Screening Assessment for the Challenge Peroxide, [1,3(or 1,4)-phenylenebis(1-methylethylidene)]bis[(1,1-dimethylethyl) (PBMBDP)
Chemical Abstracts Service Registry Number 25155-25-3
Environment Canada
Health Canada
February 2009
Table of Contents
Synopsis
Pursuant to section 74 of the Canadian Environmental Protection Act, 1999 (CEPA 1999), the Ministers of the Environment and of Health have conducted a screening assessment on Peroxide, [1,3(or1,4)-phenylenebis(1-methylethylidene)]bis[(1,1-dimethylethyl) (PBMBDP), Chemical Abstracts Service Registry Number 25155-25-3. This substance was identified as a high priority for screening assessment and included in the Challenge because it was found to meet the ecological categorization criteria for persistence, bioaccumulation potential and inherent toxicity to non-human organisms and is believed to be in commerce in Canada.
The substance PBMBDP was not considered to be a high priority for assessment of potential risks to human health, based upon application of the simple exposure and hazard tools developed by Health Canada for categorization of substances on the Domestic Substances List. Therefore, this assessment focuses on information relevant to the evaluation of ecological risks.
PBMBDP is an organic substance that is used in Canada and elsewhere in polymer processing as an initiator for polymerization. The substance is not naturally produced in the environment. PBMBDP was not manufactured in Canada in 2006, although between 10 000 and 100 000 kg of PBMBDP were imported into Canada in 2006.
Based on reported use patterns and certain assumptions, more than half of the substance is transformed during the processing phase, while substantial amounts are ultimately sent to waste management facilities. Small proportions may be released to water (0.4%). PBMBDP is not soluble in water and has a tendency to partition to particles because of its hydrophobic nature. For these reasons, PBMBDP would likely be found almost entirely in sediments and is not expected to be significantly present in other media.
PBMBDP does not meet the criteria for persistence, but does meet the criteria for bioaccumulation as set out in the Persistence and Bioaccumulation Regulations.
A realistic worst case predicted environmental concentration is more than an order of magnitude lower than the predicted no-effects concentrations for aquatic organisms.
This substance will be included in the upcoming Domestic Substances List (DSL) inventory update initiative. In addition and where relevant, research and monitoring will support verification of assumptions used during the screening assessment.
Based on the information available, it is concluded that PBMBDP does not meet any of the criteria set out in section 64 of CEPA 1999.
Introduction
The Canadian Environmental Protection Act, 1999 (CEPA 1999) (Canada 1999) requires the Minister of the Environment and the Minister of Health to conduct screening assessments of substances that have met the categorization criteria set out in the Act to determine whether these substances present or may present a risk to the environment or human health. Based on the results of a screening assessment, the Ministers can propose to take no further action with respect to the substance, to add the substance to the Priority Substances List (PSL) for further assessment, or to recommend that the substance be added to the List of Toxic Substances in Schedule 1 of the Act and, where applicable, the implementation of virtual elimination.
Based on the information obtained through the categorization process, the Ministers identified a number of substances as high priorities for action. These include substances that
- met all of the ecological categorization criteria, including persistence (P), bioaccumulation potential (B) and inherent toxicity to aquatic organisms (iT), and were believed to be in commerce; and/or
- met the categorization criteria for greatest potential for exposure (GPE) or presented an intermediate potential for exposure (IPE), and had been identified as posing a high hazard to human health based on classifications by other national or international agencies for carcinogenicity, genotoxicity, developmental toxicity or reproductive toxicity.
The Ministers therefore published a notice of intent in the Canada Gazette, Part I, on December 9, 2006 (Canada 2006), that challenged industry and other interested stakeholders to submit, within specified timelines, specific information that may be used to inform risk assessment, and to develop and benchmark best practices for the risk management and product stewardship of those substances identified as high priorities.
The substance Peroxide, 1,3(or1,4)-phenylenebis(1-methylethylidene)]bis[(1,1-dimethylethyl) (PBMBDP) had been found to be persistent, bioaccumulative and inherently toxic to aquatic organisms and is believed to be in commerce in Canada. The Challenge for PBMBDP was published in the Canada Gazette on August 18, 2007 (Canada 2007). A substance profile was released at the same time. The substance profile presented the technical information available prior to December 2005 that formed the basis for categorization of this substance. As a result of the Challenge, submissions of information were received (Environment Canada 2008a).
Although PBMBDP was determined to be a high priority for assessment with respect to the environment, it did not meet the criteria for GPE or IPE and high hazard to human health based on classifications by other national or international agencies for carcinogenicity, genotoxicity, developmental toxicity or reproductive toxicity. Therefore, this assessment focuses principally on information relevant to the evaluation of ecological risks.
Under CEPA 1999, screening assessments focus on information critical to determining whether a substance meets the criteria for defining a chemical as toxic as set out in section 64 of the Act, where
"64. [...] a substance is toxic if it is entering or may enter the environment in a quantity or concentration or under conditions that
- have or may have an immediate or long-term harmful effect on the environment or its biological diversity;
- constitute or may constitute a danger to the environment on which life depends; or
- constitute or may constitute a danger in Canada to human life or health.”
Screening assessments examine scientific information and develop conclusions by incorporating a weight-of-evidence approach and precaution as required under CEPA 1999.
This screening assessment includes consideration of information on chemical properties, hazards, uses and exposure, including the additional information submitted under the Challenge. Data relevant to the screening assessment of this substance were identified in original literature, review and assessment documents, stakeholder research reports and from recent literature searches, up to June 2008. Key studies were critically evaluated; modelling results may have been used to reach conclusions. When available and relevant, information presented in hazard assessments from other jurisdictions was considered. The screening assessment does not represent an exhaustive or critical review of all available data. Rather, it presents a summary of the critical information upon which the conclusion is based.
This screening assessment was prepared by staff in the Existing Substances Programs at Health Canada and Environment Canada and incorporates input from other programs within these departments. The draft of this screening assessment was subject to a 60-day public comment period. While external comments were taken into consideration, the final content and outcome of the screening assessment remain the responsibility of Health Canada and Environment Canada. The critical information and considerations upon which the assessment is based are summarized below.
Substance Identity
For the purposes of this document, this substance will be referred to as PBMBDP, which has been derived from the name Peroxide, [1,3(or1,4)-phenylenebis(1-methylethylidene)]bis[(1,1-dimethylethyl).
| Chemical Abstracts Service Registry Number (CAS RN) |
25155-25-3 |
|---|---|
| DSL name | Peroxide, [1,3(or 1,4)-phenylenebis(1-methylethylidene)]bis[(1,1-dimethylethyl) |
| National Chemical Inventories (NCI) namesTable note a | Peroxide, 1,1'-[1,3(or 1,4)-phenylenebis(1-methylethylidene)]bis[2-(1,1-dimethylethyl) (TSCA) Peroxide, [1,3(or 1,4)-phenylenebis(1-methylethylidene)]bis[(1,1-dimethylethyl)(PICCS, ASIA-PAC, NZIoC) [1,3(or 1,4)-Phenylenebis(1-methylethylidene)]bis[tert-butyl] peroxide (EINECS) Peroxide, [1,3(or 1,4)-phenylenebis(1-methylethylidene)]bis[(1,1-dimethylethyl)- (AICS) [1,3(or 1,4)-Phenylenebis(1-methylethylidene)]bis[(1,1-dimethylethyl) peroxide (ECL) |
| Other names | Vul-Cup Vul-Cup R Vul-Cup 40KE CCRIS 4588 EINECS 246-678-3 [1,3(or 1,4)-Phenylenebis(1-methylethylidene)]bis[(1,1-dimethylethyl) peroxide Bis(tert-butyldioxyisopropyl)benzene Bis (1-t-butylperoxy-1-methylethyl) benzene Bis t-butyldioxyisopropylbenzene (Phenylenediisopropylidene)bis(tert-butylperoxide) Peroxide, (phenylenediisopropylidene)bis(tert-butyl alpha,alpha'-Bis(tert-butylperoxy)diisopropylbenzene 1,3-bis(3-tert-butylperoxypropyl)benzene |
| Chemical group (DSL stream) |
Discrete organics |
| Chemical sub-group | Dialkyl peroxides |
| Chemical formula | C20H34O4 |
| Chemical structure | 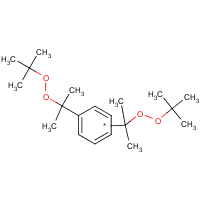 |
| Simplified Molecular Input Line Entry Specification (SMILES) | CC(c1ccc(cc1)C(OOC(C)(C)C)(C)C)(OOC(C)(C)C)C |
| Molecular mass | 338.49 g/mol |
Physical and Chemical Properties
Table 2 summarizes the modelled and experimental physical and chemical properties of PBMBDP that are relevant to its environmental fate. Few experimental values have been identified.
