Screening Assessment for the Challenge
This page has been archived on the Web
Information identified as archived is provided for reference, research or recordkeeping purposes. It is not subject to the Government of Canada Web Standards and has not been altered or updated since it was archived. Please contact us to request a format other than those available.
Archived
Phenol, 2,4,6-tris(1,1-dimethylethyl)-
(2,4,6-tri-tert-butylphenol)
Chemical Abstracts Service Registry Number
732-26-3
Environment Canada
Health Canada
November 2008
Table of Contents
Pursuant to section 74 of the Canadian Environmental Protection Act, 1999 (CEPA 1999), the Ministers of the Environment and of Health have conducted a screening assessment on phenol, 2,4,6-tris(1,1-dimethylethyl)- (2,4,6-tri-tert-butylphenol), Chemical Abstracts Service Registry Number 732-26-3. This substance was identified as a high priority for screening assessment and included in the Ministerial Challenge because it was found to meet the ecological categorization criteria for persistence, bioaccumulation potential and inherent toxicity to non-human organisms and is known to be in commerce in Canada.
The substance 2,4,6-tri-tert-butylphenol was not considered to be a high priority for assessment of potential risks to human health, based upon application of the simple exposure and hazard tools developed by Health Canada for categorization of substances on the Domestic Substances List. Therefore, this assessment focuses on information relevant to the evaluation of ecological risks.
The substance 2,4,6-tri-tert-butylphenol is an antioxidant which can be used as a fuel, oil, gasoline or lubricant additive. The only use in Canada is as a fuel additive. The substance is not naturally produced in the environment. Although it was not reported to be manufactured in Canada above the reporting threshold, total imports were reported to be in a quantity between 10 000 - 100 000 kg in the year 2000. Voluntary submissions received in 2007 reported a quantity between 1 000 and 10 000 kg of this substance being imported into Canada in the calendar year 2006. This substance was also reported to be used below the reporting threshold. Quantities of 2,4,6-tri-tert-butylphenol imported into Canada, along with its use as a fuel additive, indicate that this chemical may potentially be released into the Canadian environment. Although the information gathered to date indicates that the only current use of the substance is as a fuel additive, 2,4,6-tri-tert-butylphenol has been used in the past in Canada as a lubricant additive, and this is a recognized use for the substance elsewhere. As a result, the calculations used to make this assumption for release include a minor use of the substance as a lubricant additive.
Based on certain assumptions and reported use patterns, most of 2,4,6-tri-tert-butylphenol may be destroyed through the combustion of fuel/oil. Small proportions are estimated to be released to water (0.3%), air (1.6%) and soil (0.1%). There is also a proportion estimated to be transferred to waste disposal sites (4.8%). Given this substance's physical and chemical characteristics, it is expected to strongly adsorb to soil and sediment. This substance is not likely to be metabolized (given its highly branched chemical structure) and will have a tendency to partition to lipids (fat) of organisms because of its hydrophobic nature.
Based on its physical and chemical properties, 2,4,6-tri-tert-butylphenol does not degrade quickly in the environment; it is expected to be persistent in water, soil and sediments. Empirical and modelled data indicate that this substance also has the potential to accumulate in organisms and may biomagnify in trophic food chains. The substance has been determined to meet the persistence and bioaccumulation criteria as set out in the Persistence and Bioaccumulation Regulations. In addition, acute aquatic toxicity values suggest that the substance is highly hazardous to aquatic organisms.
Given that long-term risks associated with persistent and bioaccumulative substances cannot at present be reliably predicted, quantitative risk estimates have limited relevance. A conservative response to uncertainty is justified based upon the bioaccumulative and persistent nature of this substance.
In addition and where relevant, research and monitoring will support verification of assumptions used during the screening assessment.
Based on the information available, it is proposed that 2,4,6-tri-tert-butylphenol meets one or more of the criteria set out in section 64 of CEPA 1999.
The Canadian Environmental Protection Act, 1999 (CEPA 1999) (Canada 1999) requires the Minister of the Environment and the Minister of Health to conduct screening assessments of substances that have met the categorization criteria set out in the Act to determine whether these substances present or may present a risk to the environment or human health. Based on the results of a screening assessment, the Ministers can propose to take no further action with respect to the substance, to add the substance to the Priority Substances List (PSL) for further assessment, or to recommend that the substance be added to the List of Toxic Substances in Schedule 1 of the Act and, where applicable, the implementation of virtual elimination.
Based on the information obtained through the categorization process, the Ministers identified a number of substances as high priorities for action. These include substances that
- met all of the ecological categorization criteria, including persistence (P), bioaccumulation potential (B) and inherent toxicity to aquatic organisms (iT), and were believed to be in commerce in Canada; and/or
- met the categorization criteria for greatest potential for exposure (GPE) or presented an intermediate potential for exposure (IPE), and had been identified as posing a high hazard to human health based on classifications by other national or international agencies for carcinogenicity, genotoxicity, developmental toxicity or reproductive toxicity.
The Ministers therefore published a notice of intent in the Canada Gazette, Part I, on December 9, 2006 (Canada 2006), that challenged industry and other interested stakeholders to submit, within specified timelines, specific information that may be used to inform risk assessment, and to develop and benchmark best practices for the risk management and product stewardship of those substances identified as high priorities.
The substance 2,4,6-tri-tert-butylphenol was identified as a high priority for assessment of ecological risk as it was found to be persistent, bioaccumulative and inherently toxic to aquatic organisms and is believed to be in commerce in Canada. The Challenge for this substance was published in the Canada Gazette on May 12, 2007 (Canada 2007). A substance profile was released at the same time. The substance profile presented the technical information available prior to December 2005 that formed the basis for categorization of this substance. As a result of the Challenge, and the survey notice issued on November 17, 2001 (Canada 2001), several submissions of information were received for this substance pertaining to the manufacture, import, use, and release of this substance in Canada.
Although 2,4,6-tri-tert-butylphenol was determined to be a high priority for assessment with respect to the environment, it did not meet the criteria for high hazard to human health based on the application of simple exposure and hazard tools developed by Health Canada for categorization of substances on the DSL. Therefore, this assessment focuses principally on information relevant to the evaluation of ecological risks.
Under CEPA 1999, screening assessments focus on information critical to determining whether a substance meets the criteria for defining a chemical as toxic as set out in section 64 of the Act, where
"64. [...] a substance is toxic if it is entering or may enter the environment in a quantity or concentration or under conditions that
(a) have or may have an immediate or long-term harmful effect on the environment or its biological diversity;
(b) constitute or may constitute a danger to the environment on which life depends; or
(c) constitute or may constitute a danger in Canada to human life or health."
