Screening assessment alkyl aryl phosphites
Official title: Screening Assessment Alkyl Aryl Phosphites
Chemical Abstracts Service Registry Numbers
15647-08-2, 25550-98-5
Environment and Climate Change Canada
Health Canada
February 2019
Cat. No.: En14-358/2019E-PDF
ISBN 978-0-660-08836-5
Synopsis
Pursuant to section 74 of the Canadian Environmental Protection Act, 1999 (CEPA), the Minister of the Environment and the Minister of Health have conducted a screening assessment of two substances referred to collectively under the Chemicals Management Plan as the Alkyl Aryl Phosphites Group. These two substances were identified as priorities for assessment as they met categorization criteria under subsection 73(1) of CEPA. The Chemical Abstracts Service Registry Numbers (CAS RNFootnote 1 ), their Domestic Substances List (DSL) names and their common names or acronyms are listed in the table below.
| CAS RN | DSL name | Common names (acronym) |
| 15647-08-2 | Phosphorous acid, 2-ethylhexyl diphenyl ester | 2-Ethylhexyl diphenyl phosphite; (EHDPP) |
| 25550-98-5 | Phosphorous acid, diisodecyl phenyl ester | Diisodecyl phenyl phosphite; (DIDPP) |
In 2011, there were no reports of manufacture for EHDPP (CAS RN 15647-08-2) above the reporting threshold of 100 kg in response to a survey issued pursuant to a CEPA section 71 notice, and between 100 and 1000 kg of EHDPP were imported into Canada. There were no reports of manufacture for DIDPP (CAS RN 25550-98-5) above the reporting threshold of 100 kg in 2008, and between 28 200 and 82 000 kg of DIDPP were imported into Canada for the same year. Both substances are primarily used as a secondary antioxidant for processing polymeric or plastic materials, and residual levels may be present in final products and manufactured items.
The ecological risks of EHDPP and DIDPP were characterized using the ecological risk classification of organic substances (ERC) approach which employs multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. Hazard profiles based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity are established. Metrics considered in the exposure profiles include potential emission rate, overall persistence, and long-range transport potential. A risk matrix is used to assign a low, moderate or high level of potential concern for substances on the basis of their hazard and exposure profiles. The ERC identified EHDPP and DIDPP as having low potential to cause ecological harm.
Considering all available lines of evidence presented in this screening assessment, there is low risk of harm to the environment from EHDPP and DIDPP. It is concluded that EHDPP and DIDPP do not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
The risks to human health for EHDPP and DIDPP were characterized on the basis of available health effects and exposure information. General population exposure to both substances is mainly by contact with manufactured plastic items. The dermal and oral routes (i.e., mouthing behaviour of infants) were considered to be the primary routes of exposure. To inform the human health effects assessment for these substances, data on analogues triphenyl phosphite (TPP, 101-02-0) and triisodecyl phosphite (TIDP, 25448-25-3) were taken into consideration. In laboratory studies in rats conducted with TPP, the critical health effects for EHDPP and DIDPP were increased mortality and reduced pup body weight in the presence of significant maternal toxicity (e.g., ataxia). On the basis of a comparison of exposure estimates and critical effect levels identified in health effects studies, margins of exposure were considered to be adequate to address uncertainties in the human health effects and exposure databases.
On the basis of the information presented in this screening assessment, it is concluded that EHDPP and DIDPP do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
Therefore, it is concluded that EHDPP and DIDPP do not meet any of the criteria set out in section 64 of CEPA.
1. Introduction
Pursuant to section 74 of the Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999), the Minister of the Environment and the Minister of Health have conducted a screening assessment of two substances (2-ethylhexyl diphenyl phosphite (EHDPP) and diisodecylphenylphosphite (DIDPP)) referred to collectively under the Chemicals Management Plan as the Alkyl Aryl Phosphites Group, to determine whether these two substances present or may present a risk to the environment or to human health. These two substances were identified as priorities for assessment as they met categorization criteria under subsection 73(1) of CEPA (ECCC, HC [modified 2017]).
The ecological risks of EHDPP and DIDPP were characterized using the ecological risk classification of organic substances (ERC) approach (ECCC 2016a). The ERC describes the hazard of a substance using key metrics including mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity and considers the possible exposure of organisms in the aquatic and terrestrial environments on the basis of such factors as potential emission rates, overall persistence, and long-range transport potential in air. The various lines of evidence are combined to identify substances as warranting further evaluation of their potential to cause harm to the environment or as having a low likelihood of causing harm to the environment.
This screening assessment includes consideration of information on chemical properties, environmental fate, hazards, uses and exposures, including additional information submitted by stakeholders. Relevant data were identified up to December 2016. Empirical data from key studies as well as some results from models were used to reach conclusions. When available and relevant, information presented in assessments from other jurisdictions was considered.
This screening assessment was prepared by staff in the CEPA Risk Assessment Program at Health Canada and Environment and Climate Change Canada and incorporates input from other programs within these departments. The ecological portion of this assessment is based on the ERC document (published July 30, 2016), which has undergone external review, and was subject to a 60-day public comment period. Additionally, the draft of this screening assessment published October 28, 2017 was subject to a 60-day public comment period. While external comments were taken into consideration, the final content and outcome of this screening assessment remain the responsibility of Health Canada and Environment and Climate Change Canada.
This screening assessment focuses on information critical to determining whether substances meet the criteria as set out in section 64 of CEPA, by examining scientific information and incorporating a weight of evidence approach and precaution.Footnote 2 The screening assessment presents the critical information and considerations on which the conclusions are based.
2. Identity of substances
The Chemical Abstracts Service Registry Numbers (CAS RNFootnote 3 ), Domestic Substances List (DSL) names and acronyms for the substances in the Alkyl Aryl Phosphites Group are presented in Table 2‑1. A list of additional chemical names (e.g., trade names) is available from the National Chemical Inventories (NCI 2016).
| CAS RN (acronym) | DSL name (common name) | Chemical structure and molecular formula | Molecular weight (Da) |
|---|---|---|---|
| 15647-08-2 (EHDPP) | Phosphorous acid, 2-ethylhexyl diphenyl ester (2-ethylhexyl diphenyl phosphite) | 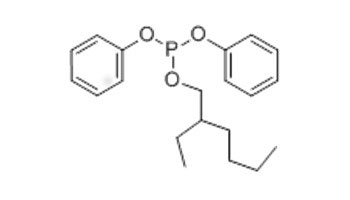 C20H27O3P |
346.4 |
| 25550-98-5 (DIDPP) | Phosphorous acid, diisodecyl phenyl ester (diisodecyl phenyl phosphite) | 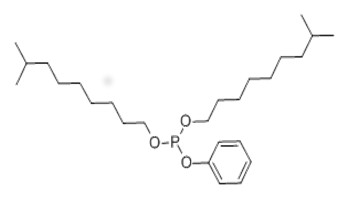 C26H47O3P |
438.62 |
Information found on substance identity indicates that substances described as diisodecyl phenyl phosphite often contain other phosphite impurities. The European Chemicals Bureau (ECB 2008) in a description of DIDPP states that “the name [diisodecyl phenyl phosphite] reflects the number of moles of isodecanol reacting with triphenyl phosphite rather than describing the exact structure of what is present.” Composition of the substance is described as triphenyl phosphite (2% w/w), diphenylisodecyl phosphite (23% w/w), diisodecyl phenyl phosphite (DIDPP) (51% w/w) and triisodecyl phosphite (21% w/w) (ECB 2008). Furthermore, the manufacture of DIDPP is described under a test plan submitted to the US EPA High Production Volume Challenge Program (General Electric Company 2001). DIDPP is manufactured by reacting triphenyl phosphite with isodecanol. The reaction does not occur cleanly and the reaction product is a mixture of triphenyl phosphite, diphenylisodecyl phosphite, diisodecyl phenyl phosphite and triisodecyl phosphite. The ratio of each element in the mixture is controlled by the reaction conditions as well as the molar ratios of triphenyl phosphite and isodecanol used in the reaction. DIDPP is not further purified and the commercial substance is typically used as 50% to 70% pure with lesser amounts of triisodecyl and triphenyl phosphites present (General Electric Company 2001).
