Science Approach Document Biomonitoring-based Approach 2 for Barium-containing Substances Molybdenum-containing Substances Silver-containing Substances Thallium-containing Substances Inorganic Tin-containing Substances
Table of Contents
- Synopsis
- 1. Introduction
- 2. Science Approach
- 3. Application of Biomonitoring Approach 2: Barium-containing substances
- 4. Application of Biomonitoring Approach 2: Molybdenum-containing substances
- 5. Application of Biomonitoring Approach 2: Silver-containing substances
- 6. Application of Biomonitoring Approach 2: Thallium-containing substances
- 7. Application of Biomonitoring Approach 2: Inorganic Tin-containing substances
- 8. Overall Summary
- References
- Appendices
List of Tables
- Table 3-1. Substance identities
- Table 3-2. Concentrations of total barium in urine (µg/g creatinine) in U.S. population
- Table 4-1. Substance identities
- Table 4-2. Concentrations of total molybdenum in whole blood (µg/L) in Canadians
- Table 4-3. Concentrations of total molybdenum in urine (µg/g creatinine) in Canadians and U.S. population
- Table 4-4. Summary of whole blood and urinary BEs associated with exposure guidance value (IOM UL) and OECD SIDS with UF
- Table 5-1. Substance identities
- Table 5-2. Concentrations of total silver in whole blood (µg/L) in Canadians
- Table 6-1. Substance identities
- Table 6-2: Concentrations of total thallium in urine (µg/g creatinine) in Canadians and U.S. population
- Table 7-1. Substance identities
- Table 7-2. Concentrations of total tin in urine (µg/g creatinine) in the U.S. population and the Quebec Region
List of Figures
- Figure 3-1. Comparison of median (bar) and 95th percentile (whiskers) concentrations of urinary barium (µg/g creatinine) with the biomonitoring equivalent; 246 µg/g creatinine for the US EPA RfD (IRIS 2005); indicated by a solid line. Biomonitoring data are for both males (M) and females (F) combined, except where sex is indicated. aPaschal et al. 1998, bCDC 2015
- Figure 4-1. Comparison of median (bar) and 95th percentile (whiskers) concentrations of whole blood molybdenum (µg/L) with the BEs; 5.04 µg/L and 27.9 µg/L, based on the IOM UL (IOM 2001) and the OECD SIDS NOAEL (OECD 2013) with a UF of 100; indicated by solid and hatched lines respectively. Biomonitoring data are for both males and females combined. aLiang 2016, bHealth Canada 2013a and cAFN 2013
- Figure 4-2. Comparison of median (bar) and 95th percentile (whiskers) concentrations of urinary molybdenum (µg/g creatinine) with the BEs; 1326 µg/g creatinine and 7516 µg/g creatinine, based on the IOM UL (IOM 2001) and the OECD SIDS NOAEL (OECD 2013) with a UF 100; indicated by solid and hatched lines respectively. Biomonitoring data are for both males and females combined. aHealth Canada 2013a, bAFN 2013 and cCDC 2015
- Figure 5-1. Comparison of the median (bar) and the 95th percentile (whiskers) of the concentrations of whole blood silver (µg/L) with the biomonitoring equivalent; 0.4 µg/L for the US EPA RfD (IRIS 1991); indicated by a solid line. Biomonitoring data are for both males and females combined. aMedian was below the detection limit and the values plotted represents half the LOD of 0.05 µg/L. bMedian was below the detection limit and the value plotted represents half the LOD of 0.05 µg/L and the 95th percentile was too unreliable to be published. cLiang 2016 dHealth Canada 2013a
- Figure 6-1. Comparison of the median (bar) and the 95th percentile (whiskers) of the concentrations of urinary thallium (µg/g creatinine) with the HBM-I of 5 µg/L in urine (German Federal Environment Agency, GFEA 2011), units converted to 6.4 µg/g creatinine; indicated by a solid line. Biomonitoring data are for both males (M) and females (F) combined. aHealth Canada 2013a, bCDC 2015
- Figure 7-1. Comparison of median (bar) and 95th percentile (whiskers) concentrations of urinary tin (µg/g creatinine) with the biomonitoring equivalent; 26 µg/g creatinine for the TDI (RIVM 2009); indicated by a solid line. Biomonitoring data are for both males (M) and females (F) combined, except where sex is indicated. aPaschal et al. 1998, bCDC 2015, cINSPQ 2004b - whiskers represent the 97.5th percentile
Synopsis
This Science Approach Document (SciAD) presents a quantitative biomonitoring-based approach to identify substances of low concern for human health which were identified as priorities for assessment as they met categorization criteria under subsection 73(1) of CEPA and/or were considered a priority based on other human health concerns.
This biomonitoring-based approach considers available Canadian and U.S. human biomonitoring data based on the analysis of the substance or moiety in whole blood, serum and/or urine. When biomonitoring data indicate that general population exposure is below the biomonitoring guidance value, the substances or moieties are considered to be of low concern with respect to human health.
The application of this biomonitoring-based approach indicates that barium-containing substances, molybdenum-containing substances, silver-containing substances, thallium-containing substances and inorganic tin-containing substances, would be of low concern with respect to human health at current levels of exposure.
An assessment of these substances conducted under section 74 of CEPA will be published at a later date.
A consultation period on this SciAD is being provided to the public who will have an opportunity to provide comments and additional information in advance of this approach being applied in Screening Assessment Reports. The publication of this scientific approach will assist the government in addressing substances that are likely of low concern in an efficient and effective manner.
1. Introduction
Following categorization of substances on the Domestic Substances List (DSL), which was completed in 2006, approximately 4300 of the 23 000 existing substances were identified for assessment. Among the remaining substances to be addressed under the Chemicals Management Program (CMP), there are 17 inorganic substances containing barium (Ba), molybdenum (Mo), silver (Ag), thallium (Tl) and tin (Sn) that were identified as priorities for assessment as they met categorization criteria under subsection 73(1) of Canadian Environmental Protection Act, 1999(CEPA) and/or were considered a priority based on human health concerns (Environment Canada, Health Canada [modified 2007]). The names and identities of the specific substances in each metal group are detailed in the corresponding chapters.
The purpose of this document is to provide the opportunity to review and comment on the human biomonitoring data approach and the results of its application prior to the publication of screening assessments under section 68 or 74 of CEPA. The publication of the scientific approach and results in the SciAD will assist the government in addressing substances that may be of low concern to human health in a more effective manner.
This document does not represent an exhaustive or critical review of all available data, but provides a summary of the approach and the results obtained. Relevant data were identified up to March 2016. This assessment only considers human health effects and exposure associated with the barium, molybdenum, silver, thallium and (inorganic) tin moieties, and does not address other elements that may be present in certain substances that may release these elements (such as gold).
Results are intended to form the basis for the human health portion of screening assessments that will be published subsequently, in conjunction with the assessment of potential ecological risks.
This SciAD was prepared by staff in the Existing Substances Program at Health Canada. It has undergone external written peer review and/or consultation. Comments were received from Lynne Haber and Andrew Maier from Toxicology Excellence for Risk Assessment (TERA) and Judy LaKind from LaKind Associates. Although all comments received were taken into consideration, the final content and outcome of the SciAD remain the responsibility of Health Canada.
The scientific approach used for this assessment is described in Section 2. The critical information and considerations upon which the assessment of each metal grouping is based are presented in the corresponding chapters.
2. Science Approach
Large population health surveys such as the Canadian Health Measures Survey (CHMS) and the National Health and Nutrition Examination Survey (NHANES) in the United States (U.S.) provide valuable data on the prevalence and concentrations of chemicals in the general population. Total concentrations of a metal moiety in blood or urine provide a biologically relevant, integrated measure of exposures that may occur across multiple routes (e.g., oral, dermal and inhalation) and sources (e.g., environmental media, diet, and frequent or daily use). Biomonitoring data are increasingly being relied upon as a state-of-the-art tool for exposure assessment of environmental chemicals (Choi et al. 2015).
Health Canada has utilized biomonitoring data to evaluate many substances including triclosan, cobalt and selenium (Environment Canada, Health Canada 2012, 2014, 2015). With the availability of high-quality biomonitoring data for inorganic substances in Canada and the U.S., a biomonitoring approach has been applied to evaluate many of the inorganic substances in the CMP when there was adequate human biomonitoring data and an existing human biomonitoring guidance value (e.g., biomonitoring equivalents (BEs), German human biomonitoring values) or adequate pharmacokinetic data available to derive a human biomonitoring guidance value. Where applicable, the BEs are derived by conversion of a risk assessment-based chronic reference dose or a point of departure (POD) from a critical study into a steady-state concentration of a given biological matrix (i.e., blood or/and urine) using kinetic data or regression correlation; while the HBM-I value used in this report was based on human biomonitoring data from epidemiology studies.
2.1 Exposure Assessment
Biomonitoring data from the CHMS (Health Canada 2010; Health Canada 2013a), the First Nations Biomonitoring Initiative (FNBI) (AFN 2013), the Étude sur l'établissement de valeurs de référence d'éléments traces et de métaux dans le sang, le sérum et l'urine de la population de la Grande Région de Québec (hereinafter referred to as the Quebec Region study) (INSPQ 2004a) and the NHANES from the United States (CDC 2015) were examined for exposure trends by age, sex, sub-populations of interest (e.g., children, pregnant women, on-reserve First Nation Peoples), geography and time. There is highest confidence in the use of data representative of the Canadian population from the CHMS to characterize exposure to Canadians, followed by population-level US data (NHANES) and then from smaller Canadian studies (i.e., FNBI, MIREC-CD Plus, Quebec Region study).
The biomonitoring data presented in this document were used as surrogate exposure data for the specific CAS RNsFootnote1 which are being evaluated. There are limited specific CAS RN exposure data, thus data on the total metal moiety was considered to be an acceptable, although protective, surrogate as total metal moiety biomonitoring data include exposures from all bioavailable forms of the moiety. For each metal moiety, the relevant toxicokinetic data were evaluated to ensure that the available biomonitoring data were adequate to assess exposure. This approach aligns with the key principles and risk assessment guidance for evaluating metals (ICMM 2007; US EPA 2007).
The biomonitoring studies considered in this approach are described as follows:
The Canadian Health Measures Survey (CHMS) is a national survey carried out by Statistics Canada, which collects information from Canadians about their general health (Health Canada 2010; 2013a). This survey was designed to be nationally representative and includes a biomonitoring component; metals were measured in whole blood and urine of approximately 5,500 to 7,000 Canadians per survey cycle. The CHMS is not a targeted survey, and thus does not target individuals with high metal exposure or living near point sources of exposure. A broad range of metals were measured in CHMS cycle 1 (2007 to 2009, ages 6 to 79 years) and cycle 2 (2009 to 2011, ages 3 to 79 years); pregnant women were included in all cycles of this study. Cycle 1 and 2 datasets include both fasting and non-fasting individuals.
The First Nations Biomonitoring Initiative (FNBI), conducted in 2011, is a cross-sectional study that measured metals in blood and urine of adults from 15 rural or isolated First Nations communities south of the 60º parallel (AFN 2013). The study had 503 adult participants from 20 to 99 years of age; pregnant women and individuals undergoing chemotherapy were excluded from this study.
The national pregnancy cohort study Maternal-Infant Research on Environmental Chemicals (MIREC) recruited 2000 pregnant women from Vancouver, Toronto, Hamilton, Kingston, Montreal and Halifax (Arbuckle et al. 2013). Breast milk samples were analysed for metals. MIREC-child development (CD) Plus study builds on MIREC, whereby a subset of children from the mothers participating in MIREC were invited to participate in a follow up study. Data collection for the study was completed in March 2015; blood from approximately 500 children less than 5 years of age was analysed for metals.
The National Health and Nutrition Examination Survey (NHANES) is a series of surveys designed to collect data related to the health and nutritional status of the U.S. population. The surveys are conducted by the U.S. Center for Disease Control's National Center for Health Statistics. In 1999, NHANES became a continuous survey, collecting data in 2-year cycles. The sampling plan follows a complex, stratified, multistage, probability-cluster design to select a representative sample of the civilian, non-institutionalized population in the U.S. based on age, gender and race/ethnicity (CDC 2009). NHANES measures metals in blood and urine from approximately 2,500 people per cycle, aged 6 years and up. Pregnant women are included in the survey. In the absence of CHMS data, NHANES is considered to be an acceptable surrogate to estimate exposure levels in Canadians as it offers the most comprehensive data in North America. NHANES data has been used previously as a surrogate for Canadian exposure with lead and triclosan (Health Canada 2013b; Environment Canada, Health Canada 2012). This dataset includes both fasting and non-fasting individuals.
The Quebec Region study was conducted by the Institut national de santé publique du Québec (INSPQ) in 2004 and measured metals in blood, serum and urine in adults aged 18 to 65 years (INSPQ 2004a). Individuals were selected across urban, semi-urban and rural areas in and around Québec City, QC. Approximately 500 people participated in the study; pregnant women and people with severe chronic diseases were excluded. Data from this study was incorporated into this approach (when available) when there were no CHMS (nationally representative) data.
2.2 Health Effects Assessment
The health effects assessment approach focussed on the use of existing hazard characterizations of metal moieties from risk assessments conducted by other international organizations. A comprehensive literature search was conducted to identify risk assessment-derived exposure guidance values (e.g., references doses (RfDs), tolerable daily intakes (TDIs)) or points of departure (PODs) from critical toxicity studies (e.g., no-observed-adverse-effect level (NOAEL), lowest-observed-adverse-effect level (LOAEL)), and any new health effects studies which could impact selection of the critical health effect. The literature search included risk assessment reports from national and international authorities or significant health effect studies published in scientific publications up to February 2016. The oral route is considered to be the predominant route of exposure and the focus of the literature review was, accordingly, on chronic oral endpoints.
The metal moieties in this approach, considered as surrogates for the specific CAS RN substances currently being evaluated, were previously assessed internationally through the US Agency for Toxic substances and Disease Registry (ATSDR), Danish Environment Protect Agency (Danish EPA), European Food Safety Authority (EFSA), Joint FAO/WHO Expert Committee on Food Additives (JECFA), National Institute for Public Health and the Environment, Netherlands (RIVM), OECD Cooperative Chemicals Assessment Programme, Institute of Medicine (IOM), US EPA Integrated Risk Information System (IRIS), WHO Drinking Water Programme. These organizations publish various assessment documents which are specific to their assessment objectives, such as Toxicological Profile by ATSDR, Evaluation of Health Hazards by Danish EPA, Panel Evaluation by EFSA, Toxicological Evaluation by JECFA, and Screening Information Dataset (SIDS) or Initial Assessment Profile (SIAP) by OECD SIAR. These assessments undergo rigorous review and approval processes. Health Canada considered these assessments to be reliable hazard characterizations.
This approach to the health effects assessment has been used throughout the CMP. An existing hazard characterization approach supports greater efficiency in assessing remaining CMP priorities, by reducing duplication of effort for substances that have previously been assessed by other organizations. In addition, a literature search on the health effects data available on each individual substance in the grouping was conducted Since none of the individual substances were more toxic than the metal moiety data on the metal moieties were used as a surrogate for CAS RN specific health effects data.
2.3 Biomonitoring Guidance Values
Most of the available toxicity studies in experimental animals and human epidemiology studies report external doses via specific routes (e.g., oral, dermal or inhalation). Hence, there is no direct method available for interpretation of measured concentrations of chemicals in blood or urine in biomonitoring studies in the context of potential health risk to the general population. It is not possible to directly compare an external dose, expressed as an intake in mg/kg bw/day to blood concentrations (e.g., mg/L) or concentrations of chemical in urine (e.g., mg/L or mg/g creatinine) (Hays et al. 2008).
The results of human clinical studies or animal toxicity studies that measured blood concentrations associated with known doses of chemicals are not currently available for the majority of substances that have biomonitoring data. Therefore, approaches have been developed to convert intake levels into internal concentrations, or vice versa (Hays et al. 2008). Human biomonitoring guidance values are values used to interpret biomonitoring data. There are two types of human biomonitoring guidance values considered in the current approach: BE and German human biomonitoring values (HBM). A BE is defined as the concentration or range of concentrations of a chemical or its metabolites in a biological medium (blood, urine, or other medium) that is consistent with an existing health-based exposure guidance value such as a reference dose (RfD) or Tolerable Daily Intake (TDI) (Hays et al. 2008). HBM values are health-related biological exposure limits established by the German Human Biomonitoring Commission (Angerer et al. 2011). Germany has two levels of HBM values, HBM-I and HBM-IIFootnote2. Health Canada considered the use of HBM-I values in this science approach, which are more conservative in nature than HBM-II values. An HBM-I value describes the concentration of the biomarker of exposure below which, according to the Commission's current assessment, no adverse health effect should be expected. Thus, no action would be needed from a health protection perspective if concentrations are below the HBM-I value (Angerer et al. 2011). Based on the definitions, the BE and HBM-I values are functionally identical (Angerer et al. 2011). However, the approaches used in the derivation of BE values and HBM-I value (for thallium) are different.
A comprehensive literature search was conducted to identify existing human biomonitoring guidance values. For the metal moieties in the current assessment, only thallium had a publicly available biomonitoring guidance value (a HBM-I value developed by the German Human Biomonitoring Commission (GFEA 2011)). For the remainder of the metal moieties (i.e. barium, molybdenum, silver and inorganic tin), BE values were derived in collaboration with external contractors (Summit Toxicology, USA and Dr. Kannan Krishnan of University of Montreal). Several requirements need to be fulfilled in the BE derivation approach, including the identification of health-based exposure guidance values or points of departure (PODs) from a toxicity study (or studies) with appropriate UFs, availability of pharmacokinetic data, and the identification of suitable biomarkers of exposure, such as the substance measured in blood and/or urine.
The BE values were derived from the exposure guidance values (e.g., RfD, TDI) or PODs (NOAELs) identified in the health effects assessment. For existing exposure guidance values, the adequacy of uncertainty factors (UFs) applied by the corresponding regulatory authority were evaluated during the BE derivation. The UFs pertain to the potential database deficiency, and interspecies and intraspecies variability. If the UFs used in the selected exposure guidance values were deemed appropriate, additional adjustments on UFs were not conducted. For the PODs selected from critical studies, UFs of 10x10 for interspecies and intraspecies variations were applied. Additional database uncertainty factors were considered where appropriate.
A literature search was conducted to identify the kinetic properties (absorption, distribution, metabolism and elimination) of the moieties that dictate the relationship between the external and internal environment. Simple kinetic relationships or complex physiologically-based pharmacokinetic models (PBPK) can be used in the BE derivation approach. The kinetic data are also important in determining the adequacy of the biomarker of exposure. Identification of a suitable biomarker of exposure is a crucial step in the derivation of BE. If a suitable biomarker of exposure (i.e., the moiety measured in a biological matrix such as whole blood, plasma, serum or urine) is identified, the concentrations of the biomarker of exposure will be a direct reflection of the magnitude and variation of external exposures to the chemical (e.g., oral exposure; oral intake) in population based sampling (Hays et al. 2008). Some of the criteria used in determining the adequacy of the biomarker are absorption fraction, distribution in the body, retention, blood half-life, route of elimination and elimination half-life. For essential nutrients, such as molybdenum, homeostasis was also taken into account when evaluating the suitability of a matrix as a biomarker of exposure.
For BE derivation of barium, molybdenum, silver, and inorganic tin, a forward dosimetry approach was applied. A forward dosimetry approach is where an external dose (RfD or POD) is converted to a steady state internal biomarker concentration (Hays et al. 2008). Several different forward dosimetry approaches were used in the BE derivation. For barium and tin, steady state urinary BE values were derived using a mass balance equation described in Appendix B (Poddalgoda et al. 2016a,b). For molybdenum, steady state whole blood and urine BE values were derived using correlations between intake levels and blood or urine concentrations based on data obtained from a human case study (Hays et al. 2016). The steady-state whole blood BE value for silver was derived using an existing PBPK model (Aylward et al. 2016).
In all cases, when deriving BEs, blood or urine concentrations are initially derived for human-equivalent POD (HEP, which is obtained from dividing POD with interspecies UF) (BEPOD) and then apply intraspecies UF to derive BE associated with exposure guidance value.
2.4 Uncertainties of Science Approach
Confidence in use of biomonitoring data as a measure of exposure for this science approach is high as blood and or urine concentrations of total moiety are available for all of these metal moieties from the CHMS and/or NHANES. CHMS and NHANES are nationally representative population health surveys that include males, females, children, adults and pregnant women, and are weighted to be representative of the Canadian and US population. In addition, for some of these metal moieties, there are studies targeting specific subpopulations of interest, which are not included in the CHMS, such as on-reserve First Nation Peoples in the FNBI and young children in MIREC-CD Plus.
