Screening Assessment Alkyl Sulfates and α-Olefin Sulfonate Group Chemical Abstracts Service Registry Numbers 139-96-8, 151-21-3, 2235-54-3 and 68439-57-6
Table of Contents
- Synopsis
- 1. Introduction
- 2. Identity of substances
- 3. Physical and chemical properties
- 4. Sources and uses
- 5. Potential to cause ecological harm
- 6. Potential to cause harm to human health
- 7. Conclusion
- References
- Appendix A. Dermal and inhalation exposures from cosmetics and cleaning products
- Appendix B. Oral exposure from dish detergent left as residue on dinnerware, toothpaste, and use of non-prescription drugs and natural health products formulated as capsules/tablets
List of tables
- Table 2-1. Substance identities of the four substances in the Alkyl Sulfates and α-Olefin Sulfonate Group
- Table 3-1. Range of key physical and chemical properties for the four substances in the Alkyl Sulfates and α-Olefin Sulfonate Group
- Table 4-1. Summary of information submitted pursuant to section 71 survey of CEPA
- Table 5-1. Ecological risk classification results for the four substances in the Alkyl Sulfates and α-Olefin Sulfonate Group
- Table 6-1. Input values used to predict surface water concentration of the lauryl sulfate salts and sodium C14-16 olefin sulfonate
- Table 6-2. Summary of estimates of dermal exposures for adults from use of cosmetics
- Table 6-3. Summary of estimates of inhalation exposures of adults from use of cleaning products with trigger spray and cosmetics
- Table 6-4. Relevant exposure for the lauryl sulfate salts, ammonium lauryl sulfate and TEA lauryl sulfate, as well as resulting MOEs based on the critical effect level (NOAEL) of 79 mg/kg bw per day
- Table 6-5. Relevant exposure values for sodium lauryl sulfate, as well as resulting MOEs based on the critical effect level (NOAEL) of 86 mg/kg bw per day
- Table 6-6. Relevant exposure values for sodium C14-16 olefin sulfonate, as well as resulting MOEs based on the critical effect level (NOAEL) of 195 mg/kg bw per day
- Table B-1. Oral exposure parameter assumptions.
Synopsis
Pursuant to section 74 of the Canadian Environmental Protection Act, 1999 (CEPA), the Minister of the Environment and the Minister of Health have conducted a screening assessment of four substances referred to collectively as the Alkyl Sulfates and α-Olefin Sulfonate Group. The substances in this group were identified as priorities for assessment as they met categorization criteria under subsection 73(1) of CEPA. The Chemical Abstracts Service Registry Numbers(CAS RNFootnote1), their Domestic Substances List names and their common names are listed in the table below.
| CAS RN | Domestic Substances List name | Common name |
|---|---|---|
| 139-96-8 | Sulfuric acid, monododecyl ester, compound with 2,2',2"-nitrilotris[ethanol] (1:1) | Triethanolamine (TEA) lauryl sulfate |
| 151-21-3 | Sulfuric acid monododecyl ester sodium salt | Sodium lauryl sulfate |
| 2235-54-3 | Sulfuric acid, monododecyl ester, ammonium salt | Ammonium lauryl sulfate |
| 68439-57-6[a] | Sulfonic acids, C14-16-alkane hydroxy and C14-16-alkene, sodium salts | Sodium C14-16 olefin sulfonate |
a This CAS RN is a UVCB (unknown or variable composition, complex reaction products, or biological materials).
All four substances in this group are anionic surfactants and do not occur naturally in the environment. They are primarily found in cleaning products (e.g. laundry, dishwashing, and household products) and in other products available to consumers (e.g. shampoos, toothpastes, soaps, bubble bath products). Sodium lauryl sulfate can also be found in food packaging materials and is an approved food additive with a limited number of permitted uses in a small number of food categories. In 2011, all substances, with the exception of TEA lauryl sulfate, were manufactured in Canada in quantities ranging from 100 to 1 000 000 kg. In the same year, all four substances were imported into Canada in quantities ranging from 10 000 to 2 240 000 kg.
The ecological risks of the substances in the Alkyl Sulfates and α-Olefin Sulfonate Group were characterized using the ecological risk classification of organic substances (ERC). The ERC is a risk-based approach that employs multiple metrics for both hazard and exposure based on weighted consideration of multiple lines of evidence for determining risk classification. Hazard profiles are established based principally on metrics regarding mode of toxic action, chemical reactivity, food web–derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Metrics considered in the exposure profiles include potential emission rate, overall persistence, and long-range transport potential. A risk matrix is used to assign a low, moderate or high level of potential concern for substances based on their hazard and exposure profiles. The ERC identified the four substances in this assessment as having low-to-moderate potential to cause ecological harm.
Considering all available lines of evidence presented in this screening assessment, there is a low risk of harm to organisms and the broader integrity of the environment from TEA lauryl sulfate, sodium lauryl sulfate, ammonium lauryl sulfate and sodium C14-16 olefin sulfonate. It is concluded that TEA lauryl sulfate, sodium lauryl sulfate, ammonium lauryl sulfate and sodium C14-16 olefin sulfonate do not meet the criteria under paragraph 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
Exposures to the substances from drinking water and from use of cleaning products and cosmetics were estimated for the general population of Canada. Additionally, exposures to sodium lauryl sulfate were estimated based on its presence as a non-medicinal ingredient in natural health products and non-prescription drugs formulated as capsules/tablets and toothpastes.
TEA lauryl sulfate, sodium lauryl sulfate, and ammonium lauryl sulfate were grouped together on the basis of structural similarity and a read-across approach was used to characterize their health effects. Sodium C14-16 olefin sulfonate was addressed separately. The liver is the target organ for systemic toxicity for alkyl sulfates with certain chain lengths following oral administration. Liver effects, however, were not observed for sodium C14-16 olefin sulfonate. Developmental effects were observed for sodium C14-16 olefin sulfonate in some laboratory studies, but not in others.
The margins of exposure comparing critical effect levels and levels to which the general population may be exposed were considered adequate to address uncertainties in the health effects and exposure databases for TEA lauryl sulfate, sodium lauryl sulfate, ammonium lauryl sulfate and sodium C14-16olefin sulfonate.
Based on the adequacy of the margins between critical effect levels and estimated exposure and on information presented in this screening assessment, it is concluded that TEA lauryl sulfate, sodium lauryl sulfate, ammonium lauryl sulfate and sodium C14-16 olefin sulfonate do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is concluded that TEA lauryl sulfate, sodium lauryl sulfate, ammonium lauryl sulfate and sodium C14-16 olefin sulfonate do not meet any of the criteria set out in section 64 of CEPA.
1. Introduction
Pursuant to section 74 of the Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999), the Minister of the Environment and the Minister of Health have conducted a screening assessment of four substances referred to collectively as the Alkyl Sulfates and α-Olefin Sulfonate Group to determine whether these substances present or may present a risk to the environment or to human health. The substances in this group include triethanolamine (TEA) lauryl sulfate, sodium lauryl sulfate, ammonium lauryl sulfate and sodium C14-16 olefin sulfonate, and they were identified as priorities for assessment as they met categorization criteria under subsection 73(1) of CEPA (ECCC, HC [modified 2007]).
The substances in the Alkyl Sulfates and α-Olefin Sulfonate Group were previously reviewed internationally through the Cooperative Chemicals Assessment Programme of the Organisation for Economic Cooperation and Development (OECD), and a Screening Initial Data Set (SIDS) Initial Assessment Report (SIAR) is available. These assessments undergo rigorous review and endorsement processes by international governmental authorities. Health Canada and Environment and Climate Change Canada are active participants in this process and consider these assessments to be reliable. Additional health effects studies for sodium C14-16 olefin sulfonate were identified in Health Canada's proposed regulatory decision document on EXIT ISP (PMRA 2005). OECD SIAR for the category of alkyl sulfates, alkane sulfonates and α-olefin sulfonates (OECD 2007) and the Health Canada document on EXIT ISP (PMRA 2005) are used to inform the characterization of health effects in this assessment. TEA lauryl sulfate, sodium lauryl sulfate and ammonium lauryl sulfate were sub-grouped together on the basis of their structural similarity; sodium C14-16 olefin sulfonate was addressed separately.
The ecological risk of TEA lauryl sulfate, sodium lauryl sulfate, ammonium lauryl sulfate and sodium C14-16olefin sulfonate was characterized using the ecological risk classification of organic substances (ERC) (ECCC 2016a). The ERC describes the hazard of a substance using key metrics, including mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity and considers the possible exposure of organisms in the aquatic and terrestrial environments based on factors such as potential emission rates, overall persistence and long-range transport potential in air. The various lines of evidence are combined to identify substances as warranting further evaluation of their potential to cause harm to the environment or as having a low likelihood of causing harm to the environment.
This screening assessment includes consideration of information on chemical properties, environmental fate, hazards, uses and exposures, including additional information submitted by stakeholders. Relevant data were identified up to June 2016.
This screening assessment was prepared by staff in the CEPA Risk Assessment Program at Health Canada and Environment and Climate Change Canada and incorporates input from other programs within these departments. The ecological portion of this assessment is based on the ERC document which was subject to an external peer-review. Additionally, the ERC document (published July, 2016) and the draft of this screening assessment (published December, 2016) were subject to a 60-day public comment period. While external comments were taken into consideration, the final content and outcome of the screening assessment remain the responsibility of Environment and Climate Change Canada and Health Canada.
This screening assessment focuses on information critical to determining whether the substances meet the criteria as set out in section 64 of CEPA. It examines scientific information and develops a conclusion by incorporating a weight-of-evidence approach and precaution. Footnote2 The screening assessment presents the critical information and considerations that form the basis of the conclusion.
2. Identity of substances
The Chemical Abstracts Service Registry Numbers (CAS RNFootnote3), Domestic Substances List (DSL) names and common names for the individual substances in the Alkyl Sulfates and α-Olefin Sulfonate Group are presented in Table 2-1.