Details regarding the synthesis of EHDPP were not available. However, information for an associated substance that is listed as a multi-constituent substance, the components of which are listed as 2-ethylhexyl diphenyl phosphite, bis(2-ethylhexyl) phenyl phosphite and triphenyl phosphite, was submitted to the European Chemicals Agency (ECHA) under the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH). Details regarding the proportions of each component are not provided (ECHA 2007-2015a). On the basis of this limited information, there is an assumption that EHDPP follows a similar manufacture process to DIDPP whereby triphenyl phosphite is reacted with 2-ethylhexanol and the reaction does not proceed cleanly, resulting in EHDPP and other phosphite esters impurities.
For the current assessment, the structures outlined in Table 2-1 are constituents of the reaction product that is the basis for this assessment.
2.1 Selection of analogues
Data on analogues were considered in the human health assessment of DIDPP and EHDPP. The analogues selected were structurally similar, having common organophosphite functionality and similar alkyl or aryl side chains. The analogues also have relevant empirical data that could be used to read-across to substances with limited empirical data (Table 2-2).
| CAS RN (Chemical name) [acronym] | Chemical structure | Molecular weight (Da) |
|---|---|---|
| 101-02-0 (Triphenyl phosphite) [TPP] | 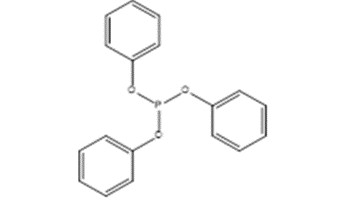 |
310.3 |
| 25448-25-3 Triisodecyl phosphite [TIDP] | 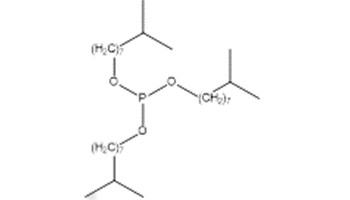 |
502.8 |
Phosphites can undergo hydrolysis when exposed to humidity in air or other moisture, with rates of hydrolysis tending to decrease with increasing molecular weight (Faring 2003). As a result, the analogues also have the potential to yield similar degradation products through hydrolysis.
The results of a water solubility test indicate that DIDPP undergoes rapid hydrolysis at pH 4, 7 and 10 (ECHA 2007-2016b). DIDPP will hydrolyze to phosphorous acid, phenol and isodecanol. The analogues considered in the health effects assessment yield hydrolysis products that overlap with those of DIDPP. TIDP hydrolyzes to phosphorous acid and isodecanol, while TPP hydrolyzes to phosphorous acid and phenol (Figure 2-1). As a result, the health effects for TPP and TIDP are used to inform the health effects assessment of DIDPP.

Figure 2-1
The analogues considered in the human health effects assessment yield hydrolysis products that overlap with those of DIDPP. The analogue TIDP hydrolyzes to phosphorous acid and isodecanol, while the analogue TPP hydrolyzes to phosphorous acid and phenol.
Figure 2‑1. Hydrolysis products of DIDPP and the selected analogues TIDP and TPP used in the human health risk assessment
EHDPP is expected to hydrolyze to phosphorous acid, phenol and 2-ethylhexanol. The analogues considered yield similar hydrolysis products that overlap those of EHDPP. TIDP and TPP are used to inform the health effects assessment of EHDPP. TIDP does not hydrolyze to the same alcohol as EHDPP (isodecanol vs. 2-ethylhexanol). However, the alcohols are similar with respect to structure and physical chemical properties.
3. Physical and chemical properties
A summary of physical and chemical properties of EHDPP and DIDPP is presented in Tables 3-1 and 3-2, respectively. Additional physical and chemical properties are presented in ECCC (2016b). A summary of physical and chemical properties of the analogues is available in Appendix A. When experimental information was limited or not available for a property, quantitative structure-activity relationship (QSAR) models were used to generate predicted values for the substance using the structures depicted in Table 2-1.
| Property | Value or range | Type of data | Key reference(s) |
| Physical state | Liquid | Experimental | (ECHA c2007-2015a) |
| Melting point (°C) | < -20 | Experimental | (ECHA c2007-2015a) |
| Boiling point (°C) | > 224a | Experimental | (ECHA c2007-2015a) |
| Vapour pressure (Pa) (at 25°C) | 3.45 × 10−5 | Calculatedb | (MPBPWIN 2008) |
| Henry’s law constant (Pa·m3/mol) (at 25°C) | 1.08 | Calculatedc | (HENRYWIN 2008) |
| Water solubility (mg/L) (at 25°C) | 2.38 × 10-3 | Calculated | (WSKOWWIN 2010) |
| log Kow (dimensionless) | 7.54 | Calculated | (KOWWIN 2010) |
Abbreviations: Kow, octanol–water partition coefficient.
a Dossier indicates that hydrolysis and decomposition are expected to occur prior to boiling point.
b Modified Grain Method.
c Bond Method.
| Property | Value or range | Type of data | Key reference(s) |
| Physical state | Liquid | Experimental | (ECHA c2007-2015b) |
| Melting point (°C) | < -20 | Experimental | (ECHA c2007-2015b) |
| Boiling point (°C) | 379a | Experimental | (ECHA c2007-2015a) |
| Vapour pressure (Pa) (at 25°C) | 2.07 × 10−7 to 6.99 × 10-5 | Calculatedb | (MPBPWIN 2008) |
| Henry’s law constant (Pa·m3/mol) (at 25°C) | 0.531 | Calculatedc | (HENRYWIN 2008) |
| Water solubility (mg/L) (at 25°C) | 2.11 × 10-8 to 2.33 × 10-4 | Calculatedd | (ECHA c2007-2015b) |
| log Kow (dimensionless) | 9.32 | Calculated | (ECHA c2007-2015b) |
Abbreviations: Kow, octanol–water partition coefficient.
a Dossier indicates that decomposition is likely to have occurred.
b Modified Grain Method.
c Bond Method.
d Dossier states results were indicative of rapid hydrolysis.
