Risk management approach for benzophenone
Official title: Risk Management Approach for Methanone, Diphenyl- (Benzophenone)
Chemical Abstracts Service Registry Number (CAS RN): 119-61-9
Environment and Climate Change Canada
Health Canada
January 2021
Summary of proposed risk management
This document outlines the proposed risk management actions for methanone, diphenyl-, herein referred to as benzophenone. In particular, the Government of Canada is proposing the following risk management actions to address human health concerns:
- Measures to reduce exposures to benzophenone from certain cosmetics by describing it as a prohibited or restricted ingredient on the Health Canada Cosmetic Ingredient Hotlist. The Hotlist is used to communicate that certain substances may not be compliant with requirements of the Food and Drugs Act or provisions of the Cosmetic Regulations; and
- A measure to reduce the concentrations of benzophenone to a maximum of 0.1 % (w/w) or 1,000 mg/kg in certain exterior and interior paint, stain and/or coating products that are available to consumers in Canada.
If available, information on the following items should be provided to the contact identified in section 8 of this document, within 60 days following the publication of this document, to inform risk management decision-making:
- Current quantities and concentrations of benzophenone used in exterior and interior paint, stain and coating products that are available to consumers;
- Current quantity and product types that would be impacted by the proposed risk management, i.e. exterior and/or interior paint, stain and coating products that are available to consumers in Canada with a final benzophenone concentration above 0.1 % w/w;
- Potential alternative substances to benzophenone for use in exterior and interior paint, stain and coating products that are available to consumers; and
- Socio-economic and technical impacts and benefits associated with the proposed risk management for benzophenone.
The risk management actions outlined in this Risk Management Approach document may evolve through consideration of assessments and risk management actions published for other Chemicals Management Plan (CMP) substances as required to ensure effective, coordinated, and consistent risk management decision-making.
Note: The above summary is an abridged list of actions proposed to manage this substance and to seek information on identified gaps. Refer to section 3 of this document for more complete details in this regard. It should be noted that the proposed risk management actions might evolve through consideration of additional information obtained from the public comment period, literature and other sources.
1. Context
The Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999) provides the authority for the Minister of Environment and the Minister of Health (the ministers) to conduct assessments to determine if substances are toxic to the environment and/or harmful to human health as set out in section 64 of CEPAFootnote 1, Footnote 2, and if so, to manage the associated risks.
As part of the Chemicals Management Plan (CMP), the ministers are assessing and will manage, where appropriate, the potential health and ecological risks associated with approximately 1,550 substances (Canada 2016). The substance methanone, diphenyl-, Chemical Abstracts Service Registry Number (CAS RN)Footnote 3 119-61-9, referred to throughout this document as benzophenone, is included in the CMP.
2. Issue
2.1 Screening assessment report conclusion
Health Canada and Environment and Climate Change Canada conducted a screening assessment of benzophenone in Canada. A notice summarizing the scientific considerations of the draft screening assessment for this substance was published in the Canada Gazette, Part I, on August 4, 2018 (Canada 2018a).
On the basis of the information available, the screening assessment concludes that benzophenone is harmful to human health under section 64(c) of CEPA because it is entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health (Canada 2021).
It is concluded that benzophenone is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity, or that constitute or may constitute a danger to the environment on which life depends under sections 64(a) or (b) of CEPA, respectively (Canada 2021).
The exposure sources of concern identified in the screening assessment are from the use of nail polishes exterior and interior paints, as well as stains (refer to section 5).
Of note, the proposed risk management actions described in this document may be subject to change. For further information on the screening assessment for benzophenone, refer to the benzophenone screening assessment.
2.2 Recommendation under CEPA
On the basis of the findings of the screening assessment conducted pursuant to CEPA, the ministers recommend that benzophenone be added to the List of Toxic Substances in Schedule 1 of the ActFootnote 4 as per section 77(2) of CEPA. According to section 91 of CEPA, a proposed regulation or instrument establishing “preventative control actions” must be published in the Canada Gazette, Part I, within 24 months of the recommendation to add the substance to Schedule I. Once proposed, the ministers have a further 18 months to finalize the regulation or instrument. If needed, additional regulations or instruments can be developed within that period or afterwards.
