Screening Assessment for the Challenge Phenol, 4,4'-(3H-1,2-benzoxathiol-3-ylidene)bis[2,6-dibromo-3-methyl-,S,S-dioxide, monosodium salt (PBTBO)
Chemical Abstracts Service Registry Number 62625-32-5
Environment Canada
Health Canada
August 2009
Table of Contents
Synopsis
Pursuant to section 74 of the Canadian Environmental Protection Act, 1999 (CEPA 1999), the Ministers of the Environment and of Health have conducted a screening assessment of Phenol, 4,4'-(3H-1,2-benzoxathiol-3-ylidene)bis[2,6-dibromo-3-methyl-,S,S-dioxide, monosodium salt (PBTBO), Chemical Abstracts Service Registry Number 62625-32-5. This substance was identified as a high priority for screening assessment and included in the Challenge because it had originally been found to meet the ecological categorization criteria for persistence, bioaccumulation potential and inherent toxicity to non-human organisms and is believed to be in commerce in Canada.
The substance PBTBO was not considered to be a high priority for assessment of potential risks to human health, based upon application of the simple exposure and hazard tools developed by Health Canada for categorization of substances on the Domestic Substances List. Therefore, this assessment focuses on information relevant to the evaluation of ecological risks.
PBTBO is an organic substance that can be used as an analytical reagent in laboratories. A total quantity of 2363 kg was imported into Canada in 2006. Based on possible uses of this substance, it could end up in water bodies. Since PBTBO is expected to be highly soluble in water, is not volatile and does not have a tendency to bind to particles (based on data for an analogue chemical), it could be found in surface water but not in sediments.
Based on its physical and chemical properties, PBTBO does not degrade quickly in the environment. It is therefore expected to be persistent in water and soil. PBTBO does not have the potential to accumulate in organisms. This substance has been determined to meet the persistence criteria but not the bioaccumulation criterion as set out in the Persistence and Bioaccumulation Regulations. In addition, it is not highly hazardous to aquatic organisms (LC50/EC50 greater than 1 mg/L).
For this screening assessment, a reasonable worst-case exposure scenario was selected in which a facility (user of the substance) discharges PBTBO into the aquatic environment. The predicted environmental concentration in water was a few orders of magnitude below predicted no-effect concentrations calculated for fish, daphnids and algae. Thus, this substance is not anticipated to cause ecological harm in the aquatic environment.
This substance will be included in the upcoming Domestic Substances List inventory update initiative. In addition and where relevant, research and monitoring will support verification of assumptions used during the screening assessment.
Based on the information available, it is concluded that PBTBO does not meet any of the criteria set out in section 64 of CEPA 1999.
Introduction
The Canadian Environmental Protection Act, 1999 (CEPA 1999) (Canada 1999) requires the Minister of the Environment and the Minister of Health to conduct screening assessments of substances that have met the categorization criteria set out in the Act to determine whether these substances present or may present a risk to the environment or human health. Based on the results of a screening assessment, the Ministers can propose to take no further action with respect to the substance, to add the substance to the Priority Substances List (PSL) for further assessment, or to recommend that the substance be added to the List of Toxic Substances in Schedule 1 of the Act and, where applicable, the implementation of virtual elimination.
Based on the information obtained through the categorization process, the Ministers identified a number of substances as high priorities for action. These include substances that
- met all of the ecological categorization criteria, including persistence (P), bioaccumulation potential (B) and inherent toxicity to aquatic organisms (iT), and were believed to be in commerce in Canada; and/or
- met the categorization criteria for greatest potential for exposure (GPE) or presented an intermediate potential for exposure (IPE), and had been identified as posing a high hazard to human health based on classifications by other national or international agencies for carcinogenicity, genotoxicity, developmental toxicity or reproductive toxicity.
The Ministers therefore published a notice of intent in the Canada Gazette, Part I, on December 9, 2006 (Canada 2006a), that challenged industry and other interested stakeholders to submit, within specified timelines, specific information that may be used to inform risk assessment, and to develop and benchmark best practices for the risk management and product stewardship of those substances identified as high priorities.
The substance Phenol, 4,4- (3H-1,2-benzoxathiol-3-ylidene)bis[2,6-dibromo-3-methyl-,S,S-dioxide, monosodium salt was identified as a high priority for assessment of ecological risk as it had been found to be persistent, bioaccumulative and inherently toxic to aquatic organisms and is believed to be in commerce in Canada. The Challenge for this substance was published in the Canada Gazette on November 17, 2007 (Canada 2007). A substance profile was released at the same time. The substance profile presented the technical information available prior to December 2005 that formed the basis for categorization of this substance. As a result of the Challenge, information pertaining to the quantity in commerce of the substance was received.
Although Phenol, 4,4- (3H-1,2-benzoxathiol-3-ylidene)bis[2,6-dibromo-3-methyl-,S,S-dioxide, monosodium salt was determined to be a high priority for assessment with respect to the environment, it did not meet the criteria for GPE or IPE, and was not identified as posing a high hazard to human health based on classifications by other national or international agencies for carcinogenicity, genotoxicity, developmental toxicity or reproductive toxicity. Therefore, this assessment focuses principally on information relevant to the evaluation of ecological risks.
Under CEPA 1999, screening assessments focus on information critical to determining whether a substance meets the criteria for defining a chemical as toxic as set out in section 64 of the Act, where
"64. [...] a substance is toxic if it is entering or may enter the environment in a quantity or concentration or under conditions that
- have or may have an immediate or long-term harmful effect on the environment or its biological diversity;
- constitute or may constitute a danger to the environment on which life depends; or
- constitute or may constitute a danger in Canada to human life or health."
Screening assessments examine scientific information and develop conclusions by incorporating a weight-of-evidence approach and precaution.
This screening assessment includes consideration of information on chemical properties, hazards, uses and exposure, including the additional information submitted under the Challenge. Data relevant to the screening assessment of this substance were identified in original literature, review and assessment documents, stakeholder research reports and from recent literature searches, up to May 2008. Key studies were critically evaluated; modelling results may have been used to reach conclusions. When available and relevant, information presented in hazard assessments from other jurisdictions was considered. The screening assessmentdoes not represent an exhaustive or critical review of all available data. Rather, it presents the most critical studies and lines of evidence pertinent to the conclusion.
This screening assessment was prepared by staff in the Existing Substances Program at Health Canada and Environment Canada and it incorporates input from other programs within these departments. This assessment has undergone external written peer review/consultation. Additionally, a draft of this screening assessment was subject to a 60-day public comment period. While external comments were taken into consideration, the final content and outcome of the screening risk assessment remain the responsibility of Health Canada and Environment Canada. The critical information and considerations upon which the assessment is based are summarized below.
