Draft Screening Assessment - Triazines and Triazole Group
Official Title: Draft screening assessment - Triazines and triazoles group
Chemical abstracts service registry numbers
61-82-5, 2893-78-9, 3089-11-0
Environment and Climate Change Canada
Health Canada
April 2019
Synopsis
Pursuant to section 68 or 74 of the Canadian Environmental Protection Act, 1999 (CEPA), the Minister of the Environment and the Minister of Health have conducted a screening assessment of three of six substances, which were originally referred to under the Chemicals Management Plan as the Triazoles Group and the Cyanurates Group. The two groups have since been merged and are referred to collectively as the Triazines and Triazole Group. These substances were identified as priorities for assessment as they met categorization criteria under subsection 73(1) of CEPA or were considered a priority on the basis of other human health concerns. Two of the six substances were determined to be of low concern through another approach, and the proposed decisions for these substances are provided in a separate report1 . The other substance will be assessed individually in a future screening assessment2 . The three substances addressed in this screening assessment will hereinafter be referred to as the Triazines and Triazole Group.
| CAS RNa | DSL Name | Common Name |
|---|---|---|
| 61-82-5 | 1H-1,2,4-triazol-3-amine | Amitrole |
| 2893-78-9 | 1,3,5-triazine-2,4,6(1H,3H,5H)-trione, 1,3-dichloro-, sodium salt | Sodium dichloroisocyanurate (NaDCC) |
| 3089-11-0b | 1,3,5-triazine-2,4,6-triamine, N,N,N’,N’,N’’,N’’-hexakis(methoxymethyl)- | Hexa(methoxymethyl)melamine |
a The Chemical Abstracts Service Registry Number (CAS RN) is the property of the American Chemical Society and any use or redistribution, except as required in supporting regulatory requirements and/or for reports to the Government of Canada when the information and the reports are required by law or administrative policy, is not permitted without the prior, written permission of the American Chemical Society.
b This substance was not identified under Subsection 73(1) of CEPA but was included in this assessment because it was considered a priority on the basis of other human health concerns.
None of the substances in the Triazines and Triazole Group have been identified as naturally occurring. According to information submitted pursuant to surveys under CEPA section 71 and via targeted stakeholder follow-up, amitrole, NaDCC, and hexa(methoxymethyl)melamine were not reported to be manufactured above the reporting threshold in reporting year 2008 or 2011. These three substances were reported to be imported into Canada in total annual quantities ranging from <100 to 1,000,000 kg, in reporting year 2008 or 2011. In Canada, amitrole was not reported to be present in any products with commercial or consumer use above the reporting threshold. In Canada, amitrole is registered as a herbicide. In 2014, the Pest Management Regulatory Agency (PMRA) conducted a re-evaluation of amitrole, and as a result, the agency implemented a phase-out of its use as a herbicide, with the exception of its use on spruce bareroot nursery stock (seedbeds). In April 2018, PMRA initiated a special review of amitrole following the non-renewal (no longer approved) of pesticidal uses in the European Union. NaDCC has uses in multiple pest control products (e.g., algicides, bactericides, slimicides, and a sanitizer). In addition, NaDCC can be used in a variety of other products, including water treatment products, cleaning products, and disinfectants. Hexa(methoxymethyl)melamine may be used as a crosslinking agent, which can be used in food packaging materials and other commercial applications.
The ecological risks of the substances in the Triazines and Triazole Group were characterized using the ecological risk classification of organic substances approach (ERC), which is a risk-based approach that employs multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. Hazard profiles are based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Metrics considered in the exposure profiles include the potential emission rate, overall persistence, and long-range transport potential. A risk matrix is used to assign a low, moderate or high level of potential concern for substances on the basis of their hazard and exposure profiles. Based on the outcome of the ERC analysis, the three substances in the Triazines and Triazole Group are considered unlikely to be causing ecological harm.
Considering all available lines of evidence presented in this screening assessment, there is low risk of harm to the environment from amitrole, NaDCC, and hexa(methoxymethyl)melamine. It is proposed to conclude that amitrole, NaDCC, and hexa(methoxymethyl)melamine do not meet the criteria under paragraphs 64(a) or (b) of CEPA, as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity, or that constitute or may constitute a danger to the environment on which life depends.
From a human health perspective, the health effects of concern for amitrole include reproductive toxicity and carcinogenicity. There were limited substance-specific health effects data available for NaDCC. A structurally similar chemical substance, sodium cyanurate/cyanuric acid, was used as an analogue for read-across. NaDCC and sodium cyanurate/cyanuric acid were reviewed internationally through the Joint Food and Agriculture Organization(FAO)/World Health Organization (WHO) Expert Committee on Food Additives (JECFA). The JECFA identified health effects of concern for sodium cyanurate, including effects on the urinary tract and heart in laboratory studies, which were considered the critical effects for NaDCC.
There were limited substance- specific health effects data for hexa(methoxymethyl)melamine. A structurally similar chemical substance, melamine, was used as an analogue for read-across. In laboratory studies with melamine, effects on the bladder and urinary system were considered the critical effects in this assessment, and there is also potential carcinogenicity.
Given the lack of consumer uses of amitrole in Canada, the potential risk to human health is considered to be low. The margins of exposure between levels of exposure of the Canadian general population from non-pesticidal use of NaDCC in water treatment tablets and cleaning products and critical effect levels were considered adequate to address uncertainties in the health effects and exposure databases. Similarly, for hexa(methoxymethyl)melamine, margins between levels of exposure of the general population from its theoretical presence in drinking water and critical effect levels were considered adequate to address uncertainties in the health effects and exposure databases.
On the basis of the information presented in this draft screening assessment, it is proposed to conclude that amitrole, NaDCC, and hexa(methoxymethyl)melamine do not meet the criteria under paragraph 64(c) of CEPA, as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore proposed to conclude that amitrole, NaDCC, and hexa(methoxymethyl)melamine do not meet any of the criteria set out in section 64 of CEPA.
1. Introduction
Pursuant to section 68 or 74 of the Canadian Environmental Protection Act, 1999 (CEPA) (Canada, 1999), the Minister of the Environment and the Minister of Health have conducted a screening assessment of three of six substances, which were originally referred to under the Chemicals Management Plan as the Triazoles Group and the Cyanurates Group, to determine whether these substances present or may present a risk to the environment or to human health. The two groups have since been merged and are referred to collectively as the Triazines and Triazole Group. The substances in this group were identified as priorities for assessment as they met categorization criteria under subsection 73(1) of CEPA or were considered a priority on the basis of other human health concerns (ECCC, HC [modified 2017]).
Two of the six substances (the CAS RNs3 101-37-1 and 288-88-0) were considered in the Ecological Risk Classification of Organic Substances (ERC) and the Threshold of Toxicological Concern (TTC)–based Approach for Certain Substances science approach documents (ECCC 2016a; Health Canada 2016), and were identified as being of low concern to both human health and the environment. As such, they are not further addressed in this report. Conclusions for these two substances are provided in the Substances Identified as Being of Low Concern on the basis of the Ecological Risk Classification of Organic Substances and the Threshold of Toxicological Concern (TTC)–based Approach for Certain Substances Draft Screening Assessment (ECCC, HC 2017). Another of the six substances, 1,3,5-triazine, hexahydro-1,3,5-trinitro- (CAS RN 121-82-4), will be part of a future screening assessment. The three substances addressed in this screening assessment will hereinafter be referred to as the Triazines and Triazole Group.
NaDCC and two structurally similar substances have been reviewed internationally through the Joint FAO/WHO Expert Committee on Food Additives (JECFA, 2004), and there is an existing assessment available. Amitrole and NaDCC have been evaluated in Canada through Health Canada’s Pest Management Regulatory Agency (PMRA) (Health Canada 2006, 2014) and an analogue of hexa(methoxymethyl)melamine used in this assessment was evaluated by Environment and Climate Change Canada (ECCC) and Health Canada (HC) under the Chemicals Management Plan (CMP) (ECCC, HC 2016). These assessments undergo rigorous review (including peer-review). Health Canada considers these assessments to be reliable, and they were used to inform the health effects characterization in this screening assessment. In addition, there were other international reviews or classifications available for the substances in the Triazines and Triazole Group from the European Food Safety Authority (EFSA 2014), the US Environmental Protection Agency (US EPA 1988, 1992, 1996, 2006), the European Commission (EC 2001), the European Chemicals Agency (EU 2008, 2016), the US National Toxicology Program (NTP 2016) and the International Agency for Research on Cancer (IARC 2001, 2018) that were used to inform the health effects characterization in this screening assessment.