| Type | Value | Temperature (°C) | Reference | |
|---|---|---|---|---|
| Melting point (ºC) | Experimental | 41 | Arkema 2007a | |
| Melting point (ºC) | Modelled | 113.26 | MPBPWIN 2000 |
|
| Boiling point (ºC) | Modelled | 350.78 | MPBPWIN 2000 |
|
| Vapour pressure (Pa) | Modelled | 0.00228 (1.71 × 10-5 mm Hg) |
25 | MPBPWIN 2000 |
| Henry's Law constant (Pa·m3/mol) |
Modelled | 9.94 (9.8 × 10-5 atm·m3/mole) |
25 | HENRYWIN 2000 |
| Log Kow (Octanol-water partition coefficient) (dimensionless) |
Modelled | 7.34 | 25 | KOWWIN 2000 |
| Log Koc (Organic carbon partition coefficient) (dimensionless) |
Modelled | 6.273 | 25 | PCKOCWIN 2000 |
| Water solubility (mg/L) | Modelled | 0.0039 | 25 | WSKOWWIN 2000 |
Sources
Organic peroxide initiators were not manufactured in Canada in 2000 and approximately 300 000 kg of dialkyl peroxides were used in the Canadian polymer resin manufacturing process in 2000 (ChemInfo Services Inc. 2002).
Response to a survey notice pursuant to section 71 of CEPA 1999 indicated that PBMBDP was not manufactured in Canada in 2006. Eight companies met the 100-kg reporting threshold and reported importing the substance into Canada in a total quantity for the eight companies of between 10 000 and 100 000 kg (Environment Canada 2008a).
It is not known how much PBMBDP is imported into Canada in finished articles (e.g., as residues in polymeric materials).
Elsewhere, PBMBDP has been identified as a United States high production volume (HPV) chemical, with total use reported under the Inventory Update Rule within the range of 455 to 4545 tonnes per year for 1990, 1994, 1998 and 2002. For 1986, the reported total use was within the range of 4545 to 22 725 tonnes (US EPA 2002). PBMBDP has also been identified as an OECD HPV chemical.
Uses
Information on uses of PBMBDP in Canada was received in response to the CEPA 1999 section 71 notice for the 2006 calendar year (Environment Canada 2008a). Uses include use as a polymer and cross-linking agent.
PBMBDP is used to produce polymers that are used in products such as wire and cable insulation, pipes and hoses, shoe soles, roller coverings, mouldings and foamed articles with closed-cell structures (Arkema 2008).
In these uses, the peroxide bonds are broken to produce reactive radicals that initiate polymerization.
One use reported under the section 71 survey, which cannot be presented in this screening assessment as it is confidential business information, results in the presence of significant amounts of PBMBDP in some finished products (Environment Canada 2008a). This information was taken into consideration in the assessment.
Releases to the Environment
PBMBDP is not naturally produced in the environment.
Mass Flow Tool
To estimate potential release of the substance to the environment at different stages of its life cycle, a Mass Flow Tool was used. Empirical data concerning releases of specific substances to the environment are seldom available. Therefore, for each identified type of use of the substance, the proportion and quantity of release to the different environmental media are estimated, as is the proportion of the substance chemically transformed or sent for waste disposal. Assumptions and input parameters used in making these estimates are based on information obtained from a variety of sources including responses to regulatory surveys, Statistics Canada, manufacturers' websites and technical databases. Of particular relevance are emission factors, which are generally expressed as the fraction of a substance released to the environment, particularly during its manufacture, processing, and use associated with industrial processes. Sources of such information include emission scenario documents, often developed under the auspices of the Organisation for Economic Co-operation and Development (OECD), and default assumptions used by different international chemical regulatory agencies. It is noted that the level of uncertainty in the mass of substance and quantity released to the environment generally increases towards the end of the life cycle. Unless specific information on the rate or potential for release of the substance from landfills and incinerators is available, the Mass Flow Tool does not quantitatively account for releases to the environment from disposal.
| Fate | Proportion of the mass (%) | Major life cycle stage involved |
|---|---|---|
| Released to soil | 0.0 | n/aTable note c |
| Released to air | 0.0 | n/a |
| Released to sewerTable note d | 0.4 | Processing |
| Chemically transformed | 57.0 | Processing |
| Transferred to waste disposal sites (e.g., landfill, incineration) |
42.6 | Waste management |
Results indicate that for PBMBDP over half of the substance (57%) present in commerce is lost by transformation during processing. Because of a use, reported as confidential business information under the section 71 survey, in which the substance is not lost by transformation, an estimated 43% remains in manufactured items and is eventually transferred to waste disposal sites. The calculations assume that there is no release of the substance from these sites, although long-term releases may be possible. PBMBDP has a high Log Koc of 6.273, so it will adhere strongly to particulate organic matter and will be quite immobile in landfills. A small fraction of solid waste is incinerated, which is expected to result in transformation of the substance. Based largely on information contained in OECD emission scenario documents for processing and uses associated with this type of substance, it is estimated that 0.4% of PBMBDP may be released to sewers.
Although no information is available on the quantity of imported consumer products containing PBMBDP, it is anticipated that the quantities of releases to the various environmental media would not be significantly different from those estimated here because losses resulting from consumer use of such products are expected to be very small. However, the quantities sent for waste management would be higher if importation of these products were taken into consideration.
Environmental Fate
Based on its physical and chemical properties (Table 2) and the results of the Level III fugacity modelling (Table 4), PBMBDP is expected to partition to sediment, air, soil or water, depending on the compartment of release.
| Fraction of substance partitioning to air (%) | Fraction of substance partitioning to water (%) | Fraction of substance partitioning to soil (%) | Fraction of substance partitioning to sediment (%) | |
|---|---|---|---|---|
| Substance released to air (100%) | 32.9 | 0.5 | 37.7 | 28.9 |
| Substance released to waster (100%) | 0.0 | 1.8 | 0.0 | 98.2 |
| Substance released to soil (100%) | 0.0 | 0.0 | 99.9 | 0.1 |
As indicated by the Mass Flow Tool results presented in Table 3, the largest direct environmental release of PBMBDP is to sewers during processing and so the 100% release scenario to water seems to be the most relevant for Canada. PBMBDP released to water is expected to strongly adsorb to suspended solids and sediments, according to its very high log Koc value of ~ 6.3 (Table 2) and this is reflected in Level III fugacity modelling results.
Persistence and Bioaccumulation Potential
Environmental Persistence
PBMBDP oxidizes in air with a predicted half-life value of 1.57 days (AOPWIN 2000).
As mentioned above, the direct release of PBMBDP to the environment would be to surface water through sewers (Table 3). Once in water, the fate analysis presented in Table 4 indicates that this substance would partition mainly into sediments (98.2%) and to a much lower extent to remain in water (1.8%). According to the same analysis, PBMBDP is not expected to partition to air or soil if released to water. Therefore, the potential for persistence of PBMBDP will be assessed for the aquatic compartment only.
While peroxides are generally considered to be reactive because of the nature of the peroxide bond, there are clear differences in the level of reactivity among different categories of organoperoxides.
PBMBDP belongs to the dialkyl peroxide category. Dialkyl peroxides are among the most stable of all the commercially available organoperoxides, with a shelf half-life of at least one year at their recommended storage temperature of less than 38°C (ATOFINA 2001). There are also expected to be significant differences in levels of reactivity between conditions found in industrial settings where peroxides are reactive and those encountered in the various media in the natural environment.