2.3 Public comment period on the draft screening assessment and the risk management scope
The draft screening assessment for benzophenone and its associated Risk Management Scope document summarizing the proposed risk management options under consideration at that time were published on August 4, 2018 (Canada 2018b). Interested stakeholders were invited to submit comments on both documents during a 60-day comment period. Comments received on the draft screening assessment report and the Risk Management Scope were taken into consideration in the development of this document. A summary of responses to public comments received is available.
3. Proposed risk management
Section 3 presents the health and risk management objectives, as well as the proposed actions to achieve them. For more information on the context and rationale for these actions, please consult sections 4 and 5.
Following the publication of this Risk Management Approach document, additional information submitted during the public comment period or obtained from other sources will be considered, along with the information presented in this document, in the instrument(s) selection and development processFootnote 5. The risk management actions outlined in this document may evolve through consideration of assessments and risk management actions published for other CMP substances to ensure effective, coordinated, and consistent risk management decision-making.
3.1 Proposed human health objective
Proposed human health objectives are quantitative or qualitative statements of what should be achieved to address human health concerns.
The proposed human health objective for benzophenone is to reduce exposure of the general population to benzophenone to levels that are protective of human health.
3.2 Proposed risk management objectives
Proposed risk management objectives set quantitative or qualitative targets to be achieved by the implementation of risk management regulations, instruments and/or tools for a given substance or group of substances.
The proposed risk management objectives for benzophenone are:
- to help reduce exposure to benzophenone contained in certain cosmetic products to levels that are protective of human health; and
- to help reduce exposure to benzophenone contained in certain exterior and interior paint, stain and/or coating products that are available to consumers to levels that are protective of human health.
To achieve the proposed risk management objectives and to work towards achieving the proposed human health objective, the proposed risk management actions for benzophenone will focus on these sources of greatest concern.
3.3 Proposed risk management actions under consideration
3.3.1 Cosmetic products
The Government of Canada will consider measures to reduce exposures to benzophenone from certain cosmetics by describing benzophenone as a prohibited or restricted ingredient on the Health Canada Cosmetic Ingredient HotlistFootnote 6.
Natural or non-prescription health products were not identified as a concern in the current assessment. However, to prevent the potential for increased exposure to benzophenone in these products, which may be similar to cosmetics, Health Canada will consider aligning the requirements of the Natural Health Products Ingredients Database (NHPID) with those described for benzophenone on the Health Canada Cosmetic Ingredient Hotlist.
3.3.2 Paints and coatings
The risk management scope document published with the draft screening assessment indicated that the proposed risk management option being considered was a measure to reduce the concentration of benzophenone to a maximum of 0.1 % (w/w) or 1,000 mg/kg in paint and/or coating products that are available to consumers in Canada.
Currently, paint, stain and coating products available to consumers must meet the labelling and packaging requirements set out in the Consumer Chemicals and Containers Regulations, 2001 (CCCR, 2001), made under the Canada Consumer Product Safety Act (CCPSA). Specifically, they must display labelling in both official languages in the form of hazard symbols, warning statements, instructions and a first-aid statement including a list of hazardous ingredients. Child-resistant packaging is required for products classified as "toxic" under the CCCR, 2001. The requirements set out in the CCCR, 2001 are minimum requirements, and manufacturers and importers are encouraged to add any further information they consider necessary to fully inform users of the hazards of their products as long as it does not disclaim or contradict the required information. For example, product labels may also include additional safety and handling instructions such as the use of personal protective equipment and applying the product under well-ventilated conditions; however, these instructions are not mandatory and may not always be followed by the consumer.
To further reduce exposures, the proposed risk management action being considered for benzophenone at this time is to work with experts and industry stakeholders involved with these products (e.g., manufacturers of the products and related trade organizations) to develop a code of practice under section 55 of CEPA that will meet the risk management objective. The proposed code of practice would be used to recommend a maximum final concentration of 0.1% (w/w) or 1,000 mg/kg benzophenone in certain exterior and interior paint, stain and/or coating products available to consumers. This concentration was selected as an appropriate health-protective limit based on the current exposure assumptions and data in the health effects database. Recommendations for additional labelling may also be included. The selected measure would improve the protection of human health by facilitating a reduction in both dermal and inhalation exposures to the general public from benzophenone during application of paint, stain and coating products available to consumers that contain benzophenone.
The impacted products will be confirmed following further analysis on levels of benzophenone in these products, and in consultation with stakeholders. If it is determined that a code of practice under CEPA cannot sufficiently achieve the risk management objective, regulatory actions under CEPA or the CCPSA may be proposed. Note that if a code of practice is adopted, additional regulatory actions may be considered at a later date, depending on the measured success of the code of practice in achieving the risk management objective.
