Draft screening assessment ketones group
Official title: Draft screening assessment ketones group
Chemical Abstracts Service Registry Numbers
78-93-3, 107-87-9, 108-10-1, 110-12-3, 123-42-2, 513-86-0, 123-54-6, 431-03-8, 600-14-6, 141-79-7
Environment and Climate Change Canada
Health Canada
January 2019
Synopsis
Pursuant to section 68 or 74 of the Canadian Environmental Protection Act, 1999 (CEPA), the Minister of the Environment and the Minister of Health have conducted a screening assessment of ten substances referred to collectively as the Ketones Group. Substances in this group were identified as priorities for assessment as they met categorization criteria under subsection 73(1) of CEPA or were considered a priority on the basis of other human health concerns. The Chemical Abstracts Service Registry Numbers (CAS RNFootnote 1 ), their Domestic Substances List (DSL) names and their common names and acronyms are listed in the table below.
CAS RN |
Subgroup |
Domestic Substances List name |
Common name (acronym) |
|---|---|---|---|
78-93-3 |
1 |
2-Butanone |
Methyl ethyl ketone (MEK) |
107-87-9 |
1 |
2-Pentanone |
Methyl propyl ketone (MPK) |
108-10-1 |
2 |
4-Methyl-2-pentanone |
Methyl isobutyl ketone (MIBK) |
110-12-3 |
2 |
2-Hexanone, 5-methyl |
Methyl isoamyl ketone (MIAK) |
123-42-2 |
2 |
4-Hydroxy-4-methyl-2-pentanone |
Diacetone alcohol (DAA) |
431-03-8 |
3 |
2,3-Butanedione |
Diacetyl |
513-86-0 |
3 |
2-Butanone, 3-hydroxy |
Acetoin |
600-14-6a |
3 |
2,3-Pentanedione |
2,3-Pentanedione (2,3-PD) |
123-54-6a |
Individual |
2,4-Pentanedione |
2,4-Pentanedione (2,4-PD) |
141-79-7 |
Individual |
4-Methyl-3-penten-2-one |
Mesityl oxide (MO) |
aThis substance was not identified under subsection 73(1) of CEPA but was included in this assessment as it was considered a priority on the basis of other human health concerns.
All ten substances in the Ketones Group are commercially produced and are also naturally present in the environment in various plants and/or food items or produced by microbes and other organisms. Several of the ketones are also produced endogenously in humans including MEK, diacetyl and acetoin. MEK, MPK and MIBK have been detected in breast milk. According to information reported in response to surveys under section 71 of CEPA, only DAA (23 000 kg) and 2,3-PD (1200 kg) were reported to be manufactured in Canada in 2011. Reported imports in Canada for these ketones ranged between 100 kg (for acetoin) and 6 000 000 kg (for MEK) in 2011. In the same year, no Canadian manufacturing or importing activities were reported for MO above the reporting threshold of 100 kg.
In general, ketones are primarily used as solvents in various products including products available to consumers such as paints, coatings and adhesives, and in numerous industrial applications as chemical intermediates and solvents among others. They may also be used as food flavouring agents, in cosmetics and as formulants in pest control products.
The ecological risks of the substances in the Ketones Group were characterized using the Ecological Risk Classification of organic substances (ERC). The ERC is is a risk-based approach that employs multiple metrics for both hazard and exposure based on weighted consideration of multiple lines of evidence for determining risk classification. Hazard profiles are established based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Metrics considered in the exposure profiles include potential emission rate, overall persistence, and long-range transport potential. A risk matrix is used to assign a low, moderate or high level of potential concern for substances based on their hazard and exposure profiles. The ERC identified the ten substances in this assessment as having low potential to cause ecological harm.
Considering all available lines of evidence presented in this draft screening assessment, there is low risk of harm to the environment from MEK, MPK, MIBK, MIAK, DAA, diacetyl, acetoin, 2,3-PD, 2,4-PD, and MO. It is proposed to conclude that MEK, MPK, MIBK, MIAK, DAA, diacetyl, acetoin, 2,3-PD, 2,4-PD, and MO do not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
Several of these ketones have been previously reviewed internationally; these reviews and assessments were used to inform the health effects characterization in this screening assessment.
For the human health risk assessment, eight of the substances in this group have been addressed under three subgroups with the remaining two substances addressed individually. For subgroup 1, the critical health effects include developmental effects for MEK and decreased body weight gain for both MEK and MPK. The general population in Canada is exposed to MEK and MPK from air and from food (primarily natural occurrence), and from products available to consumers including cosmetics, paints and do-it-yourself products for MEK and paint products for MPK. A comparison of levels of MEK and MPK that Canadians can be exposed to in environmental media and food with levels associated with adverse effects in laboratory studies results in margins that are considered adequate to address uncertainties in exposure and health effects data used to characterize risk. However, the margins between exposures to MEK in some products available to consumers, namely lacquer and adhesive remover, paint products and PVC cement/primer and critical health effect levels are considered potentially inadequate to account for uncertainties in the exposure and health data used to characterize risk. Given the low acute toxicity of MPK and absence of developmental effects via inhalation, there are no concerns related to the presence of MPK in products available to consumers.
For subgroup 2 (MIBK, MIAK and DAA), the International Agency for Research on Cancer (IARC) considers MIBK to be in group 2B (“possibly carcinogenic to humans”), with “sufficient evidence” of carcinogenicity in laboratory animals. For non-cancer effects, effects on the liver and kidney as well as developmental effects were observed in laboratory studies. The general population of Canada may be exposed to MIBK, MIAK and DAA from environmental media and food (primarily from their natural occurrence), and from the use of products available to consumers, including cosmetics, markers, paints and do-it-yourself products. A comparison of estimated levels of exposure to MIAK and DAA and critical effect levels results in margins that are considered to be adequate to address uncertainties in exposure and health effects data used to characterize risk. However, for MIBK, the resulting margins associated with the use of various paint and wood lacquer products are considered to be potentially inadequate.
For subgroup 3 (diacetyl, 2,3-PD and acetoin), diacetyl was carcinogenic in laboratory studies. Non-cancer effects have also been observed such as effects on the respiratory tract for diacetyl. The general population of Canada is primarily exposed to diacetyl, 2,3-PD and acetoin from food (due to natural occurrence and use as a flavouring agent), and to diacetyl and 2,3-PD from use of a limited number of products available to consumers including cosmetics and air fresheners, respectively. A comparison of estimated levels of exposure to diacetyl, 2,3-PD and acetoin and critical effect levels results in margins that are considered to be adequate to address uncertainties in exposure and health effects data used to characterize risk.
The available health effects information on 2,4-PD indicates general systemic toxicity and developmental effects. 2,4-PD has shown some potential for genotoxicity but is not expected to be carcinogenic. The general population of Canada may be exposed to 2,4-PD from food (natural occurrence), and from the use of a limited number of products available to consumers, such as specialty coating products. Margins for levels of 2,4-PD in food are considered adequate. A comparison of estimated levels of exposure to 2,4-PD from use of a coating applied to a large surface area such as a trailer or a boat, and critical effect levels results in margins that are considered potentially inadequate to address uncertainties in exposure and health effects data used to characterize risk.
Canadians may be exposed to MO from its presence in air and food. MO is not expected to be carcinogenic or genotoxic. General systemic toxicity has been associated with exposure to MO in laboratory studies. Comparison of estimated levels of exposure to MO in environmental media and food and critical effect levels results in margins that are considered to be adequate to address uncertainties in exposure and health effects data used to characterize risk.
Therefore, on the basis of the information presented in this draft screening assessment, it is proposed to conclude that MEK, MIBK, and 2,4-PD meet the criteria under paragraph 64(c) of CEPA as they are entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
However, it is proposed to conclude that MPK, MIAK, DAA, diacetyl, 2,3-PD, acetoin and MO do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
Therefore, it is proposed to conclude that MEK, MIBK, and 2,4-PD meet one or more of the criteria set out in section 64 of CEPA.
Therefore, it is proposed to conclude MPK, MIAK, DAA, diacetyl, 2,3-PD, acetoin and MO do not meet any of the criteria set out in section 64 of CEPA.
MEK, and 2,4-PD are proposed to meet the persistence criteria but not the bioaccumulation criteria as set out in the Persistence and Bioaccumulation Regulations of CEPA.
MIBK is proposed to not meet the persistence or bioaccumulation criteria as set out in the Persistence and Bioaccumulation Regulations of CEPA.
1. Introduction
Pursuant to section 68 or 74 of the Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999), the Minister of the Environment and the Minister of Health have conducted a screening assessment of ten substances referred to collectively as the Ketones Group to determine whether these substances present or may present a risk to the environment or to human health. The substances in this group were identified as priorities for assessment as they met categorization criteria under subsection 73(1) of CEPA or were considered a priority on the basis of other human health concerns (ECCC, HC [modified 2017]).
The ecological risks of the substances in the Ketones Group were characterized using the ecological risk classification of organic substances (ERC) (ECCC 2016a). The ERC describes the hazard of a substance using key metrics including mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity and considers the possible exposure of organisms in the aquatic and terrestrial environments based on factors including potential emission rates, overall persistence and long-range transport potential in air. The various lines of evidence are combined to identify substances as warranting further evaluation of their potential to cause harm to the environment or as having a low likelihood of causing harm to the environment.
Some substances are assessed in subgroups due to similarities in chemical structure, properties and/or toxicity. Given the potential for these substances to be used in similar ways and applications, the potential for risk to human health is assessed using similar exposure assumptions across the group.
Some substances in the Ketones Group currently being evaluated have been reviewed internationally through the Organisation for Economic Cooperation and Development (OECD) Cooperative Chemicals Assessment Programme. OECD assessments undergo rigorous review (including peer-review) and endorsement by international governmental authorities. Health Canada and Environment and Climate Change Canada are active participants in this process, and consider these assessments reliable. Some of the substances have also been reviewed by the International Programme on Chemical Safety (IPCS), the United States Environmental Protection Agency (US EPA), the International Agency for Research on Cancer (IARC), and the US National Toxicology Program (NTP). Reviews conducted by these institutions are used to inform the health effects characterization in this screening assessment.
This draft screening assessment includes consideration of information on chemical properties, environmental fate, hazards, uses and exposures, including additional information submitted by stakeholders. Relevant data were identified up to April 2017. Additional data were submitted up to September 2017. Empirical data from key studies as well as some results from models were used to reach proposed conclusions. When available and relevant, information presented in assessments from other jurisdictions was considered.
Diacetyl, 2,3-PD and acetoin have been detected in measurable quantities in vaping products. Vaping products (also known as electronic cigarettes) may represent an additional source of exposure to these substances. The assessment of risk to the general population from this use, including risk relative to that associated with conventional cigarettes, and possible options to mitigate risk associated with these products is being addressed through a separate legislative and regulatory framework.
This draft screening assessment was prepared by staff in the CEPA Risk Assessment Program at Health Canada and Environment and Climate Change Canada and incorporates input from other programs within these departments. The human health portions of this assessment have undergone external review and/or consultation. Comments on the technical portions relevant to human health were received from Theresa Lopez, Jennifer Flippin and Joan Garey (TetraTech Inc.), and from D.L. Morgan (National Toxicology Program, National Institute of Environmental Health Sciences, USA). . The ecological portion of this assessment is based on the ERC document (published July 30, 2016), which was peer-reviewed and subject to a 60-day public comment period. While external comments were taken into consideration, the final content and outcome of the screening assessment remain the responsibility of Health Canada and Environment and Climate Change Canada.
This draft screening assessment focuses on information critical to determining whether substances meet the criteria as set out in section 64 of CEPA by examining scientific information and incorporating a weight of evidence approach and precautionFootnote 2 . This draft screening assessment presents the critical information and considerations on which the proposed conclusions are based.
2. Identity of substances
The ten substances assessed in this screening assessment are ketones with a general formula shown in Figure 1. The ketones in this assessment have been divided into three subgroups based on their chemical structure, properties and/or toxicity and two individual assessments.

Figure 1. General formula for ketones
The Chemical Abstracts Service Registry Numbers (CAS RNFootnote 3), Domestic Substances List (DSL) names and common names and/or acronyms for the individual substances in the Ketones Group are presented in Table 2‑1. A list of additional chemical names (e.g., trade names) is available from the National Chemical Inventories (NCI 2015).
Subgroup |
CAS RN |
DSL name (common name or acronym) |
Chemical structure and molecular formula |
Molecular weight (g/mol) |
|---|---|---|---|---|
1 |
78-93-3 |
2-butanone (methyl ethyl ketone; MEK) |
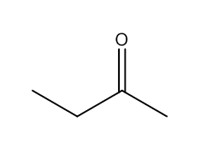 |
72.11 |
1 |
107-87-9 |
2-pentanone (methyl propyl ketone; MPK) |
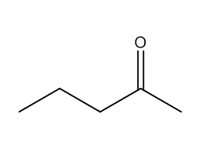 |
86.13 |
2 |
108-10-1 |
4-methyl-2-pentanone (methyl isobutyl ketone; MIBK) |
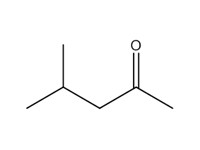 |
100.16 |
2 |
110-12-3 |
2-hexanone, 5-methyl- (methyl isoamyl ketone; MIAK) |
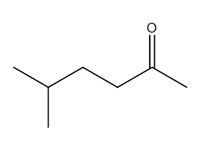 |
114.19 |
2 |
123-42-2 |
4-hydroxy-4-methyl-2-pentanone (diacetone alcohol; DAA) |
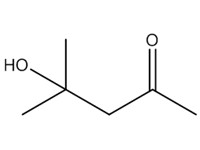 |
116.16 |
3 |
431-03-8 |
2,3-butanedione (diacetyl) |
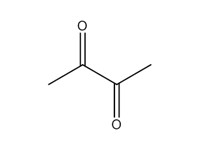 |
86.09 |
3 |
600-14-6 |
2,3-pentanedione (2,3-PD) |
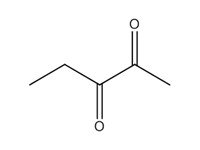 |
100.12 |
3 |
513-86-0 |
2-butanone, 3-hydroxy- (acetoin) |
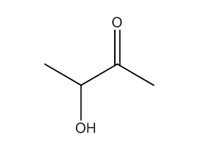 |
88.11 |
Individual |
123-54-6 |
2,4-pentanedione (2,4-PD) |
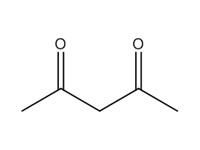 |
100.12 |
Individual |
141-79-7 |
4-methyl-3-penten-2-one (mesityl oxide; MO) |
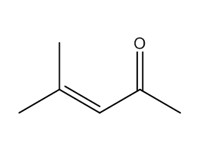 |
98.14 |
2.1 Selection of analogues and use of (Q)SAR models
A read-across approach using data from analogues and the results of (quantitative) structure-activity relationship ((Q)SAR) models, where appropriate, have been used to inform the human health assessments. Analogues were selected that were structurally similar and/or functionally similar to substances within this group (similar physical-chemical properties, toxicokinetics) and that had relevant empirical data that could be used to read across to substances with limited empirical health effects data. Details of the read-across data chosen to inform the human health assessments of each subgroup and individual are further discussed in the relevant sections of this report. Information on the identities and chemical structures of the analogues used to inform this assessment is presented in Table 2‑2. The applicability of (Q)SAR models was determined on a case-by-case basis.
Subgroup or substance being assessed |
CAS RN |
DSL or other name (common name or acronym) |
Chemical structure and molecular formula |
Molecular weight (g/mol) |
|---|---|---|---|---|
Subgroup 1 |
78-92-2 |
2-butanol | 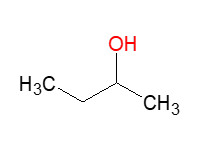 |
74.1 |
MO |
110-93-0 |
6-methyl-5-heptene-2-one (MHE) |
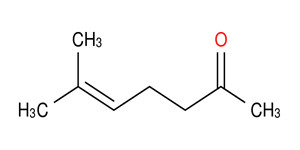 |
126.2 |
3. Physical and chemical properties
A summary of physical and chemical property data for the substances in the Ketones Group are presented in Table 3‑1 to 3-4. Additional physical and chemical properties are presented in ECCC (2016b).
Property |
MEK |
MPK |
Reference |
|---|---|---|---|
Physical state |
colourless liquid |
colourless liquid |
HSDB |
Melting point (°C) |
-85.9 |
-76.9 |
OECD 1997a, ChemIDplus 1993- |
Vapour pressure (Pa) |
10 332 |
4720 |
OECD 1997a, ChemIDplus 1993- |
Henry’s law constant (Pa·m3/mol) |
5.85 |
8.47 |
ATSDR 1992, ChemIDplus 1993- |
Water solubility (mg/L) |
276 000 |
43 000 |
OECD 1997a, ChemIDplus 1993- |
Log Kow (dimensionless) |
0.29 |
0.91 |
OECD 1997a, ChemIDplus 1993- |
Log Koc (dimensionless) |
0.55 |
1.87 [estimated] |
ATSDR 1992, HSDB 1983- |
Abbreviations:Kow, octanol–water partition coefficient; Koc, organic carbon–water partition coefficient
Property |
MIBK |
MIAK |
DAA |
Reference |
|---|---|---|---|---|
Physical state |
colourless liquid |
colourless, clear liquid |
colourless liquid |
OECD 1996, HSDB 1983- |
Melting point (°C) |
-84.7 |
-74 |
-44 |
OECD 1996, ChemIDplus 1993- |
Vapour pressure (Pa) |
2653 |
769 |
228 |
ChemIDplus 1993- |
Henry’s law constant (Pa·m3/mol) |
14.0 [estimated] |
16.2 [estimated] |
0.026 [estimated] |
ChemIDplus 1993- |
Water solubility (mg/L) |
19 000 |
5 400 |
1 000 000 |
ChemIDplus 1993- |
Log Kow (dimensionless) |
1.31 |
1.88 |
-0.34 [estimated] |
ChemIDplus 1993- |
Log Koc (dimensionless) |
2.08 [estimated] |
2.40 [estimated] |
1.32 [estimated] |
HSDB 1983- |
Abbreviations: Kow, octanol–water partition coefficient; Koc, organic carbon–water partition coefficient
Property |
Diacetyl |
2,3-PD |
Acetoin |
Reference |
|---|---|---|---|---|
Physical state |
greenish-yellow liquid |
Dark yellow to green yellow liquid |
Slightly yellow liquid or crystals |
HSDB 1983-, CDC 2016 |
Melting point (°C) |
-2.40 |
-29.38 [estimated] |
15.0 |
ChemIDplus 1993-, EPI Suite |
Vapour pressure (Pa) |
7572 |
4146 [estimated] |
359 [estimated] |
ChemIDplus 1993-, EPI Suite |
Henry’s law constant (Pa·m3/mol) |
1.35 |
0.674 [estimated] |
1.04 [estimated] |
ChemIDplus 1993-, EPI Suite |
Water solubility (mg/L) |
200 000 |
66 700 (at 15 deg C) |
1 000 000 |
ChemIDplus1993- |
Log Kow (dimensionless) |
-1.34 |
-0.85 [estimated] |
-0.36 [estimated] |
ChemIDplus 1993- |
Log Koc (dimensionless) |
-0.28 [estimated] |
-0.004 [estimated] |
0.3 [estimated] |
EPI Suite; HSDB 1983- |
Abbreviations: Kow, octanol–water partition coefficient; Koc, organic carbon–water partition coefficient
Property |
2,4-PD |
MO |
Reference |
|---|---|---|---|
Physical state |
colourless or slightly yellow liquid |
oily, colourless to light-yellow liquid |
HSDB 1983- |
Melting point (°C) |
-23.0 |
-59.0 |
ChemIDplus 1993- |
Vapour pressure (Pa) |
395 |
1095 |
ChemIDplus 1993- |
Henry’s law constant (Pa·m3/mol) |
0.238 [estimated] |
3.72 [estimated] |
ChemIDplus 1993- |
Water solubility (mg/L) |
166000 |
28 900 |
ChemIDplus 1993- |
Log Kow (dimensionless) |
0.34 – 0.4 |
1.2 – 1.7 |
ChemIDplus 1993-, OECD 2001, OECD 1997b |
Log Koc (dimensionless) |
1.54 [estimated] |
1.04 |
EPI Suite; OECD 1997b |
Abbreviations: Kow, octanol–water partition coefficient; Koc, organic carbon–water partition coefficient
4. Sources and uses
All ten substances in the Ketones Group are naturally present in the environment in various plants and/or food items or produced by microbes and other organisms but may also be synthetically produced (Burdock 2010, VCCEP 2003, O’Donoghue 2012a,b). MEK, diacetyl and acetoin are also produced endogenously in humans (VCCEP 2003, WHO 1999a, NTP 2007a).
All of the substances in the Ketones Group, except MEK, have been included in a recent survey issued pursuant to section 71 of CEPA (Environment Canada 2012). Methyl ethyl ketone was surveyed pursuant to a CEPA section 71 in 2001. Reported manufactured quantities for MEK ranged between 1 million and 10 million kg in the year 2000; however, manufacturing of this substance in Canada ceased in 2002 (Environment Canada 2001). Reported import quantities of MEK into Canada from the year 2000 were greater than 10 million kg. According to the Canadian International Merchandise Trade Database (CIMT), between 2011 and 2016, annual average imports of MEK into Canada were approximately 4.9 million kg (CIMT 2017). Table 4‑1 presents a summary of the reported total manufacture and total import quantities for the Ketones Group.
Common name |
Total manufacturea (kg) |
Total importsa (kg) |
|---|---|---|
MEK |
- |
6 042 865 (data for 2011; CIMT) |
MPK |
- |
1 097 844 |
MIBK |
- |
1 241 783 |
MIAK |
- |
35 906 |
DAA |
23 000 |
265 529 |
Diacetyl |
- |
1 430 |
2,3-PD |
1 200 |
- |
Acetoin |
- |
100 – 1 000 |
2,4-PD |
- |
100 000 – 1 000 000 |
MO |
- |
- |
a Values reflect quantities reported in response to surveys conducted under section 71 of CEPA (Environment Canada 2012) except for MEK. See survey for specific inclusions and exclusions (Schedules 2 and 3).
Table 4‑2 presents a summary of the major uses of Ketones Group according to information reported pursuant to section 71 surveys under CEPA (Environment Canada 2001, 2013) and Table 4‑3 presents additional uses identified in Canada.
Major Uses |
Subgroup 1a |
Subgroup 2 |
Subgroup 3 |
Individual |
|---|---|---|---|---|
Paints and Coatings |
MEK, MPK |
MIBK, MIAK, DAA |
N/A |
2,4-PD |
Food and Beverage |
N/A |
N/A |
2,3-PD, Acetoin |
N/A |
Agricultural Products, mixtures or manufactured items (non-pesticidal) |
MEK |
N/A |
Diacetyl |
N/A |
Adhesives and Sealants |
MEK, MPK |
MIBK, DAA |
N/A |
2,4-PD |
Ink, Toner and Colourants |
MEK, MPK |
MIBK, DAA |
N/A |
N/A |
Automotive, Aircraft and Transportation |
MEK, MPK |
MIBK, DAA |
N/A |
N/A |
Plastic and Rubber materials not otherwise covered |
N/A |
MIBK |
N/A |
2,4-PD |
Electrical and Electronics |
N/A |
MIBK, DAA |
N/A |
N/A |
Floor Coverings |
MEK |
MIBK |
N/A |
N/A |
Cleaning and Furnishing Care |
MEK |
DAA |
N/A |
N/A |
Personal Care |
N/A |
DAA |
2,3-PD, Acetoin |
N/A |
Toys, Playground and Sporting Equipment |
N/A |
DAA |
N/A |
N/A |
Otherb |
MEK, MPK |
MIBK, DAA |
Diacetyl |
N/A |
Abbreviations: N/A, Not Applicable.
a Results for MEK are from uses in 2000 and may no longer be relevant.
b Other refers to minor uses and/or uses that cannot be disclosed as a result of confidentiality claims.
Use |
Subgroup 1 |
Subgroup 2 |
Subgroup 3 |
Individual |
|---|---|---|---|---|
Food additivea |
MEK |
N |
N |
N |
Food packaging materials a |
MEK, MPK |
MIBK, DAA |
N |
2,4-PD |
Incidental additivea |
MEK |
N |
N |
N |
Internal Drug Product Database as medicinal or non-medicinal ingredients in final Pharmaceutical, Disinfectant or Veterinary drug products in Canadab |
N |
N |
N |
N |
Natural Health Products Ingredients Databasec |
MEK, MPK |
MIBK, DAA |
Diacetyl, Acetoin, 2,3-PD |
MO |
Licensed Natural Health Products Database as medicinal or non-medicinal ingredients in natural health products in Canadad |
MEK |
MIBK |
N |
N |
List of Prohibited and Restricted Cosmetic Ingredientse |
N |
N |
N |
N |
Notified to be present in cosmetics, based on notifications submitted under the Cosmetic Regulations to Health Canadaf |
MEK |
DAA |
N |
N |
Formulant in pest control products registered in Canadag |
MEK |
MIBK, MIAK, DAA |
Diacetyl, Acetoin, 2,3-PD |
N |
Abbreviations: N, No
a Personal communication, e-mail from Food Directorate (FD), Health Canada (HC) to Existing Substances Risk Assessment Bureau (ESRAB), Health Canada (HC), dated Aug. 18, 2016; unreferenced)
b DPD [modified 2016]; Personal communication, e-mail from Therapeutic Products Directorate (TPD), HC to ESRAB, HC, dated August 3, 2016; unreferenced)
c NHPID [modified 2018]
d LNHPD [modified 2018]
e Health Canada [modified 2015a]
f Personal communication, e-mail from Consumer Product Safety Directorate (CPDS), HC to ESRAB, HC, dated August 5 and 8, 2016; unreferenced)
g Personal communication, e-mail from Pest Management Regulatory Agency (PMRA), HC to ESRAB, HC, dated July 28, 2016; unreferenced)
In general, ketones are primarily used as solvents in various products including products available to consumers, and in numerous industrial applications as chemical intermediates and solvents among others (O’Donoghue 2012a,b, Kirk-Othmer). MEK is listed as a permitted food additive in natural extractives and in spice extracts as prescribed in Health Canada’s List of Permitted Carrier or Extraction Solvents, incorporated by reference in its respective Marketing Authorization issued under the Food and Drugs Act. MEK, MPK, MIBK, DAA, and 2,4-PD may be used in non-food contact food packaging applications in Canada. MEK is also used as a solvent in non-food contact cleaners in the food industry. In addition, 7 of the ketones in this group were identified as potentially being used as food flavouring agents (personal communication, email from FD, HC to ESRAB, HC, dated Aug 18, 2016; unreferenced).
MEK and MIBK are listed in the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) guideline as class 3 residual solvents (solvent with low toxic potential) used in the manufacture of pharmaceuticals. The final drug product is permitted to contain up to 5000 ppm of residual solvent (personal communication, e-mail fromBiologics and Genetic Therapies Directorate, HC to ESRAB, HC, dated 2016 Aug 5; unreferenced).
MEK is classified as a natural health product (NHP) substance, with a medicinal role, falling under Schedule 1, item 2 (an isolate) to the Natural Health Products Regulations, as well as with a non-medicinal role for topical use as a denaturant or oral use as a flavour enhancer. MPK, 2,3-PD, and MO are also listed with a non-medicinal role for oral use as flavour enhancers; MIBK is listed with a non-medicinal role for oral use as a flavour enhancer or topical use as a denaturant; DAA is listed with a non-medicinal role for topical use as fragrance ingredient or solvent. Acetoin is listed with a non-medicinal role for oral use as flavour enhancer or for topical use as a fragrance ingredient. Only MEK and MIBK are listed in the Licensed Natural Health Products Database as being present in a limited number of currently licensed topical NHPs in Canada (NHPID [modified 2018]; LNHPD [modified 2018]; personal communication, e-mail from Natural and Non-prescription Health Products Directorate, HC to ESRAB, HC, dated Aug 16, 2016, unreferenced).
Based on notifications submitted under the Cosmetic Regulations to Health Canada, MEK and DAA are used in certain cosmetics in Canada, primarily in nail care products (personal communication, e-mail from CPSD, HC to ESRAB, HC, dated August 5 and 8, 2016, unreferenced). According to publicly available sources, diacetyl was identified in cosmetics in Canada (SDS 2008a, Canada Beauty Supply 2017), but there are no products notified with this ingredient.
MEK, MPK, MIBK, MIAK, DAA and 2,4-PD are used in products available to consumers including liquid and spray paints and coatings, automotive care products, do-it-yourself (DIY products) such as paint removers, adhesives and pipe sealants (Environment Canada 2012, Health Canada 2016, HPD 1993-). MEK, MPK and MIBK have been identified in emissions from various building materials (e.g., wood, carpet, insulation) and products available to consumers (e.g., paint, automotive cleaners, caulking) in Canada by the National Research Council of Canada (Won and Lusztyk 2011, Don and Yeng 2012, Won et al. 2013, Won et al. 2014, Won 2015).
MEK was identified in several different children’s products including tents/tunnels (Hansen et al. 2004), slimy toys (Svendsen et al. 2005), rubber figures and speed markers (Glensvig and Ports 2006) in Denmark. Under the state of Washington’s Children’s Product Safety Act (WSDE 2016), MEK was detected in various products intended for children 12 years and younger including kids’ crafts, baby furniture, baby bibs, pacifiers/teething rings, children’s toys and games, baby and children’s bedding and clothing as well as footwear and camping gear (WSDE 2016). MEK’s presence in these products was primarily as a contaminant but was also present as an adhesive, binding agent, coloration/pigment/dye/ink, component of plastic resin or polymer process, hardening, manufacturing additive, preservative, protective coating, reinforcement/strength, and as a solvent (WSDE 2016). MEK has also been measured in animal care products (Nylén et al. 2004), and adult toys (Nilsson et al. 2006) in Denmark.
Other sources of these ketones include vehicle exhaust (MEK, diacetyl) (IPCS 1993), cigarette smoke (MEK, diacetyl, 2,3-PD), as well as flavoured e-cigarette liquids (diacetyl, 2,3-PD, acetoin) (personal communication e-mails from Tobacco Control Directorate, HC to ESRAB, HC, dated Aug. 15-18, 2016 and Nov 16, 2017 unreferenced) .
MO was not reported to be manufactured or imported into Canada in 2011 (Environment Canada 2013). Two of the ten substances, MEK and MIBK, are reportable under the National Pollutant Release Inventory (NPRI). Table 4‑4 summarizes the various types of releases from 2011 to 2015 (NPRI 2011-2015a,b).
Substance |
On-site releases to air |
On-site releases to water |
On-site releases to land |
Disposal on-site |
Disposal off-site |
Off-site recycling |
|---|---|---|---|---|---|---|
MEK |
1105 – 1362 |
5.4 – 20 |
0 – 0.149 |
0.802 – 46 |
863 – 1563 |
1441 – 2616 |
MIBK |
199 – 243 |
0.025 – 1.9 |
0 – 0.049 |
1.3 – 20 |
105 – 207 |
243 – 316 |
5. Environmental fate and behaviour
5.1 Environmental persistence
MEK, DAA, diacetyl, 2,3-PD, and 2,4-PD may be persistent in air, but are not expected to be persistent in water, sediment or soil according to models used in ERC (ECCC 2016b). MPK, MIBK, MIAK, acetoin and MO are not expected to be persistent in air, water, sediment or soil according to models used in ERC (ECCC 2016b).
5.2 Potential for bioaccumulation
Based on low Kow and low bioconcentration factors (ECCC 2016b) MEK, MPK, MIBK, MIAK, DAA, diacetyl, acetoin, 2,3-PD, 2,4-PD, and MO are not expected to significantly bioaccumulate in organisms.
6. Potential to cause ecological harm
6.1 Characterization of ecological risk
The ecological risks of the substances in the Ketones Group were characterized using the Ecological Risk Classification of organic substances (ERC) (ECCC 2016a). The ERC is a risk-based approach that considers multiple metrics for both hazard and exposure based on weighted consideration of multiple lines of evidence for determining risk classification. The various lines of evidence are combined to discriminate between substances of lower or higher potency and lower or higher potential for exposure in various media. This approach reduces the overall uncertainty with risk characterization compared to an approach that relies on a single metric in a single medium (e.g., LC50) for characterization. The following summarizes the approach, which is described in detail in ECCC (2016a).
Data on physical-chemical properties, fate (chemical half-lives in various media and biota, partition coefficients, and fish bioconcentration), acute fish ecotoxicity, and chemical import or manufacture volume in Canada were collected from scientific literature, from available empirical databases (e.g., OECD QSAR Toolbox), and in response to surveys under section 71 of CEPA, or they were generated using selected quantitative structure-activity relationship (QSAR) or mass-balance fate and bioaccumulation models. These data were used as inputs to other mass-balance models or to complete the substance hazard and exposure profiles.
Hazard profiles were established based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Exposure profiles were also composed of multiple metrics including potential emission rate, overall persistence, and long-range transport potential. Hazard and exposure profiles were compared to decision criteria in order to classify the hazard and exposure potentials for each organic substance as low, moderate, or high. Additional rules were applied (e.g., classification consistency, margin of exposure) to refine the preliminary classifications of hazard or exposure.
A risk matrix was used to assign a low, moderate or high classification of potential risk for each substance based on its hazard and exposure classifications. ERC classifications of potential risk were verified using a two-step approach. The first step adjusted the risk classification outcomes from moderate or high to low for substances which had a low estimated rate of emission to water after wastewater treatment, representing a low potential for exposure. The second step reviewed low risk potential classification outcomes using relatively conservative, local-scale (i.e., in the area immediately surrounding a point-source of discharge) risk scenarios, designed to be protective of the environment, to determine whether the classification of potential risk should be increased.
ERC uses a weighted approach to minimize the potential for both over and under classification of hazard and exposure and subsequent risk. The balanced approaches for dealing with uncertainties are described in greater detail in ECCC 2016a. The following describes two of the more substantial areas of uncertainty. Error with empirical or modeled acute toxicity values could result in changes in classification of hazard, particularly metrics relying on tissue residue values (i.e., mode of toxic action), many of which are predicted values from QSAR models. However, the impact of this error is mitigated by the fact that overestimation of median lethality will result in a conservative (protective) tissue residue used for critical body residue (CBR) analysis. Error with underestimation of acute toxicity will be mitigated through the use of other hazard metrics such as structural profiling of mode of action, reactivity and/or estrogen binding affinity. Changes or errors in chemical quantity could result in differences in classification of exposure as the exposure and risk classifications are highly sensitive to emission rate and use quantity. The ERC classifications thus reflect exposure and risk in Canada based on what is believed to be the current use quantity, and may not reflect future trends.
