-generation reproductive toxicity study, rats (12 females and 6 males/group) received 0 or 3% sodium cyclamate in the diet, equal to 0 or 1500 mg/kg bw/day and F2 adults were mated twice; dams delivered offspring after the first mating but were sacrificed before parturition after the second mating (Ferrando and Huchet 1968). Fertility was not affected in this study for two generations. After the first F2 mating, there was a decreased number of F2 surviving pups at postnatal day (PND) 10 and weaning (2 versus 14 in the control group). This may be due to the excessive dose level administered to the animals. No developmental effects were noted after the second mating. However, treated F2 males had decreased body weight and testicular degeneration (4/6 males). The two surviving F3 males (1500 mg/kg bw/day) showed testicular atrophy (age at sacrifice not reported). There were two other dose groups, in which sodium cyclamate was administered via drinking water at 0.8% and 1.6%, but effects in these groups were not used due to inconsistencies in the dosing protocol (unclear as to whether treated and untreated drinking water were administered separately or concurrently) (JECFA 1970; Bopp et al. 1986; HSDB 2012). A no observed adverse effect level (NOAEL) could not be determined in this study.
Developmental toxicity studies in which rabbits and rats were administered sodium cyclamate orally at doses up to 250 mg/kg bw/day did not show any evidence of embryotoxic or teratogenic effects (Bein et al. 1967, Fritz and Hess 1968; US Food and Drug Research Laboratories 1969 cited in JECFA 1970). Other acute gavage developmental studies in mice and rats were not used due to excessive dose levels (equivalent to 2150 mg/kg bw and above) (Tanaka 1964a,b cited in JECFA 1970; Bopp et al. 1986).
In a human volunteer study conducted over 30.4 weeks (7 months), men (8/group aged 25 to 39) ingested capsules containing sodium cyclamate at 0 (sucrose vehicle), 5, 10 or 16 g/day (equivalent to 0, 71, 141, or 226 mg/kg bw/day, based on Health Canada [1994]). The initial high dose was lowered to an unspecified level below 141 mg/kg bw/day due to decreased dosing over the period of the study as a result of persistent diarrhea. Soft stools (persistent diarrhea) appeared within the first two weeks in 2/8 men dosed with 141 mg/kg bw/day and 7/8 men dosed with 226 mg/kg bw/day, which persisted throughout the study. Hematology and clinical chemistry parameters, as well as sperm parameters (concentration and motility measured in semen every 2 weeks) were not affected in this study (Wills et al. 1981).
In another human volunteer study limited by self-identified (unmeasured) food intake, it was suggested that sperm parameters and fertility were not correlated with cyclamate ingestion based on CHA urinary excretion (Serra-Majem et al. 2003).
No studies examining effects via the dermal or inhalation routes of exposure were identified.
Overall, while testicular effects were identified after repeated daily doses beyond the average lifespan in a limited 24-year monkey study at 500 mg/kg bw/day and in a limited reproductive study in the second generation at 1500 mg/kg bw/day, the latter suggests that fertility would not be affected for two generations. Further, based on a human volunteer study wherein there was persistent diarrhea beginning at 141 mg/kg bw/day within the first two weeks of daily consumption during a 7-month study, it is considered unlikely that humans would continuously consume higher daily amounts of sodium cyclamate for a relatively longer duration to effect these potential changes. The limited oral studies with sodium cyclamate suggest that it is not expected to be of genotoxic or carcinogenic concern.
Health effect studies using CHA
In a specialized 13-week dietary study that specifically examined testicular effects, Sprague-Dawley rats (100 males/group) were administered 0, 68.5, 137, 274 or 411 mg/kg bw/day CHA HCl in the diet, equivalent to 0, 50, 100, 200 or 300 mg/kg bw/day CHA (Brune et al. 1978 [unpublished report], as cited in Bopp et al. 1986). Two control groups were included: ad libitum and pair-fed. The pair-fed control groups were added to help separate testicular effects from body weight effects. At the end of the study, the body weights of the male rats in all CHA-treated groups were significantly decreased in comparison to the ad libitum control animals. There was decreased body weight gain in the 200 and 300 mg/kg bw/day groups during the first 14 days of the study, but only in the 300 mg/kg group over the entire study, when compared with their respective pair-fed groups. The investigators performed detailed analysis of testicular scores for all animals in the study. Histopathological findings were observed in the testes (degenerative changes in the tubules, giant cell formation, and testicular atrophy in some animals), of which the tubular changes were statistically significant at 200 and 300 mg/kg bw/day compared to both the free-fed and pair-fed control groups, on the basis of their testicular scores. Testicular weights were also significantly lower in the 200 and 300 mg/kg bw/day groups when compared to the free-fed control group. However, a significant effect was only seen at the highest dose when compared to the pair-fed control group. The study authors identified a NOAEL of 100 mg/kg bw/day on the basis of the testicular effects observed at higher doses. Health Canada supports the selection of the NOAEL based on examination of the original unpublished report. No other measures of toxicity were examined in the study.
In another 13-week dietary study that specifically conducted a detailed examination of testicular effects, CHA HCl was fed in the diet of male Wistar rats (15 treated plus 10 controls) at doses of 0 or 400 mg CHA/kg bw/day for 1, 3, 7, 9 or 13 weeks; there were no pair-fed controls in this study (Creasy et al. 1990). Food consumption and body weights were decreased throughout the study. Following histological analysis, no testicular effects were observed after week 1, but during the rest of the study, the authors reported that gradual and persistent damage to testicular tissue occurred at 400 mg/kg bw/day. Male animals (4/15) had vacuolation of Sertoli cell cytoplasm along with localized loss of spermatocytes and spermatogonia in the testes at 3 weeks, all males showed Sertoli cell vacuolation and germ cell populations showed mild to moderate degeneration and depletion in some tubules at 7 weeks, and germ cell depletion was observed in 75% of testicular tubules with disruption of the germinal epithelium at 9 weeks. By 13 weeks, 10/15 males showed generalized germ cell degeneration and depletion, some tubules were shrunken with Sertoli cells remaining in the lining and some spermatids were multinucleated and showed degenerated round morphology. The authors also established primary cell cultures from the testicular tissue isolated from 28-day old Wistar rats and exposed the cells in vitro to 0, 0.1, 1, 3 or 10 mM CHA HCl for 24, 48 or 72 hours. Analysis of the cells using microscopy showed Sertoli and germ cell vacuolation became more extensive with time at doses of 3 mM or greater. Taken together, the authors concluded that the spermatogonial effects were secondary to CHA-mediated Sertoli cell damage (Creasy et al. 1990).
Another assessment of testicular tissue was performed by Roberts et al. (1989) for MF1 mice and Wistar and dark agouti (DA) rats administered CHA HCl in the diet at doses equivalent to 0 or 400 mg CHA/kg bw/day for 3, 7 or 13 weeks. CHA treatment did not affect food consumption, body weight, testicular weight, tissue morphology or sperm morphology in mice. In rats, significantly decreased food consumption and body weight gain were observed, testicular weights were decreased and slight germ cell degeneration and depletion were observed beginning at 3 weeks, but progressing to 75% to 100% of seminiferous tubules affected at 13 weeks, with DA rats (15/15 males) showing more extensive effects (larger proportion of tubules affected) than Wistar rats (6/15 males). Unlabelled CHA measured in the plasma and testes were lower in mice than rats over the 13 weeks (0.5 versus 6 or 3.5 µg/ml in plasma, 6 versus 40 or 30 µg/g in testes, in mice versus Wistar or DA rats, respectively). Creasy et al. (1990), discussed above, examined the same Wistar rats from this study but also included assessments at 1 and 9 weeks and conducted detailed histopathology of the testes.
In a 13-week dietary study, rats (15/sex/group) were fed a diet containing 0, 600, 2000 or 6000 ppm CHA HCl for 13 weeks, equivalent to 0, 41, 143 or 468 mg/kg bw/day, respectively (0, 30, 105 or 342 mg/kg bw/day CHA respectively) (Gaunt et al. 1974). At doses equal to or greater than 143 mg CHA HCl/kg bw/day, body weight gain was significantly reduced (9% to 20% and 26% to 34% decreases at the mid and high doses compared to controls), which was accompanied by significantly reduced food and water consumption. This effect was due in part to the ad libitum feeding, since the extent of decreased body weight gain at the high dose was less (14% to 26% decrease compared to controls) in a satellite group of pair fed animals (5/sex/control and high dose only). The only histological finding that could be related to treatment was reduced spermatogenesis and tubular atrophy at 143 and 468 mg/kg bw/day (in 4/11 and 18/20 males, respectively), which was accompanied with significantly decreased relative testes weights (0 and 17% decreases, respectively). In the pair-fed group, relative testes weights were significantly decreased by 49% at 468 mg/kg bw/day, but histopathological analyses were not conducted on pair-fed animals. The authors and ECHA (2017) identified a NOAEL of 41 mg CHA HCl/kg bw/day (30 mg CHA/kg bw/day) on the basis of the testes effects observed at higher dose levels.
