Screening Assessment
Aromatic Azo and Benzidine-based Substance Grouping
Certain Aromatic Amines
Environment and Climate Change Canada
Health Canada
May 2016
Table of Contents
List of Tables
- Table 2-1. Identity of the 16 Aromatic Amines
- Table 2-2. Definitions of seven ecological subsets of aromatic amines based on structure
- Table 2-3. Identity and structure of the 16 substances in seven ecological subsets
- Table 2-4. Identity of the analogues identified to inform the physical and chemical properties, environmental fate and ecotoxicity of substances in the Aromatic Amines subgroup
- Table 4-1. Annual import quantity ranges of surveyed Aromatic Amines for the years 2005, 2008 and 2010
- Table 5-1. Summary of empirical biodegradation data for Aromatic Amines in aerobic aqueous medium, by ecological subset
- Table 5-2. Summary of empirical BCF data in the Aromatic Amines subgroup
- Table 6-1. Sediment toxicity data for the Aromatic Amines subgroup
- Table 6-2. Available empirical chronic and acute soil toxicity studies for the Aromatic Amines subgroup
- Table 6-3. Aquatic CTVs selected for each substance in the Aromatic Amine Subgroup, by ecological subset
- Table 6-4. Aquatic PNECs representing the 10 aromatic amines found to be in commerce in Canada by ecological subset
- Table 6-5. A summary of parameter values used in the consumer release from cosmetic scenario
- Table 6-6. Aquatic PEC results for the consumer release of Aromatic Amines from cosmetics use scenario
- Table 6-7. Values of aquatic exposure parameters used for the cosmetics formulation scenario
- Table 6-8. Aquatic PEC results for industrial release of Aromatic Amines from the section 71 identified personal care and cosmetics formulation scenario
- Table 6-9. Values of aquatic exposure parameters used for the tire manufacture scenario
- Table 6-10. Aquatic PEC results for industrial release of Aromatic Amines from the tire manufacture scenario
- Table 7-1. Aromatic Amines with potential for exposure of the general population of Canada 57
- Table 7-2. Summary of results for 2,4-diaminotoluene, o-toluidine, o-anisidine and 1,3-diaminobenzene measured in the third extraction from new polyamide cooking utensils
- Table 7-3. Summary of results from textile and leather products that contain 2,4-diaminotoluene, 2-naphthylamine, o-toluidine, 4-chloroaniline and o-anisidine
- Table 7-4. Summary of textile and leather products tested by Health Canada that detected certain Aromatic Amines
- Table 7-5. Carcinogenicity and genotoxicity classifications of Aromatic Amines of certain national and international agencies
- Table 7-6. Estimated exposures to 2-naphthylamine from textiles
- Table 7-7. MOEs for daily exposure to 2-naphthylamine
- Table 7-8. MOEs for daily exposure to o-toluidine
- Table 7-9. Estimated exposures to 2,4-diaminotoluene from textiles
- Table 7-10. MOEs for daily exposure to 2,4-diaminotoluene
- Table 7-11. Estimated exposures to 4-chloroaniline from textiles
- Table 7-12. MOEs for acute dermal exposures to 4-chloroaniline
- Table 7-13. MOEs for daily exposure to 4-chloroaniline
- Table 7-14. Estimated exposures to o-anisidine from textiles
- Table 7-15. MOEs for daily exposure to o-anisidine
- Table 7-16. Summary of upper-bounding estimates of dermal exposure to p-aminophenol via use of cosmetic products
- Table 7-17. Summary of p-aminophenol in vitro genotoxicity data
- Table 7-18. Summary of p-aminophenol in vivo genotoxicity data
- Table 7-19. MOEs for daily exposure to p-aminophenol
- Table 7-20. MOEs for acute (per event) exposure to p-aminophenol
- Table 7-21. MOEs for acute and daily exposure to 1,3-diaminobenzene
- Table 7-22. Summary of upper-bounding estimated exposures to Red Lake Amine C deriving from use of Pigment Red 53:1 in face paint and lip cosmetic products via the oral route
- Table 7-23. Summary of upper-bounding estimated exposures to Red Lake Amine C deriving from use of Pigment Red 53:1 in face paint, hair dye and mascara via the dermal route
- Table 7-24. MOEs for daily exposure to Red Lake C Amine
Synopsis
Pursuant to sections 68 or 74 of the Canadian Environmental Protection Act, 1999 (CEPA 1999), the Minister of the Environment and of Health have conducted a screening assessment on 16 Aromatic Amines. These substances constitute a subgroup of the Aromatic Azo and Benzidine-based Substance Grouping being assessed as part of the Substance Groupings Initiative of the Government of Canada’s Chemicals Management Plan (CMP) based on structural similarity and applications. Substances in this Grouping were identified as priorities for assessment as they met the categorization criteria under subsection 73(1) of CEPA 1999 and/or were considered as a priority based on other human health concerns.
The Chemical Abstracts Service Registry Number (CAS RN)Footnote[1], Domestic Substances List (DSL) names and common names of the 16 substances in the Aromatic Amines subgroup are presented in the following table.
| CAS RN | DSL name | Common name used in this assessment |
|---|---|---|
| 88-53-9Footnote Table 0[b] | Benzenesulfonic acid, 2-amino-5-chloro-4-methyl- | Red Lake C Amine |
| 90-04-0Footnote Table 0[a], [b] | Benzenamine, 2-methoxy- | o-Anisidine |
| 91-59-8[a], [b] | 2-Naphthalenamine | 2-Naphthylamine |
| 95-51-2 | Benzenamine, 2-chloro- | 2-Chloroaniline |
| 95-53-4[a], [b] | Benzenamine, 2-methyl- | o-Toluidine |
| 95-76-1 | Benzenamine, 3,4-dichloro- | 3,4-Dichloroaniline |
| 95-80-7[a], [b] | 1,3-Benzenediamine, 4-methyl- | 2,4-Diaminotoluene |
| 100-01-6[b] | Benzenamine, 4-nitro- | 4-Nitroaniline |
| 106-47-8[a], [b] | Benzenamine, 4-chloro- | 4-Chloroaniline |
| 106-49-0[b] | Benzenamine, 4-methyl- | p-Toluidine |
| 108-45-2 | 1,3-Benzenediamine | 1,3-Diaminobenzene |
| 123-30-8 [b] | Phenol, 4-amino- | p-Aminophenol |
| 156-43-4 [b] | Benzenamine, 4-ethoxy- | p-Phenetidine |
| 540-23-8 [b] | Benzenamine, 4-methyl-, hydrochloride | p-Toluidine hydrochloride |
| 541-69-5 [b] | 1,3-Benzenediamine, dihydrochloride | 1,3-Diaminobenzene dihydrochloride |
| 615-05-4[a], [b] | 1,3-Benzenediamine, 4-methoxy- | 2,4-Diaminoanisole |
Global, anthropogenic sources of aromatic amines include biomass and fossil fuel combustion, chemical synthesis, coal gasification plants, aluminum smelting, wastewater treatment plants, drinking water plants, refineries and production facilities, dye houses and chemical factories. The 16 Aromatic Amines considered in this assessment are industrial chemicals primarily used as chemical intermediates in synthesis of pigments, dyes, pesticides, drugs and rubber products, as well as in laboratory chemicals.
No manufacturing activity of any of the 16 Aromatic Amines in Canada was reported above the 100 kg/year threshold, according to recent surveys under section 71 of CEPA 1999. Seven of the Aromatic Amines have been reported as being imported into Canada above the 100 kg/year survey reporting threshold. An additional two Aromatic Amines were reported as being imported into Canada below the 100kg/yr reporting threshold.
Environment
The 16 Aromatic Amines are soluble in water. In terms of potential releases to water, sediment and soil, taking into consideration the physical and chemical properties of these substances, aromatic amines will bind to dissolved organic matter, particulate matter and sediment over time; however, water is considered the primary route of exposure. Available experimental and modelled data regarding the abiotic and biotic degradation of the 16 Aromatic Amines indicate that these substances are persistent in water, sediment and soil. Information on the log octanol–water partition coefficients and fish bioconcentration factors indicates that these substances are not likely to bioconcentrate or bioaccumulate in aquatic organisms.
There is a wide range of acute and chronic aquatic toxicity data for the aromatic amines (median effective concentrations [EC50] or median lethal concentrations [LC50]: 0.0004–418 mg/L). The toxicity of substituted aniline compounds is dependent on their mode of action, the type of substituents (chloro-, methyl-, etc.), the number of substituents (mono-, di-, etc.) and their position (ortho-, meta-, para-). Aquatic invertebrates (Daphnia) were more sensitive than other organisms to aromatic amines. Limited toxicity data were available for terrestrial and sediment-dwelling organisms.
Aquatic exposure scenarios were developed to represent the potential major environmental releases due to industrial and consumer activities involving the aromatic amines. Predicted environmental concentrations were calculated for the aquatic environment for those section 71 identified substances released from tire manufacturing, tire wear, personal care and cosmetics formulation and consumer use of personal care and cosmetics. The probability that the predicted environmental concentration of aromatic amines would exceed the substances’ predicted no-effect concentration was low (~5% or less) for all four scenarios, meaning that low risk of adverse effects to aquatic organisms is expected as a result of these industrial and consumer activities, respectively.
Considering all available lines of evidence presented in this Screening Assessment, there is low risk of harm to organisms and the broader integrity of the environment from the 16 Aromatic Amines. It is concluded that these Aromatic Amines do not meet the criteria under paragraphs 64(a) or 64(b) of CEPA 1999, as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
Human Health
This human health assessment focuses on substances that are reported above the reporting threshold of 100 kg/year in the recent surveys conducted under section 71 of CEPA 1999 or for which available information indicates potential exposure to the general population of Canada. Potential exposure of the general population of Canada was characterized for nine of the 16 substances included in this assessment: 2-naphthylamine, o-toluidine, 2,4-diaminotoluene, 4-chloroaniline, 3,4-dichloroaniline, o-anisidine, p-aminophenol, 1,3-diaminobenzene and Red Lake C Amine. Exposure of the general population in Canada to one or more of the nine Aromatic Amines from the use of certain consumer products, such as cooking utensils, textiles and cosmetics, was estimated. No robust Canadian data on concentrations of these nine Aromatic Amines in environmental media were identified. With the exception of p-aminophenol, section 71 data indicate low volumes of use of these nine Aromatic Amines in Canada, therefore exposures from environmental media are generally not expected for these substances. For p-aminophenol, environmental media are not considered a significant source of exposure considering the direct exposure from use of this substance in cosmetic products.
Exposures were not expected for the remaining seven aromatic amines in this subgroup; this includes those not reported under section 71 and those with no other information identified to support exposure.
Carcinogenicity was considered to be the health effect of concern for six of the nine aromatic amines for which exposure was characterized. 2-Naphthylamine, o-toluidine, 2,4-diaminotoluene, 4-chloroaniline and o-anisidine are classified as known or possible human carcinogens by the International Agency for Research on Cancer (Group 1 or 2B) and the European Union (Category 1A or 1B). Carcinogenicity was not identified as an endpoint of concern for p-aminophenol, 1,3-diaminobenzene or Red Lake C Amine; therefore, critical non-cancer health effect levels were selected for risk characterization.
Four substances (2-naphthylamine, 2,4-diaminotoluene, 4-chloroaniline and o-anisidine) were detected in some imported textile and leather products in a study conducted by Health Canada in 2012. Margins between estimates of exposure of the general population from dermal contact with textiles as well as mouthing of textiles by infants and critical effect levels are considered adequate to address uncertainties in the health effects and exposure databases.
Available information indicates that residual o-toluidine, 2,4-diaminotoluene, o-anisidine, 4-chloroaniline and 1,3-diaminobenzene may migrate to foods being prepared with polyamide cooking utensils. Margins between the estimated daily oral exposure from use of polyamide cooking utensils and critical effect levels are considered adequate to address uncertainties in the health effects and exposure databases.
Exposures to p-aminophenol, 1,3-diaminobenzene, 4-chloroaniline and Red Lake C Amine were identified from use of certain cosmetic products. The margins between exposure estimates and critical effect levels for each of these substances were considered adequate to address uncertainties in the health effects and exposure databases.
o-Toluidine was identified at low levels in breast milk from a small sample of Canadian women. The margin between estimated daily intake of o-toluidine for non-formula-fed infants via breast milk and the critical effect level is considered adequate and does not indicate a concern at these low levels of exposure.
For the remaining seven Aromatic Amines (2,4-diaminoanisole, 2-chloroaniline, p-toluidine, p-toluidine hydrochloride, 4-nitroaniline, p-phenetidine and 1,3-diaminobenzene dihydrochloride), no information was identified to support current exposure to the general population of Canada, therefore risk to human health for these substances is not expected.
Some of the Aromatic Amines in this assessment have effects of concern based on potential carcinogenicity. While available information does not indicate a risk to human health for Canadians at current levels of exposure, there may be a concern if exposures were to increase.
Based on the information presented in this Screening Assessment, it is concluded that the Aromatic Amines evaluated in this assessment do not meet the criteria under paragraph 64(c)of CEPA 1999, as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
Overall Conclusion
It is concluded that the Aromatic Amines evaluated in this assessment do not meet any of the criteria set out in section 64 of CEPA 1999.
1. Introduction
Pursuant to sections 68 or 74 of the Canadian Environmental Protection Act, 1999 (CEPA 1999) (Canada 1999), the Minister of the Environment and the Minister of Health conduct screening assessments of substances to determine whether these substances present or may present a risk to the environment or to human health.
The Substance Groupings Initiative is a key element of the Government of Canada’s Chemicals Management Plan (CMP). The Aromatic Azo and Benzidine-based Substance Grouping consists of 358 substances that were identified as priorities for assessment, as they met the categorization criteria under section 73 of CEPA 1999 and/or were considered as a priority based on human health concerns (Environment Canada and Health Canada 2007). Some substances within this Substance Grouping have been identified by other jurisdictions as a concern due to the potential cleavage of the azo bonds, which can lead to the release of aromatic amines that are known or likely to be carcinogenic.
While many of these substances have common structural features and similar functional uses as dyes or pigments in multiple sectors, significant diversity within the substance group has been taken into account through the establishment of subgroups. Subgrouping based on structural similarities, physical and chemical properties, and common functional uses and applications accounts for variability within this Substance Grouping and allows for subgroup-specific approaches in the conduct of screening assessments. This Screening Assessment considers 16 substances that belong to the Aromatic Amines subgroup. An additional two substances 4,4’-MDA (CAS RN 101-77-9) and MBOCA (CAS RN 101-14-4) were also initially included in the Aromatic Amines subgroup but are not considered in this assessment. 4,4’-MDA is being evaluated as part of the MDI/MDA Grouping initiativeFootnote[2], and a separate assessment process is being followed for MBOCA to better align with international activities on this substance.
Aromatic amines may be produced by cleavage of the azo bond in aromatic azo and benzidine-based substances. Some aromatic amines, commonly referred to as EU22 aromatic aminesFootnote[3], as well as associated azo dyes, are restricted in other countries (EU 2006). Information on the subgrouping approach for the Aromatic Azo and Benzidine-based Substance Grouping under Canada’s CMP, as well as additional background information and regulatory context, is provided in Environment Canada and Health Canada (2013a).
Screening assessments focus on information critical to determining whether substances meet the criteria as set out in section 64 of CEPA 1999, by examining scientific information to develop conclusions by incorporating a weight of evidence approach and precaution.Footnote[4]
This Screening Assessment includes consideration of information on chemical properties, environmental fate, hazards, uses and exposure, including additional information submitted by stakeholders. Relevant data were identified up to October 2013. Empirical data from key studies as well as some results from models were used to reach conclusions. When available and relevant, information presented in assessments from other jurisdictions was considered. The Screening Assessment does not represent an exhaustive review of all available data. Because the high human health effects and ecological hazard of many of these aromatic amines is well established and previously subject to in-depth assessments by other organizations, the intent of this Screening Assessment is to characterize risk at predicted levels of exposure in Canada, based on primary uses. As such, it presents the most critical studies and lines of evidence pertinent to the conclusion.
This Screening Assessment was prepared by staff in the Existing Substances Programs at Health Canada and Environment Canada and incorporates input from other programs within these departments. The ecological and human health portions of this assessment have undergone external written peer review and/or consultation. Comments on the technical portions relevant to the environment were received from Dr. Harold Freeman (North Carolina State University, USA) and Dr. Gisela Umbuzeiro (University of Campinas, Brazil). Comments on the technical portions relevant to human health were received from Dr. David Josephy (University of Guelph, Canada), Dr. Michael Bird (University of Ottawa, Canada) and Dr. Kannan Krishnan (University of Montreal, Canada). Additionally, the draft of this Screening Assessment was subject to a 60-day public comment period. While external comments were taken into consideration, the final content and outcome of the Screening Assessment remain the responsibility of Health Canada and Environment Canada.
The critical information and considerations upon which the Screening Assessment is based are given below.
2. Identity of Substances
This Screening Assessment focuses on 16 substances that belong to the Aromatic Amines subgroup that are part of the Aromatic Azo and Benzidine-based Substance Grouping (Table 2-1). This subgroup is based on structural similarity and includes substances that were identified as postulated azo bond cleavage products of substances included in the Aromatic Azo and Benzidine-based Substance Grouping. For the purpose of this Screening Assessment, the 16 aromatic amines listed in Table 2-1 are collectively referred to as Aromatic Amines.
| CAS RN | DSL name | Common name used in this report |
|---|---|---|
| 88-53-9 | Benzenesulfonic acid, 2-amino-5-chloro-4-methyl- | Red Lake C Amine |
| 90-04-0 | Benzenamine, 2-methoxy- | o-Anisidine |
| 91-59-8 | 2-Naphthalenamine | 2-Naphthylamine |
| 95-51-2 | Benzenamine, 2-chloro- | 2-Chloroaniline |
| 95-53-4 | Benzenamine, 2-methyl- | o-Toluidine |
| 95-76-1 | Benzenamine, 3,4-dichloro- | 3,4-Dichloroaniline |
| 95-80-7 | 1,3-Benzenediamine, 4-methyl- | 2,4-Diaminotoluene |
| 100-01-6 | Benzenamine, 4-nitro- | 4-Nitroaniline |
| 106-47-8 | Benzenamine, 4-chloro- | 4-Chloroaniline |
| 106-49-0 | Benzenamine, 4-methyl- | p-Toluidine |
| 108-45-2 | 1,3-Benzenediamine | 1,3-Diaminobenzene |
| 123-30-8 | Phenol, 4-amino- | p-Aminophenol |
| 156-43-4 | Benzenamine, 4-ethoxy- | p-Phenetidine |
| 540-23-8 | Benzenamine, 4-methyl-, hydrochloride | p-Toluidine hydrochloride |
| 541-69-5 | 1,3-Benzenediamine, dihydrochloride | 1,3-Diaminobenzene dihydrochloride |
| 615-05-4 | 1,3-Benzenediamine, 4-methoxy- | 2,4-Diaminoanisole |
Aromatic amines are organic compounds that contain at least one amino group attached directly to an aryl moiety (Woo and Lai 2012), such as a phenyl or naphthyl group. In order to simplify the analysis and the presentation of the large dataset available for the Aromatic Amines subgroup for the ecological assessment, the substances in the Aromatic Amines subgroup were organized into the seven ecological structural subsets based on functional groups shown in Table 2-2. This further subgrouping does systematicaly indicate that significant differences exist between the seven subsets in terms of physical and chemical properties, persistence, bioaccumulation potential or toxicity.
| Ecological subset | Definition |
|---|---|
| 1 | Methylated and oxy- aromatic amines (one methyl, methoxy or ethoxy functional group; n = 5) |
| 2 | Phenol amines (one hydroxyl functional group; n = 1) |
| 3 | Benzenediamines (two amino functional groups; n = 4) |
| 4 | Chlorinated aromatic amines (one or two chloro functional groups; n = 3) |
| 5 | Nitroanilines (one nitro functional group; n = 1) |
| 6 | Naphthalenamines (one naphthalene functional group; n = 1) |
| 7 | Sulfonic aromatic amines (one sulfonic functional group; n = 1) |
The identities of the individual substances in this Screening Assessment are presented by ecological structural subset in Table 2-3. A list of other chemical names (e.g., trade names) is available from the National Chemical Inventories (NCI 2012).
| Ecological subset | Substance | Description of critical functional groups | Structure | Molecular weight (g/mol) |
|---|---|---|---|---|
| 1 | o-Toluidine | Amino group (1), methyl group (1) (ortho position) |  |
107.2 |
| 1 | p-Toluidine | Amino group (1), methyl group (1) (para position) | 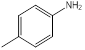 |
107.2 |
| 1 | p-Toluidine hydrochloride | Amino group (1), methyl group (1) (para position) | 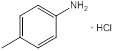 |
143.62 |
| 1 | o-Anisidine | Amino group (1), methoxy group (1) (ortho position) |  |
123.16 |
| 1 | p-Phenetidine | Amino group, ethoxy group (1) (para position) | |
137.18 |
| 2 | p-Aminophenol | Amino group (1), alcohol group (1) (para position) | 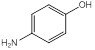 |
109.13 |
| 3 | 1,3-Diaminobenzene | Amino groups (2) |  |
108.14 |
| 3 | 2,4-Diaminotoluene | Amino groups (2), methyl group (1) |  |
122.17 |
| 3 | 1,3-Diaminobenzene dihydrochloride | Amino groups (2) | |
181.06 |
| 3 | 2,4-Diaminoanisole | Amino groups (2), methoxy group (1) | |
138.17 |
| 4 | 2-Chloroaniline | Amino group (1), chloro- group (1) (ortho position) | 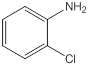 |
127.57 |
| 4 | 4-Chloroaniline | Amino group (1), chloro- group (1) (para position) | 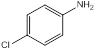 |
127.57 |
| 4 | 3,4-Dichloroaniline | Amino group (1), chloro- groups (2) |  |
162.02 |
| 5 | 4-Nitroaniline | Amino group (1), nitro- group (1) (para position) | |
138.13 |
| 6 | 2-Naphthylamine | Amino group (1), naphthyl group (1) | |
143.19 |
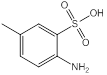
| 7 | Red Lake C Amine | Amino group (1), methyl group (1), chloro- group (1), sulfonic group (1) |  |
221.66 |
2.1 Selection of Analogues and Use of (Q)SAR Models
Guidance on the use of read-across approaches has been prepared by various organizations, such as the Organisation for Economic Co-operation and Development (OECD 2014). It has been applied in various regulatory programs, including the European Union’s (EU) Existing Substances Programme. The general method for analogue selection and the use of (quantitative) structure–activity relationship ((Q)SAR) models is provided in Environment Canada and Health Canada (2013a). For characterization of human health effects, except for characterization of the health effects of 3,4-dichloroaniline and Red Lake C Amine, analogues were not required (see section 7.3 for details). Accordingly, the remaining sections pertain to analogues in the context of the ecological assessment.
Analogues used to inform the ecological assessment were selected based on the availability of relevant empirical data pertaining to physical and chemical properties, persistence, bioaccumulation and ecotoxicity. Such data were used as read-across data for those aromatic amines that lacked empirical data, where appropriate, or to support the weight of evidence of existing empirical information. Although analogue data are used preferentially to fill data gaps for the substances in this assessment, (Q)SAR models were used to generate predictions, and their applicability was evaluated on a case-by-case basis.
Acceptable analogue candidates were identified within the Aromatic Amines subgroup for 2 of the 16 substances. In ecological structural subset 1, p-toluidine is used as an analogue for p-toluidine hydrochloride for physical and chemical properties, persistence, bioaccumulation and ecotoxicity. In ecological structural subset 3, 1,3-diaminobenzene is used as an analogue for 1,3-diaminobenzene dihydrochloride for the same parameters. Both p-toluidine hydrochloride and 1,3-diaminobenzene dihydrochloride are in a crystalline form that behaves as a salt and will release p-toluidine and 1,3-diaminobenzene, respectively, when in solution.
1-Naphthalenamine (Chemical Abstracts Service Registry Number [CAS RN] 134-32-7) was selected as a suitable analogue for 2-naphthylamine to evaluate its persistence and bioaccumulation potential. The two substances are structurally identical, with the only difference residing in the position of the amino functional group on the naphthyl group. The substances 4B Acid (CAS RN 88-44-8) and 2B Acid (CAS RN 88-51-7) were selected as suitable analogues for Red Lake C Amine. 4B Acid is used to evaluate the persistence and ecotoxicity of Red Lake C Amine, while 2B Acid is used to fill data gaps in physical and chemical properties: octanol–water partition coefficient (log Kow) and vapour pressure.
A list of the various analogues used to inform this assessment is presented in Table 2-4, along with an indication of the parameters for which potential read-across data are available.
| Common name (CAS RN) |
Ecological subset | Chemical structure and formula | MW (g/mol) | Parameters to be used in the read-across approach |
|---|---|---|---|---|
| 1-Naphthalenamine (CAS RN 134-32-7) |
Naphthalen-amines (subset #6) |
C10H9N |
143.19 | Degradation, bioaccumulation |
| 4B Acid (CAS RN 88-44-8) |
Sulfonic aromatic amines (subset #7) |
C7H9NO3S |
187.22 | Degradation, ecotoxicity |
| 2B Acid (CAS RN 88-51-7) |
Sulfonic aromatic amines (subset #7) |
C7H8ClNO3S |
221.66 | Log Kow, vapour pressure |
3. Physical and Chemical Properties
Physical and chemical properties determine the overall characteristics of a substance and are used to determine the suitability of different substances for different applications. Such properties also play a critical role in determining the environmental fate of substances (including their potential for long-range transport), as well as their toxicity to humans and non-human organisms.
Several physical and chemical properties--namely, melting point, water solubility, size, log Kow, Vapour pressure, Henry’s Law constant and acid dissociation constant (pKa)--are important in terms of ecological and human health assessment. The experimental and modelled (when appropriate) data on physical and chemical properties relevant to the environmental fate and ecotoxicity of the seven ecological structural subsets of the Aromatic Amines subgroup and their analogues are presented in Environment Canada (2014a). Pivotal values, including either single mean data points (e.g., melting point and decomposition) or a range of values, have been chosen to represent the properties of each ecological structural subset.
Generally, the 16 Aromatic Amines have low molar weights (107.2–221.66 g/mol). These substances are moderately to highly soluble (6.4–238 000 mg/L) in water due to the presence of one or multiple solubilizing functional groups, such as the amino functional group. Most of the 16 Aromatic Amines are weak bases (pKa values of less than 5.5) that will be protonated at low pH but will be found in their neutral form under environmentally relevant pH (7–9). Given their hydrophilicity and ionic character, as demonstrated by low to moderate pKa values, the 16 Aromatic Amines tend to have low to very low experimental log Kow and distribution coefficient (log D) values. However, differences have been identified between the seven ecological structural subsets, as illustrated by the range of log Kow values (-0.89–2.69), with the chlorinated aromatic amines and naphthalenamines ecological structural subsets having the greatest log Kow values. Most Aromatic Amines in this subgroup may be considered non-volatile, as indicated by their low vapour pressure (less than 0.01 Pa) and negligible Henry’s Law constant (less than 0.01 Pa·m3/mol) values. However, a few substances from ecological structural subsets 1 and 4 may be considered semi-volatile to fully volatile, based on their vapour pressures (1.4–53 Pa) and Henry’s Law constants (0.0114–0.25 Pa·m3/mol).
4. Sources and Uses
4.1 Sources
Globally, anthropogenic sources of aromatic amines include biomass and fossil fuel combustion, chemical synthesis, coal gasification plants, aluminum smelting, wastewater treatment plants, drinking water plants, refineries and production facilities, dye houses and chemical factories (Börnick et al. 1996; Jurado-Sanchez et al 2012; Van Aken and Agathos 2002; OECD 2004a; OECD 2005a; ECJRC 2006; ECJRC 2008; API 2011; Ge et al. 2011).
In recent years (2005 to present), all 16 substances in the Aromatic Amines subgroup have been included in surveys issued pursuant to section 71 of CEPA 1999. These surveys collected information on manufacturing and import activities in Canada with a reporting threshold of 100 kg/year (Canada 2006, 2009, 2011). A summary of information collected by these surveys is presented in Table 4-1. None of the substances were manufactured in Canada.
| Ecological structural subset | Substance | 2005 annual import quantity range (kg)Footnote Table 4-1[a] | 2008 annual import quantity range (kg)Footnote Table 4-1[b] | 2010 annual import quantity range (kg)Footnote Table 4-1[c] |
|---|---|---|---|---|
| 1 | o-Toluidine | – | 100–1 000 | – |
| 1 | p-Toluidine | – | 0–100 | – |
| 1 | p-Toluidine hydrochloride | – | – | Not reported |
| 1 | o-Anisidine | – | 0–100 | – |
| 1 | p-Phenetidine | – | 0–1 000 | – |
| 2 | p-Aminophenol | – | 10 000–100 000 | – |
| 3 | 1,3-Diaminobenzene | – | 1 000–10 000 | – |
| 3 | 2,4-Diaminotoluene | – | Not reported | – |
| 3 | 1,3-Diaminobenzene dihydrochloride | Not reported | – | Not reported |
| 3 | 2,4-Diaminoanisole | Not reported | – | Not reported |
| 4 | 2-Chloroaniline | – | Not reported | – |
| 4 | 4-Chloroaniline | 100–1 000 | – | Not reported |
| 4 | 3,4-Dichloroaniline | – | Not reported | – |
| 5 | 4-Nitroaniline | – | – | Not reported |
| 6 | 2-Naphthylamine | 100–1 000 | – | Not reported |
| 7 | Red Lake C Amine | – | – | 1 000–10 000 |
Some Aromatic Amines have been identified as residuals in the manufacturing process of other chemicals, impurities or degradation products of other chemicals or may be formed via reductive cleavage of azo bonds in azo dyes and pigments (Environment Canada and Health Canada 2013a). For example, o-toluidine has been measured in wastewater effluent from a plant in South Korea manufacturing inorganic pigments such as chrome yellow pigment and molybdate red pigment (Jo et al. 2008). o-Toluidine and p-toluidine have been identified as metabolites from the degradation of nitroaromatic explosives by white rot fungi (Van Aken and Agathos 2002). 2,4-Diaminotoluene can be formed by hydrolysis of 2,4-toluene diisocyanate under certain conditions (ECJRC 2008). o-Anisidine and o-toluidine were detected in tattoo inks which were present likely as residuals or breakdown products of azo colourants (CVUA 2011; Hauri 2011; Danish EPA 2012; RAPEX 2012).
4-Chloroaniline and 3,4-dichloroaniline may be found as residues in food from the use of pesticidal products or by chemical biotransformation in environmental media (BUA 1994; Wittke et al. 2001; ECJRC 2006).
In Canada, there is one registered pesticide product containing diflubenzuron (personal communication, email from Pest Management Regulatory Agency [Health Canada] to Existing Substances Risk Assessment Bureau [Health Canada], dated 2015; unreferenced), the residues of which can be metabolized in part to 4-chloroaniline in vivo (US EPA 1997; EFSA 2012).
In Canada, there are no registered uses of propanil (personal communication, email from Pest Management Regulatory Agency [Health Canada] to Existing Substances Risk Assessment Bureau [Health Canada], dated 2014; unreferenced).In the United States, primarily in California and the mid-southern states, propanil is registered as a selective post-emergent use herbicide applied at the 3-4 leaf stage of weeds and is used on approximately 50–70% of the rice grown in the USA (US EPA 2006). Consequently, rice imported from certain regions of the United States may contain propranil residues and its degradation products 3,4-dichloroaniline.
Other potential contributers to environmental 3,4-dichloroaniline in Canada are the registered herbicides diuron (5 end-use products) and linuron (5 end-use products) (personal communication, email from Pest Management Regulatory Agency [Health Canada] to Existing Substances Risk Assessment Bureau [Health Canada], dated 2014; unreferenced), both of which can undergo biotransformation in part to 3,4-dichloroaniline.
Additionally, both 4-chloroaniline and 3,4-dichloroaniline may be found as manufacturing residuals in the antibacterial substance triclocarban (Barber et al. 2006a,b; ECJRC 2006; Halden 2006; CalEPA 2010a). Similarly, 4-chloroaniline can also be found in consumer products containing chlorhexidine as a residual of synthesis and also as a degradation product (IPCS 2003; Zong 2011).
Some Aromatic Amines that have been identified in cigarette smoke include 2-naphthylamine, p-toluidine, o-toluidine and o-anisidine (personal communication, email from Controlled Substances and Tobacco Directorate [Health Canada] to Existing Substances Risk Assessment Bureau [Health Canada], dated 2013; unreferenced; Pieraccini et al. 1992; Luceri et al. 1993; Stabbert et al. 2003; Goniewicz and Czogala 2005; Saha et al. 2009).
4.2 Uses
Information regarding uses of Aromatic Amines was collected from data submitted in response to surveys (Canada 2006, 2009, 2011), internal Health Canada databases and publicly available information.
Overall, no intentional and direct uses were identified for Aromatic Amines in food, food packaging, pharmaceuticals, veterinary drugs, biologics, natural health products or pest control products in Canada (personal communications, emails from Risk Management Bureau [Health Canada] to Existing Substances Risk Assessment Bureau [Health Canada], dated 2011; unreferenced).
Ten Aromatic Amines are included on the List of Prohibited and Restricted Cosmetic Ingredients (more commonly referred to as the Cosmetic Ingredient Hotlist or simply the Hotlist), an administrative tool that Health Canada uses to communicate to manufacturers and others that certain substances, when present in a cosmetic, may contravene the general prohibition found in section 16 of the Food and Drugs Act or a provision of the Cosmetic Regulations (Health Canada 2014a). Aromatic Amines that are on the Cosmetic Ingredient Hotlist are identified in the specific sections below.
Specific uses for each Aromatic Amine are described below.
4.2.1 p-Aminophenol
In Canada, p-aminophenol is used as a cosmetics ingredient and as a laboratory agent, according to publicly available information and data submitted in response to a section 71 survey (Canada 2009; Environment Canada 2009; P&G 2009a-d; P&G 2010ab; P&G 2012; P&G 2013). Based on notifications submitted to Health Canada under the Cosmetic Regulations, this substance was identified as an ingredient in oxidative (permanent) hair dyes, nail polish and body lotions (personal communications, emails from Consumer Product Safety Directorate [Health Canada] to Existing Substances Risk Assessment Bureau [Health Canada], dated 2011 and 2013; unreferenced). Furthermore, p-aminophenol is listed as a component of hair dye products sold in Canada, at a concentration of 0–5% (P&G 2009a-d; P&G 2010ab; P&G 2012; P&G 2013).
Historically, p-aminophenol was widely used as a developer in black-and-white film. Today, p-aminophenol, as a consumer product, is mostly used in oxidative (permanent) hair dyes. In the EU, p-aminophenol is reported as being used as a hair dye precursor at a maximum concentration of 1.8% (SCCS 2011).
4.2.2 o-Anisidine
In Canada, one use of o-anisidine was reported confidentially in response to a section 71 survey (Canada 2009; Environment Canada 2009), which has been taken into consideration in this Screening Assessment.
Based on publicly available information, o-anisidine is used as a chemical intermediate in the production of numerous dyes and pigments (e.g., Direct Red 72, Disperse Orange 29, Direct Yellow 44, Direct Red 24 and Acid Red 4) (IARC 1999). It is also used as an intermediate in the production of pharmaceuticals, including the expectorant guaiacol, which is an active ingredient in products available for sale in Canada (IARC 1999; DPD 2010; NTP 2011a). It can be used as a corrosion inhibitor for steel and as an antioxidant for polymer captan resins (HSDB 1983– ; IARC 1999).
4.2.3 2-Chloroaniline
No information regarding the use of 2-chloroaniline in Canada was submitted in response to a section 71 survey (Canada 2009; Environment Canada 2009). This substance is considered as a prohibited ingredient on Health Canada’s Cosmetic Ingredient Hotlist (under “Aniline (CAS RN 62-53-3), its salts and its halogenated and sulfonated derivatives”).
Based on publicly available information, 2-chloroaniline may be used in laboratory research and commercially as a chemical intermediate in the production of rubber chemicals, dyes, pigment and colouring agents and pesticides (Ullmann’s Encyclopedia 2010), in addition to petroleum solvents and fungicides (Lewis 2001).
4.2.4 4-Chloroaniline
In Canada, 4-chloroaniline was formerly used as a soap and cleaning compound, based on information submitted in response to a section 71 survey (Canada 2006; Environment Canada 2006). This substance is considered as a prohibited ingredient on Health Canada’s Cosmetic Ingredient Hotlist (under “Aniline (CAS RN 62-53-3), its salts and its halogenated and sulfonated derivatives”).
Globally, 4-chloroaniline may be used as a chemical intermediate in the production of dyes (e.g., Vat Red 32), azoic coupling agents, pigments (e.g., Pigment Green 10), pest control products, pharmaceuticals, children’s toys and clothing, cosmetics, soaps, and cleaning products(IPCS 2003).
4-Chloroaniline may be used in the manufacture of chlorhexidine (IPCS 2003). Chlorhexidine and its salt forms (acetate, gluconate and hydrochloride) are broad-spectrum antiseptics used for sterilization, cleaning skin and hands, treating plaque and gingivitis (in certain mouthwash products), and disinfecting wounds and are generally effective against a wide variety of bacteria and yeasts (Environment Canada and Health Canada 2013b). 4-Chloroaniline may be present in products containing chlorhexidine as residual and due to hydrolysis of chlorhexidine during storage (IPCS 2003; Zong 2011).
4-Chloroaniline may be used to synthesize triclocarban, an antimicrobial agent and preservative, and may be present as a residual or following environmental degradation (TCC 2002; IPCS 2003).
4.2.5 3,4-Dichloroaniline
No information regarding the use of 3,4-dichloroaniline in Canada was submitted in response to a section 71 survey (Canada 2009). This substance is considered as a prohibited ingredient on Health Canada’s Cosmetic Ingredient Hotlist (under “Aniline (CAS RN 62-53-3), its salts and its halogenated and sulfonated derivatives”). Based on publicly available information, 3,4-dichloroaniline may be used in laboratory research and commercially as a chemical intermediate in the production of dyes, pigments and pesticides (Lewis 1993). This substance may also be used in the manufacture of triclocarban and may be present as a residual (IPCS 2003).
4.2.6 2,4-Diaminoanisole
No information regarding the use of 2,4-diaminoanisole in Canada was submitted in response to a section 71 survey (Canada 2011; Environment Canada 2012). This substance is considered as a prohibited ingredient on Health Canada’s Cosmetic Ingredient Hotlist (Health Canada 2014a).
