Draft screening assessment phosphonic acid, [[(phosphonomethyl)Imino]bis[2,1-ethanediylnitrilobis(methylene)]]tetrakis-
Official title: Draft screening assessment phosphonic acid, [[(phosphonomethyl)Imino]bis[2,1-ethanediylnitrilobis(methylene)]]tetrakis-
Chemical Abstracts Service Registry Number 15827-60-8
Environment and Climate Change Canada Health Canada
March 2019
Synopsis
Pursuant to section 68 of the Canadian Environmental Protection Act, 1999 (CEPA), the Minister of the Environment and the Minister of Health have conducted a screening assessment of phosphonic acid, [[(phosphonomethyl)imino]bis[2,1-ethanediylnitrilobis(methylene)]]tetrakis-, hereinafter referred to as DTPMP. The Chemical Abstracts Service Registry Number (CAS RNFootnote 1) for DTPMP is 15827-60-8. This substance was identified as a priority for assessment on the basis of other human health concerns.
DTPMP was included in a survey issued pursuant to section 71 of CEPA. There were no reports of manufacture of DTPMP in Canada in the 2011 reporting year. DTPMP was reported as being imported into Canada with a total quantity of 333 656 kg for commercial uses only including water treatment, laundry and dishwashing, paints and coatings, oil and gas extraction, construction and building materials, paper products, ink, toner and colourants, photographic supplies, and in a variety of care products (i.e., fabric, cleaning and furnishing care, personal care, apparel and footwear care, and air care).
DTPMP is used in some permanent hair dye products and was identified as a non-medicinal ingredient in an over-the-counter drug (i.e., an ophthalmic solution).
The ecological risk of DTPMP was characterized using the ecological risk classification of organic substances (ERC), which is a risk-based approach that employs multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. Hazard profiles are based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Metrics considered in the exposure profiles include potential emission rate, overall persistence, and long-range transport potential. A risk matrix is used to assign a low, moderate or high level of potential concern for substances on the basis of their hazard and exposure profiles. Based on the outcome of the ERC analysis, DTPMPis considered unlikely to cause ecological harm.
Considering all available lines of evidence presented in this draft screening assessment, there is low risk of harm to the environment from DTPMP. It is proposed to conclude that DTPMP does not meet the criteria under paragraphs 64(a) or (b) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
For the general population of Canada, potential exposures to DTPMP were estimated from the use of permanent hair dyes and ophthalmic solutions.
The critical effect for risk characterization was determined to be potential perturbations of iron and calcium homeostasis based on a laboratory study. Margins between estimates of exposure and critical effect levels observed in laboratory studies are considered adequate to address uncertainties in the health effects and exposure databases.
Considering all information presented in this draft screening assessment, it is proposed to conclude that DTPMP does not meet the criteria under paragraph 64(c) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
Therefore, it is proposed to conclude that DTPMP does not meet any of the criteria under section 64 of CEPA.
1. Introduction
Pursuant to section 68 of the Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999), the Minister of the Environment and the Minister of Health have conducted a screening assessment of phosphonic acid, [[(phosphonomethyl)imino]bis[2,1-ethanediylnitrilobis(methylene)]]tetrakis-, hereinafter referred to as DTPMP, to determine whether this substance presents or may present a risk to the environment or to human health. This substance was identified as a priority for assessment on the basis of other human health concerns.
The ecological risk of DTPMP was characterized using the ecological risk classification of organic substances (ERC) (ECCC 2016a). The ERC describes the hazard of a substance using key metrics, including mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity, and considers the possible exposure of organisms in the aquatic and terrestrial environments on the basis of such factors as potential emission rates, overall persistence, and long-range transport potential in air. The various lines of evidence are combined to identify substances as warranting further evaluation of their potential to cause harm to the environment or as having a low likelihood of causing harm to the environment.
DTPMP has been reviewed internationally through the Organisation for Economic Co-operation and Development (OECD) Cooperative Chemicals Assessment Programme and an OECD Screening Information Dataset (SIDS) Initial Assessment Report (SIAR) is available (OECD 2004). These assessments undergo rigorous review (including peer-review) and endorsement by international governmental authorities. Health Canada and Environment and Climate Change Canada are active participants in this process and consider these assessments reliable. The OECD SIAR was used to inform the health effects characterization in this screening assessment.