Screening assessments examine scientific information and develop conclusions by incorporating a weight of evidence approach and precaution.
This screening assessment includes consideration of information on chemical properties, hazards, uses and exposure, including the additional information submitted under the Challenge. Data relevant to the screening assessment of this substance were identified in original literature, review and assessment documents, stakeholder research reports and from recent literature searches, up to October 2007 for the ecological sections of the document. Key studies were critically evaluated; modelling results may have been used to reach conclusions. When available and relevant, information presented in hazard assessment from other jurisdictions was considered. The screening assessment does not represent an exhaustive or critical review of all available data. Rather, it presents the most critical studies and lines of evidence pertinent to the conclusion.
This screening assessment was prepared by staff in the Existing Substances Programs at Environment Canada and incorporates input from other programs within the department and at Health Canada . Additionally, the draft of this screening assessment was subject to a 60-day public comment period. The critical information and considerations upon which the assessment is based are summarized below.
For the purposes of this document, this substance will be referred to as 2,4,6-tri-tert-butylphenol, which has been derived from the Philippine Inventory of Chemicals and Chemical Substances (PICCS) inventory name.
| Chemical Abstracts Service Registry Number (CAS RN) | 732-26-3 |
| Name on Domestic Substances List (DSL) | Phenol, 2,4,6-tris(1,1-dimethylethyl)- |
| Inventory names 1 | Phenol, 2,4,6-tris(1,1-dimethylethyl)- (TSCA, ENCS, AICS, PICCS, ASIA-PAC) 2,4,6-Tri-tert-butylphenol (DSL, EINECS, PICCS) 2,4,6-Tris(1,1-dimethylethyl)phenol (ECL) 2,4,6-TRI-TERT-BUTYL PHENOL (PICCS) |
| Other names | 2,4,6-Tri-t-butylphenol; 2,4,6-Tri-tert-butyl-1-hydroxybenzene; 2,4,6-Tris(tert-butyl)phenol; Alkofen B; NSC 14459; P 23; P 23 (phenol); Phenol, 2,4,6-tri(1,1-dimethylethyl)-; Phenol, 2,4,6-tri-tert-butyl-; TM 02; Tri-tert-butylphenol; Voidox |
| Chemical group (DSL Stream) |
Discrete organics |
| Major chemical class or use | Phenols |
| Major chemical sub-class | Alkylphenols |
| Chemical formula | C18H30O |
| Chemical structure | 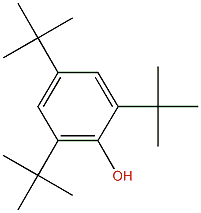 |
| SMILES | Oc(c(cc(c1)C(C)(C)C)C(C)(C)C)c1C(C)(C)C |
| Molecular mass | 262.44 g/mol |
[1] National Chemical Inventories (NCI). 2006: AICS (Australian Inventory of Chemical Substances); ASIA-PAC (Asia-Pacific Substances Lists); ECL (Korean Existing Chemicals List); EINECS (European Inventory of Existing Commercial Chemical Substances); ENCS (Japanese Existing and New Chemical Substances); PICCS (Philippine Inventory of Chemicals and Chemical Substances); and TSCA (Toxic Substances Control Act Chemical Substance Inventory).
Table 2 contains experimental and modelled physical and chemical properties of 2,4,6-tri-tert-butylphenol that are relevant to its environmental fate.
| Property | Type | Value | Temperature (°C) | Reference |
|---|---|---|---|---|
| Melting point (°C) | Experimental | 131 | -- | PhysProp 2006 |
| Modelled | 104.33 | -- | MPBPWIN 2000 | |
| Boiling point (°C) | Experimental | 278 | -- | PhysProp 2006 |
| Modelled | 324.5 | -- | MPBPWIN 2000 | |
| Density (kg/m3) | No information available | |||
| Vapour Pressure (Pa) | Modelled | 0.03 (2E-4 mm Hg) |
25 | MPBPWIN 2000 |
| Modelled | 0.27 (2E-4 mm Hg) |
-- | HENRYWIN 2000 | |
| Henry's Law Constant (Pa-m3/mol) | Modelled | 0.70-0.98 ((6.9-9.7) ×10-6 atm-m3/mole ) |
25 | HENRYWIN 2000 |
| log Kow (octanol-water partition coefficient; dimensionless) | Experimental | 60.6 | -- | MITI (1992) |
| Modelled | 6.39 | -- | KOWWIN 2000 | |
| log Koc (organic carbon-water partition coefficient; dimensionless) | Modelled | 5.01 - 5.12 | -- | PCKOCWIN 2000 |
| Water solubility (mg/L) | Experimental | 35 | 15 - 25 | MITI 1992 |
| Modelled | 0.512 | 25 | WSKOWWIN 2000 | |
| Other solubilities (g/L) | No information available | |||
| pKa (acid dissociation constant; dimensionless) | Experimental | 12.2 | 25 | Serjeant & Dempsey 1979 |
[1] If different, values in brackets represent the original values as reported by the authors or as estimated by the models.
The substance 2,4,6-tri-tert-butylphenol is not naturally produced in the environment.
Information was voluntarily submitted for this substance as part of the Challenge for the calendar year 2006 (Environment Canada 2007a). This information indicated that fewer than ten companies imported a combined total of between 1000-10 000 kg of this substance into Canada in the year 2006, whether alone, in a product, in a mixture or in a manufactured item. In addition, less than ten companies reported using 2,4,6-tri-tert-butylphenol (whether alone, in a product, in a mixture or in a manufactured item) in a quantity below the prescribed threshold (that is, in a quantity greater than or equal to 1000 kg at a concentration greater than 50%, or in a quantity greater than or equal to 10 000 kg at any concentration). There were no reports voluntarily received on the manufacture of 2,4,6-tri-tert-butylphenol in Canada. Fewer than ten companies indicated stakeholder interest in this substance.
As a result of a survey conducted under section 71 of CEPA 1999 for the year 2000 (Canada 2001), it was determined that the substance 2,4,6-tri-tert-butylphenol was not manufactured in Canada in the year 2000 in a quantity meeting the 100 kg reporting threshold (Environment Canada 2001). Less than ten companies reported importing a total of between 10 000 and 100 000 kg of 2,4,6-tri-tert-butylphenol into Canada in 2000. The survey did not require companies to report whether low concentration products (at a concentration less than 1% w/w) were manufactured or imported into Canada.
The quantity reported to be manufactured, imported or in commerce in Canada during the calendar year 1986 was between 1 000 000 and 10 000 000 kg (Environment Canada 1988). There were fewer than four notifiers for the calendar years 1984 to1986. Compared with the survey conducted in 2001, there appears to be a decreasing trend in the quantity of this substance either manufactured, imported or in commerce during this time.