Substance Identity
For the purposes of this document, this substance will be referred to as PBTBO.
| Chemical Abstracts Service Registry Number (CAS RN) | 62625-32-5 |
|---|---|
| Domestic Substances List (DSL) name | Phenol, 4,4- (3H-1,2-benzoxathiol-3-ylidene)bis[2,6-dibromo-3-methyl-,S,S-dioxide, monosodium salt |
| National Chemical Inventories (NCI) namesTable note a | Phenol, 4,4'-(2,2-dioxido-3H-1,2-benzoxathiol-3-ylidene)bis[2,6-dibromo-3-methyl-, monosodium salt (TSCA, PICCS, ASIA-PAC, NZloC) sodiuma-(3,5-dibromo-2-methyl-4-oxo-2,5-cyclohexadienylidene)-a-(3,5-dibromo-4-hydroxyphenyl)toluenesulphonate (EINECS) PHENOL, 4,4'-(3H-1,2-BENZOXATHIOL)-3-YLIDENE)BIS[ 2,6-DIBROMO-3-METHYL, S,S-DIOXIDE, MONOSODIUM SALT (PICCS) |
| Other names | None |
| Chemical group (DSL Stream) | Discrete organics |
| Major chemical class or use | Triarylmethane dyes |
| Major chemical sub-class | Brominated phenolsulfophthaleins |
| Chemical formula | C21H13Br4O5S.Na |
| Chemical structure | 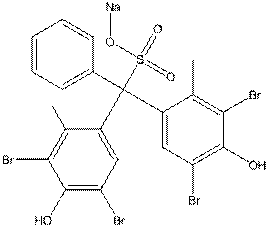 |
| Simplified Molecular Input Line Entry System (SMILES) | O=S(O[Na])(C(C1=CC(Br)=C(O)C(Br)=C1C)(C2=CC(Br)=C(O)C(Br)=C2C)C3=CC=CC=C3)=O |
| Molecular mass | 722.02 g/mol |
It should be noted that PBTBO is a structural isomer of phenol, 4,4'-(3H-2,1-benzoxathiol-3-ylidene)bis[2,6-dibromo-3-methyl-, S,S-dioxide, monosodium salt (CAS RN 67763-24-0). According to the NCI, the common name of this latter substance is bromocresol green sodium salt. However, in many instances (e.g., online chemicals databases, material safety data sheets, etc.), the name bromocresol green sodium salt is used as a synonym for both CAS numbers (i.e. 62625-32-5 and 67763-24-0), which may be misleading. Special attention was given to make sure that the information reported in this assessment report pertains specifically to CAS RN 62625-32-5.
The structure of PBTBO is often shown with a closed benzoxathiole ring and with the sodium ion associated with one of the phenol groups. However, once in water, the benzoxathiole ring will hydrolyze to form an ionic sulfonate group. The sodium ion will hence be associated with the sulfonate group. The SMILES for the neutral form of this structure (i.e. sodium atom replaced with an hydrogen atom) was used as input to the various models used in this assessment.
Physical and Chemical Properties
Table 2 contains modelled physical and chemical properties of PBTBO that are relevant to its environmental fate. Given the lack of experimental data for this substance, a search was conducted and a close structural analogue for which experimental data were available was identified. This analogue is bromophenol blue (CAS RN 115-39-9), a substance that is also assessed as part of the Challenge. Information on the chemical identity of bromophenol blue is provided in Table 3.
A key study reporting experimental data for some physical and chemical properties of the analogue bromophenol blue was critically evaluated to make sure that the results could be used to support conclusions. The review (Robust Study Summary) of this study is included in Appendix 1. This study was found as a result of recent literature searches.
When available, experimental data from analogues are preferred to modelled data for the substance being assessed, especially if the models do not provide accurate predictions. Given the scarcity of experimental data for both PBTBO and its analogue, bromophenol blue, quantitative structure-activity relationship (QSAR) models were used to generate data for physical and chemical properties of PBTBO. These models (except WSKOWWIN 2000) are mainly based on fragment addition methods, i.e., they rely on the structure of a chemical. Since these models only accept the neutral form of a chemical as input (in SMILES form), the modelled values shown in Table 2 are for the undissociated form of PBTBO.
| Type | Value | Temperature (°C) | Reference | |
|---|---|---|---|---|
| Physical state | Not available | Not available | Not available | Not available |
| Decomposition point (°C) | AnalogueTable note b | 279 | PhysProp 2006 | |
| Decomposition point (°C) | Modelled | 308.21 | MPBPWIN 2000 | |
| Boiling point (°C) | Modelled | 704.68 | MPBPWIN 2000 | |
| Vapour pressure (Pa) | ModelledTable note c | 1.38 × 10-17 (1.04 × 10-19 mm Hg) |
25 | MPBPWIN 2000 |
| Henry's Law constant (Pa·m3/mol) | Modelled | 2.23 × 10-16 (2.20 × 10-21atm·m3/mol) |
25 | MPBPWIN 2000 |
| Log Kow (Octanol-water partition coefficient) (dimensionless) | AnalogueTable note b (ionized form) | -3.07 | 25 | Franco et al. 1999 |
| Log Kow (dimensionless) | Modelled | 6.32 | KOWWIN 2000 | |
| Log Koc (Organic carbon-water partition coefficient - L/kg) (dimensionless) | AnalogueTable note b Table note c Table note d (ionized form) | -2.91 to -2.02 | 25 | Franco et al. 1999 |
| Log Koc (L/kg) (dimensionless) | Modelled | 4.755 | KOCWIN 2000 (Kow method) | |
| Water solubility (mg/L) | AnalogueTable note b | 4000 | Not available | O'Neil 2001 |
| Water solubility (mg/L) | ModelledTable note c Table note d Table note e | greater than 100 000 | 25 | WSKOWWIN 2000 |
| pKa (Acid dissociation constant) (dimensionless) | Modelled pKa1 pKa2 pKa3 | 6.27 5.55 -0.5Table note f |
ACD 2005 |
Because it is a salt, PBTBO will dissociate in water and will be found in an ionized form. Furthermore, based on modelled pKa values (-0.5 to 6.27), PBTBO is expected to be fully ionized at environmentally relevant pH values (6-9). This is further supported by the fact that the colour change of the pH indicator bromcresol green (a structural isomer) is reported to occur in the pH range 3.8-5.4 (pH-meter.Info 2005). The pKa for bromcresol green is thus expected to be at the mid-point of this range (pH 4.6). The pKa values for PBTBO were not modelled and therefore not considered during categorization.
