Science Approach Document
Biomonitoring-based Approach 1 for Beryllium Vanadium, trichlorooxo Vanadium oxide
Chemical Abstracts Service Registry Numbers
7440-41-7
7727-18-6
11099-11-9
Health Canada
September 2016
Table of Contents
List of Tables and Figures
- Table 3-1. Canadian and U.S. biomonitoring data for beryllium: whole blood and serum data are presented in µg/L and urinary data in µg/g creatinine
- Table 3-2. Summary of Canadian manufacturing and imports of beryllium
- Table 3-3. Canadian biomonitoring data for vanadium: urinary data are presented in µg/g creatinine
- Table 3-4. Summary of Canadian manufacturing and imports of vanadium-containing substances
- Table 6-1. Hazard classifications by other international agencies for beryllium and vanadium
Synopsis
This Science Approach Document (SciAD) presents a qualitative biomonitoring-based approach to identify substances of low concern for human health which were identified as priorities for assessment as they met categorization criteria under subsection 73(1) of CEPA.
This biomonitoring-based approach considers available Canadian and U.S. biomonitoring data based on the analysis of the substance or moiety in whole blood, serum, and/or urine. When biomonitoring data indicate that general population exposure is limited or unlikely, substances or moieties are considered to be of low concern with respect to human health. To determine if exposure is limited or unlikely, a number of metrics are taken into consideration, including the prevalence of exposure across the population, the magnitude of the biomarker concentration (if detected at the upper tails of the exposure distribution), the toxicokinetic properties of the substance or moiety, and the use pattern of the substance.
The application of this biomonitoring-based approach indicates that beryllium (CAS RN 7440-41-7) and two vanadium-containing substances, vanadium, trichlorooxo- (hereinafter referred to as trichlorooxo vanadium) (CAS RN 7727-18-6) and vanadium oxide (CAS RN 11099-11-9), would be of low concern with respect to human health at current levels of exposure.
An assessment of these substances conducted under section 74 of CEPA will be published at a later date.
A consultation period on this SciAD is being provided to the public who will have an opportunity to provide comments and additional information in advance of this approach being applied in Screening Assessment Reports. The publication of this scientific approach will assist the government in addressing substances that are likely of low concern in an efficient and effective manner.
1. Introduction
Following the categorization of substances on the Domestic Substances List (DSL), which was completed in 2006, approximately 4300 of the 23 000 substances on the DSL were identified for assessment. Among these substances, beryllium (CAS RNFootnote1 7440-41-7) and two vanadium substances (trichlorooxo vanadium, CAS RN 7727-18-6 and vanadium oxide, CAS RN 11099-11-9) remain to be addressed under the Chemicals Management Program (CMP). These substances were identified as priorities for assessment as they met categorization criteria under subsection 73(1) of CEPA (Canada 1999, ECCC, HC [modified 2007]). These substances are being evaluated through a biomonitoring-based approach, as detailed in this science approach document (SciAD).
The purpose of this document is to provide stakeholders and the public with the opportunity to review and comment on the current approach (Biomonitoring-based approach 1) and the results of its application to beryllium, trichlorooxo vanadium, and vanadium oxide prior to publication of screening assessments under section 68 or 74 of CEPA. The publication of the scientific approach and results in the SciAD will assist the government in addressing substances that may be of low concern to either human health or the environment in an efficient and effective manner.
Vanadium pentoxide (CAS RN 1314-62-1) has been previously assessed under the CMP (ECHC, 2010) and is therefore considered to be outside the scope of this science approach. It was concluded that vanadium pentoxide is a substance that may be entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health on the basis of the carcinogenic potential of vanadium pentoxide, for which there may be a probability of harm at any level of exposure, together with potential for general population exposure.The International Agency for Research on Cancer (IARC) has classified vanadium pentoxide as a Group 2B carcinogen (i.e., possibly carcinogenic to humans) based on sufficient evidence of lung cancer in mice following inhalation exposure. The two vanadium-containing substances assessed in this SciAD, trichlorooxo vanadium (CAS RN 7727-18-6) and vanadium oxide (CAS RN 11099-11-9), are not chemically similar to vanadium pentoxide (CAS RN 1314-62-1) and are not likely to transform into vanadium pentoxide under physiological conditions. Thus, the conclusion for vanadium pentoxide is not relevant to the two vanadium containing substances in this SciAD.