3.4 Performance measurement and evaluation
Performance measurement evaluates the ongoing effectiveness and relevance of the actions taken to manage risks from toxic substancesFootnote 7. The aim is to determine whether the human health objectives have been met and whether there is a need to revisit the risk management approach for that substance, to ensure that risks are managed effectively over time. To achieve this, the Government of Canada will review the effectiveness of the risk management actions for benzophenone.
The Government of Canada plans to measure the effectiveness of the risk management actions by collecting and analyzing data including data on benzophenone prevalence in cosmetics, as well as compliance of adopting the concentration reductions in the relevant paints, stains and/or coatings products available to consumers in order to measure progress towards meeting the risk management objectives.
The results of performance measurement and evaluation will be used to inform whether further risk management action is warranted and will be made available to Canadians along with recommendations for further action, if applicable.
3.5 Risk management information gaps
In order to make informed decisions on the proposed risk management, implicated stakeholders are invited to provide further information on the following:
- Current quantities (kilograms) and concentrations (percent weight per weight (%w/w)) of benzophenone used in both exterior and/or interior paint, stain and coating products that are available to consumers in Canada;
- Current quantities and product types that would be impacted by the proposed risk management, i.e., the number of exterior and/or interior paint, stain and coating products that are available to a consumer in Canada containing a final benzophenone concentration above 0.1 % w/w;
- Potential alternative substances to benzophenone for the use in exterior and/or interior paint, stain and coatings that are available to consumers; and,
- Socio-economic and technical impacts and benefits associated with the proposed risk management for benzophenone.
Should stakeholders have further information to help address these gaps, they should provide it ideally on or before March 31,2021 to inform the risk management decision-making process, within the timelines (and to the contact) identified in section 8 of this document.
4. Background
4.1 General information on benzophenone
Benzophenone is an organic substance (aromatic ketone), which occurs naturally in the environment and is also synthetically manufactured (Canada 2021). It was evaluated by Health Canada and Environment and Climate Change Canada as part of the CMP.
4.2 Current uses and identified sectors
Responses to a 2008 survey conducted under section 71 of CEPA indicated that less than 1,000 kg of benzophenone were manufactured in Canada, and 35,000 to 135,000 kg of benzophenone were imported into Canada (Canada 2009).
According to notifications submitted under the Cosmetic Regulations to Health Canada, benzophenone is present in certain cosmetic products such as nail polishes, fragrances, makeup, and hair products. Benzophenone has been identified as a component in some printing inks used in a limited number of food packaging materials that have no direct contact with food. Benzophenone may also be used as a food flavouring agent. Benzophenone is listed in the Natural Health Products Ingredients Database (NHPID) with a non-medicinal role for oral use as flavour enhancer only, as well as with a tolerable daily intake of 0.03 mg/kg bw/day consistent with the European Food Safety Authority (EFSA 2009, 2017). Although benzophenone is currently listed in the Licensed Natural Health Products Database (LNHPD) as being present in a limited number of currently licensed natural health products (NHPs); based on communications with the associated licence holders, it is rather benzophenone derivatives (i.e., benzophenone-1, benzophenone-2, or benzophenone-3) that are present in these products (LNHPD 2018; NHPID 2019). Benzophenone is permitted and used as a formulant in pest control products (Canada 2021).
Non-confidential uses for benzophenone reported in the 2008 survey include its function as an additive in paints and coatings, as a fragrance ingredient, as a photosensitive substance in inks, as a toner and colourant, as a laboratory substance for medical devices, as an industrial photoinitiator, and in adhesives and sealants (Canada 2009). Based on publically-available safety data sheets, benzophenone may also be present in exterior and interior paints, as well as in stains for decks, deck crack fillers and auto-related cleaning products available to the general population of Canada (Canada 2021).
5. Exposure source and identified risk
General population exposure to benzophenone may occur from indoor air, dust and food. Products available to consumers, including cosmetics, exterior and interior paints as well as stains, are also sources of general population exposure in Canada.