This draft screening assessment includes consideration of information on chemical properties, environmental fate, hazards, uses and exposure, including additional information submitted by stakeholders. Relevant data were identified up to March 2018. Empirical data from key studies, as well as some results from models were used to reach proposed conclusions. When available and relevant, information from other jurisdictions was considered.
This draft screening assessment was prepared by staff in the CEPA Risk Assessment Program at Health Canada and Environment and Climate Change Canada, based on a draft developed by staff at SLR International Corporation, and incorporates input from other programs within these departments. The ecological portion of this assessment is based on the ERC document (published July 30, 2016), which was subject to an external review as well as a 60-day public comment period. While external comments were taken into consideration, the final content and outcome of the screening assessment remain the responsibility of Environment and Climate Change Canada and Health Canada.
This draft screening assessment focuses on information critical to determining whether a substance meets the criteria as set out in section 64 of CEPA, by examining scientific information and incorporating a weight-of-evidence approach and precaution.Footnote 2 The draft screening assessment presents the critical information and considerations on which the proposed conclusion is based.
2. Substance identity
The Chemical Abstracts Service Registry Number (CAS RNFootnote 3 ), Domestic Substances List (DSL) name and abbreviation for phosphonic acid, [[(phosphonomethyl)imino]bis[2,1-ethanediylnitrilobis(methylene)]]tetrakis-, herein after referred to as DTPMP, are presented in Table 2‑1.
| CAS RN | DSL name (abbreviation) | Chemical structure and molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
| 15827-60-8 | Phosphonic acid, [[(phosphonomethyl)imino]bis[2,1-ethanediylnitrilobis(methylene)]]tetrakis- (DTPMP) | 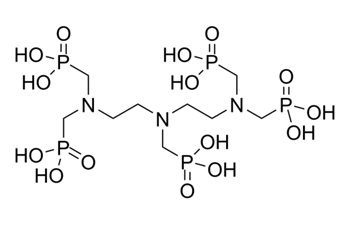 C9H28 N3O15P5 C9H28 N3O15P5 |
573.2 |
3. Physical and chemical properties
A summary of physical and chemical properties of DTPMP is presented in Table 3‑1. Additional physical chemical properties are reported in ECCC 2016b.
| Property | Value | Type of data | Reference |
|---|---|---|---|
| Melting point (oC) | 449.8 | Measured | ECHA 2017a |
| Boiling point (oC) | sublimates | Measured | ECHA 2017a |
| Water solubility (mg/L) | 500 000 | Modelled | OECD 2004 |
| Density (g/mL) | 1.4 | Estimated | ECHA 2017a |
| Vapour pressure (Pa) | 1.67 x 10-10 | Estimated | OECD 2004 |
| Henry’s law constant (atm ∙ m3/mol) | 7.3 x 10-18 | Estimated | OECD 2004 |
| log Kow (dimensionless) | -3.4 | Measured | ECHA 2017a |
| log Ksediment-water (dimensionless) | 3.06 | Measured | ECHA 2017b |
Abbreviations: Kow, octanol-water partition coefficient
4. Sources and uses
DTPMP does not occur naturally in the environment.
DTPMP was included in a survey issued pursuant to a CEPA section 71 notice (Canada 2012). In Canada, DTPMP was not reported to be manufactured above the reporting threshold of 100 kg during the 2011 calendar year, while total import quantities during that same period were reported to be 333 656 kg (Environment Canada 2013).
DTPMP is often employed as a chelating agent in bleaching systems where it can complex with metal ions and prevent scale development on surfaces. Various salts of this acid are used to adjust the pH of the solution, but the activity of the salts is the same as the acid (OECD 2004).
In Canada, DTPMP is reported to be used for commercial purposes only as an additive in industrial water treatment, and is used in laundry and dishwashing, paints and coatings, as a scale inhibitor in oil and gas extraction, some wood construction and metal materials, paper products, ink, toner and colourants, photographic supplies, and in a variety of care products (i.e., fabric, cleaning and furnishing, personal, apparel and footwear, and air) (Environment Canada 2013, TDS 2018).
