Screening assessment benzoates
Official title: Screening assessment benzoates
Chemical Abstracts Service Registry Numbers
93-58-3, 93-89-0, 120-50-3, 120-55-8, 136-60-7, 614-33-5, 8024-05-3, 27138-31-4, 68052-23-3
Environment and Climate Change Canada Health Canada
February 2019
Cat. No.: En14-356/2019E-PDF
ISBN 978-0-660-27792-9
Synopsis
Pursuant to sections 68 or 74 of the Canadian Environmental Protection Act, 1999 (CEPA), the Minister of the Environment and the Minister of Health have conducted a screening assessment of 9 of 10 substances referred to collectively under the Chemicals Management Plan as the Benzoates Group. These 9 substances were identified as priorities for assessment as they met categorization criteria under subsection 73(1) of CEPA or were considered a priority on the basis of other human health concerns. The tenth substance was subsequently determined to be of low concern for risk to ecological or human health and the decision for this substance is provided in a separate report.Footnote 1 Accordingly, this screening assessment addresses the 9 substances listed in the table below which will hereinafter be referred to as the Benzoates Group.
| CAS RNa | Domestic Substances List name | Common name |
|---|---|---|
| 93-58-3 | Benzoic acid, methyl ester | Methyl benzoate |
| 93-89-0b | Benzoic acid, ethyl ester | Ethyl benzoate |
| 120-50-3b | Benzoic acid, 2-methylpropyl ester | Isobutyl benzoate |
| 120-55-8 | Ethanol, 2,2’-oxybis-, dibenzoate | Diethylene glycol dibenzoate |
| 136-60-7 | Benzoic acid, butyl ester | Butyl benzoate |
| 614-33-5 | 1,2,3-Propanetriol, tribenzoate | Tribenzoin |
| 8024-05-3c | Oils, tuberose | Tuberose oil |
| 27138-31-4 | Propanol, oxybis-, dibenzoate | Dipropylene glycol dibenzoate |
| 68052-23-3 | 1,3-Pentanediol, 2,2,4-trimethyl-, dibenzoate | Trimethylpentanediyl dibenzoate |
a The Chemical Abstracts Service Registry Number (CAS RN) is the property of the American Chemical Society and any use or redistribution, except as required in supporting regulatory requirements and/or for reports to the Government of Canada when the information and the reports are required by law or administrative policy, is not permitted without the prior written permission of the American Chemical Society.
b This substance was not identified under subsection 73(1) of CEPA but was included in this assessment as it was considered a priority on the basis of other human health concerns.
c This substance is a UVCB (unknown or variable composition, complex reaction products, or biological materials).
All 9 substances in this assessment were included in a survey issued pursuant to a CEPA section 71 notice. Reported imported quantities ranged from 1 000 to 10 000 000 kg for 5 of the substances. Manufacturing and import activities were not reported for the 4 remaining substances.
Tribenzoin, tuberose oils, and methyl, ethyl, butyl, and isobutyl benzoates are used as food flavouring agents globally and are present in products available to consumers in Canada. These substances are also used as fragrance ingredients in household cleaning products and cosmetics. In addition to anthropogenic sources, methyl, ethyl, butyl, and isobutyl benzoates are naturally present in foods such as apples, bananas, sweet cherries, papayas, beer, cider, and cocoa.
Diethylene glycol dibenzoate, dipropylene glycol dibenzoate, and trimethylpentanediyl dibenzoate were identified in products including caulking, paint, and adhesives, as well as cosmetics and natural health products. Diethylene glycol dibenzoate and dipropylene glycol dibenzoate have also been identified as components in the manufacture of food packaging materials.
The ecological risks of the 9 benzoates in this screening assessment were characterized using the ecological risk classification of organic substances (ERC) approach, which is a risk-based approach that employs multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. Hazard profiles based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity are established. Metrics considered in the exposure profiles include potential emission rate, overall persistence, and long-range transport potential. A risk matrix is used to assign a low, moderate or high level of potential concern for substances on the basis of their hazard and exposure profiles. The ERC identified the nine benzoates in this screening assessment as having low potential to cause ecological harm.
Considering all available lines of evidence presented in this screening assessment, there is low risk of harm to the environment from the 9 benzoates addressed in this screening assessment. It is concluded that substances in the Benzoates Group do not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
The characterization of the health effects in this assessment takes into consideration empirical evidence that benzoates readily hydrolyze into benzoic acid, which is then further metabolized into hippuric acid and subsequently excreted. Accordingly, the evaluation of the benzoate esters in this assessment focuses on health effects data for benzoic acid and benzyl derivatives considered to metabolize to benzoic acid. Taking into consideration the assessments of other jurisdictions which have concluded that these substances and other similar substances show low toxicity, and given that the substances in this assessment metabolize to benzoic acid, the potential risk to human health is considered to be low.
On the basis of the information presented in this screening assessment, it is concluded that the substances in the Benzoates Group do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
Therefore, it is concluded that the substances in the Benzoates Group do not meet any of the criteria set out in section 64 of CEPA.
1. Introduction
Pursuant to sections 68 or 74 of the Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999), the Minister of the Environment and the Minister of Health have conducted a screening assessment of 9 of 10 substances, referred to collectively under the Chemicals Management Plan as the Benzoates Group, to determine whether these substances present or may present a risk to the environment or to human health. These 9 substances were identified as priorities for assessment as they met categorization criteria under subsection 73(1) of CEPA or were considered a priority on the basis of other human health concerns (ECCC, HC [modified 2017]).
One other substance, 1,3-benzenedicarboxylic acid (CAS RNFootnote 2 121-91-5) was considered in the ecological risk classification of organic substances (ERC) and the Threshold of Toxicological Concern (TTC)-based Approach for Certain Substances science approach documents (ECCC 2016a; Health Canada 2016) and was identified as being of low concern to both human health and the environment. As such, this substance is not further addressed in this report. Conclusions for this substance are provided in the Substances Identified as Being of Low Concern based on the Ecological Risk Classification of Organic Substances and the Threshold of Toxicological Concern (TTC)-based Approach for Certain Substances Screening Assessment (ECCC, HC 2018).
The other 9 substances are addressed in this screening assessment and will hereinafter be referred to as the Benzoates Group.
The ecological risks of the 9 substances in the Benzoates Group were characterized using the ERC approach (ECCC 2016a). The ERC describes the hazard of a substance using key metrics including mode of action, chemical reactivity, food-web derived internal toxicity thresholds, bioavailability, and chemical and biological activity, and it considers the possible exposure of organisms in the aquatic and terrestrial environments on the basis of factors including potential emission rates, overall persistence and long-range transport potential in air. The various lines of evidence are combined to identify substances as warranting further evaluation of their potential to cause harm to the environment or as having a low likelihood of causing harm to the environment.
The substances in the Benzoates Group are all esters of benzoic acid. The health effects assessment of the Benzoates Group has been separated into three subgroups: simple alkyl benzoates, dibenzoates and tribenzoates. The characterization of the hazard of all three subgroups is strongly linked with empirical evidence that members of all three subgroups will readily hydrolyze into benzoic acid, which is then further metabolized into hippuric acid and subsequently excreted (JECFA 1997; JECFA 2002a; OECD SIDS 2001; US EPA 2010; EFSA 2012; REACH 2016). As a consequence, the toxicological evaluation of the benzoate esters in this assessment is consistent with assessments from other jurisdictions in its focus on toxicological data relevant for benzoic acid and benzyl derivatives assumed to transform in vivo readily into benzoic acid, to identify key studies and critical endpoints.
Some substances in the Benzoates Group as well as benzoic acid and relevant benzyl derivatives were reviewed internationally through the Cooperative Chemicals Assessment Programme of the Organisation for Economic Cooperation and Development (OECD) and the Joint FAOFootnote 3 /WHOFootnote 4 Expert Committee on Food Additives (JECFA). These assessments undergo rigorous review (including peer review) and endorsement by international governmental authorities. Health Canada and Environment and Climate Change Canada are active participants in this process and consider these assessments reliable. Assessments have also been conducted by a number of other international bodies, including WHO, JECFA, the European Food Safety Authority (EFSA), and the European Commission Scientific Committee on Consumer Products (SCCP). These assessments (e.g., OECD SIDS Initial Assessment Report [SIAR], WHO Concise International Chemical Assessment Document [CICAD], JECFA reports) will be used to inform the health effects characterization in this screening assessment.
Taking into consideration the assessments of other jurisdictions (e.g., JECFA, OECD), which have concluded that these substances and other similar substances show low toxicity, and given that the substances in this grouping metabolize to benzoic acid, the potential risk to human health is considered to be low.
This screening assessment includes consideration of information on chemical properties, hazards, uses, and exposures, including additional information submitted by stakeholders. Relevant data were identified up to March 2017. Empirical data from key studies were used to reach conclusions. When available and relevant, information presented in assessments from other jurisdictions was considered.
This screening assessment was prepared by staff in the CEPA Risk Assessment Program at Health Canada and Environment and Climate Change Canada and incorporates input from other programs within these departments. The ecological portion of this assessment is based on the ERC document (published July 20, 2016), which was peer reviewed and subject to a 60-day public comment period. The human health portions of this assessment have undergone external review and/or consultation. Comments on the technical portions relevant to human health were received from Tetra Tech Inc. (Jennifer Flippin, Theresa Lopez, and Katherine Super). Additionally, the draft of this screening assessment (published December 30, 2017) was subject to a 60-day public comment period. While external comments were taken into consideration, the final content and outcome of the screening assessment remain the responsibility of Environment and Climate Change Canada and Health Canada.
This screening assessment focuses on information critical to determining whether substances meet the criteria as set out in section 64 of CEPA by examining scientific information and incorporating a weight–of-evidence approach and precaution.Footnote 5 This screening assessment presents the critical information and considerations on which the conclusions are based.
2. Identity of substances
The CAS RN, Domestic Substances List (DSL) names and common names for the individual substances in the Benzoates Group are presented in Table 2‑1. A list of additional chemical names (e.g., trade names) is available from the National Chemical Inventories (NCI 2015).