The four substances within this group are anionic surfactants. TEA lauryl sulfate, sodium lauryl sulfate and ammonium lauryl sulfate are alkyl sulfates and salts of lauryl sulfate; they have the same C12 alkyl chain with different counter-ions (i. e., triethanolamine, sodium and ammonium). Sodium C14-16olefin sulfonate is an α-olefin sulfonate; it is a mixture composed of mono-unsaturated alkene sulfonates and hydroxyalkane sulfonates, with the double bond and hydroxyl group located at various positions along a C14 or C16 alkyl chain and having sodium as the counter-ion.
| CAS RN | DSL name (common name) | Chemical structure and molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
| 139-96-8 | Sulfuric acid, monododecyl ester, compound with 2,2',2"-nitrilotris[ethanol] (1:1) (Triethanolamine (TEA) lauryl sulfate) |
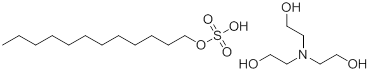 C12H26·SO4·C6H15NO3 |
415.59 |
| 151-21-3 | Sulfuric acid monododecyl ester sodium salt (Sodium lauryl sulfate) |
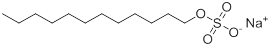 C12H26·SO4·Na |
288.38 |
| 2235-54-3 | Sulfuric acid, monododecyl ester, ammonium salt (Ammonium lauryl sulfate) |
 C12H26·SO4·NH4 |
283.43 |
| 68439-57-6[a] | Sulfonic acids, C14-16-alkane hydroxy and C14-16-alkene, sodium salts (Sodium C14-16 olefin sulfonate) |
NA C14-16=/OH·SO3·Na |
298.42 – 344.49 |
Abbreviations: NA, not available.
a This CAS RN is a UVCB (unknown or variable composition, complex reaction products, or biological materials).
3. Physical and chemical properties
No experimental values of vapour pressure were identified in the literature. However, considering the ionic character of these salts, it is expected that they have low vapour pressure and do not evaporate into air. They are very soluble in water (> 105 mg/L) and completely dissociate in the aquatic compartment. As surfactants, they have a tendency to concentrate at hydrophilic and hydrophobic boundaries rather than to equilibrate between phases. It is therefore difficult to accurately measure or model the octanol-water partition coefficient for these ionizing substances.
Key physical and chemical properties of these four substances are summarized in Table 3-1. Additional physical and chemical properties are presented in ECCC (2016b).
| Property | Value or range | Type of data | Key references |
|---|---|---|---|
| Melting point (°C) | ≥ 72 | Experimental | OECD 2007, Lide 2005, CHRIP c2008 |
| Boiling point (°C) | ≥ 388 | Modelled | EPI Suite c2000-2010 |
| Vapour pressure (Pa) | ≤ 5.87 × 10−6 | Modelled/ calculated | ECHA c2007-2015, EPI Suite c2000-2010, OECD 2007 |
| Henry's Law constant (Pa·m3/mol) | ≤ 0.0667 | Calculated | EPI Suite c2000-2010, OECD 2007 |
| Water solubility (mg/L) | ≥ 130 000 | Experimental | OECD 2007, ECHA c2007-2015, Dreger et al. 1944 |
4. Sources and uses
TEA lauryl sulfate, sodium lauryl sulfate, ammonium lauryl sulfate and sodium C14-16 olefin sulfonate are anionic surfactants and do not occur naturally in the environment. Sources of these four substances are industrial activities and products available to consumers.
On the basis of information submitted pursuant to section 71 of CEPA 1999 regarding commercial activity in Canada under Phase 2 of the DSL Inventory Update, manufacture and/or import quantities of these four substances have been reported and are summarized in Table 4-1 (Environment Canada 2013).
| Common name | Range of manufacture quantity (kg) | Range of import quantity (kg) |
|---|---|---|
| TEA lauryl sulfate | None | 10 000 – 100 000 |
| Sodium lauryl sulfate | 100 000 – 1 000 000 | 1 123 920 |
| Ammonium lauryl sulfate | 100 000 – 1 000 000 | 82 385 |
| Sodium C14-16 olefin sulfonate | 100 – 1000 | 2 239 453 |
a Values reflect quantities reported in response to a survey conducted under section 71 of CEPA (Environment Canada 2013). See survey for specific inclusions and exclusions (schedules 2 and 3).
TEA lauryl sulfate, sodium lauryl sulfate, ammonium lauryl sulfate and sodium C14-16 olefin sulfonate are used in a number of products available to consumers.
In Canada, all four substances are used in a number of cleaning products including general purpose cleaners/degreasers, multi-purpose cleaners, multi-surface cleaners, hard-surface floor cleaners, carpet cleaners, upholstery cleaners, toilet bowl cleaners, shower cleaners, glass cleaners, food surface cleaners, garbage disposal cleaners/deodorizers and jewellery cleaners (MSDS 1996, 2006a,b, 2007a, 2012a,b, 2013a,b,c,d, 2014a,b, 2015a,b,c,d,e, date unknown). They are also used in dishwashing detergents, laundry detergents and fabric stain removers (MSDS 2007b,c, 2010a,b, 2012c, 2013e,f, 2014c, 2015f). According to the information available, sodium lauryl sulfate has a broader use pattern than the other substances.
All four substances are also used in a variety of cosmetics, including shampoos and conditioners, cleansers and soaps, shaving creams, hair dyes, hair products, body and face moisturizers, tanning products and make-up removers (Household Product Database 1993–, MSDS 2006c,d,e,f,g,h, 2007d,e,f, 2008a,b, 2009, 2010c,d,e,f,g, 2011, 2012d,e, 2014d,e, 2015g,h,i,j, personal communications, emails from Consumer Product Safety Directorate, Health Canada, to Risk Management Bureau, Safe Environments Directorate, Health Canada, 2015; unreferenced).
TEA lauryl sulfate, ammonium lauryl sulfate and sodium C14-16 olefin sulfonate are listed in the Natural Health Products Ingredients Database (NHPID) as having a non-medicinal role for use as an emulsifying agent or surfactant, as ane mulsifying agent or surfactant – cleansing agent in topical products, and as a surfactant – cleansing agent in topical products, respectively (NHPID [modified 2016]). They are listed in the Licensed Natural Health Products Database (LNHPD [modified 2016]) as being present as non-medicinal ingredients in products such as shampoos, skin cleansers and moisturizers.
Sodium lauryl sulfate is also listed in the NHPID as having a non-medicinal role for use as a detergent, emulsifying agent, lubricant, skin penetrant, solubilizing agent, surfactant, surfactant – cleansing agent or wetting agent (NHPID [modified 2016]). It is listed in the LNHPD as a non-medicinal ingredient in a variety of products, including toothpastes, mouthwashes, face creams, massage creams, shampoos, skin cleansers and products formulated as capsules/tablets (LNHPD [modified 2016]).
The four substances in this group are also used as non-medicinal ingredients in non-prescription drugs, such as shampoos and skin cleansers (DPD [modified 2015], personal communications, emails from Therapeutic Products Directorate, Health Canada, to Risk Management Bureau, Safe Environments Directorate, Health Canada, 2015; unreferenced). Sodium lauryl sulfate is used as an emulsifying agent, modified-release agent, penetration enhancer, solubilising agent or capsules/tablets lubricant in non-prescription and prescription drugs (EMA 2015, personal communications, emails from Therapeutic Products Directorate, Health Canada, to Risk Management Bureau, Safe Environments Directorate, Health Canada, 2015; unreferenced). Sodium lauryl sulfate and sodium C14-16 olefin sulfonate are found in disinfectants, and ammonium lauryl sulfate is found in a small number of pet shampoos (MSDS 2007g, personal communications, emails from Therapeutic Products Directorate, Health Canada, to Risk Management Bureau, Safe Environments Directorate, Health Canada, 2015; unreferenced).
In Canada, sodium lauryl sulfate is an approved food additive with a limited number of permitted uses in a small number of food categories (i. e., as a whipping agent in egg whites and in gelatin intended for marshmallow compositions) as listed in the List of Permitted Food Additives With Other Generally Accepted Uses, which is incorporated by reference into its associated Marketing Authorization, issued under the authority of the Food and Drugs Act (Canada [1978]) (personal communications, emails from Food Directorate, Health Canada, to Risk Management Bureau, Safe Environments Directorate, Health Canada, 2015; unreferenced). It has also been identified as being used in a limited number of food processing aids and in the manufacture of different types of food packaging materials in Canada (personal communications, emails from Food Directorate, Health Canada, to Risk Management Bureau, Safe Environments Directorate, Health Canada, 2015; unreferenced).
In Canada, all four substances in this group have been identified for use as components of incidental additives in products used in food processing plants, including hand treatments, cleaners, sanitizers and disinfectants, release agents, lubricants and odor control agents (personal communications, emails from Food Directorate, Health Canada, to Risk Management Bureau, Safe Environments Directorate, Health Canada, 2015; unreferenced).
All four substances are listed on the Canadian Pest Management Regulatory Agency (PMRA) Pesticide Formulants List. Sodium lauryl sulfate and sodium C14-16 olefin sulfonate are also listed on PMRA's List of Active Pesticide Ingredients.
Uses were also identified for these substances in automotive care products and pet care products (Household Products Database 1983- ). Sodium lauryl sulfate was also identified for use as an additive for plastics and lattices and in paints and lacquers (OECD 1995).
5. Potential to cause ecological harm
5.1 Characterization of ecological risk
The ecological risks of substances in the Alkyl Sulfates and α-Olefin Sulfonate Group were characterized using the ecological risk classification of organic substances (ERC) (ECCC 2016a). The ERC is a risk-based approach that considers multiple metrics for both hazard and exposure based on weighted consideration of multiple lines of evidence for determining risk classification. The various lines of evidence are combined to discriminate between substances of lower or higher potency and lower or higher potential for exposure in various media. This approach reduces the overall uncertainty with risk characterization compared to an approach that relies on a single metric in a single medium (e. g., LC50) for characterization. The following summarizes the approach, which is described in detail in ECCC (2016a).