Elsewhere, 2,4,6-tri-tert-butylphenol has been identified as a High Production Volume (HPV) chemical under the United States Environmental Protection Agency's HPV Challenge Program (U.S. EPA 2007a,b) and is included on the OECD's list of HPV chemicals (OECD 2004a). This substance is included on the Oslo-Paris (OSPAR) Commission's list of chemicals for priority action (OSPAR 2007; ICES 2007), but is considered a low production volume chemical (OSPAR 2006). Information provided by OSPAR Contracting Parties indicated possible uses at 33 tonnes in Denmark and 1 tonne in Norway (OSPAR 2006). The Swedish Product Register included 19 registrations of products containing 2,4,6-tri-tert-butylphenol, which corresponded to a use of 1 tonne in 2001 (OSPAR 2006).
Information submitted in response to the section 71 survey notice (Canada 2001; Environment Canada 2001) and information voluntarily submitted in response to the Challenge (Environment Canada 2007a) indicates that 2,4,6-tri-tert-butylphenol is currently used in Canada as a fuel, oil, gasoline additive. However, the information gathered to date indicates that the only known use of the substance is as a fuel additive.
The use pattern codes and corresponding applications noted by companies for the 2001 survey included:
- 1 - destructive uses: this application includes, but is not limited to, feedstock, fuels (e.g., gasoline, kerosene, gas oil, fuel oil, petroleum gas, non-mineral oil, etc.), fuel additives (e.g., anti-fouling agents, antiknock agents, deposit modifiers, fuel oxidizers, etc.), chemical intermediates (e.g., monomers, prepolymers, etc.); and
- 4 - dispersive uses where the substance is released into the environment or is used in the environment: this application includes, but is not limited to, pesticides, fertilizers, salt for de-icing, solvents, cutting fluids, aerosol propellants, hydraulic fluids, lubricants and additives, cleaning/washing agents and additives (e.g., detergents, soaps, dry-cleaning solvents, optical brighteners in detergents, etc.), plant protection products, agricultural products, explosives (e.g., blasting agents, detonators, incendiaries, etc.).
The use pattern codes and corresponding applications noted by companies for the 2007 survey included:
- 09 - antioxidant, corrosion inhibitor, tarnish inhibitor, scavenger, antiscaling agent; and
- 25 - fuel, fuel additive.
Information on the use of 2,4,6-tri-tert-butylphenol that was received from the section 71 survey notice (Environment Canada 2001) indicates that all of this substance was reported for use in Canada as a fuel and oil/lubricant additive in the year 2000. It was also stated in the section 71 that 2,4,6-tri-tert-butylphenol is used as a lubricant additive. However, it was later confirmed that the only known use of this substance in Canada is as a fuel additive, and it does not seem to be currently used as a lubricant additive in Canada. Use codes were reported for both destructive uses and dispersive uses (where the substance is released into the environment or is used in the environment). Given these uses, the substance may be destroyed (i.e., through the combustion of fuel and oil) or released to the environment or used in the environment.
There is some uncertainty about the current use pattern of 2,4,6-tri-tert-butylphenol within the European Union (EU). Five possible applications have been identified in the EU (OSPAR 2006), including: as a chemical intermediate in the production of antioxidants used in rubber and plastic, as a lubricating agent in the transport sector, as a by-product in the production of 4-tert-butylphenol, as an additive for gasoline and fuel oil distillate, and use in the offshore sector.
Importation, manufacture and use of substance 2,4,6-tri-tert-butylphenol is banned in Japan (NITE 2007).
The substance 2,4,6-tri-tert-butylphenol is not reported to be naturally produced in the environment. In response to a section 71 notice (Environment Canada 2001), fewer than ten companies importing 2,4,6-tri-tert-butylphenol reported a release of the substance in the year 2000, corresponding to 20 kg of the substance released to air (based on estimates of vapour losses). The section 71 survey did not gather release information from users of the substance and because of the use of the chemical as a fuel and oil/lubricant additive, it is possible that environmental releases to the environment (as reported above) were underestimated in the 2001 survey.
As a result of the Challenge, several voluntary submissions were received for 2,4,6-tri-tert-butylphenol (Canada 2007, Environment Canada 2007a). Fewer than ten companies reported release of this substance (whether alone, in a product, in a mixture or in a manufactured item) and fewer than ten companies reported transferring the substance to an off-site waste-management facility (including the substance contained in a mixture, in a product or in a manufactured item) for the 2006 calendar year. It should be noted that companies were requested to round figures to the nearest kilogram, and in the above instances, the releases or transfers are considered to be each less than 0.5 kg of the substance.
Given the uses of this substance noted earlier, the substance may be destroyed through the combustion of fuel. Other uses (that are non-destructive) may result in the release of this substance into the Canadian environment from sources such as the transport and use of gasoline for home uses or recreational activities. Incidental spills with respect to recreational activities (e.g., boating) may result in the substance being dispersed if released into the water compartment. However, given the substance's low Henry's Law constant and its high sorptive capacity, releases to the environment would likely result in partitioning to soil and sediments. The disposal of consumer and industrial products containing 2,4,6-tri-tert-butylphenol may also lead to the transfer of this substance to landfills. The compartments to which this substance may be released include air, soil and water.
Mass Flow Tool (MFT)
To estimate potential releases of the substance to the environment at different stages of its life cycle, a Mass Flow Tool was developed (Environment Canada 2007b). Empirical data concerning releases of specific substances to the environment are seldom available. Therefore, for each identified type of use of the substance, the proportion and quantity of release to the different environmental media are estimated, as is the proportion of the substance chemically transformed or sent for waste disposal. Unless specific information on the rate or potential for release of the substance from landfills and incinerators is available, the Mass Flow Tool does not quantitatively account for releases to the environment from disposal.
