Screening Assessment for the Challenge
This page has been archived on the Web
Information identified as archived is provided for reference, research or recordkeeping purposes. It is not subject to the Government of Canada Web Standards and has not been altered or updated since it was archived. Please contact us to request a format other than those available.
Archived
Butanamide, 2,2'-[(3,3'-dimethoxy[1,1'-biphenyl]-4,4'-diyl)bis(azo)]bis[N-(2-methylphenyl)-3-oxo-
Chemical Abstracts Service Registry Number
7147-42-4
Environment Canada
Health Canada
September 2011
Table of Contents
- Synopsis
- Introduction
- Substance Identity
- Physical and Chemical Properties
- Sources
- Uses
- Releases to the Environment
- Environmental Fate
- Persistence and Bioaccumulation Potential
- Potential for Bioaccumulation
- Potential to Cause Ecological Harm
- Potential to Cause Harm to Human Health
- Conclusion
- References
- Appendix 1: Summary of QSAR results for BPAOPB and potential azo cleavage products
Pursuant to section 74 of the Canadian Environmental Protection Act, 1999 (CEPA 1999), the Ministers of the Environment and of Health have conducted a screening assessment on butanamide, 2,2'-[(3,3'-dimethoxy[1,1'-biphenyl]-4,4'-diyl)bis(azo)]bis[N-(2-methylphenyl)-3-oxo- (herein referred to as BPAOPB), Chemical Abstracts Service Registry Number[1] 7147-42-4.
This substance was identified as a high priority for screening assessment and included in the Challenge initiative under the Chemicals Management Plan because it had been found to meet the ecological categorization criteria for persistence, bioaccumulation potential and inherent toxicity to non-human organisms and is believed to be in commerce in Canada. The substance BPAOPB was not considered to be a high priority for assessment of potential risks to human health, based upon application of the simple exposure and hazard tools developed for categorization of substances on the Domestic Substances List.
BPAOPB is a disazo diarylide organic pigment that exists as a powder. Such pigments are used primarily as colour pigments in printing inks and plastics, and to a lesser extent in coatings. This substance does not naturally occur in the environment. As a result of industry surveys conducted pursuant to section 71 of CEPA 1999, total manufacturing of BPAOPB was reported between 100 and 1000 kg in Canada in 2006, while no imports or uses were reported for that year.
Based on reported use patterns and certain assumptions related to pigments in general, releases of BPAOPB to the Canadian environment resulting from its manufacture are estimated to be less than 1% to air and 4% to wastewater. It is estimated that 2% is transferred to waste disposal sites. There are no experimental data available on the physical and chemical properties of this substance. Given the data available for other disazo diarylide pigments identified as suitable analogues, BPAOPB is believed to be present in the environment as a chemically stable, non-volatile, solid particle that has very low water solubility. As a result, it would be found in sediments if released to surface waters, and would tend to remain in soils if released to terrestrial environments.
Biodegradation studies for disazo diarylide pigment analogues along with modelled data for BPAOPB suggest that little degradation of BPAOPB would occur in aerobic environments (i.e., water, sediment, soil). Given the physical and chemical properties of BPAOPB and other disazo diarylide pigments (i.e., solid particle nature, high molecular weight, large cross-sectional diameter, low water solubility, low octanol solubility), and bioconcentration studies on one analogue pigment, it is expected that BPAOPB has a low potential to accumulate in the lipid tissues of organisms. Therefore, BPAOPB is persistent but not bioaccumulative in accordance with criteria set out in the Persistence and Bioaccumulation Regulations. In addition, experimental toxicity data for other pigments identified as analogues suggest that the substance does not cause acute harm to aquatic organisms.
For this screening assessment, a conservative environmental release scenario was selected in which an industrial operation discharges BPAOPB into the aquatic environment through a single wastewater treatment plant. The upper end of the reporting range of 1000 kg per year was used to conservatively estimate releases and concentrations in the aquatic environment. The predicted environmental concentration in water for this substance was below the predicted no-effect concentration for sensitive aquatic organisms, resulting in a risk quotient of much lower than 1.
Based on the ecological information available, it is concluded that BPAOPB is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
For the human health assessment, exposure of the general population to BPAOPB from environmental media is considered to be negligible. Exposures from use of consumer products were not identified. No empirical health effects data were available for BPAOPB. Based on metabolism information and information on health effects of analogues, the hazard potential of BPAOPB is expected to be low. Based on low hazard potential of BPAOPB and expected negligible exposure to the general population, the potential risks to human health for this substance are considered to be low. It is concluded that BPAOPB is not a substance entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
Based on the information available, it is concluded that BPAOPB does not meet any of the criteria set out in section 64 of CEPA 1999.
This substance will be considered for inclusion in theDomestic Substances List inventory update initiative. In addition and where relevant, research and monitoring will support verification of assumptions used during the screening assessment.
The Canadian Environmental Protection Act, 1999 (CEPA 1999) (Canada 1999) requires the Minister of the Environment and the Minister of Health to conduct screening assessments of substances that have met the categorization criteria set out in the Act to determine whether these substances present or may present a risk to the environment or to human health.
Based on the information obtained through the categorization process, the Ministers identified a number of substances as high priorities for action. These include substances that
- met all of the ecological categorization criteria, including persistence (P), bioaccumulation potential (B) and inherent toxicity to aquatic organisms (iT), and were believed to be in commerce in Canada; and/or
- met the categorization criteria for greatest potential for exposure (GPE) or presented an intermediate potential for exposure (IPE), and had been identified as posing a high hazard to human health based on classifications by other national or international agencies for carcinogenicity, genotoxicity, developmental toxicity or reproductive toxicity.
The Ministers therefore published a notice of intent in theCanada Gazette, Part I, on December 9, 2006 (Canada 2006a), that challenged industry and other interested stakeholders to submit, within specified timelines, specific information that may be used to inform risk assessment, and to develop and benchmark best practices for the risk management and product stewardship of those substances identified as high priorities.
The substance butanamide, 2,2'-[(3,3'-dimethoxy[1,1'-biphenyl]-4,4'-diyl)bis(azo)]bis[N-(2-methylphenyl)-3-oxo- had been identified as high priority for assessment of ecological risk as it had been found to be persistent, bioaccumulative and inherently toxic to aquatic organisms and is believed to be in commerce in Canada. The Challenge for this substance was published in the Canada Gazette on May 31, 2008 (Canada 2008a, 2008b). A substance profile was released at the same time. The substance profile presented the technical information available prior to December 2005 that formed the basis for categorization of this substance. As a result of the Challenge, submissions of information pertaining to the manufacture, uses and properties of this substance were received.
Although butanamide, 2,2'-[(3,3'-dimethoxy[1,1'-biphenyl]-4,4'-diyl)bis(azo)]bis[N-(2-methylphenyl)-3-oxo- was determined to be a high priority for assessment with respect to the environment, it did not meet the criteria for GPE or IPE and high hazard to human health based on classifications by other national or international agencies for carcinogenicity, genotoxicity, developmental toxicity or reproductive toxicity.
Screening assessments focus on information critical to determining whether a substance meets the criteria as set out in section 64 of CEPA 1999. Screening assessments examine scientific information and develop conclusions by incorporating a weight-of-evidence approach and precaution.[2]
This final screening assessment includes consideration of information on chemical properties, hazards, uses and exposure, including the additional information submitted under the Challenge. Data relevant to the screening assessment of this substance were identified in original literature, review and assessment documents, stakeholder research reports and from recent literature searches, up to July 2010. Available studies were critically evaluated; modelling results have also been used to reach conclusions. When available and relevant, information presented in hazard assessments from other jurisdictions was considered. This final screening assessment does not represent an exhaustive or critical review of all available data. Rather, it presents the most critical studies and lines of evidence pertinent to the conclusion.
This final screening assessment was prepared by staff in the Existing Substances Programs at Health Canada and Environment Canada and incorporates input from other programs within these departments. The ecological portion of this assessment has undergone external written peer review/consultation. Comments on the technical portions relevant to human health were received from scientific experts selected and directed by Toxicology Excellence for Risk Assessment (TERA) and included comments by Dr. Larry Claxton, Dr. Bernard Gadagbui, Dr. Pertti Hakkinen, Dr. Glenn Talaska, and Dr. Pam Williams. Additionally, the draft of this screening assessment was subject to a 60-day public comment period. While external comments were considered, the final content and outcome of the screening assessment remain the responsibility of Health Canada and Environment Canada. Approaches used in the screening assessments under the Challenge have been reviewed by an independent Challenge Advisory Panel.
The critical information and considerations upon which this final assessment is based are summarized below.
This organic pigment has been ascribed neither a Colour Index name (C.I.; CII 2002) nor a commercial name. For the purposes of this document, the substance butanamide, 2,2'-[(3,3'-dimethoxy[1,1'-biphenyl]-4,4'-diyl)bis(azo)]bis[N-(2-methylphenyl)-3-oxo- will be referred to as BPAOPB, derived from the Domestic Substances List (DSL) name. Information on this substance’s identity is shown in Table 1 below.
Table 1. Substance identity for BPAOPB
| Chemical Abstracts Service Registry Number (CAS RN) | 7147-42-4 |
| DSL name | Butanamide, 2,2'-[(3,3'-dimethoxy[1,1'-biphenyl]-4,4'-diyl)bis(azo)]bis[N-(2-methylphenyl)-3-oxo- |
| National Chemical Inventories (NCI) names[1] | Butanamide, 2,2'-[(3,3'-dimethoxy[1,1'-biphenyl]-4,4'-diyl)bis(azo)]bis[N-(2-methylphenyl)-3-oxo-(AICS, ASIA-PAC, DSL, TSCA) 2,2'-[(3,3'-Dimethoxy[1,1'-biphenyl]-4,4'-diyl)bis(azo)]bis[N-(2-methylphenyl)-3-oxobutyramide](DSL, ECL, EINECS) |
| Other names | NSC 16674 |
Chemical group (DSL Stream) |
Discrete organics |
| Major chemical class or use | Disazo diarylide organic pigment |
| Major chemical sub-class | Secondary aromatic amines, azo compounds, acetoacetanilide dimethoxybiphenyl |
| Chemical formula | C36H36N6O6 |
| Chemical structure | 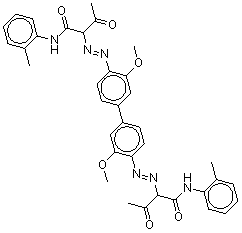 |
| SMILES[2] | O=C(Nc(c(ccc1)C)c1)C(N=Nc(c(OC)cc(c(ccc(N=NC(C(=O)C)C(=O)Nc(c(ccc2)C)c2)c3OC)c3)c4)c4)C(=O)C |
| Molecular mass | 648.72 g/mol |
[2] Simplified Molecular Input Line Entry System
The pigment industry synthesizes organic pigments that it considers to have low to very low solubilities (i.e., < 1 mg/L and < 0.01 mg/L, respectively) in nearly all solvents (Herbst and Hunger 2004; Lincke 2003). This arises from its desire to produce colourants that will retain their colour for a long time and in various types of substrates. Low solubility is enhanced by designing colourants that have strong interactive forces between molecules. This is achieved by the introduction of substituents like –CONH– in the molecule (Herbst and Hunger 2004; Lincke 2003). The resulting intermolecular bonding, in turn, generates a crystal structure that lends stability to organic pigments (Lincke 2003).