Additional uses of DTPMP in Canada are listed in Table 4-1.
| Use | Details |
|---|---|
| Incidental food additivea | Component in dishwashing detergent |
| Internal drug product database as medicinal or non-medicinal ingredients in final pharmaceutical, disinfectant or veterinary drug products in Canadab | Non-medicinal ingredient in ophthalmic solutions used to lubricate dry eyes |
| Notified to be present in cosmetics, based on notifications submitted under the Cosmetic Regulations Health Canadac | Notified as present in certain permanent hair dyes |
| Listed on PMRA pesticide formulants listd | Listed, however, not currently used in any registered products |
a Personal communication, email from the Food Directorate, Health Canada, to the Risk Assessment Bureau, Health Canada, dated January 24, 2018; unreferenced.
b Personal communication, email from Therapeutic Products Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated January 2, 2018; unreferenced
c Personal communication, email from the Consumer Product Safety Directorate, Health Canada, to the Risk Assessment Bureau, Health Canada, dated January 24, 2018; unreferenced.
d Personal communication, email from Pest Management Regulatory Agency, Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated December 29, 2017; unreferenced.
5. Potential to cause ecological harm
5.1 Characterization of ecological risk
The ecological risk of DTPMP was characterized using the ecological risk classification of organic substances (ERC) approach (ECCC 2016a). The ERC is a risk-based approach that considers multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. The various lines of evidence are combined to discriminate between substances of lower or higher potency and lower or higher potential for exposure in various media. This approach reduces the overall uncertainty with risk characterization compared to an approach that relies on a single metric in a single medium (e.g., LC50) for characterization. The following summarizes the approach, which is described in detail in ECCC (2016a).
Data on physical-chemical properties, fate (chemical half-lives in various media and biota, partition coefficients, and fish bioconcentration), acute fish ecotoxicity, and chemical import or manufacture volume in Canada were collected from the scientific literature, available empirical databases (e.g., OECD QSAR Toolbox), and from responses to surveys conducted under section 71 of CEPA, or they were generated using selected quantitative structure-activity relationship (QSAR) or mass-balance fate and bioaccumulation models. These data were used as inputs to other mass-balance models or to complete the substance hazard and exposure profiles.
Hazard profiles were based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Exposure profiles were also based on multiple metrics including potential emission rate, overall persistence, and long-range transport potential. Hazard and exposure profiles were compared to decision criteria in order to classify the hazard and exposure potentials for each organic substance as low, moderate, or high. Additional rules were applied (e.g., classification consistency, margin of exposure) to refine the preliminary classifications of hazard or exposure.
A risk matrix was used to assign a low, moderate or high classification of potential risk for each substance on the basis of its hazard and exposure classifications. ERC classifications of potential risk were verified using a two-step approach. The first step adjusted the risk classification outcomes from moderate or high to low for substances that had a low estimated rate of emission to water after wastewater treatment, representing a low potential for exposure. The second step reviewed low risk potential classification outcomes using relatively conservative, local-scale (i.e., in the area immediately surrounding a point-source of discharge) risk scenarios, designed to be protective of the environment, to determine whether the classification of potential risk should be increased.
ERC uses a weighted approach to minimize the potential for both over and under classification of hazard, exposure and subsequent risk. The balanced approaches for dealing with uncertainties are described in greater detail in ECCC 2016a. The following describes two of the more substantial areas of uncertainty. Error with empirical or modeled acute toxicity values could result in changes in classification of hazard, particularly metrics relying on tissue residue values (i.e., mode of toxic action), many of which are predicted values from QSAR models. However, the impact of this error is mitigated by the fact that overestimation of median lethality will result in a conservative (protective) tissue residue value used for critical body residue (CBR) analysis. Error with underestimation of acute toxicity will be mitigated through the use of other hazard metrics such as structural profiling of mode of action, reactivity and/or estrogen binding affinity. Changes or errors in chemical quantity could result in differences in classification of exposure as the exposure and risk classifications are highly sensitive to emission rate and use quantity. The ERC classifications thus reflect exposure and risk in Canada according to what is believed to be the current use quantity and may not reflect future trends.
Critical data and considerations used to develop the substance-specific profiles for DTPMP, and the hazard, exposure and risk classification results, are presented in ECCC (2016b).
According to information considered under ERC, DTPMP was classified as having a moderate exposure potential, due to moderate use quantities, a high margin of exposure and a critically long overall persistence, but as having a low hazard potential. Accordingly, DTPMP was classified as having a low potential for ecological risk. On the basis of current use patterns, this substance is unlikely to result in concerns for the environment in Canada.
