Assessment - Commercial naphthenic acids group
Official title: Assessment - Commercial Naphthenic Acids Group
Chemical Abstracts Service Registry Numbers:
- 1338-24-5
- 61789-36-4
Environment and Climate Change Canada
Health Canada
January 2024
Cat. No.: En84-341/2023E-PDF
ISBN 978-0-660-49147-9
Synopsis
Pursuant to section 68 of the Canadian Environmental Protection Act, 1999 (CEPA), the Minister of the Environment and the Minister of Health have conducted an assessment of two substances referred to collectively under the Chemicals Management Plan (CMP) as the Naphthenic Acids and Salts Group, hereinafter referred to as the Commercial Naphthenic Acids Group.
The Chemical Abstracts Service Registry Numbers (CAS RNFootnote 1), their Domestic Substances List (DSL) names and their common name are listed in the table below.
| CAS RN | DSL name | Common name |
|---|---|---|
| 1338-24-5a | Naphthenic acids | N/A |
| 61789-36-4a | Naphthenic acids, calcium salts | Calcium naphthenates |
Abbreviation: N/A, not applicable
a The substance bearing this CAS RN is a UVCB (unknown or variable composition, complex reaction products, or biological materials).
This assessment addresses two commercial naphthenic acids obtained via the extraction of petroleum distillates: naphthenic acids (CAS RN 1338-24-5), hereinafter referred to by its CAS RN, and naphthenic acids, calcium salts (CAS RN 61789-36-4), hereinafter referred to as calcium naphthenates. Nineteen other commercial naphthenic acids and commercial salts of naphthenic acids have been or are being addressed through various approaches under the Chemicals Management PlanFootnote 2. Commercial naphthenic acids differ from complex mixtures of naphthenic acids present as a by-product in oil sands process-affected water (OSPW) generated from oil sands mining, extraction and processing of bitumen. OSPW naphthenic acids differ in source, composition, properties and use compared to commercial naphthenic acids. OSPW naphthenic acids do not have associated CAS RNs and are not included on the DSL; therefore, they were not subject to categorization of the DSL and are not considered in this assessment. Activities to better understand OSPW naphthenic acids have been initiated under the Canada-Alberta Oil Sands Monitoring program, and are being pursued by Environment and Climate Change Canada, notably targeting the presence and effects of naphthenic acids in tailing ponds’ seepage. In addition, Environment and Climate Change Canada has added naphthenic acid fraction compounds (which includes diverse polar organic compounds present in bitumen and OSPW) and their salts to the National Pollutant Release Inventory, beginning with the 2020 reporting year. This addition does not include naphthenic acids and their salts used solely in the context of commercial mixtures.
In Canada, CAS RN 1338-24-5 and calcium naphthenates were not reported to be manufactured above the reporting threshold in 2011 in response to a survey issued pursuant to section 71 of CEPA. Import quantities reported in the survey were in the range of 100 000 kg to 1 000 000 kg for CAS RN 1338-24-5 and between 1000 kg and 10 000 kg for calcium naphthenates.
In Canada and internationally, CAS RN 1338-24-5 is mainly found in lubricants and greases, and in paints and coatings that are intended for professional/industrial use only. Lubricants and greases containing CAS RN 1338-24-5 are used primarily in the industrial, transportation and aviation sectors while paints and coatings containing CAS RN 1338-24-5 are used in the automotive and industrial sectors. CAS RN 1338-24-5 has also been identified as a component of inks used in the manufacture of polymeric coatings used to package some foods. No use of calcium naphthenates in products available to consumers was identified.
The ecological risks of CAS RN 1338-24-5 and calcium naphthenates were characterized using the ecological risk classification of organic substances (ERC), which is a risk-based approach that employs multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. Hazard profiles are based primarily on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Metrics considered in the exposure profiles include potential emission rate, overall persistence, and long-range transport potential. A risk matrix is used to assign a low, moderate or high level of potential concern for substances based on their hazard and exposure profiles. Based on the outcome of the ERC analysis, CAS RN 1338-24-5 and calcium naphthenates are considered unlikely to be causing ecological harm.
Considering all available lines of evidence presented in this assessment, there is low risk of harm to the environment from CAS RN 1338-24-5 and calcium naphthenates. It is concluded that CAS RN 1338-24-5 and calcium naphthenates do not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
CAS RN 1338-24-5 and calcium naphthenates were not identified as posing a high hazard to human health on the basis of absence of classifications by other national or international agencies for carcinogenicity, genotoxicity, developmental toxicity or reproductive toxicity. In addition, exposure of the general population to CAS RN 1338-24-5 and calcium naphthenates through environmental media, food, or the use of products available to consumers is expected to be minimal and the potential risk to human health is considered to be low.
The human health assessment took into consideration those groups of individuals within the Canadian population who, due to greater susceptibility or greater exposure, may be more vulnerable to experiencing adverse health effects. For the commercial naphthenic acids group, these subpopulations were considered; however, exposures were expected to be minimal based on use patterns and therefore were not quantified.
Considering all the information presented in this assessment, it is concluded that CAS RN 1338-24-5 and calcium naphthenates do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore concluded that CAS RN 1338-24-5 and calcium naphthenates do not meet any of the criteria set out in section 64 of CEPA.
1. Introduction
Pursuant to section 68 of the Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999), the Minister of the Environment and the Minister of Health have conducted an assessment of naphthenic acids (CAS RN 1338-24-5), and naphthenic acids, calcium salts (CAS RN 61789-36-4), hereinafter referred to by its CAS RN and calcium naphthenates, respectively, to determine whether these substances present or may present a risk to the environment or to human health. These substances were identified as priorities as they met categorization criteria as described in ECCC, HC (modified 2017). The two substances were referred to collectively under the Chemicals Management Plan as the Naphthenic Acids and Salts Group, and are hereinafter referred to as the Commercial Naphthenic Acids Group.
