Screening Assessment for the Challenge
This page has been archived on the Web
Information identified as archived is provided for reference, research or recordkeeping purposes. It is not subject to the Government of Canada Web Standards and has not been altered or updated since it was archived. Please contact us to request a format other than those available.
Archived
2,9,11,13-Tetraazanonadecanethioic acid, 19-isocyanato-11-(6-isocyanatohexyl)-10,12-dioxo-, S-[3-(trimethoxysilyl)propyl] ester
Chemical Abstracts Service Registry Number
85702-90-5
Environment Canada
Health Canada
January 2011
Table of Contents
- Synopsis
- Introduction
- Substance Identity
- Physical and Chemical Properties
- Sources
- Uses
- Releases to the Environment
- Environmental Fate
- Persistence and Bioaccumulation Potential
- Potential to Cause Ecological Harm
- Potential to Cause Harm to Human Health
- Conclusion
- References
- Appendix 1: Persistence, Bioaccumulation, Toxicity (PBT)
- Appendix 2: Estimated concentrations of TIDTE in environmental media using ChemCAN version 6.00 (ChemCAN 2003)
- Appendix 3: Dermal exposure estimate of boat joint sealant scenario
- Appendix 4: Summary of (Q)SAR Results for the Health Assessment on TIDTE
Pursuant to section 74 of the Canadian Environmental Protection Act, 1999 (CEPA 1999), the Ministers of the Environment and of Health have conducted a screening assessment on 2,9,11,13-tetraazanonadecanethioic acid, 19-isocyanato-11-(6-isocyanatohexyl)-10,12-dioxo-, S-[3-(trimethoxysilyl)propyl] ester, Chemical Abstracts Service Registry Number (CAS RN)[*] 85702-90-5. In this assessment, this chemical will be referred to by its derived acronym, TIDTE. This substance was identified as a high priority for screening assessment and included in the Challenge initiative under the Chemicals Management Plan because it had been found to meet the ecological categorization criteria for persistence, bioaccumulation potential and inherent toxicity to non-human organisms and is believed to be in commerce in Canada.
TIDTE, was not considered to be a high priority for assessment of potential risks to human health, based upon application of the simple exposure and hazard tools developed for categorization of substances on the Domestic Substances List.
TIDTE is an organic substance not naturally produced in the environment; nor is it reported to be manufactured in Canada. In each of 2005 and 2006, there were between 100 and 1000 kg of this substance imported into the country in products.
Based on the reported use as a component in marine adhesive and sealant products, TIDTE is expected to cross-link to polymers in the formulation. Thus the majority of TIDTE (99%) is considered to undergo chemical transformation in the formulated product matrix, making it unavailable for release to the environment. Residue of adhesive and sealant in product containers is assumed to be disposed of in landfill sites.
TIDTE is unlikely to be released to the environment in more than very low quantities. Any of the substance that is released to the environment, it is anticipated to hydrolyze rapidly in water (forming amines, silanol, and methanol), as well as in the presence of moisture in other environmental media. Thus although ultimate degradation (the metric used for categorization) is expected to be slow (e.g. the silanol – one of the hydrolysis products – is expected to degrade very slowly in the environment), TIDTE undergoes rapid primary degradation.
New model predictions of bioaccumulation that take account of potential for metabolic transformation show that neither TIDTE nor its hydrolysis products are bioaccumulative in aquatic organisms. Therefore, it is concluded that the substance does not meet the persistence or bioaccumulation criteria as set out in the Persistence and Bioaccumulation Regulations of the Canadian Environmental Protection Act, 1999.
In addition, based on the modelled toxicity data, neither TIDTE nor its hydrolysis products are likely to have high potential to harm aquatic organisms. Considering this, and in view of the low likelihood of release of TIDTE to any environmental compartment, it is concluded that TIDTE is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitutes or may constitute a danger to the environment on which life depends.
The potential for exposure of the general population to TIDTE from environmental media is expected to be negligible. Exposure of the general population to TIDTE in consumer products is expected to be low.
There were no empirical health effects data identified for TIDTE or its analogues. Based principally on the low solubility and high reactive nature of TIDTE, quantitative structure-activity relationship (QSAR) predictions, as well as a European Commission classification, the primary hazard concern for TIDTE is skin and respiratory tract sensitization. However, as exposure of the general population to TIDTE is expected to be low to negligible, the risk to human health is expected to be low. It is concluded that TIDTE is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
Based on the information available, it is therefore concluded that TIDTE does not meet any of the criteria set out in section 64 of the Canadian Environmental Protection Act, 1999.
This substance will be considered for inclusion in the Domestic Substances List inventory update initiative. In addition and where relevant, research and monitoring will support verification of assumptions used during the screening assessment.
The Canadian Environmental Protection Act, 1999 (CEPA 1999) (Canada 1999) requires the Minister of the Environment and the Minister of Health to conduct screening assessments of substances that have met the categorization criteria set out in the Act to determine whether these substances present or may present a risk to the environment or to human health.
Based on the information obtained through the categorization process, the Ministers identified a number of substances as high priorities for action. These include substances that
- met all of the ecological categorization criteria, including persistence (P), bioaccumulation potential (B) and inherent toxicity to aquatic organisms (iT), and were believed to be in commerce in Canada; and/or
- met the categorization criteria for greatest potential for exposure (GPE) or presented an intermediate potential for exposure (IPE) and had been identified as posing a high hazard to human health based on classifications by other national or international agencies for carcinogenicity, genotoxicity, developmental toxicity or reproductive toxicity.
The Ministers therefore published a notice of intent in the Canada Gazette, Part I, on December 9, 2006 (Canada 2006), that challenged industry and other interested stakeholders to submit, within specified timelines, specific information that may be used to inform risk assessment, and to develop and benchmark best practices for the risk management and product stewardship of those substances identified as high priorities.
The substance, 2,9,11,13-tetraazanonadecanethioic acid, 19-isocyanato-11-(6-isocyanatohexyl)-10,12-dioxo-, S-[3-(trimethoxysilyl)propyl] ester (TIDTE), had been identified as a high priority for assessment of ecological risk as it had been found to be persistent, bioaccumulative and inherently toxic to aquatic organisms in the DSL Categorization, and is believed to be in commerce in Canada. The Challenge for this substance was published in the Canada Gazette on June 20, 2009 (Canada 2009). A substance profile was released at the same time. The substance profile presented the technical information available prior to December 2005 that formed the basis for categorization of this substance. As a result of the Challenge, submissions of information pertaining to uses of the substance were received (Environment Canada 2010a).
Although TIDTE was determined to be a high priority for assessment with respect to the environment, it did not meet the criteria for GPE or IPE or high hazard to human health based on classifications by other national or international agencies for carcinogenicity, genotoxicity, developmental toxicity or reproductive toxicity.
Screening assessments focus on information critical to determining whether a substance meets the criteria set out in section 64 of CEPA 1999. Screening assessments examine scientific information and develop conclusions by incorporating a weight-of-evidence approach and precaution.[2]
This final screening assessment includes consideration of information on chemical properties, hazards, uses and exposure, including the additional information submitted under the Challenge. Data relevant to the screening assessment of this substance were identified in original literature, review and assessment documents, stakeholder research reports and from recent literature searches, up to February 2010 for the ecological sections and human health exposure section of the document. Key studies were critically evaluated; some modelling results were used to reach conclusions. The final screening assessment does not represent an exhaustive or critical review of all available data. Rather, it presents the most critical studies and lines of evidence pertinent to the conclusion.
When available and relevant, information presented in hazard assessments from other jurisdictions was considered. The final screening assessment does not represent an exhaustive or critical review of all available data. Rather, it presents the most critical studies and lines of evidence pertinent to the conclusion.
This final screening assessment was prepared by staff in the Existing Substances Programs at Health Canada and Environment Canada and incorporates input from other programs within these departments. The ecological portion of the assessment has undergone external written peer review/consultation. Additionally, the draft of this screening assessment was subject to a 60-day public comment period. While external comments were taken into consideration and considered during consultation, the final content and outcome of the screening assessment remain the responsibility of Health Canada and Environment Canada. Approaches used in the screening assessments under the Challenge have been reviewed by an independent Challenge Advisory Panel.
The critical information and considerations, upon which the final assessment is based, are summarized below.
For the purposes of this document, this substance will be referred to as TIDTE, derived from the Domestic Substances List (DSL) inventory name.