4. Sources and uses
EHDPP and DIDPP have been included in surveys issued pursuant to a CEPA section 71 notice (Environment Canada 2009, 2013). Follow-up with industry stakeholders on quantity and uses was conducted up to January 2017. Table 4‑1 presents a summary of the total manufacture and import quantities for the substances in the Alkyl Aryl Phosphites Group.
| Common name | Total manufacturea (kg) | Total importsa (kg) | Reporting year | Survey reference |
| EHDPP | 0 | 100–1000 | 2011 | Environment Canada 2013 |
| DIDPP | 0 | 28 200–82 000 | 2008 | Environment Canada 2009 |
a Values reflect quantities reported in response to the survey[s] conducted under section 71 of CEPA (Environment Canada 2009, 2013). See surveys for specific inclusions and exclusions (schedules 2 and 3).
Both EHDPP and DIDPP are used as a secondary antioxidant (i.e., process stabilizer) for processing polymeric or plastic materials (e.g., polyvinyl chloride), primarily in non-food contact plastics (Sheftel 2000; General Electric Company 2001; Stevenson et al. 2002; Galata Chemicals 2010, 2013).
Information reported through surveys issued pursuant to a CEPA section 71 notice (Environment Canada 2013) confirms the potential use of DIDPP in plastic materials. DIDPP was also reported under the use codes “adhesive and sealants,” “paints and coatings,” and “foam seating and bedding.” Additional uses for both EHDPP and DIDPP in Canada are summarized in Table 4-2. DIDPP can also be used in industrial coating products in a pneumatic spray form at a concentration up to 1% by weight, but such use was not expected to be a product available to consumers (Andek 2015). The use of DIDPP in lubricants and greases has been reported in Europe (ECHA, c2007-2015b; Mathy 2015) but no such use has been confirmed in the Canadian market.
| Use | EHDPP | DIDPP |
| Food additive | N | N |
| Food packaging materialsa | Yb | N |
| Formulant in pest control products registered in Canadac | Yd | Y |
Abbreviations: N = No; Y = Yes.
a Personal communication, email from the Food Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated Oct. 2016; unreferenced.
b Has been identified in a non-food contact layer in a limited number of food packaging materials (e.g., laminates).
c Personal communication, email from Risk Management Bureau, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated Oct. 2016; unreferenced.
d Historical use only (Personal communication, email from Risk Management Bureau, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated Oct. 2016; unreferenced).
5. Potential to cause ecological harm
5.1 Characterization of ecological risk
The ecological risks of EHDPP and DIDPP were characterized using the ecological risk classification of organic substances (ERC) approach (ECCC 2016a). The ERC is a risk-based approach that considers multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. The various lines of evidence are combined to discriminate between substances of lower or higher potency and lower or higher potential for exposure in various media. This approach reduces the overall uncertainty with risk characterization compared to an approach that relies on a single metric in a single medium (e.g., LC50) for characterization. The following summarizes the approach, which is described in detail in ECCC (2016a).
Data on physical-chemical properties, fate (chemical half-lives in various media and biota, partition coefficients, and fish bioconcentration), acute fish ecotoxicity, and chemical import or manufacture volume in Canada were either collected from scientific literature, from available empirical databases (e.g., OECD QSAR Toolbox) and from responses to surveys issued pursuant to a CEPA section 71 notice or were generated using selected QSAR or mass-balance fate and bioaccumulation models. These data were used as inputs to other mass-balance models or to complete the substance hazard and exposure profiles.
Hazard profiles were established principally on the basis of metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Exposure profiles were also established using multiple metrics, including potential emission rate, overall persistence, and long-range transport potential. Hazard and exposure profiles were compared to decision criteria in order to classify the hazard and exposure potentials for each organic substance as low, moderate, or high. Additional rules were applied (e.g., classification consistency, margin of exposure) to refine the preliminary classifications of hazard or exposure.
A risk matrix was used to assign a low, moderate or high classification of potential risk for each substance on the basis of its hazard and exposure classifications. ERC classifications of potential risk were verified using a two-step approach. The first step adjusted the risk classification outcomes from moderate or high to low for substances that had a low estimated rate of emission to water after wastewater treatment, representing a low potential for exposure. The second step reviewed low risk potential classification outcomes using relatively conservative, local-scale (i.e., in the area immediately surrounding a point-source of discharge) risk scenarios, designed to be protective of the environment, to determine whether the classification of potential risk should be increased.
ERC uses a weighted approach to minimize the potential for both over- and under-classification of hazard, exposure and subsequent risk. The balanced approaches for dealing with uncertainties are described in greater detail in ECCC 2016a. The following describes two of the more substantial areas of uncertainty. Error in empirical or modeled acute toxicity values could result in changes in classification of hazard, particularly metrics relying on tissue residue values (i.e., mode of toxic action), many of which are predicted values from QSAR models. The impact of this error is mitigated, however, by the fact that overestimation of median lethality will result in a conservative (protective) tissue residue used for critical body residue (CBR) analysis. Error in underestimation of acute toxicity will be mitigated through the use of other hazard metrics such as structural profiling of mode of action, reactivity and/or estrogen binding affinity. Changes or errors in chemical quantity could result in differences in classification of exposure as the exposure and risk classifications are highly sensitive to emission rate and use quantity. The ERC classifications thus reflect exposure and risk in Canada based on what is believed to be the current use quantity, and may not reflect future trends.
Critical data and considerations used to develop the substance-specific profiles for EHDPP and DIDPP, and the hazard, exposure and risk classification results, are presented in ECCC (2016b).
The hazard and exposure classifications for EHDPP and DIDPP are summarized in Table 5-1.
| Substance | ERC hazard classification | ERC exposure classification | ERC risk classification |
| EHDPP | low | low | low |
| DIDPP | high | low | moderate |
Given the low hazard and low exposure classifications according to the information considered under ERC for EHDPP, this substance was classified as having a low potential for ecological risk. It is therefore unlikely that it results in concerns for the environment in Canada.
According to information considered under ERC, DIDPP was classified as having a high hazard potential because of its reactive mode of action and moderate food web hazard assessment factor. In addition, structural alerts from the OECD toolbox identified DIDPP as being a potential DNA binder. However, these modelled outputs from ERC are not supported by the information in empirical studies identified in the human health portion of this assessment, which indicate that DIDPP is negative for genotoxicity and undergoes rapid hydrolysis. Therefore, although DIDPP was classified by ERC as having a moderate potential for ecological risk, fluctuations in use patterns are unlikely to result in a significant increase in risk. This substance is unlikely to result in concerns for the environment in Canada.
6. Potential to cause harm to human health
6.1 Exposure assessment
Environmental media and food
No monitoring data were identified for either EHDPP or DIDPP in air, water, soil, dust or food in Canada or elsewhere. Exposure, if any, to EHDPP from its use in food packaging applications is expected to be negligible. The releases of these two substances are not reported under the National Pollutant Release Inventory (NPRI 2011-2015). Given the annual amounts imported into Canada in 2008 and 2011 and their relatively low volatility, low water solubility and propensity to undergo rapid hydrolysis when exposed to moisture, the presence of EHDPP and DIDPP in the environmental media is expected to be minimal.
