Proposed Risk Management Approach for Ethyloxirane (1,2-Epoxybutane) Chemical Abstract Service Registry Number (CAS RN) 106-88-7
Environment Canada
Health Canada
July 2008
- Issue
- Background
- Why we need action
- Current used and industrial sectors
- Presence in the canadian environment and exposure sources
- Overview of existing actions
- Considerations
- Proposed objectives
- Proposed risk management
- Consultation approach
- Next steps / Proposed timeline
- References
This proposed risk management approach document builds on the previously released risk management scope document for ethyloxirane, and outlines the proposed control actions for this substance. Stakeholders are invited to submit comments on the content of this proposed risk management approach or provide other information that would help to inform decision making. Following this consultation period, the Government of Canada will initiate the development of the specific risk management instrument(s) where necessary. Comments received on the proposed risk management approach will be taken into consideration in developing the instrument(s). Consultation will also take place as instrument(s) are developed.
The Canadian Environmental Protection Act, 1999(CEPA 1999) (Canada 1999) requires the Minister of the Environment and the Minister of Health (the Ministers) to categorize all substances on the Domestic Substances List (DSL). Categorization involves identifying those substances on the DSL that a) are considered to be persistent (P) and/or bioaccumulative (B), based on the criteria set out in the Persistence and Bioaccumulation Regulations, and “inherently toxic” (iT) to humans or other organisms, or b) present, to individuals in Canada, the greatest potential for exposure (GPE). In addition, the Act requires the Ministers to conduct screening assessments of substances that meet the categorization criteria. The assessment further determines whether the substance meets the definition of “toxic” set out in section 64 of CEPA 1999.
In December 2006, the Challenge identified 193 chemical substances through categorization which became high priorities for assessment due to their hazardous properties and their potential to pose risks to human health and the environment. In February 2007, the Ministers began publishing, for industry and stakeholder comment, profiles of batches containing 15 to 30 high-priority substances. New batches are released for comment every three months.
In addition, the mandatory information–gathering provisions under section 71 of CEPA 1999 are being used under the Challenge to gather specific information where it is required. The information that is collected through the Challenge will be used to make informed decisions and appropriately manage any risks that may be associated with these substances.
The substance ethyloxirane, Chemical Abstract Service (CAS) Registry Number 106-88-7 referred to throughout this document by “ethyloxirane,” was included in Batch 1 of the Challenge under the Chemicals Management Plan.
A Notice summarizing the scientific considerations of a final screening assessment report was published by Environment Canada and Health Canada in the Canada Gazette, Part I, for ethyloxirane on July 5, 2008, under subsection 77(6) ofCEPA 1999. The final screening assessment report (Canada, 2008) concluded that ethyloxirane is entering or may be entering the environment in a quantity or a concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
Ethyloxirane has been classified by the International Agency for Research on Cancer (IARC) as “possibly carcinogenic to humans.” The European Commission has classified ethyloxirane as a Category 3 carcinogen (“causes concern for humans owing to possible carcinogenic effects”). These classifications were based on evidence of carcinogenicity in rodents. Ethyloxirane was found to be genotoxic in vitroand in a limited number of in vivo assays. When there is evidence that a substance for which the critical health effect is assumed to have no threshold, i.e., it is a genotoxic carcinogen, it is assumed that there is a probability of harm to human health at any level of exposure, therefore indicating that the substance meets the criterion in paragraph 64(c) of CEPA 1999. The draft screening assessment report concluded that the margin between estimated ambient exposure in the general environment and the critical effect level associated with non-neoplastic effects in experimental animals was considered adequate to protect human health.
The final screening assessment report also concluded that ethyloxirane does meet the criteria for persistence but does not meet the criteria for bioaccumulation, as defined by thePersistence and Bioaccumulation Regulations (Canada 2000) made under CEPA 1999. The presence of ethyloxirane in the environment results primarily from human activity.
For further information on the final screening assessment report conclusion for ethyloxirane, refer to the final screening assessment report, available at http://www.chemicalsubstanceschimiques.gc.ca/challenge-defi/batch-lot_1_e.html.
Following a screening assessment of a substance under section 74 of CEPA 1999, a substance may be found to meet the criteria under section 64 ofCEPA 1999. The Ministers can propose to take no further action with respect to the substance, add the substance to the Priority Substances List (PSL) for further assessment, or recommend the addition of the substance to the List of Toxic Substances in Schedule 1 of CEPA 1999. Under certain circumstances, the Ministers must make a specific proposal either to recommend addition to the List of Toxic Substances or to recommend the implementation of virtual elimination (or both). In this case, the Ministers proposed to recommend the addition of ethyloxirane to the List of Toxic Substances in Schedule 1 ofCEPA 1999. As a result, the Ministers will develop a regulation or instrument respecting preventive or control actions to protect the health of Canadians and the environment from the potential effects of exposure to this substance.
