Screening Assessment for the Challenge
This page has been archived on the Web
Information identified as archived is provided for reference, research or recordkeeping purposes. It is not subject to the Government of Canada Web Standards and has not been altered or updated since it was archived. Please contact us to request a format other than those available.
Archived
Naphthalene
Chemical Abstracts Service Registry Number
91-20-3
Environment Canada
Health Canada
July 2008
Table of Contents
- Synopsis
- Introduction
- Substance Identity
- Physical and Chemical Properties
- Sources
- Uses
- Releases to the Environment
- Persistence and Bioaccumulation Potential
- Environmental Fate
- Potential to Cause Ecological Harm
- Potential to Cause Harm to Human Health
- Conclusions
- References
- Appendix 1: Upper-bounding estimates of daily intake of naphthalene for the general population in Canada
- Appendix 2: Summary of health effects information for naphthalene
- Appendix 3: Overview of reported human health effects of naphthalene
The Ministers of the Environment and of Health have conducted a screening assessment of naphthalene, Chemical Abstracts Service Registry Number (CAS RN) 91-20-3, a substance identified in the categorization of the Domestic Substances List as a high priority for action under the Ministerial Challenge. Naphthalene was identified as a high priority as it was considered to pose greatest potential for exposure to individuals in Canada (GPE) and had been classified by other agencies on the basis of carcinogenicity. While the substance did meet the ecological categorization criteria for inherent toxicity to aquatic organisms, it did not meet the criteria for persistence or bioaccumulation. Therefore, the focus of this assessment of naphthalene relates to human health aspects.
Based on a survey conducted under Section 71 of CEPA 1999, in 2000 Canadian companies reported manufacturing more than 52 000 000 kg of naphthalene, and importing more than 150 000 000 kg into the country. Naphthalene has a wide variety of industrial uses and is also a component of a variety of consumer products.
Population exposure to naphthalene in Canada is expected to be predominantly via inhalation of indoor air, accounting for more than 95% of the total daily intake across age groups. Dermal exposure from contact with consumer products containing naphthalene can contribute to general population exposure.
Based principally on weight of evidence based assessments by several international and national agencies, a critical effect for the characterization of risk to human health is carcinogenicity, based on the observation of respiratory tract tumours in rodents. Naphthalene was also genotoxic in some assays. Therefore, although the mode of induction of tumours has not been fully elucidated, it cannot be precluded that the tumours observed in experimental animals resulted from direct interaction with genetic material. In addition, the margins of exposure for noncancer effects and exposure to the general population via inhalation may not be adequate to account for uncertainties in the databases on exposure and effects.
On the basis of the carcinogencity of naphthalene, for which there may be a probability of harm at any level of exposure, as well as the potential inadequacy of the margin of exposure for non-cancer effects, and applying a precautionary approach, it is concluded that naphthalene be considered to be a substance that may be entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is concluded that this substance is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity, or that constitute or may constitute a danger to the environment on which life depends. Additionally, naphthalene does not meet criteria for persistence and bioaccumulation potential as set out in the Persistence and Bioaccumulation Regulations.
This substance will be included in the Domestic Substances List inventory update initiative, to be launched in 2009. In addition and where relevant, research and monitoring will support verification of assumptions used during the screening assessment and, where appropriate, the performance of potential control measures identified during the risk management phase.
Based on the information available, naphthalene meets one or more of the criteria set out in section 64 of the Canadian Environmental Protection Act, 1999.
The Canadian Environmental Protection Act, 1999 (CEPA 1999) (Canada 1999) requires the Minister of the Environment and the Minister of Health to conduct screening assessments of substances that have met the categorization criteria set out in the Act to determine whether these substances present or may present a risk to the environment or human health. Based on the results of a screening assessment, the Ministers can propose to take no further action with respect to the substance, to add the substance to the Priority Substances List (PSL) for further assessment, or to recommend that the substance be added to the List of Toxic Substances in Schedule 1 of the Act and, where applicable, the implementation of virtual elimination.
Based on the information obtained through the categorization process, the Ministers identified a number of substances as high priorities for action. These include substances that
- met all of the ecological categorization criteria, including persistence, bioaccumulation potential and inherent toxicity (PBiT) to aquatic organisms, and were believed to be in commerce; and/or
- met the categorization criteria for greatest potential for human exposure (GPE) or presented an intermediate potential for exposure (IPE), and had been identified as posing a high hazard to human health based on classifications by other national or international agencies for carcinogenicity, genotoxicity, developmental toxicity or reproductive toxicity.
The Ministers therefore published a notice of intent in the Canada Gazette, Part I, on December 9, 2006 (Canada 2006), that challenged industry and other interested stakeholders to submit, within specified timelines, specific information that may be used to inform risk assessment and to develop and benchmark best practices for the risk management and product stewardship of those substances identified as the highest priorities.
The substance naphthalene was identified as a high priority for assessment of human health risk because it was considered to present GPE and had been classified by other agencies on the basis of carcinogenicity. The Challenge for naphthalene was published in the Canada Gazette on February 3, 2007 (Canada 2007a). A substance profile was released at the same time. The substance profile presented the technical information available prior to December 2005 that formed the basis for categorization of this substance. As a result of the Challenge, submissions of information were received.
Although naphthalene was determined to be a high priority for assessment with respect to human health and it also met the ecological categorization criterion for inherent toxicity for aquatic organisms, it did not meet the criteria for potential for persistence or bioaccumulation. Therefore, this assessment focuses principally on information relevant to the evaluation of risks to human health.
Under CEPA 1999, screening assessments focus on information critical to determining whether a substance meets the criteria for defining a chemical as toxic as set out in section 64 of the Act, where
“64. [...] a substance is toxic if it is entering or may enter the environment in a quantity or concentration or under conditions that
(a) have or may have an immediate or long-term harmful effect on the environment or its biological diversity;
(b) constitute or may constitute a danger to the environment on which life depends; or
(c) constitute or may constitute a danger in Canada to human life or health.”
Screening assessments examine scientific information and develop conclusions by incorporating a weight of evidence approach and precaution.
This screening assessment includes consideration of information on chemical properties, hazards, uses and exposure, including the additional information submitted under the Challenge. Data relevant to the screening assessment of this substance were identified in original literature, review and assessment documents, stakeholder research reports and from recent literature searches, up to January 2007. Key studies were critically evaluated; modelling results may have been used to reach conclusions. Evaluation of risk to human health involves consideration of data relevant to estimation of exposure (non-occupational) of the general population, as well as information on health hazards (based principally on the weight of evidence assessments of other agencies that were used for prioritization the substance). Decisions for human health are based on the nature of the critical effect and/or margins between conservative effect levels and estimates of exposure, taking into account confidence in the completeness of the identified databases on both exposure and effects, within a screening context. The screening assessment does not represent an exhaustive or critical review of all available data. Rather, it presents a summary of the critical information upon which the conclusion is based.
This screening assessment was prepared by staff in the Existing Substances Programs at Health Canada and Environment Canada and incorporates input from other programs within these departments. This assessment has undergone external written peer review/consultation. Comments on the technical portions relevant to human health were received from Meridian Environmental Inc. and Starodub & Associates. While external comments were taken into consideration, the final content and outcome of the screening risk assessment remain the responsibility of Health Canada and Environment Canada. Additionally, the draft of this screening assessment was subject to a 60-day public comment period. The critical information and considerations upon which the assessment is based are summarized below.
| CAS Registry Number | 91-20-3 |
| DSL name | Naphthalene |
| Inventory names[1] | Naphthalene (TSCA, EINECS, ENCS, AICS, ECL, SWISS, PICCS, ASIA-PAC, NZIoC) NAPHTHALENE SCALES (PICCS) |
| Other names | Albocarbon; Camphor Tar; Dezodorator; Mighty 150; Mighty RD1; Moth Balls; Moth flakes; Naftalen; Naphtalene; Naphthalin; Naphthaline; Naphthene; Tar Camphor; White tar |
| Chemical group | Discrete organics |
| Chemical sub-group | Aromatic |
| Chemical formula | C10H8 |
| Chemical structure | 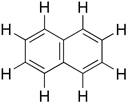 |
| SMILES | c(c(ccc1)ccc2)(c1)c2 |
| Molecular mass | 128.18 g/mol |
| The primary focus of this screening assessment report is on the uses of naphthalene as a discrete substance rather than its presence in complex mixtures such as petroleum-based streams and products | |
[1] Source: National Chemical Inventories (NCI), 2007: AICS (Australian Inventory of Chemical Substances); ECL (Korean Existing Chemicals List); EINECS (European Inventory of Existing Chemical Substances); ELINCS (European List of Notified Chemical Substances), ENCS (Japanese Existing and New Chemical Substances); PICCS (Philippine Inventory of Chemicals and Chemical Substances); TSCA (Toxic Substances Control Act Chemical Substance Inventory); ASIA-PAC (Combined Inventories from the Asia-Pacific Region); and NZIoC (New Zealand Inventory of Chemicals)
A summary of the physical and chemical properties of naphthalene is presented in Table 2.
| Property | Type | Value | Temperature (°C) |
Reference |
|---|---|---|---|---|
| Melting Point (°C) |
Experimental | 80.5 | - | Weast et al. 1985 |
| Boiling Point (°C) |
Experimental | 218 | - | Weast et al. 1985 |
| Density (kg/m3) |
Experimental | 1.145 | 20 | Weast et al. 1985 |
| Vapour pressure (Pa) |
Experimental | 11.6 | 25 | US EPA 1982e |
| Henry's Law constant (Pa•m3 /mol) |
Experimental | 46.6 (4.6 × 10-4 atm·m3/mol) |
- | Weast et al. 1985 |
| Log Kow (Octanol-water partition coefficient) (dimensionless) |
Experimental | 3.29 | - | Weast et al. 1985 |
| Log Koc (Organic carbon-water partition coefficient) (dimensionless) |
Experimental | 2.97 | - | Weast et al. 1985 |
| Water solubility (mg/L) |
Experimental | 31.7 | 25 | Weast et al. 1985 |
| Other solubilities (g/L) |
Experimental (alcohol) |
20 | 20–25 | GDCh-Advisory Committee 1989 |
| Experimental (benzene) |
1130 | 41 | GDCh-Advisory Committee 1989 | |
| Experimental (toluene) |
910 | 41 | GDCh-Advisory Committee 1989 |
Naphthalene is found in eroded geologic deposits of lignite, anthracite, bituminous and sub-bituminous coal. Based on a survey conducted under Section 71 of CEPA 1999, in 2000 Canadian companies reported manufacturing more than 52 000 000 kg of naphthalene, and importing more than 150 000 000 kg into the country (Environment Canada 2006).
The primary focus of this screening assessment report is on the uses of naphthalene as a discrete substance rather than its presence in complex mixtures such as petroleum-based streams and products.
According to submissions made under section 71 of CEPA 1999, from the Challenge questionnaire submission and other data voluntarily submitted (Environment Canada 2006), naphthalene is reported to be used in the petroleum sector as an oilfield chemical, solvent, refinery cleaner, fuel additive and feedstock. Other non-petroleum stream uses that were reported included its use as a solvent and intermediate in automotive paint manufacturing and driveway sealants, in pest control products such as moth repellants, as a chemical intermediate in the manufacture of pharmaceutical, agricultural and construction products, and as a feedstock for the production of naphthalene sulfonate plasticizers and surfactants. Consumer product uses of naphthalene may include some commercially available paints, stains and coatings (NIH 2007).
In Canada, four end-use products containing naphthalene are registered for use as moth repellents under the Pest Control Products Act (PMRA 2007). Naphthalene was reported in two notifications of cosmetic products (wig glue removers) filed with Health Canada under the Cosmetic Regulations of the Food and Drug Act (Health Canada, Product Safety Programme; personal communication, September 2007; unreferenced). Naphthalene is also a component of tobacco combustion products.
The National Pollutant Release Inventory reports that 120 tonnes of naphthalene were released by Canadian industries in 2005, with 34 out of 73 facilities reporting releases. The top four releasing facilities were located in Ontario (NPRI 2007).
Persistence
As a polycyclic aromatic hydrocarbon (PAH), naphthalene was included in a risk assessment of PAHs that was previously conducted under CEPA’s Priority Substances Assessment Program (Canada 1994). In that assessment PAHs in general were considered persistent in the environment, however naphthalene was identified as one of the more labile substances.
