Draft assessment for terpenes and terpenoids - Phenylpropanoids and aldehydes group
Official title: Draft Assessment - Terpenes and Terpenoids - Phenylpropanoids and Aldehydes Group
Environment and Climate Change Canada
Health Canada
February 2024
Synopsis
Pursuant to section 68 of the Canadian Environmental Protection Act, 1999 (CEPA), the Minister of the Environment and the Minister of Health have conducted an assessment on 12 substances referred to under the Chemicals Management Plan as the Phenylpropanoids and Aldehydes Group. The Chemical Abstracts Service Registry Numbers (CAS RNsFootnote 1), their Domestic Substances List (DSL) names, their common names, and their subgroupings used in this assessment are listed in the table below.
| CAS RN | Subgroup | DSL name | Common name |
|---|---|---|---|
| 8006-78-8a | Individual (Phenylpropanoids) | Oils, bay | Bay oil |
| 8016-88-4a | Individual (Phenylpropanoids) | Oils, tarragon | Tarragon oil |
| 8022-96-6a | Phenylpropanoids subgroup 1 (Phenylpropanoids) | Oils, jasmine | Jasmine oil |
| 8024-43-9a | Phenylpropanoids subgroup 1 (Phenylpropanoids) | Perfumes and essences, jasmin | Perfumes and essences of jasmin |
| 8024-08-6a | Individual (Aldehydes) | Oils, violet | Violet oil |
| 80-54-6 | Aldehydes subgroup 2 (Aldehydes) | Benzenepropanol, 4-(1,1-dimethylethyl)-α-methyl- | Lilial |
| 91-51-0 | Aldehydes subgroup 2 (Aldehydes) | Benzoic acid, 2-[[3-[4-(1,1-dimethylethyl)phenyl]-2-methylpropylidene]amino]-, methyl ester | Verdantiol |
| 37677-14-8 | Aldehydes subgroup 2 (Aldehydes) | 3-Cyclohexene-1-carboxaldehyde, 4-(4-methyl-3-pentenyl)- | Myrac-aldehyde |
| 52474-60-9 | Aldehydes subgroup 2 (Aldehydes) | 3-Cyclohexene-1-carboxaldehyde, 1-methyl-3-(4-methyl-3-pentenyl)- | Myrmac-aldehyde |
| 52475-86-2 | Aldehydes subgroup 2 (Aldehydes) | 3-Cyclohexene-1-carboxaldehyde, 1-methyl-4-(4-methyl-3-pentenyl)- | Myrmac-carboxaldehyde |
| 65405-84-7 | Aldehydes subgroup 2 (Aldehydes) | Cyclohexenebutanal, α,2,2,6-tetramethyl- | Cetonal |
| 66327-54-6 | Aldehydes subgroup 2 (Aldehydes) | 3-Cyclohexene-1-carboxaldehyde, 1-methyl-4-(4-methylpentyl)- | Vernaldehyde |
a This substance is a UVCB (substance of unknown or variable composition, complex reaction products, or biological materials).
All of the substances in the Phenylpropanoids and Aldehydes Group were included in a survey issued pursuant to a CEPA section 71 survey (Canada 2012). With the exception of lilial, none of the substances in this group were reported to be manufactured or imported into Canada in quantities greater than the reported threshold of 100 kg during the 2011 reporting year (Environment Canada 2013). For lilial, 910 kg was reported to be manufactured in Canada in 2008, and 24 460 kg was reported to be imported into Canada during the same calendar year (Environment Canada 2013).
The substances in the Phenylpropanoids and Aldehydes Group are generally used as fragrance ingredients in cosmetics, drugs including natural health products (NHPs), cleaning products, and air fresheners, including do-it-yourself (DIY) use of these substances to create some of these products. Some of them are also present in pest control products as formulants. In addition, some of them occur naturally in food and may be used as food flavouring agents.
The ecological risks of substances in the Phenylpropanoids and Aldehydes Group were characterized using the ecological risk classification of organic substances (ERC) approach, which is a risk-based approach that employs multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. Hazard profiles are based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Metrics considered in the exposure profiles include potential emission rate, overall persistence, and long-range transport potential. A risk matrix is used to assign a low, moderate, or high level of potential concern for substances on the basis of their hazard and exposure profiles. Based on the outcome of the ERC analysis, the substances in the Phenylpropanoids and Aldehydes Group are considered unlikely to be causing ecological harm.
Considering all available lines of evidence presented in this draft assessment, there is low risk of harm to the environment from substances in the Phenylpropanoids and Aldehydes Group. It is proposed to conclude that the 12 substances in the Phenylpropanoids and Aldehydes Group do not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
For the human health risk assessment, 9 of the 12 substances in this group have been addressed under two subgroups, due to similarities in chemical structure, properties, and/or toxicity, while the remaining 3 substances were addressed individually. An impact on human health resulting from exposure to these substances from environmental media is not expected due to the low quantities submitted in response to a CEPA section 71 survey or estimated exposures from environmental monitoring and modelling. Where applicable, exposures were characterized for the use of cosmetics, drugs including NHPs, possible use as food flavouring agents, cleaning products, air fresheners, and DIY products containing the phenylpropanoids and aldehydes, and were expected to be predominately by the dermal and inhalation routes.
For bay oil, the risk characterization was based on methyl eugenol, a component of bay oil. The critical health effect was genotoxic carcinogenicity in laboratory animals. Methyl eugenol is described as a restricted ingredient on the Cosmetic Ingredient Hotlist. The Cosmetic Ingredient Hotlist describes it as being permitted only as a naturally occurring component in botanical extracts, with maximum permitted concentrations in the final product listed for different product types. In this assessment, it has been assumed that these restrictions are met and that bay oil is the only contributor of methyl eugenol. Margins of exposure (MOEs) to bay oil from food, cosmetics, and a respiratory air spray or inhaler stick (NHP) are considered adequate to address uncertainties in the health effects and exposure data used to characterize risk. A comparison of the critical health effect to the estimated level of exposure to bay oil from its use in making a DIY bath oil product is considered adequate to address uncertainties in the health effects and exposure data used to characterize risk. The MOEs to bay oil from its use in DIY products such as in aromatic diffusers or body moisturizer, with the critical health effect, are considered potentially inadequate to account for uncertainties in the health effects and exposure data used to characterize risk.
For tarragon oil, the risk characterization was based on one of its main components, methyl eugenol, and two structurally similar compounds, estragole and elemicin, which were assumed in this assessment to have the same cancer potency as methyl eugenol. The critical health effect was genotoxic carcinogenicity in laboratory animals. The MOEs to tarragon oil from food (based on its potential use as a flavouring agent), digestive aid capsules (NHP), facial cleanser, and soap are considered adequate to address uncertainties in the health effects and exposure data used to characterize risk. However, the MOEs between the critical effect level and the estimates of daily exposure from body moisturizer, body fragrance, and facial moisturizer are considered potentially inadequate to account for uncertainties in the health effects and exposure data used to characterize risk. In addition, for exposures to tarragon oil from its use in DIY products such as in aromatic diffusers, massage oil, bath oil product, or body moisturizer, the MOEs are considered potentially inadequate to account for uncertainties in the health effects and exposure data used to characterize risk.
For phenylpropanoids subgroup 1 (jasmine oil, perfumes and essences of jasmin), hazard information was based on jasmine extract. The critical health effect was female reproductive toxicity in laboratory animals. A comparison of the critical health effect to estimated levels of exposure to phenylpropanoids subgroup 1 from food (based on its potential use as a flavouring agent), hair conditioner, body cleanser, topical treatment cream (NHP), facial sun protection powder (NHP), de-stress roll-on (NHP), lipstick, hair styling product, antiperspirant/deodorant, temporary hair colour, and sunscreen (children of 2 years and older) (NHP) resulted in MOEs that are considered adequate to address uncertainties in the health effects and exposure data used to characterize risk. In addition, MOEs for jasmine oil from its use in a DIY bath oil product are considered adequate to address uncertainties in the health effects and exposure data used to characterize risk. The MOEs derived from the use of jasmine oil in an aerosol all-purpose cleaner, all-purpose floor cleaner, aerosol laundry conditioner, or a liquid laundry detergent are considered adequate to address uncertainties in the health effects and exposure data used to characterize risk. However, the MOEs between the critical effect levels and the estimates of daily exposure from body moisturizer, body fragrance, facial moisturizer/acne treatment (NHP), sunscreen (children who are 6 to 12 months old) (NHP), or antiseptic skin cleanser (NHP) are considered potentially inadequate to account for uncertainties in the health effects and exposure data used to characterize risk. In addition, the MOEs derived from the use of jasmine oil in DIY products such as in aromatic diffusers, massage oil, body moisturizer, or facial steamer are considered potentially inadequate to account for uncertainties in the health effects and exposure data used to characterize risk.
Hazard information for 2,4-hexadienal, the read-across analogue of 2,6-nonadienal, one of the main components of violet oil, was used to inform the hazard assessment of violet oil. Critical health effects in laboratory animals of mild to moderate forestomach epithelial hyperplasia were used to characterize risk. The MOEs to violet oil from food (based on its potential use as a flavouring agent), eye moisturizer, hair conditioner, facial cleanser, body moisturizer, massage oil (people who are 9 years and above), lipstick, body fragrance, or its use in DIY products such as a bath oil product or body moisturizer are considered adequate to address uncertainties in the health effects and exposure data used to characterize risk. However, the MOEs derived from the use of violet oil in a massage oil (children who are 8 years and below) and the use of violet oil in DIY products, such as those used in aromatic diffusers or facial steamers, are considered potentially inadequate to account for uncertainties in the health effects and exposure data used to characterize risk.
For aldehydes subgroup 2 (lilial, verdantiol, myrac-aldehyde, myrmac-aldehyde, myrmac-carboxaldehyde, cetonal, vernaldehyde), hazard information for lilial was used as a read-across analogue to inform the hazard assessment for all the other substances in the aldehydes subgroup. Critical health effects of developmental toxicity in laboratory animals were used to characterize risk. The MOEs between the critical effect levels and the estimates of exposure to lilial from environmental media, body cleanser, hair conditioner (wash-off), face makeup, nail polish, nail polish remover, depilator product, spray antiperspirant/deodorant, bath product, acne treatment (NHP), antiseptic skin cleanser (NHP), temporary hair colour, or facial sunless tanning product are considered to be adequate to account for uncertainties in the health effects and exposure data used to characterize risk. In addition, the MOEs between the critical effect levels and the estimates of exposure to lilial from a carpet deodorizer are considerate to be adequate to account for uncertainties in the health effects and exposure data used to characterize risk. However, the MOEs between the critical effect levels and the estimates of daily exposure to lilial from cosmetics, solid gel air freshener, or a liquid plug-in air freshener (1 year old children) are considered potentially inadequate to account for uncertainties in the health effects and exposure data used to characterize risk.
For myrac-aldehyde, myrmac-aldehyde, myrmac-carboxaldehyde, cetonal, and vernaldehyde, the MOEs between the critical effect level and the estimates of daily exposure from cosmetics, as well as air fresheners and cleaning products, are considered adequate to account for uncertainties.
Since there were no identified sources of exposure to the general population for verdantiol, a qualitative approach to risk characterization was taken, and the risk to human health from verdantiol was considered to be low.
The human health assessment for each substance took into consideration those groups of individuals within the Canadian population who, due to greater susceptibility or greater exposure, may be more vulnerable to experiencing adverse health effects. Certain subpopulations are routinely considered throughout the assessment process, such as infants, children, and people of reproductive age. For instance, age-specific exposures are routinely estimated, and developmental and reproductive studies are evaluated for potential adverse health effects. These subpopulations that have potential for higher exposure and those who may be more susceptible were taken into account in the risk assessment outcomes.
On the basis of the information presented in this draft assessment, it is proposed to conclude that bay oil, tarragon oil, jasmine oil, perfumes and essences of jasmine, violet oil, and lilial meet the criteria under paragraph 64(c) of CEPA as they are entering or may enter the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health and that verdantiol, myrac-aldehyde, myrmac-aldehyde, myrmac-carboxaldehyde, cetonal, and vernaldehyde do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
Therefore, it is proposed to conclude that bay oil, tarragon oil, jasmine oil, perfumes and essences of jasmin, violet oil, and lilial meet one or more of the criteria set out in section 64 of CEPA and that verdantiol, myrac-aldehyde, myrmac-aldehyde, myrmac-carboxaldehyde, cetonal, and vernaldehyde do not meet any of the criteria set out in section 64 of CEPA.
It is also proposed that lilial meets the persistence criteria but not the bioaccumulation criteria as set out in the Persistence and Bioaccumulation Regulations of CEPA.
1. Introduction
Pursuant to section 68 of the Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999), the Minister of the Environment and the Minister of Health have conducted an assessment on 12 of 76 substances, referred to collectively under the Chemicals Management Plan (CMP) as the Terpenes and Terpenoids Group, to determine whether these 12 substances present or may present a risk to the environment or to human health. These 12 substances were identified as priorities for assessment as they met categorization criteria or were considered a priority through other mechanisms (ECCC, HC [modified 2017]).
Of the other substances in the Terpenes and Terpenoids Group, 33 have been assessed in terms of risk to ecological and human health, and the decisions for these substances are provided in separate reports.Footnote 2 Chemical Abstracts Service Registry Number (CAS RN) 91-51-0 was identified as a priority for assessment as it met categorization criteria and is being included in this assessment because its chemical properties are similar to those of other priority substances included herein. Decisions on the remaining substances will be communicated in separate assessments.
The 12 substances addressed in this assessment will hereinafter be referred to as the Phenylpropanoids and Aldehydes Group. Some substances are assessed in subgroups due to their similarities in chemical structure, properties, and/or toxicity. Given the potential for these substances to be used in similar ways and applications, the potential for risk to human health is assessed using similar exposure assumptions across the subgroups.
The ecological risks posed by the substances in the Phenylpropanoids and Aldehydes Group were characterized using the ecological risk classification of organic substances (ERC) approach (ECCC 2016a). The ERC describes the hazard of a substance using key metrics, including mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity, and considers the possible exposure of organisms in the aquatic and terrestrial environments on the basis of such factors as potential emission rates, overall persistence, and long-range transport potential in air. The various lines of evidence are combined to identify substances as warranting further evaluation of their potential to cause harm to the environment or as having a low likelihood of causing harm to the environment.
Some substances in the Phenylpropanoids and Aldehydes Group or the read-across analogues used in this assessment have been reviewed by the United States Environmental Protection Agency (US EPA), the Australian National Industrial Chemicals Notification and Assessment Scheme (NICNAS), European Chemicals Agency (ECHA), European Food Safety Authority (EFSA), Joint FAO/WHO Expert Committee on Food Additives (JECFA), European Scientific Committee on Consumer Safety (SCCS), or the World Health Organization (WHO). Reviews conducted by these institutions have been used to inform the health effects characterization in this assessment.
Sabinene and phytol, which are main components of tarragon oil and jasmine oil, respectively, have been identified as possible ingredients in vaping products (US EPA 2019), which may represent an additional source of exposure to tarragon oil and jasmine oil. Vaping products (such as electronic cigarettes and vaping devices containing cannabis) are being addressed through separate legislative frameworks (HC [modified 2020]).
This draft assessment includes consideration of information on chemical properties, environmental fate, hazards, uses, and exposures, including additional information submitted by stakeholders. Relevant data were identified up to September 2020. Empirical data from key studies as well as some results from models were used to reach proposed conclusions.
This draft assessment was prepared by staff in the CEPA Risk Assessment Program at Health Canada (HC) and Environment and Climate Change Canada (ECCC) and incorporates input from other programs within these departments. The human health portion of this assessment has undergone external peer review and/or consultation. Comments on the technical portions relevant to human health were received from Jennifer Flippin, Theresa Lopez, and Joan Garey, all affiliates of Tetra Tech. The ecological portion of this assessment is based on the ERC document (published July 30, 2016), which was subject to an external review as well as a 60-day public comment period. While external comments were taken into consideration, the final content and outcome of this draft assessment remain the responsibility of HC and ECCC.
Assessments focus on information critical to determining whether substances meet the criteria as set out in section 64 of CEPA by examining scientific information, including information, if available, on subpopulations who may have greater susceptibility or greater exposure, vulnerable environments and cumulative effectsFootnote 3, and by incorporating a weight-of-evidence approach and precaution.Footnote 4 This draft assessment presents the critical information and considerations on which the proposed conclusions are based.
2. Identity of substances
The CAS RNs and Domestic Substances List (DSL) names for the discrete substances and representative substances for UVCBs (unknown or variable composition, complex reaction products, or biological materials) in the Phenylpropanoids and Aldehydes Group that were used to inform the human health assessments are presented in Tables 2-1 and 2-2, respectively. The substances in this assessment have been divided into two subgroups based on their chemical structure, properties, and/or toxicity, and three individual substances.
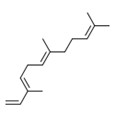
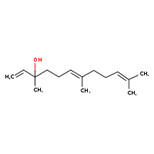
Terpenes are simple hydrocarbons consisting of repeating five-carbon isoprene units (Figure 2-1).
Terpenoids are a modified class of terpenes with different functional groups and oxidized methyl groups moved or removed at various positions. Both terpenes and terpenoids are classified according to the number of isoprene units they contain (Caputi and Aprea 2011; Perveen 2018). Monoterpenes contain two isoprene units. The prefixes di-, tri-, and tetra- refer to two, three, and four monoterpene units, respectively. Furthermore, sesquiterpenes and sesterpenes contain three and five isoprene units, respectively. Phenylpropanoids are characterized as having a chain of three carbon atoms attached to a benzene ring, and aldehydes contain the –CHO functional group and are considered as partially oxidized primary alcohols (Tisserand and Young 2014).

Long description
The Figure 2-1 presents the structural formula of the isoprene (2-methyl-1,3-butadiene) molecule unit on the left (black) and the line (skeletal) formula on the right (blue). Both representations show a double bond between the first two carbons (C1 and C2) and a second double bond between the last two carbons (C3 and C4).
These substances are the components of essential oils found in a wide variety of plants. Essential oils are mixtures of volatile, organic compounds originating from a single botanical source and contribute to the flavour and fragrance of a plant. These plant- derived essential oils have many components that can be extracted from different parts of the plant (e.g., leaves, seeds, stems, flowers, roots, fruits, woods, barks, grass, gum, tree blossoms, bulbs, and flower buds) (Tisserand and Young 2014). In addition, the concentration of these major components can be affected by different factors such as origin of the plant, species, temperature, soil, and geography. Essential oils extracted from plants of the same genus and species can be chemically different even though their origin is the same.
| Subgroupa | CAS RN | DSL name (common name) | Chemical structure or representative chemical name(s), structure(s), and their range of concentration(s) in the essential oil and molecular formula |
|---|---|---|---|
| Individual | 8006-78-8 | Oils, bayb (bay oil) |
|
| Individual | 8016-88-4 | Oils, tarragonb (tarragon oil) |
|
| 1 |
8022-96-6 8024-43-9 |
Oils, jasmineb (Jasmine oil) Perfumes and essences, jasminb (Perfumes and essences of jasmin) |
|
“Trace” is defined by less than 1%.
a The Phenylpropanoids Group was assessed under one subgroup and two individual substances. Phenylpropanoids subgroup 1 includes jasmine oil and perfumes and essences of jasmin due to their similar composition.
b This substance is a UVCB. These materials are derived from natural sources or complex reactions and cannot be characterized in terms of constituent chemical compounds because their composition is too complex or variable. A UVCB is not an intentional mixture of discrete substances and is considered a single substance.
c Concentration range of the main component(s) for Pimenta racemosa essential oil captured from Kim et al. (2008) and Bowles (2003).
d Concentration range of the main component(s) for Pimenta racemosa essential oil captured from McHale et al. (1977), Tucker et al. (1991), Jirovetz et al. (2007a), and Kim et al. (2008).
e Concentration range of the main component(s) for Pimenta racemosa essential oil captured from McHale et al. (1977), Tucker et al. (1991), Jirovetz et al. (2007a), and Kim et al. (2008).
f Concentration range of the main component(s) for Pimenta racemosa essential oil captured from Tucker et al. (1991), Abaul et al. (1995). Kim et al. (2008), Jirovetz et al. (2007a).
g Concentration range of the main component(s) for Artemisia dracunculus essential oil captured from Kordali et al. (2005), Lopes-Lutz et al. (2008), and Obolskiy et al. (2011).
h Concentration range of the main component(s) for Artemisia dracunculus essential oil captured from Kordali et al. (2005) and Obolskiy et al. (2011).
i Concentration range of the main component(s) for Artemisia dracunculus essential oil captured from Sayyah et al. (2004) and Obolskiy et al. (2011).
j Concentration range of the main component(s) for Artemisia dracunculus essential oil captured from Obolskiy et al. (2011).
k Concentration range of the main component(s) for Artemisia dracunculus essential oil captured from Ayoughi et al. (2011) and Obolskiy et al. (2011).
l Concentration range of the main component(s) for Jasminium grandiflorum essential oil captured from Jirovetz et al. (2007b), Bera et al. (2015), Wei et al. (2015).
m Concentration range of the main component(s) for Jasminium grandiflorum essential oil captured from Braun et al. (2009), Prakash et al. (2012), Bera et al. (2015), and Wei et al. (2015).
n Concentration range of the main component(s) for Jasminium grandiflorum essential oil captured from Prakash et al. (2012) and Bera et al. (2015).
o Concentration range of the main component(s) for Jasminium grandiflorum essential oil captured from Jirovetz et al. (2007b) and Wei et al. (2015).
p Concentration range of the main component(s) for Jasminium grandiflorum essential oil captured from Tisserand and Young (2014), and Wei et al. (2015).
q Concentration range of the main component(s) for Jasminium grandiflorum essential oil captured from Bera et al. (2015) and Wei et al. (2015).
r Concentration range of the main component(s) for Jasminium grandiflorum essential oil captured from Prakash et al. (2012) and Bera et al. (2015).
| Subgroupa | CAS RN | DSL name (common name) | Chemical structure or representative chemical name(s), structure(s), and their range of concentration(s) in the essential oil and molecular formula |
|---|---|---|---|
| Individual | 8024-08-6 | Oils, violetb (violet oil) |
|
| 2 | 80-54-6 | Benzenepropanol, 4-(1,1-dimethylethyl)-α-methyl- (lilial) |
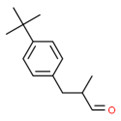 C14H20O |
| 2 | 91-51-0 | Benzoic acid, 2-[[3-[4-(1,1-dimethylethyl)phenyl]-2-methylpropylidene]amino]-, methyl ester (verdantiol) |
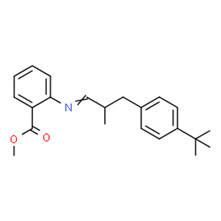 C22H27 |
| 2 | 37677-14-8 | 3-Cyclohexene-1-carboxaldehyde, 4-(4-methyl-3-pentenyl)- (myrac-aldehyde) |
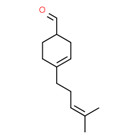 C13H20O |
| 2 | 52474-60-9 | 3-Cyclohexene-1-carboxaldehyde, 1-methyl-3-(4-methyl-3-pentenyl)- (myrmac-aldehyde) |
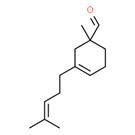 C14H22O |
| 2 | 52475-86-2 | 3-Cyclohexene-1-carboxaldehyde, 1-methyl-4-(4-methyl-3-pentenyl)- (myrmac-carboxaldehyde) |
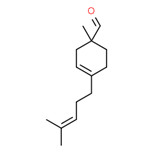 C14H22O |
| 2 | 65405-84-7 | Cyclohexenebutanal, α,2,2,6-tetramethyl-(cetonal) |
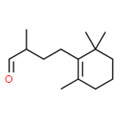 C14H24O |
| 2 | 66327-54-6 | 3-Cyclohexene-1-carboxaldehyde, 1-methyl-4-(4-methylpentyl)-(vernaldehyde) |
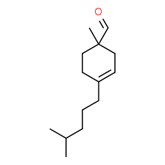 C14H24O |
a The Aldehydes Group was assessed under one subgroup and one individual substance. Aldehydes subgroup 2 includes hazard information for lilial used to inform the risk characterization. Lilial is a discrete substance in aldehydes subgroup 2 and was identified as a read-across analogue for verdantiol, myrac-aldehyde, myrmac-aldehyde, myrmac-carboxaldehyde, cetonal, and vernaldehyde. Verdantiol seems to be an impurity and a possible metabolite of lilial because verdantiol can be hydrolyzed in lilial.
b This substance is a UVCB.
c Concentration range of the main component(s) for Viola odorata captured from Cu et al. (1992) and Saint-Lary et al. (2014).
2.1 Selection of analogues and use of (Q)SAR models
A read-across approach using data from analogues or components of the target substances, where appropriate, has been used to inform the human health assessments. Analogues were selected from among a large list of substances similar in structure and/or with characteristics similar to those of substances within this group (e.g., in terms of physical-chemical properties, toxicokinetics). However, because the majority of the substances were data poor, the choice of analogues was driven by the presence of relevant empirical health effects data.
Selection was based on assessments carried out using the Organisation for Economic Co-operation and Development (OECD) (quantitative) structure-activity relationship [(Q)SAR] Toolbox version 4.3 (OECD 2019). Details of the read-across data chosen to inform the human health assessments of the phenylpropanoids and aldehydes are further discussed in the relevant sections of this assessment. Information on the identities and chemical structures of the analogues used to inform the human health assessment of the phenylpropanoids and aldehydes (i.e., violet oil) is presented in Table 2.3.
| Subgroup or substance being assessed | CAS RN for analogue | Common name | Chemical structure, molecular formula and SMILES | Molecular weight (g/mol) |
|---|---|---|---|---|
| Violet oil | 142-83-6 | 2,4-Hexadienal |
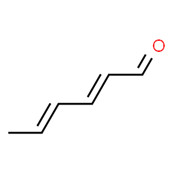 C6H8O |
96.13 |
3. Physical and chemical properties
A summary of physical and chemical property data of the substances in the Phenylpropanoids and Aldehydes Group is presented in Table 3-1. Where experimental information was limited or not available for a property, data from analogues were used for read-across and/or (Q)SAR models (OECD 2019) were used to generate predicted values for the substance. Additional physical and chemical properties are reported in ECCC (2016b).
| Substance(s) | Common name (CAS RN) of representative constituent(s) | Molecular weight (g/mol)a | Water solubility (mg/L)a | Vapour pressure (Pa)a | Log Kowa |
|---|---|---|---|---|---|
| Bay oil | Eugenol (97-53-0) | 164.21 | 2460 | 1.26(M) | 2.27 |
| Bay oil | Chavicol (501-92-8) | 134.18 | 1110(M) | 3.04(M) | 2.91(M) |
| Bay oil | beta-Myrcene (123-35-3) | 136.24 | 5.6 | 268 | 4.17 |
| Bay oil | Methyl eugenol (93-15-2) | 178.23 | 500 | 4.46 | 3.03(M) |
| Tarragon oil | Methyl eugenol (93-15-2) | 178.23 | 500 | 4.46 | 3.03(M) |
| Tarragon oil | Estragole (140-67-0) | 148.21 | 178 | 22 (M) | 3.47(M) |
| Tarragon oil | Sabinene (3387-41-5) | 136.24 | 2.49(M) | 981(M) | 4.69(M) |
| Tarragon oil | Terpinolene (586-62-9) | 136.24 | 9.5 | 99 | 4.47 |
| Tarragon oil | Elemicin (487-11-6) | 208.26 | 133.2(M) | 0.28(M) | 2.90(M) |
| Tarragon oil | cis-Anethole (25679-28-1) | 148.21 | 111 | 8.46(M) | 3.39(M) |
| Tarragon oil | trans-Anethole (4180-23-8) | 148.21 | 111 | 8.46(M) | 3.39(M) |
|
Jasmine oil Perfumes and essences of jasmin |
Benzyl acetate (140-11-4) | 150.18 | 3100(E) | 23.6(E) | 1.96(E) |
|
Jasmine oil Perfumes and essences of jasmin |
Benzyl benzoate (120-51-4) | 212.25 | 15.39(M) | 0.03(E) | 3.97(E) |
|
Jasmine oil Perfumes and essences of jasmin |
alpha-Farnesene (502-61-4) | 204.36 | 1.05 x 10-2 (M) | 3.33(M) | 7.10(M) |
|
Jasmine oil Perfumes and essences of jasmin |
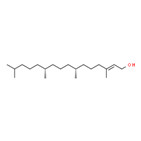
Phytol (150-86-7) | 296.54 | 3.27 x 10-3(M) | 4.30 x 10-4 (M) | 8.32(M) |
|
Jasmine oil Perfumes and essences of jasmin |
Isophytol (505-32-8) | 296.54 | 7.53 x 10-3(M) | 1.88 x 10-3 (M) | 8.23(M) |
|
Jasmine oil Perfumes and essences of jasmin |
Nerolidol (7212-44-4) | 222.37 | 7.67(M) | 0.08(M) | 5.68(M) |
|
Jasmine oil Perfumes and essences of jasmin |
Linalool (78-70-6) | 154.25 | 1590(E) | 21(E) | 2.9(E) |
| Violet oil | Linoleic acid (2197-37-7) | 280.45 | 3.77 x 10-2(M) | 1.16 x 10-4 | 7.05 |
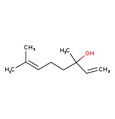
| Violet oil | 2,6- Nonadienal (557-48-2) | 138.21 | 318.8(M) | 31.1(M) | 2.84(M) |
| Violet oil | Palmitic acid (57-10-3) | 256.43 | 0.04 | 5.07 x 10-5 | 7.17 |
| Violet oil | 1-Octadecene (112-88-9) | 252.48 | 1.26 x 10-4(M) | 8.99 x 10-3(M) | 9.04(M) |
| Lilial | N/A | 204.31 | 7.86(M) | 0.477(M) | 4.36(M) |
| Verdantiol | N/A | 337.46 | 0.028(M) | 4.0 x 10-5(M) | 6.35(M) |
| Myrac-aldehyde | N/A | 192.30 | 4.35(M) | 0.783(M) | 4.73(M) |
| Myrmac-aldehyde | N/A | 206.33 | 1.51(M) | 0.349(M) | 5.19(M) |
| Myrmac-carboxaldehyde | N/A | 206.33 | 1.51(M) | 0.349(M) | 5.19(M) |
| Cetonal | N/A | 208.35 | 1.44(M) | 0.834(M) | 5.20(M) |
| Vernaldehyde | N/A | 208.35 | 1.25(M) | 0.605(M) | 5.27(M) |
Abbreviations: N/A, not available; Kow, octanol-water partition coefficient ; (M) Modelled; (E) Experienced
a US EPA (2012a)
4. Sources and uses
All of the substances in the Phenylpropanoids and Aldehydes Group were included in a survey issued pursuant to a CEPA section 71 survey (Canada 2012). With the exception of lilial, none of the substances in this group were reported to be manufactured or imported into Canada in quantities greater than the reported threshold of 100 kg during the 2011 reporting year (Environment Canada 2013).
For lilial, 910 kg was reported to be manufactured in Canada in 2008, and 24 460 kg was reported to be imported into Canada during the same calendar year (Environment Canada 2013). Table 4-1 presents a summary of information submitted in response to a CEPA section 71 survey.
| Substance | Total manufacture (kg)a | Total imports (kg)a | Reporting year |
|---|---|---|---|
| Bay oil | NR | NR | 2011 |
| Tarragon oil | NR | NR | 2011 |
| Jasmine oil | NR | NR | 2011 |
| Perfumes and essences of jasmin | NR | NR | 2011 |
| Violet oil | NR | NR | 2011 |
| Lilial | 910 | 24 460 | 2008 |
| Verdantiol | NR | NR | 2011 |
| Myrac-aldehyde | NR | NR | 2011 |
| Myrmac-aldehyde | NR | NR | 2011 |
| Myrmac-carboxaldehyde | NR | NR | 2011 |
| Cetonal | NR | NR | NA |
| Vernaldehyde | NR | NR | 2011 |
Abbreviations: NR, no reports above the reporting threshold of 100 kg; NA, not applicable, and this substance was not included in the section 71 surveys
a Values reflect quantities reported in response to a CEPA section 71 survey (Canada 2012). See surveys for specific inclusions and exclusions (Schedules 2 and 3).
Information submitted in response to a CEPA section 71 survey indicated uses of lilial as an odour agent in cleaning and furnishing care, laundry and dishwashing, personal care, air care, apparel and footwear care, pet care, automotive care, and in lubricants and greases (Environment Canada 2013). Information submitted in response to a CEPA section 71 survey also indicated uses of violet oil, myrac-aldehyde, and vernaldehyde in imported personal care productsFootnote 5 (Environment Canada 2013).
In addition, for myrac-aldehyde, myrmac-aldehyde, myrmac-carboxaldehyde, cetonal, and vernaldehyde, an industry submission reported that these substances are used as a fragrance ingredient at low concentrations in cosmetics, air fresheners, and cleaning products.
Additional uses for the Phenylpropanoids and Aldehydes Group are outlined in Table 4-2.
| Substance | Food flavouring agenta | Incidental additivesFootnote 6,a | Natural Health Products Ingredients Database | Licensed Natural Health Products Databaseb | Cosmeticsc | Formulant PCPsd |
|---|---|---|---|---|---|---|
| Bay oil | Y | N | Y | Y (MI, NMI) | Y | Y |
| Tarragon oil | Y | N | Y | Y (MI, NMI) | Y | Y |
| Jasmine oil | Y | N | Y | Y (MI, NMI) | Y | N |
| Perfumes and essences of jasmin | N | N | Y | Y (MI, NMI) | N | N |
| Violet oil | Y | N | Y | Y (MI, NMI) | Y | N |
| Lilial | N | Y (Component in hand soaps, no direct food contact) | Y | Y (NMI) | Y | Y |
| Verdantiol | N | N | N | N | N | N |
| Myrac-aldehyde | N | N | N | N | N | Y |
| Myrmac-aldehyde | N | N | N | N | Y | Y |
| Myrmac-carboxaldehyde | N | N | N | N | N | N |
| Cetonal | N | N | N | N | N | N |
| Vernaldehyde | N | N | N | N | N | Y |
Abbreviations: PCPs, pest control products; Y, yes, this use was reported for this substance; N, no, this use was not reported for this substance; MI, medicinal ingredient; NMI, non-medicinal ingredient
a Personal communication, emails from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2015, 2017, and 2020; unreferenced.
b Listed in the Licensed Natural Health Products Database as being present as a medicinal or non-medicinal ingredient in natural health products (NHPs) in Canada. Personal communication, email from the Natural and Non-prescription Health Products Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2021; unreferenced.
c Notified to be present in cosmetics based on notifications submitted under the Cosmetic Regulations to Health Canada. Personal communication, emails from the Consumer and Hazardous Products Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2017 and 2020; unreferenced.
d Formulant in PCPs registered in Canada. Personal communication, emails from the Pest Management Regulatory Agency, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2015 and 2020; unreferenced.
Do-It-Yourself (DIY) products
Certain terpene and terpenoid substances that have aromatic properties within the Phenylpropanoids and Aldehydes Group are currently available in the Canadian market (as “essential oils”) at a concentration of up to 100%. It is therefore possible that these undiluted substances are purchased and used by consumers to make DIY products. DIY products that may result in high consumer exposures include aromatic diffuser (known as aromatherapy by consumers), massage oil, bath oil product, body moisturizer, and facial steamer. Consequently, uses of undiluted substances in DIY products are evaluated in this assessment. Parameters for estimating dermal and inhalation exposures to DIY products are available in Appendix B.
Bay oil
Bay oil is a UVCB that is steam distilled from Pimenta racemosa, a tree that belongs to the Myrtaceae family. The tree is also known as the West Indian Bay tree, bay rum tree, and cilimnet; and while it is native to the Caribbean region, it is also cultivated in many warm parts of the world (Moharram et al. 2018). Stated otherwise, any extract from Pimenta racemosa to the Myrtaceae family is considered to be included in this definition.
Bay oil is used in a number of products available to consumers such as moisturizer, hair conditioner, bath product, and body cleanser. Based on notifications submitted under the Cosmetic Regulations to Health Canada, bay oilFootnote 7 is used in more than 90 products in Canada, with the majority (approximately 94%) having concentrations less than or equal to 3% (personal communication, emails from the Consumer and Hazardous Products Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2020; unreferenced). There are also NHPs that contain bay oil as an MI (medicinal ingredient) or as an NMI (non-medicinal ingredient) acting as a fragrance ingredient (LNHPD [modified 2021]; personal communication, email communication from the Natural and Non-prescription Health Products Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2021; unreferenced).
In Canada, bay oil is also reported to be used as a formulant in pest control products (PCPs) (personal communication, email from the Pest Management Regulatory Agency, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2020; unreferenced).
Bay oil (myrcia oil) is listed as being generally recognized as safe (GRAS) under Title 21 Part 182 of the United States (US) Code of Federal Regulations (21CFR182.20). Bay leaves West Indian oil (Pimenta acris Kostel; P. racemosa) is listed as number 2122 in the Flavor and Extract Manufacturers Association (FEMA)’s Flavor Ingredient Library (FEMA 2020).
Bay oil is also identified as a fragrance ingredient used in consumer goods by the International Fragrance Association (IFRA 2017).
Bay oil has reported uses internationally as a flavouring agent in a variety of foods (CoE 2000; Burdock 2010). It does not have any specific food status in Europe; however, it is listed in the United States Food and Drug Administration (US FDA) Substances Added to Food Inventory as a flavouring agent or adjuvant (EC 2008; US FDA 2018). No definitive information is available concerning the potential use of bay oil as a food flavouring agent in Canada; however, since the substance is identified as a food flavouring agent internationally, it is possible that the substance is present as a flavouring agent in foods sold in Canada (personal communication, email from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2020; unreferenced).
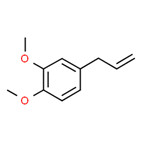
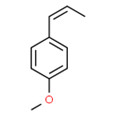
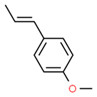
Tarragon oil
Tarragon oil is a UVCB that is steam distilled from Artemisia dracunculus, a plant that belongs to the Asteraceae family. The plant has various common names such as estragon, dragon sage-wort, false tarragon, and dragon wormwood. It is cultivated in many countries, including Iran (Raeisi et al. 2012). Unless explicitly specified, any extract from Artemisia dracunculus to the Asteraceae family is considered to be included in this definition.
Tarragon oil is used in some products available to consumers such as moisturizer, fragrances, massage oil, and antiperspirant/deodorant. Based on notifications submitted under the Cosmetic Regulations to Health Canada, tarragon oil or Artemisia dracunculus (tarragon) oil is used in approximately 30 products, with the majority (approximately 80%) having concentrations less than or equal to 3% (personal communication, email from the Consumer and Hazardous Products Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada 2020; unreferenced). There are also NHPs that contain tarragon oil as an MI, or as an NMI for the purpose of flavour enhancer, fragrance ingredient, or skin-conditioning agent (LNHPD 2021; personal communication, email communication from the Natural and Non-prescription Health Products Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2021; unreferenced).
In Canada, tarragon oil is also reported to be used as a formulant in PCPs (personal communication, email from the Pest Management Regulatory Agency, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2020; unreferenced).
Estragole (esdragol, esdragon, tarragon), estragon (tarragon), and tarragon are listed as being GRAS under Title 21 Part 182 of the US 21CFR182.20. Tarragon (Artemesia dracunculus L.) is listed as number 3043 in the FEMA’s Flavor Ingredient Library (FEMA 2020). Estragon oil (Artemisia dracunculus L.; tarragon oil) is listed with FEMA number 2412 (FEMA 2020).
Annex III to Regulation (EC) No 1334/2008 sets out substances which should not be added to food, and maximum levels (MLs) for certain substances in the European Union (EU), which are naturally present in flavourings and in food ingredients with flavouring properties. The MLs must not be exceeded as a result of the use of flavourings and/or food ingredients with flavouring properties in and on those foods in the EU. Annex III prohibits the addition of estragole to foods, as such, and sets out the MLs for estragole in certain compound foodsFootnote 8 resulting from its natural presence in flavourings and/or food ingredients with flavouring properties used in the foods. Tarragon oil is composed of approximately 0% to 82% estragole, and the amount of estragole in foods from the use of tarragon oil or other flavouring preparations would be subject to the MLs in Regulation (EC) No 1334/2008 in the EU.
Tarragon oil is identified by IFRA (2017) as a fragrance ingredient used in consumer goods.
Tarragon oil has reported uses internationally as a flavouring agent in a variety of foods (CoE 2000; Burdock 2010). Tarragon oil does not have any specific food status in Europe; however, it is listed in the US FDA Substances Added to Food Inventory as a flavouring agent or adjuvant (EC 2008; US FDA 2018). No definitive information is available concerning the potential use of tarragon oil as a food flavouring agent in Canada; however, since the substance is identified as a food flavouring agent internationally, it is possible that the substance is present as a flavouring agent in foods sold in Canada (personal communication, email from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2020; unreferenced).
