Screening Assessment for the Challenge Acetamide, N,N-dimethyl-
Chemical Abstracts Service Registry Number 127-19-5
Environment Canada
Health Canada
August 2009
Table of Contents
- Synopsis
- Introduction
- Substance Identity
- Physical and Chemical Properties
- Sources
- Uses
- Releases to the Environment
- Environmental Fate
- Persistence and Bioaccumulation Potential
- Potential to Cause Ecological Harm
- Potential to Cause Harm to Human Health
- Conclusion
- References
- Appendix 1. Estimates of total and media-specific daily intakes of DMAc for various age groups
- Appendix 2. Predicted concentrations of DMAc in environmental media
- Appendix 3. Upper-bounding estimates of exposure to DMAc emitted from linoleum flooring assembly and a medium-density fibreboard shelving unit
- Appendix 4. Summary of health effects information and associated lowest effect levels for DMAc
- Appendix 5: Estimated margins of exposure for DMAc
Synopsis
The Ministers of the Environment and of Health have conducted a screening assessment of acetamide, N,N-dimethyl- (DMAc), Chemical Abstracts Service Registry Number 127-19-5. This substance was identified in the categorization of the Domestic Substances List as a high priority for action under the Ministerial Challenge. DMAc was identified as a high priority as it was considered to pose intermediate potential for exposure to individuals in Canada and had been classified by the European Commission on the basis of developmental toxicity. The substance did not meet the ecological categorization criteria for persistence, bioaccumulation potential or inherent toxicity to aquatic organisms. Therefore, the focus of this assessment of DMAc relates to human health risks.
In response to a notice issued under section 71 of CEPA 1999, DMAc was not manufactured in Canada in 2006 above the reporting threshold of 100 kg. The total quantity imported into Canada in the same calendar year was reported to be in the range of 1000-10 000 kg. Less than 100 kg of DMAc was reported to be released to air, water or land in the 2006 calendar year. The principal uses of DMAc include polymer dissolution in the man-made fibre production industry, photoresist stripping in the manufacture of electronic components, production solvent in the pharmaceutical, photography and cosmetic industries, feedstock in the coating industry and sealant applications in aircraft.
Population exposure to DMAc from the general environment is expected to be low based on very limited information on concentrations in environmental media and the results of fugacity modelling. DMAc is used primarily in industrial settings, and consumer exposure to DMAc is not expected to be significant. Based on its uses, any resulting population exposure to residual DMAc is expected to be predominantly via indoor air.
The health effects associated with exposure to DMAc are primarily developmental toxicity and liver toxicity, based on observations in experimental animals. The margins between upper-bounding estimates of exposure from environmental media (drinking water and indoor air) and consumer products (textiles and building materials) and levels associated with effects in experimental animals are considered to be adequately protective.
On the basis of the adequacy of the margins between conservative estimates of exposure to DMAc and critical effect levels in experimental animals, it is concluded that DMAc is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
On the basis of the low ecological hazard and reported releases of DMAc, it is concluded that this substance is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends. DMAc does not meet the criteria for persistence or bioaccumulation as set out in the Persistence and Bioaccumulation Regulations.
This substance will be included in the upcoming Domestic Substances List inventory update initiative. In addition and where relevant, research and monitoring will be undertaken to confirm assumptions used during the screening assessment.
Based on the information available, it is concluded that DMAc does not meet any of the criteria set out in section 64 of CEPA 1999.
Introduction
The Canadian Environmental Protection Act, 1999 (CEPA 1999) (Canada 1999) requires the Minister of the Environment and the Minister of Health to conduct screening assessments of substances that have met the categorization criteria set out in the Act to determine whether these substances present or may present a risk to the environment or to human health. Based on the results of a screening assessment, the Ministers can propose to take no further action with respect to the substance, to add the substance to the Priority Substances List (PSL) for further assessment or to recommend that the substance be added to the List of Toxic Substances in Schedule 1 of the Act and, where applicable, the implementation of virtual elimination.
Based on the information obtained through the categorization process, the Ministers identified a number of substances as high priorities for action. These include substances that
- met all of the ecological categorization criteria, including persistence (P), bioaccumulation potential (B) and inherent toxicity to aquatic organisms (iT), and were believed to be in commerce in Canada; and/or
- met the categorization criteria for greatest potential for exposure (GPE) or presented an intermediate potential for exposure (IPE) and had been identified as posing a high hazard to human health based on classifications by other national or international agencies for carcinogenicity, genotoxicity, developmental toxicity or reproductive toxicity.
The Ministers therefore published a notice of intent in the Canada Gazette, Part I, on December 9, 2006 (Canada 2006), which challenged industry and other interested stakeholders to submit, within specified timelines, specific information that may be used to inform risk assessment and to develop and benchmark best practices for the risk management and product stewardship of those substances identified as high priorities.
The substance acetamide, N,N-dimethyl- was identified as a high priority for assessment of human health risk because it was considered to present IPE and had been classified by another agency on the basis of developmental toxicity. The Challenge for this substance was published in the Canada Gazette on February 16, 2008 (Canada 2008). A substance profile was released at the same time. The substance profile presented the technical information available prior to December 2005 that formed the basis for categorization of this substance. As a result of the Challenge, submissions of information pertaining to the substance were received (Environment Canada 2008b).
Although acetamide, N,N-dimethyl- was determined to be a high priority for assessment with respect to human health, it did not meet the criteria for persistence, bioaccumulation potential or inherent toxicity to aquatic organisms. Therefore, this assessment focuses principally on information relevant to the evaluation of risks to human health.
Under CEPA 1999, screening assessments focus on information critical to determining whether a substance meets the criteria for defining a chemical as "toxic" as set out in section 64 of the Act, where
"64. [...] a substance is toxic if it is entering or may enter the environment in a quantity or concentration or under conditions that
- have or may have an immediate or long-term harmful effect on the environment or its biological diversity;
- constitute or may constitute a danger to the environment on which life depends; or
- constitute or may constitute a danger in Canada to human life or health."
Screening assessments examine scientific information and develop conclusions by applying a weight of evidence approach and precaution.
This screening assessment includes consideration of information on chemical properties, hazards, uses and exposure, including the additional information submitted under the Challenge. Data relevant to the screening assessment of this substance were identified in original literature, review and assessment documents and stakeholder research reports and from recent literature searches up to November 2008. Key studies were critically evaluated; modelling results may have been used to reach conclusions. Evaluation of risk to human health involves consideration of data relevant to estimation of exposure (non-occupational) of the general population, as well as information on health hazards (based principally on the weight of evidence assessments of other agencies that were used for prioritization of the substance). Decisions for human health are based on the nature of the critical effect and/or margins between conservative effect levels and estimates of exposure, taking into account confidence in the completeness of the identified databases on both exposure and effects, within a screening context. The screening assessment does not represent an exhaustive or critical review of all available data. Rather, it presents a summary of the critical information upon which the conclusion is based.
This screening assessment was prepared by staff in the Existing Substances Programs at Health Canada and Environment Canada, and it incorporates input from other programs within these departments. The assessment has undergone external written peer review by Joan Strawson, Mike Jayjock and Katherine Walker of Toxicology Excellence for Risk Assessment. Although external comments were taken into consideration, the final content and outcome of the screening risk assessment remain the responsibility of Health Canada and Environment Canada. Additionally, the draft of this screening assessment was subject to a 60-day public comment period.
The critical information and considerations upon which the assessment is based are summarized below.
Substance Identity
For the purposes of this document, this substance will be referred to as DMAc (which has been derived from the National Chemical Inventory name). Information on the identity of DMAc is summarized in Table 1.
| Chemical Abstracts Service Registry Number (CAS RN) | 127-19-5 |
|---|---|
| Domestic Substances List (DSL) name | Acetamide,N,N-dimethyl- |
| National Chemical Inventories (NCI) namesTable note a | Acetamide, N,N-dimethyl- (AICS, ASIA-PAC, DSL, NZIoC, PICCS, SWISS, TSCA) N,N-Dimethylacetamide (DSL, ECL, EINECS, ENCS, PICCS) |
| Other names | Acetdimethylamide; Acetic acid dimethylamide; Dimethylacetamide; Dimethylamide acetate; DMA; DMAA; DMAc;N,N-Dimethylethanamide; NSC 3138 |
| Chemical group | Organics |
| Chemical sub-group | Amides |
| Chemical formula | C4H9NO |
| Chemical structure | 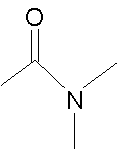 |
| Simplified Molecular Line Input Entry System (SMILES) | O=C(N(C)C)C |
| Molecular mass | 87.12 g/mol |
Physical and Chemical Properties
Table 2 contains experimental and modelled physical and chemical properties of DMAc that are relevant to its environmental fate.
| Type | Value | Descriptor | Reference | |
|---|---|---|---|---|
| Melting point (°C) | Experimental | -20.0 | - | PhysProp 2008 |
| Boiling point (°C at 101.3 kPa) | Experimental | 165.00 | - | PhysProp 2008 |
| Density (kg/m3 at 25°C) | Experimental | 936.6 | - | Lide 2000 |
| Vapour pressure (Pa at 25°C) | Experimental | 267 | High | Daubert and Danner 1989 |
| Henry's Law constant (Pa·m3/mol at 25°C) | Experimental | 1.33 × 10-3 | Very low | Taft et al. 1985 |
| Log Kow (octanol-water partition coefficient at 25°C) (dimensionless) | Experimental | -0.77 | Very low | Hansch et al. 1995 |
| Log Koc (organic carbon partition coefficient) (dimensionless) | Modelled | 0.969 | Very low | PCKOCWIN 2000 |
| Water solubility (mg/L) | Experimental | 1 000 000 (completely miscible at 20°C) | Very high | Monsanto 1960 |
| pKa (acid dissociation constant at 25°C) (dimensionless) | Experimental | 0.25 | - | Wada and Takenaka 1971Table note b |
Sources
DMAc is an anthropogenic compound and has not been identified to occur naturally. Production of DMAc involves the reaction of a stoichiometric excess of dimethylamine with acetic acid, acetic anhydride, methylacetate or the azeotrope of methylacetate and methanol in a closed system (OECD 2001; Watts and Larson 2002).
In response to a notice issued under section 71 of CEPA 1999, DMAc was not manufactured in Canada in the 2006 calendar year above the reporting threshold of 100 kg (Environment Canada 2008a). In the same calendar year, importation was reported at a total quantity between 1000 and 10 000 kg (Environment Canada 2008a).
Uses
In response to a notice issued under section 71 of CEPA 1999, total use of DMAc in Canada in the 2006 calendar year was reported to be 1000-10 000 kg (Environment Canada 2008a). According to recent submissions made under section 71 of CEPA 1999 and information derived from other sources, including the scientific and technical literature, DMAc is used strictly for industrial purposes (OECD 2001; Environment Canada 2008a). DMAc is employed most regularly in applications requiring high temperatures for resin solvation or activation of chemical reactions (Watts and Larson 2002). In fact, its principal use is the dissolution of polymers in the man-made fibre production industry (OECD 2001). These polymers include polyacrylonitrile, cellulose derivatives, styrenes, vinyl resins, including polyvinyl fluoride (PVF), linear polyesters, aramid fibres and polyurethane (DuPont 1998, 2008a; Jung et al. 2007). DMAc may occur as a manufacturing residue in some of the end products containing these polymers. PVF may be a component of the interior liner of flexible bulk containers used to transport water, wine and juice (email from Food Directorate, Health Canada, October 24, 2008; unreferenced). Aramid fibres are used in fabrics designed for heat-resistant applications, disposable medical clothing and medical bandages and dressings (DuPont 1998). Acrylic fibre is used in the production of spandex and elastane synthetic fibres, in addition to acrylic carpet, wigs, sleeping bags, sweaters, knit shirts and unprocessed and processed fabrics (Armstrong et al. 1980; Jung et al. 2007).