6. Potential to cause harm to human health
6.1 Exposure assessment
DTPMP is mainly used as a chelating agent in water treatment and for bleaching applications in industrial settings. The major route of environmental exposure is expected to be from industrial releases. DTPMP is not expected to partition to air as it has a very low vapour pressure (1.67 x 10-10 Pa), and potential releases to the environment are expected to be predominantly to surface water. DTPMP is expected to partition strongly to sediment and sludge yielding very low dissolved concentrations in surface water after wastewater treatment (Nowack 2002). In Europe, wastewater treatment plant effluents yield mean water stream concentrations of DTPMP and similar phosphonates of 0.25 µg/L (Jaworska et al. 2002). Owing to DTPMP’s high affinity to bind to wastewater sludge and river sediment, and very low vapour pressure, exposure to the general population of Canada to DTPMP from environmental media is expected to be minimal.
In Canada, DTPMP is reported to be used in paints and coatings, paper products, ink, toner and colourants, photographic supplies, construction materials, and in a variety of care products that are intended for industrial/professional use only (i.e., fabric, cleaning and furnishing care, personal care, apparel and footwear care, and air care) (Environment Canada 2013). Although there is indication that DTPMP may be used in other countries in household detergents and cleaning products (HERA 2004), no evidence of this use in Canada has been identified.
DTPMP may be used as a component in an incidental additive (dishwashing detergent) used in food processing establishments; however, direct contact with food is not expected as the use of the detergent is followed by a potable water rinse (personal communication, email from the Food Directorate, Health Canada, to the Risk Assessment Bureau, Health Canada, dated January 24, 2018; unreferenced).
Products available to Canadian consumers that have the potential to result in exposure to DTPMP include certain ophthalmic solutions and permanent hair dye.
DTPMP is used as a stabilizing agent in a limited number of ophthalmic solutions sold in Canada, at a strength of 0.06 mg/drop (personal communication, email from the Therapeutic Products Directorate, Health Canada, to the Risk Assessment Bureau, Health Canada, dated January 3, 2018; unreferenced). This product is intended to be placed directly on the eye as a treatment for dry eye.
Exposure to a component of ophthalmic solutions occurs by absorption via the cornea, and conjunctival tissue. No consumer exposure models were identified to estimate exposure from the use of ophthalmic solutions. Therefore, a review of ophthalmic dosing by Farkouh et al. (2016) was used to identify reasonable assumptions for exposure via eye drops. Farkouh et al. (2016) reports that the conjunctival sac has the capacity to retain about 10 μL, significantly less than the standard volume of commercial drop dispensers which range from 25-50 μL, with the remaining liquid draining out of the eye and onto the eyelid or cheek. Exposure was not estimated for the portion of the eye drop that overflows the eye as it is assumed an individual would wipe away this excess liquid immediately.
On the basis of product directions, an upper bound exposure scenario for treatment of dry eye can be represented as a consumer dispensing 2 drops per eye, 5 times daily. After adjusting the dosage for the portion of the drop remaining in the eye (40%), the estimated intake of DTPMP would be 0.48 mg. This represents a systemic exposure of 6.8 µg/kg bw/day for an adult, and 8 µg/kg bw/day for a teen; however, this scenario represents a short-term treatment plan that should be discontinued after 7 days, according to product directions. See Appendix A for the parameters used in the calculations.
To estimate daily exposure to DTPMP from ongoing use of eye drops, a therapeutic regime of 2 drops dispensed per eye, twice daily, was considered. This exposure scenario results in an estimated exposure of 2.7 µg/kg bw/day for an adult, and 3.2 µg/kg bw/day for a teen.
DTPMP was also identified in certain permanent hair dyes intended for tinting facial hair (e.g., eyebrows, beards) (personal communication, email from the Consumer Product Safety Directorate, Health Canada, to the Risk Assessment Bureau, Health Canada, dated January 24, 2018; unreferenced). As a conservative estimate, exposure was calculated for the use of this substance in a permanent hair dye intended to be used on the scalp. DTPMP concentrations are identical for both types of dye products and it is assumed the consumer wears gloves for the scalp treatment, while an application brush is supplied with the facial treatment kit. Only the parameter for surface area (which is lower for the facial hair scenario) would differ when calculating exposure estimates for either hair dye product. The difference in frequency of use between product types is not relevant as exposure is estimated on a per event basis.
