Risk of transmission of HIV viral load suppression

 Download this article as a PDF
Download this article as a PDFPublished by: The Public Health Agency of Canada
Issue: CCDR: Volume 49-11/12, November/December 2023: HIV and Other Sexually Transmitted and Blood-Borne Infections
Date published: November/December 2023
ISSN: 1481-8531
Submit a manuscript
About CCDR
Browse
Volume 49-11/12, November/December 2023: HIV and Other Sexually Transmitted and Blood-Borne Infections
Rapid Communication
Risk of sexual transmission of HIV in the context of viral load suppression
Pascal Djiadeu1, Housne Begum1, Stacy Sabourin1, Stephan Gadient1, Chris Archibald1, Marc-André LeBlanc1, Andrea Chittle1, Annie Fleurant1, Joseph Cox1
Affiliation
1 Centre for Communicable Diseases and Infection Control, Public Health Agency of Canada, Ottawa, ON
Correspondence
Suggested citation
Djiadeu P, Begum H, Sabourin S, Gadient S, Archibald C, LeBlanc M-A, Chittle A, Fleurant A, Cox J. Risk of sexual transmission of HIV in the context of viral load suppression. Can Commun Dis Rep 2023;49(11/12):457–64. https://doi.org/10.14745/ccdr.v49i1112a01
Keywords: HIV, viral load, sexual transmission, serodiscordance, viral suppression, gbMSM
Abstract
Background: In 2018, the Public Health Agency of Canada (PHAC) published a systematic review to calculate the risk of sexual transmission of human immunodeficiency virus (HIV) in the context of antiretroviral therapy (ART). In 2022, PHAC commissioned the Canadian Agency for Drugs and Technologies in Health (CADTH) to conduct a rapid review of evidence published since 2017. We undertook a meta-analysis of relevant studies from these two reviews.
Methods: Studies from the rapid review that adequately assessed exposure (HIV viral load) and outcome (HIV seroconversion) were included and assessed for risk of bias (RoB) and certainty of evidence. Results were pooled to estimate the risk of HIV transmission per 100 person-years.
Results: Three studies from the rapid review were eligible for inclusion and one was excluded after RoB assessment. In the remaining studies examining risk among people living with HIV who take ART and maintain a suppressed viral load (fewer than 200 copies/mL, measured every 4–6 months), no sexual transmissions of HIV were observed. The pooled incidence estimate based on these studies, and one from the 2018 PHAC review, was zero transmissions/100 person-years (95% CI: 0.00–0.10). No studies in the rapid review provided data on the risk of sexual transmission of HIV in situations of varying levels of viral load.
Conclusion: This update highlights the consistency of evidence since the 2018 PHAC review. There remains no evidence of HIV transmission to sexual partners when a person living with HIV is on ART and maintains a suppressed viral load.
Introduction
Human immunodeficiency virus (HIV) is a retrovirus that progressively destroys CD 4+ lymphocytes, which are crucial to immune system functioning. If not treated, HIV can progress to acquired immunodeficiency syndrome (AIDS). Human immunodeficiency virus can be transmitted through exposure to blood, semen, vaginal fluid, rectal fluid and human milkFootnote 1Footnote 2. In Canada, the annual number of new diagnosed cases of HIV infection has remained relatively stable since 2012, with 1,472 cases reported in 2021Footnote 3Footnote 4. As of 2020, an estimated 90% of persons living with HIV in Canada had been diagnosed and were aware of their infection. Of those diagnosed, 87% were estimated to be on treatment, and an estimated 95% of persons on treatment had a suppressed viral load of fewer than 200 copies/mL Footnote 4. Viral load is the measure of the amount of HIV ribonucleic acid circulating in the blood. In 2020, it was estimated that 77% of new HIV infections occurred through sexual transmission Footnote 4. Among people living with HIV, higher viral load levels are associated with increased risk of sexual transmission of HIVFootnote 5Footnote 6Footnote 7Footnote 8.
