Draft screening assessment - Methane, dimethoxy- (dimethoxymethane)
Official title: Draft screening assessment - Methane, dimethoxy- (Dimethoxymethane)
Chemical Abstracts Service Registry Number 109-87-5
Environment and Climate Change Canada
Health Canada
July 2019
Synopsis
Pursuant to section 68 of the Canadian Environmental Protection Act, 1999 (CEPA), the Minister of the Environment and the Minister of Health have conducted a screening assessment of methane, dimethoxy-, hereinafter referred to as dimethoxymethane. The Chemical Abstracts Service Registry Number (CAS RNFootnote 1 ) for dimethoxymethane is 109-87-5. Dimethoxymethane was identified as a priority for assessment on the basis of other human health concerns.
Dimethoxymethane occurs naturally in a limited number of food products. In the calendar year of 2011, there were no reports of manufacture or import of dimethoxymethane into Canada above the reporting threshold of 100 kg from a survey conducted under section 71 of CEPA (Environment Canada 2013). In Canada, it is primarily used as a solvent in products available to consumers including cosmetics, cleaning products, paints and coatings, spray adhesives, and batteries.
The ecological risk of dimethoxymethane was characterized using the ecological risk classification of organic substances (ERC), which is a risk-based approach that employs multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. Hazard profiles are based principally on metrics regarding mode of toxic action, chemical reactivity, foodweb-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Metrics considered in the exposure profiles include potential emission rate, overall persistence and long-range transport potential. A risk matrix is used to assign a low, moderate or high level of potential concern for substances on the basis of their hazard and exposure profiles. Based on the outcome of the ERC analysis, dimethoxymethane is considered unlikely to be causing ecological harm.
Considering all available lines of evidence presented in this draft screening assessment, there is low risk of harm to the environment from dimethoxymethane. It is proposed to conclude that dimethoxymethane does not meet the criteria under paragraphs 64(a) or (b) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
Dimethoxymethane is considered to be of low hazard potential to humans given that no adverse effects or organ-specific toxicity were observed at inhalation exposures as high as 10 068 ppm (31 334 mg/m3) in rats, and considering the available information indicating a lack of genotoxic, mutagenic or developmental effects. As dimethoxymethane is considered to be of low hazard potential, the risk to human health is considered to be low.
On the basis of the information presented in this draft screening assessment, it is proposed to conclude that dimethoxymethane does not meet the criteria under paragraph 64(c) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore proposed to conclude that dimethoxymethane does not meet any of the criteria set out in section 64 of CEPA.
1. Introduction
Pursuant to section 68 of the Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999), the Minister of the Environment and the Minister of Health have conducted a screening assessment of methane, dimethoxy-, hereinafter referred to as dimethoxymethane, to determine whether this substance presents or may present a risk to the environment or to human health. This substance was considered a priority for assessment on the basis of other human health concerns (ECCC, HC [modified 2017]).
The ecological risk of dimethoxymethane was characterized using the ecological risk classification of organic substances (ECCC 2016a). The ERC describes the hazard of a substance using key metrics including mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity and considers the possible exposure of organisms in the aquatic and terrestrial environments on the basis of such factors as potential emission rates, overall persistence and long-range transport potential in air. The various lines of evidence are combined to identify substances as warranting further evaluation of their potential to cause harm to the environment or as having a low likelihood of causing harm to the environment.
This draft screening assessment includes consideration of information on chemical properties, environmental fate, hazards, uses and exposures, including additional information submitted by stakeholders. Relevant data were identified up to August, 2018. Empirical data from key studies as well as results from models were used to reach proposed conclusions. When available and relevant, information presented in assessments from other jurisdictions was considered.
This draft screening assessment was prepared by staff in the CEPA Risk Assessment Program at Health Canada and Environment and Climate Change Canada and incorporates input from other programs within these departments. Comments on the technical portions relevant to human health were received from Theresa Lopez, Jennifer Flippin and Joan Garey (Tetra Tech). The ecological portion of this assessment is based on the ERC document (published July 30, 2016), which was subject to an external review as well as a 60-day public comment period. While external comments were taken into consideration, the final content and outcome of the screening assessment remain the responsibility of Health Canada and Environment and Climate Change Canada.
This draft screening assessment focuses on information critical to determining whether the substance meets the criteria as set out in section 64 of CEPA by examining scientific information and incorporating a weight of evidence approach and precaution.Footnote 2 This draft screening assessment presents the critical information and considerations on which the proposed conclusion is based.