The ecological risks of substances in the Triazines and Triazole Group were characterized using the ERC approach (ECCC 2016a). The ERC describes the hazard of a substance using key metrics, including the mode of toxic action, chemical reactivity, food web–derived internal toxicity thresholds, bioavailability, and chemical and biological activity, and considers the possible exposure of organisms in the aquatic and terrestrial environments on the basis of such factors as potential emission rates, overall persistence, and long-range transport potential in air. The various lines of evidence are combined to identify substances as warranting further evaluation of their potential to cause harm to the environment or as having a low likelihood of causing harm to the environment.
This draft screening assessment includes the consideration of information on chemical properties, environmental fate, hazards, uses, and exposures, including additional information submitted by stakeholders. Relevant data for the health assessment were identified up to April 2018. Empirical data from key studies, as well as some results from models, were used to reach the proposed conclusions. When available and relevant, information presented in assessments from other jurisdictions was considered.
This draft screening assessment was prepared by staff in the CEPA Risk Assessment Program at Health Canada and Environment and Climate Change Canada and incorporates input from other programs within these departments. The ecological portion of this assessment is based on the ERC document (published July 30, 2016), which was subject to an external review as well as a 60-day public comment period. While external comments were taken into consideration, the final content and outcome of the screening assessment remain the responsibility of Health Canada and Environment and Climate Change Canada.
This draft screening assessment focuses on information critical to determining whether substances meet the criteria as set out in section 64 of CEPA by examining scientific information and incorporating a weight of evidence approach and precaution4 . This draft screening assessment presents the critical information and considerations on which the proposed conclusions are based.
2. Identity of substances
The CAS RN, Domestic Substances List (DSL) names, and common names for the individual substances in the Triazines and Triazole Group are presented in Table 2‑1.
| CAS RN | DSL name (common name) | Chemical structure and molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
| 61-82-5 | 1H-1,2,4-triazol-3-amine (amitrole) | 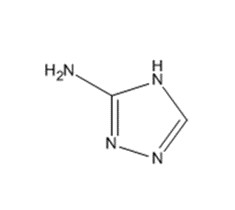 C2H4N4 C2H4N4 |
84.1 |
| 2893-78-9 | 1,3,5-triazine-2,4,6(1H,3H,5H)-trione, 1,3-dichloro-, sodium salt (NaDCC) | ![ClN1C(=O)[N-]C(=O)N(Cl)C1=O.[Na+]](/content/dam/eccc/images/pded/trizazines-triazoles/20190326-table2.1-image2.jpg) C3Cl2N3NaO3 C3Cl2N3NaO3 |
219.9 |
| 3089-11-0 | 1,3,5-triazine-2,4,6-triamine, N,N,N’,N’,N’’,N’’-hexakis(methoxymethyl)- (hexa[methoxymethyl]melamine) | 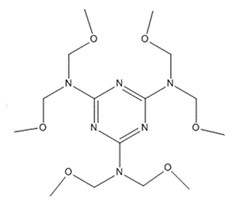 C15H30N6O6 |
390.4 |
2.1 Selection of analogues and use of (Q)SAR models
A read-across approach using data from analogues and the results of (quantitative) structure–activity relationship ([Q]SAR) models, where appropriate, has been used to inform the human health assessment. Analogues were selected that were structurally similar and/or functionally similar to substances within this group (similar physical-chemical properties, toxicokinetics) and that had relevant empirical data that could be used to read-across to substances with limited empirical data. The applicability of (Q)SAR models was determined on a case-by-case basis. Details of the read-across data and (Q)SAR models chosen to inform the human health assessments of the Triazines and Triazole Group are further discussed in the relevant sections of this report and in Appendix B.
Information on the identities and chemical structures of the analogues used to inform this assessment is presented in Table 2‑2.
| CAS RN | DSL name (common name) | Chemical structure and molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
| 108-78-1 | 1,3,5-triazine-2,4,6-triamine (melamine) |  C3H6N6 C3H6N6 |
126.1 |
| 108-80-5 | 1,3,5-triazine-2,4,6(1H,3H,5H)-trione (cyanuric acid) |
 C3H3N3O3 C3H3N3O3 |
129.1 |
| 2624-17-1 | 1,3,5-triazine-2,4,6(1H,3H,5H)-trione, monosodium salt (sodium cyanurate) |
![O=C1NC(=O)NC(=O)[N-]1.[Na+]](/content/dam/eccc/images/pded/trizazines-triazoles/20190326-table2.2.3.jpg) C3H2N3NaO3 C3H2N3NaO3 |
151.1 |
Melamine (CAS RN 108-78-1) was used as an analogue to inform the human health assessment of hexa(methoxymethyl)melamine. It was considered appropriate based on structural similarities, including a melamine core, and chemical similarities, including a similar capacity to be metabolized.
Two of the selected analogues, cyanuric acid (CAS RN 108-80-5) and sodium cyanurate (CAS RN 2624-17-1), which were selected for NaDCC, are the corresponding acid and salt of one another. The analogues are both structurally similar to NaDCC, but they are unchlorinated. The unchlorinated acid, cyanuric acid, is the material that reaches the gastrointestinal tract after metabolites of NaDCC contact saliva (JECFA, 2004). This acid, as well as its corresponding salt, were used to inform the human health assessment conducted by the JECFA for NaDCC (JECFA, 2004).
3. Physical and chemical properties
A summary of physical and chemical property data of the substances in the Triazines and Triazole Group is presented in Table 3-1. Additional physical and chemical properties are presented in ECCC (2016b).
| Property | Range | Type of data | Key references |
|---|---|---|---|
| Vapour pressure (Pa) | 1.94 × 10−12– 5.87 × 10−5 |
Experimental/ modelleda | ECHA, 2017a; EPI Suite, c2000–2012 |
| Water solubility (mg/L) | 149.3–2.80 × 105 | Experimental/ modelleda | ECHA, 2017a; EPI Suite, c2000–2012 |
| Log Kow (dimensionless) | -0.97–1.61 | Experimental/ modelleda | ECHA, 2017a; EPI Suite, c2000–2012 |
Abbreviations: Kow, octanol–water partition coefficient.
aModelled values were used to inform certain physical and chemical properties of NaDCC and hexa(methoxymethyl)melamine.
4. Sources and uses
All of the substances in the Triazines and Triazole Group have been included in surveys issued pursuant to CEPA section 71 notices (Environment Canada, 2009, 2013). Table 4‑1 presents a summary of information reported on the total annual manufacture and total annual import quantities for the substances in the Triazines and Triazole Group.
| Common name | Total manufacture (kg) | Total imports (kg) | Survey reference |
|---|---|---|---|
| Amitrole | <100 | <100 | Environment Canada, 2009b |
| NaDCC | <100 | 100,000–1,000,000 | Environment Canada, 2013 |
| Hexa(methoxymethyl)melamine | <100 | 100,000–1,000,000 | Environment Canada, 2013 |
aValues reflect quantities reported in response to surveys conducted under section 71 of CEPA (Environment Canada, 2009, 2013). See surveys for specific inclusions and exclusions (schedules 2 and 3).
bVolumes were updated based on targeted stakeholder follow-ups in 2018.
According to information submitted pursuant to a section 71 survey under CEPA and via targeted stakeholder follow-up, amitrole was not reported to be present in any products with commercial or consumer use above the reporting threshold (Environment Canada, 2009; personal communication, emails from stakeholders to the Existing Substances Risk Assessment Bureau, HC, 2018, unreferenced). In Canada, amitrole is present in a registered herbicide, but the product has not been sold in Canada over the past two years and is not expected to be sold in the foreseeable future (personal communication, emails from the Pest Management Regulatory Agency, HC, and from a stakeholder, to the Existing Substances Risk Assessment Bureau, HC, 2018, unreferenced). The PMRA has conducted a re-evaluation of amitrole and, as a result, has implemented a phase-out of its pesticidal use with the exception of spruce bareroot nursery stock (seedbeds) (Health Canada, 2014). In addition, the PMRA has recently initiated a special review of amitrole under subsection 17(2) of the Pest Control Products Act (personal communication, email from the Pest Management Regulatory Agency, HC, to the Existing Substances Risk Assessment Bureau, HC, 2018, unreferenced). Amitrole was not found in the Drug Product Database (DPD [modified 2017]). Amitrole is not a permitted food additive in Canada (personal communication, emails from the Food Directorate to the Existing Substances Risk Assessment Bureau, Health Canada, 2015, 2017, unreferenced). Cosmetic uses were not identified for amitrole in Canada, and amitrole is not listed in the Natural Health Products Ingredients Database (NHPID) or the Licensed Natural Health Products Database (LNHPD) (personal communication, emails from the Consumer Product Safety Directorate, HC, to the Existing Substances Risk Assessment Bureau, Health Canada, 2015, 2017, unreferenced; NHPID [modified 2018]; LNHPD [modified 2018]). Amitrole has been identified in other non-pesticidal uses, including lubricants for engines, which are expected to be specialized uses not relevant to consumers (e.g., SDS, 2015a, 2015b). No consumer uses of amitrole in Canada were identified.
