Screening assessment 1-nitropropane
Official title: Screening assessment Propane, 1-nitro- (1-Nitropropane)
Chemical Abstracts Service Registry Number: 108-03-2
Environment and Climate Change Canada
Health Canada
February 2022
Cat. No.: En84-282/2021E-PDF
ISBN 978-0-660-40031-0
Synopsis
Pursuant to section 68 of the Canadian Environmental Protection Act, 1999 (CEPA), the Minister of the Environment and the Minister of Health have conducted a screening assessment of propane, 1-nitro-, hereinafter referred to as 1-nitropropane. The Chemical Abstracts Service Registry Number (CAS RNFootnote 1) for 1-nitropropane is 108-03-2. This substance was considered a priority on the basis of human health concerns.
According to information submitted in response to a CEPA section 71 survey, between 1000 kg and 10 000 kg of 1-nitropropane was imported in 2011, but it was not manufactured in Canada above the reporting threshold of 100 kg. Reported uses in Canada include use in paints and coatings. It is also a solvent in markers and cosmetic nail brush cleaners.
The ecological risk of 1-nitropropane was characterized using the ecological risk classification of organic substances (ERC), which is a risk-based approach that employs multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. Hazard profiles are based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Metrics considered in the exposure profiles include potential emission rate, overall persistence, and long-range transport potential. A risk matrix is used to assign a low, moderate or high level of potential concern for substances on the basis of their hazard and exposure profiles. Based on the outcome of the ERC analysis, 1-nitropropane is considered unlikely to be causing ecological harm.
Considering all available lines of evidence presented in this screening assessment, there is low risk of harm to the environment from 1-nitropropane. It is concluded that 1-nitropropane does not meet the criteria under paragraphs 64(a) or (b) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
1-Nitropropane was reviewed internationally through the Organisation for Economic Co-operation and Development (OECD) Cooperative Chemicals Assessment Programme and the Screening Information Dataset Initial Assessment Profile was used to inform the health effects section of this screening assessment. The main effect of concern for 1-nitropropane was reproductive and developmental toxicity.
The estimated exposure of the general population in Canada to 1-nitropropane through environmental media and food is negligible. General population exposure to 1-nitropropane can occur from its use as a solvent in marker ink, spray paint primers, and in cosmetic nail brush cleaners. The margins between estimated inhalation exposure from use of products available to consumers to 1-nitropropane and the critical effect levels are considered adequate to address uncertainties in the health effects and exposure databases. The risk to human health from incidental oral exposure to markers is considered to be low.
Considering all the information presented in this screening assessment, it is concluded that 1-nitropropane does not meet the criteria under paragraph 64(c) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore concluded that 1-nitropropane does not meet any of the criteria set out in section 64 of CEPA.
1. Introduction
Pursuant to section 68 of the Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999), the Minister of the Environment and the Minister of Health have conducted a screening assessment of 1-nitropropane to determine whether this substance presents or may present a risk to the environment or to human health. This substance was considered a priority on the basis of human health concerns.
The ecological risk of 1-nitropropane was characterized using the ecological risk classification of organic substances (ERC) approach (ECCC 2016a). The ERC describes the hazard of a substance using key metrics, including mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity, and considers the possible exposure of organisms in the aquatic and terrestrial environments on the basis of such factors as potential emission rates, overall persistence, and long-range transport potential in air. The various lines of evidence are combined to identify substances as warranting further evaluation of their potential to cause harm to the environment or as having a low likelihood of causing harm to the environment.
The substance currently being evaluated has been reviewed internationally through the Organisation for Economic Co-operation and Development (OECD) Cooperative Chemicals Assessment Programme, and a Screening Information Dataset (SIDS) Initial Assessment Profile (SIAP) is available. These assessments undergo rigorous review (including peer-review) and endorsement by international governmental authorities. Health Canada and Environment and Climate Change Canada are active participants in this process, and consider these assessments to be reliable. The OECD SIAP was used to inform the health effects characterization in this screening assessment.