In the only available laboratory biodegradation study (Table 5), no biodegration of PBMBDP was detected in a closed-bottle test of ready biodegradability using ultra-pure water (OECD 301D) (Arkema 2007b). In the test protocol, degradation is determined by analysis of dissolved oxygen and comparing the disappearance of dissolved oxygen with the theoretical oxygen demand. The test is a measure of ultimate degaradation - the breakdown of the test substance to carbon dioxide and water. The disappearance of the substance is not directly measured and there is no analysis undertaken for the formation of degradation products. A negative result in a ready biodegradation test does not necessarily mean that a chemical will not be biodegraded under environmentally relevant conditions.
| Medium | Fate process | Value (range if applicable) |
Endpoint | Reference |
|---|---|---|---|---|
| Water | Biodegradation (OECD 301D) |
0% after 84 days | % Biodegradation | Arkema 2007b |
In a risk assessment of tertiary butyl hydroperoxide (CAS RN 75-91-2), a hydroperoxide, the Netherlands Chemical Substances Bureau reported that the substance was not appreciably degraded in abiotic degradation tests. Half-lives for primary degradation ranged from 170 to 6900 days in 10-day tests in ultra-pure water and from 36 to 45 days in 10-day tests with sterilized sludge (Chemical Substances Bureau 2004). The substance was not readily biodegradable in the modified Sturm test or the closed-bottle test, both of which measure ultimate degradation, but the substance was biodegraded in 1-hr activated sludge tests, with primary degradation half-lives of 18-24 minutes (Chemical Substances Bureau 2004). These results indicate that tertiary butyl hydroperoxide, and perhaps other organic peroxides, are relatively resistant to abiotic degradation in pure water and are not readily biodegradable, but that they can be biodegraded under more favourable conditions. It should be noted that in hydroperoxides the peroxide bond is at the end of the molecule, where it is more accessible to attack than in dialkyl peroxides, where the peroxide bond is closer to the centre of the molecule.
Other studies with similar substances indicate that organoperoxide substances may in fact not be persistent under environmental conditions.
Di-tert butyl peroxide (CAS RN 110-05-4) is structurally related to PBMBDP and has been found to photolyze to form tert-butoxy radicals at low temperatures (HSDB 2006). This indicates that PBMBDP may also be subject to photolysis when exposed to light; however, the rate of this process is not known.
In a submission by industry, another dialkyl peroxide, dicumyl peroxide (CAS RN 80-43-3), showed 0% biodegradation over 15 days, 18% biodegradation over 28 days, and 60% degradation over 57 days, in a ready biodegradability closed-bottle test (OECD Guideline 301D). Under the test conditions, an acclimation period of at least 15 days was required, after which degradation/elimination of the substance occurred (OPPSD 2008a). It is thus possible that biodegradation of PBMBDP would also occur, given sufficient acclimation time.
Empirical biodegradation data (NITE 2002) for another dialkyl peroxide, (1,1,4,4-tetramethyl-1,4-butanediyl)bis[(1,1-dimethylethyl)peroxide], CAS RN 78-63-7, shows only 4% primary biodegradation over 28 days in a ready biodegradation test (OECD Guideline 301C) as measured by gas chromatography (GC) analysis. This demonstrates that this substance, and perhaps other dialkyl peroxides, can be quite resistant to hydrolysis and biodegradation under the test conditions. However, the same substance was almost completely removed in an inherent biodegradability semi-continuous activated sludge (SCAS) test in which the substance was exposed to high concentrations of sewage sludge microorganisms for 8 weeks (OPPSD 2008a). This shows that the substance, and perhaps other similar organoperoxide substances, can undergo biodegradation under conducive conditions. It should be noted that the SCAS test is carried out under conditions that are highly favourable to biodegradation and that the test includes loss from solution by adsorption to solids.
In a sediment/water degradation test, carried out under anaerobic conditions, total recovery of another dialkyl peroxide, (1,1,4,4-tetramethyl-2-butyne-1,4-diyl)bis[(1,1-dimethylethyl)peroxide], CAS RN 1068-27-5, was reduced by 86.7% by day 16, with a half-life of 6 days using pseudo-first order kinetics (OPPSD 2008b). After introduction into the system, the substance partitioned mostly to the sediment, with 82.8% in the sediment and 17.3% in water on day 0. Recovery from the sediment was reduced from 82.8% on day 0 to 9.7% on day 16, while recovery from water decreased from 17.3% on day 0 to 3.6% on day 16. The test system contained sediment and water from a pond. Each test container held 30 g of dry weight equivalent of sediment and enough water to obtain a 5-cm layer of water over the sediment. Each container was spiked with the test substance dose solution delivered directly to the water layer. The final nominal concentration of the test substance was 1 ppm (1 mg/kg) based on the dry weight of the sediment. Dosing was carried out under a nitrogen atmosphere to avoid air exposure. Incubation was carried out for up to 16 days at a temperature of 25 ± 1.0°C. The study did not investigate the nature of degradation products.
In a similar study, the half-life of (3,3,5-trimethylcyclohexylidene)bis[(1,1-dimethylethyl)peroxide] (CAS RN 6731-36-8) was 4.3 days in a 14-day sediment/water degradation test, carried out under anaerobic conditions (OPPSD 2008c). The substance was not detected in water at the end of the study, and 10.4% of the applied substance remained in sediment extracts. An apparent breakdown product was 3,3,5-trimethylcyclohexanone (CAS RN 873-94-9).
In a hydrolysis test, residues of (3,3,5-trimethylcyclohexylidene)bis[(1,1-dimethylethyl)peroxide] (CAS RN 6731-36-8) were non-detectable after 5 days incubation at 50°C at pH 4 and 7. At pH 9, residues dropped to 26% of the initial application. The half-life was 2.6 days at 50°C. Using the Arrhenius equation, the estimated half-life at 25 °C and pH 9 would be 9.7 days (OPPSD 2008d).
Although experimental data on the degradation of PBMBDP and analogue substances are available, quantitative structure-activity relationships (QSARs) were also applied using degradation models. Modelling indicates that PBMBDP would be persistent in water and sediment. However, the modelled values are considered to be of lower reliability as no chemicals of structural comparability to PBMBDP are contained in their training sets. Indeed, these fragment-based models do not consider the peroxide bond, which can be reactive in some substances. Given that experimental data are available and given that the modelled values are of lower reliability, the latter are given a low relative weight in the assessment of the environmental persistence of PBMBDP.
Dialkyl peroxides have a low water solubility and they tend to partition to particulate matter if released into natural waters such as lakes or rivers. Therefore, the potential persistence of these substances in sediment is of particular concern. In general, the half-life of a substance in sediment is estimated to be about 4 times longer than that in water (Boethling et al. 1995), so a substance with a half-life in water of 13 weeks would meet the persistence criteria for sediment (half-life in sediment greater than or equal to 365 days) as set out in the Persistence and Bioaccumulation Regulations (Canada 2000). Information submitted to Environment Canada by industry states that the reactivity of organic peroxides in the presence of metals such as copper, iron and manganese is likely to be a significant factor limiting the stability of these substances in soils and sediments. This could mean that for organoperoxides the half-life in some sediments may be significantly less than four times the half-life in water. The results of the sediment/water degradation test (OPPSD 2008b), cited above, carried out with another dialkyl peroxide, tends to indicate that the PBMBDP may not be persistent in sediments.
In summary, PBMBDP and other similar organoperoxides tend to be persistent in standard ready biodegradability tests, but there is evidence that they can break down fairly quickly under environmental conditions more favourable to degradation.
Persistence Conclusion
Different lines of evidence were presented above to assess the persistence of PBMBDP in an aquatic environment. The weight of evidence based on the above-described data indicates that PBMBDP does not meet the persistence criteria for air (half-life greater than or equal to 2 days), water (half-life greater than or equal to 182 days) or sediments (half-life greater than or equal to 365 days) as set out in the Persistence and Bioaccumulation Regulations (Canada 2000). In general, the half-life of a substance in soil is estimated to be the same as that in water (Boethling et al. 1995), Therefore, it is concluded that PBMBDP does not meet the persistence criteria for soil (half-life greater than or equal to 182 days).
