Draft screening assessment acetic acid
Official title: Draft screening assessment acetic acid
Chemical Abstracts Service Registry Numbers 64-19-7
Environment and Climate Change Canada Health Canada
July 2019
Synopsis
Pursuant to section 74 of the Canadian Environmental Protection Act, 1999 (CEPA), the Minister of the Environment and the Minister of Health have conducted a screening assessment of acetic acid. The Chemical Abstracts Service Registry Number (CAS RNFootnote 1 ) for acetic acid is 64-19-7. This substance was identified as a priority for assessment as it met categorization criteria under subsection 73(1) of CEPA.
Acetic acid may be present in food products as vinegar (which is dilute aqueous acetic acid). Acetic acid is also a permitted food additive, and may be a component in incidental additives and food packaging materials. Acetic acid is also notified as present in self-care products (i.e., products available for purchase without a prescription from a doctor, and fall into one of three broad categories: cosmetics, natural health products, and non-prescription drugs) and pest control products. In Canada, it is also present in certain products available to consumers, such as household cleaners, pet shampoos, and silicone sealants.
The ecological risk of acetic acid was characterized using the ecological risk classification of organic substances (ERC), which is a risk-based approach that employs multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. Hazard profiles are based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Metrics considered in the exposure profiles include potential emission rate, overall persistence, and long-range transport potential. A risk matrix is used to assign a low, moderate or high level of potential concern for substances on the basis of their hazard and exposure profiles. Based on the outcome of ERC analysis, acetic acid is considered unlikely to be causing ecological harm.
Considering all available lines of evidence presented in this draft screening assessment, there is low risk of harm to the environment from acetic acid. It is proposed to conclude that acetic acid does not meet the criteria under paragraphs 64(a) or (b) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
On the basis of the available information, no serious adverse effects or organ-specific toxicity were observed in experimental animals. Effects observed were primarily associated with site-of-contact effects, and loss of appetite. Given the low hazard potential of this substance the risk to human health is considered to be low.
On the basis of the information presented in this draft screening assessment, it is proposed to conclude that acetic acid does not meet the criteria under paragraph 64(c) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore proposed to conclude that acetic acid does not meet any of the criteria set out in section 64 of CEPA.
1. Introduction
Pursuant to section 74 of the Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999), the Minister of the Environment and the Minister of Health have conducted a screening assessment of acetic acid to determine whether the substance presents or may present a risk to the environment or to human health. This substance was identified as a priority for assessment as it met categorization criteria under subsection 73(1) of CEPA (ECCC, HC [modified 2017]).
The ecological risk of acetic acid was characterized using the ecological risk classification of organic substances (ERC) approach (ECCC 2016a). The ERC describes the hazard of a substance using key metrics, including mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity, and considers the possible exposure of organisms in the aquatic and terrestrial environments on the basis of such factors as potential emission rates, overall persistence, and long-range transport potential in air. The various lines of evidence are combined to identify substances as warranting further evaluation of their potential to cause harm to the environment or as having a low likelihood of causing harm to the environment.
This draft screening assessment includes consideration of information on chemical properties, environmental fate, hazards, uses and exposures, including additional information submitted by stakeholders. Relevant data were identified up to October 2018. Empirical data from key studies as well as results from models were used to reach proposed conclusions. When available and relevant, information presented in assessments from other jurisdictions was considered.
This draft screening assessment was prepared by staff in the CEPA Risk Assessment Program at Health Canada and Environment and Climate Change Canada and incorporates input from other programs within these departments. The ecological portion of this assessment is based on the ERC document (published July 30, 2016), which was subject to an external review as well as a 60-day public comment period. While external comments were taken into consideration, the final content and outcome of this draft screening assessment remain the responsibility of Health Canada and Environment and Climate Change Canada.
This draft screening assessment focuses on information critical to determining whether substances meet the criteria as set out in section 64 of CEPA by examining scientific information and incorporating a weight of evidence approach and precaution.Footnote 2 This draft screening assessment presents the critical information and considerations on which the proposed conclusion is based.
