Screening Assessment for the Challenge
This page has been archived on the Web
Information identified as archived is provided for reference, research or recordkeeping purposes. It is not subject to the Government of Canada Web Standards and has not been altered or updated since it was archived. Please contact us to request a format other than those available.
Archived
1,2-Benzenedicarboxylic acid, di-C7-11-branched and linear alkyl esters
Chemical Abstracts Service Registry Number
68515-42-4
Environment Canada
Health Canada
November 2009
- Synopsis
- Introduction
- Substance Identity
- Physical and Chemical Properties
- Sources
- Uses
- Releases to the Environment
- Environmental Fate
- Persistence and Bioaccumulation Potential
- Potential to Cause Ecological Harm
- Potential to Cause Harm to Human Health
- Conclusion
- References
- Appendix 1: Upper-bounding inhalation exposure estimates for DHNUP volatilization from end products
- Appendix 2: Summary of health effects information for DHNUP
The Ministers of the Environment and of Health have conducted a screening assessment of 1,2-benzenedicarboxylic acid, di-C7-11-branched and linear alkyl esters (DHNUP), Chemical Abstracts Service Registry Number 68515-42-4. This substance was identified in the categorization of the Domestic Substances List (DSL) as a high priority for action under the Challenge. DHNUP was identified as a high priority because it was considered to pose intermediate potential for exposure of individuals in Canada and had been classified by the European Commission on the basis of reproductive and developmental toxicity. Although DHNUP met the ecological categorization criterion for inherent toxicity to aquatic organisms, it did not meet the criteria for bioaccumulation potential or persistence.
In response to a notice issued under section 71 of the Canadian Environmental Protection Act, 1999(CEPA 1999), DHNUP was reported to be manufactured in a quantity in the range of 100 000–1 000 000 kg in 2006. The total quantity imported into Canada in the same calendar year was reported to be in the range of 10 000 000–100 000 000 kg. Manufacturing activity involving DHNUP has decreased significantly in Canada subsequent to the 2006 reporting year, and annual importation activity was estimated to have declined by over 90%. This reduction in the use of DHNUP in Canada occurred largely due to the decreased availability of the upstream plasticizer alcohols required for its synthesis. It is not known if the decline in DHNUP quantities in Canadian commerce is temporary or permanent. DHNUP’s principal use that has been identified as ongoing in Canada subsequent to the 2006 reporting year was as a plasticizer for PVC (PolyVinyl Chloride).
Population exposure to DHNUP through environmental media is expected to be negligible based on minimal environmental releases of DHNUP in Canada during the 2006 calendar year as identified in responses to a notice issued under section 71 of CEPA 1999. Population exposure to DHNUP in indoor air through end products is expected to be low, based on expected use patterns and conservative exposure estimates.
The health effects associated with exposure to DHNUP are primarily reproductive and developmental toxicity and liver toxicity, based on observations in experimental animals. The margins between conservative upper-bounding estimates of exposure from indoor air (from potential off-gassing of current or historical products containing DHNUP) and levels associated with effects in experimental animals are considered to be adequately protective.
Ecological exposure scenarios were developed based on the most recent (post-2006) information on commercial use to estimate releases into the aquatic environment from industrial operations and resulting aquatic concentrations. Environmental concentrations are estimated to be below those that would harm sensitive aquatic organisms. This indicates that the substance is unlikely to cause ecological harm in the aquatic environment.
On the basis of the adequacy of the margins between exposure to DHNUP in indoor air and critical effect levels in experimental animals, it is concluded that DHNUP is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
Based on the information available, it is concluded that DHNUP is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends. DHNUP does not meet the persistence or bioaccumulation criteria as set out in the Persistence and Bioaccumulation Regulations.
This substance will be included in the upcoming Domestic Substances List inventory update initiative. In addition and where relevant, research and monitoring will support verification of assumptions used during the screening assessment.
Based on the information available, it is concluded that 1,2-Benzenedicarboxylic acid, di-C7-11-branched and linear alkyl esters does not meet any of the criteria set out in section 64 of CEPA 1999.
The Canadian Environmental Protection Act, 1999(CEPA 1999) (Canada 1999) requires the Minister of the Environment and the Minister of Health to conduct screening assessments of substances that have met the categorization criteria set out in the Act to determine whether these substances present or may present a risk to the environment or to human health.
Based on the information obtained through the categorization process, the Ministers identified a number of substances as high priorities for action. These include substances that
- met all of the ecological categorization criteria, including persistence (P), bioaccumulation potential (B) and inherent toxicity to aquatic organisms (iT), and were believed to be in commerce in Canada; and/or
- met the categorization criteria for greatest potential for exposure (GPE) or presented an intermediate potential for exposure (IPE) and had been identified as posing a high hazard to human health based on classifications by other national or international agencies for carcinogenicity, genotoxicity, developmental toxicity or reproductive toxicity.
The Ministers therefore published a notice of intent in the Canada Gazette, Part I, on December 9, 2006 (Canada 2006), which challenged industry and other interested stakeholders to submit, within specified timelines, specific information that may be used to inform risk assessment and to develop and benchmark best practices for the risk management and product stewardship of these substances identified as high priorities.
The substance 1,2-benzenedicarboxylic acid, di-C7-11-branched and linear alkyl esters (DHNUP) was identified as a high priority for assessment of human health risk because it was considered to present IPE and had been classified by other agencies on the basis of reproductive and developmental toxicity. The Challenge for this substance was published in the Canada Gazette on May 31, 2008 (Canada 2008). A substance profile was released at the same time. The substance profile presented the technical information available prior to December 2005 that formed the basis for categorization of this substance. As a result of the Challenge, submissions of information pertaining to the substance were received.
Although DHNUP met the ecological categorization criterion for inherent toxicity to aquatic organisms, it did not meet the criteria for bioaccumulation potential or persistence.
Screening assessments focus on information critical to determining whether a substance meets the criteria for defining a chemical as toxic as set out in section 64 of CEPA1999. Screening assessments examine scientific information and develop conclusions by incorporating a weight of evidence approach and precaution.
In addition to the technical information presented in the substance profile, this screening assessment considers any new information on chemical properties, hazards, uses and exposure identified after December 2005, including the additional information submitted under the Challenge as well as information received during follow-up communications with industry on section 71 survey submissions. Data relevant to the screening assessment of this substance were identified in original literature, review documents and stakeholder research reports and from literature searches up to February 2009. Key studies were critically evaluated; modelling results may have been used to reach conclusions. Evaluation of risk to human health involves consideration of data relevant to estimation of exposure (non-occupational) of the general population, as well as information on health hazards (based principally on the weight of evidence assessments of other agencies that were used for prioritization of the substance). Decisions for human health are based on the nature of the critical effect and/or margins between conservative effect levels and estimates of exposure, taking into account confidence in the completeness of the identified databases on both exposure and effects, within a screening context. The screening assessment does not represent an exhaustive or critical review of all available data. Rather, it presents a summary of the critical information upon which the conclusion is based.
This screening assessment was prepared by staff in the Existing Substances Programs at Health Canada and Environment Canada and incorporates input from other programs within these departments. The ecological and human health portions of this assessment have undergone external written peer review/consultation. Comments on the technical portions relevant to human health were received from the National Industrial Chemicals Notification and Assessment Scheme, Australia, and scientific experts selected and directed by Toxicology Excellence for Risk Assessment (TERA), including Susan Griffin (US Environmental Protection Agency [EPA]), Donna Vorhees (The Science Collaborative) and Lynne Haber (TERA). Additionally, the draft of this screening assessment was subject to a 60-day public comment period. Although external comments were taken into consideration, the final content and outcome of the screening risk assessment remain the responsibility of Health Canada and Environment Canada.
The critical information and considerations upon which the assessment is based are summarized below.
For the purposes of this document, this substance will be referred to as DHNUP, which has been derived from the name di(heptyl, nonyl, undecyl) phthalate. The substance identity information is summarized in Table 1.
Table 1. Substance identity for DHNUP
| Chemical Abstracts Service Registry Number (CAS RN) | 68515-42-4 |
| DSL name | 1,2-Benzenedicarboxylic acid, di-C7-11-branched and linear alkyl esters |
| NCI names | 1,2-Benzenedicarboxylic acid, di-C7-11-branched and linear alkyl esters (AICS, ASIA-PAC, EINECS, NZIoC, PICCS) Dialkyl phthalate (C7-11) branched and linear (PICCS) Phthalate ester (PICCS) |
| Other names | 711P; D711P; Di-711-phthalate; Dialkyl(C7-11-branched and linear) phthalate; Di(heptyl, nonyl, undecyl) phthalate; Di(heptyl, nonyl, undecyl) phthalate (mixed isomers); Phthalic acid, dialkyl (C7-C11) ester; Santicizer 711 |
| Chemical group (DSL stream)1 | UVCB Organic |
| Major chemical class or use | Phthalate ester |
| General chemical formula (minimum to maximum number of atoms) | C22H34O4–C30H50O4 |
| General structural formula | 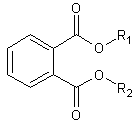 R1 = C7H15 or C9H19 or C11H23R2 = C7H15 or C9H19 or C11H23R1 and R2 can be linear or branched |
| Molecular mass | 362 – 474 g/mol |
| Representative chemical formula1 | C24H38O4 |
| Representative chemical structure used to run the estimation model1 |
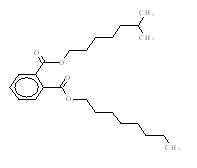 |
| Representative SMILES used to run the estimation model1 | O=C(c1ccccc1C(=O)OCCCCCC(C)C)OCCCCCCCC |
| Abbreviations: AICS, Australian Inventory of Chemical Substances; ASIA-PAC, Asia-Pacific Substances Lists; CAS RN, Chemical Abstracts Service Registry Number; DSL, Domestic Substances List; EINECS, European Inventory of Existing Commercial Chemical Substances; NCI, National Chemical Inventories; NZloC, New Zealand Inventory of Chemicals; PICCS, Philippine Inventory of Chemicals and Chemical Substances; SMILES, simplified molecular input line entry specification. Source: NCI (2006) 1 This substance is a UVCB (Unknown or Variable Composition, Complex Reaction Products, or Biological Materials). In other words, it is not a discrete chemical, and thus it may be characterized by a variety of structures. Use of the representative structure and SMILES notation, as selected by the Syracuse Research Corporation, was limited to the domain of physicochemical property modelling (EPIsuite 2009). As this representative structure is a hypothetical construct, it was not considered applicable to modelling human health toxicological properties based upon specific structure–activity relationships. |
|
DHNUP, Chemical Abstracts Service Registry Number (CAS RN) 68515-42-4, is a UVCB (Unknown or Variable Composition, Complex Reaction Products, or Biological Materials), a mixture of phthalates containing the following six components:
| CAS RN | Name |
|---|---|
| 3648-20-2 | 1,2-Benzenedicarboxylic acid, diundecyl ester, linear only |
| 68515-44-6 | 1,2-Benzenedicarboxylic acid, diheptyl ester, branched and linear |
| 68515-45-7 | 1,2-Benzenedicarboxylic acid, dinonyl ester, branched and linear |
| 111381-89-6 | 1,2-Benzenedicarboxylic acid, heptyl nonyl ester, branched and linear |
| 111381-90-9 | 1,2-Benzenedicarboxylic acid, heptyl undecyl ester, branched and linear |
| 111381-91-0 | 1,2-Benzenedicarboxylic acid, nonyl undecyl ester, branched and linear |
It should be noted that these components, except for CAS RN 3648-20-2, are themselves mixtures of branched and linear compounds.