There are some uncertainties associated with the use of biomonitoring data and derived BEs; these are described below. The urine samples collected in all of the biomonitoring surveys and studies considered in this approach were spot urine samples. For the purposes of this approach, it is assumed that spot urine samples are representative of steady-state exposure concentrations across the population. The half-life and frequency of oral exposure for the metal moiety were taken into account when considering the steady-state assumption. For reasonably large population samples, such as CHMS and NHANES, it is reasonable to assume that the population distribution appropriately captures the variability in biomarker concentrations, even for short half-life substances. However, the availability of population level 24-hour urine sample data would allow for the validation of this assumption.
There is inherent uncertainty in using spot sample data and two approaches are typically used to adjust the spot urine sample data into daily values, either adjusting by 24 hour urine volume or by 24 hour creatinine excretion. Creatinine adjustment is the most common method used in biomonitoring assessments and is the method selected for this approach. Creatinine excretion varies by gender and can influence observations made about the biomarker concentration data.
An additional limitation of using spot urine samples (versus 24 hour urine collection) is the variation of concentration of the element of interest that could result due to variability of the hydration status of the individual. This variability is overcome by normalizing the result either by creatinine concentration of the individual spot urine sample or specific gravity. Creatinine normalized urine concentration data were evaluated in this assessment as specific gravity is not available for many of the biomonitoring datasets.
The BEs derived in this assessment can be updated or replaced if the exposure guidance values for moieties are updated. The data available for deriving BE values vary in quality and robustness across the metal moieties. The exposure guidance values used in the derivation of BEs have incorporated uncertainty factors to account for uncertainties in the hazard and exposure data. While the exposure guidance values are accepted where possible, the appropriateness of the derived uncertainty factors is evaluated in each case to ensure a consistency in risk characterization approach. Following are the general uncertainties associated with BE derivation and interpretation of BE data. The BE values or HBM-I value do not represent diagnostic criteria for health assessment at the individual level, but they are useful tools to interpret population-level biomonitoring data. The BE values should only be used for the interpretation of exposure in the general population and should not be used for occupational exposure or for individuals (LaKind et al. 2008). The HBM-I value can be used in the interpretation of exposure in the general population (Angerer et al. 2011).
3. Application of Biomonitoring Approach 2: Barium-containing substances
3.1 Identity of Substances
The Chemical Abstracts Service Registry Numbers (CAS RN), Domestic Substances List (DSL) names and common names for the individual substances in the barium-containing substance group are presented in Table 3-1.
| CAS RN | DSL name (common name) | Molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
| 513-77-9 | Carbonic acid, barium salt (1:1) (Barium carbonate) |
BaCO3 | 197.33 |
| 7727-43-7 | Sulfuric acid, barium salt (1:1) (Barium sulfate) |
BaSO4 | 233.39 |
| 10361-37-2 | Barium chloride (Barium chloride) |
BaCl2 | 208.23 |
| 17194-00-2 | Barium hydroxide (Barium hydroxide lime) |
Ba(OH)2 | 171.34 |
3.2 Sources and Uses
Barium is a naturally occurring element that is present in environmental media in Canada. Total barium has been measured in drinking water distribution systems, household dust, indoor and outdoor air, the Health Canada Total Diet Study and breast milk as part of several research initiatives undertaken by Health Canada and Environment and Climate Change Canada (Arbuckle et al. 2013; Health Canada 2003; NAPS 2011; Rasmussen et al. 2016; Tugulea et al. 2016). Overall, in Canada barium is found in low to moderate concentrations in drinking water (median 22 μg/L, range 2.50 - 510 μg/L, n=97), household dust (median 277 μg/g, 95th percentile 528 μg/g, n=1025) and air samples (outdoor NAPS PM2.5 median 0.94 ng/m3, range 0.04 - 18.89 ng/m3, n=910; indoor air PM2.5 median 1.06 ng/m3, 95th percentile 4.71 ng/m3) (NAPS 2011; Rasmussen et al. 2016; Tugulea et al. 2016). Food is the primary source of barium exposure for the Canadians, and average dietary intake for all age groups is 8.82 μg/kg bw/day based on data from the Total Diet Study (1993-99). Toddlers and preschoolers between 1-4 years of age have the highest dietary intake (on a per body weight basis) at 25.25 μg/kg bw/day; dietary intakes of barium decline with age. Barium is also present in breast milk, a source of exposure for nursing infants. Average and 95th percentile intakes of 0.21 μg/kg bw/day and 0.24 μg/kg bw/day, respectively, were derived based on measured concentrations in breast milk from 2001 Canadian mothers between 2008 and 2011 as part of the core MIREC study (2016 email from the Bureau of Chemical Safety, Food Directorate, to the Existing Substances Risk Assessment Bureau, Health Canada, unreferenced; Arbuckle et al. 2013).
Barium is also present in a range of products in Canada, including: drugs (personal communication, emails from the Risk Management Bureau, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated September 29, 2015; unreferenced); natural health products (LNHPD [modified 2014]; NHPID [modified 2015]), cosmetics (Health Canada 2015), pesticides (PMRA 2010; emails from the Pesticide Management Regulatory Agency, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated March 1, 2016; unreferenced), toys (CPCat 2014), consumer products (CPCat 2014; Household Products Database 1993-), food packaging and as incidental additives in food (personal communication, emails from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated March 14, 2016; unreferenced).
3.3 Exposure Assessment
Adequacy of biomarker of exposure:
Barium can be detected in blood, urine, feces and biological tissues and background levels of barium in blood, urine and feces vary with daily intake and route of administration (ATSDR 2007). Solubility of barium compounds is the major determinant of absorption (ATSDR 2007). Urine is the main matrix used to measure barium levels in biomonitoring studies. The percent absorption of barium in humans, depending on the compound, is likely to be in the range of 3 to 60% (Leggett 1992; ATSDR 2007). Available data indicate that absorption of barium decreases with age (ICRP 1993). Barium is rapidly transported via the circulatory system and is distributed principally to bones. Barium is also distributed in soft tissues. Out of the total body burden, greater than 90% of barium is found in bones and teeth (ATSDR 2007). The biological half-life of barium in bones in rodents was reported in the range of 90-120 days (WHO 1990). Even though the studies by Tipton et al. (1969) and Stoewsand et al. (1988) suggested that the fecal excretion of barium is much more important than urinary excretion pathway, barium is also eliminated via urine. In non-occupationally exposed healthy humans at steady-state (assuming 100% oral intake), approximately 91%, 6% and 3% of the outputs are associated with the feces, sweat, and urine, respectively (WHO 1990). Based on examination of human data from around the world, Schroeder et al. (1972) concluded that about 90% and 2.3% of ingested barium is excreted in the feces and urine, respectively. Studies have shown that more than 70% of intravenously (i.v.) administered barium is excreted through feces and urine within 3 days and 90% of the initial dose is excreted within 2 weeks (ATSDR 2007). Based on these studies, the half-life of barium excretion in urine is less than 3 days. A steady state exposure can be expected even with a relatively short half-life because people are exposed to barium multiple times during the day though ingestion of food. Therefore, total barium concentrations in urine are considered to be an adequate biomarker for both short-term and chronic exposure.
Biomonitoring data:
Though barium was not measured in large-scale Canadian biomonitoring studies such as the CHMS, FNBI, MIREC CD-Plus or the Quebec Region study, barium urine concentrations were measured in the U.S. population (CDC 2015). These data offer the most comprehensive data in North America and are considered to be acceptable surrogate data to estimate exposure levels in Canadians in the absence of Canadian data.
Barium is not an essential element for human health but since it is naturally occurring and prevalent in environmental media and food, barium is widely detected in the U.S. population in over 99% of survey participants in 2011-12 (CDC 2014). Population-level concentrations of barium in urine (creatinine adjusted) are presented in Table 3-2 below. Further details on age, sex and subpopulations are presented in Appendix A-1.
Barium concentrations in urine have been measured in NHANES III (1988-94) and seven consecutive cycles of CDC-NHANES up to 2012 (Paschal et al. 1998, CDC 2015). Several trends can be observed based on these NHANES surveys. Median creatinine adjusted urine concentrations of barium were lower in NHANES III (1988-94) than in the NHANES continuous surveys (1999 onward), but overall, urine concentrations have remained relatively stable since 1999. The highest creatinine adjusted urinary barium concentrations are found in children (presented in Table 3-2 below) and concentrations decrease as a function of age. At median creatinine adjusted concentrations, children aged 6 – 11 are statistically significantly higher than teens aged 12 -19 years. Barium concentrations in urine are slightly higher in females than males; this difference is statistically significant at the median but not at the 95th percentile concentration.
| Study Population | Sampling year(s) | Age Years |
Sex | n | Median (95% CI) |
95th percentile (95% CI) |
|---|---|---|---|---|---|---|
| NHANESa U.S. population |
2011-12 | 6 and older | M+F | 2502 | 1.38 (1.27-1.47) |
6.27 (5.20-6.66) |
| NHANESa U.S. population |
2011-12 | 6-11 | M+F | 398 | 2.18 (1.70-2.61) |
6.78 (5.98-8.18) |
n = sample size, CI = confidence interval, M = males, F = females
a CDC 2015
3.4 Derivation of Biomonitoring Equivalents (BEs)
The health effects dataset associated with barium exposure, the risk-based exposure guidance values and the approaches used in BE derivation are summarized below.
The health effects of barium have previously been assessed by other international organizations (ATSDR 2007, Danish EPA 2013, SCHER 2012, Health Canada 1990, NTP 1994, OECD 2005, 2008, IRIS 2005, WHO 2015).
Studies in rats and mice indicate that renal effects are the critical health effects of barium exposure in animals (Danish EPA 2013). In a study conducted by McCauley et al. (1985), glomerular alterations consisting of fused podocytes and thickening of the capillary basement membrane were found in uninephrectomized Sprague Dawley rats, Dahl salt-sensitive rats, and Dahl salt-resistant rats exposed to Ba at 150 mg Ba/kg bw/day in drinking water for 16 weeks. In 13-week drinking water studies in rodents (NTP 1994), significant increases in the absolute and relative kidney weights were observed in female rats exposed to Ba at 115 mg Ba/kg bw/day and in male rats at 200 mg Ba/kg bw/day; in mice, mild to moderate nephropathy (characterized as tubule dilatation, regeneration and atrophy) was observed in all the males exposed to Ba at 450 mg Ba/kg bw/day and in 90% of the females exposed to barium at 495 mg Ba/kg bw/day. A significant increase in the incidence of nephropathy occurred at a dose level of 160 mg Ba/kg bw/day (NOAEL=75 mg Ba/kg bw/day) in mice in a 2-year drinking water study (NTP 1994). In the absence of contrary data, the kidney was considered as the most sensitive target of toxicity in animals following intermediate- or chronic duration oral exposure.
In humans, equivocal data have been reported for cardiovascular disease and possible association with elevated barium levels in drinking water. In a population-based study found significant increases in mortality rate from cardiovascular disease among residents 65 years of age and older living in unspecified Illinois communities with high levels of barium in the drinking water (2 to 10 mg Ba/L) compared with low barium communities (less than 0.2 mg Ba/L; Brenniman et al. 1979). However, the results reported by Brenniman et al. (1979) were not confirmed in a further cross-sectional study by Brenniman and Levy (1985) of cardiovascular disease prevalence in 1175 adult residents of West Dundee, Illinois (with a mean barium concentration in drinking water of 7.3 mg/L and a range of 2 to 10 mg/L).
By modeling the incidence of nephropathy in mice from the NTP 2-year drinking water study, a point of departure (BMDL05) of 63 mg Ba/kg bw/day was identified by the US EPA (IRIS 2005). Using this POD and applying a composite UF of 300 (10x for intraspecies, 10x for interspecies variation, 3x for the lack of reproductive and developmental toxicity studies), US EPA (IRIS 2005) derived a RfD of 0.2 mg Ba/kg bw/day. By using the same BMDL05 and applying similar UFs, WHO (2015) and SCHER (2012) derived a TDI of 0.21 mg/kg bw/day, and ATSDR (2007) derived a chronic-duration MRL of 0.2 mg Ba/kg bw/day for barium from oral route of exposure. The US EPA IRIS assessment (2005) was selected as the basis for risk characterization for human exposure to barium.
A BE was derived from the US EPA chronic RfD (IRIS 2005). The mass balance approach as explained in Hays et al. (2010) was used in the derivation of the steady state urinary barium concentration, creatinine adjusted basis (µg/g creatinine) that is consistent with an RfD of 0.2 mg/kg bw per day (US EPA 2005). Based on human data, the urinary excretion fraction was considered as 0.023 (Schroeder et al.1972). Thus, the steady state urinary BE derived for the chronic RfD (IRIS 2005) is 246 µg/g creatinine. Details of the derivation are outlined in Appendix B-1.
3.5 Results of the Approach
Figure 3-1 represents the key biomonitoring exposure values and hazard values for barium used for the determination of risk.
Estimated exposure to Canadians is based on creatinine adjusted total barium concentrations in urine in the U.S. population; NHANES data (2011-12) are considered to be acceptable surrogates to estimate exposure levels in Canadians in the absence of Canadian data. The US EPA RfD is derived from a BMDL05 of 63 mg/kg bw/day based on the risk of nephropathy from a 2 year drinking water study in mice and an UF of 300 (IRIS 2005). Exposure to total barium is lower than the creatinine adjusted urinary equivalent of the US EPA RfD 0.2 mg/kg bw/day for which a BE of 246 µg/g creatinine has been derived. Based on the information presented here, the barium-containing substances in this assessment are of low concern to the health of the general public in Canada at current levels of exposure.
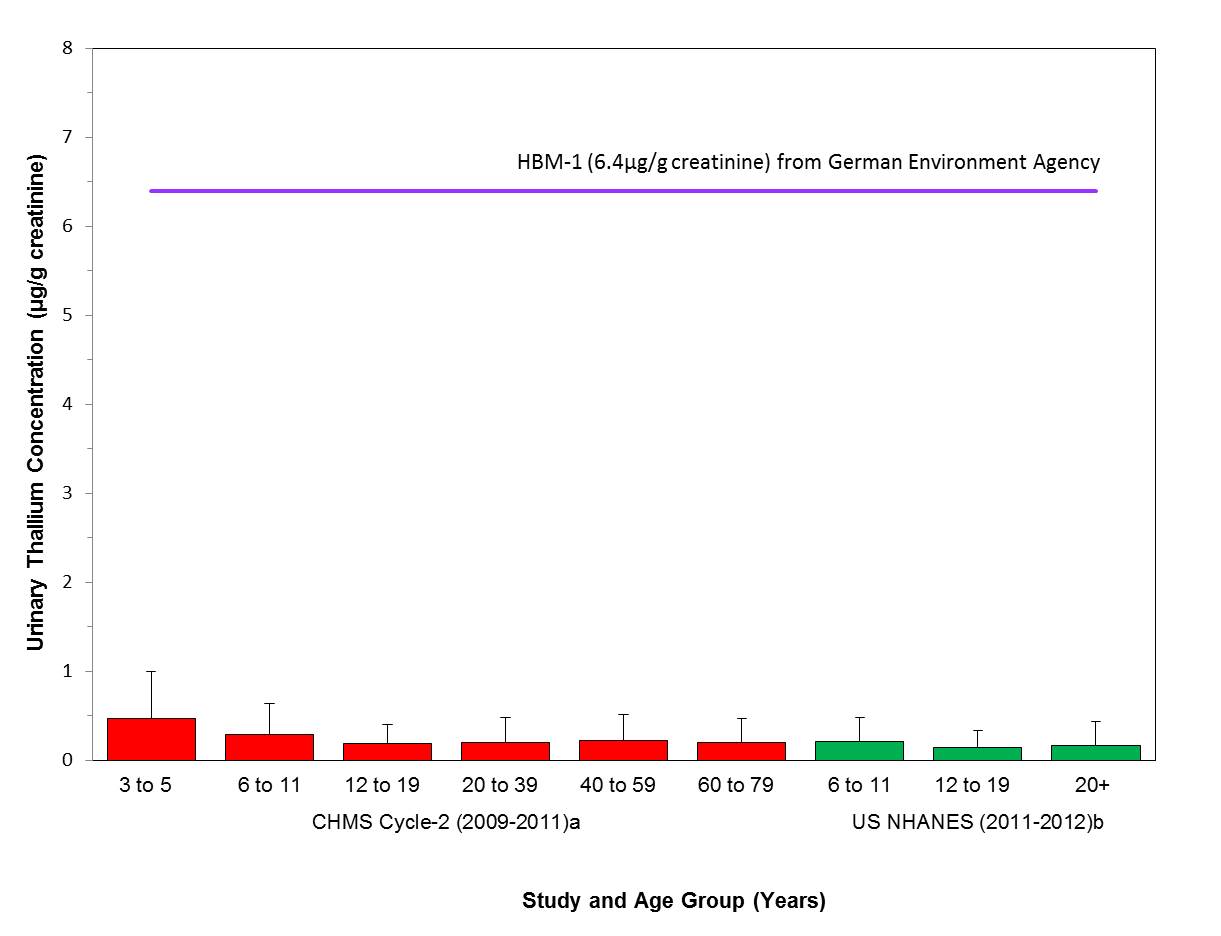
Long description for figure 3-1
Comparison of median (bar) and 95th percentile (whiskers) concentrations of urinary barium (µg/g creatinine) with the biomonitoring equivalent; 246 µg/g creatinine for the US EPA RfD (IRIS 2005); indicated by a solid line. Biomonitoring data are for both males (M) and females (F) combined, except where sex is indicated. aPaschal et al. 1998, bCDC 2015
3.6 Uncertainties
The US EPA RfD (IRIS 2005), has incorporated uncertainty factors to account for uncertainties in the hazard and exposure data. There are no biomonitoring data from the CHMS or for children under 6 years of age and children under 6 have the highest dietary intake, however, the 10x uncertainty factor for intraspecies variation in the RfD does account for variability within the population as differences in dietary intake are less than a factor of 2.
The hazard dataset for barium is robust and has been reviewed by various international and national organizations. The point of departures of critical health effects and the uncertainty factors determined for the guideline value derivation by various international organizations are identical. Although there are available human data, due to the inconsistency in observations, they are not considered as adequate for guideline value generation by various international organizations.
There is some uncertainty related to the use of in urine as a biomarker of exposure because the primary route of barium excretion is feces and urinary excretion is significantly lower. The US EPA RfD is 300 times lower than the point of departure in the rodent study; therefore this margin does not suggest physiological limitations for urinary excretion of barium at the RfD.
Uncertainties and limitations include the use of the results of the study by Schroder et al. (1972) which provided an overall value of urinary excretion fraction for barium in humans, even though individual studies conducted several decades ago (reliability unknown) and appear to suggest variability of the excretion fraction during different dose levels and regimens.
4. Application of Biomonitoring Approach 2: Molybdenum-containing substances
4.1 Identity of Substances
The Chemical Abstracts Service Registry Numbers (CAS RN), Domestic Substances List (DSL) names and common names for the individual substances in the molybdenum-containing substance group are presented in Table 4-1.
| CAS RN | DSL name (common name) |
Molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
| 1313-27-5 | Molybdenum oxidea (Molybdenum trioxide) |
MoO3 | 143.94 |
| 1317-33-5 | Molybdenum sulfide (Molybdenum disulphide) |
MoS2 | 160.07 |
a This substance was not identified under subsection 73(1) of CEPA but was included in this assessment as it was considered as a priority based on other human health concerns.
4.2 Sources and Uses
Molybdenum is a naturally occurring element that is present in environmental media in Canada. Total molybdenum has been measured in drinking water distribution systems, household dust, indoor and outdoor air, the Health Canada Total Diet Study and breast milk as part of several research initiatives undertaken by Health Canada and Environment and Climate Change Canada (Arbuckle et al. 2013; Health Canada 2003; NAPS 2011; Rasmussen et al. 2015; Rasmussen et al. 2016; Tugulea et al. 2016). Overall, in Canada concentrations of molybdenum are low in air samples (outdoor NAPS PM2.5 median concentration 0.10 ng/m3, range 0.01 - 19.77 ng/m3, n=910; indoor PM2.5 median 0.14 ng/m3, 95th percentile 1.99 ng/m3), household dust (median 2.8 µg/g, 95th percentile 8.0 µg/g, n=1025) and drinking water (median 0.50 μg/L, range 0.50-10.00 μg/L, n=97) in Canada (NAPS 2011; Rasmussen et al. 2015; Rasmussen et al. 2016; Tugulea et al. 2016). Food is the primary source of molybdenum exposure (OECD 2013, CDC 2009). Average dietary intake for all age groups is 2.7 μg/kg bw/day. Infants between 2-3 months have the highest dietary intake at 12.5 µg/kg bw/d and dietary intakes of molybdenum decline with age (on a per body weight basis). Molybdenum is also present in breast milk, a source of exposure for nursing infants. Average and 95th percentile intakes of 0.28 μg/kg bw/day and 0.33 μg/kg bw/day, respectively, were derived based on measured concentrations in breast milk from 2001 Canadian mothers between 2008 and 2011, as part of the core MIREC study (2016 email from the Bureau of Chemical Safety, Food Directorate, to the Existing Substances Risk Assessment Bureau, Health Canada, unreferenced, Arbuckle et al. 2013).