Products available to consumers
On the basis of information gathered from the literature search and reported through surveys issued pursuant to a CEPA section 71 notice (Environment Canada 2009, 2013), EHDPP and DIDPP may be present in manufactured items made of plastic that are available to consumers. The dermal and oral routes (via mouthing behaviour of infants) are the predominant routes of exposure from the use of these articles/items.
Limited information indicates that the amount of antioxidants in the original phosphite form remaining in final plastic manufactured items is approximately 1 ppm to 1% by weight, depending on the polymer materials (Schwope et al. 1990; Siró et al. 2006). Phosphite antioxidants are often added to thermoplastics at levels from 250 ppm up to 5% during thermal processing, to react with free radicals (e.g., hydroperoxide) and to control the aging of polymers caused by oxygen or heat (Carlsson et al. 2001; Stevenson et al. 2002; Crompton 2007). A combination of alkyl and aryl functional groups in the phosphite substances provides a balance between stability against hydrolysis and reactivity as an antioxidant (Jakupca 2010). As a conservative approach, 1% by weight was used to represent the residual levels of EHDPP or DIDPP in final products for estimating the potential exposure via consumer uses.
It is recognized that migration of an antioxidant may occur as it is designed to slow oxidative degradation of plastics by reacting with an oxidizing agent in the external phase (Spatafore and Pearson 1991; Marcato et al. 2003; Bhunia et al. 2013). Antioxidants are often not tightly bound to polymeric macromolecules, and they have a tendency to migrate to the contact surface or leach into contact media over time (Sheftel 2000). Several factors affect the migration potential in the polymer matrix, such as the types of polymers, the initial concentration and molecular weight of the migrant, and the contact media, contact temperature and contact time (Schwope et al. 1990; Sheftel 2000; Jakupca 2010).
Migration data on EHDPP and DIDPP from plastic materials are not available. However, migration data of other antioxidants were identified on the basis of similarity in molecular weight, initial concentrations in the polymers, water solubility and contact media, with values ranging from 0.91 µg/m2-hr to 13.4 µg/m2-hr (Schwope et al. 1990; Saito et al. 2007; Chen et al. 2009). Despite structural dissimilarity, the upper limit value of 13.4 µg/m2-hr, derived from migration of 3,4-di-t-butyl-4-hydroxytoluene in rigid polyvinyl chloride into stagnant water at 40°C for 10 days, was used as a conservative value to represent the migration of EHDPP and DIDPP from plastic materials.
Exposure scenarios were developed for infants (0 to 18 months) and adults contacting plastic objects on a regular or daily basis through various activities. For infants, such activities included holding a plastic article, being changed on a plastic change pad multiple times a day, and playing on a plastic mat; for adults, activities included wearing plastic gloves or sitting on a couch upholstered in plastic material. These scenarios are consistent with the plastic-contact scenarios previously described by Environment Canada and Health Canada (2015a, 2015b, 2015c). The dermal and oral routes are identified as the primary relevant exposure routes. Conservative exposure estimates derived for daily exposure of adults and children to plastic products are summarized in Table 6-1. Although EHDPP and DIDPP have high estimated log Kow values (> 7), dermal absorption of 100% was conservatively used to derive the estimates of systemic exposure by the dermal route with an exposure duration of 3 or 4 hours/day.
Intermittent exposures to these two substances via other potential consumer uses (e.g., adhesive and sealants) reported in surveys issued pursuant to a CEPA section 71 notice (Environment Canada 2009, 2013) were also considered. Given the concentrations, use amounts, and use frequency, the exposure estimates are expected to be lower than those from contacting plastic objects.
| Product scenario | Conc. (wt%) a | Age group | Exposure estimate |
| Mouthing manufactured plastic items intended for infants (0–18 months)b | 1%c | Infant | 0.0017 µg/kg-bw/day |
| Dermal contact with plastic itemsd | 1%c | Infant | 1.31 µg/kg-bw/day |
| Dermal contact with plastic itemse | 1%c | Adult | 0.52 µg/kg-bw/day |
a Concentration of EHDPP and/or DIDPP
b Daily exposure = (migration rate x surface area mouthed x contact time)/body weight, assuming surface area of 10 cm2 mouthed, body weight of 15.5 kg for infants of 0 to 18 months, mouthing time of 2 hours, and migration rate of 13.4 µg/m2-hr.
c Based on the upper limit of antioxidants remaining in final plastic products, as per the literature.
d Daily exposure = (migration rate x contact surface area x contact time)/body weight, assuming dermal absorption of 100%, contact surface area of 1840 cm2 (50% of the total surface area for infants of 0 to 5 months, as specified by Health Canada (1995)), body weight of 7.5 kg, contact time of 4 hours/day, and migration rate of 13.4 µg/m2-hr.
e Daily exposure = (migration rate x contact surface area x contact time)/body weight, assuming dermal absorption of 100%, contact surface area of 9100 cm2 (50% of the total surface area for adults of 20 years and above, as specified by Health Canada (1995)), contact time of 3 hours/day, and migration rate of 13.4 µg/m2-hr.
6.2 Health effects assessment
There are limited studies for DIDPP and EHDPP. Additional data on analogues have been used to inform the human health effects assessment (analogues rationale described in Section 2.1).
Toxicokinetics
Aryl alkyl phosphites undergo hydrolysis in the body to produce one molecule of phosphorous acid and three molecules of alcohol (e.g., phenol, isodecanol or 2-ethylhexanol; see Section 2.1). The alkylated phosphites are expected to hydrolyze more rapidly than the aryl phosphites because the central phosphite of the alkylated phosphites is less hindered. Because the toxicokinetics of EHDPP and DIDPP have not been studied, this report will focus on what is known about the analogue TPP. The distribution of TPP has been described in chickens (measurements made using gas chromatography; Konno et al. 1989). Following a single intravenous injection of 50 mg/kg×bw, the half-life of TPP in the plasma is ~30 minutes and it is cleared in approximately 4 hours. After a single intravenous injection of 100 mg/kg×bw, TPP can be detected in the liver, fat, leg muscle, sciatic nerve, cerebrum, spinal cord, cerebellum and plasma (tissues ordered from highest to lowest TPP content 1 hour following injection). The amount of TPP in these tissues decreases in a time-dependent manner (measurements were taken at 1, 6, 24 and 48 hours). After 48 hours, TPP remains in only three tissues: fat, sciatic nerve and leg muscle. Six hours following a single injection of 25, 50 or 100 mg/kg×bw, the amount of TPP in each tissue is dose-dependent. Detailed studies of TPP content in individual muscles have revealed that levels of TPP are higher in ‘red’ muscle and lower in ‘white’ muscle. Elimination of TPP from the body was not described.