Based on publicly available information, 2,4-diaminoanisole is generally used as an intermediate in the manufacture of dyes and pigments (O’Neil 2006). This substance was used extensively in hair dyes and in the dyeing of furs until the late 1970s (IARC 2001).
4.2.7 1,3-Diaminobenzene
In Canada, 1,3-diaminobenzene is used for tire filling, according to data submitted in response to a section 71 survey (Canada 2009; Environment Canada 2009). Based on notifications submitted to Health Canada under the Cosmetic Regulations, this substance was identified as an ingredient in oxidative (permanent) hair dyes, at a maximum concentration of 1% (personal communication, email from Consumer Product Safety Directorate [Health Canada] to Existing Substances Risk Assessment Bureau [Health Canada], dated 2011; unreferenced).
1,3-Diaminobenzene is used globally as a component in the manufacture of engineering polymers, aramid fibres, epoxy resins, wire enamel coatings and polyurea elastomers (DuPont 2011). It can also be used in the production of a large number of commercial dyes that can be used to colour various materials, including textiles, leather, paper and inks (IARC 1978).
4.2.8 1,3-Diaminobenzene dihydrochloride
No information regarding the use of 1,3-diaminobenzene dihydrochloride in Canada was submitted in response to section 71 surveys (Canada 2006, 2011).
Based on publicly available information, this substance is used primarily as an analytical reagent indicator for nitrate (IARC 1978). It is also used in hair dye (IARC 1978).
4.2.9 2,4-Diaminotoluene
No information regarding the use of 2,4-diaminotoluene in Canada was submitted in response to a section 71 survey (Canada 2009). This substance is considered as a prohibited ingredient on Health Canada’s Cosmetic Ingredient Hotlist (Health Canada 2014a).
Globally, 2,4-diaminotoluene is used primarily for the production of 2,4-toluene diisocyanate, which is subsequently used to make polyurethanes. This substance is also an intermediate in the synthesis of dyes (e.g., Direct Oxidation Black, Basic Brown 4, Direct Brown 31, Sulphur Orange 1 and Oxidation Base 20) that can be used for textiles, fur, leather, biological stains and indicators, spirit varnishes and wood stains (US EPA 1986; NTP 2011b). 2,4-Diaminotoluene can also be used in photographic developing and the production of impact resins, polyamides, antioxidants, hydraulic fluids, urethane foams and fungicide stabilizers (US EPA 1986; Layer 2000; NTP 2011b). Historically, this substance was used as a coupler in oxidative hair dyes (IARC 2010a), but became prohibited in hair dyes in the EU in 1983 (European Commission 1983).
4.2.10 2-Naphthylamine
2-Naphthylamine was reported by one company to be imported in a quantity between 100 and 1000 kg in an anthracene/coal tar feedstock for carbon black production in 2005 (Canada 2006); it was recently confirmed that this company no longer uses 2-naphthylamine, and no information regarding the use of this substance in Canada was submitted in response to a recent section 71 survey (Canada 2011). This substance is considered as a prohibited ingredient on Health Canada’s Cosmetic Ingredient Hotlist (Health Canada 2014a). The commercial use and manufacture of 2-naphthylamine were banned in the USA and the EU in the early 1970s and 1998, respectively.
Globally, this substance is now used only in laboratory research (IARC 2010a). Before the above regulatory prohibitions, it was used commercially as an intermediate in the synthesis of dyes and as an antioxidant in the rubber industry (IARC 2010a).
4.2.11 4-Nitroaniline
No information regarding the use of 4-nitroaniline in Canada was submitted in response to a section 71 survey (Canada 2011). Based on publicly available information, 4-nitroaniline is generally used as an intermediate in the manufacture of antioxidants, antiozonants, gas additives, dyes and pigments (NTP 1993; Health Council of the Netherlands 2008).
4.2.12 p-Phenetidine
In Canada, p-phenetidine is used as a laboratory substance and for food and beverage applications, according to data submitted in response to a section 71 survey (Canada 2009; Environment Canada 2009).p-Phenetidine is not present in the Lists of Permitted Food Additives incorporated by reference under their associated Marketing Authorizations, issued under the authority of the Food and Drugs Act (Health Canada 2013b); thus, it is not permitted for direct addition to foods sold in Canada. However, the monograph for ethoxyquin set out in the Food Chemicals Codex (Institute of Medicine [U.S.] 2003) sets a maximum limit of 3.0% for p-phenetidine as an organic impurity in ethoxyquin. Ethoxyquin is on the List of Permitted Food Additives with Other Generally Accepted Uses as a permitted food additive in paprika and ground chili pepper to promote colour retention, at a maximum level of 100 parts per million (ppm) (Health Canada 2013b). As a result, p-phenetidine could be present as an organic impurity in paprika and ground chili pepper at a level of 3 ppm (personal communication, email from Food Directorate [Health Canada] to Risk Management Bureau [Health Canada], dated 2013; unreferenced).
4.2.13 Red Lake C Amine
In Canada, one use of Red Lake C Amine was confidentially reported in response to a section 71 survey (Canada 2011; Environment Canada 2012), which has been taken into consideration in this Screening Assessment. This substance is considered as a prohibited ingredient on Health Canada’s Cosmetic Ingredient Hotlist (under Toluidines [CAS RN 26915-12-8] and Aniline [CAS RN 62-53-3]) (Health Canada 2014a). Based on publicly available information, Red Lake C Amine can be used as a precursor in the synthesis of pigments (Naganuma et al. 1983; Hart et al. 1986).
4.2.14 o-Toluidine
In Canada, o-toluidine is used as a laboratory agent and in tire manufacturing, according to data submitted in response to a section 71 survey (Canada 2009; Environment Canada 2009). This substance is also used in a gas detector kit for military purposes (personal communication, email from Department of National Defence to Risk Management Bureau [Health Canada], dated 2011; unreferenced). o-Toluidine is considered as a prohibited ingredient on Health Canada’s Cosmetic Ingredient Hotlist (under Toluidines [CAS RN 26915-12-8]) (Health Canada 2014a).
Globally, o-toluidine is used as an intermediate primarily in the chemical synthesis of herbicides, rubber chemicals, resin hardeners, dye and pigment intermediates, fungicide intermediates and pharmaceutical intermediates (OECDa 2004).
4.2.15 p-Toluidine and p-toluidine hydrochloride
In Canada, p-toluidine is used as a laboratory agent, according to data submitted in response to a section 71 survey (Canada 2009; Environment Canada 2009), and no commercial use of p-toluidine hydrochloride in Canada was reported. Both substances are considered as prohibited ingredients on Health Canada’s Cosmetic Ingredient Hotlist (under Toluidines [CAS RN 26915-12-8]) (Health Canada 2014a).
Globally, p-toluidine and its hydrochloride salt are used as intermediates in chemical processes, such as the manufacturing of 4B Acid (intermediate for pigments) and of other pigments, dyes, pesticides and pharmaceuticals (OECD 2005a).
4.3 Measured Environmental Concentrations
Aromatic amines may be released to the environment from a variety of anthropogenic sources, as listed previously (see Section 4.1). Little monitoring data was identified for Canada but monitoring data from other countries are available for a number of substances in the subgroup. However, in some cases the sources responsible for the aromatic amine measurements in the environment may not be significant for Canada.
4.3.1 Air
In a region of Turkey where emissions are expected to be high due to solid coal wastes and where coal is used for central heating, p-toluidine was measured in outdoor air samples at concentrations of 2.33 and 5.21 ng/m3 in the summer and winter of 2007, respectively (Akyüz 2007, 2008). During the summer and winter of 2006–2007, p-toluidine, 4-chloroaniline, 2,4-dichloroaniline, 4-nitroaniline, 2-naphthylamine and p-aminophenol were measured in ambient air samples (0.42–2.78 ng/m3) and in airborne particulate samples (0.68–9.54 ng/m3) collected in the same province of Turkey (Akyüz 2008).
p-Aminophenol, o-toluidine and p-toluidine were measured in total suspended atmospheric aerosol particle samples collected in March–May 2011 in Finland at the Station for Measuring Forest Ecosystem-Atmosphere Relations (SMEAR II) at concentrations ranging from non-detected to 0.37 ng/m3 (Ruiz-Jiménez et al. 2012).
2-naphthylamine was measured on the site of an aluminum smelter in Japan (Roussel et al. (1991).
4.3.2 Water and sediment
o-Toluidine has been measured in surface water samples collected in the River Alster (approximately 1 µg/L), the River Au (0.5 µg/L) and the River Pinnau (0.3 µg/L) in Germany in the 1970s (exact date not available) (Neurath et al. 1977). o-Toluidine was detected in a river in Germany (highest concentration of 1.8 µg/L) serving as a sewer for an urban and industrial area with several million inhabitants (OECD 2004a). The substance was also detected occasionally in the rivers Bilina and Elbe in Germany and the Czech Republic downstream of chemical factories located in the Czech Republic (Börnick et al. 1996; OECD 2004a).
p-Aminophenol has been measured in rain water (0.63 µg/L) and river water (4.08 µg/L) near San Luis in Argentina where a number of industrial activities related to food, metallurgy, plastic manufacturing and other chemical manufacturing occur (Stege et al. 2009).
Results of a 1979 monitoring program in the Netherlands revealed the presence of 2-chloroaniline, 4-chloroaniline, 3,4-dichloroaniline, o-toluidine and p-toluidine in the river Rhine and its tributaries (Wegman and De Korte 1981). In fact, 3,4-dichloroaniline was periodically detected in a number of German and Dutch river water samples (n = 20) between 1995 and 1997 at concentrations of up to 0.68 µg/L (ECJRC 2006), and these levels were attributed to 3,4-dichloroaniline production and processing to 3,4-dichlorophenylisocyanate (Rhine), or release during manufacturing and agricultural application of the herbicides linuron and diuron (ECJRC 2006). More recent European studies have reported average 3,4-DCA concentrations from 25 ng/L to 6.8 ug/L in surface waters adjacent to agricultural areas, and were associated with degradation of herbicide precursors (Claver et al. 2006; Silva et al. 2012). The US Geological Survey (USGS) also targeted 3,4-dichloroaniline as part of its water monitoring activities, along with the precursor phenylurea pesticide, diuron. Surface water levels of 68 to 310 ng/L were reported in areas of Georgia and Texas where diuron was known to be used (Thurman et al. 1999; Hladik and Calhoun 2012). Other USGS studies reported 3,4-dichloroaniline concentrations in filtered wastewater effluent ranging from 21 to 280 ng/L (Morace 2012). Concentrations of 3,4-dichloroaniline in wastewater treatment wetlands inflow and outflow were shown to be 150 and 340 ng/L respectively (Barber et al. 2006a). The presence of 3,4-dichloroaniline in wastewater effluent suggests that sources other than phenylurea pesticide precursor degradation may exist. Furthermore, the US EPA reported a high prevalence of 4-chloroaniline in wastewater biosolids (US EPA 2009c), which may be attributed to down-the-drain consumer product uses. While no recent Canadian monitoring data were identified for the 16 aromatic amines, there is uncertainty whether the US experience cited in the studies above are relevant to Canada
In Spain, it has been considered that aromatic amines may be produced during the chlorination of drinking water contaminated by aromatic amines, likely as a result of the use of pesticides in agricultural areas located along the main water source (Jurado-Sánchez et al. 2012). In a Spanish drinking water treatment plant system, the chlorination of raw water containing aniline (maximum average 11 ng/L), 3-chloroaniline (maximum average 2.7 ng/L), 3,4-dichloroaniline (maximum average 3.4 ng/L) and N-nitrosodiethylamine (maximum average 1.5 ng/L) resulted in the production of a series of up to four new aromatic amines, including 4-chloroaniline (maximum average 11 ng/L) (Jurado-Sánchez et al. 2012). In addition, increased concentrations of aniline, 3-chloroaniline and 3,4-dichloroaniline were also observed after chlorination (Jurado-Sánchez et al. 2012). The author notes that the concentrations measured in drinking water may be treated as conservative based on the relatively low quality of the raw water processed at the plant (Jurado-Sánchez et al. 2012). Elsewhere, naphthalenamines, including 2-naphthylamine, have been measured in surface water and sediment in Japan (Hasegawa et al. 1993).
4.3.3 Soil
3,4-Dichloroaniline has been measured in agricultural soils in Germany (ECJRC 2006). Triclocarban can degrade in the soil column to produce both 4-chloroaniline and 3,4-dichloroaniline (Gledhill 1975; Miller et al. 2010; Kwon and Xia 2012).
5. Environmental Fate and Behaviour
The environmental fate of chemicals describes the processes by which chemicals become distributed and are transformed in the environment. In this section, some general characteristics of the substances considered in this Screening Assessment will be discussed with respect to their environmental fate in different compartments in an effort to understand how organisms come into contact with the substances in a particular medium, the persistence of the substances in environmental compartments, and their degradation, distribution among media, migration in groundwater, removal from effluents by standard wastewater treatment methods and bioaccumulation in organisms.
While mass balance fate models such as the Equilibrium Criterion model (EQC 2011) may be used for aromatic amines, these models generally underestimate the binding properties of aromatic amines (ECJRC 2002) and the importance of the fate processes of sorption and desorption to particulate matter, soil and sediment under certain conditions (Chen and Nyman 2009), as explained in Environment Canada and Health Canada (2013a). Therefore, the environmental fate and compartmentalization of these substances will be discussed qualitatively using information on physical and chemical properties.
5.1 Water and Sediment
Aromatic amines are moderately to highly water soluble, indicating that they are likely to be found primarily in the hydrosphere if released to the environment. Low to moderate volatilization from surface waters is expected for the substances in this subgroup based on their Henry’s Law constants (BUA 1992, 1994; ECJRC 2002, 2006; OECD 2004a, OECD 2005a, 2010; US EPA 2009a, b).
Aromatic amines are known to bind to dissolved organic matter (Lee et al. 1993), natural surfaces, such as particulate matter and sediment (Weber et al. 2001; Colon et al. 2002), and likely to sludge from wastewater treatment systems. Sorption of aromatic amines to sediment and presumably other natural surfaces is generally characterized by initial fast removal of the amines in solution followed by a much slower removal rate (Weber et al. 2001; Colon et al. 2002). The sorption mechanisms involved include reversible physical processes, such as the sorption of protonated aromatic amine species through cation exchange, and irreversible processes, such as covalent binding with constituents of the sediment matrix (Weber et al. 2001; Colon et al. 2002). Sorption kinetics of aromatic amines is also influenced by the positions of their functional groups. A study of the sorption of 21 anilines with substituents in the ortho, meta and para positions found that the sorption of ortho-substituted aromatic amines onto sediment was significantly less than the sorption of the meta- and para-substituted aromatic amines (Colon et al. 2002).
Over time, aromatic amines sorbed to particulate matter in the water column are expected to settle out to bed sediments. Aromatic amines irreversibly bound to sediment will gradually be buried.
5.2 Soil
When considering only their moderate to high water solubility and relatively low organic carbon–water partition coefficients (log Koc), aromatic amines could be expected to have low to high mobility in soils (US EPA 2008a, 2009a, b).
However, the log Koc does not take into account chemisorption, and, as seen with sediment, many aromatic amines have been found to bind both reversibly and irreversibly to organic and inorganic components of soil (Graveel et al. 1985; Al-Bashir et al. 1994a; Lee et al. 1997; Cowen et al. 1998; Li and Lee 1999; Li et al. 2000; Donaldson and Nyman 2006). Reversible sorption processes include cation exchange, hydrophobic interactions by London–van der Waals forces, and dipole–dipole or induced-dipole attractions, whereas irreversible processes include covalent binding (notably quinone and phenolic functional groups) and mineral-catalyzed transformation reactions (i.e., involving manganese oxide) (Lee et al. 1997; Li and Lee 1999; Li et al. 2000). As discussed for sediment, binding of aromatic amines to soil is generally biphasic, comprising fast and slower binding phases (Lee et al. 1997). A study on the sorption of 3,4-dichloroaniline on agricultural soils with distinct texture, organic matter content and cation exchange capacities determined that sorption was primarily correlated to organic matter content (Droulia et al. 2011). Soil pH also plays an important role in soil sorption capacity, as increasing soil pHs from 6.2 and 5.3 to 7.8 reduced their sorption capacity by up to 50% (Droulia et al. 2011). 4- chloroaniline was found to be readily bound to soil residues upon release to soil Freitag et al. 1984).
A sorption study conducted with 2,4-diaminotoluene on sandy loam and silt loam under aerobic and anaerobic conditions over a period of 8 hours showed that sorption was initially rapid, yielding sorption coefficients (Kd values) of 11.4–21.3 (Cowen et al. 1998). When normalized to soil organic content, this led to Koc values of 713–1346 (Cowen et al. 1998). Low desorption of 2,4-diaminotoluene was consistent with the formation of complexes with humic materials or other irreversible soil binding processes (Cowen et al. 1998).
Based on the strong binding affinity of aromatic amines in soil and the covalent bonds that may form with organic matter, these substances are expected to be relatively immobile in this medium (BUA 1992; OECD 2004a, 2005a; ECJRC 2006, 2008).
5.3 Air
Aromatic amines may be emitted to the atmosphere by a wide range of anthropogenic and natural sources, such as the ocean, industrial activity, combustion, biomass burning and vegetation (Ge et al. 2011). Once released in the atmosphere, aromatic amines may be subjected to various physical, chemical and photochemical processes and/or interactions (Ge et al. 2011).
Semi-volatile or volatile aromatic amines may exist in the gas phase, where they may react with oxidants, such as hydroxyl radicals (OH-), ozone (O3) and nitrogen oxides (NOx), to form aerosols, as observed with a number of aliphatic amines (Murphy et al. 2007). Reaction rates with hydroxyl radicals are typically the fastest and constitute the most important removal process in the gas phase; reactions with ozone are considered negligible (Ge et al. 2011). The photooxidation of aliphatic amines with nitrogen oxides is relatively slow and may yield N-nitroso compounds (Ge et al. 2011); it is unclear whether this process is of importance to aromatic amines. Finally, a study conducted by Ketseridis et al. (1976), in which α-naphthylamine and anilines, among other compounds, were measured in aerosol samples collected from six geographic locations, including three from Atlantic air masses and three over the European continent, showed fairly constant concentrations at all locations, indicating that these compounds and possibly other aromatic amines might be formed in the atmosphere.
Due to their generally high water solubility, aliphatic amines are expected to dissolve in aqueous aerosols, as hypothesized by Sellegri et al. (2005) for dimethylamine and trimethylamine. Such a behaviour is also expected from substances of the Aromatic Amines subgroup, indicating that aromatic amines dissolved in aqueous aerosols would subsequently be removed from the atmosphere by wet deposition processes. Given their low volatility and physicochemical preference for partitioning to other media, it is not expected that water-soluble aromatic amines will be subject to long-range atmospheric transport.
5.4 Environmental Persistence
To characterize the environmental persistence of substances in the Aromatic Amines subgroup, empirical and modelled data for these substances were considered for both aerobic and anaerobic conditions. In addition, the structural analogues 1-naphthalenamine (CAS RN 134-32-7) and 4B Acid (CAS RN 88-44-8) were considered to inform on the persistence of 2-naphthylamine and Red Lake C, respectively, due to limited empirical datasets.
5.4.1 Abiotic degradation
5.4.1.1 Hydrolysis and autooxidation
None of the Aromatic Amines in this subgroup contains functional groups expected to undergo hydrolysis, as indicated in published reports on these substances (BUA 1992; OECD 2004a, 2005a, 2010; ECJRC 2002, 2006; US EPA 2008a, 2009a, b) and modelled data (HYDROWIN 2010).
p-Aminophenol is relatively unstable in water (Lerner 2011) and may oxidize to polymeric structures (OECD 2010). Half-lives of 7.67 days in purified water at 100 mg/L (pH 7, 25°C) and 7.23 hours in dechlorinated water at 1 mg/L (pH 7.3–7.6, 24°C) are reported (OECD 2010), but the original study was not available for review. Ready oxidation is also reported for unsubstituted phenylenediamines, including 1,3-diaminobenzene (Stahl et al. 1990). Oxidative degradation yields half-lives of 3200 and 8100 hours at initial concentrations of 2.5 and 25 mg/L, respectively (Stahl et al. 1990).
5.4.1.2 Photodegradation
Some of the substances in the Aromatic Amines subgroup are known to be unstable in air. Specifically, p-aminophenol has been observed to be unstable in air and easily undergoes oxidation to pink/purple-coloured products (Mitchell et al. 2004). Naphthalenamines are known to change to a red colour in air, and the photooxidation of 2-naphthylamine adsorbed onto particles yields 2-amino-4-(2′-naphthylimino)-1,4-naphthoquinone and dibenzo[a,h]phenazine (Hasegawa et al. 1993).
In addition, photodegradation in air by hydroxyl radicals has been identified as a significant removal process for substances that may exist in this medium (OECD 2004a, 2005a, 2010; US EPA 2009a, 2009b). Results from AOPWIN (2010) indicate that all substances in the subgroup are expected to have half-lives ranging from 0.053 to 0.795 day, 4-nitroaniline being the substance with the longest half-life. These results indicate that none of the substances in the Aromatic Amines subgroup is expected to persist in the atmosphere. Some aromatic amines, such as 2-chloroaniline, 3-chloroaniline and 3,4-dichloroaniline, may undergo direct photolysis in aqueous solution (Othmen and Boule 1997).
5.4.2 Biotic degradation
Empirical biodegradation data related to the persistence of Aromatic Amines in the aquatic environment are available for most substances. A summary of the empirical biodegradation data available for 13 substances in the subgroup and two analogue substances in aerobic aqueous medium is presented in Table 5-1. More detail is available in Environment Canada (2014b). No empirical data were found for p-toluidine hydrochloride, 1,3-diaminobenzene dihydrochloride or 2,4-diaminoanisole. Empirical data for p-toluidine and 1,3-diaminobenzene will be used as surrogate data for the first two compounds. Empirical data from compounds within ecological subset 3 as well as modelled data will be used to conclude on the biodegradation potential of 2,4-diaminoanisole.
| Ecological structural | CAS RN | Test typeFootnote Table 5-1 [a],Footnote Table 5-1[b] | Number of studiesFootnote Table 5-1 [c] | Degradation range observed (%) | Conclusion |
|---|---|---|---|---|---|
| 1 | 95-53-4 | Ready biodegradation | 4 | 5 – greater than 90 | Readily biodegradable |
| 1 | 95-53-4 | Inherent biodegradation | 5 | 92–97.7 | Inherently biodegradable |
| 1 | 106-49-0 | Ready biodegradation | 2 | 32–68 | Possibly readily biodegradable |
| 1 | 106-49-0 | Inherent biodegradation | 3 | 94–97.7 | Inherently biodegradable |
| 1 | 90-04-0 | Ready biodegradation | 5 | less than 10–86 | Possibly readily biodegradable |
| 1 | 90-04-0 | Inherent biodegradation | 1 | 98 | Inherently biodegradable |
| 1 | 156-43-4 | Ready biodegradation | 3 | 0 – greater than 90 | Possibly readily biodegradable |
| 1 | 156-43-4 | Inherent biodegradation | 2 | 71 – greater than 98 | Inherently biodegradable |
| 2 | 123-30-8 | Ready biodegradation | 2 | 0–6 | Not readily biodegradable |
| 2 | 123-30-8 | Inherent biodegradation | 1 | 87 | Inherently biodegradable |
| 3 | 108-45-2 | Ready biodegradation | 1 | 2 | Not readily biodegradable |
| 3 | 108-45-2 | Inherent biodegradation | 2 | 14–60 | Possibly not inherently biodegradable |
| 3 | 95-80-7 | Ready biodegradation | 4 | 0–52 | Not readily biodegradable |
| 3 | 95-80-7 | Inherent biodegradation | 4 | 49–100 | Inherently biodegradable |
| 4 | 95-51-2 | Ready biodegradation | 2 | 0–2.7 | Not readily biodegradable |
| 4 | 95-51-2 | Inherent biodegradation | 3 | 0–98 | Inherently biodegradable |
| 4 | 106-47-8 | Ready biodegradation | 1 | 0 | Not readily biodegradable |
| 4 | 106-47-8 | Inherent biodegradation | 8 | 0–97 | Inherently biodegradable |
| 4 | 95-76-1 | Ready biodegradation | 2 | 0 | Not readily biodegradable |
| 4 | 95-76-1 | Inherent biodegradation | 2 | 6–82 | Inherently biodegradable |
| 5 | 100-01-6 | Ready biodegradation | 2 | 0–2.9 | Not readily biodegradable |
| 5 | 100-01-6 | Inherent biodegradation | 3 | 0 – greater than 95 | Inherently biodegradable |
| 6 | 91-59-8 | Ready biodegradation | 0 | na | na |
| 6 | 91-59-8 | Inherent biodegradation | 1 | 40–89.6 | Inherently biodegradable |
| 6 | 134-32-7 | Ready biodegradation | 3 | 0–6 | Not readily biodegradable |
| 6 | 134-32-7 | Inherent biodegradation | 2 | 0 – greater than 80 | Inherently biodegradable |
| 7 | 88-53-9 | Ready biodegradation | 1 | 0 | Not readily biodegradable |
| 7 | 88-53-9 | Inherent biodegradation | 3 | 0–15 | Not inherently biodegradable |
| 7 | 88-44-8 | Ready biodegradation | 1 | 0–29 | Not readily biodegradable |
| 7 | 88-44-8 | Inherent biodegradation | 0 | NA | NA |
5.4.2.1 Ecological subset 1: Methylated and oxy-aromatic amines
Most substances from ecological subset 1 may qualify as readily biodegradable, and all are inherently biodegradable. o-Toluidine and p-toluidine are generally considered readily biodegradable and inherently biodegradable (OECD 2004a, 2005a; US EPA 2009a). Two Japanese studies unavailable for review concluded that neither toluidine is readily biodegradable (CHRIP ©2002–2012). However, several other experimental results indicate that o-toluidine and p-toluidine are readily biodegradable (Brown and Laboureur 1983; MITI 1992; European Commission ©2000a; Bayer 2001; OECD 2004a, 2005a; US EPA 2009a) and inherently biodegradable (Pitter 1976; HSDB 1983– ; Matsui et al. 1988; European Commission ©2000a, b; Bayer 2001; US EPA 2009a). A similar conclusion was also reached for m-toluidine (CAS RN 108-44-1) (Bayer 2001; US EPA 2009a). Based on these results, p-toluidine hydrochloride is also considered readily and inherently biodegradable. A toxicity test conducted with p-toluidine on activated sludge yielded a 3-hour median effective concentration (EC50) of 100 mg/L (Yoshioka et al. 1986; OECD 2005a), indicating that the substance may not inhibit biodegradation.
o-Anisidine is considered inherently biodegradable, but it is considered readily biodegradable only under certain conditions (ECJRC 2002). Two tests (ECJRC 2002) show degradation levels above the pass levels of biodegradation of 60% for theoretical oxygen demand according to OECD protocols; two other results are slightly below or oscillate near the pass level (CITI 1992; CHRIP ©2002–2012), while results from another experiment are clearly below the pass level (less than 10%) (Kool 1984). The variation observed with regard to the ready biodegradability status of o-anisidine could mean that ready biodegradation of o-anisidine depends on the inoculum used (ECJRC 2002). However, o-anisidine may not inhibit biodegradation, based on a 3-hour EC50 value of 800 mg/L for activated sludge originating from publicly-owned wastewater treatment system (ECJRC 2002). Another potential explanation is that metabolites produced under specific conditions may be persistent or require some degree of microbial adaptation in order to be degraded. Indeed, modelling results from CPOPs (2012) identified o-aminophenol (CAS RN 95-55-6) as a primary metabolite of o-anisidine. o-Aminophenol is known to undergo a variety of cyclization and condensation reactions (Mitchell et al. 2004) and is easily oxidized to o-benzoquinone (Urano and Kato 1986). Urano and Kato (1986) showed that o-aminophenol was degraded in two steps (possible degradation to o-benzoquinone followed by catechol) but was not readily biodegradable, as confirmed by other test results (European Commission ©2000c). However, ready biodegradability of the analogue p-anisidine (CAS RN 104-94-9) was observed by Brown and Laboureur (1983) in 10 out of 12 experiments. Therefore, while not readily biodegradable, o-anisidine may biodegrade under certain conditions. Empirical biodegradation data available for p-phenetidine (CAS RN 156-43-4) from OECD 301A and 301E test results showed ready biodegradation in 7 out of 12 tests (Brown and Laboureur 1983). Non-ready biodegradation results were associated with protocol OECD 301E, which employs a relatively low concentration of microorganism compared with OECD 301A. Neither o-toluidine nor p-toluidine was found to be degraded under anaerobic conditions in an aquifer slurry over a period of 10 months (HSDB 1983– ; Kuhn and Suflita 1989). Similar results are anticipated for other substances of this ecological structural subset.
5.4.2.2 Ecological subset 2: Phenol amines
Empirical biodegradation data available for p-aminophenol indicate that the substance is inherently biodegradable, but not readily biodegradable. A ready biodegradation test done by the Japanese government showed minimal degradation of p-aminophenol (only 6%) over 28 days; however, all of the substance was transformed into large molecular weight (greater than 1000 g/mol) polymerized structures (OECD 2010). Results from Urano and Kato (1986) showed that p-aminophenol was not biodegraded but oxidized to insoluble p-benzoquinone. As p-aminophenol is known to be unstable and to rapidly oxidize to coloured products in air (Mitchell et al. 2004), it is hypothesized that p-aminophenol is not readily biodegradable in the environment but is instead oxidized to other compounds that may be persistent. Results from Pitter (1976) show that the substance is inherently biodegradable, which indicates that the transformation products might be biodegradable under specific conditions.
5.4.2.3 Ecological subset 3: Benzenediamines
Empirical biodegradation test results available for 2,4-diaminotoluene and 1,3-diaminobenzene are consistent with each other and indicate that the substances are not readily biodegradable but can be considered inherently biodegradable. Results from six ready biodegradability test studies showed a maximum biodegradation rate of 51% over 28 days, whereas the other test results were below 14% for a similar period of time (CITI 1992; ECHA ©2007–2013; ECJRC 2008). Also, 1,3-diaminobenzene was not found to be biodegradable over a period of 20 days in the aerobic treatment stage of a wastewater treatment plant according to OECD protocol 303A (ECHA ©2007–2013). Most other study test results indicate that both substances are inherently biodegradable (Pitter 1976; ECHA ©2007–2013; ECJRC 2008) or biodegradable to a certain extent under specific conditions (Matsui et al. 1988). Similar conclusions are anticipated for 1,3-diaminobenzene dihydrochloride. Modelled results from DS TOPKAT (©2005–2009), CPOPs (2012) and EPI Suite (2012) for 2,4-diaminoanisole all indicate that the substance is unlikely to be biodegradable. Stahl et al. (1990) indicated that phenylenediamines oxidize readily in solution, suggesting that persistent metabolites or metabolites inhibiting biodegradation may be produced.
5.4.2.4 Ecological subset 4: Chlorinated aromatic amines
The abundant empirical data on the persistence of chloroanilines suggest that they are poorly biodegradable (ECJRC 2006; US EPA 2008a). Empirical results from biodegradation studies (n = 5) for all three chloroanilines in this ecological structural subset indicate that o-chloroaniline, p-chloroaniline and 3,4-dichloroaniline cannot be considered readily biodegradable (Rott et al. 1982; CITI 1992; CHRIP ©2002–2012; ECJRC 2006; ECHA ©2007–2013). Many studies conducted with acclimated activated sludge showed limited biodegradation, indicating that chloroanilines may degrade only under specific conditions (Rott et al. 1982; HSDB 1983– ; Lyons et al. 1985; CITI 1992; BUA 1994; European Commission ©2000d; ECJRC 2006; ECHA ©2007–2013). It is noted that biodegradability was observed on limited occasions under specific conditions (Pitter 1976; HSDB 1983– ; BUA 1994; ECHA ©2007–2013). The persistence of chloroanilines is further supported by modelled results from DS TOPKAT (©2005–2009), CPOPs (2012) and EPI Suite (2012), which consider these substances to have low biodegradability. p-Chloroaniline and o-chloroaniline are formed in sediment as a result of 3,4-dichloroaniline biodegradation under anaerobic conditions (ECJRC 2006). Biodegradation of p-chloroaniline and o-chloroaniline under anaerobic conditions is limited, as indicated by a maximum degradation of 34% over a 30-day period (HSDB 1983– ; Govind et al. 1991; ECJRC 2006).
5.4.2.5 Ecological subset 5: Nitroanilines
4-Nitroaniline is not readily biodegradable but may biodegrade under specific conditions.
One biodegradation study showed an absence of degradation over a period of 14 days, indicating that 4-nitroaniline is not readily biodegradable (CITI 1992). Identical results have been observed for its isomers 2-nitroaniline (CAS RN 88-74-4) and 3-nitroaniline (CAS RN 99-09-2). Additional test results indicate that 4-nitroaniline may be inherently biodegradable under specific conditions (European Commission ©2000e), but may also persist (Pitter 1976). The presence of nitro groups around the ring prevents oxidation, rendering nitro aromatic compounds resistant to biodegradation (Symons and Bruce 2006). Therefore, 4-nitroaniline is considered non-biodegradable.
5.4.2.6 Ecological subset 6: Naphthalenamines
2-Naphthylamine is not believed to be readily biodegradable but may be considered inherently biodegradable under specific conditions. There are limited biodegradation data for 2-naphthylamine, but it may be degraded under certain conditions. A biodegradation study using adapted activated sludge inoculum under aerobic conditions showed that 2-naphthylamine could be significantly degraded (40% and 89.6%) over a period of 7 days in a static or a continuous system, respectively (Fochtman and Eisenberg 1979). Biodegradation data for the analogue 1-naphthalenamine (CAS RN 134-32-7) indicate that it is not readily biodegradable, as illustrated by a maximum degradation rate of 6% over 28 days (CITI 1992; European Commission ©2000e). 1-Naphthalenamine may be degraded by adapted aerobic sludge under certain conditions (European Commission ©2000e), but may also be recalcitrant (Pitter 1976). Additionally, 1-naphthalenamine and 2-naphthylamine were 86% and 87% biodegraded, respectively, in flooded soil under aerobic conditions over a 200-day period (Al-Bashir et al. 1994b).
5.4.2.7 Ecological subset 7: Sulfonic aromatic amines
Empirical biodegradation data available for Red Lake C Amine show that the substance is poorly biodegradable. Red Lake C Amine and its analogue 4B Acid are not readily biodegradable, as indicated by a maximum degradation rate of 0% over a period of 28 days (CITI 1992; European Commission ©2000f) and 29% over a period of 14 days (CHRIP ©2002–2012). Other test results of studies that investigated the inherent biodegradability of Red Lake C Amine also found little degradation (less than or equal to 15%) (European Commission ©2000f).
5.4.3 Summary of persistence in the environment
Generally, there is much variability in the empirical biodegradation data available for the Aromatic Amines in the subgroup. The results discussed previously indicate that biodegradation of these Aromatic Amines is mostly related to the type of inoculum used, suggesting that bacterial inhibition, not chemical structure, would be the cause of the low biodegradability observed in some tests with some substances. With the exception of substances from ecological subset 1, which may readily biodegrade, substances from ecological subsets 2, 3, 4, 5, 6 and 7 are moderately or poorly biodegradable. Therefore, it is expected that these substances may have relatively long residence times in water until sorption processes with dissolved organic matter, particulate matter or other surfaces take place. Due to their moderate to high water solubility and the fact that these substances could stay in the water medium for long periods of time, they may disperse widely. Eventually, due to electrostatic interactions with particulate matter, they may also disperse widely in sediment. In sediment and soil, biodegradation is also expected to be slow under aerobic and anaerobic conditions and will be further slowed by sorption processes. Short residence times in air are expected to result in low potential for long-range atmospheric transport.
5.5 Potential for bioaccumulation
In this assessment, a variety of lines of evidence have been used to determine the bioaccumulation potential of Aromatic Amines. The number of experimental data for traditional bioaccumulation metrics such as bioconcentration factor (BCF) is moderate. In addition, the use of (Q)SAR bioaccumulation modelling was pursued for Aromatic Amines.
5.5.1 Octanol–water partition coefficient
Aromatic Amines have relatively high water solubility (6.4–238 000 mg/L), and the limited number of experimental data for these substances indicate low log Kow values (−0.89 to 2.69), which would suggest a very low bioaccumulation potential, according to equilibrium partitioning theory (BUA 1992, 1994; ECJRC 2002, 2006, 2008; OECD 2004a, 2005a, 2010; CPMA 2006 ; US EPA 2008a, 2009a, b).
5.5.2 Aquatic bioconcentration factor (BCF)
Empirical bioconcentration studies are available for a number of Aromatic Amines in this subgroup--namely, p-toluidine, p-aminophenol, 1,3-diaminobenzene, 2,4-diaminotoluene, 2-chloroaniline, 4-chloroaniline, 3,4-dichloroaniline and 4-nitroaniline--and the analogue substance 1-naphthalenamine (Korte 1978; Geyer et al. 1984; Kalsch et al. 1991; Zok et al. 1991; Tsuda et al. 1993; de Wolf et al. 1994; NITE 2000). Reported BCFs were between 0.8 and 226 L/kg for fish (Table 5-2), whereas for algae, the reported BCFs for 4-chloroaniline were between 260 and 1200, thus indicating that these substances are not likely to bioconcentrate in aquatic organisms. Experimental BCF values and additional details are provided in Environment Canada (2014c). No experimental BCF data were available for the sulfonic aromatic amine ecological structural subset 7; therefore, modelled data were generated, as (Q)SAR models were deemed acceptable for these substances based on their simple chemical structures. Additional details are available in Environment Canada (2014c).
| Ecological structural subset | Test organism | Experimental concentration range (mg/L) | BCF range (L/kg) |
|---|---|---|---|
| 1 | Fish | 0.01–0.1 | less than 1.3–13 (n = 2) |
| 2 | Fish | 0.000 15–0.015 | 39–46 (n = 2) |
| 3 | Fish | 0.001–2 | 4.6–91 (n = 5) |
| 4 | Fish | 0.000 2–25 | 0.8–226 (n = 22) |
| 4 | Algae | 0.05 | 260–1 200 (n = 2) |
| 5 | Fish | 0.000 2–0.5 | 3.6–10 (n = 4) |
| 6 | Fish | 0.02–0.2 | 9.1–54 (n = 2) |
| 7 | NA | NA | NA |
A study conducted by Zok et al. (1991) on nine anilines, including 2-chloroaniline, 4-chloroaniline, 3,4-dichloroaniline and 4-nitroaniline, determined that all compounds, with the exception of 4-nitroaniline, were metabolized and transformed to acetanilides. Biotransformation of meta-substituted anilines was faster than that of ortho- and para-substituted anilines (Zok et al. 1991). Similar transformation to the corresponding acetanilide was observed for 4-chloroaniline in a study by de Wolf et al. (1994).
