Science Approach Document:
Ecological Risk Classification of Organic Substances
Environment and Climate Change Canada
July 2016
Table of Contents
- Synopsis
- 1. Introduction
- 2. Basis of Approach to Ecological Risk Classification of Organic Substances
- 3. Data Collection and Generation
- 4. Profiling
- 5. Preliminary Classification
- 6. Examination and Adjustment of Preliminary Classification
- 7. Risk Classification Matrix
- 8. Ecological Risk Classification Results
- 9. Assessment of Risk Classification Uncertainty
- 10. Conclusion
- References
- Appendicies
- Appendix A. Summary of local exposure screening scenarios
- Appendix B. Substances classified as higher potential of risk to the environment
- Appendix C. Substances classified as moderate potential risk to the environment
- Appendix D: Substances classified as posing a lower relative risk to the environment
List of Figures
List of Tables
Synopsis
Environment and Climate Change Canada (ECCC) has characterized organic substances from the third phase of the Chemicals Management Plan (CMP) for their potential to cause ecological harm. The ecological risk classification of organic substances (ERC) ranked 640 substances into three levels of concern based on their relative anticipated potential to pose a risk to the environment. These 640 substances had met the categorization criteria under subsection 73(1) of the Canadian Environmental Protection Act, 1999 (CEPA), or were considered a priority based on other human health or ecological concerns.
This Science Approach Document presents the ERC approach and the results of its application to 640 organic substances. A period of consultation on the Science Approach Document is being provided to the public who will have an opportunity to comment and provide additional information in advance of this information being applied in Screening Assessments. This publication of the scientific approach and results in a Science Approach Document will assist the government in addressing substances that may be of low concern to either human health or the environment in a more effective manner and identifies substances of relatively higher concern that will require more detailed evaluation.
The ERC involved the use of empirical and modelled data to classify substances as warranting further evaluation of their potential to cause harm to the environment or as having a low likelihood of causing ecological harm. The ERC was applied using data collected during categorization, through the Domestic Substances List (DSL) Inventory Updates carried out under authority of section 71 of CEPA and through other recent data-gathering activities. The ERC describes the hazard or potency of a substance using key parameters, including mode of action, chemical reactivity, internal toxicity thresholds, bioavailability, and chemical activity and bioactivity. The possible exposure of organisms in the aquatic and terrestrial environments is characterized based on factors including potential emission rates, overall persistence and long-range transport potential in air. Hazard and exposure profiles were developed for individual substances based on multiple metrics. Use of a weight-of-evidence approach based on multiple lines of evidence to classify hazard, exposure, and risk reduces the overall uncertainty associated with the classification outcomes. Additional rules were applied (e.g. classification consistency, margin of exposure) to refine the preliminary classification of hazard or exposure.
A risk matrix was used to assign a low, moderate or high classification of potential concern for each substance based on its hazard and exposure classifications. Organic substances classified as having higher potential risk concern were generally those characterized as being more potent and having a greater potential for widespread continuous exposure. Substances classified as having low potential risk concern generally had short residence times in the environment, do not undergo long-range transport and are expected to only demonstrate baseline toxicity.
Initial ERC outcomes of potential risk concern were adjusted using a two-step approach. The first step decreased the risk classification outcomes for substances which had a low regional rate of emission to water after wastewater treatment, representing a low potential for exposure. The second step reviewed low classification outcomes using relatively conservative, local-scale (i.e., in the area immediately surrounding a point-source of discharge) risk scenarios designed to be protective of the environment; substances for which a potential local risk was identified were reclassified to a higher level.
Based on inherent hazard properties and current use patterns and quantities in commerce, 40 substances were classified as being of high potential ecological concern, 92 substances were classified as being of moderate ecological concern, and 508 substances were classified as being of low ecological concern. Substances classified as high ecological concerns will undergo further ecological assessment. Some of the substances of moderate ecological concern (58 of 92 substances) have similarities to substances that were classified as having a high potential for ecological concern and will therefore also undergo further assessment as part of those groups. The remainder of the substances of moderate ecological concern and of low ecological concern (542 substances in total) are not expected to pose an ecological risk based on current information, and further assessment work is not required at this time. The approach and results for these 542 substances will form the basis, in conjunction with any other relevant information that becomes available after the publication of this Science Approach Document, for the conclusions in Screening Assessment Reports that will be published at a later time. Substances which were classified as low or moderate concern primarily on the basis of current low exposures may be subject to follow-up or tracking of use pattern information to inform future priority-setting.
1. Introduction
Following categorization of substances on the Domestic Substances List (DSL), which was completed in 2006, approximately 4300 of the 23 000 substances on the DSL were identified for additional assessment activity. Among the remaining substances are 640 organic substances remaining to be addressed under the Chemicals Management Program (CMP). The 640 substances met the categorization criteria for persistence or bioaccumulation and inherent toxicity to human or non-human organisms, or for greatest potential for exposure to humans under subsection 73(1) of Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999), or were identified as having health effects of concern based on classifications by other national or international agencies for carcinogenicity, genotoxicity, developmental toxicity or reproductive toxicity, or as having other ecological concerns. The 640 substances, which include 448 discrete organic substances and 192 organic substances of Unknown or Variable Composition, Complex Reaction Products and Biological Materials (UVCBs), were evaluated through the ecological risk classification of organic substances (ERC). The approach described in this report was not applied broadly to petroleum substances, polymers or inorganic substances, but in those few cases where such substances were considered, these substances may additionally be addressed in other activities (e.g., an organometallic substance considered in the ERC may also be addressed in an assessment of the metal moiety).
The ERC was applied to 640 organic substances using data collected during DSL categorization, through the DSL Inventory Updates and from other sources. The approach involved the use of empirical and modelled data to identify substances warranting more detailed evaluation of their potential to cause harm to the environment, and those expected to have a low likelihood of causing ecological harm.
The purpose of this document is to provide stakeholders and the public with the opportunity to review and comment on the ERC approach and the results of its application. It is also an opportunity to provide additional information to inform revisions to the use of the approach prior to these results forming the basis, in conjunction with any other relevant information that becomes available after the publication of the Science Approach Document, before proposing conclusions through the publication of screening assessments under section 68 or 74 of CEPA. The publication of the scientific approach and results in the Science Approach Document will assist the government in addressing substances that may be of low concern to either human health or the environment in a more effective manner and identifies substances of relatively higher concern that require more detailed evaluation.
The ERC approach includes consideration of information on chemical properties, environmental fate, hazards, uses and exposure. Most of the substances had data on reported commercial quantities received through submissions of information in response to notices under section 71 of CEPA regarding commercial activity in Canada (DSL Inventory Update). Empirical data (where available) as well as results from models were used to inform substance-specific decisions.
This document does not represent an exhaustive or critical review of all available data, but provides a summary of the approach and the results obtained. For the substances identified as having a low and in some cases moderate likelihood of causing harmful ecological effects, results are intended to form the basis for the ecological portion of screening assessments that will be published subsequently, in conjunction with the assessment of potential human health risks. The basis of the classification pertaining to some of the substances in ERC may be subsequently updated and new data considered as part of future assessments.
This document was prepared by staff in the CEPA Risk Assessment Program at Environment and Climate Change Canada (ECCC). The document has undergone external written peer review and/or consultation. Comments on the technical portions of the document were received from Dr. Jon Arnot (ARC Arnot Research and Consulting) and Mr. Geoff Granville (GCGranville Consulting Corp.). While external comments were taken into consideration, the final content and outcome of the report remain the responsibility of Environment and Climate Change Canada.
2. Basis of Approach to Ecological Risk Classification of Organic Substances
The ERC is a risk-based approach that employs multiple metrics for both hazard (potency) and exposure based on weighted consideration of multiple lines of evidence determining risk classification. Unlike categorization of the DSL, where hazard profiles were typically based on one modelled or empirical 96-h median lethal effects endpoint (e.g. LC50) for a daphid or fish, hazard profiles are established using various approaches such as consideration of mode of toxic action, reactivity and food web-derived internal toxicity thresholds. Exposure profiles are also composed of multiple metrics including overall persistence, emission rate and long-range transport. The various lines of evidence are combined to identify substances of higher potency and increased potential for exposure in various media. This approach reduces the overall uncertainty with risk classification compared to one that relies on a single metric in a single medium (e.g., LC50) for classification.
The ERC is illustrated in Figure 1. Empirical data were collected and model data were generated for 640 organic substances (section 3) to create hazard and exposure profiles (sections 4.1 and 4.2, respectively). In parallel, structural or functional substance groups (see appendices) were assigned to maximize the effectiveness and efficiencies in the risk classification processes. Substance-specific profiles were then compared to decision criteria for preliminary classification of hazard and exposure (sections 5.1 and 5.2, respectively). If there were insufficient data, or if a UVCB substance could not be suitably represented by a single chemical structure, a manual expert judgement-based approach (section 5.3) to classification was used. The preliminary classifications of hazard and exposure were examined and were adjusted, as required, according to specific rules and use of judgement (section 6). A risk matrix was then used to classify levels of expected concern according to risk (section 7). Substances were further adjusted to minimize the potential for both over- and underestimation of risk classification results (section 7.1). This included a review of the substances initially identified as having a low potential of ecological harm using relatively conservative, local-scale (i.e., in the area immediately surrounding a point-source of discharge) exposure estimation. Final risk classifications (low, moderate, and high potential for ecological harm) and identification of potential need for tracking of future use patterns were then determined for each of the 640 organic substances (section 8).
Critical data and considerations used to create substance-specific profiles and classifications associated with hazard, exposure and risk, as well as identification of potential need for tracking of future use patterns, are presented in ECCC 2016a.
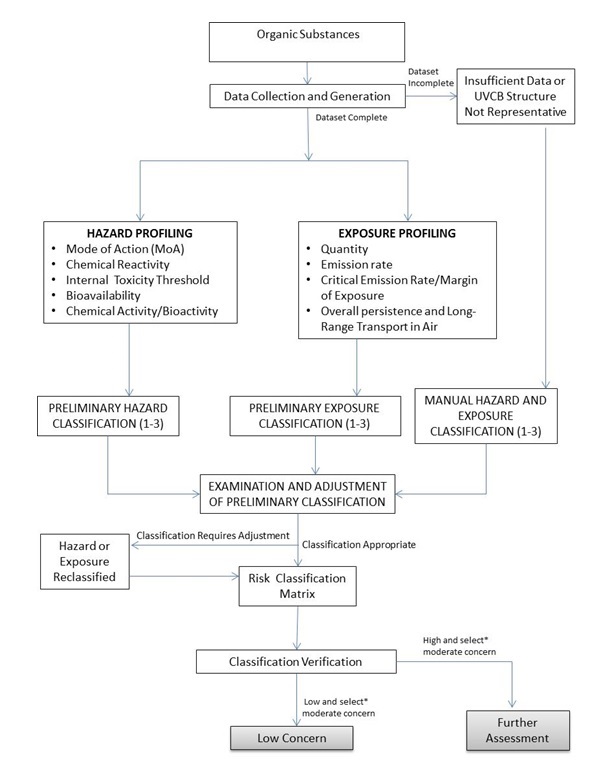
Long description for figure
Figure 1: Overview of the Ecological Risk Classification of organic substances. The figure illustrates the data collection and decision steps which occur throughout the process. In the first step data is collected and generated for each of the organic substances. If sufficient data is available the substance moves on to the hazard and exposure profiling step. For those substances with an incomplete dataset, or where no representative structure for a UVCB substance is available, the profiling step is skipped and the hazard and exposure classifications are manually generated.
The hazard of a substance is profiled using the following hazard metrics: mode of action, chemical reactivity, internal toxicity threshold, bioavailbility, and chemical and biological activity of the substance. The exposure of a substance is profiled using the following exposure metrics: quantity, emission rate, critical emission rate/margins of exposure, overall persistence and long-range transport potential in air of the substance. A numerical classification is assigned for the hazard and exposure of each substance using scores of one to three to represent lower, moderate, and higher level of hazard or exposure potential. The preliminary classifications of hazard and exposure are examined and adjusted, as required, according to specific rules and use of judgement.
A risk matrix is used to classify levels of expected concern based on hazard and exposure classifications. Substances were further adjusted to minimize the potential for both over and underestimation of risk classification results. Final risk classifications (low ecological concern or more detailed assessment required) were then determined for each of the organic substances.
* Some moderate concern substances will be further assessed because a similar substance has been classified as a high ecological concern.
3. Data Collection and Generation
Physical-chemical properties, fate (e.g., chemical half-lives in various media and biota, partition coefficients, and fish bioconcentration), acute fish ecotoxicity, and chemical import and manufacture volumes in Canada were collected from recent section 71 surveys (Canada 2009, Canada 2012); from available scientific literature or empirical databases (e.g., OECD QSAR Toolbox), or were generated using selected Quantitative Structure-Activity Relationship (QSAR) or mass-balance and bioaccumulation models. These data were required as inputs to other mass-balance models or to complete the substance profiles. For UVCB substances, a representative chemical structure was chosen to represent the substance. In many cases, a conservative representative chemical structure was chosen to represent an entire UVCB substance (e.g., where variation of the UVCB components was predictable). Individual structures deemed inappropriate for modeling (some UVCB representative structures or discrete organics such as organometallics) did not use modeling. A manual ranking of hazard and exposure classification was required in these cases. Ionogenic organic chemicals (IOCs) comprise about 20% of the 640 organics. Very few measured data are available for IOCs and, hence, assessment uncertainty may be greater for these substances than for the neutral substances. IOCs were identified and modelled using approaches and necessary simplifying assumptions described in Arnot (2014). Substances having complete and appropriate data sets were then subject to chemical profiling involving both empirical and modeling approaches.
Mass-balance modeling involved two fate models. The Risk Assessment IDentification And Ranking (RAIDAR) model was used for determining the fate of a substance in representative aquatic and terrestrial environments and food webs based on separate water or soil emissions. RAIDAR combines mass-balance environmental fate and food web bioaccumulation models to estimate the potential for an organic chemical to deliver a toxic internal dose to a target receptor on a steady-state basis in a regional-scale evaluative environment of 100,000 km2 (Arnot et al. 2006; Arnot and Mackay 2008). An updated RAIDAR Ver.2.0 (RAIDAR-IONIC) was applied to better address the fate and bioaccumulation for IOCs (Armitage et al. 2013, Arnot 2011). While the revised RAIDAR model seeks to improve the simulation for IOCs in the environment, it is recognized that significant data gaps exist and, hence, uncertainty in evaluating appreciably dissociated IOCs is generally assumed to be greater than evaluating neutral organic chemicals with the model. A second fate model, SimpleTreat (Struijs et al. 1991), was applied to determine the removal of a substance in a model wastewater treatment system (WWTS) from biodegradation (reaction) as well as adsorption to primary and secondary sludge. Losses to reaction were considered completely removed. Adsorption of substances to sludge was addressed by evaluating terrestrial hazard to account for land-applied biosolids. The quantity of substance lost from wastewater to air due to volatilization was not considered removed from the environment and was subject to subsequent atmospheric fate considerations (e.g., long-range transport).
Chemical profiling using various QSAR models and expert systems was then conducted on all organic substances with acceptable 2D chemical structures represented by an alpha-numeric formula known as the Simplified Molecular-Input Line-Entry System (SMILES). Further details are given under the hazard and exposure profiling sections.
4. Profiling
4.1 Hazard Profiling
Profiling of hazard was conducted to determine the potency of a chemical via five primary metrics: (1) mode of toxic action, (2) chemical reactivity, (3) internal toxicity thresholds (i.e., critical body residues), (4) bioavailability and (5) chemical/biological activity. The aim of hazard profiling is to identify chemicals that are bioavailable and present a high hazard either intrinsically due to their non-baseline potency (toxicodynamics) or extrinsically because of their fate and behaviour in food webs (toxicokinetics). Effect concentrations for chemicals that only exert baseline toxicity (or narcosis) as a mode of action are generally better understood and more predictable in ecological sciences than effect concentrations for chemicals that exert more specific modes of toxic action or reactivity (Mackay et al. 2009). Effect concentrations for chemicals with more specific modes of action (i.e. chemicals that also exert a specific mechanism of toxicity such as acetylcholinesterase inhibition in addition to baseline toxicity) or reactivity (e.g., electrophiles) are less reliably predicted. Aquatic concentrations or tissue residues associated with non-baseline effects can be lower than concentrations or residues associated with baseline toxicity. This difference in toxicity is sometimes referred to as "excess toxicity" or the "toxic ratio" (e.g., Maeder et al. 2004, Arnot 2014). Discriminating between baseline behaviour and non-baseline behaviour is thus useful for classifying hazardous substances simply because non-baseline substances are more potent than chemicals that only exert a toxic baseline toxicity. Hence, exposures required to exert a toxic effect can be lower for chemicals that exert additional, non-baseline modes of toxic action. A similar concept is advocated by the OECD (2014) and others as a strategy for grouping chemicals of common reactivity (e.g., Wu et al. 2010, Mackay et al. 2014a). The profiling approach also minimizes the chance of underestimating hazard classification from limited ecotoxicity data based on a single medium of exposure (e.g., water) because internal concentrations (body or tissue residues) are also considered and hence exposures and potential risks of chemicals that exhibit bioaccumulation or biomagnification in food webs (higher internal concentrations at higher trophic levels) are more appropriately evaluated.
4.1.1 Mode of toxic action
Just over half of the substances on the DSL have been predicted, using quantum chemical descriptors, to have a baseline, narcotic mode of toxic action (MoA), while approximately one third were predicted to have a specific MoA, with the remaining portion having an undefined MoA (Dimitrov et al. 2003). In the ERC, MoA was profiled using more than one approach and utilizing more recent advances to determining MoA than those described in 2003 work by Dimitrov and co-authors. Structural profiling using two rule-based systems contained in the OECD Toolbox (v3.3) was first applied. The Verharr profiler, which is based on the ToxTree software (Verharr et al. 1992, Verharr 2000, Enoch 2008) and OASIS MoA profiler developed by the Laboratory of Mathematical Chemistry (e.g., Dimitrov et al. 2003) determine MoA bins based on pass/fail structural rules. Mode of action was also determined using tissue residue toxicity ratios. Toxicity ratio refers to the difference in concentration between a baseline toxicant and a chemical exerting a more specific MoA (e.g., Maeder et al. 2004, Arnot 2014). A median critical body residue (CBR) associated with acute lethality of 3.0 mmol/kg is used for the acute threshold and 0.3 mmol/kg is used as a chronic threshold for baseline narcosis (McCarty and Mackay 1993, Esher et al. 2011, McCarty et al. 2013). Thus, if 3.0 mmol/kg divided by the acute CBRLC50 is greater than 10, a chemical is expected to be more potent than a narcotic chemical because the concentration in the tissue necessary to cause death is lower. Since MoA is determined using toxicity ratios based on fish bioconcentration factors (BCF), the toxicity data, and thus extrapolation to other species, remains uncertain. Additional structure-based metrics are combined with toxicity ratios to help increase the certainty of MoA across species (see section 5). Similar MoA considerations have been used to propose ecological Thresholds of Toxicological Concern (eco-TTC) for various modes of action. Eco-TTCs have been advocated as a useful approach to screening and prioritization of chemicals (de Wolf et al. 2005, Williams et al. 2011, Belanger et al. 2015).
Chemical activities (Mackay et al. 2009, Mackay et al. 2014a), here defined as the fraction of maximum solubility in water resulting in acute lethality in fish, were also calculated and used as a qualitative check on MoA as well as on the quality of aquatic toxicity data; i.e., activities greater than 1 indicate toxicity predictions or empirical results above maximum solubility of a chemical in water. A chemical activity of 0.01 to 0.1 has been associated with baseline narcosis (Mackay et al. 2009, Mackay et al. 2014a). Finally, the MoATox database from the USEPA (Baron et al. 2015) was incorporated to provide empirical insight into mode of toxic action, where information was available.
4.1.2 Chemical reactivity
Chemical reactivity is a broad term that refers to the ability of a chemical to undergo changes according to the environmental system in which it resides. Here, we refer to reactivity as a chemical's potential to undergo interactions with biological tissues that are outside the domain of baseline interactions, which are non-specific weak interactions with cell membrane surfaces (Dimitrov et al. 2003). In essence, profiling for chemical reactivity provides a subcellular "mechanism of action" level of investigation since many of these reactions can involve binding and/or disruptions of biologically relevant macromolecules (e.g., protein, DNA, RNA). Profiling of covalent interactions with protein and DNA was conducted using mechanistic profilers in the OECD QSAR Toolbox (v3.3). Protein and DNA binding profilers in the Toolbox developed by the OECD and Laboratory of Mathematical Chemistry (LMC) were applied. These profilers work by applying mechanistic rules governing covalent (electrophilic/nucleophilic substitution or addition) or other interactions with protein or DNA. Greater detail can be found in the meta-information in the OECD QSAR Toolbox for each of the above profilers (OECD, QSAR Toolbox (v3.3)). Protein binding is typically associated with skin sensitization in mammals but has been shown to be indicative of a non-passive mechanism of uptake and distribution in a broad suite of organisms (Princz et al. 2014) as well as having a moderate to strong correlation with aquatic toxicity, albeit with a limited sample size in some cases (Bonnell and Kuseva, unpublished). DNA binding can lead to genetic damage through, for example, adduct formation and is a well-known initiating event for genotoxicity.