In a two-year dietary study investigating chronic toxicity and carcinogenicity, rats (48/sex/group) were fed diets containing 0, 600, 2000 or 6000 ppm CHA HCl, equivalent to 0, 24, 82 or 300 mg/kg bw/day in males and 0, 35, 120 or 400 mg/kg bw/day in females (0, 18, 60 or 219 mg/kg/day in males and 0, 26, 88 or 322 mg/kg/day in females of CHA) (Gaunt et al. 1976). At all dose levels, there were statistically significant dose-related reductions in body weight, which were related to significantly reduced food and water intakes throughout the study. In females, relative brain and ovary weights were significantly increased at all doses, and relative thyroid weights were also increased at all doses (significant at the mid and high doses). In males, relative brain weights were non-significantly increased at the mid and high dose, whereas serum albumin levels were significantly dose-dependently increased, serum urea levels were significantly decreased and total leucocytes were significantly dose-dependently decreased at all doses. At the highest dose, female animals exhibited slight anemia and reduced production of normally concentrated urine while males showed significantly increased testicular changes (atrophy, tubules with few spermatids, calcium deposits in tubules). “Tubules with few spermatids” was also significantly increased at the mid dose. In addition, an increased incidence of histopathological changes in the lungs (alveoli with foamy macrophages) was also observed in both sexes at the highest dose. ECHA (2017) established a lowest observed adverse effect level (LOAEL) of 18 mg/kg bw/day based on decreased body weight and increased relative ovary, thyroid and brain weights in females. However, the reduction in body weight and associated changes in organ weights was attributed to the decreased food consumption. For this assessment, the NOAEL from this study is identified to be 60 mg/kg bw/day based on significantly increased testicular changes (atrophy, tubules with few spermatids, calcium deposits in tubules) at 219 mg CHA/kg bw/day.
In an 80 week dietary study, mice (48 males, 50 females/dose) were fed CHA HCl at doses of 0, 300, 1000 or 3000 ppm, equivalent to 0, 40, 140 or 400 mg/kg bw/day (0, 29, 102, or 293 mg/kg bw/day of CHA, respectively). No effects on food/water intake, hematology, mortality, histopathological changes in the testes were identified (Hardy et al. 1976). At doses equal to or greater than 140 mg/kg bw there was a significant decrease in body weight gain of male animals. Histopathological changes that were statistically significant and potentially treatment-related were increased incidences of liver cell vacuolation/polyploidy and pulmonary leucocyte infiltration/deposits in female animals receiving 400 mg/kg bw/day. The authors determined a NOAEL of 140 mg/kg bw/day (102 mg/kg bw/day of CHA) based on liver effects in females at the highest dose. ECHA (2017) reported the same NOAEL, but determined that there was insufficient information in the registration dossier to conclude on a NOAEL.
On the basis of available information, ECHA (2017) determined that CHA is unlikely to be genotoxic. In vitro, CHA did not increase the frequency of chromosomal aberrations in Chinese Hamster ovary cells or in rat bone marrow cells (Brusick et al. 1989; Dick et al. 1974; ECHA 2017) or increase DNA damage/repair/synthesis in rat hepatocytes (Brusick et al. 1989). Although there were some equivocal results in vivo in chromosomal aberration assays (in the ova, spermatogonia or bone marrow cells when administered orally or as an intraperitoneal injection to Chinese hamster, rats and mice), most reports were negative for CHA at doses from 50 to 68 mg/kg bw (ECHA 2017; JECFA 1982).
In a 2-year multi-generation reproductive toxicity study, CHA HCl was administered at the equivalent doses of 0, 15, 50, 100, or 150 mg CHA/kg bw/day in the diet to rats (30/sex/group) (Oser et al. 1976). The parental animals (F0) were mated six times to generate six litters (L1-L6), with a one-week rest period between matings. After a 13-week post-weaning period, 15 pairs of rats from the first litters (L1) of each generation (F1-F4) were mated twice to produce the next generation. Fifteen pairs of rats from the second litters (L2) were also mated and half of the offspring were examined for birth defects while the other half were kept until weaning. In the parental generation at the end of 2 years, there was decreased body weight at 50 mg/kg bw/day and above in females (13% to 25%), and at 100 mg/kg bw/day and above in males (19% to 23%). However, the authors attributed these differences to variations in food consumption because food efficiency was not affected during the first 12 weeks of growth when all 5 parental generations were analyzed (statistical analyses were not reported for the remainder of the study). No further information on body weight was provided for the F1 to F4 parental generations after 12 weeks. No significant histopathological changes were observed. At 150 mg/kg bw/day, there were testicular effects (F0 males, abnormal germinal epithelium and atrophy), decreased fertility (F0 dams, 4th and 5th matings), reduced litter size (average of 5 successive litters from F0 dams) and weanling BW at PND28 (average of first litters from 5 successive generations), and increased resorptions (F4). There were no malformations. ECHA (2017) established a NOAEL of 15 mg/kg bw/day for systemic toxicity based on decreased body weight in females at the LOAEL of 50 mg/kg bw/day. In this study, the significance of the decreased body weights are confounded by the lack of controlled food consumption. ECHA (2017) also established a NOAEL of 100 mg/kg bw/day for reproductive toxicity based on “growth retardation due to the lower food consumption”, slight reduction of litter size and weaning weight, and significant higher incidence of testicular atrophy in adult males at the LOAEL of 150 mg/kg bw/day.
Many of the studies discussed above showed effects on testes such as decreased testes weight, testicular atrophy, degeneration of tubuli, and reduced spermatogenesis, although some of them did not follow standard test guidelines. The data, however, show a concern for fertility, which is covered by the EU classification as Repr. Cat. 2. (ECHA 2017).
In a developmental toxicity study, CHA HCl was administered to groups of 25 rats or mice by gavage at 0, 14, 42, 140 mg/kg bw/day (equivalent approximately to 0, 10, 30 or 100 mg/kg bw/day CHA) from days 6 through 15 of gestation (Lorke and Machemer 1983). No effects were reported in mice. In rats at 100 mg CHA/kg bw/day, there was reduced placental weight (16%) and fetal weight (16%) in the presence of maternal decreased body weight gain (30% during treatment, 8% during the entire pregnancy period). There were no effects on implantations, resorption rate, or other fetal parameters (sex ratio, variations and malformations). ECHA (2017) identified a NOAEL of 30 mg/kg bw/day for both maternal and developmental toxicity.
No studies were identified using the dermal routes of exposure. For the inhalation route, a short-term study was identified which reported a lack of neurobehavioural effects in men and women after three to four hour exposures to various concentrations of CHA (up to 10 ppm or 41 mg/m3) (Juran et al. 2012), but this was not used for risk characterization since no other measures of toxicity were examined. Although repeated-dose inhalation studies in experimental animals were available (e.g., Watrous and Schulz 1950; Lomonova 1965), they were associated with methodological limitations or contained insufficient details on study design.
6.3 Characterization of risk to human health
The sodium cyclamate database is limited and although testicular effects were observed in the 24-year monkey study using sodium cyclamate, it was not considered as a critical study for various reasons, including a limited number of test animals (3 to 8 animals/sex/dose group). Since CHA is a metabolite of sodium cyclamate in humans, with potential testicular effects occurring after shorter durations and lower doses than those observed for sodium cyclamate, and there is evidence to show that it may be better absorbed and more widely distributed throughout the blood and tissues of the human body than cyclamate, studies using CHA or its analogue, CHA HCl, were selected for use in the risk assessment of sodium cyclamate.
For daily exposures, the 13-week oral feeding study in rats conducted by Brune et al. (1978) with CHA was selected as the critical toxicity study due to the large sample size (100 males/group), control for food consumption by including pair-fed control and treatment groups at all 4 treatment dose levels, and detailed analyses of testicular scores for all animals in the study. A NOAEL of 100 mg/kg bw/day was identified on the basis of the testicular effects observed at 200 mg/kg bw/day and above, based on Health Canada’s assessment of the original unpublished report.
The 13-week dietary study in rats conducted by Gaunt et al. (1974) with CHA suggests a lower NOAEL and LOAEL (30 and 105 mg/kg bw/day, respectively) for testicular effects, but it used fewer animals/dose (15/sex/group), the analysis of testicular effects was not as detailed as that of Brune et al. (1978), and the pair-feeding portion of this study analyzed a much higher dose (342 mg/kg bw/day of CHA versus controls) compared to the pair-feeding components of the Brune et al. (1978) study. The 2-year dietary study in rats conducted by Gaunt et al. (1976) was not selected as a critical study also because food consumption was not controlled, and the dose spacing in this study resulted in a higher LOAEL (219 mg/kg bw/day) and lower NOAEL (60 mg/kg bw/day) for similar testicular changes than those in the Brune et al. (1978) study.
The NOAEL of 400 mg/kg bw/day identified after the first week of testing in the 13-week dietary study in rats conducted by Creasy et al. (1990) was considered to be appropriate as the critical endpoint for characterization of risk from intermittent exposure. In this study there were adequate numbers of animals/group per time point (15 males/group plus 10 controls) and there was histopathological analysis of the most sensitive organ (testes) at 5 different periods (1, 3, 7, 9 or 13 weeks) during the study, of which the 1 week exposure period was considered most applicable for intermittent exposure estimates (the dose of 400 mg/kg bw/day was determined to be the LOAEL after 3 to 13 weeks of testing).