5.5.3 Modelling BCF and BAF
The BCF and bioaccumulation factor (BAF) of substances in the Aromatic Amines subgroup were estimated using both structure-based models and a three trophic level kinetic mass balance model. BCF and BAF modelling results for the Aromatic Amines subgroup are provided in Environment Canada (2014c). BCFs obtained from the linear regression model ranged between 2.818 and 27.542 L/kg. BCFs obtained from the mass balance model ranged between 0.935 and 19.14 L/kg, whereas the BCFs corrected for mitigating factors ranged between 2.355 and 56.156 L/kg. The modelled data are consistent with the empirical data for BCFs (Environment Canada 2014c). The chlorinated amines (more specifically, 3,4-dichloroaniline), which have higher log Kows than the other substances in the subgroup, are responsible for the upper range limits in both the modelled and empirical BCFs.
At a log Kow of 2.7, the predicted bioavailable fraction of substances in the Aromatic Amines subgroup in the water column (excluding loss from volatilization), according to mass balance fish models, is 100%, which suggests that uptake from water via the gills is a very relevant exposure for substances in the Aromatic Amines subgroup. It also suggests that the dietary uptake of these substances does not contribute a significant proportion to the overall uptake of these chemicals when both water and diet are taken into account in determining the BAF. Hence, BCF and BAF values obtained from BCFBAF Sub-models 2 and 3 (BCFBAF 2010) for middle trophic level fish are identical (Environment Canada 2014c).
5.5.4 Other factors for assessing bioaccumulation potential
Due to the limited empirical bioaccumulation data available for Aromatic Amines, available data on water solubility, molecular weight and cross-sectional diameter are considered in order to determine bioaccumulation potential. Given their moderate to high water solubility, ionic nature and high degree of dissociation under typical environmental conditions, the lipid partitioning tendency of these substances is expected to be limited. Also, bioaccumulation data resulting from exposures of organisms to these substances in soil and sediment are minimal and limited, in large part due to the moderate to high water solubility of these substances (Environment Canada and Health Canada 2013a).
In general, the Aromatic Amines in this subgroup are relatively hydrophilic, medium-sized molecules with intermediate molecular weight (107.2–221.66 g/mol). The minimum and maximum cross-sectional diameters for Aromatic Amines range from 0.805 to 1.325 nm. These characteristics suggest that molecular dimensions may also restrict the rate of uptake of these substances when crossing cell membranes in fish from water, thereby reducing their bioaccumulation potential (CPOPs 2012).
5.5.5 Summary of bioaccumulation potential
The 16 Aromatic Amines are expected to have a low bioaccumulation potential, based on their low observed bioconcentration in empirical tests, supported by their physical and chemical properties (i.e., low log Kow, ionized at relevant environmental pH, intermediate molecular weight, large cross-sectional diameters and moderate to high water solubility). The low potential for bioaccumulation of these substances suggests that there may also be low potential for internal concentrations in organisms to reach levels that could cause adverse effects. Potential for adverse effects is discussed further in the following section.
6. Potential to Cause Ecological Harm
6.1 Ecological Effects Assessment
In order to provide the best possible weight of evidence for assessing the ecological effects of substances in the Aromatic Amines subgroup, empirical data were considered, as well as modelled data as appropriate. Generally, a large number of acute and/or chronic toxicity data were available for multiple trophic levels (fish, invertebrates and algae) in the aquatic environment for most substances in the subgroup. Empirical toxicity data for sediment were limited to 4-chloroaniline and 3,4-dichloroaniline. Empirical soil toxicity data were identified for o-toluidine, 2-chloroaniline, 4-chloroaniline, 3,4-dichloroaniline and 4-nitroaniline.
Ecotoxicity value predictions generated using (Q)SAR models were also considered in the weight of evidence for all substances; however, only the predictions generated for substances without empirical data or missing endpoints (i.e., chronic toxicity) are presented in this report to fill data gaps.
6.1.1 Empirical studies for the aquatic compartment
Numerous aquatic toxicity studies are available for all substances in the Aromatic Amines subgroup, with the exception of p-toluidine hydrochloride, 1,3-diaminobenzene dihydrochloride and 2,4-diaminoanisole. Empirical values from p-toluidine and 1,3-diaminobenzene are used as analogue data for p-toluidine hydrochloride and 1,3-diaminobenzene dihydrochloride, respectively. Modelled and empirical data from the other benzenediamine substances, 1,3-diaminobenzene and 2,4-diaminotoluene, are used to characterize the ecotoxicity of 2,4-diaminoanisole. Empirical acute and chronic toxicity data for invertebrates and algae from the structural and functional analogue substance 4B Acid are used to fill data gaps for Red Lake C Amine. Additionally, modelled data are used to fill data gaps for a number of substances for which fish and/or invertebrate empirical chronic toxicity data are not available. The most sensitive empirical acute and chronic toxicity endpoints identified in available studies and reports, as well as modelled data used to fill data gaps for all trophic levels, are presented in Appendix A empirical and modelled toxicity dataset consulted for this subgroup is available in Environment Canada (2014d).
According to Verhaar et al. (1992), the 16 substances in the Aromatic Amines subgroup broadly classify as less inert compounds or polar narcotic chemicals (i.e., class 2) sharing a common mode of action via polar narcosis. Polar narcosis occurs as a result of hydrophobic (van der Waals) interactions and hydrogen bonding, which affect membrane constituents, causing a general and reversible disruption of cell membrane functioning (Nendza and Wenzel 2006; Newman and Clements 2008). Polar narcosis is dependent on hydrophobicity (log Kow) and the polarity of the functional groups. However, compounds within a chemical class can be associated with several modes of action within a species or may act by the same mode of action in some organisms but by dissimilar modes in others (Bradbury 1995; Nendza and Wenzel 2006). Toxicity data for Pseudokirchneriella subcapitata and Vibrio fischeri for 28 aromatic amines, including 2-chloroaniline, 4-chloroaniline, 3,4-dichloroaniline, o-toluidine and p-toluidine, were observed to depend on characteristics other than simply log Kow, implying the presence of other modes of action (Aruoja et al. 2011). In fathead minnow, some aromatic amines have been associated with non-polar narcosis, polar narcosis and oxidative phosphorylation uncoupling (Bradbury 1995), whereas 3,4-dichloroaniline has been classified as a non-polar narcotic (Russom et al. 1997). In vitro assays on baker’s yeast (Saccharomyces cerevisiae) determined that 4-chloroaniline potentially had an uncoupling of oxidative phosphorylation / inhibition of respiratory electron transport chain mode of action (Nendza and Wenzel 2006). In Daphnia magna, aromatic nucleophilic substitution was identified as a second mode of action in addition to polar narcosis for o-anisidine, o-toluidine, p-toluidine, 2-chloroaniline, 4-chloroaniline, 3,4-dichloroaniline and 2-naphthylamine (Zhang et al.2013). Another reactive mode of action (electrophilicity) was identified for 1,3-diaminobenzene, 2,4-diaminotoluene and p-aminophenol in Daphnia magna, involving irreversible covalent bonding with protein through Michael addition (Enoch et al. 2011; Zhang et al. 2013). This mode of action was also idenfied for p-aminophenol in another trophic level in Tetrahymena pyriformis (Cronin et al. 2002; Zhang et al. 2013), indicating that it may be the predominant mode of action for this substance in most trophic levels. These chemicals may be metabolically or abiotically transformed through oxidation to reactive quinone or quinone imine (Enoch et al. 2008; Schwöbel et al. 2011; Zhang et al. 2013).
While exceptions exist for some species, as previously indicated, the Aromatic Amines in this subgroup may be grouped into two main chemical classes: polar narcotics or reactive chemicals. p-Aminophenol is the lone reactive chemical for multiple trophic levels, whereas all the other substances qualify as polar narcotics whose toxicity is mostly driven by lipophilicity and log Kow. Therefore, while the empirical and modelled toxicity data gathered for the Aromatic Amines subgroup are presented by ecological structural subset, toxicity may not significantly differ between subsets, depending on the mode of action.
The toxicity of substituted aniline compounds is also dependent on the type of substituents (chloro-, methyl-, etc.), the number of substituents (mono-, di-, etc.) and their position (ortho-, meta-, para-) (Stahl et al. 1990; Sun et al. 2004; Aruoja et al. 2011). The relative position of radicals on the benzene ring modifies toxicity to aquatic species, but the mechanism responsible for the difference has not been determined (Sun et al. 2004). The presence of multiple substituents, however, is also known to cause steric hindrance, which may decrease the reactivity of chemical substances. Most Aromatic Amines in this subgroup are suspected or confirmed carcinogens to mammals (BUA 1992, 1994; ECJRC 2002, 2006; OECD 2004a, 2005a, 2010); in some cases, genotoxic effects have been observed in fish (ECJRC 2006). The toxicity of substituted anilines to invertebrates and fish may be reduced by binding and sorption to dissolved or particulate matter. Indeed, the toxicity of 4-chloroaniline to zebrafish has been shown to be significantly reduced by the presence of dissolved humic materials, likely through binding and adsorption (Lee et al. 1993).
6.1.1.1 Ecological subset 1: Methylated and oxy- aromatic amines
As expected, based on their similar mode of action by polar narcosis and similar log Kows (0.979–1.65), the available empirical data for the five substances constituting this subset are fairly consistent across all three trophic levels. The two methylated amines, p-toluidine and o-toluidine, exhibit slightly more toxicity to fish and invertebrates than o-anisidine and p-phenetidine because of their higher lipophilicity. The substances are moderately toxic to fish based on the acute and chronic toxicity values for o-toluidine (96-hour no-observed-effect concentration [NOEC] = 31.6 mg/L; 21-day NOEC = 12.5 mg/L) (CHRIP ©2002–2012; OECD 2004a), p-toluidine (48-hour median lethal concentration [LC50] = 42 mg/L; 30-day NOEC = 0.6; 30-day lowest-observed-effect concentration [LOEC] = 1.2 mg/L) (Tonogai et al. 1982; CHRIP ©2002–2012), o-anisidine (96-hour LC50 = 200 mg/L; 14-day LC50 = 165 mg/L) (Canton et al. 1985; CHRIP ©2002–2012) and p-phenetidine (48-hour LC0 = 20 mg/L; 14-day LC50 = 35.3 mg/L) (European Commisssion ©2000g; ECOSAR 2012). It is noted that ECOSAR (2012) chronic values for fish (ChV) are predicted to be low for all four compounds (ChV = 0.087–0.22 mg/L). The substances are slightly more toxic to invertebrates, based on acute 48-hour LC50 values of 0.12 mg/L and 0.52 mg/L for p-toluidine and o-toluidine, respectively (OECD 2004a, 2005a), a 48-hour EC50 of 6.8 mg/L for o-anisidine (Canton et al. 1985) and a 24-hour EC50 of 170 mg/L for p-phenetidine (OECD 1999). Chronic toxicity values for p-toluidine (21-day EC50 = 0.021) and o-toluidine (21-day EC50 = 0.066 mg/L) (CHRIP ©2002–2012) in invertebrates are approximately one order of magnitude above toxicity values for o-anisidine (21-day NOEC = 0.25 mg/L, but 21-day EC50 = 1.3 mg/L) (ECJRC 2002) and p-phenetidine (21-day LOEC = 0.6 mg/L) (OECD 1999). None of the data available suggests that any of these four compounds is highly toxic to algae, with the exception of a low 14-day EC50 value of 0.203 mg/L identified for p-toluidine (Gaur 1988).
6.1.1.2 Ecological subset 2: Phenol amines
As indicated previously, p-aminophenol is classified as a reactive chemical with a mode of action by electrophilicity in a number of species (Cronin et al. 2002; Enoch et al. 2011; Zhang et al. 2013). This reactive mode of action is supported by the empirical toxicity data indicating that p-aminophenol is highly toxic to fish, invertebrates and algae, despite its low log Kow values (i.e., −0.09 to 0.04). The high aquatic toxicity of this compound may be explained by the metabolism or oxidation of p-aminophenol to toxic quinones (Cronin et al. 2002; Zhang et al. 2013). Low short-term acute toxicity values (96-hour LC50 = 0.93 mg/L; 2-hour LC20 = 0.06 mg/L) (Holdway et al. 1991; CHRIP ©2002–2012) and long-term chronic values (30-day NOEC = 0.064 mg/L; 30-day LOEC = 0.13 mg/L) (CHRIP ©2002–2012) are observed for fish. Similar results are observed for invertebrates for acute toxicity (48-hour EC50s = 0.098, 0.24 mg/L) (Kühn et al. 1989; OECD 2010) and chronic toxicity (21-day NOEC = 0.055 mg/L; 21-day EC50 = greater than 0.21 mg/L) (CHRIP ©2002–2012). Low toxicity values are also observed for algae (72-hour EC50 = 0.1, 0.17 mg/L) (CHRIP ©2002–2012). p-Aminophenol was found to inhibit cell number, chlorophyll a, total carbohydrate production, 14CO2 uptake and nitrate reductase and nitrogenase activities in Chlorella vulgaris, Nostoc linckia and Nostoc muscorumat a concentration of 2 mg/L (Megharaj et al. 1991; DeLorenzo et al. 2001). In addition to its elevated toxicity to fish and invertebrates, p-aminophenol may also be mutagenic and teratogenic (Yoshida et al. 1998; Sun et al. 2004). p-Aminophenol was observed to induce deoxyribonucleic acid (DNA) damage in kidney cells of carp (Cyprinus carpio) that were exposed for 5 days at a low concentration (0.008 mg/L) using the comet assay (Sun et al. 2004). A proposed mechanism could involve oxidative metabolism and subsequent conjugation with glutathione (Sun et al. 2004).
6.1.1.3 Ecological subset 3: Benzenediamines
As previously indicated, two benzenediamines in the subgroup--namely, 1,3-diaminobenzene and 2,4-diaminotoluene--have been classified as reactive chemicals with a mode of action by electrophilicity in Daphnia magna (Zhang et al. 2013). With regard to invertebrates, all three benzenediamines showed moderate acute toxicity (48-hour EC50 greater than or equal to 1.6 mg/L) (CHRIP ©2002–2012; ECJRC 2008; ECOSAR 2012), but elevated chronic toxicity. The most sensitive chronic values for all three substances were below 1 mg/L for 1,3-diaminobenzene (21-day EC50 = 0.62 mg/L), 2,4-diaminotoluene (21-day EC50 = 0.62 mg/L) and 2,4-diaminoanisole (ChV = 0.072 mg/L) (CHRIP ©2002–2012; ECOSAR 2012). For fish, the toxicity data available for all three benzenediamines showed much lower toxicity, potentially indicating another mode of action for this trophic level. Indeed, empirical and modelled toxicity values for 1,3-diaminobenzene (96-hour LC50 = 1618 mg/L; 96-hour LC50 greater than 100 mg/L) (Stahl et al. 1990; CHRIP ©2002–2012), 2,4-diaminotoluene (96-hour LC50 = 219 mg/L) (ECJRC 2008) and 2,4-diaminoanisole (96-hour LC50 = 1500–1800 mg/L) (DS TOPKAT ©2005–2009; ECOSAR 2012) for fish are all well above 100 mg/L. Modelled chronic toxicity values using ECOSAR (2012) also indicate low toxicity of these compounds, as indicated by the ChV values for 1,3-diaminobenzene (162.3 mg/L), 2,4-diaminotoluene (69.6 mg/L) and 2,4-diaminoanisole (179.7 mg/L). It is noted that other isomers of 1,3-diaminobenzene, such as 1,2-diaminobenzene (CAS RN 95-54-2) and especially 1,4-diaminobenzene (CAS RN 106-50-3), are more reactive and exhibit greater toxicity to fish, daphnids and algae than 1,3-diaminobenzene, as they are more susceptible to undergo autooxidation to form reactive compounds (Stahl et al. 1990; Zhang et al. 2013). The three benzenediamines are moderately toxic to algae, based on empirical toxicity values greater than 1 mg/L for 1,3-diaminobenzene (96-hour EC50 = 2.4 mg/L) and 2,4-diaminotoluene (96-hour EC50 = 9.54 mg/L) (Dodard et al. 1999; CHRIP ©2002–2012). A 96-hour EC50 value of 2.93 mg/L for 2,4-diaminoanisole for algae is consistent with the modelled data (ECOSAR 2012). However, chronic toxicity values predicted for all three compounds for algae are indicative of much higher toxicity (ChV = 0.045–0.058 mg/L) (ECOSAR 2012).
6.1.1.4 Ecological subset 4: Chlorinated aromatic amines
2-Chloroaniline, 4-chloroaniline and 3,4-dichloroaniline are mainly considered polar narcotics, whose toxicity is mainly linked to their log Kow values. However, aromatic nucleophilic substitution was also identified as a second mode of action in Daphnia magna (Zhang et al. 2013). The empirical toxicity data available for 2-chloroaniline indicate that the substance may be considered moderately toxic to fish (acute 96-hour EC50 = 3.2 mg/L; chronic 30-day LOEC = 3.9 mg/L) (Canton et al. 1985; CHRIP ©2002–2012) and algae (72-hour EC10 = 6 mg/L) (Kühn and Pattard 1990), but highly toxic to invertebrates (acute 48-hour EC50 = 0.46 mg/L; chronic 21-day EC50 = 0.043 mg/L) (Canton et al. 1985; CHRIP ©2002–2012). The substance 4-chloroaniline, in contrast, may be considered highly toxic to fish (acute 96-hour LC50 = 2.4 mg/L; chronic 56-day LOEC = 1 mg/L) (Julin and Sanders 1978; Bresch 1991) and algae (96-hour EC10 = 0.4 mg/L) (Geyer et al. 1984) and very highly toxic to invertebrates (acute 48-hour LC50 = 0.05 mg/L; chronic 21-day NOEC = 0.0032 mg/L; 21-day EC50 = 0.01 mg/L) (ECOTOX 2000; CHRIP ©2002–2012). Zebrafish embryos exposed to 4-chloroaniline showed abnormal development and pigmentation after exposure to concentrations above 5 mg/L (Burkhardt-Holm et al. 1999). Temporary alterations in liver and gill cells of embryonic and larval zebrafish were observed following exposure to 4-chloroaniline of 0.05 and 0.5 mg/L, respectively; disturbance of respiration in erythrocytes was identified as an additional mode of action (Burkhardt-Holm et al. 1999). 3,4-Dichloroaniline may be considered very highly toxic to fish (acute 0.8-day LOEC = 0.5 mg/L; chronic 42-day LOEC = 0.002 mg/L) (Schäfers and Nagel 1991; Hendriks and Stouten 1993) and algae (21-day EC50 = 0.003 mg/L) (Jak et al. 1998), and very highly toxic to invertebrates (acute 3-day LC50 = 0.0146 mg/L; chronic 21-day EC50 = 0.0004 mg/L) (Jak et al. 1998; Barata and Baird 2000). 3,4-Dichloroaniline was shown to significantly affect reproduction of Ceriodaphnia dubia (Rose et al. 2002). 3,4-Dichloroaniline also causes endocrine effects in fish (ECJRC 2006).
6.1.1.5 Ecological subset 5: Nitroanilines
An isomer to 4-nitroaniline, 3-nitroaniline (CAS RN 99-09-2), was determined to be a polar narcotic in Daphnia magna, with nucleophilic substitution identified as a second mode of action (Zhang et al. 2013). Similar modes of action are also anticipated for 4-nitroaniline. Acute empirical toxicity data indicate that 4-nitroaniline is moderately toxic to fish (96-hour NOEC = 10 mg/L; 96-hour LC50 = 45 mg/L) and invertebrates (48-hour NOEC = 10 mg/L; 48-hour EC50 = 20 mg/L) (Solutia Inc., 2004). The chronic toxicity value (ChV) for invertebrates (0.03 mg/L) (ECOSAR 2012) indicates that 4-nitroaniline may be considered highly toxic under chronic exposure conditions. Modelled chronic data for fish show a ChV of 0.144 mg/L (ECOSAR 2012), indicating high toxicity to fish if exposed to this substance chronically. Finally, 4-nitroaniline may be considered moderately toxic to algae (72-hour NOEC = 0.94 mg/L; 72-hour EC50 = 43 mg/L) (CHRIP ©2002–2012).
6.1.1.6 Ecological subset 6: Naphthalenamines
2-Naphthylamine is a polar narcotic in Daphnia magna,with nucleophilic substitution identified as a second mode of action (Zhang et al. 2013). 2-Naphthylamine shows a moderate acute toxicity to fish (96-hour LC50 = 3.9 mg/L) (CHRIP ©2002–2012). While no empirical data exist regarding the chronic toxicity of 2-naphthylamine to fish, chronic fish toxicity values from (Q)SAR model ECOSAR (2012) (ChV = 0.041 mg/L) indicate that the substance may be highly toxic to fish if chronic exposure to the substance occurs. 2-Naphthylamine is also expected to be highly toxic to invertebrates, based on acute (48-hour EC50 = 0.84 mg/L) and chronic (21-day EC50 = 0.029 mg/L) exposure to the substance (CHRIP ©2002–2012). A similar conclusion is also reached for algae, based on a 72-hour EC50 of 0.43–0.5 mg/L (CHRIP ©2002–2012).
6.1.1.7 Ecological subset 7: Sulfonic aromatic amines
No mode of action was identified in the literature for Red Lake C Amine. Empirical toxicity data for Red Lake C Amine are limited. The available data indicate that the substance has low acute toxicity to fish (96-hour LC50 = 6350 mg/L) (European Commission ©2000f). Empirical data for the analogue substance Acid 4B indicate moderate acute and chronic toxicity to invertebrates (48-hour EC50 = greater than 10 mg/L; 21-day NOEC = 3.2 mg/L) and moderate to low toxicity to algae (72-hour NOEC = 10 mg/L) (CHRIP ©2002–2012).
6.1.2 Empirical toxicity data for other environmental compartments
Sediment and soil toxicity data are available for a number of substances in the Aromatic Amines subgroup. Toxicological data for sediment-dwelling organisms have only been identified for 4-chloroaniline and 3,4-dichloroaniline (see Table 6-1).
| Ecologicalsubset | CAS RN | Organism type | Type of test (duration) | Endpoint and value (mg/L) | Reference |
|---|---|---|---|---|---|
| 4 | 95-76-1 | Invertebrate (fly, Chironomus riparius) | Acute (96 h) | LC50 = 0.004 mg/L | ECOTOX 2000 |
| 4 | 95-76-1 | Invertebrate (worm, Tubifex tubifex) | Acute (48 h) | LC50 = 11 mg/L | ECJRC 2006 |
| 4 | 95-76-1 | Invertebrate (worm, Lumbriculus variegatus) | Chronic (14 d) | NOEC = 5 mg/kg | Oetken et al. 2000 |
| 4 | 95-76-1 | Invertebrate (fly, Baetis rhodani) | Chronic (28 d) | LOEC = 0.07 mg/L | Girling et al. 2000 |
| 4 | 106-47-8 | Invertebrate (Chironomus plumosus) | Acute (48 h) | EC50 = 43 mg/L | Julin and Sanders 1978 |
Soil toxicity studies are available for p-toluidine (CAS RN 106-49-0), 2-chloroaniline (CAS RN 95-51-2), 4-chloroaniline (CAS RN 106-47-8), 3,4-dichloroaniline (CAS RN 95-76-1) and 4-nitroaniline (CAS RN 100-01-6). Substance specific endpoints are reported in Table 6-2.
Results of acute and chronic toxicity tests on invertebrates or plants indicate that all five substances have moderate to low toxicity.
| Ecological Subset | CAS RN | Organism type (species) | Type of test (duration) | Endpoint and value (mg/L) | Reference |
|---|---|---|---|---|---|
| 1 | 106-49-0 | Plant (Chinese cabbage) | Acute (5 d) | EC50 = 102.2 | Feng et al. 1996 |
| 4 | 95-76-1 | Plant (Chinese cabbage, Brassica rapa var. amplexicaulis) | Acute (5 d) | EC50 =14.1 mg/L | Feng et al. 1996 |
| 4 | 95-76-1 | Plant (lettuce, Lactuca sativa) | Acute (7 d) | NOEC = 1 mg/kg | ECOTOX 2000 |
| 4 | 95-76-1 | Invertebrate (worm, Eisenia fetida ssp. andrei) | Chronic (14 d) | EC50 = 130 mg/kg | Van Gestel and Van Dis 1988 |
| 4 | 95-76-1 | Plant (lettuce, Lactuca sativa) | Chronic (14 d) | NOEC = 1 mg/kg | ECOTOX 2000 |
| 4 | 95-76-1 | Plant (lettuce, Lactuca sativa) | Chronic (14 d) | EC50 = 10 mg/kg | Hulzebos et al. 1993 |
| 4 | 106-47-8 | Invertebrate (Eisenia fetida) | Chronic (28 d) | LC50 = 540 mg/kg | ECOTOX 2000 |
| 4 | 106-47-8 | Invertebrate (Eisenia fetida) | Chronic (14 d) | LC50 = 180 mg/kg | ECOTOX 2000 |
| 4 | 106-47-8 | Plant (Brassica rapa) | Chronic (14 d) | LC50 = 66.5 mg/kg | ECOTOX 2000 |
| 4 | 106-47-8 | Plant (Brassica rapa) | Chronic (14 d) | LC50 = 200 mg/kg | ECOTOX 2000 |
| 4 | 106-47-8 | Plant (Chinese cabbage) | Acute (5 d) | EC50 = 39.4 mg/kg | Feng et al. 1996 |
| 4 | 106-47-8 | Plants (n = 9) | Chronic (14 d) | EC50 = 1000 mg/kg | ECOTOX 2000 |
| 4 | 106-47-8 | Plant (Sinapis alba) | Chronic (14 d) | EC50 = 100 mg/kg | ECOTOX 2000 |
| 4 | 106-47-8 | Plant (Vicia faba major) | Chronic (14 d) | LC50 = 140 mg/kg | ECOTOX 2000 |
| 4 | 95-51-2 | Plant (Vicia faba major) | Acute (36 h) | EC10 = 153 | BUA 1994 |
| 4 | 95-51-2 | Plant (Vicia faba major) | Acute (36 h) | EC50 = 549 | BUA 1994 |
| 5 | 100-01-6 | Plant (Chinese cabbage) | Acute (5 d) | EC50 = 43.6 | Feng et al. 1996 |
6.1.3 Derivation of the aquatic and terrestrial predicted no-effects concentrations (PNECs)
Many substances in this subgroup share a common mode of action, and one critical toxicity value (CTV) was initially considered to represent all Aromatic Amines in the subgroup in the aquatic environment. However, differences of several orders of magnitude exist between substances due to differences in the number of substituents, substituent type and position of the substituent and mode of action. Therefore, aquatic CTVs were selected for each substance in the Subgroup. CTVs of substances known to be in commerce in Canada were in turn used to calculate predicted no-effect concentrations (PNECs). For choosing a CTV, readily available studies with the lowest toxicity values were reviewed (Environment Canada 2014d). Chronic endpoints were preferred since the substances in the subgroup are likely to be found in the environment at low concentrations when released in the environment. While EC50s or LOECs are usually the preferred endpoint, NOECs were selected for 4-nitroaniline and Red Lake C Amine due to the scarcity of empirical data. Preference was given to data from empirical studies over modelled data, and to chronic data over acute data, however, since no empirical data was available for 2,4-diaminoanisole, a modelled chronic toxicity value (ChV) for invertebrates was chosen a CTV. Invertebrate data was chosen because modelled values for other trophic levels seem to overestimate toxicity when compared to empirical values for other substances structurally similar in ecological subset 3. The list of CTVs is presented in Table 6-3.
| Ecological Subset (CAS RN) | Organism (species) | Type of test (duration) | CTV (mg/L) | Reference |
|---|---|---|---|---|
| 1 (106-49-0 and 540-23-8) | Invertebrate (Daphnia magna) | Chronic (21 d) | EC50 = 0.021 | CHRIP ©2002–2012 |
| 1 (95-53-4) | Invertebrate (Daphnia magna) | Chronic (21 d) | EC50 = 0.066 | CHRIP ©2002–2012 |
| 1 (90-04-0) | Invertebrate (Daphnia magna) | Chronic (21 d) | EC50 = 1.3 | CHRIP ©2002–2012 |
| 1 (156-43-4) | Invertebrate (Daphnia magna) | Chronic (21 d) | LOEC = 0.6 | European Commission ©2000g |
| 2 (123-30-8) | Algae (Pseudokirch-neriella subcapitata) | Chronic (72 h) | EC50 = 0.1 | CHRIP ©2002–2012 |
| 3 (108-45-2 and 541-69-5) | Invertebrate (Daphnia magna) | Chronic (21 d) | EC50 = 0.62 | CHRIP ©2002–2012 |
| 3 (95-80-7) | Invertebrate (Daphnia magna) | Chronic (21 d) | EC50 = 0.81 | CHRIP ©2002–2012 |
| 3 (615-05-4) | Invertebrate (Daphnia magna) | Chronic | ChV = 0.072 | ECOSAR 2012 |
| 4 (95-51-2) | Invertebrate (Daphnia magna) | Chronic (21 d) | EC50 = 0.043 | CHRIP ©2002–2012 |
| 4 (106-47-8) | Invertebrate (Daphnia magna) | Chronic (21 d) | EC50 = 0.01 | CHRIP ©2002–2012 |
| 4 (95-76-1) | Invertebrate (Bosmina sp.) | Chronic (21 d) | EC50 = 0.0004 | Jak et al. 1998 |
| 5 (100-01-6) | Algae (Pseudokirch-neriella subcapitata) | Chronic (72 h) | NOEC = 0.94 | CHRIP ©2002–2012 |
| 6 (91-59-8) | Invertebrate (Daphnia magna) | Chronic (21 d) | EC50 = 0.029 | CHRIP ©2002–2012 |
| 7 (88-44-8) | Invertebrate (Daphnia magna) | Chronic (21 d) | NOEC = 3.2 | CHRIP ©2002–2012 |
To calculate PNECs, an assessment factor (AF) of 10 was applied to each CTV, considering that:
- the toxicity datasets are represented by all major taxonomic groups of organisms for most substances;
- each of the taxonomic groups is usually represented by multiple species;
- the toxicity values are the lowest values from the entire datasets of the subsets they represent;
- the datasets are often extensive (containing tens or hundreds of entries); and
- most studies are long-term tests.
This AF is applied to account for extrapolating laboratory results to species in the field.
Therefore, the aquatic PNEC for each substance in commerce in Canada can be calculated as PNEC = CTV / AF. The resulting PNECs are presented in Table 6-4.
| Ecological subset (CAS RN) | Organism (species) | Type of test (duration) | CTV (mg/L) | PNEC (mg/L) |
|---|---|---|---|---|
| 1 (95-53-4) | Invertebrate (Daphnia magna) | Chronic (21 d) | EC50 = 0.066 | 0.0066 |
| 1 (106-49-0) | Invertebrate (Daphnia magna) | Chronic (21 d) | EC50 = 0.021 | 0.0021 |
| 1 (90-04-0) | Invertebrate (Daphnia magna) | Chronic (21 d) | EC50 = 1.3 | 0.13 |
| 1 (156-43-4) | Invertebrate (Daphnia magna) | Chronic (21 d) | LOEC = 0.6 | 0.06 |
| 2 (123-30-8) | Algae (Pseudokirch-neriella subcapitata) | Chronic (72 h) | EC50 = 0.1 | 0.01 |
| 3 (108-45-2) | Invertebrate (Daphnia magna) | Chronic (21 d) | EC50 = 0.62 | 0.062 |
| 4 (95-51-2) | Invertebrate (Daphnia magna) | Chronic (21 d) | EC50 = 0.043 | 0.0043 |
| 6 (91-59-8) | Invertebrate (Daphnia magna) | Chronic (21 d) | EC50 = 0.029 | 0.0029 |
| 7 (88-53-9) | Invertebrate (Daphnia magna) | Chronic (21 d) | NOEC = 3.2 | 0.32 |
For terrestrial organisms, the chronic 14-day NOEC of 1 mg/kg for lettuce (Lactuca sativa) for 3,4-dichloroaniline (ECOTOX 2000) is selected as the CTV for the whole Aromatic Amines subgroup. The PNEC is then derived by dividing this value by an AF of 100 to account for differences in interspecies and intraspecies variability and to account for the limited number of data points available. Therefore, a PNEC of 0.01 mg/kg was calculated for the subgroup.
There was insufficient sediment toxicity data available from which to select a CTV to represent specific substances or the Subgroup as a whole therefore no PNEC was calculated for aromatic amines in the sediment compartment.
6.1.4 Ecological effects summary
Based on lines of evidence involving empirical and read-across aquatic ecotoxicity data, it may be concluded that some Aromatic Amines may cause harm to aquatic organisms at low concentrations (i.e., PNECs ranging from 2.1 × 10−3 – 0.32 mg/L).
6.2 Ecological Exposure Assessment
6.2.1 Releases to the environment
As few to no data on measured environmental concentrations (in water, soil or sediment) of the Aromatic Amines in Canada have been identified, environmental concentrations were estimated from other available information. Anthropogenic releases of a substance to the environment depend upon various losses that occur during the manufacture, industrial, consumer or commercialFootnote[5] use and disposal of a substance. In order to estimate releases to the environment occurring at different stages of the life cycle of the Aromatic Amines, Environment Canada compiled information on the relevant sectors and product lines as well as emission factorsFootnote[6] to wastewater, land and air at different life cycle stages in order to identify the life cycle stages that are the largest contributors to environmental concentrations. Recycling activities and transfer to waste disposal sites (landfill, incineration) were also considered. However, releases to the environment from disposal were not quantitatively accounted for unless reliable specific information on the rate of (or potential for) release from landfills and incinerators was available.
In general, wastewater is a common point of entry of a substance to water through the discharge of wastewater effluent and a potential point of entry into soil through the subsequent land application of biosolids. This information is used to further develop exposure scenarios to estimate resulting environmental concentrations.
Factors relevant to the life cycle stages of these substances have been considered, uncertainties have been recognized and assumptions have been made, subject to the availability of information. Exposure scenarios for the uses or media of concern have been developed, including the determination of applicable predicted environmental concentrations (PECs).
6.2.2 Identification of important exposure scenarios
The aromatic amines in this Subgroup are not manufactured in Canada, according to the data collected from regulatory surveys (Canada 2006, 2009, 2011). They were imported and used primarily in the production of the following section 71 identified products:
- Personal care and cosmetics
- Tires
- Pigments
Based on this use pattern, the following two industrial and two consumer release scenarios are identified as the important potential sources of environmental releases for the section 71 identified substances known to be in commerce:
- Consumer release from the use of personal care and cosmetics
- Personal care and cosmetics formulation
- Tire manufacture
- Tire wear
No quantitative exposure characterization is provided for pigment manufacture. This is because the aromatic amines are used as intermediates to manufacture organic pigments according to results from the 2012 survey (Environment Canada 2010). The residual amounts of one (o-anisidine) of these Aromatic Amines in the resulting pigments were reported to be in the range of 10–50 mg/kg (ECJRC 2002, p. 9). The maximum amount of residual aromatic amines is estimated to be 0.5 kg/day, assuming that the pigment manufacture occurs 300 days/year and the residual aromatic amines are at the upper end concentration of the reported range (50 mg/kg) and present in all organic pigments produced in Canada, which totalled 3 million kilograms in 2010 (CEH 2010, p. 53). Since only a small fraction of this daily amount is lost to wastewater during equipment cleaning along with the pigments and as the wastewater is treated both on-site and off-site, the release to the aquatic environment is not expected to be significant.
6.2.3 Derivation of predicted environmental concentrations (PECs)
The water column is considered as an important environmental compartment for the Aromatic Amines after they are discharged from industrial operations and then released to receiving water via wastewater treatment systems, given their moderate to high water solubilities and low log Kow values. The removal by wastewater sludge sorption is less than 10%, as estimated by ASTreat (2006), so the exposure in soil via biosolids land application is limited. Once released to the receiving water, these substances remain primarily in the aqueous phase due to their hydrophilic nature. Therefore, the aquatic compartment is selected for exposure calculations.
6.2.3.1 Consumer release from the use of section 71 identified personal care and cosmetics
A consumer release scenario is developed to estimate the aquatic exposure to the aromatic amines released from the use of section 71 identified personal care and cosmetics. This scenario is based on a large number of sites (1077), and each site is served by one publicly-owned wastewater treatment system. These sites account for more than 70% of the Canadian population and are therefore considered to sufficiently reflect the consumer release conditions in Canada for cosmetics.
The scenario is characterized by three parameters: (1) emission factor to wastewater (E); (2) wastewater treatment removal (R); and (3) per capita daily dilution water volume (B). Their respective values are summarized in Table 6-5.
| Parameter | Value | Distribution |
|---|---|---|
| Emission factor to wastewater (E) | 100% | Not applicable |
| Wastewater treatment removal (R) | 3.3% | Not applicable |
| Per capita daily dilution water volume (B) | 277–69 613 L/d per person | Discrete |
The emission factor to wastewater (sewer) is not known and is conservatively assumed to be 100%.
The wastewater treatment removal is calculated as an average between three different levels (no or preliminary, primary, and secondary) of publicly owned wastewater treatment systems found for the 1077 sites. The removal of each treatment type is estimated by models, except no or preliminary treatment, which is assumed to have zero removal. The average is weighted by the proportion of each treatment type and considered to represent the overall release mitigation for the aromatic amines through publicly owned wastewater treatment systems in Canada.
The per capita daily dilution water volume is the amount of water available at a site for the dilution of chemicals released on a per capita and daily basis near the discharge point of a publicly owned wastewater treatment system. This parameter is derived as a discrete distribution, with one value at each of the 1077 sites.
The aquatic PEC (μg/L) can then be estimated for a given per capita daily use quantity of the aromatic amines (A in mg/day per person) by the equation:
PEC = [A × E × (1 - R)] / B
The per capita daily use quantity of the aromatic amines is determined as a range (0.806–8.06 mg/day per person), based on the import quantity range from regulatory surveys (Environment Canada 2006; Environment Canada 2009), the Canadian population and the number of annual release days. The distribution of this range is not known; as a first approximation, it is assumed to be a uniform distribution.