Critical data and considerations used to develop the substance-specific profiles for the substances in the Ketones Group, and the hazard, exposure and risk classification results are presented in ECCC (2016b).
The hazard and exposure classifications for the substances in the Ketones Group are summarized in Table 6‑1.
Substance |
ERC hazard classification |
ERC exposure classification |
ERC risk classification |
|---|---|---|---|
MEK |
low |
high |
low |
MPK |
low |
low |
low |
MIBK |
low |
low |
low |
MIAK |
low |
low |
low |
DAA |
low |
high |
low |
Diacetyl |
low |
low |
low |
Acetoin |
low |
low |
low |
2,3-PD |
low |
low |
low |
2,4-PD |
low |
low |
low |
MO |
low |
low |
low |
On the basis of low hazard and low exposure classifications according to information considered under ERC, MPK, MIBK, MIAK, diacetyl, acetoin, 2,3-PD, 2,4-PD, and MO were classified as having a low potential for ecological risk. It is therefore unlikely that these substances will result in concerns for the environment in Canada.
According to information considered under ERC, MEK and DAA have a high exposure potential due to high reported use quantities in combination with a long half life in air. MEK and DAA were classified as having a low hazard potential and a low potential for ecological risk. It is therefore unlikely that these substances will result in concerns for the environment in Canada.
7. Potential to cause harm to human health
7.1 Assessment of subgroup 1 (MEK, MPK)
7.1.1 Exposure assessment of subgroup 1 (MEK, MPK)
Environmental media
MEK was monitored by the National Air Pollution Surveillance (NAPS) program, where mean concentrations measured between 2006 and 2010, from various sites across Canada ranged from 0.20 to 5.7 µg/m3 with 95th percentile concentrations ranging from 0.53 to 19.3 µg/m3 (ECCC 2017c). Ambient air concentrations for MEK and MPK were also measured in five Canadian air studies conducted in Windsor, Regina, Halifax, Edmonton and Ottawa (Health Canada 2010a, b, 2012, 2013, Zhu et al. 2005).
MEK concentrations in ambient air from these Canadian studies ranged from < 0.03 to 39.06 µg/m3 with geometric mean and 95th percentile concentrations ranging from 0.41 to 1.77 µg/m3 and from 0.78 to 5.53 µg/m3, respectively (Zhu et al. 2005; Health Canada 2010a, b, 2012, 2013). Ambient air concentrations for MPK from the five Canadian studies ranged from 0.002 to 14.38 µg/m3 with geometric mean and 95th percentile concentrations ranging from 0.055 to 0.49 µg/m3 and from 0.22 to 1.25 (Health Canada 2010a, b, 2012, 2013). The highest 95th percentile concentrations were used to estimate general population exposures (5.53 µg/m3 for MEK from the Windsor study, and 1.25 µg/m3 for MPK from the Regina study).
MEK and MPK were measured in the national Canadian indoor air study conducted in 2009-2011 as part of cycle 2 of the Canadian Health Measures Survey (CHMS). MEK was detected in 99% of the samples with a geometric mean concentration of 1.14 µg/m3 (weighted data at the household level) and a 95th percentile concentration of 9.76 µg/m3. MPK was detected in 97% of the samples with a geometric mean concentration (weighted data at the household level) of 0.36 µg/m3 and a 95th percentile concentration of 1.58 µg/m3 (Zhu et al. 2013).
Indoor air concentrations for MEK and MPK were also measured across the same five Canadian studies referred to above. Geometric mean concentrations of MEK in indoor air ranged from 1.51 to 9.81 µg/m3 with 95th percentile values ranging from 9.9 to 51.63 µg/m3 (Zhu et al. 2005; Health Canada 2010a, b, 2012, 2013). Geometric mean MPK concentrations in indoor air ranged from 0.089 to 2.77 µg/m3 with 95th percentile values ranging from 1.34 to 12.77 µg/m3 (Health Canada 2010a, b, 2012, 2013).
As a conservative approach, the highest 95th percentile concentrations of MEK and MPK across all indoor air studies were used to estimate general population exposures (51.63 µg/m3 for MEK from the Windsor study, and 12.77 µg/m3 for MPK from the Regina study).
MEK and MPK were also measured in personal air in the Windsor, Ontario air study (Health Canada 2010a). Personal air samples take into account exposures to MEK from both indoor and outdoor air from various locations including the home, office and during transit. The geometric mean and 95th percentile concentrations for MEK in the summer were 8.79 µg/m3 and 28.22 µg/m3, respectively which were higher than the samples collected during the winter (geometric mean of 4.24 µg/m3 and 95th percentile of 11.44 µg/m3). The geometric mean personal air concentration of MPK in the summer and winter were 2.91 µg/m3 and 1.44 µg/m3, respectively. The 95th percentile concentrations for MPK were 12.65 µg/m3 and 6.30 µg/m3 in the summer and winter, respectively (Health Canada 2010a).
MEK and MPK have very high water solubilities, but, based on their high vapour pressures and moderate Henry’s Law Constants, they are expected to rapidly volatilize from water (HSDB 1983-). In addition, MEK and MPK are expected to biodegrade in water making water unlikely to be a major source of human exposure (VCCEP 2003, HSDB 1983-). In 2000, MEK was not detected above the limit of detection of 0.99 µg/L in potable water (sample size not known) from Montreal, QC (Bernier 2000). No other Canadian data on the presence of MEK in water were identified. MEK was detected in a limited number of studies in the U.S. with low detection frequencies (0.5-0.8% of samples) and concentrations ranging from 0.6 to 340 µg/L (Grady and Casey, 2001, Delzer and Ivahnenko 2003, Grady 2003). No data were identified on the presence of MPK in water in Canada. MPK was detected in drinking water in Ottumwa, Iowa at a concentration of 0.1 µg/L (0.1 ppb) and was identified but not quantified in drinking water from 5 other US cities, and in England (HSDB 1983-). As a conservative approach, in order to estimate potential drinking water exposures to MEK and MPK, the detection limit of 0.99 µg/L from the Montreal study and the data from Iowa (0.1 µg/L) for MEK and MPK, respectively, were used.
For soils, one Canadian study was identified in which Golder Associates (1987) surveyed levels of MEK in soil in two parkland areas in the vicinity of southern Ontario petroleum refineries. A mean concentration of 5.78 µg/g (maximum: 25 µg/g) was found in 19 of 30 soil samples in which MEK was detected. Using the maximum concentration of MEK in soil resulted in general population exposures below 1 ng/kg-bw per day for all age groups; therefore exposure to MEK from soil is considered to be negligible. No information on the presence of MPK in soil or sediment was identified for Canada or elsewhere. ChemCAN was used to derive potential soil concentrations of MPK using the volume data from Table 4-1 (i.e., 1 097 844 kg). The estimated concentration of MPK in soil was 1.1 ng/kg and resulted in intakes less than 1 ng/kg-bw per day for the general population of Canada, which are considered to be negligible.
Estimates of exposure for MEK and MPK from environmental media ranged from 9.1 µg/kg-bw per day for adults (60 years and older) to 27.6 µg/kg-bw per day for toddlers (6 months to 4 years) and 2.3 µg/kg-bw per day for adults (60 years and older) to 6.8 µg/kg-bw per day for toddlers (6 months to 4 years), respectively (Health Canada 2018).
Food
MEK and MPK in food or as volatiles derived from food have been measured in most food groups primarily as a result of their natural occurrence in plants or from the production by microbes (i.e., fermentation). In addition, MEK and MPK are noted to be used as flavouring agents in food including baked goods, fats/oils, frozen dairy, gelatins/puddings, non-alcoholic beverages, and soft candy (Burdock 2010). In Canada, MEK is used as a food additive in natural extractives and in spice extracts; however, it is expected to be a minor contributor of MEK compared to the natural occurrence in foods. MEK (5 out of 12 samples) and MPK (4 out of 12 samples) were detected but not quantified in breast milk (Pellizari et al. 1982).
The Joint FAO/WHO Expert Committee on Food Additives (JECFA) evaluated a group of 39 saturated aliphatic acyclic secondary alcohols, ketones and related saturated and unsaturated esters used as flavouring substances including MEK and MPK (WHO 1999b). As part of that evaluation, the Committee estimated the per capita intake of MEK and MPK from their use as a food flavouring agent to be 0.6 and 0.7 µg/kg bw per day respectively for the US population and 2 µg/kg bw per day respectively for the European population (see Appendix A for more details).
Estimates of exposure for MEK and MPK based on their natural occurrence in foodFootnote 4 ranged from 66 µg/kg-bw per day for 14-18 year olds to 185 µg/kg-bw per day for 1 year olds and 68 µg/kg-bw per day for 14-18 year olds to 216 µg/kg-bw per day for 1 year olds, respectively (see Appendix A for more details).
Products available to consumers
MEK
Based on notifications submitted under the Cosmetic Regulations to Health Canada, MEK is used in certain cosmetic products in Canada such as face moisturizer and in various nail care products including base-coats, top-coats, nail polish, nail polish remover, nail adhesive, nail brush cleaner, nail hardener, nail cream, products to reduce drying time, and nail repair (personal communication, e-mail dated from CPSD, HC to ESRAB, HC, dated Aug. 2016 and April 2017,unreferenced). The function of MEK in these products is as a solvent or as a perfuming agent (European Commission 2017).
Inhalation exposure concentrations were derived for certain sentinel products (top-coat, nail polish and nail polish remover) which represent the highest exposures when compared to similar products using ConsExpo Web (2016). Table 7‑1 summarizes the range of MEK concentrations for the various products along with the associated inhalation exposure estimates. Only exposure estimates for adults and toddlers are shown; however, they represent the range of potential exposures for all age groups. Details on the method and parameters used to estimate inhalation and dermal exposures to MEK from cosmetics are available in Appendix B.
Product scenario |
Max Concentrationa |
Mean event concentration (mg/m3) | Mean concentration on day of exposure (mg/m3) | 7-hr TWAb (mg/m3) |
|---|---|---|---|---|
Top-coat |
55.7% |
140 |
1.8 |
6.0 |
Nail polish (adult/teen) |
35% |
190 |
4.6 |
15.8 |
Nail polish (toddler) |
35% |
65 |
1.6 |
5.4 |
Nail polish remover (adult/teen) |
84%c
|
280
|
1.6 |
5.3 |
Nail polish remover (toddler) |
76.4% |
220 |
1.2 |
4.1 |
Abbreviations: N/A, Not Applicable
a Personal communication, emails from CPSD, HC to ESRAB, HC, Aug. 2016 and April 2017; unreferenced.
b Seven-hour time-weighted average (TWA) concentratrions were derived for all product scenarios to match up with the exposure durations of the critical effects study used to characterize risk. 7-hr TWA = mean event concentration (mg/m3) x exposure duration (min) / (7 x 60 min)
c Product not anticipated to be used by young children (personal communication, emails from CPSD, HC to ESRAB, HC, dated April 2017; unreferenced)..
Although dermal exposure would be expected to contribute to the overall exposure during use of products available to consumers, the primary route is considered to be inhalation. Wilkinson and Williams (2001) measured a dermal absorption of less than 1% for MEK in a non-occluded human in vitro study. Given the high volatility and low dermal absorption of MEK, dermal exposure is considered to be minimal in comparison to that of inhalation; therefore, only inhalation estimates are presented.
Children’s products
MEK was identified in several different children’s products in the United States and Denmark including pacifiers and teething rings (WSDE 2016), as well as slimy toys (Svendsen et al. 2005). Only oral and inhalation exposure estimates are presented since they resulted in the highest exposures and would account for any possible dermal exposures.
The potential oral exposure from mouthing toys or children’s objects containing MEK was estimated using a pacifier and teether as sentinel exposure scenarios. MEK was identified as a contaminant in pacifiers/teethers by the WSDE (2016) with concentrations ranging from equal to or greater than 100 ppm to less than 500 ppm. Estimated oral exposures using the approach outlined in Appendix B ranged from 110 to 232 µg/kg-bw/day for toddlers (6 months to 4 years old) and from 91 to 300 µg/kg-bw/day for infants (0 to 6 months old).
Limited data were available regarding the migration of MEK from products. According to Svendsen et al. (2005), MEK did not migrate into artificial sweat and saliva from slimy toys with concentrations of 2.3% and 9%. The breathing zone concentration for the slimy toys was estimated to be 0.079 and 0.098 µg/m3 (Svendsen et al. 2005), lower than indoor air concentrations presented in the environmental media section. Nilsson et al. (2006) analyzed MEK in adult toys in headspace analyses and artificial sweat (pH of 4.5 and 6.5). Concentrations of MEK ranged 174 – 13016 ng/180 min in headspace analyses, from 12 – 49 µg/dm2 in artificial sweat with a pH of 4.5, and 17 µg/dm2 in artificial sweat with a pH of 6.5.
Other products
MEK is found in a limited number of currently licensed topical natural health products in Canada with a non-medicinal role including hand antiseptics meant primarily for use in medical facilities and in a facial cleanser (LNHPD modified 2016). The sentinel scenarios for cosmetics are considered to address any exposures to MEK from use of natural health products.
MEK is also used in numerous products available to consumers. Only product scenarios that result in the highest levels of potential exposure to MEK by the inhalation routes are presented in Table 7‑2. Potential inhalation exposures were estimated using ConsExpo Web (ConsExpo 2016). Appendix B summarizes the details on the parameters used in each model.
Product scenario |
MEK conc. |
Mean event conc. (mg/m3) | Mean conc. on day of exposure (mg/m3) | 7-hr TWAa (mg/m3) |
|---|---|---|---|---|
Lacquer removal |
10 – 40% |
920 – 3600 |
38 – 150 |
131 – 514 |
Adhesive removal |
100% |
3600 |
530 |
1843 |
Paint Thinner (floor coating) |
100% diluted to 3% in coating |
840 |
35 |
120 |
Liquid paint (solvent-rich) for truck bed |
20% |
210 |
20 |
176 |
Spray paint |
1 – 52% |
56 – 2800 |
0.98 - 48 |
3 – 167 |
PVC cement/ primer |
5 – 100% |
9.3 – 190 |
1.5 – 31 |
5 – 109 |
Multipurpose adhesive |
15 – 80% |
44 – 230 |
2.4 – 13 |
8 – 44 |
Abbreviations: conc., concentration
a Seven-hour time-weighted average (TWA) concentratrions were derived for all product scenarios to match up with the exposure durations of the critical effects study used to characterize risk. 7-hr TWA = mean event concentration (mg/m3) x exposure duration (min) / (7 x 60 min)
MPK
MPK was not identified in cosmetics or children’s products but was identified in several paint products. Table 7‑3 summarizes inhalation exposure estimates for products available to consumers containing MPK using ConsExpo Web (ConsExpo 2016). Similar to MEK, although dermal exposure could contribute to the overall exposure during use of products available to consumers, the primary route is considered to be inhalation; therefore, only inhalation estimates are presented.
Product scenario |
MPK conc. |
Mean event conc. (mg/m3)
|
Mean conc. on day of exposure (mg/m3)
|
6-hr TWAa (mg/m3) |
|---|---|---|---|---|
Liquid paint for steel (high-solid) |
1 – 10% |
87 – 870 |
8 – 80 |
32 – 319 |
Spray paint |
2 – 13% |
90 – 570 |
1.2 – 8 |
8 – 49 |
Abbreviations: conc., concentration
a Six-hour time-weighted average (TWA) concentratrions were derived for all product scenarios to match up with the exposure durations of the critical effects study used to characterize risk. 6-Hr TWA = Mean event conc. x exposure duration / 6 x 60 min
7.1.2 Health effects assessment of subgroup 1 (MEK and MPK)
MEK and MPK are structurally similar monoketones differing in the chain length by only one carbon. Based on the similarity in the effects of exposure to MEK and 2-butanol, as well as the finding that 2-butanol is rapidly metabolized to MEK in rats, 2-butanol is used as an analogue to inform assessment of this group. Toxicity data on 2-butanol have been used to read-across to MEK or MPK where required (see Table 7-4).
7.1.2.1 MEK
MEK has been reviewed by OECD (1997a) and US EPA IRIS (2003a). These reviews provide a basis for the health effects characterization in this draft screening assessment. Literature searches were conducted from a year prior to the US EPA IRIS (2003a) report to April 2017. No health effect studies that would impact the risk characterization (i.e. result in different critical endpoints or lower points of departure than those stated in US EPA IRIS 2003a) were identified.
Toxicokinetics
Orally administered MEK has been found to be extensively absorbed from the GI tract of rodents and rapidly eliminated (Dietz et al. 1981; reviewed in EPA IRIS 2003a). Due to its high blood/air solubility ratio it is also well-absorbed in both humans and rats upon inhalation exposure. Similarly, it was found to be rapidly absorbed upon dermal exposure (Munies and Wurster, 1965; reviewed in EPA IRIS 2003a). The available information indicates that the metabolism of MEK is similar in humans and laboratory animals with 2-butanol and 2,3-butanediol as the major metabolites (Perbellini et al., 1984; Liira et al., 1988, 1990a; reviewed in EPA IRIS 2003a). In humans, MEK appears to form endogenously, as it has been identified as a minor but normal constituent of urine, as a constituent in the serum and urine of diabetics, and in expired air (WHO 1992; reviewed in EPA IRIS 2003a).
Carcinogenicity and genotoxicity
For MEK, the US EPA (IRIS 2003a) did no identify concerns related to carcinogenicity or genotoxicity for MEK.
Repeated dose toxicity
The reported health effects of MEK were primarily related to absolute and/or relative organ weight increases (most frequently the liver) at high concentration (Nilson and Toftgard, 1980; Cavender et al., 1983; Toxigenics Inc., 1981; reviewed in US EPA IRIS 2003a).
Reproductive and Developmental toxicity
There are no studies that evaluated reproductive toxicity potential of MEK by any route of exposure (Cox et al., 1975; reviewed by US EPA IRIS 2003a; ECHA c2007-2017f, US EPA AcToR 2015).
In an inhalation developmental toxicity study, groups of 10 virgin Swiss CD-1 mice and 33 sperm plug-positive (GD 0) females were exposed to mean concentrations of 0, 398, 1,010 and 3,020 ppm (0, 1174, 2980 and 8909 mg/m3) MEK by inhalation for 7 hours/day on GD 6–15 (Schwetz et al., 1991, as reviewed in EPA IRIS 2003a). A slight but statistically significant dose-related increase absolute liver weight was observed in dams at 3,020 ppm (increase of approximately 7% when compared with the control). There was a statistically significant decrease in mean foetal weight (5%, per litter) at 3,020 ppm in males and a 4% decrease for all foetuses combined. There was also a positive trend for an increased incidence of foetuses with misaligned sternebrae with increasing exposure level (incidences were 31/310, 27/260, 49/291, and 58/323 for the control through 3,020 ppm (8909 mg/m3) exposure groups, respectively). Other non-significant developmental effects (cleft palate, fused ribs, missing vertebrae and syndactyly) were observed in exposed groups but not in controls. The NOAEC for both maternal and developmental adverse effects was considered to be 1,010 ppm (2980 mg/m3),while the developmental and maternal LOAECs were established at 3,020 ppm (8909 mg/m3), based on the decreased foetal weight among males, increased incidence of misaligned sternebrae, and an increased relative liver weight in dams. Similarly, Deacon et al. (1981, as cited in US EPA IRIS 2003a) reported foetal toxicity (increased incidence of extra ribs) and maternal toxicity (decreased body weight gain) at 3005 ppm (8865 mg/m3) (considered to be the LOAEC) in a rat developmental study, with a NOAEC of 1002 ppm (2955 mg/m3). Based on the data for misaligned sternebrae, the US EPA (2003) derived a LEC10Footnote 5 (95% lower confidence on the concentration associated with a 10% extra risk) of 5202 mg/m3 for intermittent exposure (7 hours per day) and LECHECFootnote 6 (human equivalent concentration adjusted for continuous exposure) of 1517 mg/m3.
Similarly, a more recent inhalation developmental toxicity study in rats also showed developmental toxicity with a NOAEC of 2949 mg/m3; decreased foetal body weight was observed at 2000 ppm (5899 mg/m3), while decreased maternal body weight was also noted at 11797 mg/m3 and above (Saillenfait et al. 2006). Other developmental study reported developmental effects in the absence of maternal toxicity at 7723 mg/m3 with a NOAEC of 1126 ppm (3322 mg/m3) (Schwetz et al. 1974, as cited in US EPA IRIS 2003a).
No oral developmental toxicity studies were reported for MEK hence, a read-across from the oral reproductive and developmental toxicity study with 2-butanol to MEK was used. In this multigeneration drinking water study, male and female Wistar rats were exposed to 2-butanol concentrations of 0, 0.3, 1.0, or 3.0% (equivalent to doses of 0, 538, 1644, and 5089 mg/kg bw per day (male rats) and 0, 594, 1771, and 4571 mg/kg bw per day (female rats) for 8 weeks before mating. Because increased mortality and decreased body weight occurred in the F1A litters at the 3% dose level, the high-dose was reduced to 2% (average daily intake of 3384 mg/kg-bw/day in males and 3122 mg/kg-bw/day in females for the remaining of the study). F0 females were mated again and F1A pups mated to produce litters F2. A NOAEL of 1771 mg/kg bw per day for 2-butanol for both maternal and developmental effects was established based on decreased F1B foetal weights and decreased F1A and F2 pup body weights and decreased body weight gain in dams at 3122 mg/kg bw per day (Cox et al. 1975; reviewed in US EPA IRIS 2003a). The US EPA (2003) estimated a lowest effective dose (LED)05 (95% lower confidence limit on the effective dose, ED) of 639 mg/kg bw per day for MEK (adjustment based on the molecular weights from the LED05 of 657 mg/kg bw per day for 2-butanol).
No dermal developmental toxicity studies were identified for MEK or the analogue 2-butanol.
7.1.2.2 MPK
MPK has not been assessed by other agencies. The following health assessment is based on the information identified from ECHA registration dossier (ECHA c2007-2017c) and US EPA HPVIS (US EPA 2001a).
Toxicokinetics
No quantitative information on the absorption of MPK via any route of exposure was identified. MPK is structurally similar to MEK and its metabolite 2-butanol, therefore absorption of MPK is expected to be similarly rapid and extensive.
Carcinogenicity and genotoxicity
No oral, dermal or inhalational carcinogenicity studies were reported for MPK. No positive (Q)SAR model predictions or presence of structural alerts for genotoxicity or carcinogenicity were found. In addition, MPK was not genotoxic in several in vitro assays (i.e., Ames tests, chromosomal aberration test and mouse lymphoma assay; ECHA c2007-2017c).
Repeated dose toxicity
In an oral repeat dose toxicity study, CD male rats were administered MPK in the drinking water at concentrations of 0, 0.25%, 0.5% and 1.0% MPK (equivalent to doses of 0, 144, 250 and 454 mg/kg bw per day) for a period of 13-months). The only effect reported was a slight decreased body weight gain (9%) at 1.0% at the highest dose tested (454 mg/kg bw per day). There we no effects on clinical signs, organ weights or histology and no pathological changes were observed in the central or peripheral nervous systems (Bingham et al., 2001 as cited in HSDB 1983-).
With respect to inhalation exposure, the critical effect levels were derived from inhalation studies in rats, in which NOAECs of 5000 and 5300 mg/m3 were identified after 35-21 days and 13 weeks exposure, respectively (Anonymous 1999a, Eastman Chemical Company 1999; ECHA c2007-2017c). In the combined inhalation reproductive/developmental toxicity study, SD rats were exposed up to 5000 mg/m3 MPK 6h/d for 35-48 days (females) until GD19 and for 51-days (males) (ECHA c2007-2017c, EPA AcToR 2015). Although slight neurotoxicity in dams was observed at a lower concentration (2500 mg/m3), this effect appears to be transient and similar effects were not observed at similar concentrations in other studies. No other significant effects were reported. The other short-term study revealed only a slight enlargement of hepatocytes in one animal after two 16-hour periods and two 20-hour periods on 4 consecutive days at a dose of 1074 mg/m3 (Eastman Kodak Company 1977, as cited in US EPA 2001a).
For the dermal route, no suitable study was identified.
Reproductive and developmental toxicity
In a prenatal developmental toxicity study female SD rats were exposed to concentrations 0, 250, 750 and 1500 ppm (equivalent to 0,880, 2650, 5300 mg/m3) of MPK from GD 6-19 (ECHA c2007-2017c). No effects on mean body weights, body weight gains, net body weights, net body weight gains, gravid uterine weights, or food consumption in any test substance-exposed group were observed. No adverse effects on maternal animals or effects on intrauterine growth, survival, and foetal morphology were observed at any exposure level. Based on these observations, the study authors identified a NOAEC of 1500 ppm (5300 mg/m3) (the highest exposure level evaluated) for maternal toxicity and embryo/foetal development.
Similarly, no developmental toxicity was reported up to the highest concentration tested (i.e. NOAEC of 5000 mg/m3) in the combined inhalation reproductive/developmental toxicity study decribed above. The results of these studies would suggest that MPK is not a developmental toxicantIn addition, based on OECD toolbox in silico prediction tools, there is no structural alert, indicating that MPK has any potential for inducing developmental toxicity.
No oral or dermal reproductive or developmental toxicity data are available for MPK.
Chemical name |
2-Butanol |
MEK |
MPK |
|---|---|---|---|
Role |
Analogue |
Target chemical |
Target chemical |
CAS# |
78-92-2 |
78-93-3 |
107-87-9 |
Chemical structure |
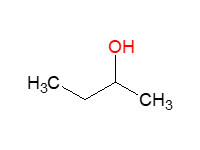 MW: 74.12 Log Kow: 0.61 |
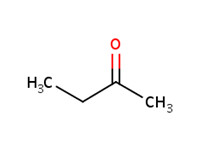 MW: 72.11 Log Kow: 0.29 |
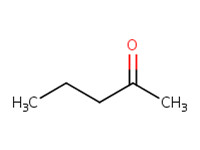 MW: 86.13 Log Kow: 0.91 |
Toxicokinetics & metabolism |
Rapidly converted to MEK |
Rapidly absorbed via oral route |
NA |
Acute toxicity |
N/A |
Oral LD50 = 3460 mg/kg bw Dermal LD50 >6400-8000 mg/kg bw |
Oral LD50 = >1600-<3200 mg/kg bw Dermal LD50 = 8000 mg/kg bw |
Short term inhalation studies |
N/A |
NOAEC = 590 mg/m3 (human study)
LEC5= 5202 mg/m3 (intermittent exposure)/ LECHEC=1517 mg/m3 (continuous exposure) (from developmental study)
|
NOAEC = 1074 mg/m3 (No effect after 4 exposures) NOAEC = 5300 mg/m3 (14-d developmental study) |
Repeat dose toxicity (Oral) |
NOAEL = 1771 mg/kg bw per day (8-week; decreased BW gain in dams) |
NOAEL = 1723 mg/kg bw per day (read-across from 2-butanola)
|
NOAEL/LOEL = 454 mg/kg bw per day (13-month; 9% decreased body weight gain) |
Repeat dose toxicity (Inhalation) |
N/A |
NOAEC = 14870 mg/m3 (decreased body weight gain, increased liver, kidney weights, decreased brain weight; absence of central/peripheral neural histopathology) (90-day) |
NOAEC = 5300 mg/m3 (13-week)
NOAEC = 5000 mg/m3 (35-51-day) |
Repeat dose toxicity (Dermal) |
NA |
NA |
NOAEL = 454 mg/kg bw/d (from MPK oral study) |
Reproductive and/or developmental toxicity (oral) |
LED05-dev = 657 mg/kg bw per day (multigeneration 8-week; NOAELdev-mat = 1771 mg/kg bw/d; decreased foetal weight, with evidence of retarded skeletal maturation; decreased body weight in dams)
|
LED05 = 639 mg/kg bw per day (NOAEL = 1723 mg/kg bw per day) (read-across from 2-butanola) |
LED05 = 763 mg/kg bw per day (read-across from 2-butanol a) |
Reproductive and/or developmental toxicity (inhalation) |
N/A |
NOAELdev-mat = 2955 mg/m3 (GD6-15; decreased foetal weight among males, increased incidence of misaligned sternebrae and increased relative liver weight in dams) LEC10= 5202 mg/m3 (intermittent exposure)/ LECHEC=1517 mg/m3 (continuous exposure) |
NOAECdev = 5000 mg/m3
NOAECmat = 5000 mg/m3 (35-51-day until GD19)
NOAECdev= 5300 mg/m3 (GD6-19) |
Reproductive and/or developmental toxicity (dermal) |
N/A |
LED05 = 639 mg/kg bw per day (read-across from 2-butanol oral study a) |
LED05 = 763 mg/kg bw per day ( read-acrossfrom 2-butanol oral studya) |
Genotoxicity |
Not genotoxic |
Not genotoxic |
Not genotoxic |
Carcinogenicity |
N/A |
Negative/ Inconclusive (EPA 2003). However, unlikely based on structural alerts, (Q)SAR. |
Not expected to be carcinogenic (read across from MEK) |
Abbreviations: NA, Not Available; N/A, Not Applicable; Log Kow, octanol-water partition coefficient; MW, molecular weight (g/mol)
a molar adjustment calculated from read-across value. Molecular weights are 74.12 g/mol for 2 butanol, 72.11 g/mol for MEK and 86.13 g/mol for MPK.
7.1.3 Risk characterization of subgroup 1 (MEK and MPK)
MEK
In the range of adverse effects in animals resulting from repeated inhalation exposure to MEK, there is consistent evidence in studies in rats and mice that developmental effects are the critical health effect, which occurred mostly in the presence of slight maternal toxicity. Based on the available studies, the US EPA (2003a) selected the study in mice by Schwetz et al (1991) with a LEC10 for intermittent exposure (7h/day) of 5,202 mg/m3 and a LECHEC for continuous exposure of 1517 mg/m3, based on the incidence of misaligned sternebrae (US EPA IRIS 2003a). Therefore, these values were considered to be the most appropriate points of departure to use for risk characterization for MEK.
In the absence of oral repeat dose toxicity studies for MEK, the reproductive and developmental drinking water toxicity study of 2-butanol in rats (Cox et al., 1975 as cited in US EPA IRIS 2003a) was selected to characterize risk by the oral and dermal routes. The US EPA (2003) estimated a LED05 (95% lower confidence limit on the effective dose, ED) of 639 mg/kg-bw/day for MEK (adjustment based on the molecular weights from the LED05 of 657 mg/kg-bw/day for 2-butanol) based on decreased pup survival and decreased neonatal body weight in presence of maternal toxicity.
The predominant source of exposure to MEK from environmental media and food for the general population is through the diet and to a lesser extent from indoor air. Based on the available data, it is expected that the majority of the dietary exposure to MEK results from its natural occurrence in foods.
The general population of Canada may also be exposed to MEK when using various products available to consumers containing the substance including nail products, paints and do-it-yourself products, primarily through inhalation.Table 7‑5 provides relevant exposure values and effect levels for critical health effects as well as the resultant margins of exposure (MOEs) for the characterization of risk for MEK.
Exposure Scenario |
Exposure |
Critical effect level |
Critical health effect |
MOE |
|---|---|---|---|---|
Total exposure from environmental media |
9.1 – 27.6 µg/kg-bw/day |
LED05 = 639b mg/kg-bw/day |
Developmental study for 2-butanol; decreased foetal and pup weights, and body weight gain in dams |
> 22 986 |
Food flavouring agent |
0.6 – 2.0 µg/kg-bw/day |
LED05 = 639b mg/kg-bw/day |
Developmental study for 2-butanol; decreased foetal and pup weights, and body weight gain in dams |
> 319 500 |
Nail products (inhalation) |
7-hr TWAa = 4.1 – 15.8 mg/m3 |
LEC10 = 5 202 mg/m3 (Intermittent exposure) |
increased incidence of misaligned sternebrae in foetus |
329 – 1 269 |
Pacifiers and teethers (oral) |
0.09 – 0.30 mg/kg-bw/day |
LED05 = 639b mg/kg-bw/day |
Developmental study for 2-butanol; decreased foetal and pup weights, and body weight gain in dams |
2 130 – 7 100 |
Lacquer remover (inhalation) |
7-hr TWA = 131 – 514 mg/m3 |
LEC10 = 5 202 mg/m3 (Intermittent exposure) |
Increased incidence of misaligned sternebrae in foetus |
10 – 40 |
Adhesive remover (inhalation) |
7-hr TWA = 1843 mg/m3 |
LEC10 = 5 202 mg/m3 (Intermittent exposure) |
Increased incidence of misaligned sternebrae in foetus |
3 |
Paint thinner, floor coating (inhalation) |
7-hr TWA = 120 mg/m3 |
LEC10 = 5 202 mg/m3 (Intermittent exposure) |
Increased incidence of misaligned sternebrae in foetus |
43 |
Liquid paint (solvent-rich) for truck bed (inhalation) |
7-hr TWA = 176 mg/m3 |
LEC10 = 5 202 mg/m3 (Intermittent exposure) |
Increased incidence of misaligned sternebrae in foetus |
30 |
Spray paint (inhalation) |
7-hr TWA = 3 – 167 mg/m3 |
LEC10 = 5 202 mg/m3 (Intermittent exposure) |
Increased incidence of misaligned sternebrae in foetus |
31 – 1 743 |
PVC cement/primer (inhalation) |
7-hr TWA = 5 – 109 mg/m3 |
LEC10 = 5 202 mg/m3 (Intermittent exposure) |
Increased incidence of misaligned sternebrae in foetus |
48 – 10 40 |
Multipurpose adhesives |
7-hr TWA = 8 – 44 mg/m3 |
LEC10 = 5 202 mg/m3 (Intermittent exposure) |
Increased incidence of misaligned sternebrae in foetus |
118 – 650 |
Abbreviations: TWA, time-weighted average
a Seven-hour time-weighted average (TWA) concentratrions were derived for all product scenarios to match up with the exposure durations of the critical effects study used to characterize risk.
b Molar adjustment calculated from read-across value. Molecular weights are 74.12 g/mol for 2 butanol, 72.11 g/mol for MEK and 86.13 g/mol for MPK.
MOEs for exposure to MEK in environmental media and food (flavouring agent) are considered adequate to address any uncertainties in the health effects and exposure databases. Additional intake of MEK from its natural occurrence in food was not identified as a concern for human health. Furthermore, the JECFA (WHO 1999b) concluded "No safety concern with the estimated levels of intake as flavouring substances" based on the dietary exposure estimates for MEK.
