Screening assessment - Cyclohexane, 5-isocyanato-1-(isocyanatomethyl)-1,3,3-trimethyl- (Isophorone diisocyanate)
Official Title: Screening assessment Cyclohexane, 5-isocyanato-1-(isocyanatomethyl)-1,3,3-trimethyl-
(Isophorone diisocyanate)
Chemical Abstracts Service Registry Number: 4098-71-9
Environment and Climate Change Canada
Health Canada
April 2019
Cat. No.: En14-372/2019E-PDF
ISBN 978-0-660-30315-4
Synopsis
Pursuant to section 68 of the Canadian Environmental Protection Act, 1999 (CEPA), the Minister of the Environment and the Minister of Health have conducted a screening assessment of cyclohexane, 5-isocyanato-1-(isocyanatomethyl)-1,3,3-trimethyl-, hereinafter referred to as isophorone diisocyanate (IPDI). The Chemical Abstracts Service Registry Number (CAS RNFootnote 1) for IPDI is 4098-71-9. This substance is among those substances identified as priorities for assessment on the basis of other human health concerns.
IPDI does not occur naturally in the environment. It is used primarily as a monomer to make various polymers, such as polyurethanes. According to information submitted in response to a survey under CEPA section 71, there was no manufacture of IPDI in Canada in 2011. A total of 111 104 kg of IPDI was imported into Canada in 2011. IPDI has been reported to be found in paints and coatings, adhesives and sealants, and floor coverings.
The ecological risk of IPDI was characterized using the ecological risk classification of organic substances (ERC), which is a risk-based approach that employs multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. Hazard profiles are based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Metrics considered in the exposure profiles include potential emission rate, overall persistence, and long-range transport potential. A risk matrix is used to assign a low, moderate or high level of potential concern for substances on the basis of their hazard and exposure profiles. Based on the outcome of the ERC analysis, IPDI is considered unlikely to be causing ecological harm.
Considering all available lines of evidence presented in this screening assessment, there is low risk of harm to the environment from IPDI. It is concluded that IPDI does not meet the criteria under paragraphs 64(a) or (b) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
The general population is not expected to be exposed to IPDI via environmental media, food or drinking water. IPDI may be used in a small number of automotive paint hardeners available to consumers. Air concentrations of IPDI from the do-it-yourself use of these products were modelled and compared with the critical health effect levels for IPDI. Changes in the nasal cavity and larynx indicative of airway irritation were identified as the critical health effect for IPDI. The resultant margins of exposure are considered adequate to address uncertainties in the health effects and exposure databases.
On the basis of the information presented in this screening assessment, it is concluded that IPDI does not meet the criteria under paragraph 64(c) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
Therefore, it is concluded that IPDI does not meet any of the criteria set out in section 64 of CEPA.
1. Introduction
Pursuant to section 68 of the Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999), the Minister of the Environment and the Minister of Health have conducted a screening assessment of cyclohexane, 5-isocyanato-1-(isocyanatomethyl)-1,3,3-trimethyl-, hereinafter referred to as isophorone diisocyanate (IPDI), to determine whether this substance presents or may present a risk to the environment or to human health. This substance is among those substances identified as priorities for assessment on the basis of other human health concerns (ECCC, HC [modified 2017]).
The ecological risk of IPDI was characterized using the ecological risk classification of organic substances (ERC) approach (ECCC 2016a). The ERC describes the hazard of a substance using key metrics, including mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity, and considers the possible exposure of organisms in the aquatic and terrestrial environments on the basis of such factors as potential emission rates, overall persistence and long-range transport potential in air. The various lines of evidence are combined to identify substances as warranting further evaluation of their potential to cause harm to the environment or as having a low likelihood of causing harm to the environment.
IPDI was reviewed internationally through the Organisation for Economic Co-operation and Development (OECD) Cooperative Chemicals Assessment Programme, and an OECD Screening Information Dataset Initial Assessment Report (SIAR) is available. The OECD assessments undergo rigorous review (including peer-review) and endorsement by international governmental authorities. Health Canada and Environment and Climate Change Canada are active participants in this process, and consider these assessments reliable. The OECD SIAR will be used to inform the health effects characterization in this screening assessment.