Acute toxicity
DIDPP: The oral LD50 is >5000 mg/kg×bw (comparable to OECD Guideline 401, in rat; no mortality was observed during the study) (Gabriel 1980). The inhalation LC50 and dermal LD50 were >11.7 mg/L (comparable to OECD Guideline 403, in rat) and >2000 mg/kg×bw (comparable to OECD Guideline 402, in rabbit), respectively (Gabriel 1980). The local lymph node assay for skin sensitization (OECD Guideline 429, in mouse) indicated that DIDPP is a weak skin sensitizer (ECHA 2007-2015b).
TIDP [analogue]: The oral LD50 for TIDP is >5000 mg/kg×bw (comparable to OECD Guideline 401, in rat), the inhalation LC50 is >12.6 mg/L (comparable to OECD Guideline 403, in rat) (Gabriel, 1980), and the dermal LD50 is >5000 mg/kg×bw (comparable to OECD Guideline 402, in rabbit) (Gabriel, 1980).
TPP [analogue]: The oral LD50 for TPP is >1590 mg/kg-bw in males and 1630 mg/kg-bw in females (comparable to OECD Guideline 401, in rat), the inhalation LC50 is >6.7 mg/L (comparable to OECD Guideline 403, in rat), and the dermal LD50 is >2000 mg/kg×bw, but <5000 mg/kg×bw (comparable to OECD Guideline 402, in rabbit) (Gabriel, 1980).
Genotoxicity and carcinogenicity
DIDPP: Available data indicate that DIDPP is not genotoxic. DIDPP produced negative results in the in vitro bacterial reverse mutation assay (+/-S9) (Van Goethem, 1980a) and in vivo micronucleus assay (Richold et al. 1981a).
TIDP [analogue]: Available data indicate that TIDP is not genotoxic. It produced negative results in the in vitro bacterial reverse mutation assay (+/-S9) and the DNA-repair suspension assay (Van Goethem, 1980b and 1981, respectively). It also tested negative in the in vivo micronucleus assay (Richold et al. 1981b).
TPP [analogue]: Available data indicate that TPP is not genotoxic. It produced negative results in the in vitro bacterial reverse mutation assay (+/-S9) (Van Goethem, 1980c; Zeiger et al., 1987) and the DNA-repair suspension assay (Van Goethem, 1980b). It also tested negative in the in vivo micronucleus assay (Richold et al. 1981c).
No carcinogenicity studies have been conducted for DIDPP, EHDPP or the analogues TPP and TIDP. Phenol, a hydrolysis product of DIDPP, EHDPP and TPP, was tested in the two-year cancer bioassay and was found to be non-carcinogenic in male and female mice and rats (NTP 1980). The health effects of phenol also were summarized in a previous assessment conducted by the Government of Canada, which concluded that phenol was not carcinogenic in animals (Environment Canada, Health Canada 2000). Phenol is classified as Group 3 (i.e., insufficient evidence to classify) by the International Agency for Research on Cancer (IARC 1989).
Repeated-dose studies
DIDPP: The neurotoxic potential of DIDPP was tested in white leghorn hens. Hens were exposed to 0, 100, 1000 or 4000 mg/kg×bw/day DIDPP by oral gavage for up to 4 weeks (5 days/week). No adverse effects were observed in the neurotoxic esterase assay (US EPA OPPTS guideline: 40 CFR 798.6450, modified). However, adverse effects were observed during the subchronic delayed neurotoxicity study (US EPA OPPTS guideline: 40 CFR 798.6560, modified), including increased mortality (1000 and 4000 mg/kg×bw/day), decreased body weight (4000 mg/kg×bw/day), and distal and peripheral neuropathy in 2/10 animals (4000 mg/kg×bw/day) (PubChem 2007- ).
EHDDP: The effect of subchronic exposure to EHDDP was tested in a repeat-dose study (Bol’shakov and Baranov 1979; study summarized in Sheftel 2000). Rats were exposed to 85 mg/kg×bw/day EHDDP for 45 days (route of exposure not specified). Observed adverse effects included effects on the central nervous system, excretorial function of the liver, anemia and decreased body weight gain.
TIDP [analogue]: The effect of subchronic exposure to TIDP was tested in a combined repeat-dose and reproduction/developmental toxicity study (OECD Guideline 422, modified). Male and female Sprague-Dawley rats (n = 10 per sex per dose group) were exposed to 0, 50, 250 and 1000 mg/kg×bw/day of TIDP in corn oil by oral gavage. The parental (F0) males were exposed for 28 days and the F0 females for 7 to 9 weeks [2 weeks of prebreed exposure, 2 weeks of mating and 3 to 5 weeks of gestation and lactation each (F0 females only)]. Detailed observations included body weight, food consumption, hematology, clinical chemistry, urinalysis, neurobehavioural examination, gross pathology and histopathology, all of which revealed no treatment-related effects for TIDP in either F0 or F1 animals (NOAEL = 1000 mg/kg×bw/day = top dose tested) (Tyl et al. 2005).
TPP [analogue]: The effect of subchronic exposure to TPP was tested in a combined repeat-dose and reproduction/developmental toxicity study (OECD Guideline 422, modified). Male and female Sprague-Dawley rats (n = 10 per sex per dose group) were exposed to 0, 5, 15 and 40 mg/kg×bw/day of TPP in corn oil by oral gavage. Parental (F0) males were exposed for 28 days (4 weeks) and F0 females for approximately 70 days (10 weeks) (prebreed through to lactation). Post-weaning F1 pups (both sexes) were directly dosed for an additional 49 days (7 weeks) until they reached sexual maturity. Detailed examinations of the F0 and F1 animals revealed no adverse effects in the 5 mg/kg×bw/day (low) and 15 mg/kg×bw/day (mid) dose groups; further, the extended dosing of the F1 pups demonstrated that they proceeded through to sexual maturity normally (acquisition of puberty in both sexes and andrological parameters in adult F1 males were unaffected; actual mating ability of the F1 generation was not evaluated). However, systemic toxicity was observed in the 40 mg/kg×bw/day (high) dose group in both the F0 and F1 animals. Specifically, F0 animals in this group presented with ataxia (which indicates neural dysfunction and presents as lack of coordination of movement of skeletal muscles), foot splay and decreased body weight gain, effects which increased in severity over time. Toxicity observed in the F1 offspring of this group included increased mortality (postnatal day, PND 0-4) and reduced pup body weights per litter starting on PND 7-21. These F1 pups were terminated at PND 22 due to increased mortality (PND 0-4) and failure to thrive (PND 7-21). The NOAEL of the study was determined to be 15 mg/kg/bw (LOAEL = 40 mg/kg×bw/day). The critical effect for F0 animals was identified to be ataxia. The critical effect for F1 animals was identified to be mortality and failure to thrive in the presence of significant maternal toxicity (Tyl et al. 2004).
The neurological effects of TPP have been investigated in a number of animal models, including ferret (Tanaka et al. 1990), chicken (Carrington and Abou-Donia 1988; Konno et al. 1989; Katoh et al. 1990; Fioroni et al. 1995) and rats (Lehning et al. 1996; Veronesi et al. 1986; Padilla et al. 1987; Katoh et al. 1990), as well as in in vitro neural cell culture models (Padilla et al. 1987; Knoth-Anderson et al. 1992). These studies were all conducted at doses that exceeded those used for the OECD 422 study described above and consistently showed that exposure to TPP causes widespread neurodegeneration. TPP-induced delayed neurotoxicity is thought to be distinct from the ‘organophosphate-induced delayed neurotoxicity’ (OPIDN) because it produces a distinct pattern of CNS degeneration (Lehning et al. 1996; Veronesi et al. 1986).