Although the developmental toxicity study in rats gavaged with CHA HCl (Lorke and Machemer 1983; ECHA 2017) demonstrated decreased maternal body weight, placental weight, and fetal weight at 100 mg CHA/kg bw/day, all other developmental parameters were not affected. This study was not selected for evaluation of intermittent exposures because it is unclear that decreased weights would be expected after a single or few exposures.
The critical health effect endpoints identified in the studies conducted with CHA were converted to an equivalent dose of cyclamic acid (this is because sodium cyclamate dissociates to cyclamic acid in the gastro-intestinal tract). The conversion formula accounted for relative molecular weights, and taking into account the 63% of cyclamate available to be converted to CHA in humans and the assumed conversion rate of cyclamate to CHA in the gut of humans (30%). This is aligned with the approach of JECFA (1982) and Health Canada in their calculation of the ADI of 11 mg/kg bw/day, expressed as cyclamic acid.
Table 6‑4 provides all relevant exposure and hazard values for sodium cyclamate, as well as resultant margins of exposure (MOEs), for determination of risk.
| Exposure Scenario (age group with highest estimate) | Systemic Exposurea | Critical effect level | Critical health effect endpoint | MOE |
|---|---|---|---|---|
| Daily oral intake from drinking water (infants aged 0 to 5 months) | 0.00054 mg/kg bw/day | NOAEL adj.b = 1058 mg/kg bw/day of cyclamic acid in 13-week rat study. | NOAEL of 100 mg/kg bw/day was selected based on the testicular effects observed at 200 and 300 mg CHA/kg bw/day. | 1 960 000 |
| Daily oral intake from table-top sweetener, eaters only (adult males, 19+ years) | 6.53 mg/kg bw/day (mean); 12.63 mg/kg bw/day (90th percentile) | NOAEL adj.b = 1058 mg/kg bw/day of cyclamic acid in 13-week rat study. | NOAEL of 100 mg/kg bw/day was selected based on the testicular effects observed at 200 and 300 mg CHA/kg bw/day. | 162 (mean); 84 (90th percentile) |
| Daily oral exposure from use as non-medicinal ingredient for the purpose of sweetening (adults, 19+ years) | 2.84 mg/kg bw/day | NOAEL adj.b = 1058 mg/kg bw/day of cyclamic acid in 13-week rat study. | NOAEL of 100 mg/kg bw/day was selected based on the testicular effects observed at 200 and 300 mg CHA/kg bw/day. | 373 |
| Daily oral exposure from use as non-medicinal ingredient for the purpose of sweetening (toddlers, 1 year) | 1.36 mg/kg bw/day | NOAEL adj.b = 1058 mg/kg bw/day of cyclamic acid in 13-week rat study. | NOAEL of 100 mg/kg bw/day was selected based on the testicular effects observed at 200 and 300 mg CHA/kg bw/day. | 778 |
| Daily buccal exposure to mouthwash (teens, 14-18 years) | 0.69 mg/kg bw/day | NOAEL adj.b = 1058 mg/kg bw/day of cyclamic acid in 13-week rat study. | NOAEL of 100 mg/kg bw/day was selected based on the testicular effects observed at 200 and 300 mg CHA/kg bw/day. | 1 530 |
| Daily inhalation exposure to respirator solution to treat bronchospasm (children, 5-8 years) | 0.034mg/kg bw/day | NOAEL adj.b = 1058 mg/kg bw/day of cyclamic acid in 13-week rat study. | NOAEL of 100 mg/kg bw/day was selected based on the testicular effects observed at 200 and 300 mg CHA/kg bw/day. | 31 100 |
| Intermittent oral exposure from chest congestion relief syrup (children,12-13 years) | 14.3 mg/kg bw/day | NOAEL adj.c = 4230 mg/kg bw/day of cyclamic acid, no effects observed after 1 week in a rat study. | NOAEL of 400 mg/kg bw/day of CHA was selected based on lack of testicular effects in males at 1 week. | 296 |
| Intermittent buccal exposure to buccal anesthetic solution (toddlers, 2-3 years) | 7.2 mg/kg bw/day | NOAEL adj.c = 4230 mg/kg bw/day of cyclamic acid, no effects observed after 1 week in a rat study. | NOAEL of 400 mg/kg bw/day of CHA was selected based on lack of testicular effects in males at 1 week. | 588 |
| Intermittent dermal exposure from topical anesthetic solution for skin pain relief (children, 3-8 years) | 0.38 mg/kg bw/dayd | NOAEL adj.c = 4230 mg/kg bw/day of cyclamic acid, no effects observed after 1 week in a rat study. | NOAEL of 400 mg/kg bw/day of CHA was selected based on lack of testicular effects in males at 1 week. | 11 100 |
Abbreviation: MOE, Margin of Exposure; NOAEL, no observed adverse effect level.
a Exposure estimates are calculated on a sodium cyclamate basis (only the daily oral intake from food is calculated as cyclamic acid). However, comparison of sodium cyclamate exposure estimates to the adjusted NOAELs does not significantly affect the MOE calculations because the molecular weight of cyclamic acid (179.2 g/mol) is close to that of sodium cyclamate (201.2 g/mol).
b Adjusted NOAEL of 1058 mg/kg bw/day was calculated from NOAEL of 100 mg/kg bw/day based on the following formula: 100 mg/kg bw per day X 2 [rounded integer from 179.2(the MW of cyclamic acid ÷ 99.2 (MW of CHA)] ÷ [0.63 (as in 63% of cyclamate is available to be converted to CHA) ÷ 0.3 (the rate of conversion of cyclamate to CHA)] = 1058 mg/kg bw/day.
c Adjusted NOAEL of 4230 mg/kg bw/day was calculated from NOAEL of 400 mg/kg bw/day based on the following formula: 400 mg/kg bw per day X 2 [rounded integer from 179.2 (the MW of cyclamic acid ÷ 99.2 (MW of CHA)] ÷ [0.63 (as in 63% of cyclamate is available to be converted to CHA) ÷ 0.3 (the rate of conversion of cyclamate to CHA)] = 4230 mg/kg bw/day.
d Assumes 100% dermal absorption.
The MOEs for sodium cyclamate from table-top sweetener are considered adequate to address uncertainties in the health effects and exposure databases. The mean exposure level for the adult male population (6.53 mg/kg bw/day) is below the ADI and results in a MOE of 162. Although the MOE is 84 for the 90th percentile of adult male consumers of sodium cyclamate, this is considered adequate for multiple reasons. Considerations include i) table-top sweeteners containing sodium cyclamate must be labelled to state that they should be used only on the advice of a physician; ii) there was no evidence of effects on sperm parameters (including motility) in various studies with sodium cyclamate or CHA; iii) no reproductive or developmental toxicity was observed in animals orally dosed with up to 250 mg/kg bw/day of sodium cyclamate; iv) repeated daily ingestion of sodium cyclamate in human males greater or equal to 140 mg/kg bw/day may result in persistent diarrhea within two weeks, which would be expected to deter males from high and/or prolonged consumption; and v) available market share data suggest that sodium cyclamate-containing sweeteners' share of the table-top sweetener market has declined significantly over the past decade (see Appendix C). As such, the MOEs for sodium cyclamate are considered adequate to address uncertainties in the health effects and exposure databases.
The maximum intakes for oral exposure to mouthwash, as well as from its use as non-medicinal ingredient for the purpose of sweetening in natural health products, ranging from 0.69 to 2.84 mg/kg bw/day, while the maximum intake for inhalation exposure via a respirator solution to treat bronchospasm was 0.0342 mg/kg bw/day. These values are lower than the ADI of 11 mg/kg bw/day, expressed as cyclamic acid, calculated for sodium cyclamate by Health Canada and JECFA (1982) (and lower than 7 mg/kg bw/day calculated by the SCF 2000) (see Appendix E). The ADI is based on a 100-fold uncertainty factor applied to the adjusted NOAEL of 1058 mg/kg bw/day used in the table above. Thus, both on the basis of comparison to the ADI and of comparison to critical effect levels from a 13-week rat study (using CHA with conversion to an equivalent dose of sodium cyclamate), the resulting margins are considered adequate to address uncertainties in the health effects and exposure databases.
For intermittent oral exposure to chest congestion relief syrup and intermittent oral or dermal exposure to a topical anesthetic, comparison of the NOAEL from a one-week rat study (using CHA with conversion to sodium cyclamate) to the conservative estimates of exposure from these products containing sodium cyclamate show MOE ranges of 296 to 11 000. The resulting margins are considered to be adequate to address uncertainties in the health effects and exposure databases.
Although the MOEs for sodium cyclamate from table-top sweetener are considered adequate to address uncertainties in the health effects and exposure databases, many of the studies were conducted before standard guidelines were established and some of them lacked several parameters that affected their study quality. A discussion for selection of critical studies for CHA follows.