This screening assessment includes consideration of information on chemical properties, hazards, uses, and exposures, including additional information submitted by stakeholders. Relevant data were identified up to July 2017. Empirical data from key studies, as well as some results from models, were used to reach conclusions. When available and relevant, information presented in assessments from other jurisdictions was considered.
This screening assessment was prepared by staff in the CEPA Risk Assessment Program at Health Canada and Environment and Climate Change Canada and incorporates input from other programs within these departments. The ecological portion of this assessment is based on the ERC document (published July 30, 2016), which was subject to an external review as well as a 60-day public comment period. Additionally, the draft of this screening assessment (published March 3, 2018) was subject to a 60-day public comment period. While external comments were taken into consideration, the final content and outcome of the screening assessment remain the responsibility of Health Canada and Environment and Climate Change Canada.
This screening assessment focuses on information critical to determining whether a substance meets the criteria as set out in section 64 of CEPA, by examining scientific information and incorporating a weight-of-evidence approach and precautionFootnote 2. It presents the critical information and considerations on which the conclusion is based.
2. Identity of substance
The substance isophorone diisocyanate (IPDI) is an organic chemical belonging to a substance class known as isocyanates. Information regarding the identity of this isocyanate is summarized in Table 2‑1.
| CAS RNa | Domestic Substances List (DSL) name (common name) |
Chemical structure and molecular formula | Molecular weight (g/mol) |
| 4098-71-9 | Cyclohexane, 5-isocyanato-1-(isocyanatomethyl)-1,3,3-trimethyl- (isophorone diisocyanate) | 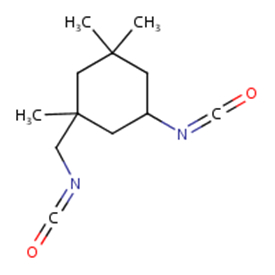 C12H18N2O2 C12H18N2O2 |
222 |
a The Chemical Abstracts Service Registry Number (CAS RN) is the property of the American Chemical Society, and any use or redistribution, except as required in supporting regulatory requirements and/or for reports to the Government of Canada when the information and the reports are required by law or administrative policy, is not permitted without the prior written permission of the American Chemical Society.
3. Physical and chemical properties
A summary of physical and chemical properties of IPDI is presented in Table 3‑1 (based on OECD 2006). Additional physical chemical properties are presented in ECCC (2016b).
| Property | Value | Type of data | Reference |
|---|---|---|---|
| Melting point (oC) | -60 | experimental | Sax and Lewis (1987), as cited in OECD 2006 |
| Boiling point (oC) | 310 | experimental | Auer (1989); INRS (1988), as cited in OECD 2006 |
| Vapour pressure (Pa) (20oC) | 0.06 | experimental | Bayer AG (1994), as cited in OECD 2006 |
| Henry’s law constant (Pa m3/mol) | 0.94 | predicted | Degussa AG (2006), as cited in OECD 2006 |
| Water solubility (mg/L) (23oC) | 15 | experimental | Infracor GmbH (2000), as cited in OECD 2006 |
| Density (g/mL) (20oC) | 1.06 | experimental | Auer (1989); INRS (1988), as cited in OECD 2006 |
| n-octanol/water partition coefficient (log Kow) | 4.75 | predicted | Degussa AG (2006), as cited in OECD 2006 |
4. Sources and uses
IPDI does not occur naturally in the environment. It is manufactured from 3-aminomethyl-3,5,5-trimethylcyclohexylamine by reaction with either phosgene or urea (OECD 2006). IPDI was included in a survey under section 71 of CEPA (Canada 2012). In the 2011 calendar year, there were no reports of manufacture in Canada above the reporting threshold of 100 kg (Environment Canada 2013). A reported total quantity of 111 104 kg of IPDI was imported into Canada, either alone or in a mixture or product at a concentration equal to or above 0.1% by weightFootnote 3. The worldwide production volume of IPDI is estimated to be in the order of 25 to 35 million kg per year (OECD 2006). In the United States, the national production volume for IPDI was 4.5 to 22.6 million kg for the year 2015 (CDAT [modified 2017]).
