Screening Assessment for the Challenge
This page has been archived on the Web
Information identified as archived is provided for reference, research or recordkeeping purposes. It is not subject to the Government of Canada Web Standards and has not been altered or updated since it was archived. Please contact us to request a format other than those available.
Archived
Peroxide, (3,3,5-trimethylcyclohexylidene)bis[(1,1-dimethylethyl)]
Chemical Abstracts Service Registry Number
6731-36-8
Environment Canada
Health Canada
July 2008
Table of Contents
Pursuant to section 74 of the Canadian Environmental Protection Act, 1999 (CEPA 1999), the Ministers of the Environment and of Health have conducted a screening assessment on peroxide, (3,3,5-trimethylcyclohexylidene)bis[(1,1-dimethylethyl)] (DBTMC), Chemical Abstracts Service Registry Number 6731-36-8. This substance was identified as a high priority for screening assessment and included in the Ministerial Challenge because it had been found to meet the ecological categorization criteria for persistence, bioaccumulation potential and inherent toxicity to non-human organisms and it is believed to be in commerce in Canada.
The substance DBTMC was not considered to be a high priority for assessment of potential risks to human health, based upon application of the simple exposure and hazard tools developed by Health Canada for categorization of substances on the Domestic Substances List (i.e., it did not meet the criteria of both being considered to present greatest or intermediate potential for exposure and having been classified by another national or international regulatory agency on the basis of carcinogenicity, genotoxicity, developmental toxicity or reproductive toxicity). Therefore, this assessment focuses on information relevant to the evaluation of ecological risks.
DBTMC is an organic substance that is used in Canada and elsewhere in polymer processing and is used in the hardening of polyester resins. The substance is not naturally produced in the environment. DBTMC was not manufactured in Canada in 2006, while between 10 000 and 100 000 kg of DBTMC were imported into Canada during the same period.
Based on certain assumptions and reported use patterns, most of the substance is transformed during the processing phase. Small proportions may be released to water (0.04%). This substance is not soluble in water and has a tendency to partition to particles because of its hydrophobic nature. For these reasons, DBTMC would likely be found almost entirely in sediments and is not expected to be significantly present in other media.
DBTMC is not expected to meet the persistence criterion as set out in the Persistence and Bioaccumulation Regulations, but it is predicted to have a potential to accumulate in organisms.
Predicted environmental concentrations are two orders of magnitude lower than the predicted no-effects concentrations for aquatic organisms. This indicates a low probability of risk in the aquatic environment.
This substance will be included in the Domestic Substances List inventory update initiative, to be launched in 2009. In addition and where relevant, research and monitoring will support verification of assumptions used during the screening assessment.
Based on the information available, DBTMC does not meet any of the criteria set out in section 64 of the Canadian Environmental Protection Act, 1999.
The Canadian Environmental Protection Act, 1999 (CEPA 1999) (Canada 1999) requires the Minister of the Environment and the Minister of Health to conduct screening assessments of substances that have met the categorization criteria set out in the Act to determine whether these substances present or may present a risk to the environment or human health. Based on the results of a screening assessment, the Ministers can propose to take no further action with respect to the substance, to add the substance to the Priority Substances List (PSL) for further assessment, or to recommend that the substance be added to the List of Toxic Substances in Schedule 1 of the Act and, where applicable, the implementation of virtual elimination.
Based on the information obtained through the categorization process, the Ministers identified a number of substances as high priorities for action. These include substances that
- met all of the ecological categorization criteria, including persistence (P), bioaccumulation potential (B) and inherent toxicity to aquatic organisms (iT), and were believed to be in commerce in Canada; and/or
- met the categorization criteria for greatest potential for exposure (GPE) or presented an intermediate potential for exposure (IPE), and had been identified as posing a high hazard to human health based on classifications by other national or international agencies for carcinogenicity, genotoxicity, developmental toxicity or reproductive toxicity.
The Ministers therefore published a notice of intent in the Canada Gazette, Part I, on December 9, 2006 (Canada 2006), that challenged industry and other interested stakeholders to submit, within specified timelines, specific information that may be used to inform risk assessment, and to develop and benchmark best practices for the risk management and product stewardship of these substances identified as high priorities.
The substance peroxide, (3,3,5-trimethylcyclohexylidene)bis[(1,1-dimethylethyl) was identified as a high priority for assessment of ecological risk as it was found to be persistent, bioaccumulative and inherently toxic to aquatic organisms and is believed to be in commerce in Canada. The Challenge for peroxide, (1,1,4,4-tetramethyl-2-butyne-1,4-diyl)bis[(1,1-dimethylethyl was published in the Canada Gazette on February 3, 2007 (Canada 2007a). A substance profile was released at the same time. The substance profile presented the technical information available prior to December 2005 that formed the basis for categorization of this substance. As a result of the Challenge, submissions of information were received.
Although peroxide, (1,1,4,4-tetramethyl-2-butyne-1,4-diyl)bis[(1,1-dimethylethyl was determined to be a high priority for assessment with respect to the environment, it did not meet the criteria for GPE or IPE and high hazard to human health based on classifications by other national or international agencies for carcinogenicity, genotoxicity, developmental toxicity or reproductive toxicity. Therefore, this assessment focuses principally on information relevant to the evaluation of ecological risks.
Screening assessments under CEPA 1999 focus on information critical to determining whether a substance meets the criteria for defining a chemical as toxic as set out in section 64 of the Act, where
“64. [...] a substance is toxic if it is entering or may enter the environment in a quantity or concentration or under conditions that
(a) have or may have an immediate or long-term harmful effect on the environment or its biological diversity;
(b) constitute or may constitute a danger to the environment on which life depends; or
(c) constitute or may constitute a danger in Canada to human life or health.”
Screening assessments examine scientific information and develops conclusions by incorporating a weight of evidence approach and precaution.
This screening assessment includes consideration of information on chemical properties, hazards, uses and exposure, including the additional information submitted under the Challenge. Data relevant to the screening assessment of this substance were identified in original literature, review and assessment documents, stakeholder research reports and from recent literature searches up to May 2008. Key studies were critically evaluated; modelling results may have been used to reach conclusions. When available and relevant, information presented in hazard assessment from other jurisdictions was considered. The screening assessment does not represent an exhaustive or critical review of all available data. Rather, it presents the most critical studies and lines of evidence pertinent to the conclusion.
This screening assessment was prepared by staff in the Existing Substances Programs at Health Canada and Environment Canada and incorporates input from other programs within these departments. Additionally, the draft of this screening assessment was subject to a 60-day public comment period. The critical information and considerations upon which the assessment is based are summarized below.
For the purposes of this report, this substance will be referred to as DBTMC, which has been derived from the name 1,1-di-tert-butylperoxy-3,3,5-trimethylcyclohexane.
