Screening Assessment for the Challenge
This page has been archived on the Web
Information identified as archived is provided for reference, research or recordkeeping purposes. It is not subject to the Government of Canada Web Standards and has not been altered or updated since it was archived. Please contact us to request a format other than those available.
Archived
2,7-Naphthalenedisulfonic acid, 3-[[2,2'-dimethyl-4'-[[4-[[(4-methylphenyl)sulfonyl]oxy]phenyl]azo][1,1'-biphenyl]-4-yl]azo]-4-hydroxy-, disodium salt
(Acid Red 111)
Chemical Abstracts Service Registry Number
6358-57-2
Environment Canada
Health Canada
September 2011
Table of Contents
- Synopsis
- Introduction
- Substance Identity
- Physical and Chemical Properties
- Sources
- Uses
- Releases to the Environment
- Environmental Fate
- Persistence and Bioaccumulation Potential
- Potential to Cause Ecological Harm
- Potential to Cause Harm to Human Health
- Conclusion
- References
- Appendix 1: Summary of QSAR results for Acid Red 111 and potential azo cleavage products
Pursuant to section 74 of the Canadian Environmental Protection Act, 1999 (CEPA 1999), the Ministers of the Environment and of Health have conducted a screening assessment on 2,7-naphthalenedisulfonic acid, 3-[[2,2'-dimethyl-4'-[[4-[[(4-methylphenyl)sulfonyl]oxy]phenyl]azo][1,1'-biphenyl]-4-yl] azo]-4-hydroxy-, disodium salt (herein referred to as Acid Red 111), Chemical Abstracts Service Registry Number[1] 635857-2. This substance was identified as a high priority for screening assessment and included in the Challenge initiative under the Chemicals Management Plan because it had been found to meet the ecological categorization criteria for persistence, bioaccumulation potential and inherent toxicity to non-human organisms and is believed to be in commerce in Canada. Acid Red 111 was not considered to be a high priority for assessment of potential risks to human health, based upon application of the simple exposure and hazard tools developed for categorization of substances on the Domestic Substances List.
Acid Red 111 is a synthetic dye that is used as a colourant primarily in the textile industry. Other applications of this substance include paper, leather, plastics, inks, and paints. This substance does not naturally occur in the environment. As a result of industry surveys conducted pursuant to section 71 of CEPA 1999, companies reported a combined total import of 100–1000 kg of this substance into Canada in both 2005 and 2006.
Based on reported use patterns and certain assumptions related to dyes in general, releases of Acid Red 111 to the Canadian environment during the formulation and consumer use of products containing this substance are estimated to be 15% to wastewater and 85% transferred to waste disposal sites (landfill and incineration). Acid Red 111 is an azo dye with two sulfonic acid groups, which dictate its adsorption characteristics and impart high water solubility. Dyes have an inherently high affinity to substrates, and a potentially large proportion can be removed during wastewater treatment as a result of such substances being adsorbed to biosolids.
Information on other sulfonated azo dyes with a naphthalene ring, as well as results of quantitative structure-activity relationship modelling, suggests that Acid Red 111 is persistent in aerobic environments (i.e., water, soil, sediment). Degradation of Acid Red 111 under anaerobic or reducing conditions may occur relatively rapidly, but would be limited to specific environments (e.g., deep layers of sediments), with potentially harmful metabolites being formed as a result of cleavage of its azo bonds. However, in these situations exposure to aquatic organisms would be limited. The high water solubility of this substance, as well as other physical and chemical properties (e.g., large molecular size), suggests that it has a low potential to accumulate in the lipid tissues of organisms. Therefore, Acid Red 111 meets the persistence criteria but does not meet the bioaccumulation criteria as set out in the Persistence and Bioaccumulation Regulations. In addition, experimental toxicity data for Acid Red 111 and three other sulfonated acid dyes of similar molecular structure suggest that this substance does not cause acute harm to aquatic organisms at low concentrations.
For this screening assessment, a conservative environmental release scenario was selected in which an industrial operation discharges this substance into the aquatic environment through a single wastewater treatment plant. The upper end of the reporting range of 1000 kg was used to conservatively estimate releases and concentrations in the aquatic environment. The predicted environmental concentration in water for this substance (0.03 mg/L) was below the predicted no-effect concentration (0.04 mg/L) for sensitive aquatic organisms, resulting in a risk quotient of 0.7.
Based on the ecological information available, it is concluded that Acid Red 111 is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
For the human health assessment, the potential for exposure of the general population to Acid Red 111 from environmental media is expected to be negligible. Exposure to Acid Red 111 from consumer products (e.g., fabrics, textiles, personal apparel), is expected to be negligible as Acid Red 111 is predominantly used as a colourant in textiles that are not frequently used by the general population. Empirical data pertaining to the health effects of Acid Red 111 were limited. However, information from analogues of Acid Red 111, and from potential azo cleavage products, indicates that there may be concern for genotoxicity and carcinogenicity. Although the potential high hazard of Acid Red 111 is recognized, on the basis of information that indicates that general population exposure is expected to be negligible due to the nature of its use and application, risk to human health is considered to be low. It is concluded that Acid Red 111 is not a substance entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
Because Acid Red 111 is listed on the Domestic Substances List, its import and manufacture in Canada are not subject to notification under subsection 81(1). Given the potential health hazards of this substance, there is concern that new activities that have not been identified or assessed could lead to this substance meeting the criteria set out in section 64 of the Act. Therefore, it is recommended to amend the Domestic Substances List, under subsection 87(3) of the Act, to indicate that subsection 81(3) of the Act applies with respect to this substance so that new manufacture, import or use of this substance is notified and undergoes ecological and human health risk assessments.
Based on the information available, it is concluded that Acid Red 111 does not meet any of the criteria set out in section 64 of CEPA 1999.
The Canadian Environmental Protection Act, 1999 (CEPA 1999) (Canada 1999) requires the Minister of the Environment and the Minister of Health to conduct screening assessments of substances that have met the categorization criteria set out in the Act to determine whether these substances present or may present a risk to the environment or to human health.
Based on the information obtained through the categorization process, the Ministers identified a number of substances as high priorities for action. These include substances that
- met all of the ecological categorization criteria, including persistence (P), bioaccumulation potential (B) and inherent toxicity to aquatic organisms (iT), and were believed to be in commerce in Canada; and/or
- met the categorization criteria for greatest potential for exposure (GPE) or presented an intermediate potential for exposure (IPE), and had been identified as posing a high hazard to human health based on classifications by other national or international agencies for carcinogenicity, genotoxicity, developmental toxicity or reproductive toxicity.
The Ministers therefore published a notice of intent in theCanada Gazette, Part I, on December 9, 2006 (Canada 2006a), that challenged industry and other interested stakeholders to submit, within specified timelines, specific information that may be used to inform risk assessment, and to develop and benchmark best practices for the risk management and product stewardship of those substances identified as high priorities.
The substance 2,7-naphthalenedisulfonic acid, 3-[[2,2'-dimethyl-4'-[[4-[[(4-methylphenyl)sulfonyl]oxy]phenyl]azo][1,1'-biphenyl]-4-yl]azo]-4-hydroxy-, disodium salt (which will be referred to as Acid Red 111 for the purposes of this document) had been identified as a high priority for assessment of ecological risk as it had been found to be persistent, bioaccumulative and inherently toxic to aquatic organisms and is believed to be in commerce in Canada. The Challenge for this substance was published in the Canada Gazette on May 31, 2008 (Canada 2008a, 2008b). A substance profile was released at the same time. The substance profile presented the technical information available prior to December 2005 that formed the basis for categorization of this substance. As a result of the Challenge, submissions of information pertaining to the properties, persistence, hazards and uses of Acid Red 111 were received.
Although Acid Red 111 was determined to be a high priority for assessment with respect to the environment, it did not meet the criteria for GPE or IPE and high hazard to human health based on classifications of other national or international agencies for carcinogenicity, genotoxicity, developmental toxicity or reproductive toxicity.
Screening assessments focus on information critical to determining whether a substance meets the criteria as set out in section 64 of CEPA 1999. Screening assessments examine scientific information and develop conclusions by incorporating a weight-of-evidence approach and precaution.[2]
This final screening assessment includes consideration of information on chemical properties, hazards, uses and exposure, including any information submitted under the Challenge. Data relevant to the screening assessment of this substance were identified in original literature, review and assessment documents, stakeholder research reports and from recent literature searches, up to July 2010. Key studies were critically evaluated; modelling results have also been used to reach conclusions. When available and relevant, information presented in hazard assessments from other jurisdictions was considered. This final screening assessment does not represent an exhaustive or critical review of all available data. Rather, it presents the most critical studies and lines of evidence pertinent to the conclusion.
This final screening assessment was prepared by staff in the Existing Substances Programs at Health Canada and Environment Canada and incorporates input from other programs within these departments. The ecological portion of this assessment has undergone external written peer review/consultation. Comments on the technical portions relevant to human health were received from scientific experts selected and directed by Toxicology Excellence for Risk Assessment (TERA) and included comments by Dr. Larry Claxton, Dr. Bernard Gadagbui, Dr. Pertti Hakkinen, Dr. Glenn Talaska, and Dr. Pam Williams. Additionally, the draft of this screening assessment was subject to a 60-day public comment period. While external comments were considered, the final content and outcome of the screening assessment remain the responsibility of Health Canada and Environment Canada. Approaches used in the screening assessments under the Challenge have been reviewed by an independent Challenge Advisory Panel.
The critical information and considerations upon which this final assessment is based are summarized below.
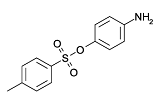
For the purposes of this document, 2,7-naphthalenedisulfonic acid, 3-[[2,2'-dimethyl-4'-[[4-[[(4-methylphenyl)sulfonyl]oxy]phenyl]azo][1,1'-biphenyl]-4-yl]azo]-4-hydroxy-, disodium salt will be referred to as Acid Red 111, its Colour Index name (Colour Index Constitution Number: 23268; CII 2002). Information on this substance’s identity is shown in Table 1 below.
| Chemical Abstracts Service Registry Number (CAS RN) | 6358-57-2 |
| DSL name | 2,7-Naphthalenedisulfonic acid, 3-[[2,2'-dimethyl-4'-[[4-[[(4-methylphenyl)sulfonyl]oxy]phenyl]azo][1,1'-biphenyl]-4-yl]azo]-4-hydroxy-, disodium salt |
| National Chemical Inventories (NCI) names[1] | 2,7-Naphthalenedisulfonic acid, 3-[[2,2'-dimethyl-4'-[[4-[[(4-methylphenyl)sulfonyl]oxy]phenyl]azo][1,1'-biphenyl]-4-yl]azo]-4-hydroxy-, disodium salt (AICS, ASIA-PAC, PICCS, TSCA); disodium 3-[[2,2'-dimethyl-4'-[[4-[[(p-tolyl)sulphonyl] oxy]phenyl]azo][1,1'-biphenyl]-4-yl]azo]-4-hydroxynaphthalene-2,7-disulphonate(EINECS); Acid Red 111 (ENCS); C.I. acid red 111 (ECL, PICCS) |
| Other names | Acid Scarlet F 3GL; C.I. 23266; C.I. Acid Red 111, disodium salt; Coomassie Fast Scarlet B; Coomassie Scarlet B; Kayanol Milling Scarlet FGW; Levanol Fast Scarlet FGN; Nylosan Scarlet F 3GL; Ortol Scarlet FG; Polar Scarlet GS; Sandolan Milling Scarlet N-GWL; Stenolana Brilliant Scarlet 2GL; Sulfonine Scarlet GWL; Sulfonine Scarlet GWLN; Supranol Fast Scarlet FGN |
Chemical group (DSL Stream) |
Discrete organics |
| Major chemical class or use | Disazo dyes |
| Major chemical sub-class | Aromatic amines, benzene sulfonates, sulfonaphthalenes, naphthols |
| Chemical formula | C37H28N4Na2O10S3 |
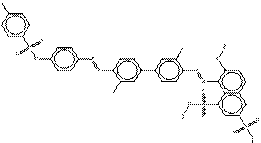
| Chemical structure[2] |  |
| SMILES[3] | Cc1ccc(cc1)S(=O)(=O)Oc2ccc(cc2)N=Nc3ccc(c(C)c3)c4ccc(cc4C) N=Nc5c(S(=O)(=O)O[Na])cc6cc(S(=O)(=O)O[Na])ccc6c5O |
| Molecular mass | 830.82 g/mol |
[1]National Chemical Inventories (NCI). 2007: AICS (Australian Inventory of Chemical Substances); ASIA-PAC (Asia-Pacific Substances Lists); ECL (Korean Existing Chemicals List); EINECS (European Inventory of Existing Commercial Chemical Substances); ENCS (Japanese Existing and New Chemical Substances); PICCS (Philippine Inventory of Chemicals and Chemical Substances); and TSCA (Toxic Substances Control Act Chemical Substance Inventory).
