Screening Assessment for the Challenge
This page has been archived on the Web
Information identified as archived is provided for reference, research or recordkeeping purposes. It is not subject to the Government of Canada Web Standards and has not been altered or updated since it was archived. Please contact us to request a format other than those available.
Archived
Methanesulfonamide, N-[2-[(2,6-dicyano-4-methylphenyl)azo]-5-(dipropylamino)phenyl]
(DADM)
Chemical Abstracts Service Registry Number
72968-82-2
Environment Canada
Health Canada
March 2010
- Synopsis
- Introduction
- Substance Identity
- Identification of Analogue Substances and Estimation of Physical and Chemical Properties
- Sources
- Uses
- Releases to the Environment
- Environmental Fate
- Persistence and Bioaccumulation Potential
- Potential to Cause Ecological Harm
- Potential to Cause Harm to Human Health
- Conclusions
- References
- Appendix 1: Robust Study Summary
- Appendix 2: PBT Model Inputs Summary Table
Pursuant to section 74 of the Canadian Environmental Protection Act, 1999 (CEPA 1999), the Ministers of the Environment and of Health have conducted a screening assessment on mmethanesulfonamide, N-[2-[(2,6-dicyano-4-methylphenyl)azo]-5-(dipropylamino)phenyl] (DADM), Chemical Abstracts Service Registry Number 7296882-2. This substance was identified as a high priority for screening assessment and included in the Challenge because it had been found to meet the ecological categorization criteria for persistence, bioaccumulation and inherent toxicity to non-human organisms and was believed to be in commerce in Canada .
The substance DADM was not considered to be a high priority for assessment of potential risks to human health, based upon application of the simple exposure and hazard tools developed by Health Canada for categorization of substances on the Domestic Substances List.
The substance was previously used in Canada as a dye and a printing ink based on use codes from the 1986 Domestic Substances List (DSL). It is not naturally produced in the environment. No companies reported manufacturing, importing or using this substance in Canada above the reporting thresholds in 2006, however four companies did have a stakeholder interest in the substance. In the assessment, the reporting threshold for manufacture and import of 100 kg was used to build scenarios and capture the highest potential quantity of DADM in use in Canada .
Based on reported use patterns and certain assumptions, most of DADM would be expected to end up in solid waste disposal sites, and a significant proportion may be released to sewer water (17.1%). The substance is not expected to be soluble in water; instead, it is expected to partition to particles because of its hydrophobic nature. For these reasons, after release to water, DADM would likely end up mostly in sediments. It is not expected to be volatile, hence it is neither significantly present in air nor subject to long-range atmospheric transport.
Based on prediction of its physical and chemical properties, DADM is expected to degrade slowly under aerobic conditions in the environment (in water, sediment and soil). Because of lack of experimental data relating to bioaccumulation potential, new data for an analogue of the substance were used in the assessment. This resulted in the prediction that the substance has a low potential for bioaccumulation in the environment. DADM is therefore concluded to meet the persistence criteria but does not meet the bioaccumulation criterion as set out in the Persistence and Bioaccumulation Regulations. In addition, experimental toxicity data for DADM and chemical analogues suggest that the substance has a low to moderate potential to cause acute harm to aquatic organisms.
For this screening assessment, two very conservative exposure scenarios were selected representing industrial and consumer use-related releases to the aquatic environment. The first scenario simulated discharge of DADM to the aquatic environment following use of the dye by an industrial operation. The second scenario simulated the release of DADM to the aquatic environment due to consumer use (in this case, washing of dyed clothing). The predicted environmental concentrations ( PECs) in water were well below the predicted no-effect concentrations (PNECs) calculated for sensitive aquatic species.
Although the potential hazard of DADM is recognized, on the basis of information which indicates that the substance is not manufactured in or imported into Canada in amounts above the reporting threshold, the likelihood of exposure in Canada is considered to be low; hence the risk to human health is likewise considered to be low.
Based on the information available, it is concluded that DADM does not meet any of the criteria set out in section 64 of the Canadian Environmental Protection Act, 1999.
Because this substance is listed on the Domestic Substances List, its import and manufacture in Canada are not subject to notification under subsection 81(1) of CEPA 1999. Given the potential hazardous properties of this substance, there is concern that new activities that have not been identified or assessed could lead to this substance meeting the criteria set out in section 64 of the Act. Therefore, it is recommended that the Domestic Substances List be amended, under subsection 87(3) of the Act, to indicate that subsection 81(3) of the Act applies with respect to this substance so that new manufacture, import or use of this substance is notified and undergoes ecological and human health risk assessments.
In addition and where relevant, research and monitoring will support verification of assumptions used during the screening assessments.
The Canadian Environmental Protection Act, 1999(CEPA 1999) (Canada 1999) requires the Minister of the Environment and the Minister of Health to conduct screening assessments of substances that have met the categorization criteria set out in the Act to determine whether these substances present or may present a risk to the environment or human health.
Based on the information obtained through the categorization process, the Ministers identified a number of substances as high priorities for action. These include substances that
- met all of the ecological categorization criteria, including persistence (P), bioaccumulation potential (B) and inherent toxicity to aquatic organisms (iT), and were believed to be in commerce in Canada ; and/or
- met the categorization criteria for greatest potential for exposure (GPE) or presented an intermediate potential for exposure (IPE), and had been identified as posing a high hazard to human health based on classifications by other national or international agencies for carcinogenicity, genotoxicity, developmental toxicity or reproductive toxicity.
The Ministers therefore published a notice of intent in the Canada Gazette, Part I, on December 9, 2006 (Canada 2006a), that challenged industry and other interested stakeholders to submit, within specified timelines, specific information that may be used to inform risk assessment, and to develop and benchmark best practices for the risk management and product stewardship of those substances identified as high priorities.
The substance methanesulfonamide, N-[2-[(2,6-dicyano-4-methylphenyl)azo]-5-(dipropylamino)phenyl] (DADM) was identified as a high priority for assessment of ecological risk as it had been found to be persistent, bioaccumulative and inherently toxic to aquatic organisms and was believed to be in commerce in Canada.
The Challenge for this substance was published in the Canada Gazette on August 30, 2008 (Canada 2008). A substance profile was released at the same time. The substance profile presented the technical information available prior to December 2005 that formed the basis for categorization of this substance. As a result of the Challenge, submissions of information pertaining to the toxicity of the substance were received.
The substance DADM was not considered to be a high priority for assessment of potential risks to human health, based upon application of the simple exposure and hazard tools developed by Health Canada for categorization of substances on the Domestic Substances List. Therefore this assessment focuses principally on information relevant to the evaluation of ecological risks.
Screening assessments focus on information critical to determining whether a substance meets the criteria set out in section 64 of CEPA 1999. Screening assessments examine scientific information and develop conclusions by incorporating a weight-of-evidence approach and precaution. This screening assessment includes consideration of information on chemical properties, hazards, uses and exposure, including the additional information submitted under the Challenge. Data relevant to the screening assessment of this substance were identified in original literature, review and assessment documents, stakeholder research reports and from recent literature searches, up to April 2009 for ecological sections and December 2009 for human health sections of the document. Key studies were critically evaluated; modelling results may have been used to reach conclusions. When available and relevant, information presented in hazard assessments from other jurisdictions was considered.
The screening assessment does not represent an exhaustive or critical review of all available data. Rather, it presents the most critical studies and lines of evidence pertinent to the conclusion
This screening assessment was prepared by staff in the Existing Substances Programs at Health Canada and Environment Canada and incorporates input from other programs within these departments. The ecological portion of this assessment has undergone external written science review and consultation. Additionally, the draft of this screening assessment was subject to a 60-day public comment period. While external comments were taken into consideration, the final content and outcome of the screening risk assessment remain the responsibility of Health Canada and Environment Canada.
The critical information and considerations upon which the final assessment is based are summarized below.
For the purposes of this document, this substance will be referred to as DADM, derived from Japanese Existing and New Chemical Substances (ENCS) inventory name.
Table 1. Substance identity for DADM
| Chemical Abstracts Service Registry Number (CASRN) | 72968-82-2 |
| DSL name | Methanesulfonamide, N-[2-[(2,6-dicyano-4-methylphenyl)azo]-5-(dipropylamino)phenyl]- |
| National Chemical Inventories(NCI) names1 | Methanesulfonamide, N-[2-[(2,6-dicyano-4-methylphenyl)azo]-5-(dipropylamino)phenyl]-(TSCA, AICS, PICCS, ASIA-PAC) N-[2-[(2,6-dicyano-p-tolyl)azo]-5-(dipropylamino)phenyl]methanesulphonamide(EINECS, ECL) 2'-[(2,6-Dicyano-4-methylphenyl)azo]-5'-(dipropylamino)methanesulfonanilide(ENCS) Methanesulfonamide,n-[2-[(2,6-dicyano-4-methylphenyl)azo]-5-(diprophylamino)phenyl]-(PICCS) |
| Other names | Benzenamine, N,N-dipropyl-3-[(methylsulfonyl)amino]-4-[(2,6-dicyano-4-methylphenyl)azo]- |
| Chemical group (DSL Stream) | Discrete organics |
| Major chemical class or use | Disperse azo dye |
| Major chemical sub-class | Azo compound, secondary aromatic amine, sulfonamide, benzonitrile, tertiary amine |
| Chemical formula | C22H26N6O2S |
| Chemical structure |  |
| SMILES2 | CS(=O)(=O)Nc1cc(N(CCC)CCC)ccc1N=Nc2c(C#N)cc(C)cc2C#N |
| Molecular mass | 438.55 g/mol |
| 1 National Chemical Inventories (NCI). 2006: AICS (Australian Inventory of Chemical Substances); ASIA-PAC (Asia-Pacific Substances Lists); ECL (Korean Existing Chemicals List); EINECS (European Inventory of Existing Commercial Chemical Substances); ENCS (Japanese Existing and New Chemical Substances); PICCS (Philippine Inventory of Chemicals and Chemical Substances); and TSCA (US Environmental Protection Agency Toxic Substances Control Act Chemical Substance Inventory). 2 Simplified Molecular Input Line Entry System |
|
Few experimental data are available for DADM.
At the Environment Canada-sponsored Quantitative Structure-Activity Relationship (QSAR) Workshop in 1999 (Environment Canada 2000), Environment Canada and other invited modelling experts identified many structural classes of pigment and dyes as “difficult to model” using QSARs. The physical and chemical properties of many of the structural classes of dyes and pigments are not amenable to model prediction because they are considered “out of the model domain of applicability” (e.g., structural and/or property parameter domains). Therefore, to determine the domain of applicability, Environment Canada reviews the applicability of QSAR models to dyes and pigments on a case-by-case basis.
It is considered inappropriate to use QSAR models to predict most of the physical and chemical properties of DADM. Consequently, analogues were identified and “read-across” data were considered when determining the approximate physical and chemical properties for the substance.
An analogue is a chemical which is structurally similar to the substance under assessment and is therefore expected to have similar physical and chemical properties, similar behaviour in the environment, and/or similar toxicity. Where there are experimental data for a given parameter for an analogue substance, these can be used directly or with adjustment as an estimate of that parameter for the substance under assessment.
To find acceptable analogues, a review of data for several disperse monoazo dyes was performed (Anliker et al. 1981; Anliker and Moser 1987; Baughman and Perenich 1988; ETAD 2005; Brown 1992; Sijm et al. 1999; Safepharm Laboratories Ltd 1990; Sandoz 1975). These monoazo compounds generally have structural similarities to DADM but also share other important attributes that contribute to their fate in the environment such as high molecular weights--generally > 400 g/mol, similar cross-sectional diameters (1.35–1.90 nm), solid particulate structures, decomposition at greater than 120°C (to 270°C), and “dispersibility” in water (i.e., not truly “soluble”). In addition, they have a negligible vapour pressure and are stable under environmental conditions, as they are designed to be so.
To predict environmental fate and effects of DADM, experimental data for monoazo substances in particular - CAS RN 68385-96-6, Disperse Blue 165 (CASRN 41642-51-7), and Disperse Orange 30 (CAS RN 5261-31-4) - were considered in this assessment.
The substance CAS RN 68385-96-6 is extremely similar in chemical structure to DADM, having similar molecular weights and minimum-maximum cross-sectional diameters (see Table 2). These two chemicals contain the same functional groups and differ only by having an ethyl rather than a propyl moiety on the terminal nitrogen. It is anticipated that the two chemicals have very similar ecological toxic potentials; therefore ecotoxicological data for CAS RN 68385-96-6 are used to characterize the toxicity for DADM.
Disperse Blue 165 is also considered an analogue of DADM, with similar chemical structure and a close molecular weight (see Table 2). There is experimental information on the water solubility for Disperse Blue 165, which was used for estimating the water solubility of DADM according to the Experimental Value Adjustment (EVA) method in the WATERNT program of EPISUITE v4.0. The EVA method uses empirical water solubility information for an analogue and adjusts the prediction of parameter for the target chemical (DADM) by comparing the structures of the analogue and the target chemical.
Disperse Orange 30 is a monoazo compound. This chemical contains some different functional groups, which makes it not as structurally close to DADM as the other analogues (see Table 2). However, such differences are not anticipated to produce significantly different environmental behaviours and toxicities due to the similarities between monoazo disperse dyes outlined above. Also, it is anticipated that using read-across data from Disperse Orange 30 may be conservative, as modelling data suggest that due to the structural differences it has a somewhat higher octanol-water partition coefficient than DADM (see Table 3). Utilizing read-across data from a chemical with a higher octanol-water partition coefficient is conservative because this characteristic can be associated with bioaccumulative substances. Therefore, experimental data of Disperse Orange 30 are considered in assessing bioaccumulation and ecological toxicity for DADM.