Data on physical-chemical properties, fate (chemical half-lives in various media and biota, partition coefficients, fish bioconcentration), acute fish ecotoxicity, and chemical import or manufacture volume in Canada were collected from scientific literature, from available empirical databases (e. g., OECD QSAR Toolbox), and from responses to surveys under section 71 of CEPA, or they were generated using selected quantitative structure-activity relationship (QSAR) or mass-balance fate and bioaccumulation models. These data were used either as inputs to other mass-balance models or to complete the substance hazard and exposure profiles.
Hazard profiles were established based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Exposure profiles were also composed of multiple metrics, including potential emission rate, overall persistence and long-range transport potential. Hazard and exposure profiles were compared to decision criteria in order to classify the hazard and exposure potentials for each organic substance as low, moderate or high. Additional rules were applied (e. g., classification consistency, margin of exposure) to refine the preliminary classifications of hazard or exposure.
A risk matrix was used to assign a low, moderate or high classification of potential risk for each substance based on its hazard and exposure classifications. ERC classifications of potential risk were verified using a two-step approach. The first step adjusted the risk classification outcomes from moderate or high to low for substances that had a low estimated rate of emission to water after wastewater treatment, representing a low potential for exposure. The second step reviewed low risk potential classification outcomes using relatively conservative, local-scale (i. e., in the area immediately surrounding a point-source of discharge) risk scenarios, designed to be protective of the environment, to determine whether the classification of potential risk should be increased.
ERC uses a weighted approach to minimize the potential for both over and under classification of hazard and exposure and subsequent risk. The balanced approaches for dealing with uncertainties are described in greater detail in ECCC 2016a. The following describes two of the more substantial areas of uncertainty. Error in empirical or modeled acute toxicity values could result in changes in classification of hazard, particularly metrics relying on tissue residue values (i. e., mode of toxic action), many of which are predicted values from QSAR models. However, the impact of this error is mitigated by the fact that overestimation of median lethality will result in a conservative (protective) tissue residue value used for critical body residue (CBR) analysis. Error of underestimation of acute toxicity will be mitigated through the use of other hazard metrics such as structural profiling of mode of action, reactivity and/or estrogen binding affinity. Changes or errors in chemical quantity could result in differences in classification of exposure as the exposure and risk classifications are highly sensitive to emission rate and use quantity. The ERC classifications thus reflect exposure and risk in Canada based on what is believed to be the current use quantity and may not reflect future trends.
Critical data and considerations used to develop the substance-specific profiles for the four substances in the Alkyl Sulfates and α-Olefin Sulfonate Group and the hazard, exposure and risk classification results are presented in ECCC (2016b).
The hazard and exposure classifications for the four substances in the Alkyl Sulfates and α-Olefin Sulfonate Group are summarized in Table 5-1.
| CAS RN | ERC hazard classification | ERC exposure classification | ERC risk classification |
|---|---|---|---|
| TEA lauryl sulfate | low | low | moderate (based on potential for local-scale exposure) |
| Sodium lauryl sulfate | moderate | low | moderate (based on potential for local-scale exposure) |
| Ammonium lauryl sulfate | low | low | low |
| Sodium C14-16 olefin sulfonate | high | low | moderate |
Sodium C14-16 olefin sulfonate was classified as having high hazard potential based on mode of toxic action (i. e. above baseline toxicity (above narcosis)) and tissue residue-based metrics, but low exposure potential. Sodium C14-16olefin sulfonate was classified as having a moderate potential for ecological risk. On the basis of low exposure potential, this substance is unlikely to result in concerns for organisms or the broader integrity of the environment in Canada.
Sodium lauryl sulfate was classified as having a moderate hazard potential based on internal toxicity threshold metrics. The substance has a low exposure potential based on current use patterns with greater potential for local-scale exposures. This substance was classified as having a moderate potential for ecological risk; however, on the basis of low exposure potential, this substance is unlikely to result in concerns for organisms or the broader integrity of the environment in Canada.
Based on low hazard and low exposure potential ammonium lauryl sulfate was classified as having a low potential for ecological risk. TEA lauryl sulfate also had a low hazard and low exposure potential based on current use patterns with greater potential for local-scale exposures; therefore, this substance was classified as having a moderate potential for ecological risk. Based on low exposure potentials, ammonium lauryl sulfate and TEA lauryl sulfate are unlikely to result in concerns for organisms or the broader integrity of the environment in Canada.
6. Potential to cause harm to human health
6.1 Exposure assessment
Environmental media
The lauryl sulfate salts and sodium C14-16 olefin sulfonate are not expected to be released to air given their very low vapour pressure and high water solubility. Volatilization would be negligible from either dry or moist soil surfaces or from surface waters given that they are found in a protonated form in the environment (OECD 2007).
These substances enter the environment primarily via wastewater as a result of their use in products available to consumers, including cosmetics and household cleaning products.
A number of monitoring studies are summarized for alkyl sulfates and α-olefin sulfonates in OECD (2007). Alkyl sulfates were measured in raw sewage, wastewater treatment plant effluent (below 10 µg/L), receiving surface waters (mostly below 5 µg/L, maximum of 10.2 µg/L) and their sediments (0.021 to 0.0035 mg/kg dry weight). α-Olefin sulfonates were monitored at seven locations of four rivers (median of 0.04 µg/L and 0.06 µg/L for C14-substances and sum of C14-C18 substances, respectively) near two Japanese metropolitans.
In a more recent study in the Faroe Islands, Iceland, and Greenland, sodium lauryl sulfate was measured in wastewater treatment plant influent (less than or equal to 0.0079 µg/L), effluent (less than or equal to 0.0056 µg/L) and sludge (210 to 3100 µg/kg dry weight), as well as in receiving waters (less than or equal to 4.1 × 10−3 µg/L) and sediment (less than or equal to 93 µg/kg dry weight); it was detected in 35 out of the 41 samples investigated (Huber et al. 2016).
Given the absence of Canadian-specific surface monitoring or drinking water data, a down-the-drain scenario, using the EAU Drinking Water Spreadsheet, was used to derive the concentration of the substances in surface water for potential ingestion through drinking water (Health Canada 2015). The values used to predict the concentration of the substances are provided in Table 6-1.
| Parameters | Lauryl sulfate salts | Sodium C14-16 olefin sulfonate |
|---|---|---|
| Canadian population | 34 755 634 | 34 755 634 |
| Total annual usage | 3 306 305 kg | 2 240 453 kg |
| Estimated removal by an activated sludge wastewater treatment plant | 94.5% (as a worst-case scenario) (OECD 2007) | 70% (as a worst-case scenario) (OECD 2007) |
On the basis of these input values, the surface water concentration for the lauryl sulfate salts and sodium C14-16 olefin sulfonate is estimated to be 2.62 × 10−3 and 9.67 × 10−3 mg/L, respectively, which results in intake estimates from drinking water of 2.79 × 10−4 mg/kg bw per day (infants 0-0.5 years) for lauryl sulfate salts and 1.03 × 10−3 mg/kg bw per day (infants 0-0.5 years) for sodium C14-16 olefin sulfonate.
These drinking water exposure estimates are considered conservative and are higher than those derived by the OECD (2007) and HERA (2002) for the alkyl sulfates, alkane sulfonates and α-olefin sulfonates group (2.0 × 10−4 mg/kg bw per day) and for C12 alkyl sulfates (4.52 × 10−5 mg/kg bw per day), respectively.
Food
Of the four substances in this group, only sodium lauryl sulfate would be expected to be found in food due to its use as an approved food additive and in the manufacture of certain food packaging materials. Although sodium lauryl sulfate is an approved food additive, it is permitted only in a limited number of foods and under conditions of use that are described by Health Canada's List of Permitted Food Additives With Other Generally Accepted Uses. Dietary exposure from these limited food additive uses is expected to be low. There are also a limited number of food processing aids that contain sodium lauryl sulfate; however, it is expected that such use would result in no or negligible residues in or on the finished food (personal communications, emails from Food Directorate, Health Canada, to Risk Management Bureau, Safe Environments Directorate, Health Canada, 2015; unreferenced).
Sodium lauryl sulfate has been identified as being used in the manufacture of a number of different types of food packaging materials in Canada; however, exposure from these uses is expected to be negligible (personal communications, emails from Food Directorate, Health Canada, to Risk Management Bureau, Safe Environments Directorate, Health Canada, 2015; unreferenced).
In Canada, all four substances within this group have been identified for use as components of incidental additives in products used in food processing plants, including hand treatments, cleaners, sanitizers and disinfectants, release agents, lubricants and odor control agents (personal communications, emails from Food Directorate, Health Canada, to Risk Management Bureau, Safe Environments Directorate, Health Canada, 2015; unreferenced).
Based on consideration of the above information, exposure of the general population to sodium lauryl sulfate through food in Canada is expected to be low.
Products available to consumers
Exposures to sodium lauryl sulfate, ammonium lauryl sulfate, TEA lauryl sulfate and sodium C14-16 olefin sulfonate were estimated for cleaning products, cosmetics, natural health products and non-prescription drugs. Only those scenarios that resulted in the highest exposure for each of the routes of exposure are presented in this section. Additional details of these exposure scenarios are summarized in Appendices A and B.
Exposure via the dermal route was evaluated for a number of products and estimates are provided in Table 6-2. Based on the studies described in section 6. 2 Health Effects Assessment, dermal absorption was assumed to be 1% for this group.
| Substance | Cosmetic scenario | Concentration (% w/w)[a] | Per application systemic exposure (mg/kg bw) | Daily systemic exposure (mg/kg bw per day) |
|---|---|---|---|---|
| Sodium lauryl sulfate | Body cream | 0.1 – 4 | 0.00062 – 0.025 | 0.00068 – 0.027 |
| Sodium lauryl sulfate | Hair perm/straightener | ≤ 10 | ≤ 0.11 | NA |
| Ammonium lauryl sulfate | Hair shampoo | ≤ 70 | ≤ 0.012 | ≤ 0.013 |
| Ammonium lauryl sulfate | Permanent hair dye (wash-in) | ≤ 30 | ≤ 0.42 | NA |
| TEA lauryl sulfate | Hair shampoo | ≤ 40 | ≤ 6.7 × 10−3 | ≤ 7.3 × 10−3 |
| Sodium C14-16 olefin sulfonate | Body cream | ≤ 10 | ≤ 0.062 | ≤ 0.068 |
NA, not applicable
a Concentrations are based on notifications submitted under the Cosmetic Regulations to Health Canada (2016 email from Consumer Product Safety Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada; unreferenced).