Table 1. Substance identity for TIDTE
| Chemical Abstracts Service Registry Number (CAS RN) | 85702-90-5 |
| DSL name | 2,9,11,13-Tetraazanonadecanethioic acid, 19-isocyanato-11-(6-isocyanatohexyl)-10,12-dioxo-, S-[3-(trimethoxysilyl)propyl] ester |
| National Chemical Inventories (NCI) names1 | 2,9,11,13-Tetraazanonadecanethioic acid, 19-isocyanato-11-(6-isocyanatohexyl)-10,12-dioxo-, S-[3-(trimethoxysilyl)propyl] ester (TSCA, DSL, PICCS, ASIA-PAC, NZIoC) A mixture of: S-(3-trimethoxysilyl)propyl 19-isocyanato-11-(6-isocyanatohexyl)-10,12-dioxo-2,9,11,13-tetraazanonadecanethioate, S-(3-(trimethoxysilyl)propyl 17-isocyanato-9-(isocyanatohexyl-aminocarbonyl)-10-oxo-2,9,11-triazaheptadecanethioate(REACH) |
| Other names | Not applicable |
Chemical group (DSL Stream) |
Discrete organics |
| Major chemical class or use | Isocyanates, alkyl silanes |
| Major chemical sub-class | Isocyanates, thiocarbamates, biuret, methoxysilanes, aliphatic amines, secondary amines, tertiary amines |
| Chemical formula | C29H54N6O8SSi |
| Chemical structure | 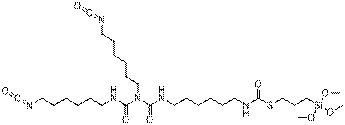 |
| SMILES2 | O=C(N(C(=O)NCCCCCCNC(=O)SCCC[Si](OC)(OC)OC)CCCCCCN=C(=O))NCCCCCCN=C=O |
| Molecular mass | 674.93 g/mol |
2 Simplified Molecular Input Line Entry System.
No experimental physical and chemical property data are available for TIDTE (Table 2). Structural analogues of TIDTE were determined using the SciFinder and ChemID websites. However, no empirical physical-chemical property data were identified for these TIDTE analogues.
Quantitative structure-activity relationship (QSAR) models were used to estimate the key physical and chemical properties of TIDTE, and predictions are summarized in Table 2 below. These models (except WSKOWWIN 2008) are mainly based on fragment addition methods, i.e., they rely on the structure of a chemical.
Table 2. Estimation of physical and chemical properties for TIDTE
| Property | Value1 | Temperature (°C) |
Reference |
|---|---|---|---|
| Melting point (ºC) | 349.84 | MPBPWIN 2008 | |
| Boiling point (ºC) | 861.44 | MPBPWIN 2008 | |
| Density (kg/m3) | Not available | ||
| Vapour pressure (Pa) | 1.73 × 10-19 (1.3 × 10-21 mm Hg) |
25 | EPIWIN 2008 |
| Henry’s Law constant (Pa·m3/mol) |
1.16 × 10-16 (1.14 × 10-21 atm·m3/mol) |
25 | HENRYWIN 2008 |
| Log KOW (octanol-water partition coefficient) (dimensionless) | 6.62 | KOWWIN 2008 | |
| Log KOC (organic carbon-water partition coefficient) (dimensionless) | 4.40 | KOCWIN 2008 | |
| Log KOA (octanol-air partition coefficient) (dimensionless) | 25.95 | KOAWIN 2008 | |
| Water solubility (mg/L) | 1.14 × 10-4 | 25 | WSKOWWIN 2008 |
| pKa (acid dissociation constant) (dimensionless) | 13.23 | ACD/pKaDB 2008 | |
| Maximum diameter (nm)2 | 1.74–3.43 | CPOPs 2008 | |
2 Values represent the range of possible maximum molecular diameters (Dmax) estimated by CPOPs.
According to model predictions, the substance is estimated to have very low vapour pressure (1.73 × 10-19 Pa), very low Henry’s Law constant (1.16 × 10-16Pa·m3/mol), high log KOW (6.62), low water solubility (1.14 × 10-4 mg/L), and a large molecular size (a maximum cross-sectional diameter of 1.74–3.43 nm).
TIDTE is not reported to be naturally produced in the environment.
The quantity reported to the DSL as being manufactured, imported or in commerce in Canada during the 1986 calendar year was between 1000 and 10 000 kg.
Recent information was collected through surveys conducted for the 2005 and 2006 calendar years by means of Canada Gazette notices issued pursuant to section 71 of CEPA 1999 (Canada 2006). These notices required submission of data on the Canadian manufacture and import of TIDTE. In the notice for 2006, data were also required on the use quantity of the substance.
There was no manufacturing activity of TIDTE reported in either 2005 or 2006 in Canada. However, between 100 and 1000 kg of TIDTE was reported to be imported by fewer than four companies into the country in each of 2005 and 2006, as a component in readyto-use consumer/commercial products. Using the Declaration of Stakeholder Interest form associated with the section 71 survey for 2006, four companies reported a stakeholder interest for this substance.
Elsewhere, TIDTE has been reported to the United States Toxic Substances Control Act (TSCA) Chemical Substances Inventory in a range of 4 500 to 225 000 kg (10 000 to 500 000 lb), manufactured or processed in the USA in 1990, 1994, and 1998 (US EPA 1986–2002); there were no reports in 2002.
The following DSL use codes were identified for TIDTE during the DSL nomination period (1984–1986): Formulation component; Adhesive and Sealant Production.
Information on uses for the 2005 and 2006 calendar years was also obtained as part of the response to the CEPA 1999 section 71 notices (Canada 2006, 2009).
TIDTE is used as a component in marine adhesives and sealants, which are mainly applied on plastic and metallic objects either above or under the water line. However, there is no information to determine the quantity for each use pattern. The products are designed to bind to the surface of treatment in a stable manner.
TIDTE was not notified as an ingredient in cosmetic products in Canada (CNS 2010) and is not on the Cosmetic Ingredient Hotlist, Health Canada’s administrative list of ingredients that are intended to be prohibited or restricted for use in cosmetics in Canada (Health Canada 2009). TIDTE is not currently present in Canada as a formulant in pesticide products, as it is not listed in the Pest Management Regulatory Agency List of Formulants (Health Canada 2007; March 2010 email from Pest Management Regulatory Agency, Health Canada to Existing Substances Risk Assessment Bureau, Health Canada; unreferenced). It is not listed as an approved food additive under Division 16 of the Food and Drug Regulations (Canada 1978). TIDTE was not identified to be used in food packaging applications or in incidental additives (January 2010 email from Food Directorate, Health Canada, to Risk Management Bureau, Health Canada; unreferenced). TIDTE is not listed in the Drug Product Database, the Therapeutic Products Directorate’s Non-Medicinal Ingredient Database, the Natural Health Products Ingredients Database or the Licensed Natural Health Products Database as a medicinal or non-medicinal ingredient present in final pharmaceutical products, natural health products or veterinary drugs (November 2009 to January 2010 emails from Therapeutic Products Directorate, Natural Health Products Directorate and Veterinary Drugs Directorate, Health Canada, to Risk Management Bureau, Health Canada; unreferenced).
Other uses of TIDTE have not been identified.
In general, releases of a substance to the environment depend upon various losses of the substance from its manufacture, industrial use, and/or consumer/commercial use. There are seven types of losses for a substance: (1) discharge to wastewater; (2) emission to air; (3) loss to land; (4) chemical transformation; (5) disposal to landfill; (6) disposal by recycling; and (7) disposal by incineration. These losses are estimated based on regulatory survey data, industry data and data published by different organizations.
To assist in estimating these losses, a spreadsheet (Mass Flow tool) was used to incorporate all data and assumptions required for the estimation (Environment Canada 2010b). Unless specific information on the rate or potential for release of the substance from landfills and incinerators is available, the Mass Flow tool does not quantitatively account for releases to the environment from waste disposal sites.
According to the information received from the section 71 survey and DSL use codes for TIDTE, the substance is a component in ready-to-use adhesive and sealant products. The majority of the substance is expected to cross-link to polymers in formulations and bind to the cured sealant matrix, which is in turn bound to the surfaces to which it is applied. In this form, some (relatively minor) losses will be through discarding of the treated materials to waste disposal sites.
There is possible release of the adhesion matrix to water, due to mechanical separation of the sealant from the treated objects. However, direct release of free TIDTE molecules (i.e. unreacted residual TIDTE) from the sealant matrix to water is expected to be very limited. Releases to the environment by other means are less likely.
For the adhesive and sealant products, in which TIDTE is not yet reacted, a potential release is through disposal of commercial containers with residues to waste disposal sites, although the amount is expected to be minor. Emission to air, loss to land, or disposal by recycling are considered unlikely.
The losses estimated for TIDTE over its life cycle are presented in Table 3 (Environment Canada 2010b). In general, the majority of TIDTE (99%) is expected to undergo chemical transformation via reacting with other components in product formulation, while release of the substance itself to the environment (surface water) is conservatively estimated to be as much as 1%.
Table 3. Estimated losses of TIDTE during its life cycle
| Type of loss | Proportion (%) | Pertinent life cycle stages |
|---|---|---|
| Water | 1 | Consumer/commercial use |
| Air emission | 0 | -- |
| Land | 0 | -- |
| Chemical transformation | 99 | Consumer/commercial use |
| Landfill | < 1% | Consumer/commercial use |
| Recycling | 0 | -- |
| Incineration | 0 | -- |
As is described in more detail in the Environmental Persistence section, TIDTE is not stable in air (photo-oxidation in the gas phase) or water (hydrolysis is expected to occur quickly – in a matter of hours – with the main products being a silanol, methanol, and amines). Based on its physical and chemical properties alone – in particular, low water solubility and high K ow and Koc (Table 2) – TIDTE itself would be expected to predominantly reside in soil or sediment; however, the results of fugacity modelling suggest otherwise. The Level III fugacity model (EQC) has been used to predict the environmental fate of TIDTE, using half-lives (t1/2) in environmental media as t1/2 air = 4 hrs, t1/2 water = 24 hrs, t1/2 soil = 2160 hrs (equivalent to 90 days), and t1/2 sediment = 24 hrs. These results (see Table 4 below) represent the partitioning of the substance in a hypothetical evaluative environment resulting from intermedia partitioning, and loss by both advective transport (out of the modelled region) and degradation/transformation processes. The partitioning values shown in Table 4 represent the net effect of these processes under conditions of continuous release when a non-equilibrium “steady-state” has been achieved.