There is variation regarding the nomenclature used to represent this mixture. In Europe, the composite CAS RN 68515-42-4 is used most commonly to represent DHNUP. However, CAS RNs in the list above are also made reference to in Europe (European Commission 2003). In the United States, DHNUP is more frequently represented by listing together all the individual CAS RNs provided above. The US EPA considers the commercial form of DHNUP to be a mixture of the above six CAS RNs and not described by CAS RN 68515-42-4 (US EPA 1990).
Although the composition of DHNUP is variable, proton and 13C nuclear magnetic resonance analysis performed on a typical sample of DHNUP found a predominantly linear composition, with approximately 30% branched aliphatics (Ernes et al. 1984). Greater than two-thirds of the branching occurred as a methyl group attached beta to the ester linkage (Ernes et al. 1984). Another review of the commercial substance DHNUP revealed an equal composition mixture of the six phthalate components (ExxonMobil Biomedical Sciences, Inc. 2006). The overall content of C4-C6 isomer impurities in the DHNUP mixture is approximately 10% due to the contribution of these impurities from some of the six components (ExxonMobil Biomedical Sciences, Inc. 2006). It should be noted that some common names or trade names for DHNUP may also represent different CAS RNs for similar mixtures of phthalates or individual phthalates. For example, the common name 711P may refer to DHNUP or to 1,2-benzenedicarboxylic acid, (C7,C11) ester, branched and linear (CAS RN 111381-90-9) (ExxonMobil Biomedical Sciences, Inc. 2006). The components of DHNUP, except for CAS RN 111381-90-9, are known to be commercially sold as separate products (ExxonMobil Biomedical Sciences, Inc. 2006).
It is notable that, due to the variable composition of DHNUP, no single discrete molecular structure may be considered truly representative of DHNUP, and thus use of the representative structure provided in Table 1 was limited to physicochemical property modelling.
Table 2 contains experimental and modeled physical and chemical properties of DHNUP that are relevant to its environmental fate.
Table 2. Physical and chemical properties for DHNUP
| Property | Type | Value1 | Descriptor | Reference |
|---|---|---|---|---|
| Abbreviations: Koc, organic carbon partition coefficient; Kow, octanol–water partition coefficient. 1 Values in parentheses represent the original values as reported by the authors or as estimated by the models. 2 The test chemical used was Palatinol®711P, a trade name representing the following six CAS RNs: 85507-79-5, 68515-44-6, 68515-45-7, 111381-89-6, 111381-90-9 and 111381-91-0. It is notable that one of the components of DHNUP, diundecyl phthalate, linear only (CAS RN 3648-20-2), is replaced by diundecyl phthalate, branched and linear (CAS RN 85507-79-5), in Palatinol® 711P. For the purposes of this assessment, Palatinol® 711P is considered to be equivalent to DHNUP. * Values used as model inputs. |
||||
| Melting point (°C) | Experimental | -57* | - | ECB 20002 |
| Modelled | 78 | – | MPBPWIN 2000 | |
| Boiling point (°C at 0.7 kPa) | Experimental | 235–278 | - | ECB 20002 |
| Modelled | 424 | – | MPBPWIN 2000 | |
| Density (kg/m3 at 20°C) | Experimental | 969–973 | - | ECB 20002 |
| Vapour pressure (Pa) | Modelled | 9.2 × 10-5*at 25°C | Low | MPBPWIN 2000 |
| Experimental | <10 at 20°C (<0.1 hPa at 20°C) |
Low | ECB 20002 | |
| Henry’s Law constant (Pa·m3/mol at 25°C) |
Modelled | 0.86 (8.5 × 10-6 atm·m3/mol) (Group method) |
Moderate | HENRYWIN 2000 |
| 1.2 (1.2 × 10-5 atm·m3/mol) (Bond method) |
Moderate | HENRYWIN 2000 |
||
| Log Kow | Experimental | 4.8 | Moderate | ECB 20002 |
| Modelled | 8.5* | Very high | KOWWIN 2000 | |
| Log Koc | Modelled | 5.2 | Very high | PCKOCWIN 2000 |
| Water solubility (mg/L) | Experimental | 0.1* (at 20°C) | Low | ECB 20002 |
| Modelled | 2.1 × 10-3 (at 25°C) | Very low | WSKOWWIN 2000 | |
DHNUP is an anthropogenic mixture of linear and branched phthalates that has not been identified to occur naturally. Production of ortho phthalate esters involves the sequential addition of a stoichiometric excess of linear and branched alcohols to phthalic anhydride (Stanley et al. 2003). The esterification reaction is catalysed by acidic conditions and occurs in heated kettles subjected to agitation and water removal (Stanley et al. 2003).
In response to a notice issued under section 71 of CEPA 1999, DHNUP was reported to be manufactured in Canada at a quantity in the range of 100 000–1 000 000 kg in 2006 (Environment Canada 2008a). Importation activities (whether alone, in a mixture, in a product or in manufactured items above the reporting threshold of 100 kg) were reported at a quantity between 10 000 000 and 100 000 000 kg in 2006 (Environment Canada 2008a). Several submissions were made for components of DHNUP as individual substances or as components of other phthalate mixtures, and these data were considered. However, in cases where the components did not constitute part of the DHNUP mixture, the data were not used in the exposure assessment of DHNUP (Environment Canada 2008a). Information provided by stakeholders, either in conjunction with the survey submissions or during further follow-up communications, indicates that manufacture of DHNUP has decreased significantly since 2006 and that importation has decreased by more than 90%. The decrease in manufacture of DHNUP occurred largely because of the decreased availability of upstream plasticizer alcohols required for its synthesis, resulting from increased prices of feedstocks used in the production of linear oxo alcohols, which led to the substitution of other phthalates in end products (BASF 2006; Bizzari et al. 2007; Environment Canada 2008a). While the decline in DHNUP quantities in Canadian commerce may persist at least until the termination of a plasticizer alcohol production contract in 2013, the long-term trend is unknown but may depend upon the development of the cost gap between linear and branched plasticizer alcohols (BASF 2006).
Regarding recent trends in commerce, Canadian consumption of linear phthalates in general, represented by C7-C11 phthalates as the majority market component, is expected to decrease at an annual average growth rate of −12.9% between 2005 and 2010 (Bizzari et al. 2007). This negative growth rate may be largely attributable to the market-based reduction of DHNUP observed in Canada for some applications (Environment Canada 2008a). Worldwide consumption of linear phthalates is expected to decrease at an annual average growth rate of −22.9% for the same period (Bizzari et al. 2007). Plasticization of vinyl resins using high molecular weight plasticizers such as DHNUP represents the highest volume use of phthalate esters worldwide and 80–90% of worldwide plasticizer consumption in general (Stanley et al. 2003; Bizzari et al. 2007).
In response to a notice issued under section 71 of CEPA 1999, total use of DHNUP in Canada in the 2006 calendar year was reported to be in the range 1 000 000–10 000 000 kg (Environment Canada 2008a). As defined in the notice under section 71 of CEPA 1999, use activity excludes any distribution, repackaging or sale of DHNUP but may include, but is not limited to, use of DHNUP in a chemical reaction, in the formulation of a mixture or in the maintenance and cleaning of equipment. Some submitters indicated that DHNUP is no longer used for some applications in Canada after the reporting year, with an estimated decline in total annual use activity of greater than 90% (Environment Canada 2008a). Currently, usage of DHNUP in Canada totals between 100,000 and 1,000,000 kg/year, with no more than approximately 200,000 kg/year used at any single facility.
According to recent submissions made under section 71 of CEPA 1999 and information derived from other sources, including the scientific and technical literature, DHNUP is used principally for plasticizing applications (Environment Canada 2008a). DHNUP’s principal use that has been identified as ongoing in Canada subsequent to the 2006 reporting year was as a plasticizer for PVC (PolyVinyl Chloride) (Environment Canada 2008a). As a plasticizer, DHNUP is compatible with several polymer resins, including copolymer and homopolymer vinyl resins, nitrile, chlorinated and styrene-butadiene rubber, cellulosics, neoprene, polyurethane, acrylic latex, alkyd resins and rosin-modified polyester resins of maleic anhydride and glycerine (TDS 1998; US Patent 1998, 2000, 2001; MSDS 2000b, 2003a, b, c; Stanley et al. 2003).
Several Health Canada partner groups indicated that use of DHNUP was not identified in their respective areas. DHNUP is not anticipated to be present in cosmetic products in Canada, as it is not listed as an ingredient in the Cosmetic Notification System database (CNS 2008); however, DHNUP is not currently prohibited or regulated in cosmetic products in Canada, as it is not listed on the Health Canada cosmetic ingredient hotlist (Health Canada 2007). There are no registered pesticides that contain DHNUP as an active ingredient or formulant in Canada (PMRA 2007), and DHNUP is not listed as an approved food additive under the Index of Food Additives contained within the Food and Drug Regulations(Canada 1978). Use of DHNUP in food packaging applications in Canada has not been reviewed (2008 email from Food Directorate, Health Canada, to Existing Substances Division, Health Canada; unreferenced). DHNUP is not present as an ingredient in natural health products (2008 email from Natural Health Products Directorate, Health Canada, to Existing Substances Division, Health Canada; unreferenced), pharmaceutical products (2008 emails from Therapeutic Products Directorate, Health Canada, to Existing Substances Division, Health Canada; unreferenced) or veterinary drugs (2008 email from Veterinary Drugs Directorate, Health Canada, to Existing Substances Division, Health Canada; unreferenced). The manufacture of medical devices in Canada is not known to involve the use of DHNUP (2008 email from Medical Devices Bureau, Health Canada, to Existing Substances Division, Health Canada; unreferenced). DHNUP was not detected in a survey of over 70 soft vinyl children’s products carried out by the Product Safety Program of Health Canada in 2007 (2009 email from Healthy Environments and Consumer Safety Branch, Health Canada, to Existing Substances Division, Health Canada; unreferenced). The Controlled Products Regulations under the Hazardous Products Act do not provide a minimum reporting limit for DHNUP concentration on Material Safety Data Sheets accompanying workplace chemicals as specified on the Ingredient Disclosure List (Canada 1988).