There is also potential for dermal exposure to sodium lauryl sulfate and sodium C14-16 olefin sulfonate from use of automotive care products and pet care products, and to TEA lauryl sulfate from use of pet care products. Such exposures are less than the exposures presented above.
Exposures via the oral route to ammonium lauryl sulfate, sodium lauryl sulfate and sodium C14-16 olefin sulfonate were evaluated for a number of products; estimates are provided below.
Oral exposure to ammonium lauryl sulfate and sodium C14-16 olefin sulfonate from dish detergent left as residue on dinnerware was estimated. The estimates for this oral exposure to ammonium lauryl sulfate (5%) ranged from 2.96 × 10−4 mg/kg bw per day (for adults) to 1. 35 × 10−3 mg/kg bw per day (for toddlers), while estimates for sodium C14-16 olefin sulfonate (10%) ranged from 5.92 × 10−4 mg/kg bw per day (for adults) to 2.71 × 10−3 mg/kg bw per day (for toddlers).
Potential oral exposures to sodium lauryl sulfate were estimated for non-prescription drugs and natural health products formulated as capsules/tablets and toothpastes for adults, children and/or toddlers.
Estimates for non-prescription drugs range from 0.106 to 0.21 mg/kg bw for adults and from 0.035 to 0.14 mg/kg bw for children. The ranges provided correspond to taking one capsule/tablet a day to the maximum recommended daily dose (personal communication, emails from the Risk Management Bureau, Safe Environments Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated June 16, 2015; unreferenced).
Estimates for natural health products formulated as capsules/tablets were 0.21 mg/kg bw per day and 0.48 mg/kg bw per day for adults and children, respectively. These estimates correspond to taking the maximum recommended daily dose of the capsules/tablets containing the highest concentration of sodium lauryl sulfate available to the general population of Canada (LNHPD [modified 2016], personal communication, emails from Health Products and Food Branch, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated May 2016; unreferenced).
Sodium lauryl sulfate is found in a number of toothpastes, including brands specifically for children and toddlers (Household Product Database 1993-, MSDS 2015k,l,m, LNHPD [modified 2016]). The maximum concentration in child- and toddler-specific toothpastes is 2% (MSDS 2015k), which corresponds to an estimated intake of 0.77 mg/kg bw per day in toddlers.
Exposure via inhalation was also considered for some products, but was not considered significant relative to other routes. Estimates of inhalation exposure from use of cleaning products with a trigger spray and from use of certain cosmetics are presented in Table 6-3.
| Substance | Exposure scenario | Concentration (% w/w)[a] | Per application exposure (mg/kg bw) | Daily exposure (mg/kg bw per day) |
|---|---|---|---|---|
| Sodium lauryl sulfate | All purpose cleaner | 5 | 2.39 × 10−3 | 2.38 × 10−3 |
| Ammonium lauryl sulfate | Glass cleaner spray | 3 | 2.06 × 10−3 | NA |
| Sodium C14-16 olefin sulfonate | Bathroom cleaning spray | 5 | 9.11 × 10−3 | NA |
| Sodium C14-16 olefin sulfonate | Hairspray | ≤ 0.3 | NA | ≤ 1.73 × 10−3 |
NA, not applicable
6.2 Health effects assessment
OECD (2007) summarizes the health effects literature related to TEA lauryl sulfate, sodium lauryl sulfate, ammonium lauryl sulfate and sodium C14-16 olefin sulfonate (CAS RNs 151-21-3, 2235-54-3, 139-96-8 and 68439-57-6, respectively) [as part of the alkyl sulfates, alkane sulfonates and α-olefin sulfonates category]. Additional studies on sodium C14-16 olefin sulfonate were identified in Health Canada's proposed regulatory decision document on EXIT ISP (PMRA 2005). OECD (2007) and PMRA (2005) were largely used to inform the hazard section of this screening assessment, including selection of effect levels for critical endpoints [i. e., no observed adverse effects level (NOAELs) and/or lowest observed adverse effects level (LOAELs)].
A literature search was conducted from the year prior to the OECD (2007) to 2015, and no additional health effects studies, which could result in points of departure lower than those identified by OECD (2007) and PMRA (2005), were identified.
Based on structural similarity and the subgroupings used in OECD (2007), TEA lauryl sulfate, sodium lauryl sulfate, and ammonium lauryl sulfate were sub-grouped and were used for read-across. Under environmental conditions, the surfactant (i. e., lauryl sulfate) and counter-ion (i. e., Na+, NH4+, or TEA+) are expected to dissociate; the latter has very low systemic toxicity and is not expected to affect the chemical reactivity and hazard classification for the purpose of this screening assessment (OECD 2007, AGDH 2015). Sodium C14-16 olefin sulfonate was addressed separately.
No inhalation studies were identified for any of the relevant toxicological endpoints.
The substances in this group are well absorbed after ingestion, but absorption through intact skin is poor, which is consistent with the ability of anionic surfactants to bind to the skin surface (OECD 2007, PMRA 2005).
Dermal absorption of sodium lauryl sulfate is reported to be 0.5% or less in in vivo studies conducted in guinea pigs and rats, while early studies with isolated human skin was unable to detect dermal penetration (OECD 1995, 2007). Human skin was also reported to be three times less permeable than rat skin in in vitro studies, and transdermal penetration was only apparent after prolonged exposure, which was considered to be related to the substance's irritating effects (OECD 1995).
Although no CAS RN-specific dermal absorption data was identified for sodium C14-16 olefin sulfonate, studies identified for another α-olefin sulfonate (CAS RN 30965-85-6) reported a dermal absorption of 0.6% or less. However, dermal absorption increased to 50% when the stratum corneum was removed (Minegishi et al. 1977).
Following absorption, these substances are distributed mainly to the liver and are subsequently metabolized by cytochrome P450. The metabolites are then rapidly excreted in the urine; excretion of the lauryl sulfate salts is complete within 6 hours of oral exposure, while α-olefin sulfonates were rapidly eliminated from the whole body within 24 hours (OECD 2007).
Acute oral toxicity studies for certain substances in the Alkyl Sulfates and α-Olefin Sulfonate Group reported acute toxicity values ranging from slight to moderate (OECD 2007).
Acute dermal toxicity studies for certain substances in the Alkyl Sulfates and α-Olefin Sulfonate Group reported acute toxicity values ranging from low to moderate. There is evidence that acute toxicity increases when skin is abraded (OECD 2007).
Substances within this group can cause skin irritation.
Sodium lauryl sulfate is considered the most irritating alkyl sulfate. It is moderately to severely irritating in rabbits under semi-occlusive and occlusive conditions when tested up to a concentration of 25%. In contrast, it is only moderately irritating in humans at a concentration of 20% under occlusive conditions, and OECD (2007) considers 20% as the threshold concentration for irritative effects of alkyl sulfates in humans. Different counter-ions did not significantly influence the degree of skin irritation.
Sodium C14-16 olefin sulfonate was irritating to the skin of rabbits when tested at a concentration of 40% according to OECD Test Guideline 404.
When 0, 40, 200, 1000 or 5000 ppm (corresponding to 0, 3, 17, 86, 430 mg/kg bw per day) of sodium lauryl sulfate was administered in the diet of rats for 90 days, a NOAEL of 86 mg/kg bw per day and a LOAEL of 430 mg/kg bw per day were established based on increases in absolute liver weights in females. No other effects were observed at the LOAEL (Walker et al. 1967). A similar NOAEL of 90 mg/kg bw per day was established when sodium lauryl sulfate was administered by gavage in a 28-day rat study (Henkel KGaA 1987). Although gastrointestinal irritation was the primary effect following administration of the substance by gavage, the liver is considered the only target organ of systemic toxicity for alkyl sulfates with chain lengths between C12 and C18 following oral administration. The gastrointestinal effects are confined to the gavage route of exposure and are consistent with the primary irritant properties of alkyl sulfates and the bolus effect after gavage administration (OECD 2007).
Two 90-day oral studies were identified for sodium C14-16 olefin sulfonate, where rats were exposed to 0, 40, 200 or 1000 mg/kg bw per day or 0, 50, 150 or 500 mg/kg bw per day from the diet. For both studies, the highest dose tested was considered the NOAEL (PMRA 2005). In a more comprehensive study, male and female rats were exposed to 0, 1000, 2500 or 5000 ppm (corresponding to 0, 39-57, 96-132 or 195-259 mg/kg bw per day) of sodium C14-16 olefin sulfonate in their diet for two years. The high dose was considered the NOAEL. At the NOAEL, a slight reduction in food intake (females) and a transient, but significant reduction in body weight gain (both sexes) between weeks 14 and 26 of the study were observed (Lion Co. 1975, Hunter and Benson 1976).
No dermal repeated-dose study was identified for the lauryl sulfate salts in this group.
Consistent with the poor dermal absorption reported for sodium C14-16 olefin sulfonate, no adverse effects were reported in rats or mice dermally exposed to the substance for up to two years or in mice dermally exposed to the substance during gestational day 0 to 14 (PMRA 2005). Similarly, only mild to moderate skin irritation was observed when rabbits were dermally exposed to 100 mg/kg bw per day of sodium C14-16 olefin sulfonate for 90 days (PMRA 2005).
Substances in this group are not considered to be carcinogenic or genotoxic (OECD 2007).