Based on its physical and chemical properties (Table 2) and the empirical degradation data for naphthalene presented below (Table 3), naphthalene does not meet the persistence criteria (half-lives in soil and water ≥ 182 days, in sediments ≥ 365 days and in air ≥ 2 days) set out in the Persistence and Bioaccumulation Regulations (Canada 2000).
| Medium | Fate process | Degradation value | Degradation endpoint/units |
Reference |
|---|---|---|---|---|
| Air | · OH radical reaction | 24.0 | Half-life, hours | Güsten et al. 1984 |
| Air | · OH radical reaction | 19.0 | Half-life, hours | Klöpffer et al. 1986 |
| Air | · OH radical reaction | 8.9 | Half-life, hours | Atkinson and Aschmann 1987 |
| Air | · OH radical reaction | 8.2 | Half-life, hours | Biermann et al. 1985 |
| Air | · OH radical reaction | 8.0 | Half-life, hours | Masclet and Mouvier 1988 |
| Air | · OH radical reaction | 8.0 | Half-life, hours | Biermann et al. 1985 |
| Air | · OH radical reaction | 7.4 | Half-life, hours | Atkinson and Aschmann 1986 |
| Soil | Degradation | Approx. 2 | Half-life, days | Park et al. 1990a, 1990b |
| Sediment | Biodegradation | 0.125–>88 | Half-life, days | ATSDR 2005 |
| Sludge | Biodegradation | 2 | Biodegradation, % | MITI 1992 |
Bioaccumulation
The experimental data (Table 4) indicate that the substance naphthalene does not meet the bioaccumulation criteria--bioconcentration factor (BCF) / bioaccumulation factor (BAF) ≥ 5000--as set out in the Persistence and Bioaccumulation Regulations (Canada 2000).
| Test organism | Endpoint | Value wet wt L/kg |
Reference |
|---|---|---|---|
| Arenicola marina | BCF | 4.07 | Lyes 1979 |
| Leucisus idus melanotus | BCF | 30.20 | Freitag et al. 1985 |
| Chlorella fusca | BCF | 128.82 | Geyer et al. 1984 |
| Daphina pulex | BCF | 131.83 | Southworth et al. 1978 |
| Pimephales promelas | BCF | 426.58 | Veith et al. 1979 |
| Daphnia magna | BCF | 50 | Eastmond et al. 1984 |
| Lepomis macrochirus | BCF | 310 | McCarthy and Jimenez 1985 |
| Lepomis macrochirus | BCF | 320 | McCarthy and Jimenez 1985 |
Based on moderate vapour pressure, logKoc and water solubility values, as well as the results of Level III fugacity modelling (Table 5), there is the indication that naphthalene will remain in the media to which it was released.
| Substance released to: | Fraction of substance partitioning into each compartment medium (%) | |||
|---|---|---|---|---|
| Air | Water | Soil | Sediment | |
| Air (100%) | 90.5 | 4.81 | 4.44 | 0.218 |
| Water (100%) | 2.19 | 93.5 | 0.107 | 4.23 |
| Soil (100%) | 0.143 | 0.379 | 99.5 | 0.0172 |
| Air, water, soil (33.3% each) | 1.03 | 12.8 | 85.6 | 0.578 |
As indicated earlier, naphthalene does not meet the persistence or bioaccumulation criteria as set out in the Persistence and Bioaccumulation Regulations (Canada 2000). Experimental ecotoxicological data indicate that naphthalene poses a high hazard for aquatic organisms. LC50s of 96 to 680 μg/L have been reported for fish (Black et al. 1983; Rice and Thomas 1989; Milleman et al. 1984; US EPA 1992). For non-mammalian organisms in other media, toxicity values of 18665 µg/kg in sediment and 205 µg/kg in soil can be derived through equilibrium partitioning from an acute toxicity LC50 of 1000 µg/L for Daphnia pulex (Trucco, 1983), using the experimental Koc of 2.97 and a fraction of organic carbon of 0.2 (Mackay, 1991). The National Pollutant Release Inventory reports releases by Canadian industries of 120 tonnes of naphthalene in 2005, mainly to air where it is not persistent (NPRI 2007).
Ecological effects of naphthalene and exposure of biota were considered as part of previous ecological components of the Priority Substances List assessments of polycyclic aromatic hydrocarbons (PAHs) (Government of Canada 1994) and creosote-impregnated waste materials (Government of Canada 1993). As a result of those assessments, Polycyclic aromatic hydrocarbons and Creosote-impregnated waste materials from creosote-contaminated sites have been added to Schedule 1 (List of Toxic Substances) of CEPA 1999. Those assessments had considered ecological impacts of exposure to total PAHs rather than to individual components such as naphthalene. In that context, ecologically-relevant releases had been found to be related to sources of total PAHs, rather than to sources of individual commercial components. Since ecological exposure and impacts are expected to be associated with total PAHs, it is not considered at this time that naphthalene, as an individual commercial compound, is likely to cause ecological harm. Should evidence be identified in the future that indicates that naphthalene on its own may have ecological impacts, revision of this conclusion may be warranted.
Exposure Assessment
Appendix 1 presents upper-bounding estimates of intake for each age group in the general population of Canada, based upon maximum identified concentrations in environmental media. The upper-bounding estimates of exposure to naphthalene for the general population range from 25.84 µg/kg-bw (kilogram of body weight) per day in the 60+ years age group to 78.01 µg/kg-bw/day in the 0.5 to 4 year age group. Inhalation of indoor air was the greatest source of intake of naphthalene, accounting for more than 95.0 % of the total daily intake across all age groups. A Health Canada survey of homes in Windsor, Canada in 2005 and 2006 (Health Canada 2008) identified maximum indoor air concentrations of 158.050 µg/m3. The corresponding mean and 90th percentile values from this study were significantly lower (6.778 and 9.405 µg/m3 respectively). Another Health Canada study conducted in Ottawa, Canada (Zhu et al. 2005) reported maximum indoor air concentrations of 144.44 µg/m3. The corresponding arithmetic mean and 90th percentile values from this study were also significantly lower (3.87 and 4.75 µg/m3 respectively. The maximum value from the Windsor survey was considered to be more appropriate for use in deriving upper-bounding estimates of intake than the maximum value of 398.70 µg/m3 as reported in an earlier Canadian survey (Fellin et al. 1992), as it is considered to be more representative of current exposures. However, the Windsor survey was conducted on non-smoking homes and since cigarette smoke is a source of naphthalene, smoking households may potentially have higher indoor air concentrations than those reported above for non-smoking homes. Therefore it is considered appropriate to use the maximum values in deriving upper-bounding estimates of exposure.
Measured values of indoor air and ambient air are believed to generally capture emissions from consumer products and other sources such as migration of volatile organic compounds from attached garages (Batterman et al. 2007). It is notable that air concentrations of 520–820 µg/m3 were reported in a limited study which simulated moth ball use in 3 homes (EURAR 2003). Relevance of this data to the Canadian use pattern for moth balls is uncertain.
Dermal exposure to naphthalene from use of consumer products containing naphthalene can contribute to general population exposure.
Confidence in the general population exposure assessment is moderate to high given the completeness of the dataset, critical study design and thoroughness and quantity of literature currently available. Confidence in the estimates of exposure from use of consumer products is low due to uncertainties in the assumptions used.
Health Effects Assessment
Appendix 2 contains a summary of the available health effects information for naphthalene in laboratory animals. An overview of health effects reported in humans is presented in Appendix 4.
The International Agency for Research on Cancer (IARC 2002) has classified naphthalene as “possibly carcinogenic to humans” (Group 2B) on the basis of “inadequate evidence” in humans and “sufficient evidence” in experimental animals for determination of carcinogenicity. The European Commission (EURAR 2003) has classified naphthalene as Category 3 for carcinogenicity (“causes concerns for humans owing to possible carcinogenic effects”). It was noted by the European Commission that a satisfactory assessment of carcinogenic effects in humans was not possible based on inadequate available information and that evidence for carcinogenicity from appropriate animal studies was insufficient to classify the substance in Category 2 (“regarded as if carcinogenic to humans”) (EURAR 2003). The National Toxicology Program (NTP) has classified naphthalene as being “reasonably anticipated to be a human carcinogen based on sufficient evidence from studies in experimental animals” (NTP 2004). The United States Environmental Protection Agency (US EPA 1998) has classified naphthalene as Group C (“possible human carcinogen”) based on inadequate human carcinogenicity data and limited evidence of carcinogenicity after oral and inhalation exposures in experimental animals. These classifications were based on increased incidence of neoplastic effects observed in both mice and rats exposed to naphthalene via inhalation. Groups of male and female B6C3F1 mice exposed to up to 30 ppm (157 mg/m3) naphthalene for 104 weeks (NTP 1992a) had a significant increase in incidence of bronchioloalveolar adenoma (females only). Increased incidences of bronchioloalveolar adenoma and carcinoma in males were not statistically significant (NTP 1992a). In a screening study summarized in IARC (2002), female A/J mice were exposed via inhalation to up to 30 ppm (157 mg/m3) naphthalene for 6 months. A significant increase in the number of adenomas per tumour-bearing mouse, but not adenomas per mouse, was reported (Adkins et al. 1986). However IARC (2002) noted that this particular strain of mouse is highly susceptible to lung tumours. Groups of male and female F344/N rats exposed to up to 60 ppm (314 mg/m3) naphthalene for 105 weeks had increased incidence of olfactory epithelium neuroblastoma and respiratory epithelium adenoma (NTP 2000). IARC (2002) reported that these nasal tumours were rare, and had not been observed in the NTP historical control database for two-year inhalation studies or the NTP database of studies conducted via all routes of exposure. IARC (2002) reports that the utility of carcinogenicity studies via the oral route in rats as well as by injection in mice and rats (Schmähl 1955; LaVoie et al. 1988; Knake 1956) was limited for evaluation of the carcinogenicity of naphthalene.
Assays investigating mutagenicity in vitro provide little evidence for induction of gene mutations; however IARC (2002) noted that positive results were obtained in assays investigating micronucleus formation, chromosomal aberrations and chromosomal recombinations in vitro which are consistent with clastogenic potential. Similarly, the European Commission (EURAR 2003) stated that results of an in vitro assay indicate that naphthalene was clastogenic, but that assays were negative for induction of sister chromatid exchange in vitro. Assays were negative in two in vivobone-marrow micronucleus tests and an in vivo rat liver unscheduled DNA synthesis test.
Although a mode of action analysis is beyond the scope of a screening assessment, nongenotoxic mechanisms have been proposed but not fully elucidated for the carcinogenicity of naphthalene (IARC 2002; EURAR 2003). IARC (2002) notes that, for mice, higher rates of metabolism leading to cytotoxic metabolites and increased cell turnover may lead to tumour development. Similarly, the European Commission (EURAR 2003) indicates that, for the rat nasal tissue, tumour development is considered to be the result of chronic tissue injury, for which an identifiable threshold will exist. The European Commission (EURAR 2003) concluded that uncertainty exists concerning the relevance of the rat nasal effects to human health, but that it is not possible to dismiss the rat nasal olfactory data as being of no relevance for humans. IARC (2002) stated that there is no full understanding of the formation of these nasal tumours, particularly the neuroblastomas. The US EPA (2005) is currently assessing the mode of action of the carcinogenicity of naphthalene. In addition, the US EPA Reregistration Eligibility Decision (RED) document for naphthalene is expected to be made publicly available in July 2008 (US EPA 2008). An industry research program is also in place from 2007-2011, where efforts are focussed on defining the mode of action for tumourgenicity (Naphthalene Council Inc.; letter dated March 12, 2008; unreferenced).
The following text presents a summary of noncancer effects observed in laboratory animals at the lowest levels of exposure via inhalation, the predominant route of exposure for the general population.
The National Toxicology Program (NTP) conducted two-year inhalation studies with mice and rats. Groups of male and female B6C3F1 mice were exposed to approximately 0, 52 or 157 mg/m3 naphthalene. Non-neoplastic effects included inflammation of the nose and lungs, metaplasia of the olfactory epithelium and hyperplasia of the nasal respiratory epithelium (NTP 1992a). Groups of male and female F344/N rats were exposed to approximately 0, 52, 157 or 314 mg/m3 naphthalene. Non-neoplastic effects observed in rats included atypical hyperplasia, atrophy, chronic inflammation and hyaline degeneration of the olfactory epithelium as well as hyperplasia, squamous metaplasia, hyaline degeneration, goblet cell hyperplasia and glandular hyperplasia of the respiratory epithelium (NTP 2000). For both the rat and mouse studies, a lowest-observed-(adverse)-effect concentration (LO(A)EC) of 52 mg/m3 was identified.