This assessment addresses two commercial naphthenic acids obtained via the extraction of petroleum distillates. Nineteen other commercial naphthenic acids and commercial salts of naphthenic acid substances that met categorization criteria have been addressed through various approaches, as outlined in Tables 1-1 and 1-2.
| CAS RN | Domestic Substances List name | Approach under which the substance is addressed |
|---|---|---|
| 12001-85-3 | Naphthenic acids, zinc salts | Zinc and its Compounds screening assessment (ECCC, HC 2019a)(based on the substance contributing to zinc moiety) |
| 1338-02-9 | Naphthenic acids, copper salts | Copper and its Compounds screening assessment (ECCC, HC 2019b)(based on the substance contributing to copper moiety) |
| 61788-56-5 | Naphthenic acids, lithium salts | Rapid Screening of Substances Identified from Phase Two of the Domestic Substances List Inventory Update (ECCC, HC 2016a) |
| 61788-69-0 | Naphthenic acids, chromium salts | Approach for a Subset of Substances Prioritized during Categorization That Have Already Been Addressed (Environment Canada, Health Canada 2015) (based on chromium having been previously assessed) |
| 61788-71-4 | Naphthenic acids, nickel salts | Rapid Screening of Substances of Lower Concern (Environment Canada, Health Canada 2013) |
| 61789-34-2 | Naphthenic acids, cadmium salts | Rapid Screening of Substances from Phase One of the Domestic Substances List Inventory Update (Environment Canada, Health Canada 2014) |
| 61789-51-3 | Naphthenic acids, cobalt salts | Cobalt and Cobalt-Containing substances screening assessment (ECCC, HC 2017a) (based on the substance contributing to cobalt moiety) |
| 61790-14-5 | Naphthenic acids, lead salts | Approach for a Subset of Substances Prioritized during Categorization That Have Already Been Addressed (Environment Canada, Health Canada 2015) (based on lead having been previously addressed) |
| 61790-20-3 | Naphthenic acids, rare earth salts | Rapid Screening of Substances Identified from Phase Two of the Domestic Substances List Inventory Update (ECCC, HC 2016a) |
| 68815-09-8 | Naphthenic acids, vanadium salts | Rapid Screening of Substances from Phase One of the Domestic Substances List Inventory Update (Environment Canada, Health Canada 2014) |
| 85736-59-0 | Naphthenic acids, bismuth salts | Rapid Screening of Substances Identified from Phase Two of the Domestic Substances List Inventory Update (ECCC, HC 2016a) |
| 68514-63-6 | Naphthenic acids, cerium(4+) salts | Rapid Screening of Substances Identified from Phase Two of the Domestic Substances List Inventory Update (ECCC, HC 2016a) |
| CAS RN | Domestic Substances List name | Approach under which the substance is addressed |
|---|---|---|
| 61790-54-3 | Naphthenic acids, compds. with N-tallow alkyltrimethylenediamines | Rapid Screening of Substances Identified from Phase Two of the Domestic Substances List Inventory Update (ECCC, HC 2016a) |
| 64754-89-8 | Naphthenic acids (petroleum), crude | Rapid Screening of Substances Identified from Phase Two of the Domestic Substances List Inventory Update (ECCC, HC 2016a) |
| 64755-04-0 | Naphthenic acids, reaction products with polyethylenepolyamines | Second phase of polymer rapid screening (ECCC, HC 2017b) |
| 68139-87-7 | Fatty acids, tall-oil, compds. with diethylenetriamine-naphthenic acid reaction products | Rapid Screening of Substances Identified from Phase Two of the Domestic Substances List Inventory Update (ECCC, HC 2016a) |
| 68553-60-6 | Naphthenic acids, vanadyl complexes | Rapid Screening of Substances Identified from Phase Two of the Domestic Substances List Inventory Update (ECCC, HC 2016a) |
| 68606-78-0 | Naphthenic acids, esters with polytriethanolamine | Rapid Screening Assessment: Polymers Identified from Phase Two of the Domestic Substances List Inventory Update (ECCC, HC 2016b) |
| 68956-65-0 | Naphthenic acids, polymers with ethylenimine, compds. with linoleic acid dimer | Rapid Screening Assessment: Polymers Identified from Phase Two of the Domestic Substances List Inventory Update (ECCC, HC 2016b) |
Commercial naphthenic acids differ from complex mixtures of naphthenic acids present as a by-product in oil sands process-affected water (OSPW) generated from oil sands mining, extraction and processing of bitumen. OSPW naphthenic acids differ in composition, properties and use compared to commercial naphthenic acids. One of the primary objectives of the Chemicals Management Plan (CMP) has been to address the existing substances identified as priorities for assessment following the Government of Canada’s categorization of the substances on the Domestic Substances List (DSL), as required under CEPA. The naphthenic acids on the DSL are refined or commercial naphthenic acids and their salts that have associated CAS RNs. OSPW naphthenic acids do not have associated CAS RNs and are not included on the DSL; therefore, they were not subject to categorization and are not considered further in this assessment. Activities to better understand OSPW naphthenic acids were initiated under the Canada-Alberta Oil Sands Monitoring programFootnote 3, and are being pursued by Environment and Climate Change Canada, which notably targets the presence and effects of naphthenic acids in tailing ponds’ seepage. In addition, Environment and Climate Change Canada added naphthenic acid fraction compounds and their salts to the National Pollutant Release Inventory (NPRI), beginning with the 2020 reporting year. The addition includes classically-defined naphthenic acidsFootnote 4 and diverse polar organic compounds present in bitumen and OSPW, but does not include naphthenic acids and their salts used solely in the context of commercial mixtures.
The ecological risks of CAS RN 1338-24-5 and calcium naphthenates were characterized using the ecological risk classification of organic substances (ERC) approach (ECCC 2016a). The ERC describes the hazard of a substance using key metrics including mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity, and considers the possible exposure of organisms in the aquatic and terrestrial environments on the basis of such factors as potential emission rates, overall persistence and long-range transport potential in air. The various lines of evidence are combined to identify substances as warranting further evaluation of their potential to cause harm to the environment or as having a low likelihood of causing harm to the environment.
This assessment includes consideration of information on chemical properties, environmental fate, hazards, uses and exposures. Relevant data were identified up to February 2021. Empirical data from key studies as well as some results from models were used to reach conclusions. When available and relevant, information from other jurisdictions was considered.