| CAS RN (acronym) | DSL name (common name) | Chemical structure and molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
| 93-58-3 | Benzoic acid, methyl ester (Methyl benzoate) | 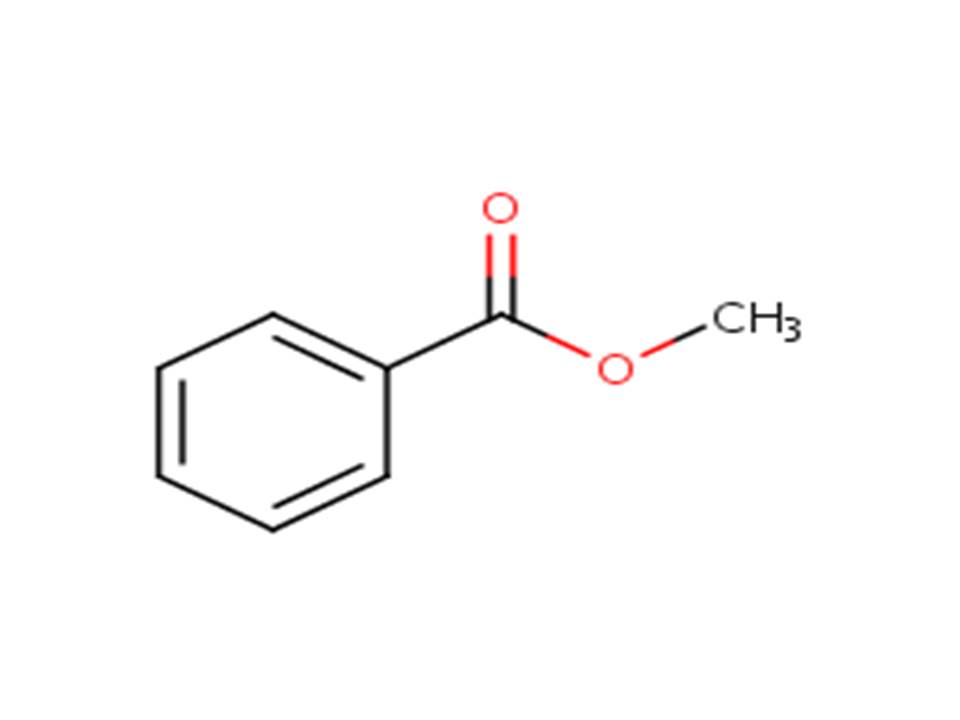 |
136.15 |
| 93-89-0 | Benzoic acid, ethyl ester (Ethyl benzoate) | 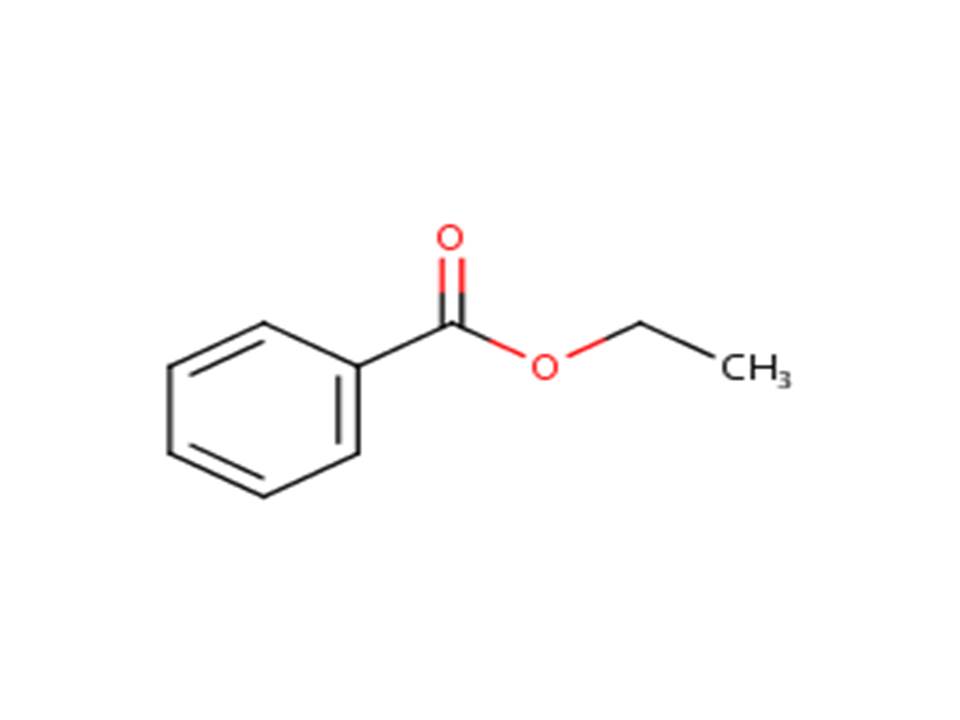 |
150.18 |
| 136-60-7 | Benzoic acid, butyl ester (Butyl benzoate) | 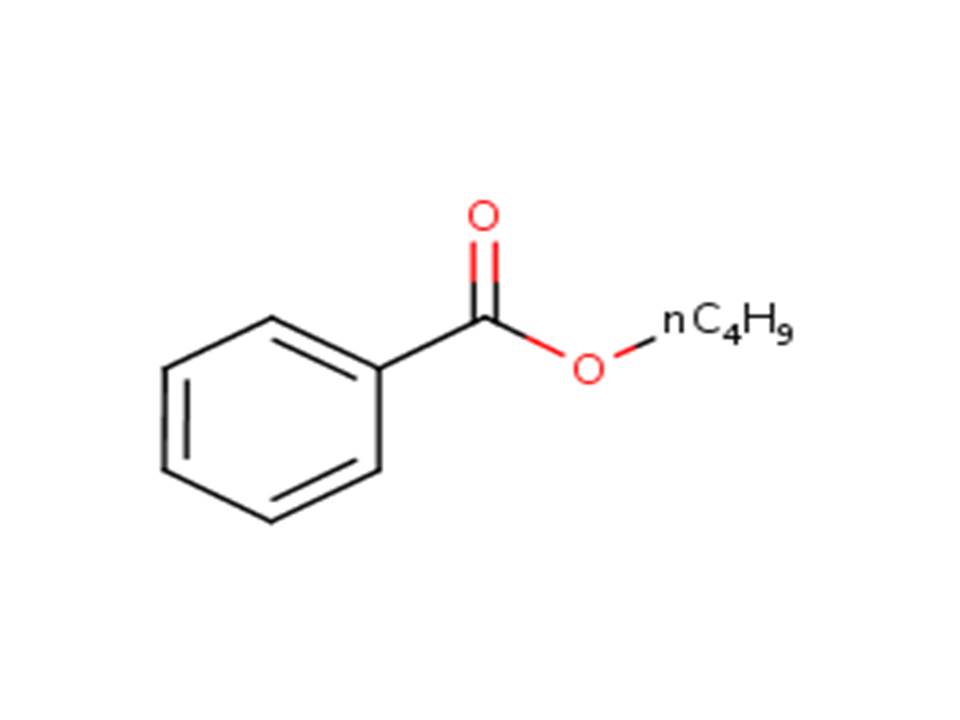 |
178.23 |
| 120-50-3 | Benzoic acid, 2-methylpropyl ester (Isobutyl benzoate) | 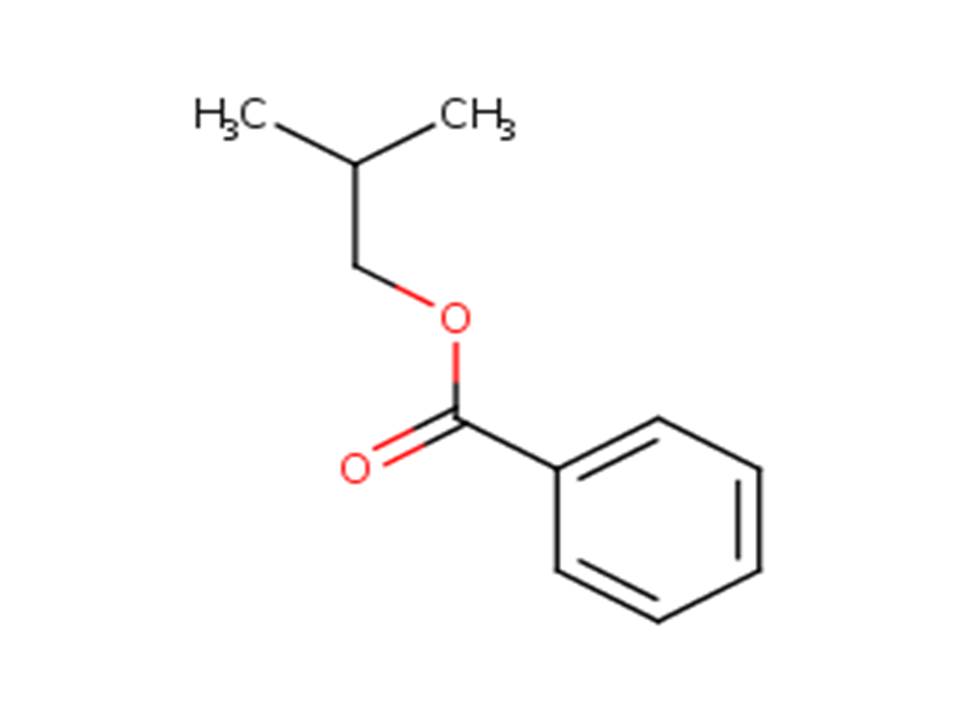 |
178.23 |
| 120-55-8 (DEGDB) | Ethanol, 2,2’-oxybis-, dibenzoate (Diethylene glycol dibenzoate) | 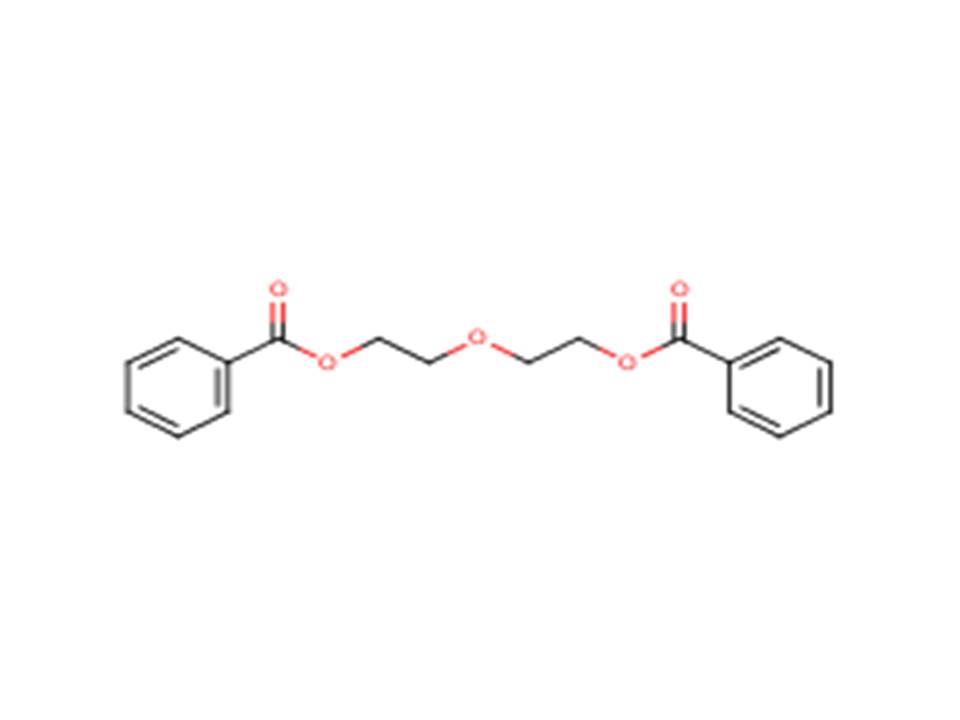 |
314.34 |
| 614-33-5 | 1,2,3-Propanetriol, tribenzoate (Tribenzoin) | 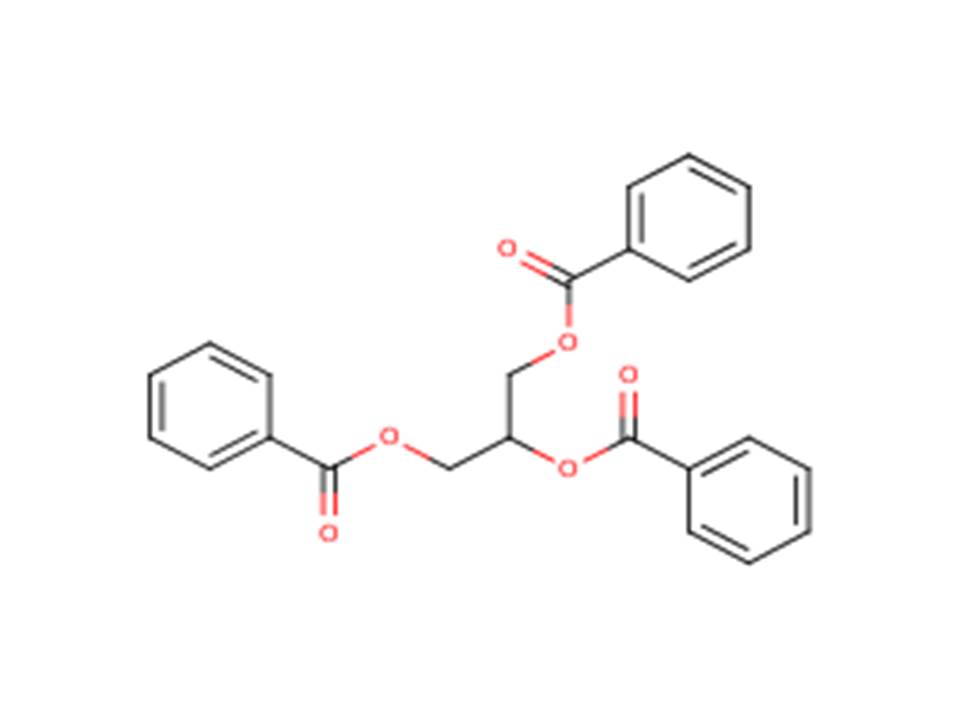 |
404.42 |
| 8024-05-3 | Oils, tuberose (Tuberose oil) | 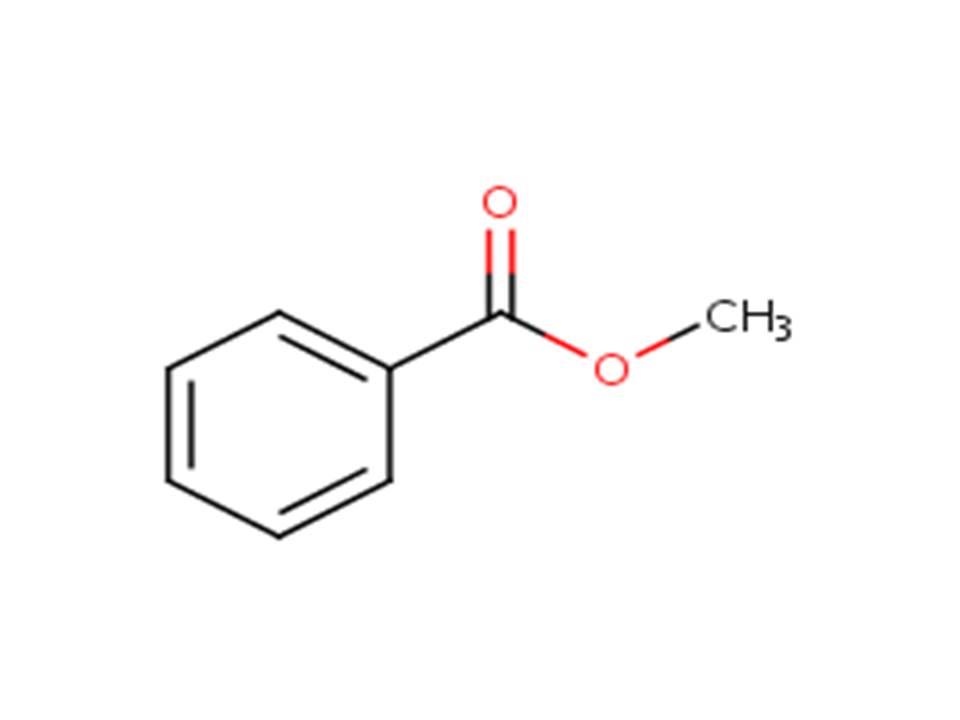 |
N/A |
| 27138-31-4 (DPGDB) | Propanol, oxybis-, dibenzoate (Dipropylene glycol dibenzoate | 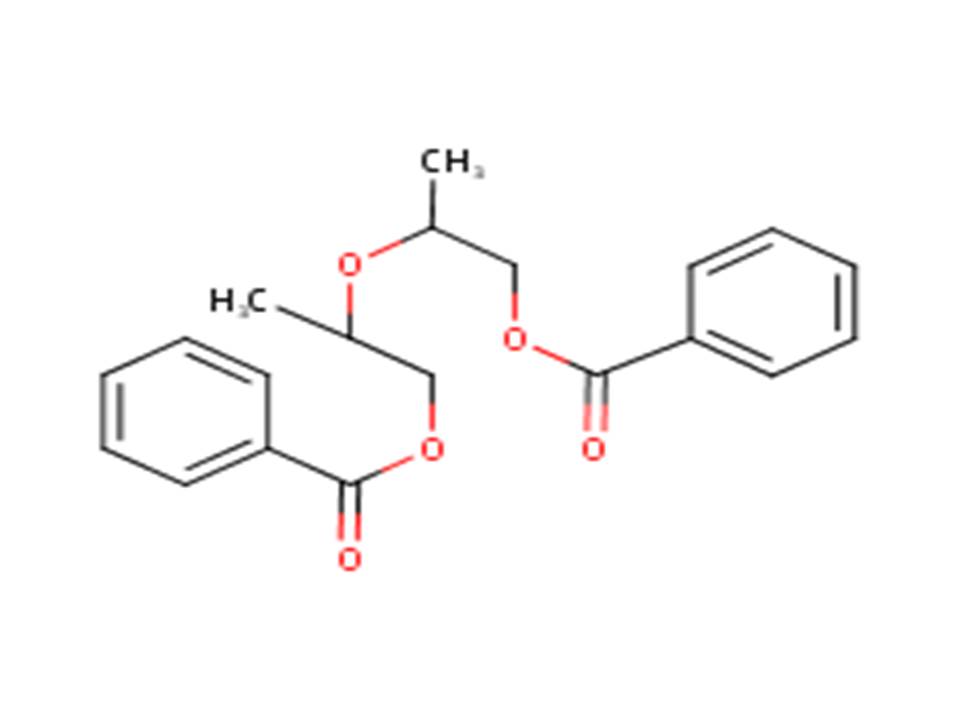 |
342.39 |
| 68052-23-3 (TMPD) | 1,3-Pentanediol, 2,2,4-trimethyl-, dibenzoate (Trimethylpentanediyl dibenzoate) | 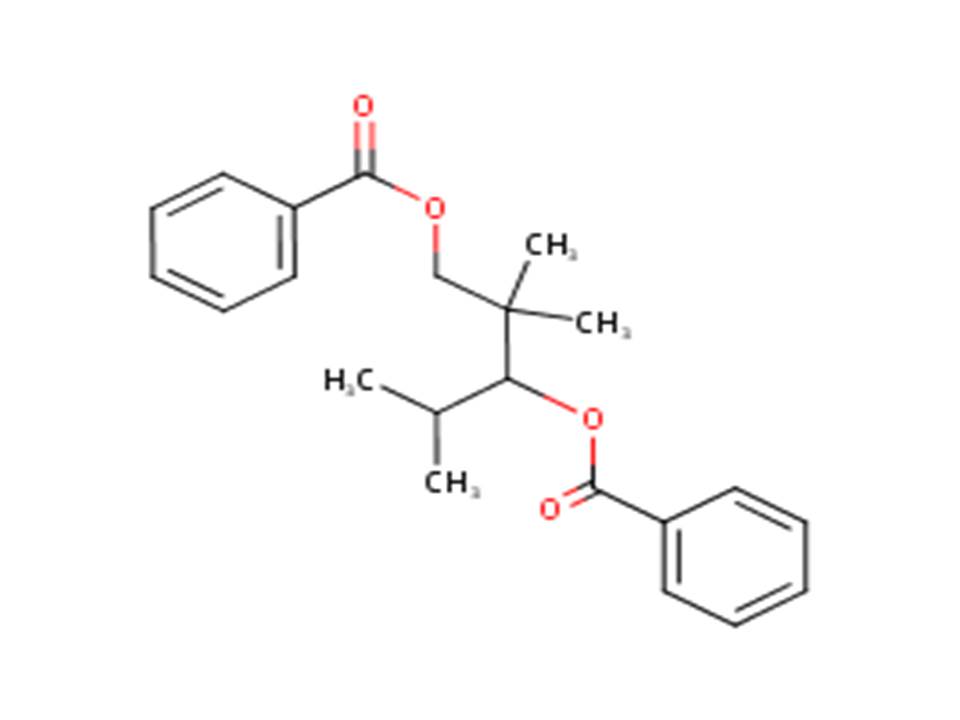 |
354.44 |
Abbreviations: N/A, not applicable
For the purposes of the assessment, the Benzoates Group has been separated into 3 subgroups: simple alkyl benzoates, dibenzoates, and tribenzoates. The simple alkyl benzoates subgroup is comprised of substances that contain a simple alkyl ester (i.e., methyl, ethyl, butyl, and isobutyl) of benzoic acid. The dibenzoates covered in this assessment are benzoate derivatives of ethylene/propylene glycols, and the tribenzoate substance is a triester of glycerol and benzoic acid. The 3 subgroups and substances within each are presented in Table 2‑2.
| CAS RN (acronym) | Common name | Subgroup |
|---|---|---|
| 93-58-3 | Methyl benzoate | simple alkyl benzoates |
| 93-89-0 | Ethyl benzoate | simple alkyl benzoates |
| 120-50-3 | Isobutyl benzoate | simple alkyl benzoates |
| 136-60-7 | Butyl benzoate | simple alkyl benzoates |
| 8024-05-3 | Tuberose oil | simple alkyl benzoates |
| 120-55-8 (DEGDB) | Diethylene glycol dibenzoate | dibenzoates |
| 27138-31-4 (DPGDB) | Dipropylene glycol dibenzoate | dibenzoates |
| 68052-23-3 (TMPD) | Trimethylpentanediyl dibenzoate | dibenzoates |
| 614-33-5 | Tribenzoin | tribenzoates |