DHNUP was introduced worldwide in the early 1970s (Ernes et al. 1984). The following uses of DHNUP were identified as global or historical in nature. For vinyl applications, DHNUP plasticizes polyvinyl chloride (PVC) coatings of mine brattice cloth and metal coils (US Patent 2001; SpecialChem S.A. 2009). Industrially, DHNUP plasticizes polyurethane prepolymers for foam applications (US Patent 1998). For automotive use, DHNUP is used as a low-volatility sealant in addition to functioning as a plasticizer in primerless urethane adhesive, glass and transmission adhesive, vibration-damping coatings and exterior trim (TDS [date unknown], 1998; US Patent 2000; Patrick 2005; MSDS 2007; Environment Canada 2008a). In terms of construction materials, DHNUP has been used as a plasticizer in elastomeric roof and barrier coatings, geomembranes, tarpaulins, flashing cement, wood filler, wood and stone hardener, caulk, sanding sealer and high-solids lacquer (TDS 1998; MSDS 2000a, b, 2001, 2003a, b, c, 2008a, b, c). Finally, DHNUP has been reported to be used to plasticize high-end luggage (TDS 1998).
In responses to a notice issued under section 71 of CEPA 1999, 1000 to 10 000 kg of DHNUP were reported to be released to air and less than 10 kg were reported to be released to water in the 2006 calendar year (Environment Canada 2008a). Section 71 data also indicate some transfers of DHNUP to hazardous and non-hazardous waste facilities occurred in the 2006 calendar year (Environment Canada 2008a). DHNUP is not reportable to the National Pollutant Release Inventory (NPRI 2007) or to the US Toxics Release Inventory Program (TRI 2006); therefore, no release information was available from these sources.
Based on its physical and chemical properties (Table 2), the results of Level III fugacity modelling (Table 3) suggest that DHNUP will reside predominantly in soil or sediment, depending on the compartment of release.
Table 3. Results of the Level III fugacity modelling for DHNUP (EQC 2003)
| Substance released to: | Fraction of substance partitioning to each medium (%) | |||
|---|---|---|---|---|
| Air | Water | Soil | Sediment | |
| Air (100%) | 8.6 | 2.3 | 69.2 | 20 |
| Water (100%) | 0 | 10.2 | 0 | 89.8 |
| Soil (100%) | 0 | 0 | 100.0 | 0 |
Environmental Persistence
An assessment of the persistence of phthalates was submitted by the Phthalate Esters Panel (2004) of the American Chemistry Council. Empirical and modelled data (using BIOWIN 2000) demonstrated that none of the discrete organic phthalates are persistent in water or soil. Estimated half-lives in water and soil did not exceed 37.5 days (Phthalate Esters Panel 2004). Environment Canada (2006) reviewed the submission and agreed with the assessment concerning the persistence of the discrete organic phthalates. The categorization of UVCB phthalates was conducted similarly to that of the discrete organics. Therefore, it is expected that none of the phthalates that make up the DHNUP mixture would be persistent in water or soil (Environment Canada 2006). Using a water:sediment half-life extrapolation ratio of 1:4 (Boethling et al. 1995), the half-life of the phthalates in sediments would be <365 days. Additionally, in a laboratory study using anaerobic sediment taken from a river in Taiwan, Chang et al. (2005) reported a half-life of 25.7 days for another phthalate, di(2-ethylhexyl) phthalate (DEHP), under optimal conditions of 30°C and pH 7. At 20°C and pH 7, the half-life was 31.5 days. This indicates that phthalates can be biodegraded under anaerobic conditions.
Klamer et al. (2005), in a study of contaminants in North Sea sediments near the Netherlands, reported concentrations of DEHP of 3.27 and 3.34 mg/kg dry weight at two offshore locations. This indicates that the substance might be at least somewhat persistent in marine sediments.
DHNUP is expected to react with hydroxyl radicals in the atmosphere, with an estimated half-life in air of about 6 hours (AOPWIN 2000).
DHNUP does not meet the persistence criteria in air, water, soil or sediment (half-life in air ³2 days, half-lives in soil and water =182 days, half-life in sediment =365 days) as set out in the Persistence and Bioaccumulation Regulations (Canada 2000).
Potential for Bioaccumulation
The Phthalate Esters Panel (2004) submitted an assessment demonstrating that most phthalates have experimental bioaccumulation factors (BAFs) and bioconcentration factors (BCFs) below 5000 L/kg, as they are readily metabolized by fish. The available data covered a wide range of structures with alkyl substituents. Environment Canada (2006) reviewed the submission and agreed with the assessment, concluding that DHNUP would also be expected to be metabolized in higher organisms and would not bioaccumulate.
A study of the distribution of dialkyl phthalate esters in a marine aquatic food web at Vancouver, British Columbia, showed that the substances did not biomagnify. In fact, the lipid equivalent concentrations of the high molecular weight phthalate esters declined significantly with increasing trophic position, likely because of increased metabolic transformation of the substances in the higher organisms (Mackintosh et al. 2004). Some of the dialkyl phthalate esters included in this study were very similar to DHNUP, with alkyl chain lengths of seven to nine carbon atoms.
DHNUP does not meet the bioaccumulation criteria (BCF, BAF ≥5000) as set out in the Persistence and Bioaccumulation Regulations (Canada 2000).
Ecological Effects
Although phthalates may have low “true” water solubilities, they possess the ability to form suspensions that may cause adverse effects through physical contact with Daphnia at concentrations that are very low, but still exceed their solubility. For the purposes of categorization, Environment Canada (2006) did not differentiate between mortality caused by a physical effect of suspended micro-droplets and mortality resulting from an internal effect caused by the dissolved substance. Available toxicity and water solubility information suggests that the high molecular weight phthalates, including a number of UVCBs such as DHNUP, form these suspensions and are able to elicit effects at concentrations below 0.1 mg/L (Rhodes et al. 1995). Therefore, these substances are considered to have the potential to harm aquatic organisms at relatively low concentrations (Environment Canada 2006). In the study by Rhodes et al. (1995) the 21-day no-observed-effect concentration (NOEC) for di-(heptyl, nonyl, undecyl) phthalate (711P) was 0.094 mg/L, based on survival of Daphnia magna. The 21-day lowest-observed-effect concentration (LOEC) was 0.19 mg/L. Rhodes et al. (1995) also studied the effect of phthalate esters on rainbow trout, Oncorhynchus mykiss. In a 152-day (120-day post-hatch) early life-stage study, 711P had no effect on survival or growth at the highest concentration tested, 0.41 mg/L.
No information about the effects of DHNUP on sediment-dwelling organisms was identified, but there is information for some other similar phthalate esters. In a 28-day sediment toxicity study with the midge (Chironomus riparius), Brown et al. (1996) reported that DEHP and di-isodecyl phthalate had no effect on the development of midges, based on emergence times and numbers and sex of emerging adults, up to the highest sediment concentration tested, 10 000 mg/kg dry weight. Similarly, Call et al. (2001) found that di-isononyl phthalate, di-isodecyl phthalate and a commercial mixture of C7, C9 and C11 isophthalate esters had no effect on survival or growth of Chironomus tentans or Hyalella azteca at the single sediment concentrations tested for each substance (2090–2900 mg/kg dry weight) after 10-day exposures. These results indicate that the phthalate esters are not highly toxic to benthic organisms.
Ecological Assessment
The approach taken in this assessment was to examine the available scientific information and develop conclusions based on a weight of evidence approach and using precaution, as required under CEPA 1999. Lines of evidence considered include results from a risk quotient (RQ) calculation as well as information on the persistence, bioaccumulation, inherent toxicity, sources and fate of the substance.
Based on the available information, DHNUP does not persist in the environment and is not bioaccumulative, as defined in the Persistence and Bioaccumulation Regulations (Canada 2000).
DHNUP can potentially enter the Canadian environment from several different sources: from facilities where the substance is manufactured, from facilities where the substance is used, and from the rinsing and cleaning of shipping containers.
No data concerning concentrations of this substance in water in Canada have been identified. Therefore, environmental concentrations are estimated from available information, including estimated substance quantities, release rates, and size of receiving water bodies. As DHNUP is used by industrial facilities and could be released to water, Environment Canada’s Industrial Generic Exposure Tool – Aquatic (IGETA) was employed to estimate a conservative substance concentration in a generic water course receiving industrial effluents (Environment Canada 2008b).
The generic scenario is designed to provide exposure estimates based on conservative assumptions regarding the amount of substance processed and released, the number of processing days, the sewage treatment plant removal rate, and the size of the receiving watercourse. The tool models an industrial-release scenario based on loading data from sources such as industrial surveys and knowledge of the distribution of industrial discharges in the country, and calculates a predicted environmental concentration (PEC).
The PEC for DHNUP was calculated based on a use quantity of 200,000 kg for a single facility at which DHNUP is assumed to be manufactured and incorporated into a resin. It is assumed that 0.015% is being released over a period of 250 days, with a sewage treatment plant removal efficiency of 83.7% (primary and secondary treatment)(ASTreat 2006) and a dilution factor of 10 in receiving water. The equation and inputs used to calculate the PEC in the receiving water course are described in Environment Canada (2008c).
The water PEC for DHNUP, resulting from industrial releases, is 0.0006 mg/L.
The predicted no-effects concentration (PNEC) was 0.094 mg/L, the 21-d NOEC for Daphnia magna reported by Rhodes et al. (1995). Because harm was caused by physical entrapment of organisms in a surface film, rather than a physiological mechanism, no assessment factor was applied to the NOEC to derive the PNEC. This concentration is above the substance’s modeled water solubility of 0.002 mg/L (Table 2) but is about the same as the experimental water solubility of 0.1 mg/L reported in ECB (2000). Rhodes et al. (1995) noted that for phthalate esters, standard phase separation techniques do not provide separation of micelles/micro-droplets from the water solution, so exposure concentrations reported in some ecotoxicology studies may be greater than the “true” water solubility.