Table 2. Structural comparison between DADM, the three identified analogues, CAS RN 68385-96-6, Disperse Blue 165 and Disperse Orange 30
| CAS RN (Common name) | Chemical Structure | Molecular Mass (g/mol) | M CSD 1Min-Max Cross-Sectional Diameter (nm) 1 | Available Empirical Data |
|---|---|---|---|---|
| 1 Based on range of maximum diameters (Dmax) for conformers calculated using CPOPs 2008. | ||||
| 72968-82-2 (DADM) |
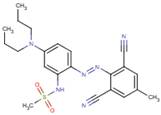 |
438.55 | 1.44–1.90 | Aquatic toxicity |
| 68385-96-6 |  |
410.49 | 1.41–1.77 | Aquatic toxicity |
| 41642-51-7 (Disperse Blue 165) |
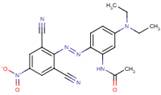 |
405.42 | 1.35–1.82 | Melting point, water solubility, octanol solubility |
| 5261-31-4 (Disperse Orange 30) |
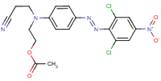 |
450.28 | 1.75–1.98 | Melting point, vapour pressure, log Kow, water solubility, aquatic toxicity |
Data on other physical and chemical properties, however, were lacking for both DADM and its analogues (listed in Table 2); therefore, other azo dye substances with some structural similarity were used to characterize some physical and chemical parameters in a read-across approach. For example, values for a particular parameter (e.g., the vapour pressure) were “read-across” from the data from a series of azo dye substances. These values were summarized in Table 3 and subsequently used for further modeling and lines of evidence in this assessment.
Table 3. Physical and chemical properties of DADM and other azo dyes
| Chemicals | Type1 | Value | Temperature (°C) | Reference |
|---|---|---|---|---|
| 1 The extrapolated values used for DADM are based on available experimental evidence from other dye analogues found in the literature. 2 The phrase “melting point” is used, but this may be better referred to as a decomposition point because dyes are known to char at high temperatures (greater than 200°C) rather than melt. 3 Boiling point is generally not applicable for dyes. For powder dyes, charring or decomposition occurs at high temperatures instead of boiling. For liquids and pastes, boiling will only occur for the solvent component, while the unevaporated solid will decompose or char (ETAD 1995). 4 Solubilities of azo dyes at 25 and 80°C were used by Baughman and Perenich (1988) to calculate Henry’s Law constants for these dyes. These values are presented here as a range to illustrate the expected Henry’s Law constant for DADM. 5 Experimental Value Adjustment (EVA) is an option provided by EPIWIN. The calculation is based on the experimental data of an analogue with justification according to structural difference between the analogue and the subject chemical. 6 Log Koc values are based on calculations by Baughman and Perenich (1988) using a range of measured solubilities for commercial dyes and an assumed melting point of 200°C. |
||||
| Melting point (ºC)2 | ||||
| Disperse Orange 30 | Experimental | 126.9–128.5 | ETAD 2005 | |
| Read-across for azo dyes | Experimental | 117–225 | Anliker and Moser 1987 | |
| Read-across for azo dyes | Experimental | 74–236 | Baughman and Perenich 1988 | |
| Boiling point (ºC)3 – not applicable | ||||
| Vapour pressure (Pa) | ||||
| Read-across for azo dyes | Experimental | 5.33×10-5 to 5.33x×10-12 (4×10-14 to 4×10-7 mm Hg) |
25 | Baughman and Perenich 1988 |
| Henry’s Law constant (Pa·m3/mol) | ||||
| Read-across for azo dyes | Calculated4 | 10-8 to 10-1 (10-13 to 10-6 atm·m3/mol) |
Baughman and Perenich 1988 | |
| Log Kow (Octanol-water partition coefficient) (dimensionless) | ||||
| Disperse Orange 30 | Experimental | 4.2 | Brown 1992 | |
| DADM | Experimental Value Adjustment5 based on the log Kow for Disperse Orange 30 | 3.8 | EPIWIN 2007 | |
| Read-across for azo dyes | Experimental | 1.79–5.07 | Baughman and Perenich 1988 | |
| Read-across for azo disperse dyes | Experimental | >2–5.1 | Anliker et al. 1981; Anliker and Moser 1987 | |
| Log Koc (Organic carbon-water partition coefficient) (dimensionless) | ||||
| Read-across for azo dyes | Calculated6 | 3.4–4.2 | Baughman and Perenich 1988 | |
| Water solubility (mg/L) | ||||
| Disperse Orange 30 | Experimental | 0.07 | Brown 1992 | |
| Disperse Blue 165 | Experimental | 0.0058–1.3 | Sijm et al. 1999 | |
| DADM | Experimental Value Adjustment based on the water solubility for Disperse Blue 165 | 0.00075–0.17 | EPIWIN 2007 | |
| Read-across for azo disperse dyes | Experimental | <0.01 | Anliker and Moser 1987 | |
| Read-across for azo dyes, | Experimental | 1.2×10-5 to 35.5 (4×10-11 to 1.8×10-4 mol/L) |
Baughman and Perenich 1988 | |
| n-octanol solubility (mg/L) | ||||
| Disperse Orange 30 | Experimental | 576 | ETAD 2005 | |
| Disperse Blue 165 | Experimental | 225 | Sijm et al. 1999 | |
| Read-across for azo disperse dyes | Experimental | 81–2430 | 20 | Anliker and Moser 1987 |
| pKa(Acid dissociation constant) (dimensionless) | ||||
| DADM | Modelled | 9.03 (acid form) 1.69 (base form) | ACD/pKaDB 2005 | |
DADM is not reported to be naturally produced in the environment.
The quantity of DADM reported during development of the Domestic Substances List (DSL) to be manufactured, imported or in commerce in Canada during the 1986 calendar year was 10 000–100 000 kg (Environment Canada 1988).
Recent information was collected through an industry survey conducted for the 2005 and 2006 calendar years under the Canada Gazette notices issued pursuant to section 71 of CEPA 1999 (Canada 2006b and Canada 2008). These notices required submission of data on the Canadian manufacture and import of DADM at the reporting threshold (i.e. 100 kg for manufacture/import). In the notice for 2006, data were also required on the use quantity of this substance.
Results of a CEPA section 71 notice for the 2005 calendar year indicated that DADM was not in commerce at the reporting threshold.
Information gathered through a similar CEPA section 71 notice for the 2006 calendar year also indicates that DADM was not manufactured in, imported into or used in Canada in a quantity meeting the prescribed thresholds. Four companies however identified themselves as having a stakeholder interest in this substance.
Given the use of this substance in other countries, it is possible that the substance is entering the Canadian market as a component of manufactured items and consumer products. DADM is commercially available from Lanxess as Bayscript®Special Red T (CBG 2009) and is used for inks, inkjet inks and printing inks (Lanxess ©2007–2009). However, information obtained from the s71 survey and other information sources did not indicate that it was present in these types of products in Canada . Available information is currently not sufficient to derive a quantitative estimate of the importance of this source either. For the purpose of assessing DADM, the reporting threshold of 100 kg was used throughout this screening assessment as a conservative estimate of the potential mass of this substance in use in Canada .
No information on uses was received in response to the CEPA section 71 notices for the 2005 and 2006 calendar years ( Canada 2006b and 2008). The following DSL use code was identified for the substance during the DSL nomination period (1984–1986): 85 – Pigment, Dye and Printing Ink (Environment Canada 1988).
DADM has been identified as used in the “apparel and other textile products” industry sector according to the National Occupational Exposure Survey (NIOSH 2002) in the United States . The United States Environmental Protection Agency listed the substance in a Federal Register notice (US EPA 2002) that targeted substances used under North American Industry Classification System (NAICS) codes 325 and 32411, chemical manufacturing and petroleum refineries, respectively.
DADM has been subject to the United States Toxic Substances Control Act inventory update rule and has been notified at quantities ranging from 10 000 to 500 000 pounds in 1986, 1990, 1994 and 1998 reporting cycles (US EPA 1986–2002). For the last inventory update (2002), DADM did not meet reporting requirements (US EPA 1986–2002).
This chemical was also reported in Sweden and Denmark in the years 2000–2004 (SPIN 2006); however, information on exact use and quantities of DADM in those countries was not open to the public.
Use of this substance other than as stated above has not been identified.
Mass Flow
To estimate potential releases of a substance to the environment at different stages of its life cycle, a Mass Flow Tool was developed (Environment Canada 2008a). Empirical data concerning releases of specific substances to the environment are seldom available. Therefore, for each identified type of use of the substance, the proportion and quantity of release to the different environmental media are estimated, as is the proportion of the substance chemically transformed or sent for waste disposal.
Assumptions and input parameters used in making the release estimates are based on information obtained from a variety of sources including responses to regulatory surveys, Statistics Canada, manufacturers’ websites, technical databases and documents, and professional knowledge and assumptions. Of particular relevance are emission factors, which are generally expressed as the fraction of a substance released to the environment, particularly during its manufacture, processing, and use associated with industrial processes. Sources of such information include emission scenario documents, often developed under the auspices of the Organisation for Economic Co-operation and Development (OECD), and default assumptions used by different international chemical regulatory agencies. It is noted that the level of uncertainty in the mass of substance and quantity released to the environment generally increases toward the end of the life cycle.
Since there was no report of use, import or manufacture of DADM in Canada in 2006 at or above the reporting thresholds specified in the CEPA section 71 notices, the release of this substance to the Canadian environment is expected to be very low. The Mass Flow Tool was nevertheless applied to estimate the fraction of DADM that could be released to the environment. Although there is uncertainty about the extent of current use in textile applications, this use has been assumed for Mass Flow Tool calculations, because it is considered to represent a reasonable worst-case (relatively high release) scenario for this substance.
Based on Statistics Canada information and an analysis by Industry Canada (2008), it is recognized that dyes may be imported in manufactured articles. Consequently, the ratio of textiles manufactured in Canada to imported textiles of 30:70 has been used to estimate the amount of dye imported in finished textiles (Industry Canada 2008; Environment Canada 2008b). This import quantity was included in the Mass Flow Tool calculations as well as in the exposure scenarios developed further.
Results indicate that DADM would be expected to be found largely in waste disposal sites (80.5%), due to the eventual disposal of manufactured items containing it (see Table 4). Mass Flow Tool calculations do not quantitatively account for releases of the substance to the environment from waste disposal sites (such as landfills, incinerators) unless specific information on the rate or potential for release is available. No such information has been identified for DADM. A small fraction of solid waste is incinerated, which would be expected to result in transformation of the substance. Based largely on information contained in OECD emission scenario documents for processing and uses associated with this type of substance, it is estimated that 17.1% of DADM may be released to wastewater. Although not considered in the Mass Flow Tool, the substance may be applied to agricultural soils and pasture lands in Canada as a component of biosludge, which is commonly used for soil enrichment.
Table 4. Estimated releases and losses of DADM to environmental media, chemical transformation during life cycle and transfer to waste disposal sites, based on the Mass Flow Tool
| Fate | Proportion of the Mass (%)1 | Major Life Cycle Stage Involved2 | |
|---|---|---|---|
| 1 For DADM, information from the following OECD emission scenario documents was used to estimate releases to the environment and the distribution of the substance as summarized in this table: Textile Manufacturing Wool Mills (OECD 2004), and Adhesive Formulation (OECD 2007). Specific assumptions used in the derivation of these estimates are summarized in Environment Canada 2008b. 2 Applicable stage(s): production, formulation, industrial use, consumer use, service life of article/product, waste disposal. 3 Wastewater before any form of treatment, either on-site industrial or off-site municipal wastewater treatment. |
|||
| Released to receiving media: | |||
| To soil | 0.0 | n/a3 | |
| To air | 0.0 | n/a | |
| To wastewater3 | 17.1 | Manufacturing of products and consumer use | |
| Chemically transformed | 2.5 | Waste disposal | |
| Transferred to waste disposal sites (e.g., landfill) | 80.5 | Waste disposal | |
Based on the above, sewer water is expected to be the medium receiving the greatest proportion of DADM emitted during product processing and consumer use (laundry). It is anticipated that the majority of the substance bound in products will ultimately be sent to landfills for disposal.
As indicated by the results of the Mass Flow Tool (Table 4), DADM would be expected to be released to wastewater during industrial processing and consumer use (laundry). The moderate to high log Kow value (Environmental Value Adjustment of 3.8 for DADM and 1.8 to 5.1 from read-across data) and high log Koc values (read-across of 3.4 to 4.2) (see Table 2) indicate that the substance would have affinity for solids. However, the log Koc is a calculated value (see footnote 6 below Table 3) and the adsorption potential of disperse particulate dye structures is generally not well understood; therefore the degree to which this particular behaviour applies to DADM is uncertain.
According to aerobic biodegradation models, DADM is expected to biodegrade slowly (see Table 5 below).
Given its estimated pKa values (9.03 acid, 1.69 base), it is unlikely that ionization will have a significant impact on the partitioning or water solubility of the substance. Instead, when released into water, DADM is expected to be mostly present as a particulate solid or as a neutral molecule adsorbed to suspended particles and eventually sink to surface bed sediments. Razo-Flores et al. (1997) have stated that due to the recalcitrant nature of azo dyes in the aerobic environment, they eventually end up in anaerobic sediments due to sediment burial, or in shallow aquifers (groundwater). Yen et al. (1991) observed that an azo analogue was transformed under anaerobic conditions in sediment via hydrolysis and reduction, and concluded that most azo dyes would likely not persist in anaerobic sediment systems. Although it has been reported that reduction rates of aromatic azo compounds in sediment are decreased by sorption (Jafert and Wolfe 1987), there is likely significant potential for degradation of azo dyes in anaerobic sediments.
The rate of volatilization from the surface of water is proportional to the Henry’s Law constant (Baughman and Perenich 1988). Baughman and Perenich (1988) also state that volatilization from aquatic systems will not be an important loss process for dyes, which agrees with the low to negligible read-across Henry’s Law constant value (10-8 to 10-1Pa•m3/mol, Table 3). Transfer to air due to the loss of dyes from moist and dry soil surfaces is not likely to be significant, as indicated by very low read-across vapour pressures for azo dyes (5.33×10-12 to 5.33×10-5 Pa) (Table 3). These data are consistent with the physical state (solid particle) of DADM, which makes it an unlikely candidate for volatilization.
Environmental Persistence
No experimental degradation data for DADM have been identified.
According to the Ecological and Toxicological Association of Dyes and Organic Pigments Manufacturers, with some exceptions, dyes are considered essentially nonbiodegradable under aerobic conditions (ETAD 1995). Repeated evaluation of ready and inherent biodegradability using accepted screening tests (see OECD Guidelines for Testing Chemicals) have confirmed this for such chemicals (Pagga and Brown 1986; ETAD 1992). Based on the chemical structure of DADM, there is no reason to suspect that biodegradation will be other than that of dyes generally (ETAD 1995).