[2] Source: chemBlink (2009)
[3] Simplified Molecular Input Line Entry System
Acid Red 111 is an anionic azo dye; the azo bond (–N=N–) being the part of the molecule that produces colour (Danish EPA 1999). This substance also contains two sulfonic acid groups, which contribute to its high water solubility. In addition to chemical structure, dyes may be classified according to their industrial applications and the methods by which they are applied to the substrate of interest (e.g., acid dyes) (ETAD 1995). This classification system tends to reflect groupings based on physical and chemical behaviour. A brief discussion of the uses of this dye can be found later in this document under the Uses section.
Commercially available dyes are formulated to contain additional chemicals to maintain the desired properties of the dye and to ensure their effectiveness in the dyeing process. Powdered dyes (like Acid Red 111) require de-dusting agents (e.g., hydrocarbon oils, polyalkylene glycol ethers), and commonly contain diluents to standardize dye strength, wetting agents and bacteriostats (ETAD 1995). The content of the active dye can therefore vary among formulations and is often confidential business information. Most sulfonated dyes are not produced or sold in pure form (ETAD 1995), which is likely one of the reasons why physical and chemical data on this substance are lacking.
Few experimental data are available on the physical and chemical properties of Acid Red 111. At the Environment Canada-sponsored Quantitative Structure-Activity Relationship (QSAR) Workshop in 1999, invited modelling experts identified many structural classes of pigments and dyes as “difficult to model” using QSARs (Environment Canada 2000). The physical and chemical properties of many of the structural classes of pigments and dyes are not amenable to model prediction because they are considered “out of the model domain of applicability” (e.g., structural and/or property parameter domains). Therefore, to determine potential utility, the domains of applicability of QSAR models to pigments and dyes are reviewed on a case-by-case basis.
For this assessment it is considered that QSAR models used to predict physical and chemical properties that lack comparable substances to Acid Red 111 in their domain of applicability, may produce results with a high degree of uncertainty. Consequently, a read-across approach has been used to determine the approximate physical and chemical properties of this substance based on similar acid dyes with available experimental data. Due to the paucity of information, acceptable analogous substances for the purposes of this assessment were chosen. These substances include Acid Red 114, Direct Black 38 and Benzoic acid, 5-[[4'-[(2-amino-8-hydroxy-6-sulfo-1-naphthalenyl)azo]-2,2'-dichloro[1,1'-biphenyl]-4-yl]azo]-2-hydroxy-, disodium salt (which will be referred to as BAHSS). These substances all have at least two azo bonds with 1 to 3 sulfonic acid groups and 1 naphthalene ring, and are relatively large molecules. They are therefore expected to behave similarly to Acid Red 111 in the environment and to demonstrate similar toxicities in the aquatic environment as a function of bioavailability and chemical reactivity. Additional analogues have been chosen for the human health assessment, where data exist (see Potential to Cause Harm to Human Health section for further rationale and discussion).
Table 2 shows available information on the physical and chemical properties of Acid Red 111 and relevant analogues identified for use in the ecological portion of this assessment. These properties are relevant to the environmental fate of Acid Red 111 and were subsequently considered in evaluating various lines of evidence in this assessment.
| Chemical | Type | Value | Temperature (°C) | Reference |
|---|---|---|---|---|
| Physical state | ||||
| Acid Red 111[1] | Red powder | 25 | Study Submission 2007 | |
| Direct Black 38 | Grey-black powder | 25 | NIEHS 2008 | |
| Decomposition (°C) | ||||
| Acid Red 111[1] | Experimental | 170–190 | Study Submission 2007 | |
| Acid Red 114 | Experimental | ≥185 | MITI 1992 | |
| Azo dyes | Read-across[2] | >300 | ETAD 1995 | |
| Density (kg/m3) | ||||
| Acid Red 111[1] | Experimental, (bulk) |
390 | Study Submission 2007 | |
| Henry’s Law constant (Pa·m3/mol) | ||||
| Acid Red 111 | Calculated[3] | 1.4–4.2 ×10-11 | 25 | HENRYWIN 2008 |
| Acid Red 114 | Calculated[3] | ≤1.7 ×10-9 | 25 | HENRYWIN 2008 |
| Direct Black 38 | Calculated[3] | 8.4 ×10-12 | 25 | HENRYWIN 2008 |
| Log Kow(octanol-water partition coefficient) (dimensionless) | ||||
| Azo dyes | Read-across[2] | <3 | 25 | ETAD 1995 |
| Water solubility (mg/L) | ||||
| Acid Red 111[1] | Experimental | 25 000 | 80 | Study Submission 2007 |
| Experimental | 65 000 | MSDS 2003 | ||
| Experimental | 20 000 | 100 | Rosi Chemical 2000 | |
| Acid Red 114 | Experimental | >500 | 25 | MITI 1992 |
| Direct Black 38 | Experimental | 93 000 | 15–25 | Isik and Sponza 2004 |
| Azo dyes with one or more sulfonic acid groups | Read-across2 | Readily soluble | ETAD 1995 | |
[1] These physical and chemical properties are for the formulated product identified in the company’s Material Safety Data Sheet (MSDS).
[2] The extrapolated values used for substances in the sulfonated acid dyes group are based on evidence on sulfonated acid dyes submitted to Environment Canada under theNew Substance Notification Regulations and/or available evidence from other sulfonated acid dye analogues (e.g., ETAD 1995).
[3] Calculated using the following physical and chemical properties: water solubility (WS), vapour pressure (VP) and molecular weight (MW), using the following formula: (VP/WS)MW. For Acid Red 111, water solubility at 20 000 to 65 000 mg/L and upper range limit of 10-8 Pa for vapour pressure were used in the calculation. For Acid Red 114, water solubility at >500 mg/L and upper range limit of 10-8Pa for vapour pressure were used in the calculation. For Direct Black 38, water solubility at 93 000 mg/L and upper range limit of 10-8 Pa for vapour pressure were used in the calculation.
In general, sulfonated pure dyes whose properties have been studied have high melting points (usually above the 250–300°C range), whereas powder dyes have slightly lower melting points (> 200°C). In this case, the experimental melting points of Acid Red 111 and analogues are between 170ºC and 190ºC. In all these cases, the substances decompose (char) at these levels (ETAD 1995). Therefore, a determination of boiling point is not meaningful for Acid Red 111.
Structural information for Acid Red 111 is presented in Table 3 along with that of other sulfonated acid dyes that are considered to be acceptable analogues for the purposes of ecological assessment. The molecular mass for these substances ranges from 696.43 to 830.82 g/mol. The maximum diameters range from a minimum of 0.5–3.0 nm to a maximum of 3.3–3.5 nm. Available empirical data for these analogues (e.g., toxicity) are used in the weight of evidence to support the ecological assessment of Acid Red 111.
| Common name (CAS RN) |
DSL name | Structure of analogue | Molecular mass (g/mol) | Range of Maximum Diameter[2](Dmax); nm |
|---|---|---|---|---|
| Acid Red 111 (6358-57-2) |
2,7-Naphthalene- disulfonic acid, 3- [[2,2'-dimethyl-4'- [[4-[[(4-methylphenyl) sulfonyl]oxy]phenyl] azo][1,1'-biphenyl]- 4-yl]azo]-4-hydroxy-, disodium salt |
 |
830.82 | 3.0–3.5 |
| Acid Red 114 (6459-94-5) |
1,3-Naphthalene- disulfonic acid, 8- [[3,3'-dimethyl-4'- [[4-[[(4-methylphenyl) sulfonyl]oxy]phenyl] azo][1,1'-biphenyl]- 4-yl]azo]-7-hydroxy-, disodium salt |
 |
830.82 | 0.5–3.4 |
| Direct Black 38 (1937-37-7) |
2,7-Naphthalene- disulfonic acid, 4- amino-3-[[4'-[(2,4- diaminophenyl)azo] [1,1'-biphenyl]-4-yl] azo]-5-hydroxy-6- (phenylazo)-, disodium salt |
 |
781.7 | 2.2–3.3 |
| BAHSS (71215-83-3) |
Benzoic acid, 5-[[4'-[(2-amino-8-hydroxy-6-sulfo-1-naphthalenyl)azo]-2,2'-dichloro[1,1'-biphenyl]-4-yl]azo]-2-hydroxy-, disodium salt | 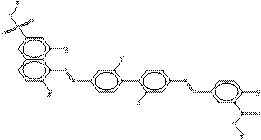 |
696.43 | 0.5–3.4 |
[1]Structures for analogues and azo cleavage products used for human health assessment found in Table 8.
[2] Based on a range of maximum cross-sectional diameters (Dmax) for conformers calculated using CPOPs (2008).
Acid Red 111, Acid Red 114 and Direct Black 38 are large, highly soluble sulfonated acid dyes. BAHSS is a monosulfonated dye. Direct Black 38 contains three azo groups in its molecule, whereas Acid Red 111, Acid Red 114 and Direct Black 38 contain only two azo groups. Molecular weights of all these substances are reasonably similar and water solubility (apart from the >500 mg/L value for Acid Red 114) appear to be within an order of magnitude. These values suggest that these substances are very soluble in water.
Acid Red 111 is not naturally occurring in the environment.
Recent information was collected through industry surveys conducted for the years 2005 and 2006 under Canada Gazettenotices issued pursuant to section 71 of CEPA 1999 (Canada 2006b, 2008b). These notices required submission of data on the Canadian manufacture and import of this substance. In the notice for 2006, data were also required on use quantities of Acid Red 111. In association with the section 71 notices for 2005 and 2006, companies that did not meet the mandatory reporting requirements but that had an interest in this substance were invited to identify themselves as stakeholders.
One company reported importing 300 kg of Acid Red 111 into Canada in 2006 and fewer than four companies reported importing a combined total between 100 kg and 1000 kg in 2005. No companies reported manufacturing Acid Red 111 above the 100-kg/year threshold in either year and no companies reported using Acid Red 111 above the 1000-kg/year threshold in 2006. One company has reported a stakeholder interest in Acid Red 111 (Environment Canada 2006, 2008a).
The quantity of Acid Red 111 reported to be manufactured, imported or in commerce in Canada during the 1986 calendar year (during the development of the DSL) was 100–1000 kg (Environment Canada 1988). Acid Red 111 is in the European Inventory of Existing Chemical Substances (EINECS) but has not been reported by the European Union as either a high or low production volume (HPV or LPV) chemical (ESIS c1995–2010). The national aggregate production volume for Acid Red 111 in the United States was 4500–226 800 kg (i.e., 10 000–500 000 pounds) in the 2002 reporting cycle under the US Environmental Protection Agency’s Inventory Update Reporting program (US EPA 2005). Acid Red 111 was also used in Sweden from 1999 to 2006 and in Denmark from 2002 to 2006 (SPIN 2008).
Products containing Acid Red 111 may enter Canada even if they are not identified as such in the section 71 survey because they may be imported unknowingly in manufactured items, or in quantities below the 100-kg reporting threshold for the survey.