Table 6‑5 provides all relevant exposure and hazard values for CHA, as well as resultant MOEs, for determination of risk.
| Exposure scenario (age group with highest estimate) | Systemic exposure | Critical effect level | Critical health effect endpoint | MOE |
|---|---|---|---|---|
| Daily oral intake from food and beverages based on potential use from incidental additives (adults, 19+ years) | 0.0057 mg/kg bw/day | NOAEL = 100 mg/kg bw/day of CHA in 13-week rat study. | NOAEL of 100 mg/kg bw/day was selected based on the testicular effects observed at 200 and 300 mg CHA/kg bw/day. | 17 500 |
| Daily oral intake from drinking water (infants aged 0 to 5 months) | 0.0017 mg/kg bw/day | NOAEL = 100 mg/kg bw/day of CHA in 13-week rat study. | NOAEL of 100 mg/kg bw/day was selected based on the testicular effects observed at 200 and 300 mg CHA/kg bw/day. | 58 800 |
| Daily inhalation and dermal exposure to aerosol hairsprays (adults, 19+ years) | 0.014 mg/kg bw/day | NOAEL = 100 mg/kg bw/day of CHA in 13-week rat study. | NOAEL of 100 mg/kg bw/day was selected based on the testicular effects observed at 200 and 300 mg CHA/kg bw/day. | 7 140 |
| Per event inhalation and dermal exposure to aerosol hairsprays (children, 4-8 years) | 0.027 mg/kg bw | NOAEL = 400 mg/kg bw/day of CHA in first week of 1 to 13-week rat study. | NOAEL of 400 mg/kg bw/day was selected based on lack of testicular effects in males at 1 week. | 15 000 |
| Per event inhalation exposure to firespace gel fuel canister (toddlers, 1 year)a | 3 mg/kg bw | NOAEL = 400 mg/kg bw/day of CHA in first week of 1 to 13-week rat study. | NOAEL of 400 mg/kg bw/day was selected based on lack of testicular effects in males at 1 week. | 133 |
Abbreviation: MOE, Margin of Exposure; NOAEL, no observed adverse effect level.
a When this product is used indoors (e.g., by an adult), toddlers can be exposed to CHA released in the indoor air.
As shown in Table 6‑5, for both daily and per event inhalation and dermal exposure to aerosol hairsprays and firespace gel fuel canister, the resulting margins are considered to be adequate to address uncertainties in the health effects and exposure databases. The resulting margins from daily oral exposure from food and beverages, and drinking water are also considered to be adequate to address uncertainties in the health effects and exposure databases.
While exposure of the general population to CHA is not of concern at current levels, this substance is considered to have a health effect of concern on the basis of its potential hazard, due to its classification as Globally Harmonized System Repr 2 (Suspected human reproductive toxicant) (ECHA 2017). Sodium cyclamate is considered to have the same potential concerns, based on the use of CHA as an analogue. Therefore, there may be a concern for human health if exposures to sodium cyclamate or CHA were to increase.
6.4 Uncertainties in evaluation of risk to human health
The key sources of uncertainty are presented in the table below.
| Key source of uncertainty | Impact |
|---|---|
| No Canadian data for sodium cyclamate or CHA levels in drinking water, air, soil or dust. | +/- |
| Consumer loyalty to particular brands or types of artificial sweeteners in Canada is unknown.a | +/- |
| Aggregate exposures of products containing sodium cyclamate were not estimated because these products (including food products) are not expected to account for a significant market share in Canada. | +/- |
| Since few consumers reported consuming sodium cyclamate, use of ‘eaters only’ has a significant influence on dietary estimates of sodium cyclamate. | + |
| High amounts of sodium cyclamate consumed by certain respondents have a significant influence on the 90th percentile exposure estimates. | + |
| There is variability in the percentage absorption and the conversion rate of cyclamate to CHA. | +/- |
| No dermal absorption data for sodium cyclamate or CHA. | + |
| No repeated dose dermal or inhalation toxicity study for sodium cyclamate or CHA. | +/- |
| No good quality carcinogenicity, reproductive or developmental oral studies with sodium cyclamate. | +/- |
+ = uncertainty with potential to cause over-estimation of risk; - = uncertainty with potential to cause under-estimation of exposure risk; +/- = unknown potential to cause over or under estimation of risk.
a While brand loyalty could not be assessed in the CCHS survey because brands were not reported, only 8% of respondents reported consuming two different table-top sweeteners (e.g. sucralose and cyclamate) and less than 1% of respondents reported consuming more than two table-top sweeteners.
7. Conclusion
Considering all available lines of evidence presented in this screening assessment, there is low risk of harm to the environment from sodium cyclamate and CHA. It is concluded that sodium cyclamate and CHA do not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
Considering all the information presented in this screening assessment, it is concluded that sodium cyclamate and CHA do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
Therefore, it is concluded that sodium cyclamate and CHA do not meet any of the criteria set out in section 64 of CEPA.
References
Arbeláez P, Borrull F, Pocurull E, Marcé RM. 2015. Determination of high-intensity sweeteners in river water and wastewater by solid-phase extraction and liquid chromatography-tandem mass spectrometry. J Chromatogr A. 1393:106-14.
Baines J, DiNovi M. 2010. Cyclamic acid and its salts: Dietary exposure assessment. Pp. 29-56 in JECFA (2010).
Berset JD, Ochsenbein N. 2012. Stability considerations of aspartame in the direct analysis of artificial sweeteners in water samples using high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS). Chemosphere. 88(5):563-9.
Bickel MH, Burkard B, Meier-Strasser E, Van Den Broek-Boot M. 1974. Entero-bacterial formation of cyclohexylamine in rats ingesting cyclamate. Xenobiotica 4(7):425-439.
Bopp BA, Sonders RC, Kesterson JW. 1986. Toxicological aspects of cyclamate and cyclohexylamine. Cril Rev Toxicol. 16(3):213-306.
Brusick D, Cifone M, Young R, Benson S. 1989. Assessment of the genotoxicity of calcium cyclamate and cyclohexylamine. Environ Mol Mutagen. 14(3):188-199.
Buss NE, Renwick AG, Donaldson KM, George CF. 1992. The metabolism of cyclamate to cyclohexylamine and its cardiovascular consequences in human volunteers. Toxicol Appl Pharmacol. 115(2):199-210.
Calorie Control Council. 2013. International regulatory status: Worldwide status of cyclamate. Atlanta (GA): Calorie Control Council.
Canada. 1978a. Food and Drug Regulations, Part E, Cyclamate and Saccharin Sweeteners. Canada Gazette Part II, vol. 112, no. 10, p. 2208.
Canada. 1978b. Food and Drug Regulations, C.R.C., c.870.
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C. 1999, c.33. Canada Gazette Part III, vol. 22, no. 3.
Canada. 2012. Canadian Environmental Protection Act, 1999: Notice with respect to certain substances on the Domestic Substances List [PDF]. Dept. of the Environment, Government of Canada. Canada Gazette, Part I, vol. 146, no. 48, Supplement.
Canadian Chemical Producers Association. 2003. Reducing emissions 2003 emissions inventory and five year projections. Report No.: 12.
Carswell TS, Morrill HL. 1937. Cyclohexylamine and dicyclohexylamine. Ind Eng Chem. 29(11):1247-1251.
ChemCAN. 2003. Level III fugacity model of 24 regions of Canada. Version 6.00. Peterborough (ON): Trent University, Canadian Centre for Environmental Modelling and Chemistry.
ChemSpider [database]. 2015. London (UK): Royal Society of Chemistry.
Codex Alimentarius. 2018. General Standard for Food Additives Online. CODEX STAN 192-1995. Last Revision 2018.
Collings AJ. 1989. Metabolism of cyclamate and its conversion to cyclohexylamine. Diabetes Care. 12(1):50-55.
[ConsExpo Web] Consumer Exposure Web Model. 2018. Bilthoven (NL): Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment].
Creasy DM, Ford GR, Gray TJ. 1990. The morphogenesis of cyclohexylamine-induced testicular atrophy in the rat: in vivo and in vitro studies. Exp Mol Patho. 52(2):155-169.
Dick CE, Schniepp ML, Sonders RC, Wiegand RG. 1974. Cyclamate and cyclohexylamine: lack of effect on the chromosomes of man and rats in vivo. Mutat Res. 26(3):199-203.
[ECCC] Environment and Climate Change Canada. 2016a. Science approach document: ecological risk classification of organic substances. Ottawa (ON): Government of Canada.
[ECCC] Environment and Climate Change Canada. 2016b. Supporting documentation: data used to create substance-specific hazard and exposure profiles and assign risk classifications in the Ecological Risk Classification of organic substances. Gatineau (QC): ECCC. Information in support of the science approach document: ecological risk classification of organic substances. Available from: substances@ec.gc.ca.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. [modified 2017 Mar 12]. Categorization of chemical substances. Ottawa (ON): Government of Canada.
[ECHA] European Chemicals Agency. c2007-2017. Registered substances database; search results for CAS RN 108-91-8 [database]. Helsinki (FI): ECHA. [updated 2018 Aug 15; accessed 2018 Oct 4].