Potential for Bioaccumulation
No experimental bioaccumulation data for PBMBDP have been identified.
Since no experimental bioaccumulation factor (BAF) or bioconcentration factor (BCF) data for PBMBDP were available, a predictive approach was applied using available BAF and BCF models as shown in Table 6 below. According to the Persistence and Bioaccumulation Regulations (Canada 2000) a substance is bioaccumulative if its BCF or BAF is greater than or equal to 5000, however measures of BAF are the preferred metric for assessing bioaccumulation potential of substances. This is because BCF may not adequately account for the bioaccumulation potential of substances via the diet, which predominates for substances with log Kow greater than ~4.0 (Arnot and Gobas 2003). Kinetic mass-balance modelling is in principle considered to provide the most reliable prediction method for determining the bioaccumulation potential because it allows for metabolism correction as long as the log Kow of the substance is within the log kow domain of the model.
Modelled log Kow values for PBMBDP (Table 2) indicate that this chemical has the potential to bioaccumulate in the environment.
Estimates from bioconcentration and bioaccumulation models are presented in Table 6. The modified GOBAS BAF middle trophic level model produced a bioaccumulation factor (BAF) of 3 758 374 L/kg. However, this model does not take into account the potential metabolism of PBMBDP. The OASIS forecast and BCFWIN models are not considered to be reliable as they do not take into account potential for metabolism and no chemicals of structural comparability to PBMBDP are contained in their training sets. Significant metabolism of PBMBDP is expected, as presented below.
| Test organism |
Endpoint | Value wet wt | Reference |
|---|---|---|---|
| Fish | BAF | 3 758 374 L/kg | GOBAS BAF T2MTL (Arnot and Gobas 2003) |
| Fish | BCF | 23 988 L/kg | Gobas BCF T2LTL (Arnot and Gobas 2003) |
| Fish | BCF | 36 308 L/kg | OASIS Forecast 2005 |
| Fish | BCF | 22 336 L/kg | BCFWIN 2000 |
New experimental information has been submitted indicating that two dialkyl peroxides, (1,1,4,4-tetramethyl-1,4-butanediyl)bis[(1,1-dimethylethyl)peroxide], CAS RN 78-63-7, and (1,1,4,4-tetramethyl-2-butyne-1,4-diyl)bis[(1,1-dimethylethyl)peroxide], CAS RN 1068-27-5, are metabolised in in vitro tests (OPPSD 2008a). However, these substances were estimated to have bioaccumulation factors (BAFs) greater than 5000 using the Arnot-Gobas kinetic model v1.11 even when metabolism was included. In addition, the increased structural complexity of PBMBDP suggests that it is likely to have a slower rate of metabolism when compared with these two dialkyl peroxides.
Information pertinent to the bioaccumulation potential of PBMBDP was submitted (Society of the Plastics Industry 2008). In an in vitro measurement of the metabolism of the substance in trout liver S9 fraction, the measured loss rate (µmoles of parent compound lost per gram of protein per hour) was 1.05. The submitter extrapolated this rate to a whole-body metabolic rate using the approach of Cowan-Ellsberry et al. (2008) according to the weight of the middle trophic level fish in the Arnot-Gobas model (184 g) at 15°C giving a metabolic rate constant (kmet) of 0.29 (1/day). The submitter used this metabolic rate in the Arnot and Gobas bioaccumulation model (Arnot and Gobas 2003) giving an estimated BCF of 120 and BAF of 1905.
Estimates of uncertainty have not currently been performed for kmet based on in vitro assays. Cowan-Ellsberry et al. (2008) suggests that for acceptance of in vitro methods, understanding of uncertainty of these methods and testing on more types of chemicals should be performed to evaluate the various assumptions used in their approach. Han et al. (2007) also indicate that uncertainty of model parameters should be understood for the hepatocyte method. For organoperoxides previously assessed under the Government of Canada's Challenge program (Batch 1), in vitro S9 metabolism values were also generated for CAS RN 78-63-7, CAS RN 1068-27-5 and CAS RN 6731-36-8 by OPPSD. For comparison purposes, Table 7 lists the results of the in vitro S9 tests according to the rate at which the parent chemical is transformed (µmoles parent lost per gram of protein per hour). These values represent the measured metabolic rates for each substance before any in vitro to in vivoscale up or normalization routines. It can be seen that for organoperoxide substances, the rate of in vitro metabolism can vary by a factor of ~3 which also translates to a factor of ~3 difference in the kmet. These differences may be a function of varying test conditions which may be reduced upon standardization of testing protocols. Nonetheless, the value of 1.05 µmoles parent lost per gram of protein per hour for PBMBDP is significantly higher than any of the other peroxides tested. It would be expected that the in vitro rate of loss of parent would be comparable among this class as the bioavailability and reactivity of the peroxide bond is also comparable. Measured BCF values available for the organoperoxides in Table 7 also suggest that, for this class of compounds, in vivo bioaccumulation is not well correlated with the in vitro rate of metabolism. Finally, the model calculated BCF of 120 using a kmet of 0.29 is a factor of ~29 to ~110 times lower than the observed BCF for CAS RN 6731-36-8 which has a comparable log kow and is likely to have a comparable level of structural hindrance as PBMBDP. In fact, for all peroxides assessed under the Challenge Program, the model derived BCF values corrected using S9 kmet, when compared with empirical values, compare more equally when kmet is less than or equal to ~0.1.
It cannot therefore be confidently concluded that CAS RN 25155-25-3 presents a low level of bioaccumulation potential according to the results of S9 testing and scale up to kmet values. It is believed that further validation of these methods is required to understand method uncertainty and the domain of applicability.
| CAS RN | Structure | Log Kow | Measured Loss Rate S9 (µmoles parent lost/gm protein × hour) |
Empirical BCF at steady state (NITE data) |
|---|---|---|---|---|
| 78-63-7 | 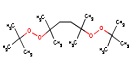 |
6.6 | 0.34 | 2250 and 3690 |
| 1068-27-5 | 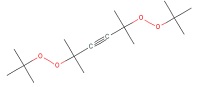 |
5.8 | 0.61 | 2250 and 3690Table note e |
| 6731-36-8 |  |
7.6 | 0.43 | 3500 and 9860 4960 and 13200 3750Table note f and 4922Table note f |
| 25155-25-3 | 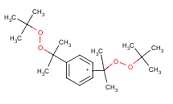 |
7.3 | 1.05 | Not available |
A more recent submission from OPPSD (OPPSD 2008b) suggested that the NITE BCF data for CAS RN 6731-36-8 (NITE 2002) were invalid due to co-elution of the metabolite 3,3,5-trimethylcyclohexanone (CAS RN 873-94-9) at the same time as the parent compound when analyzed using column chromatography. OPPSD suggests the BCF is overestimated because the analytical technique used by NITE could not distinguish between the parent and metabolite in fish tissues. The OPPSD submission suggests that if an alternative chromatographic method had been used (i.e., Stabil-Wax column), the metabolite would have been resolved and would have accounted for about 70% of the apparent BCF reported by NITE.
In reviewing these data and the information used to support the OPPSD conclusion regarding the NITE BCF data it is found that the OPPSD has not satisfactorily demonstrated that the analytical method used by NITE invalidates these data. The alternative chromatographic method suggested by the OPPSD could also promote hydrolysis of the parent compound in the column. To satisfactorily demonstrate the OPPSD method validity, a series of runs with internal standards using the parent and metabolite would be required. Therefore, the BCF data for CAS RN 6731-36-8 as well as CAS RN 78-63-7 have been used for subsequent analysis of the bioaccumulation potential of CAS RN 25155-25-3.