This screening assessment includes consideration of information on chemical properties, environmental fate, hazards, uses and exposures, including additional information submitted by stakeholders. Relevant data were identified up to January 2019. Empirical data from key studies as well as results from models were used to reach conclusions. When available and relevant, information presented in assessments from other jurisdictions was considered.
This screening assessment was prepared by staff in the CEPA Risk Assessment Program at Health Canada and Environment and Climate Change Canada and incorporates input from other programs within these departments. The human health portions of this assessment have undergone external review. Comments on the technical portions relevant to human health were received from Theresa Lopez, Jennifer Flippin and Joan Garey (TetraTech Inc.). The ecological portion of this assessment is based on the ERC document (published July 30, 2016), which was subject to an external review as well as a 60-day public comment period. Additionally, the draft of this screening assessment (published December 7, 2019) was subject to a 60-day public comment period. While external comments were taken into consideration, the final content and outcome of this screening assessment remain the responsibility of Health Canada and Environment and Climate Change Canada.
This screening assessment focuses on information critical to determining whether substances meet the criteria as set out in section 64 of CEPA by examining scientific information and incorporating a weight of evidence approach and precaution.Footnote 2 This screening assessment presents the critical information and considerations on which the conclusion is based.
2. Substance identity
The Chemical Abstracts Service Registry Numbers (CAS RN), Domestic Substances List (DSL) name, and molecular structure for 1-nitropropane are presented in Table 2‑1.
| CAS RN | DSL name (common name) | Molecular structure and formula | Molecular weight (g/mol) | Reference |
|---|---|---|---|---|
| 108-03-2 | Propane, 1-nitro- (1-nitropropane) | 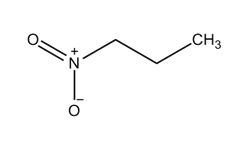 C3H7NO2 C3H7NO2
|
89.09 | ChemIDplus 1993- |
3. Physical and chemical properties
A summary of experimental physical and chemical property data of 1-nitropropane is presented in Table 3‑1. Additional physical and chemical properties are reported in ECCC (2016b).
| Property | Value | Key reference(s) |
|---|---|---|
| Physical state | liquid | OECD 2010 |
| Melting point (°C) | -104 | OECD 2010 |
| Vapour pressure (Pa) | 1 300 | OECD 2010 |
| Water solubility (mg/L) | 15 000 at 25 ˚C | OECD 2010 |
| Log Kow (dimensionless) | 0.79 | OECD 2010 |
Abbreviations: Kow, octanol-water partition coefficient.
4. Sources and uses
According to information submitted in response to a CEPA section 71 survey (Canada 2012), in 2011, 1-nitropropane was not manufactured in Canada above the reporting threshold of 100 kg and between 1000 kg and 10 000 kg were imported into Canada (Environment Canada 2013).Footnote 3
According to information submitted in response to a CEPA section 71 survey, 1-nitropropane was reported to be used in paints and coatings in Canada (Environment Canada 2013). 1-Nitropropane is also used as a solvent in metallic markers (SDS 2012), spray paint primer (SDS 2017) and in cosmetic nail brush cleaners (SDS 2016).
No additional uses for 1-nitropropane were identified in Canada. However, it is known to be used internationally as a solvent for cellulose acetate, vinyl resin, synthetic rubber, fats, oils and waxes, a gasoline additive and an additive in one or more types of tobacco products (OECD 2010; NTP 2018). It is also listed as a solvent in the European Commission's Cosmetic Ingredient database (CosIng 2018).
5. Potential to cause ecological harm
5.1 Characterization of Ecological Risk
The ecological risk of 1-nitropropane was characterized using the ecological risk classification of organic substances (ERC) approach (ECCC 2016a). The ERC is a risk-based approach that considers multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. The various lines of evidence are combined to discriminate between substances of lower or higher potency and lower or higher potential for exposure in various media. This approach reduces the overall uncertainty with risk characterization compared to an approach that relies on a single metric in a single medium (e.g., median lethal concentration) for characterization. The following summarizes the approach, which is described in detail in ECCC (2016a).