In a survey conducted under CEPA section 71, IPDI was reported to be used in paints and coatings, adhesives and sealants, and floor coverings. These uses are consistent with the global uses of IPDI. Globally, IPDI is used primarily as an intermediate or monomer to make various polymers, such as polyurethanes or other polymers comprising urethane functions, particularly coatings, varnishes and impregnation for cars, floors, leather, cans and coils, and special adhesives (OECD 2006). It may also be used in food packaging materials (personal communication, email from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated March 2017; unreferenced).
5. Potential to cause ecological harm
5.1 Characterization of ecological risk
The ecological risk of IPDI was characterized using the ecological risk classification of organic substances (ERC) approach (ECCC 2016a). The ERC is a risk-based approach that considers multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. The various lines of evidence are combined to discriminate between substances of lower or higher potency and lower or higher potential for exposure in various media. This approach reduces the overall uncertainty with risk characterization compared to an approach that relies on a single metric in a single medium (e.g., LC50) for characterization. The following summarizes the approach, which is described in detail in ECCC (2016a).
Data on physical and chemical properties, fate (chemical half-lives in various media and biota, partition coefficients, and fish bioconcentration), acute fish ecotoxicity, and chemical import or manufacture volume in Canada were collected from the scientific literature, from available empirical databases (e.g., OECD QSAR Toolbox) and from responses to surveys conducted under CEPA section 71, or they were generated using selected quantitative structure-activity relationship (QSAR) or mass-balance fate and bioaccumulation models. These data were used as inputs to other mass-balance models or to complete the substance hazard and exposure profiles.
Hazard profiles were based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Exposure profiles were also based on multiple metrics including potential emission rate, overall persistence, and long-range transport potential. Hazard and exposure profiles were compared to decision criteria in order to classify the hazard and exposure potential for each organic substance as low, moderate, or high. Additional rules were applied (e.g., classification consistency and margin of exposure) to refine the preliminary classifications of hazard or exposure.
A risk matrix was used to assign a low, moderate or high classification of potential risk for each substance on the basis of its hazard and exposure classifications. ERC classifications of potential risk were verified using a two-step approach. The first step adjusted the risk classification outcomes from moderate or high to low for substances that had a low estimated rate of emission to water after wastewater treatment, representing a low potential for exposure. The second step reviewed low risk potential classification outcomes using relatively conservative, local-scale (i.e., in the area immediately surrounding a point-source of discharge) risk scenarios designed to be protective of the environment, to determine whether the classification of potential risk should be increased.
ERC uses a weighted approach to minimize the potential for both over and under classification of hazard, exposure and subsequent risk. The balanced approaches for dealing with uncertainties are described in greater detail in ECCC (2016a). The following describes two of the more substantial areas of uncertainty. Error with empirical or modeled acute toxicity values could result in changes in classification of hazard, particularly metrics relying on tissue residue values (i.e., mode of toxic action), many of which are predicted values from QSAR models. However, the impact of this error is mitigated by the fact that overestimation of median lethality will result in a conservative (protective) tissue residue value used for critical body residue (CBR) analysis. Error with underestimation of acute toxicity will be mitigated through the use of other hazard metrics, such as structural profiling of mode of action, reactivity and/or estrogen binding affinity. Changes or errors in chemical quantity could result in differences in classification of exposure as the exposure and risk classifications are highly sensitive to emission rate and use quantity. The ERC classifications thus reflect exposure and risk in Canada on the basis of what is believed to be the current use quantity, and may not reflect future trends.
Critical data and considerations used to develop the substance-specific profiles for IPDI and the hazard, exposure and risk classification results are presented in ECCC (2016b).
