Draft assessment - Substituted Phenols Group
Official title: Draft assessment - Substituted Phenols Group
Environment and Climate Change Canada
Health Canada
January 2024
Synopsis
Pursuant to sections 68 of the Canadian Environmental Protection Act, 1999 (CEPA), the Minister of the Environment and the Minister of Health have conducted an assessment of 15 substances referred to collectively under the Chemicals Management Plan as the Substituted Phenols Group. The Chemical Abstracts Service Registry Numbers (CAS RNsFootnote 1 ) and Domestic Substances List (DSL) names of these substances are listed in the table below.
| CAS RN | DSL name |
|---|---|
| 85-60-9 | Phenol, 4,4'-butylidenebis[2-(1,1-dimethylethyl)-5-methyl- |
| 96-69-5a | Phenol, 4,4'-thiobis[2-(1,1-dimethylethyl)-5-methyl- |
| 96-76-4a | Phenol, 2,4-bis(1,1-dimethylethyl)- |
| 98-54-4a | Phenol, 4-(1,1-dimethylethyl)- |
| 118-82-1 | Phenol, 4,4'-methylenebis[2,6-bis(1,1-dimethylethyl)- |
| 128-37-0 | Phenol, 2,6-bis(1,1-dimethylethyl)-4-methyl- |
| 128-39-2 | Phenol, 2,6-bis(1,1-dimethylethyl)- |
| 1843-03-4a | Phenol, 4,4',4''-(1-methyl-1-propanyl-3-ylidene)tris[2-(1,1-dimethylethyl)-5-methyl - |
| 2082-79-3 | Benzenepropanoic acid, 3,5-bis(1,1-dimethylethyl)-4-hydroxy-, octadecyl ester |
| 4221-80-1 | Benzoic acid, 3,5-bis(1,1-dimethylethyl)-4-hydroxy-, 2,4-bis(1,1-dimethylethyl)phenyl ester |
| 6386-38-5 | Benzenepropanoic acid, 3,5-bis(1,1-dimethylethyl)-4-hydroxy-, methyl ester |
| 35958-30-6 | Phenol, 2,2'-ethylidenebis[4,6-bis(1,1-dimethylethyl)- |
| 36443-68-2 | Benzenepropanoic acid, 3-(1,1-dimethylethyl)-4-hydroxy-5-methyl-, 1,2-ethanediylbis(oxy-2,1-ethanediyl) ester |
| 41484-35-9a | Benzenepropanoic acid, 3,5-bis(1,1-dimethylethyl)-4-hydroxy-, thiodi-2,1-ethanediyl ester |
| 61788-44-1a | Phenol, styrenated |
a This CAS RN is a UVCB (unknown or variable composition, complex reaction products, or biological materials).
All substances in this group have been subject to at least one survey issued pursuant to section 71 of CEPA since 2008. Imports in Canada above the 100 kg reporting threshold and within the range of 1 000 kg to 10 000 000 kg have been reported for all substances; manufacturing in Canada above the 100 kg threshold and within the range of 100 kg and 1 000 kg was reported for three substances in the group. Based on information reported in these surveys, these substances are used in a variety of industrial, commercial and consumer applications, including in lubricant and fuel additives, plastic and rubber additives, and in paints and coatings, personal care products, as a component in the manufacture of food packaging materials, plastics and rubber products, adhesives and sealants, and fabric and textiles.
Available data and model predictions indicate that one substance (CAS RN 98-54-4) can undergo some degradation in the environment and that the other 14 substances do not readily degrade in the environment. Two substances in this group (CAS RNs 118-82-1 and 61788-44-1) are expected to have high potential for bioaccumulation, while the other 13 substances are not expected to significantly bioaccumulate in organisms.
The ecological risks associated with four substances in the Substituted Phenols Group were characterized using the ecological risk classification of organic substances (ERC), which is a risk-based approach that employs multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining the ecological risk classification. Based on the outcome of the ERC analysis, these four substances (CAS RNs 85-60-9, 2082-79-3, 6386-38-5, and 41484-35-9) are considered unlikely to be causing ecological harm.
Of the remaining 11 substances, the ecological effects assessment was conducted on the basis of available empirical toxicity data for the aquatic compartment or using the critical body residue (CBR) approach. Aquatic predicted no-effect concentrations (PNECs) were calculated for nine substances, and the PNECs calculated suggest that they are capable of causing adverse effects to aquatic organisms below their solubility limit. Among these nine substances, the potential for endocrine effects via estrogen receptor binding was identified for CAS RN 98-54-4 and the monostyrenated phenol component of the UVCB substance CAS RN 61788-44-1; both are non-hindered phenols. The two additional substances (CAS RNs 4221-80-1 and 1843-03-4) are not expected to demonstrate any adverse effect on aquatic organisms at or below their water saturation levels. Empirical sediment toxicity data is not available for any of the 11 substances, and empirical soil toxicity data is only available for five substances (CAS RNs 96-76-4, 118-82-1, and 128-39-2, and for the analogues of CAS RNs 35958-30-6 and 36443-68-2). Given the uncertainties associated with using the CBR approach for derivation of PNECs for sediment and soil organisms, PNECs for sediment organisms were not calculated, and PNECs for soil organisms were only developed using empirical soil toxicity data.
An environmental exposure assessment was conducted for these 11 substances by considering the major industrial applications and the reported import quantities of these substances. Given that few companies reporting manufacturing and that reported quantities were small, manufacturing sources were not considered in the development of exposure scenarios. The aquatic compartment is considered to be the key receiving environmental compartment; therefore, the ecological risk characterization focused on this compartment. Exposure in soil was also estimated for five substances for which PNECs were derived. The aquatic and soil predicted environmental concentrations (PECs) were initially calculated based on generic assumptions reflecting different industrial sectors. Where generic scenarios indicated risk, refinements were further applied to inform the potential for environmental exposure. Risk quotients were calculated by comparing the PECs with the PNECs for the aquatic and soil compartments, and outcomes were considered as key lines of evidence in the ecological risk characterization.
Considering all available lines of evidence presented in this draft assessment, there is risk of harm to the environment from CAS RNs 118-82-1, 128-37-0, 36443-68-2, and 61788-44-1. It is proposed to conclude that CAS RNs 118-82-1, 128-37-0, 36443-68-2, and 61788-44-1 meet the criteria under paragraph 64(a) of CEPA as they are entering or may enter the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity. However, it is proposed to conclude that CAS RNs 118-82-1, 128-37-0, 36443-68-2, and 61788-44-1 do not meet the criteria under paragraph 64(b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger to the environment on which life depends. It is also proposed to conclude that CAS RNs 85-60-9, 96-69-5, 96-76-4, 98-54-4, 128-39-2, 1843-03-4, 2082-79-3, 4221-80-1, 6386-38-5, 35958-30-6, and 41484-35-9 do not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
With respect to human health, potential exposure of the general population of Canada to substances in this group can occur through drinking water, food, potential use of the substances as a component in the manufacture of food packaging materials, and products available to consumers. Specifically, exposure to CAS RN 128-37-0 may occur through the use of cosmetics, paints, plastics and rubber, and lubricants. Exposure to CAS RN 2082-79-3 may occur from use of plastics and rubber, paints, and cosmetics. In addition, exposure to CAS RNs 96-76-4, 98-54-4, 118-82-1, 128-37-0, 128-39-2, and 41484-35-9 may occur from use of lubricants and automotive products. Liver and thyroid effects were reported for CAS RNs 128-37-0, 35958-30-6, and 36443-68-2. The critical health effects for CAS RN 35958-30-6 were determined to be testis toxicity and liver and thyroid effects in males and females, respectively, based on information from the analogue CAS RNs 88-24-4 and 119-47-1. Liver, spleen, and adrenal effects were reported for CAS RN 1843-03-4. Liver effects as well as altered hematological parameters were reported for CAS RNs 85-60-9, 96-76-4, 118-82-1, 128-39-2, and 2082-79-3. CAS RN 96-76-4 effects were read-across from CAS RN 128-39-2. It was also noted that CAS RNs 96-69-5, 98-54-4, and 35958-30-6 displayed reproductive and or developmental toxicities. Finally, no toxicological effects were identified for CAS RNs 4221-80-1, 6386-38-5, 41484-35-9, and 61788-44-1. Comparison of critical effects levels with levels of the substances in this group to which the general population may be exposed (through drinking water, food and food packaging, use of products available to consumers) resulted in margins of exposure that are considered adequate to account for uncertainties in the health effects and exposure databases.
The human health assessment took into consideration those groups of individuals within the Canadian population who, due to greater susceptibility or greater exposure, may be more vulnerable to experiencing adverse health effects. These subpopulations were taken into account in the risk assessment outcomes of certain substances in the Substituted Phenols Group.
Considering all the information presented in this draft assessment, it is proposed to conclude that the 15 substances in the Substituted Phenols Group do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute a danger in Canada to human life or health.
It is therefore proposed to conclude that CAS RNs 118-82-1, 128-37-0, 36443-68-2, and 61788-44-1 meet one or more of the criteria set out in section 64 of CEPA and that the other 11 substances in the Substituted Phenols Group do not meet any of the criteria set out in section 64 of CEPA.
It is also proposed that CAS RNs 118-82-1 and 61788-44-1 meet the persistence and bioaccumulation criteria, while CAS RNs 128-37-0 and 36443-68-2 meet the persistence but not the bioaccumulation criteria as set out in the Persistence and Bioaccumulation Regulations of CEPA.
1. Introduction
Pursuant to section 68 of the Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999), the Minister of the Environment and the Minister of Health have conducted an assessment of 15 substances, referred to collectively under the Chemicals Management Plan as the Substituted Phenols Group, to determine whether these substances present or may present a risk to the environment or to human health. Thirteen substances in the Substituted Phenols Group were identified as priorities for assessment as they met the categorization criteria or were prioritized through other mechanisms (ECCC, HC [modified 2017]). The other two substances in this group did not meet the categorization criteria; however, they were included in this assessment because they were determined to be priorities as a result of the approach described for the Identification of Risk Assessment Priorities (IRAP) (ECCC, HC 2015).
The ecological risks of 4 of the 15 substances in the Substituted Phenols Group were characterized using the ecological risk classification of organic substances (ERC) approach (ECCC 2016a; Appendix A). According to data considered in the ERC approach (ECCC 2016b), Chemical Abstract Services Registry Numbers (CAS RNs) 85-60-9, 2082-79-3, 6386-38-5, and 41484-35-9 were identified as having low potential to cause ecological harm. These results are considered in support of the conclusions made under section 64 of CEPA in this assessment.
CAS RNs 85-60-9, 96-69-5, 96-76-4, 98-54-4, 128-37-0, 128-39-2, 2082-79-3, 6386-38-5, 41484-35-9, and 61788-44-1 have been previously assessed by at least one of the following organizations: the United States (US) Environmental Protection Agency (EPA), the European Food Safety Authority (EFSA), the Organisation for Economic Co‑operation and Development (OECD), the Joint FAO/WHO Expert Committee on Food Additives (JECFA), or the European Chemicals Agency (ECHA). These assessments undergo rigorous review (typically including peer review) and endorsement. Health Canada considers these international assessments to be reliable. Furthermore, OECD Screening Information Dataset (SIDS) Initial Assessment Reports (SIARs) undergo rigorous review (including peer review) and endorsement by international governmental authorities. Health Canada and Environment and Climate Change Canada are active participants in this process and consider these assessments to be reliable. These assessments were used to inform this assessment.
This draft assessment considers information on chemical properties, environmental fate, hazard, uses, and exposure, and includes information independently identified in literature, generated with models, and submitted by stakeholders. Relevant data were identified up to April 2022. Empirical data from key studies as well as results from models were used to reach proposed conclusions. When available and relevant, information presented in assessments from other jurisdictions was considered.
This draft assessment was prepared by staff in the CEPA Risk Assessment Program at Health Canada and Environment and Climate Change Canada, and incorporates input from other programs within these departments. The ecological and human health portions of this assessment have undergone external review and/or consultation. Comments on the technical portions relevant to the environment were received from Dr. Valérie Langlois of the Institut national de la recherche scientifique, Geoff Granville of GCGranville Consulting Corp., and Dr. Connie Gaudet. Comments on the technical portions relevant to human health were received from Tetra Tech Inc. (Theresa Lopez, Jennifer Flippin, and Dr. Joan Garey). For four of the substances, the ecological portion of this assessment is based on the ERC document (published July 30, 2016), which was subject to an external peer review as well as a 60-day public comment period. While external comments were taken into consideration, the final content and outcome of this assessment remain the responsibility of Health Canada and Environment and Climate Change Canada.
Assessments focus on information critical to determining whether substances meet the criteria as set out in section 64 of CEPA by considering scientific information, including information, if available, on subpopulations who may have greater susceptibility or greater exposure, vulnerable environments and cumulative effectsFootnote 2 , and by incorporating a weight-of-evidence approach and precautionFootnote 3 . This draft assessment presents the critical information and considerations on which the proposed conclusions are based.
2. Identity of substances
Substance identity information, including the CAS RNs and Domestic Substances List (DSL) names, chemical structures, and molecular weights for the 15 substances in this group are presented in Table 2-1 and Table 2-2.
Of the 15 substances in this group, 14 are discrete chemicals, including 1 non-hindered phenol (CAS RN 98-54-4) and 13 partially or fully hindered phenols with one or two tert-butyl groups adjacent to the hydroxyl (-OH) group on the phenyl ring (Table 2-1). Other substituents in the para or meta position(s) relative to the -OH group may also contribute to steric hindrance, although to a lesser extent.
| CAS RN | DSL name | Chemical structure and molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
| 85-60-9 | Phenol, 4,4'-butylidenebis[2-(1,1-dimethylethyl)-5-methyl- |  C26H38O2 |
382.59 |
| 96-69-5a | Phenol, 4,4'-thiobis[2-(1,1-dimethylethyl)-5-methyl- | 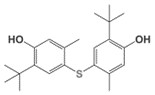 C22H30O2S |
358.54 |
| 96-76-4a | Phenol, 2,4-bis(1,1-dimethylethyl)- |  C14H22O |
206.33 |
| 98-54-4a | Phenol, 4-(1,1-dimethylethyl)- |  C10H14O |
150.22 |
| 118-82-1 | Phenol, 4,4'-methylenebis[2,6-bis(1,1-dimethylethyl)- | 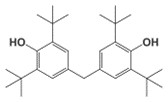 C29H44O2 |
424.67 |
| 128-37-0 | Phenol, 2,6-bis(1,1-dimethylethyl)-4-methyl- |  C15H24 |
220.36 |
| 128-39-2 | Phenol, 2,6-bis(1,1-dimethylethyl)- |  C14H22O |
206.33 |
| 1843-03-4a | Phenol, 4,4',4''-(1-methyl-1-propanyl- 3-ylidene)tris[2-(1,1-dimethylethyl)-5-methyl - |  C37H52O3 |
544.82 |
| 2082-79-3 | Benzenepropanoic acid, 3,5-bis(1,1-dimethylethyl)-4-hydroxy-, octadecyl ester | 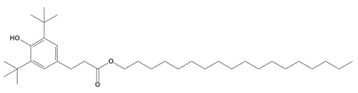 C35H62O3 |
530.88 |
| 4221-80-1b | Benzoic acid, 3,5-bis(1,1-dimethylethyl)-4-hydroxy-, 2,4-bis(1,1-dimethylethyl)phenyl ester | 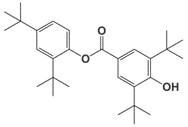 C29H42O3 |
438.65 |
| 6386-38-5 | Benzenepropanoic acid, 3,5-bis(1,1-dimethylethyl)-4-hydroxy-, methyl ester |  C18H28O3 |
292.42 |
| 35958-30-6 | Phenol, 2,2'-ethylidenebis[4,6-bis(1,1-dimethylethyl)- |  C30H46O2 |
438.70 |
| 36443-68-2b | Benzenepropanoic acid, 3-(1,1-dimethylethyl)-4-hydroxy-5-methyl-, 1,2-ethanediylbis(oxy-2,1-ethanediyl) ester | 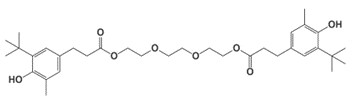 C34H50O8 |
586.77 |
| 41484-35-9a | Benzenepropanoic acid, 3,5-bis(1,1-dimethylethyl)-4-hydroxy-, thiodi-2,1-ethanediyl ester |  C38H58O6S |
642.94 |
a This substance was prioritized through other mechanisms.
b This substance was determined to be a priority as a result of the approach described for the Identification of Risk Assessment Priorities (IRAP).
The 15th substance (phenol, styrenated, CAS RN 61788-44-1) is an unknown or variable composition, complex reaction products, or biological material (UVCB) substance. This substance is comprised of at least five components, including one non-hindered phenol and four partially or fully hindered phenols (Table 2-2). Assessment of the UVCB substance with regard to its potential for persistence, bioaccumulation, effects on organisms, and potential exposure and hazard to human health is based on empirical data as well as model predictions for both the substance and its individual components.
| CAS RN | DSL name | Chemical structure and molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
| 1988-89-2 | Phenol, 4-(1-phenylethyl)- Monostyrenated phenol |
 C14H14O |
198.27 |
| 4237-44-9 26857-99-8 |
Phenol, 2-(1-phenylethyl)- Monostyrenated phenol |
 C14H14O |
198.27 |
| 2769-94-0 25640-70-4 | 2,4-distyrenated phenol Distyrenated phenol |
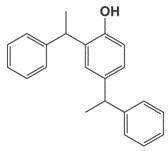 C22H22O |
302.42 |
| 4237-28-9 | 2,6-distyrenated phenol Distyrenated phenol |
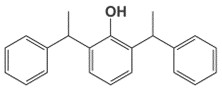 C22H22O |
302.42 |
| 18254-13-2 | Phenol, 2,4,6-tris(1-phenylethyl)- Tristyrenated phenol |
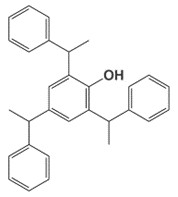 C30H30O
C30H30O |
406.57 |
The relative proportions of mono-, di-, and tri-styrenated phenol vary in commercial products under the same CAS RN. Brooke et al. (2009) has reported compositions of each component in a number of products; ranges of all components are summarized in Table 2-3. Distyrenated phenol and tristyrenated phenol components represent a large fraction of the composition in this UVCB substance.
| Component | Proportion (%) |
|---|---|
| Monostyrenated phenol | 2–15 |
| Distyrenated phenol | 23–52 |
| Tristyrenated phenol | 43–70 |
| Other minor components (styrene dimer, styrene, phenol) | <1.0 |
2.1 Selection of analogues and use of (Q)SAR models
A read-across approach using data from analogues and the results of (quantitative) structure-activity relationship ((Q)SAR) models, where appropriate, has been used to inform the ecological and human health assessments. Selected analogues were structurally similar to substances within this group and had relevant empirical data that could be used to read across to substances with limited empirical data. The applicability of (Q)SAR models was determined on a case-by-case basis. The read-across data used to inform the ecological and human health assessments of substances in this group are summarized in Table 2-4.
| CAS RN for analogue | Chemical name | Chemical structure | Physical-chemical properties | Fate | Ecotoxicity | Human health |
|---|---|---|---|---|---|---|
| 88-24-4 | 6,6'-di-tert-butyl-4,4'-diethyl-2,2'-methylenediphenol |  |
N/A | N/A | N/A | Yes |
| 119-47-1 | 2,2′-Methylenebis(6-tert-butyl-4-methylphenol) MBMBP) |
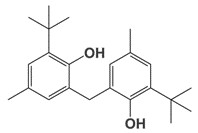 |
Yes | Yes | Yes | Yes |
| 68512-30-1a | Phenol, methylstyrenated (MSP) |
 |
Yes | Yes | Yes | N/A |
Abbreviation: N/A, not applicable
a This substance is a UVCB. Read-across was conducted between certain components in this substance and CAS RN 61788-44-1 on the basis of chemical structural similarity.
Since no empirical data were identified for CAS RN 35958-30-6, CAS RNs 88-24-4 and 119-47-1 were used as analogues to inform the assessment of this substance. The analogues were considered to be appropriate for read-across based on structural similarities; they have the same ortho orientation of the hydroxyl moieties relative to the alkyl bridge as that of CAS RN 35958-30-6, as well as similar tert-butyl substituents in the meta position to the alkyl bridge. In addition, they have similar physical-chemical properties as CAS RN 35958-30-6 (Appendix D). CAS RN 119-47-1 has an OECD SIDS (OECD 2003) and has been previously assessed by Environment Canada and Health Canada (EC, HC 2009).
Phenol, methylstyrenated (MSP) (CAS RN 68512-30-1) was identified as an analogue for the UVCB substance (CAS RN 61788-44-1). Components in both substances are structurally similar, except for the methyl group on styrene for MSP. Physical-chemical properties and data for the fate and ecotoxicity of components in MSP are considered in the assessment where there is a lack of empirical data for the relevant components in CAS RN 61788-44-1.
3. Physical and chemical properties
The 15 substances in the Substituted Phenols Group have diverse physical-chemical properties. Summaries of key physical and chemical properties for each of these substances are presented in Table 3-1, while more details are presented in ECCC (2023). Read-across data from analogues and predictions from the available (Q)SAR models were used when empirical data were limited or not available.
| CAS RN | Melting point (°C) | Vapour pressure (Pa) | Water solubility (mg/L) | log KOW | log KOC | Henry’s law constant (Pa·m3/mol) |
|---|---|---|---|---|---|---|
| 85-60-9 | 209.5b,c (a) | <1.3×10-8 b | <0.004b | 6.4b | 5.5b | ~0.0012 |
| 96-69-5 | 159.5b,d,e (a) | 2.9×10-8 d (m) | 0.028b | 5.2b | 4.0d (m) | 3.7×10-4 |
| 96-76-4 | 56.7b,d (a) | 0.64g | 34b (g) | 5.0b (g) | 3.6b (g) | 3.9 |
| 98-54-4 | 99.2b,d | 4.2h (g) | 610b | 3.3b,I,j | 2.9d (m) | 1.04 |
| 118-82-1 | 155.2b (a) | 4.2×10-7 b (g) | 3.1×10-4 b (g) | 7.4b | 5.4d (m) | 0.55 |
| 128-37-0 | 70b,d (a) | 0.52b,g (g) | 0.73b (g) | 5.1b,d | 3.9d (m) | 157 |
| 128-39-2 | 38b,d (a) | 1.0b | 3.21b,d (g) | 4.7b (g) | 3.6b (g) | 64.9 |
| 1843-03-4 | 186.5b,d (a) | 1.6×10-6 b (g) | 1.1×10-7 d (m) | 8.5b | 7.5b | 8100 |
| 2082-79-3 | 52b,k (a) | 2.5×10-7 b,j,l | 0.0029b,k | 13.4d (m) | 8.5d (m) (g) | 0.047 |
| 4221-80-1 | 197.1b (a) | 2.5×10-8 d (m) | 1.76×10-5 d (m) (g) | 9.1d (m) | 6.6d (m) (g) | 0.62 |
| 6386-38-5 | 66b (a) | 6.1×10-4 b | 2.2b | 5.06d (m) | 4.3b | 0.08 |
| 35958-30-6 (read-across from CAS RN 119-47-1) | 162m | 3.9×10-9 d,n (m) (g) | 0.012b,o (g) | 6.3d | 5.2d (m) | 1.4×10-4 |
| 36443-68-2 | 77.5b (a) | 4.0×10-8 b | 0.10b | 4.7b | 3.6d (m) | 2.3×10-4 |
| 41484-35-9 | 65.6b (a) | 1.3×10-9 b (m) | 5.4x10-7 d (m) (g) | 10.4d (m) | 7.6d (m) (g) | 1.58 |
| 61788-44-1p | 90.0d (m) | 0.0065d (m) | 231q | 3.7q (read-across from mono-methyl-styrenated phenol in MSP) | 3.1d (m) | 5.6×10-3 |
| 61788-44-1r | 159.1d (m) | 7.8×10-8 d (read-across from dimethyl-styrenated phenol in MSP) | 0.67q | 6.2q | 4.5d (m) | 3.5×10-5 |
| 61788-44-1s | 223.1d (m) | 8.7×10-10 d (m) | 0.0071q | 7.8q | 5.4d (m) | 5.0×10-5 |
Abbreviations: KOW, octanol-water partition coefficient; KOC, organic carbon-water partition coefficient
a Expanded tables of physical-chemical values for each CAS RN and associated references are provided in ECCC (2023). For the purpose of modelling, in cases where multiple values were available for a certain property, a geometric mean of these values was calculated as suitable for a CAS RN (marked with “g”). For the melting point, data identified do not range widely; therefore, mathematic means were calculated for each CAS RN (marked with “a”). In cases where there is a lack of empirical data, the modelled or estimated values were considered (marked with “m”). Henry’s law constant was calculated based on the water solubility and the vapour pressure.
b ECHA c2007-2017
c Hawley 2007
d EPI Suite c2000-2012
e Lide 2008
f OECD QSAR Toolbox 2017
g Perry and Green 1984
h Chao et al. 1983
i EC 2008
j Hansch et al. 1995
k OECD 2006
l Neely and Blau 1985
m Spectrum 2009
n ChemIDplus 1993-
o HSDB 1983-
p CAS RN 61788-44-1 is a UVCB substance; values reported are for the monostyrenated phenol component.
q Brooke et al. 2009
r CAS RN 61788-44-1 is a UVCB substance; values reported are for the distyrenated phenol component.
s CAS RN 61788-44-1 is a UVCB substance; values reported are for the tristyrenated phenol component.
4. Sources and uses
CAS RNs 98-54-4 and 128-37-0 have been reported to occur naturally in the environment (PubChem 2004-; Bouftira et al. 2007; Babu and Wu 2008; Aourahoun et al. 2014; Usman et al. 2016; Gharbi et al. 2017). None of the other substances in the Substituted Phenols Group have been identified as occurring naturally in the environment.
Each of the substances in this group was included in at least one survey issued pursuant to section 71 of CEPA (EC 2009, 2013; ECCC 2017). As reported manufacture quantities were low (<1000 kg per year), the manufacture of substituted phenols is not considered to be a major activity in Canada. Table 4‑1 summarizes total manufacture and import quantities reported in Canada for substances in the Substituted Phenols Group (EC 2009, 2013; ECCC 2017).
| CAS RN | Total manufacture (kg/year)a | Total imports (kg/year)a | Reporting year |
|---|---|---|---|
| 85-60-9 | Not reported | 10 000–100 000 | 2008 |
| 96-69-5 | Not reported | 10 000–100 000 | 2011 |
| 96-76-4 | Not reported | 1 000–10 000 | 2011 |
| 98-54-4 | Not reported | 10 000–100 000 | 2011 |
| 118-82-1 | Not reported | 10 000–100 000 | 2008 |
| 128-37-0 | 100–1 000 | 100 000–1 000 000 | 2011 |
| 128-39-2 | 100–1 000 | 100 000–1 000 000 | 2008 |
| 1843-03-4 | Not reported | 10 000–100 000 | 2011 |
| 2082-79-3 | Not reported | 1 000 000–10 000 000 | 2011 |
| 4221-80-1 | Not reported | 10 000–100 000 | 2008, 2016b |
| 6386-38-5 | <100 | 10 000–100 000 | 2008 |
| 35958-30-6 | Not reported | 1 000–10 000 | 2008 |
| 36443-68-2 | Not reported | 10 000–100 000 | 2008, 2016b |
| 41484-35-9 | Not reported | 100 000–1 000 000 | 2011 |
| 61788-44-1 | 100–1 000 | 1 000–10 000 | 2011 |
a Values reflect quantities reported in response to the surveys conducted under section 71 of CEPA (EC 2009, 2013; ECCC 2017). See surveys for specific inclusions and exclusions (Schedules 2 and 3).
b For the substances that were included in multiple surveys, the ranges of total imports represent the sum of import quantities reported by all companies in either survey.