The calculated MOEs for exposure to pacifiers and teethers, nail products and multipurpose adhesives are considered adequate; however, inhalation exposure to MEK from the other products available to consumers (i.e., lacquer remover, adhesive remover, paint thinner, liquid paint and spray paint), are considered potentially inadequate to account for uncertainties in the databases.
MPK
No carcinogenicity studies were identified for MPK. However, there were no positive (Q)SAR model predictions or presence of structural alerts for carcinogenicity. In addition, MPK was not genotoxic in several in vitro assays. In the only available oral toxicity study, minimal effects (slight decreased body weight gain) was reported in rats at the highest dose of MPK administered in the drinking water for 13 months (Bingham et al., 2001, HSDB 2015b); the NOEAL/LOEL is considered to be 454 mg/kg bw per day). With respect to inhalation exposure, no adverse effects, other than slight transient neurotoxicity in dams during exposure, were observed in studies in which NOAECs of 5000 and 5300 mg/m3 were identified (ECHA c2007-2017c).
The predominant source of exposure to MPK from environmental media and food for the general population is through the diet. Based on the available data, it is expected that the majority of the dietary exposure to MPK results from its natural occurrence in foods.
The only consumer uses identified for MPK were for paint products.Comparison of the estimates of exposure to MPK from environmental media (2.3 – 6.8 µg/kg bw/day) and from its use as a flavouring agent in food (0.7 – 2.0 µg/kg bw/day) with the NOAEL/LOEL of 454 mg/kg-bw per day resulted in MOEs greater than 66 765, which are considered adequate to address any uncertainties in the health effects and exposure databases. Additional intake of MPK from its natural occurrence in food was not identified as a concern for human health. Furthermore, the JECFA (WHO 1999b) concluded "no safety concern with the estimated levels of intake as flavouring substances" based on the dietary exposure estimates for MPK. Given the low hazard potential of MPK via inhalation, risk to human health related to the presence of MPK in products available to consumers is considered to be low.
7.1.4 Uncertainties in evaluation of risk to human health for MEK and MPK
The key sources of uncertainty are presented in the table below. There is some uncertainty in the estimates of inhalation exposure from use in products available to consumers, with respect to the range of concentrations in the various products, the location of the activity as well as the defaults used in the ConsExpo Web (ConsExpo 2016). Confidence is high that use of the maximum concentrations and the high end of the range of product amounts from these types of products does not underestimate potential population exposures.
There is some uncertainty regarding the toxicity of MEK following long term oral exposure, since no data are available; however, the use of 2-butanol, a major metabolite of MEK, was considered an appropriate analogue for use in risk characterization.
Key sources of uncertainty |
Impact |
|---|---|
Assumption that dermal exposures are minimal in comparison to inhalation exposures, given the high volatility of the substances; however, |
- |
Lack of chronic oral or inhalation study for MEK or MPK. |
- |
The use of 2-butanol as read-across to assess repeated dose toxicity and reproductive/developmental toxicity for MEK |
+/- |
Selection of a conservative effect level from a repro/devo study for risk characterization of an acute inhalation exposure scenario for MPK (paint products). |
+ |
+ = uncertainty with potential to cause over-estimation of exposure/risk;
- = uncertainty with potential to cause under-estimation of exposure risk;
+/- = unknown potential to cause over or under estimation of risk.
7.2 Assessment of subgroup 2 (MIBK, MIAK, and DAA)
7.2.1 Exposure assessment of subgroup 2 (MIBK, MIAK, and DAA)
Environmental media
MIBK was monitored by the National Air Pollution Surveillance (NAPS) program. Mean concentrations of MIBK between 2006 and 2010 from various sites across Canada ranged from 0.014 to 0.29 µg/m3 with 95th percentile concentrations ranging from 0.08 to 1.05 µg/m3 (ECCC 2017). Geometric mean ambient air concentrations of MIBK from the five Canadian air studies referred to in section 7.1.1 ranged from <0.016 to 0.185 µg/m3, and 95th percentile concentrations ranging from 0.086 to 0.470 µg/m3 (Zhu et al. 2005; Health Canada 2010a, b, 2012, 2013). The highest 95th percentile concentration (0.470 µg/m3 from the Windsor 2005 study) was used to estimate general population exposures. No information or data on MIAK and DAA in ambient air were identified in Canada or elsewhere.
MIBK was measured in the CHMS Cycle 2 Indoor Air Study and was detected in approximately 96% of the samples. The geometric mean concentration of MIBK from this study was 0.235 µg/m3 with a 95th percentile concentration of 1.62 µg/m3 (weighted data at the household level) (Zhu et al. 2013). Geometric mean MIBK concentrations from the five Canadian air studies ranged from 0.14 to 1.33 µg/m3 with 95th percentile concentrations ranging 0.33 to 13.82 µg/m3 (Zhu et al. 2005; Health Canada 2010a, b, 2012, 2013). The highest 95th percentile concentration was used to estimate general population exposures (13.82 µg/m3 from the Windsor 2005 study). MIAK was also measured in the CHMS Cycle 2 Indoor Air study but was only detected in 1.45% of samples (limit of detection of 0.19 µg/m3). The geometric mean and 95th percentile MIAK concentration in indoor air was 0.10 µg/m3 and 0.11 µg/m3, respectively (weighted data at the household level). (Zhu et al. 2013). MIAK was also measured in 24 indoor air samples from a Quebec field study with a geometric mean concentration of 0.23 µg/m3 and a maximum concentration of 1.51 µg/m3 (Won and Lustyk 2011). This maximum indoor air concentration was used to estimate general population exposures. No empirical data on DAA in indoor air were identified in Canada or elsewhere. Based on the very high water solubility and low Henry’s Law Constant, air is not expected to be a significant source of population exposure for DAA.
Personal air samples were also measured for MIBK in the Windsor study (Health Canada 2010a). The geometric mean personal air concentration of MIBK in the summer and winter were 1.04 µg/m3 and 0.23 µg/m3, respectively. The 95th percentile concentrations for MIBK were 7.96 µg/m3 and 0.99 µg/m3 in the summer and winter, respectively (Health Canada 2010a).
No concentrations for MIBK, MIAK and DAA in drinking water in Canada were identified. No further information on the presence of MIAK in water elsewhere was identified; however, MIBK was detected in 3 out of 646 samples in a study conducted in the US with measured concentrations ranging from 16-20 µg/L (Grady and Casey 2001). MIBK was also measured in several surface water studies (Benfenati et al. 1992, Hall, 1987, Sheldon and Hites, 1978, Bianchi and Varney, 1998, U.S. EPA 2002). In addition, elevated concentrations of MIBK were found in groundwater, effluent and leachate in the vicinity of landfill sites, oil reprocessing facilities and sewage treatment plants (Mutch et al., 1983; Sawhney and Kozloski, 1984; Sabel and Clark, 1984; Canter and Sabatini, 1994; Paxeus, 1996; U.S. EPA, 1998). The maximum value of 20 µg/L measured in the US was used to estimate general population exposures to MIBK from drinking water and resulting general population intakes ranged from 0.1 µg/kg-bw per day (adults 60 years and older) to 2.1 µg/kg-bw per day (formulat fed infants 0 to 6 months old). ChemCan was used to derive concentrations of MIAK and DAA in water using volume data from Table 4-1. Drinking water concentrations for MIAK and DAA were 0.15 ng/L and 46.6 ng/L, respectively resulting in general population intakes.
No information or data on MIBK, MIAK and DAA in soil were identified in Canada. DAA was qualitatively detected in sediment in a lake in Saskatchewan, Canada (HSDB 1983). ChemCan was used to derive concentrations of MIBK, MIAK and DAA in soil using volume data from Table 4-1. Soil concentrations for MIBK, MIAK and DAA were 1.9 ng/kg, 0.3 ng/kg, and 7 pg/kg respectively; therefore, exposure to these substances from soil is considered to be negligible (intakes less than 1 ng/kg-bw per day).
Estimates of exposure for MIBK, MIAK and DAA from environmental media ranged from 2.5 µg/kg-bw per day for adults (60 years and older) to 7.5 µg/kg-bw per day for toddlers (6 months to 4 years), 0.3 µg/kg-bw per day for adults (60 years and older) to 0.9 µg/kg-bw per day for toddlers (6 months to 4 years), and 0.001 µg/kg-bw per day for adults (60 years and older) to 0.005 µg/kg-bw per day for formula fed infants (0 to 6 months old), respectively (Health Canada 2018).
Food
MIBK was detected in various food items as a result of its natural occurrence including in beer, brandy, chicken, fruit, olive oil, eggs, coffee and cow’s milk (Nijssen et al. 1963-2016, studies, Burdock 2010). MIBK is also cited as being used as a flavouring ingredient in baked goods, frozen dairy, gelatins/puddings, meat products, non-alcoholic beverages and soft candy (Burdock 2010). MIBK was detected (2 out of 12 samples) but not quantified in breast milk (Pellizari et al. 1982).
The Joint FAO/WHO Expert Committee on Food Additives (JECFA) evaluated a group of 39 saturated aliphatic acyclic secondary alcohols, ketones and related saturated and unsaturated esters, including MIBK, used as flavouring substances (WHO 1999b). As part of that evaluation, the Committee estimated the per capita intake of MIBK from its use as a food flavouring agent to be 0.03 µg/kg bw per day for the US population, and 0.12 µg/kg bw per day for the European population (see Appendix A for more details).
MIAK was identified in a few food items including coffee (0.5 µg/g) and papaya (0.001 µg/g) (Nijssen et al. 1963-2016), in roasted filberts, fried bacon, cooked beef and cooked pork (HSDB 1983-). DAA was detected in various food items including fruit, vegetables, dairy, honey, nuts, eggs, chicken and alcoholic beverages (Nijssen et al. 1963-2016, study).
Quantitative exposure estimates for MIBK, MIAK and DAA from its natural occurrence in foodFootnote 7 ranged from 0.4 µg/kg-bw per day for 4 to 8 year olds to 32 µg/kg-bw per day for adults 19 years and older for MIBK, from 0.001 µg/kg-bw per day for 1 year olds to 3 µg/kg-bw per day for adults 19 years and older for MIAK, and from 0.04 µg/kg-bw per day for 6 to 12 month olds to 4 µg/kg-bw per day for 2-3 year olds for DAA.
Products available to consumers
MIBK
MIBK was identified as being present as a non-medicinal ingredient in rubbing alcohol, licensed as an NHP, meant for topical use with a concentration of 0.98% (personal communication, e-mail from Natural and Non-Prescription Health Products Directorate, Health Canada to Existing Substances Risk Assessment Bureau, Health Canada, dated Aug 16, 2016 unreferenced). ConsExpo Web (2016) was used to estimate inhalation exposures to MIBK from use of this product for toddlers and adults. Parameters used in the model are outlined in Appendix B. The mean event concentration, regardless of age group for this exposure scenario was estimated to be 0.19 mg/m3 and the mean concentration on the day of exposure was estimated to be 2.7E-4 mg/m3. Although dermal exposure could contribute to the overall exposure during use of products available to consumers, the primary route is considered to be inhalation in light of the high volatility of MIBK; therefore, only inhalation exposure estimates are presented.
MIBK is used in a variety of products available to consumers that may result in exposure to the general population of Canada. Only product scenarios that result in the highest levels of potential exposure to MIBK by the inhalation are presented in Table 7‑7. Incidental oral as well as inhalation exposures for toddlers and children (representhighest exposed age groups) from use of dry erase markers were also estimated (seeTable 7‑7). Per event incidental oral exposure estimates from the use of dry erase markers were 0.48 and 0.97 mg/kg bw for children and toddlers, respectively. Potential exposures were estimated using ConsExpo Web (ConsExpo 2016) or relevant algorithms; see Appendix B for details.
Product scenario |
MIBK conc. |
Mean event conc. (mg/m3) |
Mean conc. on day of exposure (mg/m3) | 6-hr TWAa (mg/m3) |
|---|---|---|---|---|
Wood lacquer |
1 – 10% |
270 – 2600 |
11 – 110 |
45 – 433 |
Liquid paint (solvent-rich) for truck bed liners, appliances |
13% |
130 |
12 |
48 |
Spray paint |
0.1 – 30% |
5.3 – 1200 |
0.09 – 21 |
0.4 – 83 |
Filler/putty from tube (automotive) |
1 – 8% |
6.7 – 38 |
0.3 – 1.6 |
1 – 6 |
Dry erase marker |
30% |
3.6 |
0.11 |
0.45 |
a Six-hour time-weighted average (TWA) concentratrions were derived for all product scenarios to match up with the exposure durations of the critical effects study used to characterize risk. 6-Hr TWA = Mean event conc. x exposure duration / 6 x 60 min.
MIAK
MIAK was identified in several brands of spray paints, and in automotive repair coating pen (Health Canada 2016, HPD 1993-). Use of spray paints containing 1 - 10% MIAK represents the sentinel scenario. The mean event concentration derived from ConsExpo Web (ConsExpo 2016) for an adult using a spray paint containing MIAK ranged from was 41 - 280 mg/m3 while the mean event concentration on the day of exposure ranged from 0.7 to 4.8 mg/m3. Six-hour TWA concentrations ranged from 3 – 19 mg/m3 (see Appendix B for more details). Although dermal exposure could contribute to the overall exposure during use of products available to consumers, the primary route is considered to be inhalation in light of the high volatility of MIAK, only inhalation estimates are presented.
DAA
Based on notifications submitted under the Cosmetic Regulations to Health Canada, DAA is used as a solvent or as an odour masking agent in certain cosmetic products in Canada such as eyeliner stickers and in various nail care products including base-coats, top-coats, nail polish, nail polish remover, and nail hardener (personal communication, emails from CPSD, HC to ESRAB, HC, dated Aug. 2016 and April 2017, unreferenced).
Inhalation exposure estimates were derived for certain sentinel products (base-coat, top-coat, nail polish and nail polish remover) which represent the highest exposures when compared to similar products. Table 7‑8 summarizes the concentration range for the various product scenarios along with the associated inhalation exposure estimates. Only exposure estimates for adults and toddlers are shown; however, they represent the range of potential exposures for all age groups. Details on the method and parameters used to estimate inhalation exposures to cosmetics are available in Appendix B.
Dermal exposure to DAA from use of these products is also possible and unlike the other ketones described thus far, is more likely given its lower vapour pressure and high water solubility. A human in vitro dermal absorption study was identified and found that the amount of DAA that penetrated the skin after 10-, 60-minutes, and 24 hours was 0.04%, 0.15%, and 5.71% of a 25 mg/cm2 dose, respectively (Fasano and McDougal 2008; ECHA c2007-2017a). Table 7‑8 includes the estimated systemic dermal exposures assuming 6% dermal absorption through the skin.
Product scenario |
Maximum concentrationa |
Mean event concentration (mg/m3) |
6-Hr TWA (mg/m3) |
Systemic dermal exposure (mg/kg-bw/event) |
|---|---|---|---|---|
Nail polish (adults/teens) |
10% |
3.5 |
0.34 |
0.014 – 0.016 |
Nail polish (toddler) |
10% |
3.1 |
0.30 |
0.023 |
Eyeliner sticker (adults/teens) |
30% |
0.67 |
1.3 |
0.007 – 0.008 |
a Personal communication, emails from CPSD, HC to ESRAB, HC, dated Aug. 2016 and April 2017, unreferenced.
DAA was identified in pipe thread sealants, spray products including paints, cleaners, and automotive products, and paint thinners (used to dilute lacquers and clean brushes). Table 7‑9 summarizes inhalation and dermal exposure scenarios for paint and DIY products available to consumers containing DAA using ConsExpo Web (ConsExpo 2016).
Product scenario |
DAA conc. |
Mean event conc. (mg/m3) |
Internal inhalation dose on day of exposure (mg/kg-bw/day) |
Sytemic dermal exposure (mg/kg-bw/day) |
|---|---|---|---|---|
Pipe thread sealant |
20 – 30% |
35 – 51 |
1.7 – 2.5 |
0.012 – 0.018 |
Automotive choke cleaner (spray) |
1 – 30% |
13 – 110 |
0.04 – 0.32 |
0.013 – 0.38 |
Paint/marker remover (spray) |
10% |
81 |
0.32 |
0.13 |
Spray paint |
1 – 5% |
21 – 72
|
0.11 – 0.36 |
0.007 - 0.066 |
Paint thinner (for epoxy paint) |
5 – 10% |
55 – 71
|
0.46 – 0.60 |
0.09 – 0.17 |
DAA was also identified in permanent markers (Health Canada 2016, HPD 1993-). These products may be used by young children, and therefore, incidental oral and dermal exposures were estimated. A concentration of 100% was assumed based on limited data available on the quantity of DAA in markers (SDS 2008b, SDS 2012a, SDS 2014). Per event incidental oral exposure estimates from the use of permanent markers were per event incidental oral exposure estimate from the use of permanent markers was 1.6 and 3.2 mg/kg bw for children and toddlers, respectively. The daily systemic dermal exposure estimates, assuming dermal absorption of 6%, ranged from 0.002 mg/kg-bw/day for adults to 0.005 mg/kg-bw/day for a child (toddlers assumed to not use permanent markers on a daily basis). Any inhalation exposures to DAA from use of permanent markers are considered to be included in the conservative oral or dermal exposure estimates (details in Appendix B).
7.2.2 Health effects assessment of subgroup 2 (MIBK, MIAK, and DAA)
MIBK and MIAK are structurally similar molecules, differing in chain length by only one methyl group. Biologically they are likely to have similar interactions and metabolites in the body. DAA has been included in this subgroup as it is a primary metabolite of MIBK. It is likely to have similar metabolic clearance and is possibly responsible for some of the biological effects seen following MIBK exposure. Effects common to all three chemicals included decreased body weight, increased kidney and liver weights, and central nervous system (CNS) depression. While no data on the metabolism of MIAK was identified, the structural similarities to MIBK suggest it would have analogous metabolites and metabolic rates.
7.2.2.1 MIBK
MIBK has been reviewed by OECD (1996), IPCS (1996), US EPA IRIS (2003b), IARC (2013) and NICNAS (2017). These reviews provide a basis for the health effect characterisation in this draft screening assessment. A literature search has been conducted from a year prior to the OECD SIAR report (1996) and updated to January 2017. Based on a 2-year inhalation carcinogenicity study by NTP (2007b) in two mammalian species (rat and mouse), IARC classified MIBK as group 2B (“possibly carcinogenic to humans”) with sufficient evidence of carcinogenicity in experimental animals (IARC Monograph Vol. 101, 2013). Whereas the IARC report was the basis for characterization of cancer effects, the US EPA IRIS document was the basis for characterization of non-cancer effects of MIBK
Toxicokinetics and metabolism
MIBK is rapidly absorbed following oral, inhalation and dermal exposure (Duguay and Plaa, 1995; Hjelm et al., 1990; Hjelm et al., 1991 as cited in EPA IRIS 2003b and NTP 2007b). The major metabolite detected in plasma of rats administered MIBK by gavage was DAA, with somewhat lesser amounts of 4-methyl-2-pentanol (DiVincenzo et al., 1976; Duguay and Plaa, 1995 as cited in NTP 2007b and EPA IRIS 2003b). However, 4-methyl-2-pentanol was the major metabolite (about twice as much as DAA) detected in the lung of rats following inhalation exposure. In humans, inhalation exposure to concentrations of 10, 100, or 200 mg/m3 MIBK for 2 hours resulted in a pulmonary retention of about 60% with mean blood clearance of 1.6L/h/kg and about 0.04% of the total dose was excreted unchanged in the urine within 3 hours post-exposure (Hjelm et al., 1990 as cited in NTP 2007b). With respect to dermal exposure, the percutaneous uptake rate of MIBK in exposed guinea pigs ranged from 0.11 to 2.0 μmol/min/cm and averaged 1.1 μmol/min/cm (Hjelm et al., 1991 as cited in EPA IRIS 2003b).
Carcinogenicity and genotoxicity
In a 2-year inhalation study in male and female mice and rats, MIBK increased the incidences of hepatocellular adenoma, and hepatocellular adenoma and carcinoma combined in male and female mice, as well as that of renal tubule adenoma and renal tubule adenoma and carcinoma combined in male rats, and caused two rare renal malignant mesenchymal tumours in high-dose female rats at a concentration of 7374 mg/m3 (NTP 2007b, IARC 2013). Two rate renal malignant mesenchymal tumours and increased incidence of mononuclear cell leukemia were also observed at the highest dose in rats.In rats, the incidence of renal effects, including chronic nephrophathy, was significantly increased, with an increasing trend in severity. Although the pathological changes in male rats were consistent with the spectrum of α2u-globulin induced nephropathy and hyaline droplet formation has been reported in MIBK exposed male rats in shorter term studies (e.g., Phillips et al, 1987 and Nemec et al, 2004, as cited in NTP, 2007b), in light of the observation of nephropathy also in female rats, the NTP (2007b) stated that the renal tumours may arise independently of a α2u-globulin mechanism. Further, IARC (2013) determined that the relevance of the kidney tumours in rats to humans could not be excluded. The chronic inhalation LOAEC non-cancer effects for MIBK was established at 1843 mg/m3, based on minimal to mild nephropathy in the 2-year bioassay (NTP 2007b).
Subsequent to the publication of the NTP (2007b) report and the IARC (2013) Monograph, recent studies have suggested that the MIBK-induced kidney and liver tumors occurred in rodents by mechanisms such as alpha2u-globulin nephropathy and constitutive androstane receptor (CAR)-mediated mode of action, respectively that are not relevant to humans (Hughes et al. 2016; Borghoff et al. 2015). In a review of these data, NICNAS (2017) has concluded that, while the evidence supports the liver tumours in mice arising from activation of the CAR, a mechanism other than that involving α2u-globulin may be responsible for the renal tumours in rats; this information, along with the observation of mononuclear leukaemia in male rats and the renal mesenchymal tumours in female rats, support that the conclusion that the tumours in rats are relevant to humans and are sufficient to classify MIBK as a Category 2 carcinogen according to the GHS.
Based on numerous in vivo and in vitro studies MIBK is not considered genotoxic (NTP 2007b; IARC 2013).).
Repeated dose toxicity
Repeated inhalation exposure to MIBK has been associated with effects on liver and kidney weights, biochemical parameters or central nervous system in rats and/or mice at concentrations starting to 410 mg/m3 (Phillips et al 1987; MacEwen et al. 1971; David et al. 1999, all as cited in EPA IRIS 2003b). However, according to the US EPA IRIS (2003b), there were no clear toxicological continuum of severity and/or marked progression of response with increasing concentration or any treatment-related corroborative gross pathologies or histopathological lesions and the observed effects were not considered to be clearly adverse and were therefore considered to be of uncertain relevance to effects in humans after chronic exposures.
In an oral subchronic toxicity study, male and female rats were administered a dose of 0, 50, 250 or 1000 mg/kg-bw/day MIBK by gavage for 13 weeks (MAI, 1986 as cited in US EPA IRIS 2003b and WHO 1990). Alterations in a range of clinical chemistry parameters suggestive of hepatic and renal effects were observed in males and/or females at the highest dose; increased absolute and relative kidney weights were also observed in both sexes at 250 mg/kg bw/day and above. There was an increased incidence of mild nephropathy in males at 1000 mg/kg bw/day. No effects were noted at 50 mg/kg-bw/day, which the WHO (1990) and IARC (2013) considered to be the NOEL. However, the US EPA (US EPA IRIS 2003b) considered 1000 mg/kg bw/day to be a NOAEL, suggesting that the effects observed were difficult to interpret and may not be biologically adverse in light of the absence of clear histopathological changes. The NOAEL for an oral drinking water study was also considered by the US EPA to be around 1000 mg/kg-bw/day, based on uncertainty regarding biological adversity of the observed renal effects (Carnegie-Mellon Institute of Research, 1977a, b as cited in US EPA IRIS 2003b).
In light of the uncertainties regarding the adversity of the effects observed in the subchronic oral studies, for characterization of risk following longer term oral exposure, the LOAEC of 1843 mg/m3 from the more recent, comprehensive inhalation study by the NTP (2007b) is extrapolated to an oral dose of 101 mg/kg bw/day; route to route extrapolation is considered appropriate for the critical internal effects in the kidneys of male and female rats. In addition, NICNAS (2017) recently determined the renal effects observed in the 2 year study in rats to be relevant to humans.
In a dermal study, MIBK was applied to the tails (lower 2/3) of an unspecified number of male white rats daily (4 h/day) in doses of 300 or 600 mg/kg for 4 months. These doses induced morphological changes to the skin, brain, liver, adrenal gland, spleen and testes and a reduction in the number of spermatocytes, spermatids and spermatozoa (Malysheva, 1988 as cited in NTP 2007b).
Short-term and acute toxicity
Short-term inhalation studies were conducted in rats and mice. Effects observed were limited to the liver and kidney. The lowest NOAEL was considered to be 410 mg/m3 based on increased relative kidney weight and hyaline droplet-related tubular nephrosis in males animals (Hazleton Laboratories, Inc. 1968 as cited in EPA IRIS 2003b; Dodd et al., 1982; Phillips et al., 1987 as cited in NTP 2007b and IPCS 1990; MacEwen at al., 1971; Vernot et al., 1971 as cited in EPA IRIS 2003b). Similarly to the longer term studies, the observed effects were not considered to be clearly adverse and were therefore considered to be of uncertain relevance to effects in humans.
MIBK has been shown to be of low toxicity following acute oral, dermal, and inhalation exposure (Smyth et al., 1951 & 1956; Batyrova, 1973; RTECS, 1987; Zakhari, 1977). Neurological effects (principally behavioural changes) were observed in several of the studies described above; however, effects were generally transient or attenuated with prolonged exposure and occurred at concentrations higher than those associated with liver and kidney endpoints. In several studies with human volunteers exposed to up to 200 ppm, MIBK caused reversible irritation and CNS symptoms (US EPA IRIS 2003b).
Reproductive and developmental toxicity
In an inhalation reproductive and developmental toxicity study, groups of 35 pregnant F344 rats and 30 CD-1 mice were exposed GD 6-15 to airborne MIBK concentrations of 0, 300, 1000 or 3000 ppm (0, 1229, 4106 and 12292 mg/m3) (Tyl et al., 1987, as cited in EPA IRIS 2003b and IPCS 1990). In rats, signs of maternal toxicity were observed at 12292 mg/m3, including decreased body weight gain and food consumption (which returned to normal upon cessation of exposure) and increased relative kidney weight. Maternal body weight was not affected in mice although there was increased mortality and absolute and relative liver weights in dams exposed to 12292 mg/m3 MIBK. Other maternal effects, including hypoactivity, ataxia and lacrimation, were observed at 12292 mg/m3. In rats, foetal body weights were decreased at 1229 and 12292 mg/m3, but not at 4106 mg/m3; the study authors attributed this observation to differential litter size and not exposure related. Foetal body weights were also reduced in mice at the highest concentration. At 12294 mg/m3, delays in some skeletal ossification parameters were noted in both rats and mice. Based on this study, the EPA IRIS (2003b) established a NOAEC of 4106 mg/m3 and LOAEC of 12292 mg/m3 for maternal effects and delayed foetal skeletal ossification in rats and mice. A NOAELHEC of 1026 mg/m3 was also derived for continuous exposure (LOAELHEC = 3073 mg/m3). In a 2 generation study in SD rats, signs of CNS depression in pups and transient decreased body weight and food consumption in parents were observed at 8200 mg/m3, with a NOAEC of 4100 mg/m3 (Nemec et al., 2004, as cited in IARC, 2013).
7.2.2.2 MIAK
MIAK has not been assessed by other agencies. The following hazard assessment is based on the information identified from ECHA registration dossier (ECHA c2007-2017b) and US EPA HPVIS (US EPA 2001b).
Toxicokinetics
It has been reported that clearance of MIAK following oral administration is slower than that following inhalation exposure. Results of an in vitro dermal absorption study (OECD TG 428) indicate that dermal absorption is moderate (ECHA c2007-2017b).
Carcinogenicity and genotoxicity
No chronic studies were identified for MIAK. MIAK was not genotoxic in a variety of in vitro studies.
Repeated dose toxicity
In a sub-chronic inhalation study, SD rats were exposed to MIAK vapour at concentrations of 0, 200, 1000 and 2000 ppm (equivalent to 0, 934, 4670 and 9340 mg/m3), for a total 69 exposures spanning 96 days. There were no significant changes in body weight, hematology, serum clinic chemistry or gross pathology. Dose-dependent statistically significant increases of absolute and relative liver weights were seen in males and females at concentrations of 4670 and 9340 mg/m3 . Absolute and relative kidney weights were also increased in males at both these concentrations, and relative kidney weights were elevated in females at the highest dose. Histopathology revealed hyaline droplet formation in the kidneys of male rats, hepatocytic hypertrophy in livers and tubular epithelium regeneration in kidneys in both males and females exposed to MIAK at the two highest concentrations. Overall, the NOEC was determined to be 200 ppm (934 mg/m3) based on organ weight changes and histological changes observed in the livers and kidneys of both males and females at 4670 mg/m3 or higher (Katz et al. 1986; ECHA c-2007-2017b).
With respect to oral exposure, in the only repeated dose oral study identified, only a very high dose was tested (2000 mg/kg-bw/day), which was associated with a wide range of effects (mainly kidney and liver effects) (ECHA c-2007-2017b).
In light of the limitations of the only repeated dose oral study, an oral NOEL of 52 mg/kg bw/dayFootnote 8 was derived using route-to-route extrapolation from the inhalation study, based on the similar effects on liver and kidney by both routes of exposure.
No dermal repeat dose toxicity study was reported for MIAK.
Short term and acute toxicity
Exposure of male and female SD rats to 1000 or 2000 ppm (4670 or 9340 mg/m3) MIAK via the inhalation route for a total of 12 exposures over 16 days resulted in slight, dose-dependent increase in absolute and relative liver weights in rats with no corresponding effects on serum clinical chemistries or histopathology. At 9340 mg/m3, renal hyaline droplet formation was noted in males as was mineralization involving the heart in females (Anonymous 1983; Katz et al. 1986 as cited in ECHA c-2007-2017b). Based on these observations, the authors determined a NOAEC of 1000 ppm (4670 mg/m3).
Systemic effects were also observed in the developmental study decribed below at lower doses. dams exposed to MIAK for 14 days at 3503 mg/m3 and above, showed significant body weight changes and neurological effects (i.e., reduced reactivity to stimuli).The NOAEC for systemic toxicity was considered to be 380 ppm (1775 mg/m3) (ECHA c-2007-2017b).
Reproductive and developmental toxicity
In a reproductive/developmental toxicity screening test, MIAK did not affect a range of parameters investigated in parents or offspring; the substance was therefore not considered to be a reproductive toxicant at up to the highest test concentration of 5000 mg/m3 (ECHA c-2007-2017b).
In a prenatal developmental toxicity test on MIAK, Groups of bred female SD rats were exposed to MIAK at via whole body inhalation at concentrations of 0, 380, 750 or 1500 ppm (equivalent to 0, 1775, 3503 or 7005 mg/m3) from GD 6 through 19. In the 1500 ppm group, significant decreases of mean body weights, mean body weight gains and corresponding mean food consumption were observed in dams throughout the exposure period, while reduced reactivity to a noise stimulus was noted at concentrations of 750 or 1500 ppm. The NOAEC for maternal toxicity was considered to be 380 ppm (1775 mg/m3). No exposure related external, visceral, skeletal malformations or developmental variations were seen in foetuses up to doses of 1500 ppm (7005 mg/m3). However, significantly reduced foetal weights were seen in the 1500 ppm group compared with the control group. Therefore, the NOAEC for developmental toxicity was considered to be 750 ppm (3503 mg/m3) (ECHA c-2007-2017b).
7.2.2.3 DAA
DAA has been reviewed by OECD (2000). This review provides the basis for the health effects characterization in this draft screening assessment. A literature search has been conducted from a year prior to the OECD SIAR report (2000) until January 2017.
Toxicokinetics and metabolism
The low molecular weight, log Kow value, and physical state of DAA favour its absorption via various routes of exposure (oral, dermal, and inhalation); available data suggest that absorption by the oral and inhalation routes is extensive In an in vitro dermal absorption study the skin penetration was found to be 0.04, 0.15 and 5.71 % of the dose (25mg/cm2) after 10 min, 60 min and 24 h, respectively (Fasano and McDougal 2008).
Carcinogenicity and genotoxicity
No carcinogenicity studies on DAA have been identified. DAA was reported to be non-genotoxic in various in vitro Ames tests, mammalian gene mutation assays and mammalian chromosome aberration tests (OECD 2000, ECHA c-2007-2017a).
Repeated dose toxicity
In a combined repeat dose-reproductive/developmental toxicity screening test, DAA was administered daily for 45 days by gavage to male and female SD rats at doses of 0, 30, 100, 300, or 1000 mg/kg bw/day (MHW, 1997 as cited in OECD 2000). Decreased locomotor activity and stimulation response were observed at 300 and 1000 mg/kg bw/day in both sexes while altered haematological and blood chemistry parameters were noted in males at 1000 mg/kg bw/day. In males, renal effects were observed, including hyaline droplet formation at 100 mg/kg bw/day or greater, basophilic tubules at 300 mg/kg bw/day or more and dilatation of distal tubules at 1000 mg/kg bw/day. At 300 and 1000 mg/kg bw/day, females showed dilatation of the distal tubules and fatty degeneration of the proximal tubular epithelium in the kidneys. The OECD considered to NOAELs for repeat dose toxicity to be 30 mg/kg bw/day in males and 100 mg/kg bw/day in females. However, given the hyaline droplet formation considered to be specific to male rats, the NOAEL for male rats was considered to be 100 mg/kg bw/dayIn an inhalation study, Wistar rats were exposed to DAA daily for 6 weeks to concentrations of 0, 233, 1041 and 4685 mg/m3 (Butterworth et al. 1980 as cited in ECHA c2007-2017a). The authors identified a NOAEC of 4685 mg/m3 and a NOEC of 1041 mg/m3. Only liver weight changes, not associated with histological alterations, were observed at 1041 mg/m3. At 4685 mg/m3, increased liver and kidney weights as well as rat-specific eosinophilic hyaline droplets in the proximal tubular cells were observed in males. OECD considered the middle concentration to be a NOAEC (reported as 1035 mg/m3) (SHELL Research Ltd, as cited in OECD 2000).
No repeated-dose dermal studies were available for DAA. Therefore, the oral NOAEL of 100 mg/kg-bw/day will be used to inform the risk from dermal exposure..