2. Substance identity
The Chemical Abstracts Service Registry Numbers (CAS RNFootnote 3 ), Domestic Substances List (DSL) name, and molecular structure for acetic acid are presented in Table 2-1.
| CAS RN | DSL name | Chemical structure and molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
| 64-19-7 | Acetic acid | 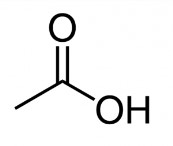 C2H4O2 |
60.05 |
3. Physical and chemical properties
A summary of physical and chemical property data of acetic acid is presented in Table 3‑1. Additional physical and chemical properties are reported in ECCC 2016b.
| Property | Range | Key reference(s) |
|---|---|---|
| Melting point (°C) | 17 | Rumble 2018 |
| Boiling point (°C) | 117.9 | Rumble 2018 |
| Density (g/mL) | 1.051 | Rumble 2018 |
| Vapour pressure (Pa) | 2.07 × 103 – 2.09 × 103 | Daubert and Danner 1985; Rumble 2018 |
| Water solubility (mg/L) | 6.029 × 105 | Yalkowsky et al. 2010 |
| pKa (dimensionless) | 4.756 | Serjeant and Dempsey 1979 |
| Log Kow (dimensionless) | -0.17 | Hench et al. 1995 |
| Henry’s law constant (Pa·m3/mol) | 1.01 × 10−2 – 2.53 × 10−2 | Johnson et al. 1996; Servant et al. 1991 |
Abbreviations: Kow, octanol-water partition coefficient; pKa, acid dissociation constant
4. Sources and uses
Acetic acid is identified as a high production volume chemical by the Organization for Economic Co-operation and Development (OECD) (OECD 2004) and the US EPA (2018). The substance has not been included in surveys issued pursuant to a CEPA section 71 notice. Total import quantities for acetic acid from 2014 to 2017 were obtained from the Canadian international merchandise trade (CIMT) database and are summarized in Table 4-1.
| Reporting year | Total imports (kg) |
|---|---|
| 2014 | 32 626 722 |
| 2015 | 28 841 415 |
| 2016 | 30 322 299 |
| 2017 | 27 215 900 |
Uses of acetic acid in products available to Canadian consumers were identified from a search of publicly available safety data sheets (SDS). When added to products available to consumers, typically acetic acid functions as a pH adjuster or buffering agent, or disinfectant. Identified uses are described in Table 4-2. Acetic acid in the form of white vinegar is also commonly used directly in a variety of household applications, including as a surface and glass cleaner.
| Uses | Reference |
|---|---|
| Household cleaner | SDS 2015a |
| Pet shampoo | SDS 2013 |
| Silicone sealant | SDS 2015b; SDS 2016 |
| Water treatment (swimming pools) | SDS 2015c |
| Waterproofing for fleece | HPD 2018 |
Acetic acid may be present in food products as vinegar (which is dilute aqueous acetic acid), as a permitted food additive, and may be a component in incidental additives and food packaging materials. Acetic acid is also notified as present in self-care productsFootnote 4 and pest control products. Details for these uses are presented in Table 4-3.
| Use | Details | |
|---|---|---|
| Food ingredienta | Vinegar is dilute aqueous acetic acid, the concentration of which must not be less than 4.1% and not more than 12.3% | |
| Food additiveb | Permitted for use as a food additive as per the List of Permitted pH Adjusting Agents, Acid-Reacting Materials and Water Correcting Agents, and the List of Permitted Preservatives | |
| Incidental additiveb | Component in cleaners, sanitizers and lubricants used in food processing establishments | |
| Food packaging materialsc | Component in the manufacturing of inner layer films in multilayer structures | |
| Medicinal or non-medicinal ingredients in disinfectant, human or veterinary drug productsd | Notified as present in certain pharmaceuticals including antibiotics, anticonvulsants, antifungals, antihistamines, antineoplastic, antivirals, hematopoietic, hemodialysis solutions, neuromuscular blocking agents, and disinfectants for agents used on objects | |
| Medicinal or non-medicinal ingredients in licensed natural health productse | Notified as present in products including certain vitamin supplements, acne creams, medicated shampoos, topical treatments for warts, and probiotics. | |
| Present in cosmetics, based on notifications under the Cosmetic Regulationsf | Notified as present in certain cleansers, deodorants, douches, exfoliants, hair products, non-permanent makeup, massage products, mouth wash, and nail polish | |
| Active ingredient or formulant in registered pest control productsg | Approved formulant or active ingredient in pest control products |
a Personal communication, email from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated July 25, 2018; unreferenced.
b Personal communication, email from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated July 16, 2018 and December 27, 2018; unreferenced.
c Personal communication, email from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated July 16, 2018; unreferenced.
d Personal communication, email from Therapeutic Products Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated July 11 2018; unreferenced).
e LNHPD (modified 2018).
f Personal communication, email from Consumer Product Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated June 20, 2018; unreferenced
g Personal communication, email from Pest Management Regulatory Agency, Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated July 25, 2018; unreferenced.