Limited information is available to address potential temporal variability in annual domestic industrial use quantities of substituted phenols, such as results from multiple surveys conducted over the years under section 71 of CEPA (EC 2009, 2013; ECCC 2017) or voluntary surveys. International data sources strongly indicate quantity variability. International trends indicate that the substances are used in large quantities globally and that increases and spikes in use are plausible (CDR 2016; SPIN c2017). In some years, the amount of substituted phenols used in a country may increase by hundreds of thousands to millions of kilograms compared to the previous year. Overall, the use of substituted phenols across multiple industrial sectors in North America has increased by 1.4% from 2014 to 2017 (Chinn et al. 2018).
The substances in the Substituted Phenols Group are primarily used as antioxidants in multiple industrial sectors. Based on their reported uses, the substances are mainly found in the following product types: lubricants, fuels, plastic products, rubber products, and paints and coatings (EC 2009, 2013; ECCC 2017). Lower quantities are used in personal care products, as a component in the manufacture of food packaging materials, adhesives and sealants, and fabric and textiles (EC 2009, 2013; ECCC 2017). Additional uses in Canada are presented in Table 4-2.
| Use | CAS RN |
|---|---|
| Food additivea | 128-37-0 |
| Incidental additiveb | 118-82-1, 6386-38-5, 128-39-2 |
| Food packaging materialsc | 85-60-9, 96-69-5, 96-76-4, 128-37-0, 1843-03-4, 2082-79-3, 4221-80-1, 6386-38-5, 35958-30-6, 36443-68-2, 41484-35-9 |
| Medicinal or non-medicinal ingredients in disinfectant, human, or veterinary drug productsd | 128-37-0 |
| Natural Health Products Ingredients Databasee | 98-54-4, 128-37-0 |
| Non-medicinal ingredient in natural health productsf | 128-37-0 |
| List of Prohibited and Restricted Cosmetic Ingredientsg | 98-54-4 |
| Notified to be present in cosmetics under the Cosmetic Regulationsh | 128-37-0, 2082-79-3, 4221-80-1, 35958-30-6 |
| Formulant in registered pest control productsi | 128-37-0, 2082-79-3, 6386-38-5 |
a Personal communication from the Food Directorate (FD) of Health Canada (HC) to the Existing Substances Risk Assessment Bureau (ESRAB), HC, dates ranging from March 2017 to Feb 2019; unreferenced.
b Personal communication from the FD of HC to the ESRAB, HC, dates ranging from March 2017 to Feb 2019; unreferenced.
c Personal communication from the FD of HC to the ESRAB, HC, dates ranging from March 2017 to Feb 2019; unreferenced.
d DPD [modified 2016]
e NHPID [modified 2022]
f LNHPD [modified 20121
g Health Canada [modified 2018]
h Personal communication from the Consumer and Hazardous Products Safety Directorate (CHPSD) of HC to the ESRAB, HC, dates ranging from March 2017 to Feb 2019; unreferenced.
I Personal communication from the Pest Management Regulatory Agency (PMRA) of HC to the ESRAB, HC, dated March 2017; unreferenced.
According to publicly available information, including product material safety data sheets (MSDSs), CAS RNs 118-82-1 (MSDS 2016) and 128-39-2 (MSDS 2015) may be found in automotive care products in Canada. CAS RN 128-37-0 may also be found in air fresheners (MSDS 2014a), glow sticks (MSDS 2010a), hunting accessories (MSDS 2014b), and other do-it-yourself (DIY) products (MSDS 2014c) in Canada. CAS RN 98-54-4 may also be found in fillers, colouring or odour agents, and surface treatment products (AGDH 2016). CAS RN 36443-68-2 may be used in fabrics and textiles (US EPA 2018), while CAS RN 61788-44-1 may be found in electronics (MSDS 2014d) and wood epoxy glue (MSDS 2013b) in Canada.
5. Releases to the environment
In general, point source releases of substances in the Substituted Phenols Group are expected to occur during their use in various industrial applications. Surface water is expected to be the main receiving compartment following release through wastewater treatment systems (WWTSs).Footnote 4 After entering surface water, these substances may partition to sediment to some extent, depending on their water solubility and partition coefficients. These substances may also enter soil from WWTS biosolids, which are commonly used for soil enrichment.
Of the substances in the group, CAS RN 128-37-0 is the only substance reportable to the National Pollutant Release Inventory (NPRI) (NPRI 1994-2017). NPRI provides information on releases and transfers of key pollutants in Canada. In 2017, one facility reported 1 kg of on-site releases of CAS RN 128-37-0.
Details on exposure characterization resulting from industrial releases are presented in section 7.2 of this assessment.
6. Environmental fate and behaviour
6.1 Environmental distribution
A Level III fugacity model (New EQC 2011) was used to characterize the mass-balance distribution of substances in the Substituted Phenols Group between various environmental media. Model outcomes of predicted environmental distribution if released to water are summarized in Table 6-1, while predictions for releases to other environmental compartments are presented in ECCC (2023).
According to the model results, if released to water (that is, the main expected receiving compartment), all of these substances are expected to remain in water or adsorb to sediment to varying extents (Table 6-1). Partitioning between these two compartments varies depending on water solubility and potential for adsorption to particles. Volatilization of these substances from surface water is expected to be minor.
If released to soil, all of these substances are expected to remain in the soil compartment. Volatilization of these substances from soil is not expected. Although direct releases to soil are not anticipated, indirect releases may result from the land application of biosolids from WWTSs.
All of the substances in the Substituted Phenols Group possess low to moderate vapour pressure. If released to air, substances with moderate vapour pressure can be expected to have some presence in air.
| CAS RN | Partitioning in air (%) | Partitioning in water (%) | Partitioning in soil (%) | Partitioning in sediment (%) |
|---|---|---|---|---|
| 85-60-9 | Negligible | 11 | Negligible | 89 |
| 96-69-5 | Negligible | 74 | Negligible | 26 |
| 96-76-4 | Negligible | 86 | Negligible | 13 |
| 98-54-4 | Negligible | 97 | Negligible | 3 |
| 118-82-1 | Negligible | 13 | 1 | 87 |
| 128-37-0 | 2 | 75 | Negligible | 23 |
| 128-39-2 | 1 | 86 | Negligible | 13 |
| 1843-03-4 | Negligible | 4 | Negligible | 96 |
| 2082-79-3 | Negligible | 4 | Negligible | 96 |
| 4221-80-1 | Negligible | 6 | Negligible | 94 |
| 6386-38-5 | Negligible | 58 | Negligible | 42 |
| 35958-30-6 | Negligible | 17 | Negligible | 83 |
| 36443-68-2 | Negligible | 87 | Negligible | 13 |
| 41484-35-9 | Negligible | 4 | Negligible | 96 |
| 61788-44-1a | Negligible | 95 | Negligible | 5 |
| 61788-44-1b | Negligible | 46 | Negligible | 55 |
| 61788-44-1c | Negligible | 13 | Negligible | 87 |
a CAS RN 61788-44-1 is a UVCB substance; values reported are for the monostyrenated phenol component.
b CAS RN 61788-44-1 is a UVCB substance; values reported are for the distyrenated phenol component.
c CAS RN 61788-44-1 is a UVCB substance; values reported are for the tristyrenated phenol component.
6.2 Environmental persistence
Empirical degradation data in air have not been identified for substances in the Substituted Phenols Group. Model half-lives for this compartment range from 0.6 to 10.8 hours (EPI Suite c2000-2012; details are presented in ECCC 2023), suggesting rapid degradation in air.
For soil, empirical biodegradation data have been identified for four substances (CAS RNs 96-76-4, 98-54-4, 118-82-1, and 128-37-0). Reported half-lives are below 50 days (ECCC 2023). No data were identified for sediment.
Empirical degradation and biodegradation data in water were identified for most of the substances in the Substituted Phenols Group. Analogues were used in a few instances to fill data gaps, and models (EPI Suitec2000 2012 and CATALOGIC 2016) were used to provide supplemental information. A summary of key empirical data in water for the 14 discrete substances is presented in Table 6-2. More details are compiled in ECCC (2023). Data for the UVCB substance CAS RN 61788-44-1 are presented in Table 6-3.
| CAS RN | Method | Key degradation data | Potential for degradation |
|---|---|---|---|
| 85-60-9 | OECD Guideline 301B | 28-day degradation = 2–12% (CO2 evolution) |
No rapid degradation |
| 96-69-5 | OECD Guideline 301B | 35-day degradation = 1% (CO2 evolution) |
No rapid degradation |
| 96-76-4 | OECD Guideline 302C | 28-day degradation = 0% (O2 consumption) |
No rapid degradation |
| 98-54-4 | OECD Guideline 301B | 28-day degradation = 58.5–63.5% (CO2 evolution) |
Ready biodegradation in water |
| 118-82-1 | OECD Guideline 301C | 28-day degradation = 0% (BOD) |
No rapid degradation |
| 128-37-0 | OECD Guideline 301C | 28-day degradation = 4.5% (BOD) |
No rapid degradation |
| 128-39-2 | OECD Guideline 301B | 28-day degradation = 1–5% (CO2 evolution) |
No rapid degradation |
| 1843-03-4 | OECD Guideline 301B | 28-day degradation = 12% (CO2 evolution) |
No rapid degradation |
| 2082-79-3 | OECD Guideline 301B | 28-day degradation = 32–35% (inorganic C analysis) |
No rapid degradation |
| 4221-80-1 | OECD Guideline 301B | 28-day degradation <20% (CO2 evolution) |
No rapid degradation |
| 6386-38-5 | OECD Guideline 301B | 28-day degradation = 3–8% (O2 evolution) |
No rapid degradation |
| 35958-30-6 | OECD Guideline 301C | 28-day degradation = 0% (O2 consumption) (read-across data from the analogue substance) |
No rapid degradation |
| 36443-68-2 | OECD Guideline 301B | 28-day degradation = 3–8% (CO2 evolution) |
No rapid degradation |
| 41484-35-9 | OECD Guideline 301B | 28-day degradation = 2–7% (CO2 evolution) |
No rapid degradation |
Abbreviation: BOD, biological oxygen demand
For CAS RN 61788-44-1, empirical data have been identified only for the monostyrenated phenol component. QSAR models have been used to fill the data gaps. A summary of empirical data and model predictions for this UVCB substance is presented in Table 6-3. More details are compiled in ECCC (2023).
| Component | Key degradation data | Reference |
|---|---|---|
| Monostyrenated phenol | 28-day degradation = 0% (O2 consumption; OECD Guideline 301C) | ECHA c2007-2017 |
| Distyrenated phenol | 2.48 (BIOWIN Sub-model 3) 3.32 (BIOWIN Sub-model 4) 0 (BIOWIN Sub-model 5) 0.03 (BIOWIN Sub-model 6) |
EPI Suite c2000-2012a |
| Tristyrenated phenol | 2.20 (BIOWIN Sub-model 3) 3.11 (BIOWIN Sub-model 4) 0 (BIOWIN Sub-model 5) 0 (BIOWIN Sub-model 6) |
EPI Suite c2000-2012a |
a As specified in EPI Suite (c2000-2012), result classifications for BIOWIN Sub-model 3 and 4 are: 5 = hours, 4 = days, 3 = weeks, 2 = months, 1 = longer; result classifications for BIOWIN Sub-model 5 and 6 are: ≥0.5 = readily degradable, <0.5 = not readily degradable.
Considering all of the above, the available degradation data indicate that CAS RN 98-54-4 will degrade rapidly in water and soil. The other 13 discrete substances are unlikely to undergo rapid degradation, particularly in the water compartment, and are thus expected to persist and have long residence times in the environment. Given that the empirical data for monostyrenated phenol and model predictions for distyrenated phenol and tristyrenated phenol components have not indicated rapid degradation, CAS RN 61788-44-1 is therefore expected to persist in the environment.
6.3 Potential for bioaccumulation
Empirical bioaccumulation data have been identified for most of the substances in the Substituted Phenols Group. Analogue data and model predictions (Arnot and Gobas 2004; Arnot et al. 2008a,2008b; CATALOGIC 2016; EAS-E Suite (Ver.097 - BETA, released June 2023); EPI Suite c2000-2012 were used to fill data gaps.
Substances with log KOW values greater than 9 (that is, CAS RNs 2082-79-3, 4221-80-1, and 41484-35-9) will be strongly adsorbed to solid particles under natural conditions and are therefore unlikely to be bioavailable for uptake by aquatic or terrestrial organisms. These substances are outside the domains of the bioaccumulation models; however, given their low water solubilities and log KOW values exceeding 9, their potential for bioaccumulation is considered to be very low.
For the other 12 substances with a log KOW value less than 9, key empirical data and model predictions are summarized in Tables 6-4 and 6-5 for the 11 discrete substances and the UVCB substance, respectively. More detailed data are compiled in ECCC (2023). In addition, metabolism rates (kM) have been estimated for these substances using the applicable QSAR models, with consideration of measured log KOW and bioconcentration factors (BCF) if available.
The available data suggest that, of the 11 discrete substances with a log KOW less than 9, CAS RN 118-82-1 has high potential for bioaccumulation in organisms, while the other 10 substances are not expected to possess high potential for bioaccumulation in organisms (Table 6-4).
| CAS RN | log KOW | BCF (L/kg) | BAFa (L/kg) | kMa (1/day) |
|---|---|---|---|---|
| 85-60-9 | 6.4 | 228b (modelled) | 445b | 1.2b |
| 96-69-5 | 5.2 | 11c,d (empirical) | 168e | 2.4e |
| 96-76-4 | 5.0 | 436d,f (empirical) | 408e | 0.91e |
| 98-54-4 | 3.2 | 68d,f (empirical) | 94e | 1.3e |
| 118-82-1 | 7.4 | 9000d,f (empirical) | 2.31×105 b,e | 0.22e |
| 128-37-0 | 5.2 | 2500d,g (empirical) | 3970e | 0.10e |
| 128-39-2 | 4.7 | 436d (empirical) | 415e | 0.83e |
| 1843-03-4 | 8.5 | 28h (modelled) | 198h | 0.28b,i,j |
| 6386-38-5 | 5.5 | 100b (modelled) | 104b | 2.14b |
| 35958-30-6 | 6.3 | 841d (read-across from CAS RN 119-47-1) | 2213e | 0.37e |
| 36443-68-2 | 4.7 | 8d (empirical) | 17e | 55.5e |
Abbreviations: KOW, octanol-water partition coefficient; BCF, bioconcentration factor; BAF, bioaccumulation factor; kM, metabolism rate constant.
a All BAFs and kMs were calculated using models since no valid empirical values for this endpoint were identified for substances in this group.
b EPI Suite c2000-2012
c US EPA 2010
d ECHA c2007-2017
e Arnot et al. 2008a, 2008b
f J-CHECK c2010-
g OECD 2002
h Arnot et al. 2004
i EAS-E Suite (Ver.097 - BETA, release June 2023)
j CATALOGIC 2016>
For the UVCB substance (CAS RN 61788-44-1), identified empirical data and model predictions for all components have been compiled in Table 6-5. Available data indicate a lower bioaccumulation potential for the monostyrenated phenol component. However, a high measured BCF value and a few moderate-to-high modelled BCF and bioaccumulation accumulation factor (BAF) values have been reported for distyrenated phenol and tristyrenated phenol components; the predicted kMs for both of these components are also low. These data suggest a high bioaccumulation potential associated with these two components. It is noted that distyrenated phenol and tristyrenated phenol components represent a large fraction of the composition (23–52% and 43–70%, respectively) of this UVCB substance (see Table 2-3); therefore, this substance (CAS RN 61788-44-1) is expected to possess high potential for bioaccumulation in organisms.
| Component | log KOW | BCF (L/kg) | BAFa (L/kg) | kMa (1/day) |
|---|---|---|---|---|
| Monostyrenated phenol | 5.2 | 190b,c (read-across from monomethylstyrenated phenol in MSP) |
178d | 2.0d |
| Distyrenated phenol | 6.2 | 613e (modelled) | 5 591e | 0.31f |
| Tristyrenated phenol | 7.8 | 10 395g (empirical) | 3.9×106 d | 0.0025d |
Abbreviations: KOW, octanol-water partition coefficient; BCF, bioconcentration factor; BAF, bioaccumulation factor; kM, metabolism rate.
a All BAFs and kMs are calculated using models since no valid empirical values for this endpoint were identified for any component in CAS RN 61788-44-1.
b J-CHECK c2010-
c ECHA c2007-2017
d Arnot et al. 2008a, 2008b
e Arnot and Gobas 2004
f EAS-E Suite (Ver.097 - BETA, release June 2023)
g Brooke et al. 2009
6.4 Summary of environmental persistence and potential for bioaccumulation
Of 14 discrete substances in the Substituted Phenols Group, CAS RN 98-54-4 is expected to undergo rapid degradation in the environment and to possess low potential for bioaccumulation in organisms. CAS RN 118-82-1 is considered to persist in the environment and to be highly bioaccumulative in organisms. The remaining 12 discrete substances in the group are expected to persist in the environment, with low to moderate potential for bioaccumulation in organisms.
For the UVCB substance (CAS RN 61788-44-1), the monostyrenated phenol component is expected to persist in the environment but not to bioaccumulate in organisms. However, both the distyrenated phenol and tristyrenated phenol components are expected to persist in the environment and to be highly bioaccumulative in organisms, and these two components represent a large fraction of the composition of this UVCB substance (Table 2-3). Therefore, CAS RN 61788-44-1 is considered to persist in the environment and to bioaccumulate in organisms.
7. Potential to cause ecological harm
Using the ecological risk classification of organic substances (ERC) approach (ECCC 2016a, 2016b), 4 of the 15 substances in the Substituted Phenols Group were characterized as having a low potential for ecological risk. More information on the ERC approach is summarized in Appendix A. The hazard and exposure classifications for these four substituted phenols are presented in Table 7-1.
| CAS RN | ERC hazard classification | ERC exposure classification | ERC risk classification |
|---|---|---|---|
| 85-60-9 | high | low | low |
| 2082-79-3 | low | high | low |
| 6386-38-5 | low | low | low |
| 41484-35-9 | low | moderate | low |
According to information considered under the ERC, CAS RN 85-60-9 was classified as having a low exposure potential. However, it was classified as having a high hazard potential due to structural alerts from the OECD QSAR Toolbox (2014), which identified it as being a potential endocrine receptor binder. This substance was also profiled to have a high potential to cause adverse effects in aquatic food webs given its bioaccumulation potential. Initially, CAS RN 85-60-9 was classified as having a moderate potential for ecological risk; however, the risk classification was decreased to a low potential for ecological risk following the adjustment of risk classifications based on reported quantities (see section 7.1.1 of the ERC approach document, ECCC 2016a). The potential for effects and how they may manifest in the environment were not further investigated due to the low exposure of this substance. On the basis of current use patterns, CAS RN 85-60-9 is unlikely to be resulting in concerns for the environment in Canada.
According to information considered under the ERC, CAS RN 2082-79-3 was classified as having a high exposure potential on the basis of a long overall persistence and a large reported use quantity according to information submitted in response to a CEPA section 71 survey (EC 2013). However, this substance was classified as having a low hazard potential and was consequently classified as having a low potential for ecological risk. Although the reported quantities result in a high exposure potential, considering its low hazard potential, CAS RN 2082-79-3 is unlikely to be resulting in concerns for the environment in Canada.
On the basis of its low hazard and low exposure classifications according to information considered under the ERC, CAS RN 6386-38-5 was classified as having a low potential for ecological risk. It is unlikely that this substance is resulting in concerns for the environment in Canada.
According to information considered under the ERC, CAS RN 41484-35-9 was classified as having a moderate exposure potential on the basis of a long overall persistence and moderate reported quantities according to information submitted in response to a CEPA section 71 survey (EC 2013). This substance was classified as having a low hazard potential and was consequently classified as having a low potential for ecological risk. Considering the moderate exposure potential and low hazard potential, CAS RN 41484-35-9 is unlikely to be resulting in concerns for the environment in Canada.
It is noted that substances with log KOW values of 8.2 or less represent the most bioavailable forms. For log KOW values as high as 9, a low to moderate degree of laboratory bioconcentration has been observed for some long-chain phthalate esters and cyclic siloxanes, albeit using solvents during testing. As a precautionary measure, substances with log KOW values less than 9 were assumed to be bioavailable for the purposes of evaluating ecological risk. One substance (CAS RN 4221-80-1) in the Substituted Phenols Group had a reported log KOW value of greater than 9. The available toxicity data suggest that CAS RN 4221-80-1 will not cause any effects on aquatic organisms at or below its water saturation level (ECCC 2023). Although this substance has been identified as being of potential ecological concern using the ERC approach, it is not expected to be bioavailable, and the available toxicity data suggest that the substance will not cause any effects on organisms at or below its water saturation limit. Therefore, its risk to the environment is not further characterized.
Given the above, the ecological risk characterization in this assessment focused on these 10 substances: CAS RNs 96-69-5, 96-76-4, 98-54-4, 118-82-1, 128-37-0, 128-39-2, 1843-03-4, 35958-30-6, 36443-68-2, and 61788-44-1.
7.1 Ecological effects assessment
7.1.1 Mode/mechanism of action (MoA)
The manner in which a chemical reacts with biological tissues and exerts toxicity (that is, its MoA) is relevant to the overall determination of its hazard potential. From the ecotoxicological perspective, a distinction can be made regarding potencies of substances with narcotic MoA and non-narcotic MoAs. A narcotic MoA is associated with non-specific interference of a chemical with cell membranes and is also referred to as baseline toxicity. A non-narcotic MoA is referred to as excess toxicity (reactive and specifically acting) and is generally associated with the potential for long-term effects to occur at lower tissue concentrations.
The identification of MoAs as being either narcotic or non-narcotic for substances in the Substituted Phenols Group was investigated using QSAR models (TEST 2016; OECD QSAR Toolbox 2017) as well as calculations of critical body residue (CBR)Footnote 5 and chemical activity (CA).Footnote 6 Since certain phenols may have effects on the endocrine system by simulating the activity of natural estrogen, estrogen receptor (ER) binding potential was assessed separately using a combination of the available in vitro data and (Q)SAR predictions (Ogawa et al. 2006; OECD QSAR Toolbox 2017; OECD 2018; Webster et al. 2019).
A summary of MoA and ER binding determinations is included in Table 7-2. Ten substances in the Substituted Phenols Group were identified as having a narcotic MoA. A consensus of available data confirmed the ER binding potential for the non-hindered phenol substances in the group (CAS RN 98-54-4 and the monostyrenated phenol component of the UVCB substance CAS RN 61788-44-1) (Ogawa et al. 2006; OECD QSAR Toolbox 2017; OECD 2018; Webster et al. 2019). ER binding potential was considered when determining the assessment factors (AFs) used to derive the predicted no-effects concentrations (PNECs) for these two substances, given that the potential non-narcotic MoA is not reflected in the consensus MoA determination.
The remainder of the group consists of partially- and fully-hindered phenols (that is, with substituents bonded to one or both positions ortho to the hydroxyl group, respectively). The (Q)SAR data identified three partially-hindered phenols (CAS RNs 85-60-9, 96-69-5, and 96-76-4) as strong or very strong ER binders; however, in vitro data indicated that these substances are likely to have negligible ER binding potential (Ogawa et al. 2006; OECD 2018; Webster et al. 2019). The remaining substances were identified as non-binders due to steric hindrance of binding sites or high molecular weight, which reduce their ER binding potential.
| CAS RN | Consensus MoAa,b,c | Consensus (Q)SAR / In vitro ER binding potentiald |
|---|---|---|
| 96-69-5 | Narcosis | Non-bindere |
| 96-76-4 | Narcosis | Non-bindere |
| 98-54-4 | Narcosis | ER binding potential |
| 118-82-1 | Narcosis | Non-binder |
| 128-37-0 | Narcosis | Non-binder |
| 128-39-2 | Narcosis | Non-binder |
| 1843-03-4 | Narcosis | Non-binder |
| 35958-30-6 | Narcosis | Non-binder |
| 36443-68-2 | Narcosis | Non-binder |
| 61788-44-1 | Narcosis | ER binding potentialf |
Abbreviations: MoA, mode/mechanism of action; (Q)SAR, (quantitative) structure-activity relationship; ER, endocrine receptor
a (Q)SAR models used included Verharr Class (OECD QSAR Toolbox 2017), Toxicity Estimation Software Tool (TEST 2016), and OASIS (OECD QSAR Toolbox 2017)
b CBR threshold for a non-narcotic MoA is <0.3 mmol/kg.
c CA threshold for a non-narcotic is <0.01 (unitless).
d Ogawa et al. 2006, OECD QSAR Toolbox 2017, OECD 2018, Webster et al. 2019.
e For this substance, there was a lack of consensus between (Q)SAR models (ER binding potential) and in vitro data (no ER binding potential); however, the in vitro results were applied.
f The OECD QSAR Toolbox (2017) ER binding alert is based on results for the monostyrenated phenol component of this UVCB substance.
However, it is noted that, during metabolism in organisms, hydroxylation may occur on the alkyl branch of the alkyl phenol moiety in a substituted phenol substance. The effects associated with metabolites will therefore not be captured via the modelling of parent compounds. Consequently, chronic toxicity data, which better reflect any potential for long-term reproductive and developmental effects associated with endocrine disruption, are preferred in the effects characterization.
7.1.2 Ecological effects on aquatic, sediment, and soil organisms
Since releases to air are not expected and these substances are not expected to reside appreciably in air (due to their low-moderate vapour pressure), effects assessment in air was not conducted.
Acute and chronic fish and invertebrate data, along with algae data, were identified for the majority of substances in the Substituted Phenols Group; details of these data and references are compiled in ECCC (2023). Based on the available toxicity and water solubility information, CAS RNs 96-69-5, 96-76-4, 98-54-4, 128-37-0, 128-39-2, 36443-68-2, and 61788-44-1 were predicted to be moderately to highly toxic in the aquatic compartment. The acute toxicity to aquatic organisms is at or below the level of 1 mg/L, and the chronic toxicity is at or below the level of 0.1 mg/L (ECCC 2023).