The majority of organic pigments generally do not exist as individual molecules but are principally particles in the submicron range. The pigment powder is typically composed of primary particles (i.e., the crystal lattice of a pigment), aggregates and agglomerates. Manufacturers usually provide the physical specifications of their pigments, which include the average particle size of the pigment powder. In doing so, users can determine which pigment is the most appropriate to colour their product(s), since performance is chiefly controlled by the particle size distribution (Herbst and Hunger 2004).
No experimental physical and chemical data are available for BPAOPB. Its structure indicates that it is a disazo diarylide organic pigment (two identical arylide molecules are joined at the same carbon ring) (Herbst and Hunger 2004).Variations in the hue arise from differences in the atoms arranged around the outer (end) carbon rings.
At the Environment Canada-sponsored Quantitative Structure-Activity Relationship (QSAR) Workshop in 1999, invited modelling experts identified many structural classes of pigments and dyes as “difficult to model” using most QSARs (Environment Canada 2000). The physical and chemical properties of many of the structural classes of pigments and dyes are often not amenable to model prediction because they are typically considered “out of the model domain of applicability” (e.g., structural and/or property parameter domains). Therefore, to determine potential utility, the domains of applicability of QSAR models to pigments and dyes are evaluated on a case-by-case basis.
For this assessment it is considered that QSAR models used to predict physical and chemical properties that lack comparable substances to BPAOPB in their domain of applicability, may produce results with a high degree of uncertainty. Consequently, a read-across approach has been used to determine the approximate physical and chemical properties of this substance based on similar disazo diarylide pigments with available experimental data. Due to the paucity of information for BPAOPB, acceptable analogous substances for the purposes of this assessment were chosen. For the ecological assessment, these substances include Pigment Yellow 12 (CAS RN 6358-85-6), Pigment Yellow 13 (CAS RN 5102-83-0), Pigment Yellow 14 CAS RN 5468-75-7) and Pigment Yellow 83 (CAS RN 5567-15-7), all of which are relatively large molecules having two azo bonds and two identical arylide molecules joined at the same carbon ring. They are therefore expected to behave similarly to BPAOPB in the environment and to demonstrate similar toxicities in the aquatic environment as a function of bioavailability and chemical reactivity. Additional analogues have been chosen for the human health assessment, where relevant data for human health endpoints exist (see Potential to Cause Harm to Human Health section for further rationale and discussion).
Pigments have high molecular weights (i.e., generally >300 g/mol), are solid particles, decompose at temperatures greater than 220oC (diarylide pigments are susceptible to thermal breakdown at temperatures above 200oC), and have extremely low solubility in water (Danish EPA 1999). In addition, these substances generally have limited solubility inn-octanol, have a negligible vapour pressure and are stable under environmental conditions, as would be expected from their intended use as pigments.
Table 2 shows available information on the physical and chemical properties of BPAOPB and relevant analogues identified for use in the ecological portion of this assessment. These properties are relevant to the environmental fate of BPAOPB and were subsequently considered in evaluating various lines of evidence in the ecological assessment. The calculated values for vapour pressure and Henry’s Law constant were included for some of the substances based on experimental data inputs (i.e., water solubility and decomposition or melting point) to EPIsuite (2008). These calculations provide a general indication (rather than an accurate estimate) that these substances have negligible vapour pressure and negligible Henry’s Law constant.
Table 2. Physical and chemical properties for BPAOPB and its relevant analogues
| Chemical | Type | Value | Temperature (°C) | Reference |
|---|---|---|---|---|
| Physical state | ||||
| BPAOPB | Powder | 25 | Study Submission 2008 | |
| Pigment Yellow 13 | Powder | 20 | European Commission 2009a | |
| Pigment Yellow 14 | Solid | 20 | European Commission 2000b | |
| Pigment Yellow 83 | Solid | 20 | European Commission 2000c | |
| Decomposition[1](ºC) | ||||
| Pigment Yellow 12 | Experimental | 317 and >350 | European Commission 2000d; US EPA 2006 | |
| Pigment Yellow 13 | Experimental | >320 and 350 | European Commission 2000a; US EPA 2006 | |
| Pigment Yellow 14 | Experimental | 360 | NPIRI 2000 | |
| Pigment Yellow 83 | Experimental | >320 and 400 | European Commission 2000c; Anliker and Moser 1987 | |
| Bulk Density (kg/m3) | ||||
| BPAOPB | Calculated | 1240 | ACD/pKaDB 1994–2008 | |
| Pigment Yellow 12 | Experimental | 1370 | US EPA 2006 | |
| Pigment Yellow 13 | Experimental | 1360 and 1450 | 20 | Clariant 2005; European Commission 2000a |
| Pigment Yellow 14 | Experimental | 1450 | 20 | US EPA 2006 |
| Pigment Yellow 83 | Experimental | 1370 | 20 | US EPA 2006 |
| Vapour pressure (Pa) | ||||
| BPAOPB | Calculated | 1.96×10-25 | 25 | ACD/pKaDB 1994–2008 |
| Pigment Yellow 12 | Calculated[2] | 3.2×10-19 | 25 | MPBPVP 2008 |
| Pigment Yellow 13 | Calculated[2] | 6.13×10-21 | 25 | MPBPVP 2008 |
| Pigment Yellow 83 | Calculated[2] | 5.84×10-23 | 25 | MPBPVP 2008 |
| Henry’s Law constant (Pa·m3/mol) | ||||
| Pigment Yellow 12 | Calculated[2] | 5.04×10-13 | 25 | HENRYWIN 2008 |
| Pigment Yellow 13 | Calculated[2] | 5.26×10-15 | 25 | HENRYWIN 2008 |
| Pigment Yellow 83 | Calculated[2] | 5.37×10-18 | 25 | HENRYWIN 2008 |
| Log Kow(Distribution coefficient; dimensionless) | ||||
| Pigment Yellow 12 | Calculated | 2.09 | CPMA 2009 | |
| Calculated | 6.8 | Anliker and Moser 1987 | ||
| Pigment Yellow 13 | Calculated | 1.44 | CPMA 2009 | |
| Water solubility (mg/L) | ||||
| Pigment Yellow 12 | Experimental | 5×10-5 – 5×10-4 | 25 | Anliker and Moser 1987 |
| Experimental[3] | 0.0004 | 25 | CPMA 2009 | |
| Pigment Yellow 13 | Experimental[3] | 0.0008 | 25 | CPMA 2009 |
| Pigment Yellow 14 | Experimental | Not soluble | 20 | European Commission 2000b |
| Pigment Yellow 83 | Experimental | 3×10-6 – 2×10-5 | 25 | Anliker and Moser 1987 |
| Experimental[4] | ≤0.02 | 25 | Clariant 2003 | |
| Experimental | 0.0089 | 25 | US EPA 2006 | |
| Azo pigments | Experimental | <0.004 | Danish EPA 1999 | |
| Octanol solubility (mg/L) | ||||
| Pigment Yellow 12 | Experimental[3] | 0.049 | CPMA 2009 | |
| Pigment Yellow 13 | Experimental[3] | 0.022 | 25 | CPMA 2009 |
| Pigment Yellow 14 | Experimental | 0.085 | European Commission 2000b; Clariant 2003 | |
| Pigment Yellow 83 | Experimental | 0.02 | Clariant 2003 | |
[2] Calculated using EPIsuite (2008) and the Modified Grain Method with user inputs defined as the experimental decomposition point (or melting point) and water solubility for each substance, including 317oC and 0.0004 mg/L for Pigment Yellow 12, 350oC and 0.0008 mg/L for Pigment Yellow 13, and 320oC and 0.0089 mg/L for Pigment Yellow 83.
[3] Recent advances have allowed for the analysis of pigments to much lower detection limits than 20 µg/L.
[4] Older detection limit: 20 µg/L.
The structure of BPAOPB is presented in Table 3 along with structural information on the Pigment Yellow substances considered to be acceptable analogues for the ecological assessment – they differ amongst themselves only by the substitution of their central aniline rings (e.g., methyl, choloro, methoxy). Available empirical data for these analogues will be used as part of the weight of evidence in the ecological assessment of BPAOPB, as these substances are expected to have similar physical and chemical properties, similar behaviour in the environment, and similar toxicity as a function of bioavailability and chemical reactivity.
Table 3. Structural analogues of BPAOPB considered for ecological assessment[1]
| Common Name (CAS RN) |
Structure of analogue | Molecular mass (g/mol) |
|---|---|---|
| BPAOPB (7147-42-4) |
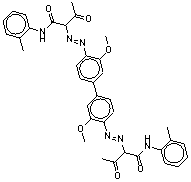 |
648.72 |
| Disazo diarylide pigments[2] | ||
| C.I. Pigment Yellow 12 (C.I. 21090) |
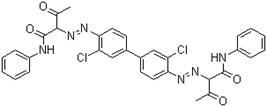 |
629.50 |
| C.I. Pigment Yellow 13 (C.I. 21100) |
![Pigment Yellow 13, 2,2'-[(3,3'-Dichloro[1,1'-biphenyl]-4,4'-diyl)bis(azo)]bis[N-(2,4-dimethylphenyl)-3-oxo-butanamide, C.I. 21100, CAS #: 5102-83-0](/content/dam/eccc/migration/ese-ees/23B13562-06E6-4CAF-8A78-56C62A0425FF/X-2011090914425264010.jpg) |
685.61 |
| C.I. Pigment Yellow 14 (5468-75-7) (C.I. 21095) |
![Pigment Yellow 14, 2,2'-[(3,3'-Dichloro[1,1'-biphenyl]-4,4'-diyl)bis(azo)]bis[N-(2-methylphenyl)-3-oxobutyramide], C.I. 21095, Permanent Yellow G, CAS #: 5468-75-7](/content/dam/eccc/migration/ese-ees/23B13562-06E6-4CAF-8A78-56C62A0425FF/X-2011090914425232814.jpg) |
657.56 |
| C.I. Pigment Yellow 83 (5567-15-7) (C.I. 21108) |
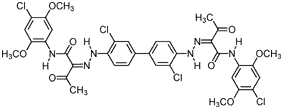 |
818.50 |
[2] Source: chemBlink 2009
BPAOPB is a very large molecule, as indicated by its maximum crosssectional diameter--calculated to range from a minimum of 2.2 and a maximum of 2.8 nm (CPOPs 2008). Molecular mass for acceptable analogues (Table 3) ranged from 629.50 to 818.50 g/mol. Minimum-maximum diameters ranged from 1.9 to 3.3 nm for these disazo diarylide pigments (CPOPs 2008). They also contain Cl atoms in their molecules, whereas BPAOPB does not. The percent structure similarity of the analogues with BPAOPB was calculated to be 86 to 95% (AIEPS 2003–2007).
BPAOPB is not naturally occurring in the environment.
Recent information was collected through industry surveys conducted for the years 2005 and 2006 under Canada Gazettenotices issued pursuant to section 71 of CEPA 1999 (Canada 2006b; Canada 2008b). In 2006, companies reported manufacturing between 100 and 1000 kg in total of BPAOPB. No companies reported importing BPAOPB above the 100 kg/year threshold or using BPAOPB above the 1000 kg/year threshold. In 2005, no companies reported importing or manufacturing BPAOPB above the 100 kg/year threshold. Five other companies reported a stakeholder interest in BPAOPB for the two years combined (Environment Canada 2006, 2008a).