The data and approach used for assessing the bioaccumulation potential of the peroxides DBTMC (CAS RN 6731-36-8) and the peroxide DMHBP (CAS RN 78-63-7) in Batch 1 of The Challenge Program were judged as suitable for read-across for PBMBDP (i.e., PBMBDP has comparable log kow, predicted water solubility and comparable if not lower structural hindrance to metabolism than DBTMC, but a cross-sectional diameter more comparable to DMHBP). In addition to the reported BCF values for DBTMC and DMHBP from NITE (see above Table 7), in vivo metabolic rate constants for DBTMC ranged from 0.0002 to 0.04 with a median of ~0.003 1/days derived according to the method of Arnot et al. (2008a) and normalized to the body weight of the middle trophic level fish (184 g) at 15°C as outlined by Arnot et al (2008b). The in vivo normalized rate constants for DMHBP using the same process ranged from 0.01 to 0.16 with a median of ~0.04 (1/days). The in vivo-based rate constants were judged to provide a more certain measure of metabolism rate than in vitro estimates as outlined previously and they resulted in predicted BCFs in close agreement with empirical BCFs for DBTMC and DMHBP. Using the median metabolic rate constants of ~0.003 from DBTMC and 0.04 from DMHBP, the metabolism corrected BCF for PBMBDP ranges from 1862 to 16218. and the BAF for PBMBDP ranges from 199526 to 3388441.
Bioaccumulation Conclusion
There is conflicting evidence regarding the bioaccumulation potential of PBMBDP. In vitro data, when scaled up toin vivo conditions would predict a lack of significant bioaccumulation potential. Read-across in vivo BCF data and predicted BCF and BAF values would suggest otherwise. In vitro methods are not fully validated as of yet nor are the uncertainties involved with using these data in a regulatory program fully understood. The domain of applicability of in vitro techniques and scale-up models also needs to be elucidated for full consideration in chemical assessment. The in vitro data were considered in the overall weight of evidence, but accordingly, were given less weight compared with in vivo evidence.
When evaluating the available evidence, there is consistency between the in vivo based estimation methods and empirical read-across bioaccumulation data. Although organoperoxides may not be within the structural domain of the Arnot-Gobas model, this model is based on first principles of bioaccumulation wherein the most important boundaries are the mechanistic (passive diffusion) and global parameter (i.e., log Kow and molecular weight) domains. PBMBDP is well within the boundaries of these domains and although there is model uncertainty associated with these estimates, it is expected that PBMBDP is highly bioaccumulated by biota, perhaps mostly from dietary exposures.
Therefore, the in vivo bioaccumulation evidence indicates that PBMBDP meets the bioaccumulation criteria (BCF, BAF greater than or equal to 5000) as set out in the Persistence and Bioaccumulation Regulations (Canada 2000).
Potential to Cause Ecological Harm
Ecological Effects Assessment
No data have been found regarding levels of PBMBDP in the environment. It was estimated that 0.4% of the quantity used at a polymer manufacturing facility may be released in liquid effluents. A very conservative predicted environmental concentration (PEC) was calculated using the following equation:
PEC = I × L × (1-R) × 1000 / D × (F + S) × 86 400
where
- PEC
- = Predicted environmental concentration (mg/L)
- I
- = Maximum mass imported into (or manufactured in) an industrial complex linked with a discharge point (100 000 kg/year)
- L
- = Losses by processing (0.004)
- R
- = Removal rate of the sewage treatment plant (STP) (0.92) (SimpleTreat 3.0 1997)
- 1000
- = Conversion of units (kg/m 3 to mg/L)
- D
- = Days of release of the substance from site (250 days/year
- F
- = Flow of the receiving watercourse (0.65 m 3/s) (Environment Canada 2008b)
- S
- = Flow of the effluent from the STP (0.04 m 3/s) (Environment Canada 2008b)
- 86 400
- = Conversion of units (days to seconds)
Based on this equation, the PEC in receiving waters is 0.002 mg/L.
Ecological Effects Assessment
Because few empirical data were available, a range of aquatic toxicity predictions were obtained from the various QSAR models considered (Table 8a). The modelled values in this table may not be reliable as no chemicals of structural comparability to PBMBDP are contained in their training sets.
| Test organism | Type of test | Endpoint | Value (mg/L) |
Reference |
|---|---|---|---|---|
| Daphnid | Acute | Lethal concentration affecting 50% of the test population (LC50) (48-h) |
0.081 | ECOSAR 2004 |
| Fathead minnow | Acute | LC50 (96-h) | 621.6 | OASIS Forecast 2005 |
| Fathead minnow | Acute | LC50 (96-h) | 0.96283 | AIES 2003-2005 |
| Fish | Acute | LC50 (96-h) | 2.444 | ECOSAR 2004 |
| Fish | Acute | LC50 (14-d) | 0.01 | ECOSAR 2004 |
| Test organism | Type of test |
Endpoint | Value (mg/L) |
Reference |
|---|---|---|---|---|
| Pimephales promelas (Fathead minnow) |
Acute | LC50 (96-hr) | greater than 20 | Eastman Kodak 1988 |
| Poecilia reticulata Guppy) | Acute | LC50 (96-hr) | 750 | Arkema 2007b |
Two empirical aquatic toxicity results were identified for PBMBDP (Table 8b). An acute aquatic toxicity result - a 96-hr LC50 of 750 mg/L for the guppy, Poecilia reticulata, reported in a material safety data sheet (Arkema 2007b) - indicates that the substance is of very limited toxicity. However, this value is about five orders of magnitude above the estimated water solubility of the substance. Additionally, a 96-hr LC50 of greater than 20 mg/L was reported for the fathead minnow, Pimephales promelas in an unpublished study (Eastman Kodak 1988).The modelled data for acute aquatic toxicity range from 0.01 to greater than 600 mg/L. There is therefore uncertainty about the potential magnitude of the aquatic toxicity of PBMBDP.
Empirical aquatic toxicity data are available for other dialkyl peroxides. A 96-hr LC50 of 4.5 mg/L was reported for (1,1,4,4-tetramethyl-1,4-butanediyl)bis[(1,1-dimethylethyl)peroxide] (CAS RN 78-63-7) for the rice fish Oryzias latipes (NITE 2002), which QSAR modelling had indicated could have LC50s as low as 0.042 mg/L (ECOSAR 2004).
For another dialkyl peroxide, (1,1,4,4-tetramethyl-2-butyne-1,4-diyl)bis[(1,1-dimethylethyl)peroxide] (CAS RN 1068-27-5), two aquatic toxicity studies found significant losses of the test substance from solution during the test period. A study of the effects on the freshwater green algaPseudokirchneriella subcapitata found an EC50of 6.17 mg/L and a no-observed-effects concentration (NOEC) of 1.88 mg/L. These values are based on the measured concentration at the beginning of the test. After 72 hours, it was found that the concentration was below the detection limit (0.081 mg/L). In a 48-hr Daphnia toxicity test, an EC50 for immobility could not be determined as immobility was not observed at any of the concentrations. The highest concentration tested was 5.31 mg/L as measured at the beginning of the test and corresponded to a measured concentration of 0.375 mg/L after 48 hours (Environment Canada 2006).
To derive a predicted no-effect concentration (PNEC), a critical toxicity value of 4.5 mg/L, the 96-hr LC50 for rice fish, was selected as the lowest value from the empirical toxicity data available for dialkyl peroxides discussed above. This study (NITE 2002) was reviewed and found acceptable (Appendix 1). The value of 4.5 mg/L was divided by an assessment factor of 100 to account for interspecies and intraspecies variability in sensitivity, to estimate a long-term no-effects concentration from a short-term LC50 and to account for uncertainty in laboratory to field extrapolation. It is noted that chronic toxicity of these substances may be significantly lower than acute toxicity levels due to bioaccumulation. This gives a PNEC of 0.045 mg/L, which is within an order of magnitude of the substance's estimated water solubility. There is significant uncertainty about the water solubility of PBMBDP, as the value of 0.0039 is a modelled estimate.
Characterization of Ecological Risk
The approach taken in this ecological screening assessment was to examine various supporting information and develop conclusions based on a weight-of-evidence approach and precaution as required under CEPA 1999. Particular consideration was given to risk quotient analysis, persistence, bioaccumulation, toxicity, sources and fate in the environment.
PBMBDP is determined to be bioaccumulative, based on estimated bioaccumulation factors (BAFs). However, it has been determined not to be persistent in accordance with the Persistence and Bioaccumulation Regulations of CEPA 1999 (Canada 2000), based on the rapid breakdown in laboratory studies of inherent degradability and in vitro metabolism and the rapid disappearance of a closely related dialkyl peroxide in laboratory toxicity studies.