According to information submitted pursuant to a section 71 survey under CEPA, NaDCC is used for water treatment, and for laundry and dishwashing with the potential for consumer use (Environment Canada, 2013). NaDCC is an active ingredient in pest control products, primarily in swimming pool algicides and bactericides (personal communication, emails from the Pest Management Regulatory Agency, HC, to the Existing Substances Risk Assessment Bureau, Health Canada, 2017, unreferenced). In Canada, NaDCC may be used in incidental additives as a component in the formulations of closed recirculating water treatment products where the treated water will not come in contact with food. It may also be used as a component in dish detergents and food contact surfaces cleaners and sanitizers, which are followed by a potable water rinse, and in sanitizers for food contact surfaces without a potable water rinse in Canada (personal communication, emails from the Food Directorate, HC, to the Existing Substances Risk Assessment Bureau, HC, 2018, unreferenced). In Canada, NaDCC is an active ingredient in various disinfectants in food premises, hospital/health care facilities, barns, and institutional/industrial settings based on the internal Drug Product Database (DPD [modified 2017]; personal communication, emails from the Therapeutic Products Directorate, HC, to the Existing Substances Risk Assessment Bureau, HC, 2018, unreferenced). NaDCC is not permitted in food additives in Canada (personal communication, emails from the Food Directorate to the Existing Substances Risk Assessment Bureau, Health Canada, 2015, 2017; unreferenced). Cosmetic uses were not identified for NaDCC in Canada, and NaDCC is not listed in the Natural Health Products Ingredients Database or the Licensed Natural Health Products Database (personal communication, emails from the Consumer Product Safety Directorate, HC, to the Existing Substances Risk Assessment Bureau, Health Canada, 2015, 2017, unreferenced; NHPID [modified 2018]; LNHPD [modified 2018]). NaDCC can be used in a variety of cleaning products, drinking water treatments, and disinfectants (e.g., SDS, 2010, 2015c, 2017a, 2017b).
Hexa(methoxymethyl)melamine is used in paints and coatings, and in automotive, aircraft, and transportation applications, but with no reported consumer use (Environment Canada, 2013). Hexa(methoxymethyl)melamine is not on the PMRA List of Active Pesticide Ingredients or the PMRA Pesticide Formulants List (personal communication, emails from the Pest Management Regulatory Agency, HC, to the Existing Substances Risk Assessment Bureau, Health Canada, 2015, unreferenced). In Canada, hexa(methoxymethyl)melamine may be used in food packaging materials as a component in adhesives (non-food contact), interior can coatings, and filters (for juice). Use of the substance as a component in incidental additives has not been identified. Additionally, hexa(methoxymethyl)melamine is not a permitted food additive in Canada (personal communication, emails from the Food Directorate, HC, to the Existing Substances Risk Assessment Bureau, Health Canada, 2015, 2017, unreferenced). Hexa(methoxymethyl)melamine was not found in the Drug Product Database (DPD [modified 2017]). Cosmetic uses were not identified for hexa(methoxymethyl)melamine in Canada, and hexa(methoxymethyl)melamine is not listed in the Natural Health Products Ingredients Database or the Licensed Natural Health Products Database (personal communication, emails from the Consumer Product Safety Directorate, HC, to the Existing Substances Risk Assessment Bureau, Health Canada, 2015, 2017, unreferenced; NHPID [modified 2018]; LNHPD [modified 2018]). Hexa(methoxymethyl)melamine can be used as a crosslinking agent, may be found in polymer mixtures, and may be used to synthesize other substances. Hexa(methoxymethyl)melamine may be used to promote various desirable properties such as increasing the thermal stability and strength of final products (e.g., Dsikowitzky and Schwarzbauer, 2015; Jeon, 2013; Liu, He, and Yang, 2017; Rahman, Kim, and Lee, 2009). Internationally, hexa(methoxymethyl)melamine may be found in textiles, paints and coatings, automobiles, plastic and rubber products, foam products, adhesives, resins, and other uses that are specialized or industrial (e.g., Cakic et al., 2015; Danish EPA, 2005; Dsikowitzky and Schwarzbauer, 2015; Jeon, 2013; Kailasam et al., 2010; Lee et al., 2014; Pathak, Khanna, and Sinha, 2007; SDS, 2012, 2014). Some reported uses submitted pursuant to section 71 surveys under CEPA are not included above due to confidentiality.
5. Potential to cause ecological harm
5.1 Characterization of ecological risk
The ecological risks of the substances in the Triazines and Triazole Group were characterized using the ecological risk classification of organic substances (ERC) approach (ECCC 2016a). The ERC is a risk-based approach that considers multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. The various lines of evidence are combined to discriminate between substances of lower or higher potency and lower or higher potential for exposure in various media. This approach reduces the overall uncertainty with risk characterization compared to an approach that relies on a single metric in a single medium (e.g., median lethal dose [LC50]) for characterization. The following summarizes the approach, which is described in detail in ECCC (2016a).
Data on physical-chemical properties, fate (chemical half-lives in various media and biota, partition coefficients, and fish bioconcentration), acute fish ecotoxicity, and chemical import or manufacture volume in Canada were collected from scientific literature, from available empirical databases (e.g., OECD QSAR Toolbox 2016), and from responses to surveys conducted under section 71 of CEPA or were generated using selected (quantitative) structure-activity relationship ([Q]SAR) models or mass-balance fate and bioaccumulation models. These data were used as inputs to other mass-balance models or to complete the substance hazard and exposure profiles.
Hazard profiles were established principally on metrics regarding the mode of toxic action, chemical reactivity, food web–derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Exposure profiles were also based on multiple metrics, including the potential emission rate, overall persistence, and long-range transport potential. Hazard and exposure profiles were compared to decision criteria in order to classify the hazard and exposure potentials for each organic substance as low, moderate, or high. Additional rules were applied (e.g., classification consistency, margin of exposure) to refine the preliminary classifications of hazard or exposure.
A risk matrix was used to assign a low, moderate or high classification of potential risk for each substance on the basis of hazard and exposure classifications. ERC classifications of potential risk were verified using a two-step approach. The first step adjusted the risk classification outcomes from moderate or high to low for substances that had a low estimated rate of emission to water after wastewater treatment, representing a low potential for exposure. The second step reviewed low risk potential classification outcomes using relatively conservative, local-scale (i.e., in the area immediately surrounding a point source of discharge) risk scenarios, designed to be protective of the environment, to determine whether the classification of potential risk should be increased.
ERC uses a weighted approach to minimize the potential for both over-and under-classification of hazard, exposure and subsequent risk. The balanced approaches for dealing with uncertainties are described in greater detail in ECCC (2016a). The following describes two of the more substantial areas of uncertainty. Error in empirical or modelled acute toxicity values could result in changes in the classification of hazard, particularly metrics relying on tissue residue values (i.e., mode of toxic action), many of which are predicted values from (Q)SAR models. The impact of this error is mitigated, however, by the fact that an overestimation of median lethality will result in a conservative (protective) tissue residue value used for critical body residue (CBR) analysis. Error in the underestimation of acute toxicity will be mitigated through the use of other hazard metrics, such as structural profiling of mode of action, reactivity, and/or estrogen binding affinity. Changes or errors in chemical quantity could result in differences in classification of exposure, as the exposure and risk classifications are highly sensitive to emission rate and use quantity. The ERC classifications thus reflect exposure and risk in Canada on the basis of what is believed to be the current use quantity, and may not reflect future trends.
Critical data and considerations used to develop the substance-specific profiles for the substances in the Triazines and Triazole Group, and the hazard, exposure, and risk classification results, are presented in ECCC (2016b).
The hazard and exposure classifications for the three substances in the Triazines and Triazole Group are summarized in Table 5-1.
| Substance | ERC hazard classification | ERC exposure classification | ERC risk classification |
|---|---|---|---|
| Amitrole | low | low | low |
| NaDCC | high | low | low |
| Hexa(methoxymethyl)melamine | low | high | low |
On the basis of low hazard and low exposure potential, amitrole was classified as having a low potential for ecological risk. According to information considered under ERC, amitrole was found to have a reactive mode of action, which is supported by the re-evaluation conducted by the PMRA (Health Canada 2014) which states that birds and small wild mammals may be at risk in and around the site of application of amitrole when used as a pesticide due to the consumption of contaminated food items. However, the potential effects and how they may manifest in the environment were not further investigated in the ecological portion of this screening assessment due to the low exposure of this substance from non-pesticidal uses. Considering current use patterns, it is unlikely that this substance is resulting in concerns for the environment in Canada.