Table 1. Substance Identity
| Chemical Abstracts Service Registry Number (CAS RN) | 6731-36-8 |
| Name on Domestic Substances List (DSL) | Peroxide, (3,3,5-trimethylcyclohexylidene)bis[(1,1-dimethylethyl) |
| Other Inventory Names | Peroxide, 1,1'-(3,3,5-trimethylcyclohexylidene)bis[2-(1,1-dimethylethyl) (TSCA) Di-tert-butyl 3,3,5-trimethylcyclohexylidene diperoxide (EINECS) |
| Other names | 1,1-Bis(tert-butylperoxy)-3,5,5-trimethylcyclohexane; 1,1-Di(t-butylperoxy)-3,3,5-trimethylcyclohexane; 3,3,5-Trimethyl-1,1-bis(tert-butylperoxy)cyclohexane; Interox TMCH 401C, Link-Cup TMCH, Luperco 231G, 231G40, 231XL, 231XLP; Luperox 231, 231-50, 231XL; Lupersol 230XL, 231, L 231; Perhexa 3M, 3M40, Sanperox CY 1.1; Trigonox 29, 29-40B-PD, 29/40, 29/40MB, 29A, 29B50, 29B75, 29B90, 29C75, Tx 29, 29B50, Varox 231XL |
| Chemical group | Discrete organics |
| Chemical sub-group | Diperoxyketals |
| Chemical formula | C17H34O4 |
| Chemical structure | 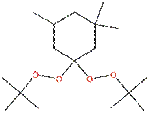 |
| SMILES | O(OC(C)(C)C)C(OOC(C)(C)C)(CC(CC1(C)C)C)C1 |
| Molecular mass | 302.46 g/mol |
Table 2 contains experimental and modelled physical and chemical properties of DBTMC that are relevant to its environmental fate.
Table 2. Physical and chemical properties for DBTMC
| Property | Type | Value | Temperature (°C) |
Reference |
|---|---|---|---|---|
| Melting point (ºC) |
Experimental | -20 90 (decomposition temp.) |
Acros Organics MSDS 2004 Morioka and Yamada 1993 |
|
| Modelled | 86.3 | MPBPWIN 2000 | ||
| Boiling point (ºC) |
Modelled | 306.87 312.2 |
MPBPWIN 2000 ACD 2007 |
|
| Vapour pressure (Pa) |
Modelled | 0.09 0.13 |
25 |
MPBPWIN 2000 ACD 2007 |
| Henry's Law constant (Pa·m3/mol) |
Modelled | 152.7 (0.001507 atm·m3/mol) |
25 | HENRYWIN 2000 |
| Log Kow (Octanol-water partition coefficient) (dimensionless) |
Modelled | 7.56 6.92 |
25 25 |
KOWWIN 2000 ACD 2007 |
| Log Koc (Organic carbon-water partition coefficient) (L/kg) |
Modelled | 5.53 5.14 |
25 | PCKOCWIN 2000 ACD 2007 |
| Water solubility (mg/L) |
Modelled | 0.004118 0.082 |
25 25 |
WSKOWWIN 2000 ACD 2007 |
Most of the physical and chemical properties in the above table were generated using quantitative structure-activity relationship (QSAR) models, and there are uncertainties related to the use of these models. For instance, the applicability domain of a model may not cover the entire structure of a given chemical, thus lowering the reliability of predictions.
Organic peroxide initiators were not manufactured in Canada in 2000. Peroxyketals made up the smallest class of organic peroxides that were used in the polymer resin manufacturing process in Canada, with approximately 100 000 kg of peroxyketals were used in the Canadian polymer resin manufacturing process in 2000 ( ChemInfo Services Inc. 2002).
Response to a survey notice pursuant to section 71 of CEPA 1999 indicated that DBTMC was not manufactured in Canada in 2006 in a quantity meeting the 100 kg reporting threshold. Eight companies met the 100 kg reporting threshold and reported importing the substance into Canada in a total quantity, for the eight companies, between 10 000 and 100 000 kg. One company reported importing the substance at a quantity below 100 kg (Environment Canada 2007a).
It is not known how much DBTMC is imported into Canada in finished articles, for example, as residues in polymeric materials.
Elsewhere, DBTMC was reported to the US Environmental Protection Agency under the US Inventory Update Rule for use between 4.5 and 225 tonnes per year from 1990 to 1998, which increased to 225 to 455 tonnes in 2002 (U.S. EPA 2002). DBTMC is a European Union (EU) Low Production Volume Chemical, indicating that production within the EU is estimated to be in the order of 10 tonnes per year. The database for Substances in Preparations in Nordic Countries indicates that it was used in Sweden, Norway and Denmark from 1999 to 2004 (SPIN Database 2000). In Japan, it was found that 108 tonnes of DBTMC were manufactured or imported in 2004. Results from Japan’s 2001 Ministry of Economy, Trade and Industry survey show that 100 to 1000 tonnes were manufactured or imported (NITE website).
Information on uses of DBTMC in Canada was received in response to the CEPA section 71 Notice for the 2006 calendar year. Reported uses include the followings: polymer additive, polymer crosslinking agent, catalyst, accelerator, initiator, activator, adhesive, binder, sealant, and filter.
Published literature indicates that DBTMC is used as an initiator for the polymerization of monomers as well as for the hardening of insatiated polyester resins and the cross-linking of polymers. It can be used as a polymerisation initiator for plastics and in rubber processing for the production of window seals and automotive seals, hoses, and soles of shoes. It may also be used for the curing of some resins for applications ranging from boat hulls and swimming pools to bodywork parts (Arkema 2006). In these uses, the peroxide bonds are broken to produce reactive radicals that initiate polymerization.
DBTMC is not naturally produced in the environment.
Mass flow tool
To estimate potential release of the substance to the environment at different stages of its life cycle, a mass flow tool was used. Empirical data concerning releases of specific substances to the environment are seldom available. Therefore, for each identified type of use of the substance, the proportion and quantity of release to the different environmental media are estimated, as is the proportion of the substance chemically transformed or sent for waste disposal. Assumptions and input parameters used in making these estimates are based on information obtained from a variety of sources including responses to regulatory surveys, Statistics Canada, manufacturers’ websites and technical databases. Of particular relevance are emission factors, which are generally expressed as the fraction of a substance released to the environment, particularly during its manufacture, processing, and use associated with industrial processes. Sources of such information include emission scenario documents, often developed under the auspices of the Organisation for Economic Co-operation and Development (OECD), and default assumptions used by different international chemical regulatory agencies. It is noted that the level of uncertainty in the mass of substance and quantity released to the environment generally increases further down the life cycle.Unless specific information on the rate or potential for release of the substance from landfills and incinerators is available, the Mass Flow Tool does not quantitatively account for releases to the environment from disposal.
Table 3. Estimated releases and losses of DBTMC to environmental media,transformation and distribution to management processes, based on the Mass Flow Tool [1]
| Fate | Proportion of the mass (%) | Major life cycle stage involved |
|---|---|---|
| Releases to receiving media: | ||
| To soil | 0.0 | |
| To air | 0.0 | Rubber vulcanization |
| To sewer [2] | 0.04 | Transport and handling |
| Chemically transformed | 99.58 | |
| Transferred to waste disposal sites (e.g., landfill, incineration) | 0.37 | Waste management |
[2] I.e., wastewater before any treatment.
The tool results indicate that the substance is amlost all (about 99.6%) lost by transformation, mostly during the processing phase at polymer manufacturing facilities, where the peroxide bonds in the substance are broken to form reactive radicals that initiate polymerization. About 0.4% may end up in waste disposal sites as a result of handling and cleaning processes and disposal of off-spec product. A small fraction of solid waste is incinerated, which is expected to result in transformation of the substance. Based largely on information contained in OECD emission scenario documents for processing and uses associated with this substance, it is estimated that 0.04% DBTMC may be released to sewers.
Based on the above, the largest release of DBTMC to the ambient environment is to sewers from transportation and handling.
Based on its physical and chemical properties (Table 2) and the results of Level III fugacity modelling (Table 4), DBTMC is expected to reside predominantly in sediment, soil and air, with a small fraction in water, depending on the compartment of release.