This assessment was prepared by staff in the CEPA Risk Assessment Program at Health Canada and Environment and Climate Change Canada, based on a draft developed by staff at Sanexen Environmental Services Incorporated, and incorporates input from other programs within these departments. The ecological portion of this assessment is based on the ERC document (published July 30, 2016), which was subject to an external review as well as a 60-day public comment period. Additionally, the draft of this assessment (published August 18, 2018) was subject to a 60-day public comment period. While external comments were taken into consideration, the final content and outcome of this assessment remain the responsibility of Health Canada and Environment and Climate Change Canada.
Assessments focus on information critical to determining whether a substance meets the criteria as set out in section 64 of CEPA by considering scientific information, including information, if available, on subpopulations who may have greater susceptibility or greater exposure, vulnerable environments and cumulative effectsFootnote 5, and incorporating a weight of evidence approach and precaution.Footnote 6 This assessment presents the critical information and considerations on which the conclusions are based.
2. Identity of substances
The CAS RNs and DSL names for CAS RN 1338-24-5 and calcium naphthenates are presented in Table 2‑1. These substances are UVCBs (unknown or variable composition, complex reaction products or biological materials). UVCBs are derived from natural sources or complex reactions. A UVCB is not an intentional mixture of discrete substances and is considered a single substance. The complexity and variability of their compositions can make them difficult to fully and consistently characterize.
CAS RN 1338-24-5 is a complex mixture of carboxylic acids with varying numbers of carbons (typically from 6 to 16). The carboxylic acids are principally monobasic with the general formula RCOOH where R represents a (cyclo)alkane moiety. This moiety can be acyclic (typically highly branched) or include a single or multiple fused rings (typically cyclopentane and/or cyclohexane). Aromatic, olefinic, hydroxy and dibasic acids are present as minor components in such mixtures (Brient et al. 2000).
Calcium naphthenates can be represented by the general formula Ca2+(‑OOCR)2, where one calcium atom forms bonds with two naphthenic acid molecules.
Commercial naphthenic acids, which are obtained via the extraction of petroleum distillates, are distinct from naphthenic acids present as a by-product of OSPW generated from oil sands mining and extraction by their respective differences in compositionFootnote 7 , propertiesFootnote 8 , and sources and uses in Canada (Brient et al. 2000).
| CAS RN | DSL name (acronym/synonym) | UVCB general formula | Example of chemical structure a |
|---|---|---|---|
| 1338-24-5 | Naphthenic acids (NAs) | RCOOH or CnH2n+zO2 | 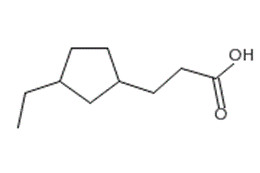
|
| 61789-36-4 | Naphthenic acids, calcium salts (calcium naphthenates) | Ca2+(‑OOCR)2 | ![C1=CC=C2C=C(C=CC2=C1)C(=O)[O-].C1=CC=C2C=C(C=CC2=C1)C(=O)[O-].[Ca+2]](/content/dam/eccc/images/pded/naphthenic-acids-and-salts/Table2-1Figure2.jpg) |
Abbreviations: UVCB, unknown or variable composition, complex reaction products or biological materials; n, carbon number; R, alkylated chain, acyclic or including a single ring or condensed rings (typically cyclopentane and/or cyclohexane); z, number of hydrogen atoms lost to ring formations (that is, z = 0: acyclic naphthenic acid, z = ‑2: 1-ring naphthenic acid, z = -4: 2-rings naphthenic acid, etc.).
a Chemical structures obtained from ChemIDplus (1993- ). The chemical structure provided for CAS RN 1338-24-5 is one example of a structure corresponding to a monocyclic naphthenic acid with formula C10H18O2 (many other structures are possible for this formula). For CAS RN 61789-36-4, the structure (Ca[C11H7O2]2) is the only one to be displayed by suppliers of calcium naphthenates.
3. Physical and chemical properties
Commercial naphthenic acids are complex mixtures of carboxylic acids produced from petroleum distillates. Their composition varies depending on the crude oil composition and on the degree of refining (Brient et al. 2000; API 2012).
As the physical and chemical properties of CAS RN 1338-24-5 and calcium naphthenates depend on their composition, the parameter values determined experimentally reflect the composition of the tested mixture and may not be representative of another test material. Similarly, values measured or predicted for individual components are not representative of the properties of the mixture. Consequently, no single value, either empirical or predicted, was selected as representative of either substance; rather their properties are described qualitatively below. Additional physical and chemical properties used to develop the substance-specific profiles for CAS RN 1338-24-5 and calcium naphthenates for assessing environmental risk are reported in ECCC (2016b).
CAS RN 1338-24-5 can generally be described as including viscous liquid components with light yellow to dark amber colour; phenolic and sulfur impurities are responsible for their characteristic odour (Brient et al. 2000). These components are weak acids with pKa values of 5 to 6. Their water solubility is low and varies with the pH (that is, due to a greater proportion of ionized constituents in alkaline solutions, solubility is increased by at least two orders of magnitude as compared with neutral solutions) (CEATAG 1998; Brient et al. 2000). CAS RN 1338-24-5 includes components which are completely soluble in organic solvents and oils. With their dual hydrophilic (carboxyl group) and hydrophobic (non‑polar aliphatic group) ends, CAS RN 1338-24-5 components are surfactants and will concentrate at aqueous/non-aqueous interfaces (Armstrong 2008). Based on its components with low vapour pressure and boiling point, CAS RN 1338-24-5 may be characterized as being semi-volatile.
Calcium naphthenates (commercial preparations at 4 to 5% [w/w] of Ca) are liquid at room temperature (CMA 1983). They are insoluble in water, but extremely soluble in non-polar solvents (CMA 1983; US EPA 1983; Lewis 2007). Based on their negligible vapour pressure, calcium naphthenates are not expected to be volatile.
4. Sources and uses
In Canada, both substances in the Commercial Naphthenic Acids Group have been included in a survey issued pursuant to section 71 of CEPA. CAS RN 1338-24-5 and calcium naphthenates were not reported to be manufactured above the reporting threshold of 100 kg in 2011, while import quantities were reported in the range of 100 000 to 1 000 000 kg for CAS RN 1338-24-5 and of 1000 to 10 000 kg for calcium naphthenates (Environment Canada 2013).Footnote 9 In the United States, CAS RN 1338-24-5 production quantities were reported to be in the range of 450 000 to 4 500 000 kg in 2016, with no data available for calcium naphthenates (CDAT [modified 2014]). In the European Union, CAS RN 1338-24-5 manufacture and/or import quantities were reported to be in the range of 100 000 to 1 000 000 kg per year, with no data available for calcium naphthenates (ECHA 2016a).