Since the per capita daily use quantity (A) and the per capita daily dilution water volume (B) are each given as a distribution, the aquatic PEC is calculated as a probability distribution, as presented in Table 6-6. This calculation is performed using Crystal Ball, a commercial software program for probabilistic analysis.
| Percentile | PEC (μg/L) |
|---|---|
| 0th | 0.01 |
| 5th | 0.19 |
| 10th | 0.28 |
| 25th | 0.51 |
| 50th | 0.93 |
| 75th | 1.86 |
| 90th | 5.93 |
| 95th | 8.93 |
| 100th | 23.4 |
6.2.3.2 Section 71 Identified Personal care and cosmetics formulation
A generic formulation scenario is developed to estimate the aquatic exposure to the aromatic amines released from the formulation of section 71 identified personal care and cosmetics in Canada. In this scenario, the aquatic exposure conditions associated with the formulation are characterized by three parameters: (1) emission factor to wastewater prior to any wastewater treatment; (2) removal of the aromatic amines by wastewater treatment (on-site industrial, off-site publicly owned or both); and (3) daily dilution water volume near the discharge point of a wastewater treatment system. Under these conditions and for a given quantity of the aromatic amines used, the aquatic exposure to the aromatic amines is estimated by the equation:
PEC = [Q × E × (1 - R) × 109] / V
where:
- PEC:
- predicted aquatic environmental concentration, μg/L
- Q:
- daily use quantity of aromatic amines at a site, kg/d
- E:
- emission factor of aromatic amines to wastewater prior to any wastewater treatment, %
- R:
- removal of aromatic amines by industrial and/or publicly owned wastewater treatment systems, %
- V:
- daily dilution water volume near the discharge point of an industrial or publicly owned wastewater treatment system, L/d
- 10 9:
- conversion factor from kg to μg
The values of the three exposure parameters are derived based on both the scientific literature and Environment Canada’s in-house data (see Table 6-7 for details). The emission factor to wastewater (E) is determined to be in the range of 0.3–2.5%, resulting from formulation equipment cleaning, according to the European Chemicals Bureau’s Technical Guidance Document on Risk Assessment (Part II) (ECJRC 2003). As a first approximation, the range is assumed to follow a uniform distribution.
| Parameter | Generic scenario | High-exposure scenario | Low-exposure scenario |
|---|---|---|---|
| Emission factor to wastewater (%) | 0.3–2.5 (uniform distribution) | 0.3–2.5 (uniform distribution) | 0.3–2.5 (uniform distribution) |
| Wastewater treatment removal (%) | 5.5 | 5.0 | 8.0 |
| Daily dilution water volume (million L/d) | 151; 250; 497; 540; 2 541; 27 868 (discrete distribution) | 151 | 27 868 |
The wastewater treatment removal (R) is calculated as an average of 5.5% for the wastewater treatment systems used at six possible personal care and cosmetics formulation sites. These sites were identified from survey data (Environment Canada 2006; Environment Canada 2009) and Environment Canada’s National Pollutant Release Inventory database (NPRI 2006). They are used to characterize the wastewater treatment removal for the generic formulation scenario.
The same six sites are also used to characterize the daily dilution water volume (V) for the generic formulation scenario. This parameter is calculated by multiplying the daily discharge rate of the treated wastewater by the dilution factor of the receiving water. It is derived as a discrete distribution, in the range of 151–27 868 million litres per day, with one value at each of the six sites.
An aquatic PEC cumulative probabilistic distribution is then calculated for an estimated daily use quantity of the aromatic amines. This quantity is determined to be less than 100 kg/day, according to the European Chemicals Bureau’s Technical Guidance Document on Risk Assessment (Part II, ECJRC 2003, Table B2.1 for personal/domestic formulation, p. 252) and data submitted from industry (personal communication, email from industry stakeholder to Environment Canada, dated February 2014; unreferenced). Crystal Ball, a commercial software program, is used for the calculation. A large number (20 000) of PECs are generated by varying the emission factor and the daily dilution water volume over their determined ranges, with the daily use quantity and wastewater treatment removal fixed at their respective single values. These PECs are then sorted by their magnitudes and arranged as a function of the cumulative percentage or probability of occurrence--i.e., a cumulative probabilistic distribution, as shown in Table 6-8.
| Percentile | Generic scenario PEC (μg/L) | High-exposure scenario PEC (μg/L) | Low-exposure scenario PEC (μg/L) |
|---|---|---|---|
| 0th | 0.0049 | 0.75 | 0.0040 |
| 5th | 0.020 | 1.61 | 0.0085 |
| 10th | 0.038 | 2.18 | 0.012 |
| 25th | 0.29 | 3.23 | 0.017 |
| 50th | 1.28 | 5.39 | 0.028 |
| 75th | 2.97 | 8.06 | 0.042 |
| 90th | 5.70 | 10.6 | 0.055 |
| 95th | 7.35 | 12.1 | 0.064 |
| 100th | 13.9 | 15.0 | 0.079 |
A high-exposure scenario and a low-exposure scenario are also provided to give further details on the aquatic exposure resulting from the personal care and cosmetics formulation. The values of the three exposure parameters (emission factor, wastewater treatment removal and daily dilution water volume) for the two scenarios are summarized in Table 6-7 above.
The daily use quantity and the emission factor to wastewater represent two significant areas of uncertainties for the aquatic exposure distributions derived. The values of the two parameters (less than 100 kg/day for the daily use quantity and 0.3–2.5% for the emission factor) are based on the European Chemicals Bureau’s Technical Guidance Document on Risk Assessment (Part II, ECJRC 2003) and data submitted from industry (personal communication, email from industry stakeholder to Environment Canada, dated February 2014; unreferenced).
6.2.3.3 Tire Wear
The aquatic PEC resulting from tire wear is conservatively estimated. The discharge of runoff from roads to the aquatic environment is identified as the aquatic exposure pathway for chemicals present in tire wear particles (Blok 2005). According to this pathway, the amount of the aromatic amines lost to roads via tire wear is conservatively estimated based on a maximum concentration of the aromatic amines in tires and the mass of tire rubber lost to roads. All of this amount is then conservatively assumed to enter the aquatic environment via runoff, without considering a certain fraction ending up in soil. The aquatic PECs are conservatively determined as the concentrations of the aromatic amines in runoff by ignoring the dilution of receiving water bodies. These PECs are found to be in the range of 0.005–0.2 μg/L for the 11 largest cities in Canada.
6.2.3.4 Tire manufacturing
A generic tire manufacture scenario is developed to estimate the aquatic exposure to the aromatic amines released from tire manufacture in Canada. In this scenario, the aquatic exposure conditions associated with tire manufacture are characterized by three parameters: (1) emission factor of the aromatic amines to wastewater prior to any wastewater treatment; (2) removal of the aromatic amines by wastewater treatment (on-site industrial, off-site publicly owned or both); and (3) daily dilution water volume near the discharge point of a wastewater treatment system. Under these conditions and for a given quantity of the aromatic amines used, the aquatic exposure to the aromatic amines is estimated by the equation:
PEC = [Q × E × (1 - R) × 109] / V
where:
- PEC:
- predicted aquatic environmental concentration, μg/L
- Q:
- daily use quantity of aromatic amines at a site, kg/d
- E:
- emission factor of aromatic amines to wastewater prior to any wastewater treatment, %
- R:
- removal of aromatic amines by industrial and/or publicly owned wastewater treatment systems, %
- V:
- daily dilution water volume near the discharge point of an industrial or publicly owned wastewater treatment system, L/d
- 10 9:
- conversion factor from kg to μg
The aquatic exposure conditions are derived based on both the scientific literature and Environment Canada’s in-house data (see Table 6-9). The emission factor (E) is determined to be in the range of 0.056–1%, according to measured data reported by the European Tyre and Rubber Manufacturers’ Association (ETRMA 2010) and estimates found in an OECD emission scenario document on additives in the rubber industry (OECD 2004b). As a first approximation, this range is assumed to follow a uniform distribution.
| Parameter | Generic scenario | High-exposure scenario | Low-exposure scenario |
|---|---|---|---|
| Emission factor to wastewater (%) | 0.056–1 (uniform distribution) |
0.056–1 (uniform distribution) | 0.056–1 (uniform distribution) |
| Wastewater treatment removal (%) | 47.5 | 71.9 | 74.4 |
| Daily dilution water volume (million L/d) | 1; 8; 10; 11; 61; 384; 550 (discrete distribution) | 1 | 61 |
The wastewater treatment removal (R) is calculated as an average of 47.5% for the wastewater treatment systems in relation to seven tire manufacture sites identified from a survey report (Cheminfo 2012). These sites are used to characterize the wastewater treatment removal for the generic tire manufacture scenario.
The same seven sites are also used to characterize the daily dilution water volume (V) for the generic tire manufacture scenario. This parameter is calculated by multiplying the daily discharge rate of the treated wastewater by the dilution factor of the receiving water. It is derived as a discrete distribution in the range of 1–550 million litres per day, with one value at each of the seven sites.
An aquatic PEC cumulative probabilistic distribution is then calculated for an estimated daily use quantity of the aromatic amines. For three of the seven sites identified, the annual use quantity of the Aromatic Amines is found to be in the range of 100–1000 kg/year per site (Environment Canada 2009). The daily use quantity is thus estimated to be in the range of 0.274–2.74 kg/day, considering the number of annual operation days to be 365 days/year (Cheminfo 2012). As a first approximation, the range is assumed to follow a uniform distribution. This range is considered to represent the higher end of use quantities in the tire manufacture and used in the aquatic PEC calculations to capture high exposure resulting from high use quantities.
Crystal Ball, a commercial software program, is used for the calculation. A large number (20 000) of PECs are generated by varying the daily use quantity, the emission factor and the daily dilution water volume over their determined ranges, with the wastewater treatment removal fixed at its average value. These PECs are then sorted by their magnitudes and arranged as a function of the cumulative percentage or probability of occurrence--i.e., a cumulative probabilistic distribution, as shown in Table 6-10.
| Percentile | Generic scenario PEC (μg/L) | High-exposure scenario PEC (μg/L) | Low-exposure scenario PEC (μg/L) |
|---|---|---|---|
| 0th | 0.0002 | 0.048 | 0.0007 |
| 5th | 0.0025 | 0.31 | 0.0044 |
| 10th | 0.0047 | 0.47 | 0.0067 |
| 25th | 0.015 | 0.92 | 0.011 |
| 50th | 0.15 | 1.9 | 0.023 |
| 75th | 0.62 | 3.4 | 0.042 |
| 90th | 1.99 | 4.9 | 0.060 |
| 95th | 5.20 | 5.8 | 0.071 |
| 100th | 14.2 | 7.9 | 0.097 |
A high-exposure scenario and a low-exposure scenario are also analyzed to provide further details on the aquatic exposure resulting from tire manufacture. The values of the three exposure parameters (emission factor, wastewater treatment removal and daily dilution water volume) for the two scenarios are summarized in Table 6-9. An aquatic PEC distribution is generated for each scenario by applying the daily use quantity range (0.274–2.74 kg/day), as given in Table 6-10.
The emission factor to wastewater is the most significant area of uncertainty. The values found for this parameter differ greatly between different sources. It is not known whether this difference results from different tire manufacture operations or from different emission factor determination methods. To avoid missing any valid data points, all the values found from different sources are taken into consideration and covered under a range (0.056–1%). In addition, the weight of each data point within the range or the distribution of the range is not known. As a first approximation, the range is assumed to follow a uniform distribution.
6.3 Characterization of Ecological Risk
The approach taken in this ecological screening assessment was to examine various supporting information and develop proposed conclusions based on a weight of evidence approach and using precaution, as required under CEPA 1999. Lines of evidence considered include information on physical and chemical properties, environmental fate, ecotoxicity and sources of the substances, as well as results from risk analyses, which are outlined below.
6.3.1 Risk analysis
Risk analyses compare the PECs with the appropriate PNEC values in order to evaluate potential risks.
For the aquatic environment, multiple PNECs were calculated (see Section 6.1). However, only the PNECs calculated for substances o-toluidine and p-aminophenol were compared to the scenarios used in the PEC calculation as these two substances were used in much greater quantities. A PNEC of 6.6 µg/L, was selected for the substance o-toluidine involved in the tire manufacturing and tire wear scenarios. A PNEC of 10 µg/L was derived for p-aminophenol representing the consumer release from cosmetic use and the cosmetic formulation scenarios (see Section 6.2). These PNEC were then compared with the respective PEC or PEC distributions outlined in the previous section (see Section 6.2), for sites using aromatic amines for cosmetic products and in tires. The probability that the PEC is above the PNEC is less than 5% for the consumer release scenario of cosmetics, approximately 5% for the cosmetics formulation scenario, and less than 5% for the tire manufacture scenario. For the tire wear scenario, the PEC is not expected to exceed the PNEC. These results indicate low probability of harm to aquatic organisms from aromatic amines in these four types of release scenarios.
No risk analysis was performed for the other compartments, because data were insufficient for determining a soil or sediment PNEC. No monitoring data were available, and the substances are not within the domain of applicability of the exposure model for estimating environmental concentrations using equilibrium partitioning.
6.3.2 Consideration of lines of evidence and conclusion of ecological risk characterization
To facilitate the ecological assessment, the 16 aromatic amines of the Subgroup were further separated into seven ecological subsets based on structural commonalities. Most of the Aromatic Amines (other than some of the substances in ecological subset 1 Methylated and oxy- aromatic amines) in this assessment are not expected to occur naturally in the environment.
No manufacturing activity of any of the 16 Aromatic Amines takes place in Canada. Seven of the Aromatic Amines have been reported as being imported into Canada above the 100 kg/year survey reporting threshold. An additional two Aromatic Amines were reported as being imported into Canada but they were below the 100kg/yr reporting threshold.
Aromatic Amines are generally moderately to highly water soluble, indicating that they are likely to be found primarily in the hydrosphere if released to the environment. Low to moderate volatilization from surface waters is expected for the substances in this subgroup, based on their Henry’s Law constants. Therefore, long-range atmospheric transport is not anticipated to be of concern.
Aromatic Amines are expected to have a low bioaccumulation potential, based on low observed bioconcentration in empirical tests and their physical and chemical properties (i.e., low log Kow, ionized at relevant environmental pH, intermediate molecular weight, large cross-sectional diameters and moderate to high water solubility). The low potential for bioaccumulation of these substances suggests that there may also be low potential for internal concentrations in organisms to reach levels that could cause adverse effects. With the exception of a few substances from ecological subset 1 (Methylated and oxy- aromatic amines), which may be considered biodegradable, aromatic amines are moderately or poorly biodegradable, and it is expected that these substances may have relatively long residence times in water until sorption processes with dissolved organic matter, particulate matter or other surfaces take place. Due to their moderate to high water solubility and the fact that these substances could stay in the water medium for long periods of time, they may disperse widely. Eventually, due to electrostatic interactions with particulate matter, they may also disperse widely in sediment. In sediment and soil, biodegradation is also expected to be slow under aerobic and anaerobic conditions and will be further slowed by sorption processes. Aromatic amines are not persistent in the air and this medium was not investigated further.
Based on empirical aquatic ecotoxicity data, it may be concluded that Aromatic Amines are hazardous to aquatic organisms at low concentrations (0.0004 to418 mg/L). Aquatic invertebrates were more sensitive than other organisms to Aromatic Amines; sensitivities also increased with increased exposure periods. Limited toxicity data were available for terrestrial and sediment-dwelling organisms.
Few to no data concerning concentrations of these substances in the Canadian environment have been identified. Based on their uses and hazard characteristics, the aquatic environment is considered the environmental compartment of most concern for the Aromatic Amine. Exposure analyses of the consumer release from cosmetic use, cosmetics formulation, tire wear and tire manufacturing processes for substances in commerce were conducted as these scenarios were anticipated to present the highest potential ecological risk related to industrial releases to the environment for these substances. Using a probabilistic approach, the PECs were compared with the PNECs for water. The probability that the PECs of certain Aromatic Amines exceeded the PNECs was low (0 to approximately 5%) for the aquatic compartment in all four scenarios.
Considering the lines of evidence presented in this ecological assessment, there is low risk of harm to organisms and the broader integrity of the environment from the 16 substances in the Aromatic Amines Subgroup
6.3.3 Uncertainties in evaluation of ecological risk
While water was found to be the key medium of interest soil and sediment also hold some importance due to potential adsorption and electrostatic interactions. The lack of available effects data for Aromatic Amines in soil and sediment is therefore a source of uncertainty; however direct releases of aromatic amines to these media in Canada weren’t identified.
The lack of monitoring or measured environmental concentrations of these substances in Canada and a lack of information on the identity and the use pattern of parent substances that may transform or degrade into aromatic amines resulted in the need to evaluate risk based on predicted concentrations in water as a result of releases from consumer use or near industrial point sources resulting from the direct use of aromatic amines. Conservative assumptions were made when using models to estimate concentrations in receiving water bodies.
Given the use of some of these substances in other countries, it is possible that they may enter the Canadian market as components of manufactured items and/or consumer products. However, it is anticipated that the proportions of these substances released to the various environmental media would not be significantly different from those estimated here, given the conservative assumptions used in the exposure analyses.
7. Potential to Cause Harm to Human Health
This human health assessment focuses on substances that are in commerce above the section 71 reporting threshold or for which available information indicates their presence in Canada. Potential exposures of the general population of Canada were characterized for nine substances: o-toluidine, o-anisidine, p-aminophenol, 2,4-diaminotoluene, 1,3-diaminobenzene, 4-chloroaniline, 3,4-dichloroaniline, 2-naphthylamine and Red Lake C Amine (Table 7-1). For the remaining seven Aromatic Amines, based on available information, exposure of the general population of Canada was not expected. In the following section, overarching key information is summarized for both exposure assessment and health effects assessment, followed by sections for individual Aromatic Amines in the order presented in Table 7-1.
| Substance | Environmental media | Breast milk | Textiles | Cooking utensils | Cosmetics | Tattoos |
|---|---|---|---|---|---|---|
| 2-Naphthylamine | – | – | X | – | – | – |
| o-Toluidine | – | X | – | X | – | X |
| 2,4-Diaminotoluene | – | – | X | X | – | – |
| 4-Chloroaniline | X | – | X | X | X | – |
| 3,4-Dichloroaniline | X | – | – | – | – | – |
| o-Anisidine | – | – | X | X | – | X |
| p-Aminophenol | – | – | – | – | X | – |
| 1,3-Diaminobenzene | – | – | – | X | X | – |
| Red Lake C Amine | – | – | – | – | X | – |
7.1 Exposure Assessment
A number of studies and surveys were identified that report Aromatic Amines in environmental media, food and consumer products (see Appendices B–F, H and J). A limited number of these studies were conducted in Canada. Among the studies identified, critical studies that were used in characterizing exposures and reported more than one Aromatic Amine are summarized below. The critical studies that reported only one Aromatic Amine are discussed later in the corresponding individual substance sections.
7.1.1 Environmental Media
As mentioned previously in Section 4.3.2, a number of studies (mostly in Europe and United States) were identified that reported measured concentrations of Aromatic Amines in environmental media (see Appendix B). However, there were no common critical studies reporting more than one Aromatic Amine that were considered applicable for characterizing human exposures in Canada. For 2-naphthylamine, there was one indoor air study (Otson et al. 1994), conducted by Health Canada, that was considered relevant to the substance-specific exposure characterization. Similarly, a study conducted in Spain measured chloroanilines deriving chloramination of raw water containing pesticides (Jurado-Sánchez et al. 2012). More details on these studies as well as the exposure characterization are presented in respective subsections of Section 7.4 (2-naphthylamine in Section 7.4.1, 4-chloroaniline in Section 7.4.4 and 3,4-dichloroaniline in Section 7.4.5).
7.1.2 Biomonitoring
One small study conducted in Canada analyzed human milk from 31 lactating mothers for monocyclic aromatic amines, including o-toluidine and p-toluidine, via solid-phase microextraction coupled with gas chromatography/mass spectrometry (DeBruin et al. 1999). p-Toluidine was not detected in the human milk samples (limit of detection [LOD] not established). o-Toluidine was present in 77% (i.e., 24) of the samples above the LOD (0.01 part per billion [ppb]); concentrations ranged from 0.01 to 0.26 ppb. No significant differences were observed in the levels of o-toluidine found in human milk of 7 smokers compared with 24 non-smokers, who reported no recent exposure to second-hand smoke. None of the mothers reported occupational exposures to aromatic amines. An upper-bounding estimate of exposure of breastfed infants to o-toluidine from human milk was derived (see Appendix G) and is presented in Section 7.4.2. Aside from the study by Debruin et al. (1999), no other Canadian biomonitoring data for these 16 Aromatic Amines were identified.
While limited human biomonitoring data were identified for these Aromatic Amines in the Canadian population, several studies conducted outside of Canada were found. Human biomonitoring studies, primarily from European countries, reported a high prevalence of Aromatic Amines (e.g., 2-naphthylamine, o-toluidine, p-toluidine, o-anisidine, 4-chloroaniline and 3,4-dichloroaniline) detected in urine of non-occupationally exposed, non-smoking individuals (Teass et al. 1993; Falter et al. 1994; Riffelmann et al. 1995; Ward et al. 1996; Branner et al. 1998; Richter et al. 2001; Wittke et al. 2001; Riedel et al. 2006; Kütting et al. 2009; Lindner et al. 2011; Weiss and Angerer 2002; Turci et al. 2006; NTP 2013a). Because aromatic amines are rapidly eliminated through urine, their presence in urine is indicative of recent exposure. However, repeated detection in urine samples across a large time span and within various populations is suggestive of ongoing aromatic amine exposure in those populations studied. Since the exposure sources were not characterized for the aromatic amines measured in the European biomonitoring studies, it is uncertain whether these exposure data are representative of, or applicable to the Canadian population. Overall, due to the lack of Canadian biomonitoring data, the aggregate exposure to these substances in the Canadian population remains an uncertainty.
7.1.3 Consumer Products
Cooking Utensils
In a study conducted in Ireland, 84 black polyamide cooking utensils (e.g., spatulas, slotted spoons) purchased from various retail locations (McCall et al. 2012) were analyzed for release of primary aromatic amines, including 2,4-diaminotoluene, o-anisidine, 1,3-diaminobenzene, o-toluidine and 4-chloroaniline during use. The contact area of the utensil was immersed in 3% acetic acid simulant solutions and left for 2 hours at 100°C. This was repeated two additional times to simulate repeated use. The results of the study showed migration of 2,4-diaminotoluene and o-toluidine from the spatulas and slotted spoons; results for the third extraction are summarized in Table 7-2. o-Anisidine, 1,3-diaminobenzene and 4-chloroaniline were not detected in the food simulant samples (LOD not specified). The authors indicated that primary aromatic amines migrate from these utensils primarily due to incomplete polymerization. Significant variation in migration levels was observed from identical utensils and among different types of cooking utensils.
A similar study, conducted by Trier and co-workers (2010), analyzed the migration of 20 primary aromatic amines, including 1,3-diaminobenzene, 2,4-diaminoanisole, 4-chloroaniline, 2,4-diaminotoluene, o-anisidine and o-toluidine. 136 black polyamide cooking utensils from Danish retail shops and importers were analyzed. Samples of utensils were immersed in a 3% acetic acid food simulant and left for 30 minutes to 4 hours (depending on the type of utensil and intended use conditions) at 100°C. The migration test was repeated two additional times to simulate repeated use, and the extract samples were analyzed for primary aromatic amines. Migration of o-toluidine, 2,4-diaminotoluene, 1,3-diaminobenzene, 4-chloroaniline and o-anisidine from these utensils to the food simulant was found in the third migration test (see Table 7-2); 2,4-diaminoanisole was not detected in the food simulant samples. Cooking utensils previously used by Danish consumers were also analyzed for migration of amines; only o-anisidine and 2,4-diaminotoluene were found, and the migration levels were lower than those for the new utensils.
In both studies, the frequency of detection of the Aromatic Amines of interest was less than 10%, and therefore the use of the median concentration was considered an appropriate conservative metric for use in characterizing potential exposure of the general population in this Screening Assessment. Conservative estimates of potential exposures to 2,4-diaminotoluene, o-toluidine, o-anisidine, 1,3-diaminobenzene and 4-chloroaniline from polyamide cooking utensils are derived for the general population of Canada based on the median migration level. For o-toluidine and 2,4-diaminotoluene, the median migration level from the McCall’s study was used because it was higher than that from the study by Trier et al. See Appendix C for more details on the exposure scenarios; estimated intakes are presented in later sections.
| Substance | LOD | Number detectedFootnote Table 7-2 [a](frequency) | Maximum concentration (µg/kg) | Median concentrationFootnote Table 7-2 [b](µg/kg) | Average concentration[b](µg/kg) |
|---|---|---|---|---|---|
| o-ToluidineFootnote Table 7-2 [c] | 0.5 | 3 (4%) | 5.8 | 0.5 | 0.6 |
| o-ToluidineFootnote Table 7-2 [d] | 0.3 | 7 (5%) | 23 | 0.3 | 0.6 |
| 2,4-Diaminotoluene[c] | 1.18 | 5 (6%) | 2780 | 1.0 | 70 |
| 2,4-Diaminotoluene[d] | 0.4 | 11 (8%) | 500 | 0.4 | 4.9 |
| o-Anisidine[d] | 0.4 | 8 (6%) | 22 | 0.4 | 0.7 |
| 1,3-Diaminobenzene[d] | 0.3 | 2 (1%) | 11 | 0.3 | 0.4 |
| 4-Chloroaniline[d] | 0.4 | 2 (1%) | 2 | 0.4 | 0.4 |
Textile and Leather Products
Six Aromatic Amines--namely, 2,4-diaminotoluene, 2-naphthylamine, o-toluidine, 4-chloroaniline, 2,4-diaminoanisole and o-anisidine--are EU22 aromatic amines. Despite EU legislation and associated restrictions, product monitoring and testing data in Europe and Japan showed the presence of these Aromatic Amines, except for 2,4-diaminoanisole, in textile and leather products (EurAzos 2007; Kawakami et al. 2010; RAPEX 2012; see Table 7-3).
RAPEX is the EU rapid alert system shared by EU member states that facilitates the rapid exchange of information on products posing a serious risk to the health and safety of consumers. The operation procedures for RAPEX are described within the EU Product Safety Directive 2001/95/EC (EU 2001), which imposes a general safety requirement on any product put on the market for consumers. The EU22 aromatic amines listed in Appendix 8 of Regulation (EC) No 1907/2006 (EU 2006) are monitored as part of the RAPEX alert system (RAPEX 2012). Alerts on certain EU22 aromatic amines detected in textiles at quantities above the limit of 30 mg/kg were found in the RAPEX database.
The EurAzos project (EurAzos 2007) is a one-time European enforcement project conducted in 2007, similar to the RAPEX alert system, which aimed to assess the compliance of textile and leather products in the European market with the above-noted EU Product Safety Directive 2001/95/EC (EU 2001) for EU22 aromatic amines (EU 2006). There were nine violations reported among 361 textile and leather products analyzed, in which the concentrations of EU22 aromatic amines were found to be above the EU limit of 30 mg/kg, four of which pertained to o-anisidine, o-toluidine and 2,4-diaminotoluene (see Appendix E for details). The country of origin for these products was not reported.
A Japanese study surveyed 86 textile products purchased at retail stores in Japan between January and March 2009 and analyzed for 26 aromatic amines that can be released from azo dyes (Kawakami et al. 2010). In addition to the EU22 aromatic amines, 2,4-xylidine, 2,6-xylidine, aniline and 1,4-phenylenediamine were included in the study. In total, 117 samples of 86 textile products were analyzed for the amount of aromatic amines released from azo dyes when extracted under reductive conditions. The British standard methods (BS EN 14362-1:2003, BS EN 14362-2:2003) (ECS 2012) adopted by the European Committee of Standardization were used, with slight modifications, depending on the type of material. The method without solvent extraction was used for 77 samples composed of natural fibres (e.g., cellulosic and protein-based fibres), whereas the method with solvent extraction was used for 40 samples composed of synthetic fibres (e.g., polyester). Both sample processing methods were conducted for “mixed-fibre samples.” 4-Chloroaniline, o-anisidine, o-toluidine, 2,4-diaminotoluene and 2-naphthylamine were found in products in this study at low concentrations (see Appendix E for details).
| Aromatic Amine | Products | Reported concentration range (mg/kg) |
|---|---|---|
| 2-Naphthylamine | 1 bed linen; 1 towel; 1 textile coaster | 0.071 (towel) – 142.2 (bed linen) |
| o-Toluidine | 6 textile clothing items; 3 handkerchiefs; 1 unknown; 3 placemats | 0.19 (handkerchief –shirt) – 407 (textile clothing item) |
| 4-Chloroaniline | 4 handkerchiefs; 9 textile clothing items; 6 placemats; 1 unknown; 3 socks; 3 towels; 1 wrist band and supporter | 0.01 (placemat) – 576 (textile clothing item) |
| 2,4-Diaminotoluene | 5 textile clothing items; 1 pair of shoes; 1 placemat; 2 toys | 30 (textile clothing item) – 860 (textile toy) |
| o-Anisidine | 7 textile clothing items; 1 accessory case; 1 handkerchief; 10 placemats; 1 towel; 1 toy | 0.017 (placemat) – greater than 30 (textile clothing item) |
Additionally, product testing conducted by Health Canada on 66 samples of imported and domestic textile and leather products for EU22 aromatic amines focussed on children’s toys, leather slippers, children’s clothing and woollen items purchased in retail stores in Ottawa, Ontario, in August 2012 (Health Canada 2013a). Four Aromatic Amines, 2-naphthylamine, 2,4-diaminotoluene, 4-chloroaniline and o-anisidine, were found in some of the products tested (see Table 7-4). The investigation also screened for o-toluidine and 2,4-diaminoanisole, which were not detected in any of the samples above the LOD of 1.1 mg/kg and 1.6 mg/kg, respectively.
| Substance | Products | Frequency of detectsFootnote Table 7-4 [b] | Concentrations (mg/kg) |
|---|---|---|---|
| 2-Naphthylamine | 2 children’s clothing; 1 woollen item |
5% of the textile samples tested | greater than 1.6 (LOD) and less than 4.7 (LOQ) |
| 2,4-Diaminotoluene | 1 children’s toy | 2% of the textile samples | 4.2 |
| o-Anisidine | 3 children’s clothing; 1 children’s toy |
7% of the textile samples | greater than 0.9 (LOD) and less than 2.6 (LOQ) |
| 4-Chloroaniline | 1 children’s leather slippers; 1 children’s toy; 3 children’s clothing |
7% of the textile samples; 14% of the leather samples tested | greater than 1.8 (LOD) and less than 5.5 (LOQ) |
Levels of 2-naphthylamine, 2,4-diaminotoluene, 4-chloroaniline and o-anisidine in the products were all below the EU limit of 30 mg/kg. It is expected that these substances, when present, were either residuals of the dye manufacturing process or breakdown products of azo dyes that were reduced under the test conditions. Given that these substances were found in textile products in the Canadian market (see Table 7-4), estimates of dermal exposure (i.e., from skin contact with textiles such as clothing) of the general population in Canada were derived. Oral exposure resulting from mouthing of textile objects by infants was also considered, and estimates of exposure were derived.
The percentage of detects of a given Aromatic Amine in textile samples ranged from 2% to 7% in the Health Canada study, indicating that not all products made of textile in the Canadian market contain these substances. This study may not be representative of the entire consumer textiles market in Canada; therefore, exposures were estimated assuming that there is a 10% probability that a given Aromatic Amine (i.e., 2-naphthylamine, 2,4-diaminotoluene, 4-chloroaniline or o-anisidine) appears in products made of textile in Canada. This adjustment factor is similar to the 8% used in the Danish assessment in estimating exposures to aromatic amines and azo dyes from textile garments in the Dutch market (Zeilmaker et al. 1999). Other surveys showed similar findings in certain cases (Danish EPA 1998; EurAzos 2007; Kawakami et al. 2010).
Exposures to 2-naphthylamine, 4-chloroaniline and o-anisidine in textile products were estimated based on the respective limits of quantification (LOQs) from the Health Canada study. Exposures to 2,4-diaminotoluene were estimated using the maximum measured concentration, 4.2 mg/kg. These amines are found at higher concentrations in textile materials in non-domestic markets (see Table 7-3); however, Canadian data were used to derive estimated exposures in this Screening Assessment because the data are considered more representative of products with which the general population of Canada may be in contact. Given that higher concentrations are measured in products in non-domestic markets, which may be exported to Canada, more conservative metrics (i.e., LOQs and maximum concentrations) are selected for deriving exposure estimates.
Dermal and oral exposure estimates for these Aromatic Amines from contact with textile products are presented in the substance-specific sections below. Further details are provided in Appendix E.
Since the Health Canada study also found 4-chloroaniline in a leather product, dermal exposure to 4-chloroaniline from contact with leather products was estimated and is presented in a substance-specific section below.
Generally, there is very limited information on non-EU22 aromatic amines in consumer products. A recent study, conducted by the Federal Food Safety and Veterninary Office, the Official Food Control Authority of the Canton Bern and FRIEDLIPARTNER AG in Switzerland, was identified that investigated non-EU22 aromatic amines in 153 samples of clothing textiles that were purchased from clothing retail outlets in Switzerland (Brüschweiler et al. 2014). The samples were analyzed for 22 non-EU22 amines, including 7 Aromatic Amines within this Screening Assessment (i.e., 4-aminophenol, p-phenetidine, p-toluidine, 1,3-diaminobenzene, 2-chloroaniline, 3,4-dichloroaniline, 4-nitroaniline); the LOD ranged from 0.05 to 0.5 mg/kg and the LOQ ranged from 0.2 to 2 mg/kg. 2-Chloroaniline, 3,4-dicholoroaniline and 1,3-diaminobenzene were not detected in any of the samples. 4-nitroaniline was also not detected however reduction of the –NO2 moiety may have occurred yielding p-phenylenediamine which was detected in 8 samples. 4-Aminophenol, p-phenetidine and p-toluidine were found in 3, 1 and 2 samples, respectively. An earlier study by the Danish EPA (1998) with a smaller sample size of 56 also reported detection of non-EU22 aromatic amines in textiles including four amines in this assessment: p-toluidine (5 samples), 2-chloroaniline (3 samples), 1,3-diaminobenzene (1 sample) and p-phenetidine (1 sample). Since these data are not specific to the Canadian market, exposure to these non-EU22 Aromatic Amines from textiles was not characterized.
Other Sources
Based on analytical testing and surveys of tattoo inks conducted in Europe (CVUA 2011; Hauri 2011; Danish EPA 2012; RAPEX 2012), o-toluidine and o-anisidine were found in several tattoo inks; 2-naphthylamine was found in only one. However, notwithstanding EU regulations that restrict EU22 amines in tattoos and permanent make-up (Council of Europe 2008), such amines were found in tattoo inks, likely as residuals or as breakdown products of azo colourants (upon reductive cleavage of the azo bond), as indicated in the analytical testing and surveys in Europe described above (see Appendix F). Permanent tattoos are considered to be a potential source of systemic exposure to these substances for individuals, as they are injected into the dermis, below the epidermal–dermal junction, at a depth of 1–2 mm (Lea and Pawlowski 1987; Sperry 1992). The potential systemic exposure to aromatic amines from injected tattoo inks may occur from their presence as a residual and/or following degradation of an azo colourant in the ink. Temporal exposure could include an acute phase immediately post-injection, a short-term phase associated with lymphatic removal, and potential long-term exposure from the stable tattoo (Danish EPA 2012). Although there is high uncertainty specifically regarding any long-term systemic exposure from tattoos (Danish EPA 2012), upper-bounding estimates of the acute and short-term exposure to o-toluidine and o-anisidine from tattoo inks were derived (Appendix F), since these two substances were found in tattoo inks manufactured in the US (RAPEX 2012), which may also be present in the Canadian market.
Some of the 16 Aromatic Amines have also been identified as present in cigarette smoke. 2-Naphthylamine, p-toluidine, o-toluidine and o-anisidine have been determined to be present at concentrations of 6.03–12.8 (2-naphthylamine), 31.36–62.65 (p-toluidine), 37.19–93.47 (o-toluidine) and 1.98–4.83 (o-anisidine) mg/kg nicotine in mainstream smoke of cigarettes sold in Canada (personal communication, email from Controlled Substances and Tobacco Directorate [Health Canada] to Existing Substances Risk Assessment Bureau [Health Canada], dated 2013; unreferenced). Several studies have also been identified that report the presence of some of these Aromatic Amines in cigarette smoke (Pieraccini et al. 1992; Luceri et al. 1993; Stabbert et al. 2003; Goniewicz and Czogala 2005; Saha et al. 2009). The level of exposure to these substances from cigarettes is dependent on smoking habits; therefore, people who smoke or live with smokers may have higher levels of exposure to these Aromatic Amines. A number of risk management activities related to tobacco are in place. For example, regulations have been made pursuant to the Tobacco Act, regarding the manufacture, sale, labelling and promotion of tobacco products.
7.2 Health Effects Assessment
Carcinogenicity and genotoxicity are the critical health effects of potential concern for Aromatic Azo and Benzidine-based Substances (Environment Canada and Health Canada 2013a). Existing carcinogenicity and genotoxicity classifications of certain national and international agencies for the 16 Aromatic Amines in this assessment are shown in Table 7-5.
| Substance | IARCFootnote Table 7-5 [a] (2013) | EU carcinogenicityFootnote Table 7-5 [b] (ESIS ©1995–2012) | EU mutagenicity[b] (ESIS ©1995–2012) | US EPA carcinogenicityFootnote Table 7-5 [c] |
|---|---|---|---|---|
| 2-Naphthylamine | Group 1 | Category 1A “EU22” |
– | Group A (US EPA 1988) |
| o-Toluidine | Group 1 | Category 1B “EU22” |
– | Group B2 (US EPA 2013) |
| 2,4-Diaminotoluene | Group 2B | Category 1B “EU22” |
Category 2 | – |
| 4-Chloroaniline | Group 2B | Category 1B “EU22” |
– | Likely to be carcinogenic to humans (US EPA 2008a) |
| o-Anisidine | Group 2B | Category 1B “EU22” |
Category 2 | – |
| 2,4-Diaminoanisole | Group 2B | Category 1B “EU22” |
Category 2 | – |
| p-Toluidine and p-toluidine hydrochloride | – | Category 2 | – | Suggestive evidence of carcinogenic potential (US EPA 2012) |
| p-Aminophenol | – | – | Category 2 | – |
| p-Phenetidine | – | – | Category 2 | – |
| 1,3-Diaminobenzene and 1,3- diaminobenzene dihydrochloride | Group 3 | – | Category 2 | – |
| Red Lake C Amine | – | – | – | – |
| 2-Chloroaniline | – | – | – | – |
| 3,4-Dichloroaniline | – | – | – | – |
| 4-Nitroaniline | – | – | – | “Not likely to be genotoxic” (US EPA 2009b) |
As shown in Table 7-5, the first six Aromatic Amines have been classified as carcinogens by international agencies (International Agency for Research on Cancer [IARC], EU, US EPA). Unless otherwise indicated in this Screening Assessment, a review of the available health effects information indicates that the critical effect level for cancer was considered protective of non-cancer effects.