One substance in the Benzoates Group, tuberose oil (CAS RN 8024-05-3), is considered a substance of unknown or variable composition, complex reaction products or biological materials (UVCB). Available information on the composition of tuberose oil, while very limited, indicates numerous potential constituent chemicals, with very few of the identified chemicals found to be present at levels comprising more than 5% to 10% (F&CT 2000; COE 2007; Rakthaworn et al. 2009). Rakthaworn et al. (2009) looked at the yields of tuberose oils from tuberose plant extracts obtained using various extraction techniques, and further analyzed their composition. The main components detected were esters of benzoic acid, in particular methyl benzoate, with compositions ranging from 30% to 44%. Similarly, Surburg and Panten (2016) found that methyl benzoate is used in perfume bases in tuberose extracts and that benzyl benzoate has been identified as a constituent chemical at relatively high concentrations in tuberose concretes and absolutes. The main constituents of tuberose absolute are cited in Surburg and Panten (2016) as esters of benzoic acid.
The subgrouping of the 9 benzoate substances is based on structural similarity and supports characterization of health effects of this group. Specifically, information on benzoic acid (see Table 2‑3), a metabolite of benzoate hydrolysis, informed the characterization of risk to human health.
| CAS RN | DSL name | Chemical structure and molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
| 65-85-0 | Benzoic acid | 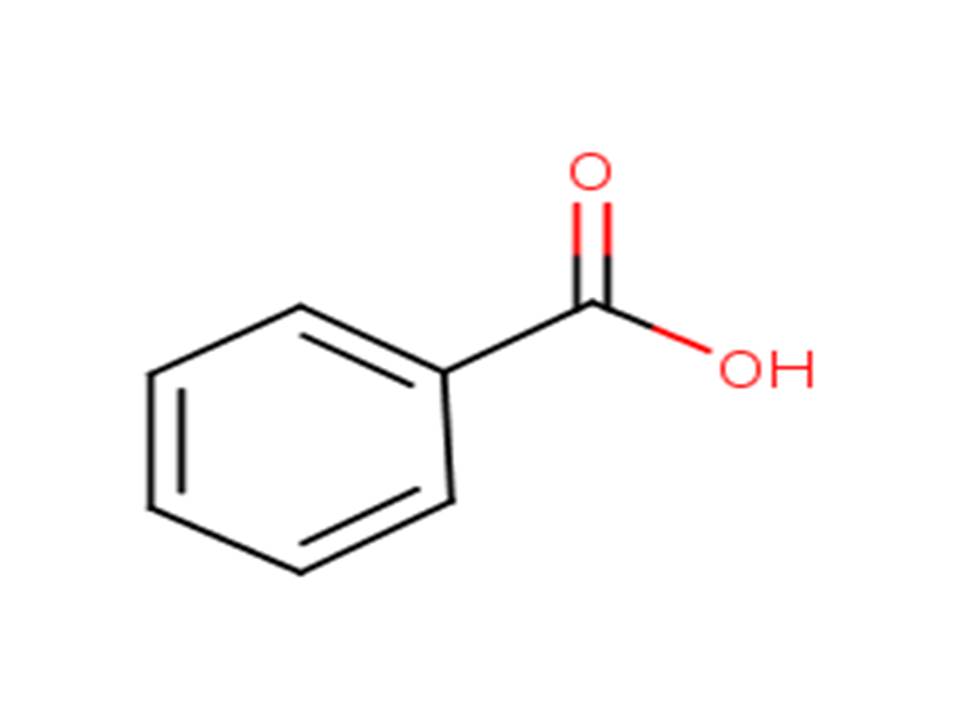 |
122.12 |
2.1 Selection of analogues and use of (Q)SAR models
A read-across approach using data from analogues and the results of (Q)SAR models, where appropriate, has been used to inform the ecological and human health assessments. Analogues were selected that were structurally and/or functionally similar to substances within this group (e.g., based on physical and chemical properties and toxicokinetics) and that had relevant empirical data that could be used to read across to substances that had limited or no empirical data to inform hazard characterization. The applicability of (Q)SAR models was determined on a case-by-case basis. Details of the read-across data and (Q)SAR models chosen to inform the ecological and human health assessments of the Benzoates Group, where applicable, are further discussed in the relevant sections of this report.