Data on physical-chemical properties, fate (chemical half-lives in various media and biota, partition coefficients, and fish bioconcentration), acute fish ecotoxicity, and chemical import or manufacture volume in Canada were collected from the scientific literature, from available empirical databases (e.g., OECD QSAR Toolbox 2014), from responses to CEPA section 71 surveys, or they were generated using selected (quantitative) structure-activity relationship ([Q]SAR) or mass-balance fate and bioaccumulation models. These data were used as inputs to other mass-balance models or to complete the substance hazard and exposure profiles.
Hazard profiles were based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Exposure profiles were also based on multiple metrics, including potential emission rate, overall persistence, and long-range transport potential. Hazard and exposure profiles were compared to decision criteria in order to classify the hazard and exposure potentials for each organic substance as low, moderate, or high. Additional rules were applied (e.g., classification consistency, margin of exposure) to refine the preliminary classifications of hazard or exposure.
A risk matrix was used to assign a low, moderate or high classification of potential risk for each substance on the basis of its hazard and exposure classifications. ERC classifications of potential risk were verified using a two-step approach. The first step adjusted the risk classification outcomes from moderate or high to low for substances that had a low estimated rate of emission to water after wastewater treatment, representing a low potential for exposure. The second step reviewed low risk potential classification outcomes using relatively conservative, local-scale (i.e., in the area immediately surrounding a point source of discharge) risk scenarios, designed to be protective of the environment, to determine whether the classification of potential risk should be increased.
ERC uses a weighted approach to minimize the potential for both over- and under- classification of hazard and exposure, and of subsequent risk. The balanced approaches for dealing with uncertainties are described in greater detail in ECCC (2016a). The following describes two of the more substantial areas of uncertainty. Error with empirical or modelled acute toxicity values could result in changes in classification of hazard, particularly metrics relying on tissue residue values (i.e., mode of toxic action), many of which are predicted values from (Q)SAR models (OECD QSAR Toolbox 2014). However, the impact of this error is mitigated by the fact that overestimation of median lethality will result in a conservative (protective) tissue residue value used for critical body residue analysis. Error with underestimation of acute toxicity will be mitigated through the use of other hazard metrics such as structural profiling of mode of action, reactivity and/or estrogen binding affinity. Changes or errors in chemical quantity could result in differences in classification of exposure as the exposure and risk classifications are highly sensitive to emission rate and use quantity. The ERC classifications thus reflect exposure and risk in Canada on the basis of what is estimated to be the current use quantity, and may not reflect future trends.
Critical data and considerations used to develop the substance-specific profile for 1-nitropropane and the hazard, exposure and risk classification results are presented in ECCC (2016b).
On the basis of low hazard and low exposure classifications according to information considered under ERC, 1-nitropropane was classified as having a low potential for ecological risk. It is unlikely that 1-nitropropane is resulting in concerns for the environment in Canada.
6. Potential to cause harm to human health
6.1 Exposure assessment
No monitoring data were identified for 1-nitropropane in any environmental media or food in Canada or elsewhere. The level III fugacity model known as ChemCAN (2003) was used to derive predicted environmental concentrations of 1-nitropropane in Canada using the upper-end volume data from imported quantities (i.e., 10 000 kg) (Environment Canada 2013). The estimated concentrations from air, water, and soil were 2.2 x 10-4 µg/m3, 1.4 x 10-3 µg/L and 8.0 x 10-3 ng/g, respectively. On the basis of these concentrations, the estimated exposure to 1-nitropropane for the general population of Canada from environmental media is considered to be negligible.
1-Nitropropane was identified in a limited number of products available to consumers such as permanent metallic markers, cosmetic nail brush cleaners and spray paint primers (SDS 2012, 2016, 2017). Inhalation and incidental oral exposures to 1-nitropropane in markers were estimated for 2- to 3-year olds who represent the highest exposures to Canadians, and thus were considered protective of other potential age groups who may be exposed to this product. Inhalation exposures to 1-nitropropane in spray paint primers and cosmetic nail brush cleaners were estimated for adults. Dermal exposure was not estimated for 1-nitropropane; it is associated with a high vapour pressure and is likely to evaporate before significant amounts of dermal absorption can take place (OECD 2010). Table 6-1 presents estimated oral and inhalation exposures from marker ink and inhalation exposures from spray paint primers and cosmetic nail brush cleaners. Details on the method and parameters used to estimate these exposures are found in Appendix A.
