Screening Assessment for the Challenge
This page has been archived on the Web
Information identified as archived is provided for reference, research or recordkeeping purposes. It is not subject to the Government of Canada Web Standards and has not been altered or updated since it was archived. Please contact us to request a format other than those available.
Archived
1,2-Benzenedicarboxylic acid, bis(2-methoxyethyl) ester
Chemical Abstracts Service Registry Number
117-82-8
Environment Canada
Health Canada
November 2009
- Synopsis
- Introduction
- Substance Identity
- Physical and Chemical Properties
- Sources
- Uses
- Releases to the Environment
- Environmental Fate
- Persistence and Bioaccumulation Potential
- Potential to Cause Ecological Harm
- Potential to Cause Harm to Human Health
- Conclusion
- References
- Appendix 1: Upper-bounding estimates of daily intake of DMEP from indoor dust by the general population in Canada
- Appendix 2: Upper-bounding estimates of dermal exposure to DMEP from indoor dust by the general population in Canada
- Appendix 3: Summary of health effects information for DMEP
The Ministers of the Environment and of Health have conducted a screening assessment of 1,2-benzenedicarboxylic acid, bis(2-methoxyethyl) ester (di(methoxyethyl)phthalate, DMEP), Chemical Abstracts Service Registry Number 117-82-8. This substance was identified in the categorization of the Domestic Substances List (DSL) as a high priority for action under the Challenge. DMEP was identified as a high priority as it was considered to pose an intermediate potential for exposure of individuals in Canada and had been classified by the European Commission on the basis of reproductive and developmental toxicity. The substance did not meet the ecological categorization criteria for persistence, bioaccumulation or inherent toxicity to aquatic organisms. Therefore, the focus of this assessment of DMEP relates principally to human health risks.
According to information reported under section 71 of the Canadian Environmental Protection Act, 1999(CEPA 1999), DMEP was not manufactured or imported in a quantity equal to or greater than the 100 kg reporting threshold or used in a quantity equal to or greater than the 1000 kg reporting threshold in Canada in 2006. Historically, DMEP was used as a plasticizer and as a paint/coating additive in Canada. The general global applications of DMEP have included its use as a plasticizer and solvent.
Based on limited information on concentrations in environmental media and results from a survey under section 71 of CEPA 1999, exposure of the general population via the environment is expected to be low. No current presence of DMEP in consumer products in the Canadian marketplace was identified. The health effects associated with exposure to DMEP are primarily developmental and reproductive toxicity, based on limited study data on the substance and supported by the toxicological database for its metabolites. The margins between upper-bounding estimates of total daily intake of DMEP for the general population in Canada and exposure levels associated with critical health effects in experimental animals are considered to be adequately protective.
On the basis of the adequacy of the margins between conservative estimates of exposure to DMEP from environmental media, using the concentrations in indoor dust as a surrogate for exposure from soil/dust, and exposure levels associated with critical health effect in exposed experimental animals, it is concluded that DMEP is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
DMEP does not meet the criteria for persistence or bioaccumulation as set out in the Persistence and Bioaccumulation Regulations. On the basis of low ecological hazard and probable low exposure in the environment, based on the very low usage of DMEP in Canada, it is concluded that this substance is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
Based on available information, it is concluded that 1,2-Benzenedicarboxylic acid, bis(2-methoxyethyl) ester is currently not entering, nor is it likely to enter, the environment. Therefore, it is concluded that it does not meet any of the criteria set out in section 64 of CEPA 1999.
Because this substance is listed on the Domestic Substances List, its import and manufacture in Canada are not subject to notification under subsection 81(1). Given the hazardous properties of this substance, there is concern that new activities that have not been identified or assessed could lead to this substance meeting the criteria set out in section 64 of the Act. Therefore, it is recommended to amend the Domestic Substances List, under subsection 87(3) of the Act, to indicate that subsection 81(3) of the Act applies with respect to the substance so that new manufacture, import or use of this substance is notified and undergoes ecological and human health risk assessments.
The Canadian Environmental Protection Act, 1999(CEPA 1999) (Canada 1999) requires the Minister of the Environment and the Minister of Health to conduct screening assessments of substances that have met the categorization criteria set out in the Act to determine whether these substances present or may present a risk to the environment or to human health.
Based on the information obtained through the categorization process, the Ministers identified a number of substances as high priorities for action. These include substances that
- met all of the ecological categorization criteria, including persistence (P), bioaccumulation potential (B) and inherent toxicity to aquatic organisms (iT), and were believed to be in commerce; and/or
- met the categorization criteria for greatest potential for exposure (GPE) or presented an intermediate potential for exposure (IPE) and had been identified as posing a high hazard to human health based on classifications by other national or international agencies for carcinogenicity, genotoxicity, developmental toxicity or reproductive toxicity.
The Ministers therefore published a notice of intent in the Canada Gazette, Part I, on December 9, 2006 (Canada 2006), which challenged industry and other interested stakeholders to submit, within specified timelines, specific information that may be used to inform risk assessment and to develop and benchmark best practices for the risk management and product stewardship of those substances identified as high priorities.
The substance 1,2-benzenedicarboxylic acid, bis(2-methoxyethyl) ester (di(methoxyethyl)phthalate, DMEP), was identified as a high priority for assessment of human health risk because it was considered to present IPE and had been classified by another agency on the basis of reproductive and developmental toxicity.
The Challenge for DMEP was published in the Canada Gazette on May 31, 2008 (Canada 2008). A substance profile was released at the same time. The substance profile presented the technical information available prior to December 2005 that formed the basis for categorization of this substance. As a result of the Challenge, submissions of stakeholder interest were received.
Although DMEP was determined to be a high priority for assessment with respect to human health, it did not meet the criteria for potential for persistence, bioaccumulation or inherent toxicity to aquatic organisms. Therefore, this assessment focuses principally on information relevant to the evaluation of risks to human health.
Screening assessments focus on information critical to determining whether a substance meets the criteria for defining a chemical as toxic as set out in section 64 of CEPA1999. Screening assessments examine scientific information and develop conclusions by incorporating a weight of evidence approach and precaution.
This screening assessment includes consideration of information on chemical properties, hazards, uses and exposure, including the additional information submitted under the Challenge. Data relevant to the screening assessment of this substance were identified in original literature, review and assessment documents and stakeholder research reports and from recent literature searches, up to January 2009 for the exposure section of the document and up to October 2008 for the health effects section. Key studies were critically evaluated; modelling results may have been used to reach conclusions. Evaluation of risk to human health involves consideration of data relevant to estimation of exposure (non-occupational) of the general population, as well as information on health hazards (based principally on the weight of evidence assessments of other agencies that were used for prioritization of the substance). Decisions for human health are based on the nature of the critical effect and/or margins between conservative effect levels and estimates of exposure, taking into account confidence in the completeness of the identified databases on both exposure and effects, within a screening context. The screening assessment does not represent an exhaustive or critical review of all available data. Rather, it presents a summary of the critical information upon which the conclusion is based.
This screening assessment was prepared by staff in the Existing Substances Programs at Health Canada and Environment Canada and incorporates input from other programs within these departments. The ecological and human health portions of this assessment have undergone external written peer review/consultation. Comments on the technical portions relevant to human health were received from the National Industrial Chemicals Notification and Assessment Scheme (NICNAS, Australia) and scientific experts selected and directed by Toxicology Excellence for Risk Assessment (TERA), including Susan Griffin (US Environmental Protection Agency), Donna Vorhees (The Science Collaborative) and Lynne Haber (TERA). Additionally, the draft of this screening assessment was subject to a 60-day public comment period. Although external comments were taken into consideration, the final content and outcome of the screening risk assessment remain the responsibility of Health Canada and Environment Canada.
The critical information and considerations upon which the assessment is based are summarized below.
For the purposes of this document, this substance will be referred to as DMEP, derived from the name di(methoxyethyl)phthalate. DMEP is a clear, light-coloured, oily liquid with a mild aromatic odour (HSDB 2002; NICNAS 2008a). Its substance identity information is summarized in Table 1.
Table 1. Substance identity for DMEP
| Chemical Abstracts Service Registry Number (CAS RN) | 117-82-8 |
| DSL name | 1,2-Benzenedicarboxylic acid, bis(2-methoxyethyl) ester |
| National Chemical Inventories (NCI) names1 | 1,2-Benzenedicarboxylic acid, bis(2-methoxyethyl) ester (AICS, ASIA-PAC, ENCS, SWISS, TSCA) Bis(2-methoxyethyl) phthalate (EINECS) |
| Other names | Bis(methoxyethyl) phthalate Di(methoxyethyl)phthalate Dimethyl glycol phthalate Kesscoflex MCP 2-Methoxyethyl phthalate Methyl glycol phthalate NSC 2147 Phthalic acid, bis(2-methoxyethyl) ester |
| Chemical group (DSL stream) | Discrete organics |
| Major chemical class or use | Phthalate ester |
| Chemical formula | C14H18O6 |
| Chemical structure | 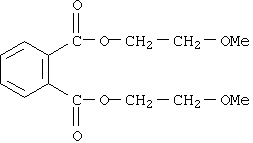 |
| SMILES | O=C(OCCOC)c(c(ccc1)C(=O)OCCOC)c1 |
| Molecular mass | 282.3 g/mol |
| 1 Abbreviations: AICS, Australian Inventory of Chemical Substances; ASIA-PAC, Asia-Pacific Substances Lists; CAS RN, Chemical Abstracts Service Registry Number; DSL, Domestic Substances List; EINECS, European Inventory of Existing Commercial Chemical Substances; ENCS, Existing and New Chemical Substances; NCI, National Chemical Inventories; SMILES, simplified molecular input line entry specification; SWISS, Swiss Giftliste 1 and Inventory of Notified New Substances; TSCA, Toxic Substances Control Act Chemical Substance Inventory. Source: NCI 2006 | |
Table 2 contains experimental and modelled physical and chemical properties of DMEP that are relevant to its environmental fate. There is uncertainty about some of the physical and chemical properties of DMEP. For example, estimated Log Kowvalues up to 2.9 and water solubility values as low as 900 mg/L have been reported.