3. Physical and chemical properties
A summary of physical and chemical properties of the substances in the Benzoates group are presented in Tables 3-1 to 3-3, with the range in values indicated for each property. When experimental information was limited or not available for a property, predicted values for the substance were used. Additional physical and chemical properties data are presented in ECCC 2016b.
| Property | Range | Key references |
|---|---|---|
| Melting point (°C) | -34 to 11 | ChemIDplus 1993-a,b, EPI Suite 2000 |
| Vapour pressure (Pa) | 1.3–51 | ChemIDplus 1993-a,b, EPI Suite 2000 |
| Henry’s law constant (Pa·m3/mol) | 3.28–10.1 | ChemIDplus 1993-a,b, EPI Suite 2000 |
| Water solubility (mg/L) | 59–2100 | ChemIDplus 1993-a,b, EPI Suite 2000 |
| log Kow (dimensionless) | 2.1–3.8 | ChemIDplus 1993-a,b, EPI Suite 2000 |
Abbreviations: Kow, octanol–water partition coefficient
| Property | Range | Key references |
|---|---|---|
| Melting point (°C) | 11–57 | ChemIDplus 1993-c,d, EPI Suite 2000 |
| Vapour pressure (Pa) | 6.1 × 10−5–11 | ChemIDplus 1993-c,d, EPI Suite 2000 |
| Henry’s law constant (Pa·m3/mol) | 3.04 × 10−7–5.53 | ChemIDplus 1993-c,d, EPI Suite 2000 |
| Water solubility (mg/L) | 0.21–68 | ChemIDplus 1993-c,d, EPI Suite 2000 |
| log Kow (dimensionless) | 3.0–6.0 | ChemIDplus 1993-c,d, EPI Suite 2000 |
Abbreviations: Kow, octanol–water partition coefficient
| Property | Value | Key references |
|---|---|---|
| Melting point (°C) | 76 | ChemIDplus 1993e |
| Vapour pressure (Pa) | 4.0 × 10−6 | EPI Suite 2000 |
| Henry’s law constant (Pa·m3/mol) | 6.18 × 10−3 | EPI Suite 2000 |
| Water solubility (mg/L) | 0.26 | EPI Suite 2000 |
| log Kow (dimensionless) | 4.73 | EPI Suite 2000 |
Abbreviations: Kow, octanol–water partition coefficient
4. Sources and uses
Simple alkyl benzoates are naturally present in foods such as apples, bananas, sweet cherries, papayas, beer, cider, and cocoa (FooDB 2004). Tuberose oil is extracted and refined from the flowers of Polianthes tuberosa for use in perfumery as a source of aroma compounds (Rakthaworn et al. 2009). The global supply of tuberose oil appears to come entirely from Polianthes tuberosa extracts.
All four substances in the dibenzoate and tribenzoate Groups are commercially produced and do not occur naturally in the environment.
All 9 substances in this assessment have been included in a survey issued pursuant to CEPA section 71 notice (Canada 2012), and their reported total manufacture and total import quantities for 2011 are summarized in Table 4‑1.
| Common name | Total manufacturea (kg) | Total importsa,b (kg) |
|---|---|---|
| Methyl benzoate | N/R | N/R |
| Ethyl benzoate | N/R | N/R |
| Butyl benzoate | N/R | 1 000–10 000 |
| Isobutyl benzoate | N/R | N/R |
| Tuberose oil | N/R | N/R |
| Diethylene glycol dibenzoate | N/R | 1 000 000–10 000 000 |
| Dipropylene glycol dibenzoate | 1 500 | 1 100 000 |
| Trimethylpentanediyl dibenzoate | N/R | 10 000–100 000 |
| Tribenzoin | N/R | 1 000–10 000 |
Abbreviations: N/R – In a survey conducted under section 71 of CEPA 1999, no submissions reporting manufacturing/import quantities received.
a Values reflect quantities reported in response to the survey conducted under section 71 of CEPA (Environment Canada 2013). See survey for specific inclusions and exclusions (Schedules 2 and 3).
b The reporting of imported quantities as ranges is intentional and is designed to mask confidential business information.
Simple alkyl benzoates
While no definitive information is available concerning the presence of the simple alkyl benzoates as food flavourings in Canada, these substances may be present in foods sold in Canada as a result of their natural occurrence or possible use as food flavouring agents (personal communication from Food Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated November 2016; unreferenced). The United States permits the use of methyl, ethyl, and isobutyl benzoates as synthetic food flavouring substances and adjuvants (21 CFR 172.515; US FDA 2016a). Tuberose oil is also considered an essential oil, oleoresin, and natural extractive and is generally recognized as safe for use in food (US FDA 2016d). The European Union permits methyl, ethyl, butyl, and isobutyl benzoates to be used as flavouring agents in food (EFSA 2012).
Based on notifications submitted under the Cosmetic Regulations to Health Canada, methyl benzoate, ethyl benzoate, and tuberose oil are used in certain cosmetic products in Canada (summarized in Table 4‑2) (personal communication from Consumer Product Safety Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated November 2016; unreferenced).
Methyl, ethyl, and isobutyl benzoates are also identified as formulants in pest control products, including insect repellents, insecticides, arachnicides, and sanitizers (personal communication from Pest Management Regulatory Agency to Existing Substances Risk Assessment Bureau, Health Canada, November 2016; unreferenced).
All substances in the simple alkyl benzoates subgroup may be used as fragrance ingredients in household cleaning products, but the specific types of products and concentrations in products are not specified (Clorox 2017; IFRA 2011). Butyl benzoate may also be used as a dye carrier during textile dyeing operations (HSDB 1983- ). However, no information was identified to determine the extent to which this use may occur in Canada. Table 4‑2 provides a summary of potential uses in Canada for simple alkyl benzoates.
Methyl benzoate is listed in the Licensed Natural Health Products Database (LNHPD) as a non-medicinal ingredient, in two discontinued licensed topical natural health products (personal communication from Natural and Non-prescription Health Products Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated June 2018; unreferenced).
| Use | 93-58-3 | 93-89-0 | 120-50-3 | 136-60-7 | 8024-05-3 |
|---|---|---|---|---|---|
| Cleaning productsa | Y | Y | Y | N | N |
| Food flavouring agentsb | Y | Y | Y | Y | Y |
| Notified to be present in cosmetics under the Cosmetic Regulationsc | Y | Y | N | N | Y |
| Formulant in registered pest control productsd | Y | Y | Y | N | N |
| Textilese | N | N | N | Y | N |
Abbreviations: Y= use was reported for this substance; N= use was not reported for this substance
a Cleaning products include the consumer code categories for cleaning and furnishing care and/or laundry and dishwashing (Canada 2012; Clorox 2017; Environment Canada 2013; IFRA 2011).
b Personal communication from Food Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated November 2016; unreferenced).
c Based on notifications submitted under the Cosmetic Regulations Health Canada (personal communications, emails from Consumer Product Safety Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated February 2017; unreferenced.
d Personal communications, emails from Pest Management Regulatory Agency to Existing Substances Risk Assessment Bureau, Health Canada, dated November 2016; unreferenced.
e HSDB 1983- .
Dibenzoates
In Canada DPGDB and DEGDB have been identified as components of adhesives used in the manufacture of cartons and corrugated boxes to package some foods (personal communication from Food Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated November 2016; unreferenced).
Based on notifications submitted under the Cosmetic Regulations to Health Canada, DPGDB and TMPD are used in certain cosmetic products in Canada (personal communication from Consumer Product Safety Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated November 2016; unreferenced).
DEGDB and DPGDB are identified as formulants in pest control products intended for remedial wood preservation (personal communication from Pest Management Regulatory Agency to Existing Substances Risk Assessment Bureau, Health Canada, dated November 2016; unreferenced).
All dibenzoates are identified as plasticizers in products available to consumers, including adhesives and sealants, paints and coatings, and plastic and rubber materials (SDS 2011; SDS 2014a; SDS 2014b; SDS 2015a; TDS 2015), as well as in commercial inks, toners and colourants (Environment Canada 2013; Pubchem 1988; TDS 2015). Specifically, DPGDB and DEGDB are present in caulking and sealant materials at concentrations up to 10% (SDS 2014c; SDS 2015b), and TMPD is used in the automotive manufacturing process (Environment Canada 2013). DPGDB may be used as an additive in paints and coatings intended for indoor waterproofing (concentration 6%) and in paint found in craft kits intended for children, at concentrations up to 7% (Environment Canada 2013; SDS 2011; SDS 2014b). TMPD was reported in paints with no consumer use (Environment Canada 2013) and DEGDB was identified for use in automotive underbody coatings (Eastman 2016; SDS 2014a).
DEGDB and DPGDB were also identified in lubricants and greases, but no evidence of consumer use was identified (Environment Canada 2013). DPGDB was also identified as a plasticizer in certain cement floor resurfacing agents and as an anti-adhesive in mould making resins. However, these uses are limited to industrial settings (Environment Canada 2013).
DPGDB is listed in the LNHPD as being present as a non-medicinal ingredient in a limited number of topical sunscreen products in Canada (personal communication from Natural and Non-prescription Health Products Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated November 2016; unreferenced). Table 4‑3 provides a summary of potential uses in Canada for dibenzoates.
| Use | 120-55-8 | 27138-31-4 | 68052-23-3 |
|---|---|---|---|
| Food packaging materialsa | Y | Y | N |
| Medicinal or non-medicinal ingredients in licensed natural health productsb | N | Y | N |
| Notified to be present in cosmetics under the Cosmetic Regulationsc | N | Y | Y |
| Formulant in registered pest control productsd | Y | Y | N |
| Products available to consumerse | Y | Y | Y |
Abbreviations: Y= use was reported for this substance; N= use was not reported for this substance
a Personal communications, email from Food Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated November 2016; unreferenced.
b Personal communications, email from Natural and Non-prescription Health Products Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated November 2016; unreferenced.
c Based on notifications submitted under the Cosmetic Regulations Health Canada (personal communications, emails from Consumer Product Safety Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated November 2016 and February 2017; unreferenced).
d Personal communications, emails from Pest Management Regulatory Agency to Existing Substances Risk Assessment Bureau, Health Canada, dated November 2016; unreferenced).
e Environment Canada 2013, Eastman 2016, SDS 2011, SDS 2014a, SDS 2015-a,b, TDS 2013, TDS 2015.
Tribenzoates
The European Union permits tribenzoin to be used as a flavouring agent in food (EFSA 2012). No information is available concerning its potential use as a food flavouring agent in Canada (personal communication from Food Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated February 2017; unreferenced).
Based on notifications submitted under the Cosmetic Regulations to Health Canada, tribenzoin is used in certain products in Canada (personal communication from Consumer Product Safety Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated March 2017; unreferenced).
Tribenzoin is not identified as a formulant or active ingredient in pest control products registered in Canada (personal communication from Pest Management Regulatory Agency to Existing Substances Risk Assessment Bureau, Health Canada, dated November 2016; unreferenced).
Tribenzoin is not listed in the Therapeutic Products Directorate’s internal Drug Product Database as a medicinal or non-medicinal ingredient in disinfectant, human or veterinary drug products in Canada (personal communications from Therapeutic Products Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated November 2016; unreferenced), nor is it listed in the LNHPD as being present in currently licensed natural health products in Canada (personal communication from Natural and Non-prescription Health Products Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated November 2016; unreferenced).
5. Potential to cause ecological harm
5.1 Characterization of ecological risk
The ecological risks of the 9 benzoate substances in this screening assessment were characterized using the ecological risk classification of organic substances (ERC) approach (ECCC 2016a). The ERC is a risk-based approach that considers multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. The various lines of evidence are combined to discriminate between substances of lower or higher potency and lower or higher potential for exposure in various media. This approach reduces the overall uncertainty with risk characterization compared to an approach that relies on a single metric in a single medium (e.g., LC50) for characterization. Since tuberose oil is a UVCB substance and could not be suitably represented by a single chemical structure, a manual judgement-based approach to classification was used. The following summarizes the approach, which is described in detail in ECCC (2016a).
Data on physical-chemical properties fate (chemical half-lives in various media and biota, partition coefficients, fish bioconcentration), acute fish ecotoxicity, and chemical import or manufacture volume in Canada were collected from scientific literature, from available empirical databases (e.g., OECD QSAR Toolbox), and from responses to surveys under CEPA section 71 or were generated using selected quantitative structure-activity relationship (QSAR) or mass-balance fate and bioaccumulation models. These data were used as inputs to other mass-balance models or to complete the substance hazard and exposure profiles.