[ECHA] European Chemicals Agency. 2017. ECHA CORAP Substance evaluation conclusion and evaluation report for cyclohexylamine. Substance Evaluation Conclusion document prepared by the Belgian Federal Public Service Health, Food Chain Safety and Environment Risk Management Service. 56 pp.
Edwards QA, Kulikov SM, Garner-O'Neale LD, Metcalfe CD, Sultana T. 2017. Contaminants of emerging concern in surface waters in Barbados, West Indies. Environ Monit Assess. 189(12):636.
Eichelbaum M, Hengstmann JH, Rest HD, Brecht T, Dengler HJ. 1974. Pharmacokinetics, cardiovascular and metabolic actions of cyclohexylamine in man. Arch Toxicol. 31:243-263.
Environment Canada. 2013. DSL Inventory Update data collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain substances on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
[EPI Suite] Estimation Program Interface SuiteTM [estimation model]. c2000-2012. Estimation Program Interface for Microsoft Windows Ver. 4.11. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
[EU] European Union. 2008. Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006. Off J Eur. Union L 353:1–1355.
Ferrando MR, Huchet B. 1968. [Study of possible activity of sodium cyclamate on the rat in the course of three generations]. Bulletin de L’Académie Nationale de Médicine 153:36-41. [in French]
Ficheux AS, Chevillotte G, Wesolek N, Morisset T, Dornic N, Bernard A, Bertho A, Romanet A, Leroy L, Mercat AC, et al. 2016. Consumption of cosmetic products by the French population. Second part: Amount data. Food Chem Toxicol. 90:130-141.
Gan Z, Sun H, Feng B, Wang R, Zhang Y. 2013. Occurrence of seven artificial sweeteners in the aquatic environment and precipitation of Tianjin, China. Water Res. 47(14):4928-37.
Gan Z, Sun H, Yao Y, Zhao Y, Li Y, Zhang Y, Hu H, Wang R. 2014. Distribution of artificial sweeteners in dust and soil in China and their seasonal variations in the environment of Tianjin. Sci Total Environ. 488-489:168-75.
Gaunt IF, Sharratt M, Grasso P, Landsdown AB, Gangolli SD. 1974. Short-term toxicity of cyclohexylamine hydrochloride in the rat. Food Cosmet Toxicol. 12(5-6):609-624.
Gaunt IF, Hardy P, Gangolli SD, Butterworth KR. 1976. Long-term toxicity of cyclohexylamine hydrochloride in the rat. Food Cosmet Toxicolo. 14(4):255-67.
Golberg L, Parekh C, Patti A, Soike K. 1969. Cyclamate degradation in mammals and in vitro. Toxicol Appl Pharmacol. 14:654.
Gomez-Berrada MP, Ficheux AS, Rakotomalala S, Roudot AC, Ferret PJ. 2017. Probabilistic exposure assessment of sun care products. Food Chem Toxicol. 108:314-325.
Hardy J, Gaunt IF, Hooson J, Hendy RJ, Butterworth KR. 1976. Long-term toxicity of cyclohexylamine hydrochloride in mice. Food Cosmet Toxicol. 14(4):269-276.
Health Canada. 1994. Human health risk assessment for priority substances. Ottawa (ON): Health Canada.
Health Canada. 2010. Guidelines for Incidental Additive Submissions – Guideline No.4. Bureau of Chemical Safety, Food Directorate. Ottawa (ON): Health Canada.
Health Canada. 2015. Food consumption table derived from Statistics Canada, Canadian Community Health Survey, Cycle 2.2, Nutrition (2004), Share file. Ottawa.
Health Canada. 2017. Water consumption table derived from Statistics Canada, Canadian Community Health Survey, Cycle 2.2, Nutrition (2004). Share file. Ottawa.
Health Canada. 2018a. Backgrounder document on default values for breast milk and formula intakes. Unpublished report. Ottawa (ON): Government of Canada.
Health Canada. 2018b. Backgrounder document on updated default body surface areas for use in ESRAB assessments. Unpublished report. Ottawa (ON): Government of Canada.
Health Council of the Netherlands. 2001. Committee on Updating of Occupational Exposure Limits. Cyclohexylamine; Health-based Reassessment of Administrative Occupational Exposure Limits. The Hague (NL): Health Council of the Netherlands. 35 pp. No. 2000/15OSH/021.
[HSDB] Hazardous Substances Data Bank [database]. 2005. Cyclohexylamine, CAS RN: 108-91-9. Bethesda (MD): US National Library of Medicine. [revised 2005 Aug 23; accessed 2018 Oct 4]. Search “cyclohexylamine”.
[HSDB] Hazardous Substances Data Bank [database]. 2012. Cyclamate, CAS RN: 100-88-9 (Includes data on Sodium cyclamate, CASRN 139-05-9). [revised 2012 Jan 6; accessed 2018 April 25 and Sept. 18]. Search “cyclamate”.
[IARC] International Agency for Research on Cancer. 1980. Cyclamates (cyclamic acid, sodium cyclamate, calcium cyclamate, cyclohexylamine and dicyclohexylamine), pp. 55-110 in IARC Monographs on the evaluation of carcinogenic risk of chemicals to humans, Volume 22: Some Non-nutritive sweetening agents. Lyon (FR): IARC Working Group on the Evaluation of Carcinogenic Risk of Chemicals to Humans.
[IARC] International Agency for Research on Cancer. 1999. Cyclamates. Pp. 195-222 in IARC Monographs on the evaluation of carcinogenic risks to humans, Volume 73: Some chemicals that cause tumours of the kidney or urinary bladder in rodents and some other substances. Lyon (FR): IARC Working Group on the Evaluation of Carcinogenic Risks to Humans.
[JECFA] Joint FAO/WHO Expert Committee on Food Additives. 1970. Toxicological evaluation of some extraction solvents and certain other substances. Geneva (CH): World Health Organization. FAO Nutrition Meetings Report Series No. 48A; WHO/FOOD ADD/70.39 11 pp.
[JECFA] Joint FAO/WHO Expert Committee on Food Additives. 1982. Evaluation of certain food additives and contaminants. Geneva (CH): World Health Organization. Report No. TRS 683-JECFA 26/27. 52 pp.
[JECFA] Joint FAO/WHO Expert Committee on Food Additives. 2010. Safety evaluation of certain food additives. Prepared by the 71st meeting of the JECFA. WHO Food Additives Series 62. Geneva (CH): World Health Organization. 284 pp.
Juran SA, van Thriel C, Kleinbeck S, Schaper M, Falkenstein M, Iregren A, Johanson G. 2012. Neurobehavioral performance in human volunteers during inhalation exposure to the unpleasant local irritant cyclohexylamine. Neurotoxicology. 33(5):1180-1187.
Lange FT, Scheurer M, Brauch HJ. 2012. Artificial sweeteners-a recently recognized class of emerging environmental contaminants: a review. Anal Bioanal Chem. 403(9):2503-18.
Lawrence JF. 2003. Cyclamates. In: Caballero B, Finglas P, Toldra F, editors. Encyclopedia of Food Sciences and Nutrition, 2nd ed. Cambridge (MA): Academic Press. 1712-1714 pp.
Lomonova GV. 1965. Toxicity of cyclohexylamine and dicyclohexylamine. Fed Proc 24: T96-8 (translation from Gig Trud Prof Zabol 1963; 7(11):51-6). [cited in Health Council of the Netherlands 2001].
Lorke D, Machemer L. 1983. The effect of cyclohexylamine on the embryo following oral administration to rats and mice. Tox Letters 17:137-143.
[MSDS] Material Safety Data Sheet. 2018. SunJel Fume-free Firespace gel fuel canister, pure gel. Salt Lake City (UT): Thatcher Company, Inc. [accessed 2018 Nov 18].
National Research Council. 2007. Cyclohexylamine. In: Acute exposure guideline levels for selected airborne chemicals, Volume 5. Washington (DC): National Academies Press. 92-144 pp.
[Nielsen] AC Nielsen Company of Canada. 2005-2017. Item ranking reports of 2005, 2010, 2014, 2015, and 2017.
OECD QSAR Toolbox. [Read-across tool]. 2014. Version 3.3. Paris (FR): Organisation for Economic Co-operation and Development, Laboratory of Mathematical Chemistry.
Oser BL, Carson S, Cox GE, Vogin EE, Sternberg SS. 1975. Chronic toxicity study of cyclamate: saccharin (10:1) in rats. Toxicology. 4(3):315-30.
Oser BL, Carson S, Cox GE, Vogin EE, Sternberg SS. 1976. Long-term and multigeneration toxicity studies with cyclohexylamine hydrochloride. Toxicology. 6(1):47–65.
Parekh C, Goldberg EK, Goldberg L. 1970. Fate of Sodium Cyclamate-14C in the Rhesus Monkey (M. mulatta). Abstract no. 26 in Abstracts of papers for the ninth annual meeting of The Society of Toxicology, Atlanta, Georgia; March 15-19, 1970. Toxicol App Pharmacol. 10:282.
[PhysProp] Interactive PhysProp Database [database]. c2013. Syracuse (NY): SRC, Inc. [accessed 2018 Oct 4].