In 2018, the Public Health Agency of Canada (PHAC) published a systematic review to calculate the risk of sexual transmission of HIV Footnote 9. The 2018 PHAC review found that the overall risk of sexual transmission of HIV when the partner living with HIV was taking antiretroviral therapy (ART) with varying levels of viral load was 0.22 transmissions per 100 person-years (PY) (pooled 95% confidence interval [CI]: 0.14–0.33), across heterosexual and gay, bisexual and other men who have sex with men (gbMSM) serodiscordant couples. Furthermore, the review determined that the overall risk when a person living with HIV was taking ART and had a suppressed viral load (defined as fewer than 200 copies/mL measured every 4–6 months) was zero transmissions per 100 PY (pooled 95% CI: 0.00–0.28).
In 2022, PHAC commissioned the Canadian Agency for Drugs and Technologies in Health (CADTH) to carry out a rapid review of new evidence published since the 2018 PHAC review. The CADTH rapid review focussed on the risk of sexual transmission of HIV when a person living with HIV is taking ART (with varying levels of viral load) or is taking ART and has a suppressed viral load Footnote 10.
The CADTH rapid review identified 15 studies published between 2017 and 2022 that were relevant to the research questions, including one systematic review and 14 non-randomized studies Footnote 10. The rapid review did not evaluate the certainty of the evidence of each study, but rather described their strengths and limitations narratively. This rapid communication includes further analyses of studies included in the CADTH rapid review and provides an updated risk of sexual transmission of HIV when a person living with HIV is taking ART.
Methods
Relevant studies from the CADTH rapid review were first identified based on the use of valid measures of exposure (viral load testing) and outcome (phylogenetic linkage of observed seroconversions to the partner living with HIV). Included studies were further evaluated for risk of bias (RoB) and certainty of evidence using the Quality in Prognosis Studies instrument and Grading of Recommendations Assessment, Development, and Evaluation (GRADE) criteria, respectivelyFootnote 11Footnote 12. Results from retained studies were pooled using a random-effects model to calculate pooled estimates of the risk of HIV transmission per 100 PY with 95% CIs. Analyses were done using R studio with the meta package: Meta-Analysis Package (v2.4-0)Footnote 13Footnote 14.
As in the 2018 PHAC review, HIV transmission risk was characterized using criteria defined by the Canadian AIDS Society (Appendix, Table A1) Footnote 15.
Results
Risk of bias and certainty of evidence of studies included in the CADTH rapid review
Regarding the risk of sexual transmission of HIV when a person living with HIV takes ART (with varying levels of viral load), only two studies were of potential relevance (Appendix, Table A2)Footnote 16Footnote 17.
The article by Nyombayire et al., Footnote 16 had methodologic limitations, including a high RoB (Appendix, Table A3) and a very low certainty of evidence (Appendix, Table A4). The article by Bavinton et al., Footnote 17 found no phylogenetically linked HIV transmissions when the partner living with HIV had varying levels of viral load and the partner did not use HIV pre-exposure prophylaxis (PrEP), but the article had only 5.8 PY of relevant follow-up. The certainty of evidence in this article was evaluated as very low (Appendix, Table A5). The RoB was high due to the lack of information on those who chose not to participate in the study, limited viral load reporting, no validation of ART adherence and considerable loss to follow up. In addition, not all reported transmissions were phylogenetically linked to the partner living with HIV. Given the above stated limitations, neither article was considered to add meaningful information to the 2018 PHAC review conclusions for this question.
Regarding the risk of sexual transmission of HIV when a person living with HIV takes ART and has a suppressed viral load (fewer than 200 copies/mL measured every 4–6 months), the CADTH rapid review found two observational studies among gbMSM (Table A2) that met the inclusion criteria, both of which were follow-up studies to work previously included in the 2018 PHAC reviewFootnote 17Footnote 18. The RoB was evaluated as moderate for the article by Bavinton et al., Footnote 17 and low for Rodger et al., Footnote 18 (Table A3), while the certainty of evidence on this question for both studies was evaluated as high (Table A5).