2. Substance identity
The Chemical Abstracts Service Registry Number (CAS RN), Domestic Substances List (DSL) name and common name for the substance are presented in Table 2-1.
| CAS RN | DSL name (common names) | Chemical structure and molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
| 109-87-5 | methane, dimethoxy- (dimethoxymethane, methylal) | 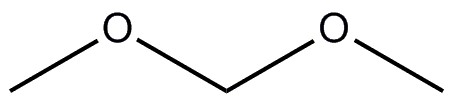 C3H8O2 C3H8O2 |
76.09 |
3. Physical and chemical properties
A summary of relevant experimental physical and chemical property values for dimethoxymethane is presented in Table 3-1. Additional physical and chemical properties are presented in ECCC (2016b).
| Property | Value | Key reference(s) |
|---|---|---|
| Physical state | Colourless liquid with a pungent odour | ECHA c2007-2017 |
| Melting point (°C) | -105 | ECHA c2007-2017; ChemIDplus 1993-; Budavari 1989 |
| Vapour pressure (Pa) | 5.31 x 104 (at 25°C) | ChemIDplus 1993- |
| Henry’s law constant (Pa·m3/mol) | 9.22 (at 20°C) | ECHA c2007-2017 |
| Water solubility (mg/L) | 3.33 x 105 (at 20°C) | Verschueren 1983 |
| Log Kow (dimensionless) | 0.18 | ICSC c1996-2011 |
| Log Koc (dimensionless) | 5.55 | ECHA c2007-2017 |
Abbreviations: Kow, octanol-water partition coefficient; Koc, organic carbon-water partition coefficient
4. Sources and uses
Dimethoxymethane is naturally present in a limited number of food products, such as cooked shrimp and strawberries (Shye et al. 1988; Teranishi et al. 1963; personal communication, emails from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2018; unreferenced).
Dimethoxymethane was included in a survey issued pursuant to a CEPA section 71 notice (Canada 2012). In the calendar year of 2011, there were no reports of manufacture or import into Canada above the reporting threshold of 100 kgFootnote 3 (Environment Canada 2013).
Based on a search of publicly available Safety Data Sheets of products available to consumers, Canadian uses for dimethoxymethane include cleaning products, paints and coatings, spray adhesives, and batteries (SDS 2014a, 2014b, 2015, 2017a, 2017b).
According to notifications submitted under the Cosmetic Regulations to Health Canada, dimethoxymethane is used as a solvent in certain cosmetic products for hair styling and hair conditioning (emails from the Consumer Product Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2018; unreferenced). Dimethoxymethane may also be used as an ingredient in certain cleaners used in Canadian food processing facilities (personal communication, emails from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2018; unreferenced).
Globally, dimethoxymethane is used as a reactant and solvent in plastic and resin manufacturing in the US (US EPA 2016). It is also found in coating products, air care products, fuels, lubricants and greases, and is a permitted food flavouring agent in the EU (ECHA c2007-2017, EC 2012).
5. Potential to cause ecological harm
5.1 Characterization of ecological risk
The ecological risk of dimethoxymethane was characterized using the ecological risk classification of organic substances (ERC) approach (ECCC 2016a). The ERC is a risk-based approach that considers multiple metrics for both hazard and exposure on the basis of weighted consideration of multiple lines of evidence for determining risk classification. The various lines of evidence are combined to discriminate between substances of lower or higher potency and lower or higher potential for exposure in various media. This approach reduces the overall uncertainty with risk characterization compared to an approach that relies on a single metric in a single medium (e.g., median lethal concentration [LC50]) for characterization. The following summarizes the approach, which is described in detail in ECCC (2016a).
Data on physical-chemical properties, fate (chemical half-lives in various media and biota, partition coefficients, and fish bioconcentration), acute fish ecotoxicity, and chemical import or manufacture volume in Canada were collected from scientific literature, from available empirical databases (e.g., OECD QSAR Toolbox 2016), and from responses to surveys under section 71 of CEPA, or they were generated using selected quantitative structure-activity relationship (QSAR) or mass-balance fate and bioaccumulation models. These data were used as inputs to other mass-balance models or to complete the substance hazard and exposure profiles.