This SciAD does not represent an exhaustive or critical review of all available data; rather, it presents the studies deemed most critical and the lines of evidence pertinent to this evaluation. Relevant data up to February 2016 are incorporated into this evaluation.
This SciAD was prepared by staff in the Existing Substances Program at Health Canada.
The critical information and considerations upon which the SciAD are based are given below.
2. Basis of Biomonitoring-based Approach 1
Large population health surveys such as the Canadian Health Measures Survey (CHMS) and the National Health and Nutrition Examination Survey (NHANES) in the United States (U.S.) provide valuable data on the prevalence and concentrations of chemicals in the general population. Total concentrations of a substance in blood or urine provide a biologically relevant, integrated measure of exposures that may occur across multiple routes (e.g., oral, dermal and inhalation) and pathways (including environmental media, diet, and frequent or daily use products to which they were exposed). Biomonitoring data are increasingly being recognized as a state-of-the-art tool for characterization of exposure to environmental chemicals (Choi et al. 2015). These biomonitoring surveys or studies utilize state of the art instrumentation for analysis, including inductively coupled plasma mass spectrometer (ICP-MS). ICP-MS is specific and sensitive, resulting in low limits of detection.
This biomonitoring approach utilizes biomonitoring data from several large scale population-level biomonitoring programs and studies to determine the prevalence of exposure within the general population of Canada. In instances where the proportion of biomarker detection (i.e., the metal moiety in blood or urine) is limited across the population and the limit of detection is sufficiently low, the substance is considered for this approach. If there are detections of the biomarker at the upper tails of the exposure distribution, the magnitude of the biomarker concentration is considered as well (determination made on a substance by substance basis).
In the next step of this approach, relevant toxicokinetic data (i.e., absorption, distribution, half-life, metabolism, and excretion data by relevant routes of exposure for the general public and in biological matrices for which there is biomarker data) are evaluated to ensure that the available biomonitoring data are adequate biomarkers of exposure. The route of exposure associated with harmful effects of the chemical, indicated by hazard classifications, is also taken into account when evaluating the adequacy of the biomarker (e.g., inhalation route and carcinogenicity classifications).
Subsequently, information on CAS RN-specific major uses, quantities of manufacture, and imports reported pursuant to a section 71 survey (Canada 2009) are used to confirm either limited or no potential for exposure to the Canadian general population. An emphasis is placed on determining if the substance is likely to be present in products used by consumers on an infrequent basis, as these exposures are not likely to be captured in biomonitoring studies, and identifying potential routes of consumer exposure (e.g., inhalation, dermal). If the uses indicate a low likelihood of exposure to the general population based on uses in products, then the substance is determined to be a good candidate for this approach.
In this approach biomonitoring data are used as surrogate exposure data for a specific CAS RNs. The use of biomonitoring data on the total metal moiety is considered to be an acceptable, although protective, surrogate as total metal moiety biomonitoring data include exposures from all bioavailable forms of the moiety, from all sources of exposure.
If the results of biomonitoring surveys indicate the presence of measurable quantities of the moiety beyond the upper tails of the exposure distribution, if the biomarker is not considered to be adequate to assess exposure, or if there are significant uses in products that would not be captured by the biomonitoring data (e.g., due to infrequent or specialized use), then the substance is not a good candidate for this approach. Rather, the substance would be evaluated using a different approach which could include a quantitative biomonitoring-based approach such as comparison of biomonitoring data with human biomonitoring guidance values (Angerer et al. 2011).