Table 4. Results of the Level III fugacity modelling (EQC 2003)
| Substance released to: | Percentage of substance partitioning into each compartment | |||
|---|---|---|---|---|
| Air | Water | Soil | Sediment | |
| Air (100%) | 1.2 | 0.1 | 98.7 | 0.01 |
| Water (100%) | < 0.01 | 89.5 | < 0.01 | 10.5 |
| Soil (100%) | < 0.01 | < 0.01 | 100 | < 0.01 |
In the unlikely event of release to air, a very small amount of the substance is expected to reside in this environmental compartment. Based on the negligible vapour pressure of 1.73 × 10-19 Pa (modelled) and Henry’s Law constant of 1.16 × 10-16 Pa·m3/mol (modelled), TIDTE is considered non-volatile. Therefore, if released solely to air, the vast majority of the substance (~99%) will tend to be deposited to soil with only a very small fraction residing in the other environmental media (see Table 4 above).
If released into water (most likely scenario), the rapid hydrolysis of TIDTE is expected to play a very important role in driving the partitioning in environmental media. Even though the substance is characterized with a high estimated log Kocvalue of 4.4, the equilibrium between water and sediment is mainly driven by the rapid degradation of TIDTE in the aquatic environment, and the majority of the substance is expected to end up residing in water. Volatilization from water surfaces is expected to be an unimportant fate process based upon this compound’s estimated Henry’s Law constant. Thus, if water is a receiving medium, TIDTE is expected to mainly reside in water (~90%) and, to some extent (~10%), partition to sediment (see Table 4 above).
In the unlikely event of release to soil, TIDTE is expected to have high adsorptivity to soil surfaces (i.e., expected to be immobile based upon its estimated log Koc). Volatilization from moist soil surfaces is expected to be negligible, based upon its very low Henry’s Law constant (estimated as 1.16 × 10-16 Pa·m3/mol) and high log KOA (estimated as 25.95). This chemical may not volatilize from dry soil surfaces either, based upon its vapour pressure. Therefore, if released to soil, TIDTE will reside entirely in this environmental compartment, which is illustrated by the results of the Level III fugacity modelling (see Table 4 above).
Environmental Persistence
No experimental degradation data for TIDTE (either alone or in adhesive and sealant formulations) have been identified.
Although release of free TIDTE molecules (i.e., not in a formulation) to the environment is unlikely, the potential for degradation of such molecules is considered in this assessment. Predictive QSAR models were used for this purpose as no empirical data were available. Given the nature of the use of the substance in Canada, as well as the ecological importance of the water compartment and the fact that most of the available persistence models apply to water, persistence in water was primarily examined. Table 5 summarizes the results for degradation of TIDTE.
Table 5. Modelled predictions for degradation of TIDTE
| Fate process | Model and model basis | Model result and prediction | Extrapolated half-life (days) |
|---|---|---|---|
| Air | |||
| Atmospheric oxidation | AOPWIN 20081 | t1/2 = 0.17 day | < 2 |
| Ozone reaction | AOPWIN 20081 | n/a2 | n/a |
| Water | |||
| Hydrolysis | HYDROWIN 20081 | ~ 1 day (pH 4-9) |
< 182 |
| Primary biodegradation | |||
| Biodegradation (aerobic) | BIOWIN 20081 Sub-model 4: expert survey (qualitative results) |
3.073 “biodegrades relatively fast” |
< 182 |
| Ultimate biodegradation | |||
| Biodegradation (aerobic) | BIOWIN 20081 Sub-model 3: expert survey (qualitative results) |
1.663 “biodegrades very slowly” |
> 182 |
| Biodegradation (aerobic) | BIOWIN 20081 Sub-model 5: MITI linear probability |
-0.304 “biodegrades very slowly” |
> 182 |
| Biodegradation (aerobic) | BIOWIN 20081 Sub-model 6: MITI non-linear probability |
0.004 “biodegrades very slowly” |
> 182 |
| Biodegradation (aerobic) | TOPKAT 2004 Probability |
0.00 “biodegrades very slowly” |
> 182 |
| Biodegradation (aerobic) | CATABOL c2004–2008 % BOD (biological oxygen demand) |
23.45 “biodegrades slowly” |
> 182 |
2 Model does not provide an estimate for this type of structure.
3 Output is a numerical score from 0 to 5.
4 Output is a probability score.
5 The result is closer to 20% cut-off (“biodegrades slowly”) than the 40% cut-off (“may biodegrade fast”); therefore, the extrapolated half-life is expected to be >182 days.
In air, a predicted atmospheric oxidation half-life value of only 0.17 day (see Table 5) demonstrates that this substance is likely to be rapidly oxidized. The substance is not expected to react with other photo-oxidative species in the atmosphere, such as O 3, nor is it likely to degrade via direct photolysis. Therefore, it is expected that reactions with hydroxyl radicals will be the most important fate process in the dry atmosphere for TIDTE. With a half-life of 0.17 day based on reactions with hydroxyl radicals, the substance is considered not persistent in air according to the criterion (half-life in air ≥ 2 days is considered persistent) as set out in the Persistence and Bioaccumulation Regulations (Canada 2000).
According to the model predictions, it is expected that TIDTE has potential for relatively rapid primary biodegradation, which is consistent with the hydrolysis potential of the substance in the aquatic environment. Indeed, TIDTE is a chemically active compound, containing functional groups of isocyanate and alkoxy silane. As all isocyanates, this substance can hydrolyze quickly in water and produce alkyl amines. Modelling (CATABOL c2004–2008) suggests that the following amines can be produced during the hydrolysis process of TIDTE:
Anime 1

Anime 2

Anime 3

Anime 4
The hydrolysis rate of isocyanates increases and the hydrolysis half-life, therefore, decreases with electron-withdrawing substituents, e.g. 9 minutes (25°C) for methyl isocyanate and 55 seconds (water/dioxane solution) for phenyl isocyanate (HSDB 2007).
The alkoxysilane component of TIDTE can hydrolyze rapidly as well. Hydrolysis of alkoxysilanes is both acid and base-catalyzed (Osterholtz and Pohl 1992; Mill and Tse 1989); however, the rates are slowest near pH=7. Hydrolysis half-lives were examined for a number of different alkoxysilanes, and results indicated times varying from only 0.04 to 504 minutes, in the range of pH 5 to 9 (Mill and Tse 1989). Hydrolysis of methoxysilane in TIDTE is expected to produce methanol and a silanol (as illustrated below).

Due to rapid hydrolysis, the estimated overall half-life of TIDTE is less than 1 day, which is much less than the criterion (half-life in water ≥ 182 days is considered persistent as set out in the Persistence and Bioaccumulation Regulations) (Canada 2000). Therefore, TIDTE is not considered to be persistent in water.
Since TIDTE is also expected to undergo rapid hydrolysis in other environmental compartments containing water – sediment and soils, the half-lives of the substance in these two environmental media are also anticipated to be short (i.e., much less than 365 days and 182 days, respectively). Therefore, TIDTE itself is not expected to be persistent in either sediment or soils.
The results from all ultimate biodegradation models (see Table 5) show that a long-half-life is required to complete mineralization of TIDTE. These predictions only consider the hydrolysis of TIDTE as an intermediate step en route to complete mineralization. Although ultimate degradation was the persistence metric used for categorization, this assessment takes into consideration the properties (including potential for toxicity) of the hydrolysis products of TIDTE. Thus, predictions of ultimate biodegradation were not used to determine the persistence of TIDTE in the environment.
Therefore, based on the above considerations, TIDTE does not meet the persistence criteria in air, water, soil, or sediment as set out in the Persistence and Bioaccumulation Regulations(Canada 2000).
Potential for Bioaccumulation
Since TIDTE is a rapidly hydrolysable substance, its bioaccumulation potential and those of its principal hydrolysis products were considered in this assessment. The reason for predicating the bioaccumulation potential of the parent compound (given its fast hydrolysis) was for the exposures under continuous-release situations (e.g., continuous release of TIDTE to water where the parent compound may be present in small amounts).
Since no experimental bioaccumulation factor (BAF) and/or bioconcentration factor (BCF) data for TIDTE were available, a predictive approach was applied using available BAF and BCF models as shown in Table 6 below. According to the Persistence and Bioaccumulation Regulations (Canada 2000), a substance is bioaccumulative if its BCF or BAF is > 5000; however measures of BAF are the preferred metric for assessing bioaccumulation potential of substances. This is because BCF may not adequately account for the bioaccumulation potential of substances via the diet, which predominates for substances with log Kow> ~4.0 (Arnot and Gobas 2003). Kinetic mass-balance modelling is in principle considered to provide the most reliable prediction method for determining the bioaccumulation potential because it allows for correction for metabolic transformation as long as the log Kow of the substance is within the log Kow domain of the model.