Molybdenum is also present in a range of products including: drugs (DPD [modified 2015]), natural health products (LNHPD [modified 2014]; NHPID [modified 2015]), cosmetics (personal communication, emails from the Consumer Product Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated February 24, 2016; unreferenced), as a formulant in pesticides (PMRA 2010; emails from the Pesticide Management Regulatory Agency, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated February 29, 2016; unreferenced), toys (CPCat 2014), consumer products (CPCat 2014, Household Products Database 1993-), food packaging and as incidental additives in food (personal communication, emails from the Foods Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated March 14, 2016; unreferenced).
4.3 Exposure Assessment
Adequacy of biomarker of exposure:
Molybdenum is an essential nutrient for human health, therefore detection in biological fluids is not only expected, but it is necessary for good health. Molybdenum has been measured in different biological fluids and tissues (e.g., urine, whole blood and serum) depending on the purpose of investigation. In a human dosing study, Turnlund and Keyes (2004) demonstrated that molybdenum in plasma and urine were well correlated with dietary intakes. Urine is the predominant route of elimination, with approximately 90% of an oral dose being eliminated via urine with a half-life of less than 12 hours (Turnlund and Keyes, 2004), indicating urine is a good matrix for assessing exposures to molybdenum. In plasma, elimination has been described with a bi-exponential function with mean half-lives of 30 minutes and 6.6 hours (Werner et al. 2000). Accordingly, the half-life of elimination of molybdenum following an oral dose is relatively short in both plasma and urine. Hence, both matrices are expected to have similar sensitivity as biomarkers of exposure with regard to elimination half-life. A steady state exposure can be expected even with a relatively short half-life because people are exposed to molybdenum multiple times during the day though ingestion of food. When BE values were derived for urine and blood, blood BE values showed a narrow margin between nutritional and toxicological effects. Compared to blood BEs, the urinary BE values for nutrition and toxicity had a wider margin. The reason for this observation is likely due to homeostasis. The blood concentrations are highly regulated through homeostasis and do not vary as much as urine concentrations with different intakes of molybdenum (Hays et al. 2016). Also, the kidney is considered the primary site of molybdenum homeostatic regulation because stable isotope studies indicated molybdenum retention at low molybdenum intakes and rapid excretion at high intakes (IOM 2001). Hence, total molybdenum concentrations in urine may be a more reliable biomarker than the molybdenum in blood for investigating molybdenum exposure from a toxicity perspective. Both biomarkers were considered in this analysis.
Biomonitoring data:
Total molybdenum concentrations have been measured primarily in whole blood and/or urine in many population-level biomonitoring surveys including, but not limited to, the CHMS and NHANES, as well as in studies targeting specific sub-populations of interest such as the FNBI and MIREC-CD Plus.
Population-level concentrations of molybdenum in the whole blood of Canadians are presented in the Table 4-2 below. Further details on age, sex and subpopulations are presented in Appendix A-2. Molybdenum was detected in over 99% of Canadians. Several trends can be observed in the concentrations of molybdenum in blood. The highest molybdenum whole blood concentrations are found in children less than 3 years of age (presented in Table 4-2 below, Liang 2016). There is a continuous decline throughout childhood, adolescence and adulthood until around 60 years of age when concentrations increase slightly (Health Canada 2010; 2013a). Molybdenum concentrations in blood are slightly higher in females than males but not statistically significant, and concentrations in pregnant women in the CHMS are similar or slightly higher than women of childbearing age (Health Canada 2010; 2013a; Walker et al. 2016). Blood molybdenum concentrations measured in the FNBI are similar to those measured in the CHMS (AFN 2013). Blood molybdenum concentrations are similar between CHMS cycle 1 and cycle 2 (Health Canada 2010; 2013).
| Study/Population | Sampling year(s) | Age years |
Sex | n | Median (95% CI) |
95th percentile (95% CI) |
|---|---|---|---|---|---|---|
| CHMS Cycle 1a Canadian population |
2007-09 | 6 - 79 | M+F | 5319 | 0.66 (0.64 - 0.68) |
1.38 (1.31 - 1.46) |
| CHMS Cycle 2b Canadian population |
2009-11 | 3 - 79 | M+F | 6070 | 0.64 (0.62-0.66) |
1.5 (1.4 – 1.5) |
| MIREC-CD Plusc Children |
2013-14 | less than or equal to 3 | M+F | 214 | 1.25 | 2.78 |
n = sample size, CI = confidence interval, M = males, F = females
a Health Canada 2010
b Health Canada 2013a
c Liang 2016
Population-level concentrations of molybdenum in the urine (creatinine adjusted) of Canadians are presented in the Table 4-3 below. Further details on age, sex and subpopulations are presented in Appendix A-2. Several trends can be observed in the creatinine adjusted concentrations of molybdenum in urine. Similar to blood, the highest concentrations are in the youngest age group measured (3 to 5 years of age) (Health Canada 2013a). There is a continuous decline in molybdenum urine concentrations throughout childhood, adolescence, and adulthood until around 60 years of age when concentrations increase slightly (Health Canada 2010; 2013a). Molybdenum concentrations in urine (creatinine adjusted) are slightly higher in females than males (Health Canada 2010; 2013a). Urine molybdenum concentrations measured in the FNBI are similar to those measured in the CHMS (AFN 2013). Urine molybdenum concentrations are similar between CHMS cycle 1 and cycle 2 (Health Canada 2010; 2013a).
Molybdenum concentrations in urine have also been measured in NHANES from 1999 to 2012 (CDC 2015). Concentrations have been stable over this 13 year period and are similar to those measured in the CHMS. Age and sex trends observed in the U.S. population are the same as observed in the two cycles of the CHMS, whereby urine molybdenum concentrations are highest in the youngest age groups measured and decrease with age and females have slightly higher urinary molybdenum concentrations (creatinine adjusted) than men (CDC 2015).
| Study/Population | Sampling year(s) | Age years |
Sex | n | Median (95% CI) |
95th percentile (95% CI) |
|---|---|---|---|---|---|---|
| CHMS Cycle 1a Canadian population |
2007-09 | 6-79 | M+F | 5479 | 43.20 (41.82 - 44.58) |
121.58 (112.49 - 130.67) |
| CHMS Cycle 2b Canadian population |
2009-11 | 3-79 | M+F | 6291 | 42 (40 - 45) |
140 (120 - 160) |
| NHANESc U.S. population |
2011-12 | 6 and older | M+F | 2502 | 41.0 (38.6-44.4) |
130 (118-148) |
| CHMS Cycle 2b Canadian population |
2009-11 | 3-5 | M+F | 572 | 140 (130 - 150) |
490E (310 - 680) |
n = sample size, CI = confidence interval, M = males, F = females, E = use data with caution
a Health Canada 2010
b Health Canada 2013a
c CDC 2015
4.4 Derivation of Biomonitoring Equivalents (BEs)
The health effects dataset associated with molybdenum exposure, the risk-based exposure guidance values and the approaches used in BE derivation are summarized below.
The health effects of molybdenum-containing substances have previously been assessed by other international organizations (IRIS 1992, IOM 2001, WHO 2011a, EFSA 2013, DECOS 2013, OECD 2013). At physiologically relevant concentrations and pH, the only molybdenum species present is the molybdate [MoO4]2- anion (OECD SIDS 2013). Therefore, the toxicological effects of molybdenum-containing substances in this group are attributed to the common moiety, the molybdate anion.
In animals, the administration of supplemental dietary molybdenum (as sodium molybdate) via drinking water for 6 weeks was associated with a prolonged estrus cycle, decreased gestational weight gain in pups and several adverse effects on embryogenesis in female Sprague-Dawley rats (Fungwe et al. 1990). These effects were not observed at 0.9 mg Mo/kg bw/day, but were reported at a dose level of 1.6 mg/kg bw/day. Schroeder and Mitchener (1971) evaluated the effect of a single dose of molybdate in drinking water (10 mg/L) on reproduction in mice over three generations. The daily molybdenum intake was later estimated as 1.5 mg Mo/kg bw/day by Vyskocil and Viau (1999) and they reported some early deaths of offspring, dead litters, maternal deaths and failure to breed. Taking together these observations, IOM (2001) determined that the effects of molybdenum on reproduction and fetal development in rats and mice were the most sensitive effects. Therefore, the NOAEL of 0.9 mg Mo/kg bw/day from Fungwe et al. (1990) was used to set the Tolerable Upper Intake Level (UL= 2000 μg/day) of molybdenum for adults using uncertainty factors (UF) of 10 for extrapolating from experimental animals to humans and 3 for intraspecies variation based on molybdenum is an essential element, therefore, similar pharmacokinetics of molybdenum among humans were expected.
However, in studies reviewed by OECD in its Initial Assessment Report (OECD 2013), health effects were not observed in rats in reproductive tissues and sperm quality was not affected after 90-day of exposure to sodium molybdate (dihydrate) in diet at up to 60 mg Mo/kg bw/day (Murray et al. 2014a). Similarly, the abovementioned developmental health effects were not reported in a prenatal developmental toxicity study in rats exposed to Sodium molybdate (dihydrate) in diet at up to 40 mg Mo/kg bw/day (Murray et al. 2014b). A NOAEL of 17 mg Mo/kg bw/day was determined by the OECD based on reduced body weight gain and kidney effects in rats observed at top dose level of 60 mg Mo/kg bw/day in the 90-day study. Therefore, the UL derived by IOM (2001) and the NOAEL determined by the OECD SIDS assessment report (OECD 2013) are both selected as the basis for deriving biomonitoring guidance values in this assessment.
Various epidemiologic studies involving occupationally-exposed populations associate inhalation or ingestion of molybdenum to gout-like symptoms in the joints (high amounts of uric acid may crystallize in the joint) and increased blood levels of uric acid. However, due to combined exposure with other potentially toxic compounds in most workplaces or other food sources, it is difficult to conclude whether the observed symptoms were caused by molybdenum (Health Council of the Netherlands 2013). As well, the OECD SIDS assessment report (OECD 2013) concluded that the available human data was of insufficient quality and reliability to use quantitatively in a risk assessment. Therefore, the chronic Reference Dose (RfD) of 0.005 mg Mo/kg bw/day for oral exposure derived by IRIS (1992) based on human serum uric acid levels and gout-like symptom reported in a cross-sectional epidemiological study was not considered as a suitable exposure guidance value for deriving biomonitoring guidance values in this assessment. The details of the approaches used in the derivation of whole blood and urine BEs are described in Hays et al. (2016).
Whole blood and urine BEs associated with toxicity were derived for the IOM UL and the lowest NOAEL identified for molybdenum in the OECD SIDS assessment profile from a 90-day toxicity study (Murray et al. 2014a) with a UF of 100. The UF of 100 was used as a conservative approach; but an UF of 30 would be sufficient as the molybdenum kinetic parameters among individuals are considered to be similar. The whole blood and urine BE values that are relevant for the interpretation of available population-level biomonitoring studies are summarised in Table 4-4. As explained in Hays et al. (2016), the derivation of plasma and urinary BEs was based on the linear relationships observed for molybdenum dietary intake (µg/day) versus plasma molybdenum levels (µg/L) or daily urinary molybdenum excretion (µg/day) reported in a human dosing study by Turnlund and Keyes (2004). In this study, dietary molybdenum intake levels ranged from 22 to 1490 µg/day. The data from Turnlund and Keyes (2004) were considered ideal for deriving the BEs as the study consisted of durations of exposure sufficiently long to reach steady-state metabolic balance for molybdenum. A factor of 0.9 was used when converting plasma BEs into whole blood BEs (Hays et al. 2016). Standard creatinine excretion rate and body weight for adults were used in order to convert daily urinary molybdenum excretion (µg/day) into a creatinine adjusted daily urinary molybdenum concentration (µg/g creatinine) as these parameters were not reported in Turnlund and Keys (2004).
| Organization (reference) | Exposure guidance value (mg/kg bw/day) | Whole blood BE (µg/L) | Urinary BE (µg/g creatinine) |
|---|---|---|---|
| IOM (2001) | 0.03a | 5.04 | 1326 |
| OECD (OECD SIDS 2013) | 0.17b | 27.9 | 7516 |
a tolerable upper intake level (UL) (based on a NOAEL of 0.9 mg/kg bw/day and a UF of 30)
b lowest NOAEL with UF of 100
4.5 Results of the Approach
Figure 4-1 and 4-2 represent the key biomonitoring exposure values and hazard values for molybdenum used for the determination of risk.
Exposure to total molybdenum in the Canadian population, characterized by whole blood and urine concentration data, are lower than the whole blood and urine equivalents of the IOM UL of 0.03 mg/kg bw/day (based on a NOAEL of 0.9 mg/kg bw/day and a UF of 30) and a NOAEL of 17 mg/kg bw/day with a UF of 100 from a sub-chronic toxicity study reviewed by the OECD in their SIDS assessment (OECD 2013) (BE values for whole blood are 5.04 and 27.9 µg/L, respectively and BE values for urine are 1326 and 7516 µg/g creatinine, respectively). Based on the information presented here, the molybdenum-containing substances in this assessment are of low concern to the health of the general public in Canada at current levels of exposure.
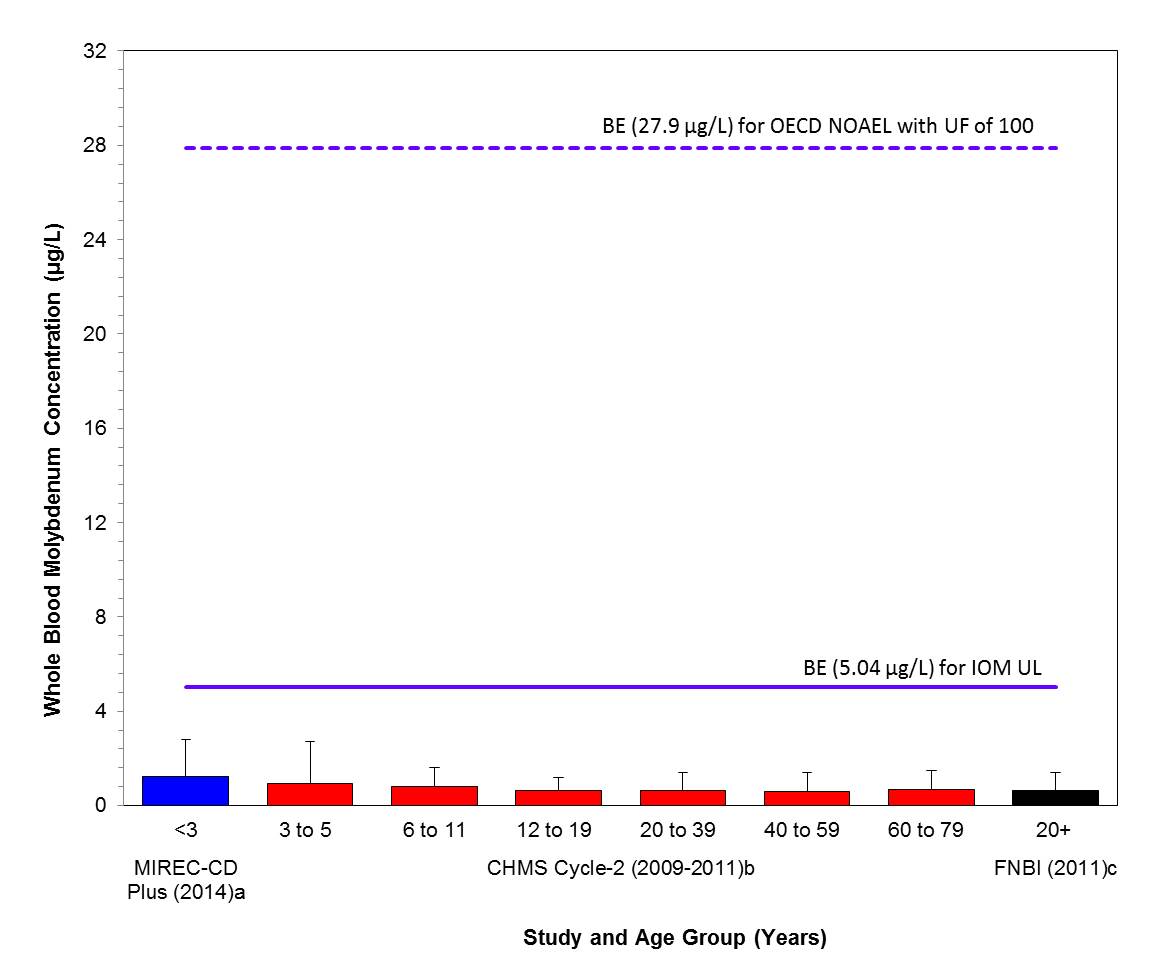
Long description for figure 4-1
Comparison of median (bar) and 95th percentile (whiskers) concentrations of whole blood molybdenum (µg/L) with the BEs; 5.04 µg/L and 27.9 µg/L, based on the IOM UL (IOM 2001) and the OECD SIDS NOAEL (OECD 2013) with a UF of 100; indicated by solid and hatched lines respectively. Biomonitoring data are for both males and females combined. aLiang 2016, bHealth Canada 2013a and cAFN 2013
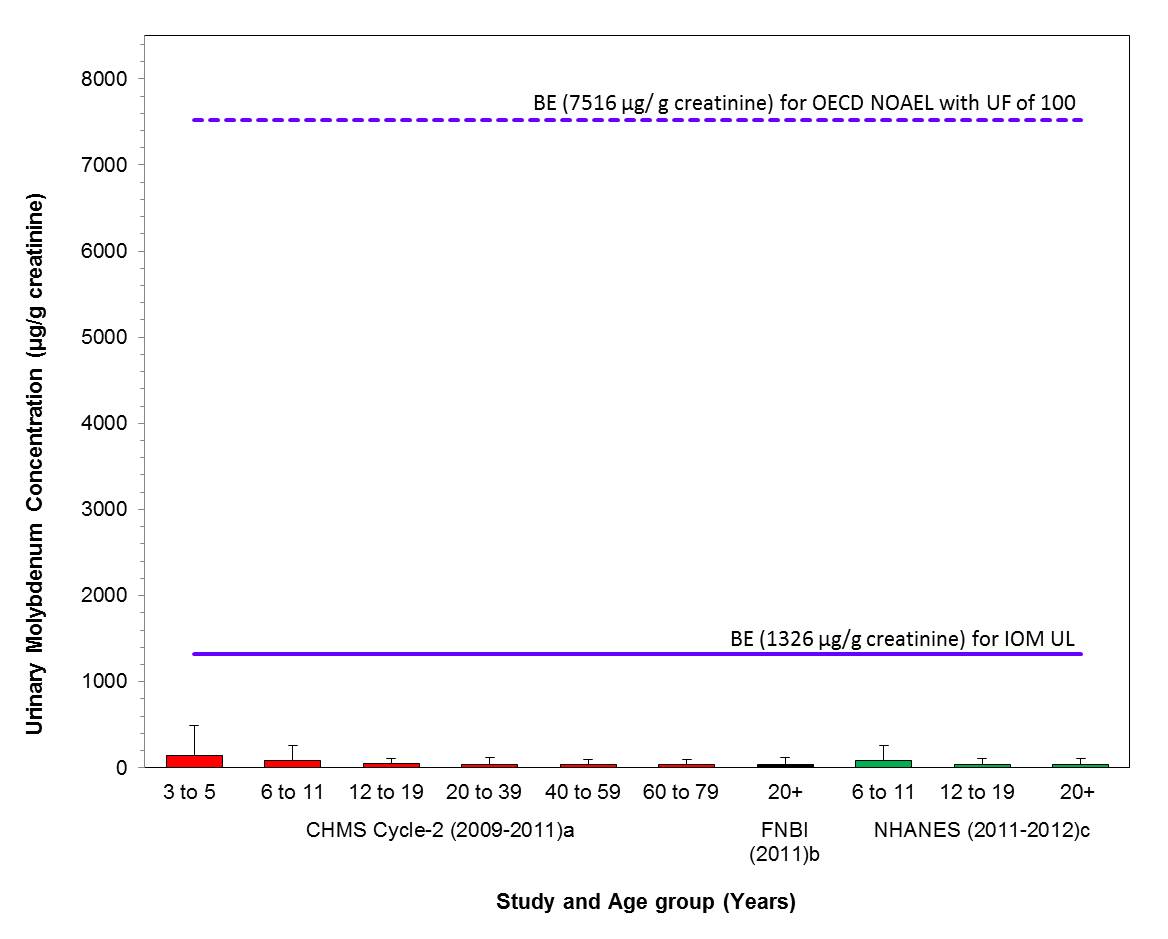
Long description for figure 4-2
Comparison of median (bar) and 95th percentile (whiskers) concentrations of urinary molybdenum (µg/g creatinine) with the BEs; 1326 µg/g creatinine and 7516 µg/g creatinine, based on the IOM UL (IOM 2001) and the OECD SIDS NOAEL (OECD 2013) with a UF 100; indicated by solid and hatched lines respectively. Biomonitoring data are for both males and females combined. aHealth Canada 2013a, bAFN 2013 and cCDC 2015
4.6 Uncertainties
The hazard dataset for molybdenum has been reviewed by the OECD's Cooperative Chemicals Assessment Program (COCAM). The OECD COCAM (OECD 2013) has concluded that "adequate screening-level data are available to characterize the human health hazard" of the molybdenum moiety and the conclusion was endorsed by the member state countries including Canada. In addition, the IOM UL (IOM 2001) and the OECD POD (OECD SIDS NOAEL-OECD 2013), which were used in risk characterization have incorporated uncertainty factors to account for uncertainties in the hazard and exposure data.