A risk quotient analysis, integrating very conservative estimated potential exposures with potential adverse environmental effects, was performed for the aquatic medium. A risk quotient (PEC/PNEC) was calculated to determine whether there is potential ecological risk in Canada.
The equation presented above was used to estimate the PEC of 0.002 mg/L.
A PNEC of 0.045 mg/L was calculated, as described above.
The resulting risk quotient (PEC/PNEC) = 0.002/0.045 = 0.04.
When PBMBDP is released into a water body, it partitions into suspended particulate matter and to bottom sediments, where sediment-dwelling organisms would be exposed to the substance. Because no environmental monitoring data or toxicity data specific to sediment-dwelling organisms are available, an equilibrium partitioning approach could be used to calculate a sediment PEC and PNEC based on the aquatic compartment values presented above. This would result in a risk quotient (PEC/PNEC) for the sediment compartment that is about the same as for the aquatic compartment, 0.04.
Based on the above evidence it is unlikely that PBMBDP is causing harm to populations of aquatic organisms in Canada.
Uncertainties in Evaluation of Ecological Risk
Some of the physical and chemical properties in Table 2 were generated using quantitative structure-activity relationship (QSAR) models, and there are uncertainties related to the use of these models.
There is uncertainty about the persistence of PBMBDP in air, water, soil and sediments under environmental conditions. Some tests indicate that this substance and some other types of organoperoxides are not readily biodegradable. Other tests, including those designed to measure metabolism and toxicity, indicate that PBMBDP and some other types of organoperoxides disappear from water quite quickly. Some of these studies report the detection of breakdown products, which indicates that the substances are degrading and that the disappearance from water is not due entirely to partitioning to particulate material or container walls or loss to air through volatilization. Models indicate that PBMBDP would be persistent in water, soil and sediment, but these models do not account for the peroxide bond, which can be reactive in some substances.
There is some uncertainty about the magnitude of bioaccumulation of PBMBDP as discussed in the bioaccumulation section of this assessment report.
No information is available on the quantity of importation of consumer products containing PBMBDP. However, because losses resulting from consumer use of such products are expected to be very small, had it been possible to account for releases from imported products, it is expected that the total quantities of PBMBDP released to environmental media would not be significantly different from those estimated.
There is little laboratory information available about the acute toxicity of PBMBDP to aquatic organisms. The two acute toxicity values that are available for fish are both higher than the selected critical toxicity value of 4.5 mg/L, based on a toxicity study using a closely-related substance. This indicates that the PNEC is a conservative value. Acute toxicity estimates for PBMBDP generated by models vary by several orders of magnitude. There is no information available about the chronic toxicity of PBMBDP to aquatic organisms.
Conclusion
Based on the information presented in this draft screening assessment, it is concluded that PBMBDP is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
It is therefore concluded that PBMBDP does not meet the definition of "toxic" as set out in section 64 of CEPA 1999. It is further concluded that PBMBDP does not meet the criteria for persistence, but does meet the criteria for bioaccumulation as set out in the Persistence and Bioaccumulation Regulations (Canada 2000).
References
[AIES] Artificial Intelligence Expert System. 2003 - 2005. Version 1.25. Ottawa (ON): Environment Canada. Model developed by Stephen Niculescu. Available from: Environment Canada, Existing Substances Division, New Substances Division, Ottawa, K1A 0H3.
[AOPWIN] Atmospheric Oxidation Program for Windows [Estimation Model]. 2000. Version 1.91. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. http://www.epa.gov/oppt/exposure/pubs/episuite.htm
Arkema. 2007a. Luperox. Organic Peroxides. Accessed December 7, 2007. http://www.luperox.com
Arkema. 2007b. LUPEROX F40 MFF Material safety data Sheet. Arkema, Inc. January 2, 2007. 8 pp.
Arkema. 2008. Luperox® Organic Peroxides. Overview. http://www.arkema-inc.com/index.cfm?pag=1077
Arnot JA, Mackay D, and Bonnell M. 2008a. Estimating Metabolic Biotransformation Rates in Fish from Laboratory Data. Envirion. Toxicol. Chem. 27(2): 341-351.
Arnot JA, MacKay D, Parkerton T, Bonnell M. 2008b. A database of fish biotransformation rate constants. Environ. Toxicol. Chem. (in press). http://www.setacjournals.org/perlserv/?request=get-abstract&doi=10.1897/08-058.1&ct=1
Arnot JA, Gobas FAPC. 2003. A generic QSAR for assessing the bioaccumulation potential of organic chemicals in aquatic food webs. QSAR Comb Sci 22(3):337-345.
ATOFINA. 2001. Organic Peroxides. Product Bulletin. Dialkyl peroxides. ATOFINA Chemicals, Inc., Philadelphia, PA. 9 pp.
[BCFWIN] BioConcentration Factor Program for Windows [Estimation Model]. 2000. Version 2.15. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. http://www.epa.gov/oppt/exposure/pubs/episuite.htm
Boethling RS, Howard PH, Beauman JA, Larosche ME. 1995. Factors for intermedia extrapolations in biodegradability assessment. Chemosphere 30(4): 741 - 752.
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C., 1999, c. 33. http://canadagazette.gc.ca/partIII/1999/g3-02203.pdf
Canada. 2000. Canadian Environmental Protection Act: Persistence and Bioaccumulation Regulations, P.C. 2000-348, 23 March 2000, SOR/2000-107. http://canadagazette.gc.ca/partII/2000/20000329/pdf/g2-13407.pdf
Canada, Dept. of the Environment, Dept. of Health. 2006.Canadian Environmental Protection Act, 1999: Notice of intent to develop and implement measures to assess and manage the risks posed by certain substances to the health of Canadians and their environment. Canada Gazette, Part I, vol. 140, no. 49, p. 4109-4117. http://canadagazette.gc.ca/partI/2006/20061209/pdf/g1-14049.pdf
Canada, Dept. of the Environment. 2007. Canadian Environmental Protection Act, 1999: Notice with respect to Batch 3 Challenge substances. Canada Gazette, Part I, vol. 141, no. 33, p. 2379-2394. http://canadagazette.gc.ca/partI/2007/20070818/pdf/g1-14133.pdf
Chemical Substances Bureau. 2004. Risk Assessment. Tertiary Butyl Hyrdoperoxide (TBHP). Final draft of 9 April 2004. R319_0404_env. Chemical Substance Bureau. Bilthoven, The Netherlands. 70 pp.
Cheminfo Services Inc. 2002. Use of Initiators in the Canadian Polymer Resin Manufacturing and Polymer Resin Processing Sectors.
Cowan-Ellsberry CE, Dyer SD, Erhardt S, Bernhard MJ, Roe AL, Dowty ME, Weisbrod AV. 2008. Approach for extrapolating in vitro metabolism data to refine bioconcentration factor estimates. Chemosphere 70: 1804-1817.
Eastman Kodak. 1988. Acute aquatic effects of Vul-Cup © R on the fathead minnow, Pimephales promelas. Study No. EN-402-QCN006-1. Eastman Kodak Company, Rochester, New York. Une 27, 1988.
[ECOSAR] Ecological Structural Activity Relationships [Internet]. 2004. Version 0.99h. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. http://www.epa.gov/oppt/exposure/pubs/episuite.htm
Environment Canada, 2006. Confidential information received following Categorization.
Environment Canada. 2007. Assumptions, limitations and uncertainties of the Mass Flow Tool for 1,3(or 1,4)-phenylenebis(1-methylethylidene)]bis[(1,1-dimethylethyl)peroxide], Chemical Abstracts Service Registry Number 25155-25-3. Available on request from the Existing Substances Division, Environment Canada.
Environment Canada. 2008a. Data for Batch 3 substances collected underCanadian Environmental Protection Act, 1999, Section 71: Notice with respect to Batch 3 Challenge substances. Data prepared by: Environment Canada, Existing Substances Program.