Analogue summary
Aryl phosphites appear to exhibit greater toxicity than alkyl phosphites (this observation has also been reported elsewhere (Toscano and Coleman 2012)). The results of acute and repeat-dose studies described above indicate that the relative toxicities of the substances described in this document can be ordered in this manner: TPP>EHDPP>DIDPP>TIDP (Table 6-2).
| Substance: | TIDP | DIDPP | EHDPP | TPP |
|---|---|---|---|---|
| Analogue or target: | Analogue | Target | Target | Analogue |
| Toxicity: | TIDP<<<TPP | DIDPP<<TPP | EHDPP<TPP | =TPP |
| Oral LD50 (mg/kg×bw): | >5000 | >5000 | n.d. | 1590 |
| NOAEL (mg/kg×bw/day): | >1000 | n.d. | n.d.a | 15 |
Abbreviations: n.d. = no data
a No NOAEL was established; however effects were observed in a single dose study at 85 mg/kg-bw/day EHDDP for 45 days (route of exposure not specified) [Bol’shakov and Baranov (1979); study summarized in Sheftel (2000)].
6.3 Characterization of risk to human health
The risk characterization uses data for the analogue triphenyl phosphite (TPP). Toxicity for TPP occurs at lower effect levels compared to DIDPP and is thought to occur at lower effect levels compared to EHDPP. The use of effect levels from TPP is considered conservative for risk characterization for DIDPP and EHDPP. Table 6-3 provides the relevant exposure and hazard values for the alkyl aryl phosphites, as well as resultant margins of exposure (MOEs), for determination of risk to human health.
| Exposure scenario | Systemic exposure (µg/kg×bw/day) | Critical effect level (mg/kg×bw/day) | Critical health effect | MOE |
|---|---|---|---|---|
| Mouthing manufactured plastic items intended for infants (0-18 months) | 0.0017 | 15 a-1000b | Effects in F1 offspring in presence of significant maternal toxicity: increased mortality (PND 0-4) and reduced pup body weights per litter (PND 7-21)a | >1000000 |
| Dermal contact with plastic articles (adults) | 0.52 | 15 a-1000b | Neurological effects (i.e., ataxia)a | 28900->1000000 |
| Dermal contact with plastic articles (infants) | 1.31 | 15 a-1000b | Effects in F1 offspring in presence of significant maternal toxicity: increased mortality (PND 0-4) and reduced pup body weights per litter (PND 7-21)a | 11400-760000 |
Abbreviations: MOE = margins of e2xposure; PND = postnatal day.
a NOAEL for TPP (ECHA 2007-2015a).
b NOAEL for TIDP – Note: No treatment-related effects were noted at the highest dose.
The MOEs listed above are considered adequate to account for uncertainties in the health effects and exposure databases.
6.4 Uncertainties in evaluation of risk to human health
The key sources of uncertainty are presented in the table below (Table 6-4).
| Key source of uncertainty | Impacta |
|---|---|
| In the absence of data on residual levels and migration specific to EHDPP and DIDPP in polymeric materials, using 1 wt% as the residual level and 13.4 µg/m2-hr as the migration rate is expected to over-estimate the exposure from contacting plastic objects. | + |
| In the absence of dermal absorption data for EHDPP and DIDPP, 100% of dermal absorption used in the exposure estimates can over-estimate the exposure given the high log Kow estimated for both substances. | + |
| TPP was used to derive the critical effects and critical effect levels; therefore, the toxicity of DIDPP and EHDPP are expected to be over-estimated. Limited studies were available for DIDPP and EHDPP. | + |
a “+” Likely to increase conservatism (i.e., likely to be overly-protective); “-” Likely to decrease conservatism; “+/-” Impact on the assessment not known.
The additional data on residual levels, migration rates and/or dermal absorption of EHDPP and DIDPP in polymeric materials would reduce the uncertainties associated with the risk characterization of the Alkyl Aryl Phosphites Group. It is expected that such additional data would result in increased margins of exposure over those presented in this assessment report.
The addition of sub-chronic and chronic studies on the toxicological effects of EHDPP and/or DIDPP (in animals or humans) to the health effects database would reduce the uncertainties associated with the risk characterization of the Alkyl Aryl Phosphites Group. Because this screening assessment report relied on data for the analogue TPP, which is expected to be more toxic than either EHDPP or DIDPP, it is expected that additional studies on either of the target chemicals would result in increased margins of exposure over those presented in this assessment report.
7. Conclusion
Considering all available lines of evidence presented in this screening assessment, there is low risk of harm to the environment from EHDPP and DIDPP. It is concluded that EHDPP and DIDPP do not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
On the basis of the information presented in this screening assessment, it is concluded that EHDPP and DIDPP do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
Therefore, it is concluded that EHDPP and DIDPP do not meet any of the criteria set out in section 64 of CEPA.
References
Aird RB, Cohn WE, Weiss S. 1940. Convulsive action of triphenyl phosphite. Proc Soc Exper Biol Med. 45:306-309.
Andek 2015. Safety Data Sheet for Cocoon 111, Cocoon 550 and Cocoon 560.
Bhunia K, Sablani SS, Tang, J, Rasco B. 2013. Migration of chemical compounds from packaging polymers during microwave, conventional heat treatment, and storage. Compr Rev Food Sci Food Saf. 12:523-545.
Canada. 1999. Canadian Environmental Protection Act, 1999.. S.C. 1999, c. 33. Canada Gazette Part III, vol. 22, no. 3.
Carlsson DJ, Krzymien ME, Deschênes L, Mercier M, Vachon C. 2001. Phosphite additives and their transformation products in polyethylene packaging for gamma-irradiation. Food Addit Contam. 18(6):581-591.
Carrington CD, Abou-Donia MB. 1988. Triphenyl phosphite neurotoxicity in the hen: inhibition of neurotoxic esterase and of prophylaxis by phenylmethylsulfonyl fluoride. Arch Toxicol. 62:375-80.
Chen SJ, Ma YJ, Wang J, Chen D, Luo XJ, Mai BX. 2009. Brominated flame retardants in children’s toys: concentration, composition, and children’s exposure and risk assessment. Environ Sci Technol. 43(11):4200-4206.
Crompton R. 2007. Determination of additives in polymers and rubbers. Rapra Technology. UK.
[ECB] European Chemicals Bureau. 2008. ECB summary fact sheet: TC NES subgroup on identification of PBT and VPVP substances: results of the evaluation of the PBT/VPVB properties of Diisodecyl phenyl phosphite. PBT List No. 46. [accessed 2017 Feb 23].
[ECCC, HC] Environment and Climate Change Canada, Health Canada. [modified 2017 Mar 12]. Categorization. Ottawa (ON): Government of Canada. [accessed 2017 Feb 10].