Total molybdenum concentration in urine is a suitable biomarker of exposure for assessing potential toxicity because there is a linear relationship between intake levels and urine excretion rates, and there is a large margin between urinary BEs for nutritionally sufficient and for toxic levels of exposure (Hays et al. 2016). There were no data to generate a relationship between intake levels and whole blood concentrations, thus plasma was used a surrogate. While plasma molybdenum levels also showed a clear linear relationship between intake levels and plasma levels in a human dosing study, the confidence in whole blood and plasma molybdenum levels as suitable biomarkers of excess molybdenum exposure is lower because the derived whole blood and plasma BE values for nutrient adequacy and excess exposure demonstrate that a relatively constant plasma level is maintained, even with a broad range of intake levels. This is likely due to efficient homeostatic mechanisms that maintain nutritionally sufficient molybdenum concentrations in the body. Whole blood molybdenum concentrations can be used as a biomarker of exposure; however, urine is a better biomarker.
There is also uncertainty when interpreting urinary molybdenum concentrations in infants, as infants have a lower urinary excretion fraction than that of adults as observed by Sievers (2001). Since the Turnlund and Keyes study was based on healthy males, there is some uncertainty in interpreting blood or urinary molybdenum levels associated with sub-populations such as pregnant women. While there are limited toxicokinetic data for humans to characterize the potential kinetic differences in these subpopulations, IOM (2001) concluded that pharmacokinetics of molybdenum is expected to similar among humans.
5. Application of Biomonitoring Approach 2: Silver-containing substances
5.1 Identity of Substances
The Chemical Abstracts Service Registry Numbers (CAS RN), Domestic Substances List (DSL) names and common names for the individual substances in the silver-containing substances group presented in Table 5-1.
| CAS RN (acronym) |
DSL name (common name) |
Molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
| 7440-22-4 | Silver (Silver) |
Ag | 107.87 |
| 7761-88-8 | Nitric acid silver(1+) salt (Silver nitrate) |
AgNO3 | 169.87 |
| 7783-90-6 | Silver chloride (Silver chloride) |
AgCl | 143.32 |
| 7785-23-1 | Silver bromide (Silver bromide) |
AgBr | 187.77 |
| 10294-26-5 | Sulfuric acid, disilver(1+) salt (Silver Sulfate) |
Ag2SO4 | 313.82 |
| 20667-12-3 | Silver oxide (Silver oxide) |
Ag2O | 231.74 |
| 21548-73-2 | Silver sulfide (Silver sulfide) |
Ag2S | 247.81 |
5.2 Sources and Uses
Silver is a naturally-occurring element that is present in environmental media in Canada. Total silver has been measured in drinking water distribution systems, household dust, indoor and outdoor air and breast milk (Arbuckle et al. 2013; NAPS 2011; Rasmussen et al. 2016; Tugulea et al. 2016). Overall, Canadian data demonstrate that concentrations of are low in air (outdoor NAPS PM2.5 median 0.01 ng/m3, range 0 - 0.86 ng/m3, n=910; indoor PM2.5median 0.04 ng/m3, 95th percentile 0.42 ng/m3), drinking water (median 0.05 μg/L, range 0.05-0.20 μg/L, n=97) and dust (median 1.84 μg/g, 95th percentile 9.33 μg/g, n=1025) (NAPS 2011; Rasmussen et al. 2016; Tugulea et al. 2016). There are no Canadian data on the concentrations of silver in foods available or estimated dietary intakes for Canadians. However, in France, silver has been measured as part of the Second French Total Diet Study and has been used to estimate dietary intakes for the French population (ANSES 2011). Mean estimated dietary intakes for adults and children in France were 1.29 and 1.60 µg/kg bw/day respectively (ANSES 2011). In France, the main contributors to adult dietary intakes were molluscs and crustaceans, and for children, primary contributors were milk and water. Based on studies conducted in Canada, silver is also present in breast milk as a contaminant and a source of exposure for nursing infants. Average and 95th percentile intakes of 0.024 μg/kg bw/day and 0.029 μg/kg bw/day, respectively, were derived by Health Canada based on measured concentrations in breast milk from 2001 Canadian mothers between 2008 and 2011 as part of the core MIREC study (2016 email from the Bureau of Chemical Safety, Food Directorate, to the Existing Substances Risk Assessment Bureau, Health Canada, unreferenced, Arbuckle et al. 2013).
In 1990, the ATSDR identified food and drinking water as the primary sources of silver exposure for the general public, however, since that time, there has been an increased use of silver nanoparticles in products available to North American consumers. This change in the overall use pattern of silver has likely had an impact on the primary sources of exposure, with consumer products playing an increasingly important role as exposure sources (Benn et al. 2010).
Silver is used in a range of products in Canada, including: drugs (DPD [modified 2015]), natural health products (LNHPD [modified 2014]; NHPID [modified 2015]), cosmetics (Health Canada 2015, personal communication, emails from the Consumer Product Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated February 24, 2016; unreferenced), pesticides (emails from the Pesticide Management Regulatory Agency, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated March 1, 2016; unreferenced), toys (CPCat 2014), consumer products (CPCat 2014; Household Products Database 1993-), food additives (CPCat 2014; Health Canada [modified 2006]), food packaging and as incidental additives in food (personal communication, emails from the Foods Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated March 14, 2016; unreferenced).
5.3 Exposure Assessment
Adequacy of biomarker of exposure:
Silver has been detected in blood, urine, feces, hair and other biological specimens (ATSDR 1990). Blood and urine are the main matrices used to measure silver levels in biomonitoring studies. Silver has low gastrointestinal absorption when exposed through the oral route. In animal studies, approximately 5% and 10% absorption has been reported in rats and dogs, respectively (US EPA 1980). The amount absorbed from the gastrointestinal tract (GI) varies with the transit time. The transit time can vary from 8 hrs in mice and rats to 24 hrs in dogs, monkeys and humans (ATSDR 1990). Once absorbed, there is a low retention in the body. During environmental exposure, 0 to 10% of exposed silver was reported to be retained in humans, mainly in liver and skin (WHO 2003). The biological half-life of silver stored in liver of humans ranges from a few days to 50 days (WHO 2003). The liver also plays an important role in silver excretion; most of the absorbed silver undergoes a first-pass effect through the liver resulting in biliary excretion, thereby reducing systemic distribution to body tissues (ATSDR 1990, WHO 2003). In mice, rats, monkeys, and dogs, 90 to 99% of exposed silver was excreted via feces within 2 to 4 days of dosing (ATSDR 1990, WHO 2003). Available data indicate that a low proportion of absorbed and retained silver is excreted in urine (Jiménez-Lamana et al. 2014). Hence, urine is not considered to be a reliable biomarker of exposure for silver for this assessment. In addition, there were insufficient data on urinary silver to validate the PBPK model predictions of urinary output associated with oral intake (Aylward et al. 2016). Silver is measurable in blood and blood serves as the sensitive biomonitoring matrix. In contrast to urine, there is a robust blood database to support the validation of the model predictions for blood silver concentrations. Hence, total silver concentrations in blood are considered to be reliable biomarkers for evaluating silver exposure from all sources.
Biomonitoring Data:
Population-level concentrations of total silver in the whole blood of Canadians are presented in Table 5-2 below. Further population details (e.g., age, sex, pregnancy) are presented in Appendix A-3. The detection frequency of silver in Canadians (aged 3 to 79) was 54% (Health Canada 2013a); for children less than 3 years of age it was approximately 92% Liang 2016). The detection frequency dropped to 39% for children aged 3 to 5 and remained around 40% through adolescence until adulthood where silver was detected in over 60% of the samples analysed (Health Canada 2013a). Similar to detection frequency, whole blood silver concentrations were also highest in children under 3 (presented in Table 5-2 below), followed by decline in childhood and adolescence (aged 3 to 19) and then increased again in adulthood (20 and older). Silver concentrations in blood are higher in females than in males; but are only statistically significant at the 95thpercentile (Health Canada 2013a). Although not statistically significant, the whole blood silver levels of pregnant women in the CHMS were lower than levels in non-pregnant women of childbearing age (Health Canada 2013a; Walker et al. 2016).
| Survey Population | Sampling year(s) | Age years |
Sex | n | Median (95% CI) |
95th percentile (95% CI) |
|---|---|---|---|---|---|---|
| CHMS Cycle 2a Canadian population |
2009-11 | 3 - 79 | M+F | 6070 | 0.066 (less than LOD-0.088) |
0.27 (0.22 – 0.31) |
| MIREC-CD Plusb children |
2013-14 | less than or equal to 3 | M+F | 214 | 0.205 | 0.259 |
n = sample size, CI = confidence interval, M = males, F = females, less than LOD = less than the limit of detection where LOD = 0.05 µg/L
a Health Canada 2013a
b Liang 2016
5.4 Derivation of Biomonitoring Equivalents (BEs)
The health effects dataset associated with ionic silver exposure, the risk-based exposure guidance values and the approaches used in BE derivation are summarized below.
The health effects of silver have previously been evaluated by other international organizations (ATSDR 1990, Australian Government 2011, EFSA 2016, IRIS 1991, WHO 2011b). Some recent reviews mainly focus on nanosilver, however, nanosilver was considered beyond the scope of this assessment. With respect to carcinogenicity, EPA's Office of Water classified silver as a Group D, not classifiable as to carcinogenicity in humans (US EPA 1993).
The limited information available indicates that ionic silver is non-mutagenic in bacteria but it is genotoxic and clastogenic in mammalian cells in vitro (Butler et al. 2015). No information is available on the genotoxic potential of ionic silver in vivo (EFSA 2016).
In an oral one-generation reproductive toxicity study with silver acetate in drinking water at dose levels of 0, 0.4, 4 or 40 mg silver acetate/kg bw/day (equivalent to 0, 0.26, 2.6 or 26 mg Ag/kg bw/day) in rats, parental males were exposed for 10 weeks prior to mating and parental females for 2 weeks prior to mating. The F1-pups were sacrificed on postnatal day (PND) 26. A NOAEL of 0.26 mg Ag/kg bw/day for developmental effects (based on the increases in the number of runts, pup death and decreased weight gain of pups) was observed. The NOAEL for fertility was 2.6 mg Ag/kg bw/day (EFSA 2016). However, these health effects were not reported in a NTP study in pregnant rats exposed to a higher dose level of silver acetate in diet during gestation only. In a prenatal developmental toxicity study, time-mated female rats were dosed with silver acetate via gavage from gestational day (GD) 6 to 19 at 0, 10, 30 and 100 mg/kg bw/day (equivalent to 0, 6.5, 19.4 and 64.6 mg Ag/kg bw/day). The NOAELs were identified as 6.5 mg Ag/kg bw/day for decreased maternal body weight and greater than or equal to 64.6 mg Ag/kg bw/day for the absence of fetal developmental toxicity (NTP 2002).
Maternal exposures to silver acetate also induced immunological alterations in pups in the above mentioned one-generation reproductive toxicity study. Changes in phenotypic markers of splenocytes in PND 26 pups from mid- and high-dosed dams and reduced concanavalin A response in pups from low-dosed dams were reported (EFSA 2016). A corresponding LOAEL of 0.26 mg Ag/kg bw/day was estimated by EFSA 2016 from this study.
Argyria or argyrosis, characterized by blue or blue-greyish staining of the skin and mucous membranes, is the principle observable change associated with long-term exposure to ingestion or occupational inhalation of high doses of metallic silver or ionisable silver compounds (EFSA 2016). However argyria, is not associated with pathological damage in a specific target organ (EFSA 2016).
Argyria-like symptoms were consistently observed in rats exposed to silver at high dose levels. After exposure to silver via drinking-water for 218 days at 57 mg Ag/kg bw/day as silver nitrate, or at 68 mg Ag/kg bw/day as silver chloride, rats exhibited a slight greyish pigmentation of the eyes, which later intensified (Olcott 1950). Increased pigmentation of different organs, including the eye, was also observed in rats after lifetime ingestion of silver nitrate at 32 mg Ag/kg bw/day (Olcott 1947).
Argyria has been observed in the therapeutic realm since the mid-19th century. Gaul and Staud (1935) described 70 cases of argyria in patients undergoing chronic therapy in which intravenous (i.v.) doses of silver arsphenamine were administered over a 2 to 9.75 year period. The lowest i.v. dose that was associated with argyria in this study was 1 g (total dose) in one patient . By dividing with the oral retention factor of 0.04, adult body weight of 70 kg and 25,500 days of a 70-year lifetime, an oral dose of 0.014 mg Ag/kg bw/day was derived as the LOAEL by IRIS (1991). By applying an UF of 3 to account for minimal effects with no associated pathology in a human subpopulation which has exhibited an increased tendency for development of argyria, a Reference Dose (RfD) of 0.005 mg Ag/kg bw/day for protecting against argyria was derived by US EPA (IRIS 1991). This RfD is consistent with the health based silver drinking water concentration of 0.1 mg Ag/L (calculated as 0.005 mg Ag/kg bw/day) as determined by the Australian government (Australia Government 2011). This was based on an oral daily dose level of 0.4 mg Ag/day which was converted from a no-effect level of 10 g for human lifetime exposure (Hill and Pillsbury 1939). Similarly, an oral no-adverse effect level for argyria of total life time intake of 10 g silver for human lifetime exposure was also estimated by the WHO (2011b) based on human case reports. The reference dose of 0.005 mg Ag/kg bw/day, as derived by the US EPA (IRIS 1991), was selected as the basis for characterizing risk to human health in this assessment. This reference dose is similar to that which is derived from the Australian Government (2011) drinking water assessment. While the endpoint of argyria may not be considered as adverse, the use of the reference dose generated by the IRIS 1991 assessment is considered protective of other health effects noted in the toxicological database.
The details of the approaches used in the derivation of whole blood BEs are explained in Aylward et al. (2016).
A physiologically-based pharmacokinetic model was used to estimate BE values for the US EPA RfD of 0.005 mg Ag/kg bw/day, which was based on a human i.v. dosing study (Bachler et al. 2013, IRIS 1991). To estimate the BEPOD, the PBPK model was used to simulate daily i.v. administration of ionic silver at the POD dose (i.e. 0.014 µg/kg bw/day). The resulting steady state BEPOD was then divided by an intra-species UF of 3 as identified by the US EPA's RfD to derive the BE associated with the RfD. The resulting whole blood BE for ionic silver associated with US EPA RfD was 0.4 µg/L.
5.5 Results of the Approach
Figure 5-1 represents the key biomonitoring exposure values and hazard values for silver used for the determination of risk. Exposure to total silver in the Canadian population, based on total silver concentrations whole blood, is lower than the whole blood equivalent (0.4 µg/L) of the US EPA RfD of 0.005 mg/kg bw/day for ionic silver (IRIS 1991). The RfD of 5 µg Ag/kg bw/day is based on a LOAEL of 0.014 mg Ag/kg bw/d with a UF of 3 to account for sensitive subpopulations for protection against argyria. Based on the information presented here, the silver-containing substances in this assessment are of low concern to the health of the general public in Canada at current levels of exposure.
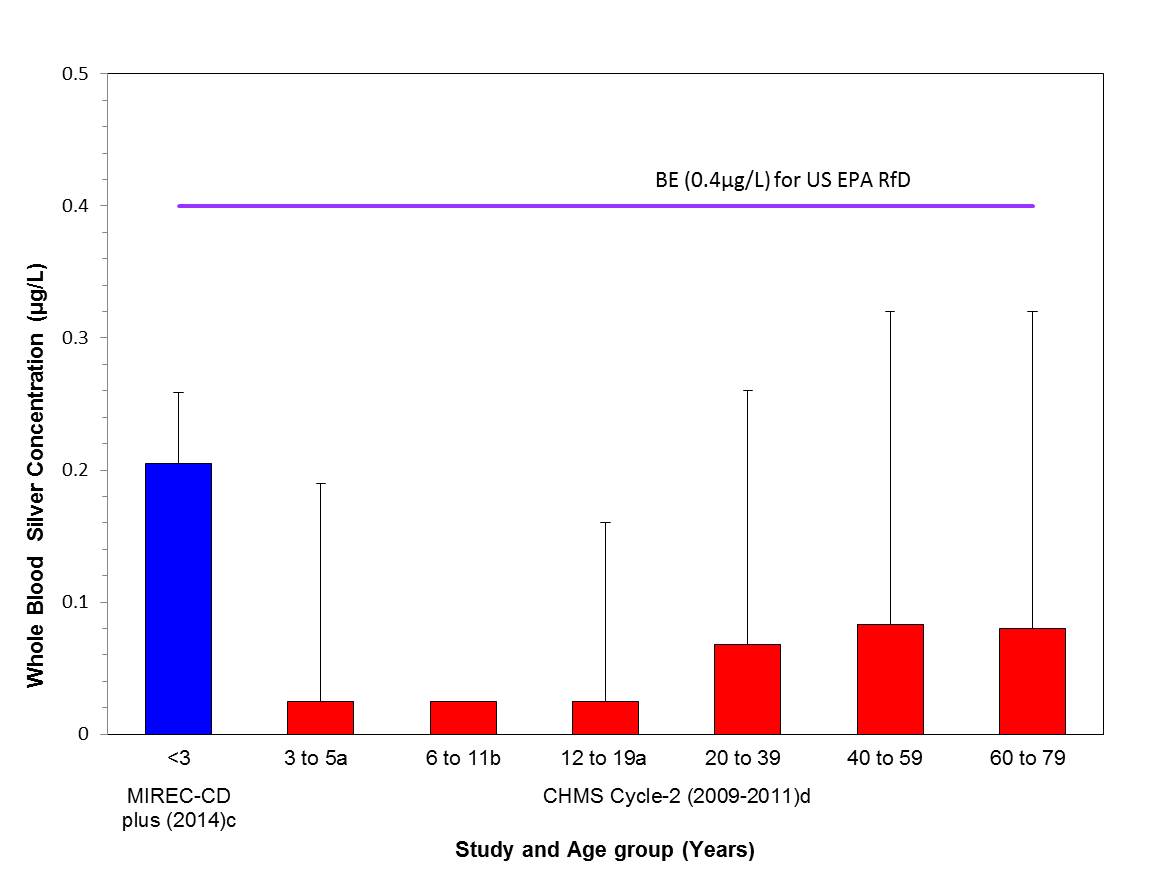
Long description for figure
Comparison of the median (bar) and the 95th percentile (whiskers) of the concentrations of whole blood silver (µg/L) with the biomonitoring equivalent; 0.4 µg/L for the US EPA RfD (IRIS 1991); indicated by a solid line. Biomonitoring data are for both males and females combined. aMedian was below the detection limit and the values plotted represents half the LOD of 0.05 µg/L. bMedian was below the detection limit and the value plotted represents half the LOD of 0.05 µg/L and the 95th percentile was too unreliable to be published. cLiang 2016 dHealth Canada 2013a
5.6 Uncertainties
The hazard dataset for silver is robust and has been reviewed by various international and national organizations. The guideline values determined by international authorities for protecting against argyria, the principle manifestation of chronic silver exposure, were commonly based on human case reports. The calculated guideline values or the estimated no-effect levels for silver lifetime exposure were consistent across different organizations. The US EPA RfD (IRIS 1991), which was used in the risk characterization, has incorporated uncertainty factors to account for uncertainties in the hazard and exposure databases. The confidence in the reference dose was further strengthened by the similar risk characterization for drinking water conducted by the Australian government (Australia Government 2011).