5. Potential to cause ecological harm
5.1 Characterization of ecological risk
The ecological risk of acetic acid was characterized using the ecological risk classification of organic substances (ERC) approach (ECCC 2016a). The ERC is a risk-based approach that considers multiple metrics for both hazard and exposure, on the basis of weighted consideration of multiple lines of evidence for determining risk classification. The various lines of evidence are combined to discriminate between substances of lower or higher potency and lower or higher potential for exposure in various media. This approach reduces the overall uncertainty with risk characterization compared to an approach that relies on a single metric in a single medium (e.g., median lethal does [LC50]) for characterization. The following summarizes the approach, which is described in detail in ECCC (2016a).
Data on physical-chemical properties, fate (chemical half-lives in various media and biota, partition coefficients, and fish bioconcentration), acute fish ecotoxicity, and chemical import or manufacture volume in Canada were collected from the scientific literature, from available empirical databases (e.g., OECD QSAR Toolbox 2016), and from responses to surveys conducted under section 71 of CEPA, or they were generated using selected quantitative structure-activity relationship (QSAR) or mass-balance fate and bioaccumulation models. These data were used as inputs to other mass-balance models or to complete the substance hazard and exposure profiles.
Hazard profiles were based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Exposure profiles were also based on multiple metrics, including potential emission rate, overall persistence, and long-range transport potential. Hazard and exposure profiles were compared to decision criteria in order to classify the hazard and exposure potentials for each organic substance as low, moderate, or high. Additional rules were applied (e.g., classification consistency, margin of exposure) to refine the preliminary classifications of hazard or exposure.
A risk matrix was used to assign a low, moderate or high classification of potential risk for each substance on the basis of its hazard and exposure classifications. ERC classifications of potential risk were verified using a two-step approach. The first step adjusted the risk classification outcomes from moderate or high to low for substances that had a low estimated rate of emission to water after wastewater treatment, representing a low potential for exposure. The second step reviewed low risk potential classification outcomes using relatively conservative, local-scale (i.e., in the area immediately surrounding a point-source of discharge) risk scenarios, designed to be protective of the environment, to determine whether the classification of potential risk should be increased.
ERC uses a weighted approach to minimize the potential for both over and under classification of hazard and exposure, and of subsequent risk. The balanced approaches for dealing with uncertainties are described in greater detail in ECCC 2016a. The following describes two of the more substantial areas of uncertainty. Error with empirical or modeled acute toxicity values could result in changes in classification of hazard, particularly metrics relying on tissue residue values (i.e., mode of toxic action), many of which are predicted values from (Q)SAR models. However, the impact of this error is mitigated by the fact that overestimation of median lethality will result in a conservative (protective) tissue residue value used for critical body residue (CBR) analysis. Error with underestimation of acute toxicity will be mitigated through the use of other hazard metrics such as structural profiling of mode of action, reactivity and/or estrogen binding affinity. Changes or errors in chemical quantity could result in differences in classification of exposure as the exposure and risk classifications are highly sensitive to emission rate and use quantity. The ERC classifications thus reflect exposure and risk in Canada on the basis of what is believed to be the current use quantity, and may not reflect future trends.
Critical data and considerations used to develop the substance-specific profiles for acetic acid, and the hazard, exposure and risk classification results, are presented in ECCC (2016b).
According to information considered under ERC, acetic acid was classified as having a low hazard potential. Acetic acid was classified as having a high exposure potential on the basis of having a long half-life in air and high use quantities. As a result of the low hazard classification acetic acid was classified as having a low potential for ecological risk. On the basis of current use patterns, this substance is unlikely to be resulting in concerns for the environment in Canada.
6. Potential to cause harm to human health
6.1 Health effects assessment
WHO (1998) summarized the health effects literature for acetic acid as part of a safety evaluation on the saturated aliphatic acyclic linear primary alcohols, aldehydes and acids food additives of chain length C1-18. Acetic acid was also evaluated by EFSA (2008; 2009; 2012; 2013) and additional information was available from notifications to ECHA under REACH (ECHA c2007-2017). It should be noted that available information on acetic acid, in particular for sub-chronic and chronic exposures, is focused primarily on acetic acid at concentrations consistent with its use in foods and self-care products (e.g., acetic acid concentrations of around 3% to 10%).