Assumptions and input parameters used in making the release estimates are based on information obtained from a variety of sources including responses to regulatory surveys, Statistics Canada, manufacturers' websites and technical databases and documents. Of particular relevance are emission factors, which are generally expressed as the fraction of a substance released to the environment, particularly during its manufacture, processing, and use associated with industrial processes. Sources of such information include emission scenario documents, often developed under the auspices of the Organization for Economic Cooperation and Development (OECD), and default assumptions used by different international chemical regulatory agencies. It is noted that the level of uncertainty in the mass of substance and quantity released to the environment generally increases toward the end of its life-cycle.
| Fate | Proportion of the mass (%)[1] | Major life cycle stage involved[2] |
|---|---|---|
| Released to receiving media: | ||
| To soil | 0.1 | Consumer use |
| To air | 1.6 | Formulation and Consumer use |
| To Sewer[3] | 0.3 | Formulation |
| Chemically transformed | 93.2 | Consumer use (e.g., fuel combustion) |
| Transferred to wastedisposal sites (e.g., landfill, incineration) | 4.8 | Waste disposal |
[1] For 2,4,6-tri-tert-butylphenol, information from the following OECD emission scenario documents was used to estimate releases to the environment and the distribution of the substance as summarized in this table: Canada 2003, OECD 2004b, NGGIC 2006. Values presented for release to environmental media do not account for possible mitigation measures that may be in place in some locations (e.g., partial removal by sewage treatment plants). Specific assumptions used in the derivation of these estimates are summarized in Environment Canada 2008a
[2] Applicable stage(s): production-formulation-industrial use-consumer use-service life of article/product-waste disposal.
[3] Wastewater before any form of treatment
Results predict that 2,4,6-tri-tert-butylphenol releases to soil, air, and water (totalling 2%) may occur during the processing, distribution and use of this substance as a fuel additive, oil and possibly as an oil/lubricant additive. The main loss is predicted to be transformation (93.2%) associated with fuel/oil combustion. A proportion (4.8%) is also estimated to be transferred to waste disposal sites (e.g., a landfill) as a result of this substance's possible use as an oil/lubricant and possible use as a lubricant additive (i.e., packaging wastes, used oil).
When using this tool, the assumption is made that this substance behaves similarly to gasoline when used as a fuel additive. Based on existing regulations (Canada 2003) and a worst-case scenario, it is assumed that loss by transformation during combustion is 99.6%. The above estimates take into account possible releases related to bag handling and blending processes. Since additives are processed with gasoline at the refinery before filling up road tankers for regional distribution, it is assumed that there are no releases during this stage. Accidental spills have not been considered during tanker transport of gasoline.
The estimated releases and losses predicted using this tool do not take into account transport and home uses of gasoline (e.g., lawn mowers, generators) or recreational activities (e.g., boats). These uses may result in incidental releases of this substance into various environmental media. In addition, releases at the gas station are expected to occur, including fugitive emissions and accidental spills at the pump with potential runoff into sewers. It should be noted that although the information gathered to date indicates that the only current use of the substance is as a fuel additive, and this is a recognized use for the substance elsewhere. Therefore, this potential minor use has been included in the calculations to estimate potential releases and losses.
As a result of the above releases and losses predicted by the Mass Flow Tool and the section 71 data discussed previously, it is estimated that a portion (2%) of the 2,4,6-tri-tert-butylphenol that is in commerce in Canada is being released to the environment.
Based on its physical-chemical properties (Table 2) and the results of the Equilibrium Criterion (EQC) model (EQC 2003; Table 4), 2,4,6-tri-tert-butylphenol is expected to partition predominantly to soil and sediment.
| Substance release to: | Percentage of substance partitioning into each compartment |
|||
|---|---|---|---|---|
| Air | Water | Soil | Sediment | |
| Air (100%) | 0 | 0 | 100 | 0 |
| Water (100%) | 0 | 2 | 4 | 94 |
| Soil (100%) | 0 | 0 | 100 | 0 |
The relatively high acid dissociation constant (pKa) of 12.2 indicates that half of the chemical will be dissociated at pH 12.2. In water bodies at environmentally relevant pH (6-9), 100% will be undissociated. This indicates that the partitioning of 2,4,6-tri-tert-butylphenol is by passive diffusion of the neutral chemical into organic materials including the lipid phases of organisms, and that little partitioning will occur through other sorption mechanisms. The relatively low proportion of dissociated chemical also indicates that partitioning behaviour predicted on the basis of the log Kow and log Koc values are likely to be relevant.
A vapour pressure of 0.03 Pa (i.e., moderate to low volatility) and a low Henry's Law constant of 0.7-0.98 Pa·m3/mol indicate that this substance is not likely to remain in air (0%). This substance is predicted to be rapidly oxidized in air, as indicated by an atmospheric oxidation half-life of 0.67 days (Table 5b). Due to its high sorptive capacity, 2,4,6-tri-tert-butylphenol will partition to solid media, as illustrated in Table 4.
When 2,4,6-tri-tert-butylphenol is released into water, it is expected to strongly adsorb to suspended solids and sediment based upon its estimated Log Koc value of 5.01-5.12. Volatilization from water surfaces is expected to be an unimportant fate process based upon this compound's estimated Henry's Law constant. Thus, if water is a receiving medium, 2,4,6-tri-tert-butylphenol is expected to mainly partition into sediment and, to a small extent, remain in water (Table 4).
If released to soil, 2,4,6-tri-tert-butylphenol is expected to have high adsorptivity to soil (i.e., it is expected to be immobile) based upon its estimated log Koc. Volatilization from moist soil surfaces would be an unimportant fate process. This substance is practically non-biodegradable according to the Japanese MITI test (MITI 1992). Therefore, if released to soil, 2,4,6-tri-tert-butylphenol will remain inthis environmental compartment (Table 4).
Environmental Persistence
The EQC model indicates partitioning of 2,4,6-tri-tert-butylphenol into soil and sediment (see Table 4 above). Once released into the environment, 2,4,6-tri-tert-butylphenol appears to be persistent in the environment, mainly in water, soil, and sediment (Tables 5a and 5b).
Table 5a presents the empirical biodegradation data (MITI 1992) that show 0% biodegradation over 28 days in a ready-biodegradation test for 2,4,6-tri-tert-butylphenol. This test indicates that the half-life in water is longer than 182 days (6 months) and that the substance is considered to persist in that environmental compartment. This result is consistent with what would be expected for the substance given its highly branched chemical structure (hindered phenol).
| Medium | Fate Process | Degradation Value | Endpoint/Units | Reference |
|---|---|---|---|---|
| Water | Biodegradation | 0 | Biodegradation, % | MITI 1992 |
Since few experimental data on the degradation of 2,4,6-tri-tert-butylphenol are available, a QSAR-based weight-of-evidence approach (Environment Canada 2007c) was also applied using the degradation models shown in Table 5b below.