Reproductive and developmental toxicity
In the combined repeat dose toxicity study with the reproduction/developmental toxicity screening test described above, there was a non-statistically significant decrease in reproductive parameters (fertility index, number of implantations and implantation index) and in developmental parameters (number of pups born, delivery index, live birth index, number of pups alive and viability index) at the highest dose of 1000 mg/kg bw/day. Although all of these changes were not statistically significant, it was considered that DAA could cause reproductive /developmental effects at 1000 mg/kg bw/day. Based on this the authors considered a NOAEL of 300 mg/kg bw/day for reproductive and developmental effects, with a parental NOAEL of 100 mg/kg bw/day as described above (MHW 1997 as cited in OECD 2000). No effect was observed in another developmental rat study up to dose of 1000 mg/kg-bw/day in rats (ECHA c2007-2017a).
In the absence of inhalation and dermal reproductive/developmental studies on DAA, the oral NOAEL of 300 mg/kg bw/day for reproductive/developmental effects was used.
Chemical name |
MIBK |
MIAK |
DAA |
|---|---|---|---|
Role |
Target chemical |
Target chemical |
Target chemical |
CAS# |
108-10-1 |
110-12-3 |
123-42-2 |
Chemical structure |
 MW = 100.16 |
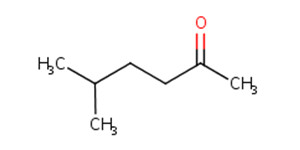 MW = 114.18 |
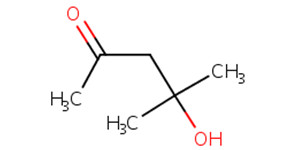 MW = 116.16 |
Acute toxicity Oral |
Rat LD50 = 2080 to 4570 mg/kg-bw Mice LD50 = 1900-2850 mg/kg bw
|
Rat LD50 = 5657 mg/kg bw | Rat LD50 = 3002 mg/kg bw |
Acute toxicity Inhalation |
Rat LC50 = 8200 to 16 400 mg/m3 Mice LC50 (1.25h) = 20 500 to 74 200 mg/m3
|
Rat LC50 (6h)= 17806 mg/m³ NOEC for non-lethal effects = 7486 mg/m3 |
Rat LC0 (4h) = 7600 mg/m3 Rat (6 h) =7230 mg/m³ air based (no effects up to) |
Acute toxicity Dermal |
Rabbit LD50 = >20 000 mg/kg bw |
NA |
Rat LD50 (24h) > 1875 mg/kg bw
|
Short-term (14d) Inhalation |
NOAEC = 410 mg/m³ (increased relative kidney weight and hyaline droplet-related tubular nephrosis) |
NOAEC = 1775 mg/m3 (maternal toxicity in developmental toxicity study) |
NOAEC = 968 mg/m3 (from oral 15-day developmental study) |
Short-term dermal (14d) |
NA |
NOEL = 137 mg/kg bw/day (from inhalation developmental study) |
NOEL = 1000 mg/kg bw/day (from oral 15-day developmental study)
|
Repeat dose toxicity (Oral) |
LOEL/NOAEL = 250 mg/kg bw / day (13-week; Hepatic and renal effects at 1000 mg/kg-bw/d)
LOAEL = 101 mg/kg-be/day (2-year; chronic nephropathy in females) |
NOEL = 52 mg/kg bw/day (from inhalation NOAEC) |
NOAEL = 100 mg/kg/day (45-day; kidney changes and altered hematological and blood chemistry) |
Repeat dose toxicity (Inhalation) |
LOAEC = 410 mg/m³ (increased incidence in renal tubule hyperplasia and chronic nephropathy (female) and mineralization of renal papilla (male))
LOAEC = 1843 mg/m3 (2-year; chronic nephropathy in females) |
NOAEC = 934 mg/m3 (96-day; increased liver and kidney weight and hepatocytic hypertrophy in liver and tubular epithelium regeneration in kidneys in both males and females at 4670 mg/m3) |
NOAEC = 1035 mg/m3 (6-week; liver weight changes without histopathological alterations at 4685 mg/m3) |
Repeat dose toxicity (Dermal) |
LOAEL = 300 mg/kg bw (4-month; lowest dose tested; morphological changes in several tissues)
LOEL/NOAEL = 250 mg/kg bw/day (from MIBK oral) |
NOEL = 52 mg/kg bw/day (from inhalation NOAEC) |
NOAEL = 100 mg/kg/day (from oral NOAEL) |
Developmental and/or Reproductive toxicity (Oral) |
NOAEL = 259 mg/kg bw / day (read-across from DAA) |
NOAECdev = 271 mg/m3 (R2R from inhalation NOAEC) NOAECmat= 137 mg/m3 (from inhalation NOAEC) |
NOAELdevo = 300 mg/kg/day (reproductive and developmental effects at 1000 mg/kg-w/d NOAELmat = 100 mg/kg/day (kidney changes and altered hematological and blood chemistry) |
Developmental and/or Reproductive toxicity (Inhalation) |
NOAECdev/mat of 4106 mg/m3 (based on transient decreased body weight and food consumption and changes to reproductive organs weights in parents and on acute CNS depressive effects in pups) |
NOAECdev = 3503 mg/m3 (reduced foetal weight at 3503 mg/m3) NOAECmat= 1775 mg/m3 (decreases body weight gain and corresponding food consumption at 3505 mg/m3) |
NOAECdevo = 968 mg/m3 (from oral 45-day NOAEL) NOAECdevo/mat = 3182 mg/m3 (from oral study GD6-20) |
Developmental and/or Reproductive toxicity (dermal) |
NOAEL = 259 mg/kg bw / day (read-across from DAA) |
NOAECdev = 271 mg/m3 (from inhalation NOAEC) NOAECmat= 137 mg/m3 (from inhalation NOAEC) |
NOAELdevo = 300 mg/kg/day NOAELmat = 100 mg/kg/day |
Genetic toxicity |
Negative |
Negative |
Negative |
Carcinogenicity |
Positive |
Possible |
Possible |
Abbreviations: MW, molecular weight (g/mol); NA, not available
7.2.3 Risk characterization of subgroup 2 (MIBK, MIAK, and DAA)
MIBK
MIBK is classified as a Category 2B carcinogen (“possibly carcinogenic to humans”) by IARC (2013), based on increased incidences of tumors in 2 year studies in rats and mice. Although some evidence suggested that the liver and kidney tumors may not be relevant to human, recent analyses by NICNAS (2017) have concluded that, while the evidence supports the liver tumours in mice arising from activation of the constitutive androstane receptor (CAR), a mechanism other than that involving α2u-globulin may be responsible for the renal tumours in rats. This information, along with the observation of mononuclear leukaemia in male rats and the renal mesenchymal tumours in female rats, support the conclusion that the tumours in rats are relevant to humans and are sufficient to classify MIBK as a Category 2 carcinogen according to the GHS (NICNAS 2017). However, MIBK is not expected to be genotoxic (NTP 2007b; IARC 2013).
The chronic inhalation LOAEC for non-cancer effects was established at 1843 mg/m3, based on minimal to mild nephropathy in the 2-year bioassay (NTP 2007b).
The available data from short-term MIBK inhalation studies in animals indicate that developmental effects were the critical health effects. A NOAEC of 4106 mg/m3 was identified for developmental effects in mice and rats (Tyl et al. 1987 as cited in US EPA IRIS 2003b). The corresponding calculated NOAECHEC for developmental effects was 1026 mg/m3 for continuous exposure.
For the oral route, in light of the uncertainties regarding the adversity of the effects observed in the subchronic oral studies, for characterization of risk following longer term oral exposure, the LOAEC from the more recent, comprehensive inhalation study by the NTP (2007) is extrapolated to an oral dose of 101 mg/kg bw/day; route to route extrapolation is considered appropriate for the critical effects in the kidneys of male and female rats. In addition, NICNAS (2017) recently determined the renal effects observed in the 2 year study in rats to be relevant to humans.
In the only dermal study available, MIBK induced morphological changes to the skin and multiple organs in rats administered doses of 300 mg/kg-bw/day and higher for four months (Malysheva, 1988 as cited in NTP 2007b). Although only limited details were available for this study, the critical effect level of 300 mg/kg bw/day is supported by the effects observed in rats administered 250 mg/kg bw/day the 13 week oral study.
The predominant source of exposure to MIBK from environmental media and food for the general population is through the diet. Based on the available data, it is expected that the majority of the dietary exposure to MIBK results from its natural occurrence in foods.
MIBK is used in a number of products available to consumers, including rubbing alcohol, dry erase markers and a range of paint and do-it-yourself products. Table 7‑11 provides all the relevant exposure values and the critical health effects as well as the resultant MOEs for the characterization of risk for MIBK.
Exposure Scenario |
Exposure |
Critical effect level |
Critical health effect endpoint |
MOE |
|---|---|---|---|---|
Environmental media |
2.5 – 7.5 µg/kg-bw/day |
LOEL/NOAEL = 101 mg/kg/day |
Renal effects in rats (hyperplasia, nephropathy, mineralization) in 2 year study (R2R from inhalation study) |
13 467 – 40 400 |
Food flavouring |
0.03 – 0.12 µg/kg-bw/day |
LOEL/NOAEL = 101 mg/kg/day |
Renal effects in rats (hyperplasia, nephropathy, mineralization) in 2 year study (R2R from inhalation study) |
≥ 850 000 |
Rubbing alcohol (inhalation) |
6-hr TWAa = 0.001 mg/m3
|
NOAEC = 4106 mg/m3 |
Skeletal variations in mice and rats and reduced foetal body weight and increased foetal death in mice |
4 106 000 |
Wood lacquer (inhalation) |
6-hr TWAa = 45 – 433 mg/m3 |
NOAEC = 4106 mg/m3 |
Skeletal variations in mice and rats and reduced foetal body weight and increased foetal death in mice |
9 – 91 |
Liquid paint (solvent-rich) for trucks (inhalation) |
6-hr TWAa = 48 mg/m3 |
NOAEC = 4106 mg/m3 |
Skeletal variations in mice and rats and reduced foetal body weight and increased foetal death in mice |
86 |
Spray paint (inhalation) |
6-hr TWAa = 0.4 – 83 mg/m3 |
NOAEC = 4106 mg/m3 |
Skeletal variations in mice and rats and reduced foetal body weight and increased foetal death in mice |
49 – 10 265 |
Filler/putty from tube (inhalation) |
6-hr TWAa = 1 – 6 mg/m3 |
NOAEC = 4106 mg/m3 |
Skeletal variations in mice and rats and reduced foetal body weight and increased foetal death in mice |
684 – 4 106 |
Dry erase markers (per event) [oral] |
0.48 mg/kg-bw (child) 0.97 mg/kg-bw (toddler) |
LOEL = 300 mg/kg/day
LOEL/NOAEL = 250 mg/kg/day |
Morphological changes in several tissues at lowest dose tested in a 4-month dermal study.
Hepatic and renal effects at 1000 mg/kg-bw/d in a 13-week oral study. |
309 – 625
258 – 520 |
Dry erase markers [inhalation] |
6-hr TWAa = 0.45 mg/m3
|
NOAEC = 4106 mg/m3 |
Skeletal variations in mice and rats and reduced foetal body weight and increased foetal death in mice |
9 124 |
a Six-hour TWA concentratrions were derived for all product scenarios to match up with the exposure durations of the critical effects study used to characterize risk.
Calculated MOEs for exposure to MIBK in environmental media and food (flavouring agent) are considered adequate to address any uncertainties in the health effects and exposure databases. Additional intake of MIBK from its natural occurrence in food was not identified as a concern for human health. Furthermore, the JECFA (WHO 1999b) concluded "No safety concern with the estimated levels of intake as flavouring substances" for MIBK used as a flavouring agent based primarily on it being “metabolized to inoccuous products”. The MOEs for inhalation exposure to MIBK in some products available to consumers, namely wood lacquer, liquid paint, and spray paints are considered potentially inadequate in light of the severity of the observed effects (i.e., developmental toxicity) and uncertainty regarding the adversity of effects observed at lower concentrations.
MIAK
No chronic studies were identified for MIAK.MIAK was not genotoxic in a variety of studies.
Subchronic exposure to MIAK induced liver and kidney effects (organ weight and histological changes) in rats exposed to concentrations of 4670 mg/m3 or higher. The NOEC was determined to be 934 mg/m3 (Katz et al. 1986; ECHA c-2007-2017b). With respect to oral exposure, in the only repeated dose oral study identified only a very high dose was tested (2000 mg/kg-bw/day), which was associated with a wide range of effects. Therefore, an oral NOEL of 52 mg/kg bw/day was derived using route-to-route extrapolation from the inhalation study, given the similarity effects on liver and kidney by both routes of exposure.
With respect to short-term inhalation exposure to MIAK, a NOAEC of 1775 mg/m3 was used to characterize the risk based on maternal systemic effects observed at 3503 mg/m3 and above in a developmental toxicity study (reduced reactivity to noise stimulus concentrations, decreased body weights, body weight gains and food consumption ) (ECHA c-2007-2017b).
The predominant source of exposure to MIAK from environmental media and food is from its natural presence in certain foods, and to a lesser extent in indoor air.
MIAK is also used in a few paint products available to consumers. Table 7‑12 provides all relevant exposure estimates and critical effect values, as well as resulting MOEs.
Exposure Scenario |
Exposure |
Critical effect level |
Critical health effect endpoint |
MOE |
|---|---|---|---|---|
Environmental media |
0.3 – 0.9 µg/kg-bw/day |
NOEL = 52 mg/kg/day |
Route-to-route extrapolation from NOEC = 934 mg/m3 |
≥ 57 778 |
Spray paint (inhalation) |
6-hr TWAa = 3 – 19 mg/m3 |
NOAEC = 1775 mg/m3 |
Significant decreases of mean body weights, mean body weight gains and corresponding mean food consumption in dams at 7005 mg/m3, while reduced reactivity to a noise stimulus at 3503 mg/m3 |
93 – 592 |
a Six-hour TWA concentratrions were derived for the spray paint scenario to match up with the exposure durations of the critical effect study used to characterize risk.
Calculated MOEs for exposure to MIAK in environmental media are considered adequate to address any uncertainties in the health effects and exposure databases. Additional intake of MIAK from its natural occurrence in food was not identified as a concern for human health. The MOEs for inhalation exposure to MIAK in spray paints available to consumers is considered adequate to address uncertainties in the health effects and exposure databases.
DAA
No chronic studies were identified for DAA. An oral NOAEL of 100 mg/kg-bw/day for the systemic toxicity (kidney changes and altered hematological and blood chemistry in rats), from the reproductive/developmental study, is used to address the risk to chronic exposure to DAA (MHW, 1997 as cited in OECD 2000; ECHA c-2007-2017a). In the absence of dermal studies, route-to-route extrapolation using the oral NOAEL is used to characterize the risk to dermal exposition of DAA.
In the only inhalation study in animals identified, only liver weight changes not associated with histological alterations were observed at 1041 mg/m3 (reported as 1035 mg/m3 by OECD 2000) in a 6-week inhalation study (Butterworth et al. 1980 as cited in ECHA c2007-2017a).
The reproductive and developmental NOAEL was established at 300 mg/kg-bw/day based on effects on numerous reproductive and developmental parameters at 1000 mg/kg-bw/day. In order to be protective of the developing foetus and children, a critical effect level of 300 mg/kg-bw/day is used to characterize risk for short-term use of products available to consumers. In the absence of dermal or inhalation reproductive/developmental studies on DAA, the oral NOAEL of 300 mg/kg bw/day was used.
The primary source of exposure to DAA from environmental media and food is from its natural occurrence in various food items.
DAA is used in a range of products available to consumers, including nail care products and permanent markers, pipe thread sealant, floor coatings, thinner, and spray products including paint removers and automotive cleaners.
Table 7‑13 provides all relevant exposure estimates and critical effect values, as well as resulting margins of exposure (MOEs). Although limited, available dermal absorption data suggest that DAA is not extensively absorbed through the skin (less than 1% to approximately 6% in humans); therefore, the dermal estimates below incorporate a 6% dermal absorption value. Given that the same oral health effects study is being used to characterize risks from both potential dermal and inhalation exposures to DAA from use of products available to consumers, these exposure estimates were combined.
Exposure Scenario |
Exposure |
Critical effect level |
Critical health effect endpoint |
MOE |
|---|---|---|---|---|
Cosmetics (inhalation) |
6-hr TWA = 0.3 – 1.3 mg/m3 | NOAEC = 1035 mg/m3 |
Liver weight changes without histopathological alterations at 4685 mg/m3 |
796 – 3 450 |
Cosmetics (dermal) |
0.007 – 0.016 mg/kg-bw/day |
NOAEL = 100 mg/kg/day |
Kidney changes and altered hematological and blood chemistry |
6 250 – 14 286 |
Paint/DIY products (inhalation and dermal) |
0.053 – 2.5 mg/kg-bw/day |
NOAEL = 300 mg/kg/day |
Reproductive and developmental effects |
120 – 5 660 |
Permanent markers (per event) [oral] |
1.6 mg/kg-bw (child) 3.23 mg/kg-bw (toddler) |
NOAEL = 300 mg/kg/day |
Reproductive and developmental effects at 1000 mg/kg-bw/day |
93 – 188 |
Permanent markers (daily) [dermal] |
0.00244 – 0.05 mg/kg-bw/day |
NOAEL = 100 mg/kg/day |
Kidney changes and altered hematological and blood chemistry |
1 250 – 2 500 |
Calculated MOEs for exposure to DAA in environmental media, and from use of products available to consumers, including the assumption of 100% DAA in permanent markers and the non-statistically significant changes in reproductive and developmental parameters used to characterize the risk associated with use of markers and paint/DIY products, calculated margins of exposure are considered adequate to address uncertainties in the health effects and exposure databases. Additional intake of DAA from its natural occurrence in food was not identified as a concern for human health.
7.2.4 Uncertainties in evaluation of risk to human health for MIBK, MIAK and DAA
The key sources of uncertainty are presented in the table below. Confidence is high that use of the maximum concentrations and the high end of the range of product amounts from these types of products do not underestimate general population exposures.
For MIBK, although the MOEs for chronic exposures are based on non-cancer effects, confidence is high that they are also protective of the carcinogenicity of MIBK in light of a likely non-genotoxic mode of action.
For MIAK, the MOE for use of spray paints is considered adequate in light of the minimal maternal effects in the critical developmental toxicity effects study and the conservativeness of exposure estimate.
In terms of the risk from use of products available to consumers for DAA, confidence in the adequacy of the margins is high in light of the conservativeness associated with the dermal absorption value and the non-statistically significant effects on reproductive and developmental parameters at the critical effect level.
Key sources of uncertainty |
Impact |
|---|---|
Assumption that dermal exposures are minimal for MIBK and MIAK in comparison to inhalation exposures, given the high volatility of the substances; however, confidence is high that exposure is overestimated in light of the conservative nature of the exposure models. |
+ |
Assumption that DAA is present in permanent markers at a concentration of 100% |
+ |
Lack of chronic studies for MIAK or DAA. |
- |
There are uncertainties associated with the selection of effect levels for characterization of risk of MIBK from inhalation and oral exposure. |
- |
Use of route-to-route extrapolation 96-day inhalation study for both oral and dermal exposure scenario for MIAK. |
+/- |
No short-term inhalation study was available for DAA. Route-to-route from oral study was applied. |
+/- |
+ = uncertainty with potential to cause over-estimation of exposure/risk;
- = uncertainty with potential to cause under-estimation of exposure risk;
+/- = unknown potential to cause over or under estimation of risk.
7.3 Assessment of subgroup 3 (diacetyl, 2,3-PD, and acetoin)
7.3.1 Exposure assessment of subgroup 3 (diacetyl, 2,3-PD, and acetoin)
Environmental media
No empirical data were identified for diacetyl, 2,3-PD or acetoin in air, water and soil in Canada.
Diacetyl has been identified as a by-product of ozone disinfection, and was detected in drinking water and in surface water (HSDB 1983-). No data were identified regarding the presence of diacetyl in soil or sediment; however, based on its low log Koc, diacetyl is not expected to be present in this media.
Only one ambient air study was identified that detected 2,3-PD in air in Roseville, California, in the summer and winter at concentrations of 11.1 and 8.1 ng/cm3, respectively (HSDB 1983-). No other environmental concentration data were identified elsewhere.
Based on its physical and chemical properties, acetoin may be present in air and water but is not likely to be present in soil and sediment. Based on its moderate Henry’s Law Constant, acetoin is expected to volatilize from water. The half-life in air is approximately 37 hours (HSDB 1983-).
ChemCAN was run to derive potential environmental concentrations of diacetyl, 2,3-PD, and acetoin for Canada using the upper-end volume data from Table 4‑1. These concentrations were used to estimate exposure to diacetyl, 2,3-PD, and acetoin from environmental media for the general population of Canada. All resulting exposures were less than 2.5 ng/kg-bw/day. Based on the the information presented above, exposure to these substances from environmental media is not expected.
Food
Diacetyl, 2,3-PD and acetoin are naturally present in various food items and may also be used as flavouring ingredients (Nijssen et al. 1963-2016, Burdock 2010). Diacetyl is being replaced in some products by 2,3-PD, acetoin and other diketones with a similar “butter” aroma and taste (Gaffney et al. 2015, Doepker et al. 2012, CDC 2016).
The Joint FAO/WHO Expert Committee on Food Additives (JECFA) evaluated a group of aliphatic acyclic and alicyclic alpha-diketones and related alpha-hydroxyketones including diacetyl, 2,3-PD and acetoin (WHO 1999a). As part of that evaluation, the Committee estimated the per capita intake for diacetyl, 2,3-PD and acetoin from their use as food flavouring agents. For the U.S. population, per capita intake estimates are 133 µg/kg-bw/day, 1 µg/kg-bw/day, and 29 µg/kg-bw/day, respectively. For the European population, per capita intake estimates are 56 µg/kg-bw/day, 4 µg/kg-bw/day, and 46 µg/kg-bw/day, respectively (WHO 1999a) (see Appendix A for more details).
Quantitative exposure estimates for diacetyl, 2,3-PD and acetoin from its natural occurrence in foodFootnote 9 ranged from 281 µg/kg-bw per day for 14 to 18 year old to 1625 µg/kg-bw per day for 1 year olds for diacetyl, from 6.5 µg/kg-bw per day for 6 to 12 month olds to 208 µg/kg-bw per day for adults 19 years and older for 2,3-PD, and from 161 µg/kg-bw per day for 14 to 18 year olds to 369 µg/kg-bw per day for 2-3 year olds for acetoin (see Appendix A for details).
The estimates of daily intake for diacetyl from its natural occurrence in food are based primarily on the high level in pasteurized milk identified in the literature (approximately 30 µg/g) (De Leonardis et al. 2013). Higher levels of diacetyl have been reported in raw milk and ultra-high temperature milk (UHT) (Macciola et al. 2008, De Leonardis et al. 2013). However, other studies reported diacetyl concentrations in milk ranging from 0.0002 to 0.024 µg/g (Nijssen et al. 1963-2016, Toso et al. 2002, Shimoda et al. 2000, Valero et al. 2001, Imhof et al. 1995). Assuming levels of diacetyl in milk in Canada are similar to those in Europe, the highest value in pasturized milk (semi-skim) from the De Leonardis et al. (2013) study was selected. The authors explain that the analytical method that was used in this study extracts the diacetyl that is linked primarily to the proteins and lactose found in the milk. The other studies used headspace techniques which only capture the volatile portion of the substance in the food (De Leonardis et al. 2013).
In addition to oral exposures to these substances, it is possible that, for certain food items such as microwaved popcorn, inhalation exposures may also be important. According to Rosati et al. (2007), diacetyl and acetoin were measured in a chamber air study with concentrations in the chamber air ranging from 0.02 to 5.8 mg/m3 and 0.01 to 4.2 mg/m3 for diacetyl and acetoin, respectively. The average amount of diacetyl emitted from a bag of microwaved popcorn was 778.9 µg/bag (Rosati et al. 2007). Based on information from Rosati et al. (2007) and an approach outlined in Zhu et al. (2001), for diacetyl, this converts to approximately 0.03 mg/m3 in a standard room 1 hour after popping the popcorn (maximum of 0.04 mg/m3 during the first hour after popping) (see Appendix C). Diacetyl and 2,3-PD have also been measured in coffee roasting plants (McCoy et al. 2017) and diacetyl was measured in a study designed to simulate exposures that could occur in a small coffee shop (Pierce et al. 2015)
Products available to consumers
Diacetyl was not listed in any cosmetics notified to Health Canada; however, it was identified in a hair styling product available in Canada at 0.1-1% (SDS 2008a). 2,3-PD was identified in certain air fresheners for home care, and in fragrant oils which can be used as an air freshener (SDS 2016a, 2015 a,b). Table 7‑15 summarizes the estimated inhalation exposures for products available to consumers containing diacetyl or 2,3-PD. Although dermal exposure could contribute to the overall exposure during use of products available to consumers, the primary route is considered to be inhalation; therefore, only inhalation estimates are presented. Acetoin was not identified in any other products available to consumers in Canada.
Product scenario |
Concentration range |
Mean event concentration (mg/m3) |
Mean concentration on day of exposure (mg/m3) |
|---|---|---|---|
Hair styling product (diacetyl) |
0.1 – 1% |
0.24 – 2.4 |
0.002 – 0.016 |
Air freshenera (2,3-PD) |
0.1-5% |
0.0031 – 0.064 |
0.0011 – 0.035 |
a Includes use of essential oils as air fresheners, as well as plug-in and gel type air fresheners.
7.3.2 Health effects assessment of subgroup 3 (diacetyl, 2,3-PD, and acetoin)
Diacetyl and 2,3-PD are alpha-diketones and their structures are identical except for an additional methyl group on 2,3-PD. Diacetyl, 2,3-PD and acetoin are all used in the food flavouring industry. The similarity in the structures and functional groups in diacetyl and 2,3-PD is reflected in their comparable physicochemical properties and similar buttery flavour and sensory sensation. 2,3-PD is expected to be similarly readily bioavailable via the oral, dermal, and inhalation routes as diacetyl and the impact of the additional methyl group in 2,3-PD is expected to be minimal. Both chemicals exhibit portal-of-entry effects to respiratory tract following inhalation exposure. Following oral exposure diacetyl is rapidly metabolized in liver to acetoin and 2,3-butanediol. For 2,3-PD the OECD QSAR Toolbox v4 in vivo and in vitro rat liver metabolism simulator predicted metabolites 2-hydroxy-3-pentanone, 3-hydroxy-2-pentanone and 2,3-pentanediol (among others) that were similar in structure to metabolites of diacetyl. Based on this information, diacetyl, acetoin and 2,3-PD were treated as a group and toxicity data for these substances were used to read-across within the group.
7.3.2.1 Diacetyl
Diacetyl has been reviewed by IPCS (1999), EFSA (2004), SCOEL (2014), CDC (2016). These reviews provide a basis for the health effect characterisation in this draft screening assessment. A literature search has been conducted from a year prior to the CDC report (2016) up to July 2017 and significant new information is included to support risk characterisation.
Toxicokinetics
Diacetyl can be generated endogenously and is a metabolite of acetaldehyde in mammals (IPCS 1999; SCOEL 2014). It is anticipated that methyl ketones are principally metabolized by oxidation of the terminal methyl group at low concentrations. At higher levels, diacetyl is reduced to acetoin, 2,3-butanediol and then conjugated with glucuronic acid and excreted. A computational fluid dynamics-physiologically based pharmacokinetic model has been developed to compare diacetyl absorption and tissue concentrations in the rat and human respiratory tracts (Gloede et al. 2011). The model estimated that the bronchiolar tissue concentrations of diacetyl in the human during light exercise exceeded those in the rat by 20 to 40 fold. Further dosimetry modeling indicated that rat inhalation toxicity studies under-predict the risk of bronchiolar injury in the human (Cichocki and Morris 2017).
Carcinogenicity and chronic toxicity
Diacetyl has not been classified by any agency on the basis of carcinogenicity or other health effects. However, recent NTP studies (NTP 2017a) demonstrated some evidence of carcinogenic activity in 2-year inhalation studies. Groups of Wistar Han rats and B6C3F1/N mice were exposed to diacetyl vapor by whole body inhalation at concentrations of 0, 12.5, 25, or 50 ppm (0, 45, 90, 179 mg/m3) 6 hours per day, 5 days per week for 105 weeks. In rats, there were increases in the incidences of squamous cell carcinomas of the nasal cavity in males and females and in the combined incidence of squamous cell carcinomas and papilloma in the nasal cavity of males at 179 mg/m3. In mice, the incidence of adenocarcinoma of the nose was increased in females at this concentration. NTP concluded that “There was some evidence of carcinogenic activity of diacetyl in male and female rats. There was no evidence of carcinogenic activity of diacetyl in male B6C3F1/N mice exposed to 12.5, 25, or 50 ppm. There was equivocal evidence of carcinogenic activity of diacetyl in female B6C3F1/N mice based on the occurrences of adenocarcinoma of the nose” (NTP 2017a).
Exposure to diacetyl also resulted in increased incidences of non-cancer effects in the nose, larynx, trachea and lung of mice at all concentrations tested and in rats at 25 and 50 ppm, with more severe effects on the respiratory system occurring at higher concentrations in mice and rats. In rats, the nasal lesions with significantly increased incidences included suppurative inflammation, respiratory epithelium hyperplasia and squamous metaplasia, olfactory epithelium atrophy, respiratory metaplasia, and necrosis (males), turbinate hyperostosis, and fibrosis of the lamina propria. Significantly increased incidences of chronic active or suppurative inflammation and epithelium hyperplasia were also observed in the larynx, trachea, lung and eye at 50 ppm. At 25 ppm, significantly increased incidences were observed for squamous epithelium hyperplasia in larynx (male and female), epithelium regeneration in trachea (male) and epithelium hyperplasia in lung (female). In mice, similar effects were observed at 12.5 ppm and also included metaplasia of the respiratory epithelium, atrophy of olfactory epithelium and turbinate atrophy in the nose, and squamous epithelium hyperplasia in the larynx. Survival rate was moderately decreased in females at 25 ppm and significantly decreased in males at 50 ppm. At the end of the study, mean body weights were decreased for both males and females at 50 ppm compared with controls. Thus a chronic inhalation LOAEC of 12.5 ppm (45 mg/m3) is considered based on these observations of non-cancer effects observed at 12.5 ppm in mice and 25 ppm in rats (NTP 2017a).
Available information indicated that diacetyl is mutagenic in a variety of in vitro test (More et al. 2012, Kato et al. 1989, Bjeldanes and Chew 1979; Marnett et al. 1985; Shane et al. 1988; Dorado et al. 1992; Aeschbacher et al. 1989, reviewed in EFSA 2004 and NTP 2017a). However, diacetyl was negative in the SOS-chromotest in E. coli (Von der Hude et al. 1988) and did not induce chromosome aberration in S. cerevisiae (Zimmermann and Mohr 1992).
Repeat dose toxicity
A profile of effects similar to that seen in the chronic bioassay was observed in subchronic inhalation studies in Wistar Han rats and B6C3F1/N mice. A NOAEC of 12.5 ppm (45 mg/m3) was derived based on significant increase in incidence of nonneoplastic lesions in the respiratory tracts (nose, larynx, trachea and lung) of mice and rats, primarily in the 50 and 100 ppm group after 14 weeks. At 25 ppm, increased incidences of squamous metaplasia of the respiratory epithelium were observed in male and female rats along with increased degeneration of the olfactory epithelium in female rats. In mice, there were increased incidences of necrosis in respiratory epithelium in males and females at 25 ppm; increased hyperplasia and chronic active inflammation were also observed in larynx in females (NTP 2017a). Similar effects were reported in an earlier study in mice exposed diacetyl for 6 or 12 weeks to 25 ppm or more; the lowest concentration tested (25 ppm or 90 mg/m3) was considered to be the LOAEC, based on the significant epithelial injury and peribronchial lymphocytic inflammation (Morgan et al. 2008).
In an oral repeat dose toxicity study, male and female CFE rats were administered 0, 10, 30, 90 or 540 mg/kg-bw/day of diacetyl in water by oral intubation for 90 days. At the highest dose of 540 mg/kg-bw/day, rats had decreased body weight gain and increased water consumption. Effects observed included anaemia, an increased leucocyte counts and an increase in relative weights of brain, liver, kidney, adrenals and pituitary glands (organ weight increases were greater than changes likely to be associated with decreased body weight). Ulcers in both squamous and glandular parts of the stomach mucosa were observed. No adverse effects were noted in lower dose groups. However, slight, but not statistically significant, anaemia and increased relative weights of some organs were noted at lower doses in the absence of histopathological changes in the stomach. The authors concluded that the NOAEL of diacetyl in this study was 90 mg/kg-bw/day, although it is not clear whether the effects observed at 540 mg/kg-bw/day may have been secondary to the ulcer formation (Colley et al. 1969; reviewed in IPCS 1999; SCOEL 2014).
The results of another repeated-dose oral study suggest that diacetyl may induce neurological and reproductive effects (Bawazir 2016). Daily administration of 25 mg/kg-bw/day diacetyl to rats via oral tube during 4-week study resulted in changes in levels of several neurotransmitters in different areas of the brain as well as a decrease in serum testosterone levels. Histological changes in the testes, associated with a significant decrease in mature sperm and tubular deficit. However, the published account of this study was very limited and only one dose was tested.
Short term and acute toxicity
Acute and short-term exposures to diacetyl vapour caused a wide range of nasal and olfactory lesions in rodents (Morgan et al 2008, Hubbs et al. 2002; Larsen et al. 2009; Morris and Hubbs 2009), affected sensory neurons (Goravanahally et al. 2014) and led to airway hyporeactivity (Zaccone et al. 2013).
Hubbs et al. (2008), exposed male Hla:(SD)CVF rats (6/exposure group) to diacetyl at concentrations over a 6 hour period as continuous exposure at time weighted average (TWA) concentrations of 0, 99.3, 198.4, or 294.6 ppm (0, 355, 710 or 1055 mg/m3), as 4 brief, intense exposures at TWA concentrations of 0, 122, 225, or 365 ppm (0, 437, 806 or 1307 mg/m3) or as a single 15 minute pulse exposure to a TWA concentration of 92.9 ppm (333 mg/m3) . Both pulsed and continuous exposure patterns caused epithelial injuries including epithelial necrosis and suppurative/fibrinosuppurative inflammation in the nose, larynx, trachea, and bronchi. The most severe effect was observed in the nose. The larynx and trachea were affected at diacetyl concentrations of 225 ppm or higher and bronchi were affected at concentrations of 294.6 ppm or more. In rats exposed to the single 15 minute pulse exposure (TWA of 92.9 ppm), there were significant histological changes (i.e., necrosis) in the nose. The authors concluded that the NOAEC for acute inhaled diacetyl was less than 93 ppm (333 mg/m3), considered here to be the LOAEC.