Hazard profiles were established primarily on the basis of mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Exposure profiles were based on multiple metrics, including potential emission rate, overall persistence, and long-range transport potential. Hazard and exposure profiles were compared to decision criteria in order to classify the hazard and exposure potentials for each organic substance as low, moderate, or high. Additional rules were applied (e.g., classification consistency, margin of exposure) to refine the preliminary classifications of hazard or exposure. However, in the case of the tuberose oil, hazard and exposure could not be fully profiled because of the lack of a representative structure to estimate needed properties and the lack of empirical data for these properties. Therefore, manual classification of hazard and exposure was performed through examination of the UVCB constituents and information obtained from section 71 surveys under CEPA, and decisions were based on consideration of similar substances and application of expert judgement.
A risk matrix was used to assign a low, moderate or high classification of potential risk for each substance on the basis of its hazard and exposure classifications. ERC classifications of potential risk were verified using a two-step approach. The first step adjusted the risk classification outcomes from moderate or high to low for substances that had a low estimated rate of emission to water after wastewater treatment, representing a low potential for exposure. The second step reviewed low risk potential classification outcomes using relatively conservative, local-scale (i.e., in the area immediately surrounding a point-source of discharge) risk scenarios, designed to be protective of the environment, to determine whether the classification of potential risk should be increased.
ERC uses a weighted approach to minimize the potential for both over- and under-classification of hazard, exposure, and subsequent risk. The balanced approaches for dealing with uncertainties are described in greater detail in ECCC (2016a). The following describes two of the more substantial areas of uncertainty. Error in empirical or modeled acute toxicity values could result in changes in classification of hazard, particularly metrics relying on tissue residue values (i.e., mode of toxic action), many of which are predicted values from QSAR models. The impact of this error is mitigated, however, by the fact that overestimation of median lethality will result in a conservative (protective) tissue residue used for critical body residue (CBR) analysis. Error in underestimation of acute toxicity will be mitigated through the use of other hazard metrics such as structural profiling of mode of action, reactivity and/or estrogen binding affinity. Changes or errors in chemical quantity could result in differences in classification of exposure as the exposure and risk classifications are highly sensitive to emission rate and use quantity. The ERC classifications thus reflect exposure and risk in Canada based on what is believed to be the current use quantity, and may not reflect future trends.
Critical data and considerations used to develop the substance-specific profiles for the 9 substances in the Benzoates Group and the hazard, exposure and risk classification results are presented in ECCC (2016b).
The hazard and exposure classifications for the 9 substances in the Benzoates Group are summarized in Table 5-1.
| Common name | ERC hazard classification | ERC exposure classification | ERC risk classification |
|---|---|---|---|
| Methyl benzoate | low | low | low |
| Ethyl benzoate | low | low | low |
| Isobutyl benzoate | low | low | low |
| Diethylene glycol dibenzoate | low | low | low |
| Butyl benzoate | low | low | low |
| Tribenzoin | low | low | low |
| Tuberose oil | low | high | low |
| Dipropylene glycol dibenzoate | low | low | low |
| Trimethylpentanediyl dibenzoate | low | moderate | low |
Tribenzoin was classified as having a low hazard potential despite modelled data indicating that it has a high potential for reactivity with biological tissues. However, these modelled outputs are not supported by the information from empirical studies identified in the human health portion of this assessment, which indicate that tribenzoin is metabolized to benzoic acid, a substance that has low hazard properties in humans. There is some uncertainty regarding the metabolic differences between aquatic systems and humans; however, considering the above information and information characterizing tribenzoin and benzyl derivatives in the health effects section, fluctuations in use patterns are unlikely to result in a significant increase in risk.
According to information considered under the ERC approach, tuberose oil was classified as having a high exposure potential due to large use quantities and a long overall persistence. Trimethylpentanediyl dibenzoate was classified as having a moderate exposure potential due to moderate use quantities and a long overall persistence. Methyl benzoate, ethyl benzoate, isobutyl benzoate, diethylene glycol dibenzote, butyl benzoate and dipropylene glycol dibenzoate were classified as having low exposure potential. These eight substances were classified as having low hazard potential and therefore, low potential for overall ecological risk. It is therefore unlikely that these substances result in concerns for the environment in Canada.
6. Potential to cause harm to human health
6.1 Exposure assessment
Environmental media and food
No Canadian or recent international data for levels in ambient air, drinking water, food, or soil were identified for the 9 substances in this assessment.
Empirical indoor air measurements were identified for methyl, ethyl and isobutyl benzoates from residential homes across Canada as part of the Canadian Health Measures Survey: Cycle 2 (Patry-Parisien et al. 2013). These substances were detected at low frequencies and at low concentrations in indoor air (maximum concentrations ranged from 0.18 to 0.54 µg/m3 and detection frequencies ranged from 0.03% to 6%). Therefore, potential exposure to simple alkyl benzoates may occur as a result of their presence in indoor air. For dibenzoates, DEGDB was not detected in a Canadian study of residences with asthmatic children (NRC 2011).
Some of the simple alkyl benzoates included in this assessment occur naturally in certain foods (see Sources and Uses section). In addition, all simple alkyl benzoates and tribenzoin may be added to food as flavouring agents; however, no definitive information is available concerning the potential use of these substances as food flavouring agents in Canada. There is therefore the potential for dietary exposure to simple alkyl benzoates and tribenzoin from their possible use as food flavouring agents and/or from consuming foods that naturally contain them.
In Canada, DPGDB and DEGDB have been identified as components of adhesives used in the manufacture of cartons and corrugated boxes to package certain foods. However, there is no direct contact of the packaging adhesive with those foods (personal communication from Food Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated November 2016; unreferenced).
Products available to consumers
According to notifications submitted under the Cosmetic Regulations to Health Canada, methyl and ethyl benzoate, tuberose oils, DPGDB, TMPD, and tribenzoin are present in cosmetics in Canada (personal communication from Consumer Product Safety Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated March 2017; unreferenced). There is therefore the potential for dermal exposure to the aforementioned substances from the use of certain cosmetic products.
Do-it-yourself products available to Canadian consumers that may contain substances in the dibenzoates subgroup include caulking and paint intended for indoor use (Environment Canada 2013; SDS 2015b; SDS 2014c; SDS 2014b). DPGDB is also present in paints packaged in craft kits intended for children at a concentration of 7% (Environment Canada 2013; SDS 2011). Methyl, ethyl, butyl, and isobutyl benzoates are used as fragrance ingredients in unspecified household cleaning products (Clorox 2017; IFRA 2011). There is therefore the potential for dermal and/or inhalation exposure of the general population of Canada from use of these types of products.
Other products, outlined in the Sources and Uses section (inks, toners, colourants, etc.), containing substances in the Benzoates Group are primarily used in industrial settings or in commercial products and are not expected to result in exposure to consumers (Environment Canada 2013; HSDB 1983- ; SDS 2014a; TDS 2015b).
Elsewhere, two of the benzoate compounds (isobutyl benzoate and ethyl benzoate) were detected in a small proportion of electronic cigarette liquids in a German study (Hutzler et al. 2014).
6.2 Health effects assessment
For the health effects assessment, the Benzoates Group has been separated into three subgroups. However, the hazard characterization is consistent with empirical evidence that members of all three subgroups will readily hydrolyze into benzoic acid, which is then further metabolized into hippuric acid and subsequently excreted (JECFA 1997; JECFA 2002a; OECD SIDS 2001; US EPA 2010; EFSA 2012; ECHA 2000a). Accordingly, the evaluation of the benzoate esters in this assessment focuses on health effects data for benzoic acid and for benzyl derivatives considered to metabolize to benzoic acid. Taking into consideration the assessments of other jurisdictions (JECFA, OECD), which have concluded that these substances and other similar substances show low toxicity, and given that the substances in this grouping metabolize to benzoic acid, the potential risk to human health is considered to be low.
Toxicokinetics
The absorption, distribution, metabolism, and elimination (ADME) of benzoate esters has been described in a number of assessments conducted internationally,Footnote 6 as well as in Cosmetic Ingredient Reviews (CIR) (Becker et al. 2012) and Flavor and Extract Manufacturers Association (FEMA) Generally Recognized as Safe (GRAS) assessments (e.g., Adams et al. 2005).
These assessments often look at benzyl derivatives more broadly, including benzyl alcohol, benzaldehyde, benzyl acetate, and benzoic acid and its salts (e.g., sodium benzoate). Benzyl derivatives, which include benzoate esters, are rapidly absorbed through the gut and hydrolyzed in the liver. These substances are frequently grouped together in assessments as it is generally accepted that they all metabolize to benzoic acid and the corresponding alcohol (e.g., methyl benzoate to benzoic acid and methanol) (JECFA 1997; JECFA 2001; JECFA 2002a; Becker et al. 2012). Benzoic acid is then conjugated with glycine, primarily in the liver, and excreted within 24 hours, predominantly as hippuric acid (JECFA 2001). EFSA (2012) discussed the toxicokinetics of benzyl derivatives and concluded that after oral administration more than 95% of benzoic acid is absorbed, metabolized and rapidly excreted. Major metabolites identified include hippuric acid (70.2% to 84.2%), benzoic acid (0.4% to 12.8%), and benzoyl glucuronide (0.7% to 1.8%) (OECD 2011; EFSA 2012).
For benzoates, the empirical evidence of dermal absorption and skin metabolism, although limited, indicates that benzoates will be systemically available and subject to enzyme-driven hydrolysis (Xia et al. 2007; Becker et al. 2012; ECHA 2015a).
Dibenzoates and tribenzoates
The toxicokinetics of some members of the dibenzoates and tribenzoates has also been evaluated. Tribenzoin (CAS RN 614-33-5) is anticipated to hydrolyze rapidly to benzoic acid (JECFA 2001; JECFA 2002a). The substances in the dibenzoates subgroup were not explicitly considered by either JECFA or EFSA as they do not have food additive uses; however, a supporting substance, propylene glycol dibenzoate (CAS RN 19224-26-1), was considered to follow the same metabolic pathways as the other benzoate esters (JECFA 2002a; JECFA 2002b; EFSA 2012). More information on the toxicokinetics of dibenzoates is provided below.
As part of a submission under Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) for both DEGDB and DPGDB (ECHA 2000a), the toxicokinetics of DEGDB was observed in CD-1 rats administered via oral gavage with a single low dose of 50 mg/kg and a high dose of 750 mg/kg. According to the study, the major metabolites identified were hippuric acid, benzoic acid, and diethylene glycol monobenzoate. The major metabolite in urine was hippuric acid, which represented 94% to 97% of the 50 mg/kg dose and 85% to 88% of the 750 mg/kg dose. At the low dose, a glucuronide of benzoic acid was identified at 1%, whereas at the high dose it was observed at 6% to 9%. The authors therefore concluded that the metabolism of DEGDB proceeded via hydrolysis to form benzoic acid, which was further conjugated with glycine (generating the hippuric acid metabolite) and to a lesser extent via a glucuronic acid pathway (ECHA 2000a). An older study on the toxicokinetics of DPGDB (i.e., technical grade Benzoflex 9-88 plasticizer administered to rats) also reported that the substance is rapidly metabolized and excreted, with 70% of the dose excreted via urine (within 48 hours) as hippuric acid and 10% excreted via feces (Butz et al. 1982).
For TMPD, no toxicokinetics information was available. However, using the metabolic simulator model OASIS TIMES (TIMES 2016), it was predicted in a rat in vivo simulation that this substance would follow metabolic pathways consistent with the other dibenzoates (i.e., DEGDB and DPGDB) in this subgroup, and the benzoates class of compounds more generally. OASIS TIMES identified the two primary metabolites as being hippuric acid and glucuronide derivatives of benzoic acid (e.g., benzoyl glucuronide). Modeling work done on DEGDB in OASIS TIMES indicated a similar metabolic profile for this substance.
6.2.1 Hazard characterization of benzoic acid
The health effects of benzoic acid have been reviewed and assessed, alone and with other benzyl derivatives, by a number of international organizations, including JECFA (1996, 2001), WHO (2005) and the OECD (2001). In addition, the US EPA, the European Commission’s Scientific Committee for Food, and the Scientific Committee on Consumer Products (SCCP) have all undertaken assessments on benzoic acid and sodium benzoate (US EPA 2010; SCF 1994; SCF 2002; SCCP 2005).