Based on the quantitative solubility value available for the analogue (4000 mg/L) and given the fact that PBTBO is a salt with a pKa that is relatively low, this substance is highly soluble in water. Regarding other physical and chemical properties, the analogue data shown in Table 2 suggest that PBTBO does not bioaccumulate in organisms, does not bind to particles and is highly mobile in soil. Its volatility cannot be assessed because the modelled values for vapour pressure and Henry's Law constant are probably not reliable (see below). However, given its ionized state under environmental pH, this substance will likely have a low volatility. A more detailed discussion of how physical and chemical properties influence the environmental fate of this substance is presented later in this report.
As seen from Table 2, many models do not perform well in estimating the physical chemical properties of PBTBO. In particular, the modelled log Kow and log Kocvalues differ from the experimental values measured for the structural analogue by many orders of magnitude. This is most likely because the chemical structures of ionisable substances like PBTBO are poorly represented in the training set of some the models used. Because modelled data are less reliable, the analogue data presented in Table 2 for bromophenol blue were used as appropriate in this assessment.
| Chemical Abstracts Service Registry Number (CAS RN) | 115-39-9 |
|---|---|
| Domestic Substances List (DSL) name | Phenol, 4,4'- (3H-2,1-benzoxathiol-3-ylidene)bis[2,6-dibromo-,S,S-dioxide |
| Major chemical class or use | Triarylmethane dyes |
| Major chemical sub-class | Phenolsulfophthaleins |
| Chemical formula | C19H10Br4O5S |
| Chemical structure | 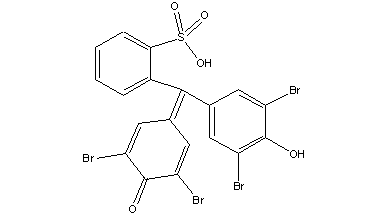 |
| SMILES | OS(C1=CC=CC=C1/C(C2=CC(Br)=C(O)C(Br)=C2)=C(C=C3Br)/C=C(Br)C3=O)(=O)=O |
| Molecular mass | 669.96 g/mol |
Sources
PBTBO is not reported to be naturally produced in the environment.
Information gathered through a CEPA section 71 notice for the 2006 calendar year indicates that 2363 kg of PBTBO were imported into Canada. During the same year, no companies reported manufacturing or using this substance in Canada in a quantity exceeding the prescribed thresholds (i.e., 100 kg for manufacture and 1000 kg for use). A total of six Canadian companies identified themselves as stakeholders, i.e., as having an interest in this substance (Environment Canada 2007). A similar CEPA section 71 notice for the 2005 calendar year also indicated that PBTBO was imported into Canada in the range of 1001-100 000 kg. For that year, five Canadian companies had identified themselves as stakeholders (Canada 2006b).
The quantity reported to be manufactured, imported or in commerce in Canada during the 1986 calendar year was 100 kg. The number of notifiers for the 1984 to 1986 calendar years was fewer than four.
Uses
No information on uses was received in response to the CEPA section 71 notice for the 2006 calendar year (Environment Canada 2007). A search in the open literature found the following possible uses for PBTBO: acid-base indicator and analytical reagent in microbiological, chemical and biochemical testing applications. However, uncertainty partly remains about these uses since this CAS RN (62625-32-5) is frequently confused with its isomer, bromocresol green sodium salt (CAS RN 67763-24-0) (see section Substance Identity in this report).
Releases to the Environment
A total quantity of 2363 kg were imported into Canada in 2006 (Environment Canada 2007). Based on information received through the section 71 notice, this quantity is likely sold to various clients located across the country. Since PBTBO is most likely used as a laboratory reagent, releases of this substance to the Canadian environment could be dispersive yet very low. Indeed, the kinds of facilities using this type of substance (e.g., research centres, academic institutions) are located across Canada. They are expected to use very small quantities (drops) of this type of reagent.
Mass Flow
To estimate potential releases of PBTBO to the environment at different stages of the life cycle of chemical substances, a Mass Flow Tool was developed (Environment Canada 2008a). Empirical data concerning releases of specific substances to the environment are seldom available. Therefore, for each identified type of use of the substance, the proportion and quantity of release to the different environmental media were estimated, as was the proportion of the substance chemically transformed or sent for waste disposal (Table 4). Assumptions and input parameters used in making the release estimates were based on professional judgment.
This substance is used as a solution. The solution can be imported or prepared on site starting from a pure liquid substance (Environment Canada 2007). If the solution is prepared on site, releases can occur during this process. A value of 2% was chosen to represent losses during handling of the solution. Such spills were assumed to go down the drain. After being used in research facilities and similar institutions, it is assumed that the solution containing PBTBO will not be tre ated as a hazardous waste. Therefore, it will probably be disposed of down the drain and sent to sewer. As a conservative scenario, 100% of the discarded solution was assumed to reach the sewer. Assuming that the entire mass of PBTBO imported into Canada during a year is prepared/used, the results in Table 4 indicate that 2363 kg of this substance would be released to sewers (based on data from 2006).
| Life cycle step analysis for PBTBO | Formulation into a product imported into Canada (kg) | Industrial useTable note g of the product imported into Canada (kg) | Consumer use of the product imported into Canada (kg) | Total use of the product imported into Canada (kg) |
|---|---|---|---|---|
| SewerTable note h | 47 | 2316 | 0 | 2363 |
| Soil | 0 | 0 | 0 | 0 |
| Air | 0 | 0 | 0 | 0 |
| Chemically transformed | 0 | 0 | 0 | 0 |
| Sent to waste disposal | 0 | 0 | 0 | 0 |
| Total | 47 | 2316 | 0 | 2363 |
Environmental Fate
Based on its physical and chemical properties (Table 2) and potential mode of entry in the environment, PBTBO is expected to mainly be found in water. The ionizing nature of PBTBO suggests that the partitioning behaviour of this chemical will be better predicted using the log Kow and log Kocvalues available for the ionized rather than neutral form of the structural analogue found (Table 2).
The Mass Flow Tool assumes that when PBTBO is used as an analytical reagent in laboratories, it is probably disposed of down the drain. From there, it will reach sewage treatment plants (STPs). Assuming no degradation in these plants, it will likely stay in waste water and will not partition to sewage sludge based on its expected high water solubility (Table 2; analogue data) and low log Kow and log Koc values for ionized forms (Table 2; less than 1, analogue data). Similarly, once released to receiving water, PBTBO will mainly remain in the water column rather than partition into the sediments, given its expected high solubility in water and low log Kow and log Koc values for the ionized form. PBTBO will not volatilize to air from water based on its tendency to ionize at ambient pH in water.
Persistence and Bioaccumulation Potential
Environmental Persistence
The preceding information on the likely pattern of release and subsequent environmental fate of PBTBO suggests that it will mostly be found in water, but not in sediments or soil. This substance is also not expected to be present in air.