Public Health Agency of Canada analysis and pooled risk of sexual transmission of eligible studies
Two studies provided additional evidence regarding the risk of sexual transmission of HIV for gbMSM couples when the person living with HIV has a suppressed viral load. In these studies, no sexual transmissions of HIV that were phylogenetically linked were reportedFootnote 17Footnote 18. The estimated incidence was zero transmissions/100 PY (95% CI: 0.00–0.23) for the article by Rodger et al., Footnote 18 and zero transmissions/100 PY (95% CI: 0.00–1.59) for the article by Bavinton et al., Footnote 17. Data from these studies were pooled to estimate an incidence of zero transmissions/100 PY (95% CI: 0.00–0.11) (Appendix, Figure A1).
The 2018 PHAC review included only one article Footnote 19 that provided data on the risk of HIV transmission for heterosexual couples where the partner living with HIV has a suppressed viral load. The estimated incidence was zero transmissions/100 PY (95% CI: 0.00–0.46) Footnote 9Footnote 19. No articles in the CADTH rapid review provided additional data for this population.
To update the 2018 PHAC review results for a combined (heterosexual and gbMSM) estimate of the risk of sexual transmission when a person living with HIV has a suppressed viral load, we pooled the results of Bavinton et al., Footnote 17 and Rodger et al., Footnote 18Footnote 19. This resulted in an incidence estimate of zero transmissions/100 PY (95% CI: 0.00–0.10) (Figure A1). With additional data, there is more precision around the estimated incidence, so that the 95% CI of 0.00 to 0.28 documented in the 2018 PHAC review Footnote 9 is now 0.00 to 0.10.
Discussion
The 2023 PHAC analysis of relevant studies from the CADTH rapid review did not provide any new evidence to alter the conclusions from the 2018 PHAC review related to the risk of sexual transmission of HIV when a person living with HIV takes ART (with varying levels of viral load). Therefore, the risk of HIV transmission in this situation remains categorized as low, as per Canadian AIDS Society guidelines (Table A1). Future work is needed to determine more precise transmission risk estimates for situations involving varying levels of viral load.
Regarding the risk of sexual transmission of HIV when a person living with HIV takes ART and has a suppressed viral load of fewer than 200 copies/mL measured every 4–6 months, the CADTH rapid review found two updated studies among gbMSM. These studies, in addition to a single study on heterosexual couples, identified in the 2018 PHAC review, allowed an update of the meta-analysis from the 2018 PHAC review, resulting in more precision for the estimated risk of sexual transmission (zero transmissions/100 PY; 95% CI: 0.00–0.10). This updated review offers additional support to the conclusions of the 2018 PHAC review, further documenting no confirmed cases of sexual HIV transmission when a person living with HIV maintains a suppressed viral load. The risk of HIV transmission in this situation remains categorized as negligible, as per Canadian AIDS Society guidelines (Table A1). Communicating this message has the potential to reduce HIV-associated stigma and support increased engagement across the HIV care continuum, with benefits for individuals and communities.
Conclusion
This meta-analysis of updated articles derived a more precise estimate of the risk of sexual transmission of HIV when a person living with HIV is taking ART and maintains a suppressed viral load (fewer than 200 copies/mL, measured every 4–6 months). With five years of additional data, the conclusion of the 2018 PHAC review is strengthened. There remains no evidence of HIV transmission to sexual partners when a person living with HIV is on ART and maintains a suppressed viral load.