Hazard profiles were based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Exposure profiles were also based on multiple metrics, including potential emission rate, overall persistence, and long-range transport potential. Hazard and exposure profiles were compared to decision criteria in order to classify the hazard and exposure potentials for each organic substance as low, moderate, or high. Additional rules were applied (e.g., classification consistency, margin of exposure) to refine the preliminary classifications of hazard or exposure.
A risk matrix was used to assign a low, moderate or high classification of potential risk for each substance on the basis of its hazard and exposure classifications. ERC classifications of potential risk were verified using a two-step approach. The first step adjusted the risk classification outcomes from moderate or high to low for substances which had a low estimated rate of emission to water after wastewater treatment, representing a low potential for exposure. The second step reviewed low risk potential classification outcomes using relatively conservative, local-scale (i.e., in the area immediately surrounding a point-source of discharge) risk scenarios, designed to be protective of the environment, to determine whether the classification of potential risk should be increased.
ERC uses a weighted approach to minimize the potential for both over and under classification of hazard and exposure, and of subsequent risk. The balanced approaches for dealing with uncertainties are described in greater detail in ECCC 2016a. The following describes two of the more substantial areas of uncertainty. Error with empirical or modelled acute toxicity values could result in changes in classification of hazard, particularly metrics relying on tissue residue values (i.e., mode of toxic action), many of which are predicted values from (Q)SAR models (OECD QSAR Toolbox 2016). However, the impact of this error is mitigated by the fact that overestimation of median lethality will result in a conservative (protective) tissue residue used for critical body residue (CBR) analysis. Error with underestimation of acute toxicity will be mitigated through the use of other hazard metrics such as structural profiling of mode of action, reactivity and/or estrogen binding affinity. Changes or errors in chemical quantity could result in differences in classification of exposure as the exposure and risk classifications are highly sensitive to emission rate and use quantity. The ERC classifications thus reflect exposure and risk in Canada on the basis of what is believed to be the current use quantity, and may not reflect future trends.
Critical data and considerations used to develop the substance-specific profiles for dimethoxymethane, and the hazard, exposure and risk classification results are presented in ECCC (2016b).
On the basis of low hazard and low exposure classifications according to information considered under ERC, dimethoxymethane was classified as having a low potential for ecological risk. It is unlikely that this substance is resulting in concerns for the environment in Canada.
6. Potential to cause harm to human health
6.1 Exposure assessment
As dimethoxymethane is considered to be of low hazard potential (see Section 8.2), quantitative estimates of exposure to the general population were not derived.
Dimethoxymethane was detected in less than 1% of Canadian residential indoor air samples, with an upper limit concentration of approximately 0.09 µg/m3 in a study by Zhu et al. (2013).
There are no data on the presence of dimethoxymethane in Canadian waters. Dimethoxymethane was qualitatively detected in US drinking water and surface water, and was measured in US wastewater effluents at levels up to 1.6 ppm (HSDB 1983-).
Dietary exposure to dimethoxymethane as an incidental food additive due to its potential presence in certain surface cleaners used in food processing facilities is not expected given that the cleaner use is followed by a potable water rinse (personal communication, emails from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2018; unreferenced). Dimethoxymethane is naturally present in a limited number of food products and dietary exposure from the ingestion of certain foods that contain dimethoxymethane is expected to be low.
Dermal exposure may result from the use of certain cosmetics (e.g., hair spray and hair conditioner) and other products available to consumers (e.g., cleaning products, paints and coatings, and adhesives) that contain dimethoxymethane. Dimethoxymethane is associated with a high vapour pressure, and the use of these products, as well as other aerosol products such as spray adhesives, may also result in inhalation exposure.
6.2 Health effects assessment
A Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) dossier for dimethoxymethane is available.
Following the approach outlined in the science approach document for substances with low potential for human health hazard (HC 2017), dimethoxymethane is considered to have a low hazard potential due to the lack of adverse effects or organ-specific toxicity at inhalation exposures as high as 10 068 ppm (31 334 mg/m3), with the available information indicating a lack of genotoxic, mutagenic or developmental effects (as detailed below).
Toxicokinetics
Dimethoxymethane is expected to be rapidly cleared from the human body after exposure. Dogs treated with the substance by intravenous injection excreted 87% of the injected dose in the first 7 hours, mainly by exhalation, and no test substance was detected in blood 24 hours after respiratory arrest, with an estimated whole-body elimination half-life of 2 hours (Virtue 1951).