According to information considered under ERC, NaDCC was classified as having a low exposure potential. NaDCC was classified as having a high hazard potential on the basis of the agreement between the reactive mode of action and elevated toxic ratio, both of which suggest that this chemical is likely of high potency. NaDCC was classified as having a low potential for ecological risk following the adjustment of risk classification based on a low potential for local-scale exposures. On the basis of current use patterns, it is unlikely that this substance is resulting in concerns for the environment in Canada.
According to information considered under ERC, hexa(methoxymethyl)melamine was classified as having a high exposure potential on the basis of overall persistence and large reported use volume. The ERC classified this substance as having a low hazard potential and subsequently having a low potential for ecological risk. It is unlikely that this substance is resulting in concerns for the environment in Canada.
6. Potential to cause harm to human health
6.1 Exposure assessment
Potential exposures to substances in the Triazines and Triazole Group from environmental media, food, and products available to consumers are presented in this section. Additional details regarding the exposure scenarios are summarized in Appendix A.
6.1.1 Environmental media
There were no environmental monitoring data identified in Canada for the substances in the Triazines and Triazole Group. Potential exposure to the general population through environmental media from amitrole is expected to be minimal at this time, as the volume in commerce is below the reporting threshold of 100 kg (Environment Canada, 2009; personal communication, emails from stakeholders to the Existing Substances Risk Assessment Bureau, HC, 2018, unreferenced).
For NaDCC, the Environmental Assessment Unit (EAU) Drinking Water Spreadsheet was used to estimate potential exposure to the general population through environmental media from non-pesticidal uses, which could result in down-the-drain releases by consumers (Health Canada, 2015). The upper bound of the total annual volume reported in Canada (i.e., 1,000,000 kg) was used as input into a consumer (i.e., down-the-drain) release scenario. This volume accounts for all the uses reported pursuant to a CEPA section 71 survey, and as such is expected to be a conservative input for assessing non-pesticidal uses only. Other inputs used include a total estimated removal percent of the substance by wastewater treatment plants of 61.5% (ECCC, 2016b), an emission factor of 100% (as a conservative assumption), and defaults from Health Canada (2015). The theoretical intakes of drinking water by the general population for this scenario were estimated to range from 5.9 × 10-4 mg/kg bw per day for formula-fed infants to 1.2×10−4 mg/kg bw per day for adults based on an estimated water concentration of 5.5 μg/L. The theoretical intake values estimated as a result of releases from industrial activities (with consideration of volume used, removal percentage, number of release days per year, releases being solely to wastewater, and river flow rate) were lower than those of the consumer release scenario.
Internationally, hexa(methoxymethyl)melamine has been detected in various waters. For example, the substance was detected in German rivers, ranging from <10 to 880 ng/L, likely as a result of release of industrial wastewaters (e.g., from coating and automotive sectors) and in the Rhine River up to 6.5 μg/L (Dsikowitzky and Schwarzbauer, 2015). In another study, hexa(methoxymethyl)melamine was detected in river systems with concentrations up to 6.16 μg/L in Germany (Eberhard et al., 2015). Tousova and colleagues (2017) have also reported concentrations of hexa(methoxymethyl)melamine in European waters with a median concentration of 27.8 ng/L. The conservative assumption was made that the highest measured concentration of 6.5 μg/L may be reflective of certain Canadian surface waters receiving industrial discharges due to its similar use in the coating and automotive sectors in Canada. Without adjusting for removal by drinking water treatment, intake values for drinking water are estimated to range from 6.9 × 10-4 mg/kg bw per day for formula-fed infants to 1.4 × 10−4 mg/kg bw per day for adults.
6.1.2 Food
The use of NaDCC in sanitizers for food contact surfaces without a potable water rinse results in a worst-case, theoretical intake estimate of 2.29 µg/kg bw per day (personal communication, emails from the Food Directorate, HC, to the Existing Substances Risk Assessment Bureau, HC, 2018, unreferenced).
Hexa(methoxymethyl)melamine may be used as a crosslinking or curing agent in the manufacture of can coatings. Because the substance reacts with the other polymeric components of the coating, it becomes bonded to the polymeric backbone, and only residual levels of unreacted substance are expected to migrate. As such, the estimated probable daily intake from this use is 0.0045 µg/kg bw per day for a 70-kg individual (personal communication, emails from the Food Directorate, HC, to the Existing Substances Risk Assessment Bureau, Health Canada, 2017, unreferenced). Hexa(methoxymethyl)melamine is also used as a component in filters used in the manufacture of juices; however, because of the high packaging-to-food ratio, exposure from this source is negligible. Exposure from the use of hexa(methoxymethyl)melamine in non-food contact adhesives is not expected (personal communication, emails from the Food Directorate, HC to the Existing Substances Risk Assessment Bureau, HC, 2018, unreferenced).
6.1.3 Products available to consumers
Products containing amitrole available to consumers in Canada have not been identified. Therefore, exposure to amitrole by the Canadian general population from consumer uses is not expected.
Potential exposures to NaDCC from non-pesticidal products available to consumers by the Canadian general population were considered, and the exposure scenarios that resulted in the highest exposures are presented here. NaDCC in water dissociates into hypochlorous acid (HClO, which is the source of free available chlorine) and the stabilizer isocyanuric acid (which reduces the degradation of chlorine from sunlight) (JECFA, 2004; Pinto and Rohrig, 2003). Table 6‑1 presents estimated exposures from NaDCC from water treatment tablets and cleaning abrasive powders. For the water treatment exposure scenario, long-term use would more likely consist of using only one tablet (or less) per litre. However, the use of two tablets per litre of water was assumed as a conservative approach for daily exposure. Although exposures may occur from floor cleaning liquids (SDS, 2010), such exposures were estimated to be less than the scenarios in Table 6-1.
| Exposure scenario | Age group | Product concentration | Oral exposure | Dermal exposurea | Inhalation exposure | Total exposure |
|---|---|---|---|---|---|---|
| Water treatment tabletsb | Infants to adults | 17% | 1.8 mg/kg bw per day for formula-fed infants to 0.36 mg/kg bw per day for adults |
N/A | N/A | 1.8 mg/kg bw per day for formula-fed infants to 0.36 mg/kg bw per day for adults |
| Cleaning abrasive powder: application exposurec | Adults | 1.5% | N/A | 0.83 mg/kg bw per event | Negligible | 0.83 mg/kg bw per event |
Abbreviation: bw, body weight; N/A, not applicable.
aDermal absorption were assumed to be 100% (relative to oral absorption).
bSDS, 2015c.
cSDS, 2017a.
Exposures to hexa(methoxymethyl)melamine are expected to be limited to specialized or industrial uses that would not result in exposure to the Canadian general population.
6.2 Health effects assessment
In Canada, the PMRA reviewed amitrole (Health Canada 2014). Internationally, the JECFA (JECFA 2004) summarized the health effects information and characterized hazard related to NaDCC and structurally similar substances, sodium cyanurate and cyanuric acid. The PMRA also evaluated NaDCC in their Re-evaluation Decision Document (Health Canada 2006) using the US Environmental Protection Agency (EPA) Reregistration Eligibility Document (RED) for Chlorinated Isocyanurates (US EPA 1992) as part of their basis. The US EPA indicated that the chlorinated isocyanuarates do not appear to induce significant acute, sub-chronic, or chronic toxicity. The US EPA also indicated that the available toxicity data suggest that these compounds do not meet their toxicity criteria for requirement of post-application/re-entry and/or mixer/loader/applicator exposure monitoring data (US EPA 1992). For hexa(methoxymethyl)melamine, Environment and Climate Change Canada and Health Canada (ECCC, HC 2016) summarized the health effects information and characterized hazard related to melamine, a structurally similar substance. Therefore, these assessments inform the health effects assessment for the three respective substances, including the selection of critical effects and points of departure.
Literature searches were conducted up to April 2018 for all three substances and for the three structurally similar substances, sodium cyanurate, cyanuric acid, and melamine. No health effect studies, which would impact the risk characterization (i.e., result in different critical endpoints or more conservative points of departure than those stated in Health Canada 2014; JECFA 2004; and ECCC, HC 2016), were identified.
6.2.1 Substance-specific hazard data for risk characterization
There were limited chemical-specific health effects data for some substances in the Triazines and Triazole group. Analogues were considered by Health Canada and the JECFA based on similarities in their physical and chemical properties, metabolism, and/or structure to the target chemicals (see Appendix B). The chemical-specific data are presented first, followed by analogue data used to inform the health effects characterization of substances in the Triazines and Triazole group.