According to the screening assessment of benzophenone, the exposure sources of concern were from the use scenarios of nail polish, exterior and interior paint as well as stain (Canada 2019). Combined dermal and inhalation exposures were estimated to be 0.049–0.091 mg/kg bw/event from the use of nail polish containing 5% benzophenone and 0.068 mg/kg bw/event from the use of interior paint containing 0.3% benzophenone. Dermal exposure was estimated to be 0.067 mg/kg bw/event from the use of exterior paint and stain containing 0.3% benzophenone. Note that inhalation exposure from the use of exterior products was not quantified due to highly variable outdoor conditions.
Benzophenone has been reviewed by the International Agency for Research on Cancer (IARC, 2013) and EFSA (2009, 2017). These reviews provide a basis for the health effects characterization in the draft screening assessment. Based on the available information, a derived benchmark dose for a 10% effect (BMDL10) of 3.1 mg/kg bw/day for non-cancer kidney effects and of 19 mg/kg bw/day for kidney cancer derived by EFSA (2009) from the chronic oral carcinogenicity study in rats were selected as the most appropriate points of departure to characterize risks from chronic daily dermal exposure (Canada 2021).
To characterize risk of benzophenone associated with short-term dermal exposure, the no-observed-adverse-effect level (NOAEL) of 5 mg/kg bw/day for maternal health effects associated with early termination of pregnancy and reductions in maternal body weight from the oral developmental toxicity study was used for adults and teens, and the NOAEL of 20 mg/kg bw/day for non-cancer kidney and liver effects from a 28-day oral toxicity study was used for children, teens and adults in light of the absence of short-term toxicity investigations. A dermal absorption value of 44% for benzophenone, determined in monkeys under unoccluded conditions, was applied to the dermal estimates for route to route extrapolation from the dermal to oral route (Canada 2021).
Margins of exposure comparing effect levels observed in laboratory animals and combined estimates of dermal and/or inhalation exposures from the use scenarios of nail polish, exterior and interior paint as well as stains are considered inadequate to address uncertainties in the health effects and exposure databases for benzophenone (Canada 2021).
The calculated margins of exposure for all other scenarios assessed, including from indoor air, dust, food, plastic uses and from other products available to consumers, were considered adequate to address uncertainties in the health effects and exposure databases (Canada 2021). Therefore, risk management for these scenarios is not being proposed at this time.
6. Risk management considerations
6.1 Alternatives
Alternative cosmetic products are available that do not use benzophenone. For products that use benzophenone as a UV absorber (i.e., to protect formulations from UV damage), substances are available with a similar function to protect against the effects of UV light (EU CosIng database). However, their feasibility as alternatives for specific cosmetic products such as nail polish is not known.
The paint, coating and solvent industry adopts two stabilization methods to protect products from light damage. The first method is using competitive UV absorbers, such as benzophenone, to protect in the wavelength range 290-350 nm and the second method is using radical scavengers to trap radicals formed during polymer degradation (Freitag and Stoye, 2008). There are substances with similar functions to benzophenone that may be used to protect products from light damage; however, their feasibility as alternatives is currently unknown.
Benzophenone also provides certain performance properties such as dirt pick-up resistance for water-based paints and coating products. Based on publically available patent applications, some research is underway to develop alternatives to benzophenone for this particular function due to the general desire in the industry to move towards no- or low-VOC formulations (Valspar, 2014; Hibben et al. 2017; 2019).
6.2 Socio-economic and technical considerations
Socio-economic factors will be considered in the selection process for regulation and/or instrument respecting preventive or control actions, and in the development of the risk management objective(s) as per the guidance provided in the Treasury Board document Assessing, Selecting, and Implementing Instruments for Government Action (Treasury Board of Canada Secretariat TBS, 2007). In addition, socio-economic factors will also be considered in the development of regulation(s), instrument(s) and/or tool(s) as to address risk management objective(s) as identified in the Cabinet Directive on Regulation (TBS 2018), Red Tape Reduction Action Plan (TBS 2012) and the Red Tape Reduction Act (Canada 2015).
7. Overview of existing risk management
7.1 Related Canadian risk management context
Food and Drugs Act (F&DA)
Food
The safety of chemicals used in food packaging materials is subject to the provisions of section 4(1)(a) of the F&DA and Division 23 of the Food and Drug Regulations. Benzophenone is not currently included on Health Canada’s Lists of Permitted Food Additives; therefore, it is not an approved food additive in foods sold in Canada. The safety of chemicals used as food flavouring agents is subject the provisions of section 4(1)(a) of the F&DA.