The European Commission (EURAR 2003) reported that naphthalene was administered via inhalation in a well-conducted unpublished study (Huntingdon Research Centre 1993a), 6 hours/day, 5 days a week for 13 weeks to male and female rats at dose levels of approximately 0, 10, 50 or 3000 mg/m3. Effects in the olfactory epithelium at the 10 mg/m3 dose level (LOEC) were reported to include slight disorganization, mild erosion, minimal atrophy, rosette formation, occasional degenerate cells, loss of Bowman’s glands and minimal hyperplasia (EURAR 2003). The European Commission (EURAR 2003) noted that a no-observed-adverse-effects concentration (NOAEC) could not be identified for local effects.
The European Commission (EURAR 2003) reported that naphthalene was administered via inhalation in a second well-conducted unpublished study (Huntingdon Research Centre 1993b), 6 hours/day, 5 days a week for 4 weeks to groups of male and female rats at dose levels of approximately 0, 5, 15, 50, 150 or 370 mg/m3. A lowest-observed-adverse-effect-concentraion (LOAEC) of 5 mg/m3 was identified by the European Commission, where observed effects in the nasal olfactory epithelium included local effects with signs of proliferative repair; a NOAEC could not be identified (EU RAR 2003).
Confidence in the toxicological database is considered to be moderate to high, as a number of studies exist for acute, short-term and chronic durations, via inhalation, dermal and oral routes.
Characterization of Risk to Human Health
Based principally on the weight of evidence-based assessments of several international and national agencies (IARC 2002; EURAR 2003; US EPA 1998; NTP 2004), a critical effect for characterization of risk to human health is carcinogenicity. Although a mode of action for induction of tumours has not been fully elucidated, it cannot be precluded that the tumours observed involved direct interaction with genetic material.
With respect to noncancer effects, the lowest identified concentrations at which effects were observed in animals were 5 mg/m3, in a 4-week inhalation study with rats (Huntingdon Research Centre 1993b) and 10 mg/m3, in a 13-week inhalation study with rats (Huntingdon Research Centre 1993a). These effect concentrations are 32 to 63 times higher, respectively, than the upper-bounding concentration of naphthalene in indoor air (158.050 μg/m3) in Canada (Health Canada 2008). Comparison of the same effect levels to the 90th percentile concentrations measured in indoor air (9.405 μg/m3 – Health Canada 2008) result in margins of exposure of 532 to 1063. These margins may not be adequate to account for uncertainties in the database on exposure and effects. For example, the indoor air survey (Health Canada, 2008) was conducted in non-smoking homes and smoking households may potentially have higher indoor air concentrations of naphthalene. Additionally, dermal exposure from use of consumer products could contribute to exposure to the general population.
Uncertainties in Evaluation of Risk to Human Health
There were uncertainties in the assumptions used in the estimates of exposure from the use of consumer products. Estimates were, however, considered to be conservative. Estimates of intake did not address potential intake from breast milk, in which naphthalene has been detected, but not quantified (Pellizzari et al. 1982).
The lack of developmental and reproductive studies by the predominant route of exposure in the general population (i.e., inhalation) is a limitation in the effects database. The scope of this screening assessment does not take into account potential interspecies differences in sensitivity to naphthalene. Uncertainty exists regarding the mode of action for tumour induction from exposure to naphthalene. Data suggest nongenotoxic mechanisms may play a role. There is uncertainty regarding the relevance to human health of nasal tumours in rats exposed chronically by inhalation (IARC 2002; EURAR 2003). The scope of this screening assessment does not take into consideration a thorough analysis of the mode of action for tumour induction from exposure to naphthalene or thorough review of individual study design.
Based on the available information, it is concluded that naphthalene is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity, or that constitute or may constitute a danger to the environment on which life depends.
On the basis of the carcinogenicity of naphthalene, for which there may be a possibility of harm at any level of exposure, as well as the potential inadequacy of the margins of exposure for non-cancer effects, and applying a precautionary approach, it is concluded that naphthalene be considered as a substance that may be entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore concluded that naphthalene does not meet the criteria in paragraph 64a and 64b of CEPA 1999, but it does meet the criteria in paragraph 64c of CEPA 1999. Additionally, naphthalene does not meet criteria for persistence and bioaccumulation potential as set out in the Persistence and Bioaccumulation Regulations.
Adkins B Jr, Van Stee EW, Simmons JE, Eustis SL. 1986. Oncogenic response of strain A/J mice to inhaled chemicals. J Toxicol Environ Health 17:311-322 [cited in IARC 2002].
[AEAB] Alberta Environmental Appeal Board. 2002. Alberta Environmental Appeal Board - Costs Decisions. Date of publication June 14, 2002. Appeal No. 01-090-CD.
Alberta Environment. 2005. Alberta Ambient Air Quality Objectives Work Plan 2005-2008. Environmental Policy Branch. Alberta Environmental. Report ISBN No. 0-7785-4554-7.
Alberta Environmental. 2006. Wabamun Lake oil spill August 2005: Data report for water and sediment quality in the pelagic area of the lake (August 4-5 to September 15, 2005). Environmental Monitoring and Evaluation Branch, Environmental Assurance Division. Report ISBN: 0-7785-4589-X, April 2006.
Anziulewicz JA, Dick HJ, Chiarulli EE. 1959. Transplacental naphthalene poisoning. Am J Obstet Gynecol 78:519-521 [cited in IARC 2002].
Atkison R, Aschmann SM. 1986. Kinetics of the reactions of naphthalene, 2-methylnaphthalene, and 2,3-dimethylnaphthalene with OH radicals and with O3 at 295 ± 1 K. Int. J. Chem. Kinet. 18(5):569-573 [cited in GDCh-Advisory Committee 1989].
Atkison R, Aschmann SM. 1987. Kinetics of the gas-phase reactions of alkylnaphthalenes with O3, N2O5, and OH radicals at 298 ± 1 K. Atmos. Environ. 21:2323-2326. [cited in GDCh-Advisory Committee 1989].
[ATSDR] Agency for Toxic Substances and Disease Registry. 2005. Toxicological profile for naphthalene, 1-methylnaphthalene, and 2-methylnaphthalene. Washington (DC): US Department of Health and Human Services, Public Health Service.
Austin CC, Wang D, Ecobichon DJ, Dussault G. 2001. Characterization of volatile organic compounds in smoke at municipal structural fire. J Toxicol Environ Health A 63(6):437-458.
Bagchi M, Bagchi D, Dalmoori J, Ye X, Stohs SJ. 1998a. Naphthalene-induced oxidative stress and DNA damage in cultured macrophage J774A1 cells. Free Radic Biol Med 25:137-143 [cited in IARC 2002].
Bagchi D, Bagchi M, Balmoori J, Vuchetich PJ, Stohs SJ. 1998b. Induction of oxidative stress and DNA damage by chronic administration of naphthalene to rats. Res Commun Mol Pathol Pharmacol 101(3):249-257 [cited in IARC 2000].
Bagchi D, Balmoori J, Bagchi M, Ye X, Williams CB, Stohs SJ. 2000. Role of p53 tumor suppressor gene in the toxicity of TCDD, endrin, naphthalene, and chromium (VI) in liver and brain tissues of mice. Free Radic Biol Med 28(6):895-903 [cited in IARC 2002; ATSDR 2005].
Balch GC, Metcalfe CD, and Huestis SY. 1995. Identification of potential fish carcinogens in sediment from Hamilton harbour, Ontario Canada. Environ Toxicol Chem 14(1):79-91.
Barfknecht TR, Naismith RW, Matthews RJ. 1985. Rat hepatocyte primary culture/DNA repair test. PH 311-TX-008-85, 5601-56-1 (unpublished). Submitted to U.S. EPA by Texaco, Inc. Office of Toxic Substances. Report microfiche No.: 0TS0513638 [cited in ATSDR (2005); cited as Pharmakon 1985c in EURAR 2003].
Batterman S, Jia C, Hatzivasilis G. 2007. Migration of volatile organic compounds from attached garages to residences: a major exposure source. Environ Res 104(2):224-240.
Bell RW, Chapman RE, Kruschel BD, Spencer MJ, Smith KV, Lusis MA. 1991. The 1990 Toronto personal exposure pilot (PEP) study. Report ISBN No.: 0-7729-7962-6.
Benoit FM, LeBel GL, Williams DT. 1979. Polycyclic aromatic hydrocarbon levels in Eastern Ontario drinking waters, 1978. Bull Environ Contam Toxicol 23:774-778 [cited in ATSDR 2005].
Biermann H, Mac Leod H, Atkinson R, Winer AM, Pitts JN. 1985. Kinetics of the gas-phase reactions of the hydroxyl radical with naphthalene, phenanthrene, and anthracene. Environ. Sci. Technol. 19(3):244-248. [cited in GDCh-Advisory Committee 1989].
Black, J.A., Birge, W.J., Westerman, A.G. und Franics, P.C. 1983. Comparative aquatic toxicology of aromatic hydrocarbons. Fundamental Applied Toxicology 3:353-358.
Bos RP, Theuws JL, Jongeneelen FJ, Henderson PT. 1988. Mutagenicity of bi-, tri- and tetra-cyclic aromatic hydrocarbons in the "taped-plate assay" and in the conventional Salmonella mutagenicity assay. Mutat Res 204:203-206 [cited in EURAR 2003; ATSDR 2005].
Bright DA, Healey N. 2003. Contaminant risks from biosolids land application: contemporary organic comtaminant levels in digested sewage sludge from five treatment plants in Greater Vancouver, British Columbia. Environ Pollut 126(1):39-49.
Canada. 1993. Priority substances list assessment report: Creosote-impregnated wate materials. Ottawa (ON): Canada, Environment Canada; Canada, Health Canada.
Canada. 1994. Polycyclic aromatic hydrocarbons. Priority substances list assessment report. Priority Substance Assessment Program.
Priority substances list assessment report: Polycyclic aromatic hydrocarbons. 1994. Ottawa (ON): Canada, Environment Canada; Canada, Health Canada.
Canada. 1999. Canadian Environmental Protection Act, 1999 = Loi canadienne sur la protection de l’environnement, 1999. Statutes of Canada = Statuts du Canada, Chapter 33. Act assented to September 14, 1999. Ottawa: Queen’s Printer. Available at Canada Gazette Part III, Vol. 22, no. 3.
Canada. 2000. Persistence and Bioaccumulation Regulations (SOR/2000-107). Canada Gazette Vol. 134. [cited 2006 Aug].
[Canada] Environment Canada, Health Canada. 2006. Canadian Environment Protection Act 1999. Statutes of Canada. Ottawa: Public Works and Government Services Canada. Canada Gazette Part I, Vol. 140, no. 49.
[Canada] Environment Canada, Health Canada. 2007. Substance profile for the challenge naphthalene CAS No. 91-20-3.
Chen W, Schopflocher D, Fowler BR, White J, Prepas E, Prince E, Gabos S. 1999. Polycyclic aromatic hydrocarbons in sediment following forest fires. Organohalogen compounds 43: 417-420.
Cho V, Chichester C, Morin D, Plopper C, Buckpitt A. 1994b. Covalent interactions of reactive naphthalene metabolites with proteins. J Pharmacol Exp Ther 269:881-889 [cited in IARC 2002].
Chuang JC, Mack GA, Kuhlman MR, Wilson NK. 1991. Polycyclic aromatic hydrocarbons and their derivatives in indoor and outdoor air in an eight-home study. Atmos Environ 25B(3):369-380 [cited in ATSDR 2005].
Chuang JC, Callahan PJ, Menton RG, Gordon SM. 1995. Monitoring methods for polycyclic aromatic hydrocarbons and their distribution in house dust and track in soil. Environ Sci Technol 29:494-500 [cited in ATSDR 2005].
Chuang JC, Callahan PJ, Lyu CW, Wilson NK. 1999. Polycyclic aromatic hydrocarbon exposures of children in low-income families. J Expo Anal Environ Epidemiol 9(2):85-98 [cited in ATSDR 2005; US EPA 2003].