Amitrole
Internationally, amitrole has been classified for carcinogenicity by the International Agency for Research on Cancer (IARC) as Group 3 (not classifiable as to its carcinogenicity to humans), by the US National Toxicology Program (NTP) as “Reasonably Anticipated to be a Human Carcinogen,” and by the US EPA as category B2 (probable human carcinogen) (IARC 2001; NTP 2016; US EPA 1988). Amitrole has been classified for reproductive toxicity as a Reproductive Category 2 (suspected) under the European Chemical Agency (ECHA) Globally Harmonised System (GHS) (EU 2008) and as reproduction category 1B (presumed) under the European Food Safety Authority (EFSA 2014) and EU (2016). Amitrole has also been reviewed in a Reregistration Eligibility Decision (RED) and by the Cancer Assessment Review Committee of the US EPA (1996, 2006) and the European Commission (EC 2001). In Canada, Health Canada’s PMRA re-evaluated the potential risks of amitrole (Health Canada 2014).
A quantitative risk assessment for tumorigenicity was conducted by the PMRA and a cancer unit risk (q1*) of 0.328 (mg/kg bw per day)–1 was derived on the basis of thyroid follicular cell tumours in male rats (Health Canada 2012, 2014).
NaDCC
As there were limited chemical-specific hazard data available for NaDCC, a breakdown product and its corresponding sodium salt (cyanuric acid and sodium cyanurate, respectively) were selected as analogues by the JECFA (2004). Critical endpoints and corresponding effect levels for sodium cyanurate and cyanuric acid to be used for risk characterization, as cited directly from JECFA (2004), can be found in section 6.2.2.
In contact with water, NaDCC is hydrolyzed to release free chlorine and creates equilibrium with chlorinated and non-chlorinated isocyanurates. Chlorinated isocyanurates react rapidly with saliva in the mouth to release free chlorine until there is no detectable chlorinated species remaining. The unchlorinated cyanuric acid is the material that reaches the gastrointestinal tract (Oxychem, 1997, 2000 as cited in JECFA 2004).
Acute studies were conducted with rats and rabbits. Mortality was observed at 1671 mg/kg bw and above orally and dermally. Short-term studies were conducted in rats from 59 days to 13 weeks. Developmental toxicity of NaDCC was assessed in dopamine-deficient (DD) mice with test animals gavaged on gestation days (GDs) 6 to 15 with 0, 25, 100, or 400 mg/kg bw per day (Gargus, Phipps, and Gluck 1984; Gargus, Phipps, and Ralph 1985; Hammond et al. 1986; Tani et al. 1980, as cited in JECFA 2004).
Hexa(methoxymethyl)melamine
This section provides the critical effects and corresponding effect levels for hexa(methoxymethyl)melamine. As there were limited chemical-specific hazard data for hexa(methoxymethyl)melamine, a structurally similar substance, melamine, was selected as an analogue for read-across. Melamine was selected as the most suitable analogue with available hazard data for read-across to hexa(methoxymethyl)melamine on the basis of similarities in chemical structure (OECD QSAR Toolbox 2016) and physical-chemical properties. Critical endpoints and corresponding effect levels for melamine to be used for risk characterization, as cited directly from ECCC, HC (2016), can be found in Section 6.2.2.
The US EPA (2007) characterized the toxicity of hexa(methoxymethyl)melamine in their hazard characterization using a mixture of hexa(methoxymethyl)melamine at 29% and a methylated melamine-formaldehyde polymer (CAS RN 68002-20-0) at 71% as the test material. The lowest no-observed-adverse-effect level (NOAEL) for the mixture from the repeat-dose toxicity studies was 250 mg/kg bw/day. The NOAEL for the mixture for reproductive and developmental effects was 500 mg/kg bw/day. The NOAEL of 250 mg/kg bw/day, if adjusted to account for only 29% of the mixture being composed of hexa(methoxymethyl)melamine (conservatively assuming all the toxicity of the mixture could be the result of hexa[methoxymethyl]melamine), would be 72.5 mg/kg bw/day. Due to the potential for confounding from the presence of the polymer on the mixture toxicity, particularly given its presence at 71% of the mixture and given that it may be a formaldehyde releaser (ECHA 2017b), the use of analogue data from melamine was preferred.
6.2.2 Read-across/analogue hazard data for risk characterization
There was a lack of health effects data identified for NaDCC and hexa(methoxymethyl)melamine for repeat-dose toxicity. Sodium cyanurate and cyanuric acid were determined to be appropriate analogues for NaDCC, as they were identified as such by the JECFA (JECFA 2004). Melamine was determined to be an appropriate analogue for hexa(methoxymethyl)melamine based on similarities in chemical structure and physical chemical properties. As such, these substances were used as surrogates where critical health effects data were required for risk characterization.
Sodium Cyanurate/ Cyanuric Acid
Sodium cyanurate and cyanuric acid were included as part of the group evaluated by the JECFA (2004a) and the committee also considered the toxicological data associated with sodium cyanurate and cyanuric acid to inform the hazard assessment of NaDCC because any residues of intact NaDCC in drinking water would be rapidly converted to cyanuric acid on contact with saliva. Sodium cyanurate is the corresponding salt of cyanuric acid.
This section provides the critical endpoints and corresponding effect levels for sodium cyanurate and cyanuric acid to be used for risk characterization, as cited directly from the JECFA (2004).
A chronic/carcinogenicity study was conducted in Charles River CD1 rats with sodium cyanurate. Test animals were exposed via drinking water for 2 years to 0, 400, 1,500, 2,400, or 5,375 mg/L (equivalent to 0, 26, 77, 154, or 371 mg/kg bw per day). The critical effect level and corresponding hazard endpoint was an NOAEL of 2,400 mg/L (154 mg/kg bw per day), based on lesions of the urinary tract and heart in males at the highest tested dose. There appeared to be no increase in tumour incidence (International Research and Development Corporation 1985, as cited in JECFA 2004). This chronic/carcinogenicity study was selected by JECFA as the key study for assessing risks from NaDCC as a drinking water disinfectant used for routine use and emergency management (JECFA 2004). In a two-year mouse study, there were no treatment-related changes in haematological, clinical chemistry, or urine analysis parameters, or incidence of tumour or histopathological lesions observed in B6C3F1 mice exposed to sodium cyanurate via drinking water up to the highest tested dose of 5,375 mg/L (1,523 mg/kg bw per day) (Serota et al. 1986 as cited in JECFA 2004).
For sodium cyanurate and cyanuric acid, oral acute studies were conducted in mice, rats, and rabbits with acute toxicity (lethality) values between 1,500 mg/kg-bw and 10,000 mg/kg-bw. Short-term studies were conducted using sodium cyanurate in drinking water for 13 weeks in mice and rat at 1,500 and 145 mg/kg bw per day, respectively. A reproductive study was done in rats for a minimum of 100 days of exposure before mating. Developmental studies were conducted via gavage in rats and rabbits. There were no treatment-related effects observed in foetuses for any study (Aldridge et al. 1985; Consultox Laboratories Ltd. 1974; Laughlin et al. 1982; Rajasekaran et al. 1981; Rodwell 1990; Serota et al. 1982; Tice 1997, as cited in JECFA 2004). Sodium cyanurate was not found to have mutagenic activity (JECFA 2004). Although these short-term studies were considered by JECFA in its evaluation of risks from the use of NaDCC as a drinking water disinfectant, they were not selected as the key study (JECFA 2004).
Melamine
Melamine was determined to be an appropriate analogue for hexa(methoxymethyl)melamine and was used as a surrogate where critical health effects data were required for risk characterization.
This section provides critical effects and corresponding effect levels for melamine, as cited in ECCC, HC (2016c).
A lowest-observable-adverse-effect level (LOAEL) was identified in a 13-week feeding study in rats, in which a dose-dependent increase in the incidence of bladder calculi and increased calcareous deposits in the kidney (not dose-related) were observed in animals fed melamine at all doses tested, the lowest one being 63 mg/kg bw per day (US NTP 1983 and Melnick et al. 1984, as cited in ECCC, HC 2016). WHO (2009, as cited in ECCC, HC 2016c) calculated a benchmark dose (BMD) and its lower confidence limit (BMDL10), based on this 13-week oral study, of 44.6 and 35 mg/kg bw per day, respectively, for a 10% increased incidence of the observed effects (urolithiasis occurrence and incidence of hyperplasia of the bladder epithelium).
Five carcinogenicity studies have been conducted in rats and one in mice; in all cases, melamine was administered through the feed of the animals. In four of the rat studies, bladder tumours or papillomas were observed at doses ranging from 263 to 1,200 mg/kg bw/day. In the one rat study where tumours were not observed, male and female Fischer 344 rats had been exposed to melamine in the diet for 24–30 months at doses of 5 to 100 mg/kg bw per day (Hazleton Laboratories 1983). No carcinogenic effects were observed in a two-year mouse feeding study at melamine doses of 327 to 1,065 mg/kg bw per day (ECCC, HC 2016c).