There are no empirical degradation data available for PBTBO or for a structural analogue. ETAD (1995) states that, with some exceptions, dyes may be considered essentially non-biodegradable under aerobic conditions. Repeated evaluation of ready- and inherent-biodegradability using accepted screening tests (e.g., OECD tests) have confirmed this characteristic (Pagga and Brown 1986, ETAD 1992). Based on the chemical structure of PBTBO, there is no reason to suspect that its biodegradation would be significantly different from that of other dyes described in ETAD (1995).
Due to the lack of experimental data, the persistence of PBTBO was examined using the predictive QSAR models for biodegradation shown in Table 5 below. Given the ecological importance of the water compartment, the fact that most of the available models apply to water and the fact that PBTBO is expected to be released and remain in this compartment, biodegradation in water was primarily examined. Although the degradation models are structure-based and only consider the neutral form of PBTBO, most of the modelled values (Table 5) are considered to be reliable, as some chemicals of structural comparability to PBTBO are contained in their training sets.
| Fate process | Model and model basis | Result | Interpretation | Extrapolated half-life (days) | Extrapolation reference and/or source |
|---|---|---|---|---|---|
| Biodegradation (aerobic) | BIOWIN 2000 Sub-model 1: Linear probability |
0.37 | Does not biodegrade fast in water | n/a | n/a |
| Biodegradation (aerobic) | BIOWIN 2000 Sub-model 2: Non-linear probability |
0.023 | Does not biodegrade fast in water | n/a | n/a |
| Biodegradation (aerobic) | BIOWIN 2000 Sub-model 3: Expert Survey (ultimate biodegradation) |
1.07 | Recalcitrant to biodegradation in water | 180 720 | US EPA 2002 Aronson et al. 2006 |
| Biodegradation (aerobic) | BIOWIN 2000 Sub-model 4: Expert Survey (primary biodegradation) |
2.19 | Primary biodegradation in months in water | 60 120 | US EPA 2002 Aronson et al. 2006 |
| Biodegradation (aerobic) | BIOWIN 2000 Sub-model 5: MITI linear probability |
-0.37 | Does not biodegrade fast in water | greater than 60 | Aronson et al. 2006 |
| Biodegradation (aerobic) | BIOWIN 2000 Sub-model 6: MITI non-linear probability |
0.00 | Does not biodegrade fast in water | greater than 60 | Aronson et al. 2006 |
| Biodegradation (anaerobic) | BIOWIN 2000 Sub-model 7: Linear probability |
-0.53 | Does not biodegrade fast | n/a | n/a |
| Biodegradation | BIOWIN 2000 Overall conclusion |
No | Not ready biodegradable in water | n/a | n/a |
| Biodegradation (aerobic) | TOPKAT 2004 Probability (MITI 1) |
Out of acceptable domain | n/a | n/a | n/a |
| Biodegradation (aerobic) | CATABOL 2004 -2008 % biological oxygen demand (BOD) (OECD 301C) |
Out of acceptable domain | n/a | n/a | n/a |
The results from Table 5 show that most of the probability models (BIOWIN 2 ,5, 6 and 7) suggest that PBTBO does not biodegrade rapidly. In fact, all probability results except BIOWIN 1 are less than 0.3, the cut-off suggested by Aronson et al. (2006) to identify substances as having a half-life greater than 60 days (based on the MITI probability models), and all are less than 0.5, the cut-off suggested by the model developers for slow biodegradation. It is suggested by the US EPA (2002) that the half-life result from the primary survey model (BIOWIN 4) of "months" corresponds to a half-life of approximately 60 days and by Aronson et al. (2006) that it corresponds to a half-life of 120 days. It is suggested by the US EPA (2002) that the ultimate survey model (BIOWIN 3) result, "recalcitrant," corresponds to a half-life of approximately 180 days and by Aronson et al. (2006) that is corresponds to a half-life of 720 days. The substance is also not expected to degrade rapidly under anaerobic conditions. The overall conclusion from BIOWIN is "not ready-biodegradable." Other ultimate degradation models (CATABOL and TOPKAT) did not produce any acceptable results, as the substance was out of their respective domains of applicability.
When the results of the probability models and the overall BIOWIN conclusion are considered, there is model consensus, suggesting that the biodegradation half-life of PBTBO in water is greater than 182 days.
Using an extrapolation ratio of 1:1 for a water: soil biodegradation half-life (Boethling et al. 1995), the biodegradation half-life in soil is also greater than 182 days. This indicates that PBTBO would be persistent in soil. However, this substance is not expected to reach this medium.
Overall, the empirical data on dyes from ETAD (1992, 1995) as well as the modelled data (Table 5) demonstrate that PBTBO meets the persistence criteria in water (half-life greater than or equal to 182 days) as set out in the Persistence and Bioaccumulation Regulations (Canada 2000). This substance is not expected to be found in soil, air or sediments.
Potential for Bioaccumulation
There are no experimental bioaccumulation factor (BAF) or bioconcentration factor (BCF) data available for PBTBO or for a structural analogue. Since PBTBO is a salt, it is expected that this substance will exist in an ionized form in the environment. The experimental log Kow value for the ionized form of the analogue bromophenol blue is -3.07 (Table 2), indicating a low potential for bioaccumulation. Ionization was not considered during categorization with respect to bioaccumulation potential.
Since no experimental BAF or BCF data for PBTBO were available, a predictive approach was applied using available BAF and BCF models as shown in Table 6 below. The experimental log Kow value for the ionized form of the analogue bromophenol blue was used as input for the models. The modelled bioaccumulation values do not take into account the metabolic potential of the substance; therefore these bioaccumulation values may be over-estimated. However, as the predicted bioconcentration and bioaccumulation factors for PBTBO were low, this would not influence the bioaccumulation conclusion.
The modified Gobas BAF middle trophic-level model for fish predicted a BAF of less than 1 L/kg, indicating that PBTBO does not have the potential to bioconcentrate and biomagnify in the environment.
The results of BCF model calculations provide additional evidence to support the low bioconcentration potential of this substance. The very low BCF value of 3.16 is a default value recommended by the BCFWIN model for compounds having a log Kow less than 1; therefore, this result is not a model-generated BCF calculated specifically for PBTBO.
| Test organism | Endpoint | Value wet weight (L/kg) | Reference |
|---|---|---|---|
| Fish | BAF | less than 1 | Gobas BAF T2MTL (Arnot and Gobas 2003) |
| Fish | BCF | less than 1 | Gobas BCF middle trophic level (Arnot and Gobas 2003) |
| Fish | BCF | 3.16 | BCFBAF 2000 |
| Fish | BCF | Out of acceptable domain | Baseline BCF model (Dimitrov et al. 2005) |
Based on the experimental log Kow data for the analogue bromophenol blue and on the modelled values for PBTBO, it is concluded that PBTBO does not meet the bioaccumulation criteria (BCF or BAF greater than or equal to 5000) as set out in the Persistence and Bioaccumulation Regulations (Canada 2000).