No empirical toxicity data for sediment species were identified for any substance in this group.
Soil toxicity data were identified for CAS RNs 96-76-4, 118-82-1, and 128-39-2 as well as the analogues for CAS RNs 35958-30-6 and 36443-68-2. Details of the ecotoxicity data are presented in ECCC (2023).
7.1.3 Approach for the ecological effects assessment
The preferred effects assessment approach for substances in this group involves selecting a critical toxicity value (CTV) from the set of available empirical toxicity data (or read-across from analogues for substances lacking empirical data). A PNEC for the relevant environmental compartment is then extrapolated from the CTV through the application of an assessment factor (AF).
An AF is derived as the product of endpoint standardization (FES), species variation (FSV), and MoA (FMOA) factors (that is, AF = FES × FSV × FMOA). An endpoint standardization factor (FES) is used to account for extrapolations from the toxicity endpoint reported in a study to a long-term, sub-lethal, no-effect endpoint. A species variation factor (FSV) is determined on the basis of the number of different species in major groups of organisms (group identities vary by environmental compartment) for which empirical data are available in the data set. A MoA factor (FMOA) is applied to address a known or suspected non-narcotic MoA.
In cases where PNECs could not be calculated based on a CTV, mainly due to the limited empirical toxicity data, PNECs were extrapolated using a CBR approach. The CBR approach assumes that a substance may demonstrate a lethal effect when its accumulation in an organism’s tissue reaches a critical concentration. For neutral narcotic chemicals, the internal concentrations causing death have been shown to be fairly constant at about 2 mmol/kg to 8 mmol/kg for acute exposures and 0.2 mmol/kg to 0.8 mmol/kg for chronic exposures (McCarty 1986; Van Hoogen and Opperhuizen 1988; McCarty and Mackay 1993; McCarty et al. 1985, 1991, 2013). The critical concentration (expressed as a CBR50 value) can be calculated using the bioaccumulation potential in an environmental medium and the environmental exposure required to cause a lethal effect on organisms (expressed as an LC50-external value).
As most of the substances in this group are expected to have a narcotic MoA, an approximate chronic CBR50 value of 0.3 mmol/kg (the median value for the range of 0.2 mmol/kg to 0.8 mmol/kg described in the previous paragraph) is used to extrapolate a narcotic LC50-external value. Relevant measures of the bioaccumulation potential are presented by BAF for a certain environmental compartment (e.g., BAF for the aquatic compartment). A PNEC can be derived through the application of an AF and standardization to mass-based units (e.g., µg/L) while taking into consideration the molecular weight.
7.1.4 Predicted no-effect concentrations for aquatic organisms
Toxicity data below the water solubility values for each substance were used to select CTVs to calculate aquatic PNEC values. For substances where the available toxicity data exceeded water solubility, the CBR approach was used instead.
Aquatic CTVs below the respective water solubility limits for these six substances were identified. A summary of the data set variety, selection of CTVs, determination of Afs, and extrapolation of PNECs for individual substances is presented in Table 7-3. Details of the ecotoxicity data are presented in ECCC (2023).
Table 7‑3. Summary of critical toxicity values and predicted no-effect concentrations for substances with reported aquatic toxicity endpoints below water saturation levels.
| CAS RN | Major groups of aquatic organisms covered in the data set | Species covered in the data set | CTV (µg/L) (reference) | AF (FES, FSV, FMoA) | Aquatic PNEC (µg/L) |
|---|---|---|---|---|---|
| 96-69-5 | 3 | 7 | 14-day LC50 = 54 on fish (ECHA c2007-2017) |
10 (10, 1, 1) | 5.4 |
| 96-76-4 | 3 | 4 | 48-hour EC50 = 500 (mobility) on Daphnia magna (ECHA c2007-2017) |
20 (10, 2, 1) | 25 |
| 98-54-4 | 3 | 7 | 128-day NOEC = 10 (growth rate, secondary sexual characterization, time to hatch) on fish (ECHA c2007-2017) | 2 (1, 1, 2) | 5 |
| 128-37-0 | 3 | 6 | 21-day NOEC = 23 (immobilization) on Daphnia magna (ECHA c2007-2017) |
10 (5, 2, 1) | 2.3 |
| 128-39-2 | 3 | 6 | 21-day NOEC = 35 (reproduction, and growth) on Daphnia magna (ECHA c2007-2017) | 2 (1, 2, 1) | 17.5 |
| 36443-68-2 | 3 | 7 | 32-day NOEC = 5.5 (mortality) on Daphnia magna (ECHA c2007-2017) | 5 (5, 1, 1) | 1.1 |
| 61788-44-1a | 1 | 1 | 21-day NOEC = 115 (reproduction and parental immobilization) on Daphnia magna (Brooke et al. 2009) | 100 (1, 50, 2) | 1.2 |
Abbreviations: CTV, critical toxicity value; AF, assessment factor; FES, endpoint-standardization factor; FSV, species variation factor; FMoA, mode of action factor; PNEC, predicted no-effect concentration; LC50, median lethal concentration; EC50, the concentration of a substance that is estimated to cause some effect on 50% of the test organisms; NOEC, no observed effect concentration
a The toxicity data for a distyrenated phenol were used to represent the toxicity for all components in the UVCB substance (CAS RN 61788-44-1); however, the identity of the distyrenated phenol was not specified (Brooke et al. 2009).
The CBR approach was used to back-calculate aquatic PNEC values for three substances with toxicity endpoints that were not applicable as they exceeded water solubility limits. The PNECs calculated for CAS RNs 118-82-1 and 35958-30-6 using the CBR approach were similar in magnitude to their water solubility limits (Table 7-4) and considered appropriate for use in the risk characterization. The PNEC derived from the CBR approach for CAS RN 1843-03-4 was more than an order of magnitude higher than its water solubility limit, suggesting that this substance is unlikely to demonstrate effects at or below its water solubility limit.
| CAS RN | Chronic CBR50 for narcotic substances (mmol/kg) | BAF (L/kg) | AF (FES, Fsv, FMoA) | Molecular weight (g/mol) | PNEC (µg/L) | Water solubility (µg/L) |
|---|---|---|---|---|---|---|
| 118-82-1 | 0.3 | 2.31×105 | 5 (5, 1, 1) | 424.67 | 0.11 | 0.31 |
| 35958-30-6 | 0.3 | 2213 | 5 (5, 1, 1) | 438.7 | 11.9 | 12 |
Abbreviations: BAF, bioaccumulation factor; AF, aAssessment factor; FES, endpoint-standardization factor; FSV, species variation factor; FMoA, mode of action factor; PNEC, predicted no-effect concentration
As discussed previously, of 11 substances in the group, CAS RN 4221-80-1 possesses a log KOW above 9 and is not expected to be bioavailable to organisms. For CAS RN 1843-03-4, the reported toxicity data for this substance exceeded its water solubility; the aquatic PNEC calculated using the CBR approach also exceeded its water solubility. This substance is not expected to cause adverse effects on organisms at or below its water solubility.
The aquatic PNECs for the other nine substances are summarized in Table 7-5 and were either derived from the empirical toxicity data or calculated using the CBR approach.
| CAS RN | Aquatic PNEC (µg/L) derived using available empirical data | Aquatic PNEC (µg/L) derived using the CBR approacha |
|---|---|---|
| 96-69-5 | 5.4 | Not applicable |
| 96-76-4 | 25 | Not applicable |
| 98-54-4 | 5 | Not applicable |
| 118-82-1 | Could not calculateb | 0.11 |
| 128-37-0 | 2.3 | Not applicable |
| 128-39-2 | 17.5 | Not applicable |
| 35958-30-6 | Could not calculateb | 11.9 |
| 36443-68-2 | 1.1 | Not applicable |
| 61788-44-1 | 1.2 | Not applicable |
Abbreviations: PNEC, predicted no-effect concentration; CBR, critical body residue
a Aquatic PNEC was derived using the CBR approach in instances where the available toxicity data exceeded the water solubility limits.
b The reported toxicity data for this substance exceeded its water solubility.
7.1.5 Predicted no-effect concentrations for sediment-dwelling organisms
It is noted that the CBR thresholds are based on literature that focuses on the aquatic compartment; therefore, the PNECs for sediment organisms are not calculated for the ecological risk assessment.
7.1.6 Predicted no-effect concentrations for soil-dwelling organisms
A summary of the selection of CTVs, determination of AFs, and extrapolation of PNECs for these five substances is presented in Table 7-6. These data suggest low to moderate toxicity to soil-dwelling organisms. The CBR approach is not used to calculate soil PNECs for the remaining eight substances that have no soil toxicity data identified.
| CAS RN | CTV (mg/kg dw) (reference) | AF (FES, FSV, FMoA) | Soil PNEC (mg/kg dw) |
|---|---|---|---|
| 96-76-4 | 21-day NOEC = 37 (seeding emergence, survival) on plant (onion, Allium cepa) (ECHA c2007-2017) |
1 (1,1,1) | 37 |
| 118-82-1 | 28-day LC50 = 89 on soil invertebrate (springtail, Folsomia candida) (ECHA c2007-2017) |
50 (10,5,1) | 1.78 |
| 128-39-2 | 28-day IC50 = 52 (juvenile production) on soil invertebrate (springtail, Folsomia candida) (ECCC 2016c) |
50 (10,5,1) | 1.04 |
| 35958-30-6 | 56-day NOEC = 1000 (reproduction) on soil invertebrate (earthworm, Eisenia fetida) (read-across from CAS RN 119-47-1) (ECHA c2007-2017) |
2 (1,2,1) | 500 |
| 36443-68-2 | 28-day LC50 = 926 on soil invertebrate (springtail, Folsomia candida) (read-across from CAS RN 41484-35-9) (ECCC 2016c) |
50 (10,5,1) | 18.52 |
Abbreviations: CTV, critical toxicity value; AF, assessment factor; FES, endpoint-standardization factor; FSV, species variation factor; FMoA, mode of action factor; PNEC, predicted no-effect concentration; NOEC, no-observed-effect concentration; LC50, median lethal concentration; IC50, the concentration of a substance that is estimated to cause growth inhibition on 50% of the test organisms
7.2 Ecological exposure assessment
Since releases to air are not expected and these substances are not expected to reside appreciably in air (due to their low-moderate vapour pressure), exposure assessment in air was not conducted.
For the aquatic compartment, exposure was estimated based on the available information. The aquatic predicted environmental concentrations (PECs) were calculated for a variety of scenarios. PECs in soil were calculated for the five substances that have available empirical toxicity data.
Exposure estimates in sediment and soil for the remaining substances are considered to be supporting information due to the elevated uncertainty associated with the CBR estimates in these media. The calculated PECs are presented in ECCC (2023).
The primary sources of data that contributed to the exposure assessment were surveys issued pursuant to CEPA section 71 (EC 2009, 2013; ECCC 2017); however, other sources of information were also considered, such as additional information submitted by industry, information from producers and importers of gasoline in Canada pursuant to the requirements of the Fuels Information Regulations, No. 1 (as these substances are fuel additives), and publicly available information on the activities of companies reporting the import of substituted phenols. The information considered from CEPA section 71 survey data reflect the reporting years 2008, 2011, or 2016. For the purposes of this ecological exposure assessment, it was assumed that the survey data were reflective of current industrial use patterns for the substances, unless more recent information was available (for example, additional information submitted by industry).
Substituted phenols serve primarily as antioxidants in a wide variety of industrial, commercial, and consumer applications. Based on the reported uses of the substances (EC 2009, 2013; ECCC 2017; information received under the Fuels Information Regulations, No. 1), substituted phenols are mainly used in the industrial formulation of the following products in Canada: lubricant and fuel additives, plastic and rubber additives, lubricants, fuels, plastic products, rubber products, paints and coatings, and personal care products (see section 4 for further information). A scenario was also developed to cover the use of personal care products. Table 7-7 identifies the relevant exposure scenarios associated with each CAS RN on the basis of reported information as well as the relative contribution (% in the sector by mass) represented by each CAS RN. Reporting companies were associated with relevant industrial sectors based on the information submitted in response to CEPA section 71 surveys, as well as other relevant information that could be identified relating to line of business. Since not all reporting companies are necessarily industrial users of the substances, only companies that are believed to be involved in industrial activities using the substance, such as formulators (that is, not distributors), were considered. When available, information on customers of the distributors was also considered. If a substance was not reported to be used in the given application, it was excluded from the corresponding exposure scenario with no PEC calculated (that is, blanks in Table 7-7).
CAS RN 35958-30-6 was reported to be imported by Canadian distributors, but it was not considered in the exposure analysis as no client information is available (that is, imported quantities could not be linked to specific users in Canada). Concentrations were nonetheless calculated using standard parameters, assuming that this substance could be used in any sector identified for these substituted phenols; however, these values are not presented due to the high uncertainty associated with them. None of the calculated PECs for CAS-RN 35958-30-6 exceeded the aquatic PNEC.
| CAS RN | ES 1a | ES 2b | ES 3c | ES 4d | ES 5e | ES 6f | ES 7g | ES 8h |
|---|---|---|---|---|---|---|---|---|
| 96-69-5 | n/a | 22 | n/a | 26 | n/a | n/a | n/a | n/a |
| 96-76-4 | 10 | n/a | n/a | n/a | n/a | n/a | n/a | 6 |
| 98-54-4 | n/a | n/a | n/a | n/a | n/a | 69 | n/a | <1 |
| 118-82-1 | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a |
| 128-37-0 | 5 | 33 | 3 | 26 | n/a | 31 | 100 | 89 |
| 128-39-2 | 85 | 7 | 97 | 8 | n/a | n/a | n/a | 5 |
| 1843-03-4 | n/a | 34 | n/a | 37 | n/a | n/a | n/a | n/a |
| 35958-30-6i | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| 36443-68-2 | n/a | 4 | n/a | 3 | n/a | n/a | n/a | n/a |
| 61788-44-1 | n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a |
Abbreviations: ES, exposure scenario; n/a, not applicable
a Formulation of lubricant and fuel additives
b Formulation of plastic and rubber additives
c Formulation of lubricants
d Formulation of plastic products
e Formulation of rubber products
f Formulation of paints and coatings
g Formulation of personal care products
h Formulation of fuel
i CAS RN 35958-30-6 was reported to be imported by Canadian distributors, but imported quantities could not be linked to specific industrial users.
Table 7-7 shows that many of the substances in the Substituted Phenols Group are used within the same industrial sectors in Canada. Additional information from MSDSs and SDSs show that multiple substituted phenols can be used within the same product. This, and the fact that all substances in the group commonly function as antioxidants, indicates that it is likely that certain substituted phenols may be used interchangeably within some product formulations. However, due to differences in physical-chemical properties, wastewater removal rates, and high variability in product formulations amongst similar product types, the exposure analysis was done on a substance-by-substance basis. These scenarios reflect only sectors of higher use quantities, and a substance may be involved in a sector that is not noted in Table 7-7.
Although releases of substituted phenols from the use of formulated products containing these substances are possible, the only use scenario covered quantitatively in this ecological exposure assessment pertains to the release from use of personal care products. This is mainly due to a lack of specific information related to the magnitudes, frequencies, and dispersive natures of releases from the use of lubricants, fuels, paints and coatings, and plastic and rubber products that contain substituted phenols. Additionally, the quantity of substituted phenols within final industrial, commercial, and consumer applications is lower at individual points of release than the quantity that is expected to be handled at individual locations during industrial formulation activities, which require large amounts of the raw substances in a single location. Lastly, many of the products that contain these substances (for example, lubricants) are collected at end-of-life according to provincial requirements and subsequently undergo specialized waste treatment, which is expected to reduce environmental releases. There is an absence of data relating to the release of substituted phenols during the end-of-life and recycling of plastic and rubber products. As a result, these potential sources are not addressed in this ecological exposure assessment.
Information received from industry indicates that two substituted phenols (CAS RNs 98-54-4 and 61788-44-1) are also present in protective and marine coatings. These coatings are only imported into Canada (that is, no formulation in Canada) and are associated with a variety of professional applications. Reported quantities associated with marine coatings are minimal. For CAS RN 98-54-4, reported quantities associated with protective coatings are higher; however, aquatic releases from the use of these coatings are expected to be small. Given the description of the products, uses are expected to be smaller-scale projects, such as maintenance and repairs, that lead to dispersed releases rather than concentrated releases at industrial locations. On the basis of this information, PECs were not calculated for this scenario.
Generic PECs in the aquatic compartment (section 7.2.3, Table 7-8) and in soil for the five substances with empirical toxicity data were calculated for each CAS RN reported to be imported by companies associated with each of the industrial sectors of interest. In cases where the generic PECs indicated possible risk to the environment, further analysis (that is, refinement) was conducted using specific parameters associated with the industrial facilities of companies that reported imports of substituted phenols into Canada. Given the uncertainty associated with some parameters used in the generic scenarios, this additional analysis served to refine the risk analysis. Additional descriptions and details of the exposure scenarios and associated input parameters for all environmental compartments of interest are presented in ECCC (2023). Generic PECs in soil for the remaining substances and in sediment were also calculated and are presented in ECCC (2023).
7.2.1 Environmental monitoring data
No monitoring data in environmental media (for example, surface water, sediment, soil, ambient air) were identified for substances in the Substituted Phenols Group in Canada. However, measured concentrations in influent, effluent, and biosolid samples from selected Canadian WWTSs have been reported for eight substances in the group. These substances were detected in samples collected from both influent and effluent streams at primary, secondary, and lagoon WWTSs (Lu et al. 2019). Median influent concentrations for seven of the substances ranged from 7.5 ng/L to 746 ng/L. The median influent concentration for CAS RN 96-69-5 could not be calculated due to a low detection frequency. However, the low detection frequency reported for CAS RN 96-69-5 is based on 12 monitored Canadian WWTSs that may not receive influents from relevant industrial users. Median effluent concentrations for the eight substances were all below method quantification limits; however, maximum values ranged from below quantification to 520 ng/L (Lu et al. 2019).
7.2.2 Predicted environmental concentration (PEC) equations
7.2.2.1 Aquatic medium
To inform the exposure characterization and subsequent risk characterization of substituted phenols in the aquatic compartment, PECs were calculated for a variety of scenarios. Releases to surface water may result either from direct discharges from an industrial facility into receiving water or from indirect entry via industrial discharge to the sewer, followed by treatment at a WWTS. The PEC values calculated for each exposure scenario consider the variability in physical-chemical properties and WWTS removal rates of each CAS RN.
The environmental exposures to surface waters (PECaquatic) resulting from industrial releases were estimated using the following equation:
where,
PECaquatic = aquatic predicted environmental concentration (μg/L);
109 = conversion factor from units of kg to μg;
Q = substance quantity used annually at a site (kg/year);
L = losses to wastewater (fraction);
R = wastewater treatment system (WWTS) removal efficiency for the substance (fraction);
N = number of annual release days (days/year); and
D = daily dilution volume of receiving water body (L/day).
The aquatic PECs represent potential concentrations of the substances in the receiving water body near the discharge point of a WWTS or facility discharging directly to a receiving water body.
Due to limitations of the available WWTS monitoring data (see section 7.2.1), the WWTS removal efficiency was calculated for each substance using the SimpleTreat 3.1 model (SimpleTreat 3.1 2003) and using the STP-EX model (c2000-2013) for facilities that discharge wastewater to lagoon WWTSs.
Daily dilution volumes were calculated by multiplying the effluent flow of a WWTS or facility discharging to a receiving water body by the dilution factor of the receiving water body. In all cases, aquatic PECs were derived using a dilution factor that was based on the 10th percentile low flow of the receiving water body and capped at a maximum dilution factor of 10 to represent exposures close to the point of discharge.
A different PEC calculation approach was used to estimate exposure for down-the-drain releases of personal care products containing substituted phenols. Aquatic PECs resulting from this use were estimated using the Consumer Release Aquatic Model (CRAM 2017). CRAM is a Canadian, population-based probabilistic model used to estimate environmental exposure resulting from wastewater treatment facility releases of chemicals present in products available to consumers that are released down-the-drain.
7.2.2.2 Soil medium
The PEC in soils (PECsoil) represents the potential concentration of substances in agricultural soils where WWTS biosolids may be applied. The soil PEC after 10 years of biosolids application, which takes into account biodegradation as a loss mechanism, is calculated by iterating the equations below. Concentrations were determined on a yearly basis immediately after application and at the end of the year (after degradation has occurred but prior to the subsequent application) over a 10-year period. In this ecological exposure assessment, only the PECsoil for the five substances with empirical toxicity data are presented.
At the beginning of the year (directly after application):
(nore that )
At the end of the year (after degradation):
where,
PECbeginning = predicted environmental concentration in soil at the beginning of the year after application of biosolids (before degradation) (mg/kg);
PECend = predicted environmental concentration in soil at the end of the year (after degradation), prior to subsequent application of biosolids (mg/kg);
t = years of biosolids land application (y), varying from 1 to 10 years;
Cs = concentration of the substance in biosolids (mg/kg dry weight);
A = annual biosolids land application rate (kg/m2-y);
d = soil mixing depth (m);
r = dry soil density (kg/m3); and
BioDeg = biodegradation half-life value in soil (days).
Measured soil half-life values were available for three of the five substances with empirical soil toxicity data. Where there was a lack of empirical degradation data for soil, degradation data for the aquatic compartment from the model predictions were used to fill the data gaps. For substituted phenols that are not expected to undergo rapid degradation in soil, 182 days was considered to be a representative estimate of soil half-life for the given substance. Half-lives ranging from 3 to 182 days were estimated for these five substituted phenols.
A summary of the key parameters used in the calculation of soil PECs for all exposure scenarios is provided in ECCC (2023).
7.2.3 Aquatic PEC results
A generic approach was used to derive PECs for the aquatic compartment for CAS RNs that were reported to be used within a given industrial sector. PECs produced using this approach represent the estimated concentrations of substituted phenols emitted from a typical Canadian industrial formulation facility within the different identified sectors. The approach assumes that industrial effluents are discharged to a WWTS that offers biological treatment (that is, secondary WWTS), which is the most common treatment type in Canada. In cases where there was information indicating widespread use of on-site treatment technologies in a particular industrial sector, this type of treatment was considered and appropriate removal rates were applied.
When determining the substance quantity used annually at a generic site (Q), the average of the quantities of each substituted phenol reported by individual companies within a particular industrial sector was used. Note that small reported quantities (for example, less than 100 kg) were excluded from the average calculations. To reflect the composition of the UVCB substance (CAS RN 61788-44-1), proportions of 5%, 52%, and 43% were applied to the annual use quantity of the substance at a site (Q) for the monostyrenated, distyrenated, and tristyrenated components, respectively. The maximum reported proportion of the distyrenated phenol component was selected since this component has the highest ecotoxicity.
The daily dilution volume applied in each generic exposure scenario was obtained from a distribution of daily dilution volumes, where available, for facilities associated with the specific industrial sector. These distributions reflect Canadian industrial users and their associated locations.
The generic aquatic PECs are summarized in Table 7-8. As mentioned above, when a substance was not reported to be imported within a given industrial sector in Canada, the PEC was not calculated.
| CAS RN | ES 1a | ES 2b | ES 3c | ES 4d | ES 5e | ES 6f | ES 7g |
|---|---|---|---|---|---|---|---|
| 96-69-5 | n/a | 5.5 | n/a | 11 | n/a | n/a | n/a |
| 96-76-4 | 0.90 | n/a | n/a | n/a | n/a | n/a | n/a |
| 98-54-4 | n/a | n/a | n/a | n/a | n/a | 3.0 | n/a |
| 118-82-1 | n/a | n/a | 0.022 | n/a | n/a | n/a | n/a |
| 128-37-0 | 0.25 | 1.5 | 0.20 | 4.0 | n/a | 0.35 | 0.053 |
| 128-39-2 | 2.2 | 2.5 | 1.9 | 6.0 | n/a | n/a | n/a |
| 1843-03-4 | n/a | 1.0 | n/a | 3.9 | n/a | n/a | n/a |
| 35958-30-6h | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| 36443-68-2 | n/a | 2.4 | n/a | 6.9 | n/a | n/a | n/a |
| 61788-44-1 (monostyrenated phenol) | n/a | n/a | n/a | n/a | 0.12 | n/a | n/a |
| 61788-44-1 (distyrenated phenol) | n/a | n/a | n/a | n/a | 0.57 | n/a | n/a |
| 61788-44-1 (tristyrenated phenol) |
n/a | n/a | n/a | n/a | 0.34 | n/a | n/a |
* Formulation of fuel was not included in this table as all Canadian petroleum refineries treat wastewater on site and discharge directly to associated receiving waters; thus, the PECs were calculated on a specific basis.
Abbreviations: ES, exposure scenario; n/a, not applicable
a Formulation of lubricant and fuel additives
b Formulation of plastic and rubber additives
c Formulation of lubricants
d Formulation of plastic products
e Formulation of rubber products
f Formulation of paints and coatings
g Formulation of personal care products
h CAS RN 35958-30-6 was reported to be imported by Canadian distributors, but imported quantities could not be linked to specific industrial users.
In cases where the generic PECs indicated possible risk to the environment, further analysis (that is, refinement) was conducted using specific parameters associated with the industrial facilities of companies that reported imports of substituted phenols into Canada. Given the uncertainty associated with some parameters used in the generic PECs calculation, this additional analysis served to refine the exposure characterization. Additional descriptions and details of the exposure scenarios and associated input parameters for all environmental compartments of interest are presented in ECCC (2023). For four CAS RNs (96-69-5, 128-37-0, 128-39-2, and 1843-03-4), calculated generic PECs were above their associated toxicity threshold (that is, PNECs) in the aquatic compartment. Further investigation was conducted for these CAS RNs to refine the risk analysis, where PECs in water and soil (sediment PECs are included in supporting information; ECCC 2023) for specific facilities of companies that reported importing these substances were estimated.
Quantities used in this additional analysis reflect the amount of each substituted phenol CAS RN reported by individual companies (EC 2009, 2013; ECCC 2017; additional information submitted by industry; and information received under the Fuels Information Regulations, No. 1). Quantities reported in these data sources are typically associated with companies, not specific facilities. Using publicly available information, the relevant Canadian industrial facilities of reporting companies were identified. In instances where a company had multiple facilities engaged in activities specific to the particular exposure scenario, it was assumed that the entire reported substance quantity could be used at any of the given facilities (that is, the entire quantity was assigned to each facility). The daily dilution volume applied in each specific PEC calculation was the product of the WWTS effluent flow and the dilution factor specific to the location of the facilities. In cases where there was knowledge of facility-specific on-site treatment technologies, appropriate removal rates were applied. PECs were calculated for the five CAS RNs and are summarized in Table 7-9. As above, the PEC was only calculated for a substance if it was reported to be imported within the given industrial sector in Canada. Ranges indicate that multiple facilities were associated with the scenario for the given substance.
| CAS RN | ES 1a | ES 2b | ES 3c | ES 4d | ES 6e | ES 7f | ES 8g |
|---|---|---|---|---|---|---|---|
| 96-69-5 | n/a | 0.11–2.0 | n/a | 0.037–2.7 | n/a | n/a | n/a |
| 128-37-0 | 0.032 | 0.011–6.2 | 0.0010–1.2 | 0.0057–3.1 | 0.00011–0.32 | 0.000085–0.0061 | 0.42–0.79 |
| 128-39-2 | n/a | n/a | n/a | n/a | n/a | n/a | 0.030–12 |
| 1843-03-4 | n/a | 0.0050–7.1 | n/a | 0.10–10 | n/a | n/a | n/a |
| 36443-68-2 | n/a | 0.01–28 | n/a | 0.01–5.6 | n/a | n/a | n/a |
* The exposure scenario for formulation of rubber (ES 5) is not included in this table as none of the CAS RNs subject to refinements were reported to be involved in the formulation of rubber.