Cosmetics
Benzophenone is present in cosmetics based on notifications submitted under the Cosmetic Regulations; it is not currently included on Health Canada’s Cosmetic Ingredient Hotlist.
Natural Health Products (NHPs)
NHPs are regulated under the Natural Health Products Regulations. Oral use of benzophenone as a non-medicinal ingredient for the purpose of flavour enhancer in NHPs is permitted in Canada, with a tolerable daily intake of up to 0.03 mg/kg bw/day. Benzophenone, as opposed to some of its derivatives, is not listed as a medicinal ingredient in Health Canada’s Sunscreen Monograph (Canada 2013).
Medical devices
Any potential use of benzophenone as a component of medical devices is subject to biocompatibility testing as part of the assessment of device safety and effectiveness as per the Medical Devices Regulations of the F&DA (Canada 2018a).
Pest Control Products Act (PCPA)
Benzophenone is a permitted formulant in pest control products regulated under the PCPA.
Canada Consumer Product Safety Act (CCPSA)
Final end-use paint, stain and coating products available to consumers must meet labelling and packaging requirements set out in the Consumer Chemicals and Containers Regulations, 2001 (CCCR 2001).
7.2 Pertinent international risk management context
7.2.1 United States
Federal Food, Drug and Cosmetic Act (FD&C Act)
Food
Benzophenone was previously listed as a food additive permitted for direct addition to food for human consumption as a synthetic flavouring substance and adjuvant (21CFR 172.515) and was permitted for use in certain rubber articles that may come in contact with food as part of food packaging or processing (21CFR177.2600; United States Food and Drug Administration [US FDA]). However, in 2018, the US FDA ruled on a food additive petition submitted in 2015 by amending the food additive regulations to no longer authorize the use of benzophenone as a synthetic flavouring substance for use in food or as a plasticizer in rubber articles intended for repeated use in contact with food. While the US FDA’s scientific analysis has determined that benzophenone does not pose a risk to public health under the conditions of its intended use, the substance is being removed from the food additive regulation under the Delaney Clause which requires that the US FDA cannot approve the use of any food additive that has been found to induce cancer in humans or animals at any dose. (US FDA 2018). Benzophenone is not approved for use in printing inks for cardboard or paperboard food packaging materials.
Cosmetics
Benzophenone is not currently included on the US FDA’s List of Prohibited and Restricted Ingredients from use in cosmetics. However, it is currently one of the substances included in a bill submitted to U.S. Congress in 2018 and again in September 2019, proposing that the FD&C Act be amended to include it as a prohibited ingredient for use in personal care products (United States 2019). The timeline for review of this bill is not publically available. Benzophenone is not listed as a permitted active ingredient in sunscreen drug products (as specified in 21CFR 352; US FDA).
Federal Insecticide, Fungicide and Rodenticide Act (FIFRA)
Benzophenone is listed as an inert ingredient on the US EPA’s Fragrance Ingredient List, and is therefore approved for use in pesticide products as a fragrance for non-food use only. As an approved fragrance, it is subject to the requirements under the US EPA’s Pesticide Fragrance Notification Pilot Program (United States 2015).
Toxic Substances Control Act (TSCA)
Benzophenone is listed on the Toxic Substances Control Act Inventory with a regulatory flag “T”, indicating that it is a substance subject to testing requirements for high production volume chemicals (as specified in 40CFR 799; US EPA).
Other
There are no specific restrictions for benzophenone in paint and coatings in the United States; however, consumer paint and coating products are subject to the applicable labelling and information requirements under the Fair Packaging and Labeling Act (FPLA), the Federal Hazardous Substances Act (FHSA), and the Consumer Product Safety Act (CPSA).
The California Environmental Protection Agency listed benzophenone as ‘Known to the State to Cause Cancer’ (Proposition 65 List) in 2012, based on the International Agency for Research on Cancer (IARC) classification. IARC concluded that “there is sufficient evidence in experimental animals for carcinogenicity of benzophenone” based on chronic oral studies in rats and mice and classified the substance as “possibly carcinogenic to humans” (Group 2B); however, there were no relevant studies identified for humans.
7.2.2 European Union
Food Flavouring Substances and Food Contact Materials Regulations
Benzophenone is listed as a permitted flavouring substance used or intended for use in or on foodstuffs as specified in Regulation (EU) No. 872/2012 (European Union 2012). It is also authorized to be used as an additive or polymer production aid in food contact materials with a specific migration limit of 0.6 mg/kg food as specified in Regulation (EU) No. 10/2011 (European Union 2011).