According to information considered under ERC, IPDI was classified as having a moderate hazard potential on the basis of a reactive mode of action and a moderate potential to cause adverse effects in aquatic food webs given its bioaccumulation potential. However, the potential effects and how they may manifest in the environment were not further investigated due to the low exposure of this substance. Considering current use patterns, IPDI is unlikely to be resulting in concerns for the environment in Canada.
6. Potential to cause harm to human health
6.1 Health effects assessment
OECD (2006) summarizes the health effects literature and characterized the hazard for IPDI. It was used to inform the health effects characterization in this screening assessment and to provide critical endpoints and corresponding effect levels for IPDI in risk characterization. A literature search was conducted from the year prior to the OECD assessment to March 2017. No health effects studies which could impact the risk characterization (i.e., result in different critical endpoints or lower points of departure than those stated in OECD) were identified. Key studies for risk characterization from the OECD SIAR are presented below.
A short-term, repeated-dose toxicity study was conducted in Wistar rats via the inhalation route of exposure following OECD Test Guideline 412. Test animals were exposed at doses of 0.24, 1.05, or 4.1 mg/m3 for 6 hours/day, 5 days/week for 4 weeks (10 animals per sex and dose group). The respiratory tract was the target organ, with clinical signs of respiratory tract irritation (nostrils: red encrustations, stridor, nasal discharge, breathing sounds, and hypothermia). The NOAEC and LOAEC were determined to be 0.24 and 1.05 mg/m3, respectively, on the basis of histopathological changes in nasal cavity and larynx (Bayer AG 2003, as cited by OECD 2006). At 4.1 mg/m3, the pharynx, trachea, and lungs were affected; lesions in the lung and trachea were reversible within the 4 weeks of recovery. However, lesions in the nasal cavity, pharynx, and larynx still remained in some animals with minimal or slight severity.
In an acute inhalation irritation experiment (Pauluhn 2004), several isocyanates including IPDI were assessed in Wistar rats during 6-hour nose-only exposures. In contrast to some pulmonary irritants, IPDI was considered an upper airway irritant. For a single, 6-hour exposure, effects elicited by IPDI were limited to upper airway irritation, which fully resolved within days following cessation of exposure. A NOAEC of 8 mg/m3 was derived by the study authors for IPDI on the basis of indications of upper airway irritation at a concentration of 26 mg/m3.
The European Commission has classified IPDI as a Category 1 respiratory sensitizer (EC 2008). Multiple cases of sensitization to the respiratory tract due to IPDI exposure in occupational settings, including spray painting and polyurethane production, have also been reported (Tyrer 1979 as cited by OCED 2006; Clarke and Aldons 1981 as cited by OCED 2006; Germanaud et al. 2003, as cited by OECD 2006). More recent publications confirm IPDI as a respiratory or skin sensitizer in the auto repair occupational exposure settings (Bello et al. 2008, Liippo and Lammintausta 2008; Aalto-Korte et al. 2012; Reeb-Whitaker et al. 2012; CNESST 2017). To be protective of respiratory effects including airway irritation and development of sensitization, a threshold limit value of 45 µg/m3 (0.005 ppm) has been determined by ACGIH for daily exposures for workers (ACGIH 2012). For acute exposures, respiratory effects are considered to be limited to upper airway irritation.
6.2 Exposure assessment
No reports of measured concentrations of IPDI in environmental media or food in Canada were identified. Although IPDI is not manufactured in Canada, releases of IPDI into the environment may occur during the manufacture of downstream products. The National Pollutant Release Inventory reported total on-site releases (air, water and land) of IPDI from industrial activities in Canada of 10 and 219 kg in 2014 and 2015[P[1] , respectively (NPRI [modified 2016]). However, IPDI readily hydrolyzes upon contact with water to form its respective amine, isophoronediamine (IPDA) (OECD 2006), and concentrations in environmental media are therefore expected to be negligible. IPDI has been identified as being used as a component in the manufacture of some food packaging materials (coatings, adhesives, casings, and paper) in Canada. As IPDI is expected to rapidly hydrolyze to IPDA, exposure to IPDI from food packaging applications are expected to be negligible (personal communication, email from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated March 2017; unreferenced).