Abbreviations: ES, exposure scenario; n/a, not applicable
a Formulation of lubricant and fuel additives
b Formulation of plastic and rubber additives
c Formulation of lubricants
d Formulation of plastic products
e Formulation of paints and coatings
f Formulation of personal care products
g Formulation of fuel
Substituted phenols may also be released to surface water from the use of personal care products via release down-the-drain. Aquatic PECs resulting from this use were estimated using the CRAM (CRAM 2017). From the reported uses of these substances, the primary substituted phenol found in this product type is CAS RN 128-37-0, and the annual quantity imported into Canada for this purpose is approximately 25 000 kg. Given the challenges associated with reporting quantities of substances contained within imported products, this quantity is likely an underestimate. The 90th percentile value of the modelled PEC distribution is 0.32 µg/L for a quantity of 25 000 kg of CAS RN 128-37-0.
7.2.4 Soil PEC results
PECsoil were derived using a similar approach as described above for the aquatic medium, where generic PECs were derived, followed by more specific PECs when refinements were necessary. PECsoil are developed as an extension of the aquatic scenarios, that is, based on the same inputs for quantity, loss, removal, and location of facilities. Generic and refined soil PECs are summarized in Tables 7-10 and 7-11, respectively.
| CAS RN | ES 1a | ES 2b | ES 3c | ES 4d |
|---|---|---|---|---|
| 96-76-4 | 0.12 | n/a | n/a | n/a |
| 118-82-1 | n/a | n/a | 0.010 | n/a |
| 128-39-2 | 0.91 | 0.64 | 1.7 | 1.1 |
| 35958-30-6e | n/a | n/a | n/a | n/a |
| 36443-68-2 | n/a | 0.29 | n/a | 0.073 |
* Formulation of fuel was not included in this table as all Canadian petroleum refineries treat wastewater on site and discharge directly to associated receiving waters; thus, the PECs were calculated on a specific basis.
Abbreviations: ES, exposure scenarios; n/a, not applicable
a Formulation of lubricant and fuel additives
b Formulation of plastic and rubber additives
c Formulation of lubricants
d Formulation of plastic products
e CAS RN 35958-30-6 was reported to be imported by Canadian distributors, but imported quantities could not be linked to specific industrial users.
| CAS RN | ES 1a | ES 2b | ES 3c | ES 4d | ES 8e* |
|---|---|---|---|---|---|
| 128-39-2 | 0.0082-0.039 | 0.0064-0.053 | 0.025 | 0.0088-0.073 | n/a* |
* Facilities considered in the formulation of fuel scenario are direct dischargers, and their biosolids are not applied to agricultural lands.
Abbreviations: ES, exposure scenarios; n/a, not applicable
a Formulation of lubricant and fuel additives
b Formulation of plastic and rubber additives
c Formulation of lubricants
d Formulation of plastic products
e Formulation of fuel
7.3 Characterization of ecological risk
The approach taken in this ecological screening assessment was to examine the assessment information and propose conclusions using a weight-of-evidence approach and precaution. Risk characterization focused on releases of 10 substances to surface water resulting from identified uses as well as on releases of 5 of these 10 substances to soil. One additional substance (CAS RN 4221-80-1) in the Substituted Phenols Group had a reported log KOW value of greater than 9. The available toxicity data suggest that CAS RN 4221-80-1 will not cause effects on aquatic organisms at or below its water saturation level (ECCC 2023). Secondary or indirect lines of evidence were considered when available, including reliable classifications of hazard or fate characteristics made by other regulatory agencies.
7.3.1 Risk quotient analysis
As discussed above, of 11 substances in the group, CAS RN 4221-80-1 possesses a log KOW value above 9 and is not expected to be bioavailable to organisms. Therefore, the risk quotient (RQ) analysis focuses on the remaining 10 substances in the group. RQs were derived by comparing the various estimates of exposure (PECs; see the Ecological Exposure Assessment section 7.2) with ecotoxicity information (PNECs; see the Ecological Effects Assessment section 7.1) to inform whether there is potential for ecological harm in Canada. RQs were calculated by dividing the PEC by the PNEC for the relevant compartments and associated exposure scenario; an RQ value close to or above 1 suggests that the predicted environmental exposure poses or may pose a risk to organisms.
It is noted that the recommended approach for determining a RQ for UVCB substances uses a concentration addition methodology irrespective of the MoA of the mixture components (Backhaus and Faust 2012). However, in this case and in consideration of the toxicity profile of the components of UVCB CAS RN 61788-44-1, emphasis was placed on the most toxic representative component, that is, distyrenated phenol, at its highest relative proportion of 52%, rather than applying an additive RQ approach.
Tables 7-12 and 7-13 present the aquatic and soil RQs, respectively, that resulted from the generic exposure analysis and refinements (shown in brackets) for substances reported to be used in the major identified industrial sectors. Bolded values indicate cases where the RQ is above 1.
| CAS RN | ES 1a | ES 2b | ES 3c | ES 4d | ES 5e | ES 6f | ES 7g | ES 8h |
|---|---|---|---|---|---|---|---|---|
| 96-69-5 | n/a | 1.0 (0.020–0.37) |
n/a | 2.0 (0.0069–0.51) |
n/a | n/a | n/a | n/a |
| 96-76-4 | 0.036 | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| 98-54-4 | n/a | n/a | n/a | n/a | n/a | 0.59 | n/a | n/a |
| 118-82-1 | n/a | n/a | 0.20 | n/a | n/a | n/a | n/a | n/a |
| 128-37-0 | 0.11 (0.014) |
0.65 (0.0050-2.7) |
0.085 (0.00045–0.54) |
1.7 (0.0025–1.3) |
n/a | 0.15 (0.000049–0.14) |
0.023 (0.000037–0.0027) |
(0.18–0.34) |
| 128-39-2 | 0.13 | 0.14 | 0.11 | 0.34 | n/a | n/a | n/a | (0.0017–0.66) |
| 1843-03-4i | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| 35958-30-6j | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| 36443-68-2i | n/a | 2.2 (0.0091–26) |
n/a | 6.3 (0.0091–6.2) |
n/a | n/a | n/a | n/a |
| 61788-44-1 | n/a | n/a | n/a | n/a | 0.48 | n/a | n/a | n/a |
Abbreviations: ES, exposure scenario; n/a, not applicable
a Formulation of lubricant and fuel additives
b Formulation of plastic and rubber additives
c Formulation of lubricants
d Formulation of plastic products
e Formulation of rubber products
f Formulation of paints and coatings
g Formulation of personal care products
h Formulation of fuel scenario was analyzed on a specific basis only as all Canadian petroleum refineries treat wastewater onsite and discharge directly to associated receiving waters.
i RQ could not be determined as the CAS RN does not have an associated aquatic PNEC value.
j CAS RN 35958-30-6 was reported to be imported by Canadian distributors, but imported quantities could not be linked to specific industrial users.
| CAS RN | ES 1a | ES 2b | ES 3c | ES 4d | ES 8e |
|---|---|---|---|---|---|
| 96-76-4 | 0.0033 | n/a | n/a | n/a | n/a |
| 118-82-1 | n/a | n/a | 0.0058 | n/a | n/a |
| 128-39-2 | 0.88 (0.0079–0.038) |
0.62 (0.0062–0.051) |
1.7 (0.024**) |
1.1 (0.0085–0.070) |
n/a* |
| 35958-30-6f | n/a | n/a | n/a | n/a | n/a |
| 36443-68-2 | n/a | 0.016 | n/a | 0.0039 | n/a |
Abbreviations: ES, exposure scenario; n/a, not applicable
a Formulation of lubricant and fuel additives
b Formulation of plastic and rubber additives
c Formulation of lubricants
d Formulation of plastic products
e Formulation of fuel
f CAS RN 35958-30-6 was reported to be imported by Canadian distributors, but imported quantities could not be linked to specific industrial users.
*Facilities considered in the formulation of fuel scenario are direct dischargers, and their biosolids are not applied to agricultural lands.
**A range is not provided since only one facility falling under this scenario is associated with the production of biosolids that are expected to be applied to agricultural land.
In general, the sites that were determined to potentially pose a risk in the local receiving environment (that is, RQ greater than 1) were sites associated with a small receiving water body, which offers limited dilution. The uncertainties associated with the substance quantities assigned to each site and the identification of industrial facilities associated with companies, as stated in section 7.2.3, should also be noted when considering the outcomes of this analysis. Companies associated with sites identified as having potential risk were contacted to obtain more detailed information on their use of substituted phenols; however, responses were only received in some cases.
In addition to the above RQ, the modelled (CRAM 2017) RQ resulting from the use of CAS RN 128-37-0 in personal care products is estimated at 0.14. This is likely an underestimate due to the challenges associated with reporting quantities of specific substances within imported products.
7.3.2 Consideration of the lines of evidence
To characterize the ecological risk of long-chain aliphatic amines, technical information for various lines of evidence was considered (as discussed in the relevant sections of this report) and qualitatively weighted. The key lines of evidence supporting the assessment conclusion are presented in Table 7-14, with an overall discussion of the weight of evidence provided in section 7.3.3. The level of confidence refers to the combined influence of data quality and variability, data gaps, causality, plausibility, and any extrapolation required within the line of evidence. The relevance refers to the impact of the line of evidence in determining the potential to cause harm in the Canadian environment. Qualifiers used in the analysis ranged from low to high, with the assigned weight having five possible outcomes.
| Line of evidence | Level of confidencea | Relevance in ecological risk assessmentb | Weight assignedc |
|---|---|---|---|
| Persistence in the environment | high | high | high |
| Bioaccumulation in aquatic organisms | high | high | high |
| MoA and other non-apicald data | high | high | high |
| Aquatic PNECs | moderate-high | moderate-high | moderate-high |
| Aquatic CBR | moderate | moderate | moderate |
| Soil PNECse | moderate-high | moderate-high | moderate-high |
| Aquatic PECs | low | high | moderate |
| Soil PECse | low | high | moderate |
| Aquatic RQs | low | high | moderate |
| Soil RQse | low | high | moderate |
Abbreviations: PNEC, predicted no-effect concentration; CBR, critical body residue; PEC, predicted environmental concentration; RQ, risk quotient; MoA, mechanism/mode of action
a Level of confidence is determined according to data quality, data variability, data gaps, and whether the data are fit for purpose (that is, plausible and show causality).
b Relevance refers to the impact of the evidence in the ecological risk assessment.
c Weight is assigned to each line of evidence according to the combined level of confidence and relevance in the ecological risk assessment.
d Non-apical endpoints are those other than mortality, growth, reproduction (that is, those endpoints identified with population-level effects).
e Conducted only for five substances (CAS RNs 96-76-4, 118-82-1, 128-39-2, 35958-30-6, and 36443-68-2) that have empirical soil toxicity data identified.
7.3.3 Weight of evidence for determining potential to cause ecological harm
The potential of ecological harm from these substances was based on consideration of the key lines of evidence as well as the relative weight and confidence associated with each of them. The aquatic compartment is identified as the primary receiving environmental compartment. Given that releases may occur from the use of WWTS biosolids for soil enrichment, the soil compartment was also considered.
Persistence and bioaccumulation lines of evidence are highly relevant in the ecological risk assessment of substances in this group. Given the available robust empirical data and reliable outcomes from QSAR models, there is high confidence associated with the persistence and bioaccumulation analyses and lines of evidence. Substances in this group that are characterized by higher water solubilities and/or general bioavailability possess high toxicity to aquatic organisms, with adverse effects demonstrated at low exposure concentrations (that is, CAS RNs 96-69-5, 96-76-4, 98-54-4, 118-82-1, 128-37-0, 128-39-2, 35958-30-6, 36443-68-2, and 61788-44-1, with PNECs calculated to be in the range of 0.11 µg/L–25 µg/L). The available aquatic toxicity data were critically evaluated to derive substance-specific aquatic PNECs. Aquatic CBR-derived PNECs were also calculated and used in instances where effects data were at or above water solubility limits (for example, for CAS RNs 118-82-1 and 35958-30-6). MoA was considered in the derivation of the PNECs; two substances (CAS RNs 98-54-4 and 61788-44-1) were found to have potential for estrogenic effects. Aquatic PNECs were assigned a moderate-to-high confidence weighting, depending on the robustness and data availability for each substance. Substances in the group, characterized by high log KOW values and low water solubilities, did not exhibit adverse effects at or below their water saturation levels (that is, CAS RNs 1843-03-4 and 4221-80-1). Aquatic PNECs were therefore not derived for these substances. Due to the uncertainty associated with some parameters used in the exposure analysis (see section 7.3.4), the level of confidence in the calculated PECs and RQs is low.
Consideration of key lines of evidence associated with each substance in the group is provided below.
CAS RN 96-69-5 is expected to persist in the environment; however, it does not possess high potential for bioaccumulation in organisms. Some RQs were found to exceed 1 in multiple generic scenarios in the aquatic compartment and, as a result, further analysis considering specific company and facility information was conducted to refine risk analyses. Refinements to the multiple generic PECs result in RQs below 1. Considering available lines of evidence, CAS RN 96-69-5 has a low potential for causing ecological harm in Canada at current levels of exposure. While exposure of CAS RN 96-69-5 to the Canadian environment is unlikely to be of concern, this substance is considered to have ecological effects of concern at low concentrations, such as the lethal effect due to long-term exposure. As such, there may be a concern if exposures to this substance were to increase.
CAS RN 96-76-4 is expected to persist in the environment, but it does not possess high potential for bioaccumulation in organisms. Considering available lines of evidence, including aquatic PNECs and the environmental exposure from the generic scenarios, a low potential for harm to the environment was identified for this substance. Considering all these lines of evidence, CAS RN 96-76-4 has a low potential for causing ecological harm.
CAS RN 98-54-4 is not expected to persist in the environment and does not possess high potential for bioaccumulation in organisms. However, there are indications that this substance has potential for estrogenic effects and endocrine activity that may lead to sub-lethal chronic adverse effects in exposed organisms. Risk characterization for one generic exposure scenario had an RQ of below 1. Considering available lines of evidence, CAS RN 98-54-4 is not expected to cause ecological harm in Canada at current levels of exposure. While exposure of CAS RN 98-54-4 to the Canadian environment for the substance’s current uses is unlikely to be of concern, this substance is considered to have ecological effects of concern at low concentrations, including estrogenic activity and the potential for endocrine effects. As a result, there may be a concern if exposures to this substance were to increase.
CAS RN 118-82-1 is expected to persist in the environment, which may lead to long-term exposure, and is also highly bioaccumulative in organisms. It is noted that this substance possesses moderate-to-high toxicity to aquatic and soil organisms, demonstrating adverse effects at very low exposure levels. However, long-term exposures could not be sufficiently accounted for in the quantitative analysis presented in the assessment. Considering available lines of evidence, including moderate-to-high aquatic and soil toxicity as well as long residence time in the environment and its potential for bioaccumulation, there is potential for harm to the environment from CAS RN 118-82-1.
CAS RN 128-37-0 is expected to persist in the environment. The available evidence shows a moderate potential for bioaccumulation. The substance possesses high toxicity to aquatic organisms; the aquatic PNEC was calculated as 2.3 µg/L. An RQ exceeding 1 was identified in a generic scenario in the aquatic compartment, and further analysis considering specific company and facility information was conducted to refine the risk analysis. Refinements were conducted using the specific analysis to reflect uses at specific industrial sites. Several refined RQs exceed 1, and multiple RQs approach 1 in the aquatic compartment. Considering available lines of evidence, there is potential for harm to the environment from CAS RN 128-37-0.
CAS RN 128-39-2 is expected to persist in the environment and may cause long-term exposure to aquatic organisms. However, it is not expected to be highly bioaccumulative in organisms. Considering the aquatic PNEC and the environmental exposure in the generic scenarios, no RQ exceeding 1 was identified for this substance. Considering available lines of evidence, CAS RN 128-39-2 has a low potential for causing ecological harm.
CAS RN 1843-03-4 is expected to persist in the environment; however, it is not expected to be highly bioaccumulative in organisms. Values of the toxicity endpoints are above its water solubility limit. The substance has not demonstrated adverse effects in aquatic organisms at or below its water saturation level. Considering available lines of evidence, CAS RN 1843-03-4 has a low potential for causing ecological harm.
CAS RN 4221-80-1 is expected to persist in the environment; however, it is not expected to be highly bioaccumulative in organisms. Moreover, it possesses a log KOW above 9 and is not expected to be bioavailable. No effects to aquatic organisms have been observed in the available studies at or below its water saturation level. Considering available lines of evidence, CAS RN 4221-80-1 has a low potential for causing ecological harm.
CAS RN 35958-30-6 is expected to persist in the environment. The available evidence shows a moderate potential for bioaccumulation. This substance was reported to be imported by Canadian distributors; however, due to a lack of client information, the substance was not included in the exposure analysis as imported quantities could not be linked to industrial users in Canada. However, to estimate whether potential environmental harm could be resulting from the use of this substance, concentrations using generic parameters were calculated for each exposure scenario, assuming that this substance would be used by all of the sectors. None of the estimated environmental concentrations exceeded the PNEC value for this substance in any media. However, given the significant uncertainty of these exposure estimates, the estimated environmental concentrations are not presented in the ecological risk assessment. Considering available lines of evidence, CAS RN 35958-30-6 has a low potential for causing ecological harm.
CAS RN 36443-68-2 is expected to persist in the environment. The available evidence shows a low potential for bioaccumulation. The substance possesses high toxicity to aquatic organisms; the aquatic PNEC was calculated to be 1.1 µg/L. An RQ exceeding 1 was identified in a generic scenario in the aquatic compartment. Refinements were conducted to reflect uses at specific industrial sites, and refined RQs for all sites exceed 1 in the aquatic compartment. Considering available lines of evidence, there is potential for harm to the environment from CAS RN 36443-68-2.
CAS RN 61788-44-1 is expected to persist in the environment, which may lead to long-term exposure to aquatic organisms. It is also highly bioaccumulative (as indicated by the high bioaccumulation potential seen in most components) in organisms, and it possesses high toxicity to aquatic organisms, with adverse effects observed at very low exposure concentrations; the aquatic PNEC is calculated as 1.2 µg/L. There is also indication of estrogenic activity and potential for endocrine effects with respect to the monostyrenated component of this substance. These long-term exposures could not be sufficiently accounted for in the quantitative analysis presented in the assessment. Considering available lines of evidence, including moderate-to-high aquatic and soil toxicity as well as long residence time in the environment and its potential for bioaccumulation, there is potential for harm to the environment from CAS RN 61788-44-1.
7.3.4 Sensitivity of conclusion to key uncertainties
An uncertainty is associated with using the CBR approach to calculate aquatic PNECs for some substances in the group where aquatic PNECs derived from empirical toxicity data exceeded water solubility. The CBR approach was used in characterizing the risks of these substances in the aquatic compartment. CBR thresholds for narcotic substances are well established in the literature; however, as both the chronic CBR50 value and the modelled BAFs were used to apply the CBR approach, the extrapolated aquatic PNEC values represent generic estimates. In addition, when applying the CBR approach to calculate aquatic PNECs, referencing values are based on literature and model predictions that focus on the aquatic compartment. Hence, the confidence is considerably higher than the CBR applications for sediment and soil compartments.
There are a number of uncertainties associated with the exposure characterization, most of which are driven by the limited availability of data and the potential variability (based on variability seen in other jurisdictions) in annual use quantities. The generic PEC approach was applied to account for the uncertainty associated with a variety of parameters used in the analysis, including the following: use quantity of substances in this group and location of industrial users. Scenarios were based on reported import quantities; however, limited information was available on specific users of these substances, including information on specific quantities used by individual facilities and locations of the user facilities.
The exposure characterization involved the use of distributions and average values for certain parameters in order to address some of the above-mentioned uncertainties. Averages were used for use quantities in the generic scenarios in order to mitigate some of the uncertainty associated with submitted information (which varied in age, reporting year, reporting format, etc.); therefore, limiting the weight of any single reported value. It is also likely that in some cases the quantity used in calculations may underestimate actual usage. It is noted that this approach only considers substances that were reported to be used in specific industrial sectors. It is possible that substances in this group may become more broadly used (that is, in additional sectors) or have their respective sectoral use quantities increased. Uncertainty relating to quantities is also supported by international use quantity information, which indicates that many of these substances are used globally and that use quantities may vary significantly from year-to-year (CDR 2016; SPIN c2017).
It should be noted that the environmental exposure analysis and risk characterization considered each substance in this group individually. Therefore, the cumulative exposure from this group was not considered. If multiple substances are contained in the same products, multiple products containing these substances are used simultaneously; or, if multiple facilities located in same area use these substances, releases of these substances could result in cumulative effects on organisms and present a higher risk. However, at this time there are insufficient data to calculate the cumulative risk potential.
Release to air, which could be relevant for substances with moderate vapour pressure, was not quantified in this ecological risk assessment. However, there is a lack of ecotoxicity data in air to support a quantitative risk analysis for this compartment.
8. Potential to cause harm to human health
The human health assessment took into consideration those groups of individuals within the Canadian population who, due to greater susceptibility or greater exposure, may be more vulnerable to experiencing adverse health effects. These subpopulations were taken into account in the risk assessment outcomes of certain substances in the Substituted Phenols Group.
8.1 Assessment of CAS RN 85-60-9
8.1.1 Exposure assessment
No Canadian environmental monitoring data were available for CAS RN 85-60-9. Due to its negligible vapour pressure and low water solubility (Table 3-1), exposure from environmental media that could impact the health of the general population is considered to be negligible.
No monitoring data were identified for the substance’s presence in food in Canada. However, CAS RN 85-60-9 may be used as a component in food packaging materials in Canada. The probable daily intake (PDI) was estimated as 0.000049 mg/kg bw/day for the general population (1 year of age and older) (personal communication from the FD of HC to the ESRAB, HC, dates ranging from March 2017 to Feb 2019; unreferenced).
No data were identified for the use of CAS RN 85-60-9 in products available to consumers. All uses reported pursuant to a CEPA section 71 survey were for commercial use only (EC 2009). Therefore, exposure from products available to consumers is not expected.
8.1.2 Health effects assessment
CAS RN 85-60-9 was previously evaluated by the US EPA under the High Production Volume (HPV) Challenge Program (US EPA 2010). The key study was a 90-day repeated-dose study in which Carworth Farms rats were exposed 0, 50, 500, or 5000 ppm in diet (equivalent to 0, 3, 30, or 300 mg/kg bw/day in males and 0, 2.5, 25, or 250 mg/kg bw/day in females; n=6/dose). The no-observed-adverse-effect levels (NOAELs) for a systemic toxicity of 2.5 mg/kg bw/day in females and 3 mg/kg bw/day in males were based on yellowish livers, increased relative liver weight, and some fatty infiltration occurring at the next dose tested.
8.1.3 Characterization of risk to human health
On the basis of the available empirical data, the NOAEL of 2.5 mg/kg bw/day based on liver effects in females was the critical health effect that was compared to the PDI from CAS RN 85-60-9’s potential use as a component in the manufacture of food packaging materials (0.000049 mg/kg bw/day for the general population [1 year of age and older]). The resulting margin of exposure (MOE) was determined to be adequate (MOE = 51 000) to address uncertainties in the health effects and exposure databases.
8.2 Assessment of CAS RN 96-69-5
8.2.1 Exposure assessment
No Canadian environmental monitoring data were available for CAS RN 96-69-5. Drinking water estimates were generated using the highest PEC (0.011 mg/L) modelled for the presence in surface water (Table 7-8). The highest daily intake estimate of exposure from drinking water was 0.0014 mg/kg bw/day (0 to 5 months; Appendix B, Table B-1).
No monitoring data were identified for the presence in food in Canada. CAS RN 96-69-5 may be used as a component in the manufacture of food packaging materials in Canada. The PDI was estimated to be negligible for this substance (personal communication from the FD of HC to the ESRAB, HC, dates ranging from March 2017 to Feb 2019; unreferenced).
No data were identified for the use of CAS RN 96-69-5 in products available to consumers. Therefore, exposure from products available to consumers is not expected.
8.2.2 Health effects assessment
CAS RN 96-69-5 was previously evaluated by the US EPA under the HPV Challenge Program (US EPA 2010). The key study for systemic toxicity was a 2-year repeated-dose study in which F344/N rats (n=15/dose/sex) were exposed orally in diet to 0, 20, 40, or 100 mg/kg bw/day. The NOAEL for systemic toxicity was 20 mg/kg bw/day, identified on the basis of liver toxicity and altered hematological parameters at the next dose tested. The key study for reproductive and developmental toxicity was a multi-generational study in which pregnant New Zealand white rabbits were exposed by oral gavage to 0, 0.2, 2, and 20 mg/kg bw/day (n=13/dose). The critical effect for maternal toxicity was anorexia and spontaneous abortion, which occurred at 20 mg/kg bw/day (this was accompanied by an increase in embryo death and decrease in litter size at 20 mg/kg bw/day). Thus, the NOAEL for maternal toxicity was 2 mg/kg bw/day. The NOAEL for developmental toxicity was 0.2 mg/kg bw/day, which was based on a decrease in mean weight of offspring in the mid-dose group. However, this reduction in body weight was not observed in the rabbits of the high-dose group that did not abort; therefore, a dose response could not be established for this endpoint.
8.2.3 Characterization of risk to human health
On the basis of the available empirical data, the NOAEL of 0.2 mg/kg bw/day for developmental toxicity identified in the multigenerational study was identified as the critical health effect for comparison to the highest estimate of daily exposure (0.0014 mg/kg bw/day, 0- to 5-month-olds, from environmental media). The resulting MOE was determined to be adequate (MOE = 139) to address uncertainties in the health effects and exposure databases.
8.3 Assessment of CAS RN 96-76-4
8.3.1 Exposure assessment
Limited environmental monitoring data relevant to current exposures in Canada have been identified for CAS RN 96-76-4. In Canada, a maximum concentration of 0.29 µg/L was reported in all samples (n=2) of natural bottled mineral water collected from 27 countries, including Canada (Guart et al. 2014). The estimated daily intake for CAS RN 96-76-4 from bottled water is expected to be negligible (<2.5 ng/kg bw/day). It was also reported in surface water in Slovakia (Slobodnik et al. 2012) and groundwater in the United Kingdom (UK) (White et al. 2016). Drinking water estimates were generated using the highest PEC (0.9 µg/L) modelled for the presence in surface water (Table 7-8). The highest daily intake estimate of exposure from drinking water was 0.00012 mg/kg bw/day (0 to 5 months; Appendix B, Table B-1).