Potential to Cause Ecological Harm
Ecological Effects Assessment
A - In the Aquatic Compartment
As mentioned earlier, PBTBO would tend to remain in water if it was released in this environmental compartment. In addition, it is expected to be persistent in this compartment. Therefore, this substance could be of concern for ecological effects in aquatic ecosystems.
As a salt, PBTBO will be found in an ionized form in aquatic ecosystems. Given its expected high water solubility, aquatic organisms could be exposed. However, since it is believed to have low affinity for lipids (see Table 6), it should not accumulate to a significant extent in tissues of exposed organisms.
There are no acceptable experimental aquatic toxicity data available for PBTBO or for a structural analogue. Therefore, modelled data were used to estimate its potential for aquatic toxicity (Table 7). The ECOSAR model was run using the experimental analogue values shown in Table 2 for water solubility and log Kow (ionized form). It was not possible to enter these values in the other models used (OASIS Forecast and AIEPS) as these models only accept chemical structure as input data. OASIS Forecast predicts a log Kow value based on the structure entered (neutral form) and uses it to estimate toxicity. AIEPS is a probabilistic neural network based predictive model that uses structural fragments and presence or absence of atoms to determine similarity between the substance being modeled and those in the training set. It then calculates a prediction for three acute endpoints (fathead minnow, Daphnia magna and Pseudokirchneriella subcapitata).
| Organism | Type of test | Endpoint | Value (mg/L) | Reference |
|---|---|---|---|---|
| Fish | Acute (96 hours) | The concentration of a substance that is estimated to be lethal to 50% of the test organisms (LC50) | greater than water solubility limitTable note i | ECOSAR 2004 |
| Fish | Acute (96 hours) | LC50 | 0.68 | OASIS Forecast 2005 |
| Fish | Acute (96 hours) | LC50 | 0.0299 | AIEPS 2003-2007 |
| Daphnia | Acute (48 hours) | LC50 | greater than water solubility limitTable note i | ECOSAR 2004 |
| Daphnia | Acute (48 hours) | LC50 | 16.2 | AIEPS 2003-2007 |
| Algae | Acute (96 hours) | The concentration of a substance that is estimated to have some toxic sublethal effect on 50% of the test organisms (EC50) | 167.7 | ECOSAR 2004 |
| Algae | Acute (96 hours) | EC50 | 12.01 | AIEPS 2003-2007 |
The aquatic toxicity predictions obtained from the ECOSAR model are reliable to some extent. Indeed, both the log Kow (analogue data) and molecular weight of PBTBO are covered by the domain of this model (cut-off of 7.0 for log Kow and 1000 g/mol for molecular weight). However, the closest analogues contained in the training set of ECOSAR are chlorophenols, suggesting that the values predicted for the toxicity of PBTBO are uncertain. Nevertheless, these modelled results suggest that PBTBO is not highly hazardous to aquatic organisms (acute LC/EC50 greater than 1.0 mg/L), as observed for numerous substances having a very low log Kow.
The result from the OASIS Forecast model suggests that PBTBO is highly hazardous (LC50 less than or equal to 1.0 mg/L); however, this value is not considered reliable. Indeed, the discrepancy between this value and the ECOSAR predictions is partly due to the fact that a modelled log Kow value for the neutral molecule is used by OASIS Forecast in calculations. As it can be seen in Table 2, this value is not representative of the partitioning behaviour of the ionized form of the molecule. In addition, the chemical structure of PBTBO is not well covered by the training set used by OASIS. Similarly, the AIEPS model did not provide reliable predictions as the similarity index indicated that most of chemicals in the training set were less than 60% similar to PBTBO.
Given the high persistence of PBTBO in the environment (see Table 5), chronic exposure is likely to occur. However, considering the likely low acute toxicity of this substance and its low bioaccumulation potential, its chronic aquatic toxicity is also expected to be low.
B - In Other Environmental Compartments
No ecological effects studies were found for this compound in media other than water (e.g., sediment, soil). However, PBTBO is not expected to be found in these media.
Ecological Exposure Assessment
There are no environmental monitoring data available for this substance. Based on the information received in response to a CEPA section 71 notice (Environment Canada 2007), and based on a life-cycle analysis (Table 4), up to 2363 kg of PBTBO could reach the aquatic environment in Canada annually. Even though PBTBO does not meet the bioaccumulation potential criteria prescribed by the Persistence and Bioaccumulation Regulations (Canada 2000), it can persist in the environment and, depending on the level of exposure, could potentially cause harm to the environment. This was investigated by conducting a quantitative evaluation of exposure associated with the release of this chemical to aquatic ecosystems.
Environmental concentrations were estimated from available information, including estimated substance quantities, potential release rates, and characteristics of possible receiving water bodies. Environment Canada's Industrial Generic Exposure Tool - Aquatic (IGETA) was employed to estimate the substance concentration in a generic watercourse receiving industrial effluents (Environment Canada 2008b). This tool represents a highly conservative scenario in which the substance is released by a single facility at a single point in a watercourse. The generic scenario is designed to provide estimates of concentrations based on conservative assumptions regarding the amount of chemical used and released, the number of days it is used, the sewage treatment plant removal rate, and the size of the receiving watercourse. The tool models an industrial-release scenario using loading estimates based on data from industrial surveys and knowledge of the distribution of industrial discharges across the country. It calculates a predicted environmental concentration (PEC) assuming instantaneous dilution in a small receiving water. The equation and inputs used to calculate the PEC in the receiving watercourse are described in Environment Canada (2008c). The total quantity of 2316 kg potentially released to aquatic ecosystems (Table 4) was used as a highly conservative estimate of the quantity of substance released annually from a single facility. Other key parameter values were as follows: 261 processing days (working days only, based on expected uses), no removal at sewage treatment plants (worst-case scenario) and 0.65 m3/s as the flow of the receiving watercourse (15th percentile of the distribution of receiving watercourse flows in the country). The resulting PEC was calculated to be 0.15 mg/L.
Characterization of Ecological Risk
The approach taken in this ecological screening assessment was to examine various supporting information and develop conclusions based on a weight-of-evidence approach and using precaution as required under CEPA 1999. Lines of evidence considered include results from a conservative risk quotient calculation, as well as information on persistence, bioaccumulation, toxicity, sources and fate of the substance.