Acetic acid is rapidly metabolized to water and carbon dioxide, is produced endogenously and reacts with enzymes to form acetyl-CoA, and is utilised as an energy source in the body (WHO 1998; EFSA 2008, 2009). Acute oral LD50 values from gavage studies are >3000 mg/kg bw (WHO 1998; EFSA 2008). The acute dermal and inhalation toxicity is associated with irritation effects and corrosivity at higher concentrations of acetic acid than are present in food and products available to consumers (ECHA c2007-2017; EFSA 2012; NIOSH 1992). Concentrations of 10% to 25% acetic acid are considered to be irritant, and concentrations greater than 25% are considered corrosive (EFSA 2012; NIOSH 1992). Site of contact effects are not a focus of this assessment as chemical products used by consumers are already regulated vis-a-vis these types of effects (Canada 2001).
Acetic acid is listed on the Generally Recognized As Safe (GRAS) list of food additives in the USA (US FDA 2018). The WHO, under the Joint FAO/WHO Expert Committee on Food Additives (JECFA), also assigned an Accepted Daily Intake (ADI) for acetic acid of “not limited” in its most recent review (WHO 1998). An ADI of “not limited” is assigned to substances considered to have very low toxicity, in particular, those considered normal constituents of food or metabolites in humans (WHO 1974). In EFSA (2013) it was concluded that “based on the widespread presence of acetic acid in human foods, together with the fact that it is a normal metabolite in humans and animals, the establishment of an acceptable daily intake (ADI) and acute reference dose (ARfD) for oral intake of acetic acid by consumers is not considered necessary. In an assessment of the safety and efficacy of acetic acid as a preservative for animal feed (all species), EFSA (2012) concluded that a maximum concentration of 2500 mg/kg of acetic acid in complete animal feed, and 1000 mg/L of acetic acid in drinking water are safe and that these levels be set based on available toxicity studies conducted on dogs and chickens.
Repeated dose toxicity
WHO (1998) specified a NOEL equivalent to 350 mg/kg bw/day acetic acid from a study in male rats administered sodium acetate orally (via gavage) for 63 days (Pardoe 1952). Lamb and Evard (1919) conducted a study on pigs administered acetic acid in feed starting at 155 mg/kg bw/day, with doses increased every 10-30 days, up to the final dose of 450 mg/kg bw/day. The pigs were then maintained on 450 mg/kg bw/day for an additional 3 months, with no treatment-related effects observed. The study pre-dates established guidelines and has several limitations based on the study design (e.g., pre-dates relevant guidelines, small number of animals from same litter). In a study reported in EFSA (2008) by Wysokinska (1952) rats were administered 10% acetic acid via gavage for 90 days at 0 and 1500 mg/kg bw/day. At 1500 mg/kg bw/day there were a number of effects observed, including, but not limited to, gastric lesions, mortality, decreased body weight gain, decreased urine pH, reduced blood cell counts and haemoglobin content. The results of the study were determined to be insufficient for the purposes of deriving a NOAEL (EFSA 2008).
In a more recent study, Kondo et al. (2001) evaluated the toxicity of acetic acid as part of an investigation into the potential effects of vinegar on blood pressure. In the study, groups of 6 spontaneously hypertensive rats were administered 6% acetic acid mixed in with their diets, either via a prepared acetic acid solution, or a commercial rice vinegar product, for 8 weeks. A control group was also included in the study. Only one dose was administered which was estimated to be equivalent to 290 mg/kg bw/day of acetic acid by the study authors. A number of parameters were measured by study authors, including blood pressure, heart rate, body weight and parameters related to the diet (i.e., food and water consumption). Urine was collected every two weeks and at the end of the study, blood was collected for determination of renin, angiotensin II, aldosterone, and prostaglandin E2 levels. In addition, the heart, aorta and kidney were removed for measurements of angiotensin II measurements. There were decreases observed in plasma renin, angiotensin and aldosterone levels in treated rats, however, the decreases in angiotensin levels were not significant in the acetic acid solution or rice vinegar treated groups. The decreases in aldosterone levels were significant in the rice vinegar treatment group, but not the acetic acid solution treated group. The study authors attributed the decreases in the aldosterone levels observed in rice vinegar treated animals to the presence of amino acid and other brewing products that comprised the commercial rice vinegar product, as opposed to acetic acid. Decreases in the plasma renin levels were significant in both the acetic acid solution and rice vinegar treatment groups. The study authors concluded that acetic acid promoted calcium absorption which prevented the release of renin/ led to a reduction in renin activity, which resulted in decreased renin levels in the treated groups in this study. Fluctuations in blood pressure in both treated groups and controls were also observed. The study authors reported that no adverse effects were observed in treated animals administered 290 mg/kg bw/day (Kondo et al. 2001). The EFSA assessment of acetic acid (EFSA 2008) also indicated that this study was the “best approximation for a NOAEL in rats” based on the available animal studies.