No adverse effects on reproduction were identified for the substances in this group (OECD 2007).
Potential effects on development were investigated in several studies (OECD 2007). Developmental effects were not observed in the absence of maternal toxicity.
6.3 Characterization of risk to human health
No evidence for carcinogenicity or genotoxicity was observed in the available empirical data for the lauryl sulfate salts or sodium C14-16 olefin sulfonate. Therefore, characterization of risk in this screening assessment is based on non-cancer effects.
The 13-week rat diet study for sodium lauryl sulfate was identified as the most relevant study for risk characterization for both acute and daily exposure to the lauryl sulfate salts. A NOAEL of 86 mg/kg bw per day was derived based on an increase in absolute liver weight in females at the LOAEL of 430 mg/kg bw per day (Walker et al., 1967). The critical effect levels are in the same range as those identified in a 4-week rat gavage study (NOAEL = 90 mg/kg bw per day, LOAEL = 270-540 mg/kg bw per day) for the same substance (Henkel KGaA 1987). They are also used to read-across to the other salts of lauryl sulfate (i. e., ammonium and TEA) given that the salts will dissociate in aqueous environments and that very low systemic toxicity is associated with the counter-ions (AGDH 2015).
For sodium C14-16 olefin sulfonate, the two-year rat diet study was identified as the most appropriate study for risk characterization for both acute and daily exposure. The highest dose, 195 mg/kg bw per day, was considered the study's NOAEL. A slight reduction in food intake (in females only) and a transient, but significant reduction in body weight gain (in both sexes) between weeks 14 and 26 of the study was observed at this dose (Lion Co. 1975, Hunter and Benson 1976). This NOAEL is also considered protective of the developmental effects (i. e., cleft palate) observed in mice at 300 mg/kg bw per day. These effects were not observed in rats or rabbits (Palmer et al. 1975a,b).
The subchronic rat diet study for sodium lauryl sulfate and the chronic rat diet study for sodium C14-16 olefin sulfonate were used to characterize risk from both per event and daily exposure to the relevant substances. Although there are no studies for chronic durations for the lauryl sulfate salts, the substances are not expected to remain in the body due to the rapid excretion of the metabolites in urine (OECD 2007).
Tables 6-4, 6-5 and 6-6 provide the relevant estimates of exposure and effect levels for the lauryl sulfate salts and sodiumC14-16 olefin sulfonate, as well as the resulting margins of exposure (MOEs). In Table 6-4, exposure estimates and the critical effect level of 86 mg/kg bw per day were converted to lauryl sulfate equivalents because the critical effect level was based on a study using sodium lauryl sulfate.
| Substance(s) | Exposure scenario | Systemic exposure[a] | MOE(s) |
|---|---|---|---|
| Lauryl sulfate salts (i. e., sodium, ammonium and TEA) | Drinking water (daily, oral) | Infants: 2.56 × 10−4 mg/kg bw per day | 308 600 |
| TEA lauryl sulfate | Hair shampoo (daily, dermal) | Adults: ≤ 4.66 × 10−3 mg/kg bw per day | 16 950 |
| Ammonium lauryl sulfate | Oral exposure from dish detergent left as residue on dinnerware (daily, oral) | Toddlers: ≤ 1.26 × 10−3 mg/kg bw per day | 62 700 |
| Ammonium lauryl sulfate | Hair shampoo (daily, dermal) | Adults: ≤ 0.012 mg/kg bw per day | 6 580 |
| Ammonium lauryl sulfate | Permanent hair dye (wash-in) (acute, dermal) | Adults: ≤ 0.39 mg/kg bw | 200 |
| Ammonium lauryl sulfate | Bathroom cleaning spray (acute, inhalation) | Adults: 1.93 × 10−3 mg/kg bw | 40 900 |
a Converted to lauryl sulfate equivalents.
These MOEs are considered adequate to address uncertainties in the health effects and exposure databases.
Exposure scenario |
Estimated exposure | MOE(s) |
|---|---|---|
| Natural health products formulated as capsules/tablets (daily, oral) | Children: 0.48 mg/kg bw per day Adults: 0.21 mg/kg bw per day |
180 410 |
| Toothpaste (daily, oral) | Toddlers: ≤ 0.77 mg/kg bw per day | 110 |
| Non-prescription drugs formulated as capsules/tablets (acute, oral) | Adults: 0.106 to 0.21 mg/kg bw | 400 – 800 |
| Body cream (daily, dermal) | Adults: ≤ 0.027 mg/kg bw per day | 3 200 |
| Hair perm/straightener (acute, dermal) | Adults: ≤ 0.11 mg/kg bw | 860 |
| All purpose cleaner (acute, inhalation) | Adults: 2.39 × 10−3 mg/kg bw | 36 000 |
These MOEs are considered adequate to address uncertainties in the health effects and exposure databases.
| Exposure scenario | Estimated exposure | MOE(s) |
|---|---|---|
| Drinking water (daily, oral) | Infants: 1.03 × 10−3 mg/kg bw per day | 189 000 |
| Oral exposure from dish detergent left as a residue on dinnerware (daily, oral) | Toddlers: ≤ 2.71 × 10−3 mg/kg bw per day | 71 900 |
| Body cream (daily, dermal) | Adults: ≤ 0.068 mg/kg bw per day | 2 900 |
| Hairspray (daily, inhalation) | Adults: ≤ 1.73 × 10−3 mg/kg bw per day | 112 700 |
| Bathroom cleaning spray (acute, inhalation) | Adults: 9.11 × 10−3 mg/kg bw | 21 400 |
These MOEs are considered adequate to address uncertainties in the health effects and exposure databases.
The risk characterization for the lauryl sulfate salts is considered conservative for a number of reasons, including the fact that the critical effect level is based on a NOAEL rather than a LOAEL and that the critical effect is associated with low severity (i. e., increase in absolute liver weight in females at the LOAEL for sodium lauryl sulfate). Conservative default values and algorithms and use of maximum concentrations (e. g., maximum recommended daily dose of the capsules/tablets containing the highest concentration of sodium lauryl sulfate available to the general population of Canada) were used in estimating exposures. In addition, alkyl sulfates are usually used in conjunction with other surfactants when formulated in products available to consumers; these mixed surfactant systems form micelles that typically lead to a reduction in the irritation potential of the mixture, compared to the irritation potential of the individual ingredients (Dillarstone and Paye 1993, Effendy and Maibach 2006, Paye et al. 2006).
6.4 Uncertainties in evaluation of risk to human health
There is uncertainty in the estimated daily intakes of lauryl sulfate salts and sodium C14-16 olefin sulfonate from drinking water due to the absence of substance-specific monitoring studies and the lack of measured concentrations in Canadian surface water or drinking water. However, confidence is high that actual exposures to these substances in Canadian drinking water would be lower than the exposures estimated based on modelled concentrations in surface water.
Due to the lack of or limited health effects data for relevant routes and durations of exposure for the lauryl sulfate salts and sodium C14-16 olefin sulfonate, route-to-route extrapolation was required, and/or use of effect levels from studies with a longer or shorter duration of exposure than the exposure scenarios was applied. The resulting MOEs, however, were considered to be adequate to address these uncertainties.
7. Conclusion
Considering all available lines of evidence presented in this screening assessment, there is low risk of harm to organisms and the broader integrity of the environment from TEA lauryl sulfate, sodium lauryl sulfate, ammonium lauryl sulfate and sodium C14-16 olefin sulfonate. It is concluded that TEA lauryl sulfate, sodium lauryl sulfate, ammonium lauryl sulfate and sodium C14-16 olefin sulfonate do not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
Based on the information presented in this screening assessment, it is concluded that TEA lauryl sulfate, sodium lauryl sulfate, ammonium lauryl sulfate and sodium C14-16 olefin sulfonate do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
Therefore, it is concluded that TEA lauryl sulfate, sodium lauryl sulfate, ammonium lauryl sulfate and sodium C14-16 olefin sulfonate do not meet any of the criteria set out in section 64 of CEPA.
References
[AGDH] Australian Government Department of Health. 2015. Human health tier II assessment for sodium, ammonium and potassium lauryl sulfate. National Industrial Chemicals Notification and Assessment Scheme (NICNAS). Inventory multi-tiered assessment and prioritization. [accessed 2015 May]. http://www. nicnas. gov. au/chemical-information/imap-assessments/imap-group-assessment-report?assessment_id=184.
Canada. [1978]. Food and Drug Regulations. C. R. C. , c. 870. http://laws-lois. justice. gc. ca/eng/regulations/c. r. c. ,_c. _870/index. html.
Canada. 1999. Canadian Environmental Protection Act, 1999. S. C. 1999, c. 33. Canada Gazette Part III, vol. 22, no. 3. http://laws-lois. justice. gc. ca/eng/acts/C-15. 31/.
Canada, Dept. of the Environment. 2012. Canadian Environmental Protection Act, 1999: Notice with respect to certain substances on the Domestic Substances List. Canada Gazette, Part I, vol. 146, no. 48, Supplement. http://www. gazette. gc. ca/rp-pr/p1/2012/2012-12-01/pdf/g1-14648. pdf.
[ConsExpo] Consumer Exposure Model [Internet]. 2006. Version 4.1. Bilthoven (NL): Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment]. http://www. rivm. nl/en/healthanddisease/productsafety/ConsExpo. jsp#tcm:13-42840.
Dillarstone A, Paye M. 1993. Antagonism in concentrated surfactant systems. Contact Dermatitis. 28(3):198 [cited in OECD 2007].
[DPD] Drug Product Database [database]. [modified 2015 Jul 17]. Ottawa (ON): Health Canada. [accessed 2015 Oct]. http://webprod5. hc-sc. gc. ca/dpd-bdpp/index-eng. jsp.
Dreger EE, Klein G, Miles G, Shedlovsky L, Ross J. 1944. Sodium Alcohol Sulfates. Properties Involving Surface Activity. Ind Eng Chem. 36(7):610–617. [cited in ECHA 2015].