A risk quotient analysis, integrating conservative estimates of exposure with ecological effects information, was performed for the aquatic medium to determine whether there is potential for ecological harm in Canada. The generic exposure scenario presented above yielded a predicted environmental concentration (PEC) of 0.15 mg/L. A predicted no-effect concentration (PNEC) was derived by selecting the lowest critical toxicity value (CTV) for a given type of organism (an EC50 or the water solubility limit of the substance; see Table 7) and by dividing it by an assessment factor of 1000 (to account for interspecies and intraspecies variability in sensitivity, for using modelled toxicity data to represent field conditions, and to estimate a long-term no-effects concentration from a short-term LC50). The PEC calculated with IGETA was then used together with the PNEC to calculate the risk quotient.
The calculated risk quotient for each of the three model organism types is lower than one (Table 8). Given that IGETA provides a conservative estimate of exposure and in view of the large assessment factor used to estimate chronic effect threshold (PNEC), the results indicate a low potential for ecological harm resulting from local exposure to a point-source industrial release of PBTBO to the aquatic environment.
| Organism | CTV (mg/L) | PNEC (mg/L) | PEC (mg/L) | Scenario | Risk quotient (PEC/PNEC) |
|---|---|---|---|---|---|
| Fish | 4000 | 4 | 0.15 | IGETA model: discharge to a watercourse from a plant | 0.037 |
| Daphnia | 4000 | 4 | 0.15 | IGETA model: discharge to a watercourse from a plant | 0.037 |
| Algae | 168 | 0.168 | 0.15 | IGETA model: discharge to a watercourse from a plant | 0.89 |
In summary, the information gathered suggests that PBTBO is not causing ecological harm when released in the Canadian environment. After its release, this substance will mainly be found in water, where it is expected to persist. However, it is not expected to bioaccumulate in organisms. This substance is expected to exhibit low acute toxicity to aquatic organisms. A risk quotient analysis based on an highly conservative industrial scenario shows that aquatic organisms are not at risk.
Uncertainties in Evaluation of Ecological Risk
For PBTBO, there are no experimental data for physical and chemical properties, degradation, bioaccumulation factors or ecotoxicity. Gaps in available experimental data were largely filled through the use of data from a structural analogue (bromophenol blue) as well as through the use of QSAR-based models. While there are uncertainties associated with the use of these models to estimate chemical and biological characteristics, the approaches used allowed meaningful interpretation of the information.
Conclusion
Based on the information presented in this screening assessment, it is concluded that PBTBO is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
It is therefore concluded that PBTBO does not meet the definition of toxic as set out in section 64 of CEPA 1999. Additionally, PBTBO meets the criteria for persistence as set out in the Persistence and Bioaccumulation Regulations (Canada 2000) but does not meet the criteria for bioaccumulation potential as set out in the same regulations.
References
[ACD] Advanced Chemistry Development, Inc. 2005. ACD/pKa DB. ACD/Labs Release 9.00. Product Version 9.0. Copyright 1994-2005. http://www.acdlabs.com/
[AIEPS] Artificial Intelligence Expert Predictive System. 2003-2007. Version 2.05. Ottawa (ON): Environment Canada, Existing Substances Division, New Substances Division. Model developed by Stephen Niculescu. Available from: Environment Canada, Existing Substances Division, New Substances Division.
Arnot JA, Gobas FAPC. 2003. A generic QSAR for assessing the bioaccumulation potential of organic chemicals in aquatic food webs. QSAR Comb Sci 22(3): 337-345.
Aronson D, Boethling B, Howard P, W Stiteler W. 2006. Estimating biodegradation half-lives for use in chemical screening. Chemosphere 63: 1953-1960.
[BCFBAF] BioConcentration Factor Program for Windows [Estimation Model]. 2000. Version 3.00. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. [cited 2008 May]. http://www.epa.gov/oppt/exposure/pubs/episuite.htm
[BIOWIN] Biodegradation Probability Program for Windows [Estimation Model]. 2000. Version 4.10. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. [cited 2009 May]. http://www.epa.gov/oppt/exposure/pubs/episuite.htm
Boethling, RS, Howard, PH, Beauman, JA, Larosche, ME 1995. Factors for intermedia extrapolations in biodegradability assessment. Chemosphere 30(4): 741-752.
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C., 1999, c. 33, part 5, s. 77. Canada Gazette. Part III. vol. 22, no. 3. http://canadagazette.gc.ca/partIII/1999/g3-02203.pdf
Canada. 2000. Canadian Environmental Protection Act: Persistence and Bioaccumulation Regulations, P.C. 2000-348, 23 March, 2000, SOR/2000-107, Canada Gazette. Part II, vol. 134, no. 7, p. 607-612. http://canadagazette.gc.ca/partII/2000/20000329/pdf/g2-13407.pdf
Canada, Dept. of the Environment, Dept. of Health. 2006a.Canadian Environmental Protection Act, 1999: Notice of intent to develop and implement measures to assess and manage the risks posed by certain substances to the health of Canadians and their environment. Canada Gazette, Part I, vol. 140, no. 49, p. 4109-4117. http://canadagazette.gc.ca/partI/2006/20061209/pdf/g1-14049.pdf
Canada, Dept. of the Environment, Dept. of Health. 2006b.Canadian Environmental Protection Act, 1999: Notice with respect to selected substances identified as priority for action. Canada Gazette, Part I, vol. 140, no. 9, p. 435-459. http://canadagazette.gc.ca/partI/2006/20060304/pdf/g1-14009.pdf
Canada, Dept. of the Environment, Dept. of Health. 2007.Canadian Environmental Protection Act, 1999: Notice of fourth release of technical information relevant to substances identified in the Challenge. Canada Gazette, Part I, vol. 141. no. 46, p. 3192-3197. http://canadagazette.gc.ca/partI/2007/20071117/pdf/g1-14146.pdf
CATABOL. [Computer Model]. c2004-2008. Version 5.10.2. Bourgas (BG): Bourgas Prof. Assen Zlatarov University, Laboratory of Mathematical Chemistry. http://oasis-lmc.org/?section=software&swid=1
Dimitrov S, Dimitrova N, Parkerton T, Comber M, Bonnell M, Mekenyan O. 2005. Base-line model for identifying the bioaccumulation potential of chemicals. SAR QSAR Environ Res 16(6):531-554.
[ECOSAR] Ecological Structural Activity Relationships [Internet]. 2004. Version 1.00. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. [cited 2009 May]. http://www.epa.gov/oppt/exposure/pubs/episuite.htm
Environment Canada. 2007. Data for Batch 4 substances collected under the Canadian Environmental Protection Act, 1999, Section 71: Notice with respect to certain Batch 4 Challenge substances. Data prepared by: Environment Canada, Existing Substances Program.