To account for receptor-mediated effects, profiling of estrogen and androgen receptor (ER and AR) binding was conducted. The OECD QSAR Toolbox structural rule-based ER profiler, which is based on the work of Dimitrov et al. (2005), Serafimova et al. (2007) and Mekenyan et al. (2009), was used. These are generally considered to be precautionary models in that binding results do not necessarily reflect adverse outcomes. AR interactions were profiled using the TIMES® software suite (v2.27.16) from the Laboratory of Mathematical Chemistry based on Fang et al. (2003) and Todorov et al. (2011). Only ER binding was used directly to classify hazard in the ERC, because the domain of applicability of AR binding is restricted and many chemicals were identified as being out of the model domain. Thus, in silico AR binding affinities were considered as additional supporting information. However, the ERC also includes the in vitro assay Endocrine Disruption Knowledge Base (EDKB) from the USFDA (Ding et al. 2010). Similar to the MOATox from the USEPA, EDKB is used as supporting empirical in vitro binding information for those organic substances contained in its database.
4.1.3 Internal toxicity threshold
The advantages of using a tissue residue approach for ecological risk assessment have long been advocated in the ecological scientific literature beginning with McCarty and Mackay (1993) and more recently by Mackay et al. (2014b). To summarize, it is preferable to compare the relative toxicity of substances using an internal dose metric rather than an external media concentration metric since concentrations external to the organism ignore the toxicokinetics of a substance in the exposed organism. Greater detail on tissue residue approaches, including applications within risk assessments (Sappington et al. 2010), can be found in a series of six 2010 Society of Environmental Toxicology and Chemistry (SETAC) Pellston Workshop papers (Integrated Environmental Assessment and Management 2011, Esher et al. 2011).
Tissue residue thresholds (CBRs) for acute lethality were employed to calculate toxicity ratios as discussed earlier in the section on mode of toxic action. CBRs are also calculated to determine food web Hazard Assessment Factors (HAF) in the RAIDAR model (v2.0). RAIDAR HAFs are estimated in aquatic and terrestrial food webs based on a default emission rate (1 kg/hr) to each of these media independently. HAFs are numerical values calculated as the ratio between the dose in representative food web organisms based on a unit default emission rate (CU) and the dose associated with acute lethality (CT), which can be indicative of a narcotic (~3 mmol/kg) or non-narcotic mode of action (less than 3 mmol/kg). The HAF can be equated to a combined persistence, bioaccumulation, toxicity metric (Arnot and Mackay 2008) because HAFs integrate unit emission rate-based chemical fate (i.e., persistence), food web bioaccumulation and toxicity (hazard data) into a single value. HAFs are independent of the actual chemical emission rate but span several orders of magnitude for the organic substances characterized. HAFs are used directly in the ERC as a hazard metric. Details on how HAFs are calculated can be found in Arnot and Mackay (2008) and, specifically as it pertains to the substances being addressed in this report, in ARC (2014). All tissue residue-based metrics (i.e., HAFs, toxicity ratios) accounted for biotransformation of a substance in the target receptor.
4.1.4 Bioavailability
Bioavailability relates the quantity of a substance absorbed by the organism compared to the quantity of substance to which the organism is exposed. Currently, no aquatic QSAR model is able to provide reliable estimates of aquatic toxicity for substances with a log Kow above eight primarily due to the lack of observed acute or chronic effects at or above this log Kow. In addition, Kelly et al. (2004), Arnot and Gobas (2006) and Arnot and Quinn (2014) have shown that, for higher log Kow substances, dietary assimilation efficiency, bioconcentration, bioaccumulation and biomagnification factors in non-human organisms decrease above ~log Kow of 8.0. Thus, a simple rule of log Kow or log D (the log dissociation constant is applied to ionisable substances) greater than 10 was used to indicate very low aquatic and terrestrial bioavailability both internal and external to an organism. Log D accounts for the dissociation of ionizable chemicals at pH 7. A log Kow or log D of 10 was used to account for a two order of magnitude error with estimating the log Kow or Log D given that there are currently no acceptable empirical log Kow values greater than 10.
4.1.5 Chemical activity and bioactivity
The thermodynamic equilibrium criterion of chemical activity can be used to help identify substances acting via a baseline MoA as well as more specific MoAs (Mackay et al. 2009). Here we define activity to be the fraction of solubility in water associated with median lethal effects (LC50) in aquatic organisms. An activity in water of 0.01 to 0.1 has been associated with baseline narcosis (Mackay et al. 2014a). Calculated activities below just 0.01 could suggest subtle chronic effects and activities far below 0.01 could suggest a MoA more potent than baseline narcosis. Activities greater than one indicate a potential error with the ecotoxicity data as solubility limits in water have been exceeded. Chemical activity in the ERC was calculated for substances with log Kow greater than 2 (very soluble substances are outside the domain of this approach, as activities would appear unrealistically low) and was used as supporting information for MoA profiling but, more importantly, to verify the quality of ecotoxicity data used in the ERC. Due to the lack of existing empirical data, many LC50 values and most water solubility values had to be estimated using QSARs and, hence, chemical activity estimates can be quite uncertain as well. Therefore, activities were not used to directly classify the potency of organic substances and were applied on a case-by-case basis, depending on reliability as determined using professional judgement.
Bioactivity is a broad definition to mean the effect of a chemical on any living tissue. Bioactivity can be determined using high-throughput in vitro assays such as those developed by the USEPA under the Toxcast and Tox21 programs. ToxCast screens chemicals in over 700 high-throughput assays that cover a range of high-level cell responses and approximately 300 signaling pathwaysFootnote 1. A hit of "activity" in either of these databases for any of the 640 organic substances in the report means that some activity has been observed in one of the many in vitro assays used for bioactivity. This does not represent a definitive adverse outcome, but another type of in vitro level alert (e.g., oxidative stress, mitotic arrest, impaired enzyme functioning). Consequently, the ERC uses both the Toxcast 2014 and Tox21 bioactivity databasesFootnote 2 as lookup information only. Given the difficulty in relating bioactivity to adverse outcomes, bioactivity is considered as supporting information to help determine overall reactivity of a chemical but is not used directly to classify hazard.
Results of the hazard profiling are compared to the decision criteria used to determine hazard classification (see Figure 1) as described later in this document.
4.2 Exposure Profiling
An exposure profile was created for all substances based on selected metrics. The purpose of the exposure profile is to determine the probability of ecological receptors coming into contact with an organic substance released to the aquatic or terrestrial environment in Canada. Similar to the hazard profile, multiple metrics are used to weigh this probability, and are discussed below. A weighted approach to exposure profiling was used to address the uncertainty associated with reliance on a single quantitative estimation of chemical release to define exposure to organisms. This helps to mitigate the possibility of over or underestimation of risk classification from relying on a single metric (Stahl and Cimorelli 2013).
4.2.1 Quantity
Data on quantity of a substance in commerce (kg/yr) were gathered for all 640 organic substances. Quantity data consist of chemical import and/or manufacture volume in Canada from recent section 71 surveys (Canada 2009, Canada 2012). Quantity data for most of the substances came from Phase 2 of the DSL Inventory Update (Canada 2012). In general, higher chemical quantities can be related to a higher probability of widespread exposure upon release to the environment.
4.2.2 Emission rate
Emission rates (kg/yr) to the aquatic environment were calculated based on Phase 2 DSL Inventory Update (Canada 2012) volumes described above after determining percentage removal in a modelled waste water treatment system (WWTS). Removal in WWTS, as a function of biodegradation and adsorption to biosolids, was estimated using the SimpleTreat model (Struijs et al. 1991). WWTS removal was used to determine the emission rate to water after treatment as well as the fraction of chemical quantity that could be applied to agricultural lands in association with biosolids. The ERC conservatively assumes that 100% of the chemical quantity reported to be in commerce can be released to a WWTS, without consideration of the fraction actually released as a function of a substance's use pattern (which would typically be considerably less than 100%).
4.2.3 Critical emission rate and margin of exposure
Estimated rate of emission to water after WWTS removal was compared to the critical emission rate to water generated by the RAIDAR model. The critical emission rate is the rate of emission to water (kg/yr) that could result in a risk (internal body burden) to the most sensitive aquatic receptor identified in the RAIDAR model (including various representative species in the food web). The ratio of these two emissions provides a margin of exposure (MoE) and is similar to the concept of an MoE used in human health studies.
4.2.4 Overall persistence and long-range transport in air
Overall persistence (Pov) is the sum of chemical half-lives in all media weighted by the mass fraction of the chemical in the medium as determined using a multimedia fate model (Webster et al. 1998; Klasmeier et al. 2006; Wegmann et al. 2009) and does not consider advection out of a model environment as removal from the environment. Pov has been advocated by many environmental chemists (e.g., Webster et al. 1998, Gouin et al. 2000; Pennington 2001, Mackay et al. 2014b) and the OECD (e.g., OECD 2004) as a preferred metric for screening chemicals for persistence, rather than medium-specific half-lives. Essentially, advection is ‘turned off' as Pov only includes the reaction (degradation) rate of a chemical. Pov was calculated for all substances using the RAIDAR model assuming 100% release of the substance to water, given that water represents the predominant entry point into the environment for industrial chemicals.
The long-range transport potential (LRTP) in air was determined using calculated or observed air half-lives and air-water partition coefficients. Combining these substance-specific properties identifies those substances expected to partition to air and potentially undergo long-range transport. The OECD LRTP and Pov Screening Tool (v2.2) was considered as a possible model to determine long-range transport in air. However, as the model cannot account for air transport as a function of release to water (as is the case with the majority of industrial chemical releases) and given the strong correlation of longer air half-life and higher CTD, air half-life and air-water partition coefficients were used. Use of the air half-life is considered a precautionary approach because the deposition of the substance, which could limit the travel distance in air, is not taken into account. Long-range transport in water was not considered, owing to the difficulties of identifying a suitable evaluative receiving water body for environments across Canada (e.g., the Great Lakes or rivers of varying sizes and depths).
5. Preliminary Classification
Hazard and exposure profiles for each organic substance were compared to decision criteria in order to classify the organic substances. A numerical score was given to the hazard and exposure profile of each substance using scores of one to three to represent lower, moderate, and higher level of hazard or exposure potential. While some of the criteria below overlap in concept (e.g., HAF and toxicity ratio), they use different algorithms and are applied for different purposes (MoA vs food web hazard). They are also applied in a step-wise manner to avoid double counting of metrics. This approach provides a precautionary fail-safe mechanism for classification. Classification was dependent on the number and type of metrics triggered in each profile. The preliminary classifications, particularly low hazard classifications, were subject to further examination in subsequent steps, as described in section 6.
5.1 Preliminary Hazard Classification Criteria
Hazard Class 3 (high hazard) was given to higher potency substances fitting any of the following criteria:
- A positive result for estrogen binding qualified as "very strong or strong binding" (section 4.1.2);
- A toxicity ratio greater than 10 and a mode of action profiled as "reactive unspecified" (section 4.1.1); or
- A RAIDAR aquatic HAFFootnote 3 value greater than 10-3 (range for all substances is 10-11 to ~50), and a log Kow or log D less than 10 (sections 4.1.3 and 4.1.4).
Hazard Class 3 substances are mostly substances that are identified to potentially exert "non-baseline toxicity" or "excess toxicity to baseline", and also include some polar and non-polar narcotics (approximately 17% of Hazard Class 3 substances) with high food web accumulation potential. Hazard Class 3 substances are profiled to be the most reactive and potentially the most potent of the 640 organics.
Hazard Class 2 (moderate hazard) was given to moderate potency substances which fit any of the following criteria:
- A positive result for estrogen binding quantified as moderate binding (section 4.1.2); or
- A RAIDAR aquatic HAFFootnote 4 value between 10-3 and 10-6 and a log Kow or log D less than 10 (sections 4.1.3 and 4.1.4).
Toxicity ratios for these Hazard Class 2 substances are generally less than 10 suggesting a baseline mode of action. No Hazard Class 2 substances have toxicity ratios greater than 10 and an unspecified mode of action. However, many profile as "reactive unspecified" (~38%) suggesting some uncertainty with the MoA. Thus, Hazard Class 2 substances may still have some degree of potency greater than baseline and remain of moderate hazard concern.
Hazard Class 1 (low hazard) was given to lower potency substances if none of the above classification rules were triggered in the hazard profile and consequently meet the following criteria:
- Are profiled to exhibit weak or no binding to estrogen receptors (section 4.1.2);
- Do not have consistent specific mode of action classification and do not have toxicity ratio greater than 10 (section 4.1.1); and
- Have a RAIDAR aquatic HAFFootnote 5 of less than 10-6, or have with a log Kowgreater than 10 (sections 4.1.3 and 4.1.4)
Structural alerts for MoA using the OASIS profiler suggest that ~25% of Hazard Class 1 substances have unknown MoAs. If Verharr MoA results are also considered in parallel with the OASIS profiler, only ~14% of the Hazard Class 1 substances have an unidentified MoA according to structural alerts only. Examination of these chemicals reveals them to be mainly weak acids, ethers, short chain esters, alcohols, amides and ketones; these groups of substances are generally not considered to present significant ecological concern except in exceptional circumstances (such as high acute exposures resulting from spills).
5.2 Preliminary Exposure Classification Criteria
Exposure Class 3 (high exposure potential) was given to substances having the greatest spatial and temporal scale of potential exposure in the environment that fit any of the following criteria:
- A half-life in air greater than 2 days and log Kawgreater than 10-06; or
- A Pov greater than 60 daysFootnote 6 and a substance quantity reported at 100 000 kg/yr or greater.
The substances in Exposure Class 3 are expected to have a longer reaction residence time in the environment (i.e., longer Pov), may undergo long-range transport in air or have been imported or manufactured in higher tonnages in Canada. Therefore, the spatial and temporal scale of potential exposure in the environment for these chemicals is the highest.
Exposure Class 2 (moderate exposure potential) was given to substances having the next greatest spatial and temporal scale of potential exposure that fit either of the following criteria:
- A Pov greater than 60 days and substance quantity reported between 10 000 - 100 000 kg/yr; or
- A Pov between 21 days and 60 days and quantity reported is greater than 100 000 kg/yr.
Classification at this level captures substances with longer reaction residence time (i.e. longer Pov) but lower quantities, or shorter reaction residence time but greater quantities. This class thus does not present a spatial and/or temporal extent of potential exposure as high as an Exposure Class 3 substance. Exposure Class 2 substances are not expected to undergo long-range transport in air.
Exposure Class 1 (low exposure potential) was given to substances having the lowest spatial and temporal scale of exposure that fit any of the following criteria:
- A Pov less than 21 days and a reported quantity less than 100,000 kg/yr; or
- A Pov greater than 60 days and reported quantity is less than 10,000 kg/yr.
Classification at this level captures substances with various combinations of overall persistence and chemical quantity not captured in Exposure Class 2 or 3. Substances in Exposure Class 1 present a low spatial and/or temporal extent of potential exposure and are not expected to undergo long-range transport in air.
The EAF (exposure assessment factor) from the RAIDAR model was considered for use in exposure classification as an alternative to use of the HAF. EAF is an integrated persistence and bioaccumulation property. However, these properties are already integrated into the HAF (along with internal toxicity). Also, analysis comparing the two approaches shows the use of HAF in the classification scheme to be more precautionary.
5.3 Manual Classification
In several instances, a manual classification of hazard and exposure was required due to insufficiency of available data. Primarily this was due to (i) the lack of a two-dimensional structure or lack of a representative structure (e.g., some UVCB biologicals) for the substance, or (ii) the substance was outside of the model domain of applicability, or (iii) lack of empirical data to fulfill other the data requirements for the ERC. The manual approach was mainly applied to the 192 UVCBs. Manual classification involved consideration of read-across empirical data from close analogues. All hazard low-concern UVCBs were closely checked for classification consistency (see section 6) and were still subject to adjustment of risk classification (section 7.1).
6. Examination and Adjustment of Preliminary Classification
Preliminary classification of hazard and exposure as discussed above relies quite heavily on modeled parameters, given the limited empirical data available for most of the organic substances addressed. It is recognized that there is some degree of uncertainty in these model-based classifications, even though empirical evidence has been used where available and multiple metrics for hazard and exposure have been considered. The following additional rules were applied to reduce the potential for both over and under classification of hazard or exposure. Preliminary classification could be adjusted based on any one of the rules outlined below. These rules were applied outside of the preliminary classification step to allow for use of judgement when applying them (such as when a rule is only intended to be applied to a subset of substances) and to give them additional weight. In most cases, the impact of these rules affects preliminary Hazard Class 1 (lower concern) substances and thus is precautionary and minimizes potential underestimation of risk of low hazard substances. In this context, the first four rules below take precedence over the fifth.
- Classification Consistency: Classification results for substance groups were examined more closely in cases where a single CAS RN within the group appeared to be an outlier compared with the other structurally similar members of the group. If there was sufficient rationale to adjust the outlier result (e.g., due to model error), the differing classification was adjusted to be consistent with the group.
- Special Classes: To allow for potential error with current predictive approaches for a potent class of highly ionized substances outside of model domain of applicability, quaternary ammonium compounds were manually classified as, at a minimum, Hazard Class 2. Higher results (i.e., Hazard Class 3) were maintained. Similar considerations were given to nitro musks.
- Potent Reactivity (section 4.1.2): If both protein and DNA binding alerts were triggered, substances were manually classified as, at a minimum, Hazard Class 2.
- Terrestrial Hazard (section 4.1.3): If the RAIDAR soil HAF was greater than 10-4, substances were manually classified as, at a minimum, Hazard Class 2. This rule considers soil hazards from the application of biosolids. This soil HAF threshold was selected in order to equate it to a preliminary Hazard Class 2 triggered by the aquatic HAF criterion.
- Margin of Exposure (section 4.2.3): For baseline substances, if the MoE, as explained in section 4.1, was greater than 10,000, hazard classification was lowered to Hazard Class 1. This rule was applied to the preliminary hazard classification and not the preliminary exposure classification because MoE is calculated using the aquatic HAF and is therefore most sensitive to the HAF (i.e., a substance has lower hazard because it cannot reach critical levels in biota associated with adverse effects).
7. Risk Classification Matrix
Following hazard and exposure classification based on multiple criteria (sections 5.1 to 5.3) and adjustment based on additional judgement rules (section 6), a risk matrix was used to classify level of potential for risk as high, moderate, or low. Table 7.1 lists the possible risk outcomes from combinations of hazard and exposure classifications. Following this step, risk classification outcomes were adjusted to account for possible overestimation of potential risk (section 7.1.1) or underestimation of potential risk (section 7.1.2).
| Hazard Class 1 | Hazard Class 2 | Hazard Class 3 | |
|---|---|---|---|
| Exposure Class 1 | Low | Low | Moderate |
| Exposure Class 2 | Low | Moderate | High |
| Exposure Class 3 | Low | Moderate | High |
Organic substances of higher risk concern are generally characterized as being more potent from a hazard perspective and having a greater potential for widespread continuous exposure. There are relatively few of these substances among the 640 organics. Substances of higher risk concern generally have moderate to high tonnage, have longer reaction residence times in the environment (months or longer), may be transported over long distances in air, and are potentially very potent substances beyond baseline toxicity. Substances in the low risk classification generally have a relatively short predicted reaction residence time in the environment (less than months, often days), do not undergo long range transport in air and are generally baseline substances (e.g., alcohols, esters, acids, alkanes) with lower reactivity. Organic substances classified as being of low risk concern are generally characterized as being less potent from a hazard perspective and having a lower potential for widespread continuous exposure. There are a relatively high proportion of these substances among the 640 organics. Additionally, there are a relatively high proportion of low exposure (Exposure Class 1) substances regardless of hazard classification.
7.1 Adjustment of Risk Classification
Adjustment of risk classification involved examination of all classifications to determine potential for both over and underestimation of risk.