Naphthenic acids occur naturally in crude oil. Commercial naphthenic acids, however, are not extracted from crude petroleum, but rather, they are recovered from the extraction of petroleum distillates such as jet fuel, kerosene, and diesel during oil processing. This recovery reduces corrosion in the refinery and improves the petroleum distillates characteristics (Brient et al. 2000). It also provides a source of crude naphthenic acids that can be further refined to produce CAS RN 1338-24-5 (McKee et al. 2014). Commercial naphthenic acids sold by the petroleum industry are available in various grades of purity and are marketed by acid number, impurity level and colour (Brient et al. 2000; API 2003).
Salts of commercial naphthenic acids, such as calcium naphthenates, do not occur naturally. Calcium naphthenates can be produced either intentionally (through the interaction of calcium with naphthenic acids from the petroleum industry) or unintentionally (in oil production facilities, calcium naphthenates deposits occur and affect crude oil productivity) (Havre 2002; Mohammed 2010).
In Canada, CAS RN 1338-24-5 is used in lubricants and greases, and as an intermediate (Environment Canada 2013). Material safety data sheets (MSDSs) for products in Canada report CAS RN 1338-24-5 concentrations ranging from 0.1 to 5% (w/v) in lubricants and greases; these products are used in the industrial, transport and aviation sectors to lubricate specialized equipment and machinery and are exposed to atypical conditions (for example, severe vibrations, high temperatures) (MSDS 2005; MSDS 2012a,b; MSDS 2013d; MSDS 2015b,c,d; MSDS 2017c,d). Other MSDSs indicate that CAS RN 1338-24-5 is also used in paints and coatings (concentrations ranging from 0.5 to 25% [w/v]) used in the automotive and industrial sectors; these products have various purposes such as automobile refinishing, sealing and protecting concrete pavements, and tank linings (MSDS 2013a; MSDS 2015a; MSDS 2017a). CAS RN 1338-24-5 may also be present as a component in a mixture or product where it functions as an adhesive or sealant agent (that is, promote bonding between other substances or adhesion to substances) (Environment Canada 2013).
Uses of calcium naphthenates and additional uses of CAS RN 1338-24-5 in Canada are listed in Table 4-1.
| Use | CAS RN 1338-24-5 | Calcium naphthenates |
|---|---|---|
| Food packaging materialsa | Y (component in inks) | N |
| Formulant in pest control products registered in Canadab | Y (7 remedial wood preservatives and one insecticide) | Y (antiouling paints) |
Abbreviations: Y, yes this use was reported for this substance; N, no this use was not reported for this substance.
a Personal communication, email from the Food Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada; dated July, 2015; unreferenced
b PMRA 2010; Personal communication, emails from the PMRA, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada; May 16, 2017 (NAs) and November 22, 2016 (calcium naphthenates); unreferenced; MSDS 2017b
Internationally, other major uses identified for CAS RN 1338-24-5 include lacquers and varnishes, construction materials, colouring agents, metalworking fluids, hydraulic fluids, corrosion inhibitors, adhesives or sealants, and biocides/fungicides (MSDS 2009a,b; MSDS 2010a; MSDS 2011; MSDS 2013b,c; MSDS 2014a,b; CPCat 2014; ECHA 2017; SPIN 2017). For calcium naphthenates, major uses identified internationally include use in lubricants and greases, and in paints, lacquers and varnishes (function as a drying accelerant during the drying process for oleoresinous paints) (CMA 1983; US EPA 1983; Hansen et al. 1987; MSDS 2008; MSDS 2010b; MSDS 2012c; SPIN 2017).
5. Potential to cause ecological harm
5.1 Characterization of ecological risk
The ecological risks of CAS RN 1338-24-5 and calcium naphthenates were characterized using the ecological risk classification of organic substances (ERC) (ECCC 2016a). The ERC is a risk-based approach that considers multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. The various lines of evidence are combined to discriminate between substances of lower or higher potency and lower or higher potential for exposure in various media. This approach reduces the overall uncertainty with risk characterization compared to an approach that relies on a single metric in a single medium (for example, median lethal concentration) for characterization. Since CAS RN 1338-24-5 and calcium naphthenates are UVCB substances and could not be suitably represented by single chemical structures, a manual judgment-based approach to classification was used. The following paragraphs in this section summarize the approach, which is described in detail in ECCC (2016a).
Hazard profiles were established based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Exposure profiles were also composed of multiple metrics, including potential emission rate, overall persistence and long-range transport potential. Hazard and exposure profiles were compared to decision criteria to classify the hazard and exposure potentials for each organic substance as low, moderate or high. Additional rules were applied (for example, classification consistency, margin of exposure) to refine the preliminary classifications of hazard or exposure. However, in the case of CAS RN 1338-24-5 and calcium naphthenates, hazard and exposure could not be fully profiled due to the lack of a representative structure to estimate needed properties, and the lack of empirical data for these properties. Therefore, manual classification of hazard and exposure was performed by examining the UVCB constituents, analyzing information submitted in response to a CEPA section 71 survey, and making decisions on the basis of consideration of similar substances and/or application of expert judgment.
A risk matrix was used to assign a low, moderate or high classification of potential risk for each substance based on its hazard and exposure classifications. ERC classifications of potential risk were verified using a two-step approach. The first step adjusted the risk classification outcomes from moderate or high, to low for substances that had a low estimated rate of emission to water after wastewater treatment, representing a low potential for exposure. The second step reviewed low risk potential classification outcomes using relatively conservative, local-scale (that is, in the area immediately surrounding a point source of discharge) risk scenarios, designed to be protective of the environment, to determine whether the classification of potential risk should be increased.