Reproductive and developmental toxicity
In groups of CD‐1 mice and albino Wistar rats administered diacetyl by gavage on days 6‐15 of gestation at doses of up to 1600 mg/kg-bw/day, no effects were seen on maternal survival, weight, or reproductive parameters or on foetal survival or microscopic appearance of external, skeletal, or soft tissues. Similarly, no maternal or developmental effects were observed in hamsters administered doses up to 1600 mg/kg-bw/day on gestation days 6-10 (US FDA 1973; reviewed in WHO 1999a; EFSA 2004).
Human studies
In several epidemiological studies, inhalation of diacetyl vapours and other ketones by workers at a microwave popcorn manufacturing plant or in the flavouring manufacturing companies has been consistently associated with the development of diverse respiratory impairment conditions such as obliterative bronchiolitis (OB), a rare disease of airway epithelial injury and airway fibrosis (Kreiss et al. 2002; Parmet and Von Essen 2002; van Rooy et al. 2007; CDC 2016). Several case reports, case series and cross-sectional studies have also been reviewed by EU (SCOEL 2014) and CDC (2016).
7.3.2.2 2,3-Pentanedione
2,3-Pentanedione (2,3-PD) has been reviewed by WHO/IPCS (1999), EFSA (2004) and CDC (2016). These reviews provide a basis for the health effect characterisation in this draft screening assessment. A literature search has been conducted from a year prior to the CDC (2016) up to July 2017 and significant new information is included to support risk characterisation.
Toxicokinetics
Zaccone et al. (2015) showed that 2,3-PD could be metabolized to acetoin and 2,3-hydroxy-3-pentanone, respectively, by human airway epithelium.
Carcinogenicity and genotoxicity
No carcinogenicity data were identified for 2,3-PD. Two studies showed that 2,3-PD was not mutagenic in Salmonella (Kim et al. 1987; Aeschbacher et al. 1989; reviewed in WHO1999 and EFSA 2004, 2016). 2,3-PD did not cause the formation of micronuclei in either rats or mice of both sexes (NTP 2017b).
Repeated dose toxicity
In a repeated dose inhalation study, male and female Wistar Han rats and B6C3F1 mice were exposed to 2,3-PD by inhalation at concentrations of 0, 6.25, 12.5, 25, 50, or 100 ppm (0, 26, 51, 102, 205 or 409 mg/m3) 6 hours/day, 5 days/week for 14 weeks. Similar results to those reported in the subchronic study with diacetyl were observed. At 50 and 100 ppm, clinical observations were noted including abnormal breathing, sneezing and eye abnormality. Significantly increased incidences of non-neoplastic lesions occurred in the respiratory tracts of male and female rats, including epithelium hyperplasia in the nose and metaplasia in the larynx. At 25 ppm and above, the incidences of respiratory epithelium hyperplasia in the nose were significantly increased in males, while the incidences of respiratory epithelium metaplasia in the larynx were significantly increased in females. In mice, there were significant decreased body weight gain and changes in internal organ weights of both sexes (in the absence of histopathological alterations) at 50 ppm (205 mg/m3) and higher, along with increased incidences of non-neoplastic lesions of the respiratory tract. At 25 ppm, incidences of respiratory epithelium metaplasia and regeneration were significantly increased in male mice. The NTP considered the NOAEC for respiratory tract effects in both rats and mice to be 12.5 ppm (51 mg/m3) and the NOAEC for non-respiractory tract effects in mice to be 25 ppm (102 mg/m3) (NTP 2017b).
No oral repeat dose toxicity study was reported for 2,3-PD. Therefore, route-to-route extrapolation of the NOAEC for non-respiratory tract effects was carried out from the subchronic inhalation study on 2,3-PD to obtain a NOAEL of 24 mg/kg bw/day (see appendix D). The NOAEL from the oral subchronic study with diacetyl was also used in a read-across manner to estimate a NOAEL of 105 mg/kg-bw/day for 2,3-PD, as support for hazard characterization .
Acute and short term repeated dose toxicity
Acute and short-term exposure to 2,3-PD for up to two weeks induced a range of histopathological lesions (including necrotizing rhinitis, tracheitis, mucosal inflammation, squamous metaplasia and regenerative changes, epithelial atrophy, exudate and lymphoid deposits) in nose, larynx, trachea and/or lungs of mice and rats at all concentrations tested. (Morgan et al. 2012; Hubbs et al. 2012; Zaccone et al. 2013; NTP 2017a).
7.3.2.3 Acetoin
Acetoin has been reviewed by WHO/IPCS (1999), EFSA (2004, 2016). These reviews provide a basis for the health effect characterisation in this draft screening assessment. A literature search has been conducted from a year prior to EFSA (2016) up to July 2017 and some information was identified in NTP draft technical report on carcinogenicity of diacetyl (NTP 2017a).
Toxicokinetics
Acetoin is metabolised primarily via oxidation at low concentration in vivo and by reduction to 2,3-butanediol at high concentration (EFSA 2004; NTP 2007a).
Carcinogenicity and chronic toxicity
In a screening test for pulmonary carcinogenicity of food additives, in which a limited number of tissues were evaluated, no increase in lung tumors was observed in strain A mice administered acetoin by intraperitoneal injection 3 times per week for 7 weeks (Stoner et al. 1973).
Genotoxicity
Several studies indicated that acetoin was not mutagenic in Salmonella or E. coli (EFSA 2004; WHO 1999a; NTP 2017b).
Repeat dose toxicity
In an oral study, male and female CFE rats (15/group) were administered acetoin in the drinking water at concentrations of 0, 750, 3000, or 12000 ppm (equivalent to 0, 85, 330, or 1300 mg/kg-bw/day) for 13 weeks (Gaunt et al. 1972). At concentrations of 750 and 3000 ppm, no significant effects were observed with respect to body weight gain, haematological findings, serum chemistry, renal cell excretion, urinary concentration tests, organ weights or histopathology. At the highest concentration, body weights were significantly decreased in males from week 5 and relative liver weight was significantly increased at weeks 2, 6, and 13; females showed these effects after 13 weeks. These effects were not accompanied by any histopathologic changes and probably due to metabolic load from acetoin intake. Small but statistically significant decreases in hemoglobin concentration and erythrocyte counts were also observed at the high dose in both sexes. The authors concluded that the NOAEL was 3000 ppm in the drinking water (equivalent to 330 mg/kg-bw/day), based on the effects of decreased body weight gain and haematologic effects (Gaunt et al. 1972, reviewed in IPCS 1999).
Male and female Wistar Han rats and B6C3F1/N mice were exposed to acetoin by whole body inhalation at concentrations of up to 800 ppm (2883 mg/m3) 6 hours per day, 5 days/week for 14 weeks. No exposure related effects on survival, body weights, organ weights, clinical pathology, or histopathology were observed in either species following exposure to acetoin concentrations up to the highest concentration tested (i.e.c 800 ppm or 2883 mg/m3) (NTP 2017b).
Chemical name |
Diacetyl |
Acetoin |
2,3-PD |
|---|---|---|---|
Role |
Target chemical |
Target chemical |
Target chemical |
CAS# |
431-03-8 |
513-86-0 |
600-14-6 |
Chemical structure |
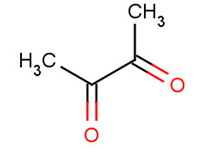 MW = 86.09 |
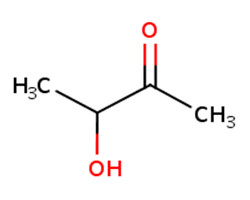 MW = 88.10 |
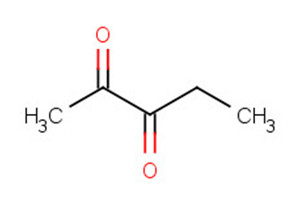 MW = 100.12 |
Acute toxicity (Lethality) |
Oral LD50 = 3000 mg/kg in rats |
Oral LD50 > 5000 mg/kg |
Oral LD50 = 3000 mg/kg in rats |
Short term toxicity (inhalation, 6h, systemic effects) |
LOAEC = 92.9 ppm (333 mg/m3) |
NA |
NOAEC = 112 ppm (459 mg/m3) (respiratory & olfactory cytotoxicity). |
Repeated dose toxicity (inhalation, < 2 wks) |
LOAEC = 100 ppm (358 mg/m3) the lowest test concentration in rats. LOAEC = 50 ppm (176 mg/m3) (read across from 2,3-PD) |
NA |
LOAEC = 50 ppm (205 mg/m3) (histopathological changes in rats and mice, 10-12 days). |
Subchronic toxicity (90-day oral) |
NOAEL = 90 mg/kg-bw/d |
NOAEL= 330 mg/kg-bw/d (drinking water, (decreased body weight and anemia) |
NOAEL = 105 mg/kg bw/d (read-across from diacetyl) NOAEL = 24 mg/kg-bw/day (from non-respiratory tract effects in 90-day inhalation study) |
Subchronic toxicity (90-day inhalation) |
NOAEC = 12.5 ppm (45 mg/m3) |
NOAEC = 800 ppm (2883 mg/m3) the highest test concentration (NTP 2017b) |
NOAEC = 12.5 ppm (51 mg/m3) (histopathological effects in respiratory system in rats and mice; 14 weeks) |
Subchronic toxicity (dermal) |
NOAEL = 90 mg/kg bw/d (from oral exposure) |
NOAEL = 330 mg/kg bw/d (Extrapolation from oral) |
NOAEL = 105 mg/kg bw/d (read-across from diacetyl) |
Chronic toxicity (inhalation) |
LOAEC =12.5 ppm ( 45 mg/m3) (squamous epithelium hyperplasia in larynx, trachea and lung) |
NA |
NA |
Developmental toxicity (Oral) |
NOAEL = 1600 mg/kg bw/d |
NOAEL = 1640 mg/kg bw/d (read-across from diacetyl) |
NA |
Genetic toxicity |
Likely to be genotoxic |
Genotoxic potential is low |
Likely to be genotoxic (read across from diacetyl) |
Carcinogenicity |
Some evidence for carcinogenicity in chronic animal studies |
Low potential |
Some evidence for carcinogenicity in chronic animal studies (read across from diacetyl) |
Abbreviations: NA, Not available; MW, molecular weight (g/mol)
7.3.3 Risk characterization of subgroup 3 (diacetyl, 2,3-PD, and acetoin)
Diacetyl
Although diacetyl has not been classified by any agency on the basis of carcinogenicity or other health effects, recent NTP studies (2017a) demonstrated some evidence of carcinogenic activity in 2-year inhalation studies in mice and rats, with significant increases in incidences of nasal tumours at 179 mg/m3. Evidence also indicates that diacetyl is likely genotoxic. Chronic exposure to diacetyl also resulted in increased incidences of non-cancer effects in the nose, larynx, trachea and lung of mice at all concentrations tested (LOAEC of 45 mg/m3), with more severe effects on the respiratory system occurring at higher concentrations in mice and rats. A similar spectrum of respiratory tract effects were also observed in subchronic, short term and acute studies in rodents. Both pulsed and continuous exposure to diacetyl for as short a duration as 15 minutes caused epithelial injuries, with the NOAEC for acute inhaled diacetyl considered to be less than 333 mg/m3 (Hubbs et al. 2008).
The available database in regards to the toxicity of oral exposure to diacetyl is more limited. In a subchronic study in rats, effects on haematological parameters and several organ weights were noted at 540 mg/kg bw/day (NOAEL of 90 mg/kg bw/day), which may have been secondary to the observed stomach ulceration (Colley et al. 1969; reviewed in IPCS 1999; EFSA 2004; SCOEL 2014). In light of the limitations of the other oral studies identified (Bawarzi 2016; US FDA 1973; reviewed in IPCS 1999; EFSA 2004) and the systemic oral NOAEL of 330 mg/kg-bw/day reported for acetoin (a metabolite of diacetyl which has been demonstrated to be less potent than diacetyl in studies involving similar protocols), in a drinking water study (Gaunt et al., 1972), it is suggested that the true NOAEL for diacetyl could potentially be between 90 and 330 mg/kg-bw/day.
Several lines of evidence demonstrate that the effects observed in inhalation studies in laboratory animals are relevant to humans and support the conclusion of the epidemiologically based quantitative risk assessment for diacetyl (CDC 2016), based on reports of the development of diverse respiratory impairment in workers at a microwave popcorn or flavouring manufacturing plantsFootnote 10 (Kreiss et al. 2002; Parmet and Von Essen 2002; van Rooy et al. 2007). However, dosimetry modelling estimates suggest that tissue concentrations of diacetyl in the respiratory tract of humans might be 20 to 40-fold greater than those in exposed rodents (Gloede et al. 2011) and that bioassay data might underestimate the risk to humans (Cichocki and Morris 2017).
The predominant source of exposure to diacetyl for the general population is through the diet. The estimated intake from the use of diacetyl as a possible flavouring agent in foods (56 – 133 µg/kg-bw/day) is less than intakes that have been estimated from natural occurrence (–281 - 1625 µg/kg-bw/day) for this substance. The JECFA (WHO 1999a) concluded "No safety concern at current levels of intake when used as a flavouring agent; secondary components do not raise a safety concern" based on the dietary exposure estimates for substances in this flavouring grouping. Considering the true NOAEL for diacetyl is likely between 90 and 330 mg/kg-bw/day and effects are likely linked to ulcer formations in the stomach, the calculated margins of exposure (≤ 54 and 199 and higher) are considered adequate to account for uncertainties in the databases.
Given the wide use of diacetyl as a flavoring agent including in microwave popcorn, potential inhalation exposures were calculated. Diacetyl was also found in a hair styling product that can be purchased in Canada. Table 7‑17 provides all relevant exposure values and the critical health effects for diacetyl as well as the resulting MOEs for the characterization of risk.
Exposure Scenario |
Exposure |
Critical effect level |
Critical health effect endpoint |
MOE |
|---|---|---|---|---|
Inhalation of microwave popcorn |
Conc. After 15 min. = 0.04 mg/m3 |
LOAEC = 333 mg/m3 |
Nasal and laryngeal effects (necrosis/inflammation in epithelium) |
8325 |
Cosmetics (inhalation) |
6-hr TWA = 0.008 – 0.064 mg/m3 |
LOAEC = 45 mg/m3 |
Nasal effects (exudate and atrophy of the olfactory epithelium) |
703 – 5 625 |
The MOEs for inhalation exposure to diacetyl from microwave popcorn and from its use in a hair styling product available to consumers, are considered adequate to account for uncertainties in the databases.
2,3-PD
No carcinogenicity data were identified for 2,3-PD..
Based on preliminary results of a subchronic inhalation study in rats and mice (NTP 2017b), similar to observations with diacetyl, the respiratory tract (the nasal passage and larynx in particular) is the target of 2,3-PD induced toxicity. The incidences of non-neoplastic lesions were significantly increased in rats and mice at concentrations of 102 mg/m3 and above (NOAEC = 51 mg/m3). Similar results were reported in shorter term studies with a LOAEC of 205 mg/m3, the lowest concentration tested (Morgan et al., 2012).
Although effects observed in mice and rats exposed to 2,3-PD were located mainly to the respiratory tract, systemic effects (decreased mean body weight gain and organ weights) were also observed at 205 mg/m3 and higher in the absence of histopathological alterations. Therefore, with respect to oral exposures, route-to-route extrapolation from the subchronic inhalation study, resulting in a NOAEL of 24 mg/kg-bw/day for non-respiratory tract effects, and read-across from the subchronic oral study with diacetyl, resulting in a NOAEL of 105 mg/kg-bw/day, were used to characterize the risk of ingested 2,3-PD.
The predominant source of exposure to 2,3-PD for the general population is through the diet primarily from its natural occurrence in foods. The estimated intake from the use of 2,3-PD as a possible flavouring agent in foods (1 – 4 µg/kg-bw/day ) is less than intakes that have been estimated from natural occurrence for this substance (6.5 – 208 µg/kg-bw/day). Although the MOE associated with an oral NOAEL of 24 mg/kg-bw/day based on route-to-route extrapolation is low, in light of the absence of the histopathological changes in internal organs and the conservative approaches used in estimating exposure to 2,3-PD naturally occurring in food, the calculated MOEs, ranging from 115 to 16 154, are considered adequate to address uncertainties in the health effects and exposure databases. The MOEs would be slightly lower, but still considered adequate, if the estimated intake from the use of 2,3-PD as a possible flavouring agent in foods was combined with the natural occurrence in food. Furthermore, the JECFA (WHO 1999a) concluded "No safety concern at current levels of intake when used as a flavouring agent; secondary components do not raise a safety concern" based on the dietary exposure estimates for 2,3-PD.
2,3-PD was also identified in products available to consumers, including certain continuous air fresheners for home care, and in fragrant oils which can be used as an air freshener. Comparison of the 6-hr TWA estimated exposures from use of air fresheners containing 2,3-PD (0.004 – 0.14 mg/m3) with the NOAEC of 51 mg/m3 resulted in MOEs ranging from 364 – 12 750 which are considered adequate to account for uncertainties in the databases.
Acetoin
A limited number of toxicity studies were available for acetoin. Acetoin did not promote lung tumors in a limited screening test for carcinogenicity of food additives in mice (Stoner et al. 1973). Acetoin is not expected to be genotoxic.
To characterize the risk associated with oral exposure to acetoin, a NOAEL of 330 mg/kg-bw/day was used, based on changes in body weight and hematological parameters in a 13-week study (Gaunt et al. 1972, reviewed in IPCS 1999).
Based on a preliminary account of the results of an inhalation study, acetoin did not induce any effects in mice exposed to concentrations of up to 800 ppm (2883 mg/m3) for 14 weeks (NTP 2017b), which is considered to be the NOAEC.
The predominant source of exposure to acetoin for the general population is through the diet primarily from its natural occurrence in foods. The estimated intake from the use of acetoin as a possible flavouring agent in foods (29 – 46 µg/kg-bw/day) is less than intakes that have been estimated from natural occurrence for this substance (0.5 – 3.2 mg/kg-bw/day). On the basis of the conservative approaches used in estimating exposure to acetoin in food, calculated margins of exposure, ranging from 102 – 11 379, are considered adequate to address uncertainties in the health effects and exposure databases. Furthermore, the JECFA (WHO 1999a) concluded "No safety concern at current levels of intake when used as a flavouring agent; secondary components do not raise a safety concern" based on the dietary exposure estimates for substances in this flavouring grouping. In addition, potential inhalation exposures to acetoin in microwave popcorn were considered. Comparison of the conservative estimates of air concentrations of acetoin from popping microwave popcorn (10 – 4200 µg/m3) with the NOAEC of 2883 mg/m3 resulted in MOEs ranging from 686 – 288 300 which are considered adequate to account for uncertainties in the databases.
7.3.4 Uncertainties in evaluation of risk to human health for diacetyl, 2,3-PD and acetoin
The key sources of uncertainty are presented in the table below. For diacetyl and 2,3-PD, while there is confidence that they induce adverse health effects following inhalation exposure, there is greater uncertainty regarding the toxicity of these substances following long term oral exposure, since ingestion in food is an important source of population exposure due to the natural occurrence of these diketones in food and their use as food flavouring agents. For acetoin, although the hazard database is limited, there is sufficient evidence to indicate that it is of lower toxicity than diacetyl; therefore there is confidence that risks to human health in Canada are likely to be low.
There is uncertainty on the natural presence of diacetyl in milk consumed by Canadians as well as the estimated daily intake derived for diacetyl based on a higher end concentration given the wide range in diacetyl concentrations in milk reported in the literature.
Key sources of uncertainty |
Impact |
|---|---|
Absence or limited Canadian monitoring data in foods. |
+/- |
For the natural occurrence of diacetyl, 2,3-PD and acetoin in food, where a range of concentrations were available from the literature, maximum values were chosen except in the case of outliers. |
+ |
The potential inhalation exposures to diacetyl when cooking with foods containing the substance, both natural sources and as a food flavouring agent. |
- |
Assumption that dermal exposures are minimal for diacetyl in comparison to inhalation exposures, given the high volatility of the substance; |
- |
Although direct evidence is limited, it was considered that hair styling products containing diacetyl may be available to the general population in Canada. |
+ |
There are no chronic toxicity studies for acetoin and 2,3-PD and the overall database on acetoin is limited. |
- |
The database on the oral toxicity of diacetyl, 2,3-PD and acetoin is limited. |
+/- |
Uncertainties related to the relevance to humans of diacetyl-induced carcinogenicity and other respiratory toxicity in rats and mice via inhalation and the potential greater sensitivity of humans. |
- |
There are no dermal or inhalation absorption data identified. |
+/- |
The use of diacetyl as read-across to assess data gap for 2,3-PD |
+/- |
+ = uncertainty with potential to cause over-estimation of exposure/risk;
- = uncertainty with potential to cause under-estimation of exposure risk;
+/- = unknown potential to cause over or under estimation of risk.
7.4 Assessment of individual substance 2,4-PD
7.4.1 Exposure assessment
Environmental media
No data were identified on the presence of 2,4-PD in indoor or outdoor air, drinking water, or soil, in Canada or elsewhere. Based on its physical and chemical properties, if released into the environment, 2,4-PD is expected to be distributed mainly to water (~90%), and to a lesser extent into air (~10%); however, its moderate Henry’s Law Constant and vapour pressure, indicate that 2,4-PD is likely to volatilize from water (HSDB 1983-, OECD 2001). 2,4-PD is likely to have high mobility in soil and not likely to adhere to sediment or suspended particles in water (HSDB 1983-).
The level III fugacity model known as ChemCAN (2003) was run to derive potential environmental concentrations of 2,4-PD for Canada using the upper-end volume data from Table 4‑1 (i.e., 1 000 000 kg). The estimated concentration from air, water, soil and sediment was 5.4 ng/m3, 48.9 ng/L, 0.007 ng/g, and 0.003 ng/g, respectively. These concentrations were used to estimate exposure to 2,4-PD from environmental media for the general population of Canada. Concentrations of 2,4-PD derived from ChemCAN for soil and sediment were all in the nanogram range with resulting exposures less than 1 ng/kg/day and are therefore considered to be negligible.
Estimates of exposure for 2,4-PD from environmental media ranged from 0.002 µg/kg-bw per day for breast fed infants (0 to 6 months old) to 0.007 µg/kg-bw per day for formula fed infants (0 to 6 months old) (Health Canada 2018).
Food
2,4-PD was identified as a volatile in a few food items including beef, chicken, coffee, mushrooms, papaya and passion fruit (Nijssen et al. 1963-2016). Quantitative data were only available for roasted chicken (0.004 ppm) and passion fruit juice (<0.01 ppm) and was used to estimate potential exposures to 2,4-PD from food for the general population of Canada. Quantitative exposure estimates for 2,4-PD from its natural occurrence in foodFootnote 11 ranged from 0.02 µg/kg-bw per day for adults 19 years and older to 0.14 µg/kg-bw per day for 2 to 3 year olds (see Appendix A for details).
Products used by consumers
2,4-PD is used as an additive in paints and coatings (OECD 2001, Environment Canada 2012, SDS 2012b). Most of these products were identified as being for commercial or industrial use only; however, some may be used by consumers (Environment Canada 2012, SDS 2012b). Inhalation and dermal exposure estimates were derived using ConsExpo Web (ConsExpo 2016) for coating a large surface such as a truck trailer or a boat containing 1 – 5% 2,4-PD (SDS 2012b, 2016b). Mean event concentrations ranged from 75 – 320 mg/m3, mean event concentrations on the day of exposure ranged from 3 – 13 mg/m3, and 6-hr TWA concentration ranged from 13 – 53 mg/m3. Dermal exposure estimates were 0.04 – 0.2 mg/kg-bw/day.
7.4.2 Health effects assessment
2,4-PD has been reviewed by OECD (2001) and EFSA (2004). These reviews provide a basis for the health effect characterisation in this draft screening assessment. A literature search has been conducted from a year prior to the OECD (2001) SIAR report up to June 2017 and significant new information is included to support risk characterisation.
Toxicokinetics
EFSA (2004) noted that the substance was expected to be readily absorbed from the gastrointestinal tract and widely distributed and metabolized, based on the structure and physicochemical characteristics. No toxicokinetic information was identified for dermal exposure of 2,4-PD.
Carcinogenicity/chronic toxicity
In a two-year inhalation carcinogenicity study, F344 rats and B6D2F1 mice were exposed to 2,4-PD vapors at target concentrations of 0, 100, 200 or 400 ppm (0, 417, 834 or 1668 mg/m3) for 6 hours/day, 5 days/week for 2 years (JBRC, 2010a,b). In rats, non-neoplastic lesions were mainly located in the nasal cavity in both sexes at 200 ppm and above, including squamous metaplasia of the respiratory epithelium, inflammation hyperplasia of the transitional epithelium, and atrophy of the olfactory epithelium. Similarly, nasal cavity effects observed in mice included squamous metaplasia, eosinophilic change, ulcer, necrosis and transitional cell hyperplasia in the respiratory epithelium; atrophy, respiratory metaplasia, eosinophilic change, necrosis in the olfactory epithelium; and respiratory metaplasia in the sub-mucosal gland at 200 and 400 ppm, while exudate and atrophy in the olfactory epithelium occurred at all concentrations. Alterations in biochemical parameters were also noted in males and females at 200 and 400 ppm. Based on the nasal lesions, the study authors identified 100 ppm (417 mg/m3) to be the NOAEC in rats and the LOAEC in mice (JBRC 2010a,b). There were no significantly increased incidences of neoplastic lesions in the extensive range of tissues examined from exposed animals.
Genotoxicity
The potential genotoxicity of 2,4-PD has been reviewed in OECD (2001) and EFSA (2004). Overall, 2,4-PD was not consistently mutagenic in vitro but showed a clastogenic potential (chromosomal aberrations and sister chromatid exchanges) notably in the absence of metabolic activation (Ballantyne and Cawley 2001; OECD 2001). In vivo, a clastogenic potential (micronuclei) was observed in mice exposed via i.p. administration, but not in rats or mice exposed via inhalation, suggesting a route dependence (Ballantyne and Cawley 2001; OECD 2001). Oral administration of 2,3-PD did not induce DNA damage in tissues of rats (ECHA c2007-2017d) or chromosomal aberrations in spermatogonia of mice (OECD 2001), although inhalation exposure resulted in a slight transient dominant lethal effect in rats (Tyl et al. 1989). However, EFSA (2004) concluded that the use of 2,4-PD as a flavouring substance is not acceptable due to its genotoxic (clastogenic) potential.
Repeated dose toxicity
In a dermal study, New Zealand White rabbits were exposed to 2,4-PD at doses of 0, 244, 975 and 1463 mg/kg bw/day by occluded contact daily for 9 days (Ballantyne 2001, as cited in OECD 2001). In the high dose group (1463 mg/kg bw/day) approximately 50% of animals of either sex died. At the middle and high doses, 2,4-PD caused signs of hypoactivity, prostration, salivation, tremors, gasping, convulsions, cyanosis (as derived from blue cutis of the nasal area), reduced body weight gain and food consumption, hemorrhages and neuronal degeneration. Based on systemic effects, the author established a NOAEL of 244 mg/kg bw/day and a LOAEL of 975 mg/kg bw/day.
With respect to oral exposure, based on limited data, gavage administration of doses of 500 mg/kg bw/day to rats for 2 weeks resulted in a range of systemic effects in the bladder, lungs, eyes, thymus, liver, kidneys, heart and lymph nodes, while all animals administered 1000 mg/kg bw/day died; the NOAEL was considered to be 100 mg/kg bw/day (Eastman Kodak 1979, as cited in OECD 2001). Other gavage studies by these investigators are cited in EFSA (2004) include a 126 day study in rats (NOAEL < 200 mg/kg bw/day) and a 14 day study in rabbits (NOAEL = 250 mg/kg bw/day); however, details provided are insufficient for evaluation.
Inhalation exposure of rats to 0, 100, 300 or 650 ppm (0, 417, 1217 or 2711 mg/m3) 2,4-PD for 14 weeks induced a wide range of systemic effects at 300 ppm and above and high mortality at 2711 mg/m3. Based on hematological, clinical and urinary chemical effects in the 1217 mg/m3 group and severe effects at 2711 mg/m3, including histopathological effects in the brain and thymus, the authors established a NOAEC of 417 mg/m3 for this study (Dodd et al. 1986, as cited in OECD 2001). In a two week study by the same authors, the NOAEC and LOAEC were determined to be 200 ppm (834 mg/m3) and 400 ppm (1668 mg/m3), respectively, based on reduced organ weights and nasal necrosis or inflammation at higher concentrations (OECD 2001).
Since no long-term oral study were available, a route-to-route extrapolation from the inhalation NOAEC of 417 mg/m3 from the study above was carried out to obtain an oral NOAEL of 23 mg/kg-bw/day (see appendix D).
Reproductive and developmental toxicity
In the sub-chronic inhalation study in male and female F344 rats exposed to 2,4-PD at concentrations of up to 2711 mg/m3, no significantly pathological findings were noted in testes and epididymis of males or in uterus, cervix and ovaries of females (Dodd et al. 1986, as cited in OECD 2001). No studies were identified in which potential effects of exposure to 2,4-PD on reproductive performance were investigated.
In an inhalation developmental toxicity study, pregnant F344 rats were exposed to concentrations of 0, 53, 202 or 398 ppm (0, 217, 827 or 1629 mg/m3) 2,4-PD for 6 hours/day through gestation days 6-15 (Tyl et al. 1990). No effects were observed on number of corpora lutea, viable implants per litter, pre- or post-implantation losses or foetal sex ratio. Significantly reduced foetal body weights per litter (approximately 10%) were reported for males, females and all foetuses at 398 ppm (1,629 mg/m3). At this concentration, a consistent pattern of reduced foetal ossification, skeletal variations and reduced maternal weight was also reported. At 202 ppm (827 mg/m3), there was a slight but statistically significant reduction (approximately 3%) in foetal body weights per litter in males only. The authors concluded the NOEC to be 53 ppm for both maternal and developmental toxicity (Tyl et al. 1990). Likewise, the OECD (2001) considered 53 ppm (217 mg/m3) to be the developmental NOAEC. However, in light of the minimal decrease in body weight in male foetuses only, which is unlikely to be considered adverse, the NOAEC for developmental and maternal toxicity is considered in this assessment to be 202 ppm (827 mg/m3).
7.4.3 Risk characterization of 2,4-PD
2,4-PD was not carcinogenic in rats and mice exposed via inhalation. Non-cancerc lesions were mainly located in the nasal cavity (JBRC 2010a, b). However, clastogenic effects were reported for 2,4-PD, leading EFSA to conclude that the use of 2,4-PD as a flavouring substance is not acceptable (2004).
Systemic and developmental toxicities are identified as critical health effects for characterizing risk for shorter term inhalation exposure to 2,4-PD. The NOAEC for systemic and developmental toxicity in rats was established at 827 mg/m3. A NOAEC of 200 ppm (834 mg/m3) from a 14-day study (Dodd et al., 1986, as cited in OECD 2001) supports the selection of this critical effect concentration for risk characterization. With respect to longer term exposure, various systemic effects were observed in the 14-week study and the NOAEC was established at 417 mg/m3 (Dodd et al. 1986, as cited in OECD 2001).
With respect to oral exposure, based on limited data, the short-term gavage study with a NOAEL of 100 mg/kg bw/day (Eastman Kodak 1979, as cited in OECD 2001) and a route-to-route extrapolation from the developmental inhalation study for longer term exposure, resulting in a NOAEL of 23 mg/kg-bw/day, were considered.
Dermal exposure to 2,4-PD also produced a range of effects in rabbits administered 500 mg/kg-bw/day; therefore, a NOAEL of 244 mg/kg-bw/day (Ballantyne 2001, as cited in OECD 2001) was used to characterize the risk associated with dermal exposure to 2,4-PD.
The general population of Canada is exposed to 2,4-PD in small amounts through environmental media and the diet as a result of its natural occurrence in foods.
2,4-PD is also used as an additive in paints and coatings primarily for commercial or industrial use; however, some are available to consumers. Table 7‑19 provides all relevant exposure values and the critical health effects for 2,4-PD as well as the resulting MOEs for the characterization of risk.
Exposure Scenario |
Exposure |
Critical effect level |
Critical health effect endpoint |
MOE |
|---|---|---|---|---|
Environmental media |
0.002 – 0.007 µg/kg-bw/day |
NOAEL = 100 mg/kg-bw/day NOAEL = 23 mg/kg-bw/day |
Systemic toxicity in a 2-week oral study Route-to-route extrapolation from 14-week inhalation study |
> 3 million |
Coating for large surface (inhalation) |
6-hr TWA = 13 – 53 mg/m3 |
NOAEC = 827 mg/m3 |
Developmental effects (reduced foetal weight in foetuses and reduced foetal ossification, skeletal variations) and reduced maternal weight. |
16 – 64 |
Coating for large surface (dermal) |
0.04 – 0.2 mg/kg-bw/day |
NOAEL = 244 mg/kg-bw/day |
Distended bladder, congested lungs, clouding of cornea, thymic necrosis, hepatocyte swelling and congestion, nephrosis, lymphadenitis of mesenteric lymph nodes and inflammation of the heart from a 9-day dermal study. |
1 220 – 6 100 |
Calculated MOEs for exposure to 2,4-PD from environmental media are considered adequate to address uncertainties in the health effects and exposure databases. Additional intake of 2,4-PD from its natural occurrence in food was not identified as a concern for human health. However, the MOEs for exposure to 2,4-PD in products available to consumers, namely floor coating, is considered potentially inadequate to account for uncertainties in the exposure and health effects databases.
7.4.4 Uncertainties in evaluation of risk to human health
The key sources of uncertainty are presented in the table below. In light of the evidence of the genotoxicity of 2,4-PD, additional data from longer term studies would be helpful to increase confidence that potential risks from prolonged exposures would be low.
Key source of Uncertainty |
Impact |
|---|---|
Absence or limited Canadian monitoring data in foods. |
+/- |
Details on the specific types of products available to consumers that contain 2,4-PD ; however, use of high-end product amounts and maximum concentrations likely overestimates exposures to products available to consumers. |
+ |
There are no chronic oral or dermal studies or subchronic oral studies for 2,4-PD |
+/- |
+ = uncertainty with potential to cause over-estimation of exposure/risk;
- = uncertainty with potential to cause under-estimation of exposure risk;
+/- = unknown potential to cause over or under estimation of risk.