| Exposure scenario | Maximum concentration (% by weight) | Per event exposure | Daily exposure (mg/kg bw/day) |
|---|---|---|---|
| Marker ink – 2- to 3-year olds (oral) | 40a | 1.33 mg/kg bw | N/A |
| Marker ink – 2- to 3-year olds (inhalation) | 40a | 5.1 mg/m3 | 0.06d |
| Cosmetic nail brush cleaner – adult (inhalation) | 100b | 9 mg/m3 | 0.04d |
| Spray paint primer – adult (inhalation) | 2.5c | 27 mg/m3 | 0.1d |
Abbreviations: N/A, not applicable.
a Sexton and Godbout 2003
b SDS 2016
c SDS 2017
d Details on the method and parameters used to estimate these exposures are found in Appendix B
6.2 Health effects assessment
As part of the Short-Chain Nitroparaffins Category, 1-nitropropane was reviewed by the OECD in 2010, and that review was used to inform the health effects characterization in this screening assessment.
A literature search was conducted from the year prior to the OECD SIDS Initial Assessment Meeting (SIAM) (i.e., January 2009) to September 2018. No health effects studies, which could impact the risk characterization (i.e., result in different critical endpoints or lower points of departure than those stated in OECD 2010), were identified. No hazard classification has been identified for 1-nitropropane internationally.
1-Nitropropane did not induce gene mutations in studies with bacteria. In vivo, 1-nitropropane did not induce micronuclei in rat or mouse bone marrow assays but was positive for micronuclei in rat liver cells (OECD 2010). In a chronic inhalation toxicity study, 1-nitropropane had no effect on tumour incidences in male and female rats exposed to 100 ppm 1-nitropropane for 21.5 months (OECD 2010). However, firm conclusions on carcinogenicity were not possible due to limited study design.
A short-term, combined repeated-dose and reproductive/developmental screening study was conducted in rats, via inhalation. Both sexes of rats were exposed for 14 days prior to mating, during mating, and for females, through gestational day 19 at 0, 24, 48 or 96 ppm (equivalent to 0, 88, 180 or 350 mg/m3) (12/sex/concentration) for 6 hours/day for 7 days/week. No systemic effects other than slightly decreased body weights (by 6.9%) associated with decreased food consumption in animals at the highest dose tested were observed in the study. Local histopathologic changes in the nasal tissues (predominantly in females) were observed at 48 ppm (180 mg/m3) and 96 ppm (350 mg/m3) (OECD 2010).
The reproductive/developmental no observed adverse effect concentration (NOAEC) was determined to be 48 ppm (180 mg/m3), based on a decrease in litter sizes and mean number of pups born live at 96 ppm (350 mg/m3) (OECD 2010).
A short-term, repeated-dose study was also conducted in rats via the oral (gavage) route. Rats were dosed with 1-nitropropane for 28 days at 0, 10, 30 or 100 mg/kg-bw/day (5/sex/dose). The critical effect level and corresponding hazard endpoint was a no observed adverse effect level (NOAEL) of 30 mg/kg-bw/day based on clinical findings of ataxia, salivation and hunched posture consistent with toxicity to the nervous system at 100 mg/kg-bw/day. These effects started to manifest on day 15 of the study with the observation of increased salivation. At 100 mg/kg bw/day, there were also significant increases in absolute and relative brain weights in the absence of corresponding morphologic changes, changes in clinical chemistry and haematology parameters and an increase in methaemoglobin (OECD 2010).
6.3 Characterization of risk to human health
On the basis of the assessment of 1-nitropropane by the OECD, a NOAEC of 180 mg/m3 based on decreased litter sizes and mean number of pups born live at 350 mg/m3, in a combined repeated-dose reproductive/developmental inhalation toxicity screening test, was selected as the most relevant endpoint for characterization of risk from inhalation exposure (OECD 2010).