The quantity of BPAOPB reported to be manufactured, imported or in commerce in Canada during the 1986 calendar year (during the development of the DSL) was 1000 to 10 000 kg (Environment Canada 1988). BPAOPB is listed in the European Inventory of Existing Commercial Chemical Substances (EINECS) but has not been reported as either a high production volume (HPV) or a low production volume (LPV) chemical (ESIS c1995–2010). The national aggregate production volume for BPAOPB in the United States was 4535–226 797 kg (i.e., 10 000–500 000 pounds) in the 1986 reporting cycle. However, no reports were received for subsequent reporting cycles (US EPA 2005).
Products containing BPAOPB may enter Canada even if they are not identified as such in the section 71 survey because they may be imported unknowingly in manufactured items, or in quantities below the 100-kg reporting threshold for the survey.
Information on uses for the 2006 calendar year was gathered for BPAOPB (Environment Canada 2008a). The uses reported for this substance include colourant, pigment, stain, dye and ink.
The group of disazo diarylide pigments consists of about 30 azo pigments (including BPAOPB) that were developed around 1940. They provide mostly yellow, orange and red hues. Their main applications are in printing inks and plastics, and to a lesser extent in coatings, and are generally not used in artists’ colours since diarylide pigments have significantly reduced lightfastness (Herbst and Hunger 2004; MacEvoy 2008).
BPAOPB is not listed as a permitted food additive under theFood and Drug Regulations nor has it been identified for use in food packaging applications (Canada 1978 and 2009 email from Health Products and Food Directorate Branch, Health Canada to Existing Substances Risk Assessment Bureau, Health Canada; unreferenced). BPAOPB is not listed in the Food and Drugs Regulations under section C.01.040.2 as a colouring agent permitted in drugs (Canada 1978). BPAOPB is not listed in the Drug Products Database, the Therapeutic Products Directorate's internal Non-Medicinal Ingredients Database, the Natural Health Products Ingredients Database or the Licensed Natural Health Products Database as a medicinal or non-medicinal ingredient present in pharmaceutical drugs, natural health products or veterinary drugs (DPD 2010; NHPID 2010; LNHPD 2010).
The European Commission REACH (Registration, Evaluation, Authorisation and Restriction of Chemical substances) regulation (European Commission 2006), specifically Point 43 of Annex XVII, restricts certain “Azocolourants and Azodyes” in certain textiles that may release one of 22 aromatic amines by cleavage of the azo bond. While BPAOPB could theoretically be reductively cleaved to one of the 22 European Commission restricted aromatic amines (i.e., 3,3'-dimethoxybenzidine CAS RN 119-90-4), based on the available information, the current global use pattern of this substance does not at this time indicate its presence in textiles and leather products.
Releases of BPAOPB are not reported as part of Environment Canada’s National Pollutant Release Inventory (Environment Canada 2010). The total manufacture of BPAOPB in Canada in 2006 was reported in the range of 100–1000 kg (Environment Canada 2008a). Therefore, releases of this substance to the Canadian environment are expected to be low.
Mass Flow
A method has been developed by Environment Canada to estimate a substance’s losses during different stages of its life cycle, including its fate within a finished product or article (Environment Canada 2008b). This method consists of a life cycle analysis and a spreadsheet tool (Mass Flow Tool or MFT) that integrates information on the manufacturing, importation and use data available for the substance. Starting with an identified mass of the substance, each life cycle stage is subsequently evaluated until all of the mass is accounted for. Relevant factors are considered, uncertainties recognized and assumptions may be made during each stage, depending on information available. The estimated losses represent the complete mass balance of the substance over the life cycle of the substance and include releases to wastewater and other receiving compartments (land, air), chemical transformation, transfer to recycling activities and transfer to waste disposal sites (landfill, incineration). However, unless specific information on the rate or potential for release of the substance from landfills and incinerators is available, the method does not quantitatively account for releases to the environment from disposal. Ultimately, the estimated losses provide a first tier in the exposure analysis of a substance and help to estimate environmental releases and focus exposure characterization later in the assessment.
In general, releases of a substance to the environment depend upon various losses from its manufacture, industrial use and consumer/commercial use. These losses can be grouped into seven types: (1) discharge to wastewater; (2) emission to air; (3) loss to land; (4) chemical transformation; (5) disposal to landfill; (6) loss to incineration; and (7) disposal through recycling (i.e., recycling is deemed a loss and not considered further). They are estimated using regulatory survey data, industry data and data published by different organizations. The discharge to wastewater refers to raw wastewater prior to any treatment by public or private wastewater systems. In a similar manner, the loss via chemical transformation refers to changes in a substance’s identity that may occur within the manufacture, industrial use, and consumer/commercial use stages, but excludes those during waste management operations such as incineration and wastewater treatment. The loss to land includes unintentional transfer or leakage to soil or paved/unpaved surfaces during the substance’s use and service life (e.g., from the use of agricultural machinery or automobiles). The loss to land, however, does not include transfers subsequent to a substance’s use and service life (e.g., land application of biosolids and atmospheric deposition).
The losses estimated for BPAOPB over its life cycle (based on conservative assumptions) are presented in Table 4 (Environment Canada 2009a), and are based on manufacturing quantities in Canada as reported by industry to the section 71 notice.
Table 4. Estimated losses of BPAOPB during its life cycle using the MFT
| Type of loss | Proportion[1] (%) | Pertinent life cycle stages[2] |
|---|---|---|
| Wastewater | 4.0 | Production, formulation |
| Land | 0 | |
| Air emission | 0.1 | Production, formulation |
| Chemical transformation | 0 | |
| Landfill and/or incineration | 2.0 | Waste disposal |
| Total | <100[3] |
[2] Potential applicable stage(s): production, formulation, industrial use, consumer use, service life of article/product, waste disposal.
[3] The product was not confirmed to be used in Canada in 2006 (Environment Canada 2008a). Therefore, life cycle stages of consumer use, service life of article/product, and waste disposal were not considered. Consequently, the total proportion of the mass is less than 100%.
Results summarized in Table 4 suggest that releases of BPAOPB related to its manufacture are estimated to be less than 1% to air, 4% to wastewater and 2% to waste disposal (Environment Canada 2009a), with no losses to land or losses due to chemical transformation expected during this life cycle stage. The Mass Flow Tool does not quantify releases from a landfill and does not consider the effectiveness of wastewater treatment. There were companies that reported manufacturing this substance; information submitted indicated that a quantity of BPAOPB would be transferred to an off-site waste management facility (Environment Canada 2008a).
This substance is expected to be used in some specialty consumer products. No information is available on the type or quantity of products containing BPAOPB that may be imported into Canada (below the reporting threshold). The quantities sent to landfills could be higher if importation of these items was taken into consideration, but available information is currently not sufficient to derive a quantitative estimate for such releases.
BPAOPB is a powder with very limited water solubility, as indicated by the water solubility of other disazo diarylide pigments (see Table 2). When released into water, this substance is expected to be mostly present as particles or adsorbed to other suspended solids and would be expected to eventually sink to bed sediments. Releases of BPAOPB to air are possible but expected to be very low and this substance would not be expected to remain in this compartment. The very low calculated vapour pressure is consistent with the fact that it is a large and complex molecule (Baughman and Perenich 1988; Danish EPA 1999). This pigment is not expected to volatilize at environmentally realistic temperatures.
The particulate character of BPAOPB should have a key influence on its fate in the environment. Its density, together with its chemical stability and low aqueous solubility, suggest that it will partition by gravity to sediments if released to surface waters, and will tend to remain in soils if released to terrestrial environments.
Because of its very low solubility in water and large molecular size, this pigment may be considered not available for uptake by organisms and not available for biodegradation. In addition, when incorporated into the materials it is destined to colour, this pigment is probably no longer in a form easily available to biota (Danish EPA 1999; Herbst and Hunger 2004).
Environmental Persistence
There are no experimental degradation data available for BPAOPB. Limited information on biodegradation studies for Pigment Yellow 12, Pigment Yellow 13 and Pigment Yellow 83 suggest that these substances are not readily biodegradable (Table 5a).
Table 5a. Empirical data for biodegradation of analogues of BPAOPB
| Substance | Method | Degradation value[1] | Degradation endpoint | Test duration | Reference |
|---|---|---|---|---|---|
| Pigment Yellow 12 (6358-75-7) |
OECD TG 301C | 0% | Biodegradation | 14 days | CHRIP c2011 |
| Pigment Yellow 13 (5102-83-0) |
OECD TG 301C | 0% | Biodegradation | 28 days | Madsen 1995 |
| Pigment Yellow 83 (5567-15-7) |
OECD TG 301C | 6% | Biodegradation | 28 days | CHRIP c2011 |
It is expected that the characteristics imparted to pigments would result in these substances being persistent in the environment. The Color Pigments Manufacturers Association, Inc. (CPMA 2003) has indicated that pigments are designed to be durable or persistent in the environment in order to provide colour to finished coatings, inks and paints.
Organic pigments are typically used in combination with a resin, and the pigment/resin mixture may (e.g., in the case of paints and printing inks) or may not (e.g., in plastics such as polyethylene) also contain solvents (Study Submission 2008). Jaffe (1996) has stated that once a pigment is incorporated into a matrix (e.g., plastic), it is expected to be durable and withstand the combined chemical and physical stresses of weather, solar radiation, heat, water and industrial pollutants.
Regarding abiotic degradation, the Pigment Yellow analogues chosen in this assessment have a calculated half-life for photo-oxidation of 1.7–4.5 hours (indirect reaction with OH-radical). This characteristic is consistent with the point that they are generally not used in artists’ colours since diarylide pigments have significantly reduced lightfastness (Herbst and Hunger 2004; MacEvoy 2008). They are expected to be hydrolytically stable, as indicated in a study on Pigment Yellow 83 that did not detect hydrolysis in a 56-day experiment (European Commission 2000c).
Therefore, BPAOPB may not be available for aerobic biodegradation if released to water, due to its very low solubility in water and its encapsulation in a resin. Direct contact with biota probably does not occur when the pigment is incorporated in paints or printing inks, and therefore it is not expected that the pigment would be susceptible to biotic degradation.
The environmental persistence of disazo diarylide pigments such as BPAOPB in anoxic environments is an important area of uncertainty. Azo dyes are reported to be degraded in anoxic waters via anaerobic reduction of the azo bond (-N=N-), which results in potentially harmful aromatic amines (Danish EPA 1999). However, no documentation has been found regarding the potential for anaerobic degradation of azo pigments in aqueous environments. In principle, the pigment crystal would have to dissolve first, which would release its constituent molecules to the aqueous medium and make the azo bonds available for biotic reduction (Danish EPA 1999). However, it is expected that only a very small proportion of the pigment may be reduced in this manner given its very low solubility that would limit the availability of the molecules for biotic reduction.