| Medium | Fate Process | Degradation Value | Degradation Endpoint | Reference |
|---|---|---|---|---|
| Air | Atmospheric oxidation | 0.6684 | Half-life (days) | AOPWIN 2000 |
| Air | Ozone reaction | Not reactive | Half-life (days) | AOPWIN 2000 |
| Water | Biodegradation | 60 | Half-life (days) | BIOWIN 2000, Ultimate survey |
| Water | Biodegradation | 0.0497 | Probability | BIOWIN 2000, MITI Non-linear Probability |
| Water | Biodegradation | 0.2186 | Probability | BIOWIN 2000, MITI Linear Probability |
| Water | Biodegradation | 0.002 | Probability | BIOWIN 2000, MITI Linear Probability |
| Water | Biodegradation | 1.4 | BOD MITI 301C (in all domains of applicability) (%) | CATABOL C2004-2008; CPOPs 2008; Jaworska et al. 2002 |
| Water | Hydrolysis | n/a[1] | Half-life (days) | HYDROWIN 2000 |
| Soil | Biodegradation | ≥ 182 days | Half-life (days) | Based on the half-life in water[2] |
| Sediment | Biodegradation | ≥ 728 days | Half-life (days) | Based on the half-life in water[2] |
[1] The result is n/a (not available) as a hydrolysis rate could not be estimated for this type of compound using the HYDROWIN model. Based on the chemical structure, this substance would not be expected to undergo hydrolysis in the environment (i.e., no hydrolysable groups present).
[2] Values were derived from the empirical half-life in water ≥ 182 days using the extrapolation factors of Boethling et al. (1995): t1/2 water : t1/2 soil : t1/2 sediment = 1:1:4.
In air, a predicted atmospheric oxidation half-life value of 0.67 days (Table 5b) demonstrates that this chemical is likely to be rapidly oxidized. The compound is not expected to react with other photo-oxidative species in the atmosphere, such as O3. Therefore, it is expected that reactions with hydroxyl radicals will be the most important fate process in the atmosphere for 2,4,6-tri-tert-butylphenol. With a half-life of 0.67 days via reactions with hydroxyl radicals, 2,4,6-tri-tert-butylphenol is considered not persistent in air.
Although the predicted half-life of 2,4,6-tri-tert-butylphenol in water is 60 days, the overall conclusion from the BIOWIN model based on weighting of the survey and probability sub-models was "not readily biodegradable" (Table 5b). Furthermore results for two other models (TOPKAT and CATABOL) indicate that biodegradation is likely to be slow. The empirical biodegradation data (MITI 1992; Table 5a), which is considered a more reliable result, confirms the latter prediction. Therefore, the half-life of this substance in water is likely longer than 182 days. Thus, this substance is considered to be persistent in this environmental compartment.
The weight of evidence based on the empirical and modelled data described above indicates that 2,4,6-tri-tert-butylphenol meets the persistence criteria in water, soil and sediment (half lives in soil and water ≥ 182 days and half life in sediment ≥ 365 days), but does not meet persistence criteria in air (half-life in air ≥ 2 days) as set out in the Persistence and Bioaccumulation Regulations (Canada 2000).
Potential for Bioaccumulation
Experimental and modelled log Kow values for 2,4,6-tri-tert-butylphenol suggest that this chemical has the potential to bioaccumulate in the environment (see Table 2 above).
The experimental bioconcentration factor (BCF) in fish is reported to range from 4320 to 23200 L/kg at a test concentration of 1 mg/L and 4830 to 16000 L/kg at 10 mg/L (Table 6a).
| Test Organism | Endpoint | Value wet weight (L/kg) | Concentration used in the test | Reference |
|---|---|---|---|---|
| Fish | BCF | 4320 to 23200[1] | 1 mg/L | MITI 1992 |
| Fish | BCF | 4830 to 16000[1] | 10 mg/L | MITI 1992 |
[1] These values represent a range of the 10 fish tested.
Since few experimental bioaccumulation factor (BAF) and bioconcentration factor (BCF) data for 2,4,6-tri-tert-butylphenol were available, a QSAR-based weight-of-evidence approach (Environment Canada 2007c) was applied using the BAF and BCF models shown in Tables 6b and 6c below.
The Arnot-Gobas model (2003) can be used to predict bioaccumulation of this substance, while taking into account potential metabolism using a metabolic rate constant (kM). Predicted BCFs and BAFs for middle trophic level (MTL) fish are shown in Table 6b. Uncertainty analysis was used to determine confidence factors around the metabolic rate constant (kM), accounting for variability in modelled and measured data as described in Arnot et al. (2008).
| Experimental metabolic rate kM (d-1)[1] |
Log Kow used in modelling[4] | Arnot-Gobas BCF wet weight (L/kg) |
Arnot-Gobas BAF wet weight (L/kg) |
|---|---|---|---|
| 3.83E-05 (2.5%)[2] | 6.1 | 51 209 | 1 177 255 |
| 6.89E-04 (average) | 6.1 | 42 144 | 965 994 |
| 1.53E-02 (97.5%)[3] | 6.1 | 8467 | 103 106 |
[1] Middle Trophic Level (MTL) as the representative trophic level for fish; normalized (corrected to the body weight of the fish used in the model).
[2] Lower percentile confidence factor.
[3] Upper percentile confidence factor.
[4] Based on range of reported experimental and predicted log Kow values (see Table 2).
The calculated kM values in Table 6b are based on in vivo experiments and suggest that the rate of metabolism for this compound is quite low (≤ 0.02 at best). The empirical MITI (1992) data agree most closely with the BCF calculated using the upper percentile confidence factor of the kM values. Results of predicted BCFs and BAFs using the Arnot-Gobas model indicate that 2,4,6-tri-tert-butylphenol has the potential to bioconcentrate and biomagnify in the environment.
Additional modelled data for the bioaccumulation of 2,4,6-tri-tert-butylphenol are shown in Table 6c.
| Test Organism | Endpoint | Value wet weight (L/kg) | Reference |
|---|---|---|---|
| Fish | BCF | 19 952 (in all domains of applicability) |
BBM 2008 (all mitigating factors) |
| Fish | BCF | 3282 | BCFWIN 2000 |
Metabolic rules in the BBM model forbid the biotransformation of this hindered phenol and thus no metabolic mitigation is used. The BCF value estimated using the BBM model is in general agreement with available empirical data. The modelled BCF from BCFWIN underestimates the BCF compared with the empirical data from MITI (1992) by a factor of ~4. This difference is likely due to the compounds used in the model's training set having a similar log Kow but having greater metabolic potential than 2,4,6-tri-tert-butylphenol.
The weight of evidence based on empirical and modelled data described above indicates that 2,4,6-tri-tert-butylphenol meetsthe bioaccumulation criterion (BCF or BAF 5000) as set out in the Persistence and Bioaccumulation Regulations (Canada 2000).