The final screening assessment report did not conclude that ethyloxirane meets the conditions set out in subsection 77(4) ofCEPA 1999. As a result, ethyloxirane will not be subject to the virtual elimination provisions under CEPA 1999 and will be managed using a lifecycle approach, to prevent or minimize its release into the environment.
Ethyloxirane is part of the chemical grouping discrete organics and the chemical sub-grouping epoxides.
Table 1 presents other names, trade names, chemical groupings, the chemical formula, the chemical structure, and the molecular mass for ethyloxirane.
Table 1. Identity of Ethyloxirane
| CAS Registry Number | 106-88-7 |
|---|---|
| DSL Name | Ethyloxirane |
| Other names1 | 1-Butylene oxide; (±)-2-Ethyloxirane; (±)-Ethyloxirane; a-Butylene oxide; 1,2-Butene oxide; 1,2-Butylene epoxide; 1,2-Butylene oxide, stabilized; 1-Butene oxide; 2-Ethyloxirane; Butene 1,2-epoxide; Butylene oxide; DL-1,2-Epoxybutane; Epoxybutane; Ethylethylene oxide; Ethyloxirane; NSC 24240 |
| Chemical group | Discrete organics |
| Chemical sub-group | Epoxides |
| Chemical formula | C4H8O |
| Chemical structure | 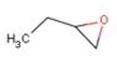 |
| SMILES | O(C1CC)C1 |
| Molecular mass | 72.12 g/mol |
Based principally on the weight-of-evidence based assessments ofIARC and the EU, a critical effect for characterization of risk to human health for ethyloxirane is carcinogenicity. Although the mode of induction of tumours has not been elucidated, ethyloxirane was genotoxic in in vitro and in a limited number ofin vivo assays; therefore, a mode of action for carcinogenicity involving direct interaction with genetic material cannot be precluded. With respect to non-cancer effects, the margin between the critical non-neoplastic effect level (i.e., 150 mg/m3) and the modelled concentration of ethyloxirane in ambient air (i.e. in the range of 10-10mg/m3) is very large and considered adequate to account for uncertainties in the database (Canada 2008).
In recent information gathered under CEPA 1999 through a section 71 notice, four Canadian companies reported the import of this substance in 2006 in a quantity ranging from 10 000 to 100 000 kg per year (Canada 2007). There are no manufacturers of ethyloxirane in Canada, although 18 companies reported stakeholder interest as part of the information submitted under the Challenge program.
According to submissions made under section 71 ofCEPA1999, from the Challenge questionnaire submission and other data voluntarily submitted (Canada 2007), as well as from other sources including the scientific and technical literature, ethyloxirane is used principally as a stabilizer in industrial solvents that in turn are primarily used for vapour degreasing and ultrasonic and cleaning solvents (U.S. EPA 1980; HSBD 2006). Ethyloxirane is also used for secondary cleaning in the semiconductor industry (HSIA 1994). Concentrations of ethyloxirane in these products are generally low; data submitted under the Challenge program indicated a range of < 0.1–15%, the latter level reported for stabilizers and boosters. These types of cleaners are mainly for industrial purposes and are designed to remove oils, lubricants, adhesives, inks and tars from a variety of metal, welded, machined, molded and diecast surfaces, as well as reinforced fibreglass and plastics (HSIA 1994). Other applications include its use in the production of pharmaceuticals (USEPA1980). Ethyloxirane is used by the coating industry to manufacture higher-molecular-weight polyesters for auto refinish (Canada 2007). Also, ethyloxirane may be used as a stabilizer in the production of n-propyl bromide (Canada 2007).
Other potential uses of ethyloxirane identified through searches of publicly available scientific and technical literature include its use as a chemical intermediate (non-disperse use) for synthesis in closed systems of fuel additives and defoamers (OECD 2001). Ethyloxirane has also been reported for use as an acid scavenger for chlorine-containing materials and for use as a corrosion inhibitor (HSDB 2006) and may also be used for secondary cleaning in the semiconductor industry (HSIA 1994).