A 21-day dermal toxicity study in New Zealand White rabbits (4 males/4 females per group, exposed 5 days a week for 3 weeks) with benzoic acid administered at 0, 100, 500 or 2 500 mg/kg bw/d identified a NO(A)EL at the highest dose tested, i.e., 2 500 mg/kg bw/d. With the exception of minor dermal irritation noted in one rabbit in the high dose group, no treatment-related effects were observed (IRDC 1981). Low acute dermal and inhalation toxicity of these compounds is anticipated on the basis of available data (OECD 2001). EFSA (2016) also undertook a re-evaluation of benzoic acid and its salts and found that benzoic acid exhibited a low acute toxicity and that the results of available short-term and subchronic oral studies “indicated that the toxicity of benzoic acid and its salts is low with no marked target organ toxicity.” Benzoic acid is slightly irritating to the skin and irritating to the eyes but is not considered dermally sensitizing (OECD 2001; SCCP 2005; WHO 2005).
In repeat dose chronic studies, the toxicity of benzoic acid was observed to be low. In a four-generation study by Kieckebusch and Lang (1960), reported as a key study by various international organizations (e.g., OECD 2001; SCF 2005; WHO 2005), 20 rats per sex per group were fed diets containing 0.5% or 1.0% benzoic acid. The estimated intake based on diets containing 0.5% and 1% benzoic acid was 375 mg/kg/d and 750 mg/kg/d, respectively. The NOAEL in this study is reported as 750 mg/kg/day (1.0% benzoic acid in diet). However, other reviewers (e.g., SCF 2005; WHO 2005; EFSA 2016) indicate that this value of 750 mg/kg/d represents a NOAEL on a body-weight-per-day basis of 500 mg/kg bw/d. The OECD report on this study indicates that in all 4 generations, no effects on growth or organ weights were found, and there were no histopathological findings in animals from the third generation (OECD 2001).Footnote 7 Overall, the OECD (2001) determined that a NOAEL of 800 mg/kg/day could be derived on the basis of the results from a variety of repeat dose oral chronic studies available in its Benzoate SIAR grouping; a NOAEL of >1 000 mg/kg/day was identified as suitable for salts of benzoic acid. In conclusion, the OECD (2001) stated that “...benzoic acid and its salts exhibit very low repeat dose toxicity.”
The OECD (2001) concluded in its assessment of benzoates, including benzoic acid, that “…the compounds exhibit no carcinogenicity.” JECFA (1996, 2001) reviewed the results of oral chronic and carcinogenicity studies for mice and rats for a number of benzyl derivatives (all assumed to metabolize readily to benzoic acid in vivo) and determined that “…the data reviewed were sufficient to demonstrate lack of carcinogenic potential” (JECFA 2001).
A number of reviews on the in vivo and in vitro genotoxicity data of both benzoic acid and its related benzyl derivatives have been conducted by a number of agencies (e.g., SCF 1994; JECFA 1996; JECFA 2001; OECD 2001; SCCP 2005; WHO 2005; EFSA 2016). For these substances, OECD (2001) indicated that all chemicals showed no mutagenic activity in in vitro Ames tests and mixed/equivocal chromosomal responses in vitro. However, no genotoxicity was observed in a number of in vivo assays, and it was concluded on the basis of available information that the substances are not mutagenic or clastogenic in in vitro and in vivo assays (OECD 2001). JECFA (2001) also noted that benzoic acid was not mutagenic in the Ames assay, but that some clastogenicity (weak) was observed. However, this result was not reproduced in in vivo assays. EFSA re-evaluated the genotoxicity data, in particular the weakly positive clastogenicity results, and looked at studies not reviewed in previous assessments. It concluded that benzoic acid and its salts did not show genotoxic effects (EFSA 2016).
EFSA (2016) reviewed the available information on reproductive and developmental toxicity of benzoic acid and its salts and considered the previously mentioned four-generation reproductive toxicity study (Kieckebusch and Lang, 1960) to be the key study. The NO(A)EL derived from this study was 500 mg/kg bw/d, which was the highest dose tested, for both parental and offspring animals, with no effects on growth, fertility, lactation or survival observed at this dose (EFSA 2016). The OECD (2001), SCCP (2005), and WHO (2005) also considered this to be the key study for reproductive and developmental toxicity, and the OECD noted that at the low dose, administration led to a prolongation of life relative to controls. OECD (2011) concluded that available data was sufficient to “demonstrate lack of reprotoxic potential” for benzoates. Similarly, the WHO (2005) considered that available information “supports the notion that benzoic acid is unlikely to have adverse reproductive effects at dose levels not toxic to the mother.”
The WHO (2005) concluded that developmental effects, including embryo/fetal toxicity, as well as malformations, were only observed at doses that induced severe maternal toxicity. Studies involving rats, mice, hamsters, and rabbits, administering benzoic acid and related substances orally via diet or gavage, had NOAELs reported up to 1400 mg/kg bw/d. All of these studies indicated that the NOAEL for developmental effects was the same as the NOAEL for maternal toxicity. The OECD (2001) concluded that the “compounds exhibit no developmental toxicity” and indicated that a NOEL of 500 mg/kg/day for this endpoint was appropriate.
On the basis of evidence from data on benzoic acid and related substances, the short-term and subchronic toxicity is low. A 21-day dermal toxicity study identified a NO(A)EL of 2 500 mg/kg bw/d, the highest dose tested.
In summary, on the basis of the available data, the systemic (chronic) and reproductive and developmental toxicity of these substances is also considered to be low. Using data for benzoic acid on long-term repeat dose toxicity and reproductive and developmental toxicity, the NOAEL for benzoic acid, its salts and related substances, was determined to be 500 mg/kg bw/d. This value is based on the four-generation study by Kieckebusch and Lang (1960), a pivotal study used and evaluated by a number of international organizations (e.g., JECFA 2001; OECD 2001; SCCP 2005; EFSA 2016). It should be noted as well that the NOAEL of 500 mg/kg bw/d in this study represents the highest dose tested. Furthermore, the weight of evidence also indicates that these substances are not genotoxic and there are no indications of carcinogenic potential.
Finally, JECFA indicated, as part of its safety evaluation of benzyl derivatives in the WHO Technical Report Series (909), that substances that readily metabolized to benzoic acid, which it noted is endogenous in humans, would “therefore not be expected to be of safety concern” (JECFA 2002a).
6.2.2 Simple alkyl benzoates
The health effects characterization of the simple alkyl benzoates in this group is based primarily on the health effects data presented for benzoic acid and related substances found in section (6.2.1). However, there are some substance-specific data available for members of this subgroup (primarily methyl and sodium benzoate) which further supports the use and application of read-across from benzoic acid.
Methyl benzoate was assessed as part of the US EPA’s screening-level hazard characterization of benzyl derivatives (benzyl and benzoate esters). In this assessment, the US EPA indicated that acute toxicity from oral, inhalation, and dermal routes was low and that methyl benzoate was not anticipated to be genotoxic. JECFA (2001) and EFSA (2012) also identified negative results for methyl benzoate in in vitro genotoxicity assays. On the basis of read-across for the subcategory, repeat dose toxicity was also evaluated to be low, with NOAELs generally at 500 mg/kg bw/d or higher (up to highest dose tested) for systemic effects (changes in body weight at the next greater dose level) (US EPA 2010).
Limited information is available for tuberose oil. As noted in section 2.0, esters of benzoic acid were noted as significant constituents of tuberose oil and therefore addressed within the characterization of health effects for benzoic acid and related substances. The Council of Europe (2007) evaluated tuberose oil as a natural source of flavourings and indicated that methyl benzoate was one of the major constituents. The Council did not identify any toxicological data for tuberose oil itself, but rather indicated that decisions from JECFA for a number of important constituents had “no safety concern at current levels of intake when used as a flavouring agent” (COE 2007). The Council listed tuberose oil as a Category 5 flavourant, which “contain source materials which have not been fully evaluated due to lack of data, but which have been categorized as temporarily acceptable on the basis of available information” (COE 2007). Tuberose oil is listed specifically under the US FDA Part 182- SUBSTANCES GENERALLY RECOGNIZED AS SAFE- Sec. 182.20 Essential oils, oleoresins (solvent-free), and natural extractives (including distillates) list (US FDA 2016d).
6.2.3 Dibenzoates
The characterization of health effects for benzoic acid and its related substances presented in section 6.2.1 is considered relevant to these substances. DPGDB and DEGDB are currently on the European Union’s Community Rolling Action Plan (CoRAP) as “suspected reprotoxic” for 2018. The justification document indicates that this is based on some notifications in the Classification and Labelling (C&L) inventory (ECHA 2015b). The reproductive and developmental studies considered in the justification document are addressed in the following section, along with other available relevant data.
DPGDB
Information was submitted to the US High Production Volume (HPV) program for DPGDB using data for Benzoflex 9-88, which was reported at approximately 90% DPGDB, with the remaining 10% comprised of di and mono-benzoate propylene glycol derivatives. Based on data submitted to the US EPA in 2001, under its HPV Challenge program, DPGDB has low acute oral and dermal toxicity. DPGDB was also found to be not genotoxic in in vitro mutagenicity and clastogenicity assays (US EPA 2017; ECHA 2016).
In a repeat dose study, a NOAEL of 1 000 mg/kg bw/d was observed in rats administered Benzoflex 9-88 orally for 13 weeks. This represents a mid-dose in the study, and higher doses of 1 750 mg/kg bw/d and 2 500 mg/kg bw/d were tolerated; however, decreases in body weights were more pronounced relative to controls (Huntingdon Life Sciences 1999). In a two-generation reproductive toxicity study conducted following OECD guideline 416, doses of 0, 1 000, 3 300 or 10 000 ppm (50, 165 or 500 mg/kg bw/d) were administered orally in food to male and female rats for 38 weeks (HC 1994). A NOEL of 500 mg/kg bw/d (high dose) was observed for both the parental (F0) and offspring (F1) according to the study authors. At the high dose, a statistically significant decrease in spleen weights, both absolute and relative to body weight, in the F2 generation was observed, as well as a slight decrease in body weight gain in the F1 and F2 generations, but these effects were not considered by the study authors to be toxicologically significant. The study authors cite a NOAEL for survival and growth of offspring to be 10 000 ppm (500 mg/kg bw/d) (Huntingdon Life Sciences 2001). While the effects on absolute and relative spleen weights in both sexes were statistically significant at the high dose and thus potentially an indication of a LOAEL for this study, there were no other effects observed at this dose. Microscopic examinations of the F1 and F2 offspring did not observe any treatment-related effects. In addition, pathological assessment and sperm analysis for the F0 and F1 adults showed no adverse effects of treatment on the estrous cycle, mating performance, fertility or fecundity (Huntingdon Life Sciences 2001). As a consequence, a NOAEL of 500 mg/kg bw/d is considered appropriate for this study.
In a developmental study submitted in 2001 to US EPA under its HPV program and considered within ECHA’s CoRAP justification (ECHA 2015b), rats were exposed daily during gestation days 6-19 to DPGDB (test substance 94.84% DPGDB). DPGDB was administered via gavage at doses of 0, 250, 500 or 1 000 mg/kg bw/d and a NOEL of 1 000 mg/kg bw/d was observed for maternal toxicity. With respect to offspring, the study authors indicated that there was an increase in cervical ribs observed at 1 000 mg/kg bw/d, which resulted in their identification of 500 mg/kg bw/day as the NOAEL for developmental toxicity. The study authors also considered the increase in cervical ribs observed at 500 mg/kg bw/day to be within the recent background control data, and found that at 1 000 mg/kg bw/day, the number of fetuses exhibiting this effect was small (10/155). In addition to the incidence of increased cervical ribs at the low (2/155) and mid dose (2/155), considered by the study authors to be within range of recent historical background data, there was also no clear dose-response relationship observed. Therefore the incidence of this effect was not considered to be treatment related. There were no incidences of increased cervical ribs observed in the control group (Huntingdon Life Sciences 2000).
In the Huntingdon Life Sciences (2000) study report summaries provided to both the US EPA (2017) and REACH (ECHA 2000b), the authors also identified a greater number of fetuses with incomplete ossification of the 5th and 6th sternebrae at both mid and high dose. The authors could not discount this being potentially a treatment-related effect; however, the available study summaries did not provide further information on either the incidence or significance of this effect in the treated groups. The authors considered these effects to be transient in nature and were of the view that they did not represent permanent structural change and were therefore of lesser long-term toxicological importance. The authors considered the incomplete ossification of sternebrae at 500 mg/kg bw/d to not be adverse and, as a consequence, indicated a NOEL of 250 mg/kg bw/d (Huntingdon Life Science 2000, ECHA 2000b).