Reproductive/developmental toxicity
No multi-generation reproductive toxicity studies were identified for acetic acid. EFSA (2008) indicated that these tests were not determined to be necessary “considering that all humans are exposed from various foods throughout life and that acetic acid is a physiological metabolite in all living organisms” (EFSA 2008). Furthermore, EFSA (2008) cited that additional multi-generation, postnatal or developmental toxicity studies are not required based on “human exposure to orally ingested acetic acid from various foods and the lack of evidence that such exposure is related to fertility problems and developmental deficiencies in humans”.
The developmental toxicity of acetic acid has been studied in mice, rats and rabbits (Morgareidge 1974). All 3 species were administered 5% acetic acid via gavage at doses of 0, 16, 74.3, 345 and 1600 mg/kg bw/day, during gestation days (GD) 6 to 15 for mice and rats which were sacrificed at GD 20, or during GDs 6 to 18 for rabbits which were sacrificed at GD 29. While acetic acid was administered to rabbits as part of this study, it was concluded, based on the study results, that rabbits were not an adequate animal model for this study design/test substance due to the high sensitivity of its gastrointestinal bacterial flora (Morgareidge 1974; EFSA 2008, 2013).
In rats, groups of 25 or 27 pregnant animals were administered acetic acid by oral gavage from days 6 through day 15 and were monitored on a daily basis for clinical signs and food consumption. Body weights were measured on days 0, 6, 11, 15 and 20 of pregnancy. At necropsy, (day 20) macroscopic examinations of the reproductive tract were conducted, and determinations were made on the number of implantation and resorption sites, live and dead foetuses, and foetal body weights. All foetuses were examined for external anomalies, with 2/3 and 1/3 of foetuses examined for skeletal and soft tissue abnormalities, respectively. The study found that no test related effects or abnormalities were observed at any dose (Morgareidge 1974).
In mice, the same examinations for developmental toxicity in the dams and measurements in the foetuses were conducted. A decrease in body weight was observed in the groups treated with the two highest doses (345 and 1600 mg/kg bw/day), and the decrease was observed to be dose-dependent. There were no effects on foetal weights at any dose level. There were, however, slight increases in the number of litters containing dead foetuses and incomplete ossification at the highest dose (1600 mg/kg bw/day). The authors reported that, in the absence of effects observed at these doses on foetal weight and litter size, these effects were a result of adverse effects observed in the dams. The study reported a NOAEL of 74 mg/kg bw/day in dams based on reduced body weight gain at the next dose of 345 mg/kg bw/day. Dam body weight gains were reduced by slightly over 9% at 345 mg/kg bw/day, and approximately 20% at 1600 mg/kg bw/day (Morgareidge 1974). Decreases in body weight gain at the two highest doses in this study are consistent with decreases in body weight gains (as well as food consumption, which was not reported in this study) observed in other studies at doses above 300 mg/kg bw/day (Wysokinska 1952; EFSA 2008, 2012, 2013)
Carcinogenicity
There is no evidence of genotoxicity for acetic acid. WHO (1998) examined the genotoxicity of acetic acid, along with related substances, and concluded that the low pH conditions in a number of the in vitro assays resulted in false-positives. Consequently, there are some in vitro test results for acetic acid with positive genotoxicity results (Morita et al. 1990; Sipi et al. 1992). However, results for acetic acid from other in vitro assays conducted at higher, more physiologically relevant pH levels have produced negative results (Morita et al. 1990). EFSA (2013) concluded that, while there was insufficient information on the genotoxicity of acetic acid in vivo, it was “unlikely to be mutagenic in vivo for sufficiently buffered systems”. There is limited information on the carcinogenicity of acetic acid, and the studies that are available, have limitations (e.g., not guideline compliant). A 32 week dermal study in CD-1 mice, with acetic acid applied once a week at 30 mg/animal, did not identify any carcinogenic effects (Slaga et al. 1975). In an oral gavage study in rats, 3% acetic acid administered 3 times per week for 8 months was not found to induce tumours. However, hyperplasia was observed in all animals in the forestomach and oesophagus, which the authors attributed to localized, site-of-contact effects (Alexandrov et al. 1989). EFSA (2013) also reviewed these studies, and concluded that, based on these results and on the results from genotoxicity assays, acetic acid has “no carcinogenic potential”.