Pitkin RM, Reynolds WA, Filer LJ. 1969. Cyclamate and cyclohexylamine: transfer across the hemochorial placenta. Proc Soc Exp Biol Med. 132(3):993-995.
Pitkin RM, Reynolds WA, Filer LJ. 1970. Placental transmission and fetal distribution of cyclamate in early human pregnancy. Am Journal Obstet Gynecol. 108(7):1043-1050.
PubChem [database]. 2004-. Bethesda (MD): US National Library of Medicine, National Center for Biotechnology Information. [accessed 2018 Sep 4].
Renwick AG, Thompson JP, O'Shaughnessy M, Walter EJ. 2004. The metabolism of cyclamate to cyclohexylamine in humans during long-term administration. Toxicol Appl Pharmacol. 196(3):367-80.
Renwick AG, Williams RT. 1972a. The fate of cyclamate in man and other species. Biochem J. 129:869-879.
Renwick AG, Williams RT. 1972b. The metabolites of cyclohexylamine in man and certain animals. Biochem J. 129:857-867.
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment]. 2006. Cosmetics fact sheet: to assess the risks for the consumer: updated version for ConsExpo 4. Bilthoven (NL): RIVM. Report No.: 320104001/2006. [accessed 2018 Oct 4].
Roberts A, Renwick AG, Ford G, Creasy DM, Gaunt I. 1989. The metabolism and testicular toxicity of cyclohexylamine in rats and mice during chronic dietary administration. Toxicol Appl Pharmacol. 98(2):216-29.
Sang Z, Jiang Y, Tsoi YK, Leung KS. 2014. Evaluating the environmental impact of artificial sweeteners: a study of their distributions, photodegradation and toxicities. Water Res. 52:260-74.
[SCCS] Scientific Committee on Consumer Safety. 2015. The SCCS’s notes of guidance for the testing of cosmetic ingredients and their safety evaluation, 9th revision. Revised version of 25 April 2016. Report No. SCCS/1564/15. 151 pp.
[SCF] Scientific Committee on Food. 2000. Revised opinion on cyclamic acid and its sodium and calcium salts. Report No. SCF/CS/EDUL/192 final. 13 March 2000. European Commission Health & Consumer and Hazardous Protection Directorate-General. 8 pp.
Scheurer M, Brauch HJ, Lange FT. 2009. Analysis and occurrence of seven artificial sweeteners in German waste water and surface water and in soil aquifer treatment (SAT). Anal Bioanal Chem. 394(6):1585-94.
Serra-Majem L, Bassas L, García-Glosas R, Ribas L, Inglés C, Casals I, Saavedra P, Renwick AG. 2003. Cyclamate intake and cyclohexylamine excretion are not related to male fertility in humans. Food Additi Contam. 20(12):1097-1104.
Spoelstra J, Schiff SL, Brown SJ. 2013. Artificial sweeteners in a large Canadian river reflect human consumption in the watershed. PLoS One. 8(12):e82706.
Spoelstra J, Senger ND, Schiff SL. 2017. Artificial Sweeteners Reveal Septic System Effluent in Rural Groundwater. J Environ Qual. 46(6):1434-1443.
Statistics Canada 2015. Canadian Community Health Survey (CCHS) 2015 Share File. Ottawa, ON. Canada. Estimates generated using SAS 9.3 [GRID] through SAS EG 5.1.
Takayama S, Renwick AG, Johansson SL, Thorgeirsson UP, Tsutsumi M, Dalgard DW, Sieber SM. 2000. Long-term toxicity and carcinogenicity study of cyclamate in nonhuman primates. Toxicol Sci. 53:33-39.
Thorgeirsson UP, Dalgard DW, Reeves J, Adamson RH. 1994. Tumor incidence in a chemical carcinogenesis study of nonhuman primates. Reg Tox Pharm. 19:130-151.
Tran NH, Hu J, Li J, Ong SL. 2014. Suitability of artificial sweeteners as indicators of raw wastewater contamination in surface water and groundwater. Water Res. 48:443-56.
[USA] United States of America. 1970. Federal Register, 35, 13644, August 27, 1970.
[US EPA] US Environmental Protection Agency. 1988. Integrated Risk Information System (IRIS) Cyclohexylamine; CASRN 108-91-8 [PDF]. Washington (DC): Office of Research and Development.
[US EPA] US Environmental Protection Agency. Chemistry Dashboard [database]. Sodium cyclamate. [updated 2018 Sept 13; accessed Sept 18, 2018].
[US FDA] US Food and Drug Administration. 1980. Cyclamate, Commissioner's Decision. Federal Register, Volume 45, No. 181, FR 61474, September 16, 1980.
[US NTP] United States National Toxicology Program. 2016. Report on Carcinogens, 14th ed. Research Triangle Park (NC): US Department of Health and Human Services, Public Health Service.
van Eyk AD. 2015. The effect of five artificial sweeteners on Caco-2, HT-29 and HEK-293 cells. Drug Chem Toxicol. 38(3):318-327.
Wallace WC, Lethco EJ, Brouwer EA. 1970. The metabolism of cyclamates in rats. J Pharmacol Exp Ther. 175(2):325-330.
Watanabe Y, Bach LT, Van Dinh P, Prudente M, Aguja S, Phay N, Nakata H. 2016. Ubiquitous detection of artificial sweeteners and iodinated X-ray contrast media in aquatic environmental and wastewater treatment plant samples from Vietnam, the Philippines, and Myanmar. Arch Environ Contam Toxicol. 70(4):671-81.
Watrous RM, Schulz HN. 1950. Cyclohexylamine, p-chloronitrobenzene, 2-aminopyridine: toxic effects in industrial use. Ind Med Surg. 19:317-320. [cited in National Research Council 2007].
Wills JH, Serrone DM, Coulston F. 1981. A 7-month study of ingestion of sodium cyclamate by human volunteers. Reg Tox Pharm. 1:163-176.
Yang YY, Zhao JL, Liu YS, Liu WR, Zhang QQ, Yao L, Hu LX, Zhang JN, Jiang YX, Ying GG. 2018. Pharmaceuticals and personal care products (PPCPs) and artificial sweeteners (ASs) in surface and ground waters and their application as indication of wastewater contamination. Sci Total Environ. 616-617:816-823.
Appendices
Appendix A. Summary of health effects and read-across approach for sodium cyclamate and CHA
| Chemical name | Cyclohexylamine hydrochloride (CHA HCl) | Cyclohexylamine (CHA) | Sodium cyclamate |
|---|---|---|---|
| Role | Analogue | Analogue and Target substance | Target substance |
| CAS# | 4998-76-9 | 108-91-8 | 139-05-9 |
| Chemical structure |  |
 |
![C1CCC(CC1)NS(=O)(=O)[O-].[Na+]](/content/dam/eccc/images/pded/sodium-cyclamate-cyclohexylamine/20220223-a1c.jpg) |
| Molecular weight (g/mol) | 135.6 | 99.2 | 201.2 |
| Vapour pressure (Pa at 25°C) | No data | 1430 | 1.70 x 10-8 [modelled] |
| Water solubility (mg/L) | 8.3 x 105 (17) | “miscible” | 1.0 x 106 [modelled] |
| Log Kow (dimensionless) | No data | 3.7 | -1.61 [modelled] |
| Toxico-kinetics and metabolism | No data |
CHA better absorbed from gastrointestinal tract and more widely distributed throughout tissues of body than cyclamate (Bopp et al. 1986; HSDB 2012). CHA metabolized in kidney to form minor metabolites: Cyclohexanone, cyclohexanol, trans-cyclohexane-1,2-diol and N-hydrocyclo-hexylamine, including minor metabolites, excreted in urine (Gaunt et al. 1974; Golberg et al. 1969; IARC 1999). Urine excretion in men ingesting sodium cyclamate for 7 months was 88% cyclamate and 17% CHA (relative mean percentages) (Wills et al. 1981). |
Sodium cyclamate salts dissociates to cyclamate in rats; some of it is absorbed from the gastrointestinal tract of all mammals (Collings 1989). Cyclamate can then be transformed to CHA by microbes in GI tract of all mammals (IARC 1999). Cyclamate and expired air and < 3% recovered CHA, including minor metabolites, excreted in urine (Gaunt et al. 1974; Golberg et al. 1969; IARC 1999). Urine excretion in men ingesting sodium cyclamate for 7 months was 88% cyclamate and 17% CHA (relative mean percentages) (Wills et al. 1981). |
| Repeat dose toxicity (oral) |
NOAEL = 137 mg/kg bw/day; 13-wk rat study in rats based on the testicular effects observed at 274 and 411 mg/kg bw/day (Brune et al. 1978 [unpublished report], as cited in ECHA 2017; Bopp et al. 1986). NOAEL = 546.8 mg/kg bw/day (HTD) based on lack of testicular effects in males at 1 week in 1 to 13-wk rat study (Creasy et al. 1990). |
Read-across from CHA HCl | Read-across from CHA |
| Long-term toxicity (oral) | NOAEL = 82 mg/kg bw/day based on testicular changes (atrophy, tubules with few spermatids, calcium deposits in tubules) at 300 mg/kg bw/day in 2-yr rat study (Gaunt et al. 1976 cited in Bopp et al. 1986). | Read-across from CHA HCl: (Gaunt et al. 1976 cited in Bopp et al. 1986). |
NOAEL = 100 mg/kg bw/day; 24-yr monkey study, based on testicular effects observed at 500 mg/kg bw/day after 23 years (Takayama et al. 2000). NOAEL = 1120 mg/kg bw/day; 2-yr rat, based on bladder carcinomas at 2500 mg/kg bw/day. Bladder carcinomas may have been related to saccharin treatment (US NTP 2016).a |
| Reproductive Toxicity (oral) | NOAEL = 100 mg/kg bw/day based on “growth retardation due to the lower food consumption”, slight reduction of litter size and weaning weight, and significant higher incidence of testicular atrophy in adult males at the LOAEL of 150 mg/kg bw/day; 2-yr multi-generation study in rats (Oser et al. 1976; ECHA 2017). | Read-across from CHA HCl | LOAEL = 1500 mg/kg bw/day (HTD); 3-generation rat study, based on testicular degeneration in F2 and F3 adult males and effects in F3 offspring (decreased pup survival from birth to weaning) (Ferrando and Huchet 1968). |
| Develop-mental Toxicity (oral) | NOAEL = 42 mg/kg bw/day; rat developmental study, based on reduced placental and fetal weights in the presence of decreased maternal body weight gain at 140 mg/kg bw/day (Lorke and Machemer 1983 cited in ECHA 2017). | Read-across from CHA HCl | NOAEL = 250 mg/kg bw/day in rat and rabbit developmental toxicity studies (HTD) (Bein et al. 1967, Fritz. and Hess 1968 and US Food and Drug Research Laboratories cited in JECFA 1970). |
| Genetic toxicity | NR | Negative in vitro and in vivo (chromosomal aberration and dominant lethal studies were mostly negative at oral or intraperitoneal doses of 50 to 150 mg/kg bw in rats, mice and Chinese hamsters [JECFA 1982; Bopp et al. 1986; ECHA 2017]). | Equivocal in vitro; Negative in vivo |
| Carcino-genicity (oral) | NR | No evidence of cancer effects based on available data. | Not classifiable (IARC Group 3) (IARC 1999). |
Abbreviation: NR, Read-across not required for risk characterization; NOAEL, no observed adverse effect level; HTD, highest tested dose; LTD, lowest tested dose; OTD, only tested dose; h, hours, wk, week.