The multi-compartment PBPK model used here was based on data from both animal and human datasets and validated against other available studies. The PBPK model generally well predicted the human blood concentrations in workers occupationally exposed to silver. However, there are uncertainties regarding the oral absorption fraction assumed, as absorption data are lacking in humans. The available PBPK model is structured only for adult physiology. As a result, predictions relevant for specific subpopulations, such as children or pregnant women, have higher uncertainty than the adults in the general population.
6. Application of Biomonitoring Approach 2: Thallium-containing substances
6.1 Identity of Substances
The Chemical Abstracts Service Registry Numbers (CAS RN), Domestic Substances List (DSL) names and common names for the individual substances in the thallium-containing substances group are presented in Table 6-1.
| CAS RN (acronym) |
DSL name (common name) |
Molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
| 563-68-8 | Acetic acid, thallium(1+) salt (Thallium(I) acetate) |
C2H3O2Tl | 266.00 |
| 7791-12-0 | Thallium chloride (Thallous chloride) |
TlCl | 239.83 |
6.2 Sources and Uses
Thallium is a naturally occurring element that is present in environmental media in Canada. Total thallium has been measured in drinking water distribution systems, household dust, indoor and outdoor air, the Health Canada Total Diet Study and breast milk as part of several research initiatives undertaken by Health Canada and Environment and Climate Change Canada (Arbuckle et al. 2013; Health Canada 2003; NAPS 2011; Rasmussen et al. 2016; Tugulea et al. 2016). Overall, in Canada the concentrations of thallium are low in drinking water (median 0.025 μg/L, range 0.025 -0.050 μg/L, n=97), dust (median 0.10 μg/g, 95th percentile 0.20 μg/g, n=1025) and air samples (outdoor NAPS PM2.5 median 0.01 ng/m3, range 0-0.10 ng/m3, n=910; indoor PM2.5 median 0.004 ng/m3, 95th percentile 0.021 ng/m3) (NAPS 2011; Rasmussen et al. 2016; Tugulea et al. 2016). Food is the primary source of thallium exposure for Canadians, where the average dietary intake for all age groups is 0.029 μg/kg bw/day. Toddlers and preschoolers between 1-4 years of age have the highest dietary intake at 0.088 μg/kg bw/day (on a per body weight basis), while dietary intakes of thallium decline with age. Thallium is also present in breast milk as a contaminant and a source of exposure for nursing infants. Average and 95th percentile intakes of 0.0089 μg/kg bw/day and 0.011 μg/kg bw/day, respectively, were derived based on measured concentrations in breast milk from 2001 Canadian mothers between 2008 and 2011 as part of the core MIREC study (2016 email from the Bureau of Chemical Safety, Food Directorate, to the Existing Substances Risk Assessment Bureau, Health Canada, unreferenced, Arbuckle et al. 2013).
Thallium is present in some products in Canada, including pharmaceuticals (DPD [modified 2015]), natural health products (LNHPD [modified 2014]; NHPID [modified 2015]), consumer products (CPCat 2014) and food packaging (personal communication, emails from the Foods Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated March 14, 2016; unreferenced).
6.3 Exposure Assessment
Adequacy of biomarker of exposure:
Thallium has been measured in blood, urine and hair for a variety of purposes, including monitoring for occupational exposure (GFEA 2011). Thallium intake through the oral route is highly absorbed in the GI tract. Studies in rats suggest 100% absorption (ATSDR 1992). Thallium absorbed through any route (oral, inhalation, dermal) will quickly distribute through the blood and be absorbed rapidly into cells and tissues. Thus, absorbed thallium spends - little time in the blood and therefore thallium concentrations in blood may not be a good marker of short- or long-term exposure. The highest concentrations of thallium following exposures are found in the intestines, liver, kidneys, heart, brain and muscles (ATSDR 1992; Galvan-Arzate and Santamaria 1998; Rodriguez-Mercano et al. 2013, GFEA 2011). Based on data from animal studies, thallium concentrations in the brain become elevated over time, whereas the other organs reach steady state (Galvan-Arzate and Santamaria 1998). Thallium as thallous sulfate has been shown to cross the placenta and distribute to the fetus within 15 minutes following intraperitoneal injection (ATSDR 1992). Thallium is cleared primarily through the urine and to a lesser extent the feces. In humans, renal excretion seems to be much more important than in animals. Based on human studies, the majority of thallium dose (~70%) is recovered in the urine (IPCS 1996). A steady state exposure in urine can be expected because the general population people are exposed to thallium daily though ingestion of food. The biological half-life of thallium in the human body has been reported to be anywhere from 1 to 30 days depending on the dose (Galvan-Arzate & Santamaria, 1998; Barclay et al., 1953). The concentrations of total thallium in urine are considered as a reliable biomarker of exposure because thallium is rapidly and well absorbed from the GI tract, the excretion is mainly via kidney with a half- life ranging from 1 to 30 days and the impairment of renal function is not expected at environmental exposure levels (Galvan-Arzate 1998).
Biomonitoring data:
Population-level concentrations of thallium in the urine (creatinine adjusted) of Canadians are presented in the Table 6-2 below. Further details on age, sex and time trends are presented in Appendix A-4. Thallium was detected in 99% of Canadians in the CHMS. Urinary thallium concentrations are relatively stable across the Canadian population. Females have slightly higher concentrations than males, but not statistically significant (Health Canada 2013a). There are no statistically significant differences in thallium between different age groups, including children; however, there is a slight non-significant decrease in urinary thallium concentrations in the oldest age group measured in the CHMS (60 to 79 years) (Health Canada 2013a).
Thallium concentrations in urine have also been measured in NHANES III and six consecutive cycles of CDC-NHANES up to 2012. Urine concentrations declined substantially from the first data point (1988-1994) and the start of the NHANES continuous surveys in 1999 (Paschal et al. 1998, CDC 2015). There was a slight decline in thallium concentrations from 1999 to 2006 at which time concentrations stabilized in the American population (CDC 2015). Age and sex trends observed in the Americans are similar to those observed in Canadians, whereby urine thallium concentrations (creatinine adjusted) are similar across age groups and slightly higher in females than males (CDC 2015). Thallium concentrations are slightly higher in Canadians than Americans.
| Study Population | Sampling year(s) | Age Years |
Sex | n | Median (95% CI) |
95th percentile (95% CI) |
|---|---|---|---|---|---|---|
| CHMS Cycle 2a Canadian population |
2009-11 | 3-79 | M+F | 6291 | 0.21 (0.20 - 0.23) |
0.55 (0.49-0.61) |
| NHANESb U.S. population |
2011-12 | 6 and older | M+F | 2502 | 0.167 (0.156 -0.178) |
0.425 (0.369 - 0.497) |
n = sample size, CI = confidence interval, M = males, F = females
a Health Canada 2013a
b CDC 2015
6.4 Human Biomonitoring Value (HBM-I)
The health effects dataset associated with thallium exposure, the risk-based exposure guidance values and HBM-I established by the German Federal Environmental Agency are summarized below.
The health effects induced by thallium have been reviewed by various international organizations (ATSDR 1992, IPCS 1996, UBA 2011, IRIS 2009).
In humans, the combination of gastroenteritis, polyneuropathy (burning feet syndrome) and alopecia is considered as the classic symptoms of thallium acute exposure. However, animal studies have shown that alopecia can be considered as a critical effect with subchronic exposure (over several months) (UBA 2011). The US EPA has not identified a final RfD for thallium, due to poor quality of the overall database. Instead, a "candidate" RfD was derived based on a 90-day oral rat toxicity study for thallium (I) sulfate reviewed by MRI (1988), where alopecia was identified as the critical effect (IRIS 2009). When the rats were given thallium (I) sulfate via gavage at 0, 0.008, 0.04, or 0.20 mg Tl/kg bw/day for 90 days, hair follicle atrophy in female rats was observed at the top dose level. The mid-dose of 0.04 mg Tl/kg bw/day (as NOAEL) and BMDL10 of 0.01 mg Tl/kg bw/day for the incidence of alopecia in female rats were selected as the PODs for RfD derivation. By applying total uncertainty factors of 3000 (10 for interspecies extrapolation, 10 for intraspecies extrapolation, 3 for extrapolation from a subchronic to chronic study and 10 for database deficiencies), the US EPA (IRIS 2009) yielded thallium RfDs of 1×10-5 mg Tl/kg bw/day for hair follicle atrophy or 3×10-6 mg Tl/kg bw/day for alopecia. However, the US EPA (IRIS 2009) indicated low confidence in the reported data and conclusions from this subchronic study. Limitations noted included dose selection was likely too low and that there were inconsistencies with the interpretation and reporting of data regarding instances of alopecia. In addition, the study author has concluded that the alopecia in female rats were not biologically significant. The alopecia observed in male rats did not show a dose-related increase. By way of comparison, the lowest exposure associated with acute thallium-related toxicity in humans is approximately 4 mg/kg (IRIS 2009), a dose 20-fold higher than the LOAEL from MRI (1988), and exposures reported to be lethal to humans are as low as 6 mg/kg (IPCS, 1996) which is a dose 30-fold higher than the LOAEL from MRI (1988).
Neurological effects of thallium have been reported both in animal toxicity studies and in human studies. The symptoms of relatively high doses of chronic thallium exposure in humans include sleep disorders, tiredness, weakness, nervousness, headache, other psychiatric alterations, and neurological and muscular problems (Kazantzis 2007, IRIS 2009).
Two epidemiology studies from Germany have investigated thallium toxicity in the general population and workers that were exposed to thallium at higher levels than background exposure. An epidemiological study by Brockhaus et al. (1981) investigated a group of the general population (n=1200) who lived in the vicinity of a thallium-emitting cement factory in Lengerich, Germany. In this study, soil samples showed decreasing thallium load as a factor of increasing distance from the factory as well as the direction of the wind. The surrounding population was studied for signs of elevated exposure and symptoms associated with thallium overload. Based on urinary samples, it was found that the nearby inhabitants had an increased renal excretion with a range of less than 0.1 to 76.5 µg/L and a mean of 5.2 µg/L. It was determined that the increase in exposure resulted mainly from the consumption of homegrown fruits and vegetables that were contaminated by thallium-containing dust. In total, over 80% of the exposed subjects had urinary thallium levels above 1 µg/L which was considered to be the upper normal limit of exposure. Two separate, non-exposed control samples taken from both rural and urban areas yielded mean urinary levels of 0.4 and 0.3 µg/L, respectively. No correlations were found between urinary thallium levels and symptoms such as hair loss, and dermal or gastrointestinal (GI) distress, which are all typical of thallium overload. Contrary to general expectations, a negative correlation was found between thallium and hair loss. A positive association was observed, however, between thallium levels and general reported symptoms such as insomnia, fatigue, weakness, nervousness, headache, psychological disorders and neurological and muscular symptoms (Brockhaus et al.1981).
In another investigation of thallium-exposed workers, 128 men who worked in cement production in Middle and Lower Fanconia, Germany submitted urine samples for examination. Thallium urine levels ranged between less than 0.3 to 6.3 µg/g creatinine and no adverse health effects attributable to thallium were seen in any of the men (Schaller et al.1980). Based on the results of this study, GFEA (2011) concluded that "the first negative effects are associated with a 15-fold increase in the normal renal excretion rate".
According to the conclusion of above two epidemiology studies, the WHO-IPCS expert group state in the Environmental Health Criteria 182 for Thallium stated that "renal thallium excretion rates less than 5 µg/L of urine have no detrimental health effects", while the health relevance of 5 and 500 µg/L of urine remains unclear. The thallium concentrations in urine greater than 500 µg/L are associated with clinical manifestation of poisoning (IPCS 1996).
The German Federal Environmental Agency has derived a human biomonitoring value-level 1 (HBM-I) for thallium and the details of the derivation are explained in GFEA 2011. The HBM-I value describes the concentration in the body matrix of a substance below which, according to the Commissions' current assessment, no adverse health effect should be expected (Angerer et al. 2011). The HBM-I value for thallium is based on the same two independent studies (Brockhaus et al.1981, Schaller et al. 1980) which also formed the basis of the WHO-IPCS conclusion. GFEA (2011) derived the HBM-I value directly as the urinary levels associated with no adverse health effects in the above two study populations. Taking into account the conclusion of the WHO-IPCS expert group, the HBM Commission derived a HBM-I value for thallium in urine of 5 µg/L (6.4 µg/g creatinine; creatinine correction is based on the criteria presented in Hays et al. 2010), below which adverse health effects associated with thallium exposure in the general population is not expected.
6.5 Results of the Approach
Figure 6-1 represents the key biomonitoring exposure values and hazard values for thallium used for the determination risk.
Exposure to total thallium in the Canadian population, based on total thallium creatinine adjusted concentrations in urine, is lower than the urine HBM-I exposure guidance value of 5 µg/L (GFEA 2011) equivalent to 6.4 µg/g creatinine when adjusted to a constant creatinine based concentration. The Canadian population exposures are also noted to be below the US EPA RfD (IRIS 2009), even though this value was not selected for risk characterization in this document. The HBM-I value describes the concentration below which no adverse health effects, such as hair loss, GI tract distress, dermal effects and neurological effects should be expected. Based on the information presented here, the thallium-containing substances in this assessment are of low concern to the health of the general public in Canada at current levels of exposure.

Long description for figure 6-1
Comparison of the median (bar) and the 95th percentile (whiskers) of the concentrations of urinary thallium (µg/g creatinine) with the HBM-I of 5 µg/L in urine (German Federal Environment Agency, GFEA 2011), units converted to 6.4 µg/g creatinine; indicated by a solid line. Biomonitoring data are for both males (M) and females (F) combined. aHealth Canada 2013a, bCDC 2015
6.6 Uncertainties
There are no biomonitoring data for children under 3 years of age and children aged 1 to 4 years have the highest dietary intakes (per body weight basis). Although there is a decline with age in estimated dietary intakes, there does not appear to be any age-related trends with thallium creatinine-adjusted urine concentrations. However, data on children were considered by the WHO-IPCS expert group. According to WHO-IPCS (1996), "there are no good data to suggest that infants or pregnant women are more sensitive to effects of thallium than the general population".
The hazard dataset for thallium is relatively robust and it has been reviewed by some international authorities. Uncertainty exists in the thallium health effects assessment, as the limited number of studies of the genotoxicity of thallium compounds provides inconsistent evidence of genotoxic potential and the evidence for the carcinogenicity of thallium is inadequate for classification. The only guideline value derived by IRIS (2009) for alopecia effect is based on a subchronic animal study but with low confidence in the reported data and its conclusions. However, since the EPA RfD is several orders of magnitude below other health effects noted in the animal database, there is confidence in the use of the RfD for this risk assessment.
The HBM-I value is based on epidemiological studies in the vicinity of industrial sources and occupational exposure studies. While the potential confounding factors such as smoking on health outcomes cannot be excluded, there is confidence in the exposures detailed in the report since they were measured urine values. Occupational studies have limitations when setting dose-response relationships and exposure characterization as individuals can be exposed to multiple substances at higher levels than non-occupational exposures. However, the studies used in this assessment directly measured thallium excretion in urine, and as such there is confidence in the measurements of exposure. While international organizations have considered urine as the suitable biomarker of exposure, the mechanism of thallium-related renal toxicity at high doses is less understood adding some uncertainty regarding the suitability of the biomarker at high exposures(i.e., thallium in urine).
7. Application of Biomonitoring Approach 2: Inorganic Tin-containing substances
7.1 Identity of Substance
The Chemical Abstracts Service Registry Numbers (CAS RN), Domestic Substances List (DSL) names and common names for the individual substances in the tin containing substance group are presented in Table 7-1. This chapter focusses on inorganic tin as no organotin substances were identified as having met categorization or as priorities for further review under CEPA (see table 7-1 below). Organic forms of tin (organotins) are more toxic than inorganic tin.
| CAS RN (acronym) |
DSL name (common name) |
Molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
| 1345-24-0 | C.I. Pigment Red 109 (Gold Stannate) |
Unspecified | Not Applicable |
| 7440-31-5 | Tin (Tin) |
Sn | 118.71 |
7.2 Sources and Uses
Tin is a naturally-occurring element that is present in environmental media in Canada. Total tin has been measured in drinking water distribution systems, household dust, and indoor and outdoor air as part of several research initiatives undertaken by Environment and Climate Change Canada (NAPS 2011; Rasmussen et al. 2016; Tugulea et al. 2016). Overall, in Canada concentrations of tin are low in drinking water (median 0.50 μg/L, range 0.50-2.00 μg/L, n=96), dust (median 20 μg/g, 95th percentile 75.7 μg/g, n=1025) and air samples (outdoor NAPS PM2.5 median 0.10 ng/m3, range 0.01-2.09 ng/m3, n=910; indoor PM2.5 median 0.58 ng/m3, 95th percentile 3.52 ng/m3)(NAPS 2011; Rasmussen et al. 2016; Tugulea et al. 2016). The major source of tin exposure is through dietary intake, which highly depends on the type and amount of canned foods and beverages consumed (ATSDR 2005; WHO 2005). There are no current Canadian measurements of inorganic tin concentrations in food or breast milk. In 2006, JECFA estimated dietary intakes to be less than 16 up to 233 µg/kg bw/day based on tin concentration data from the 1990s (JECFA 2006, based on 60 kg bw). More recently, average and 97.5th percentile dietary intakes of tin were estimated to be 15 and 61 µg/kg bw/day in Ireland (Food Safety Authority of Ireland 2011, based on 60 kg bw). Mean and 95th percentile intakes in France were estimated to be 3.9 and 17.0 µg/kg bw/day for adults and 7.3 and 31.9 µg/kg bw/day for children (ANSES 2011). Compotes, cooked fruit and fruit were the primary contributors to dietary intake in France.
Tin is also present in a range of products in Canada, including: drugs (DPD [modified 2015]), natural health products (LNHPD [modified 2014]; NHPID [modified 2015]), cosmetics (personal communication, emails from the Consumer Product Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated February 24, 2016; unreferenced), as a formulant or active ingredient in pesticides (personal communication, emails from the Pest Management Regulatory Agency, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated February 29, 2016; unreferenced), toys (CPCat 2014), consumer products (CPCat 2014; Household Products Database 1993-), food packaging, and as incidental additives in food (personal communication, emails from the Foods Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated March 14, 2016; unreferenced). The maximum level established by Health Canada for tin as a contaminant and adulterating substance in canned food is 250 mg/kg (ppm) (Health Canada 2016).
7.3 Exposure Assessment
Adequacy of biomarker of exposure:
Tin is not an essential element for human health. In general, inorganic tin absorption from the GI tract is low in humans and experimental animals. Most studies have reported less than 5% absorption of inorganic tin although some report absorption as high as 20% absorption (WHO 2004). In humans, available data suggest that GI absorption of inorganic tin decreases with increasing intake (ATSDR 2005). The GI absorption is also influenced by the oxidation state where tin(II) has a greater absorption than tin(IV) (2.8% and 0.6%, respectively) (WHO 2004). Inorganic tin distributes mainly to bones, but also to the lungs, liver, kidneys, spleen, lymph nodes, tongue and skin. In mammals, tin cations do not appear to be rapidly oxidized or reduced during absorption and systemic transportation (WHO 2005). Absorbed tin is predominantly eliminated via kidney while only a small fraction is eliminated via bile (WHO 2004). A human metabolic study (Johnson & Gregor 1982) showed that the fraction of tin excreted via urine (and feces) is dependent upon the exposure dose. This study reported a urinary excretion fraction (FUE) for basal diet (0.1 mg Sn/day) of 26%, and a FUE for a human supplemented dose study (50 mg Sn/day) of 0.25% (Johnson & Gregor 1982). When subjects were fed a test diet containing 50 mg tin daily, a greater proportion was excreted in feces than urine and less was absorbed compared to the subjects fed control diet. The average fecal excretion was 55% of the daily intake in the basal group and 97% in the supplemented group. In humans, tin shows a multi-phased elimination pattern in urine with a half-life of greater than 25 days (Nordic Expert Group 2002). This relatively longer half-life for urinary elimination in humans supports the steady state exposure assumption, even if infrequent exposure occurs through consumption of canned food, which is the predominant source of exposure for the general population. Hence, total inorganic tin concentration in urine is an adequate biomarker of tin exposure in humans.