To estimate exposure to DTPMP from hair dyes, dermal absorption of DTPMP was estimated using a maximum skin flux (Jmax) value as reported in Williams et al. (2016). See Appendix A for equations and parameters used in the calculations. The Jmax value for DTPMP was calculated to be 1.20 × 10-6 mg/cm2/h, which results in an estimated systemic exposure of 0.26 µg/kg bw/event to DTPMP from the use of hair dye for adults, and 0.3 µg/kg bw/event for teens. Owing to the very low vapor pressure of DTPMP (1.67 x 10-10 Pa), inhalation exposure during hair dye applications was not estimated.
6.2 Health effects assessment
DTPMP is not identified as posing a high hazard to human health based on classifications by other national or international agencies for carcinogenicity, genotoxicity, developmental toxicity or reproductive toxicity. Phosphonic acid compounds including DTPMP and its sodium salts have been reviewed internationally (OECD 2004), and that review was used to inform the health effects characterization in this assessment. A literature search was conducted from 2004 (the year OECD published their report) to March 2018, and no additional health effects studies were identified which could result in critical effect levels lower than those identified in OECD (2004).
The REACH registration dossier (ECHA, 2017b) includes a summary of a toxicokinetic study on DTPMP in which three male rats were given a single oral dose of DTPMP and feces and urine were collected for 72 hours after exposure. Organs were also analyzed for DTPMP. After 24 hours, 94% of DTPMP was recovered in the feces. After 72 hours, 98% of the dose was recovered in the feces. This indicates only 2% oral absorption in this rat gavage study.
The critical effect DTPMP was identified as potential perturbations of iron and calcium homeostasis (in the absence of any concurrent alteration of calcium plasma levels). This effect was observed at a LOAEL of 842 mg/kg bw/d for the mid dose male group, resulting in a NOAEL of 83 mg/kg bw/d for this 90-day oral feeding study on rats (OECD 2004; ECHA 2017b). Some haematological effects and increases in total bone density were also observed in the same study. Some evidence of equivocal fetotoxicity was identified in another study, but the results were not replicated in a developmental study; NOAELs from both studies were higher than those identified for the iron and calcium homeostasis effects of 83 mg/kg bw/day (OECD 2004). The OECD (2004) review did not identify any concerns for carcinogenicity or genotoxicity.
The OECD (2004) review notes that there is no apparent difference in acute toxicity between the acids and their sodium salts, and that these compounds have very limited uptake to the body, with low absorption following both oral and dermal exposures.
6.3 Characterization of risk to human health
DTPMP is not a naturally occurring substance and no reports of occurrences in environmental media in Canada were identified. DTPMP has a very low vapour pressure and is not expected to remain in the atmosphere if released to the environment. If released to water, DTPMP is expected to strongly partition to sediment, yielding very low dissolved concentrations in surface water. Therefore exposure to the general population of Canada to DTPMP from environmental media is not expected, and the potential risk to human health is considered to be low.
Exposure to the general population to DTPMP is expected as a result of the substance being present in a limited number of pharmaceutical and cosmetic products. To calculate margins of exposure (MOEs) the NOAEL of 83 mg/kg bw/day from a 90-day feeding study (OECD 2004) was selected as there was no data available for the dermal endpoint. The use of a sub-chronic study as an endpoint for episodic exposure to hair dye is conservative. The critical effects in this study included potential perturbations of iron and calcium homeostasis. Consideration of the reported oral absorption for this substance of 2% (ECHA 2017b) results in an adjusted point-of-departure of 1.7 mg/kg bw/day.
Table 6-1 compares the estimated systemic exposure (see Section 6.1) to the adjusted critical effect level identified in OECD (2004) for DTPMP present in ophthalmic solution and permanent hair dye. MOEs are only shown for teens as exposures for teens were higher than those for adults due to their lower body weight, resulting in lower MOEs.
| Exposure scenario | Systemic exposure | Critical effect level (mg/kg bw/day) | Critical health effect endpoint | MOE |
|---|---|---|---|---|
| Ophthalmic solution (short-term use)a | 8 × 10-3 mg/kg bw/day | NOAEL of 1.7b | potential perturbations of iron and calcium homeostasis | 212 |
| Permanent hair dye (per event)c | 3.0 × 10-4 mg/kg bw/event | NOAEL of 1.7b | potential perturbations of iron and calcium homeostasis | 5600 |
a 2 drops per eye, 5 times per day, for 7 days. See Appendix A for details.
b Based on a NOAEL of 83 mg/kg bw/day from a 90-day feeding study (OECD 2004) adjusted for reported oral absorption of 2% (ECHA 2017b)
c 9 times per year. See Appendix A for details.