The key steps for BA1 are outlined in Figure 1 below:
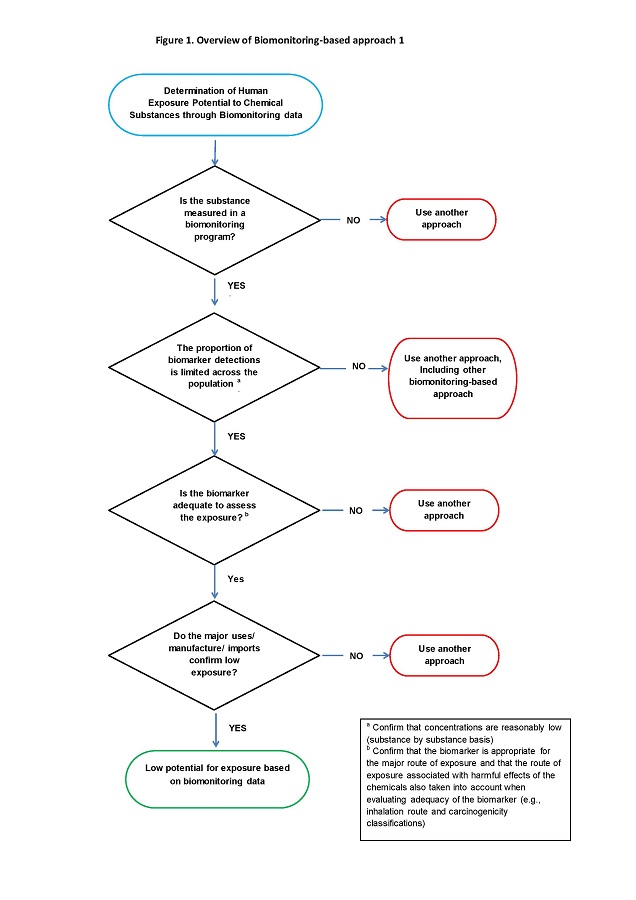
Long description for figure 1
Figure 1 is a flow diagram of the various steps taken to determine if a substance is suitable for the Biomonitoring-based approach 1. In the determination of human exposure potential to chemical substances through biomonitoring data, the first question is "Is the substance measured in a biomonitoring program?” If the answer is "No", then another approach is used. However, if the answer is "Yes", then the next question is “Is the proportion of biomarker detections limited across the population? Confirm that concentrations are reasonably low on a substance by substance basis”. If the answer is "No", then another approach is used, including another biomonitoring-based approach.If the answer is "Yes" then the next question is “Is the biomarker adequate to assess the exposure; confirm that the biomarker is appropriate for the major route of exposure and that the route of exposure associated with harmful effects of the chemicals is also taken into account when evaluating adequacy of the biomarker (e.g., inhalation route and carcinogenicity classifications)”. If the answer is "No", then another approach is used. If the answer is "Yes", then the next question is "Do the major uses/manufacture/imports confirm low exposure?” If the answer is "No", then another approach is used. If the answer is "Yes" the substance is considered to have “low potential for exposure based on biomonitoring data”.
The biomonitoring data presented in this document were used as surrogate exposure data for the specific CAS RNs which are being evaluated. There is very limited availability of CAS RN-specific exposure data, thus data on the total metal moiety were considered to be an acceptable, although conservative surrogate as total metal moiety biomonitoring data include exposures from all bioavailable forms of the moiety.
The biomonitoring studies considered in this approach are described below:
The Canadian Health Measures Survey (CHMS) is a national survey carried out by Statistics Canada, which collects information from Canadians about their general health (Health Canada 2010; 2013). This survey was designed to be nationally representative and includes a biomonitoring component; metals were measured in whole blood and urine of approximately 5,500 to 7,000 Canadians per survey cycle. As this survey was designed to be nationally representative, the population-weighted data represent 96.3% of the Canadian population. The CHMS is not a targeted survey, and thus does not target individuals with high metal exposure or who live near point sources of exposure. A broad range of metals were measured in CHMS cycle 1 (2007 to 2009, ages 6 to 79 years) and cycle 2 (2009 to 2011, ages 3 to 79 years); pregnant women were included in this study. This dataset included both fasting and non-fasting individuals.
The First Nations Biomonitoring Initiative (FNBI), conducted in 2011, is a cross-sectional study that measured metals in adults from 15 rural or isolated First Nations communities south of the 60th parallel (AFN 2013). The study had 503 adult participants from 20 to 99 years of age; pregnant women and individuals undergoing chemotherapy were excluded from this study. The results of the FNBI are presented at a national aggregate level.
The national pregnancy cohort study Maternal-Infant Research on Environmental Chemicals (MIREC) recruited 2000 pregnant women from Vancouver, Toronto, Hamilton, Kingston, Montreal and Halifax (Arbuckle et al. 2013). MIREC-child development (CD) Plus study builds on MIREC, whereby a subset of children from the mothers participating in MIREC were invited to participate in a follow-up study. Data collection for the study was completed in March 2015; blood from approximately 500 children of less than 5 years of age was analysed for metals.