The postulated mode of action for carcinogenicity starts with localized tissue irritation, to a threshold mechanism of reactive hyperplasia progressing to bladder neoplasia. There was inadequate evidence in humans for carcinogenicity but sufficient evidence in experimental animals—as such, the IARC is in preparation to classify melamine as a Group 2B carcinogen (IARC 2018). As such, melamine is possibly carcinogenic to humans. Available information indicates that melamine is not genotoxic (WHO 2009, as cited in ECCC, HC 2016c).
6.3 Characterization of risk to human health
Overall exposure of the general population to amitrole is expected to be minimal based on current use information, and the potential risk to human health is considered to be low.
Table 6‑2 provides all relevant exposure estimates from non-pesticidal uses and critical effect levels for NaDCC, as well as resulting margins of exposure.
| Exposure scenario | Estimated exposure | Critical effect level | Study type and duration | Critical effect | MOE |
|---|---|---|---|---|---|
| Water treatment tablets (daily, oral, infants to adults)a | 1.8 mg/kg bw per day for infants to 0.36 mg/kg bw per day for adults |
NOAEL (oral) = 220 mg/kg bw/day)b | 2-year oral chronic rat study | Lesions of the urinary tract and heart in males | 122 for infants to 611 for adults |
| Cleaning abrasive powder: application exposure (per event, dermal, adults) | 0.83 mg/kg bw per event | NOAEL (oral)=220 mg/kg bw/day | 2-year oral chronic rat study | Lesions of the urinary tract and heart in males | 265 for adults |
Abbreviations: bw, body weight; MOE, margin of exposure; NOAEL, no-observed-adverse-effect level.
aDrinking water exposures estimated from the consumer release scenario were less than those from the use of water treatment tablets.
bThe NOAEL for sodium cyanurate was 154 mg/kg bw per day, equivalent to 220 mg/kg bw per day as anhydrous NaDCC.
The JECFA considered the safety of NaDCC in relation to its possible use as a disinfectant for drinking water in emergency situations, and for routine use in some water supplies. The Committee concluded that studies of the toxicity of sodium cyanurate were appropriate for assessing the safety of sodium dichloroisocyanurate, because any residues of intact sodium dichloroisocyanurate in drinking water would be rapidly converted to cyanuric acid on contact with saliva. The JECFA identified the critical effect for risk characterization as lesions of the urinary tract and heart in male rats from a two-year study with sodium cyanurate. The NOAEL for sodium cyanurate was 154 mg/kg bw per day, equivalent to 220 mg/kg bw per day as anhydrous NaDCC.
No repeated-dose dermal toxicity studies were identified for NaDCC or sodium cyanurate. The oral two-year study in rats that was the basis for the oral NOAEL of 220 mg/kg bw per day was used for the characterization of risk from dermal exposure. The use of a chronic study for risk characterization was considered to be a conservative approach, as the dermal exposures to this substance are intermittent, short-term exposures. Furthermore, the US EPA Reregistration Eligibility Decision (RED) noted that sub-chronic dermal studies were not required to be submitted by industry for re-registration due to a lack of toxicity in sub-chronic oral studies at doses above use concentrations (US EPA 1992).
The calculated margins of exposure for NaDCC are considered adequate to address uncertainties in the health effects and exposure databases.
Table 6‑3 provides all relevant exposure estimates and critical effect levels for hexa(methoxymethyl)melamine, as well as resultant margins of exposure, for determination of risk.
| Exposure Scenario | Estimated Exposure | Critical Effect Level | Study Type and Duration | Critical Effect | MOE |
|---|---|---|---|---|---|
| Drinking water (daily, oral, infants to adults) | 6.9×10−4 mg/kg bw per day for infants to 1.4×10−4 mg/kg bw per day for adults | BMDL10 (oral) = 35 mg/kg bw/day |
13-week oral rat study | Increased urolithiasis and hyperplasia of the bladder epithelium | >50,000 for infants to 250,000 for adults |
Abbreviations: BMDL10, benchmark dose lower confidence limit; bw, body weight; MOE, margin of exposure.
WHO (2009) calculated a BMD and its lower confidence limit (BMDL10) for melamine, based on 13-week oral study with the lowest LOAEL, for a 10% increased incidence of the observed effects (urolithiasis occurrence and incidence of hyperplasia of the bladder epithelium). The BMDL10 of 35 mg/kg bw per day was supported by ECCC, HC 2016c
No repeated-dose dermal toxicity studies were identified for melamine, and the oral 13-week feeding study in rats for melamine (which is the basis for the BMDL10) was used for the characterization of risk from both oral and dermal exposure to hexa(methoxymethyl)melamine.
The US EPA (2007) characterized the toxicity of hexa(methoxymethyl)melamine in their hazard characterization using mixture data. Due to the potential for confounding from the presence of the polymer (particularly being at 71% of the mixture), the use of analogue data from melamine is considered appropriate. Furthermore, the use of a BMDL10 of 35 mg/kg bw/day is more conservative than the endpoints in the mixture data. As such, the use of a point of departure based on melamine is considered a conservative approach.
The IARC is in preparation to classify melamine as a Group 2B carcinogen (IARC 2018). Use of the BMDL10 is considered protective of the cancer endpoint based on the suspected mode of action leading to cancer; namely, irritation followed by hyperplasia followed by neoplasia.
The calculated margins of exposure for hexa(methoxymethyl)melamine are considered adequate to address uncertainties in the health effects and exposure databases.
Although exposure of the general population from amitrole is expected to be minimal based on current-use information, this substance is considered to have health effects of concern on the basis of its carcinogenicity and reproductive toxicity. Therefore, there may be a concern for human health if exposures were to increase.
Although exposure of the general population from hexa(methoxymethyl)melamine is not of concern at current levels, this substance is considered to have health effects of concern on the basis of the carcinogenicity of its analogue melamine. Therefore, there may be a concern for human health if such exposures were to increase.
6.4 Uncertainties in evaluation of risk to human health
There are some uncertainties with respect to the exposure and health effects database. It was assumed that environmental monitoring data for hexa(methoxymethyl)melamine identified from international sources may be relevant to Canada and intake estimates for the general population from this source of exposure were generated accordingly for hexa(methoxymethyl)melamine. Environmental modelling for NaDCC was used when monitoring data were unavailable. There is uncertainty from the extrapolation of health effects data from oral toxicity studies to the dermal route of exposure. The selection of sodium cyanurate/cyanuric acid and melamine as analogues for assessing the respective hazard potential of NaDCC and hexa(methoxymethyl)melamine is associated with uncertainty.
7. Conclusion
Considering all available lines of evidence presented in this draft screening assessment, there is low risk of harm to the environment from amitrole, NaDCC, and hexa(methoxymethyl)melamine. It is proposed to conclude that amitrole, NaDCC, and hexa(methoxymethyl)melamine do not meet the criteria under paragraphs 64(a) or (b) of CEPA, as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity, or that constitute or may constitute a danger to the environment on which life depends.
On the basis of the information presented in this draft screening assessment, it is proposed to conclude that amitrole, NaDCC, and hexa(methoxymethyl)melamine do not meet the criteria under paragraph 64(c) of CEPA, as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore proposed to conclude that amitrole, NaDCC, and hexa(methoxymethyl)melamine do not meet any of the criteria set out in section 64 of CEPA.
References
Cakic S. M., Ristic I. S., Cincovic M. M., et al. 2015. “Glycolyzed poly (ethylene terephthalate) waste and castor oil-based polyols for waterborne polyurethane adhesives containing hexamethoxymethyl melamine” [Webpage]. Prog Org Coat. 78:357–68.
Canada. 1999. Canadian Environmental Protection Act, 1999. [PDF] S.C., 1999, c. 33. Canada Gazette, Part III, vol. 22, no. 3.
[Danish EPA] Danish Environmental Protection Agency. 2005. “Screening for health effects from chemical substances in textile colorants” [PDF]. Survey of Chemical Substances in Consumer Products, No. 57 2005. Danish Technological Institute.
[DPD] “Drug product database” [database]. [modified 2017 Nov. 3]. Ottawa (ON): Health Canada. [accessed 2018 Jan. 3].
Dsikowitzky L., Schwarzbauer J. 2015. “Hexa(methoxymethyl)melamine: An emerging contaminant in German rivers” [Webpage]. Water Environ Res. 87:461–69.
Eastman Kodak. 1991. Letter from Eastman Kodak Company to UEPA submitting Enclosed Material Safety Data Sheet and toxicity report on Hexamethoxymethylmelamine with attachments. New York. 6. Document No. 86-920000010.
Eberhard S., Foht S., Potouridis T., et al. 2015. “High concentrations of hexamethoxymethylmelamine (HMMM) in selected surface waters in southern Hesse” [PDF]. Jahrg. 1:7–10.
[ECCC] Environment and Climate Change Canada. 2016a. Science approach document: ecological risk classification of organic substances. Ottawa (ON): Government of Canada.