7.1.1 Adjusting for low regional emissions
To address possible overestimation of risk, the risk classification outcome (from section 7) for substances which had a regional rate of emission to water (section 4.2.2) estimated to be less than 1 000 kg/yr after wastewater treatment was adjusted to low risk based on low potential for exposure. The likelihood of risk is very low at this rate of emission to a regional environment (defined as 100,000 km2 in RAIDAR) (Van Leuween and Vermeire 2007). In addition, the aquatic emission rate (assumed to be 100% release in the region over the substance's life-cycle) used in the ERC is very conservative in most cases and, as such, is precautionary. All low concern substances were nonetheless subject to the near-field exposure analysis outlined below.
More than 90% of the low risk substances have reported annual quantities less than 10,000 kg/yr, with the majority having less than 5,000 kg/yr, reported under phase two of the DSL inventory update. This adjustment for low regional emissions affected 9% of the 640 organics substances in the ERC.
7.1.2 Accounting for near-field exposure
Given that exposure and hazard classification is, in part, based on regional scale model results, local field exposures may not be fully accounted for in classification of potential for risk. To account for this situation, an additional near-field risk-based evaluation of all substances classified as low risk was performed to address the higher concentrations that may occur close to the point of discharge of a substance in the aquatic environment. In general, a conservative (precautionary) risk scenario similar to that used in rapid screening assessments (Canada 2013, Canada 2014, Canada 2015) was employed as described below.
The aquatic release scenario for near-field exposure involved applying a generic scenario to estimate local aquatic exposure. While the generic aquatic exposure scenario has been developed to be conservative overall, the level of conservatism applied to individual parameters was selected to be moderate, since it is recognized that:
- a high level of conservatism applied to each parameter can easily compound into an excessively conservative overall exposure scenario;
- it is very unlikely that each parameter would be "worst case" at the same time; and
- interdependence of some parameters exists.
The equation and parameters used in this scenario are given in Appendix A. In brief, the scenario estimates exposure (predicted environmental concentration (PEC)) based on releases from an assumed, representative industrial facility that is manufacturing or using the substance. Based on the use codes and North American Industry Classification System (NAICS) codes provided in the DSL inventory update submissions, a generic emission factor of 2% (low), 25% (medium) or 100% (high) was associated to the notified quantity. In order to do so, all use codes and NAICS were rated for their potential release, based on professional judgement. All undefined codes (U999) were rated manually after reviewing the description provided by the notifier. Assigned emission factors for each of the NAICS and use codes are presented in ECCC, 2016b. Wastewater removal rates were estimated for all the substances of interest based on the physical/chemical properties from categorization and using the SimpleTreat model (Struijs et al. 1991). In situations where it was not possible to calculate a removal rate (e.g. due to lack of data, or for substances falling outside of the domain of applicability of the model), a default value of 0% removal was conservatively used.
A predicted no effect concentration (PNEC) value was derived using data collected or estimated during categorization. Where updated values were judged to be more appropriate, the toxicity values may have been modified from the categorization value using new empirical or predicted (QSAR) acute ecotoxicity values. An application factor of 10 for baseline narcotic substances and 100 for reactive unspecified substances was applied. Risk quotients (RQs) were then determined by comparing PEC with PNEC.
The following considerations were taken into account in this analysis to minimize the potential for overestimating risk classification with this analysis. Specifically, the following checks were applied:
- empirical or modeled inherent aquatic toxicity data available from the DSL Categorization were updated with more recent values (mainly empirical) where applicable;
- chemical activity was applied to ensure activities for ecotoxicity values were less than 1;
- mode of toxic action profile was considered when deriving assessment factors (AFs);
- calculated PECs were not permitted to exceed the maximum solubility in a receiving waterbody; and
- given the conservative design of the local field analysis, risk quotients between 1-10 calculated for only one notifier were deemed uncertain evidence for concern and did not provide sufficient justification for adjustment of the ecological risk classification.
- Available use pattern information for 13 substances with release quantities below 1000kg (see section 7.1.1) and risk quotients above 10 were manually reviewed. This resulted in one substance being adjusted from a low potential to cause ecological harm to moderate potential to cause ecological harm. Additional use pattern information for the other 12 substances is proposed to be collected.
After application of the local scale risk analysis, there is 94% agreement between the local scale RQ approach and the risk classification matrix approach for the low risk substances. The remaining 6% of substances (where risk classification had been underestimated) were reclassified to a moderate level of risk.
8. Ecological Risk Classification Results
Appendices B to D list the risk classification outcomes for all 640 organic substances by CAS RN according to their revised ecological risk classification results. Table 8.1 summarizes the risk classifications for the 640 substances.
A total of 39 substances representing 14 chemical groups and one individual substance were classified as having higher risk potential (Appendix B). Substances were grouped based on similarity of chemical structures (e.g., hindered phenols) or similar types of use (e.g., flame retardants).
Ninety-two substances were classified as having moderate risk potential (Appendix C), 58 of which are associated with the 14 groups of higher risk potential substances. For reasons of precaution and potential for substitution, these 58 moderate risk substances will undergo more detailed assessment with the 40 high risk substances, resulting in a total of 98 organic substances being proposed for more detailed assessment. Appendix C lists these substances and their proposed groups. Given the lower likelihood of risk compared with the moderate concern substances associated with high risk groups, use patterns of the 34 remaining moderates will be tracked and their priority status re-evaluated if new information becomes available.
A total of 508 substances were classified as having low risk potential (Appendix D). More detailed assessment of these substances is not proposed at this time. However, information associated with these substances (e.g., phys-chem, ecotoxicity, tissue residue) may be used in a read-across manner during assessment of substance groups under the CMP. Data from low risk substances can be used to inform the assessment of the larger group, including the potential for cumulative risk of that group. Additionally, substances classified as "low risk" with high classification of hazard (Class 3) and those that trigger other hazard alerts, but which are currently used in low volume in Canada (225 substances), are proposed to be identified for additional tracking of use patterns and their priority status re-evaluated if new information becomes available.
Various mechanisms exist to inform the priority status of these substances based on consideration of use pattern and other types of information, including but not limited to:
- DSL Inventory Updates
- Voluntary reporting
- Environmental monitoring
- Information submitted under s. 70 of CEPA
- International activities
- National Pollutant Release Inventory (NPRI)
| Risk classification | Count | Percent (%) |
|---|---|---|
| low (use pattern data is proposed to be collected for 225 of these substances) | 508 | 80 |
| moderate (use pattern to be tracked; priority to be re-evaluated if new information becomes available) | 34 | 5 |
| moderate (to be included in group for assessment with substances classified as high potential for ecological risk) | 58 | 9 |
| high | 40 | 6 |
| Total | 640 | 100 |
Critical data and considerations used to create substance-specific profiles and classifications associated with hazard, exposure and risk, as well as identification of potential need for tracking of future use patterns, are presented in ECCC 2016a.
9. Assessment of Risk Classification Uncertainty
Uncertainties have been identified in this document and their impact considered during the design of the ERC. The ERC generally reflects a realistically conservative or precautionary approach where consistency of metrics adds to a weight of evidence for classification. A weighted approach helps minimize the potential for both over and under classification of hazard and exposure and subsequent risk classification. A balanced approach to dealing with uncertainty has been used. For example, values of central tendency rather than those from extremes of a distribution have been used (e.g., median LC50 values, median CBRs). Although empirical values were used where available, many physicochemical input parameters for fate modeling (e.g., RAIDAR) required the use of QSAR-derived values. Appropriate best available science modeling practices have been applied when selecting these values and, although median values were generally used, uncertainty estimates have been generated for some parameters (e.g., RAIDAR HAFs). Also, concepts of adequate margin of exposure have been incorporated via the ratio of critical to actual (assumed) emission rates to address the uncertainty associated with the use of some metrics (e.g., Pov, RAIDAR HAF) estimated at a regional environmental scale. Furthermore, adjustment for potential risks associated with greater exposure near points of discharge to the aquatic environment was applied, as necessary. Nonetheless, uncertainties with hazard and exposure classification remain, the more significant of which are discussed below.
9.1 Hazard Uncertainty
Hazard classification required the generation of multiple metrics, some of which are very sensitive to median values of lethal aquatic toxicity data (e.g., CBR, HAF, chemical activity, toxicity ratios). Error with empirical or modeled acute toxicity values could result in significant changes in classification of hazard, particularly metrics relying on tissue residue values (e.g., mode of toxic action and the RAIDAR HAF), many of which are predicted values from QSAR models. However, the impact of this error is mitigated by the fact that overestimation of median lethality will result in a conservative tissue residue used for CBR analysis. Error with underestimation of acute toxicity will be mitigated through the use of other hazard metrics such as structural profiling of mode of action and/or reactivity and/or estrogen binding affinity. Although employing a mode of action-driven tissue residue threshold of toxicological concern (TTC) approach mitigates the error of classifying chemicals based on external concentrations (i.e., by avoiding the illusion of hydrophobicity), it is acknowledged that some uncertainty with hazard classification remains due to the fact that median lethal aquatic toxicity data were still required to generate some of the metrics used in the hazard classification (there are few reliable LC50 data for high Kow substances and there are uncertainties with models estimating internal concentrations at high Kow due to errors in Kow).
Results from profiling of chemical reactivity, bioactivity and binding affinity are also subject to uncertainty when extrapolating to a final adverse outcome. However, because these are based on mechanistic rules, which are in turn derived from first principle chemical interactions, they generally suggest a potential for interaction with biological tissues which may or may not be realized depending on the fate and toxicokinetics of the substance. Further in vitro or in vivo testing would be necessary to confirm reactivity or bioactivity. Thus, accepting the reactivity profiling results (i.e., binding affinities) de facto reduces underestimation of chemical potency, but will likely result in overestimations of hazard. Given this uncertainty, some activity metrics (e.g., bioactivity, androgen binding) were considered as supporting information only. Also, protein and DNA binding affinity must have been supported by more than one profiler in the OECD QSAR Toolbox and were then only applied after preliminary classification using other hazard metrics (this essentially impacts only the classification of low hazard). Finally, RAIDAR values such as the HAFs and critical emission rates have estimated bounds of uncertainty. Median values have been used here to avoid overly conservative values.
Few measured data were available for ionogenic substances; hence, assessment uncertainty may generally be greater for these substances than for the neutral substances.
9.2 Exposure Uncertainty
Exposure classification required the generation of multiple metrics, some of which (e.g., emission rate, margin of exposure) are very sensitive to the reported annual quantity in commerce. Changes in chemical quantity could result in significant changes in classification of exposure; i.e., the exposure and risk-based classifications are highly sensitive to uncertainties in emission rate and use quantity estimates. The ERC classification thus represents current exposure and risk in Canada and may not reflect future trends. This is primarily why use patterns of moderate concern substances not identified for more detailed assessment and all low risk substances with a high hazard classification are proposed to be tracked. Fluctuation of, and uncertainty with quantity in commerce are also primary reasons for approaching exposure classification as a probability of organism exposure using multiple metrics. It is also the reason that the HAF was selected from RAIDAR because it relies on a default rate of emission and is thus independent of reported chemical quantity. In addition, a local scale risk-based screening procedure ensures that substances with short residence time and travel distance are properly classified.
Exposure classification is also sensitive to the prediction of long-range transport in air. The ERC assesses air transport based predominantly on chemical half-life in air. Some classes of substances may undergo transport in air based on sorption to fine particles in air (e.g., certain organic flame retardants). The half-life approach used in the ERC, although well correlated with long-range air transport, cannot account for this transport process and thus far-field concerns may be underestimated for some classes of organic substances. Efforts are underway by Environment Climate Change Canada and others to improve models to better estimate this type of transport (e.g., Lui et al. 2014, Zhang et al. 2016), but these are not currently available.
10. Conclusion
Based on inherent hazard properties and current use patterns and quantities in commerce, 40 substances were classified as being of high potential ecological concern, 92 substances were classified as being of moderate ecological concern, and 508 substances were classified as being of low ecological concern. Substances classified as high ecological concerns will undergo further ecological assessment. Some of the substances of moderate ecological concern (58 of 92 substances) have similarities to substances that were classified as having a high potential for ecological concern and will therefore also undergo further assessment as part of those groups. The remainder of the substances of moderate and low ecological concern (542 substances in total) are not expected to pose an ecological risk based on current information, and further assessment work is not required at this time. The approach and results for these 542 substances will form the basis, in conjunction with any other relevant information that becomes available after the publication of this Science Approach Document, for the conclusions in Screening Assessment Reports that will be published at a later time. Further follow-up or tracking of information may be done for the 34 moderate concern substances that are not currently planned for additional detailed assessment as well as for 225 substances which were classified as low concern primarily on the basis of current low exposures, to inform whether further activity is required in the future.
References
ARC. 2014. Parameterization and Application of the RAIDAR Model to Support Prioritization and Assessment of Substances. Report prepared by ARC Arnot Research & Consulting Inc., Toronto, ON, for the Existing Substances Division, Environment Canada, Gatineau, QC, Canada.
Armitage JM, Arnot JA, Wania F, Mackay D. 2013. Development and evaluation of a mechanistic bioconcentration model for ionogenic organic chemicals in fish. Environ. Toxicol. Chem. 32(1): 115-128.
Arnot JA. 2011. Updating the RAIDAR and FHX models to aid in the prioritization and assessments of chemicals including ionisable substances. Draft Technical Report for Health Canada.; Health Canada: Ottawa, ON, March 25, 2011; p 39.
Arnot JA, Gobas FAPC. 2006. A review of bioconcentration factor (BCF) and bioaccumulation factor (BAF) assessments for organic chemicals in aquatic organisms. Environ. Rev. 14(4):257-297.
Arnot JA, Mackay D. 2008. Policies for chemical hazard and risk priority setting: Can persistence, bioaccumulation, toxicity and quantity information be combined? Environ. Sci. Technol. 42(13): 4648-4654.
Arnot JA, Mackay D, Webster E, Southwood J. 2006. Screening level risk assessment model for chemical fate and effects in the environment. Environ. Sci. Technol. 40:2316-2323.
Arnot JA, Quinn CL. 2014. Development and evaluation dietary bioaccumulation test data for organic chemicals in fish. Environ. Sci. Technol. 49(8): 4783–4796.
Baron MG, Lilavois CR, Martin TM. 2015. MOATox: A comprehensive mode of action and acute toxicity database for predictive model development. Aquat. Toxicol. 161: 102-107.
Belanger S, Sanderson H, Embry M, DeZwart D, Farr B, Gutsell S, Haider M, Sternberg R, Wilson P. 2015. It is Time to Develop Ecological Thresholds of Toxicological Concern to Assist Environmental Hazard Assessment. Environ. Toxicol. Chem. 34(12):2864-2869.
Bonnell M, Kuseva C. 2015. Correlation analysis of RC50 protein binding and acute algal, daphnid and fish toxicity using the OECD QSAR Toolbox endpoint correlation function. (unpublished).
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C. 1999, c.33. Canada Gazette Part III, vol. 22, no. 3. http://laws-lois.justice.gc.ca/eng/acts/C-15.31/.
Canada. 2009. Canadian Environmental Protection Act, 1999: Notice with respect to certain inanimate substances (chemicals) on the Domestic Substances List. Canada Gazette, Part 1, vol. 143, no. 40.. Available from: http://www.gazette.gc.ca/rp-pr/p1/2009/2009-10-03/html/notice-avis-eng.html#d101
Canada. 2012. Canadian Environmental Protection Act, 1999: Notice with respect to certain inanimate substances (chemicals) on the Domestic Substances List, Vol. 146, No. 48, 1 December, 2012. Available from: http://www.gazette.gc.ca/rp-pr/p1/2012/2012-12-01/html/sup-eng.html
Canada. 2013. Rapid Screening of Substances of Low Concern. Available from: http://www.chemicalsubstanceschimiques.gc.ca/plan/approach-approche/rapid-eng.php
Canada. 2014. Rapid Screening of Substances from Phase One of the Domestic Substances List Inventory Update. Available from: http://www.chemicalsubstanceschimiques.gc.ca/plan/approach-approche/rapid-eng.php
Canada. 2015. Rapid Screening of Substances from Phase Two of the Domestic Substances List Inventory Update. Avalailable from: http://www.chemicalsubstanceschimiques.gc.ca/plan/approach-approche/rapid-eng.php
Cimorelli A, Stahl C. 2013. Avoiding "proofiness": Addressing uncertainty in environmental characterization. Integr. Environ. Assess. Manage. 10(1): 138-141.
de Wolf W, Siebel-Sauer A, Lecloux A, Koch V, Holt M, Feijtel T, Comber M, Boeije G. 2005. Mode of action and aquatic exposure thresholds of no concern. Environ. Toxicol. Chem. 24(2):479-485.
Dimitrov S, Dimitrova G, Pavlov T, Dimitrova D, Patlevisz G, Niemela J, Mekenyan O. 2005. A stepwise approach for defining the applicability domain of SAR and QSAR models. J. Chem. Inf. Model. 45(4): 839-849.
Dimitrov SD, Mekenyan OG, Sinks GD, Schultz TW. 2003. Global modeling of narcotic chemicals: ciliate and fish toxicity. J. Mol. Structr. 622(1-2): 62-70.
Ding D, Xu L, Fang H, Hong H, Perkins R, Harris S, Bearden ED, Shi L, Tong W. The EDKB: an established knowledge base for endocrine disrupting chemicals. BMC Bioinformatics 2010, 11 Suppl 6:S5.
Enoch SJ, Hewitt M, Cronin MTD, Azam S, Madden JC. 2008. Classification of chemicals according to mechanism of aquatic toxicity: An evaluation of the implementation of the Verhaar scheme in Toxtree. Chemosphere 73(73): 243-248.
[ECCC] Environment and Climate Change Canada. 2016a. Gatineau (QC): ECCC. Data used to create substance-specific hazard and exposure profiles and assign risk classifications. Available from: substances@ec.gc.ca.
[ECCC] Environment and Climate Change Canada. 2016b. Gatineau (QC): ECCC. Assigned emission factors for NAICS and Substance Function Codes. Information in support of Ecological Risk Classification of Organic Substances. Available from: substances@ec.gc.ca.
Escher BI, Ashauer R, Dyer S, Hermens JLM, Lee J-H, Leslie HA, Mayer P, Meador JP, Warne MSJ. 2011. Crucial role of mechanisms and modes of toxic action for understanding tissue residue toxicity and internal effect concentrations of organic chemicals. Integr Environ Assess Manag. 7 (1): 28-49
Fang M, Tong W, Branham S, Moland CLS, Dial L, Hong H, Xie Q, Perkins R, Owens W, Sheehan DM. 2003. Study of 202 Natural, Synthetic, and Environmental Chemicals for Binding to the Androgen Receptor. Chem. Res. Toxicol., 16(10): 1338 -1358.
Gouin T, Mackay D, Webster E, Wania F. 2000. Screening Chemicals for Persistence in the Environment. Environ. Sci. Technol. 34 (5): 881–884.
Integrated Environmental Assessment and Management. 2011. 7(1): 1-140.
Kelly BC, Gobas FA, McLachlan MS. 2004. Intestinal absorption and biomagnification of organic contaminants in fish, wildlife and humans. Environ Toxicol Chem 23(10):2324-2336.
Klasmeier J, Matthies M, MacLeod M, Fenner K, Scheringer M, Stroebe M, Le Gall AC, McKone TE, van de Meent D, Wania F. 2006. Application of multimedia models for screening assessment of long-range transport potential and overall persistence. Environ. Sci. Technol. 40(1):53–60.
Liu Y, Liggio J, Harner T, Jantunen L, Shoeib M, Li S-M. 2014. Heterogeneous OH initiated oxidation: a possible explanation for the persistence of organophosphate flame retardants in air. Environ. Sci. Technol. 48(2): 1041-1048.
Mackay D, Arnot JA, Petkova EP, Wallace KB, Call DJ, Brooke LT, Veith GD 2009. The physicochemical basis of QSARs for baseline toxicity. SAR QSAR Environ. Res. 20(3-4): 393-414.
Mackay D, Hughes DM, Romano ML, Bonnell M. 2014b. The role of persistence in chemical evaluations. Integrated Environ Assessment Management 10(4):588-594.
Mackay, D., McCarty, L.S. and J.A. Arnot. 2014a. Relationships between exposure and dose in aquatic toxicity tests for organic chemicals. Environ. Toxicol. Chem. 33(9): 2038-46.
Maeder V, Escher BI, Scheringer M, Hungerbühler K. 2004. Toxic ratio as an indicator of the intrinsic toxicity in the assessment of persistent, bioaccumulative, and toxic chemicals. Environ. Sci. Technol. 38(13): 3659-3666.
McCarty LS, Arnot JA, Mackay D. 2013. Evaluation of critical body residue for acute narcosis in aquatic organisms. Environ Toxicol Chem 32(10): 2301-2314.