The availability of health effects information for the Aromatic Amines in this assessment is variable. 2-Naphthylamine and o-toluidine have been shown to cause bladder cancer in humans, while other Aromatic Amines in this assessment cause multisite tumours in multiple animal species; variation in tumour type and distribution is attributed to interspecies and intraspecies toxicokinetic differences (IARC 2012). Aromatic amine–induced lesions are considered to result from genotoxic and cytotoxic (proliferative) effects. However, DNA lesions do not always correlate with carcinogenic potency. For example, aniline exhibits low genotoxic potential but has been shown to induce tumours in the spleen (IARC 2010a; Neumann 2010). Increased turnover of damaged erythrocytes as a result of methemoglobin formation is hypothesized to culminate in splenic vascular congestion, inflammation and fibrosis, which may give rise to sarcoma formation in the spleen (Neumann 2010). However, both primary tumours of the spleen as well as those of vascular origin (hemangiosarcoma) are considered rare in humans (Story and Gibb 1952).
Generally, health effects of aromatic amines are considered to have a mechanism of action that involves a primary amine attached to an aromatic moiety. Metabolic activation involves two main pathways that lead to the generation of reactive intermediates known to damage DNA and proteins, as depicted in Neumann (2010).
The critical step in aromatic amine bioactivation is oxidation. The most important organ is the liver, where P450 enzymes (including 1A2, 1A1, 1B1 and 4B1) catalyze the oxygen-dependent monooxygenase reaction that oxidizes the aromatic amine to the corresponding hydroxylamine (N-hydroxy form); although most prominent in the liver, activation may also occur in other organs. In addition to hepatic P450 enzymes, other oxidizing enzymes, including lung peroxidase, granulocyte myeloperoxidase, mammary gland lactoperoxidases and ubiquitously expressed prostaglandin H synthase, may also activate aromatic amines through reductive peroxidation, in which aromatic amines are “co-oxidized” to nitrogen-centred free radicals capable of binding macromolecules (Eling et al. 1990; Thompson et al. 1992; IARC 2012).
The hydroxylamine metabolite can be further activated by conjugation reactions that generate reactive species bearing good leaving groups, such as acetate. Humans express two forms of the enzyme arylamine N-acetyltransferase: NAT1 and NAT2. Each of these enzymes may catalyze two possible reactions. N-acetylation reactions convert aromatic amines into an acetylamides which are usually deactivated and excreted. Conversely, O-acetylation reactions (catalyzed by O-acetyltransferase, OAT) produce reactive N-acetoxy metabolites, as spontaneous decomposition of the N-acetoxy metabolite generates a reactive nitrenium ion (Josephy and Novak 2013). The nitrenium ion reacts readily with nucleophilic sites on proteins and DNA, giving rise to bulky covalent adducts that may constitute pre-mutagenic lesions (IARC 2010a).
While epidemiological evidence supporting a relationship between acetylation capacity and aromatic amine sensitivity is equivocal, humans that exhibit a slow acetylator phenotype may be at increased risk (Kadlubar et al. 1988). Several studies have demonstrated some association between slow NAT2 status, especially in combination with occupational or cigarette smoking–related exposure to aromatic amines, and bladder cancer risk (Sanderson et al. 2007). In addition, increased aromatic amine sensitivity in dogs is attributed to a non-acetylator phenotype (Neumann 2010). The NAT2 gene is the locus of the extensively studied human drug (e.g., isoniazid) acetylation polymorphism, and approximately half of the population is estimated to consist of NAT2 “slow acetylators” (Sim et al. 2012). Also, the NAT1 isozyme is thought to play a lesser role in metabolism of most aromatic amine drugs and carcinogens (Walraven et al. 2008).
Conjugation with sulfate, glucuronic acid or phosphate may be alternative pathways for hydroxylamine activation. Although conjugates of glucuronic acid are generally more stable, all conjugates may be hydrolysed in low-pH environments, including the urinary bladder, to generate the nitrenium ion (IARC 2010a; Kaivosaari et al. 2011).
Another important mechanism of toxicity in humans involves the oxidation of hemoglobin by the N-hydroxylamine metabolite to form methemoglobin. Normally, endogenous hemoglobin-oxidizing substances (oxidative stress) are thought to oxidize approximately 3% of circulating hemoglobin each day. Two primary reducing systems, including cytochrome b5-methemoglobin reductase and nicotinamide adenine donucleotide phosphate (NADPH)-methemoglobin reductase, maintain methemoglobin levels at or below 1% (Wright et al. 1999; Skold et al. 2011). Compared with activity levels in human erythrocytes, the activity of the latter reducing enzyme system is reported to be 5 and 10 times greater than in the rat and mouse, respectively (Nair et al. 1986). Because rodents possess the ability to reduce methemoglobin more effectively, aromatic amine doses that produce subclinical levels of methemoglobin in rodents may actually induce clinical methemoglobemia in humans.
Also, the oxidation of hemoglobin by N-hydroxy amines in turn generates an N-nitroso form that exhibits considerable affinity for hemoglobin polypeptide protein thiol residues. The resultant hemoglobin adducts are considered a reliable long-term indicator of exposure, as the average lifespan of human erythrocytes is 120 days (Neumann 2010).
While common aromatic amine activation steps are well studied, overarching health effects uncertainties exist. The availability of epidemiological and experimental studies that considered dermal exposure was generally lacking across many of the substances assessed, and this represents a source of uncertainty, as potential adverse effects resulting specifically from dermal exposures could not be characterized.
7.3 Substance-Specific Assessments on Aromatic Amines
Substance-specific assessments are presented in this section, starting with the nine Aromatic Amines for which there is potential for human exposure in Canada (Sections 7.3.1–7.3.9), followed by a summary of health effects information on the remaining seven substances for which, based on available information, exposure to the general population of Canada is not expected.
7.3.1 2-Naphthylamine
Exposure Assessment
A number of studies were identified that investigated levels of 2-naphthylamine in indoor and outdoor air, surface water and contaminated soil (see Appendix B). All of these studies were carried out in countries other than Canada. One Canadian study, by Health Canada, conducted a retrospective determination of airborne volatile organic compounds in the stored air samples from a previous Canadian survey of volatile organic compounds in indoor air from 757 randomly selected Canadian residences (Otson et al. 1994). Aliquots of stored air samples were pooled to form composite samples, which were subsequently analyzed by gas chromatography/mass spectrometry. 2-Naphthylamine was not detected in the composite samples above the LOD (not reported). A limitation of the use of composite samples is that the range and distribution of 2-naphthylamine across the different households could not be determined.
Based on uses of products that may contain 2-naphthylamine as an impurity and the overall limited quantity of this substance in Canada (see Section 4.1; Environment Canada 2006), exposure of the general population from releases of this amine to water and soil is not expected.
2-Naphthylamine was detected in 3 of 66 textile samples in Health Canada’s product testing (Health Canada 2013a) above the LOD (1.6 mg/kg sample), but below the LOQ (4.7 mg/kg sample). Dermal and oral exposures to 2-naphthylamine from textiles were estimated based on the LOQ of 4.7 mg/kg textile (see Table 7-6 for estimated exposures and Appendix E for details). Dermal absorption was conservatively assumed to be 100%. An in vitro dermal absorption study was identified for this substance using human skin (Lüersen et al. 2006). Twenty-four hours after a dermal application of 15 µg/cm2, 54.05% of the applied dose was measured in the receptor fluid. This study was not used in this assessment due to the uncertainty associated with the lack of reporting of the skin-bound portion of 2-naphthylamine; however, the results indicate significant dermal absorption of 2-naphthylamine.
| Exposure route | Exposure scenario | Age group | Estimated acute exposure (mg/kg-bw) |
Estimated daily exposureFootnote Table 7-6 [a] (mg/kg-bw per day) |
|---|---|---|---|---|
| DermalFootnote Table 7-6 [b] | Textiles – baby sleeper | Infants | 0.038 | 1.9 × 10−4 |
| Dermal[b] | Textiles – personal apparel | Adults | 0.024 | 1.2 × 10−4 |
| Oral | Mouthing of textile objects | Infants | Not applicable | 2.5 × 10−5 |
Health Effects Assessment
Carcinogenicity and Genotoxicity
2-Naphthylamine has been classified as a carcinogen by several international agencies (presented in Table 7-5). There is clear evidence to support the carcinogenic potential of 2-naphthylamine based on bladder tumour incidence in exposed humans as well as experimental animals: monkeys, dogs, rabbits, rats and hamsters. Liver and lung tumours have also been reported in mice treated by intraperitoneal and subcutaneous routes. Limited information indicates that dermal exposures have not resulted in tumour development. No inhalation carcinogenicity bioassays, reproductive or developmental studies were identified in the available literature. A rationale for selection of the critical effect levels for derivation of MOEs is given below.
Available information from case reports and epidemiological studies indicates a strong relationship between 2-naphthylamine exposure and bladder cancer development in occupationally exposed humans. International case reports since the 1960s have reported elevated bladder cancer incidence among 2-naphthylamine-exposed workers in France, Italy, Japan and Great Britain (Billiard-Duchesne 1960; Vigliani and Barsotti 1962; Tsuji 1962; Tsuchiya et al. 1975). Cumulative bladder cancer incidence was reported to be 25% in British coal tar dye workers (Goldwater et al. 1965). In Japan, 10.3% of 1085 workers involved in 2-naphthylamine and benzidine synthesis and handling developed occupation-related bladder cancer (Shinka et al. 1991). In another report, 20% of Japanese workers involved in aromatic amine production developed uroepithelial cancer (Hamasaki et al. 1996). Cohort studies have shown that both bladder cancer incidence and mortality are elevated in occupationally exposed individuals. Bladder cancer incidence has been reported to be significantly elevated in British, American, Polish and Russian rubber and chemical industry workers (Case et al. 1954; Mancuso and el-Attar 1967; Szeszenia-Dabrowska et al. 1991; Bulbulyan et al. 1995; Veys 2004). Significantly elevated mortality rates have also been reported in Italian, American and Japanese cohorts (Decarli et al. 1985; Delzell et al. 1989; Morinaga et al. 1990; Naito et al. 1995; Cassidy et al. 2003). However, 2-naphthylamine-associated tumours in a Georgia, USA, cohort were reported to involve the lungs and prostate rather than the urinary bladder (Schulte et al. 1985a,b, 1986; Axtell et al. 1998).
It has been proposed that the carcinogenic potential of 2-naphthylamine may be influenced by the expression of prostaglandin H synthase in the urinary bladder. While the presence of prostaglandin H synthase in a modified Ames assay did not increase mutagenic events in TA98, TA100 and TA102 strains of Salmonella typhimurium, bladder transitional epithelial microsomes containing prostaglandin H synthase–activated 2-naphthylamine caused protein and DNA binding at levels greater than that associated with hepatic microsomal activation (Wise et al. 1984; Sarkar et al. 1992).
2-Naphthylamine exposure has been shown to increase tumour incidence in a range of bioassays in experimental animals. Oral administration in rats, dogs, rabbits, hamsters and monkeys has resulted in significantly increased incidences of bladder tumours, with the lowest lowest-observed-adverse-effect level (LOAEL) of 5.4 mg/kg body weight (kg-bw) per day reported in the Beagle dog (Bonser et al. 1952, 1956; Saffiotti et al. 1967; Hadidian et al. 1968; Conzelman et al, 1969; Harrison et al. 1969; Conzelman and Moulton 1972; Romanenko and Martynenko 1972; Hicks and Chowaniec 1977; Radomski et al. 1977; Rigotti et al. 1977; Purchase et al. 1981; Hicks et al. 1982). However, oral doses beginning at 23 mg/kg-bw per day (LOAEL) have induced hepatomas in mice (Bonser et al. 1952; Yoshida et al. 1979). Chronic subcutaneous injection of 2-naphthylamine in mice has been reported to induce lung tumours, subcutaneous sarcomas and hepatomas, from doses beginning at 1 mg/kg-bw per day (LOAEL) (Bonser et al. 1956; Walters et al. 1967; Radomski et al. 1971). Mice chronically treated with 2-naphthylamine via intraperitoneal injection developed lung tumours from doses starting at 250 mg/kg-bw per day (LOAEL) (Theiss et al. 1981; Stoner et al. 1986). No relevant dermal exposure studies were identified in the available literature.
The key study was conducted by Conzelman and Moulton (1972). Beagle dogs were administered 0, 6.25, 12.5, 25 or 50 mg/kg-bw per day in hard lactose-gelatin capsules, 6 days/week for up to 26 months (equivalent to 0, 5.4, 10.7, 21.4 and 42.9 mg/kg-bw per day). While negative controls (n = 4) did not develop tumours, all treated dogs developed invasive transitional carcinoma, invasive squamous carcinoma, papillary carcinoma or a combination thereof. Invasive transitional carcinoma was observed in 2 of 9 (22%) dogs administered 6.25 mg/kg-bw per day, 2 of 10 (20%) administered 12.5 mg/kg-bw per day, 5 of 10 (50%) administered 25 mg/kg-bw per day and 2 of 5 (40%) administered 50 mg/kg-bw per day. Invasive squamous carcinoma was observed in 1 of 9 (11%) dogs administered 6.25 mg/kg-bw per day, 2 of 10 (20%) administered 12.5 mg/kg-bw per day, 3 of 10 (30%) administered 25 mg/kg-bw per day and 2 of 5 (40%) administered 50 mg/kg-bw per day. Furthermore, papillary carcinoma was observed in 0 of 9 (0%) dogs administered 5.4 mg/kg-bw per day, 1 of 10 (10%) given 10.7 mg/kg-bw per day, 3 of 10 (30%) administered 21.4 mg/kg-bw per day and 4 of 5 (80%) administered 42.9 mg/kg-bw per day. Other studies have also reported pathological lesions in the urinary bladders of treated dogs, which included preneoplastic, hyperplastic papilloma (early), papilloma (late), squamous metaplasia (early), squamous metaplasia (late), invasive transitional carcinoma, invasive squamous carcinoma and papillary carcinoma (Hueper et al. 1938; Bonser 1956; Harrison et al. 1969; Romanenko and Martynenko 1972; Radomski et al. 1977; Purchase et al. 1981).
The in vitro and in vivo genotoxic potential of 2-naphthylamine has been previously assessed by IARC (2010a). This substance is considered weakly mutagenic towards Salmonella typhimurium and Chinese hamster ovary cells, but not hamster V79 cells. Positive results in bacteria were observed in the presence of metabolic conversion and activation; however, some mammalian cell assays were positive in the absence of added activating factors. Evidence for mutagenesis in yeast cells is considered inconclusive. Evidence for in vivogenotoxicity includes the induction of recessive lethals in Drosophila melanogaster, micronucleus formation in mice administered high doses of 2-naphthylamine and DNA fragmentation in the rodent liver; however, 2-naphthylamine does not induce sister chromatid exchange events in the mouse, and evidence for sperm abnormalities in that species is equivocal (IARC 2010a).
Other Health Effects
Exposure to 2-naphthylamine in humans is reported to cause methemoglobinemia and cyanosis, shortness of breath on exertion, hematuria and increased peripheral blood lymphocytic activity ex vivo (Kumar et al. 1981; HSDB 1983– ; ChemIDplus 1993– ). Reproductive and developmental toxicity studies in humans and experimental animals have not been identified.
Risk Characterization
Exposure of the general population in Canada to 2-naphthylamine is not expected from environmental media.
Exposure of the general population in Canada to2-naphthylamine via consumer products is expected to occur predominantly through use of textile products Daily dermal exposures of adults and infants resulting from skin contact with textile clothing were estimated to be 1.2 × 10−4 and 1.9 × 10−4 mg/kg-bw per day, respectively. Daily oral exposure of infants from mouthing of textile objects was estimated to be 2.5 × 10−5 mg/kg-bw per day.
The critical effect level protective of both cancer and non-cancer effects associated with oral 2-naphthylamine administration is a LOAEL of 5.4 mg/kg-bw per day, which is based on a chronic dietary study in Beagle dogs (Conzelman and Moulton 1972); the animals exhibited invasive, transitional and squamous carcinoma in the urinary bladder at the lowest dose administered, 5.4 mg/kg-bw per day. A critical effect level associated with the dermal route of exposure was not identified. Oral toxicity data were used in the absence of dermal data; as a conservative precautionary assumption absorption by the oral and dermal routes was considered equivalent.
Comparison of the estimated daily oral exposure of infants to 2-naphthylamine via mouthing of textile products with the critical effect level (LOAEL of 5.4 mg/kg-bw per day) results in a margin of exposure (MOE) of 216 000. Comparison of estimated daily dermal exposures from wearing personal apparel with the same critical effect level results in MOEs of 28 400 for infants and 45 000 for adults. These MOEs are considered adequate to address uncertainties in the health effects and exposure databases (Table 7-7). The confidence in the risk characterization is high due to the use of a conservative dermal absorption factor (i.e.,100%).
Although dermal acute exposure scenarios were identified for 2-naphthylamine (e.g., personal apparel contact in adults and children), the available information does not indicate that 2-naphthylamine demonstrates high acute toxicity. Consequently, the risk for the general population is considered to be low.
| Exposure route | Exposure scenario (age group) | Estimated daily exposureFootnote Table 7-7 [a] (mg/kg-bw per day) |
Critical effect level: Oral feeding LOAEL (mg/kg-bw per day) | MOEs |
|---|---|---|---|---|
| DermalFootnote Table 7-7 [b] | Textiles – Personal apparel (adult) | 1.2 × 10−4 | 5.4 | 45 000 |
| Dermal[b] | Textiles – Baby sleeper (infant) | 1.9 × 10−4 | 5.4 | 28 400 |
| Oral | Mouthing of textile objects (infant) | 2.5 × 10−5 | 5.4 | 216 000 |
7.3.2 o-Toluidine
Exposure Assessment
In response to a previous section 71 survey, between 100 and 1 000 kg of o-toluidine was imported into Canada in 2008 (Environment Canada 2009). Based on the limited volume in commerce and its uses, exposure of the general population via releases of o-toluidine to the environment not expected.
o-Toluidine is used in tire manufacturing in Canada (Environment Canada 2009). Concentrations of this amine, if present in the final product, are expected to be very low. Therefore, human exposure to o-toluidine in tires through their service life and recycled uses is not expected.
Based on the maximum measured concentration (0.26 ppb) of o-toluidine in the breast milk of lactating mothers in Canada (DeBruin et al. 1999), the upper-bounding daily intake was estimated to be 0.03 µg/kg-bw per day for breastfed infants (0–6 months of age). While the presence of o-toluidine in breast milk of the mothers is indicative of recent exposures, specific sources and exposure levels could not be ascertained from this study.
Based on the two independent studies that reported migration of o-toluidine from certain polyamide cooking utensils in Ireland and Denmark (Trier et al. 2010; McCall et al. 2012), potential exposure of the general population of Canada has been estimated via use of cooking utensils. Using the higher median migration level of o-toluidine found between these studies (0.0005 µg/g food; McCall et al. 2012), potential exposure to o-toluidine from the use of polyamide cooking utensils was estimated to range from 7 × 10−4 µg/kg-bw per day (12 years of age and above) to 2.3 × 10−3µg/kg-bw per day (toddlers 0.5–4 years of age).
Conservative acute exposure to residual o-toluidine in tattoo ink was estimated to range from 0.0021 to 0.019 mg/kg-bw. Daily exposure through short-term release of amines due to gradual cleavage of azo colourants by metabolism and photodegradation was estimated to range from 4.3 × 10−7 to 4.1 × 10−7 mg/kg-bw per day. These exposure estimates are considered upper-bounding due to the underlying conservative assumptions used in their derivation (see Appendix F). Due to the high uncertainty associated with these exposure estimates, the risk from tattoo exposure has not been estimated (see Section 7.5.1 Uncertainty).
Health Effects Assessment
Carcinogenicity and Genotoxicity
o-Toluidine has recently been reclassified by IARC as Group 1, carcinogenic to humans (Table 7-5) (IARC 2012), based on strong epidemiological evidence. Occupational o-toluidine inhalation and dermal exposures are associated with a significantly increased risk of bladder cancer. The carcinogenicity of o-toluidine administered to animals via the oral route has been clearly demonstrated, with the distribution and types of tumours varying across species. A rationale for selection of the critical effect levels for derivation of MOEs is given below.
There is strong epidemiological evidence to support the carcinogenic potential of o-toluidine in humans. Occupational exposures have been described in three large cohorts involving workers at a New York state chemical plant, chemical production factory workers in North Wales and individuals employed at a dyestuff factory in Italy. While specific exposure doses have not been quantified in epidemiological studies, the results clearly demonstrate significantly increased incidence of, and mortality from, bladder cancer among exposed workers. Ward et al. (1991) reported that bladder cancer incidence among workers with known exposures was significantly higher than that of non-occupationally exposed individuals living in New York state. Incidence among those possibly exposed was also significantly elevated. Exposure durations of 10 years or more were positively associated with cancer incidence. Also, 10–20 years from the date of first employment in an exposed workplace elevated the standardized incidence ratio, and the elevation in standardized incidence ratio was statistically significant when time from first employment in an exposed department was 20 years or more. Data from this study were later re-evaluated by Carreón et al. (2010), and similar conclusions were drawn. In a cohort of 2 160 male production workers at a chemical production factory in North Wales (exposed to vulcanization inhibitors and accelerators, antioxidants and proprietary products for the rubber industry), Sorahan (2008) demonstrated that o-toluidine exposures within the cohort were associated with the highest bladder cancer mortality. Furthermore, the relative risk of developing bladder cancer was significantly elevated in workers positioned in departments with known o-toluidine exposures for employment durations of 0.1–4.9 years and 5 or more years. In Italy, bladder cancer incidence in factory workers was evaluated by Rubino et al. (1982). In a cohort of 868 male workers (80% had also been exposed to benzidine, naphthylamines or both), mortality resulting from bladder cancer (n = 36; 4.1%) was significantly higher than in the general population. Elevated mortality rates were also associated with lung, larynx and esophageal neoplasms within the worker cohort. Also, mortality caused by all tumours significantly increased with time from first exposure.
o-Toluidine-induced carcinogenicity appears to exhibit route specificity in experimental animals. While there is strong evidence to support carcinogenic potential in orally administered animals, subcutaneous treatments have not demonstrated the same effect. Outbred Syrian Golden hamsters administered 52 weekly subcutaneous injections of o-toluidine (approximately 261 mg/kg-bw per day) in peanut oil did not exhibit an increased incidence of tumours of any kind (Hecht et al. 1983). Studies have not been conducted via the dermal route, and the potential for carcinogenicity by this route of administration is uncertain for o-toluidine. Experiments involving excised skin mounted on diffusion cells have demonstrated 15% absorption of the applied dose over 7 hours and 50% over 24 hours. However, investigators have demonstrated an association between enhanced o-toluidine absorption with use of skin barrier creams (Korinth et al. 2008).
An NCI (1979b) study demonstrated the carcinogenicity of o-toluidine in Fischer 344 rats. Fifty male and 50 female rats were orally administered 0, 3 000 or 6 000 ppm o-toluidine hydrochloride in feed ad libitum for 101–104 weeks (males: low and high doses approximately 88 and 175 mg/kg-bw per day; females: low and high doses approximately 139 and 278 mg/kg-bw per day). Male rats exhibited a significant dose-related increase of fibromas in subcutaneous skin tissues; osteosarcoma, myxosarcoma, fibrosarcoma, sarcoma and lipoma in subcutaneous tissues at both dose levels; sarcomas of multiple organs and fibrosarcomas of multiple organs at the high dose; and mesotheliomas in multiple organs or the tunica vaginalis (serous covering of the testes) at the low but not the high dose. Female rats developed osteosarcomas of multiple organs at the high dose; mammary gland fibroadenomas and adenomas at both doses; transitional cell bladder carcinomas at the high but not the low dose; spleen sarcomas (angiosarcoma) at the high dose; and sarcomas of multiple organs (sarcoma, fibrosarcoma, angiosarcoma or osteosarcoma) at the high but not the low dose.
As part of the same study, 50 male and 50 female B6C3F1 mice were administered 0, 1 000 or 3 000 ppm o-toluidine hydrochloride in feed ad libitum for 101–104 weeks (males: low and high doses approximately 64 and 191 mg/kg-bw per day; females: low and high doses approximately 66 and 199 mg/kg-bw per day). Tumour type in this species did not exhibit the large degree of variability observed in rats; most tumours developed in the liver (hepatocellular carcinoma or adenoma). The incidence of combined hepatocellular carcinoma and adenoma was significantly elevated in females that received the high but not the low dose. While not statistically significant, biologically relevant hemangioma and hemangiosarcoma lesions were also observed at multiple sites in high- and low-dose males (NCI 1979b).
Other chronic feeding studies in rats have also demonstrated tumorigenic potential. Weisburger et al. (1978) reported a positive carcinogenic response in all Charles River CD rats treated with o-toluidine. Males developed subcutaneous fibromas and fibrosarcomas, pituitary and adrenal adenomas, as well as hemangiosarcomas and hemangiomas in abdominal viscera. Female mice exhibited a significant increase in vascular tumours. NTP (1996) reported lesions in the testes, epididymis, liver, kidney, spleen and urinary bladder of chronically administered male F344/N rats. Seminiferous tubule degeneration was also reported in 5–10% of treated rats. Mild diffuse hyperplasia was observed in the transitional bladder epithelium, as were significant changes in the spleen; increased absolute and relative spleen weights (in the presence of total body weight reductions), white granular plaques on the capsular surface, capsular fibrosis, fibrotic areas with lymphocyte and hematopoietic cells, and increased hemosiderin concentrations were observed. Splenic changes were attributed to hemoglobin denaturation, Heinz body formation and increased erythrocyte destruction (NTP 1996).
o-Toluidine is generally considered genotoxic in vitro and in vivo. NTP (2013a) reported that o-toluidine binds DNA, causes DNA and chromosomal damage and induces mutations in vitro. OECD (2004a) supported o-toluidine-induced clastogenicity and mutagenicity in vitro, based on reports of micronucleus formation in human lymphocytes and induction of chromosomal aberrations in several cell systems. Based on in vivo evidence, IARC (2012) concluded that o-toluidine induces DNA lesions in multiple organs. NTP (2013a) considered o-toluidine capable of causing DNA and chromosomal damage and single-stranded DNA breaks. In the OECD (2004a) report, o-toluidine was considered clastogenic in vivo, based on evidence of sister chromatid exchange and unscheduled DNA synthesis induction in rats.
Other Health Effects
o-Toluidine may have adverse effects on reproduction. Doses of 272 mg/kg-bw per day for 26 weeks produced testicular atrophy and seminiferous tubule degeneration in male rats (NTP 1996). NCI (1979b) reported significantly elevated mesotheliomas in the tunica vaginalis (testicular serous covering) of male rats at low (88 mg/kg-bw per day) but not high doses. o-Toluidine exposure may also lead to developmental effects, but the severity and types of effects have not been characterized. Progeny from male and female rats dermally administered 0, 8 or 80 mg/kg-bw via the tail skin were evaluated. Offspring of treated females and males exhibited reduced relative organ weights: kidneys, ovaries, hearts and lungs (Malysheva et al. 1983). Furthermore, OECD (2004a) concluded that while none of the available studies on reproductive or developmental toxicity was suitable for review, o-toluidine was considered a potential reproductive and developmental toxicant because of its genotoxic and carcinogenic properties. In addition, the German Commission for the Investigation of Health Hazards of Chemical Compounds in the Work Area (the MAK Commission) has categorized o-toluidine as a Class 3A germ cell mutagen (DFG 2012b).
Other adverse non-cancer effects following repeated oral exposures include increased spleen weight, splenic congestion and hematopoiesis in male rats (Short et al. 1983). Methemoglobin formation, bladder wall thickening and urothelial hyperplasia have also been reported in male and female rats (EI Dupont de Nemours & Co. Inc. 1994). In male Fischer 344 rats administered 225 mg/kg-bw per day via oral gavage once daily for up to 5, 10 or 20 days, mean spleen weight was significantly increased from control values in animals dosed at all three intervals, and the splenic changes were associated with congestion, hemosiderosis and hematopoiesis (Short et al. 1983).
Acute studies in mice, rats, cats, rabbits and guinea pigs demonstrated similar adverse effects, such as anesthesia, increased diuresis, cyanosis, dose-related increases in methemoglobinemia, pulmonary congestion, rapid respiration rate, laboured breathing, reddish-brown nasal discharge, lethargy and physical exhaustion, regardless of exposure route (oral, dermal, inhalation or intraperitoneal) (All in OECD SIDS 2004a: Smyth et al. 1962; Bayer 1978; Weisburger et al. 1978; BASF AG 1979; Price et al. 1979; DuPont Chem 1981; Senczuk and Rucinska 1984). The lowest reported median lethal dose (LD50) values for oral, dermal and intraperitoneal administration of o-toluidine were 750, 3250 and 113 mg/kg-bw, respectively.
Risk Characterization
A potential source of exposure to o-toluidine in Canada was identified to be from human milk for breastfed infants. Using the maximum measured concentration of o-toluidine in human breast milk (0.26 μg/kg) the upper-bounding daily intake was estimated to be 0.03 µg/kg-bw per day for breastfed infants (less than 6 months old). Potential daily oral exposure to o-toluidine from use of polyamide cooking utensils was conservatively estimated to range from 7 × 10−7mg/kg-bw per day (12 years of age and above) to 2.3 × 10−6 mg/kg-bw per day (toddlers 0.5–4 years of age).
The critical effect level is a LOAEL of 64 mg/kg-bw per day, which is based on a chronic dietary study in male mice; animals administered o-toluidine exhibited hemangioma and hemangiosarcoma at multiple sites.
Comparison of the upper-bounding estimate of oral exposure to o-toluidine in infants from breast milk intake with the critical effect level (LOAEL of 64 mg/kg-bw per day) results in an MOE of over 1 million. Likewise, comparison of the upper-bounding estimate of daily oral exposure to o-toluidine from use of cooking utensils with the same critical effect level also results in an MOE over 1 million (Table 7-8). These MOEs are considered adequate to address uncertainties in the health effects and exposure databases.
| Exposure route | Exposure | Upper-bounding daily exposure (mg/kg-bw per day) |
Critical effect level: Oral LOAEL (mg/kg-bw per day) |
MOEs |
|---|---|---|---|---|
| Oral | Breast milk (infant 0–0.5 year) |
3 × 10−5 | 64 | 2.13 × 106 |
| Oral | Cooking utensils | 2.3 × 10−6 | 64 | 2.8 × 107 |
7.3.3 2,4-Diaminotoluene
Exposure Assessment
No manufacture or import activity of 2,4-diaminotoluene was reported in Canada in response to a section 71 survey (Canada 2009; Environment Canada 2009). No information related to releases or measured concentrations of this substance in the Canadian environment was identified (NPRI 2006). Consequently, based on the available information, exposure of the general population in Canada to 2,4-diaminotoluene from environmental media is not expected.
Based on the two independent studies that reported migration of 2,4-diaminotoluene from certain black polyamide cooking utensils in Ireland and Denmark (Trier et al. 2010; McCall et al. 2012), potential exposure of the general population of Canada has been estimated via use of cooking utensils. Using the higher median migration level of 2,4-diaminotoluene between the two studies (0.001 µg/g food; McCall et al. 2012), potential daily exposure to 2,4-diaminotoluene from the use of polyamide cooking utensils was estimated to range from 1.3 × 10−3 µg/kg-bw per day (seniors 60 years of age and older) to 4.7 × 10−3 µg/kg-bw per day (toddlers 0.5–4 years of age).
2,4-Diaminotoluene was detected in 1 of 66 textile samples in product testing (Health Canada 2013a). Dermal and oral exposures to 2,4-diaminotoluene from textiles were estimated based on the maximum detected concentration (4.2 mg/kg) (see Table 7-9). Dermal absorption was assumed to be 24% in the estimation of dermal exposure. This is based on a dermal absorption study that was identified for 2,4-diaminotoluene. 14C-radiolabelled 2,4-diaminotoluene in acetone was placed on a human forearm for 24 hours; urine samples were collected over a 5-day period and analyzed for radiolabel. Results showed a 24% ± 16% dermal absorption (corrected for incomplete recovery) for 2,4-diaminotoluene (Marzulli et al. 1981). This represents the mean ± 2 standard deviations in a test group size between three and six subjects (not specified).
| Exposure route | Exposure scenario | Age group | Estimated acute exposure (mg/kg-bw) | Estimated daily exposureFootnote Table 7-9 [a] (mg/kg-bw per day) |
|---|---|---|---|---|
| DermalFootnote Table 7-9 [b] | Textiles – baby sleeper | Infants | 0.008 | 4.1 × 10−5 |
| Dermal[b] | Textiles – personal apparel | Adults | 0.005 | 2.5 × 10−5 |
| Oral | Mouthing of textile products | Infants | Not applicable | 2.2 × 10−5 |
Health Effects Assessment
Carcinogenicity and Genotoxicity
2,4-Diaminotoluene has been classified by IARC as Group 2B, possibly carcinogenic to humans (Table 7-5). While no epidemiological studies or case reports describing any diaminotoluene isomers have been identified in the available literature, data from animal studies clearly identify cancer and non-cancer effects. There is strong evidence to support the carcinogenic potential of 2,4-diaminotoluene in animals following oral and subcutaneous, but not dermal, exposures. A rationale for selection of the critical effect levels for derivation of MOEs is given below.
Chronic 2,4-diaminotoluene feeding studies have demonstrated mammary tumour development in female rats, subcutaneous fibromas in male rats, lung adenomas in male rats, as well as vascular tumours in male and female mice (Ito et al. 1969; Stula and Aftosmis 1976 in, ECJRC 2008); Weisburger et al. 1978; Cardy 1979). More recently, Morton et al. (2002) observed renal carcinoma in orally dosed male Eker rats. Injection site fibrosarcomas were also reported in rats treated via subcutaneous injection (Steinhoff and Dycka, 1981 in, ECJRC 2008).
The NCI (1979a) study in rats and mice exemplifies the variability in 2,4-diaminotoluene-induced tumours in experimental animals. Groups of 50 male and 50 female rats were administered 2,4-diaminotoluene in feed ad libitum. Excessive body weight depression was observed and necessitated dose adjustments during the study. Low-dose groups (males and females) were given a time-weighted average dose of 79 ppm (approximately 4.0 mg/kg-bw per day) for 103 weeks. High-dose females were given a time weighted average dose of 171 ppm (approximately 9.0 mg/kg-bw per day) for 84 weeks, and males were given a time weighted average dose of 176 ppm (approximately 9.0 mg/kg-bw per day) for 79 weeks. The authors reported statistically significant increased incidences of subcutaneous fibroma in both males and females. In males, 19 of 50 animals (38%) in the high dose group and 15 of 50 animals (30%) in the low dose group developed tumours, while only 1 of 20 controls (5%) exhibited this tumour type. In females, 10 of 50 (20%) in the high dose group developed tumours and 0 of 20 (0%) of control animals develop this tumour type. While 2,4-diaminotoluene induced hepatic lesions (hepatocellular carcinoma or neoplastic nodules) in males in a dose-dependent manner, this effect was less robust. Zero of 20 (0%) and 10 of 50 (20%) males developed tumours in the control and high-dose groups, respectively. Nevertheless, the trend in hepatocellular carcinoma or neoplastic nodules was statistically significant in males and females. In addition, mammary tumours (adenoma or carcinoma) were observed in both males and females, but incidence rates were statistically significant only in females: 1 of 20 (5%), 38 of 50 (76%) and 41 of 50 (82%) in the control, low-dose and high-dose groups, respectively.
In the same study, groups of 50 male and 50 female B6C3F1 mice were administered either 100 or 200 ppm 2,4-diaminotoluene in feed ad libitum for 101 weeks (approximate low and high doses in both males and females: 13 and 26 mg/kg-bw per day). Twenty male and female mice were used as controls. All mice in both the low- and high-dose groups exhibited reduced body weight gain and tissue mass; wasting was frequently observed in treated animals. Forty-three of 50 (86%) males in the high-dose group, 45 of 50 (90%) in the low-dose group and 18 of 20 (90%) control animals survived until the end of the study. Thirty-nine of 50 (78%) females in the high-dose group, 40 of 50 (80%) in the low-dose group and 15 of 20 (75%) in the control group survived until study completion. Target organs identified in this species were liver, bone marrow and hematopoietic tissues, lung and vasculature. The trend of hepatocellular carcinomas was significant in females. The incidence was significantly elevated in the low- and high-dose groups: 0 of 19 (0%), 13 of 47 (28%) and 18 of 46 (39%) in the control, low-dose and high-dose groups, respectively. Hematopoietic tumours (lymphoma and leukemia) were reported in 2 of 20 (10%) control males, 15 of 50 (30%) low-dose males and 8 of 49 (16%) high-dose males. In females, 2 of 19 (11%) controls, 29 of 47 (62%) low-dose animals and 11 of 46 (24%) high-dose animals developed hematopoietic tumours. Although not statistically significant, increased numbers of lung tumours (alveolar and bronchiolar carcinomas) were observed in 0 of 20 (0%) controls, 9 of 50 (18%) low-dose and 6 of 49 (12%) high-dose males (NCI 1979a).