ERC uses a weighted approach to minimize the potential for both over- and under- classification of hazard and exposure, and of subsequent risk. The balanced approaches for dealing with uncertainties are described in greater detail in ECCC (2016a). The following describes two of the more substantial areas of uncertainty. Error in empirical or modelled acute toxicity values could result in changes in classification of hazard, particularly metrics relying on tissue residue values (that is, mode of toxic action), many of which are predicted values from (Q)SAR models (OECD QSAR Toolbox 2014). However, the impact of this error is mitigated by the fact that overestimation of median lethality will result in a conservative (protective) tissue residue value used for critical body residue analysis. Error of underestimation of acute toxicity will be mitigated through the use of other hazard metrics such as structural profiling of mode of action, reactivity and/or estrogen binding affinity. Changes or errors in chemical quantity could result in differences in classification of exposure as the exposure and risk classifications are highly sensitive to emission rate and use quantity. The ERC classifications thus reflect exposure and risk in Canada on the basis of what is estimated to be the current use quantity and may not reflect future trends.
Critical data and considerations used to develop the substance-specific profiles for CAS RN 1338-24-5 and calcium naphthenates and the hazard, exposure, and risk classification results are presented in ECCC (2016b).
The hazard and exposure classifications for CAS RN 1338-24-5 and calcium naphthenates are summarized in Table 5-1.
| Substance | ERC hazard classification | ERC exposure classification | ERC risk classification |
|---|---|---|---|
| CAS RN 1338-24-5 | moderate | low | low |
| Calcium naphthenates | low | low | low |
According to information considered under ERC, CAS RN 1338-24-5 was classified as having a low exposure potential. Although CAS RN 1338-24-5 was initially classified as having low hazard potential, the classification was revised to a moderate hazard potential based on a high terrestrial food web hazard assessment factor. Acute aquatic toxicity studies also identified fish and invertebrate species as sensitive to exposure from CAS RN 1338-24-5 (Kinley et al. 2016). In addition, Marentette et al. (2015) determined that CAS RN 1338-24-5 was more hazardous (EC50 = 2 mg/L, nominal concentration) than naphthenic acid fraction compounds (NAFCs) derived from OSPW (EC50 = 5-12 mg/L, nominal concentration). Similarly, Bartlett et al. (2017) also reported that CAS RN 1338-24-5 was 30-, 4-, and 120-fold more hazardous than NAFCs derived from OSPW in Hyalella azteca (freshwater amphipod), Vibrio fischeri (marine bacterium), and Lampsilis cardium (freshwater mussel larvae), respectively. On the basis of low exposure potentials, however, CAS RN 13338-24-5 is unlikely to be resulting in concerns for the environment in Canada.
On the basis of low hazard and low exposure classifications according to ERC, calcium naphthenates were classified as having a low potential for ecological risk. It is therefore unlikely that these substances are resulting in concerns for the environment in Canada.
6. Potential to cause harm to human health
6.1 Exposure assessment
Considering reported uses in Canada, their low solubility in water, and their limited volatility, significant releases of CAS RN 1338-24-5 and calcium naphthenates to the environment are not expected. Accordingly, environmental media is not expected to be a significant source of exposure to the general population.
In Canada, CAS RN 1338-24-5 is mainly found in lubricants and greases, and in paints and coatings that are intended for professional/industrial use only (based on the MSDSs for these products). These products are not available to consumers, and thus, exposure to CAS RN 1338-24-5 from products is not expected.
In Canada, CAS RN 1338-24-5 has been identified as a component of inks used in the manufacture of polymeric coatings used to package some foods. However, there is no direct contact of the packaging with those foods and exposure via this source is expected to be negligible (personal communication, email from the Food Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated July, 2015; unreferenced).
In Canada, calcium naphthenates are used in antifouling paints which are regulated under the Pest Control Products Act.
6.2 Health effects assessment
CAS RN 1338-24-5 and calcium naphthenates were not identified as posing a high hazard to human health on the basis of absence of classifications by other national or international agencies for carcinogenicity, genotoxicity, developmental toxicity, or reproductive toxicity. They are also not on the European Chemicals Agency's Candidate List of Substances of Very High Concern for Authorisation (ECHA 2017). A limited number of laboratory studies provide information regarding the toxicity of CAS RN 1338-24-5 and calcium naphthenates; no toxicological data is available for the inhalation route. This information is summarized below.
CAS RN 1338-24-5
There are no data available on the carcinogenic potential for CAS RN 1338-24-5, and in vitro (that is, bacterial reverse mutation assay) and in vivo (that is, micronucleus test) assay results did not indicate genotoxicity (McKee et al. 2014). CAS RN 1338-24-5is not acutely toxic (oral and dermal LD50 ≥ 3000 mg/kg (Rockhold 1955; Rubinskaya 1974; Pennisi and Lynch 1977; Exxon 1979; Bio/dynamics Inc 1979; Exxon 1987). It can also induce slight to moderate dermal sensitization.
The overall no-effect level (NOEL) for systemic effects is 100 mg/kg-bw per day. This is based on a combined short-term and reproductive/developmental oral toxicity study where male and female Sprague-Dawley rats were administered CAS RN 1338-24-5 daily by gavage for 28 days (males; 14 days prior and after mating) or up to 53 days (females; 14 days prior to mating until lactation day 3) at doses of 100, 300 or 900 mg/kg-bw per day (McKee et al. 2014). Histological and organ weight changes observed at 100 mg/kg‑bw per day and above were not considered adverse by the authors (that is, statistically significant findings considered as minimal, within the historical control data at the testing facility or not considered as being associated with pathological changes by the authors).
In this study, the overall no observed adverse effect level (NOAEL) for reproductive and developmental effects is 100 mg/kg-bw per day. At 300 mg/kg-bw per day and above, a significant reduction in the number of live pups/litter, and a dose-related increase of epididymis and testes relative weights were observed. The developmental effects observed in the study at 300 mg/kg-bw per day were observed in the absence of maternal toxicity. At 900 mg/kg-bw per day, there was a significant reduction in the number of offspring born per litter and in survival (those who survived had significantly lower body weights than those in the control groups). There was also a substance-related decrease in the numbers of corpora lutea and implantation sites however changes were not significant. The significant reduction in absolute uterine weight at 100 mg/kg-bw per day and above was considered related to the reduced (not significant) body weight gain and was not associated with any gross, histopathological, or clinical pathology changes. The study authors also noted that there were no apparent treatment-related effects on mating, frequency of mating, time to mate, mating success, or gestational period length (McKee et al. 2014).