[ECCC] Environment and Climate Change Canada. 2016a. Science approach document: ecological risk classification of organic substances. Ottawa (ON): Government of Canada.
[ECCC] Environment and Climate Change Canada. 2016b. Data used to create substance-specific hazard and exposure profiles and assign risk classifications in the Ecological Risk Classification of organic substances. Gatineau (QC). Available from: substances@ec.gc.ca
[ECHA] European Chemicals Agency. c2007-2015a. Registered substances database; search results for CAS RN [101-02-0]. Helsinki (FI): ECHA. [updated 2016 Oct 19; accessed 2017 Jan 25].
[ECHA] European Chemicals Agency. c2007-2015b. Registered substances database; search results for CAS RN 25550-98-5. Helsinki (FI): ECHA. [updated 2016 Oct 19; accessed 2017 Jan 25].
[ECHA] European Chemicals Agency. c2007-2015c. Registered substances database; search results for CAS RN 15647-08-2. Helsinki (FI): ECHA. [updated 2016 Oct 19; accessed 2017 Jan 25].
Environment Canada, Health Canada. 2000. Canadian Environmental Protection Act: Priority Substances List assessment report: Phenol. Ottawa (ON): Environment Canada, Health Canada. [accessed 2017 Feb 2].
Environment Canada. 2009. DSL Inventory Update data collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain inanimate substances (chemicals) on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
Environment Canada. 2013. DSL Inventory Update data collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain substances on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
Environment Canada, Health Canada. 2015a. State of the Science Report Phthalate Substance Grouping: 1,2-Benzenedicarboxylic acid, Diisononyl ester 1,2-Benzenedicarboxylic acid, di-C8-10-branched alkyl esters, C9-rich (Diisononyl Phthalate; DINP). Ottawa (ON): Environment Canada, Health Canada. [accessed 2017 Feb 9].
Environment Canada, Health Canada. 2015b. State of the Science Report Phthalate Substance Grouping: Medium-Chain Phthalate Esters. Ottawa (ON): Environment Canada, Health Canada. [accessed 2017 Feb 9].
Environment Canada, Health Canada. 2015c. State of the Science Report Phthalate Substance Grouping: Long-chain Phthalate Esters. Ottawa (ON): Environment Canada, Health Canada. [accessed 2017 Feb 9].
Faring LO. 2003. Ashless antiwear and extreme-pressure additives. In: Rudnick LR, editor. Lubricant additives: chemistry and applications. CRC Press. p. 223-257.
Fioroni F, Moretto A, Lotti M. 1995. Triphenylphosphite neuropathy in hens. Arch Toxicol. 69:705-11.
Gabriel KL. 1980. Unpublished report no. 80-2010A: Summary of results of acute toxicity studies for Tenneco Chemicals Inc., Saddle Brook, NJ from Biosearch Inc., Philadelphia, PA [cited in General Electric Company 2001].
Galata Chemicals. 2010. Weston® EHDP liquid phosphite heat stabilizer. Technical information.
Galata Chemicals. 2013. WESTON® PDDP phosphite ester phosphite antioxidant. Technical Information.
General Electric Company. 2001. U.S. High Production Volume (HPV) Chemical Challenge Program: Justification, test plan, and robust summaries: Phosphite isodecyl/phenyl chemical category: Phosphorous acid, triisodecyl ester (CAS# 25448-25-3), phosphorous acid, diisodecyl phenyl ester (CAS# 25550-98-5), phosphorous acid, isodecyl diphenyl ester (CAS# 26544-23-0), phosphorous acid, triphenyl ester (CAS# 101-02-0), Prepared for US Environmental Protection Agency. Washington (DC). [accessed 2017 Jan 27].
Health Canada. 1995. Investigating human exposure to contaminants in the environment: a handbook for exposure calculations. Unpublished report. Ottawa (ON): Government of Canada.
[HENRYWIN] Henry’s Law Constant Program for Microsoft Windows [estimation model]. 2008. Ver. 3.20. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Jakupca M. 2010. Polymer stabilizers: current challenges and future trends. Dover Chemical Corporation.
[IARC] IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. 1989. Some organic solvents, resin monomers and related compounds, pigments and occupational exposures in paint manufacture. IARC Monogr Eval Carcinog Risks Hum. 47:264-87.
Katoh K, Konno N, Yamauchi T, Fukushima M. 1990 Effects of age on susceptibility of chickens to delayed neurotoxicity due to triphenyl phosphite. Basic Clin Pharmacol Toxicol. 66:387-92.
Knoth-Anderson J, Veronesi B, Jones K, Lapadula DM, Abou-Donia MB. 1992. Triphenyl phosphite-induced ultrastructural changes in bovine adrenomedullary chromaffin cells. Toxicol Appl Pharmacol, 112:110-9.
Konno N, Katoh K, Yamauchi T, Fukushima M. 1989. Delayed neurotoxicity of triphenyl phosphite in hens: pharmacokinetic and biochemical studies. Toxicol Appl Pharmacol. 100:440-50.
[KOWWIN] Octanol-Water Partition Coefficient Program for Microsoft Windows [estimation model]. 2010. Ver. 1.68. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Lehning EJ, Tanaka D Jr, Bursian SJ. 1996. Triphenyl phosphite and diisopropylphosphorofluoridate produce separate and distinct axonal degeneration patterns in the central nervous system of the rat. Fundam Appl Toxicol. 29:110-8.
Marcato B, Guerra S, Vianello M, Scalia S. 2003. Migration of antioxidant additives from various polyolefinic plastics into oleaginous vehicles. Int J Pharm. 257. 217-225.
Mathy Universal. 2015. Safety Data Sheet for Mathé classic Motorenöl-Zusata SAE 30.
[MPBPWIN] Melting Point Boiling Point Program for Microsoft Windows [estimation model]. 2008. Ver. 1.43. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
[NCI] National Chemical Inventories. Issue 1. Columbus (OH): American Chemical Society, Chemical Abstracts Service. [accessed 2017 Feb 10].
[NPRI] National Pollutant Release Inventory [database on the Internet]. 2011-2015. Gatineau (QC): Environment and Climate Change Canada. [accessed 2016 Dec].
[NTP] National Toxicology Program (US). 1980. Bioassay of Phenol for Possible Carcinogenicity (Technical Report Series No. 203). Research Triangle Park (NC): US Department of Health and Human Services, National Toxicology Program.
[NTP] National Toxicology Program (US). Testing Status on Agents at NTP: 2-Ethylhexanol. [updated 2017 Jan 18; accessed 2017 Feb 2].
Padilla SS, Grizzle TB, Lyerly D. 1987. Triphenyl phosphite: in vivo and in vitro inhibition of rat neurotoxic esterase. Toxicol Appl Pharmacol. 87:249-56.
[PMC] Phosphite Manufacturers Consortium. 2006. Submission to U.S. High Production Volume (HPV) Chemical Challenge Program: Additional Robust Summaries for Isodecyl/Phenyl Phosphite Category. Washington (DC).