McCarty LS, Mackay D. 1993. Enhancing ecotoxicological modeling and assessment: critical body residues and modes of toxic action. Environ Sci Technol 27(9):1719-1728.
Mekenyan O, Serafimova R. 2009. Mechanism-Based Modeling of Estrogen Receptor Binding Affinity A COREPA Implementation. CRC Press, pp. 259-293.
[OECD] Organisation for Economic Co-operation and Development. 2004. Guidance Document On The Use Of Multimedia Models For Estimating Overall Environmental Persistence And Long-Range Transport. (Series on Testing and Assessment No.45). Report No. ENV/JM/MONO(2004)5, JT00160339. Paris (FR): OECD.
[OECD]Toolbox [Read-across tool]. 2014. Version 3.3. Paris (FR): Organisation for Economic Cooporation and Development, Environment Directorate. Available from: http://www.oecd.org/chemicalsafety/risk-assessment/theoecdqsartoolbox.htm
Pennington D. 2001. An evaluation of chemical persistence screening approaches. Chemosphere. 44: 1589-1601.
Princz J, Bonnell M, Ritchie E, Velicogna J, Robidoux P-Y, Scroggins R. 2014. Estimation of the bioaccumulation potential of a non-chlorinated bisphenol and an ionogenic xanthene dye to Eisenia andrei in field-collected soils, in conjunction with predictive In silico profiling. Environ. Toxicol. Chem. 33(2): 308-316.
Sappington KG, Bridges TS, Bradbury SP, Erickson RJ, Hendriks KA, Lanno RP, Meador JP, Mount DR, Salazar MH, Spry DJ. 2010. Application of the Tissue Residue Approach in Ecological Risk Assessment. Integrated Environmental Assessment and Management. 7(1): 116–140
Serafimova, R, Todorov M, Nedelcheva D, Pavlov T, Akahori Y, Nakai M, Mekenyan O. 2007. QSAR and mechanistic interpretation of estrogen receptor binding. SAR QSAR Environ. Res. 18(3-4): 1-33.
Stahl CH, Cimorelli AJ. 2013. A Demonstration of the Necessity and Feasibility of Using a Clumsy Decision Analytic Approach on Wicked Environmental Problems. Integrated Environmental Assessment and Management 9(1): 17-30.
Struijs J, Stoltenkamp J, Van de Meent D. 1991. A Spreadsheet-based Box Model to Predict the Fate of Xenobiotics from a Municipal Wastewater Treatment Plant. Wat. Res. 25(7): 891-900.
[TIMES] TIssue MEtabolism Simulator [prediction module]. 2014. Ver. 2.27.15. Bourgas (BG): University "Prof. Dr. Assen Zlatarov", Laboratory of Mathematical Chemistry. http://oasis-lmc.org/products/software/times.aspx
Todorov M, Mombelli E, Ait-Aissa S, Mekenyan O. 2011. Androgen Receptor Binding Affinity: A QSAR Evaluation. SAR and QSAR in Environmental Research 22(3-4): 265-291.
Van Leeuwen CJ, Vermeire TG (eds). 2007. Risk assessment of chemicals: an introduction, Second edition. Springer Dordrecht, The Netherlands. ISBN 978-1-4020-6101-1 (handbook), ISBN 978-1-4020-6102-8 (e-book).
Verhaar HJM, Solbe J, Speksnijder J, van Leeuwen CJ and Hermens JLM. 2000. Classifying environmental pollutants: Part 3. External validation of the classification system. Chemosphere 40: 875-883
Verhaar HJ M, Van Leeuwen CJ, Hermens JLM. 1992. Classifying environmental pollutants. 1. Structure-activity relationships for prediction of aquatic toxicity. Chemosphere 25: 471-491.
Webster E, Mackay D, Wania F. 1998. Evaluating environmental persistence. Environ. Toxicol. Chem. 17(11):2148-2158.
Wegmann F, Cavin L, MacLeod M, Scheringer M, Hungerbuler K. 2009. The OECD software tool for screening chemicals for persistence and long range transport potential. Environ. Model. Software. 24(2): 228–237.
Williams ES, Berninger JP, Brooks BW. 2011. Application of chemical toxicity distributions to ecotoxicology data requirements under REACH. Environ. Toxicol. Chem. 30(8):1943-1954.
Wu S, Blackburn K, Amburgey J, Jaworska J, Federla T. 2010. A framework for using structural, reactivity, metabolic and physicochemical similarity to evaluate the suitability of analogs for SAR-based toxicological assessments. Regulatory Toxicology and Pharmacology 56(1): 67-81.
Zhang X, Sühring R, Serodio D, Bonnell M, Sundin N, Diamond M. 2016. Novel flame retardants: Estimating the physicalechemical properties and environmental fate of 94 halogenated and organophosphate PBDE replacements. Chemosphere 144: 2401-2407.
Appendices
Appendix A. Summary of local exposure screening scenarios
- A conservative predicted environmental concentration (PEC) resulting from the release of the substance to the aquatic environment from an industrial point source is calculated as shown in the following equation. Parameters used in this exposure scenario are described in the table below.
PEC (mg/L) = (Qty × Release × (1 – Wastewater Removal))/(Duration × (River flow + Wastewater flow))) × (1000/86400) - The aquatic predicted no-effect concentration (PNEC) is derived from the Critical Toxicity Value (CTV) as shown:
Aquatic PNEC (mg/L) = CTV/AF - The PEC is compared to the PNEC to determine a risk quotient (PEC / PNEC). If the risk quotient is greater than one, this indicates that the conservatively estimated concentration in water exceeds the aquatic estimated no-effect level and that there may exist a potential to cause harm in the aquatic ecosystem. A value below one indicates that concentrations that may cause an effect to sensitive aquatic organisms are not reached and therefore harm to aquatic organisms is unlikely under this scenario.
- A risk quotient was determined for each notifier to the DSL Inventory Update (phases one or two) associated with the substances of interest.
| Abbrev. | Parameter | Value | Units | Notes |
|---|---|---|---|---|
| Qty | quantity of substance reported by notifier | quantity from Inventory Update (or other) | kg/year | substance-specific |
| Release | release of substance during industrial process | 2% (low) 25% (medium) 100% (high) |
default value based on analysis of reported use and NAICS codes | |
| Wastewater Removal | Wastewater Treatment System (WWTS) removal efficiency | Value from SimpleTreat model Default value when removal could not be estimated by model = 0 |
substance-specific | |
| Duration | duration over which substance is released | 150 | days/year | assumes variable or discontinuous use of substance over a year |
| Wastewater flow | WWTS flow rate | 0.04 | m3/s | 10th percentile of municipal WWTS flow rates in Canada |
| River flow | flow of receiving watercourse | 1.84 | m3/s | 15th percentile of the distribution of receiving watercourse flows in the country (based on the distribution of the 50th percentile of flow rates); weighted by number of industries releasing to the receiving watercourse |
| - | factor combining conversion from kg to mg and m3 to L | 1000 | ||
| - | conversion factor from seconds to days | 86400 | ||
| CTV | critical toxicity value | Value from categorization or more recent source of data | mg/L | substance-specific |
| AF | assessment factor | 10 or 100 | To account for acute-to-chronic; inter-species variability. A default value of 10 is used for baseline narcotic substances and 100 for reactive unspecified substances |
Appendix B. Substances classified as higher potential of risk to the environment
| CAS RN | Domestic Substances List name | CMP Chemical Group | Hazard Ranking | Exposure Ranking |
|---|---|---|---|---|
| 112-69-6 | 1-Hexadecanamine, N,N-dimethyl- | Aliphatic amines | 3 | 2 |
| 61790-59-8 | Amines, hydrogenated tallow alkyl, acetates | Aliphatic amines | 3 | 2 |
| 25167-32-2 | Benzenesulfonic acid, oxybis[dodecyl-, disodium salt | Alkyl aryl sulfonates/LABS and derivatives | 3 | 2 |
| 68411-30-3 | Benzenesulfonic acid, C10-13-alkyl derivs., sodium salts | Alkyl aryl sulfonates/LABS and derivatives | 3 | 2 |
| 68411-32-5 | Benzenesulfonic acid, dodecyl-, branched | Alkyl aryl sulfonates/LABS and derivatives | 3 | 3 |
| 68608-26-4 | Sulfonic acids, petroleum, sodium salts | Alkyl aryl sulfonates/LABS and derivatives | 3 | 3 |
| 68649-00-3 | Benzenesulfonic acid, mono-C9-17-branched alkyl derivs., compds. with 2-propanamine | Alkyl aryl sulfonates/LABS and derivatives | 3 | 2 |
| 70146-13-3 | Benzenesulfonic acid, oxybis[decyl-, disodium salt | Alkyl aryl sulfonates/LABS and derivatives | 3 | 2 |
| 70775-94-9 | Sulfonic acids, C10-18-alkane, Ph esters | Alkyl aryl sulfonates/LABS and derivatives | 3 | 2 |
| 3147-75-9 | Phenol, 2-(2H-benzotriazol-2-yl)-4-(1,1,3,3-tetramethylbutyl)- | Benzotriazoles & benzothiazoles | 3 | 2 |
| 28777-98-2 | 2,5-Furandione, dihydro-3-(octadecenyl)- | Carboxylic acid anhydrides | 3 | 3 |
| 32072-96-1 | 2,5-Furandione, 3-(hexadecenyl)dihydro- | Carboxylic acid anhydrides | 3 | 3 |
| 11145-3a | Alkylamine salt of complex phosphate ester | Dithiophosphate Alkyl Esters | 3 | 2 |
| 58965-66-5b | Benzene, 1,2,4,5-tetrabromo-3,6-bis(pentabromophenoxy)- | Flame Retardants | 3 | 2 |
| 68937-41-7 | Phenol, isopropylated, phosphate (3:1) | Flame Retardants | 3 | 2 |
| 96-69-5b | Phenol, 4,4'-thiobis[2-(1,1-dimethylethyl)-5-methyl- | Hindered phenols | 3 | 2 |
| 96-76-4b | Phenol, 2,4-bis(1,1-dimethylethyl)- | Hindered phenols | 3 | 2 |
| 4221-80-1 | Benzoic acid, 3,5-bis(1,1-dimethylethyl)-4-hydroxy-, 2,4-bis(1,1-dimethylethyl)phenyl ester | Hindered phenols | 3 | 2 |
| 61788-44-1b | Phenol, styrenated | Hindered phenols | 3 | 2 |
| 25619-56-1 | Naphthalenesulfonic acid, dinonyl-, barium salt | Naphthalene sulfonic acids and salts | 3 | 2 |
| 60223-95-2 | Naphthalenedisulfonic acid, dinonyl- | Naphthalene sulfonic acids and salts | 3 | 2 |
| 81-14-1 | Ethanone, 1-[4-(1,1-dimethylethyl)-2,6-dimethyl-3,5-dinitrophenyl]- | Nitro musks | 3 | 2 |
| 81-15-2 | Benzene, 1-(1,1-dimethylethyl)-3,5-dimethyl-2,4,6-trinitro- | Nitro musks | 3 | 2 |
| 140-66-9 | Phenol, 4-(1,1,3,3-tetramethylbutyl)- | Octylphenol and ethoxylates | 3 | 2 |
| 61789-77-3 | Quaternary ammonium compounds, dicoco alkyldimethyl, chlorides | Quaternary ammonium compounds | 3 | 3 |
| 61789-80-8 | Quaternary ammonium compounds, bis(hydrogenated tallow alkyl)dimethyl, chlorides | Quaternary ammonium compounds | 3 | 3 |
| 68308-67-8 | Quaternary ammonium compounds, ethyldimethylsoya alkyl, Et sulfates | Quaternary ammonium compounds | 3 | 2 |
| 68511-92-2 | 9-Octadecenoic acid (Z)-, reaction products with diethylenetriamine, cyclized, di-Et sulfate-quaternized | Quaternary ammonium compounds | 3 | 2 |
| 70955-34-9 | Fatty acids, tall-oil, reaction products with 2-[(2-aminoethyl)amino]ethanol, di-Et sulfate-quaternized | Quaternary ammonium compounds | 3 | 2 |
| 71011-24-0 | Quaternary ammonium compounds, benzyl(hydrogenated tallow alkyl)dimethyl, chlorides, compds. with bentonite | Quaternary ammonium compounds | 3 | 2 |
| 71011-26-2 | Quaternary ammonium compounds, benzyl(hydrogenated tallow alkyl)dimethyl, chlorides, compds. with hectorite | Quaternary ammonium compounds | 3 | 2 |
| 8016-81-7 | Tall-oil pitch | Resins & rosins | 3 | 2 |
| 8050-09-7 | Rosin | Resins & rosins | 3 | 3 |
| 9007-13-0 | Resin acids and Rosin acids, calcium salts | Resins & rosins | 3 | 2 |
| 61790-51-0 | Resin acids and Rosin acids, sodium salts | Resins & rosins | 3 | 2 |
| 120-54-7 | Piperidine, 1,1'-(tetrathiodicarbonothioyl)bis- | Thiocarbamates | 3 | 3 |
| 548-62-9 | Methanaminium, N-[4-[bis[4-(dimethylamino)phenyl]methylene]-2,5-cyclohexadien-1-ylidene]-N-methyl-, chloride | Triarylmethanes | 3 | 2 |
| 569-64-2 | Methanaminium, N-[4-[[4-(dimethylamino)phenyl]phenylmethylene]-2,5-cyclohexadien-1-ylidene]-N-methyl-, chloride | Triarylmethanes | 3 | 2 |
| 1324-76-1 | Benzenesulfonic acid, [[4-[[4-(phenylamino)phenyl][4-(phenylimino)-2,5-cyclohexadien-1-ylidene]methyl]phenyl]amino]- | Triarylmethanes | 3 | 2 |
| 88-85-7 | Phenol, 2-(1-methylpropyl)-4,6-dinitro- | NA | 3 | 3 |
Table Notes
Abbreviation: CAS RN, Chemical Abstracts Service Registry Number; CMP, Chemical Management Plan; NA, not available
aConfidential Domestic Substance List (CDSL) substance(s).
bExposure ranking of this substance was increased following application of the classification consistency rule (see section 6).
Appendix C. Substances classified as moderate potential risk to the environment
| CAS RN | Domestic Substances List name | CMP Chemical Group | Hazard Ranking | Exposure Ranking | Action* |
|---|---|---|---|---|---|
| 103-11-7a | 2-Propenoic acid, 2-ethylhexyl ester | Acrylates/ methylacrylates | 1 | 1 | track |
| 141-32-2 | 2-Propenoic acid, butyl ester | Acrylates/ methylacrylates | 3 | 1 | track |
| 124-30-1a | 1-Octadecanamine | Aliphatic amines | 2 | 1 | assess in group |
| 61788-46-3a | Amines, coco alkyl | Aliphatic amines | 1 | 1 | assess in group |
| 61790-60-1 | Amines, tallow alkyl, acetates | Aliphatic amines | 3 | 1 | assess in group |
| 61791-55-7b | Amines, N-tallow alkyltrimethylenedi- | Aliphatic amines | 3 | 1 | assess in group |
| 68155-39-5 | Amines, C14-18 and C16-18-unsatd. alkyl, ethoxylated | Aliphatic amines | 2 | 1 | assess in group |
| 68479-04-9b | 1,3-Propanediamine, N-[3-(tridecyloxy)propyl]-, branched | Aliphatic amines | 3 | 1 | assess in group |
| 68783-25-5 | Amines, N,N,N'-trimethyl-N'-tallow alkyltrimethylenedi- | Aliphatic amines | 3 | 1 | assess in group |
| 27178-16-1 | Hexanedioic acid, diisodecyl ester | Aliphatic diesters | 2 | 3 | track |
| 26264-05-1a | Benzenesulfonic acid, dodecyl-, compd. with 2-propanamine (1:1) | Alkyl aryl sulfonates/LABS and derivatives | 2 | 1 | assess in group |
| 28519-02-0 | Benzenesulfonic acid, dodecyl(sulfophenoxy)-, disodium salt | Alkyl aryl sulfonates/LABS and derivatives | 2 | 2 | assess in group |
| 61789-86-4 | Sulfonic acids, petroleum, calcium salts | Alkyl aryl sulfonates/LABS and derivatives | 2 | 2 | assess in group |
| 61789-87-5 | Sulfonic acids, petroleum, magnesium salts | Alkyl aryl sulfonates/LABS and derivatives | 2 | 2 | assess in group |
| 61790-48-5 | Sulfonic acids, petroleum, barium salts | Alkyl aryl sulfonates/LABS and derivatives | 2 | 2 | assess in group |
| 68584-22-5a | Benzenesulfonic acid, C10-16-alkyl derivs. | Alkyl aryl sulfonates/LABS and derivatives | 1 | 1 | assess in group |
| 68584-24-7 | Benzenesulfonic acid, C10-16-alkyl derivs., compds. with 2-propanamine | Alkyl aryl sulfonates/LABS and derivatives | 2 | 2 | assess in group |
| 68783-96-0 | Sulfonic acids, petroleum, calcium salts, overbased | Alkyl aryl sulfonates/LABS and derivatives | 3 | 1 | assess in group |
| 90218-35-2 | Benzenesulfonic acid, dodecyl-, branched, compd. with 2-propanamine | Alkyl aryl sulfonates/LABS and derivatives | 2 | 3 | assess in group |
| 95-38-5 | 1H-Imidazole-1-ethanol, 2-(8-heptadecenyl)-4,5-dihydro- | Alkyl imidazolines and salts | 3 | 1 | track |