6.2 Exposure assessment
There is no data on the presence of acetic acid in Canadian water or ambient air. Between 1994 and 2004 the National Research Council (NRC) of Canada tested the emission rates of 90 volatile organic compounds, including acetic acid, from 58 building materials. Acetic acid was emitted from 55% of the materials tested. NRC (2011) reported the occurrence of acetic acid from a field study of indoor air and house dust in 50 homes in Quebec City (QC). Acetic acid was detected in indoor air in more than half of the homes tested (median concentration, 2.2 µg/m3; n = 48, detection limit not reported) but was not reported in house dust (NRC 2011).
Dietary exposure to the general population to acetic acid is expected to occur as the substance is used as vinegar (4.1% to 12.3% aqueous acetic acid) and is a food additive permitted for use in a variety of foods at levels consistent with good manufacturing practices. Acetic acid may also be used as a component in incidental additives (cleaners and sanitizers) used on food contact surfaces without a subsequent potable water rinse; however, exposure from this use is considered negligible. Exposure from its potential use as a component in food packaging materials, where it is used as a chemical reagent in the manufacturing of inner layer films in multilayer structure, is not expected as there is no direct contact with food (personal communication, emails from the Food Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated July 16-25, 2018; unreferenced).
Oral and/or dermal exposure to the general population to acetic acid may result from the use of certain self-care products, and products available to consumers that contain acetic acid. The concentrations of acetic acid in products available to consumers identified in Table 4-2 was reported to be 6% or less. Products listed in Table 4-2 and certain self-care products (e.g., skin exfoliants, medicated shampoos, deodorants, makeup and nail polish) are applied topically while many of the medications and licensed natural health products listed in Table 4-3 are to be consumed orally. Due to the high vapour pressure of acetic acid, the use of these products in a cream, liquid or aerosol form may also result in inhalation exposures.
As acetic acid is considered to be of low hazard potential (see Section 6.1), quantitative estimates of exposure to the general population were not derived.
6.3 Uncertainties in evaluation of risk to human health
| Key sources of uncertainty | Impact |
|---|---|
| Studies are not available on the magnitude and/or importance of effects on hormone levels from acetic acid exposure observed in spontaneously hypertensive rats to non-hypertensive individuals | +/- |
| There are no carcinogenic studies on acetic acid of adequate duration, and the studies that exist have limitations (e.g. study design, GLP compliance) | +/- |
| There are no multi-generation reproductive studies for acetic acid | +/- |
+ = uncertainty with potential to cause over-estimation of exposure/risk,
- = uncertainty with potential to cause under-estimation of exposure risk,
+/- = unknown potential to cause over- or under-estimation of risk.
6.4 Characterization of risk to human health
On the basis of the available information, no serious adverse effects or organ-specific toxicity were observed in experimental animals. Effects observed were primarily associated with site-of-contact effects, and loss of appetite. This is in line with the approach for identifying substances with low human health hazard potential described in Health Canada (2017).
Given the low hazard potential of this substance, quantitative exposure estimates were not derived and the risk to human health is considered to be low.
7. Conclusion
Considering all available lines of evidence presented in this draft screening assessment, there is low risk of harm to the environment from acetic acid. It is proposed to conclude that acetic acid does not meet the criteria under paragraphs 64(a) or (b) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
On the basis of the information presented in this draft screening assessment, it is proposed to conclude that acetic acid does not meet the criteria under paragraph 64(c) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore proposed to conclude that acetic acid does not meet any of the criteria set out in section 64 of CEPA.
References
Alexandrov VA, Novikov AI, Zabezhinsky MA, Stolyarov VI and Petrov AS. 1989. The stimulating effect of acetic acid, alcohol and thermal burn injury on oesophagus and forestomach carcinogenesis induced by n-nitrososarcosin ethyl ester in rats. Cancer Letters Vol 47, pp179-185. (as referenced in ECHA c2007-2017).