As part of the registration requirements under REACH, ECHA requested that the Registrant carry out additional toxicity studies on CAS RN 1338-24-5 (ECHA 2016b). As a result, the Registrant carried out a 90-day oral toxicity study in rats (OECD 408), as well as an oral pre-natal developmental toxicity study in rats (OECD 414) and the data were added to the registration dossier in 2019. The results from these studies (Unnamed Study Report 2018a, 2018b as cited in ECHA c2007-2017) were reviewed, and the study authors did not identify effect levels more conservative than the values described above (for example, McKee et al 2014).
Calcium naphthenates
A carcinogenicity study was available (dermal study in mice), however, the study authors noted that tumour formation in the group administered calcium naphthenates may have been influenced by local tissue damage (Shell Research Limited 1986; Shell Oil Co 1987). Calcium naphthenates were not genotoxic in several in vitro mutagenicity and clastogenicity assays (that is, bacterial reverse mutation and gene conversion assays, mouse lymphoma forward mutation test and chromosome aberration assay) (Shell Toxicology Laboratory [Tunstall] 1982; Seifried et al. 2006). In dermal studies, neither systemic toxicity nor adverse effects on reproduction/development (no significant changes in testes weights of exposed males) was observed (Shell Research Limited 1983); calcium naphthenates were found to suppress active sebaceous glands after repeated dermal exposure (Shell Research Limited 1987). Calcium naphthenates are not acutely toxic (oral LD50 ≥ 5 mL/kg in rats) (Rockhold 1955; Shell Toxicology Laboratory [Tunstall] 1977). Calcium naphthenates are not expected to have sensitizing properties.
6.3 Characterization of risk to human health
Exposure of the general population to CAS RN 1338-24-5 and calcium naphthenates through environmental media, food, or the use of products available to consumers is expected to be minimal. As a result of the expected minimal exposure, the potential risk to human health is considered to be low, and a hazard characterization was not considered necessary at this time.
The human health assessment took into consideration those groups of individuals within the Canadian population who, due to greater susceptibility or greater exposure, may be more vulnerable to experiencing adverse health effects. For the commercial naphthenic acids group, these subpopulations were considered; however, exposures were expected to be minimal based on use patterns and therefore were not quantified.
6.4 Uncertainties in evaluation of risk to human health
There are some uncertainties in the health effects database due to the lack of toxicological data. However, there is sufficient data to inform on the level of exposure of CAS RN 1338-24-5 and calcium naphthenates to the general population in Canada. Given that exposure to the general population in Canada is expected to be minimal, a qualitative approach to risk characterization is considered appropriate for this assessment.
7. Conclusion
Considering all available lines of evidence presented in this assessment, there is low risk of harm to the environment from CAS RN 1338-24-5 and calcium naphthenates. It is concluded that CAS RN 1338-24-5 and calcium naphthenates do not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
Considering all the information presented in this assessment, it is concluded that CAS RN 1338-24-5 and calcium naphthenates do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore concluded that CAS RN 1338-24-5 and calcium naphthenates do not meet any of the criteria set out in section 64 of CEPA.
References
[API] American Petroleum Institute. 2003. Robust summary of information on reclaimed substances: naphthenic acid (201-14906B).
[API] American Petroleum Institute. 2012. Naphthenic acids category analysis and hazard characterization. Submitted to the US EPA by The American Petroleum Institute Petroleum HPV Testing Group. May, 2012.
Armstrong SA. 2008. Dissipation and phytotoxicity of oil sands naphthenic acids in wetland plants. Thesis submitted to the College of Graduate Studies and Research in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Toxicology Graduate Program University of Saskatchewan Saskatoon, Saskatchewan Canada.
Bartlett AJ, Frank RA, Gillis PL, Parrott JL, Marentette J, Headley JV, Peru K, Hewitt LM. 2017. Toxicity of naphthenic acids to invertebrates: Extracts from oil sands process-affected water versus commercial mixtures. Environ Poll. 227:271-279.
Bio/dynamics Inc. 1979. Acute dermal toxicity study in rabbits. Project No: 5424-78.
Brient JA, Wessner PJ, Doyle MNN. 2000. Naphthenic acids. In: Kirk-Othmer encyclopedia of chemical technology. c.1999-2014 by John Wiley and Sons, Inc.
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C. 1999, c.33. Canada Gazette Part III, vol. 22, no. 3.
[CDAT] Chemical Data Access Tool [modified Jun 2014]. Chemical Data Reporting (CDR) non-confidential information on the production and use of chemicals manufactured or imported into the United States. [accessed 2017 Apr 26].
[CEATAG] Conrad Environmental Aquatics Technical Advisory Group. 1998. Naphthenic acids background information discussion report.
Clemente JS, Prasad NG, MacKinnon MD Fedorak PM. 2003. A statistical comparison of naphthenic acids characterized by gas chromatography-mass spectrometry. Chemosphere. 50(10):1265-1274.
[CMA] Chemical Manufacturers Association. 1983. Comments of the naphthenate metal soaps program panel on the interagency testing committee's recommendations on the need for additional testing on calcium, cobalt, and lead naphthenates. As presented in Chemical Manufacturers Association (1983) cover letter from G.V. Cox, CMA TG S. Newburg-Rinn EPA on the naphthenate metal soaps program panel with enclosure (EPA/OTS 0512190).
[CPCat] Chemical and Product Categories [database]. 2014. Ver. 04. Washington (DC): US Environmental Protection Agency. [updated 2014 May 21; accessed 2015 Sep 30]. [Database described in Dionisio KL, Frame AM, Goldsmith MR, Wambaugh JF, Liddell A, Cathey T, Smith D, Vail J, Ernstoff AS, Fantke P, et al. 2015. Exploring consumer exposure pathways and patterns of use for chemicals in the environment. Toxicol Rep. 2:228-237].
[ChemIDPlus] ChemIDPlus [database] (1993- ). Bethesda (MD): US National Library of Medicine. [updated 2012 Nov 26; accessed 2017 Apr 26].
[ECCC] Environment and Climate Change Canada. 2016a. Science approach document: ecological risk classification of organic substances. Ottawa (ON): Government of Canada.