Persistence in water was also examined using predictive QSAR models for biodegradation. These models are considered acceptable for use in this situation as they are based on chemical structure, and the disazo structure is represented in the training sets of all the BIOWIN models used, thereby increasing the reliability of the predictions (Environment Canada 2007). Water was the primary focus, given the ecological importance of the water compartment, the fact that most of the available models apply to water, and the fact that BPAOPB is expected to be released to this compartment. Table 5b summarizes the results of available QSAR models for aerobic degradation in water.
Table 5b. Modelled data for biodegradation of BPAOPB
| Fate process | Model and model basis | Model result and prediction | Expected half-life (days) |
|---|---|---|---|
| Water | |||
| Primary biodegradation | |||
| Biodegradation (aerobic) | BIOWIN 2008[1] Sub-model 4: Expert Survey (qualitative results) |
3.19[2] (biodegrades fast) |
<182 |
| Ultimate degradation | |||
| Biodegradation (aerobic) | BIOWIN 2008[1] Sub-model 3: Expert Survey (qualitative results) |
0.75[2] (biodegrades very slowly) |
≥182 |
| Biodegradation (aerobic) | BIOWIN 2008[1] Sub-model 5: MITI linear probability |
-0.33[3] (biodegrades very slowly) |
≥182 |
| Biodegradation (aerobic) | BIOWIN 2008[1] Sub-model 6: MITI non-linear probability |
0.0[3] (biodegrades very slowly) |
≥182 |
| Biodegradation (aerobic) | TOPKAT 2004 Probability |
n/a[4] | |
| Biodegradation (aerobic) | CATABOL c2004–2008 % BOD[5] |
n/a[4] | |
[2] Output is a numerical score from 0 to 5
[3] Output is a probability score
[4] n/a: not available (out of model domain)
[5] BOD: Biological oxygen demand
Regarding the aerobic biodegradation of BPAOPB, the ultimate degradation model results (i.e., BIOWIN sub-models 3, 5, 6) suggest that this substance degrades to mineral components slowly in water, with expected half-lives of =182 days. The result of the primary biodegradation model (BIOWIN 4) suggests that this substance is subject to a rapid rate of primary biodegradation and the half-life is expected to be less than 182 days. Results from this model suggest that some primary biodegradation may occur within 182 days, but that significant transformation of the molecule is not expected. In considering its structure, this substance contains similar structural features to those associated with chemicals that are not easily biodegraded. Therefore, considering the model results (particularly for ultimate degradation), the biodegradation data for Pigment Yellows 12, 13, and 83, and the structural features of BPAOPB, the weight of evidence suggests that the aerobic biodegradation half-life of BPAOPB is =182 days.
Using an extrapolation ratio of 1:1:4 for a water:soil:sediment biodegradation half-life (Boethling et al. 1995), and using the model-estimated ultimate biodegradation half-life in water of =182 days, the ultimate biodegradation half-life in aerobic soils is also expected to be =182 days and the half-life in aerobic sediments is expected to be =365 days. In anaerobic sediment conditions, there is the possibility that solubilitylimited azo reduction may occur. However, given the characteristics of this insoluble pigment, it is expected that only a very small proportion of the pigment may be available to an organism.
Based on the weight of evidence provided by the available literature and the modelled data described above (see Tables 5a and 5b), BPAOPB is considered to meet the persistence criteria in water, soil and sediment (half-lives in aerobic soil and water =182 days and half-life in aerobic sediment =365 days) as set out in the Persistence and Bioaccumulation Regulations (Canada 2000).
In this assessment, various lines of evidence have been used to determine the bioaccumulation potential of BPAOPB, which is different from the Kow-based QSAR approach used during categorization. The model-derived log Kow value for this substance, which would have influenced the categorization results for bioaccumulation potential (and toxicity), has been further evaluated and is not considered a reliable indicator of bioaccumulation potential. The CPMA (2009) calculated the log Kow values for Pigment Yellow 12 and Pigment Yellow 13 as 2.09 and 1.44, respectively, based on their experimental solubilities in octanol and water. These values indicate that these pigments have a low bioaccumulation potential.
No experimental bioaccumulation factor (BAF) and/or bioconcentration factor (BCF) data for BPAOPB were available. Limited information on experimental studies for the analogue Pigment Yellow 12 suggests that this substance has a low bioaccumulation potential. For Pigment Yellow 12, bioconcentration factors were determined over a 6-week period for Cyprinus carpio ranging from 0.38 to 3.2 at concentrations of 0.100 ppm and from 2.4 to 5.4 at concentrations of 0.010 ppm of the pigment (CHRIP c2011).
Table 6. Empirical data for the bioconcentration of Pigment Yellow 12
| Test organism | Experimental concentration (mg/L) | Endpoint (BCF, L/kg) |
Reference |
|---|---|---|---|
| Common carp (Cyprinus carpio) |
0.1 | 0.38–3.2 | CHRIP c2011 |
| 0.01 | 2.4–5.4 |
These results suggest that this analogue has a very limited affinity for the lipid phase of living organisms. By extension, BPAOPB is therefore also expected to present a low bioaccumulation potential.
Due to the very low water solubility of BPAOPB, its large molecular size, and the processes required to incorporate it into a matrix (e.g., plastic), it is expected that exposure to and subsequent potential uptake into biota would be low. Recent investigations relating fish BCF data and molecular size parameters (Dimitrov et al. 2002, 2005) suggest that the probability of a molecule crossing cell membranes as a result of passive diffusion declines significantly with increasing maximum diameter (Dmax). The probability of passive diffusion decreases appreciably when the maximum diameter is greater than ~1.5 nm and much more so for molecules having a maximum diameter of greater than 1.7 nm. Sakuratani et al. (2008) have also investigated the effect of cross-sectional diameter on passive diffusion in a BCF test set of about 1200 new and existing chemicals. They observed that substances that do not have a very high bioconcentration potential (BCF < 5000) often have a Dmax of >2.0 nm and an effective diameter (Deff) >1.1 nm.
However, as Arnot et al. (2010) have noted, there are uncertainties associated with the thresholds proposed by Dimitrov et al. (2002, 2005) and Sakuratani et al. (2008) since the BCF studies used to derive them were not critically evaluated. Arnot et al. (2010) point out that molecular size influences solubility and diffusivity in water and organic phases (membranes), and larger molecules may have slower uptake rates. However, these same kinetic constraints apply to diffusive routes of chemical elimination (i.e., slow in = slow out). Thus, significant bioaccumulation potential may remain for substances that are subject to slow absorption processes, if they are slowly biotransformed or slowly eliminated by other processes. Consequently, when evaluating bioaccumulation potential, molecular size information should be considered with care and used together with other relevant lines of evidence in a weight-of-evidence approach.
BPAOPB is a relatively large molecule with a high molecular weight (649 g/mol) and a range of maximum diameters (Dmax) from 2.2 to 2.8 nm (CPOPs 2008), comparable to some of the values cited above. These characteristics suggest that the uptake rate of this substance may be slower compared to that of smaller more compact substances, thus mitigating the overall bioconcentration potential.
Based on its physical and chemical properties (i.e., solid particle nature, high molecular weight, large cross-sectional diameter, low water solubility) and those of relevant analogue pigments (i.e., high decomposition points, high molecular weights, large cross-sectional diameters, low water solubilities, low solubilities in octanol, experimental BCF data), BPAOPB is expected to have a low bioaccumulation potential. Considering the available evidence, BPAOPB does not meet the bioaccumulation criterion (BCF or BAF =5000) as set out in the Persistence and Bioaccumulation Regulations (Canada 2000).
Ecological Effects Assessment
In the Aquatic Compartment
Various lines of evidence have been used to determine the toxicological potential of BPAOPB, which is different from the primarily Kow-based QSAR approach used during categorization. The available data to support this assessment are discussed below.
There were no experimental toxicity data available for BPAOPB; however, some data were identified for the disazo diarylide pigment analogues (Table 7).
Table 7. Experimental aquatic toxicity data for acceptable analogues of BPAOPB
| Organism | Type of test | Endpoint | Value (mg/L) |
Reference |
|---|---|---|---|---|
| Pigment Yellow 12 | ||||
| Rice fish Oryzias latipes |
Acute 48 hours |
LC50 | >420 | CHRIP c2011 |
| Golden orfe[1] Leuciscus idus |
Acute 48 hours |
LC50 | >500 (35% solution) |
European Commission 2000d |
| Acute 96 hours |
LC50 | 10–100 (81% solution) |
||
| Zebrafish[1] Brachydanio rerio |
Acute 48 hours |
LC50 | 5–10 (63% test substance + Tween80) |
European Commission 2000d |
| Acute 96 hours |
LC50 | 7.1 (63% test substance + Tween80) |
European Commission 2000d | |
| Water flea[2] Daphnia magna |
Acute 72 hours |
EC50(immobilization) | >100 (98% test substance) |
US EPA 2006 |
| Alga[3] Selenastrum capricornutum |
Acute 72 hours |
EC50 | 100 | CPMA 2009 |
| Pigment Yellow 13 | ||||
| Water flea[2] Daphnia magna |
Chronic 21 days |
EC50 (immobilization;reproduction) |
>100 | Hoechst 1999 |
| Pigment Yellow 83 | ||||
| Rainbow trout[4] Oncorhynchus mykiss |
Acute 48 hours |
LC50 | 18[*] | Hamburger et al. 1977 |
| Golden orfe[4] Leuciscus idus |
Acute 48 hours |
LC50 | 45 | Hamburger et al. 1977 |
| Common minnow[4] Phoxinus phoxinus |
Acute 48 hours |
LC50 | 45 | Hamburger et al. 1977 |
| Zebrafish Danio rerio |
Acute 96 hours |
EC50 (growth) |
>100 | US EPA 2006 |
| Alga Selenastrum capricornutum |
Acute 72 hours |
EC50 (growth) |
190 | US EPA 2006 |
[2] Only one concentration was tested, i.e., 100 mg/L (which is above the solubility limit of the substance).
[3] As reported by CPMA; no other study details were provided.
[4] This study used ethylene glycol as solvent and concentrations were unmeasured.
LC50 = The concentration of a substance that is estimated to be lethal to 50% of the test organisms; in this case, the value given represents the amount of pigment loaded into the test system, as the concentration dissolved in the water is not known, but may be assumed to be the water solubility limit.
EC50 = The concentration of a substance that is estimated to cause some effect on 50% of the test organisms; in this case, the value given represents the amount of pigment loaded into the test system, as the concentration dissolved in the water is not known, but may be assumed to be the water solubility limit.
[*] The critical toxicity value used to derive a probable no effects concentration.
Experimental studies are available for Pigment Yellows 12, 13, and 83 for fish, daphnids and alga. Many studies do not report effects at the highest concentrations tested. In effect, the concentrations reported in Table 7 represent the loading levels of the test substance, as they are all above the reported solubility limit for the substance (which is less than 8 µ/L for these substances) and concentrations were not measured. Thus, actual LC50 or EC50 values may be lower than this loading level reported, as the actual concentration dissolved in water that may cause an effect is not known.
The European Commission (2000d) data for Pigment 12 were not considered reliable due to the uncertainty around the purity of the substance and/or formulant(s) used. In the case of the zebra fish studies, a solubilizer (Tween 80) was used, which would promote the bioavailability of the substance. Test situations where solubilizers were used (also reported by Hamburger et al. 1977) may represent a worst-case scenario where the pure substance was tested.