DMAc is not anticipated to be present in cosmetic products in Canada, as it is not listed as an ingredient in the Cosmetic Notification System database (CNS 2008); however, DMAc is not currently prohibited or regulated in cosmetic products in Canada, as it is not listed on the Health Canada cosmetic ingredient hotlist (Health Canada 2007). There are no registered pesticides that contain DMAc as an active ingredient or formulant in Canada (PMRA 2007), and DMAc is not listed as an approved food additive under the Index of Food Additives contained within the Food and Drug Regulations (Canada 1978). The Controlled Products Regulations under the Hazardous Products Act require DMAc to be disclosed on Material Safety Data Sheets accompanying workplace chemicals when it is present at a minimum concentration of 1% by weight as specified on the Ingredient Disclosure List (Canada 1988). DMAc is listed as a Class 2 residual solvent (solvent to be limited) in pharmaceutical products, natural health products and veterinary medicinal products with a concentration limit of 1090 ppm (where the maximum daily dose of the product does not exceed 10 g) or a permitted daily exposure of 10.9 mg/day (Health Canada 1999). As a non-medicinal ingredient, DMAc is present in three chemotherapeutic drugs administered intravenously for cancer treatment (email from Therapeutic Drugs Directorate, Health Canada, October 7, 2008; unreferenced). DMAc has also been recognized as an impurity in an anthelmintic drug administered orally to sheep; the impurity is present at a level of 0.05 mL DMAc/mL drug (Health Canada 2006).
Other industrial uses of DMAc, some pertaining to Canada, include use as an electrolytic solvent, reactor solvent and catalyst in the pharmaceutical and cosmetic industry and as a purification and crystallization solvent (Watts and Larson 2002). As a solvent, DMAc is involved in several organic syntheses, including elimination, halogenation, cyclization, alkylation, interesterification and phthaloylation reactions (DuPont 2008a). As a methylating agent, DMAc is used to make products throughN-, O- and S-alkylation reactions, including agrichemicals, finishes, fragrances, dyes and pesticides (DuPont 2008b). In the photography industry, DMAc is used as a solvent in the production of X-ray and photoresist stripping compounds (OECD 2001). These photoresist strippers are involved in the manufacture of integrated semiconductor electronic components (Environment Canada 2008a). DMAc is used as a feedstock in the coating and lacquer industry (Verschueren 2001). Determination of plasticizer concentrations in plastics may employ DMAc as an analytical solvent (Wypych 2004). In the separation and purification of 1,3-butadiene from crude C4 streams, DMAc may be employed as an extraction solvent (Sun and Wristers 2002). DMAc is a separating agent in styrene-butadiene rubber latex production (Scorecard 2005). Finally, DMAc is a sealant in aircraft windshield sealants, fuel tank sealants and other aircraft sealing applications relevant primarily to Canada's Air Force (email from Department of National Defence, June 24, 2008; unreferenced).
Releases to the Environment
In response to a notice issued under section 71 of CEPA 1999, less than 100 kg of DMAc was released to air, water or land in the 2006 calendar year (Environment Canada 2008a). The details concerning the distribution of releases among environmental media and specific locations of releases are considered confidential business information. Section 71 data also indicate some transfers of DMAc to hazardous waste facilities occurring in the 2006 calendar year (Environment Canada 2008a). Emissions from hazardous waste facilities may occur, as indicated by detection of DMAc in air surrounding a liquid waste impoundment (location not provided) in one study (Guzewich et al. 1983). DMAc is not reportable to the National Pollutant Release Inventory (NPRI 2007) or to the United States Toxics Release Inventory Program (TRI 2006); therefore, no release information is available from these sources.
Environmental Fate
Based on its physical and chemical properties (Table 2), the results of Level III fugacity modelling (Table 3) suggest that DMAc will reside predominantly in water or soil, depending on the compartment of release.
| Substance released to: | Fraction of substance partitioning to air (%) | Fraction of substance partitioning to water (%) | Fraction of substance partitioning to soil (%) | Fraction of substance partitioning to each sediment (%) |
|---|---|---|---|---|
| Air (100%) | 38.6 | 12.3 | 49.1 | 0.0 |
| Water (100%) | 0.0 | 99.9 | 0.0 | 0.1 |
| Soil (100%) | 0.2 | 9.2 | 90.6 | 0.0 |
Persistence and Bioaccumulation Potential
Environmental Persistence
Table 4 presents empirical data for the persistence of DMAc.
| Medium | Fate process | Degradation value | Endpoint (units) | Reference |
|---|---|---|---|---|
| Air | Photodegradation | 1 | Photodegradation half-life (days) | Atkinson 1989 |
| Water | Biodegradation | 80-100 | Biodegradation (%) | NITE 2002 |
In air, the empirical half-life value of 1 day for photodegradation by atmospheric oxidation (see Table 4) demonstrates that DMAc is likely to be rapidly oxidized. The substance is not expected to react with other photo-oxidative species in the atmosphere, such as ozone, nor is it likely to degrade via direct photolysis. Therefore, it is expected that reactions with hydroxyl radicals will be the most important fate process in the atmosphere for DMAc. With a half-life of 1 day via reactions with hydroxyl radicals, DMAc is considered to be not persistent in air.
In water, a predicted hydrolysis half-life of greater than 1 year (HYDROWIN 2000) demonstrates that this chemical is likely to be slowly hydrolysed. However, other fate processes in water need to be considered to determine the overall level of persistence in this medium. In water, biodegradation appears to be an important fate process for DMAc. Table 4 presents the empirical biodegradation data (NITE 2002), which show between 80% and 100% biodegradation over 14 days, measured indirectly (biochemical oxygen demand) and directly (total organic carbon, gas chromatography), in a ready biodegradation test for DMAc (MITI-II; Organisation for Economic Co-operation and Development [OECD] Test Guideline 302C). This test indicates that the ultimate biodegradation half-life in water is much shorter than 182 days (6 months) and that the substance is considered to not persist in that environmental compartment.
This empirical biodegradation information is supported by results of available quantitative structure-activity relationship (QSAR) models for biodegradation in water (BIOWIN 2000; TOPKAT 2004; CATABOL c2004-2008). The overall conclusion from BIOWIN (2000) is that DMAc is readily biodegradable. Other ultimate degradation models (TOPKAT 2004; CATABOL c2004-2008) also predict that DMAc undergoes relatively rapid mineralization.
Using an extrapolation ratio of 1:1:4 for water:soil:sediment biodegradation half-lives (Boethling et al. 1995), the half-life in soil is less than 182 days and the half-life in sediment is expected to be less than 365 days. This indicates that DMAc is not expected to be persistent in soil or sediment.
Based on the empirical and modelled data, DMAc does not meet the persistence criteria in water, soil, sediment and air (half-lives in soil and water 182 days, half-life in sediment 365 days and half-life in air 2 days) as set out in the Persistence and Bioaccumulation Regulations (Canada 2000).
Potential for Bioaccumulation
Experimental and modelled log Kow values for DMAc suggest that this chemical has low potential to bioaccumulate in the environment (see Table 2 above).
As no experimental bioaccumulation factor (BAF) or bioconcentration factor (BCF) data were available for DMAc, a predictive approach was applied using available BAF and BCF models, as shown in Table 5. Metabolism information for this substance was not available, nor was it considered in the BAF and BCF models.
| Test organism | Endpoint | Value (L/kg wet weight) | Reference |
|---|---|---|---|
| Fish | BAF | 1.00 | Arnot and Gobas 2003 (Gobas BAF middle trophic level) |
| Fish | BCF | 1.00 | Arnot and Gobas 2003 (Gobas BCF middle trophic level) |
| Fish | BCF | 9.88 | OASIS Forecast 2005 |
| Fish | BCF | 3.16 | BCFWIN 2000 |
The modified Gobas BAF middle trophic level model for fish predicted a BAF of 1.00 L/kg, indicating that DMAc does not have the potential to bioconcentrate and biomagnify in the environment. The results of BCF model calculations provide additional evidence supporting the low bioconcentration potential of this substance. Based on the available modelled values, DMAc does not meet the bioaccumulation criteria (BCF or BAF 5000) as set out in the Persistence and Bioaccumulation Regulations (Canada 2000).
Potential to Cause Ecological Harm
Ecological Effects Assessment
Aquatic Compartment
There is experimental and modelled evidence that DMAc does not cause acute harm to aquatic organisms at low concentrations (LC50 and EC50 values are much greater than 1 mg/L; see Tables 6 and 7).
| Test organism | Type of test | Endpoint | Value (mg/L) | Reference |
|---|---|---|---|---|
| Alga (Scenedesmus subspicatus) | Acute (72 h) | Concentration of a substance that is estimated to cause some toxic sublethal effect on 50% of the test organisms (EC50) | greater than 500 | BASF 1988 |
| Water flea (Daphnia magna) | Acute (24 h) | EC50 | greater than 500 | BASF 1988 |
| Water flea (Daphnia magna) | Acute (48 h) | EC50 | greater than 1000 | Adema and van den Bos Bakker 1987 |
| Amphipod (Chaetogammarusmarinus) | Acute (96 h) | LC50 | greater than 1000 | Adema and van den Bos Bakker 1987 |
| Mosquito fish (Gambusia affinis) | Acute (48 h) | Concentration of a substance that is estimated to be lethal to 50% of the test organisms (LC50) | 13 300 | Wallen et al. 1957 |
| Golden orfe (Leuciscus idus) | Acute (96 h) | LC50 | greater than 500 | BASF 1979 |
| Fathead minnow (Pimephales promelas) | Acute (24 h) | LC50 | 1500 | Geiger et al. 1990 |
| Test organism | Type of test | Endpoint | Value (mg/L) | Reference |
|---|---|---|---|---|
| Fish | Acute (96 h) | LC50 | 568 | AIES 2003-2005 |
| Fish | Acute (96 h) | LC50 | 747Table note c | TOPKAT 2004 |
| Fish | Acute (96 h) | LC50 | 7 015 | OASIS Forecast 2005 |
| Fish | Acute (96 h) | LC50 | 1741 | ECOSAR 2004 |
| Fish | Acute (96 h) | LC50 | 27 798 | ASTER 1999 |
| Daphnia magna | Acute (48 h) | EC50 | 377 | ECOSAR 2004 |
| Daphnia magna | Acute (48 h) | EC50 | 4 538 800 | TOPKAT 2004 |
| Algae | Acute (96 h) | EC50 | 1.13 | ECOSAR 2004 |
There are many experimental studies for the toxicity of DMAc to aquatic organisms. Most, however, report the result as a lower limit (e.g., EC50 greater than 500 mg/L) as opposed to a specific number. The studies that report a specific value (e.g., Wallen et al., 1957, LC50 = 13 300 mg/L) have not been subject to a Robust Study Summary.
A range of aquatic toxicity predictions were obtained from the various QSAR models considered.
These empirical and model results indicate that the substance is not highly hazardous to aquatic organisms (i.e., acute LC50, EC50 greater than 1.0 mg/L).
Other Environmental Compartments
There are a limited number of experimental data for the toxicity of DMAc to soil-dwelling organisms. The test result shown in Table 8 suggests that DMAC has some potential to harm earthworms (OECD 2001).
| Test organism | Type of test | Endpoint | Value (mg/cm2 filter paper) | Reference |
|---|---|---|---|---|
| Worm (Annelida) (Eisenia fetida) | Unknown | LC50 | 0.01-0.1 | Roberts and Dorough 1984 |
Ecological Exposure Assessment
Presence in the Environment
No monitoring data relating to the presence of DMAc in environmental media (air, water, soil, sediment) in Canada have been identified. Some monitoring data relating to the presence of DMAc in environmental media in other countries have been identified:
- In a 1983 study (Guzewich et al. 1983), reported in the OECD Screening Information Data Set (SIDS) Initial Assessment Report (SIAR), DMAc was detected in 1982 at all of six air sampling sites within a mile radius of a hazardous liquid waste impoundment. The location of the impoundment was not given. The mean DMAc concentration ranged from 9.6 to 11 ng/m3.
- In a 1993 study (Kadokami et al. 1993), concentrations of DMAc in natural waters in the Kitakyushu area of Japan were reported. The highest concentration of DMAc found in river and reservoir water was 93 ng/L.
Modelled Concentration in Water
Given the current uses, physical and chemical properties and fate upon release, the releases of DMAc of most ecological concern are those to smaller bodies of water.