It has also been reported that there is an electronic degreaser marketed in Canada that contains ethyloxirane at a concentration of less than 1%, but that this is not available to the general public (Health Canada 2008a). In addition, it has been reported that ethyloxirane is present at a concentration of less than 2% as a stabilizer in an alternative solvent for dry-cleaning, now available to dry cleaners in Canada. This solvent is currently in use by one Canadian dry-cleaning facility and it is anticipated that there will be further interest by other Canadian dry cleaners because this solvent is a more cost-effective solvent than traditional dry-cleaning solvents and also because it is not necessary to purchase new equipment in order to convert to this alternative solvent (Health Canada 2008b).
Ethyloxirane is not manufactured in Canada, and domestic supply is met by imports from the United States. Emissions of ethyloxirane into the environment may occur during the formulation and use of the hydrocarbon solvents in which ethyloxirane is acting as a stabilizer, or during the use and production of other products that may contain ethyloxirane. Production and processing of ethyloxirane normally occur in closed systems, but no monitoring data on emissions are available (Canada, 2008).
Fugitive emission or venting during the handling, transport or storage of ethyloxirane could also be a source of emission to the atmosphere. Direct release to the environment from the use of the hydrocarbon solvent is possible; however, only a small fraction of the total production of ethyloxirane would likely be released to the environment from disposal, as it is mainly used as a chemical intermediate (US EPA 1980). There are no current NPRI release data for ethyloxirane; the latest report in 2002 indicated releases of 0 tonnes (NPRI 2007). In recent information gathered under section 71 of CEPA 1999, companies reported the release of ethyloxirane in 2006 in a quantity of less than 50 kg, the cut-off quantity for reporting (Canada 2007; Canada 2008).
No data were available for concentrations of ethyloxirane in environmental media or food in Canada or elsewhere. Based upon information submitted under section 71 of CEPA 1999, ethyloxirane is used mainly for industrial applications in closed systems. The draft screening assessment report predicts, using modelled concentrations in the environment based upon the 50-kg per-year reporting limit for data submitted under section 71 ofCEPA 1999, that air would be the primary medium of exposure for ethyloxirane. Based on this modelled release, the predicted concentrations in all media are very low, resulting in negligible exposure (Canada 2008). This would pertain to possible releases related to industrial facilities and transportation. There may also be a potential for exposure from certain dry-cleaning applications (Health Canada 2008a).
Ethyloxirane is subject to:
- the Pest Control Products Act, as the substance was previously a List 2 formulant under the Pest Control Products Act and Regulations, but was removed from the list in June, 2007; indicating that it is no longer used as a formulant in pesticides in Canada;
- Canada's Cosmetic Ingredient Hotlist, as the substance is a prohibited ingredient not to be used in cosmetics sold in Canada (Health Canada 2007); and
- CEPA 1999'sEnvironmental Emergency Regulations, proposed addition.
British Columbia has set a soil guideline of 350 µg/g for residential/agricultural sites and 3 500 µg/g for industrial sites, and a drinking water standard of 210 µg/l (Province of British Columbia 1996).
Few countries appear to have guidelines in place for ethyloxirane. Under the National Model Regulations for the Control of Workplace Hazardous Substances (NOHSC: 1005(1994)), Australia has set occupational concentration cut-off levels associated with various health end-points ranging from 1% to 25%, at and above which ethyloxirane is classified as a hazardous substance (Australia 2008). The United States also has various reporting requirements regarding ethyloxirane.
No information is available on alternative chemicals or substitutes.
No information is available on alternative technologies and/or techniques.
Where information was available, socio-economic factors have been considered, at least in a qualitative manner, in the selection process for an instrument respecting preventive or control actions, and in the development of the risk management objective(s). Socio-economic factors will also be considered in the development of regulations, instrument(s) and/or tool(s) as identified in theCabinet Directive on Streamlining Regulation (Treasury Board of Canada Secretariat 2007) and the guidance provided in the Treasury Board document Assessing, Selecting, and Implementing Instruments for Government Action.
In screening assessments, potential exposure of the general population, including infants and children, is estimated. To the extent possible, based on available data, exposure to ethyloxirane from multiple routes (i.e., inhalation, ingestion and contact on the skin) and possible sources (ambient air, indoor air, drinking water, food, beverages -- including breast milk and formula for infants -- soil, and in some instances consumer products) is estimated. Infants and children's exposure is characterized by their unique physiology (e.g., intake of air, food and water relative to body size) and generally known behaviour characteristics (e.g., crawling versus walking, mouthing activity).