a Rats fed a 10:1 mixture of sodium cyclamate:sodium saccharin in the diet.
Appendix B. Estimates of daily human exposure to sodium cyclamate and CHA in drinking water
Table B-1 summarizes the estimates of daily intake of sodium cyclamate and CHA from drinking water, as well as the parameters used in the estimation. Exposure from other environmental media such as air, dust or soil are expected to be negligible.
| Substance | Route of exposure | 0 to 5 monthsa (formula fed)b | 6 to 11 monthsc | 1 yrd | 2 to 3 yre | 4 to 8 yrf | 9 to 13 yrg | 14 to 18 yrh | Greater than or equal to 19 yearsi |
|---|---|---|---|---|---|---|---|---|---|
| Sodium cyclamate | Drinking Waterj | 0.54 | 0.34 | 0.13 | 0.12 | 0.09 | 0.07 | 0.07 | 0.08 |
| CHA | Drinking Waterk | 1.70 | 1.09 | 0.43 | 0.37 | 0.30 | 0.23 | 0.23 | 0.27 |
a Assumed to weigh 6.3 kg (Health Canada 2015)
b Exclusively for formula-fed infants, assumed to drink 0.826 L of water per day (Health Canada 2018a), where water is used to reconstitute formula. See footnote on drinking water for details.
c Assumed to weigh 9.1 kg (Health Canada 2015) and to drink 0 L of water per day (Health Canada 2017). For formula-fed infants, assumed to drink 0.764 L of water per day (Health Canada 2018a), where water is used to reconstitute formula. See footnote on drinking water for details.
d Assumed to weigh 11.0 kg (Health Canada 2015) and to drink 0.36 L of water per day (Health Canada 2017)
e Assumed to weigh 15 kg (Health Canada 2015) and to drink 0.43 L of water per day (Health Canada 2017).
f Assumed to weigh 23 kg (Health Canada 2015) and to drink 0.53 L of water per day (Health Canada 2017).
g Assumed to weigh 42 kg (Health Canada 2015) and to drink 0.74 L of water per day (Health Canada 2017).
h Assumed to weigh 62 kg (Health Canada 2015) and to drink 1.09 L of water per day (Health Canada 2017).
i Assumed to weigh 74 kg (Health Canada 2015) and to drink 1.53 L of water per day (Health Canada 2017).
j Drinking water intake was estimated using the maximum concentration of sodium cyclamate (4.1 µg/L) measured in groundwater from domestic wells in southern portion of Nottawasaga River watershed in Ontario, Canada (Spoelstra et al. 2017). Sodium cyclamate was measured at the minimum concentration of 0.09 µg/L and median concentration of 0.17 µg/L (estimated from figures) with a detection frequency of 8% from 59 samples.
k No monitoring data of drinking water in Canada were identified. The predicted environmental concentration (PEC) of 13 µg/L was calculated using in-house environmental modelling tool based on the total reported import quantity of CHA (Environment Canada 2013) and assuming wastewater treatment system removal of 83.13% from ECCC ERC report and daily release to wastewater of 4% (3% as residue containers, 1% as transfer line/process vessel residues). This PEC was selected for deriving daily intake estimate from drinking water (Health Canada, NSACB, Environmental Assessment Unit Workbook, In house model unpublished).
Appendix C. Additional details pertaining to the estimation of daily human exposure to cyclamic acid from the consumption of table-top sweeteners containing sodium cyclamate
Food consumption data from the Canadian Community Health Survey (CCHS) (Statistics Canada 2015) were employed in the exposure assessment. This survey distinguishes between five different table-top sweeteners: aspartame, sucralose, saccharin, steviol glycosides and cyclamate. Consumption estimates were generated based on "Sweetener, cyclamate" specifically (food code 403802). As described in Section 6.1.1, the quantity of sodium cyclamate-containing table-top sweetener consumed was converted to a quantity of sodium cyclamate consumed based on its presence therein at 32% by mass, and in turn converted to a quantity of cyclamic acid using the relative molecular weights of sodium cyclamate and cyclamic acid (Equation 1).
Cyclamic acid = sweetener (g) × 32% × 1000 mg/g × (179.234/201.216) ÷ body weight (kg)
Equation 1: Calculation of exposure to cyclamic acid (mg/kg bw per day) based on consumption of sodium cyclamate-based table-top sweetener (g/day)
None of the approximately six thousand respondents below age 19 reported consumption of sodium cyclamate, thus exposure estimates were only calculated for adults age 19 and over, among whom approximately 2% reported consumption of table-top sweeteners containing sodium cyclamate. As inclusion of the non-consumers, representing more than 98% of adult respondents, would greatly dilute exposure estimates, they were not considered in the exposure assessment.
Single-day consumption estimates from a 24-hour dietary recall survey provide insight about exposure on the specific survey day and are often considered appropriate for use in drawing conclusions about the health significance of this exposure. However, some assessments require that an adjustment procedure be carried out in order to generate estimates more reflective of typical, long-term dietary exposure. Approximately one third of respondents in the CCHS survey were surveyed on a second, non-consecutive day in order to provide a means to measure day-to-day variability in consumption, which is required to calculate usual intakes.
There are two sources of variation in the survey data: 'within-person' variation, the difference in what a given person eats on one day versus another day, and 'between-person' variation, the difference in what one person eats versus what another person eats. The latter is desirable to retain; for instance, it is needed to examine the exposure of high consumers, that is, the 90th percentile of exposure in the present assessment. In order to estimate usual intakes over a longer period of time, it is necessary to remove the within-person variation while preserving the between-person variation.
Usual intakes for cyclamic acid were generated using the US National Cancer Institute (NCI) adjustment method implemented through the SAS programming language (Statistical Analysis System, Version 9.3 accessed through SAS Enterprise Guide 5.1, SAS Institute). This statistical method for determining usual intakes can be used for both ubiquitously and episodically consumed foods.