Biomonitoring data:
Total tin concentrations in urine were measured as part of the NHANES (NHANES III and NHANES 2011-12) and the Quebec Region study (INSPQ 2004b). Tin was not included for analysis in the CHMS, FNBI, or MIREC CD-Plus. Therefore, in the absence of Canadian nationally representative biomonitoring data, tin concentrations from NHANES and the Quebec Region study were considered in this assessment and are presented in Table 7-2. Further details on age, sex and time trends are presented in Appendix A-5. Tin was detected in up to 85% of study participants in NHANES (CDC 2014). Tin urine concentrations in the U.S. population offer the most comprehensive data in North America and are considered to be acceptable surrogate data to estimate exposure levels in Canadians.
Several trends relating to geographic location, age and sex can be observed in the creatinine adjusted urinary concentrations of tin. Overall, creatinine adjusted tin urinary concentrations measured in Canadians in the Quebec Region (collected in 2001) are similar but slightly higher than concentrations measured in the U.S. population in NHANES (2011-12). In the NHANES survey, of the age groups analysed (6-11, 12-19, 20+), children aged 6 to 11 had statistically significantly higher creatinine adjusted urinary concentrations than teens and adults. This age group was not included in the Quebec Region study. Overall, statistically significantly higher concentrations were observed in females compared to males in NHANES and the Quebec Region study.
When compared with a data point from NHANESIII (1988-94), concentrations of urinary tin measured in the U.S population in 2011-12 showed a marked decrease over that 24 year period. This is possibly due to the decreased use of unlacquered or partially lacquered cans for food storage over time.
| Study/Population | Sampling year(s) | Age years |
Sex | n | Median (95% CI) |
95th percentile (95% CI) |
|---|---|---|---|---|---|---|
| NHANESa U.S. population |
2011-12 | 6 and older | M+F | 2502 | 0.624 (0.567-0.678) |
4.50 (3.76-5.33) |
| NHANESa U.S. population |
2011-12 | 6-11 | M+F | 398 | 1.60 (1.44-1.73) |
7.91 (6.11-12.1) |
| Quebec Region Studyb | 2001 | 18- 65 | M+F | 363 | 0.76 | 5.89c |
n = sample size, CI = confidence interval, M = males, F = females
a CDC 2015
b INSPQ 2004a (Data were converted to µg/g creatinine using the molecular weights of tin (118.7 g/mol) and creatinine (113 g/mol)
c 97.5th percentile (95th percentile not available)
7.4 Derivation of Biomonitoring Equivalents (BEs)
The health effects dataset associated with metallic and inorganic tin exposure, the risk-based exposure guidance values and the approaches used in BE derivation are summarized below.
The health effects of metallic tin and inorganic tin have previously been assessed by other international organizations (ATSDR 2005, IPCS 2005, DECOS 2005, EFSA 2005, JECFA 1982, 2011, RIVM 2009, WHO 2004).
After reviewing additional literature and reviews published since 1991, including the evaluations of EFSA (2005), ATSDR (2005) and JECFA (2006), the Netherlands National Institute for Public Health and the Environment (RIVM 2009) concluded that intermediate duration oral exposure to various inorganic tin compounds for 4-13 weeks in rats resulted in haematological effects, decreased body weight gain and histopathological changes in liver and kidney at doses levels equal or greater than 66 mg/kg bw/day (De Groot et al. 1973 a,b). The NOAEL was determined as 32 mg Sn/kg bw/day by ATSDR (2005) and EFSA (2005) for haematological effects from a 13-week rat oral study by De Groot et al. (1973b), where the rats were exposed to SnCl2 at 0, 9.5, 32, 95, and 315 mg Sn/kg bw/day in their diet. By applying an uncertainty factor of 100 (10 for animal to human extrapolation, 10 for human variability) to this NOAEL, ATSDR (2005) derived an intermediate-duration oral Minimal Risk Level (MRL) of 0.3 mg Sn/kg bw/day for inorganic tin.
For chronic exposure, there were no significant non-cancer effects observed in rats or mice exposed up to SnCl2 at 35 to 89 mg Sn/kg bw/day in mice or 203 to 348 mg Sn/kg bw/day in rats for two years (NTP 1982). In another chronic study by Sinkeldam et al. (1981), rats were exposed to tin in diets at 0, 200, 400 and 800 ppm (equivalent to 0, 10, 20, 40 mg Sn/kg bw/day) for 115 weeks. Haemoglobin and haematocrit values were decreased at all dose levels at weeks 4 and 13, but in the second year of the study, these parameters were similar to the control. A decreased food efficiency and slight tin accumulation in bone were reported in the high dose group (NOAEL= 20 mg Sn/kg bw/day). By applying a standard uncertainty factor of 100 (10 for interspecies and 10 for intraspecies variation) to the NOAEL of 20 mg Sn/kg bw/day from this study, a TDI of 0.2 mg Sn/kg bw/day was derived by RIVM (2009). The selection of this NOAEL is further strengthened by the NOAEL of 32 mg Sn/kg bw/day for haematological effects as indicated above in an intermediate duration study in rats by De Groot et al. (1973) (ATSDR 2005; EFSA 2005). In contrast, in a short-term study in mice, El-makawy et al. (2008) reported pregnancy failure at 20 mg Sn/kg bw/day. However, the effects on fertility were not noted in a multi-generation reproductive toxicity study conducted at higher dose levels nor in other species (Sinkeldam et al. 1979b). Hence, the RIVM TDI (RIVM 2009) will be used as the basis for deriving BE in this assessment.
Chronic inhalation of Sn(IV) dust in humans can cause a benign form of pneumoconiosis, without impairment of pulmonary function (ATSDR, 2005, cited in RIVM 2009).
A BE was derived for the TDI established by RIVM (2009) (Poddalgoda et al. 2016b). The mass balance approach as explained in Hays et al. (2010) was used in the derivation of the steady state urinary tin concentration for both volume basis (µg/L) and creatinine adjusted (µg/g creatinine) basis that are consistent with a TDI of 0.2 mg Sn/kg bw/day (RIVM 2009). Based on human data, the urinary excretion fraction (FUE) was considered as 0.25% (Johnson & Gregor 1982). The steady state urinary BE derived from the TDI is 26 µg/g creatinine.
7.5 Results of the Approach
Figure 7-1 represents the key biomonitoring exposure values and hazard values for tin used for the determination of risk. Estimated exposure to Canadians is based on creatinine adjusted total tin concentrations in urine in the U.S. population and the Quebec Region study; NHANES data are considered to be acceptable surrogates to estimate exposure levels in Canadians. Exposure to total tin is lower than the creatinine adjusted urinary equivalent of the RIVM TDI of 0.2 mg/kg bw/day (RIVM 2009) (BE of 26 µg/g creatinine). The TDI is based on a NOAEL of 20 mg/kg bw/day for an increase in tin accumulation in bones and a decrease in feed efficiency observed in a 2-year rat feeding study. Based on the information presented here, the inorganic tin-containing substances in this assessment are of low concern to human health in Canada at current levels of exposure.
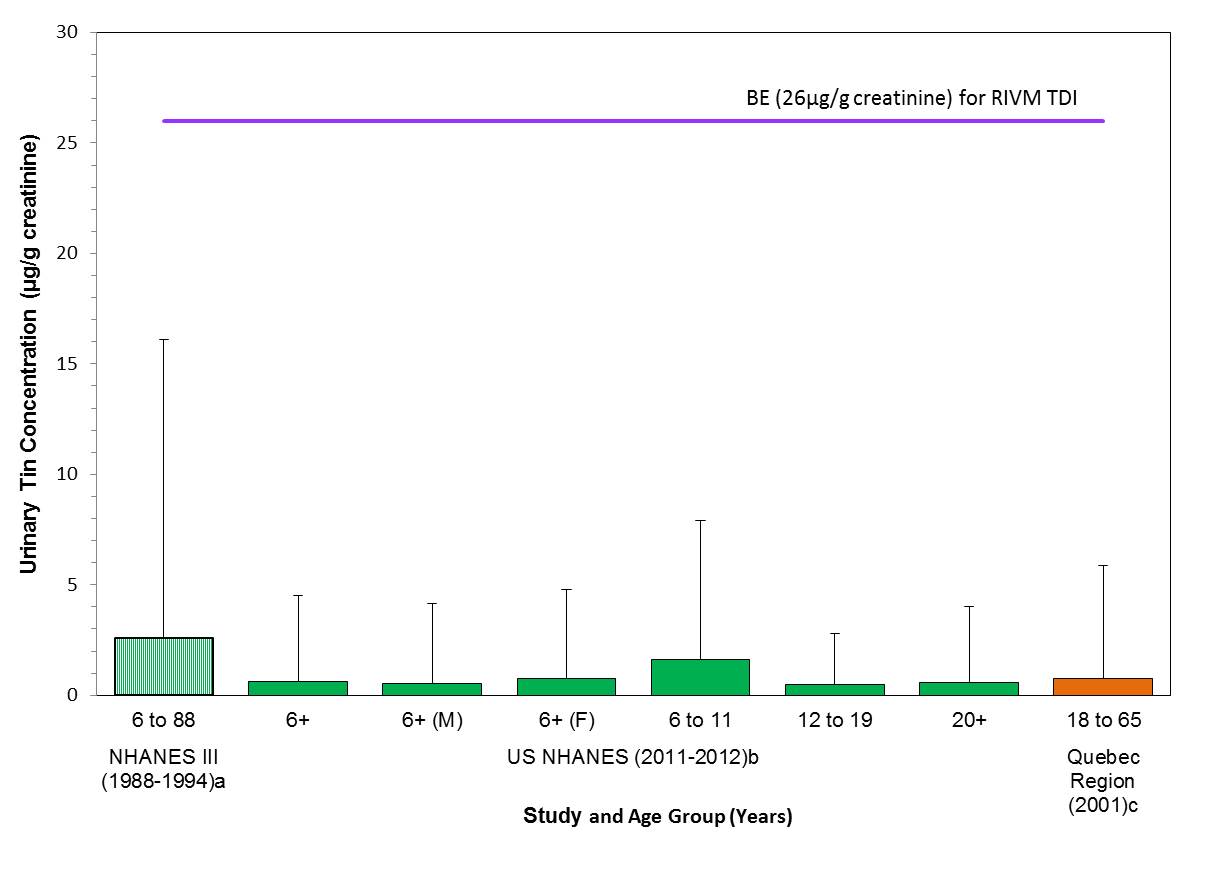
Long description for figure 7-1
Comparison of median (bar) and 95th percentile (whiskers) concentrations of urinary tin (µg/g creatinine) with the biomonitoring equivalent; 26 µg/g creatinine for the TDI (RIVM 2009); indicated by a solid line. Biomonitoring data are for both males (M) and females (F) combined, except where sex is indicated. aPaschal et al. 1998, bCDC 2015, cINSPQ 2004b - whiskers represent the 97.5th percentile
7.6 Uncertainties
The RIVM TDI (RIVM 2009), which was used in risk characterization, has incorporated uncertainty factors to account for uncertainties in the hazard and exposure data. There are no biomonitoring data from the CHMS or for children under 6 years of age, however, the 10x uncertainty factor for intraspecies variation in the TDI does account for variability within the population (RIVM 2009).
The hazard dataset for inorganic tin is robust and has been reviewed by various international and national organizations. Although the guideline value for oral chronic inorganic tin exposure was only derived by RIVM (2009), this guideline value based critical health effect level was further strengthened by the critical health effect level for intermediate duration of exposure as determined by other international organizations for their guideline value generation.
Overall, the availability of internal dose and kinetic data in the animal species forming the basis of the assessment could improve the overall confidence in the current BE. The BE derivation for inorganic tin illustrates the critical importance of choosing and using the FUE relevant to the dose range, particularly where there are data suggestive of dose-dependent absorption (Johnson & Gregor 1982). In this case, the actual FUE associated with the TDI is likely somewhere between 26% and 0.25%; therefore the FUE which would result in the most protective BE was used for the derivation. Further details on the selection of the FUE and the impact on the BE are presented in Poddalgoda et al. (2016b).
8. Overall Summary
The outcome of applying the biomonitoring-based approach 2 to the barium-containing substances, molybdenum-containing substances, silver-containing substances, thallium-containing substances and inorganic tin-containing substances identified in this science approach document indicate that they are of low concern to the health of the general population in Canada, at current levels of exposure.
References
[AFN] Assembly of First Nations. 2013. First Nations Biomonitoring Initiative National Results. (2011). Assembly of First Nations. [accessed 2016 January].
Angerer J, Aylward L, Hays S, Heinzow B, Wilhem M. 2011. Human biomonitoring assessment values: Approaches and data requirements. Int J Hyg Environ Health. 214(5):348-60.
[ANSES] French Agency for food, environmental and occupational health and safety. 2011. Second French Total Diet Study (TDS 2) Report 1 Inorganic contaminants, minerals, persistent organic pollutants, mycotoxins and phytoestrogens. ANSES Opinion. June 2011. Accessed March 2016.
Arbuckle TE, Fraser WD, Fisher M, Davis K, Liang CL, Lupien N, Bastien S, Velez MP, von Dadelszen P, Hemmings DG, Wang J, Helewa M, Taback S, Sermer M, Foster W, Ross G, Fredette P, Smith G, Walker M, Shear R, Dodds L, Ettinger AS, Weber JP, D'Amour M, Legrand M, Kumarathasan P, Vincent R, Luo ZC, Platt RW, Mitchell G, Hidiroglou N, Cockell K, Villeneuve M, Rawn DF, Dabeka R, Cao XL, Becalski A, Ratnayake N, Bondy G, Jin X, Wang Z, Tittlemier S, Julien P, Avard D, Weiler H, Leblanc A, Muckle G, Boivin M, Dionne G, Ayotte P, Lanphear B, Séguin JR, Saint-Amour D, Dewailly E, Monnier P, Koren G, Ouellet E. 2013. Cohort profile: the maternal-infant research on environmental chemicals research platform. Paediatr Perinat Epidemiol. 27(4):415-25.
[ATSDR] Agency for Toxic Substances and Disease Registry. 1990. Toxicological Profile For Silver. Updated webinfo on Jan 2015.
[ATSDR] Agency for Toxic Substances and Disease Registry. 1992. Toxicological Profile For Thallium.
[ATSDR] Agency for Toxic Substances and Disease Registry. 2005. Toxicological Profile for Tin and Tin Compounds. U.S. Department of Health and Human Services, Public Health Service. August.
[ATSDR] Agency for Toxic Substances and Disease Registry. 2007. Toxicological Profile for Barium and Barium Compounds. ATSDR, US Department Of Health and Human Services, Public Health Service.
Australian Government. 2011. Australian Drinking Water Guidelines 6. Vol 1.
Aylward LL, Bachler G, von Goetz N, Hays AM, Poddalgoda D, Nong A. 2016. Biomonitoring equivalents for interpretation of silver biomonitoring data in a risk assessment context. Int J of Hygiene and Environmental Health.
Bachler G, von Goetz N, Hungerbühler K. A physiologically based pharmacokinetic model for ionic silver and silver nanoparticles. Int J Nanomedicine. 2013(8):3365-3382.
Barclay RK, Pencock WC, Karnofsy DA. 1953. Distribution and excretion of radioactive thallium in the chick embryo, rat, and man. J Pharmacol Exp Ther 107:178-187
Benn T, Cavanaugh B, Hristovski K, Posner JD, Westerhoff P. 2010. The release of nanosilver from consumer products used in the home. J. Environ. Qual. 39:1875–1882
Brenniman GR, Namekata T, Kojola WH, et al. 1979. Cardiovascular disease death rates in communities with elevated levels of barium in drinking water. Environ Res 20:318-324. [cited in ATSDR 2007].
Brenniman, G.R. and Levy, P.S. Epidemiological study in Illinois drinking water supplies. In: Advances in modern environmental toxicology. Vol. IX. Princeton Publishing Co., Princeton, NJ. p. 231 (1985).[cited in ATSDR 2007]
Brockhaus A, Dolgner R, Ewers U, Krämer U, Sod-demann H, Wiegand H. 1981. Intake and health effects of thallium among a population living in the vicinity of a cement plant emitting thallium containing dust. Int Arch Occup Environ Health (48):375–389. [cited in UBA 2011].
Butler KS, Peeler DJ, Casey BJ, Dair BJ and Elespuru RK, 2015. AgNPs: correlating nanoparticle size and cellular uptake with genotoxicity. Mutagenesis 30, 577–591.[cited in EFSA 2016].
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C., 1999, c. 33.
[CDC] Centers for Disease Control and Prevention. 2009. Fourth National Report on Human Exposure to Environmental Chemicals. U.S. Department of Health and Human Services. Atlanta (GA): CDC. [accessed 2016 February].
[CDC] Centers for Disease Control and Prevention. 2014. Metals - Urine Data. Laboratory Data files. National Health and Nutrition Examination Survey 2011-2012.
[CDC] Centers for Disease Control and Prevention. 2015. Fourth National Report on Human Exposure to Environmental Chemicals. Updated Tables, February 2015. U.S. Department of Health and Human Services. Atlanta (GA): CDC. [accessed 2016 January].
Choi J, Morck TA, Joas A, Knudsen L. 2015. Major national human biomonitoring programs in chemical exposure assessment. AIMS Enviorn Sci 2(3):782-802.
[CPCat] Chemical and Product Categories [database]. 2014. Ver. 04. Washington (DC): US Environmental Protection Agency. [updated 2014 May 21; accessed 2016 Jan]. [Database described in Dionisio KL, Frame AM, Goldsmith MR, Wambaugh JF, Liddell A, Cathey T, Smith D, Vail J, Ernstoff AS, Fantke P, et al. 2015. Exploring consumer exposure pathways and patterns of use for chemicals in the environment. Toxicol Rep. 2:228-237. .
[Danish EPA] Danish Environment Protect Agency. 2013. Barium, inorganic water-soluble compounds. Evaluation of health hazards and proposal of health based quality criteria for soil and drinking water Environmental Project No. 1516.
[DECOS] Dutch Expert Committee on Occupational Standards. 2005. Tin and inorganic tin compounds; Health-based recommended occupational exposure limit. The Hague: Health Council of the Netherlands, 2005; publication no. 2005/06OSH.
[DECOS] Dutch Expert Committee on Occupational Standards. 2013. Molybdenum and molybdenum compounds. Health Council of the Netherlands.[Accessed 2016 February 03].
De Groot AP, Feron VJ, Til HP. 1973a. Short-term toxicity studies on some salts and oxides of tin in rats, Fd. Cosmet Toxicol., 11, 19-30 [cited in JECFA 1982].
De Groot A P, Til H P, Willems MI. 1973b. Short-term (90 day) toxicity of tin in rats on semi-purified diets with excessive or marginal iron content. Unpublished report (No. R 4264) submitted to the World Health Organization by Centraal Instituut voor Voedingsonderzoek. [cited in JECFA 1982]
[DPD] Drug Product Database [database]. [modified 2015 Jul 17]. Ottawa (ON): Health Canada. [accessed 2016 Jan 20].
[EFSA] European Food Safety Authority. 2005. Opinion of the Scientific Panel on Dietetic Products, Nutrition and Allergies on a request from the Commission related to the Tolerable Upper Intake Level of Tin (Request N° EFSA-Q-2003-018) (adopted on 6 July 2005). [accessed 2016 May 6].
[EFSA] European Food and Safety Authority. 2013. Scientific Opinion on Dietary Reference Values for molybdenum. [updated 2003 October; accessed 2016 February 03].
[EFSA] European Food Safety Authority. 2016. ANS Panel (EFSA Panel on Food Additives and Nutrient Sources Added to Food). Scientific opinion on the re-evaluation of silver (E 174) as food additive, EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS), European Food Safety Authority (EFSA), Parma, Italy [accessed 2016 May 6].
El-Makawy AI, Girgis SM, Khalil WK. 2008 Developmental and genetic toxicity of stannous chloride in mouse dams and fetuses. Mutat Res. 657(2):105-10.
Environment Canada, Health Canada. [modified 2007 Apr 20]. Categorization. Ottawa (ON): Government of Canada. [assessed 2016 Jan].
Environment Canada, Health Canada. 2012. Preliminary Assessment. Triclosan. Ottawa (ON): Environment Canada, Health Canada.
Environment Canada, Health Canada. 2014. Draft Screening Assessment. Cobalt and Cobalt-Containing Substances. Ottawa (ON): Environment Canada, Health Canada. [accessed 2016 Feb].
Environment Canada, Health Canada. 2015. Draft Screening Assessment. Selenium and its compounds. Ottawa (ON): Environment Canada, Health Canada. [accessed 2016 Feb]..
Food Saftey Authority of Ireland. 2011. Report on a Total Diet Study carried out by the Food Safety Authority of Ireland in the perod 2001 – 2005. Monitoring and Surveillance Series. Food Safety Authority of Ireland. Dublin: Ireland.