No monitoring data were identified for the presence in food in Canada. CAS RN 96-76-4 may be used as a component in food packaging materials in Canada. The PDI was estimated to be negligible for this substance (personal communication from the FD of HC to the ESRAB, HC, dates ranging from March 2017 to Feb 2019; unreferenced).
Potential exposure from use of products available to consumers was estimated. Details are presented in Appendix C. Uses of this substance in lubricants, greases, fuels, and related products available to consumers were reported in response to a CEPA section 71 survey (EC 2013, ECCC 2017). The per event exposure from use of a DIY oil change on a motor vehicle was estimated. Dermal exposure was estimated to be 0.0023 mg/kg bw (per event) for an adult (19+ years).
8.3.2 Health effects assessment
CAS RN 96-76-4 was previously evaluated by the US EPA as part of the HPV Challenge Program (US EPA 2009). This substance was grouped together with CAS RN 128-39-2. The grouping justification for these two substances was based on the “di- and tri-substituted mixed alkylphenol” category defined by the US EPA (2009), which contained seven substances (CAS RNs 128-39-2, 96-76-4, 120-95-6, 2772-45-4, 2416-94-6, 17540-75-9, and 732-26-3) and was based on similar structural, physical-chemical, and toxicological properties. The critical effect level was taken from a 28-day repeated-dose study in which Wistar rats (n=5/sex/dose) were exposed to CAS RN 128-39-2 at 0, 15, 100, or 600 mg/kg bw/day by oral gavage. The NOAEL for systemic toxicity was 100 mg/kg bw/day on the basis of increased liver weight and related histopathological changes observed at the highest dose tested.
In a combined reproductive and repeated-dose study, with protocols similar to those of OECD Test Guideline (TG) 415 and OECD TG 408, respectively, Sprague-Dawley rats were exposed to 0, 50, 150, or 300 mg/kg bw/day of CAS RN 96-76-4 in diet (ECHA c2007-2015a). A NOAEL of 150 mg/kg bw/day was reported for body weight loss; however, this may have been due to palatability issues with the treated food since reduced food consumption was observed in both males and females at the mid and high doses. Liver effects observed were found to be fully reversible. Reproductive capability was deemed to be unimpaired in the parental (P0) animals. There was a reduction in the mean number of F1 pups born at the highest dose; however, this was associated with evidence of maternal neglect (that is, no milk in the stomach and non-removal of placenta or fetal membranes). There was a dose-dependent reduction in food consumption in the F1 pups, with a related decrease in body weight. A NOAEL of 150 mg/kg bw/day was determined for body weight in the F1 pups. No effects were reported for the F2 pups.
8.3.3 Characterization of risk to human health
On the basis of the available empirical data, the NOAEL of 100 mg/kg bw/day (a read-across value from CAS RN 128-39-2, based on increased liver weight and related histopathological changes observed at the next dose tested in a 28-day study) was identified as the critical health effect for comparison to the highest estimate of potential exposure (from use of a DIY oil change product, 0.0023 mg/kg bw/day, adults 19+ years). The resulting MOE was determined to be adequate (MOE = 43 000) to address uncertainties in the health effects and exposure databases.
8.4 Assessment of CAS RN 98-54-4
8.4.1 Exposure assessment
No Canadian environmental monitoring data were available for CAS RN 98-54-4. Internationally, it was reported in indoor and outdoor air in California, United States, at 32 and 3.4 ng/m3, respectively (Rudel et al. 2010). It was also reported in indoor air (median 36.3 ng/m3) in Japan (Saito et al. 2004). It was not detected in surface water in Denmark (Long et al. 2014); however, it was reported in surface and storm water in France (Zgheib et al. 2012; Colin et al. 2014) and surface water in China (Liu et al. 2017b). It was also reported in groundwater in California, United States, (Mohler et al. 2013) and Vietnam (Duong et al. 2015).
Since no Canadian environmental monitoring data were found, drinking water estimates were generated using the highest PEC (0.0030 mg/L) modelled for the presence in surface water (Table 7-8). The highest daily intake estimate of exposure from drinking water was 0.00039 mg/kg bw/day (0 to 5 months; Appendix B, Table B-1). The contribution from indoor and ambient air is expected to be negligible (<2.5 mg/kg bw/day).
CAS RN 98-54-4 is recognized as a food flavouring agent internationally; therefore, it is possible that it is used for this purpose in foods sold in Canada.
The JECFA evaluated CAS RN 98-54-4 as a food flavouring agent and estimated the corresponding per capita intake from this use to be 0.01 µg/day (0.0002 µg/kg bw/day) for the US population (International Organization of the Flavor Industry 1995; Lucas et al. 1999; both cited in WHO 2001).
Only sparse qualitative information is available on the natural occurrence of CAS RN 98-54-4 in foods. It is found in Origanum (Coridothymus capitatus [L.] Richb.) and honey (Nijssen 1963-2018). Although no information on the concentrations occurring in these foods was identified, naturally occurring CAS RN 98-54-4 is expected to contribute a negligible amount to the overall dietary exposure to this substance (personal communication from the FD of HC to the ESRAB, HC, dates ranging from March 2017 to Feb 2019; unreferenced).
Potential exposure from products available to consumers was estimated. Details are presented in Appendix C. Potential consumer exposure from DIY motor vehicle applications was estimated. The highest exposure estimate was for a DIY fuel additive use on a motor vehicle. Dermal exposure was estimated at 0.013 mg/kg bw (per event) for an adult (19+ years). Additional potential use scenarios for this substance were considered (DIY oil change, use of specialized marine epoxy, and use of automotive paint hardener) but resulted in lower exposure estimates than the DIY fuel additive.
8.4.2 Health effects assessment
CAS RN 98-54-4 was previously evaluated by ECHA (2012), the US EPA (2009), and the European Commission (EC 2008), and is the subject of an OECD (2000) SIDS. In a combined repeated-dose and reproductive toxicity screening study (OECD TG 422), Sprague-Dawley rats (n=13/sex/dose) were exposed to 0, 20, 60, or 200 mg/kg bw/day of CAS RN 98-54-4 by oral gavage. The NOAEL for the parental generation was 60 mg/kg bw/day based on respiratory distress in females and altered blood parameters in males at the next dose tested; however, the severity of these effects was considered to be equivocal. No effects on fertility were observed at any of the doses tested (ECHA 2012) in a two-generation reproduction toxicity study (OECD TG 416) in which rats were exposed to 0, 800, 2500, or 7500 ppm (0, 70, 200, or 600 mg/kg bw/day) in their diet (ECHA 2012). A NOAEL of 70 mg/kg bw/day was identified for reproductive and developmental toxicity. The maternal NOAEL (70 mg/kg bw/day) was based on decreased body weight gain, reduced food consumption, and reduced ovary and adrenal gland weights in the P0 generation. The offspring NOAEL (70 mg/kg bw/day) was based on reduced pup body weight and litter weight in F1 and F2 generations.
8.4.3 Characterization of risk to human health
In the absence of data on the actual use, if any, of CAS RN 98-54-4 as a flavouring agent in foods sold in Canada, the JECFA per capita intake estimate for the US population is an acceptable surrogate for possible Canadian dietary exposure to this substance from this use in food for the general population (1 year of age and older) (International Organization of the Flavor Industry 1995; Lucas et al. 1999; both cited in JECFA 2001). The JECFA concluded there was “no safety concern at estimated levels of intake.”
On the basis of the available empirical data, the NOAEL of 70 mg/kg bw/day (for reproductive and developmental toxicity) was identified as the critical health effect for comparison to the highest potential exposure estimate, that is, from use of a DIY fuel additive (0.013 mg/kg bw/day, adults 19+ years). The resulting MOE was determined to be adequate (MOE = 5400) to address uncertainties in the health effects and exposure databases.
8.5 Assessment of CAS RN 118-82-1
8.5.1 Exposure assessment
No Canadian environmental monitoring data were available for CAS RN 118-82-1. Due to its negligible vapour pressure and low water solubility (Table 3-1), exposure from environmental media that could impact the health of the general population is considered to be negligible.
CAS RN 118-82-1 may be a component in incidental additives (for example, lubricants) used in food processing establishments in Canada. However, there is no direct food contact expected and exposure of the general population from this source is not expected (personal communication from the FD of HC to the ESRAB, HC, dates ranging from March 2017 to Feb 2019; unreferenced).
Potential exposure from use of products available to consumers was estimated. Details are presented in Appendix C. CAS RN 118-82-1 is reported to be used in lubricants (MSDS 2016). Potential consumer exposure from DIY automotive applications (oil change) was considered to occur mainly via the dermal route and was estimated to be 0.023 mg/kg bw/day for an adult (19+ years).
8.5.2 Health effects assessment
CAS RN 118-82-1 is not genotoxic in the Ames assay, HPRT assay, chromosome aberration assay, or the mitotic recombination assay. Two repeated-dose studies have been conducted; one 28-day study in rats (OECD TG 207) and one 6-month to 2-year study in beagles (OECD TG 452). These studies reported liver (rat and dog) and thyroid (rat) effects that were fully reversible following a recovery period. In a two-generation reproductive toxicity study (OECD TG 416), rats were exposed to 0, 15, 60, 100, 500, or 3000 ppm in their diet. No effects on fertility, offspring viability, or offspring morphology were observed at any of the doses tested. In a combined chronic toxicity / carcinogenicity study (OECD TG 453), rats were exposed for 2 years to 0, 15, 60, 100, 500, or 3000 ppm through their diet, which is equivalent to 0.55, 2.14, 3.56, 18.1, or 107 mg/kg bw/day in males and 0.67, 2.63, 4.35, 22.2, or 138 mg/kg bw/day in females (n=25-50/sex/dose). No tumours occurred at any dose. However, an increased incidence and severity of hepatic lesions was observed at 18.1 mg/kg bw/day (males) and 22.2 mg/kg bw/day (females). Therefore, the NOAEL in this study is 100 ppm (which is equivalent to 3.56 mg/kg bw/day in males and 4.35 mg/kg bw/day in females). These results are from data submitted to the ECHA under the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) regulation (ECHA c2007-2017).
8.5.3 Characterization of risk to human health
The highest potential exposure estimate is from a DIY oil change scenario (0.023 mg/kg bw/day, adults 19+ years). No MOE was calculated for reproductive toxicity because no toxicological effects were reported at the highest dose tested. On the basis of empirical data, the NOAEL of 3.56 mg/kg/day (increased incidence and hepatic lesions for males) was identified as a health effect for comparison to the potential exposure estimates. The resulting MOE was determined to be adequate (MOE = 155) to address uncertainties in the health effects and exposure database.
8.6 Assessment of CAS RN 128-37-0
8.6.1 Exposure assessment
Environmental media and food
No Canadian environmental monitoring data were available for CAS RN 128-37-0. Internationally, it was reported in indoor air in Japan with a range of 292 ng/m3 to 3510 ng/m3 and a median concentration of 550 ng/m3 (Kanazawa et al. 2010). It was also reported in dust with a range of 0.76 μg/g to 7.34 μg/g and a median concentration of 4.31 μg/g in the United States (Wang et al. 2016) and elsewhere internationally (Kanazawa et al. 2010; Papadopoulos et al. 2013; Bamai et al. 2014; Liu et al. 2017a). However, CAS RN 128-37-0 was not detected in 11 soil samples in Portugal (Fernandes et al. 2014) and 9 soil samples in Colombia (Hernández et al. 2012). CAS RN 128-37-0 was reported in surface waters in the United States with a maximum reported concentration of 35 ng/L (Baker et al. 2014) and elsewhere internationally (Gomez et al. 2012; Hernández et al. 2012; Slobodnik et al. 2012; O’Brien et al. 2016; Allinson et al. 2015; Polidoro et al. 2017). It was also reported in surface water and groundwater in Spain (Cabeza et al. 2012; Pitarch et al. 2016).
Given the lack of Canadian environmental monitoring data, drinking water estimates were generated using the highest PEC (0.0062 mg/L) modelled for the presence in surface water (Table 7-9).
CAS RN 128-37-0 has been reported as naturally occurring, emitted by fungi associated with the pre-processing storage of olives (Gharbi et al. 2017). It is an antioxidant that, in Canada, may be used as a food additive (preservative) in various foods (personal communication from the FD of HC to the ESRAB, HC, dates ranging from March 2017 to Feb 2019; unreferenced). In some cases, it may be added to food via the food’s packaging. The List of Permitted Preservatives sets out the foods to which it may be added and its maximum level of use in each food. These maximum levels of use are applied to CAS RN 128-37-0 singly or, when it is used in combination with butylated hydroxyanisole, propyl gallate, or tertiary butyl hydroquinone, to the total of these four antioxidants. The maximum level of use, that is, the maximum concentration that may be in the food, must be respected whether CAS RN 128-37-0 has been directly added to the food or indirectly added via its food packaging.
Canadian dietary exposure to CAS RN 128-37-0 from its use as a food preservative was estimated by multiplying consumption of the foods permitted to contain it by the amount of CAS RN 128-37-0 in those foods. Food consumption was based on individual one-day “eaters only” food intakes reported by respondents to the Canadian Community Health Survey (Statistics Canada 2015). The amount of CAS RN 128-37-0 assumed to be present in the foods was based on the highest levels of use reported by industry for permitted uses of CAS RN 128-37-0 (levels used ranged from 0.0000003% in partially defatted beef fatty tissue to 0.02% in fats and oils). Foods containing a low level of CAS RN 128-37-0, and that would make a negligible contribution to dietary exposure, were excluded from the exposure assessment. The mean and 90th percentile dietary exposures were estimated in this manner for various age groups (Appendix B, Table B-2). CAS RN 128-37-0 may also be used as a component in the manufacture of other food packaging materials, such as adhesives and coatings, in which case the PDI was estimated to be negligible for this substance (personal communication from the FD of HC to the ESRAB, HC, dates ranging from March 2017 to Feb 2019; unreferenced).
Daily intake estimates of CAS RN 128-37-0 from environmental media and food are shown in Appendix B (Table B-3). The highest estimated intake for CAS RN 128-37-0 was 0.15 mg/kg bw/day (1- to 3-year-olds) in the general population.
Products available to consumers
Potential exposure from products available to consumers was estimated. Details are presented in Appendix C. Estimates for uses that result in the highest level of potential oral, inhalation, or dermal exposure (referred to as sentinel scenarios) are presented in Table 8-1. For estimated potential exposures via the dermal route, dermal absorption was considered to be 0.41% (see description below).
Additional potential use scenarios for CAS RN 128-37-0 were considered (for example, as a non-medicinal ingredient in natural health products and other non-prescription drugs; in cosmetics [such as body cleanser, exfoliant, facial moisturizer, makeup remover, shaving product, shampoo, hair conditioner, hair colour, makeup, and foot cream]; as well as dermal exposure to plastics/rubber, wall paints, children’s paints, inks, candles, cleaning products, adhesives and sealants, and hunting bait], but these resulted in lower exposure estimates than those presented in Table 8-1.
| Product scenario | Concentration (%) | Route of exposureab | Per event exposure (mg/kg bw) |
Daily exposure (mg/kg bw/ day) c |
|---|---|---|---|---|
| Lipstick (14–18 years) | 10%d | Oral | N/A | 0.089 |
| Mouthing plastic/rubber (0–5 months) | N/A | Oral | N/A | 0.0048 |
| Air freshener (1 year) | 30%e | Inhalation | 0.23 | 0.23 |
| Wall paint (19+ years) | 2%f | Dermal | 0.004 | N/A |
| Sunscreen lotion (6–11 months) | 1%g | Dermal | N/A | 0.019 |
| Body lotion (0–5 months) | 1%d | Dermal | N/A | 0.01 |
| Aggregate exposure to cosmetics (14–19+ years)h | N/Ai | Dermal | N/A | 0.0071 |
| DIY motor oil change (19+ years) | 1%j | Dermal | 0.000097 | N/A |
Abbreviation: N/A, not applicable
a For estimated potential exposures via the dermal route, dermal absorption was 0.41% for CAS RN 128-37-0.
b For all substances in this group, an oral absorption of 100% was assumed.
cThese values take into account the assumed daily frequency of use, so for ConsExpo estimates, the year-averaged daily exposure value was used. See Appendix C for more details on models and parameters used.
d Personal communication from the CHPSD of HC to the ESRAB, HC, dates ranging from March 2017 to Feb 2019; unreferenced.
e MSDS 2014a
f Malshe and Sikchi 2004
g Personal communication from the Therapeutic Products Directorate (TPD) of HC to the ESRAB, HC, dates ranging from March 2017 to Feb 2019; unreferenced.
h Given the large number (approximately 8000) and variety of cosmetics applied to the skin that are reported to contain CAS RN 128-37-0, an estimate of combined exposure to CAS RN 128-37-0 from multiple dermally applied products was considered. Combined exposure was estimated from the potential daily use of body moisturizer, body soap (solid), facial makeup (solid), antiperspirant/deodorant, and fragrance product.
i See Appendix C for concentrations.
j MSDS 2010b
Dermal absorption potential
For the estimation of systemic exposure from potential dermal exposure to CAS RN 128-37-0, a dermal absorption of 0.41% was used based on in vitro experiments using pig skin (Central Toxicology Laboratory 1998 as cited in Lanigan and Yamarik 2002). In this in vitro dermal absorption study, CAS RN 128-37-0 was tested on the skin of 6- to 8- week-old pigs. CAS RN 128-37-0 was applied to the skin membranes in corn oil (200 µl/cm2) for 30 minutes, flushed and left uncovered. Samples of the receptor fluid were collected at multiple time points and repeated applications of adhesive strips were used to assess the exposure of the stratum corneum. At the end of the experiment, 0.07% of the applied dose of CAS RN 128-37-0 was extracted from the receptor chamber, 0.29% was recovered from the epidermis and dermis, and a mean total of 0.05% was calculated as recovered from the stratum corneum, for a total of 0.41% (Central Toxicology Laboratory 1998 as cited in Lanigan and Yamarik 2002).
Two additional studies were considered for dermal absorption of CAS RN 128-37-0. In an in vivo study, [14C]-CAS RN 128-37-0 (10% in Labrafil) was applied to guinea pigs, where 10% remained on the skin, and <4% of the radioactivity was excreted (Courtheoux et al. 1986). This study was considered to be an overestimate of dermal absorption because it used a vehicle (Labrafil) that is used as a bioavailability enhancer and solubilizer (Delongeas et al. 2010). In addition, this study did not report certain parameters such as dermal load, exposure durations, and quality control measures, such as recoveries. In an in vitro study, radiolabelled CAS RN 128-37-0 (5 µg/cm2) was applied in acetone to the excised skin of female fuzzy rats; the fraction of the skin radioactivity present in the stratum corneum was ~13% (Bronaugh et al. 1989). This study was also considered to be an overestimate of dermal absorption as it used a vehicle (acetone) that is known to disrupt the skin barrier for rat skin, therefore leading to an overestimate of dermal absorption (Rissmann et al. 2009).
In a 2002 review paper, the reviewers concluded that CAS RN 128-37-0 primarily remains on the skin and is slowly absorbed with minimal systemic exposure compared to the oral route (Lanigan and Yamarik 2002).
8.6.2 Health effects assessment
CAS RN 128-37-0 has been evaluated by JECFA (WHO 1995), the OECD (2002) SIDS, and EFSA (2012). Health Canada participated in the JECFA assessment of CAS RN 128-37-0, which established an acceptable daily intake (ADI) of up to 0.3 mg/kg bw for CAS RN 128-37-0, and considers the ADI developed as suitable. The ADI was calculated with a NOAEL of 25 mg/kg bw/day, which is based on a long-term, repeated-dose, oral toxicity study performed with Wistar rats exposed to 0, 25, 100, or 500 mg/kg bw/day (22-month treatment duration, with in utero exposure; Price 1994, unpublished) and the application of a 100-fold uncertainty factor. The critical lesions observed in this study were hepatic enzyme induction and thyroid hyperactivity, which occurred at the two highest doses.
In a two-year rodent cancer bioassay, CAS RN 128-37-0 was found to be non-carcinogenic (NTP 1979). However, follow-up work suggested that it may act as a tumour promoter (Olsen et al. 1986 as cited in EFSA 2012); therefore, a BMDL10 (benchmark dose lower confidence limit) for hepatocellular carcinoma was established at 247 mg/kg bw/day (EFSA 2012).
8.6.3 Characterization of risk to human health
Table 8-2 provides relevant exposure estimates and critical health effect levels as well as resultant MOEs for the characterization of risk to human health from exposures to CAS RN 128-37-0.
| Exposure scenario | Systemic exposure (mg/kg bw/day)ab | Critical effect levelc (mg/kg bw/day) | Critical health effect endpoint | MOE |
|---|---|---|---|---|
| Daily oral exposure from environmental media and food (1–3 years) | 0.15 | 25 | Induction of liver enzymes and thyroid hyperactivity at the next dose tested in a repeated-dose study | 170 |
| Daily dermal exposure from sunscreen lotion (6–11 months) | 0.019 | 25 | Induction of liver enzymes and thyroid hyperactivity at the next dose tested in a repeated-dose study | 1 300 |
| Daily dermal exposure from body lotion (0–5 months) | 0.01 | 25 | Induction of liver enzymes and thyroid hyperactivity at the next dose tested in a repeated-dose toxicity study | 2 500 |
| Daily dermal combined exposure from cosmetics (14–19+ years) | 0.0071 | 25 | Induction of liver enzymes and thyroid hyperactivity at the next dose tested in a repeated-dose study | 3 500 |
| Air freshener (inhalation; 1 year old) | 0.23 | 25 | Induction of liver enzymes and thyroid hyperactivity at the next dose tested in a repeated-dose study | 107 |
| DIY motor oil change (dermal 19+ years) | 0.000097 | 25 | Induction of liver enzymes and thyroid hyperactivity at the next dose tested in a repeated-dose toxicity study | 258 000 |
| Wall paint (dermal 19+ years) | 0.004 | 25 | Induction of liver enzymes and thyroid hyperactivity at the next dose tested in a repeated-dose toxicity study | 6 300 |
Abbreviation: MOE, margin of exposure
a For estimated potential exposures via the dermal route, dermal absorption was 0.41% for CAS RN 128-37-0 on the basis of experimental data (see description in text).
b For all substances in this group, an oral absorption of 100% was assumed.
c NOAEL
The MOEs are considered adequate to address uncertainties in the health effects and exposure databases and to be protective against adverse effects that occur at doses greater than 25 mg/kg bw/day.
8.7 Assessment of CAS RN 128-39-2
8.7.1 Exposure assessment
No Canadian environmental monitoring data have been identified for CAS RN 128-39-2. Internationally, it was reported in surface water in Slovakia (Slobodnik et al. 2012) and in groundwater in the UK (White et al. 2016). However, it was not detected in groundwater in Guiana, Guadeloupe, Martinique, Réunion, and Mayotte (Vulliet et al. 2014).
Drinking water estimates were generated using the highest PEC (12 µg/L) modelled for the presence in surface water (Table 7-9). The highest daily intake estimate of exposure from drinking water was 0.0015 mg/kg bw/day (0-5 months; Appendix B, Table B-1).
CAS RN 128-39-2 has not been identified as being used as a component in the manufacture of food packaging materials in Canada (personal communication from the FD of HC to the ESRAB, HC, dates ranging from March 2017 to Feb 2019; unreferenced). CAS RN 128-39-2 may be a component in incidental additives (for example, lubricants) used in food processing establishments in Canada. However, there is no direct food contact expected, and exposure from this source is not expected (personal communication from the FD, HC to the ESRAB, HC dates ranging from March 2017 to Feb 2019; unreferenced).
Potential exposure from products available to consumers was estimated. Details are presented in Appendix C. Use in lubricants and greases available to consumers was reported in a survey conducted pursuant to section 71 of CEPA (EC 2009, ECCC 2017). CAS RN 128-39-2 was also identified in a fuel additive (MSDS 2015). Potential consumer exposures from DIY automotive applications (oil change, fuel additive) were estimated. The highest per event exposure was for a DIY fuel additive use on a motor vehicle. Dermal exposure was estimated at 0.13 mg/kg bw (per event) for an adult (19+ years).
8.7.2 Health effects assessment
CAS RN 128-39-2 was previously evaluated by the US EPA as part of the HPV Challenge Program (US EPA 2009) and by the OECD (1994) as a SIDS. The US EPA based their critical effect level for systemic toxicity on a 28-day repeated-dose study in which Wistar rats (n=5/sex/dose) were exposed to CAS RN 128-39-2 at 0, 15, 100, or 600 mg/kg bw/day by oral gavage. The NOAEL was 100 mg/kg bw/day on the basis of increased liver weight and related histopathological changes observed at the highest dose tested.
8.7.3 Characterization of risk to human health
On the basis of the available empirical data, the NOAEL of 100 mg/kg bw/day for increased liver weight and related histopathological changes in a 28-day study was identified as the critical health effect for comparison to the highest potential exposure, DIY fuel additive (0.13 mg/kg bw, adults 19+ years). The resulting MOE was determined to be adequate (MOE = 770) to address uncertainties in the health effects and exposure databases.
8.8 Assessment of CAS RN 1843-03-4
8.8.1 Exposure assessment
No Canadian environmental monitoring data were available for CAS RN 1843-03-4. Due to its low vapour pressure and negligible water solubility (Table 3-1), exposure from environmental media that could impact the health of the general population is considered to be negligible.
No monitoring data were identified for the presence in food in Canada. CAS RN 1843-03-4 may be used as a component in the manufacture of food packaging materials in Canada. The PDI was estimated as 0.0018 mg/kg bw/day for the general population (1 year of age and older) (personal communication from the FD of HC to the ESRAB, HC, dates ranging from March 2017 to Feb 2019; unreferenced).
No data were identified for the use of CAS RN 1843-03-4 in products available to consumers. No consumer uses were reported in a survey conducted pursuant to section 71 of CEPA (EC 2013, ECCC 2017). Therefore, exposure from products available to consumers is not expected.
8.8.2 Health effects assessment
CAS RN 1843-03-4 is not genotoxic in the Ames assay (OECD TG 471), the in vitro mammalian chromosome aberration test (OECD TG 473), or the in vitro mammalian cell gene mutation test (OECD TG 476). There are no two-year cancer bioassays or any multigenerational reproductive/developmental studies for this substance. The key study is a 13-week repeated-dose study in male and female rats that were exposed to 0, 100, 500, or 5000 ppm in their diet (equivalent to 0, 6, 30, or 300 mg/kg bw/day in males and 0, 5, 25, or 250 mg/kg bw/day in females). NOAELs of 25 mg/kg bw/day in females and 30 mg/kg bw/day in males were identified on the basis of a relative reduction in liver weights, and a relative increase of spleen and adrenal weights. Decreases in body weights were also observed but not considered to be statistically or toxicologically significant. These results are from data submitted to the ECHA under the REACH (ECHA c2007-2015b).