| 27136-73-8 | 1H-Imidazole-1-ethanol, 2-(heptadecenyl)-4,5-dihydro- | Alkyl imidazolines and salts | 3 | 1 | track |
| 68442-97-7a | 1H-Imidazole-1-ethanamine, 4,5-dihydro-, 2-nortall-oil alkyl derivs. | Alkyl imidazolines and salts | 2 | 1 | track |
| 139-96-8a | Sulfuric acid, monododecyl ester, compd. with 2,2',2''-nitrilotris[ethanol] (1:1) | Alkyl Sulfates and Olefin Sulfonate | 1 | 1 | track |
| 151-21-3a | Sulfuric acid monododecyl ester sodium salt | Alkyl Sulfates and Olefin Sulfonate | 2 | 1 | track |
| 68439-57-6 | Sulfonic acids, C14-16-alkane hydroxy and C14-16-alkene, sodium salts | Alkyl Sulfates and Olefin Sulfonate | 3 | 1 | track |
| 25550-98-5 | Phosphorous acid, diisodecyl phenyl ester | Alkyl/aryl Phosphites | 3 | 1 | track |
| 81-77-6 | 5,9,14,18-Anthrazinetetrone, 6,15-dihydro- | Anthraquinones | 3 | 2 | track |
| 90-30-2 | 1-Naphthalenamine, N-phenyl- | Aromatic Amines | 2 | 3 | track |
| 101-14-4 | Benzenamine, 4,4'-methylenebis[2-chloro- | Aromatic Amines | 3 | 1 | track |
| 101-96-2 | 1,4-Benzenediamine, N,N'-bis(1-methylpropyl)- | Aromatic Amines | 2 | 2 | track |
| 793-24-8 | 1,4-Benzenediamine, N-(1,3-dimethylbutyl)-N'-phenyl- | Aromatic Amines | 2 | 3 | track |
| 5285-60-9 | Benzenamine, 4,4'-methylenebis[N-(1-methylpropyl)- | Aromatic Amines | 3 | 1 | track |
| 120-78-5 | Benzothiazole, 2,2'-dithiobis- | Benzotriazoles & benzothiazoles | 2 | 3 | assess in group |
| 149-30-4 | 2(3H)-Benzothiazolethione | Benzotriazoles & benzothiazoles | 3 | 1 | assess in group |
| 2492-26-4 | 2(3H)-Benzothiazolethione, sodium salt | Benzotriazoles & benzothiazoles | 3 | 1 | assess in group |
| 3846-71-7b | Phenol, 2-(2H-benzotriazol-2-yl)-4,6-bis(1,1-dimethylethyl)- | Benzotriazoles & benzothiazoles | 2 | 2 | assess in group |
| 3896-11-5 | Phenol, 2-(5-chloro-2H-benzotriazol-2-yl)-6-(1,1-dimethylethyl)-4-methyl- | Benzotriazoles & benzothiazoles | 3 | 1 | assess in group |
| 36437-37-3 | Phenol, 2-(2H-benzotriazol-2-yl)-4-(1,1-dimethylethyl)-6-(1-methylpropyl)- | Benzotriazoles & benzothiazoles | 3 | 1 | assess in group |
| 70321-86-7 | Phenol, 2-(2H-benzotriazol-2-yl)-4,6-bis(1-methyl-1-phenylethyl)- | Benzotriazoles & benzothiazoles | 3 | 1 | assess in group |
| 85-44-9a | 1,3-Isobenzofurandione | Carboxylic acid anhydrides | 1 | 1 | assess in group |
| 26544-38-7a | 2,5-Furandione, dihydro-3-(tetrapropenyl)- | Carboxylic acid anhydrides | 2 | 1 | assess in group |
| 68784-12-3 | 2,5-Furandione, dihydro-, mono-C15-20-alkenyl derivs. | Carboxylic acid anhydrides | 2 | 3 | assess in group |
| 11105-8c | Phosphorothioic acid, dialkyl ester, alkylamine salt | Dithiophosphate Alkyl Esters | 3 | 1 | assess in group |
| 61789-01-3 | Fatty acids, tall-oil, epoxidized, 2-ethylhexyl esters | Epoxides & glycidyl ethers | 3 | 1 | track |
| 78-51-3 | Ethanol, 2-butoxy-, phosphate (3:1) | Flame Retardants | 3 | 1 | assess in group |
| 115-86-6a | Phosphoric acid, triphenyl ester | Flame Retardants | 1 | 1 | assess in group |
| 29761-21-5 | Phosphoric acid, isodecyl diphenyl ester | Flame Retardants | 2 | 2 | assess in group |
| 56803-37-3 | Phosphoric acid, (1,1-dimethylethyl)phenyl diphenyl ester | Flame Retardants | 2 | 2 | assess in group |
| 65652-41-7 | Phosphoric acid, bis[(1,1-dimethylethyl)phenyl] phenyl ester | Flame Retardants | 3 | 1 | assess in group |
| 68527-01-5b | Alkenes, C12-30 α-, bromo chloro | Flame Retardants | 3 | 1 | assess in group |
| 68527-02-6a | Alkenes, C12-24, chloro | Flame Retardants | 1 | 1 | assess in group |
| 98-54-4a | Phenol, 4-(1,1-dimethylethyl)- | Hindered phenols | 1 | 1 | assess in group |
| 118-82-1 | Phenol, 4,4'-methylenebis[2,6-bis(1,1-dimethylethyl)- | Hindered phenols | 3 | 1 | assess in group |
| 128-37-0a | Phenol, 2,6-bis(1,1-dimethylethyl)-4-methyl- | Hindered phenols | 2 | 1 | assess in group |
| 128-39-2a | Phenol, 2,6-bis(1,1-dimethylethyl)- | Hindered phenols | 2 | 1 | assess in group |
| 1843-03-4 | Phenol, 4,4',4''-(1-methyl-1-propanyl-3-ylidene)tris[2-(1,1-dimethylethyl)-5-methyl- | Hindered phenols | 3 | 1 | assess in group |
| 35958-30-6 | Phenol, 2,2'-ethylidenebis[4,6-bis(1,1-dimethylethyl)- | Hindered phenols | 3 | 1 | assess in group |
| 36443-68-2 | Benzenepropanoic acid, 3-(1,1-dimethylethyl)-4-hydroxy-5-methyl-, 1,2-ethanediylbis(oxy-2,1-ethanediyl) ester | Hindered phenols | 3 | 1 | assess in group |
| 67774-74-7 | Benzene, C10-13-alkyl derivs. | Linear Alkyl Benzenes (LAB) and Derivatives | 2 | 2 | track |
| 68648-87-3a | Benzene, C10-16-alkyl derivs. | Linear Alkyl Benzenes (LAB) and Derivatives | 1 | 1 | track |
| 25322-17-2 | Naphthalenesulfonic acid, dinonyl- | Naphthalene sulfonic acids and salts | 3 | 1 | assess in group |
| 57855-77-3 | Naphthalenesulfonic acid, dinonyl-, calcium salt | Naphthalene sulfonic acids and salts | 3 | 1 | assess in group |
| 68425-61-6 | Naphthalenesulfonic acid, bis(1-methylethyl)-, compd. with cyclohexanamine (1:1) | Naphthalene sulfonic acids and salts | 3 | 1 | assess in group |
| 1338-02-9a,d | Naphthenic acids, copper salts | Naphthenic acids and salts | 1 | 1 | track |
| 1338-24-5a | Naphthenic acids | Naphthenic acids and salts | 2 | 1 | track |
| 12001-85-3a,d | Naphthenic acids, zinc salts | Naphthenic acids and salts | 1 | 1 | track |
| 118-96-7 | Benzene, 2-methyl-1,3,5-trinitro- | Nitrobenzenes | 2 | 2 | track |
| 121-14-2 | Benzene, 1-methyl-2,4-dinitro- | Nitrobenzenes | 2 | 2 | track |
| 1326-03-0 | Xanthylium, 9-(2-carboxyphenyl)-3,6-bis(diethylamino)-, molybdatetungstatephosphate | Pigments and Dyes | 3 | 1 | track |
| 5521-31-3 | Anthra[2,1,9-def:6,5,10-d'e'f']diisoquinoline-1,3,8,10(2H,9H)-tetrone, 2,9-dimethyl- | Pigments and Dyes | 2 | 2 | track |
| 8005-03-6 | C.I. Acid Black 2 | Pigments and Dyes | 3 | 1 | track |
| 66241-11-0 | C.I. Leuco Sulphur Black 1 | Pigments and Dyes | 2 | 2 | track |
| 75627-12-2 | Xanthylium, 3,6-bis(ethylamino)-9-[2-(methoxycarbonyl)phenyl]-2,7-dimethyl-, molybdatesilicate | Pigments and Dyes | 3 | 1 | track |
| 106276-80-6 | Benzoic acid, 2,3,4,5-tetrachloro-6-cyano-, methyl ester, reaction products with p-phenylenediamine and sodium methoxide | Pigments and Dyes | 3 | 1 | track |
| 139-07-1 | Benzenemethanaminium, N-dodecyl-N,N-dimethyl-, chloride | Quaternary ammonium compounds | 3 | 1 | assess in group |
| 139-08-2 | Benzenemethanaminium, N,N-dimethyl-N-tetradecyl-, chloride | Quaternary ammonium compounds | 3 | 1 | assess in group |
| 68391-01-5 | Quaternary ammonium compounds, benzyl-C12-18-alkyldimethyl,chlorides | Quaternary ammonium compounds | 2 | 2 | assess in group |
| 68391-05-9 | Quaternary ammonium compounds, di-C12-18-alkyldimethyl, chlorides | Quaternary ammonium compounds | 3 | 1 | assess in group |
| 68424-85-1 | Quaternary ammonium compounds, benzyl-C12-16-alkyldimethyl,chlorides | Quaternary ammonium compounds | 2 | 3 | assess in group |
| 68953-58-2 | Quaternary ammonium compounds, bis(hydrogenated tallow alkyl)dimethyl, salts with bentonite | Quaternary ammonium compounds | 3 | 1 | assess in group |
| 8002-26-4 | Tall oil | Resins & rosins | 3 | 1 | assess in group |
| 8050-15-5 | Resin acids and Rosin acids, hydrogenated, Me esters | Resins & rosins | 3 | 1 | assess in group |
| 8050-28-0 | Rosin, maleated | Resins & rosins | 3 | 1 | assess in group |
| 8052-10-6 | Tall-oil rosin | Resins & rosins | 3 | 1 | assess in group |
| 118-56-9 | Benzoic acid, 2-hydroxy-, 3,3,5-trimethylcyclohexyl ester | Salicylates | 3 | 1 | track |
| 10703-2c | Substituted alkylphenol, calcium salt | Substituted alkyl (dodecyl) phenols | 3 | 1 | track |
| 137-26-8 | Thioperoxydicarbonic diamide ([(H2N)C(S)]2S2), tetramethyl- | Thiocarbamates | 3 | 1 | assess in group |
| 2390-59-2 | Ethanaminium, N-[4-[bis[4-(diethylamino)phenyl]methylene]-2,5-cyclohexadien-1-ylidene]-N-ethyl-, chloride | Triarylmethanes | 3 | 1 | assess in group |
| 2390-60-5 | Ethanaminium, N-[4-[[4-(diethylamino)phenyl][4-(ethylamino)-1-naphthalenyl]methylene]-2,5-cyclohexadien-1-ylidene]-N-ethyl-, chloride | Triarylmethanes | 3 | 1 | assess in group |
| 3844-45-9 | Benzenemethanaminium, N-ethyl-N-[4-[[4-[ethyl[(3-sulfophenyl)methyl]amino]phenyl](2-sulfophenyl)methylene]-2,5-cyclohexadien-1-ylidene]-3-sulfo-, hydroxide, inner salt, disodium salt | Triarylmethanes | 2 | 2 | assess in group |
| 10685-2c | Substituted dimercaptodithiazole | NA | 3 | 1 | track |
| 1843-05-6 | Methanone, [2-hydroxy-4-(octyloxy)phenyl]phenyl- | NA | 3 | 1 | track |
Table Notes
* For reasons of precaution and potential for substitution, substances which are associated with groups of higher risk potential substances will undergo more detailed assessment with the high risk substances. The remaining moderates have been identified for additional tracking of use patterns and their priority status re-evaluated if new information becomes available.
Abbreviation: CAS RN, Chemical Abstracts Service Registry Number; CMP, Chemical Management Plan; NA, not available
aSubstance had initially been classified as a low risk concern, but was adjusted to moderate risk classification based on application of the near-field exposure scenario (see section 7.1.2). This adjustment is not reflected in the exposure ranking.
bExposure ranking of this substance was revised following application of the classification consistency rule (see section 6).
cConfidential Domestic Substance List (CDSL) substance(s).
dMetal moieties will be assessed in future inorganic assessments.
Appendix D. Substances classified as posing a lower relative risk to the environment
| CAS RN | Domestic Substances List name | CMP Chemical Group | Hazard Ranking | Exposure Ranking | Action |
|---|---|---|---|---|---|
| 136-51-6 | Hexanoic acid, 2-ethyl-, calcium salt | 2-EHA and derivatives | 1 | 1 | |
| 7425-14-1 | Hexanoic acid, 2-ethyl-, 2-ethylhexyl ester | 2-EHA and derivatives | 1 | 1 | |
| 79-10-7 | 2-Propenoic acid | Acrylates/methylacrylates | 1 | 1 | track |
| 79-41-4 | 2-Propenoic acid, 2-methyl- | Acrylates/methylacrylates | 1 | 1 | track |
| 97-88-1 | 2-Propenoic acid, 2-methyl-, butyl ester | Acrylates/methylacrylates | 1 | 1 | track |
| 122-68-9 | 2-Propenoic acid, 3-phenyl-, 3-phenylpropyl ester | Acrylates/methylacrylates | 1 | 1 | track |
| 7534-94-3 | 2-Propenoic acid, 2-methyl-, 1,7,7-trimethylbicyclo[2.2.1]hept-2-yl ester, exo- | Acrylates/methylacrylates | 2 | 1 | track |
| 24448-20-2 | 2-Propenoic acid, 2-methyl-, (1-methylethylidene)bis(4,1-phenyleneoxy-2,1-ethanediyl) ester | Acrylates/methylacrylates | 2 | 1 | track |
| 43048-08-4 | 2-Propenoic acid, 2-methyl-, (octahydro-4,7-methano-1H-indene-5,?-diyl)bis(methylene) ester | Acrylates/methylacrylates | 2 | 1 | track |
| 57-55-6 | 1,2-Propanediol | Alcohols | 1 | 1 | |
| 67-56-1 | Methanol | Alcohols | 1 | 3 | |
| 67-63-0 | 2-Propanol | Alcohols | 1 | 1 | |
| 71-23-8 | 1-Propanol | Alcohols | 1 | 1 | |
| 71-36-3 | 1-Butanol | Alcohols | 1 | 1 | |
| 71-41-0 | 1-Pentanol | Alcohols | 1 | 1 | |
| 75-65-0 | 2-Propanol, 2-methyl- | Alcohols | 1 | 3 | |
| 77-99-6 | 1,3-Propanediol, 2-ethyl-2-(hydroxymethyl)- | Alcohols | 1 | 1 | |
| 78-83-1 | 1-Propanol, 2-methyl- | Alcohols | 1 | 1 | |
| 87-66-1 | 1,2,3-Benzenetriol | Alcohols | 1 | 1 | |
| 96-23-1 | 2-Propanol, 1,3-dichloro- | Alcohols | 2 | 1 | track |
| 100-51-6 | Benzenemethanol | Alcohols | 1 | 1 | |
| 104-76-7 | 1-Hexanol, 2-ethyl- | Alcohols | 1 | 1 | track |
| 107-18-6 | 2-Propen-1-ol | Alcohols | 1 | 1 | |
| 108-11-2 | 2-Pentanol, 4-methyl- | Alcohols | 1 | 1 | |
| 108-46-3 | 1,3-Benzenediol | Alcohols | 1 | 1 | |
| 108-93-0 | Cyclohexanol | Alcohols | 1 | 1 | |
| 111-27-3 | 1-Hexanol | Alcohols | 1 | 1 | |
| 111-87-5 | 1-Octanol | Alcohols | 1 | 1 | |
| 112-30-1 | 1-Decanol | Alcohols | 1 | 1 | |
| 112-53-8 | 1-Dodecanol | Alcohols | 1 | 1 | |
| 112-72-1 | 1-Tetradecanol | Alcohols | 2 | 1 | track |
| 122-97-4 | Benzenepropanol | Alcohols | 1 | 1 | |
| 124-41-4 | Methanol, sodium salt | Alcohols | 1 | 1 | |
| 143-08-8 | 1-Nonanol | Alcohols | 1 | 1 | |
| 8027-33-6c | Alcohols, lanolin | Alcohols | 3 | 1 | track |
| 36653-82-4 | 1-Hexadecanol | Alcohols | 2 | 1 | |
| 67762-30-5 | Alcohols, C14-18 | Alcohols | 2 | 1 | track |
| 68603-15-6 | Alcohols, C6-12 | Alcohols | 1 | 1 | |
| 100-52-7 | Benzaldehyde | Aldehydes | 1 | 1 | |
| 124-13-0 | Octanal | Aldehydes | 1 | 1 | |
| 124-19-6 | Nonanal | Aldehydes | 1 | 1 | |
| 1334-78-7 | Benzaldehyde, methyl- | Aldehydes | 1 | 1 | |
| 8024-06-4c | Oils, vanilla | Aldehydes | 3 | 1 | track |
| 174333-80-3c | Benzaldehyde, 2-hydroxy-5-nonyl, oxime, branched | Aldehydes | 3 | 1 | track |
| 103-83-3 | Benzenemethanamine, N,N-dimethyl- | Aliphatic amines | 1 | 1 | track |
| 107-15-3 | 1,2-Ethanediamine | Aliphatic amines | 1 | 1 | track |
| 108-91-8 | Cyclohexanamine | Aliphatic amines | 1 | 1 | track |
| 111-40-0 | 1,2-Ethanediamine, N-(2-aminoethyl)- | Aliphatic amines | 1 | 1 | track |
| 112-90-3c | 9-Octadecen-1-amine, (Z)- | Aliphatic amines | 3 | 1 | track |
| 124-40-3 | Methanamine, N-methyl- | Aliphatic amines | 1 | 1 | track |
| 58713-21-6 | 1,3,5,7-Tetraazatricyclo[3.3.1.13#,7]decane, hydrochloride | Aliphatic amines | 1 | 1 | track |
| 61789-79-5 | Amines, bis(hydrogenated tallow alkyl) | Aliphatic amines | 1 | 1 | track |
| 68955-53-3 | Amines, C12-14-tert-alkyl | Aliphatic amines | 1 | 1 | track |
| 80939-62-4 | Amines, C11-14-branched alkyl, monohexyl and dihexyl phosphates | Aliphatic amines | 1 | 1 | track |
| 90367-27-4 | Ethanol, 2,2’-[[3-[(2-hydroxyethyl)amino]propyl]imino]bis-, N-tallow alkyl derivs. | Aliphatic amines | 1 | 1 | track |
| 91745-52-7c | Amines, coco alkyl, hydrochlorides | Aliphatic amines | 3 | 1 | track |
| 103-24-2 | Nonanedioic acid, bis(2-ethylhexyl) ester | Aliphatic diesters | 1 | 1 | track |
| 100-37-8 | Ethanol, 2-(diethylamino)- | Alkanolamines | 1 | 1 | |
| 102-71-6 | Ethanol, 2,2’,2’’-nitrilotris- | Alkanolamines | 1 | 1 | |
| 111-42-2 | Ethanol, 2,2’-iminobis- | Alkanolamines | 1 | 1 | track |
| 122-20-3 | 2-Propanol, 1,1’,1’’-nitrilotris- | Alkanolamines | 1 | 1 | |
| 141-43-5 | Ethanol, 2-amino- | Alkanolamines | 1 | 1 | |
| 61791-31-9 | Ethanol, 2,2’-iminobis-, N-coco alkyl derivs. | Alkanolamines | 1 | 1 | |
| 61791-44-4 | Ethanol, 2,2’-iminobis-, N-tallow alkyl derivs. | Alkanolamines | 1 | 1 | track |
| 127-68-4 | Benzenesulfonic acid, 3-nitro-, sodium salt | Alkyl aryl sulfonates/LABS and derivatives | 1 | 2 | track |