IPDI was identified as an ingredient in a small number of enamel hardeners for two-component automotive paints available for use by consumers in Canada as a do-it-yourself (DIY) application. The concentration of IPDI in these products is generally low, ranging from 0.1% to 1% (MSDS 2013; MSDS 2014). As consumers may not wear protective equipment when using products, estimates of air concentrations of IPDI were generated for a DIY scenario involving the mixing of a paint hardener containing IPDI with automotive paint. The resulting air concentrations are presented in Table 6-1 below.
When the hardener (containing IPDI) is mixed with the automotive paint, the two components react chemically and hardening occurs as the polymers are formed (RIVM 2007). IPDI is highly reactive and the majority will be consumed during polymerization, however some proportion will become airborne. Airborne IPDI not consumed during polymerization will be generated during the mixing and not during application as it will have already reacted. It is conservatively assumed that all IPDI present in the hardener becomes airborne during mixing. The frequency and duration of this activity is expected to be low, less than once per year for a period of a few days. The mixing event should only take a few minutes to complete. Several exposure durations were modelled to align with the different exposure durations in the animal studies. Further details on the model inputs are presented in Appendix A.
There is potential for incidental dermal exposure to IPDI during use of this product, however, the inhalation route of exposure was associated with the critical effect for risk characterization. As such, dermal exposures were not modelled.
| Product scenario | Mean air concentration per event (mg/m3)a | Mean air concentration per 6 hours (mg/m3)b | Mean air concentration on the day of exposure (mg/m3)c |
|---|---|---|---|
| Use of automotive paint hardener with automotive paint (two-component paint) | 0.65 | 0.0090 | 0.0022 |
a during the 5-minute event
b modelled airborne IPDI generated during the 5-minute event, amortized over 6-hours to match the duration of the animal study
c modelled airborne IPDI generated during the 5-minute event, amortized over 24-hours to match daily exposure
6.3 Characterization of risk to human health
Table 6-2 provides all relevant exposure and critical health effect levels for IPDI, as well as resultant margins of exposure (MOE) for the determination of risk. The exposure scenario is considered to be restricted to a 5-minute period. In the available health effects database, exposures to IPDI for such limited durations of exposure would result in recoverable upper airway irritation only at very high air concentrations (26 mg/m3). On the basis of the duration of exposure for the critical health effect, the mean air concentration amortized over 6 hours and on the day of exposure was considered the most appropriate exposure metric for risk characterization to harmonize with the study duration.
| Exposure scenario | Air concentrationa | Critical effect level | Critical health effect endpoint | MOE |
|---|---|---|---|---|
| DIY use of automotive paint hardener with automotive paint | Mean air concentration over 6 hours (mg/m3) 0.0090 mg/m3 | NOAEC 8 mg/m3 | Based on upper airway irritation in animals exposed to a single 6-hour exposure | 888 |
| DIY use of automotive paint hardener with automotive paint | Mean concentration on day of exposure 0.0022 mg/m3 | NOAEC 0.24 mg/m3 | Based on histopathological changes in nasal cavity and larynx, exposure 6 hours/day, 5 days/week for 4 weeksb | 427 |
Abbreviations: NOAEC, no-observed-adverse-effect-concentration.
a As modelled in ConsExpo Web (2016) for general population exposure.
b As reported by OECD 2006.
Further, given the vapour pressure of the compound and the saturated air concentration (5.4 mg/m3), it is not possible to produce air concentrations of IPDI approaching the acute NOAEC (of 8 mg/m3) for this consumer exposure scenario.
These margins are considered adequate to address uncertainties in the health effects and exposure databases.
6.4 Uncertainties in evaluation of risk to human health
On the basis of the animal data, human data, and well-known reactivity of diisocyanates, respiratory sensitization is an endpoint of concern following repeated exposures. However, it is unlikely that this endpoint will be relevant for this DIY exposure scenario for IPDI in Canada as it has a very limited use pattern given that the number and type of products are limited in scope and are used less than once per year for a period of a few days. Also, the available data suggest that health effects would be limited to upper airway irritation after acute exposures. Furthermore, for the most part, the safety data sheets that accompany the products recommend adequate ventilation or the use of respiratory protection.