The National Health and Nutrition Examination Survey (NHANES) is a series of surveys designed to collect data related to the health and nutritional status of the U.S. population (CDC 2015). The surveys are conducted by the U.S. Center for Disease Control’s National Center for Health Statistics. In 1999, NHANES became a continuous survey, collecting data in 2-year cycles. The sampling plan follows a complex, stratified, multistage, probability-cluster design to select a representative sample of the civilian, non-institutionalized population in the U.S. based on age, gender and race/ethnicity (CDC 2009). NHANES measures metals in blood and urine from approximately 2,500 people per cycle, aged 6 years and up; pregnant women are included in the survey. This dataset included both fasting and non-fasting individuals (CDC 2013). In the absence of CHMS data, NHANES is considered to be an acceptable surrogate to estimate exposure levels in Canadians as it offers the most comprehensive data in North America.
The Quebec Region study was conducted by the Institut national de santé publique du Québec (INSPQ) in 2004 and measured metals in blood, serum and urine of adults aged 18 to 65 years (INSPQ 2004). Individuals were selected across urban, semi-urban and rural areas in and around Québec City, QC. Approximately 500 people participated in the study; pregnant women and people with severe chronic diseases were excluded. Data from this study was incorporated into this approach (when available) when there was an absence of CHMS (nationally representative) data.
3. Application of Biomonitoring-based Approach 1
Beryllium
Total beryllium concentrations in urine were measured as part of NHANES III (conducted from 1988 to 1994, Paschal et al. 1998) and six consecutive cycles of NHANES (1999 onward) (CDC 2015). The Quebec Region study (INSPQ 2004) measured total beryllium in whole blood, serum and urine of study participants. Beryllium was not included for analysis in the CHMS, FNBI or MIREC CD-Plus; therefore, in the absence of Canadian nationally representative biomonitoring data, beryllium concentrations from NHANES and the Quebec Region study were analyzed.
A summary of the biomonitoring data for total beryllium is provided in Table 3-1. Total beryllium was detected in the U.S. population from 1988 to 1994; however, from 1999 to 2010, approximately 16,000 individual samples were analysed and there were no detections of total beryllium. As a result of this low detection rate, the CDC discontinued measuring beryllium in NHANES in 2010 (CDC 2015). Furthermore, beryllium was not detected in any matrix (whole blood, serum, urine) in the Quebec Region study at the median or at the 97.5th percentile.
| Study or Survey / Population (Sampling Years) |
Matrix | Sample size (age years) |
LOD (µg/L) |
Median | 95th percentile | % Detected |
|---|---|---|---|---|---|---|
| Quebec Region studya/Canadian population (2001) | Whole Blood | 472 (18 to 65) |
0.45 | ND | ND* | 0 |
| Quebec Region studya /Canadian population (2001) | Serum | 471 (18 to 65) |
0.45 | ND | ND* | 0 |
| Quebec Region studya/Canadian population (2001) | Urine | 363 (18 to 65) |
0.45 | ND | ND* | 0.3 |
| NHANES-IIIb/U.S. population (1988-1994) |
Urine | 496 (6 to 88) |
0.1 | 0.13 | 0.62 | 67 |
| NHANESc/U.S. population (1999-2000) |
Urine | 2,465 (6+) |
0.004 | ND | ND | less than or equal to 5 |
| NHANESc/U.S. population (2001-2002) |
Urine | 2,689 (6+) |
0.004 | ND | ND | less than or equal to 5 |
| NHANESc/U.S. population (2003-2004) |
Urine | 2,558 (6+) |
0.005 | ND | ND | less than or equal to 5 |
| NHANESc/U.S. population (2005-2006) |
Urine | 2,576 (6+) |
0.002 | ND | ND | less than or equal to 5 |
| NHANESc/U.S. population (2007-2008) |
Urine | 2,627 (6+) |
0.002 | ND | ND | less than or equal to 5 |
| NHANESc/U.S. population (2009-2010) |
Urine | 2,848 (6+) |
0.0017 | ND | ND | less than or equal to 5 |
Abbreviations: ND, Not Detected
*97.5th percentile
a INSPQ 2004
b NHANES-III/U.S. (Paschal et al. 1998)
c CDC 2015
Toxicokinetic data were reviewed to ensure that the biomarkers measured in the biomonitoring studies were adequate, taking into consideration relevant routes of exposure for the general public, the biological matrices for which there is biomarker data and potential routes of exposure associated with hazard classifications, as appropriate (see Appendix A for a summary of hazard classifications). The focus of the toxicokinetic data review is on the oral route of exposure as this would be the predominant route of potential intake for the general population. The hazard classifications for beryllium are based on the inhalation route in an occupational setting; however, the inhalation route, and hence these classifications, are not considered to be relevant for the general population of Canada.