For water, the exposure model IGETA (Environment Canada 2008b, c) was used to conservatively estimate local exposure in the vicinity of a potential source of release. IGETA inputs were as follows:
- the mass of DMAc: the maximum quantity (10 000 kg) of the range (1000-10 000 kg) reported in the section 71 survey to have been used in 2006 was used as a conservative value, and it was all assumed to be used at one facility;
- the percentage of DMAc released in the facility effluent, as a function of the total quantity of DMAc used at the facility: 5% (default value);
- the removal rate of the sewage treatment plant (61.6 %);
- the number of working days at the facility per year: 250 (default value).
Using these inputs and making the conservative assumption that effluents discharge into a small generic receiving water body, IGETA calculated the predicted environmental concentration (PEC) of DMAc in water to be 1.29 × 10-2 mg/L.
Characterization of Ecological Risk
The critical toxicity value (CTV) for this assessment is the lowest acceptable modelled or experimental acute value--a modelled 48 hour EC50 of 377 mg/L for immobility in Daphnia magna. An assessment factor of 100 is applied to account for uncertainty in extrapolating from a measure of acute to chronic effects and from laboratory to field conditions and for intraspecies and interspecies variations in sensitivity, giving a predicted no-effect concentration (PNEC) of 3.77 mg/L.
Therefore, the quotient for risk to aquatic species is calculated as follows:
Risk quotient = PECaq / PNECaq
= 0.013 mg/L / 3.77 mg/L
= 0.0034
As this calculated risk quotient is significantly less than 1, it is predicted that there is low risk to pelagic organisms from exposure to DMAc.
DMAc is furthermore predicted to be not persistent in any medium, nor is it expected to be bioaccumulative. No current Canadian monitoring data were found for evaluation of levels or trends in environmental concentrations of DMAc. In considering the use pattern and release information, however, it is predicted that DMAc would be released in relatively small quantities, mainly to air or water. The modelled toxicity data suggest that DMAc is not highly hazardous to aquatic organisms, and the conservatively calculated risk quotient (described above) indicates that concentrations of DMAc are unlikely to cause harm to sensitive aquatic organisms. Experimental toxicity data suggest that DMAc has some potential to cause harmful effects in soil-dwelling organisms. However, given the relatively large dilution capacity of the atmosphere and the current use pattern data, exposure to DMAc for soil-dwelling organisms will likely be low.
Based on these considerations, DMAc is unlikely to be causing ecological harm in Canada.
Uncertainties in Evaluation of Ecological Risk
No experimental bioaccumulation data were found, and models were consequently used to estimate the bioaccumulation potential.
There were uncertainties associated with the release scenario used to derive the PEC, which were addressed by making conservative assumptions.
Based on the predicted partitioning behaviour of this chemical, the significance of soil as a medium of exposure is not well addressed by the effects data available. Indeed, the effects data identified apply primarily to pelagic aquatic exposures. However, exposure of soil-dwelling organisms to DMAc is expected to be low.
Potential to Cause Harm to Human Health
Exposure Assessment
There were no empirical data identified regarding measured concentrations of DMAc in environmental media in Canada.
Monitoring data in other locations were identified for drinking water, surface water, outdoor air and indoor air and are used as surrogates for Canadian-specific data in order to estimate exposure, as described below. However, no monitoring data were identified for DMAc in soil, sediment, food or beverages, regardless of location. Residual levels of DMAc in food are not currently monitored by the Canadian Food Inspection Agency (email from Canadian Food Inspection Agency, November 10, 2008; unreferenced).
An indoor air study of a high-security data centre located in the United States indicated a maximum DMAc concentration of 4700 µg/m3 (1300 ppb), measured after the receipt of air quality complaints of occupants (Yocom et al. 1984; emails from Dr. Nasrat Hijazi May 28, 2008, and November 10, 2008; unreferenced). This office setting was used as a surrogate for residential data, as indoor air values for a home setting could not be identified. This value was not considered representative of general exposure, as correction of an air balance issue through ventilation system changes reduced the maximum air concentration of DMAc to 34 µg/m3 (9.3 ppb) (Yocom et al. 1984; email from Dr. Nasrat Hijazi, May 28, 2008; unreferenced). The lower concentration was used to estimate intake of DMAc from indoor air (see Appendix 1).
DMAc was detected at all of six outdoor air sampling sites within a mile-radius of a hazardous liquid waste impoundment (location not provided), at mean levels of 11-9600 ng/m3 (Guzewich et al. 1983). These levels were averaged for the months of June and August 1982 in order to avoid upwind/downwind air flow bias (Guzewich et al. 1983). As some transfers of DMAc to hazardous waste facilities occurred in the 2006 calendar year, these air levels are considered applicable to a Canadian setting and are conservative on the basis of the close proximity of the sampling sites to the evaporation lagoon, in addition to the measurements having occurred during summer months when higher temperatures augment evaporation rates (Guzewich et al. 1983; Environment Canada 2008a). The maximum outdoor air level of 9600 ng/m3 was not considered representative of general exposure, as the value was measured within a few feet north of the evaporation lagoon (Guzewich et al. 1983). An outdoor air level of 930 ng/m3 was measured at a distance of 300 feet and was used to estimate intake of DMAc from outdoor air (see Appendix 1). This distance coincides best with the regulatory minimum setback distances identified for residential property lines and organic soil conditioning sites in Ontario and septic stabilizing lagoons and biosolids land application and storage sites in Nova Scotia (Guzewich et al. 1983; Ontario 1990; NSEL 2004, 2006).
A study of river water in Japan revealed a maximum DMAc concentration of 0.093 µg/L (Kadokami et al. 1993); however, in another study, DMAc was not detected in Japanese river water above the detection limit of 0.02 µg/L (Kawata et al. 2001). A study performed in New Jersey using the Total Exposure Assessment Methodology (TEAM) revealed no detectable DMAc in one drinking water sample using a detection limit of 1 ng/g (Wallace et al. 1984). A study performed in Japan revealed no detectable DMAc in tapwater and well water with a detection limit of 0.044 µg/L (Kadokami et al. 1993). The drinking water detection limit of the TEAM study, 1 µg/L (1 ng/g) (Wallace et al. 1984), was used as the most conservative estimate of the DMAc concentration in drinking water for the intake estimate (see Appendix 1), whereas the maximum concentration of DMAc in surface water of 0.093 µg/L (Kadokami et al. 1993) was used as the most conservative estimate of the DMAc concentration in surface water for the purposes of ChemCAN modelling.
As no monitoring studies have been identified regarding DMAc levels in soil or sediment, release data obtained from information reported under section 71 of CEPA 1999 were used to model soil and sediment concentrations. ChemCAN, a Canadian-specific environmental exposure model, predicted negligible concentrations of DMAc in soil and sediment (ChemCAN 2003). Appendix 2 displays the predicted concentrations of DMAc for each environmental compartment.
Although adding to the knowledge base for DMAc, additional monitoring studies identified were not used to quantify exposure. DMAc was qualitatively detected in samples derived from a sedimentation tank at the Werdhozl sewage treatment plant of Zurich, Switzerland, on an unspecified date (Hangartner 1979). A TEAM study revealed no detectable DMAc in breathing-zone air, whereas DMAc was detected in some exhaled breath samples (Wallace et al. 1984). A study in Japan revealed mean DMAc levels in seawater, rain water and sewage plant effluent of 0.052 µg/L, 0.64 µg/L and 0.59 µg/L, respectively (Kadokami et al. 1993). In addition, two biomonitoring studies demonstrated the high permeability of skin to DMAc vapour (Maxfield et al. 1975; Nomiyama et al.2000).
The maximum daily intake of DMAc was estimated as approximately 18 µg/kg body weight (kg-bw) per day for toddlers between the ages of 0.5 and 4 years. Intake of DMAc from indoor air was the predominant source of environmental exposure at a maximum DMAc concentration of 34 µg/m3 (Yocom et al. 1984). Appendix 1 displays the estimated total multimedia intakes for different age groups.
In regards to estimating exposure to DMAc present in consumer products, DMAc may occur as an unintended manufacturing residue in several products, as described below.
DMAc may be present as residual solvent in some textile articles. However, through blending of fibres with cotton or wool and the application of bleaching and wet-dry treatment processes, DMAc levels diminish below 0.01% by weight in textile end products (Armstrong et al. 1980; OECD 2001). High vapour pressure leads to rapid evaporation of residual DMAc post-manufacture, further contributing to a reduction of DMAc levels prior to textile articles reaching consumers (DEPA 2005). Sweat simulation tests of a 150 g elastane-containing bathing suit worn for 3 h led to an uptake of 2.1 µg DMAc/kg-bw (Bayer et al. 1998). Perspiration fastness tests of 100% acrylic socks and T-shirts worn for 10 h led to respective maximum uptake levels of 0.30 and 1.3 µg DMAc/kg-bw (Bayer et al. 1998). The textile-related uptakes from Bayer et al. (1998) were adjusted to the Health Canada-specific default adult body weight. As such, textile-related consumer exposure is considered minimal in comparison with estimated environmental exposure (OECD 2001). This conclusion is consistent with the findings of another study that although residual DMAc could be detected at levels higher than 0.01% by weight in some commercial acrylic fibre products, diffusion of DMAc could not be detected under conditions of expected use, including apparel exposed to hand laundering, dry cleaning and synthetic perspiration and wig fibre exposed to hand shampooing (Armstrong et al. 1980).
With regards to carpeting and carpet undercushion, although a U.S. study using undercushion samples obtained in 1993 detected emissions of DMAc (Schaeffer et al. 1996), the substance was not detected in more recent emission studies specific to the Canadian context (IA-QUEST 2008), and therefore an exposure scenario was not conducted for this use. In the U.S. study, 96-h specific mass emissions of DMAc from carpet cushions in the presence and absence of overlying carpet were measured at 65 mg/m2 and 90 mg/m2, respectively (Schaeffer et al. 1996). Carpet cushion refers to a layer underlying the carpet installed in most residences for comfort and temperature insulation (Schaeffer et al.1996). The presence of carpet lowered the DMAc emission rate, as the carpet served the function of a diffusion barrier. Greater DMAc emissions were observed for cushion samples obtained directly from manufacturers rather than retailers, suggesting losses due to off-gassing during transport, handling and storage (Schaeffer et al. 1996). More recent Canadian-specific data relevant to DMAc emissions from carpet and carpet cushion have been obtained from the National Research Council of Canada's (NRCC) Indoor Environment Research Program (IA-QUEST 2008). No DMAc emissions were identified from eight carpet samples; six samples were obtained from local retail outlets in 1999, and two samples were obtained from renovation sites in 2004 (IA-QUEST 2008). The lack of detectable DMAc emissions from the carpet samples is consistent with the findings of another study, which did not detect DMAc emissions at a detection limit of 0.1 ppm from acrylic carpet samples enclosed in sealed containers that simulated closed rooms (Armstrong et al. 1980). In addition, no DMAc emissions were identified from three samples of undercushion, two of which were obtained from a local retail outlet in 1998 (IA-QUEST 2008; email from National Research Council Canada, December 11, 2008; unreferenced).
However, the Indoor Environment Research Program was able to identify DMAc emissions from a medium-density fibreboard shelving unit and a flooring assembly composed of linoleum tile, adhesive and plywood (IA-QUEST 2008). Maximum DMAc emission rates of 0.095 12 mg/m2 per hour and 0.068 76 mg/m2 per hour were measured in 2003 for the shelving unit and flooring assembly, respectively (IA-QUEST 2008). An emissions scenario, presented in Appendix 3, conducted for a bedroom containing flooring composed of linoleum assembly with a three-bracket wall shelving unit revealed a maximum DMAc indoor air concentration of 9.8 µg/m3 occurring 4.9 h after installation. As the air concentration displayed rapid first-order decay after 4.9 h to approximately 0.6 µg/m3 by 100 h, a margin of exposure (MOE) calculated on the basis of the maximum concentration would be conservative over a short-term exposure period of several days.