Ecological Effects Assessment
In the Aquatic Compartment
There is experimental and modelled evidence that 2,4,6-tri-tert-butylphenol causes harm to aquatic organisms at relatively low concentrations (Tables 7a and 7b).
| Test organism | Type of test | Endpoint | Value (mg/L) | Reference |
|---|---|---|---|---|
| Fish | Acute (96 hours) | LC50[1] | 0.06[*] | Geiger et al. (1990) |
[1] LC50 - The concentration of a substance that is estimated to be lethal to 50% of the test organisms.
[*] Categorization pivotal iT value.
| Test organism | Type of test | Endpoint | Value (mg/L) | Model/reference |
|---|---|---|---|---|
| Fish | Acute (96 hours) | LC50[1] | NP[3] | TOPKAT 2004 |
| 0.076 | ECOSAR 2004 (phenols) | |||
| NP | U.S. EPA 1999 | |||
| 0.18 (in domain) | TIMES 2007; CPOPs 2008 | |||
| 0.078 | AIES 2003-2005 | |||
| Fish | Chronic (14 days) | LC50 | 0.053 | ECOSAR 2004 (Neutral Org. SAR) |
| Daphnia | Acute (48 hours) | LC50 | NP | ECOSAR 2004 (phenols) |
| EC50[2] | NP | TOPKAT 2004 | ||
| Algae | Acute (96 hours) | EC50 | 0.017 | ECOSAR 2004 (phenols) |
[1] LC50 - The concentration of a substance that is estimated to be lethal to 50% of the test organisms.
[2] EC50 - The concentration of a substance that is estimated to cause a sublethal effect on 50% of the test organisms.
[3] NP - Not predictable or not a reliable prediction.
A range of aquatic toxicity predictions was obtained from the various QSAR models (Table 7b). These results and the experimental information (Table 7a) indicate that the substance has the potential to be highly hazardous to aquatic organisms (i.e., acute LC/EC50 ≤ 1.0 mg/L).
In Other Environmental Compartments
No ecological effects studies were found for this compound in soil or sediment. The U.S. EPA (2007b) has reported an acute oral toxicity (LD50) value of 1610 mg/kg-bw in rats and a no-observed adverse effect level (NOAEL) of 1.5 mg/kg-bw/day in rats (based on liver effects, kidney weights and increases in cholesterol and platelets).
Ecological Exposure Assessment
No monitoring data on the presence of 2,4,6-tri-tert-butylphenol in environmental media (air, water, soil, sediment) have been found.
Characterization of Ecological Risk
Evidence that a substance is highly persistent and bioaccumulative as defined in the Persistence and Bioaccumulation Regulations of CEPA 1999 (Canada 2000), when taken together with the potential for environmental release or formation and the potential for toxicity to organisms, provides a significant indication that it may be entering the environment under conditions that may have harmful long-term ecological effects (Environment Canada 2006). Substances that are persistent remain in the environment for a long time after being released, increasing the potential magnitude and duration of exposure. Substances that have long half-lives in mobile media (air and water) and partition into these media in significant proportions have the potential to cause widespread contamination. Releases of small amounts of bioaccumulative substances may lead to high internal concentrations in exposed organisms. Highly bioaccumulative and persistent substances are of special concern, since they may biomagnify in food webs, resulting in very high internal exposures, especially for top predators.
The importation volumes of 2,4,6-tri-tert-butylphenol into Canada along with its broad use indicate the potential for releases into the Canadian environment. Although the substance may be destroyed through the combustion of fuel and oil, other non-destructive uses could result in releases into the Canadian environment. However, a consultant study confirmed the use as a fuel additive as being the main use of this substance in Canada (Environment Canada, 2008b). Results from the mass flow tool estimate that of 2% of the total mass of the substance in commerce is released into the environment, the vast majority (93.2%) being lost by transformation (i.e., fuel combustion). Both empirical and modelled data indicate that once this substance is released into the environment it will remain in water, sediment and soil for a long time because of its stability in the environment. Due to its lipophilic character and persistence, the empirical and modelled data suggest that it will likely bioaccumulate and may biomagnify in trophic food chains. Both empirical and modelled data have also demonstrated the potential for relatively high toxicity to aquatic organisms. This information suggests that 2,4,6-tri-tert-butylphenol has the potential to be released into the environment and cause ecological harm in Canada.
Uncertainties in Evaluation of Ecological Risk
There is uncertainty regarding the risk that 2,4,6-tri-tert-butylphenol may pose now or in the future. Typically, quantitative risk estimates (i.e., risk quotients or probabilistic analyses) are important lines of evidence when evaluating a substance's potential to cause environmental harm. However, when risks for persistent and bioaccumulative substances such as 2,4,6-tri-tert-butylphenol are estimated using such quantitative methods, they are highly uncertain and are likely to be underestimated. Given that long term risks associated with persistent and bioaccumulative substances cannot at present be reliably predicted, quantitative risk estimates have limited relevance. Furthermore, since accumulations of such substances may be widespread and are difficult to reverse, a conservative response to uncertainty is justified.
Some physical-chemical properties were generated for 2,4,6-tri-tert-butylphenol using QSAR models, which have inherent uncertainties related to their use (e.g., in or out of domains of applicability). However, this substance (as a neutral organic) was modelled well by available QSARs. Predicted and observed data are generally in good agreement and applicability domains (where specified in a model) have been satisfied. There is some uncertainty regarding the water solubility for this substance as there is a difference of approximately a 3 order of magnitude between the experimental (35 mg/L) and modelled (0.267 mg/L) values. The 35 mg/L value is given stronger weight than the 0.267 mg/L as observed values are favoured over predicted ones. The physical-chemical properties for this substance (e.g., high sorptive capacity) and the results of EQC modelling confirm that soil and sediment are the media of concern for this substance. In addition, the convergence of both empirical and modelled data with respect to persistence, bioaccumulation and toxicity to aquatic organisms increases confidence in the results overall. However, there are no data available relating to the potential effects of this substance as a result of exposure of organisms in soil or sediment.
The section 71 data provides information on importation and use above a reporting threshold, but there is still concern for the importation and use of the substance below the reporting threshold, especially for a substance that is persistent, bioaccumulative and toxic. In using the mass flow tool, associated uncertainties and assumptions were discussed previously regarding major life cycle stages. One of the major assumptions is that this substance behaves similarly to gasoline (i.e., when used as a fuel additive, its rate of combustion is assumed to be similar to gasoline). It is important to note that this tool did not take into account the transport and use of gasoline for home uses (e.g., lawn mowers, generators) and recreational activities (e.g., boats), which has the potential to result in incidental releases of this substance to various environmental compartments. Also, there is some uncertainty regarding the uses of this substance in Canada. The only known current use of the substance in Canada is as a fuel and oil additive (Environment Canada 2008). Since 2,4,6-tri-tert-butylphenol has been used in the past in Canada as a lubricant additive, and this is a recognized use for the substance elsewhere, as well as the lack of a targeted Section 71 survey for this substance, the lubricant use was incorporated into the mass flow tool.