Table 4. Results of the Level III fugacity modelling (EPIWIN 2004)
| Substance released to: | Fraction of substance partitioning to each medium (%) | |||
|---|---|---|---|---|
| Air | Water | Soil | Sediment | |
| Air (100%) | 37 | 0.6 | 13 | 49 |
| Water (100%) | 0.003 | 1.3 | 0.001 | 98.7 |
| Soil (100%) | 6×10-5 | 0.002 | 99.8 | 0.2 |
According to the mass flow tool results presented in Table 3, the largest direct environmental release of DBTMC is to sewers during processing and so the 100% release scenario to water seems to be the most relevant for Canada. The fraction of DBTMC released to water is expected to strongly adsorb to suspended solids and sediments, according to its very high log Kow value of ~7 (Table 2) and Level III fugacity modeling results.
Persistence
As mentioned above, the only direct release of DBTMC to the environment could be to surface water through sewers (Table 3). Once in water, the fate analysis presented in Table 4 indicates that this substance would partition almost exclusively into sediments (99%), and to a much lower extent to water (1%). According to the same analysis, DBTMC is not expected to partition to air or soil if released to water. Therefore, the potential for persistence of DBTMC will be assessed for the aquatic compartment only.
While peroxides are generally considered to be reactive because of the nature of the peroxide bond, there are differences in the level of reactivity among different categories of organoperoxides, and even among different substances within a category.
Peroxyketals like DBTMC are quite stable at their recommended storage temperature of < 30°C, with a shelf half-life of at least one year (ATOFINA 2001). However, storing conditions does not reflect the transformation pathways that can exist in the natural environment, such as hydrolysis, photolysis and biodegradation.
Regarding hydrolysis, DBTMC does not contain functional groups expected to react with water. As for photolysis, there are no data on the absorption spectrum of DBTMC or for a chemical analogue. There are experimental data available to assess the biodegradation of DBTMC and other organic peroxides. Overall, these data suggest that DBTMC would not persist in water.
First, in a ready biodegradability closed bottle test (OECD Guideline 301D) conducted with DBTMC, 2% and 37% ultimate biodegradation had occurred at days 28 and 112, respectively (Study Submission 2006a). In this test, the peroxide bonds may have been initially cleaved to form products such as tertiary butanol and 3,3,5-trimethylcyclohexanone followed by biodegradation of these breakdown products. Data from the NITE database (2002) for DBTMC indicates 12% and 8% primary biodegradation over 28 days in a ready-biodegradation test (OECD Guideline 301C) as measured by gas chromatography analysis and by ultraviolet-visible spectroscopy, respectively.
Studies addressing biodegradation were also available for other organic peroxides. Even though these results are given a lower weight, they are still used as additional lines of evidence to assess the potential for persistence of DBTMC. First, in a closed bottle test (OECD Guideline 301D), a dialkyl peroxide, dicumyl peroxide (CAS RN 80-43-3), showed 18% and 60% biodegradation at days 28 and 57, respectively (OPPSD 2008). Another dialkyl peroxide, peroxide, (1,1,4,4-tetramethyl-1,4-butanediyl)bis[(1,1-dimethylethyl) (CAS RN 78-63-7), was nearly completely degraded after 8 weeks in a semi-continuous activated sludge (SCAS) test (OPPSD 2008). In a ready-biodegradation test (OECD Guideline 301C), this chemical, however, showed only 4% ultimate biodegradation over 28 days (NITE 2002).
In a risk assessment of tertiary butyl hydroperoxide (CAS RN 75-91-2), a hydroperoxide, the Netherlands Chemical Substances Bureau reported that this substance was not appreciably degraded in abiotic degradation tests. In these tests, half-lives for primary degradation ranged from 170 to 6900 days in 10-day tests in ultra-pure water and from 36 to 45 days in 10-day tests with sterilized sludge (Chemical Substances Bureau 2004). The substance was not readily biodegradable in the modified Sturm test or the closed bottle test, both of which measure ultimate degradation, but the substance was biodegraded in 1-hour activated sludge tests, with primary degradation half-lives of 18–24 minutes (Chemical Substances Bureau 2004). These results show that this hydroperoxide does not undergo hydrolysis and that it has a strong tendency to sorb to organic matter. The results also show that this peroxide can undergo primary degradation within minutes. However, it is resistant to ultimate degradation. It should be noted that in hydroperoxides, the peroxide bond is at the end of the molecule, where it is more accessible to attack than in diperoxyketal peroxides, where the peroxide bond is closer to the centre of the molecule.
Other types of studies were available to assess biodegradation which suggest that DBTMC is not persistent. Firstly, in an in vitro metabolism study using a trout liver S9 enzyme fraction (OPPSD 2008), DBTMC was metabolised rapidly under conditions of incubation and it also degraded rapidly in controls in which the S9 enzyme fraction was denatured. Two identical studies conducted with other organic peroxides (CAS RN 1068-27-5; peroxide, (1,1,4,4-tetramethyl-2-butyne-1,4-diyl)bis[(1,1-dimethylethyl)peroxide] and CAS RN 78-63-7; peroxide, (1,1,4,4-tetramethyl-1,4-butanediyl)bis[(1,1-dimethylethyl)) also found that these substances were degraded quickly (OPPSD 2008). More precisely, the reported half-life in the controls in one of these studies was 1.89 hours. The results of the metabolism studies indicate that DBTMC, as well as other organic peroxides, may undergo both biotic and abiotic degradation reactions quickly in the environment, and therefore would not be persistent.
Secondly, in two laboratory toxicity tests conducted with a dialkyl peroxide (CAS RN 1068-27-5), the measured concentration of this substance in water decreased from 3.76 mg/L to < 0.081 mg/L after 72 hours, and from 5.31 mg/L to 0.375 mg/L after 48 hours (Study Submission 2006b and 2006c). Considering the breakdown of this substance in one of the metabolism studies cited above, this disappearance may have partly resulted from the degradation of the substance.
Although experimental data on the degradation of DBTMC and analogue substances are available, QSARs were also applied using degradation models. Modelling indicates that DBTMC would be persistent in water and sediment. However, the modeled values are considered to be of lower reliability as no chemicals of structural comparability to DBTMC are contained in their training sets. Indeed, these fragment-based models do not consider the peroxide bond, which can be reactive in some substances. Given that experimental data are available and given that the modeled values are of lower reliability, the latter are given a very low weight in the assessment of the environmental persistence of DBTMC.
The potential for persistence of DBTMC in sediment is of particular concern since this substance would partition mainly to this environmental compartment should it be released to surface water (Table 4). Information submitted to Environment Canada states that the reactivity of organic peroxides in the presence of metals such as iron and manganese should prevent their accumulation in soils and sediments (Challenge Submission 2008). These metals are indeed abundant in these matrices.
Different lines of evidence were presented above to assess the persistence of DBTMC, should it be released in an aquatic environment. Based on these lines of evidence, it is concluded that DBTMC does not meet the persistence criteria for water (half-life ≥ 182 days) or sediments (half-life ≥ 365 days) as set out in the Persistence and Bioaccumulation Regulations (Canada 2000).