[ECCC] Environment and Climate Change Canada. 2016b. Gatineau (QC): Data used to create substance-specific hazard and exposure profiles and assign risk classifications in the Ecological Risk Classification of organic substances. Gatineau (QC). Available on request from: substances@ec.gc.ca.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2016c. “Draft screening assessment: Certain organic flame retardants substance grouping. 1,3,5-Triazine-2,4,6-triamine (melamine): Chemical abstracts service registry number 108-78-1.” Ottawa (ON): Government of Canada. [accessed 2017 Sept. 9].
[ECCC, HC] Environment and Climate Change Canada, Health Canada. [modified 2007 Apr. 20]. “Categorization of chemical substances” [Webpage]. Ottawa (ON): Government of Canada. [accessed 2016 Nov. 25].
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2017. Draft screening assessment: substances identified as being of low concern using the ecological risk classification of organic substances and the threshold of toxicological concern (TTC)-based approach for certain substances. Ottawa (ON): Government of Canada.
[ECHA] European Chemicals Agency. 2017a. “Troclosene sodium, CAS RN 2893-78-9” [Webpage]. [accessed 2015 Sep].
[ECHA] European Chemicals Agency. 2017b. “Investigation report: Formaldehyde and formaldehyde releasers” [PDF]. [accessed 2018 July 17].
[EFSA] European Food Safety Authority. 2014. “Conclusion on the peer review of the pesticide risk assessment of the active substance amitrole.” EFSA J. 12(7):3742.
Environment Canada. 2009. DSL Inventory Update data collected under the Canadian Environmental Protection Act, 1999, Section 71: Notice with respect to certain inanimate substances (chemicals) on the Domestic Substances List [PDF]. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
Environment Canada. 2013. DSL Inventory Update data collected under the Canadian Environmental Protection Act, 1999, Section 71: Notice with respect to certain substances on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
[EPI Suite] Estimation Program Interface Suite for Microsoft Windows [estimation model]. c2000–2012. Ver. 4.11. Washington (DC): U.S. Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
[EC] European Commission. 2001. “Amitrole.” Commission Directive 91/414/EEC of 22 March 2001. Annex I. Official Journal of the European Union. 6836/VI/97-final. European Commission.
[EU] European Union. 2008. “Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006”. Off J Eur Union L. 353:1–1355.
[EU] European Union. 2016. “Commission Implementing Regulation (EU) 2016/871 of 1 June 2016 concerning the non-renewal of approval of the active substance amitrole, in accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market, and amending Commission Implementing Regulation (EU) No 540/2011.” [PDF] Off J Eur Union L 145/4.
Haskell Laboratory. 1991. Letter to USEPA regarding the enclosed studies on N’N’N’N’N’N’-hekakis(methoxymethyl)-1,3,5-triazine-2,4,6-triamine with attachments (sanitized). Confidential submitting organization. Document No. 869200003895. Report No. 106-72.
Health Canada. 1998. “Exposure factors for assessing total daily intake of priority substances by the general population of Canada.” Unpublished report. Ottawa (ON): Health Canada, Environmental Health Directorate.
Health Canada. 2006. “Re-evaluation decision document RRD2006-17: Sodium dichloro-s-triazinetrione and trichloro-s-triazinetrione” [PDF]. Ottawa (ON): Health Canada, PMRA. [accessed 2018 June 6].
Health Canada. 2012. “Proposed re-evaluation decision document PRVD2012-01, amitrole”. Ottawa (ON): Health Canada, PMRA. [accessed 2017 Sept. 9].
Health Canada. 2014. “Re-evaluation decision document RVD2014-02, amitrole” [PDF]. Ottawa (ON): Health Canada, PMRA. [accessed 2017 Sept. 9].
Health Canada. 2015. Environmental Assessment Unit drinking water spreadsheets. [Excel format]. Ottawa (ON): Health Canada. [cited 2017 Sept. 5].
Health Canada. 2016. “Science approach document: Threshold of toxicological concern (TTC)-based approach for certain substances” [PDF]. September 2016. 54 pp.
Health Canada. 2017. Supporting document for the Screening Assessment, Certain Organic Flame Retardants Substance Grouping; 1,3,5-Triazine-2,4,6-triamine (Melamine): Human Health Supplementary Data. Ottawa (ON): Environment and Climate Change Canada. Available on request from: substances@ec.gc.ca
[IARC] International Agency for Research on Cancer. 2001. “IARC monographs on the evaluation of carcinogenic risks to humans” [PDF]. Volume 79. Some Thyrotropic Agents. pp. 381-410.
[IARC] International Agency for Research on Cancer. 2018. “Agents classified by the IARC monographs, volumes 1-121.” [PDF] Volume Sup. 7, 73, 119. Year: In prep. [accessed 10 May 2018].
[JECFA] Joint FAO/WHO Expert Committee on Food Additives. 2004. “Evaluation of certain food additives and contaminants” [PDF]. Geneva (CH): World Health Organization. (WHO Food Additive Series 52). Sixty-first report of the JECFA.
Jeon GS. 2013. “On characterizing microscopically the adhesion interphase for the adhesion between metal and rubber compound part I. Effect of hexamethoxymethylmelamine in rubber compound” [Webpage]. J Adhes Sci Technol. 27(15):1666-1680.
Kailasam K, Jun Y, Katekomol P, et al. 2010. “Mesoporous melamine resins by soft templating of block-co-polymer mesophases” [Webpage]. Chem Mater. 22:428–434.
Lee Y, Kim H, Schwartz S, et al. 2014. “Synthesis and characterization of silicone-modified polyester as a clearcoat for automotive pre-coated metals” [Webpage]. Prog Org Coatings. 77:184–193.
Liu Y, He J, Yang R 2017. “The synthesis of melamine-based polyether polyol and its effects on the flame retardancy and physical–mechanical property of rigid polyurethane foam” [PDF]. J Mater Sci. 52:4700–4712.
[LNHPD] Licensed Natural Health Products database [database]. [modified 2018 Feb. 6]. Ottawa (ON): Health Canada. [accessed 2016 Nov 25].
[NHPID] Natural Health Products Ingredients Database [database]. [modified 2018 Nov. 9]. Ottawa (ON): Health Canada. [accessed 2016 Nov 25].
[NTP] National Toxicology Program (US). 2016. Report on carcinogens, fourteenth edition. Amitrole: CAS no. 61-82-5 [PDF]. Research Triangle Park (NC): US Department of Health and Human Services, National Toxicology Program.
OECD QSAR Toolbox [read across tool]. 2016. Ver. 3.2.0.103. Paris (FR): Organisation for Economic Co-operation and Development, Laboratory of Mathematical Chemistry.
Pathak SS, Khanna AS, Sinha TJM. 2007. “HMMM cured corrosion resistance waterborne ormosil coating for aluminum alloy” [Webpage]. Prog Org Coat. 60:211–218.
Pellerin E, Macey K. 2001. “Canadian PHED tables version 7.” Ottawa (ON): Pest Management Regulatory Agency, Health Canada. August 21, 2001 [unpublished report].
Pinto G, Rohrig B. 2003. “Use of chloroisocyanuarates for disinfection of water: Application of miscellaneous general chemistry topics” [Webpage]. J Chem Educ. 80(1):41–44.
Rahman MM, Kim H, Lee W. 2009. “Properties of crosslinked waterborne polyurethane adhesives with modified melamine: Effect of curing time, temperature, and HMMM content.” [PDF] Fiber Polym. 10(1):6–13.
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment (NL)]. 2018. “Cleaning products fact sheet default parameters for estimating consumer exposure, updated version 2018” [PDF]. Bilthoven (NL): RIVM.
[SDS] Safety Data Sheet. 2010. “Material Safety Data Sheet: Spic & Span Floor Cleaner with Bleach—Powder Packets” [PDF]. Procter&Gamble. [accessed 2017 Dec. 20].
[SDS] Safety Data Sheet. 2012. “LORD PCD40558 Marking Ink”. Lord Corporation.
[SDS] Safety Data Sheet. 2014. “2,4,6-Tris[bis(methoxymethyl)amino]-1,3,5-triazine” [PDF]. TCI America. [accessed 2017 Sept. 9].
[SDS] Safety Data Sheet. 2015a. “ROYCO 560 PRF-23699F HTS” [PDF]. Anderol Specialty Lubricants. [accessed 2017 Sept. 9].
[SDS] Safety Data Sheet. 2015b. “ROYCO 555 DOD-PRF-85734” [PDF]. Anderol Specialty Lubricants. [accessed 2017 Sept. 9].
[SDS] Safety Data Sheet. 2015c. “Aquatabs” [PDF]. Medentech. [accessed 2017 Dec. 20]. Available upon request.
[SDS] Safety Data Sheet. 2017a. “Cleansing Powder” [PDF]. Old Dutch. [accessed 2017 Dec. 20]. Available upon request.