On the basis of available information, the increased incidence in the number of fetuses with incomplete ossification in the study is representative of a LOEL of 500 mg/kg bw/d, with a NOEL of 250 mg/kg bw/d. The effect, when viewed along with other available information, is not considered to be toxicologically significant, particularly in the absence of evidence of adverse effects observed in offspring in a two-generation reproductive toxicity study at these doses. With NOAELs and LOAELs in repeat dose chronic and reproductive toxicity studies observed at 1 000 mg/kg bw/d, respectively, along with evidence that DPGDB is rapidly metabolized and excreted via the benzoic acid to hippuric acid (and derivatives) pathway, a NOAEL of 500 mg/kg bw/d is considered both appropriate and consistent with critical effect levels for benzoic acid.
DEGDB
DEGDB was also found to be not genotoxic in in vitro mutagenicity and clastogenicity assays in studies submitted under REACH. In repeat dose tests the toxicity was observed to be low. In a 13 week oral study with administered doses 0, 250, 1 000, 1 750 or 2 500 mg/kg bw/d, a NOAEL of 1 000 mg/kg bw/d was identified based on no findings of toxicological significance at or below this dose level. Dosages of 1 750 or 2 500 mg/kg bw/d were tolerated but treatment-related changes in blood parameters, minor treatment-related pathology and/or adverse effects on bodyweight gain were observed (ECHA 1999). A two-generation reproductive toxicity study was also conducted following OECD guideline 416, administering doses of 0, 1 000, 3 300 or 10 000 ppm (50, 165 or 500 mg/kg bw/d) orally in food to male and female rats. The study authors concluded a NOAEL of 10 000 ppm (500 mg/kg bw/d) for the parental (F0) generation. The study authors also indicated that no abnormal findings were apparent at necropsy in the F0, F1 or F2 generation animals, and no adverse effects were observed in detailed histopathological examination of the tissues of both males and females. In addition, no treatment-related effects were observed from litter parameters in both generations of offspring and in their survival to weaning, nor in organ weight assessment of F0 and F1 parent animals. The spermatogenesis and histopathology assessments in F0 and F1 generation parents also did not identify any treatment-related effects on the testes or other reproductive organs (ECHA 2001). While treatment-related effects include statistically significant changes in spleen weights in females from the F2 generation at the high dose, in addition to potential effects on F2 body weights, on the basis of available information, a NOAEL of 500 mg/kg bw/d is considered appropriate based on no further evidence of adverse treatment-related effects from the study.
In a developmental study cited from the REACH dossier (ECHA 2000c) for DEGDB, female Sprague-Dawley rats were administered doses of 0, 250, 500 or 1 000 mg/kg bw/d by oral gavage daily between gestation days 6 to 19. Results for DEGDB were very similar to what was observed in the dietary study for DPGDB, with no observed adverse effects for maternal toxicity at the highest dose (1 000 mg/kg bw/d). Treatment-related effects at 500 mg/kg bw/d included an increase in the incidence of incomplete ossification; however, the study authors considered the occurrence to be similar to historical control data and did not consider it an adverse treatment-related effect. The authors indicated that there was a “clearer increase in the incidence of incomplete ossification, principally affecting the cranial centres, sacrocaudal vertebral arches, 5th/6th sternebral centres and pelvic bones” at the high dose, relative to the control group in this study. A slight increase in the incidence of cervical ribs was also observed in the high dose group. At 250 and 500 mg/kg bw/day, the incidence and distribution of skeletal anomalies did not indicate any obvious adverse effect of treatment. The study authors concluded that a NOAEL of 500 mg/kg bw/d was suitable for fetal growth and development and identified a NOAEL of 1 000 mg/kg bw/d for maternal toxicity (ECHA 2000c).
On the basis of available information, an increased incidence in the number of fetuses with incomplete ossification was observed at 500 mg/kg bw/d. However, the occurrence of this effect is considered within historical control occurrence rates. A LOEL of 1 000 mg/kg bw/d is considered appropriate on the basis of an increased incidence of treatment-related skeletal effects, relative to the control group in this study, observed in the high dose group. Additionally, the effect, when viewed along with other available information, is not considered to be toxicologically significant, particularly in the absence of adverse effects observed in offspring in a two-generation reproductive toxicity study at the mid dose level. NOAELs and LOAELs for DEGDB in repeat dose chronic and reproductive toxicity studies were observed at 1 000 mg/kg bw/d, respectively, very similar to data for DPGDB. This, along with evidence that DEGDB is rapidly metabolized and excreted via the benzoic-acid-to-hippuric-acid (and derivatives) pathway, indicates that a NOAEL of 500 mg/kg bw/d is considered both appropriate and consistent with critical effect levels observed for benzoic acid.
6.2.4 Tribenzoates
Tribenzoin (CAS 614-33-5) was included in the assessment of benzyl derivatives by JECFA, which concluded that as a benzoate ester, it would be hydrolyzed and yield benzoic acid. As a consequence, the toxicity of benzoic acid and related substances is considered appropriate to characterize the health effects of tribenzoin (JECFA 1997). JECFA (2002b) finalized the evaluation from JECFA (1997) and concluded that there was no safety concern identified for its use in food as a flavouring agent. Furthermore, it was noted that benzoic acid was identified as a hydrolysis product, in addition to glycerol (JECFA 2002b).
There is a limited amount of toxicological data available on tribenzoin identified in the literature. One unpublished study by Carson (1972), submitted to WHO and evaluated by JECFA (2001), was available in which 15 male and 15 female weanling rats were administered tribenzoin in the diet at an average daily intake of 0, 120, 600 or 2600 mg/kg bw/d for 90 days. On the basis of weekly measurements of body weight and food consumption, the males in the high dose group were observed to have a depressed growth rate and efficiency of food use. No other treatment-related effects were observed (e.g., haematological, blood chemical values were normal), and there was no evidence of treatment-related gross or histological lesions (JECFA 2001).
6.3 Characterization of risk to human health
The available information on the toxicokinetics of simple alkyl benzoates, dibenzoates, and tribenzoates indicates that they are rapidly metabolized and excreted from the body via the same pathways as the other benzyl derivatives (i.e., metabolized to benzoic acid). Furthermore, several lines of evidence identified a NO(A)EL of 500 mg/kg bw/d (highest dose tested) for benzoic acid as being applicable for the other benzoate substances in this assessment. This is consistent with a number of short- and long-term repeat dose studies, as well as reproductive and developmental toxicity studies, for related benzyl derivatives.
For dibenzoates, while there are developmental toxicity studies which indicate a potential for incomplete ossification at 500 mg/kg bw/d, this effect, when viewed along with other available information, is not considered to be toxicologically significant, particularly in the absence of evidence of adverse effects observed in offspring in a two-generation reproductive toxicity study at these doses. Therefore, for dibenzoates, consistent with the other benzoates in this grouping, long-term repeat dose and reproductive toxicity studies identify a NOAEL of 500 mg/kg bw/d or greater.
The JECFA concluded that methyl, ethyl, and isobutyl benzoates, and tribenzoin would not present a safety concern at estimated levels of use as food flavouring agents (JECFA 2002a). EFSA (2009) conducted a critical review of the JECFA’s approach and agreed with its conclusion of “No safety concern with the estimated levels of intake of ethyl-, methyl- and isobutyl-benzoate as flavouring substances.” In the absence of European production volumes, the EFSA panel did not conclude on tribenzoin.
Considering all the information outlined above (indication of metabolism to benzoic acid by all of the substances in this grouping, a NO(A)EL of 500 mg/kg bw/d at the highest dose tested and conclusions by international organizations), it is evident that the substances in this grouping exhibit low hazard properties, and the potential risk to human health is considered to be low.
6.4 Uncertainties in evaluation of risk to human health
The key sources of uncertainty are presented in the table below.
| Key source of uncertainty | Impact |
|---|---|
| The health effects characterization for substances in this group is based primarily on toxicological data for benzoic acid and related benzyl derivatives and considers common metabolic pathways and transformation products. | +/- |
| The availability of longer term studies via dermal routes of exposure are limited. | +/- |
| The health effects assessment of tuberose oils used toxicological data for benzoates (i.e., benzoic acid), with esters of benzoic acid being identified as the primary components of this substance. | +/- |
+ = uncertainty with potential to cause over-estimation of exposure/risk;
- = uncertainty with potential to cause under-estimation of exposure risk;
+/- = unknown potential to cause over or under estimation of risk.
7. Conclusion
Considering all available lines of evidence presented in this screening assessment, there is low risk of harm to the environment from the substances in the Benzoates Group. It is concluded that the substances in the Benzoates Group do not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
On the basis of the information presented in this draft screening assessment, it is concluded that the substances in the Benzoates Group do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
Therefore, it is concluded that the substances in the Benzoates Group do not meet any of the criteria set out in section 64 of CEPA.
Reference
Adams TB, Cohen SM, Doull J, Feron VJ, Goodman JI, Marnett LJ, Munro IC, Portoghese PS, Smith RL, Waddell WJ, Wagner BM; Expert Panel of the Flavor and Extract Manufacturers Association. 2005. The FEMA GRAS assessment of benzyl derivatives used as flavor ingredients. Food Chem Toxicol. 43:1207-1240.
Butz RG, Atallah YH, Yu CC, Calo CJ. 1982. Environmental Safety Assessment of Dipropylene Glycol Dibenzoate. Environ Toxicol Chem. 1(4):337-346.
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C. 1999, c. 33. Canada Gazette Part III, vol. 22, no. 3.
Canada, Dept. of the Environment. 2012. Canadian Environmental Protection Act, 1999: Notice with respect to certain substances on the Domestic Substances List [PDF]. Canada Gazette, Part I, vol. 146, no. 48, Supplement.
Carson S. 1972. 90-Day studies with glyceryl tribenzoate in rats. Food and Drug Research Laboratories, Inc. Lab. No. 0732. April 10, 1972. Unpublished report submitted to WHO by Flavor and Extracts Manufacturers Association (FEMA) of the United States. Reported in JECFA 2001.
ChemIDplus [database]. 1993-a. Bethesda (MD): US National Library of Medicine. Search results for CAS RN 93-58-3. [accessed 2016 Feb 22].
ChemIDplus [database]. 1993-b. Bethesda (MD): US National Library of Medicine. Search results for CAS RN 93-89-0. [accessed 2016 Feb 22].
ChemIDplus [database]. 1993-c. Bethesda (MD): US National Library of Medicine. Search results for CAS RN 120-55-8. [accessed 2016 Feb 22].
ChemIDplus [database]. 1993-d. Bethesda (MD): US National Library of Medicine. Search results for CAS RN 614-33-5. [accessed 2016 Feb 22].
ChemIDplus [database]. 1993-e. Bethesda (MD): US National Library of Medicine. Search results for CAS RN27138-31-4. [accessed 2016 Feb 22].
[Clorox] The Clorox Company. 2017. Fragrance ingredients list. Oakland (CA): The Clorox Company. [accessed 2017 Jan 17].
[COE] Council of Europe. 2007. Natural Sources of Flavourings- Report No. 2. The Committee of Experts on Flavouring Substances. Council of Europe Publishing, Belgium. pp. 147-148.
[DFC] Dictionary of Food Compounds [database]. 2004- . Boca Raton (FL): CRC Press LLC. [accessed 2017 Mar 3].
Eastman. 2016. Eastman non-phthalate plasticizers [PDF]. Kingsport (TN): Eastman Chemical Company. [accessed 2017 Mar 12].
[ECCC] Environment and Climate Change Canada. 2016a. Science approach document: ecological risk classification of organic substances. Ottawa (ON): Government of Canada.
[ECCC] Environment and Climate Change Canada. 2016b. Gatineau (QC): Data used to create substance-specific hazard and exposure profiles and assign risk classifications in the Ecological Risk Classification of organic substances. Available from: substances@ec.gc.ca.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. [modified 2017 Mar 12]. Categorization. Ottawa (ON): Government of Canada.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2015. Identification of Risk Assessment Priorities: results of the 2015 review. Ottawa (ON): Government of Canada.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2018. Screening assessment substances identified as being of low concern using the ecological risk classification of organic substances and the threshold of toxicological concern (TTC)-based approach for certain substances Ottawa (ON): Government of Canada.
Environment Canada. 2013. DSL Inventory Update data collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain substances on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
[ECHA] European Chemicals Agency. 1999. DEGDB, CAS RN 120-55-8. Industry Submission to ECHA, Updated 6 January 2016. Exp Key Repeated dose toxicity: oral. 001. [accessed 2017 May].