Acid dyes are used in the textile industry for dyeing natural fibres and synthetics (e.g., wool, silk, nylon, polyesters, acrylic and rayon). The sulfonic acid groups react with the cationic amide groups in the fibres (ETAD 1995). To a lesser extent, these dyes are used in other applications such as leather, plastics, inks and paints (CII 2002; Danish EPA 1999; ETAD 1995).
Additional information-gathering on trade names identifed for Acid Red 111 (i.e., Lanasyn Scarlet F-3GL 130, Nylosan Scarlet F-3GL 130, Sandolan Milling Scarlet or Levanol Fast Scarlet F) indicates that this is a textile dye (bright yellowish red) used to dye polyamide and wool-based items (Clariant 2008). However, a follow-up of information received through the CEPA section 71 survey indicated that specific uses of Acid Red 111 in Canada are for manufacturing of textiles used in a limited range of specialty products (Environment Canada 2008a).
In Canada, Acid Red 111 is not listed as a permitted food additive under the Food and Drug Regulations nor has it been identified for use in food packaging applications (Canada 1978; 2009 email from Food Directorate, Health Canada to Existing Substances Risk Assessment Bureau, Health Canada, unreferenced).
In Canada, Acid Red 111 is not listed in the Food and Drugs Regulations under section C.01.040.2 as a colouring agent permitted in drugs (Canada 1978). In addition, Acid Red 111 is not listed in the Drug Products Database, the Therapeutic Products Directorate's internal Non-Medicinal Ingredients Database, the Natural Health Products Ingredients Database nor the Licensed Natural Health Products Database as a medicinal or non-medicinal ingredient present in pharmaceutical drugs, natural health products or veterinary drugs (DPD 2010; 2008 email from Therapeutic Products Directorate, Health Canada, to Risk Management Bureau, Health Canada, unreferenced; NHPID 2010; LNHPD 2010).
Acid Red 111 does not occur naturally in the environment. This substance is not reportable to Environment Canada’s National Pollutant Release Inventory (NPRI; Environment Canada 2010).
A method has been developed by Environment Canada to estimate a substance’s losses during different stages of its life cycle, including its fate within a finished product or article (Environment Canada 2008b). This method consists of a life cycle analysis and a spreadsheet tool (Mass Flow Tool or MFT) that integrates information on the manufacturing, importation and use data available for the substance. Starting with an identified mass of the substance, each life cycle stage is subsequently evaluated until all of the mass is accounted for. Relevant factors are considered, uncertainties recognized and assumptions may be made during each stage, depending on information available. The estimated losses represent the complete mass balance of the substance over the life cycle of the substance and include releases to wastewater and other receiving compartments (land, air), chemical transformation, transfer to recycling activities and transfer to waste disposal sites (landfill, incineration). However, unless specific information on the rate or potential for release of the substance from landfills and incinerators is available, the method does not quantitatively account for releases to the environment from disposal. Ultimately, the estimated losses provide a first tier in the exposure analysis of a substance and help to estimate environmental releases and focus exposure characterization later in the assessment.
In general, releases of a substance to the environment depend upon various losses from its manufacture, industrial use and consumer/commercial use. These losses can be grouped into seven types: (1) discharge to wastewater; (2) emission to air; (3) loss to land; (4) chemical transformation; (5) disposal to landfill; (6) loss to incineration; and (7) disposal through recycling (i.e., recycling is deemed a loss and not considered further). They are estimated using regulatory survey data, industry data and data published by different organizations. The discharge to wastewater refers to raw wastewater prior to any treatment by public or private wastewater systems. In a similar manner, the loss via chemical transformation refers to changes in a substance’s identity that may occur within the manufacture, industrial use, and consumer/commercial use stages, but excludes those during waste management operations such as incineration and wastewater treatment. The loss to land includes unintentional transfer or leakage to soil or paved/unpaved surfaces during the substance’s use and service life (e.g., from the use of agricultural machinery or automobiles). The loss to land, however, does not include transfers subsequent to a substance’s use and service life (e.g., land application of biosolids and atmospheric deposition).
The losses estimated for Acid Red 111 over its life cycle (based on conservative assumptions) are presented in Table 4 (Environment Canada 2009a). Acid Red 111 is not manufactured in Canada above reporting thresholds, so estimated losses are based on import quantities reported in 2006.
| Type of loss | Proportion[1] (%) | Pertinent life cycle stages[2] |
|---|---|---|
| Wastewater | 14.6 | Formulation, consumer use |
| Land | 0 | |
| Air emission | 0 | |
| Chemical transformation | 0 | |
| Incineration | 2.6 | Consumer/commercial use |
| Landfill | 82.8 | Consumer/commercial use |
| Total | 100 |
[1]Information from the following OECD emission scenario documents was used to estimate releases to the environment for which the distribution of releases of this substance is summarized above: textile manufacturing wool mills (OECD 2004); adhesive formulation (OECD 2007). Values presented for release to environmental media do not account for possible mitigation measures that may be in place at some locations. Specific assumptions used in the derivation of these estimates are summarized in Environment Canada (2009a).
[2] Potential applicable stage(s): production, formulation, industrial use, consumer use, service life of article/product, waste disposal.
Results summarized in Table 4 (Environment Canada 2009a) suggest that releases of Acid Red 111 related to formulation and consumer use (i.e., the service life of products containing the substances) are estimated to be 15% in industrial wastewater. Assumptions made during this step include losses during container handling (0.3%), the dyeing process emissions in wastewater (estimated dye fixation rate of 87%), the cleaning of transfer lines and vessels, and potential releases associated with consumer use during laundering of articles. Releases that occur to wastewater during the dyeing of textiles at the manufacturing site are considered a non-dispersive use. Releases to wastewater from laundering of articles during the life of the textile would be considered a widely dispersive use. The majority of Acid Red 111 is estimated to be lost through waste disposal (incineration and landfill ~85%). The industrial use scenario assumes that this substance is used as a dyeing agent for textiles.
Dyes have an inherently high affinity to substrates, with fixation levels ranging from 85 to 98% for acid dyes with more than one sulfonic acid group (ETAD 1995). With respect to wastewater treatment, most of the adsorption/desorption research on dyes has been done using activated sludge or carbon (ETAD 1995), with dyestuffs generally being adsorbed to the extent of 40–80% (Clarke and Anliker 1980). Although dyes are not easily biodegraded aerobically, combined removal through biodegradation and adsorption on waste treatment sludge has been shown to exceed 95% in some cases (ETAD 1995). Some portion of dyes may still end up in the aquatic environment depending on their chemical structure and the properties of the dye product (Danish EPA 1999).
Acid Red 111 may be released to wastewater, as well as transferred to waste disposal sites (as described previously using the MFT). If released to the aquatic environment, this substance’s adsorption characteristics would be dictated by its two sulfonic acid groups, which impart high water solubility. Other factors such as increasing molecular size, hardness of the water and salinity, as well as decreasing pH, are thought to favour some sorption of azo dyes to suspended solids (Danish EPA 1999; NLM 2006). Acid dyes have a high fixation rate with positively charged substrates and can adsorb to positively charged particulates (e.g., nitrogen-containing particles) and have the potential to settle out to bed sediments or wastewater treatment plant sludge (ETAD 1995). It has been stated generally that, due to the recalcitrant nature of azo dyes in aerobic environments, they eventually end up in anaerobic sediments, shallow aquifers and groundwater (Razo-Flores et al. 1997).
Biosolids from wastewater treatment plants would contain dyes as a result of the removal processes (ETAD 1995), and dyes may end up being indirectly applied to soil as a result of biosolids application to soils or its deposition in landfills. In soil, the ionic nature of these dyes may result in ion-exchange processes with clay that would retard leaching (NLM 2006). Volatilization from dry or moist soil surfaces is expected to be an unimportant fate process based upon the low estimated vapour pressures for azo dyes in general (Danish EPA 1999; NLM 2006).
Given the intended use in aqueous-based treatments, Acid Red 111 is not expected to be released to air and is not expected to partition to this compartment, based on a very low calculated Henry’s Law constant of 1.4 to 4.2 x 10-11Pa•m3/mol). Moreover, air is not considered to be a carrying medium for dyes (including acid dyes), as these substances exhibit low or negligible volatility (Brown and Hamburger 1987; Danish EPA 1999; ETAD 1995).
Environmental Persistence
Dyes must have a high degree of chemical and photolytic stability in order to be useful, so most are generally considered non-degradable under environmentally relevant aerobic conditions (Danish EPA 1999; ETAD 1995). Studies applying commonly accepted screening tests (e.g., OECD guidelines) for ready and inherent biodegradability have confirmed this point (ETAD 1992; Pagga and Brown 1986). Abiotic degradation, including photolysis and hydrolysis, is not thought to play a significant role in the environmental fate of azo dyes (Danish EPA 1999), although one study showed strongly accelerated photo decomposition of azo dyes in the presence of natural humic materials (Brown and Anliker 1988).
Biotic degradation of azo dyes may take place relatively rapidly under anaerobic or reducing conditions (Baughman and Weber 1994; Danish EPA 1999; ETAD 1995; Isik and Sponza 2004; Yen et al. 1991). Permeability of the bacterial cell wall has been found to be the rate-limiting step in the reduction process (Danish EPA 1999). Azo dyes have a high tendency to cleave at the azo bond with the formation of aromatic amines (Danish EPA 1999; Hunger 2005). The carcinogenic potential of aromatic amines varies considerably with molecular structure, with carcinogenic breakdown products being associated with the moieties of benzidine, aniline, toluene or naphthalene. However, the formation of such metabolites in deep anoxic sediments would typically not result in exposure to aquatic organisms. Total mineralization or further degradation of these metabolites could take place if they are transferred (e.g., by sediment resuspension) to aerobic environments (Danish EPA 1999; Isik and Sponza 2004). Aromatic amines may also be present as impurities in commercially available azo dyes, although the metabolic cleavage of azo dyes is the main source of these compounds (Danish EPA 1999).
There are no empirical data on the persistence of Acid Red 111. Empirical tests on Acid Red 114 and the analogue Direct Black 38 (Table 5a) showed that they do not readily undergo aerobic biodegradation.
| Substance | Method | Degradation value[1] | Degradation endpoint | Test Duration | Reference |
|---|---|---|---|---|---|
| Acid Red 114 |
OECD TG 301C | 0% | Biodegradation | 42 days | CHRIP c2011 |
| Direct Black 38 (1937-37-7) |
OECD TG 301C | 0% | Biodegradation | 28 days | CHRIP c2011 |
[1] % biodegradation at a given concentration of the test substance
In addition to the experimental data, a QSAR-based weight-of-evidence approach (Environment Canada 2007) was applied using the biodegradation models shown in Table 5b. These models are considered acceptable for use as they are based on chemical structure, and the disazo structure is represented in the training sets of all the BIOWIN models used, thereby increasing the reliability of the predictions. Given the ecological importance of the water compartment, the fact that most of the available models apply to water and the fact that Acid Red 111 is expected to be released to this compartment, aerobic biodegradation in water was primarily examined. Acid Red 111 does not contain functional groups expected to undergo hydrolysis.
| Fate process | Model and model basis |
Model result and prediction | Extrapolated half-life (days) |
|---|---|---|---|
| Water | |||
| Primary biodegradation | |||
| Biodegradation (aerobic) | BIOWIN 2008[1] |
2.48[2] (biodegrades somewhat slowly) |
<182 |
| Ultimate biodegradation | |||
| Biodegradation (aerobic) | BIOWIN 2008[1] Sub-model 3: Expert Survey (qualitative results) |
0.98[2] (biodegrades very slowly) |
≥182 |
| Biodegradation (aerobic) | BIOWIN 2008[1] Sub-model 5: MITI linear probability |
-1.34[3] (biodegrades very slowly) |
≥182 |
| Biodegradation (aerobic) | BIOWIN 2008[1] Sub-model 6: MITI non-linear probability |
0.0[3] (biodegrades very slowly) |
≥182 |
| Biodegradation (aerobic) | TOPKAT 2004 Probability |
n/a[4] | |
| Biodegradation (aerobic) | CATABOL c2004–2008 % BOD[5] |
16.8 (biodegrades very slowly) |
≥182 |
[1]EPIsuite (2008); model run using SMILES notation from Table 1
[2] Output is a numerical score from 0 to 5
[3] Output is a probability score
[4] n/a: not available (out of model domain)
[5] BOD: Biological oxygen demand
The results in Table 5b reveal that the majority of aerobic biodegradation models (i.e., the ultimate biodegradation models BIOWIN 3, 5, 6, and CATABOL) suggest this dye biodegrades very slowly, if at all. The half-life results from the primary survey model (BIOWIN 4) equates to “weeks to months,” indicating that this substance undergoes a somewhat slow rate of primary biodegradation but that the half-life is expected to be less than 182 days.