| 61789-85-3c | Sulfonic acids, petroleum | Alkyl aryl sulfonates/LABS and derivatives | 3 | 1 | track |
| 68584-25-8 | Benzenesulfonic acid, C10-16-alkyl derivs., compds. with triethanolamine | Alkyl aryl sulfonates/LABS and derivatives | 1 | 1 | track |
| 71486-79-8 | Benzenesulfonic acid, mono-C15-30-branched alkyl and di-C11-13-branched and linear alkyl derivs., calcium salts, overbased | Alkyl aryl sulfonates/LABS and derivatives | 1 | 2 | track |
| 21652-27-7c | 1H-Imidazole-1-ethanol, 2-(8-heptadecenyl)-4,5-dihydro-, (Z)- | Alkyl imidazolines and salts | 3 | 1 | track |
| 31135-57-6c | 1H-Benzimidazolesulfonic acid, 2-heptadecyl-1-[(sulfophenyl)methyl]-, disodium salt | Alkyl imidazolines and salts | 3 | 1 | track |
| 67633-57-2 | 1H-Imidazolium, 1-ethyl-4,5-dihydro-1-(2-hydroxyethyl)-2-isoheptadecyl-, ethyl sulfate (salt) | Alkyl imidazolines and salts | 2 | 1 | track |
| 68122-86-1 | Imidazolium compounds, 4,5-dihydro-1-methyl-2-nortallow alkyl-1-(2-tallow amidoethyl), Me sulfates | Alkyl imidazolines and salts | 1 | 1 | track |
| 68966-38-1c | 1H-Imidazole-1-ethanol, 4,5-dihydro-2-isoheptadecyl- | Alkyl imidazolines and salts | 3 | 1 | track |
| 74-88-4c | Methane, iodo- | Alkyl or aryl halides | 3 | 1 | track |
| 74-96-4 | Ethane, bromo- | Alkyl or aryl halides | 1 | 1 | track |
| 75-00-3 | Ethane, chloro- | Alkyl or aryl halides | 1 | 3 | track |
| 77-47-4c | 1,3-Cyclopentadiene, 1,2,3,4,5,5-hexachloro- | Alkyl or aryl halides | 3 | 1 | track |
| 106-94-5 | Propane, 1-bromo- | Alkyl or aryl halides | 1 | 1 | track |
| 126-99-8 | 1,3-Butadiene, 2-chloro- | Alkyl or aryl halides | 1 | 1 | |
| 156-60-5 | Ethene, 1,2-dichloro-, (E)- | Alkyl or aryl halides | 1 | 3 | |
| 630-20-6 | Ethane, 1,1,1,2-tetrachloro- | Alkyl or aryl halides | 1 | 1 | |
| 2235-54-3 | Sulfuric acid, monododecyl ester, ammonium salt | Alkyl Sulfates and Olefin Sulfonate | 1 | 1 | track |
| 15647-08-2 | Phosphorous acid, 2-ethylhexyl diphenyl ester | Alkyl/aryl Phosphites | 1 | 1 | track |
| 81-48-1c | 9,10-Anthracenedione, 1-hydroxy-4-[(4-methylphenyl)amino]- | Anthraquinones | 3 | 1 | track |
| 2379-79-5c | Anthra[2,3-d]oxazole-5,10-dione, 2-(1-amino-9,10-dihydro-9,10-dioxo-2-anthracenyl)- | Anthraquinones | 3 | 1 | track |
| 2475-45-8c | 9,10-Anthracenedione, 1,4,5,8-tetraamino- | Anthraquinones | 3 | 1 | track |
| 4051-63-2c | [1,1’-Bianthracene]-9,9’,10,10’-tetrone, 4,4’-diamino- | Anthraquinones | 3 | 1 | track |
| 6408-72-6c | 9,10-Anthracenedione, 1,4-diamino-2,3-diphenoxy- | Anthraquinones | 3 | 1 | track |
| 13676-91-0c | 9,10-Anthracenedione, 1,8-bis(phenylthio)- | Anthraquinones | 3 | 1 | track |
| 14233-37-5c | 9,10-Anthracenedione, 1,4-bis[(1-methylethyl)amino]- | Anthraquinones | 3 | 1 | track |
| 15791-78-3c | 9,10-Anthracenedione, 1,8-dihydroxy-4-[[4-(2-hydroxyethyl)phenyl]amino]-5-nitro- | Anthraquinones | 3 | 1 | track |
| 17418-58-5c | 9,10-Anthracenedione, 1-amino-4-hydroxy-2-phenoxy- | Anthraquinones | 3 | 1 | track |
| 19286-75-0c | 9,10-Anthracenedione, 1-hydroxy-4-(phenylamino)- | Anthraquinones | 3 | 1 | track |
| 19720-45-7c | 9,10-Anthracenedione, 1,4-bis[(2-methylpropyl)amino]- | Anthraquinones | 3 | 1 | track |
| 28173-59-3c | Carbonic acid, 2-[(1-amino-9,10-dihydro-4-hydroxy-9,10-dioxo-2-anthracenyl)oxy]ethyl phenyl ester | Anthraquinones | 3 | 1 | track |
| 72391-24-3c | Benzenesulfonic acid, [[(chloroacetyl)amino]methyl][4-[[4-(cyclohexylamino)-9,10-dihydro-9,10-dioxo-1-anthracenyl]amino]phenoxy]methyl-, monosodium salt | Anthraquinones | 3 | 1 | track |
| 74499-36-8c | 9,10-Anthracenedione, 1,4-diamino-, N,N'-mixed 2-ethylhexyl and Me and pentyl derivs. | Anthraquinones | 3 | 1 | track |
| 57-97-6 | Benz[a]anthracene, 7,12-dimethyl- | Arenes | 1 | 1 | track |
| 98-82-8 | Benzene, (1-methylethyl)- | Arenes | 1 | 1 | |
| 632-51-9 | Benzene, 1,1’,1’’,1’’’-(1,2-ethenediylidene)tetrakis- | Arenes | 1 | 1 | |
| 29036-02-0c | Quaterphenyl | Arenes | 3 | 1 | track |
| 38640-62-9 | Naphthalene, bis(1-methylethyl)- | Arenes | 2 | 1 | |
| 64800-83-5 | Benzene, ethyl(phenylethyl)- | Arenes | 1 | 1 | |
| 68398-19-6 | Benzene, ethyl(phenylethyl)-, mono-ar-ethyl deriv. | Arenes | 1 | 1 | |
| 68953-80-0 | Benzene, mixed with toluene, dealkylation product | Arenes | 1 | 3 | track |
| 68987-42-8 | Benzene, ethylenated, residues | Arenes | 1 | 1 | |
| 86-30-6 | Benzenamine, N-nitroso-N-phenyl- | Aromatic Amines | 1 | 1 | track |
| 91-66-7 | Benzenamine, N,N-diethyl- | Aromatic Amines | 1 | 1 | track |
| 95-54-5c | 1,2-Benzenediamine | Aromatic Amines | 3 | 1 | track |
| 95-55-6c | Phenol, 2-amino- | Aromatic Amines | 3 | 1 | track |
| 121-69-7 | Benzenamine, N,N-dimethyl- | Aromatic Amines | 1 | 1 | track |
| 122-39-4 | Benzenamine, N-phenyl- | Aromatic Amines | 2 | 1 | track |
| 134-09-8 | Cyclohexanol, 5-methyl-2-(1-methylethyl)-, 2-aminobenzoate | Aromatic Amines | 1 | 1 | track |
| 3081-14-9c | 1,4-Benzenediamine, N,N’-bis(1,4-dimethylpentyl)- | Aromatic Amines | 3 | 1 | track |
| 13680-35-8 | Benzenamine, 4,4’-methylenebis[2,6-diethyl- | Aromatic Amines | 1 | 1 | track |
| 63449-68-3 | 2-Naphthalenol, 2-aminobenzoyl ester | Aromatic Amines | 1 | 1 | track |
| 93-58-3 | Benzoic acid, methyl ester | Benzoates | 1 | 1 | |
| 93-89-0 | Benzoic acid, ethyl ester | Benzoates | 1 | 1 | |
| 120-50-3 | Benzoic acid, 2-methylpropyl ester | Benzoates | 1 | 1 | |
| 120-55-8 | Ethanol, 2,2’-oxybis-, dibenzoate | Benzoates | 2 | 1 | |
| 121-91-5 | 1,3-Benzenedicarboxylic acid | Benzoates | 1 | 1 | |
| 136-60-7 | Benzoic acid, butyl ester | Benzoates | 1 | 1 | |
| 614-33-5 | 1,2,3-Propanetriol, tribenzoate | Benzoates | 1 | 1 | track |
| 8024-05-3 | Oils, tuberose | Benzoates | 1 | 3 | |
| 27138-31-4 | Propanol, oxybis-, dibenzoate | Benzoates | 2 | 1 | track |
| 68052-23-3d | 1,3-Pentanediol, 2,2,4-trimethyl-, dibenzoate | Benzoates | 1 | 2 | |
| 95-31-8 | 2-Benzothiazolesulfenamide, N-(1,1-dimethylethyl)- | Benzotriazoles & benzothiazoles | 2 | 1 | track |
| 95-33-0 | 2-Benzothiazolesulfenamide, N-cyclohexyl- | Benzotriazoles & benzothiazoles | 1 | 1 | track |
| 4979-32-2 | 2-Benzothiazolesulfenamide, N,N-dicyclohexyl- | Benzotriazoles & benzothiazoles | 2 | 1 | track |
| 21564-17-0c | Thiocyanic acid, (2-benzothiazolylthio)methyl ester | Benzotriazoles & benzothiazoles | 3 | 1 | track |
| 29385-43-1 | 1H-Benzotriazole, 4(or 5)-methyl- | Benzotriazoles & benzothiazoles | 1 | 1 | track |
| 80584-90-3c | 1H-Benzotriazole-1-methanamine, N,N-bis(2-ethylhexyl)-4-methyl- | Benzotriazoles & benzothiazoles | 3 | 1 | track |
| 80595-74-0c | 1H-Benzotriazole-1-methanamine, N,N-bis(2-ethylhexyl)-5-methyl- | Benzotriazoles & benzothiazoles | 3 | 1 | track |
| 94270-86-7c | 1H-Benzotriazole-1-methanamine, N,N-bis(2-ethylhexyl)-ar-methyl- | Benzotriazoles & benzothiazoles | 3 | 1 | track |
| 85-42-7 | 1,3-Isobenzofurandione, hexahydro- | Carboxylic acid anhydrides | 1 | 1 | |
| 108-31-6 | 2,5-Furandione | Carboxylic acid anhydrides | 1 | 3 | |
| 552-30-7 | 5-Isobenzofurancarboxylic acid, 1,3-dihydro-1,3-dioxo- | Carboxylic acid anhydrides | 1 | 1 | |
| 79-09-4 | Propanoic acid | Carboxylic acids | 1 | 3 | track |
| 107-92-6 | Butanoic acid | Carboxylic acids | 1 | 1 | |
| 112-05-0 | Nonanoic acid | Carboxylic acids | 1 | 1 | |
| 144-62-7 | Ethanedioic acid | Carboxylic acids | 1 | 1 | |
| 64754-95-6b | Castor oil, hydrogenated, lithium salt | Inorganic - Lithium | 1 | 1 | |
| 76-03-9 | Acetic acid, trichloro- | Chloroacetic acids | 2 | 1 | |
| 79-43-6 | Acetic acid, dichloro- | Chloroacetic acids | 1 | 1 | |
| 101-37-1 | 1,3,5-Triazine, 2,4,6-tris(2-propenyloxy)- | Cyanurates | 2 | 1 | |
| 2893-78-9c | 1,3,5-Triazine-2,4,6(1H,3H,5H)-trione, 1,3-dichloro-, sodium salt | Cyanurates | 3 | 1 | track |
| 60-00-4 | Glycine, N,N’-1,2-ethanediylbis[N-(carboxymethyl)- | EDTA and salts | 1 | 1 | |
| 64-02-8 | Glycine, N,N’-1,2-ethanediylbis[N-(carboxymethyl)-, tetrasodium salt | EDTA and salts | 1 | 1 | |
| 15708-41-5 | Ferrate(1-), [[N,N’-1,2-ethanediylbis[N-(carboxymethyl)glycinato]](4-)-N,N’,O,O’,O#N,O#N#’]-, sodium, (OC-6-21)- | EDTA and salts | 1 | 1 | |
| 21265-50-9 | Ferrate(1-), [[N,N’-1,2-ethanediylbis[N-(carboxymethyl)glycinato]](4-)-N,N’,O,O’,O#N,O#N#’]-, ammonium, (OC-6-21)- | EDTA and salts | 1 | 2 | |
| 101-90-6 | Oxirane, 2,2’-[1,3-phenylenebis(oxymethylene)]bis- | Epoxides & glycidyl ethers | 2 | 1 | track |
| 106-92-3 | Oxirane, [(2-propenyloxy)methyl]- | Epoxides & glycidyl ethers | 2 | 1 | |
| 556-52-5 | Oxiranemethanol | Epoxides & glycidyl ethers | 2 | 1 | |
| 1139-30-6 | 5-Oxatricyclo[8.2.0.04#,6]dodecane, 4,12,12-trimethyl-9-methylene-, [1R-(1R,4R,6R,10S)]- | Epoxides & glycidyl ethers | 2 | 1 | track |
| 2210-79-9c | Oxirane, [(2-methylphenoxy)methyl]- | Epoxides & glycidyl ethers | 3 | 1 | track |
| 2451-62-9 | 1,3,5-Triazine-2,4,6(1H,3H,5H)-trione, 1,3,5-tris(oxiranylmethyl)- | Epoxides & glycidyl ethers | 2 | 3 | track |
| 28768-32-3c | Oxiranemethanamine, N,N’-(methylenedi-4,1-phenylene)bis[N-(oxiranylmethyl)- | Epoxides & glycidyl ethers | 3 | 1 | track |
| 61788-72-5c | Fatty acids, tall-oil, epoxidized, octyl esters | Epoxides & glycidyl ethers | 3 | 1 | track |
| 66072-38-6c | Oxirane, 2,2’,2’’-[methylidynetris(phenyleneoxymethylene)]tris- | Epoxides & glycidyl ethers | 3 | 1 | track |
| 68082-35-9c | Fatty acids, soya, epoxidized, Me esters | Epoxides & glycidyl ethers | 3 | 1 | track |
| 120547-52-6c | Oxirane, mono[(C12-13-alkyloxy)methyl] derivs. | Epoxides & glycidyl ethers | 3 | 1 | track |
| 79-20-9 | Acetic acid, methyl ester | Esters | 1 | 1 | |
| 102-76-1 | 1,2,3-Propanetriol, triacetate | Esters | 1 | 1 | |
| 106-70-7 | Hexanoic acid, methyl ester | Esters | 1 | 1 | |
| 109-60-4 | Acetic acid, propyl ester | Esters | 1 | 3 | |
| 110-19-0 | Acetic acid, 2-methylpropyl ester | Esters | 1 | 3 | |
| 111-55-7 | 1,2-Ethanediol, diacetate | Esters | 1 | 1 | |
| 111-82-0 | Dodecanoic acid, methyl ester | Esters | 1 | 1 | |
| 122-79-2 | Acetic acid, phenyl ester | Esters | 1 | 1 | |
| 577-11-7c | Butanedioic acid, sulfo-, 1,4-bis(2-ethylhexyl) ester, sodium salt | Esters | 3 | 1 | track |
| 623-42-7 | Butanoic acid, methyl ester | Esters | 1 | 1 | |
| 1119-40-0 | Pentanedioic acid, dimethyl ester | Esters | 1 | 1 | |
| 3234-85-3 | Tetradecanoic acid, tetradecyl ester | Esters | 1 | 1 | |
| 6846-50-0 | Propanoic acid, 2-methyl-, 2,2-dimethyl-1-(1-methylethyl)-1,3-propanediyl ester | Esters | 2 | 1 | track |
| 25265-77-4 | Propanoic acid, 2-methyl-, monoester with 2,2,4-trimethyl-1,3-pentanediol | Esters | 1 | 1 | |
| 68990-53-4 | Glycerides, C14-22 mono- | Esters | 1 | 1 | |
| 70657-70-4 | 1-Propanol, 2-methoxy-, acetate | Esters | 1 | 1 | |
| 60-29-7 | Ethane, 1,1’-oxybis- | Ethers | 1 | 1 | |
| 101-84-8 | Benzene, 1,1’-oxybis- | Ethers | 1 | 1 | |
| 115-10-6 | Methane, oxybis- | Ethers | 1 | 3 | |
| 34590-94-8 | Propanol, 1(or 2)-(2-methoxymethylethoxy)- | Ethers | 1 | 1 | |
| 110-71-4 | Ethane, 1,2-dimethoxy- | Ethylene glycol ethers | 1 | 1 | |
| 111-46-6 | Ethanol, 2,2’-oxybis- | Ethylene glycol ethers | 1 | 1 | |
| 111-90-0 | Ethanol, 2-(2-ethoxyethoxy)- | Ethylene glycol ethers | 1 | 1 | |
| 111-96-6 | Ethane, 1,1’-oxybis[2-methoxy- | Ethylene glycol ethers | 1 | 1 | |
| 112-07-2 | Ethanol, 2-butoxy-, acetate | Ethylene glycol ethers | 1 | 1 | |
| 112-27-6 | Ethanol, 2,2’-[1,2-ethanediylbis(oxy)]bis- | Ethylene glycol ethers | 1 | 1 | |
| 112-34-5 | Ethanol, 2-(2-butoxyethoxy)- | Ethylene glycol ethers | 1 | 1 | |
| 112-49-2 | 2,5,8,11-Tetraoxadodecane | Ethylene glycol ethers | 1 | 1 | |
| 112-60-7 | Ethanol, 2,2’-[oxybis(2,1-ethanediyloxy)]bis- | Ethylene glycol ethers | 1 | 1 | |
| 97-53-0 | Phenol, 2-methoxy-4-(2-propenyl)- | Eugenol and Isoeugenol derivatives | 1 | 1 | |
| 120-11-6 | Benzene, 2-methoxy-1-(phenylmethoxy)-4-(1-propenyl)- | Eugenol and Isoeugenol derivatives | 1 | 1 | |
| 120-24-1 | Benzeneacetic acid, 2-methoxy-4-(1-propenyl)phenyl ester | Eugenol and Isoeugenol derivatives | 1 | 1 | |
| 84696-47-9 | Rose, Rosa canina , ext. | Eugenol and Isoeugenol derivatives | 1 | 1 | |
| 112-38-9 | 10-Undecenoic acid | Fatty acids and salts | 1 | 1 | |
| 463-40-1c | 9,12,15-Octadecatrienoic acid, (Z,Z,Z)- | Fatty acids and salts | 3 | 1 | track |
| 8001-20-5 | Tung oil | Fatty acids and salts | 1 | 3 | |
| 8002-65-1c | Margosa oil | Fatty acids and salts | 3 | 1 | track |
| 53980-88-4 | 2-Cyclohexene-1-octanoic acid, 5(or 6)-carboxy-4-hexyl- | Fatty acids and salts | 2 | 1 | track |
| 61788-89-4 | Fatty acids, C18-unsatd., dimers | Fatty acids and salts | 1 | 1 | |
| 61790-12-3d | Fatty acids, tall-oil | Fatty acids and salts | 2 | 1 | track |
| 61790-44-1c | Fatty acids, tall-oil, potassium salts | Fatty acids and salts | 3 | 1 | track |
| 68139-89-9c | Fatty acids, tall-oil, maleated | Fatty acids and salts | 3 | 1 | track |
| 68476-03-9c | Fatty acids, montan-wax | Fatty acids and salts | 3 | 1 | track |
| 68551-42-8b | Fatty acids, C6-19-branched, manganese salts | Fatty acids and salts | 1 | 1 | |
| 68647-55-2 | Fatty acids, tall-oil, esters with triethanolamine | Fatty acids and salts | 1 | 1 | |
| 68647-58-5b,c | Aluminum, benzoate hydrogenated tallow fatty acid iso-Pr alc. complexes | Fatty acids and salts | 3 | 1 | track |
| 68783-36-8b,d | Fatty acids, C16-22, lithium salts | Fatty acids and salts | 2 | 1 | track |
| 68783-37-9b,d | Fatty acids, C16-18, lithium salts | Fatty acids and salts | 2 | 1 | track |
| 68937-90-6 | Fatty acids, C18-unsatd., trimers | Fatty acids and salts | 1 | 1 | |
| 73138-45-1 | Fatty acids, montan-wax, ethylene esters | Fatty acids and salts | 1 | 1 | |
| 90028-66-3c | Evening primrose, Oenotherabiennis, ext. | Fatty acids and salts | 3 | 1 | track |
| 92044-87-6 | Fatty acids, coco, 2-ethylhexyl esters | Fatty acids and salts | 1 | 1 | |
| 112-84-5c | 13-Docosenamide, (Z)- | Fatty amides | 2 | 3 | |
| 120-40-1 | Dodecanamide, N,N-bis(2-hydroxyethyl)- | Fatty amides | 1 | 1 | |
| 142-78-9 | Dodecanamide, N-(2-hydroxyethyl)- | Fatty amides | 1 | 1 | |
| 301-02-0c | 9-Octadecenamide, (Z)- | Fatty amides | 2 | 2 | |
| 11053-1a | Fatty acids compounded with ethylenediamine | Fatty amides | 1 | 1 | |
| 11555-8a | Fatty acids, reaction products with maleic anhydride and triethanolamine | Fatty amides | 1 | 1 | |
| 11556-0a | Fatty acids, reaction products with maleic anhydride | Fatty amides | 1 | 1 | |
| 11557-1a | Fatty acids, reaction products with maleic anhydride and oleylamine | Fatty amides | 1 | 1 | |