The principal route of exposure to 1-nitropropane for the general population is expected to be via inhalation during use of products available to consumers.
Table 6‑2 provides relevant exposure and critical health effect values for 1-nitropropane, as well as resultant margins of exposure, for determination of risk from inhalation exposure from use of permanent markers, spray paint primers and in nail brush cleaner products available to consumers.
| Exposure scenario | Estimated exposure | Critical effect level | Critical health effect endpoint | MOE |
|---|---|---|---|---|
| Marker ink –2- to 3-year olds (inhalation) | 0.06 mg/kg bw/day | Adjusted internal dose = 38.73 mg/kg bw/day (NOAEC equal to 180 mg/m3) | Decreases in litter size, and number of pups born live | 645 |
| Brush cleaner -adult (inhalation) | 0.04 mg/kg bw/day | Adjusted internal dose = 38.73 mg/kg bw/day (NOAEC equal to 180 mg/m3) | Decreases in litter size, and number of pups born live | 968 |
| Spray paint primer – adult (inhalation) | 0.1 mg/kg bw/day | Adjusted internal dose = 38.73 mg/kg bw/day (NOAEC equal to 180 mg/m3) | Decreases in litter size, and number of pups born live | 387 |
Abbreviations: MOE, Margin of Exposure
a To address the differences in exposure duration between the critical effect study and the actual use pattern of the products available to consumers containing 1-nitropropane, both the NOAEC and the estimated exposure concentrations were adjusted to a continuous exposure scenario to more accurately characterize potential risk (Appendix B).
On the basis of the conservative parameters used in modelling exposure to products available to consumers, the calculated margins for spray paint primer, cosmetic nail brush cleaner and permanent metallic markers are considered adequate to address uncertainties in the health effects and exposure databases.
Incidental oral ingestion of permanent marker ink by 2- to 3-year olds may be a source of exposure to 1-nitropropane; however, based on the hazard information for this substance, health effects following single occasional exposure via the oral route are not expected to occur and the risk to human health from incidental oral exposure to permanent marker ink is considered to be low.
6.4 Uncertainties in evaluation of risk to human health
There is uncertainty in the exposure characterization due to a lack of empirical data on environmental concentrations of 1-nitropropane; however, the use of upper-end volume imported quantities used to predict environmental concentrations resulted in negligible exposures. There is also uncertainty in the exposure characterization from marker ink because there are no data available on the exact concentration of 1-nitropropane in marker ink. There is uncertainty in the effects observed in the reproductive/developmental study due to limited study design. However, the endpoint is consistent with effects seen in other members of the Short-Chain Nitroparaffins Category for reproductive/developmental toxicity and thus considered appropriate and conservative for risk characterization for all age groups.
7. Conclusion
Considering all available lines of evidence presented in this screening assessment, there is low risk of harm to the environment from 1-nitropropane. It isconcluded that 1-nitropropane does not meet the criteria under paragraphs 64(a) or (b) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
Considering all the information presented in this screening assessment, it is concluded that 1-nitropropane does not meet the criteria under paragraph 64(c) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore concluded that 1-nitropropane does not meet any of the criteria set out in section 64 of CEPA.
References
Bide RW, Armour SJ, Yee E. 2000. Allometric respiration/body mass data for animals to be used for estimates of inhalation toxicity to young adult humans. J Appl Toxicol. 20(4):273-290.
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C. 1999, c.33. Canada Gazette Part III, vol. 22, no. 3.
Canada, Dept. of the Environment. 2012. Canadian Environmental Protection Act [PDF], 1999: Notice with respect to certain substances on the Domestic Substances List. Canada Gazette, Part I, vol. 146, no. 48, Supplement.
ChemIDplus [database]. 1993- . Bethesda (MD): US National Library of Medicine. [accessed 2018 Aug 28].
ChemCAN [level III fugacity model of 24 regions of Canada]. 2003. Version 6.00. Peterborough (ON): Trent University, Canadian Centre for Environmental Modelling and Chemistry.
[ConsExpo Web] Consumer Exposure Web Model. 2016. Bilthoven (NL): Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment].