8.8.3 Characterization of risk to human health
On the basis of the available empirical data, the NOAEL of 25 mg/kg bw/day for a relative reduction in liver weights and a relative increase of spleen and adrenal weights was the critical health effect that was compared to the highest daily exposure from the substance’s potential use as a component in the manufacture of food packaging materials (0.0018 mg/kg bw/day for the general population [1 year of age and older]). The resulting MOE was determined to be adequate (MOE = 14 000) to address uncertainties in the health effects and exposure databases.
8.9 Assessment of CAS RN 2082-79-3
8.9.1 Exposure assessment
No Canadian environmental monitoring data were available for CAS RN 2082-79-3. Due to its negligible vapour pressure and low water solubility (Table 3-1), exposure from environmental media that could impact the health of the general population is considered to be negligible.
No monitoring data were identified for the presence in food in Canada. CAS RN 2082-79-3 may be used as a component in the manufacture of food packaging materials in Canada. The PDI was estimated to be negligible for this substance (personal communication from the FD of HC to the ESRAB, HC, dates ranging from March 2017 to Feb 2019; unreferenced).
Potential exposure from products available to consumers was estimated. Details are presented in Appendix C. Estimates for uses that result in the highest level of potential oral or dermal exposure (referred to as sentinel scenarios) are presented in Table 8-3. Due to the negligible vapour pressure of this substance, exposure via the inhalation route is not expected. For estimated potential exposures via the dermal route, a dermal absorption factor of 100% was assumed.
Additional potential use scenarios for this substance were considered (for example, dermal exposure to plastic/rubber, fabric/textiles and furniture, adhesives and sealants, cosmetics such as bath products, makeup, shampoo, hair conditioner, and nail polish as well as stamp pads, body markers, and candles), but these resulted in lower exposure estimates than those presented in Table 8-3.
| Product scenario | Concentration (%) | Route of exposurea | Per event exposure (mg/kg bw) |
Daily exposure (mg/kg bw/ day) b |
|---|---|---|---|---|
| Mouthing plastic/rubber (0–5 months) | N/A | Oral | N/A | 0.0048 |
| Face moisturizer (19+ years) | 0.1%c | Dermal | N/A | 0.041 |
Abbreviation: N/A, not applicable
a Oral and dermal absorption were assumed to be equivalent.
b These values take into account the assumed daily frequency of use, so for ConsExpo estimates, the year-averaged daily exposure value was used. See Appendix C for more details on models and parameters used.
c Personal communication from the CHPSD of HC to the ESRAB, HC, dates ranging from March 2017 to Feb 2019; unreferenced.
8.9.2 Health effects assessment
CAS RN 2082-79-3 was previously evaluated by the OECD (2006) as a SIAR. It was concluded that this substance has low hazard potential.
For systemic toxicity, two studies are described (OECD 2006). In one study, rats were exposed by oral gavage to 0, 5, 30, 100, or 300 mg/kg bw/day (n=5/sex/dose) in a 28-day repeated-dose oral toxicity study (OECD TG 407). The NOAEL was 30 mg/kg bw/day based on liver effects (increased liver weight and enzymes). In the second study, dogs (n=5/sex/dose) were exposed for 90 days to 0, 1 000, 3 000, or 10 000 ppm in diet (equivalent to 0, 32, 92, or 295 mg/kg bw/day in males and 0, 35, 97, or 336 mg/kg bw/day in females). The NOAELs for this study are 32 mg/kg bw/day in males and 35 mg/kg bw/day in females on the basis of increased liver weight and alkaline phosphatase.
For reproductive and developmental toxicity, two studies are described (OECD 2006). In one study, rats were exposed in diet to 0, 500, 1500, or 5000 ppm (equivalent to 0, 32, 96, or 315 mg/kg bw/day in males and 0, 39, 111, or 373 mg/kg/day in females) for two generations (10 to 12 weeks pre-mating, during mating, during gestation, and until weaning of the offspring). The NOAELs for parental toxicity were 96 (M) or 111 (F) mg/kg bw/day. There was no reproductive toxicity up to the highest dose tested; indeed, no effects were observed on mating, pregnancy rate, or duration of gestation. NOAELs for developmental toxicity were identified as 32 (M) or 39 (F) mg/kg bw/day; however, the effects observed were not consistent between the F1 and F2 generations and could not be determined to be treatment-related. Further, no effects were observed up to the highest dose tested in another developmental toxicity study (OECD TG 414) in which rats and mice were exposed to 0, 150, 500, or 1000 mg/kg bw/day by oral gavage.
8.9.3 Characterization of risk to human health
Table 8-4 provides relevant exposure estimates and critical health effect levels as well as resultant MOEs for the characterization of risk to human health from exposures to CAS RN 2082-79-3.
| Exposure scenario | Exposure estimate (mg/kg bw/day) |
Critical effect levela (mg/kg bw/day) | Critical health effect endpoint | MOE |
|---|---|---|---|---|
| Daily oral exposure from mouthing plastic/rubber (0–5 months) | 0.0048 | 30 | Liver effects, including increased liver weight and liver enzymes | 6250 |
| Daily dermal exposure from face moisturizer (19+ years) | 0.041 | 30 | Liver effects, including increased liver weight and liver enzymes | 730 |
Abbreviation: MOE, margin of exposure
a NOAEL
The MOEs are considered adequate to address uncertainties in the health effects and exposure databases.
8.10 Assessment of CAS RN 4221-80-1
8.10.1 Exposure assessment
No Canadian environmental monitoring data were available for CAS RN 4221-80-1. Due to its negligible vapour pressure and low water solubility (Table 3-1), exposure from environmental media that could impact the health of the general population is considered to be negligible.
CAS RN 4221-80-1 may be used as a component in the manufacture of food packaging materials in Canada. Exposure is not expected from this source, because it does not involve direct food contact (personal communication from the FD of HC to the ESRAB, HC, dates ranging from March 2017 to Feb 2019; unreferenced).
CAS RN 4221-80-1 is reported to be used in some nail polishes in Canada (personal communication from the CHPSD of HC to the ESRAB, HC, dates ranging from March 2017 to Feb 2019; unreferenced). Due to its negligible vapour pressure (Table 3-1), inhalation exposure to this substance from the use of nail polish is expected to be negligible.
8.10.2 Health effects assessment
CAS RN 4221-80-1 has no reported toxicity. It is not genotoxic and carcinogenicity was not observed up to 8 mg/kg bw/day (highest dose tested). No effects were seen up to the highest dose tested in multiple repeated-dose OECD TG studies, including: (1) a 28-day rat study (OECD TG 407) in which no effects were seen up to 2263 mg/kg bw/day; (2) a 13-week rat study (OECD TG 408) in which no effects were seen up to 1687 mg/kg bw/day; (3) a 13-week beagle study (OECD TG 409) in which no effects were seen up to 691 and 704 mg/kg bw/day in males and females, respectively; and (4) a prenatal developmental toxicity study conducted in mouse and rat (OECD TG 414) in which no effects were seen up to 3000 mg/kg bw/day. These results are from data submitted to the ECHA under the REACH (ECHA c2007-2015c).
8.10.3 Characterization of risk to human health
No health effects were observed at the highest doses tested in toxicity studies; therefore, this substance is considered to be of low hazard potential and, as a result, characterization of exposure potential was not considered to be warranted. The risk for human health is considered low.
8.11 Assessment of CAS RN 6386-38-5
8.11.1 Exposure assessment
No Canadian environmental monitoring data were available for CAS RN 6386-38-5. Due to its low vapour pressure and water solubility (Table 3-1), exposure from environmental media that could impact the health of the general population is considered to be negligible.
CAS RN 6386-38-5 may be used as a component in incidental additives (for example, lubricants) used in food processing establishments in Canada. However, exposure from this source is not expected, because there is no direct food contact (personal communication from the FD of HC to the ESRAB, HC, dates ranging from March 2017 to Feb 2019; unreferenced). It may also be used as a component in the manufacture of food packaging materials in Canada. The PDI was estimated to be negligible for this substance (personal communication from the FD of HC to the ESRAB, HC, dates ranging from March 2017 to Feb 2019; unreferenced).
CAS RN 6386-38-5 has been reported to be used in fragrances (OECD 2001). Due to its low vapour pressure (Table 3-1), inhalation exposure to this substance from the use of fragrance is expected to be negligible. Dermal exposure was estimated at 0.099 mg/kg bw/day for an adult (19+ years; Appendix C).
8.11.2 Health effects assessment
CAS RN 6386-38-5 was previously evaluated by the OECD as a SIAR (OECD 2001), which concluded that there is currently no concern for health. The key study was a two-generation study in which male and female rats (n=15/dose) were exposed to 0, 10, 100, or 250 mg/kg bw/day. A NOAEL of 10 mg/kg bw/day was identified on the basis of increased liver weight and associated histopathological changes at the next dose level in the P0 generation. No effects on fertility or pregnancy were observed up to the highest dose tested. The NOAEL for developmental effects is 100 mg/kg bw/day and is based on a decrease in litter size, pup weight, and pup viability; however, these effects were only observed in the presence of parental toxicity.
8.11.3 Characterization of risk to human health
The highest potential exposure for this substance is from fragrance use (0.099 mg/kg bw/day, adults 19+ years). No MOE was calculated because no toxicological effects on reproduction or development, which are considered to be the appropriate critical health effect for the exposure scenarios, were reported at the highest dose tested. Reproduction effects were deemed to be the critical health effect as the impact on liver was found to be primarily based on increased liver weight and not as adverse as the reproduction effects.
8.12 Assessment of CAS RN 35958-30-6
8.12.1 Exposure assessment
No Canadian environmental monitoring data were available for CAS RN 35958-30-6. Due to its negligible vapour pressure and low water solubility (Table 3-1), exposure from environmental media that could impact the health of the general population is considered to be negligible.
No monitoring data were identified for the presence in food in Canada. CAS RN 35958-30-6 may be used as a component in the manufacture of food packaging materials in Canada. The PDI was estimated as 0.02 mg/kg bw/day for the general population (1 year of age and older) (personal communication from the FD of HC to the ESRAB, HC, dates ranging from March 2017 to Feb 2019; unreferenced).
Potential exposure from use of products available to consumers was estimated. Details are presented in Appendix C. Estimates for uses that result in the highest level of potential oral or dermal exposure (referred to as sentinel scenarios) are presented in Table 8-5. Due to the very low vapour pressure of this substance, exposure via the inhalation route is not expected. For estimated potential exposures via the dermal route, a dermal absorption factor of 100% was assumed.
Additional potential use scenarios for this substance were considered (for example, from face moisturizer, hair shampoo/conditioner, make-up, body wash, nail polish, and plastic/rubber), but these resulted in lower exposure estimates than those presented in Table 8-5.
| Product scenario | Concentration (%) | Route of exposurea | Per event exposure (mg/kg bw) |
Daily exposure (mg/kg bw/ day) b |
|---|---|---|---|---|
| Lipstick | 0.1%c | Oral | N/A | 0.00089 |
| Body lotion (0–5 months) | 0.02%c | Dermal | N/A | 0.051 |
Abbreviation: N/A, not applicable
a An oral and dermal absorption of 100% was assumed.
b These values take into account the assumed daily frequency of use, so for ConsExpo estimates, the year-averaged daily exposure value was used. See Appendix C for more details on models and parameters used.
c Personal communication from the CHPSD of HC to the ESRAB, HC, dates ranging from March 2017 to Feb 2019; unreferenced.
8.12.2 Health effects assessment
There are no empirical data available for CAS RN 35958-30-6; therefore, a read-across approach was used. Two analogues were selected for read-across: CAS RNs 119-47-1 and 88-24-4. The details of how they were selected are presented in section 2.1.
CAS RN 119-47-1 was previously evaluated by Environment Canada and Health Canada (EC, HC 2009)and the critical effect was identified from a 90-day study in dogs in which biochemical changes were observed at a lowest-observed-effect level of 6 mg/kg bw/day (n=1/dose/sex).
CAS RN 119-47-1 was also evaluated in an OECD SIDS (OECD 2003) and was reported to be non-genotoxic and non-carcinogenic. The critical endpoint identified in the SIAR was from a reproductive and developmental toxicity study (OECD TG 421). Sprague-Dawley rats (n=12/sex/dose) were exposed to CAS RN 119-47-1 by oral gavage (0, 12.5, 50, 200, or 800 mg/kg bw/day) before mating (males and females) and during pregnancy and lactation (females). In males, giant cell formation was observed in the testis as well as increased abnormal sperm, decreased sperm motility, and a decrease in the number of sperm in the cauda epididymis beginning at 50 mg/kg bw/day. Atrophy and degeneration of seminiferous tubules, atrophy of the testis and epididymis, and a decrease in the absolute and relative testis and epididymis weight were observed at 200 and 800 mg/kg bw/day. Therefore, the NOAEL is 12.5 mg/kg bw/day for reproductive toxicity in males, which was also identified as the critical effect level for males and used for risk characterization (see section 8.12.3). In females, the NOAEL for reproductive toxicity is 50 mg/kg bw/day on the basis of a decrease in the number of corpora lutea, implantation scars, and pups born that was observed beginning at 200 mg/kg bw/day (OECD 2003). The NOAEL for developmental toxicity is 200 mg/kg bw/day on the basis of low body weight gain of offspring and increased number of stillbirths.
CAS RN 88-24-4 was tested in a repeated-dose toxicity study with reproduction and developmental screening (OECD TG 422). These results are from data submitted to the ECHA under the REACH (ECHA c2007-2017). Sprague-Dawley rats were exposed to 0, 30, 100, or 300 mg/kg bw/day of CAS RN 88-24-4 by oral gavage. Briefly, absolute and relative liver weights were elevated in males at 30 mg/kg bw/day {male lowest-observed-adverse-effect level [LOAEL]), and absolute and relative liver and thyroid weights were elevated in females at 100 mg/kg bw/day (female NOAEL = 30 mg/kg bw/day). Histopathological changes and hepatocyte hypertrophy occurred starting at 100 mg/kg bw/day. There were two female deaths in the mid-dose group and deaths from both sexes at the high dose. Additional effects were observed beginning at the mid dose, including diarrhea, hypersalivation, altered hematological findings, altered clinical chemistry, and altered pathology. The NOAEL of 30 mg/kg bw/day was identified as the critical effect level in females and used for risk characterization (see section 8.12.3).
8.12.3 Characterization of risk to human health
Table 8-6 provides relevant exposure estimates and critical health effect levels as well as resultant MOEs for the characterization of risk to human health from exposure to CAS RN 35958-30-6. Only the highest exposure scenarios for each route of exposure (food packaging for oral and body lotion for dermal) were considered.
| Substance | Exposure scenario | Systemic exposure (mg/kg bw/day) | Critical effect levela (mg/kg bw/day) | Critical health effect | MOE |
|---|---|---|---|---|---|
| CAS RN 35958-30-6 Read-across to 119-47-1 (M) and 88-24-4 (F) |
Daily oral exposure from food packaging | 0.02 | 12.5 (M) 30 (F) |
(M) Altered sperm parameters (F) Liver and thyroid effects |
625 (M) 1500 (F) |
| CAS RN 35958-30-6 Read-across to 119-47-1 (M) and 88-24-4 (F) |
Daily dermal exposure from body lotion (0–5 months) |
0.051 | 12.5 (M) 30 (F) |
(M) Altered sperm parameters (F) Liver and thyroid effects |
250 (M) 590 (F) |
Abbreviations: F, female; M, male; MOE, margin of exposure
a NOAEL
The MOEs are considered adequate to address uncertainties in the health effects and exposure databases.
8.13 Assessment of CAS RN 36443-68-2
8.13.1 Exposure assessment
No Canadian environmental monitoring data were available for CAS RN 36443-68-2. Drinking water estimates were generated using the highest PEC (28.4 µg/L) modelled for the presence in surface water (Table 7-9). The highest daily intake estimate of exposure from drinking water was 0.0037 mg/kg bw/day (0 to 5 months; Appendix B, Table B-1).
No monitoring data were identified for the presence in food in Canada. CAS RN 36443-68-2 may be used as a component in the manufacture of food packaging materials in Canada. The PDI was estimated as 0.0011 mg/kg bw/day for the general population (1 year of age and older) (personal communication from the FD of HC to the ESRAB, HC, dates ranging from March 2017 to Feb 2019; unreferenced).
CAS RN 36443-68-2 has been reported to be used in fabrics and textiles (US EPA 2018). Exposure from mouthing fabric and dermal exposure to fabric were estimated. The highest exposure estimates were for mouthing fabric at 0.07 mg/kg bw/day for an infant (0 to 5 months; Appendix C).
8.13.2 Health effects assessment
CAS RN 36443-68-2 is not genotoxic in the Ames test, HPRT assay, cell transformation assay, or micronucleus assay. Several repeated-dose studies exist for CAS RN 36443-68-2. In a repeated-dose 90-day oral toxicity study (OECD TG 409) in which beagles (n=5/dose/sex) were exposed to 0, 10, 30, 100, or 300 mg/kg bw/day in diet, no effects were observed at the highest dose tested. In a multi-generational reproduction toxicity study (OECD TG 416), male and female rats were exposed to 0, 300, 900, or 1800 ppm in their diet (equivalent to 21–26, 60–80, or 120–160 mg/kg bw/day; n=30/dose/sex in the F0 generation; n=25/dose/sex in the F1 generation). The F0 effects included minor reductions in body weight gain, increased liver and kidney weights, and major reductions in feed intake and body weight gain during lactation (no-observed-effect level [NOEL]=21–26 mg/kg bw/day]. The F1 effects included reduced pup weight gain during lactation, slightly lower weaning indices, and delayed development at the mid and high doses (NOEL=21–26 mg/kg bw/day). The F2 effects included reduced pup weight, delayed physical development, and reduced weaning indices and the mid and high doses (NOEL=21–26 mg/kg bw/day). A combined chronic toxicity / carcinogenicity study (OECD TG 453) was reported in which male and female rats were exposed to 0, 5, 15, 50, or 100 mg/kg bw/day in their diet for 2 years. Liver cysts were observed in males at a LOAEL of 50 mg/kg bw/day, and enlarged thyroid glands (characterized by hyperplasia or cystic dilatation of the thyroid follicles) were observed in both sexes at 100 mg/kg bw/day. Thyroid gland follicular adenoma and carcinoma were observed at 100 mg/kg bw/day in both sexes. The critical effect level for CAS RN 36443-68-2 is the NOAEL of 15 mg/kg bw/day on the basis of liver and thyroid effects. These data are from submissions submitted to ECHA under the REACH (ECHA c2007-2015d).
8.13.3 Characterization of risk to human health
On the basis of the available empirical data, the NOAEL of 15 mg/kg bw/day for liver and thyroid effects was the endpoint that was compared to the highest potential exposure scenario, mouthing of fabric (0.07 mg/kg bw/day, 0 to 5 months). The resulting MOE was determined to be adequate (MOE = 200) to address uncertainties in the health effects and exposure databases.
8.14 Assessment of CAS RN 41484-35-9
8.14.1 Exposure assessment
No Canadian environmental monitoring data were available for CAS RN 41484-35-9. Due to its negligible vapour pressure and low water solubility (Table 3-1), exposure from environmental media that could impact the health of the general population is considered to be negligible.
No monitoring data were identified for the presence in food in Canada. CAS RN 41484-35-9 may be used as a component in the manufacture of food packaging materials in Canada. The PDI was estimated as 0.00033 mg/kg bw/day for the general population (1 year of age and older) (personal communication from the FD of HC to the ESRAB, HC, dates ranging from March 2017 to Feb 2019; unreferenced).
CAS RN 41484-35-9 has been reported to be a lubricant for door hinges (MSDS 2013a). Dermal exposure was estimated at 0.057 mg/kg bw (per event) for an adult (19+ years; Appendix C).
8.14.2 Health effects assessment
CAS RN 41484-35-9 has been partially evaluated by the US EPA under the HPV Challenge Program (US EPA 2003). No toxicological effects or critical effect level were identified.
8.14.3 Characterization of risk to human health
Estimates of exposure to the general population of Canada from environmental media are expected to be negligible. No MOE was calculated because no toxicological effects are associated with this substance.
8.15 Assessment of CAS RN 61788-44-1
8.15.1 Exposure assessment
No Canadian environmental monitoring data were available for CAS RN 61788-44-1. Drinking water estimates were generated using the sum of the PECs for components that were identified as making up CAS RN 61788-44-1 (1 µg/L) modelled for the presence in surface water (Table 7-8). To maintain the most conservative estimate, full values of components were used. The highest daily intake estimate of exposure from drinking water was 0.00014 mg/kg bw/day (0 to 5 months; Appendix B, Table B-1).
No monitoring data were identified for its presence in food in Canada, and it has not been identified as being used as a component in the manufacture of food packaging materials or incidental additives in Canada (personal communication from the FD of HC to the ESRAB, HC, dates ranging from March 2017 to Feb 2019; unreferenced). Therefore, dietary exposure from this source is not expected.
CAS RN 61788-44-1 has been reported to be used in wood epoxy glue (MSDS 2013b). Dermal exposure from this use was estimated to be 0.95 mg/kg bw (per event) for an adult (19+ years; Appendix C).
8.15.2 Health effects assessment
CAS RN 61788-44-1 is a UVCB that exists as a mixture of mono-, di-, and tri-styrenated phenol (the mixture is described in Tables 2-2 and 2-3). CAS RN 61788-44-1 was previously evaluated by Brooke et al. (2009) on behalf of the UK Environment Agency. The key study was a 90-day repeated-dose study in male and female rats that were exposed orally to 0, 50, 158, or 500 mg/kg bw/day. A NOAEL of 50 mg/kg bw/day was identified on the basis of increased liver weight (no related histopathological or biochemical changes were observed) at the next dose level. No other adverse effects were reported.
Reproductive and developmental toxicity were identified from data submitted to the ECHA under the REACH (ECHA c2007-2017) using read-across. The test material used was MSP. No effects on reproduction or development were seen up to the highest dose tested in the following three studies (up to 300 mg/kg bw/day).
In a combined extended one-generation reproductive toxicity / sub-chronic oral toxicity study (OECD TG 443 / OECD TG 408), Wistar rats were exposed to 0, 150, 500, or 1500 ppm (equivalent to 0, 12, 40, or 124 mg/kg bw/day) of MSP in their diet. Because no treatment-related effects in reproductive parameters were observed during mating and gestation or delivery and post-partum/lactation periods in any of the treatment groups, no effects were observed up to the highest dose tested (124 mg/kg bw/day).
In a combined repeated-dose with reproduction/developmental toxicity study (OECD TG 422), Wistar rats were exposed to 0, 300, 1250, or 5000 ppm (equivalent to 0, 24, 97, or 337 mg/kg bw/day) of MSP in diet. Pup effects observed in this study were deemed to be secondary to maternal toxicity, and no effects for reproductive toxicity were observed up to the highest dose tested (337 mg/kg bw/day).
In a prenatal developmental toxicity study (OECD TG 414), Hannover Wistar rats were exposed to 0, 60, 150, or 300 mg/kg bw/day of MSP by oral gavage. A reduction in maternal body weight gain was observed at the mid dose; however, this was not considered to be adverse. No effects on feto-, terato-, and embryotoxicity were observed up to the highest dose tested (300 mg/kg bw/day).
8.15.3 Characterization of risk to human health
The highest potential exposure for this substance from use of an epoxy glue was estimated to be 0.95 mg/kg bw (per event) for an adult. No MOE was calculated because, based on read-across, no toxicological effects on reproduction were reported, which is considered to be the appropriate critical health effect for “per event” exposure scenarios. Reproductive toxicity was considered to be the critical health effect as the NOAEL of 50 mg/kg bw/day for liver was based on increased liver weight, with no other adverse effects noted. The other health effect noted was the reproductive toxicity, which, at a dose of 124 mg/kg bw/day, showed no effects.
8.16 Uncertainties in evaluation of risk to human health
The key sources of uncertainty are presented in Table 8-7.
| Key source of uncertainty | Impact |
|---|---|
| No measured Canadian data in relevant environmental media | +/- |
| There are no sub-chronic or chronic animal studies for dermal exposure | +/- |
| There are no dermal absorption studies, other than for CAS RN 128-37-0 | + |
| There is no chemical-specific toxicity information for CAS RN 35958-30-6 | +/- |
+ = uncertainty with potential to cause overestimation of exposure/risk; - = uncertainty with potential to cause underestimation of exposure risk; +/- = unknown potential to cause over- or underestimation of risk.
9. Conclusion
Considering all available lines of evidence presented in this draft assessment, there is risk of harm to the environment from CAS RNs 118-82-1, 128-37-0, 36443-68-2, and 61788-44-1. It is proposed to conclude that CAS RNs 118-82-1, 128-37-0, 36443-68-2, and 61788-44-1 meet the criteria under paragraph 64(a) of CEPA as they are entering or may enter the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity. However, it is proposed to conclude that CAS RNs 118-82-1, 128-37-0, 36443-68-2, and 61788-44-1 do not meet the criteria under paragraph 64(b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger to the environment on which life depends. It is also proposed to conclude that CAS RNs 85-60-9, 96-69-5, 96-76-4, 98-54-4, 128-39-2, 1843-03-4, 2082-79-3, 4221-80-1, 6386-38-5, 35958-30-6, and 41484-35-9 do not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
Considering all the information presented in this draft assessment, it is proposed to conclude that the 15 substances in the Substituted Phenols Group do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore proposed to conclude that CAS RNs 118-82-1, 128-37-0, 36443-68-2, and 61788-44-1 meet one or more of the criteria set out in section 64 of CEPA and that the other 11 substances in the Substituted Phenols Group do not meet any of the criteria set out in section 64 of CEPA.
It is also proposed that CAS RNs 118-82-1 and 61788-44-1 meet the persistence and bioaccumulation criteria, while CAS RNs 128-37-0 and 36443-68-2 meet the persistence but not the bioaccumulation criteria as set out in the Persistence and Bioaccumulation Regulations of CEPA (Canada 2000).
References
Allinson G, Allinson M, Kadokami K. 2015. Combining passive sampling with a GC-MS-database screening tool to assess trace organic contamination of rivers: a pilot study in Melbourne, Australia. Water, Air, Soil Pollut. 226:230.
Aourahoun KA, Fazouane F, Benayad T, Bettache Z, Denni N. 2014. The synthetic antioxidant butylated hydroxytoluene, a naturally occurring constituent of the broom Cytisus triflorus L’Herit. J Nat Prod. 7:58-64.
Arnot JA, Gobas FAPC. 2004. A Food Web Bioaccumulation Model for Organic Chemicals in Aquatic Ecosystems. Enviro Toxicol Chem. 23(10): 2343-2355.
Arnot JA, Mackay D, Bonnell M. 2008a. Estimating metabolic biotransformation rates in fish from laboratory data. Environ Toxicol Chem. 27(2):341-351.
Arnot JA, Mackay D, Parkerton TE, Bonnell M. 2008b. A database of fish biotransformation rates for organic chemicals. Environ Toxicol Chem 27(11):2263-2270.