7.5 Assessment of MO
7.5.1 Exposure assessment
Environmental media
Mesityl oxide was measured in the CHMS Cycle 2 indoor air study and in a smaller indoor air study from Quebec (personal communication, e-mail from EHSRB, HC to ESRAB, HC, dated 2012 December, unreferenced; Won and Lusztyk 2011). MO was detected in less than 12% of samples (limit of detection 0.16 µg/m3) in the CHMS study with a geometric mean concentration of 0.10 µg/m3 and a 95th percentile concentration of 0.26 µg/m3 (weighted data at the household level) (Zhu et al. 2013). The geometric mean concentration of MO from 12 samples from the Quebec field study was 0.30 µg/m3 with a maximum of 4.15 µg/m3 (Won and Lusztyk 2011).
No Canadian data were identified on the presence of MO in water. MO was qualitatively detected in drinking water from Cincinnati, Ohio as well as in a river in Switzerland (HSDB 1983-). Based on the moderate Henry’s Law Constant and high vapour pressure, MO is likely to volatilize from water (HSDB 1983-); therefore, drinking water is not expected to be a significant source of population exposure.
No data were identified on the presence of MO in soil in Canada or elsewhere. Based on its low Log Koc, MO is likely to have high mobility in soil and is not likely to adhere to sediment or suspended particles in water (HSDB 1983-); soil is therefore not expected to be an important source of exposure.
Estimates of exposure for MO from environmental media ranged from 0.8 µg/kg-bw per day for adults (60 years and older) to 2.5 µg/kg-bw per day for toddlers (6 months to 4 years) (Health Canada 2018).
Food
Mesityl oxide is naturally occurring in various food items including nectarines, bell pepper, tomatoes, crisp bread, parmesan cheese, milk, coffee, tea, peanuts, basil and shrimp to name a few (Nijssen et al. 1963-2016, Burdock 2010). It can also be used as a food flavourant in baked goods, frozen dairy, gelatins/puddings, milk products and soft candy (Burdock 2010). Quantitative exposure estimates for MO from its natural occurrence in foodFootnote 12 ranged from 0.3 µg/kg-bw per day for 1 year olds to 1.5 µg/kg-bw per day for toddlers 2 – 3 years old (see Appendix A for details).
The Joint FAO/WHO Expert Committee on Food Additives (JECFA) evaluated a group of aliphatic secondary alcohols, ketones and related esters used as flavouring substances, including MO (WHO 2003). As part of that evaluation, the Committee estimated the per capita intake of MO from its use as a food flavouring agent to be 0.0067 µg/kg bw per day for the European population (no intake data reported for the US) (see Appendix A for details).
Products available to consumers
MO was not identified in any products available to consumers in Canada.
7.5.2 Health effects assessment of individual substance (MO)
MO has been reviewed by OECD (OECD 1997b, updated in 2010) and EFSA (EFSA 2012). These reviews provide a basis for the health effects characterization in this assessment. A literature search has been conducted from one year prior to the OECD publication until May 2017. No new information was identified on the health effects associated with exposure to MO.
Limited toxicological information is available for MO; therefore, route-to-route extrapolation for MO is supported by read-across from the analogue 6-methyl—5-heptene-2-one (MHE, CAS RN 110-93-0). MHE was the only analogue identified for the read-across of MO, based on structural similarity and data availability (TERA 2017). Both MO and MHE are unsaturated ketones; MO contains an alkene group and a ketone group on adjacent carbon atoms, while MHE contains an additional two carbons in the chain between the alkene and ketone group. This structure difference suggests that MO may be more reactive (therefore more toxic) than MHE due to the presence of α, β-unsaturated carbonyl. Toxicity data on MHE has been used to read-across to MO where required.
Carcinogenicity and genetic toxicity
No carcinogenicity data were identified for MO. The predictive model OncoLogic® identified a structural alert of alpha-beta unsaturated carbonyl function group in the molecule of MO for carcinogenic potential (TERA 2017).
MO was not mutagenic in Salmonella, E. coli or in the mouse lymphoma assay, nor did it induce micronuclei in cultured human lymphocytes or in mice administered MO by intraperitoneal injection (ECHA c2007-2017e).
Repeated dose toxicity
Two studies are available for repeated dose inhalation toxicity for MO and no data are available by either oral or dermal route.
A combined repeated dose and reproductive/developmental toxicity study was conducted in groups of SD rats exposed to MO at concentrations of 0, 31, 103 and 302 ppm (0, 124, 413 and 1211 mg/m3) for 6 hours/day, 7 days/week for a total 49 exposures for male rats and a total of 36 to 49 exposures for female rats. There were no effects on mortality, haematological or urinalysis parameters, or in gross pathology for the parental animals. However, significant reductions in feed consumption, body weight, and body weight gain, along with clinical abnormalities and nasal passage pathology, were observed in the all test groups in a concentration-dependent matter. The clinical abnormalities included increased incidence of post-exposure porphyrin nasal discharge and sialorrhea. Histopathological findings included the presence of sero-cellular exudates, chronic focal inflammation and focal metaplasia of the respiratory and olfactory epithelium of the nasal passage. These changes are considered a common response to an irritating vapour (Bernard and Faber 1992, reviewed in OECD 1997b). Thus an inhalation LOAEC of 31 ppm (124 mg/m3) was determined based on the effects on feed consumption, body weights, body weight gain and nasal passage histopathology (OECD 1997b; ECHA c2007-2017e). No inhalation NOAEC has been identified in this study.
Groups of male Wistar rats and guinea pigs of both sexes were exposed to MO at concentrations of 0, 50, 100, 250 or 500 ppm (0, 200, 400, 1000 or 2000 mg/m3) for 8 hours/day, 5 days/week for 6 weeks. Chronic conjunctivitis, nasal irritation and mild albuminuria were observed at 250 and 500 ppm. Significant concentration-related increases pathological effects were observed in kidney, lung and/or liver at 100 ppm and higher doses. The pathological changes included poor growth, congestion of the liver, dialated Bowman’s capsules, swollen convoluted tubular epithelium in the kindey and congestion of the lungs. No effects were seen at 50 ppm. The authors considered the NOAEC to be 50 ppm (200 mg/m3) on the basis of pathological changes in lung, liver and/or kidney (Smyth et al. 1942; ECHA c2007-2017e).
Reproductive and developmental toxicity
In the combined repeated dose and reproductive/developmental toxicity study described above in which groups of SD rats were exposed to MO for a total 49 days for male rats and a total of 36 to 49 days for female rats (through Day 20 of gestation), no significant effects on reproductive organs or performance or on gestation length were observed at any concentration tested .However, a reduced number of litters produced by the mating pairs was observed at 302 ppm (1211 mg/m3). No external malformations were noted; no histopathological examination was conducted in pups. The NOEC for reproductive toxicity was 103 ppm (413 mg/m3) (Bernard and Faber 1992, reviewed in OECD 1997b; ECHA c2007-2017e).
No information has been identified on the potential reproductive and developmental toxicity from oral or dermal exposure to MO.
Route to route extrapolation and read-across for hazard characterization
In light of the lack of data on the toxicity of orally administered MO, observations from inhalation studies are considered in a route-to-route extrapolation approach. In the combined repeated dose and reproductive/developmental toxicity study in rats, the critical effects mainly occurred in nasal passage (i.e., the site of contact) which the OECD (1997b) attributed to the irritative property of MO. Therefore, it is considered to be more appropriate to use the NOAEC of 50 ppm (200 mg/m3) from the 6 week inhalation study in rats in which systemic effects in the lung, liver and kidney were observed to derive an oral NOAEL for MO. The adjusted oral NOAEL for repeated dose toxicity is 15.0 mg/kg-bw/d (see appendix D).
MHE (CAS RN 110-93-0) was identified as an analogue that could be used for read-across of MO, based on the structural similarity and data availability (TERA 2017). A NOAEL of 39 mg/kg-bw/day for MO can be derived by read-across from the oral NOAEL of 50 mg/kg-bw/day from MHE (based on alterations in haematological and clinical chemistry parameters in a 90 day study with rats (OECD 2003) by adjusting for molecular weight (Table 7‑21). This read-across based NOAEL for MO is similar to the route-to-route extrapolation based NOAEL (i.e., only about 2.5-fold greater, as may be expected, based on the longer chain).
Chemical name |
MO |
6-methyl-5-heptene-2-one (MHE) |
|---|---|---|
Role |
Target chemical |
Analogue |
CAS# |
141-79-7 |
110-93-0 |
Chemical structure |
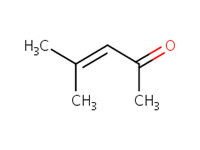 MW = 98.1 Log Kow: 1.2 – 1.7 |
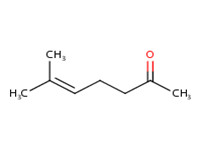 MW = 126.2 Log Kow: 2.06 |
Repeat dose toxicity (Inhalation, 49-day exposure) |
LOAEC=124 mg/m3 (reductions in body weight and body weight gain, and histopathology in nasal passage); No NOAEC was identified. |
NA |
Repeat dose toxicity (Inhalation, 6 weeks) |
NOAEC=200 mg/m3 (histopathological effects in kidney, lung and liver) |
NA |
Repeat dose toxicity (Oral) |
NOAEL=15.0 mg/kg-bw/d (R2R from inhalation 6-week study*); NOAEL = 39 mg/kg-bw/d (read-across from MHE by adjusting the molecular weight) |
NOAEL = 50 mg/kg-bw/d (increases in platelet counts in female rats in a 90-day study) LOAEL=200 mg/kg bw/d |
Developmental toxicity (Inhalation) |
No developmental toxicity was noted up to 1211 mg/m3 |
NA |
Developmental toxicity (Oral) |
No developmental toxicity was noted up to 95.2 mg/kg-bw/d (R2R from inhalation study*); NOAEL = 165 mg/kg-bw/d (read-across from MHE by adjusting the molecular weight) |
NOAEL=200 mg/kg-bw/d (maternal and prenatal developmental toxicity); LOAEL=1000 mg/kg-bw/d. No developmental up to 1000 mg/kg-bw/d |
Genetic toxicity |
Negative |
Negative |
Carcinogenicity |
NA |
NA |
Abbreviations: NA, not available; MW, molecular weight (g/mol); Log Kow, octanol-water partition coefficient
*This concentration was converted to oral dose using parameters from Health Canada (1994), with rat average body weight of 0.35kg and inhalation rate of 0.11m3/day. i.e. 200 mg/m3 X 0.11 m3/day X 8h/24h X 5 days/7 days / 0.35 kg = 15.0 mg/kg-bw/d for repeated dose toxicity; or 413 mg/m3 X 0.11 m3/day X 6h/24h / 0.35 kg = 32.5 mg/kg-bw/d for reproductive toxicity.
7.5.3 Risk characterization of MO
No carcinogenic studies on MO have been identified.
The LOAEC of 124 mg/m3 for the systemic toxicity in dams was used to characterize the risk associated with inhalation exposure to MO (OECD 1997b; ECHA c2007-2017e).Although no oral toxicity studies on MO have been identified, route to route extrapolation of the NOAEC from a 6 week inhalation study (Smyth et al. 1942; ECHA c2007-2017e) is considered appropriate to characterize potential hazards associated with ingestion of the substance. The equivalent oral NOAEL was determined to be 15 mg/kg-bw/day. This value is supported by reading across from toxicity data for an analogous substance, MHE.
The predominant source of exposure to MO for the general population is through air and the diet as a result of its natural occurrence in foods.
MO was not identified in any products available to consumers in Canada.
Comparison of the estimates of exposure to MO from environmental media (1.2 – 2.5 µg/kg bw/day) and from its use as a flavouring agent in food (0.0067 µg/kg bw/day) with the NOAEL of 15 mg/kg-bw per day resulted in MOEs greater than 6 000 which are considered adequate to address uncertainties in the health effects and exposure databases. Additional intake of MO from its natural occurrence in food was not identified as a concern for human health. Furthermore, the JECFA (WHO 2003) concluded "No safety concern at current levels of intake when used as a flavouring agent" based on the dietary exposure estimates for MO.
7.5.4 Uncertainties in evaluation of risk to human health
The key sources of uncertainty are presented in the table below. Although the database for MO is limited, in light of the large MOEs, confidence is high that risk to general population of Canada from this substance is low.
Key source of Uncertainty |
Impact |
|---|---|
Limited environmental monitoring data for MO. |
+ |
Limited hazard database for MO (especially long term data) |
- |
+ = uncertainty with potential to cause over-estimation of exposure/risk;
- = uncertainty with potential to cause under-estimation of exposure risk;
+/- = unknown potential to cause over or under estimation of risk.
8. Conclusion
Considering all available lines of evidence presented in this draft screening assessment, there is low risk of harm to the environment from MEK, MPK, MIBK, MIAK, DAA, diacetyl, 2,3-PD, acetoin, 2,4-PD and MO. It is proposed to conclude that these 10 substances do not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
On the basis of the information presented in this draft screening assessment, it is proposed to conclude that MEK, MIBK, and 2,4-PD meet the criteria under paragraph 64(c) of CEPA as they are entering or may enter the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
On the basis of the information presented in this draft screening assessment, it is proposed to conclude that MPK, MIAK, DAA, diacetyl, 2,3-PD, acetoin and MO do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
Therefore, it is proposed to conclude that MEK, MIBK, and 2,4-PD meet one or more of the criteria set out in section 64 of CEPA but that MPK, MIAK, DAA, diacetyl, 2,3-PD, acetoin and MO do not meet any of the criteria set out in section 64 of CEPA.
MEK and 2,4-PD are proposed to meet the persistence criteria but not the bioaccumulation criteria as set out in the Persistence and Bioaccumulation Regulations of CEPA.
MIBK is proposed to not meet the persistence or bioaccumulation criteria as set out in the Persistence and Bioaccumulation Regulations of CEPA.
Reference
Aeschbacher HU, Wolleb U, Löliger J, Spadone JC, Liardon R. 1989. Contribution of coffee aroma constituents to the mutagenicity of coffee. Food Chem Toxicol. 27:227-232.
Alderson M, Rattan N. 1980. Mortality of workers on the isopropyl plant and two MEK dewaxing plants. Br J Ind Med. 37:85-9.
[ATSDR] Agency for Toxic Substances & Disease Registry. 1992. Toxicological profile for 2-butatnone [PDF, 2.28MB]. Atlanta (GA): U.S. Department of Health and Human Services, Public Health Service. [Accessed 2016 November].
Ballantyne B. 2001. Systemic toxicity from repeated cutaneous contact with 2,4-pentanedione. Vet Hum Toxicol. 43:14-18.
BallantyneB,CawleyTJ. 2001. Toxicology update: 2,4-Pentanedione. J Appl Toxicol. 21(2):165-171.
Bawazir A. E. 2016. Evaluation of neurotoxicity and testicular toxicity of artificial Butter flavorings. International Journal of Pharmaceutical Research & Allied Sciences; 5(1):248-258
Benfenati E., N. Di Toro, R. Fanelli, G. Lualdi, R. Tridico, G. Stella, P. Buscaini and L. Stimilli. 1992. Characterization of organic and inorganic pollutants in the Adige river (Italy). Chemosphere 25(11): 1665-1674.
Bernier AM. 2000. Qualité de l’eau potable produite par la Ville de Montréal. Rapport annuel 2000.
Bianchi, AP and M.S. Varney. 1998. Volatile organic compounds in the surface waters of a British estuary. Part 1: Occurrence, distribution and variation. Water Research 32(2): 352-370.
Bjeldanes LF, Chew H. 1979. Mutagenicity of 1,2-dicarbonyl compounds: Maltol, kojic acid, diacetyl and related substances. Mutat Res. 67: 367-371.
Borghoff SJ, Poet TS, Green S, Davis J, Hughes B, Mensing T, Sarang SS, Lynch AM, Hard GC. 2015. Methyl isobutyl ketone exposure-related increases in specific measures of a2u-globulin (a2u) nephropathy in male rats along with in vitro evidence of reversible protein binding. Toxicology. 333:1-13.
Borkowski L. 2007. Another microwave popcorn problem. The Pump Handle: A water cooler for the public health crowd. [accessed 2017 September].
Burdock GA. 2010. Fenaroli’s handbook of flavor ingredients. 6th ed. Orlando (FL): Burdock Group.
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C. 1999, c.33. Canada Gazette Part III, vol. 22, no. 3.
Canadian Dairy Commission. 2017. The Industry, Consumption. [last modified 2017 July 12; accessed 2017 Dec 8].
Canter, L.W. and D.A. Sabatini. 1994. Contamination of public ground water supplies by superfund sites. Intern. J. Environmental Studies 46:35-57.
Cardoso DR, Bettin SM, Reche RV, Lima-Neto BS, Franco DW. 2003. HPLC-DAD analysis of ketones as their 2,4-dinitrophenylhydrazones in Brazilian sugar-cane spirits and rum. Journal of Food Composition and Analysis. 16:563-573.
Cavender FL, Casey HW, Salem H, Swenberg JA, Gralla EJ. 1983. A 90-day vapor inhalation toxicity study of methyl ethyl ketone. Fundam Appl Toxicol. 3(4):264-70.
[CCRIS] Chemical Carcinogenesis Research Information System [database]. 2012. Bethesda (MD): US National Library of Medicine. [2010-09-23; accessed 2017Oct].
[CDC] Centers for Disease Control and Prevention (US). 2016. Criteria for a recommended standard: occupational exposure to diacetyl and 2,3-pentanedione. US Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, DHHS (NIOSH) Publication No. 2016-111 Cincinnati, OH. [accessed 2017 Oct 13].
ChemCAN [level III fugacity model of 24 regions of Canada]. 2003. Version 6.00. Peterborough (ON): Trent University, Canadian Centre for Environmental Modelling and Chemistry. [cited 2016 Oct 19].
ChemIDplus [database]. 1993- . Bethesda (MD): US National Library of Medicine. [updated 2012 Nov 26; accessed 2017 Mar].
Cichocki JA, Morris JB. 2017. Inhalation dosimetry modeling provides insights into regional respiratory tract toxicity of inhaled diacetyl. Toxicology. 388:30-39.
[CIMT] Canadian International Merchandise Trade [database]. 2017. 291412 Butanone (methyl ethyl ketone), 2011-2016. Ottawa (ON): Statistics Canada. [cited 2017 May].
Colley J, Gaunt IF, Lansdown AB, Grasso P, Gangolli SD. 1969. Acute and short-term toxicity of diacetyl in rats. Food Cosmet Toxicol. 7(6):571-580.
[ConsExpo Web] Consumer Exposure Web Model. 2016. Bilthoven (NL): Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment].
David RM, Bernard LG, Banton MI, Tyler TR, Topping DC, Gill MW, O’Donoghue JL. 1999. The effect of repeated methyl iso butyl ketone vapor exposure on schedule controlled operant behavior in rats. Neurotoxicol. 20(4):583-594.
De Leonardis A, Lopez F, Nag A, Macciola V. 2013. Occurrence and persistence of diacetyl in unfermented and fermented milks. Eur Food Res Technol. 236:691-697.
Deacon MM, Pilny MD, John JA, Schwetz BA, Murray FJ, Yakel HO, Kuna RA. 1981. Embryo and fetotoxicity of inhaled methyl ethyl ketone in rats. Toxicol Appl Pharmacol. 59:620-622.
Delzer GC, Ivahnenko T. 2003. Occurrence and temporal variability of methyl tert-butyl either (MTBE) and other volatile organic compounds in select sources of drinking water: Results of the focused survey [PDF, 3.93 MB]. Water-Resources Investigations Report 02-4084. Rapid City (ND): U.S. Geological Survey.
Dietz FK, Rodriguez-Giaxola M, Traiger GJ, Stella VJ, Himmelstein KJ. 1981. Pharmacokinetics of 2-butanol and its metabolites in the rat. J Pharmacokinet Biopharm. 9(5):553-576.
DiVincenzo GD, Kaplan CJ, Dedinas J. 1976. Characterization of the metabolites of methyl n-butyl ketone, methyl iso butyl ketone and methyl ethyl ketone in guinea pig serum and their clearance. Toxicol Appl Pharmacol. 36:511-522.
Dodd DE, Garman RH, Pritts IM, Troup CM, Snellings WM, Ballantyne B. 1986. 2,4-Pentanedione: 9 -Day and 14-Week Vapor Inhalation Studies in Fischer-344 Rats. Fundam Appl Toxicol. 7:329-339.
Doepker CL, Maier A, Willis A, Hermansky SJ. 2012. Toxicology of Flavors in the Food Industry. Chapter Eighty In: Bingham E, Cohhrssen B, editiors. Patty’s Toxicology, 6th Edition, Vol. 5. New York (NY): Wiley. p. 133-168.
Dorado L, Montoya MR, Rodriguez Mellado JM. 1992. A contribution to the study of the structure mutagenicity relationship for alpha dicarbonyl compounds using the Ames test. Mutat Res. 269(2):301-306.
[DPD] Drug Product Database [database]. [modified 2015 Jul 17]. Ottawa (ON): Health Canada. [accessed 2017 Oct 13].
Duguay AB, Plaa GL. 1995. Tissue concentrations of methyl isobutyl ketone, methyl n-butyl ketone and their metabolites after oral or inhalation exposure. Toxicol Lett. 75:51-58.
[ECCC] Environment and Climate Change Canada. 2017. NAPS Annual raw data 2006-2010 [Internet]. Gatineau (QC): Environment Canada, Environmental Technology Centre [accessed 2017 February].
[ECCC] Environment and Climate Change Canada. 2016a. Science Approach Document: Ecological Risk Classification of Organic Substances. Gatineau (QC): ECCC.
[ECCC] Environment and Climate Change Canada. 2016b. Data used to create substance-specific hazard and exposure profiles and assign risk classifications in the Ecological Risk Classification of organic substances. Gatineau (QC). Available from: substances@ec.gc.ca.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2015. Identification of Risk Assessment Priorities: Results of the 2015 Review. Ottawa (ON): ECCC, HC. [accessed 2017 Oct 13].
[ECCC, HC] Environment and Climate Change Canada, Health Canada. [modified 2007 Apr 20]. Categorization. Ottawa (ON): Government of Canada. [accessed 2017 Oct 13].
ECHA] European Chemicals Agency. c2007-2017a. Registered substances database; search results for CAS RN 123-42-2. Helsinki (FI): ECHA. [updated 2017 September 21, 2017; [accessed 2017 Oct 23].
[ECHA] European Chemicals Agency. c2007-2017b. Registered substances database; search results for CAS RN 110-12-3. Helsinki (FI): ECHA. [updated 2017 June 28; [accessed 2017 Oct 13].
[ECHA] European Chemicals Agency. c2007-2017c. Registered substances database; search results for CAS RN 107-87-9. Helsinki (FI): ECHA. [updated 2017 Aug 25; [accessed 2017 Oct 13].
[ECHA] European Chemicals Agency. c2007-2017d. Registered substances database; Registered dossier for pentane-2,4-dione, CAS RN 123-54-6. Helsinki (FI): ECHA. [updated 2017 Oct 2; [accessed 2017 Oct 13].
[ECHA] European Chemicals Agency. c2007-2017e. Registered substances database; Registered dossier for 4-methylpent-3-en-2-one, CAS RN 141-79-7. Helsinki (FI): ECHA. [updated 2017 Apr 14; [accessed 2017 Oct 13].
[ECHA] European Chemicals Agency. c2007-2017f. Registered substances database; Registered dossier for Butanone, CAS RN 78-93-3. Helsinki (FI): ECHA. [updated 2017 Apr 10; [accessed 2017 Oct 23].
[EFSA] European Food Safety Authority. 2004. Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in contact with Food (AFC) on a request from the Commission related to Flavouring Group Evaluation 11 (FGE.11): Aliphatic dialcohols, diketones, and hydroxyketones from chemical group 10 [PDF, 665 KB] (Commission Regulation (EC) No 1565/2000 of 18 July 2000). EFSA, Parma, Italy. The EFSA Journal (2004) 166, 1-44.
[EFSA] European Food Safety Authority. 2012. Scientific Opinion on Flavouring Group Evaluation 204 (FGE.204): Consideration of genotoxicity data on representatives for 18 mono-unsaturated, aliphatic, α,β-unsaturated ketones and precursors from chemical subgroup 1.2.1 of FGE.19 by EFSA [PDF, 214 KB]. EFSA Journal 2012, 10(12): 2992. European Food Safety Authority (EFSA), Parma, Italy.
[EFSA] European Food Safety Authority. 2015. Scientific Opinion on the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs: Executive summary. EFSA Journal 2015. 13(1):3978.
[EFSA] European Food Safety Authority. 2016. Safety and efficacy of secondary aliphatic saturated or unsaturated alcohols, ketones, ketals and esters with a second secondary or tertiary oxygenated functional group belonging to chemical group 10 when used as flavourings for all animal species. EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP), Rychen et al., 2016.EFSA, Parma, Italy. EFSA Journal 2016, 14(11):4618-4619.
Environment Canada. 2001. Data for selected substances collected under the Canadian Environmental Protection Act, 1999. Section 71: Notice with Respect to Certain Substances on the Domestic Substances List (DSL). Data prepared by: Environment Canada, Existing Substances Program.
Environment Canada. 2012. Canadian Environmental Protection Act, 1999: Notice with respect to certain substances on the Domestic Substances List [PDF, 1.98 MB]. Canada Gazette, Part I, vol. 146, no. 48, Supplement.
Environment Canada. 2013. DSL Inventory Update data collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain substances on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
Environment Canada, Health Canada. 2002. Canadian Environmental Protection Act: Priority Substances List Health Assessment Supporting Documentation report: 2-butoxyethanol. Ottawa (ON): Government of Canada. [accessed 2017 Sept]. Unpublished.
Environment Canada, Health Canada. 2014. Approach for identification of chemicals and polymers as risk assessment priorities under Part 5 of the Canadian Environmental Protection Act, 1999 (CEPA 1999). Ottawa (ON): Environment Canada, Health Canada.
[EPI Suite] Estimation Program Interface Suite for Microsoft Windows [estimation model]. c2000-2012. Ver. 4.11. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
European Commission. 2014. Recommendation from the Scientific Committee on Occupational Exposure Limits for Diacetyl. SCOEL/SUM/149. Brussels (Belgium): Employment, Social Affairs and Inclusion, European Commission.
European Commission. 2017. Cosmetic Ingredients and Substances (CosIng) Simple Search Database [database] [updated 2017 October 20; accessed 2017 September]
Fasano WJ, McDougal JN. 2008. In vitro dermal absorption rate testing of certain chemicals of interest to the Occupational Safety and Health Administration: summary and evaluation of USEPA's mandated testing. Regul Toxicol Pharmacol. 51(2):181-194.
Ficheux A, Morisset T, Chevillotte G, Postic C, Roudot A. 2014. Probabilistic assessment of exposure to nail cosmetics in French consumers. Food Chem Toxicol 66:36.
Frantz SW, Ballantyne B, Leung HW. 1998. Acute Intravenous and Inhalation Pharmacokinetics of 2,4-Pentanedione in the Fischer 344 Rat. Toxicol Ind Health. 14(3):413-428.
Furihata C, Matsushima T. 1987. Use of in vivo/in vitro unscheduled DNA synthesis for identification of organ specific carcinogens. Crit Rev Toxicol. 17:245-277.
Furihata C, Yoshida S, Matsushima T. 1985. Potential initiating and promoting activities of diacetyl and glyoxal in rat stomach mucosa. Jpn J Cancer Res. 76(9):809-814.
Gabriel MA, Ilbawi M, Al-Khalidi UAS. 1972. The oxidation of acetoin to CO2 in intact animals and in liver mince preparation. Comp Biochem Physiol B. 41(3):493-502.
Gaffney SH, Abelmann A, Pierce JS, Glynn ME, Henshaw JL, McCarthy LA, Lotter JT, Liong M, Finley BL. 2015. Naturally occurring diacetyl and 2,3-pentanedione concentrations associated with roasting and grinding unflavored coffee beans in a commercial setting. Toxicology Reports. 2:1171-1181.
Gaunt IF, Brantom PG, Kiss IS, Grasso P, Gangolli SD. 1972. Short- term toxicity of acetoin (acetyl methyl carbinol) in rats. Food Cosmet Toxicol. 10:131-141.
Glensvig and Ports. 2006. Mapping of perfume in toys and children’s articles [PDF, 861 KB]. [accessed 2017 May]. Denmark: Danish Ministry of Environment.
Gloede E, Cichocki JA, Baldino JB, Morris JB. 2011. A Validated Hybrid Computational Fluid Dynamics Physiologically Based Pharmacokinetic Model for Respiratory Tract Vapor Absorption in the Human and Rat and Its Application to Inhalation Dosimetry of Diacetyl. Toxicol Sci. 123(1):231-246.
Golder Associates. 1987. Testing of specific organic compounds in soils in background in urban areas. Draft working paper to Shell Canada Limited and Texaco Canada Limited.
Goravanahally MP, Hubbs AF, Fedan JS, Kashon ML, Battelli LA, Mercer RR, Goldsmith WT, Jackson MC, Cumpston A, Frazer DG, Dey RD. 2014. Diacetyl Increases Sensory Innervation and Substance P Production in Rat Trachea. Toxicol Pathol. 42: 582-590.
Grady SJ, Casey GD. 2001. Occurrence and distribution of methyl tert-butyl ether and other volatile organic compounds in drinking water in the northeast and mid-Atlantic regions of the United States, 1993-98 [PDF, 7.47 MB]. Water-Resources Investigations Report 00-4228. East Hartford (CT): U.S. Geological Survey.
Grady SJ. 2003. A national survey of methyl tert-butyl ether and other volatile organic compounds in drinking-water sources: Results of the random survey [PDF, 8.99 MB]. Water-Resources Investigations Report 02-4079. East Hartford (CT): U.S. Geological Survey.
Hall, L.W., Jr., W.S. Hall, S.J. Bushong and R.L. Herman. 1987. In situ striped bass (Morone saxatilis) contaminant and water quality studies in the Potomac River. Aquatic Toxicology, 10: 73-99.
Hansen, J, Hansen OC, Pommer K. 2004. Mapping of chemical substances in consumer products. Release of chemical substances from tents and tunnels for children [PDF, 360 KB]. [accessed 2017 May]. Denmark: Danish Ministry of Environment.
Hansen PL, Tønning K, Malmgren-Hansen B, Jacobsen E. 2008. Survey and health assessment of chemical substances in hobby products for children [PDF, 372 KB]. Survey of Chemical Substances in Consumer Products, No. 93. Denmark: Danish Ministry of the Environment.
Health Canada. 1994. Human health risk assessment for priority substances [PDF, 213.5 KB]. Ottawa (ON): Minister of Supply and Services Canada. Cat. No.: En40-215/41E. [accessed 2017 May 25].
Health Canada. 1998. Exposure factors for assessing total daily intake of priority substances by the general population of Canada. Unpublished report. Ottawa (ON): Health Canada, Environmental Health Directorate.
Health Canada. 2010a. Windsor exposure assessment study (2005-2006): Data summary for volatile organic compound sampling. Ottawa (ON): Health Canada. 85 pp.
Health Canada. 2010b. Regina indoor air quality study (2007): Data summary for volatile organic compound sampling. Ottawa (ON): Health Canada. 164 pp.
Health Canada. 2012. Halifax indoor air quality study 2009: VOC sampling data summary (Draft). Ottawa (ON): Health Canada. 37 pp.
Health Canada. 2013. Edmonton indoor air quality study 2010: Volatile Organic Compounds (VOC) data summary. Ottawa (ON): Health Canada. 50 pp.
Health Canada. [modified 2015a Dec 14]. Cosmetic ingredient hotlist: list of ingredients that are prohibited for use in cosmetic products. Ottawa (ON): Health Canada, Consumer Product Safety Directorate. [accessed 2017 October 23].
Health Canada. 2015b. Food Consumption Table derived from Statistics Canada, Canadian Community Health Survey, Cycle 2.2, Nutrition (2004), Share file. Ottawa.
Health Canada 2015c. Keep calm and use hand sanitizer – how much and how often. Unpublished. Existing Substances Risk Assessment Bureau and New Substances Assessment and Control Bureau, Health Canada. Presented at Health Canada Science Forum 2015.
Health Canada. 2016. SDS Search Tool [in house database]. [last updated 2016 Sept 15; accessed 2016 July]
Health Canada. 2018. Supporting documentation: intake tables for Ketones Group. Ottawa (ON): Health Canada. Information in support of the screening assessment for the Ketones Grouping. Available from: substances@ec.gc.ca.
Hjelm EW, Boman A, Fernstrom P, Hagberg M, JohansonG. 1991. Percutaneous uptake and kinetics of methyl isobutyl ketone (MIBK) in the guinea pig. Toxicol Lett. 56:79-86.
Hjelm EW, Hagberg M, Iregren A, Lof A. 1990. Exposure to methyl isobutyl ketone: Toxicokinetics and occurrence of irritative and CNS symptoms in man. Int Arch Occup Environ Health. 62:19-26.
[HPD] Household Products Database [database]. 1993- . Bethesda (MD): US National Library of Medicine. [accessed 2017 Mar-Oct.].
[HSDB] Hazardous Substances Data Bank [database]. 1983- . Bethesda (MD): National Library of Medicine (US). [accessed 2017 October 23].
Hubbs AF, Battelli LA, Goldsmith WT, Porter DW, Frazer D, Friend S, Schwegler-Berry D, Mercer RR, Reynolds JS, Grote A, Castranova V, Kullman G, Fedan JS, Dowdy J, Jones WG. 2002. Necrosis of nasal and airway epithelium in rats inhaling vapors of artificial butter flavouring. Toxicol Appl Pharmacol. 185(2):128-135.
Hubbs AF, Cumpston AM, Goldsmith WT, Battelli LA, Kashon ML, Jackson MC, Frazer DG, Fedan JS, Goravanahally MP, Castranova V, Kreiss K, Willard PA, Friend S, Schwegler-Berry D, Fluharty KL, Sriram K. 2012. Respiratory and olfactory cytotoxicity of inhaled 2,3-pentanedione in Sprague-Dawley rats. Am J Pathol. 181(3):829-844.
Hubbs AF, Goldsmith WT, Kashon ML, Frazer D, Mercer RR, Battelli LA, Kullman GJ, Schwegler-Berry D, Friend S, Castranova V. 2008. Respiratory toxicologic pathology of inhaled diacetyl in Sprague-Dawley rats. Toxicol Pathol. 36:330-344.
Hughes BJ, Thomas J, Lynch AM, Borghoff SJ, Green S, Mensing T, Sarang SS, LeBaron MJ. 2016. Methyl isobutyl ketone induced hepatocellular carcinogenesis in B6C3F mice: A constitutive androstane receptor (CAR) mediated mode of action. Regul Toxicol Pharmacol. 81:421-429.