The US National Toxicology Program (NTP) testing status as of February 2014 (NTP 2014a) reports this substance is mutagenic in Salmonella strains, and clastogenic (causes increased sister chromatid exchange events and chromosomal aberrations) in Chinese hamster ovary cells. In 1987, the World Health Organization’s International Programme on Chemical Safety (IPCS) reported that evidence for 2,4-diaminotoluene mutagenesis was equivocal in Salmonella strains, but was positive (increased unscheduled DNA synthesis) in primary rat hepatocyte cultures, L5178Y mouse lymphoma and Chinese hamster ovary AT3-2 cells. In addition, this substance was reported to induce morphological transformations in Syrian Golden hamster embryo cells. IARC (1978) reported positive findings of 2,4-diaminotoluene-induced mutagenesis in Salmonellastrains and cell transformations in Syrian Golden hamster embryo cells. More recent in vitro reports have further supported 2,4-diaminotoluene-induced mutagenesis in Salmonella (DFG 1985; George and Westmoreland 1991; George et al. 2001; Toyoda-Hokaiwado et al. 2010). Furthermore, genotoxicity resulting in increased unscheduled DNA synthesis has also been observed in 2,4-diaminotoluene-exposed human primary hepatocytes, human-derived hepatoma cells and rat hepatocytes (Butterworth et al. 1989; Selden et al. 1994; Séverin et al. 2005). Micronucleus formation has also been observed in an exposed human-derived hepatoma cell line (Séverin et al. 2005). Induction of chromosomal aberrations in Chinese hamster ovary WBL cells as well as sister chromatid exchanges and chromosomal aberrations in Chinese hamster ovary cells were also reported (Loveday et al. 1990; Bean et al. 1992). In addition, 2,4-diaminotoluene was shown to transform Syrian Golden hamster embryo cells (LeBoeuf et al. 1996; Pant et al. 2008).
The evidence for in vivo genotoxicity of 2,4-diaminotoluene is equivocal. The NTP testing status as of February 2014 (NTP 2014a) indicates that this substance does not cause micronucleus formation in male and female B6C3F1mice (study 520516 started 1988; study 571690 started 1991). IPCS (1987) reported weak in vivo mutagenic potential in the form of sex-linked recessive lethals in Drosophila melanogaster,but 2,4-diaminotoluene was not reported to induce dominant lethals, sperm abnormalities, recessive spots or chromosomal aberrations in mice. IARC (1978) also reported weak mutagenic potential of 2,4-diaminotoluene in the Drosophila melanogaster sex-linked recessive lethal assay. More recent in vivo reports support 2,4-diaminotoluene-induced genotoxicity; DNA damage in various organs in treated (Wistar, F344, PVG) rats and (ddY) mice was observed (George and Westmoreland 1991; Sasaki et al. 1999; Sekihashi et al. 2002). Transgenic (Lac Z-Muta™Mouse and C57Bl6 Big Blue) mice and (F344 gpt delta) rats treated with 2,4-diaminotoluene also exhibited increased mutations (Hayward et al. 1995; Suter et al. 1996; Kirkland and Beevers 2006; Toyoda-Hokaiwado et al. 2010). Micronuclei were also observed in exposed F344 rats (George and Westmoreland 1991).
Other Health Effects
2,4-Diaminotoluene may have adverse effects on reproductive capability by reducing sperm production capacity; however, data on developmental effects are limited. In occupationally exposed males, reduced sperm count has been reported (Zenz 1988), although no sperm head abnormalities have been observed (Soares and Lock 1980; Topham 1980a,b). 2,4-Diaminotoluene was reported to cross the blood–testes barrier and inhibit testicular DNA synthesis (Greene et al. 1981). In female mice, doses of 150 mg/kg-bw per day decreased litter number and litter sizes (Hardin et al. 1987).
Varma et al. (1988) administered 0 or 0.03% 2,4-diaminotoluene to male Sprague-Dawley rats in feed ad libitum for 10 weeks (approximately 15 mg/kg-bw per day). Electron microscopic examination revealed degenerative changes in Sertoli cells, including cytoplasmic swelling, membrane disruption and vacuolization. In addition, following dietary exposure to approximately 31 mg/kg-bw per day for 3 weeks, sperm count was reported to be significantly less than in controls without any change in testosterone concentrations. Peritubular tissue exhibited thickening, and small vacuoles were observed in Sertoli cells.
To evaluate reproductive capacity, Thysen et al. (1985) administered 0, 0.01% or 0.03% 2,4-diaminotoluene to male Sprague-Dawley rats in feed ad libitum for 10 weeks (approximately 0, 5 and 15 mg/kg-bw per day). Treated males were each mated with two untreated virgins. All animals assigned to control and low-dose groups demonstrated an equivalent level of fertility, while animals in the high-dose group exhibited a reduced number of fertile matings. However, implantation numbers per pregnant female and resorptions were similar across all control and treated animals. Microscopic examination of testicular tissues revealed focal or diffuse hypospermatocytogenesis without interstitial cell degeneration. The lumens of seminiferous tubules in affected areas contained exfoliated cells. Also, many tubules were devoid of sperm. The authors suggested that an inhibition of spermatogenesis was responsible for the observed reduction in fertility.
Reported acute and chronic non-cancer effects in animals are similar; extensive tissue distribution of 2,4-diaminotoluene produces disturbances in multiple organ systems. Lethality study reports indicate that acute oral exposure causes increased methemoglobin concentration in blood and cyanosis, weight loss, increased diuresis and gastrointestinal tract inflammation in rats, mice and cats. Dermal lethality studies in rats also reported methemoglobinemia, petechial gastric hemorrhages, liver lesions, sedation, hyperemia of the lungs, laboured breathing, discolouration of the lungs and liver, central nervous system depression, loss of motor coordination, convulsions and enlarged adrenal glands (Bayer 1971, 1974, 1981, in ECJRC 2008; Waring and Pheasant 1976 in ECJRC 2008; Zalchari 1978 in ECJRC 2008; Weisbrod and Stephan 1983 in ECJRC 2008).
Chronic non-cancer effects commonly involve the liver. In humans, 2,4-diaminotoluene exposure has been reported to produce jaundice (Patty 1963). In male and female rats, chronic subcutaneous injections of 2,4-diaminotoluene produced cirrhosis and liver cell degeneration, as well as subcutaneous lesions related to the administration route (Steinhoff and Dycka in ECJRC, 2008). Biochemical changes associated with liver damage have also been reported: elevated serum alkaline phosphatase, alanine aminotransferase (glutamate–pyruvate transaminase) and bilirubin in orally dosed male and female rats that exhibited focal liver necrosis. Also, chronic anemia (reduced blood hemoglobin levels), leukocytosis (elevated white blood cells) and tissue accumulation of hemosiderin (intracellular iron–protein aggregates) was observed at 258 mg/kg-bw per day (Stula and Aftosmis 1976 in ECJRC 2008).
Thirty Sprague-Dawley rats were administered 2,4-diaminotoluene at a dose of 0, 8.33 or 25 mg/kg-bw via subcutaneous injection once weekly for 2 years (approximately 0, 1.2 and 3.6 mg/kg-bw per day, respectively). Sixteen of 60 (27%) animals in the high-dose group, 7 of 60 (12%) in the low-dose group and 4 of 60 (7%) controls exhibited focal hepatic necrosis and liver cirrhosis (Steinhoff and Dycka 1981 in ECJRC 2008).
Risk Characterization
The predominant sources of exposure to 2,4-diaminotoluene for the general population in Canada is considered to be contact with textiles and use of polyamide cooking utensils. MOEs based on the estimated exposures, and the associated critical effect levels, are presented in Table 7-10.
Potential daily oral exposure to 2,4-diaminotoluene from use of black polyamide cooking utensils was conservatively estimated to range from 1.3 × 10−6, mg/kg-bw per day to 4.7 × 10−6. mg/kg-bw per day. Daily exposure of infants via mouthing of textile objects was estimated to be 2.2 × 10−5 mg/kg-bw per day. Also, daily exposures of adults and infants from dermal contact with textiles in personal apparel and baby sleepers were estimated to be 2.5 × 10−5 mg/kg-bw per day, and 4.1 × 10−5 mg/kg-bw per day respectively.
The critical effect levels protective of both cancer and non-cancer effects following either oral or dermal exposure to 2,4-diaminotoluene are based on cancer effects in rats, while shorter duration of exposure at higher doses has been shown to cause reproductive effects. The critical effect level (LOAEL) for cancer effects is 4.0 mg/kg-bw per day for males and females, which was based on a 103-week dietary study in rats. At these doses, males developed subcutaneous fibromas after 71 weeks of exposure and females exhibited mammary adenomas and carcinomas after 40 weeks of exposure. Male rat reproductive effects (increased testes weight and Sertoli cell degeneration) were observed in animals administered 15 mg/kg-bw per day for 10 weeks. Also, a 3-week exposure to 31 mg/kg-bw per day reduced sperm count and increased testes weight and Sertoli cell vacuolization. Following subcutaneous injection, rats showed signs of liver toxicity and body weight reduction in a 2-year study with a similar critical effect level of 3.6 mg/kg-bw per day.
Comparison of the conservative estimate of daily exposure to 2,4-diaminotoluene from cooking utensils (4.7 × 10−6 mg/kg-bw per day) with the lowest critical effect level of 4.0 mg/kg-bw per day for cancer effects results in an MOE of 850 000 (Table 7-10), which is considered adequate to address uncertainties in the health effects and exposure databases. Comparison of the estimates of daily exposure to 2,4-diaminotoluene for adults and infants from textile materials (2.2 × 10−5 – 4.1 × 10−5 mg/kg-bw per day) with the lowest critical effect level of 4.0 mg/kg-bw per day for cancer effects results in MOEs of 98 000–180 000 (Table 7-10), which are considered adequate to address uncertainties in the health effects and exposure databases.
| Exposure route | Exposure scenario | Estimated daily exposure (mg/kg-bw per day) | Critical effect level (mg/kg-bw per day) | MOEs |
|---|---|---|---|---|
| Oral | Polyamide cooking utensils | 4.7 × 10−6 | 4.0 | 850 000 |
| Oral | Mouthing textile objects (infants)Footnote Table 7-10 [a] | 2.2 × 10−5 | 4.0 | 180 000 |
| Dermal | Textiles – baby sleeper[a],Footnote Table 7-10 [b] | 4.1 × 10−5 | 4.0 | 97 000 |
| Dermal | Textiles – personal apparel[a],[b] | 2.5 × 10−5 | 4.0 | 160 000 |
Although dermal acute exposure scenarios (i.e., personal apparel textile contact in adults and infants) were identified, 2,4-diaminotoluene is not considered acutely toxic via the oral and dermal routes as evidenced by the reported route specific LD50 value ranges in the rat of, 136 to 553, and 326 to 1200 mg/kg-bw per day respectively (ECJRC 2008). Consequently, the risk for the general population is considered low.
7.3.4 4-Chloroaniline
Exposure Assessment
Environmental Media
No Canadian empirical data on concentrations of 4-chloroaniline in environmental media were identified. Given the reported quantities of 4-chloroaniline in commerce in Canada (less than or equal to 1000 kg), exposure of the general population of Canada via direct releases to environmental media is not expected to be significant. Limited data from other jurisdictions indicate that water may be a source of exposure to 4-chloroaniline, where use patterns of precursor chemicals result in soil contamination and subsequent runoff into drinking water sources.
Limited data on 4-chloroaniline concentrations in European river water primarily associated with industrial releases were found in studies from the 1970s and 1980s (IPCS 2003). These data are not considered relevant for the Canadian context, given the limited reported quantities in commerce in Canada (see Sources). Only two relatively recent published drinking water studies (from Iran and Spain) were identified for 4-chloroaniline and other amines. The Iranian study did not find any tap water samples containing 4-chloroaniline above the detection limit (0.6 ng/L) (Djozan and Faraj-Zadeh 1995). However, a more recent study from Spain monitoring 24 amines in treated drinking water reported higher than background levels of 4-chloroaniline following rainfall and runoff from pesticide-contaminated agricultural land (Jurado-Sánchez et al. 2012). In this study, the levels of 4-chloroaniline in the raw intake water prior to treatment were all below the detection limit (0.06 ng/L); however, upon peroxidation/coagulation followed by subsequent filtration and chlorination, levels of 4-chloroaniline increased to an average concentration of 27 ng/L (range 3.4–60 ng/L), suggesting that 4-chloroaniline was formed or released during the water treatment process by an undefined and uncharacterized process. The study authors suggested that the increased levels of amine precursors (pesticides and their degradation products) in water following heavy rainfall resulted from the run-off and transport of these compounds from the soil (Jurado-Sánchez et al. 2012). These data, which are not representative of Canadian agricultural practices and conditions, demonstrate that in jurisdictions where intensive agricultural practices have resulted in contamination of soil. There exists the potential for run-off and transfer of these chemicals to surface water following heavy rainfall (Jurado-Sánchez et al. 2012). While this study suggests water as a potential source of exposure to 4-chloroaniline, the extrapolation of this information to the Canadian population is too uncertain for risk characterization.
Food
Food residues of diflubenzuron, an insecticide registered for use in Canada (PMRA 2013), the USA (US EPA 1997) and other countries (JMPR 2003), can be metabolized to 4-chloroaniline following consumption and may therefore contribute to dietary exposure to this substance (Wittke et al. 2001). In vivometabolism in mammals of diflubenzuron to 4-chloroaniline has been reported with conversion rates ranging from 2% (US EPA 1997) to 13% (EFSA 2012). The US EPA (1997), JMPR (2003) and the French Agency for Food, Environmental and Occupational Health & Safety (ANSES 2011) have independently estimated dietary intakes ranging from 0.08 to 2.0 µg/kg-bw per day, intakes representing less than 10% of the allowable daily intake of diflubenzuron. Based on the range of these intake data, and reported range of 2-13% metabolism to 4-chloroaniline, the dietary intake of 4-chloroaniline from diflubenzuron residues in food is estimated to range from 0.0016 - 0.26 µg/kg-bw per day. It is anticipated that exposures to diflubenzuron food residues in Canada are similarly low, and that current diflubenzuron food residues do not exceed the the maximum residue limit established by Health Canada under the authority of the Pest Control Products Act and Regulations. Therefore, any potential contribution of diflubenzuron to 4-chloroaniline dietary exposure is not further addressed in this assessment.
Other pesticides which may also potentially metabolize at low levels to 4-chloroaniline such as monuron, monolinuron and buturon are not registered for use in Canada (PMRA 2013). However, use of these pesticides outside of Canada may result in residues from imported foods. For example, monitoring as part of the Canadian Total Diet StudyFootnote[7] has found monolinuron at parts per billion levels in some imported foods such as in coffee (1.18 ppb) and celery (0.65 ppb) (Rawn et al. 2004). These data, although limited, indicate that agricultural uses in other jurisdictions may contribute to dietary exposures through imported foods to some pesticide residues which could also contribute to low levels of 4-chloroaniline exposure. However, these levels are well below the general maximum residue limit (GMRL) of 0.1 ppm for residues of agricultural chemicals, and pesticides, on domestically grown and imported food, unless specific MRLs are established (Canada [1978]).
Products
4-Chloroaniline was detected in five textile and leather samples (children’s clothing, toys and leather slippers) above the LOD of 1.8 mg/kg, but less than the LOQ of 5.5 mg/kg, in Health Canada’s product testing (Health Canada 2013a). Oral and dermal exposures to 4-chloroaniline in textiles were conservatively estimated based on the LOQ of 5.5 mg/kg (see Table 7-11 for exposure estimates and Appendix E for underlying assumptions). Dermal absorption of 4-chloroaniline is conservatively assumed to be 100%. An in vivo microdialysis dermal study indicated rapid absorption of 4-chloroaniline (time to reach maximum concentration of 3 hours) and metabolism to the N-acetyl metabolite during percutaneous absorption detected in the blood dialysate following topical exposure in rats (El Marbouh et al. 2000), paralleling oral and intravenous exposures (NTP 1989). An in vitrodiffusion cell study using mouse skin reported 52.5% ± 7.7% of the parent 4-chloroaniline in the receptor fluid by 24 hours after dosing; however, skin-bound residues and metabolites were not measured (Levillain et al. 1998). A comparison of acute toxicity by the oral and dermal routes suggests similar potencies (DFG 1992a; IPCS 2003), further supporting the expectation of a relatively high degree of dermal absorption of 4-chloroaniline.
| Exposure route | Exposure scenario | Age group | Estimated acute exposure (mg/kg-bw) | Estimated daily exposureFootnote Table 7-11 [a] (mg/kg-bw per day) |
|---|---|---|---|---|
| DermalFootnote Table 7-11 [b] | Textiles – baby sleeper | Infants | 0.044 | 2.2 × 10−4 |
| Dermal[b] | Textiles – personal apparel | Adults | 0.028 | 1.4 × 10−4 |
| Oral | Mouthing of textile objects | Infants | Not applicable | 2.9 × 10−5 |
Exposures to leather products are considered to be short term and intermittent. Based on the analytical method LOQ of 5.5 mg/kg, dermal exposure to 4-chloroaniline from use of leather products is estimated to range from 1.58 × 10−5 mg/kg-bw (adults) to 1.5 × 10−4 mg/kg-bw (infants).
Migration of4-chloroaniline from certain polyamide cooking utensils was reported in a study conducted in Denmark (Trier et al. 2010). Using the median migration level of 4-chloroaniline (4 × 10−4 µg/g food), estimates of potential exposure to4-chloroaniline from the use of polyamide cooking utensils were derived to range from 5 × 10−4µg/kg-bw per day (seniors 60 years of age and older) to 1.9 × 10−3 µg/kg-bw per day (toddlers 0.5–4 years of age).
Other Sources
Chlorhexidine and its salt forms (acetate, gluconate and hydrochloride) are broad-spectrum antiseptics used for sterilization, cleaning skin and hands, treating plaque and gingivitis, and disinfecting wounds and are generally effective against a wide variety of bacteria and yeasts (Environment Canada and Health Canada 2013b). The presence of 4-chloroaniline in chlorhexidine-based consumer products may occur as a residual from chlorhexidine synthesis or from hydrolysis during storage (IPCS 2003). Levels of 4-chloroaniline in chlorhexidine-based products have been reported to range from 40–240 mg/kg (Zong 2011) to greater than 1000 mg/kg (Kohlbecker 1989), while pharmaceutical grades of chlorhexidine salts from the US Pharmacopeia monographs indicate limits for 4-chloroaniline as an impurity up to 500 ppm (mg/kg) in topical formulations (USP-NF 2013).
Chlorhexidine acetate was previously evaluated in a draft assessment by the Government of Canada, in which daily dermal exposure to chlorhexidine from aftershave (0.19% chlorhexidine acetate) was estimated to be 32.2 µg/kg-bw per day (Environment Canada and Health Canada 2013b). Based on the upper-bounding estimate of 4-chloroaniline as an impurity up to 500 ppm (USP-NF 2013), the corresponding daily exposure to 4-chloroaniline from use of chlorhexidine acetate–containing aftershave is estimated to be 0.016 µg/kg-bw per day. Other chlorhexidine salts not previously characterized by the Government of Canada draft assessment were identified in additional cosmetic products (personal communication, email from Consumer Product Safety Directorate [Health Canada] to Existing Substances Assessment Bureau Health Canada], dated 2014; unreferenced). The daily applied dose from use of a skin moisturizer containing 0.2%Footnote[8] chlorhexidine digluconate and a maximum residue limit of 500 ppm 4-chloroaniline is estimated to be 0.07 µg/kg-bw per day.Footnote[9]Additional uses of chlorhexidine-based products from the Canadian Drug Product Database (DPD 2010) include several over-the-counter antibacterial hand washes, antiseptic mouthwashes and topical antiseptic products with chlorhexidine concentrations ranging from 0.003% to 20%. Estimates of exposure to residual 4-chloroaniline from these products were not derived; however, many of these uses are targeted for hospitals and/or health care facilities and are therefore not considered to represent exposures of the general population of Canada.
Triclocarban is an antimicrobial agent which is synthesized from a reaction of 3,4-dichlorophenyl isocyanate (CAS RN 102-36-3) with 4-chloroaniline (TCC 2002); consequently, 4-chloroaniline may be found as a residual in triclocarban-containing consumer products (IPCS 2003). Based on notifications submitted to Health Canada under the Cosmetic Regulations, this substance was identified as an ingredient in cosmetics on the Canadian market including uses in skin cleansers, deodorants, and bath preparations (personal communications, emails from Consumer Product Safety Directorate [Health Canada] to Existing Substances Risk Assessment Bureau [Health Canada], dated 2014; unreferenced). Triclocarban has been identified in surface waters in the USA (Halden and Paull 2005) and in residual biosolids produced during wastewater treatment (CalEPA 2010b). Levels of triclocarban in Canadian wastewater biosolids from a 2009 study indicate 100% prevalence of triclocarban across Canadian sampling sites and levels in biosolids ranging from 64 ng/g to 6700 ng/g dry weight (median, 1930 ng/g dry wt) (CCME 2009) indicating widespread down-the-drain use of this substance in Canada.There is some evidence that triclocarban can degrade in the soil column to produce both 4-chloroaniline and 3,4-dichloroaniline (Gledhill 1975; Miller et al. 2010; Kwon and Xia 2012). Although the data are limited, the available information suggests that triclocarban released into the environment and subsequently degraded can be a source of 4-chloroaniline. However, exposure from this source is uncertain and not further characterized in this Screening Assessment.
Health Effects Assessment
The toxicity dataset for 4-chloroaniline has been previously summarized in a Provisional Peer Reviewed Toxicity Values supporting document by the US EPA (2008b), a Concise International Chemical Assessment Document by the World Health Organization’s IPCS (2003) as well as reviews by IARC (1993) and the German MAK Commission (DFG 1992a). A review of 4-chloroaniline health effects data as part of an evaluation on diflubenzuron by European authorities (KEMI 2011; EFSA 2012) was also considered here. A rationale for selection of the critical effect levels for derivation of MOEs is given below.
4-Chloroaniline has been classified by IARC (2013) as a Group 2B carcinogen and by the European Commission as a Carcinogen Category 1B (ESIS 1995–2012), and it is considered by the US EPA (2008a) as likely to be carcinogenic to humans (see Table 7-5). Additional cancer evaluations include the US NTP (1989) evaluation (“clear” evidence in male rats, “some” evidence in male mice) and the German MAK Commission evaluation (Carcinogen Category 2; DFG 2012a), and 4-chloroaniline is listed on California’s Prop65, a list of substances known to cause cancer (CalEPA 2010a).
No epidemiological data examining the carcinogenic potential of 4-chloroaniline were identified (IPCS 2003; IARC 1993). However, four chronic cancer studies in rodents were available, including 78-week dietary studies in rats and mice (NCI 1979c) and 2-year gavage studies in rats and mice (NTP 1989).
In the F344 rat, increased incidences of spleen sarcomas were observed in male rats chronically administered 4-chloroaniline at a dose of 25 mg/kg-bw per day from the diet (NCI 1979c) and at 2–6 mg/kg-bw per day by oral gavage (NTP 1989). In the more recent study by the NTP (1989), the incidence of splenic sarcomas in male rats demonstrated a dose-dependent increase: 0 of 50 (0%) at 0 mg/kg-bw per day, 1 of 50 (2%) at 2 mg/kg-bw per day, 3 of 50 (6%) at 6 mg/kg-bw per day and 38 of 50 (74%) at 18 mg/kg-bw per day. The incidences at both low (1 of 50) and middle doses (3 of 50) were not statistically significant by pairwise tests; however, the incidence of these tumours was well above the average of 0.3–0.4% from the historical controls, with evidence of splenic fibrosis at the same dose. Despite the low spontaneous incidence of splenic sarcomas in historical control rats at the NTP, there is uncertainty as to whether the low-dose spleen sarcomas can be considered exposure related. However, since there were clear exposure-related non-cancer effects in the spleen at 2 mg/kg-bw per day (fibrosis, 11 of 50 [22%] versus 3 of 49 [6%] controls), it seems reasonable that the low incidence of splenic sarcomas at this dose (1 of 50, or 2%) was likely exposure related. The spleen sarcomas were associated with non-neoplastic effects at all test doses, including degenerative changes in the spleen (fibrosis and congestion), as well as evidence for oxidation of hemoglobin (methemoglobin) and increased hemolysis. These non-neoplastic effects were also observed in female rats, while only an “equivocal” increase in spleen sarcomas was observed in females at the middle and high doses (1 of 50, 2% for each dose). Nonetheless, these rates exceed the very low spontaneous incidence of splenic sarcomas in female rats from NTP historical control data at the time (0 of 297 of water gavage controls, 1 of 1961 or 0.05% of all untreated controls) (cited in NTP 1989). For the purposes of this assessment, the spleen sarcomas in male rats at the lowest tested dose are considered to be exposure related.
It should be noted that the spleen sarcoma data have been previously used by various regulatory bodies for cancer risk quantification, applying several methods, including use of a slope factor (unit risk approach; US EPA 1997), T25 method (dose causing tumours in 25% of test animals; EFSA 2012) and an MOE BMDL10 approach (lower 95% confidence limit on the benchmark dose causing a 10% increase in tumours; US EPA 2008a; EFSA 2012). However, in this Screening Assessment, the dose range of 2–6 mg/kg-bw per day for the low and middle doses for male rats (NTP 1989) was chosen as the point of departure for the carcinogenic effects of 4-chloroaniline.
In B6C3F1 mice, similar hematotoxic effects as seen in the rats were observed in both the dietary and gavage studies, except at relatively higher doses, indicating an overall greater sensitivity of rats compared with mice. Some evidence for carcinogenicity was also observed in mice, with “equivocal” evidence of increased hemangiosarcomas, primarily in spleen and liver, of both sexes in the dietary study (greater than or equal to 325 mg/kg-bw per day) (NCI 1979c). In the more recent gavage study (NTP 1989), the incidence of hepatocellular carcinomas in mice showed an apparent dose-dependent increase at all test doses in the males; however, due to the high spontaneous incidence of hepatic carcinomas from NTP historical control data for male mice of this strain (average = 18.7%, maximum = 30%), only the highest dose of 30 mg/kg-bw per day (34%) was above the maximum spontaneous tumour rate and therefore clearly related to exposure to 4-chloroaniline. Based on the chronic rat and mice studies above, the critical effect levels are considered to be a LOAEL ranging from 2 to 6 mg/kg-bw per day based on increased splenic fibrosis (male and female rats) as well as increased spleen sarcomas in male rats (NTP 1989).
The genotoxicity of 4-chloroaniline is summarized from secondary sources (DFG 1992a; IPCS 2003). Briefly, 4-chloroaniline results in reverse mutation assays in Salmonella were mixed, with different authors reporting positive and negative responses primarily from strain TA98 with S9 activation, while consistent negative results were reported without S9 activation in all strains. Conversely, 4-chloroaniline was positive for mutagenicity in five separate assays using L5178Y mouse lymphoma cells (IPCS 2003). Positive responses for chromosomal aberrations and sister chromatid exchanges were observed in Chinese hamster ovary cells, whereas mixed results were seen for unscheduled DNA synthesis in primary rat hepatocytes. The in vivo micronucleus assay was reported to be negative in CFLP mice up to 180 mg/kg-bw, whereas increased micronuclei were observed in B6C3F1mice at a dose of 300 mg/kg-bw (all studies from DFG 1992a and PCS 2003;). More recent unpublished studies on the in vivogenotoxicity of 4-chloroaniline were conducted to support a European Food Safety Authority (EFSA) evaluation for the pesticide diflubenzuron (KEMI 2011). Based on an EFSA report in which both positive mouse micronucleus assay results and evidence of single-cell DNA damage in the liver of rats (comet assay) were reported, there is some indication that 4-chloroaniline may have in vivo genotoxic potential (KEMI 2011; EFSA 2012).
The acute toxicity of 4-chloroaniline by the oral, dermal and inhalation routes has been investigated in multiple animal species in studies in which both mortality as well as other effects (methemoglobinemia, cyanosis, etc.) have been reported (DFG 1992a; IPCS 2003). Acute oral LOAELs ranging from 8 to 54 mg/kg-bw were identified for studies in cats, Beagle dogs, rats and monkeys in which a range of acute effects were observed at those doses including methemoglobin levels elevated by at least 10%in combination with reported clinical signs of cyanosis and/or methemoglobinemia. In addition, acute oral LD50 values ranging from 50 to 350 mg/kg-bw were identified based on studies in mice, rats, guinea pigs and cats.
Risk Characterization
Margins between estimates of acute dermal exposures to 4-chloroaniline via textiles and the critical effect levels (methemoglobinemia and cyanosis) result in MOEs ranging from 180 to 1900 (Table 7-12). Based on the conservativeness of the acute exposure estimate from textiles, these MOEs are considered adequate to address the uncertainties in the health effects and exposure databases.
| Consumer products | Exposure estimate (mg/kg-bw per event) |
Critical effect levels: methemoglobinemia, cyanosis (mg/kg-bw) |
MOEs |
|---|---|---|---|
| Textiles – personal apparel | 0.028 | 8–54 | 290–1900 |
| Textiles – baby sleeper | 0.044 | 8–54 | 180–1200 |
Margins between estimates of daily dermal and oral exposures to 4-chloroaniline from use of or contact with textiles (oral and dermal), polyamide cooking utensils (oral), and chlorhexidine-containing products (dermal) and the critical effect levels result in MOEs ranging from 9000 to over 1 million, which are considered adequate to address uncertainties in the health effects and exposure databases (refer to Table 7-13).
| Exposure route (via consumer products) |
Exposure estimate (µg/kg-bw per day) |
Critical effect levels: cancer LOAELs (mg/kg-bw per day) |
MOEs |
|---|---|---|---|
| Oral: Textiles – mouthing (infants) | 0.029 | 2–6 | 69 000–210 000 |
| Oral: Cooking utensils | 5 × 10−4 to 1.9 × 10−3 | 2–6 | greater than 1 million |
| DermalFootnote Table 7-13 [a] : Textiles – personal apparel | 0.14 | 2–6 | 14 000–43 000 |
| Dermal[a]: Textiles – baby sleeper | 0.22 | 2–6 | 9 000–27 000 |
| Dermal[a]: aftershave (0.19% chlorhexidine)Footnote Table 7-13 [b] | 0.016 | 2–6 | 120 000–370 000 |
| Dermal[a]: skin moisturizer (0.2% chlorhexidine digluconate[b]) | 0.07 | 2–6 | 28 500–86 000 |
7.3.5 3,4-Dichloroaniline
Exposure Assessment
Environmental Media
No Canadian empirical data on concentrations of 3,4-dichloroaniline in environmental media were identified. The information summarized below suggests that use patterns of precursor chemicals resulting in soil contamination and subsequent runoff into drinking water sources may be a potential source of 3,4-dichloroaniline exposure.
A recent study from Spain that monitored 24 amines in treated drinking water, reported higher than background levels of 3,4-dichloroaniline following rainfall and runoff from pesticide-contaminated agricultural land (Jurado-Sánchez et al. 2012). In this study, the average level of 3,4-dichloroaniline in the raw intake water was 2.8 ng/L (range 1.5–3.4 ng/L). Following peroxidation/coagulation, filtration and subsequent chlorination, levels of 3,4-dichloroaniline increased to a concentration of 119 ng/L (mean; range 0.6–190 ng/L). The authors attributed the 3,4-dichloroaniline increase to either spontaneous formation, or release during treatment by an undefined and uncharacterized process. Also, increased concentrations of amine precursors (pesticides and their degradation products) in intake water, following heavy rainfall, were thought to originate from run-off and transport of these compounds from soil (Jurado-Sánchez et al. 2012). While these data are derived from scenarios that do not necessarily represent Canadian agricultural practices, they demonstrate a potential for amine precursors to transfer to surface waters in run-off following heavy rainfall, and that such events may occur in any jurisdiction in which intensive agricultural practices has resulted in soil contamination (Jurado-Sánchez et al. 2012). While this study suggests treated drinking water may represent a potential source of 3,4-dichloroaniline exposure, extrapolation of this information to Canada is too uncertain for risk characterization in this Screening Assessment.
The phenylurea pesticides, diuron and linuron are registered in Canada for a limited number of uses (PMRA 2013), and could contribute to environmental media exposure through partial degradation to 3,4-dichloroaniline. Canadian water concentrations of diuron and linuron were measured in small streams near agricultural areas located in southern Ontario and Quebec, between 2006 and 2008 (Struger et al. 2011). This study showed a temporal trend of diuron and linuron concentrations increasing over the growing and application season up to June–July and subsequently decreasing from August to September. In 2006, diuron levels in Ontario ranged from 4.15 to 2900 ng/L, and linuron levels ranged from 107 to 240 ng/L. In 2007, diuron levels in Ontario ranged from 2.16 to 133 ng/L, and linuron levels ranged from 3.10 to 856 ng/L. In 2008, diuron levels ranged from 4.35 to 873 ng/L, and linuron levels ranged from 11.2 to 145 ng/L (Struger et al. 2011). Other Canadian monitoring data for linuron generally indicate that it is detected infrequently in water (PMRA 2012). Targeted monitoring studies in agricultural areas indicate peak linuron levels during runoff events were generally around 20 to 40 μg/L but have occasionally been detected as high as approximately 100 μg/L (PMRA 2012).The formation of 3,4-dichloroaniline as a result of environmental degradation of diuron and linuron has been documented (US EPA 2003; PMRA 2012; Sorensen et al. 2003; Giacomazzi and Cochet 2004) and US studies have reported co-localization of 3,4-dichloroaniline and diuron in surface waters near agricultural areas where diuron is used (Hladik and Calhoun 2012; Thurman et al. 1999). Based on the available information, some degradation of diuron and linuron to 3,4-dichloroaniline may occur and contribute to levels of this substance in surface water in Canada. As linuron and diuron have recently been re-evaluated by the Pest Management Regulatory Agency (PMRA) of Health Canada (personal communication, email from PMRA [Health Canada] to Existing Substances Risk Assessment Bureau [Health Canada], dated 2013; unreferenced). Exposure contributions to 3,4-dichloroaniline from registered pesticides and their degradation products are addressed under another jurisdiction of Health Canada, and therefore is not considered in this Screening Assessment.
Food
No Canadian data on concentrations of 3,4-dichloroaniline in food were identified, however, certain pesticides (e.g., linuron and diuron) in food products may be degraded to 3,4-dichloroaniline. Monitoring for linuron and diuron residues in food from the Canadian market indicates overall low levels, generally below 0.1 ppm (personal communication, email from Food Directorate [Health Canada] to Exising Substances Risk Assessment Bureau [Health Canada], dated 2014; unreferenced). As 3,4-dichloroaniline is considered only a minor metabolite of linuron and diuron in vivo, the residues of 3,4-dichloroaniline would be correspondingly much lower. The food residues for these pesticides do not generally exceed the the maximum residue limit established by Health Canada under the authority of the Pest Control Products Act and Regulations. Therefore, any potential contribution of linuron or diuron to 3,4-dichloroaniline dietary exposure is not further addressed in this assessment.
Consumer Products
Triclocarban is an antimicrobial agent which is synthesized from a reaction of 4-chlorophenyl isocyanate (CAS RN 104-12-1) with 3,4-dichloroaniline (TCC 2002); consequently, 3,4-dichloroaniline may be found as a residual in triclocarban-containing consumer products. Based on notifications submitted to Health Canada under the Cosmetic Regulations, this substance was identified as an ingredient in in cosmetics on the Canadian market including uses in skin cleansers, deodorants, and bath preparations (personal communications, emails from Consumer Product Safety Directorate [Health Canada] to Existing Substances Risk Assessment Bureau [Health Canada], dated 2014; unreferenced). Triclocarban has been identified in surface waters in the USA (Halden and Paull 2005) and in residual biosolids produced during wastewater treatment (CalEPA 2010b). Levels of triclocarban in Canadian wastewater biosolids from a 2009 study indicate 100% prevalence of triclocarban across Canadian sampling sites and levels in biosolids ranging from 64 ng/g to 6700 ng/g dry weight (median, 1930 ng/g dry wt) (CCME 2009) indicating widespread down-the-drain use of this substance in Canada.There is some evidence that triclocarban can degrade in the soil column to produce both 4-chloroaniline and 3,4-dichloroaniline (Gledhill 1975; Miller et al. 2010; Kwon and Xia 2012). Although the data are limited, the available information suggests that triclocarban released into the environment and subsequently degraded can be a source of 3,4-dichloroaniline. However, exposure from this source is uncertain and not further characterized in this Screening Assessment.
Health Effects Assessment
The health effects data for 3,4-dichloroaniline were identified in various secondary sources, including a German Chemical Society Advisory Committee on Existing Chemicals of Environmental Relevance report (BUA 1996), an International Uniform Chemical Information Database (IUCLID) dataset (European Commission ©2000d), an EU risk assessment report (ECJRC 2006) and an associated IUCLID Screening Information Data Set (BAuA 2005), as well as a review by the German MAK Commission (DFG 2013). The REACH dossier for 3,4-dichloroaniline (ECHA ©2007–2013) was also cross-referenced for data not already captured from the other reviews. The original studies were reviewed when available; otherwise, information is as reported from the secondary sources. A rationale for selection of the critical effect levels for derivation of daily MOEs is given below.
No adequate chronic toxicity studies were identified for 3,4-dichloroaniline; however, multiple acute toxicity, shorter-term toxicity and metabolism studies were available including several studies providing evidence that the herbicide propanil is efficiently metabolized to 3,4-dichloroaniline in mammals. Further, safety evaluations of propanil by the US EPA (2006) and EFSA (2011, 2013) determined that the chronic toxicity of 3,4-dichloroaniline is uncertain due to the lack of data, although it was identified as the metabolite of concern for propanil. In the context of this Screening Assessment, a chronic point of departure has been based on a read-across of relevant chloroanilines--namely, 4-chloroaniline and the herbicide propanil (the precursor of 3,4-dichloroaniline). The only chronic toxicity data available among the related chloroanilines are for 4-chloroaniline (chronic rat LOAEL of 2 mg/kg-bw per day: increased splenic fibrosis, spleen sarcoma) and for propanil (chronic rat LOAEL of approximately 7 mg/kg-bw per day as 3,4-dichloroaniline: decrease in red blood cells, decrease in packed cell volumes, increased methemoglobin, increased spleen weight and splenomegaly in females, hemosiderosis of spleen in males). Therefore, oral chronic rat LOAELs ranging from 2 to 7 mg/kg-bw per day based on the data read-across from 4-chloroaniline and propanil (3,4-dichloroaniline-equivalent doses) are considered as the chronic points of departure for 3,4-dichloroaniline
Risk Characterization
While low levels of 3,4-dichloroaniline may potentially exist in several sources (food and water residues from use of pesticide precursors, residual and environmental degradation of triclocarban), the predominant potential exposures are addressed under the PCPA or FDA. A brief summary of these other potential sources and uncertainties are provided below.
Rice and rice products imported from the US can be expected to contain some level of 3,4-dichloroaniline if the pesticide propanil is used. Exposure contributions from pesticides and their degradation products are addressed by the Pest Management Regulatory Agency, PMRA (Health Canada), and are therefore not considered in this screening assessment. Dietary exposure to 3,4-dichloroaniline from residues of the registered pesticides linuron and diuron is regulated in Canada under the authority of the Pest Control Products Act and Regulations.Exposure contributions from pesticides and their degradation products are addressed by the Pest Management Regulatory Agency, PMRA (Health Canada), and are therefore not considered in this screening assessment.