Table 2. Physical and chemical properties of DMEP
| Property | Type | Value1 | Temperature (°C) | Reference |
|---|---|---|---|---|
| Abbreviations: Koc, organic carbon partition coefficient; Kow, octanol–water partition coefficient. 1 Values in parentheses represent the original values as reported by the authors or as estimated by the models. * Value used as input for modelling. |
||||
| Melting point (°C) | Experimental | -45 | PhysProp 2006 | |
| Boiling point (°C) | Experimental | 340 | PhysProp 2006 | |
| 312.5 | ACD 2008 | |||
| Density (kg/m3) | Experimental | 1170 | 15 | NICNAS 2008a |
| Vapour pressure (Pa) | Modelled | 0.030 (2.3 × 10-4 mmHg) * | 25 | MPBPWIN 2000 |
| 0.07 (5.28 × 10-4 torr) | 25 | ACD 2008 | ||
| Henry’s Law constant (Pa·m3/mol) | Modelled | 9.96 × 10-4 | 25 | EQC 2003 |
| 2.8 × 10-8 | 25 | HENRYWIN 2000 (group method) | ||
| 5.5 × 10-6 | 25 | HENRYWIN 2000 (bond method) | ||
| Log Kow | Experimental(estimated by HPLC analysis) | 0.04* | Eastman Kodak 1984 | |
| Modelled | 2.9 | US EPA 1985 | ||
| Log Koc | Modelled | 1 | PCKOCWIN 2000 | |
| 1.6 | HSDB 2002 | |||
| 1.8 | 25 | ACD 2008 | ||
| Water solubility (mg/L) | Experimental | 8500* | 15–25 | ChemIDplus Lite 2007 |
| 9132 (distilled water) 9024 (pH 6.9 buffer) 9293 (building diluent water) | Eastman Kodak 1984 | |||
| Not stated | 900 | US EPA 1985 cited in NICNAS 2008a | ||
The main source of phthalates is anthropogenic production (IPCS 1992). DMEP is manufactured by reacting ethylene glycol monomethyl ether with phthalic anhydride (Ashford 1994). Current DMEP producers are distributed in China, Mexico and Europe (SRI Consulting 2008).
Early studies suggested the possibility of natural occurrence of phthalates in the environment (Mathur 1974), and recent studies showed that phthalates can be biosynthesized in algae (Chen 2004; Namikoshi et al. 2006). DMEP was detected in kiwi fruit (Li et al. 2002) and in the smoke from burning coal in China (Wang et al. 1997); however, it is not clear in these cases whether DMEP comes from a natural source or industrial contamination.
According to the survey conducted under section 71 of CEPA 1999, no companies reported manufacturing or importing DMEP in a quantity greater than or equal to the 100 kg reporting threshold or using DMEP in a quantity greater than or equal to the 1000 kg reporting threshold in Canada in 2006 (Environment Canada 2008). In the case of importers, the survey applied to those who import DMEP, whether alone, in a mixture, in a product or in manufactured items. Historically, DMEP was imported into Canada in a total quantity of 110 tonnes in 1986, based on information collected during Domestic Substances List (DSL) nomination (Environment Canada 1988).
Although Harris et al. considered that the European commercial usage of DMEP was negligible (Harris et al. 1997), Denmark has reported a total use of 36.7 to 111.8 tonnes in the years 2000-2007 (SPIN 2009). The annual production and/or import of DMEP in the United States in 1986–2002, reported under the Inventory Update Rule, ranged from more than 500 000 to 1 000 000 pounds (about 230–450 metric tonnes) in 1986 and from 10 000 to 500 000 pounds (about 4.5–230 tonnes) in 1990, 1994 and 1998; there were no companies reporting in 2002 in a quantity above the reporting threshold of 10 000 pounds (about 4.5 metric tonnes) and in 2005 in a quantity above the reporting threshold of 25,000 pounds ( 11.36 metric tonnes) (US EPA 2002; 2006).
No information regarding any current uses of DMEP in the Canadian marketplace has been identified. Based on the global decline of manufacture of DMEP and the information reported under the survey conducted under section 71 of CEPA 1999 (Environment Canada 2008), use of DMEP in Canada is not expected to be significant.
Uses of DMEP in food packaging, cosmetic products or pesticide products, either as an active ingredient or as a formulant, have not been notified in Canada (emails from Food Directorate, Health Products and Food Branch, Health Canada, December 1, 2008; Healthy Environments and Consumer Safety Branch, Health Canada, December 31, 2008; Pest Management Regulatory Agency, Health Canada, December 31, 2008; all unreferenced). In 2007, as part of a separate regulatory initiative to determine the use of 6 particular phthalates, the Consumer Product Safety Bureau of Health Canada sampled and tested over 70 soft vinyl children’s products. When the results were reported, other phthalates which were not the focus of the survey were also indicated when detected. DMEP was not detected in this survey conducted by Health Canada (email from Healthy Environments and Consumer Safety Branch, Health Canada, January 9, 2009; unreferenced). The Controlled Products Regulations established under the Hazardous Products Act require DMEP to be disclosed on the Material Safety Data Sheet that must accompany workplace chemicals when it is present at a concentration of 0.1% or greater as specified on the Ingredient Disclosure List (Canada 1988). Historically, DMEP was used as a plasticizer and as a paint/coating additive in Canada during the calendar years 1984–1986, based on information collected during DSL nomination (Environment Canada 1988).
The general global applications of DMEP have included its use as a plasticizer in the production of nitrocellulose, acetyl cellulose, polyvinyl acetate, polyvinyl chloride and polyvinylidene chloride intended for contact with food or drink, giving these polymeric materials good light resistance (Sheftel 2000), and as a solvent (Lewis 1993; Hathaway and Proctor 2004). DMEP can improve the durability and toughness of cellulose acetate and can be used in enamelled wire, film, high-strength varnish and adhesive (Shanghai Yancui Import and Export Co., Ltd. 2008). It can also be used in pesticide products internationally (Ash and Ash 1998).
DMEP was not found in the US Household Products Database (HPD 2008). Historically, it was reported that DMEP was primarily used in the United States as a plasticizer in cellulose ester plastics and could be used as a solvent (US EPA 1985; Lewis 1993). DMEP is prohibited to be used in cosmetic products by the European Commission (EC 2004). DMEP was detected in imported play and exercise balls and children’s toys, such as hoppers and inflatable water products, in Australia (NICNAS 2008a), in polyethylene food packaging film after ozone sterilization treatment in Austria (Steiner 1991), in the water stored for over a year in a polyethylene floppy plastic bag in France (Rudelle et al. 1995) and in T-shirts (10–30 µg/kg), diapers (10–20 µg/kg) and house carpets (10–50 µg/kg) in Germany (Pfordt and Bruns-Weller 1999). However, the Canadian data above and the declining global use of DMEP in recent years suggest that these uses are not significant to Canada.
Information reported under section 71 of CEPA 1999 indicated that there was no manufacture or import of DMEP in a quantity greater than or equal to the 100 kg reporting threshold or use of DMEP in a quantity greater than or equal to the 1000 kg reporting threshold in Canada in 2006; therefore, industrial releases are not expected to be significant (Environment Canada 2008). DMEP is not a target substance under the National Pollutant Release Inventory in Canada (NPRI 2007), the US Toxics Release Inventory (TRI 2006), the Australian National Pollutant Inventory (NPI 2007) or the Japanese Chemical Survey (JCS 2004).
The historical uses of DMEP as a plasticizer and as a solvent suggest that DMEP may be released to the environment through various waste streams (HSDB 2002).
DMEP has a high water solubility (8500 mg/L), moderate vapour pressure (0.03 Pa) and very low log Kow (0.04) and Henry’s Law constant (9.96 × 10-4Pa·m3/mol). Thus, partitioning to the soil and water compartments is potentially significant, depending on the compartment of release and the rates of partitioning relative to other fate processes, such as advection and degradation. Partitioning to air and to sediments is not expected to be significant due to the very low Henry’s Law constant and log Koc for the substance.
Based on its physical and chemical properties (Table 2), the results of Level III fugacity modelling (Table 3) suggest that DMEP would reside predominantly in water or soil, depending on the compartment of release. It should be noted that there is uncertainty about the water solubility and the octanol-water partition coefficient for DMEP. If the log Kow value is as high as 2.9, as has been reported elsewhere, partitioning to soil and sediment would be much higher and partitioning to water much lower than the values shown in Table 3.
Table 3. Results of the Level III fugacity modelling (EQC 2003) for DMEP
| Substance released to: | Fraction of substance partitioning to each medium (%) | |||
|---|---|---|---|---|
| Air | Water | Soil | Sediment | |
| Air (100%) | 0.9 | 27.3 | 71.8 | 0.0 |
| Water (100%) | 0.0 | 99.8 | 0.0 | 0.2 |
| Soil (100%) | 0.0 | 21.6 | 78.4 | 0.0 |
Environmental Persistence
Only limited empirical data regarding the persistence of DMEP were identified. A 14-day biodegradation test utilizing acclimated sludge microorganisms as the inoculum showed 60.8% degradation of the test article as measured by carbon dioxide evolution (Eastman Kodak 1984, 1985).
Additionally, the degradation of DMEP in various environmental media was predicted by available quantitative structure–activity relationship (QSAR) models. The results are summarized in Table 4.
Table 4. Modelled data for degradation of DMEP
| Fate process | Model and model basis | Model output | Expected half-life (days) |
|---|---|---|---|
| Abbreviations: MITI, Ministry of International Trade and Industry, Japan; n/a, not applicable; t½, half-life. 1 Model does not provide an estimate for this type of structure. 2 Output is a numerical score. 3 Output is a probability score. |
|||
| Air | |||
| Atmospheric oxidation | AOPWIN 2000 | t½= 6.6 h | <2 |
| Ozone reaction | AOPWIN 2000 | n/a1 | n/a |
| Water | |||
| Hydrolysis | HYDROWIN 2000 | t½ = 1.335 years (pH 7) t½ = 48.77 days (pH 8) | n/a |
| Biodegradation (aerobic) | BIOWIN 2000 Submodel 3: Expert Survey (ultimate biodegradation) | 2.82 “biodegrades fast” | <1824 |
| Biodegradation (aerobic) | BIOWIN 2000 Submodel 4: Expert Survey (primary biodegradation) | 3.92 “biodegrades fast” | <1824 |
| Biodegradation (aerobic) | BIOWIN 2000 Submodel 5: MITI linear probability | 0.83 “biodegrades fast” | <1824 |
| Biodegradation (aerobic) | BIOWIN 2000 Submodel 6: MITI non-linear probability | 0.83 “biodegrades fast” | <1824 |
In air, a predicted atmospheric oxidation half-life value of 6.6 hours (Table 4) demonstrates that DMEP is likely to be rapidly oxidized. The substance is not expected to react with other photooxidative species in the atmosphere, such as ozone. It is likely to degrade via direct photolysis. Therefore, it is expected that reactions with hydroxyl radicals will be the most important fate process in the atmosphere for DMEP. With a half-life of 6.6 hours via reactions with hydroxyl radicals, DMEP is considered to be not persistent in air.
The results from the BIOWIN models suggest a fast biodegradation rate for this substance (Table 4). The numerical score from submodel 3 indicates an ultimate biodegradation timeframe of weeks, and submodel 4 indicates a primary biodegradation timeframe of days. The probability results from submodels 5 and 6 are greater than 0.3, the cut-off suggested by Aronson et al. (2006) to identify substances as having a half-life of <60 days (based on the MITI probability models).
Using an extrapolation ratio of 1:1:4 for a water:soil:sediment biodegradation half-life (Boethling et al. 1995), the half-life of DMEP in soil is less than 182 days, and the half-life in sediments is less than 365 days. This indicates that DMEP is not expected to be persistent in soil or sediment.
Based on the empirical and modelled data, DMEP does not meet the persistence criteria in air (half-life in air of =2 days), soil or water (half-lives in soil and water =182 days) or sediment (half-life in sediment =365 days) as set out in the Persistence and Bioaccumulation Regulations (Canada 2000).
Potential for Bioaccumulation
The experimental log Kow value of 0.04 suggests that DMEP does not have the potential to bioaccumulate in the environment (Table 2).
No experimental bioaccumulation factor (BAF) or bioconcentration factor (BCF) data for DMEP were available. QSAR-modelled BAF and BCF values for DMEP are summarized in Table 5. According to the Persistence and Bioaccumulation Regulations (Canada 2000), the bioaccumulation potential of a substance is assessed principally on the basis of its BAF value. This is because the BCF does not adequately account for the bioaccumulation potential of a substance via the diet, especially for substances with a log Kow greater than about 4.0 (Arnot and Gobas 2003).
Table 5. Fish BAF and BCF predictions for DMEP
| Test organism | Endpoint | Log Kow used in model | Value (L/kg wet weight) | Reference |
|---|---|---|---|---|
| Fish | BAF | 0.04 | 0.9555 | Arnot and Gobas 2003 (Gobas BAF Middle Trophic Level) |
| Fish | BCF | 0.04 | 0.9555 | |
| 19.98 | OASIS Forecast 2005 | |||
| 1.11 | 34.12 | BCFWIN 2000 | ||
| 0.81 | 2.41 | ACD 2008 |
The modified Gobas BAF middle trophic level model for fish predicts a BAF of 0.9555 L/kg, indicating that DMEP does not have the potential to bioconcentrate and biomagnify in the environment. This estimate includes a biotransformation rate estimate (kM) of 125/day. The results of BCF model calculations provide additional evidence supporting the low bioconcentration potential of this substance. There is uncertainty about the log Kow value for DMEP, upon which BCF and BAF estimates are based, but all estimates are much less than 5000. Based on the available empirical log Kow and kinetic-based modelled bioaccumulation values, DMEP does not meet the bioaccumulation criteria (BAF, BCF = 5000) as set out in the Persistence and Bioaccumulation Regulations (Canada 2000).
DMEP was dissolved in 20-L containers of conditioned water to yield nominal concentrations of 11.7 or 117 mg/L to test acute effects in seven aquatic species (Table 6a). The high dose caused 80% and 20% mortality in Daphnia magna and Asellus intermedius, respectively. No adverse effects were observed in the other five species.
Table 6a. Empirical data for aquatic toxicity
| Test organism | Type of test | Endpoint | Value1 | Reference |
|---|---|---|---|---|
| Abbreviations: LC50, the concentration of a substance that is estimated to be lethal to 50% of the test organisms. 1 Results in parentheses are those reported in the reference. |
||||
| Fathead minnow (Pimephales promelas) | Acute | LC50 | >117 mg/L (>100 µL/L) | Eastman Kodak 1984, 1985 |
| Water flea (Daphnia magna) | 56 mg/L (48 µL/L) | |||
| Sideswimmer (Gammarus fasciatus) | >117 mg/L (>100 µL/L) | |||
| Flatworm (Dugesia tigrina) | >117 mg/L (>100 µL/L) | |||
| Snail (Helisoma trivolvis) | >117 mg/L (>100 µL/L) | |||
| Segmented worm (Lumbriculus variegatus) | >117 mg/L (>100 µL/L) | |||
| Pillbug (Asellus intermedius) | >117 mg/L (>100 µL/L) | |||
Additionally, the aquatic toxicity of DMEP was predicted from the various QSAR models (Table 6b). It should be noted that model estimates for aquatic toxicity depend on input values for log Kow and water solubility, and that there is uncertainty about these values for DMEP. However, the estimated acute toxicity values are above 1 mg/L, indicating that the substance is not highly toxic to aquatic organisms.
Table 6b. Modelled data for aquatic toxicity
| Test organism | Type of test | Endpoint | Value (mg/L) | Reference |
|---|---|---|---|---|
| Abbreviations: EC50, the concentration of a substance that is estimated to cause some toxic sublethal effect on 50% of the test organisms; LC50, the concentration of a substance that is estimated to be lethal to 50% of the test organisms. | ||||
| Fish | Acute (96 h) | LC50 | 166 | TOPKAT 2004 |
| 123 | ECOSAR 2004 | |||
| 452 | OASIS Forecast 2005 | |||
| 33 | ASTER 1999 | |||
| 4.3 | AIES 2003–2005 | |||
| Fish | Chronic (32/33 d) | Chronic value | 14 | ECOSAR 2004 |
| Daphnia | Acute (96 h) | EC50 | 27 | TOPKAT 2004 |
| Acute (48 h) | LC50 | 284 | ECOSAR 2004 | |
| Daphnia | Chronic (21 d) | Chronic value | 225 | ECOSAR 2004 |
| Green algae | Acute (96 h) | EC50 | 133 | ECOSAR 2004 |
| Green algae | Chronic | Chronic value | 25 | ECOSAR 2004 |
The empirical data shown in Table 6a indicate that DMEP is of low toxicity to aquatic organisms, with an acute toxicity >1 mg/L. The modelled data shown in Table 6b agree with the empirical data.
The potential to affect germination and the early growth of plants was determined by testing 10 and 100 µL/L (11.7 and 117 mg/L) solutions of DMEP. No adverse effects were observed in ryegrass (Lolium perenne) or lettuce (Lactuca sativa) (Eastman Kodak 1985). These results indicate that DMEP is not highly hazardous to terrestrial plants.
Some information pertaining to DMEP in the Canadian environment is presented in the section “Potential to Cause Harm to Human Health” below. DMEP was not detected in Canadian municipal sewage sludge collected from various Canadian cities from 1980 to 1985 (detection limit not reported) (Webber and Lesage 1989). DMEP was not detected (detection limit not reported) in marine surficial sediment samples or in striped seaperch (Embiotoca lateralis) fish samples from Vancouver’s Inner Harbour (Lin et al. 2003). Based on the current low usage of DMEP in Canada as indicated by section 71 survey results and its predicted low persistence and bioaccumulation potential in the environment, the ecological exposure to the substance is expected to be very low.
Based on the available information, DMEP does not persist in the environment and is not bioaccumulative, based on criteria defined in the Persistence and Bioaccumulation Regulations (Canada 2000). As the substance is not highly hazardous to aquatic organisms and terrestrial plants and exposure potential is very low, DMEP is unlikely to cause ecological harm in Canada.
Uncertainties in Evaluation of Ecological Risk
Only limited experimental data for the biodegradation of DMEP were identified. Gaps in available experimental data as well as in available values of some key physical and chemical properties were largely filled with QSAR model predictions. Although there are uncertainties associated with the use of QSAR models to estimate chemical and biological characteristics, in light of the similarity in results achieved from both experiments and QSARmodelling pertaining to the ecological toxicity of DMEP, as well as the current low usage of this substance in Canada, there is confidence that DMEP is unlikely to cause ecological harm.
There is uncertainty about the water solubility and the octanol-water partition coefficient (log Kow) for DMEP. In this assessment we have used a water solubility of 8500 mg/L and a log Kow of 0.04 for modelling purposes. Elsewhere (NICNAS 2008a), a water solubility of 900 mg/L and a log Kow up to 2.9 have been reported. Even though there is uncertainty about the octanol-water partition coefficient and the water solubility of DMEP, this does not affect the conclusion that the substance is not bioaccumulative according to the Persistence and Bioaccumulation Regulations (Canada 2000) and is not highly toxic to aquatic organisms.
Exposure Assessment
Limited data are available in regards to the concentrations of DMEP in environmental media or foods in Canada or in other countries. DMEP was detected in a natural water source (location and nature of the water source were not reported) at concentrations ranging from below the detection limit (detection limit not reported) to 0.7 µg/L (Kang and Lee 1988). In addition, DMEP was not detected in 1980 at the Inner Harbor Navigation Channel of Lake Pontchartrain, New Orleans, Louisiana (detection limit not reported) (McFall et al. 1985) or in surface water or rainwater samples in the North Rhine-Westfalia region of Germany in 1991–1992 (detection limit not reported) (Furtmann 1995).
DMEP was detected in indoor dust collected from vacuum cleaner bags (samples were sieved, and only those particles smaller than 63 µm were analysed) in a survey conducted by the Hamburg Environmental Protection Authority from 1998 to 2000 in 65 apartments in Hamburg, Germany. DMEP was detected in 49 samples to a maximum concentration of 17 mg/kg (50th percentile = 2 mg/kg; 95th percentile = 8 mg/kg). The authors speculated that the phthalates detected in indoor dust originated from the use of consumer products (Kersten and Reich 2003). Although DMEP was not detected in house dust or upholstery fabrics in Lower Saxony, Germany, in 1999, it was detected in house carpets up to concentrations of 50 µg/kg (Pfordt and Bruns-Weller 1999).
DMEP was not detected in Canadian municipal sludge (including stabilized, waste activated and raw sludge) collected from Vancouver, Edmonton, Calgary, Winnipeg, Toronto, Burlington, Halifax, Hamilton or Kitchener during 1980–1985 (detection limit not reported) (Webber and Lesage 1989). In the United Kingdom, DMEP was detected in raw sewage at concentrations of 2.82 ± 4.28 µg/L in 2001–2002. After primary treatment, the majority of DMEP was detected in the sludge at concentrations of 21.9 ± 7.54 µg/g dry weight. The concentration of DMEP in the primary effluent water was 1.61 ± 1.94 µg/L and then decreased to below the detection limit in the effluent water after further treatments (detection limit for DMEP not reported; for other phthalates in the same study, the detection limit ranged from 28.4 to 48 ng/L) (Oliver et al. 2005).
DMEP was not detected (detection limit not reported) in marine surficial sediment samples at four locations (total of 16 samples) at False Creek, a residential/industrial area of Vancouver’s Inner Harbour (Lin et al. 2003). Additionally, DMEP was not detected in sediments in the rivers Rhine, Weser, Aller and Diemel, in the North Rhine-Westfalia industrial harbours and in the West German Channels in Germany (Furtmann 1995). However, DMEP was detected in sediments from a river estuary and urban lakes in China, to a maximum concentration of 155 ng/g dry weight (Zeng et al. 2005; Liu et al. 2007).
DMEP was not detected (detection limit not reported) in striped seaperch (Embiotoca lateralis) fish samples collected from three locations (total of nine samples) at False Creek of Vancouver’s Inner Harbour (Lin et al. 2003). DMEP was detected in kiwi fruit at an unknown concentration in China (Li et al. 2002) and in plastic foil–packed nutmeg at a concentration of 10 µg/kg in Germany (Pfordt and Bruns-Weller 1999); it was not detected in plastic foil–packed almonds or hazelnuts, raw or consumer milk, creams, breast milk or glass-bottled baby foods in Germany (detection limit 10 µg/kg) (Pfordt and Bruns-Weller 1999). Additionally, DMEP was not detected in the innards of livestock (animal origin unknown), including livers of pigs, ducks, cattle and chickens and hearts of pigs and ducks, in China. The detection limit was 3.30 µg/kg (Lin et al. 2008).
Because of the lack of sufficient information on DMEP concentrations in Canadian environmental media, available environmental concentrations from other countries were reviewed with respect to the quality and relevance of the data. The concentrations of DMEP measured in indoor dust in Germany were considered relevant and appropriate for use as a surrogate for soil/dust in order to estimate the upper-bounding intake from environmental media for the general population of Canada. This is based on the rationale that the data are relatively recent and are from a country with a marketplace and historic use of consumer products that are likely similar to those in Canada. The other studies in which DMEP was detected either did not provide enough information to be used reliably or were from locations where the manufacturing and use patterns of DMEP likely differ from those in Canada. Although intake calculations from soil/dust also include exposure to outdoor soil, using DMEP levels in indoor dust as a surrogate for outdoor soil is considered conservative due to limited, if any, commercial activity in regards to DMEP in Canada in recent years and therefore likely limited release of DMEP to environmental media. Also, as DMEP is not persistent, any levels from historical uses would have largely been degraded over time.
Based on the levels of DMEP measured in indoor dust in Germany, used as a surrogate for exposure from soil/dust, the estimated total daily intake from ingestion of dust/soil for different age groups of the general population in Canada ranges from 0.01 µg/kg of body weight (kg-bw) per day for the population aged 12 and older to 0.11 µg/kg-bw per day for toddlers (aged 6 months to 4 years) (Appendix 1). Dermal exposure to DMEP from indoor dust was also estimated and ranged from 0.14 to 0.19 µg/kg-bw per day based on a 100% dermal absorption rate due to the lack of chemical-specific data (Appendix 2). Therefore, these values represent very conservative dermal exposure estimates. Although indoor dust can also be inhaled, the available information does not permit a quantitative estimate of inhalation exposure from indoor dust.
Use of consumer products containing DMEP could also be a source of exposure to DMEP. However, no submission reported under section 71 of CEPA 1999 indicated that DMEP would be present in consumer products in Canada (Environment Canada 2008).
It is possible that imported products containing DMEP may exist in the Canadian marketplace; however, available information does not permit quantification of the exposure. In addition, current information suggests that for the general population of Canada, exposure to DMEP in consumer products would not be significant.
Confidence in the exposure database is considered moderate to low, as little information is available with respect to the concentrations of DMEP in Canadian environmental media or the current use patterns of DMEP in the Canadian marketplace. However, as no company in Canada reported manufacturing, importing or using DMEP in a quantity greater than or equal to the reporting thresholds in 2006 (Environment Canada 2008), there is confidence that exposure to DMEP for the general public in Canada, both from environmental media and through possible use of consumer products containing DMEP, is not significant. Additionally, there is uncertainty as to what amount of indoor dust can be inhaled, and the total daily intake of DMEP might be higher than estimated above.
Health Effects Assessment
The available health effects information for DMEP is summarized in Appendix 3.
The European Commission has classified DMEP as a Category 2 substance with risk phrase R61 (“May cause harm to the unborn child”) and as a Category 3 substance with risk phrase R62 (“Possible risk of impaired fertility”) (ESIS 2008). This classification was based mainly on a limited dataset on DMEP, supported by the fact that DMEP is metabolized quickly to a well-characterized reproductive and developmental toxicant, 2-methoxyethanol (2-ME) (ECB 1994, 1995). A health risk assessment on 2-ME, a priority substance under CEPA 1999, was completed by the Government of Canada earlier (Canada 2002), and 2-ME has been added to the List of Toxic Substances for risk management (Environment Canada 2006).
DMEP-induced testicular effects were observed in rats following acute or 2-week gavage administration (Cassidy et al. 1983; Eastman Kodak 1985). Significant reductions in absolute and relative testis weights with seminiferous tubule atrophy and sperm degeneration and the appearance of giant spermatids were observed at 1000 mg/kg-bw per day in the 2-week study; a no-observed-adverse-effect level (NOAEL) of 100 mg/kg-bw per day for testicular effects was identified (Eastman Kodak 1985). Significantly reduced testis weights and increased abnormal sperm levels were also observed in the gavage study at a higher dose level (1500 mg/kg-bw) following single dosing (Cassidy et al. 1983). In addition, DMEP-elicited reproductive effects were studied by other routes of exposure. Significantly reduced relative testis weights were observed in mice administered DMEP by intraperitoneal (i.p.) injection for 6 weeks at a dose level of 250 mg/kg-bw per day, the only dose tested and the lowest-observed-adverse-effect level (LOAEL) (Calley et al. 1966). A significantly reduced incidence of pregnancy and reduced implantations were observed in a dominant lethal assay in mice at a dose level of 2380 mL/kg-bw (2785 mg/kg-bw, the highest dose tested), in which male mice were administered DMEP by a single i.p. injection prior to mating with females (Dillingham and Autian 1973; Singh et al. 1974). However, DMEP was not found to have estrogenic activity in vitro using a recombinant yeast screen assay (Harris et al. 1997).
Developmental effects of DMEP were observed in rats following oral (gavage) administration on gestation days 6 to 16 (Krasavage 1991). Significantly reduced pup body weight gain and slightly reduced pup survival from day 1 to 5 postpartum were observed at the lowest dose tested (60 mg/kg-bw per day, LOEL). At a higher dose level (180 mg/kg-bw per day), significantly reduced pup survival and pup body weight gain as well as pup abnormalities, including a shortened lumbosacral region, acauda and filamentous tails, were observed. At the highest dose (600 mg/kg-bw per day), complete resorption of the litters were observed in the presence of maternal toxicity (significantly reduced body weight gain and mean body weight, as well as decreased food consumption). A NOEL for maternal toxicity was identified as180 mg/kg-bw per day. In addition, single or multiple i.p. injections of DMEP during the fetal organogenesis period also induced developmental effects in rats. Significantly increased fetal resorption and fetal death, decreased fetal body weight and an increased incidence of gross and skeletal malformations and retardation as well as fetal abnormalities in brain and heart, and atrophy of the testes and kidneys were observed at 1.03 mmol/kg-bw (291 mg/kg-bw, lowest dose tested) and above; maternal effects were not examined in these studies (Singh et al. 1972, 1974; Parkhie et al. 1982; Campbell et al. 1984; Ritter et al. 1985). A no-effect level for the developmental toxicity of DMEP could not be established.
Additionally, developmental neuronal toxicity elicited by DMEP was observed in cultured chick embryos (Bower et al. 1970).
The mutagenic potential of DMEP was evidenced in the above-mentioned dominant lethal test in mice. Male ICR mice given a single i.p. injection of DMEP were mated with untreated female mice. Significantly reduced mean live fetuses per pregnancy and mean implants per pregnancy and increased early fetal death were observed in the highest dose group (2.38 mL/kg-bw, equivalent to 2785 mg/kg-bw) (Dillingham and Autian 1973; Singh et al. 1974). In addition, limited in vitro studies revealed that DMEP induced mutation in Salmonella typhimurium TA98 in the absence of metabolic activation, but negative results were obtained with S. typhimurium TA100 with or without metabolic activation and with TA98 with metabolic activation (NTP 1993).
In addition, haematological effects as well as thymic effects, exhibiting a limited dose–response relationship, were observed in a short-term oral study at 100 mg/kg-bw per day and above in male rats. The purity of the test material in this study was 78%, which may have introduced some confounding factors (Eastman Kodak 1985).
A five-generation oral study with very limited data reported did not reveal any chronic effects induced by DMEP in rats given up to 900 mg/kg diet per day (45 mg/kg-bw per day) [1], and no signs of reproductive toxicity or carcinogenicity were observed in this study (Lefaux 1968). As well, human-relevant carcinogenicity has not been recognized for 2-ME or other glycol ethers (ECETOC 2005). Although some phthalates induced various tumours in experimental animals (NICNAS 2008b), the relevance of these data to DMEP carcinogenicity and to humans is uncertain.
Few adequate studies were identified in which DMEP was administered to laboratory animals by routes that are relevant to human exposure (i.e., oral, dermal or inhalation). However, it should be noted that one of the metabolites of DMEP, 2-ME, has been intensively investigated and assessed by the Government of Canada (Canada 2002). It has been noted that DMEP rapidly underwent hydrolysis to mono-2-methoxyethyl phthalate (MMEP) and 2-ME in rats (Parkhie et al. 1982; Campbell et al. 1984; Ritter et al. 1985). 2-ME is further oxidized to methoxyacetic acid (MAA), which is the proximate teratogen (Canada 2002). DMEP as well as its metabolites can be readily transported across the placenta (Parkhie et al. 1982), and their clearance from the placenta is also rapid. However, the rat fetus appears to have little or no ability to hydrolyse DMEP to the monoester in in vivo and in vitro assays (Campbell et al. 1984; Yonemoto et al. 1984). Investigators have observed that DMEP, 2-ME and MAA showed equally potent teratogenicity at 2.07 and 4.17 mmol/kg-bw dose levels (Ritter et al. 1985) and that DMEP and 2-ME showed similar teratogenicity at the 2.49 mmol/kg-bw dose level (Campbell et al. 1984); however, intraperitoneally injected MMEP at 2.49 mmol/kg-bw did not induce significant teratogenicity in Wistar rats, and the authors speculated that the pharmacokinetics of injected MMEP might be different from those of MMEP metabolized from DMEP in vivo (Campbell et al. 1984).
The Government of Canada assessment of 2-ME considered its critical health effects to be reproductive and developmental toxicity, as well as effects on the haematological, immune and nervous systems (Canada 2002). A brief synopsis of some of the key data for 2-ME follows. 2-ME was consistently toxic to the male reproductive system in multiple species of experimental animals exposed by the oral, dermal or inhalation routes of administration (Canada 2002). Effects on the female reproductive system, such as changes in oestrous cycle and hormone levels and atrophy of reproductive organs, have also been associated with oral exposure to 2-ME (Canada 2002). The lowest oral and dermal LO(A)ELs for the reproductive toxicity of 2-ME were, respectively, 25 mg/kg-bw per day in rabbits, with a NO(A)EL of 12.5 mg/kg-bw per day (Foote et al. 1995; Berndtson and Foote 1997); and 625 mg/kg-bw per day in rats (Feuston et al. 1989). The lowest inhalation lowest-observed-(adverse-)effect concentration (LO(A)EC) was 30 parts per million (ppm) (93 mg/m3) in rabbits (Miller et al. 1983).
2-ME also consistently induced developmental toxicity, including fetal malformations, mainly in the cardiovascular system, kidney and skeletal systems, in oral, dermal and inhalation studies in several species of experimental animals (Canada 2002). The lowest oral effect level for the developmental toxicity of 2-ME was 12 mg/kg-bw per day (the lowest dose tested) in a gavage study in monkeys, evidenced by increased fetal death and resorption (Scott et al. 1989). The lowest inhalation LO(A)EC of 2-ME was identified to be 10 ppm (31 mg/m3) in rabbits, with a no-observed-(adverse-)effect concentration (NO(A)EC) of 3 ppm (9 mg/m3) (Hanley et al. 1984a, b). Developmental effects were observed following dermal application of 2-ME at approximately 48 mg/kg-bw per day or more in rats (Hellwig 1993).
2-ME-induced neurological effects were observed in rats and mice following acute or short-term inhalation exposure at concentrations of 25 ppm (78 mg/m3) or greater (Goldberg et al. 1962; Nelson et al. 1984). 2-ME-induced immunosuppression was observed in rats exposed orally at 50 mg/kg-bw per day and above (Smialowicz et al. 1992, 1993) or dermally at 300 mg/kg-bw per day and above (Williams et al. 1995). Often thymus weight decreases were observed in those studies at lower dose levels. Mice appear to be less sensitive than rats (Canada 2002). 2-ME-induced significant alterations of haematological parameters were consistently observed in experimental animals acutely or repeatedly exposed orally at 70 mg/kg-bw per day and above (NTP 1993), dermally at 1000 mg/kg-bw per day (Hobson et al. 1986) or via inhalation at 3 ppm (9.33 mg/m3) and above (Hanley et al. 1984a, b).
In addition, effects on neuronal, haematological, immunological and male reproductive systems and increased risk of spontaneous abortion were observed in human workers exposed to 2-ME, along with other chemicals or glycol ethers. However, these data were not conclusive owing to confounding exposure factors (Canada 2002).
The confidence in the toxicity dataset for DMEP is considered moderate, as some experimental data are available for the developmental and reproductive toxicity, short-term and acute toxicity, and genetic toxicity of DMEP, but not all of them were conducted by relevant exposure routes (i.e., oral, dermal and inhalation). Additionally, there is uncertainty regarding the chronic effects or carcinogenicity of DMEP, as only limited information was identified, and potential genotoxicity of DMEP was suggested in the data available. However, as DMEP shares a similar mode of action with 2-ME, a downstream metabolite of DMEP, by generating a common metabolite, MAA, there is confidence that DMEP would have a toxicity profile similar to that of 2-ME, although there may be quantitative differences in potency.
Characterization of Risk to Human Health
Based principally on the weight of evidence classification of DMEP by the European Commission as a Category 2 substance for its developmental toxicity and as a Category 3 substance for its reproductive toxicity (ESIS 2008) as well as the consideration of available relevant data, the critical effects for characterization of risk to human health for DMEP are developmental and reproductive toxicity. Therefore, margins of exposure are derived between lowest exposure levels associated with induction of these effects and conservative estimates of population exposure to DMEP.
The principal source of exposure to DMEP for the general population is expected to be indoor dust. A comparison between the lowest effect level for developmental toxicity (60 mg/kg-bw per day, gavage) (Krasavage 1991) and the maximum intake estimate (0.11 µg/kg-bw per day) based on the concentration of indoor dust (Kersten and Reich 2003) results in large margins of exposure of approximately 5 orders of magnitude.
Although minor haematological and thymic effects were observed at 100 mg/kg-bw per day in male rats orally administered DMEP in a short-term study (Eastman Kodak 1985), given the lack of a clear dose–response relationship for these effects and the low purity of test materials (78%) in this study, it was considered that this study provided only limited information regarding DMEP toxicity. However, if this study is used in a conservative estimate of margins of exposure, these margins would be in the range of 6 orders of magnitude.
As to potential dermal exposure from indoor dust, the margins between very conservative dermal exposure estimates (ranging from 0.14 to 0.19 µg/kg-bw per day) plus the total daily intake estimates (ranging from 0.01 to 0.11 µg/kg-bw per day) and the exposure levels associated with critical health effects (i.e., developmental and reproductive toxicity) or the slight haematological and thymic effects are large.
In light of only limited toxicity data available for DMEP, the exposure levels associated with critical health effects for the metabolites of DMEP, 2-ME or MAA, for which intensive toxicity investigation has been conducted (see Canada 2002 and summary for 2-ME above for details), were compared with the exposure estimates for DMEP, and the margins are still large (e.g., 5 orders of magnitude using the lowest oral LOAEL for 2-ME of 12 mg/kg-bw per day in experimental animals). These margins of exposure are considered adequate to account for uncertainties and data gaps in the database in light of the conservative nature of the estimates of daily intake and conservative selection of critical effect levels in the experimental studies.
Available data do not indicate the existence of consumer products containing DMEP in the Canadian marketplace. Therefore, exposure to DMEP via consumer products is not expected to be significant.
Uncertainties in Evaluation of Risk to Human Health
There is uncertainty regarding the actual concentrations of DMEP in Canadian environmental media owing to a lack of, or limited, recent Canadian-specific data; however, based on the information available, exposures are expected to be very low. In addition, there is uncertainty with respect to the estimates of total daily intake for the general population of Canada, based on the DMEP concentration in indoor dust measured in Germany, as inhalation of the small particulates in the dust would increase the total daily exposure to DMEP from this type of source. However, in light of the large margins of exposure, it is not anticipated that resolution of these uncertainties would have a significant impact on the conclusions. Furthermore, there is uncertainty regarding the potential presence of DMEP in some imported goods, although a survey for other phthalates conducted by the Consumer Product Safety Bureau of Health Canada in 2007 did not indicate its presence in the products tested.
There is uncertainty regarding the exposure levels associated with critical health effects, as the toxicity dataset is limited, some studies for reproductive and developmental toxicity were conducted through unconventional routes of exposure and often only one dose level was tested. However, given the large margins between the exposure levels associated with critical health effects for the metabolites of DMEP, 2-ME or MAA, for which intensive toxicity investigation has been conducted, and the exposure estimates for DMEP, there is confidence that current margins of exposure for DMEP are adequately protective for the general population of Canada. Additionally, there is some uncertainty regarding differences in sensitivity to exposure to DMEP between experimental animals and humans in view of the paucity of epidemiological data and the lack of sufficient data in animal and human systems on differences (or similarities) in toxicokinetics, toxicodynamics and mode of action. As well, there is uncertainty with respect to the potential carcinogenicity of DMEP due to lack of appropriate long-term studies (although one limited study was negative). In addition, the available information from genotoxicity tests suggests that DMEP has the potential to directly interact with genetic material.
Based on the information presented in this screening assessment, it is concluded that DMEP is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
Based upon consideration of the margins of exposure between conservative estimates of exposure to DMEP from environmental media and exposure levels associated with critical effects of DMEP (i.e., reproductive and developmental toxicity in experimental animals), it is concluded that DMEP not be considered “toxic” as defined in paragraph 64(c) of CEPA 1999: that is, DMEP is not a substance entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is concluded that DMEP does not meet the criteria in section 64 of CEPA 1999. Additionally, DMEP does not meet the criteria for persistence or bioaccumulation potential as set out in the Persistence and Bioaccumulation Regulations (Canada 2000).
[ACD] Advanced Chemistry Development. 2008. Calculated values using Advanced Chemistry Development (ACD/Labs) Software V9.04 for Solaris (© 1994–2008), presented in SciFinder database [cited 2008 Oct 16].
[AIES] Artificial Intelligence Expert System. 2003–2005. Version 1.25. Ottawa (ON): Environment Canada. Model developed by Stephen Niculescu. Available from: Environment Canada, Existing Substances Division, New Substances Division.
[AOPWIN] Atmospheric Oxidation Program for Windows [Estimation Model]. 2000. Version 1.91. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: http://www.epa.gov/oppt/exposure/pubs/episuite.htm
Arnot JA, Gobas FAPC. 2003. A generic QSAR for assessing the bioaccumulation potential of organic chemicals in aquatic food webs. QSAR Comb Sci [Internet]; 22(3): 337–345. Available from: http://www3.interscience.wiley.com/journal/104557877/home[restricted access]
Aronson D, Boethling B, Howard P, Stiteler W. 2006. Estimating biodegradation half-lives for use in chemical screening. Chemosphere 63: 1953–1960.
Ash M, Ash I. 1998. Handbook of industrial chemical additives, vol. II. 2nd ed. Endicott (NY): Synapse Information Resources Inc. p. 1498.
Ashford RD. 1994. Ashford’s dictionary of industrial chemicals. London (GB): Wavelength Publications Ltd. p. 321. [cited in HSDB 2002].
[ASTER] Assessment Tools for the Evaluation of Risk [Internet]. 1999. Duluth (MN): US Environmental Protection Agency, Mid-Continent Ecology Division. Available from:
http:www.epa.gov/med/Prods_Pubs/aster.htm [restricted access]
[BCFWIN] BioConcentration Factor Program for Windows [Estimation Model]. 2000. Version 2.15. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: http://www.epa.gov/oppt/exposure/pubs/episuite.htm
Berndtson WE, Foote RH. 1997. Disruption of spermatogenesis in rabbits consuming ethylene glycol monomethyl ether. Reprod Toxicol 11(1): 29–36.
[BIOWIN] Biodegradation Probability Program for Windows [Estimation Model]. 2000. Version 4.02. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: http://www.epa.gov/oppt/exposure/pubs/episuite.htm
Boethling RS, Howard PH, Beauman JA, Larosche ME. 1995. Factors for intermedia extrapolations in biodegradability assessment. Chemosphere 30(4): 741–752.
Bower RK, Haberman S, Minton PD. 1970. Teratogenic effects in the chick embryo caused by esters of phthalic acid. J Pharmacol Exp Ther 171: 314–324.
Calley D, Autian J, Guess WL. 1966. Toxicology of a series of phthalate esters. J Pharm Sci 55: 158–162.
Campbell J, Holt D, Webb M. 1984. Dimethoxyethylphthalate metabolism: Teratogenicity of the diester and its metabolites in the pregnant rat. J Appl Toxicol 4: 35–41.
Canada. 1988. Ingredient Disclosure List [Internet]. S.O.R./88-64. [cited 2009 Jan]. Available from: http://www.canlii.org/ca/regu/sor88-64/part274942.html
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C., 1999, c. 33. Available from: http://www.gazette.gc.ca/archives/p3/1999/g3-02203.pdf
Canada. 2000. Canadian Environmental Protection Act: Persistence and Bioaccumulation Regulations, P.C. 2000-348, 23 March 2000, SOR/2000-107. Available from: http://gazette.gc.ca/archives/p2/2000/2000-03-29/pdf/g2-13407.pdf
Canada. 2002. 2-Methoxyethanol [Internet]. Ottawa (ON): Environment Canada; Health Canada. (Priority substances list assessment report). [cited 2009 Jan]. Available from: http://www.hc-sc.gc.ca/ewh-semt/pubs/contaminants/psl2-lsp2/2_methoxyethanol/index_e.html
Canada, Dept. of the Environment, Dept. of Health. 2006. Canadian Environment Protection Act, 1999: Notice of intent to develop and implement measures to assess and manage the risks posed by certain substances to the health of Canadians and their environment. Canada Gazette. Part 1, vol. 140, no. 49, p. 4109–4116. Available from: http://www.gazette.gc.ca/archives/p1/2006/2006-12-09/pdf/g1-14049.pdf
Canada, Dept. of the Environment, Dept. of Health. 2008. Canadian Environmental Protection Act, 1999: Notice with respect to Batch 6 Challenge substances. Canada Gazette, Part I, vol. 142, no. 22, p. 1635–1740. Available from: http://www.gazette.gc.ca/rp-pr/p1/2008/2008-05-31/pdf/g1-14222.pdf
Cassidy SL, Dix KM, Jenkins T. 1983. Evaluation of a testicular sperm head counting technique using rats exposed to dimethoxyethyl phthalate (DMEP), glycerol α-monochlorohydrin (GMCH), epichlorohydrin (ECH), formaldehyde (FA), or methyl methanesulphonate (MMS). Arch Toxicol 53: 71–78.
ChemIDplus Lite [database on the Internet]. 2007. Bethesda (MD): US National Library of Medicine. [cited 2007 Apr]. Available from: http://chem.sis.nlm.nih.gov/chemidplus/chemidlite.jsp
Chen CY. 2004. Biosynthesis of di-(2-ethylhexyl) phthalate (DEHP) and di-n-butyl phthalate (DBP) from red alga--Bangia atropurpurea. Water Res 38: 1014–1018.
Dillingham EO, Autian J. 1973. Teratogenicity, mutagenicity, and cellular toxicity of phthalate esters. Environ Health Perspect 3: 81–89.
Eastman Kodak. 1984. Basic environmental profile for: bis-(2-methoxyethyl)phthalate. Environmental Sciences Section, Health and Environment Laboratories. October 31, 1984. 4 pp.
Eastman Kodak. 1985. Basic toxicity of bis(2-methoxyethyl)phthalate. Provided by Eastman Kodak Co. to Office of Toxic Substances, US Environmental Protection Agency, Washington, DC.
[EC] European Commission. 2004. Council Directive of 27 July 1976 (76/769/EEC), revised on 18 October 2004. Available from: http://www.reach-compliance.eu/english/legislation/docs/launchers/launch-76-769-EEC.htmland from http://ec.europa.eu/enterprise/cosmetics/cosing/index.cfm?fuseaction=search.details&id=29074
[ECB] European Chemicals Bureau. 1994. Summary record. Commission Working Group on the Classification and Labelling of Dangerous Substances. Meeting at ECB Ispra, 22–24 November 1994. European Commission, Directorate-General Joint Research Centre, Institute for Health and Consumer Protection, European Chemicals Bureau. ECBI/18/94-Rev. 2. Available from: http://ecb.jrc.it/classlab/SummaryRecord/1894r2_sr_CMR1194.doc
[ECB] European Chemicals Bureau. 1995. Summary record. Commission Working Group on the Classification and Labelling of Dangerous Substances. Meeting at ECB Ispra, 14–16 February 1995. European Commission, Directorate-General Joint Research Centre, Institute for Health and Consumer Protection, European Chemicals Bureau. ECBI/23/95-Rev.2. Available from: http://ecb.jrc.it/classlab/SummaryRecord/2395r2_sr_CMR0295.doc
[ECETOC] European Centre for Ecotoxicology and Toxicology of Chemicals. 2005. The toxicology of glycol ethers and its relevance to man. Technical Report No. 95. Brussels (BE): ECETOC.
[ECOSAR] Ecological Structure Activity Relationships [Internet]. 2004. Version 0.99h. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: http://www.epa.gov/oppt/exposure/pubs/episuite.htm
[Environ] ENVIRON International Corporation. 2003a. Voluntary Children’s Chemical Evaluation Program Pilot (VCCEPP)--Tier 1 assessment of the potential health risks to children associated with exposure to the commercial pentabromodiphenyl ether product and appendices [Internet]. Emerville (CA): ENVIRON International Corporation. Available from: http://www.epa.gov/oppt/vccep/pubs/chem22a.html
[Environ] ENVIRON International Corporation. 2003b. Voluntary Children’s Chemical Evaluation Program Pilot (VCCEPP)--Tier 1 assessment of the potential health risks to children associated with exposure to the commercial octabromodiphenyl ether product and appendices [Internet]. Emerville (CA): ENVIRON International Corporation. Available from: http://www.epa.gov/oppt/vccep/pubs/chem23a.html
Environment Canada. 1988. Data relating to the Domestic Substances List (DSL) 1984–1986, collected under CEPA, 1988, s. 25(1). Based on Reporting for the Domestic Substances List [guide] 1988. Data prepared by: Environment Canada
Environment Canada. 2006. CEPAEnvironmental Registry Toxic Substances List: 2-Methoxyethanol. Gatineau (QC): Environment Canada. [cited 2009 Feb]. Available from: http://www.ec.gc.ca/CEPARegistry/subs_list/Toxicupdate.cfm
Environment Canada. 2008. Data for Batch 6 substances collected under the Canadian Environmental Protection Act, 1999, Section 71: Notice with respect to Batch 6 Challenge substances. Data prepared by: Environment Canada, Existing Substances Program.
Environment Canada. 2009. Suggested approach to determining the persistence of a chemical from biodegradation data. Preliminary draft working document. Gatineau (QC): Environment Canada, Existing Substances Division.
[EQC] Equilibrium Criterion Model. 2003. Version 2.02. Peterborough (ON): Trent University, Canadian Centre for Environmental Modelling and Chemistry. Available from: http://www.trentu.ca/academic/aminss/envmodel/models/EQC2.html
[ESIS] European Chemical Substances Information System [database on the Internet]. 2008. Version 4.50. Bis(2-methoxyethyl) phthalate, CAS 117-82-8. European Chemicals Bureau (ECB). Available from: http://ecb.jrc.it/esis/
Fassett DW. 1963. Esters. In: Patty’s industrial hygiene and toxicology. 2nd ed. Vol. II: Toxicology. Fassett DW, Irish DD, editors. New York (NY): Interscience Publishers.
Feuston MH, Bodnar KR, Kerstetter SL, Grink CP, Belcak MH, Singer J. 1989. Reproductive toxicity of 2-methoxyethanol applied dermally to occluded and nonoccluded sites in male rats. Toxicol Appl Pharmacol 100: 145–161.
Foote RH, Farrel PB, Schlafer DH, McArdle MM, Trouern-Trend V, Simkin ME, Brockett CC, Giles JR, Li J. 1995. Ethylene glycol monomethyl ether effects on health and reproduction in male rabbits. Reprod Toxicol 9(6): 527–539.
Furtmann K. 1995. Phthalate analysis as a tool for environmental assessment. Anal Meth Instr 2: 254–265.
Goldberg ME, Haun C, Smyth HF Jr. 1962. Toxicologic implication of altered behaviour induced by an industrial vapour. Toxicol Appl Pharmacol 4: 148–164. [cited in Canada 2002].
Hanley TR, Young JT Jr, John JA, Rao KS. 1984a. Ethylene glycol monomethyl ether (EGME) and propylene glycol monomethyl ether (PGME): Inhalation fertility and teratogenicity studies in rats, mice and rabbits. Environ Health Perspect 57: 7–12. [cited in Canada 2002].
Hanley TR, Yano BL Jr, Nitschke KD, John JA. 1984b. Comparison of the teratogenic potential of inhaled ethylene glycol monomethyl ether in rats, mice, and rabbits. Toxicol Appl Pharmacol 75: 409–422. [cited in Canada 2002].
Harris CA, Henttu P, Parker MG, Sumpter JP. 1997. The estrogenic activity of phthalate esters in vitro. Environ Health Perspect 105: 802–811.
Hathaway GJ, Proctor NH. 2004. Proctor and Hughes’ chemical hazards of the workplace. 5th ed. Hoboken (NJ): John Wiley & Sons Inc. p. 258.
Health Canada. 1995. Investigating human exposure to contaminants in the environment: A handbook for exposure calculations. Ottawa (ON): Minister of National Health and Welfare.
Health Canada. 1998. Exposure factors for assessing total daily intake of priority substances by the general population of Canada. Unpublished report. Ottawa (ON): Health Canada, Environmental Health Directorate.
Hellwig J. 1993. Study of the prenatal toxicity of 2-methoxyethanol in rats after dermal application. Unpublished report (No. OR53/89002). Ludwigshafen (DE): BASF AG, Abteilung Toxikologie. [cited in Canada 2002].
[HENRYWIN] Henry’s Law Constant Program for Microsoft Windows [Estimation Model]. 2000. Version 3.10. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: http://www.epa.gov/oppt/exposure/pubs/episuite.htm
Hobson DW, D’Addario AP, Bruner RH, Uddin DE. 1986. A subchronic dermal exposure study of diethylene glycol monomethyl ether and ethylene glycol monomethyl ether in the male guinea pig. Fundam Appl Toxicol 6: 339–348. [cited in Canada 2002].
[HPD] Household Products Database [database on the Internet]. 2008. Bethesda (MD): National Library of Medicine (US). [updated 2008 Sep; cited 2008 Dec]. Available from: http://householdproducts.nlm.nih.gov/
[HSDB] Hazardous Substances Data Bank [database on the Internet]. 2002. Bis(2-methoxyethyl)phthalate. Bethesda (MD): National Library of Medicine (US) [updated 2002 Jan 18; cited 2009 Jan]. Available from: http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB
[HYDROWIN] Hydrolysis Rates Program for Microsoft Windows [Estimation Model]. 2000. Version 1.67. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: http://www.epa.gov/oppt/exposure/pubs/episuite.htm
[IPCS] International Programme on Chemical Safety. 1992. Diethylhexyl phthalate. Geneva (CH): World Health Organization. (Environmental Health Criteria 131). Jointly sponsored by the United Nations Environment Programme, the International Labour Organization and the World Health Organization. [cited 2009 Jan]. Available from: http://www.inchem.org/documents/ehc/ehc/ehc131.htm
[JCS] Japanese Chemical Survey. 2004. Surveyed chemical substances and their detected levels in the environment (a cumulative list for fiscal year 1974–2003). Available from: http://www.env.go.jp/chemi/kurohon/en/http2004e/03-cie/summary2004.pdf
Kang J, Lee HB. 1988. [Method for determination of twelve phthalic esters in natural water.] Huanjing Kesue 9: 49–54. [in Chinese].
Kersten W, Reich T. 2003. [Non-volatile organic substances in Hamburg indoor dust.] Reinhalt Luft 63: 85–91. [article in German with English abstract].
Krasavage WJ. 1991. Final report on Dimethoxyethyl phthalate developmental toxicity screening study in rats. Laboratory project ID: HAEL No: 90-014. 270024U, TX-91-35.
Lefaux R. 1968. Practical toxicology of plastics. Cleveland (OH): CRC Press. p. 139–140, 355–356. [English version, translated from the French by Scripta Technica Ltd.; originally published in 1964].
Lewis RJ Sr. 1993. Hawley’s condensed chemical dictionary. 12th ed. New York (NY): Van Nostrand Reinhold Co. p. 410. [cited in HSDB 2002].
Li H, Tu Z, Wang H, Liu F, Li K. 2002. [Analysis of aroma components of kiwi fruit (Actinidia chinensis Planch.) by gas chromatography–mass spectrometry.] Fenxi Ceshi Xuebao 21: 58–60. [in Chinese].
Lin Z, Ikonomou MG, Mackintosh CE, Hongwu J, Gobas FAPC. 2003. Determination of phthalate ester congeners and mixtures by LC/ESI-MS in sediments and biota of an urbanized marine inlet. Environ Sci Technol 37: 2100–2108.
Lin Z, Sun R, Zhang L, Zou X, Chen M, Tu F, Ma Y, Jiang W. 2008. [Simultaneous determination of 14 phthalate ester residues in animal innards by gas chromatography–mass spectrometry with electron impact ionization.] Se Pu [Chin J Chromatogr] 26: 280–284. [in Chinese].
Liu M, Lin Y, Zeng F, Cui K, Luo X, Zeng Z. 2007. [The distribution and composition of phthalate esters in the sediment of urban lakes in Guangzhou.] Huanjing Kexue Xuebao 27: 1377–1383. [in Chinese].
Mathur SP. 1974. Phthalate esters in the environment: Pollutants or natural products. J Environ Qual 3: 189–197.
McFall JA, Antoine SR, DeLeon IR. 1985. Organics in the water column of Lake Pontchartrain. Chemosphere 14: 1253–1265.
Miller RR, Ayres JA, Young JT, McKenna MJ. 1983. Ethylene glycol monomethyl ether. I. Subchronic vapor inhalation study with rats and rabbits. Fundam Appl Toxicol 3: 49–54.
[MPBPWIN] Melting Point Boiling Point Program for Microsoft Windows [Estimation Model]. 2000. Version 1.41. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: http://www.epa.gov/oppt/exposure/pubs/episuite.htm
Namikoshi M, Fujiwara T, Nishikawa T, Ukai K. 2006. Natural abundance 14C content of dibutyl phthalate (DBP) from three marine algae. Mar Drugs 4: 290–297.
[NCI] National Chemical Inventories [database on a CD-ROM]. 2006. Columbus (OH): American Chemical Society, Chemical Abstracts Service. [cited 2009 Jan]. Available from: http://www.cas.org/products/cd/nci/require.html
Nelson BK, Brightwell WS, Burg JR, Massari VJ. 1984. Behavioral and neurochemical alterations in the offspring of rats after maternal or paternal inhalation exposure to the industrial solvent 2-methoxyethanol. Pharmacol Biochem Behav 20: 269–279.
[NICNAS] National Industrial Chemicals Notification and Assessment Scheme. 2007. Human health hazard assessment: Bis(2-methoxyethyl) phthalate (DMEP) (CAS No. 117-82-8). April 30, 2007. Sydney (AU): Australian Government, Department of Health and Ageing. [cited 2009 Jan]. Available from: http://www.nicnas.gov.au/Industry/Existing_Chemicals/Phthalate_Hazard_Assessments/
DMEP%20hazard%20assessment%2030-4-07.pdf
[NICNAS] National Industrial Chemicals Notification and Assessment Scheme. 2008a. Existing chemical hazard assessment report: Bis(2-methoxyethyl) phthalate. Sydney (AU): Australian Government, Department of Health and Ageing. [cited 2009 Jan]. Available from: http://www.nicnas.gov.au/Publications/CAR/Other/DMEP%20hazard%20assessment.pdf
[NICNAS] National Industrial Chemicals Notification and Assessment Scheme. 2008b. Existing chemical hazard assessment report: A summary of physicochemical and human health hazard data for 24 ortho-phthalate chemicals. Sydney (AU): Australian Government, Department of Health and Ageing. [cited 2009 Mar].
[NPI] National Pollutant Inventory. 2007. Parkes (AU): Australian Government, Department of the Environment, Water, Heritage and the Arts. [cited 2009 Jan]. Available from: http://www.npi.gov.au/cgi-bin/npidbsearch.pl?proc=substance
[NPRI] National Pollutant Release Inventory [database on the Internet]. 2007. Gatineau (QC): Environment Canada. [updated 2008 Oct 13; cited 2009 Jan]. Available from: http://www.ec.gc.ca/pdb/querysite/query_e.cfm
[NTP] National Toxicology Program (US). 1993. NTP technical report on toxicity studies of ethylene glycol ethers 2-methoxyethanol, 2-ethoxyethanol, 2-butoxyethanol (CAS Nos. 109-86-4, 110-80-5, 111-76-2) administered in drinking water to F344/N rats and B6C3F1 mice. Research Triangle Park (NC): US Department of Health and Human Services, National Toxicology Program. NTP Toxicity Report Series No. 26; NIH Publication No. 93-3349. [cited in Canada 2002].
[OASIS Forecast] Optimized Approach based on Structural Indices Set [Internet]. 2005. Version 1.20. Bourgas (BG): Bourgas Prof. Assen Zlatarov University, Laboratory of Mathematical Chemistry. Available from: http://oasis-lmc.org/?section=software
Oliver R, May E, Williams J. 2005. Microcosm investigations of phthalate behavior in sewage treatment biofilms. Sci Total Environ 372: 605–614.
Parkhie MR, Webb M, Norcross MA. 1982. Dimethoxyethyl phthalate: Embryopathy, teratogenicity, fetal metabolism and the role of zinc in the rat. Environ Health Perspect 45: 89–97.
[PCKOCWIN] Organic Carbon Partition Coefficient Program for Windows [Estimation Model]. 2000. Version 1.66. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: http://www.epa.gov/oppt/exposure/pubs/episuite.htm
Pfordt J, Bruns-Weller E. 1999. [The phthalate esters as a group of chemicals with endocrine potential.] Hanover (DE): Niedersächsisches Ministerium für Ernährung, Landwirtschaft und Forsten. [in German].
[PhysProp] Interactive PhysProp Database [database on the Internet]. 2006. Syracuse (NY): Syracuse Research Corporation. [cited 2006 Mar]. Available from: http://www.syrres.com/esc/physdemo.htm
Ritter EJ, Scott WJ Jr, Randall JL, Ritter JM. 1985. Teratogenicity of dimethoxyethyl phthalate and its metabolites methoxyethanol and methoxyacetic acid in the rat. Teratology 32: 25–31.
Rudelle D, Cassard S, Hartemann P, Muller JF, Francais T, Morlot M. 1995. [Interaction between water and floppy plastic materials: Identification of organic compounds by gas chromatography–mass spectrometry.] J Eur Hydrol 26: 211–225. [in French].
Scott WJ, Fradkin R, Wittfoht W, Nau H. 1989. Teratologic potential of 2-methoxyethanol and transplacental distribution of its metabolite, 2-methoxyacetic acid, in non-human primates. Teratology 39: 363–373. [cited in Canada 2002].
Shanghai Yancui Import and Export Co., Ltd. 2008. Dimethoxyethyl phthalate. [cited 2008 Dec 16]. Available from: http://www.alibaba.com/product-gs/209059994/Dimethoxyethyl_phthalate.html
Sheftel VO. 2000. Indirect food additives and polymers: Migration and toxicity. Boca Raton (FL): CRC Press.
Singh AR, Lawrence WH, Autian J. 1972. Teratogenicity of phthalate esters in rats. J Pharm Sci 52: 51–55.
Singh AR, Lawrence WH, Autian J. 1974. Mutagenic and antifertilities of mice to di-2-ethylhexyl phthalate (DEHP) and dimethoxyethyl phthalate (DMEP). Toxicol Appl Pharmacol 29: 35–46.
Smialowicz RJ, Williams WC, Riddle MM, Andrews DL, Luebke RW, Copeland CB. 1992. Comparative immunosuppression of various glycol ethers orally administered to Fischer 344 rats. Fundam Appl Toxicol 18: 621–627.
Smialowicz RJ, Riddle MM, Williams WC. 1993. Methoxyacetaldehyde, an intermediate metabolite of 2-methoxyethanol, is immunosuppressive in the rat. Fundam Appl Toxicol 21: 1–7. [cited in Canada 2002].
[SPIN] Substances in Preparations in Nordic Countries. 2009. Online database. [cited in 2009 Sept.] Available from: http://195.215.251.229/DotNetNuke/default.aspx
SRI Consulting. 2008. Directory of chemical producers [database on the Internet]. Menlo Park (CA): SRI Consulting. [cited 2008 Nov]. Available from: http://www.sriconsulting.com [restricted access].
Steiner I. 1991. [Changes in a polyethylene food packaging film following ozone sterilization.] Dtsch Lebensmitt Rundsch 87: 107–112. [in German].
[TOPKAT] TOxicity Prediction by Komputer Assisted Technology [Internet]. 2004. Version 6.2. San Diego (CA): Accelrys Software Inc. Available from: http://www.accelrys.com/products/topkat/index.html
[TRI] Toxics Release Inventory [database on the Internet]. 2006. TRI Explorer 4.7. Washington (DC): US Environmental Protection Agency. [cited 2009 Jan]. Available from: http://www.epa.gov/triexplorer/
[US EPA] US Environmental Protection Agency. 1985. Chemical Hazard Information Profile (CHIP) draft report on dimethoxyethyl phthalate. Prepared by J.G. Smith, Oak Ridge National Laboratory, Oak Ridge, TN, under contract to US Environmental Protection Agency, Washington, DC.
[US EPA] US Environmental Protection Agency. 2002. Non-confidential IUR [Inventory Update Reporting] production volume information. [cited in 2009 Jan]. Available from: http://www.epa.gov/oppt/iur/tools/data/2002-vol.htm
[US EPA] US Environmental Protection Agency. 2006. Non-confidential IUR [Inventory Update Reporting] production volume information. [cited in 2009 Sept]. Available from: http://cfpub.epa.gov/iursearch/index.cfm?s=chem, search by CAS number.
Wang XH, Xiao TC, Yang B, Wang SR, Ou QY. 1997. [Analysis and elimination of trace organic compounds in smoke from burning coal.] Gaodeng Xuexiao Huaxue Xuebao 18: 24–28. [in Chinese].
Webber MD, Lesage S. 1989. Organic contaminants in Canadian municipal sludges. Waste Manage Res 7: 63–82.
Williams WC, Riddle MM, Copeland CB, Andrews DL, Smialowicz RJ. 1995. Immunological effects of 2-methoxyethanol administered dermally or orally to Fischer 344 rats. Toxicology 98: 215–223. [cited in Canada 2002].
Yonemoto J, Brown NA, Webb M. 1984. Effects of dimethoxyethyl phthalate, monomethoxyethyl phthalate, 2-methoxyethanol and methoxyacetic acid on post implantation rat embryos in culture. Toxicol Lett 21: 97–102.
Zeng F, Chen L, Cui K, Zhang G. 2005. [Determination of phthalate esters in sediment samples by silica gel–alumina column separation and gas chromatography.] Fenxi Huaxue 33: 1063–1067. [in Chinese]
Appendix 1: Upper-bounding estimates of daily intake of DMEP from indoor dust by the general population in Canada
| Route of exposure | Estimated intake (µg/kg-bw per day) of DMEP by various age groups | |||||||
|---|---|---|---|---|---|---|---|---|
| 0–0.5 years1,2,3 | 0.5–4 years4 | 5–11 years5 | 12–19 years6 | 20–59 years7 | 60+ years8 | |||
| Breast milk fed | Formula fed | Not formula fed | ||||||
| Soil/dust9 | 0.07 | 0.07 | 0.07 | 0.11 | 0.04 | 0.01 | 0.01 | 0.01 |
| Total intake | 0.07 | 0.07 | 0.07 | 0.11 | 0.04 | 0.01 | 0.01 | 0.01 |
Appendix 2: Upper-bounding estimates of dermal exposure to DMEP from indoor dust by the general population in Canada
| Route of exposure | Estimated intake (µg/kg-bw per day) of DMEP by various age groups | |||||
|---|---|---|---|---|---|---|
| 0–6 months1 | 0.5–4 years2 | 5–11 years3 | 12–19 years4 | 20–59 years5 | 60+ years6 | |
| Dermal exposure to dust/soil7 | 0.19 | 0.15 | 0.15 | 0.14 | 0.18 | 0.17 |
Example Calculation
| Scenario | Assumptions | Estimated exposure |
|---|---|---|
| Exposure to indoor dust | Dermal – Child 0.5–4 years of age Suggested by Environ (2003a, b) for a child less than 1 year old: Concentration of DMEP in house dust (Cdust) is 17 mg/kg (Kersten and Reich 2003). Conversion factor of 1 × 10-6 (CF1), adherence rate of dust to skin (ARdust) of 0.05 mg/cm2 per day, exposed skin surface area (Sat) (hands, arms, legs and feet) of 2890 cm2 (Health Canada 1995), exposure frequency at home (EFh) of 22 h/day, conversion factor (CF2) of 0.0417 day/h, body weight of 15.5 kg (Environ 2003a, b), and absorption factor for the dermal route (AFd) assumed to be 1. Dose rate = Cdust × CF1 × ARdust × Sat × AFd × EFh × CF2 / BW = 17 mg/kg × 1 × 10-6 kg/mg × 0.05 mg/cm2 per day × 2890 cm2 × 1 × 22 h/day × 0.0417 day/h / 15.5 kg
|
0.15 µg/kg-bw per day |
| Endpoint | Lowest effect levels1/Results |
|---|---|
| 1LC50, median lethal concentration; LD50, median lethal dose; LO(A)EL, lowest-observed-(adverse-)effect level; NO(A)EC, no-observed-(adverse-)effect concentration; NO(A)EL, no-observed-(adverse-)effect level. 2 This study was conducted by heating up the chemical to 100–200°C to generate the vapour; therefore, the animals could have inhaled aerosol in this study, and actual dose concentrations are not certain. 3 Original report did not state clearly what the actual dosage was. This dose was estimated based on the assumption that DMEP was applied to rats in diet. |
|
| Laboratory animals and in vitro | |
| Acute toxicity | Lowest oral LD50(guinea pig) = 1600 mg/kg-bw (Fassett 1963) [additional studies in guinea pigs, mice and rats, with LD50s ranging from 2750 to >4400 mg/kg-bw: Fassett 1963; Lefaux 1968; Eastman Kodak 1985] Lowest dermal LD50(guinea pig) = >10 mL/kg-bw (>11 710 mg/kg-bw) (Fassett 1963) [additional study: LD50 = >20 mL/kg-bw (>23 420 mg/kg-bw), test material purity was 78%, Eastman Kodak 1985] Lowest inhalation LC50(rat, 6 h) between 700 and 1595 ppm (Fassett 1963)2 [no additional studies identified] Lowest oral LO(A)EL (male rats) = 1500 mg/kg-bw, based on significantly reduced mean testes weights and increased abnormal sperm counts. Those effects were not observed at 1000 mg/kg-bw (Cassidy et al. 1983). [additional study by other routes of exposure (Calley et al. 1966):
|
| Short-term repeated-dose toxicity | Oral LOEL = 100 mg/kg-bw per day (lowest dose tested in male rats, 5/group, gavage, 12 treatments within 16 days; test material purity was 78%), based on slightly but significantly reduced haemoglobin and haematocrit values and minor thymic medullary haemorrhage. The authors speculated that the method of euthanasia used might have caused the thymic haemorrhage. At a higher dose level (1000 mg/kg-bw per day), relative and absolute thymus and testes weights were greatly reduced; absolute but not relative liver weights were somewhat reduced; absolute kidney weights were reduced while relative kidney weights were increased; body weight gain and food intake were significantly reduced. Significantly reduced absolute white cell counts and platelet counts were also observed at the higher dose level, along with slightly reduced red blood cell counts, decreased haemoglobin concentration and haematocrit and slightly increased granulocyte counts. In addition, thymic and testicular atrophy in seminiferous tubules, degeneration of sperm in the seminiferous tubules and epididymis, the presence of giant spermatids and atrophy of accessory sex organ in the animal dying on day 11 were observed at the higher dose, along with slight decreases in some enzyme activities, including alanine aminotransferase, aspartate aminotransferase and sorbitol dehydrogenase, and creatinine levels (Eastman Kodak 1985). [no additional studies identified] Inhalation NOEC = 145 ppm (rats, 6 h/day for 62 days). No animal deaths or any symptoms were reported (Fassett 1963).2 [no additional studies identified] No short-term dermal study was identified. [additional short-term study conducted via i.p. administration in mice: reduced testes weights were observed at 250 mg/kg-bw per day (the only dose tested), along with acute peritonitis and periportal hepatitis in the liver and extramedullary haematopoiesis in both liver and spleen; for details, see reproductive toxicity section below (Calley et al. 1966)] |
| Subchronic toxicity | No study was identified. |
| Chronic toxicity/ carcinogenicity | Oral NOEL = 900 mg/kg diet3 per day (45 mg/kg-bw per day, the highest dose tested), based on an oral study in rats with very limited experimental details. Diets containing 300, 500 and 900 mg DMEP/kg were administered to rats for up to five generations. In three cases, the growth curves were ascertained for males and females. A certain number of male animals were kept on these three diets for 21 months with a view to detecting any possible carcinogenic effects. A further five generations of rats were investigated with a 300 mg/kg diet and three generations with 500 and 900 mg/kg. The individual growth curves for five successive generations of males or females could be superimposed on those of controls. On the 900 mg/kg diet, any differences observed were not statistically significant. The weights of treated animals were similar to those of controls. No pathological symptoms were observed, nor were any lesions or anomalies found, and the weights of organs (liver, kidneys, lungs, heart and brain) showed no significant differences. Reproduction was normal. No anomalies were found in parturition or nursing with female rats of various generations. To sum up, the author stated that the substance has a low toxicity and is not carcinogenic (Lefaux 1968). |
| Reproductive toxicity | Lowest oral LOAEL = 1000 mg/kg-bw per day (male rats, 5/dose, gavage, 12 treatments over 16 days). Significant reductions in absolute and relative testis weights with seminiferous tubule atrophy and sperm degeneration and the presence of giant spermatids were observed. NOAEL = 100 mg/kg-bw per day (Eastman Kodak 1985). [additional oral study: single administration via gavage in male rats, 5/group; animals were examined on day 12. Significant reduction in testes weight and significant increases in abnormal sperm levels were observed at the 1500 mg/kg-bw dose level and above, but not at the 1000 mg/kg-bw dose level (Cassidy et al. 1983).] No reproductive toxicity study via dermal or inhalation administration was identified. [additional studies via other administration routes:
|
| Developmental toxicity | Lowest oral LOEL = 60 mg/kg-bw per day (lowest dose tested in CD pregnant rats, 10/group, gavage, on gestation days 6 –16), based on significantly reduced pup body weight gain and lightly reduced pup survival from day 1 to 5 postpartum. At mid-dose level (180 mg/kg-kw per day), significantly reduced pup survival and pup body weight gain were observed and four pups from three of the nine litters developed abnormalities including a shortened lumbosacral region, acauda and filamentous tails. At the highest dose tested (600 mg/kg-bw per day, complete resorption of the litters was observed. Maternal body weight gain and mean body weights as well as food consumption were significantly reduced at 600 mg/kg–bw per day. A NOEL for maternal toxicity = 180 mg/kg-bw per day (Krasavage 1991). [no additional oral study was identified] No developmental toxicity studies via dermal or inhalation routes of administration were identified. [additional studies via other administration routes:
|
| Genotoxicity and related endpoints: in vivo | Dominant lethal test Positive in ICR mice. In the previously mentioned study, male mice (10/group) were given a single i.p. injection at dose levels of 1.19, 1.79 and 2.38 mL/kg-bw prior to mating. Untreated females were replaced weekly during the 12-week mating period. Pregnant rats were terminated on gestation days 13–17. Males in the highest dose group (2.38 mL/kg-bw, equivalent to 2785 mg/kg-bw) exhibited 20% mortality. Significantly reduced semifertility (i.e., reduced incidence of pregnancy, mean live fetuses per pregnancy and mean implants per pregnancy) and increased early fetal death were observed in the highest dose group, indicating adverse reproductive and/or genetic effects induced by DMEP (Dillingham and Autian 1973; Singh et al. 1974). |
| Genotoxicity and related endpoints: in vitro | Mutagenicity – Ames test Positive with Salmonella typhimuriumTA98 without metabolic activation Negative with TA100 with and without activation and with TA98 with activation (NTP 1993) |
| Sensitization | DMEP was not a skin sensitizer when tested in guinea pigs (Eastman Kodak 1985) |
| Irritation | Skin irritation DMEP was slightly irritating to skin when tested with guinea pig (Eastman Kodak 1985) |
Eye irritation DMEP was slightly irritating to eye when tested with rabbits (Eastman Kodak 1985) |
|
| Humans | No data were identified. |