BCF and BAF estimates, corrected for potential biotransformation, were generated using the BCFBAF model (EPIsuite 2008). Metabolic rate constants were derived using structure activity relationships described further in Arnot et al. (2008a,b and 2009). Since metabolic potential can be related to body weight and temperature (Hu and Layton 2001, Nichols et al. 2006), the BCFBAFWIN model further normalizes the kM for a 10g fish at 15 ºC to the body weight of the middle-trophic-level fish in the Arnot-Gobas model (184 g) (Arnot et al. 2008b).
BCF and BAF estimates, corrected for potential biotransformation (BCFBAF 2008), as well as the BCF estimates from the CPOPs (2008) model, vary within 8-10 L/kg (see Table 6), suggesting a low potential for bioaccumulation.
Table 6. Modelled data for bioaccumulation for TIDTE
| Test organism | Endpoint | Value wet weight (L/kg) | Reference |
|---|---|---|---|
| Fish | BAF (middle trophic level) | 9.5 | BCFBAF 2008 |
| Fish | BCF (middle trophic level) | 10.1 | BCFBAF 2008 |
| Fish | BCF | 7.7 | CPOPs 2008 |
Another line of evidence for low bioaccumulation of TIDTE is high molecular size of this substance. Indeed, recent investigations, relating fish BCF data and molecular size parameters (Dimitrov et al. 2002, 2005), suggest that the probability of a molecule crossing cell membranes as a result of passive diffusion declines significantly with increasing maximum diameter (Dmax). The probability of passive diffusion decreases appreciably when the maximum diameter is greater than ~1.5 nm and much more so for molecules having a maximum diameter of greater than 1.7 nm. Sakuratani et al. (2008) have also investigated the effect of cross-sectional diameter on passive diffusion in a BCF test set of about 1200 new and existing chemicals. They observed that substances that do not have a very high bioconcentration potential (BCF < 5000) often have a Dmax of > 2.0 nm and an effective diameter (Deff) > 1.1 nm.
However, as Arnot et al. (2010) have noted there are uncertainties associated with the thresholds proposed by Dimitrov et al.(2002, 2005) and Sakuratani et al. (2008) since the BCF studies used to derive them were not critically evaluated. As Arnot et al. (2010) point out, molecular size influences solubility and diffusivity in water and organic phases (membranes), and larger molecules may have slower uptake rates. However, these same kinetic constraints apply to diffusive routes of chemical elimination (i.e., slow in = slow out). Thus, significant bioaccumulation potential may remain for substances that are subject to slow absorption processes, if they are slowly biotransformed or slowly diluted or eliminated by other processes. Consequently, when evaluating bioaccumulation potential molecular size information should be considered with care, and used together with other relevant lines of evidence in a weight of evidence approach.
The maximum molecular diameter of TIDTE ranges from 1.74 to 3.43 nm (CPOPs 2008), suggesting that a potential for a significantly reduced uptake rate from water and reduced in vivo bioavailability exists for this substance.
Since TIDTE can hydrolyze rapidly in water (half-life << 182 days), the bioaccumulation potentials of the hydrolysis products (silanol and amines) are also considered in the assessment. According to the model results from the Estimation Programs Interface Suite for Microsoft Windows (EPIsuite 2008), silanol is predicted to have a log Kow value of ~1.5 (KOWWIN 2008), and both the BAF and BCF values are estimated to be very low (~2 L/kg for the middletrophiclevel fish) (BCFBAF 2008). For amines (structures are presented in the Environmental Persistence section), log Kow values range within 1.3-2.7, and the BAF and BCF values for the middletrophiclevel fish are estimated to be of below 5 L/kg (BCFBAF 2008).
Therefore, the available evidence indicates that TIDTE is expected to have low bioaccumulation potential due to its physical and chemical properties (e.g., large maximum molecular diameter, low water solubility) and high biotransformation and hydrolysis rates. It is concluded that neither TIDTE nor its hydrolysis products are considered to meet the bioaccumulation criteria (BCF or BAF ≥ 5000) as set out in the Persistence and Bioaccumulation Regulations (Canada 2000).
Ecological Effects Assessment
A – In the Aquatic Compartment
Since TIDTE is a rapidly hydrolysable substance, its ecotoxicological properties as well as those of its principal hydrolysis products were considered in this assessment. The reason for predicating ecotoxicity values of the parent compound (given its fast hydrolysis) was for the exposures under continuous-release situations (e.g., continuous release of TIDTE to water where the parent compound may be present in small amounts).
There are no experimental ecotoxicity data available on TIDTE itself. Therefore, QSAR models have been used to predict its aquatic toxicity. Given the rapid hydrolysis of the substance with methoxysilane forming a silanol and methanol, and isocyanate forming amines, consideration has also been given to the hydrolysis products. QSAR model results for TIDTE are summarized in Table 7 below.
Table 7. Modelled data for aquatic toxicity of TIDTE
| Test organism | Type of test | Endpoint | Value (mg/L) | Reference |
|---|---|---|---|---|
| Fish | Acute (96 hours) | LC501 | 0.064* | ECOSAR 2009 |
| Daphnia magna | Acute (48 hours) | LC50 | 0.064* | |
| Green algae | Acute (96 hours) | EC502 | 0.185* | |
| Fish | Chronic | ChV3 | 0.005* | |
| Daphnia magna | Chronic | ChV | 0.013* | |
| Green algae | Chronic | ChV | 0.155* | |
| Daphnia magna | Acute (48 hours) | EC50 | 0.154* | |
| Daphnia magna | Acute (48 hours) | LC50 | 0.135* | CPOPs 2008 |
| Pimephales promelas | Acute | LC50 | 0.299* |
2 EC50 - The concentration of a substance that is estimated to cause some effect on 50% of the test organisms.
3 ChV – Chronic toxicity value.
* The exposure concentration is at least 10 times higher than the estimated water solubility.
It should be noted that while the aquatic toxicity of TIDTE itself is predicted to be relatively high, the estimated acute and chronic toxicity values (0.005-0.3 mg/L) are much higher than the predicted water solubility of this substance (1.14 × 10-4 mg/L). Consequently, even when aquatic organisms are exposed to saturated solutions of TIDTE, harmful effects are not likely.
The acute toxicity of the silanol (one of the hydrolysis products of TIDTE) is predicted to be much lower: modelled baseline LC50/EC50 values vary from 235 to 1250 mg/L (ECOSAR 2009), which is above silanol’s modelled water solubility value of ~12 mg/L). Chronic baseline toxicity values of 57 to 120 mg/L (ECOSAR 2009) also suggest that toxicity of silanol to aquatic organisms is low.
As methanol is also produced during hydrolysis of methoxysilane, the toxicity of methanol has also been considered in this assessment. The reported EC50s and LC50s of methanol on aquatic species are mostly above the level of 1000 mg/L (HSDB 2006); therefore, methanol is considered to be of low toxicity.
The modelled aquatic acute toxicity values (ECOSAR 2009) of other hydrolysis products – amines (structures are presented in the Environmental Persistence section) were from several mg/L to hundreds mg/L, which suggests that ecotoxicity of these substances is moderate to low.
Therefore, since TIDTE’s water solubility is well below the predicted ecotoxicity values, and the ecotoxicity of TIDTE’s hydrolysis products is not high, it may be concluded that neither TIDTE nor its hydrolysis products have a high potential to cause harm to aquatic organisms.
B – In Other Environmental Compartments
No ecological effects studies were found for this compound in soil or sediment.
Ecological Exposure Assessment
No data concerning concentrations of this substance in any environmental media in Canada have been identified; therefore, potential for environmental exposures has been characterized based on the use quantities, use pattern, features of marine adhesives and sealants, and the environmental behaviours of TIDTE and its principal hydrolysis products.
A – Industrial Release
Based on the section 71 survey, there is no manufacturing of TIDTE in Canada. The substance is imported as a component in adhesive and sealant products. Therefore, industrial release is considered to be negligible.
B – Consumer/Commercial Release
As mentioned in previous sections of this assessment, most of the TIDTE in marine adhesive and sealant formulations is incorporated into urethane polymers. The majority of this substance is expected to undergo chemical transformations in the formulated product matrix. Therefore, it can be assumed that direct release of residual/unreacted free TIDTE molecules from the weather- and salt-water-resistant sealant matrix would be negligible.
Residue of adhesive and sealant in the product containers is assumed to be disposed in landfill sites. However since releases from such sites are expected to be very limited, risks associated with releases from landfill sites are not considered in this screening assessment.
For the other environmental media, release of TIDTE to air or soil is very unlikely. Therefore, wildlife exposure to the substance is anticipated to be negligible.
Characterization of Ecological Risk
The approach taken in this ecological screening assessment was to examine various relevant information and develop conclusions based on a weight-of-evidence approach and using precaution as required under CEPA 1999. Lines of evidence considered include information on persistence, bioaccumulation, toxicity, source and fate of the substance itself, its hydrolysis products, and residual TIDTE present in the formulated product.
TIDTE has been imported into Canada in ready-to-use products. As indicated in the previous sections of this assessment, because of the nature of its uses, as well as its physical and chemical properties, TIDTE is not expected to be released to air or soil in Canada.
Releases of this substance to water are theoretically possible, but they are expected to be very small, as the release of the substance from the matrix of the formulated products and during product application is unlikely. In addition, such releases are expected to be diffuse (spread over wide areas), and the aquatic toxicity of the substance itself and its hydrolysis products is not considered to be high.