[ECCC] Environment and Climate Change Canada. 2016a. Ecological Science Approach: Ecological Risk Classification of Organic Substances. http://www.canada. ca/ese-ees/default. asp?lang=En&n=A96E2E98-1 .
[ECCC] Environment and Climate Change Canada. 2016b. Gatineau (QC): Data used to create substance-specific hazard and exposure profiles and assign risk classifications in the Ecological Risk Classification of organic substances. Gatineau (QC). Available from: eccc. substances. eccc@canada. ca.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. [modified 2007 Apr 20]. Categorization. Ottawa (ON): Government of Canada. [accessed 2016 Jan 20]. https://www.canada.ca/en/health-canada/services/chemical-substances/canada-approach-chemicals/categorization-chemical-substances. html.
[ECHA] European Chemicals Agency. c2007-2015. Registered substances database; search results for CAS RN xxxx-xx-x. Helsinki (FI): ECHA. [accessed 2015 Oct]. http://echa. europa. eu/web/guest/information-on-chemicals/registered-substances.
Effendy I, Maibach HI. 2006. Detergents. In: Chew A, Maibach HI, editors. Irritant Dermatitis. Berlin (DE): Springer-Verlag. p. 249–256 [cited in OECD 2007].
Environment Canada. 2013. DSL Inventory Update data collected under the Canadian Environmental Protection Act, 1999, section71: Notice with respect to certain substances on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
[EPI Suite] Estimation Programs Interface Suite for Microsoft Windows [Estimation Model]. c2000-2010. Version 4. 10. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. http://www. epa. gov/oppt/exposure/pubs/episuite. htm.
[EMA] European Medicines Agency. 2015. Background review for sodium laurilsulfate used as an excipient. London (UK): Committee for Human Medicinal Products. [accessed 2015 Dec]. http://www. ema. europa. eu/docs/en_GB/document_library/Report/2015/08/WC500191475. pdf.
Health Canada. 1998. Exposure factors for assessing total daily intake of priority substances by the general population of Canada. Unpublished report. Ottawa (ON): Health Canada, Environmental Health Directorate.
Health Canada. 2015. Environmental Assessment Unit Drinking Water Spreadsheets [Excel format]. Ottawa (ON): Health Canada. [cited 2016 Apr 8].
Henkel KGaA. 1987. Texapon K 12: 28-Tage-Test mit wiederholter oraler Verabreichung an Ratten. Dusseldorf (DE): Henkel KGaA. 67 p. Report No. 870121.
[HERA] Human and Environmental Risk Assessment on ingredients of European household cleaning products. 2002. Alkyl sulfates. Environmental risk assessment. [accessed 2015 Dec]. http://www. heraproject. com/files/3-E-417F36A9-DB35-F780-97A4CF8B60763C35. pdf.
Household Products Database [database]. 1993-. Bethesda (MD): US National Library of Medicine. [updated 2015 Aug; accessed 2016 Jul 18]. http://www. householdproducts. nlm. nih. gov/.
Huber S, Remberger M, Kaj L, Schlabach M, Jörundsdóttir HÓ, Vester J, Arnórsson M, Mortensen I, Schwartson R. Dam M. 2016. A first screening and risk assessment of pharmaceuticals and additives in personal care products in waste water, sludge, recipient water and sediment from Faroe Islands, Iceland and Greenland. Sci Total Environ. 562:13–25.
Hunter B, Benson HG. 1976. Long-term toxicity of the surfactant alpha-olefin sulphonate (AOS) in the rat. Toxicology. 5(3):359–370.
Lide DR, editor. 2005. CRC Handbook of Chemistry and Physics. 86th ed. Boca Raton (FL): CRC Press.
Lion Co. 1975. AOS toxicity following dietary administration to rats for two years. Huntingdon/Cambridgeshire (UK): Huntingdon Research Centre. 56 p. Report No. : LFO14/74987.
[LNHPD] Licensed Natural Health Products Database [database]. [modified 2016 Aug 10] Ottawa (ON): Health Canada. [accessed 2016 Aug]. https://health-products. canada. ca/lnhpd-bdpsnh/index-eng. jsp.
Loretz LG, Api AM, Barraj LM, Burdick J, Dressler WE, Gettings SD, Han Hsu H, Pan YHL, Re TA, Renskers KJ, Rothenstein A, Scrafford CG, Sewall C. 2005. Exposure data for cosmetic products: lipstick, body lotion, and face cream. Food Chem Toxicol. 43:279–291.
Minegishi KI, Osawa M, Yamaha T. 1977. Percutaneous absorption of alpha-olefin sulfonate (AOS) in rats. Chem Pharm Bull. 25(4):821–825.
Moore AF. 1983. Final report on the safety assessment of sodium lauryl sulfate and ammonium lauryl sulfate. J Am Coll Toxicol. 2(7):127–181.
[MSDS] Material Safety Data Sheet. 1996. Aluminum Metal Polish [Internet]. Mullins (SC): Dihoma Chemical Manufacturing, Inc. [accessed 2015 Dec 14]. http://hazard. com/msds/f2/ccj/ccjsl. html.
[MSDS] Material Safety Data Sheet. 2006a. Lysol Brand Powerons Gel Toilet Bowl Cleaner, All Scents [Internet]. Toronto (ON): Reckitt Benckiser (Canada) Inc. http://msds. canadiantire. ca.
[MSDS] Material Safety Data Sheet. 2006b. Iron Out Automatic Toilet Bowl Cleaner [Internet]. Fort Wayne (IN): Iron Out dba Summit Brands. [accessed 2015 Dec 14]. http://msds. walmartstores. com
[MSDS] Material Safety Data Sheet. 2006c. Medicated Fade Creme [Internet]. North Hellman (CA): Clear Essence Cosmetics USA. [accessed 2015 Dec 14]. http://msds. walmartstores. com
[MSDS] Material Safety Data Sheet. 2006d. Skin Beautifying Milk [Internet]. North Hellman (CA): Clear Essences Cosmetics USA. [accessed 2015 Dec 14]. http://msds. walmartstores. com
[MSDS] Material Safety Data Sheet. 2006e. Body Lotion/Hand Cream [Internet]. New York (NY): Enchante Accessories, Inc. [accessed 2015 Dec 14]. http://msds. walmartstores. com
[MSDS] Material Safety Data Sheet. 2006f. Body Scrub [Internet]. New York (NY): Enchante Accessories, Inc. [accessed 2015 Dec 14]. http://msds. walmartstores. com
[MSDS] Material Safety Data Sheet. 2006g. Queen Helene Apricot Hand and Body Lotion [Internet]. Hempstead (NY): Para Laboratories. [accessed 2015 Dec 14]. http://msds. walmartstores. com
[MSDS] Material Safety Data Sheet. 2006h. Clearasil Acne Control Deep Cleansing Scrub 150 ml/200 ml tube [Internet]. Beeston (UK): Reckitt Benckiser. [accessed 2015 Dec 14]. http://msds. walmartstores. com
[MSDS] Material Safety Data Sheet. 2007a. Fast Orange Wipes 30 CT Bucket [Internet]. Milton (CA): Permatex Canada, Inc. [accessed 2015 Dec 14]. http://msds. homehardware. ca
[MSDS] Material Safety Data Sheet. 2007b. Hero Liquid Soap with Aloe and Vitamin E [Internet]. Toronto (CA): Canadian Tire Corporation. [accessed 2015 Dec 14]. http://msds. canadiantire. ca
[MSDS] Material Safety Data Sheet. 2007c. DiDi Seven Ultra [Internet]. Toronto (CA): Interwood Marketing Group [accessed 2015 Dec 14]. http://msds. canadiantire. ca
[MSDS] Material Safety Data Sheet. 2007d. Aveeno Clear Complexion Daily Cleansing Pads [Internet]. Skillman (NJ): Wildlife Research Center, Inc. [accessed 2015 Dec 14]. http://msds. walmartstores. com
[MSDS] Material Safety Data Sheet. 2007e. Barbasol: Original [Internet]. Dublin (OH): Perio, Inc. [accessed 2015 Dec 14]. http://msds. walmartstores. com
[MSDS] Material Safety Data Sheet. 2007f. Lactovit Bath Gel [Internet]. San Juan (PR): Puerto Rico Supplies Group Inc. [accessed 2015 Dec 14]. http://msds. walmartstores. com
[MSDS] Material Safety Data Sheet. 2007g. Get Groomed Dog Beauty Products Dog Shampoo [Internet]. San Francisco (CA): SimplyShe, Inc. [accessed 2015 Dec 14]. http://msds. walmartstores. com
[MSDS] Material Safety Data Sheet. 2008a. Cosrich Bubbling Bath Fizzie [Internet]. Bloomfield (NJ): Cosrich Group, Inc. [accessed 2015 Dec 14]. http://msds. walmartstores. com
[MSDS] Material Safety Data Sheet. 2008b. Baby Body Wash & Shampoo [Internet]. Durham (NC): Secco Technologies, LLC. [accessed 2015 Dec 14]. MSDS previously obtained at http://msds. walmartstores. com/.
[MSDS] Material Safety Data Sheet. 2009. SBTC Nutritive Body Cleanser-All Fragrances [Internet]. Dallas (TX): Delicious Brands LLC. [accessed 2015 Dec 14]. MSDS previously obtained at http://msds. walmartstores. com/.
[MSDS] Material Safety Data Sheet. 2010a. Natural 2X Concentrated Laundry Detergent [Internet]. Burlington (VT): Seventh Generation, Inc. [accessed 2015 Dec 14]. http://msds. canadiantire. ca
[MSDS] Material Safety Data Sheet. 2010b. Natural Dish Liquid [Internet]. Burlington (VT): Seventh Generation, Inc. [accessed 2015 Dec 14]. http://msds. canadiantire. ca
[MSDS] Material Safety Data Sheet. 2010c. Case Tri pack Facial Aromasense Spa [Internet]. Cali (CO): Belleza Express S. A. [accessed 2015 Dec 14]. MSDS previously obtained at http://msds. walmartstores. com/
[MSDS] Material Safety Data Sheet. 2010d. Mirtha's Milk Shampoo 8 oz [Internet]. Miami (FL): Mirta de Perales, Inc. [accessed 2015 Dec 14]. http://msds. walmartstores. com
[MSDS] Material Safety Data Sheet. 2010e. Soap [Internet]. New York (NY): Townley, Inc. [accessed 2015 Dec 14]. MSDS previously obtained at http://msds. walmartstores. com/.