The risk quotient (RQ), PEC/PNEC = 0.0006/0.094 = 0.006. This indicates that DHNUP is unlikely to cause harm to aquatic organisms.
DHNUP could also enter the Canadian environment from the rinsing and cleaning of shipping containers. However, communication with industry indicates that DHNUP residues from shipping containers (tank trucks and totes) are collected by waste disposal companies. Containers are purged with alkali compounds followed by a hot water or steam rinse. The organic portion is then collected and mixed with other oily waste materials. These combined wastes are either sent to oil refineries where they are re-refined or are otherwise treated so that environmental releases are minimized.
It is concluded that DHNUP is not entering the environment at concentrations that may have a long-term harmful effect on the environment.
Uncertainties in Ecological Assessment
Gaps in available experimental data were largely filled through the use of quantitative structure–activity relationships (QSARs). For DHNUP, there were a limited number of experimental data identified for degradation, bioaccumulation and ecotoxicity, and QSARs were used to supplement them. Additionally, values for some key physical and chemical properties (e.g. Henry’s Law constant), which are used as input to the QSAR models, also had to be estimated.
To estimate the potential for DHNUP to cause ecological harm, exposure and effects were compared quantitatively using realistic worst-case RQs. There is uncertainty about the quantity of DHNUP currently used in Canada. Some former suppliers of the substance have reported that they no longer sell the product, but similar alternative substances appear to be available (BASF 2006; Environment Canada 2008a). It is not known if the decline in manufacture, importation and use quantities of DHNUP in Canadian commerce is temporary or permanent. However, if current handling practices are maintained, relatively large increases in use quantities would not pose a risk, as indicated by the low risk quotient.
The PNEC was based on a physical effect, the entrapment of Daphnia in a surface layer of undissolved material that forms under laboratory conditions at concentrations close to or above the water solubility limit. It is not known the extent to which this phenomenon may occur in natural waters, where wave action, etc., might prevent the formation of a surface layer.
Exposure Assessment
No monitoring data were identified regarding measured concentrations of DHNUP in environmental media, beverages or food, regardless of location. Literature searching was conducted for all components of DHNUP however only monitoring data that were attributable to DHNUP as a mixture were considered. Concentrations of the components of DHNUP that could not be identified as part of the mixture were not used, as the components are also sold commercially as separate substances and may occur in separate mixtures (ExxonMobil Biomedical Sciences, Inc. 2006).
Releases reported in responses to a notice issued under section 71 of CEPA 1999 were under 10 000 kg in total for the 2006 calendar year. Due to the market-based phaseout of the substance, releases are assumed to have decreased further after the end of 2006. Environmental exposure of the general population in Canada is expected to be negligible. Although some manufacturing of DHNUP occurred during the 2006 calendar year, very minimal environmental releases of phthalate esters occur during the manufacturing process as a general principle (Stanley et al. 2003). Although some disposal of DHNUP, presumably in the form of PVC edge trim waste, to non-hazardous waste facilities occurred during the 2006 calendar year, phthalate emissions from plastics buried in the soil are expected to be negligible due to ready biodegradation at the plastic surface and poor soil mobility (Stanley et al. 2003).
In regards to estimating exposure to DHNUP present in end products, DHNUP may plasticize some PVC items identified as having potential use in Canada during or after the reporting year of 2006. One such item is described below.
PVC insulation for electrical cable may contain DHNUP as a plasticizer (TDS 1998) and was a potential use in Canada during the 2006 reporting year Although no empirical studies could be identified for off-gassing of DHNUP from cable insulation, two studies involving DEHP were identified. One study of electrical cable used outdoors over an 18-year period in Romania demonstrated only 2% loss of DEHP during service life (Brebu et al. 2000). Another study performed in Sweden used a thermal acceleration aging process to approximate roughly 44 years of natural cable aging at ambient temperature (Jakubowicz et al. 1999). Only 1% loss of total extractable matter from PVC cable insulation containing 17% by weight DEHP was observed (Jakubowicz et al. 1999). As DHNUP has significantly lower volatility in comparison with DEHP, the migration rate would be accordingly decreased (TDS 1996). These two studies coincide well with the general release rate assumption of 0.05% plasticizer over service lifetime derived from observations of indoor items in Western Europe (OECD 2007). In addition, the PVC insulation is further surrounded by an external jacket composed of polyalkylene terephthalate plasticized with a different agent. Based upon the length of the time periods represented in the two empirical studies and the role of the jacket as a substantial diffusion barrier, daily inhalation exposure to DHNUP volatilized from PVC cable insulation would be expected to be negligible (US Patent 1995). In terms of dermal contact, exposure to DHNUP is expected to be negligible, as contact would occur solely with the external jacket, and handling of the cable would be anticipated to be minimal, occurring solely during installation and transport activities (US Patent 1995).
DHNUP has low volatility and long-life weatherability, ensuring a long performance lifetime for products; therefore, daily inhalation exposure is anticipated to be negligible (TDS 1996). However, off-gassing from historical products is considered due to this long service lifetime of some PVC articles (which could act as potential long-term sources of consumer exposure), with some manufactured items not present in landfills until 30 years after fabrication. Given the limited information available with which to quantify exposure to DHNUP from off-gassing of potential current or historical PVC products, the vapour pressure of pure DHNUP was used to calculate saturated DHNUP indoor air concentrations and resulting upper-bounding daily intake estimates (see Appendix 1). Based on this conservative scenario, upper-bounding intake of DHNUP from indoor air was estimated to be 8.7 µg/kg body weight (kg-bw) per day in toddlers (0.5–4 years) and 3.3 µg/kg-bw per day in adults (20–59 years). It is recognized that some high molecular weight phthalates can be associated with dust particles, increasing estimated exposure (EURAR 2003). However, with uses such as cable or wiring mentioned above, an external barrier would also reduce exposure to DHNUP (US Patent 1995).
Confidence in the assessment of environmental exposure is moderate. Although there were no literature data identified for media concentrations, environmental releases reported under section 71 of CEPA 1999 would be expected to produce negligible exposure of the general population. However, it is acknowledged that although a comprehensive literature search was conducted for DHNUP and its components as well as known trade names, since DHNUP is an undefined mixture, and also has variable nomenclature, this may limit either the ability to monitor for the substance or to identify such monitoring information. Confidence in the assessment of product exposure is high, as the use of DHNUP in electrical and communication wire insulation was Canadian specific, and uses of a current and historical nature in Canada were comprehensively identified in the submissions provided in response to a notice issued under section 71 of CEPA 1999.
Health Effects Assessment
The available information on health effects associated with DHNUP is summarized in Appendix 2. The synonyms listed in Table 1 were also used to identify toxicological data for DHNUP. Most of the in vivo studies were conducted in rats, and only a few of them were conducted in mice. These include studies on acute toxicity and in vitro genotoxicity (Ames test, mouse lymphoma L5178Y test and mouse Balb/c-3T3 cells) as well as a few studies on short-term and subchronic, chronic, and reproductive and developmental toxicity. Whereas most of the studies reported on CAS RN 68515-42-4, there are some that pertain to CAS RN 3648-20-2, which is just one of the six components of DHNUP. No toxicological data were identified for the remaining components of DHNUP.
The European Commission has classified DHNUP as Category 2 for developmental toxicity, with risk phrase R61 (“May cause harm to the unborn child”), and as Category 3 for reproductive toxicity, with risk phrase R62 (“Possible risk of impaired fertility”) (European Commission 2003, 2004; ESIS 2006). These classifications were based on data specific to DHNUP for developmental toxicity and on structure–activity relationships for reproductive toxicity.
High incidences of teratogenic and embryotoxic effects were observed following oral administration of di-711-phthalate to female rats at 1000 mg/kg-bw per day (lowest-observed-adverse-effect level [LOAEL]) during gestation. Other effects observed at this dose included reduced maternal body weight and body weight gain, increased relative liver and kidney weights and markedly reduced uterus weight. These effects were not observed at 200 mg/kg-bw per day (no-observed-adverse-effect level [NOAEL]) (BASF 1995; Hellwig et al. 1997). Another study on rats using Santicizer 711 reported a lowest-observed-effect level (LOEL) of 5000 mg/kg-bw per day based on mean fetal body weight reduction in the absence of maternal toxicity (IRDC 1981).
Reproductive toxicity in the form of slight testicular atrophy and reduced testis weights following dietary administration of CAS RN 68515-42-4 to rats for 21 days was observed at 2416 mg/kg-bw per day (LOEL). No effects were observed at 1159 mg/kg-bw per day (no-observed-effect level [NOEL]). For CAS RN 3648-20-2, the NOEL in rats was reported at 2495 mg/kg-bw per day (Lington et al. 1993).
A 2-year chronic oral toxicity study (doses 0, 15, 50 or 150 mg/kg-bw per day), aimed at observing carcinogenic effects (if any) of Santicizer 711 (ascribed CAS RN 68515-42-4 by the authors) in Fischer 344 rats, reported an approximate 10% increase in body weight as well as a non-dose-related increased incidence of pancreatic tumours in exposed males. Female rats showed an increased incidence of neoplastic changes of mammary glands at the highest dose. Additionally, up to 46% increase in incidence of mononuclear cell leukaemias was reported in all exposed males and females. However, the authors of the study did not consider mammary gland tumours, pancreatic tumours or mononuclear cell leukaemias to be substance related, as they were within the range of historical controls (reported to range between 19.6 to 50.5%) and were not dose dependent (Monsanto 1984; Thake and Houser 1984; Caldwell 1999). Although some phthalates induced various tumours in experimental animals (NICNAS 2008), the human relevance of these data is uncertain. A review paper assessing the occurrence and aetiology of mononuclear cell leukaemias in F-344 rats exposed to alkyl phthalates concluded that the increased incidence of mononuclear cell leukaemias observed in these mammals was strain specific and of little or no significance to humans (Caldwell 1999).
No data were identified on in vivo genotoxicity for either DHNUP or any of its six individual component substances. In vitro data on genotoxicity endpoints such as the Ames test (various strains of Salmonella), mouse Balb/c-3T3 cell transformation assay as well as the mouse lymphoma L5178Y test both with and without metabolic activation for CAS RN 3648-20-2 are reported to be negative (Zeiger et al. 1985; Barber et al. 2000; CCRIS 2001). For the chemical CAS RN 68515-42-4, the mouse lymphoma L5178Y test both with and without metabolic activation was found to be negative.