[IARC] International Agency for Research on Cancer. 2013. Methyl isobutyl ketone4-methyl-2-pentanone [PDF, 412 KB]. Some chemicals present in industrial and consumer products, food and drinking-water. IARC Monogr Eval Carcinog Risks Hum. 101. Lyon, France.
Imhof R, Glattle H, Bosset JO. 1995. Volatile organic compounds produced by thermophilic and mesophilic single strain dairy starter cultures. Lebensm.-Wiss. U. –Technol. 28:78-86.
[IPCS] International Programme on Chemical Safety. 1990. Environmental Health Criteria 117: Methyl isobutyl ketone. Geneva (CH): United Nations Environment Programme, International Labour Organization, World Health Organization. [accessed 2017 October 23, WHO.
[IPCS] International Programme on Chemical Safety. 1993. Environmental Health Criteria 143: Methyl ethyl ketone. Geneva (CH): United Nations Environment Programme, International Labour Organization, World Health Organization. [accessed 2017 October 23]..
[IPCS] International Programme on Chemical Safety. 1999. Safety evaluation of aliphatic acyclic and alicyclic alpha -diketones and related alpha-hydroxy ketones. Prepared by the Fifty first meeting of the Joint FAO/WHO, World Health Organization, Geneva, International Programme on Chemical Safety. (WHO Food Additive Series 42). [accessed 2017 Jan 20].
[IPCS] International Programme on Chemical Safety. 1997. Methyl isobutyl ketone. International Chemical Safety cards, International Program on Chemical Safety, Geneva: WHO.
Iregren A, Tesarz M, Wigaeus-Hjelm E. 1993. Human experimental MIBK exposure: effects on heart rats, performance, and symptoms. Environ Res. 63:101-108.
[IRIS] Integrated Risk Information System. 2003a. Toxicological review of methyl ethyl ketone in support of summary information on the Integrated Risk Information System (IRIS) [PDF, 1.07 MB]. National Center for Environmental Assessment, Washington, DC. EPA 635/R-03/009. Bethesda (MD): US National Library of Medicine. [accessed 2017 Jan 4]
[IRIS] Integrated Risk Information System. 2003b. Toxicological review of Methyl Isobutyl Ketone CAS NO. 108-10-1 in support of summary information on the Integrated Risk Information System (IRIS) [PDF, 586 KB]. National Center for Environmental Assessment, Washington, DC. EPA EPA/635/R-03/002. [accessed 2016 Aug 18].
[JBRC] Japan Bioassay Research Center. 2010a. Summary of inhalation carcinogenicity study of 2,4-pentanedione in F344 rats [PDF, 1.24 MB]. Japan Industrial Safety and Health Association. [accessed 2017 June 15].
[JBRC] Japan Bioassay Research Center. 2010b. Summary of inhalation carcinogenicity study of 2,4-Pentanedione in B6D2F1 mice [PDF, 1.24 MB]. Japan Industrial Safety and Health Association. [accessed 2017 June 15].
Juberg D, Alfano K, Coughlin RJ and Thomson KM, 2001. An observational study of object mouthing behavior by young children. Pediatrics, 107, 135–142.
Kato F, Araki A, Nozaki K, Matsushima T. 1989. Mutagenicity of aldehydes and diketones. Mutat Res. 216:366-367.
Katz GV, Renner ER Jr., Terhaar CJ. 1986. Subchronic Inhalation Toxicity of Methyl isoamyl Ketone in Rats. Fundam Appl Toxicol. 6:498-505.
Kim SB, Hayase F, Kato H. 1987. Desmutagenic effect of alpha-dicarbonyl and alpha-hydroxycarbonyl compounds against mutagenic heterocyclic amines. Mutat Res. 177:9-15.
Krasavage WJ, O’donoghue JL, Divincenzo GD. 1982. Methyl isobutyl ketone. In: Clayton GD. and Clayton FE., ed. Patty's industrial hygiene and toxicology, New York, John Wiley and Sons, Vol. 2e, pp. 4747-4751.
Kreiss K, Gomaa A, Kullman G, Fedan K, Simoes EJ, Enright PL. 2002. Clinical bronchiolitis obliterans in workers at a microwave popcorn plant. New Eng J Med. 347(5):330-338.
LaBelle CW, Brieger H. 1955. The vapor toxicity of a composite solvent and its principal components. Am Med Assoc Arch Ind Health. 12:623-627.
Larsen ST, Alarie Y, Hammer M, Nielsen GD. 2009. Acute airway effects of diacetyl in mice. Inhalation Toxicol. 21:1123-1128.
Lason AM, Speers RA, Zhu J. 1995. Chemical and sensory detection of diacetyl in strawberry juice (short communication). Die Nahrung. 39(4):323-327.
Liira J, Riihimaki V, Pfaffli P. 1988. Kinetics of methyl ethyl ketone in man: absorption,distribution and elimination in inhalation exposure. Int Arch Occup Environ Health. 60(3):195-200.
[LNHPD] Licensed Natural Health Products Database [database]. [modified 2018 Feb 06]. Ottawa (ON): Health Canada. [accessed 2017 October 23].
Lowengart RA, Peters JM, Cicioni C, Buckley J, Bernstein L, Preston-Martin S, Rappaport E. 1987. Childhood leukemia and parents' occupational and home exposures. J Natl Cancer Inst. 79(1):39-46.
Macciola V, Candela G, De Leonardis A. 2008. Rapid gas-chromatographic method for the detemination of diacetyl in milk, fermented milk and butter. Food Contol. 19:873-878.
MacEwen, JD, Vernot EH, Haun CC. 1971. Effect of 90-day continuous exposure to methyl isobutyl ketone on dogs, monkeys and rats. Aerospace Medical Research Laboratory Document No. AMRL-TR-71-65. NTIS No. AD Rep. 730291.
[MAI] Microbiological Associates, Inc. 1986. Subchronic toxicity of methyl isobutyl ketone in Sprague Dawley rats. Final Report. Study No. 5221.04. Performed by Microbiological Associates, Inc. for Research Triangle Institute. Unpublished report dated July 15, 1986.
Malysheva MV. 1988. The effect of the methods of cutaneous administration of methyl isobutyl ketone on its toxicity. Gig Sanit. 10:79-80.
Marnett LJ, Hurd HK, Hollstein MC, Levin DE, Esterbauer H, Ames BN. 1985. Naturally occurring carbonyl compounds are mutagens in Salmonella tester strain TA104. Mutat Res. 148:24-34.
McCready D, Fontaine D. 2010. Refining ConsExpo evaporation and human exposure calculations for REACH. Huamn and Ecological Risk Assessment: An International Journal. 16(4):783-800.
McCoy MJ, Hoppe Parr KA, Anderson KE, Cornish J, Haapala M, Greivell J. 2017. Diacetyl and 2,3-pentanedione in breathing zone and area air during large-scale commercial coffee roasting, blending and grinding processes. Toxicol Rep 4:113-122.
MHW (1997) Ministry of Health and Welfare: Japan, Toxicity Testing Reports of Environmental Chemicals 5, 475-498.
More SS, Raza A, Vince R. 2012. The butter flavorant, diacetyl, forms a covalent adduct with 2-deoxyguanosine, uncoils DNA, and leads to cell death. J Agric Food Chem. 60(12):3311-3317.
Morgan DL, Flake GP, Kirby PJ, Palmer SM. 2008. Respiratory toxicity of diacetyl in C57BL/6 mice. Toxicol Sci. 103(1):169-180.
Morgan DL, Jokinen MP, Price HC, Gwinn WM, Palmer SM, Flake GP. 2012. Bronchial and bronchiolar fibrosis in rats exposed to 2, 3-pentanedione vapors: implications for bronchiolitis obliterans in humans. Toxicol Pathol. 40(3):448-465.
Morris JB, Hubbs AF. 2009. Inhalation dosimetry of diacetyl and butyric acid, two components of butter flavoring vapors. Toxicol Sci. 108:173-183.
Munies R, Wurster DE. 1965. Investigation of some factors influencing percutaneous absorption-III. Absorption of methyl ethyl ketone. J Pharm Sci. 54:1281-1284.
Mutch, R.D., Jr., J. Daigler and J.H. Clarke. 1983. Clean-up of Shope’s landfill, Girard, PA. In: National Conference on Management of Uncontrolled Hazardous Waste Sites. Washington, DC. pp. 296-300.
Nelson KW, Ege JF, Ross M, Woodman LE, Silverman L. 1943. Sensory Response to Certain Industrial Solvent Vapor. J Ind Hyg Toxicol. 25:282-285.
Nemec M, Pitt J, Topping D, Gingell R, Pavkov K, Rauckman E, Harris S. 2004. Inhalation two-generation reproductive toxicity study of methyl isobutyl ketone in rats. Int J Toxicol. 23:127-143.
[NHPID] Natural Health Products Ingredients Database [database]. [modified 2018 May 07]. Ottawa (ON): Health Canada. [accessed 2017 October 23].
[NICNAS] National Industrial Chemicals Notification and Assessment Scheme. 2017. 2-Pentanone, 4-methyl-. Sydney (AU): Department of Health, National Industrial Chemicals Notification and Assessment Scheme. Human Health Tier III assessment for 2-Pentanone, 4-methyl- , CAS No. 108-10-1. [accessed 2017 Oct 13].
Nijssen LM, Ingen-Visscher CA van, Donders JJH [editors]. 1963-2016. VCF Volatile Compounds in Food online database [database]. Version 16.3. Zeist (The Netherlands): Triskelion B.V. [date updated 2016 November; accessed 2017 April].
Nilsson NH, Malmgren-Hansen B, Bernth N, Pedersen E, Pommer K. 2006. Survey of chemical substances in consumer products: Survey and health assessment of chemicals substances in sex toys [PDF, 892 KB]. Report no. 77. [accessed 2017 May]. Denmark: Danish Ministry of Environment.
[NPRI] National Pollutant Release Inventory [database]. 2011-2015a. NPRI Datasets: Methyl ethyl ketone (78-93-3). Gatineau (QC): Environment and Climate Change Canada. Search results for 78-93-3. [modified 2016 September 29; accessed 2017 February 20].
[NPRI] National Pollutant Release Inventory [database]. 2011-2015b. NPRI Datasets: Methyl isobutyl ketone (108-10-1). Gatineau (QC): Environment and Climate Change Canada. Search results for 8-10-1. [modified 2016 September 29; accessed 2017 February 20].
[NTP] National Toxicology Program (US). 2007a. Chemical information review document for artificial butter flavoring and constituents. Diacetyl(CAS No. 431-03-8) and acetoin (CAS No. 513-86-0). Supporting nomination for toxicological evaluation by the National Toxicology Program, January 2007. Prepared by Integrated Laboratory Systems, Inc. Research Triangle Park (NC): US Department of Health and Human Services, National Toxicology Program. Contract No.: N01-ES-35515.
[NTP] National Toxicology Program (US). 2007b. NTP toxicology and carcinogenesis studies of Methyl Isobutyl Ketone (CAS NO. 108–10–1) in F344/N rats and B6C3F1 mice (inhalation studies). Research Triangle Park (NC): US Department of Health and Human Services, National Toxicology Program. Report No. 538: 1–236.
[NTP] National Toxicology Program. 2017a. Draft toxicology and carcinogenesis studies of 2,3-butanedione (CAS NO. 431-03-8) in wistar han [Crl:WI (Han)] rats and B6C3F1/N mice (inhalation studies) [PDF, 21.9 MB]. Research Triangle Park (NC): US Department of Health and Human Services, National Toxicology Program. Report No.: NTP TR 593. Results only.
[NTP] National Toxicology Program. 2017b. Tox-99: Acetoin and 2,3-pentanedione, Toxicity Report Tables & Curves. Research Triangle Park (NC): US Department of Health and Human Services, National Toxicology Program. [accessed 2017 Oct 13]. Results only.
Nylén D, Borling P, Sørensen H. 2004. Survey of chemical substances in consumer products: Mapping of chemical substances in animal care products [PDF, 976 KB]. Report no. 44. [accessed 2017 May]. Denmark: Danishm Ministry of Environment.
O’Donoghue JL, Haworth SR, Curren RD, Kirby PE, Lawlor T, Moran EJ, Phillips RD, Putnam DL, Rogers-Back AM, Slesinski RS, Thilagar A. 1988. Mutagenicity studies on ketone solvents: methyl ethyl ketone, methyl isobutyl ketone, and isophorone. Mutat Res. 206:149-161.
O’Donoghue JL. 2012a. Ketones of four or five carbons. In: add editor names. Patty’s Toxicology, add ed, vol publication: John Wiley & Sons. p. 753-806.
O’Donoghue JL. 2012b. Ketones of six to thirteen carbons. In: add editor names. Patty’s Toxicology, add ed, vol publication: John Wiley & Sons. P. 807-914.
[OECD] Organization for Economic Co-operation and Development. 1996. SIDS initial assessment report: Methyl Isobutyl Ketone: CAS No. 108-10-1 [PDF, 71.4 KB]. SIAM [SIDS Initial Assessment Meeting] 5: 1996 Oct [accessed 2017 April].
[OECD] Organization for Economic Co-operation and Development. 1997a. SIDS initial assessment report: Methyl ethyl ketone: CAS No. 78-93-3 SIDS initial assessment report: Methyl ethyl ketone: CAS No. 78-93-3. SIAM [SIDS Initial Assessment Meeting] 6: 1997 June: Paris, France [accessed 2017 May 5].
[OECD] Organization for Economic Co-operation and Development. 1997b. SIDS initial assessment report: Mesityl oxide: CAS No. 141-79-7. SIAM [SIDS Initial Assessment Meeting] 6: 1997 June: Paris, France [updated 2010 November; accessed 2017 May 5].
[OECD] Organisation for Economic Co-operation and Development. 2000. SIDS initial assessment report: Diacetone alcohol, CAS No. 123-42-2. SIAM [SIDS Initial Assessment Meeting] 10; 2000 March; Japan [accessed 2017 April].
[OECD] Organisation for Economic Co-operation and Development. 2001. SIDS initial assessment report: 2,4-Pentanedione, CAS No. 123-54-6 [PDF, 465 KB]. SIAM [SIDS Initial Assessment Meeting] 13; 2001 Sept; Bern, Switzerland. [accessed 2017 Oct 13].
[OECD] Organization for Economic Co-operation and Development. 2003. SIDS initial assessment report: 6-methylhept-5-en-2-one: CAS No. 110-93-0 [PDF, 531 KB]. SIAM [SIDS Initial Assessment Meeting] 17: 2003 Novermber: Paris, France [accessed 2017 May 17].
Parmet AJ, von Essen S. 2002. Rapidly progressive, fixed airway obstructive disease in popcorn workers: a new occupational pulmonary illness. J Occup Environ Med. 44:216-218.
Paxeus, N. 1996. Organic pollutants in the effluents of large wastewater treatment plants in Sweden. Water Research 30(5): 1115-1122.
Pellizzari ED, Hartwell TD, Harris III BSH, Waddell RD, Whitaker DA, Erickson MD. 1982. Purgeable organic compunds in mother’s milk. Bull. Environm. Contam. Toxicol. 28:322-328.
Perbellini L, Brugnone F, Mozzo P, Cocheo V, Caretta D. 1984. Methyl ethyl ketone exposure in industrial workers. Uptake and kinetics. Int Arch Occup Environ Health. 54:73-81.
[P&G] Procter & Gamble. c2017a. Febreze® PLUG. [accessed 2017 Oct].
[P&G] Procter & Gamble. c2017b. Febreze Set & Refresh Rain Air Freshener 5.5 ml. [accessed 2017 Oct].
Phillips RD, Moran EJ, Dodd DE, Fowler E, Kary CD, O’Donoghue J. 1987. A 14-week vapor inhalation toxicity study of methyl isobutyl ketone. Fundam Appl Toxicol. 9:380-388.
Pierce JS, Abelmann A, Lotter JT, Comerford C, Keeton K, Finley BL. 2015. Characterization of naturally occurring airborne diacetyl concentrations associated with the preparateion and consumption of unflavoured coffee. Toxicology Reports. 2:1200-1208.
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment]. 2002. Children’s toys fact sheet: to assess the risks for the consumer [PDF, 303 KB]. Bilthoven (NL): RIVM. Report No.: 612810012/2002. [accessed 2017 April-October ].
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment (NL)]. 2006. Cosmetics fact sheet: to assess the risks for the consumer: updated version for ConsExpo 4 [PDF, 330 KB]. Bilthoven (NL): RIVM. Report No.: 320104001/2006. [accessed 2017 April-October].
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment]. 2007a. Do-it-yourself products fact sheet: to assess the risks for the consumer [PDF, 417 KB]. Bilthoven (NL): RIVM. Report No.: 320104007/2007. [accessed 2017 April-October].
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment]. 2007b. Paint products fact sheet: to assess the risks for the consumer: updated version for ConsExpo 4 [PDF, 264 KB]. Bilthoven (NL): RIVM. Report No.: 320104008/2007. [accessed 2017 April-October ].
Rosati JA, Krebs KA, Liu X. 2007. Emissions from cooking microwave popcorn. Critical Reviews in Food Science and Nutrition. 47(8):701-709.
Sabel, G.V. and T.P. Clark. 1984. Volatile organic compounds as indicators of municipal solid waste leachate contamination. Waste Management and Research 2: 119-130.
Saillenfait AM, Gallissot F, Sabate´ JP, Bourges-Abella N, Cadot R, Morel G, Lambert AM. 2006. Developmental toxicity of combined ethylbenzene and methylethylketone administered by inhalation to rats. Food Chem Toxicol. 44:1287-1298.
Sánchez-Peña CM, Luna G, García-González, Aparicio R. 2005. Characterization of French and Spanish dry-cured hams : influence of the volatiles from the muscles and the subcutaneous fat quantified by SPME-GC. Meat Science. 69:635-645.
Sawhney BL. Kozloski RP. 1984. Organic pollutants in leachates from landfill sites. J. Environ. Qual. 13(3): 349-352.
Schwetz BA, Leong BKJ, Gehring PJ. 1974. Embryo and fetotoxicity of inhaled carbon tetrachloride, 1,1-dichloroethane and methyl ethyl ketone in rats. Toxicol Appl Pharmacol. 28:452-464.
Schwetz BA, Mast TJ, Weigel RJ, Dill JA, Morrissey RE. 1991. Developmental toxicity of inhaled methyl ethyl ketone in Swiss mice. Fundam Appl Toxicol. 16:742-748.
[SCOEL] European Commission. Scientific Committee on Occupational Exposure Limits. 2014. Recommendation from the Scientific Committee on Occupational Exposure Limits for Diacetyl [PDF, 506.16 KB]. SCOEL/SUM/149. [accessed 2017 Oct 13].
[SDS] Safety Data Sheet. 2008a. La Bomba Gotas de Brillo [PDF, 88.5 KB]. San Juan (PR): Puerto Rico Merchandising. [accessed 2017 Sept 20].
[SDS] Safety Data Sheet. 2008b. Permanent markers. La Mirada (CA): Kittrich Corporation. [accessed 2016 July].
[SDS] Safety Data Sheet. 2012a. Sharpie Fine Point Marker, Shapie Ultra Fine Point Marker, etc. Oak Brook (IL): Newell Rubbermaid. [accessed 2016 July].
[SDS] Safety Data Sheet. 2012b. R-Cure 800 PCS Sour Cherry Urethane. Cornwall (ON): Valspar, Inc. [accessed 2016 July].
[SDS] Safety Data Sheet. 2014. Berol, Expo Low Odor, Expo Click, etc. Oak Brook (IL): Newell Rubbermaid. [accessed 2016 July].
[SDS] Safety Data Sheet. 2015a. Febreze Set & Refresh – Cozy Vanilla Sugar. Cincinnati (OH): Procter & Gamble, Fabric and Home Care Division. [accessed 2016 July].
[SDS] Safety Data Sheet. 2015b. Febreze Noticeables Air Freshener – Autumn Charm. Cincinnati (OH): Procter & Gamble, Fabric and Home Care Division. [accessed 2016 July].
[SDS] Safety Data Sheet. 2016a. Vanilla Custard Flavor. Scotts Valley (CA): Perfumer’s Apprentice. [accessed 2016 July].
[SDS] Safety Data Sheet. 2016b. Hardtop AX Comp A. Belle Chasse (LA): Jotun Paints, Inc. [accessed 2016 July].
[SDS] Safety Data Sheet. 2017. Epoxy Thinner. Montreal (QC): Swing Paints Ltd. [accessed 2017 Nov].
Shane BS, Troxclair AM, McMillin DJ, Henry CB. 1988. Comparative mutagenicity of nine brands of coffee to Salmonella typhimurium TA100, TA102, and TA104. Environ Mol Mutag. 11:195-206.
Sheldon LS, Hites RA. 1978. Organic compounds in the Delaware River. Environ. Sci. Technol. 12(10): 1188-1194.
Shimoda M, Yoshimura T, Ishikawa H, Hayakawa I, Osajima Y. 2000. Volatile compounds of human milk. J. Fac. Agr., Kyushu Univ. 45(1):199-206.
Silverman L, Schulte HF, First MW. 1946. Further studies on sensory response to certain industrial solvent vapors. J Ind Hyg Toxicol. 28:262-266.
Smyth HF Jr, Seaton J, Fischer L. 1942. Response of guinea pigs and rats to repeated Inhalation of vapors of mesityl oxide and isophorone. J Ind Hyg Toxicol. 24(3):46-50.
Smyth HF. 1956. Hygienic standards for daily inhalation. Am Ind Hyg Assoc J. 17:129-266.
Sparks LE, Tichenor BA, Chang J, Guo Z. 1996. Gas-phase mass transfer model for predicting volatile organic compound (VOC) emission rates from indoor pollutant sources. Indoor Air. 6:31-40.
Spirtas R, Stewart PA, Lee JS, Marano DE, Forbes CD, Grauman DJ, Pettigrew HM, Blair A, Hoover RN, Cohen JL. 1991. Retrospective cohort mortality study of workers at an aircraft maintenance facility: I. Epidemiological results. II. Exposures and their assessment.Br J Ind Med. 48(8):515-537.
Stoner GD, Shimkin MB, Kniazeff AJ, Weisburger JH, Weisburger EK, Gori GB. 1973. Test for carcinogenicity of food additives and chemotherapeutic agents by the pulmonary tumor response in strain A mice. Cancer Res. 33(12):3069-3085.
Svendsen N, Pedersen SF, Hansen OC, Pedersen E, Bernth N. 2005. Survey and release of chemical substances in “slimy” toys [PDF, 892 KB]. [accessed 2017 May]. Denmark: Danish Ministry of Environment.
[TDS] Technical Data Sheet. 2015. Klenk’s Epoxy Thinner. Montreal (QC): Wing Paints. [last modified 2015 Aug 11; accessed 2017 Nov.].
TERA 2017. Read across approach for 4-Methylpent-3-en-2-one. Toxicology Excellence for Risk Assessment (TERA) Center, Department of Environmental Health, College of Medicine, University of Cincinnati (Health Canada internal document).
Tønning K, Jacobsen E, Pedersen E, Pedersen PB. 2010. Survey of chemical substances in consumer products: Survey and health assessment of products for interior car care [PDF, 761 KB]. Report no. 105. [accessed 2017 May]. Denmark: Danish Ministry of Environment.
Toso B, Procida G, Stefanon B. 2002. Determination of volatile compounds in cows’ milk using headspace GC-MS. Journal of Dairy Research. 69:569-577.
Toxigenics. 1981. 90-Day vapor inhalation toxicity study of methyl ethyl ketone in albino rats. Submitted to Chemical Industry Institute of Toxicology, Research Triangle Park, NC. Doc. ID 878212064. Microfiche No. 205953. (Study 420-0305)
Tyl RW, Ballantyne B, Fisher LC, Tarasi DJ, Dodd DE. 1989. Dominant lethal assay of 2,4-pentanedione vapour in Fischer 344 rats. Toxicol Ind Health. 5:463-477.
Tyl RW, Ballantyne B, Pritts IM, Garman RH, Fisher LC, France KA, McNeil DJ. 1990. An evaluation of the developmental toxicity of 2, 4-pentanedione in the Fischer 344 rat by vapor exposure. Toxicol Ind Health. 6(3-4):461-474.
Tyl RW, France KA, Fisher LC, Pritts IM, Tyler TR, Phillips RD, Moran EJ. 1987. Developmental toxicity evaluation of inhaled methyl isobutyl ketone in Fischer 344 rats and CD-1 mice. Fundam Appl Toxicol. 8: 310-327.
[US EPA AcToR]. United State Environmental Protection Agency Aggregated Computational Toxicology Resource System. 2015. 2-Pentanone. CAS RN. 107-87-9, [accessed 1 February 2017].
[US EPA] US Environmental Protection Agency. 2001a. High Production Volume Information System. Washington (DC): US EPA, Office of Pollution Prevention and toxics. 2-Pentanone. Submission ID 24977107 [accessed 2017 October 23].
[US EPA] US Environmental Protection Agency. 2001b. High Production Volume Information System. Washington (DC): US EPA, Office of Pollution Prevention and toxics. 2-Hexanonem 5-methyl-. Submission ID 24977542 [Accessed 2017 October 23].
[US EPA] U.S. Environmental Protection Agency. 1988. Kin-Buc landfill. EPA/ROD/R02-88/068.
[US EPA] US Environmental Protection Agency. 2005. PARAMS model. Washington (DC): US Environmental Protection Agency. [last revised 2011 Aug 18; accessed Oct 2017].
Valero E, Villamiel M, Miralles B, Sanz J, Martinez-Castro I. 2001. Changes in flavour and volatile components during storage of whole and skimmed UHT milk. Food Chemistry. 72:51-58.
Van Engelen JGM, Park MVDZ, Janssen PJCM, Omen AG, Brandon EFA, Bouma K, Sips AJAM, Van Raaij MTM.. 2008. Chemicals in toys. A general methodology for assessment of chemical safety of toys with a focus on elements [PDF]. RIVM rpoert 320003001/2008.
Van Rooy FG, Rooyackers JM, Prokop M, Houba R, Smit LA, Heederik DJ. 2007. Bronchiolitis obliterans syndrome in chemical workers producing diacetyl for food flavorings. Am J Respir Crit Care Med. 176(5):498-504.
[VCCEP] Voluntary Children's Chemical Evaluation Program. 2003. Methyl ethyl ketone (CAS No. 78-93-3). VCCEP Submission. Prepared by: American Chemistry Council’s Ketones Panel. [accessed 2016 November].
Von der Hude W, Behm C, Gurtler R, Basler A. 1988. Evaluation of the SOS chromotest. Mutat Res. 203(2):81-94.
Wen CP, Tsai SP, Weiss NS, Gibson RL, Wong O, McClellan WA. 1985. Long-term mortality study of oil refinery workers. IV. Exposure to the lubricating dewaxing process. J Natl Cancer Inst. 74:11-18.
Wilkinson SC, Williams FM. 2001. In vitro dermal absorption of liquids. Contract Research Report 350/2001. Newcastle-upon-Tyne (UK): University of Newcastle, Department of Environmental and Occupational Medicine, Skin Research Group.
[WHO] World Health Organization. 1999a. Safety evaluation of certain food additives: Aliphatic acyclic and alicyclic alpha-diketones and related alpha-hydroxyketones. Geneva (CH): World Health Organization, International Programme on Chemical Safety. (WHO Food Additive Series 42). Prepared by the fifty-first meeting of the Joint FAO/WHO Expert Committee on Food Additives.
[WHO] World Health Organization. 1999b. Safety evaluation of certain food additives: Saturated aliphatic acyclic secondary alcohols, ketones, and related saturated and unsaturated esters. Geneva (CH): World Health Organization, International Programme on Chemical Safety. (WHO Food Additive Series 42). Prepared by the fifty-first meeting of the Joint FAO/WHO Expert Committee on Food Additives.
[WHO] World Health Organization. 2003. Safety evaluation of certain food additives: Aliphatic secondary alcohols, ketones and related esters. Geneva (CH): World Health Organization, International Programme on Chemical Safety. (WHO Food Additive Series 50). Prepared by the fifty-ninth meeting of the Joint FAO/WHO Expert Committee on Food Additives.
Won D, Lusztyk E. 2011. Data gathering on chemicals released to indoor air of residences from building materials and furnishings. Final Report. Ottawa, (ON): NRC. 158 p. Report No.: B3332.2.
Won D, Nong G, Yang W, Schleibinger H. 2013. Material Emissions Data: 52 Building Materials Tested for 124 Compounds. Ottawa (ON): National Research Council Canada. 300 p. Report No.: A1-000342.
Won D, Nong G, Yang W, Collins P. 2014. Material emissions testing: VOCs from wood, paint, and insulation materials. Ottawa (ON): National Research Council Canada. Report No.: A1-000342.2.
Won D. 2015. VOC Emissions from evaporative sources in residential garages. Ottawa (ON): National Research Council Canada. 235 p. Contract No.: A1-000342.
[WSDE] Washington State Department of Ecology. 2012-2017. [database]. Search results for CAS RN [78-93-3]. Lacey (WA): Department of Ecology State of Washington. [accessed 2016 July 4].
Wurster DE, Munies R. 1965. Factors influencing percutaneous absorption II: Absorption of methyl ethyl ketone. Journal of Pharmaceutical Sciences. 54(4):554-556.
Yue J, Zheng Y, Liu Z, Deng Y, Jing Y, Luo Y, Yu W, Zhao Y. 2015. Characterization of volatile compounds in microfiltered pasteurized milk using solid-phase microextraction and GCxGC-TOFMS. International Journal of Food Properties. 18:2193-2212.
Zaccone EJ, Goldsmith WT, Shimko MJ, Wells JR, Schwegler-Berry D, Willard PA, Case SC, Thompson JA, Fedan JS. 2015. Diacetyl and 2,3-pentanedione exposure of human cultured airway epithelial cells: Ion transport effects and metabolism of butter flavoring agents. Toxicol Appl Pharmacol. 289:542-549.
Zaccone EJ, Thompson JA, Ponnoth DS, Cumpston AM, Goldsmith WT, Jackson MC, Kashon ML, Frazer DG, Hubbs AF, Shimko MJ, Fedan JS. 2013. Popcorn flavoring effects on reactivity of rat airways in vivo and in vitro. J Toxicol Environ Health A. 76(11):669-689.
Zakhari, S. 1977. Acute oral, intraperitoneal, and inhalation toxicity of methyl isobutyl ketone in the mouse. In: L. Goldberg (ed.), Isopropanol and ketones in the environment. CRC Press, Cleveland, Ohio. pp. 101-104 (Chapter 11).
Zhu J, Cao X-L, Beauchamp R. 2001. Determination of 2-butoxyethanol emissions from selected consumer products and its application in assessment of inhalation exposure associated with cleaning tasks. Environment International. 26:589-597.
Zhu J, Newhook R, Marro L, Chan C. 2005. Selected volatile organic compounds in residential air in the city of Ottawa, Canada. Environ Sci Technol 39:3964-3971.
Zhu J, Wong SL, Cakmak S. 2013. Nationally representative levels of selected volatile organic compounds in Canadian residential indoor air: Population-based survey. Environ Sci Technol. 47:13276-13283.
Zimmermann FK, Mohr A. 1992. Formaldehyde, glyoxal, urethane, methyl carbamate, 2,3-butanedione, 2,3-hexanedione, ethyl actylate, dibromoacetonitrile, 2-hydroxypropionitrile induce chromosome loss in saccharomyces cerevisiae. Mutat Res. 270:151-166.
Appendices
Appendix A. Ketones in food
Substance Name |
U.S per capita intake (µg/day) |
U.S per capita intake (µg/kg-bw/day) |
Europe per capita intake intake (µg/day) |
Europe per capita intake intake (µg/kg-bw/day) |
|---|---|---|---|---|
MEK |
36 |
0.6 |
110 |
2 |
MPK |
42 |
0.7 |
140 |
2 |
MIBK |
2 |
0.03 |
7 |
0.12 |
Diacetyl |
8000 |
133 |
3300 |
56 |
2,3-PD |
1800 |
1 |
2800 |
4 |
Acetoin |
80 |
29 |
220 |
46 |
MO |
NA |
NA |
0.40 |
0.0067 |
Abbreviations: NA, not applicable
The JECFA per capita intake estimates (µg/day) were derived using the maximized survey-derived daily intake (MSDI) approach, assuming that the reported annual production amount for the various substances in the U.S. and Europe was consumed by just 10% of the population ("eaters only"), and that only 60% of the annual production amount was reported in the poundage surveys. A body weight of 60 kg was used to derive intake estimates in µg/kg-bw/day (International Organization of the Flavor Industry, 1995, US National Academy of Sciences, 1989, both cited in WHO 1999b).
Quantitative exposure estimates for all 10 substances were derived based on consumption data for comparable food categories from the Canadian Community Health Survey (CCHS) 2.2 Food Consumption Table (Health Canada 2015b). A summary of the data identified for each of the substances in foods from the literature, and from the Volatile Compounds in Food (VCF) database (Nijssen et al. 1963-2016) can be found in the Table A-2, Table A-3, Table A-4, and Table A-5. Quantitative data for all food items were not available; therefore, maximum reported values from the VCF database, and the literature were used for various food items to estimate potential high-end exposures to all substances from food for the general population of Canada. Estimated exposures for all ten substances from their natural occurrence in food are presented in Table A-6.