Furthermore, sabinene, one of the main components of tarragon oil, has been identified in vaping products in the United States (US EPA 2019).
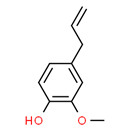
Phenylpropanoids subgroup 1 (jasmine oil, perfumes and essences of jasmin)
There are two UVCBs in phenylpropanoids subgroup 1: jasmine oil, and perfumes and essences of jasmin. Both substances are produced from the fresh flowers of several jasmine species (Jasminum spp.) of the Oleaceae family. There are more than 2000 known species of jasmine. The most common species are Jasminum officinale, J. grandiflorum, J. floribundum, J. humile, J. odoratissimum, J. paniculatum, and J. sambac. Jasmine varieties originate from India but are also well known in the Mediterranean countries of Europe, Asia, and Africa, the Comoro Islands, and China. The major suppliers of aromatic products obtained from jasmine are Egypt, Morocco, Algeria, Italy, and France (Jirovetz et al. 2007b). Unless explicitly specified, any extract from Jasminum spp. to the Oleaceae family is considered to be included in this definition.
Jasmine oil and perfumes and essences of jasmin have been identified by two different CAS RNs; however, the Toxic Substances Control Act Chemical Substance Inventory defines both as “extractives and their physically modified derivatives from Jasminum officinale, Oleaceae” (ChemIDPlus 2019). They also share similar synonyms, such as jasmine absolute or jasmin (ChemIDPlus 2019). IFRA identified both CAS RNs as identical substances (IFRA 2013). Therefore, jasmine oil and perfumes and essences of jasmin are assessed herein as the same substance. IFRA also identified CAS RN 90045-94-6, named Jasmin officinale var. grandiflorum, as being identical to jasmine oil and perfumes and essences of jasmin (IFRA 2013). The two substances will be referred to as jasmine oil.
Jasmine oil is in a number of products available to consumers such as body moisturizer, body fragrance, massage oil, makeup, and facial cleaners. Based on notifications submitted under the Cosmetic Regulations to Health Canada, jasmine oilFootnote 9 is used in approximately 550 products in Canada, with the majority (approximately 78%) having concentrations of less than or equal to 1% (personal communication, email from the Consumer and Hazardous Products Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2020; unreferenced). There are also NHPs that contain jasmine oil as an MI or as an NMI acting as a fragrance ingredient or skin-conditioning agent (LNHPD 2021; personal communication, email communication from the Natural and Non-prescription Health Products Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2021; unreferenced).
Jasmine oil may be present in cleaning products, such as liquid or gel all-purpose cleaners in pourable bottles, pump sprays or aerosols at concentrations up to 1%, liquid or gel dish detergents in pourable bottles for washing by hand or by automatic dishwashing machines at concentrations up to 1%, liquid laundry conditioners available in pourable bottles for use in washing machines or applied directly to fabric as a spray or aerosol at concentrations up to 5%, and liquid laundry detergent and detergent boosters available in pourable bottles for use in a washing machine at concentrations up to 5% (ACI 2020). Jasmine oil is also present in fragrance oil burners (MSDS 2006).
Essential oils, oleoresins (solvent-free), and natural extractives (including distillates) of jasmine (Jasminum officinale L. and other Jasminum spp.) are listed as being GRAS under Title 21 Part 182 of the US 21CFR182.20.
Jasmine oil (Jasminium grandiflorum L.) is listed as number 2600 in the FEMA’s Flavor Ingredient Library (FEMA 2020).
Jasmine oil is identified by IFRA (2017) as a fragrance ingredient used in consumer goods.
Jasmine oil has reported uses internationally as a flavouring agent in a variety of foods (CoE 2000; Burdock 2010). Jasmine oil does not have any specific food status in Europe; however, it is listed in the US FDA Substances Added to Food Inventory as a flavouring agent or adjuvant (EC 2008; US FDA 2018). No definitive information is available concerning the potential use of jasmine oil as a food flavouring agent in Canada; however, since the substance is identified to be used as a food flavouring agent internationally, it is possible that the substance is present as a flavouring agent in foods sold in Canada. There is no information available to indicate that perfumes and essences of jasmin have any direct or indirect food uses in Canada or internationally (personal communication, email from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2020; unreferenced).
Furthermore, phytol, one of the main components of jasmine oil, has been identified in vaping products in the United States (US EPA 2019).
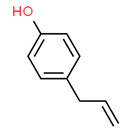
Violet oil
Violet oil is a UVCB obtained from Viola odorata, a plant that belongs to the Violaceae family. The plant is native to Asia, North Africa, and Europe. Unless explicitly specified, any extract from Viola odorata is considered to be included in this definition.
Violet oil is used in a number of products available to consumers such as body moisturizer, fragrances, facial cleanser, styling products, and lipstick. Based on notifications submitted under the Cosmetic Regulations to Health Canada, violet oilFootnote 10 is used in more than 400 products, with the majority (90%) having a concentration less than or equal to 1% (personal communication, email from the Consumer and Hazardous Products Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, March 2017; unreferenced). There are also NHPs that contain violet oil as an MI, or as an NMI acting as a fragrance ingredient (LNHPD 2021; personal communication, email from the Natural and Non-prescription Health Products Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2021; unreferenced).
Essential oils, oleoresins (solvent-free), and natural extractives (including distillates) of the violet flowers, leaves, and leaves absolute are listed as being generally recognized as safe (GRAS) under Title 21 Part 182 of the US’ 21CFR182.20. Violet leaves absolute (Viola odorata L.) is listed with the number 3110 in the FEMA’s Flavor Ingredient Library (FEMA 2020).
Violet oil is identified by IFRA (2017) as a fragrance ingredient used in consumer goods.
Violet oil has reported uses internationally as a flavouring agent in a variety of foods (Burdock 2010; CoE 2000). Violet oil does not have any specific food status in Europe; however, it is listed in the US FDA Substances Added to Food Inventory as a flavouring agent or adjuvant (EC 2008; US FDA 2018). No definitive information is available concerning the potential use of violet oil as a food flavouring agent in Canada; however, since the substance is identified as a food flavouring agent internationally, it is possible that the substance is present as a flavouring agent in foods sold in Canada (personal communication, email from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2020; unreferenced).
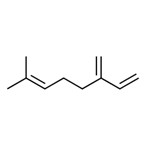
Aldehydes subgroup 2 (lilial, verdantiol, myrac-aldehyde, myrmac-aldehyde, myrmac-carboxaldehyde, cetonal, vernaldehyde)
There are seven discrete substances in aldehydes subgroup 2 (lilial, verdantiol, myrac-aldehyde, myrmac-aldehyde, myrmac-carboxaldehyde, cetonal, and vernaldehyde).
Lilial is used in a number of products available to consumers such as body moisturizer, massage oil, fragrances, hair conditioners, hair colouring products, and nail polish. Based on notifications submitted under the Cosmetic Regulations to Health Canada, lilial is used in approximately 6500 products, with the majority (90%) having concentrations less than or equal to 0.3% (personal communication, email from the Consumer and Hazardous Products Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, 2020; unreferenced). There are also NHPs that contain lilial as an NMI acting as a fragrance ingredient, in addition to non-prescription drugs such as sunscreens and makeup products with sun protection (LNHPD 2021; personal communication, email from the Therapeutic Products Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2020; unreferenced; personal communication, email from the Natural and Non-prescription Health Products Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2021; unreferenced).
In Canada, lilial, myrac-aldehyde, myrmac-aldehyde, and vernaldehyde were also reported to be used as formulants in PCPs (personal communication, email from the Pest Management Regulatory Agency, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2020; unreferenced).
In addition, lilial may be present in cleaning products (ACI 2020). Lilial is also present in air fresheners and carpet deodorizer (SDS 2016, 2017a, 2017b). Myrac-aldehyde, myrmac-aldehyde, and myrmac-carboxaldehyde are also used in air fresheners (CPID 2021).
Lilial is used as a component in incidental additives (for example, hand soaps) used in food processing establishments with no direct food contact (personal communication, email from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2020; unreferenced). There is no information available to indicate that any of the other aldehydes subgroup 2 substances have any direct or indirect food uses in Canada or internationally (personal communication, email from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2020; unreferenced).
Lilial, myrac-aldehyde, myrmac-aldehyde, myrmac-carboxaldehyde, cetonal, and vernaldehyde are primarily used as fragrance ingredients in products available to consumers in Canada and are typically present as part of fragrance mixtures at low concentrations (Environment Canada 2013).
An industry submission indicated that the substances in aldehydes subgroup 2 are used as fragrance ingredients at low concentrations and can be present in certain product categories such as cosmetics, air fresheners, and cleaning products.
5. Environmental fate and behaviour
5.1 Environmental persistence
According to models used in ERC, lilial and perfumes and essences of jasmin are expected to persist in water, sediment, and soil but are not expected to persist in air (ECCC 2016b).
According to models used in ERC, violet oil, myrmac-carboxaldehyde, and vernaldehyde are expected to persist in sediment but are not expected to persist in water, soil, or air (ECCC 2016b).
According to models used in ERC, jasmine oil is expected to persist in air but is not expected to persist in water, sediment, or soil (ECCC 2016b).
According to models used in ERC, bay oil, tarragon oil, verdantiol, myrac-aldehyde, myrmac-aldehyde, and cetonal are not expected to persist in air, water, sediment, or soil (ECCC 2016b).
5.2 Potential for bioaccumulation
Although the log Kow values for verdantiol, myrmac-aldehyde, myrmac-carboxaldehyde, cetonal, and vernaldehyde are moderate to high, the bioconcentration factors for these substances are low. As a result, verdantiol, myrmac-aldehyde, myrmac-carboxaldehyde, cetonal, and vernaldehyde are not expected to significantly bioaccumulate in organisms (ECCC 2016b).
Given their low Kow and low bioconcentration factors, bay oil, tarragon oil, jasmine oil, perfumes and essences of jasmin, violet oil, lilial, and myrac-aldehyde are not expected to significantly bioaccumulate in organisms (ECCC 2016b).
6. Potential to cause ecological harm
6.1 Characterization of ecological risk
The ecological risks of the substances in the Phenylpropanoids and Aldehydes Group were characterized using the ERC approach (ECCC 2016a). The ERC is a risk-based approach that considers multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. The various lines of evidence are combined to discriminate between substances of lower or higher potency and lower or higher potential for exposure in various media. This approach reduces the overall uncertainty associated with risk characterization compared to an approach that relies on a single metric in a single medium (for example, median lethal concentration) for characterization. Since violet oil is a UVCB substance and could not be suitably represented by a single chemical structure, a manual judgement-based approach to classification was used. The following summarizes the approach, which is described in detail in ECCC (2016a).
Data on physical-chemical properties, fate (chemical half-lives in various media and biota, partition coefficients, and fish bioconcentration), acute fish ecotoxicity, and chemical import or manufacture volume in Canada were collected from the scientific literature, available empirical databases (for example, OECD QSAR Toolbox 2014), and responses to surveys issued pursuant to section 71 of CEPA, or they were generated using selected (quantitative) structure-activity relationship [([Q]SAR] or mass-balance fate and bioaccumulation models. These data were used as inputs to other mass-balance models or to complete the substance hazard and exposure profiles.
Hazard profiles were based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Exposure profiles were also based on multiple metrics, including potential emission rate, overall persistence, and long-range transport potential. Hazard and exposure profiles were compared against decision criteria in order to classify the hazard and exposure potentials for each organic substance as low, moderate, or high. Additional rules were applied (for example, classification consistency, margin of exposure) to refine the preliminary classifications of hazard or exposure. However, in the case of violet oil, hazard and exposure could not be fully profiled because of the lack of a representative structure to estimate needed properties and the lack of empirical data for these properties. Therefore, classification of hazard and exposure was performed manually by examining the UVCB constituents, analyzing information submitted in response to a CEPA section 71 survey, and making decisions on the basis of consideration of similar substances and/or application of expert judgement.
A risk matrix was used to assign a low, moderate, or high classification of potential risk for each substance on the basis of its hazard and exposure classifications. ERC classifications of potential risk were verified using a two-step approach. The first step adjusted the risk classification outcomes from moderate or high to low for substances that had a low estimated rate of emission to water after wastewater treatment, representing a low potential for exposure. The second step reviewed low risk potential classification outcomes using relatively conservative, local-scale (that is, in the area immediately surrounding a point source of discharge) risk scenarios, designed to be protective of the environment, to determine whether the classification of potential risk should be increased.
The ERC uses a weighted approach to minimize the potential for both over- and under classification of hazard and exposure, and of subsequent risk. The balanced approaches for dealing with uncertainties are described in greater detail in ECCC (2016a). The following describes two of the more substantial areas of uncertainty. Error with empirical or modelled acute toxicity values could result in changes in the classification of hazard, particularly metrics relying on tissue residue values (that is, mode of toxic action), many of which are predicted values from (Q)SAR models (OECD QSAR Toolbox 2014). However, the impact of this error is mitigated by the fact that overestimation of median lethality will result in a conservative (protective) tissue residue value used for critical body residue analysis. Error with the underestimation of acute toxicity will be mitigated through the use of other hazard metrics such as structural profiling of mode of action, reactivity, and/or estrogen-binding affinity. Changes or errors in chemical quantity could result in differences in classification of exposure as the exposure and risk classifications are highly sensitive to emission rate and use quantity. The ERC classifications thus reflect exposure and risk in Canada on the basis of what is estimated to be the current use quantity and may not reflect future trends.
Critical data and considerations used to develop the substance-specific profiles for the substances in the Phenylpropanoids and Aldehydes Group and the hazard, exposure, and risk classification results are presented in ECCC (2016b).
The hazard and exposure classifications for the 12 substances in the Phenylpropanoids and Aldehydes Group are summarized in Table 6-1.
| Substance | ERC hazard classification | ERC exposure classification | ERC risk classification |
|---|---|---|---|
| Bay oil | low | low | low |
| Tarragon oil | low | low | low |
| Jasmine oil | low | high | low |
| Perfumes and essences of jasmin | low | low | low |
| Violet oil | low | low | low |
| Lilial | low | low | low |
| Verdantiol | low | low | low |
| Myrac-aldehyde | low | low | low |
| Myrmac-aldehyde | low | low | low |
| Myrmac-carboxaldehyde | low | low | low |
| Cetonal | low | low | low |
| Vernaldehyde | low | low | low |
On the basis of the low hazard and low exposure classifications according to information considered under the ERC, bay oil, tarragon oil, perfumes and essences of jasmin, violet oil, lilial, verdantiol, myrac-aldehyde, myrmac-aldehyde, myrmac-carboxaldehyde, cetonal, and vernaldehyde were classified as having a low potential for ecological risk. It is unlikely that these substances are resulting in concerns for the environment in Canada.
According to information considered under the ERC, jasmine oil was classified as having a high exposure potential on the basis of its critically long half-life in air. Although the current use patterns for jasmine oil result in a high exposure potential, considering its low hazard potential, jasmine oil is unlikely to be resulting in concerns for the environment in Canada.
7. Potential to cause harm to human health
For the health effects characterization of the substances in the Phenylpropanoids and Aldehydes Group, preference was given to hazard data on the oil itself. In the absence of health effects data on the oil, health effects data for the major components present in the oil of interest were considered in order to inform the risk assessment. Where there were no health effects data for the substance and/or major components in the oil, a read-across approach was taken.
Certain subpopulations have the potential for increased exposure due to differences in physical characteristics (for example, body weight, breathing rate, skin surface area). As a result of potential differences in exposure, these vulnerable populations may be at increased risk of experiencing adverse health effects. In this assessment, exposure estimates were derived for infants, toddlers, and children, who may have higher exposures per kilogram (kg) body weight (bw) than adults for certain products available to consumers, such as body lotions, sunscreens, and hand sanitizers. Additionally, where available and appropriate, reproductive and developmental studies were considered to ensure that pregnant women and their developing fetuses are protected.
7.1 Bay oil
7.1.1 Exposure assessment
Environmental media
No uses for bay oil were indicated in the information submitted in response to a CEPA section 71 survey. Monitoring data for bay oil in environmental media in Canada or elsewhere were not identified. According to information considered under the ERC, bay oil was classified as having a low ecological exposure potential (see section 6.1).
Food
No definitive information is available concerning the potential use of bay oil as a flavouring agent in foods sold in Canada. However, since bay oil is identified as a food flavouring agent internationally, it is possible that this substance is present as a flavouring agent in foods sold in Canada.
The Fenaroli’s Handbook of Flavour Ingredients reports the per capita (“individual”) estimated intake of bay oil from its use as a food flavouring agent to be 3.39 x 10-1 μg/kg bw/day on the basis of a maximized survey-derived daily intake (MSDI) approach for the US population (Burdock 2010). In the absence of data on the actual use, if any, of bay oil as a flavouring agent in foods sold in Canada, the per capita intake estimate for the US population (Burdock 2010) is an acceptable estimate of possible Canadian dietary exposure for the general population 1 year of age and older to this substance from its potential use as a food flavouring agent (personal communication, email from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2020; unreferenced).
Exposure from natural occurrence in foods:
No definitive information is available concerning the natural occurrence of bay oil in foods. However, limited dietary exposure, if any, is expected to this substance from its natural presence in foods (Nijssen et al. 2018).
Products available to consumers
Depending on the source, bay oil may contain methyl eugenol at concentrations of 0% to 2%. In cosmetics in Canada, methyl eugenol is described as a restricted ingredient on the Cosmetic Ingredient Hotlist.Footnote 11 The Cosmetic Ingredient Hotlist describes methyl eugenol as only permitted as a naturally occurring component in botanical extracts to a maximum concentration of 0.01% in fine fragrances, 0.004% in eau de toilette, 0.002% in fragrance cream, 0.0002% in other leave-on products and oral hygiene products, and 0.001% in rinse-off products (HC 2019a).
Bay oil is present in products available to consumers. To evaluate the potential exposure to bay oil from cosmetics and NHPs applied by the dermal and inhalation routes, sentinel scenarios were selected based on a combination of use frequencies and reported concentrations of bay oil in these products. The selected sentinel scenarios below represent the highest exposures, relative to other cosmetics and age groups as well as NHPs where bay oil is used as an NMI, based on identified products reported to contain this substance. Given the presence of methyl eugenol on the Cosmetic Ingredient Hotlist as being restricted in such products, it has been assumed that conditions indicated on the Cosmetic Ingredient Hotlist are met and that the exposures to bay oil are based on assuming the maximum permitted methyl eugenol Cosmetic Ingredient Hotlist concentrations in each product.
The use of 100% bay oil in DIY products such as aromatic diffuser, massage oil, bath oil product, and body moisturizer were assessed. Although the upper concentration reported for massage oil containing bay oil was 100%, massage oils are typically diluted prior to use. Therefore, the maximum concentration of bay oil in DIY massage oil was assumed to be 3% (RIVM 2006). It is reported that body products are typically diluted to concentrations of 1% to 4% (Tisserand Institute 2021). Based on this information, the maximum concentration of bay oil in DIY body moisturizer was assumed to be 3%.
In the absence of any chemical-specific dermal absorption data, a dermal absorption value of 40% for methyl eugenol was used to estimate systemic exposure from dermal exposure (EC, HC 2010). To account for the amount of product absorbed by the dermal route, the product amount available for inhalation was adjusted by 60%, except for body moisturizer. For body moisturizer, since the product amount for inhalation was adjusted for the exposed surface area, and since this value was less than 60% of the product amount, no further adjustment was made to the product amount.
Lowest and highest systemic (sum of inhalation and dermal) daily exposure estimates as well as calculated lifetime average daily doses (LADDs) of methyl eugenol in bay oil from cosmetics and NHPs are summarized in Tables 7-1 and 7-2, respectively. Lowest and highest systemic daily exposure estimates and LADDs of methyl eugenol from DIY products containing bay oil are summarized in Table 7-3.
| Product scenario | % of methyl eugenol in product | Systemic daily exposurea (mg/kg bw/day) | LADDb (mg/kg bw/day) |
|---|---|---|---|
| Fine fragrance (spray) | 0.01% | 2.66 x 10-4 (14–18 years) to 4.15 x 10-4 (4–8 years) | 2.94 x 10-4 |
| Eau de toilette | 0.004% | 1.24 x 10-4 (14–18 years) to 1.90 x 10-4 (4–8 years) | 1.31 x 10-4 |
| Fragrance cream | 0.002% | 1.06 x 10-3 (14-18 years) to 2.05 x 10-3 (0-5 months) | 1.17 x 10-3 |
| Hair conditioner (leave-on) | 0.0002% | 1.39 x 10-5 (14–18 years) to 2.34 x 10-5 (adults) | 2.12 x 10-5 |
| Body cleanser | 0.001% | 9.35 x 10-6 (adults) to 3.47 x 10-5 (0–5 months) | 1.08 x 10-5 |
a Only lowest to highest exposed age groups are presented. Exposure estimates for bay oil have been adjusted assuming that methyl eugenol is present at the maximum permitted concentrations indicated on the Hotlist.
b LADD = Lifetime average daily dose; see Appendix A for calculation details.
| Product scenario | % of bay oil in product | Systemic daily exposurea (mg/kg bw/day) | LADDb (mg/kg bw/day) |
|---|---|---|---|
| Inhaler stick | 0.06% | 1.33 x 10-5 (adults) | 1.01 x 10-5 |
| Respiratory air spray | 2% | 8.48 x 10-6 (adults) | 6.41 x 10-6 |
a Only lowest to highest exposed age groups are presented. Exposure estimates for bay oil have been adjusted for a maximum concentration of 2% methyl eugenol.
b LADD = Lifetime average daily dose; see Appendix A for calculation details.
| Product scenario | % of bay oil in the final product | Systemic daily exposurea (mg/kg bw/day) | LADDb (mg/kg bw/day) |
|---|---|---|---|
| Aroma diffuser | 100% | 2.07 x 10-2 (adults) to 3.93 x 10-2 (1 year) | 2.3 x 10-2 |
| Massage oil | 3% | 1.25 x 10-3 (adults) to 9.13 x 10-3 (0–5 months) | 1.37 x 10-2 |
| Bath oil product | 100% | 8.37 x 10-4 (adults) to 1.35 x 10-5 (9–13 years) | 7.87 x 10-4 |
| Body moisturizer | 3% | 3.18 x 10-2 (adults) to 6.13 x 10-2 (0–6 months) | 3.5 x 10-2 |
a Only lowest to highest exposed age groups are presented. Exposure estimates for bay oil have been adjusted for a maximum concentration of 2% methyl eugenol.
b LADD = Lifetime average daily dose; see Appendix B for calculation details.
7.1.2 Health effects assessment
There are no empirical health effects data available and no international assessments for bay oil.
In order to inform the health effects assessment, the hazard information available for the main components of bay oil, eugenol (46%–56%), chavicol (9%–22%), beta-myrcene (13%–26%), and methyl eugenol (0%–2%) was considered.
Eugenol was considered to be a substance of low hazard potential; therefore, the risk for human health from exposure to eugenol was considered to be low by ECCC and HC in a screening assessment (ECCC, HC 2018d).
There are no empirical health effects data available and no international assessments for chavicol.
The International Agency for Research on Cancer (IARC) considers beta-myrcene as a medium priority agent to be evaluated and classifies it in group 2B: possibly carcinogenic to humans (IARC 2014). The Office of Environmental Health Hazard Assessment (OEHHA) in California is adding Beta-Myrcene to the list of chemicals known to the state to cause cancer in laboratory animals for purposes of Proposition 65 (OEHHA 2018). Beta-myrcene is also not considered genotoxic (IARC 2014; US FDA 2018; OEHHA 2018; ECCC, HC 2020). A no-observed-adverse-effects level (NOAEL) of 250 mg/kg bw/day beta-myrcene was determined for reproductive and developmental toxicity based on a decrease in the number of implantation sites of live fetuses and an increase in the frequency of skeletal malformations as fused zygomatic, dislocated sternum, and lumbar extra ribs at 500 mg/kg bw/day in rats by ECCC and HC (2020).
The US National Toxicology Program (NTP) has classified methyl eugenol as reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity in experimental animals (NTP 2000). The IARC also evaluated methyl eugenol and concluded that there is sufficient evidence from experimental animals for its carcinogenicity (IARC 2013). The IARC therefore classified methyl eugenol as possibly carcinogenic to humans (group 2B) (IARC 2013).
Consistent with the NTP 2-year bioassay in 2005, the Government of Canada has concluded that methyl eugenol is genotoxic and carcinogenic and determined a lowest-observed-adverse-effects level (LOAEL) of 37 mg/kg bw/day, the lowest dose tested, based on the presence of liver and glandular stomach tumours in both rats and mice in both sexes (NTP 2000; EC, HC 2010).
For methyl eugenol, there is no published benchmark dose (BMD) value derived by an international agency. However, there are several BMD values available in the scientific literature based on the same standard 2-year NTP carcinogenic study (2000) and used by the Government of Canada to assess the risk of methyl eugenol (EC, HC 2010).
In a first genotoxicity and carcinogenic evaluation study of compounds that can be found in food and plant-based supplements, BMDL10 range values were obtained with the US EPA BMDS software version 2.1.2 using different models, including the Gamma, Logistic, Log-logistic, Probit, Log-probit, Multistage, Weibull, and Quantal linear model. BMD software was applied using default settings for model restrictions, risk type (extra), confidence level (95%), and basal metabolic rate (10%). Dose-response data for induction of hepatocellular carcinoma in male and female F344/N rats was calculated and adjusted for the duration of exposure (5 days instead of 7 days per week dosing regimen) (Van den Berg et al. 2011). For methyl eugenol, BMD10 values of 15.3 mg/kg bw/day to 34 mg/kg bw/day were determined for male rats and values of 48.8 mg/kg bw/day to 73.6 mg/kg bw/day were determined for female rats (Van den Berg et al. 2011).
In a second risk assessment study, the authors estimated BMDs to determine the MOEs in different herbal beverages (Suparmi et al. 2019). To determine the BMDL10, the dose-response data for induction of hepatocellular carcinoma in male and female F344/N rats were calculated and adjusted with the time of exposure (5 days instead of 7 days per week dosing regimen) (Suparmi et al. 2019). BMDL10 values were obtained by following EFSA Scientific Committee recommendations through averaging model results rather than selecting or rejecting a specific model (EFSA 2017). The approach also used EFSA’s tool for BMD analysis, which implements statistical methods for R-package PROAST. BMDL10 model averaging was performed using the default settings. The BMD10 value for male rats was determined to be 22.2 mg/kg bw/day and 66.5 mg/kg bw/day for female rats (Suparmi et al. 2019). Both values land in the range of BMD10 values determined in the previous study.
Other studies were also considered but did not follow US EPA or EFSA guidelines for cancer analysis (Rietjens et al. 2008; Smith et al. 2010).
In general, the mouse was observed to be the most sensitive species and resulted in the lowest observed doses for tumour endpoints. However, the modelling of the mouse data was found to be problematic due to the absence of a dose-response trend; as a result, data could not be fitted well to any statistical model. Because of these difficulties with the mouse data, many authors have selected dose-response data based on hepatic adenomas in male rats to derive a BMD for methyl eugenol (Rietjens et al. 2008; Smith et al. 2010; Van den Berg et al. 2011; Suparmi et al. 2019).
7.1.3 Characterization of risk to human health
The identified endpoint of concern for bay oil is genotoxic carcinogenicity for methyl eugenol with a BMDL10 of 22.2 mg/kg bw/day based on a significantly increased incidence of hepatocellular carcinoma in male rats in a dose-related manner in a 2-year NTP carcinogenicity study (2000) (Suparmi et al. 2019). To determine the risk from bay oil containing methyl eugenol, MOEs were determined by comparing the estimated LADDs resulting from product use to the BMDL10 for methyl eugenol.
LADDs and resulting MOEs for methyl eugenol in bay oil from cosmetics containing bay oil are summarized in Table 7-4, while those from food and NHPs are summarized in Table 7-5. LADDs and MOEs from DIY products containing bay oil are summarized in Table 7-6.
| Exposure scenario | LADDa (mg/kg bw/day) | MOEb |
|---|---|---|
| Systemic exposure by the dermal and inhalation routes from fine fragrance (0.01%) | 2.94 x 10-4 | >75 000 |
| Systemic exposure by the dermal and inhalation routes from eau de toilette (0.004%) | 1.31 x 10-4 | >169 000 |
| Systemic exposure by the inhalation route from fragrance cream (0.002%) | 1.17 x 10-3 | >19 000 |
| Systemic exposure by the dermal and inhalation routes from hair conditioner (leave-on) (0.0002%) | 2.12 x 10-5 | >1 000 000 |
| Systemic exposure by the dermal and inhalation routes from body cleanser (0.001%) | 1.08 x 10-5 | >2 000 000 |
a The LADDs were adjusted assuming the maximum Cosmetic Ingredient Hotlist concentrations of methyl eugenol.
b The MOEs were calculated using a BMDL10 of 22.2 mg/kg bw/day based on the carcinogenicity of methyl eugenol.
| Exposure scenario | LADDa (mg/kg bw/day) | MOEb |
|---|---|---|
| Food flavouring agent (1 year of age and older) | 6.78 x 10-6 | >3 000 000 |
| Systemic exposure by the inhalation route from an inhaler stick (0.06%) | 1.01 x 10-5 | >2 000 000 |
| Systemic exposure by the inhalation route from a respiratory air spray (2%) | 6.41 x 10-6 | >3 000 000 |
a For bay oil as a food flavouring agent, as well as in inhaler stick and respiratory air spray, the LADDs were adjusted for a maximum concentration of 2% methyl eugenol.
b The MOEs were calculated using a BMDL10 of 22.2 mg/kg bw/day based on the carcinogenicity of methyl eugenol.
| Exposure scenario | LADDa (mg/kg bw/day) | MOEb |
|---|---|---|
| Systemic exposure by the dermal and inhalation routes from DIY aromatic diffuser (100%) | 2.3 x 10-2 | 965 |
| Systemic exposure by the dermal and inhalation routes from DIY massage oil (3%) | 1.6 x 10-3 | 13 891 |
| Systemic exposure by the dermal and inhalation routes from a DIY bath oil product (100%) | 7.88 x 10-4 | 28 000 |
| Systemic exposure by the dermal and inhalation routes from DIY body moisturizer (3%) | 3.5 x 10-2 | 633 |
a For bay oil in aromatic diffuser, massage oil, bath oil product, and body moisturizer, the LADDs were adjusted for a maximum concentration of 2% methyl eugenol.
b The MOEs were calculated using a BMDL10 of 22.2 mg/kg bw/day based on the carcinogenicity of methyl eugenol.
The MOEs for bay oil from cosmetics, food flavouring agent, inhaler stick, and respiratory air spray (NHPs) are considered adequate to address uncertainties in the health effects and exposure data used to characterize risk.
The MOEs for bay oil from a DIY bath oil and a DIY massage oil are considered adequate to address uncertainties in the health effects and exposure data used to characterize risk.
For exposures to bay oil in DIY aromatic diffuser and DIY body moisturizer, the MOEs between the critical effect levels and the estimates of exposure listed in Table 7-6 are below 10 000, which accounts for uncertainties with respect to interspecies extrapolation, intraspecies extrapolation, the POD, and the adequacy of the database but are considered potentially inadequate.
7.1.4 Uncertainties in evaluation of risk to human health
The key sources of uncertainty are presented in the table below.
| Key source of uncertainty | Impact |
|---|---|
| When calculating the risk from methyl eugenol in bay oil for aromatic diffuser, massage oil, body moisturizer, and bath oil product, it was assumed that the source of bay oil contained 2% methyl eugenol. Reported methyl eugenol concentrations in bay oil can vary from 0% to 2%, depending on the source. | + |
| There are no short-term, chronic, reproductive/developmental toxicity, or carcinogenicity studies identified for bay oil. | +/- |
| Route-to-route extrapolation for bay oil was carried out for dermal and inhalation scenarios in comparison to an effect level from an oral study. | +/- |
+ = uncertainty with potential to cause overestimation of exposure/risk; - = uncertainty with potential to cause underestimation of exposure/risk; +/- = unknown potential to cause over- or underestimation of risk.
7.2 Tarragon oil
7.2.1 Exposure assessment
Environmental media
No uses were indicated in CEPA section 71 survey data for tarragon oil. No reports of monitoring for tarragon oil in environmental media in Canada or elsewhere were identified. According to information considered under the ERC, tarragon oil was classified as having a low ecological exposure potential (see section 6.1).
Food
No definitive information is available concerning the potential use of tarragon oil as a food flavouring agent in foods sold in Canada. However, since tarragon oil is known to be used as a food flavouring agent internationally, it is possible that this substance is present as a flavouring agent in foods sold in Canada.
The per capita (“individual”) estimated intake of tarragon oil as a food flavouring agent is 5.37 x 10-1 μg/kg bw/day (Burdock 2010). This estimate was based on annual production volumes reported by the food industry (NAS 1989 as cited in Burdock 2010) and assumes that only 60% of the actual volume produced was reported and that the entire amount produced was consumed by only 10% of the US population.
In the absence of data on the actual use, if any, of tarragon oil, as a flavouring agent in foods sold in Canada, the per capita intake estimates for the US population (Burdock 2010) are acceptable estimates of possible Canadian dietary exposure for the population 1 year of age and older to this substance from its use as a food flavouring agent.
Trans-anethole in breast milk was detected following ingestion of capsules containing 100 mg of trans-anethole by 15 lactating women (Hausner et al. 2008). Concentration of trans-anethole peaked ~2 to 6 hours post consumption (23.2 µg/L) and returned to baseline within 8 hours post ingestion.
Exposure from natural occurrence in foods:
No definitive information is available concerning the natural occurrence of tarragon oil in foods. Tarragon oil is obtained from plant material of the named plant source (Burdock 2010; VCF 2020). There is expected to be little dietary exposure, if any, to this substance from its natural presence in foods (Nijssen et al. 2018).
Products available to consumers
Tarragon oil is present in products available to consumers. To evaluate the potential for exposure to tarragon oil from cosmetics and NHPs applied by the dermal route, sentinel scenarios were selected based on a combination of use frequencies and reported concentrations of tarragon oil in these products. The selected sentinel cosmetic scenarios represented the highest exposures, relative to other dermally applied cosmetics as well as NHPs where tarragon oil is used as an NMI, based on identified products reported to contain this substance. Exposures to tarragon oil from the use of body moisturizer, body fragrance, facial moisturizer, facial cleanser, and soap were considered to be the sentinel scenarios for dermal applications (personal communication, email communication from the Consumer and Hazardous Product Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2020; unreferenced).
For the use of 100% tarragon oil in DIY products, the highest daily exposures are expected to occur from DIY aromatic diffuser with a reported upper concentration of 100%, the use of a body moisturizer diluted to a concentration of 3% (Tisserand institute 2021), a DIY massage oil diluted to a concentration of 3% (RIVM 2006), and a DIY bath oil product with a reported upper concentration of 100%.
Tarragon oil is also present as an NMI for the purpose of flavour enhancement in oral NHPs; oral exposure to tarragon oil was therefore quantified for such products (LNHPD 2021). The non-medicinal role of tarragon oil when used as a flavour enhancer is associated with an oral restriction of up to 0.05 mg/kg bw/day (NHPID [modified 2021]). This restriction is recommended in Tisserand and Young (2014) based on the estragole and methyl eugenol content in tarragon oil and the potential genotoxic and carcinogenic effects of estragole and methyl eugenol. When tarragon oil is used topically, this restriction is up to 0.12% (Tisserand and Young 2014).
In the absence of any chemical-specific dermal absorption data, a dermal absorption value of 40% for methyl eugenol was used to estimate systemic exposure from dermal exposure (EC, HC 2010). To account for the amount of product absorbed by the dermal route, the product amount available for inhalation was adjusted by 60%, except for body moisturizer. For body moisturizer, since the product amount for inhalation was adjusted for the exposed surface area, and since this value was less than 60% of the product amount, no further adjustment was made to the product amount.
The systemic daily exposures were estimated by summing exposures via all relevant routes for the scenario (oral, dermal, inhalation). Exposure estimates for the lowest and highest exposed age groups from products, as well as calculated LADDs, are summarized in Table 7-8.
In addition, systemic daily exposure estimates for the lowest and the highest exposed age groups and LADDs for tarragon oil from DIY products are summarized in Table 7-9.
| Product scenario | % in product | Systemic daily exposure (mg/kg bw/day)a | LADDb (mg/kg bw/day) |
|---|---|---|---|
| Body moisturizer | 0.3% | 1.59 x 10-1 (14–18 years) to 3.07 x 10-1 (0–5 months) | 1.75 x 10-1 |
| Body fragrance | 100% | 3.12 (14-18 years) to 4.68 (4–8 years) | 3.44 |
| Facial moisturizer | 0.1% | 1.05 x 10-2 (14–18 years) to 1.75 x 10-2 (adults) | 1.46 x 10-2 |
| Facial cleanser | 0.1% | 2.68 x 10-4 (14–18 years) to 3.70 x 10-4 (9–13 years) | 2.73 x 10-4 |
| Soap | 0.3% | 3.64 x 10-4 (adults) to 4.78 x 10-4 (1 year) | 3.86 x 10-4 |
| Digestive aid capsule (NHP) | 0.0026% | 1.26 x 10-4 (adults) | 9.57 x 10-5 |
a Only lowest and highest exposed age groups are presented.
b See Appendix A for calculation details.
| Product scenario | % in product | Systemic daily exposure (mg/kg bw/day)a | LADDb (mg/kg bw/day) |
|---|---|---|---|
| Aromatic diffuser | 100% | 1.09 (adults) to 1.96 (1 year) | 1.20 |
| Massage oil | 3% |
6.25 x 10-22 (adults) to 4.56 x 10-1 (0–5 months) |
8 x 10-2 |
| Bath oil product | 100% |
4.30 x 10-2 (adults) to 6.95 x 10-2 (9–13 years) |
4.05 x 10-2 |
| Body moisturizer | 3% |
1.59 (14–18 years) to 3.07 (0–5 months) |
1.75 |
a Only lowest and highest exposed age groups are presented.
b See Appendix B for calculation details.
7.2.2 Health effects assessment
There are no empirical health effects data available and no international assessments for tarragon oil, except for an unscheduled DNA synthesis (UDS) assay in rat liver (Nesslany et al. 2010) and an Ames test with Saccharomyces cerevisiae (in vitro study) (Tateo et al. 1989), which both showed positive results.
In order to inform the health effects assessment, the hazard information available for the main components of tarragon oil, methyl eugenol (2%–39%), estragole (0%–82%), sabinene (trace–39%), terpinolene (0.5%–25%), cis-anethole (51%–81%), trans-anethole (21.1%–53%), and elemicin (trace–57%) were considered.
Methyl eugenol
Methyl eugenol is determined to be a genotoxic carcinogen, and its BMDL10 is 22.2 mg/kg bw/day. Further information on the health effects of methyl eugenol is provided in the Health Effects Assessment section for bay oil (section 7.1.2).
Estragole
Estragole was determined to be a similar genotoxic carcinogen to methyl eugenol by the OEHHA, EFSA, IFRA, and European Medicines Agency (EMA) (OEHHA 1999; EFSA 2009; IFRA 2015; EMA 2020). IFRA calculated a reference dose for fragrances of 0.04 mg/kg bw/day for long-term exposure (IFRA 2015). EFSA determined BMDL10 values of between 9.2 mg/kg bw/day and 32.7 mg/kg bw/day based on the 12-month carcinogenic study from Miller et al. (1983), and the EMA used these same values for its risk assessment of estragole (EMA 2015). However, in the carcinogenic study, animals were exposed via the diet, and the authors of the study did not provide the amount of treated food consumed by animals. In addition, EFSA did not provide the justification and the calculation of the estimated daily dose of estragole that they used to derive the BMDL10 values.
In carcinogenicity oral studies, male and female preweaning CD-1 mice were administered 2.5 mmol estragole/kg bw (equivalent to 370 mg/kg bw) by gavage twice a week for 5 weeks (Miller et al. 1983). Within 11 to 14 months following the last day of exposure, a significant increase of hepatomas was observed in male mice but not in female mice (Miller et al. 1983).
In another carcinogenicity study by the same authors, adult female CD-1 mice (50/dose) were administrated 0, 2300, or 4600 mg/kg diet, 3 times per week for 12 months. A subset of animals had a 6-month recovery period without treatment (Miller et al. 1983). All animals fed with estragole showed a significantly higher number of hepatic angiosarcomas, hemangioendotheliosarcomas, and hepatomas (Miller et al. 1983). In addition, nodules in the liver were observed in both estragole groups at 12 months, but nodules gradually regressed after administration because they were reduced at 18 months (Miller et al. 1983). Livers showed various degrees of chronic inflammation, fibrosis, bile duct proliferation, and number of ceroid-laden histocytes and cellular hyperplasia and megalocytosis (Miller et al. 1983). The authors of the study did not provide a measure of the consumption of estragole by animals during the treatment, which impeded the ability to estimate daily exposure.