[CosIng] Cosmetic Ingredients & Substances [database]. Brussels (BE): European Commission. [accessed 2018 Sept 13].
[Danish EPA] Danish Environmental Protection Agency. 2008. Survey and health assessment of chemical substances in hobby products for children. Survey of chemical substances in consumer products, No 93. Copenhagen (DK): Danish EPA.
[ECCC] Environment and Climate Change Canada. 2016a. Science approach document: ecological risk classification of organic substances. Ottawa (ON): Government of Canada.
[ECCC] Environment and Climate Change Canada. 2016b. Supporting documentation: data used to create substance-specific hazard and exposure profiles and assign risk. Gatineau (QC): ECCC. Information in support of the science approach document: ecological risk classification of organic substances. Available from: substances@ec.gc.ca.
Environment Canada. 2013. DSL Inventory Update data collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain substances on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
Health Canada. 2015. Food Consumption Table derived from Statistics Canada, Canadian Community Health Survey, Cycle 2.2, Nutrition (2004), Share file. Ottawa (ON): Government of Canada.
McCready D, Fontaine D. 2010. Refining ConsExpo evaporation and human exposure calculations for REACH. Hum Ecol Risk Assess. 16(4):783-800.
[OECD] Organisation for Economic Co-operation and Development. 2010. SIDS initial assessment report: Short Chain Nitroparaffins: CAS No. 75-52-5, 79-24-3, 108-03-2 [PDF]. SIAM [SIDS Initial Assessment Meeting] 31; 20-22 October 2010. Paris (FR): OECD. [accessed 2018 Aug 13].
OECD QSAR Toolbox [Read-across tool]. 2014. Version 3.3. Paris (FR): Organisation for Economic Co-operation and Development, Laboratory of Mathematical Chemistry.
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment]. 2002. Children’s Toys Fact Sheet: to assess the risks for the consumer [PDF]. Bilthoven (NL): RIVM. Report No.: 612810012/2202. [accessed 2018 Oct 16].
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment]. 2007. Spray paint products fact sheet: to assess the risks for the consumer: updated version for ConsExpo 4 [PDF. Bilthoven (NL): RIVM. Report No.: 320104008/2007. [accessed 2019 Jan 7].
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment]. 2014. General Fact Sheet: General default parameters for estimating consumer exposure - Updated version 2014. Bilthoven (NL): RIVM. Report No.: 090013003/2014 [accessed 2019 Jan 25].
[SDS] Material Safety Data Sheet. 2012. Sharpie Metallic Permanent Marker [PDF]. Oak Brook (IL): Newell Rubbermaid, Inc.
[SDS] Material Safety Data Sheet. 2016. Kiara Sky Dip Essential Brush Cleaner [PDF]. Bakersfield (CA): Kiara Sky Professional Nails, Inc.
[SDS] Material Safety Data Sheet. 2017. Coverstain 16-Oz 6 Pk +Spray Pro Pack [PDF]. Vernon Hills (IL): Rust-Oleum Corporation.
Sexton MN, Godbout D, inventors; Sanford, L.P., assignee. 2003 Apr 1. Solvent system and ink made therefrom. United States Patent US 7,297,729.
Sparks LE, Tichenor BA, Chang J, Guo Z. 1996. Gas-phase mass transfer model for predicting volatile organic compound (VOC) emission rates from indoor pollutant sources. Indoor Air. 6:31-40.
[US EPA] US Environmental Protection Agency. 2005. PARAMS model. Washington (DC): US Environmental Protection Agency. [last revised 2011 Aug 18; accessed Oct 2017].
[US EPA] US Environmental Protection Agency. 2011. Chapter 6: Inhalation Rates. Exposure Factors Handbook 2011 Edition (Final). Washington (DC): US Environmental Protection Agency. EPA/600/R-09/052F.