When the overall results of the modelled data are considered, the weight of evidence (for models predicting ultimate biodegradation) suggests that the aerobic biodegradation halflife of this substance in water is =182 days. This finding is consistent with what would be expected for this chemical structure (i.e., few degradable functional groups, solid particle) and its intended use as a dye.
Using an extrapolation ratio of 1:1:4 for a water:soil:sediment biodegradation half-life (Boethling et al. 1995), and using the model-estimated ultimate biodegradation half-life in water of =182 days, the ultimate degradation half-life in aerobic soil is also expected to be =182 days and the half-life in aerobic sediment is expected to be =365 days. As mentioned previously, experimental evidence suggests that this dye may not persist in the deeper, anoxic layers of sediments where azo bonds are readily reduced under anaerobic conditions.
Based on the weight of evidence provided by experimental biodegradation data on Acid Red 114 and Direct Black 38, the available literature on azo dyes and the modelled data described above, Acid Red 111 meets the persistence criteria in water, soil and sediment (half-lives in aerobic soil and water ≥ 182 days and half-life in aerobic sediment ≥ 365 days) as set out in thePersistence and Bioaccumulation Regulations (Canada 2000).
Potential for Bioaccumulation
In this assessment, a variety of lines of evidence have been used to determine the bioaccumulation potential of Acid Red 111, which is different from the Kow-based QSAR approach used during categorization (that relied on outputs from QSARs using the predicted log Kow of the neutral form of the substance). As indicated in Table 2, this substance has a high water solubility (20 000–65 000 mg/L) and read-across data for azo dyes suggests relatively low log Kow values (<3.0), which would also suggest a low bioaccumulation potential. Ionic dyes (including acid dyes) are generally considered to have a very low bioaccumulation potential based on results of studies with various dyes (ETAD 1995).
Estimated and experimental log Kow values were compared with experimental bioconcentration factors (BCFs) for fish for a number of dyes (Anliker et al. 1981; Danish EPA 1999; ETAD 1995). With respect to the data for six acid dyes and one direct dye (only dye classes were reported), reported BCFs were less than 10, indicating that these very hydrophilic (ionic) dyes are not likely to bioconcentrate in aquatic organisms.
There are no empirical bioaccumulation data available for Acid Red 111. Data are available for the analogue Acid Red 114 (Table 6). Table 6 illustrates low BCF values (42–84 L/kg) for carp exposed to two different concentrations of Acid Red 114.
| Test organism | Experimental concentration (mg/L) | Endpoint (BCF, L/kg) |
Reference |
|---|---|---|---|
| Common carp (Cyprinus carpio) |
0.2 | 42–76 | MITI 1992 |
| Common carp (Cyprinus carpio) |
0.02 | 52–84 | MITI 1992 |
In addition to the experimental BCF data for Acid Red 114, available data for Acid Red 111 regarding its water solubility, molecular weight and maximum diameter have also been considered (including those for the analogues chosen) in order to determine the bioaccumulation potential of Acid Red 111.
Recent investigations relating fish BCF data and molecular size parameters (Dimitrov et al. 2002, 2005) suggest that the probability of a molecule crossing cell membranes as a result of passive diffusion declines significantly with increasing maximum diameter (Dmax). The probability of passive diffusion decreases appreciably when the maximum diameter is greater than ~1.5 nm and much more so for molecules having a maximum diameter of greater than 1.7 nm. Sakuratani et al. (2008) have also investigated the effect of cross-sectional diameter on passive diffusion in a BCF test set of about 1200 new and existing chemicals. They observed that substances that do not have a very high bioconcentration potential (BCF <5000) often have a Dmax of >2.0 nm and an effective diameter (Deff) >1.1 nm.
However, as Arnot et al. (2010) have noted, there are uncertainties associated with the thresholds proposed by Dimitrov et al. (2002, 2005) and Sakuratani et al. (2008) since the BCF studies used to derive them were not critically evaluated. Arnot et al. (2010) point out that molecular size influences solubility and diffusivity in water and organic phases (membranes), and larger molecules may have slower uptake rates. However, these same kinetic constraints apply to diffusive routes of chemical elimination (i.e., slow in = slow out). Thus, significant bioaccumulation potential may remain for substances that are subject to slow absorption processes, if they are slowly biotransformed or slowly eliminated by other processes. Consequently, when evaluating bioaccumulation potential, molecular size information should be considered with care and used together with other relevant lines of evidence in a weight-of-evidence approach.
Acid Red 111 is a relatively large molecule with a high molecular weight (830.82 g/mol) and a range of maximum diameters (Dmax) from 3.03 to 3.53 nm (CPOPs 2008), comparable to some of the values cited above. These characteristics suggest that the uptake rate of this substance may be slower compared to that of smaller more compact substances, thus mitigating the overall bioconcentration potential.
Acid Red 111 is expected to have a low bioaccumulation potential due to its physical and chemical properties (i.e., high molecular weight, large cross-sectional diameter, high water solubility), the physical and chemical properties of the relevant analogues (sulfonated dyes) used in this assessment (i.e., high molecular weights, high decomposition points, relatively large cross-sectional diameters, high water solubilities), and limited information regarding experimental log Kow values, as well as low read-across experimental BCFs. Therefore, considering the available evidence, Acid Red 111 does not meet the bioaccumulation criteria (BCF or BAF =5000) as set out in thePersistence and Bioaccumulation Regulations (Canada 2000).
Ecological Effects Assessment
In the Aquatic Compartment
A variety of lines of evidence have been used to determine the ecotoxicological potential of Acid Red 111, which is different from the primarily Kow-based QSAR approach used during categorization. The available data to support this assessment are discussed below.
Toxicological data were reported for a formulated product, Lanasyn Scarlet F-3GL 130, which contains Acid Red 111 (Study Submission 2007). The information listed in the material safety data sheet included an acute LC50 (48 hours) of approximately 11 mg/L for rainbow trout (Oncorhynchus mykiss). Study details were not reported.
Although empirical ecotoxicological data for Acid Red 111 are very limited, predictions for ecotoxicological data using QSAR models are considered unreliable, as anionic dye classes are difficult to model because the properties of these dyes fall outside the domains of applicability of the available models. Consequently, data for the acceptable analogues of Direct Black 38, Acid Red 114 and BAHSS were also considered. These data are summarized in Table 7 below.
| Test organism | Type of test | Endpoint | Value (mg/L) | Reference |
|---|---|---|---|---|
| Direct Black 38 (1937-37-7) | ||||
| Fathead minnow (Pimephales promelas) | Acute (96 hours) |
TL50 | >180 | Little and Lamb 1972 |
| Acid Red 114 (6358-57-2) | ||||
| Common carp (Cyprinus carpio) |
Acute (48 hours) |
LC50 | 4[*] | MITI 1992 |
| BAHSS (71215-83-3) | ||||
| Rainbow trout (Oncorhynchus mykiss) |
Acute (48 hours) |
LC50 | 250 | Study Submission 2005 |
TL50 – Defined by the authors as the concentration of the substance where 50% of the experimental animals survived after 96 hours.
LC50 – The concentration of a substance that is estimated to be lethal to 50% of the test organisms.
[*] The critical toxicity value used to derive a predicted no-effects concentration.
Empirical toxicity studies were available for Direct Black 38, Acid Red 114 and BAHSS. For the Direct Black 38 study, static bioassays were performed to evaluate the acute toxicity of various dyes (47 in total) to fathead minnow (Little and Lamb 1972). Bioassays were carried out according to published standard methods and data sheets for each test were prepared, including pertinent information on the test organisms, dilution water and test conditions. The experiment was designed to estimate the TL50, the concentration at which 50% of the experimental animals survived after 96 hours. However, no mortality was observed in fathead minnows exposed to Direct Black 38 at the highest concentration tested (180 mg/L). An empirical 48-hour acute toxicity test for BAHSS with rainbow trout produced a similar LC50 value of 250 mg/L.
According to the data available for these analogues, the lowest LC50 of 4 mg/L was observed for common carp in an acute toxicity study for Acid Red 114 (Table 7). This study and the other two read-across empirical toxicity values suggest that Acid Red 111 would have a moderate to low potential for toxicity.
The above results are similar to what has been reported generally for acid and direct dyes in the literature (Danish EPA 1999). The Danish EPA summarized short-term test results for zebrafish, Daphnia magna, algae and bacteria from a study by ETAD covering 47 dyes of different chemical dye classes (although specific dyes included in the study were not reported). In 96-hour LC50 tests on zebrafish, toxicity was observed for 2 acid dyes between 1–10 mg/L, for 3 acid dyes between 10–100 mg/L and 6 other acid dyes above 100 mg/L. For direct dyes, all 7 dyes tested reported toxicity for zebrafish at levels >100 mg/L. Effects were observed between 10–100 mg/L and above 100 mg/L in 48-hour EC50 tests (endpoint not specified) on D. magna for 9 acid dyes. Similar tests with direct dyes resulted in adverse effects to D. magna at levels >100 mg/L. Algal toxicity (measured in 72-hour EC50 tests) was observed in 2 cases for acid dyes below 1 mg/L, as well as above this level (for the remaining 7 acid dyes), whereas toxicity results for the 7 direct dyes tested were all above 1 mg/L. Algae appeared to be the most susceptible organisms for all dye classes tested; the effect was thought to be related to light inhibition at high dye concentrations (coloration of water can occur above 1 mg/L). Generally, bacteria were the least susceptible to most of the different classes of dyes tested, compared with the other organisms (IC50s >100 mg/L).
As mentioned previously, cleavage of the azo bond under anaerobic or reducing conditions (e.g., deep layers of sediments) is known to result in aromatic amines, some of which are known to be potentially harmful. Although such studies have not been carried out on Acid Red 111, studies using Direct Black 38 have shown breakdown to benzidine and 4-aminobiphenyl (4-ABP), which have been found to be mutagenic and carcinogenic substances (Bafana et al. 2009; Isik and Sponza 2004), and acutely toxic (LC50<1 mg/L) to some crustaceans and juvenile fish (Danish EPA 1999). However, because they are only formed in deep anoxic sediment, there is a lower likelihood that aquatic organisms are exposed to these more harmful metabolites.
Experience with over 200 acid dyes has led to the observation that the potential ecotoxicity of such substances may generally be predicted by the number of acid groups present (US EPA 2002). Some monoacid and diacid dyes have shown high to moderate toxicity (i.e., acute values <1 mg/L and <100 mg/L, respectively) to fish and other aquatic organisms. Dyes with three or more acid groups showed low toxicity (i.e., acute values >100 mg/L) towards fish and invertebrates. All acid dyes showed moderate toxicity to green algae, with further analysis suggesting that such effects may have been related to shading. For these generalizations to be applicable, the acid dyes must have some water solubility and molecular weights generally need to be near or below 1000, which is the case for Acid Red 111. Furthermore, Environment Canada has evaluated numerous acid dyes under the New Substances Notification Regulations and has generally found anionic dyes to be of low toxicity regardless of the number of acid groups, but some exceptions have been found (e.g., when a reactive functional group is not hindered).
Given the information summarized above, Acid Red 111, as an anionic dye with two sulfonic acid groups, is expected to have moderate toxicity to aquatic organisms (i.e., LC50s > 1 mg/L).
In Other Environmental Compartments
No ecological effects studies were found for Acid Red 111 in media other than water. However, this substance could end up in soil or sediment as a result of release to the aquatic environment, landfill disposal of biosolids from wastewater treatment plants, disposal of products containing these substances, or biosolids application to soils. Therefore, toxicity data for soil and sediment organisms would be desirable.
This being said, the toxicity potential is likely to be low in sediment- and soil-dwelling species, considering the low bioaccumulation potential and the physical and chemical properties of this substance. However, this cannot be substantiated due to the lack of suitable whole organism toxicity data.
Ecological Exposure Assessment
No information has been identified concerning the concentrations of Acid Red 111 in the Canadian environment (air, water, soil, sediment). A concentration in water of 0.002 mg/L of an acid red dye (unspecified) was reported in the Coosa River, USA (as summarized in Danish EPA 1999). Environmental concentrations have been estimated below for industrial releases.
Industrial Release
The aquatic exposure of Acid Red 111 is expected if the substance is released from industrial use to a wastewater treatment plant and the treatment plant discharges its effluent to a receiving water body. The concentration of the substance in the receiving water near the discharge point of the wastewater treatment plant is used as the predicted environmental concentration (PEC) in evaluating the aquatic risk of the substance. It can be calculated with the equation
Cwater-ind = [1000 × Q × L × (1 - R)] / [N × F × D]
where
Cwater-ind is aquatic concentration resulting from industrial releases, mg/L
Q is total substance quantity used annually at an industrial site, kg/year
L is loss to wastewater, fraction
R is wastewater treatment plant removal rate, fraction
N is number of annual release days, d/year
F is wastewater treatment plant effluent flow, m3/day
D is receiving water dilution factor, dimensionless
As Acid Red 111 is used industrially and is expected to be released to water, a conservative industrial release scenario is used to estimate the aquatic concentration of the substance with the help of Environment Canada’s (2009b) Industrial Generic Exposure Tool – Aquatic (IGETA). The scenario is made conservative by assuming that the total quantity of the substance used by Canadian industry is used by one single industrial facility at a small, hypothetical site and the loss to a local wastewater treatment plant is high at 15% of the total quantity resulting from textile dying processes. Such a small site is selected to have an effluent flow at the 10th percentile (3456 m3/day) of the wastewater treatment plant discharge rates across Canada. The scenario also assumes that the release occurs 150 days/year (to represent seasonal activities), the wastewater treatment plant removal rate is zero for the substance, and the receiving water dilution factor is ten. Based on the above assumptions, a total industrial use quantity of 1000 kg/year of the substance (the upper limit of the reporting range) yields a predicted environmental concentration (PEC) in water of 0.03 mg/L (Environment Canada 2009c).
Characterization of Ecological Risk
The approach taken in this ecological screening assessment was to examine various supporting information and develop conclusions based on a weight-of-evidence approach and using precaution as required under CEPA 1999. Lines of evidence considered include results from a conservative risk quotient calculation, as well as information on persistence, bioaccumulation, ecotoxicity, sources and fate of the substance.
Acid Red 111 is expected to persist in aerobic environments (i.e., water, soil, sediment), based on modelled biodegradation and general information on other acid dyes or disulfonic acid substances. Under anaerobic conditions (e.g., deep layers of sediment), this dye could undergo relatively rapid azo reduction to potentially hazardous metabolites, although exposure of aquatic organisms to these breakdown products would be limited and would likely present a low risk. Data on water solubility, molecular size and cross-sectional diameter, as well as empirical bioaccumulation data for some similar substances, suggest that Acid Red 111 has a low potential to bioaccumulate in aquatic organisms. Acid Red 111 is considered to have moderate toxicity to aquatic organisms (LC50s >1 mg/L). This substance is not present in large quantities in Canadian commerce and therefore, releases of Acid Red 111 to the Canadian environment are expected to be low.
A risk quotient analysis, integrating conservative estimates of exposure with toxicity information, was performed for the aquatic medium to determine whether there is potential for ecological harm in Canada. The industrial release scenario presented above yielded a PEC of 0.03 mg/L. A predicted no-effect concentration (PNEC) was derived from the ecotoxicity data available for relevant analogues (i.e., the most sensitive valid experimental value), as experimental data were not available for Acid Red 111. The LC50 value of 4 mg/L for an acute (48-hour) test on common carp was reported for Acid Red 114. This value was divided by an assessment factor of 100 to account for uncertainty regarding the necessary reliance on limited data from relevant analogues, interspecies and intraspecies variability in sensitivity, and extrapolation from lab to field effects. The resulting PNEC calculated for Acid Red 111 was 0.04 mg/L. The resulting risk quotient (PEC/PNEC) = 0.7. Therefore, harm to aquatic organisms is unlikely at this site.
Based on the information presented in this assessment, Acid Red 111 has a low potential to cause ecological harm in Canada.
Uncertainties in Evaluation of Ecological Risk
In general, Acid Red 111 is a data-poor substance. Information was available on the water solubility of this substance, and pertained to formulated products. Very little other physical and chemical data exist on this substance. As a result, a read-across approach using data from selected analogues was the best alternative to estimating physical and chemical properties, given that they too are sulfonated azo dyes with naphthalene rings.
This paucity of information necessitated the generation of model predictions for Acid Red 111 for biodegradation, and inference of bioaccumulative potential using available data on physical and chemical properties. The empirical toxicity study available for Acid Red 111 involved a formulated product. Therefore, available acute toxicity data from relevant analogues were also used to assess the potential toxicity of Acid Red 111. Long-term (chronic) toxicity data would be beneficial in evaluating substances such as this one that are determined to be persistent in the environment. The use of an assessment factor in determining a predicted no-effect concentration is intended to address these uncertainties. The significance of soil and sediment as possible media of exposure was not well addressed by the effects data available.
The lack of information on environmental concentrations of this substance (e.g., monitoring data) in Canada resulted in the need to evaluate risk based on predicted concentrations in water near industrial point sources. Conservative assumptions were made when using models to estimate concentrations in such receiving water bodies.
Given the use of this substance in other countries, it is possible that the substance is entering the Canadian market as a component of manufactured items and/or consumer products. Information obtained from the section 71 notice and other information sources indicated that it may be present in a limited number of these types of products in Canada. Available information is currently not sufficient to derive a quantitative estimate to help determine the importance of this source in ecological assessment. However, it is anticipated that the proportions of Acid Red 111 released to the various environmental media would not be significantly different from those estimated here, although quantities transferred to recycling and/or waste disposal may be higher. It is also recognized that releases from waste disposal sites are possible, although difficult to quantify due to the lack of data, and would contribute to overall environmental concentrations.
Exposure Assessment
From the published literature, no data were identified on measured concentrations of Acid Red 111 in environmental media (air, water, soil and sediment) in Canada or elsewhere. Under section 71 of CEPA 1999, one company reported importing about 300 kg in 2006. Environmental concentrations estimated using ChemCAN version 6 software (ChemCAN 2003) were based on the loss percentages predicted by the Mass Flow Tool (see Table 4) (Environment Canada 2009a). The percentages were applied to the total quantity of 300 kg of Acid Red 111 in Canadian commerce in 2006 (Environment Canada 2008a). The loss quantities are estimated to be 44 kg to water from wastewater, 0 kg to air from air emissions and 0 kg to soil from loss to land. The upper-bounding estimates of Acid Red 111 intake for each age group in the general population of Canada from environmental media were predicted to be negligible.
As described in the Uses section, Acid Red 111 is a bright yellowish red dye that is used primarily in the textile industry, with other potential applications including leather.
In Canada, Acid Red 111 was identified as a colourant for manufacturing textiles used in a limited range of specialty products, which could not be specified due to confidentiality requested by the submitter to the section 71 notice. These specific products, however, would not be expected to result in direct and prolonged contact with the human skin or oral cavity. Accordingly, exposure of the general population to Acid Red 111 via dermal or oral routes is expected to be negligible. Based on its physical and chemical properties, exposure via inhalation is not expected.
Health Effects Assessment
Limited empirical health effects data are available for Acid Red 111. Therefore, information on potential azo cleavage products, analogues, and properties associated with the broader chemical class has been considered. Structures and a summary of the types of data available for each are available in Table 8.
| Substance identification (CAS RN) |
Structure | Similarity from ChemID[*] | Available data considered |
|---|---|---|---|
| Acid Red 111 | |||
| Acid Red 111 C.I. 23266 (6358-57-2) |
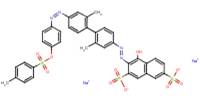 |
100 | -Ames assay -SOS test -QSAR |
| Analogues | |||
| Acid Red 99 C.I. 23285 (3701-40-4) |
 |
97 | -Ames assay |
| Acid Red 114 C.I. 23635 (6459-94-5) |
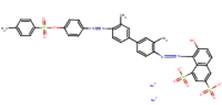 |
88 | -2-year rat bioassay -Ames assay -Mammalian cell clasotgenicity assay -Sex-linked recessive lethal assay (D. melanogaster) |
| Potential Azo Cleavage Products | |||
| 2,2'-Dimethylbenzidine or, 2,2'-DMB (84-67-3) |
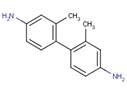 |
n/a | -Ames assay |
| Metabolite 1 2,7-Naphthalenedisulfonic acid, 3-amino-4-hydroxy, disodium salt (No CAS RN) |
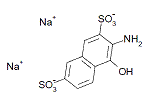 |
n/a | -QSAR |
| 2,7-Naphthalene-disulfonic acid, 4-amino-3-hydroxy-, disodium salt; analogue of metabolite 1 (42579-07-7) |
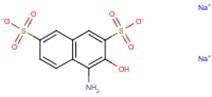 |
n/a | -Ames assay -Mouse lymphoma assay |
| Metabolite 2 (No CAS RN) |
 |
n/a | -QSAR |
[*] ChemID (2010)
n/a: not available
It has been demonstrated that azo colourants can undergo reductive cleavage mediated by azo reductase enzymes present in mammalian tissues as well as bacteria of the intestine and skin (e.g., Freeman et al. 1991; Chung et al. 2006; Platzek 1999; Golka et al. 2004; Chen 2006; Stingley et al. 2010). Accordingly, the predicted aromatic amine released by azo cleavage of Acid Red 111 (i.e., 2,2'-DMB) is also considered in this screening assessment. It is recognized that the degree of azo reduction is likely influenced by various factors (e.g., solubility of parent compound, presence/position of molecular substituents). Certain azo substances can cleave at the azo bond resulting in the formation of aromatic amines (Danish EPA 1999; Hunger 2005). The carcinogenic potential of aromatic amines varies considerably with molecular structure, with carcinogenic breakdown products being associated with moieties of benzidine, aniline, toluene or naphthalene, for example (Danish EPA 1999; Hunger 2005). Since water soluble azo dyes, as a class, have demonstrated susceptibility to azo cleavage, both in vivo and in vitro (Golka et al. 2004), it is reasonable to assume, due to its high water solubility, that Acid Red 111 could undergo azo cleavage. Therefore, in addition to the hazard data for Acid Red 111 itself, data obtained for aromatic amines predicted to be formed via Acid Red 111 cleavage are also considered. To further inform the hazard database for Acid Red 111, data from other structurally similar benzidine congener-based acid dye analogues are also considered in this assessment.
Acid Red 111
Genotoxicity and Carcinogenicity
Mutagenicity testing has been conducted in vitro in bacterial testing strains for Acid Red 111. Acid Red 111 was not mutagenic in Salmonella typhimurium strain TA98 when a preincubation procedure was used [Prival’s method - a method which is intended to mimic the metabolism of azo dyes by bacteria that can occur naturally in the gut and on the skin (Prival and Mitchell 1982)] in combination with metabolic activation by hamster liver S9 (Zhou et al. 1987). Acid Red 111 was also negative when tested against strains TA98, TA100, TA1535, and TA1538 with and without rat liver S9 (Venturini and Tamaro 1979). Additionally, Acid Red 111 did not induce DNA damage in bacterial SOS/umu testing (Reifferscheid and Heil 1996). However, these studies did not employ the Prival method, which promotes azo reductive cleavage and subsequent release of aromatic amines.
QSAR models (CASETOX 2008; DEREK 2008; TOPKAT 2004) were also used to help predict the genotoxic and carcinogenic potentials of Acid Red 111 and its potential cleavage products. Overall, mixed results were obtained for genotoxicity. For the carcinogenicity of Acid Red 111, DEREK produced a positive prediction. CASETOX predicted Acid Red 111 to be positive for carcinogenicity in male and female rats, and in male mice, but predictions were inconclusive for female mice and rodents in general. TOPKAT produced a positive prediction for carcinogenicity in female rats, but was inconclusive, or unable to model, in male rats, or in mice. QSAR predictions for the parent compound and its metabolites are summarized in Appendix 1.
Potential Azo Cleavage Products
Genotoxicity and Carcinogenicity
In the absence of empirical data for azo cleavage of Acid Red 111, it is assumed that cleavage of the azo linkage may occur and therefore exposure to this substance may also lead to exposure to several aromatic amine metabolites: 2,2'-dimethylbenzidine (2,2'-DMB, CAS RN 84-67-3 ), 2,7-naphthalenedisulfonic acid, 3-amino-4-hydroxy-, disodium salt (Metabolite 1, no CAS RN), and Metabolite 2 (no CAS RN) (see Table 8 for structures). Limited empirical data were available for 2,2'-DMB and a close structural analogue of Metabolite 1 and are presented below. QSAR results for all three predicted metabolites are summarized in Appendix 1.
Empirical health effects data for 2,2'-DMB are limited to one study of in vitro mutagenicity tested by the standard Ames assay, which was positive in S. typhimurium tester strains TA98 and TA100 when preincubated with S9 rat liver enzymes (not tested in other strains; Hinks et al. 2000). In comparison, benzidine congeners substituted by 2,2',5,5'-tetramethyl, 2,2'-dimethyl-5,5'-alkyl/alkoxy (=C3), 3,3'-alkyl/alkoxy (=C3), were non-mutagenic in the same test system (Hinks et al. 2000; Chung et al. 2006). This data suggests that 2,2'- substitution with only methyl groups does not eliminate mutagenicity of the benzidine moiety and therefore 2,2'-DMB may reasonably be considered as similar to other mutagenic benzidine congeners, including 3,3'-DMB. QSAR models (CASETOX, DEREK, TOPKAT) were also used to obtain predictions of the genotoxic and carcinogenic potential of 2,2'-DMB (Appendix 1). Predictions for carcinogenicity were positive using DEREK, predominantly positive using TOPKAT, but predominantly negative by CASETOX. Positive predictions were generated by CASETOX for models of mammalian clastogenicity, and for the mouse lymphoma mutation assay, but not for models of micronuclei formation or bacterial mutagenicity. DEREK predicted a positive outcome for bacterial mutagenicity (Ames assay), as did TOPKAT (CASETOX 2008; DEREK 2008; TOPKAT 2004).
A close structural analogue of Metabolite 1 has been tested for mutagenicity using both the standard Ames assay and the thymidine kinase heterozygous (TK+/TK-) mouse lymphoma assay. This compound was found not to be mutagenic by the standard Ames assay (Chung et al. 1981). However, positive results were obtained with this compound in the mouse lymphoma assay (Palmer et al. 1979).
Analogue Azo Dyes: Acid Red 99 and Acid Red 114
Genotoxicity and Carcinogenicity
To further inform the health effects database for Acid Red 111, empirical data from analogues were taken into consideration. Compounds with similarity scores above 80% (ChemID 2010) or a Tanimoto score of 80 (Scifinder 2010) were examined for the presence of either the 2,2'-dimethylbenzidine (2,2'-DMB; CAS RN 84-67-3) or 3,3'-dimethylbenzidine (3,3'-DMB; CAS RN 113-93-7) moieties, along with at least one naphthalene-disulfonic acid side group. The compounds Acid Red 99 (CAS RN 3701-40-4) and Acid Red 114 were identified by ChemID as having similarities of 97 and 88 percent, respectively (see Table 8).
Acid Red 99 is a soluble azo acid dye based on the same 2,2'-DMB core as Acid Red 111, and shares one identical coupling component (see Table 8). Therefore, since metabolism of these two compounds would be expected to occur in a similar manner, common aromatic amine constituents, including 2,2'-DMB, are likely to be released following azo reduction by mammalian or bacterial systems. No studies of carcinogenicity were available for Acid Red 99. However, Acid Red 99 has been tested for reverse mutagenicity in S. typhimurium with dithionite reduction (i.e., to cleave azo bonds) and was positive at all tested doses in TA100 but not in TA98 (Elliott 1980; Gregory et al. 1981). TA100 and TA98 detect different types of DNA mutations and the different sensitivity of TA100 compared to TA98 in detecting the mutagenicity of Acid Red 99 may help explain why Acid Red 111 was negative when tested for bacterial mutagenicity using Prival’s preincubation method, as only strain TA98 was tested (Zhou et al. 1987). Based on this information, it is reasonable to assume that Acid Red 111 may also be mutagenic in strain TA100 under similar test conditions.
Acid Red 114 is also a soluble azo acid dye very similar to Acid Red 111 but is based on the 3,3'- DMB, rather than 2,2'-DMB, congener. Both Acid Red 111 and Acid Red 114 are azo bonded to similar sulfonated naphthol coupling components (see Table 8). Acid Red 114 is classified by the International Agency for Research on Cancer (IARC) as a Group 2B carcinogen (possibly carcinogenic to humans) (IARC 1993). Acid Red 114 was carcinogenic in both male and female F344/N rats administered the dye in the drinking water for two years. Tumours were observed in the skin, zymbal gland and liver in male and female rats, and in the clitoral gland, lung, oral cavity, and small and large intestine in female rats (NTP 1991a). These effects are similar to what was observed in an identical study using 3,3'-DMB, the benzidine congener on which Acid Red 114 is based (NTP 1991b). Acid Red 114 is mutagenic inS. typhimurium strains TA98, TA100 and TA1538 when various reductive preincubation methods were incorporated prior to testing. However, Acid Red 114 did not induce sex-linked recessive mutations in Drosophilla melanogaster, nor did it increase incidences of sister chromatid exchange or chromosomal aberrations in rat hepatocytes or Chinese hamster ovary cells, respectively (IARC 1993). Based on: (1) similar physical-chemical properties shared by Acid Red 111 and Acid Red 114, (2) evidence that Acid Red 114 and water soluble azo dyes as a class undergo azo cleavage (Golka et al. 2004), (3) positive mutagenicity results for the benzidine congeners predicted to arise from azo cleavage of these two substances (2,2'-DMB and 3,3'-DMB, respectively), and (4) positive carcinogenicity results for Acid Red 114, it is reasonable to consider that the hazard potential of Acid Red 111 may be similar to that of Acid Red 114, namely potential genotoxicity and carcinogenicity.
Non-cancer Effects
No empirical data are available to address non-cancer health effects associated with Acid Red 111. However, investigations of short-term and sub-chronic repeated-dose toxicity have been carried out for the analogue Acid Red 114. The US National Toxicology Program (US NTP) tested Acid Red 114 for toxicity in range finding studies to determine the appropriate dosing levels for carcinogenicity testing. In a 13-day study, F344 rats (5/sex/group) were exposed to 0, 1429, 2857, or 4286 mg/kg-bw/day[3] via drinking water. At the end of the study, mean body weights were significantly lower in the mid- and high-dose groups (83% and 77%, respectively). Effects on the bone marrow characterized as hypercellularity of the sternal bone marrow were observed in 3 of 5 males and all females in the mid-dose group. The same dose group had bone marrow that was depleted of erythroid and myeloid cells. In four of five males and one female the thymus was depleted of lymphocytes (IARC 1993).
In a 13-week study performed by the US NTP, Acid Red 114 was administered to rats (10/group) at 0, 86, 171, 357, 714, or 1428 mg/kg-bw/day2 in the drinking water. In groups receiving greater than or equal to 171 mg/kg-bw/day, body weights were reduced (85–94% of control weight). Relative weights of the liver were increased in all exposed animals. Relative and absolute kidney weights were increased in females receiving 171 mg/kg-bw/day and above. There were also effects on blood parameters including decreased haematocrit, haemoglobin and erythrocyte counts in dosed females, and decreased erythrocytes in males receiving 357 mg/kg-bw/day and higher. Mild hepatocellular damage was suggested by increased enzyme levels, and this was confirmed by histopathological examinations. Other effects observed were pigmented Kupffer cells in the livers of treated females, increased incidence of reticulum-cell hyperplasia of the mesenteric lymph node in treated males and females. Chronic inflammation of the kidneys and tubular regeneration was observed more frequently in treated females than in controls (IARC 1993).
Characterization of Risk to Human Health
The potential for exposure of the general population to Acid Red 111 from environmental media is expected to be negligible. General population exposures to Acid Red 111 from consumer products (dyed textiles) are expected to be negligible due to the nature and limited range of uses.
Limited empirical health effects data were available to characterize the human health effects of Acid Red 111. However, health effects information on potential azo cleavage products and analogue azo dyes has been considered in the hazard characterization for human health. Although Acid Red 111 was not mutagenic in bacteria under reducing conditions in one test strain (TA98) in a single study, this result is not consistent with appropriately conducted mutagenicity results obtained for the 2,2'-DMB-based azo dye analogue Acid Red 99, mutagenicity studies on 2,2'-DMB itself, and data on a related 3,3'-DMB-based azo dye analogue Acid Red 114. Although neither Acid Red 111 nor its 2,2'-DMB-based azo analogue Acid Red 99 were tested for carcinogenicity, the 3,3'-DMB-based azo analogue, Acid Red 114, was carcinogenic in rats, inducing tumours in multiple organs in both sexes upon oral administration in the drinking water. Therefore, based on: (1) the likelihood of water soluble azo dyes such as Acid Red 111 to undergo azo cleavage to release component aromatic amines (i.e., 2,2'-DMB), (2) the positive mutagenicity results obtained for the two benzidine congeners (2,2'-DMB and 3,3'-DMB), (3) positive mutagenicity results for the analogue azo dyes based on these two benzidine congeners (i.e., Acid Red 99 and Acid Red 114, respectively), and (4) the evidence for carcinogenicity of Acid Red 114, a genotoxic and carcinogenic potential associated with Acid Red 111 is considered in the absence of adequate empirical data.
Although the potential high hazard of Acid Red 111 is recognized (i.e., genotoxicity, carcinogenicity), on the basis of information on confirmed uses in Canada as well as information that indicates exposure of the general population to this substance is expected to be negligible, the risk to human health is considered to be low.
Uncertainty in Evaluation of Risk to Human Health
Confidence in the toxicity database is considered low due to limited empirical health effects data available for Acid Red 111. In addition, no empirical information was available on the potential for Acid Red 111 to undergo azo cleavage, which is a major consideration in evaluating the health effects of azo compounds. In addition, the purity of Acid Red 111 used in commercial applications is unknown. Also, the scope of this screening assessment does not include a full analysis of the mode of action of Acid Red 111 or its analogues, nor does it take into account possible differences between humans and experimental species in sensitivity or potential differences in toxicity due to route of exposure. Furthermore, there is uncertainty surrounding the extrapolation of data on analogues to characterize the potential health effects associated with exposure to Acid Red 111.