There is insufficient pharmacokinetic data regarding oral exposure to beryllium in humans. However, based on animal studies, oral administration results in less than 1% of absorption and storage in the body (IPCS 2001, U.S. EPA 1991). Poor absorption is thought to result from the precipitation of soluble beryllium sulfate as an insoluble phosphate due to the high pH in the intestine (IPCS 2001). Once absorbed, beryllium is distributed throughout the body. Based on the serum levels of beryllium in accidentally exposed workers, the biological half-life in serum was estimated to be 2 to 8 weeks (ATSDR 2002). Thus, total beryllium in blood serves as an acceptable biomarker to assess beryllium exposure as it represents both recent and past exposures.
Beryllium is primarily retained in skeletal tissues, liver, and spleen, although very little of an ingested dose (0.009-0.3%) is retained in tissues (Reeves 1965, IRIS 1998). With regards to excretion, no human data after oral exposure was identified (ATSDR 2002). Based on animal studies, greater than 90% of ingested beryllium is excreted via feces (some studies with radioactive beryllium chloride indicated 98% of the administered dose is excreted via feces). While urine is the predominant excretion route for absorbed beryllium, urinary excretion remained less than 1% despite the amount ingested (Reeves 1965, ATSDR 2002). Observations from occupational exposure studies suggest that beryllium in urine may be a reflection of current exposure although it has been reported that the urinary excretion of beryllium may remain elevated several years after exposure (Leonard and Bernard 1993). While urine is also a potential biomarker of exposure, blood serves as a more sensitive biomarker as it more accurately reflects the bioavailable dose.
Use data for Beryllium (CAS RN 7440-41-7) was obtained from surveys issued pursuant to section 71 of CEPA (Canada 2009) and Table 3-2 presents a summary of the total manufacture and import quantities. The major uses according to a summary of the information reported pursuant to the section 71 survey were electrical, electronics, in connectors, and as a plating and surface treating agent. In addition, it is used in the automobile industry (CCME 2015). No products containing these substances are expected to be used by consumers, either frequently or occasionally.
| DSL name | Total Manufacture (kg) | Total Imports (kg) | Reporting Year | Survey Reference |
|---|---|---|---|---|
| Beryllium | 100 – 1,000 | 100,000-1,000,000 | 2008 | Canada 2009 |
The use data noted above support the current approach for assessing beryllium, as there are no identified consumer product uses for this substance.
Vanadium
The prevalence of total vanadium detections in the Canadian population based on the CHMS data was less than 10% (Health Canada 2010; 2013). Total vanadium was not detected in urine at median concentrations in the CHMS and FNBI; 95th percentile concentrations were above the limit of detection (LOD = 0.1 µg/L), however, these values are likely to be below the limit of quantification (LOQ). The LOQ represents the concentration at which quantitative results can be reported with a high degree of confidence; LOQ values were not reported by the studies.