Other non-cancer effects observed for DHNUP include reduction in body weight gain and an increase in relative liver and kidney weights reported in male Fischer 344 rats following oral administration of CAS RN 68515-42-4 and CAS RN 3648-20-2. The LOELs ranged from approximately 100 to 2500 mg/kg-bw per day for short-term exposure (Monsanto 1981; Shellenberger et al. 1983; BIBRA 1984, 1985; CMA 1984, 1985a, b, c; Smith et al. 2000). A 90-day subchronic toxicity study on rats for CAS RN 68515-42-4 reported the oral LOEL to be 500 mg/kg-bw per day based on a significant increase in relative liver weight in females and degenerative changes and necrosis of liver in males; however, no effects on body weight gain, food intake, haematology or urinary values were reported (IBT 1972). These studies were conducted by Industrial Bio-Test Laboratories Inc. (IBT), and no information is available as to whether these studies have been audited, reducing the confidence of the information presented.
In mice, a LOEL of 900 mg/kg-bw per day, based on increases in relative liver weight and peroxisomal beta oxidation activity following oral administration of D711P, has been reported (Smith et al. 2000). Peroxisomal proliferation was observed in both male and female rats following administration (2.5% oral concentration) of 711P and CAS RN 3648-20-2 (BIBRA 1985; Barber et al. 1987; Lin 1987). Whereas peroxisomal proliferation is considered a relevant mechanism of tumour promotion in rats, generally such is not the case for humans (Perrone et al. 1998).
Skin irritation (male mouse) and eye irritation (rabbit eye) have been reported to be negative for CAS RN 3648-20-2 (Lawrence et al. 1975). An epidemiological investigation involving two studies (that considered 15 and 128 people respectively) on the effects of dermal application of CAS RN 3648-20-2 in humans produced no evidence of dermal irritation or sensitization during either the induction or challenge phases of the study (Medeiros et al. 1999).
There is low to moderate confidence in the database on toxicity for DHNUP, as data are available for most endpoints; however, many of the studies are unpublished or limited in nature. Due to the variable nature of DHNUP, the composition of the commercial test substance likely changes between studies, increasing the uncertainty of the information available. Fetal toxicity was typically observed in the presence of maternal toxicity. Therefore, there is uncertainty as to whether maternal toxicity contributed to such effects.
Characterization of Risk to Human Health
The health effects associated with exposure to DHNUP are primarily developmental and reproductive toxicity, with effects being observed at high doses. In addition, liver toxicity was observed in experimental animals.
Based on consideration of the weight of evidence–based classification of DHNUP by the European Commission as Category 2 for developmental toxicity (regarded as if it causes developmental toxicity to humans) and as Category 3 for reproductive toxicity (causes concern for human fertility) (European Commission 2003, 2004; ESIS 2006) and consideration of available relevant data, the critical effects for characterization of risk to human health for DHNUP are developmental and reproductive toxicity. Liver effects are also observed at lower exposure levels. Therefore, margins of exposure are derived between the lowest exposure levels associated with induction of these effects and estimates of population exposure to DHNUP.
The principal source of exposure to DHNUP for the general population is considered to be indoor air. A comparison between the lowest effect levels available for developmental and reproductive toxicity in experimental animals (i.e., 1000 mg/kg-bw per day) and for slight liver toxicity in experimental animals (i.e., 100 mg/kg-bw per day) and the highest intake estimated from potential off-gassing of DHNUP from current or historical products (i.e., 8.7 µg/kg-bw per day) results in margins of exposure ranging from 11 500 to 115 000. These margins are considered adequate to account for uncertainties in the database, in light of the conservative nature of the exposure estimate and critical effect levels in studies in experimental animals.
Uncertainties in Evaluation of Risk to Human Health
The need to model some of the physicochemical properties in lieu of experimental measurements reflects uncertainty in the database. The nomenclature issue related to the varying individual phthalates or mixtures of phthalates possible under single common names or trade names complicates literature searches for DHNUP. An additional uncertainty exists in the UVCB nature of DHNUP. As the composition of DHNUP varies, any levels of individual components determined in environmental media may only partly derive from DHNUP. Thus, attribution of environmental concentrations of individual components solely to the DHNUP mixture is unwarranted. Because of this nomenclature issue, as well as the use of a number of high molecular weight phthalates of varying composition, a cumulative assessment for these compounds would allow for more representative consideration of exposures to this group of substances.
There is uncertainty in the toxicological database due to the variability of the test substance. Few studies were identified on reproductive and developmental toxicity of the mixture as a whole. The scope of this screening assessment does not take into account possible differences in sensitivity to DHNUP-induced effects between laboratory animals and humans as well as individual variability in sensitivity across the human population.
On the basis of the adequacy of the margins between exposure to DHNUP and critical effect levels in experimental animals, it is concluded that DHNUP is not a substance entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
Based on the information presented in this screening assessment, it is concluded that DHNUP is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
It is therefore concluded that DHNUP does not meet the criteria in section 64 of CEPA 1999. Additionally, DHNUP does not meet the criteria for persistence or bioaccumulation potential as set out in the Persistence and Bioaccumulation Regulations (Canada 2000).
[AOPWIN] Atmospheric Oxidation Program for Windows [Estimation Model]. 2000. Version 1.91. Washington (DC): U.S. Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: www.epa.gov/oppt/exposure/pubs/episuite.htm
[ASTreat] Activated Sludge Treatment. Computer model for sewage treatment plant removal predictions [CD-ROM]. 2006. Version 1.0. Cincinnati (OH): Procter & Gamble.
Barber ED, Astill BD, Moran EJ, Schneider BF, Gray TJB, Lake BG, Evans JG. 1987. Peroxisome induction studies on seven phthalate esters. Toxicol Ind Health 3(2): 7–24.
Barber ED, Cifone M, Rundell J, Przygoda R, Astill BD, Moran E, Mulholland A, Robinson E, Schneider B. 2000. Results of the L5178Y mouse lymphoma assay and the Balb/3t3 cell in vitrotransformation assay for eight phthalate esters. J Appl Toxicol 20(1): 69–80.
[BASF] BASFAktiengesellschaft. 1974. Unpublished report XXXIII/444. Ludwigshafen (DE): BASF, Department of Toxicology. [cited in ECB 2000].
[BASF] BASF Aktiengesellschaft. 1995. BASF report. Unpublished data. December 18, 1995. Ludwigshafen (DE): BASF, Department of Toxicology. [cited in ECB 2000].
[BASF] BASF Aktiengesellschaft. 2006. BASF, Sterling Chemicals revise toll manufacturing agreement. [cited 2009 Apr 1]. Available from: http://www2.basf.us/corporate/news2006/020706_Sterling.htm
[BIBRA] British Industrial Biological Research Association. 1984. Rat liver and liver lipid effects of representative phthalate esters. BIBRA Project No. 3.0495. OTS0000478-0.
[BIBRA] British Industrial Biological Research Association. 1985. A 21-day feeding study of diundecyl phthalate to rats: effects on the liver and liver lipids. Report No. 0495/4/84. EPA OPTS0000478-0.
[BIOWIN] Biodegradation Probability Program for Windows [Estimation Model]. 2000. Version 4.02. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: http://www.epa.gov/oppt/exposure/pubs/episuite.htm
Birch MD. 1969. Unpublished report. Younger Laboratories Inc. and Monsanto [cited in Krauskopf 1973]. [cited in ECB 2000].
Bizzari SB, Blagoev M, Kishi A. 2007. CEH marketing research report: Plasticizers [Internet]. In: Chemical economics handbook. Menlo Park (CA): SRI Consulting. Available from: http://www.sriconsulting.com/CEH/Public/Reports/576.0000/[restricted access]
Boethling RS, Howard PH, Beauman JA, Larosche ME. 1995. Factors for intermedia extrapolations in biodegradability assessment. Chemosphere 30(4): 741–752.
Brebu M, Vasile C, Antonie SR, Chiriac M, Precup M, Yang J, Roy C. 2000. Study of the natural ageing of PVC insulation for electrical cables. Polym Degrad Stab 67: 209–221.
Brown D, Thompson RS, Stewart KM, Croudace CP, Gillings E. 1996. The effect of phthalate ester plasticisers on the emergence of midge (Chironomus riparius) from treated sediments. Chemosphere 32: 2177–2187.
Caldwell DJ. 1999. Review of mononuclear cell leukaemia in F-344 rat bioassays and its significance to human cancer risk: a case study using alkyl phthalates. Regul Toxicol Pharmacol 30: 45–53.
Call DJ, Cox DA, Geiger DL, Genisot KI, Markee TP, Brooke LT, Polkinghorne CN, VandeVenter FA, Gorsuch JW, Robillard KA, Parkerton TF, Reiley MC, Ankley GT, Mount DR. 2001. An assessment of the toxicity of phthalate esters to freshwater benthos. 2. Sediment exposures. Environ Toxicol Chem 20: 1805–1815.
Canada. [1978]. Food and Drug Regulations, C.R.C., c. 870. Available from: http://laws.justice.gc.ca/en/showdoc/cr/C.R.C.-c.870///en?page=1?
Canada. 1988. Ingredient Disclosure List [Internet]. SOR/88-64. [cited 2008 Oct 9]. Available from: http://www.canlii.org/ca/regu/sor88-64/part274942.html
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C., 1999, c. 33. Available from: http://www.gazette.gc.ca/archives/p3/1999/g3-02203.pdf
Canada. 2000. Canadian Environmental Protection Act: Persistence and Bioaccumulation Regulations, P.C. 2000-348, 23 March 2000, SOR/2000-107. Available from:
http://canadagazette.gc.ca/archives/p2/2000/2000-03-29/pdf/g2-13407.pdf
Canada, Dept. of the Environment, Dept. of Health. 2006. Canadian Environmental Protection Act, 1999: Notice of intent to develop and implement measures to assess and manage the risks posed by certain substances to the health of Canadians and their environment. Canada Gazette, Part I, vol. 140, no. 49, p. 4109–4117. Available from: http://www.gazette.gc.ca/archives/p1/2006/2006-12-09/pdf/g1-14049.pdf
Canada, Dept. of the Environment. 2008. Canadian Environmental Protection Act, 1999: Notice with respect to Batch 6 Challenge substances. Canada Gazette, Part I, vol. 142, no. 22, p. 1644–1662. Available from: http://www.gazette.gc.ca/rp-pr/p1/2008/2008-05-31/pdf/g1-14222.pdf
[CCRIS] Chemical Carcinogenesis Research Information System [database on the Internet]. 2001. Diundecyl phthalate, CASRN: 3648-20-2. Bethesda (MD): National Library of Medicine (US). Available at: http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?CCRIS
Chang BV, Liao CS, Yuan SY. 2005. Anaerobic degradation of diethyl phthalate, di-n-butyl phthalate, and di-(2-ethylhexyl) phthalate from river sediment in Taiwan. Chemosphere 58: 1601–1607.