A few studies indicate that dimethoxymethane could release formaldehyde and methanol as metabolites. In an in vivo study, rats had a significant increase of methanol blood concentrations after exposure to single doses of 1600 and 3500 mg/kg bw of dimethoxymethane (Tomilina et al. 1984). However, as noted by the German MAK Commission (DFG 2013), this study provides an inadequate level of detail and is not sufficiently documented. In an in vitro study, dimethoxymethane was shown to be hydrolyzed in rat artificial gastric juice at a pH less than 2.5 to release formaldehyde and methanol (Poon et al. 2000).
However, other in vitro, ex vivo and in vivo studies showed that dimethoxymethane was rapidly absorbed as the parent substance from rat stomach, with minimal metabolism by rat liver enzymes or hydrolysis at physiological pH values. Dimethoxymethane also did not cause nasal or liver cytotoxicity (which are the known sites of contact toxicity of inhaled formaldehyde) in repeated dose rat inhalation studies. The breakdown products of dimethoxymethane (formaldehyde and methanol) were not detected in urine or exhaled air in mice, rats or dogs after exposure through various routes (Poon et al. 2000; Dahl and Hadley 1983; Weaver et al. 1951; Virtue 1951; Hofmann 1994; DFG 2013).
Repeated dose toxicity
In a sub-chronic, Organisation for Economic Co-operation and Development (OECD) guideline, nose-only inhalation study (Hofmann 1994), male and female Wistar rats (n=10 for each sex and concentration) were exposed to mean concentrations of 0, 377, 1908 or 9652 ppm (0, 1173, 5938 or 30 039 mg/m3) of dimethoxymethane for 6 hours per day, 5 days per week, for 13 weeks. The most significant observed effects were symptoms of narcosis in both males and female rats of the highest concentration group, in addition to slightly increased relative liver weights and slightly increased water intake. In female rats exposed to the highest concentration, spleen weights were slightly decreased. No macroscopic or histopathological changes were observed in any of the affected organs. No deaths or organ-specific toxicity were observed and narcotic effects were reversed before the start of the next exposure.
A sub-acute, non-guideline, whole-body inhalation study conducted with male and female Swiss mice is available (Weaver et al. 1951). However, due to several deficiencies and inadequate reporting, this study was not considered in the assessment.
One unpublished oral study in rabbits was evaluated, but was not included in the assessment due to a confidentiality claim. The results of the study support the conclusion of the health effects assessment.
Genotoxicity/Mutagenicity
Dimethoxymethane was negative in a chromosomal aberration assay in Chinese hamster ovarian cells (Young 1990) and in an in vivo micronucleus assay in mice (Ivett 1990).
Dimethoxymethane produced mixed results in one Ames test (San and Kruel 1989); positive results were found in strains TA98 and TA100 without metabolic activation, while results were negative in these two strains with metabolic activation and also in all other strains. Since the purity of the test material was not determined in this study, it is possible that the positive results were produced by the presence of impurities such as formaldehyde, which is a known mutagen and one of the reagents used in the synthesis of dimethoxymethane (DFG 2013).
A subsequent Ames test (Lambiotte et Cie SA 1996, as cited in DFG 2013), in which the purity of the test substance was verified to be 99.5%, showed negative results for all TA strains, including TA98 and TA100, with and without metabolic activation.
No carcinogenicity studies were identified. No hyperplasia or neoplastic lesions were observed in a 13-week repeated dose inhalation study in rats (Hofmann 1994).
Reproductive/Developmental toxicity
An OECD guideline, whole-body inhalation study (Lambiotte et Cie SA 1997, as cited in DFG 2013) was conducted in female rats to investigate the developmental toxicity of dimethoxymethane. Pregnant rats (n=25 for each concentration) were exposed to 0, 386, 1954 or 10 068 ppm (0, 1201, 6081 or 31 334 mg/m3) of dimethoxymethane from gestational day 6 to 15 for 6 hours per day. On gestational day 20, a post-mortem analysis was conducted on the dams, litters were counted and foetuses were examined for external and skeletal malformations. Observed maternal effects included reversible symptoms of narcosis, reduced body weight gain and increased water intake in the highest concentration group. No substance-related adverse effects were observed in the foetuses in any group.
One unpublished oral developmental toxicity study in rabbits was evaluated but was not included in the assessment due to a confidentiality claim. The results of the study support the conclusion of the health effects assessment.