In Canada, the quantity of TIDTE in commerce is very low (100 – 1 000 kg/year). The upper limit of the imported quantity of TIDTE – 1 000 kg – can be conservatively used for characterization of ecological risk of this substance. Based on the losses estimated for TIDTE over its life cycle (see Table 3) and chemical transformations in the product matrices, it may conservatively be assumed that 1% of the substance – i.e., only a maximum of 10 kg/year – is released to Canadian environment as a whole. However, the actual imported volume of TIDTE is lower than 1 000 kg/year, and the proportion that is chemically transformed is likely greater than 99%, and as a result, the actual annual release of unreacted/residual TIDTE is likely much less than 10 kg/year.
Because TIDTE has disperse use, and the amount released is likely to be less than 10 kg/year for the entire country, PECs are expected to be extremely low.
Finally, it should also be mentioned that although TIDTE is an easily hydrolysable substance with a very short half-life in water, the predicted environmental concentrations of its hydrolysis products in water are also expected to be extremely low (especially considering the very low release rate of <10 kg/year).
Taking into account all these considerations, it is concluded that the overall ecological risk of TIDTE is expected to be very low.
Uncertainties in Evaluation of Ecological Risk
A main area of uncertainty for the evaluation of TIDTE is associated with a lack of experimental data for physical and chemical properties, persistence, bioaccumulation and inherent toxicity. QSAR models were used to estimate key physical and chemical properties (i.e., water solubility, log K OW, vapour pressure) for characterizing TIDTE. These modelled parameter values were used in further modelling to predict the environmental fate, assess potentials for persistence and bioaccumulation, and evaluate toxicity and environmental risk of the substance. However, given that the functional groups in TIDTE are included in the training sets of the QSAR models, there is sufficient confidence in the model predictions to justify use of the estimated data and model predictions in the assessment.
The predicted concentrations associated with toxicity to aquatic organisms have an additional source of uncertainty in that these concentrations exceed the predicted solubility of the chemical in water. Despite this, the available data indicate that TIDTE is not highly hazardous to aquatic organisms.
There is also limited information on regional use pattern of the marine adhesive and sealant products (containing TIDTE). There is no information reporting the quantity of the products that have been applied above or under the water line. Furthermore, there is little information related to the waste disposal of the product containers. However, given the small quantity of the substance in commerce in Canada, these limitations are unlikely to have a significant influence on the overall conclusion of the assessment.
Exposure Assessment
Environmental Media
Empirical data on concentrations of TIDTE in environmental media in Canada were not identified. TIDTE is not expected to be found in food or beverages in Canada. Environmental concentrations were estimated using the loss percentages predicted by the Mass Flow tool (see Table 3) (Environment Canada 2010b). The percentages were applied to the total quantity of TIDTE in Canadian commerce in 2006. The total quantity in commerce was conservatively assumed to be up to 1 000 kg (Environment Canada 2010a). The loss quantity is estimated as 10 kg per year to surface water.
The estimated losses were used in ChemCAN, a Canada-specific environmental exposure model, to estimate concentrations in various environmental media (ChemCAN 2003). This model differs from the point source models used in the ecological assessment section of the document, which provide estimates of exposure near release points, in that it is a regional far-field level III fugacity model that is used to estimate average concentrations in various media to inform human exposure estimates. The predicted environmental concentrations are presented in Appendix 2 and were used to derive intake estimates. Conservative upper-bounding daily intakes of TIDTE for the general population in Canada were derived based on the estimated environmental concentrations, resulting in negligible exposure on the order of nanograms (10-9 g) per kg-bw (kilogram of body weight) per day.
Consumer/Commercial Products
In Canada, TIDTE is present in a marine adhesive sealant at a concentration of 1 to 2 % by weight. The Material Safety Data Sheet (MSDS) for this product identifies potential health effects including sensitization, as well as related first aid measures (3M Canada Company 2006). Marine adhesive sealant is a polyurethane substrate that cures in the presence of moisture within 24 hours of application (3M Marine 2006) forming a watertight seal above or below the waterline of boats. This substrate may be applied to a variety of joints and interfaces such as portholes, deck fittings, moldings and trunk joints (3M Marine 2006). Application procedure involves loading the adhesive sealant in a caulk gun, cutting the tip to the desired bead size and applying to the desired seal or part.
In terms of exposure from use of consumer products, due to the negligible vapour pressure of TIDTE, inhalation exposure of any TIDTE volatilized from the adhesive sealant is anticipated to be negligible. As the log KOW of TIDTE is high, there is potential for dermal absorption upon contact with the product. During the application procedure, some individuals may smooth the substrate with their finger. Using the ConsExpo v4.1 default assumptions for a joint sealant scenario to approximate a boat joint sealant scenario, exposure was estimated to be low at 2.06 µg/kg-bw per event (Appendix 3).
Health Effects Assessment
TIDTE is an alkyl isocyanate-silane compound. No empirical health effects data for TIDTE were identified. Structural analogues of TIDTE were determined using the SciFinder and ChemID websites. However, no empirical toxicity data were identified for these TIDTE analogues.
The European Commission has classified TIDTE as a R42/R43 substance – May cause sensitization by inhalation and skin contact (ECB 1991). Although the basis for this classification was not identified (a European Commission summary record for TIDTE was not located), it is likely based on the isocyanate functional group in TIDTE as isocyanate is a known skin and respiratory sensitizer (CDC 2005).
Experimental data regarding the physical and chemical properties of TIDTE were not available. Several models estimated low solubility across a wide range of pH conditions for TIDTE (Table 8). In addition, WSKOWWIN (2008) predicted a water solubility of 1.14 ´ 10-4 mg/L at 25 °C for TIDTE.
Table 8. Estimation of solubility under different pH conditions for TIDTE
| pH | ADME Boxes model Value |
ACD model Value (25 °C) |
|---|---|---|
| 1.7 (Stomach) | 1.11 ´ 10-4 mg/L | 0.11 mg/L |
| 4.6 (Duodenum) | 1.11 ´ 10-4 mg/L | 0.11 mg/L |
| 6.5 (Jejunum and Ileum) | 1.11 ´ 10-4 mg/L | 0.11 mg/L |
| 7.4 (Blood) | 1.11 ´ 10-4 mg/L | 0.11 mg/L |
| 8.0 (Colon) | 1.11 ´ 10-4 mg/L | 0.11 mg/L |
Although different water solubility values were predicted by the two models, an overall low solubility (i.e., < 0.5 mg/L) across the full pH range of biological fluids for TIDTE was consistently estimated by these models. TIDTE is a chemically active compound, containing functional groups of isocyanate and alkoxy silane, which can react with water/moisture and rapidly undergo hydrolysis. The major primary hydrolysis product of TIDTE (Figure 1) is more soluble than the parent compound with a modelled solubility of 11.81 mg/L at 25 °C (WSKOWWIN 2008).

Figure 1. Structure of the primary hydrolysis product of TIDTE
Qualitative/Quantitative structure – activity relationship model (QSAR) – based toxicity prediction for TIDTE and its primary hydrolysis product are summarized in Appendix 4. Plausible mutagenicity (based on isocyanate or isothiocyanate and thiocarbamate functional groups), chromosome damage (based on isocyanate or isothiocyanate functional groups), hepatotoxicity (based on organosilicon compound), skin, eye and respiratory irritation (based on isocyanate functional group) and skin and respiratory sensitization (based on isocyanate functional group) of TIDTE were predicted by DEREK (DEREK for Windows_12.0.0 (DEREK 2009)); and positive gene mutation in Drosophila was predicted by Leadscope (Leadscope FDA Model Applier version 1.3.2 (Leadscope Model Applier 2010)), and plausible mutagenicity (based on thiocarbamate functional group) and hepatotoxicity (based on organosilicon compound) were predicted for the primary hydrolysis product of TIDTE by DEREK. Other quantitative structure – activity relationship models could not predict toxicity for TIDTE or its primary hydrolysis product as these compounds were outside their model database (TOPKAT version 6.2 (TOPKAT 2004)), or their structures contain silicon, for which the model (CASETOX version 2.1 (CASETOX 2009)) could not recognize the structure properly.
Based on the low solubility and high reactivity nature of TIDTE and the QSAR prediction results, the potential health effects exerted by TIDTE parent compound would be expected to be skin and mucosal membrane irritation and/or sensitization, owing to the isocyanate functional group in TIDTE. After hydrolysis, the hydrolysis products become slightly soluble and less reactive. Such compounds can be further hydrolyzed/metabolized if the primary hydrolysis product is subject to conditions that may favour physiological processes. In addition, cross-links can be formed between the silanol groups in the hydrolysis compounds and generate siloxane polymers, which are also considered to be of low bioavailability. Although a qualitative SAR model, DEREK, detected a mutagenicity structure alert due to the thiocarbamate in the TIDTE hydrolysis product, there is no metabolism information to determine the relative importance of TIDTE or its primary hydrolysis product.
Methanol is also a primary hydrolysis product of TIDTE.
The confidence for the health effects assessment is considered low as no empirical health effects or physical-chemical data were identified. Available information only allows qualitative predictions of the potential genotoxicity and skin, eye and respiratory tract irritation and/or sensitization potential for this compound.