| 68153-35-5 | Ethanaminium, 2-amino-N-(2-aminoethyl)-N-(2-hydroxyethyl)-N-methyl-, N,N’-ditallow acyl derivs., Me sulfates (salts) | Fatty amides | 1 | 3 | |
| 68478-81-9 | 9-Octadecenoic acid (Z)-, reaction products with 3-(dodecenyl)dihydro-2,5-furandione and triethylenetetramine | Fatty amides | 1 | 1 | |
| 68603-42-9 | Amides, coco, N,N-bis(hydroxyethyl) | Fatty amides | 1 | 1 | track |
| 68784-17-8 | Isooctadecanoic acid, reaction products with tetraethylenepentamine | Fatty amides | 1 | 1 | |
| 71820-35-4 | Fatty acids, tall-oil, low-boiling, reaction products with 1-piperazineethanamine | Fatty amides | 1 | 1 | |
| 78-40-0 | Phosphoric acid, triethyl ester | Flame Retardants | 1 | 1 | track |
| 78-42-2 | Phosphoric acid, tris(2-ethylhexyl) ester | Flame Retardants | 2 | 1 | track |
| 298-07-7 | Phosphoric acid, bis(2-ethylhexyl) ester | Flame Retardants | 2 | 1 | track |
| 26446-73-1 | Phosphoric acid, bis(methylphenyl) phenyl ester | Flame Retardants | 2 | 1 | track |
| 64-18-6 | Formic acid | Formic acids & formates | 1 | 3 | |
| 107-31-3 | Formic acid, methyl ester | Formic acids & formates | 1 | 1 | |
| 109-94-4 | Formic acid, ethyl ester | Formic acids & formates | 1 | 1 | |
| 141-53-7 | Formic acid, sodium salt | Formic acids & formates | 1 | 3 | |
| 77-09-8 | 1(3H)-Isobenzofuranone, 3,3-bis(4-hydroxyphenyl)- | Furan and derivatives | 1 | 1 | track |
| 98-00-0 | 2-Furanmethanol | Furan and derivatives | 1 | 1 | |
| 109-99-9 | Furan, tetrahydro- | Furan and derivatives | 1 | 1 | |
| 110-00-9 | Furan | Furan and derivatives | 1 | 1 | |
| 126-33-0 | Thiophene, tetrahydro-, 1,1-dioxide | Furan and derivatives | 1 | 1 | |
| 96-45-7 | 2-Imidazolidinethione | Heterocycles | 1 | 1 | |
| 100-97-0 | 1,3,5,7-Tetraazatricyclo[3.3.1.13#,7]decane | Heterocycles | 1 | 3 | |
| 110-91-8 | Morpholine | Heterocycles | 1 | 1 | |
| 132-65-0 | Dibenzothiophene | Heterocycles | 2 | 1 | |
| 4174-09-8c | 3H-Pyrazol-3-one, 2,4-dihydro-4-[(5-hydroxy-3-methyl-1-phenyl-1H-pyrazol-4-yl)methylene]-5-methyl-2-phenyl- | Heterocycles | 3 | 1 | track |
| 28984-69-2c | 4,4(5H)-Oxazoledimethanol, 2-(heptadecenyl)- | Heterocycles | 3 | 1 | track |
| 68909-18-2 | Pyridinium, 1-(phenylmethyl)-, Et Me derivs., chlorides | Heterocycles | 1 | 1 | track |
| 4080-31-3 | 3,5,7-Triaza-1-azoniatricyclo[3.3.1.13#,7]decane, 1-(3-chloro-2-propenyl)-, chloride | Hexamethylenetetramine | 1 | 2 | |
| 51229-78-8 | 3,5,7-Triaza-1-azoniatricyclo[3.3.1.13#,7]decane, 1-(3-chloro-2-propenyl)-, chloride, (Z)- | Hexamethylenetetramine | 1 | 1 | |
| 79-74-3c | 1,4-Benzenediol, 2,5-bis(1,1-dimethylpropyl)- | Hindered phenols | 3 | 1 | track |
| 85-60-9c | Phenol, 4,4’-butylidenebis[2-(1,1-dimethylethyl)-5-methyl- | Hindered phenols | 3 | 1 | track |
| 2082-79-3 | Benzenepropanoic acid, 3,5-bis(1,1-dimethylethyl)-4-hydroxy-, octadecyl ester | Hindered phenols | 1 | 3 | track |
| 6386-38-5 | Benzenepropanoic acid, 3,5-bis(1,1-dimethylethyl)-4-hydroxy-, methyl ester | Hindered phenols | 1 | 1 | track |
| 41484-35-9 | Benzenepropanoic acid, 3,5-bis(1,1-dimethylethyl)-4-hydroxy-, thiodi-2,1-ethanediyl ester | Hindered phenols | 1 | 2 | track |
| 104-15-4 | Benzenesulfonic acid, 4-methyl- | Hydrotropes and derivatives | 1 | 1 | |
| 12068-03-0 | Benzenesulfonic acid, methyl-, sodium salt | Hydrotropes and derivatives | 1 | 1 | |
| 2422-91-5c | Benzene, 1,1’,1’’-methylidynetris[4-isocyanato- | Isocyanates | 3 | 1 | track |
| 4035-89-6c | Imidodicarbonic diamide, N,N’,2-tris(6-isocyanatohexyl)- | Isocyanates | 3 | 1 | track |
| 4098-71-9d | Cyclohexane, 5-isocyanato-1-(isocyanatomethyl)-1,3,3-trimethyl- | Isocyanates | 1 | 2 | |
| 4151-51-3c | Phenol, 4-isocyanato-, phosphorothioate (3:1) (ester) | Isocyanates | 3 | 1 | track |
| 78-93-3 | 2-Butanone | Ketones | 1 | 3 | |
| 107-87-9 | 2-Pentanone | Ketones | 1 | 3 | |
| 108-10-1 | 2-Pentanone, 4-methyl- | Ketones | 1 | 1 | |
| 110-12-3 | 2-Hexanone, 5-methyl- | Ketones | 1 | 1 | |
| 123-42-2 | 2-Pentanone, 4-hydroxy-4-methyl- | Ketones | 1 | 3 | |
| 123-54-6 | 2,4-Pentanedione | Ketones | 1 | 1 | |
| 141-79-7 | 3-Penten-2-one, 4-methyl- | Ketones | 1 | 1 | |
| 431-03-8 | 2,3-Butanedione | Ketones | 1 | 1 | |
| 513-86-0 | 2-Butanone, 3-hydroxy- | Ketones | 1 | 1 | |
| 600-14-6 | 2,3-Pentanedione | Ketones | 1 | 1 | |
| 68442-69-3 | Benzene, mono-C10-14-alkyl derivs. | Linear Alkyl Benzenes (LAB) and Derivatives | 1 | 1 | track |
| 68890-99-3 | Benzene, mono-C10-16-alkyl derivs. | Linear Alkyl Benzenes (LAB) and Derivatives | 1 | 1 | track |
| 106-02-5 | Oxacyclohexadecan-2-one | Musks (Macro/Poly cyclic) | 1 | 1 | |
| 108-94-1 | Cyclohexanone | Musks (Macro/Poly cyclic) | 1 | 1 | |
| 109-29-5 | Oxacycloheptadecan-2-one | Musks (Macro/Poly cyclic) | 1 | 1 | |
| 502-72-7 | Cyclopentadecanone | Musks (Macro/Poly cyclic) | 1 | 1 | |
| 541-91-3 | Cyclopentadecanone, 3-methyl- | Musks (Macro/Poly cyclic) | 1 | 1 | |
| 542-46-1 | 9-Cycloheptadecen-1-one, (Z)- | Musks (Macro/Poly cyclic) | 1 | 1 | |
| 1335-94-0 | Irone | Musks (Macro/Poly cyclic) | 2 | 1 | |
| 7779-30-8 | 1-Penten-3-one, 1-(2,6,6-trimethyl-2-cyclohexen-1-yl)- | Musks (Macro/Poly cyclic) | 2 | 1 | |
| 7779-50-2 | Oxacycloheptadec-7-en-2-one | Musks (Macro/Poly cyclic) | 1 | 1 | |
| 8001-04-5 | Musks | Musks (Macro/Poly cyclic) | 1 | 1 | track |
| 28645-51-4 | Oxacycloheptadec-10-en-2-one | Musks (Macro/Poly cyclic) | 1 | 1 | |
| 37609-25-9 | 5-Cyclohexadecen-1-one | Musks (Macro/Poly cyclic) | 1 | 1 | |
| 68140-48-7 | Ethanone, 1-[2,3-dihydro-1,1,2,6-tetramethyl-3-(1-methylethyl)-1H-inden-5-yl]- | Musks (Macro/Poly cyclic) | 1 | 1 | |
| 1321-69-3 | Naphthalenesulfonic acid, sodium salt | Naphthalene sulfonic acids and salts | 1 | 1 | track |
| 25638-17-9 | Naphthalenesulfonic acid, butyl-, sodium salt | Naphthalene sulfonic acids and salts | 1 | 1 | track |
| 61789-36-4 | Naphthenic acids, calcium salts | Naphthenic acids and salts | 1 | 1 | track |
| 75-05-8 | Acetonitrile | Nitriles | 1 | 1 | |
| 78-67-1 | Propanenitrile, 2,2’-azobis[2-methyl- | Nitriles | 1 | 2 | |
| 13472-08-7 | Butanenitrile, 2,2’-azobis[2-methyl- | Nitriles | 1 | 2 | track |
| 61790-28-1 | Nitriles, tallow | Nitriles | 1 | 3 | track |
| 61790-29-2d | Nitriles, tallow, hydrogenated | Nitriles | 2 | 1 | track |
| 125328-64-5 | Nitriles, rape-oil, hydrogenated | Nitriles | 1 | 2 | |
| 100-00-5 | Benzene, 1-chloro-4-nitro- | Nitrobenzenes | 1 | 1 | track |
| 872-50-4 | 2-Pyrrolidinone, 1-methyl- | NMP and NEP | 1 | 1 | |
| 2687-91-4 | 2-Pyrrolidinone, 1-ethyl- | NMP and NEP | 1 | 1 | |
| 80-15-9c | Hydroperoxide, 1-methyl-1-phenylethyl | Organic peroxides | 3 | 1 | track |
| 80-43-3c | Peroxide, bis(1-methyl-1-phenylethyl) | Organic peroxides | 2 | 2 | track |
| 133-14-2c | Peroxide, bis(2,4-dichlorobenzoyl) | Organic peroxides | 3 | 1 | track |
| 614-45-9 | Benzenecarboperoxoic acid, 1,1-dimethylethyl ester | Organic peroxides | 1 | 1 | |
| 3006-86-8d | Peroxide,cyclohexylidenebis[(1,1-dimethylethyl) | Organic peroxides | 1 | 2 | track |
| 3851-87-4 | Peroxide, bis(3,5,5-trimethyl-1-oxohexyl) | Organic peroxides | 2 | 1 | |
| 94-13-3 | Benzoic acid, 4-hydroxy-, propyl ester | Parabens | 1 | 1 | track |
| 94-18-8 | Benzoic acid, 4-hydroxy-, phenylmethyl ester | Parabens | 1 | 1 | track |
| 94-26-8 | Benzoic acid, 4-hydroxy-, butyl ester | Parabens | 1 | 1 | track |
| 99-76-3 | Benzoic acid, 4-hydroxy-, methyl ester | Parabens | 1 | 1 | |
| 120-47-8 | Benzoic acid, 4-hydroxy-, ethyl ester | Parabens | 1 | 1 | |
| 4191-73-5 | Benzoic acid, 4-hydroxy-, 1-methylethyl ester | Parabens | 1 | 1 | track |
| 4247-02-3 | Benzoic acid, 4-hydroxy-, 2-methylpropyl ester | Parabens | 1 | 1 | |
| 25155-23-1d | Phenol, dimethyl-, phosphate (3:1) | Phosphoric acid derivatives | 1 | 2 | track |
| 37310-83-1c | 9-Octadecen-1-ol, (Z)-, phosphate | Phosphoric acid derivatives | 3 | 1 | track |
| 68604-99-9c | Fatty acids, C18-unsatd., phosphates | Phosphoric acid derivatives | 3 | 1 | track |
| 68952-35-2 | Tar acids, cresylic, Ph phosphates | Phosphoric acid derivatives | 2 | 1 | |
| 111174-61-9c | Alcohols, C8-16, reaction products with phosphorus oxide (P2O5), compds. with 2-ethyl-1-hexanamine | Phosphoric acid derivatives | 3 | 1 | track |
| 119345-01-6 | Phosphorous trichloride, reaction products with 1,1’-biphenyl and 2,4-bis(1,1-methylethyl)phenol | Phosphoric acid derivatives | 1 | 1 | |
| 596-03-2 | Spiro[isobenzofuran-1(3H),9’-[9H]xanthen]-3-one, 4’,5’-dibromo-3’,6’-dihydroxy- | Pigments and Dyes | 1 | 1 | track |
| 1328-04-7b,c | C.I. Pigment Violet 5:1 | Pigments and Dyes | 3 | 1 | track |
| 1328-51-4b,c | C.I. Solvent Blue 38 | Pigments and Dyes | 3 | 1 | track |
| 2387-03-3c | 1-Naphthalenecarboxaldehyde, 2-hydroxy-, [(2-hydroxy-1-naphthalenyl)methylene]hydrazone | Pigments and Dyes | 3 | 1 | track |
| 2478-20-8 | 1H-Benz[de]isoquinoline-1,3(2H)-dione, 6-amino-2-(2,4-dimethylphenyl)- | Pigments and Dyes | 1 | 1 | track |
| 4378-61-4 | Dibenzo[def,mno]chrysene-6,12-dione, 4,10-dibromo- | Pigments and Dyes | 1 | 1 | track |
| 5718-26-3c | 1H-Indole-5-carboxylic acid, 2-[(1,5-dihydro-3-methyl-5-oxo-1-phenyl-4H-pyrazol-4-ylidene)ethylidene]-2,3-dihydro-1,3,3-trimethyl-, methyl ester | Pigments and Dyes | 3 | 1 | track |
| 6858-49-7c | Propanedinitrile, [[4-[ethyl[2-[[(phenylamino)carbonyl]oxy]ethyl]amino]-2-methylphenyl]methylene]- | Pigments and Dyes | 3 | 1 | track |
| 7576-65-0 | 1H-Indene-1,3(2H)-dione, 2-(3-hydroxy-2-quinolinyl)- | Pigments and Dyes | 2 | 1 | track |
| 12224-98-5 | Xanthylium, 9-[2-(ethoxycarbonyl)phenyl]-3,6-bis(ethylamino)-2,7-dimethyl-, molybdatetungstatephosphate | Pigments and Dyes | 1 | 1 | track |
| 13082-47-8c | Xanthylium, 9-(2-carboxyphenyl)-3,6-bis(diethylamino)-, hydroxide | Pigments and Dyes | 3 | 1 | track |
| 16294-75-0 | 14H-Anthra[2,1,9-mna]thioxanthen-14-one | Pigments and Dyes | 1 | 1 | track |
| 26694-69-9c | Xanthylium, 9-[2-(ethoxycarbonyl)phenyl]-3,6-bis(ethylamino)-2,7-dimethyl-, ethyl sulfate | Pigments and Dyes | 3 | 1 | track |
| 42373-04-6c | Thiazolium, 3-methyl-2-[(1-methyl-2-phenyl-1H-indol-3-yl)azo]-, chloride | Pigments and Dyes | 3 | 1 | track |
| 62973-79-9b,c | Xanthylium, 9-(2-carboxyphenyl)-3,6-bis(diethylamino)-, molybdatesilicate | Pigments and Dyes | 3 | 1 | track |
| 63022-09-3b,c | Xanthylium, 9-(2-carboxyphenyl)-3,6-bis(diethylamino)-, molybdatephosphate | Pigments and Dyes | 3 | 1 | track |
| 68310-07-6 | Xanthylium, 3,6-bis(ethylamino)-9-[2-(methoxycarbonyl)phenyl]-2,7-dimethyl-, molybdatephosphate | Pigments and Dyes | 1 | 1 | track |
| 68409-66-5c | Ethanaminium, N-[4-[[4-(diethylamino)phenyl][4-(ethylamino)-1-naphthalenyl]methylene]-2,5-cyclohexadien-1-ylidene]-N-ethyl-, molybdatephosphate | Pigments and Dyes | 3 | 1 | track |
| 68814-02-8b,c | Ethanaminium, N-[4-[bis[4-(diethylamino)phenyl]methylene]-2,5-cyclohexadien-1-ylidene]-N-ethyl-, molybdatephosphate | Pigments and Dyes | 3 | 1 | track |
| 80083-40-5b,c | Xanthylium, 9-[2-(ethoxycarbonyl)phenyl]-3,6-bis(ethylamino)-2,7-dimethyl-, molybdatetungstatesilicate | Pigments and Dyes | 3 | 1 | track |
| 102082-92-8c | Xanthylium, 3,6-bis(diethylamino)-9-[2-(methoxycarbonyl)phenyl]-, molybdatesilicate | Pigments and Dyes | 3 | 1 | track |
| 9015-54-7 | Protein hydrolyzates | Proteins and derivatives | 1 | 1 | |
| 92113-31-0 | Collagens, hydrolyzates | Proteins and derivatives | 1 | 1 | |
| 111174-63-1c | Protein hydrolyzates, leather, reaction products with isostearoyl chloride | Proteins and derivatives | 3 | 1 | track |
| 74-86-2 | Ethyne | PSSA2 | 1 | 1 | |
| 57-09-0c | 1-Hexadecanaminium, N,N,N-trimethyl-, bromide | Quaternary ammonium compounds | 3 | 1 | track |
| 78-21-7 | Morpholinium, 4-ethyl-4-hexadecyl-, ethyl sulfate | Quaternary ammonium compounds | 2 | 1 | track |
| 3327-22-8 | 1-Propanaminium, 3-chloro-2-hydroxy-N,N,N-trimethyl-, chloride | Quaternary ammonium compounds | 2 | 1 | track |
| 61791-34-2c | Onium compounds, morpholinium, 4-ethyl-4-soya alkyl, Et sulfates | Quaternary ammonium compounds | 3 | 1 | track |
| 71011-25-1c | Quaternary ammonium compounds, benzyl(hydrogenated tallow alkyl)dimethyl, chlorides, compds. with bentonite and bis(hydrogenated tallow alkyl)dimethylammonium chlorides | Quaternary ammonium compounds | 3 | 1 | track |
| 72102-40-0c | 1-Propanaminium, 3-amino-N-ethyl-N,N-dimethyl-, N-lanolin acyl derivs., Et sulfates | Quaternary ammonium compounds | 3 | 1 | track |
| 90459-62-4c | Octadecanoic acid, reaction products with diethylenetriamine, di-Me sulfate-quaternized | Quaternary ammonium compounds | 3 | 1 | track |
| 115340-80-2c | 1-Propanaminium, 3-amino-N-ethyl-N,N-dimethyl-, N-wheat-oil acyl derivs., Et sulfates | Quaternary ammonium compounds | 3 | 1 | track |
| 1740-19-8 | 1-Phenanthrenecarboxylic acid, 1,2,3,4,4a,9,10,10a-octahydro-1,4a-dimethyl-7-(1-methylethyl)-, [1R-(1,4aβ,10a)]- | Resins & rosins | 2 | 1 | track |
| 8046-19-3 | Storax (balsam) | Resins & rosins | 1 | 1 | track |
| 26266-77-3 | 1-Phenanthrenemethanol, dodecahydro-1,4a-dimethyl-7-(1-methylethyl)- | Resins & rosins | 1 | 1 | track |
| 68186-14-1 | Resin acids and Rosin acids, Me esters | Resins & rosins | 1 | 1 | track |
| 73138-82-6c | Resin acids and Rosin acids | Resins & rosins | 3 | 1 | track |
| 91081-53-7c | Rosin, reaction products with formaldehyde | Resins & rosins | 3 | 1 | track |
| 69-72-7 | Benzoic acid, 2-hydroxy- | Salicylates | 1 | 1 | track |
| 87-22-9 | Benzoic acid, 2-hydroxy-, 2-phenylethyl ester | Salicylates | 1 | 1 | track |
| 68917-75-9 | Oils, wintergreen | Salicylates | 1 | 1 | track |
| 84012-15-7 | Birch, Betula alba, ext. | Salicylates | 1 | 1 | track |
| 107-46-0 | Disiloxane, hexamethyl- | Siloxanes | 2 | 1 | |
| 141-62-8c | Tetrasiloxane, decamethyl- | Siloxanes | 3 | 1 | track |
| 141-63-9c | Pentasiloxane, dodecamethyl- | Siloxanes | 3 | 1 | track |
| 541-05-9 | Cyclotrisiloxane, hexamethyl- | Siloxanes | 2 | 1 | |
| 2627-95-4 | Disiloxane, 1,3-diethenyl-1,1,3,3-tetramethyl- | Siloxanes | 2 | 1 | |
| 33204-76-1c | Cyclotetrasiloxane, 2,2,4,6,6,8-hexamethyl-4,8-diphenyl-, cis- | Siloxanes | 3 | 1 | track |
| 69430-24-6 | Cyclosiloxanes, di-Me | Siloxanes | 2 | 2 | |
| 1533-45-5c | Benzoxazole, 2,2’-(1,2-ethenediyldi-4,1-phenylene)bis- | Stilbenes | 3 | 1 | track |
| 3426-43-5c | Benzenesulfonic acid, 2,2’-(1,2-ethenediyl)bis[5-[[4-methoxy-6-(phenylamino)-1,3,5-triazin-2-yl]amino]-, disodium salt | Stilbenes | 3 | 1 | track |
| 4193-55-9 | Benzenesulfonic acid, 2,2’-(1,2-ethenediyl)bis[5-[[4-[bis(2-hydroxyethyl)amino]-6-(phenylamino)-1,3,5-triazin-2-yl]amino]-, disodium salt | Stilbenes | 2 | 2 | |