Regarding overall uncertainties in this assessment, there is a lack of chronic toxicity data for 3,4-dichloroaniline. While data support the qualitatively similar effects of 3,4-dichloroaniline, 4-chloroaniline and propanil, the relative potency of 3,4-dichloroaniline remains uncertain.
There is limited evidence that environmental degradation of triclocarban may also be an exposure source of 3,4-dichloroaniline; however, there is high uncertainty due to limited data availability.
7.3.6 o-Anisidine
Exposure Assessment
Exposure to o-anisidine via environmental media for the general population of Canada is not expected due to limited commercial quantities in Canada. Exposure of the general population to o-anisidine from use of consumer products was estimated for textile products (direct and prolonged skin contact and infant mouthing) and for polyamide cooking utensils via the oral route.
o-Anisidine was detected in 4 of 66 textile samples in Health Canada’s product testing (Health Canada 2013a). Dermal and oral exposures to o-anisidine from textiles were estimated based on the LOQ of 2.6 mg/kg (see Table 7-14). Dermal absorption was conservatively assumed to be 100%; although no relevant dermal data were found, monocylic aromatic amines are expected to have high dermal absorption.
| Exposure route | Exposure scenario | Age group | Estimated acute exposure (mg/kg-bw) |
Estimated daily exposureFootnote Table 7-14 [a] (mg/kg-bw per day) |
|---|---|---|---|---|
| DermalFootnote Table 7-14 [b] | Textiles – baby sleeper | Infants | 0.021 | 1.0 × 10−4 |
| Dermal[b] | Textiles – personal apparel | Adults | 0.013 | 6.7 × 10−5 |
| Oral | Mouthing of textile objects | Infants | Not applicable | 1.3 × 10−5 |
Migration of o-anisidine from certain polyamide cooking utensils was reported in a study conducted in Denmark (Trier et al. 2010). Using the median migration level of o-anisidine (4 × 10−4 µg/g food), estimates of potential exposure to o-anisidine from the use of polyamide cooking utensils were derived to range from 5 × 10−4µg/kg-bw per day (seniors 60 years of age and older) to 1.9 × 10−3 µg/kg-bw per day (toddlers 0.5–4 years of age).
o-Anisidine has also been measured in tattoo inks and conservative estimates of acute and short-term exposure have been derived. Acute exposure to residual o-anisidine in tattoo ink was estimated to range from 0.0027 to 0.026 mg/kg-bw. Daily exposure through short-term release of amines was estimated to range from 6.3 × 10−6 to 6.1 × 10−5mg/kg-bw per day. These estimates of exposure are considered upper-bounding due to the underlying conservative assumptions used in their derivation (see Appendix F). Due to the high uncertainty associated with these exposure estimates, the risk from tattoo exposure has not been estimated (see Section 7.5.1 Uncertainty).
Health Effects Assessment
o-Anisidine has been previously assessed by IARC (1982, 1999) and the European Chemicals Bureau (ECJRC 2002). Toxicological data on the hydrochloride salt of o-anisidine were considered relevant, as the salt is expected to dissociate in physiological media to generate o-anisidine and is therefore considered to be toxicologically equivalent. A rationale for selection of the critical effect levels for derivation of MOEs is given below.
Carcinogenicity and Genotoxicity
In rats and mice administered o-anisidine (as the hydrochloride) in feed for up to 2 years, carcinomas of the urinary bladder were induced in both sexes of both species (NCI 1978). Rats were dosed with o-anisidine hydrochloride at 250 or 500 mg/kg-bw per day; the incidences of urinary bladder tumours were 52 of 54 (96%) and 52 of 52 (100%) in males and 46 of 49 (94%) and 50 of 51 (98%) in females at the low and high doses, respectively, compared with none in controls (see Appendix I for full incidence data for rats and mice). Mortalities were noted in both sexes at both dose levels, accompanied by excessive body weight deficits, suggesting that the maximum tolerated dose was exceeded. However, as a precautionary approach, quantification of cancer potency was conducted with this study.
Benchmark doses (BMD) associated with a 10% increase in tumour incidence above controls (i.e., the BMD10) and the corresponding lower limits of a one-sided 95% confidence interval (BMDL10) were derived for o-anisidine hydrochloride using the US EPA’s Benchmark Dose Software (BMDS version 2.3.1)(US EPA 2013b). The lowest calculated BMDL10 for o-anisidine hydrochloride is 5.53 mg/kg-bw per day for transitional cell papillomas or carcinomas in urinary bladder in male F344 rats (Appendix I). When adjusted for the molecular weight difference, this value corresponds to a BMDL10 of 4.3 mg/kg-bw per day for o-anisidine. As the maximum tolerated dose was exceeded in this study and as the BMDL10 fell outside the range of tested doses, the quantification of this BMDL10 is considered precautionary.
The EU derived a TC25 (defined as the chronic daily dose that will give 25% of the animals tumours at a specific tissue site, after correction for spontaneous incidence, within the standard lifespan of that species; Dybing et al. 1997) of 51.6 mg/kg-bw per day for rats (for the hydrochloride form of o-anisidine). The LTD10 (the lower tumorigenic dose associated with a 10% increase in tumour incidence above controls) for o-anisidine hydrochloride published in the Carcinogenic Potency Database is 2.85 mg/kg-bw per day for rats (CPDB 2013).
In the same study (NCI 1978), mice were dosed with o-anisidine hydrochloride at 325 or 650 mg/kg-bw per day. Body weight gain was significantly depressed in both males and females from the low- and high-dose groups. Survival was not affected in mice, and cancer of the bladder was noted as a high-dose lesion in males and females. The BMDL10 for o-anisidine hydrochloride in mice is 333 mg/kg-bw per day in males and 360 mg/kg-bw per day in females, for transitional cell papillomas or carcinomas in urinary bladder. The corresponding BMD10 values are 407 and 431 mg/kg-bw per day, respectively (see Appendix I). When the BMDL10s for o-anisidine hydrochloride are adjusted for the molecular weight difference, they are equivalent to BMDL10 values of 256 and 277 mg/kg-bw per day for o-anisidine for males and females, respectively.
In a tumour promotion study, no bladder lesions were observed in 10 male rats given o-anisidine in the diet at 85 mg/kg-bw per day for 2 weeks, then at 21 mg/kg-bw per day for 30 weeks. However, in 16 male rats that were given the initiator N-butyl N-(4-hydroxybutyl) nitrosamine in the drinking water for 4 weeks prior to receiving o-anisidine in the diet, there was a significant increase in the incidence of hyperplasia; increases in the incidences of bladder papillomas and carcinomas were not significant (Ono et al. 1992).
In vitro, reverse mutation assays in standard test strains of bacteria were negative. However, o-anisidine induced reverse mutations and DNA damage in bacterial strains with elevated N- or O-acetyltransferase, indicating that its activation occurs through acetylation. In mammalian cells, o-anisidine induced mutations, chromosomal aberrations and sister chromatid exchanges, both with and without metabolic activation. Although o-anisidine was negative in assays for DNA damage (unscheduled DNA synthesis assay) in rat hepatocytes, it induced DNA strand breaks in mouse lymphoma cells in the presence of metabolic activation factors from rat liver. o-Anisidine increased the transformation frequency of Syrian hamster embryo cells, but was negative in mouse embryo cells in transformation assays designed to detect initiators and promoters (IARC 1999; Rivedal et al. 2000; ECJRC 2002; Oda 2004; Sakai et al. 2010).
In vivo, DNA adducts were found in bladder, liver, kidney and spleen of rats given o-anisidine by intraperitoneal injection; the highest concentrations and the most persistent adducts were found in the bladder (Stiborová et al. 2005; Naiman et al. 2012). The adducts were identified as deoxyguanosine adducts derived from an N-hydroxy metabolite of o-anisidine and were similar to those obtained in vitro following activation of o-anisidine with human microsomes, suggesting that liver monoxygenase enzymes metabolize o-anisidine to generate reactive nitrenium/carbenium ions (Stiborová et al. 2005; Naiman et al. 2012). Stiborová et al. (2002) also showed that o-anisidine could be activated by peroxidases in vitro to form diimines that could react with DNA. As peroxidases are ubiquitous enzymes, the activation of o-anisidine could occur in multiple tissues, including bladder.
In an earlier study, no DNA adducts were observed in bladder or liver of mice treated with o-anisidine by gavage (Ashby et al. 1994). Mutation frequency was increased in bladder but not liver of transgenic mice given o-anisidine by gavage (Ashby et al. 1994). Some evidence for DNA damage in bladder of mice and rats was shown by Sasaki et al. (1998) and Sekihashi et al. (2002), following administration of o-anisidine by gavage. In these studies, DNA damage was also observed in the colon of both species, as well as in the rat stomach, kidney, lung and brain. However, no DNA damage was detected in any of the organs tested (including bladder) in an earlier study by Ashby et al. (1991), in which rats were treated by intraperitoneal injection or by oral gavage. Unscheduled DNA synthesis assays were also negative following gavage dosing in the rat liver and following intraperitoneal dosing in rat kidney (Tyson and Mirsalis 1985; Ashby et al. 1991). In vivo micronucleus studies were also negative in mice given o-anisidine either by gavage or by intraperitoneal injection and in rats dosed by gavage (Ashby et al. 1991).
Other Health Effects
No studies on reproductive or developmental toxicity were identified. However, in the 2-year dietary carcinogenicity studies on mice and rats, there were no effects on the reproductive organs of males or females (NCI 1978; see Section 7.4.6.2.1). In this study, there was a dose-related increase in mortality (significant at both doses) in rats and a dose-related depression of body weight (greater than 10% at both doses) in both rats and mice. In mice, there was also an increase in bladder hyperplasia, which was significant at the high dose.
The NCI (1978) study included a 7-week range-finding component, in which rats and mice were given o-anisidine (as the hydrochloride) at 1000–30 000 mg/kg in the diet (corresponding to o-anisidine doses of 38, 115, 380 and 1 150 mg/kg-bw per day in rats and 100, 300, 1 000 and 3 000 mg/kg-bw per day in mice). In rats, body weight was decreased more than 10% in the top two dose groups, and granular spleens were observed in males of all dose groups and in females in the top two dose groups. Spleens were also dark and enlarged in all animals in the top two dose groups. In mice, body weight was decreased more than 10% in the top three dose groups, and all animals in the top two dose groups had spleens that were dark and enlarged. No statistical analysis or histopathology was done during this portion of the study.
In an unpublished 28-day study cited in the EU risk assessment report (ECJRC 2002), rats were dosed daily by gavage at 0, 16, 80 or 400 mg/kg-bw per day. The EU derived a no-observed-adverse-effect level (NOAEL) of 16 mg/kg-bw per day and a LOAEL of 80 mg/kg-bw per day, based on slight hemolytic anemia and morphological changes in spleen (hemosiderosis, hyperaemia, increased hematopoiesis) in both sexes; as well as increased bilirubin and increased relative liver weight in females (Hoechst 1990).
Limited acute toxicity data were available; data were from secondary sources or lacked detail. In an oral genotoxicity study in mice and rats, Ashby et al. (1991) noted that methemoglobin increased (greater than threefold over control levels) following oral dosing at 690 mg/kg-bw. No deaths or signs of toxicity or gross pathology were reported in rats following a dermal application of 2000 mg/kg-bw per day (Hoechst 1988).
Risk Characterization
Exposure of the general population in Canada to o-anisidine is not expected from environmental media due to limited commercial quantities in Canada.
Exposure of the general population in Canada to o-anisidine via consumer products is expected to occur predominantly through use of textile products and cooking utensils. Daily dermal exposures of adults and infants resulting from skin contact with textile clothing were estimated to be 6.7 × 10−5 and 1.0 × 10−4 mg/kg-bw per day, respectively. Daily oral exposure of infants from mouthing of textile objects was estimated to be 1.3 × 10−5mg/kg-bw per day. Potential daily oral exposure to o-anisidine from use of polyamide cooking utensils was conservatively estimated to range from 5 × 10−7mg/kg-bw per day (seniors 60 years of age and older) to 1.9 × 10−6 mg/kg-bw per day (toddlers 0.5–4 years of age).
The critical effect level for chronic exposure to o-anisidine is a BMDL10 of 256 mg/kg-bw per day, based on urinary bladder tumours in male mice in a 2-year dietary study (NCI 1978).
In the same study (NCI 1978), in rats, excessive toxicity was noted in both sexes and at both dose levels; as such, the determination of a BMDL10 from this study is considered precautionary and was not used in determining MOEs.
Comparison of estimates of exposure with the precautionary BMDL10, results in MOEs of greater than 1 million (Table 7-15). Oral toxicity data were used in the absence of dermal data; as a conservative, precautionary assumption, absorption by the oral and dermal routes was considered to be equivalent. All MOEs are considered adequate to address uncertainties in the health effects and exposure databases.
| Exposure route | Consumer products | Estimated daily exposure (mg/kg-bw per day) |
Critical effect level: oral BMDL10 (mg/kg-bw per day)Footnote Table 7-15 [d] |
MOEs |
|---|---|---|---|---|
| Dermal | Textiles – baby sleeperFootnote Table 7-15 [a],Footnote Table 7-15 [b] | 1.0 × 10−4 | 256 | 2.6 × 106 |
| Dermal | Textiles – personal apparel[a],[b] | 6.7 × 10−5 | 256 | 3.8 × 106 |
| Oral | Mouthing of textile objects (infants)[a] | 1.3 × 10−5 | 256 | 2.0 × 107 |
| Oral | Cooking utensilsFootnote Table 7-15 [c] | 1.9 × 10−6 | 256 | 1.3 × 108 |
In Appendix I the calculation of the BMDL10 is presented for both rats and mice, although the maximum tolerated dose was exceeded and the dose range was limited in the rat study. The MOEs presented are based on the BMDL10 from the mouse study because of the limitations noted in the rat data set. Due to limited toxicity data on the acute endpoint, no MOEs were derived; however, acute exposures are not expected to be of concern.
7.3.7 p-Aminophenol
Exposure Assessment
No Canadian empirical data on concentration of p-aminophenol in environmental media were identified. The modelled release of p-aminophenol to water from cosmetic use and the cosmetic formulation scenarios was discussed in Section 6.2. However, exposure to p-aminophenol via air and soil is not expected. Compared to the use of cosmetic products and the resulting direct exposure (discussed below), environmental media are not considered to be a significant source of exposure for the general population in Canada.
Based on notifications submitted to Health Canada under the Cosmetic Regulations, p-aminophenol is used in hair dyes, nail polish and body cream, lotion or moisturizer in Canada (personal communications, emails from Consumer Product Safety Directorate [Health Canada] to Existing Substances Risk Assessment Bureau [Health Canada], dated 2011 and 2013; unreferenced). In addition, material safety data sheets obtained from Procter & Gamble and similar manufacturers listed p-aminophenol as a component of hair dye products sold in Canada at a concentration of up to 5% (P&G Beauty 2009a, b, c, d, 2012, b, c, 2013; TIGI 2011; JPMS 2012). These cosmetic products and the associated upper-bounding estimated exposures via the dermal route are summarized in Table 7-16. The assumptions and details for these exposure estimates are presented in Appendix J.
| Cosmetic exposure scenarioFootnote Table 7-16 [b] | Concentration (% w/w)Footnote Table 7-16 [c] |
Estimated per event exposure (mg/kg-bw) |
Estimated daily exposure (mg/kg-bw per day) |
|---|---|---|---|
| Permanent hair dye (per event) | 2.5Footnote Table 7-16 [d] | 0.212 | n/a |
| Nail polish (per event) | 10–30 | 0.004–0.013 | n/a |
| Body cream, lotion, moisturizer (infant) | less than or equal to 0.1 | less than or equal to 0.011 | less than or equal to 0.019 |
| Body cream, lotion, moisturizer (adult) | less than or equal to 0.1 | less than or equal to 0.004 | less than or equal to 0.004 |
Several studies were identified reporting dermal absorption of p-aminophenol. In human volunteers, dermal absorption of p-aminophenol was estimated to be 6%, based on recovery in urine in the 7 days following a 24-hour exposure (Bucks et al. 1990). Radiolabelled p-aminophenol was applied at a dose of 2–4 µg/cm2 in 95% ethanol solution to the ventral forearm of male volunteers. The application site received either an occlusive or non-occlusive (“protected”) covering. Urine samples were collected for 7 days after application. At 24 hours after dosing, chambers attached to the application site were removed. The application site was washed with soap, water, soap, water and water. All washes were collected for analysis. p-Aminophenol was recovered at 63% and 91% under the occlusive and non-occlusive conditions, respectively. The authours recommended percutaneous absorption of 8% and 6% for the occlusive and non-occlusive conditions, respectively. Dermal absorption in rats (including urine, feces, viscera, body and skin, excluding application site) ranged from 1.7% to 11.5% of the applied dose in the 4 days following a 30-minute dermal application of p-aminophenol (Tsomi and Kalopissis 1982). In an unpublished study in rats (Hofer et al. 1984), less than 1% of the applied radioactivity was found in urine, feces and organs following a 30-minute dermal exposure to aqueous p-aminophenol or to p-aminophenol in hair dye formulations; however, the full study is not available.
In an in vitro study using mouse skin, p-aminophenol was applied as a single dose in acetone; within 24 hours, 72% of the applied dose had been transported across the skin (Hinz et al. 1991). In an unpublished study cited in the EU’s Scientific Committee on Consumer Products review (Faith and Williams 1999), absorption across rat and human skin was much lower (10% and 0.5–6%, respectively) during a 24-hour exposure; however, the full study is not available. In another unpublished study (BMS 1997), less than 1% of the applied dose was absorbed when p-aminophenol was applied to human skin in vitro, for 30 minutes, alone or in the presence of other hair dye ingredients, including hydrogen peroxide (developer). The EU’s Scientific Committee on Consumer Safety used the BMS (1997) study, which was conducted specifically using hair dye formulations, to calculate a dermal absorption of 7.84% (SCCS 2011); however, the original data from this study were not available.
Based on the available data, a dermal absorption of 6% was selected from the human in vivo study (Bucks et al. 1990The value of 6% was selected based on the better recovery observed. The human study was the most appropriate for application to the dermal exposure scenarios for p-aminophenol. The dermal absorption value of 6% was used to estimate dermal exposure from hair dyes, nail polish, body cream, lotion and moisturizer.
Health Effects Assessment
The health effects of p-aminophenol have been previously reviewed by OECD (2010), SCCP (2005) and SCCS (2011). A rationale for selection of the critical effect levels for derivation of MOEs is given below.
Dermal exposure to p-aminophenol has been shown to produce mostly acetylated metabolites; both in vitroreconstituted epidermis and in vivo dermal exposure studies have demonstrated formation of acetaminophen (Dressler and Appelqvist 2006; Hu et al. 2009; Nohynek et al. 2005). Conversely, intraperitoneal and gavage administration in rats and rabbits resulted in approximately 5 to 45% acetaminophen conversion respectively (Bray et al. 1952; Newton et al. 1983, 1985). Acetaminophen is an approved human therapeutic drug that is authorized for sale and regulated as a drug under the Food and Drugs Act in Canada, up to a maximum daily dose of 4000 mg (C.01.021, Food and Drug Regulations) (Health Canada 2009).
Carcinogenicity and Genotoxicity
There was no increase in tumour incidence in male or female rats given p-aminophenol daily by gavage at up to 30 mg/kg-bw per day for 101 weeks (CIT 1998; ECHA 2013). The study was conducted according to OECD guidelines; however, the original study data were not available.
OECD (2010) concluded that p-aminophenol is clastogenic based on positive in vitro chromosomal aberrations and in vivo micronuclei, and SCCP (2005) concluded that clastogenic effects occur at high doses in the presence of toxic effects. SCCS (2011) supported this conclusion and added that there was “no mutagenic risk” to humans (under normal dermal use conditions), based on an indirect genotoxic mode of action similar to that reported for acetaminophen, the main dermal metabolite of p-aminophenol.
| Test Type | Positive | Negative |
|---|---|---|
| Bacterial Mutagenicity (Salmonella typhimurium strains sensitive to point mutations) | - | With and without activation (McCann et al. 1975; Garner and Nutman 1977; Degawa et al. 1979; Lavoie et al. 1979; Probst et al. 1981; Thompson et al. 1983; DeFlora et al. 1984a,b; Zeiger et al. 1988; Watanabe et al. 1991; Tomiyama et al. 2008). |
| Bacterial Mutagenicity (Escherichia coli strains sensitive to oxidative damage) | Without activation (Yoshida et al. 1998; Martinex et al. 2000) | - |
| Bacterial DNA Damage | Without activation (DeFlora et al. 1984b; Hellmer and Bolcsfoldi 1992a) | With activation (Mamber et al. 1983; DeFlora et al. 1984b; Hellmer and Bolcsfoldi 1992a) |
| Mammalian Cell Mutagenicity | TK locus – mouse lymphoma cells (Amacher and Turner 1982; Oberly et al. 1984, 1993; Majeska and Holden 1995). | HGPRT locus in Chinese hamster ovary cells (Oberly et al. 1990, 1993). HGPRT locus in CHO and mouse lymphoma cells (Majeska and Holden 1995). |
| Mammalian Cell Chromosomal Aberrations & Sister Chromatic Exchange Events | (Kirchner and Bayer 1982; Takehisa and Kanaya 1982; Holme et al. 1988; Majeska and Holden 1995; Kusakabe et al. 2002) | - |
| Mammalian Cell DNA breaks and inhibited DNA synthesis | (Andersson et al. 1982; Hayward et al. 1982; Majeska and Holden 1995) | - |
| Test Type | Positive | Negative |
|---|---|---|
| In vivo Micronucleus | Bone marrow and spleen of mice orally treated, and in the bone marrow and liver of mice treated by intraperitoneal injection (JECDB undated-a; Wild et al. 1980; Cliet et al. 1989; Sicardi et al. 1991; Benning et al. 1994) | Bone marrow of rats orally treated (CIT 1995). Bone marrow of rats treated by gavage (Hossack and Richardson 1977). |
| In vivo Sister Chromatid Exchange | - | Bone marrow of ip injected hamsters (Kirchner and Bayer 1982). |
| In vivo Dominant Lethal Mutations | - | Rats orally exposed (Burnett et al. 1989; Hellmer and Bolcsfoldi 1992b). |
| In vivo Host-mediated DNA Repair | - | Mice orally treated (Burnett et al. 1989; Hellmer and Bolcsfoldi 1992b). |
| In vivo Unscheduled DNA Synthesis (UDS) | - | Rat hepatocytes (Microtest 1989). |
| Drosophila melanogaster SLRL | - | (Eiche et al. 1990). |
| Drosophila melanogaster Somatic mutation and Recombination | (Eiche et al. 1990) | - |
Evidence for the mutagenicity of p-aminophenol is equivocal, but this aromatic amine does exhibit clastogenic potential in vivo.
Other Health Effects
In a study on the reproductive and developmental toxicity of p-aminophenol (Burnett et al. 1989), female rats were dosed in the diet for 13 weeks prior to mating, at 0, 52, 149 or 520 mg/kg-bw per day. Dams in the high-dose group (520 mg/kg-bw per day) had reduced body weights and body weight gains throughout gestation, relative to controls. There were also increased post-implantation losses and total resorptions and reduced fetal weights. The toxic effects on the fetus are likely non-specific and related to maternal toxicity.
In the same study (Burnett et al. 1989), animals of both sexes were dosed with p-aminophenol for 13 or 27 weeks (42, 120 or 420 mg/kg-bw per day in males; 52, 149 or 520 mg/kg-bw per day in females). The incidence and severity of nephropathy increased in a dose-related manner, which was more pronounced in males than in females. At the high dose (420 mg/kg-bw per day for males; 520 mg/kg-bw per day for females), significant reductions in body weights, body weight gains, feed consumption, erythrocyte counts and hemoglobin levels were observed. The LOAEL for this study is the low dose of 42 mg/kg-bw per day, based on the kidney pathology.
In another reproductive and developmental toxicity study (Harada et al. 2008), rats were administered p-aminophenol by gavage at 0, 20, 100 or 500 mg/kg-bw per day starting from 14 days before mating. Females were dosed until day 3 of lactation, and males were dosed for a total of 49 days. At the high dose of 500 mg/kg-bw per day, several treated animals died and were found to have necrosis of the kidneys. In surviving animals, kidneys and spleens were dark and showed histopathology consistent with anemia, and body weight gains at some time points were significantly decreased in both sexes. At this dose, effects on the male reproductive tract, prolonged gestation, increased rate of stillbirth and pup death were also observed; the effects on developmental parameters are likely due to parental toxicity. A NOAEL of 100 mg/kg-bw per day for developmental toxicity was identified (LOAEL of 500 mg/kg-bw per day). At 100 mg/kg-bw per day, treated animals had brown urine and decreased feed consumption; the authors and the OECD consider this the LOAEL for general toxicity (NOAEL of 20 mg/kg-bw per day).
A p-aminophenol no-observed-effect level (NOEL) of 10 mg/kg-bw per day and a lowest-observed-effect level (LOEL) of 30 mg/kg-bw per day were identified in an unpublished 90-day rat gavage study, based on a dose-related increase in kidney nephrosis in males and females (CIT undated). However, in the chronic toxicity/carcinogenicity portion of this study (see above), there were no differences in any parameter, including hematology, organ weights, gross pathology or histopathology, body weights or mortality, between controls and rats dosed at up to 30 mg/kg-bw per day for 101 weeks (ECHA 2013). SCCS (2011) used a NOAEL of 10 mg/kg-bw per day from this study in its safety assessment of p-aminophenol.
In a 28-day oral gavage study in rats (JECDB undated-a; REACH 2013), a p-aminophenol NOAEL of 20 mg/kg-bw per day was identified by the study authors and the OECD, based on kidney pathology, urinalysis and increased kidney weight at 100 mg/kg-bw per day. At 500 mg/kg-bw per day, anemia-like effects, including decreased red blood cells, hematocrit and hemoglobin, increased reticulocytes, and increased liver and spleen weights, were observed. The original study was not available.
Risk Characterization
Exposure of the general population in Canada to p-aminophenol occurs predominantly through the use of cosmetic products. Estimates of risk associated with upper-bounding cosmetic product exposure scenarios (i.e., hair dye, nail polish, body cream, lotion or moisturizer) for p-aminophenol are presented in Tables 7-17 and 7-18.
The critical effect level for p-aminophenol is a NOAEL of 20 mg/kg-bw per day, based on coloured urine and decreased feed consumption at the LOAEL of 100 mg/kg-bw per day in a 40- to 49-day gavage study in rats (Harada et al. 2008).
Comparison of upper-bounding estimates of dermal exposure to p-aminophenol from use of hair dye, nail polish and body cream, lotion or moisturizer with the critical effect level (short-term oral NOAEL of 20 mg/kg-bw per day) results in MOEs ranging from greater than or equal to 94 to 5000.
Given that the NOAEL of 20 mg/kg-bw per day in a short-term oral study was based on mild effects at the next highest dose of 100 mg/kg-bw per day and a chronic study in rats showed no effects at 30 mg/kg-bw per day, use of this NOAEL to derive MOEs for dermal per event exposures is conservative. Therefore, all of the MOEs presented in Tables 7-17 and 7-18 are considered adequate to address uncertainties in the human health and exposure databases.
Oral toxicity data were used in the absence of dermal data; as a conservative precautionary assumption, absorption by the oral and dermal routes was considered to be equivalent.
| Cosmetic product | Upper-bounding daily exposure estimate (mg/kg-bw per day) | Critical effect level: rat oral NOAEL (mg/kg-bw per day) |
MOEs |
|---|---|---|---|
| Body cream, lotion, moisturizer (infant) | less than or equal to 0.019 | 20 | greater than 1050 |
| Body cream, lotion, moisturizer (adult) | less than or equal to 0.004 | 20 | greater than 5000 |
| Cosmetic product | Upper-bounding acute exposure estimate (mg/kg-bw) |
Critical effect level: rat oral NOAEL (mg/kg-bw per day) |
MOEs |
|---|---|---|---|
| Body cream, lotion, moisturizer (infant) | less than or equal to 0.011 | 20 | greater than 1820 |
| Body cream, lotion, moisturizer (adult) | less than or equal to 0.004 | 20 | greater than 5000 |
| Nail polish | 0.004–0.013 | 20 | 1540–5000 |
| Permanent hair dye | 0.212 | 20 | 94 |
7.3.8 1,3-Diaminobenzene
Exposure Assessment
Based on information submitted in response to a section 71 survey, between 1 000 and 10 000 kg of 1,3-diaminobenzene was imported into Canada to be used for the manufacture of (industrial) tires (Environment Canada 2009). Most of the in-commerce quantity is used in tire rubber manufacture, with a negligible amount released to the environment through processing; therefore, exposure of the general population from environmental media is not expected.1,3-Diaminobenzene is incorporated within the tire matrix at low concentrations. Therefore, human exposure to 1,3-diaminobenzene in tires through their service life and recycled uses is not expected.
As identified in Section 4.2, 1,3-diaminobenzene is used as a developer in permanent hair dyes in Canada. Potential exposure to 1,3-diaminobenzene for adults using this product was estimated using ConsExpo (ConsExpo 2006; see Appendix J for underlying assumptions). The upper-bounding dermal exposure estimate for adults is 0.25 mg/kg-bw per event based on the use of hair dyes with a 1% concentration of 1,3-diaminobenzene (personal communications, emails from Consumer Product Safety Directorate [Health Canada] to Existing Substances Risk Assessment Bureau [Health Canada], dated 2011 and 2013; unreferenced).
Three studies reporting dermal absorption of 1,3-diaminobenzene were identified. Kiese et al. (1968) treated dogs with 1.5 g 1,3-diaminobenzene (approximately 176 mg/kg-bw), in various hair dye formulations, across a 20 cm × 25 cm clipped area for 3 hours. While total recovery was not addressed, the authors reported that a total of 60 mg (4%) was absorbed. More recently, Lam and Bisgaard (1989) dosed male Wistar rats with 4% weight per volume (w/v) solutions of 1,3-diaminobenzene (equivalent to 556 µmol, or 660 mg, in either 16.5 mL of 0.9% w/v saline or 16.5 mL of an aqueous 4% w/v solution of hydrogen peroxide in 0.9% saline). Following 24-hour applications of test solutions to clipped 8 cm × 8 cm areas, treated sites were washed, and urine and feces were collected during the subsequent 7 days. Percentages of absorbed dose recovered were 80.7%, 10.0% and 9.3% in urine, feces and carcass tissues, respectively; total mass recovered was reported as 99.9 ± 16.2 µmol 1,3-diaminobenzene and corresponded to 18% absorption (Lam and Bisgaard 1989). In addition, the exposed skin area was excised and soaked in a saline solution for 24 hours. Only trace amounts of 1,3-diaminobenzene were reported to have migrated into the saline solution, and less than 1% of total radioactivity was detected in the excised skin. The same authors conducted an in vivo study in which radiolabelled 1,3-diaminobenzene was applied dermally at a dose of 240 mg/kg-bw to the backs of rats (n = 7) (Bisgaard and Lam 1989). The rats were placed in metabolism cages, and urine and feces were collected over 24 hours. The animals were sacrificed after 24 hours, and the radioactivity was measured in the carcass, feces and urine. The absorption of 1,3-diaminobenzene was determined to be 18.0% ± 3.0% by mass balance analysis, which sums radioactivity in the carcass, feces and urine.
Based on the available data, a dermal absorption value of 18% was selected from the in vivo study on rats in which urine and feces were collected for 7 days after exposure (Lam and Bisgaard 1989). This study demonstrated a good recovery and thorough observation of dermal absorption for 1,3-diaminobenzene. This value is further supported by an additional in vivostudy, which also found dermal absorption to be 18% (Bisgaard and Lam 1989). Although they reported total absorption in the carcass, feces and urine, they did not report the recovery of the applied dose. Furthermore, they did not determine skin-bound residues or skin washes. While Kiese et al. (1968) reported lower absorption in dogs, the study focused only on blood concentration. In addition, the number of replicates used and how the reported total absorption of 60 mg was derived are unclear.
As discussed previously, 1,3-diaminobenzene was found to migrate from certain polyamide cooking utensils in a study conducted in Denmark (Trier et al. 2010). Using the median migration level of 1,3-diaminobenzene (0.0003 µg/g food), conservative estimates of potential exposure to 1,3-diaminobenzene from the use of polyamide cooking utensils were derived to range from 0.0004 µg/kg-bw per day (12 years of age and older) to 0.0014 µg/kg-bw per day (toddlers 0.5–4 years of age).
Health Effects Assessment
Carcinogenicity and Genotoxicity
1,3-Diaminobenzene and its salt, 1,3-diaminobenzene dihydrochloride, were considered equivalent for the purpose of this assessment. Both have been classified by IARC as, Group 3. IARC deemed all animal studies involving dermal and subcutaneous exposures to be inadequate. In addition, no adequate carcinogenicity or epidemiological studies were available for review by IARC at the time of the classification (IARC 1998) (see Table 7-5 for cancer classifications). It should be noted that structurally similar isomers have also been classified. IARC classified the para isomer as Group 2A, probably carcinogenic to humans for occupational exposures as a hairdresser or barber, and as Group 3, not classifiable as to its carcinogenicity in humans for personal use of hair colourants (IARC 1998). A rationale for selection of the critical effect levels for derivation of MOEs is given below.
No clear dose–response relationship between exposure and carcinogenicity has been demonstrated. No epidemiological studies or case reports have been identified in the available literature. While the majority of data from animal studies show no carcinogenic potency, anterior pituitary adenoma and fibrosarcoma tumours have been reported following subcutaneous injection.
Chronic oral studies in male and female rats dosed at 6–40 mg/kg-bw per day and in male rats dosed at 30–100 mg/kg-bw per day failed to demonstrate tumorigenic potential (Russfield et al. 1975; Weisburger et al. 1978; Sontag 1981; Nozaki 1991 in EC 2000i). Similarly, male and female mice administered doses of 23–520 mg/kg-bw per day did not develop tumours (Russfield et al. 1975; Weisburger et al. 1978; Sontag 1981; Amo et al. 1988). Furthermore, mice administered a 1.5% formulation once weekly for 21 months or a 0.17% formulation once weekly for 18 months did not develop treatment-related tumours (Burnett et al. 1975, 1980). Similarly, rabbits treated with a 1.5% formulation twice weekly for 13 weeks also showed no signs of toxicity (Burnett et al. 1976).
Conversely, a few investigators have reported a significant tumorigenic response following exposure. Female mice orally administered 16 or 54 mg/kg-bw per day in drinking water developed anterior pituitary adenomas (Nozaki 1991 in EC 2000i). One of five Wister-King rats administered 4.5 or 9 mg/kg-bw per day via subcutaneous injection for 11 months and 6 or 12 mg/kg-bw per day via subcutaneous injection for 5 to 11 months developed fibrosarcomas at injection sites (Saruta et al. 1962). Also, male and female rats dosed with 1.2 or 3.6 mg/kg-bw per day via subcutaneous injection for 2 years developed tumours at injection sites (Cited in EC 2000i: Habs 1980; Steinhoff and Dycka 1981).
The EU has designated 1,3-diaminobenzene as a Category 2 mutagen with risk phrase H341: suspected of causing genetic defects (EU 2008). IARC (1978) reported positive mutagenicity in Salmonella strain TA1538 (−1 frameshift) in the presence of activating microsomal fractions. Similarly, the NTP testing status of this substance as of February 2014 (NTP 2014b) also indicates that the substance is mutagenic in Salmonella(study 188858 included strains TA97, TA98, TA100, TA1535). More recent in vitro studies have also generally supported the genotoxic potential of 1,3-diaminobenzene. Shimizu and Takemura (1984) reported mutations in E. coli WP2 uvrA and WP2 uvrA/pKM cultures exposed to 1,3-diaminobenzene with activating factors. DNA damage was also reported in treated human lymphocytes, but not human–human hybridoma cells (Maeda et al. 1990; Plewa et al. 1995). Chromosomal aberrations observed in treated Chinese hamster ovary KI cells (without activation), Chinese hamster ovary WBL cells (with activation) and Chinese hamster lung CL-11 cells (with and without activation) (Lee and Lee 1986; Sofuni et al. 1990). However, no genetic damage (no induction of unscheduled DNA synthesis) was reported in primary rat hepatocytes derived from either Fischer F344 or ACI rats (Probst et al. 1981; Williams et al. 1982).
Conversely, the available evidence does not support the in vivo genotoxicity of 1,3-diaminobenzene. IARC (1978) reported that 1,3-diaminobenzene was not capable of causing mutations (dominant lethals) in Charles River rats. More recent in vivo studies do not generally support the genotoxic potential of this substance (European Commission. ©2000i).
Other Health Effects
Reproduction may be adversely impacted by oral exposure to 1,3-diaminobenzene, while developmental effects have not been demonstrated following dermal exposure. Female rats administered 0, 10, 30 or 90 mg/kg-bw per day via oral gavage during gestation days 6–15 exhibited reduced body weight gain and feed consumption at doses of 30 and 90 mg/kg-bw per day. At the high dose, increased total resorptions, decreased number of litters with living fetuses, decreased number of living fetuses, decreased number of fetuses per litter, increased number of dead fetuses, decreased fetal weights and fetal malformations were reported. Fetal lethality was attributed to reduced fetal nutrient availability secondary to decreased material feed intake (A TRDB/USEPA Technical Support Document cited in EC 2000i). Picciano et al. (1983) also reported maternal body weight gain reductions in rats following oral exposure to 1,3-diaminobenzene.
Burnett and Goldenthal (1988 – cited in EC 2000i) administered a hair dye formulation containing 1.5% 1,3-diaminobenzene to male and female Sprague-Dawley rats via the dermal route at 0.5 mL per animal, twice weekly for 6 weeks (approximately 6.1 mg/kg-bw per day). Administration was continuous across three generations of mating. Fertility, gestation, survival and live birth indices (mean numbers weaned, mean weaning weights of litters) were unaffected in all generations; mild dermatitis was reported.
Human case reports identify acute effects that are consistent with those observed in orally dosed mice, rats, rabbits and cats: edema, gastrointestinal symptoms, pulmonary dysfunction, cardiovascular and renal effects, as well as organ pigmentation (Nott 1924; Bowen 1963; Owens and Medsger 1988). However, chronic oral administration of 1,3-diaminobenzene in animals largely impacts liver and kidney function. Pigmentation of the lung, liver and thyroid has also been reported (Nozaki, 1991 in EC 2000i).