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C. 1999, c.33. Canada Gazette Part III, vol. 22, no. 3.
Canada. 2001. Consumer Chemicals and Containers Regulations. SOR/2001- 269.
Daubert TE, Danner RP. 1985. Data compilation tables of properties of pure compounds. New York (NY). Design Institute for Physical Property Data, American Institute of Chemical Engineers. [cited in EPI Suite c2000-2012].
[ECCC] Environment and Climate Change Canada. 2016a. Science approach document: ecological risk classification of organic substances. Ottawa (ON): Government of Canada.
[ECCC] Environment and Climate Change Canada. 2016b. Data used to create substance-specific hazard and exposure profiles and assign risk classifications in the Ecological Risk Classification of organic substances. Gatineau (QC). Available from: substances@ec.gc.ca.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. [modified 2017 Mar 12]. Categorization. Ottawa (ON): Government of Canada. [accessed 2018 Sep 18].
[EFSA]. European Food Safety Authority. 2008. Initial risk assessment provided by the rapporteur Member State Germany for the existing active substance Acetic Acid of the fourth state of the review programme referred to in Article 8(2) of Council Directive 91/414/EEC. Vol 3, Annex B, part 2, B.6. August 2008. [requested 2018 Nov 30].
[EFSA]. European Food Safety Authority. 2009. Calcium acetate, calcium pyruvate, calcium succinate, magnesium pyruvate magnesium succinate and potassium malate added for nutritional purposes to food supplements[PDF]. Scientific Opinion of the Panel on Food Additives and Nutrient Sources added to Food (ANS). EFSA Journal. 1088, 1-25 [accessed 2018 Nov 30].
[EFSA]. European Food Safety Authority. 2012. Scientific Opinion on the safety and efficacy of acetic acid, sodium diacetate and calcium acetate as preservatives for feed for all animal species. EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP). EFSA Journal.10(2):2571. [accessed 2018 Nov 30].
[EFSA]. European Food Safety Authority. 2013. Conclusion on the peer review of the pesticide risk assessment of the active substance acetic acid. EFSA Journal. 11(1):3060. [accessed 2018 Nov 30].
[EPI Suite] Estimation Program Interface Suite for Microsoft Windows [estimation model]. c2000-2012. Ver. 4.11. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
[ECHA] European Chemicals Agency. c2007-2017. Registered substances database; registration dossier CAS RN 64-19-7. Helsinki (FI): ECHA. [accessed 2018 October 25].
Health Canada. 2017. Science approach document for substances with low human health hazard potential. Ottawa (ON): Government of Canada.
[HPD] Household Product Database. 2018. Health and safety information on household products [database on the internet]. [accessed 2018 September 26].
Johnson BJ, Betterton EA, Craig D. 1996. Henry’s Law coefficients of formic and acetic acids [pdf]. J Atmos Chem 24: 113-119.
Kondo S, Tayama K, Tsukamoto Y, Ikeda K and Yamori Y. 2001. Antihypertensive effects of acetic acid and vinegar on spontaneously hypertensive rats. Biosci. Biotechnol. Biochem Vol 65, (12), pp 2690-2694. (as referenced in ECHA c2007-2017).
Lamb AR & Evard JM. 1919. The acid-base balance in animal nutrition. 1. The effect of certain organic and mineral acids on the growth, well-being and reproduction of swine. J Biol. Chem. Vol 37 pp 317-328. (as referenced in ECHA c2007-2017).
[LNHPD] Licensed Natural Health Products Database [database]. [modified 2018 February]. Ottawa (ON): Government of Canada. [accessed 2018 July 11].
Morgareidge, K. 1974. Teratologic evaluation of FDA 71-78 (Apple Cider Vinegar; Acetic Acid; table strength 5%) in mice, rats and rabbits. Food and Drug Research laboratories. FDABF-GRAS-241. PB234869.(As cited in EFSA 2008).
Morita, T., Takeda, K., & Okumura, K. (1990) Evaluation of clastogenicity of formic acid, acetic acid and lactic acid on cultured mammalian cells. Mutat. Res., 240(3): 195-202. (as cited in WHO 1998)
[NIOSH 1992]. National Institute of Occupational Safety and Health. Occupational Health and Safety Guideline for Acetic Acid[PDF]. Center for Disease Control and Prevention (CDC). US Department of Health and Human Services. 1992. [accessed 2018 October 25].