The MOEs calculated for use of ophthalmic solution and permanent hair dye are considered adequate to address the uncertainties in the health effects and exposure database.
6.4 Uncertainties in evaluation of risk to human health
There are some limitations in the exposure database leading to uncertainty in exposure estimates. There was no Canadian data available regarding concentrations of DTPMP in the environment, although its affinity to adsorb to sediment and sludge would indicate that exposure from release to water is unlikely. There was also no information available on concentrations of DTPMP remaining in or on products available to consumers as a result of its industrial/commercial use. Although there is evidence of the use of DTPMP in consumer products in Europe (HERA 2004), there were no uses identified in Canada. There is uncertainty with the models used to estimate exposure, but the conservative parameters used in the models were likely to overestimate exposure.
7. Conclusion
Considering all available lines of evidence presented in this draft screening assessment, there is low risk of harm to the environment from DTPMP. It is proposed to conclude that DTPMP does not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
On the basis of the information presented in this draft screening assessment, it is proposed to conclude that DTPMP does not meet the criteria under paragraph 64(c) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore proposed to conclude that DTPMP does not meet any of the criteria set out in section 64 of CEPA.
References
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C. 1999, c.33. Canada Gazette Part III, vol. 22, no. 3.
Canada, Dept. of the Environment. 2012. Canadian Environmental Protection Act, 1999: Notice with respect to certain substances on the Domestic Substances List [PDF]. Canada Gazette, Part I, vol. 146, no. 48, Supplement.
[ECCC, HC] Environment Canada, Health Canada. [modified 2017 Mar 12]. Categorization. Ottawa (ON): Government of Canada. [accessed 2018 January 22].
[ECCC] Environment and Climate Change Canada. 2016a. Science Approach Document: Ecological Risk Classification of Organic Substances. Gatineau (QC): ECCC.
[ECCC] Environment and Climate Change Canada. 2016b. Data used to create substance-specific hazard and exposure profiles and assign risk classifications in the Ecological Risk Classification of organic substances. Gatineau (QC). Available from: substances@ec.gc.ca.
[ECHA] European Chemicals Agency. 2017a. Brief profile [[(phosphonomethyl)imino]bis[ethane-2,1-diylnitrilobis(methylene)]]tetrakisphosphonic acid; CAS RN 15827-60-8. Helsinki (FI): ECHA. [updated 2018 Feb 7; accessed 2018 Jan 22].
[ECHA] European Chemicals Agency. 2017b. c2007-2017. Registered substances database; search results for CAS RN 15827-60-8. Helsinki (FI): ECHA. Substance Information: [[(phosphonomethyl)imino]bis[ethane-2,1-diylnitrilobis(methylene)]]tetrakisphosphonic acid. [updated 2017 December 11; accessed: 2018 February 12].
Environment Canada. 2013. DSL Inventory Update data collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain substances on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
Farkouh A, Frigo P, and Czejka M. 2016. Systemic side effects of eye drops: a pharmacokinetic perspective. Clinical Ophthalmology 10:2433-1441.
Health Canada. 1998. Exposure factors for assessing total daily intake of priority substances by the general population of Canada. Unpublished report. Ottawa (ON). Government of Canada.
[HERA] Human & Environmental Risk Assessment on ingredients of European household cleaning products. 2004. Phosphonates (CAS 6419-19-8; 2809-21-4; 15827-60-8) (Draft). [accessed 2018 Mar 12].
[IPCS] International Programme on Chemical Safety. 2006. Environmental Health Criteria 235: Dermal Absorption [pdf]. Geneva (CH): United Nations Environment Programme, International Labour Organization, World Health Organization. [accessed 2018 January 22].