In a carcinogenicity study, preweaning male CD-1 mice (50/dose) were exposed to 0, 4.4 or 5.2 µmol/kg bw (equivalent to 0, 651, or 769.6 µg/kg bw, respectively) in total after 4 subcutaneous injections on postnatal days (PNDs) 1, 8, 15, and 22 (Drinkwater et al. 1976). At 15 months, the number of hepatocellular carcinomas in treated animals was higher than in control animals (Drinkwater et al. 1976).
Overall, the animal laboratory studies showed a clear carcinogenic potential of estragole.
In a 90-day study, male and female F344/N rats were orally administered 0, 37.5, 75, 150, 300, or 600 mg/kg bw/day estragole by gavage for 5 days per week (NTP 2011a). No mortality was observed, but a significant decrease in mean body weight or body-weight gain was observed at 300 and 600 mg/kg bw/day in both sexes of rats. Hematological parameters showed statistically significant and dose-dependent changes in male or female rats, which included microcytic, normochromic, and nonresponsive anemia. Changes in hematological parameters were reported to intensify with duration and dose, ranging from subtle alterations at low dose exposure to statistically significant changes observed in the 300 or 600 mg/kg bw/day dose groups at study termination (week 14 of exposure). The study authors suggested that the hematological changes are indicators of ineffective erythropoiesis (NTP 2011a). The authors also reported bone marrow hyperplasia, increase in kidney weight, changes in tubular histology, degeneration of olfactory epithelium, hypertrophy of chromophobe cells in the pars distalis of the pituitary gland, atrophy of gastric glands and testes, and increase in the incidence of degeneration of germinal epithelium of testis and bilateral hypospermia at 300 and 600 mg/kg bw/day in both sexes (NTP 2011a). The level of enzymes including serum alanine aminotransferase, sorbitol dehydrogenase, and bile salt increased significantly, and these changes were consistent with histopathological lesions observed at 300 and 600 mg/kg bw/day in the liver of both sexes. Gross changes showed a significant increase in absolute and relative liver weight and discolouration followed by a histopathological evaluation of liver, which exhibited a dose-dependent increase in hepatocyte hypertrophy, oval cell hyperplasia, bile duct hyperplasia, and evidence of chronic inflammation in all dose groups in both sexes (NTP 2011a). Hepatocellular adenoma was observed in 1/10 male rats, and cholangiocarcinoma (bile duct cancer) was present in 2/10 male rats at 600 mg/kg bw/day. The study authors concluded that the liver was the primary target organ of estragole in rats (NTP 2011a).
In a 90-day study, male and female B6C3F1 mice were orally administered 0, 37.5, 75, 150, 300, or 600 mg/kg bw/day estragole by gavage for 5 days per week (NTP 2011a). Estragole treatment caused death in one male mouse at 600 mg/kg bw/day during week 9. All female mice died during week 1 after exposure to 600 mg/kg bw/day, which the study authors linked to liver necrosis caused by estragole (NTP 2011a). As was observed in rats, the study authors reported liver as the target organ in mice based on increased liver weight, hepatocyte hypertrophy, hepatocellular degeneration, oval cell hyperplasia, and necrosis in all dose groups except for 37.5 mg/kg bw/day (NTP 2011a). Statistically significant degeneration of the gastric glands of the glandular stomach, squamous hyperplasia, mineralization, and forestomach ulcer and degeneration of olfactory epithelium were observed mostly at 300 and 600 mg/kg bw/day (NTP 2011a).
Overall, the study authors concluded that liver was the primary target organ in male and female rats and mice following subchronic 90-day exposures to estragole. Estragole was also reported to show carcinogenic activity. However, it was acknowledged that this 90-day exposure study did not assess the full carcinogenic potential of estragole (NTP 2011a). It was also acknowledged in the NTP study (2011a) that ample studies have shown hepatocarcinogenic potential of estragole or structurally related substances (safrole or methyl eugenol) in rats and mice following long-term exposure.
However, the available literature cannot be used to derive a BMDL10. In the absence of adequate dose-response studies on estragole, a read-across approach was taken, and hazard information on the read-across analogue, methyl eugenol, was used to characterize the carcinogenic risk.
Results of genotoxicity testing of estragole were generally negative in Salmonella typhimurium (S. typhimurium) (Drinkwater et al. 1976; Swanson et al. 1979; Sekizawa and Shibamoto 1982; NTP 2011a) and in the Bacillus subtilis Rec assay (Sekizawa and Shibamoto 1982), but also in hamster V79 cells likely due to the complex metabolism required for bioactivation in vivo. Positive results were reported for estragole when the putative toxic metabolites, namely 1’-hydroxyestragole and allyl epoxides, were positive in mutagenicity assays with or without exogenous activation (EMA 2020).
Estragole was positive in V79 cells with the sister chromatid exchange assay and the alkaline comet assay and in two CHO cell lines with the Comet assay (Martins et al. 2012). All assays were positive without metabolic activation, suggesting that estragole, besides being metabolized to genotoxic metabolites, may also be a direct-acting genotoxic that forms DNA adducts. Several in vivo studies in adult rodents showed that the genotoxicity of estragole itself is induced by DNA adducts detected with UDS assay in liver (Phillips et al. 1984; Randerath et al. 1984; Nesslany et al. 2010; Paini et al. 2012; Suzuki et al. 2012), in lungs and kidneys (Paini et al. 2012), and in vitro in human hepatoma (HepG2) cells (Zhou et al. 2007). Estragole is considered to be genotoxic by specifically producing DNA adducts in animal models and human liver cells and is considered carcinogenic in animal models.
Estragole is an alkenylbenzene and is structurally related to methyl eugenol (IARC 2014). Chemically, estragole is 4-methoxyallylbenzene, and methyl eugenol is 3,4-dimethoxyallylbenzene. The only structural difference between estragole and methyl eugenol is that the latter contains a second ring methoxy group. Since they contain the same skeletal structure and ring substituents, both substances are expected to exhibit similar metabolic fate, pharmacokinetics, and toxicological potential. All have comparable physicochemical properties and mechanistic profiles, are bioavailable, and test positive for carcinogenicity (genotox) in OECD QSAR Toolbox (OECD QSAR Toolbox 2016). Methyl eugenol and estragole are metabolized primarily by CYP1A2 that produce similar genotoxic and carcinogenic metabolites (IARC 2014). Methyl eugenol, as estragole, is not mutagenic in bacteria but induces chromosomal aberrations in vitro and DNA adducts in the liver of rodents in vivo and in human liver cells in vitro (IARC 2014).
Sabinene
The only health effects information available for sabinene is a positive result in a bacterial reverse mutation assay using S. typhimurium strain TA 1535 in the absence or presence of metabolic activation (ECHA Registration dossier 2017). In the absence of genotoxicity, carcinogenicity, or reproductive/developmental toxicity data of sabinene, the EFSA used a NOAEL of 222 mg/kg bw/day for beta-caryophyllene (bicyclic, non-aromatic hydrocarbon group) as a supporting substance for its assessment of sabinene (EFSA 2015).
Terpinolene
Carcinogenicity studies were not identified for terpinolene.
Terpinolene was neither irritating nor a sensitizer to human skin at a concentration of 20% in petrolatum applied for 48 hours under a patch (Opdyke 1988).
In a reproductive and developmental toxicity study conducted according to OECD test guidelines, 422 male and female Sprague-Dawley rats (10/dose/sex) were orally administered 0, 800, 2500, or 5000 ppm (equivalent to 0, 54.1, 154.6, or 300.8 mg/kg bw/day, respectively) terpinolene by diet during premating and mating periods for both sexes (up to 42 days for males) and during gestation and early lactation for females only (approximately 100 days for females) (ECHA Registration dossier 2020a). Some males had a recovery period of 2 weeks. No mortality or clinical signs were observed by study authors. A significant reduction in body weight and dietary intake was observed in males and females in the 5000 ppm group and in females in the 2500 ppm group. A reduction in body weight was also observed in females in the 800 ppm group. Study authors concluded that body weight effects were a result of the low palatability of the diet (ECHA Registration dossier 2020a). No treatment-related effects were detected in mating performance, fertility, or gestation lengths by study authors. Only males in the 5000 ppm group showed an increase in liver weight (absolute and relative to body weight). Males in the 2500 and 5000 ppm groups showed centrilobular hepatocellular hypertrophy, which was reversible following 2 weeks without treatment. Reduced pup body weight at PND 7 was detected in the 5000 ppm litter group. On the basis of these results, study authors determined NOAELs for general toxicity of 151.5 and 294.6 mg/kg bw/day (2500 and 5000 ppm) for females and males, respectively, a NOAEL for maternal and developmental toxicity of 2500 ppm (corresponding to 356 mg/kg bw/day), and a NOAEL for reproductive toxicology of 5000 ppm, the highest dose tested (corresponding to 294.6 mg/kg bw/day) (ECHA Registration dossier 2020a).
Terpinolene was not genotoxic in the micronucleus assay or sister chromatid exchanges assay and had no effect on 8-oxo-2-deoxyguanosine level in cultured human blood cells (Turkez et al. 2014). Terpinolene was also not genotoxic in the gene mutation test, Ames test, chromosome aberration test, and micronucleus assay in human lymphocytes with or without metabolic activation (ECHA Registration dossier 2020a).
Cis-anethole and trans-anethole
Both anetholes are distributed and metabolized similarly in rats, rabbits, humans, and mice (Tisserand and Young 2014). Trans-anethole undergoes biotransformation by three principal pathways: O-demethylation, N-oxidation, and epoxidation. The third pathway can lead to toxic metabolites and is considered a minor pathway (3%) in humans (Tisserand and Young 2014). In rodents, trans-anethole is metabolized differently depending on dose, but this is not the case in humans (Tisserand and Young 2014).
Cis-anethole is also called (Z)-anethole and is significantly more toxic than the common (E)-anethole (trans-anethole) (Tisserand and Young 2014). Both cis- and trans-anethole are considered to be isomers of estragole (US NLM 2014). The LD50 for the oral route was determined to be 150 mg/kg for cis-anethole in rats (RTECS 2019). There are no other empirical health effects data available for cis-anethole.
In Australia, trans-Anethole was previously reviewed by NICNAS in a 2016 priority existing chemical assessment, and the only health effect reported was skin sensitization (NICNAS 2016a). A safety evaluation of trans-anethole by the WHO concluded that trans-anethole is unlikely to be genotoxic, but insufficient data were available to permit a final evaluation of the significance of the malignant liver tumours observed in rodents (WHO 1991). A NOAEL of 300 mg/kg bw/day was determined by the WHO on the basis of hepatocellular hypertrophy in rats at 600 mg/kg bw/day after a 90-day oral exposure (WHO 1999).
Male and female CD-1 mice (5/sex/dose) were orally administered 0, 60, 120, 240, 360, or 500 mg/kg bw/day trans-anethole by diet for 28 days (Newberne et al. 1999). Study authors observed that the addition of trans-anethole to the diet resulted in a very low palatability for mice in higher doses, resulting in decreased food intake and body weight gain (Newberne et al. 1999). No clinical or histopathological changes were observed (Newberne et al. 1999).
The same authors then used the previous study to establish the dose range for a 90-day study. Male and female CD-1 mice (20/sex/dose) were orally administered 0, 30, 60, 120, or 240 mg/kg bw/day trans-anethole by diet for 90 days (Newberne et al. 1999). Increased mortality was observed at doses of 60 mg/kg bw/day and higher in males and at 120 mg/kg bw/day and higher in females. Study authors attributed these deaths to “inanition syndrome” characterized by decreases in food consumption, water intake, and physical activity, presumably associated with palatability of the diet (Newberne et al. 1999). Body weight decreased weekly at doses of 120 mg/kg bw/day and greater in males and at 240 mg/kg bw/day for females, accompanied by a decrease in daily food consumption. Gross and histopathological evaluation showed a reduction in liver glycogen at doses of 30 mg/kg bw/day and greater in males and at 60 mg/kg bw/day and greater in females. Reduced kidney, brain, and spleen weight in males at the highest dose was observed and correlated with decreased cellularity in these organs (Newberne et al. 1999). An increase in hepatocellular hypertrophy in males at doses of 60 mg/kg bw/day and greater as well as enlarged livers and a dose-dependent increase in relative liver weights in all groups of treated males was observed. Study authors considered these effects to be adaptive physiological responses resulting from the known metabolic pathways of trans-anethole in rodents (Newberne et al. 1999). No other difference, especially in histopathology, was observed between any group of treated females and controls. Blood chemical analysis revealed an increase in alanine and aspartate transaminase values at doses of 120 mg/kg bw/day and greater, which, in conjunction with hepatocyte hypertrophy, suggests enzyme induction according to the study authors’ conclusion (Newberne et al. 1999). An increase in alkaline phosphatase at doses of 120 mg/kg bw/day and greater was also observed in all treated animals. With the exception of the reduction in hepatocellular glycogen, no adverse effects were observed in the liver of female mice (Newberne et al. 1999). On the basis of the lack of any evidence of histopathology, a minimal change in blood serum, and the large decrease in dietary intake as a result of the unpalatability of the food in both sexes, the study authors assigned a NOAEL of greater than 240 mg/kg bw/day (Newberne et al. 1999). Since there was no recovery time to confirm that the effects seen in the liver, kidney, brain, and spleen of males were adaptative, a LOAEL of 30 mg/kg bw/day was determined on the basis of the dose-dependent liver toxicity in male mice.
In a reproductive study, male and female Wistar rats were administered 0% or 1% trans-anethole (actual dose of trans-anethole varied from 1400 mg/kg bw/day in the earlier weeks of treatment to 700 mg/kg bw/day at the end of the pre-mating period) in diet for 70 days (WHO 1999; ECHA Registration dossier 2020b). The rats were mated on a one-to-one basis for a maximum of 15 days, with 9 pairs of rats fed control diet (group I), 9 pairs fed treated diet (group IV), 10 pairs of males fed control diet and 10 pairs of females fed treated diet (group II), and 10 pairs of males fed treated diet and 10 pairs of females fed control diet (group III). After weaning, the offspring received the same dietary treatment as both of their parents. Feeding of the appropriate diet was maintained during pre-mating, mating, gestation, and lactation. Four generations were thus produced by following the same pattern. In addition, a cross-fostering experiment was conducted by mating six control and six treated females from the F1 generation with an equal number of F1 control males. At birth, the litters of control and treated dams were exchanged, and the litters were reared by a dam from the other group (WHO 1999; ECHA Registration dossier 2020b). All groups of rats treated with trans-anethole showed reduced body weight gain. Food consumption was reduced in the treated rats during the initial weeks of the study but was mostly comparable to that of the control group for the remainder of the treatment period, except in F2 males and females, the food consumption of which was significantly lower than that of the controls throughout the pre-mating period. There was no difference in reproductive parameters such as fertility index, gestation index, and pup viability or litter size (WHO 1999; ECHA Registration dossier 2020b). No clinical signs were observed by the study authors. The pup body weights per litter were reduced for all pups reared by treated dams, regardless of the diet fed to the males or to the dams during gestation. Thus, post-natal growth was influenced by the exposure of the dams to trans-anethole during lactation but not gestation; it was suggested by the study authors that the test material may be directly toxic via the milk rather than by an effect on the quality of nutrition (WHO 1999; ECHA Registration dossier 2020b).
In a one-generation reproductive study, female Sprague-Dawley rats (10/dose) were administered 0, 35, 175, or 350 mg/kg bw/day trans-anethole by gavage for 7 days before mating, 7 days during mating with untreated males, 21 days of gestation and then parturition, and up to day 4 of lactation (around 40 days in total) (WHO 1999). The body weights and food consumption of females in the high-dose group were significantly lower during the pre-mating, gestation, and lactation periods (WHO 1999). The same trend was observed in the middle-dose group but was not significant. During lactation, some of the animals in the high-dose group appeared to be in poor condition, as indicated by clinical observations such as emaciation, pale ungroomed coat, and stained fur (WHO 1999). Mating performance and fertility were not modified by treatment; however, an increase in stillborn pups, a decrease in liveborn pups (viability index), and a decrease in pup weight in the high-dose group were observed (WHO 1999). No such effect was seen in the low- and middle-dose groups. A NOAEL was established at 175 mg/kg bw/day (WHO 1999).
In a carcinogenicity study, male and female Sprague-Dawley rats (26-78/dose) were orally administered 0, 0.25, 0.5, or 1% trans-anethole (equivalent to 0, 100, 200, or 400 mg/kg bw/day for females and 0, 120, 250, or 550 mg/kg bw/day for males, respectively) by diet for 2 years (Truhaut et al. 1989). No clinical signs or mortality related to the treatment were observed. A transient retardation of body weight gain as a result of reduced food intake during the first weeks of treatment was noticed in both sexes (Truhaut et al. 1989). No toxicological effects were observed. Some neoplastic lesions were seen in the high-dose female group, such as hepatocellular adenomas and hepatocellular carcinomas, and non-neoplastic lesions in both sexes were observed in the high-dose group, while nodular hyperplasia was observed in males in the medium-dose group (Truhaut et al. 1989).The finding of this study was reviewed by independent pathologists, all of whom agreed that there was a clear increase in hepatocellular adenoma and/or carcinoma in females in the high-dose group (1%) but not in males at any dose level (WHO 1991).
Multiple studies in vitro showed that trans-anethole was not genotoxic in Ames assay using S. typhimurium strains TA98, TA100, TA1535, TA1537, or TA1538 (Hsia et al. 1979; Swanson et al. 1979; Nestmann et al. 1980; Sekizawa and Shibamoto 1982; Mortelmans et al. 1986; Heck et al. 1989; Gorelick 1995), in chromosomal aberration assay (Gorelick 1995), and in UDS assay (Heck et al. 1989; Marshall et al. 1989; Howes et al. 1990; Müller et al. 1994). Trans-anethole was also not genotoxic in either the in vivo micronucleus test in male Swiss mice (Abraham 2001) or the in vivo UDS assay (Marshall and Caldwell 1996). Trans-anethole was positive only in the mouse lymphoma assay in the presence of metabolic activation (Heck et al. 1989; Gorelick 1995).
Elemicin
Elemicin is an alkenylbenzene that is structurally related to methyl eugenol. It differs in that it has an additional methoxy group on the fifth carbon of the phenyl ring. A search of toxicological databases revealed a lack of hazard data for elemicin, with no chronic, short-term, developmental, or other animal studies identified.
In an in vitro UDS assay, metabolism of elemicin via the 1’-hydroxylation pathway resulted in DNA adduct formation via the same mechanism as methyl eugenol (Hasheminejad and Caldwell 1994). Additionally, elemicin has been shown to cause DNA breaks in a UDS assay in turkey fetal liver (Kobets et al. 2016) and in an in vitro cultured rat hepatocyte study (Hasheminejad and Caldwell 1994). There is no other hazard information on elemicin.
A comparison of elemicin with methyl eugenol shows that both have comparable physicochemical properties and mechanistic profiles, are bioavailable, and have alerts for OECD DNA binding, for in vivo mutagenicity, and for carcinogenicity (genotox) in OECD QSAR Toolbox (OECD QSAR Toolbox 2016). Methyl eugenol was the most similar analogue proposed (78.57%) by OECD QSAR Toolbox. Given these similarities, methyl eugenol is assumed in this assessment to be a suitable analogue for elemicin.
7.2.3 Characterization of risk to human health
Methyl eugenol, estragole, and elemicin were determined to be the main components of toxicological significance for tarragon oil. All three components are considered to be genotoxic and carcinogenic, with similar modes of action and potency. A BMDL10 of 22.2 mg/kg bw/day for methyl eugenol (Suparmi et al. 2019) was selected to characterize the risk resulting from the methyl eugenol, estragole, and elemicin in tarragon oil.
Taking into consideration the reported concentrations of methyl eugenol (2%–39%), estragole (0%–82%), and elemicin (0%–57%) in tarragon oil, it was assumed that the sum of methyl eugenol, estragole, and elemicin in tarragon oil was 100%.
The LADDs and resulting MOEs for tarragon oil when used as a food flavouring agent and in products available to consumers are summarized in Table 7-10. In addition, LADDs and resulting MOEs from DIY products were calculated and are summarized in table 7-11.
| Exposure scenario | LADDa (mg/kg bw/day) | MOEb |
|---|---|---|
| Food flavouring agent (1 year of age and older) | 5.37 x 10-4 | >41 000 |
| Systemic exposure by the dermal and inhalation routes from body moisturizer (0.3%) | 1.75 x 10-1 | 127 |
| Systemic exposure by the dermal and inhalation routes from body fragrance (100%) | 3.44 | 6 |
| Systemic exposure by the dermal and inhalation routes from a facial moisturizer (0.1%) | 1.46 x 10-2 | 1563 |
| Systemic exposure by the dermal and inhalation routes from a facial cleanser (0.1%) | 2.73 x 10-4 | >80 000 |
| Systemic exposure by the dermal and inhalation routes from a soap (0.3%) | 3.85 x 10-4 | >57 000 |
| Systemic exposure by the oral route from a digestive aid capsule (NHP) (0.0026%) | 9.57 x 10-5 | >200 000 |
a Calculation details are summarized in Appendix A.
b The MOEs were calculated using a BMDL10 of 22.2 mg/kg bw/day based on the carcinogenicity of methyl eugenol.
| Exposure scenario | LADDa (mg/kg bw/day) | MOEb |
|---|---|---|
| Systemic exposure by the dermal and inhalation routes from DIY aromatic diffuser (100%) | 1.20 | 18 |
| Systemic exposure by the dermal and inhalation routes from DIY massage oil (3%) | 8 x 10-2 | 278 |
| Systemic exposure by the dermal and inhalation routes from DIY bath oil product (100%) | 4.05 x 10-2 | 549 |
| Systemic exposure by the dermal and inhalation routes from DIY body moisturizer (3%) | 1.75 | 13 |
a Calculation details are summarized in Appendix B.
b The MOEs were calculated using a BMDL10 of 22.2 mg/kg bw/day based on the carcinogenicity of methyl eugenol.
MOEs to tarragon oil from food (based on tarragon oil’s potential use as a flavouring agent), a digestive aid capsule (NHP), and as a facial cleanser and soap are considered adequate to address uncertainties in the health effects and exposure data used to characterize risk.
However, the MOEs between the critical effect levels and the estimates of daily exposure from body moisturizer, body fragrance, or facial moisturizer are below 10 000. This accounts for uncertainties with respect to interspecies extrapolation, intraspecies extrapolation, the POD, and the adequacy of the database and is considered potentially inadequate. In addition, for exposures to tarragon oil from its use in DIY aromatic diffuser, as DIY massage oil, as a DIY bath oil product, or as a DIY body moisturizer, the MOEs between the critical effect levels and the estimates of exposure listed in Table 7-11 are below 10 000, which accounts for uncertainties with respect to interspecies extrapolation, intraspecies extrapolation, the POD, and the adequacy of the database and is considered potentially inadequate.
7.2.4 Uncertainties in evaluation of risk to human health
The key sources of uncertainty are presented in the table below.
| Key source of uncertainty | Impact |
|---|---|
| Tarragon oil was considered to be comprised solely of estragole, elemicin, and methyl eugenol. | + |
| There are no short-term, chronic, reproductive/developmental toxicity, or carcinogenicity studies identified for tarragon oil. | +/- |
| A BMDL10 for methyl eugenol was selected to represent the cancer potency of methyl eugenol, estragole, and elemicin combined. | +/- |
| There were no suitable studies for the dermal or inhalation route of exposure; therefore, route-to-route extrapolation for tarragon oil was carried out for dermal and inhalation scenarios by comparing to an effect level from an oral study. | +/- |
+ = uncertainty with potential to cause overestimation of exposure/risk; - = uncertainty with potential to cause underestimation of exposure/risk; +/- = unknown potential to cause over- or underestimation of risk.
7.3 Phenylpropanoids subgroup 1 (jasmine oil, perfumes and essences of jasmin)
7.3.1 Exposure assessment
Environmental media
In consideration of the low quantity (<100 kg) of the substance submitted in response to a CEPA section 71 survey (Environment Canada 2013), exposure to this substance from environmental media is not expected. No reports of monitoring for jasmine oil in environmental media in Canada or elsewhere were identified.
Food
No definitive information is available concerning the potential use of jasmine oil as a flavouring agent in foods sold in Canada. However, since jasmine oil is identified to be used as a food flavouring agent internationally, it is possible that this substance is present as a flavouring agent in foods sold in Canada.
The Fenaroli’s Handbook of Flavour Ingredients reports the per capita (“individual”) estimated intake of jasmine oil from its use as a food flavouring agent to be 9.88 x 10-3 μg/kg bw/day on the basis of a MSDI approach for the US population (Burdock 2010).
In the absence of data on the actual use, if any, of jasmine oil as a flavouring agent in foods sold in Canada, the per capita intake estimate for the US population (Burdock 2010) is an acceptable estimate of possible Canadian dietary exposure for the general population 1 year of age and older to this substance from its potential use as a food flavouring agent (personal communication, email from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2020; unreferenced).
Exposure from natural occurrence in foods:
No definitive information is available concerning the natural occurrence of jasmine oil in foods. Therefore, low dietary exposure to this substance, if any, is expected from its natural presence in foods (Nijssen et al. 2018).
Products available to consumers
Jasmine oil is present in products available to consumers. To evaluate the potential for exposure to jasmine oil from cosmetics and NHPs applied by the dermal and inhalation routes, sentinel scenarios were selected based on a combination of use frequencies and reported concentrations of jasmine oil in these products. The selected sentinel scenarios represented the highest exposures, relative to other cosmetics as well as NHPs where jasmine oil is used as an NMI, based on identified products reported to contain these substances. Exposure to jasmine oil from the use of hair conditioner, body cleanser, body moisturizer, facial moisturizer/acne treatment (NHP), topical treatment cream (NHP), body fragrance, aerosol hair styling product, antiperspirant/deodorant, temporary hair colour, sunscreen (NHP), and antiseptic skin cleanser (NHP) were considered to be the sentinel scenarios for dermal applications (personal communication, email communication from the Consumer and Hazardous Product Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2020; unreferenced; personal communication, email communication from the Natural and Non-prescription Health Products Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2020 and 2021; unreferenced).
Jasmine oil is also present in a lipstick product. Exposure by the oral route from the use of a lipstick was quantified (personal communication, email from the Consumer and Hazardous Product Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2020; unreferenced).
For the use of 100% jasmine oil in DIY products, the highest daily exposures are expected to occur from the use of the oil in aromatic diffuser, massage oil, bath oil product, body moisturizer, and as a facial steamer, with a reported upper concentration of 100%. Although the upper concentration reported for massage oil containing jasmine oil was 100%, massage oils are typically diluted prior to use. Thus, the maximum concentration of jasmine oil in DIY massage oil was assumed in RIVM (2006) to be 3%. It is reported that body products are typically diluted to concentrations of 1% to 4% (Tisserand Institute 2021). On the basis of this information, the maximum concentration of jasmine oil in DIY body moisturizer was assumed to be 3%.
Information from the American Cleaning Institute’s website indicates potential use of jasmine oil as a fragrance ingredient in all-purpose cleaners, dish detergent, laundry conditioner, and liquid laundry detergent (ACI 2020). To assess potential exposure to jasmine oil from its use in household cleaning products, exposures from the use of jasmine oil in a liquid and aerosol all-purpose cleaner at a maximum concentration of 1%, a 5% aerosol laundry conditioner, and a 5% liquid laundry detergent for machine-washing were quantified (ACI 2020).
To calculate systemic exposure from dermal exposure to jasmine oil, a dermal absorption value of 50% was selected based on the following considerations. The dermal absorption of benzyl benzoate and benzyl acetate, which are two of the main components of jasmine oil, was determined in an in vivo study in rhesus monkeys. Values were obtained in non-occluded and occluded monkey skin. The values of the dermal absorption in unoccluded site were 34.6% and 57% for benzyl acetate and benzyl benzoate, respectively, with absorption occurring primarily during the first 24 hours after topical administration. The absorption of benzyl benzoate and benzyl acetate may be predictive of the ability of these compounds to be absorbed when applied on human skin (Bronaugh et al.1990).
To estimate systemic exposure for jasmine oil, the estimated oral, inhalation, and dermal exposures were summed, where appropriate. To account for the amount of product absorbed by the dermal route, the product amount available for inhalation was adjusted by 50%, except for body moisturizer. For body moisturizer, since the product amount for inhalation was adjusted for the exposed surface area, and this value was less than 50% of the product amount, no further adjustment was made to the product amount.
Exposure estimates for the lowest and highest exposed age groups from products available to consumers are summarized in Table 7-13. Systemic (sum of inhalation and dermal) daily exposure estimates from DIY products containing jasmine oil are summarized in Table 7-14.
| Product scenario | % in product | Route(s) of exposure | Exposure range (mg/kg bw/day)a |
|---|---|---|---|
| Hair conditioner (wash-off) | 30% | Dermal and inhalation | 5.68 x 10-2 (adults) to 6.44 x 10-2 (9–13 years) |
| Body cleanser (liquid) | 3% | Dermal and inhalation | 3.71 x 10-2 (adults) to 0.13 (0–5 months) |
| Body moisturizer | 3% | Dermal and inhalation | 2.07 (adults) to 4.78 (0–5 months) |
| Facial moisturizer/acne treatment (NHP) | 1% | Dermal and inhalation | 0.32 (adults) to 0.40 (9–13 years) |
| Topical treatment cream (NHP) | 1% | Dermal and inhalation | 0.11 (adults) to 0.14 (9–13 years) |
| Facial sun protection powder (NHP) | 0.07% | Dermal and inhalation | 2.2 x 10-3 (adults) |
| De-stress roll-on (NHP) | 0.01% | Dermal and inhalation | 3 x 10-4 (adults) |
| Lipstick | 3% | Oral | 1.78 x 10-2 (adults) to 4.40 x 10-2 (2–3 years) |
| Body fragrance | 82% | Dermal and inhalation | 3.12 (14-18 years) to 9.14 (2–3 years) |
| Aerosol hair styling product | 0.1% | Dermal and inhalation | 1.58 x 10-3 (14–18 years) to 4.26 x 10-3 (4–8 years) |
| Antiperspirant/deodorant | 2% | Dermal and inhalation | 0.11 (9–13 years) to 0.18 (14–18 years) |
| Temporary hair colour | 0.1% | Dermal and inhalation | 2.42 x 10-2 (adults) to 7.73 x 10-2 (4–8 years) |
| Sunscreen (NHP) | 0.07% | Dermal | 7.35 x 10-2 (9–13 years) to 3.32 x 10-1 (6–11 months) |
| Antiseptic skin cleanser (NHP) | 2% | Dermal and inhalation | 0.38 (14–18 years) to 1.1 (2–3 years) |
| Antiseptic skin cleanser (NHP)2 | 2% | Dermal and inhalation | 5.67 (adults) to 27.2 (2–3 years) |
| Aerosol all-purpose cleaner (application and wiping) | 1% | Dermal and inhalation | 7 x 10-2 (adults) |
| All-purpose liquid floor cleaner (mixing and loading, and application) | 1% | Dermal and inhalation | 2.53 x 10-2 (adults) |
| Post-application exposure to cleaned floors | 1% | Dermal, inhalation, and incidental oral (hand-to-mouth) | 1.16 x 10-2 (1 year) |
| Aerosol laundry conditioner | 5% | Inhalation | 2.45 x 10-2 (adults) |
| Liquid laundry detergent (machine wash) (mixing and loading, hanging laundry, and wearing recently washed clothing) | 5% | Dermal and inhalation | 1.86 x 10-1 (adults) |
| Liquid laundry detergent (machine wash) (migration from washed textiles) (all subpopulations except adults ) | 5% | Dermal, inhalation, and incidental oral (object-to-mouth) (1 year only) | 5.54 x 10-3 (14–18 years) to 9.28 x 10-3 (0–6 months) |
a Only the lowest and highest exposed age groups are presented. See Appendix A for calculation details.
b For situations of public health concern, the use of hand sanitizers among the general population may increase up to 25 uses per day (personal use by adults, increased use by children in schools and childcare facilities) (RIVM 2021a).
| Product scenario | % in product | Route(s) of exposure | Exposure range (mg/kg bw/day)a |
|---|---|---|---|
| Aromatic diffuser | 100% | Inhalation and dermal | 1.16 (adults) to 1.97 (9–13 years) |
| Massage oil | 3% | Dermal and inhalation | 6.89 x 10-1 (adults) to 4.35 (0–5 months) |
| Bath oil product | 100% | Dermal and inhalation | 1.56 x 10-1 (adults) to 2.51 x 10-1 (9–13 years) |
| Body moisturizer | 3% | Dermal and inhalation | 2.07 (adults) to 4.78 (0–5 months) |
| Facial steamer | 100% | Dermal and inhalation |
1.05 to 1.19b (adults) 3.13 to 3.47b (4–8 years) |
| Facial steamer - bystander exposure (1 year only) | 100% | Dermal and inhalation | 5.26 x 10-1 |
a Only the lowest and highest exposed age groups are presented. See Appendix A for calculation details.
b After a total of 4 hours of exposure; once the device is turned off after 20 minutes of use, it is assumed that the person remains in the room for 3 hours and 40 minutes.
7.3.2 Health effects assessment
There are limited empirical health effects data available and no international assessments for jasmine oil.
In a reproductive study, female rats (10/dose) were administered 0 or 500 mg/kg bw/day Jasmine officinale var. grandiflorum by gavage from 3 estrus cycles (approximatively 15 days), which induced a significantly longer dioestrus cycle in treated rats (Iqbal et al. 1993). Study authors did not give any details on clinical signs or effects from the treatment.
In another reproductive study from the same authors, female rats were administered 0, 250, or 500 mg/kg bw/day Jasmine officinale var. grandiflorum extract (jasmine extract) in drinking water from gestation days (GDs) 1 to 5 (15 rats/dose), or from GDs 8 to 12 (8 rats/dose), or from GDs 12 to 20 (6 rats/dose) (Iqbal et al. 1993). Animals treated from GDs 1 to 5 were euthanized at GD 10, while the other animals were euthanized at GD 20. Study authors did not give any details about the clinical state of treated animals. A dose-dependent increase in implantation loss (control: 1.42%, low dose: 48.8%, high dose: 75%) and a dose-dependent decrease in fertility (control: 100%, low dose: 92%, high dose: 60%) was observed from GDs 1 to 5. A higher fetus mortality was observed at the highest dose (12.8%) in comparison with control (1.2%). A dose-dependent increase in resorption (control: 3.44%, low dose: 7.89%, high dose: 15.35%), was observed from GDs 8 to 12. There were no significant changes in fetal weight and length, and no gross abnormalities were observed from GDs 12 to 20. However, authors of the study did not analyze the skeleton or any organs of the fetuses. A decrease in progesterone was observed at GD 5 but not at GDs 12 and 20 in all treated animals, which indicates that jasmine oil may have an endocrine-disrupting effect during early pregnancy. Study authors concluded that the decrease in progesterone levels on GD 5 may be responsible for the anti-implantation in female rats (Iqbal et al. 1993). Based on the study’s results, a LOAEL of 250 mg/kg bw/day, the lowest dose tested, was determined for female reproductive and developmental toxicity.
In order to inform the health effects assessment, the hazard information available for the main components of jasmine oil, benzyl acetate (0%–31%), benzyl benzoate (3%–21%), (E,E)-alpha-farnesene (1%–6%), linalool (7%–13%), phytol (11%–26%), isophytol (5%–12%), and nerolidol (trace–13%), have been considered.
Benzyl acetate and benzyl benzoate
There are no empirical health effects data available for benzyl acetate, and only two reproductive and developmental studies are available for benzyl benzoate.
Benzyl acetate and benzyl benzoate were included by the US EPA as benzyl esters under the chemical category “benzyl derivatives” (US EPA 2010). Benzyl acetate and benzyl benzoate are hydrolyzed to yield benzyl alcohol, which is subsequently oxidized to benzoic acid as a stable metabolite (US EPA 2010). Because they contain a benzene ring bonded directly to an oxygenated functional group that is hydrolyzed and/or oxidized to benzoic acid, these substances and other benzyl derivatives were placed in the same category (US EPA 2010). After a complete review of the toxicokinetics of benzyl acetate and benzyl benzoate, other international agencies such as EFSA, the Research Institute for Fragrance Materials (RIFM), or the Cosmetic Ingredient Review (CIR) supported the rapid and strong metabolism of both substances into benzoic acid (EFSA 2012; McGinty et al. 2012; Johnson et al. 2017).
In 2019, ECCC and HC assessed several substances belonging to the Benzoates Group, and all were esters of benzoic acid (ECCC, HC 2019). The characterization of the hazard of all three subgroups in the Benzoates Group screening assessment (that is, simple alkyl benzoates, dibenzoates, and tribenzoates) was strongly linked with empirical evidence that these substances will readily hydrolyze into benzoic acid, which is then further metabolized into hippuric acid and subsequently excreted (ECCC, HC 2019). As a consequence, and in concordance with other jurisdiction assessments, the screening assessment on the Benzoates Group concluded that these substances exhibit low hazard properties and the potential risk to human health is considered to be low (ECCC, HC 2019).
However, benzyl benzoate seems to potentially induce adverse developmental effects. Pregnant Wistar rats (5/dose) were administered 0, 25, or 100 mg/kg bw/day benzyl benzoate by gavage from GDs 0 to 20 (Koçkaya and Kιlιç 2011). A decrease in food and water consumption was measured in the animals of the high-dose group, but no changes in body weight or body weight gain were observed. A significant higher percentage of lymphocytes and a lower percentage of monocytes were observed in the high-dose group as well as a decrease in creatinine levels. An increase in aspartate aminotransferase was measured in both treated groups. Absolute heart weight in dams was higher in the high-dose group, but no histopathological changes were found. A significantly higher number of occurrences of edema in the perivascular region of the brain and vacuolarization in the cortex of dams were observed at all doses. Some significant variations in fetal skeletons, such as a shorter radius and a longer ulna, were observed in both treated groups. A significantly higher number of fetuses showed enlargement in the intermyofibrillar area of the fetal heart in both dose groups. Fetal body weight and length were significantly higher at 100 mg/kg bw/day, and the placenta size (diameter, thickness, and weight) was larger. However, the placenta was significantly smaller in fetuses in the 25 mg/kg bw/day group. At 100 mg/kg bw/day, a lower number of implantations was calculated, and a dose-dependent increase in resorptions was observed from control (4%) to low dose (12%) and was significant in the high-dose group (20%). A LOAEL of 25 mg/kg bw/day was estimated on the basis of the dose-dependent increase in resorptions, edema in the maternal brains, and fetal skeletal variations.
In another developmental study, pregnant Wistar rats (21/dose) were orally administered 0, 0.4, or 1% benzyl benzoate (equivalent to 0, 26, or 646 mg/kg bw/day as determined by the authors, respectively) by diet from GD 1 to PND 20 (ECHA registration dossier 2020c). One group of 14 animals from each dose was sacrificed at GD 20, while another group of 7 animals per dose was kept until PND 21. No clinical signs of toxicity or body weight changes were observed in dams. A decrease in implantation loss was observed in the high-dose group only but was not statistically significant. A non-significant dose-related increase in malformations such as dilation of renal pelvis, bifid apex of heart, submaxilla defect, tongue defect, cleft palate, or heterotaxia in control (1%), low- (1.2%) and high- (8.8%) dose groups was observed. Authors of the study observed a lower body weight in pups from treated dams, but this was not considered adverse by the authors because it was not statistically significant and did not follow a dose-response pattern. No other clinical changes were seen by the authors. A NOAEL of 646 mg/kg bw/day, the highest dose tested, was determined by the authors (ECHA Registration dossier 2020c). In this assessment, a LOAEL of 26 mg/kg bw/day is recommended on the basis of the decrease in body weight in postnatal pups at both doses in the absence of maternal toxicity and the increase in fetal malformations in the absence of maternal toxicity at 646 mg/kg bw/day. When compared with the previous study (Koçkaya and Kιlιç 2011), differences in the results may be related to the route of administration.
In an in vitro assay, benzyl benzoate was a weak estrogenic competitor to oestradiol but was capable of binding human estrogen receptors ERα and ERβ, and increasing the estrogen-responsive reporter gene (ERE-CAT) and endogenous estrogen-responsive pS2 gene in MCF7 cells (Charles and Darbre 2009). Benzyl benzoate was also positive in the proliferation assay in MCF7 cells (Charles and Darbre 2009). The authors of the study concluded that benzyl benzoate is estrogenic to human cells (Charles and Darbre 2009).
Linalool
Linalool was assessed by ECCC and HC as a major component of bois de rose oil, and no health effects of concern were identified. Exposure to bois de rose oil was therefore considered to be of low risk to human health (ECCC, HC 2020).
(3E,6E)-alpha-Farnesene
There are no other empirical health effects data available for (3E,6E)-alpha-farnesene.