Appendix
| Category | Description | Criteria for determining level of risk | |||||
|---|---|---|---|---|---|---|---|
| No risk | None of the practices in this category have ever been demonstrated to lead to HIV infection. There is no potential for transmission since all the basic conditions for viral transmission are not present. | Potential for transmission | None | ||||
| Evidence of transmission | None | ||||||
| Negligible risk | All the practices assigned to this risk level present a potential for HIV transmission because they involve an exchange of bodily fluids (semen, pre-ejaculate, rectal fluid, vaginal fluid, blood, or breast milk). However, the amounts, conditions and media of exchange are such that the efficiency of HIV transmission appears to be greatly diminished. There are no confirmed reports of infection from these activities. | Potential for transmission | Yes | ||||
| Evidence of transmission | None | ||||||
| Low risk | All of the practices assigned this risk level present a potential for HIV transmission because they involve an exchange of bodily fluids (semen, pre-ejaculate, rectal fluid, vaginal fluid, blood, or breast milk). There are also a few reports of infection attributed to these activities (usually through individual case studies or anecdotal reports, and usually under certain identifiable conditions). | Potential for transmission | Yes | ||||
| Evidence of transmission | Yes (under certain conditions) |
||||||
| High risk | All of the practices assigned this risk level present a potential for HIV transmission because they involve an exchange of bodily fluids (semen, pre-ejaculate, rectal fluid, vaginal fluid, blood, or breast milk). In addition, a significant number of scientific studies have repeatedly associated the activities with HIV infection. Even when the exact mechanism of transmission is not completely clear, the results of such studies conclude that activities in this category are high risk. | Potential for transmission | Yes | ||||
| Evidence of transmission | Yes | ||||||
| Study, year, country | Study design, setting and period | Population characteristics | Exposure and comparator | Clinical outcome | |||
|---|---|---|---|---|---|---|---|
Bavinton et al., 2018 Footnote 17 Australia, Brazil, Thailand |
Prospective cohort study Setting: 13 Australian clinics; 1 Brazilian clinic; 1 Thai clinic (no other information reported) Study period: May 2012–March 2016 |
HIV serodiscordant male same-sex couples/sex partners Number of participant couples, n=343 Baseline characteristics (sex partner LWH): Age, median (IQR), years 34.4 (27,7, 43.9) Sex with outside partner(s), n (%): Any=136 (40%) CLAI=59 (17%) Viral load (measure NR) in the sex partner LWH, copies/mL, n (%): <200=267 (78%) ≥200=76 (22%) Daily PrEP use by the HIV-negative partner in the past 3 months, n (%): 26 (8%) ART use at baseline in sex partner LWH, n (%): 274 (80%) ≥90% adherence to ART in the past 3 months at baseline (among 274 sex partners LWH and taking ART), n (%): 241 (88%) Condom use/CLAI in the past 3 months, n (%): Always condoms/no CLAI=156 (45%) Some condoms/CLAI=126 (37%) Always CLAI=61 (18%) Any STI, n (%): Sex partner LWH=46 (13%) HIV-negative partner=39 (11%) |
Exposure: Sex partners LWH were virally suppressed (most of whom were using ART) ART regimens: NR ART use in sex partner LWH during the follow-up, n (%): Never=6 (2%) Initiated during follow-up=85 (25%) Always=252 (73%) Viral load in sex partner LWH during the follow-up, n (%): Consistently <200 copies/mL=258 (75%) Variably >/<200 copies/mL=78 (23%) Consistently ≥200 copies/mL=7 (2%) Daily PrEP use by the HIV-negative partner anytime during the follow-up, n (%): 115 (34%) Comparator: None |