| Study or Survey / Population (Sampling Year/s) |
Sample size (age years) |
LOD (µg/L) |
Median | 95th percentile | Detected (%) |
|---|---|---|---|---|---|
| CHMS-1a/Canadian population (2007-2009) |
5479 (6 to 79) |
0.1 | ND | 0.30 (0.26-0.33) |
9.4 |
| CHMS-2b/Canadian population (2009-2011) |
6291 (3 to 79) |
0.1 | ND | 0.24 (ND-0.26) |
7.8 |
| FNBIc/Canada (2011) | 494 (20 to 99) |
0.1 | ND | 0.35 (0.25 – 0.45) |
10.9 |
Abbreviations: ND, Not Detected
a Health Canada 2010
b Health Canada 2013
c AFN 2013
Vanadium has been measured in serum, urine, fingernails and in hair depending on the purpose of investigation, however, only urinary vanadium concentrations are available for the Canadian population. There are no toxicokinetic data available for vanadium in humans when exposed through the oral route. Based on animal data, gastrointestinal (GI) absorption is rapid and can vary from less than 0.1% (within 15 min) to 16.5% (within 7 days) (ATSDR 2012). Once absorbed, vanadium is distributed in the body and can be accumulated mainly in skeletal tissues followed by the spleen and kidney. In an animal study, Edel and Sabbioni (1988) demonstrated that 0.05%, 0.01% and less than 0.01% of administered radiolabelled vanadium was found in bones, liver and other tissues, respectively, after 24 hrs which reflects very little retention in the body. More than 80% of ingested vanadium is excreted through fecal system, however, the principle route of excretion of absorbed vanadium is urine, as greater than 90% of the absorbed vanadium is eliminated via this route. Approximately 0.9% of ingested vanadium was excreted in urine, which corresponds to the low GI absorption (ATSDR 2012). It is possible that some of the absorbed vanadium is present in blood, retained in soft tissues and bones, and/or eliminated through other routes (biliary excretion, hair and nails), as these fates are not captured in urinary elimination. An elimination half-life of 11.7 days was reported for rats when exposed to vanadyl sulfate in drinking water for 3 weeks (ATSDR 2012). Since absorbed vanadium is predominantly excreted in urine with a moderately long half-life and the body retention is relatively low, urinary vanadium levels serve as a suitable biomarker of exposure to vanadium.
Use data for trichlorooxo vanadium (CAS RN 7727-18-6) and vanadium oxide (CAS RN 11099-11-9) was obtained from surveys issued pursuant to section 71 of CEPA (Canada 2009). Vanadium oxide is a by-product in processing fuel oil, combustion of fossil fuels in replacement of cement and in non-ferrous smelting. No uses were reported. Uses for trichlorooxo vanadium cannot be publically disclosed as this is considered to be confidential business information. No direct exposure uses (e.g., products available to consumers) were identified for these CAS RNs.
| DSL name | Total Manufacture (kg) | Total Imports (kg) | Reporting Year | Survey Reference |
|---|---|---|---|---|
| Trichlorooxo vanadium | 100 – 1,000 | 10,000–100,000 | 2008 | Canada 2009 |
| Vanadium oxide | 1,000,000–10,000,000 | 10,000–100,000 | 2008 | Canada 2009 |
The use data noted above, support the current biomonitoring-based approach for trichlorooxo vanadium (CAS RN 7727-18-6) and vanadium oxide (CAS RN 11099-11-9), since there are no identified consumer product uses for these substances.
4. Results of Biomonitoring-based Approach 1
Applying the current approach and based upon the available human biomonitoring data, toxicokinetic data and CAS RN specific use data, beryllium (CAS RN 7440-41-7) and the two vanadium-containing, substances (trichlorooxo vanadium (CAS RN 7727-18-6) and vanadium oxide (CAS RN 11099-11-9), would be of low concern with respect to human health at current levels of exposure.
An assessment of these compounds conducted under section 74 of CEPA will be published at a later date.
4.1 Uncertainties in the Approach
There are uncertainties associated with the use of biomonitoring data in risk assessment, including its use in the biomonitoring-based approach outlined in this document. Although the CHMS and NHANES biomonitoring data are representative of the general population, they are not targeted and therefore do not necessarily capture subpopulations which may have different exposures such as those associated with living in the vicinity of industrial facilities or children under 3 or 6 years of age.
With the specific approach outlined in this SciAD, risk is not quantified and it is possible that even limited exposures to some high hazard substances may remain of concern. This uncertainty is similar to that associated with more traditional risk characterizations where there is not a concern for human health at current levels of exposure, but there would potentially be concerns if exposures were to increase or if the nature of the exposures were to vary. In such instances, the government has mechanisms to monitor changes in the magnitude and/or nature of uses of substances of potential concern.
The urine samples tested in all biomonitoring surveys or studies considered in this science approach were collected as spot urine samples. It is assumed that spot urine samples are representative of steady-state exposure concentrations. An uncertainty related to using spot urine samples (versus 24 hour urine collection) is the variation of concentration of the biomarker of interest that could result due to variability of the hydration status or the kidney function of the individual. This variability is overcome by normalizing the result with the creatinine concentration of the individual spot urine sample; creatinine normalized urine concentration data were evaluated in this science approach.