Rats and mice chronically dosed via the oral route have inconsistently exhibited organ pigmentation, reduced body weight gain, renal effects, including increased urea and creatinine levels, and hepatic dysfunction (reduced plasma albumin and feed consumption) (Russfield et al. 1975; Weisburger et al. 1978; Amo et al. 1988; Nozaki 1991 in EC 2000i; EC 2000i). Studies that used drinking water as the exposure vehicle have generally shown adverse effects at lower doses than those that administered 1,3-diaminobenzene in feed. One explanation may be reduced bioavailability resulting from 1,3-diaminobenzene administration in an organic matrix, including foodstuffs. Nozaki (1991 cited in EC 2000i) reported decreased body weight gain, increased plasma urea, creatinine and potassium, reduced plasma albumin, chronic renal disease and eosinophilic degeneration of nasal epithelium in female Fischer 344 rats administered 40 mg/kg-bw per day in drinking water for 104 days. The same investigators demonstrated anterior pituitary adenoma formation in female BDF1 mice exposed to 6 or 54 mg/kg-bw per day in drinking water for 101 days. However, male Sprague-Dawley rats dosed with 1000 or 2000 ppm (equivalent to 75 and 150 mg/kg-bw per day) in feed for 18 months did not exhibit increased mortality, body weight gain reduction or increased tumour incidence (Cited in EC 2000i: Russfield et al. 1975; Weisburger et al).
Most 1,3-diaminobenzene metabolism studies have focused on dermal application as the most relevant exposure route in humans: exposure to hair dye preparations. In dogs, the dermally absorbed fraction of the total applied dose was estimated to be small (4%) based on quantification of colour reaction product using spectroscopic methods (Kiese et al. 1968 in EC 2000i). However, Lam and Bisgaard (1989) showed that 14C-radiolabelled 1,3-diaminobenzene (approximately 556 µmol) applied to rat skin distributed into liver and kidney tissues and that approximately 90.2 µmol (16%) of the total applied dose was recovered in urine and feces.
Consistent with the findings in the Lam and Bisgaard (1989) study, short-term dermal repeated applications in mice have resulted in hepatic and renal injury. Five male and 5 female C57Bl6 and C3Hf/He mice were dermally treated with 0, 1.1 (approximately 41 mg/kg-bw per day) or 5.7 mg/animal (approximately 204 mg/kg-bw per day), 5 days/week for 2 weeks. While no adverse effects were reported at the low dose, the high dose caused mortality in some animals after 2–3 applications; dehydration, tubular nephrosis, swollen liver and fatty degenerative changes were also associated with animals treated with the high dose (Holland et al. 1978 in EC 2000i).
However, chronic dermal administration (intended to approximate 1,3-diaminobenzene concentrations in hair dye formations) has failed to show any adverse effects (Cited in EC 2000i: Burnett et al. 1975, 1976, 1980, 1988; Weisburger et al. 1978; Holland et al. 1979).
Reports of 1,3-diaminobenzene-induced sensitization in guinea pigs are equivocal; only four out of six studies have shown a positive sensitization effect with 1,3-diaminobenzene at concentrations up to 2% (Cited in EC 2000i: Nitti et al. 1937; Dossou et al. 1985; Ishihara et al. 1985; Kurlyandskki et al. 1987 ; Shigematsu et al. 1988)(Kalish and Wood 1995), and few human patch tests have demonstrated sensitization following dermal treatment (Cited in EC 2000i: Mayer 1930; Ishihara et al. 1985; Matsunuga 1989).
Risk Characterization
Exposure of the general population in Canada to 1,3-diaminobenzene occurs predominantly through use of cooking utensils and dermal contact with permanent non-spray, wash-in hair dyes containing up to 1% 1,3-diaminobenzene. Estimated exposure concentrations are summarized in Table 7-19. Upper-bounding acute dermal exposure resulting from the application of hair dyes was estimated to be 0.25 mg/kg-bw. Potential daily oral exposures resulting from the use of polyamide cooking utensils were conservatively estimated to range from 4 × 10−7 to 1.4 × 10−6 mg/kg-bw per day.
The critical effect levels protective of acute and intermittent dermal toxicity from 1,3-diaminobenzene exposure in hair dye are a NOAEL of 41 mg/kg-bw per day and a LOAEL of 204 mg/kg-bw per day, based on minimal mortality and kidney and liver toxicity in mice treated via the dermal route, 5 days/week for 2 weeks. No studies involving exposure routes better matched to hair dye scenarios were located in the available literature; as such, a short-term repeated-dose study was chosen as a conservative surrogate for comparison. The critical effect level protective of chronic oral toxicity from 1,3-diaminobenzene leaching from polyamide cooking utensils is the highest available NOAEL of 150 mg/kg-bw per day, from two rat studies in which animals were administered 0, 1 000 or 2 000 mg/kg-bw per day (approximately 75 and 150 mg/kg-bw per day) in feed. Treated animals did not exhibit increased mortality, body weight gain reductions or increased tumour incidence at either dose level.
Comparison of the upper-bounding estimate of acute dermal exposure to 1,3-diaminobenzene from hair dyes (0.25 mg/kg-bw) with daily short-term dermal critical effect levels (NOAEL of 41 mg/kg-bw per day and LOAEL of 204 mg/kg-bw per day) results in an MOE range of 164–816, which is considered adequate to address uncertainties in the health effects and exposure databases due to the considerable level of conservatism associated with comparing acute intermittent exposure via hair dye application to a critical effect level based on repeated exposures. The likelihood of accumulation and toxicity resulting from short-term repeated-dose exposures is expected to be low, as elimination is thought to occur rapidly. A study in the rat demonstrated complete elimination of 1,3-diaminobenzene in 7 days following administration (Lam and Bisgaard 1989). Comparison of estimated daily oral exposures to 1,3-diaminobenzene from use of cooking utensils with the chronic oral critical effect level (NOAEL of 150 mg/kg-bw per day) results in MOEs ranging from 1.1 × 108 to 3.8 × 108 (Table 7-19), which are considered adequate to address uncertainties in the health effects and exposure databases.
| Exposure duration and route | Products | Estimated exposure | Critical effect levels (mg/kg-bw per day) | MOEs |
|---|---|---|---|---|
| Acute dermal | Hair dye – non-spray/ wash-in; permanent | 0.25 mg/kg-bw per event | Dermal NOAEL = 41 Dermal LOAEL = 204 (renal and hepatic injury) |
164–816 |
| Daily oral | Polyamide cooking utensils | 4 × 10−7 – 1.4 × 10−6mg/kg-bw per day | Oral NOAEL = 150 (reduced body weight gain / tumours) |
1.1 × 108 – 3.8 × 108 |
7.3.9 Red Lake C Amine
Exposure Assessment
Based on the use of Red Lake C Amine in Canada (not specified herein due to confidentiality; Canada 2011; Environment Canada 2012), release of this Aromatic Amine to the environment from this activity is expected to be minimal. Therefore exposure of the general population is Canada to Red Lake C Amine from environmental media is not expected.
Red Lake C Amine is a postulated breakdown product of three Monoazo Pigments (via reductive cleavage of the azo bond) within the Aromatic Azo and Benzidine-based Substance Grouping (Environment Canada and Health Canada 2013c). The three Monoazo Pigments are Pigment Red 53:1 (CAS RN 5160-02-1), Pigment Red 52:1 (CAS RN 17852-99-2) and Pigment Red 52:2 (12238-31-2). Oral and dermal exposures to Red Lake C Amine were estimated assuming that exposure can occur following reductive cleavage of the azo bond in the parent pigment by certain bacteria in the skin or gut (see Tables 7-20 and 7-21). Therefore, exposure to Red Lake C Amine was estimated based on the exposure to the parent pigments (see Appendix K for details of the estimations).
| Scenario | Age group | Concentration of pigment (% w/w) |
Estimated per event exposure (mg/kg-bw) |
Estimated daily exposure (mg/kg-bw per day) |
|---|---|---|---|---|
| Face paint | Toddlers | less than or equal to 15 | less than or equal to 0.51 | N/A |
| Lip balm | Toddlers | less than or equal to 0.3 | less than or equal to 4.83 × 10−4 | less than or equal to 2.83 × 10−4 |
| Lipstick | Adults | less than or equal to 0.3 | less than or equal to 1.06 × 10−4 | less than or equal to 2.53 × 10−4 |
| Scenario | Concentration of pigment (% w/w) | Estimated per event exposure (mg/kg-bw) | Estimated daily exposure (mg/kg-bw per day) |
|---|---|---|---|
| Face paint | less than or equal to 15 | less than or equal to 3.37 × 10−2 | N/A |
| Hair dye (semi-permanent) | 0.3–1 | 3.69 × 10−2 – 1.23 × 10−1 | 5.28 × 10−3–1.76 × 10−2Footnote Table 7-23 [a] |
| Mascara | less than or equal to 0.1 | less than or equal to 1.05 × 10−4 | less than or equal to 6.98 × 10−5 |
Systemic exposure to Red Lake C Amine via inhalation deriving from Pigment Red 53:1, Pigment Red 52:1 and Pigment Red 52:2 in paint products such as wall paint is expected to be lower than the estimated exposures from face paint and certain cosmetic products, as indicated in Tables 7-20 and 7-21.
Health Effects Assessment
The toxicity data for Red Lake C Amine have been obtained from the English abstract of data from the Japanese Existing Chemicals Data Base (JECDB, undated-b), summaries of unpublished studies from a IUCLID dossier (European Commission ©2000f) and additional published references. A rationale for selection of the critical effect levels for derivation of MOEs is given below.
No carcinogenicity or chronic toxicity studies were available for this substance. Red Lake C Amine was reported as negative in all available reverse mutagenicity assays in Salmonellaand E. coli strains, both with and without rat liver S9 (JECDB undated-b; Shimizu et al. 1985; ETAD 1986; European Commission ©2000f) and also negative for unscheduled DNA synthesis in primary rat hepatocytes (ETAD 1988; Williams et al. 1989). This substance was reported as positive for induction of chromosomal aberrations but not polyploidy in Chinese hamster lung cells (JECDB undated-b; Kusakabe et al. 2002). Low pH from the dissociation of the sulfonic acid moiety may have caused the cytogenetic response, as was also observed in the same study for a closely related sulfonated aromatic amine, 4B Acid. In the case of 4B Acid, a negative response for chromosomal aberrations was observed after the culture medium was buffered to neutral pH (Kusakabe et al. 2002), which led the authors to conclude that low pH likely was the cause of the observed clastogenicity, a common effect previously reported (Morita et al. 1992). While a repeated test on Red Lake C Amine was not performed at neutral pH, the apparent clastogenic effect observed was considered inconclusive by the study authors (JECDB undated-b; Kusakabe et al. 2002). In a review of the toxicity of other sulfonated aromatic amines, including specifically o-substituted sulfonated anilines such as Red Lake C Amine, the general trend indicated negative genotoxicity for these substances (Jung et al. 1992). Overall, the weight of evidence supports the conclusion of limited evidence for genotoxicity of Red Lake C Amine.
Red Lake C Amine exhibited low oral acute toxicity, with LD50s ranging from 2000 to 5000 mg/kg-bw in the rat (JECDB undated-b; European Commission ©2000f) and an LD50 of greater than 2000 mg/kg-bw in the cat (European Commission ©2000f). JECDB (undated-b) and Sakuratani et al. (2008) reported a 28-day repeated-dose study in rats (Crj:CD (SD), five of each sex per dose) orally administered 0, 100, 300 or 1000 mg/kg-bw per day (). In the English abstract of this study, no exposure-related changes were reported for body weight, feed consumption, hematological or blood parameters, organ weights, or gross and/or microscopic abnormalities. The NOAEL for this study was considered to be 1000 mg/kg-bw per day, the highest dose tested. The low toxicity of Red Lake C Amine is consistent with 4B Acid, which also showed limited toxicity in short-term studies (JECDB undated-b; Sakuratani et al. 2008). This is supported by the overall low toxicity observed for sulfonated aromatic amines in general (Jung et al. 1992).
Risk Characterization
As the acute toxicity of Red Lake C Amine is low (oral LD50 values greater than or equal to 2000 mg/kg-bw), the risk from acute exposure is not considered to be of concern (no quantification presented). For repeated exposures, the critical effect level is considered to be 1000 mg/kg-bw per day, the NOAEL from the short-term study in rats (JECDB undated-b). Comparison of upper-bounding estimates of oral and dermal exposures to Red Lake C Amine from lip balm and semi-permanent hair dye, respectively, with the critical effect level of 1000 mg/kg-bw per day from the 28-day gavage study in rats results in MOEs greater than 57 000 (Table 7-22). These MOEs are considered adequate to address uncertainties in the health effects and exposure databases.
| Exposure route | Cosmetic products | Upper-bounding daily exposure (mg/kg-bw per day) |
Critical effect level (mg/kg-bw per day) | MOEs |
|---|---|---|---|---|
| Oral | Lip balm (toddler) | 2.83 × 10−4 | NOAEL = 1000 | greater than 3.5 × 106 |
| Dermal | Hair dye (semi-permanent) |
1.76 × 10−2Footnote Table 7-24 [a] | NOAEL = 1000 | 57 000 |
7.3.10 The Remaining Seven Aromatic Amines
Based on the response to the recent surveys under section 71 of CEPA 1999 as well as other sources of information described below, exposure of the general population of Canada to the following seven Aromatic Amines is not expected: 2,4-diaminoanisole, p-toluidine, p-toluidine hydrochloride, p-phenetidine, 2-chloroaniline, 4-nitroaniline and 1,3-diaminobenzene dihydrochloride.
As exposure for the general population of Canada from these seven substances is not expected, only brief descriptions of their health effects are presented below, with the exception of 1,3-diaminobenzene dihydrochloride; this substance is considered toxicologically equivalent to 1,3-diaminobenzene (described previously).
2,4-Diaminoanisole
2,4-Diaminoanisole (as the sulfate) was tested for carcinogenic potential in one experiment in B6C3F1 mice and in two experiments in F344 rats. Thyroid gland adenomas or carcinomas were induced in both sexes of mice and rats following 78–82 weeks of dietary administration. Tumours of the skin and of the preputial, clitoral and Zymbal’s glands were also induced in rats. Other studies available found that 2,4-diaminoanisole induced tumours when administered in combination with other agents.
In mammalian cells in vitro, 2,4-diaminoanisole induced mutations, DNA damage and chromosomal aberrations. Mutagenicity (Ames) tests in bacteria were generally negative without metabolic activation, but positive with activation in frameshift-detecting strains. In vivo, 2,4-diaminoanisole was negative for dominant lethal mutations in rats and for micronuclei in bone marrow of rats and mice. However, it induced sister chromatid exchanges in mouse bone marrow and DNA damage in several organs of rats and mice, including thyroid (IARC 2001; Pomorski et al. 2002; Mattioli et al. 2005).
p-Toluidine and p-Toluidine Hydrochloride
p-Toluidine and its salt p-toluidine hydrochloride are considered toxicologically equivalent in this assessment.
A study was identified that screened for p-toluidine in breast milk of 31 lactating mothers in Canada including 7 smokers and 24 non-smokers (detection limit not established; DeBruin et al. 1999). This study did not detect p-toluidine in any of the samples tested. Based on the above, overall exposure of the general population in Canada to p-toluidine and p-toluidine hydrochloride is not expected.
In 2012, the European Commission (Carcinogen Category 2, ESIS 1995–2012) classified p-toluidine as potentially carcinogenic. At that time, the US EPA also concluded that the available information was “suggestive evidence of carcinogenic potential” (US EPA 2012b). However, the European SCOEL evaluation (SCOEL 2013) concluded that further in vivo experimental studies on genotoxicity and carcinogenicity were needed, but proposed that any carcinogenic potential attributed to p-toluidine would likely be less than that of its isomer, o-toluidine.
The genotoxicity of p-toluidine has been evaluated in a variety of in vitro and in vivo test systems (OECD 2005a). p-Toluidine does not induce point mutations in the majority of Ames tests. A positive result was obtained in an unscheduled DNA synthesis assay in primary cultures of rat hepatocytes. In the presence of metabolic activation, p-toluidine has been shown to be clastogenic, increasing chromosomal aberrations in vitro in cultured Chinese hamster lung cells. In vivo, a single intraperitoneal injection of p-toluidine increased the incidence of single-strand breaks in liver and kidney of mice. However, the applicability of these results is questionable, as the administered dose represents two-thirds of the median lethal dose, and it is unclear whether the observed response is a result of cytotoxicity or specific action on the genetic material. Negative results were obtained in the micronucleus test in mice given p-toluidine by intraperitoneal injection; however, significant toxicity and mortality were observed (OECD 2005a).
p-Phenetidine
p-Phenetidine was identified as being present in the food and beverage sector in response to a section 71 survey (Environment Canada 2008). However, it is not present in the Lists of Permitted Food Additives incorporated by reference under their associated Marketing Authorizations, issued under the authority of the Food and Drugs Act (Health Canada 2013b); thus, is not permitted for direct addition to foods sold in Canada. However, the monograph for ethoxyquin set out in the US Food Chemicals Codex (Institute of Medicine [U.S.] 2003) sets a maximum limit of 3.0% for p-phenetidine as an organic impurity in ethoxyquin. Ethoxyquin is on the List of Permitted Food Additives with Other Generally Accepted Usesas a permitted food additive in paprika and ground chili pepper to promote colour retention at a maximum level of 100 ppm (Health Canada 2013b). As a result, p-phenetidine could be present as an organic impurity in paprika and ground chili pepper at a level of 3 ppm (personal communication, email from Food Directorate [Health Canada] to Risk Management Bureau [Health Canada], dated 2013; unreferenced). Since p-phenetidine is expected to be present only as an impurity in spices, exposure of the general population of Canada to p-phenetidine is not expected.
Within the Aromatic Azo and Benzidine-based Substance Grouping (including 295 dyes and 40 pigments), two dyes, Solvent Red 3 (CAS RN 6535-42-8) and Direct Yellow 12 (2870-32-8), release p-phenetidine upon cleavage of the azo bond; however, the potential for cleavage is uncertain. The estimated exposure to Solvent Red 3 from its use in cosmetics is low (Health Canada 2013c; therefore, any hypothetical exposure to p-phenetidine from Solvent Red 3 is also expected to be low. Exposure of the general population to Direct Yellow 12 as a dye in textiles and leather products was previously estimated (Health Canada 2014b). Due to the conservatism in the exposure scenarios, any systemic exposure to p-phenetidine from Direct Yellow 12 in textiles and leather is expected to be low.
In vitro, Ames tests were negative in Salmonella strains TA98 and TA100 without activation; however, in some studies, positive results were obtained in the presence of metabolic activation. All other bacterial strains tested gave negative results (Thompson et al. 1983; Nohmi et al. 1985; Ogawa et al. 1987; Zeiger et al. 1988; JECDB undated-c). p-Phenetidine inhibited DNA synthesis in Chinese hamster lung cells without activation (Holme et al. 1988), but was negative for unscheduled DNA synthesis in hepatocytes (Thompson et al. 1983; Yoshimi et al. 1988). p-Phenetidine induced DNA strand breaks in human skin fibroblasts with activation (Andersson et al. 1982; Nordenskjöld and Moldéus 1983). It did not transform mouse embryo cells (Patierno et al. 1989). p-Phenetidine was also reported to induce mutations and chromosomal aberrations in Chinese hamster lung cells and micronucleus formation in mice; however, only limited information is available on these unpublished studies (JECDB undated-c; Hoechst 1989; Bayer 1991).
Effects following repeated-dose exposure in rats included increased spleen weight, hemosiderosis, increased extra-medullary hematopoiesis, congestion of the spleen and myeloid hyperplasia of the bone marrow, decrease in erythrocytes, hemoglobin and hematocrit, increased serum reticulocytes and Heinz bodies (Schnitzer and Smith 1966; Sato et al. 1991; Pauluhn and Mohr 2001).
Due to the conservatism in the exposure scenarios, any systemic exposure to p-phenetidine from Direct Yellow 12 in textiles and leather is expected to be low, therefore though there are limited health effects data available the potential risk is low.
2-Chloroaniline
A thorough literature search was conducted to assess the prevalence of 2-chloroaniline in the Canadian environment. No data sourced from Canadian environments were identified during this search.
Relevant information on levels of 2-chloroaniline measured in environments outside of Canada included four studies from the literature. A study by Young et al. (2011) examined trace levels of chloroanilines in environmental water samples taken from the Hudson and East rivers in New York, USA. The study observed no detectable 2-chloroaniline in samples from either location. The LOD for 2-chloroaniline was 2.4 µg/L.
A 2006 study by Akyüz and Ata examined 2-chloroaniline in river water sampled in Zonguldak, Turkey. The study observed seasonal mean concentrations of 2-chloroaniline of 95.88–192.95 ng/L. A 1981 study by Wegman and De Korte examined levels of 2-chloroaniline in the Rhine River at Lobith, the Netherlands. Concentrations of 2-chloroaniline up to 3.9 μg/L were measured, with an average concentration of 0.54 μg/L. Comparable measurements were taken in the tributaries of the Rhine River, where concentrations of 2-chloroaniline up to 1.3 μg/L were measured, with an average of 0.54 μg/L. Lastly, concentrations of 2-chloroaniline were measured in the River Meuse; lower levels, up to a maximum of 0.86 μg/L and an average of 0.15 μg/L, were measured. A 1988 paper by Scholz and Palauschek measured levels of 2-chloroaniline in combined surface water and wastewater sampled in Germany. Concentrations of 2-chloroaniline were measured in 15 of the 19 samples, ranging from 1.1 to 450 μg/L.
When present in soil, chloroanilines rapidly bind to humic components of the soil. Free chloroanilines undergo a process of microbial biodegradation; bound chloroaniline residues are desorbed and follow the same microbial biodegradation pathway at a slower rate, to the extent of almost complete mineralization under optimal conditions (Brunsbach and Reineke 1993).Therefore, exposure of the general population in Canada to 2-chloroaniline is not expected.
The health effects summary of 2-chloroaniline is based primarily on secondary sources, including an evaluation by the German MAK Commission (DFG 1992b), an industry-generated IUCLID dossier (European Commission ©2000h), information cited as part of a US NTP study (NTP 1998; Heijtmancik et al. 2002) and the publicly available literature. A brief summary of the available information is provided below.
No classifications for carcinogenicity, genotoxicity or reproductive/developmental toxicity were identified for 2-chloroaniline. The acute toxicity of 2-chloroaniline is characterized by cyanosis, lethargy, ataxia and mortality at higher doses; these effects are similar to those of other aniline derivatives, including chloroanilines, and are due to the primary erythrocyte toxicity, including oxidation of hemoglobin (methemoglobin), hemoglobin adducts, increased erythrocyte turnover (hemolysis) and damage to the spleen and compensatory erythrocyte regeneration. Acute LD50 values were reported for the oral route (mouse = 256 mg/kg-bw; rat = 1 015 mg/kg-bw), for the dermal route (rat = 1 000 mg/kg-bw; cat = 222 mg/kg-bw) and by inhalation (rat = 4 100 – 6 000 mg/m3). Levels of methemoglobin rapidly increase following acute exposure, with methemoglobin levels of up to 62% measured in cats 2 hours after oral exposure to 32 mg/kg-bw. The acute hematotoxicity of 2-chloroaniline is considered to be of relatively lower potency than 3-chloroaniline or 4-chloroaniline isomers.
No reliable chronic toxicity study was identified. However, several short-term and subchronic studies have investigated 2-chloroaniline, although many of these were unpublished studies cited in the industry IUCLID dossier (European Commission ©2000h). However, the majority of studies consistently demonstrated hematotoxicity and associated effects. The key subchronic study was conducted by the US NTP, which was a 13-week oral gavage study in rats and mice (NTP 1998). Overall, findings (hemosiderin in spleen, increased spleen weight, evidence for hemolysis, etc.) were similar to those for other chloroaniline isomers (3-chloroaniline, 4-chloroaniline); however, 2-chloroaniline was of lower potency. For both rats and mice, increases in methemoglobin were seen at levels as low as 10 – 20 mg/kg-bw per day; however, the majority of changes (increased spleen weight of at least 25% from control value, decreases in red blood cell parameters of at least 10% from control value, fibrosis of spleen of severity greater than minimal) did not occur until 80 – 160 mg/kg-bw per day for both species. In comparison, similar decreases in red blood cell parameters resulted from 4-chloroaniline treatments of 10 to 40 mg/kg-bw per day (NTP 1989).
The genotoxicity of 2-chloroaniline is similar to that of 4-chloroaniline, with generally negative results for reverse mutations in Salmonella, but positive mutagenicity observed in L5178Y mouse lymphoma cells. Mixed positive (rat) and negative (mouse) results were reported for the micronucleus assay in vivo.
4-Nitroaniline
Within the Aromatic Azo and Benzidine-based Substance Grouping (including 295 dyes and 40 pigments), 11 dyes were identified to contain a 4-nitrophenyl moiety on an azo bond. However, most of these dyes are considered not to be in commerce in Canada, except for four dyes: CAS RN 68155-63-5, CAS RN 70210-25-2, CAS RN 84878-17-1 and BANAP (CAS RN 29765-00-2).
CAS RN 68155-63-5 and CAS RN 70210-25-2 are polyazo Acid Dyes used in Canada for dyeing leather. CAS RN 84878-17-1 is a polyazo Direct Dye that is generally used as a textile and leather dye. Dermal absorption of these dyes is expected to be limited due to their large molecular sizes, and cleavage of azo bonds in these polyazo dyes may not necessarily release 4-nitroaniline (Environment Canada and Health Canada 2014). Furthermore, use of leather is considered to be short term and intermittent. BANAP (CAS RN 29765-00-2) is a monoazo Disperse Dye that is used as a textile dye. Dermal absorption of BANAP is expected to be limited, and there is uncertainty in the rate and degree of azo cleavage (Environment Canada and Health Canada 2013d). Therefore, systemic exposure to 4-nitroaniline from these dyes and their uses is not expected to be significant. Hence, exposure of the general population in Canada to 4-nitroaniline is not expected.
There was no evidence of carcinogenicity in male or female rats given 4-nitroaniline by gavage at doses up to 9 mg/kg-bw per day for 2 years (Nair et al. 1990). NTP (1993) conducted a 2-year study in mice, which were given 4-nitroaniline by gavage at a dose of 3, 30 or 100 mg/kg-bw per day. The incidence of liver hemangiosarcoma and the combined incidence of hemangioma and hemangiosarcoma at all sites were marginally increased at the high dose in males (p = 0.06). In both cases, a trend test showed significance (p = 0.033 and p = 0.026), but no pairwise comparisons were significant. No treatment-related increases in tumour incidence were observed in female mice. The NTP concluded that this was “equivocal evidence of carcinogenic activity in male mice” and “no evidence of carcinogenic activity in female mice” (NTP 1993).
In vitro, 4-nitroaniline was positive for chromosomal aberrations in mammalian cells with metabolic activation in two studies; mixed results were obtained without activation (Galloway et al. 1987; NTP 1993; Huang et al. 1995; Chung et al. 1996). Mixed results were also obtained for sister chromatid exchanges in Chinese hamster ovary cells with and without activation (Galloway et al. 1987; NTP 1993). A forward mutation assay in mouse lymphoma cells was positive without activation and negative with activation (NTP 1993). 4-Nitroaniline was negative for unscheduled DNA synthesis in primary rat hepatocytes (Thompson et al. 1983). The majority of Ames assays were negative (Chiu et al. 1978; Haworth et al. 1983; Thompson et al. 1983; Corbett et al. 1985; Pai et al. 1985; Shahin 1985; Shimizu and Yano 1986; Kawai et al. 1987; Dellarco and Prival 1989; Chung et al. 1996; Assmann et al. 1997).
All in vivo genotoxicity assays on 4-nitroaniline are negative; however, the available data are limited. Sex-linked recessive lethal assays in Drosophila were negative (Valencia et al. 1985; Zimmering et al. 1989). In an abstract, Mirsalis et al. (1983) reported that an unscheduled DNA synthesis assay in hepatocytes from male rats treated orally by gavage was negative. An unpublished study cited in US HPVIS (2012) reported no increase in micronuclei in bone marrow of male and female mice treated by intraperitoneal injection; the original study was not available (Solutia Inc. undated).
Available information indicates that 4-nitroaniline is not likely to be mutagenic or genotoxic in vivo.
Non-neoplastic effects in oral repeated-dose studies in mice and rats include increased methemoglobin, spleen pigmentation, liver hemosiderin, bone marrow hyperplasia, extra-medullary hematopoiesis in spleen and liver, and increased weights of spleen and liver (Nair et al. 1990; NTP 1993). Similar effects were observed in a 28-day inhalation study in rats (Nair et al. 1986). The hemangiomas and hemangiosarcomas observed in male mice in the 2-year gavage study are likely secondary to other effects, such as splenic congestion, extra-medullary hematopoiesis and accumulation of hemosiderin.
7.4 Overall Human Health Risk Characterization
This human health assessment focuses on substances that are reported to be available in Canada above the section 71 reporting threshold of 100 kg/year in the recent surveys or for which available information indicates evidence of their presence. Potential exposure of the general population of Canada was characterized for nine substances: 2-naphthylamine, o-toluidine, 2,4-diaminotoluene, 4-chloroaniline, 3,4-dichloroaniline, o-anisidine, p-aminophenol, 1,3-diaminobenzene and Red Lake C Amine. Exposure of the general population of Canada to one or more of the nine aromatic amines from the use of certain consumer products, such as cooking utensils, textiles and cosmetics, was estimated. Additionally, information from a single study of a small sample of Canadian women identified very low levels of o-toluidine in breast milk for which an exposure estimate to non-formula fed infants was estimated. No robust Canadian data on concentrations of these 9 aromatic amines in environmental media were identified, and, with the exception of p-aminophenol, section 71 data indicate low volumes of use of these 9 aromatic amines in Canada. Therefore, exposures to the aromatic amines from environmental media are generally considered to be low. The remaining seven aromatic amines in this subgroup were not reported under section 71 above the 100 kg/year reporting threshold and no other information was identified on these substances to support general population exposure in Canada; therefore exposures are not expected for these substances (see Section 7.3.10).
Carcinogenicity was considered to be the health effects of concern for six of the nine aromatic amines for which exposure was characterized. 2-Naphthylamine, o-toluidine, 2,4-diaminotoluene, 4-chloroaniline and o-anisidine are classified as known or possible human carcinogens by the International Agency for Research on Cancer (Group 1 or 2B) and the European Union (Category 1A or 1B Carcinogenicity was not identified as an endpoint of concern for p-aminophenol, 1,3-diaminobenzene or Red Lake C Amine; therefore, critical non-cancer health effect levels were selected for risk characterization.
Four substances (2-naphthylamine, 2,4-diaminotoluene, 4-chloroaniline and o-anisidine) were detected in some imported textile and leather products in a study conducted by Health Canada in 2012. Margins between estimates of exposure of the general population from dermal contact with textiles as well as mouthing of textiles by infants and critical effect levels are considered adequate to address uncertainties in the health effects and exposure databases.
Available information indicates that residual o-toluidine, 2,4-diaminotoluene, o-anisidine, 4-chloroaniline and 1,3-diaminobenzene may transfer to foods being prepared with polyamide cooking utensils. Margins between the estimated daily oral exposure from use of polyamide cooking utensils and critical effect levels are considered adequate to address uncertainties in the health effects and exposure databases.
Exposures to p-aminophenol, 1,3-diaminobenzene, 4-chloroaniline and Red Lake C Amine were identified from use of certain cosmetic products. The margins between exposure estimates and critical effect levels for each of these substances were considered adequate to address uncertainties in the health effects and exposure databases.
o-Toluidine was identified at low levels in breast milk from a small sample of Canadian women. The margin between estimated daily intake of o-toluidine for non-formula-fed infants via breast milk and the critical effect level is considered adequate and does not indicate a concern at these low levels of exposure.
For the remaining seven aromatic amines (2,4-diaminoanisole, 2-chloroaniline, p-toluidine, p-toluidine hydrochloride, 4-nitroaniline, p-phenetidine and 1,3-diaminobenzene dihydrochloride), no information was identified to support current exposure to these substances for the general population of Canada; therefore, risk to human health from these substances is not expected.
Some of the Aromatic Amines in this assessment have effects of concern based on potential carcinogenicity.
Based on the available information presented in this Screening Assessment, it is concluded that the 16 aromatic amines evaluated in this assessment are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore concluded that the 16 substances in the Aromatic Amines Subgroup do not meet the criteria set out in paragraph 64(c) of CEPA 1999.
7.4.1 Uncertainty
In this assessment, data from other countries were used when they were considered to be informative of potential exposure of the general population in Canada. Some level of uncertainty is recognized with this approach.
There is uncertainty in the estimated exposures from textiles, which are partly influenced by the non-uniform leaching behaviour from textile materials. The degree of leaching depends on the type of fibre, the dyes used, dyeing technology, colour intensity and after-treatment and therefore can vary considerably. Uncertainty is also recognized in the presence and levels of the Aromatic Amines in products imported into Canada due to the limited information available; product testing conducted by Health Canada included 66 samples, which may not be representative of the levels of Aromatic Amines in the textile and leather products available in Canada (Health Canada 2013a).
In all of the recent textile and leather product testing studies and surveys in Canada and elsewhere cited in this assessment (Health Canada 2013a; RAPEX 2012; EurAzos 2007; Kawakami et al. 2010), the prevalence of the Aromatic Amines was low, suggestive of these substances not being present in most imported textiles and leather products. In addition, among the tested products, the detected levels of the Aromatic Amines were variable; however, the metrics selected and used in the screening assessment to estimate exposure are considered adequate to address the uncertainties, and is further supported by indications that these substances are not present in most imported textiles and leather products.
Similarly, low incidences were also reported in the studies investigating black polyamide cooking utensils (Trier et al. 2010; McCall et al. 2012). The exposure estimates are based on conservative assumptions, including the use of foreign data in the absence of Canadian information as well as assumptions on use behaviour and the selected migration rate among highly variable data in the identified studies. Nonetheless, exposures were estimated based on conservative assumptions and therefore are considered to be conservative.
Based on the available information on the identified levels of o-toluidine and o-anisidine in tattoo inks found outside of Canada, acute and short-term exposures to these aromatic amines were conservatively estimated. Information on exposure to these substances in tattoo inks is however limited. Systemic daily exposure to aromatic amines in tattoo colourants depends partly on the degree of in vivo metabolism and photodegradation potential of azo colourants in tattoo inks and subsequent mobilization into the lymphatic system (Engel et al. 2009); information on long-term kinetics and therefore chronic exposure to aromatic amines from tattoo inks is not well characterized. Overall, there is high uncertainty regarding the systemic exposure to aromatic amines following tattoo injection (Danish EPA 2012). While the hazardous properties of o-toluidine and o-anisidine are recognized, due to the high uncertainty with respect to the exposure characterization from tattoo use, the risk from tattoo use has not been characterized in this Screening Assessment and remains an uncertainty.
Several of these Aromatic Amines have also been detected in cigarette smoke and therefore potential exposure from this source is acknowledged. However, the use of tobacco is addressed under another jurisdiction of Health Canada; therefore, the potential exposures from cigarette smoke are not considered in this assessment.
Dietary exposure to 4-chloroaniline and 3,4-dichloroaniline may occur due to in vivo metabolism following dietary consumption of residues of certain pesticides which may be found at low levels in some foods. Likewise, low levels of 4-chloroaniline and 3,4-dichloroaniline may be found in water near areas where certain registered herbicides are used following their environmental degradation.
While limited human biomonitoring data were identified for these Aromatic Amines in the Canadian population, several international studies suggest ongoing population exposure to aromatic amines. It is uncertain whether the pattern of exposure for the 16 aromatic amines identified in the European biomonitoring studies can be directly applied to the Canadian population. Therefore, in the absence of a corroborating Canadian Biomonitoring study, there remains uncertainty concerning Canadian exposure to the 16 Aromatic Amines identified in Europeans.
7.4.2 Aromatic Amines With Health Effects of Concern
Overall, human health risk from the substances in this assessment is low based on the current levels of exposure. However as indicated in previous sections, nine of the Aromatic Amines in this assessment have effects of concern based on potential carcinogenicity. A list of these substances is shown in Appendix L.
In the draft version of the Aromatic Amines assessment, 14 Aromatic Amines were identified to have human health effects of concern. Since the draft publication, more precise considerations based principally on lines of evidence for carcinogenic potential were applied to indicate substances which were considered to have effects of concern (Appendix L). As such, 6 of the 14 aromatic amines previously flagged in the draft assessment are no longer considered to meet the lines of evidence for potential carcinogenicity. Specifically, p-aminophenol, 1,3-diaminobenzene and its salt are not considered as having human health effects of concern since negative and/or equivocal carcinogenicity data are available for these substances. For p-phenetidine, 3,4-DCA and 2-CA, there is a lack of chronic data and uncertainties regarding read-across for these substances, therefore the available information does not support identifying these substances for potential carcinogenicity at this time under the Chemicals Management Plan.
8. Conclusion
Considering all available lines of evidence presented in this Screening Assessment, there is low risk of harm to organisms and the broader integrity of the environment from the 16 Aromatic Amines evaluated in this assessment. It is concluded that these Aromatic Amines do not meet the criteria under paragraphs 64(a) or 64(b) of CEPA 1999, as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
Based on the information presented in this Screening Assessment, it is concluded that the Aromatic Amines evaluated in this assessment do not meet the criteria under paragraph 64(c)of CEPA 1999, as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is concluded that the Aromatic Amines evaluated in this assessment do not meet any of the criteria set out in section 64 of CEPA 1999.
References
Appendices
- Appendix A: Ecotoxicity Values for the Aquatic Environment and Soil
- Appendix B: Summary of Available Studies Investigating Aromatic Amines in Environmental Media
- Appendix C: Estimated Exposures to Certain Aromatic Amines from Polyamide Cooking Utensils
- Appendix D: Data Availability for Certain Aromatic Amines in Foods and Food Packaging
- Appendix E: Data Availability for and Estimated Human Exposures to Certain Aromatic Amines in Textile and Leather Products
- Appendix F: Estimated Exposures to Certain Aromatic Amines in Tattoo Inks
- Appendix G: Upper-bounding Estimates of Daily Intake of o-Toluidine from Breast Milk for Breastfed Infants
- Appendix H: Estimates of Daily Intake of 4-Chloroaniline in Water by the General Population of Canada
- Appendix I: Benchmark Dose Calculations for o-Anisidine Hydrochloride
- Appendix J: Dermal Exposure Parameters for Estimating Exposure from Use of Cosmetic Products
- Appendix K:.Exposure to Red Lake C Amine Based on Uses of Parent Pigments
- Appendix L: Aromatic Amines With Effects of Concern