Outcomes: Primary HIV seroconversion in the HIV-negative partner with viral load monitoring and phylogenetic linkage demonstrated Follow-up: At least 2 clinic visits per year Viral load monitoring was every 3–6 months Total couple years of follow-up=588.4 232 person-year (with suppressed viral load and no PrEP) 5.8 person-year (with varying viral load and no PrEP) Median follow-up/couple (IQR)=1.7 (0.9, 2.2) |
|||
Rodger et al., 2019 Footnote 18 PARTNER2 UK (14 European countries) |
Single arm prospective cohort study Setting: 75 sites across 14 European countries Study period: 2010–2017 |
Gay male HIV-serodiscordant couples Inclusion criteria: both partners were ≥18 years of age, had penetrative sex with or without condoms in the month prior to enrolment, expected to have sex together again after enrolment, consent of both partners obtained Exclusion criteria (for analysis): HIV negative partner using HIV PEP or PrEP, reported no condomless sex, viral load of the sex partner LWH >200 copies/mL, absence of viral load data, absence of HIV testing in the HIV negative partner Number of participants, n=782 couples (340 of whom were from PARTNER1) Footnote 19 Age, median (IQR), years: sex partner LWH=40.0 (33.3, 46.1) HIV negative partner=37.6 (30.9, 45.3) CD 4 cell count in the sex partner LWH, n (%): >350 cells/µL, n=730 (93%) ≤350 cells/µL, n=51 (7%) Number of participants with STIs, n (%): Sex partner LWH=214 (27%) HIV negative partner=185 (24%) |
Exposure: Sex partner LWH takes suppressive ART and has viral load <200 copies/mL ART regimen: NR ART in sex partner LWH: Years on ART, median (IQR)=4.3 (1.8, 9.3) Self-reported ART adherence, n (%): ≥90%=739 (98%) Viral load in sex partner LWH at baseline: Undetectable viral load (<50 copies/mL), n (%): 754 (97%) Measured viral load: <200 copies/mL, n (%): 774 (99%) Condom use: NR, only condomless acts were included in the analysis Use of HIV PrEP in HIV-negative partner: data for participants exposed to PrEP were removed from the analyses Comparator: None |
Outcomes: Rate of phylogenetically linked HIV infections. (number of linked HIV infections/couple years of follow-up) Follow-up: 1,593 couple years Median follow-up/couple=2 years (IQR 1.1, 3.5 years) HIV negative partner: HIV testing baseline and every 6–12 months Sex partner LWH: Viral load tested baseline and every 6–12 months |
|||
Nyombayire et al., 2021 Footnote 16 Rwanda |
Prospective cohort Setting: Government clinics in Kigali Study period: 2010–2014 |
Heterosexual HIV-serodiscordant couples/sex partners Number of couples recruited n=3,777 Baseline characteristics: Number of couples with male sex partners LWH (M+/F-) n=1,947 Number of couples with female sex partners LWH (M-/F+) n=1,830 Age by sex overall, mean (SD), years: Male=35.3 (9.3) Female=29.6 (8.7) CD 4 of sex partners LWH mean (SD), (units NR)Table A2 footnote b M+/F-=472.5 (234.6) M-/F+=525.4 (269.7) Couples with current ART use in sex partner LWH at baseline, n (%): 1,684 (44.6) M+/F- couples with no contraceptive/condom use, n (%): 640 (80.7%) M-/F+ couples with no contraceptive/condom use, n (%): 570 (76.8%) |
Exposure: Sex partner LWH receiving ART ART regimen: NR ART adherence: NR Viral load in sex partner LWH across follow-up: NR Duration of ART in sex partners LWH at baseline, mean (SD) years 3.1 (2.3) Use of HIV PrEP in HIV-negative partner: NR Comparator: Sex partner LWH not receiving ART |
Outcomes: HIV seroconversion in the HIV-negative partner; virological linkage analysis (for most but not all couples with seroconversion in the HIV-negative partner) Follow-up: Quarterly clinic visits for HIV-negative partners Median (SD) follow-up, years=1.4 (1.2) |
|||
| Authors | Study participation | Study attrition | Prognostic factor measurement | Outcome measurement | Study confounding | Statistical analysis and reporting | Overall risk of bias |