[CMA] Chemical Manufacturers’ Association. 1984. A 21-day feeding study of diundecyl phthalate to rats: effects on the liver and liver lipids. TSCATS [Toxic Substances Control Act Test Submissions], Microfiche No. OTS0509538, Document Control No. 40+8526207, 4D. [cited in ECB 2000].
[CMA] Chemical Manufacturers’ Association. 1985a. 21 day feeding study of diundecyl phthalate to rats: effects on the liver and liver lipids. Project No. 3.0495.4. TSCATS [Toxic Substances Control Act Test Submissions], Microfiche No. OTS0508502, Document Control No. 40/8526195, N. [cited in ECB 2000].
[CMA] Chemical Manufacturers’ Association. 1985b. Unpublished investigation. Project No. 3.0495.2, November. [cited in ECB 2000].
[CMA] Chemical Manufacturers’ Association. 1985c. A 21-day feeding study of 711 phthalate to rats: effects on the liver lipids and liver. EPA Document No. 40-8626201, Microfiche No. OTS0509543. [cited in ECB 2000].
[CNS] Cosmetic Notification System [Proprietary Database]. 2008. Available from Health Canada, Cosmetics Division.
[ECB] European Chemicals Bureau. 2000. IUCLID dataset for 1,2-benzenedicarboxylic acid, di-C7-11-branched and linear alkyl esters (CAS RN 68515-42-4). Available from: http://ecb.jrc.it/iuclid-datasheet/68515424.pdf
Environment Canada. 2006. Response to the American Chemical Council’s Phthalate Esters Panel’s proposal regarding Environment Canada’s preliminary categorization of 93 phthalate esters. Ottawa (ON): Environment Canada. Available on request.
Environment Canada. 2008a. Data for Batch 6 substances collected under the Canadian Environmental Protection Act, 1999, Section 71: Notice with respect to Batch 6 Challenge substances. Data prepared by: Environment Canada, Existing Substances Program.
Environment Canada. 2008b. Guidance for conducting ecological assessments under CEPA, 1999: science resource technical series, technical guidance module: the Industrial Generic Exposure Tool – Aquatic (IGETA). Working document. Gatineau (QC): Environment Canada, Existing Substances Division.
Environment Canada. 2008c. IGETA report: CAS RN 68515-42-4, 2009-09-04. Unpublished report. Gatineau (QC): Environment Canada, Existing Substances Division.
[EPIsuite] Estimation Programs Interface Suite for Microsoft Windows [Estimation Model]. 2009. Version 4.0. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. [cited 2009 Apr 4]. Available from: http://www.epa.gov/oppt/exposure/pubs/episuite.htm
[EQC] Equilibrium Criterion Model. 2003. Version 2.02. Peterborough (ON): Trent University, Canadian Centre for Environmental Modelling and Chemistry. Available from: http://www.trentu.ca/academic/aminss/envmodel/models/EQC2.html
Ernes DA, Garg BK, Williams RC. 1984. A comparative study of branched and linear dialkyl phthalate plasticizer surface levels and their effects on adhesive bonds in flexible PVC. J Appl Polym Sci 29(1): 383–397.
[ESIS] European Chemical Substances Information System [database on the Internet]. 2006. CAS RN 68515-42-4. 1,2-Benzenedicarboxylic acid, di-C7-11-branched and linear alkyl esters. ESIS Version 4.50. Available from: http://ecb.jrc.it/esis/
[EURAR] European Union Risk Assessment Report: CAS No. 68515-49-1: 1,2-benzenedicarboxylic acid, di-C9-11-branched alkyl esters, C-10 rich; CAS No. 26761-40-0: di-“isodecyl” phthalate (DIDP) [Internet]. 2003. Luxembourg: Office for Official Publications of the European Communities. Report No.: EUR 20785 EN. [cited 2009 Apr 6]. 234 p. On the cover, European Commission Joint Research Centre. Available from: http://ecb.jrc.ec.europa.eu/DOCUMENTS/Existing-Chemicals/RISK_ASSESSMENT/REPORT/didpreport041.pdf
European Commission. 2003. Summary record. Meeting of the Commission Working Group on the Classification and Labelling of Dangerous Substances. Ispra, 25–27 September 2002. European Commission, Directorate-General Joint Research Centre, Institute for Health and Consumer Protection, European Chemicals Bureau. ECBI/63/02 - Rev. 5. Available from:
http://ecb.jrc.it/documents/Classification-Labelling/ADOPTED_SUMMARY_RECORDS/6302r5_sr_CMR0902.pdf
European Commission. 2004. 1,2-Benzenedicarboxylic acid, di-C7-11-branched and linear alkyl esters. Commission Directive 2004/73/EC of April 29, 2004. Annex IB. Official Journal of the European Union. 16.6.2004. L216/133. European Commission. 29th ATP. Available from:
http://ecb.jrc.it/
ExxonMobil Biomedical Sciences, Inc. 2006. High Production Volume (HPV) Chemical Challenge Program test plan for the phthalate esters category [Internet]. Prepared for: Phthalate Esters Panel, HPV Testing Group of the American Chemistry Council. Revision year: 2006; Original publication year: 2001. [cited 2008 Dec 10]. Available from: http://www.epa.gov/hpv/pubs/summaries/benzene/c13467rt3.pdf
Health Canada. 1998. Exposure factors for assessing total daily intake of priority substances by the general population of Canada. Unpublished report. Ottawa (ON): Health Canada, Environmental Health Directorate.
Health Canada. 2007. The cosmetic ingredient hotlist – March 2007 [Internet]. Ottawa (ON): Health Canada, Consumer Product Safety. [cited 2008 Oct 9]. Available from: http://www.hc-sc.gc.ca/cps-spc/person/cosmet/info-ind-prof/_hot-list-critique/hotlist-liste-eng.php
Hellwig J, Freudenberger H, Jaeckh R. 1997. Differential prenatal toxicity of branched phthalate esters in rats. Food Chem Toxicol 35(5): 501–512.
[HENRYWIN] Henry’s Law Constant Program for Microsoft Windows [Estimation Model]. 2000. Version 3.10. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: http://www.epa.gov/oppt/exposure/pubs/episuite.htm
Hirzy JW 1989. Carcinogenicity of general-purpose phthalates: structure–activity relationships. Drug Metab Rev 21: 55–63.
Howard PH, Banerjee S, Robillard KH. 1985. Measurement of water solubilities, octanol/water partition coefficients and vapor pressures of commercial phthalate esters. Environ Toxicol Chem 4: 653–661. [cited in EURAR 2003].
[IBT] Industrial Bio-Test Laboratories, Inc. 1972. Unpublished study for Monsanto Co. IBT No. B1248. [cited in ECB 2000].
[IRDC] International Research and Development Corporation. 1981. Santicizer 711; Teratology study in rats (IR-80-014). Unpublished report for Monsanto Corporation [cited in ECB 2000; Toxicology Advisory Group 2001.
Jakubowicz I, Yarahmadi N, Gevert T. 1999. Effects of accelerated and natural ageing on plasticized polyvinyl chloride (PVC). Polym Degrad Stab 66: 415–421.
Klamer HJC, Leonards PEG, Lamoree MH, Villerius LA, Åkerman JE, Bakker JF. 2005. A chemical and toxicological profile of Dutch North Sea surface sediments. Chemosphere 58: 1579–1587.
[KOWWIN] Octanol–Water Partition Coefficient Program for Microsoft Windows [Estimation Model]. 2000. Version 1.67. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: http://www.epa.gov/oppt/exposure/pubs/episuite.htm
Krauskopf LG. 1973. Studies on the toxicity of phthalates via ingestion. Environ Health Perspect 3: 61–72. [cited in ECB 2000].
Lawrence WH, Malik M, Turner JE, Singh AR, Autian J. 1975. A toxicological investigation of some acute, short-term, and chronic effects of administering di-2-ethylhexyl phthalate (DEHP) and other phthalate esters. Environ Res 9(1): 1–11.
Lin L-K. 1987. The use of multivariate analysis to compare peroxisome induction data on phthalate esters in rats. Toxicol Ind Health 3(2): 25–48.
Lington AW, Gray TJB, Evans J, Lake B, Moran B. 1993. Short-term feeding studies assessing the testicular effects of nine plasticizers in the F344 rat. Acta Pharmacol Toxicol 73(Suppl 11): 132.
Mackintosh CE, Maldonado J, Wu JH, Hoover N, Chong A, Ikonomou MG, Gobas FAPC. 2004. Distribution of phthalate esters in a marine food web: comparison to polychlorinated biphenyls. Environ Sci Technol 38: 2011–2020.
Medeiros AM, Devlin DJ, Keller LH. 1999. Evaluation of skin sensitization response of dialkyl (C6-C13) phthalate esters. Contact Dermatitis 41(5): 287–289.
[Monsanto] Monsanto Company. 1981. Unpublished study ML-79-093. [cited in ECB 2000].
[Monsanto] Monsanto Company. 1984. Unpublished study ML-79-123. [cited in Hirzy 1989; ECB 2000].