Cosmetic Products Regulations
Benzophenone is currently permitted for use in cosmetics as a UV absorber (i.e., protects the cosmetic product from the effects of UV light) as specified in the European Commission (EC) Decision 2006/257/EC Annex 1 (EC 2006). However, a proposed harmonised classification of “Carc. 2 - suspected of causing cancer” is under evaluation based on Regulation (EC) No 1272/2008 (ECHA 2019). If adopted, benzophenone use in cosmetics would likely be prohibited under the Cosmetics Regulation (EC) 1223/2009. Further, benzophenone is included in the European Commission’s data call-in for substances used in cosmetic products with potential endocrine-disrupting properties to prepare for scientific opinions to the scientific committee on consumer safety (EC 2019). In the European Union, sunscreen products are regulated as cosmetics according to Regulation (EC) 1223/2009 (European Union 2009). Benzophenone is not included in Annex VI List of UV Filters Allowed in Cosmetic Products; therefore, it is not permitted as an active ingredient in sunscreens.
Other
There are no specific restrictions for benzophenone in paints and coatings in the EU; however, consumer products are subject to general safety requirements as per the General Product Safety Directive (2001/95/EC).
7.2.3 Other jurisdictions
Benzophenone is not included on Australia’s list of sunscreening agents permitted as active ingredients in listed products (Australia 2016).
8. Next steps
8.1 Public comment period
Industry and other interested stakeholders are invited to submit comments on the content of this Risk Management Approach document or other information that would help to inform decision-making (such as outlined in section 3.2). Please submit additional information and comments prior to March 31, 2021.
Comments and information submissions on the Risk Management Approach document should be submitted to the address provided below:
Environment and Climate Change Canada
Chemicals Management Division
Gatineau Quebec K1A 0H3
Tel: 1-800-567-1999 | 819-938-3232
Fax: 819-938-5212
Email: substances@ec.gc.ca
Companies who have a business interest in benzophenone are encouraged to identify themselves as stakeholders. Stakeholders will be informed of future decisions regarding benzophenone and may be contacted for further information.
Following the public comment period on the Risk Management Approach document, the Government of Canada will initiate the development of the specific risk management instrument(s), where necessary. Comments received on the Risk Management Approach document will be taken into consideration in the selection or development of these instrument(s). Consultation will also take place as instrument(s) are developed.
8.2 Timing of actions
Electronic consultation on the Risk Management Approach: January 30, 2021 to March 31, 2021.
Publication of responses to public comments on the Risk Management Approach document: On or before March 31, 2021.
Publication of the proposed instrument(s): At the latest, 24-month from the date on which the Ministers recommended that benzophenone be added to Schedule 1 of CEPA.
Consultation on the proposed instrument(s): 60-day public comment period starting upon publication of each proposed instruments.
Publication of the final instrument(s): At the latest, 18-month from the publication of each proposed instruments.
9. References
Australia. 2016. Australian regulatory guidelines for sunscreens (ARGS). Department of Health, Therapeutic Goods Administration.
California EPA, 2016. Chemicals Known to the State to Cause Cancer or Reproductive Toxicity under the Safe Drinking Water and Toxic Enforcement Act of 1986. October 21, 2016 Proposition 65 List.
Canada. 2009. DSL Inventory Update data collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain inanimate substances (chemicals) on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
Canada. 2013. Dept. of Health. Sunscreen Monograph.
Canada. 2015. Red Tape Reduction Act.
Canada. (2016). List of Substances in the next phase of Chemicals Management Plan. Retrieved 04 2017, from Environment and Climate Change Canada.
Canada. 2018a. Dept. of the Environment, Dept. of Health. Draft Screening Assessment for methanone, diphenyl- (benzophenone).
Canada. 2018b. Dept. of the Environment, Dept. of Health. Risk Management Scope for methanone, diphenyl- (benzophenone).
Canada. 2021. Dept. of the Environment, Dept. of Health. Screening Assessment for methanone, diphenyl- (benzophenone).
[CDAT] Chemical Data Access Tool. [modified 2014 Jun]. Non-confidential 2012 Chemical Data Reporting Information: search results for CAS RN 119-61-9. Washington (DC): US Environmental Protection Agency. [accessed 2017 Feb 23].