|---|---|---|---|---|---|---|---|
| Bavinton et al., 2018, Footnote 17 | Table A3 footnote b | Table A3 footnote c | Table A3 footnote b | Table A3 footnote b | Table A3 footnote c | Table A3 footnote b | Table A3 footnote c |
| Rodger et al., 2019, Footnote 18 | Table A3 footnote b | Table A3 footnote b | Table A3 footnote b | Table A3 footnote b | Table A3 footnote c | Table A3 footnote b | Table A3 footnote b |
| Rodger et al., 2016, Footnote 19 | Table A3 footnote b | Table A3 footnote b | Table A3 footnote b | Table A3 footnote b | Table A3 footnote c | Table A3 footnote b | Table A3 footnote b |
| Nyombayire et al., 2021, Footnote 16 | Table A3 footnote b | Table A3 footnote d | Table A3 footnote b | Table A3 footnote d | Table A3 footnote d | Table A3 footnote b | Table A3 footnote d |
| Certainty assessment | Number of couples/person-years | Certainty of Evidence (GRADE) |
Number of HIV transmission per 100 person-years (95% CI) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | |||
| Outcomes: HIV incidence for unspecified sex acts (per person-years) | |||||||||
| Question 1: HIV incidence on ARTTable A4 footnote c | |||||||||
| 1Table A4 footnote d Cohort studies Footnote 16 |
Observational studies (cohort and cross-sectional) |
Very seriousTable A4 footnote d | Very seriousTable A4 footnote e | SeriousTable A4 footnote f | SeriousTable A4 footnote g | Undetected | 3,777/2,867.4 | Viral load of the Partner LWH was not reported Use of ART by Partner LWH was self-reported, and levels of adherence could not otherwise be validated Very high loss to follow up (i.e. 35%) Study power was not addressed Very low certainty of evidence (◯◯◯◯Table A4 footnote eTable A4 footnote fTable A4 footnote g) Excluded |
0.63 (0.38–1.00) |
| Certainty assessment | Number of couples/person-years | Certainty of Evidence (GRADE) |
Number of HIV transmission per 100 person-years (95% CI) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | |||
| Outcomes: HIV incidence for unspecified sex acts (per person-years) | |||||||||
| Question 1: HIV incidence on ARTTable A5 footnote c | |||||||||
| 1Table A5 footnote d Cohort study Footnote 17 |
Cohort | Not serious | Not serious | Not serious | Very seriousTable A5 footnote d | Undetected | NR/5.8 | ◯◯◯◯Table A5 footnote e Very low (Excluded) |
0.00 (0.00–63.32) |
| Question 2: HIV incidence on ART + viral load suppression + no condom useTable A5 footnote f | |||||||||
| 2Table A5 footnote g Cohort studiesFootnote 17Footnote 18 |
Cohort | Not seriousTable A5 footnote g | Not serious | Not serious | SeriousTable A5 footnote h | Undetected | 1,125/1,825.2 | ⨁⨁⨁⨁Table A5 footnote eTable A5 footnote i High |
0.00 (0.00–0.11) |
Figure A1: Pooled estimate of the risk of HIV transmission per 100 person-years among gbMSM and heterosexual serodiscordant couplesFootnote aFootnote b
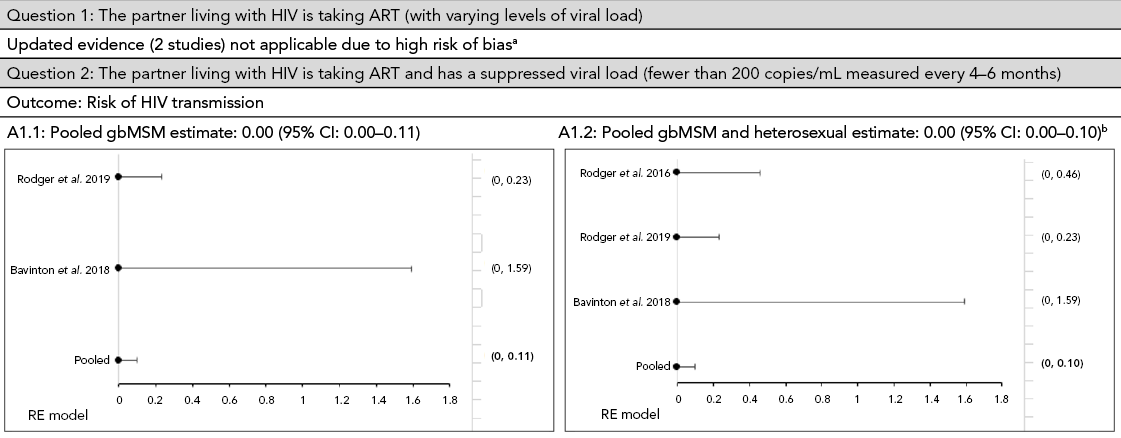
Figure 1 - Text description
This figure shows pooled incidence estimates, using a random-effect model, of the risk of HIV transmission per 100 person-years among gbMSM and heterosexual serodiscordant couples.
For question 1: "The partner living with HIV is taking ART (with varying levels of viral load)", the updated evidence on two studies was not applicable due to high risk of bias. For question 2: "The partner living with HIV is taking ART and has a suppressed viral load (fewer than 200 copies/mL measured every 4–6 months)". In A1.1, the calculated pooled gbMSM incidence estimate shows zero transmissions per 100 person-years with a 95 % confidence interval of 0.00–0.11. In A1.2, the pooled incidence estimate of gbMSM and heterosexual couples shows an overall incidence estimate of zero transmissions per 100 person-years with a 95 % confidence interval of 0.00–0.10.
Page details
- Date modified:
