Screening Assessment for The Challenge
This page has been archived on the Web
Information identified as archived is provided for reference, research or recordkeeping purposes. It is not subject to the Government of Canada Web Standards and has not been altered or updated since it was archived. Please contact us to request a format other than those available.
Archived
Spiro[isobenzofuran-1(3H),9'-[9H]xanthen]-3-one, 2',4',5',7'-tetrabromo-3',6'-dihydroxy-
(D&C Red No. 21)
Chemical Abstracts Service Registry Number
15086-94-9
Environment Canada
Health Canada
November 2008
Table of Contents
Pursuant to section 74 of the Canadian Environmental Protection Act, 1999 (CEPA 1999), the Ministers of the Environment and of Health have conducted a screening assessment on Spiro[isobenzofuran-1(3H),9'-[9H]xanthen]-3-one, 2',4',5',7'-tetrabromo-3',6'-dihydroxy- (D&C Red No. 21), Chemical Abstracts Service Registry Number 15086-94-9. This substance was identified as a high priority for screening assessment and included in the Ministerial Challenge because it was found to meet the ecological categorization criteria for persistence, bioaccumulation potential and inherent toxicity to non-human organisms and is believed to be in commerce in Canada.
The substance D&C Red No. 21 was not considered to be a high priority for assessment of potential risks to human health, based upon application of the simple exposure and hazard tools developed by Health Canada for categorization of substances on the Domestic Substances List. Therefore, this assessment focuses on information relevant to the evaluation of ecological risks.
The organic substance D&C Red No. 21 is used in Canada primarily as a dye in cosmetics. The substance is not naturally produced in the environment. Data collected pursuant to a notice published under section 71 of CEPA 1999 for the 2000 calendar year indicated that for D&C Red No. 21, one company reported potential import of the substance, however the quantities were unknown. In response to the voluntary call for new information under the Challenge, the company that reported potential import in 2000 confirmed that their importing activity was below the 100 kg threshold in 2006. In total, less than 100 kg of D&C Red No. 21 were voluntarily reported to be imported in 2006, with a few companies reporting low quantities.
Since there were no reports of import or manufacture at or above the reporting threshold of 100 kg in 2000 or 2006, releases of this substance into the Canadian environment are presumed to be very low. The substance D&C Red No. 21 will be ionized at neutral pH, and has a high solubility in water and low Log Kow. It is not volatile, and would have a tendency to remain in water when released to surface water.
Based on its predicted resistance to biodegradation, D&C Red No. 21 is expected to be persistent in the environment; however it is subject to relatively rapid primary degradation by photolysis. New experimental data relating to its partitioning between octanol and water suggest that this dye has a low potential to accumulate in the lipid tissues of organisms. The substance is thus no longer expected to meet the persistence or bioaccumulation criterion as set out in the Persistence and Bioaccumulation Regulations. In addition, new experimental toxicity data for the dye and its chemical analogues suggest that the substance has a low acute toxicity to aquatic organisms (LC50/LC50 > 1 to > 100 mg/L).
For this screening assessment, a generic conservative exposure scenario was developed to estimate releases into the aquatic environment from industrial operations and resulting aquatic concentration. No adverse effects were anticipated, as the predicted environmental concentration in water (PEC) was below predicted no-effect concentration (PNEC).
In addition and where relevant, research and monitoring will support verification of assumptions used during the screening assessment.
Based on the information available, D&C Red No. 21does not meet any of the criteria set out in section 64 of the Canadian Environmental Protection Act, 1999.
The Canadian Environmental Protection Act, 1999 (CEPA 1999) (Canada 1999) requires the Minister of the Environment and the Minister of Health to conduct screening assessments of substances that have met the categorization criteria set out in the Act to determine whether these substances present or may present a risk to the environment or human health. Based on the results of a screening assessment, the Ministers can propose to take no further action with respect to the substance, to add the substance to the Priority Substances List (PSL) for further assessment, or to recommend that the substance be added to the List of Toxic Substances in Schedule 1 of the Act and, where applicable, the implementation of virtual elimination.
Based on the information obtained through the categorization process, the Ministers identified a number of substances as high priorities for action. These include substances that
- met all of the ecological categorization criteria, including persistence (P), bioaccumulation potential (B) and inherent toxicity to aquatic organisms (iT), and were believed to be in commerce in Canada; and/or
- met the categorization criteria for greatest potential for exposure (GPE) or presented an intermediate potential for exposure (IPE), and had been identified as posing a high hazard to human health based on classifications by other national or international agencies for carcinogenicity, genotoxicity, developmental toxicity or reproductive toxicity.
The Ministers therefore published a notice of intent in theCanada Gazette, Part I, on December 9, 2006 (Canada 2006), that challenged industry and other interested stakeholders to submit, within specified timelines, specific information that may be used to inform risk assessment, and to develop and benchmark best practices for the risk management and product stewardship of those substances identified as high priorities.
The substance Spiro[isobenzofuran-1(3H),9'-[9H]xanthen]-3-one, 2',4',5',7'-tetrabromo-3',6'-dihydroxy- was identified as a high priority for assessment of ecological risk as it had been found to be persistent, bioaccumulative and inherently toxic to aquatic organisms and is believed to be in commerce in Canada. The Challenge for this substance was published in the Canada Gazette on May 12, 2007 (Canada 2007). A substance profile was released at the same time. The substance profile presented the technical information available prior to December 2005 that formed the basis for categorization of this substance. As a result of the Challenge, submissions of information pertaining to the uses of the substance were received.
Although Spiro[isobenzofuran-1(3H),9'-[9H]xanthen]-3-one, 2',4',5',7'-tetrabromo-3',6'-dihydroxy- was determined to be a high priority for assessment with respect to the environment, it did not meet the criteria for GPE or IPE and high hazard to human health based on classifications by other national or international agencies for carcinogenicity, genotoxicity, developmental toxicity or reproductive toxicity. Therefore, this assessment focuses principally on information relevant to the evaluation of ecological risks.
Under CEPA 1999, screening assessments focus on information critical to determining whether a substance meets the criteria for defining a chemical as toxic as set out in section 64 of the Act, where
"64. [...] a substance is toxic if it is entering or may enter the environment in a quantity or concentration or under conditions that
(a) have or may have an immediate or long-term harmful effect on the environment or its biological diversity;
(b) constitute or may constitute a danger to the environment on which life depends; or
(c) constitute or may constitute a danger in Canada to human life or health."
Screening assessments examine scientific information and develop conclusions by incorporating a weight of evidence approach and precaution.
This screening assessment includes consideration of information on chemical properties, hazards, uses and exposure, including the additional information submitted under the Challenge. Data relevant to the screening assessment of this substance were identified in original literature, review and assessment documents, stakeholder research reports and from recent literature searches, up to February 2008 for ecological sections of the document. Key studies were critically evaluated; modelling results may have been used to reach conclusions. When available and relevant, information presented in hazard assessment from other jurisdictions was considered. The screening assessment does not represent an exhaustive or critical review of all available data. Rather, it presents the most critical studies and lines of evidence pertinent to the conclusion.
This screening assessment was prepared by staff in the Existing Substances Programs at Health Canada and Environment Canada and incorporates input from other programs within these departments. Additionally, the draft of this screening assessment was subject to a 60-day public comment period. The critical information and considerations upon which the assessment is based are summarized below.
For the purposes of this document, this substance will be referred to as D & C Red No. 21.
| Chemical Abstracts Service Registry Number (CAS RN) | 15086-94-9 | |
| Name on Domestic Substances List (DSL) | Spiro[isobenzofuran-1(3 H),9'-[9H]xanthen]-3-one, 2',4',5',7'-tetrabromo-3',6'-dihydroxy- | |
| Inventory Names1 | 2-(3,6-dihydroxy-2,4,5,7-tetrabromoxanthen-9-yl)-benzoic acid (EINECS) 2,4,5,7-Tetrabromofluorescein (ENCS) Spiro[isobenzofuran-1(3H),9'-[9H]xanthen]-3-one, 2',4',5',7'-tetrabromo-3',6'-dihydroxy- (AICS) 2,4,5,7-tetrabromo-fluorescein aluminum lake (PICCS) Fluorescein, 2,4,5,7-tetrabromo- (PICCS) |
|
| Other names | CI Solvent Red 43; D&C Red No. 21; Eosin Acid; Eosin Y spirit soluble; C.I. 45380:2 or Japan Red 223 | |
| Chemical group (DSL stream) | Discrete organic | |
| Chemical sub-group | Xanthene Dye | |
| Chemical formula | C20H8Br4O5 | |
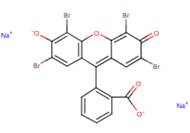
| Chemical structure |
|
|
| Simplified Molecular Input Line Entry System (SMILES) | O=C(OC(c(c(Oc1c(c(O)c(c2)Br)Br)c(c(O)c3Br)Br)c3)(c12)c4cccc5)c45 | |
| Molecular mass | 647.9 g/mol | |
[1] National Chemical Inventories (NCI). 2006: AICS (Australian Inventory of Chemical Substances); EINECS (European Inventory of Existing Commercial Chemical Substances); ENCS (Japanese Existing and New Chemical Substances); PICCS (Philippine Inventory of Chemicals and Chemical Substances).
The substance D&C Red No. 21 is a free acid that exists in two tautomeric forms; the lactonic and quinonoid forms (Table 1). Both tautomers are only lightly coloured and exist as solids. As shown in Table 2, D&C Red No. 21 has two dissociation constants, indicating that the neutral molecular form will only be the dominant form at a pH below 3.25. Under these acidic conditions, it has been found that the lactonic form is favoured. However, at higher pHs, the ionized quinonoid form is dominant. This ionized form is soluble and exhibits a much more intense colour than the neutral molecular forms (Amat-Guerri et al. 1990). It is therefore expected that at environmental pH, the soluble, dianionic quinonoid would be the predominant form present.
| Property | Type | Value | Reference |
|---|---|---|---|
| pKa (acid dissociation constant) (dimensionless) |
Modelled | pKa1=5.65 pKa2=4.84 |
ACD/pKaDB 2005 |
| Experimental | pKa1=3.25 pKa2=3.8 |
Levillain and Fompeydie 1985 |
|
| Experimental | pKa1=3.75 pKa2=6.25 |
Amat-Guerri et al. 1990 |
As a result of the ionizable nature of D&C Red No. 21, eosin, the sodium salt of D&C Red No. 21 (CAS# 17372-87-1) will serve as a suitable analogue for this substance. Lipman (1995) has suggested that D&C Red No. 21 is likely to have similar toxicological effects as eosin.
Experimental physical-chemical data were not available for D&C Red No. 21. Since the ionized form is expected to exist at environmental pH and will have different physical-chemical properties than the neutral molecular form, modelled and experimental physical-chemical data of eosin will be used in the assessment of D&C Red No. 21 (Table 3). The structure depicted in Table 3 will be used in the models throughout the assessment.
| Property | Type | Value | Temperature (°C) |
Reference |
|---|---|---|---|---|
 |
||||
| Melting point (°C) |
Modelled | 349.84 | MPBPWIN 2000 | |
| Experimental | 295.5 | PhysProp 2006 | ||
| Boiling point (°C) |
Modelled | 839.89 | MPBPWIN 2000 | |
| Density (kg/m3) |
Experimental | 2030 (2.03 g/cm3) |
20 | MSDS 2006a |
| Vapour pressure (Pa) |
Modelled | 3.386 × 10-18 (2.54 × 10-20 mm Hg) |
MPBPWIN 2000 | |
| Henry's Law constant (Pa·m3/mol) |
Modelled | 6.436 × 10-20 (6.35 × 10-25 atm·m3/mol ) |
25 | HENRYWIN 2000 |
| Log Kow (Octanol-water partition coefficient; dimensionless) |
Experimental | -0.25 | 25 | Wang et al. 2006 |
| -0.96[1] | 25 | Tonogai et al. 1982 | ||
| Modelled | -1.68 | KOWWIN 2000 | ||
| Log Koc (Organic carbon-water partition coefficient; dimensionless) |
Modelled | 4.276[2] | PCKOCWIN 2000 | |
| Water solubility (mg/L) |
Experimental | 300,000[1] | 20 | MSDS 2006a |
| Modelled | 1090 | 25 | WSKOWWIN 2000 |
|
[1] Values used in modeling fugacity, BAF and BCF.No. 21
[2] The Koc may be sensitive to pH. The estimated Koc represents a best-fit to the majority of experimental values; however, the Koc may vary significantly with pH (PCKOCWIN 2000).
Some of the physical and chemical properties in the above table were generated using quantitative structure-activity reaction (QSAR) models, and there are uncertainties related to the use of these models. The uncertainties relate to the particular physical and chemical properties being modelled, the modelling techniques, and the types of chemicals being modelled. For example, measured water solubility values for a single chemical may span two or more orders of magnitude, but the QSAR training sets are generally based on a single representative value for each chemical. This type of issue, and others not discussed here, contribute to the uncertainties associated with the use of QSARs to generate physical and chemical property data. In this case there are additional uncertainties associated with the assumption that the physical and chemical properties of eosin and D&C Red No. 21 are reasonably similar.
The predictions for eosin suggest that the ionized form of D&C Red No. 21 would have a higher water solubility and lower log Kow than the models predicted for the neutral molecular form of D&C Red No. 21 (predicted water solubility was 2.88 x 10-5mg/L and log Kow was 6.91 for D&C Red No. 21 (WSKOWWIN 2000 and KOWWIN 2000)).
In addition, two other related compounds, phloxine B (CAS RN 18472-87-2) and uranine (CAS RN 518-47-8) have reported empirical water solubilities and log Kow values in the same range as eosin (Table 4).
| Phloxine B 18472-87-2[a] |
Uranine 518-47-8[b] |
Reference | |
|---|---|---|---|
| Chemical Structure |  |
 |
|
| Molecular weight (g/mol) |
829.64 | 376.28 | |
| Water Solubility (mg/L) |
90,000 (Ref 1) | 600,000 (Ref 2) | Ref 1: MSDS 2006b Ref 2: MSDS 2004 |
| Log Kow (dimensionless) |
-0.21 (Ref 3) -0.74 (Ref 4) |
-0.28 (Ref 4) | Ref 3: Wang et al. 2006 Ref 4: Tonogai et al. 1982 |
| Log Koc (dimensionless) |
2.16 (sediment) 2.27 (soil) |
1.84 (sediment) 1.95 (soil) |
Li et al. 1998 |
[a] Eosin differs from phloxine B by one chemical feature: four additional Cl on the benzene ring of phloxine B
[b] Eosin differs from uranine by one chemical feature: there are no Br on the xanthene group of uranine
The experimental log Kow values for eosin are in agreement with the predicted value (Table 3). The study by Wang et al. (2006) did not provide a detailed description of the test methods followed; therefore it could not be evaluated for robustness. The study by Tonogai et al. (1982) was carried out according to the recommendations of the Organisation for Economic Co-operation and Development (OECD) Chemicals Testing Program, and was judged to be of satisfactory reliability. The Log Kow values measured by Tonogai et al. (1982) are thus considered the most reliable, as the Kow studies conducted by others could not be evaluated.
The predicted log Koc of eosin indicates that D&C Red No. 21 will have a high adsorptivity to soil. The model provides a note with this output that the Koc may be sensitive to pH. Others have found that anionic substances may be mobile at neutral to basic pH. Spadotto and Hornsby (2003) observed that at low pH, where acidic pesticides exist largely in neutral form, the soil sorbs much more 2,4-D as compared with sorption at high pH, where the pesticide is mostly in anionic form. They found that this observation was also supported by studies on other acidic pesticides with low acid dissociation constants (Spadatto and Hornsby 2003). This assumption is further supported by the experimental Koc values obtained by Li et al (1998) for two structurally similar substances; phloxine B and uranine (Table 4). Li et al. found that in sediment at a pH of 8, phloxine B had a log Koc of 2.16, while uranine had a slightly lower log Koc of 1.84. In soil at a pH of 7, phloxine B had a measured log Koc of 2.27, while uranine had a log Koc of 1.95. The higher Koc observed in soil is attributed to the ionic interactions which dominate in the presence of a higher mineral content of the soil relative to the sediment sample. It is therefore expected that the predicted by PCKOCWIN (2000) is overestimated and the log Koc of D&C Red No. 21 would be approximately 2.
For this screening assessment, a water solubility of 300,000 mg/L, a log Kow of -0.96 and a log Koc of 2 will be assumed for D&C Red No. 21, recognizing that the water solubility may be slightly overestimated and the log Kowmay be slightly underestimated as the values are based on the properties of a structurally similar salt.
The substance D&C Red No. 21 is not naturally produced in the environment.
Environment Canada conducted an industry survey pursuant to section 71 of CEPA 1999 requesting industrial information on manufactured and/or imported quantities, uses and releases of specified substances (Canada 2001). Any person who manufactured or imported a total quantity greater than 100 kg of a substance listed in the section 71 notice (whether alone, in a mixture or in a product) in the year 2000, was obligated to report. Data collected pursuant to this notice indicated that for D&C Red No. 21, one company reported potential import of this substance to Canada, however the quantities were unknown (Environment Canada 2001).
The substance D&C Red No. 21 was not part of the 2007 notice as data were gathered only in response to the 2001 notice when quantities reported exceeded the 100 kg threshold. However, it was included in the voluntary call for new information under the Challenge. In response to this call, the company that reported potential import in 2000 confirmed that their importing activity was below the 100 kg threshold in 2006. In total, less than 100 kg of D&C Red No. 21 were voluntarily reported to be imported in 2006, with a few companies reporting low quantities. Nine organizations identified themselves as having a stakeholder interest in the substance (Environment Canada 2007).
In 1986, there were fewer than four companies that reported handling of D&C Red No. 21 to the DSL, in a total quantity of less than 2,000 kg.
Elsewhere, D&C Red No. 21 has been identified as a European Union (EU) Low Production Volume Chemical, indicating that p roduction within the EU has been estimated to be in the order of 10 tonnes per year. In 2002, for the first time since the inception of the United States Environmental Protection Agency's (EPA) Inventory Update Reporting regulation in 1986, D&C Red No. 21 met the reporting threshold of 4.5 tonnes, with a reported use of 4.5 to 225 tonnes.
The use information reported in response to the section 71 survey (Canada 2001) has been claimed as confidential business information. However, in response to the voluntary call for new information under the Challenge, it was reported that this substance is used in small concentrations in cosmetics. Additionally, the Canadian Cosmetic, Toiletry and Fragrance Association identified themselves as being a stakeholder with respect to this substance. In 1986, the uses reported during DSL nomination included colourant, cosmetics, soap and cleaning products.
The substance D&C Red No. 21 is included in the list of approved drug colourants for internal and external use in the Canadian Food and Drug Regulations (Food and Drug Act, 1985). It is also included in the list of acceptable non-medicinal ingredients in natural health products in Canada (Health Canada 2003).
Internationally, uses of D&C Red No. 21 include:
- Cosmetics, including lipstick, lip gloss, lip pencil, eye shadow, mascara, lip balm, moisturizer, bar soap and fragrance (Environmental Working Group 2007)
- Textile dye (PubChem 1988- )
- Dye used for staining difficult tissues (StainsFile 2007)
- Colouring agent in drugs - it is permitted as a colourant in drugs and cosmetics in the U.S. and is allowed in all cosmetic products in the European Union
Information received pursuant to the section 71 Notice in 2001 indicated that one company imported this substance into Canada in 2000; however the voluntary submissions provided for 2006 confirm that the quantity imported would be less than 100 kg. Therefore releases of this substance to the Canadian environment are presumed to be very low (Environment Canada 2001 and Environment Canada 2007). Uncertainties exist in this assumption, as D&C Red No. 21 has been found to be an ingredient in numerous cosmetics that are available for purchase in Canada. However it seems that the concentrations are very low. Available information is currently not sufficient to derive a quantity estimate for this source.
As D&C Red No. 21 is used in cosmetics, the primary source of releases to the environment would occur by product disposal and by washing the cosmetics off the skin, where it would go down the drain. Given the low concentrations in the products, releases to the environment are expected to be low.
Based on its anticipated physical and chemical properties (Table 3), the results of Level III fugacity modelling (Table 5) suggest that D&C Red No. 21 is expected to partition predominantly to water and soil, depending on the compartment of release.
| Substance released to | Fraction of substance partitioning to each compartment (%) | |||
|---|---|---|---|---|
| Air | Water | Soil | Sediment | |
| Air (100%) | 0 | 3.4 | 96.6 | 0 |
| Water (100%) | 0 | 99.4 | 0 | 0.6 |
| Soil (100%) | 0 | 2.7 | 97.3 | 0 |
The low acid dissociation constants (pKa) of 3.25 and 3.8 indicate that in water bodies at environmental pH (6-9), the dianionic form will be present, therefore some partitioning of D&C Red No. 21 may occur through electrostatic sorption mechanisms. The presence of the ionized chemical also indicates that partitioning behaviour predicted based on the log Kow and log Koc values for the neutral form of the molecule are likely not relevant. The experimental log Kow and water solubility of eosin and the estimated log Koc based on analogues were used in the fugacity model. Photolysis was not included as a degradation process in the fugacity model.
If released to air, the Level III-fugacity model indicates that no amount of the substance will remain in air (see Table 5 above). A low modelled vapour pressure of 3.386 x 10-18 Pa and Henry's Law constant of 6.436 × 10-20 Pa·m3/mol indicate that D&C Red No. 21 is non-volatile. Therefore, if released solely to air, it will tend to partition mainly to soil and to a lesser extent, water (see Table 5 above).
If released into water, D&C Red No. 21 is not expected to strongly adsorb to suspended solids and sediment based upon the low value of log Koc of ~2 as estimated from two analogues. Volatilization from water surfaces is expected to be an unimportant fate process based upon this compound's estimated Henry's Law constant. As a result of the high water solubility of D&C Red No. 21, if water is a receiving medium, it is expected to mainly remain in water (see Table 5 above). There is however uncertainty in these predictions, as a study by Li et al. (1998) measured the concentration of two structurally similar xanthene dyes at a spill site. They found that the concentrations in sediment were much higher than in water suggesting that the fugacity model may overestimate the proportion of D&C Red No. 21 in water and underestimate the proportion in sediment when releases are to water - in part because the model did not take photolysis in water into account.
If released to soil, D&C Red No. 21 is expected to have low adsorptivity to soil (i.e., expected to be relatively mobile) based upon the low log Koc estimated from two analogues. Others have also found that anionic substances may be mobile in soil at neutral to basic pH (Spadatto and Hornsby 2003). Volatilization from moist soil surfaces seems to be an unimportant fate process based upon its estimated Henry's Law constant. This chemical is not likely to volatilize from dry soil surfaces based upon its vapour pressure. Therefore, if released to soil, D&C Red No. 21 is predicted to mainly remain in soil with minor partitioning into water, which is illustrated by the results of the Level III-fugacity modelling (see Table 5 above).However, based on the observed behaviour of ionisable substances discussed above, it is expected that the actual extent of the accumulation in soil when released to that medium is smaller than predicted.
Environmental Persistence
No experimental persistence data are available for D&C Red No. 21. Model predictions for eosin suggest it will undergo rapid photo-oxidation in air (half-life ≥ 0.33 days) but be resistant to biodegradation (half-life 182 days) (see Table 6 below).
| Medium | Fate process | Degradation value | Degradation endpoint/units | Reference |
|---|---|---|---|---|
| Air | Atm-oxidation | 0.3348 | Half-life, days | AOPWIN 2000 |
| Air | Ozone reaction | 0.01403 | Half-life, days | AOPWIN 2000 |
| Water | Biodegradation | 182 | Half-life, days | BIOWIN 2000, Ultimate Survey Model |
| Water | Biodegradation | 0.0002 | Probability | BIOWIN 2000, MITI Non-linear Probability |
| Soil | Biodegradation | 182 | Half-life, days | Based on the modelled half-life in water[1] |
| Sediment | Biodegradation | 728 | Half-life, days | Based on the modelled half-life in water[1] |
[1] Values were derived from the modelled half-life in water using the extrapolation factors of Boethling et al. (1995): t1/2 water : t1/2 soil : t1/2 sediment = 1:1:4.
No experimental degradation data are available for D&C Red No. 21; however primary degradation studies on other xanthene dyes are available. Xanthene dyes are photoreactive and the presence of halogens has been found to increase their photoreactivity by increasing the efficiency of their transition to the excited triplet state (Walthall and Stark 1999). Wang et al. (2006) showed that with an increasing number of halogen substituents on xanthene dyes, the singlet oxygen yields increased. Once in the excited triplet state, the dye is able to excite an oxygen molecule, which can cause degradation of the dye by attacking the double bonds in the dye molecule (Heitz 1995). Exposure of eosin to light also results in debromination, as demonstrated by the presence of the degradation product uranine (Tonogai et al. 1979).
Tonogai et al (1979) showed a 70% decrease in optical density after 10 hours of exposure of eosin (CAS RN 17372-87-1) to a low pressure mercury lamp (UV light) in water. Analysis with thin-layer chromatography revealed that the main photodecomposed product was uranine, suggesting that the four bromine atoms had been released from the dye. The study also examined photodecomposition of similar substances, phloxine B (CAS RN 18472-87-2) and uranine (CAS RN 518-47-8), and found that in 10 hours, the optical density for phloxine B had decreased by 50% while little change was found for uranine. This study did not provide enough details or follow any standard methods; therefore the reliability is uncertain. The rapid photodegradation of eosin and phloxine B is in agreement with other studies, however, others have reported that uranine also undergoes rapid photodegradation (Smart and Laidlaw 1977).
At a spill site in Hawaii, Li et al. (1998) observed that in a shallow puddle of water the concentration of phloxine B decreased from 1000 ppm to 0.076 ppm and that of uranine from 500 ppm to 0.0551 ppm over 12 days, suggesting that the half-life of both substances is less than 12 days in water (Table 7). Due to the structural similarity of eosin to phloxine B and uranine, it is thus possible that eosin could also have a half-life in water of less than 12 days. However the authors of this study acknowledge that the losses observed may not only be due to photolysis, but may in part result from washout by rain.
In another study, photolysis half-lives for phloxine B in water were measured in various pH and salt conditions (Wang et al. 1998). It was found that the half-life ranged from 10-13 minutes when exposed to sunlight, depending on the water conditions (Table 7). Photolysis rates also varied depending on the light source, occurring more slowly under VIS (365 nm) and fluorescent light (half-lives of 14-115 hours). The robust study summary used to evaluate this study is available in Appendix I.
Eosin has been shown to degrade to uranine, which is also expected to degrade by photolysis (Smart and Laidlaw 1977). Furthermore, biodegradation models used to reach the Categorization decision for uranine indicated that it was not persistent; with a predicted half life of 60 days using the Ultimate Survey Model and a biodegradation probability of 0.28 using the MITI Linear probability model (BIOWIN 2000). Furthermore, the acid form of uranine (CAS RN 2321-07-5) was observed to experience100% biodegradation after 20 days, although the original study was not available therefore the quality of the study could not be evaluated (Nalco Chemical Company 1998).
Based on these empirical results for eosin, phloxine B and uranine, it is expected that D&C Red No. 21 would also undergo relatively rapid primary degradation to uranine in water when exposed to light and in that form, would then be susceptible to ultimate degradation.
| Medium | Degradation value | Degradation endpoint/units | Reference |
|---|---|---|---|
| Uranine | |||
| Water | < 12 | Half-life, days | Li et al. 1998 |
| Soil | < 4 | Half-life, days | Alcantara-Licudine et al. 1999 |
| Phloxine B | |||
| Water | < 12 | Half-life, days | Li et al. 1998 |
| Water | < 1 | Half-life, days | Tonogai et al. 1979 |
| Water | < 1 | Half-life, days | Wang et al. 1998 |
| Soil | < 7 | Half-life, days | Alcantara-Licudine et al. 1999 |
| Sediment | 123-284 | Half-life, days | Li et al. 1998 |
Alcantara-Licudine et al. (1999) studied the dissipation of phloxine B and uranine in soil following its aerial spray on a coffee field in Hawaii. The mixture was composed of 0.68% phloxine B and 0.32% uranine so that 11.1 g and 4.8 g of each substance was applied per acre in each spray. The field was sprayed weekly for ten weeks. The soil was a silty clay loam with a slight to moderate acidity (pH < 6), suggesting that sorption might be slightly stronger in this soil compared to a more neutral soil. The half-life for uranine in the top 5 cm of soil was observed to be less than four days, while the half-life of phloxine B was approximately seven days (Table 7). The concentration of phloxine B was also measured at 5-10 cm depth in the soil, and was found to be an order of magnitude lower than the topmost layer, indicating that the loss in the upper layer is mostly due to degradation and not leaching. The concentrations of phloxine B in the lower layer also appear to decrease over time. Based on the results of this study, it would be expected that D&C Red No. 21 would also undergo photolysis in the topmost layers of soil and have a half-life ranging between four and seven days. Furthermore, Tonogai et al. (1979) have shown that eosin, a close chemical analogue to D&C Red No. 21, degrades to uranine. Therefore, this study suggests that after D&C Red No. 21 is debrominated by photodegradation, it would continue to rapidly degrade in soil. The degradation products of uranine have not been identified.
Li et al. (1998) also measured the concentration of phloxine B and uranine in sediment, following a spill. Uranine was not detected in any of the samples, which may be a result of its lower tendency to partition to sediment and subsequent photolysis in the puddle. As shown in Table 7, Li et al. (1998) observed longer half-lives for phloxine B in sediment relative to water. The longer half-life may be a result of reduced light, although the depth of the water was only 8 -10 cm and sediment samples were taken at a depth of 5 cm. The authors of this study also note, however, that some of the observed losses of these two chemicals may be due to washout by rain. Thus the significance of these data in relation to the degradation half-life of D&C Red No. 21 in sediment is uncertain.
The weight of evidence based on the data described above (particularly relating to photolysis) indicates that D&C Red No. 21 does not meet the persistence criteria for air (half-life in air ≥ 2 days), water and soil (half-life ≥ 182 days) and sediments (half-life ≥ 365 days) as set out in thePersistence and Bioaccumulation Regulations (Canada 2000)
Potential for Bioaccumulation
Experimental bioaccumulation data were not found for D&C Red No. 21. Experimental and modelled log Kow values for e osin, a close chemical analogue, range from -0.25 to -1.68, suggesting that D&C Red No. 21 does not have the potential to bioaccumulate in the environment (see Table 3 above).
The modified Gobas BAF middle trophic-level model for fish predicted a bioaccumulation factor (BAF) of 1 L/kg for eosin, suggesting that D&C Red No. 21 does not have the potential to bioconcentrate and biomagnify in the environment. The results of BCF model calculations also provide weight of evidence to support the low bioconcentration potential of this substance.
| Test organism | Endpoint | Value wet weight (L/kg) |
Reference |
|---|---|---|---|
| Fish | BAF | 1 | Gobas BAF T2MTL (Arnot and Gobas 2003) |
| Fish | BCF | 1 | Gobas BCF T2LTL (Arnot and Gobas 2003) |
| Fish | BCF | 9 | OASIS Forecast 2005 |
| Fish | BCF | 3 | BCFWIN 2000 |
Metabolism information for this substance was not available, nor was it considered appropriate to use it in the Gobas BCF/BAF and BCF models (based on structural properties and lack of appreciable biodegradation potential).
Using the eosin bioaccumulation predictions as a baseline, the BCFs and BAFs for D&C Red No. 21 would also likely be much less than 5000 and thus D&C Red No. 21 is not expected be bioaccumulative at environmental pH.
In addition, two other related compounds, phloxine B (CAS RN 18472-87-2) and uranine (CAS RN 518-47-8) have reported empirical log Kow values of less than 1, suggesting that D&C Red No. 21 would also be hydrophilic and have a low potential for bioaccumulation (Table 4).
The weight of evidence indicates that D&C Red No. 21 does not meet the bioaccumulation criteria (BCF or BAF ≥ 5000) as set out in the Persistence and Bioaccumulation Regulations(Canada 2000).
Ecological Effects Assessment
In the Aquatic Compartment
There is modelled and experimental evidence that eosin, a close chemical analogue, does not cause harm to aquatic organisms at relatively low concentrations, suggesting that D&C Red No. 21 would also exhibit low toxicity (see tables 9a and 9b below).
| Test organism | Type of test | Endpoint | Value (mg/L) | Reliability | Reference |
|---|---|---|---|---|---|
| Fish | Acute (48 hours) |
TLm[1] | 2200 | Low | Tonogai et al. 1978 |
| Fish | Acute (48 hours) no irradiation |
TLm[1] | 1800 | Low | Tonogai et al. 1979 |
| Fish | Acute (48 hours) with 10 hours irradiation |
TLm[1] | 1200 | Low | Tonogai et al. 1979 |
| Fish | Acute (48 hours) |
TLm[1] | 620 | Low | Tonogai et al. 1982 |
[1] TLm - Median Tolerance Limit: the concentration of substance at which just 50% of the test organisms are able to survive for a specified period of exposure (equivalent to a LC50).
The toxicity of xanthene dyes has been observed to increase following photoirradiation. This increased toxicity is expected to occur as a result of their excitation to triplet state, where the dye is able to excite an oxygen molecule which can react with biomolecules and also results in the release of halogen atoms (Walthall and Stark 1999 and Tonogai et al. 1979). Studies show that the toxicity of eosin to fish increases when exposed to light, although exposure at concentrations of > 100 mg/L were required to elicit observable effects (Tonogai et al. 1978; 1979; 1982). The results of these studies were deemed to be of low reliability, however, as detailed test methods were not provided. The results are nevertheless generally consistent with modelled predictions for eosin, and toxicity tests on structurally similar substances (tables 9b and 9c), suggesting that acute toxicity is unlikely when organisms are exposed to D&C Red No. 21 at relatively low concentrations (i.e., LC/EC50s > 1 mg/L).
| Test organism | Type of test |
Endpoint | Value (mg/L) |
Reference |
|---|---|---|---|---|
| Fish | Acute (96 hours) |
LC50[1] | 18 | ECOSAR 2004 |
| 5.8 | AIES 2003-2005 | |||
| Daphnia | Acute (48 hours) |
EC50[2] | 25 | ECOSAR 2004 |
[1] LC50 - The concentration of a substance that is estimated to be lethal to 50% of the test organisms.
[2] EC50 - The concentration of a substance that is estimated to cause some toxic sublethal effect on 50% of the test organisms.
In addition, empirical toxicity data are available for two other related compounds, phloxine B (CAS RN 18472-87-2) and uranine (CAS RN 518-47-8), showing the effects of the degree of halogenation on toxicity (Table 9c). As shown in Table 4, phloxine B contains more halogen substituents than eosin, while uranine does not have any halogen substituents. Based on their degree of halogenation, it is expected that the toxicity of eosin would lie in between the toxicities of these other compounds. It was found thatdaphnia is more sensitive than fish to both dyes. LC50 s to daphnia were 0.423 mg/L and 337 mg/L for phloxine B and uranine, respectively (Table 9c). The results of this study are in agreement with the modelled prediction for eosin which estimated that the toxicity to daphnia was higher than to fish (Table 9b). The TLm to fish was found to be greater than 50mg/L for both phloxine B and uranine, with uranine having lower toxicity (Table 9c). The predicted values for eosin (Table 9b) seem to overestimate the toxicity to some extent, as the predicted LC50 s are below the experimental values obtained in fish for phloxine B. The results of these studies by Tonogai et al. were also deemed to be of low reliability, as detailed test methods were not provided. Although details are lacking in several of the studies, when the trends are taken together with the modelled data, this evidence also suggests that D&C Red No. 21 would not be highly hazardous to aquatic organisms. The robust study summaries for studies that were deemed to be of satisfactory reliability can be found in Appendix I.
Walthall and Stark (1999) observed the effect of the exposure of fluorescent light to phloxine B on its acute mortality todaphnia over time. They found that after three days, the residues were no longer significantly toxic to newly exposed neonates. Based on a photoirradiation study by Tonogai et al. (1979), it is expected that the bromine atoms were removed during photodegradation and the residue was tetrachlorofluorescein. Debromination has also been identified as a degradation pathway for eosin, resulting in uranine as a residue. Therefore it would be expected that after three days of exposure to light, the residues of eosin would also exhibit low toxicity todaphnia(Tonogai et al. 1979).
| Test organism | Type of test |
Endpoint | Value (mg/L) |
Reliability | Reference |
|---|---|---|---|---|---|
| Phloxine B | |||||
| Daphnia | Acute (48 hours) |
LC50[1] | 0.423 | Satisfactory | Walthall and Stark 1999 |
| Fish | Acute (48 hours) |
TLm[2] | 190 | Low | Tonogai et al. 1978 |
| Fish | Acute (48 hours) No irradiation |
TLm[2] | 200 | Low | Tonogai et al. 1979 |
| Fish | Acute (48 hours) with 10 hours irradiation |
TLm[2] | 60 | Low | Tonogai et al. 1979 |
| Fish | Acute (48 hours) |
TLm[2] | 200 | Low | Tonogai et al. 1982 |
| Uranine | |||||
| Daphnia | Acute (48 hours) |
LC50[1] | 337 | Satisfactory | Walthall and Stark 1999 |
| Fish | Acute (48 hours) | TLm[2] | 3000 | Low | Tonogai et al. 1979 |
| Fish | Acute (96 hours) | LC50[1] | 997.1 | Satisfactory | Pouliquen et al. 1995 |
[1] LC50 - The concentration of a substance that is estimated to be lethal to 50% of the test organisms.
[2] TLm - Median Tolerance Limit: the concentration of substance at which just 50% of the test organisms are able to survive for a specified period of exposure (equivalent to LC50).
In Other Environmental Compartments
Although Level III fugacity modelling would suggest that when released to soil and air, D&C Red No. 21 will reside in soil, effects on soil organisms have not been estimated.
Ecological Exposure Assessment
No data concerning concentrations of this substance in water in Canada have been identified. Environmental concentrations are, therefore, estimated from available information, including estimated substance quantities, release rates, and receiving water bodies. Environment Canada's Industrial Generic Exposure Tool - Aquatic (IGETA) was employed to estimate the substance concentration (worst-case) in a generic water course receiving industrial effluents (Environment Canada 2008a). The generic scenario is designed to provide these estimates based on conservative assumptions regarding the amount of chemical processed and released, the number of processing days, the sewage treatment plant removal rate, and the size of the receiving watercourse. The tool models an industrial-release scenario based on loading data from sources such as industrial surveys and knowledge of the distribution of industrial discharges in the country, and calculates a predicted environmental concentration (PEC). The equation and inputs used to calculate the PEC in the receiving water course are described in the Environment Canada (2008b). A use quantity of 100 kg was assumed. Information received pursuant to the section 71 notice in 2001 indicated that one company imported this substance into Canada in 2000. However the voluntary submissions provided for 2006 confirm that the quantity imported would be less than 100 kg. Therefore releases of this substance to the Canadian environment are expected to be very low (Environment Canada 2001 and Environment Canada 2007).
The obtained predicted environmental concentration in water (PEC) resulting from this release scenario was 0.01 mg/L.
Characterization of Ecological Risk
As no experimental chronic aquatic toxicity studies were available for D&C Red No. 21, a conservative Predicted No Effects Concentration (PNEC) was estimated based on the modelled acute toxicity (to fish) of its sodium salt, eosin. The 96-hour LC50 for eosin was 5.8 mg/L. A factor of 100 was then applied to account for uncertainty in extrapolating from acute to chronic values and from laboratory results to the field and to account for physical and chemical property differences with D&C Red No. 21 (e.g., salt is more soluble). The resulting conservative PNEC is 0.058 mg/L.
For exposure resulting from industrial releases to water using a conservative release scenario, IGETA results estimate that the predicted no-effects concentration (PNEC) will not be exceeded: the resulting risk quotient (PEC/PNEC) being 0.2. This result suggests that the risks posed by D&C Red No. 21 to aquatic organisms are negligible (Environment Canada 2008b).
The scenario presented above does not consider releases from consumer uses of products containing D&C Red No. 21. However, D&C Red No. 21 is known to be present in different cosmetic products that may be on the Canadian market. Although there is currently not sufficient available information to derive a quantity estimate for this source, back calculations using the Mega Flush tool (Environment Canada's spreadsheet model for estimating down-the-drain releases from consumer uses, Environment Canada 2008c) have been performed to provide an estimate of the potential magnitude of such releases. The results indicate that around 80 000 kg would have to be used and released in the Canadian environment in order to have a risk quotient above one at one of the discharge sites.
The predicted low risk of D&C Red No. 21 to aquatic organisms is further supported by the reactive nature of this substance. Although D&C Red No. 21 is not expected to biodegrade, it is expected to undergo rapid photolysis in clear or shallow waters, which will reduce the long-term exposure and long-term toxicity to aquatic organisms. It is also expected to have low acute toxicity and not be bioaccumulative. Therefore, although exposure may be relatively widespread because of its use in cosmetics, concentrations are not expected to be of ecological concern.
Uncertainties in Evaluation of Ecological Risk
This section summarizes the key uncertainties associated with the risk assessment of D&C Red No. 21.
Eosin, the sodium salt of D&C Red No. 21, was used as an analogue throughout this assessment. However as it is a salt, it is expected to have slightly different physical and chemical properties, which creates uncertainty in the modelled predictions of fate, persistence, bioaccumulation and aquatic toxicity that are based on these estimates.
All bioaccumulation data are based on modelled estimates for analogue substances only. The lack of supporting evidence from empirical studies is a source of uncertainty in the bioaccumulation assessment.
The persistence assessment is limited by the lack of experimental biodegradation data, which necessitated the generation of model predictions and the use of data derived from analogue substances.
Empirical toxicity data were also lacking, resulting in uncertainty in the ecological effects assessment which relied on empirical data and model predictions for analogues.
For the exposure assessment, the Predicted Environmental Concentration (PEC) represents concentrations in water only, so exposure through soils is not considered. However, given the current release scenarios and quantities used in Canada, exposure is not likely to be significant at this time.
Based on the information presented in this screening assessment, it is concluded that D&C Red No. 21 is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity, or that constitute or may constitute a danger to the environment on which life depends.
It is therefore concluded that D&C Red No. 21 does not meet the definition of toxic as set out in section 64 of CEPA 1999. Additionally, D&C Red No. 21 does not meet the criteria for persistence and bioaccumulation potential as set out in thePersistence and Bioaccumulation Regulations (Canada 2000).
ACD/pKaDB [Prediction Module]. 2005. Version 9.04. Toronto (ON): Advanced Chemistry Development.
Alcantara-Licudine JP, Cunningham RT, Liquido NJ, McQuate GT, Li QX. 1999. Dissipation of Phloxine B and Uranine in protein bait sprayed in a coffee field for the suppression of Mediterranean Fruit Fly. Bull Environ Contam Toxicol 62: 344-351.
Amat-Guerri, F, Lopez-Gonzalez MMC, Sastre R, Martinez-Utrilla R. 1990. Spectrophotometric determination of ionization and isomerisation constants of Rose Bengal, Eosin Y and some derivative. Dyes and Pigments. 13: 219-232.
[AOPWIN] Atmospheric Oxidation Program for Windows [Estimation Model]. 2000. Version 1.91. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. [cited 2007 Sept 12]. Exposure Assessment Tools and Models.
Arnot JA, Gobas FAPC. 2003. A generic QSAR for assessing the bioaccumulation potential of organic chemicals in aquatic food webs. QSAR Comb Sci 22(3): 337-345.
[AIES] Artificial Intelligence Expert System. 2003-2005. Version 1.25. Ottawa (ON): Environment Canada. Model developed by Stephen Niculescu. Environment Canada, Existing Substances Division, New Substances Division, Ottawa, K1A 0H3.
[BCFWIN] BioConcentration Factor Program for Windows [Estimation Model]. 2000. Version 2.15. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. [cited 2007 Sept 12]. Exposure Assessment Tools and Models.
[BIOWIN] Biodegradation Probability Program for Windows [Estimation Model]. 2000. Version 4.02. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. [cited 2007 Sept 12]. Exposure Assessment Tools and Models.
Boethling RS, Howard PH, Beauman JA, Larosche ME 1995. Factors for intermedia extrapolations in biodegradability assessment. Chemosphere 30(4): 741-752.
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C., 1999, c. 33. Canada Gazette, Part III, Vol. 22, No. 3.
Canada. 2000. Canadian Environmental Protection Act: Persistence and Bioaccumulation Regulations, P.C. 2000-348, 23 March, 2000, SOR/2000-107, Canada Gazette, Part II, Vol. 134, No. 7.
Canada, Dept. of the Environment. 2001. Canadian Environmental Protection Act, 1999 : Notice with respect to certain substances on the Domestic Substances List (DSL). Canada Gazette, Part I, Vol. 135, No. 46.
Canada, Dept. of the Environment, Dept. of Health. 2006.Canadian Environmental Protection Act, 1999:Notice of intent to develop and implement measures to assess and manage the risks posed by certain substances to the health of Canadians and their environment. Canada Gazette, Part I, Vol. 140, No. 49.
Canada, Dept. of the Environment, Dept. of Health. 2007.Canadian Environmental Protection Act, 1999: Notice of second release of technical information relevant to substances identified in the Challenge. Canada Gazette, Part I, Vol. 141. No. 19.
[ECOSAR] Ecological Structural Activity Relationships [Internet]. 2004. Version 0.99h. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. [cited 2007 Sept 12]. Exposure Assessment Tools and Models.
Environment Canada. 2001. Data for certain substances collected undertheCanadian Environmental Protection Act, 1999, Section71:Notice with respect to certain substances on the Domestic Substances List (DSL). Data prepared by: Environment Canada, Health Canada, Existing Substances Program.
Environment Canada. 2007. Voluntary submission of data for Batch 2 substances collected undertheGovernment of Canada's Chemical Management Plan Challenge initiative. Data prepared by: Environment Canada, Existing Substances Program.
Environment Canada. 2008a. Guidance for conducting ecological assessments under CEPA, 1999: science resource technical series, technical guidance module: the Industrial Generic Exposure Tool - Aquatic (IGETA). Working document. Gatineau (QC): Environment Canada, Existing Substances Division.
Environment Canada. 2008b. IGETA report: CAS RN 15086-94-9, 2008-08-28. Unpublished report. Gatineau (QC): Environment Canada, Existing Substances Division.
Environment Canada. 2008c. Guidance for conducting ecological assessments under CEPA, 1999: science resource technical series, technical guidance module: Mega Flush consumer release scenario. Preliminary draft working document. Gatineau (QC): Environment Canada, Existing Substances Division.
Environmental Working Group`s Skin Deep Cosmetic Safety Database for D&C Red 21 [cited 2007 Oct 1].
[EQC] Equilibrium Criterion Model. 2003. Version 2.02. Peterborough (ON): Trent University, The Canadian Environmental Modelling Centre and Chemistry - EQC Model. [cited 2007 Sept 12].
Food and Drug Act. 1985 c.F-27. Food and Drug Regulations (C.R.C., c.870).
Health Canada. 2003. List of Acceptable Non-medicinal Ingredients. Drugs and Health Products, Health Canada, Ottawa, Ontario, Canada. [cited 2007 Oct 1].
Heitz JR. 1995. Pesticidal applications of photoactivated molecules. In: JR Heitz and KR Downum (eds), Light-activated pest control. Washington (DC): American Chemical Society. pp 1-16.
[HENRYWIN] Henry's Law Constant Program for Microsoft Windows [Estimation Model]. 2000. Version 3.10. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. [cited 2007 Sept 12]. Exposure Assessment Tools and Models.
[KOWWIN] Octanol-Water Partition Coefficient Program for Microsoft Windows [Estimation Model]. 2000. Version 1.67. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. [cited 2007 Sept 12]. Exposure Assessment Tools and Models.
Levillain P and Fompeydie D. 1985. Determination of equilibrium constants by derivative spectrophotometry. Application to the pKas of Eosin. Anal Chem. 57: 2561-2563.
Li QX, Voisinet Bender CJ, Alcantara-Licudine JP. 1998. Dissipation of Phloxine B and Uranine in sediment and water at a Kauai spill site. Bull Environ Contam Toxicol. 61: 426-432.
Lipman AL. 1995. Safety of xanthene dyes according to the U.S. Food and Drug Administration. In: JR Heitz and KR Downum (eds), Light-activated pest control. Washington (DC): American Chemical Society. pp 34-53.
[MPBPWIN] Melting Point Boiling Point Program for Microsoft Windows [Estimation Model]. 2000. Version 1.41. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. [cited 2007 Sept 12]. Exposure Assessment Tools and Models.
[MSDS] Material Safety Data Sheet: Basacid Yellow 226 [Internet]. 2004. Toronto, Ontario, Canada: BASF Corporation (Canada). [cited 2007 July 25]. MSDsonline - EH & S Compliance Made Simple [Restricted access].
[MSDS] Material Safety Data Sheet: Basacid Red 316 [Internet]. 2006a. Mississauga, Ontario, Canada: BASF Corporation (Canada). [cited 2007 July 25]. MSDsonline - EH & S Compliance Made Simple [Restricted access].
[MSDS] Material Safety Data Sheet: Phloxine B Certified [Internet]. 2006b. Kirwan, Old Australia: ProSciTech. [cited 2007 Aug 22]. MSDsonline - EH & S Compliance Made Simple [Restricted access].
Nalco Chemical Company. 1998. Environmental assessment for an inert tracer chemical in boiler treatment products where steam from treated boilers may contact food. Naperville (IL). [cited 2008 Aug 21].
[NCI] National Chemical Inventories [database on CD-ROM]. 2006. Columbus (OH): American Chemical Society. [cited 2006 Oct 11].National Chemical Inventories (NCI) on CD.
[OASIS Forecast] Optimized Approach based on Structural Indices Set [Internet]. 2005. Version 1.20. Bourgas (BG): Bourgas Prof. Assen Zlatarov University, Laboratory of Mathematical Chemistry. [cited 2007 Sept 12]. OASIS Software.
[PCKOCWIN] Organic Carbon Partition Coefficient Program for Windows [Estimation Model]. 2000. Version 1.66. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. [cited 2007 Sept 12]. Exposure Assessment Tools and Models.
[PhysProp] Interactive PhysProp Database [database on the Internet]. 2006. Syracuse (NY): Syracuse Research Corporation. [cited 2007 Sept 12] Interactive PhysProp Database Demo.
Pouliquen H, Algoet M, Bucht V, Le Bris H. 1995. Acute toxicity of Fluorescein to turbot (Scophthalmus Maximus). Vet Human Toxicol. 37(6): 527-529.
PubChem [database on the Internet]. 1988 - Bethesda (MD): U.S. National Library of Medicine National Center for Biotechnology Information. Eosine Yellowish-(YS) - Compound Summary (CID 11048) [cited 2007 Oct 1].
Smart PL and Laidlaw IMS. 1977. An evaluation of some fluorescent dyes for water tracing. Water Resources Research. 13(1): 15-33.
Spadotto C and Hornsby AG. 2003. Organic compounds in the environment: soil sorption of acidic pesticides: modeling pH effects. J Environ Qual. 32: 949-956.
StainsFile website. [cited 2007 January 1]. Fact Sheet - Eosinol.
Tonogai Y, Iwaida M, Tati M, Ose Y, Sato T. 1978. Biochemical decomposition of coal-tar dyes II. Acute toxicity of coal-tar dyes and their decomposed products. The Journal of Toxicological Sciences. 3: 205-214.
Tonogai Y, Ito Y, Iwaida M, Tati M, Ose Y, Sato T. 1979. Studies on the toxicity of coal-tar dyes. I. photodecomposed products of four xanthene dyes and their acute toxicity to fish. The Journal of Toxicological Sciences. 4: 115-126.
Tonogai Y, Ogawa S, Ito Y, Iwaida M. 1982. Actual survey on TLm (Median Tolerance Limit) values of environmental pollutants, especially on amines, nitriles, aromatic nitrogen compounds and artificial dyes. The Journal of Toxicological Sciences. 7: 193-203.
Walthall WK and Stark JD. 1999. The acute and chronic toxicity of two xanthene dyes, Fluorescein Sodium Salt and Phloxine B, toDaphnia pulex. Environmental Pollution. 104: 207-215.
Wang L, Cai WF, Li QX. 1998. Photolysis of Phloxine B in water and aqueous solutions. Arch Environ Contam Toxicol. 35: 397-403.
Wang H, Lu L, Zhu S, Li Y, Cai W. 2006. The phototoxicity of xanthene derivatives against Escherichia coli, Staphylococcus aureus, and Saccharomyces cerevisiae. Current Microbiology. 52: 1-5.
[WSKOWWIN] Water Solubility for Organic Compounds Program for Microsoft Windows [Estimation Model]. 2000. Version 1.41 Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. [cited 2007 Sept 12]. Exposure Assessment Tools and Models.
Robust Study Summaries Form and Instructions: Persistence in Water, Sediments, and Soil
| No | Item | Weight | Yes/No | Specify | |
|---|---|---|---|---|---|
| 1 | 1Reference: Photolysis of Phloxine B inWater and Aqueous Solutions. Wang et al. 1998. Arch. Environ. Contam. Toxicol. Vol 35, p 397-403. | ||||
| 2 | Substance identity: CAS RN | n/a | N | - | |
| 3 | Substance identity: chemical name(s) | n/a | Y | Phloxine B, or (28,48,58,78-tetrabromo-4,5,6,7-tetrachlorofluorescein-disodium salt), or D&C Red No. 28 | |
| 4 | Chemical composition of the substance | 2 | Y | - | |
| 5 | Chemical purity | 1 | Y | - | |
| Method | |||||
| 6 | Reference | 1 | Y | - | |
| 7 | OECD, EU, national, or other standard method? | 3 | N | - | |
| 8 | Justification of the method/protocol if not a standard method was used | 2 | N | - | |
| 9 | GLP (Good Laboratory Practice) | 3 | N | - | |
| Test design / conditions | |||||
| 10 | Test type (i.e. hydrolysis, biodegradation, etc.) | n/a | Y | photodegradation | |
| 11 | Test conditions type (aerobic or anaerobic) | n/a | Y | aerobic | |
| 12 | Test medium (water, sediment, or soil) | n/a | Y | water and aqueous solutions | |
| 13 | Test duration | n/a | N | duration based on halflives | |
| 14 | Sex | 1 | Y | negative | |
| 15 | Number of replicates (including controls) | 1 | Y | - | |
| 16 | Measured concentrations reported? | 3 | Y | - | |
| 17 | Analytical method / instrument | 1 | Y | - | |
| Details on Biodegradation | |||||
| 18 | Type of biodegradation (ready or inherent) reported? | 2 | - | n/a | |
| 19 | When type of biodegradation (ready or inherent) is not reported, if there is indirect information allowing to identify biodegradation type? | 1 | - | n/a | |
| 20 | Inoculum source | 1 | - | n/a | |
| 21 | Inoculum concentration or number of microorganisms | 1 | - | n/a | |
| 22 | Were inoculum pre-conditioning and pre-adaptation reported? | 1 | - | n/a | |
| 23 | Were inoculum pre-conditioning and pre-adaptation appropriate for the method used? | n/a | - | n/a | |
| 24 | Temperature | 1 | - | n/a | |
| 25 | Has percentage degradation of the reference compound reached the pass levels by day 14? | n/a | - | n/a | |
| 26 | Soil: soil moisture reported? | 1 | - | n/a | |
| 27 | Soil and sediments: background SOM (Soil Organic Matter) content reported? | 1 | - | n/a | |
| 28 | Soil and sediments: clay content reported? | 1 | - | n/a | |
| 29 | Soil and sediments:CEC (Cation Exchange Capacity) reported? | 1 | - | n/a | |
| Details on Hydrolysis | |||||
| 30 | pH values reported? | 1 | - | n/a | |
| 31 | Temperature | 1 | - | n/a | |
| 32 | Were appropriate concentrations of the substance used? | - | - | n/a | |
| 33 | If solvent was used, was it done appropriately? | - | - | n/a | |
| Details on Photodegradation | |||||
| 34 | Temperature | 1 | Y | 29 +/- 1°C | |
| 35 | Light source | 1 | Y | One 15-W ultraviolet (UV); Two 15-W visible (VIS) ; Two 15-W cool white fluorescent lamps (Sylvania, F15T12) | |
| 36 | Light spectrum (nm) | 1 | Y | 254 nm and 365 nm respectively | |
| 37 | Relative intensity based on sunlight intensity | 1 | Y | 980 µmol/m2 · s; 508 µmol/m2 · s; 16.1 µmol/m2 · s. | |
| 38 | Spectrum of a substance | 1 | N | - | |
| 39 | Indirect photolysis:sensitiser (type) | 1 | - | n/a | |
| 40 | Indirect photolysis:concentration of sensitiser | 1 | - | n/a | |
| Results | |||||
| 41 | Endpoint and value | n/a | n/a | t12 = 0.70 to 1.28 hours under 254 nm, 26.3 to 115 hours under 365 nm,, and 14.1 to 46.2 hours under and cool white fluorescent lights, in different water samples and 2% NaCl solution at 29 ± 1°C , a range of buffer pH 6-8 | |
| 42 | n/a | Y | 2',4',5'-tribromo-4,5,6,7-tetrachlorofluorescein (TBTCF) and 4',5'-dibromo-4,5,6,7-tetrachlorofluorescein (DBTCF) | ||
| 43 | Score: ... % | 60.9 | |||
| 44 | EC Reliability code: | 2 | |||
| 45 | Reliability category (high, satisfactory, low): | Satisfactory Confidence | |||
| 46 | Comments | - | |||
Robust Study Summaries Form and Instructions: Aquatic iT
| No | Item | Weight | Yes/No | Specify |
|---|---|---|---|---|
| 1 | Reference: The acute and chronic toxicity of two xanthene dyes, fluorescein sodium salt and phloxine B, to Daphnia pulex.Walthall and Stark 1999. Environmental Pollution Vol 104, Pgs 207 - 215. |
|||
| 2 | Substance identity: CAS RN | n/a | Y | 18472-87-2 |
| 3 | Substance identity: chemical name(s) | n/a | Y | phloxine B |
| 4 | Chemical composition of the substance | 2 | N | - |
| 5 | Chemical purity | 1 | N | - |
| 6 | Persistence/stability of test substance in aquatic solution reported? | 1 | Y | rapid breakdown with exposure to sunlight |
| Method | ||||
| 7 | Reference | 1 | Y | - |
| 8 | OECD, EU, national, or other standard method? | 3 | N | - |
| 9 | Justification of the method/protocol if not a standard method was used | 2 | - | n/a |
| 10 | GLP (Good Laboratory Practice) | 3 | N | - |
| Test organism | ||||
| 11 | Organism identity: name | n/a | - | Daphnia pulex |
| 12 | Latin or both Latin & common names reported? | 1 | Y | - |
| 13 | Life cycle age / stage of test organis | 1 | Y | - |
| 14 | Length and/or weight | 1 | - | n/a |
| 15 | Sex | 1 | - | n/a |
| 16 | Number of organisms per replicate | 1 | Y | 5 or 10 |
| 17 | Organism loading rate | 1 | Y | - |
| 18 | Food type and feeding periods during the acclimation period | 1 | Y | - |
| Test design / conditions | ||||
| 19 | Test type (acute or chronic | n/a | Y | both acute and chronic |
| 20 | Experiment type (laboratory or field | n/a | Y | Laboratory |
| 21 | Exposure pathways (food, water, both) | n/a | Y | water |
| 22 | Exposure duration | n/a | Y | acute exposure: 48 h; chronic exposure: 10 days |
| 23 | Negative or positive controls (specify) | 1 | Y | Negative |
| 24 | Number of replicates (including controls) | 1 | Y | 4 |
| 25 | Nominal concentrations reported? | 1 | Y | 5 |
| 26 | Measured concentrations reported? | 3 | N | - |
| 27 | Food type and feeding periods during the long-term tests | 1 | Y | - |
| 28 | Were concentrations measured periodically (especially in the chronic test)? | 1 | N | - |
| 29 | Were the exposure media conditions relevant to the particular chemical reported? (e.g., for the metal toxicity - pH, DOC/TOC, water hardness, temperature) | 3 | Y | - |
| 30 | Photoperiod and light intensity | 1 | Y | 16h:8h light dark |
| 31 | Stock and test solution preparation | 1 | Y | - |
| 32 | Was solubilizer/emulsifier used, if the chemical was poorly soluble or unstable? | 1 | - | n/a |
| 33 | If solubilizer/emulsifier was used, was its concentration reported? | 1 | - | n/a |
| 34 | If solubilizer/emulsifier was used, was its ecotoxicity reported? | 1 | - | n/a |
| 35 | Analytical monitoring intervals | 1 | N | - |
| 36 | Statistical methods used | 1 | Y | - |
| Information relevant to the data quality | ||||
| 37 | Was the endpoint directly caused by the chemical's toxicity, not by organism's health (e.g. when mortality in the control >10%) or physical effects (e.g. 'shading effect')? | n/a | Y | - |
| 38 | Was the test organism relevant to the Canadian environment? | 3 | Y | - |
| 39 | Were the test conditions (pH, temperature, DO, etc.) typical for the test organism? | 1 | Y | - |
| 40 | Does system type and design (static, semi-static, flow-through; sealed or open; etc.) correspond to the substance's properties and organism's nature/habits? | 2 | Y | - |
| 41 | Was pH of the test water within the range typical for the Canadian environment (6 to 9)? | 1 | Y | 7.4-7.8 |
| 42 | Was temperature of the test water within the range typical for the Canadian environment (5 to 27°C)? | 1 | Y | 25±0.1 C |
| 43 | Was toxicity value below the chemical's water solubility? | 3 | Y | - |
| Results | ||||
| 44 | Toxicity values (specify endpoint and value) | n/a | n/a | 48-h LC50 (95% FL) of phloxine B =0.423 (0.376±0.477) mg/L |
| 45 | Other endpoints reported - e.g. BCF/BAF, LOEC/NOEC (specify)? | n/a | - | MT50 =0.693/C; 600 mg/L, a concentration corresponding to the LC83, all individuals were dead following 10-day exposure |
| 46 | Other adverse effects (e.g. carcinogenicity, mutagenicity) reported? | n/a | N | - |
| 47 | Score : ... % | 66.7 | ||
| 48 | EC Reliability code: | 2 | ||
| 49 | Reliability category (high, satisfactory, low) : | Satisfactory Confidence | ||
| 50 | Comments | - | ||
Robust Study Summaries Form and Instructions: Aquatic iT
| No | Item | Weight | Yes/No | Specify |
|---|---|---|---|---|
| 1 | Reference: Pouliquen, H, M. Algoet, V. Buchet and H. LeBris (1995). Acute toxicity of fluorescien to turbot (Scophthalmus maximus). Vet. Human Toxicol. 37(6):527-529. | |||
| 2 | Substance identity: CAS RN | n/a | Y | 518-47-8 |
| 3 | Substance identity: chemical name(s) | n/a | Y | Fluorescein |
| 4 | Chemical composition of the substance | 2 | N | - |
| 5 | Chemical purity | 1 | N | - |
| 6 | Persistence/stability of test substance in aquatic solution reported? | 1 | N | - |
| Method | ||||
| 7 | Reference | 1 | Y | - |
| 8 | OECD, EU, national, or other standard method? | 3 | Y | - |
| 9 | Justification of the method/protocol if not a standard method was used | 2 | - | n/a |
| 10 | GLP (Good Laboratory Practice) | 3 | - | n/a |
| Test organism | ||||
| 11 | Organism identity: name | n/a | Y | Scophthalmus maximus (turbot) |
| 12 | Latin or both Latin & common names reported? | 1 | Y | - |
| 13 | Life cycle age / stage of test organis | 1 | Y | - |
| 14 | Length and/or weight | 1 | Y | - |
| 15 | Sex | 1 | - | n/a |
| 16 | Number of organisms per replicate | 1 | Y | - |
| 17 | Organism loading rate | 1 | Y | 1.25g/L |
| 18 | Food type and feeding periods during the acclimation period | 1 | Y | - |
| Test design / conditions | ||||
| 19 | Test type (acute or chronic) | n/a | Y | acute |
| 20 | Experiment type (laboratory or field | n/a | Y | Laboratory |
| 21 | Exposure pathways (food, water, both) | n/a | Y | water |
| 22 | Exposure duration | n/a | Y | 96 hour |
| 23 | Negative or positive controls (specify) | 1 | Y | Negative |
| 24 | Number of replicates (including controls) | 1 | Y | 1 |
| 25 | Nominal concentrations reported? | 1 | Y | - |
| 26 | Measured concentrations reported? | 3 | N | - |
| 27 | Food type and feeding periods during the long-term tests | 1 | - | n/a |
| 28 | Were concentrations measured periodically (especially in the chronic test)? | 1 | N | - |
| 29 | Were the exposure media conditions relevant to the particular chemical reported? (e.g., for the metal toxicity - pH, DOC/TOC, water hardness, temperature) | 3 | Y | - |
| 30 | Photoperiod and light intensity | 1 | Y | - |
| 31 | Stock and test solution preparation | 1 | Y | - |
| 32 | Was solubilizer/emulsifier used, if the chemical was poorly soluble or unstable? | 1 | - | n/a |
| 33 | If solubilizer/emulsifier was used, was its concentration reported? | 1 | - | n/a |
| 34 | If solubilizer/emulsifier was used, was its ecotoxicity reported? | 1 | - | n/a |
| 35 | Analytical monitoring intervals | 1 | N | - |
| 36 | Statistical methods used | 1 | Y | - |
| Information relevant to the data quality | ||||
| 37 | Was the endpoint directly caused by the chemical's toxicity, not by organism's health (e.g. when mortality in the control >10%) or physical effects (e.g. 'shading effect')? | n/a | Y | - |
| 38 | Was the test organism relevant to the Canadian environment? | 3 | Y | Salt water |
| 39 | Were the test conditions (pH, temperature, DO, etc.) typical for the test organism? | 1 | Y | - |
| 40 | Does system type and design (static, semi-static, flow-through; sealed or open; etc.) correspond to the substance's properties and organism's nature/habits? | 2 | Y | Static |
| 41 | Was pH of the test water within the range typical for the Canadian environment (6 to 9)? | 1 | Y | 8 |
| 42 | Was temperature of the test water within the range typical for the Canadian environment (5 to 27°C)? | 1 | Y | 14 C |
| 43 | Was toxicity value below the chemical's water solubility? | 3 | Y | - |
| Results | ||||
| 44 | Toxicity values (specify endpoint and value) | n/a | n/a | LC50=997.1mg/L |
| 45 | Other endpoints reported - e.g. BCF/BAF, LOEC/NOEC (specify)? | n/a | N | - |
| 46 | Other adverse effects (e.g. carcinogenicity, mutagenicity) reported? | n/a | N | - |
| 47 | Score : ... % | 76.9 | ||
| 48 | EC Reliability code: | 2 | ||
| 49 | Reliability category (high, satisfactory, low) : | Satisfactory Confidence | ||
| 50 | Comments | - | ||