The EFSA used beta-myrcene as a representative substance to assess the safety of acyclic terpene hydrocarbons, of which alpha-farnesene is a part (EFSA 2015). A NOAEL of 300 mg/kg bw/day beta-myrcene was determined by ECCC and HC for reproductive and developmental toxicity on the basis of an increase in the resorption rate, a decrease in the number of live fetuses, and an increase in the frequency of skeletal malformations as fused zygomatic, dislocated sternum, and lumbar extra ribs at 500 mg/kg bw/day in rats (ECCC, HC 2020).
Phytol
There are limited empirical health effects data for phytol. Phytol is an acyclic diterpene alcohol that can be used as a precursor for the manufacture of synthetic forms of vitamin E and vitamin K1 (NCBI 2019).
Phytol is classified as a tumour promotor by the Chemical Carcinogenesis Research Information System, with an unspecified mode of action in the PubChem database (NCBI 2019). In the Comparative Toxicogenomics Database, phytol interferes with genes linked to liver necrosis, hypertrophy, injury, and neoplasm as well as to hyperplasia and diseases, neoplasms, and interstitial tumour in lungs (CTD 2019).
One group of rats (6/dose) was fed with 2% phytol (approximately equivalent to 1000 mg/kg bw/day) for 40 weeks (Steinberg et al. 1966). Only one animal died at the end of the exposure period. All treated rats showed a lowest body weight. The authors did not describe any findings.
In several studies, mice and rats, males and females, were fed with 0%, 0.5%, or 1% phytol (approximately equivalent to 0, 250, and 750 mg/kg bw/day, respectively) for variable periods of time (from 19 days to 15 months) (Steinberg et al. 1966; Atshaves et al. 2004; Mackie et al. 2009). None of the animals died, but many showed hepatotoxicity effects with hypertrophy and hyperplasia or hepatocyte necrosis with early inflammation in all treated animals.
In a short-term inhalation study, Sprague-Dawley rats (5/sex/dose) were exposed to an average phytol aerosol of 5.5 mg/L for 0 or 30 minutes and 1, 2, 4, or 6 hours per day for 14 consecutive days by nose only (Schwotzer et al. 2021). The authors of the study estimated a dose range of 12.9 mg/kg bw to 155.0 mg/kg bw using a deposition fraction of 10% based on the size of the aerosol. The protocol was unexpectedly terminated on day 2 because of severe clinical signs at all times of exposure and mortality in 4-hour and 6-hour groups post exposure. All animals showed piloerection, hunched posture, and rapid respiration within the first two days. Animals exposed to phytol for 4 hours and 6 hours demonstrated severely discoloured purple lungs on gross examination. The rats in the 4-hour and above exposure duration group showed dark red discolouration. Absolute and relative lung weights were significantly increased at 4 hours and 6 hours. In addition, the respective increase was dose-related. The respiratory tract showed a dose-responsive degeneration and necrosis of epithelium within the nose/turbinates, larynx, and trachea. Pulmonary edema with fibrin, and widespread mixed cell inflammation with abundant macrophages, lymphocytes, and neutrophils were observed in the lungs of the 4- and 6-hour groups. For 0.5-, 1-, and 2-hour exposures, animals showed a mixed cell inflammation in the lungs and a distinct centriacinar distribution. The authors of the study did not assess other systemic effects; however, they estimated a LOAEL of 10.8 mg/kg bw/day for phytol based on the high level of toxicity in lungs and death at higher exposures after 2 days (Shwotzer et al. 2021). Authors of the study recommended further assessment of phytol inhalation exposure because of the lower aerodynamic diameter (0.99 µm), which is an indication of a deep lung delivery, and because the study design was based on the hypothesis of low toxicity.
Phytol was found not to be mutagenic in the Ames assay with S. typhimurium strain TA100 with and without metabolic activation and in the Drosophila wing spot test (Choi et al. 1993). However, phytol showed clastogenic effects in the Comet assay with Allium cepa (Islam et. 2017).
Multiple in vitro studies showed that not only does phytol have anti-angiogenic and anti-proliferative potential, but it also stops the cell cycle (mitosis) of lung adenocarcinoma cells (Itoh et al. 2018; Sakthivel et al. 2018).
Without further information, phytol is considered to be clastogenic.
Isophytol
Isophytol was reviewed internationally in an OECD Screening Information Data Set (SIDS) Initial Assessment Report, which considered isophytol to be of low toxicity for mammals on the basis of animal data (OECD 2003).
In a short-term study, male and female rats (12/dose/sex) were administered 0, 250, 500, or 1000 mg/kg bw/day isophytol for 28 days, with a 14-day recovery period (OECD 2003). No clinical signs or effects were observed at the low and middle doses. Fur-staining, increased kidney and spleen weights in females, hunched posture, weight loss and pallor, increased body weight in males, and some blood chemistry changes and increased liver weights in males and females were observed at the highest dose (OECD 2003). No histopathological changes were observed, and the majority of changes were no longer apparent after the recovery period (OECD 2003). On the basis of these results, a NOAEL of 500 mg/kg bw/day and a LOAEL of 1000 mg/kg bw/day were identified for isophytol (OECD 2003).
In a reproductive and developmental study, female and male rats were orally administered 0, 250, 500, or 1000 mg/kg bw/day isophytol by gavage for 10 weeks prior to mating in males and for at least 8 weeks in females (2 weeks prior to mating, 3-week gestation, 3-week lactation) (OECD 2003). At the highest dose, females showed an increase in lethargic behaviours, hunched posture, and piloerection. No significant body weight changes were observed. The absolute and relative food consumption of the females in the mid- and high-dose groups was increased during part of the pre-mating and post-mating periods, but the absolute food consumption was decreased during the complete lactation period. Absolute and relative kidney weights were significantly increased in females in the mid- and high-dose groups and only at the highest dose in males with presence of basophilic aggregates and an increase in the incidence of basophile tubules. Dilated renal tubules and general mineralization were observed in all treated animals. In the high-dose group, females showed a decrease in fertility index and conception rate and an increase in dead pups per litter, post-natal losses, and breeding losses. Post-natal losses were also increased in the low- and mid-dose groups (OECD 2003). As these values, with the exception of the high-dose group, were within the historical control range, this finding was considered to be caused by chance and not due to the treatment administered by OECD (OECD 2003). However, these values were not provided in the report by OECD for further review. Survival and general fitness of pups was reduced in the high-dose group. Pups showed pronounced clinical signs such as very small or cold body, little or no milk uptake, and death. On the basis of these results, OECD determined a LOAEL of 250 mg/kg bw/day, the lowest dose tested, for systemic toxicity in kidneys in both sexes, and a NOAEL of 500 mg/kg bw/day for maternal reproductive and developmental effects (OECD 2003).
Isophytol was not genotoxic in the Ames test with S. typhimurium strains TA97, TA98, TA100, TA102, TA103, TA1535, and TA1537, with or without metabolic activation (OECD 2003). Isophytol was also negative in the micronucleus assay in mice (OECD 2003).
Nerolidol
Nerolidol has been evaluated by JECFA for use as a food flavouring agent (WHO 2008), which concluded on the basis of the low exposure that this substance presents “no safety concern at current levels of intake when used as a flavouring agent.” Similarly, EFSA concluded that the low estimated level of intake of nerolidol as a flavouring substance presents no safety concern (EFSA 2010). There are limited empirical health effects data for nerolidol.
In a study conducted according to OECD Test Guideline 422, Wistar male and female rats (10/sex/dose) were administered 0, 1 500, 4 000, or 12 000 ppm (equivalent to 0, 100, 300, or 1 000 mg/kg bw/day, respectively) nerolidol by diet for 37 days (before mating and during mating) for males and 58 days for females (before mating, during mating, during pregnancy and lactation) (ECHA Registration dossier 2020d). A decrease in food consumption and body weight was observed in females at 4 000 and 12 000 ppm. Liver weights were increased in both sexes in the high-dose group and in females in the mid-dose group. Females also presented with central hepatocellular hypertrophy and central fatty change in the mid- and high-dose groups (ECHA Registration dossier 2020d). No effects were observed in parental fertility and reproductive parameters. A decrease in body weight in pups was observed only in the high-dose group and was a secondary effect from maternal toxicity (ECHA Registration dossier 2020d). On the basis of these results, a NOAEL of 1 500 ppm (100 mg/kg bw/day) was determined for liver toxicity in females at 4 000 ppm (300 mg/kg bw/day) (ECHA Registration dossier 2020d).
In an in vivo study, nerolidol was clastogenic in peripheral blood and liver cells in the Comet assay and the micronucleus assay from exposed Swiss mice (Pículo et al. 2010). In an in vitro study compliant with OECD Test Guideline 480, nerolidol was not mutagenic in gene mutation assay using Saccharomyces cerevisiae, but it was highly cytotoxic (Sperotto et al. 2013).
7.3.3 Characterization of risk to human health
The characterization of risk from jasmine oil considers both systemic effects resulting from the sum of all relevant routes of exposure (oral, dermal, inhalation) as well as an inhalation-specific risk.
For systemic exposures, the identified endpoint was an oral reproductive toxicity LOAEL of 250 mg/kg bw/day jasmine extract (the lowest dose tested) in rats (Iqbal et al. 1993). An increase in implantation loss, fetal mortality, and resorption, and a decrease in fertility and progesterone level were observed at 250 mg/kg bw/day and above. This POD was considered relevant for all age groups. From the critical study defined above, jasmine oil can modify the level of some reproductive hormones in adult rats following a short-duration (for example, 5 days) exposure. A dermal absorption factor of 50% was incorporated for dermal exposures.
For the inhalation route, risks were also characterized for site-of-contact effects from a component of jasmine oil (phytol) by comparing per event estimated phytol concentrations in air to 5.5 mg/L, the LOAEL that resulted in phytol-induced inflammation in the lungs and a dose-responsive degeneration and necrosis of the respiratory tract in rats (Schwotzer et al. 2021).
The combined approach is considered protective for both short- and long-term systemic effects, as well as localized effects during the exposure in question. Estimates of daily systemic exposure to jasmine oil for the highest and lowest exposed age groups and resulting MOEs from food flavouring agent, cosmetics, and other products available to consumers are summarized in Table 7-15, and daily systemic exposure estimates of jasmine oil and resulting MOEs from DIY products are summarized in Table 7-16. Estimated per event air concentrations of phytol in jasmine oil and resulting MOEs are summarized in Table 7-17.
| Exposure scenario | Systemic exposure (mg/kg bw/day)a | MOEb |
|---|---|---|
| Food flavouring agent (1 year of age and older) | 4.94 x 10-6 | >2 000 000 |
|
Dermal and inhalation exposures from hair conditioner (wash-off) (30%) (9–13 years to adults) |
5.68 x 10-2 (adults) to 6.44 x 10-2 (9–13 years) | 3 885 (9–13 years) to 4 400 (adults) |
|
Dermal and inhalation exposures from liquid body cleanser (3%) (all subpopulations) |
3.71 x 10-2 (adults) to 0.13 (0–5 months) | 1 882 (0–5 months) to 6 736 (adults) |
|
Dermal and inhalation exposures from body moisturizer (3%) (all subpopulations) |
2.07 (adults) to 4.78 (0–5 months) | 52 (0–5 months) to 121 (adults) |
| Dermal and inhalation exposures from facial moisturizer/acne treatment (NHP) (1%) (9–13 years to adults) | 0.32 (adults) to 0.40 (9–13 years) | 626 (9–13 years) to 782 (adults) |
| Dermal and inhalation exposures from topical treatment cream (NHP) (1%) (9–13 years to adults) |
0.11 (adults) to 0.14 (9–13 years) |
1 822 (9–13 years) to 2 344 (adults) |
| Dermal and inhalation exposures from facial sun protection powder (NHP) (0.07%) (adults) | 2.2 x 10-3 (adults) | 114 000 |
| Dermal and inhalation exposures from de-stress roll-on (NHP) (0.01%) (adults) | 3 x 10-4 (adults) | >800 000 |
| Oral exposure from lipstick (3%) (2–3 years to adults) | 1.78 x 10-2 (adults) to 4.40 x 10-2 (2–3 years) | 5 682 (2–3 years) to 14 015 (adults) |
|
Dermal and inhalation exposures from body fragrance (82%) (2–3 years to adults) |
3.12 (14–18 years) to 9.14 (2–3 years) | 27 (2–3 years) to 80 (14–18 years) |
|
Dermal and inhalation exposures from aerosol hair styling product (0.1%) (4–8 years to adults) |
1.58 x 10-3 (14–18 years) to 4.26 x 10-3 (4–8 years) | 58 697 (4–8 years) to 158 079 (14–18 years) |
|
Dermal and inhalation exposures from an antiperspirant/deodorant (2%) (9–13 years to adults) |
0.11 (9–13 years) to 0.18 (14–18 years) | 1 377 (14–18 years) to 2 341 (9–13 years) |
|
Dermal and inhalation exposures from temporary hair colour (0.1%) (4–8 years to adults) |
2.42 x 10-2 (adults) to 7.73 x 10-2 (4–8 years) | 3 234 (4–8 years) to 10 348 (adults) |
|
Dermal exposure from sunscreen (NHP) (0.07%) (6–11 months to adults) |
7.35 x 10-2 (9–13 years) to 3.32 x 10-1 (6–11 months) | 752 (6–11 months) to 3 401 (9–13 years) |
| Dermal and inhalation exposure from antiseptic skin cleanser (NHP) (2%) (2–3 years to adults) | 0.38 (14–18 years) to 1.09 (2–3 years) | 230 (2–3 years) to 657 (14–18 years ) |
| Dermal and inhalation exposure from antiseptic skin cleanser (NHP) (2%) (2–3 years to adults)c | 5.67 (adults) to 27.2 (2–3 years) | 9 (2–3 years) to 44 (adults) |
| Dermal and inhalation exposures from an aerosol all-purpose cleaner (1%) (adults) | 7 x 10-2 (adults) | 3 576 (adults) |
| Dermal and inhalation exposures from liquid all-purpose floor cleaner (1%) (adults) | 2.53 x 10-2 (adults) | 9 896 (adults) |
| Dermal, inhalation, and incidental oral (hand-to-mouth) exposures from post-application exposure to cleaned floor (1 year) | 1.16 x 10-2 (1 year) | 21 567 (1 year) |
| Inhalation exposure from aerosol laundry conditioner (5%) (adults) | 2.45 x 10-2 (adults) | 10 210 (adults) |
| Dermal and inhalation exposures from machine washing laundry (liquid) (mix, load, hanging, migration from clothes) (5%) (adults) | 1.86 x 10-1 (adults) | 1 341 (adults) |
|
Dermal and incidental oral exposures (1 year only) from migration from machine-washed clothes (5%) (all subpopulations except adults) |
5.54 x 10-3 (14–18 years) to 9.28 x 10-3 (0–6 months) | 26 950 (0–6 months) to 45 134 (14–18 years) |
a Exposure scenario parameters and calculations for jasmine oil are outlined in Appendix A. Dermal absorption was assumed to be 50%.
b The MOEs were calculated using the critical effect level (LOAEL = 250 mg/kg bw/day) based on female reproductive toxicity.
c For situations of public health concern, the use of hand sanitizers among the general population may increase up to 25 uses per day (personal use by adults, increased use by children in schools and child care facilities) (RIVM 2021a).
| Exposure scenario | Systemic exposure (mg/kg bw/day)a | MOEb |
|---|---|---|
| Systemic exposure by the dermal route (for the 9–13 years to adults only) and by inhalation route from DIY aromatic diffuser (100%) (all subpopulations) | 1.16 (adults) to 1.97 (14–18 years) | 127 (9–13 years) to 216 (adults) |
| Dermal and inhalation exposures from DIY massage oil (3%) (all subpopulations) | 6.89 x 10-1 (adults) to 4.35 (0–5 months) | 57 (0–5 months) to 363 (adults) |
| Dermal and inhalation exposures from DIY bath oil product (100%) (9–13 years to adults) | 1.56 x 10-1 (adults) to 2.51 x 10-1 (9–13 years) | 997 (9–13 years) to 1 606 (adults) |
| Dermal and inhalation exposures from DIY body moisturizer (3%) (all subpopulations) | 2.07 (adults) to 4.78 (0–5 months) | 52 (0–5 months) to 121 (adults) |
| Dermal and inhalation exposures from DIY facial steamer/mistc (100%) (4–8 years to adults) | 1.19 (adults) to 3.47 (4–8 years) | 72 (4–8 years) to 210 (adults) |
| Dermal and inhalation exposures from DIY facial steamer for bystander (1 year only) (100%) | 5.26 x 10-1 | 475 |
a Exposure scenario parameters and calculations for jasmine oil are outlined in Appendix A. Dermal absorption was assumed to be 50%.
b The MOEs were calculated using the critical effect level (LOAEL = 250 mg/kg bw/day) based on female reproductive toxicity.
c After a total of 4 hours of exposure; once the device is turned off after 20 minutes of use, it is assumed that the person remains in the room for 3 hours and 40 minutes.
| Product scenario | Per event air concentration (mg/m3)a | MOEb |
|---|---|---|
| Air freshener (100%) (all subpopulations) | 3.2 | >1 700 (all subpopulations) |
| Aerosol hair styling product (0.1%) (4–8 years to adults) | 2.70 x 10-3 (18 years and below) to 3.10 x 10-3 (adults) |
>1 700 000 (adults) >2 000 000 (18 years and below) |
| Aerosol laundry conditioner (5%) (adults) | 5.50 x 10-3 | >1 000 000 (adults) |
| Aerosol all-purpose cleaner (1%) (adults) | 1.50 x 10-3 | >3 000 000 (adults) |
| Liquid all-purpose cleaner (1%) (adults) | 1.72 x 10-7 | >32 100 000 000 (adults) |
a Per event air concentrations were adjusted by 50% for the maximum amount of components with toxicity in jasmine oil.
b The MOEs were calculated using the critical effect level (LOAEL = 5.5 mg/L) based on lung toxicity in rats after 2 days of exposure to phytol.
The MOEs for jasmine oil from food (based on its potential use as a flavouring agent), hair conditioner, body cleanser, topical treatment cream (NHP), facial sun protection powder (NHP), de-stress roll-on (NHP), lipstick, hair styling product, antiperspirant/deodorant, temporary hair colour, and sunscreen (2 years of age and older) (NHP) are considered adequate to address uncertainties in the health effects and exposure data used to characterize risk.
The use of jasmine oil in an aerosol all-purpose cleaner, all-purpose floor cleaner, and a liquid laundry detergent are considered adequate to address uncertainties in the health effects and exposure data used to characterize risk.
In addition, the MOEs for jasmine oil from its uses in a DIY bath oil product are considered adequate to address uncertainties in the health effects and exposure data used to characterize risk.
The MOEs between the critical effect levels and the estimates of daily exposure from body moisturizer, body fragrance, facial moisturizer/acne treatment (NHP), sunscreen (1-year-olds and 6- to 11-month-olds) (NHP), and antiseptic skin cleanser (NHP) are below 1000, which account for uncertainties with respect to interspecies extrapolation, intraspecies extrapolation, the POD, and the adequacy of the database and are considered potentially inadequate.
In addition, the use of jasmine oil in DIY products such as aromatic diffuser, massage oil, body moisturizer, or a facial steamer are below 1000, which accounts for uncertainties with respect to interspecies extrapolation, intraspecies extrapolation, the POD, and the adequacy of the database and is considered potentially inadequate to address uncertainties in the health effect and exposure data used to characterize risk.
It should be noted that a component of jasmine oil, benzyl benzoate, exhibited developmental effects in rats, such as enlargement in the intermyofibrillar area of the fetal heart and skeleton variations at the lowest dose tested (25 mg/kg/day), and was classified as a potential estrogenic substance for human cells in an in vitro study (Charles and Darbre 2009). If the jasmine oil present in products available to consumers contains greater than 10% benzyl benzoate, the current assessment may not be sufficiently protective.
The MOEs between the critical effect levels and the per event air concentration estimates from air freshener, aerosol hair styling product, aerosol laundry conditioner, aerosol all-purpose cleaner, and liquid all-purpose cleaner are considered adequate to address uncertainties in the health effects and exposure data used to characterize risk.
7.3.4 Uncertainties in evaluation of risk to human health
The key sources of uncertainty are presented in the table below.
| Key source of uncertainty | Impact |
|---|---|
| There is a degree of uncertainty associated with the dermal absorption factor used for jasmine oil, considering the in vivo dermal absorption results for benzyl benzoate and benzyl acetate. | +/- |
| The upper concentration of jasmine oil in sunscreen and antiseptic skin cleanser are conservative estimates. In the absence of any additional information, the concentration in sunscreen is based on the maximum amount of jasmine oil diluted in water, and the concentration in antiseptic skin cleanser is based on the maximum concentration of other ingredients in the product (SDS 2014b). | + |
| Jasmine oil may be used in hand sanitizers. There is uncertainty regarding the duration of increased hand sanitizer use that may occur in a situation of public health concern. | +/- |
| There are no studies via the dermal and inhalation routes. | +/- |
| No suitable studies were identified for dermal or inhalation exposure; therefore, route-to-route extrapolation from an oral study was used to determine systemic exposure. | +/- |
| Jasmine oil was considered to be a representative for perfumes and essences of jasmin in the absence of compositional information. | +/- |
| A component of jasmine oil, benzyl benzoate, exhibited developmental effects in rats at the lowest dose tested (25 mg/kg bw/day). If the jasmine oil present in products available to consumers contains greater than 10% benzyl benzoate, the current assessment may not be sufficiently protective. | - |
+ = uncertainty with potential to cause overestimation of exposure/risk; - = uncertainty with potential to cause underestimation of exposure/risk; +/- = unknown potential to cause over- or underestimation of risk. The achieved margins of exposure were considered adequate to address uncertainties in the exposure and hazard databases.
7.4 Violet oil
7.4.1 Exposure assessment
Environmental media
Given that violet oil was not reported as being manufactured or imported into Canada above the reporting threshold of 100 kg (Environment Canada 2013), exposure to this substance from environmental media is not expected. No reports of monitoring for violet oil in environmental media in Canada or elsewhere were identified.
Food
No definitive information is available concerning the potential use of violet oil as a flavouring agent in foods sold in Canada. However, since violet oil is identified as a food flavouring agent internationally, it is possible that this substance is present as a flavouring agent in foods sold in Canada.
The Fenaroli’s Handbook of Flavour Ingredients reports the “individual’ consumption intake of this substance from its use as a food flavouring agent. Individual consumption intakes are a per capita estimate of intake based on a MSDI that is based on production volumes reported by the food industry (NAS 1989 as cited in Burdock 2010).
In the absence of data on the actual use, if any, of violet oil as a food flavouring agent in Canada, the per capita intake estimate for the US population of 1.33 x 10-1 μg/kg bw/day reported in Fenaroli’s Handbook of Flavour Ingredients (Burdock 2010) is an acceptable estimate of possible Canadian dietary exposure of the general population 1 year of age and older to this substance from its potential use as a food flavouring agent (personal communication, email from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2020; unreferenced).
Exposure from natural occurrence in foods:
No definitive information is available concerning the natural occurrence of violet oil in foods. However, dietary exposure to this substance, if any, is expected from its natural presence in foods (Nijssen et al. 2018).
Products available to consumers
Violet oil is present in products available to consumers. Based on the health effects assessment of violet oil (section 7.4.2), the inhalation and oral routes of exposure are considered to be the relevant routes for risk characterization, including for dermally applied products available to consumers. The selected sentinel cosmetic scenarios represented the highest exposures, relative to other dermally applied cosmetics as well as NHPs where violet oil is used as an NMI, based on identified products reported to contain this substance. To evaluate the potential for exposure to violet oil from cosmetics applied by the dermal route, sentinel scenarios were selected based on a combination of use frequencies and reported concentrations of violet oil in these products. The selected sentinel scenarios represented the highest exposures, relative to other dermally applied cosmetics and based on identified products reported to contain this substance. Exposure to violet oil from the use of eye moisturizer, hair conditioner, facial cleanser, body moisturizer, and massage oil, and as a body fragrance were considered to be the sentinel scenarios for dermal applications (personal communication, email from the Consumer and Hazardous Product Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2017; unreferenced).
In addition, the use of 100% violet oil in DIY products such as aromatic diffuser, a bath oil product, body moisturizer, and a facial steamer was also assessed. It is reported that body products are typically diluted to concentrations of 1%–4% (Tisserand Institute 2021). On the basis of this information, the maximum concentration of violet oil in DIY body moisturizer was assumed to be 3%. Although the upper concentration reported for massage oil containing violet oil was 100%, massage oils are typically diluted prior to use. Therefore, the maximum concentration of violet oil in a DIY massage oil was assumed in RIVM (2006) to be 3%, but this scenario is covered by a consumer product massage oil at 10% (personal communication, email from the Consumer and Hazardous Product Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2017; unreferenced).
Furthermore, violet oil is present in a lipstick product. Exposure by the oral route from the use of a lipstick was quantified (personal communication, email from the Consumer and Hazardous Product Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2017; unreferenced).
The product amount available for inhalation via evaporation was adjusted by 80% to account for an estimated 20% retained via the dermal route. The 20% factor was based on in vitro human dermal absorption studies for geraniol, citronellol, linalool, and citral (Gilpin et al. 2010; ECHA Registration dossier 2018; Charles River 2019). The physical-chemical properties of 2,6-nonadienal, one of the main components of violet oil, are similar to those of the Terpenes and Terpenoids – Acyclic, Monocyclic, and Bicyclic Monoterpenes Group. The molecular weight, vapour pressure, and log KOW of 2,6-nonadienal are 138.21 g/mol, 31 Pa, and 2.84, respectively. These values are similar to those of geraniol, citronellol, linalool, and citral, which have molecular weights ranging from 152 g/mol to 156.27 g/mol, vapour pressures ranging from 4 Pa to 21 Pa, and log KOW values ranging from 2.9 to 3.5. For body moisturizer, since the product amount for inhalation was adjusted for the exposed surface area, and this value was less than 80% of the product amount, no further adjustment was made to the product amount; for hair conditioner (leave-on), only the product amount in contact with the scalp was considered available for dermal retention, and the total amount of product on the hair was considered to be potentially available for evaporation.
Exposure estimates for the lowest and highest exposed age groups from products available to consumers are summarized in Table 7-19. In addition, exposure estimates for the lowest and highest exposed age groups from DIY products are summarized in Table 7-20.
| Product scenario | % in product | Route of exposure | Exposure range (mg/kg bw/day)a |
|---|---|---|---|
| Eye moisturizer | 100% | Inhalation | 6.98 x 10-3 (adults) to 8.78 x 10-3 (14–18 years) |
| Hair conditioner (leave-on) | 100% | Inhalation | 1.82 x 10- 2 (adults) to 4.89 10- 2 (2–3 years) |
| Facial cleanser | 30% | Inhalation | 9.12 x 10-3 (14–18 years) to 1.52 x 10-3 (adults) |
| Body moisturizer | 10% | Inhalation | 1.45 x 10-2 (0–5 months) to 3.14 x 10-2 (9–13 years) |
| Massage oil | 10% | Inhalation | 4.26 x 10-2 (adults) to 88.43 x 10-2 (1 year) |
| Lipstick | 10% | Oral | 1.13 x 10-2 (adults) to 2.79 x 10-2 (2–3 years) |
| Body fragrance | 10% | Inhalation | 2.44 x 10-3 (adults) to 4.31 x 10-3 (2–3 years) |
a Only the lowest to highest exposed age groups are presented. Exposures were adjusted by 19% to account for the maximum amount of 2,6-nonadienal in violet oil, assuming that 80% of the applied dose was available for evaporation. See Appendix A for calculation details.
| Product scenario | % in product | Route of exposure | Exposure range (mg/kg bw/day)a |
|---|---|---|---|
| Aromatic diffuser | 100% | Inhalation | 1.56 x 10-1 (adults) to 3.73 x 10-1 (1 year) |
| Bath oil product | 100% | Inhalation | 2.85 x 10-2 (adults) to 4.56 x 10-2 (9–13 years) |
| Body moisturizer | 3% | Inhalation | 4.84 x 10-3 (0-5 months) to 1.05 x 10-3 (9–13 years) |
| Facial steamer | 100% | Inhalation |
0.05–0.02b (adults) 0.13–0.06b (4–8 years) |
| Facial steamer - bystander exposure (1 year only) | 100% | Inhalation | 0.10 |
a Only the lowest to highest exposed age groups are presented. Exposures were adjusted by 19% to account for the maximum amount of 22,6-nonadienal in violet oil, assuming that 80% of the applied dose was available for evaporation. See Appendix B for calculation details.
b After a total of 4 hours of exposure; once the device is turned off after 20 minutes of use, it is assumed that the person remains in the room for 3 hours and 40 minutes.
7.4.2 Health effects assessment
There are no international assessments or health effects data available for violet oil.
The only health effects studies available on violet leaf oil showed no irritation, sensitization, or phototoxicity when the substance was dermally tested on animals and human volunteers at 2% in petrolatum. They also indicated anti-inflammatory effects in acute and subacute experiments (Opdyke 1976).
In order to inform the health effects assessment, the hazard information available for the main components of violet oil—2,6-nonadienal (5%–19%), linoleic acid (0%–58%), palmitic acid (0%–17%), and 1-octadecene (0%–11%)—were considered.
2,6-Nonadienal
There are no subchronic, chronic, reproductive/developmental, or carcinogenicity animal studies identified in the literature for 2-trans-6-cis-nonadienal.
In the Ames test with S. typhimurium strain TA100, no increase in revertants was observed, with and without metabolic activation (Eder et al. 1992). However, 2,6-nonadienal significantly increased the number of sister chromatid exchanges in human blood lymphocyte and Namalva cell cultures (Dittberner et al. 1995) and induced chromosomal aberrations in Namalva cells but not in lymphocytes (Dittberner et al. 1995). A significant increase in the number of aneuploidy metaphases was noted after 2,6-nonadienal treatment (Dittberner et al. 1995). Additionally, 2,6-nonadienal induced an increase in the frequency of micronuclei in human blood lymphocyte and Namalva cells in a concentration-dependent manner (Dittberner et al. 1995). With fluorescence in situ hybridization, both Namalva cells and human lymphocytes showed a significant increase in enhanced frequencies of centromere-positive micronuclei, which generally result from impaired function of the mitotic spindle, and therefore can be classified as aneugenic effects (Dittberner et al. 1995). Without further information, 2,6-nonadienal may be considered to be aneugenic.
In the absence of hazard data for 2,6-nonadienal, a read-across approach was taken, and hazard information on the read-across analogue, 2,4-hexadienal, was used to inform the health effects assessment.
Both 2,6-nonadienal and 2,4-hexadienal are alpha, beta-unsaturated aldehydes. They are structurally similar, comprising of an aliphatic hydrocarbon chain with three double bonds and one carbonyl group. The only structural difference between the two substances is that 2,6-nonadienal (molecular formula: C9H14O) has a longer chain length than 2,4-hexadienal (molecular formula: C6H8O). The target and analogue are both naturally occurring substances in food, have been identified for use as flavouring ingredients in the food industry, and are endogenously produced from lipid peroxidation products in the body (Adams et al. 2008). 2,6-nonadienal and 2,4-hexadienal have comparable physical-chemical properties, and both have structural features associated with the potential to be highly reactive and interact with biological macromolecules such as DNA. They have the same structural alerts on QSAR Toolbox version 4.2 (OECD 2016), including DNA and protein binding alerts, in vitro and in vivo mutagenicity, and skin sensitization, making them potentially toxic and capable of modifying cellular processes.
In their US NTP report, the authors concluded that 2,4-hexadienal showed clear evidence of carcinogenic activity in male and female F344/N rats and male and female B6C3F1 mice on the basis of increased incidences of squamous cell neoplasms in the forestomach (NTP 2003). The IARC classified 2,4-hexadienal as possibly carcinogenic to humans (group 2B) based on sufficient evidence for carcinogenicity in experimental animals and the absence of hazard data in humans (IARC 2013). Genotoxicity evaluation of 2,4-hexadienal showed that it was not mutagenic when evaluated by the EFSA Expert Panel (EFSA 2018). JECFA concluded that the neoplasms of the forestomach were caused by non-genotoxic mechanisms such as the irritating effects of prolonged exposure to 2,4-hexadienal administered daily at the site of first contact (forestomach) by gavage and were not a systemic effect induced by the chemical (Nyska et al 2001; Chan et al. 2003; WHO 2004). JECFA also questioned the relevance of the induction of these tumours in rodents to cancer in humans, considering the exposure conditions used in the NTP studies (NTP 2003; WHO 2004). Moreover, the carcinogenic effect was only observed in animals exposed to high doses of 2,4-hexadienal for two years (NTP 2003).
Rats (5/sex/dose) were administered 0, 0.75, or 7.5 mg/kg bw/day 2,4-hexadienal in corn oil via gavage 6 days a week for 14 days (WHO 2004; Adams et al. 2008). No treatment-related effects were observed on clinical signs, hematology, or histopathology. The study authors established a no-observed-effects level (NOEL) of 7.5 mg/kg bw/day, the highest dose tested (WHO 2004; Adams et al. 2008).
F344/N rats (24/sex/dose) were orally administered 0 or 2.23 mg/kg bw/day 2,4-hexadienal in the diet, 7 days a week for 13 weeks (WHO 2004; Adams et al. 2008). There were no treatment-related effects on body weights or histopathology at necropsy. The authors reported a NOEL of 2.23 mg/kg bw/day, the highest dose tested (WHO 2004; Adams et al. 2008).
In a dose-range finding study, groups of male and female Fischer 344/N rats and B6C3F1 mice (5/sex/dose) were administered 2,4-hexadienal in corn oil by gavage at doses of 0, 3, 9, 27, 80, or 240 mg/kg bw/day, 5 days a week for 16 days (NTP 2003). In rats given the highest dose, mortality was observed in three males and three females before the end of the study. Rats administered 240 mg/kg bw/day had significantly lower body weight gain compared with the controls. Liver weights of female rats given the high dose were significantly greater than those of the controls (the study authors did not mention the presence of histopathological correlates). Clinical signs at the highest dose included diarrhea, ataxia, lethargy, and nasal/eye discharge in male rats, and lethargy, paleness, and abnormal breathing in female rats (NTP 2003). Gross pathological evaluation revealed necrosis and ulceration of the forestomach in most rats of both sexes at 240 mg/kg bw/day, and mild to moderate epithelial hyperplasia of the forestomach at 80 mg/kg bw/day (NTP 2003). No forestomach effect was seen in rats microscopically at 27 mg/kg bw/day. In mice, one male and one female died at the highest dose before the end of the study, and clinical signs included lethargy and ruffled fur. Marked ulceration and necrosis of the forestomach were reported in all mice at 240 mg/kg bw/day. At 80 mg/kg bw/day, there was minimal to mild epithelial hyperplasia and hyperkeratosis, but no forestomach effects were reported at lower doses in mice (NTP 2003).
In a short-term study, groups of F344/N rats (10/sex/dose) were administered 2,4-hexadienal in corn oil by gavage at doses of 0, 7.5, 15, 30, 60, or 120 mg/kg bw/day, 5 days per week for 14 weeks (NTP 2003). All animals survived until the end of treatment. Final mean body weights and body weight gains were decreased at 30, 60, and 120 mg/kg bw/day in males only, compared with the controls (NTP 2003). At the highest dose, incidences of epithelial hyperplasia, degeneration, and chronic active inflammation of the forestomach in males and females as well as incidences of nasal atrophy, osteofibrosis, and exudate in males were significantly increased (NTP 2003). In contrast to male rats, nasal lesions in females were limited to acute necrosis in one animal at 60 mg/kg bw/day and two animals at 120 mg/kg bw/day (NTP 2003).
In another short-term study, B6C3F1 male and female mice (10/sex/dose) were orally administered 2,4-hexadienal in corn oil by gavage at doses of 0, 7.5, 15, 30, 60, or 120 mg/kg bw/day, 5 days per week for 14 weeks (NTP 2003). Treatment did not affect survival or body weight gain in either sex at any dose level. Absolute and relative kidney weights were increased at 60 and 120 mg/kg bw/day (males only), absolute and relative liver weights were increased at 60 mg/kg bw/day (both sexes), and relative liver weights of females in all dose groups were increased compared to the controls. An increase in the incidence of minimal to mild epithelial hyperplasia of the forestomach (in the absence of inflammation or basal cell proliferation) was observed only in females at 120 mg/kg bw/day. The incidences of minimal to mild olfactory epithelial necrosis were significantly increased in both sexes at 120 mg/kg bw/day, whereas the incidence of olfactory epithelial atrophy was significantly increased only in males at this dose (NTP 2003).
In a carcinogenicity study, groups of F344/N male and female rats (50/sex/dose) were administered 2,4-hexadienal in corn oil by gavage at doses of 0, 22.5, 45, or 90 mg/kg bw/day, five days per week for two years (NTP 2003). Survival was not affected by the treatment. The chemical induced significant increases in the incidence of squamous cell papilloma of the forestomach in the 45 and 90 mg/kg bw/day groups in both sexes. No other significant treatment-related tumours were observed in the treated animals. The incidence of epithelial hyperplasia in the forestomach (focally extensive to diffuse thickenings of all layers of the squamous epithelium, which is considered a potential precursor lesion to neoplasia in the forestomach) was significantly increased in all dose groups for both sexes, with mild to moderate severity at 45 and 90 mg/kg bw/day, respectively. At the highest dose, males showed an increase in inflammation and cysts in the forestomach. These were interpreted by the authors to be the results of downward growths of benign hyperplastic epithelium (NTP 2003). A LOAEL of 22.5 mg/kg bw/day was identified based on the presence of a dose-response of epithelial hyperplasia in the forestomach at all doses in both sexes in rats. This critical effect level was adjusted by multiplying by 5/7 to adjust for the exposure frequency (animals were exposed for 5 days per week), resulting in a LOAEL of 16.1 mg/kg bw/day.
In a carcinogenicity study, groups of B6C3F1 male and female mice (50/sex/dose) were administered 2,4-hexadienal in corn oil by gavage at doses of 0, 30, 60, or 120 mg/kg bw/day, five days per week for two years (NTP 2003). Survival was not affected by the treatment. The incidences of squamous cell papilloma and squamous cell papilloma or carcinoma (combined) in the forestomach were significantly greater in males at 120 mg/kg bw/day and in females at 60 and 120 mg/kg bw/day. The incidence of forestomach squamous cell carcinoma alone was significantly increased in females only at the high dose. Non-neoplastic lesions of the forestomach, consisting of squamous epithelial hyperplasia, were increased in females at 60 mg/kg bw/day and in both sexes at 120 mg/kg bw/day, and the incidence of ulcers was increased in males at 120 mg/kg bw/day. Two male mice in the high-dose group exhibited squamous cell carcinoma of the tongue. The study authors justified this result by explaining that the chemical on the end of the gavage needle may have been deposited in the oral cavity during the gavage procedure or that some regurgitation of gavaged material may have occurred (NTP 2003).
Genotoxicity evaluation of 2,4-hexadienal showed that it was not mutagenic in the Ames test in S. typhimurium strains TA102 (Marnett et al. 1985), TA98, TA1535 (Florin et al. 1980; NTP 2003), TA100, and TA1537 (Florin et al. 1980), with or without metabolic activation. However, other studies using the Ames test showed that 2,4-hexadienal was positive with S. typhimurium strains TA100 (Eder et al. 1992; NTP 2003) and TA104 (Marnett et al. 1985). 2,4-Hexadienal showed weak but statistically significant positive results in an in vitro gene mutation assay at the hypoxanthine-guanine phosphoribosyl transferase locus in mouse lymphoma L5178Y cell line with metabolic activation (EFSA 2018). However, the study authors considered 2,4-hexadienal to be non-mutagenic since the results could not be compared against historical control values and could not be confirmed in other experiments (EFSA 2018). No mutagenicity was reported in the SOS chromotest with Escherichia coli (E. coli) strains PQ37 and PQ243 incubated with 2,4-hexadienal (Eder et al. 1992). However, 2,4-hexadienal induced DNA strand breaks in an alkaline elution assay using L1210 mouse leukemia cells (Eder et al. 1993). In vivo assays showed inconclusive results in micronucleus assays in bone marrow polychromatic erythrocytes of male mice and male rats administered 2,4-hexadienal intraperitoneally at doses ranging from 40 mg/kg bw/day to 160 mg/kg bw/day (mice) and from 50 mg/kg bw/day to 200 mg/kg bw/day (rats) (NTP 2003). In these assays, the trend analysis of the response over the dose ranges was significant; however, none of the mean values for the individual groups of treated animals differed significantly from the control group value (NTP 2003). Negative results were reported for micronucleus assays of peripheral blood normochromatic (mature) erythrocytes of male and female mice exposed to 2,4-hexadienal (7.5 mg/kg bw/day to 120 mg/kg bw/day) via gavage for 14 weeks (NTP 2003). Similarly, 2,4-hexadienal tested negative for potential clastogenic or aneugenic effect in an in vivo micronucleus assay with scoring in bone marrow cells and peripheral blood reticulocytes of male rats by oral gavage or intraperitoneally (EFSA 2018). 2,4-Hexadienal did not show any significant increase in cII mutant frequency when tested in a transgenic rodent gene mutation assay for its potential to induce gene mutations in mice (EFSA 2018). In accordance with the EFSA Panel conclusions, the concern for genotoxicity can be ruled out based on the results from the comprehensive battery of in vitro and in vivo tests on 2,4-hexadienal.