| 16090-02-1 | Benzenesulfonic acid, 2,2’-(1,2-ethenediyl)bis[5-[[4-(4-morpholinyl)-6-(phenylamino)-1,3,5-triazin-2-yl]amino]-, disodium salt | Stilbenes | 2 | 2 | |
| 68784-26-9 | Phenol, dodecyl-, sulfurized, carbonates, calcium salts, overbased | Substituted alkyl (dodecyl) phenols | 1 | 1 | |
| 80-54-6 | Benzenepropanal, 4-(1,1-dimethylethyl)--methyl- | Terpenes & Terpenoids | 1 | 1 | |
| 80-56-8 | Bicyclo[3.1.1]hept-2-ene, 2,6,6-trimethyl- | Terpenes & Terpenoids | 2 | 1 | |
| 87-44-5 | Bicyclo[7.2.0]undec-4-ene, 4,11,11-trimethyl-8-methylene-, [1R-(1R,4E,9S)]- | Terpenes & Terpenoids | 1 | 1 | |
| 88-84-6 | Azulene, 1,2,3,4,5,6,7,8-octahydro-1,4-dimethyl-7-(1-methylethylidene)-, (1S-cis)- | Terpenes & Terpenoids | 1 | 1 | |
| 117-98-6 | 6-Azulenol, 1,2,3,3a,4,5,6,8a-octahydro-4,8-dimethyl-2-(1-methylethylidene)-, acetate | Terpenes & Terpenoids | 2 | 1 | |
| 469-61-4 | 1H-3a,7-Methanoazulene, 2,3,4,7,8,8a-hexahydro-3,6,8,8-tetramethyl-, [3R-(3,3aβ,7β,8a)]- | Terpenes & Terpenoids | 1 | 1 | |
| 470-40-6c | Cyclopropa[d]naphthalene, 1,1a,4,4a,5,6,7,8-octahydro-2,4a,8,8-tetramethyl-, [1aS-(1a,4aβ,8aR)]- | Terpenes & Terpenoids | 3 | 1 | track |
| 471-53-4c | Olean-12-en-29-oic acid, 3-hydroxy-11-oxo, (3β,20β) | Terpenes & Terpenoids | 3 | 1 | track |
| 489-40-7c | 1H-Cycloprop[e]azulene, 1a,2,3,4,4a,5,6,7b-octahydro-1,1,4,7-tetramethyl-, [1aR-(1a,4,4aβ,7b)]- | Terpenes & Terpenoids | 3 | 1 | track |
| 489-84-9 | Azulene, 1,4-dimethyl-7-(1-methylethyl)- | Terpenes & Terpenoids | 1 | 1 | |
| 489-86-1 | 5-Azulenemethanol, 1,2,3,4,5,6,7,8-octahydro-,,3,8-tetramethyl-, [3S-(3,5,8)]- | Terpenes & Terpenoids | 1 | 1 | |
| 495-62-5 | Cyclohexene, 4-(1,5-dimethyl-4-hexenylidene)-1-methyl- | Terpenes & Terpenoids | 1 | 1 | |
| 514-51-2 | 4,7-Methanoazulene, 1,2,3,4,5,6,7,8-octahydro-1,4,9,9-tetramethyl-, [1S-(1,4,7)]- | Terpenes & Terpenoids | 1 | 1 | |
| 546-28-1 | 1H-3a,7-Methanoazulene, octahydro-3,8,8-trimethyl-6-methylene-, [3R-(3,3aβ,7β,8a)]- | Terpenes & Terpenoids | 1 | 1 | |
| 639-99-6 | Cyclohexanemethanol, 4-ethenyl-,,4-trimethyl-3-(1-methylethenyl)-, [1R-(1,3,4β)]- | Terpenes & Terpenoids | 1 | 1 | |
| 1113-21-9 | 1,6,10,14-Hexadecatetraen-3-ol, 3,7,11,15-tetramethyl-, (E,E)- | Terpenes & Terpenoids | 1 | 1 | |
| 3407-42-9 | Cyclohexanol, 3-(5,5,6-trimethylbicyclo[2.2.1]hept-2-yl)- | Terpenes & Terpenoids | 1 | 1 | track |
| 3691-12-1c | Azulene, 1,2,3,4,5,6,7,8-octahydro-1,4-dimethyl-7-(1-methylethenyl)-, [1S-(1,4,7)]- | Terpenes & Terpenoids | 3 | 1 | track |
| 3738-00-9 | Naphtho[2,1-b]furan, dodecahydro-3a,6,6,9a-tetramethyl- | Terpenes & Terpenoids | 1 | 1 | |
| 4572-09-2c | Olean-12-en-29-oic acid, 3-hydroxy-11-oxo-, (3β,20β)-, compd. with (2,5-dioxo-4-imidazolidinyl)urea (1:1) | Terpenes & Terpenoids | 3 | 1 | track |
| 4630-07-3c | Naphthalene, 1,2,3,5,6,7,8,8a-octahydro-1,8a-dimethyl-7-(1-methylethenyl)-, [1R-(1,7β,8a)]- | Terpenes & Terpenoids | 3 | 1 | track |
| 8000-27-9 | Oils, cedarwood | Terpenes & Terpenoids | 1 | 1 | |
| 8000-46-2 | Oils, geranium | Terpenes & Terpenoids | 1 | 1 | |
| 8001-61-4 | Balsams, copaiba | Terpenes & Terpenoids | 1 | 2 | |
| 8002-09-3 | Oils, pine | Terpenes & Terpenoids | 2 | 1 | |
| 8006-64-2 | Turpentine, oil | Terpenes & Terpenoids | 1 | 1 | |
| 8006-78-8 | Oils, bay | Terpenes & Terpenoids | 1 | 1 | track |
| 8006-87-9 | Oils, sandalwood | Terpenes & Terpenoids | 1 | 1 | |
| 8007-01-0 | Oils, rose | Terpenes & Terpenoids | 1 | 1 | |
| 8007-02-1 | Oils, lemongrass | Terpenes & Terpenoids | 2 | 1 | |
| 8007-08-7 | Oils, ginger | Terpenes & Terpenoids | 1 | 1 | |
| 8008-31-9 | Oils, mandarin | Terpenes & Terpenoids | 1 | 1 | |
| 8008-52-4 | Oils, coriander | Terpenes & Terpenoids | 1 | 1 | |
| 8008-57-9 | Oils, orange, sweet | Terpenes & Terpenoids | 1 | 1 | |
| 8008-93-3 | Oils, wormwood | Terpenes & Terpenoids | 1 | 1 | track |
| 8013-10-3c | Oils, cade | Terpenes & Terpenoids | 3 | 1 | track |
| 8014-19-5 | Oils, palmarosa | Terpenes & Terpenoids | 1 | 1 | |
| 8015-77-8 | Oils, bois de rose | Terpenes & Terpenoids | 1 | 1 | |
| 8016-37-3 | Oils, myrrh | Terpenes & Terpenoids | 1 | 1 | |
| 8016-85-1 | Oils, tangerine | Terpenes & Terpenoids | 1 | 1 | |
| 8016-88-4 | Oils, tarragon | Terpenes & Terpenoids | 1 | 1 | |
| 8021-28-1 | Oils, fir | Terpenes & Terpenoids | 1 | 1 | |
| 8022-56-8 | Oils, sage | Terpenes & Terpenoids | 1 | 1 | |
| 8022-96-6 | Oils, jasmine | Terpenes & Terpenoids | 1 | 1 | |
| 8023-75-4 | Oils, jonquil | Terpenes & Terpenoids | 1 | 1 | |
| 8024-08-6 | Oils, violet | Terpenes & Terpenoids | 1 | 1 | |
| 8024-43-9 | Perfumes and Essences, jasmin | Terpenes & Terpenoids | 1 | 1 | |
| 8031-03-6 | Oils, mimosa | Terpenes & Terpenoids | 1 | 2 | |
| 9005-90-7 | Turpentine | Terpenes & Terpenoids | 1 | 1 | |
| 17627-44-0 | Cyclohexene, 4-(1,5-dimethyl-1,4-hexadienyl)-1-methyl- | Terpenes & Terpenoids | 1 | 1 | |
| 22451-73-6 | 5-Azulenemethanol, 1,2,3,3a,4,5,6,7-octahydro-,,3,8-tetramethyl-, [3S-(3,3aβ,5)]- | Terpenes & Terpenoids | 1 | 1 | |
| 25428-43-7 | 3-Cyclohexene-1-methanol, ,4-dimethyl--(4-methyl-3-pentenyl)-, (R,R)-(±)- | Terpenes & Terpenoids | 1 | 1 | |
| 29350-73-0 | Naphthalene, decahydro-1,6-dimethyl-4-(1-methylethyl)-, [1S-(1,4,4a,6,8aβ)]-, didehydro deriv. | Terpenes & Terpenoids | 1 | 1 | |
| 37677-14-8 | 3-Cyclohexene-1-carboxaldehyde, 4-(4-methyl-3-pentenyl)- | Terpenes & Terpenoids | 1 | 1 | |
| 52474-60-9 | 3-Cyclohexene-1-carboxaldehyde, 1-methyl-3-(4-methyl-3-pentenyl)- | Terpenes & Terpenoids | 1 | 1 | |
| 52475-86-2 | 3-Cyclohexene-1-carboxaldehyde, 1-methyl-4-(4-methyl-3-pentenyl)- | Terpenes & Terpenoids | 1 | 1 | |
| 59056-62-1 | 2,3b-Methano-3bH-cyclopenta[1,3]cyclopropa[1,2]benzene-4-methanol, octahydro-7,7,8,8-tetramethyl-, acetate | Terpenes & Terpenoids | 2 | 1 | |
| 65113-99-7 | 3-Cyclopentene-1-butanol, ,β,2,2,3-pentamethyl- | Terpenes & Terpenoids | 1 | 1 | |
| 65405-84-7 | Cyclohexenebutanal, ,2,2,6-tetramethyl- | Terpenes & Terpenoids | 1 | 1 | |
| 66068-84-6 | Cyclohexanol, 4-(5,5,6-trimethylbicyclo[2.2.1]hept-2-yl)- | Terpenes & Terpenoids | 1 | 1 | track |
| 66327-54-6 | 3-Cyclohexene-1-carboxaldehyde, 1-methyl-4-(4-methylpentyl)- | Terpenes & Terpenoids | 1 | 1 | |
| 68608-32-2 | Terpenes and Terpenoids, cedarwood-oil | Terpenes & Terpenoids | 1 | 1 | |
| 68877-29-2 | Cyclohexanol, (1,7,7-trimethylbicyclo[2.2.1]hept-2-yl)- | Terpenes & Terpenoids | 1 | 1 | track |
| 68916-97-2 | Oils, horehound | Terpenes & Terpenoids | 1 | 1 | |
| 68917-29-3d | Terpenes and Terpenoids, clove-oil | Terpenes & Terpenoids | 2 | 1 | track |
| 68917-65-7 | Terpenes and Terpenoids, vetiver-oil | Terpenes & Terpenoids | 1 | 1 | |
| 68990-83-0 | Oils, cedarwood, Texan | Terpenes & Terpenoids | 1 | 1 | |
| 70788-30-6 | Cyclohexanepropanol, 2,2,6-trimethyl--propyl- | Terpenes & Terpenoids | 1 | 1 | |
| 70955-71-4 | Phenol, 2-methoxy-, reaction products with 2,2-dimethyl-3-methylenebicyclo[2.2.1]heptane, hydrogenated | Terpenes & Terpenoids | 1 | 1 | track |
| 84082-54-2 | Ivy, Hedera helix, ext. | Terpenes & Terpenoids | 1 | 1 | |
| 84961-67-1c | Verbena officinalis, ext. | Terpenes & Terpenoids | 3 | 1 | track |
| 90045-36-6c | Ginkgo biloba, ext. | Terpenes & Terpenoids | 3 | 1 | track |
| 90045-38-8c | Ginseng, Panaxquinquefolium, ext. | Terpenes & Terpenoids | 3 | 1 | track |
| 107898-54-4 | 4-Penten-2-ol, 3,3-dimethyl-5-(2,2,3-trimethyl-3-cyclopenten-1-yl)- | Terpenes & Terpenoids | 2 | 1 | |
| 164288-52-2 | Cork tree, Phellodendronamurense, ext. | Terpenes & Terpenoids | 1 | 1 | |
| 60-24-2 | Ethanol, 2-mercapto- | Thiols | 1 | 1 | |
| 75-18-3 | Methane, thiobis- | Thiols | 1 | 1 | |
| 150-60-7 | Disulfide, bis(phenylmethyl) | Thiols | 2 | 1 | |
| 25103-58-6 | tert-Dodecanethiol | Thiols | 2 | 1 | track |
| 71159-90-5c | 3-Cyclohexene-1-methanethiol, ,,4-trimethyl- | Thiols | 3 | 1 | track |
| 73984-93-7c | 1,3,4-Thiadiazole-2(3H)-thione, 5-(tert-dodecyldithio)- | Thiols | 3 | 1 | track |
| 632-99-5 | Benzenamine, 4-[(4-aminophenyl)(4-imino-2,5-cyclohexadien-1-ylidene)methyl]-2-methyl-, monohydrochloride | Triarylmethanes | 1 | 1 | track |
| 61-82-5 | 1H-1,2,4-Triazol-3-amine | Triazoles | 1 | 1 | |
| 121-82-4c | 1,3,5-Triazine, hexahydro-1,3,5-trinitro- | Triazoles | 3 | 1 | track |
| 288-88-0 | 1H-1,2,4-Triazole | Triazoles | 1 | 1 | |
| 3089-11-0 | 1,3,5-Triazine-2,4,6-triamine, N,N,N’,N’,N’’,N’’-hexakis(methoxymethyl)- | Triazoles | 1 | 3 | track |
| 3319-31-1 | 1,2,4-Benzenetricarboxylic acid, tris(2-ethylhexyl) ester | Trimellitates | 1 | 1 | |
| 53894-23-8 | 1,2,4-Benzenetricarboxylic acid, triisononyl ester | Trimellitates | 1 | 1 | |
| 68515-60-6 | 1,2,4-Benzenetricarboxylic acid, tri-C7-9-branched and linear alkyl esters | Trimellitates | 1 | 1 | |
| 70225-05-7 | 1,2,4-Benzenetricarboxylic acid, mixed branched tridecyl andisodecyl esters | Trimellitates | 1 | 1 | |
| 94109-09-8 | 1,2,4-Benzenetricarboxylic acid, tritridecyl ester | Trimellitates | 1 | 1 | |
| 67-97-0c | 9,10-Secocholesta-5,7,10(19)-trien-3-ol, (3β,5Z,7E)- | Vitamins & derivatives | 3 | 1 | track |
| 68-26-8 | Retinol | Vitamins & derivatives | 1 | 1 | |
| 116-31-4 | Retinal | Vitamins & derivatives | 1 | 1 | |
| 7235-40-7 | β,β-Carotene | Vitamins & derivatives | 1 | 1 | |
| 11103-57-4 | Vitamin A | Vitamins & derivatives | 1 | 1 | |
| 59-50-7 | Phenol, 4-chloro-3-methyl- | NA | 1 | 1 | |
| 62-44-2 | Acetamide, N-(4-ethoxyphenyl)- | NA | 1 | 1 | |
| 64-17-5 | Ethanol | NA | 1 | 3 | |
| 64-19-7d | Acetic acid | NA | 1 | 3 | |
| 77-73-6 | 4,7-Methano-1H-indene, 3a,4,7,7a-tetrahydro- | NA | 1 | 1 | |
| 88-19-7 | Benzenesulfonamide, 2-methyl- | NA | 1 | 1 | |
| 90-93-7c | Methanone, bis[4-(diethylamino)phenyl]- | NA | 3 | 1 | track |
| 91-44-1 | 2H-1-Benzopyran-2-one, 7-(diethylamino)-4-methyl- | NA | 1 | 1 | track |
| 91-51-0 | Benzoic acid, 2-[[3-[4-(1,1-dimethylethyl)phenyl]-2-methylpropylidene]amino]-, methyl ester | NA | 1 | 1 | |
| 92-70-6 | 2-Naphthalenecarboxylic acid, 3-hydroxy- | NA | 2 | 1 | track |
| 98-88-4 | Benzoyl chloride | NA | 2 | 1 | track |
| 100-40-3 | Cyclohexene, 4-ethenyl- | NA | 1 | 1 | |
| 101-20-2 | Urea, N-(4-chlorophenyl)-N’-(3,4-dichlorophenyl)- | NA | 2 | 1 | |
| 105-60-2 | 2H-Azepin-2-one, hexahydro- | NA | 1 | 1 | |
| 108-03-2 | Propane, 1-nitro- | NA | 1 | 1 | |
| 108-24-7 | Acetic acid, anhydride | NA | 1 | 1 | |
| 109-87-5 | Methane, dimethoxy- | NA | 1 | 1 | |
| 110-85-0 | Piperazine | NA | 1 | 1 | |
| 119-61-9 | Methanone, diphenyl- | NA | 1 | 1 | |
| 123-77-3 | Diazenedicarboxamide | NA | 1 | 1 | |
| 126-13-6 | -D-Glucopyranoside, 6-O-acetyl-1,3,4-tris-O-(2-methyl-1-oxopropyl)-β-D-fructofuranosyl, 6-acetate 2,3,4-tris(2-methylpropanoate) | NA | 1 | 1 | |
| 132-27-4 | [1,1’-Biphenyl]-2-ol, sodium salt | NA | 1 | 1 | track |
| 139-05-9 | Sulfamic acid, cyclohexyl-, monosodium salt | NA | 1 | 1 | |
| 271-89-6 | Benzofuran | NA | 1 | 1 | |
| 302-17-0 | 1,1-Ethanediol, 2,2,2-trichloro- | NA | 1 | 1 | |
| 647-42-7 | 1-Octanol, 3,3,4,4,5,5,6,6,7,7,8,8,8-tridecafluoro- | NA | 1 | 1 | |
| 4390-04-9c | Nonane, 2,2,4,4,6,8,8-heptamethyl- | NA | 3 | 1 | track |
| 5064-31-3 | Glycine, N,N-bis(carboxymethyl)-, trisodium salt | NA | 1 | 1 | |
| 5089-22-5c | Benzoxazole, 2,2’-(1,4-naphthalenediyl)bis- | NA | 3 | 1 | track |
| 8013-01-2 | Yeast, ext. | NA | 1 | 1 | |
| 8021-39-4 | Creosote, wood | NA | 1 | 1 | |
| 15827-60-8 | Phosphonic acid, [[(phosphonomethyl)imino]bis[2,1-ethanediylnitrilobis(methylene)]]tetrakis- | NA | 1 | 3 | |
| 27193-86-8c | Phenol, dodecyl- | NA | 3 | 1 | track |
| 61790-49-6 | Oils, lard, sulfurized | NA | 2 | 1 | track |
| 66071-94-1 | Corn, steep liquor | NA | 1 | 1 | |
| 68511-50-2 | 1-Propene, 2-methyl-, sulfurized | NA | 1 | 1 | track |
| 68649-11-6 | 1-Decene, dimer, hydrogenated | NA | 1 | 1 | |
| 68649-12-7 | 1-Decene, tetramer, mixed with 1-decene trimer, hydrogenated | NA | 1 | 2 | |
| 68909-20-6 | Silanamine, 1,1,1-trimethyl-N-(trimethylsilyl)-, hydrolysis products with silica | NA | 1 | 1 | |
| 68909-77-3 | Ethanol, 2,2'-oxybis-, reaction products with ammonia, morpholine derivs. residues | NA | 1 | 3 | |
| 84696-24-2c | Lotus corniculatus, ext. | NA | 3 | 1 | track |
| 129828-23-5 | Fatty acids, tall-oil, reaction products with Bu phenylmethyl phthalate, 2-(dimethylamino)ethanol, morpholine and overbased calcium petroleum sulfonates | NA | 1 | 1 |
Table Notes
* Substances that are currently used in low volume in Canada but which have high classifications of hazard or that trigger other hazard alerts, have been identified for additional tracking of use patterns and their priority status re-evaluated if new information becomes available.
Abbreviation: CAS RN, Chemical Abstracts Service Registry Number; CMP, Chemical Management Plan; NA, not available.
aConfidential Domestic Substance List (CDSL) substance(s)
bMetal moieties will be assessed in future inorganic assessments
cSubstance had initially been classified as a higher risk concern, but was adjusted to low risk classification based on low regional emissions (see section 7.1.1). In these cases, it is proposed to track use pattern of the substance.
dHazard ranking of this substance was revised following application of the classification consistency rule (see section 6).
Footnotes
- Footnote 1
- Footnote 2
- Footnote 3
-
The HAF threshold value was selected based on an examination of the HAF distribution and the HAF correlation with higher levels of inherent aquatic toxicity. A HAF of 10-3 or greater represents approximately 23% of the HAF distribution and captures more potent chemicals.
- Footnote 4
-
The threshold value was selected based on an examination of the HAF distribution and the correlation with moderate levels of inherent aquatic toxicity. A HAF between 10-3 and 10-6 represents approximately 35% of the distribution and captures more potent chemicals.
- Footnote 5
-
The threshold value was selected based on an examination of the HAF distribution and the correlation with low levels of inherent aquatic toxicity. A HAF <10-6 represents approximately 42% of the distribution and captures less potent chemicals.
- Footnote 6
-
Selection of Pov cut-off values was arbitrary, but reflect those typical of persistence criteria in some jurisdictions for water (i.e., 60 days criteria in the European Union) or reflect chronic aquatic toxicity test durations (i.e., 21 days). Single media half-lives will be lower than the Pov.