[SDS] Safety Data Sheet. 2017b. “Spa Essentials Oxidizer Spa Shock” [PDF]. Kik Holdo Company Inc. [accessed 2017 Dec. 20].
Tousova Z., Oswald P., Slobodnik J., et al. 2017. “European demonstration program on the effect-based and chemical identification and monitoring of organic pollutants in European surface waters” [Webpage]. Sci Total Environ. 601-602:1849–1868.
[US EPA] United States Environmental Protection Agency. 1988. “Evaluation of the potential carcinogenicity of amitrole (61-82-5)” [Webpage]. National Technical Reports Summary.
[US EPA] United States Environmental Protection Agency. 1992. “Re-registration eligibility decision (RED) for Chlorinated Isocyanurates.” Washington (DC): US EPA.
[US EPA] United States Environmental Protection Agency. 1996. “Re-registration eligibility decision (RED) for amitrole” [PDF]. Washington (DC): US EPA.
[U.S. EPA] United States Environmental Protection Agency. 2003. “User’s manual: Swimmer exposure assessment model (SWIMODEL) Version 3.0” [PDF]. Washington (DC): Office of Pesticides Programs, Antimicrobials Division.
[US EPA] United States Environmental Protection Agency. 2006. “Evaluation of the carcinogenic potential of amitrole (fifth review)” [PDF]. Washington (DC): US EPA.
[US EPA] United States Environmental Protection Agency. 2007. “Screening-level hazard characterization of high production volume chemicals sponsored chemical hexakis(methoxymethyl)-melamine (CAS No. 3089-11-0) [9th CI name: 1,3,5-triazine-2,4,6-triamine,N,N,N',N',N'',N''-hexakis(methoxymethyl)-].” Washington (DC): US EPA.
Versar. 1986. “Standard scenarios for estimating exposure to chemical substances during use of consumer products.” Prepared for U.S. EPA Office of Toxic Substances Exposure Evaluation Division.
Appendix A. Estimated human exposure to the Triazines and Triazole Group
Exposures were estimated on the basis of Canadian default body weights (bw), in other words, 7.5 kg of an infant, 15.5 kg of a toddler, 31.0 kg of a child, and 70.9 kg of an adult (Health Canada, 1998), and anticipated use patterns (see Table A-1). When concentrations were used to determine exposure estimates, the highest values were used as a conservative approach. Dermal and inhalation absorption were assumed to be 100% (relative to oral absorption).
| Exposure Scenario | Parameters |
|---|---|
| Water treatment tablets (NaDCC) | Amount of substance in one tablet (as): 8.5 mg, where the concentration in the tablet is 17% (one tablet treats 1 L of clear, room-temperature water without organic debris, and two tablets treat 1 L of dirty, cloudy, stained, and/or cold water according to product instructions) (SDS, 2015c). Drinking water intake (wi): 0–0.8 L/day for infant, 0.7 L/day for toddler, 1.1 L/day for child, 1.2 L/day for teenager, 1.5 L/day for adult, and 1.6 L/day for senior (Health Canada, 1998). Although the use of water treatment tablets may be on an emergency or otherwise intermittent basis, daily exposure was conservatively assumed. Estimated daily oral exposure = (2 × as × wi)/(bw × 1 L) |
| Application exposures from cleaning products (NaDCC) | Cleaning abrasive powder: Concentration (co): 1.5% (SDS, 2017a) Product amount (pa): 3.9 g (RIVM, 2018). (This is based on consideration of rubbing action resulting in the product amount that is subject to dermal exposure. Please note that scattering action, with a contact rate of 2.8 mg/min. and a release duration of 1 minute, was also considered, but its addition did not significantly change the dermal exposure estimate for this product.) Estimated dermal exposure = (co/100 × pa × unit conversion)/bw Exposure from the inhalation route was estimated based on the Pesticide Handlers Exposure Database (PHED) for cleaning products in powder form. Estimated inhalation exposure = (co/100 × pa × 56.20 μg exposure/kg ai handled × unit conversion)/bw, where 56.20 μg exposure/kg active ingredient (ai) handled is the PHED unit exposure value for mixing and loading wettable powder (Pellerin and Macey, 2001). Exposures from the inhalation route from other types of products are expected to not be a concern, given the negligible vapour pressure and non-powder/spray anticipated uses. |
Abbreviation: bw, body weight.
Appendix B. Read-across approach
| Consideration | Rationale |
|---|---|
| 1) Chemical structure. Emphasis was placed on analogues that contained a melamine or triazine core. |
Analogues that have a similar chemical structure are more likely to have similar toxicity profiles. |
| 2) Similar metabolites (predicted or observed). There were no empirical metabolism data for hexa(methoxymethyl)melamine. Metabolites were not predicted in the OECD QSAR Toolbox using the rat liver S9 metabolism and skin metabolism simulators. Metabolites were predicted using the skin metabolism simulators in OASIS Times, but not the in vivo rat liver metabolism simulator. |
Analogues that are metabolized through similar pathways to similar degradation products are more likely to have similar toxicity profiles. Analogues found that have known toxic metabolites (i.e., formaldehyde) that are not expected to result from the metabolism of the target were not considered. |
| 3) Common structural alerts. | Analogues with similar structural alerts are expected to share greater similarity in terms of toxicity. |
| 4) Similar physical-chemical properties. Emphasis was placed on chemical structures with a similar molecular weight, water solubility, vapour pressure, and log Ko/w. | Analogues with similar physical chemical properties may potentially share similar toxicological profiles and bioavailability. |
| 5) Availability of health effects data. | Only analogues with hazard data of sufficient quality and coverage of routes and durations of exposure relevant to exposure scenarios were considered applicable for read-across purposes. |
| 6) Selection and use of an analogue by reliable international review. | JECFA selected sodium cyanurate to be the representative analogue for NaDCC in their 2004 review (JECFA, 2004). |
Abbreviations: JECFA, Joint Food and Agriculture Organization/World Health Organization Expert Committee on Food Additives; Ko/w, octanol/water partition coefficient ; OECD QSAR, Organisation for Economic Co-operation and Development Quantitative Structure Activity Relationship.
| Hexa(methoxymethyl) melamine | Melamine | NaDCC |
Sodium Cyanurate | |
|---|---|---|---|---|
| CAS RN | 3089-11-0 | 108-78-1 | 2893-78-9 | 2624-17-1 |
| Structure |  |
 |
![ClN1C(=O)[N-]C(=O)N(Cl)C1=O.[Na+]](/content/dam/eccc/images/pded/trizazines-triazoles/20190326-table2.1-image2.jpg) |
![O=C1NC(=O)NC(=O)[N-]1.[Na+]](/content/dam/eccc/images/pded/trizazines-triazoles/20190326-table2.2.3.jpg) |
| MW (g/mol) | 390.44 | 126.1 b | 219.9 | 151.1 |
| Vapour pressure (Pa) | 1.4 × 10-6 | 9.4 × 10-8; 1.1 × 10-7 b | 1.9 × 10-12 | 1.88 × 10-14 |
| Henry’s law constant (atm·m3/mol) | 2.80 × 10-12 | 1.86 × 10-9 b | 3.10 × 10-12 | 8.41 × 10-15 |
| Water solubility (mg/L) | 1.49 × 102 | 4.85 × 103 b | 2.50 × 105 | 2.23 × 104 |
| LogKo/w | 1.6 | –1.14b | –0.06 | 0.62 |
| Oral LD50 (g/kg) | >5000 mg/kgc,d | 3161 mg/kg bw (male rats) and abovee | 1671 mg/kg bw (female rats) and abovef | >5000 mg/kg bw (for corresponding acid)f |
| Dermal LD50 (g/kg) | — | >1000 mg/kg bw (rabbit)e | >5000 mg/kg bwf | — |
| Genotoxicity | — | Negative | — | Negativef |
| Carcinogenicity | — | Positive | — | Negativef |
| Repeat dose toxicity (mg/kg bw/day) | — | BMDL10 (oral) = 35 mg/kg bw/day 13-week oral rat study Increased urolithiasis and hyperplasia of the bladder epithelium |
— | NOAEL (oral) = 220 mg/kg bw/day 2-year oral chronic/ carcinogenicity rat study Lesions of the urinary tract and heart in males |
Abbreviations: BMDL, lower confidence limit benchmark dose; bw, body weight; Ko/w, ; LD50, median lethal dose; MW, molecular weight; NOAEL, no-observed-adverse-effect level.
a Unless otherwise specified, data were retrieved from ECHA (2017), EPI Suite (c2000–2012), or the Health Effects section of this report.
b Health Canada (2016).
c Haskell Laboratory (1991).
d Eastman Kodak (1991).
e Health Canada (2017).
fJECFA (2004).