Based on the information presented in this screening assessment, it is concluded that 2,4,6-tri-tert-butylphenol is entering or may be entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity.
It is therefore concluded that 2,4,6-tri-tert-butylphenol meets the definition of toxic as set out in paragraphs 64a of CEPA 1999. Additionally, 2,4,6-tri-tert-butylphenol meets the criteria for persistence and bioaccumulation potential as set out in the Persistence and Bioaccumulation Regulations (Canada 2000).
[AIES] Artificial Intelligence Expert System. 2003-2005. Version 1.25. Ottawa (ON): Environment Canada. Model developed by Stefan Niculescu. Environment Canada, Existing Substances Division and New Substances Division, Ottawa, K1A 0H3.
[AOPWIN] Atmospheric Oxidation Program for Windows [Estimation Model]. 2000. Version 1.91. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Exposure Assessment Tools and Models.
Arnot JA, Gobas FAPC. 2003. A generic QSAR for assessing the bioaccumulation potential of organic chemicals in aquatic food webs. QSAR Comb Sci 22(3):337-345.
Arnot, JA, Mackay, D, Bonnell, M. 2008. Estimating metabolic biotransformation rates in fish from laboratory data. Environ. Toxicol. Chem. 27(2):341-351.
[BBM] Baseline Bioaccumulation Model. 2008. Gatineau (QC): Environment Canada, Existing Substances Division. [Model developed based on Dimitrov et al. 2005]. Available upon request.
[BCFWIN] BioConcentration Factor Program for Windows [Estimation Model]. 2000. Version 2.15. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Exposure Assessment Tools and Models.
[BIOWIN] Biodegradation Probability Program for Windows [Estimation Model]. 2000. Version 4.02. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Exposure Assessment Tools and Models.
Boethling, RS, Howard, PH, Beauman, JA, Larosche, ME. 1995. Factors for intermedia extrapolations in biodegradability assessment. Chemosphere 30(4):741-752.
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C., 1999, c. 33. Canada Gazette, Part III, Vol. 22, No. 3.
Canada. 2000. Canadian Environmental Protection Act: Persistence and Bioaccumulation Regulations, P.C. 2000-348, 23 March, 2000, SOR/2000-107, Canada Gazette, Part II, Vol. 134, No. 7.
Canada, Dept. of the Environment. 2001. Canadian Environmental Protection Act, 1999: Notice with respect to certain substances on the Domestic Substances List (DSL). Canada Gazette, Part I, Vol. 135, No. 46. Ottawa: Queen's Printer.
Canada, Dept. of the Environment. 2003. Canadian Environmental Protection Act, 1999: On-Road Vehicle and Engine Emission Regulations. Schedule 2, Regulatory Impact Analysis Statement (Table 3), p. 37. Canada Gazette, Part I, Vol. 137, No. 1. Ottawa: Queen's Printer.
Canada, Dept. of the Environment, Dept. of Health. 2006. Canadian Environmental Protection Act, 1999: Notice of intent to develop and implement measures to assess and manage the risks posed by certain substances to the health of Canadians and their environment. Canada Gazette, Part I, Vol. 140, No. 49. Ottawa: Queen's Printer.
Canada, Dept. of the Environment, Dept. of Health. 2007. Canadian Environmental Protection Act, 1999: Notice of second release of technical information relevant to substances identified in the Challenge. Canada Gazette, Part I, Vol. 141. No. 19.
[CATABOL] Probabilistic assessment of biodegradability and metabolic pathways [Computer Model]. C2004-2008. Version 5.10. Bourgas (BG): Laboratory of Mathematical Chemistry.
[CPOPs] Canadian POPs Model. 2008. Version 1.1. Gatineau (QC): Environment Canada, Existing Substances Division; Bourgas (BG): Laboratory of Mathematical Chemistry. [Model developed based on Mekenyan et al. 2005]. Available upon request.
Dimitrov, S, Dimitrova, N, Parkerton, T, Comber, M, Bonnell, M, Mekenyan, O. 2005. Baseline model for identifying the bioaccumulation potential of chemicals. SAR QSAR Environ. Res. 16:531-554.
[ECOSAR] Ecological Structural Activity Relationships [Internet]. 2004. Version 0.99h. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Exposure Assessment Tools and Models.
Environment Canada. 1988. Data relating to the Domestic Substance List (DSL) 1984 - 1986, collected under the Canadian Environmental Protection Act, 1988, s. 25(1). Collected according to: Reporting for the Domestic Substance List [reporting guide], Ministry of Supply and Services Canada, DSS cat. No. En40-364/1998E. Prepared by: Environment Canada, New Substances Division.
Environment Canada. 2001. Data collected under the Canadian Environmental Protection Act, 1999, s. 71: Notice with respect to certain substances on the Domestic Substances List (DSL). Canada Gazette, Part I, Vol. 140, No. 49. Prepared by: Environment Canada, Existing Substances Branch.
Environment Canada. 2006. Approach to ecological screening assessments under Paragraph 64(a) of CEPA 1999 for existing substances that are both persistent and bioaccumulative. In: CEPA DSL Categorization: Overview and Results [CD-ROM], released September 2006. Environment Canada, Existing Substances Division, Gatineau (QC), K1A 0H3. Available upon request.
Environment Canada. 2007a. Data for Batch 2 substances collected under the Canadian Environmental Protection Act, 1999, Section 71: Notice with respect to certain Batch 2 Challenge substances. Data prepared by: Environment Canada, Existing Substances Program.
Environment Canada. 2007b. Assumptions, limitations and uncertainties of the mass flow tool for 2,4,6-tri-tert-butlyphenol, CAS RN 732-26-3. Environment Canada, Existing Substances Division, Gatineau (QC), K1A 0H3. Internal draft document available upon request.
Environment Canada. 2007c. QSARs: Reviewed Draft Working Document, Science Resource Technical Series, Guidance for Conducting Ecological Assessments under CEPA 1999. Environment Canada, Existing Substances Division, Gatineau (QC), K1A 0H3. Internal draft document available upon request.
Environment Canada. 2008a. Guidance for conducting ecological assessments under CEPA, 1999: science resource technical series, technical guidance module: Mass Flow Tool. Preliminary draft working document. Gatineau (QC): Environment Canada, Existing Substances Division.