[ECCC] Environment and Climate Change Canada. 2016b. Supporting documentation: data used to create substance-specific hazard and exposure profiles and assign risk classifications. Gatineau (QC): ECCC. Information in support of the science approach document: ecological risk classification of organic substances. Available from: substances@ec.gc.ca.
[ECCC] Environment and Climate Change Canada. [modified 2020 Oct 9]. Canada-Alberta oil sands environmental monitoring. Ottawa (ON): Government of Canada.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2016a. Rapid Screening of Substances Identified from Phase Two of the Domestic Substances List Inventory Update. Results of the Final Screening Assessment. Ottawa (ON): Government of Canada.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2016b. Rapid Screening Assessment: Polymers Identified from Phase Two of the Domestic Substances List Inventory Update. Ottawa (ON): Government of Canada.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2017a. Screening assessment Cobalt and Cobalt-Containing substances. Ottawa (ON): Government of Canada.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2017b. Second phase of polymer rapid screening. Ottawa (ON): Government of Canada.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. [modified 2017 Mar 12]. Categorization. Ottawa (ON): Government of Canada. [accessed 2017 August 9].
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2019a. Zinc and its compounds draft screening assessment. Ottawa (ON): Government of Canada.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2019b. Copper and its compounds draft screening assessment. Ottawa (ON): Government of Canada.
[ECHA] European Chemicals Agency. c2007-2017. Registered substances database; search results for CAS RN 1338-24-5. Helsinki (FI): ECHA. [accessed 2021 February].
[ECHA] European Chemicals Agency. 2016a. Infocard for naphthenic acids. Last updated in 2016.
[ECHA] European Chemicals Agency. 2016b. Decision on testing proposal(s) set out in a registration pursuant to article 40(3) of regulation (EC) No 1907/2006 for Naphthenic acids, EC No. 700-960-7 (CAS No 1338-24-5). [accessed 2021 February].
[ECHA] European Chemicals Agency. 2017. Brief profile of naphthenic acids. Helsinki (FI): ECHA.
Environment Canada. 2013. DSL inventory update data collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain substances on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
Environment Canada, Health Canada. 2013. Rapid Screening of Substances of Lower Concern. Ottawa (ON): Government of Canada.
Environment Canada, Health Canada. 2014. Rapid Screening of Substances from Phase One of the Domestic Substances List Inventory Update. Ottawa (ON): Government of Canada.
Environment Canada, Health Canada. 2015. Approach for a Subset of Substances Prioritized during Categorization That Have Already Been Addressed. Ottawa (ON): Government of Canada.
Exxon. 1979. Acute oral toxicity of MRD-79-10 in rats, MB 79-3702.
Exxon. 1987. Toxicity statement: naphthenic acids. 87MR 1239. Exxon Biomedical Sciences, Inc. East Millstone, New Jersey.
Garcia-Garcia E, Pun J, Hodgkinson J, Perez-Estrada LA, El-Din MG, Smith DW, Martin JW, Belosevic M. Commercial naphthenic acids and the organic fraction of oil sands process water induce different effects on pro-inflammatory gene expression and macrophage phagocytosis in mice. J App Toxicol. 32(12):968-979.
Han X, Scott AC, Fedorak PM, Bataineh M, Martin JW. 2008. Influence of molecular structure on the biodegradability of naphthenic acids. Environ Sci Technol. 42(4):1290-1295.
Hansen MK, Larsen M, Cohr KH. 1987. Waterborne paints. A review of their chemistry and toxicology and the results of determinations made during their use. Scand J Work Environ Health. 13(6):473-485.
Havre TE. 2002. Formation of calcium naphthenate in water/oil systems, naphthenic acid chemistry and emulsion stability (Ph.D. thesis). Department of chemical engineering, Norwegian University of Science and Technology, Trondheim, Norway, October 2002.
Kinley CM, McQueen AD, Rodgers Jr JH. 2016. Comparative responses of freshwater organisms to exposures of a commercial naphthenic acid. Chemosphere. 153:170-178.
Lewis RJ, Sr. (Editor). 2007. Hawley’s condensed chemical dictionary, Hoboken, New Jersey, John Wiley & Sons, Inc.
Marentette JR, Frank RA, Bartlett A, Gillis P, Hewitt LM, Peru K, Headley J, Brunswick P, Shang D, Parrott J. 2015. Toxicity of naphthenic acid fraction components extracted from fresh and aged oil sands process-affected waters, and commercial naphthenic acid mixtures, to fathead minnow (Pimephales promelas) embryos. Aquat Toxicol. 164:108-117.
McKee RH, North CM, Podhasky P, Charlap JH, Kuhl A. 2014. Toxicological assessment of refined naphthenic acids in a repeated dose/developmental toxicity screening test. Int J Toxicol. 33(1-suppl): 168S-180S.
Mohammed MA. 2010. Characterization, modelling, prediction and inhibition of naphthenate deposits in oilfield production. Thesis. Heriot-Watt University.
[MSDS] Material Safety Data Sheet. 2005. Molykote BR-2 plus multi-purpose E.P. grease [PDF]. Midland, Michigan: Dow Corning Corporation. [accessed 2017 May 17].
[MSDS] Material Safety Data Sheet. 2008. Chryso®Dem 100. France: Chryso SAS. [accessed 2017 May 17].
[MSDS] Material Safety Data Sheet. 2009a. High temperature anti-corrosion coating. Dearborn (MI): Ford Motor Company. [accessed 2017 May 17].
[MSDS] Material Safety Data Sheet. 2009b. Omnigrip EP 175 part B [PDF]. Eltham, Victoria: Omnicrete Pty Ltd. [accessed 2017 May 17].
[MSDS] Material Safety Data Sheet. 2010a. Tampa (FL): Xcel. [accessed 2017 May 17].
[MSDS] Material Safety Data Sheet. 2010b. Epoxy.com #1201 zinc rich primer [PDF]. Dunnellon (FL): Epoxy Systems, Inc. [accessed 2017 May 17].
[MSDS] Material Safety Data Sheet. 2011. Pep-coat 2010 surfacing system seals and protects concrete pavements part B [PDF]. London (UK): Dantex Limited. [accessed 2017 May 17].