The confidence in the exposure assessment is low due to lack of data. Although exposure to Acid Red 111 from consumer products may potentially occur from dyed textiles, the confirmed uses in Canada are for a limited range of specialty products, which would not be expected to result in direct and prolonged contact with the human skin or oral cavity. It is possible that the substance is entering the Canadian market as a component of imported consumer products not identified through the section 71 notice or through other information sources and this represents an uncertainty in the exposure assessment.
Based on the information available, it is concluded that Acid Red 111 is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends. Additionally, Acid Red 111 meets the criteria for persistence, but does not meet the criteria for bioaccumulation potential as set out in thePersistence and Bioaccumulation Regulations (Canada 2000).
Based on the currently available information on its potential to cause harm to human health, it is concluded that Acid Red 111 is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore concluded that Acid Red 111 does not meet any of the criteria under section 64 of CEPA 1999.
Because Acid Red 111 is listed on the Domestic Substances List, its import and manufacture in Canada are not subject to notification under subsection 81(1). Given the potential health hazards of this substance, there is concern that new activities that have not been identified or assessed could lead to this substance meeting the criteria set out in section 64 of the Act. Therefore, it is recommended to amend the Domestic Substances List, under subsection 87(3) of the Act, to indicate that subsection 81(3) of the Act applies with respect to this substance so that new manufacture, import or use of this substance is notified and undergoes ecological and human health risk assessments.
Considerations for Follow-up
Acid Red 111 belongs to a group of azo substances that may metabolize to aromatic amines, which as a chemical class are known to exhibit hazardous properties, including carcinogenicity. Therefore, additional activity (e.g., research, assessment, monitoring and surveillance) to characterize the risk to human health in Canada of this broader group of azo substances may be undertaken. A Notice of Intent outlining how the Government of Canada will address this group of substances is available on theChemical Substances website.
Anliker R, Clarke EA, Moser P. 1981. Use of the partition coefficient as an indicator of bioaccumulation tendency of dyestuffs in fish. Chemosphere 10(3):263-274.
Arnot JA, Arnot M, Mackay D, Couillard Y, MacDonald D, Bonnell M, Doyle P. 2010. Molecular size cut-off criteria for screening bioaccumulation potential: Fact or fiction? Integrated Environmental Assessment and Management 6(2):210–224.
Bafana A, Chakrabarti T, Muthal P, Kanade G. 2009. Detoxification of benzidine-based azo dye by E. gallinarum: Time-course study. Ecotoxicology and Environmental Safety 72:960-964.
Baughman GL, Weber EJ. 1994. Transformation of dyes and related compounds in anoxic sediment: kinetics and products. Environ Sci Technol 28(2):267-276.
[BIOWIN] Biodegradation Probability Program for Windows [Estimation Model]. 2008. Version 4.10. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Exposure Assessment Tools and Models.
Boethling RS, Howard PH, Beauman JA, Larosche ME. 1995. Factors for intermedia extrapolations in biodegradability assessment. Chemosphere 30(4):741-752.
Brown D, Anliker R. 1988. Dyestuffs and the environment – A risk assessment. Risk Assessment of Chemicals in the Environment. Richardson M (ed). The Royal Society of Chemistry. pp 398-413. As cited in: Danish EPA 1999.
Brown D, Hamburger B. 1987. The degradation of dyestuffs: Part III – Investigations of their ultimate degradability. Chemosphere 16(7):1539-1553. As cited in: Danish EPA 1999.
Canada. 1978. Food and Drug Regulations, C.R.C., c. 870.
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C., 1999, c. 33, Canada Gazette, Part III, Vol. 22, No. 3.
Canada. 2000. Canadian Environmental Protection Act, 1999: Persistence and Bioaccumulation Regulations, P.C. 2000-348, 23 March, 2000, SOR/2000-107, Canada Gazette, Part II, Vol. 134, No. 7.
Canada, Dept. of the Environment, Dept. of Health. 2006a.Canadian Environmental Protection Act, 1999: Notice of intent to develop and implement measures to assess and manage the risks posed by certain substances to the health of Canadians and their environment. Canada Gazette, Part I, Vol. 140, No. 49.
Canada, Dept. of the Environment, Dept. of Health. 2006b.Canadian Environmental Protection Act, 1999: Notice with respect to selected substances identified as priority for action. Canada Gazette, Part I, Vol. 140, No. 9.
Canada, Dept. of the Environment, Dept. of Health. 2008a.Canadian Environmental Protection Act, 1999: Notice of sixth release of technical information relevant to substances identified in the Challenge. Canada Gazette, Part I, Vol. 142, No. 22.
Canada, Dept. of the Environment. 2008b. Canadian Environmental Protection Act, 1999: Notice with respect to Batch 6 Challenge substances. Canada Gazette, Part I, Vol. 142, No. 22.
CASETOX [Prediction module]. 2008. Version 2.0. Beachwood (OH): MultiCASE Inc. CASETOX [restricted access].
[CATABOL] Probabilistic assessment of biodegradability and metabolic pathways [Computer Model]. c2004-2008. Version 5.10.2. Bourgas (BG): Bourgas Prof. Assen Zlatarov University, Laboratory of Mathematical Chemistry. CATALOGIC.
chemBlink [Database on the Internet]. 2009. Online database of chemicals around the world: Direct Black 38. Research Triangle Park [NC]: chemBlink Headquarters, Inc., (US). [cited 2009 March].ChemBlink.
ChemCAN [Level III fugacity model of 24 regions of Canada]. 2003. Version 6.00. Peterborough (ON): Trent University, The Canadian Centre for Environmental Modelling and Chemistry[cited 2010 Jan].
[ChemID] ChemIDplus Advanced [Database on the Internet]. 2010. Structure similarity search for CAS RN 6358-57-2. Bethesda (MD): National Library of Medicine. US Department of Health and Human Services. [cited April 2010].
Chen H. 2006. Recent advances in azo dye degrading enzyme research. Curr Protein Pept Sci 7(2):101–111.
Chung KT, Fulk GE, Andrews AW. 1981. Mutagenicity testing of some commonly used dyes. Appl Env Micro 42(4):641–648.
Chung KT, Chen SC, Claxton LD. 2006. Review of theSalmonella typhimurium mutagenicity of benzidine, benzidine analogues, and benzidine-based dyes. Mut Res 612(1):58–76.
[CHRIP] Chemical Risk Information Platform [database on the Internet]. c2011. Tokyo (JP): National Institute of Technology and Evaluation, Chemical Management Centre (CMC). [cited 2011 January].
[CII] Colour Index International [database on the Internet]. 2002– . 4th Ed. Research Triangle Park (NC): American Association of Textile Chemists and Colourists. [cited 2008 January].
Clariant. 2008. The New Clariant – Textile Dyestuff World, 3rdedition. [Internet]. Clariant International AG, Muttenz. Clariant Textile Business website. [cited 2008 Dec].
Clarke EA, Anliker R. 1980. Organic dyes and pigments. Handbook of Environmental Chemistry. Springer Verlag. As cited in: Danish EPA 1999.
[CPOPs] Canadian POPs Model. 2008. Gatineau (QC): Environment Canada, Existing Substances Division; Bourgas (BG): Bourgas Prof. Assen Zlatarov University, Laboratory of Mathematical Chemistry. [Model developed based on Mekenyan et al. 2005]. Available from: Environment Canada, Ecological Assessment Division.
[Danish EPA] Danish Environmental Protection Agency. 1999.Survey of azo-colorants in Denmark. Consumption, use, health and environmental aspects. MiljØprojekt No. 509. Henriette, Danish Technological Institute, Environment, Ministry of Environment and Energy, Denmark, Danish Environmental Protection Agency, 1999. Report prepared by Øllgaard H, Frost L, Galster J, Hansen OC.
[DEREK] Deductive Estimation of Risk from Existing Knowledge [Prediction module on CD ROM]. 2008. Version 10.0.2. Cambridge (MA): Harvard University, LHASA Group. Toxicology Prediction [restricted access]
Dimitrov SD, Dimitrova NC, Walker JD, Veith GD, Mekenyan OG. 2002. Predicting bioconcentration factors of highly hydrophobic chemicals. Effects of molecular size. Pure Appl Chem 74(10):1823-1830.
Dimitrov S, Dimitrova N, Parkerton T, Comber M, Bonnell M, Mekenyan O. 2005. Base-line model for identifying the bioaccumulation potential of chemicals. SAR QSAR Environ Res 16(6):531–554.
[DPD] Drug Product Database [database on the internet]. 2010. Ottawa (ON): Health Canada. [cited 2010 June].
Elliott J. 1980. Mutagenicity of a series of benzidine congener based dyes. Vet Hum Toxicol 22(6):413–417.
Environment Canada. 1988. Data relating to the Domestic Substances List (DSL) 1984-1986, collected under CEPA, 1988, s. 25(1). Based on: Reporting for the Domestic Substances List [guide] 1988. Data compiled by: Environment Canada, Gatineau, QC.
Environment Canada. 2000. Environmental categorization for persistence, bioaccumulation and inherent toxicity of substances on the Domestic Substances List using QSARs. Unpublished final report. Gatineau (QC): Environment Canada, Existing Substances Division. On the cover: Results of an international QSAR workshop hosted by the Chemicals Evaluation Division of Environment Canada, November 11-12, 1999, Philadelphia , Pennsylvania.
Environment Canada. 2006. Data for selected substances collected under the Canadian Environmental Protection Act, 1999, Section 71:Notice with respect to selected substances identified as priority for action. Data compiled by: Environment Canada, Program Development and Engagement Division.
Environment Canada. 2007. Guidance for Conducting Ecological Assessments under CEPA, 1999: Science Resource Technical Series, Technical Guidance Module: QSARs. Reviewed Draft Working Document. Gatineau (QC): Environment Canada, Ecological Assessment Division.
Environment Canada. 2008a. Data for Batch 6 substances collected under the Canadian Environmental Protection Act, 1999, Section 71:Notice with respect to certain Batch 6 Challenge substances. Data compiled by: Environment Canada, Program Development and Engagement Division.
Environment Canada. 2008b. Guidance for conducting ecological assessments under CEPA, 1999: Science Resource Technical Series, Technical Guidance Module: Mass Flow Tool. Working document. Gatineau (QC): Environment Canada, Ecological Assessment Division.
Environment Canada. 2009a. Assumptions, limitations and uncertainties of the mass flow tool for Acid Red 111, CAS RN 6358-57-2. Internal draft document. Gatineau (QC): Environment Canada, Ecological Assessment Division.
Environment Canada. 2009b. Guidance for Conducting Ecological Assessments under CEPA, 1999: Science Resource Technical Series, Technical Guidance Module: The Industrial Generic Exposure Tool – Aquatic (IGETA). Working document. Gatineau (QC): Environment Canada, Ecological Assessment.
Environment Canada. 2009c. IGETA (Industrial Generic Exposure Tool – Aquatic) Report: CAS RN 6358-57-2. Internal draft document. Gatineau (QC): Environment Canada, Ecological Assessment Division.
Environment Canda. 2010. National Pollutant Release Inventory [NPRI; database on the Internet]. Gatineau (QC): Environment Canada.
[EPIsuite] Estimation Programs Interface Suite for Microsoft Windows [Estimation Model]. 2008. Version 4.0 Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Exposure Assessment Tools and Models.
[ESIS] European chemical Substances Information System [database on the Internet]. c1995 – 2010. Information for CAS RN 1937-37-7 and 6358-57-2. Ispra [IT]: European Commission, Joint Research Centre, Institute for Health and Consumer Protection, European Chemicals Bureau (ECB). [cited 2008 April].
[ETAD] Ecological and Toxicological Association of Dyes and Organic Pigments Manufacturers. 1992. ETAD project E3020-data summary (disperse dyes). Unpublished results summary. Submitted to Environment Canada, Existing Substances Division by ETAD in May 2008.