The data for all the analogues indicate that fish are more sensitive than daphnids. The Pigment Yellow 83 studies for rainbow trout, golden orfe, and minnow are the lowest effects concentrations reported (as cited in the US EPA ECOTOX database). The authors note that the test substance was dissolved in ethylene glycol and results indicate that Pigment Yellow 83 would have a moderate potential for toxicity (LC50s of 1 to 100 mg/L). The lowest value is an acute LC50 for rainbow trout. It is similarly expected that BPAOPB would not be acutely toxic to aquatic organisms.
The weight of evidence regarding experimental data for the disazo diarylide analogues of BPAOPB suggest that BPAOPB is not expected to cause acute harm to aquatic organisms at low concentrations (i.e., acute LC50s are greater than 1.0 mg/L).
In Other Environmental Compartments
No ecological effects studies were found for BPAOPB in media other than water. However, this substance could end up in soil or sediment as a result of release to the aquatic environment, landfill disposal of sludge from wastewater treatment plants, disposal of products containing these substances, or biosolids application to soils. Therefore, toxicity data for soil and sediment organisms would be desirable.
This being said, the toxicity potential is likely to be low in sediment- and soil-dwelling species, considering the low bioaccumulation potential and the physical and chemical properties of this substance. However, this cannot be confirmed due to the lack of suitable whole organism toxicity data.
Ecological Exposure Assessment
No information concerning the concentration of BPAOPB in environmental media (air, water, soil, sediment) in Canada or elsewhere has been identified. Environmental concentrations have been estimated below using an industrial release scenario.
Industrial Release
The aquatic exposure of BPAOPB is expected if the substance is released from industrial use to a wastewater treatment plant and the treatment plant discharges its effluent to a receiving water body. The concentration of the substance in the receiving water near the discharge point of the wastewater treatment plant is used as the predicted environmental concentration (PEC) in evaluating the aquatic risk of the substance. It can be calculated with the equation
Cwater-ind = [1000 × Q × L × (1 - R)] / [N × F × D]
where
Cwater-ind is aquatic concentration resulting from industrial releases, mg/L
Q is total substance quantity used annually at an industrial site, kg/year
L is loss to wastewater, fraction
R is wastewater treatment plant removal rate, fraction
N is number of annual release days, d/year
F is wastewater treatment plant effluent flow, m3/day
D is receiving water dilution factor, dimensionless
As BPAOPB is used industrially and is expected to be released to water, a conservative industrial release scenario is used to estimate the aquatic concentration of the substance with the help of Environment Canada’s (2009b) Industrial Generic Exposure Tool – Aquatic (IGETA). The scenario is made conservative by assuming that the total quantity of the substance used by Canadian industry is used by one single industry at a small, hypothetical site and the loss to a local wastewater treatment plant is 5% of the total quantity resulting from cleaning of container residues, transfer lines and reactors. Such a small site is selected to have an effluent flow at the 10th percentile (3456 m3/d) of the wastewater treatment plant discharge rates across Canada. The scenario also assumes that the release occurs 150 days per year (to represent seasonal activities), the wastewater treatment plant removal rate is zero for the substance, and the receiving water dilution factor is ten. Based on the above assumptions, a total industrial use quantity of 1000 kg/year of the substance (the upper limit of the reporting range) yields a PEC in water of 0.009 mg/L (Environment Canada 2009c).
Characterization of Ecological Risk
The approach taken in this ecological screening assessment was to examine various supporting information and develop conclusions based on a weight-of-evidence approach and using precaution as required under CEPA 1999. Lines of evidence considered include results from a conservative risk quotient calculation, as well as information on persistence, bioaccumulation, toxicity, sources and fate of the substance.
BPAOPB is expected to be persistent in the environment based on the available experimental data for the Pigment Yellow analogues and a category approach for pigments. This is supported by published and unpublished literature on the physical and chemical properties of pigments (e.g., particulate state, high density, chemical stability, low aqueous solubility). Pigments are expected to be durable, given their intended use as a colourant in finished products. The modelled data generated for BPAOPB also suggest that little degradation of this substance would occur in the environment. Results from bioconcentration studies for Pigment Yellow 12 indicate that this pigment likely has a low potential to accumulate in the lipid tissues of organisms. Given this experimental data, the information on the physical and chemical properties of BPAOPB (i.e., solid particle nature, high molecular weight, large cross-sectional diameter, low water solubility), and the information on its disazo diarylide pigment analogues (i.e., high decomposition points, high molecular weights, large cross-sectional diameters, low water solubilities, low solubilities in octanol), it is expected that BPAOPB has a low potential to accumulate in organisms.
A risk quotient analysis, integrating conservative estimates of exposure with toxicity information, was performed for the aquatic medium to determine whether there is potential for ecological harm in Canada. The industrial release scenario presented above yielded a PEC of 0.009 mg/L. A predicted no-effect concentration (PNEC) was derived using the lowest available toxicity value--an experimental acute LC50 for rainbow trout of 18 mg/L. An application factor of 100 was used to account for interspecies and intraspecies variability in sensitivity, to estimate a long-term no-effects concentration, and to extrapolate from lab to field studies. The resulting PNEC is calculated to be 0.18 mg/L. When compared to the conservative PEC calculated above for industrial releases to water (0.009 mg/L), the resulting risk quotient (PEC/PNEC) is 0.05, which is much lower than 1.
Overall, results of this assessment conclude that BPAOPB has a low potential to cause ecological harm in Canada.
Uncertainties in Evaluation of Ecological Risk
In the assessment of persistence, there is uncertainty as to the rate and extent to which degradation of BPAOPB occurs in anaerobic environments, and whether those degradation products (e.g., aromatic amines) could ever become biologically available. That being said, the degradation products formed in relatively deep anoxic sediments are not expected to result in significant exposure to organisms.
The experimental toxicity concentrations for aquatic organisms have an additional source of uncertainty in that reported test concentrations exceed the solubility of the chemicals in water as reported elsewhere. Many of the studies also report only the amount of the substance loaded into the test system, and the concentration dissolved in the water is unknown but may be assumed to be the water solubility limit. However, the available data suggest that the toxicity of BPAOPB is likely to be low.
The predicted partitioning behaviour of this substance suggests that soil and sediment are potentially an important media of exposure, but these media are not addressed by the effects data available. Indeed, the only effects data identified are for pelagic aquatic organisms. Therefore, toxicity data for soil- and sediment-dwelling organisms would be desirable. This being said, the toxicity potential is likely to be low in sediment- and soil-dwelling species, considering the lack of bioavailability, the low bioaccumulation potential, and the physical and chemical properties of this substance.
Uncertainties are also associated with the lack of information on environmental concentrations in Canada for BPAOPB. Although this substance has been reported to be manufactured in Canada in 2006, there were no reports of it being used, which suggests limited releases associated with the manufacture of consumer products containing BPAOPB. Although no information is available on the quantity of BPAOPB that is imported in consumer products, releases from consumer products are expected to be low and spread over wide areas, and the resulting exposure concentrations should not be significantly different from those estimated in this document.
Given the use of this substance in other countries, it is possible that the substance is entering the Canadian market as a component of manufactured items and/or consumer products. Information obtained from the section 71 survey and other information sources indicated that it may be present in a limited number of these types of products in Canada. Available information is currently not sufficient to derive a quantitative estimate to help determine the importance of this source in ecological assessment. However, it is anticipated that the proportions of BPAOPB released to the various environmental media would not be significantly different from those estimated here, although quantities transferred to recycling and/or waste disposal may be higher. It is also recognized that releases from waste disposal sites are possible, although difficult to quantify due to the lack of data, and would contribute to overall environmental concentrations.
Exposure Assessment
There were no data identified for BPAOPB in environmental media. According to information reported under section 71 of CEPA 1999, between 100 and 1000 kg of BPAOPB was manufactured in Canada in 2006. BPAOPB was not imported above the 100-kg reporting threshold and no releases of this substance were reported (Environment Canada 2008a). Environmental concentrations estimated using ChemCAN version 6 software (ChemCAN 2003) were based on the loss percentages predicted by the Mass Flow Tool as shown in Table 4 (Environment Canada 2009a). The percentages were applied to the total quantity of BPAOPB in Canadian commerce in 2006. The total quantity in commerce was conservatively assumed to be up to 1000 kg. The loss quantities are estimated to be 40 kg to water from wastewater, 1 kg to air from air emissions, and 0 kg to soil from loss to land. The upper-bounding estimates of BPAOPB intake for each age group in the general population of Canada from environmental media were predicted to be negligible.
In Canada, BPAOPB is used in organic dye and pigment manufacturing. The main application is in printing inks and plastics and to a lesser extent in coatings. BPAOPB is generally not suitable in artists’ colours since diarylide pigments have significantly reduced lightfastness (Herbst and Hunger 2004; MacEvoy 2008). No consumer products containing BPAOPB were identified in Canada, therefore the likelihood of exposure to BPAOPB of the general population in Canada from use of these products is considered to be low.
Health Effects Assessment
BPAOPB belongs to the family of azo substances, of which many may undergo reductive cleavage of the azo bond mediated by azo reductase enzymes present in mammalian tissues as well as bacteria of the intestine and skin (e.g., Golka et al. 2004; Platzek et al. 1999; Chen 2006; Stingley et al. 2010). This azo cleavage could therefore potentially result in the release of component aromatic amines. Theoretically, azo reductive cleavage of BPAOPB would release its component aromatic amines including 3,3'-dimethoxybenzidine (3,3'-DMOB; CAS RN 119-90-4), a known carcinogen (IARC 1974, 1987; NTP 1990; US DHHS 2005), as well as an amine of the original coupling component 2'-methylacetoacetanilide (no CAS RN found, no effects data, see Appendix 1 for QSAR predictions). The structures and types of data available for BPAOPB, its analogues and azo cleavage products are presented in Table 8.
Table 8: Analogues and potential azo cleavage products considered for BPAOPB in the human health effects assessment
| Substance identification (CAS RN) |
Structure | Similarity from ChemID[*] | Available data considered |
|---|---|---|---|
| BPAOPB | |||
| BPAOPB (7147-42-4) |
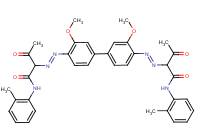 |
100% | QSAR |
| Analogues | |||
| Pigment Orange 16 C.I. 21160 (6505-28-8) |
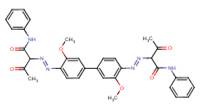 |
94% (3,3'-DMOB-based analogue) |
Ames Assay |
| Pigment Yellow 13 C.I. 21100 (5102-83-0) |
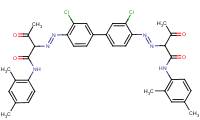 |
90% (3,3'-DCB-based analogue) |
Metabolism Data Ames Assay |
| Pigment Yellow 14 (5468-75-7) |
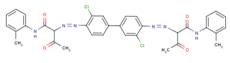 |
90% (3,3'-DCB-based analogue) |
Ames Assay |
| Pigment Yellow 17 C.I. 21105 |
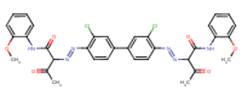 |
90% (3,3'-DCB-based analogue) |
Metabolism Data Ames Assay |
| Pigment Yellow 83 C.I. 21108 (5567-15-7) |
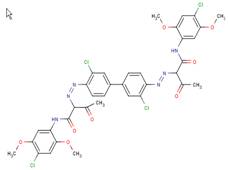 |
89% (3,3'-DCB-based analogue) |
Metabolism Data Chronic/ carcinogenicity studies |
| Pigment Yellow 12 C.I. 21090 (6358-85-6) |
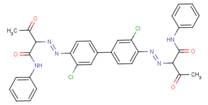 |
84%[1] (3,3'-DCB-based analogue) |
Metabolism Data Ames Assay |
| Potential Azo Cleavage Products | |||
| 3,3'-dimethoxy-benzidine, or 3,3'-DMOB (119-90-4) | 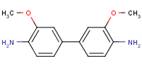 |
n/a | QSAR Chronic/ carcinogenicity studies Reproductive/ Developmental studies |
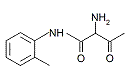
| Metabolite 1[2] (no CAS RN) |
 |
n/a | QSAR |
[2] Azo cleavage would generate an amine of the original coupling component 2-methylacetoacetanilide (CAS RN 93-68-5). No CAS RN could be identified for this potential azo cleavage product.