City of Calgary. 2003. Water works reports: Jan01-Dec 31 2003. Calgary (AB): Glenmore and Bearspaw Water Treatment Plant.
City of Toronto. 2003. Water Quality Report January-March, April-June, July-September. Toronto (ON): Toronto Works and Emergency Services, Water and Wastewater Services.
City of Toronto. 1990. The quality of drinking water in Toronto : A review of tap water, bottled water and water treated by a point-of-use device. Summary report. Department of Public Health, City of Toronto, Toronto, Ontario.
City of Toronto. 2002a. Water quality quarterly report - October-December 2002. Water and Wastewater Services Division, City of Toronto, Toronto, Ontario.
City of Toronto. 2002b. Water quality quarterly report - July-September 2002. Water and Wastewater Services Division, City of Toronto, Toronto, Ontario.
City of Toronto. 2002c. Water quality quarterly report - April-June 2002. Water and Wastewater Services Division, City of Toronto, Toronto, Ontario.
City of Toronto. 2002d. Water quality quarterly report - January-March 2002. Water and Wastewater Services Division, City of Toronto, Toronto, Ontario.
City of Toronto. 2003a. Water Quality Report January-March, April-June, July-September. Toronto (ON): Toronto Works and Emergency Services, Water and Wastewater Services.
City of Toronto. 2003b. Drinking water systems annual report for January 1, 2003 to December 31, 2003. Toronto (ON): Toronto Works and Emergency Services, Water and Wastewater Services.
Connor TH, Thiess JC, Hanna HA, Monteith DK and Matney TS. 1985. Genotoxicity of organic chemicals frequently found in the air of mobile homes. Toxicol Lett 25:33-40 [cited in IARC 2002].
Dann, T. 2002. NAPS air monitoring data on naphthalene (2002-2005). Environment Canada.
Delgado-Rodrigues A, Ortiz-Marttelo R, Graf U, Villalobos-Pietrini R, Gomez-Arroyo S. 1995. Genotoxic activity of environmentally important polycyclic aromatic hydrocarbons and their nitro derivatives in the wing spot test of Drosophila melanogaster. Mutat Res 341:235-247 [cited in IARC 2002].
Dickman M, Brindle I, Benson M. 1992. Evidence of teratogens in sediments of the Niagara River Watershed as reflected by chironomid (Diptera: chironomidae) deformities. J Great Lakes Res 18(3):467-480.
Dillon Consulting Ltd. 2006. Lower Trent River : preliminary quantitative human health risk assessment. Trenton (ON): Lower Trent Region Conservation Authority. Pp. 1-48. Report No.: 06-5735.
Djomo JE, Ferrier V, Gauthier L, Zoll-Moreux C, Marty J. 1995. Amphibian micronucleus test in vivo: Evaluation of the genotoxicity of some major polycyclic aromatic hydrocarbons found in crude oil. Mutagenesis 10:223-226 [cited in IARC 2002].
Eastmond D, Booth G, Lee M. 1984. Toxicity accumulation and elimination of polycyclic aromatic sulfur heterocycles in Daphnia magna. Arch Environ Contam Toxicol 13:105-112.
Environment Canada. 2006. Naphthalene preliminary report of Section 71 (CEPA, 1999). Notice with respect to certain substances on the Domestic Substances List (DSL) Version 2.0 December PROTECTED B Confidentiality.
EPCOR. 2005. 2005 Edmonton water performance report. Edmonton (AB): EPCOR Water Services Inc.
[EPIWIN] Estimation Programs Interface for Microsoft Windows. 2004. Version 3.12. Washington (DC): U.S. Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Epler JL, Rao TK, Guerin MR. 1979. Evaluation of feasibility of mutagenic testing of shale oil products and effluents. Environ Health Perspect 30:179-184 [cited in EURAR 2003].
[ETL] Enviro-Test Laboratories. 1991. Cayley background study: analysis of food products for target organic and inorganic parameters. Report series: 91-E1208.
[ETL] Enviro-Test Laboratories. 1992. Windsor area background study: analysis of food products for target organic and inorganic parameters. Report series: 92-E1052.
[EURAR] European Union risk assessment report: CAS: 91-20-3: naphthalene. 2003. Luxembourg : Office for Official Publications of the European Communities. Report No.: EUR 20763 EN. On the cover, European Commission Joint Research Centre. On the cover, European Commission Joint Research Centre. [European Communities, European Commission, Joint Research Centre, Institute for Health and Consumer Protection, European Chemicals Bureau].
Fellin P, Barnett SE, Tran QA. 1992. Results of a national pilot survey of airborne volatile organic compounds in Canadian residences. Downsview (ON): Concord Environmental Corporation. Report No.: CEC J2431, Vol. 1.
Florin I, Rutberg L, Curvall M, Enzell CR. 1980. Screening of tobacco smoke constituents for mutagenicity using the Ames' test. Toxicology 18:219-232 [cited in IARC 2002].
Frantz SW, Van Miller JP, Hengler WC (Bushy Run Research Center Union Carbide, Export, PA). 1986. Ninety-day (sub-chronic) dermal toxicity study with naphthalene in albino rats. Beacon (NY): Texaco, Inc. Report project No. 49-539, revised [cited in ATSDR 2005; EURAR 2003 as Bushy Run 1986]. Submitted to USEPA under section 8D of TSCA. OTS0513643.
Freeman AE, Wiesburger EK, Weisburger JH, Wolford RG, Mayak JM, Huebner RJ. 1973. Transformation of cell cultures as an indication of the carcinogenic potential of chemicals. J Natl Cancer Inst 51:799-808 [cited in IARC 2002].
Freitag D, Ballhorn L, Geyer H, Korte F. 1985. Environmental hazard profile of chemicals: an experimental method for the assessment of the behaviour of organic chemicals in the ecosphere by means of simple laboratory tests with 14 C labelled chemicals. Chemosphere 14:1589-1616. [cited in GDCh-Advisory Committee 1989].
Gabos S, Schopflocher D, Fowler BR, White J, Prepas E, Prince D, Chen W. 1998. Polycyclic aromatic hydrocarbons in water, fish, and deer liver samples following forest fires. Organohalogen Compd 43:329-333.
Gaines TB. 1969. Acute toxicity of pesticides. Toxicol Appl Pharmacol 14:515-534 [cited in ATSDR 2005].
Galloway SM, Armstrong MJ, Reuben C, Colman S, Brown B, Cannon C, Bloom AD, Makamura F, Ahmed M, Duk S, Rimpo J, Margolin BH, Resnick MA, Anderson B, Zeiger E. 1987. Chromosome aberrations and sister chromatid exchanges in Chinese hamster ovary cells: evaluations of 108 chemicals. Environ Mol Mutagen10(Suppl 10):1-175 [cited in IARC 2002].
Gatehouse D. 1980. Mutagenicity of 1,2 ring-fused acenaphthenes against S. typhimurium TA1537 and TA1538: structure-activity relationships. Mutat Res 78:121-135 [cited in IARC 2002].
GDCh-Advisory Committee. 1989. Naphthalene BUA Report 39. Germany : VCH Verlagsgesellsschaft. 155 pgs.
Germansky M, Jamall IS. 1988. Organ-specific effects of naphthalene on tissue peroxidation, glutathione peroxidases and superoxide dismutase in the rat. Arch Toxicol.61:480-483 [cited in ATSDR 2005].
Geyer H, Politzki G, Freitag D. 1984. Prediction of ecotoxicological behaviour of chemicals: relationship between n-octanol/water partition coefficient and bioaccumulation of organic chemicals by alga Chlorella. Chemosphere 13:269. [cited in GDCh-Advisory Committee 1989].
Gizyn WI. 1994. Windsor air quality study: soil and garden produce survey results. Windsor (ON): Ontario Ministry of the Environment and Energy; Windsor Air Quality Committee. Available from: NTIS, Springfield, VA; MIC-95-02281
Godek EG, Naismith RW, Matthews RJ (Pharmakon Research Internation, Inc., Waverly, PA). 1985. Ames Salmonella/microsome plate test (EPA/OECD). Beacon (NY): Texaco, Inc. Report microfiche No.: OTS0513637 (unpublished) [cited in ATSDR 2005]. Submitted to U.S. EPA, Office of Toxic Substances.
Golder Associates. 1990a. Preliminary risk assessment Canada Creosote Site, Calgary, Alta. Edmonton, AB: Alberta Environment. Report No.: 892-2803U
Golder Associates (Edmonton, AB). 1990b. Soil and groundwater investigation at the former Canada Creosote Ltd. s ite, Calgary, Alberta, Phase 2. Edmonton (AB): Alberta Environment. Report No.: 1 892-2803S.
Gollahon LS, Iyer P, Martin JE, Irwin TR. 1990. Chromosomal damage to preimplantation embryos in vitro by naphthalene. Toxicologist 274:1094 [cited in EURAR 2003; ATSDR 2005].
Greater Vancouver Regional District. 2002. Water: the Greater Vancouver water district quality control annual report. Vol. 1.
Güsten H, Klasinc L, Maric D. 1984. Prediction of the abiotic degradability of organic compounds in the troposphere. J. Atmos. Chem. 2:83-93. [cited in GDCh-Advisory Committee 1989].
Harper BL, Ramanujam VMS, Gad-El-Karim MM, Legator MS. 1984. The influence of simple aromatics on benzene clastogenicity. Mutat Res 128:105-114 [cited in IARC 2002].
Harrison EZ, Oakes SR, Hysell M, Hay A. Review Organic Chemicals in sewage sludges. Sci Total Environ 367:481-497.
Health Canada. 1998. Exposure factors for assessing total daily intake of Priority Substances by the general population of Canada. Unpublished report, December 1998. Ottawa (ON): Priority Substances Section, Environmental Health Directorate, Health Canada.
Health Canada 2008. Windsor Ontario Exposure Assessment Study 2005, 2006: VOC Sampling Data Summary (Draft). Fuels and Exposure Assessment Section, Air Health Sciences Division.
Ho YL, Ho SK. 1981. Screening of carcinogens with the prophage ?cIts857 induction test. Cancer Res 41:532-536 [cited in IARC 2002].
Ho CH, Clark BR, Guerin MR, Barkenbus BD, Rao TK, Epler JA. 1981. Analytical and biological analyses of test materials from the synthetic fuel technologies. IV. Studies of chemical structure-mutagenic activity relationships of aromatic nitrogen compounds relevant to synfuels. Mutat Res 85:335-345 [cited in IARC 2002].
Hoff RM, Chan KW. 1987. Measurement of polycyclic aromatic hydrocarbons in the air along the Niagara River. Environ Sci Technol 21:556-561.
Holmen JB, Ekesten B, Lundgren B. 1999. Naphthalene-induced cataract model in rats: a comparative study between slit and retroillumination images, biochemical changes and naphthalene dose and duration. Curr Eye Res 19:418-425 [cited in IARC 2002].
Huntingdon Research Centre. 1993a. Naphthalene 13-week inhalation study in rats. Report No.: LDA 2/930704 (unpublished) [cited in EURAR 2003].
Huntingdon Research Centre. 1993b. Naphthalene 4-week inhalation study in rats. Report No.: LDA 1/921559 (unpublished) [cited in EURAR 2003].
[IARC] Working Group on the Evaluation of Carcinogenic Risks to Humans. 2002. Naphthalene. IARC Monogr Eval Carcinog Risks Hum. 82: 367-435.
[IPCS] International Programme on Chemical Safety. 1998. Selected non-heterocyclic polycyclic aromatic hydrocarbons. Geneva (CH): World Health Organization (WHO). (Environmental health criteria 202). Jointly sponsored by the United Nations Environment Programme, the International Labour Organization, and the World Health Organization.
Isbell MA, Morin D, Boland B, Buckpitt A, Salemi M, Presley J. 2005. Identification of proteins adducted by reactive naphthalene metabolites in vitro. Proteomics 5:4197-4204.
Johnston JJ, Wong JP, Feldman SE, Ilnicki LP. 1994. Purge and trap/gas chromatography/mass spectrometry method for determining smoke contamination of foods and packaging materials. J Agric Food Chem 42:1954-1958.
Kaden DA, Hites RA, Thilly WG. 1979. Mutagenicity of soot and associated polycyclic aromatic hydrocarbons of Salmonella typhimurium. Cancer Res 39:4152-4159 [cited in IARC 2002].