Food category |
MEK (µg/g) |
MPK (µg/g) |
|---|---|---|
Beers and coolers |
0.06 |
0.02 |
Spirits and liquers |
2.00 |
1.20 |
Fruit juices |
0.20 |
0.10 |
Coffee, powder items are reconstituted |
NA |
4.70 |
Tea, including iced tea |
8.00 |
NA |
Milks |
NA |
0.10b |
Creams |
0.07 |
0.05 |
Cheeses |
67.10 |
14.71 |
Yoghurts |
7.00 |
0.01 |
Bananas |
NA |
27.00 |
Cherries |
NA |
0.01 |
Pears |
1.00 |
2.40 |
Pineapple |
NA |
0.01 |
Plums and prunes |
NA |
2.00 |
Strawberries |
NA |
3.10 |
Other fruits (blueberries, dates, kiwis, fruit salads) |
0.02 |
3.30 |
Vegetables, excluding potatoes |
6.00 |
20.00 |
Potatoes, fried |
0.13 |
0.07 |
Chicken, turkey and other birds |
0.10 |
0.23 |
Pork, fresh and ham |
0.72c |
NA |
Fish |
0.60 |
NA |
Shellfish |
NA |
0.12 |
Eggs |
0.01 |
NA |
Nuts, seeds and peanut butter |
0.10 |
7.60 |
Legumes |
0.05 |
NA |
Butter |
0.16 |
0.95 |
Other fats and spreads |
1.51 |
0.03 |
Sugars, syrups and preserves |
0.08 |
0.03 |
Savory snacks |
0.13 |
NA |
Abbreviations: NA, not available
a Data from the Volatile Compounds in Food (VCF) Database (Nijssen et al. 1963-2016) unless specified otherwise.
b Yue et al. 2015
c Sánchez-Pena et al. 2005
Food category |
MIBK (µg/g) |
MIAK(µg/g) |
DAA (µg/g) |
|---|---|---|---|
Beers and coolers |
0.12 |
NA |
NA |
Coffee, powder items are reconstituted |
6.50 |
0.50 |
NA |
Milks |
0.016b |
NA |
0.00015c |
Plums and prunes |
0.01 |
NA |
NA |
Other fruits (blueberries, dates, kiwis, fruit salads) |
0.02 |
0.001 |
0.408 |
Chicken, turkey and other birds |
0.0004 |
NA |
0.07 |
Eggs |
0.003 |
NA |
NA |
Other fats and spreads |
0.38 |
NA |
NA |
Sugars, syrups and preserves |
NA |
NA |
2.70 |
Other ingredients for recipes (e.g., spices, baking ingredients) |
NA |
NA |
28.50 |
Abbreviations: NA, not available
a Data from the Volatile Compounds in Food (VCF) Database (Nijssen et al. 1963-2016) unless specified otherwise.
b Yue et al. 2015
c Toso et al. 2002
Food category |
Diacetyl (µg/g) |
2,3-PD (µg/g) |
Acetoin (µg/g) |
|---|---|---|---|
Beers and coolers |
0.20 |
0.30 |
9.00 |
Spiritis and liqueurs |
9.77b |
0.60 |
335.00 |
Wines |
4.10 |
NA |
234.00l |
Fruit juices |
3.15c |
NA |
0.35 |
Coffee, powder items are reconstituted |
58.70 |
39.60 |
4.90 |
Tea, including iced tea |
1.00 |
2.00 |
NA |
Milks |
29.3d |
0.20j |
NA |
Creams |
30.00 |
NA |
NA |
Cheeses |
4.20 |
NA |
40.00 |
Yoghurts |
43.00e |
NA |
28.00 |
Pasta, rice, cereal grains and flours |
0.19 |
0.25 |
0.75 |
White breads |
0.92 |
0.14 |
NA |
Wholemeal breads |
1.52 |
0.14k |
1.22m |
Other breads |
0.33 |
0.04 |
NA |
Apples |
0.40 |
NA |
3.50 |
Cherries |
NA |
NA |
4.70 |
Pears |
NA |
NA |
0.11 |
Strawberries |
0.20 |
NA |
0.49 |
Other fruits (blueberries, dates, kiwis, fruit salads) |
0.60f |
NA |
22.63 |
Vegetables, excluding potatoes |
0.79g |
NA |
6.40 |
Potatoes, fried |
0.31 |
NA |
NA |
Beef |
23.00 |
6.50 |
8.40 |
Veal |
23.00 |
6.50 |
8.40 |
Chicken, turkey and other birds |
0.90 |
NA |
2.40 |
Livers and liver pates |
27.81 |
NA |
NA |
Luncheon meats, canned and cold cuts |
27.81 |
NA |
NA |
Pork, fresh and ham |
0.36 |
NA |
NA |
Fish |
0.0017 |
0.69 |
0.31 |
Shellfish |
0.10h |
NA |
0.16 |
Nuts, seeds and peanut butter |
0.09 |
0.23 |
NA |
Butter |
21.00e |
0.05e |
2.00 |
Margarines, tub |
NA |
0.01 |
NA |
Margarines, block |
NA |
0.01 |
NA |
Other fats and spreads |
0.60 |
0.01 |
0.04 |
Confectionary, chocolate bars |
0.40 |
NA |
17.00 |
Sugars, syrups and preserves |
2.60 |
NA |
26.00 |
Savory snacks |
NA |
0.20 |
NA |
Soups without vegetables |
0.00056i |
0.42i |
NA |
Gravies |
0.09 |
NA |
NA |
Seasonings, salt, pepper, vinegar |
197.00 |
NA |
1020 |
Other ingredients for recipes (e.g., spices, baking ingredients) |
238.00 |
109.00 |
951.00 |
Abbreviations: NA, not available
a Data from the Volatile Compounds in Food (VCF) Database (Nijssen et al. 1963-2016) unless specified otherwise.
b Cardoso et al. 2003
c Lawson et al. 1995
d De Leonardis et al. 2013
e Macciola et al. 2008
f Mujic et al. 2014
g Annan et al. 2005
h Yu and Chen 2010
i Giri et al. 2010
j Imhof et al. 1994
k Rychlick and Grosch 1996
l Garcia-Martinez et al. 2013
m Birch et al. 2013
Food category |
2,4-PD (µg/g) |
MO (µg/g) |
|---|---|---|
Wines |
NA |
0.00005 |
Fruit juices |
0.01 |
NA |
Other fruits (blueberries, dates, kiwis, fruit salads) |
NA |
0.01 |
Vegetables, excluding potatoes |
NA |
0.04 |
Chicken, turkey and other birds |
0.004 |
NA |
Other fats and spreads |
NA |
3.00 |
Other ingredients for recipes (e.g., spices, baking ingredients) |
NA |
2.40 |
Abbreviations: NA, not available
a Data from the Volatile Compounds in Food (VCF) Database (Nijssen et al. 1963-2016) unless specified otherwise.
Substance |
0-6 moa |
6-12 mo |
1 yr |
2-3 yrs |
4-8 yrs |
9-13 yrs |
14-18 yrs |
19+ yrs |
|---|---|---|---|---|---|---|---|---|
MEK |
0 |
112 |
185 |
174 |
130 |
82 |
66 |
67 |
MPK |
0 |
146 |
216 |
192 |
137 |
86 |
68 |
98 |
MIBK |
0 |
0.5 |
0.9 |
0.7 |
0.4 |
0.8 |
5 |
32 |
MIAK |
0 |
0 |
0.001 |
0.001 |
0.0004 |
0.04 |
0.4 |
3 |
DAA |
0 |
0.04 |
3 |
4 |
4 |
3 |
2 |
2 |
Diacetyl |
0 |
996 |
1625 |
1118 |
671 |
370 |
281 |
445 |
2,3-PD |
0 |
6.5 |
22 |
22 |
20 |
16 |
40 |
208 |
Acetoin |
0 |
173 |
346 |
369 |
293 |
189 |
161 |
308 |
2,4-PD |
0 |
0.04 |
0.1 |
0.1 |
0.08 |
0.04 |
0.03 |
0.02 |
MO |
0 |
0.3 |
1 |
2 |
1 |
0.9 |
0.7 |
0.6 |
a Infants 0 to 6 months old are assumed to be exclusively breast-fed or formula-fed.
Appendix B. Parameters used to estimate exposures.
Cosmetic exposures were estimated using ConsExpo Web (2016). Exposure estimates were calculated based on default body weights of 70.9 kg, 59.4 kg, 15.5 kg, and 7.5 kg for adults (20 years and older), adolescents (12 to 19 years old), toddlers (6 months to 4 years old), and infants (0 to 6 months old), respectively (Health Canada 1998). The estimated inhalation and dermal exposure parameters for cosmetics are described in Table B-1. Dermal intakes are only presented for DAA and 2,4-PD. Unless specified, the defaults come from the relevant ConsExpo Fact Sheet for the scenario presented.
Product (substance) |
Assumptionsa |
|---|---|
Top coat (assume put on finger and toe nails) (MEK) |
Concentration of MEK: 55.7%
Inhalation – Exposure to vapour, evaporation model, Exposure duration: 18 minutes Product amount: 0.33 g (Ficheux et al. 2014) Room volume: 1 m3 (close to the face) Ventilation rate: 1 change per hour Mass transfer coefficient: 6.401 m/hr (Sparks method) Release area mode: constant Release area: 26.2 cm2 (based on data from Ficheux et al. 2014 and assumption that both finger- and toenails are painted) Molecular weight matrix: 124 g/mol |
Nail polish (2 coats on finger and toe nails) (MEK) |
Concentration of MEK: 35%
Inhalation – Exposure to vapour, evaporation model Exposure duration: 35 minutes Product amount: 0.8 g for adults and teens, and 0.27 g on toddlers (Ficheux et al. 2014) Room volume: 1 m3 (close to the face) Ventilation rate: 1change per hour Mass transfer coefficient: 6.401 m/hr (adults), 9.082 m/hr (toddler) (Sparks method) Release area mode: constant Release area: 26.2 cm2 (adults and teens), 8.8 cm2 (toddlers) (based on data from Ficheux et al. 2014 and assumption that both finger- and toenails are painted) Molecular weight matrix: 124 g/mol |
Nail polish (2 coats on finger and toe nails) (DAA) |
Concentration of DAA: 10%
Inhalation – Exposure to vapour, evaporation model Exposure duration: 35 minutes Product amount: 0.8 g for adults and teens, and 0.27 g on toddlers (Ficheux et al. 2014) Room volume: 1 m3 (close to the face) Ventilation rate: 1change per hour Mass transfer coefficient: 5.043 m/hr (adults), 7.029 m/hr (toddler) (Sparks method) Release area mode: constant Release area: 26.2 cm2 (adults and teens), 8.8 cm2 (toddlers) (based on data from Ficheux et al. 2014 and assumption that both finger- and toenails are painted) Molecular weight matrix: 124 g/mol
Dermal: Amount on the skin (g/use): 0.16 for adults and teens and 0.06 for toddlers (Ficheux et al. 2014) Frequency (use/day): 0.18 for adults, 0.2 for teens, 0.13 for toddlers (Ficheux et al. 2014) Surface area: area around the nails = 2.7 cm2 (adults), 0.9 cm2 (toddlers) (Ficheux et al. 2014) |
Nail polish remover (MEK) |
Concentration of MEK: 84% (adults), 76.4% (toddlers)
Inhalation – Exposure to vapour, evaporation model Exposure duration: 8 minutes Product amount: 5.36 g (adults and teens) and 1.82 g (toddlers) Room volume: 1 m3 (close to the face) Ventilation rate: 1change per hour Mass transfer coefficient: 5.080 m/hr (adults), 7.209 m/hr (toddler) (Sparks method) Release area mode: constant Release area: 34 cm2 (adults and teens) and 11.6 cm2 (toddlers) (based on data from Ficheux et al. 2014 and assumption that both finger- and toenails are painted) Molecular weight matrix: 124 g/mol
|
Rubbing alcohol (MIBK) |
Concentration of MIBK: 0.98% No scenario identified in ConsExpo, used professional judgement
Inhalation – Exposure to vapour, instantaneous release model Exposure duration: 5 minutes Product amount: 0.02 g Room volume: 1 m3 (close to the face) Ventilation rate: 1 change per hour
Dermal: Surface area: assumed an area of 5 cm x 5 cm = 25 cm2 is covered Amount on the skin (g/use): adjust product amount of hand sanitizer 0.7 g (Health Canada 2015c) for 910 cm2 surface area to an area of 25 cm2 = 0.02 g |
Eyeliner stickers (DAA) |
Concentration of DAA: 30%
Dermal: Product amount (g/use): 27 mg (P95 from Ficheux et al. 2016) Surface area: 5 cm2 (professional judgement, based on 3.2 cm2 in ConsExpo and that stickers appear to cover a larger surface area)
Inhalation – Exposure to vapour, evaporation model Exposure duration: 12 hours Product amount: 27 mg/use (Loretz et al. 2005) Room volume: 1 m3 (close to the face) Mass transfer coefficient: 6.844 m/hr (Sparks method) Release area mode: constant Release area: 5 cm2 (professional judgement) Ventilation rate: 1 change per hour Molecular weight matrix: 124 g/mol |
Hair styling product (Diacetyl) |
Concentration of diacetyl: 0.1 – 1%
Frequency of use: 1 /day (professional judgement)
Inhalation – Exposure to vapour, evaporation model Exposure duration: 10 minutes Product amount: 0.25 g (according to product label, apply 2 or more drops. One drop is assumed to be 0.05 mL (reference) and assuming a density of 1 g/mL results in 0.05 g/drop. Assumed 5 drops would be used, therefore, 0.25 g) Room volume: 1 m3 Ventilation rate: 0.6 per hour Mass transfer coefficient: 5.421 m/hr (Sparks method) Release area mode: constant Release area: 637.5 cm2 (half the area of the head) Emission duration: 10 minutes Molecular weight matrix: 330 g/mol |
a Unless specified, a retention factor of 1 was used
Product (substance) |
Assumptionsa |
|---|---|
Mouthing pacifier and/or teether |
Concentration of MEK residue: 500 ug/g (WSDE 2016) Oral: where, Etoy = exposure from mouthing toy (µg/kg-bw per day) Pacifier: qproduct = amount of substance that leaches from product over 24 hours (µg) = 22500 µg (500 ug/g x 45 g)b ftime = fraction of the day that the product is mouthed (i.e., sucking time) = 0.2 (infants) and 0.32 (toddler) (Juberg et al. 2001 as cited in EFSA 2015) fsurface = fraction of the product surface that is mouthed = 0.5 (Lassen et al. 2011 as cited in EFSA 2015) Teether: qproduct = amount of substance that leaches from product over 24 hours (µg) = 68000 µg (500 ug/g x 136 g)b ftime = fraction of the day that the product is mouthed (i.e., sucking time) = 0.02 (Juberg et al. 2001) to 0.05 (Van Engelen et al. 2008 (infants and toddler) fsurface = fraction of the product surface that is mouthed = 0.5 (RIVM 2002) |
a Approach from EFSA (2015), body weights of 7.5 kg and 15.5 kg for infants (0 – 6 months old) and toddlers (6 months to 4 years old), respectively (Health Canada 1998).
b Assume all of the MEK (500 ug/g) could be released. Weight of pacifier and teether from examining product weights based on product labels.
Other products
Sentinal exposure scenarios were used to estimate the potential exposure to substances in the Ketones Group. Exposures were estimated based on the assumed weight, 70.9 kg of an adult (Health Canada 1998), and use behaviours of an adult. Exposures were estimated using ConsExpo Web (ConsExpo 2016) or algorithms (see below for more details). Scenario-specific assumptions are provided in Table B-3. The PARAMs model was used to estimate mass transfer coefficients (Sparks method) (US EPA 2005). Refer to Table B-4 for defaults used in the PARAMs model.
Exposure scenario |
Assumptions |
|---|---|
Lacquer removal (MEK) |
Concentration of MEK: 10 – 40%
Scenario: paint remover in DIY Fact Sheet (RIVM 2007a). Assume work would be done in garage.
Inhalation – Exposure to vapour, evaporation model Exposure duration: 60 minutes Product amount: 1000 g Room volume: 34 m3 (garage) Ventilation rate: 1.5 change per hour Mass transfer coefficient: 2.758 m/hr (Sparks method) Release area mode: increasing Release area: 2 m2 Molecular weight matrix: 3000 g/mol |
Adhesive removal (MEK) |
Concentration of MEK: 100%
Scenario: adhesive remover used in the house based DIY Fact Sheet (RIVM 2007a) and US EPA 2011 defaults.
Inhalation – Exposure to vapour, evaporation model Exposure duration: 215 minutes (application duration + time exposed after duration of use ~94 minutes from US EPA 2011) Application duration: 121 minutes (mean value for adhesive removers from US EPA 2011) Product amount: 200 g [based on mean amount of product used per year, 34.46 oz/yr (~1019 mL/yr) x density of MEK 0.8 g/mL divided by mean uses/year (4.22) = 193 g] (US EPA 2011) Room volume: 30 m3 Ventilation rate: 1.5 change per hour Mass transfer coefficient: 3.743 m/hr (Sparks method) Release area mode: increasing Release area: 5 m2 Molecular weight matrix: 3000 g/mol |
Paint thinner (dilute lacquer or other coating) (MEK) |
Concentration: MEK = 100% (3% diluted in product)
Scenario: general coating (floor) in Do-It-Yourself Fact Sheet (RIVM 2007a). Assume work done in garage.
The final concentration of MEK in the product was based on product information stating that lacquers should be diluted with pure MEK at no more than 4oz per gallon (3%) 4oz = 118mL 1 gallon = 3785 mL
Inhalation – Exposure to vapour, evaporation model Exposure duration: 60 minutes Product amount: 3000 g Room volume: 34 m3 Ventilation rate: 1.5 change per hour Mass transfer coefficient: MEK = 2.595 m/hr,(Sparks method) Release area mode: increasing Release area: 15 m2 Molecular weight matrix: 3000 g/mol |
Liquid paint (solvent-rich paint for truck bed) (MEK, MIBK) |
Concentration: MEK = 20%, MIBK = 13%
Scenario: Brush or roller paint with solvent-rich paint (truck bed paint) in Paint Fact Sheet (RIVM 2007b). Assume work done in garage.
Inhalation – Exposure to vapour, evaporation model Exposure duration: 132 minutes Product amount: 420 g (adjusted product amount based on surface area covered. 1000 g for 12-15 m2 to 420 g for 5 m2) Room volume: 90 m3 (garage, would need larger garage if truck being painted) (US EPA 2017)
Ventilation rate: 1.5 change per hour Mass transfer coefficient: MEK = 2.595 m/hr, MIBK = 2.115 m/hr (Sparks method) Release area mode: increasing Release area: 5 m2 Application duration: 120 minutes Molecular weight matrix: 300 g/mol |
Liquid paint for steel (high-solid paint) (MPK) |
Concentration of MPK: 1-10%
Scenario: Brush or roller paint with high-solid paint in Paint Fact Sheet (RIVM 2007b). Assume work done in garage.
Inhalation – Exposure to vapour, evaporation model Exposure duration: 132 minutes Product amount: 1300 g Room volume: 34 m3 (garage) Ventilation rate: 1.5 change per hour Mass transfer coefficient: Thibodeaux (only very slight change if use Sparks method) Release area mode: increasing Release area: 10 m2 Application duration: 120 minutes Molecular weight matrix: 550 g/mol |
Spray Paint (MEK, MPK, MIBK, MIAK, DAA) |
Concentration: MEK = 1 – 52%, MPK = 2 – 13%, MIBK = 0.1 – 30%, MIAK = 1 – 10%, DAA = 1 – 5%
Scenario: spray can scenario from Paint Fact Sheet (RIVM 2007b), but used exposure to vapour – evaporation model since substances are volatile. Assume work done in garage. Some aerosol spray cans are ~400 g in size, therefore adjusted product amount to 400 g and increased application and exposure duration by 5 minutes each.
Inhalation – Exposure to vapour, evaporation model Exposure duration: 25 minutes Product amount: 400 g Room volume: 34 m3 (garage) Ventilation rate: 1.5 change per hour Release area mode: increasing Release area: 2 m2 Application duration: 20 minutes Molecular weight matrix: 3000 g/mol Mass transfer coefficient: MEK = 3.743 m/hr, MPK = 3.346, MIBK = 3.051 m/hr, MIAK = 2.820, DAA = 2.949 (Sparks method) |
PVC cement/ primer (MEK, DAA) |
Concentration: MEK = 10 – 100%, DAA = 20 – 30%
Scenario: universal / wood glue in a bottle as per DIY Fact Sheet (RIVM 2007a)
Inhalation – Exposure to vapour, evaporation model Exposure duration: 240 minutes Product amount: 10 g Room volume: 20 m3 Ventilation rate: 0.6 change per hour Release area mode: increasing Release area: 0.04 m2 Application duration: 20 minutes Molecular weight matrix: 3000 g/mol Mass transfer coefficient: MEK = 8.064 m/hr, DAA = 5.043 (Sparks method) |
Multipurpose adhesives (MEK) |
Concentration of MEK: 70%
Scenario: tube glue (contact glue) in Do-It-Yourself Fact Sheet (RIVM 2007a)
Inhalation – Exposure to vapour, evaporation model Exposure duration: 80 minutes (based on mean minutes exposed after duration of use, ~ 70 minutes, from US EPA 2011) Product amount: 9 g Room volume: 20 m3 Ventilation rate: 0.6 change per hour Release area mode: increasing Release area: 200 cm2 Application duration: 10 minutes Molecular weight matrix: 3000 g/mol Mass transfer coefficient: MEK = 8.064 m/hr (Sparks method) |
Wood lacquer (MIBK) |
Concentration: MIBK = 1 – 10%
Scenario: general coating on a floor (in a garage) in DIY Fact Sheet (RIVM 2007a)
Inhalation – Exposure to vapour, evaporation model Exposure duration: 60 minutes Product amount: 3000 g Room volume: 34 m3 Ventilation rate: 1.5 change per hour Release area mode: increasing Release area: 15 m2 Application duration: 60 minutes Molecular weight matrix: 3000 g/mol Mass transfer coefficient: MIBK = 2.115 m/hr (Sparks method) |
Filler/putty from tube (automotive) (MIBK) |
Concentration of MIBK: 1 – 8%
Model: filler/putty from tube (in a garage) in DIY Fact Sheet (RIVM 2007a). Assume work done in garage.
Inhalation – Exposure to vapour, evaporation model Exposure duration: 60 minutes Product amount: 100 g (adjusted from 40 g based on product size and additional info from US EPA 2017) Room volume: 34 m3 (garage) Ventilation rate: 1.5 change per hour Release area mode: increasing Release area: 200 cm2 Application duration: 60 minutes Molecular weight matrix: 3000 g/mol Mass transfer coefficient: MIBK = 8.28 m/hr (Sparks method) |
Automotive choke cleaner (DAA) |
Concentration of DAA: 30%
Model: spray paint (in a garage) in Paint Fact Sheet (RIVM 2007b). Assume work done in garage.
Inhalation – Exposure to vapour, evaporation model Exposure duration: 15 minutes (13.67 min rounded up to 15 min, US EPA 2011) Product amount: 165 g (US EPA 2011) Room volume: 34 m3 (garage) Ventilation rate: 1.5 change per hour Release area mode: increasing Release area: 1 m2 Application duration: 15 minutes (US EPA 2011) Molecular weight matrix: 3000 g/mol Mass transfer coefficient: DAA = 10 m/hr (new default from RIVM) |
Paint/marker remover, spray (DAA) |
Concentration of DAA: 10%
Model: spray paint (in unknown room) in Paint Fact Sheet (RIVM 2007b).
Inhalation – Exposure to vapour, evaporation model Exposure duration: 20 minutes Product amount: 300 g Room volume: 20 m3 (garage) Ventilation rate: 0.6 change per hour Release area mode: increasing Release area: 1 m2 Application duration: 15 minutes (US EPA 2011) Molecular weight matrix: 3000 g/mol Mass transfer coefficient: DAA = 2.95 m/hr (Sparks method) |
Paint thinner (for epoxy paints) |
Concentration: DAA = 5 - 10% (SDS 2017)
Scenario: epoxy thinner, to clean brushes or surfaces. Assume this could be done where epoxy being used in the home (unspecified room)
Inhalation – Exposure to vapour, evaporation model Exposure duration: 30 minutes (Versar 1986) Product amount: 400 g (500 mL (TDS 2015)/container x 0.8 g/ml, density of paint thinner) (Versar 1986) Room volume: 20 m3 Ventilation rate: 0.6 change per hour Mass transfer coefficient: 10 m/hr (new RIVM default) Release area mode: constant Release area: 0.078 m2 Molecular weight matrix: 3000 g/mol
Dermal – instant application Product amount on skin = 2.07 g (Versar 1986) |
Markers (MIBK, DAA) |
Concentration: MIBK = 10 - 30% in dry erase markers, DAA = 60 - 100% in permanent markers
Scenario: markers (dry erase or permanent)
Inhalation: used scenario from Children’s Products Fact Sheet (RIVM 2002) – for MIBK only Product amount: 300 mg Duration: 45 minutes Room volume: 20 cm3 Ventilation Rate: 0.6 per hour Release area: 450 cm2 Molecular weight matrix: 450 g/mol Mass transfer coefficient: MIBK = 4.843 m/hr(Sparks method)
Dermal or oral (ink scenario from the Arts & Creative Materials Institute (ACMI) approacha)
Acute or per event scenario:
Intake (mg/kg-bw/event = (Concentration of substance in marker (w/w) x estimated amount of ink per exposure (50 mg) x fraction absorbed) / body weight (kg)
Amount of ink per exposure = 50 mg (Hansen et al. 2008)
Chronic scenario:
Intake (mg/kg-bw/day): ((Concentration of substance in marker (w/w) x ink laydown rate (µg/cm) x 25 cm ink line/day)/1000 µg/mg) / body weight (kg)
Ink laydown rate = 100 µg/cm (90th percentile level for ink laydown of writing instrumentsb) |
Specialty coating (2,4-PD) |
Concentration: 2,4-PD = 1 – 5%
Scenario: general coating on a truck or boat (applied in a 2-car garage) in DIY Fact Sheet (RIVM 2007a)
Inhalation – Exposure to vapour, evaporation model Exposure duration: 60 minutes Product amount: 3000 g Room volume: 90 m3 Ventilation rate: 1.5 change per hour Release area mode: increasing Release area: 15 m2 Application duration: 60 minutes Molecular weight matrix: 3000 g/mol Mass transfer coefficient: 2,4-PD = 2.266 (Sparks method) |
Essential oil as air freshener (inhalation) (2,3-PD) |
Concentration of 2,3-PD: 0.1 – 1%
Scenario: essential oil as air freshener (in a living room) in Cosmetics Fact Sheet (RIVM 2006)
Inhalation – Exposure to vapour, constant rate model Exposure duration: 240 minutes Product amount: 1.08 g Room volume: 58 m3 Ventilation rate: 0.5 changes per hour Emission duration: 180 minutes |
Plug-in air freshener (inhalation) (2,3-PD) |
Concentration of 2,3-PD: 0.1 – 1%
Scenario: plug-in air freshener
Inhalation - exposure to vapour - instantaneous release scenario
Frequency: all day, every day Exposure duration: 24 hour/day Product amount: 1 plug-in contains ~26 mL of product and can last up to 30 days (P&G c2017a; Walmart.ca c2017). Assume same amount emitted each day (26mL/30 days = ~0.9 mL or 0.9 g/day) Room volume: 20 m3 Ventilation rate: 0.6/hr |
Gel air freshener (inhalation) (2,3-PD) |
Concentration of 2,3-PD: 1 – 5%
Scenario: gel air freshener
Inhalation: exposure to vapour - instantaneous release scenario
Frequency: all day, every day Exposure duration: 24 hour/day Product amount: 1 gel ~5.5 mL of product and can last up to 30 days (P&G c2017b). Assume same amount emitted each day (5.5 mL/30 days = ~0.2 mL or 0.2 g/day) Room volume: 20 m3 Ventilation rate: 0.6/hr |
a ACMI approach (personal communication, 2009 from ACMI to ESRAB, HC; unreferenced)
b personal communication, 2009 from Duke Medical Centre to ESRAB, HC; unreferenced
Parameter |
Value |
Additional Information |
|---|---|---|
Density of air (g/cm3) |
0.0011774 |
At 25 degrees Celsius, atmospheric pressure of 760 mmHg, and relative humidity of 50% |
Viscosity of air (g/cm/s) |
1.86E-04 |
At 25 degrees Celsius |
Velocity of air (cm/s) |
10 |
(McGready and Fontaine 2010 and Sparks et al. 1996) |
Diffusivity in air (cm2/s) |
MEK: 9.19E-02 MPK: 8.22E-02 MIBK: 7.49E-02 MIAK: 6.92E-02 DAA: 7.24E-02 Diacetyl: 8.91E-02 2,3-PD: 8.02E-02 2,4_PD: 8.02E-02 |
At 25 degrees Celsius |
Length of surface for various scenarios |
Nail polish: 20 cm (adult), 7 cm (toddler) Nail polish remover: 40 cm (adult), 14 cm (toddler) Lacquer remover: 2.5 m Adhesive remover: 1 – 5 m Paint thinner (floor coating): 3 – 5 m Truck bed paint: 3 – 5 m Spray paint: 1 – 2 m Multi-purpose adhesive: 10 – 20 cm Wood finish (floor): 3 – 5 m Markers: 25 cm Graffitti remover (spray): 1 – 2 m Eyeliner stickers: 8 cm General coating: 3 – 5 m Filler/putty: 2 – 5 cm |
Values estimated taking into account release area listed in ConsExpo Fact Sheets for each specific scenario (if scenario not available in ConsExpo, used professional judgement) |
Appendix C. Inhalation exposures to diacetyl from microwave popcorn
The approach used in Zhu et al. (2001) and the Priority Substances List Assessment for 2-butoxyethanol (Environment Canada, Health Canada 2002) was used to convert the data from Rosati et al. (2007) on the emission of diacetyl from a bag of microwaved popcorn based on chamber studies to a concentration in air in a standard room. Details on the assumptions used are outlined below.
It was assumed that the emission of diacetyl from microwave popcorn follows first order decay and that there are no sink effects in the chamber. Given these assumptions, the concentration of substance in an emission chamber (or a room) is related to the emission factor as follows:
C = [(EF0*A) / (V* (N - k))]*[exp(-kt) - exp(-Nt)] (equation 1)
where: C = the concentration of diacetyl in the chamber at any time [mg/m3]
EF0 = the initial emission factor for diacetyl from the product sample [mg/m2/h]
A = the emitting surface area of the product sample [m2]
N = the number of air changes per hour in the chamber [h-1]
V = the volume of the chamber [m3]
t = the duration of the emission [h]
and k = emission decay constant [h-1]
For a slowly depleting or nearly constant emission source, the decay constant k approaches zero. When the test time t becomes infinite or a steady-state equilibrium is reached, equation 1 can be rewritten as:
C = (EF0*A) / (V* N) (equation 2)
Assumed steady-state equilibrium was achieved during product testing. For calculating the initial emission factors, equation 2 was rearranged to:
EF0 = (C * N * V) / A [mg/m2/h] = [mg/m3] [h-1][m3] / [m2] (equation 3)
where N is the number of air changes per hour in the chamber, V is the cell volume and A is the emitting surface area, and the average concentrations of diacetyl emitted from the bag of 778.9 µg/bag (Rosati et al. 2007).
Step 1: Calculate the initial emission factor using equation 3 (data from Rosati et al. 2007).
Range of concentration (mg/m3) |
N = ACH (h-1) |
V = volume of chamber (m3) |
A - emitting SAa (m2) |
EF0 (mg/m2/h) |
|---|---|---|---|---|
5.8 |
2.3 |
0.515 |
0.1 |
68.70 |
0.02 |
2.3 |
0.515 |
0.1 |
0.237 |
a Assume emitting surface are is surface area of a popcorn bag, 1000 cm2 (Borkowski 2007)
Step 2: Calculate the emission decay constant using k = EFO*A/W, where W is the total evaporable amount of the substance in the source (W = average amount emitted from microwave popcorn bag 0.779 mg)
EF0 (mg/m2/h) |
A (m2) |
W (mg) |
k (h-1) |
|---|---|---|---|
68.7 |
0.1 |
0.779 |
8.82 |
0.237 |
0.1 |
0.779 |
0.03 |
Step 3: Calculate the concentration in a standard room using equation 1
EF0 (mg/m2/h) |
A (m2) |
V (m3) |
N (h-1) |
k (h-1) |
t (h) |
Concentration (mg/m3) |
|---|---|---|---|---|---|---|
68.7 |
0.1 |
17.4 |
0.5 |
8.82 |
1 |
0.0288 |
0.237 |
0.1 |
17.4 |
0.5 |
8.82 |
1 |
0.0001 |
For 5.8 mg diacetyl/m3
Time (min) |
Conc (mg/m3) |
|---|---|
0 |
0 |
10 |
0.0328 |
20 |
0.0377 |
30 |
0.0364 |
40 |
0.0339 |
50 |
0.0313 |
60 |
0.0288 |
120 |
0.0175 |
180 |
0.0106 |
240 |
0.0064 |
300 |
0.0039 |
360 |
0.0024 |
Avg after 6 hrs |
0.02 |
For 0.02 mg/diacetyl/m3
Time (min) |
Conc (mg/m3) |
|---|---|
0 |
0 |
10 |
0.00011 |
20 |
0.00013 |
30 |
0.00013 |
40 |
0.00012 |
50 |
0.00011 |
60 |
0.00010 |
120 |
0.00006 |
180 |
0.00004 |
240 |
0.00002 |
300 |
0.00001 |
360 |
0.00001 |
Avg after 6 hrs |
0.00007 |
Appendix D. Parameters for extrapolation
Substance |
Study Parameters |
Original dose |
Converted dose |
Formula |
|---|---|---|---|---|
MIBK |
2-year; 6h/day; 5 day/week |
1843 mg/m3 |
101 mg/kg-bw/d |
1843 x 0.31a x (6h/24h)b x (5d/7d)c |
MIAK |
69 exposure spanning 96 calendar days; 6h/day; |
934 mg/m3 |
52 mg/kg-bw/d |
934 x 0.31a x (6h/24h)b x (69d/96d)d |
2.3-PD |
RA from diacetyl 90-day study |
90 mg/kg-bw/d |
105 mg/kg-bw/d |
90 x (100.12 / 86.09)e |
2.3-PD |
14-week; 6h/day; 5 day/week |
51 mg/m3 |
12 mg/kg-bw/d |
51 x 1.33f x (6h/24h)b x (5d/7d)c |
2.3-PD |
14-week; 6h/day; 5 day/week |
102 mg/m3 |
24 mg/kg-bw/d |
102 x 1.33f x (6h/24h)b x (5d/7d)c |
2.4-PD |
6h/day;GD6-15 |
827 mg/m3 |
64 mg/kg-bw/d |
827 x 0.31a x (6h/24h)b |
2.4-PD |
6h/day;5day/week; 14-week |
417 mg/m3 |
23 mg/kg-bw/d |
417 x 0.31a x (6h/24h)b x (5d/7d)c |
MO |
6-week; 8h/day; 5 day/week |
200 mg/m3 |
15 mg/kg-bw/d |
200 x 0.31a x (8h/24h)b x (5d/7d)c |
a Considering a standard rat body weight of 0.35 kg and volume of inhalation of 0.11 m3/day, resulting into a constant of 0.31 (from Health Canada 1994)
b exposure duration in a day
c duration of the study
d number of exposition
e molecular weight (substance/Read-across)
f Considering a standard mice body weight of 0.03 kg and volume of inhalation of 0.04 m3/day, resulting into a constant of 1.33 (from Health Canada 1994)