DMAc was detected in a toothbrush containing a polypropylene handle with thermoplastic elastomer component (Svendsen et al. 2004). An exposure scenario was not conducted for this use, as DMAc residuals in toothbrushes were not specifically identified in Canada.
DMAc was also detected semi-quantitatively in a latex balloon (Nilsson 2007). Semi-quantitative detection was insufficient for determining concentrations of DMAc in latex balloons, and therefore an exposure scenario was not conducted for this use.
In the polymer production industry, DMAc is a contaminant in the manufacturing process of PVF polymers. As these polymers may be used in the fabrication of the interior liner of flexible bulk containers used to transport water, wine and juice, there is potential migration of DMAc into these food items (email from Food Directorate, Health Canada, October 24, 2008; unreferenced). Tested samples of PVF film tend to have varying levels of DMAc, suggesting evolution of the latent solvent over time (Chase 2001). PVF film contains a maximum DMAc concentration of 0.5% by weight (email from Food Directorate, Health Canada, October 14, 2008; unreferenced). The results of extraction tests performed on food packaging material revealed no detectable DMAc (email from Food Directorate, Health Canada, October 24, 2008; unreferenced). Using the detection limit of 0.09 µg/L, the probable daily oral intake of DMAc was calculated as 0.000 23 µg/kg-bw per day for an adult (email from Food Directorate, Health Canada, October 24, 2008; unreferenced). This daily intake is considered negligible in comparison with estimated environmental exposure.
Finally, in one study, DMAc was detected in air at concentrations ranging between 205 and 465 µg/m3 within a 10-L bag constructed of PVF film (Ramalho 2002). The bag was used to sample indoor air in a 35-m2 living room. No DMAc was detected in the living room; therefore, the DMAc was likely an artifact of the sampling container (Ramalho 2002). As these PVF bags were designed specifically for experimental air sampling, a consumer exposure scenario was not conducted for this use.
Health Effects Assessment
The available health effects information and associated lowest-effect levels for DMAc are summarized in Appendix 4.
The European Commission has classified DMAc as Category 2 for developmental toxicity with risk phrase R61 ("May cause harm to unborn child") (ESIS [date unknown]). This classification is based primarily upon rodent studies, in which exposure to DMAc was by oral, inhalation or dermal administration. In the oral studies, the fetal malformations were observed at maternally toxic exposure levels, whereas in the studies conducted via inhalation and dermal exposure, fetal effects were observed in the absence of maternal toxicity. The details of the studies are described below and also presented in Appendix 4.
Developmental effects were observed in rabbits and rats exposed to DMAc via oral, dermal and inhalation administration. The lowest exposure levels at which developmental effects were observed were 282 mg/kg-bw per day (oral) (Merkle and Zeller 1980), 199.5 ppm (700 mg/m3) (inhalation) (BASF 1989; Klimisch and Hellwig 2000) and 500 mg/kg-bw per day (dermal) (Monsanto 1973), as described below.
When New Zealand White rabbits were exposed to 0, 94, 282 or 470 mg/kg-bw per day by oral gavage during gestation days 6-18, fetal effects, including cleft palate, fused ribs, microphthalmia (small eyes) and reduced body weight, were observed at 282 mg/kg-bw per day and above. Reduced maternal body weight was observed at all dose levels (Merkle and Zeller 1980). In oral gavage studies in pregnant rats, marked maternal and fetal effects were observed at 400 mg/kg-bw per day (Johannsen et al. 1987; DuPont Haskell Laboratory 1997).
Inhalation exposure of Himalayan rabbits to DMAc at 0, 57, 199.5 or 570 ppm (0, 200, 700 or 2000 mg/m3) for 6 h/day during gestation days 7-19 resulted in a significant increase (p less than 0.01) in skeletal variations at 199.5 ppm (700 mg/m3). The no-observed-adverse-effect concentrations (NOAECs) for maternal and fetal toxicity were greater than 570 and 57 ppm (greater than 2000 and 200 mg/m3), respectively (BASF 1989; Klimisch and Hellwig 2000). In another inhalation study in Sprague-Dawley rats, significantly reduced (p less than 0.05) fetal weight was reported at 282 ppm (1005 mg/m3). The NOAEC for both maternal and developmental endpoints was 100 ppm (356 mg/m3) (Solomon et al. 1991).
In a dermal study, when New Zealand White rabbits were exposed to 125, 250 or 500 mg DMAc/kg-bw per day (no controls were reported) via the dermal route during gestation days 6-18, fetal abnormalities (deviation of the sternum, reduced body weight, cyclopy, umbilical hernia) were observed at 500 mg/kg-bw per day, in the absence of maternal toxicity (Monsanto 1973). Stula et al. (1973) reported a maternal and fetal no-observed-adverse-effect level (NOAEL) in rats for dermal exposure to DMAc of 600 mg/kg-bw per day, whereas maternal toxicity, increased resorption and fetal malformations were reported at 1200 mg/kg-bw per day.
No reproductive toxicity was reported in male and female rats or male mice when DMAc was administered by inhalation up to 386 and 700 ppm (1375 and 2494 mg/m3), respectively (Ferenz and Kennedy 1986; Wang et al. 1989; Fairhurst et al. 1992).
No evidence of carcinogenicity or genotoxicity associated with exposure to DMAc has been reported in in vivo or in vitro assays. DMAc was not carcinogenic in a 2-year drinking water study in rats exposed to doses up to 1000 mg/kg-bw per day (Monsanto 1980; DuPont 1988). Similarly, no carcinogenic effects were reported in a 2-year inhalation study in rats and an 18-month inhalation study in mice exposed to concentrations up to 350 ppm (1247 mg/m3) (Malley et al. 1995). DMAc did not show clear genotoxic activity in a relatively wide range of in vitro and in vivo assays (Arnold et al. 1972; BASF 1976; McGregor 1981; DuPont 1988; May 1989; Monroe and Mitchell 1993; Martin [date unknown]).
In addition to developmental toxicity, liver toxicity is identified as a critical effect induced by DMAc exposure, based on observations in short-term and chronic exposure studies in experimental animals and supported by information from human occupational exposure studies.
In a chronic inhalation study, when male and female rats and mice were exposed by inhalation to DMAc at 0, 25, 100 or 350 ppm (0, 89, 356 or 1247 mg/m3) for 6 h/day, 5 days/week, for 2 years and 18 months, respectively, the lowest-observed-adverse-effect level (LOAEC) was reported as 100 ppm (356 mg/m3). This LOAEC was based on significantly increased (p 0.05) serum cholesterol and glucose levels and increased relative liver weights in female rats, whereas in male rats there was a significantly increased (p less than 0.05) hepatic focal cystic degeneration and increased hepatic peliosis. Increased relative liver weight and incidence of individual hepatocellular necrosis were observed in male mice (Malley et al. 1995). Similarly, the results of a short-term repeated-dose inhalation toxicity study in rats showed elevated serum protein levels at 30 ppm (107 mg/m3) and liver damage at 300 ppm (1069 mg/m3) (Kinney et al. 1993). In a chronic oral toxicity study, when male and female rats were exposed to 0, 100, 300 or 1000 mg DMAc/kg-bw per day in drinking water for 2 years, decreased body weight and increased liver and adrenal weights were reported at 100 mg/kg-bw per day and above (Monsanto 1980; DuPont 1988). Several other short-term and subchronic studies revealed liver toxicity associated with DMAc exposure (Horn 1961; Ferenz and Kennedy 1986; Kennedy and Sherman 1986; Wang et al. 1989).
Available limited epidemiological data suggest no clear relationship between exposure to DMAc and mortality from specified tumours among workers exposed to DMAc and acrylonitrile (Mastrangelo et al. 1993). In another cohort study, liver injury was examined among 440 workers in elastane fibre factories exposed to DMAc for up to 31 months. The exposure concentration was estimated based on the concentration of urinaryN-methylacetamide (NMA). nmA, a metabolic end product of DMAc, is used as an indicator of DMAc exposure. During the exposure period, 28 cases of DMAc-induced hepatic injury were reported. The incidence rates were 7-10 times higher in high exposure groups (greater than 30 mg NMA/g creatinine) than in low exposure groups (greater than 20 mg nmA/g creatinine), suggesting that DMAc-induced hepatic injury is dose dependent (Lee et al. 2006). In another cohort study, Corsi (1971) reported a "clear relationship between liver impairment and exposure duration" in 19 of 41 men occupationally exposed to DMAc for 2-10 years. The exposure concentrations were not reported. In contrast, no exposure-related changes in serum chemistry parameters that indicate liver injury were observed among 127 workers exposed to a 12-h time-weighted average concentration of 1.9 ppm (approximately 6.8 mg/m3) of DMAc in an acrylic fibre plant (Spies et al. 1995).
In addition, DMAc is also a mild skin and eye irritant (OECD 2001).
The confidence in the database for developmental toxicity and hepatotoxicity is moderate to high, as consistent data are available from different experimental animal species following exposure by various routes of administration. DMAc-induced hepatotoxicity is also supported by epidemiological observations.
Characterization of Risk to Human Health
Based on consideration of the weight of evidence-based classification of DMAc by the European Commission as Category 2 for developmental toxicity (ESIS [date unknown]) and consideration of available relevant data, the critical effects for characterization of risk to human health for DMAc are developmental toxicity and liver toxicity. Therefore, ratios, referred to as the margins of exposure (MOEs), are derived between the lowest exposure levels associated with induction of these effects and estimates of population exposure to DMAc. Estimated MOEs for various environmental and consumer product exposure scenarios are summarized below, with details tabulated in Appendix 5.
The principal source of exposure to DMAc for the general population is considered to be indoor air. A comparison between the lowest inhalation LOAECs available for developmental toxicity in experimental animals (i.e., 700 mg/m3) and for chronic liver toxicity in experimental animals (i.e., 356 mg/m3) and the highest concentration identified in an indoor air study after ventilation changes (i.e., 0.034 mg/m3) results in margins of exposure of approximately 10 500-20 600. These margins are considered adequate to account for uncertainties in the database, in light of the conservative nature of the exposure estimate and critical effect levels in studies in experimental animals.
Potential exposure to DMAc present in drinking water is estimated to be lower than potential indoor air exposure. Comparison of a most conservative oral intake of 0.11 µg/kg-bw per day for formula-fed infants up to 0.5 years of age from DMAc present in drinking water at a maximum concentration of 1 µg/L with the lowest oral LOEL from a chronic oral study (i.e., 100 mg/kg-bw per day) results in a margin of exposure of approximately 910 000. This margin of exposure would be adequate to protect the general population from exposure resulting from consumption of drinking water containing DMAc.
Potential exposure from consumer products containing DMAc is estimated to be lower than potential indoor air exposure. Comparison of potential residual DMAc exposure from consumer products (i.e., wearing a T-shirt, socks and bathing suit; potential exposure to DMAc volatilized from linoleum flooring assembly and medium-density fibreboard shelving) with critical effect levels in experimental animals results in margins of exposure ranging from approximately 11 000 to 81 000 (see Appendix 5). These margins of exposure would be adequate to protect the general population from exposure to consumer products containing residual DMAc.
Uncertainties in Evaluation of Risk to Human Health
Considering that releases reported under section 71 of CEPA 1999 were under 100 kg, environmental concentrations in Canada are expected to be negligible. Owing to greater reliability of experimental data in comparison with modelled data, the use of experimental air and water monitoring data derived from international sources increased confidence in the environmental exposure estimates. However, it should be noted that Canadian monitoring data were not identified in literature searches performed as recently as November 2008.
The scope of this screening assessment does not take into account possible differences in sensitivity to DMAc-induced effects between laboratory animals and humans as well as individual variability in sensitivity across the human population. However, it is noteworthy that developmental toxicity and liver toxicity were seen in multiple species of experimental animals by all exposure routes. In addition, DMAc-induced liver toxicity in experimental animals was observed for different exposure durations ranging from acute to chronic. Although available epidemiological data suggest a relationship between exposure to DMAc and liver damage among exposed workers in an occupational setting, the concentration at which these effects occurred is a considerable area of uncertainty. The possible existence of sensitive subpopulations among workers is another area of uncertainty. However, available epidemiological data suggest that there may be an inverse association between liver effects and the duration of employment, suggesting a possible healthy worker effect or development of tolerance.