Kinloch D, Kuhnlein H, Muir D. 1992. Inuit foods and diet: a preliminary assessment of benefits and risks. Sci Total Environ 122(1/2):247-278.
Kipopoulou AM, Manoli E, Samara C. 1999. Bioconcentration of polycyclic aromatic hydrocarbons in vegetables grown in an industrial area. Environ Pollut 106:369-380.
Kitchin KT, Brown JL, Kulkarni AP. 1992. Predictive assay for rodent carcinogenicity using in vivo biochemical parameters: operational characteristics and complementarity. Mutat Res 266:253-272 [cited in EURAR 2003; ATSDR 2005].
Klöpffer W, Frasnk R, Kohl E-G, Haag F. 1986. Quantitative Erfassung der photochemischen Transformationsprozesse in der Troposhäre. Chemiker-Zeitung. 110:57-61. [cited in GDCh-Advisory Committee 1989].
Knake E. 1956. Weak carcinogenic activity of naphthalene and benzene. Virchows Arch 329(2):141-176 [In German] [cited in IARC 2002].
Kojima M. 1992. Enzymatic distribution patterns of rat lenses and the changes that occur during naphthalene cataract development. Ophthalmic Res 24:73-82 [cited in ATSDR 2005].
Kowalski LA, Assi KP, Wee RKH, Madden Z. 2001. In vitro prediction of carcinogenicity using a bovine papillomavirus DNA-carrting C3H/10T½ cell line (T1). II: Results from the testing of 100 chemicals. Environ Mol Mutagen 37:231-240.
Kuhnlein H. 1989. Nutritional and toxicological components of Inuit diets in Broughton Island, Northwest Territories. Contract report. Yellowknife (NT): Berthelet E, Assistant Deputy Minister, Department of Health (NT).
Lakritz J, Chang A, Weir A, Nishio S, Hyde D, Philpot R, Buckpitt A, Plopper C. 1996. Cellular and metabolic basis of Clara cell tolerance to multiple doses of cytochrome P450-activated cytotoxicants. I: Bronchiolar epithelial reorganization and expression of cytochrome P450 monooxygenases in mice exposed to multiple doses of naphthalene. J Pharmacol Exp Ther 278:1408-1418 [cited in IARC 2002].
Landis International, Inc. 1995. Executive summaries on naphthalene toxicology, environmental, and non-target studies submitted to the US EPA. Presented at a meeting at HSE (Bootle) on November 14, 1995 by Wm. Ronald Landis (President), Valdosta, Georgia, USA [c ited in EURAR 2003].
Laroche L. 2004. Municipal drinking water levels. Annual report. Montreal (QC): Atwater and Charles-J-Des-Baillets Water Plants.
Laroche L. 2005. Municipal drinking water levels. Annual report. Montreal (QC): Atwater and Charles-J-Des-Baillets Water Plants.
LaVoie EJ, Dolan S, Little P, Wang C-X, Sugie S, Rivenson A. 1988. Carcinogenicity of quinoline, 4-and 8-methylquinoline and benzoquinolines in newborn mice and rats. Food Chem Toxicol 26:625-629 [cited in IARC 2002].
LeBel GL, Williams DT, Benoit FM. 1987. Use of large volume resin cartridges for the determination of organic contaminants in drinking water derived from the Great Lakes. In: Organic Pollutants in Water. American Chemical Society. p. 309-354 [cited in EU RAR 2003].
Lee MG, Camacho S, Buckpitt AR, Plopper CG. 2004. Injury patterns in the nasal passage from inhaled NA are related to airflow patterns and in situ metabolism of NA in Sprague Dawley rats. Toxicologist 78(1-S):349.
Lee MG, Phimister A, Morin D, Buckpitt A, Plopper C. 2005. In situ naphthalene bioactivation and nasal airflow cause region-specific injury patterns in the nasal mucosa of rats exposed to naphthalene by inhalation. J Pharmacol Exp Ther 314(1):103-110.
Lesage J, Perrault G, Durand P. 1987. Evaluation of worker exposure to polycyclic aromatic hydrocarbons. Am Ind Hyg Assoc J 48(9):743-759 [cited in EURAR 2003].
Lin CY, Boland BC, Lee YJ, Salemi MR, Morin D, Miller LA, Plopper CG, Buckpitt AR. 2006. Identification of proteins adducted by reactive metabolites of naphthalene and 1-nitronaphthalene in dissected airways of rhesus macaques. Proteomics 6:972-982.
Lyes MC. 1979. Bioavailability of a hydrocarbon from water and sediment to the marine worm Arenicola marina. Mar Biol 55:121-127. [cited in GDCh-Advisory Committee 1989].
Mackay, D. 1991. Multimedia environmental models. The fugacity approach. Lewis Publishers, Boca Raton, FL. Page 257
Mamber SW, Bryson V, Katz SE. 1984. Evaluation of the Escherichia coli K12 inductest for detection of potential chemical carcinogens. Mutat Res 130:141-151 [cited in IARC 2002].
Manitoba Conservation Authority. 2006. The Transcona community air quality monitoring survey (TCAQMS). Winnipeg (MB): Air Quality Section Programs Division. Report No.: 2006-01.
Manitoba Government. 2007. The Manitoba government regulatory informational notice.
Masclet P, Mouvier G. 1988. La chimie atmosphérique des hydrocarbures aromatiques polycycliques. Pollot. Atmos. 117: 25-31. [cited in GDCh-Advisory Committee 1989].
McCann J, Choi E, Yamasaki E, Ames BN. 1975. Detection of carcinogens as mutagens in the Salmonella/microsome test: assay of 300 chemicals. Proc Natl Acad Sci U S A 72(12):5135-5139 [cited in IARC 2002].
McCarthy JF, Jimenez BD. 1985. Reduction in bioavailability to bluegills of polycyclic aromatic hydrocarbons bound to dissolved humic material. Environ Toxicol Chem 4:511-521. [cited in IPCS 1998].
McCarthy LH, Williams TG, Stephens GR, Peddle J, Robertson K, Gregor DJ. 1997. Baseline studies in the Slave River, NWT, 1990-1994: Part I. Evaluation of the chemical quality of water and suspended sediment from the Slave River (NWT). Sci Total Environ 197:21-53.
Mersch-Sundermann V, Mochayedi S, Kevekordes S, Kern S, Wintermann F. 1993. The genotoxicity of unsubstituted and nitrated polycyclic aromatic hydrocarbons. Anticancer Res 13:2037-2044 [cited in IARC 2002].
[MESL] MacDonald Environmental Sciences Ltd. 2006. An evaluation of sediment quality conditions in the vicinity of the Maculay Point and Clover Point outfalls. Victoria (BC): British Columbia Ministry of Environment, Environmental Management Branch.
Millemann, R.E., Birge, W.J., Black, J.A., Cushman, R.M., Daniels, K.L., Franco, P.J., Giddings, J.M., McCarthy, J.F. und Stewart, A.J. 1984. Comparative acute toxicity to aquatic organisms of components of coal-derived synthetic fuels. Trans. Am. Fish Society 113:74-85.
[MITI] Ministry of International Trade and Industry (JP). 1992. Biodegradation and bioaccumulation data of existing chemicals based on the CSCL Japan. Japan : Chemical Products Safety Division Basic Industries Bureau, Ministry of International Trade & Industry. Edited by Chemicals Inspection & Testing Institute (JP).
Mortelmans K, Haworth S, Lawlor T, Speck W, Tainer B, Zeiger E. 1986. Salmonella mutagenicity tests: II. Results from the testing of 270 chemicals. Environ Mutagen 8(Suppl 7):1-119 [cited in IARC 2002].
Murano H, Kojima M, Sasaki K. 1993. Differences in naphthalene cataract formation between albino and pigmented rat eyes. Ophthalmic Res 25:16-22 [cited in ATSDR 2005].
Nakamura S, Oda Y, Shimada T, Oki I, Sugimoto K. 1987. SOS-inducing activity of chemical carcinogens and mutagens in Salmonella typhimurium TA1535/pSK1002: examination with 151 chemicals. Mutat Res 192:239-246 [cited in IARC 2002].
[NAPS] National Air Pollutants Surveillance Network. 2003, 2004, 2005. Environment Canada, Air Monitoring Data. Personal Communication with Mr. Tom Dann, Feb. 2007.
Narbonne JF, Cassand P, Alzieu P, Grolier P, Mrlina G, Calmon JP. 1987. Structure-activity relationships of the N-methylcarbamate series in Salmonella typhimurium. Mutat Res 191:21-27 [cited in IARC 2002].
NCI. 2007. National Chemical Inventories database. American Chemical Society, Chemical Abstract Service, accessed on April 2007.
[NIH] National Institutes of Health. 2007. Household products database. National Library of Medicine specialized information services. [updated 2007 April].
[NPRI] National Pollutant Release Inventory. 2007. Online database. Gatineau (QC): Environment Canada.
Ng AC, Karellas NS (1994) Windsor Air Quality Study: TAGA 6000 survey results. Windsor (ON): Ontario Ministry of the Environment, Environmental Monitoring and Reporting Branch, Windsor Air Quality Committee; Queen's Printer for Ontario. (Publication No. PIBS 3152E; ISBN 0-7778-2831-6).
[NHW] National Health and Welfare. 1980. Anthropometry report: height, weight and body dimensions; a report from Nutrition Canada . Ottawa (ON): Bureau of Nutritional Sciences, Health Promotion Directorate.
Demirjian A. 1980. Anthropometry report: height, weight and body dimensions; a report from Nutrition Canada. Ottawa: Minister of National Health and Welfare, Health and Promotion Directorate, Health Services and Promotion Branch.
[NHW] Department of National Health and Welfare. 1990. Present patterns and trends in infant feeding in Canada. Ottawa (ON). 9 pp. NHW Catalogue Number H39-199/1990E, ISBN 0-662-18397-5 [cited in Health Canada 1998].
Nohmi T, Miyata R, Yoshikawa K, Ishidate M Jr. 1985. Mutagenicity tests on organic chemical contaminants in city water and related compounds. I. Bacterial mutagenicity tests. Eisei Shikenjo Hokoku 103:60-64 [In Japanese] [cited in IARC 2002].
Nova Scotia Power Inc. 2003. Registration document by Nova Scotia Power Incorporated in support of Registration of Point Tupper Marine Coal Terminal under the Nova Scotia Environment Act.
[NTP] National Toxicology Program (US). 1980a. Subchronic toxicity study: naphthalene (C52904), B6C3F1 mice. Research Triangle Park (NC): U.S. Department of Health and Human Services, National Toxicology Program [cited in ATSDR 2005].
[NTP] National Toxicology Program (US). 1980b. Subchronic toxicity study: naphthalene (C52904), Fischer 344 rats. Research Triangle Park (NC): U.S. Department of Health and Human Services, National Toxicology Program [cited in ATSDR 2005].
[NTP] National Toxicology Program (US). 1991. Developmental toxicity of naphthalene (CAS No. 91-20-3) administered by gavage to Sprague-Dawley (CD) rats on gestational days 6 through 15. Final study report and appendix. Research Triangle Park (NC): National Toxicology Program, National Institute of Environmental Health Sciences, U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health. Report No.: TER91006 [cited in ATSDR 2005; cited as Navarro et al. 1991 in IARC 2002; EURAR 2003].
[NTP] National Toxicology Program (US). 1992a. Toxicology and carcinogenesis studies of naphthalene (CAS No. 91-20-3) in B6C3F 1 Mice (inhalation studies). Research Triangle Park (NC): National Toxicology Program. NTP Technical Report No. 410, NIH Publication No. 92-3141 [cited in IARC 2002; EURAR 2003; ATSDR 2005].
[NTP] National Toxicology Program (US). 1992b. Developmental toxicity of naphthalene (CAS No. 91-20-3) administered by gavage to New Zealand white rabbits on gestational days 6 through 9. Research Triangle Park (NC): National Toxicology Program, National Institute of Environmental Health Sciences, U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health. Report No.: TER91021 [cited in ATSDR 2005; cited as Navarroet al. 1992 in IARC 2002; EURAR 2003].