Babu B, Wu J-T. 2008. Production of natural butylated hydroxytoluene as an antioxidant by freshwater phytoplankton. J Phycol. 44(6):1447-1454.
Backhaus T, Faust M. 2012. Predictive environmental risk assessment of chemical mixtures: a conceptual framework. Environ Sci Technol 46(5):2564-2573.
Baker BH, Martinovic-Weigelt D, Ferrey M, Barber LB, Writer JH, Rosenberry DO, Kiesling RL, Lundy JR, Schoenfuss HL. 2014. Identifying non-point sources of endocrine active compounds and their biological impacts in freshwater lakes. Arch Environ Contam Toxicol. 67(3):374-388.
Bamai YA, Araki A, Kawai T, Tsuboi T, Saito I, Yoshioka E, Kanazawa A, Tajima S, Shi C, Tamakoshi A, et al. 2014. Associations of phthalate concentrations in floor dust and multi-surface dust with the interior materials in Japanese dwellings. Sci Total Environ. 468-469:147-157.
Bouftira I, Abdelly C, Sfar S. 2007. Identification of a naturally occurring 2, 6-bis (1.1-dimethylethyl)-4-methylphenol from purple leaves of the halophyte plant Mesembryanthemum crystallinum. African J Biotechnol. 6(9):1136-1139.
Bronaugh RL, Stewart RF, Storm JE. 1989. Extent of cutaneous metabolism during percutaneous absorption of xenobiotics. Toxicol Appl Pharmacol. 99(3):534-543.
Brooke D, Burns J, Cartwright C, Pearson A. 2009. Environmental risk evaluation report: Styrenated phenol [PDF]. Bristol (UK): United Kingdom Environment Agency.
Cabeza Y, Candela L, Ronen D, Teijon G. 2012. Monitoring the occurrence of emerging contaminants in treated wastewater and groundwater between 2008 and 2010. The Baix Llobregat (Barcelona, Spain). J Hazard Mater. 239-240:32-39.
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C. 1999, c.33. Canada Gazette Part III, vol. 22, no. 3.
Canada. 2000. Canadian Environmental Protection Act, 1999: Persistence and Bioaccumulation Regulations. P.C. 2000-348, 23 March, 2000, SOR/2000-107.
CATALOGIC [environmental fate and ecotoxicity model]. 2016. Ver. 5.11.17. Bourgas (BG): University “Prof. Dr. Assen Zlatarov”, Laboratory of Mathematical Chemistry.
[CDR] Chemical Data Reporting under the Toxic Substances Control Act [database]. 2016. Washington D.C. (USA): United States Environmental Protection Agency. [accessed 2019 July 10].
Chao J, Lin CT, Chuang TH. 1983. Vapor pressure of coal chemicals. J Phys Chem Ref Data. 12(4):1033-1063.
ChemIDplus. 1993-. North Syracuse (NY): SRC PhysProp Database. [updated 2017 Dec 10; accessed 2017 Dec 19].
Chinn H, Yoneyama M, Loechner U, Xu X. 2018. Antioxidants, Specialty Chemicals Update Program. London (GB): IHS Markit. 149 p. [accessed 2019 Feb 15].
Colin A, Bach C, Rosin C, Munoz J-F, Dauchy X. 2014. Is drinking water a major route of human exposure to alkylphenol and bisphenol contaminants in France? Arch Environ Contam Toxicol. 66:86-99.
ConsExpo Web [Consumer Exposure Web Model]. 2016. Bilthoven (NL): Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment].
Courtheoux S, Pechenot D, Bucks DA, Marty JP, Maibach HI, Wepierre J. 1986. Effect of repeated skin administration on in vivo percutaneous absorption of drugs. Br J Dermatol. 115 Suppl 31:49-52.
[CRAM] Consumer Release Aquatic Model. 2017. Ver 2.8.1. Gatineau (QC): Environment and Climate Change Canada, Ecological Assessment Division. Exposure modelling tool for internal use only.
Delongeas J-L, Vermeil de Conchard G, Beamonte A, Bertheux H, Spire C, Maisonneuve C, Becourt-Lhote N, Goldfain-Blanc F, Claude N. 2010. Assessment of Labrasol/Labrafil/Transcutol (4/4/2, v/v/v) as a non-clinical vehicle for poorly water-soluble compounds after 4-week oral toxicity study in Wistar rats. Regul Toxicol Pharmacol. 57(2-3):284-290.
[DPD] Drug Product Database [database]. [modified 2016 Jul 17]. Ottawa (ON): Health Canada. [accessed 2017 Nov 3].
Duong HT, Kadokami K, Chau HTC, Nguyen TQ, Nguyen TT, Kong L. 2015. Groundwater screening for 940 organic micro-pollutants in Hanoi and Ho Chi Minh City, Vietnam. Environ Sci Pollut Res. 22(24):19835-19847.
EAS-E Suite (Ver.0.97 - BETA, release June, 2023). Accessed [Insert access date]. Developed by ARC Arnot Research and Consulting Inc., Toronto, ON, Canada.
[EC] European Commission. 2008. European Union risk assessment report: p-tert-butylphenol: CAS No. 98-54-4 [PDF]. Final approved version. Luxembourg: Office for Official Publications of the European Communities. Report No.: R404 1208ENV_JH. [accessed 2017 Sep 18].
[EC, HC] Environment Canada, Health Canada. 2009. Screening assessment for phenol, 2,2'-methylenebis[6-(1,1-dimethylethyl)-4-methyl- (MBMBP): CAS No. 119-47-1. Ottawa (ON): Government of Canada.
[ECCC] Environment and Climate Change Canada. 2016a. Science approach document: ecological risk classification of organic substances. Ottawa (ON): Government of Canada.
[ECCC] Environment and Climate Change Canada. 2016b. Data used to create substance-specific hazard and exposure profiles and assign risk classifications in the ecological risk classification of organic substances. Gatineau (QC). Available from: substances@ec.gc.ca.
[ECCC] Environment and Climate Change Canada. 2016c. Evaluation of CMP organic substances from the medium priority list: comparison of toxicity of select hindered phenols and substituted phenyl amines to soil organisms. Unpublished report. Ottawa (ON): Environment Canada, Science and Risk Assessment Directorate.
[ECCC] Environment and Climate Change Canada. 2017. DSL Inventory Update Phase 3 data collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain substances on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
[ECCC] Environment and Climate Change Canada. 2023. Supporting documentation: Substituted Phenols Group. Gatineau (QC): ECCC. Information in support of the draft assessment for Substituted Phenols Group. Available from: substances@ec.gc.ca.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2015. Identification of risk assessment priorities: results of the 2015 review. Ottawa (ON): Government of Canada.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. [modified 2017 Mar 12]. Categorization. Ottawa (ON): Government of Canada.
[ECETOC] European Centre for Ecotoxicology and Toxicology of Chemicals. 2015. Defining the role of chemical activity in environmental risk assessment within the context of mode of action: practical guidance and advice [PDF]. Snowbird (UT): ECETOC. Workshop report No. 29.
[ECHA] European Chemicals Agency. c2007-2017. Registered substances database. Helsinki (FI): ECHA. [accessed 2022 Apr].
[ECHA] European Chemicals Agency. 2012. Committee for Risk Assessment (RAC). Annex 1. Background Document to the opinion of proposing harmonised classification and labelling at EU level of p-tert-butylphenol. EC number: 202-679-0. CAS number: 98-54-4. ECHA/RAC/CLH-O-0000002629-66-01/A1.
[ECHA] European Chemicals Agency. c2007-2015a. Registered substances database; search results for CAS RN [96-76-4]. Helsinki (FI): ECHA. [accessed 2019 Jan 16].
[ECHA] European Chemicals Agency. c2007-2015b. Registered substances database; search results for CAS RN [1843-03-4]. Helsinki (FI): ECHA. [accessed: 2017 Jun 8].
[ECHA] European Chemicals Agency. c2007-2015c. Registered substances database; search results for CAS RN [4221-80-1]. Helsinki (FI): ECHA. [accessed 2017 Jun 6].
[ECHA] European Chemicals Agency. c2007-2015d. Registered substances database; search results for CAS RN [36443-68-2]. Helsinki (FI): ECHA. [accessed: 2017 Jun 19].
[EFSA] European Food Safety Authority. 2012. Scientific Opinion on the re-evaluation of butylated hydroxytoluene BHT (E 321) as a food additive. EFSA Journal 2012. 10(3):2588.
[EC] Environment Canada. 2009. DSL Inventory Update data collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain inanimate substances (chemicals) on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
[EC] Environment Canada. 2013. DSL Inventory Update data collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain substances on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
[EPI Suite] Estimation Program Interface Suite for Microsoft Windows [estimation model]. c2000-2012. Ver. 4.11. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Fernandes VC, Lehotay SJ, Geis-Asteggiante L, Kwon H, Mol HGJ, van der Kamp H, Mateus N, Domingues VF, Delerue-Matos C. 2014. Analysis of pesticide residues in strawberries and soils by GC-MS/MS, LC-MS/MS and two-dimensional GC-time-of-flight MS comparing organic and integrated pest management farming. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 31(2):262-270.
Ficheux AS, Wesolek N, Chevillotte G, Roudot AC. 2015. Consumption of cosmetic products by the French population. First part: frequency data. Food Chem Toxicol. 78:159-169.
Ficheux AS, Chevillotte G, Wesolek N, Morisset T, Dornic N, Bernard A, Bertho A, Romanet A, Leroy L, Mercat AC, et al. 2016. Consumption of cosmetic products by the French population second part: amount data. Food Chem Toxicol. 90:130-141.
Gharbi I, Issaoui M, El Gharbi S, Gazzeh N-E, Tekeya M, Mechri B, Flamini G, Hammami M. 2017. Butylated hydroxytoluene (BHT) emitted by fungi naturally occurring in olives during their pre‐processing storage for improving olive oil stability. Eur J Lipid Sci Technol. 119(11):1600343.
Gómez MJ, Herrera S, Solé D, García-Calvo E, Fernández-Alba AR. 2012. Spatio-temporal evalution of organic contaminants and their transformation products along a river basin affected by urban, agricultural and industrial pollution. Sci Total Environ. 420:134-145.
Guart A, Calabuig I, Lacorte S, Borrell A. 2014. Continental bottled water assessment by stir bar sorptive extraction followed by gas chromatography-tandem mass spectrometry (SBSE-GC-MS/MS). Environ Sci Pollut Res. 21(4):2846-2855.
Hansch C, Leo A, Hoekman D. 1995. Exploring QSAR: hydrophobic, electronic, and steric constants. Washington (DC): American Chemical Society. 348 p.
Hawley GG. 2007. The condensed chemical dictionary. 15th ed. New York (NY): John Wiley and Sons, Inc. p. 200. Cited in ECHA c2007-2017 and US EPA 2010.
Health Canada. 1998. Exposure factors for assessing total daily intake of priority substances by the general population of Canada. Unpublished report. Ottawa (ON): Government of Canada.
Health Canada. 2015. Food Consumption Table derived from Statistics Canada, Canadian Community Health Survey, Cycle 2.2, Nutrition (2004), Share file. Ottawa.
Health Canada. 2017. Water Consumption Table derived from Statistics Canada, Canadian Community Health Survey, Cycle 2.2, Nutrition (2004). Share file. Ottawa.
Health Canada. 2018. Draft backgrounder document on default values for breast milk and formula intakes. Unpublished report. Ottawa (ON): Government of Canada.
Health Canada. [modified 2018]. Cosmetic Ingredient Hotlist: list of ingredients that are prohibited for use in cosmetic products. Ottawa (ON): Health Canada, Consumer Product Safety Directorate. [accessed 2020 Feb 24].
Hernández F, Portolés T, Ibáñez M, Bustos-López MC, Díaz R, Botero-Coy AM, Fuentes CL, Peñuela G. 2012. Use of time-of-flight mass spectrometry for large screening of organic pollutants in surface waters and soils from a rice production area in Colombia. Sci Total Environ. 439:249-259.
[HSDB] Hazardous Substances Data Bank [database]. 1983-. Bethesda (MD): National Library of Medicine (US). [accessed 2018 Dec].
[J-CHECK] Japan CHEmicals Collaborative Knowledge database [database]. c2010-. Tokyo (JP): National Institute of Technology and Evaluation (NITE). [accessed 2017 Sep 28].
[JECFA] Joint FAO/WHO Expert Committee on Food Additives. 2001. Evaluation of certain food additives and contaminants. Fifty-fifth report of the Joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series No. 901. Geneva (CH): World Health Organization.
Kanazawa A, Saito I, Araki A, Takeda M, Ma M, Saijo Y, Kishi R. 2010. Association between indoor exposure to semi-volatile organic compounds and building-related symptoms among the occupants of residential dwellings. Indoor Air. 20(1):72-84.
Lanigan RS, Yamarik TA. 2002. Final report on the safety assessment of BHT. Int J Toxicol. 21 Suppl 2:19-94.
Lide DR. 2008. CRC handbook of chemistry and physics. 89th ed. Boca Raton (FL): CRC Press. 2736 p.
Liu R, Lin Y, Ruan T, Jiang G. 2017a. Occurrence of synthetic phenolic antioxidants and transformation products in urban and rural indoor dust. Environ Pollut. 221:227-233.
Liu Y-H, Zhang S-H, Ji G-X, Wu S-M, Guo R-X, Cheng J, Yan Z-Y, Chen J-Q. 2017b. Occurrence, distribution and risk assessment of suspected endocrine-disrupting chemicals in surface water and suspended particulate matter of Yangtze River (Nanjing section). Ecotoxicol Environ Saf. 135:90-97.
[LNHPD] Licensed Natural Health Products Database [database]. [modified 2021 Sep 08]. Ottawa (ON): Health Canada. [accessed 2022 Apr 20].
Long M, Strand J, Lassen P, Krüger T, Dahllöf I, Bossi R, Larsen MM, Wiberg-Larsen P, Bonefeld-Jørgensen EC. 2014. Endocrine-disrupting effects of compounds in Danish streams. Arch Environ Contam Toxicol. 66(1):1-18.
Loretz LJ, Api AM, Barraj LM, Burdick J, Dressler WE, Gettings SD, Han Hsu H, Pan YHL, Re TA, Renskers KJ, et al. 2005. Exposure data for cosmetic products: lipstick, body lotion, and face cream. Food Chem Toxicol. 43(2):279-291.
Loretz L, Api AM, Barraj L, Burdick J, Davis DA, Dressler W, Gilberti E, Jarrett G, Mann S, Pan YHL, et al. 2006. Exposure data for personal care products: hairspray, spray perfume, liquid foundation, shampoo, body wash, and solid antiperspirant. Food Chem Toxicol. 44(12):2008-2018.
Lu Z, Smyth SA, De Silva AO. 2019. Distribution and fate of synthetic phenolic antioxidants in various wastewater treatment processes in Canada. Chemosphere. 219:826-835.
Mackay D, Arnot JA, Petkova EP, Wallace KB, Call DJ, Brooke LT, Veith GD. 2009. The physicochemical basis of QSARs for baseline toxicity. SAR QSAR Environ Res. 20(3-4):393-414.
Mackay D, McCarty LS, Arnot JA. 2014. Relationships between exposure and dose in aquatic toxicity tests for organic chemicals. Environ Toxicol Chem. 33(9):2038-2046.
Malshe VC, Sikchi M. 2004. Basics of paint technology. Part 1. ISBN 10: 8190329855
McCarty LS. 1986. The relationship between aquatic toxicity QSARs and bioconcentration for some organic chemicals. Environ Toxicol Chem. 5(12):1071-1080.
McCarty LS, Arnot JA, Mackay D. 2013. Evaluation of critical body residue data for acute narcosis in aquatic organisms. Environ Toxicol Chem. 32(10):2301-2314.
McCarty LS, Hodson PV, Craig GR, Kaiser KLE. 1985. The use of quantitative structure-activity relationships to predict the acute and chronic toxicities of organic chemicals to fish. Environ Toxicol Chem. 4(5):595-606.
McCarty LS, Mackay D. 1993. Enhancing ecotoxicological modeling and assessment: body residues and modes of toxic action. Environ Sci Technol. 27(9):1719-1728.
McCarty LS, Mackay D, Smith AD, Ozburn GW, Dixon DG. 1991. Interpreting aquatic toxicity QSARs: the significance of toxicant body residues at the pharmacologic endpoint. Sci Total Environ. 109-110:515-525.
Mohler RE, O’Reilly KT, Zemo DA, Tiwary AK, Magaw RI, Synowiec KA. 2013. Non-targeted analysis of petroleum metabolites in groundwater using GC×GC−TOFMS. Environ Sci Technol. 47(18):10471-10476.
[MSDS] Material Safety Data Sheet. 2010a. Glow light stick. Ningbo (CH): Ningbo Merryart Int’l Development Corp. Apr 2010. [accessed 2017 Nov 6]. [restricted access].
[MSDS] Material Safety Data Sheet. 2010b. Hy-Per Lube Oil Supplement. Seattle (WA): Hy-Per Lube Corporation. Dec 2010. [accessed 2017 Nov 6]. [restricted access].
[MSDS] Material Safety Data Sheet. 2013a. Super Lube Grease. Bohemia (NY): Synco Chemical Corporation. Jul 2013. [accessed 2017 Nov 3]. [restricted access].
[MSDS] Material Safety Data Sheet. 2013b. PC-Woody, Part A. Allentown (PA): Protective Coatings Co. Jul 2013. [accessed 2017 Nov 3]. [restricted access].
[MSDS] Material Safety Data Sheet. 2014a. Febreze NOTICEables Linen & Sky. Cincinnati (OH): The Procter & Gamble Company. Jul 2014. [accessed 2017 Nov 6]. [restricted access].
[MSDS] Material Safety Data Sheet. 2014b. Doe-In-Heat Urine. Saint-Georges (QC): Buck Expert Inc. Jun 2014. [accessed 2017 Nov 6]. [restricted access].
[MSDS] Material Safety Data Sheet. 2014c. 84125 PC Perma Poxy 5 Minute Plastic Weld (Activator). Halton Hills (ON): ITW Permatex Canada. Jun 2014. [accessed 2017 Nov 6]. [restricted access].
[MSDS] Material Safety Data Sheet. 2014d. Cylindrical batteries. Shandong (CH): Shandong RealForce Enterprises Co., Ltd. Feb 2014. [accessed 2017 Nov 6]. [restricted access].
[MSDS] Material Safety Data Sheet. 2015. Fuel stabilizer. Brampton (ON): Kleen-Flo Tumbler Industries Ltd. Jan 2015. [accessed 2017 Nov 3]. [restricted access].
[MSDS] Material Safety Data Sheet. 2016. MOBIL 1 MX4T 10W-40. Calgary (AB): Imperial Oil Downstream. Jan 2016. [accessed 2017 Nov 3]. [restricted access].
Neely WB, Blau GE, editors. 1985. Environmental exposure from chemicals. Volume 1. Boca Raton (FL): CRC Press. 256 p.
[New EQC] New Equilibrium Criterion Model. 2011. Ver. 1.00 (Beta). Peterborough (ON): Trent University, Canadian Centre for Environmental Modelling and Chemistry.
[NHPID] Natural Health Products Ingredients Database [database] [modified 2022 Apr 09]. Ottawa (ON): Health Canada. [accessed 2022 Apr 20].
Nijssen LM, Ingen-Visscher CA van, Donders JJH [eds]. 1963-2018. VCF (Volatile Compounds in Food): database, Version 16.6, 2018. Zeist (NL): Triskelion B.V.
Noguerol-Cal R, López-Vilariño JM, González-Rodríguez MV, Barral-Losada L. 2011. Effect of several variables in the polymer toys additive migration to saliva. Talanta. 85(4): 2080-2088.
[NPRI] National Pollutant Release Inventory. 1994-2017. NPRI datasets: CAS RN 137-28-0. Ottawa (ON): Government of Canada. Search results for CAS RN 137-28-0. [accessed 2019 Jul].
[NTP] National Toxicology Program (US). 1979. Bioassay of butylated hydroxytoluene (BHT) for possible carcinogenicity. CAS RN 128-37-0. National Cancer Institute, Carcinogenesis, Technical Report Series, No. 150. Report No. NCI-CG-TR-150.
O’Brien D, Lewis S, Davis A, Gallen C, Smith R, Turner R, Warne M, Turner S, Caswell S, Mueller JF, et al. 2016. Spatial and temporal variability in pesticide exposure downstream of a heavily irrigated cropping area: application of different monitoring techniques. J Ag Food Chem. 64(20):3975-3989.
[OECD] Organisation for Economic Co-operation and Development. 1994. SIDS initial assessment report: 2,6-di-tert-butylphenol. CAS No. 128-39-2. IRPTC Data Profile. UNEP Publication.
[OECD] Organisation for Economic Co-operation and Development. 2000. SIDS initial assessment report for 10th SIAM: p-tert-butyl phenol. CAS No. 98-54-4 [PDF]. SIAM [SIDS Initial Assessment Meeting] 10; 2000 March; Japan. [accessed 2016 Jan 4].
[OECD] Organisation for Economic Co-operation and Development. 2001. SIDS initial assessment profile. CAS RN 6386-38-5 [PDF]. Berne (CH): United Nations Environment Programme (UNEP). [updated 2001 Nov 09; accessed 2017 Dec 18].
[OECD] Organisation for Economic Co-operation and Development. 2002. Chemicals screening information dataset (SIDS) for initial assessment report [PDF]. 2,6-di-tert-butyl-p-cresol (BHT). CAS No. 128-37-0. Paris (FR): United Nations Environment Programme (UNEP). [updated 2004 Jan 12; accessed 2017 Dec 18].
[OECD] Organisation for Economic Co-operation and Development. 2003. SIDS initial assessment report (SIAR): 6,6'-di-tert-butyl-2,2'-methylenedi-p-cresol. CAS No. 119-47-1. UNEP Publication.
[OECD] Organisation for Economic Co-operation and Development. 2006. Summary conclusions of the SIAR for SIDS initial assessment profile. CAS RN 2082-79-3 [PDF]. Paris (FR): United Nations Environment Programme (UNEP). [updated 2006 Apr 21; accessed 2017 Dec 18].
[OECD] Organisation for Economic Co-operation and Development. 2018. Case study on the use of integrated approaches for testing and assessment (IATA) for estrogenicity of the substituted phenols. Paris (FR): OECD, Environment Directorate. (Series on Testing and Assessment No. 290; Report No.: ENV/JM/MONO(2018)26, JT03435852). [accessed 2019 Mar 5].
OECD QSAR Toolbox. [read across tool]. 2014. Ver. 3.4. Paris (FR): Organisation for Economic Co-operation and Development, Laboratory of Mathematical Chemistry.
OECD QSAR Toolbox. [read across tool]. 2017. Ver. 4.1. Paris (FR): Organisation for Economic Co-operation and Development, Laboratory of Mathematical Chemistry.
Ogawa Y, Kawamura Y, Wakui C, Mutsuga M, Nishimura T, Tanamoto K. 2006. Estrogenic activities of chemicals related to food contact plastics and rubbers tested by the yeast two-hybrid assay. Food Addit Contam. 23(4):422-430.
Papadopoulos A, Vlachogiannis D, Maggos T, Sfetsos A, Karayiannis MI. 2013. A semi-quantitative approach for analysing low-volatile organic compounds in house dust using an SFE method: significant common features and particular differences of the extracts. J Supercrit Fluids. 82:268-281.
Perry RH, Green D. 1984. Perry’s chemical engineer’s handbook. 6th ed. New York (NY): McGraw-Hill.
Pitarch E, Cervera MI, Portolés T, Ibáñez M, Barreda M, Renau-Pruñonosa A, Morell I, López F, Albarrán F, Hernández F. 2016. Comprehensive monitoring of organic micro-pollutants in surface and groundwater in the surrounding of a solid-waste treatment plant of Castellón, Spain. Sci Total Environ. 548-549: 211-220.
Polidoro BA, Comeros-Raynal MT, Cahill T, Clement C. 2017. Land-based sources of marine pollution: pesticides, PAHs and phthalates in coastal stream water, and heavy metals in coastal stream sediments in American Samoa. Mar Pollut Bull. 116(1-2): 501-507.
Price, S.C. 1994. Robens Institute; Report No. RI93/TOX/0020 as cited in OECD SIDS (2002)
PubChem [database]. 2004-. Bethesda (MD): US National Library of Medicine, National Center for Biotechnology Information. [accessed 2019 Aug].
Rissmann R, Oudshoorn MHM, Hennink WE, Ponec M, Bouwstra JA. 2009. Skin barrier disruption by acetone: observations in a hairless mouse skin model. Arch Dermatol Res. 301(8):609-613.
Rudel RA, Dodson RE, Perovich LJ, Morello-Frosch R, Camann DE, Zuniga MM, Yau AY, Just AC, Brody JG. 2010. Semi-volatile endocrine-disrupting compounds in paired indoor and outdoor air in two northern California communities. Environ Sci Technol. 44(17):6583-6590.
Saito I, Onuki A, Seto H. 2004. Indoor air pollution by alkylphenols in Tokyo. Indoor Air. 14(5):325-332.
Schmidt SN, Mayer P. 2015. Linking algal growth inhibition to chemical activity: baseline toxicity required 1% of saturation. Chemosphere. 120(1):305-308.
[SimpleTreat 3.1] Sewage Treatment Plant Removal Model version 3.1. 2003. Bilthoven (NL): National Institute for Public Health and the Environment (RIVM). Available from: National Institute for Public Health and the Environment (RIVM), Laboratory for Ecological Risk Assessment, Bilthoven, the Netherlands.
Slobodnik J, Mrafkova L, Carere M, Ferrara F, Pennelli B, Schüürmann G, von der Ohe PC. 2012. Identification of river basin specific pollutants and derivation of environmental quality standards: a case study in the Slovak Republic. TrAC Trends Anal Chem. 41:133-145.
Spectrum. 2009. Material Safety Data Sheet. 2,4-ditert-butyl-6-[1-(3,5-ditert-butyl-2-hydroxyphenyl)ethyl]phenol. CAS RN 35958-30-6. Gardena (CA): Spectrum Laboratory Products Inc. [accessed 2017 Dec 19].
[SPIN] Substances in Preparations in Nordic Countries [database]. c2017. Copenhagen (DK): Nordic Council of Ministers. [accessed 2019 Jul 10].
Statistics Canada. 2015. Canadian Community Health Survey (CCHS) 2015 Share file. Ottawa (ON). Canada. Estimates generated using SAS 9.3 [GRID] through SAS EG 5.1.
Statistics Canada. 2017. Canadian Community Health Survey – Nutrition: nutrient intakes from food and nutritional supplements. Released at 8:30 a.m. Eastern time in The Daily, Tuesday, June 20, 2017. [accessed 2017 Dec 6].
[STP-EX] Sewage Treatment Plant Expanded Model. c2000-2013. Ver. 1.0. Windsor (ON): University of Windsor, Dept. of Civil and Environmental Engineering. [Model described in Seth R, Webster E, Mackay D. 2008. Continued development of a mass balance model of chemical fate in a sewage treatment plant. Water Res. 42(3):595-604.].