Conclusion
Based upon consideration of the margins of exposure between conservative estimates of exposure to DMAc from environmental media and concentrations associated with developmental and liver effects in experimental animals, it is concluded that DMAc should not be considered "toxic" as defined in paragraph 64(c) of CEPA 1999: i.e., DMAc is not a substance entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
Based on the information presented in this screening assessment, it is concluded that DMAc is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
It is therefore concluded that DMAc does not meet the definition of "toxic" as set out in section 64 of CEPA 1999. Additionally, DMAc does not meet the criteria for persistence and bioaccumulation potential as set out in the Persistence and Bioaccumulation Regulations (Canada 2000).
References
[ACGIH] American Conference of Governmental Industrial Hygienists. 2006. TLVs and BEIs based on the documentation of the threshold limit values for chemical substances and physical agents and biological exposure indices. Cincinnati (OH): ACGIH.
Adema DMM, van den Bos Bakker GS. 1987. Aquatic toxicity of compounds that may be carried by ships (Marpol 1973, Annex II). A progress report for 1986. Report No.: R 86/326a. Eindhoven (NL): TNO (Netherlands Organisation for Applied Scientific Research). p. 1-20. [cited in OECD 2001].
[AIES] Artificial Intelligence Expert System. 2003-2005. Version 1.25. Ottawa (ON): Environment Canada. Model developed by Stephen Niculescu. Available from: Environment Canada, Existing Substances Division, New Substances Division.
[AIHAAP] American Industrial Hygiene Association Journal. 1962. Vol. 23: 95
[Anon] Anonymous. 1979. Studies on maximum allowable concentration of N,N-dimethyl-acetamide [author's translation from Chinese]. Zhonghua Yu Fang Yi Xue Za Zhi [Chin J Prev Med] 13(1): 29-31. Reported in Du Pont-Haskell Laboratory Internal DMAc Review, October 1988 [cited in OECD 2001].
[AOPWIN] Atmospheric Oxidation Program for Windows [Estimation Model]. 2000. Version 1.91. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. http://www.epa.gov/oppt/exposure/pubs/episuite.htm
Armstrong AA, Borie RD, Lanier WW. 1980. Residual solvent and its diffusion in acrylic fibers. Polym Eng Sci 20(4): 282-285.
Arnold D et al. 1972. Industrial Bio-Test Laboratories, Inc., Report IBT-E582A. Reported in Du Pont-Haskell Laboratory Internal DMAc Review, October 1988 [cited in OECD 2001].
Arnot JA, Gobas FAPC. 2003. A generic QSAR for assessing the bioaccumulation potential of organic chemicals in aquatic food webs. QSAR Comb Sci [Internet] 22(3): 337-345. [restricted access] http://www3.interscience.wiley.com/journal/104557877/home_
[ASTER] Assessment Tools for the Evaluation of Risk [Internet]. 1999. Duluth (MN): US Environmental Protection Agency, Mid-Continent Ecology Division. [cited 2006 Mar]. [restricted access] http://www.epa.gov/med/Prods_Pubs/aster.htm
Atkinson R. 1989. Kinetics and mechanisms of the gas-phase reactions of the hydroxyl radical with organic compounds. J Phys Chem Ref Data Monogr 1.
Bartsch W, Sponer G, Dietmann K, Fuchs G. 1976. Acute toxicity of various solvents in the mouse and rat. Arzneimittel-Forsch 26: 1581-1583.
[BASF] BASF Aktiengesellschaft. 1976. Unpublished data, 4/6/76. Reported in Du Pont-Haskell Laboratory Internal DMAc Review, October 1988. [cited in OECD 2001].
[BASF] BASF Aktiengesellschaft. 1977. Unpublished report ZST-No. XXIII/236-2, 24/11/1977. [cited in OECD 2001].
[BASF] BASF Aktiengesellschaft. 1979. Unpublished report 78/277. [cited in OECD 2001].
[BASF] BASF Aktiengesellschaft. 1988. Unpublished data, Ecological Laboratory. [reported in Alstoff Information Data Bank, 13 November 1992]. [cited in OECD 2001].
[BASF] BASF Aktiengesellschaft. 1989. Unpublished data, Toxicology Laboratory, ZST-No. 90R0104/8638, Gen. 1989. [cited in OECD 2001].
Bayer, DuPont, Ertisa, Fisipe, Montefibre, CIRFS. 1998. [Unpublished document concerning DMAc] [Internet]. [cited in OECD 2001]. [cited 2008 Nov 13]. http://ecb.jrc.it/classlab/1995a16.doc
[BCFWIN] BioConcentration Factor Program for Windows [Estimation Model]. 2000. Version 2.15. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. http://www.epa.gov/oppt/exposure/pubs/episuite.htm
[BIBRA] British Industrial Biological Research Association. 1989. Toxicity Profile for Dimethylacetamide. 1st ed. 1-10.
[BIOWIN] Biodegradation Probability Program for Windows [Estimation Model]. 2000. Version 4.02. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. http://www.epa.gov/oppt/exposure/pubs/episuite.htm
Boethling RS, Howard PH, Beauman JA, Larosche ME. 1995. Factors for intermedia extrapolations in biodegradability assessment. Chemosphere 30(4): 741-752.
Borm PJA, De Jong L, Vliegen A. 1987. Environmental and biological monitoring of workers occupationally exposed to dimethylacetamide. J Occup Med 29: 898-903. [cited in Finlay et al. 2001; Kennedy 2001].
Canada. [1978]. Food and Drug Regulations, C.R.C., c. 870. http://www.hc-sc.gc.ca/fn-an/legislation/acts-lois/fdr-rad/index-eng.php
Canada. 1988. Ingredient Disclosure List [Internet]. SOR/88-64. [cited 2008 Oct 9]. http://www.canlii.org/ca/regu/sor88-64/part274942.html_
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C., 1999, c. 33. http://www.gazette.gc.ca/archives/p3/1999/g3-02203.pdf
Canada. 2000. Canadian Environmental Protection Act: Persistence and Bioaccumulation Regulations, P.C. 2000-348, 23 March 2000, SOR/2000-107. http://canadagazette.gc.ca/partII/2000/20000329/pdf/g2-13407.pdf
Canada, Dept. of the Environment, Dept. of Health. 2006.Canadian Environmental Protection Act, 1999: Notice of intent to develop and implement measures to assess and manage the risks posed by certain substances to the health of Canadians and their environment. Canada Gazette, Part I, vol. 140, no. 49, p. 4109-4117. http://canadagazette.gc.ca/partI/2006/20061209/pdf/g1-14049.pdf
Canada, Dept. of the Environment, Dept. of Health. 2008. Canadian Environmental Protection Act, 1999: Notice with respect to Batch 5 Challenge substances. Canada Gazette, Part I, vol. 142, no. 7, p. 306-327. http://canadagazette.gc.ca/partI/2008/20080216/pdf/g1-14207.pdf
[CATABOL] Probabilistic assessment of biodegradability and metabolic pathways [Computer Model]. c2004-2008. Version 5.10.2. Bourgas (BG): Bourgas Prof. Assen Zlatarov University, Laboratory of Mathematical Chemistry. http://oasis-lmc.org/?section=software&swid=1
Chase RE. 2001. Properties and manufacture of Tedlar® polyvinyl fluoride film [Internet]. Attachment J, ERC Technical Report. VERL, Ford Research Laboratory. [cited 2008 Oct 17]. http://www.uscarpartnership.org/consortia&teams/ERC/Attachment%20J%20--%20Tedlar%20Manufacture.pdf
ChemCAN [Level III fugacity model of 24 regions of Canada]. 2003. Version 6.00. Peterborough (ON): Trent University, Canadian Centre for Environmental Modelling and Chemistry. [cited 2008 Oct 9]. http://www.trentu.ca/academic/aminss/envmodel/models/CC600.html
Choi TS, Woo KH, Kim JS, Park WS, Ham JH, Jung SJ, Yu JY. 2001. Toxic hepatitis induced by occupational dimethylacetamide exposure. Korean J Occup Environ Med 13(2): 164-170 (in Korean). [cited in Lee et al. 2006].
[CNS] Cosmetic Notification System. [Proprietary Database]. 2008. Ottawa (ON): Health Canada. [cited 2008 Aug].
[ConsExpo] Consumer Exposure Model [Internet]. 2006. Version 4.1. Bilthoven (NL): Rijksinstituut voor Volksgezondheid en Milieu (National Institute for Public Health and the Environment). [cited 2008 Dec 15]. http://www.rivm.nl/en/healthanddisease/productsafety/ConsExpo.jsp#tcm:13-42840
Corsi GC. 1971. [On occupational pathology caused by dimethylacetamide (with special reference to hepatic function).] Med Lav 62: 28-42 (in Italian). [cited in OECD 2001].
Daubert TE, Danner RP. 1989. Physical and thermodynamical properties of pure chemicals; data compilation. New York (NY): Hemisphere Publishing Corporation; Washington (DC): Taylor & Francis.
Drug Chem Toxicol 1986. 9: 147 (cited in RTECS).
[DEPA] Danish Environmental Protection Agency. 2005. Screening for health effects from chemical substances in textile colorants [Internet]. Copenhagen (DK): Danish Environmental Protection Agency. [cited 2008 Oct 3]. http://www2.mst.dk/common/Udgivramme/Frame.asp?http://www2.mst.dk/udgiv/publications/2005/87-7614-672-3/html/default_eng.htm
DuPont. 1984. Toxicity studies on DMF and DMAc with cover sheets and letter dated 09/24/84. EPA/OTS:Doc. 86-890000747S. Reported in TOXALL-Data Bank from DuPont-Haskell Laboratory. [cited in OECD 2001].
DuPont. 1988. Du Pont-Haskell Laboratory Internal DMAc Review, October 1988 [no further details given]. [cited in OECD 2001].
DuPont. 1998. Material Safety Data Sheet: Sontara Spunlaced Fabrics (All Types) [Internet]. Sydney (AU): DuPont (Australia) Ltd. [cited 2008 Nov 28]. http://www.conservationresources.com.au/html/home/help_info/downloads/Sontara_MSDS.pdf
DuPont. 2008a. Dimethylacetamide [Internet]. [cited 2008 Oct 9]. http://www2.dupont.com/Products/en_RU/Dimethylacetamide_en.html
DuPont. 2008b. Product list [Internet]. [cited 2008 Oct 3]. http://www2.dupont.com/Roads_Bridges_and_Tunnels/en_TR/assets/downloads/Industrial_Chemicals.en.pdf
DuPont Haskell Laboratory. 1997. (HL-1997-00203). DMAc: Developmental toxicity study in Sprague-Dawley rats. Unpublished data [cited in OECD, 2001].
[ECB] European Chemicals Bureau. Institute for Health and Consumer Protection. 2004. IUCLID (International Uniform Chemical Information Database) dataset for N,N-dimethylacetamide, 24 March 2003, updated from creation date of 12 September 2003. http://ecb.jrc.it/esis/
[ECOSAR] Ecological Structure Activity Relationships [Internet]. 2004. Version 0.99h. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. http://www.epa.gov/oppt/exposure/pubs/episuite.htm
Environment Canada. 2008a. Data for Batch 5 substances collected undertheCanadian Environmental Protection Act, 1999, Section 71: Notice with respect to Batch 5 Challenge substances. Data prepared by: Environment Canada, Existing Substances Program.
Environment Canada. 2008b. Guidance for conducting ecological assessments under CEPA, 1999: Science resource technical series, technical guidance module: the Industrial Generic Exposure Tool - Aquatic (IGETA). Working document. Gatineau (QC): Environment Canada, Existing Substances Division.
Environment Canada. 2008c. IGETA report: CAS RN 127-19-5 2008-08-07. Unpublished report. Gatineau (QC): Environment Canada, Existing Substances Division.