For beryllium, Canadian nationally representative biomonitoring data are not available, and NHANES is considered to be an acceptable surrogate. Biomonitoring data for children under 6 were not available although children are not anticipated to be exposed to the substances outlined in this approach based on the sources and uses reported (e.g., in non-ferrous smelting, electronics, or as a by-product in processing of fuel oil). For vanadium, measurements in whole blood and/or serum would increase the confidence in the vanadium results. In addition, presentation of vanadium data based on the LOQ rather than the LOD would also increase confidence in this science approach.
Despite the above-noted uncertainties, the confidence in the results of this approach for beryllium and two vanadium-containing substances is considered to be high.
5. References
[AFN] Assembly of First Nations. 2013. First Nations Biomonitoring Initiative National Results (2011). June 2013 [Internet]. Ottawa(ON): Assembly of First Nations. [assessed 2016 Jan 19].
Angerer J, Aylward L, Hays S, Heinzow B, Wilhem M. 2011. Human biomonitoring assessment values: Approaches and data requirements. Int J Hyg Environ Health. 214(5):348-60.
Arbuckle TE, Fraser WD, Fisher M, Davis K, Liang CL, Lupien N, Bastien S, Velez MP, von Dadelszen P, Hemmings DG, Wang J, Helewa M, Taback S, Sermer M, Foster W, Ross G, Fredette P, Smith G, Walker M, Shear R, Dodds L, Ettinger AS, Weber JP, D'Amour M, Legrand M, Kumarathasan P, Vincent R, Luo ZC, Platt RW, Mitchell G, Hidiroglou N, Cockell K, Villeneuve M, Rawn DF, Dabeka R, Cao XL, Becalski A, Ratnayake N, Bondy G, Jin X, Wang Z, Tittlemier S, Julien P, Avard D, Weiler H, Leblanc A, Muckle G, Boivin M, Dionne G, Ayotte P, Lanphear B, Séguin JR, Saint-Amour D, Dewailly E, Monnier P, Koren G, Ouellet E. 2013. Cohort profile: the maternal-infant research on environmental chemicals research platform. Paediatr Perinat Epidemiol. 27(4):415-25.
[ATSDR] Agency for Toxic Substances and Disease Registry. 2002. Toxicological Profile for Beryllium. Atlanta (GA): U.S. Department of Health and Human Services, Public Health Service. [assessed 2016 Jan 19].
[ATSDR] Agency for Toxic Substances and Disease Registry. 2012. Toxicological Profile for Vanadium. Atlanta (GA): U.S. Department of Health and Human Services, Public Health Service. [assessed 2016 Jan 19].
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C. 1999, c.33. Canada Gazette Part III, vol. 22, no. 3.
Canada, Dept. of the Environment. 2009. Canadian Environmental Protection Act, 1999: Notice with respect to certain inanimate substances (chemicals) on the Domestic Substances List. Canada Gazette, Part I, vol. 143, no. 40, p. 2945-2956.
[CCME] Canadian Council of Ministers of the Environment. 2015. Scientific Criteria Document for Canadian Soil Quality Guidelines for the Protection of Human Health: Beryllium. Ottawa (ON): Health Canada. ISBN 978-1-77202-017-5 PDF
[CDC] Centers for Disease Control and Prevention. 2009. Fourth National Report on Human Exposure to Environmental Chemicals. U.S. Department of Health and Human Services. Atlanta (GA): CDC. [accessed 2016 February].
[CDC] Centres for Disease Control and Prevention (U.S.). 2013. National Health and Nutrition Examination Survey: Sample Design, 2007-2010. Vital and Health Statistics, Series 2, Number 160. August 2013. Atlanta (GA): CDC, U.S. Department of Health and Human Services. [accessed 2016 June].
[CDC] Centres for Disease Control and Prevention (U.S.). 2015. Fourth National Report on Human Exposure to Environmental Chemicals. Updated Tables, February 2015. Atlanta (GA): CDC, U.S. Department of Health and Human Services. [accessed 2016 Jan 13].
Choi J, Morck TA, Joas A, Knudsen L. 2015. Major national human biomonitoring programs in chemical exposure assessment. AIMS Enviorn Sci 2(3):782-802.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. [modified 2007 Apr 20]. Categorization. Ottawa (ON): Government of Canada. [accessed 2016 Jan 19].
[ECHA] European Chemicals Agency. undated. Summary of Classification and Labelling, Beryllium [accessed 2016 Apr 21].