Environment Canada. 2008a. Guidance for conducting ecological assessments under CEPA, 1999: science resource technical series, technical guidance module: Mass Flow Tool. Preliminary draft working document. Gatineau (QC): Environment Canada, Existing Substances Division.
Environment Canada. 2008b. Guidance for conducting ecological assessments under CEPA 1999, Science Resource Technical Series, Technical Guidance Module: The Industrial Generic Exposure Tool - Aquatic (IGETA), Working document, Gatineau (QC): Environment Canada, Existing Substances Division.
Environment Canada. 2008c. IGETA report (62625-32-5 IGETA report 2008-05-30). Draft. Gatineau (QC): Environment Canada, Existing Substances Division.
ETAD (Ecological and Toxicological Association of Dyes and Organic Pigments Manufacturers). 1992. Draft Guidelines for the Assessment of Environmental Exposure to Dyestuffs.
ETAD (Ecological and Toxicological Association of Dyes and Organic Pigments Manufacturers). 1995. Health & Environmental Information on Dyes Used in Canada. An overview to assist in the implementation of the New Substances Notification Regulation under the Canadian Environmental Protection Act. Prepared by the ETAD Canadian Affiliates. July 1995. Report 7/21/95
Franco, I, Leita, L, Vischetti, C, de Nobili, M. 1999. Adsoprtion of five model organic compounds on a peat at different stages of drying. J. Soil Contamination 8(4): 423-440.
Kollig, HP 1988. Criteria for evaluating the reliability of literature data on environmental process constants. Toxicol. Environ. Chem. 17: 287-311.
[KOCWIN] Organic Carbon Partition Coefficient Program for Windows [Estimation Model]. 2000. Version 2.00. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. [cited 2009 May]. http://www.epa.gov/oppt/exposure/pubs/episuite.htm
[KOWWIN] Octanol-Water Partition Coefficient Program for Microsoft Windows [Estimation Model]. 2000. Version 1.67. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. [cited 2009 May]. http://www.epa.gov/oppt/exposure/pubs/episuite.htm
Kulichenko, SA, Fesenko, SA, Fesenko, NI. 2001. Color indicator system for acid-base titration in aqueous micellar solutions of the cationic surfactant tridecylpyridinium. J. Anal. Chem. 56(11): 1002-1006.
[MPBPWIN] Melting Point Boiling Point Program for Microsoft Windows [Estimation Model]. 2000. Version 1.43. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. [cited 2009 May]. http://www.epa.gov/oppt/exposure/pubs/episuite.htm
[NCI] National Chemical Inventories [database on CD-ROM]. 2006. Columbus (OH): American Chemical Society. [cited 2008 May]. http://www.cas.org/products/cd/nci/index.html
[OASIS Forecast] Optimized Approach based on Structural Indices Set [Internet]. 2005. Version 1.20. Bourgas, Bulgaria: Laboratory of Mathematical Chemistry. [cited 2009 May]. http://oasis-lmc.org/?section=software
[OECD] Organisation for Economic Co-operation and Development. 1995. OECD Guideline for Testing of Chemicals. Test No. 107 Partition Coefficient (n-octanol/water): Shake Flask Method [Internet]. Guideline adopted 27 July 1995. Paris (FR): OECD, Environment Directorate. http://oberon.sourceoecd.org/vl=1533603/cl=13/nw=1/rpsv/ij/oecdjournals/1607310x/v1n1/s7/p1
[OECD] Organisation for Economic Co-operation and Development. 2000. OECD Guideline for Testing of Chemicals. Test No. 106 - Adsorption - Desorption Using a Batch Equilibrium Method [Internet]. Guideline adopted 21st January 2000. Paris (FR): OECD, Environment Directorate. http://oberon.sourceoecd.org/vl=1627150/cl=27/nw=1/rpsv/ij/oecdjournals/1607310x/v1n1/s6/p1
O'Neil, M.J. (ed.). 2001. The Merck Index - An encyclopaedia of chemicals, drugs, and biologicals. 13th Edition, Whitehouse Station, NJ. Merck and Co., Inc. p. 1447.
Pagga, U, Brown, D. 1986. The degradation of dyestuffs: Part II Behaviour of dyestuffs in aerobic biodegradation tests. Chemosphere, 15(4): 478-491.
pH-meter.Info [Internet]. c2005. Marki, Poland: ChemBuddy; [cited 2008 July 24]. http://www.ph-meter.info/pH-measurements-indicators
[PhysProp] Interactive PhysProp Database [database on the Internet]. 2006. Syracuse (NY): Syracuse Research Corporation. [cited 2008 May] http://www.syrres.com/esc/physdemo.htm
[TOPKAT] Toxicity Prediction Program [Internet]. 2004. Version 6.2. San Diego (CA): Accelrys Software Inc. [cited 2008 May]. http://www.accelrys.com/products/topkat/index.html
[US EPA] US Environmental Protection Agency. 2002. PBT Profiler Methodology [Internet]. Washington (DC): US EPA, Office of Pollution Prevention and Toxics. http://www.pbtprofiler.net/methodology.asp
[WSKOWWIN] Water Solubility for Organic Compounds Program for Microsoft Windows [Estimation Model]. 2000. Version 1.41 Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. [cited 2009 May]. http://www.epa.gov/oppt/exposure/pubs/episuite.htm
Zeroual, Y, Kim, BS, Kim, CS, Blaghen, M, Lee, KM. 2006. Biosorption of bromophenol blue from aqueous solutions by Rhizopus stolonifer biomass. Water, Air, Soil Poll. 177: 135-146.
Appendix 1 - Robust Study Summary
Table A-1. Evaluation of experimental data using Kollig's approach (Kollig 1998)
Reference: Franco I, Leita L, Vischetti C, de Nobili M. 1999. Adsorption of five model organic compounds on a peat at different stages of drying. J. Soil Contamination 8(4):423-440.
Test substance: CAS RN 115-39-9; Bromophenol blue.