[EQC] Equilibrium Criterion Model. 2003. Version 2.02. Peterborough (ON): Trent University, Canadian Centre for Environmental Modelling and Chemistry. http://www.trentu.ca/academic/aminss/envmodel/models/EQC2.html
[ESIS] European Chemical Substances Information System [database on the Internet]. [date unknown]. European Chemicals Bureau (ECB). [cited 2008 Mar]. http://ecb.jrc.it/esis/
Fairhurst S, Gregg N, Cocker J, Brown R, South D, Garrod A. 1992. HSE criteria document for an OEL,N,N-dimethylacetamide. C20. London (GB): HMSO.
Ferenz RL, Kennedy GL. 1986. Reproduction study of dimethylacetamide following inhalation in the rat. Fundam Appl Toxicol 7: 132-137. [cited in OECD 2001].
Finlay C, Malley LA, Kennedy FL, Jr. 2001. Absorption of dimethylacetamide (DMAC) following application of a polymer film to the skin of rabbits. Appl Occup Environ Hyg 16: 1103-1105.
Frosch PJ, Kligman AM. 1977. Method for appraising the stinging capacity of topically applied substances. J Soc Cosmet Chem 28: 197. [cited in BIBRA 1989].
Geiger DL, Northcutt DJC, Brooke LT, editors. 1990. Acute toxicities of organic chemicals to fathead minnows (Pimephales promelas). Vol. V. Superior (WI): University of Wisconsin Press. p. 133. [cited in OECD 2001].
Guzewich DC, Bond CA, Deeter DP, Valis RJ, Vinopal JM. 1983. Air sampling around a hazardous liquid surface impoundment. In: Proceedings of the 76th Annual Meeting of the Air Pollution Control Association, 19 June 1983, Atlanta, GA, USA. Paper 24.1. Pittsburgh (PA): Air Pollution Control Association.
Hangartner M. 1979. Sampling and analysis of odorous compounds in water treatment plants. Int J Environ Anal Chem 6(2): 161-169. [cited in HSDB 2009].
Hansch C, Leo A, Hoekman D. 1995. Exploring QSAR: Hydrophobic, electronic, and steric constants. Washington (DC): American Chemical Society.
Hapke HJ, Majid H. 1983. [Hemolytic activity of solvents. 2. Effects of selected organic solvents.] Dtsch Tierarztl Wochenschr 90: 216-218 (in German).
Health Canada. 1998. Exposure factors for assessing total daily intake of priority substances by the general population of Canada. Unpublished report. Ottawa (ON): Health Canada, Environmental Health Directorate.
Health Canada. 1999. Impurities: Guideline for Residual Solvents [Internet]. International Conference on Harmonisation of Technical Requirements for the Registration of Pharmaceuticals for Human Use (ICH) / Therapeutic Products Programme guideline. Ottawa (ON): Health Canada, Therapeutic Products Programme. [cited 2008 Oct 9]. http://www.hc-sc.gc.ca/dhp-mps/prodpharma/applic-demande/guide-ld/ich/qual/q3c-eng.php
Health Canada. 2006. Current information on veterinary drugs and post-categorization of the Domestic Substances List. [Internal Document]. Prepared November 29, 2006. Ottawa (ON): Health Canada, Veterinary Drugs Directorate.
Health Canada. 2007. The cosmetic ingredient hotlist [Internet]. March 2007. Ottawa (ON): Health Canada, Consumer Product Safety. [cited 2008 Oct 9]. http://www.hc-sc.gc.ca/cps-spc/person/cosmet/hotlist-liste_e.html
Horn HJ. 1959. Unpublished summary of data, Hazleton Laboratories, 7/20/59. Reported in Du Pont-Haskell Laboratory Internal DMAc Review, October 1988. [cited in OECD 2001].
Horn HJ. 1961. Toxicology of dimethylacetamide. Toxicol Appl Pharmacol 3: 12-24 [cited in OECD 2001].
[HSDB] Hazardous Substances Data Bank [database on the Internet]. 1983- . Bethesda (MD): National Library of Medicine (US). [revised 2003 Feb 14; cited 2009 Jan 23]. http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB
[HYDROWIN] Hydrolysis Rates Program for Microsoft Windows [Estimation Model]. 2000. Version 1.67. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. http://www.epa.gov/oppt/exposure/pubs/episuite.htm
[IA-QUEST] Indoor Air Quality Emission Simulation Tool [database on the Internet]. 2008. Version 1.1. Ottawa (ON): National Research Council Canada, Institute for Research in Construction. [cited 2008 Dec 15]. http://irc.nrc-cnrc.gc.ca/ie/iaq/iaquest_e.html
[IKEA] Inter IKEA Systems B.V. 2008. Järpen: Shelf [Internet]. Designer: IKEA of Sweden. [cited 2008 Dec 16]. http://www.ikea.com/ca/en/catalog/products/20025047
Johannsen FR, Levinskas GJ, Schardein JIL. 1987. Teratogenic response of dimethylacetamide in rats. Fundam Appl Toxicol 9: 550-556. [cited in OECD 2001].
Jung SJ, Lee CY, Kim SA, Park SA, Ha BG, Kim J, Yu JY, Choi T. 2007. Dimethylacetamide-induced hepatic injuries among spandex fibre workers. Clin Toxicol (Phila) 45(5): 435-439.
Kadokami K, Sato K, Koga M. 1993. Concentrations of 14 hydrophilic chemicals in natural waters at Kitakyushu area. J Environ Chem 3(1): 15-23.
Kafyan VB. 1971. Toxicity of dimethylacetamide. Zh Eksp Klin Med 11(1): 39-42. Reported in DuPont-Haskell Laboratory Internal DMAc Review, October 1988. [cited in OECD 2001].
Katosova LD, Pavlenko GI. 1985. Cytogenetic examination of the workers of chemical industry. Mutat Res 147: 301-302. Reported in DuPont-Haskell Laboratory Internal DMAc Review, October 1988. [cited in OECD 2001].
Kawata K, Ibaraki T, Tanabe A, Yagoh H, Shinoda A, Suzuki H, Yasuhara A. 2001. Gas chromatographic-mass spectrometric determination of hydrophilic compounds in environmental water by solid-phase extraction with activated carbon fiber felt. J Chromatogr A 911: 75-83.
Kennedy GL Jr. 1986. Biological effects of acetamide, formamide and their monomethyl and dimethyl derivatives. CRC Crit Rev Toxicol 17: 129-182.
Kennedy GL Jr. 2001. Biological effects of acetamide, formamide, and their mono and dimethyl derivatives: An update. CRC Crit Rev Toxicol 31: 139-222.
Kennedy GL Jr, Sherman H. 1986. Acute and subchronic toxicity of dimethylformamide and dimethylacetamide following various routes of administration. Drug Chem Toxicol 9(2): 147-170.
Kinney LA, Burgess BA, Stula EF, Kennedy GL Jr. 1993. Inhalation studies in rats exposed to dimethylacetamide (DMAc) from 3 to 12 hours per day. Drug Chem Toxicol 16: 175-194. [cited in Kennedy 2001].
Klimisch HJ, Hellwig J. 2000. Developmental toxicity of dimethylacetamide in rabbits following inhalation exposure. Hum Exp Toxicol 19: 676-693.
Lee CY, Jung SJ, Kim SA, Park KS, Ha BG. 2006. Incidence of dimethylacetamide induced hepatic injury among new employees in a cohort of elastane fibre workers. Occup Environ Med 63: 688-693.
Lide DR. 2000. CRC handbook of chemistry and physics. 81st ed. Boca Raton (FL): CRC Press.
Malley LA, Slone TW Jr, Makovec GT, Elliott GS, Kennedy GL Jr. 1995. Chronic toxicity/oncogenicity of dimethylacetamide in rats and mice following inhalation exposure. Fundam Appl Toxicol 28: 80-93. [cited in OECD 2001].
Martin BE. [date unknown]. Unpublished data, DuPont-Haskell Laboratory, Report No. 464-76-MR No. 2389-001. [cited in OECD 2001].
Mastrangelo G, Serena R, Marzia V. 1993. Mortality from tumours in workers in an acrylic fibre factory. J Occup Med 43(3): 155-158. [cited in OECD 2001].
Maxfield ME, Barnes JR, Azar A, Trochimowicz HJ. 1975. Urinary excretion of metabolite following experimental human exposures to DMF or to DMAC. J Occup Med 17: 506-511. [cited in Fairhurst et al. 1992; Finlay et al. 2001].
May K. 1989. Dimethylacetamide: Assessment of mutagenic potential in histidine auxotrophs of Salmonella typhimurium (the Ames test). Report to Montefibre from K. May. LSR Report 89/MTR089/0568, p. 1-28. [cited in OECD 2001].
McGregor DF. 1981. Tier II mutagenic screening of 13 NIOSH priority compounds: DMAc. Report No. 33. PB83-13390-0. [cited in OECD 2001].
Merkle J, Zeller H. 1980. Studies on acetamides and formamides for embryotoxic and teratogenic activities in the rabbit [translated]. Arzneim Forsch (Drug Res) 30(9): 1557-1562. [cited in OECD 2001].
Monroe TJ, Mitchell MA. 1993. In vivo mutagenesis induced by CC-1065 and adozelesin DNA alkylation in a transgenic mouse model. Cancer Res 53: 5690-5696. [cited in OECD 2001].
[Monsanto] Monsanto Chemical Company. 1960. Technical Data Sheet: Dimethylacetamide (DMAC). St. Louis (MO): Monsanto. [cited in Riddick et al. 1986].
[Monsanto] Monsanto Chemical Company. 1973. Unpublished data: Teratogenicity study, rabbits. [reported in Alstoff Information Data Bank, 13 November 1992, and in Kennedy 1986]. [cited in OECD 2001].
[Monsanto] Monsanto Chemical Company. 1980. Unpublished data: Report No. BDN-75-61, Project No. 75-1267, January 7, 1980. [cited in DuPont 1988]. [cited in OECD 2001].
[NCI] National Chemical Inventory [database on a CD-ROM]. 2006. Columbus (OH): American Chemical Society. [cited 2006 Dec 11]. http://www.cas.org/products/cd/nci/require.html
[NHW] Department of National Health and Welfare. 1990. Present patterns and trends in infant feeding in Canada. Catalogue No. H39-199/1999E; ISBN 0-662-18397-5. Ottawa (ON): Department of National Health and Welfare. 9 p. [cited in Health Canada 1998].
Nilsson N. 2007. Analysis of chemical substances in balloons [Internet]. Survey of Chemical Substances in Consumer Products, No. 89. Copenhagen (DK): Danish Environmental Protection Agency, Danish Technological Institute. [cited 2008 Oct 9]. http://www2.mst.dk/common/Udgivramme/Frame.asp?http://www2.mst.dk/Udgiv/publications/2007/978-87-7052-662-3/html/default_eng.htm
[NITE] National Institute of Technology and Evaluation (Jpn). 2002. Biodegradation and bioconcentration of existing chemical substances under the Chemical Substances Control Law [Internet]. Tokyo (JP): NITE. [cited 2008 Mar]. http://www.safe.nite.go.jp/english/kizon/KIZON_start_hazkizon.html
Nomiyama T, Omae K, Ishizuka C, Yamauchi T, Kawasumi Y, Yamada K, Endoh H, Sakurai H. 2000. Dermal absorption ofN,N-dimethylacetamide in human volunteers. Int Arch Occup Environ Health 73: 121-126.
[NPRI] National Pollutant Release Inventory [database on the Internet]. 2007. Gatineau (QC): Environment Canada. [cited 2008 Dec 8]. http://www.ec.gc.ca/pdb/querysite/query_e.cfm
[NSEL] Nova Scotia Environment and Labour. 2004. Guidelines for land application and storage of biosolids in Nova Scotia [Internet]. [cited 2009 Jan 21]. http://www.gov.ns.ca/nse/water/docs/BiosolidGuidelines.pdf
[NSEL] Nova Scotia Environment and Labour. 2006. Guidelines for the handling, treatment and disposal of septage [Internet]. [cited 2009 Jan 21]. http://www.gov.ns.ca/nse/water/docs/SeptageGuidelines.pdf
[OASIS Forecast] Optimized Approach based on Structural Indices Set [Internet]. 2005. Version 1.20. Bourgas (BG): Bourgas Prof. Assen Zlatarov University, Laboratory of Mathematical Chemistry. http://oasis-lmc.org/?section=software
[OECD] Organisation for Economic Co-operation and Development. 2001. SIDS Initial Assessment Report for 13th SIAM (Bern, Switzerland, 6-9 November 2001). N, N-Dimethylacetamide, CAS No. 127-19-5. UNEP Publications. Includes accompanying IUCLID data set, 12 September 2003. [The date of "12-Sept. 93" featured on each page of this dataset is apparently incorrect, as references later than 1993 are included.] http://www.chem.unep.ch/irptc/sids/OECDSIDS/127-19-5.pdf
Okuda H, Takeuchi T, Senoh H, Arito H, Nagano K, Yamamoto S, Matsushima T. 2006. Developmental toxicity induced by inhalation exposure of pregnant rats toN,N-dimethylacetamide. J Occup Health 48: 154-160.