[*] ChemID (2010)
n/a: not available
BPAOPB
No empirical health effects data were identified for BPAOPB and predictions from quantitative structural activity relationship (QSAR) models (e.g., CASETOX, DEREK, TOPKAT) on genotoxicity and carcinogenicity of BPAOPB were considered equivocal (Appendix 1). Therefore, empirical health effects and metabolism data for azo analogues related to BPAOPB were also considered.
Analogues
Genotoxicity and Carcinogenicity
Structural analogues of BPAOPB were searched for within SciFinder (2010) and ChemID (2010) using all of the following criteria: containing a benzidine congener core, azo bound to a coupling component with an acetoacetanilide-derived structure, similarity score of 80% or greater, and having available health effects data. Several suitable azo pigment analogues of the diarylide pigment class were identified based on these selection criteria (see Table 8). The only suitable analogue identified based on the same 3,3'-dimethoxybenzidine (3,3'-DMOB) congener as BPAOPB was Pigment Orange 16. All other identified diarylide azo pigment analogues (Pigment Yellow 12, 13, 14, 17, and 83) are based on 3,3'-dichlorobenzidine (3,3'-DCB). The relevant health effects and metabolism data for these analogues are summarized below.
Pigment Orange 16 was positive for mutagenicity in a preliminary study using Salmonella typhimurium strain TA98; however, further study showed that this was the result of contamination. A more purified form of Pigment Orange 16 was not mutagenic with or without activation in the Ames assay, nor was it mutagenic in a modified Ames assay in which preincubation with flavin mononucleotide (FMN) was employed to facilitate cleavage of the azo bond (BF Goodrich Co. 1992).
Pigment Yellow 12 was negative in multipleSalmonella strains when tested for bacterial mutagenicity (Zeiger et al. 1987). No effects were observed in subchronic (30-day) toxicity testing (NCI 1978). In two-year cancer bioassays in rats and mice, increases in incidence of tumours in either sex of either species were not demonstrated when Pigment Yellow 12 as administered in the diet (Leuschner 1978; NCI 1978). The authors postulated that Pigment Yellow 12 was not absorbed after oral ingestion and that metabolic cleavage was unlikely. Tumour incidence was also not increased in rats or mice (50 per sex per group) that were fed diets containing 0.1, 0.3, or 0.9% Pigment Yellow 83, nor was there any increase in tumours when the test substance was spiked with 20 ppm 3,3'-DCB (Leuschner 1978, summarised in BIBRA 1996).
Pigment Yellow 13 tested negative in multiple Ames’ assays as well as in an in vitro DNA repair assay in human fibroblasts (European Commission 2000a).
Pigment Yellow 14 (technical grade) also tested negative in the Ames assay; however, the method of activation was not documented (European Commission 2000b).
Pigment Yellow 17 has been investigated for its mutagenic potential in bacterial mutagenesis studies employing Prival’s method for azo dye testing and tested negative (Prival and Mitchell 1982; Zhou et al. 1987).
Potential for Azo Bond Cleavage
No data is available to assess the metabolism of BPAOPB; however, investigations carried out on structurally analogous diarylide azo pigments have demonstrated that no, or extremely limited, azo cleavage occurs with this class of substances. Pigment Yellow 12, 13, 14, 17, and 83 all could theoretically undergo azo cleavage releasing 3,3'-DCB; however, the available data suggests this is not likely to occur. Rats exposed by inhalation to Pigment Yellow 17 did not have a detectable amount of the aromatic amine 3,3'-DCB in either blood or urine (Hofmann and Schmidt 1993). Similarly, monitoring for a period of 8 days detected none of the predicted metabolites when Pigment Yellow 12 was administered to hamsters by stomach intubation (Nony et al. 1980). However, very low levels of 3,3'-DCB-haemoglobin adducts were detected in rats fed Pigment Yellow 17 for 4 weeks. The authors calculated the release of 3,3'-dichlorobenzidine to be 0.6% of the dose (Zwirner-Baier and Neumann 1994). In a haemoglobin adduct monitoring study, 0.2% dietary Pigment Yellow 13 or Pigment Yellow 17 was administered to female Wistar rats for 4 weeks. No adducts were observed in the rats treated with Pigment Yellow 17, while only minimal amounts that slightly exceeded the limit of detection were observed in rats receiving Pigment Yellow 13 (Sagelsdorff et al. 1996). Pigment Yellow 83 fed to rats in the diet for 6 or 23 months at an average dose of 630 mg/kg-bw/day did not cause detectable amounts of 3,3'-DCB to accumulate in the urine (Leuschner 1978).
A recent review of the carcinogenicity of azo colourants concluded that the diarylide azo pigments based on 3,3'-DCB are “practically not bioavailable” (reviewed in Golka et al. 2004). Insolubility has been suggested to be the reason that metabolism of azo diarylide pigments does not occur (Golka et al. 2004). However, Decad and Snyder (1983) showed that even solubilized Pigment Yellow 12 (by addition of sulfonates on the benzene ring of the acetoacetanilide coupling component) was not appreciably absorbed in rats exposed orally, with only 0.02% of the applied dose detected in the urine and the rest of the dose excreted in the feces. It has been proposed that this low bioavailability may be due to protection against reductive cleavage of the azo bond by azo-hydrazone tautomerism. De France et al. (1986) demonstrated that benzidine-congeners that were disazo coupled to diethyl malonate (a ß-diketone) were not azo reduced following in vitro incubation with hamster liver S9 and flavin mononucleotide (FMN), nor were they mutagenic in the Prival modified Ames assay, indicating no release of the mutagenic benzidine congeners. NMR spectroscopy of these synthesized diethyl malonate azo substances verified that only the hydrazone tautomer was observed, while the azo tautomer was not detected (De France et al. 1986). It is speculated that the hydrazone tautomers, which are stabilized by ß-diketone ligands adjacent to the azo bond, may be favoured over the azo tautomer (Brown and DeVito 1993; US EPA 1979). Therefore, hydrazone tautomers stabilized by the acetoacetanilide coupling components diarylide azo pigments may also contribute to resistance of these substances to undergo metabolism by cleavage of the azo bond.
Characterization of Risk to Human Health
Due to the presence of the 3,3'-DMOB substructure within BPAOPB, genotoxicity and carcinogenicity are considered to be the critical effects that may be associated with BPAOPB. However, no empirical health effects data were available for BPAOPB itself. Results from QSAR predictions for genotoxicity and carcinogenicity were equivocal. Although BPAOPB, as an aromatic azo substance, could theoretically undergo azo cleavage to release a potentially hazardous aromatic amine (3,3'-DMOB), the available health effects and metabolic data for related diarylide azo pigment analogues demonstrates that this does not occur or is extremely limited. Insolubility and/or azo-hydrazone tautomerism may contribute to the resistance of these substances to azo cleavage (Golka et al. 2004). Therefore, based on negative mutagenicity for a 3,3'-DMOB-based diarylide azo pigment analogue (Pigment Orange 16), negative mutagenicity and carcinogenicity for multiple 3,3'-DCB-based diarylide azo pigment analogues, as well as the demonstration of no or very limited azo cleavage for the class of diarylide azo pigments, it is considered that the hazard potential for BPAOPB is likely low.
Exposure of the general population to BPAOPB from environmental media is considered to be negligible. Exposures from use of consumer products were not identified.
Therefore, based on the low hazard potential of BPAOPB, and negligible exposure to the general population, the potential risks of this substance to the general population are considered to be low.
Uncertainties in Evaluation of Risk to Human Health
No empirical health effects data were identified for BPAOPB. However, robust health effects and metabolism data for structural analogues in the same class of diarylide benzidine-based azo pigments were used to inform the hazard profile of BPAOPB. The assumption that BPAOPB does not undergo metabolism by azo cleavage to release potentially hazardous aromatic amines (i.e., 3,3'-DMOB) was a key element in characterizing hazard potential of BPAOPB. Overall, confidence in the hazard characterization of BPAOPB is considered to be moderate since adequate data were available to evaluate the metabolic and hazard potential of similar diarylide pigment analogues.
Literature data were not identified for BPAOPB concentrations in the environmental media. With the estimated losses of BPAOPB during its life cycle estimated to be less than 1 % to air, 4% in wastewater and no losses to soil (based on the Mass Flow Tool, as described in the Releases to the Environment section), confidence is high that exposure to BPAOPB from environmental media is negligible. Since no consumer products or children’s toy products containing BPAOPB were identified in Canada, the likelihood of exposure to the general population in Canada is also considered to be low.
Based on the information available, it is concluded that BPAOPB is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends. Additionally, BPAOPB meets the criteria for persistence but does not meet the criteria for bioaccumulation potential as set out in the Persistence and Bioaccumulation Regulations (Canada 2000).
Based on low hazard potential of BPAOPB and expected negligible exposure to the general population, the potential risks to human health for this substance are considered to be low. It is concluded that BPAOPB is not a substance entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore concluded that BPAOPB does not meet any of the criteria under section 64 of CEPA 1999.
Considerations for Follow-up
BPAOPB belongs to a group of azo substances that may metabolize to aromatic amines, which as a chemical class are known to exhibit hazardous properties, including carcinogenicity. Therefore, additional activity (e.g., research, assessment, monitoring and surveillance) to characterize the risk to human health in Canada of this broader group of azo substances may be undertaken. A Notice of Intent outlining how the Government of Canada will address this group of substances is available on the Chemical Substances website.
ACD/pKaDB [Prediction Module]. 1994–2008. Version 9.04. Toronto (ON): Advanced Chemistry Development.
[AIEPS] Artificial Intelligence Expert Predictive System. 2003–2007. Version 2.05. Ottawa (ON): Environment Canada, Existing Substances Division, New Substances Division. Model developed by Stephen Niculescu. Available upon request.
Anliker R, Moser P. 1987. The limits of bioaccumulation of organic pigments in fish: their relation to the partition coefficient and the solubility in water and octanol. Ecotoxicol and Environ Safety 13:43–52.
Arnot JA, Arnot M, Mackay D, Couillard Y, MacDonald D, Bonnell M, Doyle P. 2010. Molecular size cut-off criteria for screening bioaccumulation potential: Fact or fiction? Integrated Environmental Assessment and Management 6(2):210–224.