Environment Canada. 2008b. Guidance for conducting ecological assessments under CEPA, 1999: science resource technical series, technical guidance module: the Industrial Generic Exposure Tool - Aquatic (IGETA). Working document. Gatineau (QC): Environment Canada, Existing Substances Division.
[EQC] Equilibrium Crieterion Model. 2003. Version 2.02. Peterborough (ON): Trent University, Canadian Environmental Modelling Centre. http://www.trentu.ca/academic/aminss/envmodel/models/EQC2.html
Han X, Nabb DL, Mingoia RT, Yang C-H. 2007. Determination of xenobiotic intrinsic clearance in freshly isolated hepatocytes from rainbow trout (Oncorhynchus mykiss) and rat and its application in bioaccumulation assessment. Environ. Sci. Technol. 41: 3269 -3276.
[HENRYWIN] Henry's Law Constant Program for Microsoft Windows [Estimation Model]. 2000. Version 3.10. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. http://www.epa.gov/oppt/exposure/pubs/episuite.htm
[HSDB] Hazardous Substances Data Bank [database on the Internet]. 2006. Bethesda (MD): National Library of Medicine (US). http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB
[KOWWIN] Octanol-Water Partition Coefficient Program for Microsoft Windows [Estimation Model]. 2000. Version 1.67. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. http://www.epa.gov/oppt/exposure/pubs/episuite.htm
[MPBPWIN] Melting Point Boiling Point Program for Microsoft Windows [Estimation Model]. 2000. Version 1.41. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. http://www.epa.gov/oppt/exposure/pubs/episuite.htm
[NCI] National Chemical Inventories [database on CD-ROM]. 2007. Columbus (OH): American Chemical Society. http://www.cas.org/products/cd/nci/index.html
NITE (National Institute of Technology and Evaluation), Japan. 2002. Biodegradation and Bioconcentration of the Existing Chemical Substances under the Chemical Substances Control Law. Accessed October 30, 2006. http://www.safe.nite.go.jp/data/hazkizon/pk_e_kizon_data_result.home_data
[OASIS Forecast] Optimized Approach based on Structural Indices Set [Internet]. 2005. Version 1.20. Bourgas, Bulgaria : Laboratory of Mathematical Chemistry. http://oasis-lmc.org/?section=software
OECD 2004. Emission Scenario Document, "Plastics Additives", JT00166678, ENV/JM/MONO(2004)8, June 24 2004, 125 pages, Paris, France.
OECD 2006. Emission Scenario Document, "Transport and Storage of Chemicals", JT03213465, ENV/JM/EEA(2006)6, September 13 2006, 157 pages, U.K.
[OPPSD] Organic Peroxide Producers Safety Division, The Society of the Plastics Industry. 2008a. Submittal for the Environment Canada Industry Challenge Program: Comments on the Draft Screening Assessments for the Three Organic Peroxides CAS Numbers 78-63-7, 1068-27-5, and 6731-36-8 to the Executive Director, Existing Substances Division, Environment Canada. March 18, 2008. 49 pp.
[OPPSD] Organic Peroxide Producers Safety Division, The Society of the Plastics Industry. 2008b. Supplement 2: New Laboratory Data for the March 19, 2008 OPPSD Submittal for the Environment Canada DSL Challenge Program: Comments on the Draft Screening Assessments for the Three Organic Peroxides CAS RNs 78-63-7, 1068-27-5, and 6731-36-8. June 6, 2008. 5 pp.
[OPPSD] Organic Peroxide Producers Safety Division, The Society of the Plastics Industry. 2008c. Supplement 8: Final Reports (1) Anaerobic Aquatic Metabolism of CAS RN 6731-36-8 (3,3,5-trimethylcyclohexylidene)bis[(1,1-dimethylethyl)peroxide] (2) Anaerobic Aquatic Metabolism of CAS RN 1068-27-5 (1,1,4,4-tetramethyl-2butyne-1,4-diyl)bis[!1,1-dimethylethyl)peroxide] for the March 19, 2008 OPPSD Submittal for the Environment Canada DSL Challenge Program: Comments on the Draft Screening Assessments for the Three Organic Peroxides CAS RNs 78-63-7, 1068-27-5, and 6731-36-8. 105 pp.
[OPPSD] Organic Peroxide Producers Safety Division, The Society of the Plastics Industry. 2008d. Supplement 6: Progress Reports: CAS RN 6731-36-8(1) Hydrolysis at pH 2.6, 4.7, and 9 (2) Decomposition in an Anaerobic Natural Water/Sediment System for the March 19, 2008 OPPSD Submittal for the Environment Canada DSL Challenge Program: Comments on the Draft Screening Assessments for the Three Organic Peroxides CAS RNs 78-63-7, 1068-27-5, and 6731-36-8. 10 pp.
[PCKOCWIN] Organic Carbon Partition Coefficient Program for Windows [Estimation Model]. 2000. Version 1.66. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. [cited yr mon date]. http://www.epa.gov/oppt/exposure/pubs/episuite.htm
SimpleTreat 3.0. 1997. A computer program developed by The National Institute for Public Health and the Environment (RIVM) for sewage treatment plant removal predictions, released in 1997, available from Jaap Struijs, The National Institute for Public Health and the Environment (RIVM), Laboratory for Ecological Risk Assessment, PO Box 1, 3720 BA Bilthoven, The Netherlands, Tel: +31-(0)30-274-2001, Fax: +31-(0)30-274-4413, Email: j.struijs@rivm.nl
Society of the Plastics Industry. 2008. Submission to Existing Substances Division, Environment Canada, dated October 10, 2008.
[US EPA] United States Environmental Protection Agency. 2002. Toxic Substances Control Act-Inventory Update Rule (TSCA-IUR). Production Volume Information. Unpublished data, 1986, 1990, 1994, 1998, 2002. For more information on availability contact Existing Substances Division, Environment Canada, Ottawa, K1A 0H3.
[WSKOWWIN] Water Solubility for Organic Compounds Program for Microsoft Windows [Estimation Model]. 2000. Version 1.41 Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. http://www.epa.gov/oppt/exposure/pubs/episuite.htm
Appendix 1. Robust study summaries
Table A-1. Robust Study Summary for Identification No. 22875 Submission002
Reference: Study Submission. 2008. Unpublished study submitted to Environment Canada, Existing Substances Division under the Chemical Management Plan Challenge initiative. Robust Study Summary, Identification No. 22875 Submission002. NITE (National Institute of Technology and Evaluation), Japan. 2002.