[EC] European Commission, 2006. COMMISSION DECISION of 9 February 2006 amending Decision 96/335/EC establishing an inventory and a common nomenclature of ingredients employed in cosmetic products (2006/257/EC).
[EC] European Commission, 2019. Call for data on ingredients with potential endocrine-disrupting properties used in cosmetic products [accessed 2019 August 12].
[ECHA] European Chemicals Agency. 2019. CLH Report Proposal for Harmonised Classification and Labelling for Benzophenone CAS number 119-61-9 [accessed 2019 August 12].
[EU] European Union. 2009. Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products. Off J Eur Union L. 342:59-209.
[EU] European Union, 2011. COMMISSION REGULATION (EU) No 10/2011 of 14 January 2011 on plastic materials and articles intended to come into contact with food.
[EU] European Union, 2012. COMMISSION IMPLEMENTING REGULATION (EU) No 872/2012 of 1 October 2012 adopting the list of flavouring substances provided for by Regulation (EC) No 2232/96 of the European Parliament and of the Council, introducing it in Annex I to Regulation (EC) No 1334/2008 of the European Parliament and of the Council and repealing Commission Regulation (EC) No 1565/2000 and Commission Decision 1999/217/EC.
[EFSA] European Food Safety Authority. 2008. Scientific Opinion of the Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food on a request from Commission on Flavouring Group Evaluation 69, (FGE.69) Aromatic substituted secondary alcohols, ketones and related esters. The EFSA Journal. 869, 1-35. [accessed 2017 Apr 4].
[EFSA] European Food Safety Authority. 2009. Toxicological evaluation of benzophenone. Scientific opinion of the panel on food contact materials, enzymes, flavourings and processing aids (CEF). Question N° EFSA-Q-2009–411. Adopted on 14 May 2009. The EFSA Journal. 1104: 1–30. [accessed 2016 Sept 8].
[EFSA] European Food Safety Authority. 2017. Update on the date of delivery of the opinion on flavouring substances benzophenone [FL-no: 07.032] and ethyl acrylate [FL-no: 09.037] from FGE.69 and FGE.71, respectively. Question N° EFSA-Q-2016-00425. Letter dated 6 March 2017 [accessed on EFSA Register of Questions 4 April 2017].
[EFSA] European Food Safety Authority. 2017. EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids), Silano V, Bolognesi C, Castle L, Chipman K, Cravedi J-P, Engel K-H, Fowler P, Franz R, Grob K, Gürtler R, Husøy T, Kärenlampi S, Milana MR, Pfaff K, Riviere G, Srinivasan J, Tavares Poças MF, Tlustos C, Wölfle D, Zorn H, Benigni R, Binderup M-L, Brimer L, Marcon F, Marzin D, Mosesso P, Mulder G, Oskarsson A, Svendsen C, Anastassiadou M, Carfì M, Saarma S and Mennes W, 2017. Scientific Opinion on safety of benzophenone to be used as flavouring. EFSA Journal 15(11):5013, 33 pp. [accessed 2017 December 6]. https://doi.org/10.2903/j.efsa.2017.5013
Freitag, W., and Stoye, D. 2008. Paints, Coatings and Solvents. John Wiley & Sons. Chapter 5, page 168.
Hibben et al. 2017. Dirt Pick Up-Resistant Composition. United States Patent Application Publication. Pub No.: US 2017/0029654 A1 [accessed 2019 July 23].
Hibben et al. 2019. Dirt Pick Up-Resistant Composition. United States Patent Application Publication. Pub No.: US 10,221,322 B2 [accessed 2019 July 23].
[IARC] International Agency for Research on Cancer. 2013. Benzophenone. IARC Monographs on the evaluation of carcinogenic risks humans 101: 285-304. [accessed 2016 Sept 30].
[LNHPD] Licensed Natural Health Products Database [database]. Ottawa (ON): Health Canada. [accessed Dec 2016].
[NHPID] Natural Health Products Ingredients Database [database]. Ottawa (ON): Health Canada. [accessed Dec 2016].
OECD, 2009. THE 2007 OECD LIST OF HIGH PRODUCTION VOLUME CHEMICALS. ENVIRONMENT DIRECTORATE, Organisation for Economic Co-operation and Development. ENV/JM/MONO(2009)40.
[TBS] Treasury Board of Canada Secretariat. 2007. Assessing, Selecting, and Implementing Instruments for Government Action.