Physical and chemical properties measured: pKa, Kow, Koc.
| Item | Weight | Response | Mark |
|---|---|---|---|
| Could you repeat the experiment with available information? | 5 | Only partially, based on the information included in the paper. One of the study authors was contacted by Environment Canada and provided clarification on methods. | 3 |
| Is a clear objective stated? | 1 | Yes | 1 |
| Is water quality characterized or identified (distilled or deionized)? | 2 | Yes, distilled water | 2 |
| Are the results presented in detail, clearly and understandably? | 3 | Fair | 1.5 |
| Are the data from a primary source and not from a referenced article? | 3 | Yes, primary source | 3 |
| Was the chemical tested at concentrations below its water solubility? | 5 | Yes | 5 |
| Were particulates absent? | 2 | Not mentioned | 0 |
| Was a reference chemical of known constant tested? | 3 | No, but a comparison made by the Environment Canada evaluator between the data measured in this study and other published data for the same substances indicate that values found in this study are in the same order of magnitude. See the table below in the "Additional comments" section. | 1.5 |
| Were other fate processes considered? | 5 | Hydrolysis and photolysis were not considered; however, these processes are not likely to influence the fate of bromophenol blue in solution. | 5 |
| Was a control (blank) run? | 3 | Yes for the batch equilibrium experiment (Koc); N/A for determination of pKa and Kow | 3 |
| Was temperature kept constant? | 5 | Yes for batch equilibrium experiment (Koc); T° not mentioned for Kow and pKa determination. | 3 |
| Was the experiment done near room temperature (15-30°C)? | 3 | Yes (25°C) for Koc; T° not mentioned for Kow and pKa determination. | 2 |
| Is the purity of the test chemical reported (greater than 98%)? | 3 | Not reported | 0 |
| Was the chemical's identity proven? | 3 | Partially (the chemical name, molecular weight and absorption maximum provided corresponded to CAS RN 115-39-9; however, the chemical structure did not); the CAS RN of the substances tested were not provided. One of the study authors was contacted; this author indicated that the salt form of bromophenol blue (CAS 62625-28-9) was used. Environment Canada considers that the dissociated (ionized) form of the latter substance is equivalent to the dissociated form of CAS RN 115-39-9. | 2 |
| Is the source of the chemical reported? | 1 | No | 0 |
| Item | Response |
|---|---|
| Results: | pKa = 4.0 Kow (ionized form) = 0.00085 Koc (for a series of peat samples - corrected by the Environment Canada evaluator for %OC) : ionized form = 0.0012 to 0.0095 |
| Score: | 33/47 or 70% |
| EC Reliability code: | 2 |
| Reliability category (high, satisfactory, low): | Satisfactory |
| Note | Evaluated independently by three Environment Canada evaluators (May 2008) |
Additional comments (by the Environment Canada evaluators):
- The sorbent material used in this batch equilibrium experiment was peat rather than soil. Since adsorption-desorption characteristics are usually useful for assessing the behaviour of a substance in soils, soil samples would have ideally been used in this experiment. Indeed, the sorptive capacity of peat is expected to be much higher than for soils, given its high organic carbon content. However, given that adsorption could be described by Freundlich isotherms (as indicated by the 1/n values in Table 3) and given that %OC for each sample was provided, the assessor was able to calculate Koc values based on the Kfmeasured by the authors.
- The fact that the Koc values for bromophenol blue were very low, even when dried peat was used (very high sorptive capacity), indicates that the hydrophobicity of bromophenol blue is very low.
- For the batch equilibrium experiment, the test substances should have been dissolved in 0.01 M CaCl2 rather than distilled water in order to improve centrifugation and minimize cation exchange (OECD TG No 106; 2000).
- The optimal sorbent/solute ratio was determined in preliminary experiments.
- The pH of the aqueous phase before and/or after adsorption was not reported. This factor has an important influence on adsorption for ionizable substances. One of the study authors was contacted to clarify this aspect. The clarification is as follows: Batch equilibrium experiments were conducted in peat/water suspensions whose pH was that imposed by the buffering action of the peat's functional groups (pH = 4.5), as it actually happens in the field where xenobiotics are present at low concentration and the pH is imposed by the soil. To ensure that pH did not vary, the compounds were dissolved in distilled water and the pH of the solution was adjusted to that of a compound free suspension of peat in water. The adsorption isotherm therefore refers to a situation where the compound was for the most part ionized.
- Although the authors mention that they measured the water solubility of the test compounds, it does not seem that they measured it for bromophenol blue because they cite the value from the Merck Index for this property (Table 2).
- The authors did not mention how they were able to measure a Kow for both non-ionized and ionized forms of bromophenol blue (e.g., use of a buffer). Also, they did not mention the value of the pH of the aqueous phase during the measurements. The OECD TG 107 - Partition Coefficient (n-octanol/water): Shake Flask Method states that "Dissociation or association of the dissolved molecules results in deviations from the partition law (OECD TG 107; 1995). Such deviations are indicated by the fact that the partition coefficient becomes dependent upon the concentration. Measurements should be made on ionizable substances only in their non-ionized form (free acid or free base) produced by the use of an appropriate buffer with a pH of at least one unit below (free acid) or above (free base) the pK." One of the study authors was contacted to clarify this methodological aspect. The clarification is as follows: The Kow of the non-ionized form of bromophenol blue was determined in an unbuffered water solution acidified to pH 1-1.5 with 0.020 mL of concentrated HCl, while the Kow of the ionized form was determined in a solution made alkaline with a similar amount of 0.5 M NaOH. The author recognizes that this is not a standard procedure. However, she considers that measurements of Kow in buffers could be misleading because of possible formation of ionic couples, intermolecular associations at high ionic strength, etc. She also thinks that the environmental behavior of substances such as bromophenol blue, which can be expected to be present in their ionized form in the environment, cannot be predicted on the basis of the Kow of the non-ionized molecule.
- UV-Vis seems like an appropriate method to measure the concentration of the chemicals in this study given their absorption maximum and chemical structure (numerous double bonds).
- Because reference chemicals of known constant were not tested in this study, the Environment Canada evaluator conducted a search for published data in order to validate (or not) the results measured in this study. Experimental data were found in the literature for bromophenol blue and for other substances tested in this study. They show that the values found in this study for water solubility, pKa and Kow are within the same order of magnitude as those published (see table below). No data were found to validate the Koc values measured; however, there are sufficient methodological details provided in the paper and in the author's response to consider the value for the ionized form of bromophenol blue as reliable. In addition, even if an actual Koc value is not provided, a paper by Zeroual et al. 2006 does indicate that bromophenol blue does not adsorb to organic matter (fungal biomass) at pH 6, i.e., when under an ionized form.
| Water solubility (mg/L at 25°C) | pKa | Kow | |
|---|---|---|---|
| Acridine orange (CAS RN 494-38-2) | 873 (this study) vs 700 (PhysProp 2006) | - | - |
| Dinitrobenzoic acid (CAS RN 99-34-3) | 986 (this study) vs 1350 (PhysProp 2006) | 3.4 (this study) vs 2.82 (PhysProp 2006) | 11.22 (this study) vs 35.48 (PhysProp 2006) |
| Bromophenol blue (CAS RN 115-39-9) | - | 4.0 (this study) vs 4.1 (Kulichenko et al. 2001) vs 4.0 (O'Neil 2001) | - |