Baughman GL, Perenich TA. 1988. Investigating the fate of dyes in the environment. American Dyestuff Reporter 77:19–48.
BF Goodrich Co. 1992. Initial Submission: Letter from BF Goodrich Co Submitting 2 Ames Salmonella/Microsome Bioassays, One with Orange 5 & the Other with Fancon Orange 16 OD 5845. OTS0540879.
[BIBRA] BIBRA International Ltd. – Toxicology Advice and Consulting. 1996. Toxicity Profile: Pigment Yellow 83. Surrey, UK.
[BIOWIN] Biodegradation Probability Program for Windows [Estimation Model]. 2008. Version 4.10. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Exposure Assessment Tools and Models.
Boethling RS, Howard PH, Beauman JA, Larosche ME. 1995. Factors for intermedia extrapolations in biodegradability assessment. Chemosphere 30(4):741-752.
Brown MA, De Vito SC. 1993. Predicting azo dye toxicity. Critical Reviews in Environmental Science and Technology. 23(3):249–324.
Canada. 1978. Food and Drug Regulations, C.R.C., c. 870.
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C., 1999, c. 33, Canada Gazette, Part III, Vol. 22, No. 3.
Canada. 2000. Canadian Environmental Protection Act, 1999: Persistence and Bioaccumulation Regulations, P.C. 2000-348, 23 March, 2000, SOR/2000-107, Canada Gazette, Part II, Vol. 134, No. 7.
Canada, Dept. of the Environment, Dept. of Health. 2006a.Canadian Environmental Protection Act, 1999: Notice of intent to develop and implement measures to assess and manage the risks posed by certain substances to the health of Canadians and their environment. Canada Gazette, Part I, Vol. 140, No. 49.
Canada, Dept. of the Environment. 2006b. Canadian Environmental Protection Act, 1999: Notice with respect to selected substances identified as priority for action.Canada Gazette, Part I, Vol. 140, No. 9.
Canada, Dept. of the Environment, Dept. of Health. 2008a.Canadian Environmental Protection Act, 1999: Notice of sixth release of technical information relevant to substances identified in the Challenge. Canada Gazette, Part I, Vol. 142, No. 22.
Canada, Dept. of the Environment. 2008b. Canadian Environmental Protection Act, 1999: Notice with respect to Batch 6 Challenge substances. Canada Gazette, Part I, Vol. 142, No. 22.
CASETOX [Prediction module]. 2008. Version 2.0. Beachwood (OH): MultiCASE Inc. CASETOX [restricted access]
[CATABOL] Probabilistic assessment of biodegradability and metabolic pathways [Computer Model]. c2004-2008. Version 5.10.2. Bourgas (BG): Bourgas Prof. Assen Zlatarov University, Laboratory of Mathematical Chemistry. CATALOGIC.
chemBlink [Database on the Internet]. 2009. Online database of chemicals around the world: Direct Black 38. Research Triangle Park [NC]: chemBlink Headquarters Inc. (US). [cited 2009 March].ChemBlink.
ChemCAN [Level III fugacity model of 24 regions of Canada]. 2003. Version 6.00. Peterborough (ON): Trent University, The Canadian Centre for Environmental Modelling and Chemistry[cited 2010 Jan].
[ChemID] ChemIDplus Advanced [Database on the Internet]. 2010. Structure similarity search for CAS RN 7147-42-4. Bethesda (MD): National Library of Medicine, US Department of Health and Human Services. [cited 2010 June].
Chen H. 2006. Recent advances in azo dye degrading enzyme research. Curr Protein Pept Sci 7(2):101–111.
[CHRIP] Chemical Risk Information Platform [database on the Internet]. c2011. Tokyo (JP): National Institute of Technology and Evaluation, Chemical Managemet Centre (CMC). [cited 2011 January].
[CII] Colour Index International [database on the Internet]. 2002– . 4th Ed. Research Triangle Park (NC): American Association of Textile Chemists and Colourists. [cited 2008 January].
Clariant. 2003. Are pigments bioaccumulative? Presented at the ETAD Pigments Operating Committee, Workshop on Chemical Policy with Pigments as Main Issue, Workshop for the Danish Environmental Protection Agency and the Danish Industry, Copenhagen September 22, 2003.
Clariant. 2005. Colorants for the Paint Industry. Clariant GmbH, Pigments and Additives Division, Marketing Coating Business. Frankfurt, Germany. 70 pp. [cited 2009 March].
[CPMA] Color Pigments Manufacturers Association, Inc. 2003. Comments of the Color Pigments Manufacturers Association, Inc. on the Draft Guidance Manual for the Categorization of Organic and Inorganic Substances on Canada’s Domestic Substances List (‘DSL’) and Environment Canada’s Computer Generated Estimates and Empirical Data on Approximately 12,000 Discrete Organic Chemicals on the DSL. Gatineau (QC): Environment Canada, Existing Substances Division. Available upon request.
[CPMA] Color Pigments Manufacturers Association, Inc. 2009. Letter submitted to Environment Canada in response to Environment Canada’s request for information on disazo organic dyes and pigments. September 3, 2009.
[CPOPs] Canadian POPs Model. 2008. Gatineau (QC): Environment Canada, Existing Substances Division; Bourgas (BG): Bourgas Prof. Assen Zlatarov University, Laboratory of Mathematical Chemistry. [Model developed based on Mekenyan et al. 2005]. Available upon request.
[Danish EPA] Danish Environmental Protection Agency. 1999.Survey of azo-colorants in Denmark. Consumption, use, health and environmental aspects. MiljØprojekt No. 509. Henriette, Danish Technological Institute, Environment, Ministry of Environment and Energy, Denmark, Danish Environmental Protection Agency, 1999. Report prepared by Øllgaard H, Frost L, Galster J, Hansen OC.
Decad GM, Snyder CD. 1983. Fate of water-insoluble and water-soluble dichlorbenzidine-based pigments in Fischer 344 rats. J Tox Environ Health. 11:455–465.
De France BF, Carter MH, Josephy PD. 1986. Comparative metabolism and mutagenicity of azo and hydrazone dyes in the Ames test. Fd Chem Toxic 24(2):165–169.
[DEREK] Deductive Estimation of Risk from Existing Knowledge [Prediction module on CD ROM]. 2008. Version 10.0.2. Cambridge (MA): Harvard University, LHASA Group. Toxicology Prediction [restricted access]
Dimitrov SD, Dimitrova NC, Walker JD , Veith GD, Mekenyan, OG. 2002. Predicting bioconcentration factors of highly hydrophobic chemicals. Effects of molecular size. Pure Appl Chem 74(10):1823–1830.
Dimitrov S, Dimitrova N, Parkerton T, Comber M, Bonnell M, Mekenyan O. 2005. Base-line model for identifying the bioaccumulation potential of chemicals. SAR QSAR Environ Res 16(6):531–554.
[DPD] Drug Product Database [database on the internet]. 2010. Ottawa (ON): Health Canada. [cited 2010 June].
[ECOTOX] ECOTOXicology Database [database on the Internet]. 2011. Version 4.0 Washington (DC): US Environmental Protection Agency, Office of Research and Development; National Health and Environmental Effects Research Laboratory, Mid-Continent Ecology Division. [cited 2011 January].
Environment Canada. 1988. Data relating to the Domestic Substances List (DSL) 1984-1986, collected under CEPA, 1988, s. 25(1). Based on: Reporting for the Domestic Substances List [guide] 1988. Data compiled by: Environment Canada.
Environment Canada. 2000. Environmental categorization for persistence, bioaccumulation and inherent toxicity of substances on the Domestic Substances List using QSARs. Unpublished final report. Gatineau (QC): Environment Canada, Existing Substances Division. On the cover: Results of an international QSAR workshop hosted by the Chemicals Evaluation Division of Environment Canada, November 11-12, 1999, Philadelphia, Pennsylvania. Available upon request.
Environment Canada. 2006. Data for selected substances collected under the Canadian Environmental Protection Act, 1999, Section 71: Notice with respect to selected substances identified as priority for action. Data compiled by: Environment Canada, Program Development and Engagement Division.
Environment Canada. 2007. Guidance for Conducting Ecological Assessments under CEPA, 1999: Science Resource Technical Series, Technical Guidance Module: QSARs. Reviewed Draft Working Document. Gatineau (QC): Environment Canada, Ecological Assessment Division. Available upon request.
Environment Canada. 2008a. Data for Batch 6 substances collected under the Canadian Environmental Protection Act, 1999, Section 71:Notice with respect to Batch 6 Challenge substances. Data compiled by: Environment Canada, Program Development and Engagement Division.
Environment Canada. 2008b. Guidance for Conducting Ecological Assessments under CEPA, 1999: Science Resource Technical Series, Technical Guidance Module: Mass Flow Tool. Working Document. Gatineau (QC): Environment Canada, Ecological Assessment Division. Available upon request.
Environment Canada. 2009a. Assumptions, limitations and uncertainties of the mass flow tool for BPAOPB, CAS RN 7147-42-4. Internal draft document. Gatineau (QC): Environment Canada, Ecological Assessment Division. Available upon request.
Environment Canada. 2009b. Guidance for Conducting Ecological Assessments under CEPA, 1999: Science Resource Technical Series, Technical Guidance Module: The Industrial Generic Exposure Tool – Aquatic (IGETA). Working document. Gatineau (QC): Environment Canada, Ecological Assessment Division. Available upon request.
Environment Canada. 2009c. IGETA (Industrial Generic Exposure Tool – Aquatic) Report: CAS RN 7147-42-4, 2008-11-14. Internal draft document. Gatineau (QC): Environment Canada, Ecological Assessment Division. Available upon request.
Environment Canada. 2010. National Pollutant Release Inventory [NPRI; database on the Internet]. Gatineau (QC): Environment Canada.
[EPIsuite] Estimation Programs Interface Suite for Microsoft Windows [Estimation Model]. 2008. Version 4.0 Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Exposure Assessment Tools and Models.
[ESIS] European chemical Substances Information System [database on the Internet]. c1995–2010. Information for CAS RN 7147-42-4. Ispra [IT]: European Commission, Joint Research Centre, Institute for Health and Consumer Protection, European Chemicals Bureau (ECB). [cited 2008 April].
[ETAD] Ecological and Toxicological Association of Dyes and Organic Pigments Manufacturers. 1995. Health & Environmental Information on Dyes Used in Canada. An overview to assist in the implementation of the New Substances Notification Regulation under the Canadian Environmental Protection Act. Prepared by the ETAD Canadian Affiliates. July 1995. Report 7/21/95
European Commission. 2000a. IUCLID Dataset – CAS RN 5102-83-0 (Pigment Yellow 13) [Internet]. Ispra [IT]: European Commission, Joint Research Centre, Institute for Health and Consumer Protection, European Chemicals Bureau. [cited 2010 May].
European Commission. 2000b. IUCLID Dataset – CAS RN 5468-75-7 (Pigment Yellow 14) [Internet]. Ispra [IT]: European Commission, Joint Research Centre, Institute for Health and Consumer Protection, European Chemicals Bureau. [cited 2009 March].