Mean and 90th percentile eaters-only, estimates of usual dietary exposure to sodium cyclamate (as cyclamic acid) were generated both for the "general population" (including those with diabetes), and for individuals with diabetes as a subpopulation hypothesized to consume higher quantities of table-top sweeteners. These estimates are presented in Table C-1 along with consumption rates of sodium cyclamate-based table-top sweeteners. Available market share data suggest that sodium cyclamate-containing sweeteners' share of the table-top sweetener market has declined significantly over the past decade (Nielsen 2005–2017).
| Estimates | Age group | Mean (male) | Mean (female) | 90th Percentile (male) | 90th Percentile (female) |
|---|---|---|---|---|---|
| Sweetenerb consumption (g/day) | 19+ years (general) | 2.14 | 2.10 | 4.07 | 3.81 |
| Sweetenerb consumption (g/day) | 19+ years (diabetes) | 2.06 | 2.06 | 3.88 | 3.81 |
| Sweetener consumption(packets/day)c | 19+ years (general) | 2–3 | 2–3 | 5 | 4–5 |
| Sweetener consumption(packets/day)c | 19+ years (diabetes) | 2–3 | 2–3 | 4–5 | 4–5 |
| Exposure to cyclamic acid (mg/kg bw per day)d | 19+ years (general) | 6.53 | 8.00 | 12.63 | 14.98 |
| Exposure to cyclamic acid (mg/kg bw per day)d | 19+ years (diabetes) | 6.28 | 7.67 | 12.15 | 14.57 |
a The assumed cyclamate content of 32% is based on the average of three brands of cyclamate sweeteners containing 30-34% cyclamate.
b 'Sweetener' here refers to the total mass of the sweetener product, including the assumed 32% sodium cyclamate as well as the mass of carrier that comprises the remainder.
c Number of sweetener packets that consumption represents is included for ease of conceptualization of the quantity. Packets are assumed to weigh 0.8 grams each, which was found to be typical among the available products.
d Body weights are self-reported values from the survey respondents, thus varied depending on individuals.
Appendix D. Parameters used to estimate human exposure to sodium cyclamate and CHA from use of products available to consumers
Exposure from the use of products available to consumers was estimated using the in-house algorithms (for oral, buccal, and dermal routes) or ConsExpo Web (2018) (for inhalation route). Exposure estimates were calculated based on default body weights of 74 kg, 62 kg, 42 kg, 23 kg, 15 kg, 11 kg, 9.1 kg, and 6.3 kg for adults (19 years and older), teens (14 to 18 years old), children (9 to 13 years), younger children (4 to 8 years old), toddlers aged 2 to 3 years, toddlers aged 1 year, infants aged 6 to 11 months, and infants aged 0 to 5 months, respectively (Health Canada 2015). Dermal absorption of 100% was assumed in the absence of dermal absorption data. The parameters used in the estimation of exposure from the use of natural health products and drugs are described in Table D-1.
| Product scenario | Model parameters and assumptionsa |
|---|---|
|
Non-medicinal ingredient for the purpose of sweetening |
Quantity per dosage unit: 70 mgb Dosage unit: 5 mL (1 tsp)b Frequency of use: 3 times dailyb Age group: adults (19+ yrs)b |
|
Non-medicinal ingredient for the purpose of sweetening |
Concentration: 1.5%b Dose amount: 1 mLb Frequency of use: 1 time dailyb Age group: 1 to 18 yrsb |
|
Mouthwash |
Concentration: 0.5%c Ingested amount of product per application: 1700 mg/use for adults and teens, and 1000 mg/use for children (9-13 yrs) (based on mean product amount from Ficheux et al. [2016] and ingestion factor of 10% from SCCS [2015]) Frequency of use: 5 times daily for adults, teens, and children (based on professional judgement and product description)c Age group: 9 to 19+ yrsc |
|
Chest congestion relief syrup |
Concentration: 1.5%c Dose amount: 10 mL (product labels) Frequency of use: 4 times daily (for up to 7 days based on similar product labels) Age group: 12 to 19+ yrs (product labels) |
|
Buccal anesthetic solution |
Mouthwash exposure scenario (buccal) was assumed, except where otherwise indicated, based on the product description and general use information Concentration: 2%c Frequency of use: 8 times/day for adultsc and 6 times/day for teens, children, and toddlers (2-18 yrs) (based on similar product) Dose amount: Adults and teens: 15 mL (4.5 mg/kg bw or maximum of 300 mg per single dose)c Children (4-13 yrs) and toddlers (2-3 yrs): 1 mL per 5 kg bw (4 mg/kg bw; based on similar product) Ingestion rate (IR): Adults, teens, and children: 10% of dose amount (assuming mouthwash buccal exposure scenario; SCCS [2015]) Toddlers (2-3 yrs): 30% of dose amount (assuming application with a cotton tip based on similar product; IR estimated from % of cosmetic product transferred onto the skin from wipes; Ficheux et al. [2016]) Age group: 2 to 19+ yrs (similar product labels) |
|
Topical anesthetic solution for skin pain relief |
After-sun milk dermal exposure scenario was assumed based on product description and general uses (temporary relief of pain associated with minor burns, sunburns, minor cuts, scrapes, insect bites or minor skin irritations)c Concentration: 2%c Surface area: half the surface of forearms assuming sunburns on small area (adjusted from Health Canada [2018b]; based on product label ‘do not use in large quantities’) Adults (19+ yrs): 637.5 cm2 Teens (14-18 yrs): 622.5 cm2 Children (9-13 yrs): 475 cm2 Younger children (3-8 yrs): 312.5 cm2 Amount of product used per day per cm2 (Gomez-Berrada et al. 2017) Adults and teens: 0.64 mg/day/cm2 Children (10-14 yrs): 1.05 mg/day/cm2 Younger children (3-9 yrs): 1.41 mg/day/cm2 |
|
Respirator solution to treat bronchospasm |
Ingredient quantity per dose: 3 mg/dosec Exposure model, Exposure to vapour - Instantaneous release (RIVM 2006) Frequency of use: 4 times dailyc Age group: 5 to 19+ yrsc Exposure duration: 10 minute for each treatmentc Room volume: 1 m³ (formulation is administered via a respirator or nebulizer) Ventilation rate: 2 per hour Inhalation rate: 15.1 m³/day for adults, 15.9 m³/day for teens, 13.9 m³/day for children (9-13 yrs), and 11.1 m³/day for children (4-8 yrs) Absorption fraction: 100% |
Abbreviation: yrs, years
a Unless specified, a retention factor of 1 was used.
b Personal communication, e-mails from NNHPD, HC to ESRAB, HC, dated May 3, May 14, July 27, Aug 8, and Sept 18, 2018; unreferenced.
c Personal communication, e-mails from TPD, HC to ESRAB, HC, dated May 3, July 27, Aug 8, and Sept 17, 2018; unreferenced.
The parameters used in the estimate of inhalation and dermal exposure from use of products available to consumers are described in Table D-2. Exposures were estimated using ConsExpo Web (2018) or other algorithms (see below for more details).
| Product | Assumptionsa |
|---|---|
|
Hair styling product – aerosol hairspray |
Concentration of CHA: 0.3%c Frequency of use: Inhalation: Dermal: |
|
Firespace gel fuel canister |
Concentration of CHA: 0.3%d Inhalation: Limit concentration to saturated air concentration: yes |
Abbreviation: yrs, years
a Unless otherwise stated, the retention factor is assumed to be 1.
b Total surface area of half head.
c Personal communication, e-mail from CPSD, HC, to ESRAB, HC, dated December 14, 2016; unreferenced.
d MSDS (2018).
Appendix E. Sodium cyclamate acceptable daily intake (ADI)
Canada enacted regulations on cyclamate sweeteners (Canada 1978a), including sodium cyclamate, based on an internal review of cyclamic acid and its salts (see Canada 1978b for updated regulations on cyclamate sweeteners). Health Canada established an acceptable daily intake (ADI) of 11 mg/kg bw/day, expressed as cyclamic acid (personal communication, e-mail from FD, HC to ESRAB, HC, dated Oct. 24, 2018; unreferenced).
The ADI for sodium cyclamate was based on the 13-week study in which rats were administered CHA and using the NOAEL for testicular effects (Brune et al. 1978). The conversion to a cyclamic acid basis (due to the fact that sodium cyclamate dissociates to the cyclamic acid anion and the sodium cation in the gastrointestinal tract) was calculated based on the relative molecular weights (MW) of cyclamic acid and CHA, and other factors. JECFA (1982) calculated an ADI = 11 mg//kg bw/day based on [100 mg/kg bw/day X 2 (equal to the molecular weight [MW] of cyclamic acid ÷ MW of CHA) = 2 when rounded up to the integer value] ÷ [0.63 (as in 63% of cyclamate is available to be converted to CHA) ÷ 0.3 (the rate of conversion of cyclamate to CHA)] = 1058 mg/kg bw/day and applying an uncertainty factor (UF) of a 100. From their interpretation of data, JECFA assumed that 18.9% of cyclamate is converted to CHA by gut bacteria. This is conservative as most studies have shown a conversion rate of < 10% in animal and human models. Note that Health Canada calculated the same ADI value based on data available in 1976 (personal communication, e-mails from FD, HC to ESRAB, HC, dated Oct. 18 and 24, 2018; unreferenced).
SCF (2000) calculated an ADI = 7 mg/kg bw/day based on the following formula: (NOAEL X MW) ÷ (UF X conversion rate) = (100 mg/kg bw/day X 2) ÷ (32 X 0.85) = 7.35 mg/kg bw/day. SCF (2000) increased the 18.9% assumed conversion rate used by JECFA (1982) and replaced it with an overall value of 85% absorption and conversion rate. By accounting for inter-individual variability with a high conversion/absorption rate, the SCF (2000) then applied a smaller uncertainty factor, reducing it from 100 to 32.