Ontario. 1990. Environmental Protection Act: R.R.O. 1990, Regulation 347, s. 15.General - Waste Management [Internet]. [cited 2009 Jan 21]. http://www.e-laws.gov.on.ca/html/regs/english/elaws_regs_900347_e.htm#BK3
[PCKOCWIN] Organic Carbon Partition Coefficient Program for Windows [Estimation Model]. 2000. Version 1.66. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. http://www.epa.gov/oppt/exposure/pubs/episuite.htm
[PhysProp] Interactive PhysProp Database [database on the Internet]. 2008. Syracuse (NY): Syracuse Research Corporation. [cited 2008 Nov 26]. http://www.syrres.com/esc/physdemo.htm
[PMRA] Pest Management Regulatory Agency. 2007. PMRA List of Formulants [Internet]. Ottawa (ON): PMRA. [updated 2007 Jun; cited 2008 Oct 9]. http://www.pmra-arla.gc.ca/english/pdf/reg/reg2007-04-e.pdf
Ramalho O. 2002. Odor intensity of a real room field evaluation and laboratory investigations. In: Indoor Air 2002, Proceedings of the 9th International Conference on Indoor Air Quality and Climate, Monterey, CA, June 30 - July 5, 2002, Vol. 2. Santa Cruz (CA): International Academy of Indoor Air Sciences. p. 273-278.
Riddick JA, Bunger WB, Sakano WB. 1986. Organic solvents: Physical properties and methods of purification. In: Techniques of chemistry. 4th ed. New York (NY): Wiley-Interscience.
Roberts BL, Dorough HW (1984) Relative toxicities of chemicals to the earthworm Eisenia foetida. Environ Toxicol Chem 3(1): 67-78. [reported in Kennedy 1986]. [cited in OECD 2001].
[RTECS] Registry of Toxic Effects of Chemical Substances. 2009 (online database). http://ccinfoweb2.ccohs.ca/rtecs/Action.lasso?-database=rtecs&-layout=Display&-response=detail.html&-op=eq&RTECS+NUMBER=AB7700000&-search
Schaeffer VH, Bhooshan B, Chen S, Sonenthal JS, Hodgson AT. 1996. Characterization of volatile organic chemical emissions from carpet cushions. J Air Waste Manage Assoc 46: 813-820.
Scorecard [database on the Internet]. 2005. Chemical profile for dimethylacetamide (CAS Number: 127-19-5). [cited 2008 Oct 9]. http://www.scorecard.org/chemicalprofiles/summary.tcl?edf_substance_id=+127-19-5
Smyth HF Jr, Carpenter CP, Weil CS, Pozzani UC, Striegel JA. 1962. Range-finding toxicity data: List VI. Am Ind Hyg Assoc J 23: 95-107.
Solomon HM, Ferenz RL, Kennedy G Jr, Staples RE. 1991. Developmental toxicity of dimethylacetamide by inhalation in the rat. Fundam Appl Toxicol 16: 414-422. [cited in OECD 2001].
Spies GJ, Rhyne RH Jr, Evans R, Wetzel KE, Ragland DT, Turney HG, Leet TL, Oglesby JL. 1995. Monitoring acrylic fiber workers for liver toxicity and exposure to dimethylacetamide. II: Serum clinical chemistry results of dimethylacetamide-exposed workers. J Occup Environ Med 37(9): 1102-1107. [cited in OECD 2001].
Steiner WG, Himwich HE. 1964. Electroencephalographic changes following administration of N-dimethylacetamide and other antitumor agents to rabbits. Int J Neuropharmacol 3: 327-332.
Stula EF et al. 1973. Unpublished data: Pathology Report No. 60-73, DuPont-Haskell Laboratory. [cited in OECD 2001].
Sun HN, Wristers JP. 2002. Butadiene. In: Kirk-Othmer encyclopaedia of chemical technology [Internet]. [cited 2008 Nov 18]. New York (NY): John Wiley & Sons, Inc. [restricted access] http://www.mrw.interscience.wiley.com/emrw/9780471238966/kirk/article/butasun.a01/current/pdf
Svendsen N, Pedersen SF, Hansen OC, Mossing JT, Bernth N. 2004. Survey of chemical substances in toothbrushes [Internet]. In: Survey of Chemical Substances in Consumer Products, No. 42. Copenhagen (DK): Danish Environmental Protection Agency, Danish Technological Institute. p. 55-57. [cited 3 Oct 2008]. http://www.mst.dk/NR/rdonlyres/FD9A8CB8-27BD-465A-AB72-9CE678CCE1C2/0/42.pdf
Taft RW, Abraham MH, Doherty RM, Kamlet MJ. 1985. The molecular properties governing solubilities of organic nonelectrolytes in water. Nature 313: 384-386.
Thiersch JB. 1962. Effects of acetamides and formamides on the rat litter in utero. J Reprod Fert 4: 219-220.
[TOPKAT] TOxicity Prediction by Komputer Assisted Technology [Internet]. 2004. Version 6.2. San Diego (CA): Accelrys Software Inc. http://www.accelrys.com/products/topkat/index.html
[TRI] Toxics Release Inventory [database on the Internet]. 2006. TRI Explorer 4.7. Washington (DC): US Environmental Protection Agency. [cited 2009 Jan 28]. http://www.epa.gov/triexplorer/
Valentine R, Hurtt ME, Frame SR, Kennedy GL Jr. 1997. Inhalation toxicology of dimethylacetamide (DMAc) in mice and rats: Age-related effects on lethality and testicular injury. Inhal Toxicol 9: 141-156. [cited in Kennedy 2001].
Verschueren K. 2001. Handbook of environmental data on organic chemicals, Vols. 1-2. 4th ed. New York (NY): John Wiley & Sons. p. 890-891.
Wada G, Takenaka T. 1971. Basicities of formamide, acetamide, and their alkyl derivatives in aqueous solution [Internet]. Bull Chem Soc Jpn 44: 2877. [cited 2008 Dec 19]. http://www.journalarchive.jst.go.jp/jnlpdf.php?cdjournal=bcsj1926&cdvol=44&noissue=10&startpage=2877&lang=en&from=jnlabstract
Wallace LA, Pellizzari E, Hartwell T, Rosenzweig M, Erickson M, Sparacino C, Zelon H. 1984. Personal exposure to volatile organic compounds: I. Direct measurements in breathing-zone air, drinking water, food, and exhaled breath. Environ Res 35(1): 293-319.
Wallen IE, Greer WC, Lasater R. 1957. Toxicity to Gambusia affinis of certain pure chemicals in turbid water. Sewage Ind Wastes 29: 695-711. [reported in Kennedy 1986]. [cited in OECD 2001].
Wang GM, Kier LD, Pounds GW. 1989. Male fertility study onN,N-dimethylacetamide administered by the inhalation route to Sprague-Dawley rats. J Toxicol Environ Health 27: 297-305.
Watts JC, Larson PA. 2002. Dimethylacetamide. In: Kirk-Othmer encyclopaedia of chemical technology [Internet]. [cited 2008 Oct 9]. New York (NY): John Wiley & Sons, Inc. [restricted access] http://mrw.interscience.wiley.com/emrw/9780471238966/kirk/article/dimewatt.a01/current/html?hd=All,dimethylacetamide
Wypych G, editor. 2004. Handbook of plasticizers. Toronto (ON): ChemTec Publishing; Norwich (NY): William Andrew Inc. p. 515.
Yocom JE, Hijazi NH, Zoldak JJ. 1984. Use of direct analysis mass spectrometry to solve indoor air quality problems. In: Indoor air, Vol. 4. Chemical characterization and personal exposure. Stockholm (SE): Swedish Council for Building Research. p. 245-250.
Appendix 1. Estimates of total and media-specific daily intakes of DMAc for various age groups
| Route of exposure | 0-0.5 yearsTable note d Table note e Breast milk fed | 0-0.5 yearsTable note e Table note f Formula fed | 0-0.5 yearsTable note d Table note e Tablenote f Not formula fed | 0.5-4 yearsTable note g | 5-11 yearsTable note h | 12-19 yearsTable note i | 20-59 yearsTable note j | 60+ yearsTable note k |
|---|---|---|---|---|---|---|---|---|
| AirTable note l | 8.36 | 8.36 | 8.36 | 17.92 | 13.97 | 7.94 | 6.83 | 5.93 |
| Drinking waterTablenote m | 0.00 | 0.11 | 0.03 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| Food and beveragesTable note n | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| SoilTable note o | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Total intake | 8.36 | 8.47 | 8.39 | 17.94 | 13.98 | 7.95 | 6.83 | 5.94 |
Maximum total intake from all routes of exposure: 17.94 µg/kg-bw per day.
Appendix 2. Predicted concentrations of DMAc in environmental media
Concentrations predicted using ChemCAN version 6.0 (ChemCAN 2003).