[MSDS] Material Safety Data Sheet. 2010f. Prell Original Rinse Clean Shampoo [Internet]. King of Prussia (PA): Ultimark Products, LLC. [accessed 2015 Dec 14]. http://msds. walmartstores. com
[MSDS] Material Safety Data Sheet. 2010g. HairSil Accelerator Shampoo [Internet]. Clinton (MI): Universal Products. [accessed 2015 Dec 14]. http://msds. walmartstores. com
[MSDS] Material Safety Data Sheet. 2011. pHisoderm 6 0z. Anti-blemish Gel Facial Wash [Internet]. Orchard Park (NY): The Mentholatum Company. [accessed 2015 Dec 14]. http://msds. walmartstores. com
[MSDS] Material Safety Data Sheet. 2012a. Homex [Internet]. Fergus (CA): CP Industries Ltd. [accessed 2015 Dec 14]. http://msds. homehardware. ca
[MSDS] Material Safety Data Sheet. 2012b. Oven Cleaner [Internet]. Ningbo (CN): Ningbo Rejoice I/E Co. , Ltd. [accessed 2015 Dec 14]. http://msds. homehardware. ca
[MSDS] Material Safety Data Sheet. 2012c. Hartz Maximum Protection Stain & Odor Remover [Internet]. Secaucus, (NJ): The Hartz Mountain Corporation. http://msds. walmartstores. com
[MSDS] Material Safety Data Sheet. 2012d. Softsoap Liquid Hand Soap Pump Aloe Vera [Internet]. Toronto (CA): Colgate-Palmolive Canada Inc. [accessed 2015 Dec 14]. http://msds. homehardware. ca
[MSDS] Material Safety Data Sheet. 2013a. Klear-Glass Cleaning Solution. Toronto (CA): Klear-Glass of Canada, Ltd. [accessed 2015 Dec 14]. http://msds. canadiantire. ca
[MSDS] Material Safety Data Sheet. 2013b. Bref Toilet Care [Internet]. Scottsdale (AZ): The Dial Corporation. [accessed 2015 Dec 14]. http://msds. canadiantire. ca
[MSDS] Material Safety Data Sheet. 2013c. Simple Green Scrubbing Pad [Internet]. Huntington Beach (CA): Sunshine Makers, Inc. [accessed 2015 Dec 14]. https://hecsb. hres. ca/msds/ [restricted access].
[MSDS] Material Safety Data Sheet. 2013d. Ammonia-Free Glass Cleaner Concentrate [Internet]. Atlanta (GA): Zep Commercial Sales & Service. [accessed 2015 Dec 14]. http://msds. canadiantire. ca
[MSDS] Material Safety Data Sheet. 2013e. Tide To Go Stain Pen [Internet]. Cincinnati (OH): Proctor & Gamble Company. [accessed 2015 Dec 14]. http://msds. homehardware. ca
[MSDS] Material Safety Data Sheet. 2014a. Ad Jewelry Cleaner Concentrate [Internet]. Woburn (MA): Connoisseurs Products Corporation. [accessed 2015 Dec 14]. MSDS previously obtained at http://msds. walmartstores. com/.
[MSDS] Material Safety Data Sheet. 2014b. Simple Green Naturals Multi-Surface Care [Internet]. Huntington Beach (CA): Sunshine Makers Inc. [accessed 2015 Dec 14]. http://msds. walmartstores. com
[MSDS] Material Safety Data Sheet. 2014c. Lemon Suds [Internet]. Acheson (AB): Sci-Tech Inc. [accessed 2015 Dec 14]. http://msds. homehardware. ca
[MSDS] Material Safety Data Sheet. 2014d. Pert Plus 2 in 1 Shampoo & Conditioner Classic Clean [Internet]. El Paso (TX): Wildlife Research Center, Inc. [accessed 2015 Dec 14]. http://msds. walmartstores. com
[MSDS] Material Safety Data Sheet. 2014e. SkinMilk Foaming Bath [Internet]. El Paso (TX): Idelle Labs Ltd. [accessed 2015 Dec 14]. MSDS previously obtained at http://msds. walmartstores. com/.
[MSDS] Material Safety Data Sheet. 2015a. Glisten Disposer Care [Internet]. Fort Wayne (IN): Iron Out dba Summit Brands. [accessed 2015 Dec 14]. http://msds. walmartstores. com
[MSDS] Material Safety Data Sheet. 2015b. Comet Cleaner with Bleach Ready to Use [Internet]. Cincinnati (OH): Procter & Gamble Professional. [accessed 2015 Dec 14]. http://msds. walmartstores. com
[MSDS] Material Safety Data Sheet. 2015c. Lysol No Mess Automatic Toilet Bowl Cleaner Lavender [Internet]. Parsippany (NJ): Rechitt Benckiser LLC. [accessed 2015 Dec 14]. http://msds. homehardware. ca
[MSDS] Material Safety Data Sheet. 2015d. Simple Green Carpet Cleaner [Internet]. Huntington Beach (CA): Sunshine Makers Inc. [accessed 2015 Dec 14]. http://msds. walmartstores. com
[MSDS] Material Safety Data Sheet. 2015e. Simple Green Glass Cleaner [Internet]. Huntington Beach (CA): Sunshine Makers Inc. [accessed 2015 Dec 14]. http://msds. walmartstores. com
[MSDS] Material Safety Data Sheet. 2015f. Hunter Specialties/Scent-a-way Clean Rinse Laundry Detergent Fragrance Free [Internet]. Cedar Rapids (IA): Hunter's Specialties. MSDS previously obtained at http://msds. walmartstores. com/.
[MSDS] Material Safety Data Sheet. 2015g. Big Sexy Hair Shampoo [Internet]. Glendale (NY): Primary One, LLC. [accessed 2015 Dec 14]. http://msds. walmartstores. com
[MSDS] Material Safety Data Sheet. 2015h. L'Oreal Shampoo [Internet]. Glendale (NY): Primary One, LLC. [accessed 2015 Dec 14]. MSDS previously obtained at http://msds. walmartstores. com/.
[MSDS] Material Safety Data Sheet. 2015i. Organic Root Stimulator Uplifting Shampoo [Internet]. Chicago (IL): Namaste Laboratories LLC. [accessed 2015 Dec 14]. http://msds. walmartstores. com
[MSDS] Material Safety Data Sheet. 2015j. Dove Beauty Bars Gentle Exfoliating [Internet]. Englewood Cliffs (NJ): Unilever. [accessed 2015 Dec 14]. MSDS previously obtained at http://msds. walmartstores. com/.
[MSDS] Material Safety Data Sheet. 2015k. Oral-B Stages Toothpaste Princess [Internet]. Mason (OH): Procter & Gamble Company. [accessed 2015 Dec 14]. http://msds. walmartstores. com
[MSDS] Material Safety Data Sheet. 2015l. Colgate Max Fresh Toothpaste: Clear Mint [Internet]. New York (NY): Colgate-Palmolive Co. [accessed 2015 Dec 14]. http://msds. walmartstores. com
[MSDS] Material Safety Data Sheet. 2015m. Aquafresh Toothpaste [Internet]. Research Triangle Park (NC): GlaxoSmithKline. [accessed 2015 Dec 14]. http://msds. walmartstores. com
[MSDS] Material Safety Data Sheet. Date unknown. LCD Lens Cleaning Kit [Internet]. New York (NY): Merkury Innovations LLC. [accessed 2015 Dec 14]. MSDS previously obtained at http://msds. walmartstores. com/.
[NHPID] Natural Health Products Ingredients Database [Internet]. [modified 2016 Apr 18]. Ottawa (ON): Health Canada. [accessed 2016 Aug]. http://webprod. hc-sc. gc. ca/nhpid-bdipsn/search-rechercheReq. do?lang=eng.
[OECD] Organisation for Economic Co-operation and Development. 1995. SIDS Initial Assessment Report for: Sodium dodecyl sulfate. SIDS Initial Assessment Meeting; 28-30 October 1995. http://webnet. oecd. org/HPV/UI/SIDS_Details. aspx?key=f8440838-0e76-4ce5-95a9-c4ca07b90603&idx=0.
[OECD] Organisation for Economic Co-operation and Development. 2007. SIDS Initial Assessment Report for: Category of alkyl sulfates, alkane sulfonates and α-olefin sulfonates. SIDS Initial Assessment Meeting. Bonn (DE): United Nations Environment Programme (UNEP). [updated 2007 Oct 19, accessed 2015 May 5]. http://webnet. oecd. org/hpv/ui/SIDS_Details. aspx?id=f831fcb3-cdc0-458a-ad43-3226107998b0.
Paye M, Block C, Hamaide N, Hüttmann GE, Kirkwood S, Lally C, Lloyd PH, Makela P, Razenberg H, Young R. 2006. Antagonisms between surfactants: The case of laundry detergents. Tenside Surfactants Detergents. 43:290–294. [cited in OECD 2007].
[PMRA] Pest Management Regulatory Agency. 2006. Proposed Regulatory Decision Document PRDD2005-04. EXITTM ISP. Ottawa (ON): Health Canada, PMRA. Cat. No. : H113-9/2005-4E. [accessed 2016 Apr 08]. http://publications. gc. ca/collections/Collection/H113-9-2005-4E. pdf.
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu. 2006a. Cleaning products fact sheet: To assess the risks for the consumer: updated version for ConsExpo 4. Bilthoven (NL): RIVM. Report No. : 320104003/2006. http://www. rivm. nl/bibliotheek/rapporten/320104003. pdf.