Fungwe TV, Buddingh F, Demick DS, Lox CD, Yang MT, Yang SP. 1990. The role of dietary molybdenum on estrous activity, fertility, reproduction and molybdenum and copper enzyme activities of female rats. Nutr Res 10:515–524.
Galván-Arzate S, Santamaría A. Thallium toxicity. Toxicol Lett. 1998 Sep 30;99(1):1-13. Review. PubMed PMID: 9801025.
Gaul LE and Staud AH. 1935. Clinical spectroscopy. Seventy cases of generalized argyrosis following organic and colloidal silver medication including a biospectrometric analysis of ten cases. J. Am. Med. Assoc. 104(16): 1387-1390.[cited in US EPA 1991]
[GFEA] German Federal Environment Agency. 2011. Substance monograph on thallium – Reference and human biomonitoring (HBM) values for thallium in urine. Human Biomonitoring Committee of the Federal Environment Agency. Bundesgesundheitsbl 54: 516-524. With permission of Springer. Gocke E, King MT, Eckhardt K, Wild D. Mutagenicity of cosmetics ingredients licensed by the European Communities. Mutat Res 1981;90:91-109. [cited in DECOS 2005].
Hays SM, Aylward LL, LaKind JS, Bartels MJ, Barton HA, Boogaard PJ, et al. 2008. Guidelines for the derivation of Biomonitoring Equivalents: report from the Biomonitoring Equivalents Expert Workshop. Regul Toxicol Pharmacol 51(3): S4-15.
Hays SM, Aylward LL, Gagne M, Nong A, Krishnan K. 2010. Biomonitoring Equivalents for inorganic Arsenic. Regul Toxicol Pharmacol 58(1): 1-9.
Hays SM, Macey K, Poddalgoda D, Lu M, Nong A, Aylward L. 2016. Biomonitoring Equivalents for Molybdenum. Reg Toxicol and Pharmacol. 77 : 223-229.
Health Canada.1990. Guideline for Canadian Drinking Water Quality. Barium Ottawa (ON): Health Canada. [Accessed 2016 Jan 19].
Health Canada. 2003. Canadian Total Diet Study [Internet]. Ottawa (ON): Health Canada. [accessed 2016 Jan].
Health Canada. 2006. Food additives permitted for use in Canada [modified 2006 Dec 11]. Ottawa (ON): Health Canada. [Accessed 2016 Jan 19].
Health Canada. 2010. Report on Human Biomonitoring of Environmental Chemicals in Canada. Results of the Canadian Health Measures Survey cycle 1 (2007-2009). August 2010. Ottawa (ON): Health Canada. [Accessed 2016 Jan].
Health Canada. 2013a. Second Report on Human Biomonitoring of Environmental Chemicals in Canada. Results of the Canadian Health Measures Survey cycle 2 (2009-2011). April 2013. Ottawa (ON): Health Canada.
Health Canada. 2013b. Final Human Health State of the Science Report on Lead. Ottawa(ON): Health Canada [Accessed 2016 June].
Health Canada. 2015. The Cosmetic Ingredient Hotlist – December 2015 [Internet]. Ottawa (ON): Health Canada, Consumer Product Safety. [Accessed 2016 February].
Health Canada. 2016 List of Contaminants and Other Adulterating Substances in Foods. Ottawa(ON): Health Canada. [Accessed 2016 March].
Health Council of the Netherlands. 2013 Molybdenum and molybdenum compounds - Health-based recommended occupational exposure limit. The Hague: Health Council of the Netherlands, [Accessed 2016 March].
Hill WR and Pillsbury DM. 1939. Argyria, the Pharmacology of Silver. The Williams and Wilkins Co., Baltimore, Maryland.[cited in Australian Government 2011].
Household Products Database [database on the Internet]. 1993- Bethesda (MD): U.S. National Library of Medicine [updated 2015 August; Accessed 2016 January].
[ICMM] International Council on Mining and Metals. 2007. HERAG Health Risk Assessment Guidance for Metals. London(UK). ISBN 978-0-9553591-4-9. [Accessed June 2016].
[INSPQ] Institut national de santé publique du Québec. 2004a. Étude sur l'établissement de valeurs de référence d'éléments traces et de métaux dans le sang, le sérum et l'urine de la population de la grande région de Québec. Institut national de santé publique du Québec, Québec, Que. Cote: INSPQ-2004-030.
[INSPQ] Institut national de santé publique du Québec. 2004b. Supplémentai data. Étude sur l'établissement de valeurs de référence d'éléments traces et de métaux dans le sang, le sérum et l'urine de la population de la grande région de Québec. Institut national de santé publique du Québec, Québec, Que. Cote: INSPQ-2004-030.
[IOM] Institute of Medicine. 2001. Dietary Reference Intakes for Vitamin A, Aresnic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicone, Vanadium, Zinc. [Accessed 2016 February 03].
[IPCS] International Programme on Chemical Safety. 1996. Thallium. International Programme On Chemical Safety, Environmental Health Criteria 182. [Accessed 2016 Jan].
[IPCS] International Programme on Chemical Safety. 2005. Concise International Chemical Assessment Document 65. Tin and Inorganic Tin Compounds. Geneva (CH): United Nations Environment Programme; International Labour Organization; World Health Organization. [Accessed February 2016].
[IRIS] Integrated Risk Information System. 1991. Silver; CASRN 7440 - 22 - 4: U.S. Environmental Protection Agency, National Center for Environmental Assessment. [Accessed 27 July 2015].
[IRIS] Integrated Risk Information System. 1992. Molybdenum; CASRN 7439-98-7: U.S. Environmental Protection Agency, National Center for Environmental Assessment. [Accessed 2016 February 03].
[IRIS] Integrated Risk Information System. 2005. Barium and compounds; CASRN 7440-39-3: U.S. Environmental Protection Agency, National Center for Environmental Assessment [Accessed April 2016].
[IRIS] Integrated Risk Information System. 2009. Thallium (I), soluble salts; CASRN U.S Environmental Protection Agency, National Center for Environmental Assessment [Accessed April 2016].
[JECFA] Joint FAO/WHO Expert Committee on Food Additives. 1982. Toxicological evaluation of certain food additives and contaminants. Joint FAO/WHO Expert Committee on Food Additives. Cambridge, Cambridge University Press, pp. 297–319 (WHO Food Additives Series No. 17).
[JECFA] Joint FAO/WHO Expert Committee on Food Additives. 2006. EVALUATION OF CERTAIN FOOD CONTAMINANTS Sixty-fourth report of the Joint FAO/WHO Expert Committee on Food Additives WHO Geneva. WHO Technical Report Series 930.
[JECFA] Joint FAO/WHO Expert Committee on Food Additives. 2011. Working document for information and use in discussions related to contaminants and toxins in the GSCTFF.
Jiménez-Lamana J, Laborda F, Bolea E, Abad-Álvaro I, Castillo JR, Bianga J, He M, Bierla K, Mounicou S, Ouerdane L, Gaillet S, Rouanet JM, Szpunar J. 2014. An insight into silver nanoparticles bioavailability in rats. Metallomics. 6(12):2242-9.
Johnson, M.A. and Greger, J.L. (1982). Effects of dietary tin on tin and calcium metabolism of adult males. Am. J. Clin. Nutr. (35): 655-660.
LaKind JS, Aylward, LL, Brunk C, DiZio S, Dourson M, Goldstein DA, Kilpatrick ME, Krewski D, Bartels MJ, Barton HA, Boogaard PJ, Lipscomb J, Krishnan K, Nordberg M, Okino M, Tan YM, Viau C, Yager JW, Hays SM, 2008. Guidelines for the communication of Biomonitoring Equivalents: report from the biomonitoring equivalents expert workshop. Reg. Toxicol. Pharmacol. 51, S16–S26.
Leggett RW. 1992. Fractional absorption of ingested barium in adult humans. Health Phys 62(6):556-561.
[LNHPD] Licensed Natural Health Products Database [database]. [modified 2014 Feb 27]. Ottawa (ON): Health Canada. [Accessed 2016 Jan 16].
Liang CL. 2016. Descriptive statistics of metals for MIREC-CD Plus. 28/01/2016. Ottawa (ON): Population Studies Division, Health Canada [personal communication, unpublished data].
McCauley PT, Douglas BH, Laurie RD, Bull RG. 1985. Investigations into the effect of drinking water barium on rats. Environ. Health Perspect. Vol. IX, E. J. Calabrese, ed. Princeton Scien-tific Publications, Princeton, NJ. pp.197–210.[cited in US EPA 2005].
[MRI] Midwest Research Institute. 1988. External letter peer review of a report by midwest research institute, revised final report: Toxicity of Thallium (I) Sulfate (CAS No. 7446-18-6) in Sprague-Dawley Rats, Volume Two: Subchronic (90-day) Study, July 1988. Prepared for Health and Ecological Criteria Division Office of Science and Technology Office of Water U.S. Environmental Protection Agency
Murray FJ, Sullivan FM, Tiwary AK, Carey S. 2014a. 90-Day subchronic toxicity study of sodium molybdate dihydrate in rats. Regul. Toxicol. Pharmacol. 70 (3): 579–588.
Murray FJ, Tyl RW, Sullivan FM, Tiwary AK, Carey S. 2014b. Developmental toxicity study of sodium molybdate dihydrate administered in the diet to Sprague Dawley rats. Reprod. Toxicol. 49: 202–208.
[NAPS] National Air Pollution Surveillance Program. 2011. PM Speciation - WICPMS metals data (9 files). (accessed 2015 Dec 23)
[NHPID] Natural Health Products Ingredients Database [database]. [modified 2015 Nov 23]. Ottawa (ON): Health Canada. [accessed 2016 Jan 16].
[Nordic Expert Group] The Nordic Expert Group for Criteria Documentation of Health Risks from Chemicals and The Dutch Expert Committee on Occupational Standards. 2002. 130. Tin and Inorganic Tin Compounds. Stockholm, National Institute for Working Life. [Accessed 2016 February].
[NTP] National Toxicology Program. 1982. Carcinogenesis bioassay of stannous chloride. Bethesda, MD, US Department of Health and Human Services, National Institutes of Health, National Toxicology Program (DHHS Publication No. (NIH) 81-1787).(cited in WHO 2004). [Accessed 2016 February].
[NTP] National Toxicology Program. 1994. Technical report on the toxicology and carcinogenesis studies of barium chloride dihydrate (CAS No. 10326-27-9) in F344/N rats and B6C3F1 mice. NTP, Washington, DC.
[NTP] National Toxicology Program. 2002. Developmental toxicity evaluation for silver acetate (CAS NO. 563-63-3) administered by gavage to Sprague -Dawley (CD) rats on gestational days 6 through 19. NTP/NIEHS Contract NO.: N01-ES-65405 NTP Study NO.: TER-20-001. [cited in EFSA 2016].
[OECD] Organisation for Economic Co-operation and Development. 2005. Barium carbonate (CAS – 513 77 9). OECD SIDS Initial assessment report for SIAM20, Paris, France. 19-22 April 2005
[OECD] Organisation for Economic Co-operation and Development. 2008. Barium chloride, CAS No. 10361-37-2. OECD SIDS initial assessment profile. SIAM 27, 14-16 October 2008.
[OECD] Organisation for Economic Co-operation and Development. 2013. SIDS initial assessment report: Highly Soluble Molybdenum Salts, CoCam 5, 15/10/2013 March. [Accessed 2016 February 03].
Olcott CT.1947. Experimental argyrosis. III. Pigmentation of the eyes of rats following ingestion of silver during long periods of time. American journal of pathology, 1947, 23:783- 789. [cited in EFSA 2016]
Olcott CT.1950. Experimental argyrosis. V. Hypertrophy of the left ventricle of the heart. Archives of pathology, 1950, 49:138-149. [cited in EFSA 2016]
Paschal DC, Ting BC, Morrow JC, Pirkle JL, Jackson RJ, Sampson EJ, Miller DT, Caldwell KL. 1998. Trace Metals in Urine of United States Residents: Reference Range Concentrations. Environ Res 76(1):53-59. doi:10.1006/enrs.1997.3793
Poddalgoda D, Macey K, Assad H, Krishnan K. 2016a. Derivation of biomonitoring equivalent for barium for interpreting population-level urinary biomonitoring data. Manuscripts submitted.
Poddalgoda D, Macey K, Jayawardene I, Krishnan K. 2016b. Derivation of biomonitoring equivalent for tin for interpreting population-level urinary biomonitoring data. Regulatory Toxicology and Pharmacology. Manuscript accepted.
[PMRA] Pest Management Regulatory Agency. 2010. PMRA list of formulants. Ottawa (ON): Health Canada, PMRA. HC Pub. No.: 100460, Cat. No.: H114- 22/2010E. [Accessed 2016 Jan].
Rasmussen et al. 2015. Preliminary Data from the Windsor Exposure Assessment Study and the Canadian House Dust Study. October 9, 2015. Ottawa (ON): Exposure and Biomonitoring Division, Health Canada [personal communication, unpublished data].
Rasmussen et al. 2016. Preliminary Exposure Data for Five Metals from 2014-2017 CMP(3) Research. February 15, 2016. Ottawa (ON): Exposure and Biomonitoring Division, Health Canada [personal communication, unpublished data].
[RIVM] National Institute for Public Health and the Environment, Netherlands. 2009. Tiesjema B and Baars AJ. Re-evaluation of some human toxicological maximum permissible risk levels earlier evaluated in the period 1991-2001. RIVM report no. 711701092. National Institute for Public Health and the Environment, Bilthoven. [Accessed 2016 Feb].
Rodríguez-Mercado JJ, Altamirano-Lozano MA. 2013. Genetic toxicology of thallium: a review. Drug Chem Toxicol. 36(3):369-83
Schaller KH, Manke G, Raithel HJ, et al. 1980. Investigations of thallium exposed workers in cement factories. Int Arch Occup Environ Health 47:223-231
[SCHER] European Commission Scientific Committee on Health and Environmental Risks. 2012. Assessment of the tolerable daily intake of Barium. The SCHER adopted this opinion at its 16th plenary on 22 March 2012. [Accessed 2016 March].
Schroeder HA and Mitchener M. 1971. Toxic effects of trace elements on the reproduction of mice and rats.Arch Environ Health 23(2): 102-106. [cited in DECOS 2013].
Schroeder HA, Tipton IH & Nason AP. 1972. Trace metals in man. Strontium & Barum. J Chro Dis 25: 491-517.
Schroeder H, Mitchener M. 1975a. Life-term studies in rats: effects of aluminum, barium, beryllium and tungsten. J Nutr 105:421-427 [cited in IRIS 2005].
Schroeder H, Mitchener M. 1975b. Life-term effects of mercury, methyl mercury and nine other trace metals onmice. J Nutr 105:452-458 [cited in IRIS 2005].
Sievers E, Oldigs HD, Dörner K, Kollmann M, Schaub J. 2001. Molybdenum balance studies in premature male infants. Eur J Pediatr 160(2):109-13.
Sinkeldam EJ, Dreef-van der Meulen EJ, Willems MI.1981. Chronic (115 weeks) oral toxicity study with stannous chloride in rats. Report No R6372. Central Institute for Nutrition and food Research, Zeist, DECOS. Unpublished report submitted by Thomassen and Drijver-Verblifa, N.V. Deventer, Netherlands to WHO/FAO. [cited in EFSA 2005]
Sinkeldam EJ, Koeter HBWM, Willems MI.1979b. Multigeneration study with stannous chloride in rats. Report No R6281. Central Institute for Nutrition and food Research, Zeist, Netherlands. Unpublished report submitted by Thomassen and Drijver-Verblifa, N.V. Deventer, DECOS to WHO/FAO.[cited in EFSA 2005].
Stoewsand, G. S., Anderson, J. L., Tutzke, M. (1988). Deposition of Barium in the Skeleton of Rats Fed Brazil Nuts. Nutrition Reports International. 38(2): 259–262.
Tipton, I. H., Stewart, P. L., Dickson, J. (1969). Patterns of Elemental Excretion in Long Term Balance studies. Health Phys. 16(4): 455–462.
Tugulea AM et al. 2016. National survey of disinfection by-products and selected drinking water contaminants in Canadian drinking water (2009-2010) [personal communication, unpublished data].
Turnlund JR, Keyes WR. Plasma molybdenum reflects dietary molybdenum intake. J Nutr Biochem. 2004 Feb;15(2):90-5. PubMed PMID: 14972348.
[UBA] German Federal Environment Agency. 2011. Substance monograph on thallium – Reference and human biomonitoring (HBM) values for thallium in urine. Position paper by the Human Biomonitoring Committee of the Federal Environmental Agency (Kommission "Human-Biomonitoring" des Umweltbundesamtes). Bundesgesundheitsbl 2011 54:516-524. Springer-Verlag 2011 [in BE folder: 9232032_TG_thallium BE- german only_DE_EN.docx]
[US EPA] United States Environmental Protection Agency. 1980. Ambient water quality criteria for silver. Washington D.C, US Environmental Protection Agency (440/5-80-071).
[US EPA] United Sates Environmental Protection Agency. 1993, R.E.D. FACTS. Silver. Office for Prevention, Pesticides And Toxic Substances. Washington D.C. US Environmental Protection Agency. [Accessed 2016 Feb].
[US EPA] United States Environmental Protection Agency. 2007. Framework for Metals Risk Assessment. Office for the Scientific Advisor, Risk Assessment Forum. Washington D.C. US Environmental Protection Agency. [Accessed 2016 June].
Vyskocil A, Viau C. 1999. Assessment of molybdenum toxicity in humans. J Appl Toxicol 19:185–192. [cited in IOM 2001].
Walker M. 2016. Metals analysis for pregnant women in CHMS. January 20, 2016. Ottawa (ON): Environmental Health Sciences and Research Bureau, Health Canada [personal communication, unpublished data].
Werner E, Roth P, Heinrichs U, Giussani A, Cantone MC, Zilker TH, Felgenhauer N, Greim H, 2000. Internal biokinetic behaviour of molybdenum in humans studied with stable isotopes as tracers. Isot. Environ. Health Stud. 36(2):123-132
[WHO] World Health Organization. 1990. Environmental Health Criteria 107 Barium. Geneva, WHO.
World Health Organization (WHO). 2003. Silver in drinking-water. Background document for preparation of WHO Guidelines for drinking-water quality. Geneva, World Health Organization (WHO/SDE/WSH/03.04/14).
[WHO] World Health Organization. 2004. Inorganic tin in drinking-water. Background document for preparation of WHO Guidelines for drinking-water quality. Geneva, World Health Organization (WHO/SDE/WSH/03.04/115). [Accessed 2016 Feb]. [Accessed 2014-08-27]
[WHO] World Health Organization. 2005. Tin and inorganic tin compounds. Concise international chemical assessment document; 65. Geneva, World Health Organization. [Accessed 2016 August 19].
[WHO] World Health Organization. 2011a. Chemical hazards in drinking-water: Molybdenum, Molybdenum in Drinking-water, WHO/SDE/WSH/03.04/11/Rev/1. [Accessed 2016 February 03].
[WHO] World Health Organization. 2011b. Guidelines for Drinking-water Quality, Fourth Edition. [Accessed 2015 July 27].
[WHO] World Health Organization. 2015. Barium in Drinking-water, Draft background document for development of WHO Guidelines for Drinking-water Quality, 22 December 2015, Version for public review. [Accessed 2016 Feb].