[NRC] National Research Council of Canada. 2011. Chemicals management plan Health Canada moderate priorities: Data gathering on chemicals released to indoor air of residences from building materials and furnishings. Contract Report to Health Canada. Ottawa (ON): Health Canada.
[OECD] Organisation for Economic Co-operation and Development. 2004. The 2004 OECD list of high production volume chemicals [PDF]. Paris (FR): OECD, Environment Directorate. [accessed 2018 September 28].
Pardoe, S.U. (1952) Renal functioning in lead poisoning. Br. J. Pharmacol., 7: 349-357.(As cited in WHO 1998)
Rumble JR, editor. 2018. CRC Handbook of chemistry and physics. 99th edition. Cleveland (OH): CRC Press.
[SDS] Safety Data Sheet. 2013. Omega paw solutions – ITCH. [accessed 2016 April 11].
[SDS] Safety Data Sheet. 2015a. Top job basic cleaning vinegar [PDF]. [accessed 2018 September 26].
[SDS] Safety Data Sheet. 2015b. 66 BR clear RTV silicone adhesive sealant 3 oz[PDF]. [accessed 2016 June 1].
[SDS] Safety Data Sheet. 2015c. HTH natural clarifier. [accessed 2016 April 11].
[SDS] Safety Data Sheet. 2016. 66 BR clear RTV silicone adhesive sealant 3 oz. [accessed 2018 September 26].
Serjeant EP, Dempsey B. 1979. Ionization Constants of Organic Acids in Aqueous Solution. Oxford (UK): Pergamon. [cited in EPI Suite c2000-2012].
Servant J, Kouadio G, Cros B, Delmas R. 1991. Carboxylic monoacids in the air of Mayombe Forrest (Congo): Role of the forest as a source or sink [pdf]. J Atmos Chem 12: 367-380.
Sipi, P., Jarventaus, H., & Norppa, H. (1992) Sister-chromatid exchanges induced by vinyl esters and respective carboxylic acids in cultured human lymphocytes. Mutat. Res., 279: 75-82. (as referenced in WHO 1998)
Slaga T, Bowden G & Boutwell R. 1975. Acetic acid, a potent stimulator of mouse epidermal macromolecular synthesis and hyperplasia but with weak tumour-promoting ability. Nat. Cancer Inst., Vol 55, pp 983-987 (as cited in ECHA c2007-2017).
Mrogareidge, K. 1974 (Unpublished). Teratologic Evaluation of FDA 71-78 (Apple Cider Vinegar; Acetic acid; Table Strength 5%) in Mice, Rats and Rabbits. Food and Drug Laboratories, Waverly, N.Y. FDABF-GRAS-241, NTIS, PB234869; FDA 1766c (October 1973) (as cited in EFSA 2008).
[US EPA] United States Environmental Protection Agency. 2018. High production volume chemical list. [accessed 2018 September 28].
[US FDA]. United States Food and Drug Administration. 2018. Code of Federal Regulations. Title 21: Volume 3. Section § 184.1005 - Acetic acid. Part 184 – Direct Food Substances Affirmed as Generally Recognized As Safe (GRAS). Subpart B - Listing of Specific Substances Affirmed as GRAS. [47 FR 27814, June 25, 1982]. Date: 2018-04-01. [accessed 2018 October 16].
[WHO]. World Health Organization (WHO). 1974. Toxicological evaluation of some food additives including anticaking agents, antimicrobials, antioxidants, emulsifiers and thickening agents. Acetic Acid and its Potassium and Sodium Salts. WHO Food additives Series 5. Prepared for the Seventeenth meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). World Health Organization, Geneva. 1974. [accessed 2018 October 25].
[WHO]. World Health Organization (WHO). 1988. Safety Evaluation of Certain Food Additives and Contaminants. Saturated Aliphatic Acyclic Linear Primary Alcohols, Aldehydes, and Acids. WHO Food additives Series 40. Prepared for the Forty-ninth meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). World Health Organization, Geneva. 1998. [accessed 2018 October 25].
Wysokinska, Z. 1952. Comparison of the effect of lactic acid and acetic acid on the rat organism. [Porownanie Dzialania Kwasu Mlekowego I Octowego na Organizm Szczuru]. Roczniki Panstwowego ZakladuHig. 3:273-292. Polish (English summary translation as cited in EFSA 2008).
Yalkowsky SH, He Y, Jain P. 2010. Handbook of aqueous solubility data. 2nd edition. Boca Raton (FL): CRC Press.