Linoleic acid
9,12-Octadecadienoic acid, an omega-6 fatty acid also known as linoleic acid, is a polyunsaturated fatty acid that is naturally present in vegetable oils (Whelan and Fritsche 2013). Linoleic acid is the main component of evening primrose oil (70% to 77% of the oil), which was evaluated by ECCC and HC as a substance in the Fatty Acids and Derivatives Group (ECCC, HC 2018c). The available information indicates that evening primrose oil is considered to be of low hazard potential, and risk to human health is considered to be low (ECCC, HC 2018c). The US FDA has confirmed GRAS status for linoleic acid as a direct food substance used as a food flavouring agent or adjuvant (US FDA 2018). In 1998 and 2002, JECFA evaluated linoleic acid as a single substance and as a mixture combined with linolenic acid and concluded that it presented no safety concern at current levels of intake when used as a food flavouring agent (WHO 2019). NICNAS identified linoleic acid as being of low concern to human health (NICNAS 2017a).
Limited data are available on the toxicity of linoleic acid. In an OECD SIDS Initial Assessment Profile, linoleic acid was evaluated among other fatty acids including palmitic acid, another major component of violet oil (OECD 2014). Linoleic acid and palmitic acid were not identified as possessing properties indicating a hazard to human health for systemic health effects (OECD 2014).
In a study following the Chernoff/Kavlock Developmental Toxicity Screen, groups of female mice (26-30/dose) were administered 10 g/kg bw/day of linoleic acid via oral gavage on GDs 8 to 12 (OECD 2014). No reproductive or developmental effects were observed at this dose (OECD 2014).
In addition, no adverse findings were noted in a repeated-dose oral toxicity study in which a group of twenty male rats were administered linoleic acid in the diet at a dose of 1.5% (~467 mg/kg bw/day to 1970 mg/kg bw/day) for 36 weeks (OECD 2014).
Linoleic acid was not mutagenic when tested in S. typhimurium strains TA98, TA100, TA1535, TA1537, and TA97 in the presence and absence of metabolic activation (OECD 2014).
Palmitic acid
JECFA evaluated palmitic acid and concluded that it presented no safety concern at current levels of intake when used as a food flavouring agent (WHO 1999). NICNAS identified palmitic acid as being of low concern to human health (NICNAS 2017b).
A safety assessment of the Oleic Acid Group including palmitic acid by the CIR Expert Panel was published in 1987, with a conclusion that these ingredients are safe in present practices of use and concentration in cosmetics (CIR 1987). A re-evaluation of this group demonstrated no toxicity to reproductive and developmental physiology by palmitic acid (CIR 2006). Palmitic acid was evaluated by the RIFM in a fragrance ingredient safety assessment using a TTC approach for repeated-dose and reproductive toxicity endpoints, and the expert panel concluded that palmitic acid is safe to use at current levels of exposure (Api et al. 2019).
1-Octadecene
The OECD SIDS published an Initial Assessment Profile on higher olefins, including 1-octadecene, and used a category/analogue approach to assess them (OECD 2004).
In a reproduction and developmental toxicity screening test, male and female rats (12/sex/dose) were administered 0, 100, 500, or 1000 mg/kg bw/day of a test substance containing a mix of octadecene isomers (% of the test substance is unknown in the mix) by oral gavage for two weeks prior to mating and during mating (males and females), four weeks following mating for males, and during gestation and following parturition for females (OECD 2004). Based on the absence of clinical, reproductive, and developmental effects, a NOAEL of 1000 mg/kg bw/day, the highest dose tested, was identified by the study authors (OECD 2004).
The expert panel on this assessment concluded that, based on the weight of evidence from toxicity studies with olefins, category members, including 1-octadecene, are currently of low priority for further work (OECD 2004).
7.4.3 Characterization of risk to human health
2,6-Nonadienal was determined to be the main component of toxicological significance for violet oil. An adjusted LOAEL of 16.1 mg/kg bw/day was identified for 2,4-hexadienal (analogue of 2,6-nonadienal) based on the presence of a dose-dependent response in epithelial hyperplasia in the forestomach at all doses in both sexes in the 2-year carcinogenicity study in rats (NTP 2003). This was considered applicable for both the oral and inhalation routes of exposure. However, these effects were not considered relevant for the characterization of risks from the dermal route. Given the lack of any other suitable POD, dermal exposures were not considered further in this assessment.
The EFSA (2018) and WHO (2004) reported that non-genotoxic mechanisms have been proposed for the possible carcinogenicity of 2,4-hexadienal. The NTP (2003) stated that the development of tumours following administration of 2,4-hexadienal was observed in rats exposed to doses ≥45 mg/kg bw/day (females) and in mice exposed to doses of ≥60 mg/kg bw/day (females) and 120 mg/kg bw/day (males). No difference in neoplastic effects was observed between the control group and the lowest dose group, 22.5 mg/kg bw/day (NTP 2003). Therefore, the non-carcinogenic LOAEL of 16.1 mg/kg bw/day from 2,4-hexadienal used to characterize the risk for violet oil is considered sufficiently protective.
Daily exposure estimates for the highest and lowest exposed age groups and resulting MOEs are summarized in Table 7-21 for food flavouring agent and products available to consumers. In addition, daily estimates for the highest and lowest age groups and resulting MOEs are summarized in Table 7-22 for DIY products.
| Exposure scenario | Exposure range (mg/kg bw/day)a | MOE rangeb |
|---|---|---|
| Food flavouring agent (1 year and older) | 2.52 x 10-5 | >630 000 |
| Inhalation exposure from eye moisturizer (100%) (14–18 years to adults) | 6.98 x 10-3 (adults) to 8.78 x 10-3 (14–18 years) | 1836 (14–18 years) to 2307 (adults) |
| Inhalation exposure from hair conditioner (leave-on) (100%) (2–3 years to adults) | 1.82 x 10- 2 (adults) to 4.89 x 10- 2 (2–3 years) | 329 (2–3 years) to 884 (adults) |
| Inhalation exposure from facial cleanser (30%) (9–13 years to adults) | 9.12 x 10-3 (14–18 years) to 1.52 x 10-3 (adults) | 1060 (adults) to 1756 (14–18 years) |
| Inhalation exposure from body moisturizer (10%) (all subpopulations) | 1.45 x 10-2 (0–5 months) to 3.14 x 10-2 (9–13 years) | 512 (9–13 years) to 1110 (0–5 months) |
| Inhalation exposure from massage oil (10%) (all subpopulations) | 44.26 x 10-2 (adults) to 88.43 x 10-2 (1 year) | 191 (1 year) to 378 (adults) |
|
Oral exposure from lipstick (10%) (2–3 years to adults) |
1.13 x 10-2 (adult) to 2.79 x 10-2 (2–3 years) |
578 (2–3 years) to 1425 (adults) |
| Inhalation exposure from body fragrance (10%) (2–3 years to adults) | 2.44 x 10-3 (adults) to 4.31 x 10-3 (2–3 years) | 3734 (2–3 years) to 6592 (adults) |
a Exposure estimates were adjusted by 19% for the maximum amount of 2,6-nonadienal present in violet oil, assuming that 80% of the applied dose was available for evaporation (except for the exposure from food flavouring agent). Details are available in Appendix A.
b The MOEs were calculated using an adjusted non-carcinogenic LOAEL of 16.1 mg/kg bw/day for the read-across analogue 2,4-hexadienal, based on the presence of mild to moderate forestomach epithelial hyperplasia at all doses in both sexes in the 2-year carcinogenicity study in rats.
| Exposure scenario | Exposure range (mg/kg bw/day)a | MOE rangeb |
|---|---|---|
| Inhalation exposure from DIY aromatic diffuser (100%) (all subpopulations) | 1.56 x 10-1 (adults) to 3.73 x 10-1 (1 year) | 43 (1 year) to 154 (adults) |
| Inhalation exposure from DIY bath oil product (100%) (9–13 years to adults) | 2.85 x 10-2 (adults) to 4.56 x 10-2 (9–13 years) | 353 (9–13 years) to 565 (adults) |
| Inhalation exposure from DIY body moisturizer (3%) (all subpopulations) | 4.84 x 10-3 (0–5 months) to 1.05 x 10-3 (9–13 years) | 1536 (9–13 years) to 3330 (0–5 months) |
| Inhalation exposure from DIY facial steamer/mistc (100%) (4–8 years to adults) | 0.08 (adults) to 0.2 (4–8 years) | 82 (4–8 years) to 198 (adults) |
| Inhalation exposures from DIY facial steamer for bystander (100%) (1 year only) | 0.10 | 168 |
a Exposure estimates were adjusted by 19% for the maximum amount of violet oil aldehyde present in violet oil, assuming that 80% of the applied dose was available for evaporation (except for the exposure from food flavouring agent). Details are available in Appendix B.
b The MOEs were calculated using an adjusted non-carcinogenic LOAEL of 16.1 mg/kg bw/day for the read-across analogue 2,4-hexadienal, based on the presence of mild to moderate forestomach epithelial hyperplasia at all doses in both sexes in the 2-year carcinogenicity study in rats.
c After a total of 4 hours of exposure; once the device is turned off after 20 minutes of use, it is assumed that the person remains in the room for 3 hours and 40 minutes.
The MOEs to violet oil from food (based on its potential use as a flavouring agent), eye moisturizer, leave-on hair conditioner, facial cleanser, body moisturizer, massage oil (9 years and above), lipstick, and body fragrance are considered adequate to address uncertainties in the health effects and exposure data used to characterize risk.
In addition, the use of violet oil in a massage oil (8 years and younger) are below 300, which accounts for uncertainties with respect to interspecies extrapolation, intraspecies extrapolation, the POD, and the adequacy of the database and is considered potentially inadequate.
The use of violet oil as a DIY bath oil product and a DIY body moisturizer is considered adequate to address uncertainties in the health effects and exposure data used to characterize risk.
The MOEs between the critical effect level and the estimates of daily exposure from the use of violet oil in DIY aromatic diffuser, as a DIY facial steamer (during the use of the device), and once the device is turned off after 20 minutes of use are below 300, which account for uncertainties with respect to interspecies extrapolation, intraspecies extrapolation, the POD, and the adequacy of the database and are considered potentially inadequate.
7.4.4 Uncertainties in evaluation of risk to human health
The key sources of uncertainty are presented in the table below.
| Key source of uncertainty | Impact |
|---|---|
| Limited hazard data are available for violet oil and its main component, 2,6-nonadienal. The read-across analogue, 2,4-hexadienal, was used to inform the risk assessment. | +/- |
| There are no suitable dermal or inhalation toxicity studies available for 2,4-hexadienal; therefore, route-to-route extrapolation for violet oil was carried out for inhalation exposure scenarios using a critical effect level from an oral study, and a dermal POD was not determined. | +/- |
+ = uncertainty with potential to cause overestimation of exposure/risk; - = uncertainty with potential to cause underestimation of exposure/risk; +/- = unknown potential to cause over- or underestimation of risk.
7.5 Aldehyde subgroup 2 (lilial, verdantiol, myrac-aldehyde, myrmac-aldehyde, myrmac-carboxaldehyde, cetonal, vernaldehyde)
7.5.1 Exposure assessment
Environmental media
Considering the low quantity (<100 kg) of the substance submitted in response to a CEPA section 71 survey (Environment Canada 2013), exposure to verdantiol, myrac-aldehyde, myrmac-aldehyde, myrmac-carboxaldehyde, and vernaldehyde from environmental media is not expected.
In 2011, the National Research Council of Canada (NRC) examined the presence of 954 organic chemicals from four databases on building materials, indoor air, and dust samples. A subset of data from a 2010 NRC study involving indoor air and dust samples from 115 homes in Quebec City was analyzed to identify certain chemicals, including lilial. The maximum reported concentrations were 28.99 μg of lilial/g of dust and 3.76 μg/m3 in indoor air (NRC 2011). Lilial was also quantified in indoor air by examining the gas chromatographs of indoor air samples collected in two field monitoring studies measuring volatile organic compounds in indoor air at 36 homes in Ottawa in 2014 (that is, the garage study) and 54 homes in Nunavik in 2018 (that is, Nunavik study). Lilial was not detected at levels above the method detection limit (that is, 2 ng) in Ottawa, but it was detected in 9% of samples collected in Nunavik. The air concentrations of lilial from samples above the method detection limit ranged from 0.4 μg/m3 to 0.7 μg/m3 (n=5) (NRC 2019). As the air concentrations in indoor air were higher in the Quebec City study, this study was used to calculate potential exposure to lilial from indoor air.
Measured concentrations of lilial in ambient air, water, or soil were not identified in Canada. The level III fugacity model known as ChemCAN (2003) was used to derive predicted environmental concentrations using the quantities reported in Canadian commerce for 2011 for lilial (Environment Canada 2013), that is, 25 370.22 kg (manufacturing and imports). The estimated concentrations in air, water, and soil were 5.50 x 10-5 μg/m3, 3.34 x 10-3 μg/L, and 0.32 ng/g, respectively.
Exposure to lilial from environmental media was estimated using the predicted concentrations in ambient air, water, and soil, and the maximum concentrations of lilial measured in indoor air and dust in the Quebec City study (NRC 2011). Estimated exposure ranged from 6.70 x 10-4 mg/kg bw/day to 2.49 x 10-3 mg/kg bw/day for adults to 1-year-olds, respectively.
Products available to consumers
Lilial is present in products available to consumers. To evaluate the potential for exposure to lilial from cosmetics and NHPs applied by the dermal route, sentinel scenarios were selected based on a combination of use frequencies and reported concentrations of lilial in these products. The selected sentinel scenarios represented the highest exposures, relative to other dermally applied cosmetics, and NHPs based on identified products reported to contain these substances. Exposure to lilial from the use of body fragrance; liquid body cleanser; wash-off hair conditioner; massage oil; body moisturizer; face makeup; nail polish; nail polish remover; depilator; antiperspirant/deodorant; bath product; facial moisturizer; acne treatment (NHP); antiseptic skin cleanser (NHP); hair colour; hair straightening, waving, and curling product; and sunless tanning product for the face (non-SPF) were considered to be the sentinel scenarios for dermal application (personal communication, email from the Consumer and Hazardous Product Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2020; unreferenced; personal communication, email from the Natural and Non-prescription Health Products Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2021; unreferenced).
Given the widespread use of lilial in products available to consumers, it was selected as a chemical of relevance for human biomonitoring (HBM) in Germany. In the first HBM study on lilial, 2133 children and adolescents (both sexes) aged 3 to 17 years provided void urine samples that were analyzed (Murawski et al. 2020). Four main metabolites, that is, tert-butylbenzoic acid (TBBA), lysmerol, lysmerylic acid, and hydroxy-lysmerylic acid, were found in quantifiable amounts with maximum concentrations of 315, 91.1, 8.97 and 39.7 μg/L, respectively; mean concentrations of 10.21 μg/L for TBBA, 1.528 μg/L for lysmerol, and concentrations below the limit of quantification of 0.2 μg/L and 0.4 μg/L for lysmerylic acid and hydroxy-lysmerylic acid, respectively, were also found (Murawski et al. 2020). In addition, the same metabolites were measured in urine samples from 329 young adults aged 20 to 29 years. These samples were provided by the German Environment Agency to investigate exposure to lilial between 2000 and 2018 (Scherer et al. 2021). The maximum concentrations were 84.72, 14.02, 31.32, and 29.23 μg/24-hour, and mean concentrations were 13.73, 1.78, 0.99, and 3.41 μg/24-hour for TBBA, lysmerol, lysmerylic acid, and hydroxy-lysmerylic acid, respectively. A significant decline of these metabolites was observed over the sampling years (Scherer et al. 2021).
Lilial has been identified in a carpet deodorizer product (SDS 2017a). Lilial has also been identified in various air freshener products (solid gel, liquid plug-in, and air spray) (SDS 2016, 2017a, 2019) at concentration ranges from 0.1% to 5%. Exposure was quantified from a solid gel air freshener product (SDS 2016) and a liquid plug-in (SDS 2017a).
Following occlusive dermal application of lilial (6.8 mg/kg bw/day in 70% ethanol on the back skin of rats), a mean cumulative total of 14.6% of the dose was excreted in urine, 0.8% was recovered in cage washings, and 2.0% was excreted via feces (levels in expired air traps were not detectable). The remaining radioactivity in all tissues investigated was 1.2% of the initial dose (site of application). The mean total proportion of the applied dose was ~19% in excreta and tissues up to 120 hours after application (SCCS 2016; RIFM 2020).
To estimate systemic exposure for the aldehydes subgroup 2 substances, estimated oral, inhalation, and dermal exposures were summed, where appropriate. A dermal absorption factor of 15%, based on a chemical-specific human in vitro study for lilial, was used (SCCS 2019). The estimated dermal absorption value was a high-end estimate from four different formulation groups with observed dermal absorption values ranging from 8.5% to 13.5% (including skin-bound residues and the addition of two standard deviations for two groups and one standard deviation for the other two dose groups). To account for the amount of product absorbed by the dermal route, the product amount available for inhalation was adjusted by 85%, except in the case of body moisturizer. For body moisturizer, since the product amount for inhalation was adjusted for the exposed surface area, and this value was less than 85% of the product amount, no further adjustment was made to the product amount.
Estimates of systemic exposures to lilial for the lowest and highest exposed age groups are summarized in Table 7-24.
| Product scenario | % in product | Route(s) of exposure | Exposure range (mg/kg bw/day)a |
|---|---|---|---|
| Body fragrance | 44% | Dermal and inhalation | 0.54 (adults) to 1.52 (2–3 years) |
| Liquid body cleanser | 1% | Dermal and inhalation | 3.24 x 10-3 (adults) to 1.3 x 10-2 (0–5 months) |
| Hair conditioner (wash-off) | 2% | Dermal and inhalation | 5.06 x 10-3 (14–18 years) to 1.06 x 10-2 (2–3 years) |
| Massage oil | 0.3% | Dermal and inhalation | 2.58 x 10-2 (adults) to 1.38 x 10-1 (0–5 months) |
| Body moisturizer | 1% | Dermal and inhalation | 2.17 x 10-1 (adults) to 4.84 x 10-1 (0–5 months) |
| Face makeup liquid | 1% | Dermal and inhalation | 1.30 x 10-2 (14–18 years) to 2.69 x 10-2 (4–8 years) |
| Nail polish | 3% | Dermal and inhalation | 9.73 x 10-3 (adults) to 1.80 x 10-2 (2–3 years) |
| Nail polish remover | 0.1% | Dermal and inhalation | 5.86 x 10-2 (adults) to 1 x 10-2 (9–13 years) |
| Hair removal/depilator | 0.1% | Dermal and inhalation | 3.67 x 10-3 (14–18 years) to 4.46 x 10-3 (adults) |
| Antiperspirant/deodorant (solid) | 3% | Dermal and inhalation | 5.18 x10-2 (9–13 years) to 8.63 x 10-2 (14-18 years) |
| Antiperspirant/deodorant (spray) | 0.3% | Dermal and inhalation | 1.01 x 10-2 (9–13 years) to 2.67 x 10-2 (14–18 years) |
| Bath product | 3% | Dermal and inhalation | 9.18 x 10-4 (9–13 years) to 2.83 x 10-3 (14–18 years) |
| Facial moisturizer | 3% | Dermal and inhalation | 1.32 x 10-1 (14–18 years) to 2.11 x 10-1 (adults) |
| Permanent hair colour | 1% | Dermal and inhalation | 2.69 x 10-1 (adults) to 3.21 x 10-1 (14–18 years) |
| Temporary hair colour | 0.11% | Dermal and inhalation | 8.33 x 10 -3 (adults) to 2.59 x 10-2 (4–8 years) |
| Hair straightening, waving, and curling product | 11% | Dermal and inhalation | 1.62 x 10-1 (adults) to 4.31 x 10-1 (4–8 years) |
| Sunless tanning product for the face (lotion) | 1% | Dermal and inhalation | 3.35 x 10-2 (adults) to 3.94 x 10-2 (14–18 years) |
| Acne treatment (NHP) | 0.1% | Dermal and inhalation | 2.23 x 10-4 (adults) to 3.53 x 10-4 (9–13 years) |
| Antiseptic skin cleanser (NHP) | 0.007% | Dermal and inhalation | 3.56 x 10-4 (14–18 years) to 1.05 x 10-3 (2–3 years) |
| Antiseptic skin cleanser (NHP)b | 0.007% | Dermal and inhalation | 5.33 x 10-3 (adults) to 2.63 x 10-2 (2–3 years) |
| Carpet deodorizer (application) | 1% | Dermal and inhalation | 7.71 x 10-4 (adults) |
| Post-application exposure to cleaned carpets | 1% | Dermal, inhalation, and incidental oral | 1.31 x 10-2 (1 year old) |
| Solid gel air freshener | 5% | Inhalation | 5.31 x 10-2 (adults) to 2.11 x 10-1 (1 year) |
| Liquid plug-in air freshener | 3% | Inhalation | 1.37 x 10-2 (adults) to 4.87 x 10-2 (1 year) |
a Only lowest to highest exposed age groups are presented. Calculation details are in Appendix A.
b For situations of public health concern, the use of hand sanitizers among the general population may increase up to 25 uses per day (personal use by adults, increased use by children in schools and child care facilities) (RIVM 2021a)
No Canadian uses in products available to consumers, food packaging materials, or incidental additives have been identified for myrac-aldehyde, myrmac-aldehyde, myrmac-carboxaldehyde, cetonal, vernaldehyde, and verdantiol.
An industry submission indicated that lilial, myrac-aldehyde, myrmac-aldehyde, myrmac-carboxaldehyde, cetonal, and vernaldehyde may be present as fragrance ingredients in products available to consumers, and these products are present in the following categories: leave-on cosmetics (that is, less than 0.05%), rinse-off cosmetics (that is, less than 0.1%), air fresheners (that is, less than 2.5%), and cleaning products (that is, less than 0.05%). In order to evaluate the potential exposure of the general population to these substances from the use of these categories of products, representative sentinel exposure scenarios were selected. These scenarios describe the highest level of exposure to these substances from product use, taking into consideration frequency of use and concentrations. Therefore, a body moisturizer at 0.05%, a body cleanser at 0.1%, a liquid air freshener at 2.5%, and a liquid laundry detergent machine at 0.05% represent the sentinel scenarios for the leave-on cosmetics, rinse-off cosmetics, air fresheners, and cleaning products categories, respectively. Estimates of systemic exposures for the lowest and highest exposed age groups are summarized in Table 7-25.
| Product scenario | % in product | Route(s) of exposure | Exposure range (mg/kg bw/day)a | Leave-on cosmetics | 0.05% | Dermal and inhalation | 1.08 x 10-1 (adults) to 2.42 x 10-2 (0–5 months) |
|---|---|---|---|
| Rinse-off cosmetics | 0.1% | Dermal and inhalation | 1.71 x 10-3 (adults) to 4.37 x 10-3 (0–5 months) |
| Air fresheners | 2.5% | Inhalation | 1.14 x 10-3 (adults) to 4.07 x 10-3 (1 year) |
| Cleaning products | 0.05% | Dermal and inhalation | 1.66 x 10-5 (14–18 years) to 5.6 x 10-4 (adults) |
a Only lowest to highest exposed age groups are presented. Calculation details are in Appendix A.
7.5.2 Health effects assessment
There are seven discrete substances in aldehydes subgroup 2 (lilial, verdantiol, myrac-aldehyde, myrmac-aldehyde, myrmac-carboxaldehyde, cetonal, and vernaldehyde).
There is no hazard information available for verdantiol, myrmac-aldehyde, and myrmac-carboxaldehyde. For myrac-aldehyde and vernaldehyde, there is limited hazard information available consisting of skin sensitization data. Cetonal was evaluated by the NICNAS on the basis of limited available hazard information on skin sensitization and genotoxicity (NICNAS 2016b).
Myrac-aldehyde is not considered to be a skin sensitizer based on the findings from a maximization test in which 23 human volunteers did not have a sensitization reaction following exposure to 3% myrac-aldehyde in petrolatum (Fragrance raw materials monographs 1976).
Vernaldehyde is not considered to be a skin sensitizer based on the findings from a repeated-insult patch test in which human volunteers (n=52) did not have a sensitization reaction following exposure to 2% vernaldehyde in dimethyl phthalate (Ford et al. 1992).
Cetonal is considered to be a skin sensitizer in female mice based on the positive results seen in a local lymph node assay conducted according to OECD Test Guideline 429 (NICNAS 2016b). Cetonal was considered a non-sensitizer in the KeratinoSen assay since the luciferase gene was induced at cytotoxic concentrations (ECHA Registration dossier 2020d).
Cetonal showed negative results in an in vitro point mutation assay (Ames test) in S. typhimurium strains TA98, TA100, TA102, TA1535, and TA1537, with or without metabolic activation (NICNAS 2016b; ECHA Registration dossier 2020d).
In the absence of hazard data on these substances, a read-across approach was taken, and hazard information on the analogue, lilial, was used to inform the health effects assessment.
Lilial is a synthetic substance that has not been reported to occur in nature (Arnau et al. 2000), whereas the other five substances in aldehydes subgroup 2 are naturally occurring and belong to the class of terpenes. Lilial is a racemic mixture covering two enantiomers, namely (2S)-3-(4-tert-butylphenyl)-2-methylpropanal and (2R)-3-(4-tert-butylphenyl)-2-methylpropanal (SCCS 2019). The seven substances within aldehydes subgroup 2 are all aromatic aldehydes, characterized by a six carbon ring and possessing multiple methyl groups and a carbonyl group on the side chain. However, lilial possesses three double bonds in the ring structure (benzene ring), whereas the other aldehydes in aldehydes subgroup 2 only have one. In addition, the position of the carbonyl group differs for each substance. For example, the carbonyl group is present on the side chain farther away from the ring for lilial and cetonal, whereas for the other substances, it is found closer to the ring structure. Finally, myrac-aldehyde and myrmac-aldehyde both possess one double bond in the side chain, unlike the other substances in the subgroup that do not have double bonds (except for the carbonyl group). Human and animal studies show clear evidence of systemic absorption of lilial via the oral and dermal routes as well as high bioavailability via the oral route (SCCS 2016). There are no toxicokinetics data on the analogues, but they are also likely to have high bioavailability via the oral route as their physical and chemical properties are very similar to those of lilial. Target and analogues are simple aldehydes, and therefore, all have structural alerts for genotoxicity as well as in vitro and in vivo mutagenicity on QSAR Toolbox, version 4.2 (OECD 2016).
Verdantiol is a Schiff base substance synthetically prepared from an aldehyde (lilial) and an amine (methyl anthranilate). The preparation of Schiff bases is a reversible reaction, which means that verdantiol is unstable and prone to hydrolysis to regenerate the original aldehyde, lilial. The stoichiometric presence of lilial of the verdantiol is taken into account, assuming 100% dissociation as default based on the absence of data on verdantiol.
An amendment to the CLP Regulation was made on August 2020 to classify lilial as Reproductive toxicant category 1B (presumed human reproductive toxicant based on animal studies) based on evidence of reproductive toxicity, and lilial has been prohibited in cosmetics in Europe since March 2022. Lilial is also under assessment by ECHA as a potential endocrine disruptor. Furthermore, lilial is classified as a reproductive toxicant according to the harmonized classification and labelling approved by the EU and is included on their candidate list for authorization as a substance of very high concern.
Toxicokinetics
After semi-occlusive dermal application of 14C-lilial (11.37 mg of lilial in 70% ethanol on 10 cm2 of back skin) on three human volunteers for 6 hours, a mean of 1.4% of the applied dose was excreted in urine within 24 hours, and radioactivity was below the detection limit in urine samples of later time points and in all feces and blood plasma samples (SCCS 2016).
Based on multiple lines of evidence (Hunter et al. 1965; Cagen et al. 1989), the toxicity of lilial on the male reproductive tract in rats is hypothesized to be due to the formation of the metabolite p-tert-butyl-benzoic acid (TBBA), more specifically the TBBA coenzyme A (CoA) conjugate formed (McCune et al. 1982). Lilial and the metabolite TBBA is rapidly transformed to TBBA-CoA in rat hepatocytes, leading to an accumulation of stable levels of this conjugate, which was not detectable in human hepatocytes; this indicates a species-specific effect in the mechanism of toxicity of lilial where rats seem to be more sensitive than humans (Laue et al. 2017).
The possible estrogenic activity of lilial was investigated in an assay in MCF7 human breast cancer cells in vitro (Charles et al. 2009). Lilial partially displaced [3H]-estradiol from recombinant human estrogen receptors ERα and ERβ and from cytosolic estrogen receptor of MCF7 cells (Charles et al. 2009). Furthermore, lilial increased the expression of a stable integrated estrogen-responsive reporter gene and of the endogenous estrogen-responsive pS2 gene in MCF7 cells (Charles et al. 2009). On the basis of these observations, the authors concluded that lilial can induce estrogenic responses in the MCF7 human breast cancer cell line in vitro. However, no studies are available on the potential estrogenic activity of lilial in vivo.
Repeated-dose toxicity studies
In a study compliant with OECD Test Guideline 408, lilial was orally administered to female and male Albino rats (14/sex/dose) by gavage at doses of 0, 2, 5, 25, or 50 mg/kg bw/day, 5 days a week for 13 weeks (SCCS 2016; ECHA Registration dossier 2020e). The animals in the control and the high-dose groups had a post-treatment recovery of four weeks. At ≥25 mg/kg bw/day, dose-dependent increases in absolute and relative liver and adrenal weights were observed in both sexes, but these changes were reversible in the recovery (high-dose) group. Furthermore, a significant decrease in plasma cholinesterase activity (30% and 70% compared to controls) and lower plasma cholesterol levels were observed at 25 and 50 mg/kg bw/day in both sexes, but these changes were also reversible in the recovery group. There was an increased incidence of testicular atrophy and spermatoceles in the epididymis in males at 50 mg/kg bw/day. Disturbances of spermatogenesis and spermiogenesis, testicular increases in Sertoli cell-only tubules, and increased surface density in Leydig cells were described, along with a decreased density of spermatozoa, nucleated cells, and spermatoceles in the epididymis at 50 mg/kg bw/day. In the four-week recovery group, the effects on the male reproductive organs persisted after treatment. A NOAEL of 25 mg/kg bw/day for testicular toxicity was determined by the study authors (SCCS 2016; ECHA Registration dossier 2020e).
In a subchronic toxicity study, beagle dogs (3/sex/dose) were orally administered daily capsules containing 0, 4.4, 22.3, or 44.6 mg/kg bw/day lilial, 7 days a week for 13 weeks (SCCS 2016). No mortality occurred, and no significant differences in body weight gains were observed. No treatment-related effects were reported for clinical signs and chemistry. Gross pathology and histopathology revealed no specific substance-related findings; in particular, there were no alterations on reproductive organs in males or females (SCCS 2016). In a subsequent study by the same authors, in which three female beagle dogs were orally administered 200 mg/kg bw/day lilial in the form of capsules for 13 weeks, similarly to the previous study, no treatment-related effects were found (SCCS 2016; CLH 2017).
In an explorative dose-escalation study, two male dogs were treated with lilial at increasing doses of 50 μL/kg bw/day from days 1 to 7; 100 μL/kg bw/day from days 8 to 14; 200 μL/kg bw/day from days 15 to 21; 400 μL/kg bw/day from days 22 to 50; and 600 μL/kg bw/day from days 51 to 64 (corresponding to 47 mg/kg bw/day up to 564 mg/kg bw/day) (SCCS 2016; CLH 2017). This study indicated occasional vomiting in both animals, diarrhea in one animal, and body weight reduction together with an increase in glutamate dehydrogenase and alanine aminotransferase levels. Histological examinations showed multifocal inflammation in the liver and mild atrophy in seminiferous tubules in both animals (necrosis of germ cells, multinucleated giant cells in tubular lumen) (SCCS 2016; CLH 2017).
In a one-generation reproductive toxicity range-finding study, male and female Wistar rats (10/sex/dose) were orally administered 0, 400, 800, 1700, or 3400 ppm (equivalent to 14.5, 28.7, 62.6, or 119.7 mg/kg bw/day, respectively) of lilial microencapsulated in the feed during the premating (six weeks) and mating periods for both sexes and throughout gestation and lactation (14 weeks) for F0 dams only (SCCS 2016; NICNAS 2016c; CLH 2017). F0 males showed a dose-dependent decrease in body weights and body weight gains, along with a reduction in food consumption in the 3400 ppm group. Increases in relative liver weights starting at 800 ppm and decreases in relative kidney weights in the 3400 ppm group were observed in F0 males only. In F0 males, increased levels of plasma alanine aminotransferase, alkaline phosphatase, and glutamate dehydrogenase were observed at 1700 and 3400 ppm, and increased levels of plasma gamma-glutamyltransferase were observed only at 3400 ppm. Effects on male reproductive organs were observed at 1700 and 3400 ppm, including decreases in relative testes and cauda epididymis weights, diffuse testes degeneration, and aspermia of the epididymis. In the 3400 ppm male group, a decrease in seminal vesicle and prostate weights was observed as well as hyperplasia of Leydig cells. F0 dams showed decreases in body weights and body weight gains in the 800 ppm group and at higher doses during and after premating. During gestation and lactation, mean maternal body weights and body weight gain were decreased in the 800 ppm group, and food consumption was decreased during lactation compared to controls. A 2-8 fold increase in serum levels of gamma-glutamyltransferase and a decrease of between 50% and 65% in serum cholinesterase levels compared to controls were seen in all dose groups in F0 dams. No viable offspring was produced from animals in the 1700 and 3400 ppm groups. At 1700 ppm, only one of eight mated females became pregnant, and only had one implantation, which was finally resorbed (post-implantation loss). In the 400 and 800 ppm groups, non-significant increases in mean implantation losses and decreases in the mean number of delivered pups per dam were observed. Post-natal survival was non-significantly decreased between days 0 and 4 in the 400 and 800 ppm groups, and no pup mortality was observed between PNDs 4 and 21. Pup weight at birth and weaning and pup body weight gain were also reduced in the 400 and 800 ppm groups. Based on these results, the authors suggested the dose of 400 ppm (~14.5 mg/kg bw/day) as a NOAEL in males and a LOAEL in dams for systemic toxicity and determined a NOAEL of 800 ppm (~28.7 mg/kg bw/day) for male reproductive toxicity (SCCS 2016; NICNAS 2016c; CLH 2017).
In a developmental toxicity study compliant with OECD Test Guideline 414, pregnant rats (25/dose) were orally administered 0, 5, 15, or 45 mg/kg bw/day (effective doses: 0, 4.1, 12.7, or 40.7 mg/kg bw/day, respectively) lilial by gavage in olive oil from GDs 6 to 20 (SCCS 2016; NICNAS 2016c; CLH 2017). No mortality occurred, but signs of maternal toxicity were observed at 15 and 45 mg/kg bw/day. Increases in mean alanine aminotransferase levels (20% to 30% above control) and decreases in serum cholinesterase levels (20% to 45% below control) were found in the 15 and 45 mg/kg bw/day groups, and mean glutamate dehydrogenase levels were increased (79% above controls) in the 45 mg/kg bw/day dose group. Increases in absolute and relative liver weights were found at all dose levels, with histopathological findings at 15 and 45 mg/kg bw/day, and uterus weights were reduced (20% below controls) at 45 mg/kg bw/day, though not significantly. Mean food consumption was reduced (18% below controls) in the high-dose group on days 6 to 8 post coitum (p.c.) but was comparable to controls by study termination. Although no decrease in food consumption was observed in mid-dose animals, mean maternal weight gains decreased on days 6 to 8 p.c. (56% below controls) but recovered during the study period. At 45 mg/kg bw/day, maternal mean body weight loss was observed on days 6 to 8 p.c., and the mean body weight gain was reduced (25% below controls) over the entire treatment phase. Mean post-implantation losses were increased in the high-dose group: resorptions were observed at 15.1% per dam compared to 4.4%, 4.7%, and 4.9% at 0, 5 and 15 mg/kg bw/day, respectively. Signs of prenatal developmental toxicity were observed at 15 and 45 mg/kg bw/day, consisting of reduced mean fetal body weights and an increase in mean percentages of skeletal variations per litter (delays and disturbances in ossification of the skull, sternebra, and pubic girdle). Malformations were observed in 3 out of 170 high-dose group fetuses (1.8% of all high-dose group fetuses; 3 out of 23 litters affected), including anasarca, polydactyly, and cervical hemivertebra. A NOAEL of 4.1 mg/kg bw/day was determined by the authors for maternal and prenatal developmental toxicity at higher doses (SCCS 2016; NICNAS 2016c; CLH 2017).
In a modified extended one-generation reproduction toxicity study following OECD Test Guideline 443, encapsulated lilial was administered to male and female Wistar rats (35-40/sex/dose) at nominal doses of 0, 1, 3, or 10 mg/kg bw/day (equivalent to 0, 1.4, 4.5, or 15.1 mg/kg bw/day, respectively) in food (CLH 2017; SCCS 2019; RIFM 2020). F0 animals were treated with lilial for approximately two weeks prior to mating, through mating (up to two weeks), and for a maximum of six post-mating weeks (males) or gestation (three weeks) and lactation (three weeks) for females. Pups of the F1 litter were assigned to seven different cohorts (cohorts 1A, 1B, 2A, 2B, 3, 4A, and 4B) for assessment of specific (histo-) pathological examinations and were maintained on the test diet until sacrifice. Cohort 1B was selected to produce F2 pups. Cohorts 1 (A/B) were examined for reproductive toxicity, cohorts 2 (A/B) for developmental neurotoxicity, cohort 3 for developmental immunotoxicity (DIT), and cohorts 4 (A/B) for acetyl cholinesterase activities in F0 animals, PND 4 surplus pups, and PND 22 and adolescent F1 offspring. The study was terminated with the sacrifice of the F2 weanlings and F1 cohort 1B parental animals. There were no test substance-related mortalities reported. Maternal body weights were significantly reduced compared to controls in the high-dose F0 and F1 females during gestation and lactation. Absolute ovary weights (100, 99, 94, and 88 mg at 0, 1, 3, and 10 mg/kg bw/day, respectively) and relative ovary weights (100, 96, 93, and 89 mg at 0, 1, 3, and 10 mg/kg bw/day, respectively) were reduced in a dose-dependent manner in the F0 females, reaching statistical significance at the high dose. Some changes in blood and enzyme parameters were observed at 10 mg/kg bw/day, such as a prolonged prothrombin time in F0 and F1 for both sexes and increased γ-glutamyl transferase activity and reduced albumin levels in F0 and F1 females. At 10 mg/kg bw/day, higher red blood cell counts and hemoglobin values were detected in F0 females and F1 males and females. Higher hematocrit levels were also noted in F0 females at this dose. A decrease in mean serum acetylcholinesterase activities was seen in F0 females (16% in mid-dose and 21% in high-dose animals below controls) and other peripheral tissues including erythrocytes. In the F0 high-dose males, a decrease in the mean acetylcholinesterase activities of the musculus gastrocnemius (18% below controls) was observed. In the high-dose F0 females and F1 females (cohorts 1A and 1B), an increase in absolute and relative liver weights (119% and 120% above controls, respectively) was observed, associated with minimal to slight centrilobular hypertrophy and accompanied by minimal to slight apoptosis/single cell necrosis of hepatocytes. The only notable findings on reproductive parameters were slightly and non-significantly higher mean percentages of abnormal sperms (3.5% above control) in the cauda epididymis in the high-dose F0 males. Pup body weight of the high-dose F1 and F2 offspring was 14% to 16% lower than controls after birth and did not recover until weaning. In high-dose F1 females, a decrease in the mean number of implantation sites as well as a decrease in the mean number of F2 pups delivered were observed. Lower peripheral acetylcholinesterase activities in serum erythrocytes and diaphragm tissue were found in male F1 pups at PND 4 and in females at PND 76 of the high-dose group (up to 50% of the means in control animals). No corresponding clinical signs of developmental neurotoxicity were evident in male and female F1 offspring at any dose level. There were no treatment-related effects on motor activity, auditory startle habituation, or in the field observation battery following exposure to the test compound in these animals. The only notable findings in neurobehavioural testing were lower maximum amplitudes in the auditory startle response test for the high-dose F1 males only, and no corresponding effects were recorded for startle response latency. Regarding neuropathology, brain weight determination, brain length and width measurements, and brain morphometry and neuropathological examination by light microscopy did not reveal any neurotoxicological treatment-related findings. Neither T-cell dependent anti-SRBC IgM antibody response, nor absolute and relative lymphocyte cell counts in the spleen tissue showed any treatment-related changes, indicating no evidence of any DIT induced by lilial. On the basis of these results, the authors determined a NOAEL for systemic toxicity of 3 mg/kg bw/day (effective dose: 4.5 mg/kg bw/day) for the F0 and F1 parental animals as well as the adolescent animals; a NOAEL for developmental toxicity of 3 mg/kg bw/day (effective dose: 4.5 mg/kg bw/day) in the F1 and F2 progeny; and a NOAEL for reproductive performance of 10 mg/kg bw/day (effective dose: 15.1 mg/kg bw/day), the highest dose tested, in the F0 and F1 parental rats (CLH 2017; SCCS 2019; RIFM 2020).