[MPBPWIN] Melting Point Boiling Point Program for Microsoft Windows [Estimation Model]. 2000. Version 1.41. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: http://www.epa.gov/oppt/exposure/pubs/episuite.htm
[MSDS] Material Safety Data Sheet: Wood-Tex Face Grade Syn Wood/All Colors [Internet]. 2000a. Springfield (OR): Eclectic Products, Inc. [revised 2000 Jun 20; cited 2009 Feb 23]. Available from: ftp://www.woodworker.com/296-007.pdf [restricted access]
[MSDS] Material Safety Data Sheet: MEGAFlash & MEGAWallFlash Flashing Cement (Trowel Grade) [Internet]. 2000b. Phoenix (AZ): MEGA Industries Corporation. [cited 2008 Dec 8]. Available from: http://www.coolroof.com/PDF%20Files/2007/MSDS-M500%20WC500.pdf
[MSDS] Material Safety Data Sheet: Wood & Stone MEKP Hardener [Internet]. 2001. Eaton Rapids (MI): Axson® North America, Inc. [cited 2008 Dec 8]. Available from: http://www.vicintl.com/msds/axson/mekp_hard_clear.pdf
[MSDS] Material Safety Data Sheet: High Solids Aerosol Lacquer [Internet]. 2003a. El Reno (OK): Gemini Industries, Inc. [cited 2008 Jan 20]. Available from: http://www.stainsafe.com/webdocs/msds/wood/High%20Solids%20Lacquers.pdf
[MSDS] Material Safety Data Sheet: Sanding Sealer Aerosol [Internet]. 2003b. El Reno (OK): Gemini Industries, Inc. [cited 2008 Jan 20]. Available from: http://www.stainsafe.com/webdocs/msds/wood/Sanding%20Sealer.pdf
[MSDS] Material Safety Data Sheet: Flat Oil Finish Aerosol [Internet]. 2003c. El Reno (OK): Gemini Industries, Inc. [cited 2008 Jan 20]. Available from: http://stainsafe.com/webdocs/msds/wood/Flat%20Oil%20finish.pdf
[MSDS] Material Safety Data Sheet: U428 Plus Primerless to Auto Glass Urethane Adhesive [Internet]. 2007. Sarnia (ON): Dow Chemical Canada, Inc. [cited 2008 Feb 20]. Available from: http://ccinfoweb.ccohs.ca/msds/pdf/dow88/5684044.pdf
[MSDS] Material Safety Data Sheet: #400 Manufactured Home & RV Roof Coating [Internet]. 2008a. Largo (FL): Anvil Paints & Coatings, Inc. [cited 2008 Dec 8]. Available from: http://www.anvilpaints.com/MSDS/MSDS%20400%2001-09.pdf
[MSDS] Material Safety Data: #1216 Ultra Seal #2 [Internet]. 2008b. Largo (FL): Anvil Paints & Coatings, Inc. [cited 2008 Dec 8]. Available from: http://www.anvilpaints.com/MSDS/MSDS%201216%2001-09.pdf
[MSDS] Material Safety Data Sheet: #1205 Acrylic Latex Brush On Caulk [Internet]. 2008c. Largo (FL): Anvil Paints & Coatings, Inc. [cited 2008 Dec 8]. Available from: http://www.anvilpaints.com/MSDS/MSDS%201205%2001-09.pdf
[NCI] National Chemical Inventories [database on a CD-ROM]. 2006. Columbus (OH): American Chemical Society, Chemical Abstracts Service. [cited 2009 Apr 14]. Available from: http://www.cas.org/products/cd/nci/require.html
[NICNAS] National Industrial Chemicals Notification and Assessment Scheme. 2008. Existing chemical hazard assessment report: a summary of physicochemical and human health hazard data for 24 ortho-phthalate chemicals. Sydney (AU): Australian Government, Department of Health and Ageing. [cited 2009 Mar].
[NPRI] National Pollutant Release Inventory [database on the Internet]. 2007. Gatineau (QC): Environment Canada. [cited 2008 Dec 8]. Available from: http://www.ec.gc.ca/pdb/querysite/query_e.cfm
[OECD] Organisation for Economic Co-operation and Development. 2007. Emission scenario document on plastics additives. OECD Environmental Health and Safety Publications, Series on Emission Scenario Documents, No. 3. ENV/JM/MONO(2004)8_2007_update. 135 pp. Available from: http://www.olis.oecd.org/olis/2004doc.nsf/LinkTo/NT0000451A/$FILE/JT00166678.PDF
Patrick SG. 2005. Practical guide to polyvinyl chloride. Shropshire (GB): Rapra Technology Limited. p. 33–35.
[PCKOCWIN] Organic Carbon Partition Coefficient Program for Windows [Estimation Model]. 2000. Version 1.66. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: http://www.epa.gov/oppt/exposure/pubs/episuite.htm
Perrone CE, Shao L, Williams GM. 1998. Effect of rodent hepatocarcinogenic peroxisome proliferators on fatty acyl-CoA oxidase, DNA synthesis, and apoptosis in cultured human and rat hepatocytes. Toxicol Appl Pharmacol 150(2): 277–286.
Phthalate Esters Panel. 2004. Phthalate Esters Panel technical report--An assessment of PBiT of selected phthalates, trimellitates, adipates, and related monoesters on the DSL. Arlington (VA): American Chemistry Council. Available on request.
[PMRA] Pest Management Regulatory Agency. 2007. Regulatory Note REG 2007-04: PMRA list of formulants [Internet]. Ottawa (ON): Health Canada, Pest Management Regulatory Agency. [updated 2007 Jun; cited 2008 Oct 9]. Available from: http://www.hc-sc.gc.ca/cps-spc/pubs/pest/_decisions/reg2007-04/index-eng.php
Rhodes JE, Adams WJ, Biddinger GR, Robillard KA, Gorsuch JW. 1995. Chronic toxicity of 14 phthalate esters to Daphnia magnaand rainbow trout (Oncorhynchus mykiss). Environ Toxicol Chem 14(11): 1967–1976.
Shellenberger TE, Kowalski JJ, Unwin S, Grandjean C, Carter J, Hodgson JR. 1983. Comparative 28-day oral toxicity of selected phthalate esters. Toxicologist 3: 157 (Abstract No. 626). [cited in ECB 2000; Smith et al. 2000].
Smith JH, Isenberg JS, Pugh G Jr, Kamendulis LM, Ackley D, Lington AW, Klaunig JE. 2000. Comparative in vivo hepatic effects of di-isononyl phthalate (DINP) and related C7-C11 dialkyl phthalates on gap junctional intercellular communication (GJIC), peroxisomal beta-oxidation (PBOX), and DNA synthesis in rat and mouse liver. Toxicol Sci 54: 312–321.
SpecialChem S.A. 2009. Diplast® L7-11 from Lonza [Internet]. [cited 2008 Feb 20]. Available from: http://www.specialchem4coatings.com/tds/Diplast-L-7-11/Lonza/6075/index.aspx
Stanley MK, Robillard KA, Staples CA. 2003. Introduction. In: The handbook of environmental chemistry, vol. 3. Anthropogenic compounds. Part 3Q. Phthalate esters. p. 1–7. Berlin (DE): Springer-Verlag.
[TDS] Technical Data Sheet: U-428/U-428 Plus [Internet]. [date unknown]. Dayton (OH): The Dow Chemical Company, Dow Automotive. [cited 2008 Feb 20]. Available from: http://corporateportal.ppg.com/NR/rdonlyres/90E64760-495C-4B58-A382-355F11B1FBFC/0/29950889_U428_U428Plus_Technical_Data.pdf
[TDS] Technical Data Sheet: Palatinol® 711P Phthalate Plasticizer [Internet]. 1996. Mount Olive (NJ): BASF Corporation, Chemicals Division. [cited 2008 Dec 8]. Available from: http://www2.basf.us/businesses/chemicals/plasticizers/pdfs/pal-711.pdf
[TDS] Technical Data Sheet: Palatinol® 711P Phthalate Plasticizer [Internet]. 1998. Toronto (ON): BASF Canada. [cited 2009 Mar 13]. Available from: http://www2.basf.us/basf-canada/productsheets/chemical_indus/tds-711p-short.pdf
Thake DC, Houser RM. 1984. A chronic toxicity study of Santicizer 711 in Fischer 344 rats. Monsanto Corporation Report MSL-3444, March. [cited in Toxicology Advisory Group 2001].
Toxicology Advisory Group. 2001. Toxicology Advisory Group criteria document on the classification of di-C7-C11 phthalate (highly branched; di-C7-11 P; CAS No. 68515-42-4). 23 November 2001. ECBI/52/01 Add.2. Available from: http://ecb.jrc.it/classlab/5201a2_DE_Di-C7-C11-phthalate.doc
[TRI] Toxics Release Inventory [database on the Internet]. 2006. TRI Explorer 4.7. Washington (DC): US Environmental Protection Agency. [cited 2009 Feb 19]. Available from: http://www.epa.gov/triexplorer/
[US EPA] US Environmental Protection Agency. 1990. Alkyl phthalates technical amendment; Consent order [Internet]. 40 CFR Part 799. Federal Register, vol. 55, no. 20. January 30, 1990. Rules and regulations. Washington (DC): US EPA, Federal Register. [cited 2008 Dec 10]. Available from: http://www.epa.gov/oppt/chemtest/pubs/note12.pdf
[US Patent] United States Patent 5460885. Insulated electrical products and processes of forming such products [Internet]. 1995. [cited 2008 Dec 10]. Available from: http://www.freepatentsonline.com/5460885.html
[US Patent] United States Patent 5817860. Polyurethane prepolymer compositions, foams made therefrom and methods of making each thereof [Internet]. 1998. [cited 2009 Mar 12]. Available from: http://www.freepatentsonline.com/5817860.html?query=PN/5817860%2520OR%25205817860
&stemming=on
[US Patent] United States Patent 6153709. Chip resistant, vibration damping coatings for vehicles [Internet]. 2000. [cited 2009 Mar 12]. Available from: http://www.freepatentsonline.com/6153709.html?query=PN%2F6153709+OR+6153709&
stemming=on
[US Patent] United States Patent 6238772. Mine brattice cloth [Internet]. 2001. [cited 2009 Mar 12]. Available from: http://www.freepatentsonline.com/6238772.html?query=PN%2F6238772+OR+6238772&
stemming=on
WR Grace & Co. 1948. Dioctyl-phthalate. Technical brochure [cited in Krauskopf 1973]. [cited in ECB 2000].
[WSKOWWIN] Water Solubility for Organic Compounds Program for Windows [Estimation Model]. 2000. Version 1.41. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: http://www.epa.gov/oppt/exposure/pubs/episuite.htm
Younger Laboratories Inc. 1973. Unpublished report for Monsanto Company, Y-73-160. [cited in ECB 2000].