[NTP] National Toxicology Program (US). 2000. Toxicology and carcinogenesis studies of naphthalene (CAS No. 91-20-3) in F344/N rats (inhalation studies). Research Triangle Park (NC): National Toxicology Program. NTP Technical Report No. 500, NIH Publication No. 01-4434 [cited in IARC 2002; EURAR 2003; ATSDR 2005].
[NTP] National Toxicology Program (US). 2004. Report on carcinogens. 11th ed. Substance profiles: naphthalene. Research Triangle Park (NC): U.S. Department of Health and Human Services, Public Health Service, National Toxicology Program.
[NWP] Northern Wood Preservers. 1980. Northern Wood Preservers, Thunder Bay, ON and DOMTAR, New Westminster BC Data. Thunder Bay (ON): Northern Wood Preservers [cited in Health Canada 1993].
Okada T, Matsunaga K, Hayakawa R. 1985. Naphthol AS sensitisation tests in guinea pigs. Nippon Hikaishi 95:1487-1489 (HSE Translation No. 15353) [cited in EURAR 2003].
[OMOE] Ontario Ministry of Environment and Energy. 1994. Ontario Ministry of Environment and Energy "Ontario Typical Range" of chemical parameters in soil, vegetation, moss bags and snow. Version 1.0a. Toronto (ON): Phytotoxicology Section Standards Development Branch.
[OMOE] Ontario Ministry of Environment and Energy. 2000. Ontario Ministry of the Environment 2000 technical memorandum. Follow-up investigations of the U.S.E. Hickson Products fire of April 9 th, 2000. Report No.: SDB-060-3511-2000, August 2001, PIBS: 4121e.
[OMOE] Ontario Ministry of Environment and Energy. 2000. Ontario Ministry of the Environment air quality in Ontario 2000 report.
[OMOE] Ontario Ministry of Environment and Energy. 2000. Ontario Ministry of the Environment 2000 technical memorandum. Follow-up investigations of the U.S.E. Hickson Products fire of April 9 th, 2000. Report No. SDB-060-3511-2000, August 2001, PIBS: 4121e.
[OMOE] Ontario Ministry of Environment and Energy. 2002. Ontario Ministry of the Environment Air Quality in Ontario 2002 report.
[OMOE] Ontario Ministry of Environment and Energy. 2002. Atlantic Packaging Ltd. Paper Fibre Biosolids and Sound Sorb Berm, Oshawa Skeet and Gun club: results of chemical and microbiological testing.
[OMOE] Ontario Ministry of Environment and Energy. 2003. Ontario Ministry of the Environment Air Quality in Ontario 2003 report.
[OMOE] Ontario Ministry of Environment and Energy. 2004. Ontario Ministry of the Environment Air Quality in Ontario 2004 report.
[OMOE] Ontario Ministry of Environment and Energy. 2004. Great Lakes reconnaissance survey. Water and sediment quality monitoring survey harbours and embayments Lake Superior and the Spanish River. Etobicoke (ON): Water Monitoring Section, Environmental Monitoring and Reporting Branch.
Orzalesi N, Migliavacca L, Miglior S. 1994. Subretinal neovascularization after naphthalene damage to the rabbit retina. Invest Ophthalmol Vis Sci 35(2):696-705 [cited in ATSDR 2005].
Otson and Benoit. 1986. Surveys of selected organics in residential air. Indoor Air Quality in Cold Climates: Hazards and Abatement Measures. Walkinshaw DS, editor. Pittsburgh (PA):Air Pollution Control Association. p. 224-236.
Otson R, Fellin P, Tran Q. 1994. VOCs in representative Canadian residences. Atmos Environ 28(2):3563-3569.
Otson R, Williams DT, Fellin P. 1998. Contribution of traffic emissions to indoor airborne VOCs. Proceedings of the annual meeting. Air Waster Management Association 91 st annual meeting and exhibition, June 14-18, 1998. San Diego (CA). Report No.: WAB07P/1-WAB07P/12.
Otson R. 1996. I/O values for determination of the origin of some indoor organic pollutants. Proceedings of the annual meeting. Air Waster Management Association 91 st annual meeting and exhibition, June 14-18, 1998. San Diego (CA). Report No.: TP5402/1-TP5402/10.
Otson, Rein, Williams, David T., and Peter D. Bothwell. 1982. Volatile Organic Compounds in Water at Thirty Canadian Potable Water Treatment Facilitites. J. Asso. Off. Anal. Chem. 65(6):1370-1374.
[PMRA] Pest Management Regulatory Agency. 2007. PMRA Product Label Database [database on the internet].
Papciak RJ, Mallory VT. 1990. Acute toxicological evaluation of naphthalene. J Am Coll Toxicol 1(1):17-19 [cited in ATSDR 2005].
Park KS, Sims RC, Dupont RR, Doucette WJ, Matthews JE. 1990a. Fate of PAH compounds in two soil types: influence of volatilization, abiotic loss and biological activity. Environ Toxicol Chem 9(2):187-195.
Park KS, Sims RC, Dupont RR. 1990b. Transformation of PAHs in soil systems. J Environ Sci Eng 116(3):632-640.
Pellizzari E, Hartwell T, Harris B, Waddell R, Whitaker D, Erickson M. 1982. Purgeable organic compounds in mother's milk. Bull Environ Contam Toxicol 28(3):322-328.
Phimister AJ, Lee MG, Morin D, Buckpitt AR, Plopper CG. 2004. Glutathione depletion is a major determinant of inhaled naphthalene respiratory toxicity and naphthalene metabolism in mice. Toxicol Sci 82(1):268-278.
Plasterer MR, Bradshaw WS, Booth GM, Carter MW, Schuler RL, Hardin BD. 1985. Developmental toxicity of nine selected compounds following prenatal exposure in the mouse: naphthalene, p-nitrophenol, sodium selenite, dimethyl phthalate, ethylenethiourea and four glycol ether derivatives. J Toxicol Environ Health 15(1):25-38 [cited in IARC 2002; ATSDR 2005].
[PRI] Pharmakon Research International. 1985a. Primary dermal irritation study in rabbits (83/EPA): naphthalene. Waverly (PA): Pharmakon Research International, Inc. Report No.: PH 420-TX-013-84 [cited in ATSDR 2005].
[PRI] Pharmakon Research International. 1985c. Delayed contact hypersensitivity in guinea pigs: naphthalene. Waverly, PA: Pharmakon Research International, Inc. PH 424-TX-001 84. Cited in ATSDR (2005).
[PRI] Pharmakon Research International. 1986. Developmental toxicity study in rabbits: Naphthalene. Waverly, PA: Pharmakon Research International, Inc. PH 329-TX-001 85. Cited in ATSDR (2005). [Also cited as Pharmakon, 1985e in ECB, 2003].
Purchase IFH, Longstaff E, Ashby J, Styles JA, Anderson D, Leferve PA, Westwood FR. 1978. An evaluation of 6 short-term tests for detecting organic chemical carcinogens. Br J Cancer 37:873-959 [as ciited in EURAR 2003].
Rao GS, Pandya KP. 1981. Biochemical changes induced by naphthalene after oral administration in albino rats. Toxicol Lett 8:311-315 [cited in ATSDR 2005].
Rathbun WB, Holleschau AM, Murray DL, Buchanan A, Sawaguchi S, Tao RV. 1990. Glutathione synthesis and glutathione redox pathways in naphthalene cataract of the rat. Curr Eye Res 9:45-53 [cited in ATSDR 2005].
Reprotox. 1980a. Dermal tolerance after a single-dose application on the intact and abraded skin in rabbits. Reprotox Order No. 556 (9/6/80). product 104.111 (unpublished) [cited in EURAR 2003].
Reprotox 1980b. Ocular tolerance test after single-dose application in rabbits. Reprotox Order No. 557. (11/6/80), product 104.111 (unpublished) [cited in EURAR 2003].
Rice, S.D., Thomas, R.E.. 1989. Effect of pre-treatment exposures of toluene or naphthalene on the tolerance of pink salmon (Oncorhynchus gorbuscha) and kelp shrimp (Eualis suckleyi). Comparative Biochemistry and Physiology - C Pharmacology Toxicology and Endocrinology. 94(1):289-293.
Roper JC, Brown DM, Sullivan MA, Schoonhoven R, Swenberg JA, Pfaender FK. 2006. Epifluorescence microscopy and image analysis of high-level polycyclic aromatic hydrocarbon contamination in soils. Environ Toxicol Chem 25(12):3093-3100.
Rossa V, Pau H. 1988. Is the experimental naphthalene cataract a model for human senile cataract? Graefes Arch Clin Exp Ophthalmol 226:291-293 [cited in ATSDR 2005].
[RTC] Research Toxicology Center. 1999. Naphthalene unscheduled DNA synthesis (UDS) after in vivo treatment. Monitored by Rutgers VFT AG. Sponsored by International Tar Assoc. Research Toxicology Center, Rome [cited in EURAR 2003; ATSDR 2005.
[RTECS] Registry of Toxic Effects of Chemical Substances. 2006. Naphthalene (RTECS Number: QJ0525000).
Rundell JO, Guntakatta M, Matthews EJ. 1983. Criterion development for the application of BALB/c-3T3 cells to routine testing for chemical carcinogenic potential. Res J Environ Sci 27:309-324 [cited in IARC 2002].
Sakai M, Yoshida D, Mizusaki S. 1985. Mutagenicity of polycyclic aromatic hydrocarbons and quinones on Salmonella typhimurium TA97. Mutat Res 156:61-67 [cited in IARC 2002].
Sasaki JC, Arey J, Eastmond DA, Parks KK, Grosovsky AJ. 1997. Genotoxicity induced in human lymphoblasts by atmospheric reaction products of naphthalene and phenanthrene. Mutat Res 393(1-2):23-35 [cited in IARC 2002; ATSDR 2005].
Schmähl, D. 1955. Testing of naphthalene and anthracene for carcinogenic effects in rats. Z Krebsforsch 60:697-710 [In German] [cited in IARC 2002].
Sciences Inernational. 2006. Peer review of Health Canada’s “Proposed Framework for Calculating the Plausible Maximum Estimate of Near-Field (Consumer) Exposure to DSL Substances”.
Seixas GM, Andon BM, Hollingshed PG, Thilly WG. 1982. The aza-arenes as mutagens for Salmonella typhimurium. Mutat. Res 102:201-212 [cited in IARC 2002].
Sina JF, Bean CL, Dysart GR, Taylor VI, Bradley MO. 1983. Evaluation of the alkaline elution/rat hepatocyte assay as a predictor of carcinogenic/mutagenic potential. Mutat Res 113:357-391 [cited in IARC 2002; ATSDR 2005].
Shopp GM, White KL Jr., Holsapple MP, Barnes DW, Duke SS, Anderson AC, Condie LW, Hayes JR, Borzelleca JF. 1984. Naphthalene toxicity in CD-1 mice: General toxicology and immunotoxicology. Fundam Appl Toxicol 4:406-419 [cited in ATSDR 2005; EURAR 2003].
Snyder JM, King JW, Nam KS. 1996. Determination of volatile and semivolatile contaminants in meat by supercritical fluid extraction/gas chromatography/mass spectrometry. J Sci Agric 72:25-30.
Sorg RM, Naismith RW, Matthews RJ. 1985. OECD micronucleus test (MNT). Report No.: OTS0513639 (unpublished material). Submitted to USEPA under section 8D of TSCA [cited in ATSDR 2005; cited as Pharmakon 1985d in EURAR 2003].
Southworth GR, Beauchamp JJ, Schmieder PK. 1978. Bioaccumulation potential of polycyclic aromatic hydrocarbons in Daphnia pulex. Water Res 12:973-977. [cited in GDCh-Advisory Committee 1989].
Srivastava SK, Nath R. 1969. Metabolic alterations in experimental cataract. Part I. Inhibition of lactate dehydrogenase and appearance of o-diphenol oxidase in cataractous lens of naphthalene fed rabbits. Indian J Med Res 57:225-227 [cited in ATSDR 2005].
Strosher MT (Kanaskis Centre for Environmental Research). 1982. Trace organic compounds in the atmosphere near industrial developments. Report submitted to Alberta Environment under contract no. 82-9109.
Tao RV, Holleschau AM, Rathbun WB. 1991. Naphthalene-induced cataract in the rat. Ophthalmic Res 23:272-283 [cited in ATSDR 2005].