Characterization of Risk to Human Health
Based on the low solubility and high reactivity nature of TIDTE, and qualitative/quantitative structure-activity relationship (QSAR) model predictions, the primary health effects associated with TIDTE exposure are expected to be skin, eye and respiratory tract irritation and/or sensitization. The European Commission also classified TIDTE as a potential skin and inhalation sensitizer (ECB 1991).
Hydrolysis products of TIDTE can become more soluble and less reactive and can either be further hydrolyzed/metabolized or form polymers by cross-linking. However, no metabolism data were available to determine the relative importance of TIDTE or its primary hydrolysis products under physiological conditions. Although a qualitative SAR model detected a weak mutagenicity structure alert due to the thiocarbamate in the TIDTE hydrolysis product, the relevance to human exposures is uncertain.
Exposure to TIDTE from environmental media was estimated to be on the order of magnitude of nanograms (10-9 g) per kg-bw (kilogram of body weight) per day. General population exposure to TIDTE from use of consumer products, namely marine adhesive sealant, is expected to be low due to the infrequent and specialized use. Thus, exposure of the general population in Canada is expected to be low to negligible and the risk to human health is considered to be low.
Consumer use of products containing TIDTE is expected to be low. For products that fall under the Controlled Products Regulations of the Hazardous Products Act, potential health effects, including sensitization, would be identified on the Material Safety Data Sheet.
Uncertainties in Evaluation of Risk to Human Health
Uncertainty in the exposure characterization is high in the absence of empirical data. However confidence is high that the exposure to TIDTE from environmental sources is negligible. Data in the literature were not identified for concentrations of this substance in environmental media. However, quantities in commerce for the calendar year of 2006 are known and were combined with estimated loss percentages from the Mass Flow tool to model environmental concentrations. As the maximum values of the quantity in commerce ranges were used in the modeling, it is likely that the modeled results are conservative estimates of environmental exposure. Confidence in the consumer product exposure estimate is moderate. The lack of experimental physical and chemical properties for inputs in the exposure model, ConsExpo v4.1, reduced confidence in the exposure estimate.
Due to the absence of experimental health effects data and use of modelling, confidence in the determination of critical health effects is low. Although the characterization of risk is based on the parent compound and its hydrolysis products, the relative exposure to and prevalence of these compounds in mammalian systems is not known.
Based on the information available, it is concluded that TIDTE is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends. Additionally, TIDTE does not meet the criteria for persistence or bioaccumulation as set out in the Persistence and Bioaccumulation Regulations (Canada 2000).
Based on the information available, it is concluded that TIDTE is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore concluded that TIDTE does not meet any criteria as set out in section 64 of CEPA 1999.
This substance will be considered for inclusion in the Domestic Substances List inventory update initiative. In addition and where relevant, research and monitoring will support verification of assumptions used during the screening assessment.
3M Canada Company. 2006. Material Safety Data Sheet: 3M™ Marine Adhesive Sealant Fast Cure 5200 – White; PN 06520, 05220. 3M Canada Company: London (ON). [issue date: 2006 Mar 13]. Available from: http://www.compositescanada.com/pictures/brochure/629.pdf?tt=1262186318
3M Marine. 2006. Technical Data Sheet: 3M™ Marine Adhesive/Sealant Fast Cure 5200. Saint Paul (MN): 3M Marine. [date effective: 2006 Jan 4]. [cited 2010 Feb 1]. Available from: http://multimedia.3m.com/mws/mediawebserver?66666UuZjcFSLXTtlx&EoXM6EVuQEcuZgVs6EVs6E666666--
ACD/pKaDB [Prediction Module]. 2008. Version 11. Toronto (ON): Advanced Chemistry Development. [cited 2010 March]. Available from: http://www.acdlabs.com/products/phys_chem_lab/pka/
ADME Boxes [Prediction Module]. Pharma Algorithms. Copyright valid from 2001– 2008. Available from ADME ToxWEB: http://www.pharma-algorithms.com/webboxes/
[AOPWIN] Atmospheric Oxidation Program for Windows [Estimation Model].2008. Version 1.92a. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: http://www.epa.gov/oppt/exposure/pubs/episuite.htm
Arnot JA, Arnot M, Mackay D, Couillard Y, MacDonald D, Bonnell M, Doyle P. 2010. Molecular size cut-off criteria for screening bioaccumulation potential: Fact or fiction? Integrated Environmental Assessment and Management 6(2):210–224.
Arnot JA, Meylan W, Tunkel J, Howard PH, Mackay D, Bonnell M, Boethling RS. 2009. A quantitative structure-activity relationship for predicting metabolic biotransformation rates for organic chemicals in fish. Environ Toxicol Chem 28(6): 1168-1177.
Arnot JA, Mackay D, Bonnell M. 2008a. Estimating metabolic biotransformation rates in fish from laboratory data. Environ Toxicol Chem 27(2):341–351.
Arnot JA, Mackay D, Parkerton TF, Bonnell M. 2008b. A database of fish biotransformation rates for organic chemicals. Environ Toxicol Chem 27(11): 2263-2270.
Arnot JA, Gobas FAPC. 2003. A generic QSAR for assessing the bioaccumulation potential of organic chemicals in aquatic food webs. QSAR Comb Sci 22(3):337–345.
[BCFBAF] BioConcentration Factor Program for Windows [Estimation Model]. 2008. Version 3.00. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: http://www.epa.gov/oppt/exposure/pubs/episuite.htm
[BIOWIN] Biodegradation Probability Program for Windows [Estimation Model]. 2008. Version 4.10. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: http://www.epa.gov/oppt/exposure/pubs/episuite.htm
Boethling RS, Howard PH, Beauman JA, Larosche ME. 1995. Factors for intermedia extrapolations in biodegradability assessment. Chemosphere 30(4):741-752.
Canada. 1978. Food and Drug Regulations, C.R.C., c. 870. Available from: http://laws.justice.gc.ca/en/showdoc/cr/C.R.C.-c.870///en?page=1?
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C., 1999, c. 33. Canada Gazette. Part III, vol. 22, no. 3. Available from: http://www.gazette.gc.ca/archives/p3/1999/g3-02203.pdf
Canada. 2000. Canadian Environmental Protection Act, 1999: Persistence and Bioaccumulation Regulations, P.C. 2000-348, 23 March 2000, SOR/2000-107. Canada Gazette. Part II, vol. 134, no. 7, p. 607-612. Available from: http://www.gazette.gc.ca/archives/p2/2000/2000-03-29/pdf/g2-13407.pdf
Canada, Dept. of the Environment, Dept. of Health. 2006. Canadian Environmental Protection Act, 1999: Notice of intent to develop and implement measures to assess and manage the risks posed by certain substances to the health of Canadians and their environment. Canada Gazette. Part I, vol. 140, no. 49, p. 4109–4117. Available from: http://www.gazette.gc.ca/archives/p1/2006/2006-12-09/pdf/g1-14049.pdf
Canada, Dept. of the Environment, Dept. of Health. 2009. Canadian Environmental Protection Act, 1999: Notice of tenth release of technical information relevant to substances identified in the Challenge. Canada Gazette. Part I, vol. 143, no. 25, p. 1796–1812. Available from: http://gazette.gc.ca/rp-pr/p1/2009/2009-06-20/pdf/g1-14325.pdf
CASETOX [Prediction Module]. 2009. Version 2.1. Beachwood (OH): MultiCASE. [cited 2009 Dec 16]. Available from: http://www.multicase.com/products/prod03.htm [restricted access]
[CATABOL] Probabilistic assessment of biodegradability and metabolic pathways [Computer Model]. c2004-2008. Version 5.10.2. Bourgas (BG): Bourgas Prof. Assen Zlatarov University, Laboratory of Mathematical Chemistry. Available from:
[CDC] Centres for Disease Control and Prevention (US). 2005. NIOSH health hazard evaluation report. HETA #2004-0349-2970, Kewaunee Fabrications, LLC. Kewaunee (WI), Cincinnati (OH): CDC, National Institute for Occupational Safety and Health Publications Office. [cited 2010 April 09]. Available from: http://www.cdc.gov/niosh/hhe/reports
ChemCAN [Level III fugacity model of 24 regions of Canada]. 2003. Version 6.00. Peterborough (ON): Trent University, Canadian Centre for Environmental Modelling and Chemistry. [cited 2010 Feb 15]. Available from: http://www.trentu.ca/academic/aminss/envmodel/models/CC600.html
[CNS] Cosmetic Notification System [Proprietary Database]. 2010. Available from Health Canada, Cosmetics Division.
[CPOPs] Canadian POPs Model. 2008. Gatineau (QC): Environment Canada, Ecological Assessment Division; Bourgas (BG): Bourgas Prof. Assen Zlatarov University, Laboratory of Mathematical Chemistry. [Model developed based on Mekenyan et al. 2005]. Available from: Environment Canada, Ecological Assessment Division.
[DEREK] Deductive Estimation of Risk from Existing Knowledge [Prediction Module]. 2009. Version 12.0. Lhasa Limited, Leeds, UK. Available from: https://www.lhasalimited.org/derek/ [restricted access].