No reproductive toxicity studies were identified. However, no adverse effects were observed in male and female rat reproductive organs in repeated dose inhalation studies (Hofmann 1994; Lambiotte et Cie SA 1997, as cited in DFG 2013).
6.3 Characterization of risk to human health
Given that no adverse effects or organ-specific toxicity were observed at inhalation exposures as high as 10 068 ppm in rats, and given the available information indicating a lack of genotoxic, mutagenic or developmental effects, dimethoxymethane is considered to be of low hazard potential. As such, quantitative exposure estimates were not derived and the risk to human health is considered to be low.
Although there may be limitations in the health effects database, no significant uncertainties were identified as additional data is considered unlikely to impact the determination of low hazard potential for dimethoxymethane.
7. Conclusion
Considering all available lines of evidence presented in this draft screening assessment, there is low risk of harm to the environment from dimethoxymethane. It is proposed to conclude that dimethoxymethane does not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
On the basis of the information presented in this draft screening assessment, it is proposed to conclude that dimethoxymethane does not meet the criteria under paragraph 64(c) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore proposed to conclude that dimethoxymethane does not meet any of the criteria set out in section 64 of CEPA.
References
Budavari S, editor. 1989. The merck index - an encyclopedia of chemicals, drugs and biologicals. 11th ed. Rahway (NJ): Merck and Co., Inc. 949 p.
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C. 1999, c.33. Canada Gazette Part III, vol. 22, no. 3.
Canada, Dept. of the Environment. 2012. Canadian Environmental Protection Act, 1999: Notice with respect to certain substances on the Domestic Substances List [PDF]. Canada Gazette, Part I, vol. 146, no. 48, Supplement.
ChemIDplus [database]. 1993-. Bethesda (MD): US National Library of Medicine. [updated 2018 Jun 15; accessed 2018 Jul 14].
Dahl AR, Hadley WM. 1983. Formaldehyde production promoted by rat nasal cytochrome P-450-dependent monooxygenases with nasal decongestants, essences, solvents, air pollutants, nicotine, and cocaine as substrates. Toxicol Appl Pharmacol. 67(2):200-5.
[DFG] Deutsche Forschungsgemeinschaft. 2013. Dimethoxymethane [MAK Value Documentation, 2003]. The MAK collection for occupational health and safety. Wiley-VCH Verlag GmbH & Co. [accessed 2018 Apr 19].
[EC] European Commission. 2012. Commission implementing regulation (EC) No 872/2012 of 1 October 2012. Official Journal of the European Union L267/1. 161 p.
[ECCC] Environment and Climate Change Canada. 2016a. Science approach document: ecological risk classification of organic substances. Ottawa (ON): Government of Canada.
[ECCC] Environment and Climate Change Canada. 2016b. Supporting documentation: data used to create substance-specific hazard and exposure profiles and assign risk classifications. Gatineau (QC): ECCC. Information in support of the science approach document: ecological risk classification of organic substances. Available from: substances@ec.gc.ca.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. [modified 2017 Mar 12]. Categorization of chemical substances. Ottawa (ON): Government of Canada. [accessed 2018 Jul 30].
[ECHA] European Chemicals Agency. c2007-2017. Registered substances database; search results for CAS RN 109-87-5. Helsinki (FI): ECHA. [updated 2018 May 14; accessed 2018 Jul 13].
Environment Canada. 2013. DSL Inventory Update data collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain substances on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
[HC] Health Canada. 2017. Science Approach Document. Science approach document for substances with low human health hazard potential. Ottawa (ON): Health Canada. December 2017
Hofmann T (Pharma Development Corporate Toxicology Hoechst Aktiengesellschaft, Frankfurt am Main, DE). 1994. Subchronic (13-week) inhalation toxicity study of methylal in rats with cover letter dated 12/01/94. Dallas (TX): Hoechst Celanese Corporation. 495 p. Report No. 94.0647. Available from: NTIS, Springfield, VA, USA.
[HSDB] Hazardous Substances Databank. 1983-. Bethesda (MD): Search results for CAS RN 109-87-5.National Library of Medicine (US). [revised 2005 Jun 23; accessed 2018 Jun 20].
[ICSC] International Chemical Safety Cards [database]. c1996-2011. Geneva (CH): International Labour Organization. [updated 2017 Apr; accessed 2018 Jun 26].