Appendices
Appendix A: Biomonitoring Data
A-1 Barium
| Study Population | Sampling year(s) | Age years |
Sex | n | Median (95% CI) |
95th percentile (95% CI) |
|---|---|---|---|---|---|---|
| NHANES IIIa U.S. population |
1988-94 | 6-88 | M+F | 496 | 0.72 | 7.52 |
| NHANESb U.S. population |
1999-2000 | 6 and older | M+F | 2180 | 1.41 (1.28-1.54) |
6.33 (5.47-8.09) |
| NHANESb U.S. population |
2001-02 | 6 and older | M+F | 2689 | 1.49 (1.35-1.63) |
6.24 (5.28-7.27) |
| NHANESb U.S. population |
2003-04 | 6 and older | M+F | 2558 | 1.41 (1.31-1.58) |
7.10 (6.29-7.77) |
| NHANESb U.S. population |
2005-06 | 6 and older | M+F | 2576 | 1.48 (1.37-1.61) |
6.38 (5.62-7.56) |
| NHANESb U.S. population |
2007-08 | 6 and older | M+F | 2627 | 1.60 (1.51-1.72) |
7.20 (5.94-8.59) |
| NHANESb U.S. population |
2009-10 | 6 and older | M+F | 2848 | 1.55 (1.42-1.64) |
6.24 (5.60-7.43) |
| NHANESb U.S. population |
2011-12 | 6 and older | M+F | 2502 | 1.38 (1.27-1.47) |
6.27 (5.20-6.66) |
| NHANESb U.S. population |
2011-12 | 6 and older | M | 1261 | 1.17 (1.08-1.33) |
5.35 (4.11-6.34) |
| NHANESb U.S. population |
2011-12 | 6 and older | F | 1241 | 1.51 (1.39-1.67) |
6.52 (5.32-8.33) |
| NHANESb U.S. population |
2011-12 | 6-11 | M+F | 398 | 2.18 (1.70-2.61) |
6.78 (5.98-8.18) |
| NHANESb U.S. population |
2011-12 | 12-19 | M+F | 390 | 1.42 (1.24-1.78) |
6.29 (4.90-6.57) |
| NHANESb U.S. population |
2011-12 | 20+ | M+F | 1714 | 1.31 (1.20-1.43) |
5.54 (4.38-6.84) |
n = sample size, CI = confidence interval, M = males, F = females
a NHANES III Paschal et al. 1998
b CDC 2015
A-2 Molybdenum
| Study Population | Sampling year(s) | Age years |
Sex | n | Median (95% CI) |
95th percentile (95% CI) |
|---|---|---|---|---|---|---|
| CHMS Cycle 1a Canadian population |
2007-09 | 6-79 | M+F | 5319 | 0.66 (0.64 - 0.68) |
1.38 (1.31 - 1.46) |
| CHMS Cycle 1a Canadian population |
2007-09 | 6-79 | M | 2576 | 0.65 (0.64 - 0.67) |
1.35 (1.24 - 1.46) |
| CHMS Cycle 1a Canadian population |
2007-09 | 6-79 | F | 2743 | 0.67 (0.65 - 0.70) |
1.40 (1.31 - 1.49) |
| CHMS Cycle 1a Canadian population |
2007-09 | 6-11 | M+F | 910 | 0.80 (0.76 - 0.84) |
1.60 (1.51 - 1.69) |
| CHMS Cycle 1a Canadian population |
2007-09 | 12-19 | M+F | 945 | 0.65 (0.60 - 0.71) |
1.31 (1.10 - 1.52) |
| CHMS Cycle 1a Canadian population |
2007-09 | 20-39 | M+F | 1165 | 0.64 (0.60 - 0.67) |
1.38 (1.22 - 1.53) |
| CHMS Cycle 1a Canadian population |
2007-09 | 40-59 | M+F | 1220 | 0.64 (0.60 - 0.67) |
1.24 (1.17 - 1.30) |
| CHMS Cycle 1a Canadian population |
2007-09 | 60-79 | M+F | 1079 | 0.72 (0.70 - 0.74) |
1.64 (1.47 - 1.81) |
| CHMS Cycle 1 Pregnant womenb |
2007-09 | 18-49 | F | 29 | 0.67 (0.43-0.91) |
1.4 (1-1.9) |
| CHMS Cycle 2c Canadian population |
2009-11 | 3-79 | M+F | 6070 | 0.64 (0.62-0.66) |
1.5 (1.4-1.5) |
| CHMS Cycle 2c Canadian population |
2009-11 | 3-79 | M | 2940 | 0.63 (0.60-0.66) |
1.4 (1.3-1.5) |
| CHMS Cycle 2c Canadian population |
2009-11 | 3-79 | F | 3130 | 0.65 (0.62-0.68) |
1.5 (1.4-1.6) |
| CHMS Cycle 2c Canadian population |
2009-11 | 3-5 | M+F | 495 | 0.95 (0.91 - 0.98) |
2.7E (1.4 - 4.0) |
| CHMS Cycle 2c Canadian population |
2009-11 | 6-11 | M+F | 961 | 0.79 (0.75 - 0.84) |
1.6 (1.2 - 2.1) |
| CHMS Cycle 2c Canadian population |
2009-11 | 12-19 | M+F | 997 | 0.64 (0.62 - 0.67) |
1.2 (1.1 - 1.3) |
| CHMS Cycle 2c Canadian population |
2009-11 | 20-39 | M+F | 1313 | 0.64 (0.62 - 0.66) |
1.4 (1.2 - 1.6) |
| CHMS Cycle 2c Canadian population |
2009-11 | 40-59 | M+F | 1222 | 0.59 (0.56 - 0.61) |
1.4 (1.2 - 1.5) |
| CHMS Cycle 2c Canadian population |
2009-11 | 60-79 | M+F | 1082 | 0.67 (0.62 - 0.72) |
1.5 (1.4 - 1.7) |
| CHMS Cycle 2 Pregnant womenb |
2009-11 | 18-49 | F | 38 | 0.95E (0.35-1.5) |
1.7E (1.1-2.3) |
| MIREC-CD Plusd Children |
2013-14 | less than or equal to 3 | M+F | 214 | 1.25 | 2.78 |
| FNBIe First Nation Peoples |
2011 | 20+ | M+F | 473 | 0.64 (0.57-0.70) |
1.41E (0.84-1.98) |
n = sample size, CI = confidence interval, M = males, F = females, E = use data with caution
a Health Canada 2010
b Walker 2016
c Health Canada 2013a
d Liang 2016
e AFN 2013
| Study Population | Sampling year(s) | Age years |
Sex | n | Median (95% CI) |
95th percentile (95% CI) |
|---|---|---|---|---|---|---|
| CHMS Cycle 1a Canadian population |
2007-09 | 6-79 | M+F | 5479 | 43.20 (41.82-44.58) |
121.58 (112.49-130.67) |
| CHMS Cycle 1a Canadian population |
2007-09 | 6-79 | M | 2653 | 40.58 (38.97-42.19) |
117.53 (107.02-128.04) |
| CHMS Cycle 1a Canadian population |
2007-09 | 6-79 | F | 2826 | 45.93 (43.37-48.48) |
127.94 (115.75-140.14) |
| CHMS Cycle 1a Canadian population |
2007-09 | 6-11 | M+F | 1031 | 84.59 (79.41-89.78) |
217.99 (194.33-241.64) |
| CHMS Cycle 1a Canadian population |
2007-09 | 12-19 | M+F | 982 | 46.80 (42.66-50.94) |
114.07 (101.92-126.21) |
| CHMS Cycle 1a Canadian population |
2007-09 | 20-39 | M+F | 1165 | 42.44 (39.50-45.37) |
112.16 (94.91-129.41) |
| CHMS Cycle 1a Canadian population |
2007-09 | 40-59 | M+F | 1218 | 39.11 (36.67-41.55) |
107.33 (95.57-119.09) |
| CHMS Cycle 1a Canadian population |
2007-09 | 60-79 | M+F | 1083 | 43.04 (40.08-46.00) |
111.51 (99.70-123.32) |
| CHMS Cycle 2b Canadian population |
2009-11 | 3-79 | M+F | 6291 | 42 (40 - 45) |
140 (120 - 160) |
| CHMS Cycle 2b Canadian population |
2009-11 | 3-79 | M | 3028 | 39 (36 - 43) |
140 (120 - 150) |
| CHMS Cycle 2b Canadian population |
2009-11 | 3-79 | F | 3263 | 44 (41 - 46) |
150 (120 - 170) |
| CHMS Cycle 2b Canadian population |
2009-11 | 3-5 | M+F | 572 | 140 (130 - 150) |
490E (310 - 680) |
| CHMS Cycle 2b Canadian population |
2009-11 | 6-11 | M+F | 1058 | 85 (77 - 93) |
260 (190 - 330) |
| CHMS Cycle 2b Canadian population |
2009-11 | 12-19 | M+F | 1039 | 46 (41 - 50) |
110 (85 - 140) |
| CHMS Cycle 2b Canadian population |
2009-11 | 20-39 | M+F | 1319 | 42 (39 - 45) |
120 (85 - 160) |
| CHMS Cycle 2b Canadian population |
2009-11 | 40-59 | M+F | 1223 | 36 (33 - 38) |
94 (78 - 110) |
| CHMS Cycle 2b Canadian population |
2009-11 | 60-79 | M+F | 1080 | 37 (33 - 41) |
99 (90 - 110) |
| FNBIc First Nation Peoples |
2011 | 20+ | M+F | 493 | 41.10 (38.78-43.42) |
125.13 (100.42-149.84) |
| NHANESd U.S. population |
1999-2000 | 6 and older | M+F | 2257 | 41.6 (38.5-45.2) |
144 (125-171) |
| NHANESd U.S. population |
2001-02 | 6 and older | M+F | 2689 | 42.2 (40.1-45.2) |
130 (120-149) |
| NHANESd U.S. population |
2003-04 | 6 and older | M+F | 2558 | 39.2 (37.9-40.7) |
120 (107-135) |
| NHANESd U.S. population |
2005-06 | 6 and older | M+F | 2576 | 43.9 (41.8-45.7) |
132 (125-143) |
| NHANESd U.S. population |
2007-08 | 6 and older | M+F | 2627 | 46.3 (44.8-47.7) |
137 (127-152) |
| NHANESd U.S. population |
2009-10 | 6 and older | M+F | 2848 | 44.4 (41.9-47.4) |
143 (129-157) |
| NHANESd U.S. population |
2011-12 | 6 and older | M+F | 2502 | 41.0 (38.6-44.4) |
130 (118-148) |
| NHANESd U.S. population |
2011-12 | 6 and older | M | 1261 | 40.0 (36.0-43.6) |
118 (108-131) |
| NHANESd U.S. population |
2011-12 | 6 and older | F | 1241 | 43.3 (39.2-45.5) |
149 (118-157) |
| NHANESd U.S. population |
2011-12 | 6-11 | M+F | 398 | 81.7 (74.3-91.2) |
259 (185-300) |
| NHANESd U.S. population |
2011-12 | 12-19 | M+F | 390 | 43.7 (39.1-48.0) |
109 (92.4-131) |
| NHANESd U.S. population |
2011-12 | 20+ | M+F | 1714 | 38.5 (36.1-41.0) |
112 (102-123) |
n = sample size, CI = confidence interval, M = males, F = females, E = use data with caution
a Health Canada 2010
b Health Canada 2013a
c AFN 2013
d CDC 2015
A-3 Silver
| Study Population | Sampling year(s) | Age years |
Sex | n | Median (95% CI) |
95th percentile (95% CI) |
|---|---|---|---|---|---|---|
| CHMS cycle 2a Canadian population |
2009-11 | 3 -79 | M+F | 6070 | 0.066 (less than LOD-0.088) |
0.27 (0.22-0.31) |
| CHMS cycle 2a Canadian population |
2009-11 | 3 -5 | M+F | 495 | less than LOD | 0.19E (0.095-0.28) |
| CHMS cycle 2a Canadian population |
2009-11 | 6-11 | M+F | 961 | less than LOD | NP |
| CHMS cycle 2a Canadian population |
2009-11 | 12-19 | M+F | 997 | less than LOD | 0.16E (0.070-0.24) |
| CHMS cycle 2a Canadian Population |
2009-11 | 20-39 | M+F | 1313 | 0.068 (less than LOD-0.090) |
0.26 (0.21-0.30) |
| CHMS cycle 2a Canadian population |
2009-11 | 40-59 | M+F | 1222 | 0.083 (0.058-0.11) |
0.32 (0.22-0.42) |
| CHMS cycle 2a Canadian population |
2009-11 | 60-79 | M+F | 1082 | 0.080 (0.054-0.11) |
0.32 (0.28-0.36) |
| CHMS cycle 2a Canadian population |
2009-11 | 3-79 | M | 2940 | 0.060E (less than LOD-0.086) |
0.22 (0.19 - 0.24) |
| CHMS cycle 2a Canadian population |
2009-11 | 3-79 | F | 3130 | 0.074 (0.052-0.096) |
0.32 (0.27 - 0.37) |
| CHMS Cycle 2b Pregnant women |
2009-11 | 18-49 | F | 38 | 0.061E (less than LOD-0.093) |
0.29E (0.1 – 0.48) |
| MIREC-CD Plusc children |
2013-14 | less than or equal to 3 | M+F | 214 | 0.205 | 0.259 |
n = sample size, CI = confidence interval, M = males, F = females, E = use data with caution, NP = Data is too unreliable to be published, less than LOD = less than the limit of detection (LOD = 0.05 µg/L)
a Health Canada 2013a
b Walker et al. 2016
c Liang 2016
A-4 Thallium
| Study Population | Sampling year(s) | Age years |
sex | n | Median (95% CI) |
95th percentile (95% CI) |
|---|---|---|---|---|---|---|
| CHMS Cycle 2a Canadian population |
2009-11 | 3-79 | M+F | 6291 | 0.21 (0.20-0.23) |
0.55 (0.49-0.61) |
| CHMS Cycle 2a Canadian population |
2009-11 | 3-79 | M | 3028 | 0.20 (0.18-0.21) |
0.49 (0.42 - 0.57) |
| CHMS Cycle 2a Canadian population |
2009-11 | 3-79 | F | 3263 | 0.23 (0.21 - 0.24) |
0.59 (0.52 - 0.66) |
| CHMS Cycle 2a Canadian population |
2009-11 | 3-5 | M+F | 572 | 0.47 (0.41 - 0.53) |
1.0 (0.80-1.3) |
| CHMS Cycle 2a Canadian population |
2009-11 | 6-11 | M+F | 1058 | 0.29 (0.27 - 0.31) |
0.64 (0.59 - 0.70) |
| CHMS Cycle 2a Canadian population |
2009-11 | 12-19 | M+F | 1039 | 0.19 (0.18 - 0.20) |
0.40 (0.36 - 0.44) |
| CHMS Cycle 2a Canadian population |
2009-11 | 20-39 | M+F | 1319 | 0.20 (0.17 - 0.22) |
0.48 (0.39 - 0.56) |
| CHMS Cycle 2a Canadian population |
2009-11 | 40-59 | M+F | 1223 | 0.22 (0.21 - 0.23) |
0.52 (0.39 - 0.65) |
| CHMS Cycle 2a Canadian population |
2009-11 | 60-79 | M+F | 1080 | 0.20 (0.18 - 0.21) |
0.47 (0.42 - 0.52) |
| NHANES IIIb U.S. population |
1988-94 | 6-88 | M+F | 496 | 0.27 | 0.76 |
| NHANESc U.S. population |
1999-2000 | 6 and older | M+F | 2413 | 0.168 (0.162-0.176) |
0.366 (0.338-0.387) |
| NHANESc U.S. population |
2001-02 | 6 and older | M+F | 2652 | 0.156 (0.148-0.164) |
0.349 (0.337-0.365) |
| NHANESc U.S. population |
2003-04 | 6 and older | M+F | 2558 | 0.153 (0.146-0.160) |
0.350 (0.328-0.369) |
| NHANESc U.S. population |
2005-06 | 6 and older | M+F | 2576 | 0.150 (0.140-0.160) |
0.370 (0.350-0.390) |
| NHANESc U.S. population |
2007-08 | 6 and older | M+F | 2627 | 0.150 (0.140-0.160) |
0.370 (0.350-0.380) |
| NHANESc U.S. population |
2009-10 | 6 and older | M+F | 2848 | 0.150 (0.140-0.160) |
0.370 (0.350-0.400) |
| NHANESc U.S. population |
2011-12 | 6 and older | M+F | 2502 | 0.167 (0.156-0.178) |
0.425 (0.369-0.497) |
| NHANESc U.S. population |
2011-12 | 6 and older | M | 1261 | 0.155 (0.144-0.165) |
0.353 (0.312-0.385) |
| NHANESc U.S. population |
2011-12 | 6 and older | F | 1241 | 0.180 (0.168-0.193) |
0.480 (0.422-0.510) |
| NHANESc U.S. population |
2011-12 | 6-11 | M+F | 398 | 0.216 (0.200-0.241) |
0.486 (0.422-0.568) |
| NHANESc U.S. population |
2011-12 | 12-19 | M+F | 390 | 0.147 (0.130-0.169) |
0.339 (0.268-0.500) |
| NHANESc U.S. population |
2011-12 | 20+ | M+F | 1714 | 0.164 (0.154-0.175) |
0.436 (0.365-0.510) |
n = sample size, CI = confidence interval, M = males, F = females
a Health Canada 2013a
b NHANES III Paschal et al. 1998
c CDC 2015
A-5 Tin
| Study Population | Sampling year(s) | Age years |
sex | n | Median (95% CI) |
95th percentile (95% CI) |
|---|---|---|---|---|---|---|
| NHANES IIIa U.S. population |
1988-94 | 6-88 | M+F | 496 | 2.59 | 16.1 |
| NHANESb U.S. population |
2011-12 | 6 and older | M+F | 2502 | 0.624 (0.567-0.678) |
4.50 (3.76-5.33) |
| NHANESb U.S. population |
2011-12 | 6 and older | M | 1261 | 0.515 (0.436-0.576) |
4.17 (2.80-5.55) |
| NHANESb U.S. population |
2011-12 | 6 and older | F | 1241 | 0.764 (0.676-0.857) |
4.81 (3.50-5.59) |
| NHANESb U.S. population |
2011-12 | 6-11 | M+F | 398 | 1.60 (1.44-1.73) |
7.91 (6.11-12.1) |
| NHANESb U.S. population |
2011-12 | 12-19 | M+F | 390 | 0.484 (0.411-0.643) |
2.80 (2.00-3.74) |
| NHANESb U.S. population |
2011-12 | 20+ | M+F | 1714 | 0.593 (0.537-0.663) |
4.04 (3.00-5.09) |
| Quebec Region Study/Canadac |
2001 | 18- 65 | M+F | 363 | 0.76 | 5.89d |
| Quebec Region Study/Canadac |
2001 | 18- 65 | M | 111 | 0.45 | 6.52d |
| Quebec Region Study/Canadac |
2001 | 18-65 | F | 252 | 0.89 | 5.89d |
| Quebec Region Study/Canadac |
2001 | 18-39 | M+F | 104 | 0.63 | 5.89d |
| Quebec Region Study/Canadac |
2001 | 40-59 | M+F | 243 | 0.81 | 6.07d |
| Quebec Region Study/Canadac |
2001 | less than or equal to 60 | M+F | 16 | 0.75 | 3.65d |
n = sample size, CI = confidence interval, M = males, F = females
a NHANES III Paschal et al. 1998
b CDC 2015
c INSPQ 2004b (Data were converted to µg/g creatinine using the molecular weights of tin (118.7 g/mol) and creatinine (113 g/mol)
d 97.5th percentile (95th percentile not available)
Appendix B: Derivation of the Biomonitoring Equivalent
B-1. Barium
Summary
Creatinine-adjusted urinary barium concentrations for the BE and BEPOD were calculated using a mass balance approach with the following equation (as per Hays et al. 2010):
Cc = D x BW x FUE,mass/CE
Cc = creatinine-adjusted 24-hour urinary barium concentration; D = unit dose of barium;
BW = body weight for group; FUE = the mass-based urinary barium excretion fraction;
CE = 24-hour creatinine excretion rate
The BE was derived for the US Environmental Protection Agency reference dose (RfD) (US EPA, 2005). A chronic reference dose for non-carcinogenic effects (RfD) of 0.2 mg/kg bw per day was derived based on a lower confidence limit on the benchmark dose (BMDL05) of 63 mg/kg bw per day derived from a 2-year drinking water bioassay in mice where increased incidence of chemical-related nephropathy was observed in male mice (NTP 1994). An uncertainty factor of 300 was applied to account for inter-species variation (10x), intra-species variation (10x) and database limitations (3x). The urinary BE for barium based on the US EPA chronic RfD is 246 µg/g creatinine, details are outlined in table B-1-1 and B-1-2 below.
| Age Groupa | Body weight (kg)a | 24 hr creatinine excretion, g/da | Unit dose urinary barium concentration (mg/g creatinine per 1 mg/kg oral dose)a |
|---|---|---|---|
| Children, 5-11 | 32 | 0.5 | 1.472 |
| Adolescents, 11-16 | 57 | 1.2 | 1.093 |
| Men, greater than 16 | 70 | 1.5 | 1.073 |
| Women, greater than 16 | 55 | 1.2 | 1.054 |
| Average unit barium concentration (CC), all ages | - | - | 1.173 |
afrom Hays et al. 2010
| POD (BMDL05), mg/kg-d: | 63 |
|---|---|
| Interspecies UF: | 10 |
| Human Equivalent POD, mg/kg bw/day: | 6.3 |
| Human equivalent BEPOD, mg/g creatinine: (human equivalent POD x CC from Table C-1-1) | 7.39 [=6.3 * 1.173] |
| Intraspecies UF-TK: | 3.162 |
| Intraspecies UF-TD: | 3.162 |
| Additional UF: | 3 |
| urinary BE (mg/g creatinine) | 0.246 [= (7.39)/ (3.162 * 3.162 * 3)] |