| Medium | ConcentrationTable note p |
|---|---|
| AirTable note q | 658 ng/m3 |
| WaterTable note q | 180 ng/L |
| Soil | 8.5 × 10-4 ng/g solids |
| Sediment | 8.3 × 10-4 ng/g solids |
Appendix 3. Upper-bounding estimates of exposure to DMAc emitted from linoleum flooring assembly and a medium-density fibreboard shelving unit
| Consumer product scenario | Assumptions | Estimated exposure |
|---|---|---|
| Flooring assembly (linoleum tile + adhesive + plywood) and medium-density fibreboard (MDF) shelving unit | Inhalation - off-gassing of DMAc to indoor air:
|
Inhalation - maximum air concentration of 9.8 µg/m3 at 4.9 h |
Appendix 4. Summary of health effects information and associated lowest effect levels for DMAc
| Endpoint | Lowest effect levels/Results |
|---|---|
| Acute toxicity | Lowest oral median lethal dose (LD50) (dog) = greater than 940 to less than 1880 mg/kg-bw (BASF 1977) [additional studies in rats and mice: Smyth et al. 1962; Thiersch 1962; Kafyan 1971; Bartsch et al. 1976; Hapke and Majid 1983; Kennedy and Sherman 1986] Lowest dermal LD50 (guinea pig) = less than 940 mg/kg-bw (Anon 1979) [additional studies in rabbits and mice: Smyth et al. 1962; Kafyan 1971] Lowest inhalation LC50(mouse) 1470 mg/m3 (Horn 1959) [additional studies in rats and mice: Kafyan 1971; Kennedy and Sherman 1986] |
| Short-term repeated-dose toxicity | Lowest oral lowest-observed-effect level (LOEL) = 94 mg/kg-bw per day for rabbits (10-11/group) exposed to 0, 94, 282 or 470 mg/kg-bw per day by gavage during gestation days 6-18, based on maternal toxicity and reduced body weight (Merkle and Zeller 1980) [additional studies: DuPont 1984; Kennedy and Sherman 1986; Johannsen et al. 1987] Lowest dermal LOEL = greater than 500 mg/kg-bw per day (pregnant rabbits, gestation days 6-18), based on maternal toxicity (Monsanto 1973) [additional studies: Horn 1959, 1961; DuPont 1984; Kennedy and Sherman 1986] Lowest inhalation lowest-observed-effect concentration (LOEC) = 30 ppm, equivalent to 107 mg/m3 (male rats, 6 or 12 h/day, 5 days/week, for 2 weeks); increased total protein levels in the blood (possible indication of liver damage) were observed; no-observed-effect-level (NOEL) = 10 ppm, equivalent to 36 mg/m3 (Kinney et al. 1993) [additional studies: Kennedy and Sherman 1986; Solomon et al. 1991; Valentine et al. 1997; Klimisch and Hellwig 2000; Okuda et al. 2006] |
| Subchronic toxicity | Lowest oral lowest-observed-adverse-effect level (LOAEL) = 50 mg/kg-bw per day (12 rats, exposed through diet for 90 days), only dose tested; slight anemia and leukocytosis (increased white blood cells) were observed (Kennedy and Sherman 1986) [no additional studies found] Lowest dermal LOAEL = 299 mg/kg-bw per day (dogs, application of 94 or 299 mg/kg-bw per day on uncovered skin, 5 h/day, 5 days/week, for up to 6 months); rapid weight loss and slight liver effects (slightly reduced cytoplasm in hepatocytes) and mild skin inflammatory response were seen; NOAEL = 95 mg/kg-bw per day (Horn 1961) [no additional studies found] Lowest inhalation LOAEC = 103 ppm, equivalent to 367 mg/m3 (rats, 20/group, exposed to 40, 64, 103 or 195 ppm, 6 h/day, 5 days/week, for 6 months); based on significant dose-dependent increase in nasal and upper respiratory tract irritation and significant liver cell degeneration (Horn 1961) [additional studies: Ferenz and Kennedy 1986; Wang et al. 1989] Lowest intravenous or subcutaneous injection LOAEL = 200 mg/kg-bw per day (rabbits, intravenous injections of 250-2000 mg/kg-bw per day or subcutaneous injections of 250 mg/kg-bw per day, for 21 days) based on changes in electroencephalogram (EEG) at all dose levels, which indicates an effect on the central nervous system (Steiner and Himwich, 1964) |
| Chronic toxicity/ carcinogenicity | Neoplastic effects: Oral (drinking water) bioassay in rats: No carcinogenicity effects were observed when rats were exposed to 0, 100, 300 or 1000 mg/kg-bw per day by drinking water for 2 years; incidences of thymomas were 0/50, 1/50 (2%), 0/50 and 3/50 (3%), respectively, and were not considered treatment related (unpublished data from Monsanto 1980; DuPont 1988) [no additional oral studies found] Inhalation bioassay in rats and mice: No significant difference in tumour formation was observed when male and female rats and mice were exposed by inhalation to concentrations of 0, 25, 100 or 350 ppm (0, 89, 356 or 1247 mg/m3) for 6 h/day, 5 days/week, in a 2-year and an 18-month study, respectively; incidence of squamous cell papillomas in female rats and incidence of lymphomas in female mice were not considered dose related, as the highest incidence was within the average for historical controls (Malley et al. 1995) [no additional inhalation studies found] Non-neoplastic effects: Non-neoplastic oral LOEL = 100 mg/kg-bw per day when rats (50/group) were exposed to 0, 100, 300 or 1000 mg/kg-bw per day by drinking water for 2 years, based on decreased body weight and increased liver and adrenal weights; although the OECD SIAR has identified the LOAEL as 1000 mg/kg-bw per day, 100 mg/kg-bw per day is the most appropriate LOEL based on the data presented by OECD (2001) (unpublished data from Monsanto 1980; DuPont 1988) [no additional studies found] Non-neoplastic inhalation LOAEC = 100 ppm, equivalent to 356 mg/m3, when male and female rats and mice were exposed by inhalation to concentrations of 0, 25, 100 or 350 ppm (0, 89, 356 or 1247 mg/m3) for 6 h/day, 5 days/week, in a 2-year and an 18-month study, respectively, based on significantly increased serum cholesterol and glucose levels, increased relative liver weights (female rats) (p 0.05), significantly increased hepatic focal cystic degeneration (p less than 0.05) and increased hepatic peliosis in male rats; at 100 ppm, male mice showed increased relative liver weight and incidence of individual hepatocellular necrosis (Malley et al. 1995) [no additional studies found] |
| Reproductive toxicity | No reproductive effects were observed in male and female rats when exposed by inhalation to concentrations up to 1375 mg/m3 (386 ppm) (Ferenz and Kennedy 1986; Wang et al. 1989) or in male mice when exposed by inhalation to concentrations up to 2494 mg/m3 (700 ppm) (Fairhurst et al. 1992) [no additional inhalation reproductive studies found] |
| Developmental toxicity | Lowest oral LOAEL = 282 mg/kg-bw per day for New Zealand White rabbits (10-11/group) exposed to 0, 94, 282 or 470 mg/kg-bw per day by gavage during gestation days 6-18; based on fetal toxicity effects, including cleft palate, fused ribs, microphthalmia (small eyes) and reduced weight; reduced maternal body weight was observed at all dose levels (Merkle and Zeller, 1980) [additional studies: Johannsen et al. 1987; DuPont Haskel Laboratories 1997] Lowest dermal LOAEL = 500 mg/kg-bw per day for New Zealand White rabbits exposed to 125, 250 or 500 mg/kg-bw per day (no controls reported) through dermal route during gestation days 6-18; based on developmental abnormalities (deviation of the sternum, reduced body weight, cyclopy, umbilical hernia), in the absence of maternal toxicity (Monsanto 1973) [additional study in rats: Stula et al. 1973] Lowest inhalation LOAEC = 199.5 ppm, equivalent to 700 mg/m3, for Himalayan rabbits (15/group) exposed to 0, 57, 199.5 or 570 ppm (0, 200, 700 or 2000 mg/m3), 6 h/day, on gestation days 7-19; based on significantly increased skeletal variations (p less than 0.01); no maternal toxicity reported for any of the dose levels tested (BASF 1989; Klimisch and Hellwig 2000) [additional study in rats: Solomon et al. 1991] |
| Genotoxicity and related endpoints: in vivo | Chromosome aberration test (rat, male and female) Negative in rat bone marrow cells following inhalation exposure to 20 or 700 ppm (71 or 2494 mg/m3; no indication of any controls), 7 h/day for 5 days (or 1-5 days, as reported in SIDS/IUCLID dataset) (McGregor 1981) Dominant lethal assay Negative in germ cell mutations (rat, mouse, male, groups of 10) following inhalation exposure to 20 or 700 ppm (71 or 2494 mg/m3; no indication of any controls), 7 h/day for 5 days, and mated with 2 untreated females per week for 9 weeks (McGregor 1981) Negative when rats and mice exposed through dermal application of 1500 or 3000 mg/kg-bw per day (Arnold et al. 1972) Negative when mice exposed through intraperitoneal injection of 680 µL/kg-bw (BASF 1976) Sex-linked recessive lethal mutation assay Negative in Drosophila melanogasterexposed to vapour (McGregor 1981) |
| Genotoxicity and related endpoints: in vitro | Mutagenicity Negative: Ames test in Salmonella typhimurium TA98, TA100, TA1535, TA1537, TA1538 with and without activation (Martin [date unknown]; May 1989) Sister chromatid exchange Equivocal results in Chinese hamster ovary (CHO) cells (DuPont 1988) Unscheduled DNA synthesis assay Negative in human embryonic intestinal test (McGregor 1981) or in the transgenic mouse mutation assay when it was used as the "control" (Monroe and Mitchell 1993) |
| Sensitization | Negative in guinea pig (DuPont 1988) |
| Irritation | Skin irritation Rabbit: 10 mg/24 h open irritation test: mild irritation (AIHAAP 1962) [additional studies: Horn 1961; Smyth et al. 1962] Eye irritation Rabbit: 100 mg: mild irritation (Drug and Chemical Toxicol 1986)) [additional studies: Smyth et al. 1962; Kennedy and Sherman 1986] |
| Endpoint | Lowest effect levels/Results |
|---|---|
| Genotoxicity: in vivo | Statistically non-significant increase in chromosomal damage was observed in 20 workers exposed to 5-10 mg/m3 for an unspecified period (Katosova and Pavlenko 1985) |
| Short-term repeated-dose toxicity | No adverse effects were observed in 8 workers exposed to average levels of 22-79 mg/m3, 4-8 h/day for 5 days (Borm et al. 1987) |
| Chronic toxicity | A limited epidemiological (retrospective cohort) study that examined the mortality of 571 workers with at least 1 year's exposure to DMAc (exposure concentrations not given) and acrylonitrile found no clear relationship between the duration of exposure to DMAc and mortality from specified tumours (Mastrangelo et al., 1993) In a cohort study, 440 workers from 10 elastane fibre manufacturing facilities where DMAc had been used as a solvent were studied for hepatotoxicity. Exposure duration was up to 31 months (mean exposure 7.5 months), and the exposure concentration (presumably from inhalation and dermal) was estimated from urinaryN-methylacetamide (NMA). Two exposure classification cut-offs were used for urinary NMA: (i) greater than 20 mg NMA/g creatinine and (ii) greater than 30 mg NMA/g creatinine, the current American Conference of Governmental Industrial Hygienists (ACGIH)-recommended Biological Exposure Index (BEI) (ACGIH 2006). There were 28 cases of DMAc-induced hepatic injury--19 and 22, respectively, captured by cut-offs (i) and (ii). The odds ratios for hepatic injury for those exceeding cut-offs (i) and (ii) were 3.7 (95% confidence interval [CI] 1.3-10.3) (p less than 0.05) and 4.7 (95% CI 1.7-13.2) (p less than 0.01), respectively. For both classifications, an inverse association was found with duration of employment, suggestive of a healthy worker effect or the development of tolerance (Lee et al. 2006). Another case report indicated a "clear relationship between liver impairment and [DMAc] exposure duration" in 19 of 41 men occupationally exposed for 2-10 years (exposure concentrations not reported). Upper respiratory tract, gastric and nervous disturbances were also noted, although supporting data were not provided (Corsi 1971). One year's monitoring of 127 workers exposed to DMAc in an acrylic fibre plant and of 217 in-plant unexposed controls for liver function (based on serum clinical chemistry tests) and exposure (as determined by personal air monitoring for DMAc during the 12-h shifts and by biological monitoring for DMAc, NMA and acetamide levels in the urine at the end of the shifts) found no exposure-related trends in hepatic serum clinical chemistry. The mean 12-h time-weighted average exposure was 1.9 ppm (around 6.8 mg/m3), with unspecified higher excursions (Spies et al. 1995). [additional study: Choi et al. 2001] |
| Skin irritation | Mild skin irritation was reported when a volunteer was exposed through covered applications of neat DMAc, 1 h/day, for 5 days. No irritancy was reported in two volunteers exposed to 2 h/day for 5 days (Maxfield et al. 1975). [additional study: Frosch and Kligman 1977] |
Appendix 5: Estimated margins of exposure for DMAc
| Environmental medium | Route of exposure | Concentration/intake | Critical effect levels | Margin of exposure |
|---|---|---|---|---|
| Indoor air | Inhalation | 34 µg/m3 (Yocom et al. 1984) | 700 mg/m3 - developmental toxicity (BASF 1989; Klimisch and Hellwig 2000) | 20 590 |
| Indoor air | Inhalation | 34 µg/m3 (Yocom et al. 1984) | 356 mg/m3 - liver toxicity (Malley et al. 1995) | 10 470 |
| Drinking water | Oral | 0.11 µg/kg-bw per day (1 µg/L) (Wallace et al. 1984) | 100 mg/kg-bw per day (Monsanto 1980) | 910 000 |
| Fabrics: T-shirt, bathing suit, socks | Dermal | 3.7 µg/kg-bw per day (Bayer et al. 1998, converted to Health Canada reference body weight) | 299 mg/kg-bw per day (Horn 1961) | 80 800 |
| Linoleum floor assembly and medium-density fibreboard shelving unit | Inhalation | 9.8 µg/m3 (IA-QUEST 2008) | 700 mg/m3 - developmental toxicity (BASF 1989; Klimisch and Hellwig 2000) | 71 400 |
| Linoleum floor assembly and medium-density fibreboard shelving unit | Inhalation | 9.8 µg/m3 (IA-QUEST 2008) | 107 mg/m3 - increase in total protein levels in the blood as possible indication of liver damage (Kinney et al. 1993) | 10 920 |