| No. | Item | Weight | Yes/No | Specify |
|---|---|---|---|---|
| 2 | Substance identity: CAS RN | n/a | 78-63-7 | |
| 3 | Substance identity: chemical name(s) | n/a | (1,1,4,4-Tetramethyl-1,4-butanediyl)bis(1,1-dimethylethyl)peroxide | |
| 4 | Chemical composition of the substance | 2 | ||
| 5 | Chemical purity | 1 | Y | 95% |
| 6 | Persistence/stability of test substance in aquatic solution reported? | 1 | Y |
| No. | Item | Weight | Yes/No | Specify |
|---|---|---|---|---|
| 7 | Reference | 1 | Y | Japanese New Substances method |
| 8 | OECD, EU, national, or other standard method? | 3 | Y | OECD 203 |
| 9 | Justification of the method/protocol if a standard method was not used | 2 | ||
| 10 | GLP (good laboratory practice) | 3 | Y |
| No. | Item | Weight | Yes/No | Specify |
|---|---|---|---|---|
| 11 | Organism identity: name | n/a | Y | Rice fish |
| 12 | Latin or both Latin & common names reported? | 1 | Y | Oryzias latipes |
| 13 | Life cycle age / stage of test organism | 1 | Y | |
| 14 | Length and/or weight | 1 | Y | |
| 15 | Sex | 1 | N | |
| 16 | Number of organisms per replicate | 1 | Y | 7 |
| 17 | Organism loading rate | 1 | Y | 0.3 g/L |
| 18 | Food type and feeding periods during the acclimation period | 1 | Y |
| No. | Item | Weight | Yes/No | Specify |
|---|---|---|---|---|
| 19 | Test type (acute or chronic) | n/a | Y | Acute |
| 20 | Experiment type (laboratory or field) | n/a | Y | Laboratory |
| 21 | Exposure pathways (food, water, both) | n/a | Y | Water |
| 22 | Exposure duration | n/a | Y | 96 hours |
| 23 | Negative or positive controls (specify) | 1 | Y | Negative |
| 24 | Number of replicates (including controls) | 1 | N | |
| 25 | Nominal concentrations reported? | 1 | Y | 4 |
| 26 | Measured concentrations reported? | 3 | N | |
| 27 | Food type and feeding periods during the long-term tests | 1 | Y | |
| 28 | Were concentrations measured periodically (especially in the chronic test)? | 1 | N | |
| 29 | Were the exposure media conditions relevant to the particular chemical reported? (e.g., for the metal toxicity - pH, DOC/TOC, water hardness, temperature) | 3 | Y | |
| 30 | Photoperiod and light intensity | 1 | N | |
| 31 | Stock and test solution preparation | 1 | Y | |
| 32 | Was solubilizer/emulsifier used, if the chemical was poorly soluble or unstable? | 1 | Y | |
| 33 | If solubilizer/emulsifier was used, was its concentration reported? | 1 | Y | |
| 34 | If solubilizer/emulsifier was used, was its ecotoxicity reported? | 1 | N | |
| 35 | Analytical monitoring intervals | 1 | N | |
| 36 | Statistical methods used | 1 | N |
| No. | Item | Weight | Yes/No | Specify |
|---|---|---|---|---|
| 37 | Was the endpoint directly caused by the chemical's toxicity, not by organism's health (e.g., when mortality in the control is greater than 10%) or physical effects (e.g., "shading effect")? |
n/a | Y | |
| 38 | Was the test organism relevant to the Canadian environment? | 3 | Y | |
| 39 | Were the test conditions (pH, temperature, DO, etc.) typical for the test organism? | 1 | Y | |
| 40 | Does system type and design (static, semi-static, flow-through; sealed or open; etc.) correspond to the substance's properties and organism's nature/habits? | 2 | Y | |
| 41 | Was pH of the test water within the range typical for the Canadian environment (6 to 9)? | 1 | pH not cited | |
| 42 | Was temperature of the test water within the range typical for the Canadian environment (5 to 27°C)? | 1 | Y | |
| 43 | Was toxicity value below the chemical's water solubility? | 3 | N |
| No. | Item | Weight | Yes/No | Specify |
|---|---|---|---|---|
| 44 | Toxicity values (specify endpoint and value) | n/a | n/a | 96-hr LC50 = 4.5 mg/L |
| 45 | Other endpoints reported - e.g., BCF/BAF, LOEC/NOEC (specify)? | n/a | N | |
| 46 | Other adverse effects (e.g., carcinogenicity, mutagenicity) reported? | n/a | N |
| No. | Item | Specify |
|---|---|---|
| 47 | Score :... % | 70.5 |
| 48 | EC reliability code: | 2 |
| 49 | Reliability category (high, satisfactory, low) : | Satisfactory Confidence |
| 50 | Comments |
Table A-2. Robust Study Summary for Study No. EN-402-QCN006-1
Reference: Eastman Kodak. 1988. Acute aquatic effects of Vul-Cup © R on the fathead minnow, Pimephales promelas. Study No. EN-402-QCN006-1. Eastman Kodak Company, Rochester, New York. Une 27, 1988.
| No. | Item | Weight | Yes/No | Specify |
|---|---|---|---|---|
| 2 | Substance identity: CAS RN | n/a | 25155-25-3 | |
| 3 | Substance identity: chemical name(s) | n/a | Peroxide, [1,3(or 1,4)- phenylenebis(1- methylethylidene)]bis [(1,1-dimethylethyl) |
|
| 4 | Chemical composition of the substance | 2 | Y | |
| 5 | Chemical purity | 1 | Y | 96 - 100% |
| 6 | Persistence/stability of test substance in aquatic solution reported? | 1 | N |
| No. | Item | Weight | Yes/No | Specify |
|---|---|---|---|---|
| 7 | Reference | 1 | Y | |
| 8 | OECD, EU, national, or other standard method? | 3 | Y | OECD 203 |
| 9 | Justification of the method/protocol if not a standard method was used | 2 | ||
| 10 | GLP (Good Laboratory Practice) | 3 | Y |
| No. | Item | Weight | Yes/No | Specify |
|---|---|---|---|---|
| 11 | Organism identity: name | n/a | Y | Fathead minnow |
| 12 | Latin or both Latin & common names reported? | 1 | Y | Pimephales promelas |
| 13 | Life cycle age / stage of test organis | 1 | Y | Juvenile |
| 14 | Length and/or weight | 1 | N | |
| 15 | Sex | 1 | N | |
| 16 | Number of organisms per replicate | 1 | Y | 10 |
| 17 | Organism loading rate | 1 | Y | 0.5 g/L |
| 18 | Food type and feeding periods during the acclimation period | 1 | N |
| No. | Item | Weight | Yes/No | Specify |
|---|---|---|---|---|
| 19 | Test type (acute or chronic | n/a | Y | Acute |
| 20 | Experiment type (laboratory or field | n/a | Y | Laboratory |
| 21 | Exposure pathways (food, water, both) | n/a | Y | Water |
| 22 | Exposure duration | n/a | Y | 96-h |
| 23 | Negative or positive controls (specify) | 1 | Y | Negative |
| 24 | Number of replicates (including controls) | 1 | Y | 2 |
| 25 | Nominal concentrations reported? | 1 | Y | 5 |
| 26 | Measured concentrations reported? | 3 | N | |
| 27 | Food type and feeding periods during the long-term tests | 1 | N | |
| 28 | Were concentrations measured periodically (especially in the chronic test)? | 1 | N | |
| 29 | Were the exposure media conditions relevant to the particular chemical reported? (e.g., for the metal toxicity - pH, DOC/TOC, water hardness, temperature) | 3 | Y | |
| 30 | Photoperiod and light intensity | 1 | Y | |
| 31 | Stock and test solution preparation | 1 | Y | |
| 32 | Was solubilizer/emulsifier used, if the chemical was poorly soluble or unstable? | 1 | Y | |
| 33 | If solubilizer/emulsifier was used, was its concentration reported? | 1 | Y | |
| 34 | If solubilizer/emulsifier was used, was its ecotoxicity reported? | 1 | N | |
| 35 | Analytical monitoring intervals | 1 | N | |
| 36 | Statistical methods used | 1 | Y |
| No. | Item | Weight | Yes/No | Specify |
|---|---|---|---|---|
| 37 | Was the endpoint directly caused by the chemical's toxicity, not by organism's health (e.g. when mortality in the control is greater than 10%) or physical effects (e.g. 'shading effect')? | n/a | Y | |
| 38 | Was the test organism relevant to the Canadian environment? | 3 | Y | |
| 39 | Were the test conditions (pH, temperature, DO, etc.) typical for the test organism? | 1 | Y | |
| 40 | Does system type and design (static, semi-static, flow-through; sealed or open; etc.) correspond to the substance's properties and organism's nature/habits? | 2 | Y | |
| 41 | Was pH of the test water within the range typical for the Canadian environment (6 to 9)? | 1 | Y | |
| 42 | Was temperature of the test water within the range typical for the Canadian environment (5 to 27°C)? | 1 | Y | |
| 43 | Was toxicity value below the chemical's water solubility? | 3 | N |
| No. | Item | Weight | Yes/No | Specify |
|---|---|---|---|---|
| 44 | Toxicity values (specify endpoint and value) | n/a | n/a | 96-hr LC50 greater than 20 mg/L |
| 45 | Other endpoints reported - e.g. BCF/BAF, LOEC/NOEC (specify)? | n/a | Y | NOEC = 20 mg/L |
| 46 | Other adverse effects (e.g. carcinogenicity, mutagenicity) reported? | n/a | N |
| No. | Item | Specify |
|---|---|---|
| 47 | Score: ... % | 70.2 |
| 48 | EC Reliability code: | 2 |
| 49 | Reliability category (high, satisfactory, low): | Satisfactory Confidence |
| 50 | Comments |