[ECHA] European Chemicals Agency. 2000a. DEGDB, CAS RN 120-55-8. Industry Submission to ECHA, Updated 6 January 2016. Basic Toxicokinetics. [accessed 2017 May].
[ECHA] European Chemicals Agency. 2000b. DPGDB, CAS RN 27138-31-4. Industry Submission to ECHA, Updated 6 January 2016. Exp Key Developmental toxicity/teratogenicity: oral (gavage). 001. [accessed 2017 May].
[ECHA] European Chemicals Agency. 2000c. DEGDB, CAS RN 120-55-8. Industry Submission to ECHA, Updated 6 January 2016. Exp Key Developmental toxicity/teratogenicity: oral (gavage). 001. [accessed 2017 May].
[ECHA] European Chemicals Agency. 2001. DEGDB, CAS RN 120-55-8. Industry Submission to ECHA, Updated 6 January 2016. Toxicity to reproduction. [accessed 2017 May].
[ECHA] European Chemicals Agency. 2009. Data on manufacture, import, export, uses and releases of benzyl butyl phthalate (BBP) as well as information on potential alternatives to its use [PDF].
[ECHA] European Chemicals Agency. 2015a. Ethyl benzoate, CAS RN 93-89-0. Industry Submission to ECHA, Updated 6 January 2016. Basic Toxicokinetics. [accessed 2017 May].
[ECHA] European Chemicals Agency. 2015b. Justification for the selection of a substance for CoRAP inclusion: oxydipropyl dibenzoates. [PDF]
[ECHA] European Chemicals Agency. 2016. DPGDB, CAS RN 27138-31-4. Industry Submission to ECHA, [updated 2016 Jan 6]. Genetic Toxicity- Endpoint summary [accessed 2017 May].
[EFSA] European Food Safety Authority. 2012. Scientific opinion on Flavouring Group Evaluation 20, Revision 4 (FGE.20Rev4): Benzyl alcohols, benzaldeydes, a related acetal, benzoic acid, and related esters from chemical group 23 and 30. EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids. EFSA Journal 10(12):2994, 140 p. doi:10.2903/j.efsa.2012.2994.
[EFSA] European Food Safety Authority. 2016. Scientific opinion on the re-evaluation of benzoic acid (E 210), sodium benzoate (E 211), potassium benzoate (E 212) and calcium benzoate (E 213) as food additives. EFSA Panel on Food Additives and Nutrient Sources. EFSA Journal 14(3):4433, 110 p. doi:10.2903/j.efsa.2016.4433.
[EPI Suite] Estimation Program Interface Suite for Microsoft Windows [estimation model]. c2000-2012. Ver. 4.11. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
FooDB [database]. 2004. Edmonton (AB): Wishart Research Group, University of Alberta. Search compound results based on CAS RN. [accessed 2016 Feb 22].
[F&CT] Food and Chemical Toxicology. Fragrance Materials Monograph: Tuberose Concrete. Volume 38, Supplement 3,2000, pp. s231-s233.
[HSDB] Hazardous Substances Data Bank [database]. 1983- . Bethesda (MD): National Library of Medicine (US). [updated 2016 Sept; accessed 2017 Jan 22].
Health Canada. 2016. Science approach document: threshold of toxicological concern (TTC)-based approach for certain substances. Ottawa (ON): Government of Canada.
Huntingdon Life Sciences. 1999. Benzoflex 9-88. Toxicity to rats by Dietary Administration for 13 weeks with Subsequent 4-Week Recovery Period for Selected Animals [PDF]. [As referenced in Test plan and robust study summaries submitted to US EPA 2001.]
Huntingdon Life Sciences. 2000. Benzoflex 9-88. Study of Prenatal Development in the CD Rat by Oral Gavage Administration. [As referenced in Test plan and robust study summaries submitted to US EPA 2001.]
Huntingdon Life Sciences. 2001. Benzoflex 9-88. Study of Reproductive Performance in CD Rats Treated Continuously through Two Successive Generations by Dietary [PDF]. [as referenced in robust summaries and test plan submitted to US EPA 2001].
Hutzler C, Paschke M, Kruschinski S, Henkler F, Han J, Luch A. 2014. Chemical hazards present in liquids and vapors of electronic cigarettes. Arch Toxicol. 88:1295-1308.
[IFRA] International Fragrance Association. c2011–2017. List of ingredients [webpage]. Geneva: International Fragrance Association. [accessed 2017 Jan 17].
IRDC. 1981. 21-Day Dermal Toxicity Study in Rabbits. IRDC #163-675 (Unpublished study). [as cited in OECD SIAR 2000].
[JECFA] Joint FAO/WHO Expert Committee on Food Additives. 1996. Toxicological evaluation of certain food additives. Prepared by the 46th meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). Benzyl acetate, benzyl alcohol, benzaldehyde and benzoic acid and its salts. WHO Food Additives Series 37. WHO, Geneva.
[JECFA] Joint FAO/WHO Expert Committee on Food Additives. 1997. Evaluation of certain food additives and contaminants. Forty-sixth meeting of the Joint FAO/WHO Expert Meeting on Food Additives. WHO Technical Report Series, No. 868, pp. 41-43.
[JECFA] Joint FAO/WHO Expert Committee on Food Additives. 2001. Safety evaluation of certain food additives and contaminants: Benzyl derivatives. WHO Food Additive Series, No. 48.
[JECFA] Joint FAO/WHO Expert Committee on Food Additives. 2002a. Evaluation of certain food additives and contaminants [PDF]. Fifty-seventh report of the Joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series, No. 909, pp. 73–84.
[JECFA] Joint FAO/WHO Expert Committee on Food Additives. 2002b. Evaluation of certain food additives [PDF]. Fifty-ninth report of the Joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series, No. 913, pp. 118-122.
Kieckebusch W, Lang K. 1960. Tolerance of benzoic acid in chronic feeding. Arzneim-Forsch 10:1001-1003. [cited in OECD 2001, SCF 2005, WHO 2005] [In German]
[LNHPD] Licensed Natural Health Products Database [Database]. [modified 2018 Feb 06]. Ottawa (ON): Health Canada. [Accessed 2018 June 28].
[NCI] National Chemical Inventories [database on a CD-ROM]. 2015. Columbus (OH): American Chemical Society, Chemical Abstracts Service. [accessed 2017 Jan 14].
[NRC] National Research Council. 2011. Data gathering on chemicals released to indoor air of residences from building materials and furnishings. Unpublished report. Ottawa (ON): National Research Council of Canada.
[OECD] Organisation for Economic Co-operation and Development. 2001. Screening Information DataSet Benzoates [PDF].
Patry-Parisien J, Zhu J, Wong SL. 2013. Implementation of the indoor air component of cycle 2 of the Canadian Health Measures Survey (CHMS) [PDF]. Components of Statistics Canada Catalogue no. 82-003-X Health Reports.
[P&G] Procter and Gamble. Product fragrances & scents. Cincinnati (OH): Procter & Gamble. [accessed 2019 Jan 21].
PubChem [database]. 1988- . Bethesda (MD): US National Library of Medicine, National Centre for Biotechnology Information. [updated 2017 Mar 18; accessed 2017 Mar 21].
Rakthaworn P, Dilokkunanant U, Sukkatta U, Vajrodaya S, Haruethaitanasan V, Pitpiangchan P, Punjee P. 2009. Extraction methods for tuberose oil and their chemical components. Kasetsart Journal - Natural Science. 43:204-211.
[SCCP] Scientific Committee on Consumer Products. 2005. Opinion on benzoic acid and sodium benzoate [PDF]. SCCP/0891/05.
[SCF] Scientific Committee on Food. 1994. Opinion on benzoic acid and its salts. Expressed on 24 February 1994. Reports of the Scientific Committee for Food, Thirty-Fifth Series. CEC, Luxembourg, 1996, pp. 33-39.
[SCF] Scientific Committee on Food. 2002. Opinion of the Scientific Committee on Food on benzoic acid and its salts SCF/CS/ADD/CONS/48 Final. pp. 1-13.
[SDS] Safety Data Sheet. 2011. Stencil / project pain – red. Warren (NJ): Horizon Group USA, Inc. [accessed 2017 Mar 12].
[SDS] Safety Data Sheet. 2014a. Benzoflex™ 354 Plasticizer. Kingston (TN): Eastman Chemical Company. [accessed 2017 Mar 12].
[SDS] Safety Data Sheet. 2014b. DRYLOK Clear Masonry Waterproofer [PDF]. Dunmore (PA) United Gilsonite Laboratories. [accessed 2017 Mar 12].
[SDS] Safety Data Sheet. 2014c. Titebond no-run, no-drip wood glue [PDF]. Columbus (OH): Franklin International. [accessed 2017 Mar 12].
[SDS] Safety Data Sheet. 2015a. Benzoflex™ 425 Plasticizer [PDF]. Kingston (TN): Eastman Chemical Company. [accessed 2017 Mar 12].
[SDS] Safety Data Sheet. 2015b. Dynaflex 230 caulking [PDF]. Baltimore (MD): DAP Products Inc. [accessed 2017 Mar 12].
Smyth HF, Carpenter P. 1948. Further experience with the range-finding test in the industrial toxicology laboratory. J Ind Hyg Toxicol. 30:63-68.
Surberg H, Panten J. 2016. Common Fragrance and Flavor Materials: Preparation, Properties and Uses, 6th ed. Weinheim (DEU): Wiley-VCH. April 2016. 392 p.
[TDS] Technical Data Sheet. 30 Aug 2013. Uniplex 260 plasticizer [PDF]. Leverkusen (DE): LANXESS Deutschland GmbH. [accessed 2017 Mar 12].
[TDS] Technical Data Sheet. 2015a. K-FLEX 500P® Dibenzoate Plasticizer [PDF]. Kalama (WA): Emerald Kalama Chemical. [accessed 2019 Jan 21].
[TDS] Technical Data Sheet. 2015b. K-Flex 975® Dibenzoate Plasticizer [PDF]. Kalama (WA): Emerald Kalama Chemical. [accessed 2019 Jan 21].
[TIMES] TIssue MEtabolism Simulator [prediction module]. 2016. Ver. 2.27.19. Bourgas (BG): University “Prof. Dr. Assen Zlatarov”, Laboratory of Mathematical Chemistry.
[TURI] Toxics Use Reduction Institute. 2006. Five chemicals alternatives assessment study [PDF]. Lowell (MA). University of Massachusetts Lowell, Toxics Use Reduction Institute.
[US EPA] US Environmental Protection Agency. 2010. Screening-Level Hazard Characterization: Benzyl Derivatives Category. Hazard Characterization Document. U.S. Environmental Protection Agency..
[US EPA] US Environmental Protection Agency. 2017. High Production Volume Information System. Washington (DC): US Environmental Protection Agency. [accessed 2017 Apr 24].
[US FDA] US Food and Drug Administration. 2016a. Code of Federal Regulations. Title 21. Food additives permitted for direct addition to food for human consumption. Subpart F–Flavoring agents and related substances. [accessed 2017 Feb 20].
[US FDA] US Food and Drug Administration. 2016b. Code of Federal Regulations. Title 21. Indirect food additives: Adhesives and components of coatings. Subpart B–Substances for use only as components of adhesives. [accessed 2017 Feb 20].
[US FDA] US Food and Drug Administration. 2016c. Code of Federal Regulations. Title 21. Indirect food additives: Paper and paperboard components. Subpart B–Substances for use only as components of paper and paperboard. [accessed 2017 Feb 20].
[US FDA] US Food and Drug Administration. 2016d. Code of Federal Regulations. Title 21. Substances generally recognized as safe. Subpart A- General provisions. Sec. 182.20 Essential Oils, oleoresins (solvent-free), and natural extractives (including distillates). [revised 2016 Apr 1; accessed 2017 Mar 27].
[WHO] World Health Organization. 2005. Benzoic acid and sodium benzoate (Rev. 1) [PDF]. Concise International Chemical Assessment Document 26, pp. 1-48.
Xia XR, Baynes RE, Monteiro-Riviere NA, Riviere JE. 2007. An experimentally based approach for predicting skin permeability of chemicals and drugs using membrane-coated fiber array. Toxicol Appl Pharmacol. 221:320-8. As cited in ECHA REACH dossier for Methyl Benzoate (CAS RN 93-58-3). [accessed 2017 May].