In the only dermal toxicity study, undiluted lilial was applied as occlusive patches on the back of albino rats (5 males/group) for six hours, at doses of 0, 250, 500, 1000, or 2000 mg/kg bw/day for five consecutive days (SCCS 2016; NICNAS 2016c; CLH 2017). A slight decrease in body weight and marked testicular atrophy were noted at 2000 mg/kg bw/day. Seminiferous tubules with disorganization of the epithelial structure, decreases in the number of germ cells, and increases in the number of degenerating germ cells (including giant cells) were observed in combination with immature/degenerating germ cells in epididymis and the presence of spermatoceles at 2000 mg/kg bw/day. No further observations were performed to assess adverse effects other than testicular toxicity. The study authors considered the NOAEL to be 1000 mg/kg bw/day based on the effects observed on the reproductive organs at the highest dose tested (NICNAS 2016c; SCCS 2016; CLH 2017).
Rats and mice (5 males/group) were administered 50 mg/kg bw/day lilial and lysmerylic acid (metabolite of lilial) in olive oil by gavage for 1, 2, 3, 4, or 14 days, with the main focus on reproductive organ toxicity (SCCS 2016; CLH 2017). No mortality occurred, and no clinical signs were reported for both rats and mice. In rats, slight to severe testicular atrophy, with an incidence of 2/5 animals after a single application of lilial and in all animals after longer application periods, was observed. In mice, a reduction in the ratio of normal to abnormal sperm was observed after exposure to lilial for three and four days only, but no histopathological changes in the testes were noted. However, longer treatment periods did not influence this parameter or other sperm parameters, such as sperm motility and spermatid count in testes or cauda epididymis (SCCS 2016; CLH 2017).
Several explorative oral gavage studies conducted for five consecutive days in rats at doses ranging from 25 mg/kg bw/day to 400 mg/kg bw/day showed clinical signs of toxicity, body weight loss, and macroscopic changes in the liver starting from 50 mg/kg bw/day (SCCS 2016; NICNAS 2016c; CLH 2017). At the same dose level, degeneration and loss of germ cells in the seminiferous epithelium were found. However, decreased testes and kidney weights and decreased sizes of prostate and seminal vesicles became evident at higher dose levels, which were not specified in the study (SCCS 2016; NICNAS 2016c; CLH 2017).
Five male mice and five male guinea pigs administered a daily oral dose of 100 mg/kg bw/day of lilial for five consecutive days showed no signs of systemic toxicity, including testicular toxicity (SCCS 2016; NICNAS 2016c; CLH 2017).
Five male rabbits treated for 15 days via gavage with lilial at 30, 100, or 300 mg/kg bw/day showed no treatment-related findings on clinical observations, body weight, and food consumption (SCCS 2016; NICNAS 2016c; CLH 2017). At 30 mg/kg bw/day, one animal showed a moderate degeneration of the seminiferous tubules, and moderate oligospermia and moderate inflammation in the epididymis. At 100 mg/kg bw/day, reduced testes and epididymides sizes with severe diffuse degeneration of seminiferous tubules and severe atrophy plus aspermia in the left epididymis were observed. However, sperm evaluation did not reveal any treatment-related effect in this or any other treated animal, and a dose-response relationship could not be observed. These effects were not considered to be treatment-related by the study authors (SCCS 2016; NICNAS 2016c; CLH 2017).
Beagle dogs (4 males/group) were administered oral doses of lilial via gelatine capsules at 0, 40, 200, or 1000 mg/kg bw/day for two weeks (SCCS 2016; NICNAS 2016c; CLH 2017). Due to occurrences of vomitus and diarrhea at 1000 mg/kg bw/day, the high dose was lowered to 500 mg/kg bw/day starting on day 3. At the mid- and high-dose groups, increased liver weight and centrilobular hypertrophy of hepatocytes was observed. At 200 mg/kg bw/day, one dog showed degeneration of seminiferous tubules, hyperplasia of Leydig cells, and aspermia and epithelial vacuolation in the epididymis (SCCS 2016; NICNAS 2016c; CLH 2017). A follow-up study in 10 male dogs administered 200 mg/kg bw/day of lilial for two weeks led to severe body weight loss, anemia, increased liver weight, and decreased prostate and testes weight with histomorphological correlates. Altered sperm quality and reduced sperm motility were also noted (SCCS 2016; NICNAS 2016c; CLH 2017).
Two male Rhesus monkeys were orally administered lilial in food at 0 or 100 mg/kg bw/day for five days (SCCS 2016; NICNAS 2016c; CLH 2017). Only small foci in one epididymis of one animal and small hollow spaces in the epithelium of one epididymis of the other animal were observed. The testes of both animals were found to be free of lesions. According to the study authors, the findings in one epididymis of each animal do not represent a test substance-related effect since other male reproductive tissues were not affected (SCCS 2016; NICNAS 2016c; CLH 2017).
The toxicity studies on lilial show adverse reproductive effects in most animal species tested (rats, mice, dogs, rabbits, and monkeys), with the rat being the most sensitive and showing adverse effects on the male reproductive organs at lower doses compared with the other species.
Genotoxicity
Lilial showed negative results in an Ames test using S. typhimurium strains TA1535, TA1537, TA1538, TA97, TA98, TA100, and TA102, with or without metabolic activation (Di Sotto et al. 2014; SCCS 2016; NICNAS 2016c). Similarly, another bacterial reverse mutation assay on S. typhimurium strains TA98, TA100, and TA1537 and E. coli strain WP2 uvrA gave negative results in the presence and absence of S9-mix (SCCS 2019). However, increased numbers of revertants in the absence of S9-mix were observed in this reverse mutation assay for the S. typhimurium strain TA1535 in the plate incorporation test (but not in the follow-up pre-incubation test), although the authors considered lilial to be non-mutagenic in this assay since the increases observed were not reproducible, were associated with cytotoxicity, and did not show a dose trend (SCCS 2019). A mammalian cell gene mutation assay using Chinese hamster lung fibroblasts (V79) showed significant increases in mutant frequency. However, the authors concluded that lilial was not mutagenic under the study conditions since the increases observed were not dose-dependent or reproducible (ECHA Registration dossier 2020e; SCCS 2016; NICNAS 2016c). Lilial did not induce any significant increases in the mutant frequency at the TK +/- locus in L5178Y cells in another gene mutation test using a mouse lymphoma assay (ECHA Registration dossier 2020e). Lilial did not induce single-strand DNA breaks in an alkaline Comet assay in human colonic epithelial cells (Di Sotto et al. 2014). In a non-guideline micronucleus assay on human lymphocytes, lilial showed no increase in the micronuclei frequency in comparison with the control (Di Sotto et al. 2014). In contrast, lilial induced numerical and structural chromosomal aberrations in the absence of S9-mix and a concentration-dependent increase of structural aberrations in the presence of S9-mix in a mammalian chromosome aberration test using Chinese hamster ovary cells (SCCS 2016; NICNAS 2016c).
In an in vivo micronucleus study, lilial was injected intraperitoneally into mice (5/sex/dose) at a dose of 150, 300, or 600 mg/kg bw/day. An increase in the sum of micronucleated polychromatic erythrocytes in the high-dose group was observed only in males 48 (but not 24) hours post treatment. However, the study authors concluded that lilial showed no clastogenic potential as the increases observed were within the historical controls range, were not dose-dependent, and were not observed in any other dose group (ECHA Registration dossier 2020e; SCCS 2016; NICNAS 2016c).
7.5.3 Characterization of risk to human health
The critical effect level identified for the aldehydes subgroup 2 is an oral NOAEL of 4.1 mg/kg bw/day from a developmental toxicity study in rats exposed to lilial and based on adverse effects in the dams and fetuses at higher doses. This effect level is based on pathological changes in the liver and decreases in acetylcholinesterase levels in dams at 12.7 mg/kg bw/day, decreased fetal body weights and increased incidence of skeletal variations in fetuses at 12.7 mg/kg bw/day, and decreases in maternal body weight and increases in post-implantation losses at 40.7 mg/kg bw/day (SCCS 2016). The critical effect level of 4.1 mg/kg bw/day identified is supported by other studies with similar endpoints, such as an extended one-generation reproduction toxicity study in which the NOAEL was 4.5 mg/kg bw/day based on liver toxicity, decreased food consumption in F0 females and decreased maternal body weights, reduced pup weights, and decreased acetylcholinesterase activity in pups and adolescent rats at the next dose of 15.1 mg/kg bw/day (SCCS 2019).
No suitable hazard data were identified for the dermal and inhalation routes; therefore, the oral NOAEL of 4.1 mg/kg bw/day was used for characterization of risk along with route-to-route extrapolation for aldehydes subgroup 2. Daily exposure estimates for the highest and lowest exposed age groups and resulting MOEs for lilial are summarized in Table 7-26.
| Exposure scenario | Systemic exposure (mg/kg bw/day)a | MOEb |
|---|---|---|
| Inhalation and oral exposures from environmental media (that is, air, water, dust, and soil) (all subpopulations) | 6.70 x 10-4 (adult) to 2.49 x 10-3 (1 year) | 1 647 (1 year) to 6 119 (adults) |
| Dermal and inhalation exposures from body fragrance (44%) (2–3 years to adults) | 0.54 (adult) to 1.52 (2–3 years) | 3 (2–3 years) to 8 (adult) |
| Dermal and inhalation exposures from liquid body cleanser (1%) (all subpopulations) | 3.24 x 10-3 (adult) to 1.3 x 10-2 (0–5 months) | 318 (0–5 months) to 1 265 (adult) |
| Dermal and inhalation exposures from hair conditioner (wash-off) (2%) (2–3 years to adults) | 5.06 x 10-3 (14–18 years) to 1.06 x 10-2 (2–3 years) | 387 (2–3 years) to 810 (14–18 years) |
| Dermal and inhalation exposures from massage oil (0.3%) (all subpopulations) | 2.58 x 10-2 (adult) to 1.38 x 10-1 (0–5 months ) | 30 (0–5 months) to 159 (adults) |
| Dermal and inhalation exposures from body moisturizer (1%) (all subpopulations) | 2.17 x 10-1 (adult) to 4.84 10-1 (0–5 months) | 8 (0–5 months) to 19 (adults) |
| Dermal and inhalation exposures from liquid face makeup (1%) (4–8 years to adults) | 1.30 x 10-2 (14–18 years) to 2.69 x 10-2 (4–8 years) | 152 (4–8 years) to 315 (14–18 years) |
| Dermal and inhalation exposures from nail polish product (3%) (2–3 years to adults) | 9.73 x 10-3 (adult) to 1.80 x 10-2 (2–3 years) | 228 (2–3 years) to 421 (adult) |
| Dermal and inhalation exposures from a nail polish remover product (0.1%) (2–3 years to adults) | 5.86 x 10-3 (adult) to 1 x 10-2 (9–13 years) | 405 (9–13 years) to 700 (adult) |
| Dermal and inhalation exposures from depilator product (0.1%) (9–13 years to adults) | 3.67 x 10-3 (14–18 years) to 4.46. x 10-3 (adult) | 920 (adult) to 1 117 (14–18 years) |
| Dermal and inhalation exposures from solid antiperspirant/deodorant (3%) (9–13 years to adults) | 5.18 x 10-2 (9–13 years) to 8.63 x 10-2 (14–18 years) | 48 (14–18 years) to 79 (9–13 years) |
| Dermal and inhalation exposures from spray antiperspirant/deodorant (0.3%) (9–13 years to adults) | 1.01 x 10-2 (9–13 years) to 2.67 x 10-2 (14–18 years) | 153 (14–18 years) to 406 (9–13 years) |
| Dermal and inhalation exposures from bath product (3%) (all subpopulations) | 9.18 x 10-4 (9–13 years) to 2.83 x 10-3 (14–18 years) | 1 451 (14–18 years) to 4 464 (9–13 years) |
| Dermal and inhalation exposures from facial moisturizer (3%) (9–13 years to adults) | 1.32 x 10-1 (14–18 years) to 2.11 x 10-1 (adult) | 19 (adult) to 31 (14–18 years) |
| Dermal and inhalation exposures from permanent hair colour product (1%) (14–18 years to adults) | 2.69 x 10-1 (adult) to 3.21 x 10-1 (14–18 years) | 13 (14–18 years) to 15 (adult) |
| Dermal and inhalation exposures from temporary hair colour product (0.11%) (4–8 years to adults) | 8.33 x 10-3 (adult) to 2.59 x 10-2 (4–8 years) | 158 (4–8 years) to 492 (adult) |
| Dermal and inhalation exposures from hair straightening, waving, and curling product (1%) (4–8 years to adults) | 1.62 x 10-1 (adult) to 4.31 x 10-1 (4–8 years) | 10 (4–8 years) to 25 (adult) |
| Dermal and inhalation exposures from lotion sunless tanning product for the face (non-SPF) (1%) (14–18 years to adults) | 3.35 x 10-2 (adult) to 3.94 x 10-2 (14–18 years) | 104 (14–18 years) to 123 (adult) |
| Dermal and inhalation exposures from acne treatment (0.11%) (NHP) (9–13 years to adults) | 2.23 x 10-4 (adult) to 3.53 x 10-4 (9–13 years) | 11 610 (9–13 years) to 18 412 (adults) |
| Dermal and inhalation exposures from antiseptic skin cleanser product (0.007%) (NHP) (2–3 years to adults) | 3.56 x 10-4 (14–18 years) to 1.05 x 10-3 (2–3 years) | 3 904 (2–3 years) to 11 516 (14–18 years) |
| Dermal and inhalation exposures from antiseptic skin cleanser productc (0.007%) (NHP) (2—3 years to adults) | 5.33 x 10-3 (adult) to 2.63 x 10-2 (2–3 years) | 156 (2–3 years) to 770 (adults) |
| Dermal and inhalation exposures from carpet deodorizer (application) (1%) (adults) | 7.71 x 10-4 (adult) | 5 316 (adult) |
| Dermal, inhalation, and incidental oral post-application exposures from carpet deodorizer (1%) (1 year) | 1.31 x 10-2 (1 year) | 312 (1 year) |
| Inhalation exposure from solid gel air freshener (5%) (all subpopulations) | 5.31 x 10-2 (adult) to 2.11 x 10-1 (1 year) | 19 (1 year) to 77 (adults) |
| Inhalation exposure from liquid plug-in air freshener (3%) (all subpopulations) | 1.37 x 10-2 (adult) to 4.87 x 10-2 (1 year) | 84 (1 year) to 300 (adults) |
a Exposure scenario parameters and calculations for aldehydes subgroup 2 are outlined in Appendix A. Dermal absorption was assumed to be 15%.
b MOEs were calculated using the critical effect level (NOAEL = 4.1 mg/kg bw/day) based on a developmental toxicity study in rats.
c For situations of public health concern, the use of hand sanitizers among the general population may increase up to 25 uses per day (personal use by adults, increased use by children in schools and child care facilities) (RIVM 2021a).
The MOEs between the critical effect level and the estimates of exposure to lilial from environmental media, body cleanser, hair conditioner, massage oil (9 years and older), liquid face makeup, nail polish, nail polish remover, depilator product, spray antiperspirant/deodorant, bath product, acne treatment cream (NHP), antiseptic skin cleanser (NHP), temporary hair colour, facial sunless tanning product, and carpet deodorizer are considered to be adequate to account for uncertainties in the health effects and exposure data used to characterize risk.
The MOEs between the critical effect level and the estimates of daily exposure to lilial from cosmetics,Footnote 12 solid gel air freshener, and a liquid plug-in air freshener (1 year old) are below 100, which account for uncertainties with respect to interspecies extrapolation, intraspecies extrapolation, the POD, and the adequacy of the database and are considered potentially inadequate.
In addition, daily exposure estimates for the highest and lowest exposed age groups and resulting MOEs for lilial, myrac-aldehyde, myrmac-aldehyde, myrmac-carboxaldehyde, cetonal, and vernaldehyde are summarized in Table 7-27.
| Exposure scenario | Systemic exposure (mg/kg bw/day)a | MOEb |
|---|---|---|
| Dermal and inhalation exposures from leave-on cosmetics (0.05%) (all subpopulations) | 1.08 x 10-1 (adults) to 2.42 x 10-2 (0–5 months) | 169–379 |
| Dermal and inhalation exposures from rinse-off cosmetics (0.1%) (all subpopulations) | 1.71 x 10-3 (adults) to 4.37 x 10-3 (0–5 months) | 938–2 400 |
| Inhalation exposure from air fresheners product (2.5 %) (all subpopulations) | 1.14 x 10-2 (adults) to 4.07 x 10-2 (1 year) | 101 |
| Dermal and inhalation exposures from cleaning products (0.05%) (all subpopulations) | 1.66 x 10-5 (14–18 years) to 5.6 x 10-4 (adults) | 7 300–200 000 |
a Exposure scenario parameters and calculations for aldehydes subgroup 2 are outlined in Appendix A. Dermal absorption was assumed to be 15%.
b MOEs were calculated using the critical effect level (NOAEL = 4.1 mg/kg bw/day) based on a developmental toxicity study in rats.
The MOEs between the critical effect level and the estimates of exposure to lilial, myrac-aldehyde, myrmac-aldehyde, myrmac-carboxaldehyde, cetonal, and vernaldehyde from cosmetics, air fresheners, and cleaning products are considered to be adequate to account for uncertainties in the health effects and exposure data used to characterize risk.
Since there were no identified sources of exposure to verdiantiol for the general population, a qualitative approach to risk characterization was taken, and the risk to human health from verdantiol was considered to be low.
While exposure of the general population to myrac-aldehyde, myrmac-aldehyde, myrmac-carboxaldehyde, cetonal, vernaldehyde, and verdantiol are not of concern at current levels, these substances are considered to present a health effect of concern based on their potential reproductive and developmental toxicity. Therefore, there may be a concern if exposures were to increase.
7.5.4 Uncertainties in evaluation of risk to human health
The key sources of uncertainty are presented in the table below.
| Key source of uncertainty | Impact |
|---|---|
| The potential use of more than one product by a single person in a day (that is, aggregate exposure) was not considered. This may potentially underestimate exposure to some individuals. | - |
| The use of multiple aldehydes subgroup 2 substances in the same product was not considered. However, maximum concentrations were used in the risk assessment, which may be representative of the sum of multiple substances. | - |
| There are no hazard data available for myrac-aldehyde, myrmac-aldehyde, myrmac-carboxaldehyde, cetonal, or vernaldehyde. The read-across analogue and a member of aldehydes subgroup 2, lilial, was used to inform risk assessment. | +/- |
| Route-to-route extrapolation for aldehydes subgroup 2 was carried out for dermal and inhalation exposure scenarios using a critical effect level from an oral toxicity study. | +/- |
+ = uncertainty with potential to cause overestimation of exposure/risk; - = uncertainty with potential to cause underestimation of exposure risk; +/- = unknown potential to cause over- or underestimation of risk
8. Consideration of subpopulations who may have greater susceptibility or exposure
There are groups of individuals within the Canadian population who, due to greater susceptibility or greater exposure, may be more vulnerable to experiencing adverse health effects from exposure to substances. Certain populations are routinely considered throughout the assessment process, such as infants, children, and people of reproductive age. For instance, age-specific exposures are routinely estimated, and developmental and reproductive toxicity studies are evaluated for potential adverse health effects. These subpopulations with potential for higher exposure and those who may be more susceptible were taken into account in the risk assessment outcomes.
9. Conclusion
Considering all available lines of evidence presented in this draft assessment, there is low risk of harm to the environment from the substances in the Phenylpropanoids and Aldehydes Group. It is proposed to conclude that the 12 substances in the Phenylpropanoids and Aldehydes Group do not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
On the basis of the information presented in this assessment, it is proposed to conclude that bay oil, tarragon oil, jasmine oil, perfumes and essences of jasmin, violet oil, and lilial meet the criteria under paragraph 64(c) of CEPA as they are entering or may enter the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health, and that verdantiol, myrac-aldehyde, myrmac-aldehyde, myrmac-carboxaldehyde, cetonal, and vernaldehyde do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore proposed to conclude that bay oil, tarragon oil, jasmine oil, perfumes and essences of jasmin, violet oil, and lilial meet one or more of the criteria set out in section 64 of CEPA and that verdantiol, myrac-aldehyde, myrmac-aldehyde, myrmac-carboxaldehyde, cetonal, and vernaldehyde do not meet any of the criteria set out in section 64 of CEPA.
It is proposed that lilial meets the persistence criteria but not the bioaccumulation criteria as set out in the Persistence and Bioaccumulation Regulations of CEPA.
References
[21CFR182.20] Code of Federal Regulations, Title 21 – Food and Drugs, Part 182 Substances Generally Recognized as Safe, Section 182.20 Essential oils, oleoresins (solvent-free), and natural extractives (including distillates).
Abaul J, Bourgeois P, Bessiere JM. 1995. Chemical composition of the essential oils of chemotypes of Pimenta racemosa var. racemosa (P. Miller) JW Moore (Bois d'Inde) of Guadeloupe (FWI). Flavour Fragr J. 10(5):319-321.
Abraham SK. 2001. Anti-genotoxicity of trans-anethole and eugenol in mice. Food Chem Toxicol. 39(5):493-498.
[ACI] American Cleaning Institute. 2018. [database] [accessed 2018].
[ACI] American Cleaning Institute. 2020. [database] [accessed 2020 Jun].
Adams TB, Gavin CL, Taylor SV, Waddell WJ, Cohen SM, Feron VJ, Goodman J, Rietjens IMCM, Marnett LJ, Portoghese PS, et al. 2008. The FEMA GRAS assessment of α, β-unsaturated aldehydes and related substances used as flavor ingredients. Food Chem Toxicol. 46(9):2935-2967.
Api AM, Belmonte F, Belsito D, Biserta S, Botelho D, Bruze M, Burton Jr GA, Buschmann J, Cancellieri MA, Dagli ML, et al. 2019. RIFM fragrance ingredient safety assessment, palmitic acid, CAS Registry Number 57-10-3. Food Chem Toxicol. 134 Suppl:110980.
Arnau EG, Andersen KE, Bruze M, Frosch PJ, Johansen JD, Menne T, Rastogi SC, White IR, Lepoittevin JP. 2000. Identification of Lilial as a fragrance sensitizer in a perfume by bioassay-guided chemical fractionation and structure-activity relationships. Contact Derm. 43(6):351-358.
Atshaves BP, Payne HR, McIntosh AL, Tichy SE, Russell D, Kier AB, Schroeder F. 2004. Sexually dimorphic metabolism of branched-chain lipids in C57BL/6J mice. J Lipid Res. 45(5):812-830.
Ayoughi F, Barzegar M, Sahari MA, Naghdibadi H. 2011. Chemical compositions of essential oils of Artemisia dracunculus L. and endemic Matricaria chamomilla L. and an evaluation of their antioxidative effects. J Agric Sci Tech. 13(1):79-88.
Bánsághi S, Soule H, Guitart C, Pittet D, Haidegger T. 2020. Critical reliability issues of common type alcohol-based handrub dispensers. Antimicrob Resist Infect Control. 9(90).
Bera P, Kotamreddy JNR, Samanta T, Maiti S, Mitra A. 2015. Inter-specific variation in headspace scent volatiles composition of four commercially cultivated jasmine flowers. Nat Prod Res. 29(14):1328-1335.
Beyer J, Ehlers D, Maurer HH. 2006. Abuse of nutmeg (Myristica fragrans Houtt.): studies on the metabolism and the toxicologic detection of its ingredients elemicin, myristicin, and safrole in rat and human urine using gas chromatography/mass spectrometry. Ther Drug Monit. 28(4):568-575.
Bowles EJ. 2003. The chemistry of aromatherapeutic oils. 3rd ed. Online version. Crows Nest (AU): Allen & Unwin.
Braun NA, Kohlenberg B, Sim S, Meier M, Hammerschmidt F-J. 2009. Jasminum flexile flower absolute from India - a detailed comparison with three other jasmine absolutes. Nat Prod Commun. 4(9):1239-1250.
Bremmer HF, Prud’homme de Lodder LCH, van Engelen JGM. 2006. Cosmetics Fact Sheet. RIVM report 320104001/2006.
Bronaugh RL, Wester RC, Bucks D, Maibach HI, Sarason R. 1990. In vivo percutaneous absorption of fragrance ingredients in rhesus monkeys and humans. Food Chem Toxicol. 28(5):369-373.
Burdock GA. 2010. Fenaroli’s handbook of flavor ingredients. 6th ed. Boca Raton (FL): CRC Press.
Cagen SZ, Patterson DR, Wimberly HC, Lu CC, Gardiner TH. 1989. Toxicity induced by subchronic dermal exposure to paratertiary butyl benzoic acid (pt BBA) in Fischer 344 rats. J Am Coll Toxicol. 8(5):1027-1038.
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C. 1999, c.33. Canada Gazette Part III, vol. 22, no. 3.
Canada, Dept. of the Environment. 2012. Canadian Environmental Protection Act, 1999: Notice with respect to certain substances on the Domestic Substances List [PDF]. Canada Gazette, Part I, vol. 146, no. 48, Supplement.
Caputi L, Aprea E. 2011. Use of terpenoids as natural flavouring compounds in food industry. Recent Pat Food Nutr Agric. 3(1):9-16.
Chan PC, Mahler J, Peddada S, Lomnitski L, Nyska A. 2003. Forestomach tumor induction by 2,4-hexadienal in F344N rats and B6C3F1 mice. Arch Toxicol. 77(9):511-520.
Charles AK, Darbre PD. 2009. Oestrogenic activity of benzyl salicylate, benzyl benzoate and butylphenylmethylpropional (Lilial) in MCF7 human breast cancer cells in vitro. J Appl Toxicol. 29(5):422-434.
Charles River. 2019. The in vitro percutaneous absorption of radiolabelled alpha-pinene and radiolabelled citral in two test preparations through human skin (OECD 428 and SCCS). Tranent, East Lothian, United Kingdom. 109 p. Report No. 40807. Unpublished.
ChemCAN [level III fugacity model of 24 regions of Canada]. 2003. Version 6.00. Peterborough (ON): Trent University, Canadian Centre for Environmental Modelling and Chemistry.
ChemID Plus [database]. 2019. Bethesda (MD): US National Library of Medicine. Search results for CAS RN [8022-96-6] and [8024-43-9]. [accessed 2020 May].
Choi Y-H, Ahn S-J, Park K-Y, Yoo M-A, Lee W-H. 1993. Effects of phytol on the mutagenicity of UV and EMS in salmonella and drosophila mutation assaying systems. Environ Mut Carc. 13(2):92-100.
[CIR] Cosmetic Ingredient Review. 1987. Final report on the safety assessment of oleic acid, lauric acid, palmitic acid, myristic acid, and stearic acid. J Am Coll Toxicol. 6(3).
[CIR] Cosmetic Ingredient Review. 2006. Annual review of cosmetic ingredient safety assessments—2004/2005. Int J Toxicol. 25 Suppl 2:1-89.
CLH. 2017. CLP Report for 2-(4-tert-butylbenzyl)propionaldehyde.
[CoE] Council on Europe. 2000. Natural sources of flavourings - report no. 1.
[CPID] Consumer Product Ingredients Database [database]. c2021. McLean(VA): DeLima Associates. [accessed 2021 July].
[CTD] The Comparative Toxicogenomics Database. 2019. Phytol [database]. [accessed 2019 Dec].
Cu JQ, Perineau F, Gaset A. 1992. Volatile components of violet leaves. Phytochemistry. 31(2):571-573.
Di Sotto A, Maffei F, Hrelia P, Di Giacomo S, Pagano E, Borrelli F, Mazzanti G. 2014. Genotoxicity assessment of some cosmetic and food additives. Regul Toxicol Pharmacol. 68(1):16-22.
Dittberner U, Eisenbrand G, Zankl H. 1995. Genotoxic effects of the α, β-unsaturated aldehydes 2-trans-butenal, 2-trans-hexenal and 2-trans, 6-cis-rmnonadienal. Mut Res/Environ Mutagen Relat Subj. 335(3):259-265.
Drinkwater NR, Miller EC, Miller JA, Pitot HC. 1976. Hepatocarcinogenicity of estragole (1-allyl-4-methoxybenzene) and 1’-hydroxyestragole in the mouse and mutagenicity of 1’-acetoxyestragole in bacteria. J Natl Cancer Inst. 57(6):1323-1331.
[EC] European Commission. 2008. REGULATION (EC) No 1334/2008 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 16 December 2008 on flavourings and certain food ingredients with flavouring properties for use in and on foods and amending Council Regulation (EEC) No 1601/91, Regulations (EC) No 2232/96 and (EC) No 110/2008 and Directive 2000/13/EC.
[EC, HC] Environment Canada, Health Canada. 2010. Screening assessment for the challenge of benzene, 1,2-dimenthoxy-4-(2-propenyl)-(methyl eugenol). Ottawa (ON): Government of Canada.
[ECCC] Environment and Climate Change Canada. 2016a. Science approach document: ecological risk classification of organic substances. Ottawa (ON): Government of Canada.
[ECCC] Environment and Climate Change Canada. 2016b. Supporting documentation: data used to create substance-specific hazard and exposure profiles and assign risk classifications. Gatineau (QC): ECCC. Information in support of the science approach document: ecological risk classification of organic substances. Available from: substances@ec.gc.ca.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. [modified 2017 Mar 12]. Categorization of chemical substances. Ottawa (ON): Government of Canada. [accessed 2018 Mar].
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2018a. Rapid screening of substances with limited general population exposure [PDF]. Ottawa (ON): Government of Canada.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2018b. Screening assessment: substances identified as being of low concern using the ecological risk classification of organic substances and the threshold of toxicological concern (TTC)-based approach for certain substances [PDF]. Ottawa (ON): Government of Canada.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2018c. Draft screening assessment – Fatty Acids and Derivatives Group. Ottawa (ON): Government of Canada.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2018d.Screening assessment: phenol, 2-methoxy-5-(2-propenyl)-(Eugenol) and Rose, Rosa canina, ext [PDF]. Ottawa (ON): Government of Canada.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2019. Screening assessment benzoates. Ottawa (ON): Government of Canada.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2020. Draft screening assessment – Terpenes and terpenoids – Acyclic, Monocyclic, and Bicyclic Monoterpenes Group. Ottawa (ON): Government of Canada.
[ECHA] European Chemicals Agency. 2015. Biocides human health exposure methodology. First edition, October, version 1.
[ECHA Registration dossier] Europe Chemicals Agency. 2017. Registration dossier for thuj-4(10)-ene (Sabinene).
[ECHA Registration dossier] European Chemicals Agency. 2018. Registration dossier for linalool.
[ECHA Registration dossier] Europe Chemicals Agency. 2020a. Registration dossier for p-mentha-1,4(8)-diene (Terpinolene).
[ECHA Registration dossier] Europe Chemicals Agency. 2020b. Registration dossier for (E)-anethole.
[ECHA Registration dossier] Europe Chemicals Agency. 2020c. Registration dossier for benzyl benzoate.
[ECHA Registration dossier] Europe Chemicals Agency. 2020d. Registration dossier for nerolidol.
[ECHA Registration dossier] European Chemicals Agency. 2020d. Registration dossier for α,2,2,6-tetramethylcyclohexene-1-butyraldehyde (CAS No. 65405-84-7).
[ECHA Registration dossier] European Chemicals Agency. 2020e. Registration dossier for 2-(4-tert-butylbenzyl)propionaldehyde (CAS No. 80-54-6).
Eder E, Deininger C, Neudecker T, Deininger D. 1992. Mutagenicity of β‐alkyl substituted acrolein congeners in the Salmonella typhimurium strain TA100 and genotoxicity testing in the SOS chromotest. Environ Mol Mutagen. 19(4):338-345.
Eder E, Scheckenbach S, Deininger C, Huffman C. 1993. The possible role of α, β-unsaturated carbonyl compounds in mutagenesis and carcinogenesis. Toxicol Lett. 67(1-3):87-103.
[EFSA] European Food Safety Authority. 2009. Scientific Cooperation Report: Advice on the EFSA guidance document for the safety assessment of botanicals and botanical preparations intended for use as food supplements, based on real case studies [EPDF]. EFSA Journal. 7(9):2080.
[EFSA] European Food Safety Authority. 2010. Scientific opinion on flavouring group evaluation 90 (FGE.90): Consideration of aliphatic, acyclic and alicyclic terpenoid tertiary alcohols and structurally related substances evaluated by JECFA (68th meeting) structurally related to aliphatic, alicyclic and aromatic saturated and unsaturated tertiary alcohols, aromatic tertiary alcohols and their esters evaluated by EFSA in FGE.18Rev1 (2009). EFSA Journal. 8(2):1336.
[EFSA] European Food Safety Authority. 2012. Scientific opinion on the safety and efficacy of benzyl alcohols, aldehydes, acids, esters and acetals (chemical group 23) when used as flavourings for all animal species. EFSA Journal. 10(7):2785.
[EFSA] European Food Safety Authority. 2015. Scientific Opinion on flavouring Group Evaluation 78, Revision 2 (FGE.78Rev2): consideration of aliphatic and alicyclic and aromatic hydrocarbons evaluated by JECFA (63rd meeting) structurally related to aliphatic hydrocarbons evaluated by EFSA in FGE.25Rev3 [EPDF]. EFSA Journal. 13(4):4067.
[EFSA] European Food Safety Authority. 2017. Update: use of the benchmark dose approach in risk assessment. EFSA Journal. 15(1):4658.
[EFSA] European Food Safety Authority. 2018. Scientific Opinion on Flavouring Group Evaluation 203, Revision 2 (FGE.203Rev2): α, β‐unsaturated aliphatic aldehydes and precursors from chemical subgroup 1.1.4 of FGE.19 with two or more conjugated double‐bonds and with or without additional non‐conjugated double‐bonds. EFSA Journal. 16(7):e05322.
[EMA] European Medicines Agency. 2015. Public statement on the use of herbal medicinal products containing estragole [PDF]. European Medicines Agency/Committee on Herbal Medicinal Products. 137212. Amsterdam (NL): European Medicines Agency.
[EMA] European Medicines Agency. 2020. Public statement on the use of herbal medicinal products containing estragole [PDF]. European Medicines Agency/Committee on Herbal Medicinal Products. 137212. Amsterdam (NL): European Medicines Agency.
Environment Canada. 2013. DSL Inventory Update data collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain substances on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
[FEMA] Flavor and Extract Manufacturers Association. 2020. Flavor Ingredient Library.
Ficheux AS, Morriset T, Chevillotte G, Postic C, Roudot AC. 2014. Probabilistic assessment of exposure to nail cosmetics in French consumers. Food and Chemical Toxicology. 66:36-43
Ficheux AS, Chevillotte G, Wesolek N, Morisset T, Dornic N, Bernard A, Bertho A, Romanet A, Leroy L, Mercat AC, et al. 2016. Consumption of cosmetic products by the French population. Second part: amount data. Food Chem Toxicol. 90:130-141.
Ficheux AS, Wesolek N, Chevillotte G, Roudot AC. 2015. Consumption of cosmetic products by the French population. First part: frequency data. Food Chem Toxicol. 78:159-169.
Florin I, Rutberg L, Curvall M, Enzell CR. 1980. Screening of tobacco smoke constituents for mutagenicity using the Ames' test. Toxicology. 15(3):219-232.
Ford RA, Api AM, Letizia CS. 1992. Fragrance raw materials monographs: 1-Formyl-1-methyl-4-(4-methylpentyl)-3-cyclohexene. Food Chem Toxicol. 30:37S.
Fragrance raw materials monographs. 1976. Fragrance raw materials monographs: Isohexenyl cyclohexenyl carboxaldehyde. Food Cosmet Toxicol. 14:803.
Garcia-Hidalgo E, von Goetz N, Siegrist M, Hungerbühler K. 2017. Use-patterns of personal care and household cleaning products in Switzerland. Food Chem Toxicol. 99:24-39.
Gilpin S, Hui X, Maibach H. 2010. In vitro human skin penetration of geraniol and citronellol. Dermatitis. 21(1):41-48.
Gomez-Berrada M-P, Gautier F, Parent-Massin D, Ferret P-J. 2013. Retrospective exposure data for baby and children care products: an analysis of 48 clinical studies. Food Chem Toxicol. 57:185-194.
Gorelick NJ. 1995. Genotoxicity of trans-anethole in vitro. Mutat Res. 326(2):199-209.
Greenaway RE, Ormandy K, Fellows C, Hollowood T. 2018. Impact of hand sanitizer format (gel/foam/liquid) and dose amount on its sensory properties and acceptability for improving hand hygiene compliance. J Hosp Infect. 100(2):195-201.
Hall B, Tozer S, Safford B, Coroama M, Steiling W, Leneveu-Duchemin MC, McNamara C, Gibney M. 2007. European consumer exposure to cosmetic products, a framework for conducting population exposure assessments. Food Chem Toxicol. 45(11):2097-2108.
Hasheminejad G, Caldwell J. 1994. Genotoxicity of the alkenylbenzenes α− and β-asarone, myristicin and elemicin as determined by the UDS assay in cultured rat hepatocytes. Food Chem Toxicol. 32(3):223-231.
Hausner H, Bredie WLP, Mølgaard C, Petersen MA, Møller P. 2008. Differential transfer of dietary flavour compounds into human breast milk. Physiol Behav. 95(1-2):118-124.
[HC] Health Canada. 2013. Interim guidance on human health risk assessment for short-term exposure to carcinogens at contaminated sites [PDF]. Ottawa (ON): Health Canada.
[HC] Health Canada. 2016. Science approach document: threshold of toxicological concern (TTC)-based approach for certain substances. Ottawa (ON): Government of Canada.
[HC] Health Canada. 2019a. Cosmetic Ingredient Hotlist [PDF] [Internet]. Ottawa (ON): Health Canada, Consumer Product Safety.
[HC] Health Canada. 2019b. Canadian exposure factors used in human health risk assessment. Unpublished report. Ottawa (ON): Government of Canada.
[HC] Health Canada. 2019c. Self-care products and Health Canada [Internet]. Ottawa (ON): Health Canada, Government of Canada.
[HC] Health Canada. 2020. [modified 2020 Jul 14]. Vaping products regulations. Ottawa (ON): Government of Canada. [accessed 2021 Apr 22].
Heck JD, Vollmuth TA, Cifone MA, Jagannath DR, Myhr B, Curren RD. 1989. An evaluation of food flavoring ingredients in a genetic toxicity screening battery. Toxicologist. 9(1):257-272.
Howes AJ, Chan VSW, Caldwell J. 1990. Structure-specificity of the genotoxicity of some naturally occurring alkenylbenzenes determined by the unscheduled DNA synthesis assay in rat hepatocytes. Food Chem Toxicol. 28(8):537-542.
Hsia MTS, Adamovics JA, Kreamer BL. 1979. Microbial mutagenicity studies of insect growth regulators and other potential insecticidal compounds in Salmonella typhimurium. Chemosphere. 8(8):521-529.
Hunter CG, Chambers PL, Stevenson DE. 1965. Studies on the oral toxicity of p-tert-butyl benzoic acid in rats. Food Cosmet Toxicol. 3(2):289-298.
[IARC] International Agency for Research on Cancer. 2013 IARC monographs on the evaluation of carcinogenic risks to humans: some chemicals present in industrial and consumer products, food and drinking-water [PDF]. Lyon (FR): International Agency for Research on Cancer.
[IARC] International Agency for Research on Cancer. 2014. IARC monographs on the evaluation of carcinogenic risks to humans [PDF]. Report of the Advisory Group to Recommend Priorities for IARC Monographs during 2015-2019. Lyon (FR): International Agency for Research on Cancer.
[IFRA] International Fragrance Association. 2013. International Fragrance Association Standard, 43rd Amendment [PDF]. Brussels (BE): International Fragrance Association. [accessed 2020 May].
[IFRA] International Fragrance Association. 2015. International Fragrance Association Standard, 48th Amendment [PDF]. Brussels (BE): International Fragrance Association. [accessed 2020 Apr].
[IFRA] International Fragrance Association. 2017. Volume of Use Survey 2017: Transparency List. Geneva (CH): International Fragrance Association. [accessed 2018 Dec].
Iqbal M, Ghosh AKM, Saluja AK. 1993. Antifertility activity of the floral buds of Jasminum officinale var. grandiflorum in rats. Phytother Res. 7(1):5-8.