[ETAD] Ecological and Toxicological Association of Dyes and Organic Pigments Manufacturers. 1995. Health & Environmental Information on Dyes Used in Canada. An overview to assist in the implementation of the New Substances Notification Regulation under the Canadian Environmental Protection Act. Prepared by the ETAD Canadian Affiliates. July 1995. Report 7/21/95
Freeman HS, Esancy MK, Esancy JF, Claxton LD. 1991. Color, yes. Cancer, no. Chemtech 21(7):438–445.
Golka K, Kopps S, Myslak ZW. 2004. Carcinogenicity of azo colorants: influence of solubility and bioavailabilty. Toxicol Lett 151(1):203–210.
Gregory AR, Elliot J, Kluge P. 1981. Ames testing of Direct Black 38 parallels carcinogenicity testing. J Appl Toxicol 1(6):308–313.
Health Canada. 1994. Canadian Environmental Protection Act: Human Health Risk Assessments for Priority Substances. Ottawa, ON. Minister of Supply and Services Canada.
[HENRYWIN] Henry’s Law Constant Program for Microsoft Windows [Estimation Model]. 2008. Version 3.20. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Exposure Assessment Tools and Models.
Hinks D, Freeman HS, Nakpathom M, Sokolowska J. 2000. Synthesis and evaluation of organic pigments and intermediates. 1. Nonmutagenic benzidine analogs. Dyes and Pigments 44:199–207.
Hunger K. 2005. Toxicology and toxicological testing of colorants. Review of Progress in Coloration 35:76-89.
[IARC] International Agency for Research on Cancer. 1993. Occupational Exposures of Hairdressers and Barbers and Personal use of Hair Colorants; Some Hair Dyes, Cosmetic Colorants, Industrial Dyestuffs and Aromatic Amines. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 57. Lyon, France. p. 247–257.
Isik M, Sponza DT. 2004. Monitoring of toxicity and intermediates of C.I. Direct Black 38 azo dye through decolourization in an anaerobic/aerobic sequential reactor system. Journal of Hazardous Materials B114: 29-39.
Little LW, Lamb III JC. 1972. Acute toxicity of 46 selected dyes to the fathead minnow, Pimephales promelas. Chapel Hill (NC): The University of North Carolina, Department of Environmental Sciences and Engineering, School of Public Health, UNC Wastewater Research Center. Prepared for Ecology Committee, American Dye Manufacturers, Inc. September 1972. 126 pp.
[LNHPD] Licensed Natural Health Products Database. 2010. Ottawa (ON): Health Canada. [cited 2010 June].
Mekenyan G, Dimitrov SD, Pavlov TS, Veith GD. 2005. POPs: a QSAR system for creating PBT profiles of chemicals and their metabolites. SAR QSAR Environ Res 16(1-2):103-133. As cited in: CPOPs 2008.
[MITI] Ministry of International Trade & Industry (Jpn), Basic Industries Bureau, Chemical Products Safety Division. 1992. Biodegradation and bioaccumulation data of existing chemicals based on the CSCL Japan. Tokyo (Jpn): Japan Chemical Industry Ecology-Toxicology & Information Centre.
[MSDS] Material Safety Data Sheet [Internet]. 2003. Material Safety Data Sheet for Navacid Acid Scarlet FGW. S.M.S. Technology Co., Ltd. I.N.T. International Inc. [cited 2009 March].
[NCI] National Chemical Inventories [database on a CD-ROM]. 2007. Issue 1. Columbus (OH): American Chemical Society, Chemical Abstracts Service. [cited 2009 January]. National Chemical Inventory (NCI) System Requirements.
[NHPID] Natural Health Products Ingredients Database. 2010. Ottawa (ON): Health Canada. [cited 2010 June].
[NIEHS] National Institute of Environmental Health Sciences. 2008. CAS Registry Number 1937-37-7. Research Triangle Park (NC): National Toxicology Program, US Department of Health and Human Services. Last updated May 14, 2008. [cited 2009 March]. Testing Status of Agents at NTP: CAS No. 1937-37-7.
[NLM] National Library of Medicine (US). 2006. Hazardous Substances Data Bank. 2006. [database on the Internet].
[NTP] National Toxicology Program (US). 1991a. Toxicology and carcinogenesis studies of C.I. Acid Red 114 (CAS No. 6459-94-5) in F344/N rats (drinking water studies). Technical Report Series No 405. Research Triangle Park, (NC): US Department of Health and Human Services, Public Health Service, National Toxicology Program.
[NTP] National Toxicology Program (US). 1991b. Toxicology and carcinogenesis studies of 3,3'-dimethylbenzidine dihydrochloride in F344/N rats (drinking water studies). Technical Report Series No 390. 231 pp. Research Triangle Park, (NC): US Department of Health and Human Services, Public Health Service, National Toxicology Program.
[OECD] Organisation for Economic Co-operation and Development. 2004. Emission Scenario Document on Plastics Additives [Internet]. Paris (FR): OECD Environment Directorate, Environmental Health and Safety Division. ENV/JM/MONO(2004)8, JT00166678. [cited 2008 February]. Official OECD Documents.
[OECD] Organisation for Economic Co-operation and Development. 2007. Emission scenario document on adhesive formulation [Internet]. Final report. Paris (FR): OECD, Environment Directorate. Series on Emission Scenario Documents. [cited 2008 August].
Pagga U, Brown D. 1986. The degradation of dyestuffs: Part II – Behaviour of dyestuffs in aerobic biodegradation tests. Chemosphere 15(4):479-491.
Palmer KA, Sheu CW, Green S. 1979. Mutagenicity studies of R-amino salt, a metabolite of amaranth (FD&C Red No. 2) in mouse lymphoma cells heterozygous at the thymidine kinase locus and in the rat dominant lethal test. Fd Cosmet Toxicol 17:5-9.
Platzek T, Lang C, Grohmann G, Gi US, Baltes W. 1999. Formation of a carcinogenic aromatic amine from an azo dye by human skin bacteria in vitro. Hum Exp Toxicol 18(9):552-559.
Prival MJ, Mitchell VD. 1982. Analysis of a method for testing azo dyes for mutagenic activity in Salmonella typhimuriumin the presence of flavin mononucleotide and hamster liver S9. Mutat Res 97(2):103-116.
Razo-Flores E, Luijten M, Donlon BA, Lettinga G, Field JA. 1997. Complete biodegradation of the azo dye azodisalicylate under anaerobic conditions. Environ Sci Technol 31:2098-2103. As cited in: Danish EPA 1999.
Reifferscheid G, Heil J. 1996. Validation of the SOS/umu test using test results of 486 chemicals and comparison with the Ames test and carcinogenicity data. Mutat Res 369:129-145.
Rosi Chemical [Internet]. 2000. Company web site. Web page listing solubility information for various dyes. Rosi Chemical Co., Ltd. Yueqing City, Zhejiang Province, China. [cited 2009 March].
Sakuratani Y, Noguchi Y, Kobayashi K, Yamada J, Nishihara T. 2008. Molecular size as a limiting characteristic for bioconcentration in fish. J Environ Biol. 29(1):89-92.
Scifinder, Web Version. 2010. Chemcal Abstracts Service; Columbus, OH. [cited April 2010].
[SPIN] Substances in Preparations in Nordic Countries [database on the Internet]. 2008. Copenhagen (DK): Nordic Council of Ministers. [cited 2008 December].
Stingley RL, Zou W, Heinze TM, Chen H, Cerniglia CE. 2010. Metabolism of azo dyes by human skin microbiota. J Med Microbiol 59(Pt.1):108-114.
Study Submission. 2005. Unpublished study submitted to Environment Canada under the Chemicals Management Plan Challenge initiative. Toxicity Data for 71215-83-3. Gatineau (QC): Environment Canada, Program Development and Engagement Division.
Study Submission. 2007. Unpublished study submitted to Environment Canada under the Chemicals Management Plan Challenge initiative. Material Safety Data Sheet for Lanasyn Scarlet F-3GL 103. Gatineau (QC): Environment Canada, Program Development and Engagement Division.
[TOPKAT] Toxicity Prediction Program [Internet]. 2004. Version 6.2. San Diego (CA): Accelrys Software Inc.
[US EPA] United States Environmental Protection Agency. 2002. TSCA (Toxic Substances Control Act) New Chemicals Program (NCP) Chemical Categories. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics. October, 2002. pp. 2-4 (revised June, 1994). 145 p. Chemical Categories Report.
[US EPA] United States Environmental Protection Agency. 2005. Inventory Update Reporting program. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics.
Venturini S, Tamaro M. 1979. Mutagenicity of anthraquinone and azo dyes in ames Salmonella typhimurium test. Mutat Res 68:307-312.
Yen CC, Perenich TA, Baughman GL. 1991. Fate of commercial disperse dyes in sediments. Environ Toxicol Chem 10:1009-1017.
Zhou Y, You X, Ye, X. 1987. Mutagenicity of benzidines and their congener derivative dyes. Huanjing Kexue 8(2):31– 34
Carcinogenicity
| Substance Identity | DEREK (2008) | CASETOX (2008) | TOPKAT (2004) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cancer | m-rat | f-rat | m-mice | f-mice | NTP Rodent | NTP m-rat |
NTP f-rat |
NTP m-mouse |
NTP f-mouse |
|
| Acid Red 111 (CAS RN 6358-57-2) |
P | P | P | P | IC | IC | IC | P | IC | ND |
| 2,2'-DMB (CAS RN 84-67-3) |
P | N | N | N | N | IC | P | P | IC | P |
| 2-amino-naphthol-3,6-disulphonic acid (CAS RN 42579-07-7) |
ND | P | IC | IC | IC | IC | N | IC | IC | IC |
| Metabolite 2 (no CAS RN) |
ND | N | N | P | N | IC | IC | IC | N | IC |
CAS RN – Chemical Abstracts Registry Number; IC – inconclusive ; m – male; ND – not in domain of model ; f – female; ChrAb – chromosomal aberration ; NTP – National Toxicology Program; CT – CASETOX; P – positive; TK – TOPKAT; N – negative; # – in vitro test (in cultured CHO cells)
Genotoxicity
| Substance Identity | Ames | ChrAb | Micronuclei Induction | Mouse Lymphoma mutation | |||
|---|---|---|---|---|---|---|---|
| Derek | CT | TK | Derek | CT# | CT | CT | |
| Acid Red 111 (CAS RN 6358-57-2 ) | ND | IC | ND | ND | P | IC | IC |
| 2,2'-DMB (CAS RN 84-67-3) | P | N | P | ND | P | N | P |
| 2-amino-naphthol-3,6-disulphonic acid - (CAS RN 42579-07-7) |
ND | N | P | ND | P | N | IC |
| Metabolite 2 (no CAS RN) | ND | N | P | ND | IC | IC | P |
CAS RN – Chemical Abstracts Registry Number; IC – inconclusive ; m – male; ND – not in domain of model ; f – female; ChrAb – chromosomal aberration ; NTP – National Toxicology Program; CT – CASETOX; P – positive; TK – TOPKAT; N – negative; # – in vitro test (in cultured CHO cells)
Footnotes
[1] The Chemical Abstracts Service Registry Number (CAS RN) is the property of the American Chemical Society and any use or redistribution, except as required in supporting regulatory requirements and/or for reports to the government when the information and the reports are required by law or administrative policy, is not permitted without the prior, written permission of the American Chemical Society.
[2] A determination of whether one or more of the criteria of section 64 are met is based upon an assessment of potential risks to the environment and/or to human health associated with exposures in the general environment. For humans, this includes, but is not limited to, exposures from ambient and indoor air, drinking water, foodstuffs, and the use of consumer products. A conclusion under CEPA 1999 on the substances in the Chemicals Management Plan (CMP) Challenge is not relevant to, nor does it preclude, an assessment against the hazard criteria specified in the Controlled Products Regulations, which is part of the regulatory framework for the Workplace Hazardous Materials Information System (WHMIS) for products intended for workplace use. Similarly, a conclusion based on the criteria contained in section 64 of CEPA 1999 does not preclude actions being taken under other sections of CEPA or other Acts.
[3] Doses converted using Health Canada reference values (Health Canada 1994).