[DERMWIN] Dermal Permeability Coefficient Program [Estimation Model]. 2000. Version 1.43a. Washington (DC): US Environmental Protection Agency. [cited 2010 Feb 2]. Available from: http://www.epa.gov/oppt/exposure/pubs/episuite.htm
Dimitrov S, Dimitrova N, Walker J, Veith G, Mekenyan O. 2002. Predicting bioconcentration potential of highly hydrophobic chemicals. Effect of molecular size. Pure Appl Chem 74(10): 1823-1830.
Dimitrov S, Dimitrova N, Parkerton T, Comber M, Bonnell M, Mekenyan O. 2005. Base-line model for identifying the bioaccumulation potential of chemicals. SAR QSAR Environ Res 16(6): 531–554.
[ECB] European Chemicals Bureau. 1991. Commission Directive 91/326/EEC of 5 March 1991 adapting to technical progress for the thirteenth time Council Directive 67/548/EEC on the approximation of laws, regulations and administrative provisions relating to the classification, packaging and labelling of dangerous substances. Directive 91/326/EEC (ATP13).
[ECOSAR] Ecological Structural Activity Relationships Class Program for Windows [Internet]. 2009. Version 1.00. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: http://www.epa.gov/oppt/exposure/pubs/episuite.htm
Environment Canada. 2010a. Data for Batch 10 substances collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain Batch 10 Challenge substances.Data compiled by: Environment Canada, Program Development and Engagement Division.
Environment Canada. 2010b. Assumptions, limitations and uncertainties of the Mass Flow Tool for TIDTE, CAS RN 85702-90-5. Internal draft document. Gatineau (QC): Environment Canada, Ecological Assessment Division.
[EPIsuite] Estimation Programs Interface Suite for Microsoft Windows [Estimation Model]. 2008. Version 4.0. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: www.epa.gov/oppt/exposure/pubs/episuitedl.htm
[EQC] Equilibrium Criterion Model. 2003. Version 2.02. Peterborough (ON): Trent University, Canadian Environmental Modelling Centre. Available from: http://www.trentu.ca/academic/aminss/envmodel/models/EQC2.html
Health Canada. 1998. Exposure factors for assessing total daily intake of priority substances by the general population of Canada. Unpublished report. Ottawa (ON): Health Canada, Environmental Health Directorate.
Health Canada. 2007. Pest Management Regulatory Agency (PMRA) Regulatory Note REG 2007-04: PMRA list of formulants [Internet]. Ottawa (ON): Health Canada, Pest Management Regulatory Agency. [cited 2010 Mar 1]. Available from: http://www.hc-sc.gc.ca/cps-spc/pubs/pest/_decisions/reg2007-04/index-eng.php
Health Canada. 2009. The Cosmetic Ingredient Hotlist – September 2009 [Internet]. Ottawa (ON): Health Canada, Consumer Product Safety. [cited 2010 Mar 1]. Available from: http://www.hc-sc.gc.ca/cps-spc/person/cosmet/info-ind-prof/_hot-list-critique/hotlist-liste-eng.php
[HENRYWIN] Henry’s Law Constant Program for Microsoft Windows [Estimation Model]. 2008. Version 3.20. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: www.epa.gov/oppt/exposure/pubs/episuite.htm
[HSDB] Hazardous Substances Data Bank [database on the Internet]. 2006. Methanol. US National Library of Medicine. [cited 2010 February]. Available from: http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB
[HSDB] Hazardous Substances Data Bank [database on the Internet]. 2007. Hydrolysis of isocyanates. US National Library of Medicine. [quoted from EPIsuite 2008]. [cited 2010 February]. Available from: http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB
Hu TM, Layton WL. 2001. Allometric scaling of xenobiotic clearance: uncertainty versus universality. AAPS PharmSci [Internet]. (October 2010). Vol. 3(4): Article 29. Available from: http://www.aapsj.org/view.asp?art=ps030429.
[HYDROWIN] Hydrolysis Rates Program for Microsoft Windows [Estimation Model]. 2008. Version 2.00. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: www.epa.gov/oppt/exposure/pubs/episuite.htm
Industry Canada. 2008. Recreational Boats and Equipment. Available from: www.ic.gc.ca/eic/site/rb-bp.nsf/eng/home. Date Modified: 2008-11-05. Access date: October 12, 2010.
[KOAWIN] Octanol Air Partition Coefficient Program for Microsoft Windows [Estimation Model]. 2008. Version 1.10. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: www.epa.gov/oppt/exposure/pubs/episuite.htm
[KOCWIN] The Soil Adsorption Coefficient Program [Estimation Model]. 2008. Version 2.00. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: www.epa.gov/oppt/exposure/pubs/episuite.htm
[KOWWIN] Octanol-Water Partition Coefficient Program for Microsoft Windows [Estimation Model]. 2008. Version 1.67. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: www.epa.gov/oppt/exposure/pubs/episuite.htm
Mekenyan G, Dimitrov SD, Pavlov TS, Veith GD. 2005. POPs: A QSAR system for creating PBT profiles of chemicals and their metabolites. SAR QSAR Environ Res 16(1–2):103-133.
Mill T, Tse D. 1989. Environmental fate and exposure studies: Development of a SAR for hydrolysis of alkoxysilanes. SRI International, Draft Summary Report: Task 243, EPA Contract No. 68-02-4254. Menlo Park (CA): SRI International.
[MPBPWIN] Melting Point Boiling Point Program for Microsoft Windows [Estimation Model]. 2008. Version 1.43. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: www.epa.gov/oppt/exposure/pubs/episuite.htm
[NCI] National Chemical Inventories [database on CD-ROM]. 2007. Issue 1. Columbus (OH): American Chemical Society. [cited 2007 Dec 11]. Available from: http://www.cas.org/products/cd/nci/index.html
Nichols JW, Fitzsimmons PN, Burkhard LP. 2007. In vitro – in vivo extrapolation of quantitative hepatic biotransformation data for fish. II. Modeled effects on chemical bioaccumulation. Environ Toxicol Chem 26:1304-1319.
Osterholtz FD, Pohl ER. 1992. Kinetics of the hydrolysis and condensation of organofunctional alkoxysilanes: A review. J Adhes Sci Technol 6:127–49.
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu. 2007. Do-it-yourself products fact sheet: To assess the risks for the consumer [Internet]. [cited 2010 Feb 2]. Bilthoven (NL): RIVM (National Institute for Public Health and the Environment). RIVM Report 320104007/2007. Available from: http://www.rivm.nl/bibliotheek/rapporten/320104007.pdf
Sakuratani Y, Noguchi Y, Kobayashi K, Yamada J, Nishihara T. 2008. Molecular size as a limiting characteristic for bioconcentration in fish. J Environ Biol 29(1):89-92.
[TOPKAT] Toxicity Prediction by Komputer Assisted Technology [Internet]. 2004. Version 6.2. San Diego (CA): Accelrys Software Inc. Available from: http://www.accelrys.com/products/topkat/index.html
[US EPA] United States Environmental Protection Agency. 1986-2002. Toxic Substances Control Act: Inventory update reporting (TSCA-IUR). Non-confidential production volume information for 1986, 1990, 1994, 1998 and 2002 reporting cycles [CD-ROM]. Washington (DC): US Environmental Protection Agency.
[WSKOWWIN] Water Solubility for Organic Compounds Program for Microsoft Windows [Estimation Model]. 2008. Version 1.41. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: www.epa.gov/oppt/exposure/pubs/episuite.htm
Model Inputs Summary Table
TIDTE has few experimental data for physical and chemical properties. Modelled predictions from the Estimation Programs Interface Suite for Microsoft Windows (EPIsuite) on key parameters have been used in furthering modelling.
The Simplified Molecular Input Line Entry System (SMILES) of TIDTE has been used in modelling as follows:
O=C(N(C(=O)NCCCCCCNC(=O)SCCCSi](OC)(OC)OC)CCCCCCN=C(=O))NCCCCCCN=C=O
Given that TIDTE can hydrolyze rapidly in water, including moisture in other environmental media, the immediate hydrolysis product has been included in the assessment. The SMILES of the immediate hydrolysis product has been used in modelling as follows: O=C(N(C(=O)NCCCCCCNC(=O)SCCC[Si](O)(O)O)CCCCCCN)NCCCCCCN
| Physical and chemical fate | Fate | PBT profiling | Ecotoxicity | |
|---|---|---|---|---|
| Model input parameters | EPIWIN Suite (including: AOPWIN, KOWWIN, KOCWIN, BCFBAF BIOWIN and ECOSAR) | EQC (Type I chemical) | Canadian–POPs (including: CATABOL) |
TOPKAT |
| SMILES code of TIDTE | x | x | x | x |
| SMILES code of the hydrolysis product | x | x | ||
| Molecular weight (g/mol) | 674.93 | |||
| Melting point (ºC) | 349.84 | |||
| Data temperature (ºC) | 25 | |||
| Vapour pressure (Pa) | 1 × 10-11 | |||
| Log Kow (Octanol-water partition coefficient) (dimensionless) |
6.62 | |||
| Water solubility (mg/L) | 1.14 × 10-4 | |||
| Half-life in air1 (days) | 0.17 | |||
| Half-life in water (days) | 1 | |||
| Half-life in sediment2 (days) | 45 | |||
| Half-life in soil3 (days) | 1 |
2 Estimated based on primary biodegradation rate (BIOWIN 2008 sub-model 3).
3 Estimated to have the same hydrolysis rate as in water.
Appendix 2: Estimated concentrations of TIDTE in environmental media using ChemCAN version 6.00 (ChemCAN 2003)1
| Medium2 | Estimated concentration |
|---|---|
| Ambient air3 | 0.319 ng/m3 |
| Surface water4 | 0.0213 ng/L |
| Soil4 | 2.93 x 10-3 ng/g solids |
| Sediment4 | 7.89 x 10-4 ng/g solids |
2 Default inflow concentrations of 2 ng/m3 in air and 3 ng/L in water were specified by ChemCAN.
3 The oxidative degradation half-life in air was assumed to be 0.17 days (AOPWIN 2008).
4 The hydrolytic degradation half-life in the aquatic compartment was assumed to be 1 day (HYDROWIN 2008). As TIDTE is also expected to hydrolyze in sediment and soil, a 1 day half-life was also used to approximate the degradation rate in sediment and soil.