Islam MT, Streck L, Barros de Alencar MVO, Cardoso Silva SW, da Conceição Machado K, da Conceição Machado K, Júnior ALG, Jardim Paz MFC, Ferreira da Mata AMO, de Castro e Sousa JM, et al. 2017. Evaluation of toxic, cytotoxic and genotoxic effects of phytol and its nanoemulsion. Chemosphere. 177:93-101.
Itoh T, Ono A, Kawaguchi K, Teraoka S, Harada M, Sumi K, Ando M, Tsukamasa Y, Ninomiya M, Koketsu M, et al. 2018. Phytol isolated from watermelon (Citrullus lanatus) sprouts induces cell death in human T-lymphoid cell line Jurkat cells via S-phase cell cycle arrest. Food Chem Toxicol. 115:425-435.
Jirovetz L, Buchbauer G, Stoilova I, Krastanov A, Stoyanova A, Schmidt E. 2007a. Spice plants: chemical composition and antioxidant properties of Pimenta lindl. Essential oils, part: 2 Pimenta racemosa (Mill.) J.W Moore leaf oil from Jamaica. Ernahhrung/Nutrition. 31(7/8):293-300.
Jirovetz L, Buchbauer G, Schweiger T, Denkova Z, Slavchev A, Stoyanova A, Schmidt E, Geissler M. 2007b. Chemical composition, olfactory evaluation and antimicrobial activities of Jasminum grandiflorum L. absolute from India. Nat Prod Commun. 2(4):407-412.
Johnson Jr W, Bergfeld WF, Belsito DV, Hill RA, Klaassen CD, Liebler DC, Marks Jr JG, Shank RC, Slaga TJ, Snyder PW, et al. 2017. Safety assessment of benzyl alcohol, benzoic acid and its salts, and benzyl benzoate. Int J Toxicol. 36(3 Suppl):5S-30S.
Kampf G, Ruselack S, Eggerstedt S, Nowak N, Bashir M. 2013. Less and less–influence of volume on hand coverage and bactericidal efficacy in hand disinfection. BMC Infect Dis. 13:472.
Kenters N, Eikelenboom-Boskamp A, Hines J, McGreer A, Huijskens EGW, Voss A. 2020. Product dose considerations for real-world hand sanitiser efficacy. Am J Infect Control. 48(5):503-506.
Kim J, Lee Y-S, Lee S-G, Shin S-C, Park I-K. 2008. Fumigant antifungal activity of plant essential oils and components from west Indian bay (Pimenta racemosa) and thyme (Thymus vulgaris) oils against two phytopathogenic fungi. Flavour Fragr J. 23(4):272-277.
Kobets T, Duan J-D, Brunnemann KD, Etter S, Smith B, Williams GM. 2016. Structure-activity relationships for DNA damage by alkenylbenzenes in turkey egg fetal liver. Toxicol Sci. 150(2):301-311.
Koçkaya EA, Kιlιç A. 2011. Developmental toxicity of benzyl benzoate in rats after maternal exposure throughout pregnancy. Environ Toxicol. 29(1):40-53.
Kordali S, Kotan R, Mavi A, Cakir A, Ala A, Yildirim A. 2005. Determination of the chemical composition and antioxidant activity of the essential oil of Artemisia dracunculus and of the antifungal and antibacterial activities of Turkish Artemisia absinthium, A. dracunculus, Artemisia santonicum, and Artemisia spicigera essential oils. J Agric Food Chem. 53(24):9452-9458.
Laue H, Kern S, Badertscher RP, Ellis G, Natsch A. 2017. p-Alkyl-benzoyl-CoA conjugates as relevant metabolites of aromatic aldehydes with rat testicular toxicity—studies leading to the design of a safer new fragrance chemical. Toxicol Sci. 160(2):244-255.
[LNHPD] Licensed Natural Health Products Database [database]. [modified 2021 Sep 08]. Ottawa (ON): Government of Canada. [accessed 2021 Nov 09].
Lopes-Lutz D, Alviano DS, Alviano CS, Kolodziejczyk P. 2008. Screening of chemical composition, antimicrobial and antioxidant activities of Artemisia essential oils. Phytochemistry. 69(8):1732-1738.
Loretz LG, Api AM, Barraj LM, Burdick J, Dressler WE, Gettings SD, Han Hsu H, Pan YHL, Re TA, Renskers KJ, Rothenstein A, Scrafford CG, Sewall C. 2005. Exposure data for cosmetic products: lipstick, body lotion, and face cream. Food Chem Toxicol 43: 279-291.
Loretz L, Api AM, Barraj L, Burdick J, Davis DA, Dressler W, Gilberti E, Jarrett G, Mann S, Pan YHL, et al. 2006. Exposure data for personal care products: hairspray, spray perfume, liquid foundation, shampoo, body wash, and solid antiperspirant. Food Chem Toxicol. 44(12):2008-2018.
Loretz LJ, Api AM, Babcock L, Barraj LM, Burdick J, Cater KC, Jarrett G, Mann S, Pan YHL, Re TA, et al. 2008. Exposure data for cosmetic products: facial cleanser, hair conditioner, and eye shadow. Food Chem Toxicol. 46(5):1516-1524.
Macinga DR, Edmonds SL, Campbell E, Shumaker DJ, Arbogast JW. 2013. Efficacy of novel alcohol-based hand rub products at typical in-use volumes. Infect Control Hosp Epidemiol. 34(3):299-301.
Mackie JT, Atshaves BP, Payne HR, McIntosh AL, Schroeder F, Kier AB. 2009. Phytol-induced hepatotoxicity in mice. Toxicol Pathol. 37(2):201-208.
Marnett LJ, Hurd HK, Hollstein MC, Levin DE, Esterbauer H, Ames BN. 1985. Naturally occurring carbonyl compounds are mutagens Salmonella tester strain TA104. Mutat Res/Fund Molec Mech Mutagen. 148(1-2):25-34.
Marshall AD, Caldwell J. 1996. Lack of influence of modulators of epoxide metabolism on the genotoxicity of trans-anethole in freshly isolated rat hepatocytes assessed with the unscheduled DNA synthesis assay. Food Chem Toxicol. 34(4):337-345.
Marshall AD, Howes AJ, Caldwell J. 1989. Cytotoxicity and genotoxicity of the food flavour anethole in cultured rat hepatocytes. Hum Toxicol. 8:404.
Martins C, Cação R, Cole KJ, Phillips DH, Laires A, Rueff J, Rodrigues AS. 2012. Estragole: a weak direct-acting food-borne genotoxin and potential carcinogen. Mutat Res/Genet Toxicol Environ Mutagen. 747(1):86-92.
McCune SA, Durant PJ, Flanders LE, Harris RA. 1982. Inhibition of hepatic gluconeogenesis and lipogenesis by benzoic acid, p-tert.-butylbenzoic acid, and a structurally related hypolipidemic agent SC-33459. Arch Biochem Biophys. 214(1):124-133.
McGinty D, Vitale D, Letizia CS, Api AM. 2012. Fragrance material review on benzyl acetate. Food Chem Toxicol. 50 Suppl 2:S363-384.
McHale D, Laurie WA, Woof MA. 1977. Composition of West Indian bay oils. Food Chem. 2(1):19-25.
Miller EC, Swanson AB, Phillips DH, Fletcher TL, Liem A, Miller JA. 1983. Structure-activity studies of the carcinogenicities in the mouse and rat of some naturally occurring and synthetic alkenylbenzene derivatives related to safrole and estragole. Cancer Res. 43(3):1124-1134.
Moharram FA-E, Al-Gendy AA, El-Shenawy SM, Ibrahim BM, Zarka MA. 2018. Phenolic profile, anti-inflammatory, antinociceptive, anti-ulcerogenic and hepatoprotective activities of Pimenta racemosa leaves. BMC Complement Altern Med. 18(1):208.
Mortelmans K, Haworth S, Lawlor T, Speck W, Tainer B, Zeiger E. 1986. Salmonella mutagenicity tests: II. Results from the testing of 270 chemicals. Environ Mutagen. 8 Suppl 7:1-119.
[MSDS] Material Safety Data Sheet. 2006. Angel oil burner. Lynwood (IL): Hosley International.
Müller L, Kasper P, Müller-Tegethoff K, Petr T. 1994. The genotoxic potential in vitro and in vivo of the allyl benzene etheric oils estragole, basil oil and trans-anethole. Mutat Res. 325(4):129-136.
Murawski A, Fiedler N, Schmied-Tobies MIH, Rucic E, Schwedler G, Stoeckelhuber M, Scherer G, Pluym N, Scherer M, Kolossa-Gehring M. 2020. Metabolites of the fragrance 2-(4-tert-butylbenzyl) propionaldehyde (lysmeral) in urine of children and adolescents in Germany–Human biomonitoring results of the German Environmental Survey 2014–2017 (GerES V). Int J Hyg Environ Health. 229:113594.
[NCBI] US National Library of Medicine National Center for Biotechnology Information. 2019. Substance Record on PubChem: Phytol CAS Number 150-86-7 [database]. [accessed 2019 Dec].
Nesslany F, Parent-Massin D, Marzin D. 2010. Risk assessment of consumption of methylchavicol and tarragon: the genotoxic potential in vivo and in vitro. Mutat Res/Genet Toxicol Environ Mutagen. 696(1):1-9.
Nestmann ER, Lee EGH, Matula TI, Douglas GR, Mueller JC.1980. Mutagenicity of constituents identified in pulp and paper mill effluents using the Salmonella/mammalian-microsome assay. Mutat Res. 79(3):203-212.
Newberne P, Smith RL, Doull J, Goodman JI, Munro IC, Portoghese PS, Wagner BM, Weil CS, Woods LA, Adams TB, et al. 1999. The FEMA GRAS assessment of trans-anethole used as a flavouring substance. Food Chem Toxicol. 37(7):789-811.
[NHPID] Natural Health Products Ingredients Database [database]. [modified 2021 Oct 13]. Ottawa (ON): Government of Canada. [accessed 2021 Nov 09].
[NICNAS] National Industrial Chemicals Notification and Assessment Scheme. 2016a. Benzene, 1-methoxy-4-(1-propenyl)-, (E)-: Human health tier II assessment [PDF].
[NICNAS] National Industrial Chemicals Notification and Assessment Scheme. 2016b. Cyclohexenebutanal, .alpha.,2,2,6-tetramethyl-: human health tier II assessment.
[NICNAS] National Industrial Chemicals Notification and Assessment Scheme. 2016c. Benzenepropanal, 4(1,1dimethylethyl).alpha.methyl: human health tier II assessment.
[NICNAS] National Industrial Chemicals Notification and Assessment Scheme. 2017a. Search assessment: 9,12-Octadecadienoic acid, (Z,Z)-. [accessed 2020 Sep 10].
[NICNAS] National Industrial Chemicals Notification and Assessment Scheme. 2017b. Search assessment: Hexadecanoic acid. [accessed 2020 Sep 10].
Nijssen LM, Ingen-Visscher CA van, Donders JJH, editors. 1963-2018. VCF (Volatile Compounds in Food): database, version 16.6.1, 2018. Zeist (NL): Triskelion B.V.
[NRC] National Research Council of Canada. 2011. Data gathering on chemicals released to indoor air of residences of building materials and furnishings. NRC – Institute for Research on Construction. Report No. B3332.2. Prepared for Health Canada, Ottawa (ON).
[NRC] National Research Council of Canada. 2019. Investigation of sources and indoor levels of CMP3 chemicals. Phase 1 – analysis of indoor air samples from two field studies. Report No. A1-014282.1. Prepared for Health Canada, Ottawa (ON).
[NTP] National Toxicology Program. 2000. NTP technical report on the toxicology and carcinogenesis studies of methyleugenol (CAS NO. 93-15-2) in F344/N rats and B6C3F1 mice (gavage studies). Research Triangle Park (NC): US Department of Health and Human Services, National Toxicology Program. NTP Toxicity Report 491.
[NTP] National Toxicology Program. 2003. NTP technical report on the toxicology and carcinogenesis studies of 2,4-hexadienal (89% trans,trans isomer, CAS No. 142-83-6; 11% cis,trans isomer) in F344/N rats and B6C3F1 mice (gavage studies). Research Triangle Park (NC): US Department of Health and Human Services, National Toxicology Program. NTP Toxicity Report 509.
[NTP] National Toxicology Program. 2011a. NTP technical report on the 3-month toxicity studies of estragole (CAS No. 140-67-0) administered by gavage to F344/N rats and B6C3F1 mice [PDF]. Research Triangle Park (NC): US Department of Health and Human Services, National Toxicology Program. NTP Toxicity Report 82.
Nyska A, Moomaw CR, Lomnitski L, Chan P. 2001. Glutathione S-transferase Pi expression in forestomach carcinogenesis process induced by gavage-administered 2,4-hexadienal in the F344 rat. Arch Toxicol. 75(10):618-624.
O.Berk Leaders in Packaging Solutions. [date unknown]. O.Berk online product catalog – fine mist sprayers. [accessed 2018 Sep].
Obolskiy D, Pischel I, Feistel B, Glotov N, Heinrich M. 2011. Artemisia dracunculus L. (tarragon): a critical review of its traditional use, chemical composition, pharmacology, and safety. J Agric Food Chem. 59(21):11367-11384.
[OECD] Organisation for Economic Co-operation and Development. 2003. OECD SIDS Initial Assessment Report for SIAM 16: Isophytol. OECD Paris.
[OECD] Organisation for Economic Co-operation and Development. 2004. SIDS Initial Assessment Profile (SIAP): Higher Olefins Category. [accessed 2020 Aug 11].
[OECD] Organisation for Economic Co-operation and Development. 2014. SIDS Initial Assessment Profile (SIAP): Aliphatic Acids Category. CoCAM [Cooperative Chemicals Assessment Meeting] 6, 2014 September 30–October 3. [accessed 2020 Aug 11].
OECD QSAR Toolbox [Read-across tool]. 2014. Version 3.3. Paris (FR): Organisation for Economic Co-operation and Development, Laboratory of Mathematical Chemistry.
OECD QSAR Toolbox [Read-across tool]. 2016. Paris (FR): Organisation for Economic Co-operation and Development, Laboratory of Mathematical Chemistry.
OECD QSAR Toolbox [Read-across tool]. 2019. Version 4.3. Paris (FR): Organisation for Economic Co-operation and Development, Laboratory of Mathematical Chemistry.
[OEHHA] Office of Environmental Health Hazard Assessment, Reproductive and Cancer Hazard Assessment Section. 1999. Evidence on the carcinogenicity of estragole [PDF]. [accessed 2020 Apr].
[OEHHA] Office of Environmental Health Hazard Assessment. 2018. Chemical listed effective March 27, 2015 as known to the State of California to cause cancer: beta-myrcene. [accessed 2020 Apr].
Opdyke DLJ. 1976. Monographs on fragrance raw materials: violet leaf absolute. Food Cosmet Toxicol. 14:729.
Opdyke DLJ. 1988. Fragrance raw materials monographs: terpinolene. Food Cosmet Toxicol. 14 Suppl:877-878.
Paini A, Punt A, Scholz G, Gremaud E, Spenkelink B, Alink G, Schilter B, van Bladeren PJ, Rietjens IMCM. 2012. In vivo validation of DNA adduct formation by estragole in rats predicted by physiologically based biodynamic modelling. Mutagenesis. 27(6):653-663.
Perveen S. 2018. Introductory chapter: terpenes and terpenoids. In: Perveen S, Al-Taweel A, editors. Terpenes and terpenoids. London (UK): IntechOpen. [accessed 2019 Dec 18].
Phillips DH, Reddy MV, Randerath K. 1984. 32P-post labelling analysis of DNA adducts formed in the livers of animals treated with safrole, estragole and other naturally-occurring alkenylbenzenes. II. Newborn male B6C3F1 mice. Carcinogenesis. 5(12):1623-1628.
Pículo F, Macedo CG, de Andrade SF, Maistro EL. 2010. In vivo genotoxicity assessment of nerolidol. J Appl Toxicol. 31(17):633-639.
Prakash O, Sahoo D, Rout PK. 2012. Liquid CO2 extraction of Jasminum grandiflorum and comparison with conventional process. Nat Prod Commun. 7(1):89-92.
Raeisi M, Tajik H, Razavi RS, Maham M, Moradi M, Hajimohammadi B, Naghili H, Hashemi M, Mehdizadeh T. 2012. Essential oil of tarragon (Artemisia dracunculus) antibacterial activity on Staphylococcus aureus and Escherichia coli in culture media and Iranian white cheese. Iran J Microbiol. 4(1):30-34.
Randerath K, Haglund RE, Phillips DH, Reddy MV. 1984. 32P-post-labelling analysis of DNA adducts formed in the livers of animals treated with safrole, estragole and other naturally-occurring alkenylbenzenes. I. Adult female CD-1 mice. Carcinogenesis. 5(12):1613-1622.
Rietjens IMCM, Slob W, Galli C, Silano V. 2008. Risk assessment of botanicals and botanical preparations intended for use in food and food supplements: emerging issues. Toxicol Lett. 180(2):131-136.
[RIFM] Research Institute for Fragrance Materials, Inc. 2020. RIFM fragrance ingredient safety assessment, p-t-butyl-α-methylhydrocinnamic aldehyde, CAS Registry Number 80-54-6. Food Chem Toxicol. 141(Suppl 1):111430.
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment (NL)]. 2006. Cosmetics fact sheet: to assess the risks for the consumer: updated version for ConsExpo 4. Bilthoven (NL): RIVM. Report No.: 320104001/2006.
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment (NL)]. 2018. ConsExpo. 2016. ConsExpo Web consumer exposure models. Bilthoven (NL): RIVM.
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu (National Institute for Public Health and the Environment). 2018. Cleaning Products Factsheet: Default parameters for estimating consumer exposure - updated version 2018 [PDF]. Bilthoven (NL): RIVM. Report No. 2016-0179. Contains erratum d.d. 13-9-2018.
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment (NL)]. 2021a. Health risk assessment of ethanol-containing hand sanitizer [PDF]. Bilthoven (NL): RIVM. Report No.: 2021-0026.
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment (NL)]. 2021b. Air Fresheners Fact Sheet [PDF]. Bilthoven (NL): RIVM. Report No.: 2021-0233.
[RTECS] Registry of Toxic Effects of Chemical Substances. 2019. Record for anisole, p-propenyl-, cis- (25679-28-1). Hamilton (ON): Canadian Centre for Occupational Health and Safety, National Institute for Occupational Safety and Health. [updated 2019 Nov; accessed 2020 May].
Saint-Lary L, Roy C, Paris J-P, Tournayre P, Berdagué J-L, Thomas OP, Fernandez X. 2014. Volatile compounds of Viola odorata absolutes: identification of odorant active markers to distinguish plants originating from France and Egypt. Chem Biodivers. 11(6):843-860.
Sakthivel R, Mala DS, Devi KP. 2018. Phytol shows anti-angiogenic activity and induces apoptosis in A549 cells by depolarizing the mitochondrial membrane potential. Biomed Pharmacother. 105:742-752.
Sayyah M, Nadjafnia L, Kamalinejad M. 2004. Anticonvulsant activity and chemical composition of Artemisia dracunculus L. essential oil. J Ethnopharmacol. 94(2-3):282-287.
[SCCS] Scientific Committee on Consumer Safety. 2015. The SCCS notes of guidance for the testing of cosmetic ingredients and their safety evaluation [PDF]. 9th Revision. European Commission. Report No. SCCS/1564/15, Revised version of 25 April 2016. [accessed 2020 Apr].
[SCCS] Scientific Committee on Consumer Safety. 2016. Opinion on butylphenyl methylpropional (BMHCA). 12 August 2015, SCCS/1540/14, revision of 16 March 2016.
[SCCS] Scientific Committee on Consumer Safety. 2019. Opinion on the safety of butylphenyl methylpropional (p-BMHCA) in cosmetic products - submission II, preliminary version of 14 December 2017, final version of 10 May 2019, SCCS/1591/2017.
[SCF] European Commission, Scientific Committee on Food. 2001. Opinion of the Scientific Committee on Food on estragole (1-allyl-4-methoxybenzene) [PDF].
Scherer M, Petreanu W, Weber T, Scherer G, Pluym N, Kolossa-Gehring M. 2021. Human biomonitoring in urine samples from the Environmental Specimen Bank reveals a decreasing trend over time in the exposure to the fragrance chemical lysmeral from 2000 to 2018. Chemosphere. 265:128955.
Schwotzer D, Gigliotti A, Irshad H, Dye W, McDonald J. 2021. Phytol, not propylene glycol, causes severe pulmonary injury after inhalation dosing in Sprague-Dawley rats. Inhal Toxicol. 33(1):33-40.
[SDS] Safety Data Sheet. 2014a. Oil Essentials Soothe Lavender & Rose Hip. Yokneam (IL): Emilia Cosmetics Ltd.
[SDS] Safety Data Sheet. 2014b. Hand Sanitizer -Vitamin E. Toronto (CA): Apollo Health and beauty care.
[SDS] Safety Data Sheet. 2016. Fresh Products Curve Air Freshener Cucumber Melon. Toledo (OH): Fresh Products. [restricted access].
[SDS] Safety Data Sheet. 2017a. Glade® PlugIns® Scented Oil Delicate Vanilla Embrace™. Bradford (ON): S.C. Johnson and Son, Limited. [restricted access].
[SDS] Safety Data Sheet. 2017b. Air Wick Carpet Powder – Country Vanilla – 2 in 1. Sydney (AU): Reckitt Benckiser Pty Limited.
[SDS] Safety Data Sheet. 2019. AIRLIFT TROPICAL AIR FRESHENER (AEROSOL) [PDF]. Maumee (OH): Spartan Chemical Company, Inc.
[SDS] Safety Data Sheet. 2020. Febreze LIGHT PLUGS Bamboo [PDF]. Cincinnati (OH): Procter & Gamble Inc.
Sekizawa J, Shibamoto T. 1982. Genotoxicity of safrole-related chemicals in microbial test systems. Mutat Res. 101(2):127-140.
Smith B, Cadby P, Leblanc J-C, Setzer RW. 2010. Application of the margin of exposure (MoE) approach to substances in food that are genotoxic and carcinogenic: example: methyleugenol, CASRN: 93-15-2. Food Chem Toxicol. 48 Suppl 1:S89-S97.
Sperotto ARM, Moura DJ, Péres VF, Damasceno FC, Caramão EB, Henriques JAP, Saffi J. 2013. Cytotoxic mechanism of Piper gaudichaudianum Kunth essential oil and its major compound nerolidol. Food Chem Toxicol. 57:57-68.
Statistics Canada. 2017. Custom tabulation of grooming products data from the Canadian Health Measures Survey Cycle 2 (2010-2011). Prepared for Existing Substances Risk Assessment Bureau, Health Canada by Statistics Canada. Unpublished.
Steinberg D, Avigan J, Mize CE, Baxter JH, Cammermeyer J, Fales HM, Highet PF. 1966. Effects of dietary phytol and phytanic acid in animals. J Lipid Res. 7(5):684-691.
Suparmi S, Ginting AJ, Mariyam S, Wesseling S, Rietjens IMCM. 2019. Levels of methyleugenol and eugenol in instant herbal beverages available on the Indonesian market and related risk assessment. Food Chem Toxicol. 125:467-478.
Suzuki Y, Umemura T, Hibi D, Inoue T, Jin M, Ishii Y, Sakai H, Nohmi T, Yanai T, Nishikawa A, et al. 2012. Possible involvement of genotoxic mechanisms in estragole-induced hepatocarcinogenesis in rats. Arch Toxicol. 86(10):1593-1601.
Swanson AB, Chambliss DD, Blomquist JC, Miller EC, Miller JA. 1979. The mutagenicities of safrole, estragole, eugenol, trans-anethole, and some of their known or possible metabolites for Salmonella typhimurium mutants. Mutat Res. 60(2):143-153.
Tateo F, Santamaria L, Bianchi L, Bianchi A. 1989. Basil oil and tarragon oil: composition and genotoxicity evaluation. J Essent Oil Res. 1(3):111-118.
Tisserand R, Young R. 2014. Essential oil safety. 2nd ed. London (UK): Churchill Livingstone.
Tisserand Institute. 2021. How to use essential oils safely. [accessed 2021 Jul].
Truhaut R, Le Bourhis B, Attia M, Glomot R, Newman J, Caldwell J. 1989. Chronic toxicity/carcinogenicity study of trans-anethole in rats. Food Chem Toxicol. 27(1):11-20.
Tucker AO, Maciarello MJ, Adams RP, Landrum LR, Zanoni TA. 1991. Volatile leaf oils of Caribbean Myrtaceae. I. Three varieties of Pimenta racemosa (Miller) J. Moore of the Dominican Republic and the commercial bay oil. J Essent Oil Res. 3(5):323-329.
Turkez H, Aydin E, Geyikoglu F, Cetin D. 2014. Genotoxic and oxidative damage potentials in human lymphocytes after exposure to terpinolene in vitro. Cytotechnology. 67(3):409-418.
[US EPA] United States Environmental Protection Agency. 2010. Screening-level hazard characterization: Benzyl Derivatives Category [PDF]. Hazard Characterization Document. US Environmental Protection Agency.
[US EPA] United States Environmental Protection Agency. 2012a. EPI Suite- Estimation Program Interface Suite for Microsoft Windows [estimation model]. C2000-2012. Version 4.11. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
[US EPA] United States Environmental Protection Agency. 2012b. Standard Operating Procedures for Residential Pesticide Exposure Assessment [PDF]. October 2012 Washington (DC): Health Effects Division, Office of Pesticide Programs, Office of Chemical Safety and Pollution Prevention.
[US EPA] United States Environmental Protection Agency. 2019. The Chemistry Dashboard. LIST: Terpenes in vape. Research Triangle Park (NC): US Environmental Protection Agency, Chemical Safety for Sustainability Research Program.
[US FDA] United States Food and Drug Administration. 2018. Substances Added to Food (formerly EAFUS).
[US NLM] United States National Library of Medicine. 2014. Record on anethole. [updated 2014 Nov; accessed 2020 May]. Bethesda (MD): National Institutes of Health of United States.
Van den Berg SJPL, Restani P, Boersma MG, Delmulle L, Rietjens IMCM. 2011. Levels of genotoxic and carcinogenic compounds in plant food supplements and associated risk assessment. Food Nutr Sci. 2(9):989-1010.
[VCF] Volatile Compounds in Food: database. 2020. Van Dongen WD, Donders JJH, editors. Version 16.7. Reeuwijk (NL): BeWiDo B.V., 1963-2020
Wei FH, Chen FL, Tan XM. 2015. Gas chromatographic-mass spectrometric analysis of essential oil of Jasminum officinale L var Grandiflorum flower. Trop J Pharm Res. 14(1):149-152.
Whelan J, Fritsche K. 2013. Linoleic acid. Adv Nutr. 4(3):311-312.
[WHO] Joint FAO/WHO Expert Committee on Food Additives. 1991. First draft for safety evaluation of certain food additives: trans-anethole. WHO Food Additives Series: 28. Geneva (CH): World Health Organization.
[WHO] Joint FAO/WHO Expert Committee on Food Additives. 1999. Safety evaluation of certain food additives: trans-anethole. WHO Food Additives Series: 42. Geneva (CH): World Health Organization.
[WHO] Joint FAO/WHO Expert Committee on Food Additives. 2004. Safety evaluation of certain food additives and contaminants: aliphatic, alicyclic, linear, a,b-unsaturated, di- and trienals and related alcohols, acids and esters. WHO Food Additives Series: 52. Geneva (CH): World Health Organization.
[WHO] Joint FAO/WHO Expert Committee on Food Additives. 2008. Safety evaluation of certain food additives and contaminants [PDF]. Sixty-eighth meeting of the Joint FAO/WHO Expert Committee on Food additives (JECFA). WHO Food Additives Series: 59. Geneva (CH): World Health Organization.
[WHO] Joint FAO/WHO Expert Committee on Food Additives. 2019. Linoleic and linolenic acid (mixture). [accessed 2020 Sep 10].
Wilkinson MAC, Ormandy K, Bradley CR, Fraise AP, Hines J. 2017. Dose considerations for alcohol-based hand rubs. J Hosp Infect. 95(2):175-182.
Wu XM, Bennett DH, Ritz B, Cassady DL, Lee K, Hertz-Picciotto I. 2010. Usage pattern of personal care products in California households. Food Chem Toxicol. 48(11):3109-3119.
Yang X-N, Wang Y-K, Zhu X, Xiao X-R, Dai M-Y, Zhang T, Qu Y, Yang X-W, Qin H-B, Gonzalez FJ, et al. 2019. Metabolic Activation of Elemicin Leads to the Inhibition of Stearoyl-CoA Desaturase 1. Chem Res Toxicol. 32(10):1965-1976.
Zhou G-D, Moorthy B, Bi J, Donnelly KC, Randerath K. 2007. DNA adducts from alkoxyallylbenzene herb and spice constituents in cultured human (HepG2) cells. Environ Mol Mutagen. 48(9):715-721.
Appendix A. Parameters for estimating oral, dermal, and inhalation exposures to products available to consumers
Exposures to products available to consumers were estimated using ConsExpo Web (RIVM 2018). Exposure estimates were calculated using default body weights and inhalation rates of 74 kg/15.1 m3/day for adults (19 years and older); 62 kg/15.9 m3/day for 14- to 18-year-olds; 42 kg/13.9 m3/day for 9- to 13-year-olds; 23 kg/11.1 m3/day for 4- to 8-year-olds; 15 kg/9.2 m3/day for 2- to 3-year-olds; 11 kg/8.0 m3/day for 1-year-olds; 9.1 kg/5.4 m3/day for 6- to 11-month-olds; and 6.3 kg/3.7 m3/day for 0- to 5-month-olds (HC 2019b).
A dermal absorption factor of 20% was used for violet oil, 50% for phenylpropanoids subgroup 1 (jasmine oil, perfumes and essences of jasmin), 40% for bay oil and tarragon oil, and 15% for substances in aldehydes subgroup 2 (lilial, myrac-aldehyde, myrmac-aldehyde, myrmac-carboxaldehyde, cetonal, vernaldehyde). Calculated exposure estimates for violet oil and bay oil were adjusted by 19% and 2% for the maximum amount of 2,6-nonadienal and methyl eugenol in violet oil and bay oil, respectively.
When calculating the LADDs, an average lifetime of 78 years was used (HC 2013).