As part of the Challenge, the Government asked industry and interested stakeholders to submit any information on the substance that may be used to inform risk assessment, risk management and product stewardship. In particular, stakeholders were asked through a questionnaire if any of the products containing the substance were intended for use by children. Given the information received, and other data considered, it is proposed that no additional risk management actions to specifically protect children are required for this substance at this time.
An environmental or human health objective is a quantitative or qualitative statement of what should be achieved to address environmental or human health concerns identified during a risk assessment. The proposed human health objective for ethyloxirane is to minimize, to the extent practicable, exposure to this substance, and hence to minimize the associated risk to human health.
A risk management objective is a target expected to be achieved for a given substance by the implementation of risk management tool(s) and/or instrument(s). As the current exposures of Canadians to ethyloxirane were considered to be negligible under the current use conditions, the risk management objective is to prevent increases in exposure.
As required by the Government of Canada's Cabinet Directive on Streamlining Regulation,2 and criteria identified in the Treasury Board document entitled Assessing, Selecting, and Implementing Instruments for Government Action, the proposed risk management regulations, instrument(s) and/or tools(s) were selected using a consistent approach, and took into consideration the information that has been received through the Challenge and other information available at the time.
In order to achieve the risk management objective and to work towards achieving the human health objective, the risk management being considered for ethyloxirane is a provision whereby any future use of ethyloxirane would require that the federal government be notified. Additional areas of focus involve the presence of ethyloxirane in alternative dry-cleaning solvents and in pharmaceuticals.
No consumer products were identified which contained ethyloxirane. It is proposed to create a provision whereby any proposed use of ethyloxirane require that the federal government be notified.
The emerging use of ethyloxirane as a stabilizer in n-propyl bromide dry-cleaning solvents has been identified. This solvent is currently known to be used by one facility in Canada at concentrations of <2% ethyloxirane. Releases are not expected from this source due to the technical equipment specifications of machines used with this solvent and their closed-loop system design. Potential expanded use of the solvent in the dry-cleaning sector will be evaluated by the federal government to determine whether there is a potential for future risk of exposure to consumers from this source.
Issues pertaining to ethyloxirane in pharmaceuticals fall under Health Canada's Food and Drugs Act. No specific action with respect to ethyloxirane is being taken by Health Canada at this time. The number and type of pharmaceuticals for which ethyloxirane was used as an intermediary in the manufacturing process cannot be determined. However, all drug products are subject to good manufacturing practices, which require that the manufacturer must ensure that the pharmaceutical is of acceptable quality. Each formulation must undergo analysis and all substances which have significant amounts of residue (more than 0.15%) must be identified and appropriate toxicology data supplied. Impurity levels are guided by the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) recommendations.
The proposed regulation or instrument will be published in theCanada Gazette, Part I, no later than July 2010, as per the timelines legislated in CEPA 1999.
Releases of ethyloxirane will continue to be monitored under the National Pollutant Release Inventory. Other monitoring will be considered in order to assess the performance of the risk management instrument and to determine whether further action needs to be taken with respect to ethyloxirane.
The risk management scope for ethyloxirane, which summarized the proposed risk management under consideration at that time, was published on January 19, 2008, and is available at www.ec.gc.ca/TOXICS/EN/detail.cfm?par_substanceID=236&
par_actn=s1. Industry and other interested stakeholders were invited to submit comments on the risk management scope during a 60-day comment period. Comments received on the risk management scope document were taken into consideration in the development of this proposed risk management approach document.
Consultation for the risk management approach will involve publication on July 5, 2008, and a 60-day public comment period.
The primary stakeholders include:
- the dry-cleaning industry
- Health Canada and Environment Canada
Next steps / Proposed timeline
| Actions | Date |
|---|---|
| Electronic consultation on Proposed Risk Management Approach | July 5, 2008 to September 3, 2008 |
| Response to comments on the Risk Management Approach | At time of publication of proposed instrument |
| Consultation on the draft instrument | Winter 2008-2009 |
| Publication of the proposed instrument | No later than July 2010 |
| Formal public comment period on the proposed instrument | No later than summer 2010 |
| Publication of the final instrument | No later than January 2012 |
Industry and other interested stakeholders are invited to submit comments on the content of this proposed risk management approach or provide other information that would help to inform decision making. Please submit comments prior to September 3, 2008, since the Government of Canada will be moving forward with the risk management of ethyloxirane after this date. Pursuant to section 313 of CEPA 1999, any person who provides information to the Minister underCEPA 1999 may submit with the information a request that it be treated as confidential. During the development of the risk management instrument(s) and/or tool(s), there will be opportunity for consultation on the proposed instrument(s). Comments and information submissions on the proposed risk management approach should be submitted to the address provided below:
Existing Substances Division
Place Vincent Massey, 20th Floor
351 Saint Joseph Boulevard
Gatineau QC K1A 0H3
Tel: 1-888-228-0530 / 819-956-9313
Fax: 1-800-410-4314 / 819-953-4936
Email: Existing.Substances.Existantes@ec.gc.ca
Australia. 2008. Hazardous Substances Information System [database on the Internet]. Australian Safety and Compensation Council. Available from: http://hsis.ascc.gov.au/SearchHS.aspx
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C., 1999, c. 33, part#, s.#. Canada Gazette., Part III. Vol. , vol. 22, no. 3. Ottawa: Queen's Printer. Chapter 33. Available from: http://canadagazette.gc.ca/partIII/1999/g3-02203.pdf.