| Assumptions | Calculations | Exposure estimate |
|---|---|---|
Kp (permeability coefficient): 0.0073 cm/hr (DERMWIN 2000) SA (surface area of fingertip): 2 cm2 (RIVM 2007) WF (maximum weight fraction of TIDTE): 0.02 (3M Canada Company 2006) ρ (density of substrate): 1 g/cm3 (RIVM 2007) ED (exposure duration): 0.5 h (RIVM 2007) Cf (conversion factor): 1 × 106 µg / 1 g BW (adult body weight): 70.9 kg-bw (Health Canada 1998) |
Dermal intake: = (Kp)(SA)(ED)(WF)(ρ)(Cf) / (BW) = (0.0073 cm/hr)(2 cm2)(0.5 hr)(0.02)(1 g/cm3)(1 × 106 µg / 1 g) / (70.9 kg-bw) = 2.06 µg/kg-bw per event |
Acute dermal dose: 2.06 µg/kg-bw per event |
(Q)SAR predictions on carcinogenicity for TIDTE and its primary hydrolysis product
| Model/ Species | Mice | Rat | Rat | Mice | Rodent | Mammal | ||
|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | |||||
| Model Applier | ND | ND | ND | ND | ND | ND | ND | - |
| Multicase CASETOX | NA | NA | NA | NA | NA | NA | NA | - |
| TOPKAT | ND | ND | ND | ND | - | - | - | - |
| DEREK | - | - | - | - | - | - | - | NR |
NA – not applicable
‘-‘ no model available in QSAR suite
NR – no result
(Q)SAR predictions on genotoxicity for TIDTE
| Model/ endpoints |
chrom. ab. | chrom. ab. other rodent | chrom. ab. rat | micro- nucleus mice |
micro- nucleus rodent |
droso- phila |
droso- phila HT |
droso- phila SLRL |
mam. mutation | mam. mutation DL | UDS |
|---|---|---|---|---|---|---|---|---|---|---|---|
| MA | ND | ND | ND | ND | N | P | ND | P | ND | ND | ND |
| CT | NA | - | - | NA | - | NA | - | - | - | - | NA |
| TK | - | - | - | - | - | - | - | - | - | - | - |
| DEREK | P | P | P | - | - | - | - | - | NR | - | - |
CT - Multicase Casetox
TK – Topkat
ND – not in domain (model indicates query chemical to be outside of its applicability domain)
NA – not applicable
‘-‘ no model available in QSAR suite
NR – no result
P – Positive
(Continuation of previous table)
| Model/ endpoints |
UDS human lympho- cytes |
UDS rat hepa- tocytes |
mouse lymphoma mut | s. cere- visiae |
yeast | hgprt | e. coli | e. coli w | Microbial/ bacteria |
salmo- nella |
|---|---|---|---|---|---|---|---|---|---|---|
| MA | ND | ND | ND | N | N | ND | ND | ND | ND | ND |
| CT | - | - | NA | - | - | - | - | - | - | NA |
| TK | - | - | - | - | - | - | - | - | - | ND |
| DEREK | - | - | - | - | - | - | P | P | P | P |
CT - Multicase Casetox
TK – Topkat
ND – not in domain (model indicates query chemical to be outside of its applicability domain)
NA – not applicable
‘-‘ no model available in QSAR suite
NR – no result
P – Positive
(Q)SAR predictions on genotoxicity for TIDTE primary hydrolysis product
| Model/ endpoints |
chrom. ab. | chrom. ab. other rodent | chrom. ab. rat | micro- nucleus mice |
micro- nucleus rodent |
droso- phila |
droso- phila HT |
droso- phila SLRL |
mam. mutation | mam. mutation DL |
|---|---|---|---|---|---|---|---|---|---|---|
| CT | NA | - | - | NA | - | NA | - | - | - | - |
| TK | - | - | - | - | - | - | - | - | - | - |
| DEREK | NR | NR | NR | - | - | - | - | - | NR | - |
TK – Topkat
NA – not applicable
ND – not in domain (model indicates query chemical to be outside of its applicability domain)
‘-‘ no model available in QSAR suite
NR – no result
P – Positive
(Continuation of previous table)
| Model/ endpoints |
UDS | UDS human lympho- cytes |
UDS rat hepa- tocytes |
mouse lymphoma mut | s. cere- visiae |
yeast | hgprt | e. coli | e. coli w | Microbial/ bacteria |
salmo- nella |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CT | NA | - | - | NA | - | - | - | - | - | - | NA |
| TK | - | - | - | - | - | - | - | - | - | - | ND |
| DEREK | - | - | - | - | - | - | - | P | P | P | P |
TK – Topkat
NA – not applicable
ND – not in domain (model indicates query chemical to be outside of its applicability domain)
‘-‘ no model available in QSAR suite
NR – no result
P – Positive
(Q)SAR predictions on developmental toxicity for TIDTE
Model Applier
| Endpoint/ Species | Mice | Rabbit | Rat | Rodent |
|---|---|---|---|---|
| Retardation | ND | ND | ND | ND |
| Weight decrease | ND | ND | ND | ND |
| Fetal death | ND | ND | ND | ND |
| Post impl. loss | ND | ND | ND | ND |
| Pre impl. loss | ND | ND | ND | ND |
| Structural | ND | ND | ND | ND |
| Visceral | ND | - | ND | ND |
‘-‘ no model available in QSAR suite
Impl. – Implantation
Multicase Casetox
| Endpoint/Species | Hamster | Mammal | Miscellaneous |
|---|---|---|---|
| Teratogenicity | - | NA | NA |
| Developmental | NA | - | - |
NA – not applicable
(Q)SAR predictions on reproductive toxicity for TIDTE
Model Applier
| Model/ endpoint | Female | Male | ||||
|---|---|---|---|---|---|---|
| Species | mice | rat | rodent | mice | rat | rodent |
| repro | ND | ND | ND | ND | ND | ND |
| sperm | - | - | - | ND | ND | ND |
‘-‘ no model available in QSAR suite
Repro - Reproductive toxicity
Multicase Casetox
| mice | rat | rabbit | human |
|---|---|---|---|
| NA – not applicable | |||
| NA | NA | NA | NA |
(Q)SAR predictions on sensitization for TIDTE
| Model/ endpoints |
RS - dog | RS – guinea pig | RS - hamster | RS - human | RS - mammal | RS - monkey | RS - mouse | RS - primate | RS - rabbit | RS - rat | RS - rodent |
|---|---|---|---|---|---|---|---|---|---|---|---|
| DEREK | P | P | P | P | P | P | P | P | P | P | P |
(Continuation of previous table)
| Model/ endpoints |
SS - dog | SS – guinea pig | SS - hamster | SS - human | SS - mammal | SS - monkey | SS - mouse | SS - primate | SS - rabbit | SS - rat | SS - rodent |
|---|---|---|---|---|---|---|---|---|---|---|---|
| DEREK | P | P | P | P | P | P | P | P | P | P | P |
(Q)SAR predictions on sensitization for TIDTE primary hydrolysis product
| Model/endpoints | RS - dog | RS – guinea pig | RS - hamster | RS - human | RS - mammal | RS - monkey | RS - mouse | RS - primate | RS - rabbit | RS - rat | RS - rodent |
|---|---|---|---|---|---|---|---|---|---|---|---|
| DEREK | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
(Continuation of previous table)
| Model/endpoints | SS - dog | SS – guinea pig | SS - hamster | SS - human | SS - mammal | SS - monkey | SS - mouse | SS - primate | SS - rabbit | SS - rat | SS - rodent |
|---|---|---|---|---|---|---|---|---|---|---|---|
| DEREK | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
Footnotes
[2] A determination of whether one or more of the criteria of section 64 are met is based upon an assessment of potential risks to the environment and/or to human health associated with exposures in the general environment. For humans, this includes, but is not limited to, exposures from ambient and indoor air, drinking water, foodstuffs, and the use of consumer products. A conclusion under CEPA 1999 on the substances in the Chemicals Management Plan (CMP) Challenge Batches 1-12 is not relevant to, nor does it preclude, an assessment against the hazard criteria specified in the Controlled Products Regulations, which is part of regulatory framework for the Workplace Hazardous Materials Information System [WHMIS] for products intended for workplace use. Similarily, a conclusion based on the criteria contained in section 64 of CEPA 1999 does not preclude actions being taken under other sections of CEPA or other Acts.