PubChem [database]. 2007- . Bethesda (MD): US National Library of Medicine, National Center for Biotechnology Information. [updated 2017 January 28; accessed 2017 Jan 31].
Richold et al. 1981a. Unpublished report TCO 17C/81309 entitled “Micronucleus test on phenyldiisodecyl phosphite (PDDP)”, dated June 26, 1981 for Tenneco Chemicals Inc., Saddle Brook, NJ from Huntingdon Research Centre, Cambridgeshire, England [cited in General Electric Company 2001].
Richold et al. 1981b. Unpublished report TCO 17E/81311 entitled “Micronucleus test on triisodecyl phosphite (TDP)”, dated June 25, 1981, for Tenneco Chemicals Inc. Saddle Brooke, NJ from Huntingdon Research Center, Cambridgeshire, England [cited in General Electric Company 2001].
Richold et al. 1981c. Unpublished report TCO 17E/81311 entitled “Micronucleus test on triphenyl phosphite (TPP)”, dated June 26, 1981, for Tenneco Chemicals Inc. Saddle Brooke, NJ from Huntingdon Research Center, Cambridgeshire, England [cited in General Electric Company 2001].
Saito I, Onuki A, Seto H. 2007. Indoor organophosphate and polybrominated flame retardants in Tokyo. Indoor Air. 17:28-36.
Schwope AD, Goydan R, Reid RC. 1990. Methods for assessing exposure to chemical substances. Vol. 11. Methodology for estimating the migration of additives and impurities from polymeric materials. Contract No. 68-D9-0166. Prepared for US Environmental Protection Agency Office of Pesticides and Toxic Substances. Washington (DC).
Sheftel VO. 2000. Indirect food additives and polymers: migration and toxicology. Boca Raton (FL): CRC Press LLC.
Siró I, Fenyvesi E, Szente L, de Meulenaer B, Devlieghere F, Orgoványi J, Sényi J, Barta J. 2006. Release of alpha-tocopherol from antioxidative low-density polyethylene film into fatty food simulant: influence of complexation in beta-cyclodextrin. Food Addit Contam. 23(8):845-853.
Spatafore R, Pearson LT. 1991. Migration and blooming of stabilizing antioxidants in polypropylene. Polym Eng Sci. 31(22):1810-1817.
Stevenson DR, Harr ME, Jakupca MR. 2002. Phosphite ester compositions for PVC compounds. J Vinyl Addit Technol. 8(1):61-69.
Tanaka D Jr, Bursian SJ, Lehning EJ, Aulerich RJ. 1990. Exposure to triphenyl phosphite results in widespread degeneration in the mammalian central nervous system. Brain Res. 531:294-98.
Toscano WA, Coleman KP. 2012. Esters of carbonic and orthocarbonic acid, organic phosphorous, monocarboxylic halogenated acids, haloalcohols, and organic silicon. In: Patty’s industrial hygiene and toxicology. 6th ed. Hoboken (NJ): John Wiley and Sons, Inc.
Tyl RW, Myers CB, Marr MC. 2004. Modified Combined Repeated Dose Toxicity Study with the Reproductive/Developmental Toxicity Screening Test of Triphenyl Phosphite (TPPi; CAS No. 101-02-0) Administered Via Oral Gavage to CD® (Sprague-Dawley) Rats (OECD 422). RTI Identification No. 65C09165.000.400. Report to Crompton Corporation, RTI International, Research Triangle Park, NC [cited in PMC 2006; ECHA 2007-2016a].
Tyl RW, Myers CB, Marr MC. 2005. Modified Combined Repeated Dose Toxicity Study with the Reproductive/Developmental Toxicity Screening Test of Triisodecyl Phosphite (TDP) Administered Via Oral Gavage to CD® (Sprague-Dawley) Rats (OECD 422). RTI Identification No. 65C-09178.000.500. Report to Phosphite Manufacturers Consortium, RTI International, Research Triangle Park, NC [cited in PMC 2006; ECHA 2007-2016d].
Van Goethem D. 1980a. Unpublished report no 4822-E entitled “Evaluation of phenyldiisodecyl phosphite in the Salmonella/microsome (Ames) assay” dated September 30, 1980, for Tenneco Chemicals, Inc. Saddle Brook, NJ from Midwest Research Institute, Kansas City, MO [cited in General Electric Company 2001].
Van Goethem D. 1980b. Unpublished report no. 4822-E entitled “Evaluation of triisodecyl phosphite in the Salmonella/microsome (Ames) assay” dated September 30, 1981, for Tenneco Chemicals Inc., Saddle Brooke, NJ from Midwest Research Institute, Kansas City, MO [cited in General Electric Company 2001].
Van Goethem D. 1980c. Unpublished report no. 4822-E entitled “Evaluation of triphenyl phosphite in the Salmonella/microsome (Ames) assay” dated September 15, 1981, for Tenneco Chemicals Inc. Saddle Brooke, NJ from Midwest Research Institute, Kansas City, MO [cited in General Electric Company 2001].
Van Goethem D. 1981. Unpublished report no. 4822-E entitled “Evaluation of triisodecyl phosphite in the E. coli DNA repair suspension assay” dated January 19, 1981, for Tenneco Chemicals Inc. Saddle Brooke, NJ from Midwest Research Institute, Kansas City, MO [cited in General Electric Company 2001].
Veronesi B, Padilla S, Newland D. 1986. Biochemical and neuropathological assessment of triphenyl phosphite in rats. Toxicol Appl Pharmacol. 83:203-10.
[WSKOWWIN] Water Solubility for Organic Compounds Program for Microsoft Windows [estimation model]. 2010. Ver. 1.42. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Zeiger E, Anderson B, Haworth S, Lawlor T, Mortelmans K, Speck W. 1987. Salmonella mutagenicity tests: III. Results from the testing of 255 chemicals. Environ Mutagen. 9(9):1-110 [cited in General Electric Company 2001].
Appendices
Appendix A. Physical and chemical properties of analogues used in the human health assessment of EHDPP and DIDPP
| Common name | Property | Value | Key reference |
| 101-02-0 [TPP] (Triphenyl phosphite) | Vapour pressure (Pa @ 25oC) | 0.069 | (ECHA c2007-2015c) |
| 101-02-0 [TPP] (Triphenyl phosphite) | Water solubility (mg/L)a | 2.35 × 10-2 | (WSKOWWIN 2010) |
| 101-02-0 [TPP] (Triphenyl phosphite) | log Kow (dimensionless)a | 6.62 | (KOWWIN 2010) |
| 25448-25-3 [TIDP] (Triisodecyl phosphite) | Vapour pressure (Pa @ 25oC)a | 2.07 × 10-7 | (MPBPWIN 2008) |
| 25448-25-3 [TIDP] (Triisodecyl phosphite) | Water solubility (mg/L)a | 2.11 × 10-8 | (WSKOWWIN 2010) |
| 25448-25-3 [TIDP] (Triisodecyl phosphite) | log Kow (dimensionless)a | 12.31 | (KOWWIN 2010) |
Abbreviations: Kow, octanol–water partition coefficient.
a Calculated values.