Canada. 2000. Canadian Environmental Protection Act: Persistence and Bioaccumulation Regulations, P.C. 2000-348, 23 March, 2000, SOR/2000-107. Canada Gazette. Part II, vol. 134, no. 7, p. 607−612. Ottawa: Queen's Printer. Available from: http://canadagazette.gc.ca/partII/2000/20000329/pdf/g2-13407.pdf
[Canada], Dept. of the Environment. 2007. Canadian Environmental Protection Act, 1999. Notice with respect to certain substances identified in the Challenge, published in the December 9, 2006 Notice of intent to develop and implement measures to assess and manage the risks posed by certain substances to the health of Canadians and their environment. Canada Gazette, Part I, vol. 141, no. 5, p. 165–177. Ottawa: Queen's Printer. Available from: http://canadagazette.gc.ca/partI/2007/20070203/pdf/g1-14105.pdf.
[Canada], Dept. of the Environment, Dept. of Health. 2008. Screening Risk Assessment for Ethyloxirane. Screening assessment for the Challenge, ethyloxirane, Chemical Abstracts Service Registry Number 106-88-7.
Health Canada. 2007. The Cosmetic Ingredient Hotlist. List of Prohibited and Restricted Cosmetic Ingredients. Available from: http://www.hc-sc.gc.ca/cps-spc/person/cosmet/hotlist-liste_e.html
Health Canada, 2008a. Personal Communication
Health Canada 2008b. Personal Communication.
[HSDB] Hazardous Substances Databank [database on the Internet]. 2006. 1-Butene oxide. Bethesda (MD): National Library of Medicine (US). [cited 2006 Mar]. Available from:http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB
[HSIA] Halogenated Solvents Industry Alliance Inc. 1994. White paper on methyl chloroform (1,1,1-trichloroethane) [Internet]. Washington (DC). Available from: http://www.hsia.org/white_papers/111tri%20wp.html
[NPRI] National Pollutant Release Inventory [database on the Internet]. 2007. Gatineau (QC): Environment Canada. Available from: http://www.ec.gc.ca/pdb/querysite/query_e.cfm
[OECD] Organization for Economic Cooperation and Development [Internet]. 2001. SIDS Initial Assessment Report for butane 1,2-epoxy. United Nations Environment Programme Publications. [cited 2001 Mar 23]. Available from: http://www.chem.unep.ch/irptc/sids/OECDSIDS/indexcasnumb.htm.
Province of British Columbia. 1996. B.C. Reg. 375/96 Environmental Management Act, Contaminated Sites Regulation. [Provisions of the Environmental Management Act. S.B.C. 2003, c. 53, relevant to the enactment of this regulation: sections 62, 63, and 139] Available from: http://www.qp.gov.bc.ca/statreg/reg/E/EnvMgmt/EnvMgmt375_96/
375_96_11.htm#Schedule10
Treasury Board of Canada Secretariat. 2007. Cabinet Directive on Streamlining Regulation, section 4.4 [Internet]. Available from:http://www.regulation.gc.ca/directive/directive01-eng.asp
[US EPA] United States Environmental Protection Agency. 1980. Investigation of selected environmental contaminants: epoxides. Washington (DC): Office of Toxic Substances, US Environmental Protection Agency. Report No.:EPA-560/11-80-005.
Footnotes
2 Section 4.4 of the Cabinet Directive on Streamlining Regulationstates that “Departments and agencies are to: identify the appropriate instrument or mix of instruments, including regulatory and non-regulatory measures, and justify their application before submitting a regulatory proposal”.