Disperse dyes enter the aquatic system mostly as a dispersion of fine suspended particles, eventually settling to the aerobic layers of surface sediment where they will persist until sediment burial creates reducing conditions. The rate of sediment deposition and the extent of bioturbation vary from site to site, and thus it is very difficult to ascertain the residence time of dyes in aerobic sediment layers. It is likely, however, that in many cases this is greater than 365 days. Once under anaerobic or reducing conditions, azo dyes may undergo rapid degradation to substituted aromatic amine constituents as demonstrated by Yen et al. (1989), who measured reduction half-life values of 1.9–2.0 days for an azo benzothiazole dye (CAS RN 68133-69-7) in compacted sediments at room temperature. However, in deep anoxic sediment these biodegradation transformation products are not expected to present a high degree of exposure potential to most aquatic organisms. This is in part because contact of organisms with anoxic sediment is likely to be limited, and also because the amine degradation products are expected to be tightly bound to sediments, resulting in very low bioavailability (Weber et al. 2001; Colon et al. 2002). Therefore they are not likely to present an ecological concern.
Given the expected release of DADM, persistence in water was examined using predictive QSAR models for biodegradation, which are considered acceptable for use in this situation as these models are based on chemical structure and the azo structure is represented in the training sets of all the degradation models used, thereby increasing the reliability of these predictions. The following analysis applies primarily to the portion of a substance that is present in the environment in the dissolved form, recognizing that the largest proportion would likely exist in dispersed form as solid particles. DADM does not contain functional groups expected to undergo hydrolysis in aerobic environments, as dyes are designed to be stable in aqueous conditions.
Table 5 summarizes the results of available QSAR models for the degradation of DADM in water.
Table 5. Modelled data for biodegradation of DADM in water
| Fate Process | Model and Model Basis | Model Output | Expected Half-life (days) |
|---|---|---|---|
| 1 Output is a numerical score. 2 Output is a probability score. |
|||
| Biodegradation (aerobic) | BIOWIN 2000 Sub-model 3: Expert Survey (ultimate biodegradation) |
1.441 (biodegrades very slowly) | > 182 |
| Biodegradation (aerobic) | BIOWIN 2000 Sub-model 5: MITI linear probability |
-0.302 (biodegrades very slowly) | > 182 |
| Biodegradation (aerobic) | BIOWIN 2000 Sub-model 6: MITI non-linear probability |
0.02 (biodegrades very slowly) | > 182 |
| Biodegradation (aerobic) | CATABOL c2004–2008 % BOD (biological oxygen demand) |
0.012 (biodegrades very slowly) | > 182 |
The results from Table 5 indicate that all aerobic biodegradation models (BIOWIN 3, 5, 6 and CATABOL) suggest that DADM biodegrades slowly. In fact, both of the BIOWIN 5 and 6 probability results are much less than 0.3, the cut-off value suggested by Aronson et al. (2006) to identify a substance as having a half-life > 60 days (based on the MITI probability models). Furthermore, both of the other ultimate degradation models, BIOWIN 3 and CATABOL, predict that this substance will be persistent in water.
When the results of the probability and the other degradation models are considered, there is model consensus that the ultimate biodegradation half-life in water is > 182 days. This finding is consistent with what would be expected for this chemical’s structure (i.e., few degradable functional groups, sparingly soluble particle).
Using an extrapolation ratio of 1:1:4 for a water:soil:sediment biodegradation half-life (Boethling et al. 1995), the ultimate biodegradation half-life in soil is also > 182 days and the half-life in sediments is > 365 days. This indicates that DADM is persistent in soil and oxic sediment.
Based on the modelled data (see Tables 5 above), DADM meets the persistence criteria in water, soil and sediment (half-lives in soil and water = 182 days and half-life in sediment = 365 days), as set out in the Persistence and Bioaccumulation Regulations (Canada 2000).
As noted previously DADM is not expected to be volatile or significantly present in air. Hence it is not expected to be subject to long-range atmospheric transport.
Potential for Bioaccumulation
No experimental bioaccumulation data are available for DADM.
For many non-soluble dye classes, including non-soluble azo dyes, it is difficult to model the bioaccumulation potentials, and thus the results are generally unreliable. Predicted and/or empirically determined properties of dyes related to bioaccumulation (e.g., log Kow) can be of uncertain relevance or associated with a high degree of error, which reduces the utility of model predictions of BCF and BAF. In addition, monoazo dyes generally fall outside of bioaccumulation model domains of applicability. As a result, in this assessment, bioaccumulation modelling has not been used to evaluate the bioaccumulation status of DADM.
In the absence of experimental and modelled data specific to DADM, the bioconcentration factor (BCF) estimated for the azo dye Disperse Orange 30 was used to provide an indication of the bioaccumulation potential of DADM. The structural difference between DADM and Disperse Orange 30 has been noted (see Table 2). However, because of the similarities between monoazo disperse dyes outlined above, these differences are not anticipated to result in significantly different environmental behaviours. Furthermore, modelling data suggest that, due to the structural differences, Disperse Orange 30 is expected to have a somewhat higher octanol-water partition coefficient than DADM (see Table 3). Utilizing read-across data from a chemical with a higher octanol-water partition coefficient is conservative because this characteristic can be associated with bioaccumulative substances.
A bioconcentration study submitted for Disperse Orange 30 suggests that it is unlikely to accumulate in fish (Shen and Hu 2008). This test was performed according to the OECD Guidelines for Testing of Chemicals, Test No. 305B, Bioconcentration: Semi-Static Fish Test (OECD 1996). The bioconcentration of Disperse Orange 30 in zebra fish (Brachydanio rerio) was determined in a 28-day semi-static test with a test medium renewal every two days. A nominal concentration of 20 mg/L (mean measured concentration 0.028 ~ 0.28 mg/L) was used in the study to check the bioconcentration potential of the test substance. Samples from both test solutions and test organisms were taken daily from Day 26 to Day 28 of the 28-day exposure test period. Samples were prepared by extracting the lipid component from the test fish. The measured concentration of test substance, fish lipid content and BCF calculation are reported in Table 6.
Table 6. Measured concentrations of Disperse Orange 30, fish lipid content and BCF calculation
| Treatments (20 mg/L) | Sampling Time | ||
|---|---|---|---|
| Day 26 | Day 27 | Day 28 | |
| Measured concentration of the test substance in extracted solutions (mg/L) | < 0.028 | < 0.028 | < 0.028 |
| Content of the test substance in the fish lipids (mg) | < 1.68 | < 1.68 | < 1.68 |
| Fish total weight (g) | 2.07 | 2.13 | 2.53 |
| Concentration of the test substance in the fish Cf (mg/kg) | <0.81 | < 0.79 | < 0.66 |
| Measured concentration of the test substance in the water Cw (mg/L) | 0.028 ~ 0.28 | 0.028 ~ 0.28 | 0.028 ~ 0.28 |
| Fish lipid content (%) | 0.81 | 0.57 | 1.25 |
| BCF | < 100 | < 100 | < 100 |
| Average BCF | < 100 | ||
The Shen and Hu (2008) study was reviewed and considered acceptable (see Appendix 1). Lack of detection of Disperse Orange 30 in fish extracts (< 0.028 mg/L) suggests a limited solubility in lipids and/or limited potential to partition into fish tissue from aqueous systems. Assuming that the concentration in solution in the test was equal to the lowest water solubility value of 0.028 mg/L, and using the fish concentration of 0.81 mg/kg as a worst-case estimate, the BCF may be calculated to be < 100. However, there is some uncertainty associated with limit-bounded values in any study because the “true” value is not known. But given the structure and likely behavior of the analogue in aqueous systems, the low BCF result is expected.
Most disperse dyes exist as fine dispersible particles with limited truly soluble fractions. Solubility, however, can be increased by adding polar functional groups to the molecule. Disperse Orange 30 contains some of these solubilizing groups (nitroso), thus some degree of water solubility would be expected. DADM does not contain any functional groups expected to be highly ionic at relevant environmental pHs of 6–9.
While the above study serves as primary evidence to support the expectation that DADM lacks bioaccumulation potential, other research corroborates this conclusion. Anliker et al. (1981) reported experimental fish bioaccumulation values for 18 disperse monoazo dyes, performed according to test methods specified by the Japanese Ministry of International Trade and Industry (MITI). Expressed on the basis of wet body weight of the fishes, these log bioaccumulation factors ranged from 0.00 to 1.76 (Anliker et al. 1981). A lack of reporting of chemical registry numbers and chemical structures limited the utility of this study for read-across purposes for DADM. However, follow-up studies, which provided the chemical structures for the disperse dyes tested, confirmed low bioaccumulation potential for ten nitroazo dyes, with reported log bioaccumulation factors ranging from 0.3 to 1.76 (Anliker and Moser 1987; Anliker et al. 1988). Studies available from MITI also support low bioaccumulation potential for disperse azo dyes. Reported BCFs for 3 disperse azo dyes (CAS RNs 40690-89-9, 61968-52-3 and 71767-67-4) tested at a concentration of 0.01 mg/L were in the range of < 0.3 to 47 (MITI 1992). An accumulation study by Brown (1987) also showed that none of the twelve disperse dyes tested accumulated during an eight-week study with carp.
Moderate to high log Kow values for other disperse azo dyes and the modelled log Kow value for DADM itself (Table 3) are the only lines of evidence suggesting that DADM may have a high potential for bioaccumulation. In spite of the high log Kow values for Disperse Orange 30 and the azo structural analogues, evidence for bioaccumulation of disperse azo dyes is lacking (Anliker et al. 1981; Anliker and Moser 1987; Anliker et al. 1988; MITI 1992). Authors who have measured high log Kows and concomitant low bioaccumulation factors for disperse azo dyes suggest that the low accumulation factors may be a result of low absolute fat solubility (Brown 1987) or relatively high molecular weights, which may make transport across fish membranes difficult (Anliker et al. 1981; Anliker and Moser 1987). It is also likely that the lack of bioavailability and limited capacity to partition under BCF test conditions limits accumulation in fish lipids.
It has been stated by ETAD (1995) that the molecular characteristics indicating the absence of bioaccumulation are a molecular weight of > 450 g/mol and a cross-sectional diameter of > 1.05 nm. Recent investigation by Dimitrov et al. (2002), Dimitrov et al. (2005) and the BBM (2008) suggests that the probability of a molecule crossing cell membranes as a result of passive diffusion declines significantly with increasing maximum cross-sectional diameter (Dmax). The probability of passive diffusion lowers appreciably when cross-sectional diameter is > ~1.5 nm and more significantly for molecules having a cross-sectional diameter of > 1.7 nm. Sakuratani et al. (2008) have also investigated the effect of cross-sectional diameter on passive diffusion from a test set of about 1200 new and existing chemicals. They also observed that substances not having a very high bioconcentration potential often have a Dmax > 2.0 nm and an effective diameter (Deff) > 1.1 nm.
DADM has a molecular weight of close to 450 g/mol and a cross-sectional diameter in the range of 1.45 to 1.90 nm, indicating a potential for a reduced uptake rate from water and reduced in vivo bioavailability of DADM.
In addition, there is model evidence that this dye can be metabolized via N-reduction Phase I metabolism (probability = 1.0) should it get into tissues of organisms (CATABOL c2004-2008). This provides a further indication that DADM is not likely to be bioaccumulative.
Based on a lack of observed accumulation in bioconcentration tests with analogous substances and on other supporting evidence (e.g., the relatively large molecular size of the substance), DADM is considered to have a low potential for bioaccumulation. It is therefore concluded that DADM does not meet the bioaccumulation criterion (BCF or BAF > 5000) as set out in the Persistence and Bioaccumulation Regulations (Canada 2000).
Ecological Effects Assessment
A - In the Aquatic Compartment
Three toxicity studies were submitted for DADM (Study Submission 2008) to support the assessment of ecological effects (Table 7a).
Table 7a. Empirical data for aquatic toxicity of DADM and the analogue CAS RN 68385-96-6 (Study Submission 2008)
| Test Chemicals | Test Organism | Type of Test | Duration (hours) | End Point | Value (mg/L) | Reliability of the Study |
|---|---|---|---|---|---|---|
| 1 LC0 – The concentration of a substance at which there is no lethal effect on the test organisms observed within the test duration. Due to the low water solubility of the test chemical, the end point of LC0 shall be interpreted as the loading rate. 2 EC50 - The concentration of a substance that is estimated to have some toxic sublethal effect on 50% of the test organisms. |
||||||
| DADM and CAS RN 68385-96-6 | Zebra fish (Brachydanio rerio) | Acute | 96 | LC01 | > 1000 | Acceptable |
| Rainbow trout (Oncorhyncus mykiss) | Acute | 96 | LC0 | > 1000 | Acceptable | |
| DADM | Bacteria | Acute | 3 | EC502 | > 10 000 | Unacceptable |
In two toxicity studies on fish, DADM and the analogous chemical CAS RN 68358-96-6 demonstrated a 96-hour LC0 > 1000 mg/L in both zebra fish (Brachydanio rerio) and rainbow trout (Oncorhyncus mykiss) (Study Submission 2008; see Table 7a). The studies were evaluated as being of acceptable reliability, and robust study summaries (RSS) are provided in Appendix 1.
It may be noted that the reported end point LC0 > 1000 mg/L refers to a concentration much higher than the predicted water solubility of DADM. Such end points should be interpreted as the loading rate applied in the studies. Given the low water solubility of these compounds, it is expected that this loading results in a saturated solution; therefore the result may be more accurately described as an LL0 (the lethal loading rate) of more than 1000 mg/L. Although the reported mass loading data are considered not appropriate to directly derive a predicted no-effect concentration (PNEC), the lack of mortality associated with exposure to a saturated solution of DADM may be is taken into consideration in determining the PNECfor the substance.
In another toxicity study on bacteria, DADM had a 3-hour EC50> 10 000 mg/L. However, the study was deemed to be of low confidence due to lack of sufficient details in the submission (see RSS in Appendix 1).
Table 7b. Empirical data for aquatic toxicity of Disperse Orange 30
| Test Organism | Type of Test | Duration (hours) | End Point | Value (mg/L) | Reliability of the Study | Reference |
|---|---|---|---|---|---|---|
| 1 LC50 - The concentration of a substance that is estimated to be lethal to 50% of the test organisms. 2 EC50 - The concentration of a substance that is estimated to have some toxic sublethal effect on 50% of the test organisms. 3 IC50 – The concentration of a substance that is estimated to inhibit growth in 50% of the test organisms. |
||||||
| Rainbow trout (Oncorhynchus mykiss) |
Acute | 48 | LC501 | > 700 | Unacceptable | Sandoz 1975 |
| Rainbow trout (Salmo gairdneri) |
Acute | 96 | LC50 | > 100 | Low confidence | Safepharm Laboratories Ltd 1990 |
| Zebra fish | Acute | 96 | LC50 | 710 | Not available Not available | Brown 1992 |
| Daphnia magna | Acute | 48 | EC502 | 5.8 | ||
| Scenedesmus subspicatus | Acute | 72 | EC50 | 6.7 | ||
| Bacteria | Acute | n/a | IC503 | > 100 | ||
A study submitted on behalf of ETAD provides acute ecotoxicity data for fish, invertebrates, algae and bacteria for Disperse Orange 30 (Brown 1992). A 96-hour LC50 of 710 mg/L for zebra fish, a 48-hour EC50 of 5.8 mg/L for Daphnia magna, and a 72hour EC50 of 6.7 mg/L (for algal growth) for Scenedesmus subspicatushave been reported based on toxicity studies using Disperse Orange 30 (Table 7b). The original studies have not been provided to allow verification of their reliability. However, these results are consistent with those of a number of other toxicity studies with azo dyes, which report acute effect values (LC50 and EC50s) in the range of 7 to 505 mg/L for fish, invertebrates and algae (Environment Canada 1995; Brown 1992; Cohle and Mihalik 1991; Little and Lamb 1973).
Another result for Disperse Orange 30 was submitted to Environment Canada as a voluntary data submission. An LC50for rainbow trout (Oncorhynchus mykiss) was established to be > 700mg/L (Sandoz 1975). An evaluation was conducted based on the robust study summary provided in the submission, and it was concluded that the study (Sandoz 1975) was unacceptable (see Appendix 1).
An acute toxicity study with Disperse Orange 30 using rainbow trout (LC50 reported as > 100 mg/L) was also submitted to Environment Canada (Table 9) (Safepharm Laboratories Ltd. 1990). An assessment of the reliability of the study using a robust study summary was conducted, and the study was deemed to be of low confidence due to lack of details (Appendix 1).
A range of aquatic toxicity predictions for dyes and analogues were also obtained from QSAR models. However, as with bioaccumulation, these QSARecotoxicity predictions for dyes are not considered reliable because of the potential error associated with input parameters and the unique nature of disperse dyes, such as solid physical state, as well as structural and/or physical and chemical properties which fall outside of the models’ domain of applicability.
The available empirical ecotoxicity information for DADM and an analogue, with consideration of data for some other azo compounds, indicates that the substance is not likely to be highly hazardous to aquatic organisms.
B - In Other Environmental Compartments
Since DADM is expected to accumulate in sediment and may potentially enter soil from biosludge which is commonly used for soil enrichment as well as from the disposal of products that degrade and release the substance, it would be desirable to have toxicity data for sediment and soil organisms. However, no suitable ecological effects studies were found for DADM or its analogues in media other than water. Considering the toxicity data for aquatic organisms as well as the lack of bioaccumulation potential and its low bioavailability, potential for toxicity to soil-dwelling organisms is likely to be low. For the same reasons, the toxicity potential is also likely to be low in sediment-dwelling species, although this cannot be substantiated due to the lack of suitable whole-organism toxicity data. In addition, the toxicity potential of DADM in anoxic sediments will likely be low because of the low bioavailability of their anaerobic degradation products.
Ecological Exposure Assessment
No data concerning concentrations of DADM in water in Canada have been identified. Environmental concentrations are, therefore, estimated from available information, including estimated substance quantities (using the reporting threshold quantity of 100 kg for manufacture and import), release rates, and characteristics of receiving water bodies.
The Mass Flow Tool identified realistic worst-case releases to water (wastewater) from industrial use and from consumer use of products containing this substance (Table 4). To address releases from industrial activities, Environment Canada’s Industrial Generic Exposure Tool – Aquatic (IGETA) was employed to estimate a conservative substance concentration of 0.0007 mg/L in a generic watercourse receiving industrial effluents (Environment Canada 2008c). The generic scenario is designed to provide these estimates based on conservative assumptions regarding the amount of chemical processed and released, the number of processing days, sewage treatment plant removal rate, and the size of the receiving watercourse. The tool models an industrial-release scenario based on loading data from sources such as industrial surveys and knowledge of the distribution of industrial discharges in the country, and calculates a predicted environmental concentration (PEC).
The PEC (0.0007 mg/L) for DADM was calculated based on a use quantity of 100 kg for a single facility. This corresponds to the reporting threshold for manufacture and import in the section 71 notice, which was not met by any reporter in 2006. The conservative assessment assumed a primary sewage treatment plant (STP). The release to the ambient environment was estimated with IGETA, assuming that 16% was being released--over a period of 250 days (to represent annual activities and based on previous experience of Environment Canada assessing other azo dyes), with a primary removal rate at STP of 60%--into a small receiving watercourse with a flow of 0.4 m3/s. The equation and inputs used to calculate the PEC in the receiving watercourse are described in the Environment Canada (2008d).
As DADM can be found in consumer products, Mega Flush, Environment Canada’s spreadsheet model for estimating down-the-drain releases from consumer uses, was used to estimate the potential substance concentration in multiple water bodies receiving sewage treatment plant effluents to which the substance may have been released (Environment Canada 2008e). The spreadsheet model is designed to provide these estimates based on conservative assumptions regarding the amount of a substance used and released by consumers.
A scenario was run assuming a total consumer use quantity of 333 kg/year by Mega Flush consumer release scenario. This represents the cumulative quantity of DADM at the reporting threshold of 100 kg as the industrial use quantity and takes into consideration the ratio of 30:70 for textiles manufactured in Canada versus textiles imported. The following assumptions were made: the primary STP removal rate was 60%, losses from use were 10%, consumer use of the product was 365 days/year, and receiving waterflow rates at all sites were in the tenth percentile (low end). The overall effect of these parameters is to make this scenario moderately conservative. Mega Flush results an estimate of the maximum PEC as 5.1 x 10-5 mg/L (Environment Canada 2008f).
Characterization of Ecological Risk
The approach taken in this ecological screening assessment was to examine various supporting information and develop conclusions based on a weight-of-evidence approach and using precaution as required under CEPA 1999. Lines of evidence considered include results from conservative risk quotient calculations, as well as information on persistence, bioaccumulation, inherent toxicity, sources and fate of the substance.
Based on the available information, DADM is predicted to be persistent in water, soil and oxic sediment but is expected to have low bioaccumulation potential. The lack of reports of manufacture and the likely very low importation quantities of the substance into Canada , along with information on physical and chemical properties and uses, indicate a low potential for releases into the Canadian environment. If released into the environment, it is expected that the substance will be mainly discharged to surface waters, where ultimately it is expected to be transferred to sediment.
Based on data for the toxicity of DADM and analogous substances, DADM is expected to have only a low to moderate potential for acute toxicity to aquatic organisms.
As mentioned, the acute end point LC0 > 1000 mg/L refers to a concentration much higher than the predicted water solubility of DADM. The data may be interpreted as meaning that there are no lethal effects at the loading rate applied in the studies. It can be assumed that there is no effect at a concentration that is equivalent to the water solubility of the substance.
The water solubility of DADM was estimated based on the experimental data of the analogue Disperse Blue 165 (see Table 2 and 3). The high end calculated water solubility (0.17 mg/L) is consequently selected as the no-observed-effect concentration (NOEC) for DADM.
An assessment factor of 100 is usually applied to account for extrapolating from an acute short(shortterm) toxicity and from laboratory results for one species to other potentially sensitive species in the field. For DADM, an assessment factor of 10 was deemed sufficient to account for extrapolation from the limit of water solubility used as the NOEC to potential chronic effects in the environment. The PNEC for DADM is therefore determined as 0.017 mg/L. When compared to the conservative PEC calculated above using IGETA, the resulting risk quotient for industrial discharges (PEC/PNEC) is 0.0007/0.017 = 0.041.
Considering the experimental toxicity studies from other azo compounds, Disperse Orange 30 has the lowest nominal acute effect concentration (EC50) (see Table 7b). The critical toxicity value was defined using the 96-hour EC50 to D. magna at 5.8 mg/L, based on nominal concentrations. A factor of 100 was then applied to account for extrapolating from acute to chronic (longterm) toxicity and from laboratory results for one species to other potentially more sensitive species in the field. This gives a conservative PNEC of 0.058 mg/L, and the resulting risk quotient for industrial discharges (PEC/PNEC) is 0.0007/0.058 = 0.012.
Given that IGETA provides a conservative estimate of exposure, the results indicate a low potential for ecological harm resulting from local exposure to a point source industrial release.
For exposure resulting from down-the-drain releases through consumer uses (conservative scenario), Mega Flush results indicate that the PECs will not exceed the PNEC at any sites (i.e., all risk quotients < 1) (Environment Canada 2008f). This indicates that down-the-drain consumer releases of DADM are not expected to cause harm to aquatic organisms.
Given that the substance is insoluble in water and associated with the low aquatic toxicity, it is concluded that DADM is unlikely to cause ecological harm in Canada .
Uncertainties in Evaluation of Ecological Risk
Uncertainty is associated with the use of “read-across” physical and chemical properties, environmental fate data and toxicity data from analogues. This is due to a lack of empirical data for monoazo dye analogues with similar functional groups to DADM. While the chemicals identified (CAS RN 68385-96-6, Disperse Blue 165 and Disperse Orange 30) share many similarities with DADM--including being azo dyes with high molecular weights, similar cross sectional diameters, having solid particulate structures that decompose at greater than 120oC (to 270oC), and being “dispersible” in water (i.e., not truly “soluble”)--they do have some differences in functional groups. These differences in chemical structure add uncertainty because the properties, environmental fate and toxicity of DADM may be somewhat different. However, it was expected that the similarities were sufficient to justify inclusion of the data from analogues as part of the weight of evidence in the assessment of DADM.
The persistence assessment is limited by the absence of biodegradation data, which necessitated generation of model predictions. Although all model predictions have some degree of error, the biodegradation model outputs confirmed that DADM is not likely to biodegrade quickly under oxic conditions, and that DADM meets the persistence criteria as set out in the Persistence and Bioaccumulation Regulations (Canada 2000). Nevertheless, it is clear that anaerobic degradation of the bioavailable portion of azo dyes in sediments to constitutive amines is much faster (half-lives in the order of days) than aerobic biodegradation. Although the amine degradation products are not expected to be biologically available because they form only in relatively deep anoxic sediment and can be tightly bound to sediment through nucleophilic addition and oxidative radical coupling (Weber et al. 2001; Colon et al. 2002), this issue is a source of uncertainty in the toxicity assessment of DADM.
The bioaccumulation assessment for DADM was limited by the lack of empirical data and the inability of available models to reliably estimate bioaccumulation for azo dyes. Instead, the assessment relied on the use of bioaccumulation data for chemically similar azo substances.
Uncertainties are also present due to the lack of information on environmental concentrations in Canada for DADM. However the lack of reports of manufacturing and import into Canada suggests low releases of the substance into the Canadian environment. When developing the exposure scenario, assumptions about uses of DADM were based on use codes reported for DSL nomination (1984–1986) as well as the traditional uses of dyes. Among a variety of potential applications, the application with the greatest potential for releases to the environment (i.e., textile manufacturing) was used for predicting the environmental concentrations of DADM.
Uncertainties are also associated with the fraction of the substance that is assumed to be released during use (i.e., during industrial activities and use of consumer products). These uncertainties were addressed by making conservative assumptions in each of the modelling exercises.
The lack of experimental toxicity data for aquatic organisms is an additional source of uncertainty. The available toxicity studies reported an end point as LC0 > 1000 mg/L, which was a concentration much higher than the predicted water solubility of the chemical. The data should be interpreted as meaning that there is no lethal effect at the loading rate applied in the studies. In other words, it can be assumed that there is no effect at a concentration equivalent to the water solubility of the substance. However, given DADM’s low water solubility and its limited bioavailabilty due to its molecular size, this substance is not expected to be highly hazardous to aquatic organisms.
Also, regarding ecotoxicity, based on the predicted partitioning behaviour of the dyes, the significance of soil and sediment as important media of exposure is not well addressed by the effects data available. Indeed, the only effects data identified apply primarily to pelagic aquatic exposures, although the water column may not be the medium of primary long-term concern. Nevertheless, based on the relatively low aquatic toxicity of this substance, potential for harm to soil- and sediment-dwelling organisms is also expected to be low.
No activity was reported, under section 71 of CEPA 1999, for DADM above the reporting threshold of 100 kg for the reporting year 2006. Based on the available information, exposure of the general population in Canada to DADM is considered low.
Limited empirical toxicity data are available for this substance; however, DADM belongs to the class of azo substances that could exhibit similar toxicological properties, i.e., undergo enzymatic reductive cleavage into component aromatic amine metabolites which as a chemical class have carcinogenic and genotoxic potential.(Platzek et al. 1999; Golka et al. 2004; Chen 2006; Xu et al. 2007; Stingley et al. 2010, Vineis and Pirastu 1997; Benigni and Passerini 2002; Talaska 2003). Limited information on the class of substances suggests a potential concern for hazard associated with DADM. However, based on the results of a survey under section 71 of CEPA 1999, DADM is not considered to be manufactured in or imported into Canada in quantities above the reporting limits. Therefore, the likelihood of exposure to the general population of Canada is considered to be low; hence the risk to human health is likewise considered to be low.
Based on the information presented in this screening assessment, it is concluded that DADM is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
Although there may be potential hazards associated with DADM, on the basis of information indicating that this substance is not manufactured in or imported into Canada in a quantity above the reporting threshold, it is concluded that this substance is not entering the environment in a quantity or concentration or under conditions that constitute a danger in Canada to human life or health.