Appendices
Appendix A. Parameters used to estimate human exposures
Exposure estimates were calculated based on default body weights of 15 kg for 2- to 3-year olds and 74 kg for adults (Health Canada 2015). The estimated exposure parameters are described in Table A-1. Unless specified otherwise, ConsExpo Web (2016) was used to estimate exposures. The PARAMS model was used to estimate mass transfer coefficients (Sparks method) (US EPA 2005). Refer to Table A-2 for defaults used in the PARAMS model.
| Exposure scenario | Assumptions |
|---|---|
| Marker ink - 2- to 3-year olds (per event, oral) |
Scenario: "single ingestion" scenario in Children’s Toys Fact Sheet (RIVM 2002) Where: |
| Marker ink -2- to 3-year olds (per event, inhalation) |
Scenario: Ink from felt pen in Children’s Toys Fact Sheet (RIVM 2002) Inhalation: |
| Cosmetic nail brush cleaner -adult (per event, inhalation) |
Inhalation: Product amount and scenario was based on professional judgment. The cosmetic nail brush cleaner is only opened to exchange the dirty brush with the clean brush. It is assumed that 10% of the nail brush cleaner might evaporate during such an exchange. |
| Primer – adult (inhalation) |
Scenario: Spray can scenario from Paint Fact Sheet (RIVM 2007) but used exposure to vapour – evaporation model since substance is volatile. Inhalation: |
| Parameter | Value | Additional Information |
|---|---|---|
| Density of air (g/cm3) | 0.0011774 | At 25 degrees Celsius, atmospheric pressure of 760 mmHg, and relative humidity of 50% |
| Viscosity of air (g/cm/s) | 1.86E-04 | At 25 degrees Celsius |
| Velocity of air (cm/s) | 10 | (McCready and Fontaine 2010; Sparks et al. 1996) |
| Diffusivity in air (cm2/s) | 9.263E-2 | At 25 degrees Celsius |
| Length of surface for various scenarios | Spray paint: 2 m | Value estimated taking into account release area listed in ConsExpo Fact Sheet for specific scenario |
Appendix B. Equations used to adjust inhalation exposure and hazard values
Internal dose (mg/kg bw/day) = concentration (mg/m3) x RF x F X (inhalation rate (m3/day)/body weight (kg)) (equation 1)
Where, the "day” in mg/kg bw/day is defined as those days in which the test item was administered (i.e., day of exposure) and does not mean a 24-hour cycle, while ‘m3/day’ is defined as a the volume of air inhaled (or exhaled) in a 24-hour cycle.
RF is assumed to be 1 (100%), where RF is defined as the respirable fraction of substance that reaches the alveolar region of the lungs
F refers to the fraction of a day of exposure during which the test item was administered
The internal dose was calculated using a concentration of 175 mg/m3, RF of 1, F of (6h/24h), inhalation rate of 0.31m3/day and a body weight of 0.35 kg and equation 1.
The internal dose was calculated to be 38.73 mg/kg bw/day.
For exposure adjustments, the inhalation and body weight defaults from Appendix B were used to calculate internal dose for humans. The inputs in Equation 1 for concentration and inhalation rate for rats were calculated as follows.
Concentration:
The NOAEC was converted using a concentration of 48 ppm and a molecular weight of 89.09 g/mol and using equation 2.
Concentration (mg/m3) = (concentration (ppm) x Molecular weight (g/mol))/ 24.45 (L/mol) (equation 2)
Where, the volume of 1 mole of a gas or vapour at 1 atmosphere (760 torr or 760 mm Hg) and at 25°C, is 24.45 litres.
Concentration (mg/m3) = 175
Inhalation rate:
The minute volume (Vm) was calculated based on equation 3 for non-anesthetised animals (Bide et al 2000)
Vm =0.499 x BW0.809 (equation 3)
Where, BW is the body weight (kg) of the animal and Vm is the minute volume (L/min)
The minute volume (Vm) for a rat was calculated using a body weight of 0.35 kg and using equation 3.
Vm = 0.213 L/min
The inhalation rate for a rat was calculated using the calculated minute volume and equation 4.
Inhalation rate: Inhalation rate (m3/day) = Minute volume (L/min) x 1.44 (equation 4)
Where, 1L/min = 1.44 m3/day
Inhalation rate in m3/day =0.213 (L/min) x1.44 = 0.31m3/day