European Commission. 2000c. IUCLID Dataset – CAS RN 5567-15-7 (Pigment Yellow 83) [Internet]. Ispra [IT]: European Commission, Joint Research Centre, Institute for Health and Consumer Protection, European Chemicals Bureau. [cited 2011 January].
European Commission. 2000d. IUCLID Dataset – CAS RN 6358-85-6 (Pigment Yellow 12) [Internet]. Ispra [IT]: European Commission, Joint Research Centre, Institute for Health and Consumer Protection, European Chemicals Bureau. [cited 2009 March].
European Commission. 2006. Regulation (EC) No 1907/2006 of the European Parliament and of the Council (REACH).
Golka K, Kopps S, Myslak ZW. 2004. Carcinogenicity of azo colorants: influence of solubility and bioavailability. Toxicol Lett 151(1):203–210.
Hamburger B, Haberling H, Hitz HR. 1977. Comparative tests on toxicity to fish using minnows, trout, and golden orfe. Arch Fischereiwiss 28(1):45–55. As cited in ECOTOX (2011).
[HENRYWIN] Henry’s Law Constant Program for Microsoft Windows [Estimation Model]. 2008. Version 3.20. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Exposure Assessment Tools and Models.
Herbst W, Hunger K. 2004. Industrial Organic Pigments, 3rd edition. Weinheim (Germany): Wiley-VCH, Verlag GmbH & Co. KGaA. 660 p.
Hoechst MR. 1999. C.I. Pigment Yellow 13 Daphnia magnareproduction test report number 99.0405. Data provided under the HPV Challenge program. Chemical Search Results for CAS RN 5102-83-0. [cited 2009 March]. As cited in US EPA 2009. High Production Volume Information System (HPVIS).
Hofmann, T, Schmidt D. 1993. Investigation of possible metabolism of Pigment Yellow 17, a 3,3'-dichlorobenzidine-based pigment, after inhalation exposure in rats. Arch. Toxicol. 67:141–144.
IARC. 1974. Some Aromatic Amines, Hydrazine and Related Substances, N-Nitroso Compounds and Miscellaneous Alkylating Agents. IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Humans. Lyon, France, International Agency for Research on Cancer. vol. 4:286 pp.
IARC. 1987. Overall Evaluations of Carcinogenicity. IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Humans. Lyon, France, International Agency for Research on Cancer. Supplement 7:440 pp.
Jaffe EE. 1996. Pigments. In: Kroschwitz, Howe-Grant JM (eds.). Kirk-Othmer Encyclopedia of Chemical Technology, 4th ed. New York (NY): John Wiley and Sons. Vol. 19:41–78.
Leuschner F. 1978. Carcinogenicity studies on different diarylide yellow pigments in mice and rats. Toxicol Lett 2:253–260.
Lincke G. 2003. Molecular stacks as a common characteristic in the crystal lattice of organic pigment dyes. A contribution to the ‘insoluble-soluble’ dichotomy of dyes and pigments from the technological point of view. Dyes Pigments 59(1):1–24.
[LNHPD] Licensed Natural Health Products Database. 2010. Ottawa (ON): Health Canada. [cited 2010 June].
MacEvoy B. 2008. Watercolors: The World’s Finest Guide to Watercolor Painting. Synthetic Organic Pigments [Internet]. [cited April 2010].
Madsen T. 1995. Aerobic biodegradability of Pimatex Yellow 2GL-modified MITI test. Data provided under the HPV Challenge program. Chemical Search Results for CAS RN 5102-83-0. [cited 2009 March]. As cited in US EPA 2009. High Production Volume Information System (HPVIS).
Mekenyan G, Dimitrov SD, Pavlov TS, Veith GD. 2005. POPs: a QSAR system for creating PBT profiles of chemicals and their metabolites. SAR QSAR Environ Res 16(1-2):103-133. As cited in CPOPs 2008.
[MPBPVP] Melting Point Boiling Point Vapor Pressure Program for Microsoft Windows [Estimation Model]. 2008. Version 1.43. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Exposure Assessment Tools and Models.
[NCI] National Cancer Institute. 1978. Bioassay of diarylanilide yellow for possible carcinogenicity. CAS No. 6358-85-6. NCI-CG-TR-30. Carcinogenesis Technical Report Series No. 30. U.S. Department of Health, Education, and Welfare. Public Health Service. National Institutes of Health. Bethesda, Maryland. DHEW Publication No. (NIH) 78-830
[NCI] National Chemical Inventories [database on a CD-ROM]. 2007. Issue 1. Columbus (OH): American Chemical Society, Chemical Abstracts Service. [cited 2009 January]. National Chemical Inventory (NCI) System Requirements.
[NHPID] Natural Health Products Ingredients Database. 2010. Ottawa (ON): Health Canada. [cited 2010 June].
Nony CR, Bowman MC, Cairns T, Lowry LK, Tolos WP. 1980. Metabolism studies of an azo dye and pigment in the hamster based on analysis of the urine for potentially carcinogenic aromatic amine metabolites. J Anal Toxicol 4(3):132–140.
[NPIRI] National Printing Ink Research Institute. 2000. NPIRI raw materials data handbook, Volume 4 Pigments, 2nd ed. Woodbridge (NJ): National Printing Research Institute.
[NTP] United States National Toxicology Program. 1990. Toxicology and carcinogenesis studies of 3,3'-dimethoxybenzidine dihydrochloride (CAS RN 20325-40-0) in F344/N rats (drinking water studies). Technical report series No. 372. (NC): Research Triangle Park.
[OECD] Organisation for Economic Co-operation and Development. 2004. Emission Scenario Document on Plastics Additives [Internet]. Paris (FR): OECD Environment Directorate, Environmental Health and Safety Division. ENV/JM/MONO(2004)8, JT00166678. [cited 2008 October]. Official OECD Documents.
[OECD] Organisation for Economic Co-operation and Development. 2007. Emission scenario document on adhesive formulation [Internet]. Final report. Paris (FR): OECD, Environment Directorate. Series on Emission Scenario Documents. [cited 2008 October].
[OECD] Organisation for Economic Co-operation and Development. 2008. Emission Scenario Document on the Application of Radiation Curable Coatings, Inks, and Adhesives. Revised Draft. August 2008. Paris (FR): OECD Environment Directorate. 141 pages. [cited 2008 October].
Platzek T, Lang C, Grohmann G, Gi US, Baltes W. 1999. Formation of a carcinogenic aromatic amine from an azo dye by human skin bacteria in vitro. Hum Exp Toxicol 18(9):552–559.
Prival MJ, Mitchell VD. 1982. Analysis of a method for testing azo dyes for mutagenic activity in Salmonella typhimuriumin the presence of flavin mononucleotide and hamster liver S9. Mutat Res 97(2):103–16.
Sagelsdorff P, Haenggi R, Heuberger R, Joppich-Kuhn, Jung R, Weideli HJ and Joppich M. 1996. Lack of bioavailability of dichlorobenzidine from diarylide azo pigments: molecular dosimetry for haemoglobin and DNA adducts. Carcinogenesis 17(3):507-514.
Sakuratani Y, Noguchi Y, Kobayashi K, Yamada J, Nishihara T. 2008. Molecular size as a limiting characteristic for bioconcentration in fish. J Environ Biol 29(1):89–92.
Scifinder, Web Version. 2010. Chemcal Abstracts Service; Columbus, OH. [cited April 2010].
Stingley RL, Zou W, Heinze TM, Chen H, Cerniglia CE. 2010. Metabolism of azo dyes by human skin microbiota. J Med Microbiol 59(Pt.1):108–114.
Study Submission. 2008. Letter submitted to Environment Canada. Data for Batch 6 substances collected under Canadian Environmental Protection Act, 1999, Section 71: Notice with respect to Batch 6 Challenge substances. Data compiled by: Environment Canada, Program Development and Engagement Division.
[TOPKAT] Toxicity Prediction Program [Internet]. 2004. Version 6.2. San Diego (CA): Accelrys Software Inc.
[US DHHS] United States Department of Health and Human Services. 2005. Report on Carcinogens. Eleventh Edition. Dyes Metabolized to Benzidine [Internet]. Research Triangle Park (NC): US Department of Health and Human Services, Public Health Service, National Toxicology Program. [cited 2010 June].
[US EPA] US Environmental Protection Agency. 1979. Preliminary risk assessment: Phase I, Benzidine, its congeners, and their derivative dyes and pigments. Washington (DC). Office of Testing and Evaluation, Office of Pesticides and Toxic Substances.
[US EPA] US Environmental Protection Agency. 2005. Inventory Update Reporting Program. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics.
[US EPA] US Environmental Protection Agency. 2006. High Production Volume (HPV) Challenge Program. Test Plan for C.I. Pigment Yellow 14 (CAS No 5468-75-7. Washington (DC): US EPA, Office of Pollution Prevention and Toxics. Prepared by Color Pigment Manufacturers Association, Inc., Diarylide Pigments Committee. High Production Volume Information System (HPVIS).
Zeiger E, Anderson B, Haworth S, Lawlor T, Mortelmans K, and Speck W. 1987. Salmonella mutagenicity tests: III. Results form the testing of 255 chemicals. Environ Mol Mutagen 9(Suppl. 9):1–110.
Zhou Y, You X, and Ye X. 1987. Mutagenicity of benzidines and their congener derivative dyes. Huanjing Kexue 8(2):31–34. (Article in Chinese)
Zwirner-Baier and Neumann. 1994. Biomonitoring of aromatic amines IV: use of haemoglobin adducts to demonstrated the bioavailablility of cleavage products from diarylide azo pigmentsin vivo. Arch. Toxicol. 68:8–14.
Carcinogenicity
| CAS RN | DEREK (2008) | CASETOX (2008) | TOPKAT (2004) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cancer | m-rat | f-rat | m-mice | f-mice | NTP Rodent | NTP m-rat | NTP f-rat | NTP m-mouse | NTP f-mouse | |
| 7147-42-4 | P | IC | N | IC | IC | IC | ND | ND | ND | N |
| 119-90-4 | P | P | P | N | N | P | P | P | ND | ND |
| Metabolite 1 (no CAS RN) | ND | N | N | IC | IC | IC | IC | IC | N | IC |
Genotoxicity
| CAS RN | Ames | ChrAb | Micronuclei Induction | Mouse Lymphoma mutation | |||
|---|---|---|---|---|---|---|---|
| Derek | CT | TK | Derek | CT# | CT | CT | |
| 7147-42-4 | ND | N | ND | ND | N | IC | IC |
| 119-90-4 | P | P | P | ND | P | IC | P |
| Metabolite 1 (no CAS RN) | ND | N | N | ND | N | N | IC |
Footnotes
[2] A determination of whether one or more of the criteria of section 64 are met is based upon an assessment of potential risks to the environment and/or to human health associated with exposures in the general environment. For humans, this includes, but is not limited to, exposures from ambient and indoor air, drinking water, foodstuffs, and the use of consumer products. A conclusion under CEPA 1999 on the substances in the Chemicals Management Plan (CMP) Challenge is not relevant to, nor does it preclude, an assessment against the hazard criteria specified in the Controlled Products Regulations, which is part of the regulatory framework for the Workplace Hazardous Materials Information System (WHMIS) for products intended for workplace use. Similarly, a conclusion based on the criteria contained in section 64 of CEPA 1999 does not preclude actions being taken under other sections of CEPA or other Acts.