It is therefore concluded that DADM does not meet any of the criteria set out in section 64 of CEPA 1999. Additionally, DADM meets the criteria for persistence, but does not meet the criterion for bioaccumulation, as set out in the Persistence and Bioaccumulation Regulations (Canada 2000).
Because this substance is listed on the Domestic Substances List, its import and manufacture in Canada are not subject to notification under subsection 81(1) of CEPA 1999. Given the potential hazardous properties of this substance, there is concern that new activities that have not been identified or assessed could lead to this substance meeting the criteria set out in section 64 of the Act. Therefore, it is recommended that the Domestic Substances List be amended, under subsection 87(3) of the Act, to indicate that subsection 81(3) of the Act applies with respect to this substance so that new manufacture, import or use of this substance is notified and undergoes ecological and human health risk assessments.
In addition and where relevant, research and monitoring will support verification of assumptions used during the screening assessment.
Considerations for Follow-up
In light of the potential high hazard of this class of substances, additional activity (e.g., research, monitoring and surveillance, assessment) to characterize the risk to human health in Canada of this broader group of azo substances may be undertaken.
ACD/pKaDB [Prediction module]. 2005. Version 9.04. Toronto (ON): Advanced Chemistry Development. [2008-12]. Available from: http://www.acdlabs.com/products/phys_chem_lab/pka/. [restricted access]
[AIEPS] Artificial Intelligence Expert Predictive System. 2003-2007. Version 2.05. Ottawa (ON): Environment Canada , Ecological Assessment Division, New Substances Division. Model developed by Stephen Niculescu. Available from: Environment Canada , Ecological Assessment Division, New Substances Division.
Anliker R, Clarke EA, Moser P. 1981. Use of the partition coefficient as an indicator of bioaccumulation tendency of dyestuffs in fish. Chemosphere 10(3):263-274.
Anliker R, Moser P. 1987. The limits of bioaccumulation of organic pigments in fish: their relation to the partition coefficient and the solubility in water and octanol. Ecotoxicol Environ Safety 13:43-52.
Anliker R, Moser P, Poppinger D. 1988. Bioaccumulation of dyestuffs and organic pigments in fish. Relationships to hydrophobicity and steric factors. Chemosphere 17(8):1631-1644.
[AOPWIN] Atmospheric Oxidation Program for Windows [Estimation Model]. 2000. Version 1.91. Washington (DC): U.S. Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: www.epa.gov/oppt/exposure/pubs/episuite.htm
Arnot JA, Gobas FAPC. 2003. A generic QSAR for assessing the bioaccumulation potential of organic chemicals in aquatic food webs. QSAR Comb Sci 22(3): 337-345.
Aronson D, Boethling B, Howard P, Stiteler W. 2006. Estimating biodegradation half-lives for use in chemical screening. Chemosphere 63:1953-1960.
[ASTER] Assessment Tools for the Evaluation of Risk [Internet]. 1999. Duluth (MN): US Environmental Protection Agency, Mid-Continent Ecology Division. Available from: http://www.epa.gov/med/Prods_Pubs/aster.htm[restricted access]
ASTreat Model [sewage treatment plant removal model]. 2006. Version 1.0. Cincinnati (US): Procter & Gamble Company. Available from Procter & Gamble Company, P.O. Box 538707, Cincinnati, OH 45253-8707, US .
Baughman GL, Perenich TA. 1988. Fate of dyes in aquatic systems: I. Solubility and partitioning of some hydrophobic dyes and related compounds. Environ Toxicol Chem 7(3):183-199.
[BBM] Baseline Bioaccumulation Model. 2008. Gatineau (QC): Environment Canada , Existing Substances Division. [Model based on Dimitrov et al. 2005]. [2008-12]. Available upon request.
[BCFWIN] BioConcentration Factor Program for Windows [Estimation Model]. 2000. Version 2.15. Washington (DC): U.S. Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: www.epa.gov/oppt/exposure/pubs/episuite.htm
Benigni R, Passerini L. 2002. Carcinogenicity of the aromatic amines: from structure–activity relationships to mechanisms of action and risk assessment. Mutat Res 511(3): 191–206.
[BIOWIN] Biodegradation Probability Program for Windows [Estimation model]. 2000. Version 4.02. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. [cited 2009-04]. Available from: www.epa.gov/oppt/exposure/pubs/episuite.htm
Boethling RS, Howard PH, Beauman JA, Larosche ME. 1995. Factors for intermedia extrapolations in biodegradability assessment. Chemosphere 30(4):741-752.
Brown D. 1987. Effects of colorants in the aquatic environment. Ecotoxicol Environ Safety 13:139-47.
Brown D (ICI Group Environmental Laboratory, Brixham, UK ). 1992. Environmental assessment of dyestuffs. Prepared for Ecological and Toxicological Association of the Dyes and Organic Pigments Manufacturers, Basel, Switzerland . ETAD ecological sub-committee project E3020. Submitted to Environment Canada .
[ Canada ]. 1999. Canadian Environmental Protection Act, 1999. S.C., 1999, c. 33. Canada Gazette. Part III, vol. 22, no. 3. Ottawa: Queen’s Printer. Available from: http://canadagazette.gc.ca/partIII/1999/g3-02203.pdf
[ Canada ]. 2000. Canadian Environmental Protection Act, 1999: Persistence and Bioaccumulation Regulations, P.C. 2000-348, 23 March, 2000, SOR/2000-107. Canada Gazette. Part II, vol. 134, no. 7, p. 607–612. Ottawa: Queen’s Printer. Available from: http://canadagazette.gc.ca/partII/2000/20000329/pdf/g2-13407.pdf
Canada , Dept. of the Environment, Dept. of Health. 2006a. Canadian Environmental Protection Act, 1999: Notice of intent to develop and implement measures to assess and manage the risks posed by certain substances to the health of Canadians and their environment. Canada Gazette, Part I, vol. 140, no. 49, p. 4109–4117. Ottawa: Queen’s Printer. Available from: http://canadagazette.gc.ca/partI/2006/20061209/pdf/g1-14049.pdf.
Canada , Dept. of the Environment, Dept. of Health. 2006b. Canadian Environmental Protection Act, 1999: Notice with respect to selected substances identified as priority for action. Canada Gazette, Part I, vol. 140, no. 9, p. 435–459. Ottawa: Queen’s Printer. Available from: http://canadagazette.gc.ca/partI/2006/20060304/pdf/g1-14009.pdf
Canada , Dept. of the Environment, Dept. of Health. 2008. Canadian Environmental Protection Act, 1999: Notice of seventh release of technical information relevant to substances identified in the Challenge. Canada Gazette, Part I, vol. 142, no. 35. Ottawa: Queen’s Printer. Available from: http://canadagazette.gc.ca/partI/2008/20080830/html/notice-e.html#d102
[CATABOL] Probabilistic assessment of biodegradability and metabolic pathways [Computer model]. c2004-2008. Version 5.10.2. Bourgas (BG): Bourgas Prof. Assen Zlatarov University, Laboratory of Mathematical Chemistry. Available from: http://oasis-lmc.org/?section=software&swid=1
[CBG] ChemBuyersGuide [Internet]. 2009. New York (NY): ChemBuyersGuide. [cited 2009 Dec 11]. Available from: http://www.chembuyersguide.com/partners/lanxess.html
Chen H. 2006. Recent advances in azo dye degrading enzyme research. Curr Protein Pept Sci 7: 101–111.
Cohle P, Mihalik R. 1991. Early life stage toxicity of C.I. Disperse Blue 79:1 purified preecake to rainbow trout (Oncorhynchus mykiss) in a flow-through system. Final report. ABC laboratories Inc. Columbia MO
Colon D, Weber E, Baughman G. 2002. Sediment-associated reactions of aromatic amines. 2. QSARdevelopment. Environ Sci Technol 35 (12):2443–2450.
[CPOPs] Canadian POPs Model. 2008. Gatineau (QC): Environment Canada , Existing Substances Division; Bourgas (BG): Bourgas Prof. Assen Zlatarov University, Laboratory of Mathematical Chemistry. [Model developed based on Mekenyan et al. 2005]. Available upon request.
Dimitrov SD, Dimitrova NC, Walker JD, Veith GD, Mekenyan OG. 2002. Predicting bioconcentration factors of highly hydrophobic chemicals. Effects of molecular size*. Pure Appl Chem 74(10):1823-1830.
Dimitrov S, Dimitrova N, Parkerton T, Comber M, Bonnell M, Mekenyan O. 2005. Base-line model for identifying the bioaccumulation potential of chemicals. SAR QSAR Environ Res 16(6):531–554.
[ECOSAR] Ecological Structural Activity Relationships [Internet]. 2004. Version 0.99h. Washington (DC): U.S. Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: www.epa.gov/oppt/exposure/pubs/episuite.htm
Environment Canada . 1988. Data relating to the Domestic Substance List (DSL) 1984-1986, collected under CEPA, 1988, s. 25(1). Based on Reporting for the Domestic Substances List [guide] 1988. Data prepared by: Environment Canada .
Environment Canada 1995. Acute Fish Toxicity Test Submission in Fulfillment of New Substances Notification Regulations to New Substances Branch, Environment Canada under New Substance Notification Program.
Environment Canada . 2000. Chemicals Evaluation Division. Environmental Categorization for Persistence, Bioaccumulation and Inherent Toxicity of Substances on the Domestic Substances List Using QSARs. Final Report. Environment Canada . July.
Environment Canada . 2008a. Guidance for conducting ecological assessments under CEPA, 1999: science resource technical series, technical guidance module: Mass Flow Tool. Preliminary draft working document. Gatineau (QC): Environment Canada , Existing Substances Division.
Environment Canada . 2008b. Assumptions, limitations and uncertainties of the Mass Flow Tool for DADM. Internal draft document. Gatineau (QC): Environment Canada, Existing Substances Division. Available on request.
Environment Canada . 2008c. Guidance for conducting ecological assessments under CEPA, 1999: science resource technical series, technical guidance module: the Industrial Generic Exposure Tool – Aquatic (IGETA). Working document. Gatineau (QC): Environment Canada , Existing Substances Division.
Environment Canada . 2008d. IGETA report: DADM. 2009-04. Unpublished report. Gatineau (QC): Environment Canada , Existing Substances Division.
Environment Canada . 2008e. Guidance for conducting ecological assessments under CEPA, 1999: science resource technical series, technical guidance module: Mega Flush consumer release scenario. Preliminary draft working document. Gatineau (QC): Environment Canada , Existing Substances Division.
Environment Canada . 2008f. Mega Flush report: DADM. 2009-04. Unpublished report. Gatineau (QC): Environment Canada , Existing Substances Division.
[EPIsuite] Estimation Programs Interface Suite for Microsoft Windows [Estimation Model]. 2007. Version 4.0. Washington (DC): U.S. Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. [cited 2009-04]. Available from: www.epa.gov/oppt/exposure/pubs/episuite.htm
[EQC] Equilibrium Criterion Model. 2003. Version 2.02. Peterborough (ON): Trent University, Canadian Environmental Modelling Centre. Available from: http://www.trentu.ca/academic/aminss/envmodel/models/EQC2.html
[ETAD] Ecological and Toxicological Association of Dyes and Organic Pigments Manufacturers. 1992. Draft Guidelines for the Assessment of Environmental Exposure to Dyestuffs.
[ETAD] Ecological and Toxicological Association of Dyes and Organic Pigments Canadian Affiliates, Dayan J, Trebitz H, consultants. 1995. Health and environmental information on dyes used in Canada . Unpublished report submitted to Environment Canada, New Substances Division. On the cover: An overview to assist in the implementation of the New Substances Notification Regulations under the Canadian Environmental Protection Act
[ETAD] Ecological and Toxicological Association of Dyes and Organic Pigments Manufacturers. 2005. ETAD data for DSL categorization and screening submitted to Environment Canada on October 27, 2005. Study report by Intertek ASG. File Ref 2005/CC0157-001/REGIS.
Golka K, Kopps S, Myslaket ZW. 2004. Carcinogenicity of azo colorants: influence of solubility and bioavailability. Toxicol Lett 151: 203–210.
Industry Canada . 2008. Textile and Fabric Finishing [NAICS 31331]: 2004-2007 and Fabrics Coating 2004-2007. [NAICS 31332]: 2004-2007. Prepared by: Apparel and Textiles Directorate, Service Industries and Consumer Products Branch, Industry Canada ,.
Jafert CT, Wolfe NL. 1987. Degradation of selected halogenated ethanes in anoxic sediment-water systems. Environ Toxicol Chem 6:827-837.
[Lanxess] Lanxess Web Catalog [Internet]. ©2007–2009. Bayscript Special Red T. Leverkusen (DE): Lanxess Corporation. [cited 2009 Dec 10]. Available from: http://lanxess.com/brands-products/product-search/
Little LW, Lamb JC III. 1973. Acute Toxicity of 46 Selected Dyes to the Fathead Minnow, Pimephales promelas. Dyes and the Environment - Reports on Selected Dyes and Their Effects, Vol.1, American Dye Manufacturers Institute, Inc.:130
Mekenyan G, Dimitrov SD, Pavlov TS, Veith GD. 2005. POPs: A QSAR system for creating PBT profiles of chemicals and their metabolites. SAR and QSAR in Environ. Research, 16(1-2), 103-133.
[MITI] Ministry of International Trade & Industry (Jpn), Basic Industries Bureau, Chemical Products Safety Division. 1992. Biodegradation and bioaccumulation data of existing chemicals based on the CSCL Japan. Tokyo (Jpn): Japan Chemical Industry Ecology-Toxicology & Information Centre.
[NCI] National Chemical Inventories [database on CD-ROM]. 2006. Columbus (OH): American Chemical Society. Available from: http://www.cas.org/products/cd/nci/index.html
[NIOSH] National Institute for Occupational Safety and Health. 2007. National Occupational Exposure Survey (1981–1983). Estimated Numbers of Employees Potentially Exposed to Specific Agents by 2-Digit Standard Industrial Classification (SIC). (accessed 2007 Feb. 16)
http://www.cdc.gov/noes/noes1/e0579sic.html
[OECD] Organisation for Economic Co-operation and Development. 1984. OECD Guidelines for Testing of Chemicals, No. 209. Activated Sludge, Respiration Inhibition Test. Paris: OECD,
[OECD] Organisation for Economic Co-operation and Development. 1992. OECD Guidelines for Testing of Chemicals, No. 203. Fish, Acute Toxicity Test. Paris: OECD, Adopted 17th July, 1992.
[OASIS Forecast] Optimized Approach based on Structural Indices Set [Internet]. 2005. Version 1.20. Bourgas (BG): Bourgas Prof. Assen Zlatarov University, Laboratory of Mathematical Chemistry. Available from: http://oasis-lmc.org/?section=software
[OECD] Organisation for Economic Co-operation and Development. 1996. OECD Guidelines for Testing of Chemicals, No. 305B. Bioconcentration: Semi-Static Fish Test. Paris: OECD, Adopted June 1996.
[OECD] Organisation for Economic Co-operation and Development. 2004. Draft emission scenario on textile manufacturing wool mills [Internet]. Paris (FR): OECD, Environment Directorate. Report No.: ENV/JM/EEA(2004)8/1/REV, JT00175156. [cited 2008 August]. Available from: http://www.oecd.org/dataoecd/2/47/34003719.pdf
[OECD] Organisation for Economic Co-operation and Development. 2007. Emission scenario document on adhesive formulation [Internet]. Final report. Paris (FR): OECD, Environment Directorate. (Series on Emission Scenario Documents). [Cited 2008 August]. Available from: http://ascouncil.org/news/adhesives/docs/EPAFormulation.pdf
Pagga U, Brown D. 1986. The degradation of dyestuffs: Part II Behaviour of dyestuffs in aerobic biodegradation tests. Chemosphere 15(4):479-491.
Platzek T, Lang C, Grohmann G, Gi US, Baltes W. 1999. Formation of a carcinogenic aromatic amine from an azo dye by human skin bacteria in vitro. Hum Exp Toxicol 18: 552–559.
Razo-Flores E, Luijten M, Donlon B, Lettinga G, Field J. 1997. Biodegradation of selected azo dyes under methanogenic conditions. Wat Sci Tech 36(6-7): 65-72.
Safepharm Laboratories Ltd. 1990. Acute toxicity to rainbow trout. Project number 47/781. Challenge submission ID#11347
Sakuratani Y, Noguchi Y, Kobayashi K, Yamada J, Nishihara T. 2008. Molecular size as a limiting characteristic for bioconcentration in fish. J Environ Biol 29(1): 89-92.
Sandoz. 1975. Data for Batch 5 substances collected under the Canadian Environmental Protection Act, 1999, Section 71: Notice with respect to certain Batch 5 Challenge substances. Submitted to Environment Canada . Submission ID: #157
Scheringer M, MacLeod M, Wegmann F. 2006. The OECD POV and LRTP Screening Tool [Internet]. Version 2.0. Organization for Economic Cooperation and Development; Zurich (CH): Swiss Federal Institute of Technology. Distributed at OECD/UNEP Workshops on Application of Multimedia Models for Identification of Persistent Organic Pollutants, Ottawa, Canada , May 31 – June 2, 2006. Available from: http://www.sust-chem.ethz.ch/downloads/Tool2_0_Manual.pdf
Shen G, Hu S. 2008. Bioconcentration Test of C.I. Disperse Orange 30 in Fish. Prepared by Environmental Testing Laboratory, Shanghai Academy of Environmental Sciences, Shanghai, China for Dystar in the name of Ecological and Toxicological Association of the Dyes and Organic Pigments Manufacturers (ETAD) Basel, Switzerland . Report No. S-070-2007. Submitted to Environment Canada in April 2008. Challenge Submission ID#8351
Sijm DTHM, Schuurmann G, deVries PJ, Opperhuizen A. 1999. Aqueous solubility, octanol solubility, and octanol/water partition coefficient of nine hydrophobic dyes. Environ Toxicol Chem 18(6):1109-1117.
SimpleTreat [sewage treatment plant removal model]. 1997. Version 3.0. Bilthoven (NL): National Institute for Public Health and the Environment (RIVM). Available from National Institute for Public Health and the Environment (RIVM), Laboratory for Ecological Risk Assessment, PO Box 1, 3720 BA Bilthoven, The Netherlands.
[SPIN] Substances in Preparations in Nordic Countries. 2006. Database developed by the Nordic Council of Ministers. Copenhagen. Denmark . (Accessed March 2006).
http://195.215.251.229/Dotnetnuke/Home/tabid/58/Default.aspx
Stingley R, Zou W, Heinze T, Chen H, Cerniglia C. 2010. Metabolism of azo dyes by human skin microbiota. J Med Microbiol 59: 108–114.
STP Model [sewage treatment plant removal model]. 2001. Version 1.5. Peterborough (ON): Trent University, Canadian Environmental Modelling Centre. Available from: http://www.trentu.ca/academic/aminss/envmodel/models/VBSTP.html.
Study Submission, 2008. Data for Batch 7 substances collected under the Canadian Environmental Protection Act, 1999, Section 71: Notice with respect to certain Batch 7 Challenge substances. Data submitted to Environment Canada. Submission ID: #12890.
Talaska G. 2003. Aromatic amines and human urinary bladder cancer: exposure sources and epidemiology. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 21(1): 29–43.
[TaPL3] Long Range Transport and Persistence Level III model [Internet]. 2000. Version 2.10. Peterborough (ON): Trent University, Canadian Environmental Modelling Centre. Available from: http://www.trentu.ca/academic/aminss/envmodel/models/TaPL3.html.
[TOPKAT] TOxicity Prediction by Komputer Assisted Technology [Internet]. 2004. Version 6.2. San Diego (CA): Accelrys Software Inc. Available from: http://www.accelrys.com/products/topkat/index.html.
[ US EPA] United States Environmental Protection Agency. 1986–2002. Toxic Substances Control Act - Inventory Update Rule (TSCA-IUR). [Internet]. Available from: http://www.epa.gov/cgi-bin/iursrch3_2002.cgi
[ US EPA] United States Environmental Protection Agency. 2002. Fiftieth Report of the TSCA Interagency Testing Committee to the Administrator of the Environmental Protection Agency; Receipt of Report and Request for Comments. Federal Register, Vol. 67, No. 146. Available from: http://tsca-itc.syrres.com/itcrep/docs/50.pdf
Vineis P, Pirastu R. 1997. Aromatic amines and cancer. Cancer Causes Control 8(3): 346–355.
Weber E, Colon D, Baughman GL 2001. Sediment-associated reactions of aromatic amines. 1. Elucidation of sorption mechanisms. Environ Sci Tech 35(12):2470-2475.
Xu H, Heinze TM, Chen S, Cerniglia CE, Chen H. 2007. Anaerobic metabolism of 1-amino-2-naphthol-based azo dyes ( Sudan dyes) by human intestinal microflora. Appl Environ Microbiol 73: 7759–7762.
Yen CC, Perenich TA, Baughman GL. 1989. Fate of dyes in aquatic systems II. Solubility and octanol/water partition coefficients of disperse dyes. Environ Toxicol Chem 8(11):981-986.
Yen CC, Perenich TA, Baughman GL. 1991. Fate of commercial disperse dyes in sediments. Environ Toxicol Chem 10:1009-1017.
Robust Study Summary Form: Aquatic iT
| No | Item | Weight | Yes/No | Specify | ||
|---|---|---|---|---|---|---|
| 1 | Reference: Acute sludge respiration inhibition (bacteria) (Study Submission 2008) | |||||
| 2 | Substance identity: CAS RN | n/a | Y | 72968-82-2 | ||
| 3 | Substance identity: chemical name(s) | n/a | Y | Resolinrot F3BS Vorpr. 2Tr. | ||
| 4 | Chemical composition of the substance | 2 | Y | 100% of 72968-82-2 | ||
| 5 | Chemical purity | 1 | N | Not available | ||
| 6 | Persistence/stability of test substance in aquatic solution reported? | 1 | N | Not mentioned | ||
| Method | ||||||
| 7 | Reference | 1 | Y | OECD Guideline 209 (1984): Activated sludge respiration inhibition ISO 8192-1986/B | ||
| 8 | OECD, EU, national, or other standard method? | 3 | Y | |||
| 9 | Justification of the method/protocol if a non-standard method was used | 2 | ||||
| 10 | GLP (good laboratory practice) | 3 | ||||
| Test organism | ||||||
| 11 | Organism identity: name | n/a | Y | Mixed population of aquatic micro-organisms (activated sludge) | ||
| 12 | Latin or both Latin and common names reported? | 1 | N | |||
| 13 | Life cycle age/stage of test organism | 1 | N | |||
| 14 | Length and/or weight | 1 | N | |||
| 15 | Sex | 1 | N | |||
| 16 | Number of organisms per replicate | 1 | N | |||
| 17 | Organism loading rate | 1 | N | |||
| 18 | Food type and feeding periods during the acclimation period | 1 | N | |||
| Test design/conditions | ||||||
| 19 | Test type (acute or chronic) | n/a | Y | Acute | ||
| 20 | Experiment type (laboratory or field) | n/a | Y | Laboratory | ||
| 21 | Exposure pathways (food, water, both) | n/a | ||||
| 22 | Exposure duration | n/a | Y | 3 hrs | ||
| 23 | Negative or positive controls (specify) | 1 | Y | Two controls without test item were included in the test design. | ||
| 24 | Number of replicates (including controls) | 1 | Y | Two controls plus six test groups | ||
| 25 | Nominal concentrations reported? | 1 | Y | |||
| 26 | Measured concentrations reported? | 3 | Y | |||
| 27 | Food type and feeding periods during the long-term tests | 1 | n/a | |||
| 28 | Were concentrations measured periodically (especially in the chronic test)? | 1 | N | |||
| 29 | Were the exposure media conditions relevant to the particular chemical reported? (e.g., for metal toxicity – pH, DOC/TOC, water hardness, temperature) | 3 | Y | Test temperature, pH, deionzed water with synthetic sewage feed | ||
| 30 | Photoperiod and light intensity | 1 | N | |||
| 31 | Stock and test solution preparation | 1 | Y | Deionzed water with synthetic sewage feed | ||
| 32 | Was solubilizer/emulsifier used if the chemical was poorly soluble or unstable? | 1 | ||||
| 33 | If solubilizer/emulsifier was used, was its concentration reported? | 1 | ||||
| 34 | If solubilizer/emulsifier was used, was its ecotoxicity reported? | 1 | ||||
| 35 | Analytical monitoring intervals | 1 | N | |||
| 36 | Statistical methods used | 1 | N | |||
| Information relevant to the data quality | ||||||
| 37 | Was the endpoint directly caused by the chemical's toxicity, not by the organism’s health (e.g., when mortality in the control > 10%) or physical effects (e.g., “shading effect”)? | n/a | ||||
| 38 | Was the test organism relevant to the Canadian environment? | 3 | Y | |||
| 39 | Were the test conditions (pH, temperature, DO, etc.) typical for the test organism? | 1 | Y | |||
| 40 | Do system type and design (static, semi-static, flow-through; sealed or open; etc.) correspond to the substance's properties and the organism's nature/habits? | 2 | Y | |||
| 41 | Was pH of the test water within the range typical for the Canadian environment (6 to 9)? | 1 | Y | |||
| 42 | Was temperature of the test water within the range typical for the Canadian environment (5 to 27°C)? | 1 | Y | |||
| 43 | Was toxicity value below the chemical’s water solubility? | 3 | N | |||
| Results | ||||||
| 44 | Toxicity values (specify endpoint and value) | n/a | n/a | EC50 = 10 000 mg/L | ||
| 45 | Other endpoints reported - e.g., BCF/BAF, LOEC/NOEC (specify)? | n/a | n/a | |||
| 46 | Other adverse effects (e.g., carcinogenicity, mutagenicity) reported? | n/a | n/a | |||
| 47 | Score: ... % | 24/49 | ||||
| 48 | EC reliability code: | 3 | ||||
| 49 | Reliability category (high, satisfactory, low): | Low | ||||
| 50 | Comments | |||||
Robust Study Summary Form: Aquatic iT
| No | Item | Weight | Yes/No | Specify | ||
|---|---|---|---|---|---|---|
| 1 | Reference: Acute toxicity to fish (Study Submission 2008) | |||||
| 2 | Substance identity: CAS RN | n/a | Y | 72968-82-2 and 68385-96-6 | ||
| 3 | Substance identity: chemical name(s) | n/a | Y | Resolinrot F3BS Presskuchen Roh | ||
| 4 | Chemical composition of the substance | 2 | 21.25% + 63.75% + 15% (water) | |||
| 5 | Chemical purity | 1 | Y | |||
| 6 | Persistence/stability of test substance in aquatic solution reported? | 1 | ||||
| Method | ||||||
| 7 | Reference | 1 | Y | OECD Guideline 203: Fish Acute Toxicity Test | ||
| 8 | OECD, EU, national, or other standard method? | 3 | Y | |||
| 9 | Justification of the method/protocol if a non-standard method was used | 2 | ||||
| 10 | GLP (good laboratory practice) | 3 | ||||
| Test organism | ||||||
| 11 | Organism identity: name | n/a | Y | Zebra fish | ||
| 12 | Latin or both Latin and common names reported? | 1 | Y | Brachydanio rerio | ||
| 13 | Life cycle age/stage of test organism | 1 | N | |||
| 14 | Length and/or weight | 1 | Y | 2.5–3.5 cm | ||
| 15 | Sex | 1 | N | |||
| 16 | Number of organisms per replicate | 1 | Y | 10 fish per test concentration | ||
| 17 | Organism loading rate | 1 | Y | |||
| 18 | Food type and feeding periods during the acclimation period | 1 | Y | No feeding during the exposure period | ||
| Test design/conditions | ||||||
| 19 | Test type (acute or chronic) | n/a | Y | Acute | ||
| 20 | Experiment type (laboratory or field) | n/a | Y | Laboratory | ||
| 21 | Exposure pathways (food, water, both) | n/a | Y | Water | ||
| 22 | Exposure duration | n/a | Y | 96 hrs | ||
| 23 | Negative or positive controls (specify) | 1 | Y | |||
| 24 | Number of replicates (including controls) | 1 | Y | |||
| 25 | Nominal concentrations reported? | 1 | Y | |||
| 26 | Measured concentrations reported? | 3 | Y | |||
| 27 | Food type and feeding periods during the long-term tests | 1 | Y | No feeding during the exposure period | ||
| 28 | Were concentrations measured periodically (especially in the chronic test)? | 1 | N | |||
| 29 | Were the exposure media conditions relevant to the particular chemical reported? (e.g., for metal toxicity – pH, DOC/TOC, water hardness, temperature) | 3 | Y | Temperature, water hardness | ||
| 30 | Photoperiod and light intensity | 1 | Y | 16 hrs light / 8 hrs dark | ||
| 31 | Stock and test solution preparation | 1 | Y | Synthetic fresh water according to ISO 7346 with no medical pre-treatment | ||
| 32 | Was solubilizer/emulsifier used if the chemical was poorly soluble or unstable? | 1 | N | |||
| 33 | If solubilizer/emulsifier was used, was its concentration reported? | 1 | N | |||
| 34 | If solubilizer/emulsifier was used, was its ecotoxicity reported? | 1 | N | |||
| 35 | Analytical monitoring intervals | 1 | Y | The pH and oxygen values are measured at the beginning of the test and every 24 hrs after the test starts. | ||
| 36 | Statistical methods used | 1 | Y | |||
| Information relevant to the data quality | ||||||
| 37 | Was the endpoint directly caused by the chemical's toxicity, not by the organism’s health (e.g., when mortality in the control > 10%) or physical effects (e.g., “shading effect”)? | n/a | ||||
| 38 | Was the test organism relevant to the Canadian environment? | 3 | Y | |||
| 39 | Were the test conditions (pH, temperature, DO, etc.) typical for the test organism? | 1 | Y | The pH values were measured during the exposure period. | ||
| 40 | Do system type and design (static, semi-static, flow-through; sealed or open; etc.) correspond to the substance's properties and the organism's nature/habits? | 2 | Y | |||
| 41 | Was pH of the test water within the range typical for the Canadian environment (6 to 9)? | 1 | Y | |||
| 42 | Was temperature of the test water within the range typical for the Canadian environment (5 to 27°C)? | 1 | Y | 21 + 1°C | ||
| 43 | Was toxicity value below the chemical’s water solubility? | 3 | N | |||
| Results | ||||||
| 44 | Toxicity values (specify endpoint and value) | n/a | n/a | LC0 (96 h) = 1000 mg/L | ||
| 45 | Other endpoints reported - e.g., BCF/BAF, LOEC/NOEC (specify)? | n/a | N | |||
| 46 | Other adverse effects (e.g., carcinogenicity, mutagenicity) reported? | n/a | N | |||
| 47 | Score: ... % | 32/49 | ||||
| 48 | EC reliability code: | 2 | ||||
| 49 | Reliability category (high, satisfactory, low): | Satisfactory | ||||
| 50 | Comments | |||||
Robust Study Summary Form: Aquatic iT
| No | Item | Weight | Yes/No | Specify | ||
|---|---|---|---|---|---|---|
| 1 | Reference: Acute Toxicity Test to Fish (Study Submission 2008) | |||||
| 2 | Substance identity: CAS RN | n/a | Y | 72968-82-2 and 68385-96-6 | ||
| 3 | Substance identity: chemical name(s) | n/a | Y | |||
| 4 | Chemical composition of the substance | 2 | Y | 21.25% + 63.75% + 15%(water) | ||
| 5 | Chemical purity | 1 | ||||
| 6 | Persistence/stability of test substance in aquatic solution reported? | 1 | ||||
| Method | ||||||
| 7 | Reference | 1 | Y | OECD Guideline for Testing of Chemical No. 203 | ||
| 8 | OECD, EU, national, or other standard method? | 3 | Y | |||
| 9 | Justification of the method/protocol if a non-standard method was used | 2 | ||||
| 10 | GLP (good laboratory practice) | 3 | ||||
| Test organism | ||||||
| 11 | Organism identity: name | n/a | Y | Rainbow trout | ||
| 12 | Latin or both Latin and common names reported? | 1 | Y | Oncorhynchus mykiss | ||
| 13 | Life cycle age/stage of test organism | 1 | N | |||
| 14 | Length and/or weight | 1 | Y | 2.5–3.5 cm | ||
| 15 | Sex | 1 | N | |||
| 16 | Number of organisms per replicate | 1 | Y | 10 per testing group | ||
| 17 | Organism loading rate | 1 | ||||
| 18 | Food type and feeding periods during the acclimation period | 1 | Y | No feeding during the exposure period | ||
| Test design/conditions | ||||||
| 19 | Test type (acute or chronic) | n/a | Y | Acute | ||
| 20 | Experiment type (laboratory or field) | n/a | Y | Laboratory | ||
| 21 | Exposure pathways (food, water, both) | n/a | Y | Water | ||
| 22 | Exposure duration | n/a | Y | 96 hrs | ||
| 23 | Negative or positive controls (specify) | 1 | Y | |||
| 24 | Number of replicates (including controls) | 1 | Y | One control group and 3 test concentration groups | ||
| 25 | Nominal concentrations reported? | 1 | ||||
| 26 | Measured concentrations reported? | 3 | Y | |||
| 27 | Food type and feeding periods during the long-term tests | 1 | Y | No feeding during the exposure period | ||
| 28 | Were concentrations measured periodically (especially in the chronic test)? | 1 | N | |||
| 29 | Were the exposure media conditions relevant to the particular chemical reported? (e.g., for metal toxicity – pH, DOC/TOC, water hardness, temperature) | 3 | Y | |||
| 30 | Photoperiod and light intensity | 1 | Y | 16 hrs light / 8 hrs dark | ||
| 31 | Stock and test solution preparation | 1 | Y | Synthetic fresh water according to ISO 7346 with no medical pre-treatment | ||
| 32 | Was solubilizer/emulsifier used if the chemical was poorly soluble or unstable? | 1 | N | |||
| 33 | If solubilizer/emulsifier was used, was its concentration reported? | 1 | N | |||
| 34 | If solubilizer/emulsifier was used, was its ecotoxicity reported? | 1 | N | |||
| 35 | Analytical monitoring intervals | 1 | N | |||
| 36 | Statistical methods used | 1 | ||||
| Information relevant to the data quality | ||||||
| 37 | Was the endpoint directly caused by the chemical's toxicity, not by the organism’s health (e.g., when mortality in the control > 10%) or physical effects (e.g., “shading effect”)? | n/a | ||||
| 38 | Was the test organism relevant to the Canadian environment? | 3 | Y | |||
| 39 | Were the test conditions (pH, temperature, DO, etc.) typical for the test organism? | 1 | Y | |||
| 40 | Do system type and design (static, semi-static, flow-through; sealed or open; etc.) correspond to the substance's properties and the organism's nature/habits? | 2 | Y | |||
| 41 | Was pH of the test water within the range typical for the Canadian environment (6 to 9)? | 1 | Y | |||
| 42 | Was temperature of the test water within the range typical for the Canadian environment (5 to 27°C)? | 1 | Y | |||
| 43 | Was toxicity value below the chemical’s water solubility? | 3 | N | |||
| Results | ||||||
| 44 | Toxicity values (specify endpoint and value) | n/a | n/a | LC0 (96 h) = 1000 mg/L | ||
| 45 | Other endpoints reported - e.g., BCF/BAF, LOEC/NOEC (specify)? | n/a | ||||
| 46 | Other adverse effects (e.g., carcinogenicity, mutagenicity) reported? | n/a | ||||
| 47 | Score: ... % | 29/49 | ||||
| 48 | EC reliability code: | 2 | ||||
| 49 | Reliability category (high, satisfactory, low): | Satisfactory | ||||
| 50 | Comments | |||||
Robust Study Summaries Form and Instructions: Aquatic B
| No | Item | Weight | Yes/No | Specify | |
|---|---|---|---|---|---|
| 1 | Reference: Shen, Genxiang and Hu, Shuangqing. 2008. Bioconcentration Test of C.I. Disperse Orange 30 in Fish. Prepared by Environmental Testing Laboratory, Shanghai Academy of Environmental Sciences, Shanghai, China for Dystar in the name of Ecological and Toxicological Association of the Dyes and Organic Pigments Manufacturers (ETAD) Basel, Switzerland . Report No. S-070-2007. Submitted to Environment Canada in April 2008. Challenge Submission ID#8351 | ||||
| 2 | Substance identity: CAS RN | n/a | Y | 5261-31-4 | |
| 3 | Substance identity: chemical name(s) | n/a | Y | Propanenitrile, 3-[[2-(acetyloxy)ethyl][4-[(2,6-dichloro-4-nitrophenyl)azo]phenyl]amino]- | |
| 4 | Chemical composition of the substance | 2 | N | ||
| 5 | Chemical purity | 1 | N | ||
| 6 | Persistence/stability of test substance in aquatic solution reported? | 1 | N | ||
| 7 | If test material is radiolabelled, were precise position(s) of the labelled atom(s) and the percentage of radioactivity associated with impurities reported? | 2 | n/a | ||
| Method | |||||
| 8 | Reference | 1 | Y | OECD guidelines for the testing of chemicals No 305B-1996 | |
| 9 | OECD, EU, national, or other standard method? | 3 | Y | OECD | |
| 10 | Justification of the method/protocol if a non-standard method was used | 2 | |||
| 11 | GLP (good laboratory practice) | 3 | N | ||
| Test organism | |||||
| 12 | Organism identity: name | n/a | Y | Zebra fish (Brachydanio rerio) | |
| 13 | Latin or both Latin and common names reported? | 1 | Y | Both | |
| 14 | Life cycle age/stage of test organism | 1 | N | ||
| 15 | Length and/or weight | 1 | Y | Mean body length 3.91± 0.18 cm and mean body weight 0.32 ± 0.06 g | |
| 16 | Sex | 1 | N | ||
| 17 | Number of organisms per replicate | 1 | Y | 7 | |
| 18 | Organism loading rate | 1 | Y | 20 mg/L | |
| 19 | Food type and feeding periods during the acclimation period | 1 | Y | Fed a commercial fish diet until one day before start of test | |
| Test design/conditions | |||||
| 20 | Experiment type (laboratory or field) | n/a | Y | Laboratory | |
| 21 | Exposure pathways (food, water, both) | n/a | Y | Water | |
| 22 | Exposure duration | n/a | Y | 28 days | |
| 23 | Number of replicates (including controls) | 1 | Y | ||
| 24 | Concentrations | 1 | Y | 20 mg/L | |
| 25 | Food type/composition and feeding periods during the test | 1 | Y | Fish were fed two hours before water renewal | |
| 26 | If BCF/BAF derived as a ratio of chemical concentration in the organism and in water, was experiment duration equal to or longer than the time required for the chemical concentrations to reach steady state? | 3 | Y | 28 days | |
| 27 | If BCF/BAF derived as a ratio of chemical concentration in the organism and in water, were measured concentrations in both water and organism reported? | 3 | Y | ||
| 28 | Were concentrations in the test water measured periodically? | 1 | Y | On three separate days | |
| 29 | Were the exposure media conditions relevant to the particular chemical reported? (e.g., for metal toxicity – pH, DOC/TOC, water hardness, temperature) | 3 | Y | Yes every second day | |
| 30 | Photoperiod and light intensity | 1 | Y | 12:12 | |
| 31 | Stock and test solution preparation | 1 | Y | ||
| 32 | Analytical monitoring intervals | 1 | Y | Every second day for dissolved oxygen, pH and temperature | |
| 33 | Statistical methods used | 1 | Y | ||
| 34 | Was solubilizer/emulsifier used if the chemical was unstable or poorly soluble? | n/a | N | ||
| Information relevant to the data quality | |||||
| 35 | Was the test organism relevant to the Canadian environment? | 3 | Y | ||
| 36 | Were the test conditions (pH, temperature, DO, etc.) typical for the test organism? | 1 | Y | ||
| 37 | Do system type and design (static, semi-static, flow-through; sealed or open; etc.) correspond to the substance's properties and the organism's nature/habits? | 2 | Y | Semi-static | |
| 38 | Was pH of the test water within the range typical for the Canadian environment (6 to 9)? | 1 | Y | 7.22-7.84 | |
| 39 | Was temperature of the test water within the range typical for the Canadian environment (5 to 27°C)? | 1 | Y | 22-23 | |
| 40 | Was lipid content (or lipid-normalized BAF/BCF) reported? | 2 | Y | ||
| 41 | Were measured concentrations of a chemical in the test water below the chemical’s water solubility? | 3 | N | ||
| 42 | If radiolabelled test substance was used, was BCF determination based on the parent compound (i.e., not on total radiolabelled residues)? | 3 | n/a | ||
| Results | |||||
| 43 | Endpoints (BAF, BCF) and values | n/a | n/a | BCF | |
| 44 | Was BAF or BCF determined as: 1) the ratio of chemical concentration in the organism and in water, or 2) the ratio of the chemical uptake and elimination rate constants? | n/a | n/a | 1 | |
| 45 | Was BAF/BCF derived from a 1) tissue sample or 2) whole organism? | n/a | n/a | 2 | |
| 46 | Was 1) average or 2) maximum BAF/BCF used? | n/a | n/a | 1 | |
| 47 | Score: ... % | 75.0 | |||
| 48 | EC reliability code: | 2 | |||
| 49 | Reliability category (high, satisfactory, low): | Satisfactory Confidence | |||
| 50 | Comments | The present procedure is based on semi-static conditions (renewal of test solutions every 2 days). Therefore, test chemicals with very low water solubility, e.g., Disperse Orange 30, can also be characterized as to their bioconcentration potential without adding solvents or other auxiliary substances that may affect the results. | |||
Robust Study Summary Form: Aquatic iT
| No | Item | Weight | Yes/No | Specify | ||
|---|---|---|---|---|---|---|
| 1 | Reference: Sandoz 1975. Acute fish toxicity (Rainbow trout) 48hr | |||||
| 2 | Substance identity: CAS RN | n/a | Y | 5261-31-4 | ||
| 3 | Substance identity: chemical name(s) | n/a | Y | |||
| 4 | Chemical composition of the substance | 2 | N | |||
| 5 | Chemical purity | 1 | N | |||
| 6 | Persistence/stability of test substance in aquatic solution reported? | 1 | N | |||
| Method | ||||||
| 7 | Reference | 1 | Y | |||
| 8 | OECD, EU, national, or other standard method? | 3 | Y | |||
| 9 | Justification of the method/protocol if a non-standard method was used | 2 | ||||
| 10 | GLP (good laboratory practice) | 3 | Y | |||
| Test organism | ||||||
| 11 | Organism identity: name | n/a | Y | Rainbow trout | ||
| 12 | Latin or both Latin and common names reported? | 1 | Y | |||
| 13 | Life cycle age/stage of test organism | 1 | N | |||
| 14 | Length and/or weight | 1 | Y | |||
| 15 | Sex | 1 | N | |||
| 16 | Number of organisms per replicate | 1 | N | |||
| 17 | Organism loading rate | 1 | N | |||
| 18 | Food type and feeding periods during the acclimation period | 1 | N | |||
| Test design/conditions | ||||||
| 19 | Test type (acute or chronic) | n/a | Y | Acute | ||
| 20 | Experiment type (laboratory or field) | n/a | Y | Laboratory | ||
| 21 | Exposure pathways (food, water, both) | n/a | ||||
| 22 | Exposure duration | n/a | Y | 48 | ||
| 23 | Negative or positive controls (specify) | 1 | N | |||
| 24 | Number of replicates (including controls) | 1 | N | |||
| 25 | Nominal concentrations reported? | 1 | N | |||
| 26 | Measured concentrations reported? | 3 | N | |||
| 27 | Food type and feeding periods during the long-term tests | 1 | N | |||
| 28 | Were concentrations measured periodically (especially in the chronic test)? | 1 | N | |||
| 29 | Were the exposure media conditions relevant to the particular chemical reported? (e.g., for metal toxicity – pH, DOC/TOC, water hardness, temperature) | 3 | N | |||
| 30 | Photoperiod and light intensity | 1 | N | |||
| 31 | Stock and test solution preparation | 1 | N | |||
| 32 | Was solubilizer/emulsifier used if the chemical was poorly soluble or unstable? | 1 | N | |||
| 33 | If solubilizer/emulsifier was used, was its concentration reported? | 1 | ||||
| 34 | If solubilizer/emulsifier was used, was its ecotoxicity reported? | 1 | ||||
| 35 | Analytical monitoring intervals | 1 | N | |||
| 36 | Statistical methods used | 1 | N | |||
| Information relevant to the data quality | ||||||
| 37 | Was the endpoint directly caused by the chemical's toxicity, not by the organism’s health (e.g., when mortality in the control > 10%) or physical effects (e.g., “shading effect”)? | n/a | ||||
| 38 | Was the test organism relevant to the Canadian environment? | 3 | Y | |||
| 39 | Were the test conditions (pH, temperature, DO, etc.) typical for the test organism? | 1 | N | |||
| 40 | Do system type and design (static, semi-static, flow-through; sealed or open; etc.) correspond to the substance's properties and the organism's nature/habits? | 2 | N | |||
| 41 | Was pH of the test water within the range typical for the Canadian environment (6 to 9)? | 1 | N | |||
| 42 | Was temperature of the test water within the range typical for the Canadian environment (5 to 27°C)? | 1 | Y | |||
| 43 | Was toxicity value below the chemical’s water solubility? | 3 | N | |||
| Results | ||||||
| 44 | Toxicity values (specify endpoint and value) | n/a | n/a | 48HR LC50>700mg/L | ||
| 45 | Other endpoints reported - e.g., BCF/BAF, LOEC/NOEC (specify)? | n/a | ||||
| 46 | Other adverse effects (e.g., carcinogenicity, mutagenicity) reported? | n/a | ||||
| 47 | Score: ... % | 28.9 | ||||
| 48 | EC reliability code: | 4 | ||||
| 49 | Reliability category (high, satisfactory, low): | Not Satisfactory | ||||
| 50 | Comments | |||||
Robust Study Summary Form: Aquatic iT
| No | Item | Weight | Yes/No | Specify |
|---|---|---|---|---|
| 1 | Reference: Environment Canada . 1995. NSN submission. | |||
| 2 | Substance identity: CAS RN | n/a | N | |
| 3 | Substance identity: chemical name(s) | n/a | Y | |
| 4 | Chemical composition of the substance | 2 | N | |
| 5 | Chemical purity | 1 | N | |
| 6 | Persistence/stability of test substance in aquatic solution reported? | 1 | N | |
| Method | ||||
| 7 | Reference | 1 | Y | OECD 203 |
| 8 | OECD, EU, national, or other standard method? | 3 | Y | |
| 9 | Justification of the method/protocol if a non-standard method was used | 2 | Not applicable | |
| 10 | GLP (good laboratory practice) | 3 | Y | |
| Test organism | ||||
| 11 | Organism identity: name | n/a | Y | Rainbow trout |
| 12 | Latin or both Latin and common names reported? | 1 | Y | |
| 13 | Life cycle age/stage of test organism | 1 | Y | Mean length 51 mm and mean weight 1.54 g |
| 14 | Length and/or weight | 1 | Y | See above |
| 15 | Sex | 1 | Not applicable | |
| 16 | Number of organisms per replicate | 1 | Y | 10 |
| 17 | Organism loading rate | 1 | Y | |
| 18 | Food type and feeding periods during the acclimation period | 1 | Y | |
| Test design/conditions | ||||
| 19 | Test type (acute or chronic) | n/a | Y | Acute |
| 20 | Experiment type (laboratory or field) | n/a | y | Lab |
| 21 | Exposure pathways (food, water, both) | n/a | y | Water |
| 22 | Exposure duration | n/a | y | 96 hrs |
| 23 | Negative or positive controls (specify) | 1 | Y | 3 |
| 24 | Number of replicates (including controls) | 1 | Y | 2 |
| 25 | Nominal concentrations reported? | 1 | Y | 320 to 3200 mg/L |
| 26 | Measured concentrations reported? | 3 | N | |
| 27 | Food type and feeding periods during the long-term tests | 1 | Not applicable | |
| 28 | Were concentrations measured periodically (especially in the chronic test)? | 1 | N | |
| 29 | Were the exposure media conditions relevant to the particular chemical reported? (e.g., for metal toxicity – pH, DOC/TOC, water hardness, temperature) | 3 | Y | |
| 30 | Photoperiod and light intensity | 1 | Y | |
| 31 | Stock and test solution preparation | 1 | Y | |
| 32 | Was solubilizer/emulsifier used if the chemical was poorly soluble or unstable? | 1 | N | |
| 33 | If solubilizer/emulsifier was used, was its concentration reported? | 1 | ||
| 34 | If solubilizer/emulsifier was used, was its ecotoxicity reported? | 1 | ||
| 35 | Analytical monitoring intervals | 1 | Y | |
| 36 | Statistical methods used | 1 | Y | |
| Information relevant to the data quality | ||||
| 37 | Was the endpoint directly caused by the chemical's toxicity, not by the organism’s health (e.g., when mortality in the control > 10%) or physical effects (e.g., “shading effect”)? | n/a | Y | |
| 38 | Was the test organism relevant to the Canadian environment? | 3 | Y | |
| 39 | Were the test conditions (pH, temperature, DO, etc.) typical for the test organism? | 1 | Y | |
| 40 | Do system type and design (static, semi-static, flow-through; sealed or open; etc.) correspond to the substance's properties and the organism's nature/habits? | 2 | Y | |
| 41 | Was pH of the test water within the range typical for the Canadian environment (6 to 9)? | 1 | Y | |
| 42 | Was temperature of the test water within the range typical for the Canadian environment (5 to 27°C)? | 1 | Y | |
| 43 | Was toxicity value below the chemical’s water solubility? | 3 | Unknown water solubility | |
| Results | ||||
| 44 | Toxicity values (specify endpoint and value) | n/a | n/a | 96-hr LC50 |
| 45 | Other endpoints reported - e.g., BCF/BAF, LOEC/NOEC (specify)? | n/a | N | |
| 46 | Other adverse effects (e.g., carcinogenicity, mutagenicity) reported? | n/a | N | |
| 47 | Score: ... % | 77.5 | ||
| 48 | EC reliability code: | 2 | ||
| 49 | Reliability category (high, satisfactory, low): | Satisfactory Confidence | ||
| 50 | Comments | |||
Robust Study Summary Form: Aquatic iT
| No | Item | Weight | Yes/No | Specify |
|---|---|---|---|---|
| 1 | Reference: Safepharm Laboratories Ltd. 1990. Acute toxicity to rainbow trout. Project number 47/781 | |||
| 2 | Substance identity: CAS RN | n/a | Y | 5261-31-4 |
| 3 | Substance identity: chemical name(s) | n/a | Y | |
| 4 | Chemical composition of the substance | 2 | N | |
| 5 | Chemical purity | 1 | N | |
| 6 | Persistence/stability of test substance in aquatic solution reported? | 1 | N | |
| Method | ||||
| 7 | Reference | 1 | N | |
| 8 | OECD, EU, national, or other standard method? | 3 | N | |
| 9 | Justification of the method/protocol if a non-standard method was used | 2 | N | |
| 10 | GLP (good laboratory practice) | 3 | n/a | |
| Test organism | ||||
| 11 | Organism identity: name | n/a | Rainbow trout | |
| 12 | Latin or both Latin and common names reported? | 1 | Y | |
| 13 | Life cycle age/stage of test organism | 1 | Y | |
| 14 | Length and/or weight | 1 | Y | |
| 15 | Sex | 1 | n/a | |
| 16 | Number of organisms per replicate | 1 | Y | 3-10 |
| 17 | Organism loading rate | 1 | Y | 0.70 g bodyweight/L |
| 18 | Food type and feeding periods during the acclimation period | 1 | n/a since acute test | |
| Test design/conditions | ||||
| 19 | Test type (acute or chronic) | n/a | Acute | |
| 20 | Experiment type (laboratory or field) | n/a | Lab | |
| 21 | Exposure pathways (food, water, both) | n/a | Water | |
| 22 | Exposure duration | n/a | 96 hrs | |
| 23 | Negative or positive controls (specify) | 1 | Y | Positive |
| 24 | Number of replicates (including controls) | 1 | Y | Two at definitive study |
| 25 | Nominal concentrations reported? | 1 | Y | 3 |
| 26 | Measured concentrations reported? | 3 | N | |
| 27 | Food type and feeding periods during the long-term tests | 1 | n/a | |
| 28 | Were concentrations measured periodically (especially in the chronic test)? | 1 | N | |
| 29 | Were the exposure media conditions relevant to the particular chemical reported? (e.g., for metal toxicity – pH, DOC/TOC, water hardness, temperature) | 3 | Y | |
| 30 | Photoperiod and light intensity | 1 | N | |
| 31 | Stock and test solution preparation | 1 | N | |
| 32 | Was solubilizer/emulsifier used if the chemical was poorly soluble or unstable? | 1 | N | |
| 33 | If solubilizer/emulsifier was used, was its concentration reported? | 1 | n/a | |
| 34 | If solubilizer/emulsifier was used, was its ecotoxicity reported? | 1 | n/a | |
| 35 | Analytical monitoring intervals | 1 | Y | |
| 36 | Statistical methods used | 1 | N | |
| Information relevant to the data quality | ||||
| 37 | Was the endpoint directly caused by the chemical's toxicity, not by the organism’s health (e.g., when mortality in the control > 10%) or physical effects (e.g., “shading effect”)? | n/a | Y | |
| 38 | Was the test organism relevant to the Canadian environment? | 3 | Y | |
| 39 | Were the test conditions (pH, temperature, DO, etc.) typical for the test organism? | 1 | Y | |
| 40 | Do system type and design (static, semi-static, flow-through; sealed or open; etc.) correspond to the substance's properties and the organism's nature/habits? | 2 | n/a | |
| 41 | Was pH of the test water within the range typical for the Canadian environment (6 to 9)? | 1 | N | No pH given |
| 42 | Was temperature of the test water within the range typical for the Canadian environment (5 to 27°C)? | 1 | Y | |
| 43 | Was toxicity value below the chemical’s water solubility? | 3 | N | Water solubility for this substance was 0.07 |
| Results | ||||
| 44 | Toxicity values (specify endpoint and value) | n/a | 96-hr LC50 > 100 mg/L | |
| 45 | Other endpoints reported - e.g., BCF/BAF, LOEC/NOEC (specify)? | n/a | N | |
| 46 | Other adverse effects (e.g., carcinogenicity, mutagenicity) reported? | n/a | N | |
| 47 | Score: ... % | 43.6 | ||
| 48 | EC reliability code: | 3 | ||
| 49 | Reliability category (high, satisfactory, low): | Low Confidence | ||
| 50 | Comments | |||
Most models are not suitable for DADM, as it is an azo dye. Only the EPI SUITE (BIOWIN) and CPOPs (CATABOL) models have been addressed, using as input the SMILES of the subject chemical, to estimate the water solubility and predict bioaccumulation.
SMILES of DADM: CS(=O)(=O)Nc1cc(N(CCC)CCC)ccc1N=Nc2c(C#N)cc(C)cc2C#N
| Phy- sical and Che- mical Fate |
Fate | Fate | Fate | Fate | Fate | Fate | PBT Profiling | Eco- toxi- city |
|
|---|---|---|---|---|---|---|---|---|---|
| Model input parameters | EPI Suite (all models, inclu- ding: AOP- WIN, KOC- WIN, BCF- WIN BIO- WIN and ECO- SAR) |
STP (1) ASTreat (2) Simple- Treat (3) (required inputs are different depen- ding on model) |
EQC (required inputs are different if Type I vs. Type II chemical) |
TaPL3 (required inputs are different if Type 1 vs. Type 2 chemical) |
OECD POPs Tool | Arnot- Gobas BCF/BAF Model | Gobas Wolf BMF Model | Canadian POPs (including: Catabol, BCF Mitigating Factors Model, OASIS Toxicity Model) |
Arti- ficial Intel- ligence Expert System (AIEPS) / TOP- KAT/ ASTER |
| SMILES code | x (BIO- WIN only) |
n/a | n/a | n/a | n/a | n/a | n/a | x (CATA- BOL only) |
n/a |