[TBS] Treasury Board of Canada Secretariat. 2018. Cabinet Directive on Regulation. Ottawa (ON): Government of Canada.
[TBS] Treasury Board of Canada Secretariat. 2012. Red Tape Reduction Action Plan.
[US EPA] United States Environmental Protection Agency. Substance Registry Services (SRS) Database. 2016.
[US FDA] United States Food and Drug Administration. 2018. Food Additive Regulations; Synthetic Flavoring Agents and Adjuvants. [publication date October 9, 2018; accessed February 20, 2019]
United States. 2015. US Environmental Protection Agency Office of Pesticide Programs. Pesticide Fragrance Notification Pilot Program. Revised December 2015.
Environmental Defense Fund, and James Huff; Filing of Food Additive Petition. Federal Register Vol. 81, No. 1, January 4, 2016.
United States, 2016. Removal of Certain Inert Ingredients From the Approved Chemical Substance List for Pesticide Products. Federal Register Vol 81, No 240. December 14, 2016.
United States, e-CFR Title 40 Part 799 – Identification of specific chemical substance and mixture testing requirements.
United States, 2019. H.R. 4296 — 116th Congress: To amend title VI of the Federal Food, Drug, and Cosmetic Act to ensure the safe use of cosmetics, and for other purposes. 2019. [accessed September 17, 2019]
Valspar Sourcing, Inc. 2014. Water-based compositions that resist dirt pick-up. §371 U.S. National Stage of International Application No. PCT/US/2014/020719, filed Mar. 5, 2014 [accessed July 23, 2019].
[WHO] World Health Organization. 2002. Evaluation of certain food additives and contaminants: Aromatic substituted secondary alcohols, ketones, and related esters. Geneva (CH): World Health Organization, International Programme on Chemical Safety. (WHO Food Additives Series 48).
Annex A. Substance targeted for risk management
| CAS RN | DSL name(common name; acronyms; other names) | Chemical structure and molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
| 119-61-9 | Diphenylmethanone (Benzophenone; benzophenone; Ph2CO; BZPh; Diphenyl ketone) | 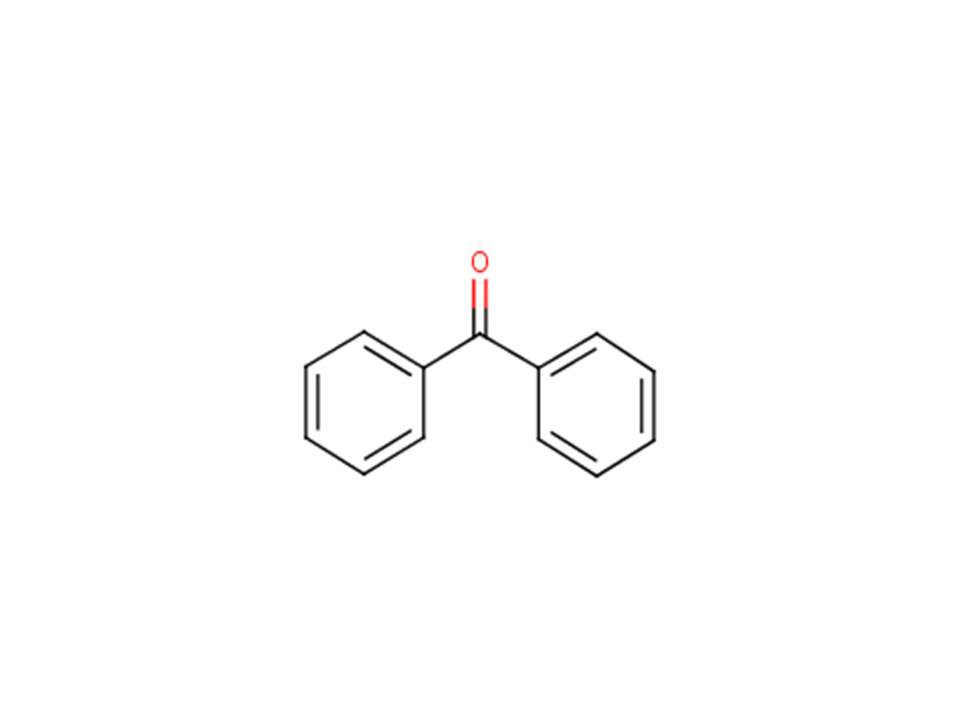 C13H10O C13H10O | 182.22 |