Tingle MD, Pirmohamed M, Templeton E, Wilson AS, Madden S, Kitteringham NR, Park BK. 1993. An investigation of the formation of cytotoxic, genotoxic, protein-reactive and stable metabolites from naphthalene by human liver microsomes. Biochem Pharmacol 46:1529-1538 [cited in IARC 2002; ATSDR 2005].
Tremblay C, Dann T. 1995. Volatile organic compounds in the 'ambient' air of the province of Quebec 1989-1993. Ottawa (ON): Environment Canada, Environmental Protection Branch.
Trucco, R.G., Engelhardt, F.R., Stacey, B. 1983. Toxicity, Accumulation and Clearance of Aromatic Hydrocarbons in Daphnia pulex. Environmental Protection (Series A) 31: 191-202
Tsuda H, Lee G, Farber E. 1980. Induction of resistant hepatocytes as a new principle for a possible short-term in vivo test for carcinogens. Cancer Res 40:1157-1164 [cited in ATSDR 2005].
Tsuruda LS, Lamé MW, Jones AD. 1995. Formation of epoxide and quinine protein adducts in B6C3F1 mice treated with naphthalene, sulphate conjugate of 1,4-dihydroxy-naphthalene and 1,4-naphthoquinone. Arch Toxicol 69:362-367 [cited in IARC 2002].
[US EPA] US Environmental Protection Agency. 1982e. Aquatic fate process data for organic priority pollutants. Washington (DC): US Environmental Protection Agency, Office of Water Regulations and Standards. Report No.: EPA440481014 [cited in ATSDR 2005].
[US EPA] US Environmental Protection Agency. 1992. Classification criteria for environmental toxicity and fate of industrial chemicals. Washington, DC. Office of Pollution Prevention and Toxics (OPPT), Chemical Control Division (CCD).
[US EPA] US Environmental Protection Agency. 1998. Toxicological review of naphthalene (CAS No. 91-20-3) in support of summary information on the Integrated Risk Information System (IRIS). Washington (DC): United States Environmental Protection Agency. 116 pp.
[US EPA] US Environmental Protection Agency. 2003. Health effects support document for naphthalene. Washington (DC): U.S. Environmental Protection Agency, Office of Water (4304T), Health and Ecological Criteria Division. EPA Doc No.: 822-R-03-005.
[US EPA] US Environmental Protection Agency. 2005. Peer consultation workshop on research needs related to the IRIS draft toxicological review of naphthalene. Final Report. Washington (DC): United States Environmental Protection Agency. Report No.: EPA/635/R-05/003.
[US EPA] US Environmental Protection Agency. 2008. Pesticide Reregistration Status Table [database on the internet]. [Updated 2008 March 28; cited 2008 May 5].
Valaes T, Doxiadis SA, Fessas P. 1963. Acute hemolysis due to naphthalene inhalation. J Pediatr 63:904-915 [cited in IARC 2002].
Van Heyningen, R. 1970. Ascorbic acid in the lens of the naphthalene-fed rabbit. Exp Eye Res 9:38-48 [cited in ATSDR 2005].
Van Heyningen R, Pirie A. 1967. The metabolism of naphthalene and its toxic effect on the eye. Biochem J 102:842-852 [cited in IARC 2002].
Van Heyningen R, Pirie A. 1976. Naphthalene cataract in pigmented and albino rabbits. Exp Eye Res 22:393-394 [cited in EURAR 2003].
Veith GD, DeFoe DL, Bergstedt BV. 1979. Measuring and estimating the bioconcentration factor of chemicals in fish. J Fish Res Board Can 36:1040-1048. [cited in GDCh-Advisory Committee 1989].
Versar Inc. 1986. Standard scenarios for estimating exposure to chemical substances during use of consumer products, Vol. I and 2. Prepared for the US Environmental Protection Agency, Office of Toxic Substances, EPA Contract No. 68-02-3968.
Vuchetich PJ, Bagchi D, Bagchi M, Hassoun EA, Tang L, Stohs SJ. 1996. Naphthalene-induced oxidative stress in rats and the protective effects of vitamin E succinate. Free Radic Biol Med 21:577-590 [cited in IARC 2002].
Weast RC, Astle MJ, Beyer WH, editors. 1985. CRC handbook of chemistry and physics: A ready-reference book of chemical and physical data. Boca Raton, FL : CRC Press, Inc. C-357, C-361 [cited in ATSDR, 2005].
Webber MD. 1994. Industrial organic compounds in selected Canadian municipal sludges and agricultural soils. Burlington (ON): Wastewater Technology Centre, RockCliffe Research Management Inc.
Webber MD, Bedford JA. 1996. Organic and metal contaminants in Canadian municipal sludges and sludge compost: supplemental report. Burlington (ON): Wastewater Technology Centre, RockCliffe Research Management Inc. 26. pp.
Webber MD, Nichols JA. 1995. Organic and metal contaminants in Canadian municipal sludges and a sludge compost. Burlington (ON): Wastewater Technology Centre, RockCliffe Research Management Inc.
West JAA, Pakehham G, Morin D, Fleschner CA, Buckpitt AR, Plopper CG. 2001. Inhaled naphthalene causes dose dependent cell cytotoxicity in mice but not in rats. Toxicol Appl Pharmacol 173(2):114-119 [cited in ATSDR 2005].
Western Canadian Coal. 2005. Brule Mine project - Brule Mine EA certificate application No. 8-211 (Section 8. Aquatic resources and Fisheries).
Williams DT, Otson R, Bothwell PD. 1982. Volatile compounds in water at thirty Canadian potable water treatment facilities. J Assoc Off Anal Chem 65(6):1370-1374.
Wilson AS, Tingle MD, Kelly MD, Park BK. 1995. Evaluation of the generation of genotoxic and cytotoxic metabolites of benzo[a]pyrene, aflatoxin B1, naphthalene and tamoxifen using human liver microsomes and human lymphocytes. Hum Exp Toxicol 14:507-515 [cited in IARC 2002].
Xu GT, Zigler JS, Lou MF. 1992b. The possible mechanism of naphthalene cataract in rat and its prevention by an aldose reductase inhibitor (AL01576). Exp Eye Res 54:63-72 [cited in ATSDR 2005].
Yamauchi T, Komura S, Yagi K. 1986. Serum lipid peroxide levels of albino rats administered naphthalene. Biochem Int 13:1-6 [cited in ATSDR 2005].
Zhu J, Newhook R, Marro L, Chan CC. 2005. Selected volatile organic compounds in residential air in the City of Ottawa, Canada. Environ Sci Technol 39:3964-3971.
Zinkham WH, Childs B. 1957. Effect of vitamin K and naphthalene metabolites on glutathione metabolism of erythrocytes from normal newborns and patients with naphthalene haemolytic anemia. Am J Dis Child 94(6):420-423 [cited in ATSDR 2005].
Zinkham WH, Childs B. 1958. A defect of glutathione metabolitsm of erythrocytes from patients with naphthalene-induced haemolytic anemia. Pediatrics 22:461-471 [cited in ATSDR 2005].
Zitko V, Stenson G, Hellou J. 1998. Levels of organochlorine and polycyclic aromatic compounds in harp seal beaters (Phoca groenlandica). Sci Total Environ 221:11-29.
Zuelzer WW, Apt L. 1949. Acute haemolytic anemia due to naphthalene poisoning. JAMA 141: 185-190. [Cited in EURAR 2003; ATSDR 2005].
Appendix 1: Upper-bounding estimates of daily intake of naphthalene for the general population in Canada
| Route of exposure | Estimated intake (µg/kg-bw per day) of naphthalene by various age groups | ||||||
|---|---|---|---|---|---|---|---|
| 0-6 months [1] | 0.5-4 years[3] | 5-11 years[4] | 12-19 years[5] | 20-59 years[6] | 60+ years[7] | ||
| formula fed[2] | not formula fed | ||||||
| Ambient air[9] | 0.52 | 0.52 | 1.11 | 0.87 | 0.49 | 0.42 | 0.37 |
| Indoor air [10] | 38.71 | 38.71 | 82.95 | 64.67 | 36.77 | 31.59 | 27.46 |
| Drinking water [11] | 0.14 | 0.05 | 0.06 | 0.05 | 0.03 | 0.03 | 0.03 |
| Food and beverages [12] | 1.05 | 1.01 | 0.79 | 0.51 | 0.42 | 0.34 | |
| Soil [13] | 9.3 × 10-3 | 3.1 × 10-2 | 2.0 × 10-2 | 9.3 × 10-3 | 9.3 × 10-3 | 9.3 × 10-3 | |
| Total intake | 39.37 | 40.33 | 85.13 | 66.37 | 37.81 | 32.46 | 28.19 |
[1]Naphthalene was identified, but not quantified, in 6 of 8 samples of breast milk in the United States (Pellizzari et al. 1982).
[2] Assumed to weigh 7.5 kg, to breathe 2.1 m3 of air per day, to drink 0.8 L of water per day (formula fed) or 0.3 L/day (not formula fed) and to ingest 30 mg of soil per day (Health Canada 1998).
[3] For exclusively formula-fed infants, intake from water is synonymous with intake from food. The highest Canadian concentration of naphthalene in water was used to reconstitute formula was based on Williams et al. 1982. Approximately 50% of non formula-fed infants are introduced to solid foods by 4 months of age and 90% by 6 months of age (NHW 1990).
[4] Assumed to weigh 15.5 kg, to breathe 9.3 m3 of air per day, to drink 0.7 L of water per day and to ingest 100 mg of soil per day (Health Canada 1998).
[5] Assumed to weigh 31.0 kg, to breathe 14.5 m3 of air per day, to drink 1.1 L of water per day and to ingest 65 mg of soil per day (Health Canada 1998).
[6] Assumed to weigh 59.4 kg, to breathe 15.8 m3 of air per day, to drink 1.2 L of water per day and to ingest 30 mg of soil per day (Health Canada 1998).
[7] Assumed to weigh 70.9 kg, to breathe 16.2 m3 of air per day, to drink 1.5 L of water per day and to ingest 30 mg of soil per day (Health Canada 1998).
[8] Assumed to weigh 72.0 kg, to breathe 14.3 m3 of air per day, to drink 1.6 L of water per day and to ingest 30 mg of soil per day (Health Canada 1998).
[9] The highest Canadian concentration of naphthalene measured in outdoor air from a large survey was in Winnipeg, Manitoba, at 14.8 µg/m3, n=443 (Manitoba Government 2007). A higher maximum was obtained from Quebec, -- however the sample size was considerably smaller. A higher value from a study in the U.S. was not used as Canadian data were available. Canadians are assumed to spend 3 hours outdoors each day (Health Canada 1998). This concentration was within the range of concentrations reported in a NAPS survey of outdoor air in Canada (NAPS 2003/04/05) and other studies e.g. Ontario 's Ministry of the Environment. The critical data were identified in a data set of studies of ambient air: Dann (2002); Otson et al . (1998); Otson and Zhu (1996); Tremblay and Dann (1995); Ng and Karellas (1994); Bell et al. (1991); Hoff and Chan (1987); Otson and Benoit (1986); NAPS (2003, 2004, 2005); Health Canada (1993, 2005, 2006); OMOE (2000, 2002, 2003, 2004); Chuang et al. (1991); Zhu et al. (2005); Manitoba Conservation Authority (2006); Alberta Environment (2005); Austin et al. (2001); Strosher (1982); Lesage et al. (1987 cited in EURAR 2003).
[10] The maximum concentration of naphthalene of 158.050 µg/m3 (range: 0.001–158.050 µg/m3; arithmetic means ranging from 1.002 to 6.778 µg/m3 depending upon year and season of sampling) in a recent indoor air survey in Windsor, Canada, based on sampling over 2 years, and 2 seasons, in 48 homes, was used to derive intake (Health Canada 2008). This value was very similar to the maximum value of 144.44 (arithmetic mean of 3.8 µg/m3) in another indoor air survey in Ottawa, Canada (Zhu et al. 2005). Maximum values from both studies were taken in homes that reported usage of mothballs. These values are lower than the maximum value of 398.70 µg/m3 reported by Fellin et al. (1992), but are considered more representative of current exposures and the analytical methodology was more robust. Canadians are assumed to spend 21 hours indoors each day (Health Canada 1998). The critical data were identified from a data set of studies of indoor air: Otson et al. (1994, 1998); Otson and Zhu (1996); Bell et al. (1991); Fellin et al. (1992); Otson and Benoit (1986); Health Canada (2008); Chuang et al. (1991); Zhu et al. (2005)
[11] The maximum Canadian concentration value (µg/L) of naphthalene in drinking water was 1.3 µg/L for 12 Great Lakes municipalities (Williams et al. 1982). This concentration falls within the range of other studies conducted on bottled water in Canada and extensive U.S. surveys. The critical data were identified in a data set of studies of drinking water: City of Toronto (1990, 2002a,b,c,d, 2003a,b); Otson et al. (1982); LeBel et al. (1987); EURAR (2003); Williams et al. (1982); Benoit et al. (1979); Laroche (2004, 2005); EPCOR (2005); Greater Vancouver Regional District (2002); City of Calgary (2003).