Zeiger E, Haworth S, Mortelmans K, Speck W. 1985. Mutagenicity testing of di(2-ethylhexyl)phthalate and related chemicals in salmonella. Environ Mutagen 7: 213–232.
| Assumptions1 | Calculations | Exposure estimates3 |
|---|---|---|
| 1 Daily air intake volumes and average body weights are derived from Health Canada (1998). Toddlers are 6 months to 4 years of age, and adults are aged 20–59 years. 2 The modelled vapour pressure was used for this scenario, as the experimental vapour pressure was not actually determined; it was only known to be less than 10 Pa at 20°C. The modelled vapour pressure is consistent with values reported for other higher molecular weight phthalates in the literature. For example, di-isodecyl phthalate has a vapour pressure of 5.1E-5 Pa at 25°C (EURAR 2003). In addition, the modelled vapour pressure of DHNUP, 9.2E-5 Pa at 25°C, is consistent with the general linear relationship observed for phthalates of log vapour pressure = -0.0145 molecular weight + 2.2147 (R2 = 0.9543) at 25°C ± 0.5°C (Howard et al. 1985). 3 These estimates are conservative, in that the vapour pressure of pure DHNUP is used to determine the air concentration. |
||
Vapour pressure: 9.2E-5 Pa at 25°C2 Exposure temperature: 25°C Universal gas constant: 8.31 J/mol/K R represents universal gas constant p represents vapour pressure T represents temperature V represents volume m represents mass M represents molecular weight Exposure duration is assumed to be constant (24 h/day), as exposure may occur indoors (PVC cable insulation) or outdoors (volatilization of DHNUP from automotive components) Adult daily air intake: 16.2 m3 Adult body weight: 70.9 kg Toddler daily air intake: 9.3 m3 Toddler body weight: 15.5 kg Uptake fraction = 1 (conservative assumption for lipophilic compounds) Note: As 21 h/day is spent indoors, exposure to DHNUP in indoor air is considered the predominant source of exposure (Health Canada 1998). |
Ideal gas law: (m/M)/V = p/RT (m/0.39057 kg/mol)/V = (9.2E-5 Pa)/(8.31 J/mol/K)(25 + 273.16 K) (m/V) = 1.45E-8 kg/m3 (m/V) = 14.5 µg/m3 As a first-tier approach, the concentration of DHNUP in air (14.5 µg/m3) was used to estimate daily intakes for both an adult and a toddler receptor. As the vapour pressure refers to a pure liquid, the actual concentration of DHNUP in air will diminish, depending upon its partial pressure above the end product as determined by its mole fraction. The thickness of the product will also impact the release rate by altering the surface area to volume ratio. Daily intake equation: (Concentration)(Daily air intake)(Uptake fraction)/(Body weight) Adult intake: (14.5 µg/m3)(16.2 m3/day)(1)/(70.9 kg) = 3.3 µg/kg-bw per day Toddler intake: (14.5 µg/m3)(9.3 m3/day)(1)/(15.5 kg) = 8.7 µg/kg-bw per day |
Adult daily intake: 3.3 µg/kg-bw per day Toddler daily intake: 8.7 µg/kg-bw per day |
| Endpoint | Lowest effect levels1/Results |
|---|---|
1 LD50, median lethal dose; LDLO, lowest lethal dose; LOAEL, lowest-observed-adverse-effect level; NOAEL, no-observed-adverse-effect level. This assessment did not consider toxicological data for CAS RNs 68515-41-3 (di-C7-9) and 68515-43-5 (di-C9-11), as these are derived from a different alcohol stream than that used for the manufacture of CAS RN 68515-42-4 (di-C7-11); moreover, the former two are only slightly branched, unlike the latter, which is highly branched. |
|
| Laboratory animals and in vitro | |
| Acute toxicity | CAS RN 68515-42-4 Oral LD50 (rat) = >64 000 mg/kg-bw (WR Grace & Co. 1948) Oral LDLO (rat) = >15 800 mg/kg-bw (Younger Laboratories Inc. 1973) Oral LD50 (mouse) = >20 000 mg/kg-bw (Birch 1969) No deaths in rat (inhalation) (Younger Laboratories Inc. 1973; BASF 1974) Dermal LDLO (rabbit) = >7940 mg/kg-bw (Younger Laboratories Inc. 1973) CAS RN 3648-20-2 LD50 (male mouse) = >100 g/kg-bw (also >100 mL/kg-bw) (Lawrence et al. 1975) No studies were identified for other CAS RNs. |
| Short-term repeated-dose toxicity | CAS RN 68515-42-4 Oral LOEL (rat) = 270 mg/kg-bw per day based on changes in rat liver enzymes. Peroxisomal proliferation was observed at the high dose (2416-2533 mg/kg-bw per day) in male rats (CMA 1984, 1985a, b, c). Oral LOEL (rat) = 1159–1185 mg/kg-bw per day based on reduced body weight gain in males, reduced testis weights at top dose and increased relative liver and kidney weights in both sexes. The rats were exposed to approximately 0, 270–281, 1159–1185 or 2416–2533 mg/kg-bw per day for 21 days (CMA 1984, 1985a, b, c). Oral LOEL (rat) = 100 and 340 mg/kg-bw per day based on changes in liver enzymes and increased relative liver weight, respectively, in both sexes of rat. The rats were exposed to 0, 100, 340 or 1000 mg/kg-bw per day for 28 days (Shellenberger et al. 1983). Oral LOEL (rat) = 750 mg/kg-bw per day based on liver discoloration. The rats were exposed to 0, 250, 500, 750, 1000 or 2000 mg/kg-bw per day for 28 days. Decreased body weights were observed in males in high-dose groups (1000 or 2000 mg/kg bw/day) (Monsanto 1981). D711P Oral (LOEL) (mouse) = 900 mg/kg-bw per day based on increases in relative liver weight (at 2 and 4 weeks) and peroxisomal beta oxidation activity (at 2 and 4 weeks, only at high dose) in male mice. Male B6C3F1 mice were given an oral dose of 0, 500 or 6000 mg/kg diet (equivalent to about 0, 75 or 900 mg/kg-bw per day) for a period of 2 or 4 weeks (Smith et al. 2000). Oral LOEL (rat) = 1200 mg/kg-bw per day based on increases in relative liver weight (at 2 and 4 weeks), peroxisomal beta oxidation activity and periportal hepatocellular replicative DNA synthesis (at 2 weeks and high dose only). Male F344 rats were given an oral dose of 0, 1000 or 12 000 mg/kg diet (equivalent to about 0, 100 or 1200 mg/kg-bw per day) for a period of 2 or 4 weeks (Smith et al. 2000). 711P Administration of 2.5% (approximately 2500 mg/kg-bw per day) of chemical in diet to male rats in a 21-day study period caused reduction in body weight gain (BIBRA 1984). CAS RN 3648-20-2 Administration of 2.5% (approximately 2500 mg/kg-bw per day) of chemical in diet to male rats in a 21-day study period caused reduction in body weight gain (BIBRA 1985). [additional studies: BIBRA 1984; Barber et al. 1987; Lin 1987] No short-term repeated-dose studies were identified for other CAS RNs. |
| Subchronic toxicity | CAS RN 68515-42-4 Oral LOEL (rat) = 500 mg/kg-bw per day based on a significant increase in relative liver weight in females and degenerative changes and necrosis of liver in males. No effects on body weight gain, food intake, haematology or urinary values were observed. The rats were fed 50, 150 or 500 mg/kg-bw per day for 90 days (IBT 1972). No relevant studies were identified for other CAS RNs. |
| Chronic toxicity/ carcinogenicity | Santicizer 7113 Rats (Fischer 344) were given daily oral doses of 0, 15, 50 or 150 mg/kg-bw for a period of 2 years. All exposed males showed an approximate 10% increase in body weight as well as a non-dose-related increased incidence of pancreatic tumours (0/68, 7/69, 12/71 and 4/71 for control, low-, mid- and high-dose groups, respectively). Females showed an increased incidence of neoplastic changes of mammary glands at the highest dose (1/55, 3/58, 2/59 and 6/61 for control, low-, mid- and high-dose groups, respectively). Additionally, up to a 46% increase in incidence of mononuclear cell leukaemias was observed in all exposed males and females (control incidences 20/72 in males and 18/70 in females; exposed males low 32/72, intermediate 28/71, high 33/72; exposed females low 27/72, intermediate 26/71, high 26/72)). However, the authors of the study did not consider these tumours to be substance related, as they were within the range of historical controls and were not dose dependent (Monsanto 1984; Thake and Houser 1984). |
| Genotoxicity and related endpoints: in vivo | No data were identified. |
| Genotoxicity and related endpoints: in vitro | CAS RN 68515-42-4 Negative in mouse lymphoma L5178Y test with and without exogenous metabolic activation (Barber et al. 2000) Negative in mouse Balb/c-3T3 cell transformation assay (Barber et al. 2000) CAS RN 3648-20-2 Negative in Ames tests in Salmonella typhimuriumstrains TA98, TA100, TA1535 and TA1537 with and without exogenous metabolic activation (Zeiger et al. 1985; CCRIS 2001) Negative in mouse lymphoma L5178Y test with and without exogenous metabolic activation (Barber et al. 2000; CCRIS 2001) Negative in mouse Balb/c-3T3 cell transformation assay (Barber et al. 2000) |
| Reproductive toxicity | CAS RN 68515-42-4 Oral NOEL (rat) = 1159 mg/kg-bw per day and LOEL = 2416 mg/kg-bw per day based on weak testicular effects in the form of slight testicular atrophy and reduced testis weights (Lington et al. 1993). Rats were fed 0, 270, 1159 or 2416 mg/kg-bw per day for 3 weeks. CAS RN 3648-20-2 Oral NOEL (rat) = 2495 mg/kg-bw per day (Lington et al. 1993). Rats were fed 0, 285, 1183 or 2495 mg/kg-bw per day for 3 weeks. |
| Developmental toxicity | di-711-phthalate2 Oral (gavage) LOAEL (rat) = 1000 mg/kg-bw per day based on teratogenic and embryonic effects and maternal toxicity. Rats were fed 0, 40, 200 or 1000 mg/kg-bw per day on days 6–15 of pregnancy. Rats in the high-dose group showed clear signs of maternal toxicity, including reduced body weight gain, reduced body weight, reduced maternal body weight (14.3%), increased relative liver and kidney weights, markedly reduced uterus weight and vaginal haemorrhages in six dams. A drastic increase in resorptions, fewer live fetuses per dam, reduced fetal body weight and skeletal malformations were also observed. The number of foetuses with malformations, variations and retardations at the high dose were 89, 70 and 58 percent, respectively. NOAEL = 200 mg/kg-bw per day (BASF 1995; Hellwig et al. 1997). Santicizer 7113 Oral (gavage) LOEL (rat) = >5000 mg/kg-bw per day based on maternal toxicity and LOEL = 5000 mg/kg-bw per day based on developmental toxicity. Rats were fed with 0, 250, 1000 or 5000 mg/kg-bw per day on days 6–19 of pregnancy. No maternal toxicity was observed; however, at the top dose, the mean fetal body weight was significantly reduced (IRDC 1981). No studies were identified for other CAS RNs. |
| Sensitization | No data were available. |
| Irritation | CAS RN 3648-20-2 Skin irritation has been reported to be negative in the male mouse (Lawrence et al. 1975). |
CAS RN 3648-20-2 Eye irritation has been reported to be negative to rabbit eye (Lawrence et al. 1975). |
|
| Humans | |
| Irritation and sensitization | CAS RN 3648-20-2 No evidence of dermal irritation or sensitization was found (Medeiros et al. 1999). |