In addition, the exposure models and inputs used to generate air concentrations for the DIY scenario are very conservative as it is assumed that 100% of the IPDI in the product is volatilized during mixing and that none remains in the mixture. This is an upper bound, as most of the IPDI monomer will be incorporated into the polymer during hardening and only a small fraction of the monomer will be available for volatilization into air during mixing.
7. Conclusion
Considering all available lines of evidence presented in this screening assessment, there is low risk of harm to the environment from IPDI. It is concluded that IPDI does not meet the criteria under paragraphs 64(a) or (b) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
On the basis of the information presented in this screening assessment, it is concluded that IPDI does not meet the criteria under paragraph 64(c) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
Therefore, it is concluded that IPDI does not meet any of the criteria set out in section 64 of CEPA.
References
Aalto-Korte K, Suuronen K, Kuuliala O, Hendriks-Eckerman M, Jolanki R. 2012. Occupational contact allergy to monomeric isocyanates. Contact Dermatitis. 67(2):78-88.
[ACGIH] American Conference of Governmental Industrial Hygienists. 2012. TLVs and BEIs, Based on the Documentation of the Threshold Limit Values for Chemical Substances and Physical Agents, Biological Exposure Indices. Cincinnati (OH): ACGIH.
Bello D, Redlich CA, Stowe MH, Sparer J, Woskie SR, Streicher RP, Hosgood HD, Liu Y. 2008. Skin exposure to aliphatic polyisocyanates in the auto body repair and refinishing industry: II. A quantitative assessment. Ann Occup Hyg 52(2):117-124.
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C. 1999, c. 33. Canada Gazette Part III, vol. 22, no. 3.
Canada, Dept. of the Environment. 2012. Canadian Environmental Protection Act, 1999: Notice with respect to certain substances on the Domestic Substances List [PDF]. Canada Gazette, Part I, vol. 146, no. 48, Supplement.
[CDAT] Chemical Data Access Tool. [modified 2017 May]. Non-confidential 2016 Chemical Data Reporting Results: search results for CAS RN 108-24-7. Washington (DC): US Environmental Protection Agency. [accessed 2017 Sep 7].
[CNESST] Commission des normes, de l'équité, de la santé et de la sécurité du travail. 2017. List of Agents Causing Occupational Asthma. Quebec (QC): CNESST. [updated 2017 Jan; accessed 2017 Jun 7].
[ConsExpo Web] Consumer Exposure Web Model. 2016. Bilthoven (NL): Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment].
[EC] European Commission, the European Parliament and the Council of the European Union. 2008. Regulation (EC) No 1272/2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006. Official Journal of the European Union. Volume 51. L 353. [accessed 2017 Jun 7].
[ECCC] Environment and Climate Change Canada. 2016a. Science approach document: ecological risk classification of organic substances. Ottawa (ON): Government of Canada.
[ECCC] Environment and Climate Change Canada. 2016b. Gatineau (QC): Data used to create substance-specific hazard and exposure profiles and assign risk classifications in the Ecological Risk Classification of organic substances. Available from: substances@ec.gc.ca.
[ECCC, HC] Environment Canada, Health Canada. [modified 2017 Mar 12]. Categorization. Ottawa (ON): Government of Canada.
Environment Canada. 2013. DSL Inventory Update data collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain substances on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
Health Canada. [modified 2016 Sep 9]. Food additives permitted for use in Canada. Ottawa (ON): Health Canada. [accessed 2015 Nov 18].
Health Canada. [modified 2015 Dec 14]. Cosmetic ingredient hotlist: list of ingredients that are prohibited for use in cosmetic products. Ottawa (ON): Government of Canada.