[ECHA] European Chemicals Agency. [modified 2016 Jan 20]. Candidate List of Substances of Very High Concern for Authorisation [Internet]. Helsinki (FI): European Chemicals Agency. [accessed 2016 Jan 21].
[ECHC] Environment Canada, Health Canada. 2010. Screening Assessment for the Challenge (Batch 9).Vanadium oxide (Vanadium pentoxide) [assessed 2016 April 07].
Edel J, Sabbioni E. 1988. Retention of intratracheally instilled and ingested tetravalent and pentavalent vanadium in the rat. J Trace Elem Electrolytes Health Dis. 2:23-30.
Health Canada. 2010. First Report on Human Biomonitoring of Environmental Chemicals in Canada. Results of the Canadian Health Measures Survey cycle 1 (2007-2009). August 2010. Ottawa (ON): Health Canada.
Health Canada. 2013. Second Report on Human Biomonitoring of Environmental Chemicals in Canada. Results of the Canadian Health Measures Survey cycle 2 (2009-2011). April 2013. Ottawa (ON): Health Canada.
[IARC] International Agency for Research on Cancer. 1993. Working Group on the Evaluation of Carcinogenic Risks to Humans. Beryllium and Beryllium compounds. IARC Monogr Eval Carcinog Risks Hum. 58:41-117.
[INSPQ] Institut national de santé publique du Québec. 2004. Étude sur l'établissement de valeurs de référence d'éléments traces et de métaux dans le sang, le sérum et l'urine de la population de la grande région de Québec. Institut national de santé publique du Québec, Québec, Que. Cote. [assessed 2016 Jan 19].
[IPCS] International Programme on Chemical Safety. 2001. Concise International Chemical Assessment Document 32: Beryllium and beryllium compounds. World Health Organization International Programme on Chemical Safety. World Health Organization. Geneva (Switzerland): [Accessed 2016 February 12].
[IRIS] Integrated Risk Information System. 1998. Beryllium and compounds; CASRN 7440-41-7: US National Library of Medicine. [accessed 2016 February 12].
Leonard L, Bernard A. 1993. Biomonitoring exposure to metal compounds with carcinogenic properties. Environ Health Perspect. 101 (3):127-33.
[NTP] National Toxicology Program. 2014. Report on Carcinogens, Thirteenth Edition. Beryllium and Beryllium Compounds. Research Triangle Park (NC): U.S. Department of Health and Human Services, Public Health Service. [assessed 2016 Jan 19].
Paschal DC, Ting BG, Morrow JC, Pirkle JL, Jackson RJ, Sampson EJ, Miller DT, and Caldwell KL. 1998. Trace Metals in Urine of United States Residents: Reference Range Concentrations. Environ Res. 76:53-59.
Reeves AL. 1965. The absorption of beryllium from the gastrointestinal tract. Arch Environ Health. 11:209-214.
[U.S. EPA] U.S. Environmental Protection Agency. 1991. Drinking water criteria document for beryllium. Cincinnati (OH): Office of Health and Environmental Assessment, Environmental Criteria and Assessment Office, Office of Drinking Water (NTIS PB92-173301).
6. Appendix
| CAS RN | DSL name | Classification for human health | Reference for classification |
|---|---|---|---|
| 7440-41-7 | Beryllium | Probable Human Carcinogen (Based on the limited evidence of carcinogenicity in humans). | U.S. EPA 2002 |
| 7440-41-7 | Beryllium | Known to be human carcinogen based on sufficient evidence of carcinogenicity from studies in humans. | NTP 2014 |
| 7440-41-7 | Beryllium | Beryllium and beryllium compounds are carcinogenic to humans; Suspected human carcinogen. |
IARC 1993 |
| 7440-41-7 | Beryllium | These classifications are based on lung cancer epidemiological studies conducted on workers in manufacturing plants. | ECHA undated |
| 7727-18-6; | Trichlorooxo Vanadium; | Trichlorooxo vanadium and vanadium oxide have not been assessed by other national or international agencies for carcinogenicity, mutagenicity or reproductive toxicity. | N/A |
| 11099-11-9 | Vanadium oxide | Trichlorooxo vanadium and vanadium oxide have not been assessed by other national or international agencies for carcinogenicity, mutagenicity or reproductive toxicity. | N/A |
None of these substances are on the Candidate List of Substances of Very High Concern for Authorisation (ECHA [modified 2016]).