| Exposure scenario | Assumptions |
|---|---|
| Eye moisturizer (violet oil) |
Concentration: 100% violet oil Product amount: 0.16 g (adults and 14–18 yrs) (Ficheux et al. 2016). Air concentrations were modelled using the ConsExpo exposure to vapour–evaporation–constant release area model. Inhalation exposure (mg/kg bw/day) = [Air concentration (mg/m3) (24 h time-weighted average) * Inhalation rate (m3/day)] ÷ Body weight Combined exposure (mg/kg bw/day) = Dermal exposure (mg/kg bw/day) + Inhalation exposure (mg/kg bw/day) |
| Hair conditioner (leave-on) (violet oil, bay oil) |
Concentration: 100% violet oil, 3% bay oil (assuming the 0.0002% maximum concentration of methyl eugenol permitted according to the Cosmetic Ingredient Hotlist) Product amount (violet oil): 1.5 g, assumed for all the subpopulations (as per manufacturer instructions) Product amount (bay oil): 13.1 g (adults); 10 g (14–18 yrs); 7.8 g (9–13 yrs and 4–8 yrs); 5.2 g (2–3 yrs) (Ficheux et al. 2016; Garcia-Hidalgo et al. 2017) Frequency: 1.1 (adults) (Loretz et al. 2008). For all other subpopulations, the mean frequency was assumed to be 1. Retention factor: 0.1 Bay oil dermal exposure (mg/kg bw/day) was calculated using the following formula: [mean product (g/application) * mean daily frequency * product concentration * dermal absorption * retention factor * conversion factor (1000 mg/g)] ÷ Body weight Air concentrations were modelled using the ConsExpo exposure to vapour–evaporation–constant release area model. Inhalation exposure (mg/kg bw/day) = [Air concentration (mg/m3) (24 h time-weighted average) * Inhalation rate (m3/day)] ÷ Body weight Bay oil: combined exposure (mg/kg bw/day) = Dermal exposure (mg/kg bw/day) + Inhalation exposure (mg/kg bw/day) A lifetime average daily dose (LADD) was also calculated for bay oil using the following formula: LADD (mg/kg bw/day) = ((combined exposure (mg/kg bw/day) 0–5 mths * time in lifestage (0.5 yr)) + (combined exposure (mg/kg bw/day) 6–12 mths * time in lifestage (0.5 yr)) + (combined exposure (mg/kg bw/day) 1 yr * time in lifestage (1 yr)) + (combined exposure (mg/kg bw/day) 2–3 yrs * time in lifestage (2 yrs)) + (combined exposure (mg/kg bw/day) 4–8 yrs * time in lifestage (5 yrs)) + (combined exposure (mg/kg bw/day 9–13 yrs * time in lifestage (5 yrs)) + (combined exposure (mg/kg bw/day) 14–18 yrs * time in lifestage (5 yrs)) + (combined exposure (mg/kg bw/day) adult (19+) * time in lifestage (59 yrs))/Average lifetime (78 yrs) |
| Hair conditioner (wash-off) (jasmine oil, lilial) |
Concentration: 30% jasmine oil, 2% lilial. Product amount (jasmine oil): 0.71 g, assumed for all the subpopulations (personal communication, email communication from the Consumer and Hazardous Products Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, February 2021; unreferenced) Product amount (lilial): 13.1 g (adults); 10 g (14–18 yrs); 7.8 g (9–13 yrs and 4–8 yrs); 5.2 g (2–3 yrs) (Ficheux et al. 2016; Garcia-Hidalgo et al. 2017) Frequency: 1.1 (adults) (Loretz et al. 2008). For all other subpopulations, the mean frequency was assumed to be 1. Dermal exposure (mg/kg bw/day) was calculated using the following formula: [mean product (g/application) * mean daily frequency * product concentration * dermal absorption * retention factor * conversion factor (1000 mg/g)] ÷ Body weight Air concentrations were modelled using the ConsExpo exposure to vapour–evaporation–constant release area model. Inhalation exposure (mg/kg bw/day) = [Air concentration (mg/m3) (24 h time-weighted average) * Inhalation rate (m3/day)] ÷ Body weight Combined exposure (mg/kg bw/day) = Dermal exposure (mg/kg bw/day) + Inhalation exposure (mg/kg bw/day) |
| Facial cleanser (violet oil, tarragon oil) |
Concentration: 30% violet oil, 0.1% tarragon oil Product amount: 3.3 g (adults and 14–18 yrs); 3.1 g (9–13 yrs) (Ficheux et al. 2016) Frequency: 1.6/day (adults) (Loretz et al. 2008); 1.2/day (14–18 yrs and 9–13 yrs) (Ficheux et al. 2015) Dermal exposure (mg/kg bw/day) was calculated using the following formula: [mean product (g/application) * mean daily frequency * product concentration * dermal absorption * retention factor * conversion factor (1000 mg/g)] ÷ Body weight Air concentrations were modelled using the ConsExpo exposure to vapour–evaporation–constant release area model. Inhalation exposure (mg/kg bw/day) = [Air concentration (mg/m3) (24 h time-weighted average) * Inhalation rate (m3/day)] ÷ Body weight Combined exposure (mg/kg bw/day) = Dermal exposure (mg/kg bw/day) + Inhalation exposure (mg/kg bw/day) For tarragon oil, a LADD was also calculated using the following formula : LADD (mg/kg bw/day) = {[combined exposure (mg/kg bw/day) 9–13 yrs * time in lifestage (5 yrs)] + [combined exposure (mg/kg bw/day) 14–18 yrs * time in lifestage (5 yrs)] + [combined exposure (mg/kg bw/day) adult (19+) * time in lifestage (59 yrs)]}/Average lifetime (78 yrs) |
| Acne treatment (NHP) (lilial) |
Concentration: 0.1% lilial Product amount: 3.3 g (adults and 14–18 yrs); 3.1 g (9–13 yrs) (Ficheux et al. 2016) Dermal exposure (mg/kg bw/day) was calculated using the following formula: [mean product (g/application) * mean daily frequency * product concentration * dermal absorption * retention factor * conversion factor (1000 mg/g)] ÷ Body weight Air concentrations were modelled using the ConsExpo exposure to vapour–evaporation–constant release area model. Inhalation exposure (mg/kg bw/day) = [Air concentration (mg/m3) (24 h time-weighted average) * Inhalation rate (m3/day)] ÷ Body weight Combined exposure (mg/kg bw/day) = Dermal exposure (mg/kg bw/day) + Inhalation exposure (mg/kg bw/day) |
| Body moisturizer (violet oil, bay oil, tarragon oil, jasmine oil, and lilial) |
Concentration: 10% violet oil, 0.3% bay oil (assuming the 0.002% maximum concentration of methyl eugenol permitted according to the Cosmetic Ingredient Hotlist), 0.3% tarragon oil, 3% jasmine oil, 1% lilial, 0.1% fragrance ingredient Product amount: 10 g (adults and 14–18 yrs); 7.7 g (9–13 yrs); 5 g (4–8 yrs); 4.1 g (2–3 yrs); 3.1 g (1 yr); 2.5 g (6–11 mths); 2 g (0–5 mths) (Ficheux et al. 2016) For all other subpopulations, the mean frequency was assumed to be 1. For bay oil and tarragon oil (calculation of LADD), a frequency of 0.8/day was used for all other subpopulations (Wu et al. 2010; Ficheux et al. 2015). For bay oil, tarragon oil, jasmine oil, and lilial, dermal exposure (mg/kg bw/day) was calculated using the following formula: [mean product (g/application) * mean daily frequency (1/day) * product concentration * dermal absorption * conversion factor (1000 mg/g)] ÷ Body weight Air concentrations were modelled using the ConsExpo exposure to vapour–evaporation–constant release area model. Product amount: adjusted for unclothed surface area of short-sleeved shirt and shorts. 4.87 g (adults); 4.65 g (14–18 yrs); 3.61 g (9–13 yrs); 2.3 g (4–8 yrs); 1.85 g (2–3 yrs); 1.45 g (1 yr); 1.16 g (0–5 mths); 0.93 g (0–5 mths). Inhalation exposure (mg/kg bw/day) = [Air concentration (mg/m3) (24 h time-weighted average) * Inhalation rate (m3/day) * Frequency (calculation of LADD only)] ÷ Body weight Combined exposure (mg/kg bw/day) = Dermal exposure (mg/kg bw/day) + Inhalation exposure (mg/kg bw/day) For bay oil and tarragon oil, LADDs were also calculated using the following formula (HC 2013): LADD (mg/kg bw/day) = ((combined exposure (mg/kg bw/day) 0–5 mths * time in lifestage (0.5 yr)) + (combined exposure (mg/kg bw/day) 6–12 mths * time in lifestage (0.5 yr)) + (combined exposure (mg/kg bw/day) 1 yr * time in lifestage (1 yr)) + (combined exposure (mg/kg bw/day) 2-3 yrs * time in lifestage (2 yrs)) + (combined exposure (mg/kg bw/day) 4–8 yrs * time in lifestage (5 yrs)) + (combined exposure (mg/kg bw/day 9–13 yrs * time in lifestage (5 yrs)) + (combined exposure (mg/kg bw/day) 14–18 yrs * time in lifestage (5 yrs)) + (combined exposure (mg/kg bw/day) adult (19+) * time in lifestage (59 yrs))/Average lifetime (78 yrs) |
| Massage oil (violet oil, lilial) |
Concentration: 10% violet oil, 0.3% lilial. Product amount: 3.2 g (adults); 2.9 g (14–18 yrs); 2.3 g (9–13 yrs); 1.9 g (4–8 yrs); 1.8 g (2–3 yrs, 1 yr, 6–11 mths, 0–5 mths) (Ficheux et al. 2016) Dermal exposure (mg/kg bw/day) was calculated using the following formula: [mean product (g/application) * mean daily frequency * product concentration * dermal absorption * conversion factor (1000 mg/g)] ÷ Body weight Air concentrations were modelled using the ConsExpo exposure to vapour–evaporation–constant release area model. Inhalation exposure (mg/kg bw/day) = [Air concentration (mg/m3) (24 h time-weighted average) * Inhalation rate (m3/day)] ÷ Body weight Combined exposure (mg/kg bw/day) = Dermal exposure (mg/kg bw/day) + Inhalation exposure (mg/kg bw/day) |
| Lipstick (violet oil, jasmine oil) |
Concentration: 10% violet oil, 3% jasmine oil Product amount: 0.022 g (all subpopulations) (Ficheux et al. 2016) Oral exposure (mg/kg bw/day) was calculated using the following formula: [mean product (g/application) * mean daily frequency (1/day) * product concentration * conversion factor ( mg/g)] ÷ Body weight |
| Body fragrance (violet oil, bay oil, tarragon oil, jasmine oil, lilial) |
Concentration: 10% violet oil, 10% bay oil (assuming 0.004% for eau de toilette and 0.01% for fine fragrance as the maximum concentration of methyl eugenol permitted according to the Cosmetic Ingredient Hotlist), 100% tarragon oil, 82% jasmine oil, 44% lilial Product amount: based on eaux de toilette, 0.33 g (adults, 14–18 yrs, and 9–13 yrs) (Ficheux et al. 2016) Frequency: 1.7/day (adults) (Loretz et al. 2006); 1.4/day (14–18 yrs and 9–13 yrs) (Statistics Canada 2017) For bay oil, tarragon oil, jasmine oil, and lilial, dermal exposure (mg/kg bw/day) was calculated using the following formula: [mean product (g/application) * mean daily frequency * product concentration * dermal absorption * conversion factor (1000 mg/g)] ÷ Body weight For the spray products, air concentrations were modelled using the ConsExpo exposure to spray–evaporation–constant release area model. For the roll-on products, air concentrations were modelled using the ConsExpo exposure to vapour–evaporation–constant release area model. Inhalation exposure (mg/kg bw/day) = [Air concentration (mg/m3) (24 h time-weighted average) * Inhalation rate (m3/day)] ÷ Body weight Combined exposure (mg/kg bw/day) = Dermal exposure (mg/kg bw/day) + Inhalation exposure (mg/kg bw/day) For bay oil and tarragon oil, LADDs were also calculated using the following formula (HC 2013): LADD (mg/kg bw/day) = ((combined exposure (mg/kg bw/day) 9–13 yrs * time in lifestage (5 yrs)) + (combined exposure (mg/kg bw/day) 14–18 yrs * time in lifestage (5 yrs)) + (combined exposure (mg/kg bw/day) adult (19+) * time in lifestage (59 yrs))/Average lifetime (78 yrs)) |
| De-stress roll-on (NHP) (jasmine oil) |
Concentration: 0.01% Product amount: 0.14 g [as per manufacturer instruction, 0.15 mL * jasmine oil density (0.947 g/mL)) Frequency :3/day (as per manufacturer instructions) Dermal exposure (mg/kg bw/day) was calculated using the following formula: [mean product (g/application) * mean daily frequency * product concentration * dermal absorption] ÷ Body weight Air concentrations were modelled using the ConsExpo exposure to vapour–evaporation–constant release area model. Product amount: as above, adjusted to account for the amount remaining on the skin surface following dermal absorption of the substance and the daily frequency. Exposure and emission duration: 24 h Combined exposure (mg/kg bw/day) = Dermal exposure (mg/kg bw/day) + Inhalation exposure (mg/kg bw/day) |
| Bath product (lilial) |
Concentration: 3% lilial Product amount: For lilial, 0.0394 g (adults), 0.0370 g (14–18 yrs), 0.0078 g (9–13 yrs) (Ficheux et al.2016), 0.051 g (4–8 yrs), 0.0055 g (2–3 yrs), 0.0041 g (1yr), 0.0034 g (6–11 mths) (Garcia-Hidalgo et al. 2017), 0.0026 g (0–5 mths) (Garcia-Hidalgo et al. 2017) Frequency: 1 (all subpopulations) Dermal exposure (mg/kg bw/day) was calculated using the following formula: [mean product (g/application) * mean daily frequency * product concentration * dermal absorption * retention factor * conversion factor (1000 mg/g)] ÷ Body weight Air concentrations were modelled using the ConsExpo exposure to vapour–evaporation–constant release area model. Inhalation exposure (mg/kg bw/day) = [Air concentration (mg/m3) (24 h time-weighted average) * Inhalation rate (m3/day)] ÷ Body weight Combined exposure (mg/kg bw/day) = Dermal exposure (mg/kg bw/day) + Inhalation exposure (mg/kg bw/day) |
| Facial moisturizer (tarragon oil, lilial) |
Concentration: 0.1% tarragon oil, 3% lilial Product amount: 1.5 g (adults and 14–18 yrs); 1.1 g (9–13 yrs) (Ficheux et al. 2016) Frequency: 2/day (adults) (Loretz et al. 2005); 1/day (14–18 yrs and 9–13 yrs) (Ficheux et al. 2015) Dermal exposure (mg/kg bw/day) was calculated using the following formula: [mean product (g/application) * mean daily frequency * product concentration * dermal absorption * conversion factor (1000 mg/g)] ÷ Body weight Air concentrations were modelled using the ConsExpo exposure to vapour–evaporation–constant release area model. Inhalation exposure (mg/kg bw/day) = [Air concentration (mg/m3) (24 h time-weighted average) * Inhalation rate (m3/day)] ÷ Body weight Combined exposure (mg/kg bw/day) = Dermal exposure (mg/kg bw/day) + Inhalation exposure (mg/kg bw/day) For tarragon oil, a LADD was also calculated using the following formula: LADD (mg/kg bw/day) = {[combined exposure (mg/kg bw/day) 9–13 yrs * time in lifestage (5 yrs)] + [combined exposure (mg/kg bw/day) 14–18 yrs * time in lifestage (5 yrs)] + [combined exposure (mg/kg bw/day) adult (19+) * time in lifestage (59 yrs)]}/Average lifetime (78 yrs) |
| Facial moisturizer/acne treatment (NHP) (jasmine oil) |
Concentration: jasmine oil 1% Product amount: 1.5 g (adults) (Ficheux et al. 2016) Dermal exposure (mg/kg bw/day) was calculated using the following formula: [mean product (g/application) * mean daily frequency * product concentration * dermal absorption * conversion factor (1000 mg/g)] ÷ Body weight Air concentrations were modelled using the ConsExpo exposure to vapour–evaporation–constant release area model. Inhalation exposure (mg/kg bw/day) = [Air concentration (mg/m3) (24 h time-weighted average) * Inhalation rate (m3/day)] ÷ Body weight Combined exposure (mg/kg bw/day) = Dermal exposure (mg/kg bw/day) + Inhalation exposure (mg/kg bw/day) |
| Facial sun protection powder (NHP) (jasmine oil) |
Concentration: 0.07% Product amount: 0.073 g (adults) (Ficheux et al. 2016) Frequency: 6/day (as per manufacture recommendations) Dermal exposure (mg/kg bw/day) was calculated using the following formula: [mean product (g/application) * mean daily frequency * product concentration * dermal absorption * conversion factor (1000 mg/g)] ÷ Body weight Air concentrations were modelled using the ConsExpo exposure to vapour–evaporation–constant release area model. Inhalation exposure (mg/kg bw/day) = [Air concentration (mg/m3) (24 h time-weighted average) * Inhalation rate (m3/day)] ÷ Body weight Combined exposure (mg/kg bw/day) = Dermal exposure (mg/kg bw/day) + Inhalation exposure (mg/kg bw/day) |
| Topical treatment cream (NHP) (jasmine oil) |
Concentration: 1% jasmine oil Product amount: 1.5 g (adults) (Ficheux et al. 2016) Frequency: 1/day (as per manufacturer recommendations) Dermal exposure (mg/kg bw/day) was calculated using the following formula: [mean product (g/application) * mean daily frequency * product concentration * dermal absorption * conversion factor (1000 mg/g)] ÷ Body weight Air concentrations were modelled using the ConsExpo exposure to vapour–evaporation–constant release area model. Inhalation exposure (mg/kg bw/day) = [Air concentration (mg/m3) (24 h time-weighted average) * Inhalation rate (m3/day)] ÷ Body weight Combined exposure (mg/kg bw/day) = Dermal exposure (mg/kg bw/day) + Inhalation exposure (mg/kg bw/day) |
| Soap (tarragon oil) |
Concentration: 0.3% tarragon oil Product amount: 1.1 g (adults and 14–18 yrs), 0.82 g (9–13 yrs), 0.53 g (4–8 yrs), 0.38 g (2–3 yrs), 0.29 g (1 yr), 0.24 g (6–11 mths), 0.18 g (0–5 mths) (Ficheux et al. 2016) Frequency: 1.2 (adults and 14–18 yrs), 1.15 (9–13 yrs and 4–8 yrs), 1.1 (2–3 yrs, 1 yr, 6–11 mths and 0–5 mths) (Ficheux et al. 2015) Dermal exposure (mg/kg bw/day) was calculated using the following formula: [mean product (g/application) * mean daily frequency * retention factor * product concentration * dermal absorption * conversion factor (1000 mg/g)] ÷ Body weight Air concentrations were modelled using the ConsExpo exposure to vapour–evaporation–constant release area model. Exposure and emission duration: 5 min Inhalation exposure (mg/kg bw/day) = [Air concentration (mg/m3) (24 h time-weighted average) * Inhalation rate (m3/day)] ÷ Body weight LADD was also calculated using the following formula: LADD (mg/kg bw/day) = {[combined exposure (mg/kg bw/day) 0–5 mths * time in lifestage (0.5 yr)] + [combined exposure (mg/kg bw/day) 6–12 mths * time in lifestage (0.5 yr)] + [combined exposure (mg/kg bw/day) 1 yr * time in lifestage (1 yr)] + [combined exposure (mg/kg bw/day) 2–3 yrs * time in lifestage (2 yrs)] + [combined exposure (mg/kg bw/day) 4–8 yrs * time in lifestage (5 yrs)] + [combined exposure (mg/kg bw/day) 9–13 yrs * time in lifestage (5 yrs)] + [combined exposure (mg/kg bw/day) 14–18 yrs * time in lifestage (5 yrs)] + [combined exposure (mg/kg bw/day) adult (19+) * time in lifestage (59 yrs)]}/Average lifetime (78 yrs) |
| Digestive aid capsule (NHP) (tarragon oil) |
Tarragon oil: 0.0026% LADD was also calculated using the following formula (HC 2013): LADD (mg/kg bw/day) = [oral exposure (mg/kg bw/day) adult (19+) * time in lifestage (59 yrs)]/Average lifetime (78 yrs) |
| Inhaler stick (NHP) (bay oil) |
Concentration: 0.06% bay oil Product amount: 1 g (adults) (calculated assuming the entire product of 1 mL is used in one day and assuming a density of 1 g/mL (1 mL * 1 g/mL) Frequency: it is assumed that a user would use the product 30 days per year (professional judgment) Inhalation exposure (mg/kg bw/day) was calculated using the following formula: [mean product (g/application) * mean daily frequency * product concentration * adjusted composition factor (2%) * conversion factor (1000 mg/g)] ÷ Body weight For bay oil, LADD was also calculated using the following formula: LADD (mg/kg bw/day) = [inhalation exposure (mg/kg bw/day) adult (19+) * time in lifestage (59 yrs)]/Average lifetime (78 yrs) |
| Respiratory air spray (NHP) (bay oil) |
Concentration: 2% bay oil Air concentrations were modelled using the exposure to spray–instantaneous release model. Product amount: 1.3 g, calculated using the upper range of volume per spray of 0.16 mL and the assumption that the maximum number of sprays per day would be 9. Exposure duration: 240 min (RIVM 2006) Inhalation exposure (mg/kg bw/day) = [Air concentration (mg/m3) (24 h time-weighted average) * Inhalation rate (m3/day) * Frequency (calculation of LADD only) (168 times/365 (year) based on 14 times per month) (RIVM 2006)] ÷ Body weight LADD was also calculated using the following formula: LADD (mg/kg bw/day) = [inhalation exposure (mg/kg bw/day) adult (19+) * time in lifestage (59 yrs)]/Average lifetime (78 yrs) |
| Antiperspirant/deodorant (solid) (tarragon oil, jasmine oil, lilial) |
Concentration: 1% tarragon oil, 2% jasmine oil, 3% lilial Product amount: 1 g (adults and 14–18 yrs); 0.4 g (9–13 yrs) (Ficheux et al. 2016) Frequency: 1.3 (adults) (Loretz et al. 2006); 1.1 (14–18 yrs and 9–13 yrs) (Wu et al. 2010; Ficheux et al. 2015) Dermal exposure (mg/kg bw/day) was calculated using the following formula: [mean product (g/application) * mean daily frequency * product concentration * dermal absorption * conversion factor (1000 mg/g)] ÷ Body weight Air concentrations were modelled using the ConsExpo exposure to vapour–evaporation–constant release area model. Exposure and emission duration: 18 h (adults); 22 h (14–18 yrs and 9–13 yrs) Inhalation exposure (mg/kg bw/day) = [Air concentration (mg/m3) (24 h time-weighted average) * Inhalation rate (m3/day)] ÷ Body weight Combined exposure (mg/kg bw/day) = Dermal exposure (mg/kg bw/day) + Inhalation exposure (mg/kg bw/day) For tarragon oil, a LADD was also calculated using the following formula: LADD (mg/kg bw/day) = {[combined exposure (mg/kg bw/day) 9–13 yrs * time in lifestage (5 yrs)] + [combined exposure (mg/kg bw/day) 14–18 yrs * time in lifestage (5 yrs)] + [combined exposure (mg/kg bw/day) adult (19+) * time in lifestage (59 yrs)]}/Average lifetime (78 yrs) |
| Antiperspirant/deodorant (spray) (lilial) |
Concentration: 0.3% lilial Product amount: 3.48 g (adults and 14–18 yrs) (Hall et al. 2007); 0.98 g (9–13 yrs) (Ficheux et al. 2016) Frequency: 1.3 (adults) (Loretz et al. 2006); 1.2 (14–18 yrs) (Ficheux et al. 2015); 1.1 (9–13 yrs) (Wu et al. 2010) Retention factor: 0.85 Dermal exposure (mg/kg bw/day) was calculated using the following formula: [mean product (g/application) * mean daily frequency * product concentration * dermal absorption * retention factor * conversion factor (1000 mg/g)] ÷ Body weight Inhalation exposure to the spray was quantified using the exposure–spray model–instantaneous release and the following parameters: Inhalation exposure (mg/kg bw/day) = [Air concentration (mg/m3) (24 h time-weighted average) * Inhalation rate (m3/day)] ÷ Body weight Combined exposure (mg/kg bw/day) = Dermal exposure (mg/kg bw/day) + Inhalation exposure (mg/kg bw/day) |
| Antiseptic skin cleanser (NHP) (jasmine oil, lilial) |
Concentration: 2% jasmine oil, lilial 0.07% Product amount: 1.5 g (all age groups, Kampf et al. 2013; Macinga et al. 2013; Bánsághi et al. 2020) Frequency: 2.9 (adults); 1.4 (14–18 yrs, 9–13 yrs, 4–8 yrs), 0.8 (adjusted to 1) (2– 3 yrs) (Wu et al. 2010) Frequency (for situations of public health concern): 25/day (all age groups, RIVM 2021a) Dermal exposure (mg/kg bw/day) was calculated using the following formula: [mean product (g/application) * mean daily frequency * product concentration * dermal absorption * conversion factor (1000 mg/g)] ÷ Body weight Air concentrations were modelled using the ConsExpo exposure to vapour–evaporation–constant rate release model. Exposure and emission duration: 20 min (all age groups) Inhalation exposure (mg/kg bw/day) = [Air concentration (mg/m3) (24 h time-weighted average) * Inhalation rate (m3/day)] ÷ Body weight Combined exposure (mg/kg bw/day) = Dermal exposure (mg/kg bw/day) + Inhalation exposure (mg/kg bw/day) |
| Body cleanser (liquid) (bay oil, jasmine oil, lilial) |
Concentration: 3% bay oil (assuming the 0.001% maximum concentration of methyl eugenol permitted according to the Cosmetic Ingredient Hotlist), 3% jasmine oil, 1% lilial Product amount: 11 g (adults and 14–18 yrs); 10.9 (9–13 yrs and 4–8 yrs); 6.7 g (2–3 yrs); 5.4 g (1 yr); 4.9 g (6–11 mths); 4.5 g (0–5 mths) (Loretz et al. 2006; Ficheux et al. 2016; Garcia-Hidalgo et al. 2017) Frequency: 1.4 (adults); 1.2 (14–18 yrs, 2–3 yrs, 1 yr, 6–11 mths, and 0–5 mths); 1 (9–13 yrs and 4–8 yrs) (Loretz et al. 2006; Ficheux et al. 2015). Frequencies less than 1 were assigned a frequency of 1. Retention factor: 0.01 Dermal exposure (mg/kg bw/day) was calculated using the following formula: [mean product (g/application) * mean daily frequency * product concentration * dermal absorption * retention factor * conversion factor (1000 mg/g)] ÷ Body weight Air concentrations were modelled using the ConsExpo exposure to vapour–evaporation–constant release area model. Inhalation exposure (mg/kg bw/day) = [Air concentration (mg/m3) (24 h time-weighted average) * Inhalation rate (m3/day)] ÷ Body weight Combined exposure (mg/kg bw/day) = Dermal exposure (mg/kg bw/day) + Inhalation exposure (mg/kg bw/day) |
| Permanent hair colour (lilial) |
Concentration: 1% lilial Product amount: 132.6 g (adults and 14–18 yrs) (Ramirez-Martinez et al. (2015) Dermal exposure (mg/kg bw/day) was calculated using the following formula: [mean product (g/application) * mean daily frequency * product concentration * dermal absorption * retention factor * conversion factor (1000 mg/g)] ÷ Body weight Air concentrations were modelled using the ConsExpo exposure to vapour–evaporation–constant release area model. Inhalation exposure (mg/kg bw/day) = [Air concentration (mg/m3) (24 h time-weighted average) * Inhalation rate (m3/day)] ÷ Body weight Combined exposure (mg/kg bw/day) = Dermal exposure (mg/kg bw/day) + Inhalation exposure (mg/kg bw/day) |
| Temporary hair colour (jasmine oil, lilial) |
Concentration: 0.1% jasmine oil, 0.11% lilial Product amount: 35 g (adults, 14–18 yrs, 9–13 yrs, and 4–8 yrs) (SCCS 2015) Dermal exposure (mg/kg bw/day) was calculated using the following formula: [mean product (g/application) * mean daily frequency * product concentration * dermal absorption * retention factor * conversion factor (1000 mg/g)] ÷ Body weight Air concentrations were modelled using the ConsExpo exposure to vapour–evaporation–constant release area model. Inhalation exposure (mg/kg bw/day) = [Air concentration (mg/m3) (24 h time-weighted average) * Inhalation rate (m3/day)] ÷ Body weight Combined exposure (mg/kg bw/day) = Dermal exposure (mg/kg bw/day) + Inhalation exposure (mg/kg bw/day) |
| Aerosol hair styling product (jasmine oil) |
Concentration: 0.1% jasmine oil Product amount: 2.6 g (adults) (Loretz et al. 2008); 2.3 g (14–18 yrs, 9–13 yrs, and 4–8 yrs) (Ficheux et al. 2016) Dermal exposure (mg/kg bw/day) was calculated using the following formula: [mean product (g/application) * mean daily frequency * product concentration * dermal absorption * retention factor * conversion factor (1000 mg/g)] ÷ Body weight Inhalation exposure to the spray was quantified using the exposure–spray model–spraying towards exposed person and the following parameters: Inhalation exposure (mg/kg bw/day) = [Air concentration (mg/m3) (24 h time-weighted average) * Inhalation rate (m3/day)] ÷ Body weight Combined exposure (mg/kg bw/day) = Dermal exposure (mg/kg bw/day) + Inhalation exposure (mg/kg bw/day) |
| Hair straightening, waving, and curling product (lilial) |
Concentration: 11% lilial Product amount: 80 g (adults and 14–18 yrs) (Bremmer et al. 2006), 76 g ( 9–13 yrs) (SA adjustment), 66 g (4–8 yrs) (SA adjustment) Dermal exposure (mg/kg bw/day) was calculated using the following formula: [mean product (g/application) * mean daily frequency * product concentration * dermal absorption * retention factor * conversion factor (1000 mg/g)] ÷ Body weight Air concentrations were modelled using the ConsExpo exposure to vapour–evaporation–constant release area model. Inhalation exposure (mg/kg bw/day) = [Air concentration (mg/m3) (24 h time-weighted average) * Inhalation rate (m3/day)] ÷ Body weight Combined exposure (mg/kg bw/day) = Dermal exposure (mg/kg bw/day) + Inhalation exposure (mg/kg bw/day) |
| Hair removal/depilator (lilial) |
Concentration: 0.1% lilial Product amount: 9.7 g (adults, 14–18 yrs and 9–13 yrs); face mask was used as a surrogate, and amount was adjusted for surface area (Ficheux et al. 2016). Dermal exposure (mg/kg bw/day) was calculated using the following formula: [mean product (g/application) * mean daily frequency * product concentration * dermal absorption * retention factor * conversion factor (1000 mg/g)] ÷ Body weight Air concentrations were modelled using the ConsExpo exposure to vapour–evaporation–constant release area model. Inhalation exposure (mg/kg bw/day) = [Air concentration (mg/m3) (24 h time-weighted average) * Inhalation rate (m3/day)] ÷ Body weight Combined exposure (mg/kg bw/day) = Dermal exposure (mg/kg bw/day) + Inhalation exposure (mg/kg bw/day) |
| Sunscreen (NHP) (jasmine oil, lilial) |
Concentration: 0.07% jasmine oil, 0.01% lilial Product amount: 18.2 g (adults and 14–18 yrs); 6.3 g (9–13 yrs and 4–8 yrs); 5.4 g (2–3 yrs, 1 yr, and 6–11 mths) (Ficheux et al. 2016) Inhalation exposure from jasmine oil and lilial was not quantified as the product is expected to be used outside. Exposure by the inhalation route is therefore expected to be minimal. Dermal exposure (mg/kg bw/day) was calculated using the following formula: [mean product (g/application) * mean daily frequency * product concentration * dermal absorption * conversion factor (1000 mg/g)] ÷ Body weight |
| Face makeup liquid (lilial) |
Concentration: 1% lilial Product amount: 0.54 g (adult) (Loretz et al. 2016); 0.41 g (14–18 yrs) (Ficheux et al. 2016); 0.39 g (9–13 yrs) (SA adjustment); 0.34 g (SA adjustment) Dermal exposure (mg/kg bw/day) was calculated using the following formula: [mean product (g/application) * mean daily frequency * product concentration * dermal absorption * conversion factor (1000 mg/g)] ÷ Body weight Air concentrations were modelled using the ConsExpo exposure to vapour–evaporation–constant release area model. Combined exposure (mg/kg bw/day) = Dermal exposure (mg/kg bw/day) + Inhalation exposure (mg/kg bw/day) |
| Nail polish (lilial) |
Concentration: 3% lilial Product amount: 0.16 g (adults,14–18 yrs and 9–13 yrs); 0.06 g (4–8 yrs and 2–3 yrs) (Ficheux et al. 2014) Dermal exposure (mg/kg bw/day) was calculated using the following formula: [mean product (g/application) * mean daily frequency * product concentration * dermal absorption * conversion factor (1000 mg/g)] ÷ Body weight Air concentrations were modelled using ConsExpo exposure to vapour–evaporation–constant release area model. Inhalation exposure (mg/kg bw/day) = [Air concentration (mg/m3) (24 h time-weighted average) * Inhalation rate (m3/day)] ÷ Body weight Combined exposure (mg/kg bw/day) = Dermal exposure (mg/kg bw/day) + Inhalation exposure (mg/kg bw/day) |
| Nail polish remover (lilial) |
Concentration: 0.1% lilial Product amount: 2.25 g (adults,14–18 yrs and 9–13 yrs); 0.76 g (4–8 yrs and 2–3 yrs) (Ficheux et al. 2014) Dermal exposure (mg/kg bw/day) was calculated using the following formula: [mean product (g/application) * mean daily frequency * product concentration * dermal absorption * conversion factor (1000 mg/g)] ÷ Body weight Air concentrations were modelled using the ConsExpo exposure to vapour–evaporation–constant release area model. Inhalation exposure (mg/kg bw/day) = [Air concentration (mg/m3) (24 h time-weighted average) * Inhalation rate (m3/day)] ÷ Body weight Combined exposure (mg/kg bw/day) = Dermal exposure (mg/kg bw/day) + Inhalation exposure (mg/kg) |
| Sunless tanning products for the face (lotion) (lilial) |
Concentration:1% lilial Product amount: 1.5 g (adults and 1–18 yrs) (Ficheux et al. 2016) Dermal exposure (mg/kg bw/day) was calculated using the following formula: [mean product (g/application) * mean daily frequency * product concentration * dermal absorption * conversion factor (1000 mg/g)] ÷ Body weight Air concentrations were modelled using the ConsExpo exposure to vapour–evaporation–constant release area model. Inhalation exposure (mg/kg bw/day) = [Air concentration (mg/m3) (24 h time-weighted average) * Inhalation rate (m3/day)] ÷ Body weight Combined exposure (mg/kg bw/day) = Dermal exposure (mg/kg bw/day) + Inhalation exposure (mg/kg bw/day) |
| Air freshener (solid gel) (lilial) |
Concentration: 5% lilial (SDS 2016) Air concentrations were modelled using the ConsExpo exposure to vapour–instantaneous release model. Inhalation exposure (mg/kg bw/day) = [Air concentration (mg/m3) (24 h time-weighted average) * Inhalation rate (m3/day)] ÷ Body weight |
| Air freshener (liquid plug-in) (subgroup 2) |
Concentration: lilial 3% (SDS 2017a), 1% myrac-aldehyde (SDS 2020) Air concentrations were modelled using the ConsExpo exposure to vapour–constant rate model. Product amount: 0.5 g/day (based on a product size of approximately 26 g and 50 days of use, assuming a density of 1 g/mL)) Inhalation exposure (mg/kg bw/day) = [Air concentration (mg/m3) (24 h time-weighted average) * Inhalation rate (m3/day)] ÷ Body weight |
| Exposure scenario | Assumptions |
|---|---|
| Mixing, loading, and application of an all-purpose floor cleaner (liquid) (adult) (jasmine oil, lilial) |
Concentration: 1% jasmine oil (ACI 2018), 1% lilial Mixing and loading (dermal) Application (dermal) Product amount: 0.36 g {16.4 g/L concentration of product in cleaning solution * 21.85 mL [based on film thickness approach of 0.01 cm * exposed surface area of 2185 cm2 for hands (910 cm2) and forearms (½ arms 2550 cm2)], 1 mL = cm3} Total dermal exposure: Dermal mixing and loading + Dermal application Mix/load (inhalation) Application (inhalation) Air concentration (time-weighted average) = {[mean event concentration (mix/load) (mg/m3) * 0.75 min] + [mean event concentration (application) (mg/m3) * 240 min]} ÷ [total time 240.75 min / (24 h * 60 min)] Inhalation exposure (mg/kg bw/day) = [Air concentration (mg/m3) (24 h time-weighted average) * Inhalation rate (m3/day)] ÷ Body weight Combined exposure (mg/kg bw/day) = Total dermal exposure (mg/kg bw/day) + Total inhalation exposure (mg/kg bw/day) |
| Exposure from contacting cleaned floors (toddler) (jasmine oil, lilial) |
Concentration: 1% jasmine oil (ACI 2018), 1% lilial Calculations are based on the US EPA Residential SOPs (2012b), Section 7. Dermal Deposited residue (mg/cm2): calculated assuming 14 g of product per 22 m2 of floor (ConsExpo Cleaning Fact Sheet RIVM 2018) * 1 000 mg/g * 1 m2/10 000 cm2 Incidental oral (that is, hand-to-mouth exposure) HR: hand residue loading (mg/cm2); calculated using the following algorithm: Faihands: 0.15 (unitless); fraction of active ingredient on hands compared to total surface residue from jazzercise study FM: 0.13 (unitless); fraction of hand mouthed per event Combined exposure = Dermal + inhalation+ Incidental oral |
| Mixing, loading, and application of an all-purpose cleaner (aerosol) (adult) (jasmine oil) |
Concentration: 1% jasmine oil (ACI 2018) Inhalation-exposure to spray-spraying Inhalation during leave-on and wiping is expected to be minimal due to the low vapour pressure of jasmine oil.
Application (dermal) Wiping (dermal) Total combined exposure: Inhalation application + Dermal application + Dermal wiping |
| Mixing and loading a liquid laundry detergent for hand-washing, and hanging hand-washed clothes (adult) (lilial) |
Concentration: 1% lilial Mixing and loading (dermal) Washing (dermal) Hanging (dermal): 80.08 mg [calculated using a film-thickness approach, 8.8 mg/mL (concentration of regular liquid in washing water) * 910 cm2 (surface area of hands) * 0.01 cm] Total dermal exposure (mg/kg bw/day) = [Dermal (mixing/loading) (mg) + Dermal (washing) (mg) + Dermal (hanging) (mg)] * dermal absorption/Body weight Mixing and loading (inhalation) Washing (inhalation) Hanging (inhalation) Air concentration (24 h TWA) = {[mean air concentration – mixing and loading (mg/m3) * time (0.75 min)] + [mean air concentration – washing (mg/m3) * time (10 min)] + [mean air concentration – hanging (mg/m3) * time (240 min)]} / {[total time (250.75 min)] * [250.75 min * (24 hrs/60 min)]} Inhalation exposure (mg/kg bw/day) = [24 h TWA air concentration (mg/m3) * daily inhalation rate (m3/day)]/Body weight (kg) Total combined exposure (mg/kg bw/day) = total dermal exposure (mg/kg bw/day) + total inhalation exposure (mg/kg bw/day) |
| Mixing and loading a liquid for machine washing, hanging machine-washed clothes, and migration from washed clothes (adult) (jasmine oil) |
Concentration: 5% jasmine oil (ACI 2018) Mixing and loading (dermal) Hanging laundry (dermal) Mixing and loading (inhalation) Hanging (inhalation) Air concentration (24 h TWA) = {[mean air concentration – mixing and loading (mg/m3) * time (0.75 min)] + [mean air concentration – hanging (mg/m3) * time (240 min)]} / {[total time (250.75 min)] * [250.75 min * (24 h/60 min)]} Inhalation exposure (mg/kg bw/day) = (24 h TWA air concentration (mg/m3) * daily inhalation rate (m3/day)/Body weight (kg) Migration from machine-washed clothes (dermal) Weight fraction of product on textile: 7.6 x 10-4 (regular liquid) (RIVM 2018) Total dermal exposure = Dermal mixing and loading + Dermal hanging washed clothing + Dermal migration from clothes Total combined exposure (mg/kg bw/day) = total dermal exposure (mg/kg bw/day) + total inhalation exposure (mg/kg bw/day) |
| Migration from washed clothes (all subpopulations except adults) (see above) (jasmine oil) |
Concentration: 5% jasmine oil (ACI 2018) Migration from machine-washed clothes (dermal): Weight fraction of product on textile: 7.6 x 10-4 (regular liquid) (RIVM 2018) Incidental oral (mouthing of washed textiles) (1–2 yrs only) Surface residue (mg/cm2): see calculation above Combined exposure (1–2 yrs only) = Dermal migration from washed clothing + Incidental oral from mouthing washed clothing or textiles |
| Toilet bowl cleaner (adult) (lilial) |
Concentration: 1% lilial Application (dermal) Dermal exposure (mg/kg bw/day) = [product amount (mg) * dermal absorption]/body weight Application (inhalation) Inhalation exposure (mg/kg bw/day): [daily air concentration (mg/m3) * daily inhalation rate (m3/day)]/body weight Combined exposure (mg/kg bw/day) = dermal exposure (mg/kg bw/day) + inhalation exposure (mg/kg bw/day) |
| Carpet deodorizer (adult) (lilial) |
Concentration: 1% lilial Assessed as a carpet cleaner Application (dermal) Dermal exposure (mg/kg bw/day) = [product amount (mg) * dermal absorption]/body weight Inhalation Inhalation exposure (mg/kg bw/day) = [daily air concentration (mg/m3) * daily inhalation rate (m3/day)]/body weight Combined exposure (mg/kg bw/day) = dermal exposure (mg/kg bw/day) + inhalation exposure (mg/kg bw/day) |
| Exposure from contacting cleaned carpets (carpet deodorizer) (toddler) (lilial) |
Concentration: 1% lilial Calculations are based on the US EPA Residential SOPs (2012), Section 7. Dermal Deposited residue (mg/cm2): calculated assuming 2 200 g of product per 22 m2 of floor * 10% to account for vacuuming (ConsExpo Cleaning Fact Sheet, RIVM 2018) * 1 000 mg/g * 1 m2/10 000 cm2 Incidental oral (that is, hand-to-mouth exposure) HR: hand residue loading (mg/cm2); calculated using the following algorithm: HR = [Faihands * Dermal exposure (mg) (calculated above)] / (SAH * 2) Faihands: 0.15 (unitless); fraction of active ingredient on hands compared to total surface residue from jazzercise study FM: 0.13 (unitless); fraction of hand mouthed per event Combined exposure (mg/kg bw/day) = Dermal + Incidental oral + inhalation (calculated using the daily air concentration from application) |
Appendix B. Parameters for estimating dermal and inhalation exposures to Do-It-Yourself products
| Exposure scenario | Assumptions |
|---|---|
| DIY aromatic diffuser (violet oil, bay oil, tarragon oil, jasmine oil) |
Concentration: 100% violet oil,100% bay oil, 100% tarragon oil, 100% jasmine oil Product amount for the dermal exposure is based on approximately 2 drops of essential oil (professional judgment) being added to the stationary device (1 drop is equivalent to 0.05 mL)* the oil density Air concentrations were modelled using an evaporation–constant rate model (RIVM 2021b). Inhalation exposure (mg/kg bw/day) = [Air concentration (mg/m3) (24 h time-weighted average) * Inhalation rate (m3/day) * Frequency (365 times/365 (year))] ÷ Body weight For bay oil and tarragon oil, LADDs were also calculated using the following formula (HC 2013): |
| DIY massage oil (bay oil, tarragon oil, jasmine oil) |
Concentration: 3% bay oil, 3% tarragon oil, 3% jasmine oil Product amount: 3.2 g (adults); 2.9 g (14–18 yrs); 2.3 g (9–13 yrs); 1.9 g (4–8 yrs); 1.8 g (2–3 yrs, 1 yr, 6–11 mths, 0–5 mths) (Ficheux et al. 2016) Frequency: jasmine oil, 1 for all subpopulations. Dermal exposure (mg/kg bw/day) was calculated using the following formula: [mean product (g/application) * mean daily frequency * product concentration * dermal absorption * conversion factor (1000 mg/g)] ÷ Body weight Air concentrations were modelled using the ConsExpo exposure to vapour–evaporation–constant release area model. Inhalation exposure (mg/kg bw/day) = [Air concentration (mg/m3) (24 h time-weighted average) * Inhalation rate (m3/day) * Frequency (as above, for LADD calculation only)] ÷ Body weight Combined exposure (mg/kg bw/day) = Dermal exposure (mg/kg bw/day) + Inhalation exposure (mg/kg bw/day) For bay oil and tarragon oil, LADDs were also calculated using the following formula (HC 2013): LADD (mg/kg bw/day) = ((combined exposure (mg/kg bw/day) 0–5 mths * time in lifestage (0.5 yr)) + (combined exposure (mg/kg bw/day) 6–12 mths * time in lifestage (0.5 yr)) + (combined exposure (mg/kg bw/day) 1 yr * time in lifestage (1 yr)) + (combined exposure (mg/kg bw/day) 2–3 yrs * time in lifestage (2 yrs)) + (combined exposure (mg/kg bw/day) 4–8 yrs * time in lifestage (5 yrs)) + (combined exposure (mg/kg bw/day 9–13 yrs * time in lifestage (5 yrs)) + (combined exposure (mg/kg bw/day) 14–18 yrs * time in lifestage (5 yrs)) + (combined exposure (mg/kg bw/day) adult (19+) * time in lifestage (59 yrs))/Average lifetime (78 yrs) |
| DIY bath oil product (violet oil, bay oil, tarragon oil, jasmine oil) |
Concentration: 100% violet oil, 100% bay oil, 100% tarragon oil, 100% jasmine oil Product amount is based on approximately 10 drops of pure essential oil being added to a bath filled with 120 L water (1 drop is equivalent to 0.05 mL), multiplied by the oil density (RIVM 2006). Dermal product amount was calculated using a film-thickness approach where the volume of water that comes into contact with skin is equal to the surface area of the entire body, except the head, multiplied by the layer thickness of liquid film on the skin (0.01 cm; ECHA 2015). Frequency: 0.29 (all subpopulations) (calculation of LADD only) (Bremmer et al. 2006) Inhalation exposure (mg/kg bw/day) = [Air concentration (mg/m3) (24 h time-weighted average) * Inhalation rate (m3/day) * Frequency (calculation of LADD only)] ÷ Body weight Combined exposure (mg/kg bw/day) = Dermal exposure (mg/kg bw/day) + Inhalation exposure (mg/kg bw/day) For bay oil and tarragon oil, a LADD was also calculated using the following formula (HC 2013): LADD (mg/kg bw/day) = {[combined exposure (mg/kg bw/day) 9–13 yrs * time in lifestage (5 yrs)] + [combined exposure (mg/kg bw/day) 14–18 yrs * time in lifestage (5 yrs)] + [combined exposure (mg/kg bw/day) adult (19+) * time in lifestage (59 yrs)]}/Average lifetime (78 yrs) |
| DIY body moisturizer (violet oil, bay oil, tarragon oil, jasmine oil) |
Concentration: 3% violet oil, 3% bay oil (assuming the 0.002% maximum concentration of methyl eugenol permitted according to the cosmetics hotlist), 3% tarragon oil, 3% jasmine oil Product amount: 10 g (adults and 14–18 yrs); 7.7 g (9–13 yrs); 5 g (4–8 yrs); 4.1 g (2–3 yrs); 3.1 g (1 yr); 2.5 g (6–11 mths); 2 g (0–5 mths) (Ficheux et al. 2016) For bay oil, tarragon oil, and jasmine oil, dermal exposure (mg/kg bw/day) was calculated using the following formula: [mean product (g/application) * mean daily frequency * product concentration * dermal absorption * conversion factor (1000 mg/g)] ÷ Body weight Air concentrations were modelled using the ConsExpo exposure to vapour–evaporation–constant release area model. Inhalation exposure (mg/kg bw/day) = [Air concentration (mg/m3) (24 h time-weighted average) * Inhalation rate (m3/day) * Frequency (calculation of LADD only)] ÷ Body weight Combined exposure (mg/kg bw/day) = Dermal exposure (mg/kg bw/day) + Inhalation exposure (mg/kg bw/day) For bay oil and tarragon oil, LADD was also calculated using the following formula (HC 2013): LADD (mg/kg bw/day) = {[combined exposure (mg/kg bw/day) 0–5 mths * time in lifestage (0.5 yr)] + [combined exposure (mg/kg bw/day) 6–12 mths * time in lifestage (0.5 yr)] + [combined exposure (mg/kg bw/day) 1 yr * time in lifestage (1 yr)] + [combined exposure (mg/kg bw/day) 2–3 yrs * time in lifestage (2 yrs)] + [combined exposure (mg/kg bw/day) 4–8 yrs * time in lifestage (5 yrs)] + [combined exposure (mg/kg bw/day 9–13 yrs * time in lifestage (5 yrs)] + [combined exposure (mg/kg bw/day) 14–18 yrs * time in lifestage (5 yrs)] + [combined exposure (mg/kg bw/day) adult (19+) * time in lifestage (59 yrs)]}/Average lifetime (78 yrs) |
| DIY facial steamer (violet oil, jasmine oil) |
Concentration: 100% violet oil, 100% jasmine oil Product amount is based on approximately 10 drops of essential oil being added to the stationary device (1 drop is equivalent to 0.05 mL) * density (RIVM 2006; HC 2015). Frequency: assumed to be 1 (adults, 14–18 yrs, 9–13 yrs, 4–8 yrs) Air concentrations were modelled using the ConsExpo exposure to vapour–constant rate model. It is assumed that 50% of the mean event concentration will be inhaled and 50% of the mean event concentration will be exposed dermally. For inhalation exposure, after 20 minutes of facial steaming, it is assumed that a person remains in a 20 m3 room for 3 hours and 40 minutes. It is assumed that a 1-year-old bystander is present in the room for 4 hours. Inhalation exposure for 20 min (mg/kg bw/day) = {Mean event concentration (mg/m3) * 0.5 * [Inhalation rate (m3/day) * exposure time (20 min) ÷ (60*24)]* room volume} ÷ Body weight Dermal product amount: mean event concentration (mg/m3) * room volume (1 m3) * 0.5 Dermal exposure (mg/kg bw/day) was calculated using the following formula: [mean product amount in air (mg/application) * mean daily frequency * dermal absorption] ÷ Body weight Combined exposure (mg/kg bw/day) = Dermal exposure (mg/kg bw/day) + Inhalation exposure (mg/kg bw/day) Secondary inhalation exposure for 3 hours and 40 minutes: Total amount inhaled in 20 min (mg) = mean event concentration (mg/m3) * 0.5 * air inhaled in 20 min (m3) Inhalation exposure (mg/kg bw/day) for bystanders (1-year-old only) = Product amount in air after 20 min spread in the 20 m3 room (mg/m3) * Inhalation rate (m3/day) * [220 min ÷ (60 min/h * 24 h/day)] ÷ Body weight |