Liippo J, Lammintausta K. 2008. Contact sensitization to 4,4'-diaminodiphenylmethane and to isocyanates among general dermatology patients. Contact Dermatitis. 59:109-14.
[MSDS] Material Safety Data Sheet. 2014. EA100S EA100SHP SLOW Acrylic Urethane Activator. Mississauga. (ON): Dominion Sure Seal Ltd [accessed 2017 June 13].
[MSDS] Material Safety Data Sheet. 2013. PF 593C Wet Look Acrylic Enamel Hardener 2.1 [PDF]. Milton (ON): Pro Form Products Ltd. [accessed 2017 Feb 5].
[NPRI] National Pollutant Release Inventory [database on the Internet]. [modified 2016 Dec 7]. Datasets: isophorone diisocyanate (CAS RN 4098-71-9). Ottawa (ON): Government of Canada.. [cited 2017 February].
[OECD] Organisation for Economic Co-operation and Development. 2006. SIDS initial assessment report: isophorone diisocyanate: CAS No. 4098-71-9. [ZIP] SIAM [SIDS Initial Assessment Meeting] 23; 2006 Oct; Jeju, Korea. [accessed 2017 Feb 7].
Pauluhn J. 2004. Pulmonary irritant potency of polyisocyanate aerosols in rats: Comparative assessment of irritant threshold concentrations by bronchoalveolar lavage. J Appl Toxicol. 24:231-247.
Reeb-Whitaker C, Whittaker SG, Ceballos DM, Weiland EC, Flack SL, Fent KW, Thomasen JM, Trelles Gaines LG, Nylander-French LA. 2012. Airborne isocyanate exposures in the collision repair industry and a comparison to occupational exposure limits. J Occup Environ Hyg. 9:329-39.
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment]. 2005. ConsExpo 4.0 Consumer Exposure and Uptake Models Program Manual [PDF]. Bilthoven (NL): RIVM. Report No.: 320104004/2005. [accessed 2017 Feb 22].
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment]. 2007. Paint products fact sheet: to assess the risks for the consumer [PDF]. Updated version for ConsExpo 4. Bilthoven (NL): RIVM. Report No.: 320104008/2007. [accessed 2016 Feb 22].
Appendix A
Exposures were estimated using the ConsExpo Web (2016) inhalation exposure to vapour, evaporation model as recommended in the ConsExpo Paint Fact Sheet for two-component paints (RIVM 2007). All recommended default values for two-component paints from the fact sheet were used.
As per the guidance in the ConsExpo Paint Fact Sheet, the mixing of the base lacquer and the hardener immediately before painting may lead to inhalation and dermal exposure. The base lacquer and the hardener are mixed and react chemically, then harden. After mixing, the mixed paint can be used for a specific time period (known as the ‘pot life’). Inhalation exposure can occur if volatile compounds evaporate during the mixing process. It is unlikely that the volatile compounds will be formed during the chemical reaction between the base lacquer and the hardener, because polymers are formed to provide the bond.
Model inputs: Inhalation exposure model: Exposure to vapour - Evaporation
Molecular weight of IPDI: 222 g/mol
Kow: log 4.75
Concentration of IPDI in enamel hardener: 1%
Body weight: 70.9 kg
Frequency: less than once per year for a period of a few days
Exposure duration: 5 minutes, default for time to mix/load paint; RIVM 2007
Product amount: 118 g
Weight fraction substance: 1%
Room volume: 1 m3, default for personal space; RIVM 2007
Ventilation rate: 0.6/hr, default for unspecified room; RIVM 2007
Inhalation rate: 13.5 m3/day, default for light exercise, RIVM 2005
Application temperature: 20°C
Vapour pressure 0.06 Pa
Molecular weight: 222 g/mol
Mass transfer coefficient 2500 m/min, default, RIVM 2007
Release area mode: constant, RIVM 2007
Release area: 95 cm2, default, RIVM 2007
Emission duration: 5 minutes, default, RIVM 2007
Product in pure form: no
Molecular weight matrix: 3000 g/mol, default, RIVM 2007
Absorption model: not applicable