Environment Canada. 2008b. Background Technical Study on Certain Challenge Substances of Interest Under the Chemicals Management Plan. Environment Canada, Oil, Gas and Energy, Gatineau (QC), K1A 0H3. Internal draft document available upon request.
[EQC] Equilibrium Criterion Model. 2003. Version 2.02. Peterborough (ON): Trent University, The Canadian Environmental Modelling Centre and Chemistry - EQC Model.
Geiger, DL, Brooke, LT, Call, DJ. 1990. Acute Toxicities of Organic Chemicals to Fathead Minnows (Pimephales promelas). Vol. 5. Center for Lake Superior Environmental Studies, University of Wisconsin-Superior, Superior, Wisconsin. 332 p.
[HENRYWIN] Henry's Law Constant Program for Microsoft Windows [Estimation Model]. 2000. Version 3.10. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Exposure Assessment Tools and Models.
[HYDROWIN] Hydrolysis Rates Program for Microsoft Windows [Estimation Model]. 2000. Version 1.67. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Exposure Assessment Tools and Models.
[ICES] International Council for the Exploration of the Sea. 2007. Report of the ICES/OSPAR Workshop on Integrated Monitoring of Contaminants and their Effects in Coastal and Open-sea Areas (WKIMON III), 16-18 January 2007, ICES Headquarters. ICES CM 2007/ACME:01. 209 pp. [cited 2007 Oct].
Jaworska, J, Dimitrov, S, Nikolova, N, Masscheleyn, P, Mekenyan, O. 2002. Probabilistic Assessment of Biodegradability Based on Metabolic Pathways: CATABOL System. In: Mekenyan, O, Schultz, TW (eds.). Proceedings of Quantitative Structure Activity Relationships in Environmental Sciences - I. 2002. SAR QSAR Environ. Res. 13:307-323.
[KOWWIN] Octanol-Water Partition Coefficient Program for Microsoft Windows [Estimation Model]. 2000. Version 1.67. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Exposure Assessment Tools and Models.
Mekenyan, OG, Dimitrov, SD, Pavlov, TS, Veith, GD. 2005. POPs: A QSAR system for creating PBT profiles of chemicals and their metabolites. SAR QSAR Environ. Res. 16(1-2):103-133.
[MITI] Ministry of International Trade & Industry (Jpn), Basic Industries Bureau, Chemical Products Safety Division. 1992. Biodegradation and bioaccumulation data of existing chemicals based on the CSCL Japan. Tokyo (Jpn): Japan Chemical Industry Ecology-Toxicology & Information Centre.
[MPBPWIN] Melting Point Boiling Point Program for Microsoft Windows [Estimation Model]. 2000. Version 1.41. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Exposure Assessment Tools and Models.
[NCI] National Chemical Inventories [database on a CD-ROM]. 2006. Issue 1. Columbus (OH). American Chemical Society, Chemical Abstracts Service Columbus, Ohio. National Chemical Inventory (NCI) System Requirements.
[NGGIC] National Greenhouse Gas Inventory Committee. 2006. Australian Methodology for the Estimation of Greenhouse Gas Emissions and Sinks 2005. Energy (Fugitive Fuel Emissions) [Internet]. Canberra (AU). Published by the Australian Greenhouse Office, Department of the Environment and Heritage, Commonwealth of Australia. ISBN 978-1-921297-14-4.
[NITE] National Institute of Technology and Evaluation (Jpn). 2007. National Institute of Technology and Evaluation database search results on laws and regulations in Japan for the Class I Specified Chemical, 2,4,6-tri-tert-butylphenol [Internet]. Class Reference Number in the Gazetted List: 3-540, under Cabinet Order No. 11, designated by Cabinet Order December 27, 2000. Tokyo (Jpn): NITE. [cited 2007 Dec].
[OECD] Organisation for Economic Co-Operation and Development. 2004a. Manual for Investigation of HPV Chemicals: The 2004 OECD List of High Production Volume Chemicals. Paris (FR): OECD, Environment Directorate. [cited 2007 Oct].
[OECD] Organisation for Economic Co-Operation and Development. 2004b. Emission Scenario Document on Lubricants and Lubricant Additives [Internet]. Paris (FR): Joint Meeting of the Chemicals Committee and the Working Party on Chemicals, Pesticides and Biotechnology. November 26, 2004. OECD Environmental Health and Safety Publications. Series on Emission Scenario Documents No. 10. ENV/JM/MONO(2004)21. JT00166913.
[OSPAR] OSPAR Commission. 2006. OSPAR Background Document on 2,4,6-tri-tert-butylphenol. Hazardous Substances Series. OSPAR Commission 2003 (Update 2006) [Internet]. Publication Number: 274/2006. ISBN 1-905859-01-5. [cited 2007 Oct].
[OSPAR] OSPAR Commission. 2007. OSPAR List of Chemicals for Priority Action (Revised 2010), Reference Number 2004-12 [Internet]. Reykjavik (IS): OSPAR Convention for the Protection of the Marine Environment of the North-East Atlantic. [cited 2007 Oct].
[PCKOCWIN] Organic Carbon Partition Coefficient Program for Microsoft Windows [Estimation Model]. 2000. Version 1.66. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Exposure Assessment Tools and Models.
[PhysProp] Interactive PhysProp Database [database on the Internet]. 2006. Syracuse (NY): Syracuse Research Corporation. [cited 2006 Mar]. Interactive PhysProp Database Demo.
Serjeant, EP, Dempsey, B. 1979. Ionisation Constants of Organic Acids in Aqueous Solutions. IUPAC Chemical Data Series. New York (NY): Pergamon Press. As cited in PhysProp 2006 [cited 2007 Oct].
[TIMES] Tissue Metabolism Simulator [Computer Model]. 2007. Version 2.25. Bourgas (BG): Laboratory of Mathematical Chemistry.
[TOPKAT] Toxicity Prediction Program [Internet]. 2004. Version 6.2. San Diego (CA): Accelrys Software Inc.
[US EPA] US Environmental Protection Agency. 1999. Assessment Tools for the Evaluation of Risk. Environmental Research Laboratory, Duluth, MN. Mid-Continent Ecology Division - Aster
[US EPA] US Environmental Protection Agency. 2007a. High Production Volume (HPV) - Sponsored Chemicals [Internet]. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics. [cited 2007 October].
[US EPA] US Environmental Protection Agency. 2007b. Screening-Level Hazard Characterization for High Production Volume Chemicals: Alkylphenols. August 2007. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics, High Production Volume Chemicals Branch, Risk Assessment Division.
[WSKOWWIN] Water Solubility for Organic Compounds Program for Microsoft Windows [Estimation Model]. 2000. Version 1.41. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Exposure Assessment Tools and Models.