Jaworska J, Van Genderen-Takken H, Hanstveit A, van de Plassche E, and Feigtel T. 2002. Environmental risk assessment of phosphonates, used in domestic laundry and cleaning agents in the Netherlands. Chemosphere 47:655-665.
Nowack B. 2002 Aminopolyphosphonate removal during wastewater treatment. Water Research 36:4636-4642.
[OECD] Organisation for Economic Co-operation and Development. 2004. SIDS initial assessment profile (SIAP): DTPMP and salts (Phosphonic Acid Compounds Group 3). SIAM [SIDS Initial Assessment Meeting], 2004 April 20-23. [accessed 2018 Mar 12].
Potts RO, and Guy RH. 1992. Predicting skin permeability. Pharmacological Research 9:663-669.
[TDS] Technical Data Sheet. 2018. PRO-200: Scale inhibitor for the oil and gas industry [PDF]. Grande Prairie (AB): Prochem Oilfield Chemicals Inc. [accessed 2018 March 14].
Williams FM, Rothe H, Barrett G, Chiodini A, Whyte J, Cronin MTD, Monteiro-Riviere NA, Plautz J, Roper C, Westerhout J, et al. 2016. Assessing the safety of cosmetic chemicals: consideration of a flux decision tree to predict dermally delivered systemic dose for comparison with oral TTC (Threshold of Toxicological Concern). Regul Toxicol Pharmacol. 76:174-186.
Appendix A - Exposure parameters for estimating exposure to products available to consumers
The maximum skin flux (Jmax) approach as conducted in Williams et al. (2016) was used to estimate dermal exposures to DTPMP across the scalp. Jmax represents the theoretical upper limit to steady-state flux of a given substance across the skin, independent of vehicle (barring potential penetration-retarding or -enhancing effects of certain formulations). Its use does not account for the presence of potentially absorbable skin-bound residues following termination of exposure. However, its use is conservative with respect to the assumptions that a given substance is present at its solubility limit in the “in-use” vehicle of a product and that the absorption is entirely steady-state (i.e., ignores slower absorption during the lag phase).
The equations used are provided below and the water solubility, log Kow and molecular weight (MW) values were obtained from Tables 2-1 and 3-1 of this screening assessment report. The permeability coefficient (Kp) is calculated using the Potts and Guy (1992) equation:
log Kp (in cm/h) = -2.71 + (0.71)(log Kow) - (0.0061)(MW, in g/mol)
DTPMP Kp = 2.4 × 10-9 cm/h
Jmax (in mg/cm2/h) = Kp (in cm/h) x water solubility (in mg/cm3)
DTPMP Jmax = 1.2 × 10-6 mg/cm2/hr.
Maximum theoretical amount absorbed per day (Qabs):
Qabs (in mg) = Jmax (in mg/cm2/h) x Surface area of skin contact (in cm2) x Exposure duration (in h)
DTPMP Qabs = 1.8 × 10-2 mg/event
On the basis of a Qabs equal to 1.8 × 10-2 mg/event, the estimated DTPMP intake from use of a hair dye for an adult would be 0.26 µg/kg bw/event, or 0.3 µg/kg bw/event for teens.
The estimated dermal exposure parameters for hair dye and ophthalmic solution are described in Table A-1.
| Exposure scenario | Model input parameter |
|---|---|
Ophthalmic solution (short-term use) |
Frequency of Use: 2 drops per eye, 5 times per day, for 7 days
Concentration: 0.06 mg/drop (Personal communication, email from Therapeutic Products Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated January 2, 2018; unreferenced)
Dosage adjustment = 40% - commercial eye droppers deliver drop sizes ranging from 25 – 50 µL, however the conjunctival sac can hold only 10 µL (Farkouh et al. 2016). Assuming dose is contained in smallest drop (25 µL), 60% of the dose will overflow the eye and not be available for absorption; the remaining 40% is available for absorption via the cornea and conjunctival tissue.
Body weight: Adult = 70.9 kg; Teen = 59.4 kg (Health Canada 1998) |
Permanent hair dye (per event) |
Duration: 24 hours (time between dye initially rinsed off and first hair wash; professional judgement)
Surface area of skin contact: ½ of scalp = 637.5 cm2 (Health Canada 1998) It is assumed that gloves are used during application and skin contact only involves the scalp.
Body weight: Adult = 70.9 kg; Teen = 59.4 kg (Health Canada 1998) |