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment (NL)]. 2006b. Cosmetics fact sheet: To assess the risks for the consumer: updated version for ConsExpo 4. Bilthoven (NL): RIVM. Report No. : 320104001/2006. http://www. rivm. nl/bibliotheek/rapporten/320104001. pdf.
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment (NL)]. 2007. Paint products fact sheet: To assess the risks for the consumer: updated version for ConsExpo 4. Bilthoven (NL): RIVM. Report No. : 320104008/2007. http://www. rivm. nl/bibliotheek/rapporten/320104008. pdf.
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment (NL)]. 2009. The ConsExpo spray model. Modeling and experimental validation of the inhalation exposure of consumers to aerosols from spray cans and trigger sprays. Bilthoven (NL): RIVM. RIVM Report 320104005/2009. http://www. rivm. nl/dsresource?objectid=1d347421-99e3-4d32-a685-6d3a70a59b1c&type=org&disposition=inline.
[SCCS] Scientific Committee on Consumer Safety. 2011. The SCCS's notes of guidance for the testing of cosmetic ingredients and their safety evaluation. 7th Revision. [Internet]. Scientific Committee on Consumer Safety. [accessed 27 Jul 2012]. http://ec. europa. eu/health/scientific_committees/consumer_safety/docs/sccs_s_004. pdf.
Statistics Canada. 2012. Canadian Health Measures Survey (CHMS). Cycle 2. 2009-2011. http://www23. statcan. gc. ca/imdb-bmdi/pub/instrument/5071_Q2_V2-eng. htm.
[US EPA] United States Environmental Protection Agency. 2006. Inert reassessment decision document for alkyl sulfates. Washington (DC): US EPA, Office of Prevention, Pesticides and Toxic Substances. [accessed 2016 Jan 18]. http://www. epa. gov/sites/production/files/2015-04/documents/alkyl. pdf.
Walker AIT, Brown VKH, Ferrigan LW, Pickering RG, Williams DA. 1967. Toxicity of sodium lauryl sulphate, sodium lauryl ethoxysulphate and corresponding surfactants derived from synthetic alcohols. Food Cosmet Toxicol. 5:763–769.
Wibbertmann A, Mangelsdorf I, Gamon K, Sedlak R. 2011. Toxicological properties and risk assessment of the anionic surfactants category: Alkyl sulfates, primary alkane sulfonates, and α-olefin sulfonates. Ecotoxicol Environ Saf. 74(5):1089–106.
Wu X, Bennett DH, Ritz B, Cassady DL, Lee K, Hertz-Picciotto I. 2010. Usage pattern of personal care products in California households. Food Chem Toxicol. 48:3109–3119.
Footnotes
Appendix A. Dermal and inhalation exposures from cosmetics and cleaning products
Exposures were estimated for different age groups based on body weights from Health Canada's exposure factors for the general population of Canada (Health Canada 1998):
Toddlers (0.5–4 years): 15.5 kg
Children (5–11 years): 31.0 kg
Adults (20–59 years): 70.9 kg
Dermal and inhalation exposures from cosmetics and cleaning products were estimated for adults using ConsExpo version 4.0 or algorithms from the model (ConsExpo 2006). Dermal absorption was conservatively assumed to be 1%. An inhalation rate of 16. 2 m3/day was assumed for adults (Health Canada 1998). Scenario-specific assumptions are provided in Table A-1. An overall retention factor of 1 was used unless otherwise specified.
| Exposure scenario | Assumptions |
|---|---|
| Body cream | Exposure frequency: 1.13/day (Loretz et al. 2005) Product amount: 4.4 g/application (mean) (Loretz et al. 2005) |
| Permanent hair dye (wash-in) | Exposure frequency: 0.02/day (7.99/year) (Statistics Canada 2012) Product amount: 100 g/application (RIVM 2006b) Overall retention factor: 0.10 (SCCS 2011) |
| Hair perm/straightener | Exposure frequency: 0.017/day (6/year) (Wu et al. 2010) Product amount: 80 g/application (RIVM 2006b) Overall retention factor: 0.10 (SCCS 2011) |
| Spray perfume (aerosol) | Exposure frequency: 1.7/day (Loretz et al. 2006) Product amount: 0.33 g/application (Loretz et al. 2006) |
| Hairspray | Frequency: 18x/month (Loretz et al. 2006) Product amount: 2.58 g/application (Loretz et al. 2006) Retention factor: 0.085 (Assuming 15% is loss from spray action and a transfer factor of 0.1 from hair to scalp) |
| All purpose cleaner (spraying away from exposed person; spraying kitchen top) |
Concentration of sodium lauryl sulfate: 5% (MSDS 2015b) Frequency: 365/year (RIVM 2006a) Exposure duration: 60 min (RIVM 2006a) Room volume: 15 m3 (kitchen) (RIVM 2006a) Ventilation rate: 2.5/h (kitchen) (RIVM 2006a) Mass generation rate: 1.6 g/sec (RIVM 2009) Spray duration: 0.41 min (RIVM 2006a) Airborne fraction: 0.006 g/g (RIVM 2009) Weight fraction non-volatile: 0.05 g/g (RIVM 2006a) Density non-volatile: 1.8 g/cm3 (RIVM 2006a) Room height: 2.5 m (standard room height) (RIVM 2006a) Inhalation cut-off diameter: 10 µm (RIVM 2006a,b) Non-respirable uptake fraction: 1 (RIVM 2006a) |
| Glass cleaner spray (spraying away from exposed person) |
Concentration of ammonium lauryl sulfate: 3% (MSDS 2013d) Frequency: 365/year (RIVM 2006a) Exposure duration: 240 min (RIVM 2006a) Room volume: 58 m3 (living room) (RIVM 2006a) Ventilation rate: 0.5/h (living room) (RIVM 2006a) Mass generation rate: 1.6 g/sec (RIVM 2009) Spray duration: 0.7 min (RIVM 2006a) Airborne fraction: 0.006 g/g (RIVM 2009) Weight fraction non-volatile: 0.05 g/g (RIVM 2006a) Density non-volatile: 1.8 g/cm3 (RIVM 2006a) Room height: 2. 5 m (standard room height) (RIVM 2006a) Inhalation cut-off diameter: 10 µm (RIVM 2006a,b) Non-respirable uptake fraction: 1 (RIVM 2006a) |
| Bathroom cleaning spray (spraying away from exposed person) |
Concentration of sodium C14-16 olefin sulfonate: 5% (MSDS 2012a) Frequency: 52/year (RIVM 2006a) Exposure duration: 25 min (RIVM 2006a) Room volume: 10 m3 (bathroom) (RIVM 2006a) Ventilation rate: 2/h (bathroom) (RIVM 2006a) Mass generation rate: 1.6 g/sec (RIVM 2009) Spray duration: 1.5 min (RIVM 2006a) Airborne fraction: 0.006 g/g (RIVM 2009) Weight fraction non-volatile: 0.1 g/g (RIVM 2006a) Density non-volatile: 1.8 g/cm3 (RIVM 2006a) Room height: 2.5 m (standard room height) (RIVM 2006a) Inhalation cut-off diameter: 10 µm (RIVM 2006a,b) Non-respirable uptake fraction: 1 (RIVM 2006a) |
Appendix B. Oral exposure from dish detergent left as residue on dinnerware, toothpaste, and use of non-prescription drugs and natural health products formulated as capsules/tablets
Oral exposure to ammonium lauryl sulfate and sodium C14-16 olefin sulfonate from dish detergent left as residue on dinnerware and to sodium lauryl sulfate from use of toothpaste was estimated using ConsExpo version 4.0 or algorithms from the model (ConsExpo 2006). The body weights used are indicated in Appendix A. Scenario-specific assumptions are provided in Table B-1.
| Substance | Exposure scenario | Assumptions |
|---|---|---|
| Ammonium lauryl sulfate and sodium C14-16 olefin sulfonate | Dish detergent left as residue on dinnerware (adults and toddlers) | The following ConsExpo default assumptions (RIVM 2006a) were used: Exposure frequency: 365/year Amount ingested: 0.00042 g Uptake fraction: 1 |
| Sodium lauryl sulfate | Toothpaste (toddlers) | The following ConsExpo default assumptions (RIVM 2006b) were used: Exposure frequency: twice a day Amount ingested (mean): 0.3 g Overall retention factor: 1 |
Oral exposure to sodium lauryl sulfate from the use of non-prescription drugs formulated as capsules/tablets was considered an acute/occasional exposure. An upper-bounding concentration of 7.5 mg per capsule/tablet was identified for adults taking pain relievers, flu medications, bismuth caplets or laxatives, which corresponds to 0.106 mg/kg bw for a single dose and 0.21 mg/kg bw for the maximum recommended daily dose. An upper-bounding concentration of 1. 1 mg per capsule/tablet was identified for children taking pain relievers, which corresponds to 0.035 mg/kg bw for a single dose and 0.14 mg/kg bw for the maximum recommended daily dose (personal communication, emails from the Risk Management Bureau, Safe Environments Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated June 16, 2015; unreferenced).
Daily exposure to natural health products formulated as capsules/tablets was also estimated for sodium lauryl sulfate. Although an upper-bounding concentration of 15.2 mg per capsule/tablet (up to three times per day) was identified for one individual calcium and vitamin D supplement for use in children, adolescents and adults, this product was not available online nor was it advertised as available in the manufacturer's catalog and was therefore not considered for daily exposure. The next highest concentration of 5 mg per capsule/tablet (three times per day) for calcium and vitamin D supplements for children and adults was therefore considered upper-bounding (LNHPD [modified 2016], personal communication, emails from Health Products and Food Branch, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated May 2016; unreferenced). Use of this concentration to estimate exposure from natural health products formulated as capsules/tablets is considered conservative as the majority of natural health products formulated as capsules/tablets provide daily doses well below the daily dose of sodium lauryl sulfate used in this scenario (i. e., 15 mg/day) and the availability of the product on the Canadian market is limited.