[TEST] Toxicity Estimation Software Tool. 2016. Ver. 4.2. Washington (DC): US Environmental Protection Agency.
Unocal. 2002. Material Safety Data Sheet: Unocal '76' Guardol 15W/40 Motor Oil [PDF]. Unocal Refining & Marketing Division. [cited 2019 Jan 31].
[US EPA] United States Environmental Protection Agency. 2003. Robust summaries for Irganox 1035, Thiodiethylene bis (3,5-di-tert-butyl-4-hydroxyhydrocinnamate). CAS No. 41484-35-9. Report No. 201-14690B.
[US EPA] United States Environmental Protection Agency. 2009. Screening-level hazard characterization. Alkylphenols category. Washington (DC): US EPA, Office of Pollution Prevention and Toxics.
[US EPA] United States Environmental Protection Agency. 2010. Screening-level hazard characterization. Bridged alkyl phenol category. Washington (DC): US EPA, Office of Pollution Prevention and Toxics.
[US EPA] United States Environmental Protection Agency. 2011a. Chapter 6: inhalation rates. Exposure factors handbook 2011 edition (final report). Washington (DC): US Environmental Protection Agency. EPA/600/R-09/052F.
[US EPA] United States Environmental Protection Agency. 2011b. Exposure factors handbook: 2011 edition (final report). Washington (DC): US Environmental Protection Agency, National Centre for Environmental Assessment.
[US EPA] United States Environmental Protection Agency. 2012. Standard operating procedures for residential pesticide exposure assessment [PDF]. Washington (DC): US Environmental Protection Agency, Office of Pesticide Programs, Health Effects Division. [accessed 2020 Feb 24].
[US EPA] United States Environmental Protection Agency. 2018. Chemical Data Reporting under the Toxic Substances Control Act. [updated 2018 Jul 24; cited 2019 Feb 6].
Usman A, Mohammad RH, Raheem D. 2016. Isolation and characterization of naturally occurring butylated hydroxytoluene from Trichilia emetica whole seeds. J Nat Prod Resour. 2(2):68-70.
Van Hoogen G, Opperhuizen A. 1988. Toxicokinetics of chlorobenzenes in fish. Environ Toxicol Chem. 7(3):213-219.
Versar. 1986. Standard scenarios for estimating exposure to chemical substances during use of consumer products. Volume II. Technical report. Washington (DC). Prepared for US EPA Office of Toxic Substances Exposure Evaluation Division. 319 p. EPA Contract No. 68-02-3968
Vulliet E, Tournier M, Vauchez A, Wiest L, Baudot R, Lafay F, Kiss A, Cren-Olivé C. 2014. Survey regarding the occurrence of selected organic micropollutants in the groundwaters of overseas departments. Environ Sci Pollut Res. 21(12):7512-7521.
Wang W, Asimakopoulos AG, Abualnaja KO, Covaci A, Gevao B, Johnson-Restrepo B, Kumosani TA, Malarvannan G, Minh TB, Moon H-B, et al. 2016. Synthetic phenolic antioxidants and their metabolites in indoor dust from homes and microenvironments. Environ Sci Technol. 50(1):428-434.
Webster F, Gagné M, Patlewicz G, Pradeep P, Trefiak N, Judson RS, Barton-Maclaren TS. 2019. Predicting estrogen receptor activation by a group of substituted phenols: an integrated approach to testing and assessment case study. Regul Toxicol Pharmacol. 106:278-291.
White D, Lapworth DJ, Stuart ME, Williams PJ. 2016. Hydrochemical profiles in urban groundwater systems: new insights into contaminant sources and pathways in the subsurface from legacy and emerging contaminants. Sci Total Environ. 562:962-973.
[WHO] World Health Organization. 1995. Evaluation of certain food additives and contaminants: forty fourth report of the Joint FAO/WHO Expert Committee on Food Additives. WHO technical report series, 859. Geneva (CH): World Health Organization. [accessed 2017 Feb 20].
[Wilson and Meridian] Wilson Scientific Consulting Inc. and Meridian Environmental Inc. 2015. Critical review of soil ingestion rates for use in contaminated site human health risk assessments in Canada. Ottawa (ON): contractor report prepared for the Contaminated Sites Division, Safe Environments Programme, Health Canada.
Wu XM, Bennett DH, Ritz B, Cassady DL, Lee K, Hertz-Picciotto I. 2010. Usage pattern of personal care products in California households. Food Chem Toxicol. 48(11):3109-3119.
Zeilmaker MJ, van Kranen HJ, van Veen MP, Janus J. 2000. Cancer risk assessment of azo dyes and aromatic amines from tattoo bands, folders of paper, toys, bed clothes, watch straps and ink. Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment].
Zgheib S, Moilleron R, Chebbo G. 2012. Priority pollutants in urban stormwater: part 1 – case of separate storm sewers. Water Res. 46(20):6683-6692.
Appendix A. The ecological risk classification of organic substances (ERC)
The ecological risks of four substituted phenols (CAS RNs 85-60-9, 2082-79-3, 6386-38-5, and 41484-35-9) were characterized using the ERC approach (ECCC 2016a). The ERC is a risk-based approach that considers multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. The various lines of evidence are combined to discriminate between substances of lower or higher potency and lower or higher potential for exposure in various media. This approach reduces the overall uncertainty of risk characterization compared to an approach that relies on a single metric in a single medium (for example, median lethal concentration) for characterization. The following summarizes the approach, which is described in detail in ECCC (2016a).
Data on physical-chemical properties, fate (chemical half-lives in various media and biota, partition coefficients, and fish bioconcentration), acute fish ecotoxicity, and chemical import or manufacture volume in Canada were collected from scientific literature from available empirical databases (for example, OECD QSAR Toolbox 2014) and from responses to surveys issued pursuant to section 71 of CEPA, or they were generated using selected quantitative structure-activity relationship (QSAR) or mass-balance fate and bioaccumulation models. These data were used as inputs to other mass-balance models or to complete the substance hazard and exposure profiles.
Hazard profiles were based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Exposure profiles were also based on multiple metrics including potential emission rate, overall persistence, and long-range transport potential. Hazard and exposure profiles were compared to decision criteria in order to classify the hazard and exposure potentials for each organic substance as low, moderate, or high. Additional rules were applied (for example, classification consistency, margin of exposure) to refine the preliminary classifications of hazard or exposure.
A risk matrix was used to assign a low, moderate, or high classification of potential risk to each substance on the basis of its hazard and exposure classifications. ERC classifications of potential risk were verified using a two-step approach. The first step adjusted the risk classification outcomes from moderate or high to low for substances that had a low estimated rate of emission to water after wastewater treatment, representing a low potential for exposure. The second step reviewed low risk potential classification outcomes using relatively conservative, local-scale (that is, in the area immediately surrounding a point-source of discharge) risk scenarios, designed to be protective of the environment, to determine whether the classification of potential risk should be increased.
ERC uses a weighted approach to minimize the potential for both over- and under-classification of hazard and exposure, and of subsequent risk. The balanced approaches for dealing with uncertainties are described in greater detail in ECCC (2016a). The following describes two of the more substantial areas of uncertainty. Error with empirical or modelled acute toxicity values could result in changes in classification of hazard, particularly metrics relying on tissue residue values (that is, mode of toxic action), many of which are predicted values from (Q)SAR models (OECD QSAR Toolbox 2014). However, the impact of this error is mitigated by the fact that overestimation of median lethality will result in a conservative (protective) tissue residue value used for critical body residue analysis. Error with underestimation of acute toxicity will be mitigated through the use of other hazard metrics such as structural profiling of mode of action, reactivity, and/or estrogen-binding affinity. Changes or errors in chemical quantity could result in differences in classification of exposure as the exposure and risk classifications are highly sensitive to emission rate and use quantity. The ERC classifications thus reflect exposure and risk in Canada on the basis of quantities reported by industry but may not reflect future trends.
Critical data and considerations used to develop the substance-specific profiles for these substances and the hazard, exposure, and risk classification results are presented in ECCC (2016b).
Appendix B. Estimates of exposure to environmental media and food of substances in the Substituted Phenols Group
| CAS RN | PEC (mg/L)a | Highest estimated daily intake (mg/kg bw/day)b,c |
|---|---|---|
| 85-60-9 | N/A | N/Ad |
| 96-69-5 | 0.011 | 0.0014 |
| 96-76-4 | 0.0009 | 0.00012 |
| 98-54-4 | 0.0030 | 0.00039 |
| 118-82-1 | 0.000022 | 0.0000029 |
| 128-37-0 | 0.004 | 0.00082 |
| 128-39-2 | 0.012 | 0.0015 |
| 1843-03-4 | 0.010 | 0.0013 |
| 2082-79-3 | N/A | N/Ad |
| 4221-80-1 | N/A | N/Ad |
| 6386-38-5 | N/A | N/Ad |
| 35958-30-6 | N/A | N/Ad |
| 36443-68-2 | 0.0284 | 0.0037 |
| 41484-35-9 | N/A | N/Ad |
| 61788-44-1 | 0.00103e | 0.00014 |
Abbreviations: PEC, predicted environmental concentration; N/A, not applicable
a PEC (mg/L) from Table 7-8, 7-9.
b Highest exposed age group 0 to 5 months. Assumed to weigh 6.3 kg (Health Canada 2015).
c Exclusively for formula-fed infants (0 to 5 months), assumed to drink 0.826 L of water per day (Health Canada 2018), where water is used to reconstitute formula.
d Due to the physical-chemical properties of these substances (Table 3-1), exposure is not expected from drinking water.
e Sum of the PECs of the components of the UVCB.
| Age group (years) | Mean (mg/kg bw/day) a | 90th percentile (mg/kg bw/day) a |
|---|---|---|
| 1 | 0.15 | 0.32 |
| 2–3 | 0.15 | 0.33 |
| 4–8 | 0.13 | 0.28 |
| 9–13 | 0.08 | 0.19 |
| 14–18 | 0.06 | 0.13 |
| 19+ | 0.04 | 0.10 |
a Personal communication from the FD of HC to the ESRAB, HC, dated Jan 2019; unreferenced.
| Route of exposure | 0 to 5 monthsa (breast milk-fed)b |
0 to 5 monthsa (formula-fed)c | 6 to 11 monthsd | 1 yeare | 2 to 3 yearsf | 4 to 8 yearsg | 9 to 13 yearsh | 14 to 18 yearsi | ≥19 yearsj |
|---|---|---|---|---|---|---|---|---|---|
| Ambient airk | N/I | N/I | N/I | N/I | N/I | N/I | N/I | N/I | N/I |
| Indoor airl | 0.00028 | 0.00028 | 0.00029 | 0.00035 | 0.0003 | 0.00023 | 0.00016 | 0.00012 | 0.000098 |
| Drinking waterm | N/I | 0.00081 | 0.00052 | 0.0002 | 0.00018 | 0.00014 | 0.00011 | 0.00011 | 0.00013 |
| Food and beveragesn | N/I | N/I | N/I | 0.15 | 0.15 | 0.13 | 0.08 | 0.06 | 0.04 |
| Soilp | N/I | N/I | N/I | N/I | N/I | N/I | N/I | N/I | N/I |
| Dustq | 0.000015 | 0.000015 | 0.000013 | 0.000014 | 6.1×10-6 | 4.6×10-6 | 2.4×10-6 | 1.5×10-7 | 1.5×10-7 |
| Total intake | 0.0003 | 0.0011 | 0.00082 | 0.15 | 0.15 | 0.13 | 0.08 | 0.06 | 0.04 |
Abbreviations: N/A, not applicable; N/I, data not identified in the literature
a Assumed to weigh 6.3 kg (Health Canada 2015), to breathe 3.7 m3 of air per day (US EPA 2011b [modified]), and to ingest 21.6 mg of dust per day (Wilson and Meridian 2015 [modified]). It is assumed that no soil ingestion occurs due to typical caregiver practices.
b Exclusively for breast milk-fed infants, assumed to consume 0.744 L of breast milk per day (Health Canada 2018), where breast milk is assumed to be the only dietary source.
c Exclusively for formula-fed infants, assumed to drink 0.826 L of water per day (Health Canada 2018), where water is used to reconstitute formula. See footnote on drinking water for details.
d Assumed to weigh 9.1 kg (Health Canada 2015), to breathe 5.4 m3 of air per day (US EPA 2011a [modified]), to ingest 7.3 mg of soil per day, and to ingest 27.0 mg of dust per day (Wilson and Meridian 2015 [modified]). For breast milk-fed infants, assumed to consume 0.632 L of breast milk per day (Health Canada 2018). For formula-fed infants, assumed to drink 0.764 L of water per day (Health Canada 2018), where water is used to reconstitute formula. See footnote on drinking water for details.
e Assumed to weigh 11.0 kg (Health Canada 2015), to breathe 8.0 m3 of air per day (US EPA 2011a [modified]), to drink 0.36 L of water per day (Health Canada 2017), to ingest 8.8 mg of soil per day, and to ingest 35.0 mg of dust per day (Wilson and Meridian 2015 [modified]).
f Assumed to weigh 15 kg (Health Canada 2015), to breathe 9.2 m3 of air per day (US EPA 2011a [modified]), to drink 0.43 L of water per day (Health Canada 2017), to ingest 6.2 mg of soil per day, and to ingest 21.4 mg of dust per day (Wilson and Meridian 2015 [modified]).
g Assumed to weigh 23 kg (Health Canada 2015), to breathe 11.1 m3 of air per day (US EPA 2011a [modified]), to drink 0.53 L of water per day (Health Canada 2017), to ingest 8.7 mg of soil per day, and to ingest 24.4 mg of dust per day (Wilson and Meridian 2015 [modified]).
h Assumed to weigh 42 kg (Health Canada 2015), to breathe 13.9 m3 of air per day (US EPA 2011a [modified]), to drink 0.74 L of water per day (Health Canada 2017), to ingest 6.9 mg of soil per day, and to ingest 23.8 mg of dust per day (Wilson and Meridian 2015 [modified]).
i Assumed to weigh 62 kg (Health Canada 2015), to breathe 15.9 m3 of air per day (US EPA 2011a [modified]), to drink 0.09 L of water per day (Health Canada 2017), to ingest 1.4 mg of soil per day, and to ingest 2.1 mg of dust per day (Wilson and Meridian 2015 [modified]).
j Assumed to weigh 74 kg (Health Canada 2015), to breathe 15.1 m3 of air per day (US EPA 2011a [modified]), to drink 1.53 L of water per day (Health Canada 2017), to ingest 1.6 mg of soil per day, and to ingest 2.6 mg of dust per day (Wilson and Meridian 2015 [modified]).
k No monitoring data of ambient air in Canada or elsewhere were identified. Canadians are assumed to spend 3 hours outdoors each day (Health Canada 1998).
l No monitoring data of indoor air in Canada were identified. The median concentration of CAS RN 128-37-0 (550 ng/m3) measured in indoor air samples in Japan (Kanazawa et al. 2010) was selected for deriving estimates of daily intake for indoor air exposure. Canadians are assumed to spend 21 hours indoors each day (Health Canada 1998).
m No monitoring data of drinking water in Canada were identified. The highest PEC of CAS RN 128-37-0 predicted (Table 7-11) was selected for deriving upper-bounding estimates of daily intake for drinking water exposure. See Table B-1.
n Estimated mean value for dietary exposure to CAS RN 128-37-0 from potential food additive use (Table B-2).
p No soil concentration data for Canada or elsewhere were identified.
q The median concentration of CAS RN 128-37-0 (4.31 ug/g) in house dust from the United States (Wang et al. 2016) was selected for deriving estimates of daily intake for dust exposure.
Appendix C. Exposure estimates to humans from products available to consumers
Sentinel exposure scenarios were used to estimate the potential exposure to substances in the Substituted Phenols Group; scenario assumptions are summarized in Table C-1. Exposures were estimated using ConsExpo Web version or algorithms from the model (ConsExpo Web 2016), except where noted otherwise. For the estimated potential exposures via the dermal route, dermal absorption was assumed to be 0.41% for CAS RN 128-37-0 and 100% for all other substances (see section 8.6). Oral absorption was assumed to be 100% for all substances. Due to the low-moderate vapour pressures of these substances, exposure from inhalation was not expected. An overall retention factor of 1 was used unless otherwise specified.
| Substance | Exposure scenario | Assumptions |
|---|---|---|
| CAS RN 128-37-0; 2082-79-3 | Mouthing plastic/rubber | Mouthing plastic (mg/kg bw/day) = Max. amount of CAS RN 128-37-0 migrating from various toysa (0.03 mg; Noguerol-Cal et al. 2011) / bw (6.3 kg; Health Canada, 2018) |
| CAS RN 36443-68-2 | Mouthing fabric | Mouthing textile (mg/kg bw/day) = Total surface area (cm2)*Area weight (mg/cm2)*Concentrations*fractional release of CAS RN 128-37-0/day / bw (kg) Default mean body weight: 6.3 kg (0–5 months; Health Canada 2015) Release of CAS RN 128-37-0/dayb: 11% (Noguerol-Cal et al. 2011) Area weight: 20 mg/cm2 (US EPA 2012); Surface area of object mouthed: 20 cm2 (Zeilmaker et al. 2000). Concentration in textile: 0.01 (unitless; CDR 2016) |
| CAS RN 128-37-0; 35958-30-6 | Lipstickc | Concentration of CAS RN 35958-30-6: 0.1%; Concentration of CAS RN 128-37-0: 10% (personal communication from the CHPSD of HC to the ESRAB, HC, dates ranging from March 2017 to Feb 2019; unreferenced). Oral: Direct product contact, direct oral intake Frequency: 2.5 times per day (Statistics Canada 2017) Amount ingested: 0.022 g (Ficheux et al. 2016) Body weight (14–18 years): 62 kg (Health Canada 2015) |
| CAS RN 28-37-0 | Sunscreen lotionc | Concentration 1% (personal communication from the TPD of HC to the ESRAB, HC, dates ranging from March 2017 to Jan 2019; unreferenced). Dermal: Direct product contact, instant application Frequency: 1.6 times per day for infants (Ficheux et al. 2015) Exposed area: 3680 cm2 Product amount: 5.4 g (Ficheux et al. 2016) Body weight (6–11 months): 9.1 kg (Health Canada 2015) |
| CAS RN 128-37-0; 35958-30-6 | Body lotionc | Concentration of CAS RN 35958-30-6: 0.02%; Concentration of CAS RN 128-37-0: 1% or 0.3%d (personal communication from the CHPSD of HC to the ESRAB, HC, dates ranging from March 2017 to Feb 2019; unreferenced). Dermal: Direct product contact, instant application Frequency: 0.8/day (0–5 months; Ficheux et al. 2015), 0.8/day (9–18 years; Wu et al. 2010), 1/day (19+ years; Ficheux et al. 2015) Exposed area: 2860 cm2 Product amount: 2 g (0–5 months; Surface area adjustment), 7.7 g (9–13 years; Surface area adjustment), 10 g (14–19+ years; Ficheux et al. 2016) Body weight: 6.3 kg (0–5 months), 42 kg (9–13 years), 62 kg (14–18 years), 74 kg (19+ years) (Health Canada 2015) |
| CAS RN 128-37-0 | Body soapc (solid) | Concentration: 1% (personal communication from the CHPSD of HC to the ESRAB, HC, dates ranging from March 2017 to Feb 2019; unreferenced). Retention factor of 0.01 was applied, giving the final weight fraction of 0.01% (professional judgment). Dermal: Direct product contact, instant application Frequency: 1.15/day (9–13 years), 1.2/day (14–19+ years) (Ficheux et al. 2015) Exposed area: 12 700 cm² (9–13 years), 16 460 cm² (14–18 years),17 500 cm² (19+ years) Product amount: 0.82 g (Surface area adjustment), 1.1 g (14–19+ years; Ficheux et al. 2016) Body weight: 42 kg (9–13 years), 62 kg (14–18 years), 74 kg (19+ years) (Health Canada 2015) |
| CAS RN 128-37-0 | Facial makeupc (solid foundation) | Concentration of CAS RN 128-37-0: 10% (personal communication from the CHPSD of HC to the ESRAB, HC, dates ranging from March 2017 to Feb 2019; unreferenced). Dermal: Direct product contact, instant application Frequency: 1/day (14–19+ years; Ficheux et al. 2015) Product amount: 0.073 g (14–19+ years; Ficheux et al. 2016) Exposed area: 370 cm² (14–18 years), 585 cm² (19+ years) Body weight: 62 kg (14–18 years), 74 kg (19+ years) (Health Canada 2015) |
| CAS RN 128-37-0 | Antiperspirant/deodorantc | Concentration of CAS RN 128-37-0: 1% (personal communication from the CHPSD of HC to the ESRAB, HC, dates ranging from March 2017 to Feb 2019; unreferenced). Dermal: Direct product contact, instant application Frequency: 1.1/day (9–18 years; Wu et al. 2010; Ficheux et al. 2015), 1.3/day (19+ years; Loretz et al. 2006) Product amount: 1 g (9–19+ years; Ficheux et al. 2016) Exposed area: 240 cm² Body weight: 42 kg (9–13 years), 62 kg (14–18 years), 74 kg (19+ years) (Health Canada 2015) |
| CAS RN 128-37-0; 6386-38-5 | Fragrance productc | Concentration CAS RN 128-37-0: 1% (personal communication from the CHPSD of HC to the ESRAB, HC, dates ranging from March 2017 to Feb 2019; unreferenced). Concentration CAS RN 6386-38-5: 0.1% (expert judgment) Body weight: 62 kg (14–18 years), 74 kg (19+ years) (Health Canada 2015) Direct product contact, instant application Frequency: 1.4/day (14–18 years; Statistics Canada 2017), 1.7/day (19+ years; Loretz et al. 2006) Dermal: Product amount: 4.3 g (14–19+ years; Ficheux et al. 2016) Exposed area: 200 cm² (14–19+ years) |
| CAS RN 2082-79-3 | Face moisturizerc | Inhalation: Inhalation rate: 15.9 m3/day (14-18 years; Health Canada 2015), 15.1 m3/day (19+ years; Health Canada 2015) Concentration: 0.1% (personal communication from the CHPSD, HC to the ESRAB, HC, dates ranging from March 2017 to Feb 2019; unreferenced). Dermal: Direct product contact, instant application Frequency: 2 times per day (Loretz et al. 2005) Product amount: 1.5 g (Ficheux et al. 2016) Exposed area: 585 cm2 Body weight (19+ years): 74 kg (Health Canada 2015) |
| CAS RN 96-76-4; 118-82-1; 128-37-0; 41484-35-9 | DIY motor oil change or lubricant for door hinges | Dermal exposure while changing your own motor oil or using a fuel additive in a personal vehicle. Using a lubricant on a door hinge. Weight fraction (WF) CAS RN 96-76-4: 0.001 (<0.1%; ECCC 2017) Weight fraction CAS RN 118-82-1e: 0.01 (1%; MSDS 2010b) Weight fraction CAS RN 128-37-0: 0.01 (1%; MSDS 2010b) Weight fraction CAS RN 41484-35-9: 0.025 (2.5% MSDS 2013a) Surface area exposed (SA): 12 cm2 (professional judgment) Density of motor oil (DSY): 0.89 g/mL (Unocal 2002) Film thickness (T) retained on skin: 15.88 × 10-3 cm (US EPA 2011b) Body weight (19+ years): 74 kg (Health Canada 2015) Estimated Exposure = (WF × SA × T × DSY) / Body weight |
| CAS RN 98-54-4; 128-39-2 | DIY, addition of fuel additive | Dermal exposure while using a fuel additive Weight fraction CAS RN 98-54-4: 0.005 (0.5%; ECCC 2017) Weight fraction CAS RN 128-39-2: 0.05 (1%–5%; MSDS 2015) Surface area exposed (SA): 12 cm2 (professional judgment) Density (DSY) = 1 g/cm3 (Versar 1986) Film thickness (T) retained on skin: 15.88 × 10-3 cm (US EPA 2011b) Body weight (19+ years): 74 kg (Health Canada 2015) Estimated exposure = (WF × SA × T × DSY) / Body weight |
| CAS RN 61788-44-1 | Wood epoxy glue (two-component filler) | Concentration: 35% (MSDS 2013b) Dermal: Direct product contact, instant application Body weight (19+ years): 74 kg (Health Canada 2015) |
| CAS RN 128-37-0 | Wall paint | Concentration: 2% (Malshe and Sikchi 2004) Dermal: Direct product contact, instant application Body weight (19+ years): 74 kg (Health Canada 2015) |
| CAS RN 128-37-0 | Air freshener | Concentration: 10%–30% (MSDS 2014a) Essential oil, air freshener scenarioc Inhalation: Exposure to vapour, constant rate Body weight (1 year): 11 kg (Health Canada 2015) Inhalation rate (1 year): 8 m3/day (US EPA 2011a) |
a Migration data for CAS RN 2082-79-3 were not available. CAS RN 128-37-0 was used as a surrogate, due to similar expected use.
b Migration data for CAS RN 36443-68-2 were not available. CAS RN 128-37-0 was used as a surrogate, due to similar expected use.
c ConsExpo Web (2016) scenario defaults were used, except for those noted here.
d A concentration of 0.3% was considered for CAS RN 128-37-0 in body lotion for the estimate of combined exposure to cosmetics. 986/991 cosmetic products reported to the CNS are <0.3%; Personal communication from the CHPSD of HC to the ESRAB, HC, dates ranging from March 2017 to Feb 2019; unreferenced.
e Concentration data for CAS RN 118-82-1 were not available in MSDS (2016). Concentration of CAS RN 128-37-0 (MSDS 2010b) was used as a surrogate, due to similar expected use.
Appendix D. Physical chemical properties of CAS RNs 35958-30-6, 119-47-1, and 88-24-4
| CAS number | 35958-30-6 | 119-47-1 | 88-24-4 |
|---|---|---|---|
| Chemical name | Tetrabutyl ethylidenebisphenol | 2,2'-Methylenebis(6-tert-butyl-p-cresol) | 6,6'-di-tert-butyl-4,4'-diethyl-2,2'-methylenediphenol |
| SMILES | CC(c1cc(cc(c1O)C(C)(C)C)C(C)(C)C)c1cc(cc(c1O)C(C)(C)C)C(C)(C)C | Cc1cc(Cc2cc(C)cc(c2O)C(C)(C)C)c(O)c(c1)C(C)(C)C | CCC1=CC(=C(C(=C1)C(C)(C)C)O)CC2=C(C(=CC(=C2)CC)C(C)(C)C)O |
| Vapor pressure (Antoine method) (mm Hg) | 7.42E-14 | 1.3E-10 | 7.875647e-7 |
| Molecular weight (Da) | 438.66524 | 340.48408 | 368.55 |
| log Kow | 9.13 | 7.97 | 8.95 |
| Boiling point (°C) | 495.8 | 444.75 | 458.9 |
| Water solubility (mgL) | 0.000107 | 0.1235 | <1 mg/L |
| Similarity (%) | 100 | 63 | 61 |