[MSDS] Material Safety Data Sheet. 2012a. Pro 1 oil supplement [PDF]. Seattle (WA): Bardahl Manufacturing Corporation [accessed 2017 May 17].
[MSDS] Material Safety Data Sheet. 2012b. Pro 2 oil treatment [PDF]. Seattle (WA): Bardahl Manufacturing Corporation [accessed 2017 May 17].
[MSDS] Material Safety Data Sheet. 2012c. Matrix® LC winter [PDF]. Rockwall (TX): The Whitmore Manufacturing Company [accessed 2017 May 17].
[MSDS] Material Safety Data Sheet. 2013a. Ful-Base®, Ful-Base® binder, Ful-Cryl® binder and Ful-Thane® binder [PDF]. Ajax (ON): Axalta Coating Systems Canada Company. [accessed 2017 May 17].
[MSDS] Material Safety Data Sheet. 2013b. Eni rustia 100/F [PDF]. Roma (IT): ENI S.p.A. [accessed 2017 May 17].
[MSDS] Material Safety Data Sheet. 2013c. Copper naphthenate, 8% Cu MSDS. Houston (TX): Sciencelab.com, Inc. [accessed 2017 May 17].
[MSDS] Material Safety Data Sheet. 2013d. Super K05 chuck grease® part # 11139101 [PDF]. San Clemente (CA): Talega Products Inc. [accessed 2017 May 17].
[MSDS] Material Safety Data Sheet. 2014a. 8% Copper NAP-ALL [PDF]. Frankin (PA): OMG Americas, INC. [accessed 2017 May 17].
[MSDS] Material Safety Data Sheet. 2014b. Rustilo DW 901. Mumbai (IN): Castrol India Ltd. [accessed 2017 May 17].
[MSDS] Material Safety Data Sheet. 2015a. BC825 light maroon [PDF]. Florham Park (NJ): BASF Corporation. [accessed 2017 May 17].
[MSDS] Material Safety Data Sheet. 2015b. Molykote® G-4700 extreme pressure synthetic grease [PDF]. Seneffe: Dow Corning Europe S.A. [accessed 2017 May 17].
[MSDS] Material Safety Data Sheet. 2015c. AeroShell grease 33 [PDF]. London (UK): Shell UK Oil Products Limited. [accessed 2017 May 17].
[MSDS] Material Safety Data Sheet. 2015d. London (UK): Shell UK Oil Products Limited. [accessed 2017 May 17].
[MSDS] Material Safety Data Sheet. 2017a. Plasite 4100/4110 part D. St. Louis (MO): Carboline Company. [accessed 2017 May 17].
[MSDS] Material Safety Data Sheet. 2017b. Technical copper naphthenate [PDF]. Memphis (TN): ISK Biocides, Inc. [accessed 2017 May 17].
[MSDS] Material Safety Data Sheet. 2017c. Vultrex EGF 1000. Mississauga (ON): Petro-Canada Lubricants Inc. [accessed 2017 May 17].
[MSDS] Material Safety Data Sheet. 2017d. Shell gadus S2 V220 00 [PDF]. Ballerup: Struers ApS. [accessed 2017 May 17].
[NPRI] National Pollutant Release Inventory (NPRI) Substance List [2016-2017]. Gatineau (QC): Environment and Climate Change Canada. [accessed 2017 Jun 16].
OECD QSAR Toolbox [Read-across tool]. 2014. Version 3.3. Paris (FR): Organisation for Economic Co-operation and Development, Laboratory of Mathematical Chemistry.
Pennisi SC, Lynch VD. 1977. Acute toxicity and subacute toxicity of naphthenic-acid. Pharmacologist. 19(2):181.
[PMRA] Pest Management Regulatory Agency. 2010. PMRA List of Formulants [PDF]. Ottawa (ON): Health Canada, PMRA. HC Pub. No.: 100460, Cat. No.: H114-22/2010E. [accessed 2017 Jun 16].
Rockhold WT. 1955. Toxicity of naphthenic acids and their metal salts. A.M.A. Arch Ind Health. 12(5):477-482.
Rubinskaya SE. 1974. TR Azerb Nauchno-Issled Inst Gig Tr Prof Zabol, 9:37-40.
Seifried HE, Seifried RM, Clarke JJ, Junghans TB, San RH. 2006. A compilation of two decades of mutagenicity test results with the Ames Salmonella typhimurium and L5178Y mouse lymphoma cell mutation assays. Chem Res Toxicol. 19(5):627-644.
Shell Oil Co. 1987. A two year cutaneous carcinogenicity study with oil additive SAP 011 and its carrier oil in female mice. SBER.87.001. Volume II of II. EPA/OTS0512234.
Shell Research Limited. 1983. Toxicity studies on oil additives: one generation reproduction study in male rabbits repeatedly treated dermaly with SAP 011 for 10 weeks. SBER. 84. 002. As presented in Shell Oil Co (1984). Toxicity studies on oil additives: one generation reproduction study in male rabbits repeatedly treated dermally with SAP 011 for 10 weeks with cover letter. EPA/OTS215260.
Shell Research Limited. 1986. A two year cutaneous carcinogenicity study with oil additive SAP 011 and its carrier oil in female mice. SBER.87.001. Volume I of II. As presented in Shell Oil Co (1987). A two year cutaneous carcinogenicity study with oil additive SAP 011 and its carrier oil in female mice with cover letter dated 031687. EPA/OTS0513224.
Shell Research Limited. 1987. Sebaceous gland suppression tests with SAP 011, and oil fraction isolated (by dialysis) from SAP 011 and a carrier oil.
Shell Toxicology Laboratory (Tunstall). 1977. Toxicology of oil additives: acute toxicity of N7OC. GRR - TLGR. 0091. 77.
Shell Toxicology Laboratory (Tunstall). 1982. Toxicity studies with additives: short-term in vitro tests for genotoxic activity with Shell additive product SAP 010, Report SBGR.82.198.
[SPIN] Substances in Preparations In the Nordic countries [database]. 2017. [database on the internet containing information on chemical substances from each of the Nordic product registers].
[US EPA] United States Environmental Protection Agency. 1983. Twelfth report of the interagency testing committee to the administrator; receipt of report and request for comments regarding priority list of chemicals (notice). Federal Register 48, (106, June 1, 1983): 24443-24452.