Ivett JL (Hazleton Laboratories America, Inc. Kensington, MD). 1990. Mutagenicity test on methylal in vivo mouse micronucleus assay. Final report. Dallas (TX): Hoechst Celanese Corporation. 20 p. Report No. NTIS/OTS 0530014. Available from: NTIS, Springfield, VA, USA.
Lambiotte et Cie SA. 1996. Methylal, bacterial mutation assay. Unpublished report. Report No. LAC 1/961982. [cited in DFG 2013].
Lambiotte et Cie SA. 1997. Methylal, a study for effects on embryo-foetal development by inhalation administration in the rat. Unpublished report. Report No. LBC 2/970964. [cited in DFG 2013].
OECD QSAR Toolbox. [read across tool]. 2016. Paris (FR): Organisation for Economic Co-operation and Development, Laboratory of Mathematical Chemistry.
[SDS] Safety Data Sheet. 2014a. Sugar Artist's acrylic [PDF]. Spring Hill (AU): AVT Paints. [accessed 2018 Jul 30].
[SDS] Safety Data Sheet. 2014b. CR lithium battery series type no.: (CR2450, CR2430, CR2330, CR2320, CR2032, CR2025, CR2016, CR1620, CR1616, CR1225, CR1220, CR1216, CR927) [PDF]. Guangdong (CN): Dongguan Tuobu Electric Co., Ltd. [accessed 2018 Jul 31].
[SDS] Safety Data Sheet. 2015. Camie 22/90 heavy duty degreaser [PDF]. Addison (IL): Camie-Campbell, Inc. [accessed 2018 Jul 30].
[SDS] Safety Data Sheet. 2017a. Loctite SF 7200 known as Loctite 7200 [PDF]. Hemel Hempstead (UK): Henkel Ltd. [accessed 2018 Jul 30].
[SDS] Safety Data Sheet. 2017b. Adhesive spray KK 100 [PDF]. Stockstadt (DE): Gunold GmbH. [accessed 2018 Jul 30].
Shye SC, Pan BS, Wu CM. 1988. Formation of volatile pyrazine compounds in shrimp (Parapenaeus fissurus). J Chinese Agri Chem Soc. 26(4): 545-551.
Poon R, Moir D, Elwin J, nadeau B, Singh A, Yagminas A, Chu I. 2000. A study of the acid lability and acute toxicity of dimethoxymethane in rats. Int J Toxicol 19:179-185.
San RH, Kruel C (Microbiological Associates, Inc., Rockville, MD). 1989. Salmonella/mammalian-microsome preincubation mutagenicity assay with a closed phase incubation system. Final report. Dallas (TX): Hoechst Celanese Corporation. 55 p. Report No. NTIS/OTS 0521278. Available from: NTIS, Springfield, VA, USA.
Teranishi R, Corse JW, McFadden WH, Black DR, Morgan Jr, AI. 1963. Volatiles from strawberries. I. Mass spectral identification of the more volatile components. J Food Sci. 28(4): 371-488.
Tomilina LA, Rotenberg YS, Mashbits FD, Komanovskaya MB, Knizhnikova LM. 1984. Methylal: Metabolism and Hygienic Standardization in a Work Environment. Gig Tr Prof Zabol. 6: 27–29
[US EPA] US Environmental Protection Agency. 2016. Chemical Data Reporting [Internet]. Washington (DC): US EPA, Office of Pollution Prevention and Toxics. Search results for CAS RN [109-87-5]. [cited Sep 2018]
Verschueren K. 1983. Handbook of environmental data of organic chemicals. 2nd ed. New York (NY): Van Nostrand Reinhold Co. 542 p.
Virtue RW. 1951. Anesthesia with methylal in dogs, mice and rats. Anesthesiology. 12(1):100-13.
Weaver FL, Hough AR, Highman B, Fairhall LT. 1951. The toxicity of methylal. Br J Ind Med. 8(4):279-83.
Young RR (Hazleton Laboratories America Inc., Kensington, MD). 1990. Mutagenicity test on methylal in the CHO/HGPRT forward mutation assay. Final report. Dallas (TX): Hoechst Celanese Corporation. 15 p. Report No. NTIS/OTS 0528332. Available from: NTIS, Springfield, VA, USA.
Zhu J, Wong SL, Cakmak S. 2013. Nationally representative levels of selected volatile organic compounds in Canadian residential indoor air: a population based survey. Environ Sci Technol. 47: 13276–13283.