[12] Estimates of intake from food are based upon concentrations in foods that are selected to represent the twelve food groups addressed in calculating intake (Health Canada 1998) Amounts of foods consumed on a daily basis by each age group are described by Health Canada (Health Canada 1998).
Dairy products: a maximum concentration of 0.25 µg/ml in dairy products was reported by the US EPA (2003). This converts to 0.24 µg/kg using a milk density of 1036.86kg/m3 at 20oC. This is the only reported level in milk.
Fats: no data were identified for the general population. A mean (n=8) concentration of 23.5 ng/g was reported for blubber from harp seals (Zitko et al. 1998),; this would result in an intake of <0.01 µg/kg-bw per day. [Based on mean body weight of 62 kg and 1:1 sex ratio (NHW 1980). Adult consumes 24.3 g blubber/person/day,based on a study of Inuit food harvest and consumption on an isolated island community on the east coast of Baffin Island, NWT in 1987-1988 (Kinloch et al. 1992; Kuhnlein 1989); 24-hour recall of food consumption was examined by interview every two months during 1987–1988, for a total of 7 surveys.]
Fruits and fruit products: no data identified.
Vegetables: a maximum concentration of 63 µg/kg naphthalene in vegetables (endive, n=3) was reported from Kipopoulou et al. (1999) cited in US EPA (2003). Several levels in other vegetable produce were available e.g. carrots,, cabbage, leeks, lettuce.
Cereal products: the maximum concentration of naphthalene in cereal products, in this case rice, was 28 µg/kg, as reported by the US EPA (2003). This was the only cereal level identified.
Meat and poultry: The maximum concentration of naphthalene in meat and poultry was 26 µg/kg for beef or fried chicken (Johnston et al. 1994). Other levels were reported for a number of other meat products. These were the only levels in meat products that were identified.
Fish: a maximum naphthalene concentration of 1.7 µg naphthalene/kg was reported in fish (Gabos et al. 1998). No other levels were identified.
Eggs: no data were identified
Foods, primarily sugar: no data were identified
Mixed dishes: no data were identified
Nuts and seeds: no data were identified
Beverages (soft drinks/alcohol/coffee/tea): no data were identified
The critical data were identified in a data set of studies of foods: Gabos et al. (1998); ETL (1991, 1992); US EPA (2003); Kipopoulou et al. (1999); Johnston et al. (1994); Snyder et al. (1996); EURAR (2003); Zitko et al. (1998).
[13] The maximum Canadian concentration of naphthalene in soil from a non-point source was reported as 310 ng/g (OMOE 1994) as a typical upper bound for Ontario soil levels. This level falls within the range identified by other studies including a study by Dillon Consulting Ltd. (2006) and others. The critical data were identified in a data set of studies of soil, sediment and sludge: Gizyn (1994); OMEE (1994); Webber (1994); OMOE (2000, 2002, 2004); Chuang et al. (1995, 1999); Golder Associates (1987, 1990a,b); Roper et al. (2006); Health Canada (1993); NWP (1980); AEAB (2002); Chen et al. (1999); McCarthy et al. (1997); Balch et al. (1995); Dickman et al. (1992); Dillon Consulting Ltd. (2006); Western Canadian Coal (2005); Alberta Environment (2006); Nova Scotia Power Inc. (2003); MESL (2006); Webber and Bedford (1996); Webber and Nichols (1995); Webber (1994); Harrison et al. (2006); Bright and Healey (2003).
| Endpoint | Lowest effect levels[1]/Results |
|---|---|
| Acute toxicity | Lowest inhalation LC50 > 340 mg/m3 (RTECS 2006) Lowest oral LD50 = 316 mg/kg in rats (RTECS 2006) Lowest dermal LD50 > 2000 mg/kg in rabbits (Landis International 1995) |
| Short-term repeated-dose toxicity | Lowest inhalation LOAEC = 5 mg/m3 (1 ppm); local effects with signs of proliferative repair in nasal olfactory epithelium of male and female rats. Animals dosed at 370 mg/m3 (71 ppm), the highest dose level, were reported by the European Commission (EURAR 2003) to have a 50% reduction in body weight associated with reduced food consumption. No no-observed-adverse-effect level (NOAEL) was identified, as the lowest-observed-adverse-effect level (LOAEC) identified by the European Commission was at the lowest exposure level tested (Huntingdon Research Centre 1993b; unpublished study). Lowest oral LOEL = 50 mg/kg/day identified by ATSDR (2005) for neurological effects in a developmental toxicity study; Sprague-Dawley rats; maternal effects: slow respiration, lethargy, apnea, inability to move at lowest dose tested; no NOEL as maternal LOEL was at lowest exposure level tested; identified as LOAEL by ATSDR (2005); body weight effects and fetotoxicity identified at higher exposure levels (NTP 1991). Lowest dermal LOEL = N/A |
| Subchronic toxicity | Lowest inhalation LOEC = 10 mg/m3 (2 ppm); damage to nasal epithelium of rats (mild atrophy, rosette formation in olfactory epithelium, loss of Bowman's glands, minimal hyperplasia; no NOAEC as LOEC was the lowest exposure level of the study (Huntingdon Research Centre 1993a; unpublished study) [Additional studies: 6-month Carcinogenicity screening assay in mice exposed to up to 30 ppm (Adkins et al. 1986)] Lowest oral LOEL = 133 mg/kg/day for decreased absolute brain, liver and spleen weights; male and female CD-1 mice exposed by gavage for 90 days; NOAEL = 53 mg/kg/day, LOAEL = 133 mg/kg/day identified by ATSDR (2005); NOAEL = 133 mg/kg/day identified by the European Commission (EURAR 2003) (Shopp et al. 1984). Lowest dermal LOEL = 1000 mg/kg/day in rats for dermal effects; NOAEL = 300 mg/kg/day, LOAEL = 1000 mg/kg/day identified by ATSDR (2005); NOAEL = 1000 mg/kg/day by the European Commission (EURAR 2003) (Frantz et al. 1986) |
| Developmental/ reproductive toxicity | No studies specifically designed to investigate developmental and/or reproductive toxicity by inhalation exposure were identified. Lowest oral LOEL = 150 mg/kg/day in rats identified by ATSDR (2005) for developmental effects (decreased maternal weight gain > 20%) without fetotoxic or teratogenic effects; IARC (2002) notes decreased fetal body weight and increased percentage of adversely affected implants per litter as significant trends, as well as an increased percentage (non-significant) of malformed fetuses per litter in next highest level, 450 mg/kg/day group; European Commission (EURAR 2003) states that this “study provides some evidence of fetotoxicity occurring at maternally toxic doses with no fetotoxicity occurring at doses which were not maternally toxic”; US EPA (1998) identifies a fetal NOAEL = 450 mg/kg (NTP 1991) [Study also utilized for LOAEL for short-term toxicity (maternal toxicity)]. [Additional Studies: oral LOEL = 200 mg/kg/day in rabbits identified by ATSDR (2005) for developmental effects in pregnant females described as: “maternal dyspnea, cyanosis, body drop, hypoactivity with no pathological aberrations”; European Commission (EURAR 2003) notes no developmental effects observed (PRI 1985, 1986); LOEL = 300 mg/kg/day in mice; identified by ATSDR (2005) for reproductive effects (> 10% maternal mortality); IARC (2002) noted that the average number of live offspring per litter was decreased; IARC (2002) suggests early embryonic absorption (Plasterer et al. 1985). [Additional studies: NOAEL = 120 mg/kg/day in rabbits; highest dose tested (NTP 1992b)] |
| Chronic toxicity/ carcinogenicity | Non-neoplastic endpoints Oral NO(A)EL = 41 mg/kg/day; rat exposed in feed to 10–20 mg/day, 6 days/week for 100 weeks; body weight of rats assumed to be 200 g (EURAR 2003); no tumours observed; IARC (2002) notes small group sizes, incomplete reporting of study (Schmähl 1955). Neoplastic endpoints: F344/N rats: 0, 52, 157, 314 mg/m3 (0, 10, 30, 60 ppm); 6 hours/day, 5 days/week for 105 weeks; significant increase in incidence of nasal olfactory neuroblastoma in males and females; rare tumour type, not observed in historical controls [0/49, 0/49, 4/48 (p=0.056), 3/48 in male rats and 0/49, 2/49, 3/49, 12/49 (p=001) in female rats at 0, 52, 157 and 314 mg/m3respectively]; significant incidence of adenoma of the respiratory epithelium of male and female rats [0/49, 6/49 (p=0.013), 8/48 (p=0.003), 15/48 (p<0.001) in male rats and 0/49, 0/49, 4/49 (p=0.053), 2/49 in female rats at 0, 52, 157,and 314 mg/m3 respectively] (NTP 2000). [Additional studies: A/J mice; inhalation, up to 30 ppm for 6 months; significant increase in number of adenomas per tumour-bearing mouse, but not adenomas per mouse (Adkins et al. 1986).] |
| Genotoxicity and related endpoints: in vivo | Micronuclei test Unscheduled DNA synthesis DNA damage DNA fragmentation Adduct formation to proteins: Neoplastic transformation: Non-mammalian wing spot test: Non-mammalian micronucleus formation: |
| Genotoxicity and related endpoints: in vitro | Mutagenicity Negative : mutation at TK and HPRT loci; human MCL-5B-lymphoblastoid cells (Sasaki et al. 1997) Negative: E. coli K12 envA - uvrB -, GY5027 envA - uvrB -, GY40451 amp R; prophage induction with activation (Ho and Ho 1981; Mamber et al. 1984) Negative: E. coli PQ37 (chromotest), SOS induction without activation (Mersch-Sundermann et al. 1993) Negative: S. typhimurium TA1535/pSK1002 (umu gene expression - SOS inducing activity) with and without activation (Nakamura et al. 1987) Negative: E. coli WP2/WP100 uvrA - recA - with activation (Mamber et al. 1984) Micronucleus Formation Chromosomal aberrations Sister chromatid exchange DNA strand breaks Unscheduled DNA synthesis Protein adduct formation DNA fragmentation Cell transformation assay T1 assay |
[1] LD50 = median lethal dose; LC50 = median lethal concentration; LO(A)EL = Lowest-observed –(adverse)-effect level; LO(A)EC = Lowest-observed-(adverse)-effect concentration; NO(A)EL = No-observed-(adverse)-effect level; NO(A)EC = No-observed-(adverse)-effect concentration; LED = Lowest effective dose; HID = Highest ineffective dose
The European Commission (EURAR 2003) reported that there were no epidemiological studies on the human health effects of naphthalene, and the only human information available derives from a limited number of early case reports that provide no quantitative data on the levels or duration of exposure. Effects observed in humans after acute exposure to naphthalene are haemolytic anaemia and cataract formation. Most human toxicity is described by the International Agency for Research on Cancer (IARC 2002) as being of an accidental or suicidal nature, resulting from inhalation of fumes containing naphthalene or ingestion of mothballs. The European Commission also notes that exposure to naphthalene vapour may also result in dermal exposure (EURAR 2003). Some of these cases of haemolytic anaemia involved babies exposed to naphthalene through diapers, clothing and blankets treated with mothballs (Anziulewicz et al. 1959; Valaes et al. 1963). Additional case reports have been presented involving human transplacental exposure of the fetus after maternal ingestion of naphthalene, resulting in haemolytic anemia of the infant (Anziulewicz et al. 1959; Zinkham and Childs 1957, 1958). Within the human population, a subset of people may have increased susceptibility to the anaemic effects of naphthalene, due to a genetically inherited deficiency in an enzyme of the red blood cell, glucose-6-phoshate dehydrogenase (IARC 2002).