Bioaccumulation
The experimental bioconcentration factor (BCF) values in fish (Table 5a) are reported to range from 3500 to 13 200 L/kg (NITE 2002; see Appendix), indicating that DBTMC has the potential to bioconcentrate in the environment. In this study, fish were exposed under flow-through conditions for 8 weeks. Test water sampling and analyses were done twice a week and test fish sampling and analyses were done every two weeks. Fish were not fed on the days of fish sampling. Information from the NITE database submitted by industry (OPPSD 2008) showed BCFs at 8 weeks to range from 4960 to 6510. These values may be considered steady-state BCFs. OPPSD (2008) points out a potential error in the calculation of the BCFs in this study. Reported values were apparently corrected for analytical recovery efficiency, but this approach may not have taken into consideration degradation of DBTMC. OPPSD (2008) conducted an analytical recovery study with spiked samples of fish homogenate and found that the organic peroxide was rapidly breaking down and therefore not available to be recovered. In the absence of detailed information about how the recovery efficiency was conducted in the NITE study it is impossible to judge whether the resulting BCF values are accurate or if they are overstated, which would be the case if degradation was not taken into account when calculating the correction factor (OPPSD 2008). If the correction factor is removed, the steady-state BCFs would range from 3750 to 4922 (OPPSD 2008). However, for calculation of the metabolism potential the uncertainty in the BCF data are taken into account using the Arnot et al. (2008) method.
Table 5a. Empirical bioaccumulation values
| Test organism | Concentration tested (mg/L) | Endpoint | Value wet wt | Reference |
|---|---|---|---|---|
| Cyprinus carpio (Common carp) | 0.2 | BCF | 3500-9860 L/kg | NITE database (2002) |
| Cyprinus carpio (Common carp) | 0.02 | BCF | 4960-13 200 L/kg | NITE database (2002) |
The steady-state BCF values from the NITE database were used to derive an in vivo-based metabolic rate constant (kM) according to the method of Arnot et al. (2008). In this method, km is derived according to the following equation:
kM = (k1φ/BCF) - (k2 + kE + kG) (1)
where:
kM = the metabolic rate constant (1/days)
k1 = the uptake rate constant (Arnot and Gobas 2003)
φ = fraction of freely dissolved chemical in water (Arnot and Gobas 2003)
BCF = the available empirical bioconcentration factor
k2 = the elimination rate constant (Arnot and Gobas 2003)
kE = fecal egestion rate constant (Arnot and Gobas 2003)
kG = growth rate constant (Arnot and Gobas 2003)
The non-corrected BCF (as suggested by OPPSD) was not used here because in effect this would be a double counting of the metabolism potential of DBMTC using this in vivo method. Therefore a geometric mean of the BCFs reported at 8 weeks (steady-state) of 5880 was used for the above calculation.
The method of Arnot et al. (2008) provides for the estimation of confidence factors (CF) for the kM to account for error associated with the in vivo data (i.e., measurement variability, parameter estimation uncertainty and model error and uncertainty with the predicted logKow). A CF of ±13.3 was calculated for the available BCF data.
Because metabolic potential can be related to body weight and temperature (e.g., Hu and Layton 2001, Nichols et al. 2006), the kM was further normalized to 15oC and then corrected for the body weight of the middle trophic level fish in the Arnot-Gobas model (0.184 g). The middle trophic level fish was used to represent overall model output as suggested by the model developer (Arnot pers. comm.) and is most representative of fish weight and size likely to be consumed by an avian or terrestrial piscivore. After normalization routines, the kM ranges from 0.0002 to 0.04.
An in vitro S9 metabolism study was reported by OPPSD (2008). Whole body fish metabolism rate constants, kmet, from this study was derived by OPPPSD using the extrapolation methods of Cowan-Ellsberry et al. (2008). The S9 kmet for arterial and portal blood flow (most realistic) was reported as 0.22 (Table 4 OPPSD 2008). Unlike the procedure of Arnot et al. (2008), estimates for kmet based on in vitro assays do not provide for the calculation of confidence factors. Cowan-Ellsberry et al. (2008) suggests that for acceptance of in vitro methods, understanding of uncertainty of these methods and testing on more types of chemicals should be performed to evaluate the various assumptions used in their approach. Han et al. (2007) also indicate that uncertainty of model parameters should be understood for the hepatocyte method. As no bounds of uncertainty could be directly estimated for the in vitro data, a one order of magnitude error (CF = ±10) was assumed for potential variability and uncertainty in the parameters used to derive the kmet. The S9 and kmet value was also normalized to the weight of the middle trophic level fish in the Arnot and Gobas model. The normalized values for kmet thus ranged from 0.14 to 1.44.
The in vivo and in vitro metabolic rate constants were used to adjust the predicted BCF and BAF values from the Arnot and Gobas model’s default of zero metabolism. The results are presented along with other QSAR estimates in Table 5b.
Table 5b. BAF and BCF predictions for DBTMC using the Arnot-Gobas kinetic model (v1.11)
| kM (1/days) | S9 kmet (1/days) | LogKow Used | Arnot-Gobas BCF | Arnot-Gobas BAF[*] | Half-Life (days) |
|---|---|---|---|---|---|
| 2.16E-04 (CF -13.3) (2.5%) | - | 7.6 | 21093 | 5862361 | 3209 |
| 2.87E-03 (median) | - | 7.6 | 5760 | 2049444 | 242 |
| 0.04 (CF +13.3) (97.5%) | - | 7.6 | 506 | 49370 | 17 |
| - | 1.44E-02 (CF -10) | 7.6 | 1357 | 276963 | 48 |
| - | 1.44E-01 | 7.6 | 143 | 4499 | 5 |
| - | 1.44E+00 (CF +10) | 7.6 | 15 | 61 | 0.5 |
Comparing the metabolic rates constants shows that there is approximately one to two orders of magnitude difference between the median values kM and kmet and at the extremes of the range. BCF values ranged from 15 to 21093 with an average of ~4812 regardless of which method was used for metabolic correction. BAF values ranged from 61 to 5862361 with an average BAF of ~1373400 regardless of metabolic correction used. Half-lives ranged from less than 1 day to years. The geometric mean steady-state BCF reported in the NITE database is 5880 which is in very good agreement with the corrected BCF of 5760 (factor = 1.02) corresponding to a metabolic rate constant of ~0.003. Greatest confidence is associated with the BAF predicted using this metabolic rate correction. The BAF corresponding to the metabolism corrected BCF of 5760 is 2049444. The S9 kmet (no CF) corrected BCF value of 143 is a factor of ~41 lower than the NITE geometric mean value of 5880 and a factor of 31 lower than the geometric mean value of 4445 if the OPPSD non-corrected BCF values are taken.
Table 5c. Additional Modelled data for bioaccumulation
| Test organism | Endpoint | Value wet wt |
Reference |
|---|---|---|---|
| Fish | BCF | 106 000 | ACD 2007 |
| Fish | BCF | 25 119 L/kg | OASIS Forecast 2005 |
| Fish | BCF | 10 965 L/kg | BCFWIN 2000 |
The modeled values in table 5c however are considered to be of lower reliability as no metabolism considerations are taken into account by these models and no chemicals of structural comparability are contained in their training sets.
According to the Persistence and Bioaccumulation Regulations (Canada 2000) measures of BAF are the preferred metric for assessing bioaccumulation potential of substances. This is because BCF does not adequately account for the bioaccumulation potential of substances via the diet, which predominates for substances with logKow > ~4.0 (Arnot and Gobas 2003). No empirical BAF were available for DBTMC consequently BAF was modelled. Kinetic mass-balance modelling was considered to provide the most reliable prediction method for determining the bioaccumulation potential of DBTMC because it allows for metabolism correction and DBTMC is within the logKow domain of the model.
Metabolism corrected BCF and BAF values range from 15 to 21093 and from 61 to 2049444, respectively, depending on the rate of metabolism. Environment Canada has analyzed these values and determined that the most reliable metabolism rate is reached when the metabolism corrected predicted BCF is in close agreement with the empirical BCF. Using this metabolic rate to correct the predicted BAF results in a BAF 2049444. Therefore, based on the available empirical and kinetic-based modelled values corrected for metabolism and considering evidence from both in vivo andin vitro techniques for metabolic potential, DBTMC meets the bioaccumulation criterion (BAF ≥ 5000) as set out in the Persistence and Bioaccumulation Regulations (Canada 2000).
A quantitative evaluation based on exposure and ecological effects was conducted for this substance as part of the weight of evidence evaluation of its potential to cause harm.
First, a predicted environmental concentration (PEC) was determined based on an analysis of exposure pathways. A predicted no-effect concentration (PNEC) was derived by selecting a critical toxicity value (CTV) from the available toxicity data and dividing this value by an assessment factor.
Ecological Exposure Assessment
No empirical data have been found regarding levels of DBTMC in the environment. It was estimated that 0.04% of the quantity used at a polymer manufacturing facility may be released in liquid effluents. A conservative predicted environmental concentration was calculated using the following equation (Environment Canada 2007c):
PEC = [I × L × (1-R) × 1000] / [D × (F + S) × 86 400]
Where:
PEC = Predicted environmental concentration (mg/L)
I = Maximum mass imported into (or manufactured in) an industrial complex linked with a discharge point (45 800 kg/year, OPPSD 2008)
L = Losses by processing (0.0008)
R = Removal rate of the sewage treatment plant (0.92) (based on Simple Treat 3.0 model results)
1000 = Conversion of units (kg/m3 to mg/L)
D = Days of release of the substance from site (250 days/year, OPPSE 2008)
F = Flow of the receiving watercourse (0.65 m3/s) (default value, Environment Canada 2007c)
S = Flow of the effluent from the sewage treatment plant (0.04 m3/s) (default value, Environment Canada 2007c)
86400 = Conversion of units (days to seconds)
Based on this equation, the PEC in receiving waters is 0.00003 mg/L.
Ecological Effects Assessment
Acute experimental data for DBTMC are presented in Table 6. A study conducted by MITI found that the 48-hour LC50 for the fish Oryzias latipes was greater than 500 mg/L. However, given that this value exceeds the estimated water solubility of DBTMC by many orders of magnitude (< 1 mg/L; Table 2), the validity of this result is questioned. In another study submitted to Environment Canada, a 48-hour EC50 of 0.13 mg/L was measured for Daphnia magna, based on nominal test concentrations (Study Submission 2008; see Appendix).
In another study submitted to Environment Canada, a 48-hour EC50 of 0.13 mg/L was measured for Daphnia magna, based on nominal test concentrations (Study Submission 2006d). In this test, a co-solvent was added to increase the substance’s solubility. The co-solvent was also tested and revealed no toxicity.
Table 6. Empirical data for aquatic toxicity
| Test Organism | Type of Test | Endpoint | Value (mg/L) | Reference |
|---|---|---|---|---|
| Oryzias latipes (Medaka fish) | Acute | LC50 | >500 | Study Submission 2008 |
| Daphnia magna (Water flea) |
Acute | EC50 | 0.13 | Study Submission 2006d |
EC50 - Concentration effecting 50% of the test population
A range of aquatic toxicity predictions (0.006 to 0.440 mg/L) were also obtained from various QSAR models. However, the modelled values are considered of low reliability as no chemicals of structural comparability to DBTMC are contained in their training sets.
In order to help characterize the ecological risk of DBTMC, a predicted no-effects concentration (PNEC) was derived. To do this, a Critical Toxicity Value (CTV) of 0.13 mg/L was chosen based on the test conducted with D. magna. This CTV was then divided by an assessment factor of 100 to account for interspecies and intraspecies variability in sensitivity, to estimate a long-term no-effects concentration from a short-term LC50 and to account for uncertainty in laboratory-to-field extrapolation. It is noted that chronic toxicity levels of this substance may be significantly lower than acute toxicity levels due to bioaccumulation. A PNEC of 0.0013 mg/L was obtained.
Characterization of Ecological Risk
The approach taken in this ecological screening assessment was to examine various supporting information and develop conclusions based on a weight of evidence approach and the precautionary principle as required under Section 76.1 of CEPA 1999. Particular consideration was given to risk quotient analysis, persistence, bioaccumulation, toxicity, sources and fate in the environment.
A mass flow tool was used to estimate the releases of DBTMC to the environment at different stages of its life cycle. The results indicate that DBTMC is mainly lost by transformation during its use in industrial operations. A low proportion is expected to end up in waste disposal sites, while an even lower proportion (0.04%) could end up in sewers. Based on this analysis, DBTMC could reach the environment through effluents from sewage treatment plants. Once released to aquatic ecosystems, DBTMC will partition mainly into sediment, while a minor proportion will stay in the water column. Based on experimental evidence available for DBTMC and for other organic peroxides, this substance has been determined not to be persistent in the environment. However, DBTMC has been determined to be bioaccumulative, based on estimated Bioaccumulation Factors (BAFs). Because it is not expected to persist in water, DBTMC should not bioaccumulate substantially in organisms if it is released in aquatic ecosystems. In addition, its low solubility in water should partly mitigate its potential hazard to aquatic organisms.
A risk quotient analysis (PEC/PNEC), integrating conservative estimated potential exposure with conservative levels for potential adverse toxic effects, was performed for the aquatic environment in Canada. A PEC of 0.00003 mg/L was estimated. A PNEC of 0.0013 mg/L was calculated, as described above. The resulting risk quotient is (PEC/PNEC) = 0.00003/0.0013 = 0.02. This value indicates that pelagic organisms would not likely be at risk should DBTMC be released in aquatic ecosystems.
If DBTMC is released into a water body, it will partition to sediments, where sediment-dwelling organisms would be exposed to the substance. Because no environmental monitoring data or toxicity data specific to sediment-dwelling organisms are available, the equilibrium partitioning approach could be used to calculate a sediment PEC and PNEC based on the aquatic compartment values presented above. The risk quotient (PEC/PNEC) for the sediment compartment would therefore be as the same as that for the aquatic compartment, 0.02. Again, this indicates that benthic organisms would not likely be at risk should DBTMC be released in aquatic ecosystems.
Uncertainties in Evaluation of Ecological Risk
There remains uncertainty about the persistence of DBTMC in air, water, soil and sediments under environmental conditions. While metabolism studies and some biodegradation tests conducted with DBTMC as well as with other types of organoperoxides suggest that these substances can disappear quickly in laboratory tests, more data specific to DBTMC would be needed in order to derive an actual half-life.
There is some uncertainty about the potential bioconcentration of DBTMC as only a single bioconcentration study was available with limited detail from the NITE database. There is also uncertainty associated with the estimation of metabolism of DBTMC, in fish as demonstrated by the range of kM and kmet. The uncertainty bounds were, however, used to determine the most reliable rate of metabolism for correction of BAF predictions for conclusion of bioaccumulation potential.
Based on the information presented in this screening assessment, it is concluded that DBTMC is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends. Similarly, it is concluded that DBTMC meet the criterion for bioaccumulation but not for persistence as set out in the Persistence and Bioaccumulation Regulations (Canada 2000).
Therefore it is concluded that DBTMC does not meet the definition of toxic as set out in paragraph 64(a) of the Canadian Environmental Protection Act, 1999.
[ACD] Advanced Chemistry Development. 2007. Calculated values using Advanced Chemistry Development (ACD/Labs) Software V9.04 for Solaris (© 1994–2007, presented in SciFinder database, searched July 25, 2007.
Acros Organics N.V. Material Safety Data Sheet. 1,1-Di-(tert-butylperoxy)-3,3,5-trimethylcyclohexane, 75% solution in aromatic free mineral spirit. 10/5/2004. MSDSonline
[AIES] Artificial Intelligence Expert System. 2003-2005. v 1.25. Ottawa (ON): Environment Canada. Model developed by Stefan Niculescu. Available from: Environment Canada, Existing Substances Division, New Substances Division, Ottawa, K1A 0H3.
Arkema. Products in Everyday Life. Accessed December 14, 2006. Terrain d'entente
Arnot JA, Gobas FAPC. 2003. A generic QSAR for assessing the bioaccumulation potential of organic chemicals in aquatic food webs. QSAR Comb. Sci. 22(3): 337–345.
Arnot JA, Mackay D, Bonnell M. 2008. Estimating metabolic biotransformation rates in fish from laboratory data. Environ. Toxicol. Chem. 27(2): 341–351.
ATOFINA. 2001. Organic Peroxides. Product Bulletin. Peroxyketals. ATOFINA Chemicals, Inc., Philadelphia, PA. 7 pp.
[BCFWIN] BioConcentration Factor Program for Windows [Estimation Model]. 2000. Version 2.15. Washington (DC): U.S. Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Brooke DN, Crookes MJ. 2007. Emission scenario document on transport and storage of chemicals [Internet]. Bristol (UK): Environment Agency. Product code:SCHO0407BMLK-E-P [cited 2008 Feb 26].
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C., 1999, c. 33, part #, s. #. Canada Gazette Part III, Vol. 22, no. 3.
Canada. 2000. Canadian Environmental Protection Act: Persistence and Bioaccumulation Regulations, P.C. 2000-348, 23 March, 2000, SOR/2000-107, Canada Gazette Part II, Vol. 134, no. 7.
Canada, Dept. of the Environment, Dept. of Health. 2006. Canadian Environmental Protection Act, 1999: Notice of intent to develop and implement measures to assess and manage the risks posed by certain substances to the health of Canadians and their environment. Canada Gazette Part I, Vol. 140, no. 49.
Canada, Dept. of the Environment, Dept. of Health. 2007.Canadian Environmental Protection Act, 1999: Notice of first release of technical information relevant to substances identified in the Challenge. Canada Gazette Part I, Vol. 141, no. 5.
Challenge Submission. 2008. Confidential communication for DMBP, CAS RN 1068-27-5 submitted to Environment Canada, Existing Substances Division under the Chemical Management Plan Challenge initiative. Received on August 2, 2007.
Chemical Substances Bureau. 2004. Risk Assessment. Tertiary Butyl Hyrdoperoxide (TBHP). Final draft of 9 April 2004. R319_0404_env. Chemical Substance Bureau. Bilthoven, The Netherlands. 70 pp.
ChemInfo Services Inc. Use of Initiators in the Canadian Polymer Resin Manufacturing and Polymer Resin Processing Sectors. March 2002. Draft Report. Toronto: Cheminfo Services Inc. 62 p. Submitted to Environment Canada, National Office of Pollution Prevention. Available on request.
Cowan-Ellsberry CE, Dyer SD, Erhardt S, Bernhard MJ, Roe AL, Dowty ME, Weisbrod AV. 2008. Approach for extrapolating in vitro metabolism data to refine bioconcentration factor estimates. Chemosphere 70:1804-1817.
[ECOSAR] Ecological Structural Activity Relationships [Estimation Model]. 2004. Version 0.99h. Washington (DC): U.S. Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Environment Canada. 2007a. Data for Batch 1 substances collected undertheCanadian Environmental Protection Act, 1999, Section71:Notice with respect to certain substances identified in the Challenge, published in the December 9, 2006 Notice of intent to develop and implement measures to assess and manage the risks posed by certain substances to the health of Canadians and their environment. Data prepared by: Environment Canada, Existing Substances Program.
Environment Canada. 2007b. Assumptions, limitations and uncertainties of the Mass Flow Tool for (3,3,5-trimethylcyclohexylidene)bis[(1,1-dimethylethyl)peroxide] (DBTMC), CAS RN 6731-36-8. Existing Substances Division, Environment Canada, Gatineau (QC). Internal Draft Document, Gatineau (QC): Environment Canada, Existing Substances Division.
Environment Canada. 2007c. Guidance for Conducting Ecological Assessments under CEPA 1999. Science Resource Technical Series. Technical Guidance Module. The Industrial Generic Exposure Tool – Aquatic (IGETA). Working document, Gatineau (QC): Environment Canada, Existing Substances Division.
[EPIWIN] Estimation Programs Interface for Microsoft Windows [Estimation Model]. 2004. Version 3.12. Washington (DC): U.S. Environmental Protection Agencyy, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Han X, Nabb DL, Mingoia RT, Yang C-H. 2007. Determination of xenobiotic intrinsic clearance in freshly isolated hepatocytes from rainbow trout (Oncorhynchus mykiss) and rat and its application in bioaccumulation assessment. Environ. Sci. Technol. 41: 3269 -3276.
[HENRYWIN] Henry’s Law Constant Program for Microsoft Windows [Estimation Model]. 2000. Version 3.10. Washington (DC): U.S. Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Hu, T-M., and W.L. Layton. 2001. Allometric scaling of xenobiotic clearance: uncertainty versus universality. AAPSPharmSci. 3(4) Article 29.
[KOWWIN] Octanol-Water Partition Coefficient Program for Microsoft Windows [Estimation Model]. 2000. Version 1.67. Washington (DC): U.S. Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Morioka I,. Yamada K. 1993. Manufacture of high-molecular-weight styrene polymers. Jpn. Kokai Tokkyo Koho, 5 pp. CODEN: JKXXAF JP 05178914 A 19930720 Heisei. Patent written in Japanese. Application: JP 91-345988 19911227. Priority: CAN 120:271468 AN 1994:271468. Cited in SciFinder 2007.
[MPBPWIN] Melting Point Boiling Point Program for Microsoft Windows [Estimation Model]. 2000. Version 1.41. Washington (DC): U.S. Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
[NCI] National Chemical Inventories database. 2007. American Chemical Society, Chemical Abstract Service, accessed April 2007.
Nichols JW, Fitzsimmons PN, Burkhard LP. 2007. In vitro – in vivo extrapolation of quantitative hepatic biotransformation data for fish. II. Modeled effects on chemical bioaccumulation. Environ. Toxicol. Chem. 26: 1304-1319.
[NITE] National Institute of Technology and Evaluation, Japan [Database]. 2002. Biodegradation and Bioconcentration of the Existing Chemical Substances under the Chemical Substances Control Law. (accessed October 30, 2006).
[OASIS Forecast] Optimized Approach Based on Structural Indices Set [Estimation Model]. 2005. Version 1.20. Bourgas, Bulgaria: Laboratory of Mathematical Chemistry.
[OECD] Organisation for Economic Co-operation and Development. 2004. Emission Scenario Document on Plastics Additives [Internet]. Paris (FR): OECD Environmental Directorate, Environmental Health and Safety Division.
[OPPSD] Organic Peroxide Producers Safety Division, The Society of the Plastics Industry. 2008.. Submittal for the Environment Canada Industry Challenge Program: Comments on the Draft Screening Assessments for the Three Organic Peroxides CAS Numbers 78-63-7, 1068-27-5, and 6731-36-8 to the Executive Director, Existing Substances Division, Environment Canada. March 18, 2008. 49 pp.
[PCKOCWIN] Organic Carbon Partition Coefficient Program for Windows [Estimation Model]. 2000. Version 1.66. Washington (DC):U.S. Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. [cited yr mon date].
[SPIN] Substances in Preparations in Nordic Countries [database on the Internet]. 2006. Copenhagen (DK): Nordic Council of Ministers. [cited 2006 Mar].
Study Submission. 2006a. Unpublished confidential study submitted to Environment Canada, Existing Substances Division. Robust Study Summary, Identification No.: 11142Submission004, available on request.
Study Submission. 2006b. Unpublished confidential study submitted to Environment Canada, Existing Substances Division. Robust Study Summary, Identification No.: 11142Submission001, available on request.
Study Submission. 2006c. Unpublished confidential study submitted to Environment Canada, Existing Substances Division. Robust Study Summary, Identification No.: 11142Submission002, available on request.
Study Submission. 2006d. Unpublished confidential study submitted to Environment Canada, Existing Substances Division. Robust Study Summary, Identification No.: 11142Submission003, available on request.
Study Submission. 2008. Unpublished study submitted to Environment Canada, Existing Substances Division under the Chemical Management Plan Challenge initiative. Robust Study Summary, Identification No.: 22875Submission001. [see Appendix].
[U.S.EPA] United States Environmental Protection Agency. 2002. Toxic Substances Control Act-Inventory Update Rule (TSCA-IUR). Production Volume Information. Unpublished data, 1986, 1990, 1994, 1998, 2002. Available on request.
[WSKOWWIN] Water Solubility for Organic Compounds Program for Microsoft Windows [Estimation Model]. 2000. Version 1.41 Washington (DC): U.S. Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
| No | Item | Weight | Yes/No | Specify | |
|---|---|---|---|---|---|
| 1 | Reference: [NITE] National Institute of Technology and Evaluation, Japan [Database]. 2002. Biodegradation and Bioconcentration of the Existing Chemical Substances under the Chemical Substances Control Law. (accessed October 30, 2006). | ||||
| 2 | Substance identity: CAS RN | n/a | 6731-36-8 | ||
| 3 | Substance identity: chemical name(s) | n/a | (3,3,5-Trimethylcyclohexylidene)bis[(1,1-dimethylethyl)peroxide] | ||
| 4 | Chemical composition of the substance | 2 | |||
| 5 | Chemical purity | 1 | Y | 97.8 | |
| 6 | Persistence/stability of test substance in aquatic solution reported? | 1 | Y | ||
| 7 | If test material is radiolabelled, were precise position(s) of the labelled atom(s) and the percentage of radioactivity associated with impurities reported? | 2 | |||
| Method | |||||
| 8 | Reference | 1 | Y | ||
| 9 | OECD, EU, national, or other standard method? | 3 | Y | Japanese method | |
| 10 | Justification of the method/protocol if not a standard method was used | 2 | |||
| 11 | GLP (Good Laboratory Practice) | 3 | |||
| Test organism | |||||
| 12 | Organism identity: name | n/a | Carp, Cyprinus carpio | ||
| 13 | Latin or both Latin & common names reported? | 1 | Y | ||
| 14 | Life cycle age / stage of test organism | 1 | N | ||
| 15 | Length and/or weight | 1 | Y | ||
| 16 | Sex | 1 | N | ||
| 17 | Number of organisms per replicate | 1 | Y | 19 | |
| 18 | Organism loading rate | 1 | Y | 5 g/L | |
| 19 | Food type and feeding periods during the acclimation period | 1 | Y | ||
| Test design / conditions | |||||
| 20 | Experiment type (laboratory or field) | n/a | Laboratory | ||
| 21 | Exposure pathways (food, water, both) | n/a | Water | ||
| 22 | Exposure duration | n/a | 8 weeks | ||
| 23 | Number of replicates (including controls) | 1 | Y | 16 | |
| 24 | Concentrations | 1 | Y | 0.2 and 0.02 mg/L | |
| 25 | Food type/composition and feeding periods during the test | 1 | Y | ||
| 26 | If BCF/BAF derived as a ratio of chemical concentration in the organism and in water, was experiment duration equal to or longer than the time required for the chemical concentrations to reach steady state? | 3 | Y | Ratio of concentration | |
| 27 | If BCF/BAF derived as a ratio of chemical concentration in the organism and in water, were measured concentrations in both water and organism reported? | 3 | Y | ||
| 28 | Were concentrations in the test water measured periodically? | 1 | Y | ||
| 29 | Were the exposure media conditions relevant to the particular chemical reported? (e.g., for the metal toxicity - pH, DOC/TOC, water hardness, temperature) | 3 | Y | ||
| 30 | Photoperiod and light intensity | 1 | N | ||
| 31 | Stock and test solution preparation | 1 | Y | ||
| 32 | Analytical monitoring intervals | 1 | Y | ||
| 33 | Statistical methods used | 1 | N | ||
| 34 | Was solubilizer/emulsifier used, if the chemical was unstable or poorly soluble? | n/a | N | ||
| Information relevant to the data quality | |||||
| 35 | Was the test organism relevant to the Canadian environment? | 3 | Y | ||
| 36 | Were the test conditions (pH, temperature, DO, etc.) typical for the test organism? | 1 | Y | ||
| 37 | Does system type and design (static, semi-static, flow-through; sealed or open; etc.) correspond to the substance's properties and organism's nature/habits? | 2 | Y | ||
| 38 | Was pH of the test water within the range typical for the Canadian environment (6 to 9)? | 1 | Y | ||
| 39 | Was temperature of the test water within the range typical for the Canadian environment (5 to 27°C)? | 1 | Y | ||
| 40 | Was lipid content (or lipid-normalized BAF/BCF) reported? | 2 | Y | ||
| 41 | Were measured concentrations of a chemical in the test water below the chemical's water solubility? | 3 | Y | ||
| 42 | If radiolabelled test substance was used, was BCF determination based on the parent compound (i.e. not on total radiolabelled residues)? | 3 | |||
| Results | |||||
| 43 | Endpoints (BAF, BCF) and values | n/a | n/a | BCFs = 3500 - 13200 L/kg | |
| 44 | BAF or BCF determined as: 1) the ratio of chemical concentration in the organism and in water, or 2) the ratio of the chemical uptake and elimination rate constants | n/a | n/a | Ratio of concentration | |
| 45 | Whether BAF/BCF was derived from a 1) tissue sample or 2) whole organism? | n/a | n/a | Whole organism | |
| 46 | Whether 1) average or 2) maximum BAF/BCF was used? | n/a | n/a | Values for 2-, 4-, 6- and 8-weeks reported | |
| 47 | Score: ... % | 90.7 | |||
| 48 | EC Reliability code: | 1 | |||
| 49 | Reliability category (high, satisfactory, low): | High Confidence | |||
| 50 | Comments | ||||
| No | Item | Weight | Yes/No | Specify | |
|---|---|---|---|---|---|
| 1 | Reference: Study Submission. 2008. Unpublished study submitted to Environment Canada, Existing Substances Division under the Chemical Management Plan Challenge initiative. Robust Study Summary, Identification No.: 22875Submission001. | ||||
| 2 | Substance identity: CAS RN | n/a | 6731-36-8 | ||
| 3 | Substance identity: chemical name(s) | n/a | (3,3,5-Trimethylcyclohexylidene)bis[(1,1-dimethylethyl)peroxide] | ||
| 4 | Chemical composition of the substance | 2 | |||
| 5 | Chemical purity | 1 | Y | 97.80 % | |
| 6 | Persistence/stability of test substance in aquatic solution reported? | 1 | Y | ||
| Method | |||||
| 7 | Reference | 1 | Y | ||
| 8 | OECD, EU, national, or other standard method? | 3 | Y | Japanese test method | |
| 9 | Justification of the method/protocol if not a standard method was used | 2 | |||
| 10 | GLP (Good Laboratory Practice) | 3 | |||
| Test organism | |||||
| 11 | Organism identity: name | n/a | Himedaka, Oryzias latipes | ||
| 12 | Latin or both Latin & common names reported? | 1 | Y | ||
| 13 | Life cycle age / stage of test organism | 1 | |||
| 14 | Length and/or weight | 1 | Y | ||
| 15 | Sex | 1 | N | ||
| 16 | Number of organisms per replicate | 1 | Y | 10 | |
| 17 | Organism loading rate | 1 | Y | 0.75 g/L | |
| 18 | Food type and feeding periods during the acclimation period | 1 | N | ||
| Test design / conditions | |||||
| 19 | Test type (acute or chronic) | n/a | Acute | ||
| 20 | Experiment type (laboratory or field) | n/a | Laboratory | ||
| 21 | Exposure pathways (food, water, both) | n/a | Water | ||
| 22 | Exposure duration | n/a | 48 hours | ||
| 23 | Negative or positive controls (specify) | 1 | N | ||
| 24 | Number of replicates (including controls) | 1 | N | ||
| 25 | Nominal concentrations reported? | 1 | N | ||
| 26 | Measured concentrations reported? | 3 | N | ||
| 27 | Food type and feeding periods during the long-term tests | 3 | Y | ||
| 28 | Were concentrations measured periodically (especially in the chronic test)? | 1 | N | ||
| 29 | Were the exposure media conditions relevant to the particular chemical reported? (e.g., for the metal toxicity - pH, DOC/TOC, water hardness, temperature) | 3 | Y | ||
| 30 | Photoperiod and light intensity | 1 | N | ||
| 31 | Stock and test solution preparation | 1 | Y | ||
| 32 | Was solubilizer/emulsifier used, if the chemical was poorly soluble or unstable? | 1 | Y | Hydrogenated castor oil | |
| 33 | If solubilizer/emulsifier was used, was its concentration reported? | 1 | Y | ||
| 34 | If solubilizer/emulsifier was used, was its ecotoxicity reported? | 1 | N | ||
| 35 | Analytical monitoring intervals | 1 | N | ||
| 36 | Statistical methods used | 1 | N | ||
| Information relevant to the data quality | |||||
| 37 | Was the endpoint directly caused by the chemical's toxicity, not by organism’s health (e.g. when mortality in the control >10%) or physical effects (e.g. 'shading effect')? | n/a | Y | ||
| 38 | Was the test organism relevant to the Canadian environment? | 3 | Y | ||
| 39 | Were the test conditions (pH, temperature, DO, etc.) typical for the test organism? | 1 | Y | ||
| 40 | Does system type and design (static, semi-static, flow-through; sealed or open; etc.) correspond to the substance's properties and organism's nature/habits? | 2 | Y | ||
| 41 | Was pH of the test water within the range typical for the Canadian environment (6 to 9)? | 1 | Y | ||
| 42 | Was temperature of the test water within the range typical for the Canadian environment (5 to 27°C)? | 1 | Y | ||
| 43 | Was toxicity value below the chemical’s water solubility? | 3 | N | ||
| Results | |||||
| 44 | Toxicity values (specify endpoint and value) | n/a | n/a | 48-h LC50 => 500 mg/L | |
| 45 | Other endpoints reported - e.g. BCF/BAF, LOEC/NOEC (specify)? | n/a | N | ||
| 46 | Other adverse effects (e.g. carcinogenicity, mutagenicity) reported? | n/a | N | ||
| 47 | Score: ... % | 58.5 | |||
| 48 | EC Reliability code: | 3 | |||
| 49 | Reliability category (high, satisfactory, low): | Low Confidence | |||
| 50 | Comments | ||||
| Item | Yes | No |
|---|---|---|
| Reference: Study Submission. 2006. Unpublished confidential study submitted to Environment Canada, Existing Substances Division. Robust Study Summary, Identification No.: 11142Submission003. |
||
| Test Substance: CAS # and name: 6731-36-8 1,1-Di-(t-butylperoxy)-3,3,5-trimethylcyclohexane | ||
| * Substance purity reported?technical product | x | |
| Persistence/stability of test substance in aquatic solution reported? presumed to be stable at test & storage condition. | x | |
| Method | ||
| References (Y/N) | ||
| *OECD, EU, national, or other standard method? | x | |
| If not a standard method, justification of the method/protocol provided? | ||
| *GLP (Good Laboratory Practice) | x | |
| Test organisms (specify common and/or Latin names) daphnia magna (water flea) | ||
| Latin or both Latin and common names reported? | x | |
| Life cycle age / stage of test organism less than 24h old | x | |
| Sex | N/A | |
| Length and/or weight of test organisms | N/A | |
| Number of test organisms per replicate? 5 | x | |
| Food type / feeding periods (acclimation/during test) ? no | x | |
| Test design / conditions | ||
| Test type – acute or chronic: acute | ||
| Experiment type (laboratory or field) specified? lab. | x | |
| System type (static, semi-static, flow-through) static | x | |
| Negative or positive controls? negative & contain co-solvent | x | |
| Number of replicates (including controls) and concentrations:) 4+2 | x | |
| Exposure pathways (food, water, both): water | x | |
| Exposure duration: 48 h | x | |
| *Measured concentrations reported? | x | |
| Exposure media conditions (temperature, pH, electrical conductivity, hardness, TOC, DOC, DO, major cations and anions; other) | x | |
| Was pH within 6-9 range? 8.0-8.1 | x | |
| Was temperature within 5-28 °C range? 19.5 - 20.2 °C | x | |
| Photoperiod and light intensity:16 h ambient light/day | x | |
| Stock and test solution preparation | x | |
| Information on emulsifiers used for poorly soluble / unstable substances Tween 80 was added as co-solvent | x | |
| Analytical monitoring intervals | x | |
| Statistical methods used | x | |
| Results | ||
| Toxicity endpoints / values / units : 48h EC50 = 0.133 mg/L | ||
| Other endpoints reported (e.g. BCF/BAF, LOEC/NOEC, etc.) (specify, but do not assess this item): NOEC < 0.1 mg/L, LOEC = 0.1 mg/L, LC100 = 0.48 mg/L *Was toxicity value below the chemical’s water solubility? WS = mg/L |
||
| Other adverse effects | N/A | |
| Score: | major items – 4/5; overall score: 19/22= 86 % | |
| EC Reliability code: | 1 | |
| Reliability category (high, satisfactory, low): | high | |
| Comments: | The WS and iT value are within 1 magnitude. A co-solvent was added to increase the substance’s solubility. The co-solvent was tested and revealed no toxicity. | |